Research Advances in the Multiscale Protein-polysaccharide Based Systems for Probiotics Delivery
-
摘要: 益生菌在食品加工过程、贮存和胃肠消化等不良环境中易大量失活,导致缺乏足够的活菌量能够顺利到达人体结肠并发挥其益生作用。作为食品中常用的天然生物聚合物材料,蛋白质和多糖可以通过非共价或共价作用组装形成宏观级-微米级-纳米级三种尺度结构的益生菌递送体系。其中,水凝胶、微粒、纳米颗粒等体系均已展现出优异的结肠靶向传输功效,但更多关于益生菌活性保持的方法和材料仍待进一步探索。蛋白质-多糖基递送体系已在酸奶、奶酪、饮料等食品中成功运用,其尺度结构决定了益生菌的靶向递送效果和发酵程度对产品品质的改善情况。未来,蛋白质-多糖基递送体系可在设计高效传输的纳米级结构、与其他生物活性物质共包埋的多功能食品、含活性益生菌的包装材料及新型健康食品等方面继续深入研究。本文以蛋白质-多糖基体系的形成机制为出发点,对常用于递送益生菌的蛋白质-多糖基体系的多尺度结构及在食品中的最新研究应用进展进行论述,以期为开发含活性益生菌的蛋白质-多糖基食品体系提供参考。Abstract: Probiotics can easily and largely lose the viability under the undesirable environment, such as food processing, storage, gastrointestinal digestion and so on, which lead to the deficiency of enough amount of viable cells reaching the human colon and exerting its beneficial effect. As the commonly used natural biopolymers in food, proteins and polysaccharides can form macro-, micro- and nano-scale triple-structured probiotics delivery systems through non-covalent or covalent interaction. Hydrogel, microparticles, nanoparticles and other systems have shown the outstanding colon-targeted delivery performance, but more about the approaches and materials of probiotics viability retention need to be further explored. Protein-polysaccharide based delivery systems have been successfully applied in food including yogurt, cheese, beverages and so on, of which scale and structure determine the colon-targeted delivery performance and the improvement level of product quality influenced by the fermentation degree of probiotics. In the future, protein-polysaccharide based delivery system can be further investigated in the aspects of designing highly-efficient-delivery nano structure, multi-functional food with co-encapsulation of other bioactives, packaging materials and novel health food within viable probiotics. With the formation mechanisms of protein-polysaccharide based system as a starting point, this article comprehensively introduces the multiscale structure of the protein-polysaccharide based system for probiotics delivery and its application in food. It can provide references for the development of the protein-polysaccharide based food system within encapsulated viable probiotics.
-
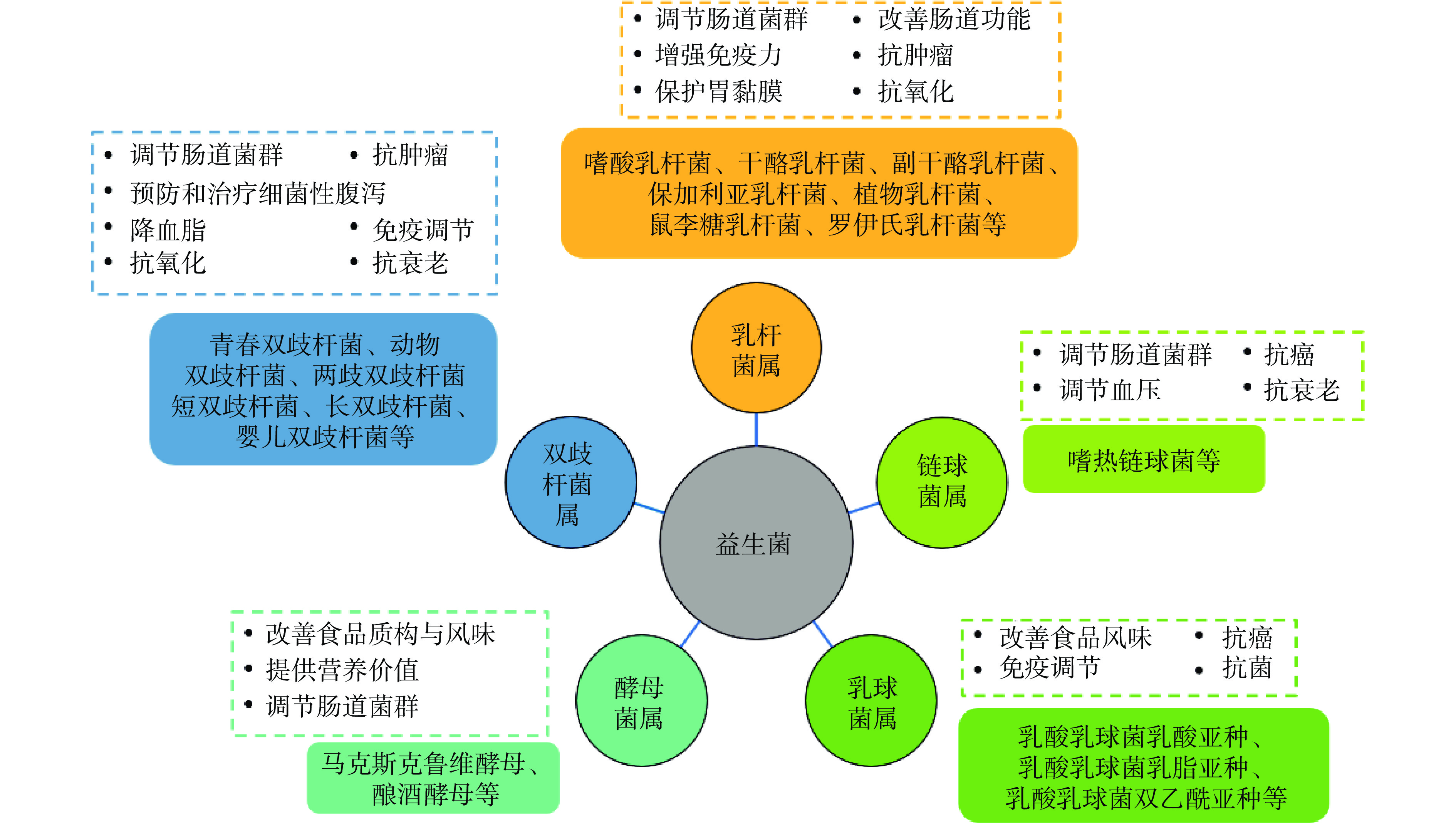
益生菌(probiotics)是一类对宿主有益的活性微生物。当达到足够数量时,益生菌可以通过调节宿主黏膜与系统免疫功能或调节肠道内菌群平衡,对宿主发挥健康作用[1]。常用于食品中的益生菌主要从发酵食品或粪便微生物菌群中分离筛选而来[2],包括了双歧杆菌属(Bifidobacterium)、乳杆菌属(Lactobacillus)、链球菌属(Streptococcus)、乳球菌属(Lactococcus)、芽孢杆菌属(Bacillus)、酵母菌属(Saccharomyces)等(图1)。益生菌必须能够顺利到达肠道内且有足够活菌量(通常应达到至少106 CFU/g以上)才能达到有效递送效果,发挥其益生作用[3]。然而,大多数益生菌对加工和储存过程中酸、氧气、热、光照、水分活度等外部环境条件[4-5],以及对人体消化道环境中的胃酸、消化酶和胆盐[6]抵抗力差,致使活菌量大幅度降低,益生作用失效[7]。
为了提高益生菌对不良环境的抵抗力和存活率,建立有效的活性益生菌递送体系是最为有效的方法之一。蛋白质和多糖是具有健康、安全、生物兼容性、生物可降解性等特性,常用于制备食品递送体系的天然生物聚合物。在特定的加工技术条件下,利用蛋白质和多糖之间的相互作用可以组装形成多尺度结构的蛋白质-多糖基益生菌递送体系,并设计出实现靶向传输目的的功能性食品,其原因主要有三:第一,蛋白质-多糖的结合使用可以协同形成更稳定、更致密、更高机械强度的凝胶网状结构[10],并互补单一生物聚合物的缺点,比如蛋白质颗粒易聚集、多糖通常乳化性较差等[11],从而提高递送体系对酸、离子强度、热等加工过程和贮存环境因素的稳定性,避免其失稳分解并释放出被包埋的益生菌;第二,复合材料的双重消化优势得以发挥,例如,蛋白质在消化过程中起到缓冲作用,阻隔了酶对益生菌的损害,而多糖为益生菌提供了物理屏障,保护它们免受酸和胆汁的侵害[12],且蛋白酶对蛋白质的水解位点可因蛋白质-多糖相互作用而大量降低[13],保证了益生菌能抵抗胃肠消化环境;第三,复合运载体系可被设计成在胃和小肠的强酸性或弱酸性条件下释放受到抑制、在结肠的弱碱性条件下释放受到促进的pH敏感性运载体系,或通过结肠内微生物分泌出的酶(还原酶、糖苷酶等)使其降解[14],从而实现在结肠中控制和持续释放益生菌的目的。本文主要对蛋白质-多糖基活性益生菌递送体系的形成机制、常见多尺度结构及在食品中的最新研究应用进展进行论述。
1. 蛋白质-多糖复合体系的形成机制
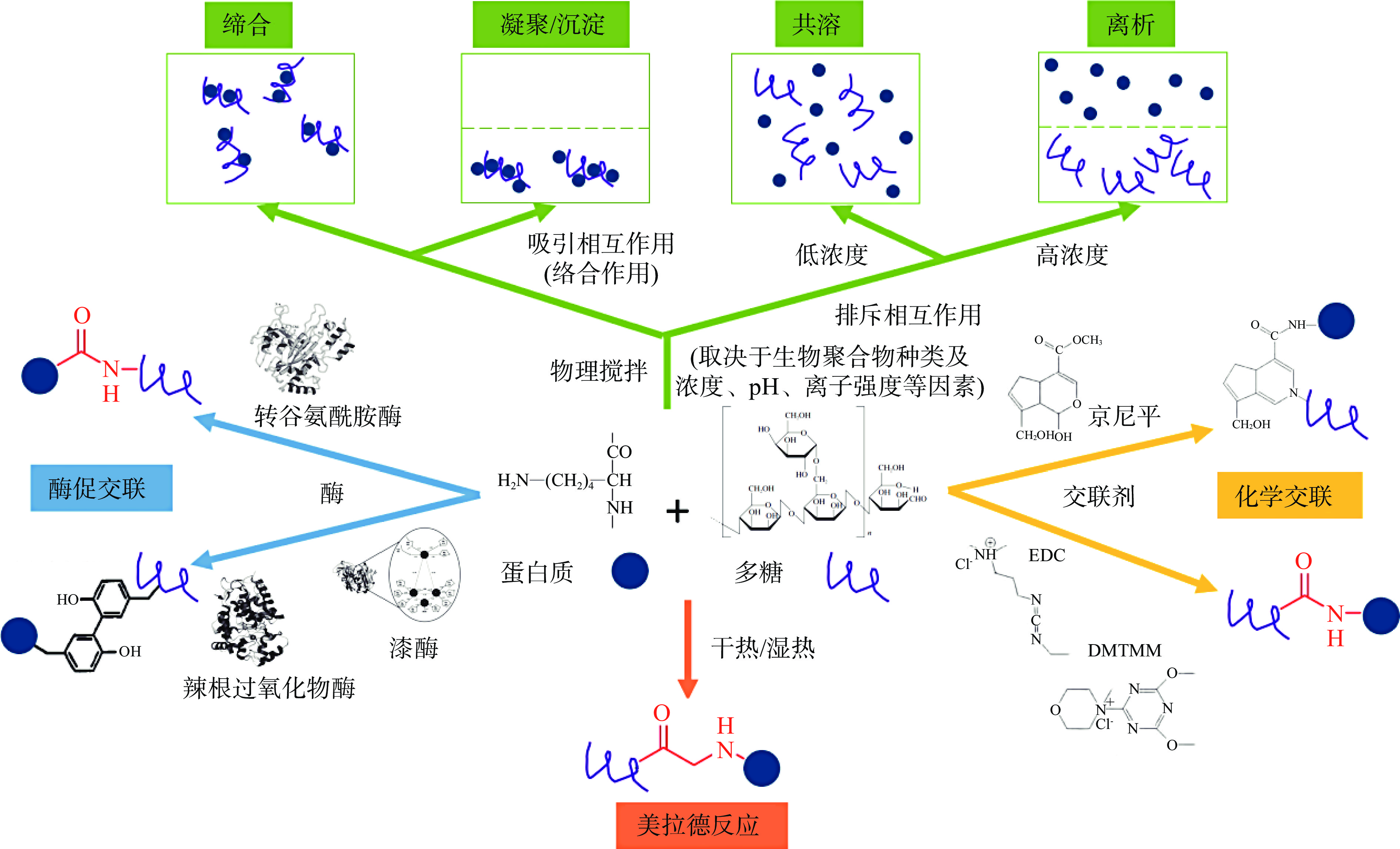
蛋白质-多糖复合体系的构建是基于这两种生物聚合物的相互作用形成的。不同来源的蛋白质和多糖可以通过静电相互作用、疏水作用、氢键、范德华力等物理方式形成非共价结合,也可以通过美拉德反应、酶促交联、化学交联等化学方式形成共价结合(图2)[15]。
1.1 非共价结合
由于蛋白质和带负电荷的多糖溶液主要以带电形式存在,蛋白-多糖非共价的结合作用主要由静电相互作用引起[11-16]:a. 反应体系pH小于蛋白质等电点时,带正电的蛋白质分子会与带负电的多糖分子发生强烈的静电吸附作用,从而产生强静电复合物并形成均相;但随着体系自由能的降低,静电复合物可进一步聚集,导致出现相分离、凝聚、或沉淀;b. 当反应体系pH大于等电点时,带负电的蛋白质分子会与带负电的多糖分子发生弱相互作用,产生弱可逆复合物,导致形成两相溶液,或在足够低的浓度下,蛋白质和多糖以单个分子存在形成单相溶液,最终表现为共溶状态(图2)。其他非共价相互作用,包括氢键、范德华力、疏水相互作用等物理方式,在一定条件下有助于蛋白质与多糖形成更强的络合[17]:比如当反应体系pH大于等电点时,蛋白质和多糖具有相似的电荷,可能会发生氢键结合;而范德华力可能发生在相近粒子彼此适当靠近,出现引力增大;加热可以使蛋白质和多糖的构象发生改变,疏水结构暴露并接触形成相互作用[18]。
1.2 共价结合
美拉德反应是制备蛋白质-多糖共价结合物的常用化学反应[19],是蛋白质分子中氨基酸侧链的氨基与多糖还原性末端的羰基之间缩合形成Schiff碱化合物、共价键交联的过程[20](图2)。该反应能实现蛋白质和带中性电荷多糖(比如葡聚糖和淀粉)的共价交联[21-22]。相对于单一天然生物聚合物和蛋白质-多糖静电复合物,通过美拉德反应形成的蛋白质-多糖共价复合物的多种功能性均得到提高,包括乳化性、起泡性、溶解性和热稳定性等[23-25],同时蛋白质-多糖基乳液的功能性质也得到改善,比如形成更小的分散液滴粒径、更大的净ζ电位、更好的稳定性[26]及在较低pH和热处理条件下的稳定性[27]。但在反应时间、反应温度等条件控制不好的情况下,美拉德反应产生的具有诱变性、致癌性和细胞毒性的化合物会导致潜在的安全问题[28]。通过控制相对温和的反应条件、添加抗氧化剂等方式可为形成安全、健康的美拉德反应蛋白质-多糖产物提供更好的途径[29]。
蛋白质-多糖酶促交联反应主要通过转谷氨酰胺酶、过氧化物酶(比如辣根过氧化物酶)、多酚氧化酶(比如漆酶)等实现[30]。转谷氨酰胺酶通过催化蛋白质多肽链中的酰基供体和氨基糖上的伯氨基发生酰基转移反应,从而将具有伯胺基的糖分子导入到蛋白质分子中形成糖基化蛋白,而过氧化物酶和多酚氧化酶可以催化多糖中的阿魏酰残基与蛋白质的酪氨酸残基交联[17](图2)。酶促交联蛋白质-多糖复合物不仅比单一天然生物聚合物具有更高的乳化性和对盐、低pH、加热和冻融处理等环境应力的抵抗力[31],而且相比美拉德反应或静电结合物,其制备的乳液对酸性条件具有更高的抵抗力[32]。
蛋白质-多糖的化学交联主要通过戊二醛、京尼平、1-乙基-3-(3-二甲胺丙基)碳二亚胺(EDC)、4-(4,6-二甲氧基三嗪-2-基)-4-甲基吗啉盐酸盐(DMTMM)等交联剂形成[33]。其中植物基天然来源、无毒、易溶于水的京尼平是对蛋白质氨基和多糖氨基进行交联[34],而EDC和DMTMM可促进蛋白质氨基和多糖羧基结合形成酰胺键,且自身不会成为交联物的一部分,转化为小分子副产物后能通过渗析等方法去除[35](图2)。但经研究表明,交联位点对蛋白-多糖共价结合物的界面性能有较大影响,基于蛋白质氨基与多糖羰基结合的美拉德交联结合物的乳化性优于蛋白质氨基与多糖氨基结合的京尼平化学交联结合物[36]。
2. 用于益生菌递送的多尺度蛋白质-多糖基体系
蛋白质-多糖复合体系的常用制备方法主要包括自上而下和自下而上两种策略[37-38]:自上而下策略的本质是利用外部机械破坏力将大结构材料的尺寸减小为小颗粒,包括研磨、喷雾/冷冻干燥、挤出、乳化(高压均质、高速剪切、超声、微流控、膜乳化)等技术,适用于生产微米级或毫米级的蛋白质-多糖复合体系;自下而上策略主要是通过控制环境条件,比如生物聚合物的类型和浓度、pH、温度、离子强度等,通过分子自组装或自组织来实现较大颗粒的形成,比如包体络合、复合凝聚、液体抗溶剂沉淀、逐层沉积、静电纺丝或静电喷涂等方法,可用于制造纳米级或微米级的蛋白质-多糖复合体系。用于递送益生菌的蛋白质-多糖复合体系尺度主要包括宏观级(>1000 μm)、微米级(1~1000 μm)和纳米级(<1 μm)(表1)。
表 1 蛋白质-多糖基活性益生菌递送体系结构及性能特征Table 1. Structure and property characteristics of protein-polysaccharide based viable probiotics delivery system递送体系 益生菌 复合物 蛋白质-多糖结合作用 制备技术 性能特征 参考文献 皮克林乳液大粒凝胶 乳酸双歧杆菌(Bifidobacterium lactis) 茶蛋白-黄原胶 − 高速剪切,3D打印技术 45 ℃和55 ℃打印温度不会影响益生菌存活率,但在65 ℃活菌数会从8.07 lg CFU/g减少至6.59 lg CFU/g;在4 ℃保存11 d,存活率没有发生显著变化;凝胶能有效提高益生菌对热处理(72 ℃,15 s或65 ℃,30 min)和模拟胃肠消化的抵抗力(存活率分别为4.79 lg CFU/g和5.88 lg CFU/g以上),而未被包埋的益生菌全部死亡(初始活菌数为8 lg CFU/g) Xu等[39] 大粒凝胶 鼠李糖乳杆菌(Lactobacillus rhamnosus) β-乳球蛋白-藻酸丙二醇酯 静电相互作用、氢键、
疏水作用自组装 实现益生菌与姜黄素共包埋;鼠李糖乳杆菌包埋率达98%以上;对比单一藻酸丙二醇酯水凝胶和β-乳球蛋白水凝胶,β-乳球蛋白-藻酸丙二醇酯水凝胶能有效提高模拟胃肠消化后的存活率(达8.92 lg CFU/mL),且4 ℃贮存4周后存活率达
9.72 lg CFU/mLSu等[40] 大粒凝胶珠 两歧双歧杆菌(Bifidobacterium bifidum) 玉米醇溶蛋白-藻朊酸盐 − 注射法 外观直径1.4~1.7 mm;当玉米醇溶蛋白添加量为7%时,最高包埋率达94.56%,活菌量>107 CFU/g,暴露于模拟胃肠液后活菌量损失<2 lg CFU/mL;在4 ℃保存32 d活菌量都
>106 CFU/g,而未包埋的或只用藻朊酸盐包埋的活菌量
分别为103 CFU/g和105 CFU/gRiaz等[41] 微粒 植物乳杆菌(Lactobacillus plantarum) 乳清分离蛋白-葡聚糖 美拉德反应
共价交联干热法,冷冻干燥 经模拟胃消化后,植物乳杆菌在乳清分离蛋白和葡聚糖
复合物包埋体系和未包埋体系中分别减少了0.89和
1.73 lg CFU/g(初始活菌数分别为8.42和8.76 lg CFU/g);
在4 ℃保存90 d后植物乳杆菌仅损失0.33 lg CFU/mLGuo等[42] 微粒 植物乳杆菌(Lactobacillus plantarum) 酪蛋白-壳聚糖-香草精/三聚磷酸盐/钙盐 − 复合凝聚,喷雾干燥 酪蛋白-壳聚糖-香草精微粒的植物乳杆菌(Lactobacillus plantarum)载量达11 lg CFU/mL,且最能抵抗模拟胃肠消化环境(损失约1.5 lg CFU/mL,而未被包埋的益生菌损失约
4 lg CFU/mL);在小鼠口服微粒4 h后能达到回肠末端和结肠,并在日服107 CFU/鼠三周后,小鼠的Th1和Th17细胞因子释放增加,表明免疫功能得到调节Peñalva等[43] 微粒 马克斯克鲁维酵母(Kluyveromyces marxianus) 乳清浓缩蛋白-水溶性壳聚糖衍生物 − 喷雾干燥 平均粒径10 µm;最适喷雾干燥出风温度为68 ℃;固形物含量对于储存期益生菌存活率有显著影响;当30%的固形物含量(乳清浓缩蛋白:水溶性壳聚糖衍生物=29:1)时,在模拟胃肠道消化后存活率为95% Braber等[44] 固体脂质微粒 嗜酸乳杆菌(Lactobacillus acidophilus),动物双歧杆菌(Bifidobacterium animals) 明胶-阿拉伯胶 静电相互
作用喷淋冷却/
冷冻干燥在25 ℃保存30 d后,喷淋冷却制备的微粒益生菌存活率为
7.2 lg CFU/g,而冷冻干燥制备的微粒为6.7 lg CFU/g;
经模拟胃肠消化后,嗜酸乳杆菌在喷淋冷却微粒中损失了
1.8 lg CFU/g,在冷冻干燥微粒中损失1.2 lg CFU/g,而未包埋的损失了2.5 lg CFU/g;经模拟胃肠消化后,双歧杆菌在喷淋冷却微粒中损失了0.5 lg CFU/g,在冷冻干燥微粒中损失
1.7 lg CFU/g,而未包埋的损失了4.2 lg CFU/gSliva等[45] 微凝胶 嗜酸乳杆菌(Lactobacillus acidophilus) 乳清分离蛋白-苦瓜多糖 静电相互作用,疏水作用,氢键 冷冻干燥 复合微凝胶的持水性、乳化性和热稳定性优于单一乳清分离蛋白微凝胶;复合微凝胶成功提高了益生菌冷冻干燥后的存活率,包埋率达98%,提高了益生菌贮存稳定性(4 ℃ 30 d达6.74~8.69 lg CFU/mL;单一微凝胶为6.35 lg CFU/mL)和经模拟胃肠消化后的存活率(8.65 lg CFU/mL;未包埋为
4.9 lg CFU/mL;单一微凝胶为6.5 lg CFU/mL)Bora等[46] 微凝胶珠 嗜热唾液链球菌(Streptococcus salivarius ssp. thermophilus),嗜酸乳杆菌(Lactobacillus acidophilus),两歧双歧杆菌(Bifidobacterium bifidum),德尔布鲁氏乳杆菌(Lactobacillus delbrueckii ssp. bulgaricus) 乳清蛋白水解物-藻朊酸盐 静电相互
作用注射法 直径约为800 μm;相对乳清浓缩蛋白,乳清蛋白水解物与藻朊酸盐形成的微凝胶珠呈现出更少的微孔结构,能更好地保护益生菌抵抗胃消化环境(酸性,4 h);经模拟胃肠消化后,凝胶中益生菌存活率超过96%,而未被包埋的益生菌存活率为37.43% Krunić等[47] 微凝胶珠 干酪乳杆菌(Lactobacillus casei) 乳清蛋白-藻朊酸盐-纤维素纳米晶体 − 复合凝聚,
注射法,
冷冻干燥双层微凝胶珠的平均粒径为0.45~0.57 mm,益生菌包埋率为83.25%~84.34%;当乳清蛋白:藻朊酸盐=1.5:1时,在4 ℃和
25 ℃保存120 d后,益生菌的活性分别达到6.72和5.97 lg CFU/g;经模拟胃、肠道消化后存活率分别为6.56和5.76 lg CFU/gZhang等[48] 微乳 植物乳杆菌(Lactobacillus plantarum) 酪蛋白-豌豆分离蛋白-结冷胶 静电相互
作用高速剪切 在4 ℃贮存21 d后,蛋白-多糖基乳液中益生菌存活率
(8.96 lg CFU/mL,相对初始值仅减少0.4 lg CFU/mL)显著
高于蛋白乳液(8.73 lg CFU/mL)和未被包埋的对照组
(7.16 lg CFU/mL);经63 ℃处理30 min后,三种样品的益生菌存活率分别为6.47、4.58和低于2 lg CFU/mL;经75 ℃加热
2 min,三者的益生菌分别损失了约3.17、2.13和5 lg CFU/mL;经模拟胃肠消化后,三者的益生菌分别损失了2.83和
3.83 lg CFU/mL及全部死亡Li等[49] 微乳 唾液乳杆菌(Lactobacillus salivarius) 乳清分离蛋白/酪蛋白酸钠-柑橘果胶 静电相互
作用高速剪切 将喷雾干燥的唾液乳杆菌悬浮在融化的无水乳脂中,然后用乳清分离蛋白或酪蛋白酸钠制备单层固/油/水(s/o/w)乳液,再在pH3时将柑橘果胶在液滴表面静电沉积形成双层乳液,包埋率达90%;在4 ℃保存20 d后,益生菌在单层乳液和双层乳液中的存活率比未包埋对照组高3倍;在63 ℃加热30 min后,未包埋益生菌全部死亡,而在单层和双层乳液中的存活率为2 lg CFU/mL;之后在微乳微胶囊化的过程中,喷雾干燥处理使益生菌降低了2 lg CFU/g,而未被包埋的降低了
5 lg CFU/gZhang等[50] 微胶囊 干酪乳杆菌(Lactobacillus casei) 酪蛋白酸钠-麦芽糊精/普多糖/加蒂胶/阿拉伯胶 − 高压均质,喷雾干燥 研究不同碳水化合物的添加对酪蛋白酸钠基低熔点脂肪微胶囊中益生菌耐胃性和储存性能的影响;在添加加蒂胶或阿拉伯胶的酪蛋白酸钠基微胶囊中,益生菌在喷雾干燥后的存活率较高(≥,0%);添加加蒂胶或阿拉伯胶后益生菌的模拟胃消化抵抗力加强,而添加麦芽糊精或普多糖后其抵抗力减弱;酪蛋白酸钠-阿拉伯胶复合微胶囊具有最高的玻璃化转变温度,且在25 ℃、水分活度0.11~0.76储存16周后具备最高益生菌存活率 Liu等[51] w/o微胶囊 植物乳杆菌(Lactobacillus plantarum) 明胶-阿拉伯胶 静电相互
作用高速剪切,复合凝聚,冷冻干燥 益生菌活菌数和包埋率分别达8.6 lg CFU/g和97.78%;经模
拟胃肠消化后益生菌存活率为80.4%,而未被包埋的益生菌存活率为25%;在8 ℃和−18 ℃保存45 d后活菌数为
7.6 lg CFU/g。Paula等[52] 纳米乳液 屎球肠菌(Enterococcus faceium) 乳清浓缩蛋白-阿拉伯胶 − 超声 在27 ℃和4 ℃保存60 d后,纳米乳液粒径从150 nm增至
500 nm,益生菌的活菌数从3.4×108 CFU/mL增长至
7.3×108 CFU/mLKrithika等[53] 注:−表示未提及。 2.1 宏观级
2.1.1 大粒凝胶/大粒凝胶珠
水凝胶(hydrogel)是通过pH、加热、冷却、盐离子、酶促交联、高压、3D打印等方式诱导形成、可保持大量水不溶解的生物聚合物分子三维交联网络体系[18, 38, 54]。其网络结构特征限制了外部环境因子(比如酶、氧气、盐等)与益生菌的接触,进而降低不良环境对益生菌的损耗。根据其粒径大小,水凝胶又可以分为大粒凝胶(macrogels)、微凝胶(microgels)、纳米凝胶(nanogels)。其中大粒凝胶已被广泛用于包埋递送益生菌[55](表1)。Yan等[56]发现经模拟胃肠消化后,副干酪乳杆菌(Lactobacillus paracasei)在所有大豆分离蛋白-甜菜果胶酶促交联大粒凝胶中的损失仅约为1 lg CFU/mL,而未被包埋的副干酪乳杆菌全部死亡,展现出结肠靶向递送副干酪乳杆菌的效果;但副干酪乳杆菌的加入降低了水凝胶的硬度、提高了膨胀率并轻微扰乱了水凝胶的有序微观结构。
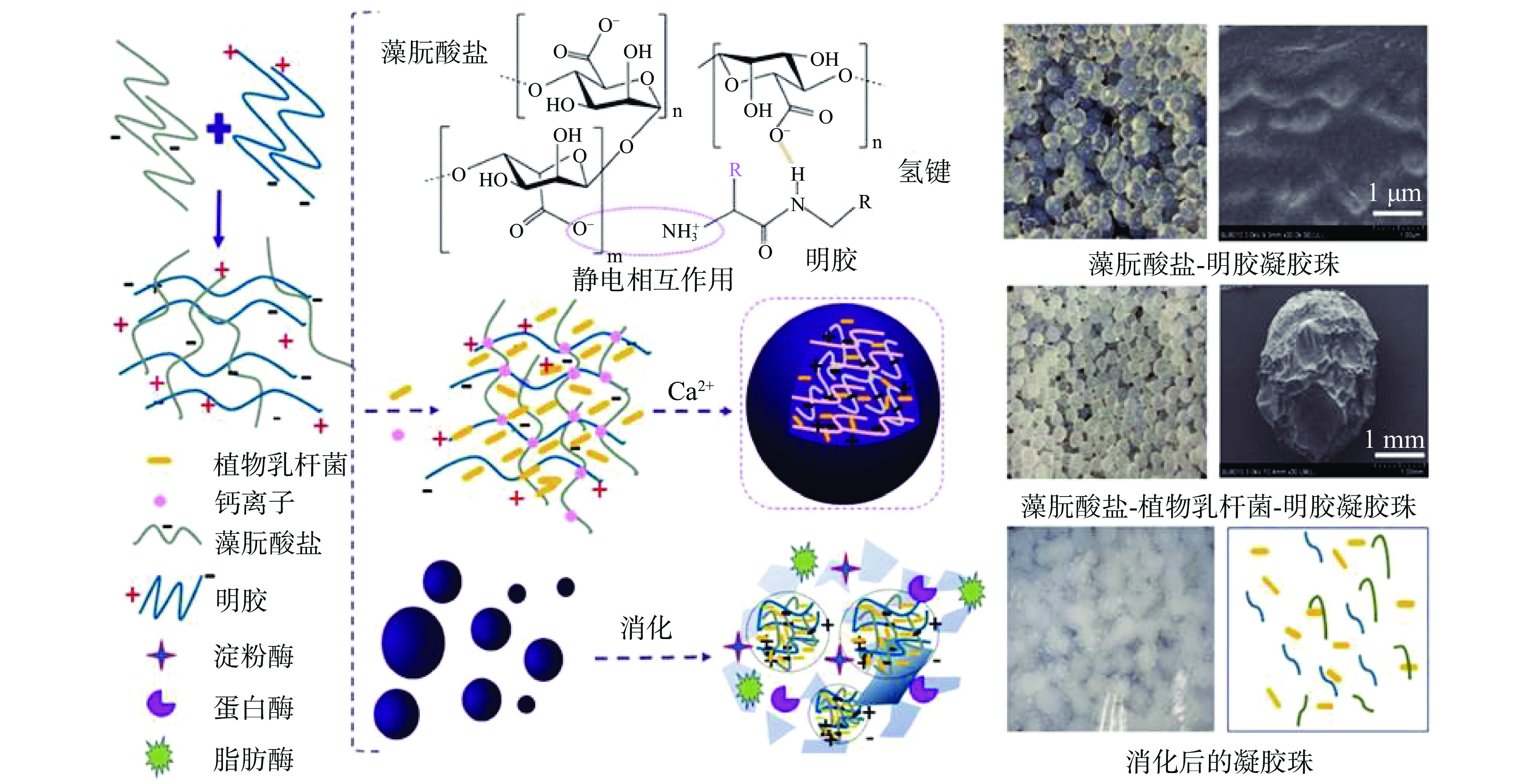
凝胶珠(hydrogel beads)是一种外观尺寸在微米级或毫米级的球形体系[57],根据尺寸又可分为大粒凝胶珠和微凝胶珠。凝胶珠可通过简单的注射法制得,比如将藻朊酸盐注入含二价阳离子(如Ca2+、Cu2+、Ba2+、Co2+)的水溶液,或将琼脂糖倒入冷水中等[58]。而蛋白质可通过非共价或共价作用与多糖进一步结合,从而改善凝胶珠的总体功效[59-60]。Ni等[61]发现相对于单一藻朊酸盐凝胶珠,基于静电和氢键结合的藻朊酸盐-明胶基大粒凝胶珠对植物乳杆菌(Lactobacillus plantarum)的耐热性(50 ℃ 5 min)和贮存稳定性(4 ℃,6 d)分别提高了8%和15%,且可以提高植物乳杆菌对胃酸的抵抗力,并在小肠中因失去分子间氢键作用而逐渐溶解,从而达到小肠靶向释放的目的;但植物乳杆菌的负电与藻朊酸盐-明胶复合物形成的静电排斥力降低了凝胶珠的强度和硬度(图3)。
综上,虽然不同结构特性的蛋白质-多糖基大粒凝胶/大粒凝胶珠通过简单的方法即可制得,且展现出优异的益生菌包封率和对环境应力的抵抗力,但尚存在一些实际应用限制:一方面益生菌的添加会对其凝胶结构和稳定性产生一定的破坏作用,另一方面通常需要通过冷冻干燥等方法以提高其稳定性、延长货架期,而益生菌在冷冻干燥过程中的存活率会下降。故更多关于蛋白质-多糖基大粒凝胶体系稳态化技术与材料的开发及其与益生菌活性保持的相关性仍有待进一步研究。
2.2 微米级
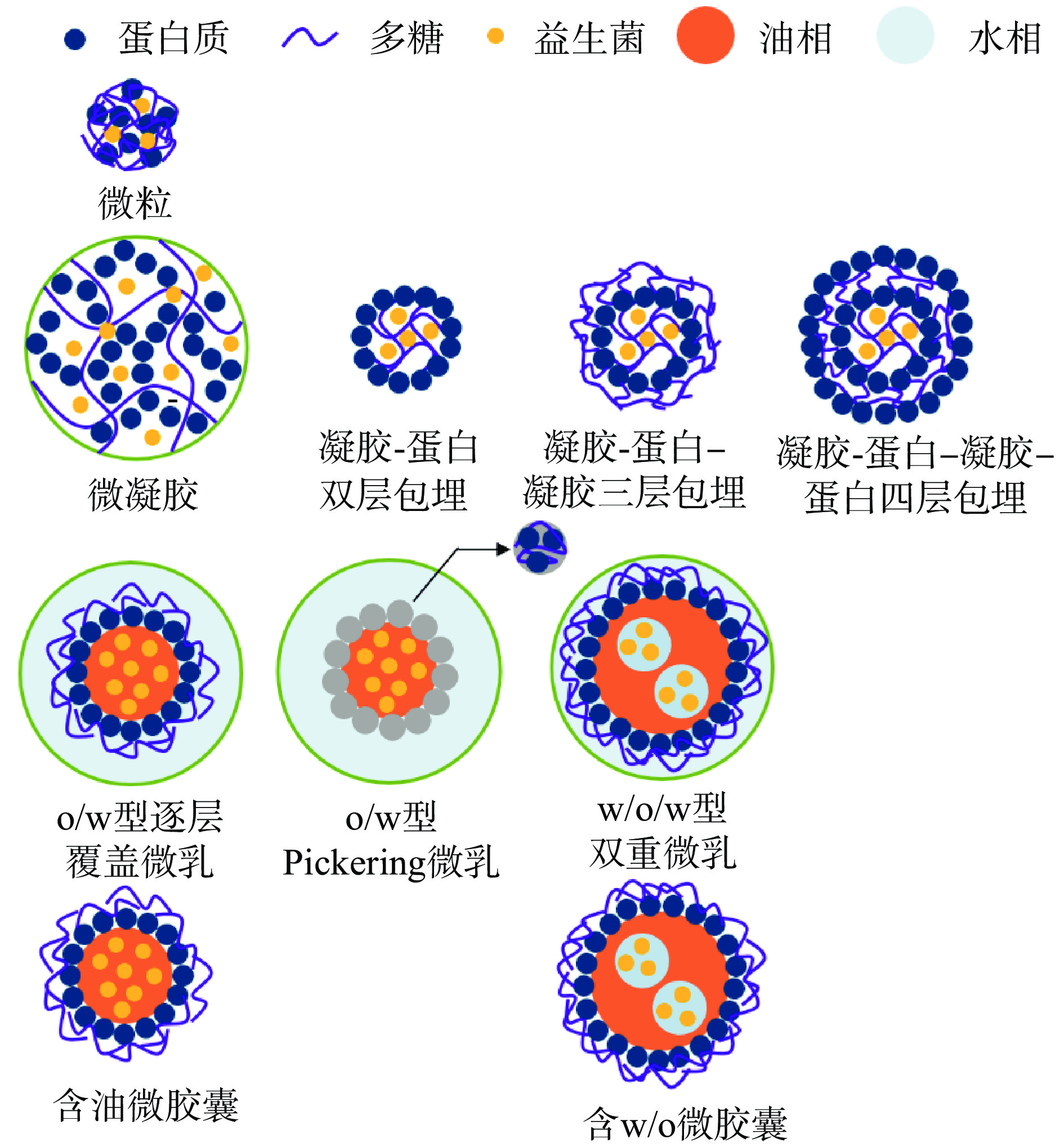
常见的微米级蛋白质-多糖基益生菌递送体系包括微粒(microparticles)、微凝胶/微凝胶珠、微乳(microemulsions)、微胶囊(microcapsules)等[37](表1,图4)。除了蛋白质-多糖复合材料自身的靶向递送特征,微粒、微乳和微胶囊壳-核结构的物理屏障作用和微凝胶网络结构的限制作用(2.1)进一步有助于实现益生菌的肠道定植。
2.2.1 微粒
相对于纳米粒子,直径为1~1000 μm的微粒具有足够空间来包埋益生菌的优势[62]。通过调节pH、温度等条件,蛋白质和多糖可通过非共价或共价结合形成具有不同结构特性的微粒。Mao等发现美拉德反应制备的大豆分离蛋白和I-卡拉胶复合物[63]比复合凝聚法制备的复合物[64]更能提高长双歧杆菌(Bifidobacterium longum)对不利环境的抵抗力:经贮存(4 ℃,30 d)、巴氏杀菌(85 ℃,30 min)和模拟胃肠消化后,前者分别损失0.4、2.05和3.12 lg CFU/mL,而后者分别损失1.62、2.71和4.16 lg CFU/mL。这可能是因为益生菌在通过复合凝聚法制备微粒的酸性条件中容易受到损耗。Ma等[65]利用复合凝聚法和化学交联法制备大豆蛋白-阿拉伯胶微粒能提高植物乳杆菌(Lactobacillus plantarum)在贮存过程(25 ℃,28 d)和模拟胃液中的存活率(分别达88%和98%以上)及模拟肠液中释放的活菌量(4 h后达6.52 CFU/g)。但不同共价交联方式对蛋白质-多糖基微粒益生菌递送的影响及其消化特性的作用机制还需继续深入研究。
2.2.2 微凝胶/微凝胶珠
与单一生物聚合物微凝胶相比,蛋白质-多糖复合微凝胶可形成更紧密的互穿双网络结构,进而表现出更好的机械性能、持水能力、耐热性[66]。通过调节混合比例、pH、离子强度等条件,带相反电荷的蛋白质和多糖可以通过静电相互作用复合凝聚形成微凝胶/微凝胶珠。Etchepare等[67]发现,相比于未包埋、藻朊酸盐单层包埋、藻朊酸盐-乳清浓缩蛋白-藻朊酸盐三层包埋和藻朊酸盐-乳清浓缩蛋白-藻朊酸盐-乳清浓缩蛋白四层包埋(图4),藻朊酸盐-乳清浓缩蛋白双层包埋的嗜酸乳杆菌(Lactobacillus acidophilu)微凝胶珠经模拟胃肠道消化后有最高的存活率,达9.19 lg CFU/g(初始活菌量约为12 lg CFU/g)。但与大粒凝胶相似(2.1.1),微凝胶/微凝胶珠的长期稳定性问题仍待进一步研究。
2.2.3 微乳
蛋白质-多糖复合物通常作为亲水性乳化剂用于稳定水包油(o/w)型或水包油包水(w/o/w)型微乳(图4)[16, 68-69]。微乳不仅是递送益生菌的有效工具[70],而且其组成成分也可能对肠道菌群产生积极或消极的影响[71]。Kan等[72]采用第二种途径制备了乳清分离蛋白-阿拉伯胶美拉德反应共轭物稳定的o/w微乳,发现该微乳可显著调节人体粪便中肠道菌群的组成和丰度,以及丰富碳水化合物代谢和胆汁酸生物合成的途径。这可能归功于美拉德偶联物的消化率较低,使得大多数肠道菌群可发酵的碳水化合物和蛋白质底物能进入结肠,进而被肠道菌群代谢、并影响宿主的健康[73]。因此,通过美拉德反应制备的蛋白质-多糖复合物具有促进人体肠道菌群健康变化的潜力,这对设计使用蛋白质-多糖复合物作为乳化剂的微乳具有重要意义。
w/o/w双重微乳不仅具有减脂、减糖、减盐、共包埋亲水和亲油性生物活性物质的优点[74],而且相比于其他微乳体系(比如o/w,油包水w/o,水包水w/w等类型),其结构更能有效保护益生菌[62]:不同于o/w微乳,益生菌可以包埋在双重微乳内层水相中,而大多数益生菌在水中的溶解性更好;另外w/o/w双重微乳包含一层油相和两层水相,故双重微乳的油相和外层水相都能保护在内层水相包埋的益生菌(图4)。Qin等[75]开发了一种在酸性条件呈水凝胶状态、在中性pH条件呈溶液状态的pH响应性w/o/w双重微乳,能有效提高植物乳杆菌(Lactobacillus plantarum)经模拟胃肠消化后的活菌数(从7.79×107 CFU/mL降至7.36×107 CFU/mL,而未被包埋的益生菌从7.81×107 CFU/mL降至0.14×107 CFU/mL)。虽然双重微乳具有提高益生菌稳定性、改善控释效果的应用前景,但双重微乳的实际应用仍存在一些问题,比如由于存在较多分散液滴及表面积,双重微乳的长期稳定性通常比较欠缺[76]。另外,PGPR存在安全食用限量(ADI 25 mg/kg)、异味及消费者对人工合成添加剂抵触等缺点[77],对双重乳液的可食用性也存在影响,故找到替代甚至超越PGPR乳化性的天然亲油性乳化剂[78]仍是双重微乳递送体系急需解决的问题。
2.2.4 微胶囊
微胶囊是一种利用天然或合成的大分子聚合物作为壳/覆盖层,包覆固体、液体或气体等核心物质,利用物理(喷雾干燥、冷冻干燥、挤出技术、分子微囊化技术等)或化学方法(复合凝聚、复合沉淀、原位聚合等)制成的微型胶囊或微粒。2.2.1中介绍的仅以蛋白质-多糖复合物为材料的微粒也属于一种微胶囊,故在此介绍以乳液为基础制备的蛋白质-多糖复合微胶囊。以o/w乳液为基础(图4)是最常见制备微胶囊的方式之一[79]。若将天然或合成的固体脂质用作o/w乳液中的油相,再采用固化手段去除水分即可制成固体脂质微粒(solid lipid microparticle)。Tasch Holkem等[80]发现添加了5%肉桂提取物的乳球浓缩蛋白-阿拉伯胶静电复合固体脂质微粒具有最高的动物双歧杆菌(Bifidobacterium animalis)包埋率(98.59%),并在7 ℃贮存120 d后获得最高存活率(9.3 lg CFU/g)。虽然含油微胶囊具有长期稳定性高、不良环境抵抗力强等特点,但以o/w乳液为基础制备微胶囊的方式更适用于疏水性强的益生菌,而对强亲水性益生菌的包埋难以达到理想效果[80]。而以w/o/w乳液为基础制备的w/o微胶囊内层水相适用于包埋大部分益生菌(图4)[81]。但在喷雾干燥、冷冻干燥等过程中w/o/w乳液结构容易受到破坏、难以获得理想的颗粒形貌[82],进而影响w/o微胶囊的递送效果。除此之外,食品工业中制备微胶囊也通常采用传统干燥方法,而这些干燥脱水过程通常对益生菌有较大的损伤[83]。
2.3 纳米级
近年来,纳米级递送体系的构建与应用前景一直是研究的热点与焦点[37]。虽然纳米颗粒(nanoparticles)、纳米凝胶、纳米纤维(nano-fibers)、纳米乳液(nano-emulsions)、纳米胶囊(nano-capsules)等纳米级体系已被证实是递送益生菌的优势载体[84-85],但目前纳米级益生菌递送体系的研究开发仍面临一定的困难与挑战[62, 86-87]:一方面难以找到合适的可用于制备纳米颗粒的生物聚合物材料(包括蛋白质-多糖复合物),另一方面益生菌的尺寸普遍较大(通常在1~10 μm)且形状各异,常难以较好地匹配纳米级递送体系。下面主要介绍可用蛋白质-多糖复合物制备的纳米纤维、纳米颗粒和纳米乳液。
2.3.1 纳米纤维
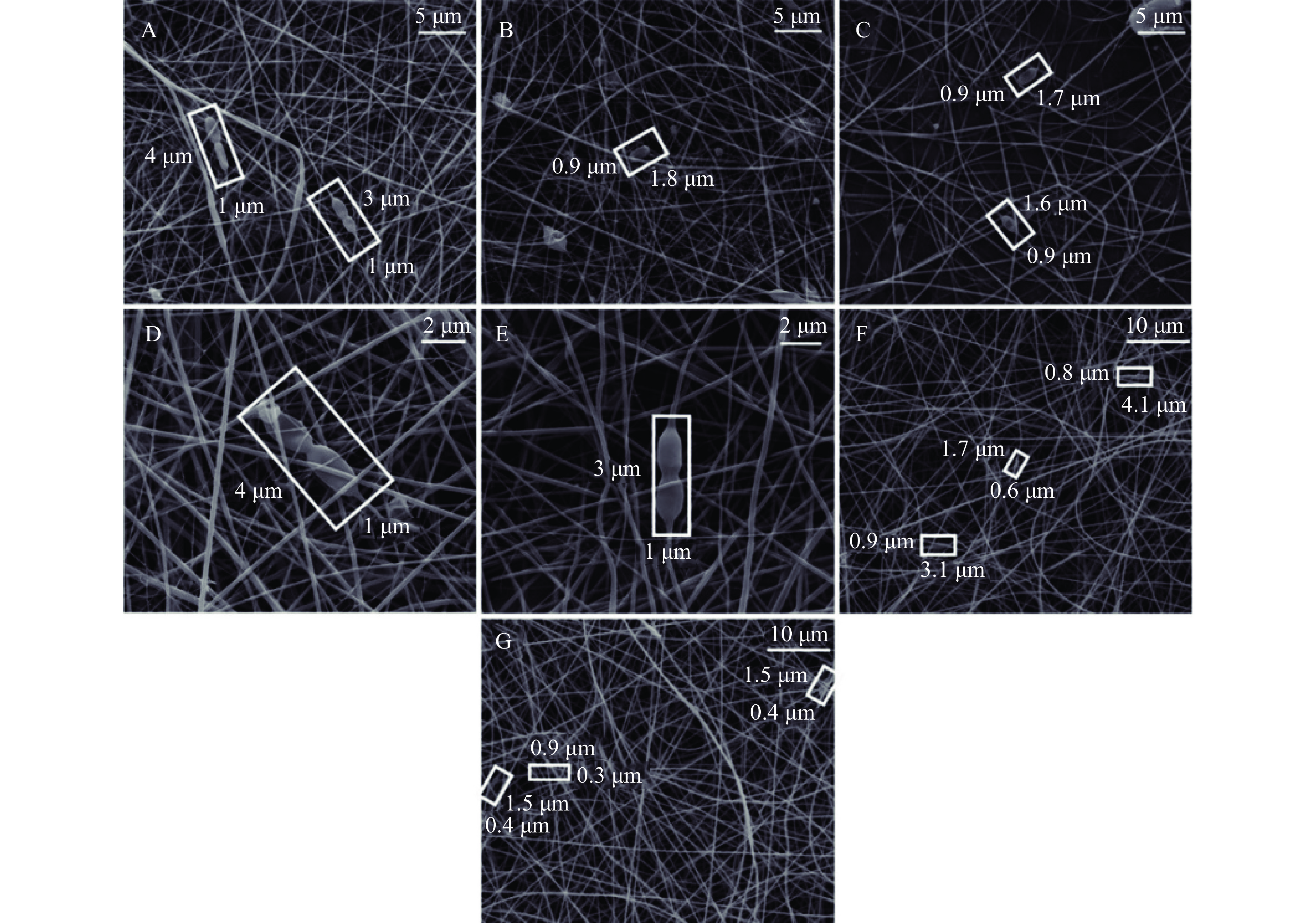
利用电流体动力学加工技术(包括静电纺丝和静电喷涂),在高压静电场下将表面带电 的生物聚合物溶液通过喷嘴喷出即可形成负载生物活性物质的纳米纤维[88]。其中制备纳米 纤维的聚合物材料溶液是最关键的因素之一。蛋白质因其静电、疏水和亲水本质可用作疏水 袋保护益生菌免受不利环境的影响[89],但溶解在水中的蛋白质因其α-螺旋、β-片二级结构、 三级结构和球状结构等三维结构在电场作用下难以拉伸和缠绕[90]。多糖的加入可以改善蛋 白质在电流体动力学加工技术中的应用限制,包括具有潜在益生元特性[91]的普鲁兰多糖[92]、 藻朊酸盐[93]等。Akkurt 等[94]发现在静电纺丝加工后,酪蛋白酸钙-普鲁兰多糖纳米纤维和酪 蛋白酸钠-普鲁兰多糖纳米纤维中鼠李糖乳杆菌(Lactobacillus rhamnosus)活菌数分别达9.5 和9.6 lg CFU/mL(分别负载9.3 和9 lg CFU/mL);经扫描电镜观察发现鼠李糖乳杆菌分布 在纳米纤维内(图5)。但更多适应于电流体动力学加工技术的蛋白质-多糖复合材料及其在 益生菌靶向递送的实际应用还有待进一步探索。
![]() 图 5 鼠李糖乳杆菌电纺纤维垫扫描电镜图[94]注:孔隙率分别为46%、47%、47%的酪蛋白酸钙-普多糖-鼠李糖乳杆菌纤维垫直径分别为(A)200 nm、(B)209 nm和(C)228 nm,放大倍数10000×;孔隙率分别为42%和48%的酪蛋白酸钙-普多糖-鼠李糖乳杆菌纤维垫直径分别为(D)127 nm和(E)125 nm,放大倍数25000×;孔隙率分别为52%和48%的酪蛋白酸钠-普多糖-鼠李糖乳杆菌纤维垫直径分别为(F)286 nm和(G)298 nm,放大倍数5000×。Figure 5. Scanning electron microscopy images of electrospun Lactobacillus rhamnosus GG (LGG) incorporated in fibrous mats[94]
图 5 鼠李糖乳杆菌电纺纤维垫扫描电镜图[94]注:孔隙率分别为46%、47%、47%的酪蛋白酸钙-普多糖-鼠李糖乳杆菌纤维垫直径分别为(A)200 nm、(B)209 nm和(C)228 nm,放大倍数10000×;孔隙率分别为42%和48%的酪蛋白酸钙-普多糖-鼠李糖乳杆菌纤维垫直径分别为(D)127 nm和(E)125 nm,放大倍数25000×;孔隙率分别为52%和48%的酪蛋白酸钠-普多糖-鼠李糖乳杆菌纤维垫直径分别为(F)286 nm和(G)298 nm,放大倍数5000×。Figure 5. Scanning electron microscopy images of electrospun Lactobacillus rhamnosus GG (LGG) incorporated in fibrous mats[94]2.3.2 纳米颗粒
利用蛋白质-多糖复合材料制备益生菌包封纳米颗粒的途径主要有两种:第一种是用抗溶剂共沉淀法[95]、纳米沉淀法[96]等方式制备天然生物聚合物纳米颗粒,但由于起步较晚,蛋白质-多糖基纳米颗粒包封益生菌的研究报道较少[85];第二种是以磷脂或胆固醇制备包封益生菌的脂质纳米颗粒,再将蛋白质和多糖进一步吸附在脂质纳米颗粒表面。Hosseini等[97]发现经模拟胃液和肠液消化后,明胶-壳聚糖-卵磷脂基脂质纳米颗粒中的鼠李糖乳杆菌(Lactobacillus rhamnosus)分别损失了1.2和1.6 lg CFU/mL,而卵磷脂基脂质纳米颗粒分别损失了1.7和3.28 lg CFU/mL,未被包埋的益生菌分别损失了4.5和5.5 lg CFU/mL。故利用蛋白质和多糖复合材料制备纳米颗粒对于提高益生菌结肠靶向递送具有重要意义。
2.3.3 纳米乳液
纳米乳液是一种热力学稳定、能有效包埋生物活性物质并提高其生物利用率的递送体系,通常在粒径50~200 nm时呈现透明,在粒径大于500 nm时呈现乳白色外观[98-99]。纳米乳液可以更容易地穿过细胞壁、进入目标细胞,具有较强的肠黏膜黏附能力和与其他代谢因子相互作用的能力,且纳米乳液液滴可以保护被包埋益生菌免受不良环境的影响,提高益生菌活性[86]。Vaishanavi等[100]发现在27 ℃贮存40 d后,大豆分离蛋白-阿拉伯胶基o/w纳米乳液中的德尔布鲁氏乳杆菌(Lactobacillus delbrueckii subsp. bulgaricus)活菌数达9.8×107 CFU/mL和8.8×108 CFU/mL(初始活菌数分别为5.4×107 CFU/mL和4.3×108 CFU/mL)。
3. 蛋白质-多糖基益生菌递送体系在食品中的应用
3.1 酸奶
酸奶是益生菌的理想载体。酸奶为益生菌的生存提供了良好的介质,且口感好,营养价值高,可以常规地纳入日常饮食中。然而,在酸奶的储存、运输和配送过程中,益生菌仍能在较低环境温度下利用残留的乳糖缓慢发酵、产生乳酸,导致酸奶酸度继续上升,并对其感官属性产生不利影响[8]。此外,如果酸奶在储存过程中pH下降过多,益生菌的活性也会降低。益生菌酸奶在储存期间的过度酸化问题可以通过不同尺度结构的蛋白质-多糖基益生菌递送体系得以改善,比如微凝胶珠[101]和微胶囊[102]均能降低酸奶的过酸化程度,且能提高益生菌在贮存过程中的存活率,并改善酸奶品质;w/o/w双重乳液具有分离发酵益生菌的作用,以避免对发酵过程干扰,同时不会对酸奶的理化性质产生影响,并提高益生菌经贮存和胃肠消化后的存活率[103]。
3.2 奶酪
因其具有相对较高的pH、脂肪含量、固体稠度和高缓冲能力,奶酪亦被认为是一种优秀的益生菌输送工具[104]。另外,益生菌也可以在奶酪成熟过程中产生蛋白酶、脂肪酶、乳糖酶、胞外多糖和/或抗菌物质,帮助产品质量属性、安全性和保质期的提高。但奶酪加工环境、长期贮存及食用消化过程都会极大影响益生菌的活菌量,而通过蛋白质-多糖复合材料进行包埋,并在奶酪中应用能提高益生菌在不良环境中的生存能力。例如,水凝胶能提高益生菌在奶酪贮存过程中的稳定性,还能限制蛋白质水解和可滴定酸度变化[105];微粒能提高益生菌在奶酪贮存和模拟胃肠消化环境中的存活率[106];微胶囊能提高益生菌在低pH、高盐离子浓度等奶酪加工环境因素和数月贮存过程中的存活率[79]。
3.3 饮料
因为令人满意的风味和营养特性,众多乳、茶、果蔬、植物蛋白等饮料都是提供益生菌的理想食品。此外,饮料中的许多营养物质均对益生菌生长起着积极作用,包括维生素、矿物质、膳食纤维和抗氧化剂等[107]。利用蛋白质-多糖基体系包埋益生菌能改善益生菌在杀菌、干燥等饮料加工工艺、数周或数月的贮存及食用消化过程中大量失活的问题,但蛋白质-多糖复合材料的筛选及益生菌递送体系尺度结构的构建十分关键,不仅决定了益生菌是否能实现结肠靶向传递,而且决定了益生菌是否发酵并对饮料的品质性能产生影响[108]:相对于多糖基水凝胶[109],蛋白质-多糖基水凝胶不仅能进一步提高益生菌在发酵、贮存及胃肠消化后的存活率[47, 61],而且因蛋白质载体具有较高的孔隙率,益生菌可从蛋白质-多糖基水凝胶中持续释放到饮料主体中并繁殖增长,进而产生更高的益生菌代谢活性而获得更高滴定酸度的饮料,同时因为蛋白质的缓冲作用,加入蛋白质-多糖基水凝胶的饮料pH更高[110];蛋白质-多糖基微粒可以分离益生菌以避免后酸化过程,进而避免对饮料pH、可滴定酸度和还原糖含量等性能的影响[111];在蛋白质-多糖基微胶囊中,由于喷雾干燥过程中蛋白质变性而导致微胶囊孔隙度和表面活性变化,饮料中营养物质在微胶囊中扩散减慢,进而减缓了益生菌的生长及饮料pH的降低,同时能提高饮料在贮存和模拟胃肠消化后的存活率[112]。
除以上食品外,蛋白质-多糖基益生菌递送体系还能在肉制品[113]、可食用膜[114]等领域中应用,并在益生菌活性保持和产品品质改善等方面发挥了重要作用。
4. 结论
综上所述,利用蛋白质和多糖之间的非共价或共价作用可以组装形成多尺度结构的蛋白-多糖基递送体系,其中水凝胶、微粒、纳米颗粒等体系均已展现出优异的靶向传输功效。但目前不同尺度结构的递送体系仍存在不同的缺陷,比如水凝胶的长期稳定性差、货架期短,益生菌在o/w型微乳的油相里溶解性差,w/o/w型双重微乳长期稳定性和亲油性乳化剂方面仍存在待改善的问题,微胶囊利用传统干燥方法制备的过程中对益生菌有很大的损耗,纳米纤维、纳米颗粒、纳米乳液等体系的材料开发和在食品中的实际应用还有待继续深入探究。蛋白质-多糖基益生菌递送体系已在酸奶、奶酪、饮料等食品中展现出优异的功能性,其尺度结构决定了益生菌的靶向传递效果及发酵程度对产品品质的影响。未来,蛋白质-多糖基益生菌递送系统的研究工作可在如下方面进一步探索:a. 结合纳米纤维、纳米颗粒、纳米乳液等纳米体系的结构特征,开发高效传输益生菌的纳米级递送体系;b. 开展益生菌与其他生物活性物质共包埋的蛋白质-多糖复合递送体系的构建机制及性能研究,开发多功能的新型食品;c. 利用蛋白质-多糖复合体系的天然性、生物相容性、生物可降解性、食用安全性及特有的营养和功能特性等优点,设计含活性益生菌的食品包装材料和低脂食物、肉类替代物等对人体健康的新型食品。
-
图 5 鼠李糖乳杆菌电纺纤维垫扫描电镜图[94]
注:孔隙率分别为46%、47%、47%的酪蛋白酸钙-普多糖-鼠李糖乳杆菌纤维垫直径分别为(A)200 nm、(B)209 nm和(C)228 nm,放大倍数10000×;孔隙率分别为42%和48%的酪蛋白酸钙-普多糖-鼠李糖乳杆菌纤维垫直径分别为(D)127 nm和(E)125 nm,放大倍数25000×;孔隙率分别为52%和48%的酪蛋白酸钠-普多糖-鼠李糖乳杆菌纤维垫直径分别为(F)286 nm和(G)298 nm,放大倍数5000×。
Figure 5. Scanning electron microscopy images of electrospun Lactobacillus rhamnosus GG (LGG) incorporated in fibrous mats[94]
表 1 蛋白质-多糖基活性益生菌递送体系结构及性能特征
Table 1 Structure and property characteristics of protein-polysaccharide based viable probiotics delivery system
递送体系 益生菌 复合物 蛋白质-多糖结合作用 制备技术 性能特征 参考文献 皮克林乳液大粒凝胶 乳酸双歧杆菌(Bifidobacterium lactis) 茶蛋白-黄原胶 − 高速剪切,3D打印技术 45 ℃和55 ℃打印温度不会影响益生菌存活率,但在65 ℃活菌数会从8.07 lg CFU/g减少至6.59 lg CFU/g;在4 ℃保存11 d,存活率没有发生显著变化;凝胶能有效提高益生菌对热处理(72 ℃,15 s或65 ℃,30 min)和模拟胃肠消化的抵抗力(存活率分别为4.79 lg CFU/g和5.88 lg CFU/g以上),而未被包埋的益生菌全部死亡(初始活菌数为8 lg CFU/g) Xu等[39] 大粒凝胶 鼠李糖乳杆菌(Lactobacillus rhamnosus) β-乳球蛋白-藻酸丙二醇酯 静电相互作用、氢键、
疏水作用自组装 实现益生菌与姜黄素共包埋;鼠李糖乳杆菌包埋率达98%以上;对比单一藻酸丙二醇酯水凝胶和β-乳球蛋白水凝胶,β-乳球蛋白-藻酸丙二醇酯水凝胶能有效提高模拟胃肠消化后的存活率(达8.92 lg CFU/mL),且4 ℃贮存4周后存活率达
9.72 lg CFU/mLSu等[40] 大粒凝胶珠 两歧双歧杆菌(Bifidobacterium bifidum) 玉米醇溶蛋白-藻朊酸盐 − 注射法 外观直径1.4~1.7 mm;当玉米醇溶蛋白添加量为7%时,最高包埋率达94.56%,活菌量>107 CFU/g,暴露于模拟胃肠液后活菌量损失<2 lg CFU/mL;在4 ℃保存32 d活菌量都
>106 CFU/g,而未包埋的或只用藻朊酸盐包埋的活菌量
分别为103 CFU/g和105 CFU/gRiaz等[41] 微粒 植物乳杆菌(Lactobacillus plantarum) 乳清分离蛋白-葡聚糖 美拉德反应
共价交联干热法,冷冻干燥 经模拟胃消化后,植物乳杆菌在乳清分离蛋白和葡聚糖
复合物包埋体系和未包埋体系中分别减少了0.89和
1.73 lg CFU/g(初始活菌数分别为8.42和8.76 lg CFU/g);
在4 ℃保存90 d后植物乳杆菌仅损失0.33 lg CFU/mLGuo等[42] 微粒 植物乳杆菌(Lactobacillus plantarum) 酪蛋白-壳聚糖-香草精/三聚磷酸盐/钙盐 − 复合凝聚,喷雾干燥 酪蛋白-壳聚糖-香草精微粒的植物乳杆菌(Lactobacillus plantarum)载量达11 lg CFU/mL,且最能抵抗模拟胃肠消化环境(损失约1.5 lg CFU/mL,而未被包埋的益生菌损失约
4 lg CFU/mL);在小鼠口服微粒4 h后能达到回肠末端和结肠,并在日服107 CFU/鼠三周后,小鼠的Th1和Th17细胞因子释放增加,表明免疫功能得到调节Peñalva等[43] 微粒 马克斯克鲁维酵母(Kluyveromyces marxianus) 乳清浓缩蛋白-水溶性壳聚糖衍生物 − 喷雾干燥 平均粒径10 µm;最适喷雾干燥出风温度为68 ℃;固形物含量对于储存期益生菌存活率有显著影响;当30%的固形物含量(乳清浓缩蛋白:水溶性壳聚糖衍生物=29:1)时,在模拟胃肠道消化后存活率为95% Braber等[44] 固体脂质微粒 嗜酸乳杆菌(Lactobacillus acidophilus),动物双歧杆菌(Bifidobacterium animals) 明胶-阿拉伯胶 静电相互
作用喷淋冷却/
冷冻干燥在25 ℃保存30 d后,喷淋冷却制备的微粒益生菌存活率为
7.2 lg CFU/g,而冷冻干燥制备的微粒为6.7 lg CFU/g;
经模拟胃肠消化后,嗜酸乳杆菌在喷淋冷却微粒中损失了
1.8 lg CFU/g,在冷冻干燥微粒中损失1.2 lg CFU/g,而未包埋的损失了2.5 lg CFU/g;经模拟胃肠消化后,双歧杆菌在喷淋冷却微粒中损失了0.5 lg CFU/g,在冷冻干燥微粒中损失
1.7 lg CFU/g,而未包埋的损失了4.2 lg CFU/gSliva等[45] 微凝胶 嗜酸乳杆菌(Lactobacillus acidophilus) 乳清分离蛋白-苦瓜多糖 静电相互作用,疏水作用,氢键 冷冻干燥 复合微凝胶的持水性、乳化性和热稳定性优于单一乳清分离蛋白微凝胶;复合微凝胶成功提高了益生菌冷冻干燥后的存活率,包埋率达98%,提高了益生菌贮存稳定性(4 ℃ 30 d达6.74~8.69 lg CFU/mL;单一微凝胶为6.35 lg CFU/mL)和经模拟胃肠消化后的存活率(8.65 lg CFU/mL;未包埋为
4.9 lg CFU/mL;单一微凝胶为6.5 lg CFU/mL)Bora等[46] 微凝胶珠 嗜热唾液链球菌(Streptococcus salivarius ssp. thermophilus),嗜酸乳杆菌(Lactobacillus acidophilus),两歧双歧杆菌(Bifidobacterium bifidum),德尔布鲁氏乳杆菌(Lactobacillus delbrueckii ssp. bulgaricus) 乳清蛋白水解物-藻朊酸盐 静电相互
作用注射法 直径约为800 μm;相对乳清浓缩蛋白,乳清蛋白水解物与藻朊酸盐形成的微凝胶珠呈现出更少的微孔结构,能更好地保护益生菌抵抗胃消化环境(酸性,4 h);经模拟胃肠消化后,凝胶中益生菌存活率超过96%,而未被包埋的益生菌存活率为37.43% Krunić等[47] 微凝胶珠 干酪乳杆菌(Lactobacillus casei) 乳清蛋白-藻朊酸盐-纤维素纳米晶体 − 复合凝聚,
注射法,
冷冻干燥双层微凝胶珠的平均粒径为0.45~0.57 mm,益生菌包埋率为83.25%~84.34%;当乳清蛋白:藻朊酸盐=1.5:1时,在4 ℃和
25 ℃保存120 d后,益生菌的活性分别达到6.72和5.97 lg CFU/g;经模拟胃、肠道消化后存活率分别为6.56和5.76 lg CFU/gZhang等[48] 微乳 植物乳杆菌(Lactobacillus plantarum) 酪蛋白-豌豆分离蛋白-结冷胶 静电相互
作用高速剪切 在4 ℃贮存21 d后,蛋白-多糖基乳液中益生菌存活率
(8.96 lg CFU/mL,相对初始值仅减少0.4 lg CFU/mL)显著
高于蛋白乳液(8.73 lg CFU/mL)和未被包埋的对照组
(7.16 lg CFU/mL);经63 ℃处理30 min后,三种样品的益生菌存活率分别为6.47、4.58和低于2 lg CFU/mL;经75 ℃加热
2 min,三者的益生菌分别损失了约3.17、2.13和5 lg CFU/mL;经模拟胃肠消化后,三者的益生菌分别损失了2.83和
3.83 lg CFU/mL及全部死亡Li等[49] 微乳 唾液乳杆菌(Lactobacillus salivarius) 乳清分离蛋白/酪蛋白酸钠-柑橘果胶 静电相互
作用高速剪切 将喷雾干燥的唾液乳杆菌悬浮在融化的无水乳脂中,然后用乳清分离蛋白或酪蛋白酸钠制备单层固/油/水(s/o/w)乳液,再在pH3时将柑橘果胶在液滴表面静电沉积形成双层乳液,包埋率达90%;在4 ℃保存20 d后,益生菌在单层乳液和双层乳液中的存活率比未包埋对照组高3倍;在63 ℃加热30 min后,未包埋益生菌全部死亡,而在单层和双层乳液中的存活率为2 lg CFU/mL;之后在微乳微胶囊化的过程中,喷雾干燥处理使益生菌降低了2 lg CFU/g,而未被包埋的降低了
5 lg CFU/gZhang等[50] 微胶囊 干酪乳杆菌(Lactobacillus casei) 酪蛋白酸钠-麦芽糊精/普多糖/加蒂胶/阿拉伯胶 − 高压均质,喷雾干燥 研究不同碳水化合物的添加对酪蛋白酸钠基低熔点脂肪微胶囊中益生菌耐胃性和储存性能的影响;在添加加蒂胶或阿拉伯胶的酪蛋白酸钠基微胶囊中,益生菌在喷雾干燥后的存活率较高(≥,0%);添加加蒂胶或阿拉伯胶后益生菌的模拟胃消化抵抗力加强,而添加麦芽糊精或普多糖后其抵抗力减弱;酪蛋白酸钠-阿拉伯胶复合微胶囊具有最高的玻璃化转变温度,且在25 ℃、水分活度0.11~0.76储存16周后具备最高益生菌存活率 Liu等[51] w/o微胶囊 植物乳杆菌(Lactobacillus plantarum) 明胶-阿拉伯胶 静电相互
作用高速剪切,复合凝聚,冷冻干燥 益生菌活菌数和包埋率分别达8.6 lg CFU/g和97.78%;经模
拟胃肠消化后益生菌存活率为80.4%,而未被包埋的益生菌存活率为25%;在8 ℃和−18 ℃保存45 d后活菌数为
7.6 lg CFU/g。Paula等[52] 纳米乳液 屎球肠菌(Enterococcus faceium) 乳清浓缩蛋白-阿拉伯胶 − 超声 在27 ℃和4 ℃保存60 d后,纳米乳液粒径从150 nm增至
500 nm,益生菌的活菌数从3.4×108 CFU/mL增长至
7.3×108 CFU/mLKrithika等[53] 注:−表示未提及。 -
[1] SÁNCHEZ B, DELGADO S, BLANCO-MÍGUEZ A, et al. Probiotics, gut microbiota, and their influence on host health and disease[J]. Molecular Nutrition and Food Research,2017,61(1):1600240. doi: 10.1002/mnfr.201600240
[2] CUNNINGHAM M, AZCARATE-PERIL M A, BARNARD A, et al. Shaping the future of probiotics and prebiotics[J]. Trends in Microbiology,2021,29(8):667−685. doi: 10.1016/j.tim.2021.01.003
[3] FLACH J, VAN DER WAAL M B, VAN DEN NIEUWBOER M, et al. The underexposed role of food matrices in probiotic products: Reviewing the relationship between carrier matrices and product parameters[J]. Critical Reviews in Food Science and Nutrition,2018,58(15):2570−2584. doi: 10.1080/10408398.2017.1334624
[4] SINGH J, VYAS A. In advances in dairy microbial products[M]. Sawston: Woodhead Publishing, 2022: 295−302.
[5] TRIPATHI M K, GIRI S K. Probiotic functional foods: Survival of probiotics during processing and storage[J]. Journal of Functional Foods,2014,9:225−241. doi: 10.1016/j.jff.2014.04.030
[6] FENG K, WEI Y, HU T, et al. Colon-targeted delivery systems for nutraceuticals: A review of current vehicles, evaluation methods and future prospects[J]. Trends in Food Science & Technology,2020,102:203−222.
[7] MIN M, BUNT C R, MASON S L, et al. Non-dairy probiotic food products: An emerging group of functional foods[J]. Critical Reviews in Food Science and Nutrition,2019,59(16):2626−2641. doi: 10.1080/10408398.2018.1462760
[8] GU Q, YIN Y, YAN X, et al. Encapsulation of multiple probiotics, synbiotics, or nutrabiotics for improved health effects: A review[J]. Advances in Colloid and Interface Science,2022,309:102781. doi: 10.1016/j.cis.2022.102781
[9] DAS T K, PRADHAN S, CHAKRABARTI S, et al. Current status of probiotic and related health benefits[J]. Applied Food Research,2022,2(2):100185. doi: 10.1016/j.afres.2022.100185
[10] RAZAVI S, JANFAZA S, TASNIM N, et al. Microencapsulating polymers for probiotics delivery systems: preparation, characterization, and applications[J]. Food Hydrocolloids, 2021: 106882.
[11] GENTILE L. Protein-polysaccharide interactions and aggregates in food formulations[J]. Current Opinion in Colloid & Interface Science,2020,48:18−27.
[12] JIANG Z, LI M, MCCLEMENTS D J, et al. Recent advances in the design and fabrication of probiotic delivery systems to target intestinal inflammation[J]. Food Hydrocolloids,2022,125:107438. doi: 10.1016/j.foodhyd.2021.107438
[13] YI J, LAM T I, YOKOYAMA W, et al. Controlled release of β-carotene in β-lactoglobulin-dextran-conjugated nanoparticles' in vitro digestion and transport with Caco-2 monolayers[J]. J Agric Food Chem,2014,62(35):8900−8907. doi: 10.1021/jf502639k
[14] YANG Z, MCCLEMENTS D J, LI C, et al. Targeted delivery of hydrogels in human gastrointestinal tract: A review[J]. Food Hydrocolloids,2023,134:108013. doi: 10.1016/j.foodhyd.2022.108013
[15] CHEN Y, SONG H, WU M, et al. Application of protein-polysaccharide complex system in the delivery of active ingredients[J]. Progress in Chemistry,2022,34(10):2267−2282.
[16] WANG J, HAN X, LI T, et al. Mechanism and application of emulsifiers for stabilizing emulsions: a review[J]. Food Science,2020,41(21):303−310.
[17] WANG S, FENG Y, WU J, et al. Formation mechanism of protein-polysaccharide multi-scale complexes and their future applications[J]. Food Science,2021,42(17):1−9.
[18] LIU K, CHEN Y Y, ZHA X Q, et al. Research progress on polysaccharide/protein hydrogels: Preparation method, functional property and application as delivery systems for bioactive ingredients[J]. Food Research International,2021,147:110542. doi: 10.1016/j.foodres.2021.110542
[19] KAN X, CHEN G, ZHOU W, et al. Application of protein-polysaccharide Maillard conjugates as emulsifiers: Source, preparation and functional properties[J]. Food Research International,2021,150:110740. doi: 10.1016/j.foodres.2021.110740
[20] KE C, LI L. Influence mechanism of polysaccharides induced Maillard reaction on plant proteins structure and functional properties: A review[J]. Carbohydrate Polymers,2023,302:120430. doi: 10.1016/j.carbpol.2022.120430
[21] SPOTTI M J, LOYEAU P A, MARANGÓN A, et al. Influence of Maillard reaction extent on acid induced gels of whey proteins and dextrans[J]. Food Hydrocolloids,2019,91:224−231. doi: 10.1016/j.foodhyd.2019.01.020
[22] CHENG Y H, MU D C, JIAO Y, et al. Microwave-assisted maillard reaction between rice protein and dextran induces structural changes and functional improvements[J]. Journal of Cereal Science,2021,97:103134. doi: 10.1016/j.jcs.2020.103134
[23] CHENG Y H, MU D C, FENG Y Y, et al. Glycosylation of rice protein with dextran via the Maillard reaction in a macromolecular crowding condition to improve solubility[J]. Journal of Cereal Science,2022,103:103374. doi: 10.1016/j.jcs.2021.103374
[24] ZHENG Y, LI Z, LU Z, et al. Structural characteristics and emulsifying properties of lotus seed protein isolate-dextran glycoconjugates induced by a dynamic high pressure microfluidization Maillard reaction[J]. LWT,2022,160:113309. doi: 10.1016/j.lwt.2022.113309
[25] LI M, WEN X, WANG K, et al. Maillard induced glycation of β-casein for enhanced stability of the self-assembly micelles against acidic and calcium environment[J]. Food Chemistry,2022,387:132914. doi: 10.1016/j.foodchem.2022.132914
[26] NAGARAJU P G, P S, DUBEY T, et al. Influence of sodium caseinate, maltodextrin, pectin and their Maillard conjugate on the stability, in vitro release, anti-oxidant property and cell viability of eugenol-olive oil nanoemulsions[J]. International Journal of Biological Macromolecules,2021,183:158−170. doi: 10.1016/j.ijbiomac.2021.04.122
[27] SETIOWATI A D, SAEEDI S, WIJAYA W, et al. Improved heat stability of whey protein isolate stabilized emulsions via dry heat treatment of WPI and low methoxyl pectin: effect of pectin concentration, pH, and ionic strength[J]. Food Hydrocolloids,2017,63:716−726. doi: 10.1016/j.foodhyd.2016.10.025
[28] ZHANG N, ZHOU Q, FAN D, et al. Novel roles of hydrocolloids in foods: inhibition of toxic maillard reaction products formation and attenuation of their harmful effects[J]. Trends in Food Science & Technology,2021,111:706−715.
[29] SEDAGHAT DOOST A, NIKBAKHT NASRABADI M, WU J, et al. Maillard conjugation as an approach to improve whey proteins functionality: A review of conventional and novel preparation techniques[J]. Trends in Food Science & Technology,2019,91:1−11.
[30] WU T, LIU C, HU X. Enzymatic synthesis, characterization and properties of the protein-polysaccharide conjugate: A review[J]. Food Chemistry,2022,372:131332. doi: 10.1016/j.foodchem.2021.131332
[31] CHEN H, JI A, QIU S, et al. Covalent conjugation of bovine serum album and sugar beet pectin through Maillard reaction/laccase catalysis to improve the emulsifying properties[J]. Food Hydrocolloids,2018,76:173−183. doi: 10.1016/j.foodhyd.2016.12.004
[32] ZHANG J, WOLF B. Physico-chemical properties of sugar beet pectin-sodium caseinate conjugates via different interaction mechanisms[J]. Foods,2019,8(6):192. doi: 10.3390/foods8060192
[33] FALSAFI S R, ROSTAMABADI H, SAMBORSKA K, et al. Protein-polysaccharide interactions for the fabrication of bioactive-loaded nanocarriers: Chemical conjugates and physical complexes[J]. Pharmacological Research,2022,178:106164. doi: 10.1016/j.phrs.2022.106164
[34] AZEREDO H M C, WALDRON K W. Crosslinking in polysaccharide and protein films and coatings for food contact–A review[J]. Trends in Food Science & Technology,2016,52:109−122.
[35] ALAVARSE A C, FRACHINI E C G, DA SILVA R L C G, et al. Crosslinkers for polysaccharides and proteins: Synthesis conditions, mechanisms, and crosslinking efficiency, a review[J]. International Journal of Biological Macromolecules,2022,202:558−596. doi: 10.1016/j.ijbiomac.2022.01.029
[36] AI C, ZHAO C, GUO X, et al. Physicochemical properties of whey protein isolate and alkaline soluble polysaccharide from sugar beet pulp conjugates formed by Maillard reaction and genipin crosslinking reaction: A comparison study[J]. Food Chemistry:X,2022,14:100358. doi: 10.1016/j.fochx.2022.100358
[37] ZHANG Q, ZHOU Y, YUE W, et al. Nanostructures of protein-polysaccharide complexes or conjugates for encapsulation of bioactive compounds[J]. Trends in Food Science & Technology,2021,109:169−196.
[38] CAO Y, MEZZENGA R. Design principles of food gels[J]. Nature Food,2020,1(2):106−118. doi: 10.1038/s43016-019-0009-x
[39] XU D, LIU Z, AN Z, et al. Incorporation of probiotics into 3D printed Pickering emulsion gel stabilized by tea protein/xanthan gum[J]. Food Chemistry,2023,409:135289. doi: 10.1016/j.foodchem.2022.135289
[40] SU J, CAI Y, ZHI Z, et al. Assembly of propylene glycol alginate/β-lactoglobulin composite hydrogels induced by ethanol for co-delivery of probiotics and curcumin[J]. Carbohydrate Polymers,2021,254:117446. doi: 10.1016/j.carbpol.2020.117446
[41] RIAZ T, IQBAL M W, SAEED M, et al. In vitro survival of Bifidobacterium bifidum microencapsulated in zein-coated alginate hydrogel microbeads[J]. J Microencapsul,2019,36(2):192−203. doi: 10.1080/02652048.2019.1618403
[42] GUO Q, TANG J, LI S, et al. Lactobacillus plantarum 21805 encapsulated by whey protein isolate and dextran conjugate for enhanced viability[J]. International Journal of Biological Macromolecules,2022,216:124−131. doi: 10.1016/j.ijbiomac.2022.06.207
[43] PEÑALVA R, MARTÍNEZ-LÓPEZ A L, GAMAZO C, et al. Encapsulation of Lactobacillus plantarum in casein-chitosan microparticles facilitates the arrival to the colon and develops an immunomodulatory effect[J]. Food Hydrocolloids,2023,136:108213. doi: 10.1016/j.foodhyd.2022.108213
[44] VANDEN BRABER N L, DÍAZ VERGARA L I, ROSSI Y E, et al. Effect of microencapsulation in whey protein and water-soluble chitosan derivative on the viability of the probiotic Kluyveromyces marxianus VM004 during storage and in simulated gastrointestinal conditions[J]. LWT,2020,118:108844. doi: 10.1016/j.lwt.2019.108844
[45] SILVA M P, TULINI F L, MATOS-JR F E, et al. Application of spray chilling and electrostatic interaction to produce lipid microparticles loaded with probiotics as an alternative to improve resistance under stress conditions[J]. Food Hydrocolloids,2018,83:109−117. doi: 10.1016/j.foodhyd.2018.05.001
[46] BORA A F M, KOUAME K J E-P, LI X, et al. Development, characterization and probiotic encapsulating ability of novel Momordica charantia bioactive polysaccharides/whey protein isolate composite gels[J]. International Journal of Biological Macromolecules,2023,225:454−466. doi: 10.1016/j.ijbiomac.2022.11.097
[47] KRUNIĆ T Ž, OBRADOVIĆ N S, RAKIN M B. Application of whey protein and whey protein hydrolysate as protein based carrier for probiotic starter culture[J]. Food Chemistry,2019,293:74−82. doi: 10.1016/j.foodchem.2019.04.062
[48] ZHANG H, WEI S, YAN J, et al. Development of double layer microcapsules for enhancing the viability of Lactobacillus casei LC2W in simulated gastrointestinal fluids[J]. LWT,2021,145:111319. doi: 10.1016/j.lwt.2021.111319
[49] 李春, 刘丽波, 张国芳, 等. 一种包埋植物乳杆菌乳剂及其制备方法和应用CN115558659A[P]. 2023-01-03. LI C, LIU L, ZHANG G, et al. Embedded lactobacillus plantarum emulsion and preparation method and application thereof. China, 115558659A[P]. 2023-01-03.
[50] ZHANG Y, LIN J, ZHONG Q. The increased viability of probiotic Lactobacillus salivarius NRRL B-30514 encapsulated in emulsions with multiple lipid-protein-pectin layers[J]. Food Research International,2015,71:9−15. doi: 10.1016/j.foodres.2015.02.017
[51] LIU H, GONG J, CHABOT D, et al. Incorporation of polysaccharides into sodium caseinate-low melting point fat microparticles improves probiotic bacterial survival during simulated gastrointestinal digestion and storage[J]. Food Hydrocolloids,2016,54:328−337. doi: 10.1016/j.foodhyd.2015.10.016
[52] PAULA D D A, MARTINS E M F, COSTA N D A, et al. Use of gelatin and gum arabic for microencapsulation of probiotic cells from Lactobacillus plantarum by a dual process combining double emulsification followed by complex coacervation[J]. International Journal of Biological Macromolecules,2019,133:722−731. doi: 10.1016/j.ijbiomac.2019.04.110
[53] KRITHIKA B, PREETHA R. Formulation of protein based inulin incorporated synbiotic nanoemulsion for enhanced stability of probiotic[J]. Materials Research Express,2019,6(11):114003. doi: 10.1088/2053-1591/ab4d1a
[54] MCCLEMENTS D J. Designing biopolymer microgels to encapsulate, protect and deliver bioactive components: Physicochemical aspects[J]. Advances in Colloid and Interface Science,2017,240:31−59. doi: 10.1016/j.cis.2016.12.005
[55] YUAN Y, YIN M, ZHAI Q, et al. The encapsulation strategy to improve the survival of probiotics for food application: From rough multicellular to single-cell surface engineering and microbial mediation[J]. Critical Reviews in Food Science and Nutrition, 2022: 1−17.
[56] YAN W, JIA X, ZHANG Q, et al. Interpenetrating polymer network hydrogels of soy protein isolate and sugar beet pectin as a potential carrier for probiotics[J]. Food Hydrocolloids,2021,113:106453. doi: 10.1016/j.foodhyd.2020.106453
[57] PUSHPAMALAR J, SATHASIVAM T, GUGLER M C. Hydrogel beads of natural polymers as a potential vehicle for colon-targeted drug delivery[J]. Methods in Molecular Biology,2021,2211:171−182.
[58] KWIECIEŃ I, KWIECIEŃ M. Application of polysaccharide-based hydrogels as probiotic delivery systems[J]. Gels, 2018, 4(2).
[59] WEI Z, HUANG Q. Assembly of protein-polysaccharide complexes for delivery of bioactive ingredients: A perspective paper[J]. Journal of Agricultural and Food Chemistry,2019,67(5):1344−1352. doi: 10.1021/acs.jafc.8b06063
[60] ALEHOSSEINI A, GOMEZ DEL PULGAR E-M, FABRA M J, et al. Agarose-based freeze-dried capsules prepared by the oil-induced biphasic hydrogel particle formation approach for the protection of sensitive probiotic bacteria[J]. Food Hydrocolloids,2019,87:487−496. doi: 10.1016/j.foodhyd.2018.08.032
[61] NI F, LUO X, ZHAO Z, et al. Enhancing viability of Lactobacillus plantarum encapsulated by alginate-gelatin hydrogel beads during gastrointestinal digestion, storage and in the mimic beverage systems[J]. International Journal of Biological Macromolecules,2023,224:94−104. doi: 10.1016/j.ijbiomac.2022.10.106
[62] GAO Y, WANG X, XUE C, et al. Latest developments in food-grade delivery systems for probiotics: A systematic review[J]. Critical Reviews in Food Science and Nutrition, 2021: 1−18.
[63] MAO L, PAN Q, HOU Z, et al. Development of soy protein isolate-carrageenan conjugates through Maillard reaction for the microencapsulation of Bifidobacterium longum[J]. Food Hydrocolloids,2018,84:489−497. doi: 10.1016/j.foodhyd.2018.06.037
[64] MAO L, PAN Q, YUAN F, et al. Formation of soy protein isolate-carrageenan complex coacervates for improved viability of Bifidobacterium longum during pasteurization and in vitro digestion[J]. Food Chemistry,2019,276:307−314. doi: 10.1016/j.foodchem.2018.10.026
[65] 马铁铮, 赵宏亮. 一种基于复合凝聚法制备益生菌微胶囊的方法与应用CN115381101A[P]. 2022-11-25. MA T, ZHAO H. Method for preparing probiotic microcapsules based on complex coacervation method and application. China, 115381101A[P]. 2022-11-25.
[66] DU M, LU W F, ZHANG Y, et al. Natural polymer-sourced interpenetrating network hydrogels: Fabrication, properties, mechanism and food applications[J]. Trends in Food Science & Technology,2021,116:342−356.
[67] DE ARAÚJO ETCHEPARE M, NUNES G L, NICOLOSO B R, et al. Improvement of the viability of encapsulated probiotics using whey proteins[J]. LWT,2020,117:108601. doi: 10.1016/j.lwt.2019.108601
[68] SUN X, WANG H, LI S, et al. Maillard-type protein-polysaccharide conjugates and electrostatic protein-polysaccharide complexes as delivery vehicles for food bioactive ingredients: formation, types, and applications[J]. Gels,2022,8(2):135. doi: 10.3390/gels8020135
[69] WANG X, LI X, XU D, et al. Comparision of heteroaggregation, layer-by-layer and directly mixing techniques on the physical properties and in vitro digestion of emulsions[J]. Food Hydrocolloids,2019,95:228−237. doi: 10.1016/j.foodhyd.2019.04.034
[70] QIN X S, GAO Q Y, LUO Z G. Enhancing the storage and gastrointestinal passage viability of probiotic powder (Lactobacillus Plantarum) through encapsulation with pickering high internal phase emulsions stabilized with WPI-EGCG covalent conjugate nanoparticles[J]. Food Hydrocolloids,2021,116:106658. doi: 10.1016/j.foodhyd.2021.106658
[71] HAN K, YAO Y, DONG S, et al. Chemical characterization of the glycated myofibrillar proteins from grass carp (Ctenopharyngodon idella) and their impacts on the human gut microbiota in vitro fermentation[J]. Food & Function,2017,8(3):1184−1194.
[72] KAN X, HU Y, HUANG Y, et al. Characterization of whey protein isolate-gum Arabic Maillard conjugate and evaluation of the effects of conjugate-stabilized emulsion on microbiota of human fecal cultures[J]. Food Hydrocolloids,2023,134:108060. doi: 10.1016/j.foodhyd.2022.108060
[73] FINOT P-A. The absorption and metabolism of modified amino acids in processed foods[J]. Journal of AOAC International,2019,88(3):894−903.
[74] CUI F, HAN S, WANG J, et al. Co-delivery of curcumin and epigallocatechin gallate in W/O/W emulsions stabilized by protein fibril-cellulose complexes[J]. Colloids and Surfaces B:Biointerfaces,2023,222:113072. doi: 10.1016/j.colsurfb.2022.113072
[75] QIN X S, LUO Z G, LI X L. An enhanced pH-sensitive carrier based on alginate-Ca-EDTA in a set-type W1/O/W2 double emulsion model stabilized with WPI-EGCG covalent conjugates for probiotics colon-targeted release[J]. Food Hydrocolloids,2021,113:106460. doi: 10.1016/j.foodhyd.2020.106460
[76] KUMAR A, KAUR R, KUMAR V, et al. New insights into water-in-oil-in-water (W/O/W) double emulsions: Properties, fabrication, instability mechanism, and food applications[J]. Trends in Food Science & Technology,2022,128:22−37.
[77] BALCAEN M, VERMEIR L, VAN DER MEEREN P. Influence of protein type on Polyglycerol Polyricinoleate replacement in W/O/W (water-in-oil-in-water) double emulsions for food applications[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects,2017,535:105−113.
[78] BALCAEN M, STEYLS J, SCHOEPPE A, et al. Phosphatidylcholine-depleted lecithin: A clean-label low-HLB emulsifier to replace PGPR in w/o and w/o/w emulsions[J]. Journal of Colloid and Interface Science,2021,581:836−846. doi: 10.1016/j.jcis.2020.07.149
[79] SHARIFI S, REZAZAD-BARI M, ALIZADEH M, et al. Use of whey protein isolate and gum arabic for the co-encapsulation of probiotic Lactobacillus plantarum and phytosterols by complex coacervation: enhanced viability of probiotic in Iranian white cheese[J]. Food Hydrocolloids,2021,113:106496. doi: 10.1016/j.foodhyd.2020.106496
[80] TASCH HOLKEM A, FAVARO-TRINDADE C S. Potential of solid lipid microparticles covered by the protein-polysaccharide complex for protection of probiotics and proanthocyanidin-rich cinnamon extract[J]. Food Research International,2020,136:109520. doi: 10.1016/j.foodres.2020.109520
[81] BELDARRAIN-IZNAGA T, VILLALOBOS-CARVAJAL R, SEVILLANO-ARMESTO E, et al. Functional properties of Lactobacillus casei C24 improved by microencapsulation using multilayer double emulsion[J]. Food Research International,2021,141:110136. doi: 10.1016/j.foodres.2021.110136
[82] SHIN M J, SHIN Y J, HWANG S W, et al. Microencapsulation of imidazole curing agent by solvent evaporation method using W/O/W emulsion[J]. Journal of Applied Polymer Science,2013,129(3):1036−1044. doi: 10.1002/app.38767
[83] RODRIGUES F J, CEDRAN M F, BICAS J L, et al. Encapsulated probiotic cells: Relevant techniques, natural sources as encapsulating materials and food applications–A narrative review[J]. Food Research International,2020,137:109682. doi: 10.1016/j.foodres.2020.109682
[84] DING X, XU Y, WANG Y, et al. Carboxymethyl konjac glucomannan-chitosan complex nanogels stabilized double emulsions incorporated into alginate hydrogel beads for the encapsulation, protection and delivery of probiotics[J]. Carbohydrate Polymers,2022,289:119438. doi: 10.1016/j.carbpol.2022.119438
[85] XU C, BAN Q, WANG W, et al. Novel nano-encapsulated probiotic agents: Encapsulate materials, delivery, and encapsulation systems[J]. Journal of Controlled Release,2022,349:184−205. doi: 10.1016/j.jconrel.2022.06.061
[86] REQUE P M, BRANDELLI A. Encapsulation of probiotics and nutraceuticals: Applications in functional food industry[J]. Trends in Food Science & Technology,2021,114:1−10.
[87] AHMAD M, GANI A, HAMED F, et al. Comparative study on utilization of micro and nano sized starch particles for encapsulation of camel milk derived probiotics (Pediococcus acidolactici)[J]. LWT,2019,110:231−238. doi: 10.1016/j.lwt.2019.04.078
[88] MENDES A C, CHRONAKIS I S. Electrohydrodynamic encapsulation of probiotics: A review[J]. Food Hydrocolloids,2021,117:106688. doi: 10.1016/j.foodhyd.2021.106688
[89] BURGAIN J, GAIANI C, CAILLIEZ-GRIMAL C, et al. Encapsulation of Lactobacillus rhamnosus GG in microparticles: Influence of casein to whey protein ratio on bacterial survival during digestion[J]. Innovative Food Science & Emerging Technologies,2013,19:233−242.
[90] AKKURT S, LIU L S, TOMASULA P M. Electrospinning of edible, food-based polymers[M]. Florida: CRC Press, 2018.
[91] KYCIA K, CHLEBOWSKA-ŚMIGIEL A, SZYDŁOWSKA A, et al. Pullulan as a potential enhancer of Lactobacillus and Bifidobacterium viability in synbiotic low fat yoghurt and its sensory quality[J]. LWT,2020,128:109414. doi: 10.1016/j.lwt.2020.109414
[92] SHEN C, DENG Z, RAO J, et al. Characterization of glycosylated gelatin/pullulan nanofibers fabricated by multi-fluid mixing solution blow spinning[J]. International Journal of Biological Macromolecules,2022,214:512−521. doi: 10.1016/j.ijbiomac.2022.06.082
[93] GHALEHJOOGHI H D, TAJIK H, SHAHBAZI Y. Development and characterization of active packaging nanofiber mats based on gelatin-sodium alginate containing probiotic microorganisms to improve the shelf-life and safety quality of silver carp fillets[J]. International Journal of Food Microbiology,2023,384:109984. doi: 10.1016/j.ijfoodmicro.2022.109984
[94] AKKURT S, RENYE J, TOMASULA P M. Encapsulation of Lactobacillus rhamnosus GG in edible electrospun mats from calcium and sodium caseinates with pullulan blends[J]. JDS Communications,2022,3(6):381−386. doi: 10.3168/jdsc.2021-0173
[95] HASANKHAN S, TABIBIAZAR M, HOSSEINI S M, et al. Fabrication of curcumin-zein-ethyl cellulose composite nanoparticles using antisolvent co-precipitation method[J]. International Journal of Biological Macromolecules,2020,163:1538−1545. doi: 10.1016/j.ijbiomac.2020.08.045
[96] JIANG F, DU C, ZHAO N, et al. Preparation and characterization of quinoa starch nanoparticles as quercetin carriers[J]. Food Chemistry,2022,369:130895. doi: 10.1016/j.foodchem.2021.130895
[97] HOSSEINI S F, ANSARI B, GHARSALLAOUI A. Polyelectrolytes-stabilized liposomes for efficient encapsulation of Lactobacillus rhamnosus and improvement of its survivability under adverse conditions[J]. Food Chemistry,2022,372:131358. doi: 10.1016/j.foodchem.2021.131358
[98] SOLANS C, IZQUIERDO P, NOLLA J, et al. Nano-emulsions[J]. Current Opinion in Colloid & Interface Science,2005,10(3):102−110.
[99] HOU X, SHENG J J. Properties, preparation, stability of nanoemulsions, their improving oil recovery mechanisms, and challenges for oil field applications–A critical review[J]. Geoenergy Science and Engineering,2023,221:211360. doi: 10.1016/j.geoen.2022.211360
[100] VAISHANAVI S, PREETHA R. Soy protein incorporated nanoemulsion for enhanced stability of probiotic (Lactobacillus delbrueckii subsp. bulgaricus) and its characterization[J]. Materials Today:Proceedings,2021,40:S148−S153. doi: 10.1016/j.matpr.2020.05.008
[101] MOGHADDAS KIA E, GHASEMPOUR Z, GHANBARI S, et al. Development of probiotic yogurt by incorporation of milk protein concentrate (MPC) and microencapsulated in gellan-caseinate mixture[J]. British Food Journal,2018,120(7):1516−1528. doi: 10.1108/BFJ-12-2017-0668
[102] LI H, LIU T, YANG J, et al. Effect of a microencapsulated synbiotic product on microbiology, microstructure, textural and rheological properties of stirred yogurt[J]. LWT,2021,152:112302. doi: 10.1016/j.lwt.2021.112302
[103] EL KADRI H, LALOU S, MANTZOURIDOU F, et al. Utilisation of water-in-oil-water (W1/O/W2) double emulsion in a set-type yogurt model for the delivery of probiotic Lactobacillus paracasei[J]. Food Research International,2018,107:325−336. doi: 10.1016/j.foodres.2018.02.049
[104] ROLIM F R L, FREITAS NETO O C, OLIVEIRA M E G, et al. Cheeses as food matrixes for probiotics: In vitro and in vivo tests[J]. Trends in Food Science & Technology,2020,100:138−154.
[105] MOGHADDAS KIA E, ALIZADEH M, ESMAIILI M. Development and characterization of probiotic UF Feta cheese containing Lactobacillus paracasei microencapsulated by enzyme based gelation method[J]. J Food Sci Technol,2018,55(9):3657−3664. doi: 10.1007/s13197-018-3294-8
[106] LIU L, CHEN P, ZHAO W, et al. Effect of microencapsulation with the Maillard reaction products of whey proteins and isomaltooligosaccharide on the survival rate of Lactobacillus rhamnosus in white brined cheese[J]. Food Control,2017,79:44−49. doi: 10.1016/j.foodcont.2017.03.016
[107] LILLO-PÉREZ S, GUERRA-VALLE M, ORELLANA-PALMA P, et al. Probiotics in fruit and vegetable matrices: Opportunities for nondairy consumers[J]. LWT,2021,151:112106. doi: 10.1016/j.lwt.2021.112106
[108] ISLAM M Z, TABASSUM S, HARUN-UR-RASHID M, et al. Development of probiotic beverage using whey and pineapple (Ananas comosus) juice: Sensory and physico-chemical properties and probiotic survivability during in-vitro gastrointestinal digestion[J]. Journal of Agriculture and Food Research,2021,4:100144. doi: 10.1016/j.jafr.2021.100144
[109] KRUNIĆ T Ž, BULATOVIĆ M L, OBRADOVIĆ N S, et al. Effect of immobilisation materials on viability and fermentation activity of dairy starter culture in whey-based substrate[J]. Journal of the Science of Food and Agriculture,2016,96(5):1723−1729. doi: 10.1002/jsfa.7278
[110] KRUNIĆ T Ž, RAKIN M B. Enriching alginate matrix used for probiotic encapsulation with whey protein concentrate or its trypsin-derived hydrolysate: Impact on antioxidant capacity and stability of fermented whey-based beverages[J]. Food Chemistry,2022,370:130931. doi: 10.1016/j.foodchem.2021.130931
[111] HERNÁNDEZ-BARRUETA T, MARTÍNEZ-BUSTOS F, CASTAÑO-TOSTADO E, et al. Encapsulation of probiotics in whey protein isolate and modified huauzontle's starch: An approach to avoid fermentation and stabilize polyphenol compounds in a ready-to-drink probiotic green tea[J]. LWT,2020,124:109131. doi: 10.1016/j.lwt.2020.109131
[112] OBRADOVIĆ N, VOLIĆ M, NEDOVIĆ V, et al. Microencapsulation of probiotic starter culture in protein–carbohydrate carriers using spray and freeze-drying processes: Implementation in whey-based beverages[J]. Journal of Food Engineering,2022,321:110948. doi: 10.1016/j.jfoodeng.2022.110948
[113] LE N T T, BACH L G, NGUYEN D C, et al. Evaluation of factors affecting antimicrobial activity of bacteriocin from lactobacillus plantarum microencapsulated in alginate-gelatin capsules and its application on pork meat as a bio-preservative[J]. Int J Environ Res Public Health,2019,16(6):1017. doi: 10.3390/ijerph16061017
[114] 刘颖, 朱金铭. 一种载有益生菌的功能化可食性膜及其制备方法CN 115399481A[P]. 2022-11-29. LIU Y, ZHU J. Functional edible film loaded with probiotics and preparation method thereof. China, 115399481A[P]. 2022-11-29.
-
期刊类型引用(3)
1. 张皓然,张琴,文丽琼,零莉,胡俊杰,岑仕宇,王勤志,陈德慰. 利用~1H NMR测定姜中姜辣素与姜黄素. 中国调味品. 2025(01): 193-200 .  百度学术
百度学术
2. 李晓娟,钟梨,彭开锋,尹军,曾新安,刘志伟,韩忠. 脉冲电场强化生姜姜酚提取及工艺优化. 现代食品科技. 2024(09): 270-277 .  百度学术
百度学术
3. 王芮,蒋起宏,周宇芳,陈慧,朱正华,刘书来,相兴伟. 贻贝多糖对胰岛素抵抗HepG2细胞糖代谢的影响. 食品工业科技. 2023(15): 385-391 .  本站查看
本站查看
其他类型引用(2)





 下载:
下载:
 下载:
下载:



