Research Progress on Carotenoids Compositions and Stability of Lycium barbarum L.
-
摘要: 类胡萝卜素是一类具有异戊二烯结构的天然色素,高度不饱和结构致使其稳定性较差,受光、热、氧等因素影响在加工和贮存过程中易发生异构化及降解。枸杞中富含类胡萝卜素,且以玉米黄素酯为主,这有别于常见果蔬中的类胡萝卜素。因此,深入了解枸杞类胡萝卜素稳定性对促进有色食品基质的综合利用具有重要意义。本文简要综述枸杞类胡萝卜素组成,重点关注加工方式(制干、机械破碎、发酵等工艺)、食品抗氧化剂(如维生素C、柠檬酸、维生素E)和环境因素(光照、贮藏温度、氧气等)对枸杞类胡萝卜素稳定性的影响,同时总结了枸杞类胡萝卜素在加工及贮藏过程中的降解途径和降解产物。通过制备脂质体、微胶囊和纳米乳液等方法富集类胡萝卜素,添加食品抗氧化剂或是食用油可延缓色素的氧化,提高枸杞类胡萝卜素的稳定性,以期为枸杞类胡萝卜素的高效利用及其特医食品、保健品研发提供参考。Abstract: Carotenoids are a class of natural pigments with an isoprene structure. Due to its highly unsaturated structure, carotenoids are unstable in vitro and prone to isomerisation and degradation during processing and storage affected by factors such as light, heat, and oxygen. Lycium barbarum L. is rich in carotenoids, mainly zeaxanthin ester, which differs from carotenoids found in common fruits and vegetables. Therefore, it is of great significance to understand the stability of carotenoids of Lycium barbarum L. to promote the comprehensive utilization of colored food matrix. This paper briefly reviews the carotenoids compositions of Lycium barbarum L., focusing on the effects of processing methods (drying, mechanical crushing, fermentation, etc.), food antioxidants (vitamin C, citric acid, vitamin E) and environmental factors (light, storage temperature, oxygen, etc.) on the stability of Lycium barbarum L. carotenoids. The degradation pathways and products of carotenoids during processing and storage in Lycium barbarum L. are summarized. Carotenoids are enriched by preparation of liposomes, microcapsules and nano-emulsion, and the addition of food antioxidants or edible oils can delay the oxidation of pigments and improve the stability of carotenoids in Lycium barbarum L., to provide references for the efficient utilization of Lycium barbarum L. carotenoids and the development of special medical foods and health products.
-
Keywords:
- Lycium barbarum L. /
- carotenoids /
- composition /
- processing methods /
- stability
-
枸杞(Lycium barbarum L.),自古以来就是我国重要的药食同源佳品,具有悠久的种植历史和食用历史,在欧洲和北美被认为是“超级食品”或“超级水果”[1]。枸杞广泛种植在我国宁夏、甘肃、青海、新疆等地,欧洲、美洲亦有分布。2023年,全国枸杞种植面积在181.3万亩,干果总产量24.15万吨,精深加工转化率达到10%左右。现代研究表明,枸杞具有抗氧化、抗衰老、改善肝损伤、调节机体免疫和肠道菌群等作用[2−3]。
类胡萝卜素是枸杞主要的呈色物质和功效物质,主要以双酯化形式存在,少量以游离态和单酯化形式存在。众多研究表明,在加工及贮藏过程中,制干、机械破碎、工艺参数及环境条件均会造成枸杞类胡萝卜素的降解与转化,且游离的类胡萝卜素更易损失[4]。类胡萝卜素降解可生成多类型衍生物,如类维生素A[5]、色素[6]、植物激素[7]、挥发性物质[8]等。因此,本文围绕枸杞类胡萝卜素,介绍近年来该成分在加工及贮藏环节稳定性的研究进展,以期为枸杞类胡萝卜素高效、广泛地应用于食品加工领域、保健品开发提供参考。
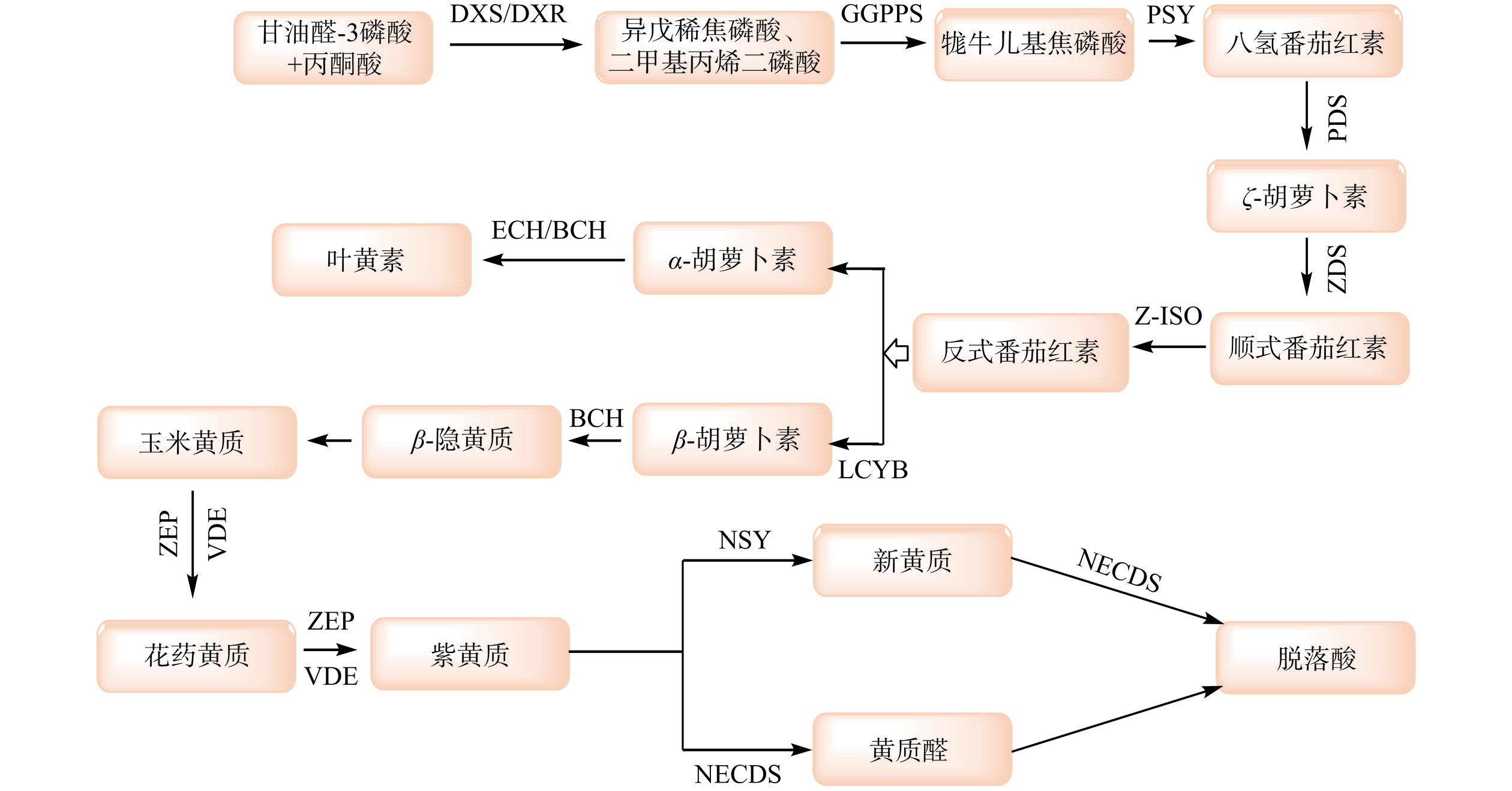
1. 枸杞类胡萝卜素及其组成
类胡萝卜素是天然的呈色物质,在自然界广泛呈现橙色、橙红色等[9]。根据两端基团不同,分为α、β、γ、δ、ε等多种类型的异构体,根据组成元素不同,分为胡萝卜素类和叶黄素类[10]。枸杞中有丰富的类胡萝卜素,干果和鲜果中的总类胡萝卜素含量分别为1.28~401.53 mg/100 g和20.36~89.91 mg/100 g[11−12]。目前,枸杞中已鉴定出游离类胡萝卜素24种、类胡萝卜素酯22种、类胡萝卜素糖基化衍生物2种(如表1所示)。在游离类胡萝卜素中,主要为β-胡萝卜素、β-隐黄质、玉米黄质。在类胡萝卜素酯中,主要以玉米黄质双棕榈酸酯为主[13],并含有13Z-zeaxanthin dipalmitate、9Z-zeaxanthin dipalmitate这两个几何异构体,以及少量的棕榈酸、硬脂酸棕榈酸、油酸棕榈酸等玉米黄质酯化物及糖苷化衍生物或甘聚糖酯化物[14−22]。
表 1 枸杞类胡萝卜素组成及离子信息Table 1. Carotenoid composition and ion information of Lycium barbarum L.组成 电离模式 MS/MS(m/z) 参考文献 phytoene
八氢番茄红素[M+H]+ 545.3,81 [14] γ-carotene
γ-胡萝卜素[M+H]+ 537.4,177.3 [14] lycopene
番茄红素[M+H]+ 537.4,81 [14] capsorubin
辣椒红素[M+H]+ 585.5,109.1 [14] phytofluene
六氢番茄红素[M+H]+ 543.5 [14] β-Citraurin
β-柠乌素[M+H]+ 433.3,341.1 [14] Lutein myristate acid ester
叶黄素肉豆寇酸酯[M+H-18]+ 761.8,533.5 [14] 5,6 epoxide lutein-hairpin-palmitate
5,6环氧叶黄素-发酸酯-棕榈酸酯[M+H-172]+ 805.4,549.4 [14] lutein dimyristate ester
叶黄素双肉豆蔻酸酯[M+H-228]+ 761.8,533.5 [14] violaxanthin dibutyrate
紫黄质二丁酸盐[M+H]+ 741.6,653.5 [14] violaxanthin palmitate
紫黄质棕榈酸酯[M+H]+ 839.8,821.8 [14] zeaxanthin-octanoate-laurate
玉米黄质-辛酸-月桂酸酯[M+H]+ 906.9,533.6 [14] zeaxanthin dilaurate ester
玉米黄质双月桂酸酯[M+H]+ 933.9,533.2 [14] zeaxanthin-laurate-myristate ester
玉米黄质-月桂酸-肉豆蔻酸酯[M+H]+ 962.7,733.5 [14] zeaxanthin dimyristic acid ester
玉米黄质双肉豆蔻酸酯[M+H]+ 990,761.8 [14] zeaxanthin-laurate-palmitate
玉米黄质-月桂酸-棕榈酸酯[M+H]+ 989.9,533.4 [14] zeaxanthin-myristo-palmitate
玉米黄质-肉豆蔻酸-棕榈酸酯[M+H]+ 1018.1,533.6 [14] zeaxanthin-palmitate-stearate ester
玉米黄质硬脂酸棕榈酸酯[M+H]+ 1074.1 [14] zeaxanthin-oleate-palmitate
玉米黄质-油酸-棕榈酸酯[M+H]+ 1071.9,789.8 [14] β-cryptoxanthin myristate ester
β-隐黄质肉豆蔻酸酯[M+H]+ 763.9,535.5 [14] neochrome palmitate
新色素棕榈酸酯[M+H-18]+ 821.7,565.5 [14] neochrome
新色素[M+H]+ 601.32 [15] auroxanthin
玉米黄二呋喃素[M+H]+ 811.7 [15] β-apo-8'-carotenl
β-阿朴胡萝卜素醛[M+H]+ 417.3,325.3 [14,17] β-cryptoxanthin palmitate
β-隐黄质棕榈酸酯[M+H]+,[M]− 791.9,791,790,535.5,535 [14,17] antheraxanthinhua
花药黄质[M+H]+,[M]− 585.5,585,584,175.4 [14,17] lutein
叶黄素[M+H]+,[M+H-18]+,[M]− 569,568,551.5,175.4 [14,17] lutein palmitate
叶黄素棕榈酸酯[M+H-18]+,[M+H]+,[M]− 807,806,789.8,789,533.5,533,551 [14,17] violaxanthin dipalmitate
紫黄质双棕榈酸酯[M+H]+ 1077.9,821. [14,18] zeaxanthin dipalmitate
玉米黄素双棕榈酸酯[M+H]+,[M+H-256]+,[M+H-2PA]+,
[M+H-PA]+,[M]−1300.1,1045.9, 1045,1044,789.7,79.6,789.5,789,533.5,533.4,533 [14,17−22] β-carotene
β-胡萝卜素[M+H]+,[M-92]+,[M]− 537.6,537.4,537,536,444,413,375,177.1 [14,17−20,22] zeaxanthin
玉米黄质[M+H]+,[M+H-18]+,[M-92]+,[M-394]+,[M]− 569.4,569.3,569,568,543,533,551.4,
469,476.4,175.1,477.5[14,17−20,22] neoxanthin
新黄质[M+H]+,[M+H-18]+,[M]− 601.4,601,600,583.4,565.5,491,393 [14,17,19−20,22] 9-or 9'-Z-β-cryptoxanthin
9或9’-Z-β-隐黄质[M+H]+ 553.4 [20] 15-or 15'-Z-zeaxanthin
15或15’-Z-玉米黄质[M+H]+ 569.4 [17,19] β-cryptoxanthin
β-隐黄质[M+H]+,[M+H-18]+ 553.4,553.3,535.4,461,497 [16,19] zeaxanthin monopalmitate
玉米黄质单棕榈酸酯[M+H]+,[M+H-PA]+,[M+PA-H]− 1062,807.7,551.4,551 [18−19] 13-or13'-Z-zeaxanthin
13或13’-Z-玉米黄质[M+H]+,[M+H-18]+ 569.4,533,551.4,469 [16,19] β-Cryptoxanthin monopalmitate
β-隐黄质单棕榈酸酯[M+H]+,[M+H-PA]+ 791.6,790,535.4 [16,18−20] 9-or 9'-Z-zeaxanthin
9或9’-Z-玉米黄质[M+H]+,[M+H-18]+ 601.4,583.4,569.4,551,533 [16−17,19−20] 13-or 13'-Z-β-carotene
13或13’-Z-β-胡萝卜素[M+H]+,[M]− 537.4,537.3,537,536 [17,19,22] 9-or 9'-Z-β-carotene
9或9’-Z-β-胡萝卜素[M+H]+,[M]− 537.6,537.4,537,536,445,444,413 [16,19−20,22] antheraxanthin dipalmitate
花药黄质双棕榈酸酯[M+H]+,[M+H-PA]+,[M]− 1061.9,1061,1060,805.6,805,549 [18,22] zeaxanthin myristate palmitate
玉米黄质肉豆蔻酸棕榈酸酯[M+H]+,[M+H-MA]+,[M+H-PA]+,[M]− 1017.9,1017,1016,789.6,789,761.6,761 [18,22] α-carotene
α-胡萝卜素[M+H]+ 537.3,399,331,135 [16,20] zeaxanthin glucomannan
玉米黄质葡甘聚糖[M+H-18]+ 965.7 [16,20] 1'-Hydroxy-γ-carotenoid glucomannan
1'-羟基-γ-类胡萝卜素葡甘聚糖[M+H-18]+ 697.5,279,97,78 [16,20] violaxanthinz
紫黄质[M+H]+,[M]− 601,600 [22] 2. 枸杞类胡萝卜素稳定性研究
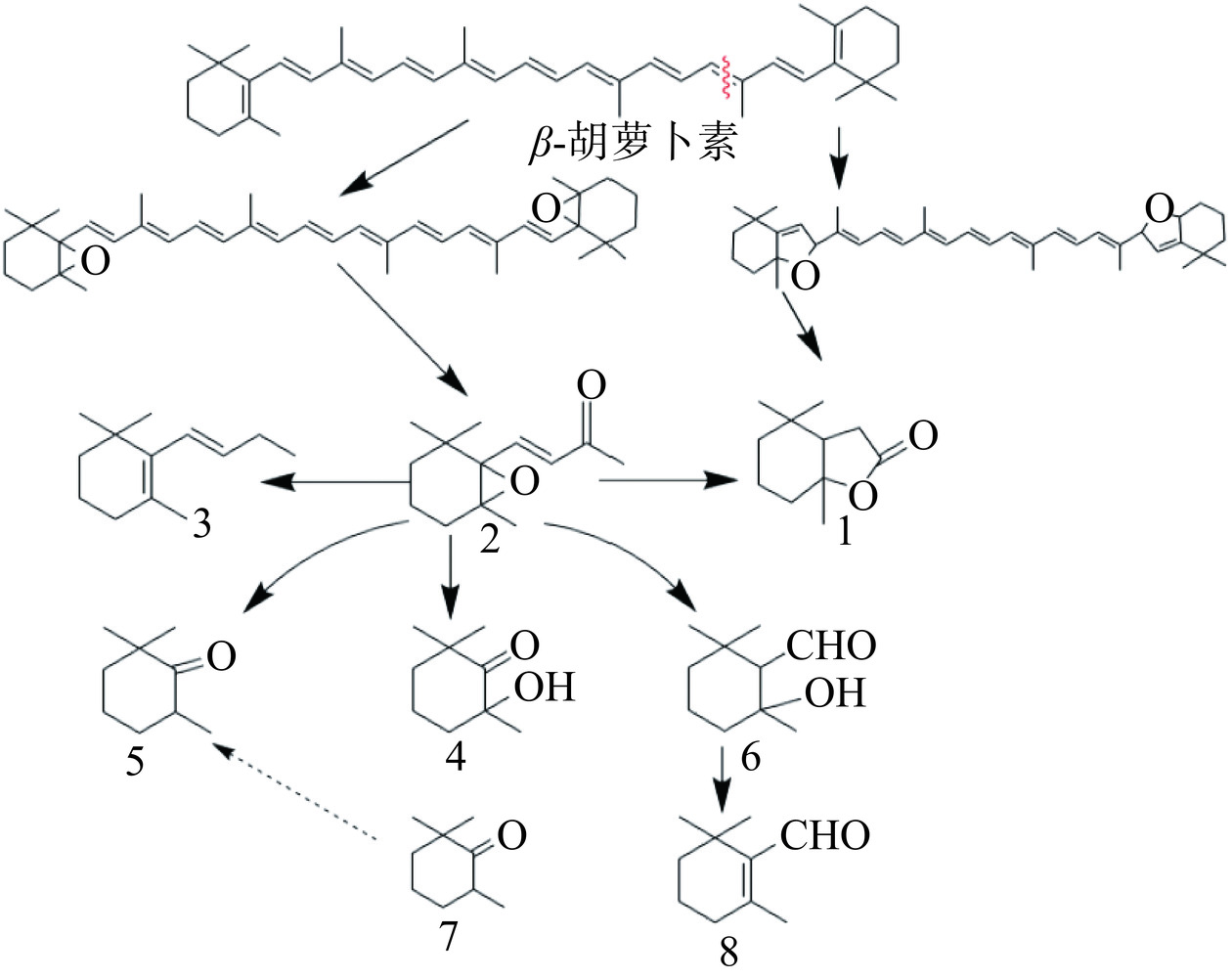
类胡萝卜素含有多个不饱和共轭双键,极易受热、光、氧的影响而发生降解和异构化[23−24]。图1所示,β-胡萝卜素是众多类胡萝卜素组分及降解产物的前体物质,在不同合成酶的作用下可生成β-隐黄质、玉米黄质、花药黄质等物质。β-胡萝卜素在自然界中呈现黄色或橙黄色,在食物加工、贮藏过程中性质并不稳定,容易发生全反式向顺式结构的转化。在热降解过程中,β-胡萝卜素上环己烯基上的双键和直链上的双键易发生周环反应,原先共轭体系两段的Π键进行断裂,重新以σ键相连,从而形成环状分子,构成共轭体系。由于体系的不稳定性,在C9~C10处发生断裂形成一分子的5,6-环氧基-β-紫罗兰酮,接着进行氧化或者还原反应,最终生成2,2,6-三甲基环己酮、异氟尔酮和β-环柠檬醛等(图2所示),从而引发食品褪色和营养成分损失。而叶黄素和玉米黄质互为同分异构体,经β-胡萝卜素羟化酶(BCH)转化而来,现阶段对叶黄素和玉米黄质的降解产物研究较少[25]。
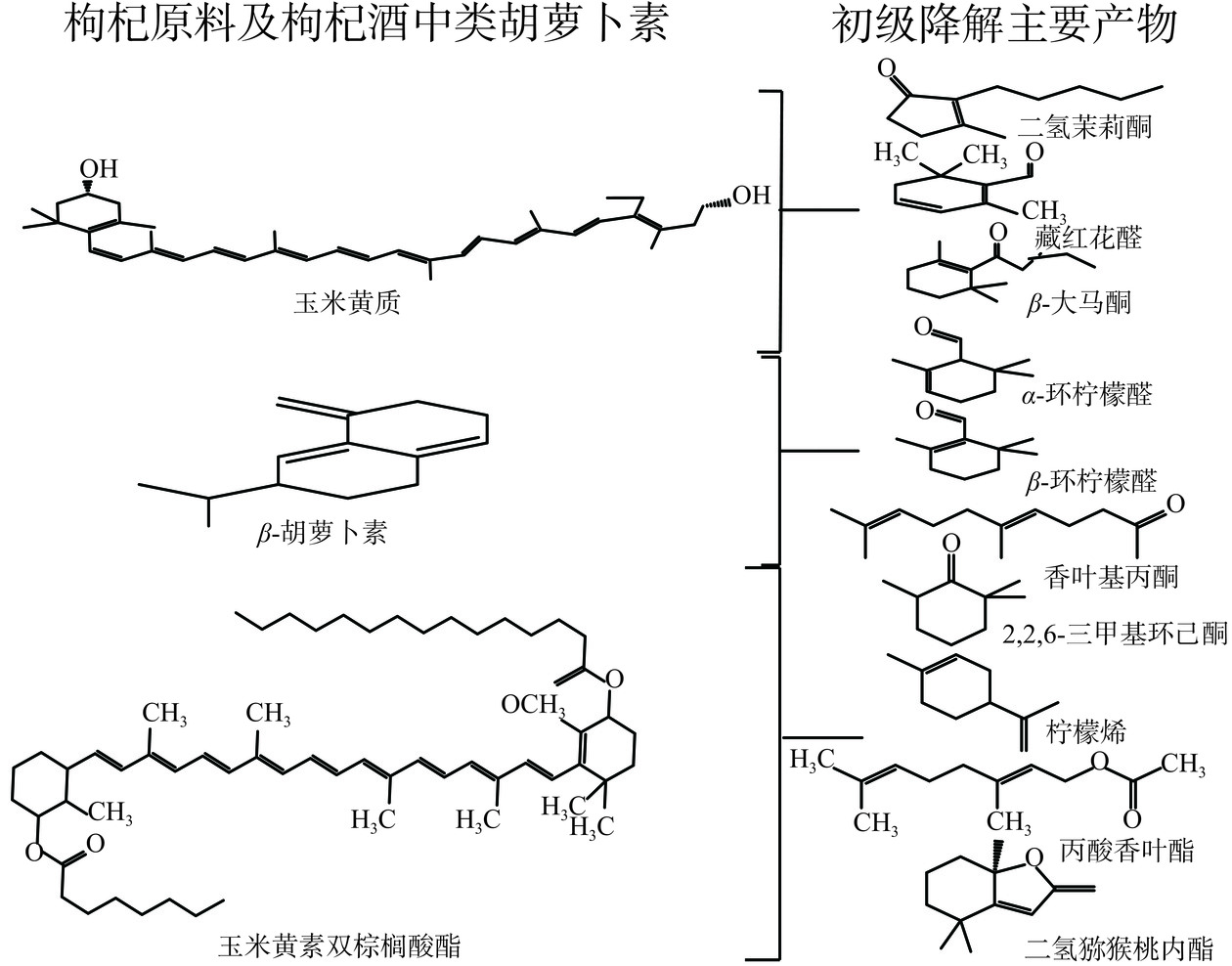
枸杞类胡萝卜素在加工、贮藏过程中,色素双键易被破坏发生反应,甲基极容易发生取代、氧化反应,从而发生降解和异构化(见图3)。最具有典型性香气的β-大马酮是由C10~C11断裂、C9去氢氧化生成,香叶基丙酮是由C7’~C8’、C13~C14断裂生成。
枸杞类胡萝卜素因组成、断裂位点不同、所受影响因素不同,可发生异构化和降解(表2所示)。异构化则多以反式向顺式转化,通常采用HPLC-MS和UPLC-MS,并结合核磁共振(nuclear magnetic resonance,NMR)等手段对这类物质进行鉴定。降解产物主要为挥发性降异戊二烯类物质,常用制备方法以顶空固相微萃取法(headspace solid-phase micro-extraction,HS-SPME)和溶剂法为主,结合气相色谱-质谱(gas chromatography-mass spectrometry,GC-MS)分析挥发性降解产物。
表 2 不同加工、贮藏条件影响下类胡萝卜素降解及其异构化产物Table 2. Carotenoids degradation and isomerization products under different processing and storage conditions类胡萝卜素 主要制备方法 仪器型号 离子源 MS 影响因素 断裂位点 异构化及降解产物 参考文献 非挥发性 挥发性 β-胡萝卜素 HS-SPME GC-MS − NH3;3.7 mL/min;250 ℃;30~
450 AUM烘干 − β-紫罗兰酮、β-环柠檬醛、二氢猕猴桃内酯 [28] 水,己烷,四氢呋喃于超声波反应器中反应 UPLC-MS APCI 20~600 nm 超声 − 15-Z-、9-Z-、di-Z-、13-Z-、β-apo-11-、β-apo-13-、β-apo-15-、β-apo-14’-、β-apo-12’-、β-apo-10’-、β-apo-8’- − [40] 吐温80乳化,蒸馏水溶解 HPLC/
HPLC-MSEI He;1.5 mL/min;
230 ℃;40~400 nm超声 − 15-Z-、13-Z、9-Z β-紫罗兰酮、异佛尔酮、2,2,6-三甲基环己酮 [41] HS-SPME GC-MS EI He;1 mL/min;
250 ℃;70 eV;20~450 AUM发酵 − α-环柠檬醛、β-环柠檬醛、香叶基
丙酮[45] 四氢呋喃溶解 HPLC-MS − N2;10 L/min;80 psi 氧气、臭氧 − 13-Z-、9-Z-、di-Z-、β-apo-13-、β-apo-14′- − [64] 玉米黄素 HS-SPME GC-MS EI He;1 mL/min;
250 ℃;70 eV;20~450 AUM发酵 − − 二氢茉莉酮、藏红花醛、β-大马酮 [45] 己烷溶解,正己烷-碘液催化,荧光灯
照射HPLC-MS APCI N2;5.0 L/min;
2500 V;20 psi;4 μA光+热 − 13-13’-Z-、9-9’-Z-、15-15’-Z-、9,13-di-Z- − [68] 玉米黄素双棕榈酸酯 HS-SPME GC-MS EI He;1 mL/min;
250 ℃;70 eV;20~450 AUM发酵 − − 2,2,6-三甲基环己酮、柠檬烯、2-辛烯醛、丙酸香叶酯、二氢猕猴桃
内酯[45] β-隐黄质 甲醇,正己烷溶解,25 ℃
照射HPLC-MS APCI N2;5.0 L/min;
2500 V;20 psi;汽化350 ℃;鞘气
400 ℃;4 μA光照 − β-β-carotene-3-one、5,6-epoxy-β-β-carotene-3-one、9'-Z-、13-13'-Z- − [63] 甲醇溶解,正己烷碘液催化,25 ℃照射 HPLC-MS APCI N2;5.0 L/min;
2500 V;20 psi;汽化350 ℃;鞘气
400 ℃;4 μA碘+光照 − β-β-carotene-3-one、5,6-epoxy-β-β-carotene-3-one、9-Z-,9'-Z-、13-Z-、13'-Z-、15-Z- − [63] 无环类胡萝卜素 HS-SPME GC-MS − NH3;3.7 mL/min;250 ℃;30~
450 AUM烘干 − − 异佛尔酮、香叶基丙酮 [28] 玉米黄质、β-胡萝卜素、玉米黄素双棕榈
酸酯HS-SPME GC-MS EI N2;1 mL/min;
250 ℃;20~
450 AMU机械破碎 C7~C8/C8~C9/
C14~C15− 2-异丙烯基-6-异丙基-3-甲基-3-乙烯基环己酮、异香叶醇、3-异佛尔酮 [33] HS-SPME GC-MS EI N2;1 mL/min;
250 ℃;20~
450 AMUpH C7~C8/C9~C10/
C9'~C10'− 2,2,6-三甲基环己酮、2-庚烯醛、β-紫罗兰酮 [33] HS-SPME GC-MS EI N2;1 mL/min;
250 ℃;20~
450 AMU高压灭菌 C1~C6/C5~C6/C7~C8/C13~C14/C6’~C7’/
C9~C10/C9’~C10’− 2,4-壬二烯醛、2,2,6-三甲基环己酮、β-环柠檬醛、香叶基丙酮、β-紫罗兰酮、2-甲基-5-(1-甲基乙基)-环已酮 [33] HS-SPME GC-MS EI N2;1.5 mL/min;
250 ℃;45~
450 AMU机械处理 C4~C5/C6~C7/C7~C8/C9~C10/C12'~C13'/
C13~C14/C14~C15− 甲基庚烯酮、异戊醇、异佛尔酮 [33] HS-SPME GC-MS EI N2;1.5 mL/min;
250 ℃;45~
450 AMU光照 C6~C7/C7~C8/C7'~C8'/C9~C10/C9'~C10'/
C12'~C13'/C13~C14/
C14~C15− 甲基庚烯酮、2,4-壬二烯醛,β-环柠檬醛、香叶丙酮、β-紫罗酮、二氢猕猴桃内酯 [33] HS-SPME GC-MS EI N2;1.5 mL/min;
250 ℃;45~
450 AMU氧化 C6~C7/C7~C8/C7'~C8'/C9~C10/C12'~C13'/
C13~C14/C14~C15− 甲基庚烯、2,4-壬二烯醛、β-环柠檬醛、香叶酰丙酮、2,2,6-三甲基环己酮、β-紫罗酮 [33] HS-SPME GC-MS EI N2;1.5 mL/min;
250 ℃;45~
450 AMUpH − − 甲基庚烯酮、辛烯、2,2,6-三甲基环己酮、β-紫罗酮 [33] 叶黄素 有机溶剂溶解 HPLC-MS APCI N2;350 ℃;4 μA;50~1200 m/z 超声(120、180、240、300 W) − 13-Z-、13'-Z-、
9-Z-、9'-Z-− [39] 甲醇溶解,
25 ℃照射HPLC–MS APCI N2;5.0 L/min;
2500 V;汽化
350 ℃;4 μA;80~
1000 m/z光照 − 9-Z-、9'-Z-、
13-13'-Z-− [62] 甲醇溶解,正己烷碘液催化,25 ℃照射 HPLC–MS APCI N2;5.0 L/min;
2500 V;汽化
350 ℃;4 μA;80~
1000 m/z碘+光照 − 15-Z-、9-Z-、9'-Z-、13-13'-Z-、di-Z-异构体 − [62] 注:−未报道。 2.1 加工方式对枸杞类胡萝卜素稳定性的影响
2.1.1 制干
制干是枸杞主要的加工方式。传统制干方式有晒干和热风烘干,新型制干方式有微波干燥、真空脉动干燥和真空冷冻干燥等,不同制干方式对枸杞类胡萝卜素含量有较大影响。热风干燥是枸杞制干方式中最常见的干燥方法,研究发现,枸杞热风制干起始阶段,玉米黄素和β-胡萝卜素含量增加,是鲜果含量的2~22倍,制干后期,两种色素含量分别比干制起始阶段增加44%和23%;玉米黄素双棕榈酸酯在制干起始阶段降幅达到40%以上,制干后期达到稳定状态[26]。真空冷冻干燥最大限度地保留了枸杞子所含有的多类型功效成分,特别是含有较高的β-胡萝卜素(0.060 mg/g)成分[27]。枸杞在烘干(40 ℃)过程中,β-胡萝卜素降解产生β-紫罗兰酮、β-环柠檬醛和二氢猕猴桃内酯,无环氧类胡萝卜素降解产生异佛尔酮和香叶基丙酮[28]。故而,在制干加工过程中,由于热效应、美拉德反应及组织内水分的迁移,类胡萝卜素常会发生复杂的降解反应产生挥发性降异戊二烯物质,因此制干是枸杞初级加工环节影响类胡萝卜素稳定性的主要因素。
2.1.2 机械破碎
机械破碎是加工过程中的重要工序。常用的破碎方式有研磨粉碎、均质、打浆等。这些破碎方式大多是基于一定的压力、剪切力、空化和湍流等力学效应应用的非热处理技术[29]。但受力作用点样品局部会短暂瞬间升温,因此,容易从不稳定位点发生断裂,导致降解[30]。通过不同机械破碎工艺对类胡萝卜素含量进行分析,结果表明,与家用搅拌器应用相比,Ultraturrax®均质化能够更好的限制亲脂性成分的降解[31]。赵璐[32]对机械破碎处理条件下枸杞汁类胡萝卜素降解产物降异戊二烯化合物进行研究,分析表明,机械破碎与手动破碎相比明显增加了降戊二烯化合物2-异丙烯基-6-异丙基-3-甲基-3-乙烯基环己酮、异香叶醇和异佛尔酮的含量。Geng等[33]发现机械破碎引起类胡萝卜素C4~C5、C6~C7、C7~C8、C9~C10、C12’~C13’、C13~C14、C14~C15键断裂,生成甲基庚烯酮、异戊醇和异佛尔酮。因此,机械破碎通常易引起类胡萝卜素C=C双键的断裂,从而导致类胡萝卜素降解生成降异戊二烯化合物。
2.1.3 超声处理
超声是常用的活性成分提取常用的辅助技术。然而,长时间的超声处理过程中,活性成分易发生氧化和降解[34],如加速多糖的聚集和分解[35−36],促使多酚[37]、蛋白[38]等食品成分的氧化降解。但目前,关于超声处理下类胡萝卜素的降解机制报道较少。Song等[39]研究了不同超声功率处理对全反式叶黄素稳定性的影响,结果表明,超声可诱导叶黄素异构化,形成13-Z-、13’-Z-、9-Z-和9’-Z-等顺式异构体。Carail等[40]研究高功率(20 kHz)超声处理对β-胡萝卜素降解的影响,已初步确定降解产物为4种Z-isomers和7种β-apo-carotenals。于奉生等[41]研究了超声对β-胡萝卜素降解的影响,发现超声处理后β-胡萝卜素生成3种异构体,分别是15-Z、13-Z、9-Z,其降解产生的挥发性产物主要为β-紫罗兰酮(27.73%)、异佛尔酮(15.15%)、2,2,6-三甲基环已酮(8.31%)等。超声是导致类胡萝卜素异构或氧化降解的主要原因,其中超声频率、功率、溶剂种类及温度对类胡萝卜素的稳定性的影响有待更深入的探究[42]。
2.1.4 发酵
发酵是果蔬非热力加工的常用技术,可最大程度减少果蔬中的营养及功效成分和风味损失,延长保质期。在发酵过程中,发酵液环境会发生变化,酵母菌在代谢产生乙醇的同时,会有许多副产物有机酸、醛、酮、酯类等物质生成,而类胡萝卜素会与代谢产物发生化学反应,生成酯类、羧酸类物质,故而使类胡萝卜素含量减少,如玉米黄质结构中的羟基(-OH)与代谢产物有机酸中的羰基(-COO-)发生化学反应生成酯类,使玉米黄质含量减少[43−44]。枸杞在发酵过程中,类胡萝卜素降解产物为发酵风味物质的形成提供了众多前体物质,其中玉米黄素双棕榈酸酯下降32.36%,降解产物为2,2,6-三甲基环己酮、柠檬烯、2-辛烯醛、丙酸香叶酯;β-胡萝卜素下降16.52%,降解产物为α-环柠檬醛、β-环柠檬醛、香叶基丙酮;玉米黄质下降15.82%,降解产生β-大马酮为枸杞酒提供了独特的玫瑰香[45]。Chen等[46]比较了未处理(GW1)、高压灭菌处理(GW2)和NXU-GQ 15菌株处理的(GW3)枸杞渣经发酵制备成枸杞酒颜色、风味和感官属性,发现GW3类胡萝卜素的降解率最高为84.73%,其次GW2为56.77%,GW1为27.25%。
2.2 食品添加剂对枸杞类胡萝卜素稳定性的影响
2.2.1 维生素C
维生素C(Vitamin C,VC)又称抗坏血酸,是一种酸性多羟化合物,也是最重要的水溶性抗氧化物[47]。抗坏血酸可与食物中所含氧发生反应,从而使初期的氧化物还原,起到稳定的效果。《国家卫生计生委关于批准β-半乳糖苷酶为食品添加剂新品种等的公告》(2015年第1号)规定抗坏血酸在果蔬汁(浆)中的最大使用量为1.5 g/kg。加工过程中添加VC,能一定程度提高类胡萝卜素的稳定性,从而起到护色作用。胡晗艳[48]分析了枸杞原汁加工过程中β-胡萝卜素的变化,研究表明,在破碎打浆过程中添加0.2%VC,对枸杞原汁中的β-胡萝卜素有保护作用。枸杞原浆生产中均会发生褐变,可通过添加维生素C来抑制褐变程度,随着维生素C抗氧剂浓度的增加,枸杞原浆中枸杞红素的保存率也随之提升,当超过0.3%时,保存稳定率达到了85.7%,枸杞原浆中的枸杞红素趋向于稳定[49]。
2.2.2 柠檬酸
柠檬酸作为酸味剂和护色剂在食品加工中较为常见。添加0.5%的柠檬酸可使类胡萝卜素含量降低[50],并促使类胡萝卜素异构化速度加快[51]。由于添加柠檬酸,改变了食品基质的pH,而类胡萝卜素稳定性受pH的影响较大,故而会导致类胡萝卜素降解及异构化。Geng等[33]用柠檬酸调节枸杞汁pH为3.3,测定枸杞汁中类胡萝卜素含量,发现总类胡萝卜素含量下降9.63%,降解形成降异戊二烯化合物甲基庚烯酮、辛烯、2,2,6-三甲基环己酮、β-紫罗兰酮。
2.2.3 维生素E
维生素E(Vitamin E,VE),是一种脂溶性抗氧化剂,常见的存在形式包括α-,β-,δ-,γ-生育酚和α-,β-,δ-,γ-生育三烯[52−53]。VE对枸杞色素具有保护作用[54],通过阻断色素过氧自由基的产生,故而对色素起到保护效果。常旋等[55]研究发现不同含量的VE均提高了β-胡萝卜素的色价保留率,且添加量为3%时饮料稳定性最佳。因此,可以在枸杞加工生产中添加VE,提高类胡萝卜素稳定性,从而保持产品色泽稳定。
此外,植物油脂可以作为绿色溶剂保存类胡萝卜素,延缓色素氧化进程,提高类胡萝卜素的稳定性[56]。Kan等[57]采用橄榄油(ON)和大豆油(SN)制备枸杞类胡萝卜素酯纳米乳液,结果表明,玉米黄素双棕榈酸酯在富含单不饱和脂肪酸的橄榄油乳液中具有良好的溶解度和生物可及性(25.67%)。Luo等[58]对比分析了不同油脂(棕榈油、大豆油、菜籽油、葵花籽油、棉籽油)对类胡萝卜素稳定性的影响,其中长链油有利于提高类胡萝卜素的稳定性,且富含类胡萝卜素的棉籽油最为稳定。同时,研究表明,脂质体、微胶囊包埋和纳米乳液等方法富集类胡萝卜素,可提高其分散性和溶解度,进而提高稳定性[59]。
2.3 环境因素对枸杞类胡萝卜素稳定性的影响
2.3.1 光照
研究表明,光照会加速色素氧化,促进类胡萝卜素降解,生产环氧化物[60]。曹有龙等[61]比较了日光直射,室内散射光照射,避光保存条件下枸杞类胡萝卜素的稳定性,发现该色素在日光直射下极不稳定,8 h后枸杞类胡萝卜素保存率仅为18.6%,而室内散射光照射和避光条件下保存率>95%。此外,光诱导类胡萝卜素产生多种顺式(Z-)异构体。Li等[62]对叶黄素进行光照处理,结果表明,发现光照条件下叶黄素异构生成9-Z-、9'-Z-、13-Z-、13'-Z-等顺式叶黄素。光照可促使β-隐黄质异构化生成β-β-carotene-3-one、5,6-epoxy-β-β-carotene-3-one、9’-Z-隐黄质、13或13’-Z-隐黄质等异构体[63]。
2.3.2 氧气
有氧条件下加速类胡萝卜素降解及异构。Henry等[64]研究了臭氧和氧气对水溶性类胡萝卜素稳定性的影响,发现13-Z和9-Z是β-胡萝卜素的主要异构体,β-胡萝卜素在13-14和13’-14’双键上裂解产生β-apo-13-carotenone和β-apo-14′-carotenal。Geng等[33]测定了氧化处理条件下枸杞汁中类胡萝卜素的降解产物,结果表明,甲基庚烯、2,4-壬二烯醛、β-环柠檬醛、香叶酰丙酮、2,2,6-三甲基环己酮、β-紫罗酮等是其主要的降解产物。由此可见,氧化产生的自由基结合在类胡萝卜素结构的共轭双键上,使类胡萝卜素降解氧化成醛、酮类物质。因此,降低氧浓度是防止类胡萝卜降解的有效方法之一。
2.3.3 pH
pH是影响色素稳定性的重要因素之一。果蔬中的色素会随pH改变而发生结构变化,从而引起色泽变化。研究报道,以芒果、杨桃为主的果汁随着pH的增加,色调角(H)逐渐减小,颜色由绿色向黄色转变[65]。枸杞类胡萝卜素在酸性条件下更易降解,这有别于常见果蔬。赵璐[32]研究表明,pH由4.8调整为3.3时,导致枸杞类胡萝卜素C7~C8、C9~C10、C9’~C10’位点断裂生成降戊二烯化合物2,2,6-三甲基环己酮、2-庚烯醛、β-紫罗兰酮,其中微带油脂青香的2-庚烯醛是主要的降解物质。当pH3.0~3.5时,枸杞总类胡萝卜素、玉米黄素、玉米黄素双棕榈酸酯含量随pH的降低而降低,β-胡萝卜素较为稳定,表明在酸性条件下,类胡萝卜素5,6-环氧化合物发生重排,形成2,5-二氢呋喃,并导致这类物质降解,β-胡萝卜素不含此结构,故较为稳定[66]。
2.3.4 贮藏温度
果蔬在贮藏期间易发生抗坏血酸、多酚、类胡萝卜素等活性物质降解。众多研究表明,枸杞在贮藏过程中,易发生腐烂、走油、变色等现象,可能归因于氧化反应、非酶促褐变反应、多糖及类胡萝卜素含量降低。然而,温度是影响枸杞营养物质变化的重要原因。β-胡萝卜素作为常见影响枸杞颜色的类胡萝卜素,枸杞在贮藏12 d后,β-胡萝卜素水平在−2 ℃(101.09 µg/g)和10 ℃(115.56 µg/g)之间不显著,与0 ℃(163.86 µg/g)有显著差异,因此贮藏温度对枸杞品质起着关键作用[67]。Xiao等[68]采用HPLC-DAD-MS技术对玉米黄质在光诱导模型系统中的热降解动力进行分析,发现,在25、35和45 ℃下的玉米黄质热降解形成13或13’-Z-、9或9’-Z-、15或15’-Z-单顺式异构体和9-dis-Z-和13-di-Z-玉米黄质的双顺式异构体。−4 ℃贮藏的枸杞果实,β-胡萝卜素、玉米黄素和玉米黄素双棕榈酸酯含量均显著高于4 ℃,是由于4 ℃枸杞果实存储蛋白基因(LbHSP21,LbOR2)表达极显著低于−4 ℃,降解酶基因(LbNCED6,LbCCD1,LbCCD4)则极显著高于−4 ℃[69]。
3. 结论与展望
枸杞中的类胡萝卜素不仅是主要的呈色物质,而且是重要的功能成分,将直接影响商品品质与价值。由于类胡萝卜素物质具有高度不饱和性质,且枸杞中的类胡萝卜素大多以酯化形式存在,因此,很大程度上限制了枸杞类胡萝卜素的加工与利用。本文详细综述了加工方式、贮藏条件对枸杞类胡萝卜素的稳定性的影响。相关研究主要集中在组分含量的变化层面,而对于枸杞中的主要类胡萝卜素物质-玉米黄素双棕榈酸酯,或以玉米黄素及其衍生物在加工、贮藏过程中成分的变化与降解研究目前较为鲜见。因此,进一步研究并提高枸杞类胡萝卜素的稳定性,揭示枸杞类胡萝卜素的转化/降解机制,加强枸杞类胡萝卜素靶向递送体系的研发,研究其消化吸收特性,对提高类胡萝卜素生物利用度及更好的发挥其生物活性具有重要意义,为枸杞及有色食物基质的精深加工生产提供理论依据。
-
表 1 枸杞类胡萝卜素组成及离子信息
Table 1 Carotenoid composition and ion information of Lycium barbarum L.
组成 电离模式 MS/MS(m/z) 参考文献 phytoene
八氢番茄红素[M+H]+ 545.3,81 [14] γ-carotene
γ-胡萝卜素[M+H]+ 537.4,177.3 [14] lycopene
番茄红素[M+H]+ 537.4,81 [14] capsorubin
辣椒红素[M+H]+ 585.5,109.1 [14] phytofluene
六氢番茄红素[M+H]+ 543.5 [14] β-Citraurin
β-柠乌素[M+H]+ 433.3,341.1 [14] Lutein myristate acid ester
叶黄素肉豆寇酸酯[M+H-18]+ 761.8,533.5 [14] 5,6 epoxide lutein-hairpin-palmitate
5,6环氧叶黄素-发酸酯-棕榈酸酯[M+H-172]+ 805.4,549.4 [14] lutein dimyristate ester
叶黄素双肉豆蔻酸酯[M+H-228]+ 761.8,533.5 [14] violaxanthin dibutyrate
紫黄质二丁酸盐[M+H]+ 741.6,653.5 [14] violaxanthin palmitate
紫黄质棕榈酸酯[M+H]+ 839.8,821.8 [14] zeaxanthin-octanoate-laurate
玉米黄质-辛酸-月桂酸酯[M+H]+ 906.9,533.6 [14] zeaxanthin dilaurate ester
玉米黄质双月桂酸酯[M+H]+ 933.9,533.2 [14] zeaxanthin-laurate-myristate ester
玉米黄质-月桂酸-肉豆蔻酸酯[M+H]+ 962.7,733.5 [14] zeaxanthin dimyristic acid ester
玉米黄质双肉豆蔻酸酯[M+H]+ 990,761.8 [14] zeaxanthin-laurate-palmitate
玉米黄质-月桂酸-棕榈酸酯[M+H]+ 989.9,533.4 [14] zeaxanthin-myristo-palmitate
玉米黄质-肉豆蔻酸-棕榈酸酯[M+H]+ 1018.1,533.6 [14] zeaxanthin-palmitate-stearate ester
玉米黄质硬脂酸棕榈酸酯[M+H]+ 1074.1 [14] zeaxanthin-oleate-palmitate
玉米黄质-油酸-棕榈酸酯[M+H]+ 1071.9,789.8 [14] β-cryptoxanthin myristate ester
β-隐黄质肉豆蔻酸酯[M+H]+ 763.9,535.5 [14] neochrome palmitate
新色素棕榈酸酯[M+H-18]+ 821.7,565.5 [14] neochrome
新色素[M+H]+ 601.32 [15] auroxanthin
玉米黄二呋喃素[M+H]+ 811.7 [15] β-apo-8'-carotenl
β-阿朴胡萝卜素醛[M+H]+ 417.3,325.3 [14,17] β-cryptoxanthin palmitate
β-隐黄质棕榈酸酯[M+H]+,[M]− 791.9,791,790,535.5,535 [14,17] antheraxanthinhua
花药黄质[M+H]+,[M]− 585.5,585,584,175.4 [14,17] lutein
叶黄素[M+H]+,[M+H-18]+,[M]− 569,568,551.5,175.4 [14,17] lutein palmitate
叶黄素棕榈酸酯[M+H-18]+,[M+H]+,[M]− 807,806,789.8,789,533.5,533,551 [14,17] violaxanthin dipalmitate
紫黄质双棕榈酸酯[M+H]+ 1077.9,821. [14,18] zeaxanthin dipalmitate
玉米黄素双棕榈酸酯[M+H]+,[M+H-256]+,[M+H-2PA]+,
[M+H-PA]+,[M]−1300.1,1045.9, 1045,1044,789.7,79.6,789.5,789,533.5,533.4,533 [14,17−22] β-carotene
β-胡萝卜素[M+H]+,[M-92]+,[M]− 537.6,537.4,537,536,444,413,375,177.1 [14,17−20,22] zeaxanthin
玉米黄质[M+H]+,[M+H-18]+,[M-92]+,[M-394]+,[M]− 569.4,569.3,569,568,543,533,551.4,
469,476.4,175.1,477.5[14,17−20,22] neoxanthin
新黄质[M+H]+,[M+H-18]+,[M]− 601.4,601,600,583.4,565.5,491,393 [14,17,19−20,22] 9-or 9'-Z-β-cryptoxanthin
9或9’-Z-β-隐黄质[M+H]+ 553.4 [20] 15-or 15'-Z-zeaxanthin
15或15’-Z-玉米黄质[M+H]+ 569.4 [17,19] β-cryptoxanthin
β-隐黄质[M+H]+,[M+H-18]+ 553.4,553.3,535.4,461,497 [16,19] zeaxanthin monopalmitate
玉米黄质单棕榈酸酯[M+H]+,[M+H-PA]+,[M+PA-H]− 1062,807.7,551.4,551 [18−19] 13-or13'-Z-zeaxanthin
13或13’-Z-玉米黄质[M+H]+,[M+H-18]+ 569.4,533,551.4,469 [16,19] β-Cryptoxanthin monopalmitate
β-隐黄质单棕榈酸酯[M+H]+,[M+H-PA]+ 791.6,790,535.4 [16,18−20] 9-or 9'-Z-zeaxanthin
9或9’-Z-玉米黄质[M+H]+,[M+H-18]+ 601.4,583.4,569.4,551,533 [16−17,19−20] 13-or 13'-Z-β-carotene
13或13’-Z-β-胡萝卜素[M+H]+,[M]− 537.4,537.3,537,536 [17,19,22] 9-or 9'-Z-β-carotene
9或9’-Z-β-胡萝卜素[M+H]+,[M]− 537.6,537.4,537,536,445,444,413 [16,19−20,22] antheraxanthin dipalmitate
花药黄质双棕榈酸酯[M+H]+,[M+H-PA]+,[M]− 1061.9,1061,1060,805.6,805,549 [18,22] zeaxanthin myristate palmitate
玉米黄质肉豆蔻酸棕榈酸酯[M+H]+,[M+H-MA]+,[M+H-PA]+,[M]− 1017.9,1017,1016,789.6,789,761.6,761 [18,22] α-carotene
α-胡萝卜素[M+H]+ 537.3,399,331,135 [16,20] zeaxanthin glucomannan
玉米黄质葡甘聚糖[M+H-18]+ 965.7 [16,20] 1'-Hydroxy-γ-carotenoid glucomannan
1'-羟基-γ-类胡萝卜素葡甘聚糖[M+H-18]+ 697.5,279,97,78 [16,20] violaxanthinz
紫黄质[M+H]+,[M]− 601,600 [22] 表 2 不同加工、贮藏条件影响下类胡萝卜素降解及其异构化产物
Table 2 Carotenoids degradation and isomerization products under different processing and storage conditions
类胡萝卜素 主要制备方法 仪器型号 离子源 MS 影响因素 断裂位点 异构化及降解产物 参考文献 非挥发性 挥发性 β-胡萝卜素 HS-SPME GC-MS − NH3;3.7 mL/min;250 ℃;30~
450 AUM烘干 − β-紫罗兰酮、β-环柠檬醛、二氢猕猴桃内酯 [28] 水,己烷,四氢呋喃于超声波反应器中反应 UPLC-MS APCI 20~600 nm 超声 − 15-Z-、9-Z-、di-Z-、13-Z-、β-apo-11-、β-apo-13-、β-apo-15-、β-apo-14’-、β-apo-12’-、β-apo-10’-、β-apo-8’- − [40] 吐温80乳化,蒸馏水溶解 HPLC/
HPLC-MSEI He;1.5 mL/min;
230 ℃;40~400 nm超声 − 15-Z-、13-Z、9-Z β-紫罗兰酮、异佛尔酮、2,2,6-三甲基环己酮 [41] HS-SPME GC-MS EI He;1 mL/min;
250 ℃;70 eV;20~450 AUM发酵 − α-环柠檬醛、β-环柠檬醛、香叶基
丙酮[45] 四氢呋喃溶解 HPLC-MS − N2;10 L/min;80 psi 氧气、臭氧 − 13-Z-、9-Z-、di-Z-、β-apo-13-、β-apo-14′- − [64] 玉米黄素 HS-SPME GC-MS EI He;1 mL/min;
250 ℃;70 eV;20~450 AUM发酵 − − 二氢茉莉酮、藏红花醛、β-大马酮 [45] 己烷溶解,正己烷-碘液催化,荧光灯
照射HPLC-MS APCI N2;5.0 L/min;
2500 V;20 psi;4 μA光+热 − 13-13’-Z-、9-9’-Z-、15-15’-Z-、9,13-di-Z- − [68] 玉米黄素双棕榈酸酯 HS-SPME GC-MS EI He;1 mL/min;
250 ℃;70 eV;20~450 AUM发酵 − − 2,2,6-三甲基环己酮、柠檬烯、2-辛烯醛、丙酸香叶酯、二氢猕猴桃
内酯[45] β-隐黄质 甲醇,正己烷溶解,25 ℃
照射HPLC-MS APCI N2;5.0 L/min;
2500 V;20 psi;汽化350 ℃;鞘气
400 ℃;4 μA光照 − β-β-carotene-3-one、5,6-epoxy-β-β-carotene-3-one、9'-Z-、13-13'-Z- − [63] 甲醇溶解,正己烷碘液催化,25 ℃照射 HPLC-MS APCI N2;5.0 L/min;
2500 V;20 psi;汽化350 ℃;鞘气
400 ℃;4 μA碘+光照 − β-β-carotene-3-one、5,6-epoxy-β-β-carotene-3-one、9-Z-,9'-Z-、13-Z-、13'-Z-、15-Z- − [63] 无环类胡萝卜素 HS-SPME GC-MS − NH3;3.7 mL/min;250 ℃;30~
450 AUM烘干 − − 异佛尔酮、香叶基丙酮 [28] 玉米黄质、β-胡萝卜素、玉米黄素双棕榈
酸酯HS-SPME GC-MS EI N2;1 mL/min;
250 ℃;20~
450 AMU机械破碎 C7~C8/C8~C9/
C14~C15− 2-异丙烯基-6-异丙基-3-甲基-3-乙烯基环己酮、异香叶醇、3-异佛尔酮 [33] HS-SPME GC-MS EI N2;1 mL/min;
250 ℃;20~
450 AMUpH C7~C8/C9~C10/
C9'~C10'− 2,2,6-三甲基环己酮、2-庚烯醛、β-紫罗兰酮 [33] HS-SPME GC-MS EI N2;1 mL/min;
250 ℃;20~
450 AMU高压灭菌 C1~C6/C5~C6/C7~C8/C13~C14/C6’~C7’/
C9~C10/C9’~C10’− 2,4-壬二烯醛、2,2,6-三甲基环己酮、β-环柠檬醛、香叶基丙酮、β-紫罗兰酮、2-甲基-5-(1-甲基乙基)-环已酮 [33] HS-SPME GC-MS EI N2;1.5 mL/min;
250 ℃;45~
450 AMU机械处理 C4~C5/C6~C7/C7~C8/C9~C10/C12'~C13'/
C13~C14/C14~C15− 甲基庚烯酮、异戊醇、异佛尔酮 [33] HS-SPME GC-MS EI N2;1.5 mL/min;
250 ℃;45~
450 AMU光照 C6~C7/C7~C8/C7'~C8'/C9~C10/C9'~C10'/
C12'~C13'/C13~C14/
C14~C15− 甲基庚烯酮、2,4-壬二烯醛,β-环柠檬醛、香叶丙酮、β-紫罗酮、二氢猕猴桃内酯 [33] HS-SPME GC-MS EI N2;1.5 mL/min;
250 ℃;45~
450 AMU氧化 C6~C7/C7~C8/C7'~C8'/C9~C10/C12'~C13'/
C13~C14/C14~C15− 甲基庚烯、2,4-壬二烯醛、β-环柠檬醛、香叶酰丙酮、2,2,6-三甲基环己酮、β-紫罗酮 [33] HS-SPME GC-MS EI N2;1.5 mL/min;
250 ℃;45~
450 AMUpH − − 甲基庚烯酮、辛烯、2,2,6-三甲基环己酮、β-紫罗酮 [33] 叶黄素 有机溶剂溶解 HPLC-MS APCI N2;350 ℃;4 μA;50~1200 m/z 超声(120、180、240、300 W) − 13-Z-、13'-Z-、
9-Z-、9'-Z-− [39] 甲醇溶解,
25 ℃照射HPLC–MS APCI N2;5.0 L/min;
2500 V;汽化
350 ℃;4 μA;80~
1000 m/z光照 − 9-Z-、9'-Z-、
13-13'-Z-− [62] 甲醇溶解,正己烷碘液催化,25 ℃照射 HPLC–MS APCI N2;5.0 L/min;
2500 V;汽化
350 ℃;4 μA;80~
1000 m/z碘+光照 − 15-Z-、9-Z-、9'-Z-、13-13'-Z-、di-Z-异构体 − [62] 注:−未报道。 -
[1] ULBRICHT C, BRYAN J K, COSTA D, et al. An evidence-based systematic review of goji (Lycium spp.) by the natural standard research collaboration[J]. Journal of Dietary Supplements, 2015, 12(2):184−240.
[2] ZENG W, CHEN L, XIAO Z, et al. Comparative study on the structural properties and bioactivities of three different molecular weights of Lycium barbarum polysaccharides[J]. Molecules,2023,28(2):701. doi: 10.3390/molecules28020701
[3] LIU P Y, ZHOU W, XU W Q, et al. The main anthocyanin monomer from Lycium ruthenicum Murray fruit mediates obesity via modulating the gut microbiota and improving the intestinal barrier[J]. Foods,2022,11(1):98.
[4] FRATIANNI A, NIRO S, ALAM M, et al. Effect of a physical pre-treatment and drying on carotenoids of goji berries (Lycium barbarum L.)[J]. LWT,2018,92:318−323. doi: 10.1016/j.lwt.2018.02.048
[5] HARRISON E H. Carotenoids, β-apocarotenoids, and retinoids:The long and the short of it[J]. Nutrients, 2022, 14(7):1411.
[6] BOUVIER F, DOGBO O, CAMARA B. Biosynthesis of the food and cosmetic plant pigment bixin (annatto)[J]. Science,2003,300(5628):2089−2091.
[7] SATHASIVAM R, RADHAKRISHNAN R, KIM J K, et al. An update on biosynthesis and regulation of carotenoids in plants[J]. South African Journal of Botany,2021,140:290−302. doi: 10.1016/j.sajb.2020.05.015
[8] CHENG G T, LI Y S, QI S M, et al. SlCCD1A enhances the aroma quality of tomato fruits by promoting the synthesis of carotenoid-derived volatiles[J]. Foods,2021,10(11):2678. doi: 10.3390/foods10112678
[9] 朱明明, 樊明涛, 何鸿举. 类胡萝卜素降解方式的研究进展[J]. 食品科学,2017,38(11):308−317. [ZHU M M, FAN M T, HE H J. Advances in methods for the degradation of carotenoids[J]. Food Science,2017,38(11):308−317.] doi: 10.7506/spkx1002-6630-201711048 ZHU M M, FAN M T, HE H J. Advances in methods for the degradation of carotenoids[J]. Food Science, 2017, 38(11): 308−317. doi: 10.7506/spkx1002-6630-201711048
[10] 许春平, 王铮, 郑坚强, 等. 类胡萝卜素降解方式的研究综述[J]. 郑州轻工业学院学报:自然科学版,2012,27(4):5. [XU C P, WANG Z, ZHENG J Q, et al. Review of research on degradation pattern of carotenoid[J]. Journal of Zhengzhou Institute of Light Industry (Natural Science),2012,27(4):5.] XU C P, WANG Z, ZHENG J Q, et al. Review of research on degradation pattern of carotenoid[J]. Journal of Zhengzhou Institute of Light Industry (Natural Science), 2012, 27(4): 5.
[11] 闫亚美, 米佳, 曹有龙, 等. 枸杞类胡萝卜素抗氧化组效关系研究[Z]. 2018. [YAN Y M, MI J, CAO Y L, et al. Study on the effect relationship of carotenoid antioxidant groups in Lycium barbarum[Z]. 2018.] YAN Y M, MI J, CAO Y L, et al. Study on the effect relationship of carotenoid antioxidant groups in Lycium barbarum[Z]. 2018.
[12] 雷建武, 米佳, 罗青, 等. 枸杞中类胡萝卜素及体外抗氧化活性研究[J]. 食品工业,2015,36(12):5−8. [LEI J W, MI J, LUO Q, et al. Composition and antioxidant activity of carotenoids from different varieties of Lycium[J]. Food Industry,2015,36(12):5−8.] LEI J W, MI J, LUO Q, et al. Composition and antioxidant activity of carotenoids from different varieties of Lycium[J]. Food Industry, 2015, 36(12): 5−8.
[13] 米佳, 罗青, 禄璐, 等. 枸杞脂溶性物质的中试提取、成分分析及主要类胡萝卜素单体的制备[J]. 食品工业科技,2022,43(11):185−191. [MI J, LUO Q, LU L, et al. Pilot extraction and component analysis of fat-soluble substances from Lycium barbarum L. and the preparation of major carotenoids monomer[J]. Science and Technology of Food Industry,2022,43(11):185−191.] MI J, LUO Q, LU L, et al. Pilot extraction and component analysis of fat-soluble substances from Lycium barbarum L. and the preparation of major carotenoids monomer[J]. Science and Technology of Food Industry, 2022, 43(11): 185−191.
[14] 王梦泽. 冻融处理对枸杞冻干果色泽稳定性的影响及机理[D]. 北京:北京林业大学, 2021. [WANG M Z. Effect and action mechanism of freeze-thaw treatment on the color stability of lyophilized goji berry[D]. Beijing:Beijing Forestry University, 2021.] WANG M Z. Effect and action mechanism of freeze-thaw treatment on the color stability of lyophilized goji berry[D]. Beijing: Beijing Forestry University, 2021.
[15] DUMONT D, DANIELATO G, CHASTELLIER A, et al. Multi-targeted metabolic profiling of carotenoids, phenolic compounds and primary metabolites in goji (Lycium spp.) berry and tomato (Solanum lycopersicum) reveals inter and intra genus biomarkers[J]. Metabolites,2020,10(10):422. doi: 10.3390/metabo10100422
[16] 马亚男. 不同处理方式的枸杞中类胡萝卜素生物可及性与抗氧化性研究[D]. 银川:宁夏大学, 2021. [MA Y N. The bioaccessibility and antioxidant activity of carotenoids in different treatments of Lycium barbarum L D]. Yinchuan:Ningxia University, 2021.
[17] HSU H J, HUANG R F, KAO T H, et al. Preparation of carotenoid extracts and nanoemulsions from Lycium barbarum L. and their effects on growth of HT-29 colon cancer cells[J]. Nanotechnology,2017,28(13):135103. doi: 10.1088/1361-6528/aa5e86
[18] YU Z, XIA M, LI X, et al. Characterization of carotenoids in Lycium barbarum fruit by using UPC2-PDA-Q-TOF-MSE couple with deep eutectic solvents extraction and evaluation of their 5α-reductase inhibitory activity[J]. Frontiers in Chemistry, 2022.
[19] INBARAJ B S, LU H, HUNG C F, et al. Determination of carotenoids and their esters in fruits of Lycium barbarum Linnaeus by HPLC−DAD−APCI−MS[J]. Journal of Pharmaceutical and Biomedical Analysis,2008,47(4-5):812−818.
[20] HU Z, MA Y, LIU J, et al. Assessment of the bioaccessibility of carotenoids in goji berry (Lycium barbarum L.) in three forms:In vitro digestion model and metabolomics approach[J]. Foods,2022,11(22):3731. doi: 10.3390/foods11223731
[21] LONG J T, FAN H X, ZHOU Z Q, et al. The major zeaxanthin dipalmitate derivatives from wolfberry[J]. Journal of Asian Natural Products Research,2020,22(8):746−753. doi: 10.1080/10286020.2019.1621855
[22] HEMPEL J, SCHÄDLE C N, SPRENGER J, et al. Ultrastructural deposition forms and bioaccessibility of carotenoids and carotenoid esters from goji berries (Lycium barbarum L.)[J]. Food Chemistry,2017,218:525−533. doi: 10.1016/j.foodchem.2016.09.065
[23] SEMITSOGLOU-TSIAPOU S, MEADOR T B, PENG B, et al. Photochemical (UV–vis/H2O2) degradation of carotenoids:Kinetics and molecular end products[J]. Chemosphere,2022,286:131697.
[24] FUSI F, ROMANO G, SPERANZA G, et al. Photon- and singlet-oxygen-induced cis–trans isomerization of the water-soluble carotenoid crocin[J]. International Journal of Molecular Sciences, 2023, 24(13):10783.
[25] XU M, CHEN T, BUTT C M. Identification of beta-carotene degradation compounds and their structural elucidation by high-resolution accurate mass spectrometry[J]. Journal of Food Science,2019,84(12):3535−3545. doi: 10.1111/1750-3841.14909
[26] 马文平, 李赫, 叶立勤, 等. 不同采收期枸杞干燥过程中主要类胡萝卜素的变化[J]. 中国农业科技,2007,40(7):1492−1497. [MA W P, LI H, YE L Q, et al. Changes of the main carotenoids pigment contents during the drying processes at different harvest stage in Lycium barbarum L. fruits[J]. Scientia Agricultura Sinica.,2007,40(7):1492−1497.] MA W P, LI H, YE L Q, et al. Changes of the main carotenoids pigment contents during the drying processes at different harvest stage in Lycium barbarum L. fruits[J]. Scientia Agricultura Sinica., 2007, 40(7): 1492−1497.
[27] 吴励萍, 卢有媛, 李海洋, 等. 不同干燥方法对枸杞子药材多类型功效成分的影响及其分析评价[J]. 中草药,2022,53(7):2125−2136. [WU L P, LU Y Y, LI H Y, et al. Analysis and evaluation of different drying methods for Lycii fructus based on multi-type functional components[J]. Chinese Traditional and Herbal Drugs,2022,53(7):2125−2136.] WU L P, LU Y Y, LI H Y, et al. Analysis and evaluation of different drying methods for Lycii fructus based on multi-type functional components[J]. Chinese Traditional and Herbal Drugs, 2022, 53(7): 2125−2136.
[28] 曲云卿. 产地和烘干过程对枸杞中挥发性物质和类胡萝卜素的影响[D]. 银川:宁夏大学, 2015. [QU Y Q. The influence of origins and drying process on volatile substances and carotenoids of wolfberry[D]. Yinchuan:Ningxia University, 2015.] QU Y Q. The influence of origins and drying process on volatile substances and carotenoids of wolfberry[D]. Yinchuan: Ningxia University, 2015.
[29] WEI B X, CAI C X, XU B G, et al. Disruption and molecule degradation of waxy maize starch granules during high pressure homogenization process[J]. Food Chemistry,2018,240:165−173. doi: 10.1016/j.foodchem.2017.07.078
[30] AHMED J, SHIVHARE U, RAGHAVAN G. Rheological characteristics and kinetics of colour degradation of green chilli puree[J]. Journal of Food Engineering,2000,44(4):239−244. doi: 10.1016/S0260-8774(00)00034-0
[31] PATSILINAKOS A, RAGNO R, CARRADORI S, et al. Carotenoid content of goji berries:CIELAB, HPLC-DAD analyses and quantitative correlation[J]. Food Chemistry,2018,268:49−56.
[32] 赵璐. 枸杞酒制作中类胡萝卜素降解产物降异戊二烯分析研究[D]. 银川:宁夏大学, 2018. [ZHAO L. Analysis of isoprene degradation products of carotenoids in wolfberry wine during fermentation process[D]. Yinchuan:Ningxia University, 2018.] ZHAO L. Analysis of isoprene degradation products of carotenoids in wolfberry wine during fermentation process[D]. Yinchuan: Ningxia University, 2018.
[33] GENG J, ZHAO L, ZHANG H. Formation mechanism of isoprene compounds degraded from carotenoids during fermentation of goji wine[J]. Food Quality and Safety,2021,5:fyaa033. doi: 10.1093/fqsafe/fyaa033
[34] KHAN M K, ABERT-VIAN M, FABIANO-TIXIER A S, et al. Ultrasound-assisted extraction of polyphenols (Flavanone glycosides) from orange (Citrus sinensis L.) peel[J]. Food Chemistry,2010,119(2):851−858. doi: 10.1016/j.foodchem.2009.08.046
[35] IIDA Y, TUZIUTI T, YASUI K, et al. Control of viscosity in starch and polysaccharide solutions with ultrasound after gelatinization[J]. Innovative Food Science & Emerging Technologies,2008,9(2):140−146.
[36] CZECHOWSKA-BISKUP R, ROKITA B, LOTFY S, et al. Degradation of chitosan and starch by 360-kHz ultrasound[J]. Carbohydrate Polymers,2005,60(2):175−184. doi: 10.1016/j.carbpol.2004.12.001
[37] QIAO L, YE X, SUN Y, et al. Sonochemical effects on free phenolic acids under ultrasound treatment in a model system[J]. Ultrasonics Sonochemistry,2013,20(4):1017−1025. doi: 10.1016/j.ultsonch.2012.12.007
[38] VILLAMIEL M, DE JONG P. Influence of high-intensity ultrasound and heat treatment in continuous flow on fat, proteins, and native enzymes of milk[J]. Journal of Agricultural and Food Chemistry,2000,48(2):472−478. doi: 10.1021/jf990181s
[39] SONG J F, LI D J, PANG H L, et al. Effect of ultrasonic waves on the stability of all-trans lutein and its degradation kinetics[J]. Ultrasonics Sonochemistry,2015,27:602−608. doi: 10.1016/j.ultsonch.2015.04.020
[40] CARAIL M, FABIANO T A S, MEULLEMIESTRE A, et al. Effects of high power ultrasound on all-E-β-carotene, newly formed compounds analysis by ultra-high-performance liquid chromatography–tandem mass spectrometry[J]. Ultrasonics Sonochemistry,2015,26:200−209. doi: 10.1016/j.ultsonch.2015.04.003
[41] 于奉生, 孙志高, 方明. β-胡萝卜素模拟体系超声降解机制研究[J]. 食品与发酵工业,2020,46(11):69−76. [YU F S, SUN Z G, FANG M. Study on ultrasound degradation mechanism of β-carotene simulation system[J]. Food and Fermentation Industries,2020,46(11):69−76.] YU F S, SUN Z G, FANG M. Study on ultrasound degradation mechanism of β-carotene simulation system[J]. Food and Fermentation Industries, 2020, 46(11): 69−76.
[42] 孙玉敬. 类胡萝卜素在超声波辅助提取中的稳定性及其定量构效关系的研究[D]. 杭州:浙江大学, 2011. [SUN Y J. The stability of carotenoids under ultrasond-assisted extraction and quantitative structure-activity relationship of carotenoids[D]. Hangzhou:Zhejiang University, 2011.] SUN Y J. The stability of carotenoids under ultrasond-assisted extraction and quantitative structure-activity relationship of carotenoids[D]. Hangzhou: Zhejiang University, 2011.
[43] 刘亚, 刘建花, 张惠玲, 等. 枸杞酒发酵主要代谢产物对类胡萝卜素降解的影响[J]. 食品科学,2017,38(14):36−41. [LIU Y, LIU J H, ZHANG H L, et al. Effect of main metabolites on carotenoids degradation during the fermentation of Chinese wolfberry wine[J]. Food Science,2017,38(14):36−41.] LIU Y, LIU J H, ZHANG H L, et al. Effect of main metabolites on carotenoids degradation during the fermentation of Chinese wolfberry wine[J]. Food Science, 2017, 38(14): 36−41.
[44] 刘建花. 不同酵母菌在枸杞酒酿造中对类胡萝卜素的影响研究[D]. 银川:宁夏大学, 2017. [LIU J H. Effects of different yeasts on carotenoids in wolfberry wins[D]. Yinchuan: Ningxia University, 2017.] LIU J H. Effects of different yeasts on carotenoids in wolfberry wins[D]. Yinchuan: Ningxia University, 2017.
[45] 田争福, 申鹏森, 赵璐, 等. 类胡萝卜素降解对枸杞酒特征香气的影响[J]. 食品与生物技术学报,2022,41(9):78−84. [TIAN Z F, SHEN P S, ZHAO L. Effect of carotenoid degradation on characteristic aroma of wolfberry wine[J]. Journal of Food and Biotechnology,2022,41(9):78−84.] doi: 10.3969/j.issn.1673-1689.2022.09.009 TIAN Z F, SHEN P S, ZHAO L. Effect of carotenoid degradation on characteristic aroma of wolfberry wine[J]. Journal of Food and Biotechnology, 2022, 41(9): 78−84. doi: 10.3969/j.issn.1673-1689.2022.09.009
[46] CHEN K Y, ZHAO L, YUE Y Y, et al. New process of goji fermented wine:Effect of goji residue degradation to generate norisoprenoid aroma compounds[J]. Food Science and Technology,2023,43:e126522. doi: 10.1590/fst.126522
[47] 谭榀新, 叶涛, 刘湘新, 等. 植物提取物抗氧化成分及机理研究进展[J]. 食品科学,2010,31(15):288−292. [TAN P X, YE T, LIU X X, et al. Research advances in antioxidant composition of botanical extracts and their action mechanisms[J]. Food Science,2010,31(15):288−292.] TAN P X, YE T, LIU X X, et al. Research advances in antioxidant composition of botanical extracts and their action mechanisms[J]. Food Science, 2010, 31(15): 288−292.
[48] 胡晗艳. 枸杞加工过程中β-胡萝卜素变化的研究[D]. 天津:天津科技大学, 2012. [HU H Y. Study on change of bete carotene in Lycium bararum L. during processing[D]. Tianjin:Tianjin University of Science and Technology, 2012.] HU H Y. Study on change of bete carotene in Lycium bararum L. during processing[D]. Tianjin: Tianjin University of Science and Technology, 2012.
[49] 沈垚垚. 维生素C及柠檬酸对于鲜枸杞原浆的抗氧化研究[J]. 食品安全导刊, 2020(36):132, 134. [SHEN Y Y. Study on antioxidant effects of vitamin C and citric acid on fresh Wolfberry pulp[J]. Food Safety Guide, 2020(36):132, 134.] SHEN Y Y. Study on antioxidant effects of vitamin C and citric acid on fresh Wolfberry pulp[J]. Food Safety Guide, 2020(36): 132, 134.
[50] DEMARCANO E, MÉNDEZ O, GONZÁLEZ J, et al. Effect of drying and citric acid treatment on carotenoids degradation in sweet potato (Ipomea batatas L.) flours[J]. Revista De La Facultad de Agronomía, Universidad del Zulia,2010,27(1):112−124.
[51] MELÉNDEZ-MARTÍNEZ A J, ESCUDERO-GILETE M L, VICARIO I M, et al. Effect of increased acidity on the carotenoid pattern and colour of orange juice[J]. European Food Research and Technology,2010,230(3):527−532. doi: 10.1007/s00217-009-1190-1
[52] EMAMI M R, JAMSHIDI S, ZAREZADEH M, et al. Can vitamin E supplementation affect obesity indices? A systematic review and meta-analysis of twenty-four randomized controlled trials[J]. Clinical Nutrition,2021,40(5):3201−3209. doi: 10.1016/j.clnu.2021.02.002
[53] REBOUL E, THAP S, PERROT E, et al. Effect of the main dietary antioxidants (carotenoids, γ-tocopherol, polyphenols, and vitamin C) on α-tocopherol absorption[J]. European Journal of Clinical Nutrition,2007,61(10):1167−1173. doi: 10.1038/sj.ejcn.1602635
[54] 张春兰. 枸杞色素的提取及稳定性研究[D]. 乌鲁木齐:新疆农业大学, 2005. [ZHANG C L. Studies on the extraction and stability of fructus Lycii pigment[D]. Urumqi:Xinjiang Agricultural University, 2005.] ZHANG C L. Studies on the extraction and stability of fructus Lycii pigment[D]. Urumqi: Xinjiang Agricultural University, 2005.
[55] 常旋, 王根女, 黄国周, 等. 维生素E对β-胡萝卜素纳米乳液性质及其饮料稳定性的影响[J]. 食品工业科技,2022,43(6):56−62. [CHANG X, WANG G, HUANG G Z. Effects of vitamin E on the properties of β-carotene nanoemulsions and beverage stability[J]. Science and Technology of Food Industry,2022,43(6):56−62.] CHANG X, WANG G, HUANG G Z. Effects of vitamin E on the properties of β-carotene nanoemulsions and beverage stability[J]. Science and Technology of Food Industry, 2022, 43(6): 56−62.
[56] 周丹红, 蔡红, 徐基贵, 等. 番茄红素在食用油中的稳定性及抗氧化机理研究[J]. 广东农业科学,2009(6):124−126. [ZHOU D H, CAI H, XU J G. Studies on the stability of lycopene and its antioxidant mechanism in the edible oil[J]. Guangdong Agricultural Sciences,2009(6):124−126].] ZHOU D H, CAI H, XU J G. Studies on the stability of lycopene and its antioxidant mechanism in the edible oil[J]. Guangdong Agricultural Sciences, 2009(6): 124−126].
[57] KAN X, YAN Y, RAN L, et al. Evaluation of bioaccessibility of Zeaxanthin dipalmitate from the fruits of Lycium barbarum in oil-in-water emulsions[J]. Food Hydrocolloids,2020,105:105781. doi: 10.1016/j.foodhyd.2020.105781
[58] LUO Y, LIU Y, GUO H, et al. Evaluation of the bioaccessibility of carotenoid esters from Lycium barbarum L. in nano-emulsions:A kinetic approach[J]. Food Research International,2020,136:109611. doi: 10.1016/j.foodres.2020.109611
[59] LI J Y, HUANG G L, QIAN H, et al. Fabrication of soy protein isolate - high methoxyl pectin composite emulsions for improving the stability and bioavailability of carotenoids[J]. Food Bioscience, 2023, 53.
[60] 赵宇慧, 魏超昆, 徐梦霞, 等. 枸杞复合糖果储藏稳定性研究[J]. 食品工业科技,2017,38(21):31−36. [ZHAO Y H, WEI C K, XU M X, et al. Study on the stability of wolfberry composite candy[J]. Food Industry Technology,2017,38(21):31−36.] ZHAO Y H, WEI C K, XU M X, et al. Study on the stability of wolfberry composite candy[J]. Food Industry Technology, 2017, 38(21): 31−36.
[61] 曹有龙, 刘兰英, 李晓莺, 等. 枸杞鲜果类胡萝卜素超声提取工艺优化及光稳定性[J]. 食品研究与开发,2014,35(5):20−22. [CAO Y L, LIU L Y, LI X Y. Ultrasonic extraction technology and stability to light of carotenoids in wolfberry fruit[J]. Food Research and Development,2014,35(5):20−22.] doi: 10.3969/j.issn.1005-6521.2014.05.006 CAO Y L, LIU L Y, LI X Y. Ultrasonic extraction technology and stability to light of carotenoids in wolfberry fruit[J]. Food Research and Development, 2014, 35(5): 20−22. doi: 10.3969/j.issn.1005-6521.2014.05.006
[62] LI D J, XIAO Y D, ZHANG Z Y, et al. Analysis of (all-E)-lutein and its (Z)-isomers during illumination in a model system[J]. Journal of Pharmaceutical and Biomedical Analysis,2014,100:33−39. doi: 10.1016/j.jpba.2014.07.018
[63] LI D J, XIAO Y D, ZHANG Z Y, et al. Light-induced oxidation and isomerization of all-trans-β-cryptoxanthin in a model system[J]. Journal of Photochemistry and Photobiology B:Biology,2015,142:51−58. doi: 10.1016/j.jphotobiol.2014.11.003
[64] HENRY L K, PUSPITASARI-NIENABER N L, JARÉN-GALÁN M, et al. Effects of ozone and oxygen on the degradation of carotenoids in an aqueous model system[J]. J Agric Food Chem,2000,48(10):5008−5013. doi: 10.1021/jf000503o
[65] MUHAMMAD ZAHIR S A, YAHAYA O K, OMAR A F. Correlating the natural color of tropical fruit juice with its pH[J]. Color Research & Application,2021,46(2):467−476.
[66] 周广志. 枸杞酒发酵对类胡萝卜素的变化影响研究[D]. 银川:宁夏大学, 2015. [ZHOU G Z. Studies of effects of wolfberry wine fermentation on carotenoids[D]. Yinchuan:Ningxia University, 2015.] ZHOU G Z. Studies of effects of wolfberry wine fermentation on carotenoids[D]. Yinchuan: Ningxia University, 2015.
[67] JATOI M A, FRUK M, BUHIN J, et al. Effect of different storage temperatures on storage life, physico-chemical and sensory attributes of goji berry (Lycium barbarum L.) fruits[J]. Erwerbs-Obstbau,2018,60(2):119−126. doi: 10.1007/s10341-017-0344-8
[68] XIAO Y D, HUANG W Y, LI D J, et al. Thermal degradation kinetics of all-trans and cis-carotenoids in a light-induced model system[J]. Food Chemistry,2018,239:360−368.
[69] 周宜洁, 李新, 马三梅, 等. 贮藏温度对鲜枸杞类胡萝卜素和氨基酸的影响及调控机制[J]. 科学通报,2022,67:385−395. [ZHOU Y J, LI X, MA S M. Effects of storage temperature on carotenoids and amino acids in fresh goji berry and the related regulation mechanism[J]. Chin Sci Bul,2022,67:385−395.] doi: 10.1360/TB-2020-1688 ZHOU Y J, LI X, MA S M. Effects of storage temperature on carotenoids and amino acids in fresh goji berry and the related regulation mechanism[J]. Chin Sci Bul, 2022, 67: 385−395. doi: 10.1360/TB-2020-1688





 下载:
下载:
 下载:
下载:



