Determination of 47 Pesticide Residues in Tea by QuEChERS Method Combined with Ultra Performance Liquid Chromatography-Tandem Mass Spectrometry
-
摘要: 建立一种QuEChERS-超高效液相色谱-串联质谱法同时测定茶叶中47种农药残留的分析方法。样品经1%乙酸乙腈提取,QuEChERS方法净化,配有电喷雾离子源(ESI±)的超高效液相色谱-串联质谱(UPLC-MS/MS),在正负离子多反应监测(MRM)模式下同时测定,基质匹配外标法定量。结果表明:47种目标化合物在一定范围内线性关系良好,相关系数(r)为0.9971~0.9996,检出限为0.001~0.006 mg/kg,定量限为0.002~0.020 mg/kg,平均回收率为73.4%~114.3%,相对标准偏差(n=6)为0.3%~19.9%。该方法灵敏、准确、稳定,可满足茶叶中47种农药残留的检测要求。
-
关键词:
- QuEChERS /
- 茶叶 /
- 农药残留 /
- 超高效液相色谱-串联质谱法(UPLC-MS/MS)
Abstract: An analysis method for simultaneous determination of 47 kinds of pesticide residues in tea was established by QuEChERS method combined with ultra performance liquid chromatography-tandem mass spectrometry. The samples were extracted by acetonitrile containing 1% acetic acid, and purified by QuEChERS method. The 47 pesticides were determined by ultra performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) with an electrospray ion source (ESI±) in positive and negative ion switching multiple reaction monitoring (MRM), and quantified by matrix-matched external standard method. Method validation revealed satisfactory linearities with correlation coefficients (r) between 0.9971 and 0.9996, and limits of detection were 0.001~0.006 mg/kg while the limits of quantitation were 0.002~0.020 mg/kg, the average recoveries at three spiked levels were in the range of 73.4%~114.3%, with relative standard deviations (n=6) of 0.3%~19.9%. The method is sensitive, accurate, stable, and can meet the requirements for determination of 47 pesticide residues in tea. -
我国茶叶有着悠久的历史,其本身具有抗氧化、抗菌、抗癌和抗炎作用,还能降低血压,减少胆固醇和心血管疾病的患病风险[1]。我国茶叶种植存在过量使用农药的情况,且使用的大多数是化学类农药[2]。此类农药作用单一无法达到大规模的病虫治理,且使害虫的抗药性不断提高,从而促使茶农使用复配农药,造成农药残留现象愈发严重[3−5]。此外,发达国家对进口产品的检验标准不断提高,而我国的茶叶因农药残留不达标而被阻止出口。茶叶中农药残留超标已成为中国茶叶出口最主要的技术性贸易壁垒[6]。目前,各国在茶叶产品中农药残留的监测重点是制定各种农药的最大残留限量(Maximum residue limit,MRL)和开发准确快速的定性、定量分析方法[7]。我国从2005年起开始对茶叶产品中农药的MRL做出强制性要求限制,但仍不及欧盟等发达国家的要求。
目前研究较为深入、使用最广泛的茶叶农药残留检测技术主要有气相色谱法、气相色谱-串联质谱法、液相色谱法、液相色谱-串联质谱法[8]等;前处理方法有超声提取法[9]、均质提取法[10]、索氏提取法、固相萃取法[11]等。大多前处理方法操作繁琐,试剂消耗量较大。QuEChERS方法是近年来国际上最新发展起来的一种快速样品前处理技术[12],因其具有快速(Quick)、简单(Easy)、便宜(Cheap)、有效(Effective)、可靠(Rugged)、安全(Safe)的特点而得名[13−16]。QuEChERS法分为提取和净化两部分:使用适宜的溶剂提取待测物,并针对杂质选用适宜的净化剂,一般常用的净化剂有N-丙基乙二胺(PSA)、十八烷基键合硅胶(C18)、石墨化碳黑(Graphitizing of carbon black,GCB)等。由于茶叶基质复杂,采用常用净化剂进行净化,净化结果不彻底,会存在基质干扰现象[17−18]。多壁碳纳米管(MWCNTs)[19−20]是一种新型碳纳米材料,由多层石墨片卷曲而成的碳纳米管,层与层之间呈无序排列,具有比表面积大、化学稳定性好、吸附能力强等优点。通过向吸附剂中加入多壁碳纳米管,改进QuEChERS法对茶叶的净化能力,液质联用法进行检测的研究鲜有报道。因此,本研究将QuEChERS技术和多壁碳纳米管净化剂相结合,采用超高效液相色谱-串联质谱法检测茶叶中农药残留,建立一种快速、简便、高效的检测茶叶中多种农残的方法,为茶叶农药的使用、监管提供技术及数据支持。
1. 材料与方法
1.1 材料与仪器
空白茶叶 购于本地有机茶社;待测茶叶 100份,包含绿茶20份、红茶20份、白茶10份、黑茶25份、花茶25份,分别购自广西各地;47种农药标准物质分别为毒虫畏、甲拌磷、甲拌磷砜、甲拌磷亚砜、甲基异柳磷、特丁硫磷、特丁硫磷砜、特丁硫磷亚砜、克百威、硫环磷、甲基硫环磷、甲胺磷、乐果、灭线磷、内吸磷、水胺硫磷、氧乐果、乙酰甲胺磷、灭多威、辛硫磷、吡虫啉、敌百虫、多菌灵、甲萘威、茚虫威、啶虫脒、噻虫胺、噻虫啉、噻虫嗪、噻嗪酮、喹螨醚、噻螨酮、乙螨唑、除虫脲、啶氧菌酯、呋虫胺、氟虫脲、唑虫酰胺、甲氨基阿维菌素甲酸盐、醚菊酯、吡唑醚菌酯、杀虫威、杀螟松、氟虫腈砜、氟虫腈、氟虫腈亚砜、氟甲腈 纯度均大于99%,德国Dr Ehrenstorfer公司;乙腈、甲酸、甲醇 色谱纯,德国默克公司;乙酸铵 色谱纯,天津市光复精细化工研究所;QuEChERS提取盐包 含6 g无水硫酸镁和1.5 g无水乙酸钠,美国Waters公司;多壁碳纳米管 MWCNTs,成都中科时代纳能科技有限公司;N-丙基乙二胺 PSA,天津博纳艾杰尔科技有限公司;十八烷基键合硅胶 C18,广州沃恩生物科技有限公司;实验室用水 超纯水。
1290超高效液相色谱仪 美国Agilent公司;TRIPLE QUAD 5500三重四极杆串联质谱仪(配备AB SCIEX 数据处理软件、电喷雾源) 美国AB SCIEX公司;MS303TS型电子天平 梅特勒-托利多仪器(上海)公司;Multi Reax涡旋仪 德国Heidolph公司;Shaker SA320振荡器 日本YAMATO公司;X3R高速冷冻离心机 美国热电Thermo公司;Gradient A10 Milli-Q超纯水机 美国Millipore公司。
1.2 实验方法
1.2.1 样品前处理
提取:精密称取粉碎茶叶样品2.000 g(精确至0.001 g)于50 mL离心管中,加入5 mL水浸泡30 min,接着加入15 mL 1% 乙酸乙腈溶液,在涡漩仪上涡漩振荡提取25 min(450次/min),然后加入QuEChERS盐析剂(1.5 g乙酸钠,6 g无水硫酸镁),立即摇匀,置于振荡器上剧烈振荡3 min(450次/min),以10000 r/min离心5 min,上清液待净化。
净化:取上清液8 mL,转移到含有1200 mg无水硫酸镁、50 mg MWCNTs、100 mg PSA和100 mg C18的净化管中,涡漩2 min(450次/min),以10000 r/min离心5 min,精密吸取上清液2 mL,氮吹至近干,甲醇定容1 mL,过0.22 μm有机滤膜,供UPLC-MS/MS分析。
1.2.2 标准溶液配制
准确称取适量标准物质,根据标准物质的溶解性选用甲醇或乙腈分别配制成1 mg/mL的标准储备液,−20 ℃保存。分别适量移取一定体积的各标准储备液于同一10 mL容量瓶中,乙腈定容至刻度,得到10 μg/mL的混合标准溶液,再精密量取1 mL混合标准溶液于100 mL容量瓶中,乙腈稀释定容至刻度得到0.1 μg/mL混合标准使用液,用于绘制标准曲线。
称取空白基质样品,按“1.2.1”处理后,得到空白基质溶液。分别精密吸取混合标准使用液0、0.02、0.05、0.1、0.5、1.0 mL,用空白基质溶液配制成浓度为0、2、5、10、50、100 ng/mL系列基质混合标准工作曲线。
1.2.3 超高效液相色谱条件
色谱柱:InertsilTM ODS-3色谱柱(3 μm,2.1 mm×150 mm);流动相:A为0.1%甲酸水,B为甲醇。梯度洗脱程序:0~1.5 min,97%A;1.5~2.5 min,85%A;2.5~9 min,50%A;9~11 min,30%A;11~13 min,2%A;13~13.1 min,97% A;13.1~15 min,97% A;流速:300 μL/min;柱温:35 ℃;进样量:2.0 μL。
1.2.4 质谱条件
离子源:电喷雾离子源(ESI);扫描方式:正、负离子同时扫描;检测模式:多反应监测(Multiple reaction monitoring,MRM);电喷雾电压:5500 V和−4500 V,雾化气(氮气)压力:55 psi;辅助气压力:60 psi;气帘气(氮气)压力:30 psi;碰撞气(氮气)压力:7 psi;离子源温度:550 ℃;扫描时间:60 ms;碰撞室入口电压:7 V;碰撞室出口电压:12 V;各目标化合物的质谱采集参数见表1。
表 1 47种农药的多反应监测扫描模式的质谱参数Table 1. MRM mass spectrometric parameters of 47 kinds of pesticides序号 化合物 CAS号 保留时间(min) 母离子(m/z) 子离子(m/z) 去簇电压(V) 碰撞能量(eV) 1 毒虫畏 470-90-6 9.69 359.0 155.0*,127.0 80 17,22 2 甲拌磷 298-02-2 11.31 261.0 75.0*,47.0 51 21,53 3 甲拌磷砜 2588-04-7 7.30 293.0 97.0*,115.0 65 50,35 4 甲拌磷亚砜 2588-05-8 5.75 277.0 199.0*,153.0 25 13,19 5 甲基异柳磷 99675-03-3 10.82 332.1 121.0*,231.0 20 43,19 6 特丁硫磷 13071-79-9 12.13 289.1 103.1*,233.0 53 13,9 7 特丁硫磷砜 56070-16-7 8.53 321.0 97.0*,115.0 75 57,39 8 特丁硫磷亚砜 10548-10-4 6.91 305.0 187.0*,130.9 57 20,38 9 克百威 1563-66-2 5.71 222.1 165.0*,123.1 70 16,29 10 硫环磷 947-02-4 4.47 256.0 140.0*,228.0 40 30,18 11 甲基硫环磷 5120-23-0 3.96 228.0 168.0*,109.0 30 20,35 12 甲胺磷 10265-92-6 2.47 142.0 94.0*,125.0 57 19,18 13 乐果 60-51-5 4.42 230.0 199.0*,125.0 56 13,29 14 灭线磷 13194-48-4 8.36 243.0 97.0*,131.0 67 43,26 15 内吸磷 8065-48-3 6.62 259.0 89.0*,61.0 48 22,45 16 水胺硫磷 24353-61-5 10.82 231.0 121.0*,109.0 100 26,38 17 氧乐果 1113-02-6 3.63 214.0 183.0*,109.0 60 16,36 18 乙酰甲胺磷 30560-19-1 3.57 184.0 143.0*,125.0 50 12,25 19 灭多威 16752-77-5 4.02 163.1 88.0*,106.0 38 12,14 20 辛硫磷 14816-18-3 11.19 299.1 129.0*,153.0 55 18,10 21 吡虫啉 138261-41-3 4.35 256.1 175.1*,209.1 45 27,22 22 敌百虫 52-68-6 5.44 256.9 109.0*,221.0 65 21,16 23 甲萘威 63-25-2 6.01 202.1 145.0*,127.0 56 15,40 24 多菌灵 10605-21-7 3.48 192.0 160.0*,132.0 80 25,41 25 茚虫威 144171-61-9 11.28 528.1 293.0*,249.0 50 18,23 26 啶虫脒 135410-20-7 4.41 223.0 126.0*,99.0 65 28,60 27 噻虫胺 210880-92-5 4.26 250.0 169.1*,132.0 35 17,21 28 噻虫啉 1119-88-49-9 4.79 253.0 126.0*,99.0 81 29,57 29 噻虫嗪 153719-23-4 4.03 292.0 211.1*,181.1 30 16,30 30 噻嗪酮 69327-76-0 10.91 306.2 201.1*,116.2 60 17,22 31 喹螨醚 120928-09-8 12.59 307.2 161.0*,131.1 30 22,60 32 噻螨酮 78587-05-0 12.36 353.1 228.0*,168.1 56 21,33 33 乙螨唑 153233-91-1 12.47 360.2 141.0*,304.0 80 42,25 34 除虫脲 35367-38-5 8.87 311.0 158.0*,141.2 45 20,49 35 啶氧菌酯 117428-22-5 10.20 368.1 205.1*,145.1 54 13,27 36 呋虫胺 165252-70-0 3.75 203.1 129.0*,157.0 35 16,11 37 氟虫脲 101463-69-8 12.12 489.0 158.1*,141.2 71 27,65 38 唑虫酰胺 129558-76-5 11.77 384.1 197.1*,171.0 40 35,33 39 甲氨基阿维菌素甲酸盐 155569-91-8 3.97 886.2 82.0*,158.1 50 110,41 40 醚菊酯 80844-07-1 13.22 394.2 177.1*,107.1 30 19,59 41 吡唑醚菌酯 175013-18-0 10.89 388.1 194.1*,163.1 50 18,36 42 杀虫威 22248-79-9 9.05 365.0 127.0*,204.0 38 18,49 43 杀螟松 122-14-5 8.70 278.1 125.0*,246.0 30 29,24 44 氟虫腈砜 120068-36-2 10.97 451.0 415.0*,282.0 −28 −26,−38 45 氟虫腈 120068-37-3 9.97 435.0 330.0*,250.0 −80 −21,−35 46 氟虫腈亚砜 120067-83-6 11.02 418.9 383.0*,314.0 −123 −18,−26 47 氟甲腈 205650-65-3 10.50 387.0 351.0*,282.0 −30 −19,−47 注:*表示定量离子。 1.3 数据处理
分别利用Analyst Software和MutiQuant 3.0.2软件(AB SCIEX)进行数据采集及处理,Excel 2013 进行数据汇总和分析。
2. 结果与分析
2.1 提取液的优化
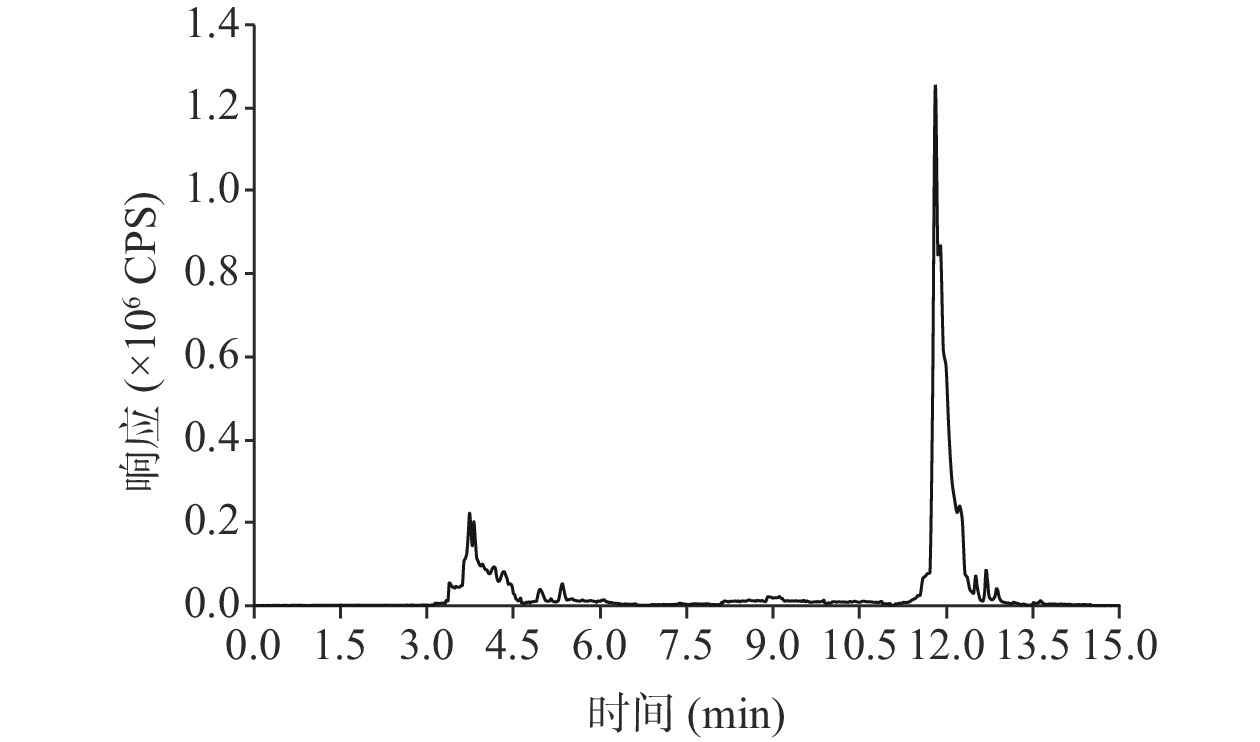
目前,农药残留的提取溶剂主要有乙腈、乙酸乙酯、丙酮、甲醇、甲苯、正己烷和石油醚[21−24]等。茶叶基质比较复杂,使用乙酸乙酯、丙酮、甲醇等作为提取溶剂时,提取液颜色很较深,提取物也更多,不利于后续净化。而乙腈作为提取溶剂,因其极性较强,对于油性物质如蜡、脂肪、油性色素等不易提取出,可减小杂质的干扰,最适于提取各种极性的农药残留,且仅加入盐便可实现与水相的分离[25]。在乙腈中加入少量有机酸,可防止部分农药分解及调节pH,因此加入1%乙酸[26]。对于含水量低的样品,通过先向样品中添加一定量的水,可弱化分析物与样品基质之间的相互作用,使待分析物更易在萃取分配过程中被充分提取。通过UPLC-MS/MS全扫描得到的总离子流图显示,乙酸乙腈提取的共萃物少(图1)。因此,本研究采用加入5 mL超纯水润湿茶叶后加入15 mL 1%乙酸乙腈作为提取溶剂。
2.2 净化条件的优化
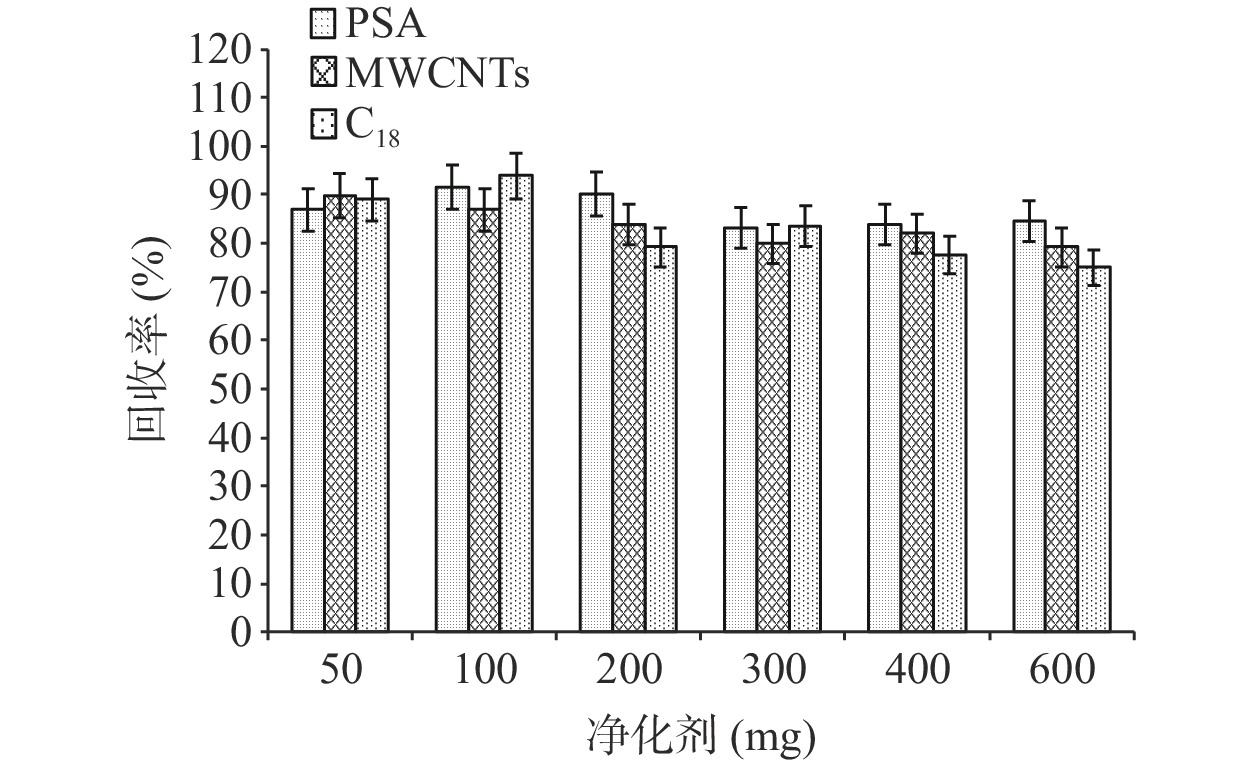
茶叶基质复杂,富含色素、茶多酚、生物碱、咖啡碱和脂肪酸等物质,水分含量极低[27]。茶叶样品经过有机溶剂提取后,样液多呈深红色,若不采取方法对提取液进行净化,直接进仪器分析,既污染色谱柱和离子源,又影响仪器的响应,干扰检测的灵敏度和准确性[28]。常见的净化剂有无水硫酸镁、MWCNTs、GCB、C18、PSA等。提取时加入无水硫酸镁能除去茶叶中的水分,保证其他净化填料的吸附效果[29],同时避免水分损害仪器和毛细柱,降低柱效。PSA的表面键合极性官能团,具有极性吸附作用和弱阴离子交换功能,对样品基质中的有机酸、极性色素、糖类等杂质吸附能力较强[30−31]。相反,C18可去除酯类和脂肪等非极性干扰物的影响。GCB具有片状结构,是一种非极性吸附剂,对含苯环官能团的化合物有较强的吸附作用,可有效去除具有平面结构的甾醇和色素杂质,但也会对平面结构的有机氯农药进行吸附,导致有机氯农药回收率降低。相比于GCB,MWCNTs的空心柱状结构对农药吸附作用很小,对色素的吸附能力更强,更易获得较高的回收率。因此本实验选取无水硫酸镁、PSA、C18、MWCNTs作净化剂,比较不同配比的净化效果。取8 mL提取液,固定加入1200 mg无水硫酸镁,分别考察PSA添加量为50、100、200、300、400、600 mg的回收率,在PSA添加量为100 mg时,各农药的平均回收率为73.2%~110.1%;固定1200 mg无水硫酸镁、100 mg PSA用量,比较MWCNTs添加量为50、100、200、300、400、600 mg的回收率,在MWCNTs添加量为50 mg时,各农药的平均回收率为68.0%~111.7%;固定1200 mg无水硫酸镁、100 mg PSA、50 mg MWCNTs用量,比较C18添加量为50、100、200、300、400、600 mg的回收率,在C18添加量为100 mg时,各农药回收率为73.4%~114.3%。最终净化配比为无水硫酸镁1200 mg、MWCNTs 50 mg、PSA 100 mg、C18 100 mg(图2)。
2.3 质谱条件的优化
将各农药配制成0.05 μg/mL的混合标准溶液。用蠕动泵直接注入质谱仪,采用正、负离子扫描模式,优化锥孔电压使母离子响应丰度最大且稳定,同时优化碰撞能量,选取响应丰度较高、易得到、出峰稳定的碎片离子作为子离子,并将母离子和子离子组成离子对。具体质谱参数见表1。
2.4 液相色谱条件的优化
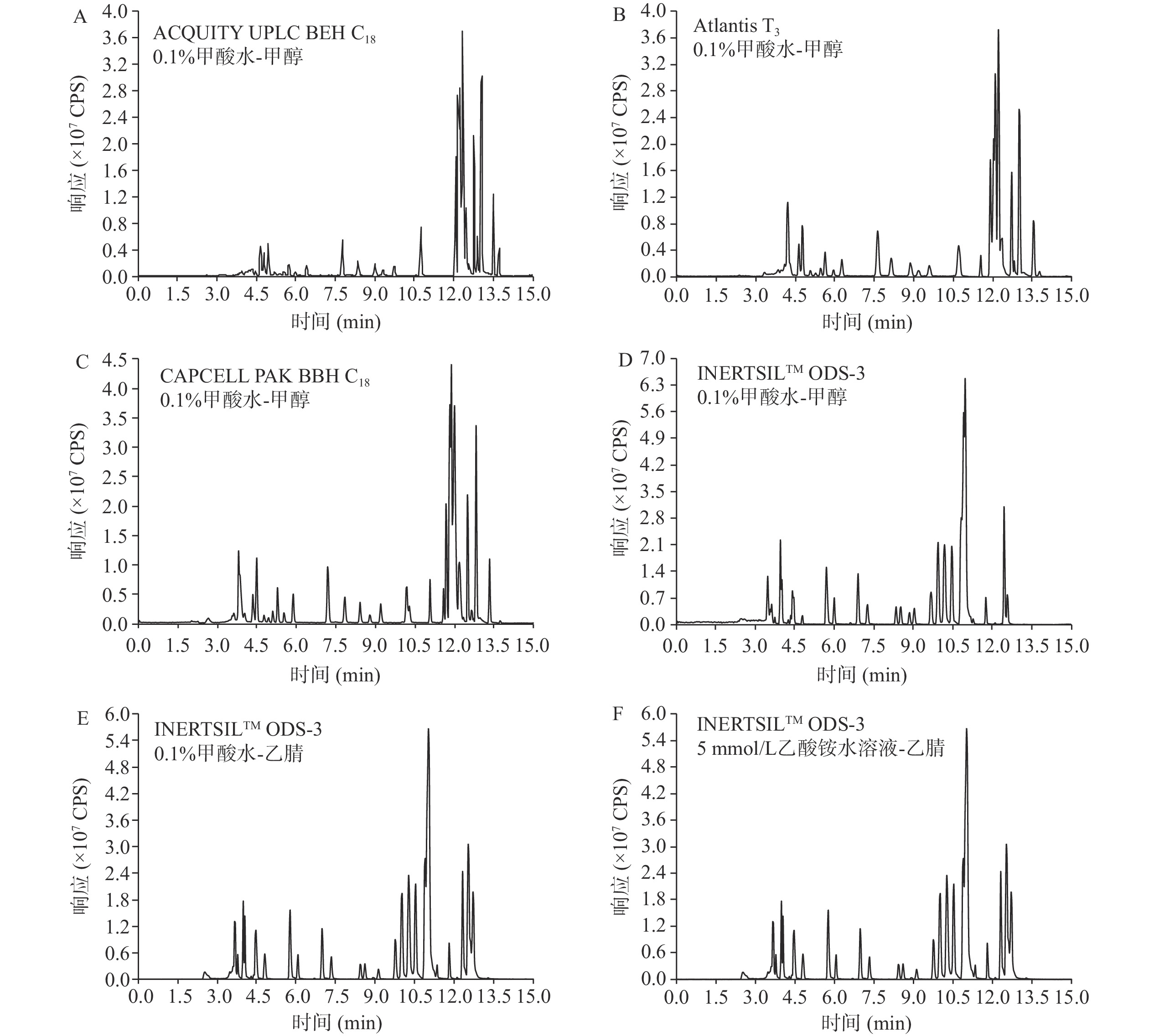
在优化的质谱条件下,考察不同色谱柱不同流动相对目标物质谱信号的影响。本实验对比了四种色谱柱,ACQUITY UPLC BEH C18色谱柱(1.7 μm,2.1 mm×150 mm)、ATLANTIS T3色谱柱(3 μm,2.1 mm×150 mm)、INERTSILTM ODS-3色谱柱(3 μm,2.1 mm×150 mm)、CAPCELL PAK BBH C18色谱柱(3 μm,2.1 mm×150 mm)。甲酸和乙酸铵具有增强目标化合物离子化程度和改善峰形的作用[14],因此,分别考察0.1%甲酸水-甲醇、0.1%甲酸水-乙腈、5 mmol/L乙酸铵溶液-乙腈几种流动相对目标化合物的影响。对比图3A~D,同种流动相下ACQUITY UPLC BEH C18色谱柱、Atlantis T3色谱柱、CAPCELL PAK BBH C18色谱柱、INERTSILTM ODS-3色谱柱,使用INERTSILTM ODS-3色谱柱的色谱图目标化合物响应均高于其它3根色谱柱;对比图3D~F,同种色谱柱中的三种流动相体系出峰均良好,间距适宜。使用INERTSILTM ODS-3色谱柱,对比0.1%甲酸水-甲醇流动相体系和5 mmol/L乙酸铵溶液-乙腈流动相体系,杀虫威、除虫脲在5 mmol/L乙酸铵溶液-乙腈流动相中出现杂峰,特丁硫磷砜在0.1%甲酸水-甲醇流动相中响应值较高。使用INERTSILTM ODS-3色谱柱,对比0.1%甲酸水-甲醇和0.1%甲酸水-乙腈流动相,提取目标化合物离子对,结果显示使用0.1%甲酸水-甲醇体系时绝大部分化合物的响应都高于0.1%甲酸水-乙腈体系。因此,选用INERTSILTM ODS-3色谱柱和0.1%甲酸水-甲醇体系。
2.5 基质效应
基质效应(Matrix effect,ME)是指样品中除待测物以外的组分,常常对分析物的分析过程有明显的干扰,并影响分析结果的准确性。基质效应可用以下公式计算:
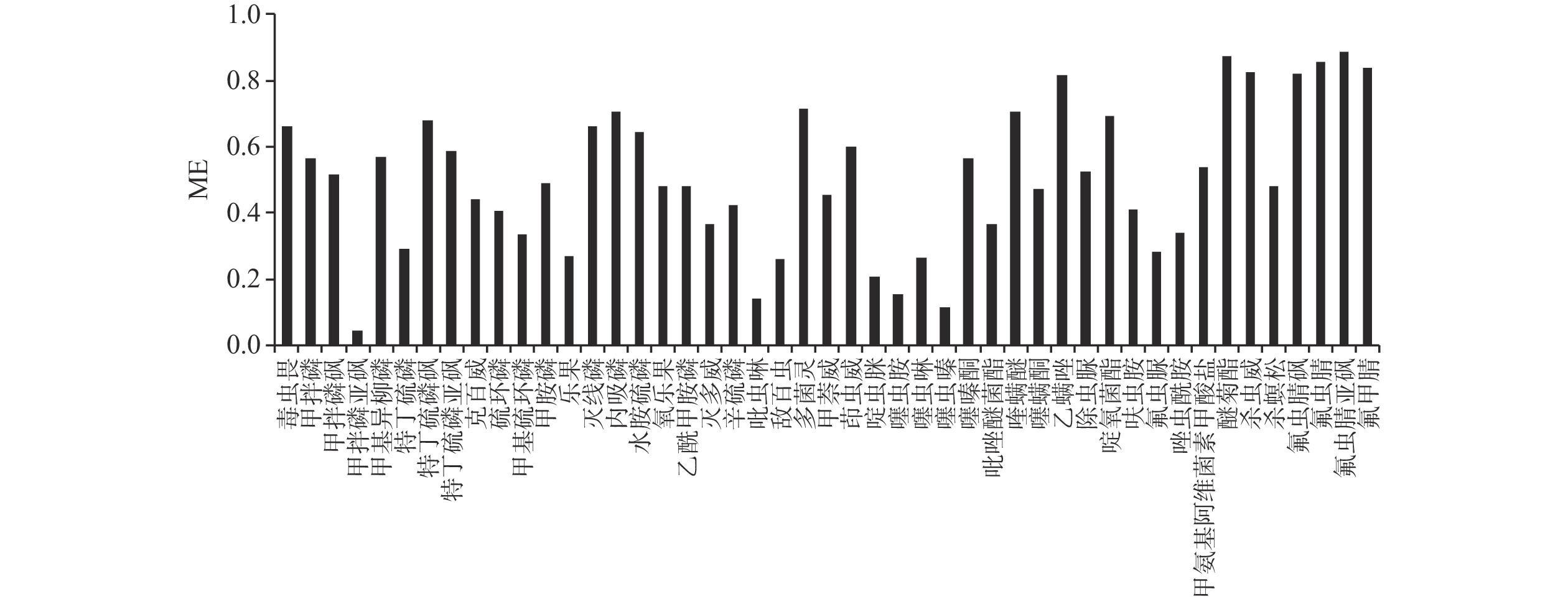
ME=BA 式中,ME为基质效应;A为溶剂标准曲线的斜率;B为基质匹配标准曲线的斜率。若ME<0.8,则表现为基质抑制效应,ME>1,则为基质增强效应,ME=0.8~1,则表现为基质效应影响不大。本实验对47种化合物的基质效应进行评估(图4),发现47种化合物的基质效应均低于1,其中85%的化合物受到基质抑制,因此本方法检测采用基质匹配曲线,以此满足农药残留的检测要求。
2.6 线性范围、检出限、定量限
在优化的实验条件下,按“1.2.2”配制6个浓度水平的基质工作溶液,上机测定,以目标物的质量浓度为横坐标,对应的定量离子峰面积为纵坐标绘制标准曲线。如表2,47种目标化合物在2~100 ng/mL范围内线性良好,相关系数为0.9971~0.9996,检出限(LOD)为0.001~0.006 mg/kg,定量限(LOQ)为0.002~0.020 mg/kg,满足农药残留的检测要求。
表 2 47种化合物的线性范围、回归方程、相关系数、检出限、定量限和食品中农药最大残留限量(MRL)Table 2. Linear ranges, regression equations, correlation coefficients, LOD, LOQ and maximum residue limits (MRL) for pesticides in food of 47 compounds化合物名称 线性范围
(ng/mL)回归方程 r 检出限
(mg/kg)定量限
(mg/kg)MRL
(mg/kg)[32]毒虫畏 2~100 Y=2.73×105X+6.01×105 0.9990 0.002 0.005 0.01 甲拌磷 2~100 Y=3.35×104X−3.14×104 0.9990 0.001 0.004 0.01 甲拌磷砜 2~100 Y=4.26×104X+1.07×104 0.9990 0.002 0.008 0.01 甲拌磷亚砜 2~100 Y=1.14×105X+1.05×105 0.9990 0.002 0.007 0.01 甲基异柳磷 2~100 Y=2.11×105X+4.54×105 0.9993 0.002 0.007 0.01 特丁硫磷 2~100 Y=2.28×104X+2.44×104 0.9990 0.002 0.008 0.01 特丁硫磷砜 2~100 Y=1.03×105X+8.77×104 0.9990 0.002 0.005 0.01 特丁硫磷亚砜 2~100 Y=2.91×105X+4.63×105 0.9992 0.002 0.006 0.01 克百威 2~100 Y=2.52×105X+2.20×105 0.9991 0.001 0.004 0.02 硫环磷 2~100 Y=1.49×105X+1.56×105 0.9994 0.001 0.005 0.03 甲基硫环磷 2~100 Y=2.69×105X+7.81×105 0.9990 0.002 0.005 0.03 甲胺磷 2~100 Y=1.99×105X+3.41×105 0.9994 0.001 0.004 0.05 乐果 2~100 Y=1.09×105X+1.27×105 0.9991 0.001 0.003 0.05 灭线磷 2~100 Y=1.47×105X+6.23×104 0.9992 0.001 0.003 0.05 内吸磷 2~100 Y=9.03×103X–3.61×103 0.9991 0.003 0.009 0.05 水胺硫磷 2~100 Y=1.35×105X+1.02×105 0.9992 0.002 0.007 0.05 氧乐果 2~100 Y=1.13×105X+1.34×105 0.9994 0.001 0.004 0.05 乙酰甲胺磷 2~100 Y=1.95×105X+3.74×105 0.9996 0.005 0.017 0.05 灭多威 2~100 Y=1.50×105X+2.77×105 0.9990 0.002 0.006 0.2 辛硫磷 2~100 Y=1.98×105X+1.93×105 0.9990 0.002 0.006 0.2 吡虫啉 2~100 Y=2.56×104X+1.17×104 0.9989 0.002 0.006 0.5 敌百虫 2~100 Y=2.76×104X+2.06×103 0.9988 0.004 0.012 2 多菌灵 2~100 Y=5.27×105X+1.87×106 0.9993 0.001 0.002 5 甲萘威 2~100 Y=1.80×105X+1.75×105 0.9989 0.002 0.006 5 茚虫威 2~100 Y=1.25×104X+4.58×103 0.9987 0.002 0.005 5 啶虫脒 2~100 Y=1.08×105X+5.19×104 0.9990 0.001 0.004 10 噻虫胺 2~100 Y=1.94×104X+5.86×103 0.9988 0.002 0.006 10 噻虫啉 2~100 Y=7.72×104X+6.16×104 0.9990 0.002 0.005 10 噻虫嗪 2~100 Y=3.32×104X+4.33×104 0.9994 0.001 0.004 10 噻嗪酮 2~100 Y=6.00×105X+3.18×106 0.9985 0.002 0.008 10 吡唑醚菌酯 2~100 Y=4.17×105X+1.22×105 0.9994 0.001 0.003 10 喹螨醚 2~100 Y=1.71×105X+5.27×105 0.9992 0.001 0.002 15 噻螨酮 2~100 Y=5.86×104X+4.60×104 0.9988 0.001 0.003 15 乙螨唑 2~100 Y=7.73×105X+9.96×106 0.9971 0.002 0.008 15 除虫脲 2~100 Y=1.13×105X+1.20×105 0.9993 0.001 0.002 20 啶氧菌酯 2~100 Y=5.48×105X+3.16×106 0.9988 0.001 0.003 20 呋虫胺 2~100 Y=4.74×104X+1.50×105 0.9993 0.006 0.020 20 氟虫脲 2~100 Y=1.94×104X–2.43×104 0.9990 0.002 0.006 20 唑虫酰胺 2~100 Y=1.49×105X+8.02×105 0.9990 0.002 0.005 50 甲氨基阿维菌素甲酸盐 2~100 Y=5.51×104X+7.96×103 0.9991 0.001 0.005 0.5 醚菊酯 2~100 Y=8.72×104X+4.83×104 0.9992 0.001 0.005 50 杀虫威 2~100 Y=1.75×105X+2.52×105 0.9994 0.001 0.003 0.01 杀螟松 2~100 Y=4.75×103X+2.88×103 0.9987 0.004 0.014 0.5 氟虫腈砜 2~100 Y=1.03×106X+9.10×106 0.9986 0.001 0.002 / 氟虫腈 2~100 Y=6.18×105X+1.10×105 0.9991 0.001 0.003 / 氟虫腈亚砜 2~100 Y=6.06×105X+4.82×106 0.9991 0.001 0.002 / 氟甲腈 2~100 Y=6.90×105X+4.50×106 0.9991 0.001 0.002 / 2.7 回收率
根据GB/T 27404-2008[33]对食品理化检测的质量控制要求,分别向空白茶叶样品添加低、中、高3水平加标回收实验,平行测试6次,结果见表3。47种化合物的平均回收率为73.4%~114.3%,相对标准偏差(RSD%)为0.3%~19.9%(n=6),方法具有良好的回收率与精密度。
表 3 47种化合物的加标回收率及相对标准偏差(n=6)Table 3. Recovery rates and relative standard deviations of 47 compounds (n=6)化合物名称 加标7.50 μg/kg 加标18.75 μg/kg 加标37.50 μg/kg 平均回收率(%) RSD(%) 平均回收率(%) RSD(%) 平均回收率(%) RSD(%) 毒虫畏 88.2 13.2 95.6 14.8 101.0 9.0 甲拌磷 92.3 12.5 96.4 15.2 100.8 11.7 甲拌磷砜 105.0 2.5 103.0 5.4 99.2 2.6 甲拌磷亚砜 101.0 12.7 99.8 3.6 99.9 4.8 甲基异柳磷 90.6 7.6 97.3 4.3 100.6 8.3 特丁硫磷 86.8 1.3 98.5 4.9 100.2 1.5 特丁硫磷砜 74.6 6.5 98.7 7.8 100.1 6.8 特丁硫磷亚砜 89.6 8.1 103.0 10.1 99.2 10.8 克百威 83.3 11.6 103.0 6.4 99.2 3.8 硫环磷 73.4 11.3 100.2 4.5 99.8 3.2 甲基硫环磷 89.6 16.2 95.7 8.7 100.9 6.2 甲胺磷 76.4 9.3 99.9 8.0 99.9 11.0 乐果 86.5 5.7 97.9 3.8 100.3 2.5 灭线磷 88.8 8.6 100.6 8.8 99.7 8.8 内吸磷 77.7 3.6 97.4 5.1 100.4 1.9 水胺硫磷 83.5 10.2 102.5 7.9 99.3 6.5 氧乐果 84.4 7.1 99.8 6.6 99.9 7.3 乙酰甲胺磷 85.2 8.5 103.3 10.3 99.2 9.0 灭多威 93.5 11.4 97.1 3.9 100.5 7.5 辛硫磷 87.7 11.9 95.2 14.4 101.1 5.4 吡虫啉 110.4 6.8 97.2 7.0 100.6 8.4 敌百虫 93.5 7.8 96.1 3.6 100.8 5.9 多菌灵 95.4 15.9 97.5 14.6 100.6 14.2 甲萘威 94.3 9.3 96.5 4.2 100.7 5.8 茚虫威 97.6 17.4 94.1 5.8 101.5 1.4 啶虫脒 100.7 14.5 97.7 1.1 100.4 1.6 噻虫胺 109.3 3.9 99.1 0.5 100.0 0.3 噻虫啉 92.0 12.2 98.2 0.9 100.3 0.9 噻虫嗪 91.5 17.9 97.9 3.8 100.4 3.4 噻嗪酮 96.3 10.9 94.3 16.8 101.5 16.0 喹螨醚 100.5 9.2 97.5 14.3 100.6 11.3 噻螨酮 96.8 15.7 104.7 17.1 98.9 1.6 乙螨唑 88.2 19.9 92.1 19.1 102.1 12.3 除虫脲 114.3 4.8 98.4 2.5 100.3 4.9 啶氧菌酯 101.8 17.3 95.0 16.8 101.2 18.2 呋虫胺 84.4 9.3 97.2 3.4 100.6 1.4 氟虫脲 101.7 2.4 103.3 9.5 99.2 4.1 唑虫酰胺 104.4 11.9 96.9 14.0 100.7 11.6 甲氨基阿维菌素甲酸盐 79.2 9.4 98.0 1.8 100.3 3.2 醚菊酯 100.9 10.4 104.7 10.6 98.9 1.1 吡唑醚菌酯 103.7 14.5 98.7 14.0 100.2 9.2 杀虫威 110.9 7.2 97.5 9.5 100.5 3.2 杀螟松 94.6 16.8 93.7 4.5 101.5 0.9 氟虫腈砜 112.0 16.5 94.4 18.3 101.3 14.6 氟虫腈 95.3 13.4 94.8 16.6 101.3 10.5 氟虫腈亚砜 94.5 10.3 95.4 15.4 101.2 11.5 氟甲腈 105.2 16.1 95.9 19.2 101.0 7.7 2.8 实际样品检测
利用本次实验建立的方法和国家标准方法GB 23200.121-2021[34]分别对广西省内销售的100份茶叶进行农药残留分析,包含绿茶20份、红茶20份、白茶10份、黑茶25份、花茶25份。部分农药超出国家标准限值,结果见表4,其余农药均为未检出。
表 4 QuEChERS方法和国家标准方法结果对比Table 4. Comparison results of QuEChERS method and national standard method序号 农药 结果(mg/kg) 本法 国家标准方法 标准偏差SD值 MRL 05绿茶 水胺硫磷 1.78 1.84 0.04 0.05 14绿茶 吡虫啉 0.65 0.67 0.01 0.5 11红茶 氧乐果 0.061 0.061 0.00 0.05 3. 结论
本文采用优化QuEChERS方法进行前处理,超高效液相色谱-串联质谱作为检测仪器,在最优的实验条件下,47种农药平均回收率为73.4%~114.3%,相对标准偏差为0.3%~19.9%(n=6),检出限为0.001~0.006 mg/kg,定量限为0.002~0.020 mg/kg,并对广西省内销售的100份茶叶进行农药残留分析,包含绿茶20份、红茶20份、白茶10份、黑茶25份、花茶25份,其中检出绿茶中水胺硫磷1.78 mg/kg、吡虫啉0.65 mg/kg,红茶中氧乐果0.061 mg/kg,其余农药均为未检出。与国家标准方法相比,标准偏差SD值满足农药残留的检测要求。该方法灵敏、准确、稳定,可满足茶叶中47种农药残留的检测要求。
-
表 1 47种农药的多反应监测扫描模式的质谱参数
Table 1 MRM mass spectrometric parameters of 47 kinds of pesticides
序号 化合物 CAS号 保留时间(min) 母离子(m/z) 子离子(m/z) 去簇电压(V) 碰撞能量(eV) 1 毒虫畏 470-90-6 9.69 359.0 155.0*,127.0 80 17,22 2 甲拌磷 298-02-2 11.31 261.0 75.0*,47.0 51 21,53 3 甲拌磷砜 2588-04-7 7.30 293.0 97.0*,115.0 65 50,35 4 甲拌磷亚砜 2588-05-8 5.75 277.0 199.0*,153.0 25 13,19 5 甲基异柳磷 99675-03-3 10.82 332.1 121.0*,231.0 20 43,19 6 特丁硫磷 13071-79-9 12.13 289.1 103.1*,233.0 53 13,9 7 特丁硫磷砜 56070-16-7 8.53 321.0 97.0*,115.0 75 57,39 8 特丁硫磷亚砜 10548-10-4 6.91 305.0 187.0*,130.9 57 20,38 9 克百威 1563-66-2 5.71 222.1 165.0*,123.1 70 16,29 10 硫环磷 947-02-4 4.47 256.0 140.0*,228.0 40 30,18 11 甲基硫环磷 5120-23-0 3.96 228.0 168.0*,109.0 30 20,35 12 甲胺磷 10265-92-6 2.47 142.0 94.0*,125.0 57 19,18 13 乐果 60-51-5 4.42 230.0 199.0*,125.0 56 13,29 14 灭线磷 13194-48-4 8.36 243.0 97.0*,131.0 67 43,26 15 内吸磷 8065-48-3 6.62 259.0 89.0*,61.0 48 22,45 16 水胺硫磷 24353-61-5 10.82 231.0 121.0*,109.0 100 26,38 17 氧乐果 1113-02-6 3.63 214.0 183.0*,109.0 60 16,36 18 乙酰甲胺磷 30560-19-1 3.57 184.0 143.0*,125.0 50 12,25 19 灭多威 16752-77-5 4.02 163.1 88.0*,106.0 38 12,14 20 辛硫磷 14816-18-3 11.19 299.1 129.0*,153.0 55 18,10 21 吡虫啉 138261-41-3 4.35 256.1 175.1*,209.1 45 27,22 22 敌百虫 52-68-6 5.44 256.9 109.0*,221.0 65 21,16 23 甲萘威 63-25-2 6.01 202.1 145.0*,127.0 56 15,40 24 多菌灵 10605-21-7 3.48 192.0 160.0*,132.0 80 25,41 25 茚虫威 144171-61-9 11.28 528.1 293.0*,249.0 50 18,23 26 啶虫脒 135410-20-7 4.41 223.0 126.0*,99.0 65 28,60 27 噻虫胺 210880-92-5 4.26 250.0 169.1*,132.0 35 17,21 28 噻虫啉 1119-88-49-9 4.79 253.0 126.0*,99.0 81 29,57 29 噻虫嗪 153719-23-4 4.03 292.0 211.1*,181.1 30 16,30 30 噻嗪酮 69327-76-0 10.91 306.2 201.1*,116.2 60 17,22 31 喹螨醚 120928-09-8 12.59 307.2 161.0*,131.1 30 22,60 32 噻螨酮 78587-05-0 12.36 353.1 228.0*,168.1 56 21,33 33 乙螨唑 153233-91-1 12.47 360.2 141.0*,304.0 80 42,25 34 除虫脲 35367-38-5 8.87 311.0 158.0*,141.2 45 20,49 35 啶氧菌酯 117428-22-5 10.20 368.1 205.1*,145.1 54 13,27 36 呋虫胺 165252-70-0 3.75 203.1 129.0*,157.0 35 16,11 37 氟虫脲 101463-69-8 12.12 489.0 158.1*,141.2 71 27,65 38 唑虫酰胺 129558-76-5 11.77 384.1 197.1*,171.0 40 35,33 39 甲氨基阿维菌素甲酸盐 155569-91-8 3.97 886.2 82.0*,158.1 50 110,41 40 醚菊酯 80844-07-1 13.22 394.2 177.1*,107.1 30 19,59 41 吡唑醚菌酯 175013-18-0 10.89 388.1 194.1*,163.1 50 18,36 42 杀虫威 22248-79-9 9.05 365.0 127.0*,204.0 38 18,49 43 杀螟松 122-14-5 8.70 278.1 125.0*,246.0 30 29,24 44 氟虫腈砜 120068-36-2 10.97 451.0 415.0*,282.0 −28 −26,−38 45 氟虫腈 120068-37-3 9.97 435.0 330.0*,250.0 −80 −21,−35 46 氟虫腈亚砜 120067-83-6 11.02 418.9 383.0*,314.0 −123 −18,−26 47 氟甲腈 205650-65-3 10.50 387.0 351.0*,282.0 −30 −19,−47 注:*表示定量离子。 表 2 47种化合物的线性范围、回归方程、相关系数、检出限、定量限和食品中农药最大残留限量(MRL)
Table 2 Linear ranges, regression equations, correlation coefficients, LOD, LOQ and maximum residue limits (MRL) for pesticides in food of 47 compounds
化合物名称 线性范围
(ng/mL)回归方程 r 检出限
(mg/kg)定量限
(mg/kg)MRL
(mg/kg)[32]毒虫畏 2~100 Y=2.73×105X+6.01×105 0.9990 0.002 0.005 0.01 甲拌磷 2~100 Y=3.35×104X−3.14×104 0.9990 0.001 0.004 0.01 甲拌磷砜 2~100 Y=4.26×104X+1.07×104 0.9990 0.002 0.008 0.01 甲拌磷亚砜 2~100 Y=1.14×105X+1.05×105 0.9990 0.002 0.007 0.01 甲基异柳磷 2~100 Y=2.11×105X+4.54×105 0.9993 0.002 0.007 0.01 特丁硫磷 2~100 Y=2.28×104X+2.44×104 0.9990 0.002 0.008 0.01 特丁硫磷砜 2~100 Y=1.03×105X+8.77×104 0.9990 0.002 0.005 0.01 特丁硫磷亚砜 2~100 Y=2.91×105X+4.63×105 0.9992 0.002 0.006 0.01 克百威 2~100 Y=2.52×105X+2.20×105 0.9991 0.001 0.004 0.02 硫环磷 2~100 Y=1.49×105X+1.56×105 0.9994 0.001 0.005 0.03 甲基硫环磷 2~100 Y=2.69×105X+7.81×105 0.9990 0.002 0.005 0.03 甲胺磷 2~100 Y=1.99×105X+3.41×105 0.9994 0.001 0.004 0.05 乐果 2~100 Y=1.09×105X+1.27×105 0.9991 0.001 0.003 0.05 灭线磷 2~100 Y=1.47×105X+6.23×104 0.9992 0.001 0.003 0.05 内吸磷 2~100 Y=9.03×103X–3.61×103 0.9991 0.003 0.009 0.05 水胺硫磷 2~100 Y=1.35×105X+1.02×105 0.9992 0.002 0.007 0.05 氧乐果 2~100 Y=1.13×105X+1.34×105 0.9994 0.001 0.004 0.05 乙酰甲胺磷 2~100 Y=1.95×105X+3.74×105 0.9996 0.005 0.017 0.05 灭多威 2~100 Y=1.50×105X+2.77×105 0.9990 0.002 0.006 0.2 辛硫磷 2~100 Y=1.98×105X+1.93×105 0.9990 0.002 0.006 0.2 吡虫啉 2~100 Y=2.56×104X+1.17×104 0.9989 0.002 0.006 0.5 敌百虫 2~100 Y=2.76×104X+2.06×103 0.9988 0.004 0.012 2 多菌灵 2~100 Y=5.27×105X+1.87×106 0.9993 0.001 0.002 5 甲萘威 2~100 Y=1.80×105X+1.75×105 0.9989 0.002 0.006 5 茚虫威 2~100 Y=1.25×104X+4.58×103 0.9987 0.002 0.005 5 啶虫脒 2~100 Y=1.08×105X+5.19×104 0.9990 0.001 0.004 10 噻虫胺 2~100 Y=1.94×104X+5.86×103 0.9988 0.002 0.006 10 噻虫啉 2~100 Y=7.72×104X+6.16×104 0.9990 0.002 0.005 10 噻虫嗪 2~100 Y=3.32×104X+4.33×104 0.9994 0.001 0.004 10 噻嗪酮 2~100 Y=6.00×105X+3.18×106 0.9985 0.002 0.008 10 吡唑醚菌酯 2~100 Y=4.17×105X+1.22×105 0.9994 0.001 0.003 10 喹螨醚 2~100 Y=1.71×105X+5.27×105 0.9992 0.001 0.002 15 噻螨酮 2~100 Y=5.86×104X+4.60×104 0.9988 0.001 0.003 15 乙螨唑 2~100 Y=7.73×105X+9.96×106 0.9971 0.002 0.008 15 除虫脲 2~100 Y=1.13×105X+1.20×105 0.9993 0.001 0.002 20 啶氧菌酯 2~100 Y=5.48×105X+3.16×106 0.9988 0.001 0.003 20 呋虫胺 2~100 Y=4.74×104X+1.50×105 0.9993 0.006 0.020 20 氟虫脲 2~100 Y=1.94×104X–2.43×104 0.9990 0.002 0.006 20 唑虫酰胺 2~100 Y=1.49×105X+8.02×105 0.9990 0.002 0.005 50 甲氨基阿维菌素甲酸盐 2~100 Y=5.51×104X+7.96×103 0.9991 0.001 0.005 0.5 醚菊酯 2~100 Y=8.72×104X+4.83×104 0.9992 0.001 0.005 50 杀虫威 2~100 Y=1.75×105X+2.52×105 0.9994 0.001 0.003 0.01 杀螟松 2~100 Y=4.75×103X+2.88×103 0.9987 0.004 0.014 0.5 氟虫腈砜 2~100 Y=1.03×106X+9.10×106 0.9986 0.001 0.002 / 氟虫腈 2~100 Y=6.18×105X+1.10×105 0.9991 0.001 0.003 / 氟虫腈亚砜 2~100 Y=6.06×105X+4.82×106 0.9991 0.001 0.002 / 氟甲腈 2~100 Y=6.90×105X+4.50×106 0.9991 0.001 0.002 / 表 3 47种化合物的加标回收率及相对标准偏差(n=6)
Table 3 Recovery rates and relative standard deviations of 47 compounds (n=6)
化合物名称 加标7.50 μg/kg 加标18.75 μg/kg 加标37.50 μg/kg 平均回收率(%) RSD(%) 平均回收率(%) RSD(%) 平均回收率(%) RSD(%) 毒虫畏 88.2 13.2 95.6 14.8 101.0 9.0 甲拌磷 92.3 12.5 96.4 15.2 100.8 11.7 甲拌磷砜 105.0 2.5 103.0 5.4 99.2 2.6 甲拌磷亚砜 101.0 12.7 99.8 3.6 99.9 4.8 甲基异柳磷 90.6 7.6 97.3 4.3 100.6 8.3 特丁硫磷 86.8 1.3 98.5 4.9 100.2 1.5 特丁硫磷砜 74.6 6.5 98.7 7.8 100.1 6.8 特丁硫磷亚砜 89.6 8.1 103.0 10.1 99.2 10.8 克百威 83.3 11.6 103.0 6.4 99.2 3.8 硫环磷 73.4 11.3 100.2 4.5 99.8 3.2 甲基硫环磷 89.6 16.2 95.7 8.7 100.9 6.2 甲胺磷 76.4 9.3 99.9 8.0 99.9 11.0 乐果 86.5 5.7 97.9 3.8 100.3 2.5 灭线磷 88.8 8.6 100.6 8.8 99.7 8.8 内吸磷 77.7 3.6 97.4 5.1 100.4 1.9 水胺硫磷 83.5 10.2 102.5 7.9 99.3 6.5 氧乐果 84.4 7.1 99.8 6.6 99.9 7.3 乙酰甲胺磷 85.2 8.5 103.3 10.3 99.2 9.0 灭多威 93.5 11.4 97.1 3.9 100.5 7.5 辛硫磷 87.7 11.9 95.2 14.4 101.1 5.4 吡虫啉 110.4 6.8 97.2 7.0 100.6 8.4 敌百虫 93.5 7.8 96.1 3.6 100.8 5.9 多菌灵 95.4 15.9 97.5 14.6 100.6 14.2 甲萘威 94.3 9.3 96.5 4.2 100.7 5.8 茚虫威 97.6 17.4 94.1 5.8 101.5 1.4 啶虫脒 100.7 14.5 97.7 1.1 100.4 1.6 噻虫胺 109.3 3.9 99.1 0.5 100.0 0.3 噻虫啉 92.0 12.2 98.2 0.9 100.3 0.9 噻虫嗪 91.5 17.9 97.9 3.8 100.4 3.4 噻嗪酮 96.3 10.9 94.3 16.8 101.5 16.0 喹螨醚 100.5 9.2 97.5 14.3 100.6 11.3 噻螨酮 96.8 15.7 104.7 17.1 98.9 1.6 乙螨唑 88.2 19.9 92.1 19.1 102.1 12.3 除虫脲 114.3 4.8 98.4 2.5 100.3 4.9 啶氧菌酯 101.8 17.3 95.0 16.8 101.2 18.2 呋虫胺 84.4 9.3 97.2 3.4 100.6 1.4 氟虫脲 101.7 2.4 103.3 9.5 99.2 4.1 唑虫酰胺 104.4 11.9 96.9 14.0 100.7 11.6 甲氨基阿维菌素甲酸盐 79.2 9.4 98.0 1.8 100.3 3.2 醚菊酯 100.9 10.4 104.7 10.6 98.9 1.1 吡唑醚菌酯 103.7 14.5 98.7 14.0 100.2 9.2 杀虫威 110.9 7.2 97.5 9.5 100.5 3.2 杀螟松 94.6 16.8 93.7 4.5 101.5 0.9 氟虫腈砜 112.0 16.5 94.4 18.3 101.3 14.6 氟虫腈 95.3 13.4 94.8 16.6 101.3 10.5 氟虫腈亚砜 94.5 10.3 95.4 15.4 101.2 11.5 氟甲腈 105.2 16.1 95.9 19.2 101.0 7.7 表 4 QuEChERS方法和国家标准方法结果对比
Table 4 Comparison results of QuEChERS method and national standard method
序号 农药 结果(mg/kg) 本法 国家标准方法 标准偏差SD值 MRL 05绿茶 水胺硫磷 1.78 1.84 0.04 0.05 14绿茶 吡虫啉 0.65 0.67 0.01 0.5 11红茶 氧乐果 0.061 0.061 0.00 0.05 -
[1] 施杰, 来庆华, 郭思聪, 等. 茶叶农药残留与检测技术[J]. 食品安全质量检测学报,2019,10(5):1243−1249. [SHI J, LAI Q H, GUO S C, et al. Pesticide residue and detection technology of tea[J]. Food Safety and Quality Detection Technology,2019,10(5):1243−1249.] SHI J, LAI Q H, GUO S C, et al. Pesticide residue and detection technology of tea[J]. Food Safety and Quality Detection Technology, 2019, 10(5): 1243−1249.
[2] 陈宗懋. 茶园有害生物绿色防控技术发展与应用[J]. 中国茶叶,2022,44(1):1−6. [CHEN Z M. Development and application of green pest control technology in tea garden[J]. China Tea,2022,44(1):1−6.] CHEN Z M. Development and application of green pest control technology in tea garden[J]. China Tea, 2022, 44(1): 1−6.
[3] 王家哲, 李英梅, 张锋, 等. 根结线虫防治药剂及抗药性研究进展[J]. 陕西农业科学,2021,67(12):74−77. [WANG J Z, LI Y M, ZHANG F, et al. Advance of research in chemical control and resistance of root-knot nematodes[J]. Shaanxi Journal of Agricultural Sciences,2021,67(12):74−77.] WANG J Z, LI Y M, ZHANG F, et al. Advance of research in chemical control and resistance of root-knot nematodes[J]. Shaanxi Journal of Agricultural Sciences, 2021, 67(12): 74−77.
[4] 王超, 李新安, 刘恩良, 等. 我国麦蚜抗药性及研究现状[J]. 环境昆虫学报,2022,44(3):626−635. [WANG C, LIX A, LIU E L, et al. Research status of wheat aphid resistance to insecticides in China[J]. Journal of Environmental Entomology,2022,44(3):626−635.] WANG C, LIX A, LIU E L, et al. Research status of wheat aphid resistance to insecticides in China[J]. Journal of Environmental Entomology, 2022, 44(3): 626−635.
[5] 张明浩, 康珊珊, 郭靖立, 等. 新烟碱类杀虫剂在农药复配中的应用进展[J]. 农药,2022,61(5):313−320. [ZHANG M H, KANG S S, GUO J L, et al. Application progress of neonicotinoid insecticides in pesticide combination[J]. Agrochemicals,2022,61(5):313−320.] ZHANG M H, KANG S S, GUO J L, et al. Application progress of neonicotinoid insecticides in pesticide combination[J]. Agrochemicals, 2022, 61(5): 313−320.
[6] 杨森, 何志华, 杨留勇. 茶叶中农药残留分析技术研究进展[J]. 安徽农业科学,2018,46(20):20−22. [YANG S, HE Z H, YANG L Y. Research progress of pesticide residues analysis techniques in tea[J]. Journal of Anhui Agricultural Sciences,2018,46(20):20−22.] YANG S, HE Z H, YANG L Y. Research progress of pesticide residues analysis techniques in tea[J]. Journal of Anhui Agricultural Sciences, 2018, 46(20): 20−22.
[7] 许祯毅, 姜咸彪. 茶叶中农药残留检测技术应用进展[J]. 福建茶叶, 2020, 42(5):8−10. [XU Z Y, JIANG X B, Application progress of pesticide residue detection technology in tea [J]. Tea in Fujian, 2020, 42(5):8−10.] XU Z Y, JIANG X B, Application progress of pesticide residue detection technology in tea [J]. Tea in Fujian, 2020, 42(5): 8−10.
[8] HUANG Y S, SHI T, LUO X, et al. Determination of multipesticide residues in green tea with a modified QuEChERS protocol coupled to HPLC-MS/MS[J]. Food Chemistry,2019,275:255−264. doi: 10.1016/j.foodchem.2018.09.094
[9] 林芳, 赵立苹. 超高效液相色谱串联质谱法测定茶叶中杀螟丹的残留[J]. 现代食品,2020(9):181−184. [LI F, ZHAO L P. Determination of cartap in tea by ultra high performance liquid chromatography-tandem mass spectrometry/mass spectrometry[J]. Modern Food,2020(9):181−184.] LI F, ZHAO L P. Determination of cartap in tea by ultra high performance liquid chromatography-tandem mass spectrometry/mass spectrometry[J]. Modern Food, 2020(9): 181−184.
[10] 胡雪艳, 彭涛, 陈辉, 等. 茶叶中10种农药残留的液相色谱-串联质谱法和气相色谱-串联质谱法测定[J]. 食品工业科技,2018,39(17):240−247,252. [HU X Y, PENG T, CHEN H, et al. Determination of ten pesticide residues in tea by liquid chromatography-tandem mass spectrometry and gas chromatography-tandem mass spectrometry[J]. Science and Technology of Food Industry,2018,39(17):240−247,252.] HU X Y, PENG T, CHEN H, et al. Determination of ten pesticide residues in tea by liquid chromatography-tandem mass spectrometry and gas chromatography-tandem mass spectrometry[J]. Science and Technology of Food Industry, 2018, 39(17): 240−247,252.
[11] 曾艳, 郎红, 杨巧慧, 等. 固相萃取-GC/LC-MS/MS测定茶叶中79种农药残留[J]. 茶叶科学,2019,39(5):576−586. [ZENG Y, LANG H, YANG Q H, et al. Determination of 79 pesticide residues in tea by solid phase extraction with GC-MS/MS and LC-MS/MS[J]. Journal of Tea Science,2019,39(5):576−586.] ZENG Y, LANG H, YANG Q H, et al. Determination of 79 pesticide residues in tea by solid phase extraction with GC-MS/MS and LC-MS/MS[J]. Journal of Tea Science, 2019, 39(5): 576−586.
[12] 付雨婷, 章慧芳, 孟信刚. 蔬菜中农药前处理技术以及检测技术研究进展[J]. 广东化工,2022,49(5):153−155. [FU Y T, ZHANG H F, MENG X G. Research progress of pesticide pretreatment and detection technology in vegetables[J]. Guangdong Chemical Industry,2022,49(5):153−155.] FU Y T, ZHANG H F, MENG X G. Research progress of pesticide pretreatment and detection technology in vegetables[J]. Guangdong Chemical Industry, 2022, 49(5): 153−155.
[13] ANASTASSIADES M, LEHOTAY S J, STAJNBAHER D, et al. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce[J]. Journal of AOAC International,2003,86(2):412−431. doi: 10.1093/jaoac/86.2.412
[14] 朱会军, 李菁华. QuEChERS方法在环境监测分析中的应用[J]. 化工管理,2021(33):142−143. [ZHU H J, LI J H. Application of QuEChERS method in environmental monitoring and analysis[J]. Chemical Enterprise Management[J],2021(33):142−143.] ZHU H J, LI J H. Application of QuEChERS method in environmental monitoring and analysis[J]. Chemical Enterprise Management[J], 2021(33): 142−143.
[15] 刘缙, 朱文涛, 董文彬, 等. QuECHERS方法在法医毒物分析中的应用研究进展[J]. 刑事技术,2020,45(4):403−407. [LIU J, ZHU W T, DONG W B, et al. Research progress of QuEChERS methodology in forensic toxicological analysis[J]. Forensic Science and Technology,2020,45(4):403−407.] LIU J, ZHU W T, DONG W B, et al. Research progress of QuEChERS methodology in forensic toxicological analysis[J]. Forensic Science and Technology, 2020, 45(4): 403−407.
[16] 曾霞, 于雅汇, 王鸟, 等. QuEChERS前处理技术在农药多残留检测中的研究进展[J]. 当代化工研究,2022(6):33−35. [ZENG X, YU Y H, WANG N, et al. Research progress of QuEChERS pretreatment technology in the detection of pesticide residues[J]. Modern Chenical Research,2022(6):33−35.] ZENG X, YU Y H, WANG N, et al. Research progress of QuEChERS pretreatment technology in the detection of pesticide residues[J]. Modern Chenical Research, 2022(6): 33−35.
[17] OZGUR G, BULENT K. Evaluation of QuEChERS sample preparation and liquid chromatography-triple-quadrupole mass spectrometry method for the determination of 109 pesticide resticide in tomatoes[J]. Food Chemistry,2015,176:319−332. doi: 10.1016/j.foodchem.2014.12.083
[18] SUN R, YANG W Q, LI Y X, et al. Multi-residue analytical methods for pesticides in teas:A review[J]. European Food Research and Technology,2021,247:1839−1858. doi: 10.1007/s00217-021-03765-3
[19] HAN Y T, ZOU N, SONG L E, et al. Simultaneous determination of 70 pesticide residues in leek, leaf lettuce and garland chrysanthemum using modified QuEChERS method with multi-walled carbon nanotubes as reversed-dispersive solid-phase extraction materials[J]. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences,2015,1005:56−64. doi: 10.1016/j.jchromb.2015.10.002
[20] GILBERT-L PEZ B, GARC A-REYES J F, LOZANO A, et al. Large-scale pesticide testing in olives by liquid chromatography-electrospray tandem mass spectrometry using two sample preparation methods based on matrix solid-phase dispersion and QuEChERS[J]. Journal of Chromatography A,2010,12(17):6022−6035.
[21] 李洁, 鞠香, 王艳丽, 等. QuEChERS-在线凝胶渗透色谱-气相色谱-串联质谱法高通量筛查动物源性食品中的多农药残留[J]. 色谱,2023,41(7):610−621. [LI J, JU X, WANG Y L, et al. High-throughput screening of multi-pesticide residues in animal-derived foods by QuEChERS-online gel permeation chromatography-gas chromatography-tandem mass spectrometry[J]. Chinese Journal Chromatography,2023,41(7):610−621.] doi: 10.3724/SP.J.1123.2022.10010 LI J, JU X, WANG Y L, et al. High-throughput screening of multi-pesticide residues in animal-derived foods by QuEChERS-online gel permeation chromatography-gas chromatography-tandem mass spectrometry[J]. Chinese Journal Chromatography, 2023, 41(7): 610−621. doi: 10.3724/SP.J.1123.2022.10010
[22] 余磊, 赵志燊. QuEChERS-GC-MS/MS法同时测定青贮玉米饲料中20种农药残留[J]. 饲料研究,2023,568(10):116−121. [YU L, ZHAO Z Y. Simultaneous determination of 20 pesticide residues in silage corn feed by QuEChERS-GC-MS/MS[J]. Feed Research,2023,568(10):116−121.] YU L, ZHAO Z Y. Simultaneous determination of 20 pesticide residues in silage corn feed by QuEChERS-GC-MS/MS[J]. Feed Research, 2023, 568(10): 116−121.
[23] 任祥红, 黄莉. LC-MS/MS检测棉花中阿维菌素残留量的研究[J]. 山西师范大学学报(自然科学版),2023,139(2):47−51. [REN X H, HUANG L. Research on determination of abamectin residue in cotton by LC-MS/MS[J]. Journal of Shanxi Normal University (Natural Science Edition),2023,139(2):47−51.] REN X H, HUANG L. Research on determination of abamectin residue in cotton by LC-MS/MS[J]. Journal of Shanxi Normal University (Natural Science Edition), 2023, 139(2): 47−51.
[24] 刘进进. QuEChERS-气相色谱-三重四极杆串联质谱法测定青贮玉米中20种农药残留[J]. 饲料研究,2023,570(12):123−127. [LIU J J. Determination of 20 pesticide residues in silage corn by QuEChERS gas chromatography triple quadrupole mass spectrometry[J]. Feed Research,2023,570(12):123−127.] LIU J J. Determination of 20 pesticide residues in silage corn by QuEChERS gas chromatography triple quadrupole mass spectrometry[J]. Feed Research, 2023, 570(12): 123−127.
[25] 朱颖洁, 曹燕卿, 毛劼, 等. QuEChERS前处理结合超高效液相色谱-串联质谱法同时检测茶叶中14种农药残留[J]. 食品工业科技,2022,43(12):283−290. [ZHU Y J, CAO Y Q, MAO J, et al. Determination of 14 oesticide residues in tea by QuEChERS combined with ultra-performance liquid chromatography-tandem mass spectrometry[J]. Science and Technology of Food Industry,2022,43(12):283−290.] ZHU Y J, CAO Y Q, MAO J, et al. Determination of 14 oesticide residues in tea by QuEChERS combined with ultra-performance liquid chromatography-tandem mass spectrometry[J]. Science and Technology of Food Industry, 2022, 43(12): 283−290.
[26] 刘相真, 叶美君, 陆小磊. GC-MS和LC-MSMS法快速测定茶叶中35种农药残留[J]. 中国茶叶加工,2019(3):48−58. [LIU X Z, YE M J, LU X L. Rapid determination of 35 pesticides residues in tea by GC-MS and LC-MSMS[J]. China Tea Processing,2019(3):48−58.] LIU X Z, YE M J, LU X L. Rapid determination of 35 pesticides residues in tea by GC-MS and LC-MSMS[J]. China Tea Processing, 2019(3): 48−58.
[27] LY T K, HO T D, BEHRA P, et al. Determination of 400 pesticide residues in green tea leaves by UPLC-MS/MS and GC-MS/MS combined with QuEChERS extraction and mixed-mode SPE clean-up method[J]. Food Chemistry,2020,326:126928.
[28] 邵林, 刘晓云, 李福敏, 等. 多壁碳纳米管结合气相色谱-电子捕获检测法同时测定茶叶中18种有机氯类农药残留[J]. 食品安全质量检测学报,2021,12(20):8136−8140. [SHAO L, LIU X Y, LI F M, et al. Simultaneous determination of 18 kinds of organochlorine pesticide residues in tea by multi walled carbon nanotubes combined with gas chromatograph-electron capture detection[J]. Journal of Food Safety and Quality,2021,12(20):8136−8140.] SHAO L, LIU X Y, LI F M, et al. Simultaneous determination of 18 kinds of organochlorine pesticide residues in tea by multi walled carbon nanotubes combined with gas chromatograph-electron capture detection[J]. Journal of Food Safety and Quality, 2021, 12(20): 8136−8140.
[29] 曾小娟, 林涛, 王莉丽, 等. QuEChERS-超高效液相色谱-串联质谱法测定茶叶中3种杀虫剂手性农药[J]. 食品安全质量检测学报,2021,12(2):602−608. [ZENG X J, LIN T, WANG L L, et al. Determination of 3 kinds of chiral pesticides in tea by QuEChERS-ultra performance liquid chromatography-tandem mass spectrometry[J]. Journal of Food Safety and Quality,2021,12(2):602−608.] ZENG X J, LIN T, WANG L L, et al. Determination of 3 kinds of chiral pesticides in tea by QuEChERS-ultra performance liquid chromatography-tandem mass spectrometry[J]. Journal of Food Safety and Quality, 2021, 12(2): 602−608.
[30] 刘华文, 苏海雁, 陆小康, 等. QuEChERS/超高效液相色谱-串联质谱法测定茶叶中28种农药残留[J]. 食品工业科技,2021,42(2):223−229,236. [LIU H W, SU H Y, LU X K, et al. Determination of 28 kinds of pesticide residues in tea by QuEChERS/ultra performance liquid chromatography-tandem mass spectrometry[J]. Science and Technology of Food Industry,2021,42(2):223−229,236.] LIU H W, SU H Y, LU X K, et al. Determination of 28 kinds of pesticide residues in tea by QuEChERS/ultra performance liquid chromatography-tandem mass spectrometry[J]. Science and Technology of Food Industry, 2021, 42(2): 223−229,236.
[31] 张聪, 周常义, 江锋, 等. 超高效液相色谱-串联质谱法测定动物性食品中10种拟除虫菊酯类农药残留[J]. 分析测试学报,2018,37(8):887−893. [ZHANG C, ZHOU C Y, JIANG F, et al. Determination of 10 pyrethroid pesticide residues in animal foods by ultra performance liquid chromatography-tandem mass spectrometry[J]. Journal of Instrumental Analysis,2018,37(8):887−893.] ZHANG C, ZHOU C Y, JIANG F, et al. Determination of 10 pyrethroid pesticide residues in animal foods by ultra performance liquid chromatography-tandem mass spectrometry[J]. Journal of Instrumental Analysis, 2018, 37(8): 887−893.
[32] 中华人民共和国国家卫生健康委员会, 中华人民共和国农业农村部, 国家市场监督管理总局. GB 2763-2021 食品安全国家标准 食品中农药最大残留限量[S]. 北京:中国农业出版社, 2021. [The People’s Republic of China, National Health Commission, Ministry of Agriculture and Rural Affairs, State Administration for Market Regulation. GB 2763-2021 National food safety standard maximum residue limits for pesticides in food[S]. Beijing:China Agriculture Press, 2021.] The People’s Republic of China, National Health Commission, Ministry of Agriculture and Rural Affairs, State Administration for Market Regulation. GB 2763-2021 National food safety standard maximum residue limits for pesticides in food[S]. Beijing: China Agriculture Press, 2021.
[33] 中华人民共和国国家质量监督检验检疫总局. GB/T 27404-2008 实验室质量控制规范 食品理化检测[S]. 北京:中国标准出版社, 2008:26−27. [General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China. GB/T 27404-2008 Criterion on quality control of laboratories-chemical testing of food[S]. Beijing:China Standards Press, 2008:26−27.] General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China. GB/T 27404-2008 Criterion on quality control of laboratories-chemical testing of food[S]. Beijing: China Standards Press, 2008: 26−27.
[34] 中华人民共和国国家卫生健康委员会, 中华人民共和国农业农村部, 国家市场监督管理总局. GB 23200.121-2021 食品安全国家标准 植物源性食品中331种农药及其代谢物残留量的测定液相色谱-质谱联用法[S]. 北京:中国农业出版社, 2021. [The People’s Republic of China, National Health Commission, Ministry of Agriculture and Rural Affairs, State Administration for Market Regulation. GB 23200.121-2021 National food safety standard determination of 331 pesticides and metabolites residues in foods of plant origin liquid chromatography-tandem mass spectrometry method[S]. Beijing:China Agriculture Press, 2021.] The People’s Republic of China, National Health Commission, Ministry of Agriculture and Rural Affairs, State Administration for Market Regulation. GB 23200.121-2021 National food safety standard determination of 331 pesticides and metabolites residues in foods of plant origin liquid chromatography-tandem mass spectrometry method[S]. Beijing: China Agriculture Press, 2021.





 下载:
下载:
 下载:
下载:
