Preliminary Study on the Mechanism of Chitosan and ε-Polylysine Inhibition against Carbibacterium divergens
-
摘要: 研究壳聚糖和ε-聚赖氨酸对肉食杆菌细胞结构和细胞保护酶的作用,探讨其对肉食杆菌的抑菌作用机制。采用肉汤稀释法测定壳聚糖和ε-聚赖氨酸对肉食杆菌的最小抑菌浓度(minimal inhibitory concentration,MIC),由细菌生长曲线、电导率、胞外核酸、胞外蛋白、碱性磷酸酶(alkaline phosphatase,AKP)活力等指标,并结合扫描电子显微镜(scanning electron microscopy,SEM)观察,综合评价壳聚糖和ε-聚赖氨酸对肉食杆菌的作用机制;通过测定细胞保护酶(超氧化物歧化酶(superoxide dismutase,SOD)和过氧化氢酶(catalase,CAT))的活力变化分析其氧化应激反应参与情况。结果表明:壳聚糖和ε-聚赖氨酸对肉食杆菌的MIC分别为0.1953和 0.1563 mg/mL,经MIC和2MIC的壳聚糖和ε-聚赖氨酸处理后,菌体细胞内成分(AKP酶、蛋白质、核酸和离子)发生渗漏,CAT和SOD酶活力呈显著下降趋势(P<0.05),且壳聚糖和ε-聚赖氨酸质量浓度与酶活力呈负相关关系。由扫描电镜观察得出,壳聚糖能使菌体变形且内容物渗出,ε-聚赖氨酸则使菌体出现褶皱和扭曲变形,细胞表面生成孔洞。综上所述,壳聚糖和ε-聚赖氨酸均能通过破坏细胞壁、损伤细胞膜、产生氧化应激反应等机制来抑制肉食杆菌的生长。Abstract: To investigate the effects of chitosan and ε-polylysine on the cell structure and cytoprotective enzymes of Carbibacterium divergens to explore their inhibition mechanism against Carbibacterium divergens, the broth dilution method was used to determine the minimum inhibitory concentrations (MICs) of chitosan and ε-polylysine against Carbibacterium divergens. Their effects on the cellular structure of Carbibacterium divergens were then assessed by several indexes, such as growth curves, electronic conductivity, extracellular nucleic acids, extracellular proteins, and alkaline phosphatase (AKP) activity, and the microstructure of bacteria were also observed by scanning electron microscopy (SEM). In addition, their role in oxidative stress was analyzed by detecting changes in catalase (CAT) activity and superoxide dismutase (SOD) activity. The results showed that the MIC of chitosan and ε-polylysine against Carbibacterium divergens was 0.1953 and 0.1563 mg/mL, respectively. The intracellular components (AKP, proteins, nucleic acids, and ions) leaked after treatment with MIC and 2MIC chitosan and ε-polylysine. The mass concentrations of chitosan and ε-polylysine were negatively correlated with enzyme activity, and the activities CAT and SOD significantly decreased (P<0.05). Scanning electron microscopy showed that chitosan could deform the cell and its contents oozed out, while ε-polylysine caused the cell to fold and distort, forming holes on the cell surface. In summary, both chitosan and ε-polylysine can inhibit the growth of Carbibacterium divergens by destroying cell wall, damaging cell membrane and generating oxidative stress reaction.
-
Keywords:
- chitosan /
- ε-polylysine /
- Carbibacterium divergens /
- cell structure /
- antibacterial
-
肉食杆菌(Carbibacterium divergens)是一种革兰氏阳性菌,耐盐性能较强,可利用碳水化合物产酸,易存在于鱼、肉和其他各种食物中产生异味并导致腐败变质。李成[1]研究发现蟹糊在−20 ℃和4 ℃贮藏温度下的优势腐败菌均为肉食杆菌属,该菌属能分解糖类产生乳酸,降低pH,其还能利用精氨酸产生氨类物质,产生不愉快气味;桂国弘等[2]研究得出肉食杆菌在保藏过程中逐渐成为冷鲜鸡的优势腐败菌,是导致挥发性盐基氮含量升高,继而发生腐败变质的主因。作为常用的天然抑菌剂,壳聚糖和ε-聚赖氨酸均具有抑菌效果好、抑菌性广等优点[3−4]。Wei等[5]提出,由于壳聚糖氨基的质子化,产生一个抑菌基团-NH3+,与细菌表面负电荷间的静电相互作用,使细胞膜破裂,难以形成细胞壁,从而达到抑菌目的[6]。ε-聚赖氨酸源于白色链霉菌的发酵产物[7],是一种高度安全的天然食品抑腐剂。其中,Hou等[8]和Wei等[9]研究表明,ε-聚赖氨酸的抑菌机制主要作用于细胞膜和细胞壁导致细胞死亡,同时还可能作用于酶。
目前除常规的热杀菌、辐照、气调包装等处理手段,在肉制品中添加天然抑菌剂是抑制肉类腐败菌生长繁殖的方法。王志琦[10]研究表明在4 ℃贮藏条件下将复配抑菌剂(0.03% ε-PL+0.08%百里香精油+0.06%肉桂醛+0.04%牛至精油)应用于阚疃板鸡成品,可显著抑制肉食杆菌属等菌群,使其货架期延长到 9 d。李新福[11]研究得出复配精油牛至+百里香(1:1,v/v)对肉食杆菌抑制作用最佳。李成[1]发现复配保鲜剂(0.01% Nisin+0.04% ε-PL+0.03%茶多酚+0.1%柠檬酸)抑制肉食杆菌的增长,有效提高腌制生食蟹糊冻藏期间的食用安全性。
本文拟通过研究壳聚糖和ε-聚赖氨酸对肉食杆菌细胞结构和细胞保护酶的作用,探讨其对肉食杆菌的抑菌作用机制。通过测定最小抑菌浓度(minimum inhibition concentration,MIC),结合微生物生长曲线,比较其抑菌作用强弱,评价其对肉食杆菌的抑菌活性;然后通过测定电导率值、胞外核酸、胞外蛋白、碱性磷酸酶(alkaline phosphatase,AKP)活力等指标,结合扫描电子显微镜观察,研究壳聚糖和ε-聚赖氨酸对肉食杆菌细胞结构的破坏作用,还通过检测超氧化物歧化酶(superoxide dismutase,SOD)和过氧化氢酶(catalase,CAT)活性变化,进一步探究壳聚糖和ε-聚赖氨酸对肉食杆菌的细胞保护酶的作用影响,初步阐述壳聚糖和ε-聚赖氨酸的抑菌机理,为其在食品抑菌防腐领域中的开发应用提供一定的理论参考。
1. 材料与方法
1.1 材料与仪器
肉食杆菌P10(Carbibacterium divergens) 为课题组早期从腐败酱卤肉鸽中分离、鉴定并保存的菌株;水溶性壳聚糖(分子量10W,脱乙酰度90.22%) 河南万邦万邦化工科技有限公司;ε-聚赖氨酸(纯度99.26%) 郑州拜纳佛生物工程股份有限公司;营养琼脂(NA)培养基、营养肉汤(NB)培养基 广州环凯微生物科技有限公司;蛋白定量测试盒、AKP测试盒、SOD测试盒、CAT测试盒 南京建成生物工程研究所;戊二醛 分析纯,上海源叶生物科技有限公司。
DDS-307电导率仪 上海仪电科学仪器股份有限公司;760CRT紫外可见光分光光度计 上海精密科学仪器有限公司;TDL80-2B台式离心机 上海安亭科学仪器厂;HITACHI SU8100扫描电子显微镜 日本Hitachi株式会社。
1.2 实验方法
1.2.1 菌株活化及菌液制备
菌株活化:将肉食杆菌接种在NB培养基中,并在37 ℃下培养24 h,反复传代培养[12]。
菌悬液制备:取活化的菌液以1%(体积分数,后同)接种量接入100 mL的营养肉汤培养基中,37 ℃静置培养12 h,培养至对数生长期,得到原菌液备用。将营养肉汤在4 ℃、4000 r/min条件下离心10 min,弃上清液,所得沉淀用无菌PBS调整菌液,通过平板计数法得出具体菌落总数,在600 nm下测定吸光度Abs(参考菌浓度-吸光度标准曲线),调整菌液浓度为107~108 CFU/mL,现配现用[11]。
1.2.2 最小抑菌浓度(MIC)
通过肉汤稀释法测定抑菌剂对肉食杆菌的最小抑菌浓度(minimal inhibitory concentration,MIC),即37 ℃下培养24 h,明显抑制微生物生长的最小浓度[13]。参考Wang等[14]方法,稍作修改。收集对数生长期的菌液,稀释至106 CFU/mL。采用二倍稀释法将壳聚糖和ε-聚赖氨酸在无菌PBS中依次稀释,使壳聚糖浓度依次为12.5、6.25、3.125、1.5625、0.7813、0.3906、0.1953、0.0977、0.04885 g/kg;ε-聚赖氨酸浓度依次为5、2.5、1.25、0.625、0.3125、0.1563、0.0781、0.0391、0.00196 g/kg。以无菌水作为对照组。37 ℃下培养24 h。随后,取0.1 mL活化后的菌液于平板上涂布均匀。以无菌水作为空白对照,将各平板于 37 ℃中倒置培养 24 h,以完全不长菌的最小浓度确定为壳聚糖和ε-聚赖氨酸对肉食杆菌作用的 MIC[15]。
1.2.3 细菌培养液的制备
参照蓝蔚青等[16]方法并做适当修改。将已制备的菌悬液按1%的接种量接种至NB培养基中,以加入MIC、2 MIC的抑菌液(壳聚糖、ε-聚赖氨酸)为实验组(MIC组、2MIC组),以不加抑菌液为对照组(CK组),37 ℃静置培养。
1.2.4 微生物生长曲线
采用紫外-可见分光光度法测定肉食杆菌的生长曲线[17]。取制备好的细菌培养液,每隔2 h测定600 nm波长处的OD值。
1.2.5 肉食杆菌细胞膜通透性的测定
通过测定电导率值,表示壳聚糖和ε-聚赖氨酸对菌体细胞膜通透性的影响,参照Liang等[18]方法并做适当修改。取制备好的细菌培养液,每隔2 h测定1 次电导率,连续12 h。
1.2.6 胞外核酸的测定
参照梅佳林等[19]方法并做适当修改。取制备好的细菌培养液,每隔2 h取样一次,连续 12 h,以4000 r/min低温离心10 min 后,取上清液,测定260 nm波长处的OD值。
1.2.7 胞外蛋白质含量的测定
参照Stec等[20]方法并做适当修改。取制备好的细菌培养液,每隔2 h取样一次,连续 12 h,以4000 r/min 低温离心10 min 后,取上清液,采用试剂盒测定细菌胞外蛋白质含量。
1.2.8 肉食杆菌细胞壁完整性的测定
通过测定碱性磷酸酶(AKP),研究壳聚糖和ε-聚赖氨酸对菌体细胞壁完整性的影响,参照Zhao等[21]方法并做适当修改。分别在0、2、4、6、8、10、12 h的细菌培养液离心后取上清液,用AKP试剂盒来测定。
1.2.9 超氧化物歧化酶(SOD)和过氧化氢酶(CAT)的测定
参照张亮亮[22]和Motavallihaghi等[23]方法,将肉食杆菌培养至对数期(107 CFU/mL),分别于0、2、4、6、8、10、12 h取制备好的细菌培养液,各取10 mL在低温环境下超声破碎,每次破碎5 s,处理2 min,4000 r/min低温离心10 min。吸取上清液,用试剂盒测定超氧化物歧化酶(SOD)活力和过氧化氢酶(CAT)活力。
1.2.10 扫描电子显微镜观察
取制备好的细菌培养液,在37 ℃、120 r/min摇床培养12 h后,将上述培养液于4 ℃、8000 r/min离心5 min,收集沉淀的细菌;将菌体重悬于2.5%戊二醛溶液中,并在4 ℃下固定24 h。弃掉上清液,用磷酸缓冲液冲洗样品;1%的锇酸溶液固定样品2 h[24],再用磷酸缓冲液冲洗样品;用30%、50%、70%、80%、90%、95%的乙醇溶液和无水乙醇对菌体依次脱水。临界点干燥,将其涂在金属载体上,喷金,在扫描电子显微镜中观察[25]。
1.3 数据处理
试验各平行3次,采用Microsoft Excel 2019(微软公司)制表,Origin 2018软件(Origin Lab公司)制图,IBM SPSS Statistics 22软件(IBM公司)做显著性分析,P<0.05表示差异显著。
2. 结果与分析
2.1 壳聚糖和ε-聚赖氨酸对肉食杆菌的最小抑菌浓度
最小抑菌浓度(MIC)是衡量和评价物质抑菌能力的主要指标之一[26],MIC值越小,抑菌效果越好,即在较低的质量浓度下,就可以抑制微生物的生长[27]。由表1和表2可知,壳聚糖和ε-聚赖氨酸对肉食杆菌的生长均有较好的抑制作用,且抑菌效果随壳聚糖和ε-聚赖氨酸浓度的增大而增强,当壳聚糖和ε-聚赖氨酸分别稀释到最小浓度0.1953和0.1563 mg/mL时,培养基上肉眼看不见细菌生长,因此,其MIC分别为0.1953和0.1563 mg/mL,而ε-聚赖氨酸对肉食杆菌的MIC低于壳聚糖对肉食杆菌的MIC,表明ε-聚赖氨酸对肉食杆菌的抑制作用强于壳聚糖。
表 1 壳聚糖对肉食杆菌的MICTable 1. MIC of chitosan against Carnobacterium divergens抑菌剂浓度(mg/mL) 0.04885 0.0977 0.1953 0.3906 0.7813 1.5625 3.125 6.25 12.5 壳聚糖 + + − − − − − − − 注:“−”表示无菌生长,“+”表示有菌生长;表2同。 表 2 ε-聚赖氨酸对肉食杆菌的MICTable 2. MIC of ε-polylysine against Carnobacterium divergens抑菌剂浓度(mg/mL) 0.00196 0.0391 0.0781 0.1563 0.3125 0.625 1.25 2.5 5 ε-聚赖氨酸 + + + − − − − − − 2.2 壳聚糖和ε-聚赖氨酸对肉食杆菌生长曲线的影响
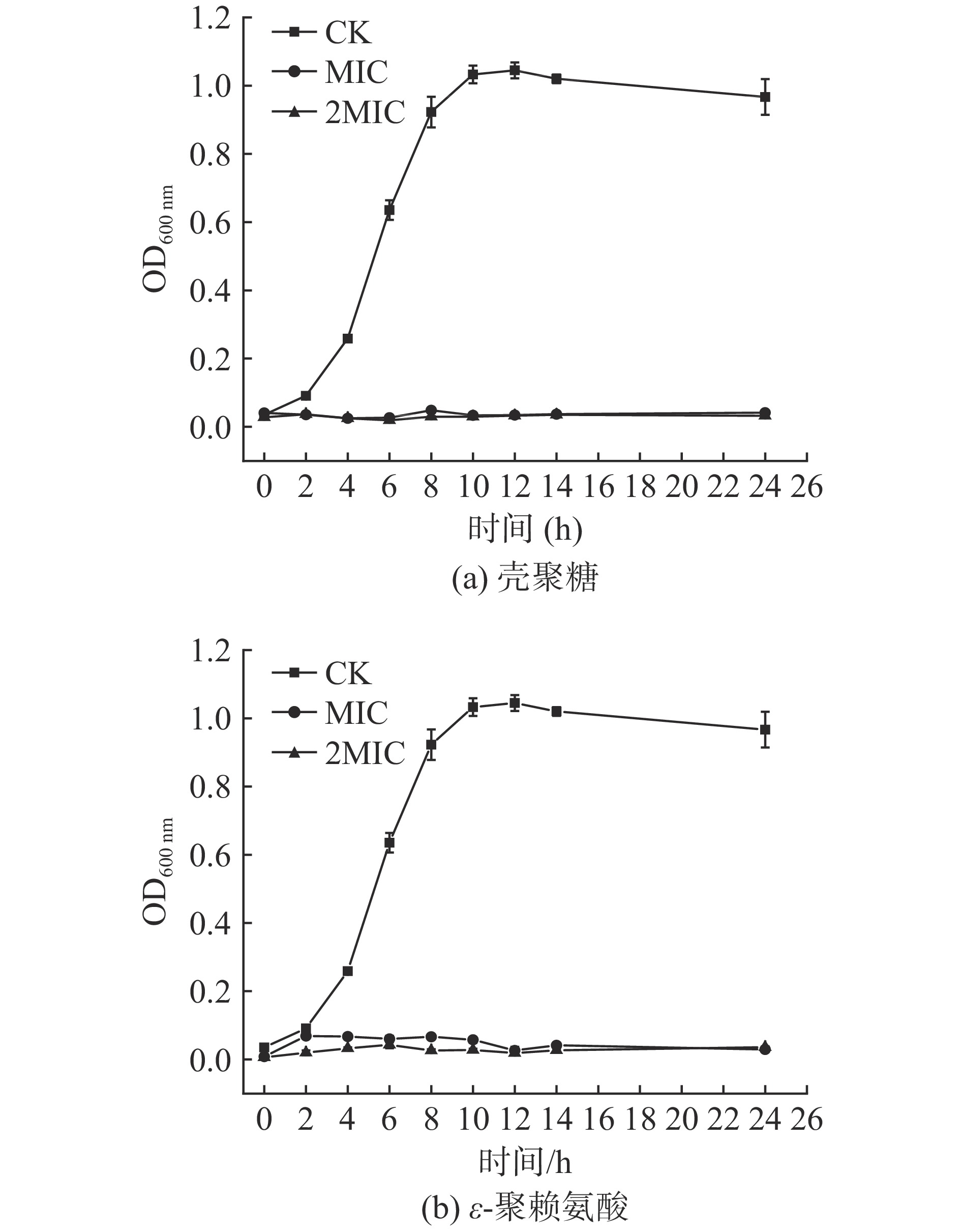
细菌的生长曲线可以直接反映其生长情况及生长速率。在一定的波长范围内,细菌悬液的浓度与吸光度值成正比[28],可通过OD值变化来评判壳聚糖和ε-聚赖氨酸对肉食杆菌的抑制作用。如图1所示,CK组的肉食杆菌呈现出典型的“S”型生长曲线特点。4~10 h为对数期,该阶段细菌迅速生长,10 h后进入稳定期。壳聚糖和ε-聚赖氨酸的MIC组和2MIC组,OD值基本处于低值波动,显著低于CK组,说明细菌几乎停止生长。加入质量浓度为MIC和2MIC的壳聚糖和ε-聚赖氨酸延缓了肉食杆菌细胞的生长周期,能有效抑制肉食杆菌的生长。
2.3 壳聚糖和ε-聚赖氨酸对肉食杆菌细胞膜通透性的影响
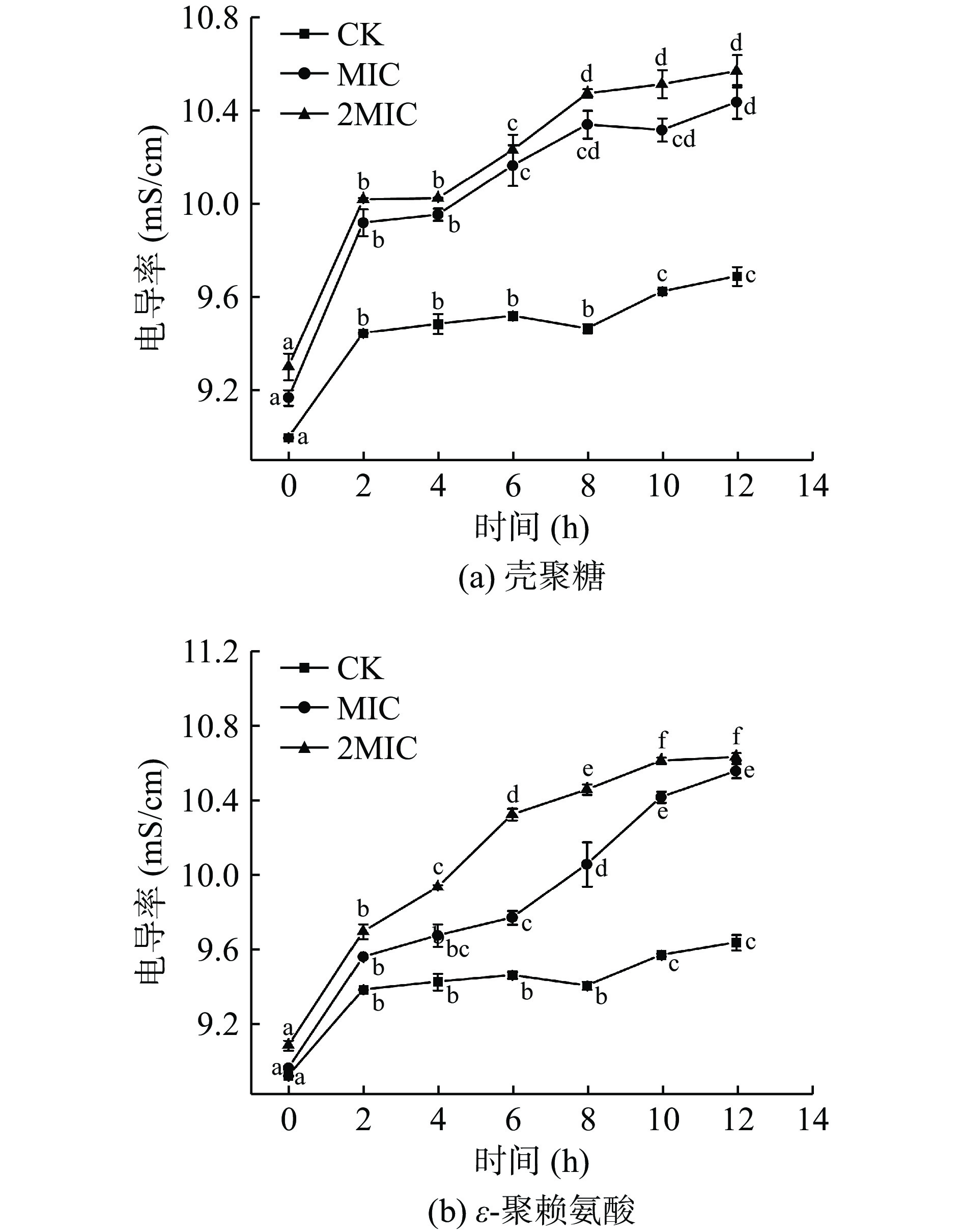
菌液电导率的变化可用来分析壳聚糖和ε-聚赖氨酸对肉食杆菌细胞膜的通透性的影响[29]。图2中,壳聚糖和ε-聚赖氨酸的MIC组和2MIC组的电导率均显著增加(P<0.05),而CK组的电导率较为稳定。正常情况下,细胞内外离子在细胞膜屏障下,会保持动态平衡,膜外环境的电导率基本保持不变。CK组菌液在2 h内的电导率增加较明显,随后维持在相对稳定状态。在壳聚糖和ε-聚赖氨酸的作用下,肉食杆菌的细胞膜被破坏,失去保护能力和屏障作用,使原本细胞内容物中的带电离子泄漏到细胞膜外,包括K+、Ca2+、Na+,导致膜外环境的电导率升高[30],图2中MIC与2MIC组菌液的电导率始终高于CK组,壳聚糖的增长速率高于ε-聚赖氨酸,其与处理液浓度呈正相关。壳聚糖和ε-聚赖氨酸可以通过损伤细胞膜,增加细胞膜的通透性,影响其正常代谢,从而抑制细菌生长[31]。
2.4 壳聚糖和ε-聚赖氨酸对肉食杆菌核酸泄漏的影响
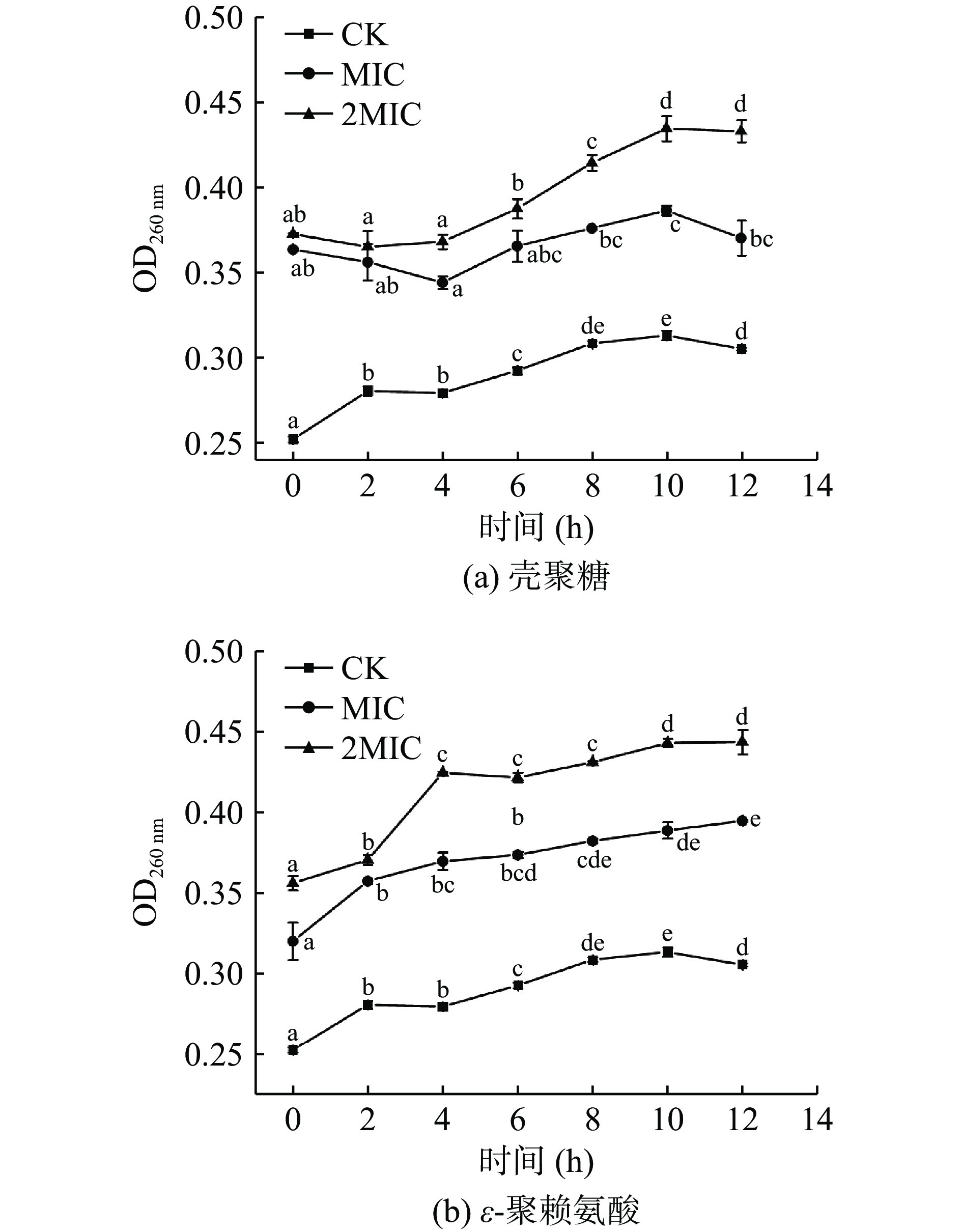
核酸具有编码合成蛋白质的功能,在细胞的生命活动中起着重要作用[32],如果细胞内核酸丢失,将会导致细胞死亡。在不同培养时间,细胞内的核酸泄漏情况如图3所示。各组的OD值均呈现出上升趋势,MIC和2MIC的壳聚糖和ε-聚赖氨酸的OD值均显著增大(P<0.05)。壳聚糖的MIC组和2MIC组OD值在4 h后明显增长,ε-聚赖氨酸处理的MIC组和2MIC组OD值在0~4 h内增长最快,之后增长速度减慢,说明壳聚糖和ε-聚赖氨酸破坏了肉食杆菌的细胞膜,导致细胞内核酸不断从细胞膜内流出,破坏强度与浓度呈正相关。因此,壳聚糖和ε-聚赖氨酸不仅破坏细胞结构,还导致胞内遗传物质的泄漏,从而影响细胞的正常生理活动。
2.5 壳聚糖和ε-聚赖氨酸对肉食杆菌胞外蛋白质量浓度的影响
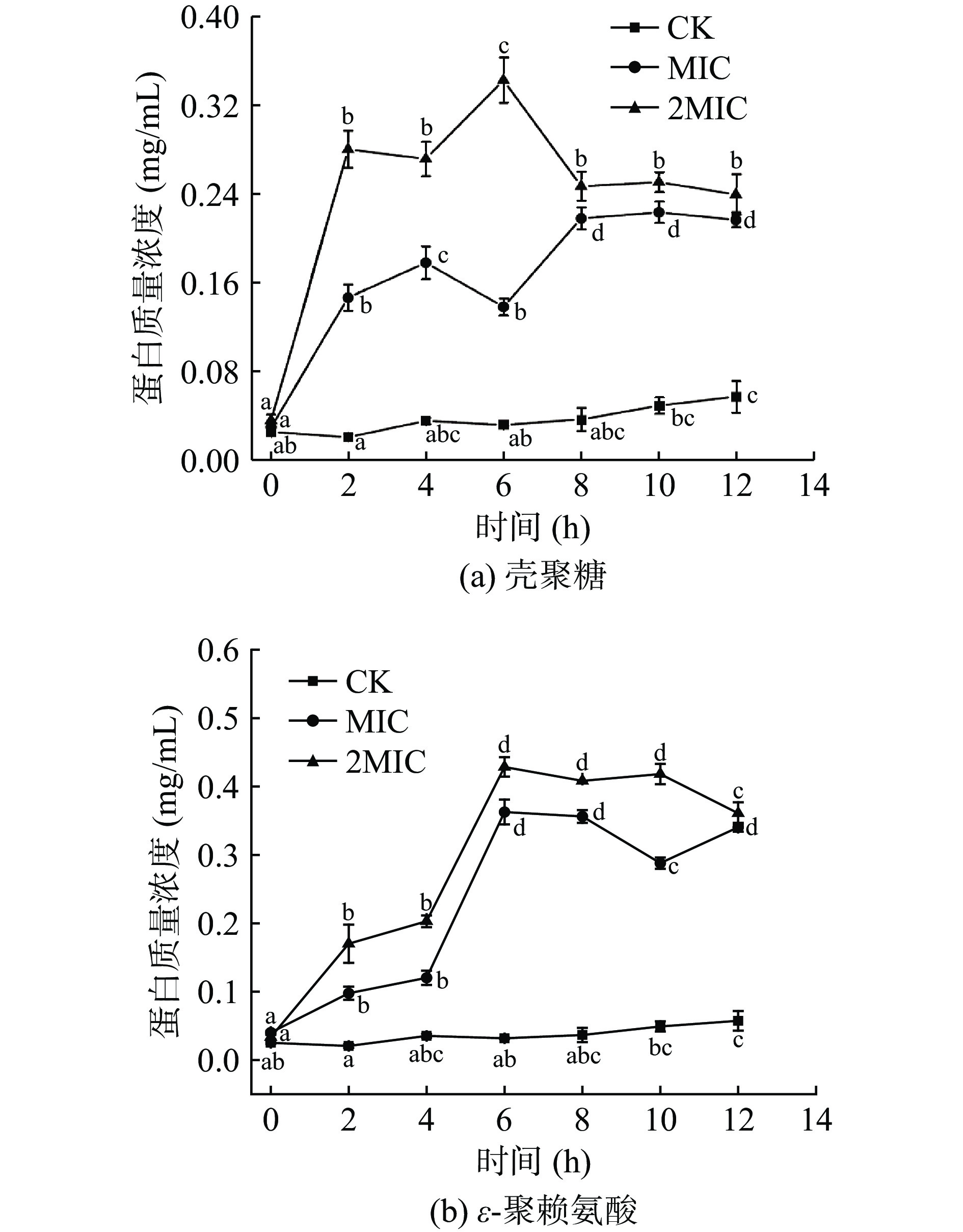
由图4可知,CK组中胞外蛋白质量浓度趋于平稳,且均低于MIC组和2MIC组的胞外蛋白质量浓度,说明壳聚糖和ε-聚赖氨酸具有损伤细胞,破坏细胞的完整性,导致胞内蛋白质大量外泄的效果。0~2 h内,壳聚糖作用菌液后的胞外蛋白质量浓度显著增加(P<0.05),4 h后有轻微波动, 2MIC组的壳聚糖在6 h有最大值为0.344 mg/mL。而ε-聚赖氨酸的MIC组和2MIC组是在4 h后胞外蛋白质量浓度迅速增加,2MIC组的ε-聚赖氨酸在6 h有最大值为0.4287 mg/mL,随后趋于稳定。两者相比之下,壳聚糖抑菌的速度远远大于ε-聚赖氨酸,可明显抑制肉食杆菌的生长繁殖。细胞膜通透性增加,胞内蛋白外泄,引起胞外蛋白迅速增加,且高浓度壳聚糖和ε-聚赖氨酸对肉食杆菌蛋白质泄漏影响更剧烈,可能导致菌体内可利用的蛋白质较少[33],菌体无法继续繁殖,甚至死亡。
2.6 壳聚糖和ε-聚赖氨酸对肉食杆菌细胞壁完整性的影响
碱性磷酸酶(AKP)主要存在于细胞壁与细胞膜间。当细胞结构被破坏时,AKP会从细胞内渗出。可通过测定AKP的活力变化来判断抑菌剂对菌体细胞壁的破坏情况[34]。由图5所示,MIC和2MIC的壳聚糖和ε-聚赖氨酸的AKP活力均显著增加(P<0.05),而CK组中的AKP活力均维持较为稳定的水平。在0~2 h时,检测到壳聚糖中MIC组和2MIC组的AKP大量渗出,随后趋于稳定,且壳聚糖2MIC组的菌液中的AKP泄漏量高于MIC组。当ε-聚赖氨酸作用于肉食杆菌时,0~2 h内,MIC组的菌液中AKP活力迅速增加且高于2MIC组,可能由于菌体受到高质量浓度的ε-聚赖氨酸后,刺激其自我修复功能,少数菌体恢复活力,继续生存繁殖[35]。壳聚糖和ε-聚赖氨酸可通过诱导细胞膜损伤而后作用在细胞壁,增加细胞壁的通透性,导致大量AKP渗漏到细胞外。壳聚糖和ε-聚赖氨酸相比之下,壳聚糖的抑菌时间更短,可能是当壳聚糖达到高浓度时,也会诱导几丁质蛋白酶,即激发某些细菌本身的几丁质蛋白酶活力,使其降解微生物自身细胞壁的几丁质,细胞壁遭到破坏,细菌被杀灭,达到抑菌作用[36]。
2.7 壳聚糖和ε-聚赖氨酸对肉食杆菌CAT活力的影响
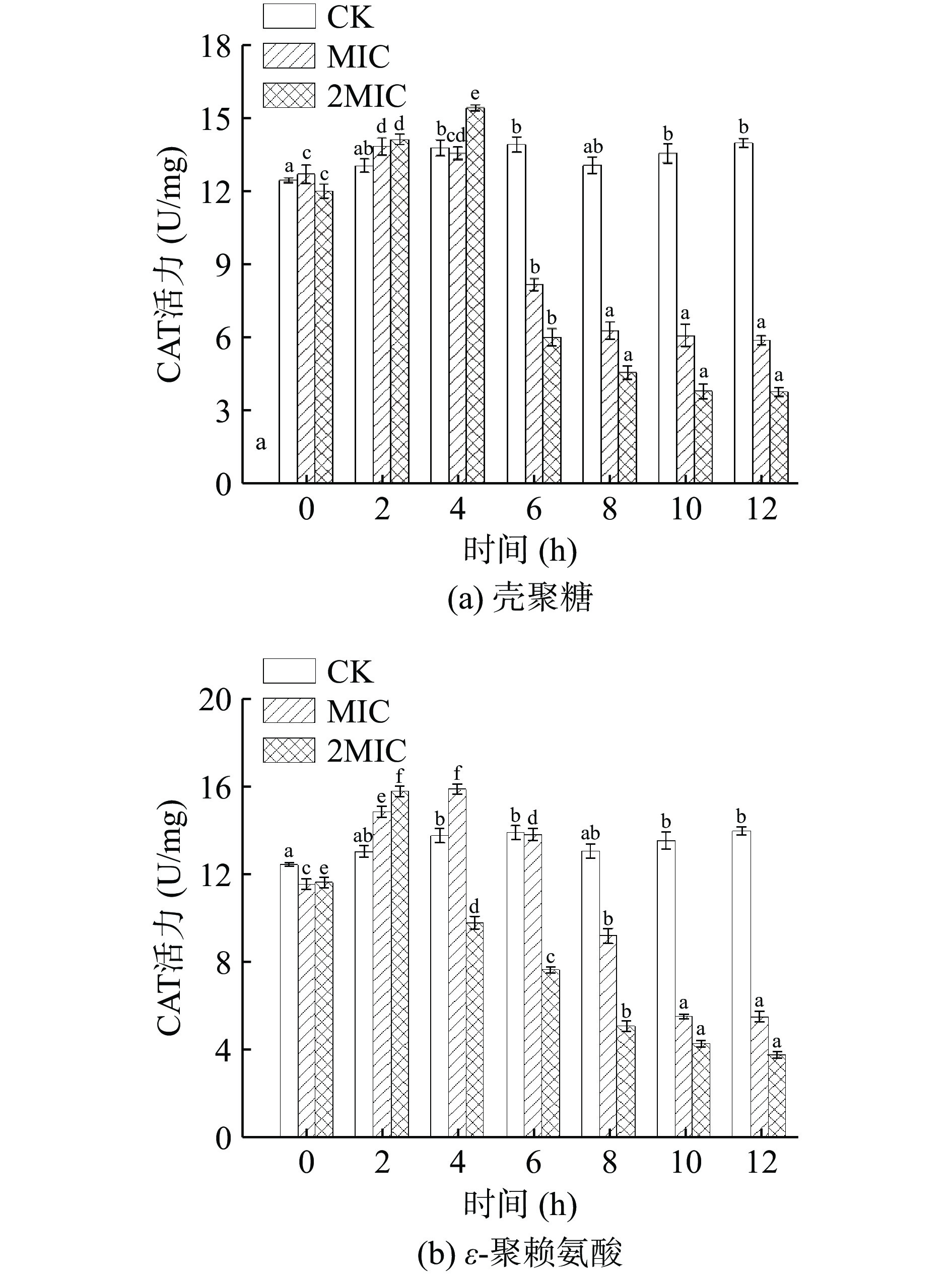
过氧化氢酶(CAT)存在于细胞的过氧化物酶体内,能将过氧化氢催化分解为氧气和水,避免机体受到氧化损伤[37]。由图6可知,CK组菌体内CAT活性较为稳定。壳聚糖和ε-聚赖氨酸处理导致肉食杆菌的CAT 活性先升高后降低。0~4 h内,壳聚糖MIC组和2MIC组的菌体CAT活力显著升高(P<0.05), CAT活力分别达到了13.58、15.44 U/mg。4 h后,菌体内CAT活性明显下降,在12 h时,CAT活力分别为5.92、3.81 U/mg。ε-聚赖氨酸MIC组和2MIC组的CAT活力迅速上升,在4 h时MIC组达到了15.93 U/mg,而2MIC组在2 h时达到最大值15.83 U/mg,随着抑菌时间的延长,在12 h时出现最小值分别为5.50、3.77 U/mg。肉食杆菌在壳聚糖和ε-聚赖氨酸的逆环境胁迫下,CAT酶活力前期呈上升趋势,可能是菌体为维持自身的氧化平衡状态,提高过氧化氧化酶活性,从而清除过量过氧化物的一种自我调节与保护作用[38]。后期呈下降趋势,可能由于高浓度的壳聚糖和ε-聚赖氨酸削弱了菌体细胞自身清除过氧化物的能力,加剧氧自由基的积累[39],细胞通透性增加,蛋白质变性严重,导致酶活性完全被抑制,造成菌体自身防御机能降低而死亡。
2.8 壳聚糖和ε-聚赖氨酸对肉食杆菌SOD活力的影响
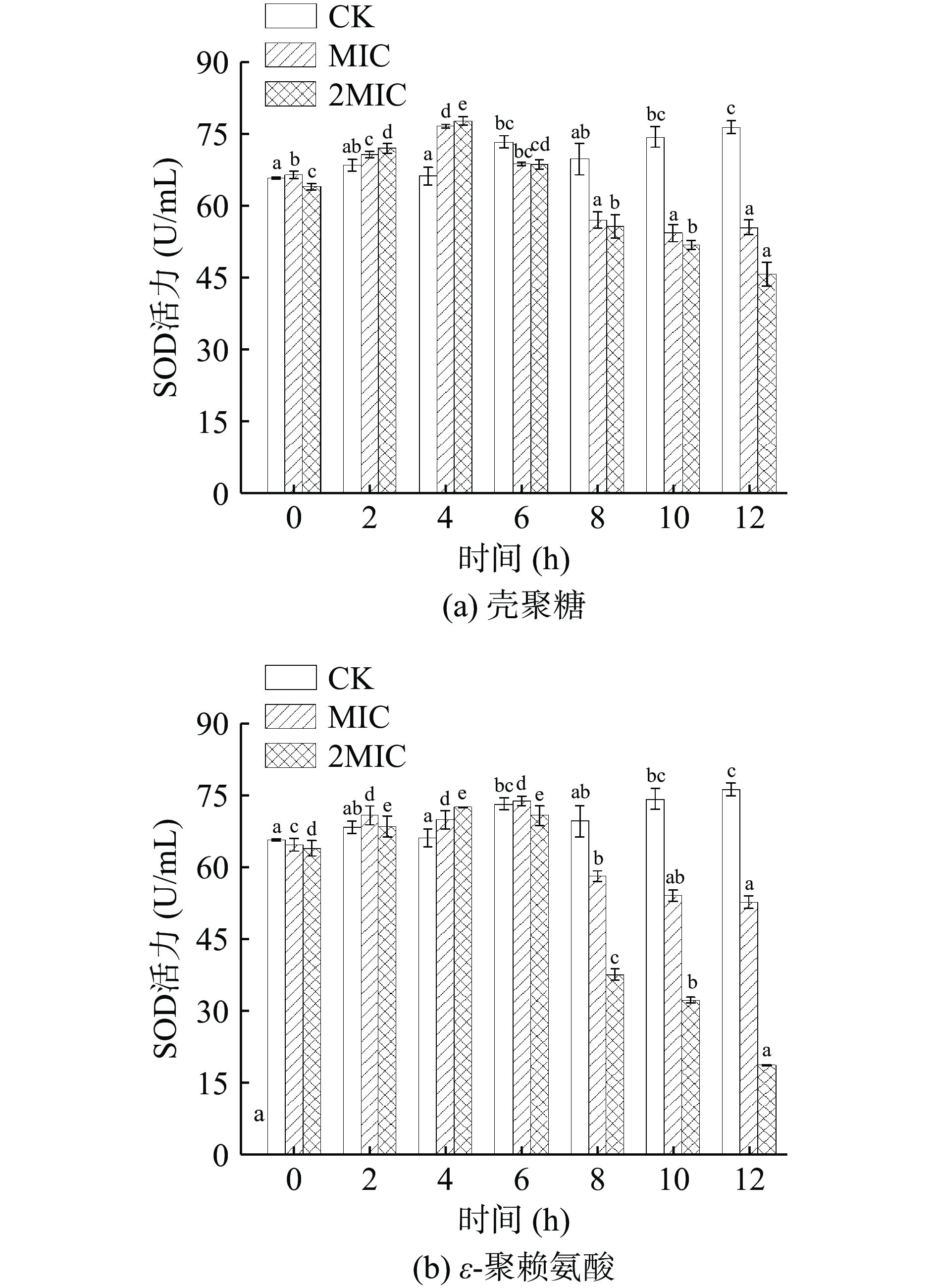
超氧化物歧化酶(SOD)是一种抗氧化金属酶,负责分解细胞体内超氧化物自由基,催化超氧阴离子(O2−)自由基歧化生成氧和过氧化氢,减少对膜的损害[40],在生物体氧化平衡中发挥关键作用。如图7所示,随着抑菌时间和抑菌剂(壳聚糖、ε-聚赖氨酸)浓度的升高,菌体细胞 SOD 活性呈现先升高,壳聚糖在4 h达到最大活性,此时MIC组和2MIC组的SOD活力分别为76.96、78.04 U/mg,4 h后呈现下降趋势。而ε-聚赖氨酸在6 h达到最大活性,此时MIC组和2MIC组的SOD活力分别为 74.35、71.17 U/mg,随后其SOD活力迅速下降。因此,前期SOD活力呈上升趋势,说明此时肉食杆菌受到壳聚糖和ε-聚赖氨酸的损害,可能胁迫作用导致细胞内超氧阴离子自由基代谢平衡被破坏[41],使SOD活力升高。随后SOD活力迅速下降,可能由于壳聚糖和ε-聚赖氨酸对菌体的影响发挥主导作用,影响了蛋白质与SOD 生成,使SOD 对超氧离子清除率下降,导致超氧阴离子自由基的积累[42],影响机体氧化应激系统的动态平衡,触发细胞凋亡或坏死性死亡。
2.9 壳聚糖和ε-聚赖氨酸对肉食杆菌细胞形态的影响
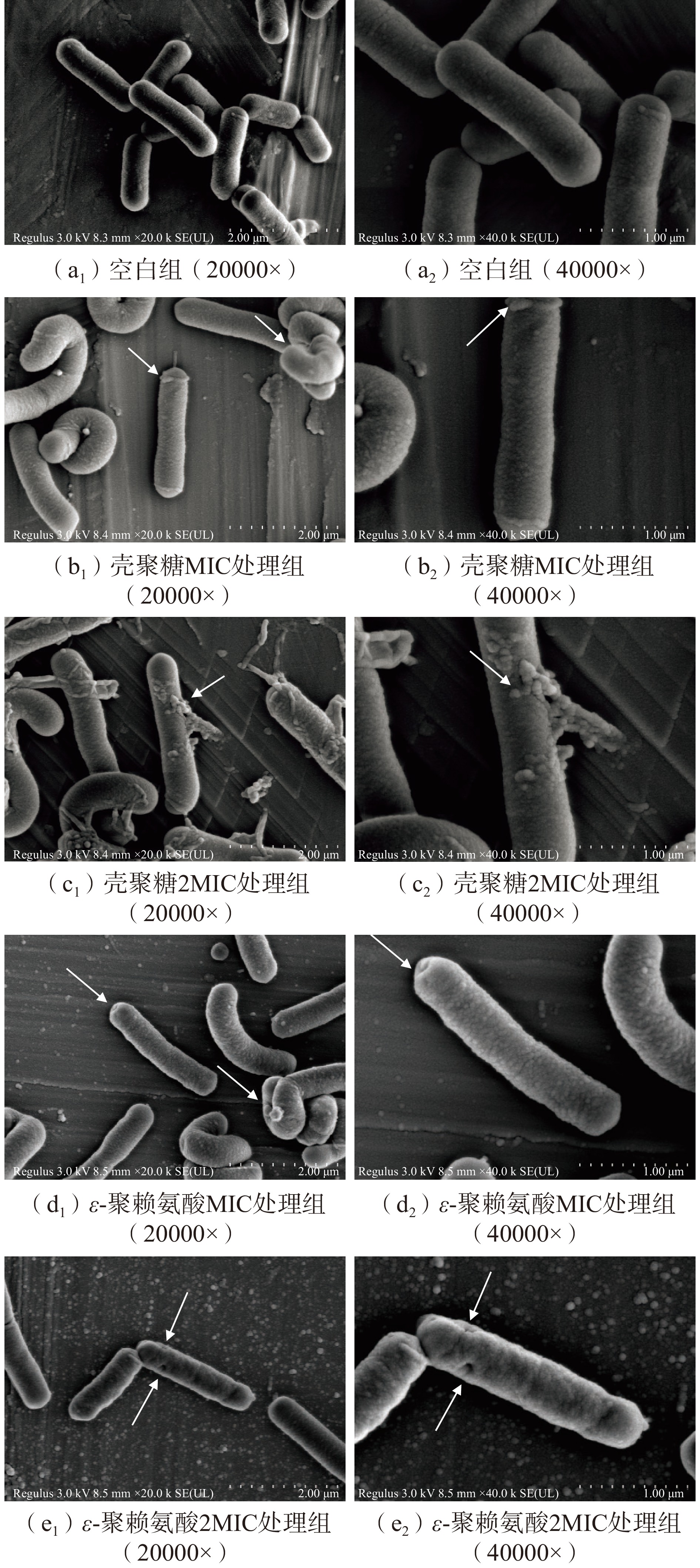
通过扫描电子显微镜对菌体进行形态观察,更直观看到壳聚糖和ε-聚赖氨酸作用后肉食杆菌的细胞形态变化,结果如图8所示。从图8中(a1)、(a2)可以看出,CK组的肉食杆菌的细胞呈正常的短棒状,细胞边界清晰,结构完整、形态饱满、表面光滑、分布均匀,没有细胞破裂或内容物溢出等现象。相比之下,壳聚糖实验组的变化则较为明显,壳聚糖的MIC组和2MIC组作用后,从图8中(b1)、(b2)可知,肉食杆菌的细胞形态发生较大变化,观察到菌体表面粗糙,细胞出现扭曲变形,图8中(c1)、(c2)的菌体变形的同时,细胞质从菌体细胞体内大量渗出,基本没有形态正常的细胞;ε-聚赖氨酸的MIC组作用肉食杆菌后(图8中(d1)、(d2)),肉食杆菌的细胞形态受到严重破坏,菌体表面粗糙,菌体发生扭曲变形,出现褶皱凹陷,ε-聚赖氨酸的2MIC组作用后(图8中(e1)、(e2)),出现褶皱和扭曲变形的现象更加明显,细胞表面粗糙不平并出现孔洞,细胞内容物外泄。细胞形态改变可能是壳聚糖和ε-聚赖氨酸破坏了细菌的细胞膜,引起胞内物质外泄所致,最终导致菌体死亡。
3. 结论
本文研究表明,壳聚糖和ε-聚赖氨酸对肉食杆菌有良好的抑菌效果,能显著减缓细菌生长速率,损伤肉食杆菌的细胞膜,使细胞内物质向外渗出,导致电导率增大,胞外核酸和蛋白含量明显增加,从而导致菌体死亡。同时,AKP活力增大,表明细胞壁完整性受损,抗氧化酶(CAT、SOD)活力降低,使菌体不能清除体内的自由基,造成菌体不可逆损伤,从而失去代谢和增殖活性。扫描电镜图显示,壳聚糖可使菌体细胞壁与细胞膜的通透性增加,核酸、蛋白质、Na+、K+等内容物发生泄漏,明显抑制细菌的正常生长,ε-聚赖氨酸能使菌体褶皱和扭曲变形的现象明显,细胞表面粗糙不平并出现孔洞,细胞内容物外泄,破坏微生物的正常生理代谢,最终导致细胞死亡。本文初步探究壳聚糖和ε-聚赖氨酸对肉食杆菌的抑菌机理,也为这两种天然抑菌剂在食品工业中的应用提供一定的理论参考。
-
表 1 壳聚糖对肉食杆菌的MIC
Table 1 MIC of chitosan against Carnobacterium divergens
抑菌剂浓度(mg/mL) 0.04885 0.0977 0.1953 0.3906 0.7813 1.5625 3.125 6.25 12.5 壳聚糖 + + − − − − − − − 注:“−”表示无菌生长,“+”表示有菌生长;表2同。 表 2 ε-聚赖氨酸对肉食杆菌的MIC
Table 2 MIC of ε-polylysine against Carnobacterium divergens
抑菌剂浓度(mg/mL) 0.00196 0.0391 0.0781 0.1563 0.3125 0.625 1.25 2.5 5 ε-聚赖氨酸 + + + − − − − − − -
[1] 李成. 腌制生食蟹糊菌相分析及防腐保鲜研究[D]. 南京:南京师范大学, 2019. [LI C. Phase analysis and preservative preservation of pickled raw crab paste[D]. Nanjing:Nanjing Normal University, 2019.] LI C. Phase analysis and preservative preservation of pickled raw crab paste[D]. Nanjing: Nanjing Normal University, 2019.
[2] 桂国弘, 杨华, 朱江群, 等. 冷鲜鸡冷藏保存过程中菌群结构变化分析[J]. 浙江农业学报,2019,31(1):47−55. [GUI G H, YANG H, ZHU J Q, et al. Study on microbial community structure in chilled chicken during cold storage[J]. Acta Agriculturae Zhejiangensis,2019,31(1):47−55.] doi: 10.3969/j.issn.1004-1524.2019.01.06 GUI G H, YANG H, ZHU J Q, et al . Study on microbial community structure in chilled chicken during cold storage[J]. Acta Agriculturae Zhejiangensis,2019 ,31 (1 ):47 −55 . doi: 10.3969/j.issn.1004-1524.2019.01.06[3] ZHANG H, ZHENG Y, LI R. Effects of chitosan-based coatings incorporated with varepsilon-polylysine and ascorbic acid on the shelf-life of pork[J]. Food Chemistry,2022,390:133206. doi: 10.1016/j.foodchem.2022.133206
[4] RISEH R S, WVATANKHAH M, HASSANISAADI M, et al. Chitosan-based nanocomposites as coatings and packaging materials for the postharvest improvement of agricultural product:A review[J]. Carbohydrate Polymers,2023,309:120666. doi: 10.1016/j.carbpol.2023.120666
[5] WEI X, LI Q, WU C, et al. Preparation, characterization and antibacterial mechanism of the chitosan coatings modified by Ag/ZnO microspheres[J]. Journal of the Science of Food and Agriculture,2020,100(15):5527−5538. doi: 10.1002/jsfa.10605
[6] 李典典, 刘然, 牛欣璐, 等. 壳聚糖及其复合保鲜技术在水产品抗菌保鲜中的研究进展[J]. 食品研究与开发,2022,43(22):208−214. [LI D D, LIU R, NIU X L, et al. Application of chitosan and its complex preservation technology in aquatic products:A review[J]. Food Research and Developent,2022,43(22):208−214.] doi: 10.12161/j.issn.1005-6521.2022.22.029 LI D D, LIU R, NIU X L, et al . Application of chitosan and its complex preservation technology in aquatic products: A review[J]. Food Research and Developent,2022 ,43 (22 ):208 −214 . doi: 10.12161/j.issn.1005-6521.2022.22.029[7] XIANG J, DABBOUR M, GAO X, et al. Influence of low-intensity ultrasound on ε-polylysine production:Intracellular ATP and key biosynthesis enzymes during Streptomyces albulus fermentation[J]. Foods,2022,11(21):3525. doi: 10.3390/foods11213525
[8] HOU Y, WANG F, TAN Z, et al. Antifungal mechanisms of ε-poly-L-lysine with different molecular weights on Saccharomyces cerevisiae[J]. The Korean Journal of Chemical Engineering,2020,37(3):482−492. doi: 10.1007/s11814-019-0466-9
[9] WEI M, GE Y, LI C, et al. Antifungal activity of ε-poly-L-lysine on Trichothecium roseum in vitro and its mechanisms[J]. Physiological and Molecular Plant Pathology,2018,103:23−27. doi: 10.1016/j.pmpp.2018.04.005
[10] 王志琦. 阚疃板鸡菌相分析及保鲜技术研究[D]. 合肥:合肥工业大学, 2022. [WANG Z Q. Study on microbial diversity analysis and fresh-keeping technology of Kantuan-sliced chicken[D]. Hefei:Hefei University of Technology, 2022.] WANG Z Q. Study on microbial diversity analysis and fresh-keeping technology of Kantuan-sliced chicken[D]. Hefei: Hefei University of Technology, 2022.
[11] 李新福. 培根加工及贮藏过程中腐败菌变化、鉴定及控制[D]. 无锡:江南大学, 2019. [LI X F. Changes, identification and control of spoilage microorganisms during bacon processing and storage[D]. Wuxi:Jiangnan University, 2019.] LI X F. Changes, identification and control of spoilage microorganisms during bacon processing and storage[D]. Wuxi: Jiangnan University, 2019.
[12] HALKAI K R, HALKAI R, PATIL S, et al. Evaluation of cytotoxic effects of fungal origin nanosilver particles on oral cancer cell lines:An in vitro:study[J]. Journal of Cancer Research and Therapeutics,2022,18(1):240−244. doi: 10.4103/jcrt.JCRT_1308_20
[13] BELANGER C R, HANCOCK R. Testing physiologically relevant conditions in minimal inhibitory concentration assays[J]. Nature Protocols,2021,16(8):3761−3774. doi: 10.1038/s41596-021-00572-8
[14] WANG X, XIONG L, YU W, et al. Evaluation of piperacillin/sulbactam, piperacillin/tazobactam and cefoperazone/sulbactam dosages in gram-negative bacterial bloodstream infections by Monte Carlo simulation[J]. Antibiotics,2023,12(2):363. doi: 10.3390/antibiotics12020363
[15] 李兆亭, 陈文学, 韩迎洁, 等. 胡椒油中萜类化合物对单增李斯特菌抑菌机理及在冷鲜肉中的应用[J]. 食品工业科技,2019,40(19):89−93. [LI Z T, CHEN W X, HAN Y J, et al. Antibacterial mechanism of terpenoids in pepper oil against Listeria monocytogenes and its application in cold meat[J]. Science and Technology of Food Industry,2019,40(19):89−93.] doi: 10.13386/j.issn1002-0306.2019.19.015 LI Z T, CHEN W X, HAN Y J, et al . Antibacterial mechanism of terpenoids in pepper oil against Listeria monocytogenes and its application in cold meat[J]. Science and Technology of Food Industry,2019 ,40 (19 ):89 −93 . doi: 10.13386/j.issn1002-0306.2019.19.015[16] 蓝蔚青, 张楠楠, 陈梦玲, 等. ε-聚赖氨酸对腐生葡萄球菌细胞结构与能量代谢的影响[J]. 食品科学,2020,41(23):56−62. [LAN W Q, ZHANG N N, CHEN M L, et al. Effect of ε-polylysine on cell structure and energy metabolism of Saprophytic staphylococcus[J]. Food Science,2020,41(23):56−62.] doi: 10.7506/spkx1002-6630-20191112-155 LAN W Q, ZHANG N N, CHEN M L, et al . Effect of ε-polylysine on cell structure and energy metabolism of Saprophytic staphylococcus[J]. Food Science,2020 ,41 (23 ):56 −62 . doi: 10.7506/spkx1002-6630-20191112-155[17] BABII C, BAHRIN L G, NEAGU A N, et al. Antibacterial activity and proposed action mechanism of a new class of synthetic tricyclic flavonoids[J]. Journal of Applied Microbiology,2016,120(3):630−637. doi: 10.1111/jam.13048
[18] LIANG C, HUANG S, GENG Y, et al. A study on the antibacterial mechanism of thymol against Aeromonas hydrophila in vitro[J]. Aquaculture International,2022,30(1):115−129. doi: 10.1007/s10499-021-00789-0
[19] 梅佳林, 李婷婷, 张星晖, 等. 芳樟醇对三文鱼源莓实假单胞菌的抑菌机理[J]. 食品科学,2022,43(9):199−206. [MEI J L, LI T T, ZHANG X H, et al. Antibacterial mechanism of linalool against Pseudomonas fragi from salmon[J]. Food Science,2022,43(9):199−206.] MEI J L, LI T T, ZHANG X H, et al . Antibacterial mechanism of linalool against Pseudomonas fragi from salmon[J]. Food Science,2022 ,43 (9 ):199 −206 .[20] STEC A, JONCA J, WALERON K, et al. Quality control of bacterial extracellular vesicles with total protein content assay, nanoparticles tracking analysis, and capillary electrophoresis[J]. International Journal of Molecular Sciences,2022,23(8):4347. doi: 10.3390/ijms23084347
[21] ZHAO X, LAN W, YANG X, et al. Inactivation effect and protective barriers damage caused to Shewanella putrefaciens by stable chlorine dioxide combined with slightly acidic electrolyzed water[J]. Journal of Food Processing and Preservation,2022,46(8):e16775.
[22] 张亮亮. 山楂不同提取物的成分分析、生物活性及抑菌机理研究[D]. 太原:山西师范大学, 2020. [ZHANG L L. Study on component analysis, biological activity and antibacterial mechanism of different extracts from hawthorn[D]. Taiyuan:Shanxi Normal University, 2020.] ZHANG L L. Study on component analysis, biological activity and antibacterial mechanism of different extracts from hawthorn[D]. Taiyuan: Shanxi Normal University, 2020.
[23] MOTAVALLIHAGHI S, KHODADADI I, GOUDARZI F, et al. The role of Acanthamoeba castellanii (T4 genotype) antioxidant enzymes in parasite survival under H2O2-induced oxidative stress[J]. Parasitology International,2022,87:102523. doi: 10.1016/j.parint.2021.102523
[24] 张莲娇. 超声与百里香油纳米乳液协同杀菌机制及其初步应用研究[D]. 杭州:浙江大学, 2021. [ZHANG L J. Synergistic antibacterial mechanism of ultrasonication with thyme essential oil nanoemulsion and their application[D]. Hangzhou:Zhejiang University, 2021.] ZHANG L J. Synergistic antibacterial mechanism of ultrasonication with thyme essential oil nanoemulsion and their application[D]. Hangzhou: Zhejiang University, 2021.
[25] 肖媛, 潘兆平, 尹春晓, 等. ε-聚赖氨酸对柑橘酸腐菌的抑菌活性及作用机制[J]. 食品科学,2020,41(19):221−229. [XIAO R, PAN Y P, YIN C X, et al. Antifungal activity and mechanism of ε-polylysine against Geotrichum citri-aurantii[J]. Food Science,2020,41(19):221−229.] XIAO R, PAN Y P, YIN C X, et al . Antifungal activity and mechanism of ε-polylysine against Geotrichum citri-aurantii[J]. Food Science,2020 ,41 (19 ):221 −229 .[26] 李远颂, 何荣荣, 蔡佳欣, 等. 芳樟醇对莓实假单胞菌的抑菌活性及机制[J]. 食品科学技术学报,2022,40(4):55−63. [LI Y S, HE R R, CAI J X, et al. Antibacterial activity and mechanism of linalool against Pseudomonas fragi[J]. Journal of Food Science and Technology,2022,40(4):55−63.] doi: 10.12301/spxb202100543 LI Y S, HE R R, CAI J X, et al . Antibacterial activity and mechanism of linalool against Pseudomonas fragi[J]. Journal of Food Science and Technology,2022 ,40 (4 ):55 −63 . doi: 10.12301/spxb202100543[27] 岳明, 阿迪拉·阿布都热西提, 尼格尔热依·亚迪卡尔, 等. 洋甘菊残渣抑菌活性及作用研究[J]. 食品研究与开发,2023,44(4):36−42. [YUE M, ADILA A, NIGARAY Y, et al. Study on the antibacterial activity and action of chamomile residues[J]. Food Research and Development,2023,44(4):36−42.] doi: 10.12161/j.issn.1005-6521.2023.04.006 YUE M, ADILA A, NIGARAY Y, et al . Study on the antibacterial activity and action of chamomile residues[J]. Food Research and Development,2023 ,44 (4 ):36 −42 . doi: 10.12161/j.issn.1005-6521.2023.04.006[28] XIONG X, LI X, ZHU Z, et al. Antibacterial and alkali-responsive cationic waterborne polyurethane based on modification of aloe emodin[J]. Chemical Research in Chinese Universities,2023,39(2):266−275. doi: 10.1007/s40242-022-2179-6
[29] LAN W, ZHANG N, LIU S, et al. ε-Polylysine inhibits Shewanella putrefaciens with membrane disruption and cell damage[J]. Molecules,2019,24(20):3727. doi: 10.3390/molecules24203727
[30] LIU F, LIU Y, SUN Z, et al. Preparation and antibacterial properties of ε-polylysine-containing gelatin/chitosan nanofiber films[J]. International Journal of Biological Macromolecules,2020,164:3376−3387. doi: 10.1016/j.ijbiomac.2020.08.152
[31] DIAO W, HU Q, ZHANG H, et al. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel ( Foeniculum vulgare Mill.)[J]. Food Control,2014,35(1):109−116. doi: 10.1016/j.foodcont.2013.06.056
[32] 舒琴. 甘露糖赤藓糖醇脂对食源性致病菌浮游态和生物膜态的抑制机理及其应用研究[D]. 杭州:浙江大学, 2022. [SHU Q. Study on the antimicrobial mechanism of mannosylerythritol lipid-A against planktonic cells and biofilm of food-borne pathogens and its application[D]. Hangzhou:Zhejiang University, 2022.] SHU Q. Study on the antimicrobial mechanism of mannosylerythritol lipid-A against planktonic cells and biofilm of food-borne pathogens and its application[D]. Hangzhou: Zhejiang University, 2022.
[33] 朱力, 王恒樑, 黄培堂. 蛋白质组学在细菌应激反应研究中的应用[J]. 生物技术通讯,2007,18(3):511−514. [ZHU L, WANG H L, HUANG P T. Screening of cholesterol-lowering lactic acid bacteria and synergic effect with Chinese herbal medicines[J]. Letters in Biotechnology,2007,18(3):511−514.] doi: 10.3969/j.issn.1009-0002.2007.03.043 ZHU L, WANG H L, HUANG P T . Screening of cholesterol-lowering lactic acid bacteria and synergic effect with Chinese herbal medicines[J]. Letters in Biotechnology,2007 ,18 (3 ):511 −514 . doi: 10.3969/j.issn.1009-0002.2007.03.043[34] SHENG J, LIU D, JING L, et al. Striatisporolide A, a butenolide metabolite from Athyrium multidentatum (Doll.) Ching, as a potential antibacterial agent[J]. Molecular Medicine Reports,2019,20(1):198−204.
[35] 杨盈. 拟穴青蟹新型抗菌肽Scyreprocin的发现与功能研究及其与SCY2在精子顶体反应中的关键作用[D]. 厦门:厦门大学, 2020. [YANG Y. Discovery and functional studies of a novel antimicrobial peptide Scyreprocin and its crucial role with SCY2 in spermacrosome reaction of mud crab Scylla paramamosain[D]. Xiamen:Xiamen University, 2020.] YANG Y. Discovery and functional studies of a novel antimicrobial peptide Scyreprocin and its crucial role with SCY2 in spermacrosome reaction of mud crab Scylla paramamosain[D]. Xiamen: Xiamen University, 2020.
[36] 陈佳新, 陈倩, 孔保华. 壳聚糖的保鲜机制及其在肉与肉类制品保鲜中应用的研究进展[J]. 肉类研究,2016,30(10):35−39. [CHEN J X, CHEN Q, KONG B H. Preservative mechanism of chitosan and its application in meat products[J]. Research on Rabbit Meat Products,2016,30(10):35−39.] doi: 10.15922/j.cnki.rlyj.2016.10.007 CHEN J X, CHEN Q, KONG B H . Preservative mechanism of chitosan and its application in meat products[J]. Research on Rabbit Meat Products,2016 ,30 (10 ):35 −39 . doi: 10.15922/j.cnki.rlyj.2016.10.007[37] BARREIRO D S, OLIVERIA R N S, PAULETA S R. Bacterial peroxidases-multivalent enzymes that enable the use of hydrogen peroxide for microaerobic and anaerobic proliferation[J]. Coordination Chemistry Reviews,2023,485:215114. doi: 10.1016/j.ccr.2023.215114
[38] ZWICK J V, NOBLE S, ELLAICY Y K, et al. AhpA is a peroxidase expressed during biofilm formation in Bacillus subtilis[J]. Microbiologyopen,2017,6(1):e403.
[39] JIANG Z, NI L, LI X, et al. Mechanistic insight into the inhibitory effect of artemisinin sustained-release inhibitors with different particle sizes on Microcystis aeruginosa[J]. Environmental Science and Pollution Research International,2022,29(58):87545−87554. doi: 10.1007/s11356-022-21534-x
[40] MONTLLOR-ALBALATE C, COLIN A E, CHANDRASEKHARAN B, et al. Extra-mitochondrial Cu/Zn superoxide dismutase (Sod1) is dispensable for protection against oxidative stress but mediates peroxide signaling in Saccharomyces cerevisiae[J]. Redox Biology,2019,21:101064. doi: 10.1016/j.redox.2018.11.022
[41] ZHANG H, HATOKO M, YIN D, et al. Antibacterial activity and biocompatibility of nanoporous titanium doped with silver nanoparticles and coated with n-acetyl cysteine[J]. Journal of Hard Tissue Biology,2018,27(4):351−358. doi: 10.2485/jhtb.27.351
[42] 郭崇婷. 黄檗果实精油抑菌活性及机理研究[D]. 沈阳:沈阳农业大学, 2016. [GUO C T. Research on inhibition action and antibacterial mechanism of Phellodendron fruit oils[D]. Shenyang:Shenyang Agricultural University, 2016.] GUO C T. Research on inhibition action and antibacterial mechanism of Phellodendron fruit oils[D]. Shenyang: Shenyang Agricultural University, 2016.
-
期刊类型引用(2)
1. 陈燕雨. 高光谱成像技术在食品掺假检测中的应用研究综述. 食品安全导刊. 2025(03): 144-147 .  百度学术
百度学术
2. 王冬,栾云霞,王欣然,贾文珅. 近红外光谱无损分析肉类品质的研究进展. 肉类研究. 2024(05): 61-70 .  百度学术
百度学术
其他类型引用(3)





 下载:
下载:

 下载:
下载:
