Types of Electrochemiluminescence Sensing Techniques and Research Progress in Food Analysis
-
摘要: 电致化学发光传感技术(Electrochemiluminescence,ECL)是一种结合了电化学和光化学的分析方法,因其可控性强、灵敏度高和响应速度快等优势在食品分析领域引起了广泛关注。ECL是通过改变发射物或共反应物的浓度,使其信号强度发生变化,从而实现对目标物质的灵敏检测。首先,该文总结了经典ECL检测体系及基于新型发射物和共反应物的检测体系,并重点介绍了新型发射物中金属纳米团簇和量子点的最新进展,举例阐述其ECL传感器的结构和检测原理。其次,综述了ECL传感器在食品分析领域中的研究进展。最后对ECL传感技术的未来发展趋势进行了展望,为食品中营养成分和污染物的检测提供参考,同时也促进该技术的进一步研究,助力未来食品检测发展。Abstract: Electrochemiluminescence (ECL) is an analytical method that combines electrochemistry and photochemistry. It has attracted a lot of attention from researchers in the field of food analysis due to its advantages of high controllability, high sensitivity and fast response time. ECL is the sensitive detection of target substances by varying the concentration of luminophores or co-reactants, resulting in changes in their signal intensity. Firstly, the classical ECL detection system and the novel luminophore and co-reactor based detection system are summarized in this paper. It also focuses on recent advances in metal nanoclusters and quantum dots as novel luminophores, and illustrates the structure and detection principle of their ECL sensors with examples. Secondly, the research progress of ECL sensors in the food analysis field. Finally, the future development trend of ECL sensor technology is predicted. It provides a reference for the detection of nutrients and contaminants in food, and also encourages further research into this technology to support the future development of food testing.
-
Keywords:
- electrochemiluminescence /
- biosensor /
- food analysis /
- luminophore /
- co-reacant
-
生物传感器是检测生物信号变化并通过传感器将其转换为可量化信号的设备,具有高灵敏度、高选择性和检测准确的特点[1]。生物传感器的种类繁多,可分为光学、温度、电化学和电致化学发光生物传感器等[2]。其中,电致化学发光(Electrochemiluminescence,ECL)也被称为电化学发光,是指带电物质之间的电子,基于电化学激发和高能级电子转移而发光的过程[3],于1964年被首次提出[4]。与化学发光法和电化学法相比,ECL能更好的控制发光时间,并具有更强的选择性。与光致发光法相比,ECL不需要额外的光源,可实现几乎为零的背景噪声[5],这使它成为生物传感技术的有力工具。ECL还具有操作简单、背景信号低和灵敏度高等优点[6],被认为是一种很有前景的生物分析技术,已广泛应用于免疫分析[7]、药品检测[8]以及环境监测[9]等领域。
ECL机制大致可以分为两种,分别是湮灭和共反应物途径[10]。在湮没途径中,氧化和还原的发射物材料是在电极上通过电化学法生成,然后二者相互反应形成发射激发态。而在共反应物途径中,共反应物通过电化学氧化或还原产生活跃的中间体,该中间体可以与氧化或还原的发光体反应形成激发态发光。共反应物途径可以产生更稳定和更强的ECL信号,这使其几乎主导了现代生物分析领域的ECL应用[11]。影响共反应物途径ECL的三个重要因素为发射物(或发光体)、共反应物和电极[12]。电致化学发光体系按照发射物性质的不同可大致划分为三类,包括最早出现的有机化合物发光体系、应用较为成熟的无机金属配合物发光体系和正在迅猛发展的纳米材料发光体系。经典ECL体系主要包括有机化合物2,3-氨基邻苯二甲酰肼/过氧化氢体系(鲁米诺/H2O2)和无机金属发射物三(2,2'-联吡啶)氯化钌(Ⅱ)/三丙胺体系(Ru(bpy)32+/TPA)[13],其具有化学性质稳定、发光效率高等特点。但由于其在发展的过程中被发现存在检测仪器复杂和ECL信号较弱等问题[14],因此基于纳米材料的新型ECL发射物和共反应物成为了研究热点。近年来,研究较多的新型发射物和共反应物为金属纳米团簇(Metal nanoclusters,MNCs)、镉量子点(CdS)、硫量子点(SQDs)和碳量子点(CQDs)[15]。
本文以经典ECL体系与新型ECL发射物和共反应物的电致化学发光传感器为切入点,举例阐述ECL传感器的结构和检测原理;并对近年来ECL传感技术在检测食品中营养成分和污染物的研究进展进行综述,最后分析了ECL传感技术面临的挑战并对未来发展趋势进行了展望。
1. 电致化学发光传感技术
1.1 经典ECL体系传感器
经典ECL体系传感器主要包括两种体系,第一种为有机化合物鲁米诺/H2O2体系,第二种为无机金属发射物钌(Ⅱ)配合物Ru(bpy)32+/TPA体系。鲁米诺作为典型的有机化合物发射物,具有无毒、价格低廉且发光效率高的优势[16],是ECL中一种应用最为广泛的发射物。在鲁米诺中引入共反应物H2O2可以显著提高ECL体系的发光效率,所形成的鲁米诺/H2O2体系是经典ECL体系之一。与直接在检测溶液中加入鲁米诺的传感器相比,将鲁米诺固定在电极表面构建出固态核酸适配体ECL传感器,不仅可以节省试剂,还能提高发光效率[17]。Cheng等[18]选择银纳米粒子(AgNPs)催化鲁米诺/H2O2体系,构建了一种用于检测卡那霉素(Kanamycin,KAN)的ECL传感器。AgNPs作为催化剂可以加速H2O2的分解并产生各种中间活性氧(ROS),从而提高体系的ECL信号强度。在检测KAN时,由于KAN与核酸适配体之间存在特异性的相互作用,ECL信号会出现明显下降的现象,以此来确定KAN的浓度。该检测体系回收率在94.4%到105.17%之间。Jiang等[19]以聚酰胺功能化的氧化锌纳米棒(ZNs-PAMAM)为载体,制备基于信号探针ZNs-PAMAM-鲁米诺-二抗(Secondary antibody,Ab2)的ECL免疫传感器,用于检测糖抗原15-3(CA15-3)。在检测CA15-3时,ZNs-PAMAM-鲁米诺-Ab2以夹心式免疫反应修饰到电极上,这使得鲁米诺与电极表面的距离缩小,即可更为有效地发射ECL信号。此外,ZNs还可以加速分解H2O2,产生ROSs加速鲁米诺的ECL反应,增强ECL信号强度,RSD为95.9%~108.6%。大多数基于鲁米诺的研究都是在碱性介质中进行的。然而在碱性条件下,当鲁米诺没有共反应物与之共同参与反应时,ECL信号会较弱甚至无法检测到。但H2O2作为共反应物存在不稳定、易分解和背景信号高的缺陷。因此需要开发更多稳定的共反应物,增强ECL信号并扩大鲁米诺的应用范围。
Ru(bpy)32+是一种传统的无机金属阳极ECL发射物,因其在水相系统中具有更高的发光率和化学稳定性,且价格低廉[20],近年来被广泛应用于DNA分析、免疫测定等领域中[21−22]。TPA为通用共反应剂,可增强Ru(bpy)32+的ECL信号强度[23],Ru(bpy)32+/TPA体系也被称为经典的ECL共反应体系。Li等[24]在Ru(bpy)32+/TPA体系中将金与二氧化硅纳米粒子修饰于玻碳电极(Au@SiO2/GCE)的表面,构建用于检测多巴胺(DA)的增强电致发光平台。经过研究得出,Au局部表面发生等离子体共振会引起电磁场增强。在电磁场被增强的优势下,Au@SiO2/GCE的ECL信号强度相较于裸GCE的信号提升了29倍,相较于Au修饰的GCE信号提升了100倍。该传感器检测限低至0.004 μmol/L,回收率在98%~104%之间。由于其优异的稳定性、水溶性以及对体系中可能存在干扰物质的抵抗力,Ru(bpy)32+/TPA是第一种也是唯一一种被正式投入商业实物应用的电致化学发光免疫传感器发光体系,在食品分析领域也同样具有的巨大潜力。
共反应物途径在现阶段的ECL领域中起着主导作用,ECL的共反应物也受到很多关注。由于TPA存在毒性和在低浓度时灵敏度较低的不足的问题[25],研究人员致力于开发取代TPA的ECL共反应物。Kitte等[26]将一种新的共反应物1-乙基-3-(3-二甲氨基丙基)碳二亚胺(1-Ethyl-3-(3-dimethylaminopropyl)-carbodiimide,EDC)引入Ru(bpy)32+电化学发光技术,并应用Ru(bpy)32+/EDC体系对H2O2进行检测,检测限为0.33 μmol/L,检测范围为0.5~200 µmol/L。Yuan等[27]通过二丁基氨基乙醇(DBAE)与丙烯酰氯的反应合成了一种新型ECL共反应物丙烯酸2-(二丁基氨基)乙酯(DBAEA),并用Ru(bpy)32+/DBAEA体系检测半胱氨酸(Cys),如图1所示。与TPA和DBAE相比,DBAEA可显著提高铂(Pt)电极上Ru(bpy)32+的ECL信号强度。经过研究得出,ECL信号强度的增加归因于烯基与铂电极之间的相互作用,使得DBAEA更容易被电氧化,检测限为1.15 μmol/L,回收率在93.5%~105.6%之间。
综上所述,将鲁米诺固定在电极上可以加快电子转移速率,缩短信号响应时间,但固定在电极上的同时不降低其ECL信号强度却是一个巨大的挑战。同时,未来可引入更多共反应物代替H2O2或研发性能更优异的共反应促进剂,进一步提高用于食品分析的ECL体系稳定性和信号强度。增强传统发射物的ECL信号通常需要在溶液中加入多种共反应物[28],然而这就无法避免ECL操作复杂化和试剂消耗快导致成本升高的问题。鉴于此,仍然需要继续寻找新的ECL体系并持续围绕经典ECL体系拓展应用的范围。
1.2 基于新型发射物和共反应物的ECL传感器
由于纳米材料的研究发展,越来越多的相关研究者看到了其旷阔前景,遂将纳米材料引入ECL发光体系。贵金属纳米团簇、铜纳米团簇、镉量子点、硫量子点和碳量子点等能增强信号强度的新型ECL发射物和共反应物备受关注。金属纳米团簇是一种多功能纳米材料,具有介于金属原子和金属纳米粒子之间的性质[29]。MNCs主要包括金纳米团簇(AuNCs)和银纳米团簇(AgNCs),其中金纳米团簇因具有稳定性高、生物相容性好等优势,已在生物成像[30]和电催化[31]领域中被广泛应用。但是,与经典的ECL发射物相比,金属纳米簇的ECL发光效率相对比较低。因此,采用不同策略来增强金属纳米簇的ECL信号强度,提高其量子产率是目前的研究热点。Zhang等[32]将金纳米团簇和二维过渡金属碳化物Ti3C2结合,形成异质结构AuNCs@Ti3C2,并将其作为发射物构建ECL生物传感器,用于检测微小RNA-155(miRNA-155)。研究显示,AuNCs通过氧原子牢固地锚定在Ti3C2表面,Au-O-Ti连接起了AuNCs和Ti3C2,改善了AuNCs因尺寸较小不利于分离与固定的不足。此外,Ti3C2作为载体不仅导电性良好,还能增加AuNCs的负载量。Huang等[33]提出了一种基于金纳米团簇和过硫酸钾(AuNCs/K2S2O8)的ECL传感体系,用于检测尼古丁(Nicotine)。通过高效液相色谱-质谱分析(High-performance liquid chromatography-mass spectrometry,HPLC-MS)研究了K2S2O8中尼古丁的变化,结果表明,K2S2O8会产生强氧化中间体SO4−·自由基阴离子。尼古丁分子的吡咯烷部分不仅很容易被氧化成烟酸,还会与SO4−·发生竞争性反应,导致ECL信号下降,检测限低至7.0×10−7 μmol/L。
铜是一种经济、高效且储量丰富的金属元素,也是人体必需的微量元素。铜纳米团簇(CuNCs)展现出的ECL性能使其可应用在传感检测和成像分析等领域[34]。Zhao等[35]将牛血清白蛋白保护的CuNCs作为发射物,肼作为共反应物构建ECL传感器,用于检测DA。Zhuang等[36]利用稀土元素铕(Eu)丰富的电子结构和显著的光学性能,提出了基于铕掺杂铜纳米团簇(Eu3+-CuNCs)的ECL生物传感器,以实现灵敏检测多巴胺。Eu3+改变了CuNCs的表面,并驱动合成Eu(Ⅲ)复合物,形成新的表面状态,以促进能量有效转移到Eu3+,从而大大增强了ECL发射强度和CuNCs的稳定性。该传感器具有很强的选择性,对DA的检测限为1×10−11 μmol/L。
量子点(Quantum dots,QDs)被称为胶体半导体纳米晶体,作为一种零维纳米材料,QDs具有优异的光学、电学性能[37]。因此,量子点在光催化[38]和生物医学影像[39]等领域中受到了极大的关注。其中,硫化镉量子点(CdS)因表面易功能化和ECL性质稳定,使其成为ECL传感中发射物的理想选择[40]。Zhao等[41]通过电沉积法合成硫化镉/硫化锌量子点(CdS/ZnS QDs),并基于CdS/ZnS QDs的电致化学发光猝灭构建了一种灵敏检测Cu2+的ECL传感器。研究结果表明,制备的CdS/ZnS QDs表现出很好的溶解性和强而稳定的阴极电泳能力,Cu2+与Cd2+和Zn2+反应生成的硫化铜会使CdS/ZnS量子点的结构被破坏,ECL强度显著降低,即CdS/ZnS量子点被Cu2+选择性淬灭。该传感器对Cu2+的检测限为9.5×10−4 μmol/L,检测范围为2.5~200 nmol/L。
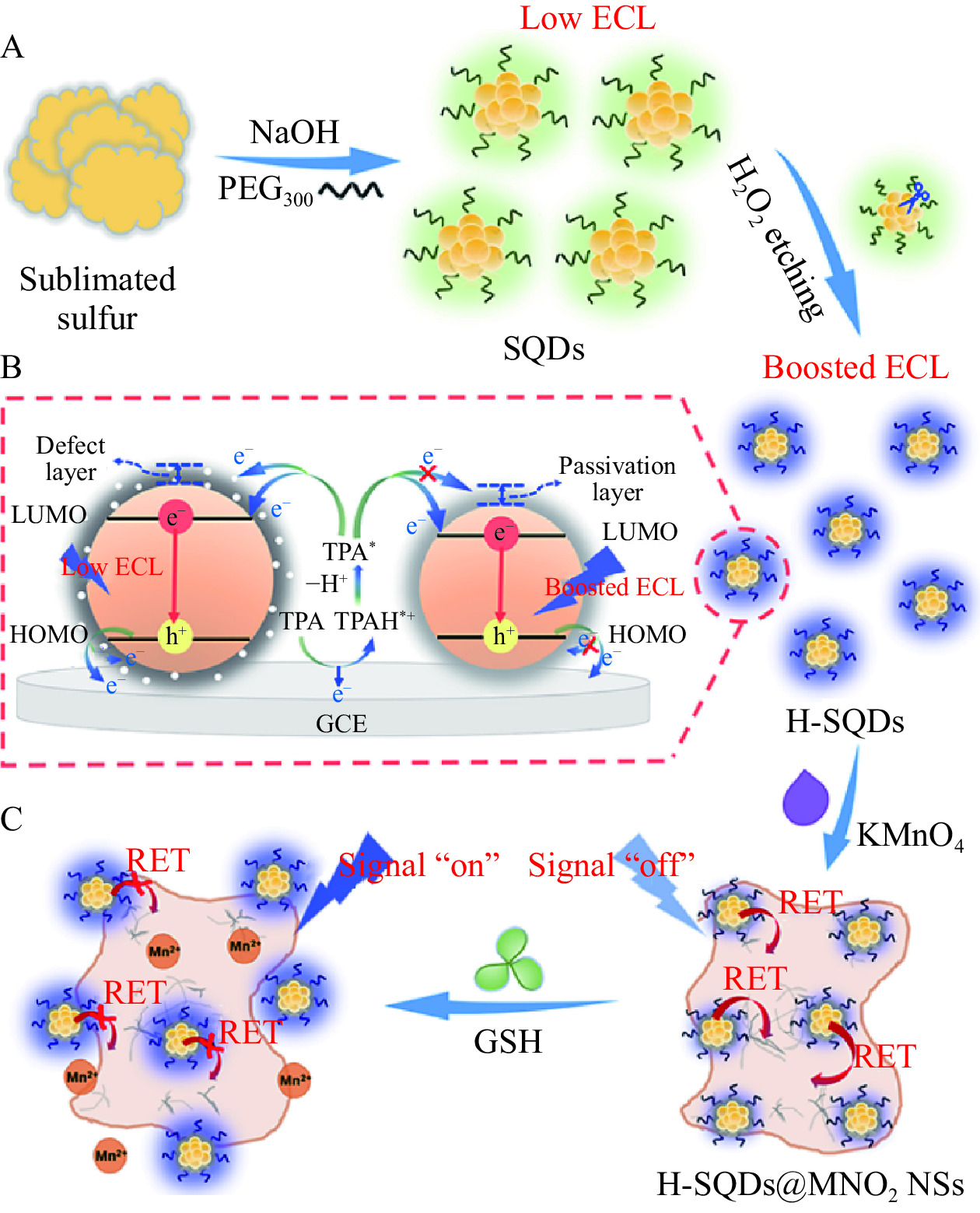
由于硫化镉量子点中包含重金属镉,其中存在不可避免的毒性,并对生物环境有着极大的威胁,所以近年来研究人员致力于开发无毒副作用或无重金属的量子点。其中硫量子点(SQDs)作为一种无金属量子点,且具有良好的化学稳定性、生物相容性和低毒性[42],被认为是有很潜力的绿色纳米材料。Hu等[43]建立了一种以SQDs作为发射物,K2S2O8为共反应物的电化学发光传感器,用于测定多巴胺。检测限达到2.5×10−5 μmol/L,检测范围为1×10−10~1×10−3 mol/L。Han等[44]将硫量子点与H2O2结合形成H-SQDs,再复合二氧化锰(MnO2),制备了H-SQDs@MnO2/GCE阳极增强型“off-on”ECL生物传感器(图2),用于对谷胱甘肽(GSH)进行灵敏检测。MnO2被用作ECL共振能量转移(Resonant energy transfer,RET)受体,也可作为识别单元来识别GSH。如图2C所示,通过在MnO2上原位锚定H-SQDs建立ECL-RET体系。H-SQDs@MnO2显示出几乎被猝灭的ECL响应,处于“信号关闭”状态。GSH则会特异性地将MnO2还原为Mn2+,并从H-SQDs@MnO2上标定下来H-SQDs,ECL响应恢复为“信号开启”状态,从而实现GSH的灵敏检测。研究表明,H-SQDs的ECL性能和光学性质得到了显著改善,这是因为其表面形成高度钝化,并且合成H-SQDs后再用H2O2进行蚀刻,电荷转移能力会增强。
碳量子点因其易于合成和修饰、高生物相容性和低毒性[45]的特点在光电催化[46]和生物成像[47]中表现出巨大潜力,有望替代重金属量子点。但CQDs量子产率低并且需要很高的激发电位,所以人们将其他元素掺杂进CQDs中,以提高CQDs的ECL性能[48]。许多研究表明,氮掺杂是促进CQDs的ECL发射的有效方法之一[49−50]。Li等[51]采用一步水热法制备出氮掺杂碳量子点(N-CQDs),并结合丝网印刷技术开发了一种基于N-CQDs的纸基ECL传感器,用于检测自来水中的Cu2+。该传感器使用N-CQDs作为发射物,K2S2O8作为共反应物,Cu2+的检测限为0.12 μmol/L,检测范围为0.01~1000 μmol/L。研究表明,使用掺杂了N的CQDs会更容易将电子注入CQDs中,并且N原子会在QDs表面产生更多的表面缺陷,在提高ECL活性的同时提供了更多的化学活性位点。近年来,CQDs成为一种很有前途的ECL共反应材料,受到了广泛关注。
综上所述,新型ECL发射物的生物传感器已经有了一定的发展,并在各个领域均具有良好的应用前景。Au、Ag金属纳米团簇虽具有优异的光学性能但成本过高,而Cu更廉价且同样具有高生物相容性,在食品分析领域备受关注。但由于Cu存在易被氧化的问题,并且制备出稳定的CuNCs较为困难,导致CuNCs在ECL传感分析中的应用相对滞后。预测今后研究人员会聚焦于研究CuNCs的稳定制备及应用方面,CuNCs有望用于构建更高效的ECL传感器。量子点在生物分析领域的探索仍处于早期阶段,却已经在ECL生物传感方面显示出巨大的潜力。目前基于碳量子点的ECL研究主要集中在应用开发上,但较少对ECL发射机制进行研究和说明。虽然开发高ECL性能的量子点仍然是巨大的挑战,但这也会有效地促进ECL传感器的持续创新发展,相信未来出现更多能够用于食品分析及各个领域的性能优异的新型发射物和共反应物。
2. 电致化学发光传感技术在食品分析中的研究进展
2.1 在检测食品营养成分中的应用
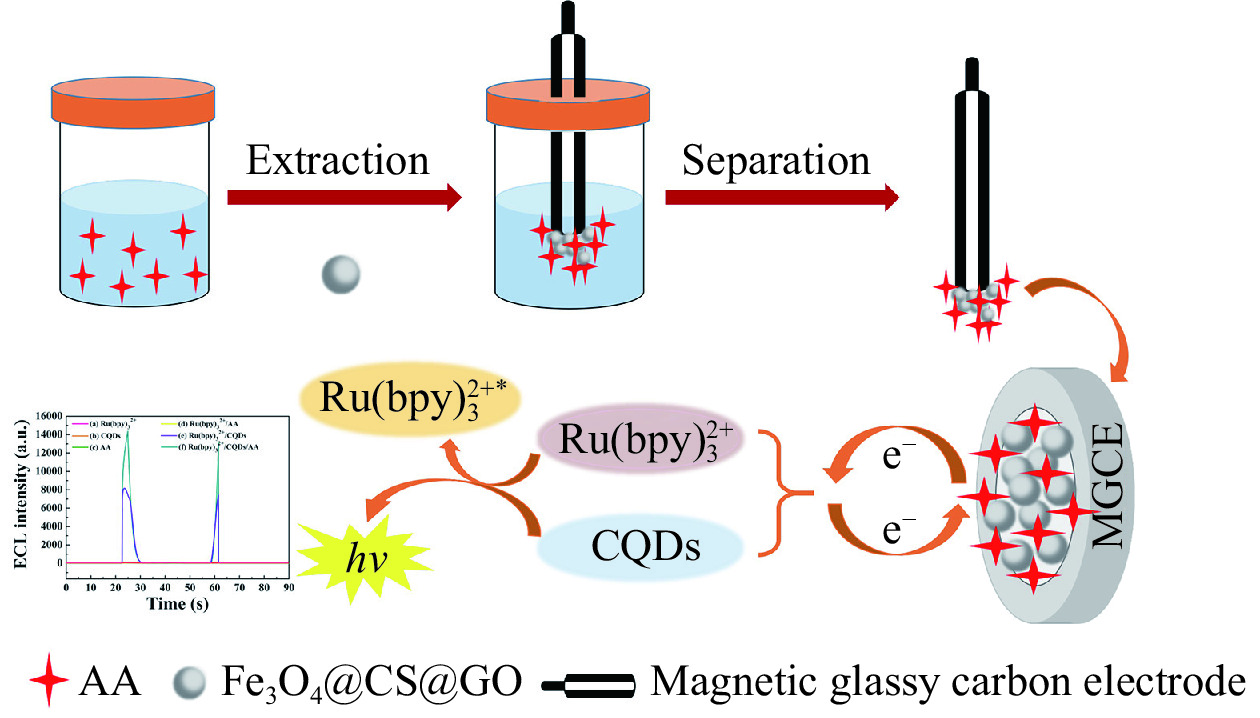
食品中的营养成分包括维生素、碳水化合物和脂类等,从食品中获取需要的营养对人体健康有着不可替代的作用。因此,对食品中的营养成分进行检测十分重要。抗坏血酸(Ascorbic acid,AA)又称维生素C,是人体不可缺少的水溶性维生素,在调节各种氧化还原代谢反应中起着重要作用[52]。研究发现,缺乏AA会导致坏血病和免疫力下降,但过量摄入AA也可能会导致腹泻、皮疹等疾病[53]。因此,开发一种准确、灵敏的AA检测方法对于食品安全具有重要意义。迄今为止,已经设计出各种传统的AA检测方法,包括高效液相色谱法、比色法和电泳法。但这些检测方法都存在设备昂贵、工艺复杂、耗时长等问题,在一定程度上受到限制。因此,电致化学发光法以其设备成本低、操作简单等优点而受到广泛关注。Wang等[54]使用内标法构建了石墨氮化碳量子点(g-CNQDs)与双共反应物K2S2O8和四丁基溴化铵(TBAB)的ECL传感器,用来检测人血清中的AA。在该传感器中,g-CNQDs作为单一发射物,在两种共反应物存在下可以产生双ECL信号,ECL信号强度会随着AA浓度的增加而降低,从而检测出AA含量。Su等[55]基于双增强Ru(bpy)32+/CQDs/AA体系,结合磁场增强固相微萃取(MFE-SPME)技术开发了一种双峰ECL传感器,可直接测定AA,如图3所示。该传感器还将四氧化三铁(Fe3O4)、壳聚糖(CS)和氧化石墨烯(GO)结合,制备出磁性纳米复合材料Fe3O4@CS@GO,并将其作为磁性吸附剂,在磁性玻碳电极(MGCE)的外磁场作用下提取AA。Fe3O4@CS@GO减少了GO的聚集,增加了CS溶解度,还提高了萃取性能。研究表明,ECL传感器出现双峰是因Ru(bpy)32+和CQDs之间的相互作用引起的。此外,在Ru(bpy)32+/CQDs体系中加入AA后,双峰ECL的信号强度明显增加,说明AA可以增强Ru(bpy)32+/CQDs体系的ECL信号强度,也进一步表明CQDs可以加速Ru(bpy)32+的电化学反应速率。该检测体系回收率为87.2%~104.0%,RSD为3.12%~4.71%。
葡萄糖(Glucose)是碳水化合物的主要成分,也是生命活动必不可缺的物质之一[56]。血液中的葡萄糖水平是临床医学中的一个重要参数,高血糖浓度会导致糖尿病,而糖尿病会诱发肾衰竭、心脏病或失明等许多疾病[57]。所以快速、准确、便捷的检测葡萄糖尤为重要。Lu等[58]使用氧化钴掺杂氧化银(Co3O4@Ag2O)和卟啉锌(TMPP-Zn)修饰电极,并构建了一种用于测定葡萄糖的增强型ECL传感器。该传感器首先使用多壁碳纳米管(MWNTs)将Ru(bpy)32+固定在GCE电极表面,然后依次将Co3O4@Ag2O和TMPP-Zn两种复合物修饰到玻碳电极表面,最终形成TMPP-Zn/Co3O4@Ag2O/Ru(bpy)32+/MWNTs/GCE修饰电极。Co3O4@Ag2O和TMPP-Zn的协同作用增强了系统的ECL性能,检测限为3.3×10−3 μmol/L,回收率在96.8%~104.4%之间。Zhang等[59]选择碲化镉(CdTe)、沸石咪唑骨架材料(ZIF-7)、羧化多壁碳纳米管(MWCNTs)、全氟磺化阳离子交换聚合物(Nafion)和葡萄糖氧化酶(GOD)修饰电极(Nafion/ZIF-7-GOD/CdTe@MWCNTs-GO/GCE),制备出用于测定血清中葡萄糖的ECL传感器。研究结果显示,电极表面覆盖的CdTe和ZIF-7作为共反应促进剂可以通过协同作用放大ECL信号;与此同时,MWCNTs和GO具有大比表面积,可作为量子点的载体以增强ECL信号强度;负载能力强的ZIF-7会固定GOD,而GOD与葡萄糖反应会产生H2O2,H2O2分解也可增强ECL信号,诸多因素共同促使ECL传感器表现出了良好的灵敏度和精确度,检测限为6.7×10−4 μmol/L。
胆固醇(Cholesterol)是血脂的一种,也是类固醇激素、胆汁酸和维生素D的主要前体物质[60]。健康人血清中总胆固醇(游离胆固醇和胆固醇酯的总和)的水平约为2.86~5.98 mmol/L,胆固醇含量过高会增加患心脏病的风险[61]。检测胆固醇的方法有比色法和荧光光谱法等,ECL相较于传统光谱技术的优势是可控制反应的空间和时间,同时信噪比和选择性得到改善,因此是检测胆固醇的有力工具。Yang等[62]以牛血清白蛋白(BSA)为保护剂,制备固定胆固醇氧化酶(ChOx)的Ag-BSA-MnO2纳米片,成功构建出了一种用于检测胆固醇的ECL传感器。研究表明,BSA是一种非导电蛋白,但在体系中加入AgNPs时会促进电子转移并增加纳米片的生物相容性。ChOx可以催化胆固醇生成H2O2并形成鲁米诺-H2O2体系;AgNPs与MnO2的协同作用提高了催化效率,从而有效放大了鲁米诺-H2O2体系的ECL信号,检测限为0.07 μmol/L。
ECL技术除了能够检测上述营养成分之外,也有研究报道显示其可以检测食品中的其他营养成分,如表1所示。尽管高效液相色谱、比色法和分光光度法等检测方法已用于测定食品中的营养成分,但它们存在样品制备步骤复杂并且分析时间相对较长的问题。因此,电致化学发光法凭借响应时间短、操作简单、以及成本效益高的优点,为开发低成本和高灵敏度的检测食品中营养成分的传感器奠定了基础。
表 1 ECL传感技术在检测食品营养成分中的应用进展Table 1. Progress in the application of ECL sensing technology in the detection of nutrient content of food检测物质 检测体系 发射物 共反应物 线性范围
(μmol/L)检测限
(μmol/L)参考文献 葡萄糖 AuNPs-CdTe QDs-CHIT/GCE CdTe QDs 共基质氧气 10~1×104 5.28 [63] 葡萄糖 Au-Co/GO/GOD 鲁米诺 H2O2 1~100 0.18 [64] 葡萄糖 GOD/AuNPs/PANi 鲁米诺 H2O2 0.1~100 0.05 [65] 胆固醇 MWCNTs-GO-Thi-Au 鲁米诺 H2O2 0.15~828 0.05 [66] 胆固醇 ChOx/hemin-GR/GCE hemin-GR K2S2O8 3.3×10−3~1.5 1×10−3 [67] 胆固醇 ChOx/CdTe-MWCNTs@rGONRs CdTe H2O2 1~1×103 0.33 [68] 谷胱甘肽 PLA@rGO/GCE 鲁米诺 聚-L-赖氨酸 1×10−3~100 7.7×10−4 [69] 谷胱甘肽 Pt@MnO2 NF/GR-CS/GCE Ru(bpy)32+ TPA 0.1~10 0.05 [70] 谷胱甘肽 AuNCs/GCE/K2S2O8 Au NCs K2S2O8 1×10−3~10 3.2×10−4 [71] 谷胱甘肽 GCE/rGO/AuNPs/AGS/SiO2/CS AGS K2S2O8 1~1×104 8.03×10−6 [72] 辣椒素 Ru(bpy)32+/辣椒素/裸GCE电极 Ru(bpy)32+ 辣椒素 0.1~100 0.094 [73] 维生素B12 三通道LIG-C-BPE-ECL 鲁米诺 H2O2 5×10−4~1 1.09×10−4 [74] 叶酸 NGQDs/BNQDs/K2S2O8 NGQDs/BNQDs K2S2O8 1×10−5~100 5.1×10−6 [75] 注:1.AuNPs—金纳米粒子;2.CHIT、CS—壳聚糖;3.Thi—亚硫氨酸;4.hemin-GR—血红素-功能化石墨烯;5.rGONRs—还原石墨烯纳米带;6.MnO2NF—二氧化锰纳米花;7.GR—石墨烯;8.AGS—硫化银镓量子点;8.LIG-C-BPE—激光诱导石墨烯-闭合双极电极。 2.2 在检测食品化学污染物中的应用
一般来说,食品中的化学污染物主要包含三个方面,分别是自然环境中存在的污染物、农药和兽药的残留污染物和非法添加剂。邻苯二酚(Catechol)常在合成染料、橡胶制品等被用作反应中间体,是环境中常见的有机污染物[76]。但邻苯二酚有剧毒,属于2B类致癌物,易通过皮肤或黏膜进入人体,即使浓度很低也会对人体的多个系统造成损害[77]。许多方法已被用于检测邻苯二酚,如分光光度法、气相色谱法和高效液相色谱法。但由于这些方法存在所需仪器复杂、污染环境等问题。电致化学发光法不仅可以节省发光试剂,还可以实现ECL仪器的小型化和现场快速检测。Peng等[78]以CdTe量子点为前驱体,通过离子交换法合成碲化银(Ag2Te)量子点,并以K2S2O8为共反应物,形成ECL传感器检测邻苯二酚,检测限为3.1×10−4 μmol/L,检测范围为1~1000 nmol/L。Liu等[79]制备基于Ru(bpy)32+和g-CNQDs的ECL传感器,用于检测茶叶中的邻苯二酚。g-CNQDs具有较大的比表面积和显著的催化作用,还在体系中起到共反应物的作用。研究结果显示,g-CNQDs与Ru(bpy)32+结合后进行电化学性质研究,出现Ru(bpy)32+的氧化峰电流增加而还原峰电流降低的现象,这意味着Ru(bpy)32+会诱导g-CNQDs进行催化氧化,从而使ECL信号增强。该传感器对邻苯二酚的检测限为2.5×10−3 μmol/L,相对标准偏差为4.6和5.1,表明ECL法对邻苯二酚的测定是可靠、有效的。环保型Ag+在替代有毒Cd+在量子点中的应用时,会使其毒性降低,因此未来有望成为ECL生物传感器在食品领域的候选材料。
丙溴磷(Profenofos,PFF)是一种广泛使用的有机磷农药,可有效防治棉花和甘蓝等多种蔬菜作物的害虫[80−81]。然而,PFF残留物会通过抑制乙酰胆碱酯酶活性对水生动物和人类产生毒性,并堆积在各个器官中,从而严重损害神经系统[82]。目前检测有机磷农药的方法主要有电化学法、比色法和荧光法等。在这些方法中,电致化学发光法具有灵敏度高、易于控制、测试时间短等优势,是检测实际样品中有机磷农药必不可少的方法。Shi等[83]使用多壁碳纳米管修饰的金纳米粒子(AuNPs@MWCNTs)来增强鲁米诺-H2O2的ECL传感体系,该ECL传感器可灵敏检测蔬菜中的丙溴磷。AuNPs@MWCNTs用于提供大比表面积以负载更多发光基团并实现ECL信号的首次放大;另合成在体系中起到催化剂作用的Au@AgNPs,加速H2O2的分解并提高电化学发光反应的效率,从而实现了输出ECL信号的二次放大。所提出的ECL传感器对丙溴磷的检测限为1.66×10−5 nmol/L,并具有3.2×10−4~3.2×103 nmol/L的良好检测范围。
腐霉利(Procymidone,PCM)作为一种有机氯农药,主要用于预防蔬菜和水果中的菌核病和灰霉病[84],长期食用PCM残留超标的蔬菜会对人体健康产生不利影响[85]。Zhang等[86]结合分子印迹技术(Molecularly imprinted polymer,MIP)和ECL技术,制备了一种检测腐霉利的MIP-ECL传感器。在该传感器中,采用一步法合成Ru(bpy)32+@ZIF-7复合材料用于ECL信号识别,ZIF-7由于孔隙率高,比表面积大,可以吸附更多的Ru(bpy)32+,并且二者协同作用还能提高ECL信号强度,Ru(bpy)32+@ZIF-7/GCE修饰电极的ECL信号相比裸GCE提升了29.1倍,对腐霉利的检测限为20 μmol/L,检测范围为1.0×10−10~1.0×10−6 mol/L,回收率为89.0%~105.5%。
除上述应用外,ECL传感技术还被用于检测其他食品污染物,如有机磷农药马拉硫磷、甲基对硫磷和毒死蜱[87]、兽药残留物苯乙醇胺A[88]和动物饲料中的非法添加剂溴布特罗[89]。基于电致化学发光传感技术仪器简单、背景信号低、灵敏度高和可快速进行检测的独特优势,如今已成为一种强大的分析技术。ECL传感技术在食品污染物分析中显示出了巨大的潜力,为未来快速检测食品中的污染物提供了新的研究方向。
3. 结论和展望
电致化学发光传感技术是生物技术领域中的一种重要分析检测方法。作为新兴的检测技术之一,电致化学发光传感技术可以很好地解决传统检测方法成本较高、使用条件相对苛刻、稳定性较差等问题。虽然ECL技术已取得巨大进展,但对于食品分析的实际应用而言,已开发的ECL传感器仍存在着一些问题。a.现有用于食品分析的ECL发射物存在环境安全隐患并且其信号强度仍有提高的空间。因此,有必要继续寻找新的高效、无毒的发射物和共反应物来提高ECL生物传感器的灵敏度和生物相容性,使其可以安全的用于食品分析。b.目前,基于新型发射物的检测方法大多只能检测一种分析物。因此,开发可同时检测多种分析物的基于新型发射物ECL传感器可以作为发展ECL技术的新方向。c.目前用于食品分析的发射物和共反应物无法满足构建稳定、经济的ECL生物传感器的需求。应继续研发不同激发电位、不同激发强度和不同激发波长的新型发射物和共反应物。也可引入其他设备或技术,进一步扩展ECL应用范围。综上,随着对电致化学发光传感技术的不断探索,其优势和潜力也不断显现出来。希望ECL技术可以引起更多研究人员的关注,设计和合成更多新型ECL生物传感器,开拓更多应用领域。
-
表 1 ECL传感技术在检测食品营养成分中的应用进展
Table 1 Progress in the application of ECL sensing technology in the detection of nutrient content of food
检测物质 检测体系 发射物 共反应物 线性范围
(μmol/L)检测限
(μmol/L)参考文献 葡萄糖 AuNPs-CdTe QDs-CHIT/GCE CdTe QDs 共基质氧气 10~1×104 5.28 [63] 葡萄糖 Au-Co/GO/GOD 鲁米诺 H2O2 1~100 0.18 [64] 葡萄糖 GOD/AuNPs/PANi 鲁米诺 H2O2 0.1~100 0.05 [65] 胆固醇 MWCNTs-GO-Thi-Au 鲁米诺 H2O2 0.15~828 0.05 [66] 胆固醇 ChOx/hemin-GR/GCE hemin-GR K2S2O8 3.3×10−3~1.5 1×10−3 [67] 胆固醇 ChOx/CdTe-MWCNTs@rGONRs CdTe H2O2 1~1×103 0.33 [68] 谷胱甘肽 PLA@rGO/GCE 鲁米诺 聚-L-赖氨酸 1×10−3~100 7.7×10−4 [69] 谷胱甘肽 Pt@MnO2 NF/GR-CS/GCE Ru(bpy)32+ TPA 0.1~10 0.05 [70] 谷胱甘肽 AuNCs/GCE/K2S2O8 Au NCs K2S2O8 1×10−3~10 3.2×10−4 [71] 谷胱甘肽 GCE/rGO/AuNPs/AGS/SiO2/CS AGS K2S2O8 1~1×104 8.03×10−6 [72] 辣椒素 Ru(bpy)32+/辣椒素/裸GCE电极 Ru(bpy)32+ 辣椒素 0.1~100 0.094 [73] 维生素B12 三通道LIG-C-BPE-ECL 鲁米诺 H2O2 5×10−4~1 1.09×10−4 [74] 叶酸 NGQDs/BNQDs/K2S2O8 NGQDs/BNQDs K2S2O8 1×10−5~100 5.1×10−6 [75] 注:1.AuNPs—金纳米粒子;2.CHIT、CS—壳聚糖;3.Thi—亚硫氨酸;4.hemin-GR—血红素-功能化石墨烯;5.rGONRs—还原石墨烯纳米带;6.MnO2NF—二氧化锰纳米花;7.GR—石墨烯;8.AGS—硫化银镓量子点;8.LIG-C-BPE—激光诱导石墨烯-闭合双极电极。 -
[1] VELUSAMY K, PERIYASAMY S, KUMAR P S, et al. Biosensor for heavy metals detection in wastewater:A review[J]. Food Chemistry Toxicol.,2022,168:113307. doi: 10.1016/j.fct.2022.113307
[2] NONTIPICHET N, KHUMNGERN S, CHOOSANG J, et al. An enzymatic histamine biosensor based on a screen-printed carbon electrode modified with a chitosan-gold nanoparticles composite cryogel on Prussian blue-coated multi-walled carbon nanotubes[J]. Food Chemistry,2021,364:130396. doi: 10.1016/j.foodchem.2021.130396
[3] XIONG X, ZHANG Y, WANG Y F, et al. One-step electrochemiluminescence immunoassay for breast cancer biomarker CA15-3 based on Ru(bpy)62+-coated UiO-66-NH2 metal-organic framework[J]. Sensors and Actuators B:Chemical,2019,297:126812. doi: 10.1016/j.snb.2019.126812
[4] ROBERT E V, EDWIN A C. Electroluminescence in solutions of aromatic hydrocarbons[J]. Journal of the American Chemical Society,1964,86(23):5350−5351. doi: 10.1021/ja01077a073
[5] LI L L, CHEN Y, ZHU J J. Recent advances in electrochemiluminescence analysis[J]. Analytical Chemistry,2017,89(1):358−371. doi: 10.1021/acs.analchem.6b04675
[6] LI Y P, ZHOU H, ZHANG J H, et al. Determination of nitrite in food based on its sensitizing effect on cathodic electrochemiluminescence of conductive PTH-DPP films[J]. Food Chemistry,2022,397:133760. doi: 10.1016/j.foodchem.2022.133760
[7] KATARINA R R, KORVA M, KNAP N, et al. Performance of the rapid high-throughput automated electrochemiluminescence immunoassay targeting total antibodies to the SARS-CoV-2 spike protein receptor binding domain in comparison to the neutralization assay[J]. Journal of Clinical Virology,2021,139:104820. doi: 10.1016/j.jcv.2021.104820
[8] LIU G, WANG P L, GAO H. Visualization analysis of lecithin in drugs based on electrochemiluminescent single gold microbeads[J]. Journal of Pharmaceutical Analysis,2021,11(4):515−522. doi: 10.1016/j.jpha.2021.02.002
[9] HE Y, YANG G M, ZHAO J W, et al. Potentially tunable ratiometric electrochemiluminescence sensing based on conjugated polymer nanoparticle for organophosphorus pesticides detection[J]. Journal of Hazardous Materials,2022,432:128699. doi: 10.1016/j.jhazmat.2022.128699
[10] CHINNADAYYALA S R, PARK J, NGOCLE, H T, et al. Recent advances in microfluidic paper-based electrochemiluminescence analytical devices for point-of-care testing applications[J]. Biosensors and Bioelectronics,2019,126:68−81. doi: 10.1016/j.bios.2018.10.038
[11] LIU D, LI W J, ZHU C X, et al. Recent progress on electrochemical biosensing of aflatoxins:A review[J]. TRAC Trends in Analytical Chemistry,2020,133:115966. doi: 10.1016/j.trac.2020.115966
[12] LIU X C, ZHAO S, TAN L L, et al. Frontier and hot topics in electrochemiluminescence sensing technology based on CiteSpace bibliometric analysis[J]. Biosensors and Bioelectronics,2022,201:113932. doi: 10.1016/j.bios.2021.113932
[13] ZHANG Y, ZHANG R, YANG X L, et al. Recent advances in electrogenerated chemiluminescence biosensing methods for pharmaceuticals[J]. Journal of Pharmaceutical Analysis,2019,9(1):9−19. doi: 10.1016/j.jpha.2018.11.004
[14] BABAMIRI B, BAHARI D, SALIMI A. Highly sensitive bioaffinity electrochemiluminescence sensors:Recent advances and future directions[J]. Biosensors and Bioelectronics,2019,142:111530. doi: 10.1016/j.bios.2019.111530
[15] YANG E L, ZHANG Y J, SHEN Y F. Quantum dots for electrochemiluminescence bioanalysis-A review[J]. Analytica Chimica Acta,2022,1209:339140. doi: 10.1016/j.aca.2021.339140
[16] TANG Y, HU X P, LIU Y W, et al. An antifouling electrochemiluminescence sensor based on mesoporous CuO2@SiO2/luminol nanocomposite and co-reactant of ionic liquid functionalized boron nitride quantum dots for ultrasensitive NSE detection[J]. Biosensors and Bioelectronics,2022,214:114492. doi: 10.1016/j.bios.2022.114492
[17] CHENG S T, XU R, YANG F Z, et al. Novel sandwich-type electrochemiluminescence aptasensor based on luminol functionalized aptamer as signal probe for kanamycin detection[J]. Bioelectrochemistry,2022,147:108174. doi: 10.1016/j.bioelechem.2022.108174
[18] CHENG S T, LIU H M, ZHANG H, et al. Ultrasensitive electrochemiluminescence aptasensor for kanamycin detection based on silver nanoparticle-catalyzed chemiluminescent reaction between luminol and hydrogen peroxide[J]. Sensors and Actuators B:Chemical,2019,304:127367.
[19] JIANG X Y, WANG H J, YUAN R, et al. Sensitive electrochemiluminescence detection for CA15-3 based on immobilizing luminol on dendrimer functionalized ZnO nanorods[J]. Biosensors and Bioelectronics,2015,63:33−38. doi: 10.1016/j.bios.2014.07.009
[20] XIANG W, LUO Y H, YUE Y, et al. Inhibiting effect of molybdenum disulfide nanosheets on cathodic Ru(bpy)32+ electrochemiluminescence in ionic liquids and its sensing application[J]. Journal of Electroanalytical Chemistry,2022,195:116364.
[21] WANG Y F, LI Y X, ZHUANG X M, et al. Ru(bpy)32+ encapsulated cyclodextrin based metal organic framework with improved biocompatibility for sensitive electrochemiluminescence detection of CYFRA21-1 in cell[J]. Biosensors and Bioelectronics,2021,190:113371. doi: 10.1016/j.bios.2021.113371
[22] LIN C Y, HUANG Q Q, HONG X, et al. Electrochemiluminescence aptasensor for vascular endothelial growth factor 165 detection based on Ru(bpy)32+/Au nanoparticles film modified electrode and double signal amplification[J]. Bioelectrochemistry,2022,146:108151. doi: 10.1016/j.bioelechem.2022.108151
[23] ZHANG P, ZHANG Y, XIONG X, et al. A sensitive electrochemiluminescence immunoassay for glycosylated hemoglobin based on Ru(bpy)32+ encapsulated mesoporous polydopamine nanoparticles[J]. Sensors and Actuators B:Chemical,2020,321:128626. doi: 10.1016/j.snb.2020.128626
[24] LI X Y, DU X Z. Surface enhanced electrochemiluminescence of the Ru(bpy)32+/tripropylamine system by Au@SiO2 nanoparticles for highly sensitive and selective detection of dopamine[J]. Microchemical Journal,2022,176:107224. doi: 10.1016/j.microc.2022.107224
[25] KITTE S A, CHAO W, LI S P, et al. Electrogenerated chemiluminescence of tris-(2, 2'-bipyridine) rutenium (Ⅱ) using N-(3-aminopropyl)diethanolamine as coreactant[J]. Analytical and Bioanalytical Chemistry,2016,408(25):7059−7065. doi: 10.1007/s00216-016-9409-z
[26] KITTE S A, BUSHIRA F A, SORETA T R. A new anodic electrochemiluminescence of tris-(2, 2′-bipyridine) ruthenium (II) with 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide as a coreactant for determination of hydrogen peroxide[J]. Microchemical Journal,2022,177:107256. doi: 10.1016/j.microc.2022.107256
[27] YUAN F, HAO K, SHENG S, et al. 2-(Dibutylamino) ethyl acrylate as a highly efficient co-reactant of Ru(bpy)32+ electrochemiluminescence for selective detection of cysteine[J]. Electrochimica Acta,2020,329:135117. doi: 10.1016/j.electacta.2019.135117
[28] FANG D D, ZHENG X Q, YI H, et al. A H2O2-free electrochemiluminescence immunosensor constructed on all-in-one bioprobe comprised of TiO2-B supported fluoro-coumarin silicon phthalocyanine for deoxynivalenol sensing[J]. Sensors and Actuators B:Chemical,2019,283:407−414. doi: 10.1016/j.snb.2018.11.148
[29] LETTIERI M, PALLADINO P, SCARANO S, et al. Copper nanoclusters and their application for innovative fluorescent detection strategies:An overview[J]. Sensors and Actuators Reports,2022,4:100108. doi: 10.1016/j.snr.2022.100108
[30] QUAN Z Y, XUE F, LI H Y, et al. A bioinspired ratiometric fluorescence probe based on cellulose nanocrystal-stabilized gold nanoclusters for live-cell and zebrafish imaging of highly reactive oxygen species[J]. Chemical Engineering Journal,2021,431:133954.
[31] SANTHOSH M, CHINNADAYYALA S R, SINGH N K, et al. Human serum albumin-stabilized gold nanoclusters act as an electron transfer bridge supporting specific electrocatalysis of bilirubin useful for biosensing applications[J]. Bioelectrochemistry,2016,111:7−14. doi: 10.1016/j.bioelechem.2016.04.003
[32] ZHANG H X, ZHUANG T T, WANG L, et al. Efficient Au nanocluster@Ti3C2 heterostructure luminophore combined with Cas12a for electrochemiluminescence detection of miRNA[J]. Sensors and Actuators B:Chemical,2022,370:132428. doi: 10.1016/j.snb.2022.132428
[33] HUANG Z N, LI Z L, XU L Y, et al. Mechanistic insight into a novel ultrasensitive nicotine assay base on high-efficiency quenching of gold nanocluster cathodic electrochemiluminescence[J]. Analytical Chemistry,2020,92:11438−11443. doi: 10.1021/acs.analchem.0c02500
[34] YAO Z X, LIU H M, LIU Y S, et al. FRET-based fluorometry assay for curcumin detecting using PVP-templated CuNCs[J]. Talanta,2021,223:121741. doi: 10.1016/j.talanta.2020.121741
[35] ZHAO M, CHEN A Y, HUANG D, et al. Cu Nanoclusters:Novel electrochemiluminescence emitters for bioanalysis[J]. Analytical Chemistry,2016,88(23):11527−11532. doi: 10.1021/acs.analchem.6b02770
[36] ZHUANG X M, GAO X Q, TIAN C Y, et al. Synthesis of europium (Ⅲ)-doped copper nanoclusters for electrochemiluminescence bioanalysis[J]. Chemical Communications,2020,56:5755−5758. doi: 10.1039/D0CC01573C
[37] WANG L, SHI X H, ZHANG Y F, et al. CdZnSeS quantum dots condensed with ordered mesoporous carbon for high-sensitive electrochemiluminescence detection of hydrogen peroxide in live cells[J]. Electrochimica Acta,2020,362:137107. doi: 10.1016/j.electacta.2020.137107
[38] OU M, TU W G, YIN S M, et al. Amino-assisted anchoring of CsPbBr3 perovskite quantum dots on porous g-C3N4 for enhanced photocatalytic CO2 reduction[J]. Angewandte Chemie International Edition,2018,57(41):13570−13574. doi: 10.1002/anie.201808930
[39] THOMAS P, SOPHIE B, VINCENT L, et al. In vivo imaging of single tumor cells in fast-flowing bloodstream using near infrared quantum dots and time-gated imaging[J]. Acs Nano,2019,13(3):3125−3131. doi: 10.1021/acsnano.8b08463
[40] LI X, XU Y, CHEN Y, et al. Dual enhanced electrochemiluminescence of aminated Au@SiO2/CdS quantum dot superstructures:Electromagnetic field enhancement and chemical enhancement[J]. ACS Applied Materials & Interfaces,2019,11(4):4488−4499.
[41] ZHAO G H, LI X J, ZHAO Y B, et al. Electrochemiluminescence assay of Cu2+ by using one-step electrodeposition synthesized CdS/ZnS quantum dots[J]. Analyst,2017,142:3272−3277. doi: 10.1039/C7AN01014A
[42] YAN F Y, XU M, XU J P, et al. Facile synthesis of high-performance sulfur quantum dots via an effective ethylenediamine-assisted acceleration strategy for fluorescent sensing[J]. Sensors and Actuators B:Chemical,2022,370:132393. doi: 10.1016/j.snb.2022.132393
[43] HU S L, QIN D M, MENG S, et al. Cathodic electrochemiluminescence based on resonance energy transfer between sulfur quantum dots and dopamine quinone for the detection of dopamine[J]. Microchemical Journal,2022,181:107776. doi: 10.1016/j.microc.2022.107776
[44] HAN T T, YANG J L, WANG Y, et al. Boosted anodic electrochemiluminescence from blue-emissive sulfur quantum dots and its bioanalysis of glutathione[J]. Electrochimica Acta,2021,381:138281. doi: 10.1016/j.electacta.2021.138281
[45] JAMPASA S, NGAMROJANANICH N, RENGPIPAT S, et al. Ultrasensitive electrochemiluminescence sensor based on nitrogen-decorated carbon dots for Listeria monocytogenes determination using a screen-printed carbon electrode[J]. Biosensors and Bioelectronics,2021,188(3):113323.
[46] ALI M, ANJUM A S, BIBI A, et al. Gradient heating-induced bi-phase synthesis of carbon quantum dots (CQDs) on graphene-coated carbon cloth for efficient photoelectrocatalysis[J]. Carbon,2022,196:649−662. doi: 10.1016/j.carbon.2022.05.040
[47] KHAN M E, MOHAMMAD A, YOON T. State-of-the-art developments in carbon quantum dots (CQDs):Photo-catalysis, bio-imaging, and bio-sensing applications[J]. Chemosphere,2022,302:134815. doi: 10.1016/j.chemosphere.2022.134815
[48] WANG C J, SUN Q, LI C X, et al. Biocompatible double emission boron nitrogen co-doped carbon quantum dots for selective and sensitive detection of Al3+ and Fe2+[J]. Materials Research Bulletin,2022,155:111970. doi: 10.1016/j.materresbull.2022.111970
[49] YANG E L, NING Z Q, YIN F, et al. Surface plasmon-enhanced electrochemiluminescence of P, N-doped carbon dots for ultrasensitive detection of BRAF gene[J]. Sensors and Actuators B:Chemical,2022,369:132288. doi: 10.1016/j.snb.2022.132288
[50] CHEN A Y, LIANG W B, WANG H J, et al. Anodic electrochemiluminescence of carbon dots promoted by nitrogen doping and application to rapid cancer cell detection[J]. Analytical Chemistry 2020, 92:1379-1385.
[51] LI R R, ZHU Z K, PAN P, et al. One-step synthesis of nitrogen-doped carbon quantum dots for paper-based electrochemiluminescence detection of Cu2+ ions[J]. Microchemical Journal,2022,174:107057. doi: 10.1016/j.microc.2021.107057
[52] FU Z J, GAO W M, YU T, et al. Study of Bi-directional detection for ascorbic acid and sodium nitrite based on Eu-containing luminescent polyoxometalate[J]. Talanta,2019,195:463−471. doi: 10.1016/j.talanta.2018.11.091
[53] PENG B, GUO Y, MA Y J, et al. Smartphone-assisted multiple-mode assay of ascorbic acid using cobalt oxyhydroxide nanoflakes and carbon quantum dots[J]. Microchemical Journal,2022,175:107185. doi: 10.1016/j.microc.2022.107185
[54] WANG H, PU G Q, DEVARAMANI S, et al. Bimodal electrochemiluminescence of G-CNQDs in the presence of double coreactants for ascorbic acid detection[J]. Analytical Chemistry,2018,90(7):4871−4877. doi: 10.1021/acs.analchem.8b00517
[55] SU L Y, MAO J, WANG S, et al. A bimodal electrochemiluminescence method based on dual-enhancement Ru (bpy)32+/CQDs/AA system combined with magnetic field enhanced solid-phase microextraction for the direct determination of ascorbic acid[J]. Journal of Electroanalytical Chemistry,2020,873:114376. doi: 10.1016/j.jelechem.2020.114376
[56] WANG D, LIANG Y, SU Y, et al. Sensitivity enhancement of cloth-based closed bipolar electrochemiluminescence glucose sensor via electrode decoration with chitosan/multi-walled carbon nanotubes/graphene quantum dots-gold nanoparticles[J]. Biosensors and Bioelectronics,2019,130:55−64. doi: 10.1016/j.bios.2019.01.027
[57] SU L, XIN C X, YANG J T, et al. A polysaccharide from Inonotus obliquus ameliorates intestinal barrier dysfunction in mice with type 2 diabetes mellitus[J]. International Journal of Biological Macromolecules,2022,214:312−323. doi: 10.1016/j.ijbiomac.2022.06.071
[58] LU J, WANG Y, SHAN X, et al. Synergistic enhancement effects of cobalt oxide doped silver oxide and porphyrin zinc on an electrochemiluminescence sensor for detection of glucose[J]. Microchemical Journal,2021,170:106716. doi: 10.1016/j.microc.2021.106716
[59] ZHANG X, LI T, WU K X, et al. High sensitivity electrochemiluminescence sensor based on the synergy of ZIF-7 and CdTe for determination of glucose[J]. Microchemical Journal,2022,177:107254. doi: 10.1016/j.microc.2022.107254
[60] ZHANG Y, WANG Y N, SUN X T, et al. Boron nitride nanosheet/CuS nanocomposites as mimetic peroxidase for sensitive colorimetric detection of cholesterol[J]. Sensors and Actuators B:Chemical,2017,246:118−126. doi: 10.1016/j.snb.2017.02.059
[61] NANTAPHOL S, CHAILAPAKUL O, WEENA S. Sensitive and selective electrochemical sensor using silver nanoparticles modified glassy carbon electrode for determination of cholesterol in bovine serum[J]. Sensors and Actuators B:Chemical,2015,207:193−198. doi: 10.1016/j.snb.2014.10.041
[62] YANG D P, GUO W W, CAI Z F, et al. Highly sensitive electrochemiluminescence biosensor for cholesterol detection based on AgNPs-BSA-MnO2 nanosheets with superior biocompatibility and synergistic catalytic activity[J]. Sensors and Actuators B:Chemical,2018,260:642−649. doi: 10.1016/j.snb.2018.01.096
[63] LIU L L, MA Q, LI Y, et al. A novel signal-off electrochemiluminescence biosensor for the determination of glucose based on double nanoparticles[J]. Biosensors and Bioelectronics,2015,63:519−524. doi: 10.1016/j.bios.2014.07.087
[64] QIAO X, WEI X X, HAO Y Q, et al. Alloy-structured Au-Co bimetallic nanoparticles-decorated graphene oxide as an efficient electrochemiluminescence sensing platform for sensitive detection of glucose in human serum[J]. Materials Letters,2019,236:476−479. doi: 10.1016/j.matlet.2018.10.165
[65] LOU F M, LU Z S, HU F X, et al. A 3D bio-platform constructed by glucose oxidase adsorbed on Au nanoparticles assembled polyaniline nanowires to sensitively detect glucose by electrochemiluminescence[J]. Journal of Electroanalytical Chemistry,2017,787:125−131. doi: 10.1016/j.jelechem.2017.01.048
[66] WU X P, CHAI Y Q, RUO Y, et al. Synthesis of multiwall carbon nanotubes-graphene oxide-thionine-Au nanocomposites for electrochemiluminescence detection of cholesterol[J]. Electrochimica Acta,2014,129:441−449. doi: 10.1016/j.electacta.2014.02.103
[67] ZHANG J J, WANG W T, CHEN S H, et al. Bi-pseudoenzyme synergetic catalysis to generate a coreactant of peroxydisulfate for an ultrasensitive electrochemiluminescence-based cholesterol biosensor[J]. Biosensors and Bioelectronics,2014,57:71−76. doi: 10.1016/j.bios.2014.01.046
[68] HUAN J, LIU Q, FEI A R, et al. Amplified solid-state electrochemiluminescence detection of cholesterol in near-infrared range based on CdTe quantum dots decorated multiwalled carbon nanotubes@reduced graphene oxide nanoribbons[J]. Biosensors and Bioelectronics,2015,73:221−227. doi: 10.1016/j.bios.2015.06.004
[69] WANG C X, CHEN L M, WANG P J, et al. A novel ultrasensitive electrochemiluminescence biosensor for glutathione detection based on poly-L-lysine as co-reactant and graphene-based poly (luminol/aniline) as nanoprobes[J]. Biosensors and Bioelectronics,2019,113:154−159.
[70] WANG H J, ZHANG R, ZHUO Y, et al. Sensitive electrochemiluminescence biosensor for glutathione using MnO2 nanoflower as novel co-reaction accelerator for Ru complex/tripropylamine system[J]. Analytica Chimica Acta,2021,1188:339181. doi: 10.1016/j.aca.2021.339181
[71] PENG H P, JIAN M L, HUANG Z N, et al. Facile electrochemiluminescence sensing platform based on high-quantum-yield gold nanocluster probe for ultrasensitive glutathione detection[J]. Biosensors and Bioelectronics,2018,105:71−76. doi: 10.1016/j.bios.2018.01.021
[72] ZHANG J Z, LIU X, LIU H X, et al. Construction of electrochemiluminescence biosensor for monitoring of glutathione released by living cancer cells[J]. Analytica Chimica Acta,2022,1226:340251. doi: 10.1016/j.aca.2022.340251
[73] LEE S J, LEE W Y. Highly sensitive determination of capsaicin with tris-(2, 2′-bipyridyl) ruthenium (Ⅱ) electrogenerated chemiluminescence[J]. Journal of Electroanalytical Chemistry,2022,910:116169. doi: 10.1016/j.jelechem.2022.116169
[74] BHAIYYA M, PATTNAIK P K, GOEL S. Simultaneous detection of Vitamin B12 and Vitamin C from real samples using miniaturized laser-induced graphene based electrochemiluminescence device with closed bipolar electrode[J]. Sensors and Actuators A:Physical,2021,331:112831. doi: 10.1016/j.sna.2021.112831
[75] LI M S, WANG C X, CHEN L M, et al. A novel electrochemiluminescence sensor based on resonance energy transfer system between nitrogen doped graphene quantum dots and boron nitride quantum dots for sensitive detection of folic acid[J]. Analytica Chimica Acta,2019,1090:57−63. doi: 10.1016/j.aca.2019.09.018
[76] XU Y Q, YU Y J, XUE S, et al. Innovative electrochemical sensor based on graphene oxide aerogel wrapped copper centered metal-organic framework to detect catechol[J]. Journal of Electroanalytical Chemistry,2021,899:115686. doi: 10.1016/j.jelechem.2021.115686
[77] HUANG Y H, CHEN J H, SUN X, et al. One-pot hydrothermal synthesis carbon nanocages-reduced graphene oxide composites for simultaneous electrochemical detection of catechol and hydroquinone[J]. Sensors and Actuators B:Chemical,2015,212:165−173. doi: 10.1016/j.snb.2015.02.013
[78] PENG Y, DONG Y P, AI M M, et al. Electrogenerated chemiluminescence of Ag2Te quantum dots and its application in sensitive detection of catechol[J]. Journal of Luminescence,2017,190:221−227. doi: 10.1016/j.jlumin.2017.05.051
[79] LIU Z M, WU H, GE X G, et al. A sensitive method to monitor catechol by using graphitic carbon nitride quantum dots as coreactants in Ru(bpy)32+-based electrochemiluminescent system[J]. Journal of Electroanalytical Chemistry,2020,860:113910. doi: 10.1016/j.jelechem.2020.113910
[80] CHATTERJEE A, BHATTACHARYA R, CHATTERJEE S, et al. Acute toxicity of organophosphate pesticide profenofos, pyrethroid pesticide λ cyhalothrin and biopesticide azadirachtin and their sublethal effects on growth and oxidative stress enzymes in benthic oligochaete worm, Tubifex tubifex[J]. Comparative Biochemistry and Physiology Part C:Toxicology & Pharmacology,2020,242:108943.
[81] HAN J, YU Y Y, WANG G J, et al. Ultrasensitive electrochemiluminescence aptasensor based on ABEI reduced silver nanoparticles for the detection of profenofos[J]. Science of the Total Environment,2022,844:157184. doi: 10.1016/j.scitotenv.2022.157184
[82] LIU F Y, ZHAO S L, LAI X D, et al. Colorimetric and fluorescent probes for the rapid detection of profenofos in farmland system[J]. Food Chemistry,2022,393:133321. doi: 10.1016/j.foodchem.2022.133321
[83] SHI X J, LIU H M, ZHANG M, et al. Ultrasensitive electrochemiluminescence aptasensor based on AuNPs@MWCNTs and Au@AgNPs for dection of profenofos residues[J]. Sensors and Actuators B:Chemical,2021,348:130663. doi: 10.1016/j.snb.2021.130663
[84] WANG W Z, GAO Z Q, QIAO C X, et al. Residue analysis and removal of procymidone in cucumber after field application[J]. Food Control,2021,128:108168. doi: 10.1016/j.foodcont.2021.108168
[85] DI S S, WANG Y H, XU H, et al. Comparison the dissipation behaviors and exposure risk of carbendazim and procymidone in greenhouse strawberries under different application method:Individual and joint applications[J]. Food Chemistry,2021,354:129502. doi: 10.1016/j.foodchem.2021.129502
[86] ZHANG X, TIAN L, SUN Z, et al. A molecule-imprinted electrochemiluminescence sensor based on self-accelerated Ru(bpy)32+@ZIF-7 for ultra-sensitive detection of procymidone[J]. Food Chemistry,2022,391:133235. doi: 10.1016/j.foodchem.2022.133235
[87] MIAO S S, WU M S, MA L Y, et al. Electrochemiluminescence biosensor for determination of organophosphorous pesticides based on bimetallic Pt-Au/multi-walled carbon nanotubes modified electrode[J]. Talanta,2016,158:142−151. doi: 10.1016/j.talanta.2016.05.030
[88] TANG Q H, CAI F D, DENG A P, et al. Ultrasensitive competitive electrochemiluminescence immunoassay for the β-adrenergic agonist phenylethanolamine A using quantum dots and enzymatic amplification[J]. Microchimica Acta,2015,182:139−147. doi: 10.1007/s00604-014-1292-8
[89] HU L Y, DONG T T, ZHAO K, et al. Ultrasensitive electrochemiluminescent brombuterol immunoassay by applying a multiple signal amplification strategy based on a PAMAM-gold nanoparticle conjugate as the bioprobe and Ag@Au core shell nanoparticles as a substrate[J]. Microchimica Acta,2017,184:3415−3423. doi: 10.1007/s00604-017-2359-0
-
期刊类型引用(3)
1. 张恩仁,何理琴,姜振锟,龙腾,操晓亮,苏明亮,周康熙,齐凌峰. 大孔吸附树脂D301促进枯草芽孢杆菌产出纤维素酶的研究. 福建轻纺. 2025(04): 22-26 .  百度学术
百度学术
2. 温冬灼,张智,张晓彤. 解淀粉芽孢杆菌BA-2原生质体的制备及纤维素酶高产突变菌株筛选. 食品安全质量检测学报. 2024(14): 139-147 .  百度学术
百度学术
3. 王文凡,刘银秀,谢晓杰,杨健,赵卓群,王敏,郑华宝. 牛粪堆肥中纤维素高效降解菌的筛选与产酶条件优化. 微生物学通报. 2023(11): 4796-4811 .  百度学术
百度学术
其他类型引用(3)






 下载:
下载:
 下载:
下载:
