Liver Toxicity of Rare Ginsenosides in Rats after 13 Weeks of Oral Exposure
-
摘要: 目的:以热转化后的人参稀有皂苷为原料,探究人参稀有皂苷90 d口服对大鼠肝毒性的研究。方法:将48只雌雄大鼠随机分为人参稀有皂苷高剂量(600 mg/kg)组、人参稀有皂苷中剂量(200 mg/kg)组、人参稀有皂苷低剂量(60 mg/kg)组与空白对照组。90 d经口灌胃处理后通过UHPLC-MS对大鼠血清进行代谢组学分析以及肝脏流式细胞凋亡综合分析对大鼠肝脏的潜在损伤。结果:雌雄大鼠高剂量组肝细胞凋亡率和对照组比较,有极显著差异(P<0.01)。代谢组学结果表明,与对照组相比,中低剂量组无显著差异(P>0.05),而高剂量雌性大鼠血清中出现了23种差异代谢物,如组氨酸、谷氨酸、脯氨酸和精氨酸等,并影响了组氨酸和尿素循环等代谢途径,血氨升高,引发肝损伤;在高剂量雄性大鼠血清中出现了10种差异代谢物,如亚油酸和花生四烯酸等,影响了α-亚麻酸和亚油酸代谢途径,高浓度的花生四烯酸表现出致炎性和毒性,可以通过血液吸收进入门静脉系统引起肝损伤。结论:高剂量的人参稀有皂苷对雌雄大鼠有轻微肝损伤,其主要原因是组氨酸和α-亚麻酸和亚油酸代谢途径的改变所导致的,对雌雄大鼠未观察到的肝脏不良反应水平均小于200 mg/kg。
-
关键词:
- 人参稀有皂苷 /
- Sprague Dawley大鼠 /
- 代谢组学 /
- 肝脏 /
- 流式细胞分析
Abstract: Objective: This study aims to evaluate the hepatotoxicity of rare ginsenosides in rats after 90 days of oral administration using heat-transformed rare ginsenosides as the primary material. Methods: A total of 48 male and female rats were randomly assigned into four groups: High-dose rare ginsenosides (600 mg/kg), medium-dose rare ginsenosides (200 mg/kg), low-dose rare ginsenosides (60 mg/kg), and a blank control group. After 90 days of oral gavage treatment, ultra-high-performance liquid chromatography-mass spectrometry (UHPLC-MS) was employed for metabolomic analysis of rat serum and flow cytometry analysis of liver apoptosis to evaluate the potential liver damage comprehensively in rats. Results: A significant difference in hepatocyte apoptotic rate was observed between the high-dose group and the control group in both male and female rats (P<0.01). Metabolomic findings revealed no significant differences in metabolites between the low-dose and medium-dose groups compared to the control group (P>0.05). However, 23 differential metabolites, such as histidine, glutamate, proline and arginine were identified in the serum of female rats in the high-dose group, affecting the histidine and urea cycle metabolic pathways and causing hyperammonemia and liver damage. Ten differential metabolites affecting the alpha-linolenic acid and linoleic acid metabolic pathways were found in male rats, such as linoleic acid and arachidonic acid. High concentrations of arachidonic acid showed inflammatory and toxic effects, which could be absorbed into the portal vein system through blood and cause liver damage. Conclusion: High-dose rare ginsenosides mainly cause slight liver damage in male and female rats mainly due to the changes of histidine, α-linolenic acid and linoleic acid metabolic pathways. Hence, no adverse liver effects were observed at doses less than 200 mg/kg in both male and female rats.-
Keywords:
- rare ginsenosides /
- Sprague Dawley rats /
- metabolomics /
- liver /
- flow cytometry analysis
-
人参是五加科、人参属多年生草本植物,其主要生物活性成分是人参皂苷,如Re、Rg1、Rb1、Rd等[1],而人参皂苷在人参属植物(人参、西洋参、三七)的根、茎和叶、及果实中皆有分布[2−3]。人参制品在亚洲地区有数千年食用历史,安全性较高,其主要活性成分人参皂苷被证实有诸多的活性作用,如抗肿瘤、抗衰老、降血糖等作用[1−2],被广泛用于医药领域和功能食品[3]。因含量低、活性好、化学合成难度大等因素,人参中含量极低的皂苷如Rg3,Rk1,F4,Rg6,Rg5和Rh4等又被称为人参稀有皂苷[4−6]。
近年来,人参皂苷的安全性引起了科研工作者的重视。人参皂苷Re、Rg1对大鼠口服慢性毒性的研究表明,无大鼠死亡,组织病理学检查均未见异常[7−8]。SD大鼠对人参稀有皂苷Rg3的口服最高安全剂量为180 mg/kg[9]。以上研究都证明了人参皂苷有较高的安全性。但在人参皂苷Rb1经肠道细菌代谢转化为人参稀有皂苷化合物K(CK)的口服慢性毒性实验中,发现CK(120 mg/kg)有明显的肝肾毒性,表现为肝肾相对重量增加,并伴有灶性肝坏死和血浆ALT、ALP升高[10]。人参皂苷类型丰富,限于人参稀有皂苷单体的制备成本,其相关的毒性研究较少。传统人参稀有皂苷是通过转化人参属植物的根获取,而人参茎和叶中皂苷的含量高于根部,且茎和叶为非药用部位,采集周期短,材料成本低[11−12]。课题组前期通过热转化人参属植物茎和叶的方法制备出了人参稀有皂苷混合物,主要含Rg3,Rk1,F4,Rg6,Rg5和Rh4等成分,不但提高了人参属植物茎和叶的利用率,也极大地降低了人参稀有皂苷的制备成本[4−5]。
传统毒理学对人参稀有皂苷毒性研究主要从血生化,血常规,体重指数和病理切片等常规方法评价,难以捕捉一些微观的生理变化。而代谢组学作为一种新兴的高通量测定技术,可以快速对大量微观内源性化合物同时进行分析,从更加微观的视角探究机体生理变化[13]。因此本研究在由课题组前期获取人参稀有皂苷混合物的基础上,对SD大鼠进行90 d经口灌胃处理,采用血生化指标,肝脏病理切片和肝脏流式细胞凋亡结合非靶向代谢组学技术综合评估其潜在肝脏毒性,以期为人参稀有皂苷食用的安全性提供数据支持。
1. 材料与方法
1.1 材料与仪器
西洋参茎叶皂苷 吉林宏州生物技术有限公司。将西洋参茎叶皂苷细粉过60目筛后,与0.1%的甲酸混合,在高压蒸汽灭菌锅中140 ℃加热2 h,得到热转化后的皂苷[4−5];SPF级SD大鼠 48只,8周龄,雌雄各半,济南朋悦实验动物繁育有限公司(批号:SCXK(鲁)2019003,370726210100415561号);乙腈(质谱级)、甲醇(质谱级)、细胞凋亡检测试剂盒(FITC Annexin V/Dead Cell Apoptosis Kit) 赛默飞世尔科技有限公司;谷草转氨酶(AST)、低密度脂蛋白胆固醇(LDL-C)、总蛋白(TP)试剂盒 南京建成生物工程研究所;4%多聚甲醛 上海桥星贸易有限公司;苏木素伊红(HE)染色试剂盒 碧云天生物技术有限公司。
Multiskan FC酶标仪、UHPLC-Q-Exactive型超高效液相质谱联用仪、Waters C18色谱柱(3.0 mm×100 mm,1.7 μm) 赛默飞世尔科技有限公司;CytoNova流式细胞仪 北京博奥晶典有限公司;CKX41倒置光学显微镜 日本Olympus有限公司。
1.2 实验方法
1.2.1 动物实验设计
动物实验伦理学申请(2020SDL147)通过后,将48只大鼠适应性喂养1周。课题组前期急毒实验测出人参稀有皂苷的LD50为6 g/kg,按照GB 15193.13-2015食品安全国家标准进行90 d经口毒性试验,以LD50的10%作为高剂量组,递减剂量组间距按照2~4倍设置中剂量和低剂量组,将48只雄性和雌性大鼠,每组6只成年SD大鼠,随机分为4组:低剂量人参稀有皂苷组(60 mg/kg)、中剂量人参稀有皂苷组(200 mg/kg)、高剂量人参稀有皂苷组(600 mg/kg)和空白(蒸馏水)对照组。高中低剂量组以5 mL/kg人参稀有皂苷灌胃,对照组予以等体积蒸馏水。正式分组后,饲养条件保持不变。禁食5 h后每日灌胃,连续90 d。所有大鼠在采血前禁食24 h,用试剂盒测量血清中的生化指标。
1.2.2 肝细胞悬液的制备与细胞凋亡检测
实验结束,处死大鼠后迅速取出肝脏组织,冰上剥离多余的脂肪,放入预先冷藏的生理盐水中漂洗,洗去红细胞,称重,然后置于洁净的培养皿中。用眼科剪将肝小叶剪成小块,将剪碎的肝小叶组织加入提前预冷的PBS(pH7.4)中进行冲洗,过200目的网筛,洗去剪碎的细胞碎片,经过网筛的过滤作用,收集细胞悬液于离心管中,1500 r/min,离心5 min,弃掉上清液,加入适量的不含EDTA的胰蛋白酶消化液,将收集的细胞沉淀在水浴锅中以37 ℃恒温消化10 min,期间不断吹打细胞。水浴结束后,用PBS重新洗涤细胞,再次离心,弃去上清液。按照细胞凋亡检测试剂盒(FITC Annexin V/Dead Cell Apoptosis Kit)说明书,将5X膜联蛋白结合缓冲液稀释成1X膜联蛋白结合缓冲液,并将下层细胞沉淀重新悬浮在1X膜联蛋白结合缓冲液中,确定细胞密度,使得细胞密度不少于1×106 cells/mL,将5 μL的FITC Annexin V和1 μL的PI工作液加入到每100 μL的细胞悬液中,轻轻混匀,使用铝箔避光孵育15 min,尽快用流式细胞仪上机分析[14]。
1.2.3 组织病理学检查
90 d灌胃结束后,对大鼠采血,解剖器官进行尸检,评估组织器官的损伤程度。这些器官和组织被摘除并称重:大脑、心脏、肾脏、肝、肺、脾脏、睾丸、附睾、子宫和卵巢。比较各组绝对脏器重量(g)。用4%多聚甲醛固定,用苏木精和伊红(HE)染色法对肝小叶组织进行染色,制作常规石蜡切片,光镜下观察肝脏组织病理切片的变化。
1.2.4 代谢组学分析
1.2.4.1 血液前处理
血清:75%甲醇=1:3(V:V)混合,12000 r/min离心5 min,取上清,按照上清:二氯甲烷=1:4(V:V)稀释,涡旋振荡30 s混匀,12000 r/min离心5 min,取下层(二氯甲烷层)200 μL,干燥挥发后,用甲醇再复溶,过0.22 μm滤膜[15−16]。
1.2.4.2 液相色谱质谱联用条件
使用UHPLC-Q-Exactive型超高效液相质谱联用仪对血液样本进行代谢组学分析,色谱柱为Waters C18柱(3.0 mm×100 mm,1.7 μm)。流动相A为0.1%的甲酸水溶液,流动相B为乙腈。梯度洗脱条件为:0 min为2% B;2 min为50% B;9 min为98% B;15 min为98% B;20 min为50% B;23 min为2% B;28 min为2% B。速率0.3 mL/min,进样体积为4 μL;质谱条件:电喷雾离子源(ESI),数据采集范围50~1200 m/z,采用Full MS-ddMS模式在正负离子模式下扫描[15−16]。
使用Compounds Discover 3.1.1对液相质谱联用系统导出的.raw文件进行进行峰对齐,峰过滤,峰提取和自动积分。搜索BioCyc、Metabolome Database、Human Metabolome Database、KEGG、Serum Metabolome Database数据库,使用保留时间RT、分子式和精确分子量等对化合物进行判定分析,通过对比高剂量组与对照组、中剂量组与对照组和低剂量组与对照组后导出Excel数据文件,后使用SIMCA-P 14.1对代谢组学数据进行分析。
1.3 数据处理
采用SPSS 25.0软件对人参稀有皂苷给药组和对照组的血清生化参数进行分析。对雌性和雄性大鼠均采用单因素方差分析(ANOVA)。当P<0.05,视为差异有统计学意义。组间差异比较采用最小显著性差异法(LSD)检验。使用SIMCA-P 14.1对代谢组学数据进行分析,通过正交偏最小二乘法-判别分析(OPLS-DA),检验R2、Q2值,得Folding Change值及P值后,筛选差异代谢物(VIP>1,P<0.05),使用MetaboAnalyst 5.0进行代谢通路富集分析[16]。
2. 结果与分析
2.1 热转化后皂苷的组成
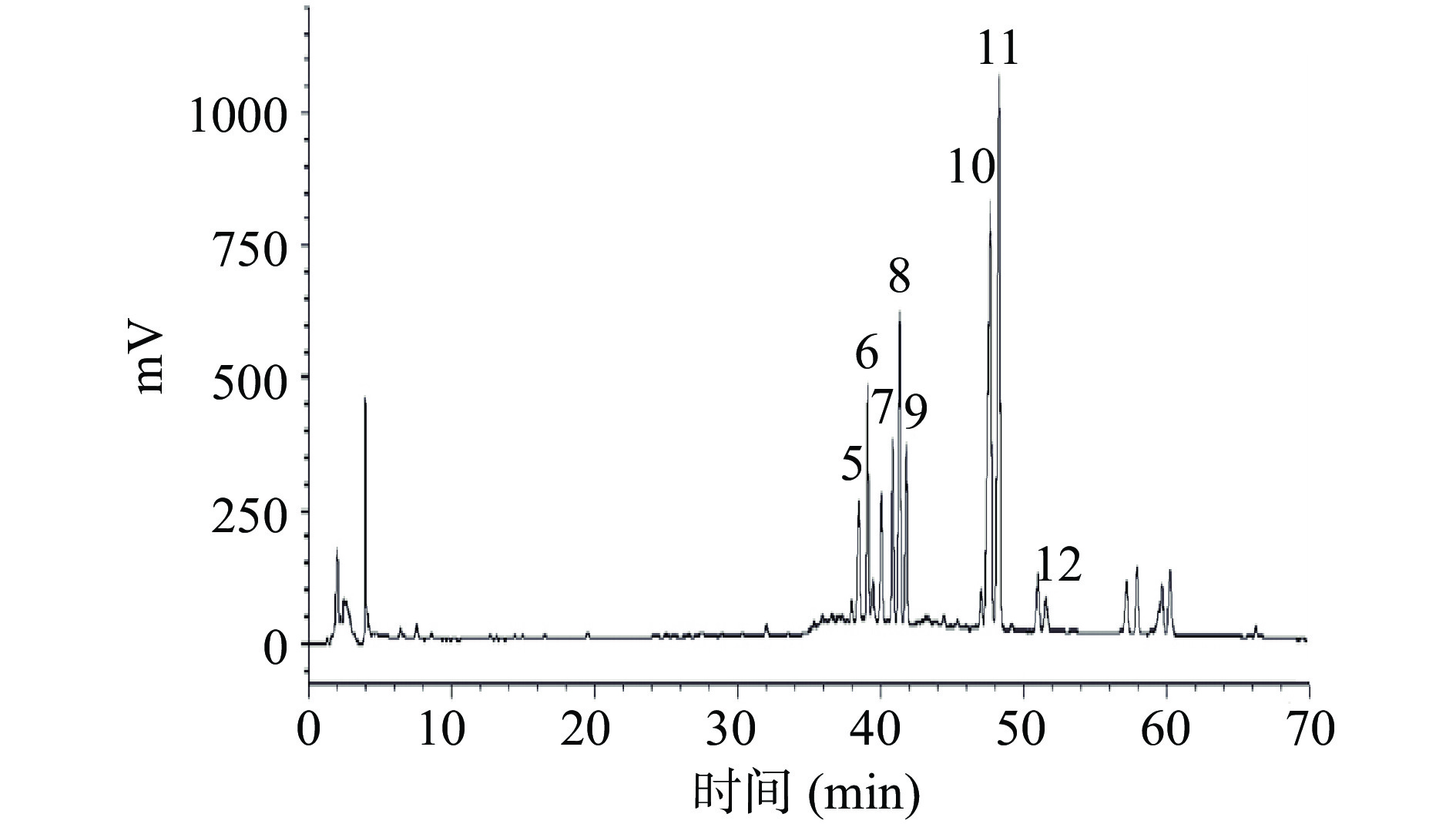
根据课题组前期研究[4−5]获得高效液相色谱分析结果如图1所示,热转化后的皂苷主要由Rg3,Rk1,F4,Rg6,Rg5和Rh4等稀有皂苷组成。人参稀有皂苷的含量占热转化后总皂苷含量的80%以上,热转化后的总皂苷总含量约为(0.83±0.02 mg/mg),人参稀有皂苷总含量约为(0.76±0.02 mg/mg)。
2.2 热转化后的人参皂苷对大鼠生理指标的影响
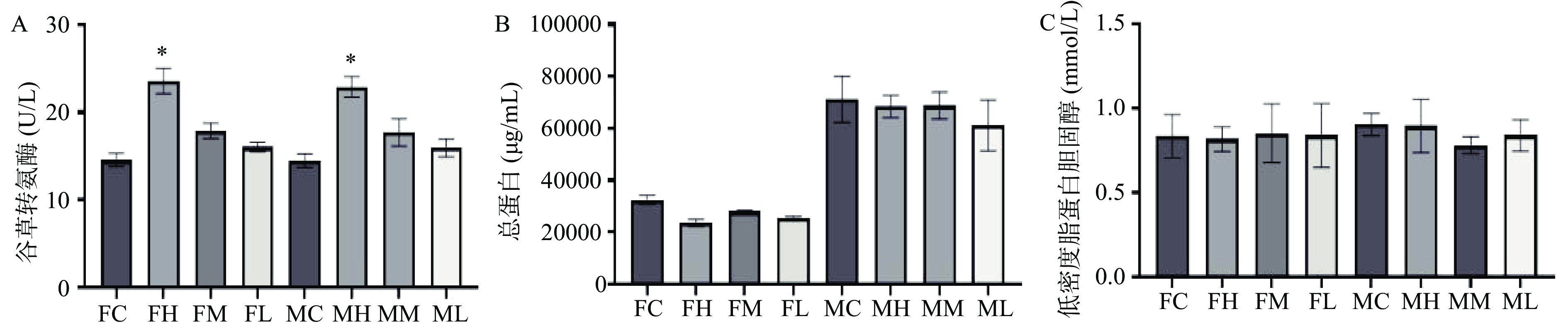
对大鼠进行90 d灌胃实验后无大鼠死亡,只有高剂量人参稀有皂苷组雌雄大鼠在给药第一周出现轻度腹泻和稀便。各组织和器官均未出现异常变色、大小异常、脓液、肿块形成或其他肉眼可见病变发展。各组雌雄大鼠绝对器官重量见表1,人参稀有皂苷给药组和空白对照组大鼠的绝对器官重量无显著性差异(P>0.05)。高剂量雌雄大鼠的肝脏绝对重量略高于对照组,但差异无统计学意义。如图2所示,肝功能指标谷草转氨酶(AST),总蛋白(TP)和低密度脂蛋白胆固醇(LDL-C)测定结果显示,高剂量组雌雄大鼠的AST水平均显著高于对照组(P<0.05)。然而,TP和LDL-C的水平在不同剂量人参稀有皂苷组和空白对照组之间,无显著性差异(图2)。高剂量组雄雌大鼠AST水平显著升高,提示肝脏代谢异常。
表 1 热转化后人参皂苷剂量对雌雄大鼠绝对脏器重量的影响(g)Table 1. Effect of ginsenoside dosage after thermal transformation on absolute organ weight in male and female rats (g)样本 雄鼠 雌鼠 空白对照组 低剂量组 中剂量组 高剂量组 空白对照组 低剂量组 中剂量组 高剂量组 脑 2.2±0.1 1.9±0.1 2.0±0.3 2.1±0.1 2.1±0.3 1.9±0.2 2.0±0.1 2.2±0.3 心 1.4±0.1 1.2±0.2 1.1±0.1 1.4±0.2 0.8±0.1 0.9±0.2 0.9±0.1 1.0±0.2 肺 1.1±0.2 0.8±0.3 0.7±0.1 0.9±0.2 0.8±0.1 0.6±0.2 0.7±0.2 0.9±0.1 肝脏 11.5±2.0 10.6±1.2 9.2±1.4 12.7±1.5 9.3±1.7 8.9±0.4 8.4±0.7 11.3±1.6 肾脏 4.2±0.3 4.0±0.2 3.8±0.4 3.6±0.5 2.9±0.4 2.8±0.5 3.1±0.2 3.0±0.1 脾脏 0.8±0.1 0.9±0.2 0.8±0.1 1.0±0.1 0.6±0.1 0.7±0.1 0.7±0.1 0.8±0.2 睾丸 3.6±0.3 3.1±0.2 3.1±0.4 3.2±0.3 − − − − 附睾 1.7±0.1 1.6±0.2 1.5±0.2 1.8±0.3 − − − − 卵巢 − − − − 0.2±0.1 0.2±0.1 0.2±0.1 0.3±0.1 子宫 − − − − 0.7±0.2 0.8±0.3 0.6±0.1 0.7±0.1 2.3 肝脏流式细胞分析
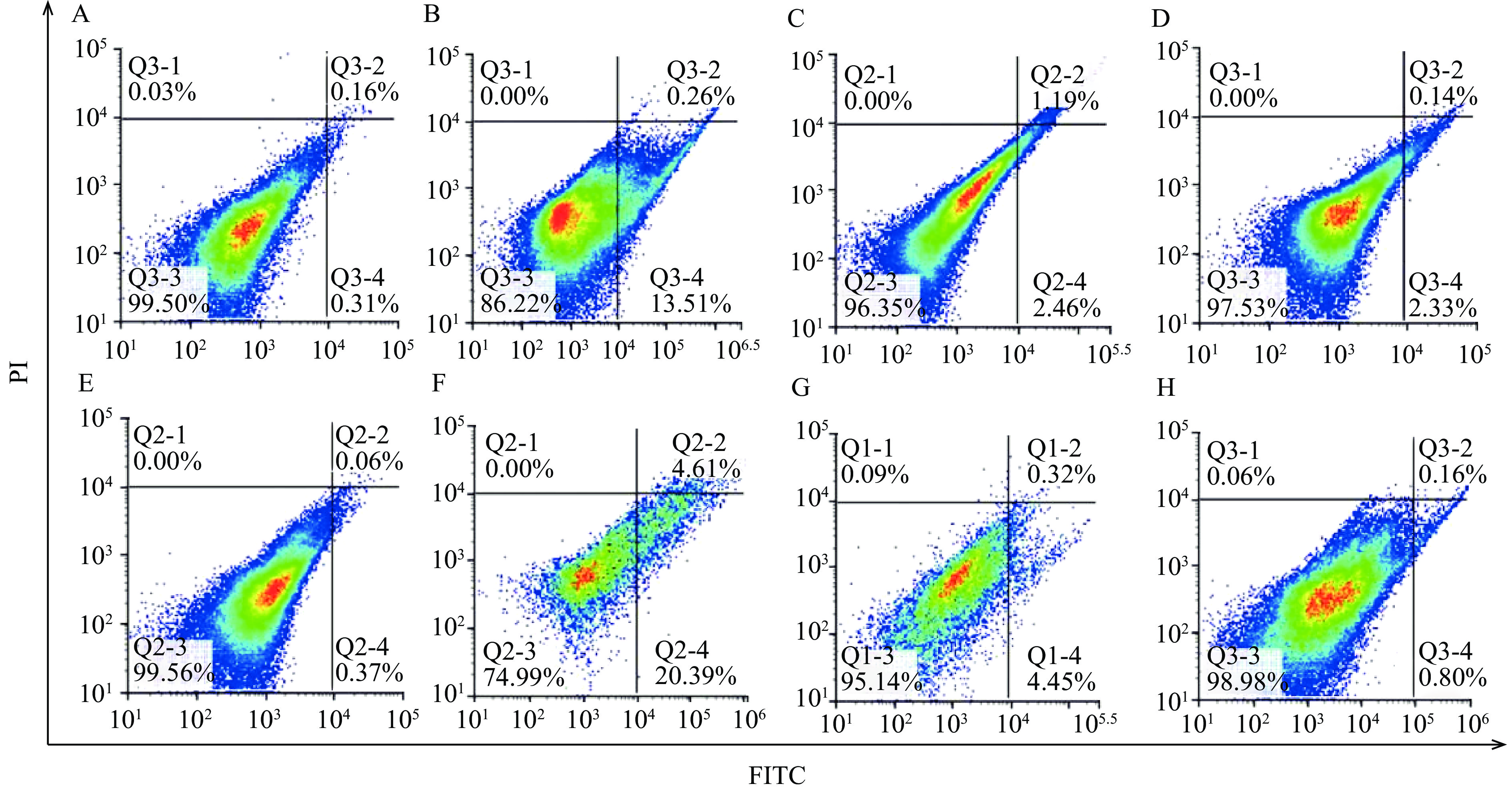
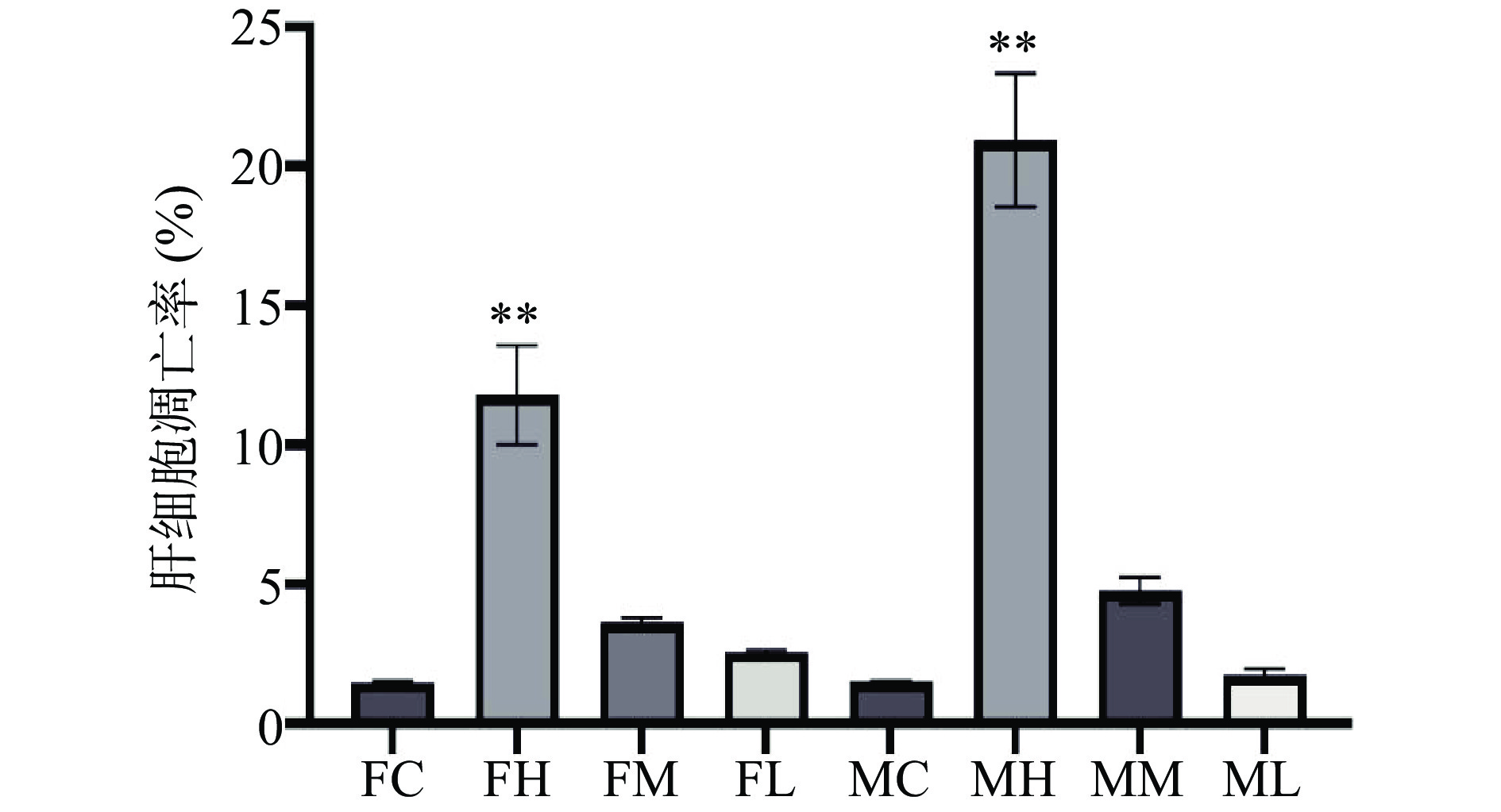
如图3所示,每个图的右上象限和右下象限之和代表细胞凋亡率(Q2+Q4)。和对照组相比,雌雄大鼠高剂量组,中剂量组,低剂量组细胞凋亡率都有所上升。如图4所示,与空白对照组相比,雌雄大鼠高剂量组肝细胞凋亡率有极显著差异(P<0.01),提示长期摄入高剂量的人参稀有皂苷可能对雌雄大鼠肝脏造成了一定损伤,促使肝细胞发生凋亡。而雌雄大鼠人参稀有皂苷中、低剂量组肝细胞凋亡率与对照组比较无显著差异(P>0.05)。课题组既往研究表明,人参稀有皂苷更容易通过破坏细胞膜系统引起细胞凋亡[5],人参稀有皂苷可以与细胞膜表面的甾醇相连,导致细胞膜的穿孔和破裂,最终导致生物膜系统的破裂[12]。临床一线抗肿瘤辅助药物人参稀有皂苷Rg3(10 mg/kg)和Rh2(80 µmol/L)在低剂量下对人正常肝细胞没有明显毒性[17−18]。尽管在安全剂量内人参皂苷没有明显的肝毒性,但过量使用人参皂苷(4000 mg/L)会抑制正常肝细胞增殖,引起肝细胞损伤[19]。本研究中使用的人参稀有皂苷混合物(主要含Rg3,Rk1,F4,Rg6,Rg5和Rh4等成分),糖基含量较少,通过细胞膜的渗透性好,更利于机体吸收[12],当剂量过高时会破坏生物膜系统导致肝细胞凋亡。中、低剂量组的肝细胞凋亡率与对照相比,无显著差异(P>0.05),说明中、低剂量组的人参稀有皂苷对肝细胞并无明显的损伤作用。
2.4 肝切片病理学检查
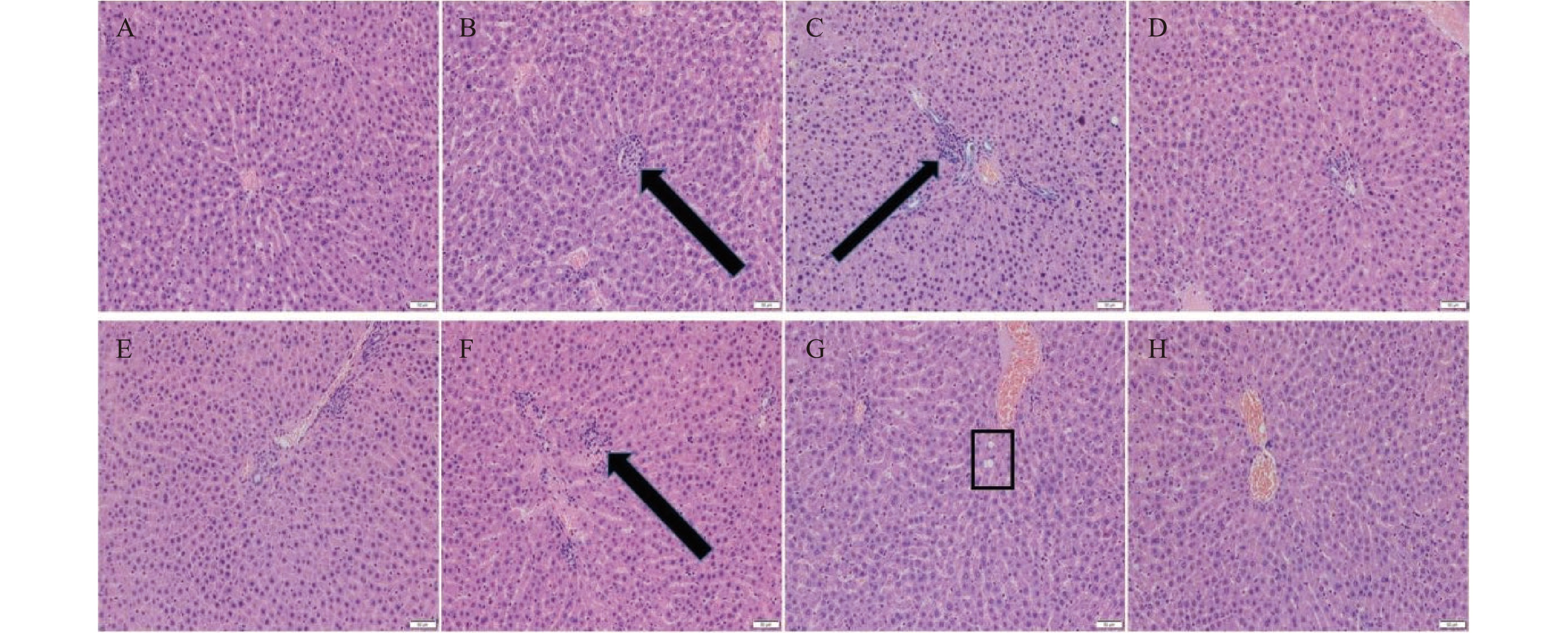
用苏木精-伊红(HE)对肝组织进行染色,制备常规石蜡切片。结果如图5所示,与空白对照组相比,高剂量组的雌雄大鼠均肝索排列紊乱,有炎症细胞浸润并伴有少量空泡;中剂量组的雌雄大鼠有少量炎症细胞和空泡;低剂量组肝脏切片显示肝索排列整齐,从中央静脉中心呈放射状排列,未见明显病理损伤。说明低剂量的人参稀有皂苷对雌雄大鼠肝脏并未造成明显病理损伤。
2.5 代谢组学研究
2.5.1 差异代谢物有监督的判别分析
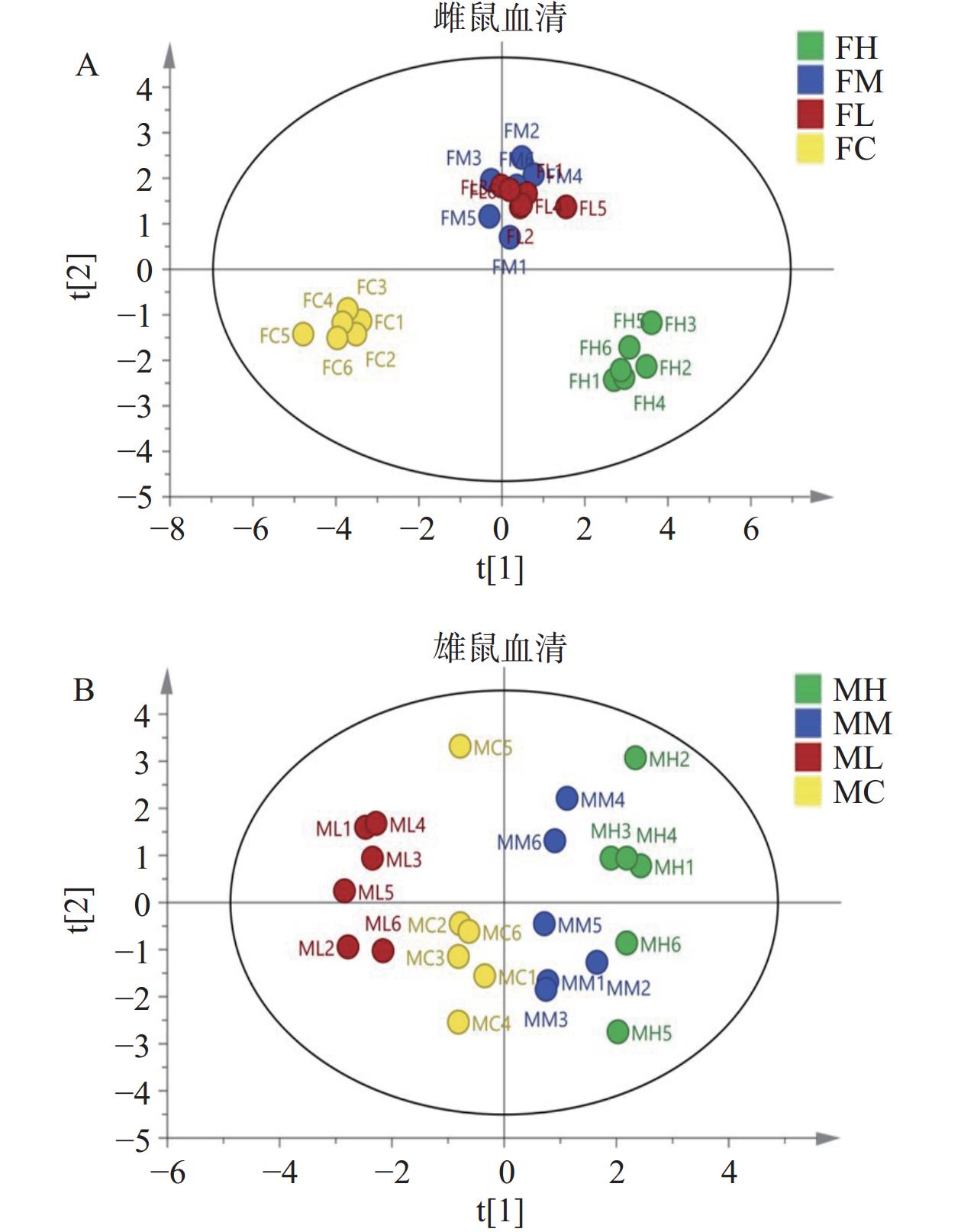
如图6所示的OPLS-DA模型,对于雌雄大鼠,空白对照组与人参稀有皂苷灌胃各组均存在差异,说明不同剂量的人参稀有皂苷对雄性和雌性大鼠的代谢有一定影响。同一组的点聚集在一起,对照组雌雄大鼠血液样本均位于X轴左侧,而人参稀有皂苷高剂量灌胃组大鼠血液样本均分散于X轴右侧。雌性大鼠高剂量灌胃组(图6A)与对照组区分明显,雄性大鼠不同剂量人参皂苷灌胃组与对照组区分明显(图6B)。
2.5.2 差异代谢物通路富集分析
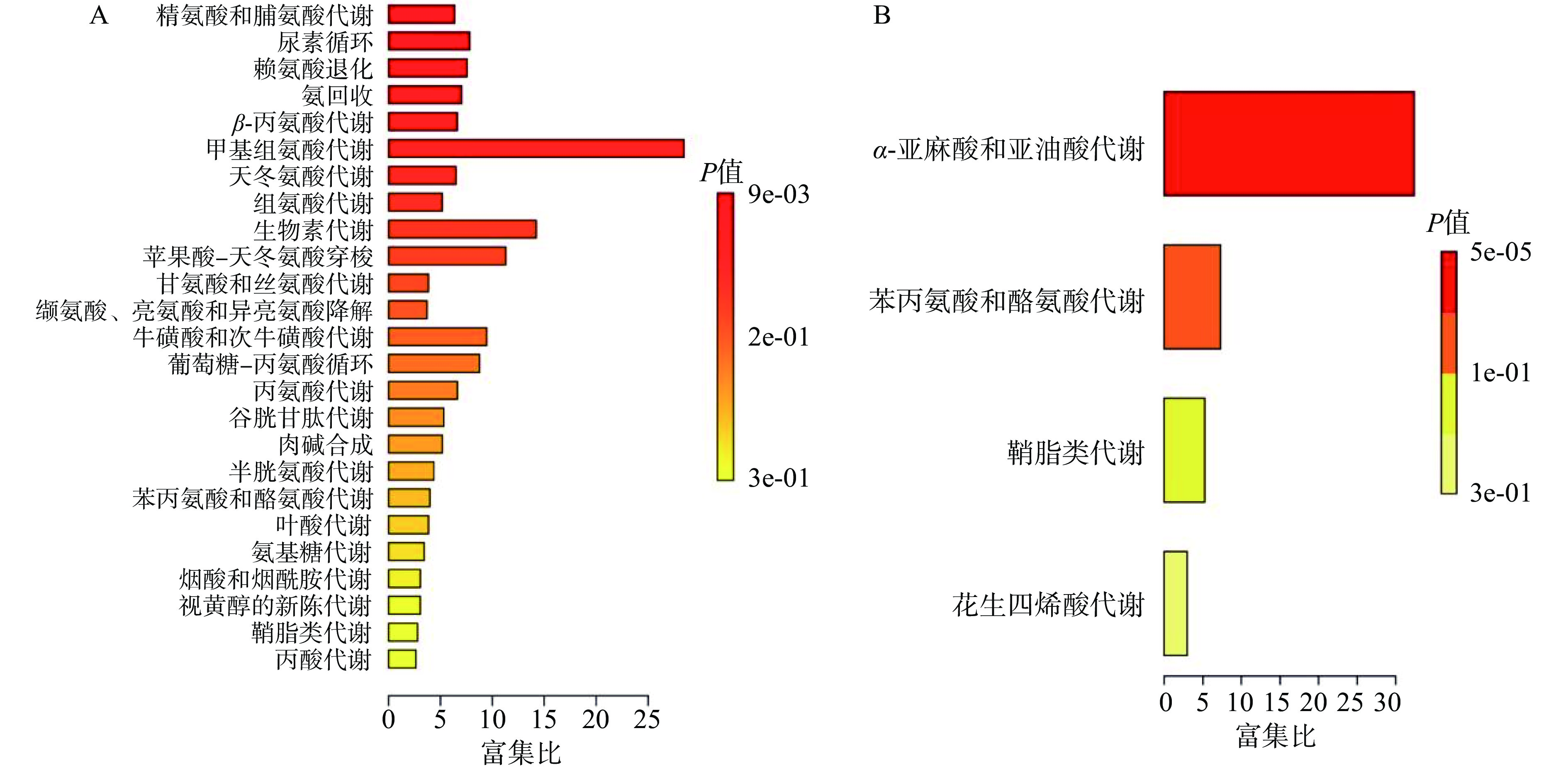
主要对差异明显的高剂量组雌雄大鼠血清与空白对照组雌雄大鼠血清进行富集分析。雌性大鼠筛选出差异化合物23种,其中11种差异化合物下调,12种差异化合物上调。雄性大鼠筛选出差异化合物10种,其中5种差异化合物下调,5种差异化合物上调。雌雄大鼠血清差异化合物见表2和表3。将差异代谢物带入MetaboAnalyst 5.0进行通路富集分析,雌鼠代谢通路中,组氨酸代谢和尿素循环等代谢途径改变明显(图7A)。雄鼠代谢通路中,α-亚麻酸和亚油酸代谢途径改变明显(图7B)。对于雌鼠,高剂量的人参稀有皂苷通过提高组氨酸代谢中差异化合物组氨酸的水平,导致氨产量增加,血氨升高,有研究表明高氨血症会引发肝性脑病,导致肝脏受损[20]。同样人参稀有皂苷提高了尿素循环代谢中差异化合物谷氨酸和精氨酸的水平,影响了尿素的合成排泄。研究表明,肝脏中尿素的合成是去除氨毒害作用的主要途径,血氨升高导致尿素循环障碍,无法将游离的氨合成尿素排出体外,导致肝功能异常[21−22]。高剂量人参稀有皂苷组AST升高和肝细胞凋亡率增加,提示人参稀有皂苷改变了上述代谢途径对肝脏产生的损伤。对于雄鼠,人参稀有皂苷提高了差异化合物亚油酸的水平进而影响了α-亚麻酸和亚油酸代谢途径。亚油酸在体内会合成花生四烯酸,而高浓度的花生四烯酸具有致炎性[23−24],且花生四烯酸在环氧化酶及脂氧化酶合体催化下产生炎症因子前列腺素PGE2和白三烯B4,后者通过破坏细胞膜稳定性,加速细胞凋亡[25−26]。高剂量组肝脏切片中有炎症细胞浸润伴有少量空泡,这些炎症因子如前列腺素PGE2和白三烯B4可以通过血液吸收进入门静脉系统引起肝脏轻度损伤[27−28]。综上,人参稀有皂苷主要影响了组氨酸代谢、尿素循环、α-亚麻酸和亚油酸等代谢途径,导致肝脏轻度受损。考虑到人参稀有皂苷在世界范围内的广泛使用,不能完全排除其过量食用存在的肝毒性风险,如人参稀有皂苷CK会增加血清肝酶水平,促使机体出现局灶性肝坏死[29−31]。因此,对热转化后的人参稀有皂苷混合物进行90 d口服毒性的研究,明确其安全食用剂量,不但可以为人参稀有皂苷的安全制备提供新思路,还可进一步扩展人参稀有皂苷的应用领域。
表 2 雌鼠血清差异化合物Table 2. Serum differential compounds in female rats化合物 中文名称 分子式 分子量 保留时间(min) P值 投影重要性分析值 差异倍数 趋势 Sphinganine 鞘氨醇 C18H39NO2 299.2822 12.16 0.00 2.48 −7.50 ↓ N-Methyl-2-pyrrolidone N-甲基-2-吡咯烷酮 C5H9NO 145.0739 5.83 0.02 2.34 −6.72 ↓ 1-Benzofuran-3-ylmethanol 1-苯并呋喃-3-基甲醇 C9H8O2 117.0791 12.20 0.00 1.92 −5.82 ↓ 2-Chromanone 2-色酮 C9H8O2 232.1057 12.23 0.00 1.82 −5.46 ↓ 11,12-EET 11,12-环氧二十碳三烯酸 C20H32O3 141.1153 13.55 0.01 1.43 −5.30 ↓ (10)-SHOGAOL (10)-鲨烯醇 C21H32O3 232.1057 11.38 0.00 1.34 −4.67 ↓ 7α-Hydroxypregnenolone 7α-羟基孕烯醇酮 C21H32O3 188.1153 11.38 0.00 1.19 −4.56 ↓ (3β,24R,24'R)-fucosterol epoxide (3β,24R,24'R)-褐藻糖醇 C29H48O2 188.1160 18.26 0.00 1.11 −4.41 ↓ Tretinoin 维A酸 C20H28O2 362.2096 13.18 0.00 1.07 −2.28 ↓ 2-Allyl Benzylalcohol 2-烯丙基苯甲醇 C10H12O 189.0630 11.78 0.00 1.02 −2.21 ↓ 4-Phenylbutanal 4-苯基丁醛 C10H12O 131.0583 12.23 0.00 1.02 −1.91 ↓ trans-Anethole 反式异戊二醇 C10H12O 169.0139 11.78 0.00 1.26 1.11 ↑ Isoleucine 异亮氨酸 C6H13NO2 117.0579 1.74 0.01 1.31 1.07 ↑ DL-Phenylalanine DL-苯丙氨酸 C9H11NO2 297.0898 5.97 0.00 1.44 1.02 ↑ Proline 脯氨酸 C5H9NO2 264.1107 1.88 0.00 1.69 1.02 ↑ D-(+)-Tryptophan D-(+)-色氨酸 C11H12N2O2 210.1250 6.54 0.01 1.17 1.26 ↑ Cytosine 胞嘧啶 C4H5N3O 270.0528 1.93 0.00 1.37 1.31 ↑ DL-Arginine DL-精氨酸 C6H14N4O2 176.0545 1.63 0.01 1.56 1.44 ↑ Allantoic acid 尿囊酸 C4H8N4O4 150.0680 1.58 0.00 1.47 1.99 ↑ L-Histidine L-组氨酸 C6H9N3O2 270.0528 1.62 0.00 1.17 2.00 ↑ DL-Lysine DL-赖氨酸 C6H14N2O2 133.0488 1.58 0.00 1.06 2.01 ↑ Taurine 牛胆素 C2H7NO3S 270.0525 1.74 0.00 1.18 2.05 ↑ L-Glutamic acid 左旋谷氨酸 C5H9NO4 176.0538 1.78 0.00 1.19 2.08 ↑ 表 3 雄鼠血清差异化合物Table 3. Serum differential compounds in male rats化合物 中文名称 分子式 分子量 保留时间(min) P值 投影重要性分析值 差异倍数 趋势 Sphinganine 鞘氨醇 C18H39NO2 299.2822 12.239 0.00 1.06 −4.37 ↓ 2-Allyl Benzylalcohol 2-烯丙基苯甲醇 C10H12O 145.0739 14.731 0.02 1.47 −3.55 ↓ 4-Phenylbutanal 4-苯基丁烷 C10H12O 131.0583 12.592 0.00 1.29 −2.51 ↓ Eicosapentanoic acid 二十碳戊酸 C20H30O2 232.1057 13.602 0.00 1.14 −1.51 ↓ 11,12-EET 11,12-环氧二十碳三烯酸 C20H32O3 232.1057 11.847 0.00 1.36 −1.05 ↓ 1-Benzofuran-3-ylmethanol 1-苯并呋喃-3-基甲醇 C9H8O2 117.0791 12.285 0.00 1.16 1.52 ↑ Arachidonic acid 花生四烯酸 C20H32O2 141.1153 5.853 0.01 1.26 1.67 ↑ Linoleic acid 亚油酸 C18H32O2 334.2142 10.474 0.00 1.45 1.78 ↑ L-Phenylalanine L-苯丙氨酸 C9H11NO2 297.0898 5.986 0.00 1.28 2.37 ↑ N-Methyl-2-pyrrolidone N-甲基-2-吡咯烷酮 C5H9NO 188.1153 12.263 0.00 1.30 3.55 ↑ 3. 结论
本研究发现,与空白对照组相比,高剂量热转化人参皂苷(600 mg/kg)肝细胞凋亡率极显著升高(P<0.01),肝切片有炎症细胞浸润伴有少量空泡;血液中代谢物如组氨酸、谷氨酸、精氨酸和亚油酸的变化影响了组氨酸代谢、尿素循环和α-亚麻酸和亚油酸等代谢途径,引发血氨升高和炎症因子产生,说明长期高剂量摄入(600 mg/kg)人参稀有皂苷对肝脏有轻微损伤。本研究结合非靶向代谢组学发现了人参稀有皂苷对大鼠潜在肝毒性影响因素,由于非靶向代谢组学定性时较为困难,也只能相对定量,结合筛选出目标代谢物,为后续研究提供了数据支持。确定了体内实验中人参稀有皂苷对雌性和雄性大鼠的未观察到的肝脏不良反应水平均小于200 mg/kg。人参稀有皂苷因其各种有益的特性而具有广泛的用途。因此,低剂量短期摄入人参稀有皂苷对机体是安全的。
-
表 1 热转化后人参皂苷剂量对雌雄大鼠绝对脏器重量的影响(g)
Table 1 Effect of ginsenoside dosage after thermal transformation on absolute organ weight in male and female rats (g)
样本 雄鼠 雌鼠 空白对照组 低剂量组 中剂量组 高剂量组 空白对照组 低剂量组 中剂量组 高剂量组 脑 2.2±0.1 1.9±0.1 2.0±0.3 2.1±0.1 2.1±0.3 1.9±0.2 2.0±0.1 2.2±0.3 心 1.4±0.1 1.2±0.2 1.1±0.1 1.4±0.2 0.8±0.1 0.9±0.2 0.9±0.1 1.0±0.2 肺 1.1±0.2 0.8±0.3 0.7±0.1 0.9±0.2 0.8±0.1 0.6±0.2 0.7±0.2 0.9±0.1 肝脏 11.5±2.0 10.6±1.2 9.2±1.4 12.7±1.5 9.3±1.7 8.9±0.4 8.4±0.7 11.3±1.6 肾脏 4.2±0.3 4.0±0.2 3.8±0.4 3.6±0.5 2.9±0.4 2.8±0.5 3.1±0.2 3.0±0.1 脾脏 0.8±0.1 0.9±0.2 0.8±0.1 1.0±0.1 0.6±0.1 0.7±0.1 0.7±0.1 0.8±0.2 睾丸 3.6±0.3 3.1±0.2 3.1±0.4 3.2±0.3 − − − − 附睾 1.7±0.1 1.6±0.2 1.5±0.2 1.8±0.3 − − − − 卵巢 − − − − 0.2±0.1 0.2±0.1 0.2±0.1 0.3±0.1 子宫 − − − − 0.7±0.2 0.8±0.3 0.6±0.1 0.7±0.1 表 2 雌鼠血清差异化合物
Table 2 Serum differential compounds in female rats
化合物 中文名称 分子式 分子量 保留时间(min) P值 投影重要性分析值 差异倍数 趋势 Sphinganine 鞘氨醇 C18H39NO2 299.2822 12.16 0.00 2.48 −7.50 ↓ N-Methyl-2-pyrrolidone N-甲基-2-吡咯烷酮 C5H9NO 145.0739 5.83 0.02 2.34 −6.72 ↓ 1-Benzofuran-3-ylmethanol 1-苯并呋喃-3-基甲醇 C9H8O2 117.0791 12.20 0.00 1.92 −5.82 ↓ 2-Chromanone 2-色酮 C9H8O2 232.1057 12.23 0.00 1.82 −5.46 ↓ 11,12-EET 11,12-环氧二十碳三烯酸 C20H32O3 141.1153 13.55 0.01 1.43 −5.30 ↓ (10)-SHOGAOL (10)-鲨烯醇 C21H32O3 232.1057 11.38 0.00 1.34 −4.67 ↓ 7α-Hydroxypregnenolone 7α-羟基孕烯醇酮 C21H32O3 188.1153 11.38 0.00 1.19 −4.56 ↓ (3β,24R,24'R)-fucosterol epoxide (3β,24R,24'R)-褐藻糖醇 C29H48O2 188.1160 18.26 0.00 1.11 −4.41 ↓ Tretinoin 维A酸 C20H28O2 362.2096 13.18 0.00 1.07 −2.28 ↓ 2-Allyl Benzylalcohol 2-烯丙基苯甲醇 C10H12O 189.0630 11.78 0.00 1.02 −2.21 ↓ 4-Phenylbutanal 4-苯基丁醛 C10H12O 131.0583 12.23 0.00 1.02 −1.91 ↓ trans-Anethole 反式异戊二醇 C10H12O 169.0139 11.78 0.00 1.26 1.11 ↑ Isoleucine 异亮氨酸 C6H13NO2 117.0579 1.74 0.01 1.31 1.07 ↑ DL-Phenylalanine DL-苯丙氨酸 C9H11NO2 297.0898 5.97 0.00 1.44 1.02 ↑ Proline 脯氨酸 C5H9NO2 264.1107 1.88 0.00 1.69 1.02 ↑ D-(+)-Tryptophan D-(+)-色氨酸 C11H12N2O2 210.1250 6.54 0.01 1.17 1.26 ↑ Cytosine 胞嘧啶 C4H5N3O 270.0528 1.93 0.00 1.37 1.31 ↑ DL-Arginine DL-精氨酸 C6H14N4O2 176.0545 1.63 0.01 1.56 1.44 ↑ Allantoic acid 尿囊酸 C4H8N4O4 150.0680 1.58 0.00 1.47 1.99 ↑ L-Histidine L-组氨酸 C6H9N3O2 270.0528 1.62 0.00 1.17 2.00 ↑ DL-Lysine DL-赖氨酸 C6H14N2O2 133.0488 1.58 0.00 1.06 2.01 ↑ Taurine 牛胆素 C2H7NO3S 270.0525 1.74 0.00 1.18 2.05 ↑ L-Glutamic acid 左旋谷氨酸 C5H9NO4 176.0538 1.78 0.00 1.19 2.08 ↑ 表 3 雄鼠血清差异化合物
Table 3 Serum differential compounds in male rats
化合物 中文名称 分子式 分子量 保留时间(min) P值 投影重要性分析值 差异倍数 趋势 Sphinganine 鞘氨醇 C18H39NO2 299.2822 12.239 0.00 1.06 −4.37 ↓ 2-Allyl Benzylalcohol 2-烯丙基苯甲醇 C10H12O 145.0739 14.731 0.02 1.47 −3.55 ↓ 4-Phenylbutanal 4-苯基丁烷 C10H12O 131.0583 12.592 0.00 1.29 −2.51 ↓ Eicosapentanoic acid 二十碳戊酸 C20H30O2 232.1057 13.602 0.00 1.14 −1.51 ↓ 11,12-EET 11,12-环氧二十碳三烯酸 C20H32O3 232.1057 11.847 0.00 1.36 −1.05 ↓ 1-Benzofuran-3-ylmethanol 1-苯并呋喃-3-基甲醇 C9H8O2 117.0791 12.285 0.00 1.16 1.52 ↑ Arachidonic acid 花生四烯酸 C20H32O2 141.1153 5.853 0.01 1.26 1.67 ↑ Linoleic acid 亚油酸 C18H32O2 334.2142 10.474 0.00 1.45 1.78 ↑ L-Phenylalanine L-苯丙氨酸 C9H11NO2 297.0898 5.986 0.00 1.28 2.37 ↑ N-Methyl-2-pyrrolidone N-甲基-2-吡咯烷酮 C5H9NO 188.1153 12.263 0.00 1.30 3.55 ↑ -
[1] SZCZUKA D, NOWAK A, ZAKŁOS-SZYDA M, et al. American ginseng ( Panax quinquefolium L.) as a source of bioactive phytochemicals with pro-health properties[J]. Nutrients,2019,11(5):1041. doi: 10.3390/nu11051041
[2] JING J J, ZHANG R Y, WANG Y H, et al. Less polar ginsenosides have better protective effects on mice infected by Listeria monocytogenes[J]. Ecotoxicology and Environmental Safety,2021,213:112065. doi: 10.1016/j.ecoenv.2021.112065
[3] LEE J W, CHOI B R, KIM Y C, et al. Comprehensive profiling and quantification of ginsenosides in the root, stem, leaf, and berry of Panax ginseng by UPLC-QTOF/MS[J]. Molecules,2017,22(12):2147. doi: 10.3390/molecules22122147
[4] ZHANG F X, TANG S J, ZHAO L, et al. Stem-leaves of Panax as a rich and sustainable source of less-polar ginsenosides:Comparison of ginsenosides from Panax ginseng, American ginseng and Panax notoginseng prepared by heating and acid treatment[J]. Journal of Ginseng Research,2021,45(1):163−175. doi: 10.1016/j.jgr.2020.01.003
[5] XUE P, YAO Y, YANG X S, et al. Improved antimicrobial effect of ginseng extract by heat transformation[J]. Journal of Ginseng Research,2017,41(2):180−187. doi: 10.1016/j.jgr.2016.03.002
[6] ZHANG Y C, ZHANG J X, LIU C, et al. Extraction, isolation, and aromatase inhibitory evaluation of low-polar ginsenosides from Panax ginseng leaves[J]. Journal of Chromatography A,2017,1483:20−29. doi: 10.1016/j.chroma.2016.12.068
[7] LU D, LIU J P, ZHAO W J, et al. Chronic toxicity of ginsenoside Re on Sprague-Dawley rats[J]. Journal of Ethnopharmacology,2012,144(3):656−663. doi: 10.1016/j.jep.2012.10.007
[8] ZHAO Q, YANG M, DENG Y P, et al. The safety evaluation of salvianolic acid B and ginsenoside Rg1 combination on mice[J]. International Journal of Molecular Sciences,2015,16(12):29345−29356. doi: 10.3390/ijms161226176
[9] LI C M, WANG Z Z, LI G S, et al. Acute and repeated dose 26-week oral toxicity study of 20 (S)-ginsenoside Rg3 in Kunming mice and Sprague-Dawley rats[J]. Journal of Ginseng Research,2020,44(2):222−228. doi: 10.1016/j.jgr.2018.10.001
[10] GAO Y L, WANG T, WANG G F, et al. Preclinical safety of ginsenoside compound K:Acute, and 26-week oral toxicity studies in mice and rats[J]. Food and Chemical Toxicology,2019,131:110578. doi: 10.1016/j.fct.2019.110578
[11] LIU J, LIU Y, WANG Y, et al. The integration of GC-MS and LC-MS to assay the metabolomics profiling in Panax ginseng and Panax quinquefolius reveals a tissue-and species-specific connectivity of primary metabolites and ginsenosides accumulation[J]. Journal of Pharmaceutical and Biomedical Analysis,2017,135:176−185. doi: 10.1016/j.jpba.2016.12.026
[12] XUE P, YANG X S, ZHAO L, et al. Relationship between antimicrobial activity and amphipathic structure of ginsenosides[J]. Industrial Crops and Products,2020,143:111929. doi: 10.1016/j.indcrop.2019.111929
[13] ZHANG Z H, WANG X Y, WANG J C, et al. Metabonomics approach to assessing the metabolism variation and endoexogenous metabolic interaction of ginsenosides in cold stress rats[J]. Journal of Proteome Research,2016,15(6):1842−1852. doi: 10.1021/acs.jproteome.6b00015
[14] 李媛媛, 张媛媛, 李俊霞, 等. 甲氨基阿维菌素苯甲酸盐和高效氯氰菊酯混配染毒致小鼠睾丸细胞遗传损伤研究[J]. 环境卫生学杂志,2020,10(4):363−366. [LI Y Y, ZHANG Y Y, LI J X, et al. Genetic damage of mouse testicular cells caused by mixed exposure to emamectin benzoate and beta-cypermethrin[J]. Journal of Environmental Hygiene,2020,10(4):363−366. doi: 10.13421/j.cnki.hjwsxzz.2020.04.006 LI Y Y, ZHANG Y Y, LI J X, et al . Genetic damage of mouse testicular cells caused by mixed exposure to emamectin benzoate and beta-cypermethrin[J]. Journal of Environmental Hygiene,2020 ,10 (4 ):363 −366 . doi: 10.13421/j.cnki.hjwsxzz.2020.04.006[15] ZHANG R Y, ZHAI Q F, YU Y, et al. Safety assessment of crude saponins from Chenopodium quinoa Willd. husks:90-day oral toxicity and gut microbiota & metabonomics study in rats[J]. Food Chemistry,2022,375:131655. doi: 10.1016/j.foodchem.2021.131655
[16] 张若愚, 闫菲, 李雪宁, 等. 藜麦皂苷灌胃后大鼠尿液代谢及肠道菌变化[J]. 现代食品科技,2022,38(8):1−9. [ZHANG R Y, YAN F, LI X N, et al. Changes of urine metabolism and gut microbiome in rats after intragastric administration of quinoa saponins[J]. Modern Food Science and Technology,2022,38(8):1−9. doi: 10.13982/j.mfst.1673-9078.2022.8.0999 ZHANG R Y, YAN F, LI X N, et al . Changes of urine metabolism and gut microbiome in rats after intragastric administration of quinoa saponins[J]. Modern Food Science and Technology,2022 ,38 (8 ):1 −9 . doi: 10.13982/j.mfst.1673-9078.2022.8.0999[17] LIU X X, MI X J, WANG Z, et al. Ginsenoside Rg3 promotes regression from hepatic fibrosis through reducing inflammation-mediated autophagy signaling pathway[J]. Cell Death & Disease,2020,11(6):454.
[18] SHI Q Q, LI J, FENG Z Q, et al. Effect of ginsenoside Rh2 on the migratory ability of HepG2 liver carcinoma cells:Recruiting histone deacetylase and inhibiting activator protein 1 transcription factors[J]. Molecular Medicine Reports,2014,10(4):1779−1785. doi: 10.3892/mmr.2014.2392
[19] 刘佳, 孔嗣强, 张明, 等. 过量人参皂苷对小鼠原代培养肝细胞的毒性实验[J]. 中医药导报,2017,23(18):33−36. [LIU J, KONG S Q, ZHANG M, et al. Toxicity experiment of excess ginseng saponin on primitive cultured mouse liver cells[J]. Guiding Journal of Traditional Chinese Medicine and Pharmacy,2017,23(18):33−36. doi: 10.13862/j.cnki.cn43-1446/r.2017.18.009 LIU J, KONG S Q, ZHANG M, et al . Toxicity experiment of excess ginseng saponin on primitive cultured mouse liver cells[J]. Guiding Journal of Traditional Chinese Medicine and Pharmacy,2017 ,23 (18 ):33 −36 . doi: 10.13862/j.cnki.cn43-1446/r.2017.18.009[20] HOLEČEK M. Histidine in health and disease:Metabolism, physiological importance, and use as a supplement[J].Nutrients,2020,12(3):848. doi: 10.3390/nu12030848
[21] 全敏, 杨松. 尿素循环障碍所致急慢性肝病的识别与治疗[J].中国实用儿科杂志,2021,36(10):758−762. [QUAN M, YANG S. Identification and treatment of acute and chronic liver diseases caused by urea cycle disorders[J]. Chinese Journal of Practical Pediatrics,2021,36(10):758−762. doi: 10.19538/j.ek2021100611 QUAN M, YANG S . Identification and treatment of acute and chronic liver diseases caused by urea cycle disorders[J]. Chinese Journal of Practical Pediatrics,2021 ,36 (10 ):758 −762 . doi: 10.19538/j.ek2021100611[22] 李敏, 陈哲晖, 周颖, 等. 尿素循环障碍的药物治疗[J]. 中国实用儿科杂志,2021,36(10):764−768. [LI M, CHEN Z H, ZHOU Y, et al. Drug treatment for urea cycle disorders[J]. Chinese Journal of Practical Pediatrics,2021,36(10):764−768. doi: 10.19538/j.ek2021100613 LI M, CHEN Z H, ZHOU Y, et al . Drug treatment for urea cycle disorders[J]. Chinese Journal of Practical Pediatrics,2021 ,36 (10 ):764 −768 . doi: 10.19538/j.ek2021100613[23] 蔡亚玮, 刘建宏, 马宁. 花生四烯酸靶向代谢组学在炎症中的研究现状[J]. 中国临床药理学杂志,2021,37(19):2721−2723,2728. [CAI Y W, LIU J H, MA N. Research status of arachidonic acid-targeted metabonomics in inflammation[J]. The Chinese Journal of Clinical Pharmacology,2021,37(19):2721−2723,2728. doi: 10.13699/j.cnki.1001-6821.2021.19.044 CAI Y W, LIU J H, MA N . Research status of arachidonic acid-targeted metabonomics in inflammation[J]. The Chinese Journal of Clinical Pharmacology,2021 ,37 (19 ):2721 −2723,2728 . doi: 10.13699/j.cnki.1001-6821.2021.19.044[24] 胡煜. 热应激通过miR-145-5p-花生四烯酸调节仔猪睾丸支持细胞自噬的分子机制[D]. 重庆:西南大学, 2021. [HU Yu. Animal genetics breeding and reproduction field:animal reproductive physiology and reproduction regulation[D]. Chongqing:Southwest University, 2021. HU Yu. Animal genetics breeding and reproduction field: animal reproductive physiology and reproduction regulation[D]. Chongqing: Southwest University, 2021.
[25] INNES J K, CALDER P C. Omega-6 fatty acids and inflammation[J]. Prostaglandins, Leukotrienes and Essential Fatty Acids,2018,132:41−48. doi: 10.1016/j.plefa.2018.03.004
[26] SIMOPOULOS A P. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity[J]. Nutrients,2016,8(3):128. doi: 10.3390/nu8030128
[27] FARHANGFAR S D, FESAHAT F, ZARE-ZARDINI H, et al. In vivo study of anticancer activity of ginsenoside Rh2-containing arginine-reduced graphene in a mouse model of breast cancer[J]. Iranian Journal of Basic Medical Sciences,2022,25(12):1442−1451.
[28] TAO T Z, CHEN F, BO L L, et al. Ginsenoside Rg1 protects mouse liver against ischemia-reperfusion injury through anti-inflammatory and anti-apoptosis properties[J]. Journal of Surgical Research,2014,191(1):231−238. doi: 10.1016/j.jss.2014.03.067
[29] 李大磊, 李延团, 韩兵, 等. 人参皂苷 CK 乳注射液对新西兰兔的长期毒性试验研究[J]. 中国中医药现代远程教育,2011,9(3):188−189. [LI D L, LI Y T, HAN B, et al. Long-term toxicity test of ginsenoside ck milk injection on new zealand rabbits[J]. Chinese Medicine Modern Distance Education of China,2011,9(3):188−189. doi: 10.3969/j.issn.1672-2779.2011.03.147 LI D L, LI Y T, HAN B, et al . Long-term toxicity test of ginsenoside ck milk injection on new zealand rabbits[J]. Chinese Medicine Modern Distance Education of China,2011 ,9 (3 ):188 −189 . doi: 10.3969/j.issn.1672-2779.2011.03.147[30] 浦滇, 李俊, 黄娜娜, 等. 人参皂苷CK对肝脏的药理作用研究概况[J]. 中国民族民间医药,2022,31(4):62−65. [PU D, LI J, HUANG N N, et al. Research progress on related effects of ginsenoside CK on liver[J]. Chinese Journal of Ethnomedicine and Ethnopharmacy,2022,31(4):62−65. PU D, LI J, HUANG N N, et al . Research progress on related effects of ginsenoside CK on liver[J]. Chinese Journal of Ethnomedicine and Ethnopharmacy,2022 ,31 (4 ):62 −65 .[31] MANCUSO C, SANTANGELO R. Panax ginseng and Panax quinquefolius:From pharmacology to toxicology[J]. Food and Chemical Toxicology,2017,107:362−372. doi: 10.1016/j.fct.2017.07.019





 下载:
下载:
 下载:
下载:



