Research Progress of the Anti-inflammatory Effects of Honey
-
摘要: 蜂蜜是由蜜蜂生产的天然产物,长期以来作为食品在世界范围内被人们普遍食用。蜂蜜具有抗炎作用,这与蜂蜜的抗菌和抗氧化功能是密切相关的。对蜂蜜抗炎功能的探究涉及两个方面:一方面基础研究探索蜂蜜生物学功能的分子机制并明确有效成分;另一方面则通过临床研究评估蜂蜜对炎症性疾病的改善效果。基础研究揭示了蜂蜜发挥抗炎作用的关键有效成分,并进一步阐明了相关的分子机制。蜂蜜的抗炎功能与其含有的黄酮类化合物、酚酸、葡萄糖氧化酶、过氧化氢和甲基乙二醛密切相关,这些有效成分主要通过调控免疫细胞功能、调节炎症细胞因子水平、抑制致病菌生长和抗氧化而发挥作用。蜂蜜能减轻消化道炎症、改善肠道菌群、缓解慢性鼻窦炎症状以及预防和治疗放射性口腔炎。本文以近5年蜂蜜相关的研究为主,综述了蜂蜜抗炎作用的机制和关键有效成分的功能,以及目前蜂蜜在炎症性疾病领域的应用。Abstract: Honey is a natural substance produced by honeybees and is widely consumed as food worldwide. Honey has anti-inflammatory effects, which are related to its antibacterial and antioxidant functions. The anti-inflammatory properties of honey can be explored from two aspects: Basic research studies that reveal the molecular mechanisms underlying the biological functions of honey and identify its effective components, and clinical research studies that evaluate the healing effects of honey in inflammatory diseases. Basic research studies are indicated that honey has anti-inflammatory properties and further elucidated the relevant molecular mechanisms. The anti-inflammatory functions of honey are highly related to its components, specifically flavonoids, phenolic acids, glucose oxidase, hydrogen peroxide, and methylglyoxal. These effective constituents exhibit anti-inflammatory effects by regulating immune cell function, affecting inflammatory cytokines, inhibiting the growth of pathogenic bacteria, and acting as antioxidants. Honey can attenuate gastrointestinal inflammation, aid the recovery of intestinal microbiota, relieve symptoms of chronic sinusitis, and prevent radiation-induced oral mucositis. Based on honey-related basic and clinical research studies conducted over the past five years, this article reviews the anti-inflammatory mechanisms of honey, the functions of its effective constituents, and the applications of honey in inflammatory diseases.
-
Keywords:
- honey /
- anti-inflammatory activity /
- mechanism /
- components /
- inflammatory diseases
-
蜂蜜是由蜜蜂从植物中采集花蜜后,经添加蜜蜂分泌物并经充分酿造储存在巢房中的天然甜味物质,长期以来被应用于食品、药品领域[1]。蜂蜜中大约含有200种成分,其中主要成分是糖类和水,此外含有蛋白质、氨基酸、维生素、矿物质、生物酶和酚类化合物[2]。蜂蜜中的单糖和二糖约占其干重的95%,其中果糖和葡萄糖的含量最高[3]。蜂蜜中氨基酸比重约占0.5%,其中脯氨酸、精氨酸、谷氨酸、半胱氨酸和天冬氨酸等含量较高[3−4]。蜂蜜中的蛋白质主要以酶的形式存在,包括葡萄糖转化酶、葡萄糖氧化酶、α-葡萄糖苷酶、淀粉酶和过氧化氢酶等[5−6]。蜂蜜具有抗炎、抗癌、免疫调节和神经保护等多种生物学活性,其中抗炎作用是其重要的生物学功能之一[7]。近年来,针对蜂蜜的抗炎功能,研究者开展了一系列基础和临床研究,逐步揭示蜂蜜的抗炎作用机制,并推动其在临床和保健领域的应用。
炎症是一种复杂的免疫过程,能清除病原体或修复受损组织。然而,巨噬细胞或其他免疫细胞的过度活跃会导致炎症失控,可能造成组织损伤和器官衰竭[8]。外源性因素如病原体感染等,通常引起急性炎症,常见表现为红肿、发热和疼痛;内源性因素引发的慢性炎症,持续时间则相对较长,最终可能导致组织损伤或纤维化[9]。蜂蜜通过调控免疫细胞功能和炎症信号通路发挥抗炎作用。蜂蜜通过影响炎症信号受体的活化过程和炎症信号转录因子的功能,进而调控一系列炎症细胞因子和炎症介质的水平,最终影响炎症反应过程[10−11]。蜂蜜也能通过其抗菌和抗氧化作用发挥抗炎功能。细菌感染经常导致急性炎症,蜂蜜中的过氧化氢和防御素-1等成分能直接作用于致病菌,抑制细菌生长,减轻炎症反应[12]。蜂蜜通过调控细胞氧化还原信号和直接清除ROS等自由基,降低氧化应激水平,进而减轻慢性炎症及其造成的组织损伤[13]。
本文基于蜂蜜调节免疫细胞功能、调控炎症信号通路、抑制致病菌生长和调节氧化应激几个方面,综述了蜂蜜抗炎作用的机制,并进一步对蜂蜜中发挥抗炎作用的关键有效成分进行了梳理,阐明各成分的功能机理,论述了其在医药和保健领域的应用潜力,为蜂蜜生物学功能机制研究和应用研究提供了理论依据。
1. 蜂蜜抗炎作用的相关机制
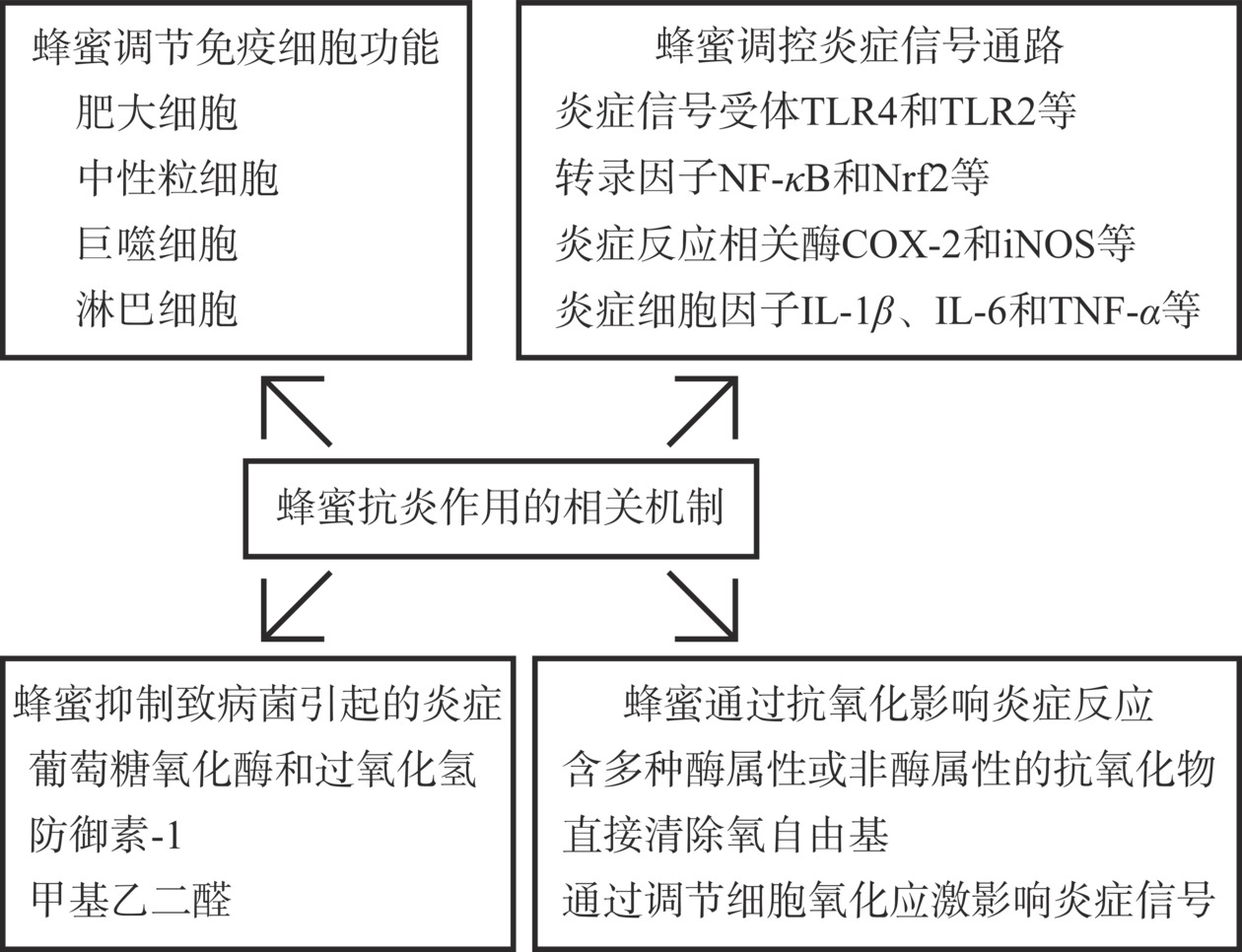
炎症反应引起免疫细胞的募集、白细胞浸润、炎症细胞因子释放、血流速度加快和毛细血管通透性增加[14]。细胞表面受体接受炎症信号后,细胞内启动炎症信号通路级联反应,调控细胞内蛋白质功能或启动下游靶基因转录,促进一系列炎症细胞因子的释放。释放的炎症因子进一步作用于附近的细胞,引起炎症的扩大[14−15]。蜂蜜通过受体脱敏、转录因子失活等机制抑制炎症信号通路,影响免疫细胞功能,并通过其抗菌和抗氧化作用,多种途径抑制炎症反应(图1)。
1.1 蜂蜜调节免疫细胞功能
蜂蜜通过调控多种免疫细胞功能进而抑制炎症反应。蜂蜜抑制肥大细胞脱颗粒过程,抑制组胺和β-己糖苷酶的释放,减轻炎症反应[16−17]。蜂蜜抑制中性粒细胞超氧化物的释放,调节其炎症细胞因子、趋化因子和基质降解酶的水平,降低其趋化性和迁移能力,抑制炎症区域内中性粒细胞的募集,缓解炎症反应对周围组织的损伤[18−20]。体外实验表明,蜂蜜对中性粒细胞的调控作用呈现浓度依赖性[20]。蜂蜜调控巨噬细胞的成熟过程和功能,在生理状态下,蜂蜜对巨噬细胞的活力和吞噬能力无抑制作用,并通过降低细胞内Caspase活性和改善线粒体呼吸作用而抑制吞噬细胞的凋亡。然而,在脂多糖(Lipopolysaccharide,LPS)诱导的强烈炎症反应下,蜂蜜抑制巨噬细胞的吞噬能力,并抑制单核细胞向巨噬细胞的转化,减轻炎症反应[21]。蜂蜜对人淋巴细胞发挥遗传保护作用,减轻X射线辐射和四氯化碳对人外周血淋巴细胞造成的基因损伤,这与蜂蜜的抗氧化作用是相关的[22−23]。
蜂蜜提取物也能调节免疫细胞功能。蜂蜜中提取的多糖,能激活小鼠淋巴结中的树突状细胞,刺激其表达主要组织相容性复合物-I(Major Histocompatibility Complex,MHC-I)和MHC-II,并提高小鼠脾脏CD3e+/CD4+和CD3e+/CD8+T细胞比例[24]。蜂蜜多糖能减轻环磷胺对小鼠免疫系统的损伤,诱导T淋巴细胞和B淋巴细胞增殖和活化,保护小鼠脾脏和胸腺[25−26]。蜂蜜的酚类提取物能保护淋巴细胞免受氧化损伤,通过螯合铁离子,维持小鼠淋巴细胞功能[27]。
1.2 蜂蜜调控炎症信号通路
蜂蜜通过调控炎症反应信号通路,影响一系列炎症细胞因子表达水平。蜂蜜通过对核因子κB(Nuclear Factor-Kappab,NF-κB)活力的抑制作用降低血清中白介素-1β(Interleukin-1β,IL-1β)和IL-6水平,并上调IL-10水平,进而发挥抗炎作用[28]。对于大肠杆菌诱导的细胞炎症反应,蜂蜜降低IL-8、Toll样受体2(Toll Like Receptor 2,TLR2)和髓样分化初级反应因子88(Myeloid Differentiation Primary Response Gene 88,MyD 88)的表达水平,抑制TLR2/MyD88复合物的形成,下调丝裂原活化蛋白激酶(Mitogen-activated Protein Kinase,MAPK)磷酸化水平,下调IL-8等炎症细胞因子水平,抑制炎症反应[29]。蜂蜜降低结肠炎性细胞因子IL-6和肿瘤坏死因子-α(Tumour Necrosis Factor alpha, TNF-α)水平,降低拟杆菌、棒状杆菌和变形杆菌丰度,缓解葡聚糖硫酸钠诱导的大鼠结肠炎[30]。蜂蜜提取物激活核因子E2相关因子2(Nuclear Factor Erythroid 2-related Factor 2,Nrf2)转录活性,上调Nrf2/HO-1信号通路,并通过下调NFκB抑制因子(Inhibitor of NF-kappaB,I-κB)磷酸化水平,抑制NF-κB的活化及下游信号通路,降低IL-1β、TNF-α和MCP-1表达水平,发挥抗炎作用[31]。
蜂蜜通过调控炎症反应相关酶的活性,进而减轻炎症。前列腺素在炎症反应过程中介导血管舒张,提高血管通透性,促进白细胞穿透血管并增强炎症反应。蜂蜜通过抑制前列腺素合成过程的关键酶,环氧化酶-2(Cyclooxygenase-2,COX-2)的活力,降低前列腺素的合成,抑制炎症反应[32]。在炎症反应过程中,经常出现诱导型一氧化氮合酶(Inducible Nitric Oxide Synthase,iNOS)上调,导致一氧化氮(Nitric Oxide,NO)水平升高,进而导致炎症反应增强。蜂蜜通过抑制TLR4/NF-κB信号通路,降低NF-κB活性,进而下调iNOS的转录过程,降低NO,抑制炎症反应[33−34]。蜂蜜通过抑制TNF-α转化酶的活力降低炎症信号TNF-α表达水平,抑制炎症反应[19]。
1.3 蜂蜜抑制致病菌生长
致病菌过度生长是引起炎症的重要因素之一,如幽门螺旋菌引起胃炎,大肠杆菌引起肠炎,金黄色葡萄球菌导致肺炎。蜂蜜有效抑制多种致病菌生长,减轻致病菌引起的感染。蜂蜜对大肠杆菌、产气肠杆菌、鼠伤寒沙门氏菌、化脓性链球菌、变形链球菌、铜绿假单胞菌、金黄色葡萄球菌均表现出抑制作用[12,35]。
蜂蜜中的葡萄糖氧化酶将葡萄糖转化为葡萄糖酸内酯,并在反应过程中形成过氧化氢,引起致病菌DNA氧化损伤进而死亡。在此过程中,过氧化氢以缓慢且连续的方式释放,既有效抑制了细菌的生长,同时不会对组织造成伤害[35−36]。致病菌形成的生物膜能保护细菌群落,防止抗菌药物的渗透并抵抗宿主免疫防御,导致致病菌顽固性感染。蜂蜜中含有的抗菌多肽防御素-1,通过抑制层粘连蛋白、弹性蛋白和纤维蛋白原表达,破坏细菌生物膜,进而抑制耐甲氧西林金黄色葡萄球菌、大肠杆菌和艰难梭菌等多种致病菌的生长[37−38]。
蜂蜜作为食品,食用后能抑制消化道致病菌的生长。此外,蜂蜜也可用于外伤,加速创口愈合和预防细菌感染。不同类型的蜂蜜抗菌效果存在一定差异。多数蜂蜜在处理体液溢出量较大的伤口时,由于其含有的过氧化氢被血液、组织液中的过氧化氢酶(Catalase,CAT)清除,会导致其抗菌功能降低。而麦卢卡蜂蜜由于甲基乙二醛含量较高且不易被体液中和,表现出更好的抗菌效果[39]。
1.4 蜂蜜通过抗氧化作用调节炎症反应
氧化应激引起炎症反应。细胞内活性氧(Reactive Oxygen Species,ROS)水平升高导致氧化应激,进而活化多种炎症相关的转录因子,如NF-κB、Nrf2和缺氧诱导因子-1α(Hypoxia-inducible Factor-1α,HIF-1α)等,激活炎症信号通路,导致一系列炎性细胞因子、趋化因子等水平升高[40]。ROS激活凋亡信号调节激酶1(Apoptosis Signal-regulating Kinase-1,ASK1),进而活化MAPK和c-Jun氨基末端激酶(c-Jun N-terminal kinase, JNK)信号通路,促进炎症反应[41]。细胞内高水平ROS也会导致部分酶的活性异常,如过氧化还原酶2(Peroxiredoxin 2,PRDX2)。PRDX2激活巨噬细胞产生并释放TNF-α,促进炎症级联反应[42]。蜂蜜的抗氧化作用能降低ROS水平,进而减轻炎症反应。
有文献采用DPPH自由基清除检测法评估了13种蜂蜜的抗氧化作用,包括麦卢卡蜂蜜、荞麦蜂蜜、油菜蜂蜜等,13种蜂蜜均表现出抗氧化活性,其中荞麦蜂蜜抗氧化活性最高,其对DPPH自由基的清除率达到53.11%,蜜露蜂蜜和麦卢卡蜂蜜对DPPH自由基的清除率分别达到41.94%和34.02%,阿拉伯树胶蜂蜜抗氧化活性最低,不同类型蜂蜜的抗氧化活性与蜂蜜中酚酸和黄酮类化合物的总含量呈正相关[43]。
蜂蜜通过下调氧化应激水平抑制炎症反应。麦卢卡蜂蜜激活AMPK/Nrf2/ARE信号通路,上调超氧化物歧化酶(Superoxide Dismutase,SOD)和CAT表达水平,进而保护细胞线粒体功能,降低成纤维细胞细胞氧化损伤水平[44]。蜂蜜多酚提取物抑制ROS诱导的细胞内JNK和IKK-β信号通路的激活,下调胰岛素受体底物1(Insulin Substrate,IRS-1)307位丝氨酸和蛋白激酶B(Protein Kinase B,PKB)473位丝氨酸磷酸化水平,降低IL-1β、IL-6和TNF-α水平,抑制细胞炎症反应[45]。口服蜂蜜提高大鼠肝脏和肾脏抗氧化酶SOD、CAT和谷胱甘肽过氧化物酶(Glutathione Peroxidase,GPx)活力,降低ROS和丙二醛水平,下调MyD88/IKKβ/NF-κB信号通路,抑制丙氨酸转氨酶和天冬氨酸转氨酶活力,减轻炎症并缓解炎症对内脏的损伤[46]。产自中国秦岭地区的中华蜜蜂蜂蜜(A. cerana honey)能有效预防小鼠急性酒精性肝损伤,提高血清抗氧化物水平,显著抑制血清和肝脏中转化生长因子β1(Transforming Growth Factor-β1,TGF-β1)水平,具有良好的保肝效果[3]。
2. 蜂蜜中发挥抗炎作用的主要成分
蜂蜜的抗炎症作用与其所含的酚类化合物和甲基乙二醛密切相关。蜂蜜中的酚类化合物主要为两种类型,即黄酮类化合物和酚酸,通过调控一系列炎症相关的转录因子和细胞因子,影响免疫细胞功能,调节炎症反应过程蜂蜜中的黄酮类化合物主要包括槲皮素、芹菜素、白杨素、高良姜素、芦丁和山奈酚等[3]。黄酮类化合物调控一系列炎症相关的细胞信号通路,调节TLR4和TLR2等炎症信号受体的激活,影响NF-κB和Nrf2等转录因子的活性,影响TNF-α、IL-1β、IL-6和IL-17等炎症细胞因子的释放,调节前列腺素等炎症介质的水平[1,3]。蜂蜜中的部分黄酮类化合物经肠道吸收后,能发挥全身性的抗炎作用,其中槲皮素、芦丁等,能直接穿透血脑屏障,进而对中枢神经系统发挥抗炎作用[47−48]。蜂蜜中黄酮类化合物的抗炎作用机制如表1所示。甲基乙二醛在麦卢卡蜂蜜中含量高,其自身的化学性质使之能与精氨酸、半胱氨酸等氨基酸残基发生反应,改变蛋白质化学键结构,影响炎症信号通路[49]。
表 1 蜂蜜中黄酮类化合物的抗炎作用机制Table 1. Anti-inflammatory mechanisms of flavonoids in honey成分 抗炎作用机制 槲皮素 槲皮素通过抑制TLR4/MyD88/NF-κB信号通路,减轻小鼠肠道和胶质细胞炎症,通过抑制TLR2信号通路限制NLRP3炎症小体的活
化[51−53]。槲皮素抑制肥大细胞脱颗粒和趋化因子的释放,限制单核细胞向巨噬细胞的转变,降低调节性T细胞比例,降低IL-6、IL-17、TNF-α、IgE、IgG1和组胺水平[54−55]。芹菜素 芹菜素通过激活过氧化物酶体增殖物激活受体(Peroxisome Proliferator-activated Receptors,PPAR-γ)和调控TRPM7-mTOR途径,降低细胞内NO合成,抑制巨噬细胞M1型极化,降低COX-2和前列腺素2的表达水平[56−58]。芹菜素提高SOD和还原型谷胱甘肽(Glutathione,GSH)浓度,降低ROS水平,下调ROS/ASK1/MAPK通路,减轻小鼠气管和肾脏炎症,抑制炎症细胞浸润[59−60]。 白杨素 白杨素通过抑制IκB的磷酸化,降低NF-κB活力,下调TNF-α、IL-1β等炎症因子的表达水平[61]。白杨素通过激活SIRT1/Nrf2信号通路,抑制中性粒细胞活化,降低肺组织中黏附分子表达,缓解大鼠肺炎及组织损伤[62]。白杨素通过上调Nrf2/ARE/HO-1信号通路,抑制大鼠神经细胞炎症[63]。 高良姜素 高良姜素通过抑制PI3K/PKB信号通路,抑制iNOS和前列腺素内过氧化物合酶2活性,降低NO和前列腺素E2水平,下调TNF-α和IL-1β表达水平,减轻因尿酸积累引起的大鼠肾炎,且不对细胞产生毒性作用[64]。 芦丁 芦丁上调蛋白磷酸酶2A(Protein Phosphatase 2A,PP2A)水平,进而抑制神经轴突内微管结合蛋白tau磷酸化水平,抑制小鼠胶质增生和神经炎症,改善小鼠认知水平[65]。 山奈酚 山奈酚诱导巨噬细胞产生髓系抑制性细胞,并在内脏脂肪组织中积累,进而发挥抗炎症作用[66]。山奈酚通过抑制p38 MAPK和JNK磷酸化,进而下调NF-κB信号通路,抑制大鼠脊髓中小胶质细胞活化,缓解大鼠神经系统炎症[67]。 2.1 黄酮类化合物
蜂蜜中的黄酮类化合物主要包括槲皮素、芹菜素、白杨素、高良姜素、芦丁和山奈酚等[3]。黄酮类化合物调控一系列炎症相关的细胞信号通路,调节TLR4和TLR2等炎症信号受体的激活,影响NF-κB和Nrf2等转录因子的活性,影响TNF-α、IL-1β、IL-6和IL-17等炎症细胞因子的释放,调节前列腺素等炎症介质的水平[11,50]。蜂蜜中的部分黄酮类化合物经肠道吸收后,能发挥全身性的抗炎作用,其中槲皮素、芦丁等,能直接穿透血脑屏障,进而对中枢神经系统发挥抗炎作用[47−48]。蜂蜜中黄酮类化合物的抗炎作用机制如表1所示。
2.2 酚酸
蜂蜜中的含量相对较高的酚酸主要包括肉桂酸及其衍生物咖啡酸、香豆酸和阿魏酸等,以及苯甲酸及其衍生物没食子酸和丁香酸等[68]。酚酸调控炎症信号通路,并缓解因炎症反应造成的组织损伤。苯甲酸能直接作用于致病菌,抑制致病菌生长,减轻因细菌感染导致的肠道炎症。蜂蜜中酚酸的抗炎作用机制如表2所示。
表 2 蜂蜜中酚酸的抗炎作用机制Table 2. Anti-inflammatory mechanisms of phenolic acids in honey成分 抗炎作用机制 肉桂酸 肉桂酸抑制含NLR家族Pyrin域蛋白3(NLR Family Pyrin Domain Containing 3,NLRP3)炎症小体信号通路,并通过抑制ASK1活力,下调MAPK、NF-κB信号通路,缓解大鼠急性胰腺炎,并减轻急性胰腺炎造成的高淀粉酶血症和高脂血症,减轻急性炎症对胰腺
腺泡细胞的损伤[69]。咖啡酸 咖啡酸降低人结肠肌成纤维细胞和结肠炎模型小鼠COX-2、前列腺素E2和丙二醛水平,抑制炎症因子IL-1β、IL-6、IL-8、TNF-α和单核细胞趋化蛋白-1水平,减轻结肠炎,并缓解炎症对结肠组织的病理损伤[70−71]。 阿魏酸 阿魏酸是蜂蜜中抗氧化能力相对较强的成分,其抗炎作用也与其抗氧化活性相关[72]。阿魏酸通过降低ROS水平,抑制NF-κB和p38 MAPK下游信号通路,影响小胶质细胞功能,下调炎症细胞因子水平,减轻炎症[73]。 苯甲酸 苯甲酸具有减轻消化道炎症,修复肠道功能和改善肠道菌群的作用。动物体内实验表明,苯甲酸抑制因大肠杆菌引起的腹泻,减轻肠道炎症,降低致病大肠杆菌数量,并提高乳酸杆菌、双歧杆菌丰度[74]。苯甲酸分子能破坏大肠杆菌细胞膜上酰基脂质链的结构,导致膜功能受损甚至崩解[75]。 没食子酸 没食子酸通过下调MAPK降低炎性细胞因子TNF-α和IL-6表达水平,并通过调节环磷酸腺苷和Ca2+影响组胺的释放[76],抑制嗜酸性粒细胞浸润,缓解小鼠过敏性鼻炎[77]。 丁香酸 丁香酸的抗炎作用与其抗氧化功能密切相关。对来自于心肌梗死患者的外周血单个核细胞,丁香酸降低其ROS和NO水平,降低脂质和蛋白质氧化水平,抑制TNF-α和IL-6表达水平[13]。 2.3 甲基乙二醛
甲基乙二醛(Methylglyoxal)在麦卢卡蜂蜜中含量较高,比在其它蜂蜜中的含量高出100倍左右[13]。麦卢卡蜂蜜中的甲基乙二醛能激活黏膜相关恒定T细胞,调节免疫系统稳态[78]。甲基乙二醛通过下调小胶质细胞表面葡萄糖转运蛋白1(Glucose Transporter 1,GLUT1)表达水平,进而抑制小胶质细胞M1极化过程,通过降低小胶质细胞CD80、CD86和MHC II表达水平而抑制其抗原呈递能力,降低趋化因子CXCL2、CCL7和CCL12的转录及蛋白分泌水平,抑制小胶质细胞对T淋巴细胞的募集[79]。
甲基乙二醛对炎症信号和免疫细胞的调节作用与其化学反应性质有关。甲基乙二醛通过与Kelch样ECH相关蛋白1(Kelch-like ECH-associated Protein 1,KEAP1)的半胱氨酸和精氨酸残基形成共价修饰,导致KEAP1二聚化[80],进而激活Nrf2转录,Nrf2与IκB启动子区域结合,促进其表达,最终抑制受NF-κB调控的炎症因子表达[79]。甲基乙二醛与T淋巴细胞表面的L-精氨酸反应,导致炎症区域内效应T细胞活力被降低,抑制T细胞增殖并降低炎症因子TNF-α和IFN-γ的释放[81]。
3. 蜂蜜治疗炎症性疾病的临床研究进展
蜂蜜对口腔炎和慢性鼻窦炎的治疗作用主要因其成分对口腔和鼻腔黏膜的直接作用。蜂蜜中的酚类化合物在肠道消化吸收程度较低,因此可在肠道聚集,减轻肠道炎症并调节肠道微生物[68]。蜂蜜对肾脏炎症、地中海贫血和关节炎等疾病的治疗作用则更复杂,涉及到其成分和代谢产物经消化吸收进入血液循环系统后发挥抗炎作用[82]。
3.1 蜂蜜减轻消化道炎症并改善肠道菌群
肠胃炎是常见的消化道炎症疾病,蜂蜜能明显减轻肠胃炎症状。有两项临床研究评估了将蜂蜜添加到口服补液盐中,对5岁以下幼儿肠胃炎的改善效果。两项研究的结果都表明,相比于单纯口服补液盐,在常规补液盐中添加蜂蜜,能更有效地改善幼儿肠胃炎症状,减少患儿的呕吐和腹泻频次,缩短肠胃炎痊愈时间[83−84]。
在美国进行的一项临床研究提示了蜂蜜对肠道微生物群的改善效果。40名胎龄≤34周,出生超过3 d的早产儿,随机分为A、B、C、D共4组,每组10人,其中A、B、C组在婴儿奶粉中分别按照5、10、15 g/d的剂量添加医用级蜂蜜,持续喂养2周,D组不添加蜂蜜。与D组相比,3个蜂蜜喂养组婴儿体重显著增加,肠杆菌数减少,双歧杆菌和乳酸杆菌数增加。该结果体现了蜂蜜对早产儿肠道吸收功能和菌群的改善效果[85]。
3.2 蜂蜜减轻糖尿病引发的肾脏炎症
肾脏炎症是糖尿病常见的并发症之一,临床研究表明,食用蜂蜜能降低糖尿病患者炎症指标。糖尿病患者按照30 g/d的剂量连续食用蜂蜜15 d,患者C反应蛋白水平降低,提示了蜂蜜的抗炎效果。食用蜂蜜的糖尿病患者胆固醇、低密度脂蛋白胆固醇和甘油三酯水平均降低[86]。由60名糖尿病肾病患者参与的一项临床研究显示,患者按照25 g/d的剂量食用含益生菌的蜂蜜12周,其炎症标志物血清C反应蛋白和氧化应激标志物血浆丙二醛显著降低,血清胰岛素和总胆固醇指标均有改善[87]。
然而,关于糖尿病患者能否通过食用蜂蜜而预防或缓解肾炎,目前仍存在争议。有临床研究表明,蜂蜜能改善糖尿病患者代谢,是一种优质的甜味替代剂。20名年龄在4~18岁的1型糖尿病患者参与了该研究,按照0.5 mL/kg/d的剂量食用蜂蜜12周,显著降低患者空腹血糖、总胆固醇、血清甘油三酯和低密度脂蛋白,增加空腹C肽和餐后2 h C肽水平[88]。而也有临床研究提示了蜂蜜对糖尿病患者的潜在风险。48名2型糖尿病受试者随机分为2组,其中一组受试者食用蜂蜜8周,对照组不食用蜂蜜。8周后,食用蜂蜜组受试者的血红蛋白A水平升高,这提示了蜂蜜可能对糖尿病患者造成风险[89]。因此,糖尿病患者食用蜂蜜仍需谨慎,需开展更多的临床试验。
3.3 蜂蜜减轻慢性鼻窦炎症状
临床研究表明,麦卢卡蜂蜜能有效缓解慢性鼻窦炎症状。在美国进行的两项临床研究评估了麦卢卡蜂蜜治疗慢性鼻窦炎的可行性,分别有42和13名患有慢性鼻窦炎且过往接受过鼻窦手术的受试者参与,使用浓度为10%的麦卢卡蜂蜜冲洗鼻腔,每天2次,持续30 d,采用Lund-Kennedy鼻内镜评分体系对效果进行评估。结果显示,与对照组相比,10%麦卢卡蜂蜜改善了受试者的鼻窦炎指标,且患者依从性较好[90−91]。在澳大利亚开展的一项临床研究显示,使用含16.5%麦卢卡蜂蜜的甲基乙二醛鼻腔冲洗液治疗后,慢性鼻窦炎患者的鼻腔细菌检出率降低,安全性高且无不良反应[92]。
3.4 蜂蜜用于预防和治疗放射性口腔炎
放射性口腔炎是放疗和化疗后常见的副作用,尤其是头颈癌患者,经放化疗后,放射性口腔炎发病率约为50%[93]。多项临床研究表明,蜂蜜漱口或局部外敷均能有效降低放射性口腔炎的发病率。一项临床研究中50名头颈癌患者随机分为两组,在放疗过程中分别接受聚维酮碘或蜂蜜治疗,聚维酮碘组按照标准操作完成,蜂蜜组使用一种多花蜂蜜漱口,分别在患者放疗前1 h、放疗后2和6 h各执行一次。结果显示,蜂蜜组患者放射性口腔炎发病率显著降低,且不影响放疗治疗效果[94]。另一项临床研究中,接受放疗治疗的头颈癌患者在口腔粘膜局部外敷蜂蜜,同时每日两次食用蜂蜜,6周后,采用常规疗法的对照组患者放射性口腔炎发病率为42%,而蜂蜜干预组患者放射性口腔炎发病率为20%[95]。
对于其它恶性肿瘤放化疗后的放射性口腔炎,蜂蜜同样表现出较好的效果。由53名急性髓系白血病患者参与的一项临床研究中,患者在化疗后使用蜂蜜水漱口,持续4周。在第3周和第4周,蜂蜜漱口患者口腔黏膜炎的严重程度明显低于对照组,同时,因化疗导致的体重下降情况也优于对照组。推测是由于蜂蜜漱口降低了放射性口腔炎的症状,患者的饮食情况较好,因而体重情况改善[96]。
3.5 蜂蜜对其它炎症相关疾病的改善效果
麦卢卡蜂蜜与omega-3脂肪酸联合用药能减轻地中海贫血患者铁过载和慢性炎症。麦卢卡蜂蜜通过铁螯合作用,降低地中海贫血患者血清铁、铁蛋白和血浆异前列腺素水平,降低炎症指标C反应蛋白水平,减轻红细胞炎症状态。麦卢卡蜂蜜通过螯合自由基和抑制脂质过氧化,进而降低氧化应激水平,改善红细胞膜脂质成分[97]。一种蜂蜜和生姜提取物的混合糖浆能有效改善膝骨关节炎症状,患者按照每次30 mL剂量,每日两次,持续服用12周,关节炎症状明显减轻,疼痛、身体运动功能和僵硬指标均明显改善[98]。
4. 结论与展望
炎症反应的成因复杂,免疫状态紊乱、致病菌感染或氧化应激失控均能诱发炎症反应。蜂蜜通过调控免疫与炎症信号通路、抗菌和抗氧化等机制,发挥抗炎作用。不同种类的蜂蜜生物学活性存在一定的差异,这主要与蜂蜜的花源、蜂型等有关。蜂蜜的抗炎作用主要与其所含的黄酮类化合物、酚酸和甲基乙二醛有关,对蜂蜜关键成分的抗炎作用开展研究,有助于揭示蜂蜜抗炎的靶点,并阐明相关的分子机制。然而,对蜂蜜单一成分的研究可能无法反映蜂蜜中多成分之间的协同作用,这是该类型研究的局限。
基于蜂蜜的抗炎作用,其在保健和临床领域的应用日趋广泛。蜂蜜作为一种长期被广泛食用的食品,安全性很高,这是其被推广应用的重要优势。蜂蜜对炎症性疾病的有效作用已被临床研究证实,但是其在人体内的代谢过程和代谢产物尚不清楚,其代谢产物在抗炎过程中发挥的作用也有待探究。因此,为了开发和推广蜂蜜在疾病治疗中的应用,仍需要进一步开展相关临床研究和代谢组学研究。此外,为了加强蜂蜜的质量保障,还需要优化蜂蜜的加工工艺,开发易操作且准确的蜂蜜质量鉴定方法。
-
表 1 蜂蜜中黄酮类化合物的抗炎作用机制
Table 1 Anti-inflammatory mechanisms of flavonoids in honey
成分 抗炎作用机制 槲皮素 槲皮素通过抑制TLR4/MyD88/NF-κB信号通路,减轻小鼠肠道和胶质细胞炎症,通过抑制TLR2信号通路限制NLRP3炎症小体的活
化[51−53]。槲皮素抑制肥大细胞脱颗粒和趋化因子的释放,限制单核细胞向巨噬细胞的转变,降低调节性T细胞比例,降低IL-6、IL-17、TNF-α、IgE、IgG1和组胺水平[54−55]。芹菜素 芹菜素通过激活过氧化物酶体增殖物激活受体(Peroxisome Proliferator-activated Receptors,PPAR-γ)和调控TRPM7-mTOR途径,降低细胞内NO合成,抑制巨噬细胞M1型极化,降低COX-2和前列腺素2的表达水平[56−58]。芹菜素提高SOD和还原型谷胱甘肽(Glutathione,GSH)浓度,降低ROS水平,下调ROS/ASK1/MAPK通路,减轻小鼠气管和肾脏炎症,抑制炎症细胞浸润[59−60]。 白杨素 白杨素通过抑制IκB的磷酸化,降低NF-κB活力,下调TNF-α、IL-1β等炎症因子的表达水平[61]。白杨素通过激活SIRT1/Nrf2信号通路,抑制中性粒细胞活化,降低肺组织中黏附分子表达,缓解大鼠肺炎及组织损伤[62]。白杨素通过上调Nrf2/ARE/HO-1信号通路,抑制大鼠神经细胞炎症[63]。 高良姜素 高良姜素通过抑制PI3K/PKB信号通路,抑制iNOS和前列腺素内过氧化物合酶2活性,降低NO和前列腺素E2水平,下调TNF-α和IL-1β表达水平,减轻因尿酸积累引起的大鼠肾炎,且不对细胞产生毒性作用[64]。 芦丁 芦丁上调蛋白磷酸酶2A(Protein Phosphatase 2A,PP2A)水平,进而抑制神经轴突内微管结合蛋白tau磷酸化水平,抑制小鼠胶质增生和神经炎症,改善小鼠认知水平[65]。 山奈酚 山奈酚诱导巨噬细胞产生髓系抑制性细胞,并在内脏脂肪组织中积累,进而发挥抗炎症作用[66]。山奈酚通过抑制p38 MAPK和JNK磷酸化,进而下调NF-κB信号通路,抑制大鼠脊髓中小胶质细胞活化,缓解大鼠神经系统炎症[67]。 表 2 蜂蜜中酚酸的抗炎作用机制
Table 2 Anti-inflammatory mechanisms of phenolic acids in honey
成分 抗炎作用机制 肉桂酸 肉桂酸抑制含NLR家族Pyrin域蛋白3(NLR Family Pyrin Domain Containing 3,NLRP3)炎症小体信号通路,并通过抑制ASK1活力,下调MAPK、NF-κB信号通路,缓解大鼠急性胰腺炎,并减轻急性胰腺炎造成的高淀粉酶血症和高脂血症,减轻急性炎症对胰腺
腺泡细胞的损伤[69]。咖啡酸 咖啡酸降低人结肠肌成纤维细胞和结肠炎模型小鼠COX-2、前列腺素E2和丙二醛水平,抑制炎症因子IL-1β、IL-6、IL-8、TNF-α和单核细胞趋化蛋白-1水平,减轻结肠炎,并缓解炎症对结肠组织的病理损伤[70−71]。 阿魏酸 阿魏酸是蜂蜜中抗氧化能力相对较强的成分,其抗炎作用也与其抗氧化活性相关[72]。阿魏酸通过降低ROS水平,抑制NF-κB和p38 MAPK下游信号通路,影响小胶质细胞功能,下调炎症细胞因子水平,减轻炎症[73]。 苯甲酸 苯甲酸具有减轻消化道炎症,修复肠道功能和改善肠道菌群的作用。动物体内实验表明,苯甲酸抑制因大肠杆菌引起的腹泻,减轻肠道炎症,降低致病大肠杆菌数量,并提高乳酸杆菌、双歧杆菌丰度[74]。苯甲酸分子能破坏大肠杆菌细胞膜上酰基脂质链的结构,导致膜功能受损甚至崩解[75]。 没食子酸 没食子酸通过下调MAPK降低炎性细胞因子TNF-α和IL-6表达水平,并通过调节环磷酸腺苷和Ca2+影响组胺的释放[76],抑制嗜酸性粒细胞浸润,缓解小鼠过敏性鼻炎[77]。 丁香酸 丁香酸的抗炎作用与其抗氧化功能密切相关。对来自于心肌梗死患者的外周血单个核细胞,丁香酸降低其ROS和NO水平,降低脂质和蛋白质氧化水平,抑制TNF-α和IL-6表达水平[13]。 -
[1] RANNEH Y, AKIM A M, HAMID H A, et al. Honey and its nutritional and anti-inflammatory value[J]. BMC Complement Med Ther,2021,21(1):30. doi: 10.1186/s12906-020-03170-5
[2] SERAGLIO S K T, SCHULZ M, BRUGNEROTTO P, et al. Quality, composition and health-protective properties of citrus honey:A review[J]. Food Res Int,2021,143:110268. doi: 10.1016/j.foodres.2021.110268
[3] CIANCIOSI D, FORBES-HERNANDEZ T Y, AFRIN S, et al. Phenolic compounds in honey and their associated health benefits:A review[J]. Molecules,2018,23(9):2322. doi: 10.3390/molecules23092322
[4] ZAMMIT Y G W, BLUNDELL R. A review on the phytochemical composition and health applications of honey[J]. Heliyon,2023,9(2):e12507. doi: 10.1016/j.heliyon.2022.e12507
[5] ESCUREDO O, SEIJO M C. Honey:Chemical composition, stability and authenticity[J]. Foods,2019,8(11):577. doi: 10.3390/foods8110577
[6] MARTINEZ-ARMENTA C, CAMACHO-REA M C, MARTÍNEZ-NAVA G A, et al. Therapeutic potential of bioactive compounds in honey for treating osteoarthritis[J]. Front Pharmacol,2021,12:642836. doi: 10.3389/fphar.2021.642836
[7] WANG H, LI L T, LIN X H, et al. Composition, functional properties and safety of honey:A review[J]. J Sci Food Agric,2023,103(14):6767−6779. doi: 10.1002/jsfa.12720
[8] SOLIER S, MÜLLER S, CAñEQUE T, et al. A druggable copper-signalling pathway that drives inflammation[J]. Nature,2023,617(7960):386−394. doi: 10.1038/s41586-023-06017-4
[9] SUZUKI K. Chronic inflammation as an immunological abnormality and effectiveness of exercise[J]. Biomolecules,2019,9(6):223. doi: 10.3390/biom9060223
[10] TALEBI M, TALEBI M, FARKHONDEH T, et al. Molecular mechanism-based therapeutic properties of honey[J]. Biomed Pharmacother,2020,130:110590. doi: 10.1016/j.biopha.2020.110590
[11] MASAD R J, HANEEFA S M, MOHAMED Y A, et al. The immunomodulatory effects of honey and associated flavonoids in cancer[J]. Nutrients,2021,13(4):1269. doi: 10.3390/nu13041269
[12] ALMASAUDI S. The antibacterial activities of honey[J]. Saudi J Biol Sci,2020,28(4):2188−2196.
[13] STEFANIS C, STAVROPOULOU E, GIORGI E, et al. Honey's antioxidant and antimicrobial properties:A bibliometric study[J]. Antioxidants (Basel),2023,12(2):414. doi: 10.3390/antiox12020414
[14] KONG P, CUI Z Y, HUANG X F, et al. Inflammation and atherosclerosis:signaling pathways and therapeutic intervention[J]. Signal Transduct Target Ther,2022,7(1):131. doi: 10.1038/s41392-022-00955-7
[15] LI X, LI C T, ZHANG W Y, et al. Inflammation and aging:signaling pathways and intervention therapies[J]. Signal Transduct Target Ther,2023,8(1):239. doi: 10.1038/s41392-023-01502-8
[16] ALANGARI A A, MORRIS K, LWALEED B A, et al. Honey is potentially effective in the treatment of atopic dermatitis:Clinical and mechanistic studies[J]. Immun Inflamm Dis,2017,5(2):190−199. doi: 10.1002/iid3.153
[17] AW YONG P Y, ISLAM F, HARITH H H, et al. The potential use of honey as a remedy for allergic diseases:A mini review[J]. Front Pharmacol,2021,11:599080. doi: 10.3389/fphar.2020.599080
[18] MINDEN-BIRKENMAIER B A, CHERUKURI K, SMITH R A, et al. Manuka honey modulates the inflammatory behavior of a dHL-60 neutrophil model under the cytotoxic limit[J]. Int J Biomater,2019,2019:6132581.
[19] ROMÁRIO-SILVA D, LAZARINI J G, FRANCHIN M, et al. Brazilian organic honey from atlantic rainforest decreases inflammatory process in mice[J]. Vet Sci,2022,9(6):268. doi: 10.3390/vetsci9060268
[20] MINDEN-BIRKENMAIER B A, MEADOWS M B, CHERUKURI K, et al. The effect of Manuka honey on dHL-60 cytokine, chemokine, and matrix-degrading enzyme release under inflammatory conditions[J]. Med One,2019,4(2):e190005.
[21] AFRIN S, GASPARRINI M, FORBES-HERNÁNDEZ T Y, et al. Protective effects of Manuka honey on LPS-treated RAW 264.7 macrophages. Part 1:Enhancement of cellular viability, regulation of cellular apoptosis and improvement of mitochondrial functionality[J]. Food Chem Toxicol,2018,121:203−213. doi: 10.1016/j.fct.2018.09.001
[22] CHENG N, WU L M, ZHENG J B, et al. Buckwheat honey attenuates carbon tetrachloride-induced liver and DNA damage in mice[J]. Evid Based Complement Alternat Med,2015,2015:987385.
[23] BAGATIR G, KAYA M, SUER I, et al. The effect of Anzer honey on X-ray induced genotoxicity in human lymphocytes:An in vitro study[J]. Microsc Res Tech,2022,85(6):2241−2250. doi: 10.1002/jemt.24081
[24] WUSIMAN A, JIANG W M, YU L, et al. Cationic polymer-modified Alhagi honey polysaccharide PLGA nanoparticles as an adjuvant to induce strong and long-lasting immune responses[J]. Int J Biol Macromol,2021,177:370−382. doi: 10.1016/j.ijbiomac.2021.02.130
[25] CAI G F, WU Y, WUSIMAN A, et al. Alhagi honey polysaccharides attenuate intestinal injury and immune suppression in cyclophosphamide-induced mice[J]. Food Funct,2021,12(15):6863−6877. doi: 10.1039/D1FO01008E
[26] CAI G, YANG Y, GU P F, et al. The secretion of sIgA and dendritic cells activation in the intestinal of cyclophosphamide-induced immunosuppressed mice are regulated by Alhagi honey polysaccharides[J]. Phytomedicine,2022,103:154232. doi: 10.1016/j.phymed.2022.154232
[27] CHENG N, WANG Y A, CAO W. The protective effect of whole honey and phenolic extract on oxidative DNA damage in mice lymphocytes using comet assay[J]. Plant Foods Hum Nutr,2017,72(4):388−395. doi: 10.1007/s11130-017-0634-1
[28] NAVAEI-ALIPOUR N, MASTALI M, FERNS G A, et al. The effects of honey on pro- and anti-inflammatory cytokines:A narrative review[J]. Phytother Res,2021,35(7):3690−3701. doi: 10.1002/ptr.7066
[29] YOU R, KWON O Y, WOO H J, et al. Hovenia monofloral honey can attenuate enterococcus faecalis mediated biofilm formation and inflammation[J]. Food Sci Anim Resour,2022,42(1):84−97. doi: 10.5851/kosfa.2021.e65
[30] ZHAO H A, CHENG N, ZHOU W Q, et al. Honey polyphenols ameliorate DSS-induced ulcerative colitis via modulating gut microbiota in rats[J]. Mol Nutr Food Res,2019,63(23):e1900638. doi: 10.1002/mnfr.201900638
[31] SUN L P, SHI F F, ZHANG W W, et al. Antioxidant and anti-inflammatory activities of safflower (Carthamus tinctorius L.) honey extract[J]. Foods,2020,9(8):1039. doi: 10.3390/foods9081039
[32] NEAMATALLAH T, EL-SHITANY N A, ABBAS A T, et al. Honey protects against cisplatin-induced hepatic and renal toxicity through inhibition of NF-κB-mediated COX-2 expression and the oxidative stress dependent BAX/Bcl-2/caspase-3 apoptotic pathway[J]. Food Funct,2018,9(7):3743−3754. doi: 10.1039/C8FO00653A
[33] GASPARRINI M, AFRIN S, FORBES-HERNÁNDEZ T Y, et al. Protective effects of Manuka honey on LPS-treated RAW 264.7 macrophages. Part 2:Control of oxidative stress induced damage, increase of antioxidant enzyme activities and attenuation of inflammation[J]. Food Chem Toxicol,2018,120:578−587. doi: 10.1016/j.fct.2018.08.001
[34] NAQVI F, DASTAGIR N, JABEEN A. Honey proteins regulate oxidative stress, inflammation and ameliorates hyperglycemia in streptozotocin induced diabetic rats[J]. BMC Complement Med Ther,2023,23(1):14. doi: 10.1186/s12906-023-03837-9
[35] OLIVEIRA A, RIBEIRO H G, SILVA A C, et al. Synergistic antimicrobial interaction between honey and phage against escherichia coli biofilms[J]. Front Microbiol,2017,8:2407. doi: 10.3389/fmicb.2017.02407
[36] NOLAN V C, HARRISON J, COX J A G. Dissecting the antimicrobial composition of honey[J]. Antibiotics (Basel),2019,8(4):251. doi: 10.3390/antibiotics8040251
[37] KOT B, SYTYKIEWICZ H, SPRAWKA I, et al. Effect of manuka honey on biofilm-associated genes expression during methicillin-resistant staphylococcus aureus biofilm formation[J]. Sci Rep,2020,10(1):13552. doi: 10.1038/s41598-020-70666-y
[38] KIM S Y, KANG S S. Anti-biofilm activities of manuka honey against Escherichia coli O157:H7[J]. Food Sci Anim Resour,2020,40(4):668−674. doi: 10.5851/kosfa.2020.e42
[39] MOLAN P, RHODES T. Honey:A biologic wound dressing[J]. Wounds,2015,27(6):141−151.
[40] MCGARRY T, BINIECKA M, VEALE D J, et al. Hypoxia, oxidative stress and inflammation[J]. Free Radic Biol Med,2018,125:15−24. doi: 10.1016/j.freeradbiomed.2018.03.042
[41] PAPACONSTANTINOU J. The role of signaling pathways of inflammation and oxidative stress in development of senescence and aging phenotypes in cardiovascular disease[J]. Cells,2019,8(11):1383. doi: 10.3390/cells8111383
[42] HUSSAIN T, TAN B, YIN Y L, et al. Oxidative stress and inflammation:What polyphenols can do for us[J]. Oxid Med Cell Longev,2016,2016:7432797.
[43] KAČÁNIOVÁ M, BOROTOVÁ P, GALOVIČOVÁ L, et al. Antimicrobial and antioxidant activity of different honey samples from beekeepers and commercial producers[J]. Antibiotics (Basel),2022,11(9):1163. doi: 10.3390/antibiotics11091163
[44] ALVAREZ-SUAREZ J M, GIAMPIERI F, CORDERO M, et al. Activation of AMPK/Nrf2 signalling by Manuka honey protects human dermal fibroblasts against oxidative damage by improving antioxidant response and mitochondrial function promoting wound healing[J]. J Funct Foods,2016,25:38−49. doi: 10.1016/j.jff.2016.05.008
[45] SAFI S Z, BATUMALAIE K, QVIST R, et al. Gelam honey attenuates the oxidative stress-induced inflammatory pathways in pancreatic hamster cells[J]. Evid Based Complement Alternat Med,2016,2016:5843615.
[46] ARIGELA C S, NELLI G, GAN S H, et al. Bitter gourd honey ameliorates hepatic and renal diabetic complications on type 2 diabetes rat models by antioxidant, anti-Inflammatory, and anti-Apoptotic mechanisms[J]. Foods,2021,10(11):2872. doi: 10.3390/foods10112872
[47] DEEPIKA, MAURYA P K. Health benefits of quercetin in age-related diseases[J]. Molecules,2022,27(8):2498. doi: 10.3390/molecules27082498
[48] PLUTA R, MIZIAK B, CZUCZWAR S J. Apitherapy in post-ischemic brain neurodegeneration of Alzheimer's disease proteinopathy:Focus on honey and its flavonoids and phenolic acids[J]. Molecules,2023,28(15):5624. doi: 10.3390/molecules28155624
[49] ZHANG X D, SCHALKWIJK C G, WOUTERS K. Immunometabolism and the modulation of immune responses and host defense:A role for methylglyoxal[J]. Biochim Biophys Acta Mol Basis Dis,2022,1868(8):166425. doi: 10.1016/j.bbadis.2022.166425
[50] SILVA B, BILUCA F C, GONZAGA L V, et al. In vitro anti-inflammatory properties of honey flavonoids:A review[J]. Food Res Int,2021,141:110086. doi: 10.1016/j.foodres.2020.110086
[51] LE K, SONG Z P, DENG J, et al. Quercetin alleviates neonatal hypoxic-ischemic brain injury by inhibiting microglia-derived oxidative stress and TLR4-mediated inflammation[J]. Inflamm Res,2020,69(12):1201−1213. doi: 10.1007/s00011-020-01402-5
[52] YE Y R, JIANG M Z, HONG X Y, et al. Quercetin alleviates deoxynivalenol-induced intestinal damage by suppressing inflammation and ferroptosis in mice[J]. J Agric Food Chem,2023,71(28):10761−10772. doi: 10.1021/acs.jafc.3c02027
[53] LUO X, BAO X Y, WENG X Z, et al. The protective effect of quercetin on macrophage pyroptosis via TLR2/Myd88/NF-κB and ROS/AMPK pathway[J]. Life Sci,2021,291:120064.
[54] LI J H, SUN Z Y, LUO G, et al. Quercetin attenuates trauma-induced heterotopic ossification by tuning immune cell infiltration and related inflammatory insult[J]. Front Immunol,2021,12:649285. doi: 10.3389/fimmu.2021.649285
[55] KE X, CHEN Z Q, WANG X Q, et al. Quercetin improves the imbalance of Th1/Th2 cells and Treg/Th17 cells to attenuate allergic rhinitis[J]. Autoimmunity,2023,56(1):2189133. doi: 10.1080/08916934.2023.2189133
[56] AL-KHAYRI J M, SAHANA G R, NAGELLA P, et al. Flavonoids as potential anti-inflammatory molecules:A review[J]. Molecules,2022,27(9):2901. doi: 10.3390/molecules27092901
[57] ADHAM A N, ABDELFATAH S, NAQISHBANDI A M, et al. Cytotoxicity of apigenin toward multiple myeloma cell lines and suppression of iNOS and COX-2 expression in STAT1-transfected HEK293 cells[J]. Phytomedicine,2020,80:153371.
[58] JI X Y, DU W, CHE W Q, et al. Apigenin inhibits the progression of osteoarthritis by mediating macrophage polarization[J]. Molecules,2023,28(7):2915. doi: 10.3390/molecules28072915
[59] WU Q J, LI W, ZHAO J, et al. Apigenin ameliorates doxorubicin-induced renal injury via inhibition of oxidative stress and inflammation[J]. Biomed Pharmacother,2021,137:111308. doi: 10.1016/j.biopha.2021.111308
[60] YU H, HUANG X, ZHU H H, et al. Apigenin ameliorates non-eosinophilic inflammation, dysregulated immune homeostasis and mitochondria-mediated airway epithelial cell apoptosis in chronic obese asthma via the ROS-ASK1-MAPK pathway[J]. Phytomedicine,2023,111:154646. doi: 10.1016/j.phymed.2023.154646
[61] ZHAO S N, LIANG M L, WANG Y L, et al. Chrysin suppresses vascular endothelial inflammation via inhibiting the NF-κB signaling pathway[J]. J Cardiovasc Pharmacol Ther,2019,24(3):278−287. doi: 10.1177/1074248418810809
[62] YANG Z P, GUAN Y F, LI J, et al. Chrysin attenuates carrageenan-induced pleurisy and lung injury via activation of SIRT1/NRF2 pathway in rats[J]. Eur J Pharmacol,2018,836:83−88. doi: 10.1016/j.ejphar.2018.08.015
[63] ZHANG Y, ZHAO J, AFZAL O, et al. Neuroprotective role of chrysin-loaded poly (lactic-co-glycolic acid) nanoparticle against kindling-induced epilepsy through Nrf2/ARE/HO-1 pathway[J]. J Biochem Mol Toxicol,2020,35(2):e22634.
[64] LU H, YAO H, ZOU R, et al. Galangin suppresses renal inflammation via the inhibition of NF-κB, PI3K/AKT and NLRP3 in uric acid treated NRK-52E tubular epithelial cells[J]. Biomed Res Int,2019,2019:3018357.
[65] SUN X Y, LI L J, DONG Q X, et al. Rutin prevents tau pathology and neuroinflammation in a mouse model of alzheimer's disease[J]. J Neuroinflammation,2021,18(1):131. doi: 10.1186/s12974-021-02182-3
[66] KITAMURA H, SAITO N, FUJIMOTO J, et al. Brazilian propolis ethanol extract and its component kaempferol induce myeloid-derived suppressor cells from macrophages of mice in vivo and in vitro[J]. BMC Complement Altern Med,2018,18(1):138. doi: 10.1186/s12906-018-2198-5
[67] LIU Z Y, YAO X Q, SUN B H, et al. Pretreatment with kaempferol attenuates microglia-mediate neuroinflammation by inhibiting MAPKs-NF-κB signaling pathway and pyroptosis after secondary spinal cord injury[J]. Free Radic Biol Med,2021,168:142−154. doi: 10.1016/j.freeradbiomed.2021.03.037
[68] BECERRIL-SÁNCHEZ A L, QUINTERO-SALAZAR B, DUBLÁN-GARCÍA O, et al. Phenolic compounds in honey and their relationship with antioxidant activity, botanical origin, and color[J]. Antioxidants (Basel),2021,10(11):1700. doi: 10.3390/antiox10111700
[69] ABOZAID O A R, MOAWED F S M, AHMED E S A, et al. Cinnamic acid nanoparticles modulate redox signal and inflammatory response in gamma irradiated rats suffering from acute pancreatitis[J]. Biochim Biophys Acta Mol Basis Dis,2020,1866(11):165904. doi: 10.1016/j.bbadis.2020.165904
[70] WAN F, ZHONG R Q, WANG M Y, et al. Caffeic acid supplement alleviates colonic inflammation and oxidative stress potentially through improved gut microbiota community in mice[J]. Front Microbiol,2021,12:784211. doi: 10.3389/fmicb.2021.784211
[71] ZIELIŃSKA D, ZIELIŃSKI H, LAPARRA-LLOPIS J M, et al. Caffeic acid modulates processes associated with intestinal inflammation[J]. Nutrients,2021,13(2):554. doi: 10.3390/nu13020554
[72] LIMA Â C O, DIAS E R, REIS I M A, et al. Ferulic acid as major antioxidant phenolic compound of the tetragonisca angustula honey collected in Vera Cruz-Itaparica Island, Bahia, Brazil[J]. Braz J Biol,2022,84:e253599.
[73] LI D, RUI Y X, GUO S D, et al. Ferulic acid:A review of its pharmacology, pharmacokinetics and derivatives[J]. Life Sci,2021,284:119921. doi: 10.1016/j.lfs.2021.119921
[74] MAO X B, YANG Q, CHEN D W, et al. Benzoic acid used as food and feed additives can regulate gut functions[J]. Biomed Res Int,2019,2019:5721585.
[75] SYNOWIEC A, ŻYłA K, GNIEWOSZ M, et al. An effect of positional isomerism of benzoic acid derivatives on antibacterial activity against Escherichia coli[J]. Open Life Sci,2021,16(1):594−601. doi: 10.1515/biol-2021-0060
[76] BAI J R, ZHANG Y S, TANG C, et al. Gallic acid:Pharmacological activities and molecular mechanisms involved in inflammation-related diseases[J]. Biomed Pharmacother,2021,133:110985. doi: 10.1016/j.biopha.2020.110985
[77] SHAHZAD S, MATEEN S, KAUSAR T, et al. Effect of syringic acid and syringaldehyde on oxidative stress and inflammatory status in peripheral blood mononuclear cells from patients of myocardial infarction[J]. Naunyn Schmiedebergs Arch Pharmacol,2020,393(4):691−704. doi: 10.1007/s00210-019-01768-2
[78] TANG J, COMPTON B J, MARSHALL A, et al. Mānuka honey-derived methylglyoxal enhances microbial sensing by mucosal-associated invariant T cells[J]. Food Funct,2020,11(7):5782−5787. doi: 10.1039/D0FO01153C
[79] WEI S L, YANG Y, SI W Y, et al. Methylglyoxal suppresses microglia inflammatory response through NRF2-IκBζ pathway[J]. Redox Biol,2023,65:102843. doi: 10.1016/j.redox.2023.102843
[80] BOLLONG M J, LEE G, COUKOS J S, et al. A metabolite-derived protein modification integrates glycolysis with Keap1-Nrf2 signalling[J]. Nature,2018,562(7728):600−604. doi: 10.1038/s41586-018-0622-0
[81] BAUMANN T, DUNKEL A, SCHMID C, et al. Regulatory myeloid cells paralyze T cells through cell-cell transfer of the metabolite methylglyoxal[J]. Nat Immunol,2020,21(5):555−566. doi: 10.1038/s41590-020-0666-9
[82] PALMA-MORALES M, HUERTAS J R, RODRÍGUEZ-PÉREZ C. A comprehensive review of the effect of honey on human health[J]. Nutrients,2023,15(13):3056. doi: 10.3390/nu15133056
[83] ABDULRHMAN M A, MEKAWY M A, AWADALLA M M, et al. Bee honey added to the oral rehydration solution in treatment of gastroenteritis in infants and children[J]. J Med Food,2010,13(3):605−609. doi: 10.1089/jmf.2009.0075
[84] ANDAYANI R P, NURHAENI N, AGUSTINI N. The effect of honey with ORS and a honey solution in ORS on reducing the frequency of diarrhea and length of stay for toddlers[J]. Compr Child Adolesc Nurs,2019,42(sup1):21−28. doi: 10.1080/24694193.2019.1577922
[85] ALY H, SAID R N, WALI I E, et al. Medically graded honey supplementation formula to preterm infants as a prebiotic:A randomized controlled trial[J]. J Pediatr Gastroenterol Nutr,2017,64(6):966−970. doi: 10.1097/MPG.0000000000001597
[86] AL-WAILI N S. Natural honey lowers plasma glucose, C-reactive protein, homocysteine, and blood lipids in healthy, diabetic, and hyperlipidemic subjects:comparison with dextrose and sucrose[J]. J Med Food,2004,7(1):100−107. doi: 10.1089/109662004322984789
[87] MAZRUEI ARANI N, EMAM-DJOMEH Z, TAVAKOLIPOUR H, et al. The effects of probiotic honey consumption on metabolic status in patients with diabetic nephropathy:A randomized, double-blind, controlled trial[J]. Probiotics Antimicrob Proteins,2019,11(4):1195−1201. doi: 10.1007/s12602-018-9468-x
[88] ABDULRHMAN M A, MAMDOUH N A, EL GUINDY W M, et al. Effects of honey supplementation on children with idiopathic dilated cardiomyopathy:A randomized single blinded controlled study[J]. World J Pharm Res,2018,7:19−34.
[89] BAHRAMI M, ATAIE-JAFARI A, HOSSEINI S, et al. Effects of natural honey consumption in diabetic patients:An 8-week randomized clinical trial[J]. Int J Food Sci Nutr,2009,60(7):618−626. doi: 10.3109/09637480801990389
[90] LEE V S, HUMPHREYS I M, PURCELL P L, et al. Manuka honey sinus irrigation for the treatment of chronic rhinosinusitis:A randomized controlled trial[J]. Int Forum Allergy Rhinol,2017,7(4):365−372. doi: 10.1002/alr.21898
[91] LEE V S, HUMPHREYS I M, PURCELL P L, et al. Manuka honey versus saline sinus irrigation in the treatment of cystic fibrosis-associated chronic rhinosinusitis:A randomised pilot trial[J]. Clin Otolaryngol,2021,46(1):168−174. doi: 10.1111/coa.13637
[92] OOI M L, JOTHIN A, BENNETT C, et al. Manuka honey sinus irrigations in recalcitrant chronic rhinosinusitis:Phase 1 randomized, single-blinded, placebo-controlled trial[J]. Int Forum Allergy Rhinol,2019,9(12):1470−1477. doi: 10.1002/alr.22423
[93] ALSUBAIE H M, ALSINI A Y, ALSUBAIE K M, et al. Glutamine for prevention and alleviation of radiation-induced oral mucositis in patients with head and neck squamous cell cancer:Systematic review and meta-analysis of controlled trials[J]. Head Neck,2021,43(10):3199−3213. doi: 10.1002/hed.26798
[94] RAO S, HEGDE S K, RAO P, et al. Honey mitigates radiation-induced oral mucositis in head and neck cancer patients without affecting the tumor response[J]. Foods,2017,6(9):77. doi: 10.3390/foods6090077
[95] MAMGAIN R K, GUPTA M, MAMGAIN P, et al. The efficacy of an ayurvedic preparation of yashtimadhu (Glycyrrhiza glabra) on radiation-induced mucositis in head-and-neck cancer patients:A pilot study[J]. J Cancer Res Ther,2018,16(3):458−462.
[96] KHANJANI POUR-FARD-PACHEKENARI A, RAHMANI A, GHAHRAMANIAN A, et al. The effect of an oral care protocol and honey mouthwash on mucositis in acute myeloid leukemia patients undergoing chemotherapy:a single-blind clinical trial[J]. Clin Oral Investig,2019,23(4):1811−1821. doi: 10.1007/s00784-018-2621-9
[97] GAMALELDIN M, ABRAHAM I, MEABED M, et al. Comparative effectiveness of adding omega-3 and Manuka honey combination to conventional therapy in preventing and treating oxidative stress in pediatric β-thalassemia major-a randomized clinical trial[J]. Eur Rev Med Pharmacol Sci,2023,27(13):6058−6070.
[98] AFSHAR F, ABDOLAHI N, AMIN G, et al. A randomized, double-blind placebo-controlled phase I clinical study on safety and efficacy of the G-Rup® syrup (a mixture of ginger extract and honey) in symptomatic treatment of knee osteoarthritis[J]. J Clin Pharm Ther,2022,47(12):2295−2301. doi: 10.1111/jcpt.13812
-
期刊类型引用(4)
1. 汪玉玲,吴晨曦,徐心如,夏道宗. 滁菊化学成分、药理作用及质量标志物预测分析. 中药新药与临床药理. 2025(02): 307-316 .  百度学术
百度学术
2. 石超峰,吴林璇,高重智,谭军,周海燕,刘志,黄红梅. 基于表型分类的珠芽魔芋种质资源综合评价. 湖南农业科学. 2024(04): 1-7 .  百度学术
百度学术
3. 叶田诚,潘松,许贺然,杨瑞雨,惠琳,王嘉宝,闫淼,梁晓曼,李馨玥,辛广. 花粉直感效应对长江1号软枣猕猴桃果实品质的影响. 中国果树. 2024(06): 47-56 .  百度学术
百度学术
4. 赵帅,王帅,李天娇,赵琳,包永睿,孟宪生. 基于“质-量”双标的菊花质量分析方法研究. 药学研究. 2024(12): 1185-1189+1224 .  百度学术
百度学术
其他类型引用(1)
-
其他相关附件
-
PDF格式
eiCertificate 42KB
-





 下载:
下载:
 下载:
下载:



