Template Synthesis of Metal Nanoclusters and Application in Food
-
摘要: 金属纳米团簇(Metal Nanoclusters,MNCs)因其独特的物理化学性质,在食品安全领域显示出极大的应用潜力。通过模板合成法,可实现MNCs特定尺寸、形状和分散性的调控,进而提升其功能特性。本文结合近年来关于金属纳米团簇的研究,主要综述了模板辅助合成金属纳米团簇的机制及其在食品污染物检测、食品包装材料、抗菌剂和食品添加剂检测方面的应用,为模板法合成金属纳米团簇提供可参考的科学依据,为金属纳米团簇在食品安全监测领域提供见解和方向。Abstract: Metal Nanoclusters (MNCs) have shown great potential applications in the field of food safety due to their unique physical and chemical properties. Through the template synthesis method, the specific size, shape and dispersion of MNCs can be regulated, thereby improving their functional characteristics. Based on the recent studies on MNCs, the mechanism of template-assisted synthesis of MNCs and their applications in the detection of food contaminants, food packaging materials, antimicrobial agents and food additives are reviewed. It provides a scientific basis for template synthesis of MNCs, and to provide insights and directions for MNCs in food safety monitoring.
-
Keywords:
- metal nanoclusters (MNCs) /
- template synthesis /
- application
-
金属纳米团簇是一种以金属为核,将不同的有机配体修饰在其表面的纳米材料,具有精确的原子组成和结构,尺寸约为1~3 nm,是介于原子和纳米颗粒之间的过渡态,其物理和化学性质具有量子尺寸效应[1]。金属纳米团簇因其具有独特的结构和性质,可在食品领域发挥重要作用。例如,金属纳米团簇可用于食品包装材料,提供抗菌活性并延长食品的保质期[2−3];此外,还可用于检测食品中的有害物质,如污染物、残留农药和重金属[4−5]。通过与这些物质的相互作用,金属纳米团簇可以发出特定的光谱信号或电化学信号,从而实现对食品质量和安全性的监测。
金属纳米团簇的尺寸通常与电子的Fermi波长相当[6]。相比传统的金属纳米颗粒,金属纳米团簇具有单一分布的尺寸和精确的结构。然而,由于其小体积且比表面积大,容易发生团聚形成大颗粒,因此合成高稳定性的金属纳米团簇至关重要。在过去的几十年里,金属纳米团簇的合成方法得到了快速发展,研究人员采用了不同的模板方法来合成高质量的金属纳米团簇,如聚合物[7]、蛋白质[8−9]、肽[10]、硫醇[11]和DNA[12]。迄今为止,已报道了多种金属纳米团簇的合成,如AuNCs、AgNCs、CuNCs、PtNCs、PdNCs或合金纳米团簇等。然而,传统的合成方法通常难以精确控制金属纳米团簇的尺寸和形态,且难以实现大规模合成。因此,如何高效简便地制备金属纳米团簇,成为当前研究的热点之一。
本论文介绍了基于不同的模板材料,如聚合物、蛋白质、肽、硫醇和DNA合成金属纳米团簇的方法,并概述了金属纳米团簇在食品污染物检测、食品包装材料、食品添加剂检测和抗菌方面的应用。通过全面总结该领域的最新研究成果,旨在深入剖析金属纳米团簇的模板合成机制,进而为开发出精准高效的食品安全监测工具奠定坚实的科学基础。
1. 模板合成金属纳米团簇机制
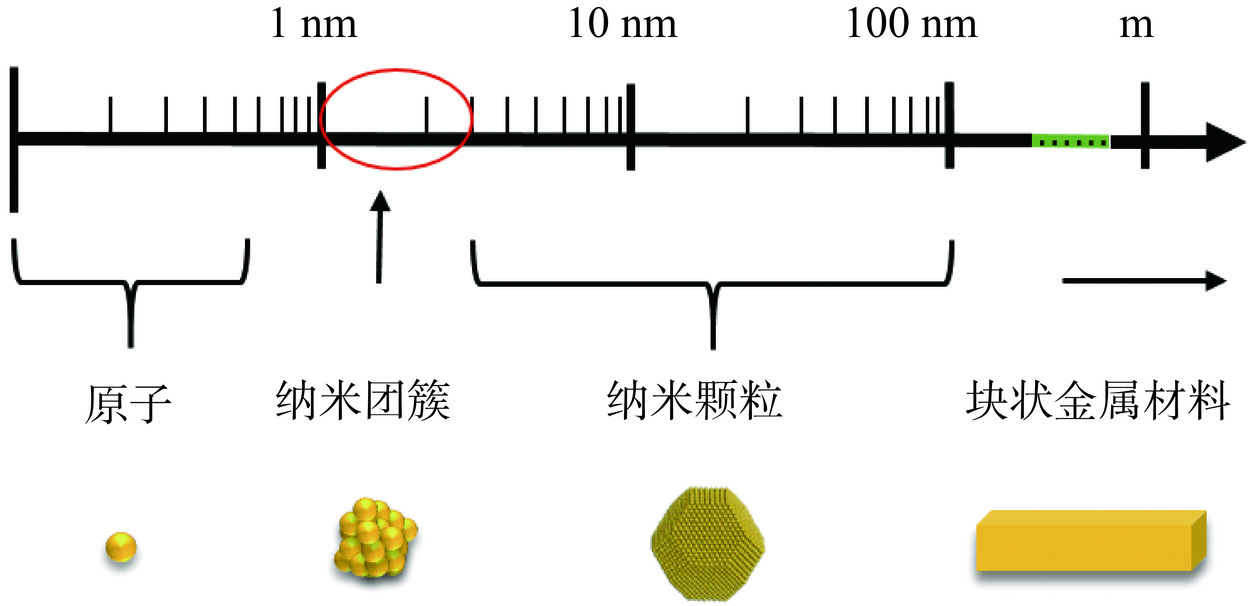
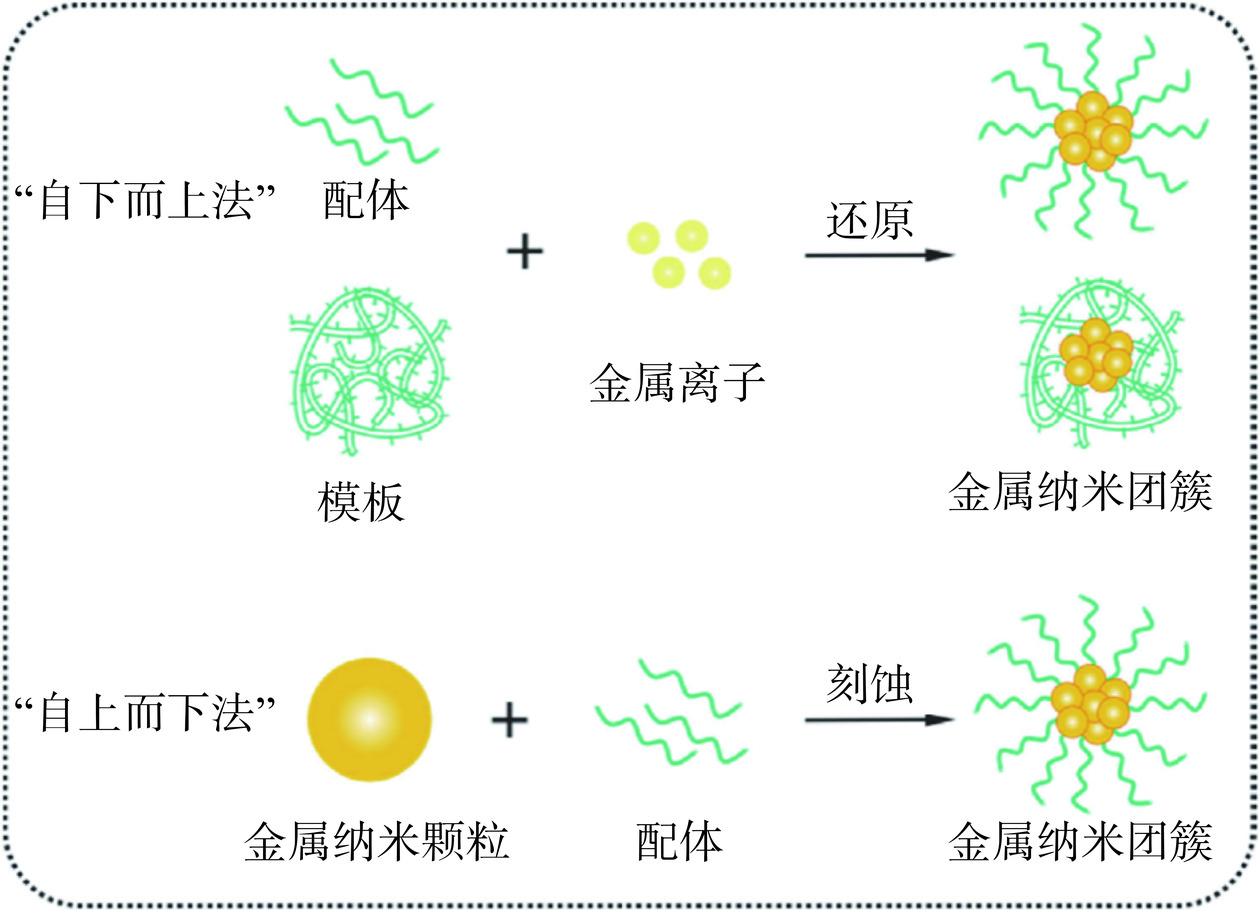
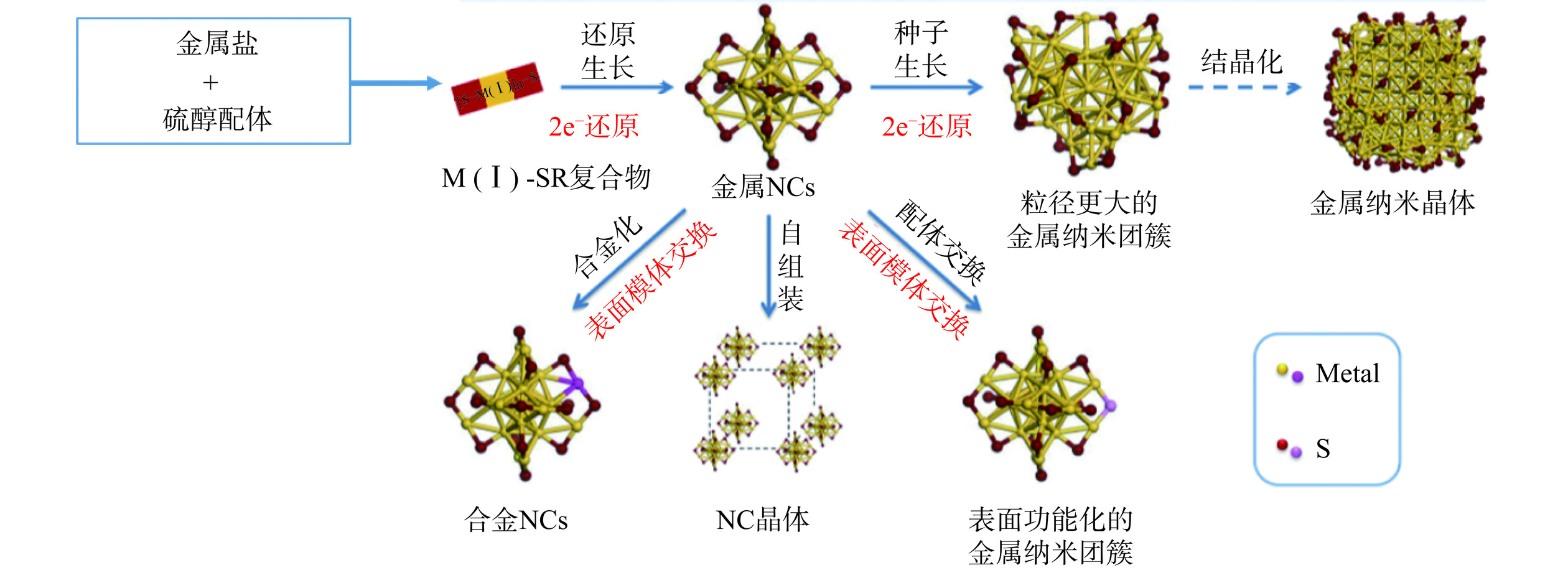
金属纳米团簇的尺寸介于原子和纳米颗粒之间(图1),因此有两种通用的纳米团簇合成机制,即自下而上法和自上而下法。自下而上法:在保护配体的作用下,通过还原辅助剂将金属离子还原为金属原子,并进一步组装成金属核[13]。但是,金属核中的金属原子由于具有零价态和高表面能,具有很强的自聚集倾向,可能会逐渐长大形成更大的纳米颗粒;自上而下法:利用刻蚀剂上的官能团(如巯基、氨基、羧基等)与金属原子的相互作用,逐步刻蚀较大的金属纳米颗粒,反复刻蚀后可生成尺寸更小的金属纳米团簇。与自下而上法相比,这种方法虽能制备出更稳定的纳米团簇,但通常产率较低,制备过程也更为复杂一些[14]。图2介绍了金属纳米团簇合成方法示意图[15]。金属纳米团簇的合成是一个复杂的过程,金属离子浓度、反应溶液的pH、模板选择、还原剂、温度和反应时间等因素都会影响金属纳米团簇的合成[16]。
2. 不同模板合成金属纳米团簇
2.1 聚合物
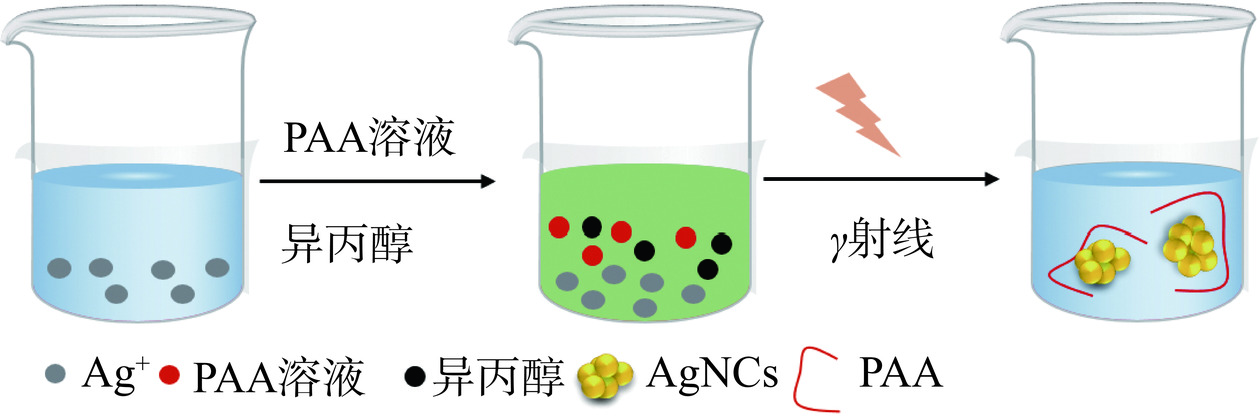
聚合物作为模板合成金属纳米团簇的主要步骤为聚合物选择、配位反应、还原剂加入(如柠檬酸、氢氯酸、抗坏血酸、硼氢化钠等)、核化和成核、形态调控和稳定性、分离和纯化。该方法可以通过选择不同的聚合物模板来实现对金属纳米团簇形貌和性质的调控,由于聚合物模板提供了多个配位位点,有助于稳定金属离子并促进团簇的形成[17]。如聚乙烯亚胺(PEI)是一种水溶性聚合物,分子链中含有大量氨基,很容易与Cu2+、Zn2+、Co2+络合,常作为配体用于合成各种材料。焦婷等[7]使用PEI作为稳定剂和抗坏血酸作为还原剂制备PEI-CuNCs。Han等[18]提供了一种简单的方法来合成银纳米团簇,在该方法中,以聚丙烯酸(PAA)为模板,在室温下通过辐照将银离子还原为银纳米团簇(PAA-AgNCs),具有良好的荧光特性和稳定性(图3)。Shi等[19]提出了一种合成发光铜纳米团簇的新策略,该策略通过在75 ℃下使用抗坏血酸还原Cu2+,并在聚乙烯基吡咯烷酮的保护下进行,得到的铜纳米团簇显示出蓝光发射,并且具有高稳定性,至少可以保持一个月,其光致发光量子产率为12%。Ge等[20]利用聚甲基丙烯酸(PMAA)在水溶液中合成了稳定的银纳米团簇,并通过硫醇(R-SH)和胺(R-NH2)配体对其实现了功能化。这种功能化的银纳米团簇在金黄色葡萄球菌和大肠杆菌上的抗菌测试揭示了其作为潜在抗菌口罩材料的应用价值。
聚合物模板的多样性和修饰性为金属纳米团簇的合成提供了丰富的选项。这种独特优势可以有效控制金属纳米团簇的尺寸、形态,并显著提高其稳定性。因此,聚合物模板极大地推动了金属纳米团簇在更多领域中的应用前景。
2.2 蛋白质和多肽
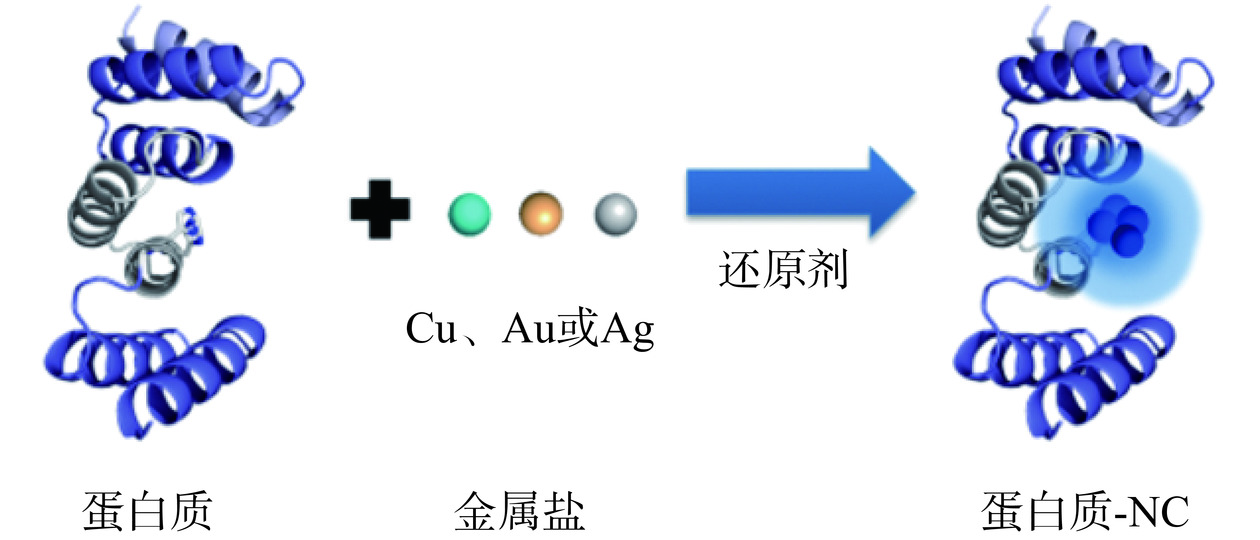
以蛋白和多肽为模板合成金属纳米团簇实质上是一种生物矿化的过程,即通过调控生物分子的结构和组成,如控制金属离子的浓度、反应pH、蛋白的结构或多肽的序列组成等得到尺寸、荧光性质以及表面化学性质不同的金属纳米团簇[21−22]。利用蛋白质和多肽可合成具有高度特定功能和多功能的金属纳米团簇,蛋白和多肽表面所具有丰富基团和结合位点便于金属纳米团簇的后期多功能化[23−24]。如Li等[25]以牛血清白蛋白(BSA)为模板合成金纳米团簇之后通过外部共价键连接二乙烯三胺五乙酸钆,因此功能化之后的纳米团簇既能够荧光成像,又可核磁共振成像。此外,研究人员陆续将具有类似结构的蛋白应用到金属纳米团簇的合成中,如胰岛素[26]、辣根过氧化物酶[27]、鸡蛋蛋白[28−29]、溶菌酶[30]、胃蛋白酶[31]、植物蛋白酶,如木瓜蛋白酶[32]、大豆蛋白[33]等。如图4总结了以蛋白质合成和稳定荧光金属纳米团簇的一般方案[34]。
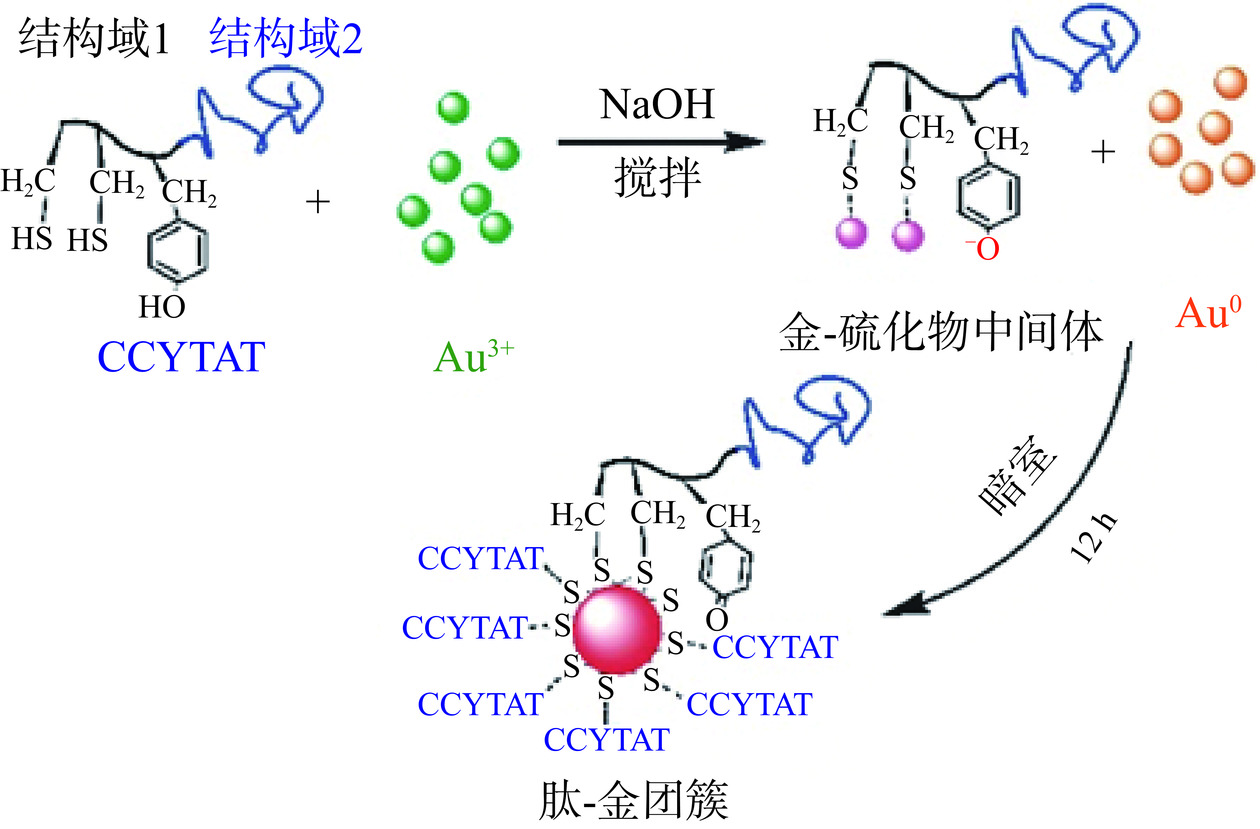
相对于蛋白质而言,多肽结构更加简单明确,分子量较小更直观体现出在合成过程中每一个特定氨基酸所起到作用,且可以根据自己的需要的性质比如尺寸大小、表面电荷、靶向等来自行设计或者直接购买商品化的多肽产品[35−36]。如Yuan等[37]研究了AuNCs荧光动力学,并利用酪氨酸(Y)的还原性和半胱氨酸(C)对金表面具有强结合亲和力,设计了“CYY”为核心序列的肽成功合成了具有高量子产率的荧光肽AuNCs;Wang等[38]以多肽序列H2N-CCYRGRKKRRQRRR-COOH(简称CCYTAT)成功合成了荧光金纳米团簇(图5)。因此选择合适的蛋白与多肽序列,合成稳定性好、产率高的金属纳米团簇, 并以此为基础结合其他技术,实现多功能化是一个良好的发展前景。
2.3 硫醇
硫醇可以作为模板用于合成原子级精确的金属纳米团簇,可以用分子式 [Mn(SR)m]q表示,其中n、m和q分别为金属原子(Au、Ag或Cu)和硫醇盐配体(SR)的数量以及单个簇中净电荷的数量。在硫醇配体作为模板合成金属纳米团簇大的过程中,硫醇分子中的巯基与金属离子相互作用,S-H键断裂,形成M(Ⅰ)-SR键[39−40],M(Ⅰ)-SR键能够避免金属纳米团簇团聚,从而赋予其稳定的结构。常用于水溶液中进行保护金属核心的硫醇类分子主要是:半胱氨酸(Cys)、6-巯基己酸(6-MHA)、二硫苏糖醇(DDT)、二氢硫辛酸(DHLA)和谷胱甘肽(GSH)等,其中谷胱甘肽(GSH)是经典的巯基化合物之一,由巯基、氨基和羧基组成,是一种有效的金属离子络合剂。在水溶液中制备金属纳米团簇的过程中,GSH作为配体并通过巯基附着在团簇的表面。因此,GSH可以保护团簇免受热力学不稳定引起的聚集,并提高生物利用度和传感性能[41]。如Zhang等[42]以GSH为稳定剂,抗坏血酸(AA)为还原剂,通过绿色一步法合成了高稳定的GSH-CuNCs。图6介绍了硫醇配体全合成金属纳米团簇示意图[43]。
2.4 DNA
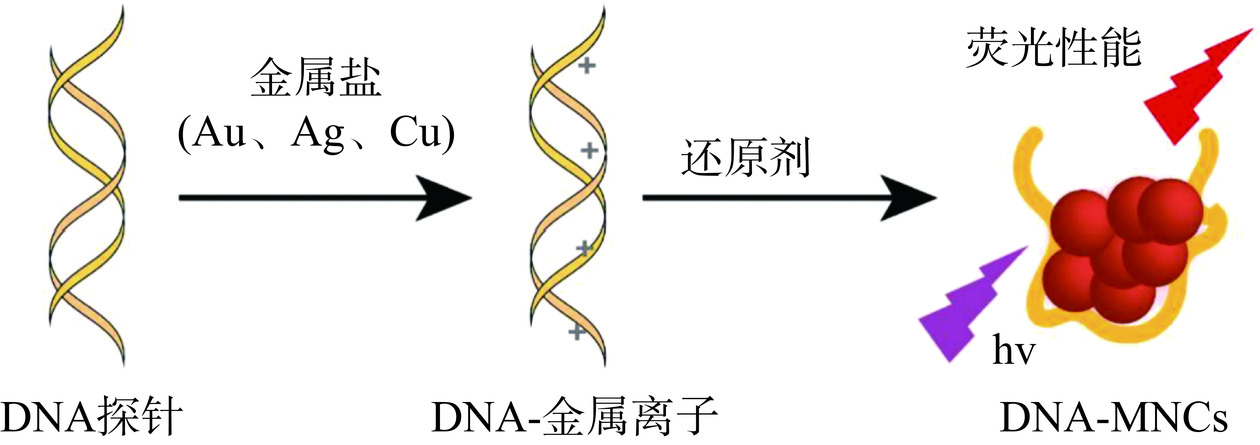
DNA是制备金属纳米团簇的模板和配体,最常用的结构包括具有一级和二级结构的单链DNA(ssDNA)[44]、双链DNA(dsDNA)[45]、三链DNA(tsDNA)[46]和DNA纳米结构[47]。DNA作为模板合成金属纳米团簇的原理是利用DNA分子具有多样性的天然序列和二级结构,通过设计和合成特定的DNA序列可以精确控制金属纳米团簇的大小、形状和组成。
DNA作为模板合成金属纳米团簇主要有3种方法:DNA模板方法:该方法以DNA分子为模板,将金属离子还原为金属纳米团簇,并沉积在DNA模板上,最终通过降解DNA模板获得DNA-MNCs[48];DNA辅助方法:该方法利用DNA分子作为辅助剂参与金属离子还原成金属纳米粒子过程中反应物浓度、pH和离子强度的调节,从而实现DNA-MNCs的形成[5];DNA自组装方法:研究表明,每一种核苷酸都具有与MNCs结合的潜力,并且DNA中的每一种核苷酸都至少包含一个金属结合位点。该方法利用DNA分子的相互吸引力,将多个DNA分子和金属离子组装成特定的DNA-MNCs结构,具有制备简单、可扩展性好的特点[49]。图7介绍了DNA作为模板合成金属纳米团簇的原理示意图[50]。
3. 金属纳米团簇在食品中的应用
金属纳米团簇在食品行业中具有广泛的应用潜力,它们可用于制备具有防菌、抗氧化等特性的纳米材料,应用于食品包装,延长食品的保鲜期并防止发生细菌感染和氧化反应[51]。此外,金属纳米团簇可以用于食品质量检测,如致病菌、农药、霉菌毒素、重金属以及食品中的其他污染物,确保食品的安全和质量[52]。然而,金属纳米团簇在食品中的应用还处于研究和探索阶段,其安全性、稳定性以及对食品质量的影响等问题需要进一步研究和评估。
3.1 食品中污染物的检测
目前,传统的外源性有害残留物分析方法主要依赖于大型仪器或设备,如高效液相色谱、胶束电动色谱法、液相色谱-电喷雾电离串联质谱、气相色谱串联质谱等[53−55],这些方法具有较高的灵敏度和可靠性。然而,由于样品前处理复杂、规模庞大,以及对专业技术人员的要求,这些方法不适合快速检测和现场污染物检测。因此,迫切需要开发准确、灵敏、快速、简便的污染物检测方法,以实现人类健康和环境保护。其中,荧光分析法作为一种快速响应、成本低、灵敏度高和抗干扰能力强的方法,近年来受到广泛关注。金属纳米团簇作为一种荧光纳米材料,由于其易于制造、良好的表面功能性、生物相容性、低毒性以及光致发光特性而引起了人们的广泛关注[56−58]。与传统的仪器方法相比,基于金属纳米团簇的污染物检测方法在定量和定性方面存在一定的局限性。例如,其分析性能通常无法与仪器参与的技术相媲美[59−61]。尽管如此,金属纳米团簇仍然具有快速检测,无需携带大型仪器或进行复杂操作即可实时检测的优点。
金属纳米团簇通过选择性地与目标污染物形成复合物或发生化学反应来增强检测信号。这些反应可以导致荧光增强、颜色变化或电流变化等直接可观测到的信号变化。因此,通过监测这些信号变化,可以确定食品样品中是否存在目标污染物及其浓度。如Liu等[62]通过监测AgNCs在10~106 CFU·mL−1的浓度范围内从蓝色到红色的可见颜色变化来检测单核细胞增生李斯特氏菌。Chen等[63]利用单一的AgNCs,研究了金属离子与AgNCs之间的相互作用,并发现了一种特征性的荧光变化模式,成功地实现了对六种金属离子(Cu2+、Co2+、Ni2+、Cr3+、Mn2+、Fe3+)的高效检测。另外,Sheikholeslami等[64]开发了一种新型的协同双金属纳米团簇,利用金和银作为发射荧光探针,在牛奶样品中同时检测四环素(TC)、氨苄西林(AMP)和磺胺醋酰胺(SAC)。因此,金属纳米团簇作为一种便捷且具有潜力的快速检测方法,为满足准确、灵敏和简便的污染物检测需求提供了新的选择。表1列举了部分基于Au、Ag、Cu金属纳米团簇在食品污染物中的检测实例。
表 1 一些金属纳米团簇在食品污染物中的检测实例Table 1. Examples of detection of some metal nanoclusters in food contaminants.污染物 MNCs 目标食品污染物 参考文献 致病菌 CuNCs 金黄色葡萄球菌 [65] AuNCs 葡萄球菌肠毒素B [66] AgNCs 葡萄球菌肠毒素A [67] AgNCs 金黄色葡萄球菌 [68] 重金属离子 AuNCs Pb2+ [69] AuNCs 血清铜 [70] AuNCs Co2+ [71] CuNCs Fe3+ [72] AuNCs Ag+ [73] CuNCs Hg2+ [74] 农药、抗生素 AuNCs–MnO2 氨基甲酸酯类杀虫剂 [75] AuNCs 有机磷农药 [76] AuNCs 2-甲基-4-氯苯氧乙酸 [77] AgNCs 土霉素(OTC) [78] AgNCs 硅酸盐 [79] CuNCs 有机磷酸盐 [80] Cd(II)-Nd(III)NCs 硝基呋喃类抗生素 [81] PEI-OVA-AuNCs 四环素类 [82] 3.2 食品包装材料
将纳米技术纳入食品包装系统,在改善阻隔性能、提供抗菌活性以及改善生物降解性和生物相容性的领域中已经取得了快速进展,在食品包装领域取得巨大进步。目前,已经开发出具有抗微生物特性的食品包装材料,用于杀死或抑制可能污染食品的病原微生物的腐败和生长。在包装材料中加入不同的金属纳米团簇,可以提高食品安全性并延长食品的保质期[51]。在抗微生物包装中使用的常见纳米结构包括基于Ag的纳米结构(AgNPs、AgNCs),其抗微生物活性来源于几种机制,例如破坏细胞膜完整性[83]、损害生物分子(例如,DNA和蛋白质)[84],调节代谢系统[85],并施加氧化应激,研究者们已经广泛探索了这些纳米结构对超过650种微生物的有效抗微生物活性,包括革兰氏阳性和革兰氏阴性细菌、真菌和病毒[86]。金属纳米团簇在食品包装材料中的应用还处于研究和探索阶段,其安全性、稳定性以及对食品质量的影响等问题需要进一步研究和评估。
3.3 抗菌剂
金属纳米团簇可以通过产生氧化应激或细胞壁穿孔等方式杀死细菌,从而作为抗菌剂使用。目前,人们广泛开发多种MNCs用于抑制和减少有害细菌的生长,其中,AgNCs由于Ag+与微生物界面间独特的化学特性而在抗菌方面的应用最广泛。如Selyawati等[87]以谷胱甘肽(GSH)修饰不同氧化状态的AgNCs(Ag+和Ag0),结果发现,具有丰富Ag+的Ag NCs对革兰氏阴性菌(P. aeruginosa、E.coli)和革当氏阳性菌(B. subrilis、S. aureus)都具有明显的抑菌作用。此外,Verma等[88]提出了一种使用金属有机框架(MOFs)作为支架的简单快速合成Ag NCs的方法。合成的AgNCs-in-MOF纳米复合材料对革兰氏阳性和革兰氏阴性细菌菌株均具有高度杀菌作用,并导致细胞壁穿孔,它还可使DNA损伤,最终导致细菌细胞死亡,而银剂量显著降低。Wang等[89]以杆菌肽为模板合成了抗菌的Au、Ag和Cu纳米团簇,其荧光分别为黄色、青色和红色。AgNCs@bacitracin要比AuNCs@bacitracin和CuNCs@bacitracin抗菌性更强,它不仅对细菌细胞膜的损伤最有效,而且诱导活性氧(ROS)的增加最大。该研究揭示了抗菌活性的机理,为高效抗菌材料的设计提供了新的思路。
3.4 食品添加剂的检测
金属纳米团簇可用于检测食品添加剂的原理是利用其特殊的光学、电学或磁学性质与目标添加剂相互作用。通过将金属纳米团簇与特定的探针分子结合,当目标添加剂存在时,会引起纳米团簇的性质发生改变,可以通过光谱、电化学或磁性等方法来检测其存在与浓度。这种检测方法具有高灵敏度、快速响应和低成本等优势,因此广泛用于食品添加剂的检测领域[52,90]。如Shankar等[91]使用牛血清白蛋白(BSA)封端的AuNCs,它具有较强的红色发光和较高的光稳定性,可选择性、灵敏地测定食品添加剂叔丁基对苯二酚(TBHQ)。另外,亚硝酸盐是一种典型的无机食品防腐剂,Chen等[92]报道了用超支化聚乙烯亚胺保护的银纳米簇(hPEI-AgNCs)检测亚硝酸盐的荧光关闭方法。对于食品添加剂的检测,金属纳米团簇具有巨大的发展潜力,为食品添加剂的高灵敏度检测提供了一种新的工具,具有良好的实际应用前景。
金属纳米团簇在检测食品添加剂方面尽管存在一些缺点,如成本高昂、金属纳米团簇的稳定性等问题,但其作为一种新型检测技术仍然具有潜力,在进一步的研究和发展中可以克服这些问题,并实现更为广泛的应用。
4. 展望
金属纳米团簇的模板合成具有重要的研究价值和应用前景。通过模板合成方法,可以制备出具有精确尺寸和组成的金属纳米团簇,为设计和调控其性质提供了可能性;金属纳米团簇在催化、光学、电子等领域显示出独特的性质,可用于催化剂、生物传感器、光电器件等高性能材料的制备;模板合成方法相对简单且可扩展,有望实现大规模制备金属纳米团簇,满足工业应用需求;金属纳米团簇在食品领域中具有广阔的应用前景,可用于食品安全检测、包装材料改进、食品质量控制等领域,金属纳米团簇在食品领域中的应用仍处于研究和开发阶段,需要进一步的研究来确保其安全性和可行性。
金属纳米团簇的模板合成具有广阔的展望,但也面临一些挑战,如高效的模板合成方法仍然需要进一步发展,以提高产率和控制尺寸、形貌及结构的准确性;对于不同金属纳米团簇材料,如铜、银、金等,需要针对其特定属性开发适用的模板合成策略;纳米团簇的表面结构和组成可能导致合成过程中的不稳定性和聚集现象,需要解决这些挑战;对于某些金属纳米团簇,如镧系和过渡金属纳米团簇,尚缺乏有效的模板合成方法;金属纳米团簇在食品应用中应注意其毒性和安全性、稳定性、生产成本、法规和监管和感知和接受度等。
-
表 1 一些金属纳米团簇在食品污染物中的检测实例
Table 1 Examples of detection of some metal nanoclusters in food contaminants.
污染物 MNCs 目标食品污染物 参考文献 致病菌 CuNCs 金黄色葡萄球菌 [65] AuNCs 葡萄球菌肠毒素B [66] AgNCs 葡萄球菌肠毒素A [67] AgNCs 金黄色葡萄球菌 [68] 重金属离子 AuNCs Pb2+ [69] AuNCs 血清铜 [70] AuNCs Co2+ [71] CuNCs Fe3+ [72] AuNCs Ag+ [73] CuNCs Hg2+ [74] 农药、抗生素 AuNCs–MnO2 氨基甲酸酯类杀虫剂 [75] AuNCs 有机磷农药 [76] AuNCs 2-甲基-4-氯苯氧乙酸 [77] AgNCs 土霉素(OTC) [78] AgNCs 硅酸盐 [79] CuNCs 有机磷酸盐 [80] Cd(II)-Nd(III)NCs 硝基呋喃类抗生素 [81] PEI-OVA-AuNCs 四环素类 [82] -
[1] JIN R C. Quantum sized, thiolate-protected gold nanoclusters[J]. Nanoscale,2010,2(3):343−62. doi: 10.1039/B9NR00160C
[2] MEI L, TENG Z. ZHU G Z, et al. Silver nanocluster-embedded zein films as antimicrobial coating materials for food packaging[J]. ACS Applied MaterialsInterfaces,2017,9(40):35297−35304. doi: 10.1021/acsami.7b08152
[3] CHENG H, CHEN L, JULIAN M D, et al. Recent advances in the application of nanotechnology to create antioxidant active food packaging materials[J]. Critical Reviews in Food Science and Nutrition,2022,64(10):11−16.
[4] MU J, YANG J L, ZHANG D W, et al. Progress in preparation of metal nanoclusters and their application in detection of environmental pollutants[J]. Chinese Journal of Analytical Chemistry,2021,49(3):319−329. doi: 10.1016/S1872-2040(21)60082-8
[5] PAN Y, HAN Z, CHEN S H, et al. Metallic nanoclusters:From synthetic challenges to applications of their unique properties in food contamination detection[J]. Coordination Chemistry Reviews,2023,478(1):214964.
[6] SHANG L, DONG S J, ULRICH N G. Ultra-small fluorescent metal nanoclusters:Synthesis and biological applications[J]. Nano Today,2011,6(4):401−418. doi: 10.1016/j.nantod.2011.06.004
[7] 焦婷, 温昊翔, 李忠平. 基于铜纳米簇荧光猝灭选择性检测盐酸阿霉素[J]. 分析化学,2022,50(2):9. [JIAO T, WEN H X, LI Z P. Selective detection of doxorubicin hydrochloride based on fluorescence quenching of copper nanoclusters[J]. Chinese Journal of Analytical Chemistry,2022,50(2):9.] JIAO T, WEN H X, LI Z P. Selective detection of doxorubicin hydrochloride based on fluorescence quenching of copper nanoclusters[J]. Chinese Journal of Analytical Chemistry, 2022, 50(2): 9.
[8] WANG B Q, GUI R J, JIN H, et al. Red-emitting BSA-stabilized copper nanoclusters acted as a sensitive probe for fluorescence sensing and visual imaging detection of rutin[J]. Talanta,2018,178:1006−1010. doi: 10.1016/j.talanta.2017.08.102
[9] XIE J P, ZHENG Y G, YING J Y. Protein-directed synthesis of highly fluorescent gold nanoclusters[J]. Journal of the American Chemical Society,2009,131(3):888−889. doi: 10.1021/ja806804u
[10] SLOCIK J M, WRIGHT D W. Biomimetic mineralization of noble metal nanoclusters[J]. Biomacromolecules,2003,4(5):1135−1141. doi: 10.1021/bm034003q
[11] CATHCART N, MISTRY P, MAKRA C, et al. Chiral thiol-stabilized silver nanoclusters with well-resolved optical transitions synthesized by a facile etching procedure in aqueous solutions[J]. Langmuir:The ACS Journal of Surfaces and Colloids,2009,25(10):5840−5846. doi: 10.1021/la9005967
[12] IVANOVA N K, KARPUSHKIN E A, LOPATINA L I, et al. DNA as a template for synthesis of fluorescent gold nanoclusters[J]. Mendeleev Communications,2023,33(3):346−348. doi: 10.1016/j.mencom.2023.04.016
[13] ESMA K, THALAPPIL P. New Routes for multicomponent atomically precise metal nanoclusters[J]. ACS Omega,2021,6(1):11−16.
[14] CUI M L, ZHAO Y, SONG Q J. Synthesis, optical properties and applications of ultra-small luminescent gold nanoclusters[J]. Trends in Analytical Chemistry, 2014:5773−5782.
[15] XIAO Y, WU Z N, YAO Q F, et al. Luminescent metal nanoclusters:Biosensing strategies and bioimaging applications[J]. Aggregate,2021,2(1):114−132. doi: 10.1002/agt2.11
[16] BARBARA C, GASPAR M J M, SEQUEIRA P J F. Encapsulation of gold nanoclusters:stabilization and more[J]. Nanoscale,2021,13(41):17199−17217. doi: 10.1039/D1NR04939A
[17] ZHENG G, PETTY T J, DICKSON M R. High quantum yield blue emission from water-soluble Au8 nanodots[J]. Journal of the American Chemical Society,2003,125(26):7780−7781. doi: 10.1021/ja035473v
[18] HAN F, LI J H, WANG W R, et al. Synthesis of silver nanoclusters by irradiation reduction and detection of Cr3+ ions[J]. RSC Advances,2022,12(51):33207−33214. doi: 10.1039/D2RA06536C
[19] SHI Y E, LUO S J, JI X J, et al. Synthesis of ultra-stable copper nanoclusters and their potential application as a reversible thermometer[J]. Dalton Transactions,2017,46(41):14251−14255. doi: 10.1039/C7DT02193C
[20] GE S, HAN Y M, SUN M L, et al. Functionalization of polymer-wrapped silver nanoclusters and potential applications as antimicrobial mask materials[J]. ACS Omega,2023,8(45):42678−42688. doi: 10.1021/acsomega.3c05454
[21] LOURDU P X, KAMALESH C, ANANYA B, et al. Protein-protected luminescent noble metal quantum clusters:An emerging trend in atomic cluster nanoscience[J]. Nano Reviews,2012,3(1):14767−14767. doi: 10.3402/nano.v3i0.14767
[22] DICKERSON B M, SANDHAGE H K, NAIK R R. Protein- and peptide-directed syntheses of inorganic materials[J]. Chemical Reviews,2008,108(11):4935−78. doi: 10.1021/cr8002328
[23] CHEVRIER M D, CHATT A, ZHANG P. Properties and applications of protein-stabilized fluorescent gold nanoclusters:Short review[J]. Journal of Nanophotonics,2012,6(1):064504. doi: 10.1117/1.JNP.6.064504
[24] GOSWAMI N, SAHA R, PAL K S. Protein-assisted synthesis route of metal nanoparticles:Exploration of key chemistry of the biomolecule[J]. Journal of Nanoparticle Research,2011,13(10):5485−5495. doi: 10.1007/s11051-011-0536-3
[25] LI M Z, Z HANG Y D, MA J L, et al. Albumin-based nanoparticle for dual-modality imaging of the lymphatic system[J]. RSC Advances,2023,13(4):2248−2255. doi: 10.1039/D2RA07414A
[26] KAWASAKI H, YOSHIMURA K, HAMAGUCHI K, et al. Trypsin-stabilized fluorescent gold nanocluster for sensitive and selective Hg2+ detection[J]. Analytical Sciences:The International Journal of the Japan Society for Analytical Chemistry,2011,27(6):591. doi: 10.2116/analsci.27.591
[27] WEN F, DONG Y H, FENG L, et al. Horseradish peroxidase functionalized fluorescent gold nanoclusters for hydrogen peroxide sensing[J]. Analytical Chemistry,2011,83(4):1193−1196. doi: 10.1021/ac1031447
[28] JOSEPH D, GECKELER. K E. Synthesis of highly fluorescent gold nanoclusters using egg white proteins[J]. Colloids and Surfaces B:Biointerfaces,2014,115:46−50. doi: 10.1016/j.colsurfb.2013.11.017
[29] TIAN J H, YAN L, SANG A, et al. Microwave-Assisted synthesis of red-light emitting Au nanoclusters with the use of egg white[J]. Journal of Chemical Education,2014,91(10):1715−1719. doi: 10.1021/ed400605y
[30] WEI H, WANG Z D, YANG L M, et al. Lysozyme-stabilized gold fluorescent cluster:synthesis and application as Hg 2+ sensor[J]. The Analyst,2010,135(6):1406−1410. doi: 10.1039/c0an00046a
[31] KAWASAKI H, HAMAGUCHI K, OSAKA I, et al. pH‐dependent synthesis of pepsin-mediated gold nanoclusters with blue green and red fluorescent emission[J]. Advanced Functional Materials,2011,21(18):3508−3515. doi: 10.1002/adfm.201100886
[32] CHEN Y F, QIAO J, LIU Q R, et al. Fluorescence turn-on assay for detection of serum D-penicillamine based on papain @AuNCs-Cu2+ complex[J]. Analytica Chimica Acta,2018,1026:133−139. doi: 10.1016/j.aca.2018.04.014
[33] LING S J, LIANG H Y, LI Z, et al. Soy protein-directed one-pot synthesis of gold nanomaterials and their functional conductive devices[J]. Journal of Materials Chemistry B,2016,4(21):3643−3650. doi: 10.1039/C6TB00616G
[34] AIRES A, LLARENA I, MOLLER M, et al. A simple approach to design proteins for the sustainable synthesis of metal nanoclusters[J]. Angewandte Chemie,2019,58(19):6214−6219. doi: 10.1002/anie.201813576
[35] HA K S, KWARK G, J UN, K W, et al. Ordered mesoporous carbon nanochannel reactors for high-performance Fischer-Tropsch synthesis[J]. Chemical Communications,2013,49(45):5141−3. doi: 10.1039/c3cc00297g
[36] SÖPTEI B, NAGY N L, BARANYAI P, et al. On the selection and design of proteins and peptide derivatives for the production of photoluminescent, red-emitting gold quantum clusters[J]. Gold Bulletin,2013,46(3):195−203. doi: 10.1007/s13404-013-0100-2
[37] YUAN M, LIAN J Q, HAN X N, et al. Real-time fluorescence dynamics in one-step synthesis of gold nanoclusters coupling with peptide motifs[J]. Colloids and Surfaces B:Biointerfaces,2022,219:112820. doi: 10.1016/j.colsurfb.2022.112820
[38] WANG Y L, CUI Y Y, ZHAO Y L, et al. Bifunctional peptides that precisely biomineralize Au clusters and specifically stain cell nuclei[J]. Chemical Communications,2012,48(6):871−873. doi: 10.1039/C1CC15926G
[39] DU P, ZHANG J, M A J Y, et al. Synthesis of copper nanoclusters and their application for environmental pollutant probes:A review[J]. Critical Reviews in Analytical Chemistry, 2022: 11−14.
[40] WANG E D, XU W W, ZHU B, et al. Understanding the chemical insights of staple motifs of thiolate-protected gold nanoclusters[J]. Small,2020,17(27):2001836.
[41] WANG Z G, CHEN B K, ROGACH L A. Synthesis, optical properties and applications of light-emitting copper nanoclusters[J]. Nanoscale Horizons,2017,2(3):135−146. doi: 10.1039/C7NH00013H
[42] ZHANG H D, TAO B B, WU N N, et al. Inter filter effect between fluorescent copper nanoclusters and Cr (VI) and its application for probing the activity of alkaline phosphatase[J]. Microchemical Journal, 2023:193.
[43] YAO Q F, CHEN T K, YUAN X, et al. Toward total synthesis of thiolate-protected metal nanoclusters[J]. Accounts of Chemical Research,2018,51(6):1338−1348. doi: 10.1021/acs.accounts.8b00065
[44] AI J, GUO W W, LI B L, et al. DNA G-quadruplex-templated formation of the fluorescent silver nanocluster and its application to bioimaging[J]. Talanta, 2012:88450-88455.
[45] MA K, SHAO Y, CUI Q H, et al. Base-stacking-determined fluorescence emission of DNA abasic site-templated silver nanoclusters[J]. Langmuir:The ACS Journal of Surfaces and Colloids,2012,28(43):15313−22. doi: 10.1021/la301957m
[46] FENG LG, HUANG Z Z, REN J S, et al. Toward site-specific, homogeneous and highly stable fluorescent silver nanoclusters fabrication on triplex DNA scaffolds[J]. Nucleic Acids Research,2012,40(16):e122. doi: 10.1093/nar/gks387
[47] PARK J, SONG J, PARK J, et al. Branched DNA-based synthesis of fluorescent silver nanocluster[J]. Bulletin of the Korean Chemical Society,2014,35(4):1105−1109. doi: 10.5012/bkcs.2014.35.4.1105
[48] XU F Z, QING T P, QING Z H. DNA-coded metal nano-fluorophores:preparation, properties and applications in biosensing and bioimaging[J]. Nano Today, 2021, 36: 101021.
[49] WANG Z G, LIU Q, LI N, et al. DNA-based nano-template directed in situ synthesis of silver nanoclusters with specific fluorescent emission:Surface-guided chemical reactions[J]. Chemistry of Materials,2016,28:8834−8841. doi: 10.1021/acs.chemmater.6b04150
[50] PANDYA A, LAD N A, SINGH P S, et al. DNA assembled metal nanoclusters:Synthesis to novel applications[J]. RSC Advances,2016,114(6):113095−113114.
[51] FAHMY M H, ELDIN S E R, SEREA A S E, et al. Advances in nanotechnology and antibacterial properties of biodegradable food packaging materials[J]. RSC Advances,2020,10(35):20467−20484. doi: 10.1039/D0RA02922J
[52] CHEN L, CHENG Z H, LUO M, et al. Fluorescent noble metal nanoclusters for contaminants analysis in food matrix[J]. Critical Reviews in Food Science and Nutrition,2021,63(19):11−19.
[53] JEYAKUMAR J M J, ZHANG M Q, THIRUVENGADAM M. Determination of mycotoxins by HPLC, LC-ESI-MS/MS, and MALDI-TOF MS in fusarium species-infected sugarcane[J]. Microbial Pathogenesis,2018,123:98−110. doi: 10.1016/j.micpath.2018.06.045
[54] NARENDERAN S T, MEYYANATHAN S N, BABU B. Review of pesticide residue analysis in fruits and vegetables. Pre-treatment, extraction, and detection techniques[J]. Food Research International,2020,133:109141. doi: 10.1016/j.foodres.2020.109141
[55] WEI J C, HU J, CAO J L, et al. Sensitive detection of organophosphorus pesticides in medicinal plants using ultrasound-assisted dispersive liquid-liquid microextraction combined with sweeping micellar electrokinetic chromatography[J]. Journal of Agricultural and Food Chemistry,2016,64(4):932−940. doi: 10.1021/acs.jafc.5b05369
[56] KANG X, ZHU M Z. Tailoring the photoluminescence of atomically precise nanoclusters[J]. Chemical Society Reviews,2019,48(8):2422−2457. doi: 10.1039/C8CS00800K
[57] LI C G, CHEN H, CHEN B, et al. Highly fluorescent gold nanoclusters stabilized by food proteins:from preparation to application in detection of food contaminants and bioactive nutrients[J]. Critical Reviews in Food Science and Nutrition,2018,58(5):689−699. doi: 10.1080/10408398.2016.1213698
[58] ZHANG L B, WANG E K. Metal nanoclusters:New fluorescent probes for sensors and bioimaging[J]. Nano Today,2014,9(1):132−157. doi: 10.1016/j.nantod.2014.02.010
[59] BIAN R X, WU X T, CHAI F, et al. Facile preparation of fluorescent Au nanoclusters-based test papers for recyclable detection of Hg2+ and Pb2+[J]. Sensors and Actuators B:Chemical,2017,241:592−600. doi: 10.1016/j.snb.2016.10.120
[60] CHEN S, KUANG Y F, ZHANG P P, et al. A dual-functional spectroscopic probe for simultaneous monitoring Cu2+ and Hg2+ ions by two different sensing nature based on novel fluorescent gold nanoclusters[J]. Sensors and Actuators B:Chemical,2017,253:283−291. doi: 10.1016/j.snb.2017.06.140
[61] GAO P, WU S, CHANG X, et al. Aprotinin encapsulated gold nanoclusters:a fluorescent bioprobe with dynamic nuclear targeting and selective detection of trypsin and heavy metal[J]. Bioconjugate Chemistry,2018,29(12):4140−4148. doi: 10.1021/acs.bioconjchem.8b00773
[62] LIU Y S, WANG J, SONG X L, et al. Colorimetric immunoassay for listeria monocytogenes by using core gold nanoparticles, silver nanoclusters as oxidase mimetics, and aptamer-conjugated magnetic nanoparticles[J]. Microchimica Acta,2018,185(8):1−7.
[63] CHEN X H, XU J M, ZHOU H M, et al. Tree-based machine learning models assisted fluorescent sensor array for detection of metal ions based on silver nanocluster probe[J]. Spectrochimica Acta Part A:Molecular and Biomolecular Spectroscopy,2023,297:122738. doi: 10.1016/j.saa.2023.122738
[64] SHEIKHOLESLAMI M N, HAMIDIPANAH Y, SALEHNIA F, et al. Multiplex detection of antibiotic residues in milk:Application of MCR-ALS on excitation-emission matrix fluorescence (EEMF) data sets[J]. Analytical Chemistry,2022,94(16):6206−6215. doi: 10.1021/acs.analchem.1c05592
[65] QING T P, LONG C C, WANG X, et al. Detection of micrococcal nuclease for identifying staphylococcus aureus based on DNA templated fluorescent copper nanoclusters[J]. Mikrochimica Acta,2019,186(4):248. doi: 10.1007/s00604-019-3363-3
[66] XIE X X, TAN F, XU A Q, et al. UV-induced peroxidase-like activity of gold nanoclusters for differentiating pathogenic bacteria and detection of enterotoxin with colorimetric readout[J]. Sensors & Actuators:B. Chemical, 2018, 279:289-297.
[67] ZHANG X Y, KHAN I M, JI H, et al. A label-free fluorescent aptasensor for detection of staphylococcal enterotoxin a based on aptamer-functionalized silver nanoclusters[J]. Polymers,2020,12(1):152−152. doi: 10.3390/polym12010152
[68] YAO S, ZHAO C, LIU Y S, et al. Colorimetric immunoassay for the detection of Staphylococcus aureus by using magnetic carbon dots and sliver nanoclusters as o-phenylenediamine-oxidase mimetics[J]. Food Analytical Methods,2020,13(4):1−6.
[69] BHAMORE J R, GUL A R, CHAE W, et al. One-pot fabrication of amino acid and peptide stabilized gold nanoclusters for the measurement of the lead in plasma samples using chemically modified cellulose paper[J]. Sensors and Actuators B:Chemical, 2020, 322:128603.
[70] CHEN Y F, QIAO J, LIU Q R, et al. Ovalbumin-stabilized gold nanoclusters with ascorbic acid as reducing agent for detection of serum copper[J]. Chinese Chemical Letters,2018,29(3):366−370. doi: 10.1016/j.cclet.2017.10.014
[71] LAKKAKULA J R, DIVAKARAN D, THAKUR M, et al. Cyclodextrin-stabilized gold nanoclusters for bioimaging and selective label-free intracellular sensing of Co2+ ions[J]. Sensors and Actuators B:Chemical,2018,262:270−281.
[72] HUANG H, LI H, FENG J J, et al. One-pot green synthesis of highly fluorescent glutathione-stabilized copper nanoclusters for Fe3+ sensing[J]. Sensors and Actuators B:Chemical, 2017, 241:292−297.
[73] HU Y L, LIU A Y, WU B C, et al. Modulating fluorescence emission of L-methionine-stabilized Au nanoclusters from green to red and its application for visual detection of silver ion[J]. Microchemical Journal,2021,166(1):106198.
[74] ZHANG C, LIANG M N, SHAO C Y, et al. Visual detection and sensing of mercury ions and glutathione using fluorescent copper nanoclusters[J]. ACS Applied Bio Materials,2023,6(3):1283−1293. doi: 10.1021/acsabm.3c00031
[75] YAN X, KONG D S, JIN R, et al. Fluorometric and colorimetric analysis of carbamate pesticide via enzyme-triggered decomposition of gold nanoclusters-anchored MnO2 nanocomposite[J]. Sensors and Actuators B:Chemical,2019,290:640-647.
[76] WEI D L, LI M W, WANG Y, et al. Encapsulating gold nanoclusters into metal–organic frameworks to boost luminescence for sensitive detection of copper ions and organophosphorus pesticides[J]. Journal of Hazardous Materials,2023,441:129890. doi: 10.1016/j.jhazmat.2022.129890
[77] NAZIR K, AHMED A, HUSSAIN S Z, et al. Development of gold nanoclusters based direct fluorescence restoration approach for sensitive and selective detection of pesticide[J]. Applied Nanoscience,2020,10(9):1−10.
[78] HOSSEINI M, MEHRABI F, GANJALI M R, et al. A fluorescent aptasensor for sensitive analysis oxytetracycline based on silver nanoclusters[J]. Luminescence:The Journal of Biological and Chemical Luminescence,2016,31(7):1339−1343. doi: 10.1002/bio.3112
[79] HU Y, LI Y P, LIAO Y W, et al. Poly (sodium-p-styrenesulfonate-enhanced fluorescent silver nanoclusters for the assay of two food flavors and silicic acid[J]. Food Chemistry,2020,318:126502. doi: 10.1016/j.foodchem.2020.126502
[80] BAGHERI H, AFKHAMI A, KHOSHSAFAR H, et al. Protein capped Cu nanoclusters-SWCNT nanocomposite as a novel candidate of high performance platform for organophosphates enzymeless biosensor[J]. Biosensors and Bioelectronics, 2017, 89(Pt 2):829-836.
[81] LIU Y, ZHAO J N, YANG X P, et al. A heptanuclear Cd (II)-Nd (III) nanocluster with NIR luminescence response to nitrofuran antibiotics[J]. Polyhedron,2023,240:116451. doi: 10.1016/j.poly.2023.116451
[82] LI M T, ZHU N W, ZHU W, et al. Enhanced emission and higher stability ovalbumin-stabilized gold nanoclusters (OVA-AuNCs) modified by polyethyleneimine for the fluorescence detection of tetracyclines[J]. Microchemical Journal,2021,169:106560. doi: 10.1016/j.microc.2021.106560
[83] BONDARENKO O M, SIHTMÄE M, KUZMIČIOVA J, et al. Plasma membrane is the target of rapid antibacterial action of silver nanoparticles in Escherichia coli and pseudomonas aeruginosa[J]. International Journal of Nanomedicine,2018,13:6779−6790. doi: 10.2147/IJN.S177163
[84] BISWAS M C, TIIMOB B J, ABDELA W, et al. Nano silica-carbon-silver ternary hybrid induced antimicrobial composite films for food packaging application[J]. Food Packaging and Shelf Life,2019,19:104−113. doi: 10.1016/j.fpsl.2018.12.003
[85] HOSEINNEJAD M, JAFARI S M, KATOUZIAN I. Inorganic and metal nanoparticles and their antimicrobial activity in food packaging applications[J]. Taylor & Francis,2018,44(2):161−181.
[86] SIDDIQUI N, BHARDWAJ A, HADA R, et al. Synthesis, characterization and antimicrobial study of poly (methyl methacrylate)/Ag nanocomposites[J]. Vacuum,2018,153:6−11. doi: 10.1016/j.vacuum.2018.03.036
[87] SETYAWATI M I, YUAN X, XIE J P, et al. The influence of lysosomal stability of silver nanomaterials on their toxicity to human cells[J]. Biomaterials,2014,35(25):6707−6715. doi: 10.1016/j.biomaterials.2014.05.007
[88] VERMA A, SINGH A, SHUKLA N, et al. Synthesis of highly stable luminescent silver nanoclusters in metal–organic framework for heightened antibacterial activity[J]. Applied Physics A,2022,128(4):1−10.
[89] WANG S S, WANG Y Y, PENG Y, et al. Exploring the antibacteria performance of multicolor Ag, Au, and Cu nanoclusters[J]. ACS Applied Materials & Interfaces,2019,11(8):8461−8469.
[90] WANG D W, WANGZ Q, WANG X B, et al. Functionalized copper nanoclusters-based fluorescent probe with aggregation-induced emission property for selective detection of sulfide ions in food additives[J]. Journal of Agricultural and Food Chemistry,2020,68(40):11301−11308. doi: 10.1021/acs.jafc.0c04275
[91] SHANKAR S, GOWTHAMAN N S K, ARUL P, et al. Ultra-sensitive and selective determination of a phenolic food additive using protein capped gold nanoclusters:A dual in-line fluorometric and colorimetric sensing probe[J]. New Journal of Chemistry,2021,45:1278−1285. doi: 10.1039/D0NJ04712K
[92] CHEN C, YUAN Z Q, CHANG H, et al. Silver nanoclusters as fluorescent nanosensors for selective and sensitive nitrite detection[J]. Analytical Methods,2016,8:2628−2633. doi: 10.1039/C6AY00214E





 下载:
下载:
 下载:
下载:



