Inhibition of Human Serum Albumin Aggregation by Polyphenols with Similar Structures in Macromolecular Crowding Environment
-
摘要: 为了揭示生理拥挤环境下巴西苏木素(Brazilin,Bra)、苏木素(Hematoxylin,Hto)和氧化苏木精(Hematein,Hte)抗蛋白质聚集能力,本文首先利用大分子拥挤试剂聚乙二醇构建了模拟拥挤环境,然后利用荧光光谱法、紫外-可见吸收光谱法、动态光散射法和原子力显微镜法研究了这三种结构类似的多酚类化合物对人血清白蛋白(HSA)聚集行为的抑制机制。结果表明,在拥挤环境中它们均能够维持HSA结构的稳定,减少形成的淀粉样纤维数量,缩短其长度,从而抑制蛋白质的聚集过程,且抑制能力依次为:Hto>Bra>Hte,此外,与体外稀溶液环境相比,拥挤试剂的存在会导致这三个抑制剂的抑制活性降低。总之,本研究表明Hto可作为潜在的蛋白质聚集抑制剂和功能性食品成分,用于淀粉样变性疾病的治疗干预。Abstract: To reveal the anti-protein aggregation ability of brazilin (Bra), hematoxylin (Hto) and hematein (Hte) under physiological crowding environment, the macromolecular crowding reagent (polyethylene glycol) was used to construct a simulated crowding environment. Then the inhibition mechanisms of these three structurally similar polyphenol compounds on the aggregation behavior of human serum albumin (HSA) were investigated by fluorescence spectroscopy, UV-vis absorption spectroscopy, dynamic light scattering (DLS) and atomic force (AFM) microscope assay. The results showed that they could maintain the stability of the HSA structure, decrease the number of amyloid fibrils and shorten the length of aggregates in crowding environment. Therefore, they inhibited the aggregation process of HSA, and the sequence of the inhibition ability was in the order of Hto>Bra>Hte. Moreover, the presence of crowding reagents could lead to a decrease in the inhibitory activity of these three inhibitors compared to in vitro dilute solution. In conclusion, this study suggested that Hto could be used as a potential protein aggregation inhibitor and a functional food ingredient for the treatment and intervention of amyloid-related diseases.
-
Keywords:
- brazilin /
- hematoxylin /
- hematein /
- human serum albumin /
- protein aggregation /
- macromolecular crowding
-
在病理条件下,蛋白质容易发生错误折叠和聚集,导致淀粉样纤维结构的形成[1−4]。此种纤维具有高度组织化的交叉β-折叠特征[5−6],能在细胞内或细胞外会形成沉积物,从而破坏组织的结构与功能,最终导致细胞的凋亡[7−8]。许多神经退行性疾病,如阿尔茨海默病、帕金森症、亨廷顿氏病等就是由不同蛋白质的淀粉样纤维化导致的。另外,一些非退行性疾病也与蛋白质的淀粉样聚集密切相关,如Ⅱ型糖尿病和全身性淀粉样变性疾病等[9]。此外,一些具有稳定构象的蛋白质在特定的体外环境中(如高温)也会形成淀粉样聚集体,如人血清白蛋白(Human serum albumin,HSA)[10−13]。故研究者常将其作为模型蛋白,用来研究蛋白质折叠/去折叠过程以及聚集行为等内容。
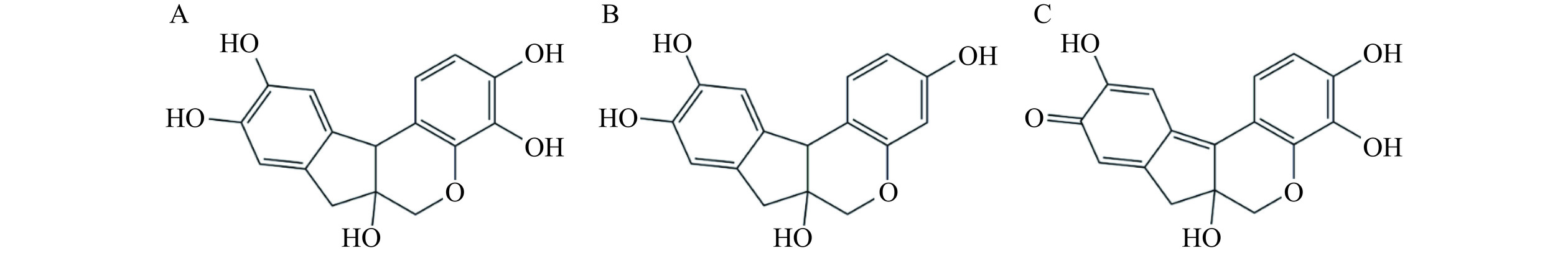
研发抑制淀粉样纤维形成的药物是当今治疗淀粉样变性疾病的主要策略之一。一些物质,如天然化合物、多肽/蛋白质、纳米颗粒等被报道具有此功能,但在这些抑制剂中,天然化合物(特别是多酚化合物)因其来源广泛、种类繁多,生物活性高且毒性低等特点而被广泛关注[14−16]。如藏红花醛[17]和表没食子儿茶素没食子酸酯[18]等天然化合物被证明具有较好的抑制HSA淀粉样聚集过程的作用。苏木素(Hematoxylin,Hto)、巴西苏木素(Brazilin,Bra)和氧化苏木精(Hematein,Hte)是三个结构非常类似的多酚化合物(图1),主要的差异是苯环上羟基基团的数目和排列位置不同。其中,与Bra相比,Hto结构上多一个羟基,而Hte则是由Hto的一个羟基被氧化成羰基而产生的。研究表明,这三个多酚化合物具有多种生物活性,如抗肿瘤、抗炎、抗氧化和降血糖等[19−20]。此外,Hto和Bra也被证明能够抑制蛋白质(如Aβ蛋白)淀粉样纤维的形成[21],但是对其结构-功能间的关系未见系统的分析。
“拥挤”是细胞内普遍存在的一大特性,即细胞中充满着成千上万种的生物大分子,如蛋白质、核酸和多糖等,它们占据细胞总体积的10%~40%(总浓度为80~400 g/L)[22−24],严重影响着细胞内各种成分的热力学性质和动力学行为。目前,绝大多数抗淀粉样蛋白纤维化的研究都聚焦于稀溶液环境中,这与真实的生物学环境相差巨大。为了更加真实地揭示抑制剂分子与淀粉样蛋白的相互作用机制以及其在细胞内的行为,必须考虑细胞内大分子拥挤环境。本文利用聚乙二醇在体外构建了模拟的大分子拥挤环境,然后研究了Hto、Bra和Hte对HSA纤维形成的抑制效果以及它们与HSA的结合对蛋白质构象的影响。综上,本研究的结果可为未来新型抗蛋白质聚集药物以及相关功能性食品的开发提供一些理论参考。
1. 材料与方法
1.1 材料与仪器
巴西苏木素 上海源叶生物科技有限公司;氧化苏木精 Acros公司;人血清白蛋白、聚乙二醇(Polyethylene glycol,PEG)、聚蔗糖70(Ficoll 70)、葡聚糖70(Dextran 70)、硫黄素T、8-苯胺基-1-萘磺酸 Sigma公司;其他试剂均为国产分析/色谱纯;实验用水为超纯水。
TU-1900双光束紫外可见分光光度计 北京普析通用仪器有限责任公司;F97 Pro荧光分光光度计 上海棱光技术有限公司;DynaPro NanoStar动态光散射仪 美国Wyatt Technology公司;Dimension icon原子力显微镜 德国Bruker公司。
1.2 实验方法
1.2.1 溶液体系的配制
PEG(分子量为1000、2000、4000、6000、8000和10000 Da)、Ficoll 70(分子量为70000 Da)和Dextran 70(分子量为70000 Da)溶液:称取50 g固体溶于超纯水中,制成500 g/L的储液,室温保存备用;HSA溶液:准确称取适量HSA(分子量为66478 Da)溶于PBS缓冲溶液(pH7.4)中,制成浓度为100和10 mmol/L储液,于4 ℃冰箱保存备用,使用时稀释至所需浓度;硫磺素T(Thioflavin T,ThT)溶液:称取适量ThT(摩尔消光系数为36500 mol−1·cm−1)溶于超纯水中,然后使用0.22 μm的滤膜进行抽滤,再根据朗伯比尔定律计算其实际浓度,避光保存;1-苯胺基-8-萘磺酸(1-Aniline-8-naphthalenesulfonate,ANS)溶液:称取适量ANS溶于PBS缓冲溶液中,制成3 mmol/L的储液,4 ℃保存备用。
1.2.2 大分子拥挤试剂对HSA内源荧光光谱的影响
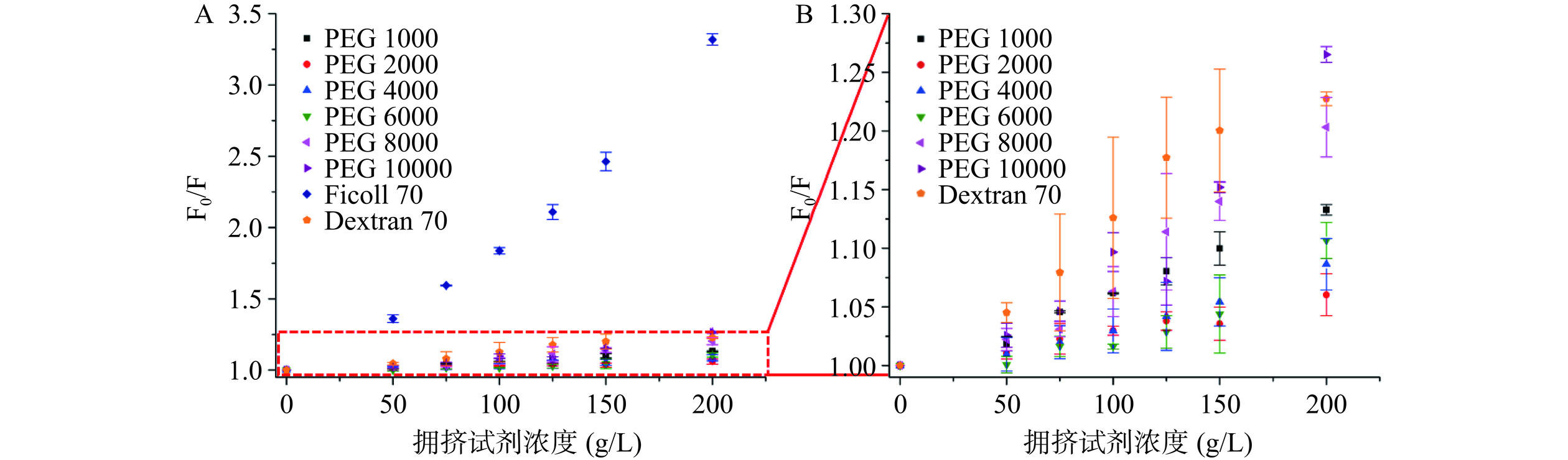
取一定量的HSA溶液(浓度为2 μmol/L)与PEG/Ficoll/Dextran溶液(浓度分别为0、50、75、100、125、150和200 g/L)进行混合,充分混匀后扫描每个样品在25 ℃ 时290~500 nm波长范围内的荧光光谱。其中,荧光光谱仪的狭缝宽度设置为10 nm,增益设置为700 V。根据Stern-Volmer方程对HSA的荧光猝灭进行分析:
F0/F=1+KSV[Q] 式中,F0表示不存在拥挤试剂时HSA的荧光强度;F表示加入拥挤试剂后HSA的荧光强度;Ksv表示Stern-Volmer猝灭常数;[Q]表示拥挤试剂的浓度。
1.2.3 HSA淀粉样纤维的制备
称取适量的HSA溶解于PBS缓冲溶液(pH7.4)中,接着用0.22 μm的滤膜进行抽滤,最后根据朗伯比尔定律确定HSA的实际浓度,保存备用。制备三组样品,第一组中HSA的浓度设为30 μmol/L,然后分别取不同体积的Bra/Hto/Hte储液加入到HSA溶液中,使得它们的终浓度分别为0、6和60 μmol/L;第二组和第三组样品中分别加入一定体积的PEG 2000/PEG 4000储液,使其终浓度为100 g/L,其余组分的设置与第一组样品保持一致。将样品充分混匀后,在温度为65 ℃、转速为150 r/min的循环振荡水浴中孵育140 h。
1.2.4 ThT荧光光谱测定
在不同时间点取出一定量孵育的HSA纤维样品,接着加入一定体积的ThT溶液,使得HSA的浓度为10 μmol/L,ThT的浓度为90 μmol/L。充分混匀后在25 ℃下避光孵育5 min,随后使用荧光光谱仪在444 nm的激发波长下,测定485 nm处的荧光强度。其中,狭缝宽度为10 nm,增益为650 V。
1.2.5 ANS荧光光谱测定
当孵育时间为140 h时,取出一定量的HSA纤维样品,并加入一定体积的ANS溶液,使得HSA的浓度为10 μmol/L,ANS的浓度为30 μmol/L,混匀后在25 ℃下避光孵育5 min,然后使用荧光光谱仪进行检测。激发波长为370 nm,扫描范围为400~600 nm,狭缝宽度为10 nm,增益为650 V。
1.2.6 DLS实验
当孵育时间为140 h时,取出一定量的HSA纤维样品,接着加入PBS溶液进行稀释,使HSA的终浓度为10 μmol/L,然后使用动态光散射仪测量每个样品的水力学半径(Rh)。
1.2.7 AFM实验
当孵育时间为140 h时,取出一定量的HSA纤维样品,加入超纯水将样品稀释500倍,取10 μL滴加到洁净的天然云母片表面,静置1 h后,用超纯水冲洗表面未吸附的样品。室温静置干燥后,使用原子力显微镜观察纤维形态,得到的图像利用Gwyddion 2.58软件进行分析。
1.2.8 紫外可见吸收光谱的测定
当大分子拥挤试剂(PEG 2000/PEG 4000的浓度为100 g/L)存在或不存在时,分别取一定量的HSA溶液和Bra/Hto/Hte储液进行混合,使得HSA的浓度为2 μmol/L,Bra/Hto/Hte的浓度分别为0、5、10、15、20、25、30、35和40 μmol/L,然后使用紫外可见分光光度计记录25 ℃下200~500 nm波长区间内样品的紫外可见吸收光谱,以相同浓度的Hto/Bra/Hte溶液作为参比溶液进行扣除。
1.2.9 三维荧光光谱的测定
按照1.2.8节中方法配制样品,其中HSA的浓度为2 μmol/L,PEG 2000/PEG 4000的浓度为100 g/L,多酚化合物的浓度分别为0和20 μmol/L,然后使用荧光光谱仪的三维波长扫描模式测定每个样品在25 ℃时的三维荧光光谱。其中,激发波长设置为200~360 nm,发射波长设置为230~600 nm。
1.3 数据处理
所有实验数据均进行三次重复实验,结果以平均值±标准差表示。使用Origin 8软件进行数据绘图,利用GraphPad Prism 5软件进行单因素方差分析,P<0.05表示数据具有显著性差异。
2. 结果与分析
2.1 不同大分子拥挤试剂对HSA构象的影响
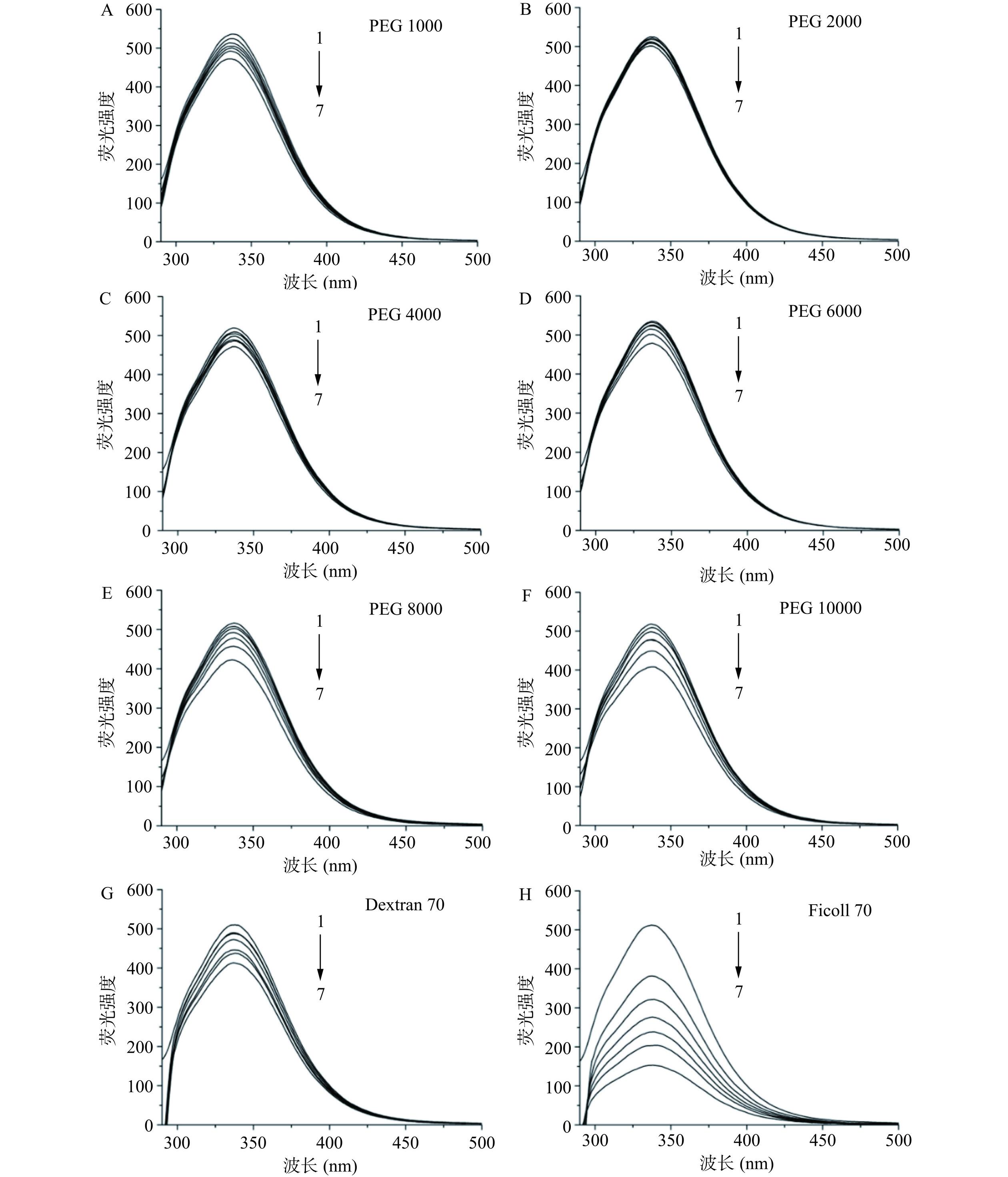
为了真实地模拟体内拥挤环境的纯物理排斥作用(大分子拥挤效应),考察了几种不同的惰性试剂对HSA构象的影响情况,即利用荧光光谱法研究8种大分子拥挤试剂(Dextran 70、Ficoll 70、PEG 1000、PEG 2000、PEG 4000、PEG 6000、PEG 8000和PEG 10000)对HSA内源荧光光谱(λ激发=280 nm)的影响。如图2所示,随着拥挤试剂浓度的增加,HSA的荧光强度呈现出不同程度的降低,即发生了荧光猝灭现象,且不同的拥挤试剂对HSA荧光强度的猝灭程度不同。
由不同拥挤试剂存在时HSA的Stern-Volmer图(图3)可以看出,在这8种拥挤试剂中,Ficoll 70对HSA荧光的猝灭最为明显,而PEG 2000和PEG 4000对HSA荧光的猝灭程度最小,故表明PEG 2000和PEG 4000对HSA构象产生的影响较小。此外,当拥挤试剂的浓度为150和200 g/L时,它们对HSA荧光强度的猝灭程度较大,而当拥挤试剂的浓度为 50 g/L 时,虽然它们对 HSA 荧光的影响较小,但是其不能很好地描述体内大分子拥挤环境的情况。因此,选择 100 g/L 的 PEG 2000 和 PEG 4000作为模拟体内大分子拥挤环境的试剂,用来开展后续实验。
2.2 拥挤环境下多酚化合物对HSA纤维化过程的影响
2.2.1 ThT荧光光谱分析
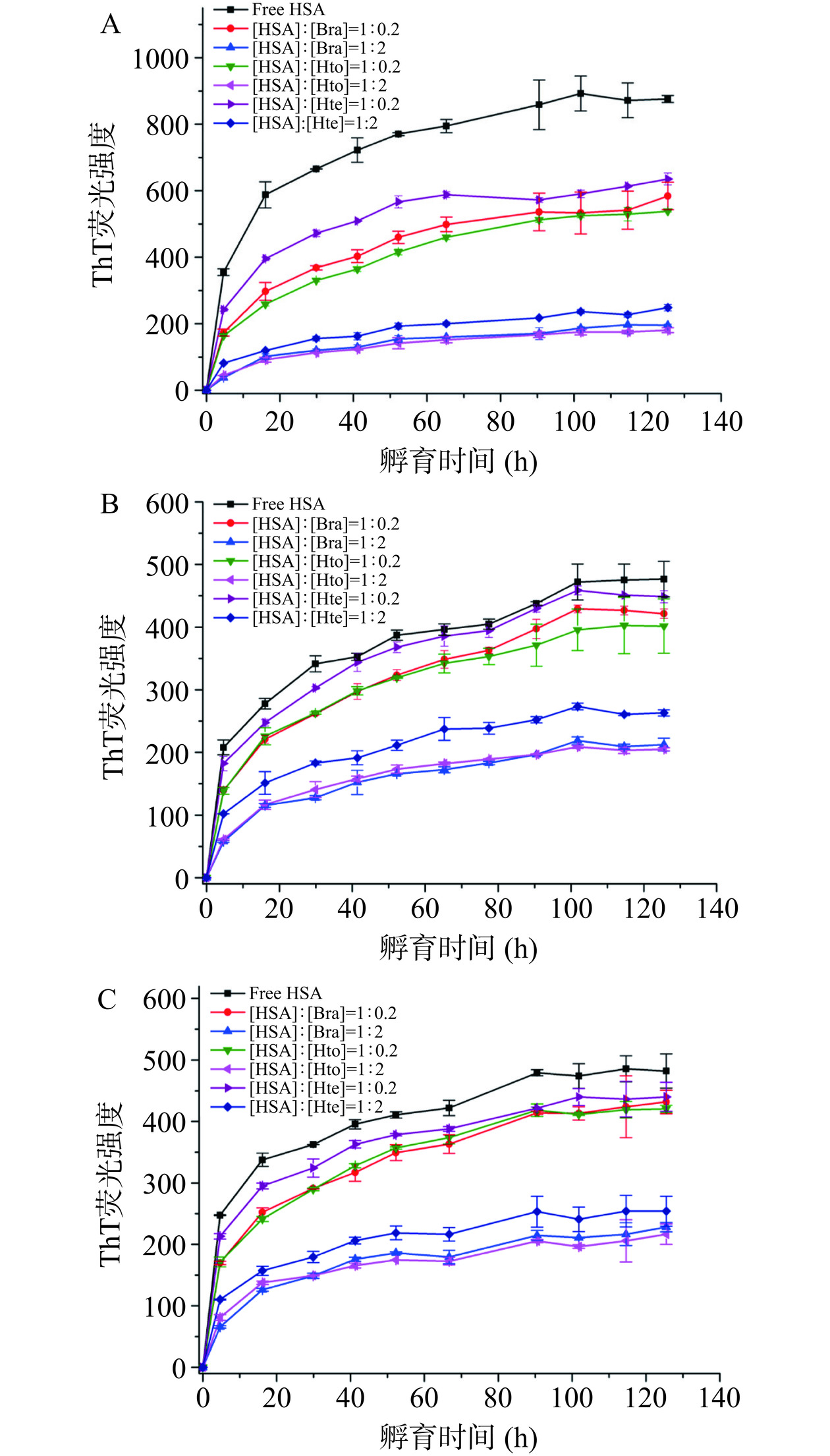
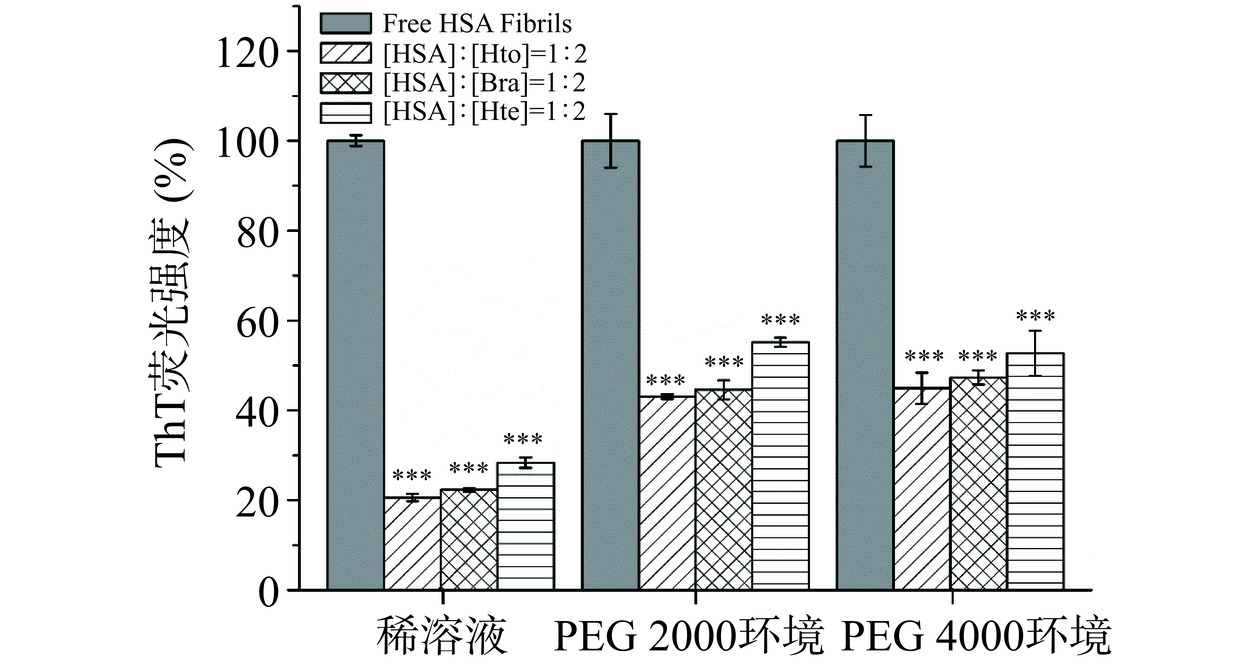
当ThT试剂结合到富含β-折叠结构的淀粉样蛋白纤维上时,其荧光强度会被显著增加。因此,可利用ThT荧光光谱法研究稀溶液环境/拥挤环境下HSA样品的聚集动力学行为以及Bra/Hto/Hte的抑制能力。由图4可知,无论是在稀溶液环境,还是在拥挤环境中,HSA均具有快速聚集的特性,即在纤维形成过程中几乎观察不到成核期的出现,这不同于大多数蛋白质的核依赖性聚集模型(会呈现出S形生长曲线)。可以发现HSA在前20 h内迅速聚集形成原纤维,随后原纤维聚合成成熟的淀粉样纤维(达到平台期),这与之前的研究报道类似[17]。当多酚化合物存在时,样品的ThT荧光强度降低,表明Hto、Bra和Hte的加入会抑制HSA蛋白淀粉样纤维的形成,且抑制能力顺序依次为:Hto>Bra>Hte。这三个多酚化合物抑制能力的不同可能是因为它们结构之间的细微变化导致其与HSA间的结合力存在着差异造成的。Zhang等[25]的研究表明,298 K时Hto-HSA体系和Bra-HSA体系的结合常数分别为(2.813±0.240)×104 mol−1和(2.705±0.055)×104 mol−1,大于Hte-HSA体系的结合常数(2.330±0.017)×104 mol−1),说明Hto/Bra与HSA的结合力强于Hte,从而使得Hto和Bra能更好地去抑制HSA的聚集行为。此外,分析这三种环境中HSA的聚集动力学曲线可知,与稀溶液环境相比,拥挤环境中HSA的ThT荧光强度明显下降,表明拥挤试剂的存在也会抑制HSA蛋白原纤维的形成。以HSA-Hto体系为例,分析拥挤试剂的加入是否会对多酚化合物的抑制能力产生影响。从图5可知,相对于稀溶液环境来说,拥挤环境中Hto降低样品ThT荧光强度的能力会变弱(相对荧光强度从20.6%(稀溶液环境)升为40.0%(PEG 2000拥挤环境)和44.9%(PEG 4000拥挤环境))。HSA-Bra体系和HSA-Hte体系也存在类似的现象。故表明拥挤环境中多酚化合物对HSA纤维化过程的抑制效果没有稀溶液环境中明显,即拥挤环境会降低多酚化合物的抑制能力,这可能与拥挤环境存在着“大分子拥挤效应”(纯物理排斥作用)有关,导致多酚化合物不易与HSA发生相互作用。
![]() 图 5 稀溶液、PEG 2000拥挤环境和PEG 4000拥挤环境中Hto/Bra/Hte存在时HSA纤维的相对ThT荧光强度注:样品孵育时间为125 h;检测体系中CHSA=10 μmol/L;三个体系中单纯HSA纤维的荧光强度设定为100%;***表示与不含Hto/Bra/Hte的HSA样品相比,P<0.001。Figure 5. Relative ThT fluorescence intensity of HSA fibrils with Hto/Bra/Hte in dilute solution, PEG 2000 crowding environment and PEG 4000 crowding environment
图 5 稀溶液、PEG 2000拥挤环境和PEG 4000拥挤环境中Hto/Bra/Hte存在时HSA纤维的相对ThT荧光强度注:样品孵育时间为125 h;检测体系中CHSA=10 μmol/L;三个体系中单纯HSA纤维的荧光强度设定为100%;***表示与不含Hto/Bra/Hte的HSA样品相比,P<0.001。Figure 5. Relative ThT fluorescence intensity of HSA fibrils with Hto/Bra/Hte in dilute solution, PEG 2000 crowding environment and PEG 4000 crowding environment2.2.2 ANS荧光光谱分析
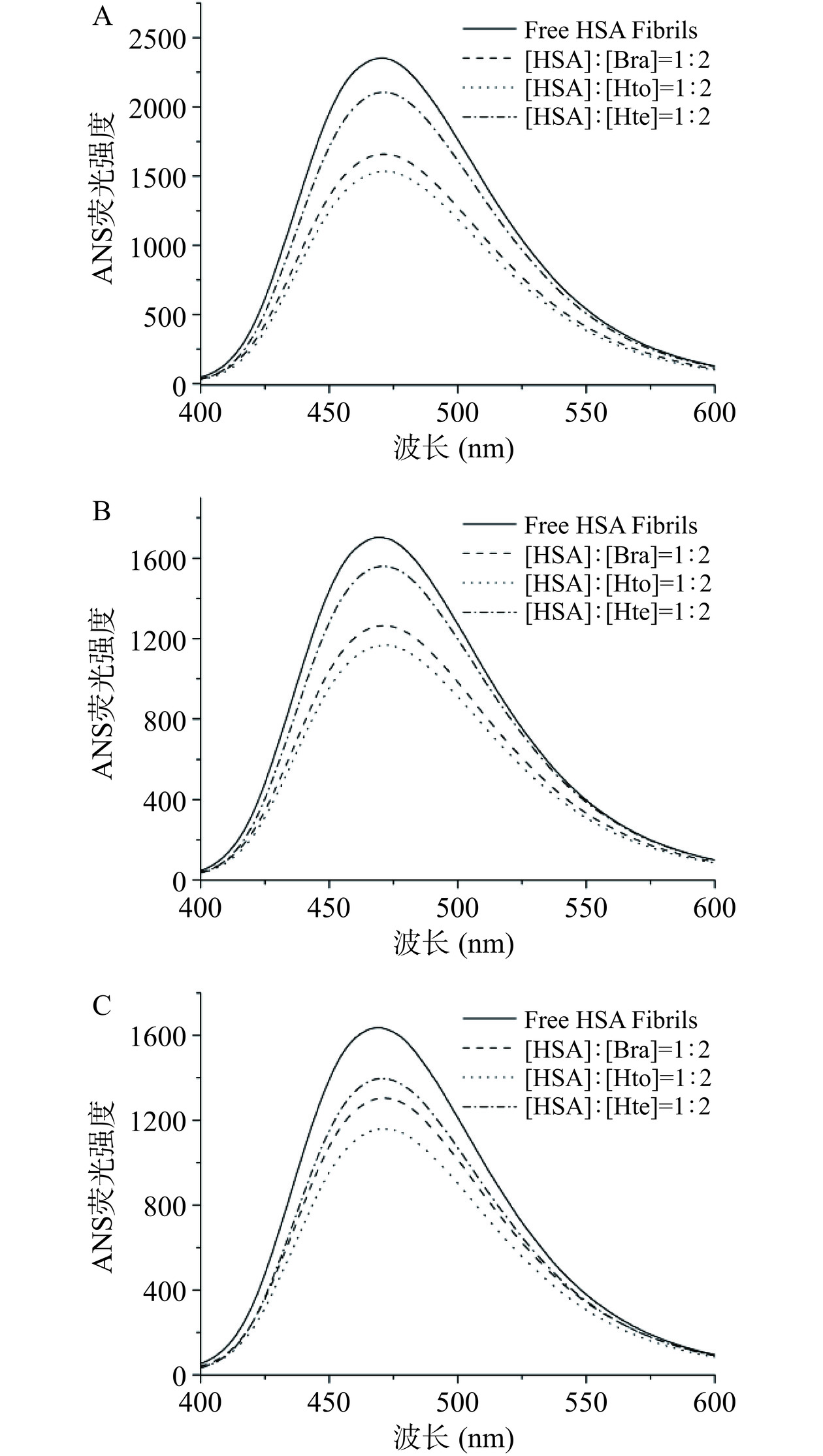
ANS作为一种外源荧光探针,当它以游离形式处在水溶液环境中时其荧光强度非常弱,但是当它与蛋白质的疏水性区域相结合时其荧光强度显著增强[26]。因此,可利用ANS荧光光谱检测拥挤环境下Bra/Hto/Hte对HSA疏水性区域的影响。如图6所示,在这三种溶液环境中,单独HSA淀粉样纤维在470 nm处均能发射出强烈的荧光,表明其疏水性区域的暴露。但是与稀溶液环境相比,PEG 2000/PEG 4000的存在使其荧光强度值明显降低,说明拥挤环境会抑制蛋白质的聚集以及保护部分疏水性区域,这与前面ThT荧光光谱实验结果相一致。此外,对于这三种多酚化合物来说,Hto/Bra/Hte的加入均降低了ANS荧光强度,且Hto和Bra的降低程度更明显,说明这三个小分子可以维持HSA结构的稳定性(降低蛋白质内部疏水区域的暴露)以及有效抑制蛋白质的聚集,且Hto和Bra抑制效果更明显。
2.2.3 动态光散射(DLS)分析
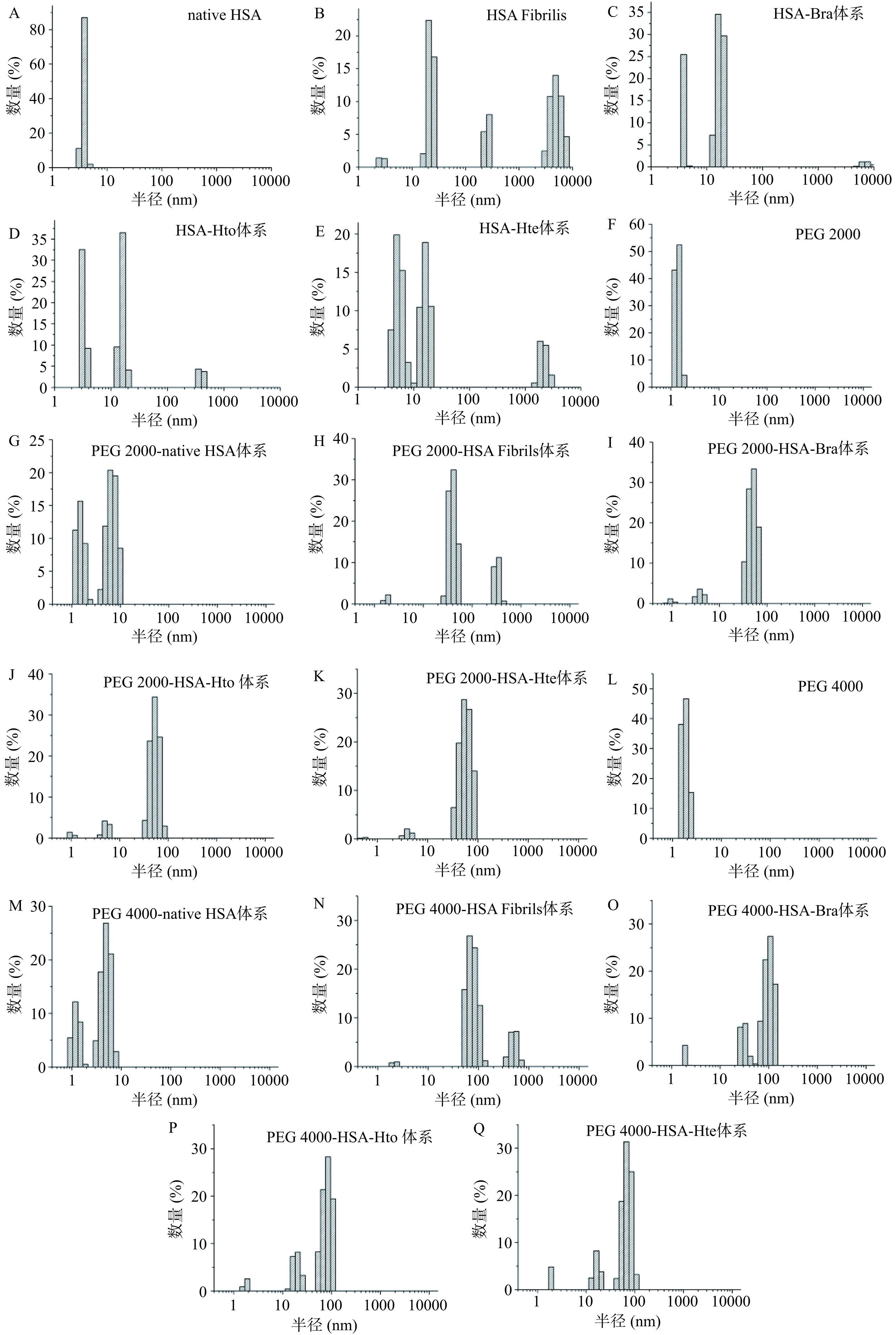
为了研究多酚化合物对HSA聚集体粒径的影响,利用动态光散射技术检测了拥挤环境下HSA样品的粒径分布情况(图7)。如图7A~E所示,在稀溶液环境中,天然HSA的平均水力学半径为3.9 nm。在65 ℃孵育140 h后HSA的平均水力学半径增加至111.9 nm,说明淀粉样蛋白纤维的形成。但当HSA与多酚化合物一起孵育140 h时,其平均水力学半径分别降至8.0(Hto)、10.1(Bra)和14.1 nm(Hte),表明这三个小分子都能改变HSA的聚集途径,使其形成粒径较小的聚集体。此外,当拥挤试剂单独存在时(图7H和7N),其也会导致HSA的平均水力学半径发生降低。对于PEG 2000来说,样品粒径从111.9 nm降至48.7 nm。PEG 4000存在时也会出现类似现象,粒径从111.9 nm降至62.4 nm,这表明稀溶液环境中HSA样品的粒径大于拥挤环境中的粒径,即拥挤试剂的存在也能够抑制HSA淀粉样纤维的形成。此外,在拥挤环境中,Hto/Bra/Hte的加入也会降低HSA样品的粒径,只是下降趋势没有在稀溶液环境中明显(对于PEG 2000来说,从48.7 nm分别降至33.0(Hto)、34.8(Bra)和43.7 nm(Hte);对于PEG 4000来说,从62.4 nm分别降至43.9(Hto)、49.3(Bra)和52.6 nm(Hte)),说明拥挤环境会对多酚化合物的抑制效果产生影响,这可能与“大分子拥挤效应”密切相关。
2.2.4 原子力显微镜(AFM)分析
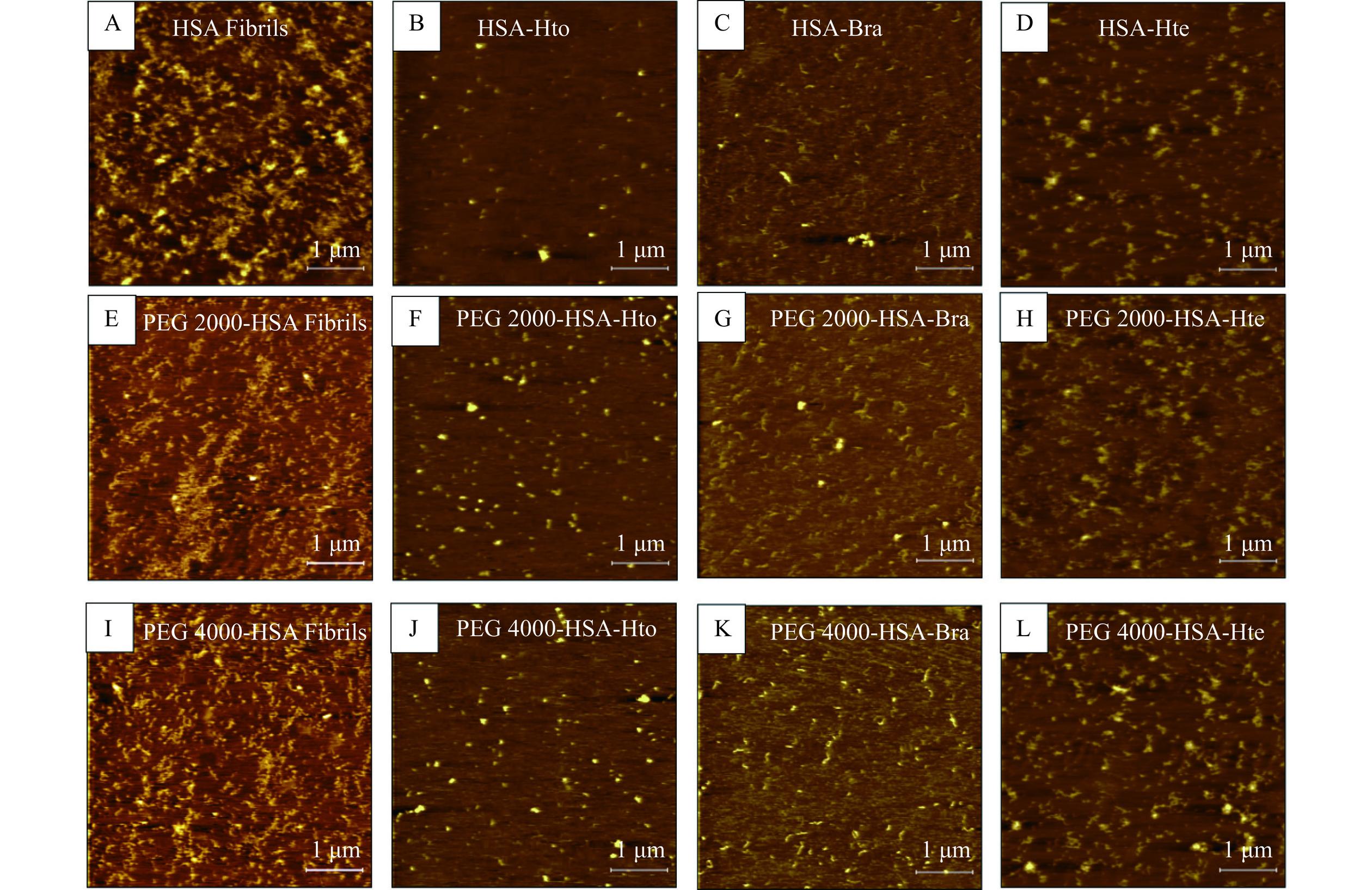
为了进一步验证三种多酚化合物对HSA淀粉样聚集体的抑制作用,利用AFM技术观察HSA纤维的形貌特征(图8)。如图8A所示,HSA发生淀粉样聚集时会形成致密缠结的纤维。而当加入Hto/Bra/Hte后,聚集体的数量减少,长度缩短。其中,Hto的影响是最明显的,即纤维长度最短且非常稀疏,表明Hto具有最优异的抗HSA淀粉样纤维化活性。此外,当样品中存在拥挤试剂时(图8E和8I),与稀溶液环境相比,纤维长度和数量表现为明显的降低,而Hto/Bra/Hte的加入会进一步降低HSA的聚集程度,这与ThT荧光光谱实验以及DLS实验结果相一致。
2.3 拥挤环境下多酚化合物对HSA结构的影响
2.3.1 紫外可见吸收光谱分析
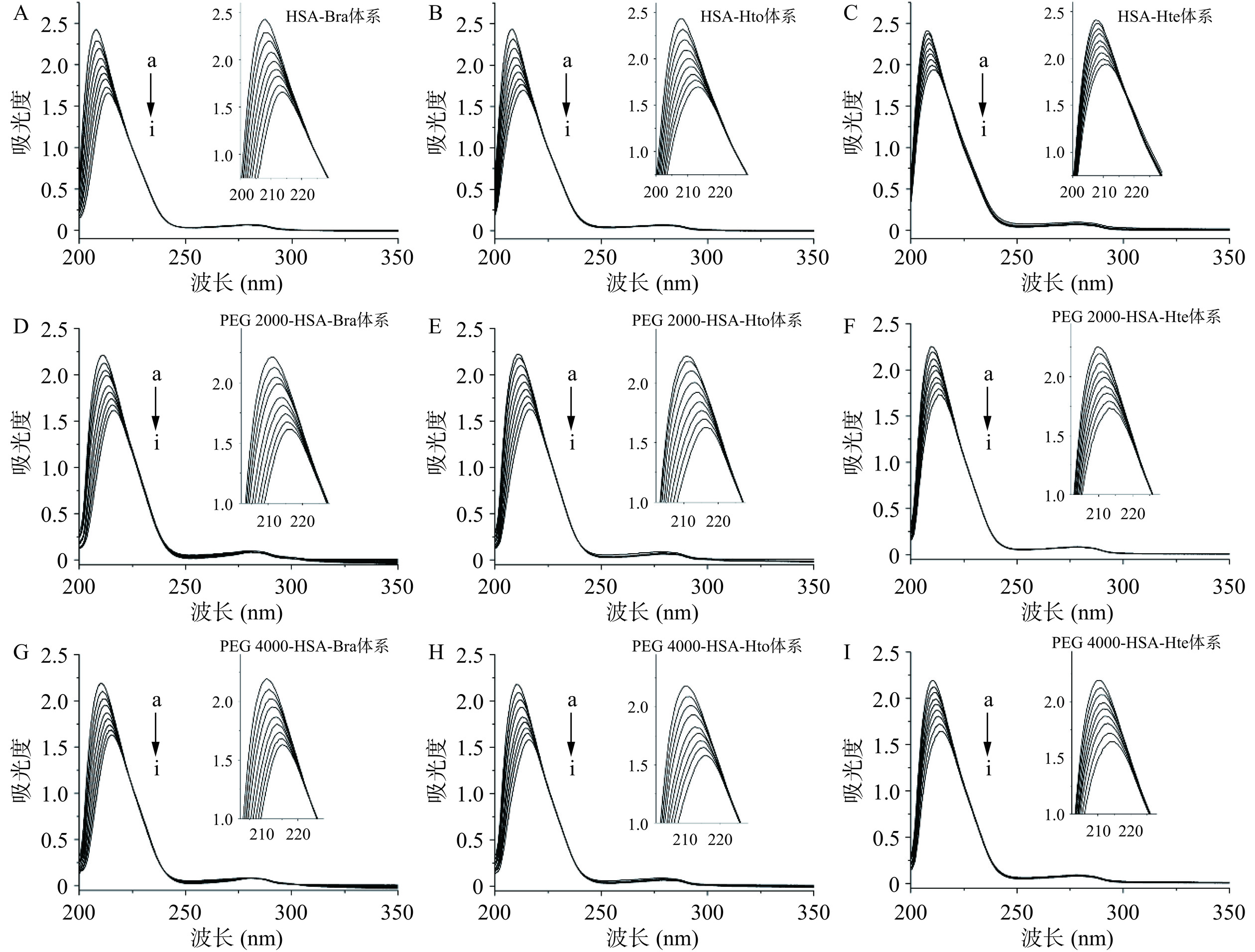
紫外可见吸收光谱法是一种简单实用的技术,可以探究小分子与蛋白质相互作用过程中蛋白质的结构变化以及复合物形成的信息。如图9示,随着多酚化合物的加入,HSA在210 nm附近的吸收峰强度明显降低,峰位置发生红移(见表1),而位于278 nm处的吸收峰基本上没有发生变化,表明无论拥挤试剂是否存在,多酚化合物都会与HSA发生相互作用,且改变HSA的空间结构。从表1可知,与Hte相比,在相同条件下Hto和Bra更容易使HSA的吸光度值降低,同时波长红移更明显,说明Hto和Bra与HSA的结合更加显著。
![]() 图 9 多酚化合物与天然HSA相互作用的紫外可见吸收光谱图注:检测体系中CHSA=2 μmol/L;多酚化合物的浓度(a~i)分别为0、5、10、15、20、25、30、35和40 μmol/L;稀溶液环境下样品的紫外可见吸收光谱图(A~C)引用于参考文献[25]。Figure 9. UV-vis absorption spectra of the interaction between polyphenol compounds and native HSA表 1 Bra/Hto/Hte与HSA相互作用时的紫外可见吸收特征值(λ=210 nm)Table 1. UV-vis absorption characteristic values for the interaction of Bra/Hto/Hte with HSA (λ=210 nm)
图 9 多酚化合物与天然HSA相互作用的紫外可见吸收光谱图注:检测体系中CHSA=2 μmol/L;多酚化合物的浓度(a~i)分别为0、5、10、15、20、25、30、35和40 μmol/L;稀溶液环境下样品的紫外可见吸收光谱图(A~C)引用于参考文献[25]。Figure 9. UV-vis absorption spectra of the interaction between polyphenol compounds and native HSA表 1 Bra/Hto/Hte与HSA相互作用时的紫外可见吸收特征值(λ=210 nm)Table 1. UV-vis absorption characteristic values for the interaction of Bra/Hto/Hte with HSA (λ=210 nm)项目 稀溶液环境 PEG 2000拥挤环境 PEG 4000拥挤环境 HSA-Bra HSA-Hto HSA-Hte HSA-Bra HSA-Hto HSA-Hte HSA-Bra HSA-Hto HSA-Hte △λ(λ40 μmol/L−λ0 μmol/L)(nm) 5.50 5.50 4.00 5.00 5.50 3.50 5.00 6.00 4.00 A40 μmol/L/A0 μmol/L 0.68 0.70 0.80 0.73 0.73 0.77 0.74 0.73 0.75 2.3.2 三维荧光光谱分析
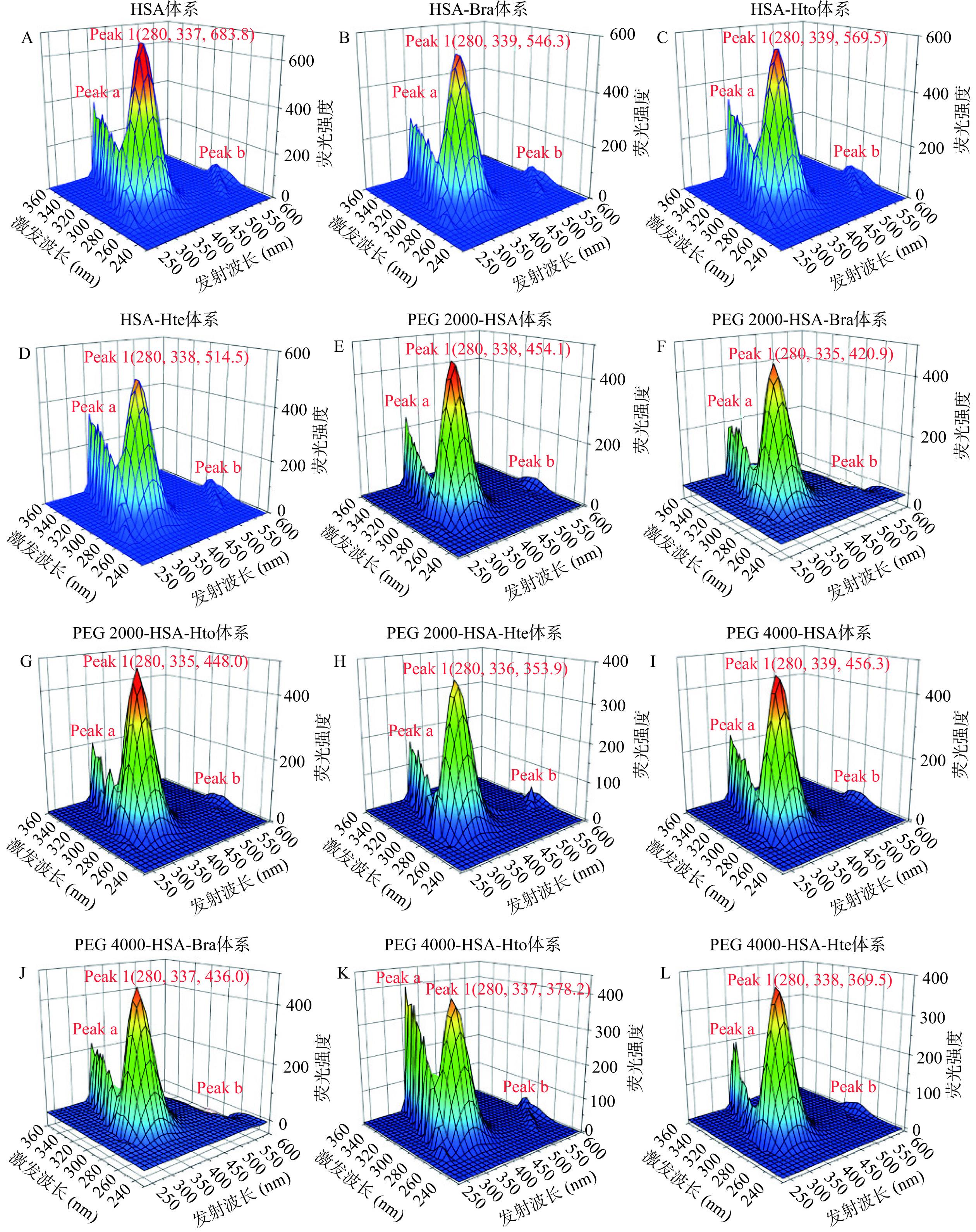
三维荧光光谱法是一种新的荧光分析技术,可为蛋白质结构的研究提供更详细、更全面的信息。图10是拥挤试剂存在和不存在时HSA-Hto/Bra/Hte体系的三维荧光光谱图。从图中可知,其中存在着三个特征峰:Peak 1(色氨酸和酪氨酸残基的荧光光谱特征)、Peak a(瑞利散射峰)和Peak b(二阶瑞利散射峰)。可以看出,无论是在稀溶液环境中还是在拥挤环境中,多酚化合物的加入都会使Peak 1的荧光强度值降低,这说明多酚化合物与HSA发生了相互作用,这与文献[27-28]的研究结果相似。但是对峰位置的变化来说却出现不一致的情况,即在稀溶液环境中Peak 1的峰位置发生了一定的红移,说明多酚化合物的加入导致HSA中色氨酸和酪氨酸残基所处微环境疏水性减小,极性增加,这可能是因为多酚化合物诱导HSA多肽链发生部分展开,促使色氨酸和酪氨酸残基暴露于亲水性环境中。而拥挤环境中峰位置却出现蓝移现象,可能是拥挤试剂存在时产生的“大分子拥挤效应”导致HSA的结构变得更加紧密[29-30],从而使得色氨酸和酪氨酸残基不易暴露于亲水性环境中,即它们所处微环境的疏水性增加。当然,产生这一现象的具体原因还需要后续的研究来进一步的解释。总之,这些结果都表明Hto/Bra/Hte与HSA之间存在着相互作用,从而导致蛋白质的构象发生一定程度的变化,这与前面紫外可见吸收光谱实验结果相一致。
![]() 图 10 稀溶液(A~D)、PEG 2000拥挤环境(E~H)和PEG 4000拥挤环境(I~L)中Bra/Hto/Hte与天然HSA相互作用的三维荧光光谱图注:检测体系中CHSA=2 μmol/L;CBra=CHto=CHte=20 μmol/L;稀溶液环境下样品的三维荧光光谱图(A~D)引用于参考文献[25]。Figure 10. Three-dimensional fluorescence spectra of HSA-Bra/Hto/Hte systems in dilute solution (A~D), PEG 2000 crowding environment (E~H) and PEG 4000 crowding environment (I~L)
图 10 稀溶液(A~D)、PEG 2000拥挤环境(E~H)和PEG 4000拥挤环境(I~L)中Bra/Hto/Hte与天然HSA相互作用的三维荧光光谱图注:检测体系中CHSA=2 μmol/L;CBra=CHto=CHte=20 μmol/L;稀溶液环境下样品的三维荧光光谱图(A~D)引用于参考文献[25]。Figure 10. Three-dimensional fluorescence spectra of HSA-Bra/Hto/Hte systems in dilute solution (A~D), PEG 2000 crowding environment (E~H) and PEG 4000 crowding environment (I~L)3. 结论
本研究分析了拥挤试剂种类和浓度对HSA构象的影响,发现100 g/L的大分子拥挤试剂PEG 2000和PEG 4000对蛋白质结构的影响最小,因此选择这两种拥挤试剂来构建模拟体内拥挤环境。在稀溶液环境和大分子拥挤环境中,Hto/Bra/Hte均可以有效地抑制HSA成熟纤维的形成,且Hto和Bra对HSA聚集的抑制效果要比Hte更明显。同时,拥挤试剂单独存在时会对HSA的淀粉样纤维聚集产生破坏作用,但是在拥挤环境中,这三个多酚化合物的抑制能力反而弱于稀溶液环境中的作用,表明“大分子拥挤效应”会影响它们的抑制活性。此外,紫外可见吸收光谱和三维荧光光谱实验表明,三种多酚化合物都能够与HSA发生相互作用,形成新的复合物,从而改变蛋白质的空间结构。总之,本研究为多酚化合物真实生物活性(特别是抗蛋白聚集能力)的探究提供了有益的参考。为了更加准确地揭示这类抑制剂在体内的药理作用,未来设计合理的动物模型开展相关的实验是非常有必要的。
-
图 5 稀溶液、PEG 2000拥挤环境和PEG 4000拥挤环境中Hto/Bra/Hte存在时HSA纤维的相对ThT荧光强度
注:样品孵育时间为125 h;检测体系中CHSA=10 μmol/L;三个体系中单纯HSA纤维的荧光强度设定为100%;***表示与不含Hto/Bra/Hte的HSA样品相比,P<0.001。
Figure 5. Relative ThT fluorescence intensity of HSA fibrils with Hto/Bra/Hte in dilute solution, PEG 2000 crowding environment and PEG 4000 crowding environment
图 9 多酚化合物与天然HSA相互作用的紫外可见吸收光谱图
注:检测体系中CHSA=2 μmol/L;多酚化合物的浓度(a~i)分别为0、5、10、15、20、25、30、35和40 μmol/L;稀溶液环境下样品的紫外可见吸收光谱图(A~C)引用于参考文献[25]。
Figure 9. UV-vis absorption spectra of the interaction between polyphenol compounds and native HSA
图 10 稀溶液(A~D)、PEG 2000拥挤环境(E~H)和PEG 4000拥挤环境(I~L)中Bra/Hto/Hte与天然HSA相互作用的三维荧光光谱图
注:检测体系中CHSA=2 μmol/L;CBra=CHto=CHte=20 μmol/L;稀溶液环境下样品的三维荧光光谱图(A~D)引用于参考文献[25]。
Figure 10. Three-dimensional fluorescence spectra of HSA-Bra/Hto/Hte systems in dilute solution (A~D), PEG 2000 crowding environment (E~H) and PEG 4000 crowding environment (I~L)
表 1 Bra/Hto/Hte与HSA相互作用时的紫外可见吸收特征值(λ=210 nm)
Table 1 UV-vis absorption characteristic values for the interaction of Bra/Hto/Hte with HSA (λ=210 nm)
项目 稀溶液环境 PEG 2000拥挤环境 PEG 4000拥挤环境 HSA-Bra HSA-Hto HSA-Hte HSA-Bra HSA-Hto HSA-Hte HSA-Bra HSA-Hto HSA-Hte △λ(λ40 μmol/L−λ0 μmol/L)(nm) 5.50 5.50 4.00 5.00 5.50 3.50 5.00 6.00 4.00 A40 μmol/L/A0 μmol/L 0.68 0.70 0.80 0.73 0.73 0.77 0.74 0.73 0.75 -
[1] PINNEY J H, HAWKINS P N. Amyloidosis[J]. Annals of Clinical Biochemistry,2012,49:229−241. doi: 10.1258/acb.2011.011225
[2] ANAND B G, PRAJAPATI K P, PUROHIT S, et al. Evidence of anti-amyloid characteristics of plumbagin via inhibition of protein aggregation and disassembly of protein fibrils[J]. Biomacromolecules,2021,22(9):3692−3703. doi: 10.1021/acs.biomac.1c00344
[3] CIUDAD S, PUIG E, BOTZANOWSKI T, et al. Aβ (1-42) tetramer and octamer structures reveal edge conductivity pores as a mechanism for membrane damage[J]. Nature Communications,2020,11(1):14. doi: 10.1038/s41467-019-13635-y
[4] HUGHES C, CHOI M L, YI J H, et al. Aβ amyloid aggregates induce sensitized TLR4 signaling causing long-term potentiation deficit and rat neuronal cell death[J]. Communications Biology,2020,3(1):7. doi: 10.1038/s42003-019-0737-3
[5] HARD T, LENDEL C. Inhibition of amyloid formation[J]. Journal of Molecular Biology,2012,421(4-5):441−465. doi: 10.1016/j.jmb.2011.12.062
[6] SUNNY L P, SRIKANTH P, SUNITHA A K, et al. Tryptophan-cardanol fluorescent nanoparticles inhibit α-synuclein aggregation and disrupt amyloid fibrils[J]. Journal of Peptide Science,2022,28(4):3374. doi: 10.1002/psc.3374
[7] GANCAR M, KURIN E, BEDNARIKOVA Z, et al. Green tea leaf constituents inhibit the formation of lysozyme amyloid aggregates:An effect of mutual interactions[J]. International Journal of Biological Macromolecules,2023,242:9.
[8] LERI M, CHAUDHARY H, IASHCHISHYN I, et al. Natural compound from olive oil inhibits S100A9 amyloid formation and cytotoxicity:Implications for preventing Alzheimer's disease[J]. ACS Chemical Neuroscience,2021,12(11):1905−1918. doi: 10.1021/acschemneuro.0c00828
[9] SHARIATIZI S, MERATAN A, GHASEMI A, et al. Inhibition of amyloid fibrillation and cytotoxicity of lysozyme fibrillation products by polyphenols[J]. International Journal of Biological Macromolecules,2015,80:95−106. doi: 10.1016/j.ijbiomac.2015.06.030
[10] JUAREZ J, TABOADA P, MOSQUERA V. Existence of different structural intermediates on the fibrillation pathway of human serum albumin[J]. Biophysical Journal,2009,96(6):2353−2370. doi: 10.1016/j.bpj.2008.12.3901
[11] TABOADA P, BARBOSA S, CASTRO E, et al. Amyloid fibril formation and other aggregate species formed by human serum albumin association[J]. Journal of Physical Chemistry B,2006,110(42):20733−20736. doi: 10.1021/jp064861r
[12] COLLINS S R, DOUGLASS A, VALE R D, et al. Mechanism of prion propagation:Amyloid growth occurs by monomer addition[J]. PLoS Biology,2004,2(10):1582−1590.
[13] SHARMA N, SIVALINGAM V, MAURYA S, et al. New insights into in vitro amyloidogenic properties of human serum albumin suggest considerations for therapeutic precautions[J]. Febs Letters,2015,589(24):4033−4038.
[14] CHAUDHURI P, PRAJAPATI K P, ANAND B G, et al. Amyloid cross-seeding raises new dimensions to understanding of amyloidogenesis mechanism[J]. Ageing Research Reviews,2019,56:18.
[15] GAUDREAULT R, MOUSSEAU N. Mitigating Alzheimer's disease with natural polyphenols:A review[J]. Current Alzheimer Research,2019,16(6):529−543. doi: 10.2174/1567205016666190315093520
[16] GHOSH P, DE P. Modulation of amyloid protein fibrillation by synthetic polymers:Recent advances in the context of neurodegenerative diseases[J]. ACS Applied Biomaterials,2020,3(10):6598−6625. doi: 10.1021/acsabm.0c01021
[17] ALI M S, AL-LOHEDAN H A, TARIQ M, et al. Modulation of amyloid fibril formation of plasma protein by saffron constituent "safranal":Spectroscopic and imaging analyses[J]. International Journal of Biological Macromolecules,2019,127:529−535. doi: 10.1016/j.ijbiomac.2019.01.052
[18] BHATTACHARYA S, PANDEY N K, ROY A, et al. Effect of (-)-epigallocatechin gallate on the fibrillation of human serum albumin[J]. International Journal of Biological Macromolecules,2014,70:312−319. doi: 10.1016/j.ijbiomac.2014.07.003
[19] COOKSEY C J. Hematoxylin in the 21st century[J]. Biotechnic & Histochemistry,2021,96(3):242−249.
[20] YIN H H, HAN Y L, YAN X, et al. Hematoxylin modulates tau-RD protein fibrillization and ameliorates Alzheimer's disease-like symptoms in a yeast model[J]. International Journal of Biological Macromolecules,2023,250:126140. doi: 10.1016/j.ijbiomac.2023.126140
[21] TU Y L, MA S, LIU F F, et al. Hematoxylin inhibits amyloid β-protein fibrillation and alleviates amyloid-induced cytotoxicity[J]. Journal of Physical Chemistry B,2016,120(44):11360−11368. doi: 10.1021/acs.jpcb.6b06878
[22] RIVAS G, FERRONE F, HERZFELD J. Life in a crowded world[J]. EMBO Reports,2004,5(1):23−27. doi: 10.1038/sj.embor.7400056
[23] ELLIS R J. Macromolecular crowding:Obvious but underappreciated[J]. Trends in Biochemical Sciences,2001,26(10):597−604. doi: 10.1016/S0968-0004(01)01938-7
[24] GORENSEK-BENITEZ A H, KIRK B, MYERS J K. Protein fibrillation under crowded conditions[J]. Biomolecules,2022,12(7):21.
[25] ZHANG C Y, GUAN J, ZHANG J X, et al. Protective effects of three structurally similar polyphenolic compounds against oxidative damage and their binding properties to human serum albumin[J]. Food Chemistry,2021,349:10.
[26] MAJID N, SIDDIQI M K, ALAM A, et al. Cholic acid inhibits amyloid fibrillation:Interplay of protonation and deprotonation[J]. International Journal of Biological Macromolecules,2022,221:900−912. doi: 10.1016/j.ijbiomac.2022.09.019
[27] ZIELENKIEWICZ W, SWIERZEWSKI R, ATTANASIO F, et al. Thermochemical, volumetric and spectroscopic properties of lysozyme-poly (ethylene) glycol system[J]. Journal of Thermal Analysis and Calorimetry,2006,83(3):587−595. doi: 10.1007/s10973-005-7417-x
[28] DAI J H, CHEN C, YIN M, et al. Interactions between gold nanoparticles with different morphologies and human serum albumin[J]. Frontiers in Chemistry,2023,11:15.
[29] JIA J, WANG Y X, LIU Y Y, et al. Exploration of interaction of canthaxanthin with human serum albumin by spectroscopic and molecular simulation methods[J]. Luminescence,2018,33(2):425−432. doi: 10.1002/bio.3430
[30] OTA C, TAKANO K. Behavior of bovine serum albumin molecules in molecular crowding environments investigated by raman spectroscopy[J]. Langmuir,2016,32(29):7372−7382. doi: 10.1021/acs.langmuir.6b01228





 下载:
下载:
 下载:
下载:



