Research Progress on the Anti-inflammatory Mechanism of Ziziphus jujuba Mill. and Its Bioactive Ingredients
-
摘要: 枣作为“药食同源”的一种干果,具有抗炎、抗氧化和抗癌等生物活性功能。抗炎是其主要活性功能之一,国内外对枣抗炎作用机制的研究多在于提取物及其活性成分,并通过体内、体外模型验证其对炎症性疾病的作用效果。枣主要的抗炎活性成分有多糖类、多酚类、三萜类和生物碱等,这些成分通过调控免疫应答反应和炎症信号、抑制脂质过氧化和改善肠道菌群等发挥作用。因此,本文重点综述了枣活性成分的种类和结构对炎症反应的影响,讨论了其通过调节免疫系统、氧化应激和菌群调节的抗炎作用途径,并总结了枣及活性成分在结肠炎、糖尿病、过敏性哮喘、阿兹海默症、肥胖和关节炎等疾病中的应用。旨在探索枣中活性成分的抗炎机制,为枣的精深加工和应用提供理论参考和依据。Abstract: Jujube, a kind of "medicine and food homology" dried fruit, shows anti-inflammatory, antioxidant, anticancer and other biological activities. Among them, anti-inflammatory is one of the main activities. Most of the researches on the anti-inflammatory mechanism of jujube mainly focused on the extracts and their bioactive substances and verified their effects on inflammatory diseases using in vivo and in vitro models. The main anti-inflammatory components of jujube include polysaccharides, polyphenols, triterpenoids and alkaloids, and play their roles by regulating immune response and inflammatory signal, inhibiting peroxidation and improving intestinal flora. Therefore, this review focuses on the effects of the types and structures of active ingredients of jujube on inflammatory response, discusses the anti-inflammatory pathway through the regulation of immune system, oxidative stress and microflora, and summarizes the applications of jujube and its active ingredients in colitis, diabetes, allergic asthma, Alzheimer's disease, obesity and arthritis. This paper aims to explore the anti-inflammatory mechanism of the bioactive ingredients in jujube, and provide theoretical reference and basis for the deep processing and applications of jujube.
-
枣(Ziziphus jujuba Mill.)是鼠李科枣属植物,有悠久的种植和食用历史。中国是最大的也是唯一的经济枣属出口国,世界市场上90%的枣和枣加工产品都源自中国[1],现有枣品种超700种,种植地区超21个省份,主要在河北、山西、山东、陕西及新疆等地。古药典《本草纲目》中记载,枣具有健脾和胃、补血益气和安神助眠的功效,常被用在食品和药品中[2]。红枣中含有总糖(43%~56%)、粗纤维(3.2%~5.1%)、维生素(132~183 mg/g)、矿物质(1.0~1.4 mg/g)、蛋白质(2.9%~4.4%)和氨基酸(1.9%~2.6%)等营养成分(以干重为基准)[3−4]。此外,红枣还含有多糖类化合物(220~561 mg/g)、多酚类化合物(0.41~8.42 mg/g)、三萜类化合物(0.39~15 mg/g)和生物碱(极少)等多种活性成分[5−6],具有抗氧化、抗炎、护肝、抗衰老等多种生物活性,其中抗炎是其主要的活性功效之一[7]。近年来,学者们对枣提取物及活性成分的抗炎活性进行了一系列研究,逐步揭示了其抗炎作用机制,并带动了相关产业的应用。
炎症反应是机体自我保护的重要机制之一,旨在清除损伤组织或病原体并促进伤口愈合[8]。炎症可分为急性炎症和慢性炎症,前者主要由外源性因素(微生物、病毒感染等)引起,发病迅速,常表现为红肿、疼痛和发热;后者主要由内源性因素(炎症介质等)引起,发病缓慢且持续,最终可能导致组织损伤或纤维化[9]。这些反应信号通过血液和淋巴传播到全身,引起或加剧相关疾病的发生,如过敏性哮喘、结肠炎、关节炎、糖尿病等[10−11],这些疾病影响了人体正常的生理活动。使用化学药物来改善炎症的同时,也要承受药物带来的不良反应,如超敏反应、心律失常、造血变化和男性乳房发育等[12]。因此,寻找天然抗炎活性物质对改善和预防炎症性疾病具有重要意义。
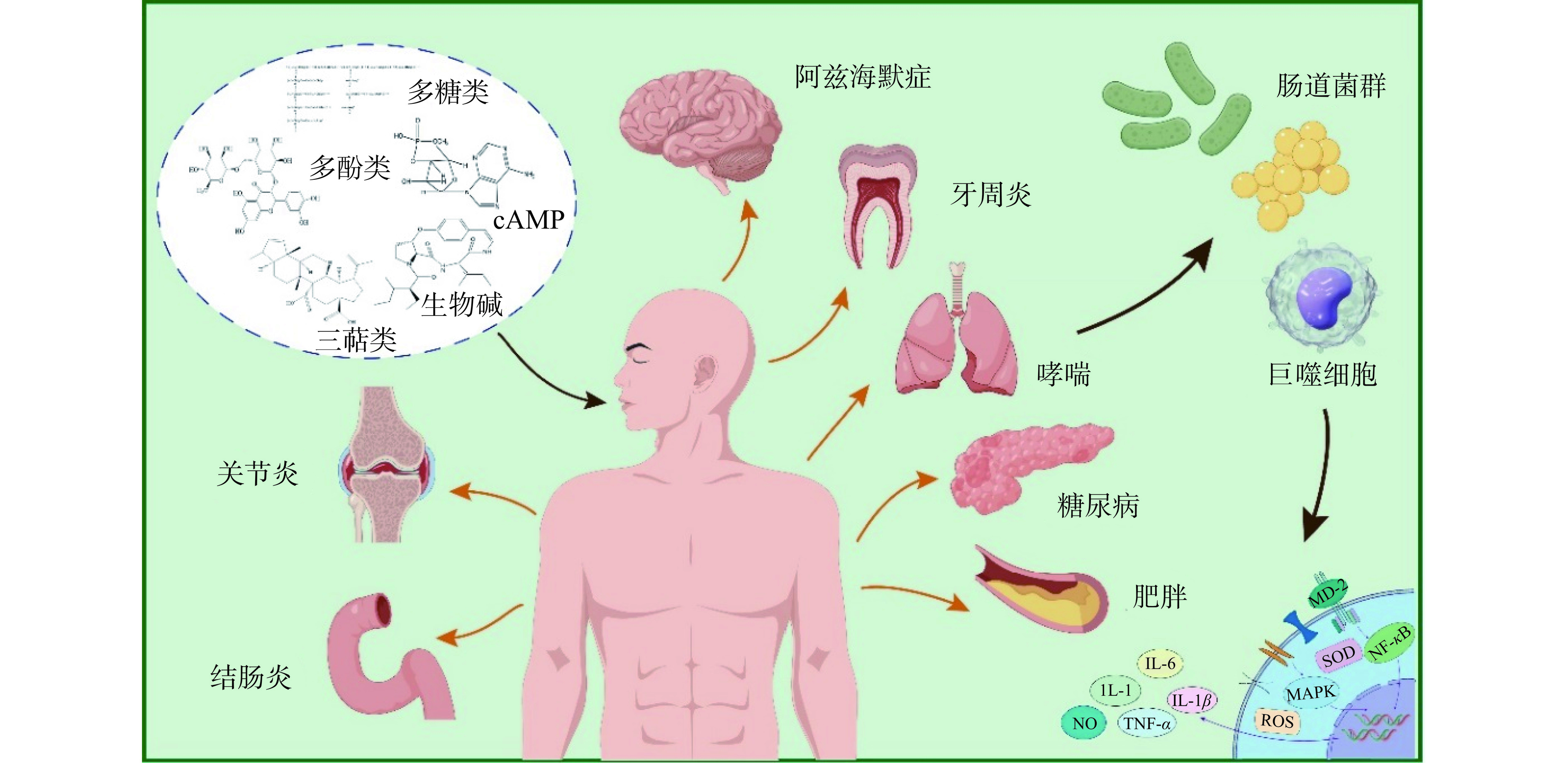
到目前为止,虽然对枣抗炎活性的研究已广泛展开,但仍然缺乏系统的整理。因此,本文综合了最新的研究成果,概述了枣的抗炎活性成分和功能,并系统归纳了枣的抗炎作用机制以及在结肠炎、过敏性哮喘、肥胖、糖尿病和关节炎等炎症性疾病中的应用,为枣的功能机制研究和应用提供参考依据(图1)。
1. 枣的抗炎生物活性成分
1.1 多糖类化合物
多糖是由10个及以上单糖通过不同类型的糖苷键连接而成的大分子聚合物,是枣中主要的活性物质[14],一般以多糖链的形式参与细胞壁的构成,发挥结构支撑和信号传导的作用[15]。枣多糖具有显著的抗炎效果,这与其分子量、单糖组成和糖苷键关系密切[16]。表1列举了枣多糖的组成成分和连接结构,并对多糖的抗炎活性进行总结整理。多糖分子量的大小影响其分子构型,从而赋予多糖不同的抗炎活性[17]。有研究表明,从灰枣中制备得到两种不同分子量的多糖(68.7 kDa和111 kDa),高分子量的多糖表现出了更大的免疫调节活性[18]。此外,单糖组成也显著影响了多糖的抗炎活性。枣多糖通常含有甘露糖、鼠李糖、半乳糖醛酸、阿拉伯糖、葡萄糖和半乳糖等,其中,甘露糖和半乳糖能被巨噬细胞特异性识别,并诱导细胞行为的动态变化[16]。糖醛酸能刺激细胞发挥更强的抗氧化活性,从而增强抗炎活性。葡萄糖能为细胞提供能量,细胞受到刺激时就会加快葡萄糖的利用。红枣多糖大多数为果胶多糖,含有半乳糖醛酸结构域,具有显著的抗炎效果,能下调炎症模型中一氧化氮(Nitrogen monoxide,NO)、活性氧(Reactive oxygen species,ROS)、白细胞介素-17(Interleukin 17,IL-17)和肿瘤坏死因子-α(Tumor necrosis factor-α,TNF-α)等细胞因子的表达,降低诱导型一氧化氮合酶(Inducible nitric oxide sythase,iNOS)和环氧化酶-2(Cyclooxygenase,COX-2)等酶活性,抑制相关通路发挥抗炎作用[19]。枣多糖结构复杂,具有分子量大、粘度高、生物利用度低等特点,虽有研究初步阐明了枣多糖的化学结构,但其结构与抗炎功能之间的联系仍不明确,对构效关系进行明确的阐述,有利于快速筛选具有较大生物活性和生物利用度的多糖,现在仍需学者们的不断努力。
表 1 枣多糖的基本结构和抗炎作用Table 1. Basic structure and anti-inflammatory effect of jujube polysaccharide序号 品种 提取方法 分子量(kDa) 单糖组成 化学结构 功能活性 参考文献 1 狗头枣 热水提取法 242 半乳糖醛酸:阿拉伯糖:半乳糖:鼠李糖:葡萄糖:木糖=4.2:1.0:0.6:0.5:0.2:0.2 β-D-Gal、α-D-GalpA、α-L-Ara和α-L-Rha 调节LPS刺激和MRSA感染下巨噬细胞产生的过度炎症反应,并正向调节口腔微生物群 [20] 2 若羌红枣 超声提取法 342 阿拉伯糖:葡萄糖:木糖:半乳糖:鼠李糖=
21.6:9.8:13.5:15.3:8.7α型和β型糖苷键,并带有吡喃糖环 诱导细胞凋亡、抗肿瘤 [21] 3 哈密大枣 热水提取法 69.8 阿拉伯糖:半乳糖:木糖:葡萄糖=56.9:20.0:8.7:8.5 →4)-α-D-GalpA (1→ 和→2,4)-α-L-Rhap (1→ 降低细胞内活性氧的产生,有抗炎活性 [22] 4 骏枣 热水提取法 153 − 1,4-D-GalpA 显著降低NO的产生,抑制NF-κB、MAPK信号通路的表达 [19] 5 木枣 碱提取法 89.9 阿拉伯糖:半乳糖:葡萄糖:鼠李糖:甘露糖=
49.67:29.01:11.43:5.38:4.51− 抑制了LPS诱导的RAW264.7细胞中促炎细胞因子的产生 [23] 6 木枣 碱提取法 9.37 鼠李糖:阿拉伯糖:木糖:甘露糖:葡萄糖:半乳糖:半乳糖醛酸=10.51:6.70:0.50:0.26:0.50:
6.75:74.691,4-α-D-GalAp 降低细胞中活性氧的含量 [24] 7 木枣 碱提取法 141 鼠李糖:阿拉伯糖:半乳糖:半乳糖醛酸=0.84:5.88:0.31:0.12 → 5)-α-L-Araf-(1 →和→3,5)-α-L-Araf-(1→ 有效清除自由基,有潜在的抗炎活性 [25] 8 木枣 超声辅助提取法 16.97 阿拉伯糖:半乳糖:葡萄糖:甘露糖:木糖=
17.36:3.29:2.68:1.05:1.001,3,5-Araf、1,3- Araf、1,5-Araf、1,4-Glcp、1-Araf和1-Glcp 降低脂质积累,改善血脂异常,抑制慢性炎症 [26] 9 木枣 热水提取法 28.94 鼠李糖:阿拉伯糖:木糖:甘露糖:半乳糖醛酸=
1:0.9:0.05:0.07:28.91,4-GalA 降低细胞内活性氧和丙二醛的产生,提高超氧化物歧化酶和过氧化氢酶的活性 [27] 10 红枣 亚临界水提取 713 阿拉伯糖:半乳糖:葡萄糖:甘露糖:鼠李糖:半乳糖醛酸=
0.14:0.08:0.09:0.16:0.15:0.34→4)-D-GalAp(1→ 显著的自由基清除能力,有潜在的抗炎活性 [28] 注:“−”:在文献中未阐述;Gal:低聚半乳糖、GalA:半乳糖醛酸、Ara:阿拉伯糖、Rha:鼠李糖、Glc:葡萄糖;单糖后缀“f”代表呋喃糖,“p”代表吡喃糖,都是单糖的存在形式。 1.2 多酚类化合物
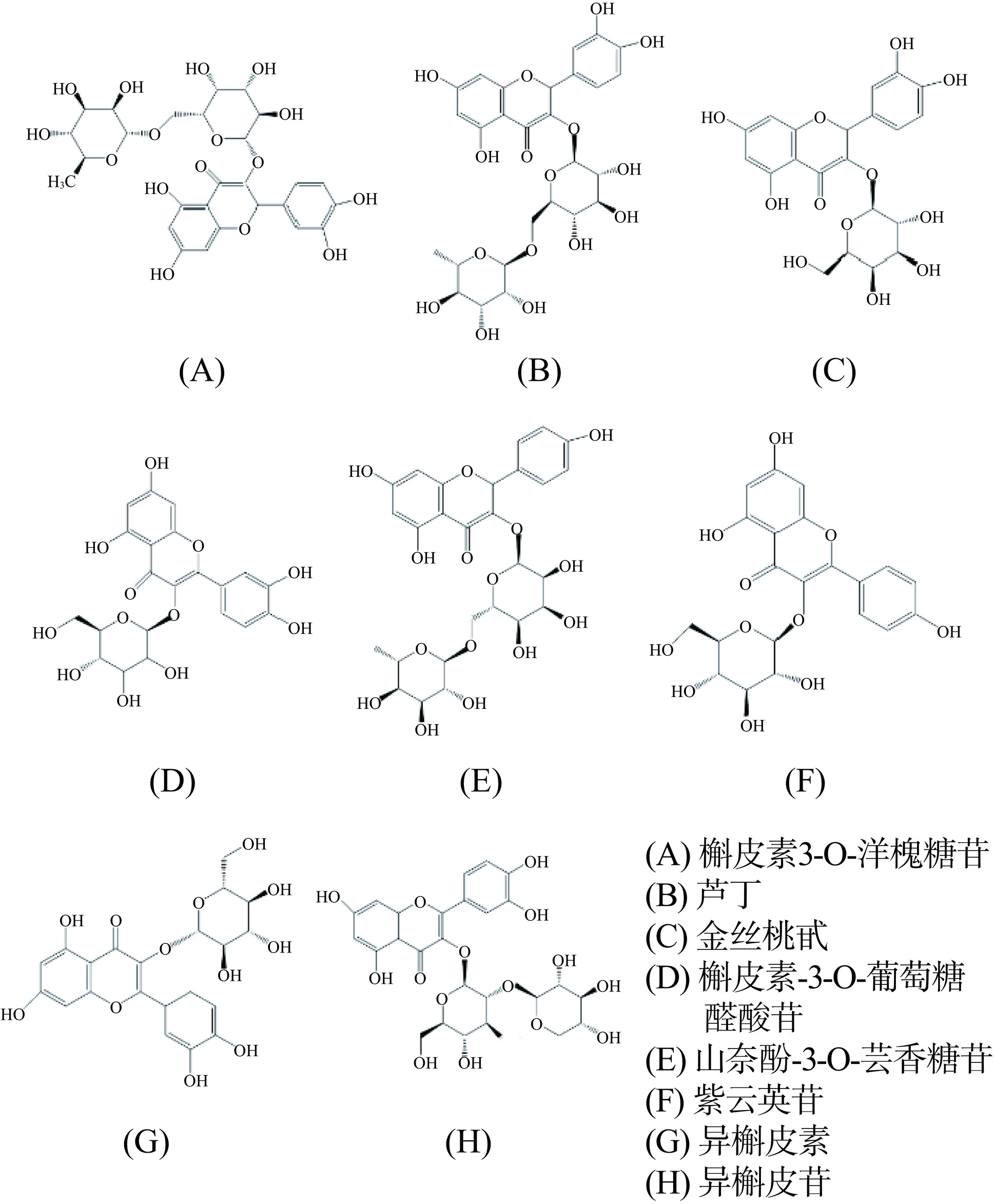
多酚是植物的次生代谢产物之一,主要分布在枣皮和枣核内,是红枣中主要的活性物质,多以与酯、糖苷结合的形式出现,具有抗氧化、抗炎和抗癌活性[29]。枣中含有多种多酚化合物,图2列举了8种枣多酚化合物[30]。多酚化合物具有共轭结构和多个羟基,有较好的抗氧化和抗炎活性。有研究表明,芦丁、儿茶素、表儿茶素是枣中主要的多酚类物质,占总多酚含量的90%[31]。肿胀是急性炎症的症状之一,口服儿茶素能抑制卡拉胶诱导的小鼠爪子肿胀现象,降低组织内TNF-α、白细胞介素-1β(Interleukin-1β,IL-1β)和白细胞介素-6(Interleukin-6,IL-6)等炎症因子的表达,并通过抑制炎症介质(组胺、前列腺素、缓激肽和血清素等)缓解由炎症引起的阵痛现象[32]。芦丁能抑制大鼠急性炎症模型中的血管扩张,降低血浆蛋白水平、中性粒细胞、白细胞、血小板和淋巴细胞的数量,降低血液中的ROS含量,抑制细胞凋亡,进而预防和改善小鼠的炎症反应[33]。此外,没食子酸能调节丝裂原活化蛋白激酶(Mitogen activated protein kinases,MAPK)、核因子κB(Nuclear factor kappa-B,NF-κB)和Toll样受体4/髓样分化因子88/TIR结构域衔接蛋白信号通路,抑制炎症细胞因子、趋化因子和粘附因子等的表达,改善炎症反应[34]。槲皮素、山奈酚等都能抑制结肠炎小鼠中NF-κB信号通路和谷胱甘肽(Glutathione,GSH)的表达,降低iNOS活性,有显著的抗炎效果[35]。儿茶素是主要的抗炎多酚物质,可以通过多靶点多通路发挥抗炎效果[19]。高脂饮食能诱导肠道微生物群紊乱和肠道炎症。阿魏酸能显著提高肠道内产短链脂肪酸菌的数量,增加短链脂肪酸的含量,并降低产内毒素相关菌和肥胖相关菌的活性,抑制结肠组织中NF-κB信号通路的表达,发挥抗炎活性[36]。枣多酚能通过多种途径发挥抗炎活性,包括抑制炎症因子的生成和调控NF-κB等相关信号通路的表达,降低组织内炎性细胞的浸润,并有效调节肠道菌群组成和肠道内容物,有显著的抗炎效果。
1.3 三萜类化合物
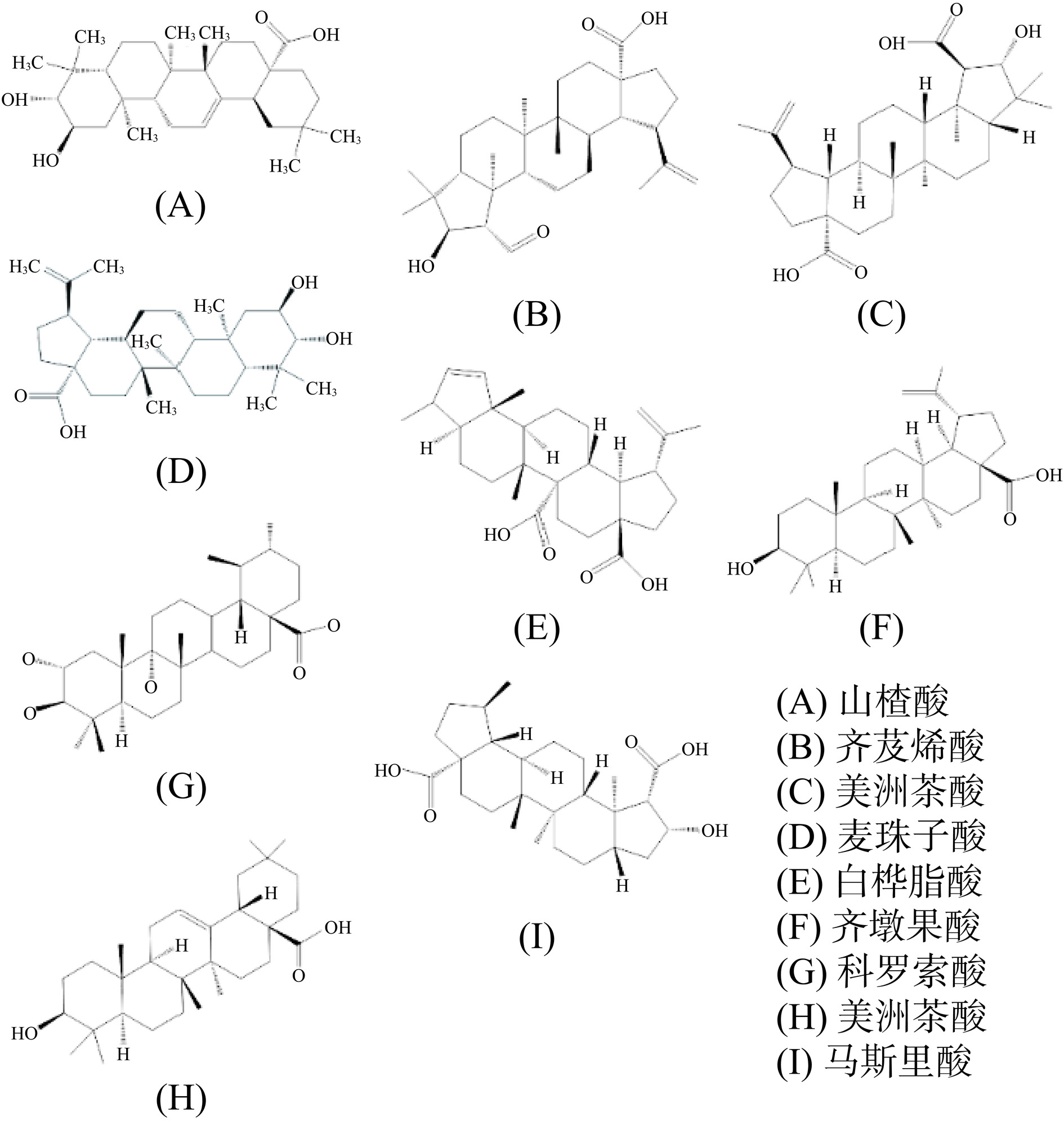
三萜类化合物是植物三大次生代谢产物之一,通常以游离状态存在于细胞中,可分为羽扇烷型、琼脂烷型、熊烷型和羊毛烷型,具有抗病毒、抗炎和抗菌等生物活性[37]。枣中的三萜类化合物主要为五环三萜类,其侧链结构(如羟基化、环氧化、环化、碳还原和双键的形成等)的差异导致了不同成分之间显著的活性差异,图3列举了枣中9种不同的三萜类化合物[38]。 Ruan等[39]从红枣中制备得到了29种三萜类化合物,研究发现在LPS诱导的RAW264.7细胞炎症模型中,熊果酸和齐墩果酸能通过抑制NO的产生发挥显著的抗炎效果,但2-O-反式-对-香豆酰-马来酸和3-氧代-乌苏-12-烯-28-酸在低浓度下对炎症反应中NO的产生无抑制效果,部分三萜类化合物还能下调IL-6和TNF-α等炎症因子的表达,降低iNOS的活性并抑制NF-κB信号通路来发挥抗炎作用。此外,白桦脂酸能降低关节炎小鼠中的关节炎症指数,缓解脚趾肿胀,增加血液流量,减少细胞凋亡,同时抑制组织内炎性细胞的浸润[40]。齐墩果酸能抑制促炎细胞因子的产生来发挥抗炎效果。熊果酸能显著降低炎症小鼠模型中IL-6、IL-1β和TNF-α的表达水平,对炎症有抑制作用[41]。山奈酚能显著降低关节炎小鼠中炎症因子的表达,并改善关节炎指数和爪子厚度,且平衡小鼠内肠道菌群及代谢物的波动[42]。皂苷A能降低高糖高脂引起的冠心病大鼠心肌组织中TNF-α、IL-1β、IL-6等炎症因子的产生,抑制NF-κB信号通路和MAPK信号通路,并改善心肌细胞凋亡[43]。总的来说,三萜类化合物能通过抑制炎症因子和相关炎症信号通路的表达来发挥抗炎活性,其活性在很大程度上受自身结构影响。
1.4 生物碱
生物碱是存在于生物体内的一类含氮的碱性有机物,具有抗镇痛、抗炎、抗氧化、抗菌和抗癌等活性功能,是植物的次生代谢产物[44]。生物碱已被证明在治疗炎症相关疾病方面具有潜力,例如癌症、关节炎和心血管疾病等[45]。Yuan等[46]从角茴香中提取得到的生物碱能显著改善卡拉胶诱导的小鼠爪子水肿情况,并从提取物中分离得到6种异喹啉类生物碱,研究发现这6种生物碱都能通过抑制脂多糖(Lipopolysaccharide,LPS)诱导的RAW264.7细胞中炎症因子(COX-2、IL-1β和TNF-α)的表达,对炎症有显著的改善作用。Wu等[47]从铁线莲中制备得到了20种生物碱,其中3种环肽生物碱对LPS诱导的RAW264.7细胞有抗炎效果。红枣中生物碱种类丰富,已知种类就有50多种,主要可分为环肽类生物碱和异喹啉类生物碱两大类[48]。有研究表明,狗牙枣中的生物碱能较好的清除自由基,具有良好的抗氧化和抗炎活性[49]。从泰国枣中分离出了6个14元环肽生物碱,这些生物碱有明显的抗菌、抗细胞毒性作用[50]。但枣中生物碱的含量较低,对红枣中生物碱的提取的文献也很少,这对生物碱的利用有较大的局限性。
1.5 核苷
环磷酸腺苷(Cyclic adenosine monophosphate,cAMP)和环磷酸鸟苷(Cyclic guanosine monophosphate,cGMP)是红枣中含量较高的核苷类物质,其总含量介于15.51~480.92 μg/g之间(以干枣为基准),也是调节物质代谢和生物体功能的主要物质[7]。有大量的研究表明,cAMP和cGMP通过调控不同的信号通路来抑制炎症反应。cGMP能通过激活蛋白激酶A,并抑制cGMP依赖性蛋白激酶的表达来抑制炎症细胞的活化和减少炎症因子的产生[51]。在哮喘小鼠中,cAMP能通过激活蛋白激酶A和转录因子CREB(cAMP响应元件结合蛋白)来抑制炎症反应,减少炎症因子(IL-6、TNF-α、IL-1β和IL-4)的释放和炎性细胞的活化[52]。采用超声辅助果胶酶提取法从骏枣中提取制备了cAMP,该物质提高了LPS诱导的巨噬细胞中IL-10的水平,减少了TNF-α的生成,表现出了显著的抗炎活性[53]。现已有核苷类药物应用到哮喘、乙型肝炎和肝硬化的临床治疗中[54]。
2. 抗炎作用机制
2.1 通过免疫系统调节炎症
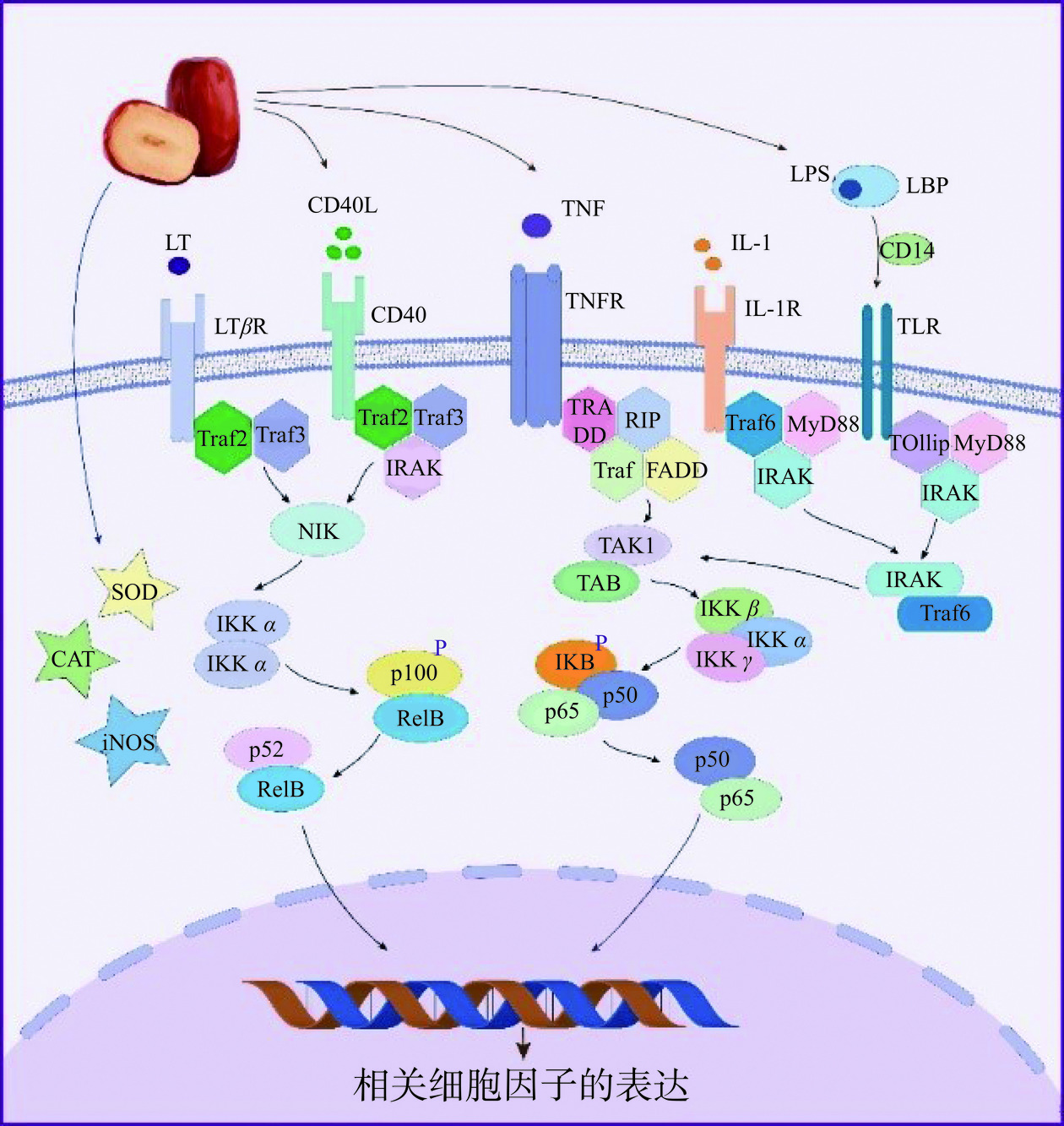
炎症反应是一个复杂的免疫过程,细胞表面受体识别到炎症信号后,会激发细胞内信号通路级联反应,调控细胞内上下游信号基因的表达,进一步增加促炎细胞因子的释放。枣活性成分能通过调节炎症因子的表达,缓解炎症反应作用程度和作用时间(图4)[55]。LPS诱导的巨噬RAW264.7细胞是经典的炎症细胞模型,从骏枣中分离纯化得到一个酸性多糖,将多糖作用到LPS诱导的巨噬RAW264.7细胞中,显著降低了细胞内NO、TNF-α、IL-17和γ-干扰素(Interferon-γ,IFN-γ)等细胞因子的表达水平[56]。枣皮多酚能通过抑制LPS诱导RAW264.7细胞中TNF-α、IL-1β、IL-6、NO和前列腺素E2(Prostaglandin E2,PGE2)的产生,从而抑制炎症反应[57]。从枣肉中提取得到的4种皂苷,均都能降低LPS诱导巨噬细胞中抗炎因子TNF-α的产生,具有抗炎活性[20]。此外,枣中的活性成分也能引起相关分子含量或性质的变化,进而调控信号通路,缓解炎症相关疾病。NF-κB是细胞内最主要的核转录因子,它参与机体的免疫应答、炎症反应、调节细胞凋亡、应激反应等各种基本细胞功能相关通路的激活。从枣皮中分离出三种多酚物质,分别是对香豆酸、儿茶素和芦丁,研究发现枣皮多酚能通过抑制LPS诱导的巨噬RAW264.7细胞中的MAPK和NF-κB信号通路,同时激活核转录因子红系2相关因子2(Nuclear factor-erythroid 2-related factor 2,Nrf2)信号通路,从而减轻氧化应激和炎症反应[57]。红枣多糖能显著抑制LPS诱导的巨噬RAW264.7细胞中NO的产生,对MAPK和NF-κB信号通路发挥抑制作用[19]。从红枣中提取得到了三萜类化合物,其显著下调了结肠炎小鼠中NF-κB信号通路中的人核因子κB抑制蛋白α(NF-kappa-B inhibitor alpha,IκB-α)和p65的蛋白表达,降低了炎症因子(IL-6和TNF-α)的表达和iNOS的活性,从而减轻了小鼠肠道内的炎症反应[58]。因此,红枣能通过调节炎症相关信号表达和酶活性来发挥抗炎作用(图4)。
2.2 通过抑制菌群调节炎症
正常机体的菌群处在稳定状态,当稳态被打破时,菌群数量比例失调,有害菌及其产生的毒素随血液进入循环系统并危害机体健康,损害细胞和组织并引起炎症反应[59]。红枣中多糖、多酚类化合物都能通过正向调节菌群,有效维持机体内环境稳态。从红枣中制备得到多酚提取物(PERJ),给溃疡性结肠炎小鼠灌胃PERJ后发现,PERJ有效改善了小鼠的疾病活动指数和脾脏指数,降低了细胞内炎症因子的表达和细胞间糖蛋白和粘蛋白的减少,并抑制了Nod样受体家族蛋白结构域3和MAPK信号通路相关蛋白的表达水平,并且正向调节小鼠肠道内菌群组成,增加厚壁菌门数量且减少变形菌门和拟杆菌属的数量[60]。炎症是结肠癌形成的必要因素之一[61]。分别给结肠癌小鼠喂食红枣粉、枣水溶性多糖和不溶性膳食纤维,结果表明,喂食红枣粉和多糖均能抑制肠道肿瘤的生长,在枣粉喂养组中,小鼠肠道内厚壁菌门和毛螺菌属的丰度明显升高,普雷沃菌科的丰度显著下降,同时肠道内丁酸的含量上升,肠道粘膜损伤有所改善,在一定程度上对结肠癌有抑制作用[62]。Ji等[63]也发现不同浓度的枣多糖能抑制结肠癌小鼠的死亡率和结肠缩短,下调结肠组织中IL-1β,IL-6和 TNF-α等炎症因子的表达,减轻结肠区域的炎症反应,并且减少肠道内有害菌数量,增加肠道有益菌的数量。Wang等[64]还发现枣多糖能调控肠道菌群中的菌种组成,抑制病原菌和脱硫弧菌等病原微生物生长,并促进巨单胞菌等有益菌的生长,具有良好的益生特性。从狗头枣中分离纯化得到一个酸性多糖,该多糖降低了LPS诱导的巨噬细胞中IL-1β、IL-6、TNF-α、粒细胞-巨噬细胞集落刺激因子、粒细胞集落刺激因子、趋化因子(C-X-C基序)配体1和细胞间黏附分子-1等促炎因子的产生,且有效抑制了变形链球菌、耐甲氧西林金黄色葡萄球菌和牙龈卟啉单胞菌在牙周的生长和粘附,并形成生物膜,抑制牙周炎的持续发生。另外,枣多糖增加了口腔共生菌和有益菌的数量,减少了致病菌的数量,且作用效果显著[20]。
2.3 通过抗氧化调节炎症
氧化应激能引起炎症反应,炎症反应本身也会引起氧化应激的发生[65]。已有研究表明,枣水提物能刺激乙醇诱导的HepG2细胞中Nrf2通路相关信号的表达,增加抗氧化防御酶(血红素加氧酶-1、NADPH醌氧化还原酶1和γ-谷氨酸-半胱氨酸连接酶)的表达,从而抑制细胞内的氧化应激和炎症反应[66]。从冬枣中分离出的结合多酚和游离多酚均能降低小鼠组织内丙二醛(Malondialdehyde,MDA)和ROS的表达水平,提高超氧化物歧化酶(Superoxide dismutase,SOD)和谷胱甘肽过氧化物酶(Glutathione peroxidase,GSH-Px)的活性,并调节MAPK、Nrf2和NF-κB信号通路,抑制氧化应激并避免炎症的发生[67]。枣果胶多糖能下调过氧化氢诱导的Caco-2细胞中ROS和MDA的表达含量,增加SOD和GSH-Px的活性,促进keap1-Nrf2的解离和Nrf2的活化,激活Nrf2-keap2-ARE信号通路,发挥抗氧化和抗炎作用[53]。枣中的生物碱也能较好的清除自由基,具有良好的抗氧化性能和抗炎活性[49]。
3. 对炎症性疾病的调节
3.1 结肠炎
肠道菌群和自身免疫系统的紊乱就易引发炎症性肠病[68]。红枣常被应于治疗结肠炎的处方中。有研究表明,枣水提物能抑制NF-κB 信号通路和促炎细胞因子的表达含量,从而延缓小鼠结肠炎的炎症反应。其中枣三萜类化合物可能是发挥了主要作用,其通过抑制结肠炎小鼠体重的减少,增加结肠长度,抑制肠道组织中磷脂酰肌醇-3-激酶/丝苏氨酸蛋白激酶/NF-κB信号通路的表达,并正向调节肠道菌群丰度,有效缓解肠道炎症[69]。Wei等[60]发现,枣多酚化合物能通过调节炎症相关通路的表达和调节肠道菌群来改善结肠炎。Ji等[63]还发现,枣多糖能增加结肠炎小鼠的结肠长度,降低其死亡率,加快肠黏膜细胞愈合速度,减少组织中促炎细胞因子的释放,并增加短链脂肪酸的浓度。此外,枣多糖还可以调控肠道内双歧杆菌属、拟杆菌属和乳酸菌属等益生菌的丰度,从而抑制肠道内的炎症反应和氧化应激,并增强小鼠的肠道屏障。
3.2 过敏性哮喘
过敏性哮喘是支气管气道内的一种持续性炎症性疾病。从红枣乙醇提取物中提取得到皂苷B,用卵清蛋白诱导小鼠过敏性哮喘,分别灌胃小鼠枣乙醇提取物和皂苷B,结果表明,乙醇提取物和皂苷B处理组均能显著抑制诱导剂引起的嗜酸性粒细胞、巨噬细胞和淋巴细胞等炎症细胞的增加,缓解过敏反应中小鼠爪子水肿和体积增加,降低组织内炎症因子表达水平,进而缓解和预防过敏性哮喘[70]。有研究表明,红枣抗过敏反应与其cAMP含量有关。从红枣中提取得到cAMP,用花生蛋白提取物建立小鼠过敏模型,cAMP处理组抑制了巨噬细胞和淋巴细胞诱导的过度免疫反应,且降低了组织内IL-4、TNF-α和IFN-γ的表达水平,从而发挥抗敏作用[53]。
3.3 糖脂异常
过量摄入高脂肪和高糖食物会导致血脂异常、糖代谢异常和肥胖等疾病,糖脂代谢异常能引起持续的全身炎症,是炎症主要的发病机制之一[71]。枣已被证明能通过抑制葡萄糖的吸收和α-葡萄糖苷酶的活性来改善葡萄糖代谢[72]。用高果糖喂养建立小鼠胰岛素抵抗和血脂异常模型,使用不同剂量的红枣多糖喂养小鼠,结果发现,实验组小鼠的血清葡萄糖、胰岛素、总胆固醇、甘油三酯、低密度脂蛋白胆固醇和高密度脂蛋白胆固醇水平显著降低,血液中胰岛素抵抗稳态模型评估、致动脉粥样硬化指数和β细胞功能有明显改善[73]。肥胖是一种自身脂肪过多引起的慢性代谢性疾病,在肥胖状态下会导致机体代谢紊乱和慢性低度炎症的发生[74]。连续给高脂肪模型小鼠喂养枣肉,结果发现枣处理组显著抑制了小鼠的体重和血糖的增长,提高了SOD、CAT和GHx抗氧化酶的活性,减少了MDA的生成,并抑制了促炎因子IL-6和TNF-α的表达,增加了抑炎因子IL-10的表达。在LPS诱导的RAW264.7细胞体外实验中,枣处理组也发挥了显著的抗炎活性[75]。
3.4 糖尿病
慢性炎症和先天免疫系统的激活是糖尿病发展的重要因素[76]。对48名不使用降糖药的2型糖尿病患者进行临床实验,连续6周食用30 g红枣干,结果显示,食用组患者血液中血糖含量、甘油三酯、总胆固醇和高密度脂蛋白胆固醇含量均下降,全身炎症和氧化应激得到有效改善[77]。Zhong等[78]用高糖高脂饮食建立糖尿病小鼠模型,使用皂苷A喂养小鼠,结果发现,处理组显著降低了小鼠空腹血糖指数和糖基化血清蛋白含量,提升了SOD、CAT和GPX等抗氧化酶的活性,抑制了ROS、MDA、BCL2-Associated X的蛋白质(Bax)、含半胱氨酸的天冬氨酸蛋白水解酶9(Ccysteinyl aspartate specific proteinase 9,Caspase9)和凋亡酶激活因子的表达水平,通过调节线粒体介导的细胞凋亡和抑制氧化应激来改善糖尿病。
3.5 阿兹海默症
阿兹海默症是一种神经系统退行性疾病,脑组织中的炎症反应伴随着阿兹海默症的发生而产生,阿兹海默症发病机制的核心因素β淀粉样蛋白也可以作为促炎因子间接激活炎症反应[79]。现有研究表明,红枣在一定程度上对阿兹海默症有改善效果,主要在于改善脑部组织的炎症反应和氧化应激,清除环境内的β淀粉样蛋白和增加神经细胞活性等。Choi等[80]发现,枣核中的黄酮类化合物能减弱H2O2诱导的PC-12神经细胞中的氧化应激,减少乳酸脱氢酶的产生,并增强PC-12细胞活性。其中,对香豆酸、芦丁、异槲皮素和山奈酚等7种酚类化合物发挥了主要作用。皂苷B能增强线虫模型中的短期学习和认知能力,并抑制脂质过氧化和内源性脂褐素的积累,从而减少β淀粉样蛋白对机体的损害,此外,在β淀粉样蛋白诱导的细胞凋亡模型中,皂苷B还可以减轻线粒体功能障碍并抑制过量ROS的产生,增加ATP合成以改善能量代谢,降低乳酸脱氢酶的释放速率,并抑制Bax和caspase3的表达,促进B淋巴细胞瘤-2基因的产生来缓解神经元细胞的凋亡和炎症反应[81]。给β淀粉样蛋白诱导的认知障碍小鼠注射皂苷A,结果发现,皂苷A显著减轻了小鼠的学习行为障碍,抑制了小鼠海马和大脑皮质中乙酰胆碱酯酶的活性,减少了MDA和NO的产生,从而抑制炎症反应相关酶和蛋白的表达[82]。
3.6 关节炎
关节炎是一种常见的慢性炎症疾病,其病理在于关节骨损伤,滑膜产生持续性炎症,导致关节疼痛、肿胀和僵硬。在卡拉胶诱导的关节炎小鼠模型中,组织内ROS和蛋白水解酶过度产生,增加了前列腺素的表达,并促进了TNF-α和IL-1β的产生,导致骨关节炎症。Shahwar等[83]使用甲醇、乙酸乙酯和氯仿从枣中制备得到三种提取物,结果发现,这三种提取物均能抑制细胞内的蛋白变性,具有潜在的抗炎效果,其中氯仿提取物(CE)的抗炎活性最强,对其进行分离纯化,得到一个五环三萜化合物((3β)-3-甲基-20(29)-烯-28-酸羟甲基酯),该化合物下调了关节炎小鼠中COX-2、NF-κB、TNF-α和IL-1β的表达,具有显著的抗炎效果。Khoramjouy等[84]还发现,在碘乙酸钠诱导关节炎小鼠模型中,分别灌胃小鼠不同量的含有1%山奈酚的枣乙醇提取物,枣处理组能显著增加关节炎小鼠的体重和总移动距离,降低关节组织中炎症细胞因子IL-1β、IL-6和TNF-α的含量,进而改善小鼠的关节炎症。这表明,枣能通过激活各种信号,抑制促炎细胞因子的产生进而改善关节炎症,这可能是枣三萜类和多酚类化合物发挥了主要作用。
4. 结论与展望
红枣性温味甘,营养物质丰富,价格低廉,产量颇丰,易贮存,在食品行业被广泛应用。红枣能通过调控免疫系统、氧化应激和肠道菌群等,对结肠炎、过敏性哮喘、肥胖和糖尿病等炎症性疾病发挥改善作用,具有较大的发展潜力和应用价值。本文对多糖、多酚、三萜类和生物碱等主要的活性成分进行研究,有助于明确枣抗炎活性成分的功能活性,揭示枣的抗炎靶点,阐明其分子机制,推动绿色食品的开发,带动枣果精细加工产业发展,进一步扩宽枣市场,解决枣生产过剩等问题。在探索枣及活性成分对相关疾病改善活性的过程中发现,食用全枣或枣全提取物的效果要优于单一活性物质,枣单一活性成分的作用效果与枣多成分之间的协同作用仍有一定差异,目前对单一物质作用机制仅限于单方向的研究,但人体是一个多向关联的整体,未来是否可以从多层次进行探究,以寻找不同成分的内在调节机制。此外,对红枣的研究多集中在多糖和多酚类化合物中,而皂苷、cAMP和cGMP等具有较大生物功能的成分因其含量较少而受到限制,未来是否可以通过探究其化学结构,采用人工合成的方式对其进行应用,这仍需要学者的不断探索。目前学者们对枣活性成分的研究大都是体内动物实验和体外细胞实验,很少有研究去验证人食用后对机体的实际作用,这对红枣的应用有一定的局限性。最后,我们发现肠道菌群在抗炎活性中发挥重要作用,但对其研究深度还远远不够,未来能否从“肠脑轴”等方向对其进行深层次作用机制的探究,进一步对作用机制进行阐述,为红枣应用提供理论基础。
-
表 1 枣多糖的基本结构和抗炎作用
Table 1 Basic structure and anti-inflammatory effect of jujube polysaccharide
序号 品种 提取方法 分子量(kDa) 单糖组成 化学结构 功能活性 参考文献 1 狗头枣 热水提取法 242 半乳糖醛酸:阿拉伯糖:半乳糖:鼠李糖:葡萄糖:木糖=4.2:1.0:0.6:0.5:0.2:0.2 β-D-Gal、α-D-GalpA、α-L-Ara和α-L-Rha 调节LPS刺激和MRSA感染下巨噬细胞产生的过度炎症反应,并正向调节口腔微生物群 [20] 2 若羌红枣 超声提取法 342 阿拉伯糖:葡萄糖:木糖:半乳糖:鼠李糖=
21.6:9.8:13.5:15.3:8.7α型和β型糖苷键,并带有吡喃糖环 诱导细胞凋亡、抗肿瘤 [21] 3 哈密大枣 热水提取法 69.8 阿拉伯糖:半乳糖:木糖:葡萄糖=56.9:20.0:8.7:8.5 →4)-α-D-GalpA (1→ 和→2,4)-α-L-Rhap (1→ 降低细胞内活性氧的产生,有抗炎活性 [22] 4 骏枣 热水提取法 153 − 1,4-D-GalpA 显著降低NO的产生,抑制NF-κB、MAPK信号通路的表达 [19] 5 木枣 碱提取法 89.9 阿拉伯糖:半乳糖:葡萄糖:鼠李糖:甘露糖=
49.67:29.01:11.43:5.38:4.51− 抑制了LPS诱导的RAW264.7细胞中促炎细胞因子的产生 [23] 6 木枣 碱提取法 9.37 鼠李糖:阿拉伯糖:木糖:甘露糖:葡萄糖:半乳糖:半乳糖醛酸=10.51:6.70:0.50:0.26:0.50:
6.75:74.691,4-α-D-GalAp 降低细胞中活性氧的含量 [24] 7 木枣 碱提取法 141 鼠李糖:阿拉伯糖:半乳糖:半乳糖醛酸=0.84:5.88:0.31:0.12 → 5)-α-L-Araf-(1 →和→3,5)-α-L-Araf-(1→ 有效清除自由基,有潜在的抗炎活性 [25] 8 木枣 超声辅助提取法 16.97 阿拉伯糖:半乳糖:葡萄糖:甘露糖:木糖=
17.36:3.29:2.68:1.05:1.001,3,5-Araf、1,3- Araf、1,5-Araf、1,4-Glcp、1-Araf和1-Glcp 降低脂质积累,改善血脂异常,抑制慢性炎症 [26] 9 木枣 热水提取法 28.94 鼠李糖:阿拉伯糖:木糖:甘露糖:半乳糖醛酸=
1:0.9:0.05:0.07:28.91,4-GalA 降低细胞内活性氧和丙二醛的产生,提高超氧化物歧化酶和过氧化氢酶的活性 [27] 10 红枣 亚临界水提取 713 阿拉伯糖:半乳糖:葡萄糖:甘露糖:鼠李糖:半乳糖醛酸=
0.14:0.08:0.09:0.16:0.15:0.34→4)-D-GalAp(1→ 显著的自由基清除能力,有潜在的抗炎活性 [28] 注:“−”:在文献中未阐述;Gal:低聚半乳糖、GalA:半乳糖醛酸、Ara:阿拉伯糖、Rha:鼠李糖、Glc:葡萄糖;单糖后缀“f”代表呋喃糖,“p”代表吡喃糖,都是单糖的存在形式。 -
[1] JI X L, HOU C Y, ZHANG X L, et al. Microbiome-metabolomic analysis of the impact of Zizyphus jujuba cv. Muzao polysaccharides consumption on colorectal cancer mice fecal microbiota and metabolites[J]. International Journal of Biological Macromolecules,2019,131:1067−1076. doi: 10.1016/j.ijbiomac.2019.03.175
[2] GAO Q H, WU C S, WANG M. The jujube (Ziziphus jujuba mill.) fruit:A review of current knowledge of fruit composition and health benefits[J]. Journal of Agricultural and Food Chemistry,2013,61(14):3351−3363. doi: 10.1021/jf4007032
[3] 王军, 张宝善, 陈锦屏. 红枣营养成分及其功能的研究[J]. 食品研究与开发,2003(2):68−72. [WANG J, ZHANG B S, CHEN J P. Study on nutritional components and functions of red jujube[J]. Food Research and Development,2003(2):68−72.] WANG J, ZHANG B S, CHEN J P. Study on nutritional components and functions of red jujube[J]. Food Research and Development, 2003(2): 68−72.
[4] 张砚垒. 不同品种红枣营养成分分析及抗氧化活性研究[D]. 泰安:山东农业大学, 2022. [ZHANG Y L. Nutritional composition analysis and antioxidant activity of different varieties of red jujube[D]. Taian:Shandong Agricultural University, 2022.] ZHANG Y L. Nutritional composition analysis and antioxidant activity of different varieties of red jujube[D]. Taian: Shandong Agricultural University, 2022.
[5] 高娅, 杨洁, 杨迎春, 等. 不同品种红枣中三萜酸及环核苷酸的测定[J]. 中成药,2012,34(10):1961−1965. [GAO Y, YANG J, YANG Y C, et al. Determination of triterpenoid acids and cyclic nucleotides in different varieties of jujube[J]. Chinese Patent Medicine,2012,34(10):1961−1965.] GAO Y, YANG J, YANG Y C, et al. Determination of triterpenoid acids and cyclic nucleotides in different varieties of jujube[J]. Chinese Patent Medicine, 2012, 34(10): 1961−1965.
[6] 朱星宇, 郭东起. 红枣关键功能成分及其生物活性的研究进展[J]. 食品研究与开发,2021,42(8):197−201. [ZHU X Y, GUO D Q. Research progress of key functional components and biological activities of Chinese jujube[J]. Food Research and Development,2021,42(8):197−201.] ZHU X Y, GUO D Q. Research progress of key functional components and biological activities of Chinese jujube[J]. Food Research and Development, 2021, 42(8): 197−201.
[7] 吴喆, 朱佳敏, 刘军, 等. 红枣主要活性成分及其功能活性研究进展[J]. 现代食品科技,2024,40(9):359−369. [WU J, ZHU J M, LIU J, et al. Research progress of main active ingredients and functional activities of red jujube[J]. Modern Food Science and Technology,2024,40(9):359−369.] WU J, ZHU J M, LIU J, et al. Research progress of main active ingredients and functional activities of red jujube[J]. Modern Food Science and Technology, 2024, 40(9): 359−369.
[8] SOLIER S, MÜLLER S, CAÑEQUE T, et al. A druggable copper-signalling pathway that drives inflammation[J]. Nature,2023,617(7960):386−394. doi: 10.1038/s41586-023-06017-4
[9] BARANWAL A, AGGARWAL P, RAI A, et al. Pharmacological actions and underlying mechanisms of catechin:A review[J]. MINI-Reviews in Medicinal Chemistry,2022,22(5):821−833. doi: 10.2174/1389557521666210902162120
[10] LIN L, HUANG C X, LIU D Q, et al. WNT2B activates macrophages via NF-κB signaling pathway in inflammatory bowel disease[J]. FASEB Journal,2024,38(6):23551. doi: 10.1096/fj.202302213R
[11] HE W J, TANG M, GU R F, et al. The role of p53 in regulating chronic inflammation and panoptosis in diabetic wounds[J]. Aging and Disease, 2024:38377027.
[12] FRANÇOISE S L, ELODIE G V, STEPHANIE C, et al. Inflammation is a major regulator of drug metabolizing enzymes and transporters:consequences for the personalization of drug treatment[J]. Pharmacology Therapeutics,2020,215:107627. doi: 10.1016/j.pharmthera.2020.107627
[13] ALSAYARI A, WAHAB S. Genus ziziphus for the treatment of chronic inflammatory diseases[J]. Saudi Journal of Biological Sciences,2021,28:6897−6914. doi: 10.1016/j.sjbs.2021.07.076
[14] 冀晓龙, 郭建行, 田静源, 等. 植物多糖降解方法及降解产物特性研究进展[J]. 轻工学报,2023,38(3):55−62. [JI X L, GUO J X, TIAN J Y, et al. Research progress on degradation methods of plant polysaccharides and properties of degradation products[J]. Journal of Light Engineering,2023,38(3):55−62.] JI X L, GUO J X, TIAN J Y, et al. Research progress on degradation methods of plant polysaccharides and properties of degradation products[J]. Journal of Light Engineering, 2023, 38(3): 55−62.
[15] KOU X H, CHEN Q, LI X H, et al. Quantitative assessment of bioactive compounds and the antioxidant activity of 15 jujube cultivars[J]. Food Chemistry,2015,173:1037−1044. doi: 10.1016/j.foodchem.2014.10.110
[16] HOU C, CHEN L S, YANG L, et al. An insight into anti-inflammatory effects of natural polysaccharides[J]. International Journal of Biological Macromolecules,2020,153:248−255. doi: 10.1016/j.ijbiomac.2020.02.315
[17] YANG L, ZHANG L M. Chemical structural and chain conformational characterization of some bioactive polysaccharides isolated from natural sources[J]. Carbohydrate Polymers,2009,76(3):349−361. doi: 10.1016/j.carbpol.2008.12.015
[18] ZOU M, CHEN Y L, WATERHOUSE S D, et al. Immunomodulatory acidic polysaccharides from Zizyphus jujuba cv. Huizao:Insights into their chemical characteristics and modes of action[J]. Food Chemistry,2018,258:35−42. doi: 10.1016/j.foodchem.2018.03.052
[19] ZHAN R, XIA L, SHAO J H, et al. Polysaccharide isolated from Chinese jujube fruit (Zizyphus jujuba cv. Junzao) exerts anti-inflammatory effects through MAPK signaling[J]. Journal of Functional Foods,2018,40:461−470. doi: 10.1016/j.jff.2017.11.026
[20] XU D, XIAO J, JIANG D Z, et al. Inhibitory effects of a water-soluble jujube polysaccharide against biofilm-forming oral pathogenic bacteria[J]. International Journal of Biological Macromolecules,2022,208:1046−1062. doi: 10.1016/j.ijbiomac.2022.03.196
[21] WU Z, LI H, WANG Y D, et al. Optimization extraction, structural features and antitumor activity of polysaccharides from Z. jujuba cv. Ruoqiangzao seeds[J]. International Journal of Biological Macromolecules,2019,135:1151−1161. doi: 10.1016/j.ijbiomac.2019.06.020
[22] YANG Y M, QIU Z C, LI L Y, et al. Structural characterization and antioxidant activities of one neutral polysaccharide and three acid polysaccharides from Ziziphus jujuba cv. Hamidazao:A comparison[J]. Carbohydrate Polymers,2021,261:117879. doi: 10.1016/j.carbpol.2021.117879
[23] JI X L, PENG Q, LI H Y, et al. Chemical characterization and anti-inflammatory activity of polysaccharides from Zizyphus jujube cv. Muzao[J]. International Journal of Food Engineering,2017,13(7):0382.
[24] LIN X M, JI X L, WANG M, et al. An alkali-extracted polysaccharide from Zizyphus jujuba cv. Muzao:Structural characterizations and antioxidant activities[J]. International Journal of Biological Macromolecules,2019,136(1):607−615.
[25] JI X L, GUO J H, DING D Q, et al. Structural characterization and antioxidant activity of a novel high-molecular-weight polysaccharide from Ziziphus jujuba cv. Muzao[J]. Journal of Food Measurement and Characterization,2022,16(3):2191−2200. doi: 10.1007/s11694-022-01288-3
[26] JI X L, LIU F, PENG Q, et al. Purification, structural characterization, and hypolipidemic effects of a neutral polysaccharide from Ziziphus jujuba cv. Muzao[J]. Food Chemistry,2018,245:1124−1130. doi: 10.1016/j.foodchem.2017.11.058
[27] LIN M, LIU K S, YIN S, et al. A novel pectic polysaccharide of jujube pomace:structural analysis and intracellular antioxidant activities[J]. Antioxidants,2020,9(2):127−132. doi: 10.3390/antiox9020127
[28] LIU X X, LIU H M, YAN Y Y, et al. Structural characterization and antioxidant activity of polysaccharides extracted from jujube using subcritical water[J]. LWT-Food Science and Technology,2020,117(1):108645.
[29] ZHANG J T, DENG J J, YANG H X. Natural polyphenols and metabolic syndrome[J]. Frontiers in Nutrition,2023,10:1190577. doi: 10.3389/fnut.2023.1190577
[30] LIU H X, GUO X H, WU J J, et al. Determination of polyphenols in Chinese jujube using ultra-performance liquid chromatography-mass spectrometry[J]. Open Chemistry,2023,21(1):0305.
[31] 刘杰超, 张春岭, 陈大磊, 等. 不同品种枣果实发育过程中多酚类物质、VC含量的变化及其抗氧化活性[J]. 食品科学,2015,36(17):94−98. [LIU J C, ZHANG C L, CHEN D L, et al. Changes of polyphenols and VC contents and their antioxidant activities in fruits of different Jujube cultivars during development[J]. Food Science,2015,36(17):94−98.] LIU J C, ZHANG C L, CHEN D L, et al. Changes of polyphenols and VC contents and their antioxidant activities in fruits of different Jujube cultivars during development[J]. Food Science, 2015, 36(17): 94−98.
[32] ALSAYED E, ABDELDAIM M. Analgesic and anti-inflammatory activities of epicatechin gallate from Bauhinia hookeri[J]. Drug Development Research,2018,79(4):157−164. doi: 10.1002/ddr.21430
[33] ADEFEGHA S A, LEAL D B R, OLIVEIRA J S, et al. Modulation of reactive oxygen species production, apoptosis and cell cycle in pleural exudate cells of carrageenan-induced acute inflammation in rats by rutin[J]. Food Function,2017,8(12):4459−4468. doi: 10.1039/C7FO01008G
[34] BAI J R, ZHANG Y S, TANG C, et al. Gallic acid:Pharmacological activities and molecular mechanisms involved in inflammation-related diseases[J]. Biomedicine Pharmacotherapy,2021,133:110985. doi: 10.1016/j.biopha.2020.110985
[35] ETTITAOU A, KABDY H, OUBELLA K, et al. Molecular docking of quercetin:A promising approach for the development of new anti-inflammatory and analgesic drugs[J]. Natural Product Research, 2024:1−10.
[36] TIAN B M, GENG Y, WANG P Y, et al. Ferulic acid improves intestinal barrier function through altering gut microbiota composition in high-fat diet-induced mice[J]. European Journal of Nutrition,2022,61(7):3767−3783. doi: 10.1007/s00394-022-02927-7
[37] GE J M, LIU Z, ZHONG Z C, et al. Natural terpenoids with anti-inflammatory activities:potential leads for anti-inflammatory drug discovery[J]. Bioorganic Chemistry,2022,124(1):105817.
[38] SONG L J, ZHANG L, XU L, et al. Optimized extraction of total triterpenoids from jujube (Ziziphus jujuba mill.) and comprehensive analysis of triterpenic acids in different cultivars[J]. Plants,2020,9(4):412−419. doi: 10.3390/plants9040412
[39] RUAN J Y, SUN F, HAO M M, et al. Structurally diverse triterpenes obtained from the fruits of Ziziphus jujuba Mill. as inflammation inhibitors by NF-κB signaling pathway[J]. Food Function,2021,12(10):4496−4503. doi: 10.1039/D1FO00117E
[40] LUO Z J, HE H, TANG T T, et al. Synthesis and biological evaluations of betulinic acid derivatives with inhibitory activity on hyaluronidase and anti-inflammatory effects against hyaluronic acid fragment induced inflammation[J]. Frontiers in Chemistry,2022,10:892554. doi: 10.3389/fchem.2022.892554
[41] ZHAO M, WU F Y, TANG Z H, et al. Anti-inflammatory and antioxidant activity of ursolic acid:A systematic review and meta-analysis[J]. Frontiers in Pharmacology,2023,14:1256946. doi: 10.3389/fphar.2023.1256946
[42] ALAM W, KHAN H, SHAH M A, et al. Kaempferol as a dietary anti-inflammatory agent:Current therapeutic standing[J]. Molecules,2020,25(18):4073. doi: 10.3390/molecules25184073
[43] FU Q, YUAN H M, CHEN J, et al. Dammarane-type saponins from Ziziphus jujube and their inhibitory effects against TNF-α release in LPS-induced RAW 246.7 macrophages[J]. Phytochemistry Letters,2016,16:169−173. doi: 10.1016/j.phytol.2016.04.010
[44] BAI R R, YAO C S, ZHONG Z C, et al. Discovery of natural anti-inflammatory alkaloids:potential leads for the drug discovery for the treatment of inflammation[J]. European Journal of Medicinal Chemistry,2021,213:113165. doi: 10.1016/j.ejmech.2021.113165
[45] TAECHAKULWANIJYA N, WEERAPREEYAKUL N, BARUSRUX S, et al. Apoptosis-inducing effects of jujube (Zao) seed extracts on human Jurkat leukemia T cells[J]. Chinese Medicine,2016,11:1−13. doi: 10.1186/s13020-015-0073-6
[46] YUAN H L, ZHAO Y L, QIN X J, et al. Diverse isoquinolines with anti-inflammatory and analgesic bioactivities from Hypecoum erectum[J]. Journal of Ethnopharmacology,2021,270(24):113811.
[47] WU Y, ZHANG Y F, ZHANG H, et al. A new cyclopeptide alkaloid from clematis florida, natural product research[J]. 2022, 36(7):1693−1699.
[48] 张砚垒, 高琳, 张仁堂. 红枣活性成分及其生物活性的研究进展[J]. 中国食物与营养,2020,26(3):9−13. [ZHANG Y L, GAO L, ZHANG R T. Research progress of active ingredients and biological activities of red jujube[J]. Chinese Food and Nutrition,2020,26(3):9−13.] ZHANG Y L, GAO L, ZHANG R T. Research progress of active ingredients and biological activities of red jujube[J]. Chinese Food and Nutrition, 2020, 26(3): 9−13.
[49] 张向前, 屈晓逸, 刘冲, 等. 红枣中生物碱的提取工艺优化及抗氧化性分析[J]. 分子植物育种,2019,17(3):972−977. [ZHANG X Q, QU X Y, LIU C, et al. Extraction process optimization and antioxidant analysis of alkaloids from jujube[J]. Molecular Plant Breeding,2019,17(3):972−977.] ZHANG X Q, QU X Y, LIU C, et al. Extraction process optimization and antioxidant analysis of alkaloids from jujube[J]. Molecular Plant Breeding, 2019, 17(3): 972−977.
[50] SHI Q Q, HAN G, LIU Y, et al. Nutrient composition and quality traits of dried jujube fruits in seven producing areas based on metabolomics analysis[J]. Food Chemistry,2022,385:132627. doi: 10.1016/j.foodchem.2022.132627
[51] BROWNER N, SELLAK H, LINCOLN T. Downregulation of cGMP-dependent protein kinase expression by inflammatory cytokines in vascular smooth muscle cells[J]. American Journal of Physiology-Cell Physiology,2004,287(1):88−96. doi: 10.1152/ajpcell.00039.2004
[52] 郑亚娟, 袁培培, 傅阳, 等. 基于cAMP/PKA/CREB信号通路探讨麻黄生物碱组分对过敏性哮喘大鼠的抗炎机制[J]. 中药新药与临床药理,2022,33(11):1453−1459. [ZHENG Y J, YUAN P P, FU Y, et al. Study on the anti-inflammatory mechanism of ephedra alkaloid components in allergic asthmatic rats based on cAMP/PKA/CREB signaling pathway[J]. New Chinese Medicine and Clinical Pharmacology,2022,33(11):1453−1459.] ZHENG Y J, YUAN P P, FU Y, et al. Study on the anti-inflammatory mechanism of ephedra alkaloid components in allergic asthmatic rats based on cAMP/PKA/CREB signaling pathway[J]. New Chinese Medicine and Clinical Pharmacology, 2022, 33(11): 1453−1459.
[53] SANG C W, BAI Q, FENG X P, et al. Optimized extraction of cAMP from jujube by ultra-high pressure technology and the anti-allergic effect for peanut allergy mouse[J]. Frontiers in Nutrition,2022,9:862900. doi: 10.3389/fnut.2022.862900
[54] 陈梅花. 核苷类药物用于慢性乙型病毒性肝炎治疗的疗效分析[J]. 现代医学与健康研究电子杂志,2023,7(19):35−37. [CHEN M H. Analysis of efficacy of nucleoside drugs in the treatment of chronic viral hepatitis B[J]. Electronic Journal of Modern Medicine and Health Research,2023,7(19):35−37.] CHEN M H. Analysis of efficacy of nucleoside drugs in the treatment of chronic viral hepatitis B[J]. Electronic Journal of Modern Medicine and Health Research, 2023, 7(19): 35−37.
[55] 朱文振, 范营营, 谢绍华, 等. 蜂蜜抗炎作用的研究进展[J]. 食品工业科技,2024,45(14):426−434. [ZHU W Z, FAN Y Y, XIE S H, et al. Research progress of anti-inflammatory effect of honey[J]. Science and Technology of Food Industry,2024,45(14):426−434.] ZHU W Z, FAN Y Y, XIE S H, et al. Research progress of anti-inflammatory effect of honey[J]. Science and Technology of Food Industry, 2024, 45(14): 426−434.
[56] XUE H K, WANG W L, BIAN J Y, et al. Recent advances in medicinal and edible homologous polysaccharides:extraction, purification, structure, modification, and biological activities[J]. International Journal of Biological Macromolecules,2022,222:1110−1126. doi: 10.1016/j.ijbiomac.2022.09.227
[57] SHEN D B, WU C, FAN G J, et al. Jujube peel polyphenols synergistically inhibit lipopolysaccharide-induced inflammation through multiple signaling pathways in RAW264.7 cells[J]. Food and Chemical Toxicology,2022,164:113062. doi: 10.1016/j.fct.2022.113062
[58] MASULLO M, MONTORO P, AUTORE G, et al. Quali-quantitative determination of triterpenic acids of Ziziphus jujuba fruits and evaluation of their capability to interfere in macrophages activation inhibiting NO release and iNOS expression[J]. Food Research International,2015,77:109−117. doi: 10.1016/j.foodres.2015.09.009
[59] CHEN J M, WANG M C, ZHANG P, et al. Cordycepin alleviated metabolic inflammation in western diet-fed mice by targeting intestinal barrier integrity and intestinal flora[J]. Pharmacological Research,2022,178(1):106191.
[60] WEI X B, MA N, YANG W, et al. Polyphenol extracts from Ziziphus jujuba mill. “junzao” attenuates ulcerative colitis by inhibiting the NLRP3 and MAPKs signaling pathways and regulating gut microbiota homeostasis in mice[J]. Molecular Nutrition Food Research,2024,68(8):2300643. doi: 10.1002/mnfr.202300643
[61] PAULINO D S M, MENDES M C S, CAMARGO J A, et al. Diacerein treatment prevents colitis-associated cancer in mice[J]. World Journal of Clinical Oncology,2020,11(9):732−746. doi: 10.5306/wjco.v11.i9.732
[62] WANG L, NAN J, LIU X D, et al. Nurturing and modulating gut microbiota with jujube powder to enhance anti-PD-L1 efficiency against murine colon cancer[J]. Journal of Functional Foods,2020,64(1):103647.
[63] JI X L, HOU C Y, GAO Y G, et al. Metagenomic analysis of gut microbiota modulatory effects of jujube (Ziziphus jujuba Mill.) polysaccharides in a colorectal cancer mouse model[J]. Food Function,2020,11(1):163−173. doi: 10.1039/C9FO02171J
[64] WANG N, LI Q Y, LIU M L, et al. Structural characterization of alkali-extracted jujube polysaccharides and their effects on the fecal microbiota in vitro[J]. LWT-Food Science and Technology,2023,184:115087. doi: 10.1016/j.lwt.2023.115087
[65] MCGARRY T, BINIECKA M, VEALE D J, et al. Hypoxia, oxidative stress and inflammation[J]. Free Radical Biology and Medicine,2018,125:15−24. doi: 10.1016/j.freeradbiomed.2018.03.042
[66] HONG S W, KIM Y H, SONG R J, et al. Jujube (Ziziphus jujuba Mill.) protects hepatocytes against alcohol-induced damage through Nrf2 activation[J]. Evidence-Based Complementary and Alternative Medicine,2020,10:1−12.
[67] SHAN S H, XIE Y, ZHAO H L, et al. Bound polyphenol extracted from jujube pulp triggers mitochondria-mediated apoptosis and cell cycle arrest of HepG2 cell in vitro and in vivo[J]. Journal of Functional Foods,2019,53:187−196. doi: 10.1016/j.jff.2018.12.017
[68] PAN Y P, ZHANG H J, LI M H, et al. Novel approaches in IBD therapy:targeting the gut microbiota-bile acid axis[J]. Gut Microbes,2024,16(1):2356284. doi: 10.1080/19490976.2024.2356284
[69] RUAN J Y, LI H M, LU M Q, et al. Bioactive triterpenes of jujube in the prevention of colorectal cancer and their molecular mechanism research[J]. Phytomedicine,2023,110:154639. doi: 10.1016/j.phymed.2022.154639
[70] NINAVE P B, PATIL S D. Antiasthmatic potential of Zizyphus jujuba Mill. and jujuboside B. -possible role in the treatment of asthma[J]. Respiratory Physiology Neurobiology, 2019, 260:28−36.
[71] KIM J M, HEO H J. The roles of catechins in regulation of systemic inflammation[J]. Food Science and Biotechnology,2022,31(8):957−970. doi: 10.1007/s10068-022-01069-0
[72] 杨丽娜, 曹媛. 炎症和自噬及其交互作用在胰岛素抵抗中作用机制的研究进展[J]. 吉林大学学报(医学版),2019,45(3):742−746. [YANG L N, CAO Y. Research progress of inflammation, autophagy and their interaction in insulin resistance[J]. Journal of Jilin University (Medical Edition),2019,45(3):742−746.] YANG L N, CAO Y. Research progress of inflammation, autophagy and their interaction in insulin resistance[J]. Journal of Jilin University (Medical Edition), 2019, 45(3): 742−746.
[73] ZHAO Y, YANG X B, REN D Y, et al. Preventive effects of jujube polysaccharides on fructose-induced insulin resistance and dyslipidemia in mice[J]. Food Function,2014,5(8):1771−1778. doi: 10.1039/C3FO60707K
[74] 钟莎莎, 刘奇, 王强, 等. 肥胖引发低度炎症导致认知障碍的机制研究进展[J]. 中国医药导报,2024,21(8):32−35. [ZHONG S S, LIU Q, WANG Q, et al. Research progress on the mechanism of cognitive impairment caused by low degree inflammation induced by obesity[J]. China Medical Review,2024,21(8):32−35.] ZHONG S S, LIU Q, WANG Q, et al. Research progress on the mechanism of cognitive impairment caused by low degree inflammation induced by obesity[J]. China Medical Review, 2024, 21(8): 32−35.
[75] GHALEM M, MURTAZA B, BELARBI M, et al. Antiinflammatory and antioxidant activities of a polyphenol-rich extract from Zizyphus lotus L fruit pulp play a protective role against obesity[J]. Journal of Food Biochemistry,2018,42(6):12689. doi: 10.1111/jfbc.12689
[76] 王佩雯, 王俭勤. 炎症在糖尿病肾脏疾病进展中的作用[J]. 中国中西医结合肾病杂志,2024,25(8):738−741. [WANG P, WANG J Q. The role of inflammation in the progression of diabetic kidney disease[J]. Chinese Journal of Integrative Nephropathy,2024,25(8):738−741.] WANG P, WANG J Q. The role of inflammation in the progression of diabetic kidney disease[J]. Chinese Journal of Integrative Nephropathy, 2024, 25(8): 738−741.
[77] FARHADNEJAD H, ASGHARI G, HEDAYATI M, et al. Effect of ziziphus jujube on cardiometabolic factors and systemic inflammation in type 2 diabetic patients:a randomized controlled trial[J]. Clinical Nutrition ESPEN,2022,49:53−60. doi: 10.1016/j.clnesp.2022.03.043
[78] ZHONG Y J, LUO R L, LIU Q, et al. Jujuboside A ameliorates high fat diet and streptozotocin induced diabetic nephropathy via suppressing oxidative stress, apoptosis, and enhancing autophagy[J]. Food and Chemical Toxicology,2022,159:112697. doi: 10.1016/j.fct.2021.112697
[79] 陈临宜, 何波, 沈志强. 老鹳草属药用植物鞣质成分及防治阿尔茨海默病研究进展[J]. 中国实验方剂学杂志,2023,29(20):257−264. [CHEN L Y, HE B, SHEN Z Q. Research progress of tannin components of Geranium and its prevention and treatment of Alzheimer's disease[J]. Chinese Journal of Experimental Formulae,2023,29(20):257−264.] CHEN L Y, HE B, SHEN Z Q. Research progress of tannin components of Geranium and its prevention and treatment of Alzheimer's disease[J]. Chinese Journal of Experimental Formulae, 2023, 29(20): 257−264.
[80] CHOI J H, AN X, LEE B H, et al. Protective effects of bioactive phenolics from jujube (Ziziphus jujuba) seeds against H2O2-induced oxidative stress in neuronal PC-12 cells[J]. Food Science and Biotechnology,2015,24:2219−2227. doi: 10.1007/s10068-015-0296-4
[81] LIU J R, ZHANG Y Q, LAI C J, et al. Multitarget protective effects of JUB on Aβ-Induced neurotoxicity and the mechanism predication using network pharmacology analysis[J]. Journal of Agricultural and Food Chemistry,2023,71(51):20724−20734. doi: 10.1021/acs.jafc.3c06430
[82] LIU Z, ZHAO X, LIU B, et al. Jujuboside A, a neuroprotective agent from semen Ziziphi spinosae ameliorates behavioral disorders of the dementia mouse model induced by Aβ1–42[J]. European Journal of Pharmacology,2014,738(5):206−213.
[83] SHAHWAR D, SHEHZADI N, KHAN M, et al. A new anti-inflammatory lupane in Ziziphus jujuba (L.) Gaertn. var. hysudrica Edgew[J]. Heliyon,2024,10(9):1−9.
[84] KHORAMJOUY M, BAYANATI M, NOORI S, et al. Effects of Ziziphus jujuba extract alone and combined with boswellia serrata extract on monosodium iodoacetate model of osteoarthritis in mice[J]. Iranian Journal of Pharmaceutical Research:IJPR,2022,21(1):9−16.





 下载:
下载:
 下载:
下载:



