Exploring the Mechanism of Hippophae Fructus Anti-obesity through Network Pharmacology and Molecular Docking
-
摘要: 目的:通过网络药理学和分子对接技术,探讨沙棘抗肥胖的活性成分、靶点和作用机制,并验证其体外抗肥胖效果。方法:使用TCMSP平台检索沙棘活性成分与靶点,收集疾病靶点,利用Venny 2.0.2得沙棘靶点与肥胖靶点的交集。通过STRING数据库平台建立药物靶点-疾病靶蛋白相互作用(PPI)网络。使用David数据库对交集靶点进行分析,得到GO富集分析和KEGG通路分析结果。通过Cytoscape 3.9.1软件构建沙棘成分-靶点-信号通路网络图。使用Autodock Dock 1.5.7和Pymol 2.2.0进行沙棘核心靶点与其成分的分子对接,并进行可视化处理。通过体外实验验证沙棘提取物的抗肥胖作用。结果:筛选得到33个沙棘活性成分,2820个疾病靶点和151个交集靶点。主要活性成分包括黄酮类、维生素类、甾醇类等,关键靶点涉及AKT1、TNF、IL6、TP53、VEGFA、CASP3等。KEGG通路富集分析得到恶性肿瘤通路、脂质与动脉粥样硬化通路、AGE-RAGE信号通路等131条信号通路。分子对接结果表明核心靶点与其对应活性成分对接结果良好。体外实验表明沙棘提取物具有抑制3T3-L1小鼠前脂肪细胞增殖的作用。结论:研究显示沙棘具有多成分、多靶点、多通路协同发挥抗肥胖作用,为其临床研究和产品开发提供了参考。Abstract: Objective: To investigate the active components, protein targets, and mechanisms underlying the anti-obesity effects of Hippophae fructus using network pharmacology and molecular docking techniques, and to validate its in vitro anti-obesity efficacy. Methods: The TCMSP platform was utilized to retrieve the active components and targets of Hippophae fructus, and disease targets were collected. Venny 2.0.2 was used to identify the intersection of targets between Hippophae fructus and obesity-related targets. The STRING database was used to establish a drug-target-disease protein interaction (PPI) network. The intersecting targets were analyzed using the David database to perform GO enrichment analysis and KEGG pathway analysis. Cytoscape 3.9.1 was used to construct a network diagram of the components of Hippophae fructus, anti-obesity targets, and related signaling pathways. Autodock Dock 1.5.7 and Pymol 2.2.0 were used to carry out molecular docking between the core targets of Hippophae fructus and its components, followed by visualization. The in vitro anti-obesity effect of Hippophae fructus extract was evaluated through cell experiments using 3T3-L1 cells. Results: A total of 33 active components, 2820 disease targets, and 151 intersection targets of Hippophae fructus were identified. The main active components included flavonoids, vitamins, and sterols, while key targets involved AKT1, TNF, IL6, TP53, VEGFA, CASP3, and others. KEGG pathway enrichment analysis revealed 131 signaling pathways, including those related to malignant tumors, lipid and atherosclerosis, and AGE-RAGE signaling. Molecular docking results demonstrated favorable binding interactions between the core targets and the corresponding active components of Hippophae fructus. The in vitro experiments indicated that Hippophae fructus extract exhibited inhibitory effects on the proliferation of 3T3-L1 pre-adipocytes. Conclusion: This study reveals that Hippophae fructus exerts anti-obesity effects through multiple components, targets, and pathways, providing valuable insights for its clinical research and product development.
-
Keywords:
- Hippophae fructus /
- anti-obesity /
- network pharmacology /
- molecular docking /
- 3T3-L1 cells
-
近年来,全球肥胖人数飞速增长,肥胖已成为全球范围日益关注的健康问题。研究表明超重和肥胖不仅会增加死亡风险[1],也是诱发糖尿病、冠心病、高血压、脂肪肝、肿瘤等疾病的高危因素[2]。目前,用于治疗肥胖的手段有运动、药物、手术、饮食干预、针灸推拿等[3−5],然而这些方法却存在着见效慢、副作用多、难以坚持等问题,因此积极有效辅助干预治疗肥胖是当今社会高度关注的问题。
沙棘别名醋柳、酸刺、沙枣,为落叶灌木、小乔木或乔木。果实为假果类,常见黄色、黄绿色、红色、浅黄色、橘红色;果型多为圆形和卵型[6]。在我国,沙棘药用历史悠久,唐朝时期藏族医药早期著作《月王药诊》中记载沙棘可助饮食开胃消化和增强体魄[7],元朝时蒙古族经典著作《饮膳正要》记载沙棘熬成膏可生津止咳[8],明朝《本草纲目》记载了沙棘用于治疗消化系统疾病的方法[9]。1977年,沙棘被收录入《中国药典》[10],1989年,沙棘被列为药食同源中药。沙棘富含黄酮、多酚、多糖、维生素和不饱和脂肪酸等活性成分,具有其抗炎、抗癌、调节免疫、抗衰老、抗氧化等作用[11]。现有研究发现,沙棘具有一定降脂减肥的作用[12],沙棘黄酮可抑制体重增加、改善肝脏脂质累积[13],沙棘多糖能激活棕色脂肪,改善机体热量,抑制体重增加和脂质累积[14]。
网络药理学以系统生物学、高通量组学、生物信息学为基础,将药理与网络生物相结合,开启了一种多靶标与多种疾病间复杂网状关系的新研究模式,赋予了网络药理学的整体性、系统性特点,为中医药的证候研究、方剂配伍、新药开发等提供了新的思路和方法[15]。分子对接是一种主要通过电场力分析受体配体的性质特征以及相互作用来预测二者之间结合模式与亲和力的一种模拟方法。在中医药领域,利用分子对接技术将小分子活性物质与相关的靶蛋白进行对接,可以从分子水平阐明中药药效成分与作用机制[16]。网络药理学与分子对接技术结合,为阐释现代中药(复方)药效物质基础和作用机制提供理论基础与技术支持[17]。
目前对沙棘降脂减肥的研究多集中于某类成分或某一通路靶点,本研究拟通过网络药理学与分子对接技术探讨沙棘多成分、多靶点、多通路降脂减肥的协同作用机制和沙棘防治肥胖潜在的药效物质基础与作用机制,为后续研究及临床应用提供参考。
1. 材料与方法
1.1 材料与仪器
沙棘药材 甘肃甘农生物科技有限公司提供,深圳清华大学研究院马骥教授鉴定为胡颓子科植物沙棘Hippophae rhamnoides L.的成熟果实;3T3-L1小鼠前脂肪细胞 上海中乔新舟生物科技有限公司;MTT 上海麦克林生化科技有限公司;PBS磷酸盐缓冲液 北京索莱宝科技有限公司;胎牛血清、DEME高糖培养基、0.25%胰酶消化液、青霉素-链霉素溶液 美国Gemini公司;小牛血清 美国Gibco公司;二甲基亚砜(DMSO)、乙醇 分析纯,四川西陇科学有限公司;石油醚 分析纯,天津市大茂化学试剂厂。
AG 135电子天平 瑞士梅特勒托利多公司;MOV-313 P热风干燥箱、MCO-15 A CO2细胞培养箱 日本SANYO公司;CK 40-32 PH倒置显微镜 日本OLYMPUS公司;冰箱 海尔集团;生物安全柜 新加坡艺思高科技有限公司;400 R离心机、Multiskan FC酶标仪 美国Thermo公司;水浴锅 群安实验仪器有限公司;CR-30 B+超纯水机 上海杲森仪器设备有限公司;液氮罐 中科美菱低温科技股份有限公司。
1.2 实验方法
1.2.1 网络药理学与分子对接
1.2.1.1 沙棘活性成分及其对应靶点筛选
使用中药系统药理学分析平台(Traditional Chinese Medicine System Pharmacological,TCMSP,https://old.tcmsp-e.com/tcmsp.php)检索沙棘的活性成分,并设置药代动力学参数口服生物利用度(oral bioavailability,OB)≥30%及类药性(drug-likeness,DL)≥0.18对活性成分初步筛选[18],获得有效成分及其相关作用靶点。将获得的靶点导入UniProt数据库(https: //www.uniprot.org/)对其名称进行校正,去除无对应靶点的蛋白质,得沙棘化合物靶点集。将最终获得的沙棘有效成分及靶点信息导入至Cytoscape 3.9.1,生成“活性成分-作用靶点”相互关系网络图。
1.2.1.2 肥胖靶点筛选
以“obesity”为关键词,对人类孟德尔遗传综合数据库(online mendelian inheritance in man,OMIM),GeneCards数据库,DisGeNET数据库,TTD(Therapeutic Target Database)靶点数据库进行检索。将四个数据库内的搜索结果进行合并去重,得到肥胖靶点。
1.2.1.3 绘制韦恩图及蛋白质相互作用(protein-protein interaction,PPI)网络
将上述所得沙棘活性成分靶点与肥胖靶点导入Venny 2.0.2(https://bioinfogp.cnb.csic.es/tools/venny/index 2.0.2.html),绘制VEEN图,得到沙棘靶点与肥胖靶点的交集靶点,即为沙棘抗肥胖的潜在靶点。将交集靶点上传至STRING数据库平台(Search tool for recurring instances of neighbouring genes,https://cn.string-db.org/),在“Multiple proteins”项下种类定义为“Homo sapiens”进行分析,设定最低互相作用评分条件为0.400,即中等置信度[minimum required interaction score:medium confidence(0.400)],隐藏游离节点,建立药物靶蛋白-疾病靶蛋白相互作用网络,结果以TSV格式文件导出。使用CytoScape 3.9.1软件对结果进行拓扑分析,计算度(degree)值,并以degree≥60为界限筛选出沙棘抗肥胖的核心靶点。
1.2.1.4 GO功能富集分析与KEGG通路富集分析
将上述所得交集靶点导入David数据库https://david.ncifcrf.gov/summary.jsp,“select identifier”项选为官方基因名official gene symbol,种属选为人Homo sapiens,进行基因本体论Gene Ontology(GO)富集分析和京都基因与基因组百科全书 Kyoto Encyclopedia of Genes and Genomes(KEGG)通路分析,得到GO功能富集分析图和KEGG通路分析图。
1.2.1.5 成分-靶点-信号通路网络的构建
将上述各项得到的沙棘活性成分、交集靶点与KEGG信号通路信息导入Cytoscape 3.9.1构建沙棘成分-靶点-信号通路网络图。
1.2.1.6 分子对接
选择沙棘抗肥胖的PPI网络中的关键靶点与其对应的潜在活性成分进行分子对接。在PDB数据库(https://www.rcsb.org/structure)中下载关键靶点的pdb格式,在TCMSP数据库中下载活性成分的mol 2格式。利用Autodock Dock 1.5.7软件进行去水加氢处理后保存,并将抗肥胖的关键靶点与相应活性成分进行分子对接。分子对接结果用Pymol 2.2.0进行可视化处理。
1.2.2 体外细胞实验
1.2.2.1 沙棘提取物制备
沙棘鲜果洗净挑拣后,50 ℃鼓风干燥。阴凉通风处密封贮存。
沙棘水提物[19]:称取15.00 g沙棘干果,加入150 mL超纯水,浸泡1 h,水浴回流1 h,放冷抽滤,收集滤液;重复提取三次,每次1 h。合并滤液,旋蒸水浴挥至浸膏状,−80 ℃冻存。
沙棘油[20]:称取120.00 g沙棘果,加1200 mL石油醚(沸程30~60 ℃)浸泡过夜,索氏回流提取至无色。提取液旋蒸、水浴挥干溶剂,得沙棘油,4 ℃保存。
1.2.2.2 MTT法检测细胞增殖
对数生长期的3T3-L1小鼠前脂肪细胞,弃去完全培养基,加入1 mL PBS清洗后,用1 mL胰酶消化2~3 min,加入3 mL完全培养基终止消化。离心后,弃去废液,加入1 mL完全培养基,使用计数板计数。调整细胞浓度为35000 个/mL,接种于96孔板,每孔加入100 μL细胞悬液。设置空白组(含100 μL完全培养基)、对照组(含细胞的100 μL细胞培养液)、药物组(沙棘水提物、沙棘油浓度分别设置为100、125、250、500 μg·mL−1),每组3个复孔。细胞培养24 h后,吸去各药物组培养基,加入含不同浓度药物的完全培养基,培养24、48、72 h。每孔加入10 μL MTT,放入培养箱培养4 h,取出,弃去培养基,每孔加入150 μL DMSO。放入酶标仪,振荡5 min,于570 nm处测其吸光度,计算细胞存活率[21]。
1.3 数据处理
使用Graph Pad Prism 9.0.0等软件进行数据统计分析,组间差异均使用单因素方差分析,P<0.05表示存在显著性差异,两两比较用LSD-T检验。
2. 结果与分析
2.1 网络药理学与分子对接
2.1.1 沙棘活性成分及其对应靶点
基于TCMSP数据库筛选符合OB≥30%、DL≥0.18值的活性成分共33个,其中22个活性成分有对应靶点(表1),沙棘活性成分对应靶点蛋白去重后共196个。绘制沙棘活性成分与靶点网络图(图1)。
表 1 沙棘活性成分表Table 1. Active components of Hippophae fructus编号 成分ID 活性成分 中文名称 OB(%) DL 1 MOL001004 pelargonidin 天竺葵色素 37.99 0.21 2 MOL010212 14-methyl-alpha-sitosterol 14-甲基-α-谷甾醇 43.49 0.78 3 MOL010241 ergostenol 麦角甾醇 35.41 0.71 4 MOL001979 Lanosterol 隐甾醇 42.12 0.75 5 MOL001420 (8S,9S,10R,13R,14S,17R)-17-[(2R,5R)-5-ethyl-6-methylheptan-2-yl]-10,13-dimethyl-
1,2,4,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-3-oneβ-植物甾醇 38 0.76 6 MOL001494 Mandenol 亚油酸乙酯 42 0.19 7 MOL001510 24-epicampesterol 22,23-二氢菜子甾醇 37.58 0.71 8 MOL002268 rhein 大黄酸 47.07 0.28 9 MOL002588 (3S,5R,10S,13R,14R,17R)-17-[(1R)-1,5-dimethyl-4-methylenehexyl]-4,4,10,13,14-pentamethyl-
2,3,5,6,7,11,12,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-3-ol齿孔醇 42.37 0.77 10 MOL002773 beta-carotene β-胡萝卜素 37.18 0.58 11 MOL000354 isorhamnetin 异鼠李素 49.6 0.31 12 MOL000358 beta-sitosterol β-谷甾醇 36.91 0.75 13 MOL000359 sitosterol 谷甾醇 36.91 0.75 14 MOL000422 kaempferol 山柰酚 41.88 0.24 15 MOL000433 Folsaeure 叶酸 68.96 0.71 16 MOL000449 Stigmasterol 豆甾醇 43.83 0.76 17 MOL000492 (+)-catechin (+)-儿茶素 54.83 0.24 18 MOL005100 5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)chroman-4-one 橙皮素 47.74 0.27 19 MOL006756 Schottenol 仙人掌甾醇 37.42 0.75 20 MOL000073 ent-Epicatechin (+)-表儿茶素 48.96 0.24 21 MOL000953 Cholestrol 胆甾醇 37.87 0.68 22 MOL000098 quercetin 槲皮素 46.43 0.28 2.1.2 肥胖靶点
通过OMIM数据库、GeneCards数据库、DisGeNET数据库、TTD数据库检索肥胖相关基因,筛选DisGeNET数据库中Score≥0.02的靶点,GeneCards数据库中Relevance>1.247的靶点,将所得的肥胖靶点合并去重,共得2971个疾病靶点。
2.1.3 韦恩图及PPI网络
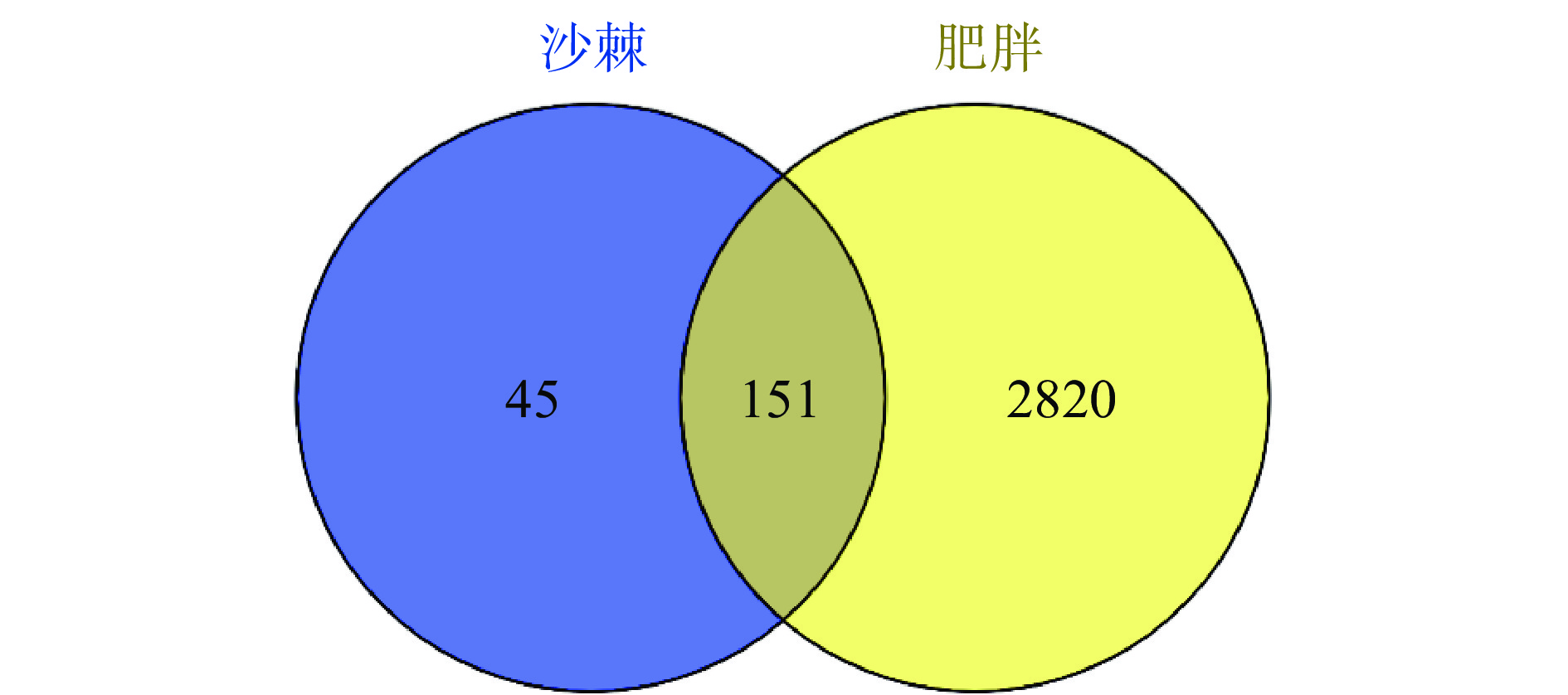
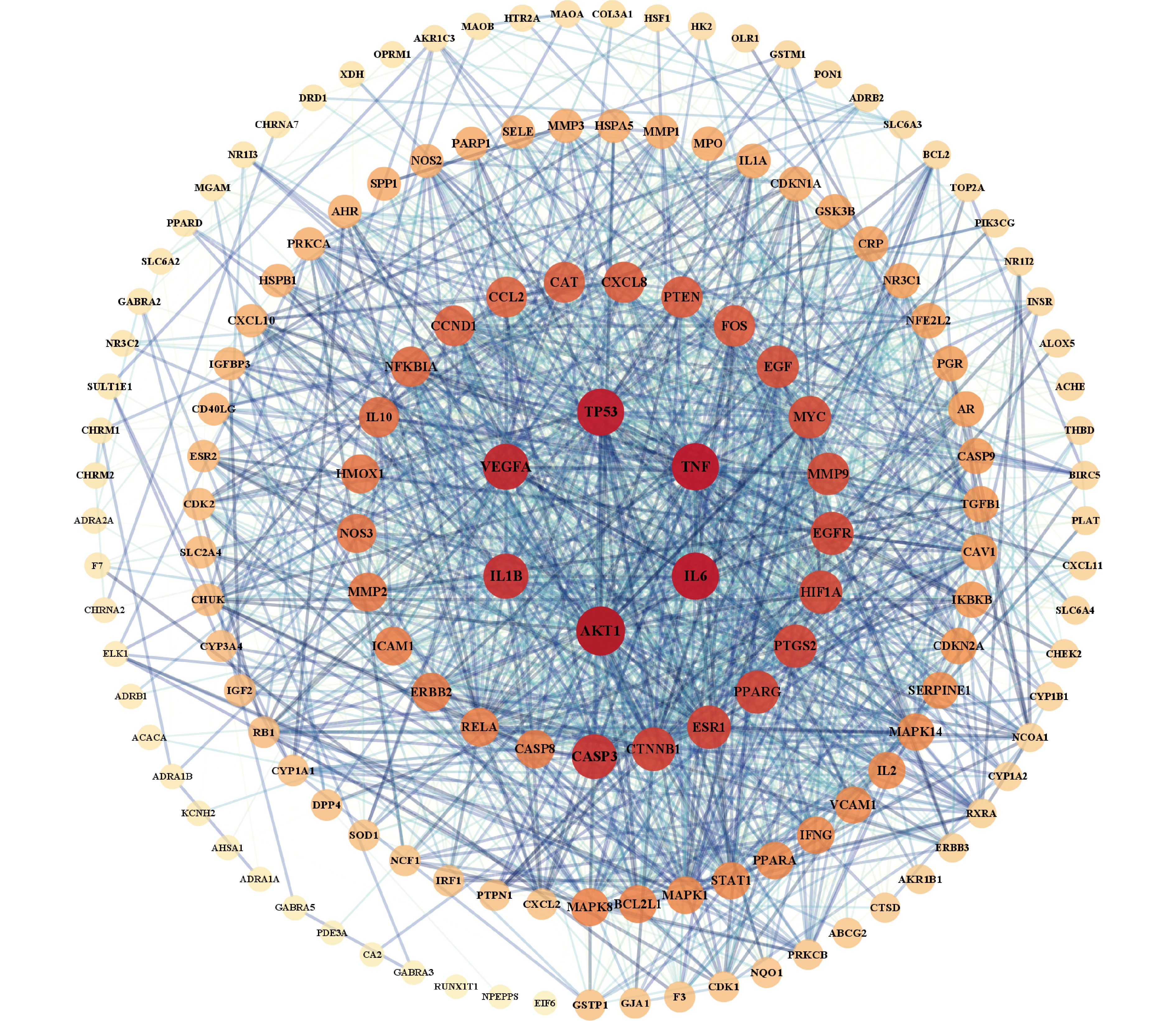
将沙棘活性成分靶点及疾病靶点导入Venny,绘制Veen图(图2)得到二者交集靶点,即得沙棘治疗肥胖的潜在靶点151个,包括TNF、IL6、CASP3、FOS、MAPK8、PPARA。将151个交集靶点导入String在线数据库,获得PPI网络图(图3),利用CytoScape 3.9.1软件进行拓扑分析。以degree≥60筛选出33个核心作用靶点:AKT1、TNF、IL6、TP53、VEGFA、IL1B、CASP3、CTNNB1、ESR1、PPARG、PTGS2、HIF1A、EGFR、MYC、MMP9、EGF、PTEN、FOS、CXCL8、CAT、CCND1、CCL2、NFKBIA、IL10、NOS3、HMOX1、MMP2、ERBB2、ICAM1、RELA、CASP8、BCL2L1、MAPK8。
2.1.4 GO功能富集分析及KEGG通路富集分析
将交集靶点呈递到David数据库进行KEGG通路富集分析和GO功能分析。
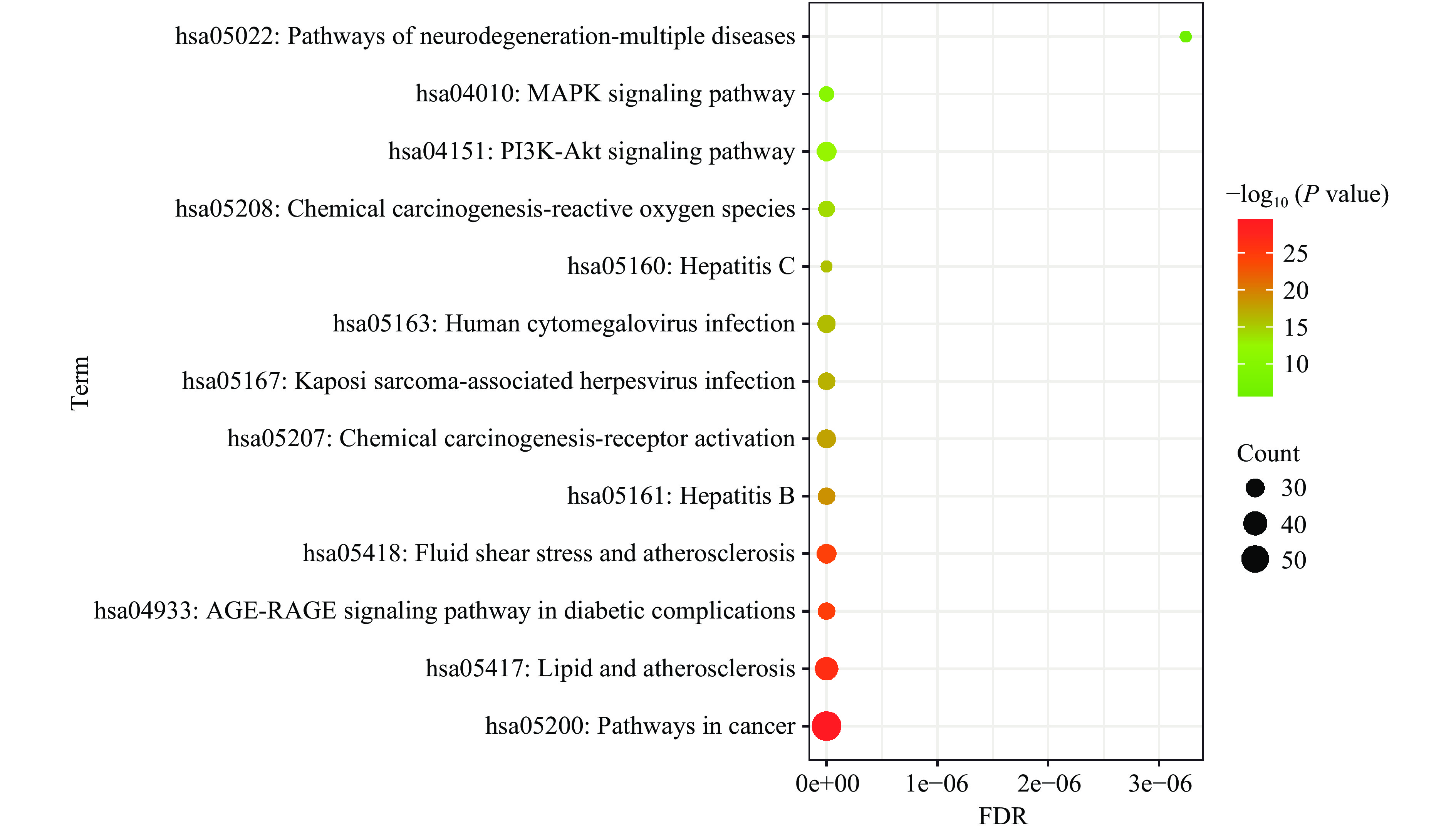
KEGG分析结果显示共有173条富集显著的信号通路,对count值≥25的信号通路进行气泡图展示,结果见图4。其中恶性肿瘤通路显示出较强关联,沙棘抗肥胖的其他相关信号通路有:脂质与动脉粥样硬化通路、糖尿病并发症中的AGE-RAGE信号通路、剪切力与动脉粥样硬化通路、乙型肝炎等。
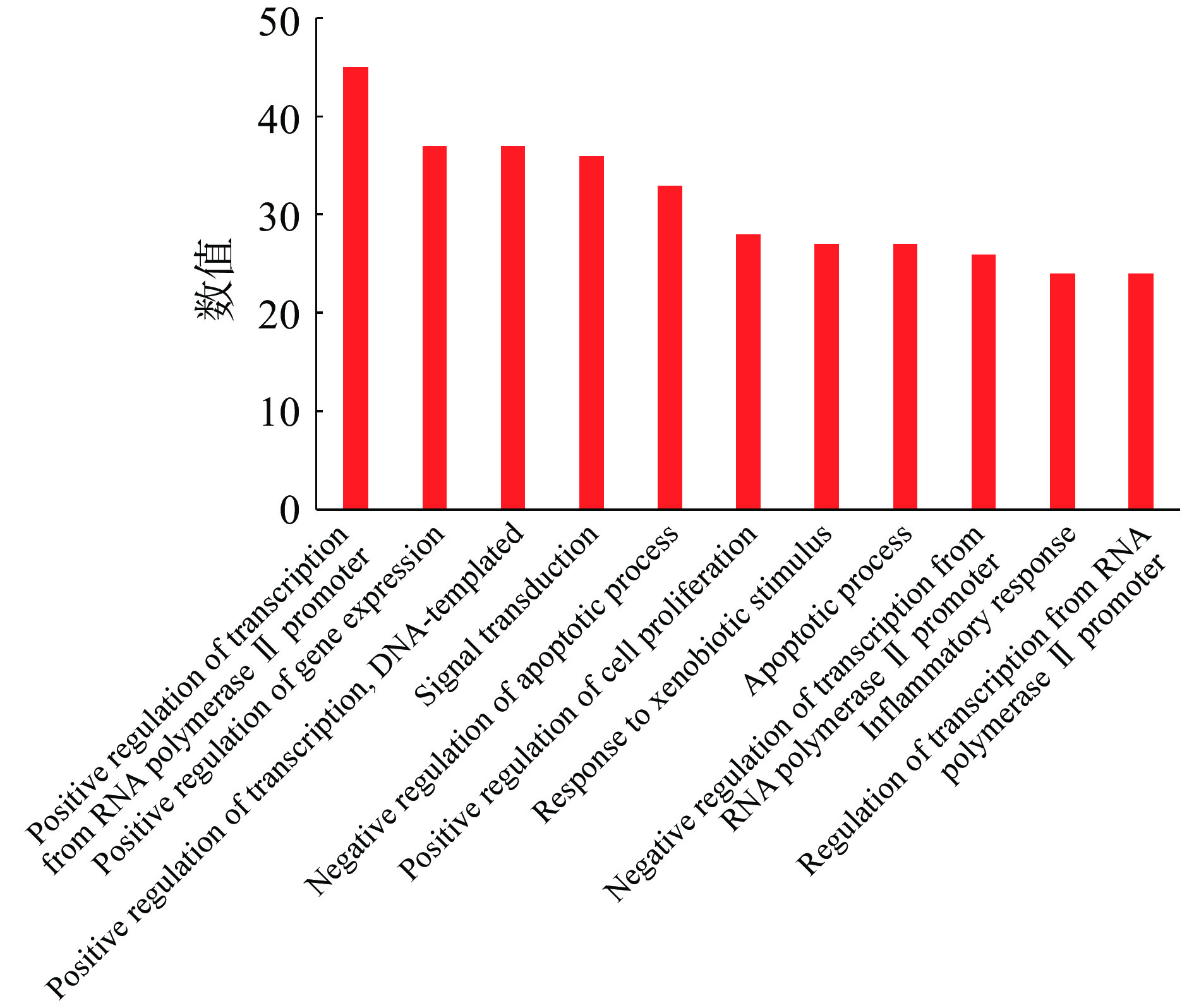
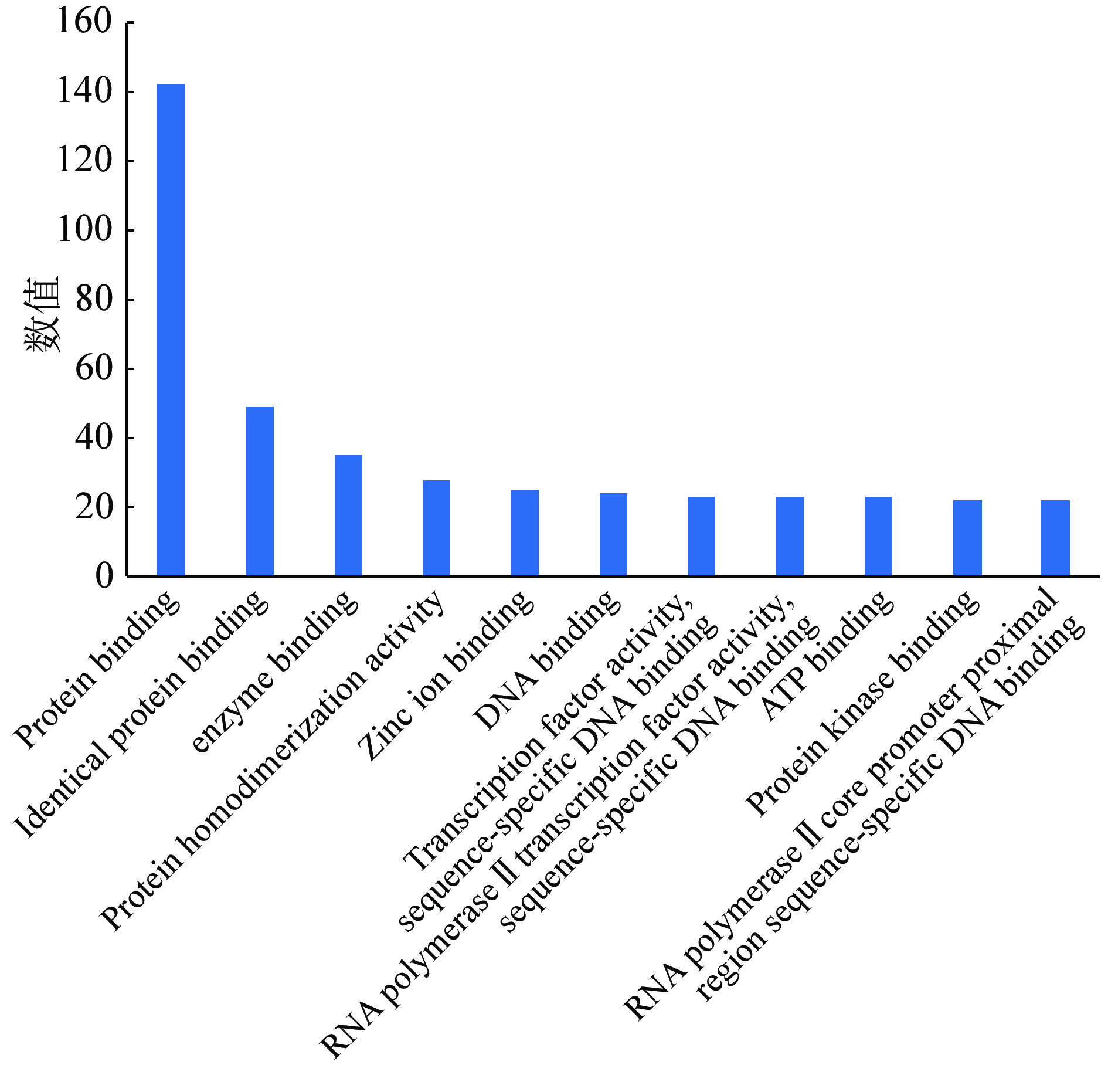
GO富集分析包括GO生物过程(biological process,BP)分析、分子功能(molecular function,MF)分析和细胞组分(cellular component,CC)分析。选取count值≥25的GO各项富集分析结果进行统计学分析,按照相关度排列。GO-BP分析结果表示沙棘主要通过RNA聚合酶Ⅱ启动子转录的正调控、基因表达的正调控、DNA转录正调控、信号转导、凋亡过程负调控等生物过程发挥抗肥胖的作用(见图5);GO-CC分析结果表示可通过细胞溶质、细胞核、质膜等细胞组分达到治疗肥胖的效果(见图6);GO-MF分析结果表示沙棘可通过蛋白结合、同蛋白结合、酶结合、蛋白质同源二聚活性等分子功能治疗肥胖(见图7)。
2.1.5 成分-靶点-信号通路网络的构建
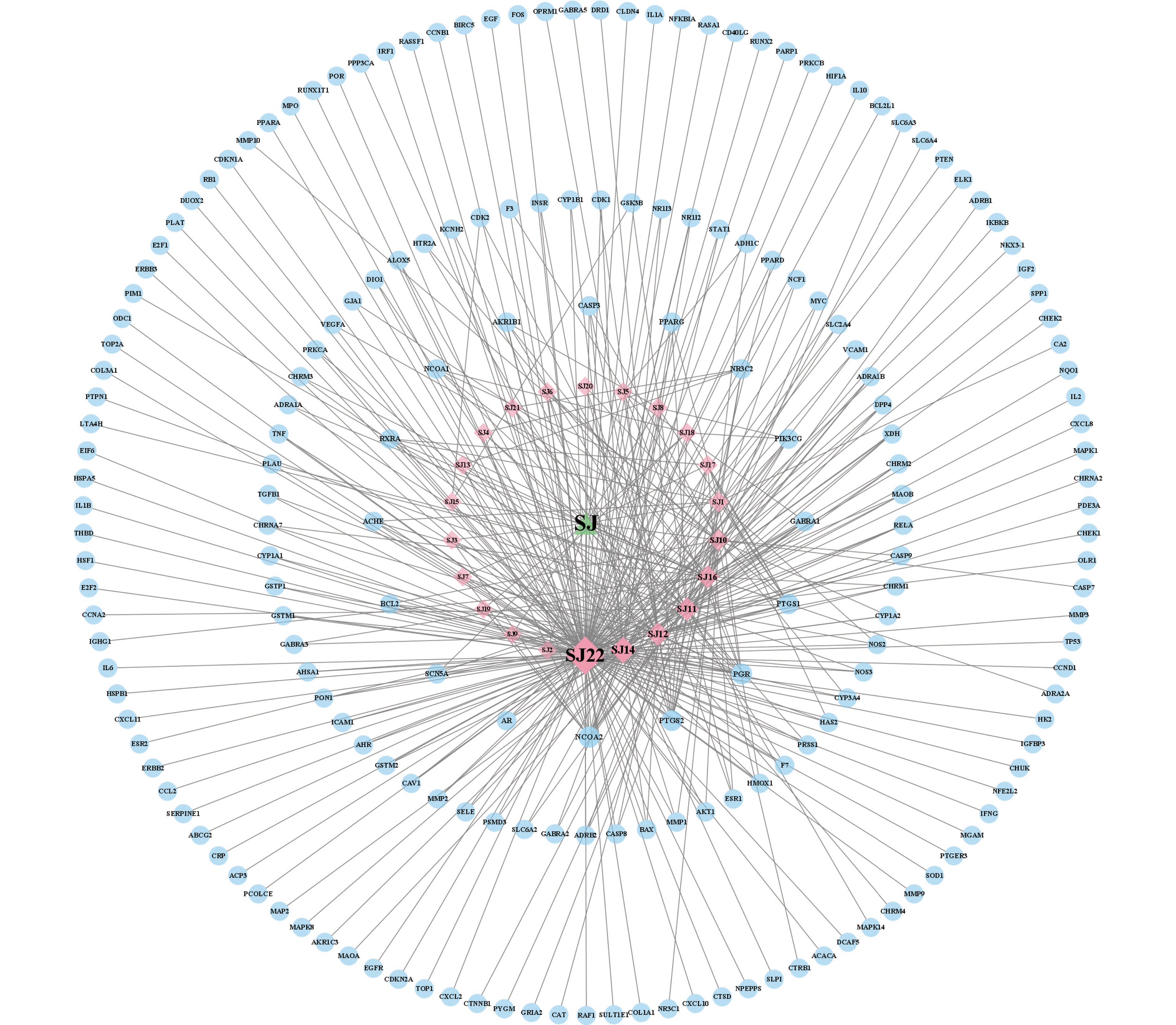
将筛选所得沙棘活性成分、药物-疾病交集靶点与KEGG信号通路信息导入Cytoscape 3.9.1软件构建沙棘成分-靶点-信号通路网络图,结果见图8。此网络图共计186个节点和724条边,其中22个红色菱形节点表示沙棘活性成分,13个紫色六边形节点表示信号通路,151个蓝色圆形节点表示交集靶点蛋白。从该图中可知活性成分SJ22(Quercetin)、SJ14(Kaempferol)、SJ12(Beta-sitosterol)、SJ11(Isorhamnetin)SJ16(Stigmasterol)、SJ10(Beta-carotene)、SJ1(Pelargonidin)可能是沙棘治疗肥胖的关键活性成分,恶性肿瘤、脂质和动脉粥样硬化、PI3K-Akt可能是关键作用通路。该分析结果展示出沙棘治疗肥胖病症过程中多成分、多靶点、多通路协同作用的特点。
2.1.6 分子对接
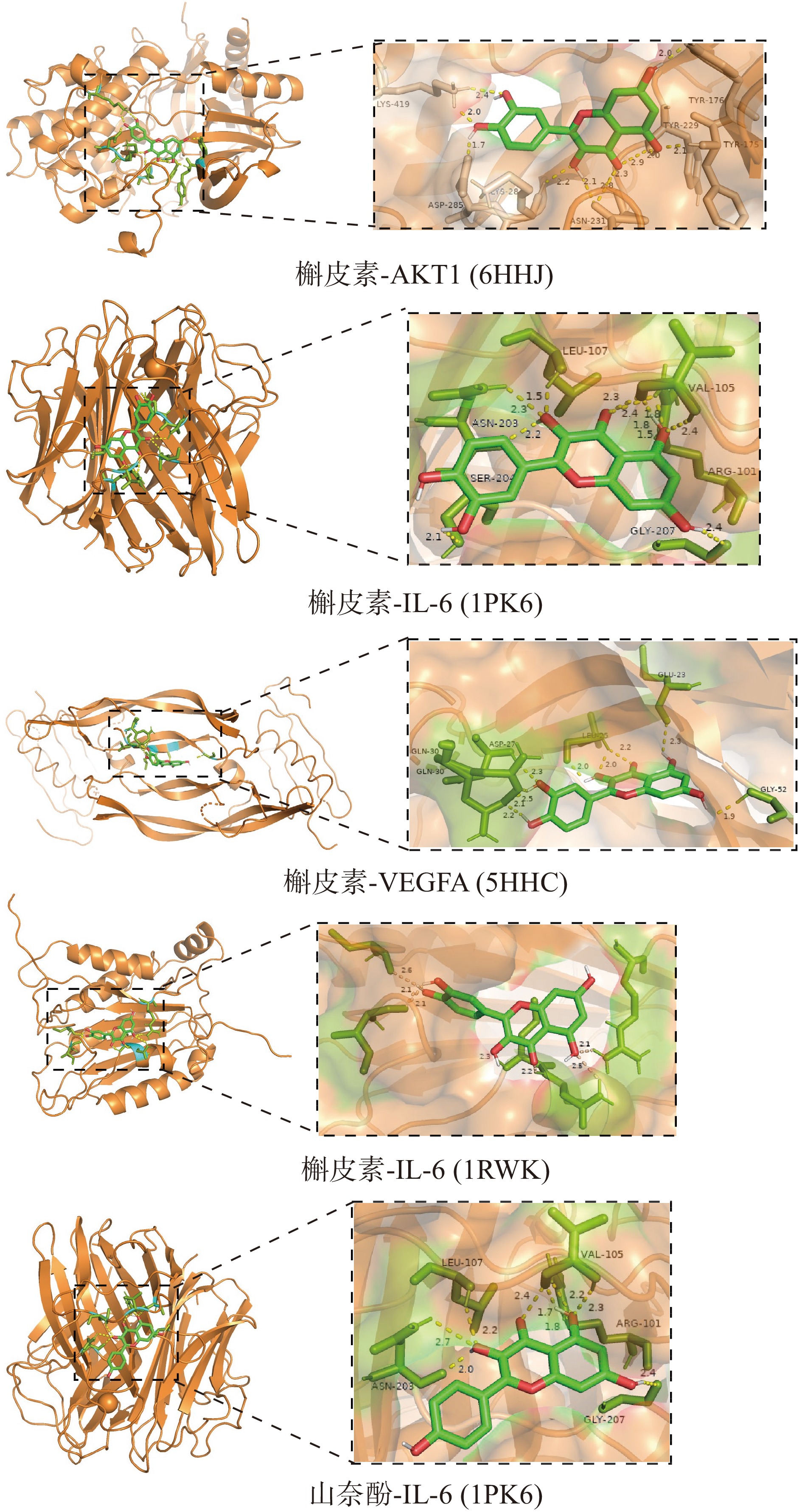
选择沙棘抗肥胖的PPI网络中度值≥95的关键靶点AKT1、IL-6、VEGFA、TNF与其对应的潜在活性成分进行分子对接验证。对接结合能见表2。结合能<0 kcal·mol−1,表明受体分子与配体分子能自发结合,结合能≤−5.0 kcal·mol−1表明受体与配体之间有较好的结合活性。结合能绝对值越高,对接能力越强,对接后分子稳定性越高,均方根偏差(RMSD)越小表示对接结果越准确。部分分子对接结果可视化见图9。
表 2 分子对接结合能Table 2. Binding energy of molecular docking编号 靶点蛋白 蛋白编号 活性成分 结合能(kcal·mol−1) 均方根偏差 1 AKT1 6HHJ quercetin −7.89 0.065 2 AKT1 6S9W kaempferol −7.69 0 3 AKT1 6S9W beta-carotene −9.72 1.570 4 IL-6 1RWK quercetin −5.93 0.050 5 TNF 1PK6 quercetin −8.07 0.263 6 TNF 1PK6 kaempferol −7.93 0.743 7 VEGFA 5HHC quercetin −7.06 0.062 8 VEGFA 5HHD beta-carotene −10.10 1.244 2.2 MTT检测沙棘提取物对3T3-L1小鼠前脂肪细胞增殖作用的影响
3T3-L1小鼠前脂肪细胞具有增殖分化的能力,是常用的体外脂肪生成模型[22]。脂肪细胞数目扩增和体积肥大是影响体内脂肪总重的主要因素,细胞数量受到细胞凋亡和增殖等活动的影响,因此抑制脂肪增殖、诱导细胞凋亡也被视为肥胖治疗手段[23]。
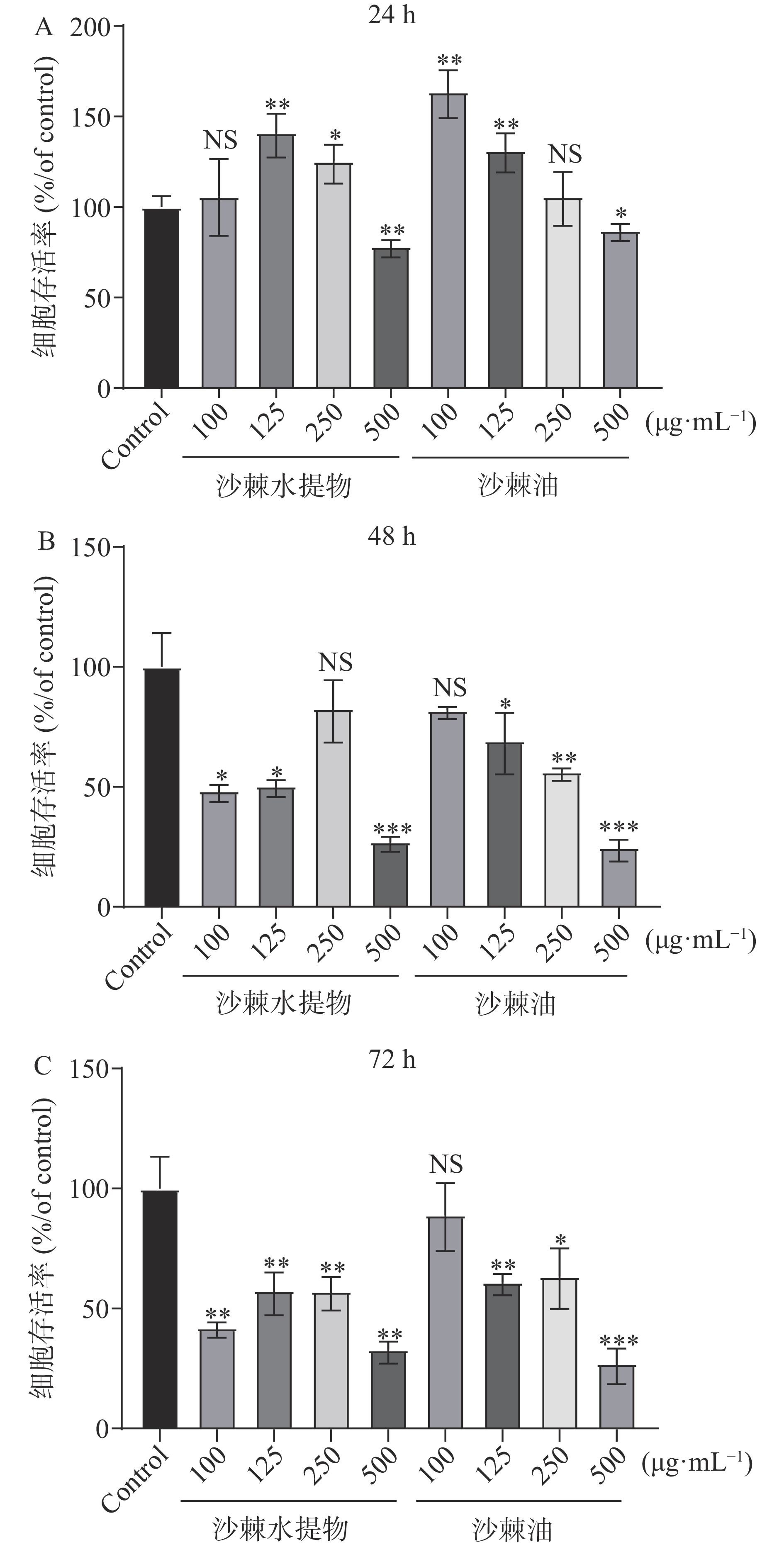
利用MTT法检测沙棘提取物不同浓度、不同作用时间对3T3-L1前脂肪细胞的增殖的影响,结果见图10。由图10A可知,与对照组相比,沙棘水提物与沙棘油作用于3T3-L1前脂肪细胞24 h后,125、250 μg·mL−1沙棘水提物与100、125 μg·mL−1沙棘油对细胞有短暂的促增殖效果(P<0.01,P<0.05),100 μg·mL−1沙棘水提物与250 μg·mL−1沙棘油无较为明显变化。250 μg·mL−1的沙棘水提物与沙棘油则表现出抑制细胞增殖的效果(P<0.01,P<0.05)。随着药物作用时间的增长至48 h(见图10B),除250 μg·mL−1的沙棘水提物与100 μg·mL−1的沙棘油外与对照组无显著性差异外,其余各组均表现出显著抑制细胞增殖的效果(P<0.05,P<0.01,P<0.001)。药物作用时间至72 h后,除100 μg·mL−1沙棘油与对照组相比无显著性差异外,其余各组均表现出显著抑制细胞增殖的效果(见图10C)(P<0.05,P<0.01,P<0.001)。
3. 讨论与结论
本研究采用网络药理学和分子对接技术探讨沙棘抗肥胖的有效成分和作用机制,并利用3T3-L1小鼠前脂肪细胞进行体外验证。
本研究筛选出22个具有对应靶点的沙棘活性成分,槲皮素、山奈酚、β-胡萝卜素、α/β-谷甾醇、麦角甾烯醇、表儿茶素可能为沙棘抗肥胖潜在活性成分。研究表明,槲皮素能改善与代谢综合征相关的异常,如肥胖、血脂异常和葡萄糖不耐受等病症[24],它能通过靶向PPARα/γ调控糖异生改善肝脏脂肪蓄积[25]。山奈酚可促进成熟脂肪细胞中Cebpa基因表达的下调和脂质积累的减少,调节3T3-L1细胞的成脂分化,进而达到抗肥胖作用,也可通过调节能量平衡、下丘脑炎症相关中枢过程、肠道菌群等途径达到抗肥胖的目的[26−28]。β-胡萝卜素是类胡萝卜素的一种,也是维生素A的主要来源。类胡萝卜素可参与脂肪组织代谢和炎症的调节,β-胡萝卜素通过β3-AR/p38 MAPK/SIRT信号通路上调调节脂肪分解和脂肪氧化,诱导3T3-L1白色脂肪细胞褐变[29]。
通过构建沙棘与疾病的靶点Veen图与PPI网络,筛选出151个沙棘抗肥胖的潜在作用靶点。AKT1是脂肪细胞分化和克隆扩增过程中极为重要的信号分子[30],周坤[31]研究表明山楂多糖、黄酮提取物能够通过PI3K/AKT通路起到调节肥胖小鼠糖脂代谢异常的作用。白色脂肪组织中的脂肪细胞以及M1巨噬细胞可分泌大量的TNF-α及IL-6等促炎因子,加快肥胖及其相关代谢病的发展[32],高脂小鼠表现出明显的体质量增加、糖脂代谢紊乱、回肠炎症水平增加[33]。IL-6因子可促进脂肪组织脂解,刺激脂质代谢[34]。严欢等[35]发现小麦麸皮多酚提取物能降低大鼠肝匀浆中IL-6、TNF-α等因子的含量,减轻肥胖引起的慢性炎症,减肥效果良好。VEGFA为血管内皮生长因子,能促进血管生成、增强血管通透性,调节脂肪组织的分化和能量代谢等过程[36],VEGFA表达下调后能够促进白色脂肪的棕色化,降低体重、增强葡萄糖清除能力以及胰岛素敏感性,并且对高脂食物诱导的肥胖具有一定的抗性[37]。
结合筛选得到的沙棘抗肥胖交集作用靶点,进行KEGG通路分析和GO功能分析,探讨其抗肥胖分子作用机制。结果提示恶性肿瘤通路富集靶点最多,是沙棘抗肥胖较为显著通路。肥胖是癌症诱发因素之一,研究发现子宫内膜癌[38]、乳腺癌[39]、结直肠癌[40]、甲状腺癌[41]等都与肥胖有密切的关系[42]。肥胖相关癌症的发生与体内生长激素和脂肪因子水平升高、肠道菌群失调、肿瘤代谢改变和慢性低度炎症等微环境变化有着密切关系[43−44],脂肪细胞分泌高水平的IL-6、TNF、脂肪因子和瘦素,组织炎症加重,促进癌细胞增殖[45]。研究人员通过癌症与肥胖等代谢疾病间的相关性研究,期望达到早期识别、应对癌症发生[46]。沙棘成分-靶点-通路图表明沙棘可通路多成分、多靶点、多通路的协同作用达到抗肥胖。将AKT1、TNF、IL-6、VEGFA四个核心靶点与对应活性成分进行分子对接验证,结果表明各结合能均<−7 kcal·mol−1,对接结果良好,有较好的结合活性。体外细胞实验表明,浓度为100、125、250 μg·mL−1的沙棘水提物与沙棘油短时间(24 h)作用于3T3-L1前脂肪细胞,促进细胞增殖。随着药物作用时间的增长(48 h),各个浓度(100~500 μg·mL−1)的沙棘水提物与沙棘油均表现出一定的抑制细胞增殖的作用(250 μg·mL−1的沙棘水提物与100 μg·mL−1的沙棘油除外)。在作用时间达到72 h时,仅100 μg·mL−1的沙棘油与对照组差异不显著,各组药物对抑制细胞增殖均有显著效果(P<0.05,P<0.01,P<0.001)。
综上所述,本研究探讨了沙棘抗肥胖的潜在有效成分和作用机制,为进一步临床研究和产品开发提供参考。
-
表 1 沙棘活性成分表
Table 1 Active components of Hippophae fructus
编号 成分ID 活性成分 中文名称 OB(%) DL 1 MOL001004 pelargonidin 天竺葵色素 37.99 0.21 2 MOL010212 14-methyl-alpha-sitosterol 14-甲基-α-谷甾醇 43.49 0.78 3 MOL010241 ergostenol 麦角甾醇 35.41 0.71 4 MOL001979 Lanosterol 隐甾醇 42.12 0.75 5 MOL001420 (8S,9S,10R,13R,14S,17R)-17-[(2R,5R)-5-ethyl-6-methylheptan-2-yl]-10,13-dimethyl-
1,2,4,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-3-oneβ-植物甾醇 38 0.76 6 MOL001494 Mandenol 亚油酸乙酯 42 0.19 7 MOL001510 24-epicampesterol 22,23-二氢菜子甾醇 37.58 0.71 8 MOL002268 rhein 大黄酸 47.07 0.28 9 MOL002588 (3S,5R,10S,13R,14R,17R)-17-[(1R)-1,5-dimethyl-4-methylenehexyl]-4,4,10,13,14-pentamethyl-
2,3,5,6,7,11,12,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-3-ol齿孔醇 42.37 0.77 10 MOL002773 beta-carotene β-胡萝卜素 37.18 0.58 11 MOL000354 isorhamnetin 异鼠李素 49.6 0.31 12 MOL000358 beta-sitosterol β-谷甾醇 36.91 0.75 13 MOL000359 sitosterol 谷甾醇 36.91 0.75 14 MOL000422 kaempferol 山柰酚 41.88 0.24 15 MOL000433 Folsaeure 叶酸 68.96 0.71 16 MOL000449 Stigmasterol 豆甾醇 43.83 0.76 17 MOL000492 (+)-catechin (+)-儿茶素 54.83 0.24 18 MOL005100 5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)chroman-4-one 橙皮素 47.74 0.27 19 MOL006756 Schottenol 仙人掌甾醇 37.42 0.75 20 MOL000073 ent-Epicatechin (+)-表儿茶素 48.96 0.24 21 MOL000953 Cholestrol 胆甾醇 37.87 0.68 22 MOL000098 quercetin 槲皮素 46.43 0.28 表 2 分子对接结合能
Table 2 Binding energy of molecular docking
编号 靶点蛋白 蛋白编号 活性成分 结合能(kcal·mol−1) 均方根偏差 1 AKT1 6HHJ quercetin −7.89 0.065 2 AKT1 6S9W kaempferol −7.69 0 3 AKT1 6S9W beta-carotene −9.72 1.570 4 IL-6 1RWK quercetin −5.93 0.050 5 TNF 1PK6 quercetin −8.07 0.263 6 TNF 1PK6 kaempferol −7.93 0.743 7 VEGFA 5HHC quercetin −7.06 0.062 8 VEGFA 5HHD beta-carotene −10.10 1.244 -
[1] CHOI M K, PARK Y M, SHIVAPPA N, et al. Inflammatory potential of diet and risk of mortality in normal-weight adults with central obesity[J]. Clinical Nutrition,2023,42(2):208−215. doi: 10.1016/j.clnu.2022.11.019
[2] 中华医学会内分泌学分会肥胖学组. 中国成人肥胖症防治专家共识[J]. 中华内分泌代谢杂志, 2011(9):711−717. [Obesity group of the endocrinology branch of the Chinese Medical Association. Expert consensus on adult obesity prevention and treatment in China[J]. Chinese Journal of Endocrinology and Metabolism, 2011(9):711−717.] Obesity group of the endocrinology branch of the Chinese Medical Association. Expert consensus on adult obesity prevention and treatment in China[J]. Chinese Journal of Endocrinology and Metabolism, 2011(9): 711−717.
[3] 张彦康, 张婷, 李雨, 等. 肥胖治疗的挑战与希望[J]. 自然杂志, 2022, 44(6):469−479. [ZHANG Yankang, ZHANG Ting, LI Yu, et al. Challenges and prospects of obesity treatment[J]. Nature Journal, 2019, 44(6):469−479.] ZHANG Yankang, ZHANG Ting, LI Yu, et al. Challenges and prospects of obesity treatment[J]. Nature Journal, 2019, 44(6): 469−479.
[4] 杨金炎, 邓小敏, 雷海玲, 等. 中医药防治肥胖症的研究概况[J]. 中医药临床杂志,2023,35(3):604−608. [YANG Jinyan, DENG Xiaomin, LEI Hailing, et al. Research overview of TCM prevention and treatment of obesity[J]. Clinical Journal of Chinese Medicine,2023,35(3):604−608.] YANG Jinyan, DENG Xiaomin, LEI Hailing, et al . Research overview of TCM prevention and treatment of obesity[J]. Clinical Journal of Chinese Medicine,2023 ,35 (3 ):604 −608 .[5] MÜLLER T D, BLÜHER M, TSCHÖP M H, et al. Anti-obesity drug discovery:Advances and challenges[J]. Nature Reviews Drug Discovery,2022,21(3):201−223. doi: 10.1038/s41573-021-00337-8
[6] 何长廷, 昂青才旦, 才曾卓玛, 等. 沙棘资源、药用状况及其对心血管疾病药理作用概述[J]. 中国野生植物资源, 2023, 42(4):1−7,17. [HE Changting, ANGQING Caidan, CAIZENG Zhuoma, et al. Resources, medicinal status and pharmacological effects of Hippophae fructus on cardiovascular diseases[J]. China Wild Plant Resources, 2019, 42(4):1−7,17.] HE Changting, ANGQING Caidan, CAIZENG Zhuoma, et al. Resources, medicinal status and pharmacological effects of Hippophae fructus on cardiovascular diseases[J]. China Wild Plant Resources, 2019, 42(4): 1−7,17.
[7] 马世林, 毛继祖. 月王药诊[M]. 上海:上海科学技术出版社, 2012. [MA Shilin, MAO Jizu. Yue Wang Pharmaceutical Diagnosis[M]. Shanghai:Shanghai Science and Technology Press, 2012.] MA Shilin, MAO Jizu. Yue Wang Pharmaceutical Diagnosis[M]. Shanghai: Shanghai Science and Technology Press, 2012.
[8] (元)忽思慧著. 饮膳正要[M]. 刘正书译. 北京:人民卫生出版社, 1986. [(Yuan) Wrote by HU Sihui. Yin Shan Zheng Yao[M]. Translated by LIU Zhengshu. Beijing:People's Health Publishing House, 1986.] (Yuan) Wrote by HU Sihui. Yin Shan Zheng Yao[M]. Translated by LIU Zhengshu. Beijing: People's Health Publishing House, 1986.
[9] (明)李时珍著. 本草纲目[M]. 武汉:崇文书局, 2008. [(Ming) Wrote by LI Shizhen. Compendium of Materia Medica[M]. Wuhan:Chongwen Bookstore, 2008.] (Ming) Wrote by LI Shizhen. Compendium of Materia Medica[M]. Wuhan: Chongwen Bookstore, 2008.
[10] 卫生部药典委员会编. 中华人民共和国药典[M]. 第1版. 北京:人民卫生出版社, 1978. [Compiled by The Pharmacopoeia Committee of the Ministry of Health. Pharmacopoeia of the People's Republic of China[M]. 1st Edition. Beijing:People's Health Publishing House, 1978.] Compiled by The Pharmacopoeia Committee of the Ministry of Health. Pharmacopoeia of the People's Republic of China[M]. 1st Edition. Beijing: People's Health Publishing House, 1978.
[11] 宁志雪, 牛广财, 朱立斌, 等. 沙棘活性成分、生理功能及开发利用研究进展[J]. 食品与机械,2021,37(11):221−227,240. [NING Zhixue, NIU Guangcai, ZHU Libin, et al. Research progress on active ingredients, physiological functions and development and utilization of Hippophae fructus[J]. Food and Machinery,2021,37(11):221−227,240.] NING Zhixue, NIU Guangcai, ZHU Libin, et al . Research progress on active ingredients, physiological functions and development and utilization of Hippophae fructus[J]. Food and Machinery,2021 ,37 (11 ):221 −227,240 .[12] 韩丽. 沙棘冻干粉对高脂饮食小鼠的降脂减肥与肠道菌群的调节作用[D]. 太原:山西大学, 2020. [HAN Li. The regulatory effects of Hippophae fructus powder on lipid reduction and intestinal flora in high-fat diet mice[D]. Taiyuan:Shanxi University, 2020.] HAN Li. The regulatory effects of Hippophae fructus powder on lipid reduction and intestinal flora in high-fat diet mice[D]. Taiyuan: Shanxi University, 2020.
[13] 艾孜古丽·木拉提. 沙棘黄酮改善高热能膳食诱导小鼠糖脂代谢紊乱及认知障碍作用与机制研究[D]. 杨凌:西北农林科技大学, 2022. [Aziguli·MULATI. Study on the effect and mechanism of Hippophae fructus flavonoids on glucolipid metabolism disorder and cognitive impairment induced by high caloric diet in mice[D]. Yangling:Northwest A&F University, 2022.] Aziguli·MULATI. Study on the effect and mechanism of Hippophae fructus flavonoids on glucolipid metabolism disorder and cognitive impairment induced by high caloric diet in mice[D]. Yangling: Northwest A&F University, 2022.
[14] MA Zhiyuan, SUN Qingyang, CHANG Lili, et al. A natural anti-obesity reagent derived from sea buckthorn polysaccharides:Structure characterization and anti-obesity evaluation in vivo[J]. Food Chemistry,2022,375:131884−131893. doi: 10.1016/j.foodchem.2021.131884
[15] ZHOU Zhuchen, CHEN Bing, CHEN Simiao, et al. Applications of network pharmacology in traditional chinese medicine research[J]. Evidence-Based Complementary and Alternative Medicine,2020,2020:1646905−1646911.
[16] 王文军, 丁一, 窦芳, 等. 分子对接在中药药效物质筛选及作用机制研究中的应用进展[J]. 中国药师,2018,21(6):1020−1023. [WANG Wenjun, DING Yi, DOU Fang, et al. Application of molecular docking in the screening of pharmacodynamic substances and mechanism of action of Traditional Chinese Medicine[J]. Chinese Pharmacists,2018,21(6):1020−1023.] WANG Wenjun, DING Yi, DOU Fang, et al . Application of molecular docking in the screening of pharmacodynamic substances and mechanism of action of Traditional Chinese Medicine[J]. Chinese Pharmacists,2018 ,21 (6 ):1020 −1023 .[17] JIAO Xinyi, JIN Xin, MA Yuanyuan, et al. A comprehensive application:Molecular docking and network pharmacology for the prediction of bioactive constituents and elucidation of mechanisms of action in component-based Chinese medicine[J]. Computational Biology and Chemistry,2021,90:107402−107409. doi: 10.1016/j.compbiolchem.2020.107402
[18] LIU Hui, WANG Jinan, ZHOU Wei, et al. Systems approaches and polypharmacology for drug discovery from herbal medicines:An example using licorice[J]. Journal of Ethnopharmacology,2013,146(3):773−793. doi: 10.1016/j.jep.2013.02.004
[19] 刘悦. 多基原民族药沙棘的多维鉴定方法研究[D]. 成都:成都中医药大学, 2016. [LIU Yue. Research on multi-dimensional identification method of multi-base original ethnic medicine Hippophae fructus[D]. Chengdu:Chengdu University of Traditional Chinese Medicine, 2016.] LIU Yue. Research on multi-dimensional identification method of multi-base original ethnic medicine Hippophae fructus[D]. Chengdu: Chengdu University of Traditional Chinese Medicine, 2016.
[20] 闫克玉, 杜紫娟. 正交试验法优化沙棘籽油的提取工艺[J]. 食品研究与开发,2010,31(4):31−34. [YAN Keyu, DU Zijuan. Optimization of extraction process of Hippophae fructus seed oil by orthogonal test[J]. Food Research and Development,2010,31(4):31−34.] YAN Keyu, DU Zijuan . Optimization of extraction process of Hippophae fructus seed oil by orthogonal test[J]. Food Research and Development,2010 ,31 (4 ):31 −34 .[21] MIAO Hui, PAN Hui, WANG Linjie, et al. Ghrelin promotes proliferation and inhibits differentiation of 3T3-L1 and human primary preadipocytes[J]. Frontiers in Physiology,2019,10:1296−1307. doi: 10.3389/fphys.2019.01296
[22] CIMAS F J, DE L C-M M Á, CIFUENTES C, et al. Effect of crocetin on basal lipolysis in 3T3-L1 adipocytes[J]. Antioxidants,2023,12(6):1254−1272. doi: 10.3390/antiox12061254
[23] JAKAB J, MIŠKIĆ B, MIKŠIĆ Š, et al. Adipogenesis as a potential anti-obesity target:A review of pharmacological treatment and natural products[J]. Diabetes Metab Syndr Obes,2021,14:67−83. doi: 10.2147/DMSO.S281186
[24] KÁBELOVÁ A, MALÍNSKÁ H, MARKOVÁ I, et al. Quercetin supplementation alters adipose tissue and hepatic transcriptomes and ameliorates adiposity, dyslipidemia, and glucose intolerance in adult male rats[J]. Frontiers in Nutrition,2022,9:952065−952074. doi: 10.3389/fnut.2022.952065
[25] ZHAO Jingqi, SUN Yantong, YUAN Cuiping, et al. Quercetin ameliorates hepatic fat accumulation in high-fat diet-induced obese mice via PPARs[J]. Food & function,2023,14(3):1674−1684.
[26] TORRES-VILLARREAL D, CAMACHO A, CASTRO H, et al. Anti-obesity effects of kaempferol by inhibiting adipogenesis and increasing lipolysis in 3T3-L1 cells[J]. Journal of Physiology and Biochemistry,2018,75(1):83−88.
[27] ROMERO-JUÁREZ P A, VISCO D B, MANHÃES-DE-CASTRO R, et al. Dietary flavonoid kaempferol reduces obesity-associated hypothalamic microglia activation and promotes body weight loss in mice with obesity[J]. Nutritional Neuroscience,2023,26(1):25−39. doi: 10.1080/1028415X.2021.2012629
[28] BIAN Yifei, LEI Jiaqi, ZHONG Jia, et al. Kaempferol reduces obesity, prevents intestinal inflammation, and modulates gut microbiota in high-fat diet mice[J]. Journal of Nutritional Biochemistry,2022,99:108840−108850. doi: 10.1016/j.jnutbio.2021.108840
[29] MUKHERJEE S, YUN J W. β-Carotene stimulates browning of 3T3-L1 white adipocytes by enhancing thermogenesis via the β3-AR/p38 MAPK/SIRT signaling pathway[J]. Phytomedicine,2022,96:153857−153865. doi: 10.1016/j.phymed.2021.153857
[30] SANCHEZ-GURMACHES J, MARTINEZ Calejman C, JUNG S M, et al. Brown fat organogenesis and maintenance requires AKT1 and AKT2[J]. Molecular Metabolism,2019,23:60−74. doi: 10.1016/j.molmet.2019.02.004
[31] 周坤. 山楂果叶多糖提取物对肥胖小鼠糖脂代谢的作用研究[D]. 西安:西北大学, 2022. [ZHOU Kun. Effects of polysaccharide extracts from Hawthorn fruits and leaves on glycolipid metabolism in obese mice[D]. Xi'an:Northwest University, 2022.] ZHOU Kun. Effects of polysaccharide extracts from Hawthorn fruits and leaves on glycolipid metabolism in obese mice[D]. Xi'an: Northwest University, 2022.
[32] 陆江, 朱道仙, 卢劲晔, 等. 高聚合度菊粉通过调节肠-脂肪组织轴改善高脂饮食诱导的犬肥胖[J/OL]. 畜牧兽医学报:1−10[2023-12-20]. http://kns.cnki.net/kcms/detail/11.1985.S.20230412.1310.006.html. [LU Jiang, ZHU Daoxian, LU Jinye, et al. Highly polymerized inulin improves high-fat diet-induced obesity in dogs by regulating the enteric-adipose tissue axis[J/OL]. Acta Veterinaria et Zootechinca Sinica:1−10[2023-12-20]. http://kns.cnki.net/kcms/detail/11.1985.S.20230412.1310.006.html.] LU Jiang, ZHU Daoxian, LU Jinye, et al. Highly polymerized inulin improves high-fat diet-induced obesity in dogs by regulating the enteric-adipose tissue axis[J/OL]. Acta Veterinaria et Zootechinca Sinica: 1−10[2023-12-20]. http://kns.cnki.net/kcms/detail/11.1985.S.20230412.1310.006.html.
[33] 蔡雪琴, 曹丽远, 周亚飞, 等. 双歧杆菌三联活菌对肥胖小鼠的改善作用及其机制[J]. 中国微生态学杂志, 2023, 35(3):304−310. [CAI Xueqin, CAO Liyuan, ZHOU Yafei, et al. Effect and mechanism of bifidobacterium triad on obesity mice[J]. Chinese Journal of Microecology, 2019, 35(3):304−310.] CAI Xueqin, CAO Liyuan, ZHOU Yafei, et al. Effect and mechanism of bifidobacterium triad on obesity mice[J]. Chinese Journal of Microecology, 2019, 35(3): 304−310.
[34] 胡满江. DHA、EPA调节小鼠胰岛素抵抗的作用差异及GPR120/PPARγ介导的机制研究[D]. 广州:南方医科大学, 2019. [HU Mangjiang. The difference of the effects of DHA and EPA on the regulation of insulin resistance in mice and the mechanism mediated by GPR120/PPARγ[D]. Guangzhou:Southern Medical University, 2019.] HU Mangjiang. The difference of the effects of DHA and EPA on the regulation of insulin resistance in mice and the mechanism mediated by GPR120/PPARγ[D]. Guangzhou: Southern Medical University, 2019.
[35] 严欢, 韩加. 小麦麸皮多酚对肥胖大鼠的减肥降脂作[J/OL]. 食品科学:1−11[2023-12-20]. http://kns.cnki.net/kcms/detail/11.2206.TS.20221230.1606.044.html. [YAN Huan, HAN Jia. Effects of wheat bran polyphenols on weight loss and lipid reduction in obese rats[J/OL]. Food science:1−11[2023-12-20]. http://kns.cnki.net/kcms/detail/11.2206.TS.20221230.1606.044.html.] YAN Huan, HAN Jia. Effects of wheat bran polyphenols on weight loss and lipid reduction in obese rats[J/OL]. Food science: 1−11[2023-12-20]. http://kns.cnki.net/kcms/detail/11.2206.TS.20221230.1606.044.html.
[36] 郑婷婷. VEGFA (164)脂肪组织特异表达小鼠模型的构建及研究[D]. 长春:东北师范大学, 2020. [ZHENG Tingting. Construction and study of adipose tissue-specific expression of VEGFA (164) in mice[D]. Changchun:Northeast Normal University, 2020.] ZHENG Tingting. Construction and study of adipose tissue-specific expression of VEGFA (164) in mice[D]. Changchun: Northeast Normal University, 2020.
[37] 金红红. VEGFA和VEGFB调节脂肪组织分化、基因表达和生物学功能的平衡[D]. 长春:东北师范大学, 2018. [JIN Honghong. VEGFA and VEGFB regulate the balance of adipose tissue differentiation, gene expression and biological function[D]. Changchun:Northeast Normal University, 2018.] JIN Honghong. VEGFA and VEGFB regulate the balance of adipose tissue differentiation, gene expression and biological function[D]. Changchun: Northeast Normal University, 2018.
[38] PETERSEN H S, BALMACEDA J, SPOOZAK L, et al. Higher baseline BMI and lower estimated median income is associated with increasing BMI after endometrial cancer diagnosis[J]. Gynecologic Oncology Reports,2022,44:101123−101128. doi: 10.1016/j.gore.2022.101123
[39] 黄维荪, 许隽颖, 晏芾. 中心性肥胖对妇女乳腺癌发病的影响因素分析[J]. 中华肿瘤防治杂志, 2023, 30(4):219−224. [HUANG Weisun, XU Junying, YAN Fu. Analysis of the influence factors of central obesity on the incidence of breast cancer in women[J]. Chinese Journal of Cancer Prevention and Treatment, 2019, 30(4):219−224.] HUANG Weisun, XU Junying, YAN Fu. Analysis of the influence factors of central obesity on the incidence of breast cancer in women[J]. Chinese Journal of Cancer Prevention and Treatment, 2019, 30(4): 219−224.
[40] 李雪, 闫雨萌, 平卫伟. 肥胖对消化道系统癌症发病影响的系统评价[J]. 中国循证医学杂志,2022,22(10):1155−1160. [LI Xue, YAN Yumeng, PING Weiwei. A systematic review of the effects of obesity on the incidence of digestive tract cancer[J]. Chinese Journal of Evidence-Based Medicine,2022,22(10):1155−1160.] LI Xue, YAN Yumeng, PING Weiwei . A systematic review of the effects of obesity on the incidence of digestive tract cancer[J]. Chinese Journal of Evidence-Based Medicine,2022 ,22 (10 ):1155 −1160 .[41] 张宏伟, 石福民, 薛荣, 等. 肥胖指标体质量指数和腰围与甲状腺癌的关系[J]. 中国耳鼻咽喉头颈外科,2022,29(6):373−377. [ZHANG Hongwei, SHI Fumin, XUE Rong, et al. Relationship between body mass index, waist circumference and thyroid cancer[J]. Chinese Journal of Otolaryngology Head and Neck Surgery,2022,29(6):373−377.] ZHANG Hongwei, SHI Fumin, XUE Rong, et al . Relationship between body mass index, waist circumference and thyroid cancer[J]. Chinese Journal of Otolaryngology Head and Neck Surgery,2022 ,29 (6 ):373 −377 .[42] HEUCHAN G N, LALLY P J, BEEKEN R J, et al. Perception of a need to change weight in individuals living with and beyond breast, prostate and colorectal cancer:A cross-sectional survey[J]. Journal of Cancer Survivorship, 2023.
[43] DYCK L, LYNCH L. Diverse effects of obesity on antitumor immunity and immunotherapy[J]. Trends in Molecular Medicine,2023,29(2):112−123. doi: 10.1016/j.molmed.2022.11.004
[44] JOVANOVIĆ M, KOVAČEVIĆ S, BRKLJAČIĆ J, et al. Oxidative stress linking obesity and cancer:Is obesity a 'radical trigger' to cancer?[J]. International Journal of Molecular Sciences,2023,24(9):8452−8473. doi: 10.3390/ijms24098452
[45] BUDEK M, NUSZKIEWICZ J, PIÓRKOWSKA A, et al. Inflammation related to obesity in the etiopathogenesis of gastroenteropancreatic neuroendocrine neoplasms[J]. Biomedicines,2022,10(10):2660−2678. doi: 10.3390/biomedicines10102660
[46] 卢小溪. 肥胖与子宫内膜癌相关性研究[D]. 北京:北京协和医学院, 2017. [LU Xiaoxi. A study on the correlation between obesity and endometrial cancer[D]. Beijing:Peking Union Medical College, 2017.] LU Xiaoxi. A study on the correlation between obesity and endometrial cancer[D]. Beijing: Peking Union Medical College, 2017.





 下载:
下载:


 下载:
下载:
