Determination of 21 Triazole Fungicides in Fruits and Vegetables by Lipophilicity-matched Chromatographic Separation-Ultra Performance Liquid Chromatography-tandem Mass Spectrometry
-
摘要: 为了提高农药残留的色谱分离效率和降低基质效应,本研究提出亲脂性匹配色谱分离,选取三唑类杀菌剂为研究对象,建立了果蔬中21种三唑类杀菌剂的超高效液相色谱-串联质谱(Ultra performance liquid chromatography-tandem mass spectrometry,UPLC-MS/MS)检测方法。样品经乙腈提取,盐析分相,分散固相萃取净化,选用与三唑类杀菌剂具有相近亲脂性的色谱柱进行分离,探究不同亲脂性烷基键合相对基质效应、回收率以及三唑杀菌剂和基质组分色谱分离的影响。结果表明,亲脂性匹配色谱分离能够提高色谱分离效率,改善三唑杀菌剂和基质组分的色谱分离,21种三唑杀菌剂的基质效应为−8.3%~4.7%,在5~250 μg/L浓度范围内线性关系良好,决定系数R2≥0.999,平均回收率为91.4%~108.1%,定量限为0.5~3.5 μg/kg。该检测方法能够有效降低基质效应,使用溶剂校准曲线进行定量即可获得满意的回收率,显著提高了检测效率,具有简便、准确、灵敏度高等特点,适用于果蔬中三唑杀菌剂的检测。所述亲脂性匹配色谱分离,为农药残留的液相色谱-串联质谱法分析中液相色谱柱的选择和基质效应的降低提供了方法参考。
-
关键词:
- 亲脂性匹配色谱分离 /
- 基质效应 /
- 三唑类杀菌剂 /
- 分散固相萃取 /
- 超高效液相色谱-串联质谱
Abstract: In order to improve the chromatographic separation efficiency of pesticide residues and reduce matrix effects, a lipophilicity-matched chromatographic separation strategy was proposed in this study. The triazole fungicides in fruits and vegetables were selected as a case study to establish the analytical method of 21 triazole fungicides with ultra performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS). The samples were extracted by acetonitrile, followed by salting out and cleaned-up with dispersive solid phase extraction, then separated on chromatographic column with similar lipophilicity to triazole fungicides. The effects of alkyl bonded phase with different lipophilicity on the matrix effects, recoveries and chromatographic separation of triazole fungicides and matrix components were explored. The results showed that this strategy could improve the chromatographic separation of triazole fungicides and matrix components. And 21 triazole fungicides had matrix effects within −8.3%~4.7% and good linear relationship within 5~250 μg/L, the determination coefficient R2≥0.999. The average recoveries ranged from 91.4% to 108.1%, while the limits of quantitation were in the range of 0.5~3.5 μg/kg. This method could effectively reduce matrix effects and provide satisfactory recoveries using solvent calibration for quantification, which could significantly improve the detection efficiency. The proposed method was simple, accurate and sensitive, and suitable for determination of triazole fungicides in fruits and vegetables. This strategy could provide a method reference for column selection and matrix effect reduction in liquid chromatography-tandem mass spectrometry analysis. -
高效液相色谱-电喷雾电离-串联质谱法(High performance liquid chromatography-electrospray ionization tandem mass spectrometry,HPLC-ESI-MS/MS)具有高的灵敏度和特异性,广泛应用于农药残留的定性定量分析[1−3]。HPLC-ESI-MS/MS在复杂基质如食品的分析过程中通常会出现基质效应(Matrix effects,MEs),严重影响分析方法的准确度、灵敏度和精密度。与目标分析物从色谱柱上共洗脱的干扰基质组分会影响目标分析物的离子化效率,引发“离子增强”或“离子抑制”作用,进而导致基质效应的产生[4−5]。
目前降低基质效应的方法主要有同位素内标法[6−8]、空白基质匹配校准法[9−10]、样品稀释[11−13]和样品前处理[14−15]等。其中,空白基质匹配校准法是目前最常用的补偿基质效应的方法,广泛应用于农药残留的测定,但对于不同的样品需要分别配制相应的空白基质标准溶液,显著增加了检测工作量[14,16],且多农残测定时,多种农药同时阴性的样品也不易获得。此外,样品前处理是一种有效的降低基质效应的方法,能够选择性除去基质组分,不同的方法如固相萃取[17−18]、液液微萃取[19]以及分散固相萃取[20−22]等已被用于样品前处理。但是,前处理过程在去除基质组分的同时,分析物也可能会受到损失,因此,样品前处理通常只能除去部分基质组分[23],需要通过液相色谱分离过程进一步分离目标分析物和基质组分,减少分析物和基质组分的共洗脱,降低基质效应[24]。然而,目前仍缺少能够提高农药残留色谱分离效率的有效策略,因而有必要发展一种新的液相色谱分离方法,用以改善农药和基质组分的色谱分离,降低基质效应。
大部分农药通常使用反相的烷基键合相色谱柱进行分离,分离过程主要依据不同化合物与烷基键合相之间疏水相互作用强度的差异来实现[25−26],色谱分离与这些化合物和烷基键合相的亲脂性密切相关。目前农药残留液质分析通常使用C18柱分离[7,22,27],但对于很多农药其色谱分离效果不理想,仍然存在显著的基质效应,这可能是由于其键合的十八烷基是高度亲脂的,其亲脂性远高于大部分农药。基于此,本研究提出亲脂性匹配色谱分离,作为一种有效的液相色谱分离策略,用以提高农药残留的色谱分离效率,改善农药和基质组分的色谱分离。本研究选取三唑类杀菌剂为研究对象,采用分散固相萃取净化,选用与三唑杀菌剂具有相近亲脂性的色谱柱进行分离,建立果蔬中21种三唑杀菌剂的UPLC-MS/MS检测方法,探究不同亲脂性烷基键合相对三唑杀菌剂和基质组分色谱分离的影响,为农药残留的UPLC-MS/MS分析提供方法参考。
1. 材料与方法
1.1 材料与仪器
实验所用橙子、黄瓜、香蕉和苹果 购自本地农贸市场;乙腈、甲醇 色谱纯,美国Honeywell公司;甲酸 色谱纯,国药集团化学试剂有限公司;QuEChERS提取盐包(4 g无水硫酸镁、1 g氯化钠、1 g柠檬酸钠和0.5 g柠檬酸氢二钠)、聚四氟乙烯微孔滤膜(0.22 μm)、石墨化碳黑(GCB)(38~120 μm)、N-丙基乙二胺(PSA)(40~63 μm) 上海安谱实验科技股份有限公司;标准物质亚胺唑(97.5%)、氯氟醚菌唑(99%) 德国Dr. Ehrenstorfer公司;标准物质粉唑醇(99.5%)、三唑醇(98.2%)、三唑酮(98.4%)、环丙唑醇(98.0%)、腈菌唑(99.6%)、氟环唑(98.0%)、灭菌唑(97.8%)、四氟醚唑(99.9%)、腈苯唑(98.5%)、氟硅唑(99.1%)、戊菌唑(99.1%)、戊唑醇(99.3%)、联苯三唑醇(1000 μg/mL)、叶菌唑(97.9%)、己唑醇(99.1%)、烯唑醇(98.0%)、丙环唑(98.5%)、苯醚甲环唑(98.3%)和种菌唑(98.8%) 购自坛墨质检科技股份有限公司。
ACQUITY UPLC-TQD超高效液相色谱-串联质谱仪(UPLC-MS/MS) 配电喷雾离子源(ESI),美国Waters公司;TG-16 高速冷冻离心机 湖南湘仪科技公司;JE1103C电子分析天平 瑞士梅特勒-托利多仪器公司;ACQUITY BEH C8色谱柱(2.1 mm×50 mm,1.7 µm)、ACQUITY BEH C18色谱柱(2.1 mm×50 mm,1.7 µm) 美国Waters公司;JYL-C022E粉碎机 九阳股份有限公司;Milli-Q超纯水机 美国Millipore公司。
1.2 实验方法
1.2.1 标准溶液配制
标准储备溶液:分别准确称取21种农药标准品各10 mg,用甲醇溶解并定容至10 mL,配制成1000 mg/L标准储备溶液,−20 ℃冰箱避光保存。
混合标准中间液:分别准确移取21种标准储备溶液各1.0 mL至100 mL容量瓶中,用乙腈稀释定容至刻度,得到10 mg/L混合标准中间液,−20 ℃冰箱避光保存。使用时用乙腈稀释成不同浓度的系列标准工作溶液,现用现配。
1.2.2 样品前处理
将样品切成小块状,放入粉碎机制成均质样品,放入密闭容器中,−18 ℃冰箱冷冻保存备用。准确称取10 g (精确至0.01 g)橙子样品至50 mL离心管中,加入10 mL乙腈后混匀,涡旋振荡1 min,加入QuEChERS提取包后立即剧烈振荡1 min,以5000 r/min离心5 min,取2 mL上清液至含有300 mg无水MgSO4、50 mg PSA和5 mg GCB的离心管中,涡旋振荡1 min,5000 r/min离心5 min,取上清液过0.22 μm PTFE滤膜,供UPLC-MS/MS测定。
1.2.3 色谱条件
色谱柱:Waters ACQUITY BEH C8色谱柱(2.1 mm×50 mm,1.7 µm);柱温:40 ℃;自动进样器温度:15 ℃;进样体积:3 μL;流速:0.2 mL/min;流动相:0.05%甲酸水溶液(A相),甲醇(B相);梯度洗脱程序见表1。
表 1 流动相梯度洗脱程序Table 1. Program of mobile phase gradient elution时间(min) A(%) B(%) 0 80 20 0.5 80 20 2.5 60 40 4.0 50 50 6.0 40 60 9.0 30 70 10.5 10 90 12 10 90 12.5 80 20 14.5 80 20 1.2.4 质谱条件
离子源:电喷雾离子源(Electron spray ionization,ESI),正离子模式;监测方式:多反应监测(Multiple reaction monitoring,MRM);毛细管电压:3.2 kV;离子源温度:100 ℃;脱溶剂温度:350 ℃;脱溶剂气N2流速550 L/h;锥孔气N2流速:50 L/h,21种杀菌剂的多反应监测质谱分析参数见表2。
表 2 21种三唑杀菌剂的多反应监测质谱分析参数Table 2. Optimized MRM parameters for the 21 triazole fungicides化合物 母离子
(m/z)子离子
(m/z)锥孔电
压(V)碰撞能量
(eV)粉唑醇 302.0 122.8*, 108.9 35 30, 31 三唑酮 294.0 68.9*, 196.9 38 21, 15 环丙唑醇 292.0 69.8*, 124.8 41 19, 26 三唑醇 296.0 69.8*, 98.9 35 22, 17 腈菌唑 289.0 69.8*, 124.9 34 20, 33 氟环唑 329.9 120.9*, 100.9 32 21, 51 灭菌唑 318.0 69.8*, 124.8 31 20, 33 四氟醚唑 371.9 158.8*, 69.9 43 26, 23 腈苯唑 337.0 125.0*, 70.0 38 29, 19 氟硅唑 316.0 165.1*, 247.1 40 27, 18 戊菌唑 284.0 158.8*, 69.8 31 23, 18 戊唑醇 308.0 124.9*, 150.8 40 32, 25 联苯三唑醇 338.2 269.1*, 251.1 30 9, 13 氯氟醚菌唑 397.9 69.8*, 181.9 50 24, 29 叶菌唑 320.0 69.8*, 124.9 37 23, 33 己唑醇 314.0 69.8*, 158.8 43 21, 30 烯唑醇 325.9 69.8*, 158.8 50 26, 25 丙环唑 341.9 68.9*, 158.8 35 23, 25 苯醚甲环唑 405.9 250.8*, 336.9 50 25, 18 种菌唑 334.0 69.8*, 124.8 36 21, 39 亚胺唑 410.9 124.8*, 170.8 48 30, 19 注:*为定量离子。 1.2.5 基质效应的评价
采用橙子、黄瓜、香蕉和苹果空白样品按照1.2.2方法进行前处理,得到空白基质溶液。分别用空白基质溶液和乙腈配制浓度为5、10、20、50、100、250 μg/L的基质匹配标准工作溶液和溶剂标准工作溶液,绘制基质匹配标准曲线和溶剂标准曲线,依据式(1)通过两种曲线斜率的比值来计算基质效应(ME)[28−29]。
ME(%)=(AB−1)×100 (1) 式中:ME为基质效应;A为基质匹配标准曲线的斜率;B为溶剂标准曲线的斜率。
当ME%≤−10%时,表现为基质抑制效应;当ME%≥10%时,则为基质增强效应;若−10%<ME%<10%,可认为基质效应不显著[30]。
1.3 数据处理
数据采集由Waters TQD MassLynx软件完成,采用仪器自带数据处理软件QuanLynx 4.1对测定结果进行定性和定量分析。加标回收试验为5次平行测定结果,以平均值±标准偏差表示。用Excel 2010进行数据分析处理,Origin 8.0绘制统计图表。
2. 结果与分析
2.1 质谱条件优化
本研究使用21种1 mg/L的三唑杀菌剂标准溶液通过针泵导入质谱仪,在正、负离子模式下分别进行扫描,结果显示21种三唑杀菌剂在电喷雾正离子模式下质谱响应更高。通过调整优化锥孔电压,使母离子响应强度达到最佳,再通过调整优化碰撞能量,使得对应的子离子响应强度达到最佳,选择受基质干扰小、相对丰度高的离子作为定量离子,得到的质谱分析参数见表2。
2.2 样品前处理
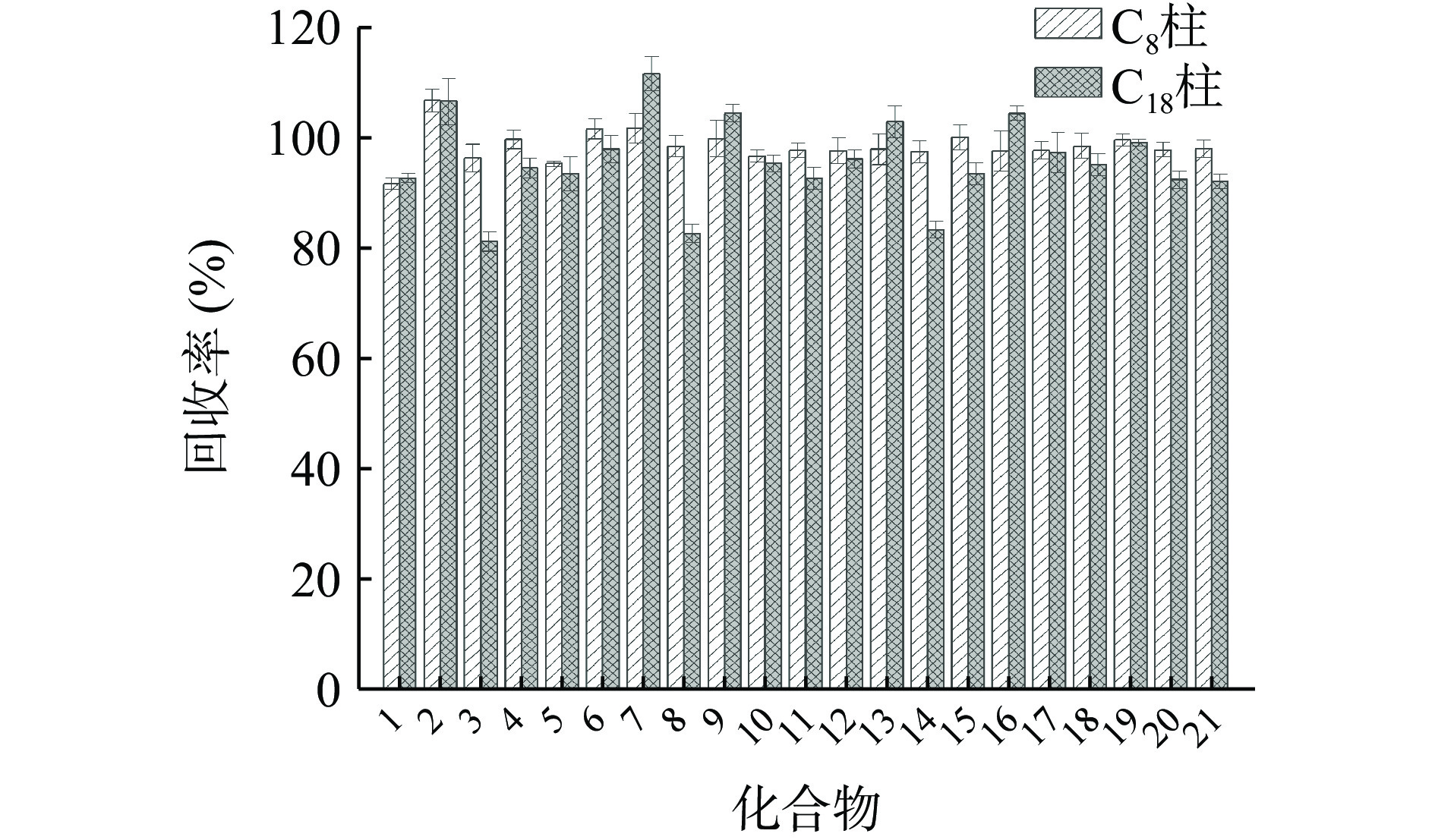
本研究选用QuEChERS方法[31−32]对果蔬样品进行前处理,该方法具有简单、快速、高效等特点,广泛应用于农药残留分析的样品前处理。果蔬样品用乙腈提取,采用柠檬酸盐缓冲体系盐析分相,离心取上清液经PSA和GCB分散固相萃取净化,去除样品提取溶液中的部分基质组分如有机酸、脂肪酸、糖类以及色素等。净化后样品溶液中仍存在其他类型的基质组分,导致显著的基质效应,由表3和图1可知,使用C18柱分离时,三唑酮、灭菌唑和氟环唑有明显的基质效应,且回收率偏低。同时,由图2空白橙子样品的全扫描色谱图可知,净化后的样品溶液中仍有多种基质组分。因而需要通过高效的色谱分离过程进一步将基质组分和目标分析物分离,减少共洗脱,从而有效降低基质效应。
表 3 21种三唑杀菌剂的保留时间和在橙子样品中的基质效应Table 3. Retention time and matrix effect for 21 triazole fungicides in orange化合物 C8柱 C18柱 基质效应(%) 保留时间(min) 基质效应(%) 保留时间(min) 粉唑醇 −8.3 5.65 −8.9 5.65 三唑酮 −3.4 7.10 −21.2 7.18 环丙唑醇 0.1 7.38 −7.9 7.51 三唑醇 4.7 7.39 1.5 7.50 腈菌唑 0.4 7.37 −7.1 7.33 氟环唑 −2.3 7.55 −14.9 7.77 灭菌唑 −0.3 7.47 −15.7 7.68 四氟醚唑 -4.5 7.90 −2.8 7.87 腈苯唑 −4.3 7.85 −4.2 8.06 氟硅唑 2.1 8.07 −3.4 8.21 戊菌唑 0.2 8.36 −5.4 8.53 戊唑醇 −0.1 8.31 −2.4 8.63 丙环唑 −3.3 8.40 −6.4 8.76 氯氟醚菌唑 2.3 8.70 2.9 9.01 联苯三唑醇 −0.6 8.79 4.5 9.21 叶菌唑 −4.2 8.69 −3.8 9.06 己唑醇 −3.4 8.83 4.2 9.05 烯唑醇 −3.2 9.18 −3.2 9.39 苯醚甲环唑 −1.8 8.99 −3.0 9.45 种菌唑 −2.4 9.55 −5.9 9.72 亚胺唑 −1.5 10.26 −8.2 10.22 2.3 亲脂性匹配色谱分离
大多数农药是非极性或低极性的,通常使用反相烷基键合相色谱柱进行分离,而农药在烷基键合相色谱柱上的保留与其亲脂性密切相关。本研究中21种三唑杀菌剂的正辛醇-水分配系数log P值为2.3~4.9[33],依据亲脂性匹配色谱分离,本研究选择与三唑杀菌剂具有相近亲脂性的C8色谱柱进行分离,C8色谱柱键合的辛烷基的log P值为4.32(辛烷)[34],与三唑杀菌剂相近。21种三唑杀菌剂的基质效应和回收率结果如表3和图1所示,所有21种三唑杀菌剂的基质效应为−8.3%~4.7%,全部处于−10%~10%范围内,基质效应不显著。采用溶剂标准工作曲线外标法进行定量,测定的回收率结果(图1)表明,21种三唑杀菌剂的平均回收率为91.8%~107.0%,具有良好的回收率。基质效应和回收率测定结果表明,该方法能够有效降低三唑杀菌剂UPLC-MS/MS分析中的基质效应,采用溶剂标准工作曲线进行定量即可获得满意的回收率。
为进一步证实亲脂性匹配色谱分离在提高农药残留色谱分离效率中的作用,本研究采用农药残留分析中最常使用的C18色谱柱作为对照,C18柱是高度亲脂的,其键合的十八烷基的log P值为9.18(十八烷)[35],远高于21种三唑杀菌剂。以0.05%甲酸水溶液和甲醇为流动相,梯度洗脱,采用溶剂标准工作曲线外标法进行定量,评价三唑杀菌剂的基质效应和回收率。结果表明(表3),三唑酮、灭菌唑和氟环唑均表现为基质抑制效应,其中仅52.4%(11种)三唑杀菌剂的基质效应处于−5%~5%范围内,而对于C8色谱柱,95.2%(20种)三唑杀菌剂的基质效应处于−5%~5%范围内。此外,回收率测定结果表明(图1),对于C18色谱柱,三唑杀菌剂的平均回收率为81.3%~111.9%。
基质效应和回收率测定结果表明,相较于高度亲脂的C18色谱柱,与三唑杀菌剂具有相近亲脂性的C8色谱柱能够更好地降低基质效应,改善回收率,表明C8色谱柱具有更高的色谱分离效率,能够有效分离三唑杀菌剂和基质组分,减少目标分析物和基质组分的共洗脱,降低基质效应。
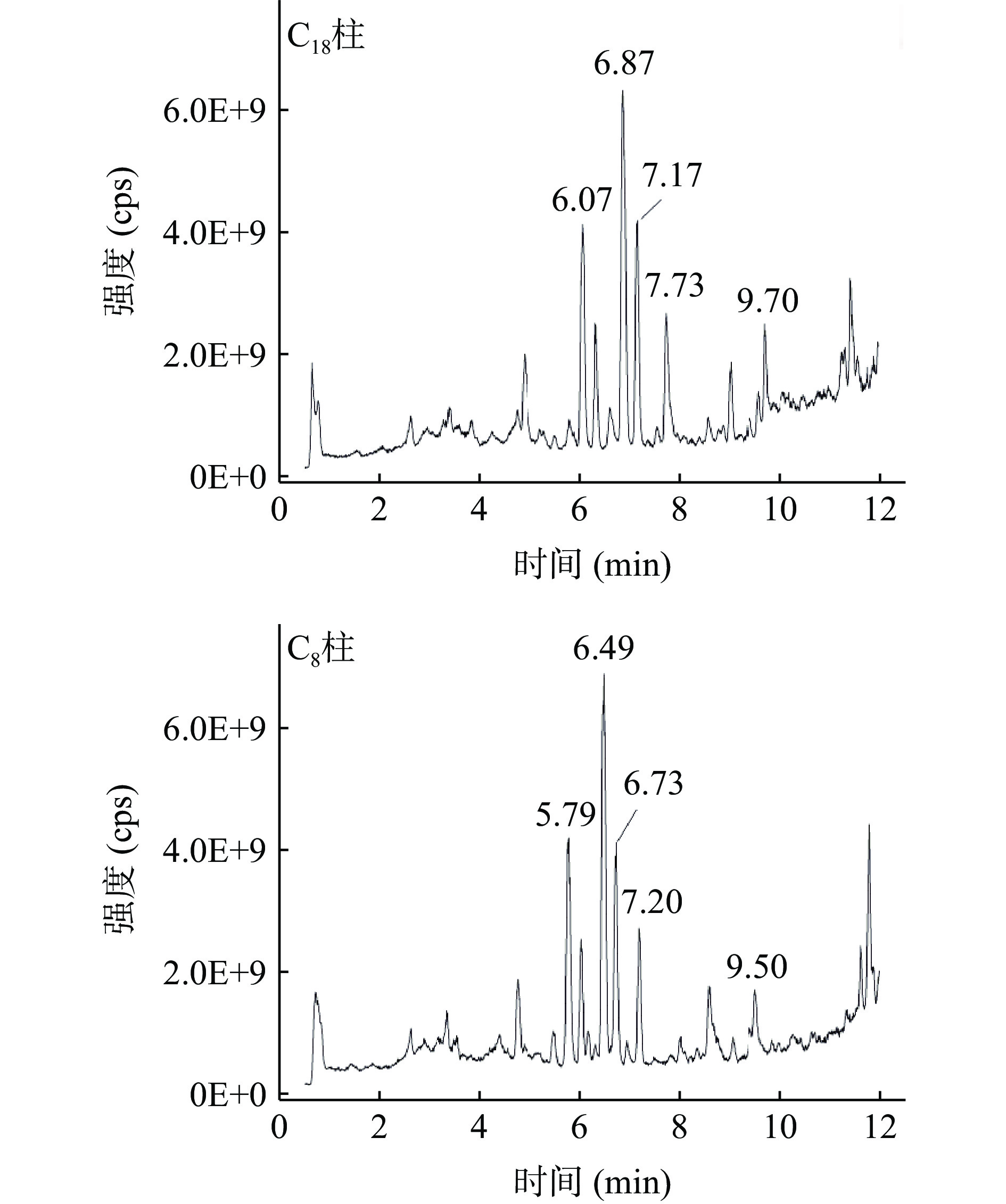
此外,本研究还通过空白橙子样品的全扫描色谱图进一步研究不同亲脂性烷基键合相色谱柱对三唑杀菌剂和基质组分的分离效果。空白橙子样品按1.2.2方法进行前处理,随后分别使用C8和C18色谱柱进行分离测定其全扫描色谱图,结果如图2所示。由图可知,对于C18柱,位于7.17 min和7.73 min的基质组分的色谱峰分别与三唑酮(7.18 min)和灭菌唑(7.68 min)、氟环唑(7.77 min)非常接近,这些基质组分和分析物从C18柱上共洗脱,导致明显的基质效应,这与表3的结果相一致,表明C18柱无法将这些分析物和基质组分有效分离,可能是由于这些基质组分和分析物与高度亲脂的十八烷基固定相之间的疏水作用大小非常接近,导致其色谱保留时间非常接近。而对于C8柱,位于6.73 min和7.20 min的基质组分的色谱峰与各分析物的保留时间有显著差别,因而各分析物没有明显的基质效应,表明C8色谱柱能够显著改善三唑杀菌剂和基质组分的色谱分离,降低基质效应,这可能是由于C8柱键合的辛烷基与三唑杀菌剂具有相近的亲脂性,基质组分和分析物与固定相之间的疏水作用大小不同,导致其色谱保留时间有显著差别。
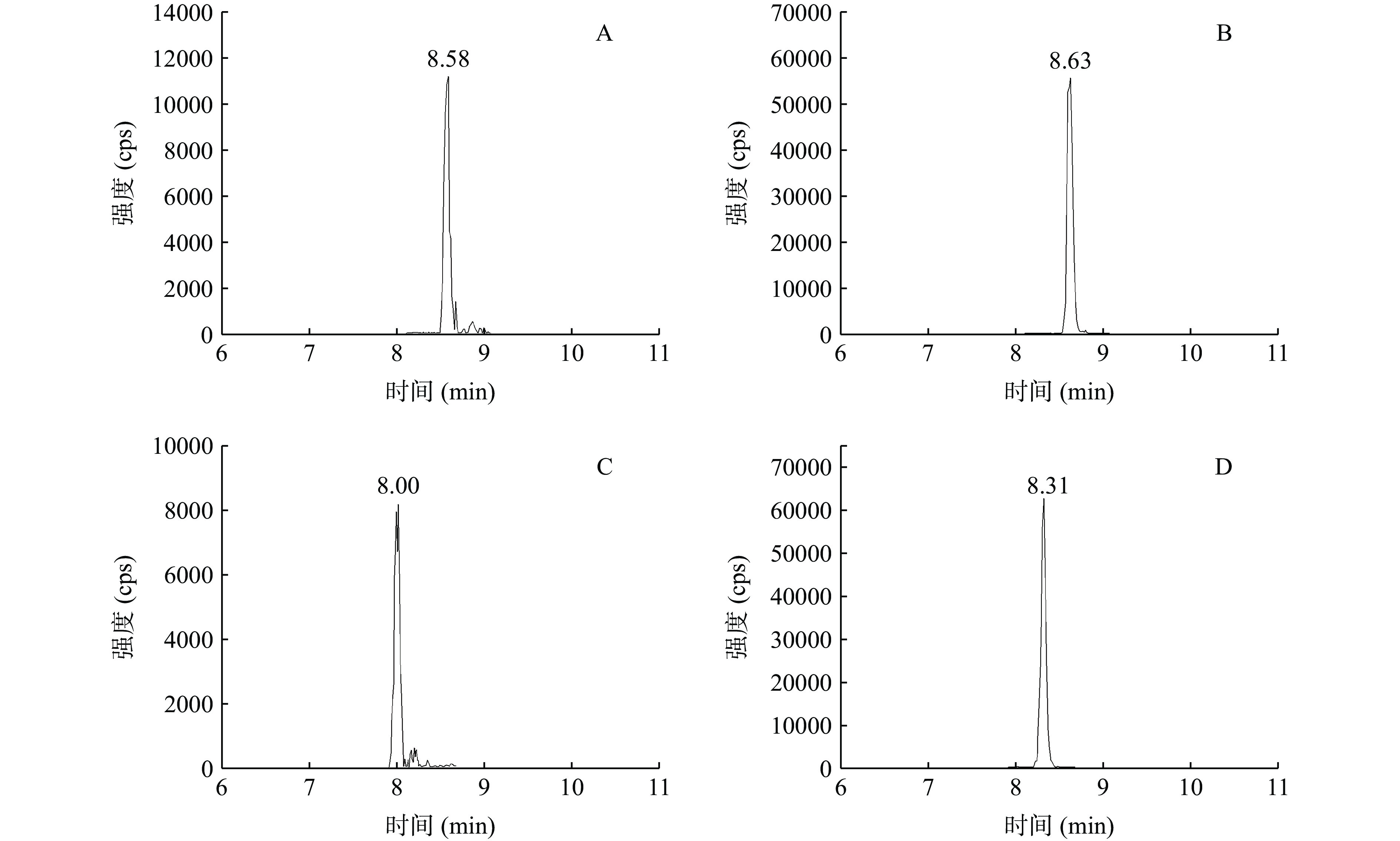
对于戊唑醇,使用C18柱分离时,其基质效应ME%为−2.4%,而平均回收率为111.9%,其基质效应和回收率之间不一致。由戊唑醇的提取离子色谱图可知,空白样品基质在8.58 min存在明显的干扰峰(图3A),与戊唑醇色谱峰(8.63 min)重叠(图3B),C18柱无法将该干扰物和戊唑醇分离,导致定量结果偏高,从而引起回收率偏高。同时,该干扰物可能对戊唑醇的离子化效率没有明显影响,使戊唑醇没有明显的基质效应。当使用C8柱分离时,该干扰物和戊唑醇的保留时间分别为8.00 min和8.31 min(图3C和3D),二者得到有效分离。这些结果也表明,C8柱具有更高的色谱分离效率,能够有效分离戊唑醇和干扰物。
上述结果证实了与三唑杀菌剂具有相近亲脂性的C8色谱柱具有更高的色谱分离效率,能够有效分离三唑杀菌剂和基质组分,将分散固相萃取和C8色谱柱分离相结合,能够有效降低果蔬中三唑杀菌剂UPLC-MS/MS测定时的基质效应,使用溶剂标准工作曲线外标法进行定量即可获得满意的回收率,显著提高了检测效率。因此,所述亲脂性匹配色谱分离是一种简单、有效的液相色谱分离策略,能够提高农药残留的色谱分离效率,降低基质效应。
2.4 方法学验证
2.4.1 方法线性范围和定量限
将混合标准中间液用乙腈进行稀释,配制成浓度为5、10、20、50、100、250 μg/L的混合标准工作液,以各分析物的质量浓度(x,μg/L)为横坐标,峰面积为纵坐标(y),绘制溶剂标准工作曲线,评价方法的线性。以定量离子信噪比(S/N)为10确定方法的定量限(Limit of quantification,LOQ)[36−37]。各分析物的溶剂校准曲线和空白橙子基质匹配校准曲线线性方程、定量限和线性范围见表4。21种三唑杀菌剂在5~250 μg/L浓度范围内线性关系良好,决定系数R2≥0.999,满足实际样品分析需要,采用10倍信噪比法确定了各分析物的定量限为0.5~3.5 μg/kg。
表 4 21种三唑杀菌剂的线性范围、定量限(LOQ)、线性方程和决定系数(R2)Table 4. Liner ranges, LOQ, regression equation and determination coefficient (R2) for 21 triazole fungicides化合物 线性范围 (µg/L) LOQ(μg/kg) 溶剂校准曲线 基质匹配校准曲线 线性方程 R2 线性方程 R2 粉唑醇 5~250 1.6 y=300.35x+33.620 0.9996 y=275.42x−17.422 0.9997 三唑酮 5~250 1.0 y=635.64x+136.53 0.9996 y=613.99x+81.598 0.9999 环丙唑醇 5~250 1.1 y=763.15x+278.65 0.9996 y=763.92x−122.85 0.9991 三唑醇 5~250 3.2 y=89.176x−61.643 0.9999 y=93.367x+51.599 0.9990 腈菌唑 5~250 0.7 y=854.80x+675.88 0.9992 y=858.10x−104.39 0.9993 氟环唑 5~250 0.8 y=927.65x+424.89 0.9999 y=905.99x−104.53 0.9995 灭菌唑 5~250 0.6 y=1257.6x+291.33 0.9995 y=1253.4x−41.490 0.9991 四氟醚唑 5~250 1.0 y=660.59x−114.96 0.9995 y=630.87x−76.457 0.9997 腈苯唑 5~250 1.0 y=384.09x+69.926 0.9999 y=367.6x+88.758 0.9997 氟硅唑 5~250 1.5 y=566.45x+270.793 0.9991 y=578.25x+140.93 0.9998 戊菌唑 5~250 1.8 y=350.60x+149.43 0.9992 y=351.32x−111.23 0.9993 戊唑醇 5~250 3.1 y=82.119x+11.768 0.9993 y=82.041x+3.023 0.9997 丙环唑 5~250 2.1 y=718.82x−790.269 0.9993 y=694.89x−332.70 0.9995 氯氟醚菌唑 5~250 0.7 y=1227.8x+881.15 0.9992 y=1256.3x−494.42 0.9992 联苯三唑醇 5~250 3.5 y=86.316x+50.360 0.9993 y=85.772x+39.333 0.9991 叶菌唑 5~250 0.6 y=1698.0x+668.40 0.9996 y=1626.8x+1046.3 0.9996 己唑醇 5~250 0.9 y=969.50x+302.88 0.9999 y=936.85x+180.38 0.9997 烯唑醇 5~250 0.9 y=955.70x+56.911 0.9996 y=925.16x−149.17 0.9997 苯醚甲环唑 5~250 1.5 y=627.38x−503.191 0.9991 y=616.03x+120.11 0.9998 种菌唑 5~250 0.5 y=1693.9x+289.57 0.9998 y=1653.3x−104.67 0.9999 亚胺唑 5~250 2.0 y=419.88x+0.462 0.9998 y=413.59−54.521 0.9993 2.4.2 准确度和精密度
通过橙子样品的加标回收实验评价该方法的准确度和精密度,目标分析物加标水平分别为10、50、200 μg/kg,每个加标水平设置5个平行样,连续测定3 d,分别计算加标回收率以及日内、日间相对标准偏差(Relative standard deviation,RSD),结果如表5所示。21种杀菌剂在三个加标水平的平均回收率为91.4%~108.1%,日内和日间精密度分别为0.7%~5.2%和1.7%~6.0%,说明该方法具有良好的准确度和精密度。
表 5 21种三唑杀菌剂的回收率和精密度Table 5. Recovery and precision for 21 triazole fungicides化合物 平均回收率(%,n=15) 日内RSD(%,n=5) 日间RSD(%,n=15) 10 50 200 10 50 200 10 50 200 μg/kg μg/kg μg/kg 粉唑醇 91.4 92.5 92.2 1.3 1.6 2.0 1.7 1.9 2.7 三唑酮 96.4 95.7 96.8 2.5 1.9 2.7 3.1 4.0 2.8 环丙唑醇 100.6 101.3 99.7 2.5 1.9 2.6 3.5 3.8 3.9 三唑醇 108.1 107.5 105.3 3.3 1.8 3.1 3.3 5.7 4.2 腈菌唑 98.5 99.7 101.1 3.8 3.2 1.6 4.6 3.7 2.3 氟环唑 98.3 97.9 100.3 2.3 1.3 1.9 4.3 2.7 4.5 灭菌唑 98.8 97.7 100.6 1.7 2.9 2.3 2.3 3.2 4.6 四氟醚唑 97.5 99.4 97.0 2.1 2.2 2.4 3.4 4.1 2.7 腈苯唑 99.1 97.3 95.7 3.6 0.7 1.7 3.8 1.8 4.5 氟硅唑 102.2 102.6 98.8 2.7 2.3 1.9 3.6 4.1 2.7 戊菌唑 99.7 98.8 97.8 3.8 1.8 2.4 3.5 2.9 1.9 戊唑醇 98.3 99.6 102.9 4.2 3.6 3.2 5.2 5.5 3.1 丙环唑 101.3 98.8 102.1 3.5 1.6 1.9 4.3 4.1 2.6 氯氟醚菌唑 98.2 101.5 103.7 3.1 1.9 2.2 3.7 3.1 2.5 联苯三唑醇 101.1 99.1 98.0 3.3 2.5 2.7 5.6 4.2 2.9 叶菌唑 101.2 97.6 98.7 2.2 2.1 1.8 3.3 4.0 3.0 己唑醇 102.0 98.3 100.5 1.9 1.1 1.5 3.6 3.0 2.1 烯唑醇 96.8 98.5 100.8 2.3 2.1 1.7 5.0 3.1 3.5 苯醚甲环唑 98.7 99.0 101.5 5.2 1.5 1.8 6.0 4.1 2.8 种菌唑 97.2 98.2 100.6 2.4 3.1 1.7 4.1 5.0 5.6 亚胺唑 101.4 99.1 99.3 1.7 2.4 2.8 3.3 3.6 4.2 2.5 其它果蔬样品的分析
为了验证该方法的实用性和普适性,采用本研究建立的方法对其他果蔬样品苹果、香蕉和黄瓜进行分析,分别评价其回收率和基质效应。结果显示(表6),三种果蔬样品的基质效应为−8.9%~9.7%,平均回收率分别是95.6%~108.9%(苹果)、102.4%~109.3%(香蕉)以及98.6%~106.8%(黄瓜),基质效应不显著,回收率良好,说明该方法对于不同果蔬样品具有普适性。
表 6 21种三唑杀菌剂在不同果蔬基质中的基质效应和加标回收率Table 6. Matrix effect and recoveries for 21 triazole fungicides in different fruits and vegetables化合物 基质效应(%) 平均回收率±相对偏差(%,n=5) 苹果 香蕉 黄瓜 苹果 香蕉 黄瓜 粉唑醇 −1.4 −2.8 1.3 104.1±1.5 103.7±1.0 100.7±0.7 三唑酮 2.5 −1.0 −2.0 108.3±2.2 109.3±1.9 99.8±2.1 环丙唑醇 −1.6 −4.2 −0.7 103.0±1.1 103.5±2.2 98.7±0.8 三唑醇 3.8 0.0 9.7 103.7±2.2 104.6±2.7 106.8±2.1 腈菌唑 −2.6 −1.0 −1.4 102.6±1.1 106.3±3.2 100.6±1.6 氟环唑 −0.8 −3.6 0.2 103.6±1.3 104.3±2.6 99.8±2.1 灭菌唑 −7.3 −1.0 −1.0 100.2±0.5 102.9±1.9 99.9±1.2 四氟醚唑 4.0 −2.2 0.9 108.9±0.8 106.2±2.6 99.3±1.8 腈苯唑 2.2 −1.0 −1.5 106.6±0.9 108.6±1.3 100.6±1.7 氟硅唑 −4.3 −1.4 −2.0 98.1±1.2 106.6±0.4 99.1±0.9 戊菌唑 5.5 −3.6 −2.5 102.2±1.2 104.4±0.8 99.1±2.0 戊唑醇 0.9 −6.5 −3.1 107.2±1.6 102.9±4.0 98.6±4.6 丙环唑 −1.5 −2.2 −1.6 105.1±0.6 105.2±1.7 99.8±1.0 氯氟醚菌唑 −2.3 −2.6 −0.6 103.7±0.6 102.4±2.0 99.7±1.9 联苯三唑醇 −7.0 2.0 −8.9 95.6±3.8 108.7±3.8 102.3±1.6 叶菌唑 −1.8 −0.5 −0.6 102.2±1.2 106.3±1.4 100.8±2.8 己唑醇 2.2 −0.5 1.4 108.7±1.1 103.8±2.0 100.4±1.5 烯唑醇 −1.5 −2.5 −0.9 103.4±1.2 103.3±0.7 99.8±1.2 苯醚甲环唑 3.2 −3.5 −0.7 107.6±2.3 108.8±1.0 100.9±2.0 种菌唑 −2.1 −3.4 −1.8 101.9±0.9 103.6±1.2 98.9±1.8 亚胺唑 2.0 −2.6 0.1 107.1±1.5 105.1±0.6 100.0±1.4 3. 结论
本研究基于亲脂性匹配色谱分离和分散固相萃取,建立了果蔬中21种三唑类杀菌剂的超高效液相色谱-串联质谱分析方法。样品经乙腈提取,分散固相萃取净化,选用与三唑杀菌剂具有相近亲脂性的C8柱进行色谱分离。结果表明,所述亲脂性匹配色谱分离能够提高色谱分离效率,改善三唑杀菌剂和基质组分的色谱分离,与分散固相萃取相结合,有效降低了果蔬中三唑杀菌剂UPLC-MS/MS测定时的基质效应,使用溶剂校准工作曲线即可获得满意的回收率,显著提高了检测效率。21种三唑杀菌剂的基质效应在−8.3%~4.7%,在5~250 μg/L质量浓度范围内线性关系良好,平均回收率为91.4%~108.1%,日内和日间RSD分别为0.7%~5.2%和1.7%~6.0%,定量限为0.5~3.5 μg/kg。该方法操作简单、结果准确、灵敏度高,适用于果蔬中三唑杀菌剂的分析检测,为果蔬中农药残留UPLC-MS/MS分析中提供了方法参考。所述亲脂性匹配色谱分离是一种简单、有效的液相色谱分离策略,对于提高农药残留色谱分离效率,降低基质效应具有重要意义。
-
表 1 流动相梯度洗脱程序
Table 1 Program of mobile phase gradient elution
时间(min) A(%) B(%) 0 80 20 0.5 80 20 2.5 60 40 4.0 50 50 6.0 40 60 9.0 30 70 10.5 10 90 12 10 90 12.5 80 20 14.5 80 20 表 2 21种三唑杀菌剂的多反应监测质谱分析参数
Table 2 Optimized MRM parameters for the 21 triazole fungicides
化合物 母离子
(m/z)子离子
(m/z)锥孔电
压(V)碰撞能量
(eV)粉唑醇 302.0 122.8*, 108.9 35 30, 31 三唑酮 294.0 68.9*, 196.9 38 21, 15 环丙唑醇 292.0 69.8*, 124.8 41 19, 26 三唑醇 296.0 69.8*, 98.9 35 22, 17 腈菌唑 289.0 69.8*, 124.9 34 20, 33 氟环唑 329.9 120.9*, 100.9 32 21, 51 灭菌唑 318.0 69.8*, 124.8 31 20, 33 四氟醚唑 371.9 158.8*, 69.9 43 26, 23 腈苯唑 337.0 125.0*, 70.0 38 29, 19 氟硅唑 316.0 165.1*, 247.1 40 27, 18 戊菌唑 284.0 158.8*, 69.8 31 23, 18 戊唑醇 308.0 124.9*, 150.8 40 32, 25 联苯三唑醇 338.2 269.1*, 251.1 30 9, 13 氯氟醚菌唑 397.9 69.8*, 181.9 50 24, 29 叶菌唑 320.0 69.8*, 124.9 37 23, 33 己唑醇 314.0 69.8*, 158.8 43 21, 30 烯唑醇 325.9 69.8*, 158.8 50 26, 25 丙环唑 341.9 68.9*, 158.8 35 23, 25 苯醚甲环唑 405.9 250.8*, 336.9 50 25, 18 种菌唑 334.0 69.8*, 124.8 36 21, 39 亚胺唑 410.9 124.8*, 170.8 48 30, 19 注:*为定量离子。 表 3 21种三唑杀菌剂的保留时间和在橙子样品中的基质效应
Table 3 Retention time and matrix effect for 21 triazole fungicides in orange
化合物 C8柱 C18柱 基质效应(%) 保留时间(min) 基质效应(%) 保留时间(min) 粉唑醇 −8.3 5.65 −8.9 5.65 三唑酮 −3.4 7.10 −21.2 7.18 环丙唑醇 0.1 7.38 −7.9 7.51 三唑醇 4.7 7.39 1.5 7.50 腈菌唑 0.4 7.37 −7.1 7.33 氟环唑 −2.3 7.55 −14.9 7.77 灭菌唑 −0.3 7.47 −15.7 7.68 四氟醚唑 -4.5 7.90 −2.8 7.87 腈苯唑 −4.3 7.85 −4.2 8.06 氟硅唑 2.1 8.07 −3.4 8.21 戊菌唑 0.2 8.36 −5.4 8.53 戊唑醇 −0.1 8.31 −2.4 8.63 丙环唑 −3.3 8.40 −6.4 8.76 氯氟醚菌唑 2.3 8.70 2.9 9.01 联苯三唑醇 −0.6 8.79 4.5 9.21 叶菌唑 −4.2 8.69 −3.8 9.06 己唑醇 −3.4 8.83 4.2 9.05 烯唑醇 −3.2 9.18 −3.2 9.39 苯醚甲环唑 −1.8 8.99 −3.0 9.45 种菌唑 −2.4 9.55 −5.9 9.72 亚胺唑 −1.5 10.26 −8.2 10.22 表 4 21种三唑杀菌剂的线性范围、定量限(LOQ)、线性方程和决定系数(R2)
Table 4 Liner ranges, LOQ, regression equation and determination coefficient (R2) for 21 triazole fungicides
化合物 线性范围 (µg/L) LOQ(μg/kg) 溶剂校准曲线 基质匹配校准曲线 线性方程 R2 线性方程 R2 粉唑醇 5~250 1.6 y=300.35x+33.620 0.9996 y=275.42x−17.422 0.9997 三唑酮 5~250 1.0 y=635.64x+136.53 0.9996 y=613.99x+81.598 0.9999 环丙唑醇 5~250 1.1 y=763.15x+278.65 0.9996 y=763.92x−122.85 0.9991 三唑醇 5~250 3.2 y=89.176x−61.643 0.9999 y=93.367x+51.599 0.9990 腈菌唑 5~250 0.7 y=854.80x+675.88 0.9992 y=858.10x−104.39 0.9993 氟环唑 5~250 0.8 y=927.65x+424.89 0.9999 y=905.99x−104.53 0.9995 灭菌唑 5~250 0.6 y=1257.6x+291.33 0.9995 y=1253.4x−41.490 0.9991 四氟醚唑 5~250 1.0 y=660.59x−114.96 0.9995 y=630.87x−76.457 0.9997 腈苯唑 5~250 1.0 y=384.09x+69.926 0.9999 y=367.6x+88.758 0.9997 氟硅唑 5~250 1.5 y=566.45x+270.793 0.9991 y=578.25x+140.93 0.9998 戊菌唑 5~250 1.8 y=350.60x+149.43 0.9992 y=351.32x−111.23 0.9993 戊唑醇 5~250 3.1 y=82.119x+11.768 0.9993 y=82.041x+3.023 0.9997 丙环唑 5~250 2.1 y=718.82x−790.269 0.9993 y=694.89x−332.70 0.9995 氯氟醚菌唑 5~250 0.7 y=1227.8x+881.15 0.9992 y=1256.3x−494.42 0.9992 联苯三唑醇 5~250 3.5 y=86.316x+50.360 0.9993 y=85.772x+39.333 0.9991 叶菌唑 5~250 0.6 y=1698.0x+668.40 0.9996 y=1626.8x+1046.3 0.9996 己唑醇 5~250 0.9 y=969.50x+302.88 0.9999 y=936.85x+180.38 0.9997 烯唑醇 5~250 0.9 y=955.70x+56.911 0.9996 y=925.16x−149.17 0.9997 苯醚甲环唑 5~250 1.5 y=627.38x−503.191 0.9991 y=616.03x+120.11 0.9998 种菌唑 5~250 0.5 y=1693.9x+289.57 0.9998 y=1653.3x−104.67 0.9999 亚胺唑 5~250 2.0 y=419.88x+0.462 0.9998 y=413.59−54.521 0.9993 表 5 21种三唑杀菌剂的回收率和精密度
Table 5 Recovery and precision for 21 triazole fungicides
化合物 平均回收率(%,n=15) 日内RSD(%,n=5) 日间RSD(%,n=15) 10 50 200 10 50 200 10 50 200 μg/kg μg/kg μg/kg 粉唑醇 91.4 92.5 92.2 1.3 1.6 2.0 1.7 1.9 2.7 三唑酮 96.4 95.7 96.8 2.5 1.9 2.7 3.1 4.0 2.8 环丙唑醇 100.6 101.3 99.7 2.5 1.9 2.6 3.5 3.8 3.9 三唑醇 108.1 107.5 105.3 3.3 1.8 3.1 3.3 5.7 4.2 腈菌唑 98.5 99.7 101.1 3.8 3.2 1.6 4.6 3.7 2.3 氟环唑 98.3 97.9 100.3 2.3 1.3 1.9 4.3 2.7 4.5 灭菌唑 98.8 97.7 100.6 1.7 2.9 2.3 2.3 3.2 4.6 四氟醚唑 97.5 99.4 97.0 2.1 2.2 2.4 3.4 4.1 2.7 腈苯唑 99.1 97.3 95.7 3.6 0.7 1.7 3.8 1.8 4.5 氟硅唑 102.2 102.6 98.8 2.7 2.3 1.9 3.6 4.1 2.7 戊菌唑 99.7 98.8 97.8 3.8 1.8 2.4 3.5 2.9 1.9 戊唑醇 98.3 99.6 102.9 4.2 3.6 3.2 5.2 5.5 3.1 丙环唑 101.3 98.8 102.1 3.5 1.6 1.9 4.3 4.1 2.6 氯氟醚菌唑 98.2 101.5 103.7 3.1 1.9 2.2 3.7 3.1 2.5 联苯三唑醇 101.1 99.1 98.0 3.3 2.5 2.7 5.6 4.2 2.9 叶菌唑 101.2 97.6 98.7 2.2 2.1 1.8 3.3 4.0 3.0 己唑醇 102.0 98.3 100.5 1.9 1.1 1.5 3.6 3.0 2.1 烯唑醇 96.8 98.5 100.8 2.3 2.1 1.7 5.0 3.1 3.5 苯醚甲环唑 98.7 99.0 101.5 5.2 1.5 1.8 6.0 4.1 2.8 种菌唑 97.2 98.2 100.6 2.4 3.1 1.7 4.1 5.0 5.6 亚胺唑 101.4 99.1 99.3 1.7 2.4 2.8 3.3 3.6 4.2 表 6 21种三唑杀菌剂在不同果蔬基质中的基质效应和加标回收率
Table 6 Matrix effect and recoveries for 21 triazole fungicides in different fruits and vegetables
化合物 基质效应(%) 平均回收率±相对偏差(%,n=5) 苹果 香蕉 黄瓜 苹果 香蕉 黄瓜 粉唑醇 −1.4 −2.8 1.3 104.1±1.5 103.7±1.0 100.7±0.7 三唑酮 2.5 −1.0 −2.0 108.3±2.2 109.3±1.9 99.8±2.1 环丙唑醇 −1.6 −4.2 −0.7 103.0±1.1 103.5±2.2 98.7±0.8 三唑醇 3.8 0.0 9.7 103.7±2.2 104.6±2.7 106.8±2.1 腈菌唑 −2.6 −1.0 −1.4 102.6±1.1 106.3±3.2 100.6±1.6 氟环唑 −0.8 −3.6 0.2 103.6±1.3 104.3±2.6 99.8±2.1 灭菌唑 −7.3 −1.0 −1.0 100.2±0.5 102.9±1.9 99.9±1.2 四氟醚唑 4.0 −2.2 0.9 108.9±0.8 106.2±2.6 99.3±1.8 腈苯唑 2.2 −1.0 −1.5 106.6±0.9 108.6±1.3 100.6±1.7 氟硅唑 −4.3 −1.4 −2.0 98.1±1.2 106.6±0.4 99.1±0.9 戊菌唑 5.5 −3.6 −2.5 102.2±1.2 104.4±0.8 99.1±2.0 戊唑醇 0.9 −6.5 −3.1 107.2±1.6 102.9±4.0 98.6±4.6 丙环唑 −1.5 −2.2 −1.6 105.1±0.6 105.2±1.7 99.8±1.0 氯氟醚菌唑 −2.3 −2.6 −0.6 103.7±0.6 102.4±2.0 99.7±1.9 联苯三唑醇 −7.0 2.0 −8.9 95.6±3.8 108.7±3.8 102.3±1.6 叶菌唑 −1.8 −0.5 −0.6 102.2±1.2 106.3±1.4 100.8±2.8 己唑醇 2.2 −0.5 1.4 108.7±1.1 103.8±2.0 100.4±1.5 烯唑醇 −1.5 −2.5 −0.9 103.4±1.2 103.3±0.7 99.8±1.2 苯醚甲环唑 3.2 −3.5 −0.7 107.6±2.3 108.8±1.0 100.9±2.0 种菌唑 −2.1 −3.4 −1.8 101.9±0.9 103.6±1.2 98.9±1.8 亚胺唑 2.0 −2.6 0.1 107.1±1.5 105.1±0.6 100.0±1.4 -
[1] 张微, 肖曼, 吴丹, 等. 固相萃取/超高效液相色谱-串联质谱法同时测定水产养殖“非药品”投入品中37种禁限兽药[J]. 分析测试学报,2022,41(12):1751−1757. [ZHANG W, XIAO M, WU D, et al. Simultaneous determination of 37 kinds of prohibited veterinary drug residues in aquacultural “non-pharmaceutical” inputs by ultra performance liquid chromatography-tandem mass spectrometry with solid phase extraction[J]. Journal of Instrumental Analysis,2022,41(12):1751−1757. doi: 10.19969/j.fxcsxb.22053001 ZHANG W, XIAO M, WU D, et al . Simultaneous determination of 37 kinds of prohibited veterinary drug residues in aquacultural “non-pharmaceutical” inputs by ultra performance liquid chromatography-tandem mass spectrometry with solid phase extraction[J]. Journal of Instrumental Analysis,2022 ,41 (12 ):1751 −1757 . doi: 10.19969/j.fxcsxb.22053001[2] IAN O, DOMINIK B, ANNETTE R, et al. Quantifying up to 90 polyphenols simultaneously in human bio-fluids by LC-MS/MS[J]. Analytica Chimica Acta,2022,1216:339977. doi: 10.1016/j.aca.2022.339977
[3] NASIRI A, JAHANI R, MOKHTARI S, et al. Overview, consequences, and strategies for overcoming matrix effects in LC-MS analysis:A critical review[J]. Analyst,2021,146:6049−6063. doi: 10.1039/D1AN01047F
[4] CHAMBERS E, WAGROWSKI D D M, LU Z, et al. Systematic and comprehensive strategy for reducing matrix effects in LC/MS/MS analyses[J]. Journal of Chromatography B,2007,852:22−34. doi: 10.1016/j.jchromb.2006.12.030
[5] QIN J, FU Y, LU Q, et al. Matrix-matched monitoring ion selection strategy for improving the matrix effect and qualitative accuracy in pesticide detection based on UFLC-ESI-MS/MS:A case of chrysanthemum[J]. Microchemical Journal,2021,160:105681. doi: 10.1016/j.microc.2020.105681
[6] CORTESE M, GIGLIOBIANCO M R, MAGNONI F, et al. Compensate for or minimize matrix effects? Strategies for overcoming matrix effects in liquid chromatography-mass spectrometry technique:A tutorial review[J]. Molecules,2020,25:3047−3077. doi: 10.3390/molecules25133047
[7] DIAS J, LÓPEZ S H, MOL H, et al. Influence of different hydrophilic interaction liquid chromatography stationary phases on method performance for the determination of highly polar anionic pesticides in complex feed matrices[J]. Journal of Separation Science,2021,44:2165−2176. doi: 10.1002/jssc.202001134
[8] LARA O F J, ROBLES M J, BRANDT S, et al. Use of dielectric barrier discharge ionization to minimize matrix effects and expand coverage in pesticide residue analysis by liquid chromatography-mass spectrometry[J]. Analytica Chimica Acta,2018,1020:76−85. doi: 10.1016/j.aca.2018.02.077
[9] CAO J, ZHENG Y, KAIUM A, et al. A comparative study of biochar, multiwalled carbon nanotubes and graphitized carbon black as QuEChERS absorbents for the rapid determination of six triazole fungicides by UPLC-MS/MS[J]. International Journal of Environmental Analytical Chemistry,2019,99(1/5):209−223. doi: 10.1080/03067319.2019.1586892
[10] QIN Y, ZHAO P, FAN S, et al. The comparison of dispersive solid phase extraction and multi-plug filtration cleanup method based on multi-walled carbon nanotubes for pesticides multi-residue analysis by liquid chromatography tandem mass spectrometry[J]. Journal of Chromatography A,2015,1385:1−11. doi: 10.1016/j.chroma.2015.01.066
[11] CHAWLA S, PATEL H K, GOR H N, et al. Evaluation of matrix effects in multiresidue analysis of pesticide residues in vegetables and spices by LC-MS/MS[J]. Journal of Aoac International,2017,100:616−623. doi: 10.5740/jaoacint.17-0048
[12] STAHNKE H, KITTLAUS S, KEMPE G, et al. Reduction of matrix effects in liquid chromatography-electrospray ionization-mass spectrometry by dilution of the sample extracts:How much dilution is needed?[J]. Analytical Chemistry,2012,84:1474−1482. doi: 10.1021/ac202661j
[13] YANG P, CHANG J S, WONG J W, et al. Effect of sample dilution on matrix effects in pesticide analysis of several matrices by liquid chromatography-high resolution mass spectrometry[J]. Journal of Agricultural and Food Chemistry,2015,63:5169−5177. doi: 10.1021/jf505168v
[14] RUTKOWSKA E, ŁOZOWICKA B, KACZYŃSKI P. Three approaches to minimize matrix effects in residue analysis of multiclass pesticides in dried complex matrices using gas chromatography tandem mass spectrometry[J]. Food Chemistry,2019,279:20−29. doi: 10.1016/j.foodchem.2018.11.130
[15] HUA S, ZHAO M, MAO Q, et al. Rapid one-step cleanup method to minimize matrix effects for residue analysis of alkaline pesticides in tea using liquid chromatography-high resolution mass spectrometry[J]. Food Chemistry,2019,299:125146. doi: 10.1016/j.foodchem.2019.125146
[16] KWONA H, LEHOTAYA S J, GEIS A L. Variability of matrix effects in liquid and gas chromatography-mass spectrometry analysis of pesticide residues after QuEChERS sample preparation of different food crops[J]. Journal of Chromatography A,2012,1270:235−245. doi: 10.1016/j.chroma.2012.10.059
[17] LIU Z, WANG J, ZHANG Z, et al. Development of magnetic solid phase extraction using magnetic amphiphilic polymer for sensitive analysis of multi-pesticides residue in honey[J]. Journal of Chromatography A,2022,1664:462789. doi: 10.1016/j.chroma.2021.462789
[18] LIU G, TIAN M, LU M, et al. Preparation of magnetic MOFs for use as a solid-phase extraction absorbent for rapid adsorption of triazole pesticide residues in fruits juices and vegetables[J]. Journal of Chromatography B,2021,1166:122500. doi: 10.1016/j.jchromb.2020.122500
[19] ZHANG R, TAN Z, HUANG K, et al. A vortex-assisted dispersive liquid-liquid microextraction followed by UPLC-MS/MS for simultaneous determination of pesticides and aflatoxins in herbal tea[J]. Molecules,2019,24:1029. doi: 10.3390/molecules24061029
[20] WANG S, LI M, LI X, et al. A functionalized carbon nanotube nanohybrids-based QuEChERS method for detection of pesticide residues in vegetables and fruits[J]. Journal of Chromatography A,2020,1631:461526. doi: 10.1016/j.chroma.2020.461526
[21] 郭礼强, 刘永强, 王乐, 等. HPLC-MS/MS法测定婴幼儿果蔬米粉中17种新烟碱类杀虫剂及代谢物[J]. 分析测试学报,2022,41(12):1773−1778. [GUO L Q, LIU Y Q, WANG L, et al. Determination of 17 neonicotinoid pesticides and their metabolites in baby fruit and vegetable rice flour by high performance liquid chromatography-tandem mass spectrometry[J]. Journal of Instrumental Analysis,2022,41(12):1773−1778. doi: 10.19969/j.fxcsxb.22060103 GUO L Q, LIU Y Q, WANG L, et al . Determination of 17 neonicotinoid pesticides and their metabolites in baby fruit and vegetable rice flour by high performance liquid chromatography-tandem mass spectrometry[J]. Journal of Instrumental Analysis,2022 ,41 (12 ):1773 −1778 . doi: 10.19969/j.fxcsxb.22060103[22] HERGUETA C M E, LÓPEZ R E, LÓPEZ R R, et al. Targeted and untargeted analysis of triazole fungicides and their metabolites in fruits and vegetables by UHPLC-orbitrap-MS2[J]. Food Chemistry,2022,368:130860. doi: 10.1016/j.foodchem.2021.130860
[23] KITTLAUS S, SCHIMANKE J, KEMPE G, et al. Assessment of sample cleanup and matrix effects in the pesticide residue analysis of foods using postcolumn infusion in liquid chromatography-tandem mass spectrometry[J]. Journal of Chromatography A,2012,1218:8399−8410.
[24] ZHAO J, PU J, WU X. Evaluation of the matrix effect of pH value and sugar content on the analysis of pesticides in tropical fruits by UPLC-MS/MS[J]. Microchemical Journal,2021,168:106375. doi: 10.1016/j.microc.2021.106375
[25] HANAI T. Quantitative explanation of retention mechanisms of hydrophobic and hydrophilic-interaction liquid chromatography-inductive effect of alkyl chain[J]. Separations,2017,4:33. doi: 10.3390/separations4040033
[26] LAYNE J. Characterization and comparison of the chromatographic performance of conventional, polar-embedded, and polar-endcapped reversed-phase liquid chromatography stationary phases[J]. Journal of Chromatography A,2002,957:149−164. doi: 10.1016/S0021-9673(02)00193-0
[27] KECOJEVIĆ I, ĐEKIĆ S, LAZOVIĆ M, et al. Evaluation of LC-MS/MS methodology for determination of 179 multi-class pesticides in cabbage and rice by modified QuEChERS extraction[J]. Food Control,2021,123:107693. doi: 10.1016/j.foodcont.2020.107693
[28] 李益丰, 张秋云, 杨洪生, 等. 免疫亲和柱净化-液相色谱-串联质谱法同时测定水产品中8种霉菌毒素[J]. 食品工业科技,2023,44(7):294−300. [LI Y F, ZHANG Q Y, YANG H S, et al. Simultaneous determination of 8 mycotoxins in aquatic products by liquid chromatography-tandem mass spectrometry coupled with immunoaffinity column clean-up[J]. Science and Technology of Food Industry,2023,44(7):294−300. doi: 10.13386/j.issn1002-0306.2022050233 LI Y F, ZHANG Q Y, YANG H S, et al . Simultaneous determination of 8 mycotoxins in aquatic products by liquid chromatography-tandem mass spectrometry coupled with immunoaffinity column clean-up[J]. Science and Technology of Food Industry,2023 ,44 (7 ):294 −300 . doi: 10.13386/j.issn1002-0306.2022050233[29] 文静, 莫楠, 张立佳, 等. 超高效液相色谱-串联质谱法测定牛乳中氯虫苯甲酰胺、氟苯虫酰胺和2甲4氯的残留[J]. 食品工业科技,2023,44(5):285−291. [WEN J, MO N, ZHANG L J, et al. Determination of chlorantraniliprole, flubendiamide and 2-methyl-4-chlorophenoxyacetic acid residues in milk by ultra performance liquid chromatography-tandem mass spectrometry[J]. Science and Technology of Food Industry,2023,44(5):285−291. doi: 10.13386/j.issn1002-0306.2022050039 WEN J, MO N, ZHANG L J, et al . Determination of chlorantraniliprole, flubendiamide and 2-methyl-4-chlorophenoxyacetic acid residues in milk by ultra performance liquid chromatography-tandem mass spectrometry[J]. Science and Technology of Food Industry,2023 ,44 (5 ):285 −291 . doi: 10.13386/j.issn1002-0306.2022050039[30] 商春锋, 周一冉, 胡国栋, 等. Sin-QuEChERS 净化技术结合液相色谱-串联质谱法检测大棚蔬菜中13种全氟烷基酸[J]. 食品安全质量检测学报,2023,14(1):90−97. [SHANG C F, ZHOU Y R, HU G D, et al. Determination of 13 kinds of perfluoroalkyl acids in greenhouse vegetables by Sin-QuEChERS purification technology and liquid chromatography-tandem mass spectrometry[J]. Journal of Food Safety and Quality,2023,14(1):90−97. doi: 10.3969/j.issn.2095-0381.2023.1.spaqzljcjs202301012 SHANG C F, ZHOU Y R, HU G D, et al . Determination of 13 kinds of perfluoroalkyl acids in greenhouse vegetables by Sin-QuEChERS purification technology and liquid chromatography-tandem mass spectrometry[J]. Journal of Food Safety and Quality,2023 ,14 (1 ):90 −97 . doi: 10.3969/j.issn.2095-0381.2023.1.spaqzljcjs202301012[31] KIM Y A, ATY A M A E, RAHMAN M M, et al. Method development, matrix effect, and risk assessment of 49 multiclass pesticides in kiwifruit using liquid chromatography coupled to tandem mass spectrometry[J]. Journal of Chromatography B,2018,1076:130−138. doi: 10.1016/j.jchromb.2018.01.015
[32] LOZOWICKA B, ILYASOVAl G, KACZYNSKI P, et al. Multi-residue methods for the determination of over four hundred pesticides in solid and liquid high sucrose content matrices by tandem mass spectrometry coupled with gas and liquid chromatograph[J]. Talanta,2016,151:51−61. doi: 10.1016/j.talanta.2016.01.020
[33] ChemSpider数据库[OL]. [2023-04-27]. http://www.chemspider.com. [ChemSpider database[OL]. [2023-04-27]. http://www.chemspider.com/. ChemSpider database[OL]. [2023-04-27]. http://www.chemspider.com/.
[34] CEVC G, BERTS I, FISCHER S F, et al. Nanostructures in n-octanol equilibrated with additives and/or water[J]. Langmuir,2018,34:6285−6295. doi: 10.1021/acs.langmuir.8b00142
[35] ZHANG Y, LI Q, DAI B, et al. A versatile polar-embedded polyphenyl phase for multimodal separation in liquid chromatography[J]. Journal of Chromatography A,2018,1553:81−89. doi: 10.1016/j.chroma.2018.04.025
[36] 韦迪哲, 王蒙, 翟文磊. 基于磁性ZIF-67纳米材料的QuEChERS-超高效液相色谱-串联质谱技术同时检测小麦中17种真菌毒素[J]. 食品安全质量检测学报,2022,13(23):7563−7572. [WEI D Z, WANG M, ZHAI W L. Simultaneous determination of 17 kinds of mycotoxins in wheat by QuEChERS-ultra performance liquid chromatography-tandem mass spectrometry based on magnetic ZIF-67 nanomaterials[J]. Journal of Food Safety and Quality,2022,13(23):7563−7572. doi: 10.3969/j.issn.2095-0381.2022.23.spaqzljcjs202223010 WEI D Z, WANG M, ZHAI W L . Simultaneous determination of 17 kinds of mycotoxins in wheat by QuEChERS-ultra performance liquid chromatography-tandem mass spectrometry based on magnetic ZIF-67 nanomaterials[J]. Journal of Food Safety and Quality,2022 ,13 (23 ):7563 −7572 . doi: 10.3969/j.issn.2095-0381.2022.23.spaqzljcjs202223010[37] 潘永波, 万娜, 王承业, 等. 快速滤过型净化结合液相色谱-质谱联用法测定海产品中19种磺胺类药物残留[J]. 食品工业科技,2023,44(7):320−328. [PAN Y B, WAN N, WANG C Y, et al. Determination of 19 sulfonamides residues in seafood by multi-plug filtration cleanup method combined with liquid chromatography-tandem mass spectrometry[J]. Science and Technology of Food Industry,2023,44(7):320−328. doi: 10.13386/j.issn1002-0306.2022060288 PAN Y B, WAN N, WANG C Y, et al . Determination of 19 sulfonamides residues in seafood by multi-plug filtration cleanup method combined with liquid chromatography-tandem mass spectrometry[J]. Science and Technology of Food Industry,2023 ,44 (7 ):320 −328 . doi: 10.13386/j.issn1002-0306.2022060288 -
期刊类型引用(3)
1. 胡潇潇,杨妍,程名玉,杜利敏,李志岭. 超高效液相色谱-四级杆飞行时间质谱法快速筛查果蔬中40种农药残留. 现代疾病预防控制. 2025(01): 62-68 .  百度学术
百度学术
2. 张波,郭二菱,黄笑晨,郭爱静,王可. 豆粉中31种杀菌剂残留的UPLC-MS/MS测定法. 职业与健康. 2024(12): 1590-1595 .  百度学术
百度学术
3. 张子豪,谭智毅,麦晓霞,林海,李全忠,刘莹峰,肖前. 固相萃取-气相色谱-串联质谱技术测定船用残渣燃料油中酚类及脂肪酸甲酯类化合物. 分析科学学报. 2024(04): 395-403 .  百度学术
百度学术
其他类型引用(0)





 下载:
下载:
 下载:
下载:



