Effects of Different Polyphenols on the Co-pigmentation of Blueberry Anthocyanin Monomers
-
摘要: 为探究不同多酚对蓝莓花色苷单体的辅色效果,本试验通过分析不同多酚对四种蓝莓花色苷溶液的颜色、吸收峰值、花色苷含量等的影响,明确适合四种花色苷单体的多酚辅色素。结果表明,绿原酸、表儿茶素可将锦葵色素-3-O-半乳糖苷(Mal-3-O-gal)、锦葵色素-3-O-阿拉伯糖苷(Mal-3-O-ara)、飞燕草色素-3-O-半乳糖苷(Del-3-O-gal)、飞燕草色素-3-O-阿拉伯糖苷(Del-3-O-ara)的吸收峰值提高了21%~37%(P<0.05),经辅色的花色苷单体a*值显著增强、L*值显著下降(P<0.05),颜色明显变红;没食子酸、香草醛对Mal-3-O-gal、Mal-3-O-ara、Del-3-O-gal的最大吸收波长、颜色、花色苷含量无显著影响(P>0.05),但可将Del-3-O-ara的吸收峰值提高了23.33%(P<0.05);阿魏酸、咖啡酸对花色苷溶液产生负面影响,颜色由红转变为偏紫色、褪色明显。因此,绿原酸、表儿茶素、没食子酸、香草醛这4种外源物质可作为蓝莓花色苷有效的辅色剂,用于提高蓝莓汁的颜色稳定性。Abstract: In order to explore the influence of different polyphenols on the co-pigmentation effects of blueberry anthocyanins, this study analyzed the effects of different polyphenols on color, absorption peak and anthocyanin content of four blueberry anthocyanin solutions, and identified the suitable polyphenol copigment for four anthocyanin monomers. The results showed that chlorogenic acid and epicatechin significantly increased the absorption peaks of malvidin-3-O-galactoside (Mal-3-O-gal), malvidin-3-O-arabinoside (Mal-3-O-ara), delphinidin-3-O-galactoside (Del-3-O-gal) and delphinidin-3-O-arabinoside (Del-3-O-ara) by 21%~37% (P<0.05). The a* values of the four anthocyanin monomers were significantly enhanced (P<0.05), while L* values were significantly decreased by chlorogenic acid and epicatechin, resulting in a significant reddening of color. Gallic acid and vanillin had no significant effect on the maximum absorption wavelengths, color, and anthocyanin content of Mal-3-O-gal, Mal-3-O-ara, and Del-3-O-gal (P>0.05), but they significantly increased the absorption peak of Del-3-O-ara by 23.33% (P<0.05). Ferulic acid and caffeic acid had an adverse effect on the anthocyanin solution, causing a color shift from red to purplish and significant fading. Therefore, chlorogenic acid, epicatechin, gallic acid and vanillin can be used as effective co-colorants for blueberry anthocyanins, which could be used in the blueberry processing industry to improve the color stability of blueberry juice.
-
Keywords:
- blueberry anthocyanin monomer /
- polyphenol compounds /
- coloration /
- stability
-
蓝莓富含花青素,其中飞燕草素和锦葵素是蓝莓中最主要的两大类花青素[1]。锦葵色素是蓝莓中的特征性花青素,它赋予了蓝莓其特有的深蓝色调[2−3]。飞燕草素在蓝莓中含量较大,但其稳定性相对较差[4]。花色苷由花青素与一个或多个糖类物质以糖苷键相连组成[5],具有抗2型糖尿病、抗心血管疾病、抗炎和抗癌等作用[6],被赋予“护眼之宝”之称[7−8]。花色苷作为蓝莓的主要呈色物质[9],使之呈现红、蓝、紫等不同的颜色[10]。在加工和贮藏过程中,花色苷的稳定性受多种因素的影响,包括花色苷的结构、pH、温度、水分含量、氧气和金属离子等[11],这些因素导致蓝莓花色苷容易降解和褪色,极大地影响了蓝莓产品如蓝莓汁的加工和贮藏性能。
提高花色苷稳定性较好的方法是辅色反应,该反应能有效提高花色苷的结构稳定性和呈色效果。酚类化合物作为辅色剂使用量较小,而且天然存在于植物体系中,其结构具有足够扩展的π共轭体系,这促进了酚类化合物通过π-π堆积相互作用与花色苷结合[12−13]。黄金萍等[14]研究表明酚类化合物的添加可以作为辅色因子提高果蔬汁中花色苷的稳定性,从而限制花色苷和VC的相互作用。Liu等[15]采用酶催化法将对香豆酸和咖啡酸接枝于蓝莓花色苷上,发现在25、40和60 ℃下贮藏期间的颜色稳定性高于天然蓝莓花色苷。Bi等[16]使用没食子酸对蓝莓复合果汁进行辅色,研究发现没食子酸提高了果汁中总花色苷和抗坏血酸的稳定性。Zhang等[17]在加速条件下研究没食子酸对蓝莓汁中花色苷稳定性和色泽的影响,结果表明没食子酸的加入使蓝莓汁呈现出更深的红色色调、颜色饱和度和花色苷稳定性。不同文献中优化出的蓝莓汁护色剂具有较大差异,推测可能是由于不同品种的蓝莓中花色苷种类和含量不同导致的。因此本文从花色苷单体出发,通过对不同花色苷单体的适宜辅色剂进行研究,采用简单体系来明确多酚与花色苷之间分子的相互作用,为在后续复杂体系中的应用奠定基础。
在已有的研究中,绿原酸(Chlorogenic acid,CA)、表儿茶素(Epicatechin,Epi)、没食子酸(Gallic acid,GA)、香草醛(Vanillin,Van)、阿魏酸(Ferulic acid,FA)、咖啡酸(Caffeic acid,CaA)已成功应用于提高花色苷的稳定性。苏帆[18]分别选用咖啡酸、阿魏酸、绿原酸和没食子酸对红肉苹果花色苷进行辅色,研究发现4种酚酸(浓度为0.01 mol/L)均能使红肉苹果花色苷产生明显的辅色效应。Zou等[19]研究发现天竺葵素-3-葡萄糖苷与酚酸(儿茶素或表儿茶素)的共色反应速率,在0.1 MPa,60 ℃下处理时,随着酚酸浓度的增加而略有增加。在类似的研究中,没食子酸、香草醛、咖啡酸均被观察到其浓度增加导致花色苷辅色效果增强[20−21]。
本文以蓝莓花色苷为原料,选取已有报道可能在花色苷辅色中有较好效果的多酚化合物,绿原酸、表儿茶素、没食子酸、香草醛、阿魏酸、咖啡酸等6种外源添加物质作为多酚辅色剂,研究其对4种蓝莓花色苷单体(锦葵色素-3-O-半乳糖苷、锦葵色素-3-O-阿拉伯糖苷、飞燕草色素-3-O-半乳糖苷、飞燕草色素-3-O-阿拉伯糖苷)的颜色、含量和吸收光谱的变化规律,评估不同多酚辅色剂对特定蓝莓花色苷单体颜色稳定性的影响,以期找到较适宜的多酚辅色剂,为实际应用中蓝莓花色苷稳定性提升提供参考依据。
1. 材料与方法
1.1 材料与仪器
锦葵色素-3-O-半乳糖苷(Mal-3-O-gal,98%)、锦葵色素-3-O-阿拉伯糖苷(Mal-3-O-ara,98%)、飞燕草色素-3-O-半乳糖苷(Del-3-O-gal,95%)、飞燕草色素-3-O-阿拉伯糖苷(Del-3-O-ara,94%) 宝鸡市辰光生物科技有限公司;绿原酸(CA)、表儿茶素(Epi)、没食子酸(GA)、阿魏酸(FA)、香草醛(Van)、咖啡酸(CA) 成都瑞芬思生物科技有限公司;柠檬酸、磷酸氢二钠 分析纯,成都科龙化工试剂厂;甲醇、甲酸 色谱纯,美国Sigma公司。
MC-01000228 pH计 成都世纪方舟科技有限公司;UV-1900紫外-可见分光光度计 深圳市华德隆科技有限公司;BSA224S-CW分析天平 赛多利斯科学仪器(北京)有限公司;BT25s十万分之一天平 德国赛多利斯Sartorius;Agilent 1160高效液相色谱仪 安捷伦科技中国有限公司。
1.2 实验方法
1.2.1 花色苷辅色溶液配置
按照Zhao等[22]的方法制备蓝莓汁模型溶液,并略微修改。本研究使用的蓝莓汁模型缓冲液由6.44 mL的0.2 mol/L磷酸氢二钠与13.56 mL的0.1 mol/L柠檬酸混合,使用磷酸氢二钠或柠檬酸将pH调节至3.6。使用模型缓冲液配制浓度为10 mmol/L的6种辅色素溶液(CA、Epi、GA、FA、Van、CaA);使用甲醇配制浓度为1 mmol/L的4种花色苷单体溶液(Mal-3-O-gal、Mal-3-O-ara、Del-3-O-gal、Del-3-O-ara),将多酚/花色苷单体溶液/模型缓冲液以体积比1:1:3混合,配置为花色苷单体/多酚摩尔比为1:10的辅色溶液[23],保证在模型溶液中花色苷单体浓度为0.2 mmol/L,避免其自缔结[24]。将混合溶液置于黑暗中静置30 min以达到平衡,所有样品设置3个平行。
1.2.2 吸收光谱测定
根据Qian等[25]的方法,准确量取20 μL待测样品,以蒸馏水做空白,于450~600 nm下对待测样品进行可见光谱扫描,得到待测样品的吸收光谱图。
1.2.3 辅色溶液颜色计算
根据Pérez-Magariño等[26]研究开发的回归模型,分别在420、520和620 nm处测定吸光度值,估计模拟溶液的CIELAB参数,具体计算过程如下:
L*=exp(4.611−0.670×A520) (1) a*=√−11.666+52.425×(A520) (2) b*=−0.711+91.194×A420−41.672×A520−54.220×A620 (3) ΔE=√(L*−L0*)2+(a*−a0*)2+(b*−b0*)2 (4) 式中:L*—样品的亮度;L0*—第0 d未处理样品亮度;a*—样品的红度;a0*—第0 d未处理样品红度;b*—样品的黄度;b0*—第0 d未处理样品黄度;ΔE的值表示样品与未添加辅色素样品比较的颜色变化程度,值为0~0.5代表变化不明显、值为0.5~1.5代表略微变化、值为1.5~3.0代表变化明显、值为3.0~6.0代表变化清晰可见,值为6.0~12.0代表变化极大[27]。
1.2.4 花色苷含量测定及计算
根据田瑶[28]的方法使用高效液相色谱仪(High Performance Liquid Chromatography,HPLC)测定花色苷含量。将辅色样品过0.45 μm微孔滤膜后上样分析。
1.2.4.1 色谱检测条件
Kromasil 100-5-C18色谱柱,柱温30 ℃,柱压范围<400 bar,流动相A:超纯水(加入5%的甲酸),流动相B:甲醇;流速:1 mL/min;检测波长:530 nm;进样量:20 μL。
按照如下梯度洗脱:0~1 min,10% B;1~15 min,10%~60% B;15~15.1 min,60%~100% B;15.1~18 min,100% B;18~20 min,100%~10% B。
1.2.4.2 定量计算方法
分别配制0.50、0.25、0.167、0.125、0.1 mmol/L浓度的花色苷单体溶液,获取各个浓度标准品的对应的峰面积数据;以标品浓度为横坐标,峰面积为纵坐标,绘制不同物质的标准曲线。将检测到的样品峰面积代入标准曲线线性方程进行计算,得到实际样本中该物质的含量数据[29]。
Y1=(0.67X+7.23)×10−4×493.44 (5) Y2=(0.65X+248)×10−4×463.41 (6) Y3=(0.77X+309)×10−4×465.39 (7) Y4=(0.62X+307)×10−4×435.36 (8) 式中:其中X代表峰面积(mAu·s);Y1是Mal-3-O-gal单体浓度(mg/L);493.44为其摩尔质量(g/mol);Y2是Mal-3-O-ara的标准曲线,463.41为其摩尔质量(g/mol);Y3是Del-3-O-gal的标准曲线,465.39为其摩尔质量(g/mol);Y4是Del-3-O-ara的标准曲线,435.36为其摩尔质量(g/mol)。
1.3 数据处理
以上所有试验重复三次,数据处理及方差分析使用Excel和SPSS 22.0软件中的单因素Tukey检验,以P<0.05为差异显著,画图使用软件Origin Pro 8.0。
2. 结果与分析
2.1 不同多酚对花色苷单体表观颜色的影响
不同多酚对花色苷单体颜色的影响如图1所示。FA、CaA辅色的四种花色苷单体,肉眼可见的由红转变为偏紫色、褪色明显。相比于未辅色Mal-3-O-gal、Mal-3-O-ara、Del-3-O-gal,CA、Epi辅色的样品肉眼可见红色增强;GA、Van辅色的样品出现略微的变红或者无明显颜色改变的现象。相比于未辅色的Del-3-O-ara,CA、Epi、GA、Van辅色的样品肉眼可见红色增强。结果表明,使用CA和Epi对花色苷溶液进行辅色时,颜色增强;FA、CaA辅色的样品,褪色比较明显。4种酚酸均使花色苷溶液产生了增色效果。这可能是因为酚酸与花色苷发生了分子间辅色,两者以非共价键和氢键结合,形成水平或垂直重叠的复合物[30],使红花色苷溶液呈色加强而产生了增色效应,这与孙晨晨等[13]的研究基本一致。
2.2 不同多酚对花色苷单体吸收光谱的影响
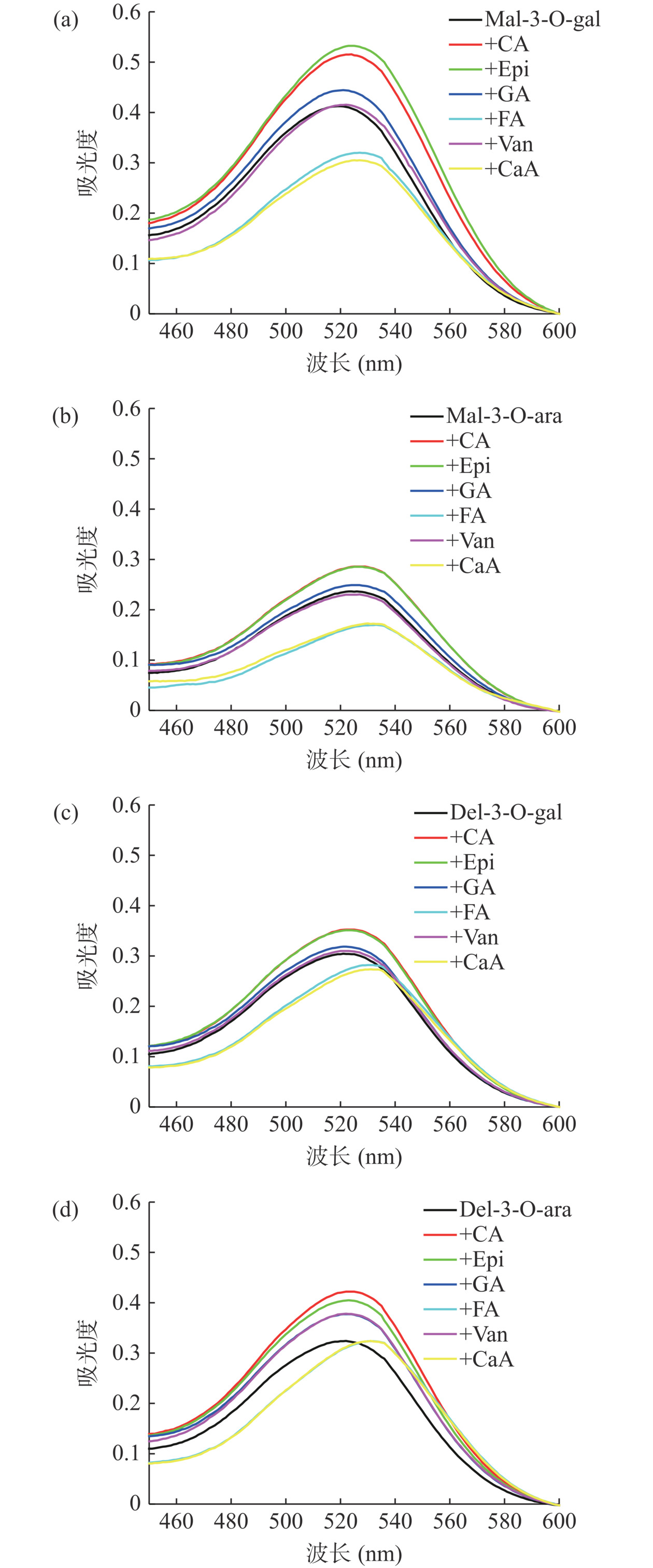
花色苷溶液中添加不同辅色剂后的吸收光谱如图2所示。
由图2可知,花色苷溶液在不同辅色剂的作用下,最大吸收波长发生了不同程度的红移。其中添加FA、CaA对上述四种花色苷溶液的红移现象影响最明显,发生红移7~11 nm。CA、Epi辅色的Mel-3-O-gal,最大吸收波长从520 nm红移至524 nm;CA、Epi辅色的Mel-3-O-ara,最大吸收波长从523 nm红移至525 nm;其余多酚辅色剂对花色苷溶液的红移现象无显著影响(P>0.05)。Eiro等[31]的研究表明FA以摩尔比100:1辅色锦葵色素-3-O-葡萄糖苷时,花色苷的最大吸收波长红移了17.6 nm。发生红移的原因可能是花色苷-辅色剂分子之间形成π-π共轭现象,产生了氢键作用,使花色苷单体极性或电子分布下降使其在可见光范围内的最大吸收波长红移[32]。总的来说,多酚辅色剂可以增强花色苷的吸收强度或波长范围,部分发生红移现象。
从表1可知,CA、Epi均提高了花色苷溶液的吸收峰值,提升幅度高达21%~37%;GA、Van辅色的Del-3-O-ara,其吸收峰值均增强了23.33%;FA、CaA降低了Mal-3-O-gal、Mal-3-O-ara的吸收峰值,下降幅度为19.51%~26.09%;其余多酚辅色剂对花色苷溶液的最大吸收峰值无显著影响。Marković等[33]的研究表明,在pH3.65体系下,FA和CaA以摩尔比20:1辅色锦葵色素-3,5-葡萄糖苷时,吸收峰值较未辅色分别增加了119%、37.5%。Lambert等[34]的研究表明在pH3.6体系下,CA、Epi、CaA以摩尔比100:1辅色锦葵色素-3-O-葡萄糖苷,吸收峰值较未辅色分别高了253%、192%、196%,且辅色效果CA>CaA>Epi。
表 1 不同多酚对花色苷单体吸收峰值、颜色、含量的影响Table 1. Effects of different polyphenols on the absorption peak, color, and content of anthocyanin monomers花色苷单体 辅色剂 吸收峰值 颜色 花色苷含量
(mg/L)L* a* b* ΔE Mal-3-O-gal CK 0.41±0.00b 76.68±0.00b 21.70±0.00b −4.01±0.23b − 96.17±3.49a +CA 0.53±0.02a 70.61±0.74c 26.43±0.56a −6.96±0.49cd 8.24±1.04a 97.37±0.05a +Epi 0.55±0.03a 69.60±1.22c 27.20±0.93a −7.41±0.77d 9.58±1.70a 96.62±6.16a +GA 0.44±0.01b 74.75±0.42b 23.23±0.33b −4.04±0.36b 2.47±0.54bc 100.42±1.79a +FA 0.33±0.03c 80.98±1.50a 18.15±1.27c −3.09±0.84ab 5.68±2.07abc 100.09±1.66a +Van 0.42±0.01b 76.07±0.58b 22.19±0.50b −5.10±0.15bc 1.39±0.53c 93.16±2.75a +CaA 0.31±0.01c 81.91±0.35a 17.36±0.30c −1.61±0.34a 7.21±0.55ab 99.87±2.47a Mal-3-O-ara CK 0.23±0.02b 86.36±1.02b 13.34±0.97b −2.79±0.10bc − 82.22±5.48b +CA 0.29±0.01a 83.30±0.43c 16.15±0.38a −3.49±0.43c 4.23±0.54b 87.72±1.49ab +Epi 0.29±0.00a 83.24±0.04c 16.20±0.03a −3.40±0.47c 4.29±0.12b 86.60±1.37ab +GA 0.25±0.00b 85.47±0.24b 14.18±0.23b −1.04±0.33a 2.13±0.46c 88.34±1.05ab +FA 0.18±0.00c 89.88±0.09a 9.82±0.09c −2.83±0.18bc 4.97±0.12ab 93.03±2.32a +Van 0.22±0.01b 86.87±0.70b 12.84±0.67b −2.07±0.09ab 1.13±0.68c 87.19±0.39ab +CaA 0.17±0.01c 90.66±0.34a 8.97±0.38c −1.00±0.37a 6.39±0.38a 95.19±0.15a Del-3-O-gal CK 0.28±0.01b 83.24±0.67a 16.20±0.59b −4.41±0.31b − 99.43±3.07b +CA 0.34±0.01a 80.12±0.57b 18.88±0.48a −5.18±0.58b 4.19±0.83a 101.20±2.62ab +Epi 0.34±0.00a 80.26±0.15b 18.77±0.13a −5.11±0.11b 4.00±0.21a 102.17±3.51ab +GA 0.30±0.00b 82.32±0.23a 17.00±0.20b −3.01±0.59a 1.91±0.24b 97.92±2.89b +FA 0.29±0.02b 83.44±0.79a 16.03±0.70b −5.06±0.28b 1.04±0.10b 108.67±0.56ab +Van 0.29±0.00b 82.91±0.12a 16.49±0.10b −4.03±0.01ab 0.59±0.11b 98.15±2.33b +CaA 0.28±0.00b 84.02±0.36a 15.50±0.32b −4.51±0.48ab 1.11±0.50b 110.96±3.07a Del-3-O-ara CK 0.30±0.03b 82.32±1.79a 17.00±1.56b −4.56±0.31ab − 90.29±7.04b +CA 0.41±0.02a 76.22±1.23b 22.07±0.98a −6.34±0.15c 8.12±1.56a 96.13±0.96ab +Epi 0.39±0.02a 77.35±1.17b 21.16±0.95a −5.86±0.14bc 6.60±1.51ab 101.66±2.89ab +GA 0.37±0.01a 78.66±0.60ab 20.09±0.49ab −3.90±0.32a 4.85±0.72ab 96.59±3.21ab +FA 0.35±0.01ab 80.69±0.31a 18.40±0.26b −5.97±0.23bc 2.58±0.46b 105.73±2.51ab +Van 0.37±0.00a 78.63±0.19ab 20.11±0.15ab −5.47±0.80abc 4.94±0.38ab 100.75±1.58ab +CaA 0.35±0.02ab 80.66±1.34a 18.43±1.13b −5.73±0.24bc 2.52±1.63b 110.83±5.67a 注:同列不同小写字母表示差异显著,P<0.05;CK:未辅色样品。 根据红移现象以及吸收峰值的大小来综合考虑多酚对花色苷辅色效果的影响。CA、Epi、FA、CaA能够使花色苷溶液的最大吸收波长发生红移,表明多酚辅色剂与花色苷之间存在相互作用,增强了其颜色的鲜艳度和稳定性。此外,CA、Epi均提高了花色苷溶液的吸收峰值,峰值越大表明多酚辅色剂与花色苷溶液之间的相互作用越强,辅色效果越好。结果表明,CA和Epi对花色苷溶液的辅色效果较好,FA和CaA辅色效果较差。FA和CaA辅色效果较差可能因为锦葵色素类与羟基肉桂酸的辅色反应是一种对熵不利的焓驱动过程,并且锦葵色素糖基部分阻碍了羟基肉桂核一侧的辅色[35];还有可能因为FA和CaA辅色花色苷使其结构转化为吡喃花色苷后产生了颜色的变化[36]。
2.3 不同多酚对花色苷单体颜色参数的影响
经多酚处理的花色苷单体颜色参数如表1所示。与对照组相比,CA、Epi辅色的花色苷溶液,其L*值显著下降、a*值显著增强(P<0.05);FA、CaA辅色的花色苷溶液,其L*值显著增强、a*值显著下降(P<0.05);GA、Van辅色的样品L*、a*值变化均不显著(P>0.05)。
总色差ΔE结合了L*、a*、b*三个指标的变化,可直观反映样品与标准颜色之间的差异程度。由表1可知,相比于其他四种多酚辅色剂,使用CA和Epi辅色样品时,ΔE值的变化更大。表明多酚对花色苷辅色的影响较大,能够更有效地影响花色苷的颜色。使用CA、Epi对花色苷进行辅色,导致溶液颜色变红、亮度降低;FA、CaA对花色苷进行辅色,导致溶液颜色变浅、亮度增大。GA、Van辅色的溶液,其L*、a*、b*值相比对照组变化较小,颜色的稳定性增强,花色苷的品质也得到了较好的保留[37]。其结论与图1所示结果相同。添加辅色剂改善了花色苷溶液的颜色特性,这反映在较低的L*以及较大的a*上,推测其原因可能是辅色素和花色苷在辅色过程中产生了增色效应[38]和红移变化,因此添加CA、Epi的花色苷溶液具有更深、更鲜艳的红色色调。
不同辅色素辅色不同的花色苷单体效果存在差异,主要与花色苷和多酚的结构有关。已有研究表明辅色效应随着花青素发色的甲氧基化和羟基化程度而增加[39−40],故锦葵色素类(B环含2个甲氧基)与飞燕草色素类(B环含3个羟基)均易发生辅色反应。多酚中的羰基氧原子和羟基与花色苷形成氢键,两个原子之间的紧密接触、环与芳香环之间π-π堆积产生的范德华力,故含有更多甲氧基或羟基的多酚与花色苷单体相互作用会形成更稳定的复合物,从而导致更大的增色[41]。
2.4 不同多酚对花色苷单体含量的影响
经多酚辅色的花色苷单体含量如表1所示,与未辅色Mal-3-O-gal相比,经6种多酚辅色的样品花色苷含量无显著变化(P>0.05)。与未辅色Mal-3-O-ara相比,经CA、Epi、GA、Van辅色的样品花色苷含量无显著变化(P>0.05),FA、CaA辅色的样品花色苷含量分别显著增加了13.15%、15.77%(P<0.05)。与未辅色Del-3-O-ara、Del-3-O-gal相比,经CA、Epi、GA、FA、Van辅色的样品花色苷含量无显著变化(P>0.05),CaA辅色的样品花色苷含量分别显著增加了22.75%、11.60%、15.77%(P<0.05)。
结果表明,花色苷含量的变化取决于多酚的种类和花色苷的类型,使用多酚对花色苷溶液进行辅色时,花色苷含量增加可能是因为发生了酰化反应[15]。Zhang等[17]的研究表明GA辅色蓝莓汁后其主要的花色苷单体芍药色素-3-O-葡萄糖苷含量显著提高了51.11%。Aleixandre-Tudó等[42]的研究表明在葡萄酒中添加CaA显著增加其花色苷含量,因为CaA与花色苷共辅色反应生成酰化花色苷,增加了其单体含量。这从侧面证实了FA、CaA辅色花色苷单体是有可能发生酰化反应[17]。
3. 结论
本研究选用6种酚类化合物(绿原酸、表儿茶素、没食子酸、阿魏酸、香草醛、咖啡酸),结合辅色效果评价其对蓝莓中主要的花色苷单体颜色稳定性的影响。绿原酸、表儿茶素、没食子酸、香草醛与蓝莓花色苷相互作用可以增加其最大吸光值和最大吸收波长,并且产生了不同程度的增色及红移效应,辅色效果显著;而阿魏酸、咖啡酸对蓝莓花色苷具有减色作用。因此,可以选择绿原酸、表儿茶素、没食子酸、香草醛辅色花色苷单体进行后续研究以明确辅色复合物的加工稳定性。
-
表 1 不同多酚对花色苷单体吸收峰值、颜色、含量的影响
Table 1 Effects of different polyphenols on the absorption peak, color, and content of anthocyanin monomers
花色苷单体 辅色剂 吸收峰值 颜色 花色苷含量
(mg/L)L* a* b* ΔE Mal-3-O-gal CK 0.41±0.00b 76.68±0.00b 21.70±0.00b −4.01±0.23b − 96.17±3.49a +CA 0.53±0.02a 70.61±0.74c 26.43±0.56a −6.96±0.49cd 8.24±1.04a 97.37±0.05a +Epi 0.55±0.03a 69.60±1.22c 27.20±0.93a −7.41±0.77d 9.58±1.70a 96.62±6.16a +GA 0.44±0.01b 74.75±0.42b 23.23±0.33b −4.04±0.36b 2.47±0.54bc 100.42±1.79a +FA 0.33±0.03c 80.98±1.50a 18.15±1.27c −3.09±0.84ab 5.68±2.07abc 100.09±1.66a +Van 0.42±0.01b 76.07±0.58b 22.19±0.50b −5.10±0.15bc 1.39±0.53c 93.16±2.75a +CaA 0.31±0.01c 81.91±0.35a 17.36±0.30c −1.61±0.34a 7.21±0.55ab 99.87±2.47a Mal-3-O-ara CK 0.23±0.02b 86.36±1.02b 13.34±0.97b −2.79±0.10bc − 82.22±5.48b +CA 0.29±0.01a 83.30±0.43c 16.15±0.38a −3.49±0.43c 4.23±0.54b 87.72±1.49ab +Epi 0.29±0.00a 83.24±0.04c 16.20±0.03a −3.40±0.47c 4.29±0.12b 86.60±1.37ab +GA 0.25±0.00b 85.47±0.24b 14.18±0.23b −1.04±0.33a 2.13±0.46c 88.34±1.05ab +FA 0.18±0.00c 89.88±0.09a 9.82±0.09c −2.83±0.18bc 4.97±0.12ab 93.03±2.32a +Van 0.22±0.01b 86.87±0.70b 12.84±0.67b −2.07±0.09ab 1.13±0.68c 87.19±0.39ab +CaA 0.17±0.01c 90.66±0.34a 8.97±0.38c −1.00±0.37a 6.39±0.38a 95.19±0.15a Del-3-O-gal CK 0.28±0.01b 83.24±0.67a 16.20±0.59b −4.41±0.31b − 99.43±3.07b +CA 0.34±0.01a 80.12±0.57b 18.88±0.48a −5.18±0.58b 4.19±0.83a 101.20±2.62ab +Epi 0.34±0.00a 80.26±0.15b 18.77±0.13a −5.11±0.11b 4.00±0.21a 102.17±3.51ab +GA 0.30±0.00b 82.32±0.23a 17.00±0.20b −3.01±0.59a 1.91±0.24b 97.92±2.89b +FA 0.29±0.02b 83.44±0.79a 16.03±0.70b −5.06±0.28b 1.04±0.10b 108.67±0.56ab +Van 0.29±0.00b 82.91±0.12a 16.49±0.10b −4.03±0.01ab 0.59±0.11b 98.15±2.33b +CaA 0.28±0.00b 84.02±0.36a 15.50±0.32b −4.51±0.48ab 1.11±0.50b 110.96±3.07a Del-3-O-ara CK 0.30±0.03b 82.32±1.79a 17.00±1.56b −4.56±0.31ab − 90.29±7.04b +CA 0.41±0.02a 76.22±1.23b 22.07±0.98a −6.34±0.15c 8.12±1.56a 96.13±0.96ab +Epi 0.39±0.02a 77.35±1.17b 21.16±0.95a −5.86±0.14bc 6.60±1.51ab 101.66±2.89ab +GA 0.37±0.01a 78.66±0.60ab 20.09±0.49ab −3.90±0.32a 4.85±0.72ab 96.59±3.21ab +FA 0.35±0.01ab 80.69±0.31a 18.40±0.26b −5.97±0.23bc 2.58±0.46b 105.73±2.51ab +Van 0.37±0.00a 78.63±0.19ab 20.11±0.15ab −5.47±0.80abc 4.94±0.38ab 100.75±1.58ab +CaA 0.35±0.02ab 80.66±1.34a 18.43±1.13b −5.73±0.24bc 2.52±1.63b 110.83±5.67a 注:同列不同小写字母表示差异显著,P<0.05;CK:未辅色样品。 -
[1] 刘彩芬, 秦公伟, 韩豪, 等. UPLC-MS/MS分析不同品种蓝莓中的花青苷[J]. 中国实验方剂学杂志,2018,24(21):62−68. [LIU C F, QIN G W, HAN H, et al. Analysis of anthocyanins in different blueberry cultivars by UPLC-MS /MS[J]. Chinese Journal of Experimental Traditional Medical Formulae,2018,24(21):62−68.] LIU C F, QIN G W, HAN H, et al. Analysis of anthocyanins in different blueberry cultivars by UPLC-MS /MS[J]. Chinese Journal of Experimental Traditional Medical Formulae, 2018, 24(21): 62−68.
[2] 张晓晓, 黄午阳, 於虹, 等. 不同种植地区蓝莓果中花色苷的分布[J]. 中国食品学报,2022,22(10):314−324. [ZHANG X X, HUANG W Y, YU H, et al. The distribution of anthocyanins in blueberry fruit from different growing locations[J]. Journal of Chinese Institute of Food Science and Technology,2022,22(10):314−324.] ZHANG X X, HUANG W Y, YU H, et al. The distribution of anthocyanins in blueberry fruit from different growing locations[J]. Journal of Chinese Institute of Food Science and Technology, 2022, 22(10): 314−324.
[3] 张念, 彭怡霖, 陈细羽, 等. 超高效液相色谱法检测植物源性食品中花青素[J]. 分析科学学报,2022,38(1):17−23. [ZHANG N, PENG Y L, CHEN X Y, et al. Determination of anthocyanins in plant origin products by ultra-high performance liquid chromatography[J]. Journal of Analytical Science,2022,38(1):17−23.] ZHANG N, PENG Y L, CHEN X Y, et al. Determination of anthocyanins in plant origin products by ultra-high performance liquid chromatography[J]. Journal of Analytical Science, 2022, 38(1): 17−23.
[4] REQUE P M, STEFFENS R S, JABLONSKI A, et al. Cold storage of blueberry (Vaccinium spp.) fruits and juice:Anthocyanin stability and antioxidant activity[J]. Journal of Food Composition and Analysis,2014,33(1):111−116. doi: 10.1016/j.jfca.2013.11.007
[5] CHEN Y, BELWAL T, XU Y, et al. Updated insights into anthocyanin stability behavior from bases to cases:Why and why not anthocyanins lose during food processing[J]. Critical Reviews in Food Science and Nutrition, 2022:1−33.
[6] WU Y Q, HAN T Y, YANG H, et al. Known and potential health benefits and mechanisms of blueberry anthocyanins:A review[J]. Food Bioscience,2023,55:103050. doi: 10.1016/j.fbio.2023.103050
[7] 覃悦, 杨雪莲, 吴永飞. 不同蓝莓品种的花青素抗氧化活性研究新进展[J]. 绿色科技,2022,24(13):72−75. [QIN Y, YANG X L, WU Y F. Research progress on antioxidant activity of anthocyanins in different blueberry varieties[J]. Journal of Green Science and Technology,2022,24(13):72−75.] QIN Y, YANG X L, WU Y F. Research progress on antioxidant activity of anthocyanins in different blueberry varieties[J]. Journal of Green Science and Technology, 2022, 24(13): 72−75.
[8] 钟绍金, 韩珊颖, 黄裕昌. 花色苷对糖尿病视网膜病变模型大鼠视网膜氧化应激损伤拮抗作用研究[J]. 现代中药研究与实践,2023,37(3):21−25. [ZHONG S J, HAN S Y, HUANG Y C. Antagonistic effect of anthocyanins on oxidative stress injury of retina in DA rat model based on Notch1/Hes-1 axis[J]. Research and Practice on Chinese Medicines,2023,37(3):21−25.] ZHONG S J, HAN S Y, HUANG Y C. Antagonistic effect of anthocyanins on oxidative stress injury of retina in DA rat model based on Notch1/Hes-1 axis[J]. Research and Practice on Chinese Medicines, 2023, 37(3): 21−25.
[9] 王二雷, 黄佳莹, 段海章, 等. 花色苷稳态化技术研究进展及应用前景[J]. 食品工业科技,2024,45(18):394−403. [WANG E L, HUANG J Y, DUAN H Z, et al. Progress on the stabilization technology of anthocyanins and the application prospects[J]. Science and Technology of Food Industry,2024,45(18):394−403.] WANG E L, HUANG J Y, DUAN H Z, et al. Progress on the stabilization technology of anthocyanins and the application prospects[J]. Science and Technology of Food Industry, 2024, 45(18): 394−403.
[10] RAKIC V, POKLAR U N. Influence of pH on color variation and stability of cyanidin and cyanidin 3-O-beta-glucopyranoside in aqueous solution[J]. CyTA-Journal of Food,2021,19(1):174−182. doi: 10.1080/19476337.2021.1874539
[11] 张志国, 姜闪. 食用玫瑰花褪色原因及控制措施研究进展[J]. 食品科学,2017,38(9):322−328. [ZHANG Z G, JIANG S. Causes and control measures of the fading of edible roses[J]. Food Science,2017,38(9):322−328.] ZHANG Z G, JIANG S. Causes and control measures of the fading of edible roses[J]. Food Science, 2017, 38(9): 322−328.
[12] KANHA N, SURAWANG S, PITCHAKARN P, et al. Copigmentation of cyanidin 3-O-glucoside with phenolics:Thermodynamic data and thermal stability[J]. Food Bioscience,2019,30:100419. doi: 10.1016/j.fbio.2019.100419
[13] 孙晨晨, 高庆超, 李亚辉, 等. 5种多酚类化合物提高紫甘蓝花色苷热稳定性及辅色机理初探[J]. 现代食品科技,2022,38(3):89−96. [SUN C C, GAO Q C, LI Y H, et al. Enhancing the thermal stability of purple cabbage anthocyanins and mechanisms of co-pigmentation using five polyphenolic compounds[J]. Modern Food Science and Technology,2022,38(3):89−96.] SUN C C, GAO Q C, LI Y H, et al. Enhancing the thermal stability of purple cabbage anthocyanins and mechanisms of co-pigmentation using five polyphenolic compounds[J]. Modern Food Science and Technology, 2022, 38(3): 89−96.
[14] 黄金萍, 吴继红, 廖小军, 等. 果蔬汁饮料中花色苷与VC相互作用研究进展[J]. 食品科学,2022,43(21):358−371. [HUANG J P, WU J H, LIAO X J, et al. Recent progress in the study on the interaction between anthocyanins and vitamin C in fruit and vegetable beverages[J]. Food Science,2022,43(21):358−371.] HUANG J P, WU J H, LIAO X J, et al. Recent progress in the study on the interaction between anthocyanins and vitamin C in fruit and vegetable beverages[J]. Food Science, 2022, 43(21): 358−371.
[15] LIU J N, ZHUANG Y H, HU Y H, et al. Improving the color stability and antioxidation activity of blueberry anthocyanins by enzymatic acylation with p-coumaric acid and caffeic acid[J]. LWT,2020,130:109673. doi: 10.1016/j.lwt.2020.109673
[16] BI X F, NING N, WANG X Q, et al. Comparison of high-pressure processing, ultrasound and heat treatments on the qualities of a gallic acid copigmented blueberry–grape–pineapple–cantaloupe juice blend[J]. International Journal of Food Science and Technology,2022,57:6948−6962. doi: 10.1111/ijfs.15919
[17] ZHANG L L, WANG W B, YUE X Y, et al. Gallic acid as a copigment enhance anthocyanin stabilities and color characteristics in blueberry juice[J]. Journal of Food Science and Technology,2020,57:1405−1414. doi: 10.1007/s13197-019-04175-w
[18] 苏帆. 酚酸辅色及微胶囊化对红肉苹果花色苷稳定性的影响[D]. 西安:陕西师范大学, 2017. [SU F. Research on effects of copigmentation with phenolic and microencapsulation on stability of anthocyanins in red-fleshed apple[D]. Xi'an:Shaanxi Normal University, 2017.] SU F. Research on effects of copigmentation with phenolic and microencapsulation on stability of anthocyanins in red-fleshed apple[D]. Xi'an: Shaanxi Normal University, 2017.
[19] ZOU H, MA Y, XU Z Z, et al. Isolation of strawberry anthocyanins using high-speed counter-current chromatography and the copigmentation with catechin or epicatechin by high pressure processing[J]. Food Chemistry,2018,247:81−88. doi: 10.1016/j.foodchem.2017.11.102
[20] LIN M H, SUN C C, GAO Q C, et al. Effect of five polyphenols on the stability of purple cabbage anthocyanins in simulated beverage systems containing L-ascorbic acid[J]. Food Packaging and Shelf Life,2023,37:101065. doi: 10.1016/j.fpsl.2023.101065
[21] 范琳琳, 王英, 黄自苏, 等. 外源添加物质对黑莓清汁花色苷稳定性和抗氧化活性的影响[J]. 食品工业科技,2019,40(4):56−61. [FAN L L, WANG Y, HUANG Z S, et al. Effects of exogenous compounds on stability and antioxidant activity of anthocyanins from blackberry juice[J]. Science and Technology of Food Industry,2019,40(04):56−61.] FAN L L, WANG Y, HUANG Z S, et al. Effects of exogenous compounds on stability and antioxidant activity of anthocyanins from blackberry juice[J]. Science and Technology of Food Industry, 2019, 40(04): 56−61.
[22] ZHAO X, DING B W, QIN J W, et al. Intermolecular copigmentation between five common 3-O-monoglucosidic anthocyanins and three phenolics in red wine model solutions:The influence of substituent pattern of anthocyanin B ring[J]. Food Chemistry,2020,326:126960. doi: 10.1016/j.foodchem.2020.126960
[23] ZOU H, MA Y, LIAO X J, et al. Effects of high pressure processing on the co-pigmentation reaction of pelargonidin-3-glucoside and catechin[J]. LWT,2019,108:240−246. doi: 10.1016/j.lwt.2019.03.080
[24] ASEN S, STEWART R N, NORRIS K H, et al. Copigmentation of anthocyanins in plant tissues and its effect on color[J]. Phytochemistry,1972,11:1139−1144. doi: 10.1016/S0031-9422(00)88467-8
[25] QIAN B J, LIU J H, ZHAO S J, et al. The effects of gallic/ferulic/caffeic acids on colour intensification and anthocyanin stability[J]. Food Chemistry,2017,228:526−532. doi: 10.1016/j.foodchem.2017.01.120
[26] PÉREZ-MAGARIÑO S, GONZÁLEZ-SANJOSÉ M L. Application of absorbance values used in wineries for estimating CIELAB parameters in red wines[J]. Food Chemistry,2003,81(2):301−306. doi: 10.1016/S0308-8146(02)00509-5
[27] NING N, WANG X Q, LI J R, et al. Effects of different antioxidants combined with high hydrostatic pressure on the color and anthocyanin retention of a blueberry juice blend during storage[J]. Food Science and Technology International,2022,29(5):35491658.
[28] 田瑶. 蓝莓中黄酮类物质的提取分离纯化及生物活性研究[D]. 哈尔滨:东北农业大学, 2012. [TIAN Y. Study on extraction, purification and bioactivity of flavonoids in blueberry[D]. Harbin:Northeast Agricultural University, 2012.] TIAN Y. Study on extraction, purification and bioactivity of flavonoids in blueberry[D]. Harbin: Northeast Agricultural University, 2012.
[29] 李琼婕, 赵哲, 王秀娟, 等. 涡旋辅助离子液体双水相萃取五味子中木脂素类化合物[J]. 食品工业科技,2022,43(4):169−177. [LI Q J, ZHAO Z, WANG X J, et al. Vortex-assisted ionic liquid aqueous two-phase extraction of lignans from schisandra chinensis[J]. Science and Technology of Food Industry,2022,43(4):169−177.] LI Q J, ZHAO Z, WANG X J, et al. Vortex-assisted ionic liquid aqueous two-phase extraction of lignans from schisandra chinensis[J]. Science and Technology of Food Industry, 2022, 43(4): 169−177.
[30] CHEN L J, HRAZDINA G. Structural aspects of anthocyanin-favonoid complex formation and its role in pant coor[J]. Phytochemistry,1981,20(2):297−303. doi: 10.1016/0031-9422(81)85111-4
[31] EIRO M J, HEINONEN M. Anthocyanin color behavior and stability during storage:Effect of intermolecular copigmentation[J]. Journal of Agricultural and Food Chemistry,2002,50(25):7461−7466. doi: 10.1021/jf0258306
[32] 赵磊, 潘飞, 周娜, 等. 提高黑米花色苷颜色稳定性辅色剂的筛选及其作用机制[J]. 食品科学,2021,42(14):16−23. [ZHAO L, PAN F, ZHOU N, et al. Screening of co-pigments to improve color stability of black rice anthocyanins and underlying mechanism[J]. Food Science,2021,42(14):16−23.] ZHAO L, PAN F, ZHOU N, et al. Screening of co-pigments to improve color stability of black rice anthocyanins and underlying mechanism[J]. Food Science, 2021, 42(14): 16−23.
[33] MARKOVIĆ D, PETRANOVIĆ N A, BARANAC J M. A spectrophotometric study of the co-pigmentation of malvin with caffeic and ferulic acids[J]. Journal of Agricultural and Food Chemistry,2000,48(11):5530−5536. doi: 10.1021/jf000038v
[34] LAMBERT S G, ASENSTORFER R E, WILLIAMSON N M, et al. Copigmentation between malvidin-3-glucoside and some wine constituents and its importance to colour expression in red wine[J]. Food Chemistry,2011,125(1):106−115. doi: 10.1016/j.foodchem.2010.08.045
[35] GALLAND S, MORA N, ABERT V M, et al. Chemical synthesis of hydroxycinnamic acid glucosides and evaluation of their ability to stabilize natural colors via anthocyanin copigmentation[J]. Journal of Agricultural and Food Chemistry,2007,55(18):7573−7579. doi: 10.1021/jf071205v
[36] 曾颖钰, 郭大三, 李旭, 等. 吡喃花色苷结构及其性质研究进展[J]. 食品科学,2022,43(13):199−209. [ZENG Y Y, GUO D S, LI X, et al. Progress in research on the structure and properties of pyranoanthocyanins[J]. Food Science,2022,43(13):199−209.] doi: 10.7506/spkx1002-6630-20210517-205 ZENG Y Y, GUO D S, LI X, et al. Progress in research on the structure and properties of pyranoanthocyanins[J]. Food Science, 2022, 43(13): 199−209. doi: 10.7506/spkx1002-6630-20210517-205
[37] 张锦钰, 郑棉文, 王锋, 等. 辅色剂对紫淮山花色苷稳定性的影响及其热降解动力学研究[J]. 激光生物学报,2020,29(1):80−86. [ZHANG J Y, ZHENG M W, WANG F, et al. Effects of copigments on the stability of purple Yam anthocyanins and their thermal degradation kinetics[J]. Acta Laser Biology Sinica,2020,29(1):80−86.] ZHANG J Y, ZHENG M W, WANG F, et al. Effects of copigments on the stability of purple Yam anthocyanins and their thermal degradation kinetics[J]. Acta Laser Biology Sinica, 2020, 29(1): 80−86.
[38] 李运奎, 张煜, 范舒悦, 等. 外源多酚添加条件下干红葡萄酒颜色和花色苷特性研究[J]. 农业机械学报,2023,54(4):399−406. [LI Y K, ZHANG Y, FAN S Y, et al. Effects of exogenous polyphenols addition on color and anthocyanins of dry red wine[J]. Transactions of the Chinese Society for Agricultural Machinery,2023,54(4):399−406.] LI Y K, ZHANG Y, FAN S Y, et al. Effects of exogenous polyphenols addition on color and anthocyanins of dry red wine[J]. Transactions of the Chinese Society for Agricultural Machinery, 2023, 54(4): 399−406.
[39] FOSSEN T, CABRITA L, ANDERSEN O M. Color and stability of pure anthocyanins influenced by pH including the alkaline region[J]. Food Chemistry,1998,63:435−440. doi: 10.1016/S0308-8146(98)00065-X
[40] CABRITA L, FOSSEN T, ANDERSEN O M. Colour and stability of the six common anthocyanidin 3-glucosides in aqueous solutions[J]. Food Chemistry,2000,68:101−107. doi: 10.1016/S0308-8146(99)00170-3
[41] BROUILLARD R, WIGAND M C, DANGLES O, et al. PH and solvent effects on the copigmentation reaction of malvin with polyphenols, purine and pyrimidine derivatives[J]. Journal of the Chemical Society, Perkin Transactions 2,1991(8):1235−1241. doi: 10.1039/p29910001235
[42] ALEIXANDRE-TUDÓ J L, ÁLVAREZ I, LIZAMA V, et al. Impact of caffeic acid addition on phenolic composition of tempranillo wines from different winemaking techniques[J]. Journal of Agricultural and Food Chemistry,2013,61(49):11900−11912. doi: 10.1021/jf402713d





 下载:
下载:
 下载:
下载:



