Effects of Extraction Methods on Structural and Functional Properties of Water-insoluble Dietary Fiber from Polygonatum sibiricum Residue
-
摘要: 以黄精渣为原料,探究不同提取方法对黄精渣水不溶性膳食纤维(IDF)理化性质和功能特性的影响。采用复合酶法(CE)、超声辅助复酶法(UCE)和酶碱法(CEA)提取IDF,并考察三种IDF的组成、理化特性、葡萄糖吸附能力、阳离子交换能力和DPPH自由基清除能力等功能特性。结果表明,超声辅助复酶法得率最高(84.68%)。三种黄精渣IDF纯度均较高,主要由纤维素、半纤维素和木质素组成,其中UCE-IDF纯度高达82.24%。UCE-IDF持水力(4.74±0.66)g/g、持油力(3.84±0.29)g/g和结合水力(3.29±0.09)g/g最优,总酚含量(7.77±0.07)mg/g最高,阳离子交换能力(0.40±0.003)mmol/g最佳,抗氧化能力最佳。CEA-IDF的膨胀力(5.33±0.11)mL/g和总黄酮含量(1.88±0.03)mg/g最高。CE-IDF的葡萄糖吸附能力(19326.67±41.63)μmol/g最佳。理化表征显示,UCE-IDF结构更加不规则且具有更高的热稳定性。红外光谱显示三种黄精渣IDF均具有纤维素类特征吸收峰。X射线结果显示三种黄精渣IDF呈纤维素I晶型。综上,三种IDF的理化和功能特性之间存在差异,UCE-IDF在持水、持油等多项理化特性中具有明显优势,且热稳定性和抗氧化活性最佳。CEA-IDF的总黄酮含量最高且膨胀力最佳。CE-IDF的葡萄糖吸附能力最佳,本实验结果可为黄精渣IDF在食品加工中的应用提供理论参考。Abstract: The effects of different extraction methods on the physical and chemical properties and functional characteristics of insoluble dietary fiber (IDF) from Polygonatum sibiricum residue were investigated. The complex enzyme method (CE), ultrasonic-assisted complex enzyme method (UCE) and enzyme-alkali method (CEA) were used to extract IDF. The composition, physicochemical properties, glucose adsorption capacity, cation exchange capacity and DPPH free radical scavenging capacity of three IDFs were analyzed. Results showed that the yield of ultrasound-assisted compound enzyme method was the highest (84.68%). Three IDFs had a higher purity and mainly consisted of cellulose, hemicellulose, and lignin. The purity of UCE-IDF was up to 82.24%. UCE-IDF had the best water holding capacity (4.74±0.66) g/g, oil holding capacity (3.84±0.29) g/g, binding capacity (3.29±0.09) g/g, the highest total phenol content (7.77±0.07) mg/g, the best cation exchange capacity (0.40±0.003) mmol/g, and the antioxidant capacity. CEA-IDF had the highest swelling capacity (5.33±0.11) mL/g and flavonoid content (1.88±0.03) mg/g. CE-IDF had the best glucose adsorption capacity (19326.67±41.63) μmol/g. Physical and chemical characterization indicated that the structure of UCE-IDF was more irregular and had higher thermal stability. The infrared spectra suggested that the three IDFs had the characteristic absorption peaks of cellulose. The X-ray results revealed that the three IDFs were cellulose I crystal. In summary, there were differences in physical and chemical properties and functional characteristics among the three IDFs. UCE-IDF showed significant advantages in several physical and chemical properties, such as water holding, oil holding thermal stability, and antioxidant activity. CEA-IDF was distinguished by its high flavonoid content and the best swelling power. CE-IDF had the best glucose adsorption capacity. The results of this study could provide a theoretical basis for the application of IDF in food processing industry.
-
黄精(Polygonatum sibiricum)是百合科黄精属植物,为传统药食同源类食品之一,含有多种活性成分[1]。目前,对黄精的研究局限于多糖、皂苷等功能活性成分方面[2],对活性成分提取后产生的大量残渣利用暂不充分。废弃黄精渣中含有大量膳食纤维,具有在食品工业应用的潜力[3]。因此,对黄精渣中膳食纤维进行提取并研究其理化特性对开发黄精膳食纤维产品具有重要意义。
膳食纤维是主要来源于植物细胞壁的非淀粉多糖。根据溶解性可分为水溶性膳食纤维(SDF)和水不溶性膳食纤维(IDF)两类。其中水不溶性膳食纤维占天然纤维的2/3,主要包括纤维素、半纤维素、木质素等,且在预防和减轻便秘、改善肠道菌群等方面有显著功效[4]。将提取活性成分后的黄精渣进行充分利用,不仅可以提高黄精的附加值,还可以减少废弃黄精渣造成的环境污染。
研究表明,对于不同膳食纤维原料有着不同的最佳提取方法和条件,并且不同提取方法会影响膳食纤维的理化性质和功能特性[5]。李建周等[6]采用酶法提取的豆渣IDF具有较高的持水力;Ma等[7]采用酶法修饰马铃薯IDF,其葡萄糖吸附能力、阳离子交换能力等功能特性显著增强,提高了马铃薯IDF在食品应用中的价值。超声辅助提取可以在一定程度上提高得率,且超声改性过程会对IDF结构与特性产生一定影响。Fan等[8]采用超声辅助酶法进行豆渣IDF提取,通过超声处理诱导豆渣IDF结构变化,结果表明其相对结晶度从55.14%降低到36.47%,并且超声处理还改善了豆渣IDF的持水、持油和膨胀力等特性;张晓娟等[9]认为采用超声辅助复酶法提取的芒果皮IDF持水力和膨胀力较高,能够降低肥胖率及相关的并发症,并且可以作为原料进行深入的改性表征研究。Ma等[10]通过碱法提取紫萝卜IDF,极大地提高了其持水力等功能特性。目前对膳食纤维的研究范围主要集中在水果、蔬菜和谷物[11−12],其他具有较高营养价值的食品还未进行开发利用,尤其是药食两用类食品。因此进行优选提取黄精渣水不溶性膳食纤维方法的研究,开发出高效绿色的黄精渣IDF提取工艺,可提高黄精综合利用率。
综上,本研究采用复合酶法、超声辅助复酶法、酶碱法提取黄精渣水不溶性膳食纤维。通过对比三种方法提取得到的水不溶性膳食纤维的得率、理化性质和功能特性,探究提取方法对黄精渣IDF特性的影响,为充分利用黄精渣、提高黄精附加值提供理论依据。
1. 材料与方法
1.1 材料与仪器
黄精渣(烘干粉碎后呈粉末状) 本课题组提取多糖后剩余黄精渣(黄精烘干粉碎后过60目筛,料液比为1:30,80 ℃水浴1 h,于4000 r/min条件下离心5 min,残渣采用蒸馏水洗涤三次,75%乙醇洗涤一次,于50 ℃下烘干后储存于干燥器中,备用);无水乙醇 天津市登科化学试剂有限公司;α-淀粉酶(1000 U/g) 山东隆科特酶制剂有限公司;木瓜蛋白酶(10万U/g) 南宁庞博生物工程有限公司。
QE-200 高速万能粉碎机 浙江屹立工贸有限公司;L530 离心机 湖南湘仪实验室仪器开发有限公司;Scientz-IID 超声破碎仪 宁波新芝生物科技股份有限公司;JA2003 电子分析天平 上海舜宇恒平科学仪器有限公司;UV-752N 紫外可见分光光度计 上海精密科学仪器有限公司;HH-2 数显恒温水浴锅 金坛市晶玻实验仪器厂;Multiskan GO 全波长酶标仪 上海旦鼎国际贸易有限公司;K9860 凯氏定氮仪 广州平展仪器有限公司;101-4A 电热鼓风干燥箱 上海捷呈实验仪器有限公司;Spectrum Two 傅立叶红外光谱分析仪 美国PerkinElmer股份有限公司;MiniFlex 600 X射线衍射仪 日本理学株式会社;JSM-7900F 扫描电镜 上海爱仪通网络科技有限公司;T6新世纪热重分析仪 北京普析通用仪器有限责任公司。
1.2 实验方法
1.2.1 黄精渣IDF的提取方法
1.2.1.1 复合酶法提取黄精渣IDF工艺
取黄精渣粉2.0 g,以料液比1:20(g/mL)加入去离子水,调节pH至6,添加α-淀粉酶(0.3%,w/w),于60 ℃水浴酶解1 h,煮沸灭酶;添加木瓜蛋白酶(0.15%,w/w),于50 ℃水浴酶解1 h,煮沸灭酶。冷却至室温后,于4000 r/min下离心10 min。舍去上清液,用去离子水洗涤两次,75%乙醇洗涤一次。将残渣放入55 ℃干燥箱内烘干至恒重,即得到复合酶法提取黄精渣水不溶性膳食纤维(CE-IDF)。
1.2.1.2 超声辅助复酶法提取黄精渣IDF工艺
取黄精渣粉2.0 g,以料液比1:20(g/mL)加入去离子水,调节pH至6,添加α-淀粉酶(0.3%,w/w),于60 ℃水浴酶解1 h,煮沸灭酶;添加木瓜蛋白酶(0.15%,w/w),于50 ℃水浴酶解1 h,煮沸灭酶;置于超声细胞破碎仪中在50 ℃下进行超声处理30 min,冷却至室温后,于4000 r/min下离心10 min。舍去上清液,用去离子水洗涤两次,75%乙醇洗涤一次。将残渣放入55 ℃干燥箱内烘干至恒重,即得到超声辅助复酶法提取黄精渣水不溶性膳食纤维(UCE-IDF)。
1.2.1.3 酶碱法提取黄精渣IDF工艺
取黄精渣粉2.0 g,以料液比1:20(g/mL)加入去离子水,调节pH至6,添加α-淀粉酶(0.3%,w/w),于60 ℃水浴酶解1 h,煮沸灭酶;加入4% 1 mol/L的NaOH溶液,于60 ℃下碱水解30 min,冷却至室温后,于4000 r/min下离心10 min。舍去上清液,用去离子水洗涤两次,75%乙醇洗涤一次。将残渣放入55 ℃干燥箱内烘干至恒重,即得到酶碱法提取黄精渣水不溶性膳食纤维(CEA-IDF)。
1.2.1.4 黄精渣IDF得率
黄精渣IDF得率计算公式如下:
IDF得率(%)=m1m2×100 (1) 式中:m1为提取得到的IDF质量,g;m2为黄精渣样品质量,g。
1.2.2 IDF理化性质表征
1.2.2.1 组成成分分析
采用范式(Van Soest)测定纤维素含量法[13],分别测定三种方法提取的黄精渣IDF的组成成分,具体含量计算公式如下。参照GB 31637-2016采用酸水解-DNS法测定淀粉含量,参照GB 5009.9-2016采用凯氏法测定蛋白质含量,参照GB 5009.4-2016采用烘干重量法测定灰分含量。
中性洗涤纤维NDF(%)=W1−W2W×100 (2) 式中:W1为玻璃坩埚和NDF重量,g ;W2为玻璃坩埚重量,g;W为样品质量,g。
酸性洗涤纤维ADF(%)=G1−G2G×100 (3) 式中:G1为玻璃坩埚和ADF重量,g ;G2为玻璃坩埚重量,g;G为样品质量,g。
半纤维素(%)=NDF(%)−ADF(%) (4) 纤维素(%)=ADF(%)−经72%硫酸处理后的残渣(%) (5) 木质素ALD(%)=经72%硫酸处理后的残渣−灰化后的灰分(%) (6) 1.2.2.2 持水力
取1.0 g黄精渣IDF于已加入10 g蒸馏水的离心管中,充分搅拌3 min后离心(4000 r/min,10 min),弃上清液,将试管内壁擦干,称量,重复测定三次,计算其持水力[14]。
持水力=m3−m4m4 (7) 式中:m3为黄精渣IDF离心后质量,g;m4为黄精渣IDF质量,g。
1.2.2.3 持油力
取1.0 g黄精渣IDF于已加入10 g花生油的离心管中,充分搅拌3 min后离心(4000 r/min,10 min),弃上清液,将试管内壁擦干,称量,重复测定三次,计算其持油力[14]。
持油力=m5−m6m6 (8) 式中:m5为黄精渣IDF离心后质量,g;m6为黄精渣IDF质量,g。
1.2.2.4 膨胀力
取1.0 g黄精渣IDF于25 mL离心管中,记录初始体积,加入15 mL蒸馏水,振荡均匀后,室温静置24 h。观察记录样品在量筒中的自由膨胀体积,重复测定三次,然后换算成每克干物质的膨胀体积来表示[14]。
膨胀力(%)=V0−V样m样×100 (9) 式中:V0为黄精渣IDF膨胀后体积,mL;V样为黄精渣IDF加入蒸馏水前体积,mL;m样为加入的黄精渣IDF质量,g。
1.2.2.5 结合水力
取0.25 g黄精渣IDF于已加入25 mL蒸馏水的离心管中,于6000 r/min下离心1 h,取沉淀,室温静置1 h后称重,记为m7,再将沉淀置于恒温干燥箱中箱烘干,称重记为m8。
结合水力(g/g)=m7−m8m9 (10) 式中:m7为静置后的质量,g;m8为烘干后的质量,g;m9为离心前黄精渣IDF的质量,g。
1.2.2.6 傅里叶红外光谱分析
三种黄精渣IDF的红外光谱测定采用溴化钾压片法[15],扫描范围为4000~500 cm−1,扫描次数为32次,分辨率为0.5 cm−1。
1.2.2.7 扫描电镜分析
将三种黄精渣IDF分散在样品台的导电胶上,进行喷金处理。分别在1 K×、2 K×和5 K×下观察并拍摄图像。
1.2.2.8 热重分析
取三种黄精渣IDF各4 mg,使用热分析仪进行热重扫描,升温速率10 ℃/min,升温范围25~600 ℃,气氛为氮气。
1.2.2.9 X射线衍射(XRD)分析
使用X-射线衍射仪对三种黄精渣IDF进行X-射线衍射扫描。分析条件为:Cu-Kα靶(λ=0.154 nm),电压40 kV,电流25 mA,扫描范围为80°,扫描速度10°/min,步长0.05°。
1.2.2.10 阳离子交换能力测定
采用桑嘉玘等[16]的方法略作修改。取1.0 g样品与10 mL 0.1 mol/L的HCl溶液混合,充分混匀,待稳定后于4 ℃冰箱放置过夜,在4000 r/min条件下进行离心酸洗5 min。将残渣于50 ℃烘箱烘干。烘干后取0.25 g和100 mL 5%的NaCl溶液混合,用0.1 mol/L的NaOH溶液进行滴定,空白样品中用蒸馏水代替HCl溶液。记录所消耗的NaOH溶液体积,阳离子交换能力计算公式如下:
阳离子交换能力(mmol/g)=(V2−V1)×0.1m10 (11) 式中:V2为样品消耗NaOH溶液体积,mL;V1为空白样品消耗NaOH溶液体积,mL;m10为称取的样品质量。
1.2.2.11 总酚、总黄酮含量测定
采用桑嘉玘等[16]的方法绘制标准曲线,并分别测定三种黄精渣IDF相应的吸光值后计算其总酚和总黄酮含量,计算公式如下。
总酚含量(mg/g)=C1V1×N×1000m11 (12) 式中:C1为总酚质量浓度,mg/L;V1为提取液体积,mL;m11为黄精渣IDF质量,g;N为稀释倍数。
总黄酮含量(mg/g)=C×V1×稀释倍数m12×V2×1000 (13) 式中:C为从标准曲线中计算得到的样品提取液中总黄酮含量,μg;V1为样品提取液总体积,mL;m12为PIDF质量,g;V2为反应中样品测定体积,mL。
1.2.2.12 DPPH自由基清除能力
参照魏依华[17]的方法并略微修改。将三种黄精渣IDF分别配成0.5、1.0、1.5、2.0、2.5 mg/mL的浓度备用。称取DPPH采用无水乙醇配制浓度为0.2 mmol/L的DPPH溶液。于10 mL棕色试剂管中加入不同浓度梯度的样品提取液2 mL,再加入2 mL 0.2mmol/L的DPPH溶液。混匀后避光反应30 min,在517 nm处测定吸光值。以70%乙醇溶液为对照。计算样品清除DPPH自由基的能力,计算公式如下。
DPPH自由基清除率(%)=[A0−(A1−A2)]A0×100 (14) 式中:A0为乙醇溶液2.0 mL与DPPH溶液2.0 mL的吸光值;A1为样品液2.0 mL与DPPH溶液2.0 mL的吸光值;A2为样品液2.0 mL与乙醇溶液2.0 mL的吸光值。
1.2.2.13 葡萄糖吸附能力测定
取0.1 g黄精渣IDF于50 mL离心管中,加入1 mg/mL葡萄糖溶液25 mL,于35 ℃水浴条件下吸附平衡1 h,于6000 r/min转速下离心20 min。取15 mL刻度试管,加入1.0 mL上清液、2.0 mL DNS试剂,沸水浴2 min,冷却后用去离子水补足到刻度,在540 nm波长下测定吸光度,计算公式如下。
葡萄糖吸附能力(µmoL/g)=(c初−c吸附)Vm样 (15) 式中:c初为加入黄精渣IDF前葡萄糖溶液浓度,mg/mL;c吸附为吸附平衡后葡萄糖溶液浓度,mg/mL;m样为倒入离心管的黄精渣IDF质量,mg;V为葡萄糖溶液体积,mL。
2. 结果与分析
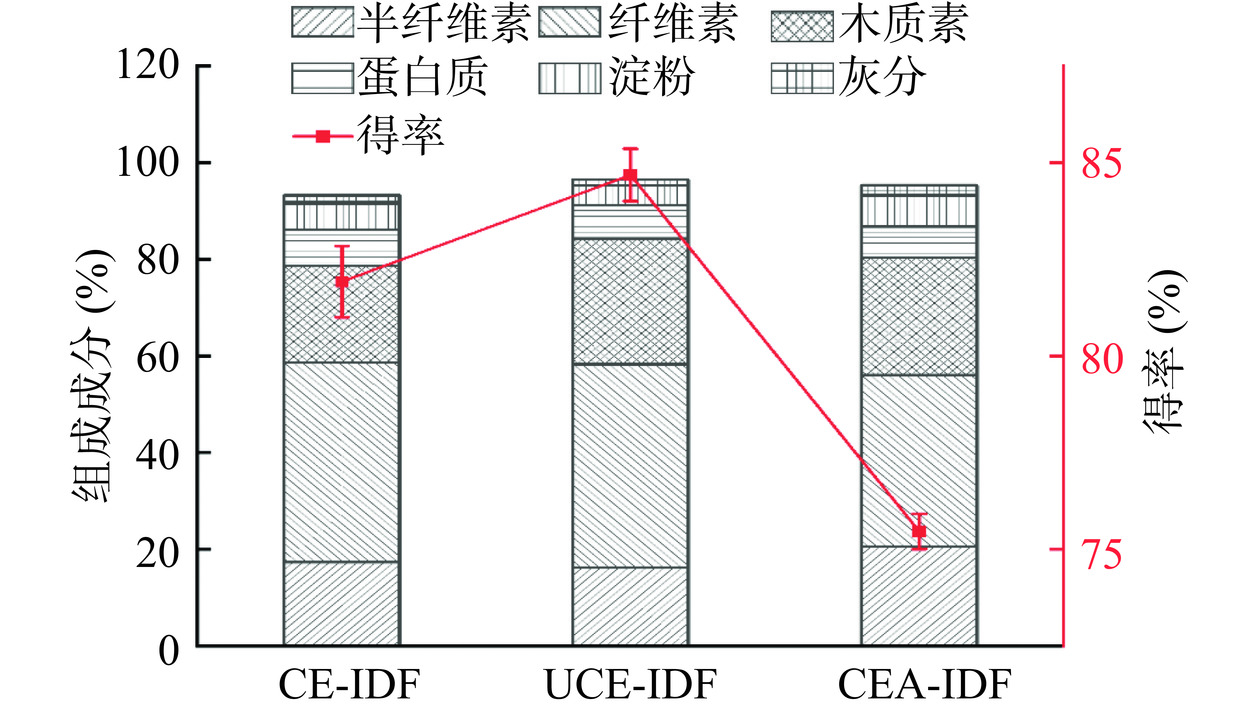
2.1 黄精渣IDF得率与组成成分分析结果
图1为三种提取方法黄精渣IDF的得率与组成成分分析的结果图。三种提取方法中超声辅助复酶法的黄精渣IDF得率最高。其中超声辅助复酶法为84.68%,复合酶法为81.92%,酶碱法为75.46%。并且可以看出,CEA-IDF半纤维素含量较高,这是因为碱法处理破坏程度较高,并且可以有效除去蛋白质和脂肪等杂质[18]。UCE-IDF的纤维素含量较高,且总不溶性膳食纤维含量高,这是由于超声作用使纤维素与蛋白质和淀粉等杂质之间的分离更彻底,所以纯度较高[19]。综合比较这三种IDF的组成成分,可以看出纤维素、半纤维素和木质素含量差异较小。但三种IDF的总不溶性膳食纤维含量均较高,表明黄精渣富含高质量的水不溶性膳食纤维,具有较好的开发应用前景,值得对其结构和功能特性进行深入探究。
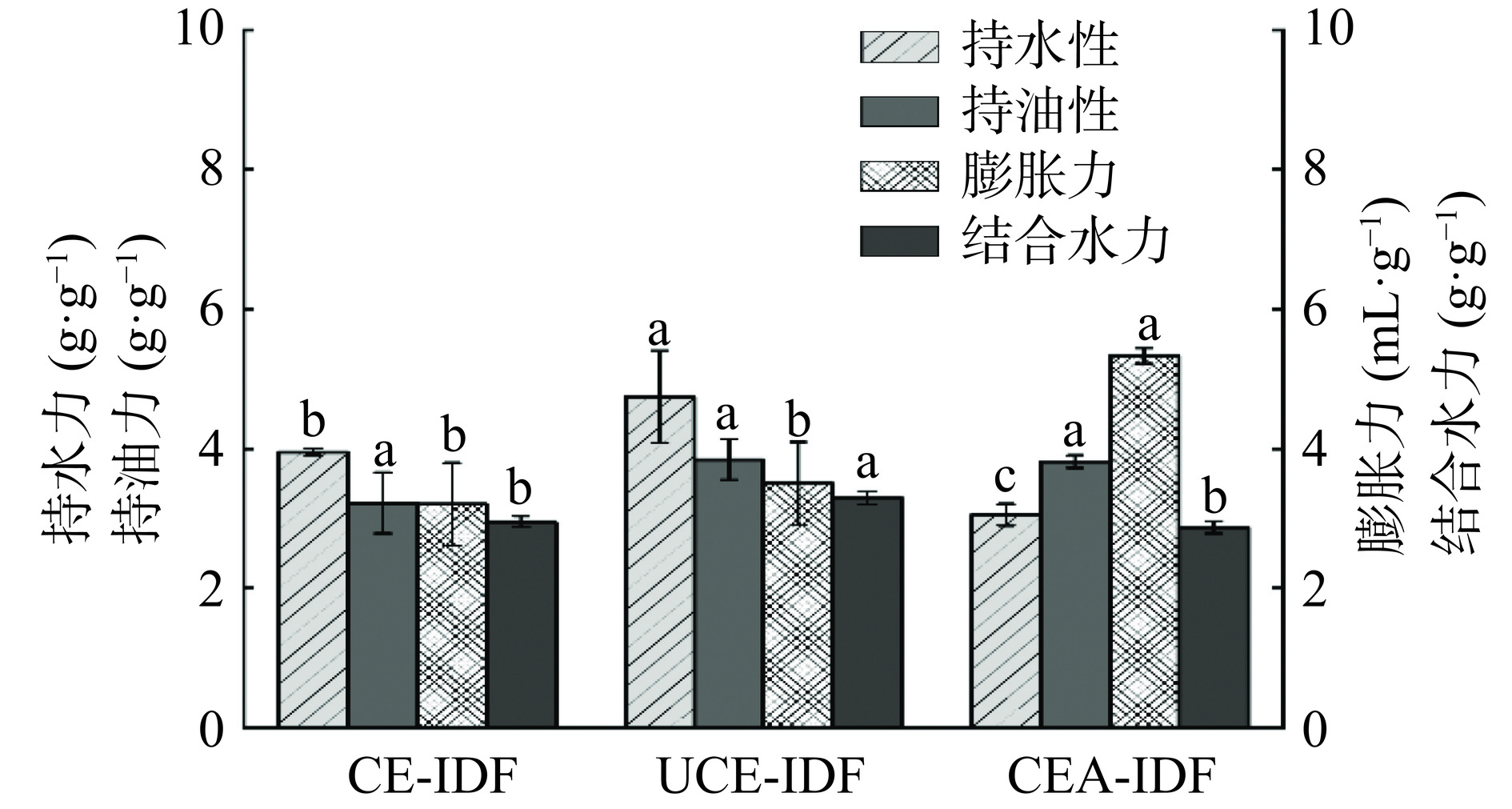
2.2 黄精渣IDF的持水力、持油力、膨胀力、结合水力结果
图2为三种提取方法对黄精渣IDF水合性质的影响结果。持水力反映的是膳食纤维在压缩或离心后保持水分的能力,可反映膳食纤维对肠道蠕动的影响。由图2可知,持水力最佳的为UCE-IDF,高达(4.74±0.66)g/g,CEA-IDF的持水力最差,这与超声破碎原料细胞壁,使纤维结构更松散有关[20]。三种黄精渣IDF持水力均高于苦荞麦壳IDF[21]的持水力。
膨胀力是指膳食纤维在溶剂中达到平衡后的体积变化,与自身结构密切相关。在膨胀力方面,CEA-IDF的膨胀力为(5.33±0.11)mL/g,高于CE-IDF(3.2±0.59)mL/g和UCE-IDF(3.5±0.59)mL/g(P<0.05),这是由于经过碱处理后,CEA-IDF颗粒变小,呈现较小片状结构,与水接触面积较大,膨胀力提高[16]。
持油力在调节脂肪代谢和降低血清胆固醇等方面发挥着重要作用,持油力较强的纤维素可以延缓脂肪吸收[22]。三种黄精渣IDF中,UCE-IDF持油力为(3.84±0.29)g/g,CEA-IDF的持油力为(3.81±0.09)g/g,均高于CE-IDF((3.22±0.44)g/g),这是由于超声处理和碱处理导致黄精渣IDF表面结构破坏较剧烈,空隙较多[5]。有研究发现高含量的木质素使得IDF具有较高的持油力,可以增强其对脂肪的吸附能力 [23]。由图1可知,UCE-IDF木质素含量最高,与此结果相符。
结合水力性能较好的膳食纤维在改善食品品质和预防肠道疾病等方面更有效[14]。在结合水力方面,UCE-IDF最佳,为(3.29±0.09)g/g,高于CE-IDF((2.96±0.08)g/g)和CEA-IDF((2.87±0.09)g/g)(P<0.05)。结合水力是膳食纤维与水的相互作用的反映,主要与组分间的结构有关,这也表明超声辅助作用可以更大程度破坏IDF结构的完整性,而不仅仅是颗粒尺寸减小[24]。
综合来看,UCE-IDF的持水力、持油力和结合水力都优于其他两种IDF,可归因于超声效应使UCE-IDF内部的分子氢键断裂,增加烃基和羧基的暴露面积,使致密的片状结构更松散,孔隙率更高,使其对水和油的结合能力较强[25];CEA-IDF的膨胀力最好,这是由于碱处理过程中使其结构和颗粒大小发生改变,导致结构聚合度下降且孔隙较多,膨胀力较好[26]。
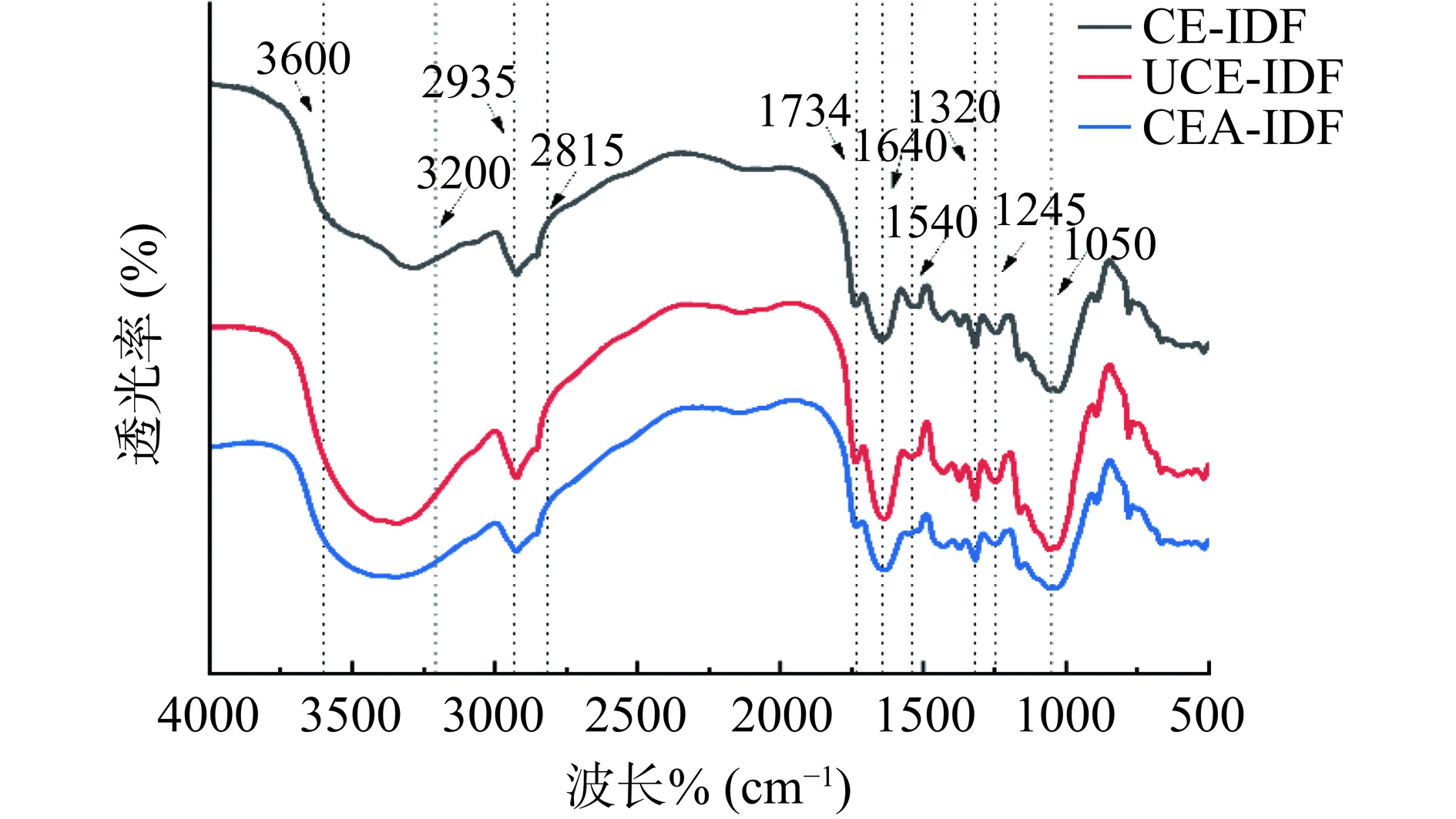
2.3 黄精渣IDF的傅里叶红外光谱分析结果
由图3可知,三种黄精渣IDF的红外光谱图具有典型的膳食纤维光谱特征。在三种黄精渣IDF的红外光谱图中,3600~3200 cm−1范围均存在O-H伸缩振动峰[27],2935 cm−1处尖锐的峰,可归属于糖类中C-H键的伸缩振动。2815 cm−1附近呈现由多糖亚甲基和甲基的-CH伸缩振动引起的较弱的吸收峰。1734 cm−1和1640 cm−1附近范围内有一个C=O振动引起的吸收峰,在1540 cm−1和1245 cm−1附近的吸收峰可归因于木质素中-CH3和-CH2的弯曲振动。在1050 cm−1处的吸收峰归属于糖类C-O-C的特征峰,这与莲叶渣-IDF[28]和人参渣-IDF[29]的研究结果基本一致,属于典型膳食纤维类化合物红外光谱特征。综上,不同提取方法得到的IDF具有相似的官能团类型。
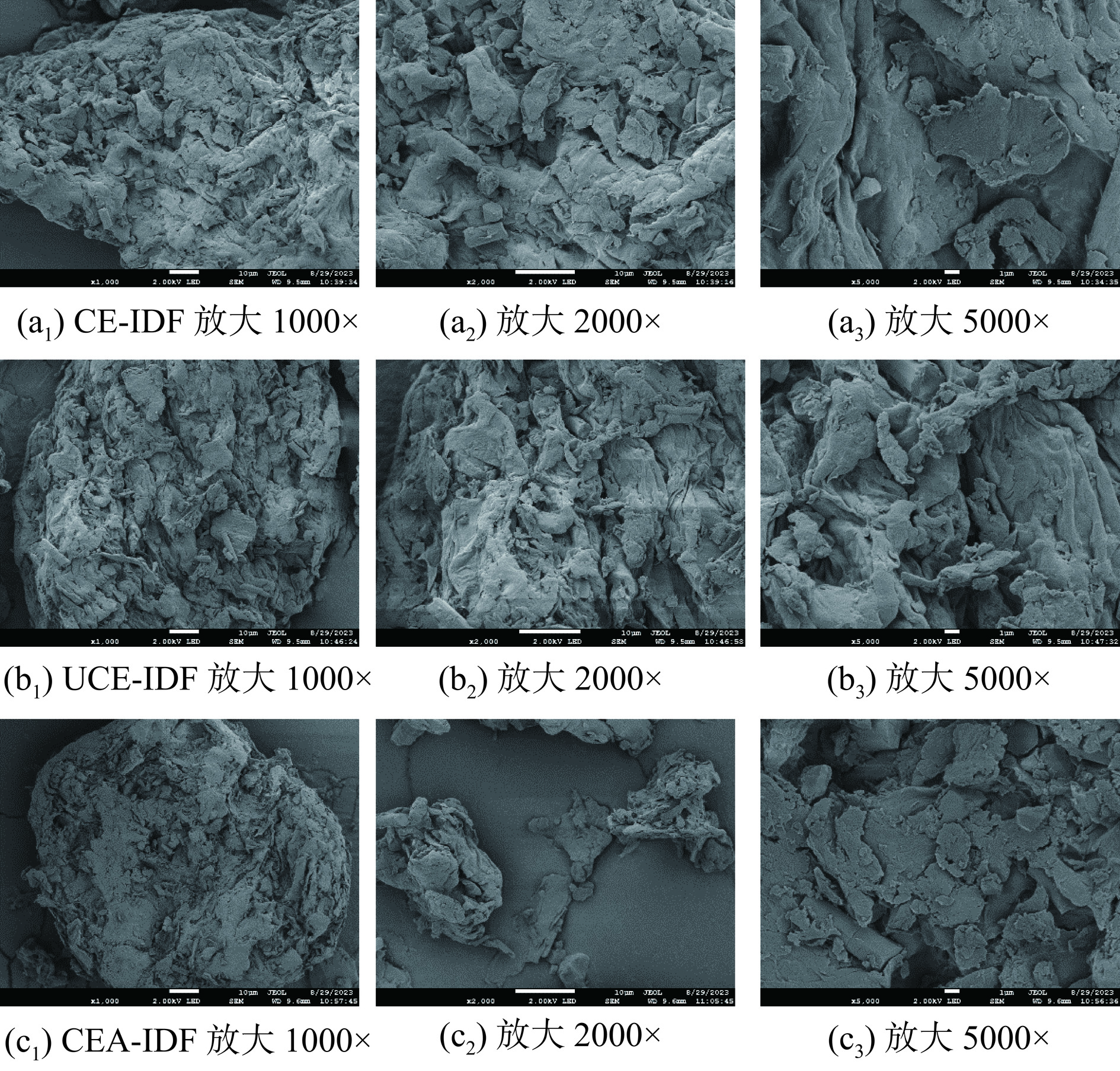
2.4 黄精渣IDF的扫描电镜分析结果
三种黄精渣IDF显微结构如图4所示。在图4a中,CE-IDF为块状且表面附着大小不一的颗粒,这可能为未分解完的淀粉和蛋白质[30]。放大后(图4a2和图4a3)观察到CE-IDF有少量褶皱,酶解使CE-IDF产生大量裂缝,使整体结构更加疏松。而图4b中,CE-IDF表面具有明显裂纹和孔洞,结构更松散。这可归因于超声辅助作用使UCE-IDF受到强烈的机械剪切和撕裂等影响,更大强度地破坏组分间的交联,使得UCE-IDF的降解效应增强[31]。此外,放大后观察(图4b2和图4b3)到UCE-IDF表面褶皱较多,更为粗糙,且具有空洞,比表面积增大,这也从侧面解释了UCE-IDF具有较高的持水力和持油力的结构基础[28]。图4c中可以看出经过碱法处理后,CEA-IDF呈现出较为规则的疏松结构并带有空腔,与CE-IDF对比可看出颗粒变小。这是由于复合酶法处理过程比较温和,使纤维结构保持的较完整[12],而碱处理可以破坏纤维基质,明显改变颗粒大小,导致松散结构[18]。综上,不同的提取方法对黄精渣IDF的微观结构会造成一定程度的影响,并可能影响其功能特性[32] 。
2.5 黄精渣IDF的热重和DSC分析结果
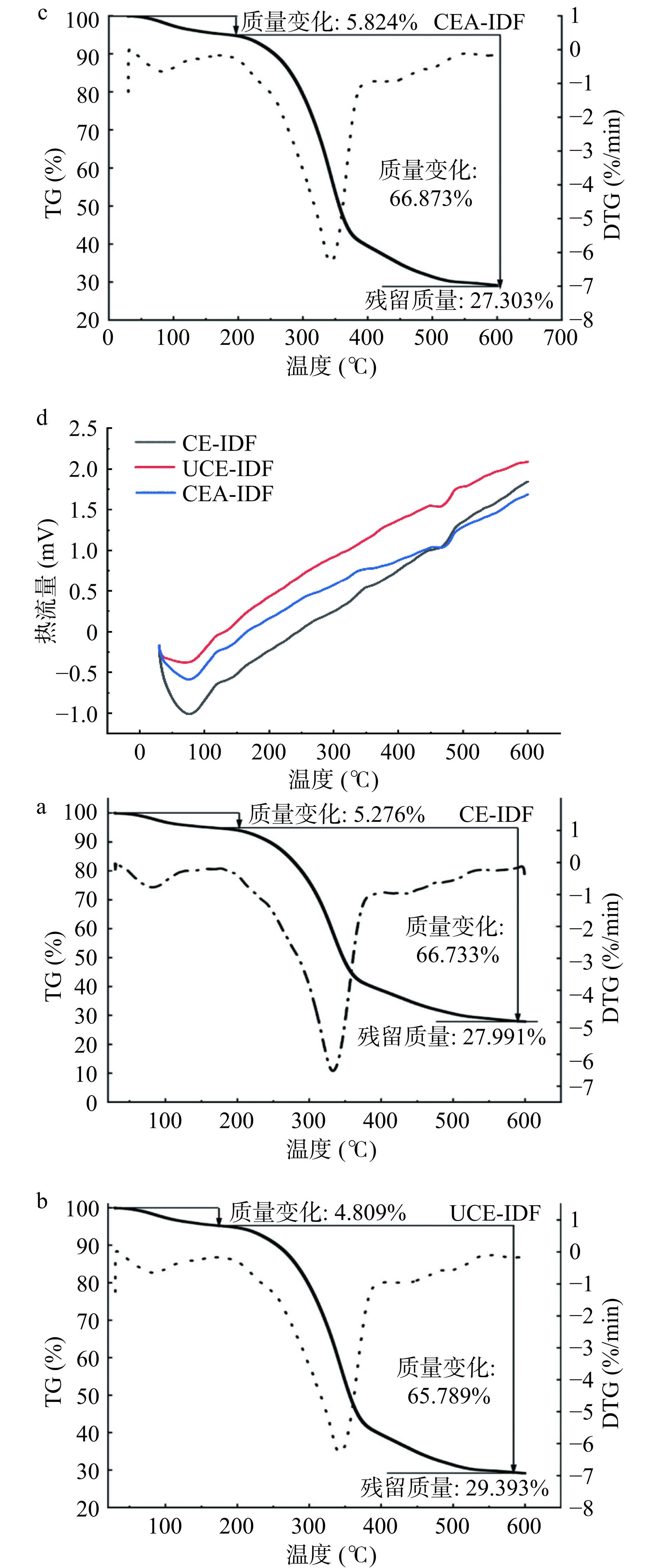
热重法(TG)是对样品热稳定性及热反应变化的表征[7]。从图5可以看出,三种黄精渣IDF均在50~600 ℃有两个质量损失阶段。CEA-IDF在总范围内质量损失最多,达到72.697%,降解速率最快;其次是CE-IDF,总范围内质量损失72.009%;UCE-IDF质量损失最少,为70.607%。这说明CEA-IDF含有相对较多的自由水和结合水,并且由于酶碱法处理破坏结构程度剧烈[18],降低了CEA-IDF对热解的阻力。第一段范围内,三种黄精渣IDF质量损失相差不大,为5.276%(CE-IDF)、4.809%(UCE-IDF)、5.824%(CEA-IDF);在第二段范围内,DTG曲线上在345℃附近出现了明显的尖峰,说明三种黄精渣IDF失重速率较快,这与自身半纤维素等成分热解有关[29],可以看出黄精渣IDF在200°C以下相对稳定。
差示扫描量热法(DSC)主要是对样品的热稳定性进行分析。三种黄精渣IDF均在118 ℃左右有一个吸热峰,这是由于水分演化,黄精渣IDF中自由水和结合水被蒸发形成的[28]。木质素的热解峰一般出现在400 ℃以上,因此400~500 ℃的峰为木质素的热解峰。CE-IDF吸热最强并在放热阶段中速率最快;CEA-IDF在后期放热阶段速率最慢;UCE-IDF在吸热阶段最低,并且在放热阶段比较稳定,这说明提取方法会影响黄精渣IDF的热稳定性[8]。
2.6 黄精渣IDF的X射线衍射(XRD)分析结果
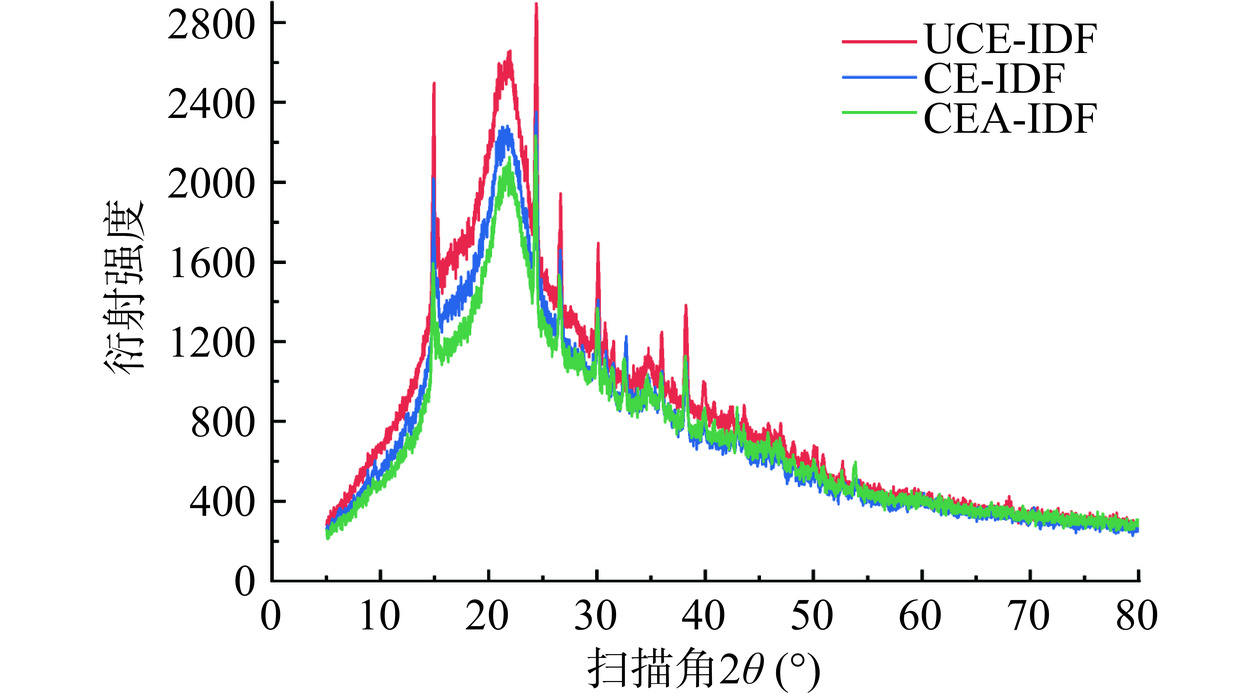
XRD衍射图谱是对样品结晶结构的反映。由图6可知,三种黄精渣IDF均在22°有明显的衍射峰,表现出纤维素I特征[33],这与人参渣-IDF[29]和凤榴-IDF[34]研究中测定得到的结果类似。但从出峰程度的强弱可以看出超声辅助提取法对黄精渣IDF结晶区造成破坏更大。CAE-IDF的衍射峰较弱,造成这种现象的原因是附着在纤维素微纤维表面的无序无定形半纤维素在碱处理过程中被优先水解[35]。
2.7 黄精渣IDF的阳离子交换能力结果
膳食纤维结构中含有大量羧基、羟基等侧链基团,具有弱酸性阳离子交换树脂的性能[7]。由图7可知,UCE-IDF的阳离子交换能力最佳,为(0.40±0.003)mmol/g,CEA-IDF((0.36±0.01)mmol/g)次之,CE-IDF((0.33±0.01)mmol/g)最低。该结果表明超声辅助有助于提高黄精渣IDF的阳离子交换能力[19]。超声空化可以较高强度的破坏IDF结构,使得IDF的羧基和羟基等基团更暴露,如糖醛酸等物质[36]。桑嘉玘等[16]研究结果表明,超声辅助复酶法提取的柚皮IDF的阳离子交换能力高于酶碱法和复合酶法,与本研究结果一致。
2.8 黄精渣IDF的总酚、总黄酮含量结果
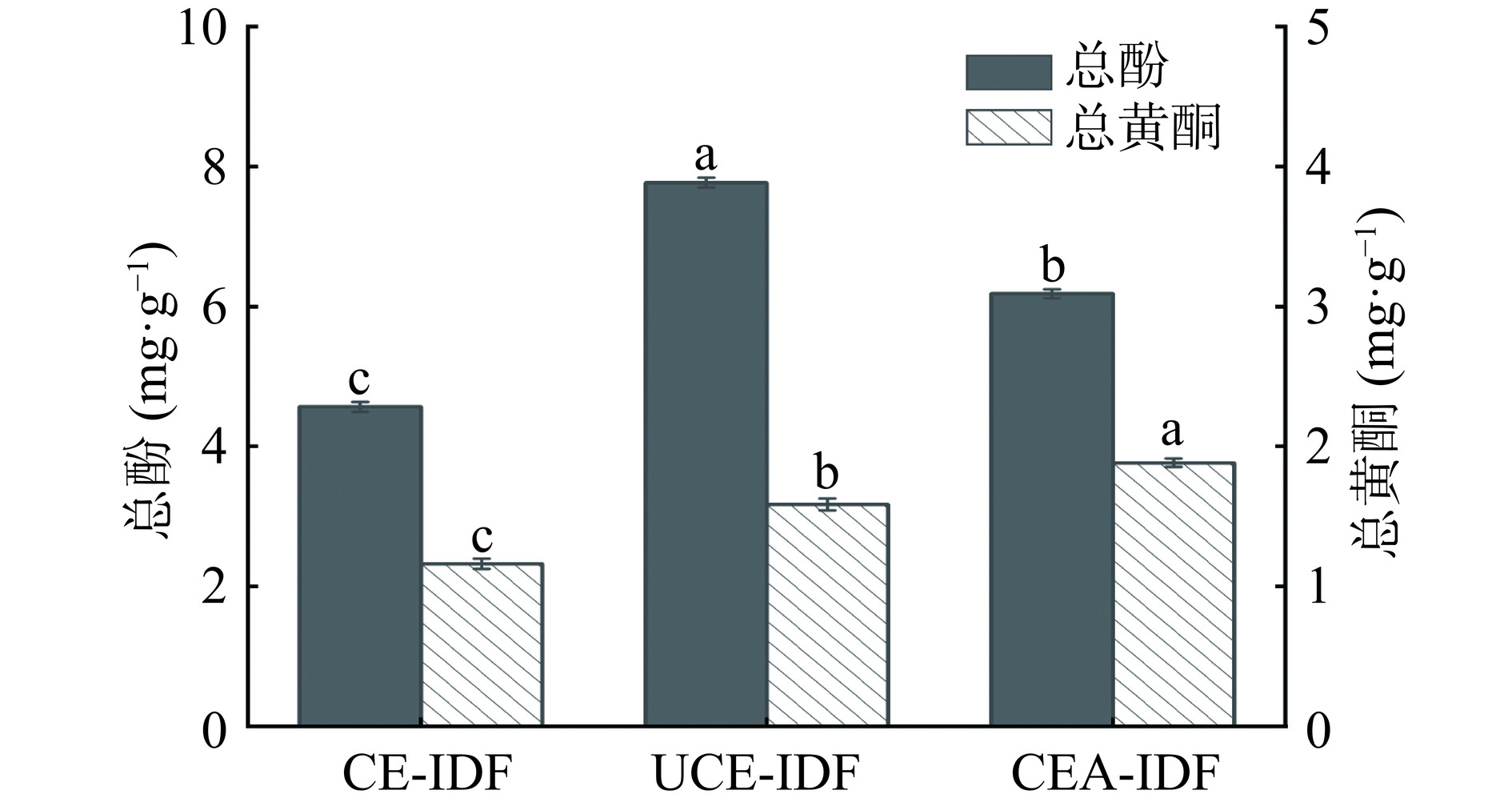
研究发现,膳食纤维的抗氧化功效与其结合的酚类和黄酮类物质密切相关[37]。如图8所示,CE-IDF、UCE-IDF和CEA-IDF的总酚含量分别为(4.57±0.07)、(7.77±0.07)和(6.18±0.07)mg/g。UCE-IDF的总酚含量最高。三种黄精渣IDF的总酚含量都远高于燕麦麸皮IDF(3.0 mg/g)[25],表明黄精渣IDF具有较高的抗氧化活性。CEA-IDF的总黄酮含量为(1.88±0.03)mg/g,高于CE-IDF((1.16±0.04)mg/g)和UCE-IDF((1.59±0.04)mg/g)。这可归因于在酶碱法提取过程,碱性环境使黄精渣IDF颗粒尺寸的破碎程度较高,促进黄酮类物质的溶出[38]。综上,IDF的总酚和总黄酮含量因提取方法的不同而有所差异。
2.9 黄精渣IDF的DPPH自由基清除能力结果
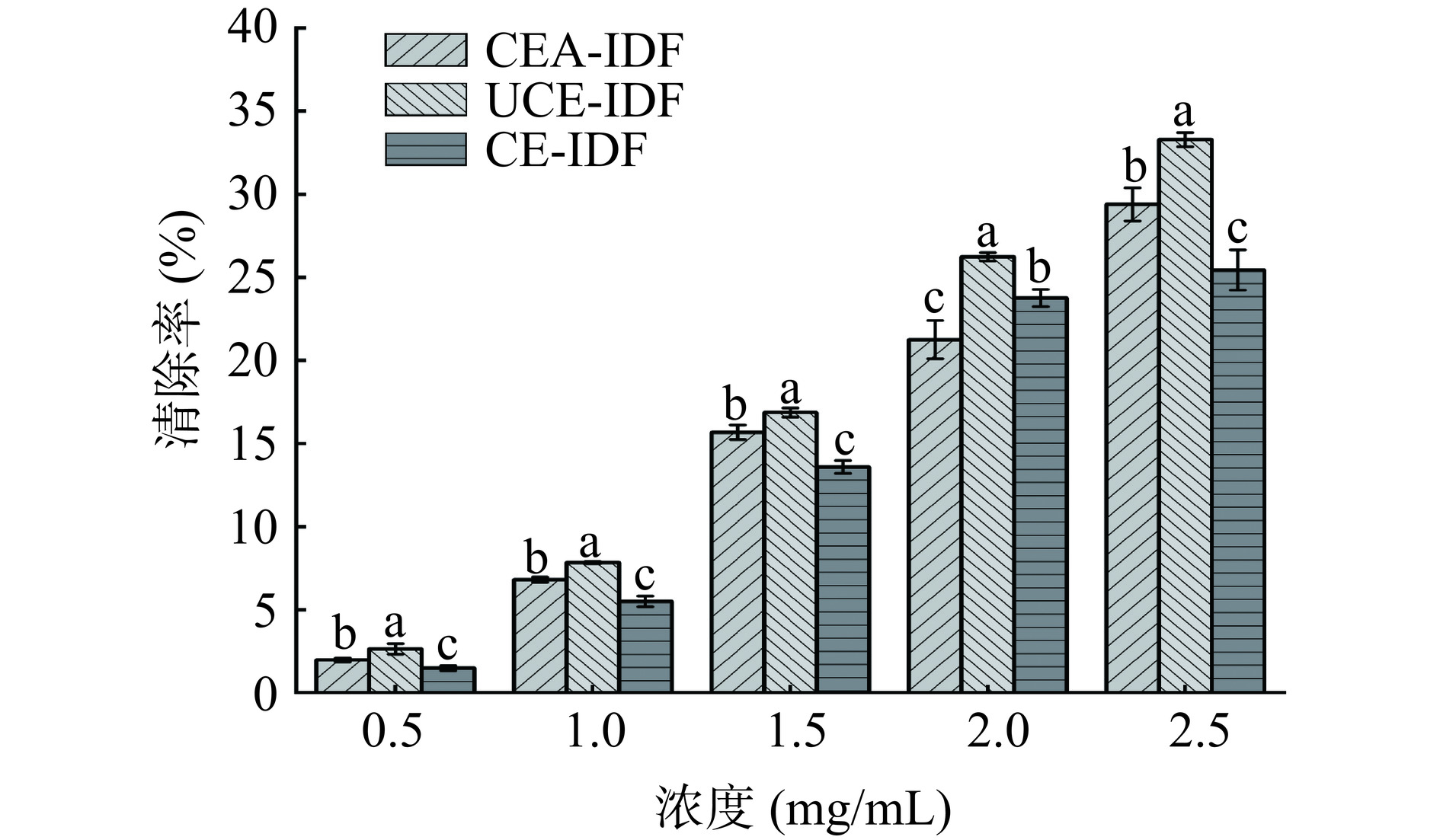
如图9所示,三种黄精渣IDF的DPPH自由基清除能力均随着浓度的增加而增强。其中在同一浓度下UCE-IDF的DPPH自由基清除能力较强,这是由于超声作用使UCE-IDF中氢键断裂,导致更多的氢离子与DPPH相结合。另外,有研究表面膳食纤维的抗氧化能力与总酚含量呈正相关[39],与本研究结果一致(图8),较高的总酚含量使得UCE-IDF具有较高的抗氧化性。其中三种黄精渣IDF不同浓度时的DPPH清除率均高于绿豆皮不溶性膳食纤维[17],表明黄精渣IDF对DPPH自由基清除效果较好,抗氧化能力较优。
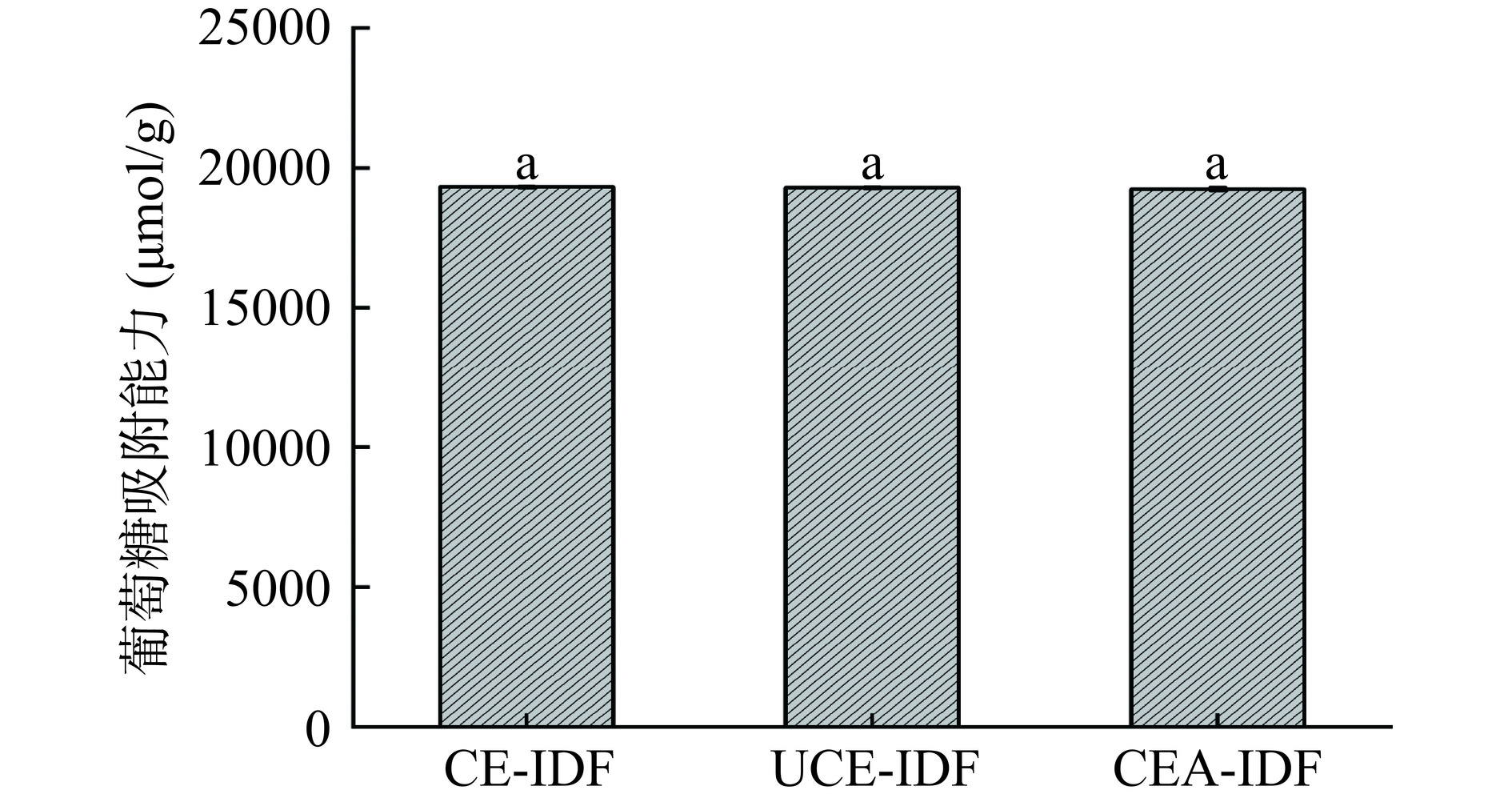
2.10 黄精渣IDF的葡萄糖吸附能力结果
由图10可知,三种黄精渣IDF的葡萄糖吸附能力无明显差异。CE-IDF、UCE-IDF和CEA-IDF的葡萄糖吸附能力分别为19326.67±41.63、19300.00±40.00和19253.33±60.10 μmol/g,这表明不同提取方法对黄精渣IDF的葡萄糖吸附作用影响较小。此外,三种黄精渣IDF的葡萄糖吸附能力均高于麦麸IDF(15700 μmol/g)[40]和橘皮IDF(129990 μmol/g)[41]。综上,黄精渣IDF与葡萄糖分子间的互相作用较强,有助于延缓葡萄糖分子的吸收,从而有利于控制血糖水平。因此,黄精渣IDF具有在糖尿病特殊食品中应用的潜力。
3. 结论
本研究采用复合酶法、超声辅助复酶法和酶碱法三种方法提取黄精渣水不溶性膳食纤维,并对三种黄精渣IDF组成、理化性质和功能特性等进行对比探究。结果表明,UCE-IDF的得率高达84.68%,均高于CE-IDF和CEA-IDF。三种提取方法对IDF的理化和功能特性影响程度不同,UCE-IDF和CEA-IDF的结构更加松散。红外光谱显示三种IDF具有纤维素类光谱特征,X射线衍射显示三种IDF均为纤维素I型衍射峰。UCE-IDF的持水力(4.74±0.66)g/g、持油力(3.84±0.29)g/g、结合水力(3.29±0.09)g/g和阳离子交换能力(0.40±0.003)mmol/g最佳,UCE-IDF的热稳定性和抗氧化活性最佳。CEA-IDF的总黄酮含量(1.88±0.03)mg/g较高且膨胀力(5.33±0.11)mL/g更佳,说明UCE-IDF和CEA-IDF在功能食品工业中具有更大的应用潜力。本研究结果可促进对黄精副产物的综合利用。
-
-
[1] LI X L, MA R H, ZHANG F, et al. Evolutionary research trend of Polygonatum species:A comprehensive account of their transformation from traditional medicines to functional foods[J]. Critical Reviews in Food Science and Nutrition,2023,63(19):3803−3820. doi: 10.1080/10408398.2021.1993783
[2] WAN P, LIU H, ZHU Y Y, et al. Effects of Polygonatum sibiricum on physicochemical properties, biological compounds, and functionality of fermented soymilk[J]. Foods,2023,12(14):2715. doi: 10.3390/foods12142715
[3] 丁政宇, 张士凯, 何子杨, 等. 响应面优化黄精渣不溶性膳食纤维酶法提取工艺及其结构表征[J]. 食品工业科技,2021,42(20):157−163. [DING Z Y, ZHANG S K, HE Z Y, et al. Optimization of enzymatic extraction process and structure characterization of insoluble dietary fiber from Polychloramphenicol residue by response surface optimization[J]. Science and Technology of Food Industry,2021,42(20):157−163.] DING Z Y, ZHANG S K, HE Z Y, et al. Optimization of enzymatic extraction process and structure characterization of insoluble dietary fiber from Polychloramphenicol residue by response surface optimization[J]. Science and Technology of Food Industry, 2021, 42(20): 157−163.
[4] YE S, SHAH B R, LI J, et al. A critical review on interplay between dietary fibers and gut microbiota[J]. Trends in Food Science & Technology,2022,124:237−249.
[5] 林伟达, 李娜, 祝子坪, 等. 不同提取方法对青钱柳叶不溶性膳食纤维理化性质和抗氧化活性的影响[J]. 食品研究与开发,2022,43(6):93−98. [LIN W D, LI N, ZHU Z P, et al. Different extraction methods on physical and chemical properties of cyclocarya paliurus leaf insoluble dietary fiber and the effects of antioxidant activity[J]. Food Research and Development,2022,43(6):93−98.] LIN W D, LI N, ZHU Z P, et al. Different extraction methods on physical and chemical properties of cyclocarya paliurus leaf insoluble dietary fiber and the effects of antioxidant activity[J]. Food Research and Development, 2022, 43(6): 93−98.
[6] 李建周, 陈晓华, 罗思诗. 豆渣中水不溶性膳食纤维的提取及性质研究[J]. 食品研究与开发,2017,38(7):29−33. [LI J Z, CHEN X H, LUO S S. Extraction and characterization of Water-insoluble dietary fiber from soybean residue[J]. Food Research and Development,2017,38(7):29−33.] LI J Z, CHEN X H, LUO S S. Extraction and characterization of Water-insoluble dietary fiber from soybean residue[J]. Food Research and Development, 2017, 38(7): 29−33.
[7] MA Q Y, MA Z Y, WANG W X, et al. The effects of enzymatic modification on the functional ingredient-Dietary fiber extracted from potato residue[J]. LWT-Food Science and Technology,2022,153:112511. doi: 10.1016/j.lwt.2021.112511
[8] FAN X J, CHANG H D, LIN Y A, et al. Effects of ultrasound-assisted enzyme hydrolysis on the microstructure and physicochemical properties of okara fibers[J]. Ultrasonics Sonochemistry,2020,69:105247. doi: 10.1016/j.ultsonch.2020.105247
[9] 张晓娟, 杨爱燕. 芒果皮中不溶性膳食纤维的提取及其理化性质研究[J]. 现代食品,2020(22):123−126,129. [ZHANG X J, YANG A Y. The extraction of insoluble dietary fiber in mango skin and its physical and chemical properties[J]. Modern Food,2020(22):123−126,129.] ZHANG X J, YANG A Y. The extraction of insoluble dietary fiber in mango skin and its physical and chemical properties[J]. Modern Food, 2020(22): 123−126,129.
[10] MA R, CHEN J N, ZHOU X J, et al. Effect of chemical and enzymatic modifications on the structural and physicochemical properties of dietary fiber from purple turnip (Brassica rapa L.)[J]. LWT-Food Science and Technology,2021,145:111313. doi: 10.1016/j.lwt.2021.111313
[11] FIDRIYANTO R, SINGH B P, MANJU K M, et al. Multivariate analysis of structural and functional properties of fibres from apple pomace using different extraction methods[J]. Food Production, Processing and Nutrition,2023,5(1):6. doi: 10.1186/s43014-022-00119-8
[12] HE Y, WANG B X, WEN L K, et al. Effects of dietary fiber on human health[J]. Food Science and Human Wellness,2022,11(1):1−10. doi: 10.1016/j.fshw.2021.07.001
[13] 陈贤情, 商晋, 宋慧芳, 等. 秸秆中纤维素/半纤维素和木质素的几种测定方法对比[C]. 中国农业工程学会. 中国农业工程学会2011年学术年会论文集. [CHEN X Q, SHANG J, SONG H F, et al. Comparison of several methods for determination of cellulose/hemicellulose and lignin in straw[C]. Chinese Society of Agricultural Engineering. Proceedings of 2011 Academic annual meeting of Chinese Society of Agricultural Engineering.] CHEN X Q, SHANG J, SONG H F, et al. Comparison of several methods for determination of cellulose/hemicellulose and lignin in straw[C]. Chinese Society of Agricultural Engineering. Proceedings of 2011 Academic annual meeting of Chinese Society of Agricultural Engineering.
[14] 胡金祥, 许程剑, 闫妮娜, 等. 松茸渣不溶性膳食纤维提取及特性研究[J]. 食品安全质量检测学报,2022,13(2):359−365. [HU J X, XU C J, YAN N N, et al. Extraction and characterization of insoluble dietary fiber from Matsutake Mushroom residue[J]. Journal of Food Safety & Quality,2022,13(2):359−365.] HU J X, XU C J, YAN N N, et al. Extraction and characterization of insoluble dietary fiber from Matsutake Mushroom residue[J]. Journal of Food Safety & Quality, 2022, 13(2): 359−365.
[15] HE Y Y, LI W, ZHANG X Y, et al. Physicochemical, functional, and microstructural properties of modified insoluble dietary fiber extracted from rose pomace[J]. Journal of Food Science and Technology,2020,57:1421−1429. doi: 10.1007/s13197-019-04177-8
[16] 桑嘉玘, 辜青青, 徐玉娟, 等. 提取方法对柚皮海绵层不溶性膳食纤维理化性质、功能及结构的影响[J]. 食品与发酵工业,2022,48(3):149−154. [SANG J Q, GU Q Q, XU Y J, et al. Extraction method of pomelo peel the sponge layer of insoluble dietary fiber physical and chemical properties, function and the influence of structure[J]. Food and Fermentation Industries,2022,48(3):149−154.] SANG J Q, GU Q Q, XU Y J, et al. Extraction method of pomelo peel the sponge layer of insoluble dietary fiber physical and chemical properties, function and the influence of structure[J]. Food and Fermentation Industries, 2022, 48(3): 149−154.
[17] 魏依华. 绿豆皮不溶性膳食纤维的改性及降血脂的研究[D]. 长春:吉林农业大学, 2022. [WEI Y H. Modification of insoluble dietary fiber from mung bean skin and hypolipidemic study[D]. Changchun:Jilin Agricultural University, 2022.] WEI Y H. Modification of insoluble dietary fiber from mung bean skin and hypolipidemic study[D]. Changchun: Jilin Agricultural University, 2022.
[18] 孟圆, 夏婷, 程艳, 等. 碱法提取普洱茶渣膳食纤维的工艺优化[J]. 食品研究与开发,2023,44(18):158−164. [MENG Y, XIA T, CHENG Y, et al. Optimization of extracting dietary fiber from Pu-erh tea residue by alkali method[J]. Food Research and Development,2023,44(18):158−164.] MENG Y, XIA T, CHENG Y, et al. Optimization of extracting dietary fiber from Pu-erh tea residue by alkali method[J]. Food Research and Development, 2023, 44(18): 158−164.
[19] 王司琪, 王佳佳, 李泊铮, 等. 提取方法对玉木耳膳食纤维结构特征和功能特性的影响[J]. 食品科学,2022,43(24):93−101. [WANG S Q, WANG J J, LI B Z, et al. Effects of extraction methods on the structural and functional characteristics of dietary fiber from Auricularia japonicum[J]. Food Science,2022,43(24):93−101.] WANG S Q, WANG J J, LI B Z, et al. Effects of extraction methods on the structural and functional characteristics of dietary fiber from Auricularia japonicum[J]. Food Science, 2022, 43(24): 93−101.
[20] 刘倩倩. 响应面优化绿豆皮不溶性膳食纤维超声辅助提取工艺[J]. 食品工业科技,2019,40(14):203−207. [LIU Q Q. Ultrasound-assisted extraction of insoluble dietary fiber from mungbean skin was optimized by response surface[J]. Science and Technology of Food Industry,2019,40(14):203−207.] LIU Q Q. Ultrasound-assisted extraction of insoluble dietary fiber from mungbean skin was optimized by response surface[J]. Science and Technology of Food Industry, 2019, 40(14): 203−207.
[21] KAUR B, PANESAR P S, THAKUR A. Extraction and evaluation of structural and physicochemical properties of dietary fiber concentrate from mango peels by using green approach[J]. Biomass Conversion and Biorefinery, 2021.
[22] 潘宇, 丁一鸣, 李向荣, 等. 苦荞麦壳不溶性膳食纤维的理化性能及其结合酚提取工艺优化[J]. 保鲜与加工,2023,23(7):27−34. [PAN Y, DING Y M, LI X R, et al. Physicochemical properties of insoluble dietary fiber from Tartary buckwheat shell and optimization of extraction process of combined phenol[J]. Storage and Process,2023,23(7):27−34.] PAN Y, DING Y M, LI X R, et al. Physicochemical properties of insoluble dietary fiber from Tartary buckwheat shell and optimization of extraction process of combined phenol[J]. Storage and Process, 2023, 23(7): 27−34.
[23] 郭芸. 燕麦麸不溶性膳食纤维的酶法提取工艺及应用的研究[D]. 天津:天津大学, 2020. [GUO Y. Study on enzymatic extraction of insoluble dietary fiber from oat bran and its application[D]. Tianjin:Tianjin University, 2020.] GUO Y. Study on enzymatic extraction of insoluble dietary fiber from oat bran and its application[D]. Tianjin: Tianjin University, 2020.
[24] 牛希, 史乾坤, 赵城彬, 等. 超声改性对燕麦膳食纤维理化性质及结构的影响[J]. 食品科学,2020,41(23):130−136. [NIU X, SHI Q K, ZHAO C B, et al. Effects of ultrasonic modification on physicochemical properties and structure of oat dietary fiber[J]. Food Science,2020,41(23):130−136.] NIU X, SHI Q K, ZHAO C B, et al. Effects of ultrasonic modification on physicochemical properties and structure of oat dietary fiber[J]. Food Science, 2020, 41(23): 130−136.
[25] ZHANG W M, ZENG G L, PAN Y G, et al. Properties of soluble dietary fiber-polysaccharide from papaya peel obtained through alkaline or ultrasound-assisted alkaline extraction[J]. Carbohydrate Polymers,2017,172:102−112. doi: 10.1016/j.carbpol.2017.05.030
[26] ZHANG Y, LIAO J S, QI J R. Functional and structural properties of dietary fiber from citrus peel affected by the alkali combined with high-speed homogenization treatment[J]. LWT-Food Science and Technology,2020,128:109397. doi: 10.1016/j.lwt.2020.109397
[27] WANG S A, SUN W L, SWALLAH M S, et al. Preparation and characterization of soybean insoluble dietary fiber and its prebiotic effect on dyslipidemia and hepatic steatosis in high fat-fed C57BL/6J mice[J]. Food & Function,2021,12(18):8760−8773.
[28] ZHENG H, SUN Y, ZHENG T, et al. Effects of shear emulsifying/ball milling/autoclave modification on structure, physicochemical properties, phenolic compounds, and antioxidant capacity of lotus (Nelumbo) leaves dietary fiber[J]. Frontiers in Nutrition,2023,10:1064662. doi: 10.3389/fnut.2023.1064662
[29] HUA M, LU J X, QU D, et al. Structure, physicochemical properties and adsorption function of insoluble dietary fiber from ginseng residue:A potential functional ingredient[J]. Food Chemistry,2019,286:522−529. doi: 10.1016/j.foodchem.2019.01.114
[30] WANG K L, LI M, WANG Y X, et al. Effects of extraction methods on the structural characteristics and functional properties of dietary fiber extracted from kiwifruit (Actinidia deliciosa)[J]. Food Hydrocolloids,2021,110:106162. doi: 10.1016/j.foodhyd.2020.106162
[31] KAUR R, PANESAR P S, RIAR C S. Green extraction of dietary fiber concentrate from pearl millet bran and evaluation of its microstructural and functional properties[J]. Biomass Conversion and Biorefinery, 2023:1−10.
[32] WANG S Q, FANG Y Q, XU Y B, et al. The effects of different extraction methods on physicochemical, functional and physiological properties of soluble and insoluble dietary fiber from rubus chingii hu. fruits[J]. Journal of Functional Foods,2022,93:105081. doi: 10.1016/j.jff.2022.105081
[33] ZHU Y L, JI X L, YUEN M, et al. Effects of ball milling combined with cellulase treatment on physicochemical properties and in-vitro hypoglycemic ability of sea buckthorn seed meal insoluble dietary fiber[J]. Frontiers in Nutrition,2022,8:820672. doi: 10.3389/fnut.2021.820672
[34] WANG D, WANG Q M, SUN Y F, et al. Effect of insoluble dietary fiber extracted from Feijoa (Acca sellowiana (O. Berg) Burret. ) supplementation on physicochemical and functional properties of wheat bread[J]. Foods,2023,12(10):2019. doi: 10.3390/foods12102019
[35] SANG J Q, LI L, WEN J, et al. Chemical composition, structural and functional properties of insoluble dietary fiber obtained from the Shatian pomelo peel sponge layer using different modification methods[J]. LWT-Food Science and Technology,2022,165:113737. doi: 10.1016/j.lwt.2022.113737
[36] LIN D R, ZHANG Q T, XIAO L J, et al. Effects of ultrasound on functional properties, structure and glycation properties of proteins:A review[J]. Critical Reviews in Food Science and Nutrition,2021,61(15):2471−2481. doi: 10.1080/10408398.2020.1778632
[37] MOCZKOWSKA M, KARP S, NIU Y, et al. Enzymatic, enzymatic-ultrasonic and alkaline extraction of soluble dietary fibre from flaxseed–A physicochemical approach[J]. Food Hydrocolloids,2019,90:105−112. doi: 10.1016/j.foodhyd.2018.12.018
[38] LI Y, YU Y S, WU J J, et al. Comparison the structural, physicochemical, and prebiotic properties of litchi pomace dietary fibers before and after modification[J]. Foods,2022,11(3):248. doi: 10.3390/foods11030248
[39] 燕文胜. 改性方法对连翘渣不溶性膳食纤维理化、结构及功能性质的影响[D]. 太原:山西师范大学, 2022. [YAN W S. Effect of modification method on physicochemical, structural and functional properties of insoluble dietary fiber of Forsythia slag[D]. Taiyuan:Shanxi Normal University, 2022.] YAN W S. Effect of modification method on physicochemical, structural and functional properties of insoluble dietary fiber of Forsythia slag[D]. Taiyuan: Shanxi Normal University, 2022.
[40] 施建斌, 隋勇, 蔡沙, 等. 麦麸及麦麸膳食纤维常规粉碎和超微粉碎物化特性比较[J]. 现代食品科技,2021,37(1):150−156,149. [SHI J B, SUI Y, CAI S, et al. Comparison of the physicochemical properties of conventional grinding and ultrafine grinding of wheat bran and wheat bran dietary fiber[J]. Modern Food Science and Technology,2021,37(1):150−156,149.] SHI J B, SUI Y, CAI S, et al. Comparison of the physicochemical properties of conventional grinding and ultrafine grinding of wheat bran and wheat bran dietary fiber[J]. Modern Food Science and Technology, 2021, 37(1): 150−156,149.
[41] LIU Y L, WANG L F, LIU F X, et al. Effect of grinding methods on structural, physicochemical, and functional properties of insoluble dietary fiber from orange peel[J]. International Journal of Polymer Science, 2016, 2016.





 下载:
下载:

 下载:
下载:



