Inhibitory Effect of Polyphenols from Arctium lappa Roots on Lipase
-
摘要: 为明确牛蒡根中多酚成分对脂肪酶的抑制作用,本文提取并富集牛蒡根中的多酚成分,采用福林-西奥卡特(Folin-Ciocalteu)法测定总酚含量;HPLC-UV法测定多酚部位中指标性成分的含量;对硝基苯基丁酸酯(p-NPB)法测定牛蒡根多酚和其指标性成分对脂肪酶的抑制效果、抑制类型,采用等效线图解法和药物联合指数(CI)测定牛蒡根中的多酚单体化合物联合使用时的抑制作用。结果表明,富集后的牛蒡根多酚部位的总多酚含量达到51.23%,5种指标性成分的含量占纯化后牛蒡根多酚含量的12.87%,牛蒡根多酚对脂肪酶半数抑制浓度IC50值为1.706 mg/mL,抑制类型为可逆性竞争型抑制。新绿原酸、隐绿原酸、咖啡酸、绿原酸和异绿原酸A对脂肪酶的IC50值分别为0.872、0.910、0.280、0.847、0.244 mg/mL。绿原酸和异绿原酸A浓度比为1:2.15时,CI值为0.549,咖啡酸和异绿原酸A浓度比为1:0.87时,CI值为0.282,对脂肪酶具有协同抑制作用。本研究为探究牛蒡根降脂功效的物质基础及其降脂的机制提供了实验依据,为牛蒡根的开发奠定基础。Abstract: In order to demonstrate the inhibitory effect of polyphenols from Arctium lappa roots (ALP) on lipase, the ALP were extracted and enriched. The content of total polyphenol was determined by Folin-Ciocalteu method, and the index components in ALP were determined by high performance liquid chromatography-UV (HPLC-UV) method. The inhibitory effect and type on lipase of ALP and its index components were determined by p-nitrophenyl butyrate (p-NPB) method. Combined inhibitory effect of them were evaluated by equivalent line diagram method and drug combination index (CI). The results showed that the total polyphenol of ALP reached 51.23%, and the contents of the five index components, i.e., neochlorogenic acid, cryptochlorogenic acid, caffeic acid, chlorogenic acid, and isochlorogenic acid A accounted for 12.87%. ALP showed reversible competitive inhibition on lipase with half maximal inhibitory concentration (IC50) value 1.706 mg/mL. The IC50 of neochlorogenic acid, cryptochlorogenic acid, caffeic acid, chlorogenic acid, and isochlorogenic acid A were 0.872, 0.910, 0.280, 0.847, 0.244 mg/mL, respectively. When the concentration ratio of chlorogenic acid and isochlorogenic acid A was 1:2.15, the CI value was 0.549, and when the concentration ratio of caffeic acid to isochlorogenic acid A was 1:0.87, the CI value was 0.282, which indicated synergistic inhibition effect on lipase. This study provides a theoretical basis for exploring the chemical basis of the lipid-lowering effect of Arctium lappa roots and its lipid-lowering mechanism, and layed a foundation for the development of Arctium lappa roots.
-
Keywords:
- Arctium lappa roots /
- polyphenol /
- lipase /
- inhibition
-
高脂血症是以总胆固醇、甘油三酯、低密度脂蛋白胆固醇含量升高,高密度脂蛋白胆固醇含量降低为主要特征的血脂代谢异常疾病[1],人体内的能量吸收和消耗长期失衡是其重要诱因[2],其正在严重危害人们的健康与生活。人体内有多种酶参与脂质的吸收与代谢,脂肪酶是最重要的、研究最深入的治疗肥胖的靶点[3]。脂肪酶负责水解约50%~70%的膳食脂肪,是调控哺乳动物脂类物质吸收的关键酶[4]。抑制消化器官中脂肪酶的活性可减少机体对膳食脂肪的消化和吸收,进而改善高脂血症的症状[5]。目前临床上常见的降血脂药为奥利司他,是一种强效的脂肪酶抑制剂,能够阻止大约人体内30%的膳食脂肪吸收[6]。其在临床上虽有良好的降脂作用,但却伴随着不可忽视的胃肠道不良反应,如腹泻和便秘等[7]。因此,从天然食品中开发有降脂作用且安全的脂肪酶抑制剂具有一定的研究意义。
牛蒡(Arctium lappa L.)为菊科(Asteraceae)牛蒡属(Arctium)植物,广泛种植于东亚各国,特别是中国、日本和韩国,为药食同源植物,具有降血糖、降血脂、降血压、保护血管内皮及改善II型糖尿病症状等作用[8−12]。牛蒡根中含有多酚类化合物如阿魏酰基/咖啡酰基奎宁酸类化合物(绿原酸、异绿原酸A、B、C)等[13]。其中,绿原酸具有抑制脂肪酶和糖脂代谢的作用[14−15]。多酚类化合物是一种重要的脂肪酶抑制剂[16],长期摄入含有多酚的食物能够有效的预防机体衰老并控制诸如糖尿病、肥胖等相关疾病的发展[17−20]。然而,现阶段对牛蒡根多酚类物质的研究主要集中在抗氧化活性和清除氧自由基的能力等[21],对其抑制脂肪酶活性和协同效应的研究较少。课题组前期实验表明结构类似物牡荆苷和异牡荆苷可协同抑制于脂肪酶,二者联合作用优于单独使用时的抑制效果,且半数抑制浓度低[22]。多酚类成分可以与脂肪酶的多个位点结合[23],牛蒡根中的多为阿魏酰基/咖啡酰基奎宁酸类化合物,为结构类似物,其可能存在协同抑制作用。因此,牛蒡根多酚抑制脂肪酶的作用值得深入研究。
本文对牛蒡多酚进行提取和富集,采用福林-西奥卡特(Folin-Ciocalteu)法测定总酚含量、HPLC-UV法测定多酚部位中指标性成分的含量、对硝基苯基丁酸酯(p-NPB)法测定牛蒡根多酚和其指标性成分对脂肪酶的抑制作用和抑制类型。采用等效线图解法和药物联合指数测定其联合使用时的抑制作用。明确牛蒡根中多酚类成分对脂肪酶的抑制作用,为深入开发牛蒡根奠定实验基础,为发挥牛蒡根在保健食品开发中的作用提供科学依据。
1. 材料与方法
1.1 材料与仪器
牛蒡 安徽毫州沁圆春堂提供,经辽宁中医药大学许亮教授鉴定为菊科牛蒡属植物牛蒡(Arctium lappa)的干燥根茎;猪胰脂肪酶(30000 U/g) 上海源叶生物公司;对硝基苯基丁酸酯 北京酷来搏科技有限公司;氯化钠 分析纯,国药集团化学试剂有限公司;无水碳酸钠 分析纯,中国上海虹光化工厂;乙醇(95%) 铁岭康泰消毒剂有限公司;磷酸 分析纯,西陇化工股份有限公司;D101大孔吸附树脂、没食子酸(≥98%)、福林试剂、奥利司他、新绿原酸(≥98%)、隐绿原酸(≥98%)、咖啡酸(≥98%)、异绿原酸A(≥98%) 标准品,北京索莱宝科技有限公司;甲醇(色谱级)、石油醚(分析纯)、正丁醇(分析纯)、氯化钙(分析纯) 天津科密欧化学试剂有限公司;乙腈 色谱级,Oceanpak Alexative Chemical;绿原酸(≥98%) 标准品,四川维克奇生物生物技术有限公司;纯净水 娃哈哈。
KHB ST-360酶标分析仪 上海科华生物工程股份有限公司;Sartorious CP225D型精密天平 北京塞多斯仪器系统有限公司;FA-1004电子天平 上海精科天平厂;RE-3000旋转蒸发仪 上海亚荣生化仪器厂;SHZ-DⅢ型循环水真空泵、HH-S型水浴锅 巩仪市予华仪器责任有限公司;HC-2518R高速冷冻离心机 安徽中科中佳科学仪器有限公司;可调式移液器 Eppendorf;UV-2100 紫外分光光度计 上海UNICO公司;XK96涡旋振荡器 江苏新康医疗器械有限公司;SHZ-82恒温振荡器 常州国华公司;Agilent 1100高效液相色谱仪 安捷伦科技有限公司;Diamonsil C18色谱柱(250 mm×4.6 mm,5 μm) 北京迪科马科技有限公司。
1.2 实验方法
1.2.1 牛蒡根多酚(ALP)的提取及纯化
参照文献[24]的方法略作修改提取并富集牛蒡根多酚,具体操作如下:取牛蒡根4500 g用煎药袋包裹,使用10倍量50%乙醇室温浸泡3次,每次24 h,于35 ℃减压浓缩至无醇味,得到牛蒡根浸膏,合并三次浸膏共1800 g。将浸膏经水分散后与石油醚按体积比1:1的比例置于分液漏斗中,振荡溶液使得两相溶液充分接触,静置1 h分取下层溶液。按上述方法处理5次,将下层溶液于25 ℃减压蒸馏除去残留的石油醚。将脱脂后的溶液与正丁醇按体积比1:1的比例置于分液漏斗中,振荡溶液使得两相溶液充分接触,静置1 h,将上层萃取液倾出,按上述方法处理5次,合并上层萃取液[25]。于60 ℃减压回收正丁醇,浓缩后得膏重188.13 g,加水分散后过D101大孔吸附树脂,上样量与大孔吸附树脂的质量比为1:10,依次以水、40%乙醇、95%乙醇洗脱,每种洗脱剂洗脱5个柱体积,收集40%乙醇洗脱部位[26],即得牛蒡根多酚(ALP)浸膏51.9 g,计算公式如下。
浸膏得率(%)=提取所得浸膏质量原料的质量×100 (1) 1.2.2 ALP中总酚含量测定
总酚含量测定采用福林-西奥卡特(Folin-Ciocalteu)法[27],没食子酸作为标准品。没食子酸标准品用甲醇溶解,配制成浓度为0.04、0.05、0.06、0.07、0.08、0.09、0.10 mg/mL的标准液,分别准确移取上述标准液0.3 mL,加入福林酚试剂1 mL充分混合,室温避光放置5 min后,加入0.1 g/mL碳酸钠溶液3 mL,蒸馏水定容至5 mL。室温避光放置1 h,在760 nm处测定吸光度。以没食子酸浓度为横坐标,吸光度为纵坐标,绘制标准曲线[28],y=6.9536x+0.0021(R2=0.9995,线性范围0.04~0.10 mg/mL)。精密称取纯化后的ALP 0.2087 g,加入9 mL甲醇充分溶解,取上清液稀释120倍,按上述方法测定吸光度,将吸光度带入标准曲线得到ALP中总酚浓度。
1.2.3 ALP化学成分分析及含量测定
利用高效液相色谱仪测定纯化后牛蒡根的化学组成。色谱柱:Diamonsil C18 (250 mm×4.6 mm,5 μm);流动相:乙腈(A)-0.1%磷酸水(B),梯度洗脱:0~20 min,11%~15%A;21~35 min,15%~22%A;36~60 min,22%A;61~70 min,22%~60%A。流速:1 mL/min;柱温:30 ℃;进样量20 μL。检测波长:327 nm[29]。以标准品浓度为横坐标、以峰面积为纵坐标绘制各标准品的工作曲线(表1)。
表 1 标准品的工作曲线Table 1. Working curves of standard products标准品 回归方程 R2 线性范围(mg/mL) 新绿原酸 y=67058x−254.47 0.9998 0.04~0.80 隐绿原酸 y=81990x+34.05 0.9997 0.0125~0.25 咖啡酸 y=88985x+2413.2 0.9994 0.05~1.0 绿原酸 y=64794x+508.64 0.9993 0.075~1.50 异绿原酸A y=82737x+3095.4 0.9993 0.075~1.50 称取ALP 10.4 mg,以1 mL甲醇溶解,过0.45 μm微孔滤膜,取续滤液进样分析,记录峰面积,代入标准曲线计算ALP中各成分含量。
1.2.4 脂肪酶抑制实验
1.2.4.1 ALP对脂肪酶的抑制作用
根据文献[30]方法稍作修改,即将p-NPB预先溶解在乙腈中,配制成5.6 μmol/mL的溶液,−20 ℃保存备用。配制含有CaCl2(1.3 mmol/L)、NaCl(150 mmol/L)的Tris-HCl缓冲液(13 mmol/L,pH=7.2),4 ℃保存备用。取适量脂肪酶溶解在Tris-HCl缓冲液中,4 ℃,3600×g离心20 min,收取上清液,配制成0.25 mg/mL的脂肪酶溶液,4 ℃保存备用,现用现配。以甲醇配制浓度为0.4、0.8、1.2、1.6、2.0 mg/mL的ALP溶液,将50 μL不同浓度的ALP溶液分别加入96孔板中,并加入130 μL脂肪酶溶液,37 ℃孵育10 min,加入20 μL 5.60 μmol/mL底物p-NPB溶液,37 ℃孵育20 min后于405 nm处读取吸光度值。空白对照组用甲醇代替ALP溶液,Tris-HCl缓冲液代替脂肪酶溶液,奥利司他为阳性对照,每组实验平行做3次,计算抑制率。
抑制率(%)=(1−C−DA−B)×100 (2) 式中:A、B、C和D分别为空白组(不加样加酶)、空白对照组(不加样不加酶)、样品组(加样加酶)和样品对照组(加样不加酶)的吸光度值。
1.2.4.2 ALP对脂肪酶的抑制类型
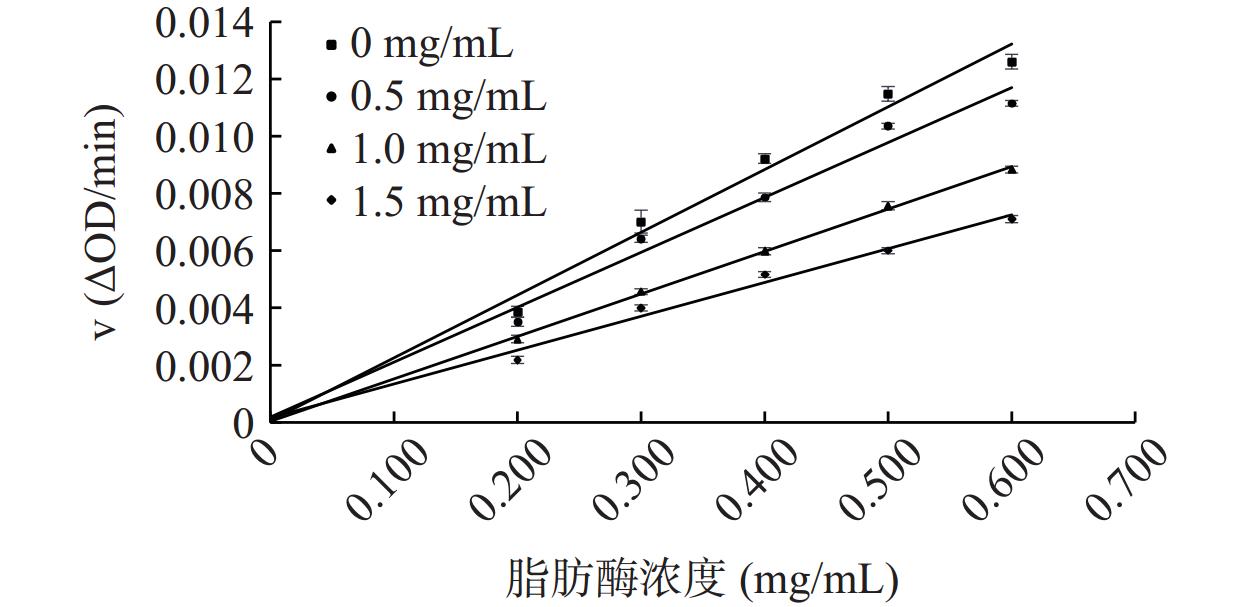
参照文献[31]的方法稍作修改,分别取50 μL 0、0.5、1.0、1.5 mg/mL ALP溶液于96孔板中,分别加入130 μL 0、0.2、0.3、0.4、0.5、0.6 mg/mL的脂肪酶溶液,37 ℃孵育10 min,加入20 μL 5.60 μmol/mL底物p-NPB溶液,37 ℃孵育20 min后于405 nm处读取吸光度值。0 mg/mL的ALP溶液用甲醇代替,0 mg/mL的脂肪酶溶液用Tris-HCl缓冲液代替,每组实验平行做3次。
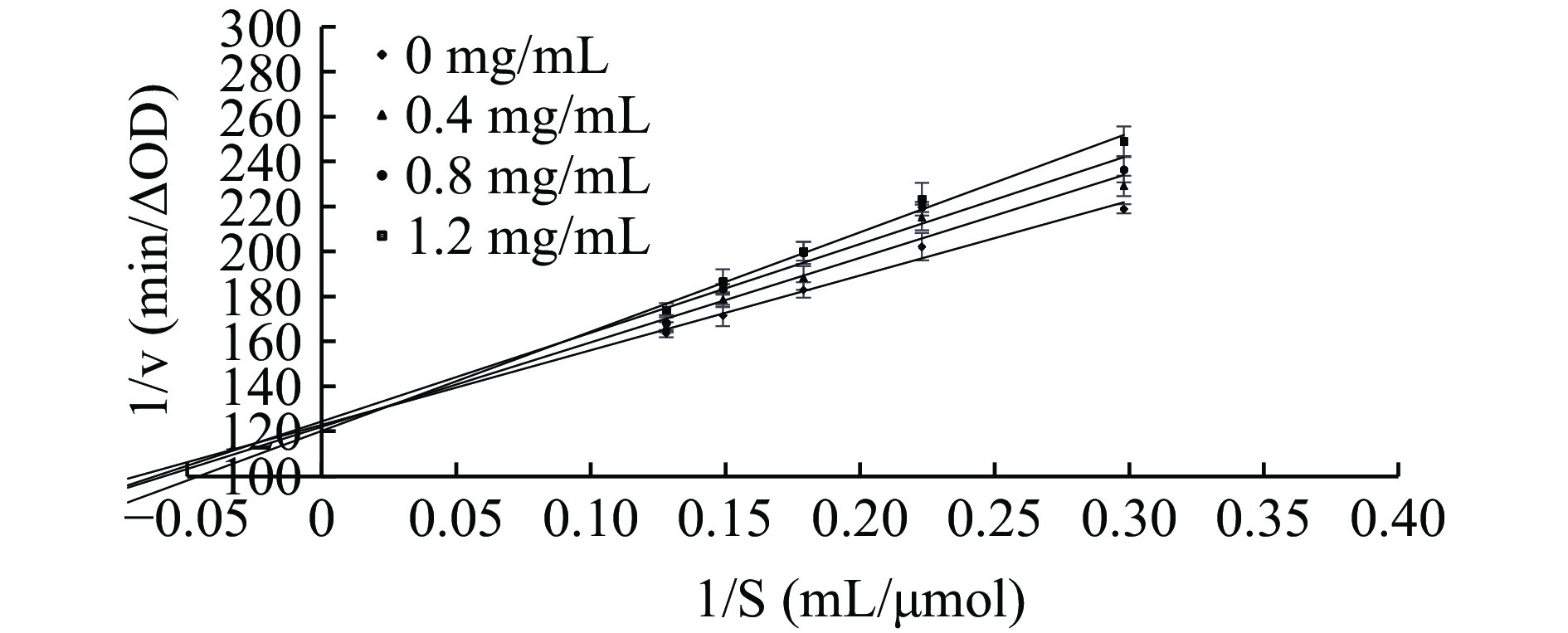
根据上述的操作步骤,固定脂肪酶浓度为0.25 mg/mL,改变底物p-NPB浓度(3.36、4.48、5.60、6.72、7.84 μmol/mL),分别测定不同浓度的牛蒡根多酚溶液(0、0.4、0.8、1.2 mg/mL)的酶促反应速率,每组实验平行做3次。以p-NPB浓度的倒数为横坐标、反应速率的倒数为纵坐标绘制 Lineweaver-Burk双倒数曲线,根据拟合得到的直线与坐标轴交点位置判断脂肪酶的抑制类型[32]。
1.2.4.3 ALP中单体化合物对脂肪酶的抑制作用
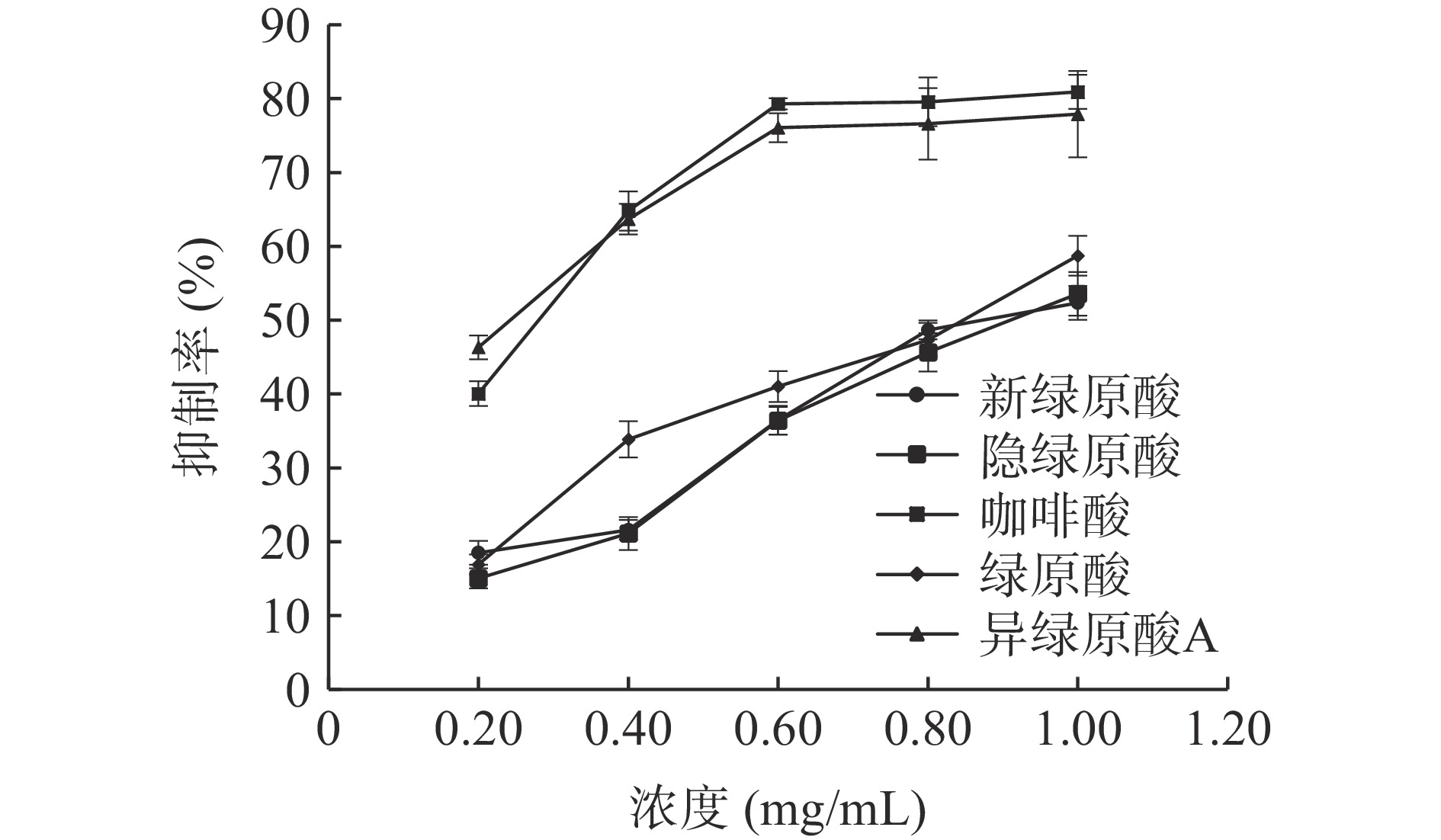
配制浓度为0.2、0.4、0.6、0.8、1.0 mg/mL的新绿原酸、隐绿原酸、咖啡酸、绿原酸和异绿原酸A溶液,测定方法同1.2.4.1。
1.2.4.4 ALP中单体化合物对脂肪酶的联合作用
选择在ALP中含量最高以及IC50值最低的单体化合物为研究对象,分别以其在药材中、ALP中的含量比以及半数抑制浓度比值为联合作用的比值,采用等效线图解法和Chou-Talalay药物联合指数(combination index,CI)进行评价两药联合作用效果。CI计算公式如下:
CI=D1Dx2+D2Dx2 (3) 式中:D1、D2分别为组合系统中产生一定抑制水平单体化合物1和2的剂量;Dx1、Dx2分别为单独添加的导致相同抑制水平单体化合物1和2的剂量。
1.3 数据处理
使用Excel 2019软件处理数据及绘制图表,所有实验数据采用3个平行样本取平均值,数据采用平均值±标准差表示。
2. 结果与分析
2.1 ALP的富集和指标性化学成分的含量测定
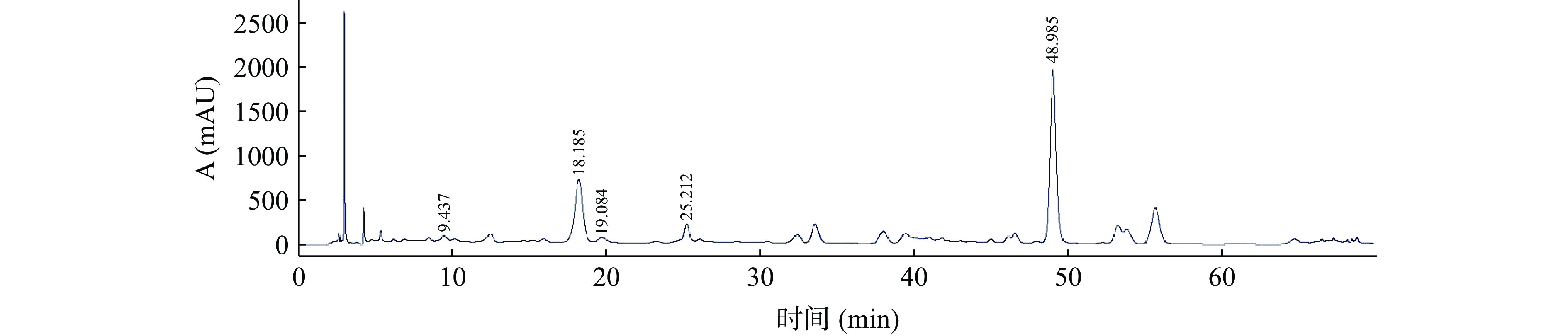
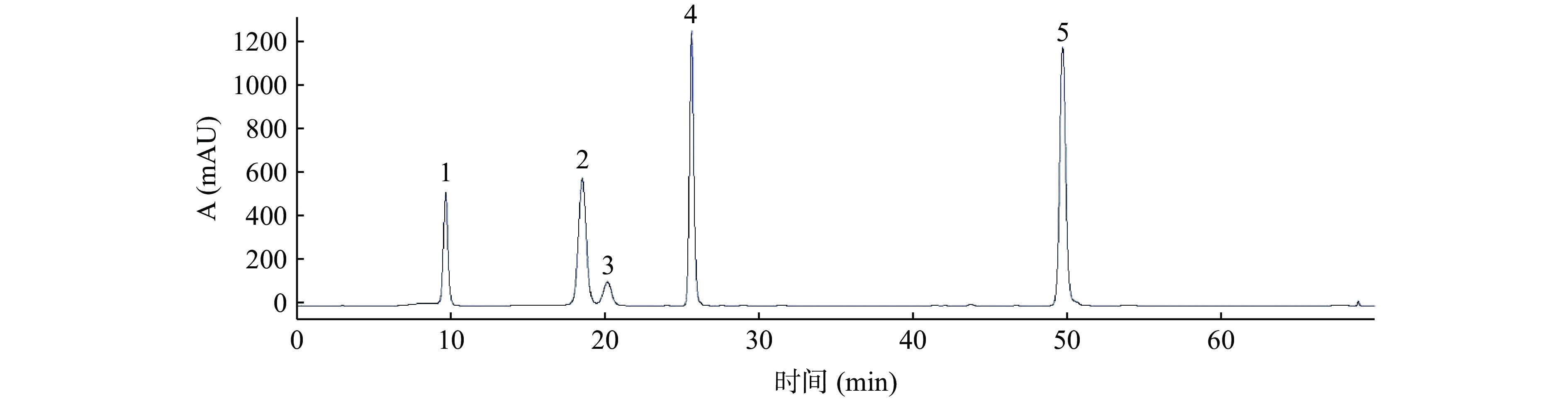
经过大孔树脂富集后的ALP浸膏得率为1.15%,多酚含量为51.23%。采用外标法测定ALP中指标性化学成分的新绿原酸、隐绿原酸、咖啡酸、绿原酸和异绿原酸A的含量分别为0.64%、0.58%、0.65%、4.5%和6.5%,5种指标性成分的含量占ALP的12.87%,浸膏得率和多酚含量与指标性成分绿原酸含量与文献相近[33]。ALP及混合标准品的液相色谱图见图1和图2。
2.2 ALP对脂肪酶的抑制作用及抑制类型
2.2.1 ALP对脂肪酶的抑制作用
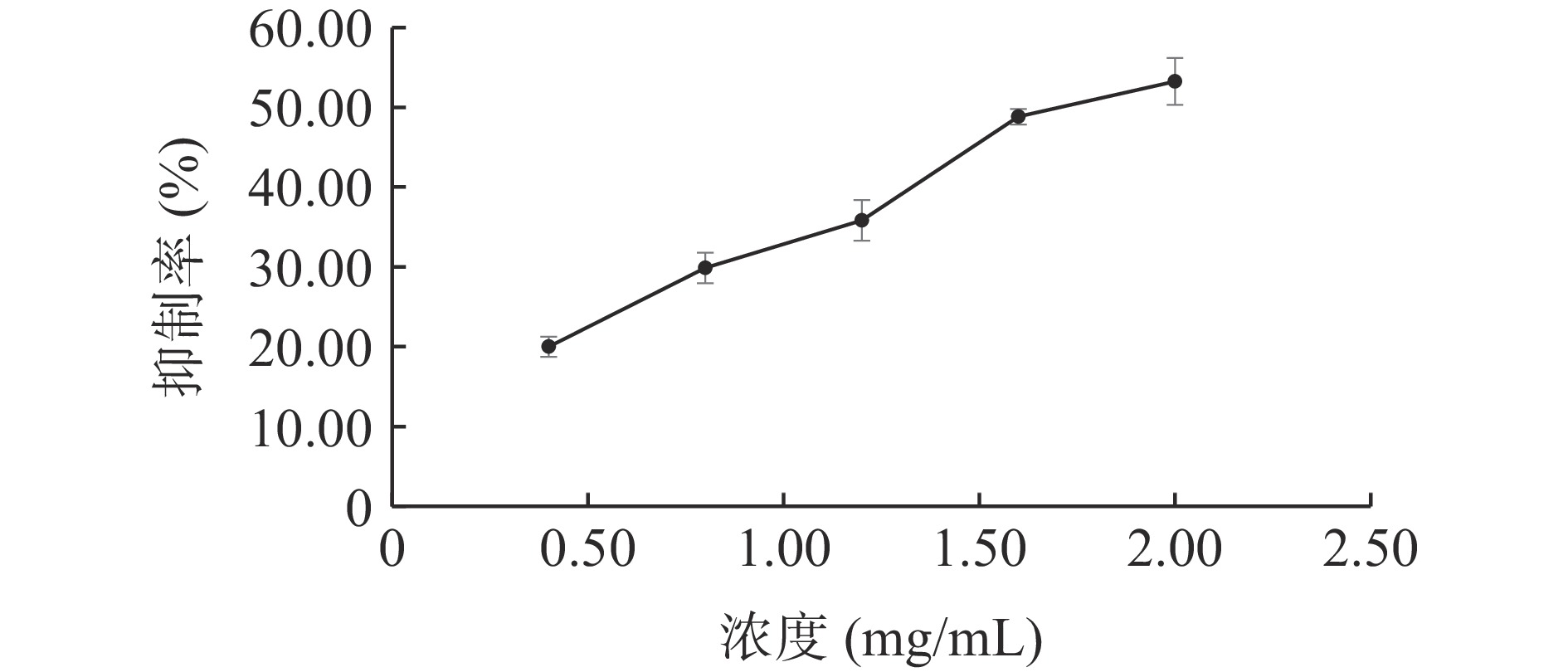
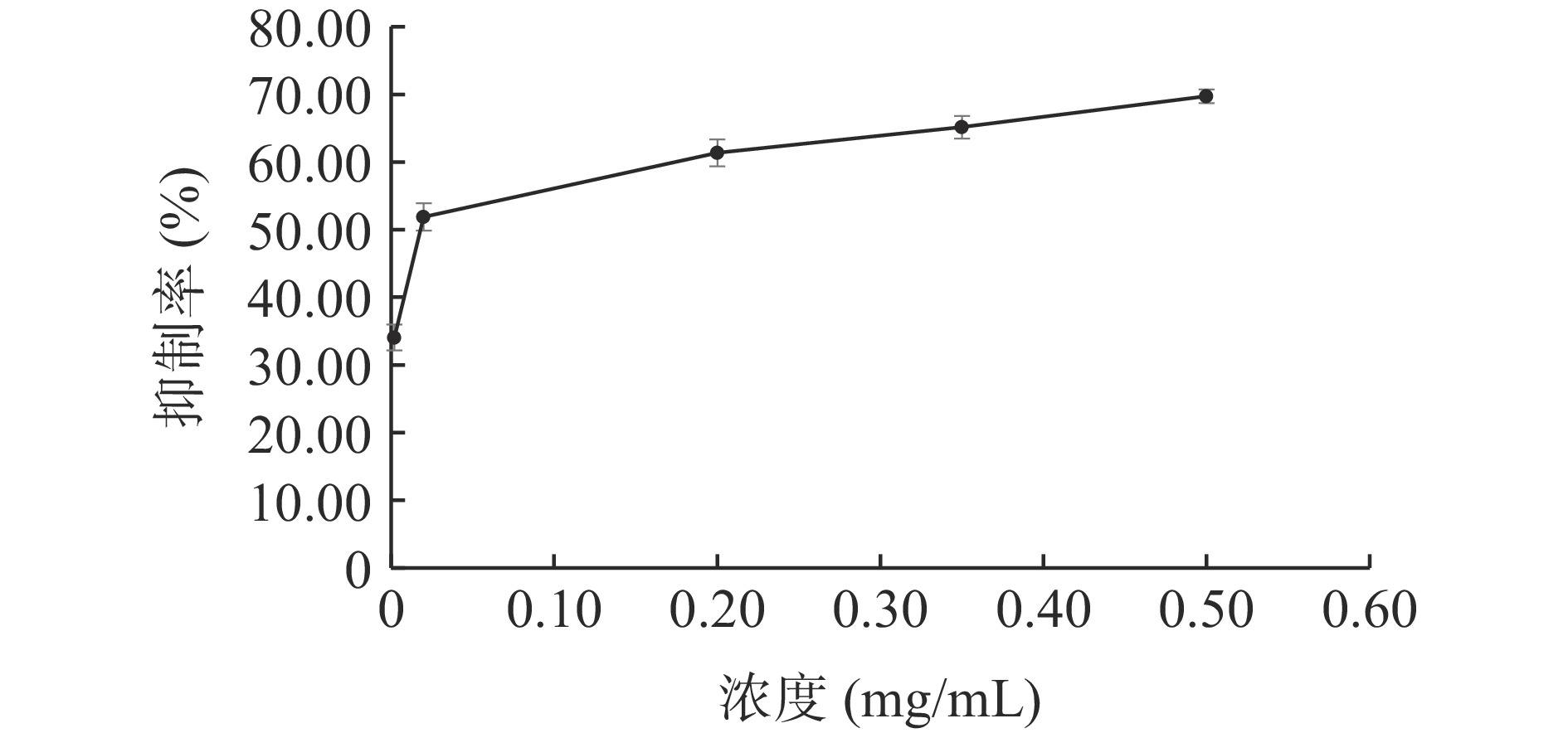
随着ALP的浓度增加,其对脂肪酶的抑制率升高(见图3),采用线性拟合方法计算得IC50值为1.706 mg/mL。阳性药奥利司他对脂肪酶的抑制结果见图4,在本实验反应体系下,奥利司他的IC50值为0.018 mg/mL。与文献[30]相比,奥利司他的IC50值与其相近,说明该体系适合用于评价脂肪酶抑制作用。实验结果表明ALP对脂肪酶活性有抑制作用,但抑制效果不如阳性药奥利司他。
2.2.2 ALP对脂肪酶的抑制类型
ALP对脂肪酶抑制动力学的结果见图5,所有直线均通过原点,斜率随ALP溶液浓度增加而减小,说明ALP对脂肪酶的抑制作用呈浓度依赖性,ALP能够可逆地抑制脂肪酶的活性。有研究表明天然植物中的多酚类化合物如秋茄果单宁对脂肪酶为可逆性抑制[34]。本文的研究结果同样表明ALP是通过降低脂肪酶的催化效率,而不是通过减少有效酶的数量来实现脂肪酶抑制作用[35]。
ALP对脂肪酶抑制的双倒数曲线结果见图6,所有直线均与y轴正方向相交,斜率随ALP溶液的浓度增加而增大。这表明ALP对脂肪酶的抑制作用属于竞争性抑制。有研究表明多酚含量占79.18%的山丹花鳞茎醇提物[36]和绿原酸单体[37]能够竞争性抑制脂肪酶活性。本文的研究结果表明了ALP能与脂肪酶的活性中心结合,占据底物p-NPB的结合位点,影响了脂肪酶与底物p-NPB的亲和度,从而抑制p-NPB的水解[38]。
2.3 ALP中单体化合物对脂肪酶的抑制作用
单体化合物新绿原酸、隐绿原酸、咖啡酸、绿原酸和异绿原酸A对脂肪酶的抑制作用随浓度增加而升高,实验结果见图7,IC50值分别为0.872、0.910、0.280、0.847、0.244 mg/mL,表明ALP中的单体化合物可以抑制脂肪酶活性。有文献表明绿原酸、异绿原酸A、新绿原酸、隐绿原酸单体化合物对脂肪酶具有明显的抑制作用,IC50值分别为0.114、0.081、0.647、0.677 mg/mL,可能是以非共价相互作用结合于脂肪酶,从而发挥对脂肪酶的抑制作用[39−40]。
2.4 ALP中单体化合物对脂肪酶的联合作用
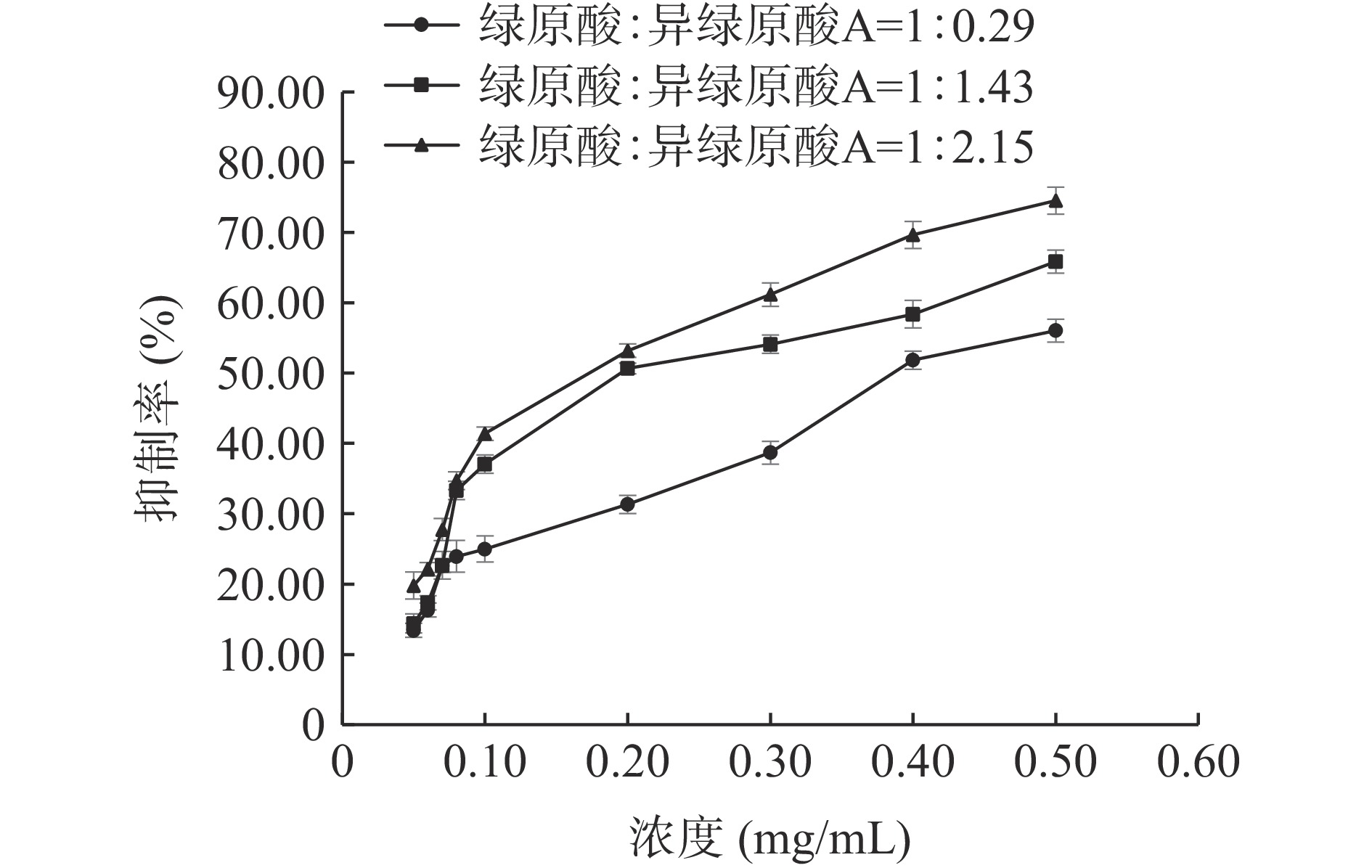
实验结果表明在ALP中异绿原酸A和绿原酸含量较高,而咖啡酸和异绿原酸A的IC50值较低,因此选择绿原酸和异绿原酸A,以及咖啡酸和异绿原酸A研究多酚化合物对脂肪酶的联合抑制作用。参考文献[41]中牛蒡根中的绿原酸和异绿原酸A、咖啡酸和异绿原酸A的含量比分别为1:2.15和1:186.67;本实验富集得到的ALP中绿原酸和异绿原酸A、咖啡酸和异绿原酸A的含量比分别为1:1.43和1:9.98;绿原酸和异绿原酸A、咖啡酸和异绿原酸A的IC50浓度比值分别为1:0.29和1:0.87。因此,分别以浓度比值1:0.29、1:1.43和1:2.15测定绿原酸和异绿原酸A联合使用对脂肪酶的抑制作用;分别以浓度比值1:0.87、1:9.98和1:186.67测定咖啡酸和异绿原酸A联合使用对脂肪酶的抑制作用。
2.4.1 绿原酸和异绿原酸A对脂肪酶的联合作用
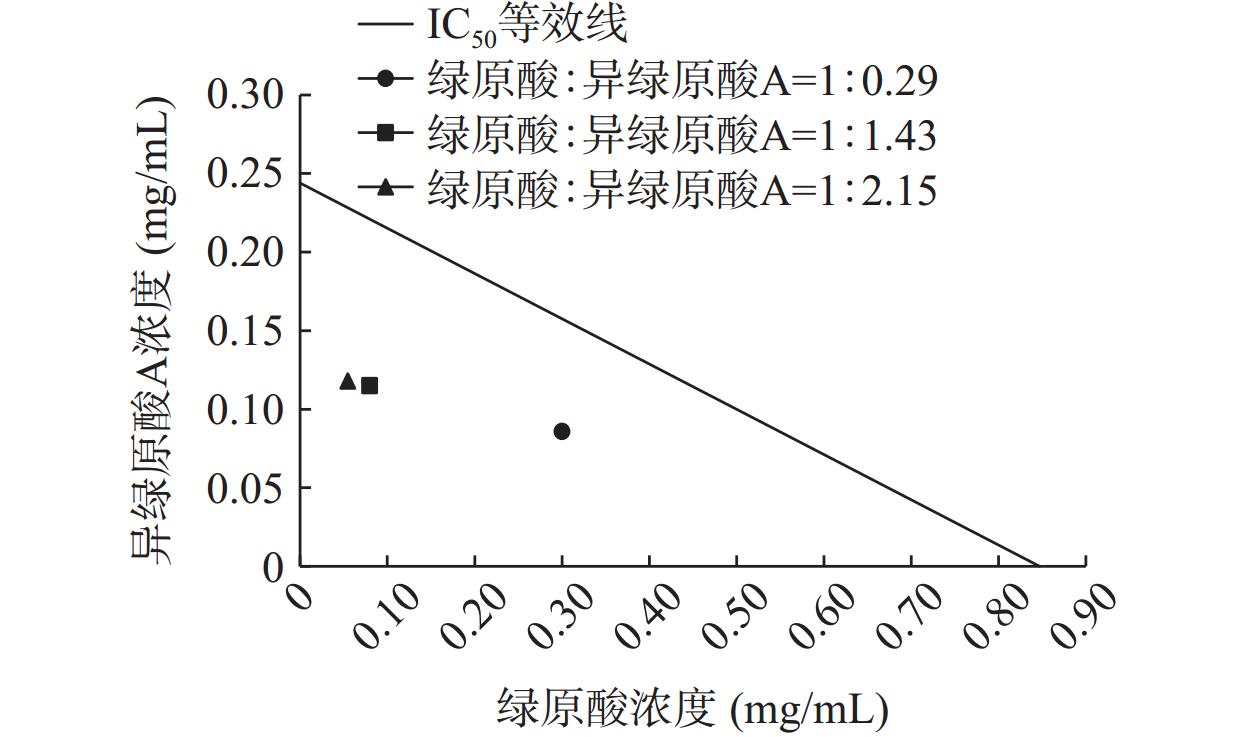
绿原酸和异绿原酸A联合使用对脂肪酶的抑制作用见图8。在测定的三组不同浓度比中,当绿原酸和异绿原酸A的浓度比为1:2.15时抑制能力最强,IC50值为0.173 mg/mL,二者联合使用对脂肪酶的抑制率较绿原酸或异绿原酸A单独使用时抑制率高。
CI<1,表示药物组合为协同作用;当CI=1,表示药物组合为相加作用;当CI>1,表示药物组合为拮抗作用。绿原酸和异绿原酸A联合使用对脂肪酶的IC50等效线实验结果见图9,绿原酸和异绿原酸A在实验选用的3个比例下对脂肪酶的抑制作用均位于等效线下方,CI值分别为0.707、0.566和0.549,均小于1。表明绿原酸和异绿原酸A联合使用对脂肪酶具有协同抑制的作用[42]。绿原酸和异绿原酸A浓度比为1:2.15时,CI值最小,具有更好的协同抑制效果。这可能是由于绿原酸与异绿原酸A对脂肪酶都属于竞争性抑制,联合使用时优势互补,增加了多酚-酶复合物的稳定性[43]。
2.4.2 咖啡酸和异绿原酸A对脂肪酶的联合作用
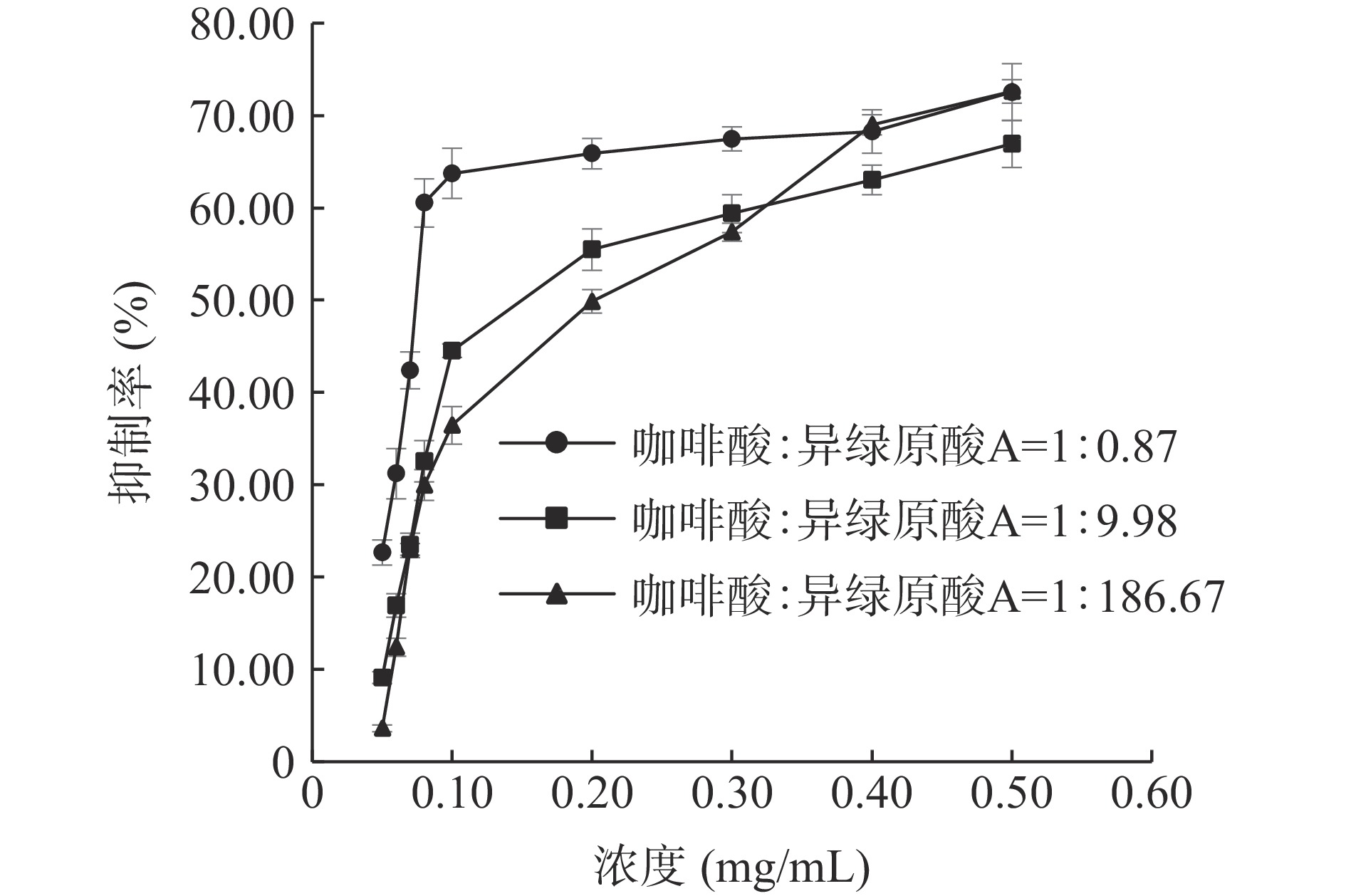
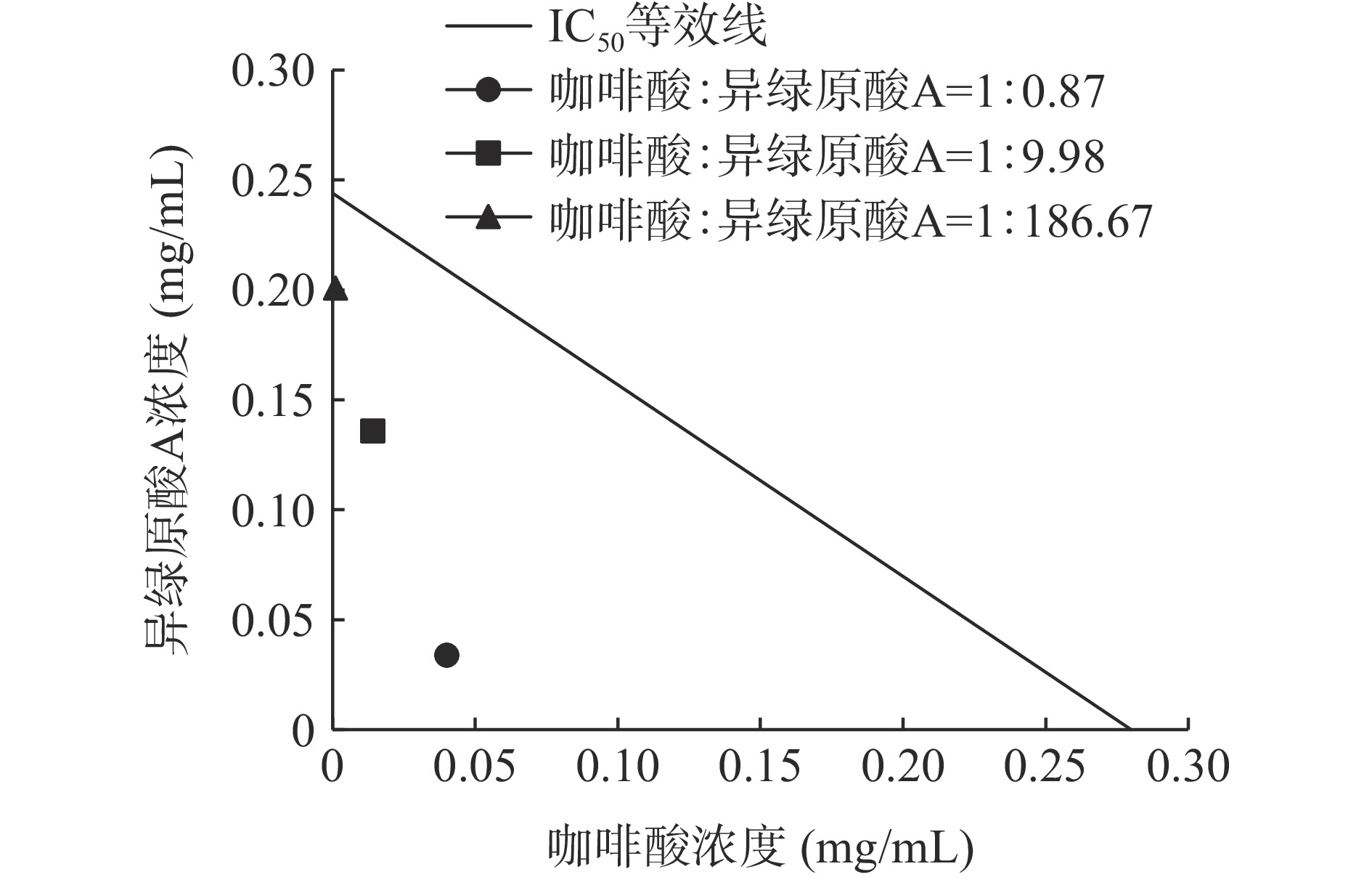
咖啡酸和异绿原酸A联合使用对脂肪酶的抑制作用见图10。在测定的三组不同浓度比中,咖啡酸和异绿原酸A的浓度比为1:0.87时抑制作用最强,IC50值为0.074 mg/mL。二者联合使用对脂肪酶的抑制率较咖啡酸或异绿原酸A单独使用时抑制率高。
咖啡酸和异绿原酸A联合使用对脂肪酶的IC50等效线的实验结果见图11,咖啡酸和异绿原酸A在3种浓度比下的剂量对均位于等效线下方,CI值分别为0.282、0.607和0.827,均小于1。咖啡酸和异绿原酸A浓度比为1:0.87时,CI值最小,具有更好的协同抑制效果。这可能是由于咖啡酸对脂肪酶产生非竞争性抑制[44],与脂肪酶非竞争性位点相互结合、抑制脂肪酶活性,随着咖啡酸的占比升高,增加了其与异绿原酸A对脂肪酶的协同抑制效果。
3. 结论
本文进行了ALP及其主要单体化合物对脂肪酶的体外抑制作用研究。结果显示经过富集后的ALP中总酚含量为51.23%,主要含有新绿原酸、隐绿原酸、咖啡酸、绿原酸和异绿原酸A等成分,其总含量占ALP 12.87%。ALP对脂肪酶起到了可逆性竞争性抑制作用,IC50值为1.706 mg/mL。各单体化合物新绿原酸、隐绿原酸、咖啡酸、绿原酸和异绿原酸A对脂肪酶的IC50值分别为0.872、0.910、0.280、0.847、0.244 mg/mL。绿原酸和异绿原酸A、咖啡酸和异绿原酸A联合使用对脂肪酶存在着协同抑制作用。
药食两用的植物尤其是中草药,在长期的临床实践中表现出了良好的安全性和较低的副作用。本文的研究结果提示多酚类化合物的组成和比例不同,对脂肪酶的抑制作用也不同。在开发利用天然植物多酚时,可通过优化提取制备工艺,调节其组成和比例,以达到较好的活性效果。本文为阐明牛蒡根的降脂机制提供了实验依据,为提高牛蒡根的利用价值提供理论基础。但本实验仅从体外证明了牛蒡根多酚对脂肪酶具有协同抑制作用,后续将会通过体内外实验验证牛蒡根多酚对脂肪酶的抑制作用,深入探究其降脂的具体作用机制。
-
表 1 标准品的工作曲线
Table 1 Working curves of standard products
标准品 回归方程 R2 线性范围(mg/mL) 新绿原酸 y=67058x−254.47 0.9998 0.04~0.80 隐绿原酸 y=81990x+34.05 0.9997 0.0125~0.25 咖啡酸 y=88985x+2413.2 0.9994 0.05~1.0 绿原酸 y=64794x+508.64 0.9993 0.075~1.50 异绿原酸A y=82737x+3095.4 0.9993 0.075~1.50 -
[1] 田英杰, 吴广, 王洪桂, 等. 益生菌干预高脂血症研究的新进展[J]. 中华高血压杂志,2022,30(5):420−424. [TIAN Yingjie, WU Guang, WANG Honggui, et al. New progress in the study of probiotic intervention in hyperlipidemia[J]. Chinese Journal of Hypertension,2022,30(5):420−424. TIAN Yingjie, WU Guang, WANG Honggui, et al . New progress in the study of probiotic intervention in hyperlipidemia[J]. Chinese Journal of Hypertension,2022 ,30 (5 ):420 −424 .[2] ANTONOPOULOS A S, OIKONOMOU E K, ANTONIADES C, et al. From the BMI paradox to the obesity paradox:The obesity-mortality association in coronary heart disease[J]. Obesity Reviews,2016,17(10):989−1000. doi: 10.1111/obr.12440
[3] SHI Y, BURN P. Lipid metabolic enzymes:Emerging drug targets for the treatment of obesity[J]. Nature Reviews Drug Discovery ,2004,3(8):695−710. doi: 10.1038/nrd1469
[4] 侯旭东, 胡情, 马丽娟, 等. 源于中药的胰脂肪酶抑制剂研究进展[J]. 药学学报,2020,55(7):1478−1493. [HOU Xudong, HU Qing, MA Lijuan, et al. Recent advances on pancreatic lipase inhibitors from Chinese herbs[J]. Acta Pharmaceutica Sinica,2020,55(7):1478−1493. HOU Xudong, HU Qing, MA Lijuan, et al . Recent advances on pancreatic lipase inhibitors from Chinese herbs[J]. Acta Pharmaceutica Sinica,2020 ,55 (7 ):1478 −1493 .[5] KUSHNER R F. Weight loss strategies for treatment of obesity[J]. Progress in Cardiovascular Diseases,2014,56(4):465−472. doi: 10.1016/j.pcad.2013.09.005
[6] GUERCIOLINI R. Mode of action of orlistat[J]. International Journal of Obesity,1997,21(Suppl 3):S12−S23.
[7] 王雨, 孙延平, 杨炳友, 等. 天然产物多糖对脂肪酶的抑制活性研究进展[J]. 化学工程师,2022,36(11):62−65. [WANG Yu, SUN Yanping, YANG Bingyou, et al. Research progress on the inhibitory activity of natural polysaccharides on lipase[J]. Chemical Engineer,2022,36(11):62−65. doi: 10.16247/j.cnki.23-1171/tq.20221162 WANG Yu, SUN Yanping, YANG Bingyou, et al . Research progress on the inhibitory activity of natural polysaccharides on lipase[J]. Chemical Engineer,2022 ,36 (11 ):62 −65 . doi: 10.16247/j.cnki.23-1171/tq.20221162[8] KWON K, KOONG H S, KANG K H. Effect of burdock extracts upon inflammatory mediator production[J]. Technology and Health Care,2016,24(3):459−469. doi: 10.3233/THC-151123
[9] RODRIGUEZ F M J, SOUZA D C R A, KRUGER L R, et al. Kinetics, composition and antioxidant activity of burdock ( Arctium lappa) root extracts obtained with supercritical CO2 and co-solvent[J]. Journal of Supercritical Fluids,2018,135:25−33. doi: 10.1016/j.supflu.2017.12.034
[10] HA M S, KIM J H, KIM Y S, et al. Effects of aquarobic exercise and burdock intake on serum blood lipids and vascular elasticity in Korean elderly women[J]. Experimental Gerontology,2018,101:63−68. doi: 10.1016/j.exger.2017.11.005
[11] 乔贝贝. 牛蒡根化学成分及其生物活性研究[D]. 南京:南京农业大学, 2019. [QIAO Beibei. Studies on chemical components and biological activities of Arctium lappa L. Root[D]. Nanjing:Nanjing Agricultural University, 2019. QIAO Beibei. Studies on chemical components and biological activities of Arctium lappa L. Root[D]. Nanjing: Nanjing Agricultural University, 2019.
[12] 刘秀峰, 王晓晖, 谢明. 牛蒡根性能的本草考证及传统食疗应用探析[J]. 中国中医药现代远程教育,2023,21(16):200−203. [LIU Xiufeng, WANG Xiaohui, XIE Ming. Herbal textual research on the performance and traditional dietotherapy application of Arctium lappa Root[J]. Chinese Medicine Modern Distance Education of China,2023,21(16):200−203. doi: 10.3969/j.issn.1672-2779.2023.16.069 LIU Xiufeng, WANG Xiaohui, XIE Ming . Herbal textual research on the performance and traditional dietotherapy application of Arctium lappa Root[J]. Chinese Medicine Modern Distance Education of China,2023 ,21 (16 ):200 −203 . doi: 10.3969/j.issn.1672-2779.2023.16.069[13] 张朋, 丁朋, 丁利, 等. 响应面法优化牛蒡根多酚的闪式提取工艺[J]. 食品工业,2023,44(7):40−43. [ZHANG Peng, DING Peng, DING Li, et al. Optimization of flash extraction process of polyphenols from burdock roots by response surface methodology[J]. Food Industry,2023,44(7):40−43. ZHANG Peng, DING Peng, DING Li, et al . Optimization of flash extraction process of polyphenols from burdock roots by response surface methodology[J]. Food Industry,2023 ,44 (7 ):40 −43 .[14] 王亚凤, 张祖湘, 何瑞杰, 等. 黄精地上部位化学成分及其胰脂肪酶抑制活性研究[J]. 天然产物研究与开发,2020,32(11):1811−1817. [WANG Yafeng, ZHANG Zuxiang, HE Ruijie, et al. Study on the chemical constituents of the aerial parts of Polygonatum sibiricum and its pancreatic lipase inhibitory activity[J]. Natural Product Research,2020,32(11):1811−1817. WANG Yafeng, ZHANG Zuxiang, HE Ruijie, et al . Study on the chemical constituents of the aerial parts of Polygonatum sibiricum and its pancreatic lipase inhibitory activity[J]. Natural Product Research,2020 ,32 (11 ):1811 −1817 .[15] 姚宏纪, 薛兆毅, 王瑞琪, 等. 绿原酸激动AMPK调节糖脂代谢的抗糖尿病作用研究[J]. 海南医学院学报,2022,28(9):654−662. [YAO Hongji, XUE Zhaoyi, WANG Ruiqi, et al. Chlorogenic acid modulates glucose and lipid metabolisms via AMPK activation in HepG2 cells and shows its anti-hyperglycemic effect on streptozocin-induced diabetic mice[J]. Journal of Hainan Medical University,2022,28(9):654−662. doi: 10.13210/j.cnki.jhmu.20211223.002 YAO Hongji, XUE Zhaoyi, WANG Ruiqi, et al . Chlorogenic acid modulates glucose and lipid metabolisms via AMPK activation in HepG2 cells and shows its anti-hyperglycemic effect on streptozocin-induced diabetic mice[J]. Journal of Hainan Medical University,2022 ,28 (9 ):654 −662 . doi: 10.13210/j.cnki.jhmu.20211223.002[16] 南敏伦, 付香婷, 司学玲, 等. 抑制脂肪酶活性成分研究进展[J]. 延边大学医学学报,2021,44(3):223−227. [NAN Minlun, FU Xiangting, SI Xueling, et al. Research progress of components inhibiting lipase activity[J]. Journal of Medical Science Yanbian University,2021,44(3):223−227. NAN Minlun, FU Xiangting, SI Xueling, et al . Research progress of components inhibiting lipase activity[J]. Journal of Medical Science Yanbian University,2021 ,44 (3 ):223 −227 .[17] GIOVANA J, DEBORA E, LUCINAN P P. Contribution of anthocyanin-rich foods in obesity control through gut microbiota interactions[J]. Biofactors,2017,43(4):557−564.
[18] MARCILLINUS Z, PETER B, ABDUL R, et al. Role of dietary polyphenols in non-communicable chronic disease prevention, and interactions in food systems:An overview[J]. Nutrition,2023,112:109349.
[19] WU G, CHENG H, GUO H, et al. Tea polyphenol EGCG ameliorates obesity-related complications by regulating lipidomic pathway in leptin receptor knockout rats[J]. The Journal of Nutritional Biochemistry, 2023, 118.
[20] MEZHIBOVSKY E, KNOWLES K A, HE Q, et al. Grape polyphenols attenuate diet-induced obesity and hepatic steatosis in mice in association with reduced butyrate and increased markers of intestinal carbohydrate oxidation[J]. Frontiers in Nutrition,2021,8:675267. doi: 10.3389/fnut.2021.675267
[21] 谢小花, 安晓婷, 陈静, 等. 牛蒡根中的功能成分及多酚类化合物提取方法研究进展[J]. 湖南文理学院学报(自然科学版),2019,31(3):25−31. [XIE Xiaohua, AN Xiaoting, CHEN Jing, et al. The research progress of the functional ingredients and the methods for extraction of polyphenols from the Burdock root[J]. Journal of Hunan University of Arts and Science(Science and Technology),2019,31(3):25−31. doi: 10.3969/j.issn.1672-6146.2019.03.005 XIE Xiaohua, AN Xiaoting, CHEN Jing, et al . The research progress of the functional ingredients and the methods for extraction of polyphenols from the Burdock root[J]. Journal of Hunan University of Arts and Science(Science and Technology),2019 ,31 (3 ):25 −31 . doi: 10.3969/j.issn.1672-6146.2019.03.005[22] 王祎. 葫芦巴及其盐制品的化学成分与降脂作用研究[D]. 沈阳:辽宁中医药大学, 2023. [WANG Yi. Study on chemical constituents and lipidlowering effect of fenugreek and its salt products[D]. Shenyang:Liaoning University of Traditional Chinese Medicine, 2023. WANG Yi. Study on chemical constituents and lipidlowering effect of fenugreek and its salt products[D]. Shenyang: Liaoning University of Traditional Chinese Medicine, 2023.
[23] BUCHHOLZ T, MELZIG M F. Polyphenolic compounds as pancreatic lipase inhibitors[J]. Planta Medica,2015,81(10):771−783. doi: 10.1055/s-0035-1546173
[24] 赵谋明, 刘晓丽, 崔春, 等. 余甘子多酚响应面法优化提取及其抗氧化活性研究[J]. 食品工业科技,2007(6):117−120. [ZHAO Mouming, LIU Xiaoli, CUI Chun, et al. Optimization of extraction and antioxidant activity of polyphenols from Phyllanthus emblica by response surface methodology[J]. Science and Technology of Food Industry,2007(6):117−120. doi: 10.3969/j.issn.1002-0306.2007.06.034 ZHAO Mouming, LIU Xiaoli, CUI Chun, et al . Optimization of extraction and antioxidant activity of polyphenols from Phyllanthus emblica by response surface methodology[J]. Science and Technology of Food Industry,2007 (6 ):117 −120 . doi: 10.3969/j.issn.1002-0306.2007.06.034[25] 许瑞如, 张秀玲, 李振, 等. 桔梗根提取物不同溶剂萃取物的抗氧化活性[J]. 食品研究与开发,2021,42(1):31−36. [XU Ruiru, ZHANG Xiuling, LI Zhen, et al. Antioxidant activity of extracts from different solvents of extract of Platycodon grandiflorum Roots[J]. Food Research and Development,2021,42(1):31−36. doi: 10.12161/j.issn.1005-6521.2021.01.006 XU Ruiru, ZHANG Xiuling, LI Zhen, et al . Antioxidant activity of extracts from different solvents of extract of Platycodon grandiflorum Roots[J]. Food Research and Development,2021 ,42 (1 ):31 −36 . doi: 10.12161/j.issn.1005-6521.2021.01.006[26] 李瑾, 王露, 郭东艳, 等. 牛蒡根总多酚纯化工艺研究[J]. 亚太传统医药,2016,12(16):34−36. [LI Jin, WANG Lu, GUO Dongyan, et al. Separation and purification of total polyphenols from burdock root[J]. Asia-Pacific Traditional Medicine,2016,12(16):34−36. LI Jin, WANG Lu, GUO Dongyan, et al . Separation and purification of total polyphenols from burdock root[J]. Asia-Pacific Traditional Medicine,2016 ,12 (16 ):34 −36 .[27] SAMARA M, NASSER A, MINGELGRIN U. Critical examination of the suitability of the Folin-Ciocalteu reagent assay for quantitative analysis of polyphenols-the case of olive-mill wastewater[J]. American Journal of Analytical Chemistry,2022,13(11):476−493. doi: 10.4236/ajac.2022.1311032
[28] 李达, 马聪玉, 吕青林, 等. 不同产地脱脂乳木果仁总酚含量测定及其生物活性[J]. 中国药科大学学报,2018,49(5):572−579. [LI Da, MA Congyu, LÜ Qinglin, et al. Total phenolic contents and bioactivities of defatted Vitellaria paradoxa kernels from different origin[J]. Journal of China Pharmaceutical University,2018,49(5):572−579. doi: 10.11665/j.issn.1000-5048.20180509 LI Da, MA Congyu, LÜ Qinglin, et al . Total phenolic contents and bioactivities of defatted Vitellaria paradoxa kernels from different origin[J]. Journal of China Pharmaceutical University,2018 ,49 (5 ):572 −579 . doi: 10.11665/j.issn.1000-5048.20180509[29] 孙小玲, 何凡. 牛蒡根RRLC指纹图谱研究[J]. 时珍国医国药,2017,28(2):382−384. [SUN Xiaoling, HE Fan. Study on RRLC fingerprint of Arctium lappa roots[J]. Lishizhen Medicine and Materia Medica Research,2017,28(2):382−384. SUN Xiaoling, HE Fan . Study on RRLC fingerprint of Arctium lappa roots[J]. Lishizhen Medicine and Materia Medica Research,2017 ,28 (2 ):382 −384 .[30] ZHANG H, WU Q, WEI X, et al. Pancreatic lipase and cholesterol esterase inhibitory effect of Camellia nitidissima Chi flower extracts in vitro and in vivo[J]. Food Bioscience,2020,37:100682. doi: 10.1016/j.fbio.2020.100682
[31] 晏幸, 肖文熙, 欧丽明, 等. 琯溪蜜柚柚皮苷纯化鉴定及其对胰脂肪酶的抑制作用[J]. 食品科学,2022,43(7):1−7. [YAN Xing, XIAO Wenxi, OU Liming, et al. Purification, identification and pancreatic lipase inhibitory effect of naringin from the peel of guanxi honey pomelo ( Citrus grandis (L.) Osbeck)[J]. Food Science,2022,43(7):1−7. doi: 10.7506/spkx1002-6630-20201228-313 YAN Xing, XIAO Wenxi, OU Liming, et al . Purification, identification and pancreatic lipase inhibitory effect of naringin from the peel of guanxi honey pomelo (Citrus grandis (L.) Osbeck)[J]. Food Science,2022 ,43 (7 ):1 −7 . doi: 10.7506/spkx1002-6630-20201228-313[32] LIU B S, CHENG X, ZHANG H C. Spectroscopic and molecular docking studies on the binding mechanism of Mobic and lipase[J]. Journal of Pharmaceutical and Biopharmaceutical Research,2019,1(2):53−60. doi: 10.25082/JPBR.2019.02.002
[33] 窦培元, 栾晓宁, 朱连连, 等. 牛蒡根中有效成分的含量测定[J]. 山西中医学院学报,2018,19(4):30−33,44. [DOU Peiyuan, LUAN Xiaoning, ZHU Lianlian, et al. Determination of active ingredients in the roots of Arctium lappa[J]. Journal of Shanxi College of Traditional Chinese Medicine,2018,19(4):30−33,44. DOU Peiyuan, LUAN Xiaoning, ZHU Lianlian, et al . Determination of active ingredients in the roots of Arctium lappa[J]. Journal of Shanxi College of Traditional Chinese Medicine,2018 ,19 (4 ):30 −33,44 .[34] 陈宗雄. 秋茄果单宁对3T3-L1脂肪细胞抑制作用的初步探究[D]. 厦门:厦门大学, 2022. [CHEN Zongxiong. Preliminary exploration of inhibitory effect of Kandelia candel fruit tannins on 3T3-L1 adipocytes[D]. Xiamen:Xiamen University, 2022. CHEN Zongxiong. Preliminary exploration of inhibitory effect of Kandelia candel fruit tannins on 3T3-L1 adipocytes[D]. Xiamen: Xiamen University, 2022.
[35] 张芳, 张鑫, 杨海山. 四氢嘧啶对酪氨酸酶的抑制作用机制及类型[J]. 中国高原医学与生物学杂志,2021,42(1):42−46. [ZHANG Fang, ZHANG Xin, YANG Haishan. Mechanism and types of inhibitory effects of ectoine on tyrosinase[J]. Chinese High Altitude Medicine and Biology,2021,42(1):42−46. ZHANG Fang, ZHANG Xin, YANG Haishan . Mechanism and types of inhibitory effects of ectoine on tyrosinase[J]. Chinese High Altitude Medicine and Biology,2021 ,42 (1 ):42 −46 .[36] 萨日那. 山丹花鳞茎醇提物对小鼠脂肪酶的抑制作用研究[D]. 锦州:锦州医科大学, 2022. [SA Rina. Study on the inhibition of lipase in mice by the Lilium pumilum album bulb alcoholic extract[D]. Jinzhou:Jinzhou Medical University, 2022. SA Rina. Study on the inhibition of lipase in mice by the Lilium pumilum album bulb alcoholic extract[D]. Jinzhou: Jinzhou Medical University, 2022.
[37] 曹琼琚. 绿原酸对草鱼冷藏过程中脂质氧化的抑制及其作用机制[D]. 武汉:华中农业大学, 2020. [CAO Qiongju. The inhibitory effect and mechanism of chlorogenic acid on lipid oxidation of grass carp (Ctenopharyngodon idellus) during chilled storage[D]. Wuhan:Huazhong Agricultural University, 2020. CAO Qiongju. The inhibitory effect and mechanism of chlorogenic acid on lipid oxidation of grass carp (Ctenopharyngodon idellus) during chilled storage[D]. Wuhan: Huazhong Agricultural University, 2020.
[38] 周一鸣, 马思佳, 蒋晴怡, 等. 苦荞中芦丁和槲皮素对淀粉消化酶的抑制能力[J]. 食品科学,2022,43(18):30−37. [ZHOU Yiming, MA Sijia, JIANG Qingyi, et al. Inhibition of starch-digesting enzymes by rutin and quercetin in tartary buckwheat[J]. Food Science,2022,43(18):30−37. doi: 10.7506/spkx1002-6630-20210820-256 ZHOU Yiming, MA Sijia, JIANG Qingyi, et al . Inhibition of starch-digesting enzymes by rutin and quercetin in tartary buckwheat[J]. Food Science,2022 ,43 (18 ):30 −37 . doi: 10.7506/spkx1002-6630-20210820-256[39] CHANG Y, ZHANG D, YANG G, et al. Screening of anti-lipase components of Artemisia argyi leaves based on spectrum-effect relationships and HPLC-MS/MS[J]. Front Pharmacol,2021,12:675396. doi: 10.3389/fphar.2021.675396
[40] MCDOUGALL G J, KULKARNI N N, STEWART D. Berry polyphenols inhibit pancreatic lipase activity in vitro[J]. Food Chemistry,2009,115(1):193−199. doi: 10.1016/j.foodchem.2008.11.093
[41] 田盛, 王祎, 吴德超, 等. 黑曲霉发酵牛蒡根的化学成分及对 α-葡萄糖苷酶和 α-淀粉酶抑制作用[J]. 食品工业科技,2022,43(10):50−55. [TIAN Sheng, WANG Yi, WU Dechao, et al. Chemical composition of Arctium lappa roots fermented by aspergillus niger and its inhibition on α-glucosidase and α-amylase[J]. Science and Technology of Food Industry,2022,43(10):50−55. doi: 10.13386/j.issn1002-0306.2021060161 TIAN Sheng, WANG Yi, WU Dechao, et al . Chemical composition of Arctium lappa roots fermented by aspergillus niger and its inhibition on α-glucosidase and α-amylase[J]. Science and Technology of Food Industry,2022 ,43 (10 ):50 −55 . doi: 10.13386/j.issn1002-0306.2021060161[42] MEHMOOD A, ZHAO L, WANG C, et al. Stevia residue extract alone and combination with allopurinol attenuate hyperuricemia in fructose−PO−induced hyperuricemic mice[J]. Journal of Food Biochemistry,2020,44(1):e13087.
[43] XU Z, CAO Q, MANYANDE A, et al. Analysis of the binding selectivity and inhibiting mechanism of chlorogenic acid isomers and their interaction with grass carp endogenous lipase using multi-spectroscopic, inhibition kinetics and modeling methods[J]. Food Chemistry,2022,382:132106. doi: 10.1016/j.foodchem.2022.132106
[44] 范金波, 王晓露, 姜海静, 等. 荧光光谱法研究咖啡酸与胰脂肪酶相互作用[J]. 食品工业科技,2017,38(2):152−155,161. [FAN Jinbo, WANG Xiaolu, JIANG Haijing, et al. Study on the interaction between caffeic acid and pancreatic lipase by fluorescence spectroscopy[J]. Science and Technology of Food Industry,2017,38(2):152−155,161. doi: 10.13386/j.issn1002-0306.2017.02.020 FAN Jinbo, WANG Xiaolu, JIANG Haijing, et al . Study on the interaction between caffeic acid and pancreatic lipase by fluorescence spectroscopy[J]. Science and Technology of Food Industry,2017 ,38 (2 ):152 −155,161 . doi: 10.13386/j.issn1002-0306.2017.02.020 -
期刊类型引用(1)
1. 汪洪涛,石雪萍,陈成,李清光,陈帆,刘文静. 牛蒡花生扇贝复合营养酱制作工艺研究. 江苏调味副食品. 2024(03): 9-13 .  百度学术
百度学术
其他类型引用(0)





 下载:
下载:
 下载:
下载:



