Exploring the Taste Characteristics and ACE-inhibitory Active Mechanism of Stropharia rugosoannulata Decapeptides Based on Virtual Screening, Molecular Docking, and Molecular Interactions
-
摘要: 为探究大球盖菇十肽的呈味特性和潜在的生物活性,以两种大球盖菇十肽(RIEDNLVIIR和SLPIKPRVPF)为研究对象,采用虚拟筛选、分子对接和分子互作技术,对两种大球盖菇十肽的呈味特性和ACE抑制活性作用机制进行预测和验证。结果显示,两种大球盖菇十肽均具有咸鲜呈味肽片段和ACE抑制活性肽片段。RIEDNLVIIR具有咸味呈味特性,SLPIKPRVPF具有鲜味呈味特性。两种大球盖菇十肽可与ACE靶标蛋白受体结合形成氢键和静电相互作用。体外活性验证结果表明,大球盖菇咸味十肽RIEDNLVIIR的ACE抑制效果较好,IC50值为0.012 mg/mL。分子互作热力学和动力学结果显示,RIEDNLVIIR与ACE受体之间结合是焓驱动反应的特异性结合。虚拟筛选活性预测结果、体外活性验证结果、及分子对接和分子互作的ACE抑制机制解析结果具有一致性。研究为理解大球盖菇十肽呈味特性和ACE抑制活性作用机制提供理论依据,为具有ACE抑制活性的大球盖菇咸鲜味十肽在健康调味品和功能产品中的应用奠定基础。Abstract: To explore the taste characteristics and potential biological activities of the decapeptides of Stropharia rugosoannulata, two decapeptides (RIEDNLVIIR and SLPIKPRVPF) were selected to predict and validate the taste-presenting properties and ACE inhibitory activity mechanism by using virtual screening, molecular docking, and molecular interactions techniques. The results showed that the two decapeptides of S. rugosoannulata all had salty and umami tastes and ACE-inhibited peptide fragments. RIEDNLVIR had a salty taste, and SLPIKPRVPF had an umami taste. Two decapeptides of S. rugosoannulata could strongly bind to ACE receptors to form hydrogen bonds and electrostatic interactions. The in vitro activity validation results showed that the salty decapeptide RIEDNLVIIR inhibited the ACE well with an IC50 value of 0.012 mg/mL. The molecular interaction thermodynamics and kinetics results showed that the binding between RIEDNLVIR and ACE receptor was the specific binding of enthalpy-driven reaction. The results of virtual screening activity prediction, in vitro activity validation, and molecular docking and molecular interactions for ACE inhibition mechanism analysis were consistent. The study provides a theoretical basis for understanding the taste characteristics and ACE inhibition mechanism of S. rugosoannulata decapeptides and lays a foundation for applying the decapeptides with ACE inhibitory activity in healthy condiments and functional products.
-
大球盖菇,又名赤松茸,是以稻秸秆为主要栽培基质生长的草腐菌,在上海郊区广泛种植,为农业废弃物的循环利用提供了新途径。大球盖菇子实体滋味鲜美,富含蛋白质、肽及氨基酸等营养物质,不仅能带来愉悦的味觉感受,在保障人体健康中也发挥着重要的营养作用和生物活性。成熟的大球盖菇子实体中蛋白含量可达到40%~50%干重,游离肽含量可达到11%~14%干重[1]。大球盖菇是开发天然风味基料和营养健康产品的优质原料。
血管紧张素转化酶(angiotensin-І converting enzyme,ACE)在诱导体内血压升高中起到关键作用,是治疗高血压、心力衰竭等疾病的理想药物靶点[2]。ACE抑制肽能够抑制ACE的活性,从而起到降血压的作用。食源性ACE抑制肽具有高生物活性、低毒性,且易于在体内代谢,不会表现出抗高血压药物常见的副作用,对患高血压、心血管疾病等特殊人群有着重要的利用价值[3]。前期研究发现,多模式超声提取和辅助酶解制备的大球盖菇肽基料具有较好的降血压、抗氧化等生物活性,愉悦的咸鲜味呈味特性[1,4-5]。将味觉活性与食盐相当的咸鲜味呈味肽,以部分替代食盐的方式应用到食品领域,可降低因食盐摄入过量引发的高血压、冠心病等心血管疾病患病风险;同时,在不影响食品风味品质与保质期等前提下减少食盐的添加量,将天然食源性咸鲜味肽作为食盐的替代品,达到“减盐不减咸”目的,在国家倡导的减盐策略执行上,有着重要的研究意义。目前,以大球盖菇为原料开发兼具ACE抑制功能活性及愉悦呈味特性的风味活性肽研究较少,肽的功能活性和呈味特性联动作用机制尚未有报道。因此,进一步挖掘大球盖菇风味活性肽,解析其活性、呈味机制,推进其在食品、药品领域的应用,仍然十分有必要。
近年来,肽组学及生物信息学方法在食品科学中的重要性与日俱增[6-7]。国内外学者已有较多的应用肽组学分析方法解析食源性肽功能活性或呈味结构基础,利用分子对接、分子互作等技术手段探究食源性肽活性作用机制[8-11]或呈味感知机制[12-15]。本研究基于前期采用肽组学等方法构建的大球盖菇十肽肽谱库(94种,http://139.224.23.107),以二级质谱峰面积排名前十、峰面积相当的两种大球盖菇十肽RIEDNLVIIR(质谱峰面积2.58×10−7)和SLPIKPRVPF(质谱峰面积2.17×10−7)为研究对象,采用虚拟筛选、分子对接和分子互作技术探究两种大球盖菇十肽的呈味特性、ACE抑制活性作用机制,为大球盖菇风味活性肽开发利用提供理论依据。
1. 材料和方法
1.1 材料与仪器
DOJINDO同仁化学ACE Kit-WST试剂盒 上海宥露生物科技有限公司;ACE受体蛋白 北京艾普希隆生物科技有限公司;Genemore G-MM-IGT生物素化试剂盒 江苏博美达生命科学有限公司;两种大球盖菇十肽 委托吉尔生化(上海)有限公司对两种大球盖菇十肽RIEDNLVIIR(RR-10)和SLPIKPRVPF(SL-10)进行合成,合成肽纯度大于98%;柠檬酸、蔗糖、异亮氨酸、氯化钠、谷氨酸钠 北京索莱宝科技有限公司;其他试剂(分析纯) 国药集团化学试剂有限公司。
TS-5000Z Insent电子舌味觉分析系统 北京盈盛恒泰科技有限责任公司;Bio-Tek Epoch 2酶标仪 美国BioTek公司;Allegra 25R高速冷冻离心机 美国Beckman公司;MS3002TS/02电子天平 梅特勒-托利多国际贸易(上海)有限公司;TA NANO ITC等温滴定量热仪 沃特世科技(上海)有限公司;ForteBio Octet Red96e Biolayer Interferometry(BLI)实时无标记分子相互作用仪 美国ForteBio公司;GE PD MiniTrap G-25预装脱盐柱 GE公司。
1.2 实验方法
1.2.1 大球盖菇十肽呈味特性及活性预测
利用BIOPEP-UWM(https://biochemia.uwm.edu.pl/biopep-uwm/)的Sensory peptide and amino acids模块,预测RR-10和SF-10的呈味特性及呈味肽片段[5]。利用BIOPEP-UWM的Bioactive peptide模块,预测RR-10和SF-10是否是潜在的ACE抑制剂及其潜在的活性肽片段[16-17]。利用BIOPEP-UWM的Enzyme(s) action模块,预测RR-10和SF-10经胃肠蛋白酶消化后潜在的活性肽片段及其抑制率[18]。采用admetSAR(http://lmmd.ecust.edu.cn/admetsar1/predict/)预测肽的血脑屏障穿透性、肠道上皮细胞Caco-2细胞穿透性和AMES toxic急性口服毒性[19]。
1.2.2 大球盖菇十肽呈味特性分析
采用TS-5000Z Insent电子舌味觉分析系统对RR-10和SF-10的呈味特性及呈味强度进行分析。准确称取0.1 g合成肽,加入100 mL纯水溶解,逐级稀释为浓度梯度1.0、0.75、0.5、0.375和0.25 mg/mL的肽溶液。以同浓度梯度的氯化钠(NaCl)和谷氨酸钠(MSG)做呈味对照[5]。
1.2.3 大球盖菇十肽体外ACE抑制活性分析
准确称取0.1 g合成肽,加入50 mL纯水溶解,逐级稀释为浓度梯度2.0、0.4、0.08、0.016和0.0032 mg/mL的肽溶液。采用DOJINDO ACE Kit-WST试剂盒对肽溶液的ACE抑制活性进行测定,分析方法同试剂盒方法。以样品浓度和抑制率做ACE抑制活性曲线,计算抑制率50%时的样品浓度(IC50)。
1.2.4 大球盖菇十肽ACE抑制机制预测
从PDB数据库中获得ACE受体蛋白(1O8A)的晶体结构作为靶标蛋白。利用分子对接技术,分析大球盖菇十肽与ACE受体结合程度。以肽-受体结合打分值、成键数量及氨基酸残基结合能为指标,筛选紧密结合的肽-受体复合物,解析肽-受体结合方式和结合位点,预测大球盖菇十肽的ACE抑制作用机制[4]。
1.2.5 大球盖菇十肽ACE抑制机制验证
采用ITC和BLI分子相互作用仪分析十肽和ACE受体之间的结合模式。
将PBS缓冲液配制的ACE受体蛋白溶液注射到ITC样品池中,样品池温度25 ℃,搅拌器转速1000 r/min,开始至第一次滴定间隔时间60 s,总注射次数20次,单次注射缓冲液配制的肽配体溶液2 μL,两次滴定时间间隔150 s,单次注射时间2 s。测量受体蛋白、肽分子结合过程中反应体系温度变化情况,ITC Nano分析软件计算受体配体结合亲和力(KD)、化学计量(N)、焓(ΔH)和熵(ΔS),对受体、配体是否有相互作用进行验证。
将PBS缓冲液配制的ACE受体蛋白溶液进行蛋白生物素化。蛋白生物素化孵育30~60 min后进行脱盐处理。BLI的4根SSA传感器固化生物素化的蛋白,4根传感器做参比电极。设置基线平衡时间60 s,负载样本时间800 s,基线平衡时间60 s程序固化受体蛋白。设置基线平衡时间60 s,结合时间120 s,解离时间120 s生物分子互作循环程序进行受体蛋白与肽分子结合、解离互作反应。使用BLI分析程序获取受体与肽配体间结合常数Kon、解离常数Koff、亲和力KD等动力学相关信息,确定ACE受体与肽配体互作结合模式。
1.3 数据处理
采用电子舌味觉分析系统软件进行肽段呈味强度数据采集及处理,试验所得数据以3次测定结果的平均值±标准差表示。肽段抑制活性采用Excel软件处理和分析。采用MOE 2019进行分子对接、结合方式和结合位点分析。采用GraphPad Prism 9软件绘制ITC和BLI分析程序获得的热力学和动力学数据。
2. 结果与分析
2.1 大球盖菇十肽呈味特性及活性预测分析结果
BIOPEP-UWM分析预测的大球盖菇十肽呈味及活性片段结果显示,RR-10具有呈咸鲜味的ED肽片段,SF-10具有呈咸味提升的RV肽片段。RR-10和SF-10均是潜在的ACE抑制剂。RR-10具有的ACE抑制活性肽片段为IE;SF-10具有的ACE抑制活性肽片段为IKP、PR、VP、KP和LP。
由BIOPEP-UWM虚拟酶切位点分析结果可知,RR-10经胃蛋白酶和胰蛋白酶连续消化后,理论水解度为11.11%。RR-10存在一个胃蛋白酶酶切位点L-V。RR-10及胃蛋白酶酶切片段RIEDNL、VIIR不能进一步被胰蛋白酶酶切,由此推测RR-10及其消化肽片段通过细胞旁转运和跨胞途径被小肠上皮细胞完整吸收。SF-10存在一个胃蛋白酶酶切位点L-P,两个胰蛋白酶酶切位点K-P和R-V。SF-10经胃蛋白酶和胰蛋白酶消化理论水解度为40%,产生的潜在抑制ACE活性肽片段PR。SF-10模拟消化后产生的活性肽片段分子量低,可通过载体介导(PepT1介导)转运到细胞内部发挥活性作用[20]。基于admetSAR预测结果可知,RR-10和SF-10均无毒(Non AMES toxic,评价数值分别为0.6671和0.7318),血脑屏障透过性(评价数值分别为0.8235和0.9928)和Caco-2细胞穿透性(评价数值分别为0.7015和0.7849)良好。
2.2 大球盖菇十肽呈味特性验证结果
大球盖菇十肽电子舌呈味分析结果显示,RR-10具有咸味和浓厚感呈味特性,SF-10具有鲜味和浓厚感呈味特性。RR-10在1.0 mg/mL时开始呈现较优的咸味呈味强度,等同于同浓度NaCl呈咸强度的1.86倍。SF-10在低浓度下即可达到较好的呈鲜效果,与同浓度MSG呈鲜强度相当;随着肽段浓度增加,呈鲜效果反而减弱(表1)。相较于MSG在一定浓度范围(0.25~1.0 mg/mL)内呈鲜强度与剂量浓度正相关的呈味特性,电子舌评价结果显示SF-10与MSG呈味机制明显不同,SF-10的呈鲜机制有待进一步解析。因此,在一定配比浓度下,RR-10可作为增咸剂、SF-10作为增鲜剂,应用于天然调味品开发。
表 1 大球盖菇十肽RR-10和SF-10呈味特性评价结果Table 1. Evaluation results of the taste characteristics of the decapeptides RR-10 and SF-10 from Stropharia rugosoannulata浓度
(mg/mL)RR-10电子舌 SF-10电子舌 余味 浓厚味 咸味 余味 鲜味 浓厚味 0.25 0.80±0.03 0.74±0.02 −18.87±0.37 0.40±0.01 4.76±0.26 0.69±0.10 0.375 0.72±0.03 0.74±0.03 −12.65±0.13 0.59±0.01 4.07±0.11 0.69±0.08 0.5 0.65±0.02 0.75±0.02 −8.77±0.17 0.77±0.02 3.62±0.19 0.69±0.09 0.75 0.60±0.01 0.73±0.01 −1.29±0.04 0.88±0.01 2.82±0.08 0.67±0.09 1.0 0.56±0.01 0.70±0.02 3.84±0.08 0.97±0.01 2.27±0.05 0.64±0.11 2.3 大球盖菇十肽体外ACE抑制活性分析结果
RR-10在配制浓度0.4 mg/mL时,其体外ACE抑制率可达到92.61%±0.51%;SF-10在2.0 mg/mL时,体外ACE抑制率为83.41%±0.49%;RR-10和SF-10均具有体外ACE抑制活性。从两种十肽体外ACE抑制率拟合曲线计算可知,RR-10和SF-10的ACE抑制IC50值分别0.012 mg/mL和0.221 mg/mL。一定浓度范围内(0.0032~2.0 mg/mL),RR-10体外ACE抑制效果优于SF-10。
2.4 大球盖菇十肽ACE抑制机制分析结果
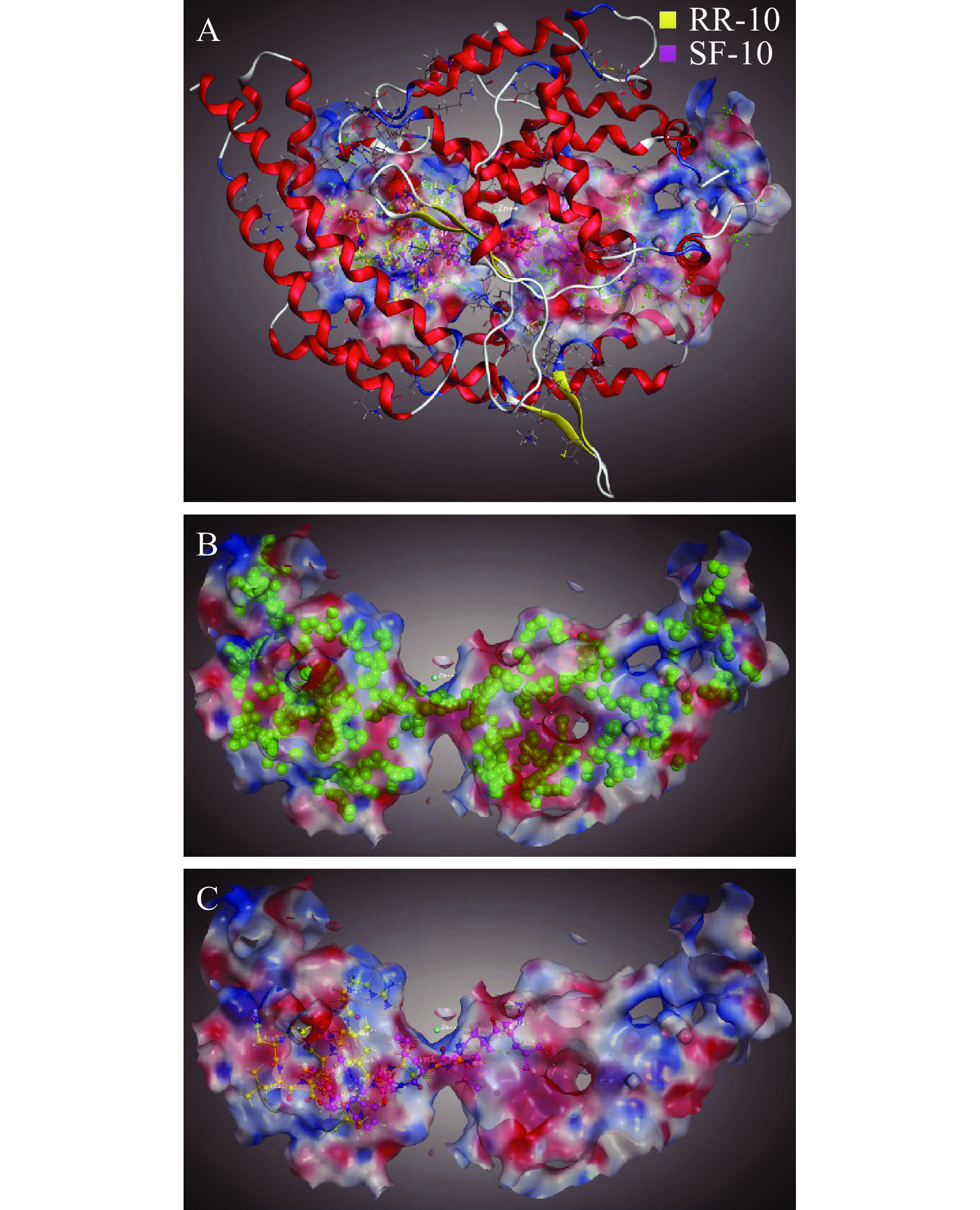
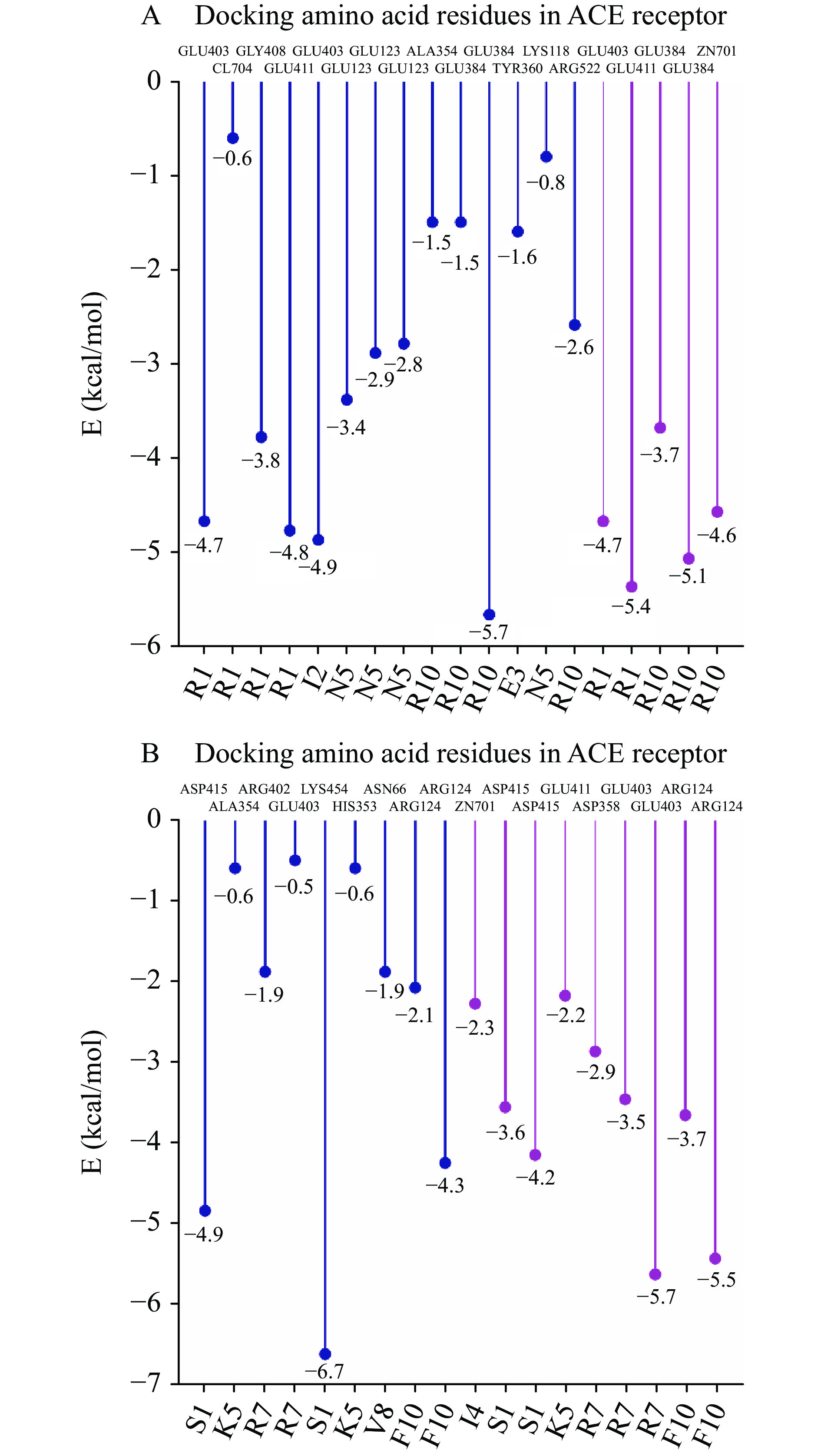
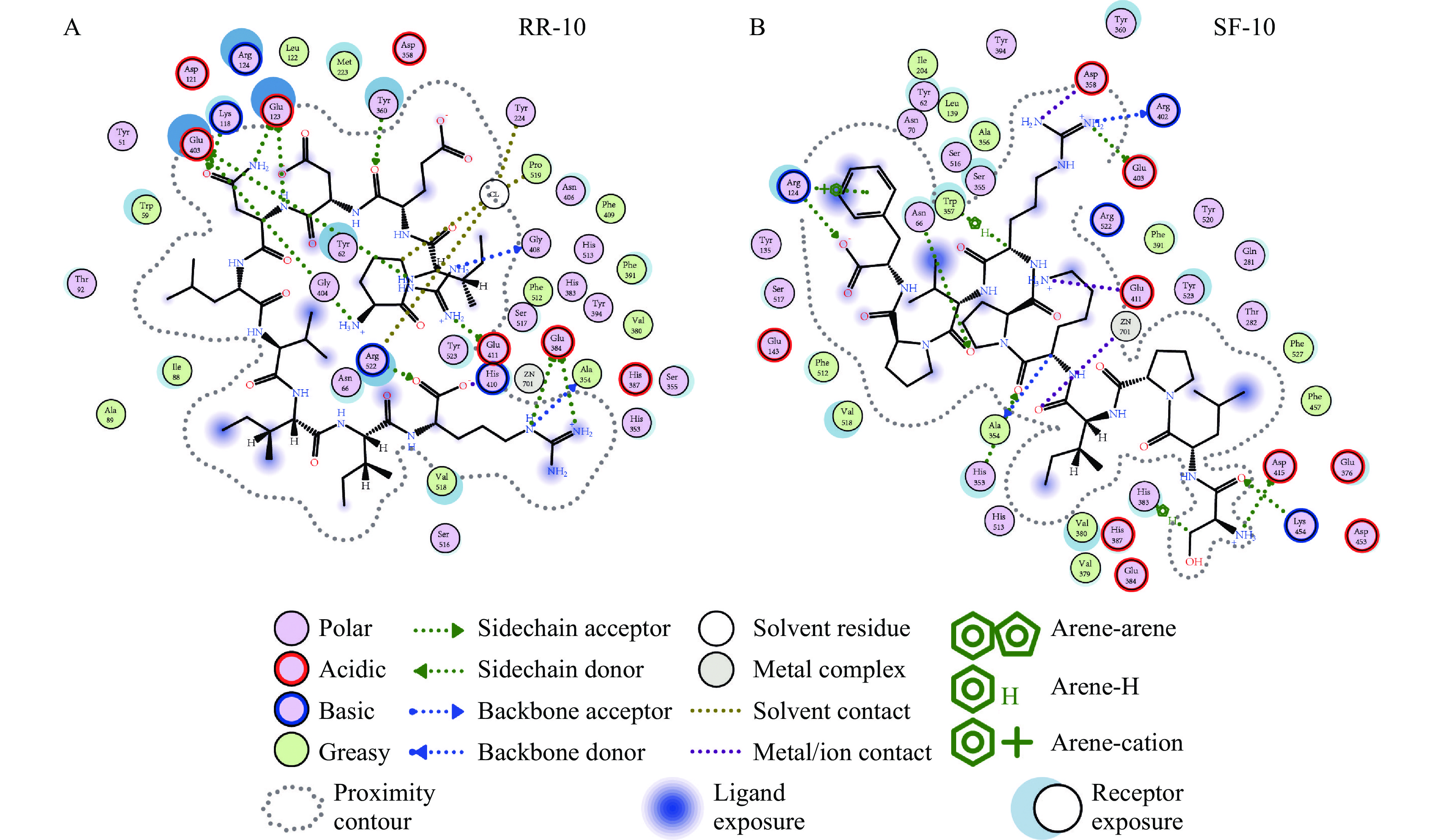
两种十肽与ACE受体分子对接结果图1~图3所示。RR-10和SF-10均可进入ACE受体的活性空腔(图1)。RR-10与ACE受体对接得分−15.05(同一肽段-受体不同构象结合得分越低,形成的复合物越稳定),RR-10与ACE的氨基酸残基GLU403、GLU411、GLU384等形成14个氢键和5个离子键。RR-10中R1、I2、E3、N5和R10是与受体形成相互作用的氨基酸残基,其中,R1和R10与受体之间形成的结合键数量多,结合键能量均较低,在维持肽-受体形成的复合物稳定性中发挥重要作用(图2A,图3A)。SF-10与ACE受体对接得分−14.69。SF-10与ACE受体形成9个氢键和8个离子键相互作用。ASP415、LYS454、GLU403、ARG124等是ACE受体中结合到SF-10的关键氨基酸残基。肽段中S1、I4、K5、R7、V8和F10均与ACE受体产生相互作用,其中,S1、R7和F10与ACE受体氨基酸残基之间形成的结合键能量低,成键数量多,是SF-10中发挥主要结合作用的氨基酸残基(图2B,图3B)。综上所述,氢键和静电相互作用是两种十肽与ACE受体的主要作用方式。肽段C、N端氨基酸残基及肽段中的R残基,易被受体识别形成结合能量较低的作用键。两种十肽可与ACE受体活性口袋氨基酸残基(ALA354、GLU384和HIS353)[19,21]、锌离子(Zn701,2.98 Å和2.08 Å)或结构域上的关键氨基酸强结合,是肽段具有ACE抑制活性的主要原因。RR-10与ACE受体活性口袋氨基酸残基、锌离子之间的结合键数量及结合强度,均高于SF-10,说明RR-10的氨基酸序列组成及空间结构,更易被ACE受体识别及结合,这可能是RR-10的ACE抑制活性高于SF-10的主要原因。
考虑到大球盖菇十肽RR-10体外ACE抑制活性、分子对接预测的ACE受体结合抑制作用效果更好,采用RR-10进行分子互作热力学和动力学层面的ACE抑制机制研究。大球盖菇十肽RR-10与ACE受体之间结合反应热力学结果如图4所示。在RR-10与ACE受体的结合反应中,热力学结合常数为1.471×10−4,焓值(ΔH)和熵值(ΔS)的变化均为负值,表明RR-10与ACE受体的结合反应是焓驱动反应。研究表明,焓驱动反应比熵驱动反应更有利于活性肽对ACE的抑制作用[9]。RR-10和ACE受体之间结合反应动力学结果显示(图5),RR-10与ACE受体之间属于特异性结合,动力学结合常数为5.61×10−4。分子互作热力学和动力学结果表明,焓驱动反应的RR-10与ACE受体特异性结合,是生物分子基团之间的氢键和静电结合,这与分子对接结果具有一致。
3. 讨论与结论
食源性活性肽因来源广泛,安全性高,靶向效果好,近十年在临床及临床前研究中比例大幅度提升[22]。活性肽在降血压[9,23-26]、降血糖[27-30]、降血脂[8,31]等保健食品和药物开发上具有良好潜力和应用前景。基于肽组学、分子对接等技术开展的食源性功能肽、肽与靶标受体蛋白互作机制研究,已取得突破性进展。Li等[25]从郫县豆瓣酱中获得的ACE抑制肽RGLSK,可与ACE受体的ALA354,TYR523和GLU384形成氢键、与Zn2+形成配位键相互作用。Ma等[32]从纹鳢水解产物中筛选出潜在的ACE抑制肽,氢键、静电和疏水相互作用是肽段与ACE受体的主要作用方式。Wei等[26]从酒糟中鉴定得到的IC50值较好(50.01 μmol/L)且含量较高的ACE抑制肽PR,与ACE受体结合为竞争性抑制结合方式。Fu等[33]从牛胶原蛋白中分离得到的ACE抑制肽,未与ACE受体口袋活性氨基酸残基结合,主要通过非竞争机制显示ACE抑制活性。本研究基于BIOPEP-UWM肽谱库等虚拟筛选及预测,获得了两种大球盖菇十肽RR-10和SF-10的特征性呈味和活性肽片段、消化特性、毒理性等理论评价结果。体外呈味及活性验证结果表明,RR-10和SF-10在一定浓度配比下,可呈现愉悦咸鲜味呈味特性;RR-10具有较优的ACE抑制活性(IC50,0.012 mg/mL)。两种大球盖菇十肽可与ACE靶标蛋白受体结合形成氢键和静电相互作用,与已报道文献研究结果具有一致性。基于分子互作热力学和动力学技术解析的大球盖菇十肽RR-10,与ACE靶标受体焓驱动反应特异性结合发挥ACE抑制活性,为食源性肽ACE抑制机制验证及解析提供方法和思路。
风味肽分子量低,能带来愉悦的味觉感受,可与食盐、谷氨酸钠等相互作用提升食品咸鲜味醇厚口感,增强滋味丰富度和协调性[34-36]。开发兼具功能活性和呈味特性的风味活性肽产品,既可在保障人体健康中发挥重要的营养作用和生物活性,亦可满足消费者对天然高品质呈味基料、风味休闲食品的需求,具有广阔的应用前景。本研究基于虚拟筛选、体外呈味及活性评价、分子对接和分子互作技术解析的大球盖菇十肽呈味特性和ACE抑制作用机制,还未与真正的味觉传感、生理作用产生直接联系。考虑到不同技术的检测原理不同,检测结果之间的相同趋势是值得关注的。未来仍需进一步开展基于感官受体、胃肠受体分子互作过程中的呈味机制和活性作用机制解析,为开发兼具功能活性及风味特性的大球盖菇风味活性肽提供理论支撑。
-
表 1 大球盖菇十肽RR-10和SF-10呈味特性评价结果
Table 1 Evaluation results of the taste characteristics of the decapeptides RR-10 and SF-10 from Stropharia rugosoannulata
浓度
(mg/mL)RR-10电子舌 SF-10电子舌 余味 浓厚味 咸味 余味 鲜味 浓厚味 0.25 0.80±0.03 0.74±0.02 −18.87±0.37 0.40±0.01 4.76±0.26 0.69±0.10 0.375 0.72±0.03 0.74±0.03 −12.65±0.13 0.59±0.01 4.07±0.11 0.69±0.08 0.5 0.65±0.02 0.75±0.02 −8.77±0.17 0.77±0.02 3.62±0.19 0.69±0.09 0.75 0.60±0.01 0.73±0.01 −1.29±0.04 0.88±0.01 2.82±0.08 0.67±0.09 1.0 0.56±0.01 0.70±0.02 3.84±0.08 0.97±0.01 2.27±0.05 0.64±0.11 -
[1] 李文, 陈万超, 马海乐, 等. 大球盖菇肽基料超声制备及其食药特性分析[J]. 食用菌学报,2022,29(3):81−94. [LI W, CHEN W C, MA H L, et al. Ultrasonic preparation of Stropharia rugosoannulata peptides and analysis of their taste characteristics and pharmacological activities[J]. Acta Edulis Fungi,2022,29(3):81−94. doi: 10.16488/j.cnki.1005-9873.2022.03.010 LI W, CHEN W C, MA H L, et al. Ultrasonic preparation of Stropharia rugosoannulata peptides and analysis of their taste characteristics and pharmacological activities[J]. Acta Edulis Fungi, 2022, 29(3): 81-94. doi: 10.16488/j.cnki.1005-9873.2022.03.010
[2] FRAGASSO G, MARANTA F, MONTANARO C, et al. Pathophysiologic therapeutic targets in hypertension: A cardiological point of view[J]. Expert Opinion on Therapeutic Targets,2012,16(2):179−93. doi: 10.1517/14728222.2012.655724
[3] XUE L, YIN R X, HOWELL K, et al. Activity and bioavailability of food protein-derived angiotensin-I-converting enzyme-inhibitory peptides[J]. Comprehensive Reviews in Food Science and Food Safety,2021,20(2):1150−1187. doi: 10.1111/1541-4337.12711
[4] LI W, CHEN W C, MA H L, et al. Structural characterization and angiotensin-converting enzyme (ACE) inhibitory mechanism of Stropharia rugosoannulata mushroom peptides prepared by ultrasound[J]. Ultrasonics Sonochemistry,2022,88:106074. doi: 10.1016/j.ultsonch.2022.106074
[5] LI W, CHEN W C, MA H L, et al. Study on the relationship between structure and taste activity of the umami peptide of Stropharia rugosoannulata prepared by ultrasound[J]. Ultrasonics Sonochemistry,2022,90:106206. doi: 10.1016/j.ultsonch.2022.106206
[6] ZHANG Y Y, DAI Z J, ZHAO X J, et al. Deep learning drives efficient discovery of novel antihypertensive peptides from soybean protein isolate[J]. Food Chemistry, 2023, 404, Part B: 134690.
[7] XIONG Y Z, GAO X C, PAN D D, et al. A strategy for screening novel umami dipeptides based on common feature pharmacophore and molecular docking[J]. Biomaterials,2022,288:121697. doi: 10.1016/j.biomaterials.2022.121697
[8] YU Z P, CAO Y X, KAN R T, et al. Identification of egg protein-derived peptides as xanthine oxidase inhibitors: Virtual hydrolysis, molecular docking, and in vitro activity evaluation[J]. Food Science and Human Wellness,2022,11(6):1591−1597. doi: 10.1016/j.fshw.2022.06.017
[9] ZHANG B Y, LIU J B, WEN H D, et al. Structural requirements and interaction mechanisms of ACE inhibitory peptides: Molecular simulation and thermodynamics studies on LAPYK and its modified peptides[J]. Food Science and Human Wellness,2022,11(6):1623−1630. doi: 10.1016/j.fshw.2022.06.021
[10] CAO S M, WANG Z X, XING L J, et al. Bovine bone gelatin-derived peptides: Food processing characteristics and evaluation of antihypertensive and antihyperlipidemic activities[J]. Journal of Agricultural and Food Chemistry,2022,70:9877−9887. doi: 10.1021/acs.jafc.2c02982
[11] SALEHABADI H, KHAJEH K, DABIRMANESH B, et al. Evaluation of angiotensin converting enzyme inhibitors by SPR biosensor and theoretical studies[J]. Enzyme and Microbial Technology,2019,120:117−123. doi: 10.1016/j.enzmictec.2018.10.010
[12] ZHANG J C, ZHANG J C, LIANG L, et al. Identification and virtual screening of novel umami peptides from chicken soup by molecular docking[J]. Food Chemistry,2023,404:134414. doi: 10.1016/j.foodchem.2022.134414
[13] ZHANG C X, MIAO Y L, FENG Y H, et al. Umami polypeptide detection system targeting the human T1R1 receptor and its taste-presenting mechanism[J]. Biomaterials,2022,287:121660. doi: 10.1016/j.biomaterials.2022.121660
[14] BU Y, LIU Y N, LUAN H W, et al. Characterization and structure-activity relationship of novel umami peptides isolated from Thai fish sauce[J]. Food & Function,2021,12(11):5027−5037.
[15] WANG W L, YANG L, NING M H, et al. A rational tool for the umami evaluation of peptides based on multi-techniques[J]. Food Chemistry,2022,371:131105. doi: 10.1016/j.foodchem.2021.131105
[16] FU Y, YOUNG J F, LØKKE M M, et al. Revalorisation of bovine collagen as a potential precursor of angiotensin I-converting enzyme (ACE) inhibitory peptides based on in silico and in vitro protein digestions[J]. Journal of Functional Foods,2016,24:196−206. doi: 10.1016/j.jff.2016.03.026
[17] CUI L, YANG G, LU S Y, et al. Antioxidant peptides derived from hydrolyzed milk proteins byLactobacillus strains: A BIOPEP-UWM database-based analysis[J]. Food Research International,2022,156:111339. doi: 10.1016/j.foodres.2022.111339
[18] YAP P G, GAN C Y. In vivo challenges of anti-diabetic peptide therapeutics: Gastrointestinal stability, toxicity and allergenicity[J]. Trends in Food Science & Technology,2020,105:161−175.
[19] 于志鹏, 樊玥, 赵文竹, 等. 鸡蛋蛋白ACE抑制肽的筛选、鉴定及其作用机制[J]. 食品科学,2020,41(12):129−135. [YU Z P, FAN Y, ZHAO W Z, et al. Identification and mechanism of action of angiotensin-і converting enzyme inhibitory peptides from hen egg proteins[J]. Food Science,2020,41(12):129−135. doi: 10.7506/spkx1002-6630-20190507-050 YU Z P, FAN Y, ZHAO W Z, et al. Identification and mechanism of action of angiotensin-і converting enzyme inhibitory peptides from hen egg proteins[J]. Food Science, 2020, 41(12): 129-135. doi: 10.7506/spkx1002-6630-20190507-050
[20] WANG B, XIE N N, LI B. Influence of peptide characteristics on their stability, intestinal transport, and in vitro bioavailability: A review[J]. Journal of Food Biochemistry,2019,43(1):e12571. doi: 10.1111/jfbc.12571
[21] RAWENDRA R D S, AISHA, CHANG C I, et al. A novel angiotensin converting enzyme inhibitory peptide derived from proteolytic digest of Chinese soft-shelled turtle egg white proteins[J]. Journal of Proteomics,2013,94:359−369. doi: 10.1016/j.jprot.2013.10.006
[22] MUTTENTHALER M, KING G E, ADAMS D J, et al. Trends in peptide drug discovery[J]. Nature Reviews Drug Discovery,2021,20(4):309−325. doi: 10.1038/s41573-020-00135-8
[23] SUO S K, ZHAO Y Q, WANG Y M, et al. Seventeen novel angiotensin converting enzyme (ACE) inhibitory peptides from protein hydrolysate of Mytilus edulis: Isolation, identification, molecular docking study, and protective function on HUVECs[J]. Food & Function,2022,13(14):7831−7846.
[24] LU Y T, WANG Y, HUANG D Y, et al. Inhibitory mechanism of angiotensin-converting enzyme inhibitory peptides from black tea[J]. Journal of Zhejiang University Science B,2021,22(7):575−589. doi: 10.1631/jzus.B2000520
[25] LI M Y, FAN W L, XU Y. Identification of angiotensin converting enzyme (ACE) inhibitory and antioxidant peptides derived from Pixian broad bean paste[J]. LWT,2021,151:112221. doi: 10.1016/j.lwt.2021.112221
[26] WEI D, FAN W L, XU Y. Identification of water-soluble peptides in distilled spent grain and its angiotensin converting enzyme (ACE) inhibitory activity based on UPLC-Q-TOF-MS and proteomics analysis[J]. Food Chemistry,2021,353:129521. doi: 10.1016/j.foodchem.2021.129521
[27] PAUL R K, NATH V, KUMAR V. Structure based virtual screening of natural compounds and molecular dynamics simulation: Butirosin as dipeptidyl peptidase (DPP-IV) inhibitor[J]. Biocatalysis and Agricultural Biotechnology,2021,35:102042. doi: 10.1016/j.bcab.2021.102042
[28] ZHANG X G, WANG R C, CHENG C L, et al. Identification of two novel dipeptidyl peptidase-IV inhibitory peptides from sheep whey protein and inhibition mechanism revealed by molecular docking[J]. Food Bioscience,2022,48:101733. doi: 10.1016/j.fbio.2022.101733
[29] WANG J, XIE Y J, LUAN Y Y, et al. Identification and dipeptidyl peptidase IV (DPP-IV) inhibitory activity verification of peptides from mouse lymphocytes[J]. Food Science and Human Wellness,2022,11(6):1515−1526. doi: 10.1016/j.fshw.2022.06.009
[30] LI M Q, BAO X, ZHANG X T, et al. Exploring the phytochemicals and inhibitory effects against α-glucosidase and dipeptidyl peptidase-IV in Chinese pickled chili pepper: Insights into mechanisms by molecular docking analysis[J]. LWT,2022,162:113467. doi: 10.1016/j.lwt.2022.113467
[31] 叶灏铎, 苗建银, 李龙星, 等. 勐库大叶茶蛋白4血脂肽的酶解制备及活性分析[J]. 食品工业科技,2022,43(9):212−221. [YE H D, MIAO J Y, LI L X, et al. Preparation and activity of hypolipidemic peptides from Mengkudayecha protein by enzymatic hydrolysis[J]. Science and Technology of Food Industry,2022,43(9):212−221. YE H D, MIAO J Y, LI L X, et al. Preparation and activity of hypolipidemic peptides from Mengkudayecha protein by enzymatic hydrolysis[J]. Science and Technology of Food Industry, 2022, 43(9): 212-221.
[32] MA T X, FU Q Q, MEI Q G, et al. Extraction optimization and screening of angiotensin-converting enzyme inhibitory peptides from Channa striatus through bioaffinity ultrafiltration coupled with LC-Orbitrap-MS/MS and molecular docking[J]. Food Chemistry,2021,354:129589. doi: 10.1016/j.foodchem.2021.129589
[33] FU Y, YOUNG J F, RASMUSSEN M K, et al. Angiotensin I-converting enzyme–inhibitory peptides from bovine collagen: Insights into inhibitory mechanism and transepithelial transport[J]. Food Research International,2016,89:373−381. doi: 10.1016/j.foodres.2016.08.037
[34] XIE X N, DANG Y L, PAN D D, et al. The enhancement and mechanism of the perception of saltiness by umami peptide from Ruditapes philippinarum and ham[J]. Food Chemistry, 2023, 405, Part A: 134886.
[35] XIA X Z, FU Y, MA L, et al. Protein hydrolysates from Pleurotus geesteranus modified by Bacillus amyloliquefaciens γ-Glutamyl transpeptidase exhibit a remarkable taste-enhancing effect[J]. Journal of Agricultural and Food Chemistry,2022,70:12143−12155. doi: 10.1021/acs.jafc.2c03941
[36] LI X P, XIE X X, WANG J X, et al. Identification, taste characteristics and molecular docking study of novel umami peptides derived from the aqueous extract of the clam Meretrix meretrix Linnaeus[J]. Food chemistry,2020,312:126053. doi: 10.1016/j.foodchem.2019.126053
-
期刊类型引用(2)
1. 仇晨晨,刘云,刘斌. 灰树花醇提物体外抗氧化及降血糖活性分析. 中国食用菌. 2025(01): 41-49 .  百度学术
百度学术
2. 陈雪梅,邵惠丽,高燕,郑真,邓碧琦,朱立俏,盛华刚. 不同产地玄参多糖的单糖组成及分子量测定. 天津中医药大学学报. 2024(12): 1082-1089 .  百度学术
百度学术
其他类型引用(1)





 下载:
下载:


 下载:
下载:



