Preventive Effect of Chimonanthus praecox Flower Extract on High-fat Diet-induced Obesity in Mice
-
摘要: 目的:探究腊梅花提取物对高脂膳食诱导小鼠肥胖的预防作用。方法:将50只C57BL/6小黑鼠分为5组,即空白对照组(NC),模型对照组(HFD),腊梅花提取物(Chimonanthus praecox flower extract,CPFE)低(LDG)、中(MDG)、高(HDG)剂量组。空白对照组给予基础维持饲料,模型对照组和剂量组给予高脂饲料。低、中、高剂量组分别给予腊梅花提取物0.2、0.4、0.8 g/(kg mb·d)进行干预,即腊梅花多酚剂量分别为24.81、49.62、99.25 mg/(kg mb·d),对照组给予等量0.9% NaCl。连续灌胃4周,测定小鼠体重、肝脏系数、血清生化指标、脏器抗氧化能力,观察肝脏组织形态学变化。结果:模型对照组与空白对照组比较,体重、肝脏系数、血清总胆固醇(Total Cholesterol,TC)、低密度脂蛋白胆固醇(Low-Density Lipoprotein Cholesterol,LDL-C)和脏器丙二醛(Malondialdehyde,MDA)水平升高(P<0.05);血清高密度脂蛋白胆固醇(High-Density Lipoprotein Cholesterol,HDL-C)和脏器谷胱甘肽过氧化物酶(Glutathione peroxidase,GSH-Px)、过氧化氢酶(Catalase,CAT),超氧化物歧化酶(Superoxide Dismutase,SOD)、谷胱甘肽(Glutathione,GSH)水平降低(P<0.05);肝脏病理结果显示,模型对照组与空白对照组相比,肝小叶结构不规则,细胞形态被破坏,出现空泡变性,且肝索不清晰,肝窦被挤压变形,表明高脂饮食诱导小鼠肥胖成功。剂量组与模型对照组相比,腊梅花提取物能够下调血清TC、LDL-C、甘油三酯(Triglyceride,TG)和脏器MDA水平,高剂量组效果更明显(P<0.05);腊梅花提取物还能使血清HDL-C水平和脏器GSH-Px、CAT、SOD、GSH水平升高,效果与腊梅花提取物浓度呈剂量效应。肝脏组织病理结果较模型对照组有所改善,可见肝脏组织形态和结构更加完整。腊梅花提取物还能降低肥胖小鼠的体质量和肝脏指数且高剂量组效果更显著。结论:腊梅花提取物具有降低血清胆固醇,增强小鼠抗氧化能力和保护肝组织细胞功能形态的作用。Abstract: Objective: To study the preventive effect of ethanolic extract from Chimonanthus praecox flower on obesity induced by high-fat diet in mice. Methods: A total of 50 C57BL/6 mice were divided into five groups of blank control, model control, low-, medium- and high-dose Chimonanthus praecox flower extract (CPFE) treatment. The mice in the blank control group were provided with a basal diet whereas those in the other groups were given a high-fat diet. The mice in the low-, medium- and high-dose groups were orally administered with CPFE at doses of 0.2, 0.4 and 0.8 g/(kg mb·d), containing 24.81, 49.62 and 99.25 mg/(kg mb·d) of Chimonanthus praecox flower polyphenols, respectively. The blank control, model control groups were administered with 0.9% NaCl. The administration lasted for 4 weeks. Then, body weight, serum biochemical indicators, organ antioxidant capacity were measured and pathological sections of liver tissue were examined. Results: The body weight, liver coefficient, serum levels of TC, LDL-C, organ levels of MDA in the model control group were significantly higher than those in the blank control group (P<0.05). The levels of organ GSH-Px, CAT, SOD, GSH in the model control group were significantly lower than those in the blank control group (P<0.05). Pathological examination showed that compared with the blank control group, the structure of liver lobules was irregular, the cell morphology was destroyed, vacuolar degeneration occurs, and the hepatic cord was not clear, and the hepatic sinuses were squeezed and deformed, indicating successful obesity induction in mice. In addition, compared with the model control group, the CPFE especially at the high dose lowered serum TC, LDL-C, TG, organ MDA in obese mice (P<0.05) and increased serum HDL-C levels, organ GSH-Px, CAT, SOD, GSH levels, and the effect was dose-effective with the concentration of CPFE. Pathological status of the organ were improved compared to the model control group, as evidenced by a more intact liver histomorphology and structure. The extract also reduced the body weight and liver index of obese mice and the effect was more pronounced in the high dose group. Conclusion: The CPFE have the effects of lowering serum cholesterol, enhancing antioxidant capacity and protecting the functional morphology of liver tissue cells in mice.
-
肥胖是指由遗传、环境等多种因素相互作用所引起的体内脂肪堆积过多和(或)分布异常、体质量增加的一种慢性代谢性疾病[1]。可导致高血压、血脂异常、内分泌代谢紊乱,同时还是癌症、肾脏疾病等的诱发因素[2]。目前的减肥方式主要有手术减肥[3]、药物减肥[4]等,但是副作用明显。人们还可以改变生活方式,通过加强身体锻炼和摄入高膳食纤维、低糖、低脂等更为健康的食物来达到减肥的目的。肥胖通常伴随着糖尿病、高胆固醇血症等并发性疾病,高脂血症一般是指血液中总胆固醇(Total Cholesterol,TC)和/或(Low-Density Lipoprotein Cholesterol,LDL-C)或甘油三酯(Triglyceride,TG)升高,还有可能伴随着高密度脂蛋白胆固醇(High-Density Lipoprotein Cholesterol,HDL-C)降低[5]。
腊梅(Chimonanthus praecox (L.) Link)为腊梅科腊梅属落叶丛生灌木[6],多分枝,皮灰白色,生长于亚热带湿润的常绿落叶阔叶混交林地带,广泛分布于中国南部,以长江流域最为丰富。腊梅的花、茎、根均可入药,常用于治疗感冒、解热镇痛、止咳、降压和改善免疫系统,功效显著[7]。植物化学研究表明腊梅花富含龙脑、桉精油和芳樟醇等精油成分[8−9],此外,腊梅提取物中还含有非挥发性的生物碱、黄酮类和多酚类等物质,具有抑菌[10]、抗炎症[11]、抗氧化[12]、降血糖及降血脂[13]等作用,其活性成分应用潜力巨大。
腊梅作为我国传统植物,种植广泛,但开发利用程度较低。王延等[14]研究了山腊梅叶挥发油对血管性痴呆大鼠认知功能障碍的影响及其机制,发现山腊梅叶挥发油可以保护脑组织免于氧化损伤,保持大鼠脑组织氧化/抗氧化平衡,减轻海马区损伤,具有改善大鼠血管性痴呆认知障碍的作用。袁青青等[15]对山腊梅叶挥发油的镇咳、平喘、祛痰、抗炎及免疫调节作用进行了研究,发现山腊梅叶挥发油能减少小鼠咳嗽次数,降低小鼠耳肿胀度,升高小鼠吞噬指数;高剂量山腊梅叶挥发油还能有效增加小鼠排痰量,升高小鼠廓清指数。艾安涛等[16]对腊梅花茶的加工工艺进行了研究,制出的腊梅花茶花香馥郁鲜灵、醇厚回甘,具有解暑生津的作用。目前国内鲜有针对腊梅花活性物质的体内研究,本实验拟探究腊梅花提取物对高脂膳食诱导氧化损伤小鼠的影响,通过考察其对小鼠血脂四项指标及体内抗氧化酶活力等的作用,为充分利用腊梅花中的天然活性物质提供可靠的理论基础。
1. 材料与方法
1.1 材料与仪器
健康雄性C57BL/6小黑鼠 SPF级,50只,合格证号[SCXK(川)2020-030],体重20±1 g,6周龄,购自四川省成都达硕动物实验中心;基础饲料、高脂饲料 成都达硕动物有限公司;腊梅花 2020年于西华大学内采摘,经西华大学食品与生物工程学院焦士蓉教授鉴定为腊梅科属灌木植物—腊梅;考马斯亮蓝 纯度85%,合肥博美试剂有限公司;牛血清蛋白 纯度99.9%,上海生物工程有限公司;肝脏丙二醛(Malondialdehyde,MDA)、过氧化氢酶(Catalase,CAT)、超氧化物歧化酶(Superoxide Dismutase,SOD)、谷胱甘肽(Glutathione,GSH)、谷胱甘肽过氧化物酶(Glutathione peroxidase,GSH-Px)含量测定试剂盒 南京建成生物研究所;石蜡 北京索莱宝科技有限公司。
7200可见光分光光度计 上海尤尼柯仪器有限公司;TGL-16冷冻离心机 四川蜀科仪器有限公司;E2000MV生物显微镜 南京尼康江南光学仪器有限公司。
1.2 实验方法
1.2.1 腊梅花提取物的制备
参考包善思等[17]的方法,将干燥腊梅花粉碎后过80目筛,60 ℃恒温干燥12 h,采用乙醇以料液比1:30 g/mL微波提取,微波功率406 W,微波时间50 s,将提取液以7000 r/min的转速离心10 min,收集上清液,浓缩冻干,得到腊梅花提取物。将冻干样品于4 ℃保存。在实验室前期研究发现腊梅提取物多酚含量为124.06 mg/g,经HPLC测定,腊梅花多酚物质中芦丁含量最高为36.53±0.64 mg/g,槲皮素含量为11.27±0.22 mg/g,没食子酸含量为4.43±0.14 mg/g,阿魏酸含量为1.62±0.04 mg/g,山奈酚含量为1.19±0.01 mg/g[18]。已有大量文献证明,这几种多酚类物质具有抗氧化、抗炎[19−21]等作用,因此以多酚含量确认灌胃剂量。
1.2.2 小鼠饲养、分组及干预
环境温度20±5 ℃,控制12 h光照/黑暗,相对湿度约为50%条件下采用普通饲料降小鼠适应性喂养一周。一周后按体质量随机分为5组(n=10),分别为空白对照组(NC)、模型组对照组(HFD)、低剂量组(LDG)、中剂量组(MDG)和高剂量组(HDG),试验期间小鼠自由摄食饮水。空白对照组基础饲料喂养,同时每天灌胃等量0.9% NaCl;模型组高脂饲料喂养,同时每天灌胃等量0.9% NaCl;低、中、高剂量组高脂饲料喂养,分别灌胃0.2、0.4、0.8 g/(kg mb·d)的腊梅花提取物,灌胃量按照李国秀[22]所提取的石榴多酚含量换算,所有实验组均连续灌胃4周。
1.2.3 小鼠解剖及组织取材
各组小鼠连续灌胃4周,每周称重,末次给药后禁食不禁水12 h,随后小鼠采用眼球取血,保留血液,解剖小鼠,将小鼠的肝脏、心脏、肺、肾脏等器官取出,用生理盐水冲洗干净,滤纸吸水后称重记录,其中肝脏分为两份,一份作为病理切片保存在10%组织固定液中,另一份及其他器官均保存在−80 ℃冰箱,作为测定酶活组织。
1.2.4 指标的测定
1.2.4.1 小鼠脏器系数的测定
根据实验期间记录的小鼠肝脏重量,按照下列公式计算肝脏系数[23]。
肝脏指数(%)=mM×100 式中:m表示肝脏质量,g;M表示小鼠体重,g。
1.2.4.2 血清生化指标的检测
小鼠眼部静脉取血,将血液置于离心管内,4 ℃冰箱保存2~3 h,然后3500 r/min离心8 min,取上清液。低密度脂蛋白胆固醇(LDL-C)、高密度脂蛋白胆固醇(HDL-C)、总胆固醇(TC)和总甘油三酯(TG)含量均按照试剂盒要求进行测定。
1.2.4.3 内脏组织的酶活测定
将小鼠内脏组织低温解冻,滤纸吸水,称重,用剪刀将组织剪碎,加入9倍质量的生理盐水,用组织研磨器充分研磨,研磨液于4 ℃,3500 r/min条件下离心10 min,取上清液即为10%组织匀浆液。MDA、GSH含量和CAT、GSH-Px、SOD酶活均按照试剂盒要求进行测定。
1.2.4.4 病理组织切片
取10%组织固定液中的肝脏及胰腺组织进行切片制作,通过脱水、透明、浸蜡、包埋、切片和HE染色等步骤制作病理切片[24],经显微镜镜检和图像采集分析,观察病理组织学变化。
1.3 数据处理
实验数据均以¯X±s表示,应用SPSS 20.0 统计软件进行独立样本t检验分析,确定两组数据有无差异。以P<0.05为显著性差异水平,以P<0.01为极显著性差异水平。
2. 结果与分析
2.1 腊梅花提取物对各组小鼠体质量和脏器系数的影响
体重变化是小鼠饲养过程中最直观的观测指标,通过检测小鼠体重可知小鼠是否正常生长或出现超重肥胖等现象。小鼠内脏器官的重量和脏器系数是小鼠的生物学体性之一,作为动物实验的重要观测指标,脏器系数也是鉴定动物遗传品质的重要依据[25],在生物医学等科学研究中,脏器系数通常被用来衡量动物的状态[26]。
由表1所示,五组小鼠初始体重无显著性差异(P>0.05),经过四周的喂养,模型对照组小鼠最终体重和肝脏质量均显著高于空白对照组(P<0.05),说明高脂膳食会引起小鼠体重和肝脏重量增加。各剂量组最终体重和肝脏质量均低于模型对照组,且存在显著性差异(P<0.05),说明CPFE可以预防高脂膳食诱导小鼠体重和肝脏质量的增加。各组肝脏系数无显著性差异(P>0.05),分析原因可能是饲养周期过短,肝脏受高脂饮食影响较轻,肝脏内脂肪积蓄不足,体重增长速度大于肝脏质量的增长速度,导致各组肝脏系数均无显著性差异。
表 1 腊梅花提取物对小鼠肝脏系数的影响Table 1. Changes of liver coefficient of mice组别/指标 初始体重(g) 最终体重(g) 肝脏重量(g) 肝脏系数(%) NC 20.75±0.23 23.06±0.68 0.96±0.22 4.16±0.64 HFD 20.94±0.59 25.55±0.75* 1.13±0.18* 4.89±0.88 LDG 20.87±0.54 24.00±0.58*# 0.92±0.11# 4.27±0.47 MDG 20.83±0.49 23.73±0.82*# 0.86±0.11*# 4.25±0.75 HDG 20.76±0.76 23.07±0.64# 1.02±0.11*# 4.53±0.28 注:*表示与正常组小鼠对比有显著性差异P<0.05; #表示与模型组小鼠对比有显著性差异P<0.05,表2同。 2.2 腊梅花提取物对各组小鼠血清生化指标的影响
本研究通过测定小鼠血清TC、TG、LDL-C、HDL-C水平分析评价腊梅花提取物的降血脂功效。胆固醇(Cholesterol)是一种人体必需的甾醇类化合物,在人体内具有重要的生理作用,维持着人体细胞和系统的正常稳态运行[27]。胆固醇分为高密度脂蛋白胆固醇(HDL-C)和低密度脂蛋白胆固醇(LDL-C),高密度脂蛋白胆固醇可以起到保护心血管的作用,被认为是“好胆固醇”,而低密度脂蛋白胆固醇偏高则会导致冠心病和粥样硬化型心脏病[28],因而被称为“坏胆固醇”。
如表2所示。模型对照组小鼠血清LDL-C、TC水平极显著高于正常组(P<0.01),说明高脂饲料引起小鼠体内血脂代谢紊乱。而剂量组的LDL-C、TC水平降低,具有统计学意义(P<0.05),说明CPFE干预有助于改善高脂小鼠血浆的LDL-C和TC值,使之接近正常水平。模型对照组HDL-C较空白对照组上升,说明高脂膳食的摄入没有导致小鼠血浆HDL-C水平下降,但是各剂量组HDL-C含量仍然高于模型组,说明CPFE的摄入可以增加HDL-C含量,且中、高剂量组HDL-C水平与HDF组具有显著性(P<0.05)。从TG水平来看,各剂量组较空白对照组和模型对照组降低,其中HDG组效果最为明显,较模型组下降了22.73%(P<0.05),说明CPFE干预能降低小鼠血浆TG水平。Ishimwe等[29]的研究表明血清中TC、LDL-C水平每降低1%,心脏病的风险将会分别降低2%、1%,说明腊梅花提取物具有降低高脂小鼠患心脏病风险的作用。
表 2 腊梅花提取物对小鼠血清四项的影响Table 2. Blood biochemical indicators组别/指标 LDL-C (nmol/L) HDL-C (nmol/L) TC (nmol/L) TG (nmol/L) 正常组 0.53±0.21 0.93±0.55 2.60±0.57 1.10±0.22 对照组 0.91±0.19* 2.10±0.31* 4.78±0.42* 1.10±0.16 低剂量组 0.69±0.14*# 2.14±0.27* 3.60±0.55*# 1.01±0.17 中剂量组 0.61±0.11*# 2.42±0.35*# 3.56±0.67*# 1.01±0.19 高剂量组 0.57±0.19# 2.73±0.15*# 3.52±0.43*# 0.85±0.18*# 2.3 腊梅花提取物对小鼠抗氧化防御系统的影响
丙二醛(MDA) 是动物体内脂质过氧化代谢产物,被用作组织损伤的指示物[30],其含量越高说明机体氧化损伤越严重。人体内酶促抗氧化系统主要包括过氧化氢酶(CAT)、超氧化物歧化酶(SOD)、和谷胱甘肽过氧化物酶(GSH-PX)等,谷胱甘肽(GSH)则是一种非酶型抗氧化剂,人体内各种抗氧化剂相互协同清除机体产生的自由基[31],维持机体各项生理活动正常运行。
2.3.1 腊梅花提取物对小鼠肝脏组织抗氧化防御系统的影响
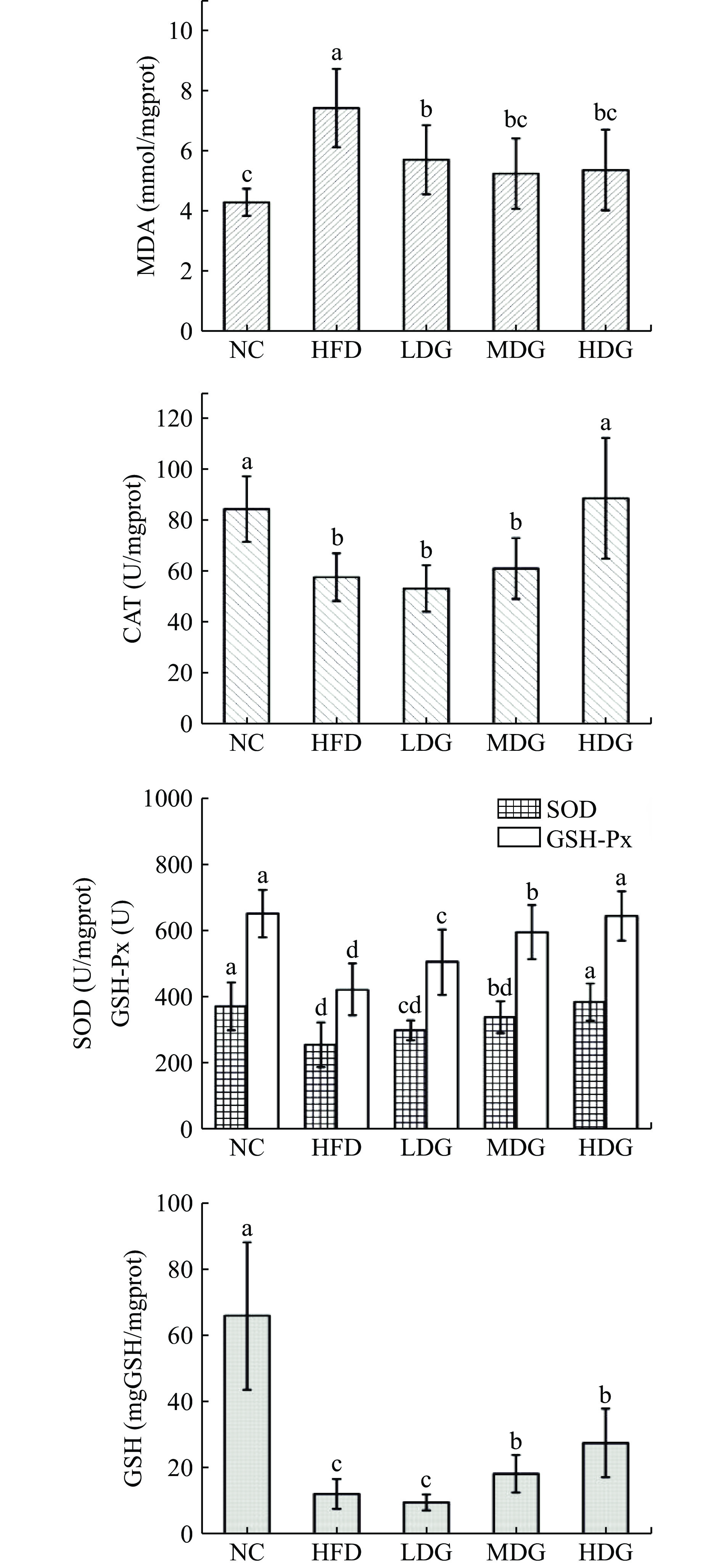
图1为CPFE和高脂膳食对肝脏抗氧化能力的影响。分析结果可知,模型对照组与空白对照组比较,MDA含量显著性升高(P<0.05),CAT、SOD、GSH-Px酶活、GSH含量呈显著性下降(P<0.05)。各剂量组MDA含量较模型对照组均有所下降,且都具有显著性(P<0.05)。肝脏抗氧化酶CAT、GSH-Px、SOD活性整体呈现出正常组>高剂量组>中剂量组>低剂量组>模型组的趋势;可以看出空白组小鼠抗氧化酶的活力最强,高脂膳食处理后的小鼠肝脏酶活下降,进一步下去可能会造成肝脏的不可逆损伤,而各剂量组的酶活较模型组呈现上升趋势,且各HDG组具有显著差异性(P<0.05)。模型组GSH水平较空白对照组显著下降(P<0.05),各剂量组GSH水平较模型对照组有所升高,且中、高剂量组呈显著性升高(P<0.01),但无法使之恢复到正常水平。
2.3.2 腊梅花提取物对小鼠肾脏组织抗氧化防御系统的影响
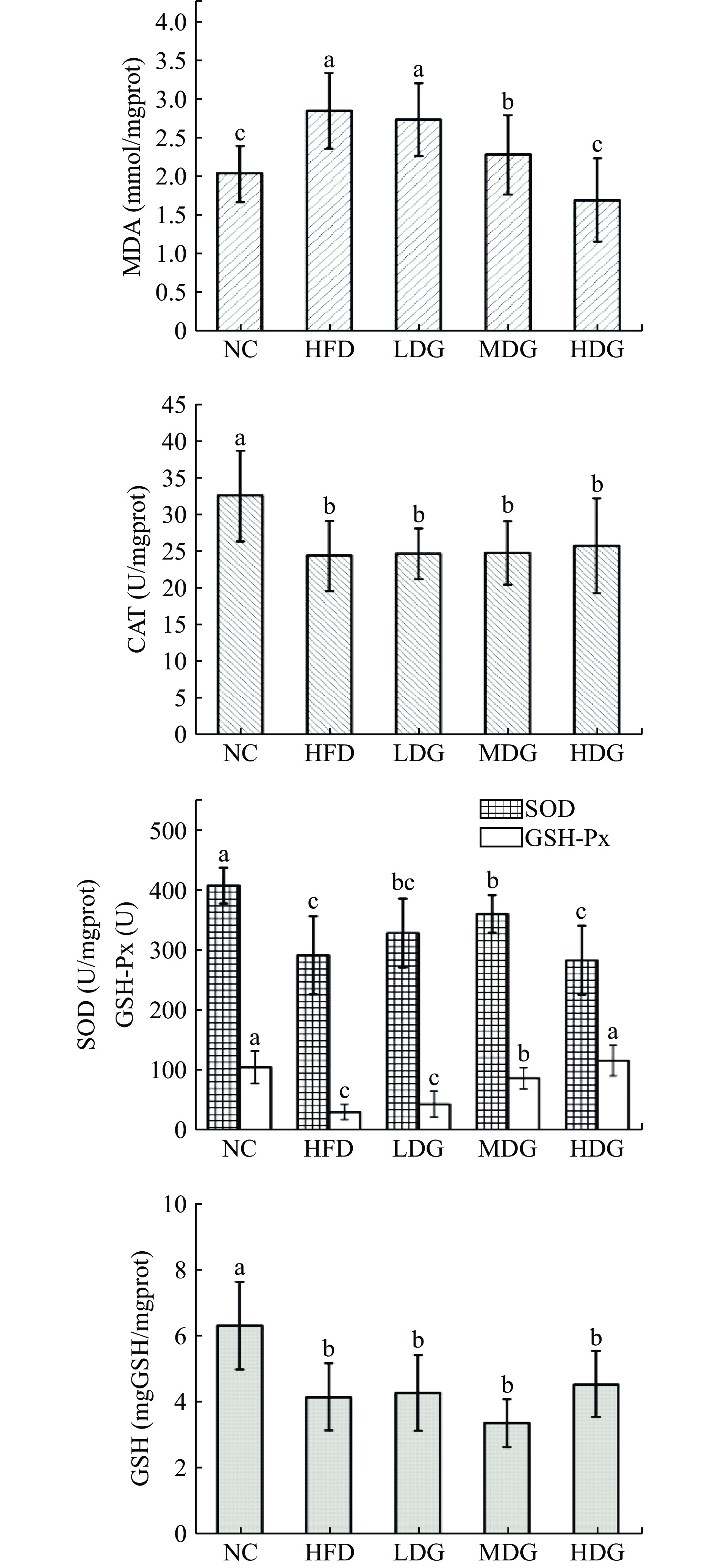
图2为CPFE和高脂膳食对肾脏抗氧化能力的影响。可看出相较于空白对照组,模型对照组肾脏MDA的含量显著上升(P<0.05),三种抗氧化酶和GSH含量显著性降低(P<0.05)。剂量组相较于模型对照组,MDA含量有所下降,HDG小鼠下降极为明显(P<0.05);与HFD相比,各剂量组CAT活力和GSH含量无明显升高(P>0.05),SOD、GSH-Px活力显著升高(P<0.05)。
2.3.3 腊梅花提取物对小鼠肺组织抗氧化防御系统的影响
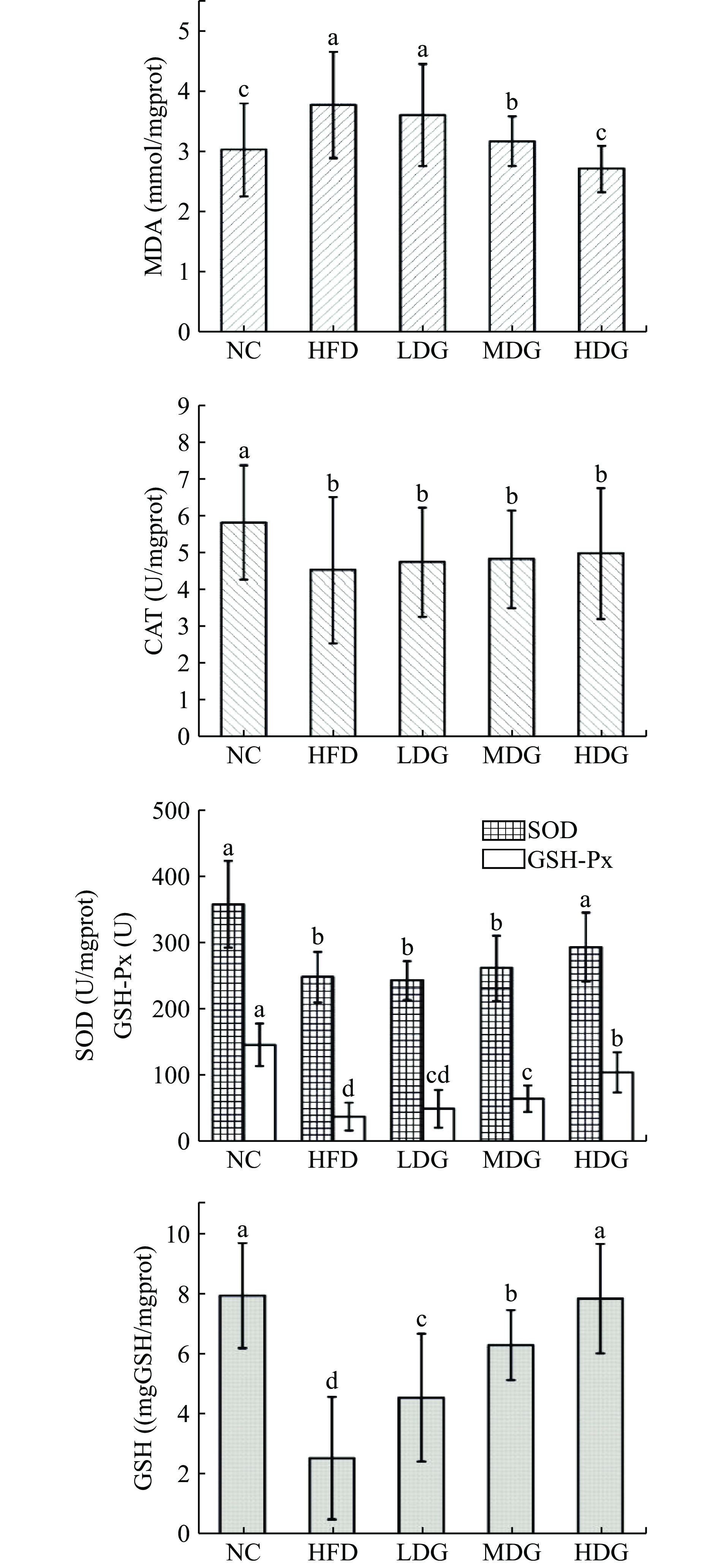
图3为CPFE和高脂膳食对肺组织抗氧化能力的影响。与空白对照组相比,模型对照组肺部的MDA含量显著上升(P<0.05),CAT、SOD、GSH-Px活力和GSH含量显著下降(P<0.05)。各剂量组MDA含量相较于模型对照组有所下降,呈剂量依赖,HDG组小鼠肺组织MDA含量最低(P<0.05);剂量组CAT活力含量较模型对照组无显著性升高(P>0.05);GSH活力较模型对照组明显升高(P<0.05),呈剂量效应,MDG、HDG组GSH活力极显著高于模型对照组(P<0.01);SOD、GSH-Px活力小幅度上升,只有HDG组与模型对照组呈显著性差异(P<0.05)。
综上所述,实验通过测定肝脏、肾脏、肺组织的SOD、GSH-Px、CAT活力与MDA、GSH含量来探究五组小鼠的体内抗氧化性差异。由实验结果可知,空白组小鼠的体内抗氧化能力最强,模型组小鼠体内抗氧化能力最弱,表明高脂膳食的摄入导致了小鼠体内抗氧化能力下降,造成了小鼠组织细胞氧化损伤,产生过氧化产物,造模成功。随着高脂饮食摄入时间的增长,小鼠体内的抗氧化能力可能会进一步下降,给小鼠带来不可逆损伤,有形成脂肪肝和肥胖型代谢疾病的可能。而CPFE干预的小鼠体内抗氧化能力强于模型组,说明CPFE发挥了抗氧化作用,其中对肝脏组织抗氧化防御机制的保护效果最明显,在CPFE的作用下,三种抗氧化酶(CAT、SOD、GSH-Px)的活力显著增强,呈剂量效应,HDG组小鼠肝脏酶活力恢复至与NC组无显著性差异(P>0.05),过氧化产物(MDA)含量较模型对照组显著降低;对增强肾脏和肺组织抗氧化能力的效果较弱,实验结果与李新月等[32]的研究一致,分析产生这种现象的主要原因可能是实验周期较短,肝脏作为动物体内的代谢和解毒枢纽,外源性物质被肠道上皮细胞吸收,经门静脉循环到血液后,再到达肝脏[33−34],分解吸收后再进入人体循环,因此药物对肝脏的影响更为显著。
2.4 腊梅提取物对小鼠肝脏组织的影响
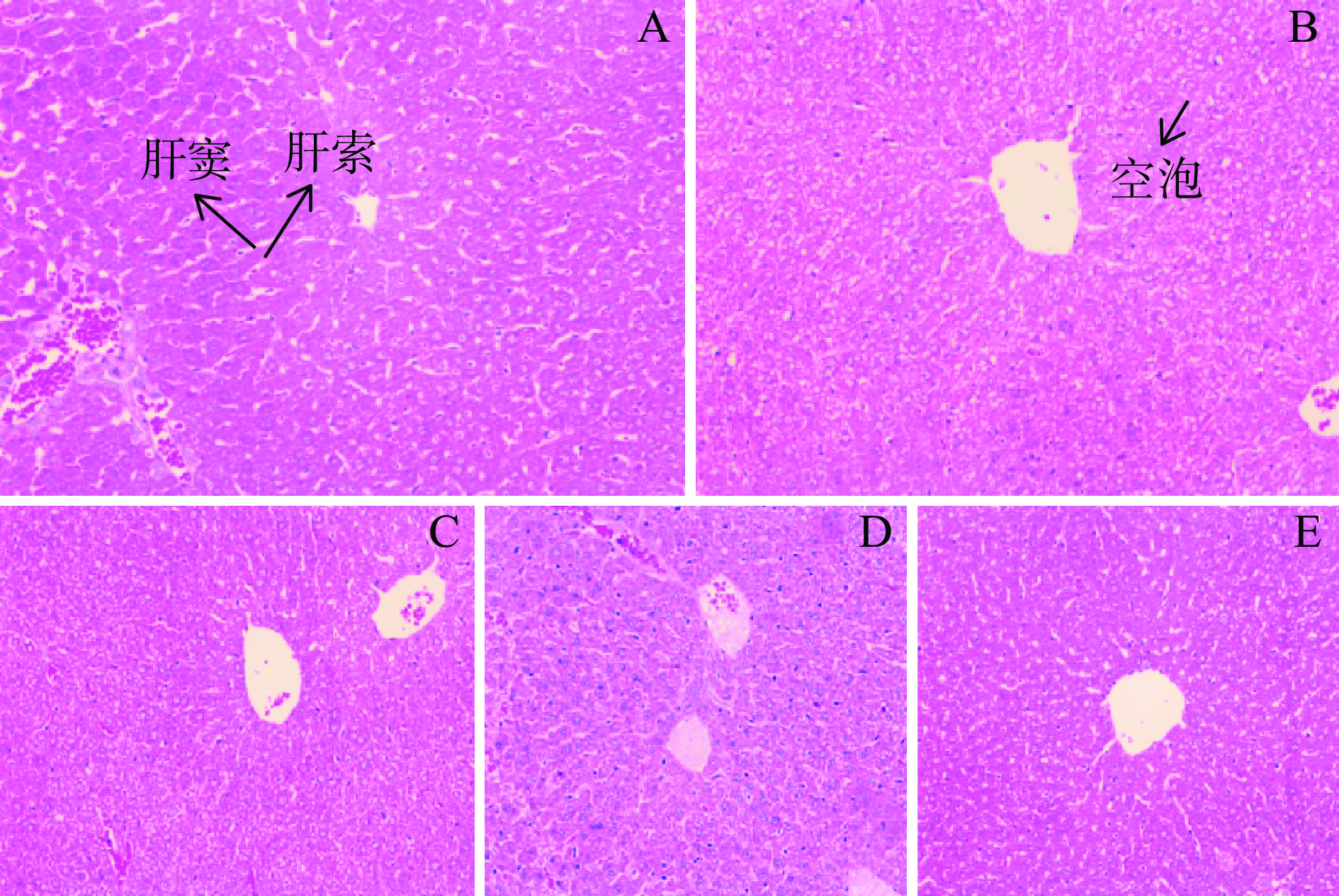
从图4A可以看出,空白对照组小鼠的肝小叶结构比较清晰,肝细胞呈放射状紧密排列在中央静脉四周;肝细胞的形态、大小都很正常,细胞质紧密包裹细胞核,连接各个肝细胞组成条索状的肝索,肝血窦正常排列在四周,细胞状态良好。模型对照组(图4B)小鼠肝小叶结构无规则,肝细胞肿胀,细胞质与细胞核之间存在空隙出现空泡变性,且肝索不清晰,肝窦被挤压变形,考虑高脂膳食造成了肝脏细胞损伤。图4C~图4E分别为LDG、MDG、HDG组小鼠肝脏切片。LDG组小鼠肝脏细胞肝窦受挤压变形,但肝索结构较清晰、细胞质较好地包裹细胞核、细胞空泡化较轻;MDG组小鼠肝细胞质紧密包裹细胞核,连接各细胞呈现清晰的肝索,部分周围分布正常肝窦,细胞受损较轻;HDG组小鼠肝细胞肝索、肝窦更加清晰,且排列规则,细胞有少量空泡变性,受损情况较模型对照组有明显减轻[35]。
由以上结果可知,包括三个剂量组在内的高脂膳食摄入组,小鼠均出现了肝脏组织形态和肝脏细胞功能损坏,出现脂肪细胞水肿变形。已经有研究表明,长期摄入高脂饮食,会导致出现动脉粥样化[36]、脂代谢和肠道菌群紊乱[37]、糖尿病[38]、高脂血症[39]等疾病。但从图4C、图4D、图4E可以看出,剂量组与模型组相比,肝组织中肝小叶结构更加完整,脂质空泡数量减少,尤其是HDG组表现出更好的效果,说明CPFE的干预能有效保护和修复肝脏组织细胞的功能和形态,减轻高脂膳食对小鼠肝脏细胞的损伤。
3. 结论
高脂膳食小鼠结束4周的腊梅提取物干预后,实验结果表明腊梅花提取物能有效减缓小鼠体重上升速率,降低小鼠肝脏系数,并且能增强小鼠体内抗氧化能力,抑制高脂膳食引起的MDA含量上升,提高了CAT、SOD、GSH-PX活力和增加了GSH的含量;同时还能改善肝脏因高脂膳食引起的脂肪肝变性,有效抑制脂肪对肝细胞的浸润出现细胞空泡;此外,血生化指标还反映了腊梅花提取物能够显著降低高脂膳食小鼠体内的TG、TC和LDL-C水平,提高HDL-C含量,改善了小鼠血脂代谢异常,基本恢复了高脂膳食破坏的细胞的代谢功能,且0.8 g/(kg mb·d)剂量组腊梅花提取物的效果最好。
综上所述,腊梅花醇提取物对高脂膳食诱导引起小鼠肥胖和血脂代谢异常、肝损伤有良好的改善作用,且腊梅花提取物对这种改善作用具有一定的量效关系,但其作用机制还不够明确,需进一步研究。此研究为腊梅花在食品、饲料、医药等领域的研究提供了理论基础,对于通过饮食预防代谢类疾病,提高机体抗氧化活性提供了可靠的理论依据。
-
表 1 腊梅花提取物对小鼠肝脏系数的影响
Table 1 Changes of liver coefficient of mice
组别/指标 初始体重(g) 最终体重(g) 肝脏重量(g) 肝脏系数(%) NC 20.75±0.23 23.06±0.68 0.96±0.22 4.16±0.64 HFD 20.94±0.59 25.55±0.75* 1.13±0.18* 4.89±0.88 LDG 20.87±0.54 24.00±0.58*# 0.92±0.11# 4.27±0.47 MDG 20.83±0.49 23.73±0.82*# 0.86±0.11*# 4.25±0.75 HDG 20.76±0.76 23.07±0.64# 1.02±0.11*# 4.53±0.28 注:*表示与正常组小鼠对比有显著性差异P<0.05; #表示与模型组小鼠对比有显著性差异P<0.05,表2同。 表 2 腊梅花提取物对小鼠血清四项的影响
Table 2 Blood biochemical indicators
组别/指标 LDL-C (nmol/L) HDL-C (nmol/L) TC (nmol/L) TG (nmol/L) 正常组 0.53±0.21 0.93±0.55 2.60±0.57 1.10±0.22 对照组 0.91±0.19* 2.10±0.31* 4.78±0.42* 1.10±0.16 低剂量组 0.69±0.14*# 2.14±0.27* 3.60±0.55*# 1.01±0.17 中剂量组 0.61±0.11*# 2.42±0.35*# 3.56±0.67*# 1.01±0.19 高剂量组 0.57±0.19# 2.73±0.15*# 3.52±0.43*# 0.85±0.18*# -
[1] BIJLSMA J W J, BERENBAUM F, LAFEBER F P J G. Osteoarthritis:An update with relevance for clinical practice[J]. Lancet,2011,377(9783):2115−2126. doi: 10.1016/S0140-6736(11)60243-2
[2] 王静, 黄庆辰, 高敏玥, 等. 连翘叶连翘苷提取物对高脂饮食诱导大鼠肥胖的预防作用[J]. 食品科学,2021,42(9):85−90. [WANG Jing, HUANG Qingchen, GAO Minyue, et al. Preventive effect of Forsythin from Forsythia suspensa leaves on obesity induced by high-fat diet in rats[J]. Food Science,2021,42(9):85−90.] WANG Jing, HUANG Qingchen, GAO Minyue, et al . Preventive effect of Forsythin from Forsythia suspensa leaves on obesity induced by high-fat diet in rats[J]. Food Science,2021 ,42 (9 ):85 −90 .[3] 张彦康, 张婷, 李雨, 等. 肥胖治疗的挑战与希望[J]. 自然杂志,2022,44(6):469−479. [ZHANG Yankang, ZHANG Ting, LI Yu, et al. Challenges and hopes in obesity intervention[J]. Chinese Journal of Nature,2022,44(6):469−479.] ZHANG Yankang, ZHANG Ting, LI Yu, et al . Challenges and hopes in obesity intervention[J]. Chinese Journal of Nature,2022 ,44 (6 ):469 −479 .[4] ALLISON D B, GADDE K M, GARVEY W T, et al. Controlled-release phentermine/topiramate in severely obese adults:A randomized controlled trial (EQUIP)[J]. Obesity (Silver Spring, Md.),2012,20(2):330−342. doi: 10.1038/oby.2011.330
[5] VERBEEK R, HOOGEVEEN R M, LANGSTED A, et al. Cardiovascular disease risk associated with elevated lipoprotein(a) attenuates at low low-density lipoprotein cholesterol levels in a primary prevention setting[J]. European Heart Journal: The Journal of the European Society of Cardiology,2018,39(27):2589−2596.
[6] 中国科学院中国植物志编辑委员会. 中国植物志[M]. 北京:科学出版社, 1998. [Editorial Committee of Flora of China, Chinese Academy of Sciences. Flora of China[M]. Beijing:Science Press, 1998.] Editorial Committee of Flora of China, Chinese Academy of Sciences. Flora of China[M]. Beijing: Science Press, 1998.
[7] 刘志雄, 刘祝祥. 超临界CO2萃取腊梅籽化学成分研究[J]. 中药材, 2008, 31(7):992−995. [LIU Zhixiong, LIU Zhuxiang. Chemical constituents from the seed of Chimonanthus praecox extracted by supercritical carbondioxide[J]. Journal of Chinese Medicinal Materials, 31(7):992−995.] LIU Zhixiong, LIU Zhuxiang. Chemical constituents from the seed of Chimonanthus praecox extracted by supercritical carbondioxide[J]. Journal of Chinese Medicinal Materials, 31(7): 992−995.
[8] KITAJIMA M, MORI I, ARAI K, et al. Two new tryptamine-derived alkaloids from Chimonanthus praecox F. concolor[J]. Tetrahedron Letters,2006,47(19):3199−3202. doi: 10.1016/j.tetlet.2006.03.047
[9] WANG W X, CAO L, XIONG J, et al. Constituents from Chimonanthus praecox (wintersweet)[J]. Phytochemistry Letters,2011,4(3):271−274. doi: 10.1016/j.phytol.2011.04.012
[10] ZHANG J W, GAO J M, XU T, et al. Antifungal activity of alkaloids from the seeds of Chimonanthus praecox[J]. Chemistry & Biodiversity,2010,6(6):838−845.
[11] SUN Q, ZHU J J, CAO F W, et al. Anti-inflammatory properties of extracts from Chimonanthus nitens Oliv. leaf[J]. PLoS ONE,2017,12(7):e0181094. doi: 10.1371/journal.pone.0181094
[12] 赵梓桐, 李景恩, 张清峰, 等. 柳叶腊梅叶乙醇提取物成分分析及其体外抗氧化研究[J]. 江西农业大学学报,2021,43(3):665−674. [ZHAO Zitong, Li Jingen, ZHANG Qingfeng, et al. A study on constituents and antioxidant activity of the alcohol extract of Chimonanthus salicifolius S. Y. Hu. leaves[J]. Acta Agriculturae Universitatis Jiangxiensis,2021,43(3):665−674.] ZHAO Zitong, Li Jingen, ZHANG Qingfeng, et al . A study on constituents and antioxidant activity of the alcohol extract of Chimonanthus salicifolius S. Y. Hu. leaves[J]. Acta Agriculturae Universitatis Jiangxiensis,2021 ,43 (3 ):665 −674 .[13] CHEN H, JIANG Y, YANG Z W, et al. Effects of Chimonanthus nitens Oliv. leaf extract on glycolipid metabolism and antioxidant capacity in diabetic model mice[J]. Oxidative medicine and cellular longevity,2017,2017:7648505.
[14] 王延, 伍沙沙, 徐婷, 等. 山腊梅叶挥发油对血管性痴呆大鼠认知障碍的改善作用[J]. 安徽农业科学,2021,49(2):170−174. [WANG Yan, WU Shasha, XU Ting, et al. Improving effect of volatile oil from Chimonanthus nitens Oliv. leaves on cognitive disorder inrats with vascular dementia[J]. Journal of Anhui Agricultural Sciences,2021,49(2):170−174.] WANG Yan, WU Shasha, XU Ting, et al . Improving effect of volatile oil from Chimonanthus nitens Oliv. leaves on cognitive disorder inrats with vascular dementia[J]. Journal of Anhui Agricultural Sciences,2021 ,49 (2 ):170 −174 .[15] 袁青青, 张行, 李媛, 等. 山腊梅叶挥发油镇咳、平喘、祛痰、抗炎及免疫调节作用研究[J]. 亚太传统医药,2018,14(12):17−19. [YUAN Qingqing, ZHANG Hang, LI Yuan, et al. Study on antitusive, antiasthmatic, expectorant, antinflammatory and immunomodulatory efects of the essentiai oil of Chimonanthus nitens Oliv leaves[J]. Asia-Pacific Traditional Medicine,2018,14(12):17−19.] YUAN Qingqing, ZHANG Hang, LI Yuan, et al . Study on antitusive, antiasthmatic, expectorant, antinflammatory and immunomodulatory efects of the essentiai oil of Chimonanthus nitens Oliv leaves[J]. Asia-Pacific Traditional Medicine,2018 ,14 (12 ):17 −19 .[16] 艾安涛, 何文毅, 潘清华, 等. 腊梅花茶加工工艺研究[J]. 广东茶业,2020,42(4):12−17. [AI Antao, HE Wenyi, PAN Qinghua, et al. Study on the processing technology of Chimonanthus praecox flower tea[J]. Guangdong Tea Industry,2020,42(4):12−17.] AI Antao, HE Wenyi, PAN Qinghua, et al . Study on the processing technology of Chimonanthus praecox flower tea[J]. Guangdong Tea Industry,2020 ,42 (4 ):12 −17 .[17] 包善思, 姚瑶, 孙博瑞, 等. 腊梅花抗氧化活性物质微波辅助提取的响应面优化研究[J]. 食品科技,2019,44(10):229−234. [BAO Shansi, YAO Yao, SUN Borui, et al. Optimization for microwave-assisted extraction of antioxidant active substances in plum blossoms by response surfaces[J]. Food Science and Technology,2019,44(10):229−234.] BAO Shansi, YAO Yao, SUN Borui, et al . Optimization for microwave-assisted extraction of antioxidant active substances in plum blossoms by response surfaces[J]. Food Science and Technology,2019 ,44 (10 ):229 −234 .[18] 杨浩, 王文杰, 雷霜江, 等. 腊梅花多酚类物质及挥发油的成分分析[J]. 食品工业,2022,43(4):302−306. [YANG Hao, WANG Wenjie, LEI Shuangjiang, et al. Analysis of polyphenols and volatile oil from Chimonanthus praecox[J]. The Food Industry,2022,43(4):302−306.] YANG Hao, WANG Wenjie, LEI Shuangjiang, et al . Analysis of polyphenols and volatile oil from Chimonanthus praecox[J]. The Food Industry,2022 ,43 (4 ):302 −306 .[19] KIOKIAS S, PROESTOS C, OREOPOULOU V. Phenolic acids of plant origin-a review on their antioxidant activity in vitro (O/W emulsion systems) along with their in vivo health biochemical properties[J]. Foods,2020,9(4):534. doi: 10.3390/foods9040534
[20] LESJAK M, BEARA I, SIMIN N, et al. Antioxidant and anti-inflammatory activities of quercetin and its derivatives[J]. Journal of Functional Foods,2018,40:68−75. doi: 10.1016/j.jff.2017.10.047
[21] GULLON B, LU-CHAU T A, MOREIRA M T, et al. Rutin:A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability[J]. Food Science & Technology,2017,67:220−235.
[22] 李国秀. 石榴多酚类物质的分离鉴定和抗氧化活性研究[D]. 西安:陕西师范大学, 2008:27−28. [LI Guoxiu. Separation and identification of polyphenols in pomegranate fruit and its antioxidant activities[D]. Xi’an:Shanxi Normal University, 2008:27−28.] LI Guoxiu. Separation and identification of polyphenols in pomegranate fruit and its antioxidant activities[D]. Xi’an: Shanxi Normal University, 2008: 27−28.
[23] 裘迪红, 黄丽华, 陈哲科, 等. 植物乳杆菌发酵乳对小鼠降胆固醇作用[J]. 食品工业科技,2018,39(15):313−316. [QIU Dihong, HUANG Lihua, CHEN Zheke, et al. Hypocholesterolemic effect of yogurt fermented with Lactobacillus plantarum on mice[J]. Science and Technology of Food Industry,2018,39(15):313−316.] QIU Dihong, HUANG Lihua, CHEN Zheke, et al . Hypocholesterolemic effect of yogurt fermented with Lactobacillus plantarum on mice[J]. Science and Technology of Food Industry,2018 ,39 (15 ):313 −316 .[24] 孙悦, 张文龙, 李楠, 等. 沙棘熊果酸对酒精性肝损伤大鼠肝FXR信号通路的影响[J]. 食品工业科技,2023,44(5):363−370. [SUN Yue, ZHANG Wenlong, LI Nan, et al. Effect of ursolic acid extracted from Hippophae rhamnoides L. on FXR signaling pathway in liver of rats with alcoholic liver injury[J]. Science and Technology of Food Industry,2023,44(5):363−370.] SUN Yue, ZHANG Wenlong, LI Nan, et al . Effect of ursolic acid extracted from Hippophae rhamnoides L. on FXR signaling pathway in liver of rats with alcoholic liver injury[J]. Science and Technology of Food Industry,2023 ,44 (5 ):363 −370 .[25] 孙建新, 安娟, 连军. 影响实验动物脏器重量及脏器系数因素分析[J]. 实验动物科学,2009,26(1):49−51. [SUN Jianxin, AN Juan, LIAN Jun. Analysis of factors affecting organ weight and organ coefficient in experimental animals[J]. Laboratory Animal Science,2009,26(1):49−51.] SUN Jianxin, AN Juan, LIAN Jun . Analysis of factors affecting organ weight and organ coefficient in experimental animals[J]. Laboratory Animal Science,2009 ,26 (1 ):49 −51 .[26] 恽时锋, 胡玉红, 田小芸. 不同品种实验兔主要脏器重量及脏器系数的研究[J]. 中国比较医学杂志,2004,14(6):34−38. [YUN Shifeng, HU Yuhong, TIAN Xiaoyun. Study on the chief organ weight and coefficients of different species of experimental rabbits[J]. Chinese Journal of Comparative Medicine,2004,14(6):34−38.] YUN Shifeng, HU Yuhong, TIAN Xiaoyun . Study on the chief organ weight and coefficients of different species of experimental rabbits[J]. Chinese Journal of Comparative Medicine,2004 ,14 (6 ):34 −38 .[27] LUO J, YANG H, SONG B L. Mechanisms and regulation of cholesterol homeostasis[J]. Nature Reviews Molecular Cell Biology,2020,21(4):225−245. doi: 10.1038/s41580-019-0190-7
[28] 隋棠. 糯米红曲对高胆固醇小鼠炎症的影响研究[D]. 合肥:安徽农业大学, 2021:6−7. [SUI Tang. Study on the effect of red yeast glutinous rice on inflammation in mice with high cholesterol[D]. Hefei:Anhui Agricultural University, 2021:6−7.] SUI Tang. Study on the effect of red yeast glutinous rice on inflammation in mice with high cholesterol[D]. Hefei: Anhui Agricultural University, 2021: 6−7.
[29] ISHIMWE N, DALIRI E B, LEE B H, et al. The perspective on cholesterol-lowering mechanisms of probiotics[J]. Molecular Nutrition and Food Research,2015,59(1):94−105. doi: 10.1002/mnfr.201400548
[30] 张秋萍, 吴霞红, 郑剑恒, 等. 生物样本中丙二醛测定方法的研究进展[J]. 理化检验(化学分册),2016,52(8):979−985. [ZHANG Qiuping, WU Xiahong, ZHENG Jianheng, et al. Progress of researches on methods for determination of malondialdehyde in biological samples[J]. Physical Testing and Chemical Analysis (Part B:Chemical Analysis),2016,52(8):979−985.] ZHANG Qiuping, WU Xiahong, ZHENG Jianheng, et al . Progress of researches on methods for determination of malondialdehyde in biological samples[J]. Physical Testing and Chemical Analysis (Part B:Chemical Analysis),2016 ,52 (8 ):979 −985 .[31] 谢小俊. 葛根黄酮对高脂膳食小鼠体内抗氧化和体外抗氧化研究[D]. 南昌:江西农业大学, 2015:5. [XIE Xiaojun. Research on the vivo-antioxidant and vitro-antioxidant effect of kudzu flavonoids in high-fet diet-fed mice[D]. Nanchang:Jiangxi Agricultural University, 2015:5.] XIE Xiaojun. Research on the vivo-antioxidant and vitro-antioxidant effect of kudzu flavonoids in high-fet diet-fed mice[D]. Nanchang: Jiangxi Agricultural University, 2015: 5.
[32] 李新月, 赵猛, 丁子康, 等. 藻蓝蛋白对环磷酰胺致小鼠肝肾损伤的保护作用[J]. 食品工业科技,2023,44(10):379−386. [LI Xinyue, ZHAO Meng, DING Zikang, et al. Protective effect of phycocyanin on cyclophosphamide- induced immunocompromised mice[J]. Science and Technology of Food Industry,2023,44(10):379−386.] LI Xinyue, ZHAO Meng, DING Zikang, et al . Protective effect of phycocyanin on cyclophosphamide- induced immunocompromised mice[J]. Science and Technology of Food Industry,2023 ,44 (10 ):379 −386 .[33] SAEEDI B J, LIU K H, OWENS J A, et al. Gut-resident Lactobacilli activate hepatic Nrf2 and protect against oxidative liver injury[J]. Cell Metabolism,2020,31(5):1−13.
[34] WANG Z Y, ZENG M M, WANG Z J, et al. Dietary polyphenols to combat nonalcoholic fatty liver disease via the gut-brain-liver axis:A review of possible mechanisms[J]. Journal of Agricultural and Food Chemistry,2021,69(12):3585−3600.
[35] 李晓东, 封德梅, 赵良功, 等. 红芪多糖HPS-3对2型糖尿病大鼠糖脂代谢和胰腺组织病理改变的影响[J]. 中药药理与临床,2012,28(1):83−86. [LI Xiaodong, FENG Demei, ZHAO Lianggong, et al. Effects of Hedysari polysacchcride-3 on metabolism of glucose and lipid and pathological changes of pancreatic tissue in experimental type-2 diabetic rats[J]. Pharmacology and Clinics of Chinese Materia Medica,2012,28(1):83−86.] LI Xiaodong, FENG Demei, ZHAO Lianggong, et al . Effects of Hedysari polysacchcride-3 on metabolism of glucose and lipid and pathological changes of pancreatic tissue in experimental type-2 diabetic rats[J]. Pharmacology and Clinics of Chinese Materia Medica,2012 ,28 (1 ):83 −86 .[36] 张丽阳, 孙军, 陈迪, 等. 苦龙胆酯苷拮抗高脂饮食所致的小鼠动脉粥样硬化[J]. 中国动脉硬化杂志,2023,31(5):399−403. [ZHANG Liyang, SUN Jun, CHEN Di, et al. Amarogentin antagonizes atherosclerosis induced by high fat diet in mice[J]. Chinese Journal of Arteriosclerosis,2023,31(5):399−403.] ZHANG Liyang, SUN Jun, CHEN Di, et al . Amarogentin antagonizes atherosclerosis induced by high fat diet in mice[J]. Chinese Journal of Arteriosclerosis,2023 ,31 (5 ):399 −403 .[37] 安玉红, 关天琪, 郭艳红, 等. 元宝枫籽油对高脂膳食小鼠脂代谢及肠道健康的影响[J]. 中国油脂,2022,47(8):103−108. [AN Yuhong, GUAN Tianqi, GUO Yanhong, et al. Effects of Acertruncatum seed oil on lipid metabolism and intestinal health of high-fat diet mice[J]. China Oils and Fats,2022,47(8):103−108.] AN Yuhong, GUAN Tianqi, GUO Yanhong, et al . Effects of Acertruncatum seed oil on lipid metabolism and intestinal health of high-fat diet mice[J]. China Oils and Fats,2022 ,47 (8 ):103 −108 .[38] 任欣, 张付龙, 秦梦园, 等. 小米饲料干预对高脂膳食联合STZ诱导糖尿病大鼠肠道菌群的影响[J]. 粮油食品科技,2022,30(4):76−84,11. [REN Xin, ZHANG Fulong, QIN Mengyuan, et al. Effect of foxtail millet intervention on gut microbiota in high-fat-diet and STZ induced diabetic rats[J]. Science and Technology of Cereals, Oils and Foods,2022,30(4):76−84,11.] REN Xin, ZHANG Fulong, QIN Mengyuan, et al . Effect of foxtail millet intervention on gut microbiota in high-fat-diet and STZ induced diabetic rats[J]. Science and Technology of Cereals, Oils and Foods,2022 ,30 (4 ):76 −84,11 .[39] 尼格尔热依·亚迪卡尔, 彭禛菲, 岳明, 等. 野山杏果肉不溶性膳食纤维对高脂血症大鼠肠道菌群及短链脂肪酸的影响[J]. 食品科技,2022,47(1):190−195,202. [YADIKAR N, PENG Zhenfei, YUE Ming, et al. Effects of insoluble dietary fiber from wild apricot flesh on intestinal flora and short chain fatty acids in hyperlipidemic rats[J]. Food Science and Technolog,2022,47(1):190−195,202.] YADIKAR N, PENG Zhenfei, YUE Ming, et al . Effects of insoluble dietary fiber from wild apricot flesh on intestinal flora and short chain fatty acids in hyperlipidemic rats[J]. Food Science and Technolog,2022 ,47 (1 ):190 −195,202 .





 下载:
下载:
 下载:
下载:



