Optimization of Ultrasound-Assisted Extraction and Decolorization Process of Polysaccharides from Mori fructus and Its Antioxidant Activity
-
摘要: 本文研究桑葚多糖超声提取工艺、树脂脱色工艺和体外抗氧化活性。以桑葚粗多糖得率为指标,通过单因素实验、正交试验考察超声提取温度、料液比、超声时间、超声功率的影响;以脱色率为指标,通过单因素实验、正交试验考察脱色时间、多糖溶液浓度、脱色温度的影响;通过ABTS法、DPPH法、邻二氮菲法、邻苯三酚法考察其抗氧化能力。结果表明,超声提取桑葚多糖的最佳工艺为:超声温度50 ℃、料液比1:30 g/mL、超声时间70 min、超声功率500 W,该条件下多糖得率为4.59%±0.25%;AB-8大孔吸附树脂脱色的最佳工艺为:脱色时间5 h、桑葚粗多糖溶液浓度4 mg/mL、脱色温度25 ℃,该条件下脱色率为62.34%±1.27%;桑葚多糖清除ABTS+自由基、DPPH自由基、羟基自由基和超氧阴离子自由基的IC50分别为0.14、0.68、0.19和3.14 mg/mL。Abstract: The ultrasound-assisted extraction, resin decolorization process and antioxidant activity of polysaccharides from Mori fructus (MFPs) were investigated in this work. The effects of ultrasonic extraction temperature, solid-liquid ratio, ultrasonic time and ultrasonic power on extraction yield of MFPs were investigated by single factor and orthogonal test. Taking decolorization rate as index, the effects of decolorization time, concentration of polysaccharide solution and decolorization temperature were investigated by single factor and orthogonal test. ABTS assay, DPPH assay, phenanthroline method and pyrogallol autoxidation reaction were used to evaluate the antioxidant activity in vitro. The results showed that the optimal ultrasonic extraction conditions were ultrasonic temperature 50 ℃, solid-liquid ratio 1:30 g/mL, ultrasonic time 70 min and ultrasonic power 500 W. Under these conditions, the extraction yield of MFPs was 4.59%±0.25%. The optimal decolorization conditions were decolorization time 5 h, concentration of MFPs solution 4 mg/mL and decolorization temperature 25 ℃. Under these conditions, the decolorization rate was 62.34%±1.27%. MFPs had strong scavenging ability on ABTS+ radical, DPPH radical, hydroxyl radical and superoxide anion radical with half inhibitory concentration (IC50) of 0.14, 0.68, 0.19 and 3.14 mg/mL, respectively.
-
桑葚(Mori fructus)是桑科(Moraceae)落叶乔木桑树的成熟果实,广泛分布于热带、亚热带和温带地区,富含氨基酸、碳水化合物、脂肪、维生素和矿物质等营养成分[1–3]。桑葚是一种食药同源水果,常作新鲜食用或制成果汁、果酒、果酱等产品,美味且营养丰富[4]。研究表明[5-6],桑葚具有治疗糖尿病、高血压、肝肾损伤以及抗炎、抗菌、抗肿瘤、神经保护、抗衰老、调节肠道菌群等功效,这使桑葚的食药需求不断增加。桑葚多糖是其重要的生物活性成分,具有治疗肝脏损伤[7–10]、免疫调节[11-12]、降血糖[13-14]、治疗心血管疾病[15]等多种生物活性。
目前,桑葚原料浪费、加工利用率不高等问题严重,难以发挥我国丰富的桑葚资源优势[16-17],研究和优化桑葚多糖提取工艺,对延长桑葚产业链,提高其附加值具有重要意义。超声波辅助提取基于其“空化效应”促进可溶性成分溶出,提高提取效率[18],已被广泛运用于天然活性物质的提取。与连续超声处理相比,间歇性超声处理具有产量更高的优势[19]。曹荣景等[20]于超声辅助提取后测得提取液中桑葚多糖含量为17.78%,Chen等[2]和祝新媛[21]分别使用超声波清洗器水提醇沉制备桑葚多糖的得率为3.13%±0.07%和3.64%±0.22%,提取液中小分子糖对测定结果的影响明显,目前未见采用超声波辅助提取兼醇沉制备桑葚多糖得率更高的报道。水提醇沉法得到的桑葚粗多糖色泽深重,给后续的分离纯化、活性鉴定带来困难,也影响其作为功能产品的外观品质[22]。多糖脱色方法主要有活性炭法、双氧水法和树脂法,前两种方法存在脱色效果差和破坏多糖结构的问题[23],大孔树脂因其强大的吸附能力、良好的保留能力、易于再生和环境友好等特点[24-25]得到越来越多的使用,目前关于桑葚多糖树脂法脱色的工艺研究罕见报道。天然来源的活性多糖具有清除自由基的作用,而其活性受到提取工艺的影响[26-27]。
本研究使用超声波细胞粉碎机代替传统超声波清洗器,采用“工作1 s/间歇1 s”循环脉冲式超声方式提高破碎效果,旨在优化超声波辅助提取桑葚多糖和树脂法脱色的工艺条件,提高桑葚多糖得率,并通过体外自由基清除实验评估其抗氧化活性,以期为桑葚多糖产业化的建立和发展提供数据支持,为桑葚作为功能性食品和天然来源药物的进一步开发利用提供实验依据。
1. 材料与方法
1.1 材料与仪器
桑葚冻干粉 西安东峰生物科技有限公司;浓硫酸、盐酸、苯酚、葡萄糖 南京化学试剂有限公司;石油醚、无水乙醇 无锡市亚盛化工有限公司;氢氧化钠 西陇化工股份公司;XDA-8、NKA-9、AB-8、D101、D301G树脂 上海源叶生物科技有限公司;氯仿、正丁醇 上海凌峰化学试剂有限公司;2,2-联氮-二(3-乙基-苯并噻唑-6-磺酸)二铵盐(ABTS) 美国Sigma试剂公司;1,1-二苯基-2-三硝基苯肼(DPPH) 索莱宝科技有限公司;邻二氮菲 梯希爱(上海)化成工业发展有限公司;邻苯三酚 上海贤鼎生物科技有限公司;三(羟甲基)氨基甲烷 生工生物工程(上海)股份有限公司;L(+)-抗坏血酸、七水合硫酸亚铁、过硫酸钾 国药集团化学试剂有限公司。
JY29-ⅡN超声波细胞粉碎机 宁波新芝生物科技有限公司;LXJ-Ⅱ型低速大容量多管离心机 上海安亭科学仪器厂;HZQ-F160全温振荡培养箱 苏州培英实验设备有限公司;PD-1C-50真空冷冻干燥机 北京博医康实验仪器有限公司;DZF-6050型真空干燥箱 上海博迅实业有限公司;ATX224分析天平 岛津有限公司;RE-5205旋转蒸发仪 上海亚荣生化仪器厂;TU-1901双光束紫外可见分光光度计 北京普析通用仪器有限公司。
1.2 实验方法
1.2.1 桑葚多糖超声提取和脱色工艺
冻干桑葚粉→石油醚脱脂→烘干→脱脂桑葚粉→超声提取→离心过滤→旋蒸浓缩→醇沉→真空干燥→桑葚粗多糖→重溶→脱色→脱蛋白→醇沉→透析→冻干→精制桑葚多糖。
称取400 g桑葚冻干粉置于2 L石油醚中,室温下搅拌3 h,重复2次,烘干得到脱脂桑葚粉[28]。称取一定质量的脱脂桑葚粉于烧杯中,加入一定量的蒸馏水,于超声细胞粉碎机中进行超声提取。提取完毕后在4000 r/min条件下离心5 min,抽滤得上清液,50 ℃旋转蒸发浓缩至1/4体积,冷却至室温后加入4倍体积工业乙醇,4 ℃醇沉过夜。4000 r/min离心5 min收集沉淀,用无水乙醇洗涤后放入50 ℃真空干燥箱中干燥5 h,即为桑葚粗多糖[29]。称取桑葚粗多糖配置一定浓度的桑葚粗多糖溶液,以1:2 g/mL固液比加入树脂,于摇床150 r/min静态脱色,收集脱色液[30]。Sevag法去除蛋白,醇沉,收集沉淀,透析,冷冻真空干燥,得到精制桑葚多糖。
1.2.2 多糖得率测定
绘制葡萄糖标准曲线[31]:精确配制0.1 mg/mL的葡萄糖标准溶液,分别吸取0、0.2、0.4、0.6、0.8、1.0 mL葡萄糖母液于试管中,用蒸馏水补足1.0 mL,加入1.0 mL苯酚溶液(5%)和5.0 mL浓硫酸,涡旋振荡混合,室温下静置反应30 min后,于490 nm处测量吸光值。
y=9.3921x+0.0495(R2=0.9999) (1) 式中:y为490 nm处的吸光值;x为葡萄糖的浓度,mg/mL;决定系数R2=0.9999。
准确称取适量桑葚粗多糖,加蒸馏水溶解,配制成0.1 mg/mL的桑葚粗多糖溶液,按上述操作测量吸光度,计算得桑葚粗多糖中的总糖含量,公式(2):
Y(%)=(A−0.0495)×109.3921×100 (2) 式中:Y为桑葚粗多糖中的总糖含量,%;A为样品溶液反应后在490 nm处的吸光值。
计算桑葚粗多糖得率,公式(3):
多糖得率(%)=Y×M2M1×100 (3) 式中:Y为桑葚粗多糖中的总糖含量,%;M2为真空干燥后桑葚粗多糖的重量,g;M1为所称取脱脂桑葚粉的重量,g。
1.2.3 超声提取工艺优化
1.2.3.1 超声提取单因素实验
分别考察不同超声提取温度、料液比、超声时间、超声功率对桑葚粗多糖得率的影响。固定料液比1:25 g/mL、超声时间30 min、超声功率400 W不变,考察超声温度分别为30、40、50、60、70 ℃对桑葚粗多糖得率的影响;固定超声温度50 ℃、超声时间30 min、超声功率400 W不变,考察料液比分别为1:10、1:20、1:25、1:30、1:40、1:50 g/mL对桑葚粗多糖得率的影响;固定超声温度50 ℃、料液比1:25 g/mL、超声功率400 W不变,考察超声时间分别为20、30、40、50、60、70、80 min对桑葚粗多糖得率的影响;固定超声温度50 ℃、料液比1:25 g/mL、超声时间60 min不变,考察超声功率分别为200、300、400、500、600、700 W对桑葚粗多糖得率的影响。
1.2.3.2 超声提取正交优化试验
依据单因素实验结果,采用L9(34)正交设计表进行正交优化,见表1。
表 1 超声提取工艺正交试验因素水平设计Table 1. Factors and levels of orthogonal experiments for ultrasonic extraction水平 因素 A超声温度
(℃)B料液比
(g/mL)C超声时间
(min)D超声功率
(W)1 40 1:20 50 400 2 50 1:25 60 500 3 60 1:30 70 600 1.2.4 桑葚多糖树脂法脱色工艺优化
1.2.4.1 不同树脂脱色性能筛选
选取5种不同极性的大孔吸附树脂,分别进行预处理。采用静态吸附法,分别称取10 g湿树脂于50 mL锥形瓶中,加入20 mL浓度为2 mg/mL桑葚粗多糖溶液,置于摇床,转速为150 r/min,温度为25 ℃,振荡脱色12 h。脱色前后,在513 nm处测量溶液吸光值,并计算脱色率[22],公式(4):
多糖脱色率(%)=A1−A2A1×100 (4) 式中:A1为脱色前513 nm处的吸光值;A2为脱色后513 nm处吸光值。
使用硫酸苯酚法,在490 nm处测量脱色前后桑葚粗多糖溶液反应显色后的吸光值,并计算多糖保留率[22],公式(5):
多糖保留率(%)=A2−0.0495A1−0.0495×100 (5) 式中:A1为脱色前反应液490 nm处的吸光值;A2为脱色后反应液490 nm处的吸光值。
1.2.4.2 脱色单因素实验
分别考察不同脱色时间、桑葚粗多糖溶液浓度、脱色温度对AB-8大孔树脂脱色效果的影响。固定桑葚粗多糖溶液浓度2 mg/mL、脱色温度25 ℃不变,考察脱色时间分别为0、0.25、0.5、0.75、1、2、3、4、5、12、17 h对多糖脱色率的影响;固定脱色时间4 h、脱色温度25 ℃不变,考察桑葚粗多糖溶液浓度分别为1、2、3、4、5、6 mg/mL对多糖脱色率的影响;固定脱色时间4 h、桑葚粗多糖溶液浓度3 mg/mL不变,考察脱色温度分别为20、25、30、40、50 ℃对多糖脱色率的影响。
1.2.4.3 脱色正交优化试验
依据单因素实验结果,采用L9(34)正交设计表进行正交优化,见表2。
表 2 脱色工艺正交试验因素水平设计Table 2. Factors and levels of orthogonal experiments for decolorization process水平 因素 A 脱色时间
(h)B 多糖溶液浓度
(mg/mL)C 脱色温度
(℃)1 3 2 25 2 4 3 30 3 5 4 35 1.2.5 抗氧化活性测定
桑葚粗多糖经树脂法脱色、Sevag法脱蛋白后再次加入4倍体积工业乙醇醇沉过夜,透析、冻干后得到桑葚多糖。分别配制不同浓度的桑葚多糖溶液和抗坏血酸(VC)溶液,其中VC作为阳性对照。
1.2.5.1 ABTS自由基清除率
参考文献方法[32-33],称取19.45 mg ABTS和7.58 mg过硫酸钾,分别准确配制5 mL溶液,按1:1混合后于4 ℃冰箱避光反应16 h,将其稀释至在734 nm处吸光值为0.700±0.020。移取2 mL样品溶液,加入2 mL ABTS工作液混合均匀,室温避光反应20 min,于波长734 nm处测吸光值。ABTS自由基清除公式为:
清除率(%)=(1−AiAc)×100 (6) 式中:Ai为加入样品溶液组的吸光值;Ac为以蒸馏水代替样品溶液的吸光值。
1.2.5.2 DPPH自由基清除率
参考文献[34-35]方法,移取2 mL样品溶液,加入0.1 mmol/L DPPH溶液2 mL,振荡混匀,室温下避光反应30 min,于波长517 nm处测定吸光值。DPPH自由基清除公式为:
清除率(%)=(1−Ai−AjAc)×100 (7) 式中:Ai为加入样品溶液组的吸光值;Aj为2 mL样品溶液加2 mL乙醇的吸光值;Ac为2 mL DPPH溶液加2 mL乙醇的吸光值。
1.2.5.3 羟基自由基清除率
参考文献[36-37]方法,移取1 mL样品溶液,加入0.1 mol/L磷酸盐缓冲液(pH7.4)1 mL、0.75 mmol/L硫酸亚铁溶液1 mL和0.75 mmol/L邻二氮菲溶液1 mL后混合均匀,加入1 mL体积分数为0.03%的H2O2溶液,振荡混匀,37 ℃水浴60 min,于波长536 nm处测定吸光值。羟基自由基清除公式为:
清除率(%)=Ai−AjAc−Aj×100 (8) 式中:Ai为加入样品溶液组的吸光值;Aj为以蒸馏水代替样品溶液的吸光值;Ac为以蒸馏水代替样品溶液和H2O2溶液的吸光值。
1.2.5.4 超氧阴离子自由基清除率
参考文献[38-39]方法,移取1 mL样品溶液,加入25 ℃预热的50 mmol/L Tris-HCl溶液(pH8.2)4 mL和10 mmol/L邻苯三酚溶液0.2 mL混匀,反应4 min后加入1滴浓盐酸终止反应,于波长325 nm处测定吸光值。超氧阴离子自由基清除公式为:
清除率(%)=Ac−AiAc×100 (9) 式中:Ai为加入样品溶液组的吸光值;Ac为以蒸馏水代替样品溶液的吸光值。
1.3 数据处理
所有实验均重复三次,结果用平均值±标准差表示,采用Origin 2021进行数据处理,采用SPSS 22进行显著性分析。
2. 结果与分析
2.1 超声提取单因素实验结果
2.1.1 超声温度选择
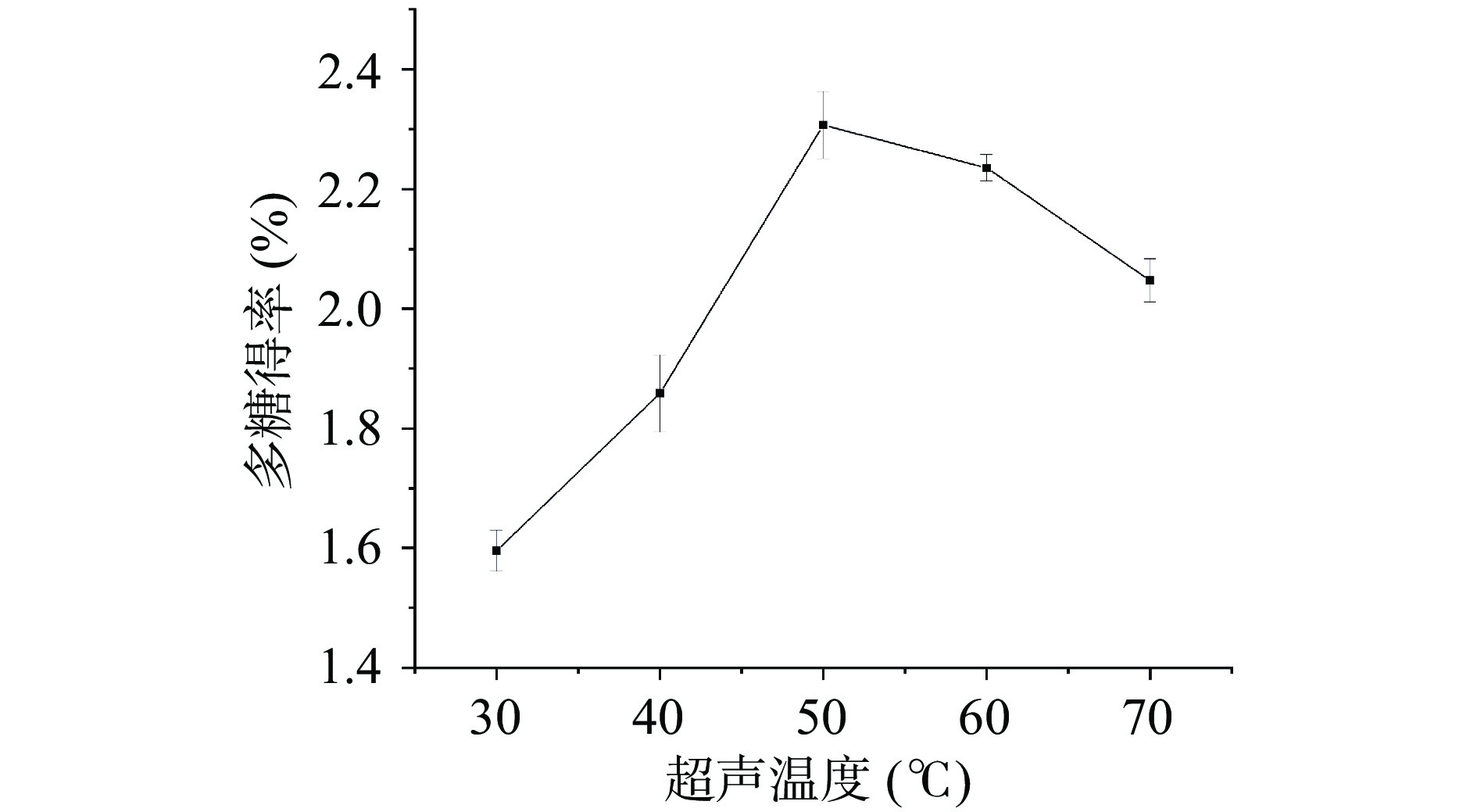
超声提取温度对桑葚粗多糖得率影响的实验结果如图1所示。随着超声提取温度的升高,桑葚粗多糖的得率逐渐上升,当超声提取温度为50 ℃时,得率达到最大值2.31%±0.06%;而后随温度继续升高,桑葚粗多糖得率开始下降。提取温度升高可增加多糖在溶剂中的溶解度和扩散系数,提高多糖得率[40],但温度过高时,可能破坏多糖结构,易引起多糖分解,不利于多糖提取。陈成等[41]和王杉杉等[42]采用超声辅助提取五味子多糖和枸杞多糖时也有类似结论。因此,选择超声温度50 ℃进行后续实验。
2.1.2 料液比选择
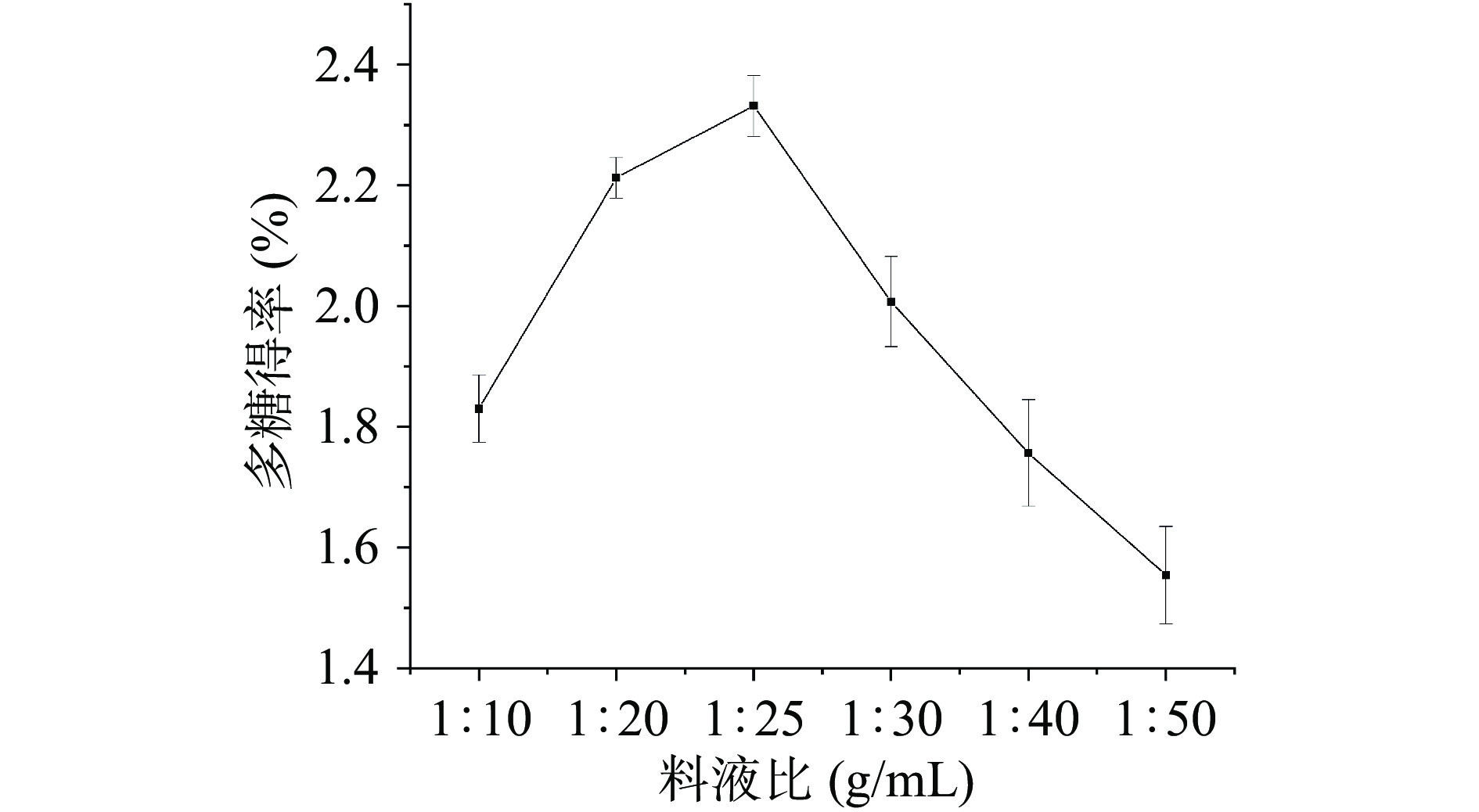
料液比对桑葚粗多糖得率影响的实验结果见图2。随着料液比的增大,桑葚多糖的得率呈上升趋势,当料液比达到1:25 g/mL时,有最高得率为2.33%±0.05%,而后得率随料液比的增大而下降。桑葚多糖为水溶性物质,随溶剂增加,固液相中的浓度差增大,多糖溶出速率增大,但当料液比过大时,超声处理的空化效应和机械振动效果受到影响,从而导致多糖得率下降[43]。帅良等[44]和冉俊枫等[45]在提取百香果果皮多糖和苦笋壳多糖时也有类似现象。因此,选择料液比1:25 g/mL进行后续实验。
2.1.3 超声时间选择
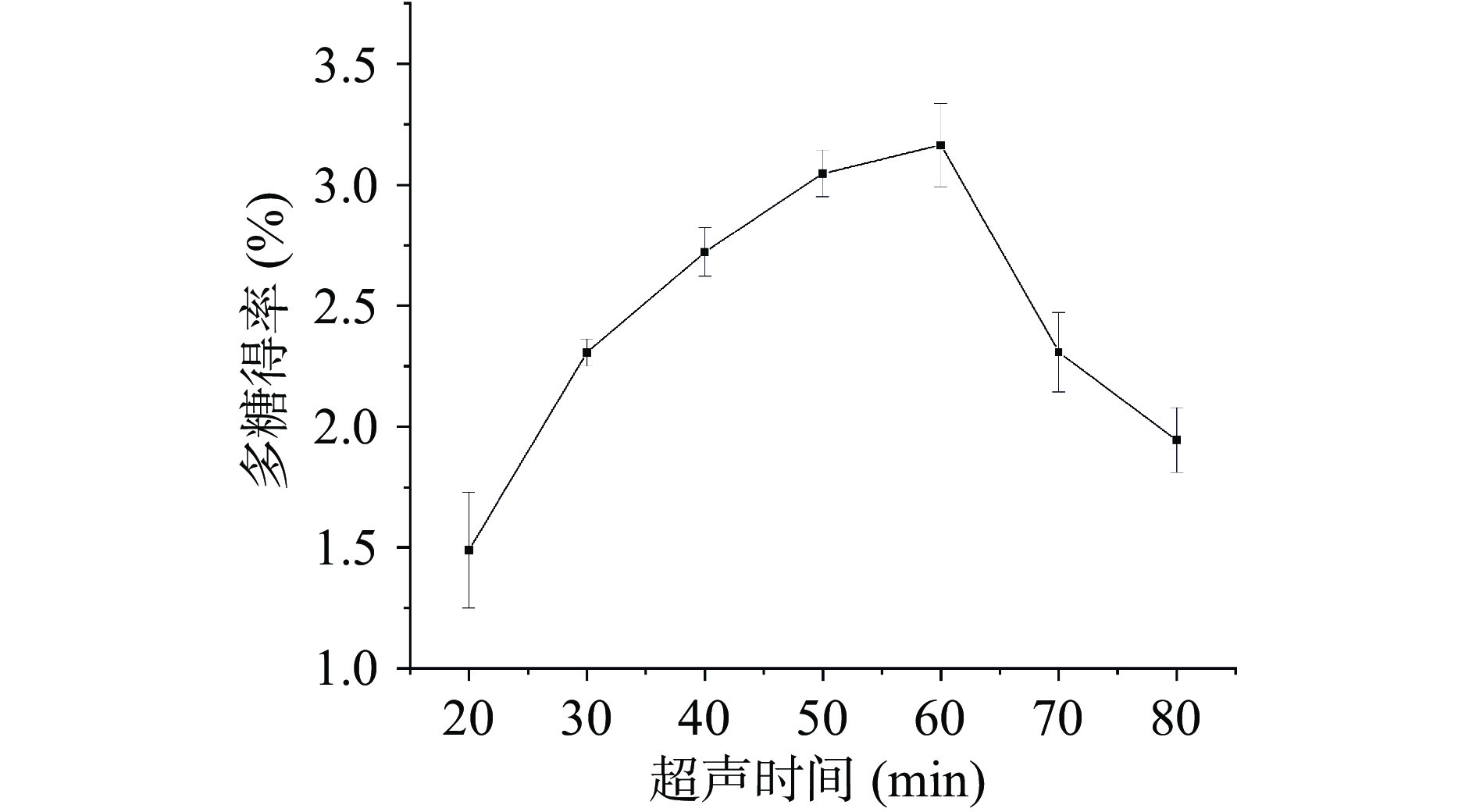
超声提取时间对桑葚粗多糖得率影响的实验结果见图3。随着超声时间的延长,桑葚多糖的得率逐渐升高,当超声时间为60 min时,得率最高为3.16%±0.17%,而后随超声时间延长得率开始下降。该这可能是因为在提取初期,超声波的“空化效应”促进多糖扩散至溶剂中,且呈时间依赖,使得多糖在溶剂中充分溶解,然而当超声时间过长时,长时间的超声波机械振动可能使提取液中溶解的多糖发生分解[43],从而导致得率降低。该现象与沈晓静等[46]和丁梁斌等[47]在提取云南小粒咖啡花多糖和鸡血藤多糖时的发现相似。因此,选择超声时间60 min进行后续实验。
2.1.4 超声功率选择
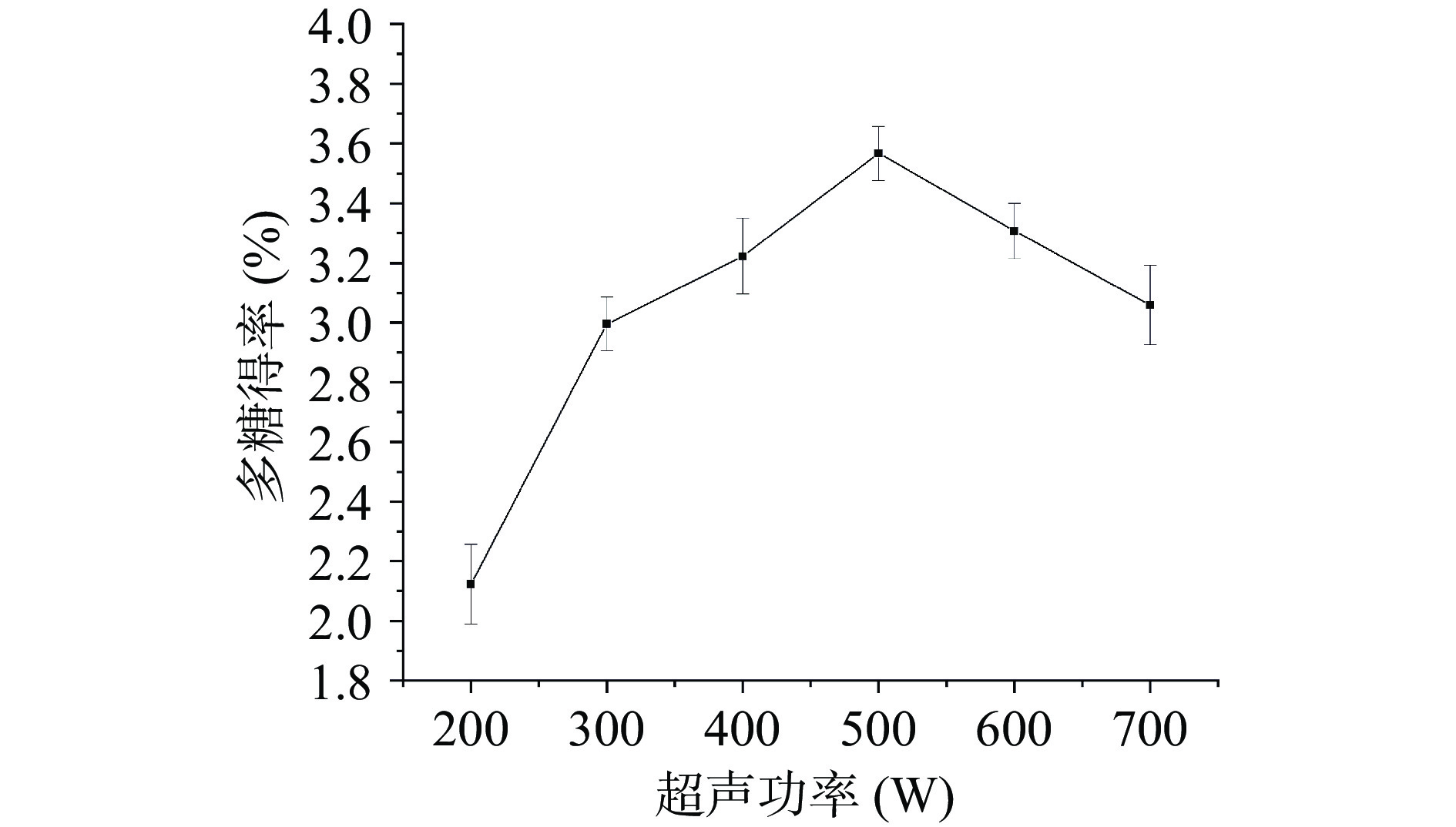
超声功率对桑葚粗多糖得率影响的实验结果如图4所示。随着超声功率的增大,桑葚粗多糖的得率先升高后下降,在超声功率为500 W时,得率达到最大,为3.57%±0.09%,随着超声功率的继续增强,桑葚粗多糖得率开始下降。超声功率的增大对于超声波的“空化效应”具有正向作用,能有效破坏细胞壁,促进多糖溶出,但当超声功率过大时,超声波带来的过强的机械振动可能破坏多糖结构,使得提取出来的多糖产生降解,导致得率下降[48]。该现象与孙宁云等[31]和卫娜等[49]在提取鸡蛋花多糖和板栗壳多糖时的结论类似。因此,选择超声功率500 W进行后续实验。
2.2 超声提取正交优化试验结果
超声辅助提取桑葚粗多糖的正交试验设计及结果如表3所示。通过极差大小确定各因素对桑葚粗多糖得率影响的主次顺序为:A(超声温度)>B(料液比)>D(超声功率)>C(超声时间)。最佳提取工艺条件为A2B3C3D2,即:超声温度50 ℃、料液比1:30 g/mL、超声时间70 min、超声功率500 W。经3次验证实验,在该条件下,桑葚粗多糖的得率可达4.59%±0.25%,相较Chen等[2]所报道的超声辅助提取桑葚粗多糖得率3.13%±0.07%有明显提升。
表 3 超声提取正交试验结果Table 3. Results of ultrasonic extraction orthogonal experiment实验号 A B C D 多糖得率(%) 1 1 1 1 1 3.26±0.06 2 1 2 2 2 3.83±0.03 3 1 3 3 3 3.78±0.04 4 2 1 2 3 4.15±0.03 5 2 2 3 1 4.46±0.06 6 2 3 1 2 4.51±0.02 7 3 1 3 2 4.23±0.04 8 3 2 1 3 4.12±0.13 9 3 3 2 1 4.13±0.01 k1 3.625 3.879 3.963 3.950 k2 4.376 4.138 4.036 4.189 k3 4.157 4.140 4.159 4.018 R 0.751 0.261 0.196 0.239 2.3 不同树脂脱色性能筛选
不同种类树脂对桑葚粗多糖溶液静态脱色的脱色率和多糖保留率如表4所示。不同种类大孔树脂对桑葚多糖的脱色效果不一,AB-8大孔树脂的脱色率最高;D301G的多糖保留率最高,但其脱色率较低,排在其后的AB-8和D101的多糖保留率无显著性差异(P>0.05)。树脂的吸附特性与其孔径、表面积和极性等物理化学性质密切相关[24],不同大孔树脂间的吸附差异可能是由于被吸附物质、树脂表面的活性位点和液相之间的分子间作用力不同所致[50]。合适的脱色树脂不仅要有很强的色素亲和性,而且对多糖的吸附能力应当很弱。结果显示树脂极性对桑葚粗多糖脱色率有较大影响,提示桑葚粗多糖中色素可能以弱极性色素为主[21,50]。综合考虑各树脂的脱色和多糖保留效果,选择AB-8大孔树脂进行后续实验。
表 4 不同树脂对桑葚多糖脱色效果的影响Table 4. Effects of different resins on decolorization of polysaccharides from Mori fructus树脂名称 极性 脱色率(%) 多糖保留率(%) XDA-8 极性 16.89±0.87e 66.85±1.66c NKA-9 极性 42.58±0.93c 53.64±1.18d AB-8 弱极性 53.19±0.66a 73.78±0.89b D101 非极性 46.95±0.35b 73.86±0.74b D301G 阴离子交换树脂 29.70±1.04d 94.11±0.21a 注:同列数据不同字母表示组间差异显著,P<0.05;相同字母表示差异不显著,P>0.05。 2.4 脱色工艺单因素实验结果
2.4.1 脱色时间选择
脱色时间对桑葚粗多糖脱色率影响的实验结果如图5所示。在0~3 h内,随脱色时间的延长,脱色率逐渐上升;在3~5 h内,脱色率基本恒定不变(P>0.05);脱色时间到达12 h时,脱色率最高为54%±1.57%,但12和5 h时的脱色率无显著性差异(P>0.05)。该结果与刘伟等[23]利用NKA-9脱色草莓多糖时的结果一致,随着脱色时间的延长,多糖脱色率逐渐上升而后达到动态平衡。但在脱色过程中,大孔吸附树脂对多糖也有相同的吸附趋势[50],为减少多糖损失,脱色时间较短为宜。综合考虑脱色率和脱色时间长短,选择脱色时间4 h进行后续实验。
2.4.2 多糖溶液浓度选择
桑葚粗多糖浓度对脱色率影响的实验结果如图6所示。随着桑葚粗多糖溶液浓度的增大,脱色率先上升后降低,在多糖溶液浓度为3 mg/mL时脱色率达到最大为56.04%±0.82%。固定大孔树脂用量,树脂中官能团所能吸附的色素总量不变,当多糖溶液浓度过低时,被吸附的色素总量减少,脱色前后溶液吸光度差别变小,脱色率较低;当多糖溶液浓度过高时,树脂可吸附的色素总量达到饱和,溶液中残存色素增加,脱色率降低。该现象与韩伟等[51]应用大孔树脂脱色蛹虫草多糖时一致。因此,选择桑葚粗多糖溶液浓度为3 mg/mL进行后续实验。
2.4.3 脱色温度选择
脱色温度对桑葚粗多糖脱色率影响的实验结果如图7所示。在20~30 ℃,脱色率随脱色温度的升高而增大,在30~50 ℃,脱色率随脱色温度的变化无显著性差异(P>0.05),脱色温度为50 ℃时,脱色率最大为54.71%±0.80%。在树脂的吸附过程中同时存在吸附和解吸两种反作用,一方面,温度的升高有利于溶质分子到达并吸附在树脂的表面和内部;另一方面,分子热运动的增加也会引起吸附的物质从树脂上解离。因此,树脂的吸附能力是在一定温度下吸附和解吸相互作用的结果[24]。该结果与Shi等[52]脱色香椿芽多糖时的现象一致。同时考虑到温度升高对多糖分解的促进作用,选择30 ℃进行后续实验。
2.5 脱色正交优化试验结果
采用AB-8大孔树脂静态法脱色桑葚粗多糖的正交试验设计及结果如表5所示。通过极差大小确定各因素对桑葚粗多糖脱色率影响的主次顺序为:C(脱色温度)>A(脱色时间)>B(桑葚多糖溶液浓度)。最佳工艺条件为A3B3C1,即:脱色时间5 h、桑葚粗多糖溶液浓度4 mg/mL、脱色温度25 ℃。在此条件下重复三次验证实验,桑葚粗多糖的脱色率为62.34%±1.27%。在最佳脱色工艺条件下进行放大实验,使用200 g AB-8大孔树脂对400 mL桑葚粗多糖溶液进行脱色处理,经3次验证实验,脱色率可达74.06%±2.62%。工艺放大后的脱色率比20 mL小试的脱色率有明显提升,这可能是由于脱色体系体积与深度增大的影响,溶质更易扩散进入树脂孔隙,使得脱色更为彻底。
表 5 脱色正交试验结果Table 5. Results of decolorization orthogonal experiment实验号 A B C 空白列 脱色率(%) 1 1 1 1 1 52.22±1.70 2 1 2 2 2 48.51±1.24 3 1 3 3 3 50.03±2.88 4 2 1 2 3 46.26±0.61 5 2 2 3 1 44.31±1.75 6 2 3 1 2 58.32±2.32 7 3 1 3 2 52.42±2.12 8 3 2 1 3 57.96±1.15 9 3 3 2 1 60.12±1.30 k1 50.253 50.300 56.167 k2 49.630 50.260 51.630 k3 56.833 56.157 48.920 R 7.203 5.897 7.247 2.6 抗氧化活性测定结果
2.6.1 ABTS+自由基清除率
ABTS与过硫酸钾反应可生成蓝绿色的稳定自由基,在734 nm处有最大吸收波长,当有抗氧化剂存在时,ABTS+自由基与之反应则变成无色。此法快速简便,被广泛应用于评价抗氧化剂清除自由基的能力。如图8所示,在测定范围内,桑葚多糖对ABTS+自由基的清除率随浓度增加先升高而后趋于平缓,在0.6 mg/mL时清除率达到99.03%±0.17%,与同浓度下VC对ABTS+自由基的清除率无显著性差异(P>0.05)。结果显示桑葚多糖对ABTS+自由基具有良好的清除能力,IC50值为0.14 mg/mL。
2.6.2 DPPH自由基清除率
DPPH是一种稳定的氮中心自由基,当有自由基清除剂存在时,DPPH的单电子被捕捉而颜色变浅,此法操作简便,重复性好,被广泛用于天然产物的体外抗氧化能力测定。如图9所示,在测定浓度范围内,桑葚多糖对DPPH自由基的清除率随浓度提高而上升,当桑葚多糖溶液浓度为1 mg/mL时,清除率最大为80.08%±0.84%,但小于同等浓度下VC对DPPH自由基的清除率91.18%±0.93%。该实验结果表明桑葚多糖对DPPH自由基具有良好的清除效果,IC50为0.68 mg/mL。
2.6.3 羟基自由基清除率
邻二氮菲可与Fe2+形成有色络合物,而H2O2与Fe2+通过Fenton反应产生的羟基自由基可将邻二氮菲-Fe2+氧化为邻二氮菲-Fe3+,若体系中存在羟基自由基清除剂,则Fenton反应产生的羟基自由基将被全部或部分清除,邻二氮菲-Fe2+络合物受到的损伤将随之减少。如图10所示,在测定浓度范围内,桑葚多糖对羟基自由基的清除能力随剂量增大而提高,在0.4 mg/mL时清除率为54.72%±1.14%,小于同等浓度下VC对羟基自由基的清除率89.61%±1.28%。结果表明,桑葚多糖在体外对羟基自由基具有较强的清除能力,IC50为0.19 mg/mL。
2.6.4 超氧阴离子自由基清除率
在弱碱性条件下,邻苯三酚能发生自氧化反应产生超氧阴离子自由基和中间有色产物,当体系中存在超氧阴离子自由基清除剂时能迅速与其反应,从而减少有色物累积。如图11所示,在测定浓度范围内,VC对超氧阴离子具有显著的清除作用,桑葚多糖对超氧阴离子自由基的清除率缓慢增加,当桑葚多糖浓度为1 mg/mL时,清除率为30.34%±0.78%。与VC相比,桑葚多糖对超氧阴离子自由基的清除能力较弱,IC50值为3.14 mg/mL,但优于甘薯渣多糖[53]清除超氧阴离子的IC50值5.832 mg/mL和苦笋壳多糖[45]清除超氧阴离子的IC50值13.58 mg/mL。这可能是由于天然活性多糖不同的水溶性、构象等因素[27],导致抗氧化能力差异。
3. 结论
本实验采用超声波细胞粉碎机提取桑葚多糖,通过单因素和正交优化实验得到最佳提取工艺为:超声温度50 ℃、料液比1:30 g/mL、超声时间70 min、超声功率500 W。该条件下桑葚粗多糖得率为4.59%±0.25%。并通过单因素和正交优化试验,得到使用AB-8大孔吸附树脂静态法脱色桑葚粗多糖的最佳条件为:脱色时间5 h、多糖溶液浓度4 mg/mL、脱色温度25 ℃。该条件下脱色率可达62.34%±1.27%。此外,体外抗氧化实验表明,桑葚多糖对ABTS+自由基、DPPH自由基、羟基自由基和超氧阴离子自由基均具有一定的清除能力,具备良好的体外抗氧化活性。后续需进一步探究桑葚多糖的体内生物活性和品质、结构鉴定方法,为桑葚多糖天然活性产品的进一步开发及功能研究提供参考,也为桑葚的产业开发与产业升级提供数据支撑。
-
表 1 超声提取工艺正交试验因素水平设计
Table 1 Factors and levels of orthogonal experiments for ultrasonic extraction
水平 因素 A超声温度
(℃)B料液比
(g/mL)C超声时间
(min)D超声功率
(W)1 40 1:20 50 400 2 50 1:25 60 500 3 60 1:30 70 600 表 2 脱色工艺正交试验因素水平设计
Table 2 Factors and levels of orthogonal experiments for decolorization process
水平 因素 A 脱色时间
(h)B 多糖溶液浓度
(mg/mL)C 脱色温度
(℃)1 3 2 25 2 4 3 30 3 5 4 35 表 3 超声提取正交试验结果
Table 3 Results of ultrasonic extraction orthogonal experiment
实验号 A B C D 多糖得率(%) 1 1 1 1 1 3.26±0.06 2 1 2 2 2 3.83±0.03 3 1 3 3 3 3.78±0.04 4 2 1 2 3 4.15±0.03 5 2 2 3 1 4.46±0.06 6 2 3 1 2 4.51±0.02 7 3 1 3 2 4.23±0.04 8 3 2 1 3 4.12±0.13 9 3 3 2 1 4.13±0.01 k1 3.625 3.879 3.963 3.950 k2 4.376 4.138 4.036 4.189 k3 4.157 4.140 4.159 4.018 R 0.751 0.261 0.196 0.239 表 4 不同树脂对桑葚多糖脱色效果的影响
Table 4 Effects of different resins on decolorization of polysaccharides from Mori fructus
树脂名称 极性 脱色率(%) 多糖保留率(%) XDA-8 极性 16.89±0.87e 66.85±1.66c NKA-9 极性 42.58±0.93c 53.64±1.18d AB-8 弱极性 53.19±0.66a 73.78±0.89b D101 非极性 46.95±0.35b 73.86±0.74b D301G 阴离子交换树脂 29.70±1.04d 94.11±0.21a 注:同列数据不同字母表示组间差异显著,P<0.05;相同字母表示差异不显著,P>0.05。 表 5 脱色正交试验结果
Table 5 Results of decolorization orthogonal experiment
实验号 A B C 空白列 脱色率(%) 1 1 1 1 1 52.22±1.70 2 1 2 2 2 48.51±1.24 3 1 3 3 3 50.03±2.88 4 2 1 2 3 46.26±0.61 5 2 2 3 1 44.31±1.75 6 2 3 1 2 58.32±2.32 7 3 1 3 2 52.42±2.12 8 3 2 1 3 57.96±1.15 9 3 3 2 1 60.12±1.30 k1 50.253 50.300 56.167 k2 49.630 50.260 51.630 k3 56.833 56.157 48.920 R 7.203 5.897 7.247 -
[1] RAMAPPA V K, SRIVASTAVA D, SINGH P, et al. Mulberries: A promising fruit for phytochemicals, nutraceuticals, and biological activities[J]. International Journal of Fruit Science,2020,20(3):s1254−s1279.
[2] CHEN C, YOU L J, ABBASI A M, et al. Optimization for ultrasound extraction of polysaccharides from mulberry fruits with antioxidant and hyperglycemic activity in vitro[J]. Carbohydrate Polymers,2015,130:122−132. doi: 10.1016/j.carbpol.2015.05.003
[3] DONG Y H, CHEN C, HUANG Q, et al. Study on a novel spherical polysaccharide from Fructus mori with good antioxidant activity[J]. Carbohydrate Polymers,2021,256:117516. doi: 10.1016/j.carbpol.2020.117516
[4] AI J, BAO B, BATTINO M, et al. Recent advances on bioactive polysaccharides from mulberry[J]. Food & Function,2021,12(12):5219−5235.
[5] BHATTACHARJYA D, SADAT A, DAM P, et al. Current concepts and prospects of mulberry fruits for nutraceutical and medicinal benefits[J]. Current Opinion in Food Science,2021,40:121−135. doi: 10.1016/j.cofs.2021.03.009
[6] WEN P, HU T G, LINHARDT R J, et al. Mulberry: A review of bioactive compounds and advanced processing technology[J]. Trends in Food Science & Technology,2019,83:138−158.
[7] DENG Q, WANG X, CHEN H, et al. Structural characterization, modification and hepatoprotective effects of polysaccharide from Mori fructus[J]. International Journal of Biological Macromolecules,2020,153:357−363. doi: 10.1016/j.ijbiomac.2020.02.300
[8] TAN X, CHEN H, ZHOU X. Study on the activity of Mori fructus polysaccharides and its derivatives against acute alcoholic liver injury in mice[J]. Journal of Carbohydrate Chemistry,2020,39(9):450−471. doi: 10.1080/07328303.2021.1895194
[9] CHEN H, XIAO R, ZHOU X. Study on the extraction, purification, partial chemical characterization and anti-alcohol liver injury activity of Mori fructus polysaccharides[J]. New Journal of Chemistry,2020,44(46):20060−20070. doi: 10.1039/D0NJ00795A
[10] BIAN L, CHEN H, ZHOU X. Untargeted lipidomics analysis of Mori fructus polysaccharide on acute alcoholic liver injury in mice using ultra performance liquid chromatography-quadrupole-orbitrap-high resolution mass spectrometry[J]. International Immunopharmacology,2021,97:107521. doi: 10.1016/j.intimp.2021.107521
[11] LIU C J, LIN J Y. Anti-inflammatory and anti-apoptotic effects of strawberry and mulberry fruit polysaccharides on lipopolysaccharide-stimulated macrophages through modulating pro-/anti-inflammatory cytokines secretion and Bcl-2/Bak protein ratio[J]. Food and Chemical Toxicology,2012,50(9):3032−3039. doi: 10.1016/j.fct.2012.06.016
[12] WANG D, LI H, LI B, et al. Systematic fractionation and immunoenhancement of water-soluble polysaccharides isolated from fruit of Morus alba L[J]. International Journal of Biological Macromolecules,2018,116:1056−1063. doi: 10.1016/j.ijbiomac.2018.05.106
[13] JIAO Y, WANG X, JIANG X, et al. Antidiabetic effects of Morus alba fruit polysaccharides on high-fat diet and streptozotocin-induced type 2 diabetes in rats[J]. Journal of Ethnopharmacology,2017,199:119−127. doi: 10.1016/j.jep.2017.02.003
[14] CHEN C, ZHANG B, FU X, et al. A novel polysaccharide isolated from mulberry fruits (Murus alba L.) and its selenide derivative: Structural characterization and biological activities[J]. Food & Function,2016,7(6):2886−2897.
[15] 李瑶, 李文林, 杨丽丽, 等. 桑椹在心血管疾病领域的药效实验研究现状分析与思考[J]. 中国中药杂志,2020,45(13):3055−3062. [LI Y, LI W L, YANG L L, et al. Status analysis and thinking on experimental study on efficacy of Mori fructus in treatment of cardiovascular diseases[J]. China Journal of Chinese Materia Medica,2020,45(13):3055−3062. LI Y, LI W L, YANG L L, et al. Status analysis and thinking on experimental study on efficacy of Mori fructus in treatment of cardiovascular diseases[J]. China Journal of Chinese Materia Medica, 2020, 45(13): 3055-3062.
[16] 王强, 王睿, 王存, 等. 桑葚多糖调节血糖代谢及体外抗氧化效果研究[J]. 食品科学,2014,35(11):260−264. [WANG Q, WANG R, WANG C, et al. Effects of mulberry polysaccharides on glucose metabolism and their antioxidant activities in vitro[J]. Food Science,2014,35(11):260−264. doi: 10.7506/spkx1002-6630-201411052 WANG Q, WANG R, WANG C, et al. Effects of mulberry polysaccharides on glucose metabolism and their antioxidant activities in vitro[J]. Food Science, 2014, 35(11): 260-264. doi: 10.7506/spkx1002-6630-201411052
[17] 包海蓉, 李柏林, 阎冬妮, 等. 桑葚的开发利用与市场营销[J]. 食品科学,2004(S1):208−211. [BAO H R, LI B L, YAN D N, et al. Development, utilization and marketing of mulberry[J]. Food Science,2004(S1):208−211. BAO H R, LI B L, YAN D N, et al. Development, utilization and marketing of mulberry[J]. Food Science, 2004(S1): 208-211.
[18] SILLERO L, PRADO R, LABIDI J. Simultaneous microwave-ultrasound assisted extraction of bioactive compounds from bark[J]. Chemical Engineering & Processing,2020,156:108100.
[19] HOMA B, FARZIN Z A, AMIR F, et al. Comparisons between conventional, microwave- and ultrasound-assisted methods for extraction of pectin from grapefruit[J]. Chemical Engineering and Processing: Process Intensification,2011,50(11−12):1237−1243. doi: 10.1016/j.cep.2011.08.002
[20] 景荣琴, 熊清平, 景怡. 响应面法优化桑葚多糖的超声波辅助提取工艺条件[J]. 天然产物研究与开发,2014,26(4):570−574. [JING R Q, XIONG Q P, JING Y. Optimization of ultrasonic-assisted extraction of polysaccharides from Fructus mori by response surface methodology[J]. Natural Product Research and Development,2014,26(4):570−574. JING R Q, XIONG Q P, JING Y. Optimization of ultrasonic-assisted extraction of polysaccharides from Fructus mori by response surface methodology[J]. Natural Product Research and Development, 2014, 26(4): 570-574.
[21] 祝新媛. 桑葚多糖的提取纯化及其对溃疡性结肠炎小鼠保护作用研究[D]. 沈阳: 沈阳农业大学, 2020 ZHU X Y. Extraction and purification of mulberry polysaccharide and its protective effect on ulcerative colitis in mice[D]. Shenyang: Shenyang Agricultural University, 2020.
[22] 夏玮, 吕庆, 张文清, 等. 大孔吸附树脂脱色桑叶多糖的研究[J]. 食品与发酵工业,2007(2):141−144. [XIA W, LÜ Q, ZHANG W Q, et al. Study on the decoloration of polysaccharides from mulberry leaves by macro-resin absorption[J]. Food and Fermentation Industries,2007(2):141−144. doi: 10.3321/j.issn:0253-990X.2007.02.032 XIA W, LÜ Q, ZHANG W Q, et al. Study on the decoloration of polysaccharides from mulberry leaves by macro-resin absorption[J]. Food and Fermentation Industries, 2007(2): 141-144. doi: 10.3321/j.issn:0253-990X.2007.02.032
[23] 刘伟, 刘倩楠, 张良, 等. 草莓多糖树脂法脱色工艺优化及其化学性质研究[J]. 食品工业科技,2020,41(10):38−46,51. [LIU W, LIU Q N, ZHANG L, et al. Optimization of decoloration process by microporous resins and its chemical properties of strawberry polysaccharides[J]. Science and Technology of Food Industry,2020,41(10):38−46,51. LIU W, LIU Q N, ZHANG L, et al. Optimization of decoloration process by microporous resins and its chemical properties of strawberry polysaccharides[J]. Science and Technology of Food Industry, 2020, 41(10): 38-46, 51.
[24] ZHEN B, CHEN X S, HAN D, et al. An alternative method for the decoloration of ɛ-poly-l-lysine eluate by macroporous resin in the separation and purification of ɛ-poly-l-lysine from fermentation broth[J]. Food and Bioproducts Processing,2015,95:332−338. doi: 10.1016/j.fbp.2014.10.006
[25] 王松柏, 秦雪梅, 郭小青, 等. 树脂对防风粗多糖脱色效果[J]. 应用化学,2005(12):1308−1311. [WANG S B, QIN X M, GUO X Q, et al. Decolorization of crude Saposhnikovia divaricata polysaccharide by resins[J]. Chinese Journal of Applied Chemistry,2005(12):1308−1311. doi: 10.3969/j.issn.1000-0518.2005.12.008 WANG S B, QIN X M, GUO X Q, et al. Decolorization of crude Saposhnikovia divaricata polysaccharide by resins[J]. Chinese Journal of Applied Chemistry, 2005(12): 1308-1311. doi: 10.3969/j.issn.1000-0518.2005.12.008
[26] LI X Y, WANG L. Effect of extraction method on structure and antioxidant activity of Hohenbuehelia serotina polysaccharides[J]. International Journal of Biological Macromolecules,2016,83:270−276. doi: 10.1016/j.ijbiomac.2015.11.060
[27] CHEN X Y, SUN WATERHOUSE D X, YAO W Z, et al. Free radical-mediated degradation of polysaccharides: Mechanism of free radical formation and degradation, influence factors and product properties[J]. Food Chemistry,2021,365:130524. doi: 10.1016/j.foodchem.2021.130524
[28] 赵喜兰. 桑葚多糖提取、纯化分离及其降糖作用的研究[J]. 食品工业科技,2011,32(2):259−260. [ZHAO X L. Study on the purification and hypoglycemic effect of polysaccharide in mulberries[J]. Science and Technology of Food Industry,2011,32(2):259−260. ZHAO X L. Study on the purification and hypoglycemic effect of polysaccharide in mulberries[J]. Science and Technology of Food Industry, 2011, 32(2): 259-260.
[29] LIAO D W, CHEN C, LIU J P, et al. Characterization and antitumor activities of polysaccharides obtained from ginger (Zingiber officinale) by different extraction methods[J]. International Journal of Biological Macromolecules,2020,152:894−903. doi: 10.1016/j.ijbiomac.2020.02.325
[30] YANG J, TONG Y P, ZHU K M, et al. Optimization of mechanochemical-assisted extraction and decoloration by resins of polysaccharides from petals of Crocus sativus L[J]. Journal of Food Processing and Preservation,2018,42(1):e13369. doi: 10.1111/jfpp.13369
[31] 孙宁云, 姚欣, 张英慧, 等. 鸡蛋花多糖提取工艺优化及生物活性研究[J/OL]. 食品工业科技: 1−18[2021-12-02]. doi: 10.13386/j. issn1002-0306.2021050198. SUN N Y, YAO X, ZHANG Y H, et al. Optimization of extraction process of polysaccharides from Plumeria rubra L. cv. Acutifolia and evaluation of biological activities[J/OL]. Science and Technology of Food Industry: 1−18[2021-12-02]. doi: 10.13386/j.issn1002-0306.2021050198.
[32] 尹明松, 丁贺辉, 潘飞兵, 等. 响应面优化超声辅助双水相提取槟榔多糖及抗氧化活性研究[J]. 食品研究与开发,2021,42(19):163−170. [YIN M S, DING H H, PAN F B, et al. Optimization of ultrasonic-assisted aqueous two-phase extraction of Areca catechu L. polysaccharide using response surface design and assessment of its antioxidant activities[J]. Food Research and Development,2021,42(19):163−170. doi: 10.12161/j.issn.1005-6521.2021.19.023 YIN M S, DING H H, PAN F B, et al. Optimization of ultrasonic-assisted aqueous two-phase extraction of Areca catechu L. polysaccharide using response surface design and assessment of its antioxidant activities[J]. Food Research and Development, 2021, 42(19): 163-170. doi: 10.12161/j.issn.1005-6521.2021.19.023
[33] 郑婷婷, 严亮, 张文杰, 等. 水碱连续提取黄皮疣柄牛肝菌粗多糖的理化性质及抗氧化活性研究[J]. 食品工业科技,2020,41(15):84−89. [ZHENG T T, YAN L, ZHANG W J, et al. Physicochemical properties and antioxidant activity of water-alkali continuous extraction of crude polysaccharides from Leccinellum crocipodium (Letellier.) watliag[J]. Science and Technology of Food Industry,2020,41(15):84−89. ZHENG T T, YAN L, ZHANG W J, et al. Physicochemical properties and antioxidant activity of water-alkali continuous extraction of crude polysaccharides from Leccinellum crocipodium (Letellier. ) watliag[J]. Science and Technology of Food Industry, 2020, 41(15): 84-89.
[34] 张莉, 柏红梅, 游敬刚, 等. 不同发酵剂菌种对蓝莓-桑葚复合酵素抗氧化活性的影响[J]. 食品科技,2021,46(6):29−34. [ZHANG L, BAI H M, YOU J G, et al. Effect of different fermentation strains on the antioxidant activity of blueberry-mulberry complex fermented liquor[J]. Food Science and Technology,2021,46(6):29−34. ZHANG L, BAI H M, YOU J G, et al. Effect of different fermentation strains on the antioxidant activity of blueberry-mulberry complex fermented liquor[J]. Food Science and Technology, 2021, 46(6): 29-34.
[35] 付金, 姚秋萍, 邓水秀, 等. 黔产皂角米多糖提取动力学及抗氧化活性研究[J]. 食品工业科技,2021,42(1):8−14. [FU J, YAO Q P, DENG S X, et al. Extraction kinetics and antioxidant activities of polysaccharides from seeds of Gleditsia sinensis in Guizhou[J]. Science and Technology of Food Industry,2021,42(1):8−14. FU J, YAO Q P, DENG S X, et al. Extraction kinetics and antioxidant activities of polysaccharides from seeds of Gleditsia sinensis in Guizhou[J]. Science and Technology of Food Industry, 2021, 42(1): 8-14.
[36] 陈红惠, 牛念拉姆. 底圩茶多糖的超声波辅助提取及其抗氧化活性[J]. 食品工业科技,2020,41(21):179−184. [CHEN H H, NIUNIAN L M. Ultrasonic extraction and antioxidant activity of polysaccharide from Dixu tea[J]. Science and Technology of Food Industry,2020,41(21):179−184. CHEN H H, NIUNIAN L M. Ultrasonic extraction and antioxidant activity of polysaccharide from Dixu tea[J]. Science and Technology of Food Industry, 2020, 41(21): 179-184.
[37] 何念武, 秦娇娇, 王新军. 超声辅助提取灰灰菜多糖工艺优化及其体外抗氧化活性[J]. 食品工业科技,2018,39(1):235−240,252. [HE N W, QIN J J, WANG X J. Optimization of ultrasonic assisted extraction technology of polysaccharide from Chenopodium album Linn and its antioxidant activity in vitro[J]. Science and Technology of Food Industry,2018,39(1):235−240,252. HE N W, QIN J J, WANG X J. Optimization of ultrasonic assisted extraction technology of polysaccharide from Chenopodium album Linn and its antioxidant activity in vitro[J]. Science and Technology of Food Industry, 2018, 39(1): 235-240, 252.
[38] 范秀萍, 吴红棉, 王娅楠, 等. 4种贝类糖胺聚糖体外清除自由基活性的比较[J]. 食品科技,2008(2):165−167. [FAN X P, WU H M, WANG Y N, et al. Free radical- scavenging activity of glycosaminoglycans from four seashells in vitro[J]. Food Science and Technology,2008(2):165−167. doi: 10.3969/j.issn.1005-9989.2008.02.048 FAN X P, WU H M, WANG Y N, et al. Free radical- scavenging activity of glycosaminoglycans from four seashells in vitro[J]. Food Science and Technology, 2008(2): 165-167. doi: 10.3969/j.issn.1005-9989.2008.02.048
[39] 蔡延渠, 董碧莲, 陈利秋, 等. 桃胶多糖体内外抗氧化作用的研究[J]. 食品工业科技,2020,41(13):53−58. [CAI Y Q, DONG B L, CHEN L Q, et al. Antioxidant activity in vivo and in vitro of polysaccharide from peach gum[J]. Science and Technology of Food Industry,2020,41(13):53−58. CAI Y Q, DONG B L, CHEN L Q, et al. Antioxidant activity in vivo and in vitro of polysaccharide from peach gum[J]. Science and Technology of Food Industry, 2020, 41(13): 53-58.
[40] VÁZQUEZ-RODRÍGUEZ B, GUTIÉRREZ-URIBE J, ANTUNES-RICARDO M, et al. Ultrasound-assisted extraction of phlorotannins and polysaccharides from Silvetia compressa (Phaeophyceae)[J]. Journal of Applied Phycology,2020,32(2):1441−1453. doi: 10.1007/s10811-019-02013-2
[41] 陈成. 五味子多糖提取工艺优化及其对α-葡萄糖苷酶抑制活性分析[J/OL]. 食品工业科技: 1−10[2021-12-02]. doi: 10.13386/j. issn1002-0306.2021090146. CHEN C. Optimization of extraction process of Schisandra chinensis polysaccharide and analysis of its inhibitory activity against α-glucosidase[J]. Science and Technology of Food Industry: 1−10[2021-12-02]. doi: 10.13386/j.issn1002-0306.2021090146.
[42] 王杉杉, 马韵升, 姚刚, 等. 超声波辅助复合酶法提取枸杞多糖工艺研究[J]. 中国酿造,2015,34(7):134−137. [WANG B B, MA Y S, YAO G, et al. Ultrasound-assisted compound enzyme extraction technology of polysaccharides from Lycium barbarum[J]. China Brewing,2015,34(7):134−137. doi: 10.11882/j.issn.0254-5071.2015.07.032 WANG B B, MA Y S, YAO G, et al. Ultrasound-assisted compound enzyme extraction technology of polysaccharides from Lycium barbarum[J]. China Brewing, 2015, 34(7): 134-137. doi: 10.11882/j.issn.0254-5071.2015.07.032
[43] SOROURIAN R, KHAJEHRAHIMI A, TADAYONI M, et al. Ultrasound-assisted extraction of polysaccharides from Typha domingensis: Structural characterization and functional properties[J]. International Journal of Biological Macromolecules,2020,160:758−768. doi: 10.1016/j.ijbiomac.2020.05.226
[44] 帅良, 廖玲燕, 段振华, 等. 百香果果皮多糖提取工艺优化及其抗氧化活性研究[J]. 食品工业科技,2020,41(18):150−156. [SHUAI L, LIAO L Y, DUAN Z H, et al. Optimization of extraction technology of polysaccharides from passion fruit peel and its antioxidant activity[J]. Science and Technology of Food Industry,2020,41(18):150−156. SHUAI L, LIAO L Y, DUAN Z H, et al. Optimization of extraction technology of polysaccharides from passion fruit peel and its antioxidant activity[J]. Science and Technology of Food Industry, 2020, 41(18): 150-156.
[45] 冉俊枫, 任艳, 田余波, 等. 苦笋壳多糖提取工艺及抗氧化活性研究[J]. 食品科技,2021,46(6):207−214. [RAN J F, REN Y, TIAN Y B, et al. Extraction optimization for polysaccharides from bamboo shoot shell of Pleioblastus amarus and the in vitro antioxidant activity[J]. Food Science and Technology,2021,46(6):207−214. RAN J F, REN Y, TIAN Y B, et al. Extraction optimization for polysaccharides from bamboo shoot shell of Pleioblastus amarus and the in vitro antioxidant activity[J]. Food Science and Technology, 2021, 46(6): 207-214.
[46] 沈晓静, 黄璐璐, 聂凡秋, 等. 云南小粒咖啡花多糖提取工艺优化及其抗氧化活性分析[J]. 食品工业科技,2022,43(4):8. [SHEN X J, HUANG L L, NIE F Q, et al. Study on optimization of extraction technology and antioxidant activity of polysaccharide from Yunnan arabica coffee flowers[J]. Science and Technology of Food Industry,2022,43(4):8. SHEN X J, HUANG L L, NIE F Q, et al. Study on optimization of extraction technology and antioxidant activity of polysaccharide from Yunnan arabica coffee flowers[J]. Science and Technology of Food Industry, 2022, 43(4): 8.
[47] 丁梁斌, 马春梅, 赵苹苹, 等. 响应面法优化酶解-超声辅助提取鸡血藤多糖工艺研究[J]. 中国食品添加剂,2021,32(7):88−96. [DING L B, MA C M, ZHAO P P, et al. Optimization of enzymatic hydrolysis-ultrasonic assisted extraction of polysaccharides from spatholobi by response surface methodology[J]. China Food Additives,2021,32(7):88−96. DING L B, MA C M, ZHAO P P, et al. Optimization of enzymatic hydrolysis-ultrasonic assisted extraction of polysaccharides from spatholobi by response surface methodology[J]. China Food Additives, 2021, 32(7): 88-96.
[48] ZHU C P, ZHAI X C, LI L Q, et al. Response surface optimization of ultrasound-assisted polysaccharides extraction from pomegranate peel[J]. Food Chemistry,2015,177:139−146. doi: 10.1016/j.foodchem.2015.01.022
[49] 卫娜, 罗至钧, 郑逸蓝, 等. 超声-微波辅助提取板栗壳多糖及其结构鉴定[J]. 食品安全质量检测学报,2021,12(16):6600−6608. [WEI N, LUO Z J, ZHENG Y L, et al. Optimization of ultrasonic-microwave synergistic extraction process of chestnut shell polysaccharides and its structural identification[J]. Journal of Food Safety & Quality,2021,12(16):6600−6608. WEI N, LUO Z J, ZHENG Y L, et al. Optimization of ultrasonic-microwave synergistic extraction process of chestnut shell polysaccharides and its structural identification[J]. Journal of Food Safety & Quality, 2021, 12(16): 6600-6608.
[50] YANG R, MENG D, SONG Y, et al. Simultaneous decoloration and deproteinization of crude polysaccharide from pumpkin residues by cross-linked polystyrene macroporous resin[J]. Journal of Agricultural and Food Chemistry,2012,60(34):8450−8456. doi: 10.1021/jf3031315
[51] 韩伟, 陈静雯. 应用大孔树脂提纯蛹虫草多糖的工艺研究[J]. 徐州工程学院学报(自然科学版),2021,36(2):7−14. [HAN W, CHEN J W. Study on purification of Cordyceps militaris polysaccharides by macroporous resin[J]. Journal of Xuzhou Institute of Technology (Natural Sciences Edition),2021,36(2):7−14. [HAN W, CHEN J W. Study on purification of Cordyceps militaris polysaccharides by macroporous resin[J]. Journal of Xuzhou Institute of Technology (Natural Sciences Edition), 2021, 36(2): 7-14.
[52] SHI Y Y, LIU T T, HAN Y, et al. An efficient method for decoloration of polysaccharides from the sprouts of Toona sinensis (A. Juss.) roem by anion exchange macroporous resins[J]. Food Chemistry,2017,217:461−468. doi: 10.1016/j.foodchem.2016.08.079
[53] 段旭, 冉军舰, 孙俊良, 等. 甘薯渣多糖的提取工艺优化、结构鉴定及其功能活性研究[J]. 食品工业科技,2022,43(8):10. [DUAN X, RAN J J, SUN J L, et al. Study on extraction process optimization, structure identification and functional activity of polysaccharide from sweet potato residue[J]. Science and Technology of Food Industry,2022,43(8):10. DUAN X, RAN J J, SUN J L, et al. Study on extraction process optimization, structure identification and functional activity of polysaccharide from sweet potato residue[J]. Science and Technology of Food Industry, 2022, 43(8): 10.
-
期刊类型引用(12)
1. 刘容旭,王语聪,刘金阳,谢宜桐,谢智鑫,种正晨,李世函,刘丹怡,韩建春. 超高压辅助酶解对汉麻分离蛋白结构和抗氧化活性的影响. 食品工业科技. 2024(04): 24-32 .  本站查看
本站查看
2. 刘佳怡,黄磊磊,王天怡,张庆芬,杨逢建. 超声协同低共熔溶剂提取紫丁香花多酚工艺优化及抗氧化活性分析. 食品工业科技. 2024(04): 171-179 .  本站查看
本站查看
3. 刘秋叶,刘辉,陈鑫,左亚杰. 布渣叶多糖的提取工艺优化及生物活性分析. 食品工业科技. 2024(04): 197-204 .  本站查看
本站查看
4. 茹巧美,任国平,王艳. 酸化甘油-超声辅助提取杨梅花青素工艺优化及其抗氧化活性研究. 食品安全质量检测学报. 2024(13): 124-132 .  百度学术
百度学术
5. 王润平,李云萍,路小彬,贺银菊,张宏福. 探究杜仲叶多糖的超声提取优化及透析纯化工艺与抗氧化性能. 饲料工业. 2024(14): 87-95 .  百度学术
百度学术
6. 陈强,王璐,徐峥嵘,罗金超,邓千千,方雨婷,李从虎,程旭. 玉米植物糖原超声辅助提取工艺优化及其生物活性评价. 食品工业科技. 2024(19): 177-186 .  本站查看
本站查看
7. 尹凯静,高婷,孙浩浩,张冬冬,张爽. 超声波辅助乙醇法提取广西武鸣沃柑皮渣中多酚物质的工艺优化. 芜湖职业技术学院学报. 2024(04): 41-46 .  百度学术
百度学术
8. 刘芸,魏宗敏,陈忠铃,孙宝山,李灵犀. 辣木叶多酚的超声提取及抗氧化活性分析. 中国食品添加剂. 2023(02): 52-60 .  百度学术
百度学术
9. 刘书伟,沈梦霞,王燕,张田田,武天明. 海绵Hyrtios erectus抗氧化产物超声提取工艺优化及其抗氧化活性分析. 食品工业科技. 2023(09): 236-243 .  本站查看
本站查看
10. 姚昕,涂勇,李海生,户芝芳,张忠. 模糊数学综合评价法优化石榴桑葚复合果糕生产工艺. 中国果菜. 2023(05): 35-41 .  百度学术
百度学术
11. 郑燕菲,韦凤,庞光沃,张贞发. 单性木兰叶多糖的超声辅助提取工艺及稳定性研究. 中国调味品. 2023(12): 188-192 .  百度学术
百度学术
12. 袁心田,陈华国,赵超,龚小见,周欣. 基于膜技术的桑葚多糖分级分离及生物活性组分筛选. 食品工业科技. 2022(24): 72-80 .  本站查看
本站查看
其他类型引用(15)








 下载:
下载:













 下载:
下载:
