Morphological Characterization and Identification of Five Pathogenic Fungi from Apricot Fruit during Postharvest Storage
-
摘要: 杏采后易受病原菌侵染,本研究通过对采后贮藏期间‘金太阳杏’和‘胭脂杏’果实发病部位的病原菌进行分离、纯化,并将纯化后的菌株回接到相应品种的健康果实上,出现与分离发病果实相同的病症,经形态学特征分析、真菌rDNA内转录间隔区(internal transcribed spacer,ITS)的序列分析并构建系统发育树,从杏果实共分离到5株病原菌。从‘金太阳杏’分离到3株病原真菌,其中325#为甜樱间座壳菌(Diaporthe eres),327#为葡萄座腔菌(Botryosphaeria dothidea),328#为出芽短梗霉(Aureobasidium pullulans),从‘胭脂杏’果实分离得到两株病原真菌,分别为326#椭圆葡萄孢菌(Botrytis elliptica)和331#灰葡萄孢菌(Botrytis cinerea)。 D. eres、B. dothidea、A. pullulans 和 B. elliptica 为杏果实上未见文献报道的病原真菌,而 B. cinerea 未有发现侵染‘胭脂杏’的报道,研究结果旨在为杏果实的生物防治措施提供一定的参考。
-
关键词:
- 杏果实 /
- 采后病害 /
- 病原真菌 /
- ITS区rDNA序列分析
Abstract: Apricot fruit is highly susceptible to infection by pathogenic fungi. In this study, the pathogenic fungi in the diseased parts of the fruits of ‘Golden Sun’ and ‘Red almond’ apricot (Prunus armeniaca L.) during postharvest storage were isolated and purified, and the purified strains were inoculated back to the healthy fruits of corresponding varieties, showing the same disease symptoms as the diseased fruits. Five fungi isolates were identified based on morphological characteristics and internal transcribed spacer (ITS) sequence analysis combined with construction of phylogenetic tree. Three isolates from ‘Golden Sun’ apricot were identified as Diaporthe eres (325#), Botryosphaeria dothidea (327#) and Aureobasidium pullulans (328#). Two isolates from ‘Red almond’ apricot were identified as Botrytis elliptica (326#) and Botrytis cinerea (331#). D. eres, B. dothidea, A. pullulans and B. elliptica were pathogenic fungi that had not been reported in the literature on apricot fruits, whereas B. cinerea had not been reported on the infection of ‘Red almond’ apricot. The present results provided references for the biological control measures of apricot fruits.-
Keywords:
- apricot fruit /
- postharvest diseases /
- pathogenic fungi /
- rDNA ITS sequence analysis
-
杏(Prunus armeniaca L.)为蔷薇科、李属植物,果实多汁肉厚,酸甜可口,营养价值丰富。杏果实中含有多种维生素、矿质元素以及丰富的β-胡萝卜素、黄酮醇、黄烷醇、总酚和三萜酸等物质,尤其是胡萝卜素的含量高于其他水果,具有较高的抗氧化活性以及防癌、抗癌功效[1-2]。但杏果实时令性较强,采后果实易软化,不耐贮藏、运输困难。杏果实水分、糖分含量高,果皮薄易受到器械损伤,导致病原菌侵染而容易发生腐烂变质,发病率高达50%,造成严重的经济损失,限制了杏果实产业的发展[3],因此,造成杏果实贮藏期间发病的病原菌种类是亟需研究的问题。
杏果实采后侵染性病害主要是由细菌和真菌引起,其中链格孢属真菌引起的黑斑病,青霉属引起的青霉病以及根霉属引起的软腐病报道较多[4-5]。有研究从采后贮藏的杏果实中分离得到链格孢菌,并对其碳源代谢指纹图谱分析发现分离到的链格孢菌可以利用的碳源在88种以上,表明其对碳源适应性较强,这可能是链格孢菌侵染范围广的原因[6];韩盛等[7]发现黑根霉、灰葡萄孢霉、链格孢霉、青霉和粉红聚端孢霉为杏采后病害主要病原菌;程元等[8]在杏果实病斑处分离获得嗜果门座孢菌;也有研究者从自然发病的杏果实中分离得到交孢链格孢、匍枝根霉和皮落青霉三种病原真菌[9]。此外,Wu等[10]研究发现尖孢镰刀菌可导致杏果实腐烂。目前,关于杏果实采后病害的防治措施主要针对链格孢属、根霉属、青霉属和曲霉属真菌[11-13],进一步明确杏果实采后病害的病原种类,可为筛选杏采后病害的有效防治措施提供理论基础。
真菌rDNA的内转录间隔区(internal transcribed spacer,ITS)具有一定的保守性,种间差异明显,通过PCR技术扩增真菌ITS区目的片段进行序列分析,可有效地将真菌进行分类鉴定[14]。有研究从产生根腐病的人参中分离得到8株病菌真菌,通过PCR扩增鉴定其种属水平[15],Solairaj 等[16]对采后腐烂葡萄进行病原真菌分离,通过形态学观察结合PCR扩增的方法鉴定出7株葡萄采后病原真菌,因此,基于PCR扩增真菌ITS区进行序列分析广泛应用于果蔬采后致病病原真菌的鉴定[17-18] 。本研究拟从采后自然发病的‘金太阳杏’和‘胭脂杏’两个品种的杏果实上分离得到丝状病原真菌,通过形态学特征和真菌rDNA ITS区序列分析,构建系统进化树,对杏果实的致病病原真菌进行鉴定,以发现更多引起杏果实采后侵染性病害的病原真菌种类,为杏果实的病害研究及开展有效的生物防治工作提供参考。
1. 材料与方法
1.1 材料与仪器
金太阳杏、胭脂杏果实 来源于北京新发地批发市场,产地为陕西省,采收期为6月。选择在室温下贮藏至发病的果实进行研究;DNA分子质量标准2×Taq PCR Master Mix、Marker Ⅶ 天根生化试剂公司;通用引物ITS-4、ITS-5 Invitrogen公司;氢氧化钠(NaOH)、三羟甲基氨基甲烷(Tris)、乙二胺四乙酸钠(EDTA)、十二烷基磺酸钠(SDS) Amersco公司;乙醇、盐酸 分析纯,北京化工厂;琼脂粉 分析纯,Biottopped公司;葡萄糖 分析纯,国药集团化学试剂有限公司;麦芽浸粉 北京奥博星生物技术有限公司。
马铃薯培养基(PDA):称取削皮后的马铃薯200 g,切片煮沸30 min后,过滤取汁,加入琼脂粉18 g、葡萄糖20 g,加水补足至1 L,121 ℃灭菌20 min;麦芽浸粉培养基(ME):麦芽浸粉20 g、琼脂粉18 g,用1 mol/L的NaOH溶液调节pH至5.5±0.2,加水补足至1 L,121 ℃灭菌20 min。
Forma ClassⅡA2生物安全柜 美国Thermo公司;Axio Image A1显微镜 德国Zeiss公司;MG96+PCR仪 杭州朗基科学仪器有限公司;CJ100净化试验台 北京赛伯乐实验仪器有限公司;MJX-250Ⅱ霉菌培养箱 广东省医疗器械厂。
1.2 实验方法
1.2.1 菌种的分离、纯化与回接
分离:取杏果实的发病部位和健康交界处组织(5 mm×5 mm)于PDA固体培养基平板上,25 ℃培养1~3 d。纯化:将分离菌边缘菌块,分别三点转接到PDA和ME固体培养基平板上,于25 ℃培养14 d,观察7 d和14 d菌落形态特征。回接:挑选健康无损伤的杏果实,用体积分数为75%的乙醇进行表面消毒,无菌接种针刺孔,将纯化后的菌块回接到相应品种杏果实伤口处,观察果实接种处是否出现与分离果实相同病症,并从该病害部位再次分离病原菌,比较分离到的病原菌与接种病原菌的菌落形态[19]。
1.2.2 菌落形态学观察
PDA培养基上的菌落经纯化后,取菌落外沿菌块,分别三点转接到PDA和ME培养基平板上,于25 ℃条件下培养14 d,观察菌落形态。采用乳酸石炭酸棉蓝染色液法,置于显微镜下观察菌落产生分生孢子及分生孢子梗的形状、色泽和分生孢子隔膜等性状[20-21]。
1.2.3 基因组DNA的提取及ITS区序列分析
采用改良后十二烷基硫酸钠-氯化苄法提取待鉴定菌株总DNA[18]。将菌体用液氮研磨至粉状,取0.1 g菌粉加入500 μL氯化苄提取液(100 mmol/L Tris-HCl,pH9.0;40 mmol/L EDTA,pH8.0),充分振荡混合后,加入100 μL SDS(10%)和300 μL氯化苄提取液,剧烈振荡,50 ℃下保温1 h后,12000 r/min,4 ℃离心10 min弃上清。加入300 μL NaAc(3 mol/L,pH5.2)混匀,冰浴15 min,12000 r/min,4 ℃离心10 min收集上清液。加入等体积300 μL无水乙醇室温沉淀20 min,12000 r/min,4 ℃离心10 min,沉淀用70%乙醇清洗一次,挥发后加入30 μL TE缓冲液(10 mmol/L Tris-HCl,1 mmol/L EDTA,pH8.0)溶解DNA。
以真菌rDNA ITS区通用引物ITS-4(5’-TCCTCCGCTTATTGATATGC-3’)和ITS-5(5’-GGAAGTAAAAGTCGTAACAAGG-3’)为PCR扩增引物。PCR体系:10 μL 2×Taq PCR Master Mix,0.8 μL ITS-4,0.8 μL ITS-5,0.5 μL DNA模板和7.9 μL ddH2O;PCR扩增条件:94 ℃预变性3 min,94 ℃变性30 s,55 ℃退火30 s,72 ℃延伸1 min,72 ℃再延伸5 min,35个循环。PCR产物由北京六合华大基因科技股份有限公司华大基因进行脱盐、纯化和双向测序。
1.3 数据处理
测序结果在NCBI网站(https://www.ncbi.nlm.nih.gov/)进行同源序列比对(blast),并且用MEGA6软件构建系统进化树。
2. 结果与分析
2.1 菌株的分离与回接结果分析
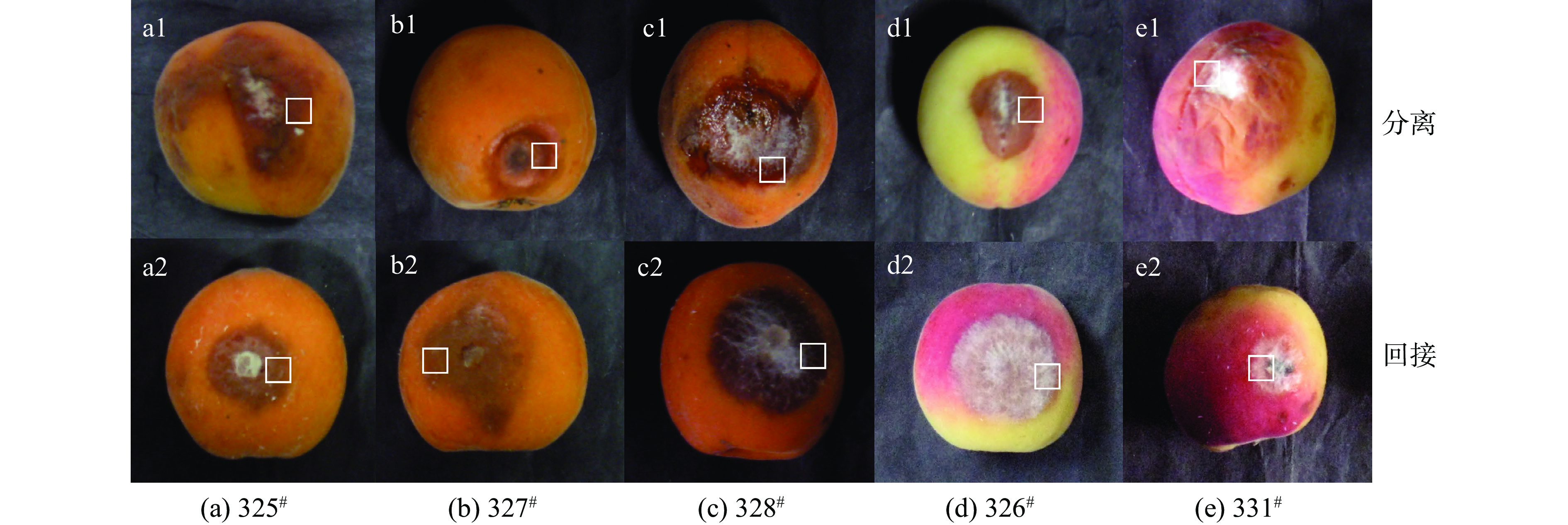
如图1所示,从采后贮藏自然发病的杏果实发病部位进行取样,在‘金太阳杏’果实上分离得到丝状真菌325#、327#、328#(图1 a1~c1),在‘胭脂杏’果实上分离得到326#、331#病原菌(图1 d1~e1),将分离到的病原菌回接到健康无伤的相应品种杏果实上,导致杏果实发病的病斑特征如图1 a2~e2,在接种部位均出现同样的病症,并能从该病害部位再次分离得到相应病原菌,因此,上述5株丝状真菌确定是杏果实致病菌。
2.2 病原菌的形态观察结果
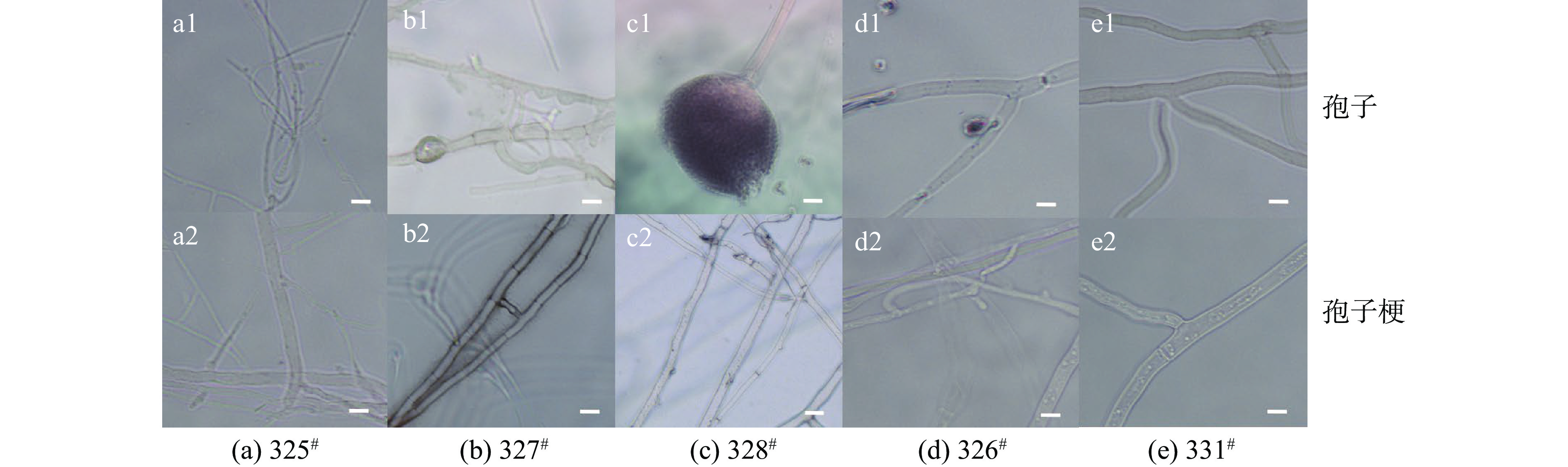
将分离到的5株丝状病原真菌,经纯化后分别三点转接至PDA和ME固体培养基上,并于25 ℃条件下培养14 d,菌落形态如图2。图2结果显示,菌株325#在PDA培养基和ME培养基上均生长旺盛,菌落质地致密,培养7 d后,菌落在PDA培养基上覆盖整个平板,菌丝呈白色(图2 a1),14 d后菌落颜色加深(图2 a2);在ME培养基上培养7 d菌丝呈浅黄褐色,具边缘不整齐的轮纹(图2 a3),14 d后菌落颜色不均,白色小点增多(图2 a4)。显微观察可以看到,325#在PDA培养基上未产孢(图3 a1),菌丝着色较浅、分枝多且内部隔膜观察明显 (图3 a2)。
菌株327#在PDA和ME培养基上长势相近,菌落形态较大,在PDA平板培养7 d时,菌丝呈灰绿色,菌丝质地紧密(图2 b1),14 d后菌落颜色变为深褐色 (图2 b2);在ME平板培养7 d时,与PDA板形态类似(图2 b3),培养至14 d,菌落颜色变为灰褐色 (图2 b4)。菌株327#表面粗糙(图3 b1),菌丝着色较深,分枝少且内部隔膜观察明显(图3 b2),分生孢子单孢,呈灰褐色。
菌株328#在PDA培养基上培养7 d时,菌落已覆盖整个平板,质地松散且菌丝较长,外观干燥,菌丝呈黄褐色,菌丝上密布黑色孢子(图2 c1),培养14 d后菌落外观无明显变化,菌丝颜色均加深(图2 c2);在ME平板上形态与PDA平板相似,生长旺盛,培养7 d后产生黑色孢子,14 d后菌丝颜色加深(图2 c3)和(图2 c4)。菌株328#孢子梗顶端膨大形成顶囊,分生孢子卵圆形,密生于顶囊之上,表面干燥,常具黑色花纹(图3 c1),菌丝着色较浅,具隔或不具隔(图3 c2)。
菌株326#生长速度较快,PDA平板培养7 d,菌丝呈白色,质地松散、成簇生长(图2 d1),14 d后菌落仍呈白色,菌丝无明显变化(图2 d2);培养7 d时,ME板上菌丝较PDA板稀疏,呈白色,继续培养至14 d,菌落颜色和菌丝无明显变化(图2 d3)和(图2 d4)。通过显微观察,326#未产生孢子,菌丝着色较浅,分枝多且内部隔膜观察明显(图3 d1)和(图3 d2)。
菌株331#在PDA培养基培养7 d时,菌落已覆盖平板,菌丝呈现浅灰褐色,质地紧密,外观干燥(图2 e1),培养至14 d,菌落颜色加深,形成菌球(图2 e2);在ME培养基上,7 d时覆盖平板,菌丝呈现灰褐色(图2 e3),14 d后,菌落颜色加深,ME板相对于PDA板形成菌球较多 (图2 e4),其显微形态与菌株326#相似。
参考《真菌鉴定手册》对相关菌株的菌落及形态描述[20],菌株325#为间座壳属真菌,327#为葡萄座腔属真菌,328#为短柄霉属真菌,326#和331#为葡萄孢属真菌。
2.3 ITS区序列分析及系统进化树构建
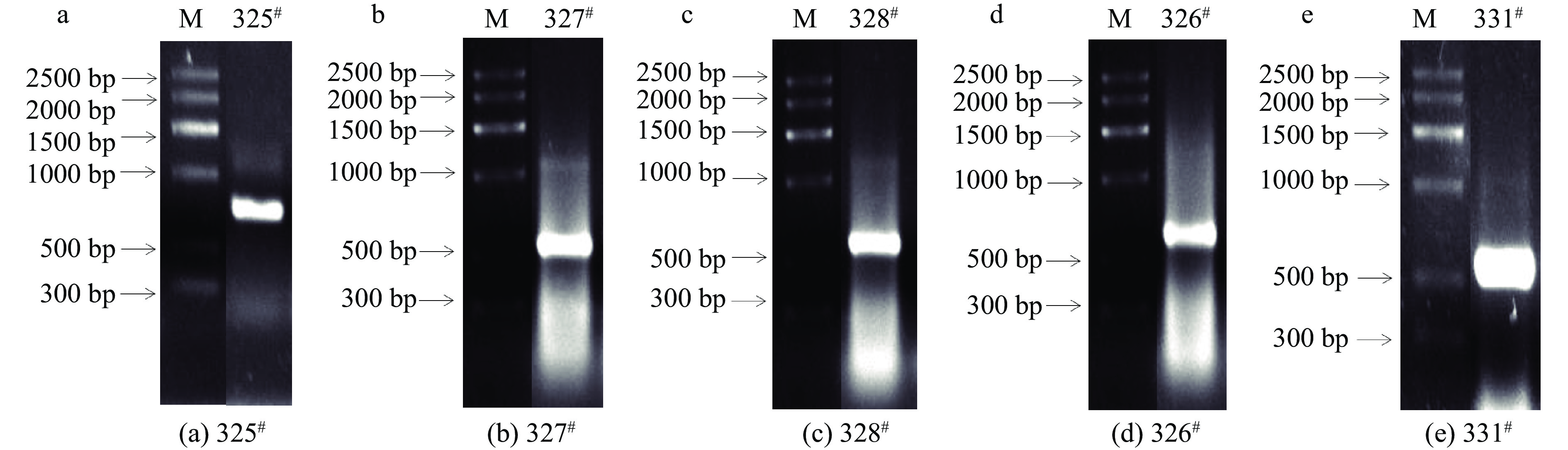
采用SDS-氯化苄方法提取总DNA,以基因组DNA为模板,ITS-4和ITS-5为引物,经PCR扩增得到ITS区目的片段,PCR产物电泳检测结果如图4,电泳条带清晰。图4结果表明,325#菌株(图4a)、327#菌株(图4b)、328#菌株(图4c)、326#菌株(图4d)和331#菌株(图4e)目的条带均在600 bp左右。
将5株杏果实病原真菌的rDNA ITS测序结果在网站NCBI上进行比对,进行同源性分析并且构建系统进化树,如图5所示,5株菌分别属于4个不同的属。由图5结果可知,菌株325#与甜樱间座壳菌(Diaporthe eres)在同一分枝上,亲缘距离小于0.01,该结果置信度达100%(图5a)。菌株327#与葡萄座腔菌(Botryosphaeria dothidea)在同一分枝上,亲缘距离小于0.02,置信度达95%(图5b)。菌株328#与出芽短梗霉(Aureobasidium pullulans)在同一分枝上,亲缘距离小于0.05,置信度达100%(图5c)。菌株326#与331#经比对均为葡萄孢属,菌株326#与椭圆葡萄孢(Botrytis elliptica)在同一分枝上,亲缘距离小于0.05,置信度达100%(图5d),菌株331#与灰葡萄孢(Botrytis cinerea)在同一分枝上,亲缘距离为零,置信度达97%(图5d)。因此,分离得到的5株杏果实病原真菌经ITS区鉴定:325#为甜樱间座壳菌(D. eres)、327#为葡萄座腔菌(B. dothidea)、328#为出芽短梗霉(A. pullulans)、326#为椭圆葡萄孢(B. elliptica)以及331#为灰葡萄孢(B. cinerea)。
3. 讨论
杏果实属于呼吸跃变型果实,采收期多为高温季节,易受微生物侵染而腐烂变质,据报道,引起杏果实采后病害的病原菌主要涉及到链格孢属、青霉属、根霉属以及葡萄孢属病原真菌等。本研究分离到1株间座壳属真菌,该属真菌分布广泛,能够引起植物发生茎溃疡病、枝枯病、果实腐烂、叶斑病以及叶片坏死等多种植物病害[22]。研究发现,甜樱间座壳菌(D. eres)可导致苹果[23]、黄桃[24]、梨[25]和葡萄腐烂[26]以及猕猴桃黑斑病[27],有研究从产生芽枯症状的蓝莓枝条分离得到甜樱间座壳菌(LNSY003:D. eres)[28]。本文从自然发病的“金太阳”杏果实上分离得到了一株甜樱间座壳菌(D. eres),但目前在杏果实采后贮藏过程中未见该菌引起病害的报道,为该菌的生物学特性及对不同果实侵染机制的研究提供新思路。
葡萄座腔菌(B. dothidea)是葡萄座腔属中常见的种类,能够侵染多种果实,引起果实腐烂。有研究者发现葡萄座腔菌(B. dothidea)是梨轮纹病与干腐病的常见病菌真菌[29],可导致芒果和猕猴桃采后病害的发生[30-31],也有研究从产生采后病害的桃果实中分离得到了葡萄座腔菌(030:B. dothidea)[32]。本研究分离得到1株葡萄座腔菌(B. dothidea)可导致杏果实采后病害的发生,目前未见该菌侵染杏果实的报道。短柄霉属病原菌也可引起果实采后病害,姚婷等[33]从采后自然发病的枣果实上分离得到1株产酶短梗霉(230#:Aureobasidium proteae),导致枣果实采后发病。本研究分离得到的出芽短梗霉(Aureobasidium pullulans)有导致葡萄和枣果实腐烂的报道[34-35],但未见有该菌引起杏果实采后病害的报道。
此外,本研究在采后贮藏期间自然发病的胭脂杏果实上分离到了两株葡萄孢属真菌。据报道,椭圆葡萄孢(Botrytis elliptica)是百合的主要致病菌[36],也可引起桃和蓝莓采后病害[19, 37],而由椭圆葡萄孢(Botrytis elliptica)引起的杏果实采后病害未见有文献报道。灰葡萄孢菌是目前报道较多的葡萄孢属真菌,是果蔬采后贮藏期间常见的致病病原真菌,导致葡萄[17]、草莓和樱桃[37]等果实灰霉病的发生,也有在伊犁李光杏果实上发现灰葡萄孢菌病原菌,但未见有侵染胭脂杏果实的报道。葡萄孢属真菌寄主范围广、防治困难,本研究在‘胭脂杏’果实上分离到的两株葡萄孢属真菌,为该属真菌对宿主营养物质的需求及病害防治研究提供参考。
4. 结论
从采后贮藏期间的‘金太阳杏’分离得到三株病原真菌(325#、327#和328#),从‘胭脂杏’果实上分离得到两株病原真菌(326#和331#),结合形态学特征及ITS区序列分析鉴定325#为甜樱间座壳菌(D. eres)、327#为葡萄座腔菌(B. dothidea)、328#为出芽短梗霉(A. pullulans)、326#为椭圆葡萄孢(B. elliptica)以及331#为灰葡萄孢(B. cinerea)。D. eres、B. dothidea、A. pullulans 以及B. elliptica均未有侵染杏果实导致其产生采后病害的报道,此外,未有 B. cinerea导致‘胭脂杏’果实采后病害的报道,因此,本文在产生采后侵染性病害的‘金太阳杏’和‘胭脂杏’果实上发现了未见报道的5株丝状病原真菌,丰富了杏果实采后病原真菌的种类,为杏果实保鲜靶标菌的选择、筛选杏果实采后病害的广谱抑菌剂以及开展有效的生物防治工作提供参考。
-
-
[1] 夏乐晗, 陈玉玲, 冯义彬, 等. 不同品种杏果实发育过程中类黄酮、总酚和三萜酸含量及抗氧化性研究[J]. 果树学报,2016,33(4):425−435. [XIA L H, CHEN Y L, FENG Y B, et al. Changes in flavonoids, total phenolics, triterpenoidic acids and antioxidant capacity during fruit development of different cultivars of apricot[J]. Journal of Fruit Science,2016,33(4):425−435. doi: 10.13925/j.cnki.gsxb.20150395 XIA L H, CHEN Y L, FENG Y B, et al. Changes in flavonoids, total phenolics, triterpenoidic acids and antioxidant capacity during fruit development of different cultivars of apricot[J]. Journal of Fruit Science, 2016, 33(4): 425-435. doi: 10.13925/j.cnki.gsxb.20150395
[2] 张俊环, 杨丽, 孙浩元, 等. 不同品种杏果实发育进程中多酚与类黄酮物质含量的变化[J]. 北方园艺,2012,24:1−5. [ZHANG J H, YANG L, SUN H Y, et al. Changes on the total phenols and flavonoids in apricot peel and pulp of different cultivars during fruit development[J]. Northern Horticulture,2012,24:1−5. ZHANG J H, YANG L, SUN H Y, et al. Changes on the total phenols and flavonoids in apricot peel and pulp of different cultivars during fruit development[J]. Northern Horticulture, 2012, 24: 1-5
[3] 张瑾, 徐秉良, 梁巧兰, 等. 杏采后病害病原菌鉴定及室内药剂筛选[J]. 植物保护,2011,37(5):118−123. [ZHANG J, XU B L, LIANG Q L, et al. Identification of the pathogens of postharvest apricot fruit diseases and indoor screening of fungicides[J]. Plant Protection,2011,37(5):118−123. ZHANG J, XU B L, LIANG Q L, et al. Identification of the pathogens of postharvest apricot fruit diseases and indoor screening of fungicides[J]. Plant Protection, 2011, 37(5): 118-123.
[4] 阿衣木古丽·艾赛提. 杏采后病原菌鉴定及采前壳寡糖和水杨酸处理对杏品质和病害的影响[D]. 乌鲁木齐: 新疆农业大学, 2014: 1−30. AYIMUGULI A S T. Identification of the pathogenic fungus from post-harvest apricot fruit and effect of preharvest oligochitosan, salicylic acid treatment on storage quality and diseases of apricot fruit[D]. Urumqi: Xinjiang Agricultural University, 2014.
[5] 郑琪, 徐秉良, 薛应钰, 等. 杏采后病害病原拮抗菌的分离筛选及鉴定[J]. 植物保护,2013,39(4):34−39, 71. [ZHENG Q, XU B L, QUE Y Y, et al. Isolation, screening and identification of antagonistic strains against apricot postharvest diseases[J]. Plant Protection,2013,39(4):34−39, 71. doi: 10.3969/j.issn.0529-1542.2013.04.008 ZHENG Q, XU B L, QUE Y Y, et al. Isolation, screening and identification of antagonistic strains against apricot postharvest diseases[J]. Plant Protection, 2013, 39(4): 34-39, 71. doi: 10.3969/j.issn.0529-1542.2013.04.008
[6] 任向峰, 姚婷, 张萌, 等. 4株水果采后链格孢属真菌的rDNA ITS区序列与碳源代谢指纹图谱差异性分析[J]. 食品科学技术学报,2018,36(1):72−78, 94. [REN X F, YAO T, ZHANG M. Comparison of rDNA ITS Sequence andcarbon metabolic fingerprinting of four Alternaria Nees isolated from fruits[J]. Journal of Food Science and Technology,2018,36(1):72−78, 94. REN X F, YAO T, ZHANG M, Comparison of rDNA ITS Sequence andcarbon metabolic fingerprinting of four Alternaria Nees isolated from fruits [J]. Journal of Food Science and Technology, 2018, 36(1): 72-78, 94.
[7] 韩盛, 玉山江·麦麦提, 潘俨, 等. 鲜杏采后病原菌鉴定及室内药剂筛选研究[J]. 新疆农业科学,2016,53(5):866−876. [HAN S, YUSHANJIANG M M T, PAN Y, et al. Identification of postharvest pathogens and screening of fungicides for fresh apricot in Xinjiang[J]. Xinjiang Agricultural Sciences,2016,53(5):866−876. HAN S, YUSHANJIANG M M T, PAN Y, et al. Identification of postharvest pathogens and screening of fungicides for fresh apricot in Xinjiang[J]. Xinjiang Agricultural Sciences, 2016, 53(5): 866-876.
[8] 程元, 淮稳霞, 姚艳霞, 等. 新疆巩留县杏果实斑点病病原菌鉴定[J]. 林业科学研究,2019,32(2):117−122. [CHENG Y, HUAI W X, YAO Y X, et al. The pathogen identification of apricot fruit spots disease in Gongliu County, Xinjiang[J]. Forest Research,2019,32(2):117−122. CHENG Y, HUAI W X, YAO Y X, et al. The pathogen identification of apricot fruit spots disease in Gongliu County, Xinjiang[J]. Forest Research, 2019, 32(2): 117-122.
[9] 吴思雅, 阿衣木古丽·艾赛提, 郑灿龙, 等. 新疆赛买提杏采后主要病原真菌的分离及鉴定[J]. 食品工业科技,2016,37(1):149−152. [WU S Y, AYIMUGULI A S T, ZHENG S L, et al. Identification of main pathogenic fungus from post-harvest “Saimaiti” apricot fruits[J]. Science and Technology of Food Industry,2016,37(1):149−152. WU S Y, AYIMUGULI A S T, ZHENG S L, et al. Identification of main pathogenic fungus from post-harvest “Saimaiti” apricot fruits[J]. Science and Technology of Food Industry, 2016, 37(1): 149-152.
[10] WU F, LI S C, MA Q L, et al. First report of Fusarium oxysporum causing fruit rot on apricot (Prunus armeniaca) in China[J]. Plant Disease,2022,106(8):2261−2261.
[11] 贾盼盼, 刘晓丹, 吝晨晨, 等. 壳寡糖对杏果实采后主要病原菌抑菌作用的研究[J]. 新疆农业科学,2012,49(2):290−295. [JIA P P, LIU X D, LIN C C, et al. Study on influencing factors of the antifungal activity on the main postharvest disease-producing fungus of apricot fruit by oligochitosan[J]. Xinjiang Agricultural Sciences,2012,49(2):290−295. JIA P P, LIU X D, LIN C C, et al. Study on influencing factors of the antifungal activity on the main postharvest disease-producing fungus of apricot fruit by oligochitosan[J]. Xinjiang Agricultural Sciences, 2012, 49(2): 290-295.
[12] CHAN Y, GUO Q, WEI J, et al. Inhibitory effect and mechanism of nitric oxide (NO) fumigation on fungal disease in Xinjiang Saimaiti dried apricots[J]. LWT-Food Science and Technology,2019,116:108507. doi: 10.1016/j.lwt.2019.108507
[13] LI Y C, MA Y Y, ZHANG T T, et al. Exogenous polyamines enhance resistance to Alternaria alternata by modulating redox homeostasis in apricot fruit[J]. Food Chemistry,2019,301:125303. doi: 10.1016/j.foodchem.2019.125303
[14] SCHOCH C L, SEIFERT K A, HUHNDORF S. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi[J]. Proceedings of the National Academy of Sciences of the United States of America,2012,109(16):6241−6246. doi: 10.1073/pnas.1117018109
[15] DURAIRAJ K, VELMURUGAN P, VEDHANAYAKISRI KA, et al. Molecular and phenotypic characterization of pathogenic fungal strains isolated from ginseng root rot[J]. Physiological and Molecular Plant Pathology,2019,105:28−33. doi: 10.1016/j.pmpp.2018.11.005
[16] SOLAIRAJ D, LEGRAND N N G, YANG Q Y, et al. Isolation of pathogenic fungi causing postharvest decay in table grapes and in vivo biocontrol activity of selected yeasts against them[J]. Physiological and Molecular Plant Pathology,2020,110:101478. doi: 10.1016/j.pmpp.2020.101478
[17] 黄津津, 姚婷, 王友升. 10株葡萄源丝状真菌分离鉴定及其致病力分析[J]. 食品科学技术学报,2018,36(4):46−54. [HUANG J J, YAO T, WANG Y S. Internal transcribed spacer region sequencing and phylogenetic analysis of 10 strains of Beauveria spp[J]. Journal of Food Science and Technology,2018,36(4):46−54. doi: 10.3969/j.issn.2095-6002.2018.04.007 HUANG J J, YAO T, WANG Y S. Internal transcribed spacer region sequencing and phylogenetic analysis of 10 strains of Beauveria spp[J]. Journal of Food Science and Technology, 2018, 36(4): 46-54. doi: 10.3969/j.issn.2095-6002.2018.04.007
[18] 王友升, 张燕, 陈玉娟. 5株桃、李果实采后褐腐病菌鉴定、rDNA ITS序列与碳源代谢指纹图谱分析[J]. 食品科学,2012,33(16):246−250. [WANG Y S, ZHANG Y, CHEN Y J, et al. Identification, rDNA ITS analysis and carbon metabolic fingerprinting of five Monilinia fructicola strains isolated from postharvest peach and plum fruits[J]. Food Science,2012,33(16):246−250. WANG Y S, ZHANG Y, CHEN Y J, et al. Identification, rDNA ITS analysis and carbon metabolic fingerprinting of five Monilinia fructicola strains isolated from postharvest peach and plum fruits [J]. Food Science, 2012(16): 246-250.
[19] 王友升, 郭晓敏, 姚子鹏, 等. 1株桃果实采后病原真菌的鉴定以及碳源代谢指纹图谱分析[J]. 北京工商大学学报(自然科学版),2011,29(1):47−53. [WANG Y S, GUO X M, YAO Z P, et al. Identification and carbon metabolic fingerprinting analysis of a pathogen isolated from postharvest peach fruit[J]. Journal of Food Science and Technology,2011,29(1):47−53. WANG Y S, GUO X M, YAO Z P, et al. Identification and carbon metabolic fingerprinting analysis of a pathogen isolated from postharvest peach fruit [J]. Journal of Food Science and Technology, 2011, 29(1): 47-53.
[20] 魏景超. 真菌鉴定手册 [M]. 上海: 上海科技出版社, 1979: 60−501. WEI J C. Fungal identification manual[M]. Shanghai Scientific & Technical Publishers, 1979: 60−501.
[21] 王宇, 郭良栋. 内生真菌EPICOCCUM NIGRUM的形态与分子鉴定[J]. 菌物学报,2004,23(4):474−479. [WANG Y, GUO L D. Morphological and molecular identification of an endophytic fungus EPICOCCUM NIGRUM[J]. Mycosystema,2004,23(4):474−479. doi: 10.3969/j.issn.1672-6472.2004.04.005 WANG Y, GUO L D. Morphological and molecular identification of an endophytic fungus EPICOCCUM NIGRUM[J]. Mycosystema, 2004, 23(4): 474-479. doi: 10.3969/j.issn.1672-6472.2004.04.005
[22] CHAISIRI C, LIU X Y, LIN Y, et al. Phylogenetic and haplotype network analyses of Diaporthe eres species in China based on sequences of multiple loci[J]. Biology-Basel,2021,10(3):179.
[23] GOS H, BRYK H, MICHALECKA M, et al. First report of Diaporthe eres a new pathogen causing rot of apples during storage period in Poland[J]. Journal of Plant Pathology,2021,103(6):393−394.
[24] XIAO Y S, HUO G H, LIU L L, et al. First report of postharvest fruit rot disease of yellow peach caused by Diaporthe eres in China[J]. Plant Disease,2021,106(7):1983−1983.
[25] BERTETTI D, GUARNACCIA V, SPADARO D, et al. First report of fruit rot in European pear caused by Diaporthe eres in Italy[J]. Plant Disease,2018,102(12):2651−2651.
[26] LORENZINI M, ZAPPAROLI G. Identification of Pestalotiopsis bicilita, Diplodia seriata and Diaporthe eres causing fruit rot in withered grapes in Italy[J]. European Journal of Plant Pathology,2018,151(4):1089−1093. doi: 10.1007/s10658-017-1416-1
[27] YAMIN D U, WANG X, GUO Y, et al. Biological and molecular characterization of seven Diaporthe species associated with kiwifruit shoot blight and leaf spot in China[J]. Phytopathologia Mediterranea,2021,60(2):177−198. doi: 10.36253/phyto-12013
[28] 严雪瑞, 王旭, 胡梦琼, 等. 蓝莓间座壳芽枯病病原菌鉴定及其生物学特性[J]. 植物病理学报,2015,45(5):556−560. [YAN X R, WANG X, HU M Q, et al. Identification and biological characteristic of blueberry Diaporthe bud blight pathogen[J]. Acta Phytopathologica Sinica,2015,45(5):556−560. YAN X R, WANG X, HU M Q, et al. Identification and biological characteristic of blueberry Diaporthe bud blight pathogen[J]. Acta Phytopathologica Sinica, 2015, 45(5): 556-560.
[29] 翟立峰. 梨轮纹病与干腐病的病原关系及轮纹病菌携带真菌病毒多样性研究[D]. 武汉: 华中农业大学, 2016. ZHAI L F. Relations among four Botryosphaeria species isolated from pear stem wart and stem canker and diversity of mycoviruses from Botryosphaeria dothidea[D]. Wuhan: Huazhong Agricultural University, 2016.
[30] 刘娜, 谢国芳, 袁孟孟, 等. 猕猴桃软腐病发病过程内生真菌多样性分析[J]. 食品科技,2020,45(4):31−36. [LIU N, XIE G F, YUAN M M, et al. Diversity of endophytic fungal in the pathogenesis of soft rot in kiwifruit[J]. Food Science and Technology,2020,45(4):31−36. doi: 10.13684/j.cnki.spkj.2020.04.006 LIU N, XIE G F, YUAN M M, et al. Diversity of endophytic fungal in the pathogenesis of soft rot in kiwifruit[J]. Food Science and Technology, 2020, 45(4): 31-36. doi: 10.13684/j.cnki.spkj.2020.04.006
[31] 孙秋玲, 杨芝霓, 唐利华, 等. 芒果蒂腐病病原葡萄座腔菌科真菌种类鉴定[J]. 植物病理学报,2022,52(6):1009−1012. [SUN Q, YANG Z N, TANG L H, et al. Identification of pathogenic Botryosphaeriaceae species from mango stem-end rot[J]. Acta Phytopathologica Sinica,2022,52(6):1009−1012. SUN Q, YANG Z N, TANG L H, et al. Identification of pathogenic Botryosphaeriaceae species from mango stem-end rot[J]. Acta Phytopathologica Sinica, 2022, 52(6): 1009-1012.
[32] 姚子鹏, 王友升, 郭晓敏, 等. 桃果实贮藏期间病原真菌的ITS rDNA序列分析与鉴定[J]. 中国食品学报,2011,11(4):172−178. [YAO Z P, WANG Y S, GUO X M, et al. ITS rDNA sequence analysis and identification of pathogens for postharvest diseases of peach fruit[J]. Journal of Chinese Institute of Food Science and Technology,2011,11(4):172−178. doi: 10.3969/j.issn.1009-7848.2011.04.026 YAO Z P, WANG Y S, GUO X M, et al. ITS rDNA sequence analysis and identification of pathogens for postharvest diseases of peach fruit[J]. Journal of Chinese Institute of Food Science and Technology, 2011, 11(4): 172-178. doi: 10.3969/j.issn.1009-7848.2011.04.026
[33] 姚婷, 丁凤兰, 王珂, 等. 5株枣果实采后病原真菌的分离鉴定及ITS区序列分析[J]. 食品科学技术学报,2022,40(5):62−70. [YAO T, DING F L, WANG K, et al. Morphological characterization and rDNA ITS sequence analysis of five pathogenic fungi isolated from infected jujube fruit[J]. Journal of Food Science and Technology,2022,40(5):62−70. doi: 10.12301/spxb202100734 YAO T, DING F L, WANG K, et al. Morphological characterization and rDNA ITS sequence analysis of five pathogenic fungi isolated from infected jujube fruit[J]. Journal of Food Science and Technology, 2022, 40(5): 62-70. doi: 10.12301/spxb202100734
[34] LORENZINI M, ZAPPAROLI G. Occurrence and infection of Cladosporium, Fusarium, Epicoccum and Aureobasidium in withered rotten grapes during post-harvest dehydration[J]. Antonie Van Leeuwenhoek International Journal of General and Molecular Microbiology,2015,108(5):1171−1180. doi: 10.1007/s10482-015-0570-8
[35] 任苗苗, 闫思远, 李嘉泓, 等. 宁夏‘灵武长枣’采后病原真菌的分离与鉴定[J]. 中国果树,2020(5):93−97, 102, 142. [REN M M, YAN S Y, LI J H, et al. Isolation and identification of postharvest pathogenic fungi from ‘Lingwuchan’ jujube in Ningxia[J]. China Fruits,2020(5):93−97, 102, 142. doi: 10.16626/j.cnki.issn1000-8047.2020.05.017 REN M M, YAN S Y, LI J H, et al. Isolation and identification of postharvest pathogenic fungi from ‘Lingwuchan’ jujube in Ningxia[J]. China Fruits, 2020(5): 93-97, 102, 142. doi: 10.16626/j.cnki.issn1000-8047.2020.05.017
[36] GAO X, CUI Q, CAO Q Z, et al. Transcriptome-wide analysis of Botrytis elliptica responsive microRNAs and their targets in Lilium Regale Wilson by high-throughput sequencing and degradome analysis[J]. Frontiers in Plant Science,2017,8:753. doi: 10.3389/fpls.2017.00753
[37] 姚婷, 陈其葳, 张燕, 等. 3株果实采后葡萄孢属真菌ITS区rDNA序列与碳源代谢指纹图谱分析[J]. 食品科学技术学报,2017,35(4):49−55. [YAO T, CHEN Q W, ZHANG Y, et al. Analysis of rDNA ITS and carbon metabolic fingerprinting of Botrytis Nees isolated from three fruits[J]. Journal of Food Science and Technology,2017,35(4):49−55. doi: 10.3969/j.issn.2095-6002.2017.04.007 YAO T, CHEN Q W, ZHANG Y, et al. Analysis of rDNA ITS and carbon metabolic fingerprinting of Botrytis Nees isolated from three fruits [J]. Journal of Food Science and Technology, 2017, 35(4): 49-55. doi: 10.3969/j.issn.2095-6002.2017.04.007
-
期刊类型引用(2)
1. 王佳,丁方莉,安宇,曾雪莹,张智慧,李思楠,徐开媛,周芳,王颖,张璐,徐炳政,孙泽堃. 芸豆-蓝靛果复合发酵液制备工艺优化及其抗氧化活性. 食品工业科技. 2025(03): 222-231 .  本站查看
本站查看
2. 王胜宇,杨梅,胡鹤宇,朱才庆,董欢欢,管咏梅,朱卫丰. 结合态酚类物质在植物生长、食品加工及人体消化过程中的释放规律研究进展. 食品工业科技. 2024(14): 408-417 .  本站查看
本站查看
其他类型引用(1)





 下载:
下载:


 下载:
下载:
