Research Progress on the Biotransformation of Rare Ginsenosides
-
摘要: 人参皂苷作为人参中的主要活性成分,被广泛应用于食品和药品中。人参皂苷因化学结构差异导致其具有不同的性质和功能。具有高活性的稀有人参皂苷在自然界中含量很低,限制了人参皂苷资源的利用。通过不同方法将高含量人参皂苷转化为稀有人参皂苷是获得稀有人参皂苷的重要途径。本文以人参皂苷的结构、药理作用为基础,综述生物修饰法对人参皂苷的糖基水解和母核结构的影响,并对稀有人参皂苷在食品、药品中应用的研究现状、未来发展趋势进行了分析,为改善人参皂苷资源应用提供理论依据。Abstract: As the main active component in ginseng, ginsenoside is widely used in food and medicine. Ginsenosides have different properties and functions due to their different chemical structures. The content of rare ginsenosides with high activity is very low in nature, which limits the utilization. It is an important way to obtain rare ginsenosides by transforming high content ginsenosides into rare ginsenosides through different methods. Based on the structure and pharmacology of ginsenosides, this paper reviews the effects of biotransformation on the glycosyl hydrolysis and parent nucleus structure of ginsenosides, and it analyzes the application status and future development trend of rare ginsenosides in food and medicine, providing a theoretical basis for improving the application of ginsenosides resources.
-
人参(Panaxginseng C. A. Meyer) 为五加科多年生草本植物,是传统名贵中药。人参皂苷是人参的主要活性成分之一,属于三萜苷类物质,由糖和苷元缩合而成。研究表明人参皂苷具有多种药理作用[1-3],口服后,大部分原人参二醇型皂苷被肠道菌群水解为人参皂苷C-K、Rg3、Rh2 和PPD[4-5],原人参三醇型皂苷主要被降解为人参皂苷F1和20(S)-原人参三醇(20(S)-PPT)[6-7]。原人参二醇型皂苷的代谢产物C-K在体内外均具有显著抗肿瘤活性,较代谢前体活性增强[8]。Rg1、Re和20(S)-PPT口服后具有很强的抗肿瘤细胞转移作用,而静脉注射给药只有20(S)-PPT有抗肿瘤作用,说明Rg1、Re口服后的抗肿瘤转移作用是由其代谢产物20(S)-PPT产生[9]。大量研究证明人参多糖皂苷经肠道菌的去糖化作用,生成的具有两个或单个糖苷键的稀有人参皂苷具有更强的生物活性[10]。
生物转化可改变人参皂苷的化学结构,有效提高人参皂苷的体内利用率,优化药物的临床使用效果,减少不良反应[11]。人参皂苷的生物转化包括糖基变化、羟基变化和双键改变。人参皂苷的糖基变化主要发生在C-3、C-6和C-21,包括糖基水解和加糖成苷(糖基化);还有一些转化在C-3、C-12和C-17侧链上连接甲基和羟基;双键的修饰主要有C-24/C-25的双键加成或氧化。本文就近年来微生物生物转化稀有人参皂苷的发展状况、母核结构变化及其衍生物的应用展开综述,期望为人参皂苷及其衍生物的结构研究和应用提供理论依据。
1. 稀有人参皂苷的结构及应用
1.1 稀有人参皂苷的结构
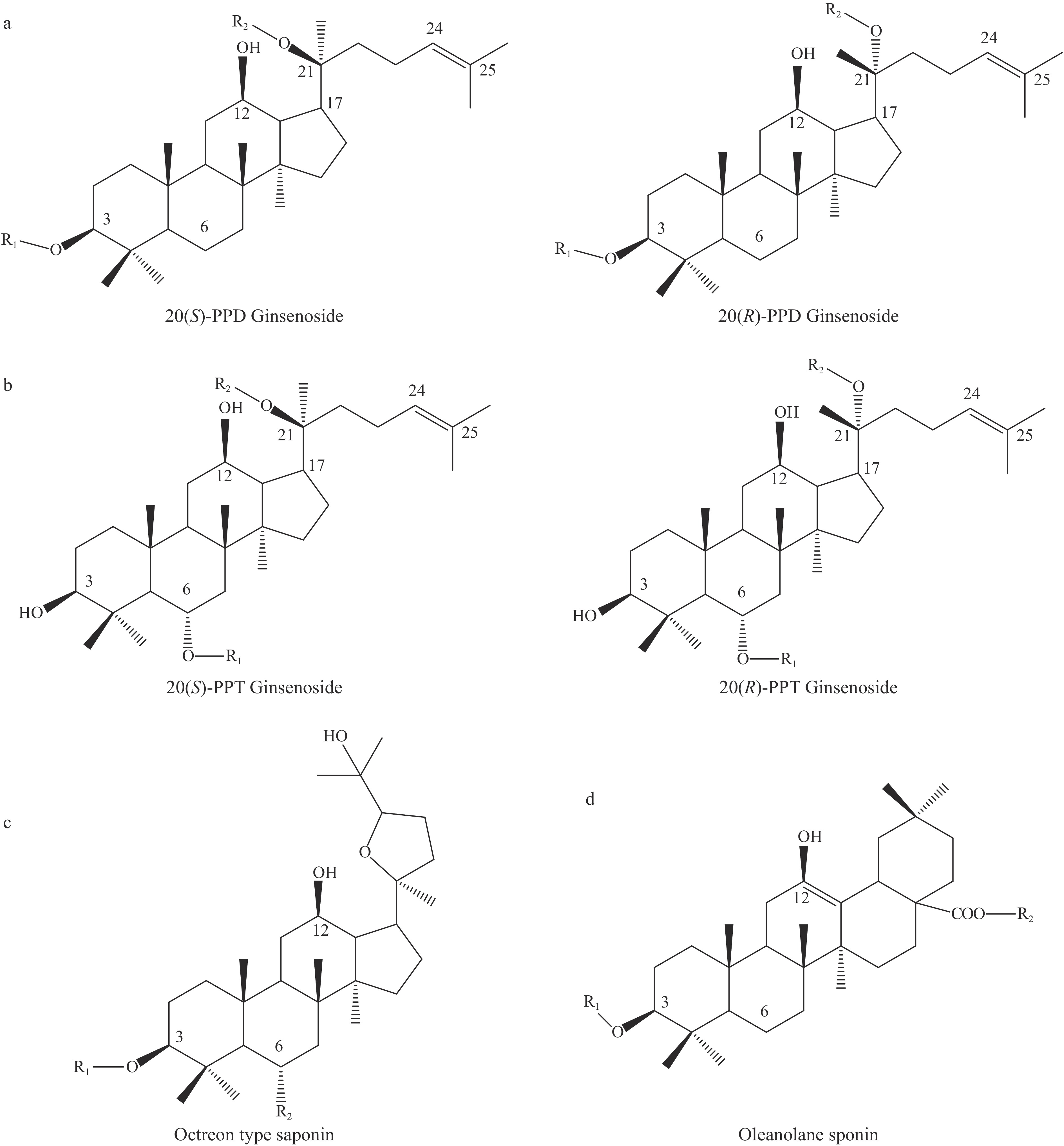
根据苷元结构的不同,人参皂苷分为四环三萜类和五环三萜类。原人参二醇型皂苷(图1a)和原人参三醇型皂苷(图1b)均属于达玛烷型四环三萜类皂苷,奥克梯隆型皂苷(图1c)是一类侧链含有呋喃环的四环三萜类皂苷;以齐墩果酸为母核的齐墩果烷型皂苷属于五环三萜类皂苷(图1d)。
稀有人参皂苷因其天然中含量较少或几乎不存在而得名,稀有人参皂苷以达玛烷型皂苷为主,在母核的C-3位、C-6位和C-20位上所连糖基不超过3个,且单个位置所连糖基数码不多于2个。稀有人参皂苷主要通过人参皂苷脱糖或脱水获得,目前已鉴定的稀有人参皂苷主要有Rg3、Rg1、Rh1、Rh2、Rh3、RT3、F1、F2、C-K、PPD、PPT等,如表1所示。
表 1 常见的稀有人参皂苷Table 1. Structures of rare ginsenosides人参皂苷 R1 R2 Rg3 -Glc-Glc -H F2 -Glc -Glc Rh2 -Glc -H C-K -H -Glc Rg1 -Glc -Glc Rh1 -Glc -H F1 -H -Glc RT3 -Xyl -Glc Rh3 -Glc − PPD -H − PPT − -H 1.2 稀有人参皂苷的应用
稀有人参皂苷主要存在于红参和黑参中,是红参、黑参生理活性不同于人参的物质基础。市面上销售的人参粉、人参压片等,只是把提取人参皂苷的原材料(人参、西洋参等)磨成粉,或者加入辅料再压成片。人参皂苷片剂和胶囊只提取出药材中的人参总皂苷,没有进一步的分离和转化。这样直接提取出来的人参皂苷进入人体后需经过特定的酶分解,才能被人体吸收。但是这种酶在人体内很少或者不存在。导致人参总苷片等在人体内生物利用率低、药理作用弱等现象。
目前国内的稀有人参皂苷制剂产品大部分含有单体成分Rh2 或Rg3,只含有一种人参皂苷单体的制剂见表2。
表 2 已在中国获批的含有稀有人参皂苷的药物Table 2. Drugs containing rare ginsenosides approved in China产品名称 代表稀有人参皂苷 功效 参一胶囊 人参皂苷Rg3 与化疗配合用药,
提高治疗癌症的疗效20(S)-人参皂苷 Rg3眼膏 人参皂苷Rg3 抗病毒 20(S)-人参皂苷 Rg3 注射液 人参皂苷Rg3 抗癌 20(S)-原人参二醇原料药 人参皂苷Rg3 抗癌 人参皂苷 CK 片 人参皂苷C-K 类风湿性关节炎 今幸胶囊 人参皂苷Rh2 对免疫力低下人群起到
增强免疫力的功效常见的稀有人参皂苷如人参皂苷C-K、Rg3和Rh2等已用于提高机体免疫的保健品或配合其他药物进行辅助治疗的临床应用中,同时,稀有人参皂苷的应用研究依然是开发人参皂苷的重要内容。研究者以20(R)-人参三醇为原料通过C-3位与叔丁氧羰基甘氨酸的酯化反应,合成了一种新的人参皂苷甘氨酸酯衍生物,并确定了该衍生物的抗肿瘤活性是20(R)-人参三醇(IC50>30 mmol/L)的100多倍,A11的体内抑制作用比20(R)-人参三醇更显著(P<0.01),同时A11呈剂量依赖性地抑制HeLa细胞的增殖、迁移和侵袭,促进其凋亡[12]。人参皂苷Rg4是一种存在于人参叶和黑参中的稀有人参三醇型皂苷,Rg4抑制炎症并对CLP诱导的脓毒症表现出保护作用[13]。Rg5与人嘌呤受体12(P2RY12)变构相互作用形成稳定的复合物,通过残基E188和R265减低其活性,使血浆中白介素(IL)-6、IL-1β和肿瘤坏死因子-α的释放减少,改善炎症反应,同时抑制静脉血栓的形成[14]。人参皂苷Rk1具有抗肿瘤[15]、调节血糖[16-17]、保护神经系统[18-19],与人参皂苷Rk5联合使用后通过增加碱性磷酸酶的活性,促进骨细胞分化和增长,从而达到治疗骨质疏松的作用[20]。随着研究的深入,将会有更多的稀有人参皂苷和其衍生物的生物活性被发掘。
2. 稀有人参皂苷生物转化的研究现状
人参皂苷转化的主要方法为化学转化法和生物转化法,化学转化法主要利用酸碱水解糖苷键,氧化加成和乙酰化等反应改变取代基;生物转化是利用微生物细胞对外源底物进行结构修饰,利用代谢过程中产生的某种或某几种酶对底物特定部位(基团)进行催化反应,从而增加活性成分的含量和药性[21]。生物转化法包括细菌转化、肠道菌群转化、真菌转化和体外酶催化,弥补了化学转化法反应条件剧烈,污染环境,易产生副产物等缺点,被广泛应用于研究和生产中。生物转化主要反应类型包括糖基水解反应、氧化还原反应等[22],其中以糖基的水解反应应用最为广泛[23],近年来C-24,C-25位的加成反应也逐渐被应用于实践生产中。
2.1 稀有人参皂苷的糖基水解
2.1.1 微生物对人参皂苷的糖基水解
人参皂苷的体内代谢途径是人参皂苷构效关系研究的重要内容,逐级脱去糖基化是人参皂苷体内主要的代谢途径,经代谢产生的稀有人参皂苷和苷元在体内有显著药理作用。将原人参二醇型皂苷Rd、F2、Rg3、C-K和Rh2与人肠道菌群离体共培养,发现人参皂苷Rd 的主要转化产物为F2、Rg3、C-K、Rh2和PPD;人参皂苷F2主要转化产物为C-K,PPD;人参皂苷Rg3主要转化产物为Rh2,PPD[24];而C-K和Rh2又可以转化为PPD[25]。通过对原三醇型皂苷Re、Rg1、Rh1、Rf、F1和R1在人肠道菌群中转化产物的分析,确定其代谢产物及转化途径,即人参皂苷Re 的转化途径为Re→Rg1/Rg2→Rh1/F1→PPT;人参皂苷Rg1转化途径为Rg1→Rh1/F1→PPT;人参皂苷Rf 的转化途径为Rf→Rh1→PPT;三七皂苷R1的代谢途径为R1→Rg1/Rg2→Rh1→PPT[26]。
单体皂苷在体外肠道菌代谢途径是脱糖的过程,在离体条件下肠道菌群对人参皂苷Rb1的水解也是一个逐级脱糖的过程[27]。人参总皂苷在体内肠道菌代谢也是如此,GUO等[28]使用广谱抗生素构建了无菌大鼠模型,验证肠道微生物群在大鼠体内转化三七皂苷(1.535 g/kg)。结果在有菌大鼠血浆中检测到人参皂苷F1、Rh2、C-K和PPT四种代谢物,而在无菌大鼠血浆中未检测到。因此推断人参皂苷体内的代谢基本规律为四糖苷→三糖苷→二糖苷→单糖苷→苷元。
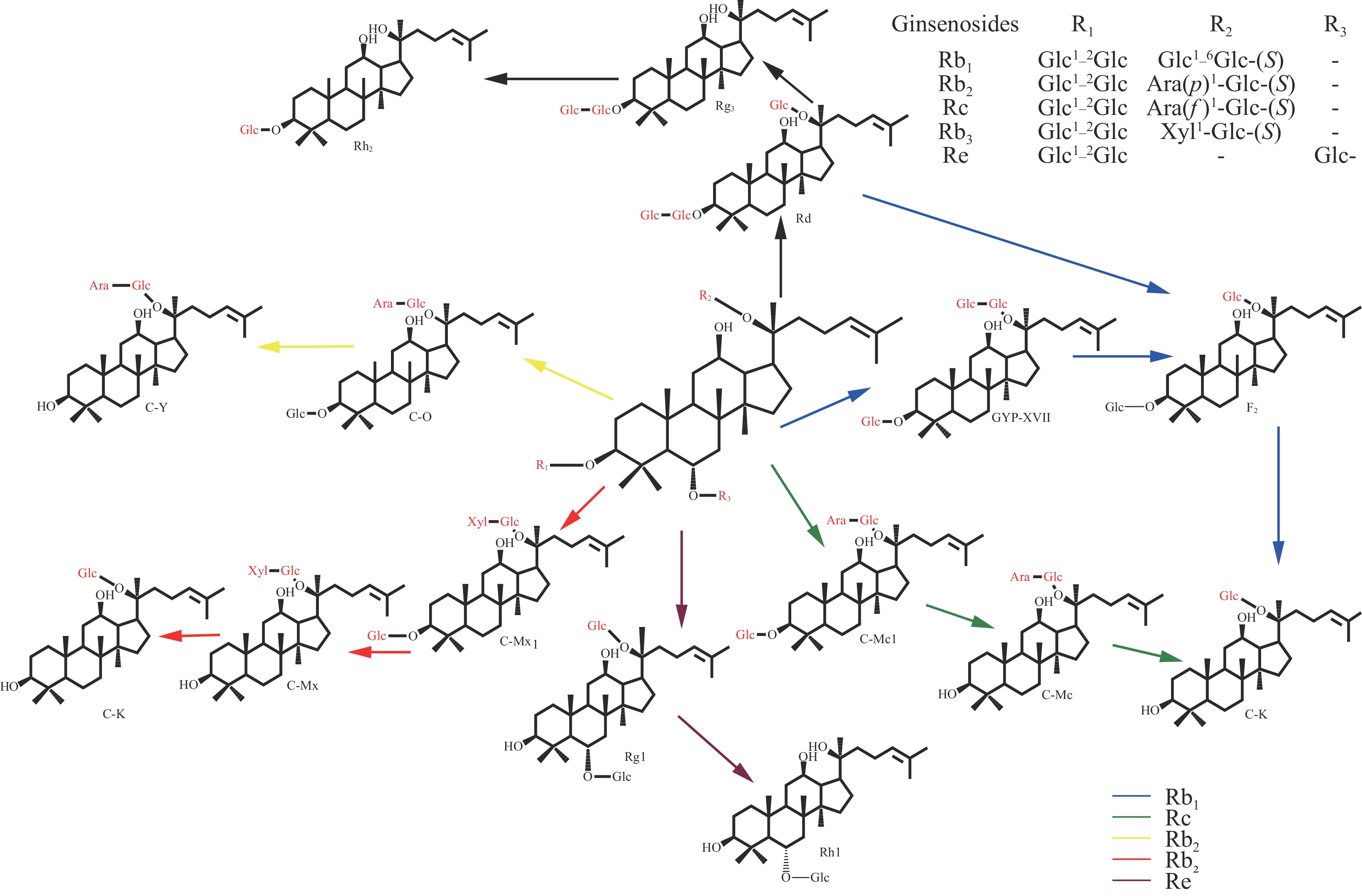
微生物中存在的酶种类较多,具有转化糖苷类化合物的潜力[29]。同时,发酵转化后脱去的糖可作为真菌生长繁殖所需的碳源,从而代谢生成更多的酶[30],因此不同微生物可作为生物催化剂在天然产物的生物转化过程中发挥重要作用,国内外学者利用细菌和真菌对人参皂苷进行了生物转化研究(图2)。
研究表明一些真菌和细菌如黑曲霉菌(Aspergillus niger)、镰刀菌(Fusarium sp.)、青霉菌(Penicillium sp.)、冬虫夏草菌(Cordyceps sinensis)和蜜环菌( Armillaria mellea)、双歧杆菌(Bifidobacterium breve)、芽孢杆菌(Bacillus sp.)、乳酸球菌(Lactococcus lactis )以及植物乳杆菌(Lactobacillus plantarumsubsp.)、地杆菌(Terrabacter sp.)、纤维菌(Cellulosimicrobium cellulans sp.)和嗜热菌(Thermotoga thermarum)均可将人参皂苷C-3或C-20上的糖基代谢水解,生成稀有人参皂苷或苷元(表3)。黑曲霉菌可将人参皂苷Rb1、Rb3依次代谢为人参皂苷Rd、F2、C-K[31-32];也可以先水解人参皂苷Rb2和Rc的3-O-Glc,然后再水解20-O-Ara,产生单一的水解途径:Rb2→C-O→C-Y→C-K、Rc→C-Mc1→C-Mc→C-K,同时主要通过Rb3→C-Mx1→C-Mx→C-K的途径水解Rb3的3-O-Glc,并通过Rb3→Rd→F2→CK的途径缓慢水解Rb3的20-O-Xly[32];青霉菌通过Rb1→Rd→F2→C-K 的途径,将人参总皂苷和人参皂苷单体水解,产生稀有人参皂苷[33-35];镰刀菌通过发酵培养将人参总皂苷转化为稀有人参皂苷C-K[36];双歧杆菌发酵液可将人参皂苷F2转化为人参皂苷C-K[37];芽孢杆菌分别水解人参皂苷Rb1母核的C-3 位和C-20 位,生成人参皂苷Rd、Rg3[37]和Gyp-ⅩⅦ、F2[38]。从乳酸球菌[39]和嗜热菌[40-41]中克隆的葡萄糖苷酶基因,在大肠杆菌中表达,产生葡萄糖苷酶可逐步水解人参皂苷Rb1的C-20和C-3位的外部和内部葡萄糖部分。反应如下:Rb1→Rd→Rg3(S)和F2→C-K。
表 3 人参皂苷的水解途径Table 3. Hydrolysis pathway of ginsenoside转化产物 菌株类型 转化途径 C-K Aspergillus niger [31-32] Rb1→Rd→F2→C-K[31,33−34,37,50−52] Penicillium sp. [33−34,52] Rb2→C-O→C-Y→C-K[32,43] Fusarium moniliforme[53] Rb3→Rd→F2→C-K[32] Rc→C-Mc-1→C-Mc→C-K[39] Bifidobacterium breve[37] 总皂苷→C-K[53] Armillaria mellea[43,50] Rd→F2→C-K[50] Lactococcus lactis[39]
Lactobacillus plantarum subsp.[51]Rg3 Cellulosimicrobium cellulans sp.[54] Rb1→Rg3[32] Lactococcus lactis[39] Rb1→Rd→Rg3[37−39,41,45,54] Thermotoga thermarum[40]
Thermotoga petrophila[41]Rb2→Rd→Rg3[40−41,45,54] Bacillus sp.[38]
Bacillus subtilis[36]
Monascus purpureus[45]Rc→Rd→Rg3[40,42,54]
总皂苷→Rg3[36]Rh1 Sphingomonas sp. [55]
Dictyoglomus thermophilum[57]Re→Rg1→Rh1[54−56]
R1→Rg1[57]Rh2 Penicillium[41] Terrabacter ginsenosidimutans[58] Fusarium proliferatum[57-59] Rb1→Rg3→Rh2[34,39] Myrothecium verrucaria[60] Rb1→Rd→Rg3→Rh2[58−59] Lactococcus lactis[51,53,56] Rg3→Rh2[56,60] F2 Bacillus sp.[38] Rb1→Rd→F2[38] Rb1→Gyp-ⅩⅦ→F2[38] 近年,食用真菌也被应用于人参皂苷的糖基水解研究中,例如冬虫夏草菌可将人参皂苷Rb1转化为稀有人参皂苷F2,转化途径为Rb1→Rd→F2[42]。蜜环菌可将人参皂苷Rb2转化为稀有人参皂苷C-Y 和C-K,转化途径为Rb2→C-Y→C-K[43],这是文献记载中首次使用担子菌将人参皂苷Rb2 转化为稀有人参皂苷C-K。
微生物转化法有其独特的优势,反应条件温和,相比于物理和化学转化法,不需要高温高压环境,降低成本;反应过程中几乎不使用有机试剂,可以最大程度保证人参皂苷的活性。微生物分泌的酶可以分解消耗掉糖、蛋白质等杂质,提高人参皂苷的浓度,增加转化率。微生物转化法原材料利用效率高,同时转化效率高,在节约原材料的同时,也提高了产量[44]。
2.1.2 酶对人参皂苷的糖基水解
菌类转化的特异性低于直接使用酶进行转化,因此需要对糖苷酶活性进行调整,以达到在转化中限制副产物的产生并提高其特异性的效果。
皂苷类化合物具有结构的不同和功能的多样性,人参皂苷结构上包括1~4个糖苷键,常见的糖基有β-葡萄糖、L-阿拉伯糖、D-木糖和L-鼠李糖,需要不同的酶相互协同作用实现生物转化。常见的酶有葡萄糖苷酶、甘露醇酶、阿拉伯糖苷酶、木糖苷酶等,主要从微生物或动植物中分离纯化获得。在酶转化人参皂苷的研究中,研究者们首先使用了天然微生物产生的酶,对人参皂苷进行了糖苷键的水解,如利用可在胞外产生β-葡萄糖苷酶的红曲霉(Monascus purpureus)对人参皂苷进行发酵培养,使人参皂苷中Rg3的质量分数达到了发酵前的2.3倍[45]。随着研究的深入,研究者从真菌和动植物中提取出相对纯净的β-葡萄糖苷酶,对人参皂苷进行糖苷水解;蒋磊等[46]利用β-葡萄糖苷酶将人参中含量较高的皂苷Re转化为人参皂苷Rg1。但从微生物和动植物体内分离的酶多为混合酶,定向转化困难且副反应产物多,增加转化产物分离纯化的难度。
随着分子生物学和基因工技术的发展,利用基因重组技术构建基因工程菌对人参皂苷进行糖基水解是一种有效地提高转化效率的途径。大肠杆菌和酵母细胞作为成熟的表达宿主,常被用作重组生物酶蛋白的首选表达载体[47]。近年来通过利用基因拼接技术将嗜热菌来源的木糖苷酶基因xln-DT重组至pET-20b质粒中pelB信号肽下游,重组质粒pelB-xln-DT转化大肠杆菌表达宿主E.coli BL21(DE3),并以此为催化剂进行三七皂苷R1的糖基水解,成功生产出人参皂苷Rg1[42]。Jeon等[48]使用重组葡萄糖苷酶(MT619),对人参皂苷Re进行转化,转化途径Re→Rg1→F1→MT1,MT1是一个新的PPT型人参皂苷。通过克隆两株芽孢杆菌的β-葡萄糖苷酶的基因,在大肠杆菌BL21(DE3) 中高效表达。用BL21(DE3)粗提物水解人参皂苷Rb1生成人参皂苷F2[38];大肠杆菌串联表达的重组酶纯度更高,可以使PPD 型人参皂苷转化为稀有人参皂苷Rh2(S)[49]。
目前研究中多使用单一酶对人参皂苷进行转化,因此,今后研究可以通过基因重组技术,将不同功能的酶在同一宿主中表达,通过不同酶的协同作用,达到产生特定需求的稀有人参皂苷及其衍生物的目的,从而提高人参资源的利用率。
2.2 微生物对人参皂苷苷元结构的修饰
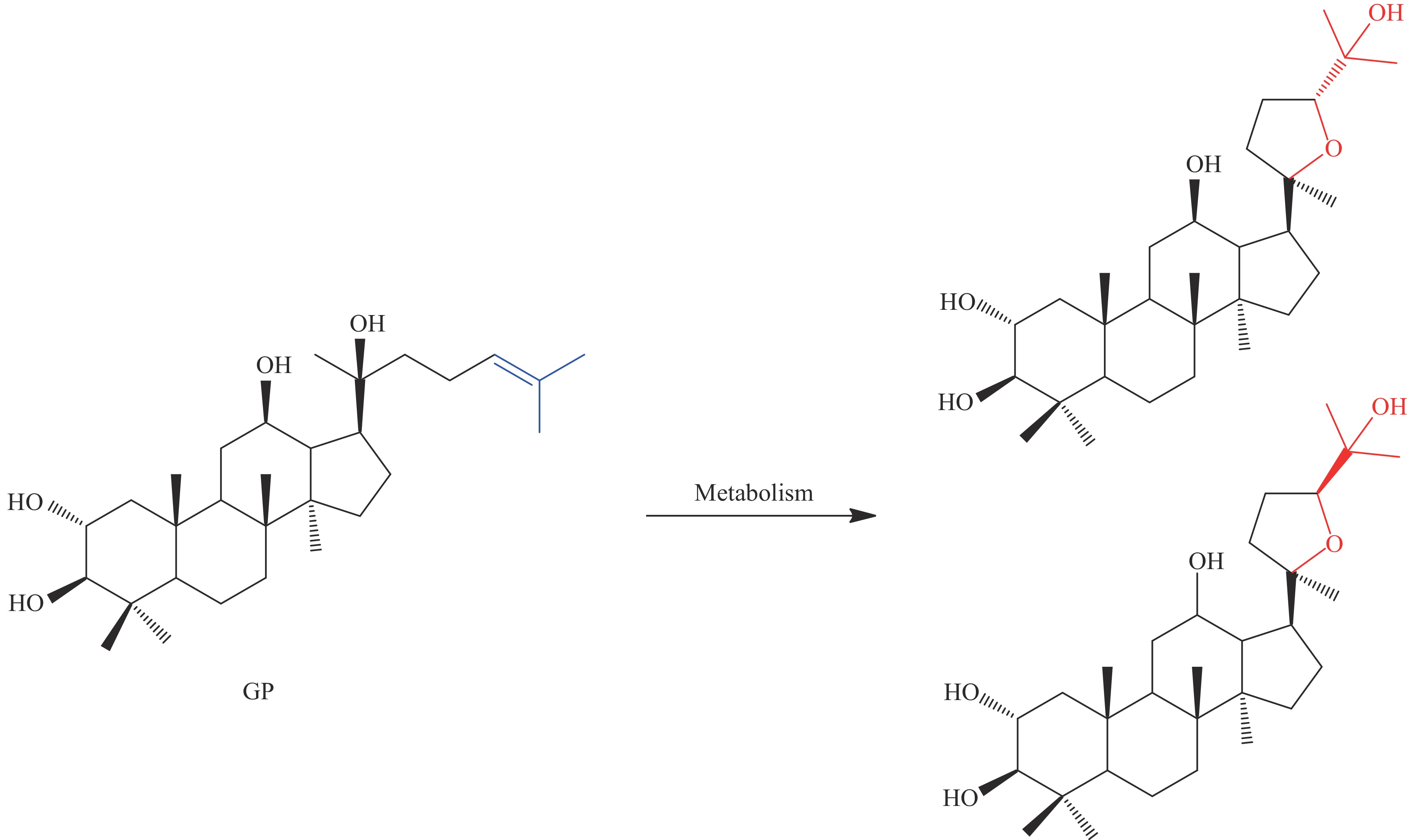
关于人参皂苷苷元的结构修饰研究主要集中于PPD型人参皂苷,获得其衍生物如25-OH-PPD、25-OCH3-PPD等。衍生方法通常为化学法和物理法,通过生物法改变皂苷母核结构的研究并不多见,目前已报导的方法主要有C24-C25位的双键水合,在大鼠体内观察到的 20S-dammar-24-en-2α,3β,12β,20-tetrol (GP) 的主要代谢途径是24,25-双键氧化生成24,25-环氧化物,其次是水解和重排生成20,24-氧化物(图3)[61]。研究者通过人参皂苷与冬夏草菌的共培养将人参皂苷Rg1转化为25-OH-(S/R)-Rh1,将三七皂苷R1转化为25-OH-20(S/R)-R2,且25-OH衍生物在转化产物中的含量远高于人参皂苷Rh1 和三七皂苷R2,使得该衍生物的分离和纯化简单易行[62-63]。使用刺毛霉(Mucor spinosus)与20(S)-原人参三醇皂苷共同培养得到了6个衍生物,研究结果证明经C-12位的脱氢反后在C-7或C-11上的进一步羟基化和C-15羰基化以及C-26位的双键重排后生成的人参皂苷的衍生物对癌细胞具有明显的抑制活性[64]。根据国内外的研究推测这可能是人参皂苷的区域选择性羟基化最有效的生物催化体系。
经试验研究发现,这类衍生物通过不同的机制在体内外发挥着不同的作用,并且生物活性比常见人参皂苷更高[65],如Rb1经过脱氢反应后生成二氢人参皂苷Dg-Rb1,可以在不影响全身参数的情况下修复受损的神经元,且Dg-Rb1的有效剂量比Rb1低10 倍[66]。人参皂苷Rh2的辛酯衍生物Rh2-O通过激活内在凋亡途径,具有比Rh2更高的抗癌活性[67]。PPD的乙酰基衍生物具有显著的抗增殖和诱导凋亡活性,并且部分衍生物的抗癌活性高于PPD[68]。将吡嗪环引入25-OH-PPD以提高其抗肿瘤活性,2-吡嗪-PPD可显著抑制胃癌细胞的增殖,并且对正常细胞(人胃上皮细胞系GES-1)几乎没有毒性[69]。苷元PPD的衍生物25-OH-PPD的抗癌活性优于人参皂苷Rg3、紫杉醇和氨甲叶酸等市场上已有的药物[70]。
因此,在保持人参皂苷原有的活性结构的前提下,通过引入极性基团和药效基团来修饰人参皂苷的苷元结构,增强人参皂苷的水溶性、靶向性和疗效,提高人参皂苷生物利用度。
2.3 人参皂苷的糖基化
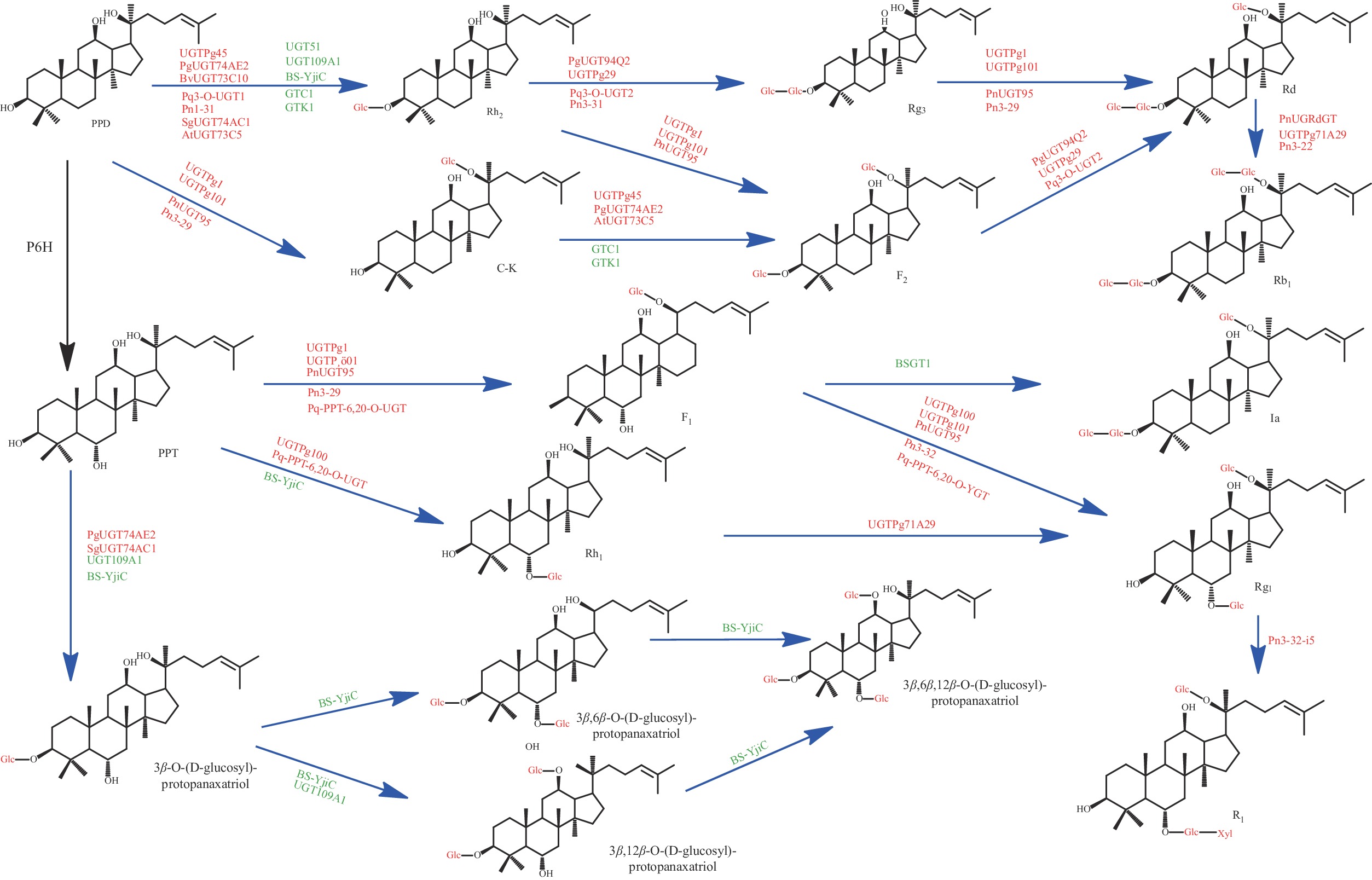
从2,3-氧化角鲨烯为起点,人参皂苷的生物合成可以分为三步:氧化角鲨烯环化酶(OSC)使2,3-氧化角鲨烯环化;再通过细胞色素(Cyt)和糖基转移酶(GT)进行羟基化和糖基化反应,最终生成人参皂苷[71]。糖基转移酶(UGT)是合成生物中生产稀有人参皂苷的最关键酶之一[72],也是人参皂苷生物合成的最后一步。将UGT基因从鼠李糖乳杆菌中克隆出来后,在大肠杆菌BL21(DE3) 中进行表达,重组UGT蛋白可将Rh2转化为两种新的人参皂苷,分别为葡萄糖基人参皂苷Rh2 和二葡萄糖基人参皂苷Rh2[73]。
三萜苷元经UGT催化生成结构不同的人参皂苷,原人参二醇型皂苷在C-3和C-20位发生糖基化,原人参三醇类皂苷则是在C-6 和C-20 位发生糖基化[78],稀有人参皂苷糖基化途径如图4所示。从拟南芥中分离到糖基转移酶73C5(UGT 73C5),将其分步加入PPD中,该糖基转移酶可选择性的将葡萄糖部分转移到PPD的C-3羟基上合成人参皂苷Rh2,达到了目前报道人参皂苷Rh2的最大产量(3.2 mg/mL)[74]。糖基转移酶74AE2(UGT 74AE2)催化葡萄糖转移到PPD和C-K的C-3羟基,分别生成Rh2和F2[75],糖基转移酶94Q2(UGT 94Q2) 可以将葡萄糖转移到Rh2 和F2,分别生成Rg3和Rd[76]。UGT51 是酿酒酵母S288c中的糖基转移酶之一[77],它能将葡萄糖转移到PPD 的C-3 羟基上,生成Rh2[47]。未来对UGTs 的研究可能会深入揭示人参皂苷糖基化的调控机制,并为人参皂苷的生产修饰提供新的途径。
作为催化人参皂苷形成的直接因素,UGTs在生物法合成人参皂苷的生产中起着至关重要的作用。大多数植物源UGTs的催化活性较低,而重组微生物来源的UGTs具有特殊的生物催化特性,如底物杂交性和在微生物底物细胞中的高表达水平,这使得它们成为生产人参皂苷的有效工具。因此,为了提高目标稀有人参皂苷的产率和产量,有必要提高UGTs的催化活性。
3. 结论及展望
研究表明稀有人参皂苷具有更高的药用价值。同时,因稀有人参皂苷的水溶性低、含量少导致其应用受到限制。不同微生物的胞内或胞外酶对人参皂苷进行水解或糖基化,有效的将多糖基人参皂苷以及低活性苷元转化为稀有人参皂苷,从而提高了人参皂苷生物利用度。
研究发现一些官能团的变化可使分子结构和性质发生变化,从而影响药物与受体的结合而影响药效,如烷基可增加化合物的脂溶性,降低解离度,增加稳定性,以药物的延长作用时间;巯基增加脂溶性,易于药物的吸收;酰胺键易与生物大分子形成氢键,易与受体结合,显示特殊的结构特异性;羟基断裂形成氢键增加化合物的水溶性,从而增加药物的活性。根据人参皂苷的结构推测在C-2、C-11、C-15、C-24、C-30位可发生羟基化反应;C-12、C-13位碳碳双键可以发生加成反应,氧化反应;同时二醇型人参皂苷通过C-12和C-24位氧化作用生成奥克梯隆型皂苷。因此,如能通过对不同微生物和酶的筛选,找到可以对人参皂苷的结构进行修饰的酶,在稀有人参皂苷已有的活性结构的基础上,添加或敲去部分基团,以提高人参皂苷的药理活性和靶向性,提高人参皂苷的生物利用率,为人参皂苷结构修饰提供了新的方向。
同时,随着食品、药品、生物化学领域技术的不断发展,研究者可利用遗传学、分子细胞学等多学科交叉融合,利用高通量筛选和基因组学技术选择或者设计出选择性高、转化率高的酶或者菌种,通过生物学手段以实现高活性稀有人参皂苷及其衍生物的工业化生产,进一步提升稀有人参皂苷及其高活性衍生物的产量,对于充分利用人参资源造福大众有着重要意义。
-
表 1 常见的稀有人参皂苷
Table 1 Structures of rare ginsenosides
人参皂苷 R1 R2 Rg3 -Glc-Glc -H F2 -Glc -Glc Rh2 -Glc -H C-K -H -Glc Rg1 -Glc -Glc Rh1 -Glc -H F1 -H -Glc RT3 -Xyl -Glc Rh3 -Glc − PPD -H − PPT − -H 表 2 已在中国获批的含有稀有人参皂苷的药物
Table 2 Drugs containing rare ginsenosides approved in China
产品名称 代表稀有人参皂苷 功效 参一胶囊 人参皂苷Rg3 与化疗配合用药,
提高治疗癌症的疗效20(S)-人参皂苷 Rg3眼膏 人参皂苷Rg3 抗病毒 20(S)-人参皂苷 Rg3 注射液 人参皂苷Rg3 抗癌 20(S)-原人参二醇原料药 人参皂苷Rg3 抗癌 人参皂苷 CK 片 人参皂苷C-K 类风湿性关节炎 今幸胶囊 人参皂苷Rh2 对免疫力低下人群起到
增强免疫力的功效表 3 人参皂苷的水解途径
Table 3 Hydrolysis pathway of ginsenoside
转化产物 菌株类型 转化途径 C-K Aspergillus niger [31-32] Rb1→Rd→F2→C-K[31,33−34,37,50−52] Penicillium sp. [33−34,52] Rb2→C-O→C-Y→C-K[32,43] Fusarium moniliforme[53] Rb3→Rd→F2→C-K[32] Rc→C-Mc-1→C-Mc→C-K[39] Bifidobacterium breve[37] 总皂苷→C-K[53] Armillaria mellea[43,50] Rd→F2→C-K[50] Lactococcus lactis[39]
Lactobacillus plantarum subsp.[51]Rg3 Cellulosimicrobium cellulans sp.[54] Rb1→Rg3[32] Lactococcus lactis[39] Rb1→Rd→Rg3[37−39,41,45,54] Thermotoga thermarum[40]
Thermotoga petrophila[41]Rb2→Rd→Rg3[40−41,45,54] Bacillus sp.[38]
Bacillus subtilis[36]
Monascus purpureus[45]Rc→Rd→Rg3[40,42,54]
总皂苷→Rg3[36]Rh1 Sphingomonas sp. [55]
Dictyoglomus thermophilum[57]Re→Rg1→Rh1[54−56]
R1→Rg1[57]Rh2 Penicillium[41] Terrabacter ginsenosidimutans[58] Fusarium proliferatum[57-59] Rb1→Rg3→Rh2[34,39] Myrothecium verrucaria[60] Rb1→Rd→Rg3→Rh2[58−59] Lactococcus lactis[51,53,56] Rg3→Rh2[56,60] F2 Bacillus sp.[38] Rb1→Rd→F2[38] Rb1→Gyp-ⅩⅦ→F2[38] -
[1] JIN HEE K, MISEON K, SUN-MI Y, et al. Ginsenoside Rh2 induces apoptosis and inhibits epithelial-mesenchymal transition in HEC1A and Ishikawa endometrial cancer cells[J]. Biomedicine & Pharmacotherapy,2017,96:871−876.
[2] JIANG Z, YANG Y , YANG Y , et al. Ginsenoside Rg3 attenuates cisplatin resistance in lung cancer by downregulating PD-L1 and resuming immune[J]. Biomed Pharm, 2017, 96. 378–383.
[3] YAO W, GUAN Y. Ginsenosides in cancer: A focus on the regulation of cell metabolisms[J]. Biomed Pharmacother, 2022, Oct 10;156: 113756.
[4] YU H, WANG Y, LIU C, et al. Conversion of ginsenoside Rb1 into six types of highly bioactive ginsenoside Rg3 and its derivatives by FeCl3 catalysis[J]. Chem Pharm Bull (Tokyo),2018,66(9):901−906. doi: 10.1248/cpb.c18-00426
[5] ZHANG J, AI Z, HU Y, et al. Remarkable impact of commercial sterilizing on ginsenosides transformation in fresh ginseng pulp based on widely targeted metabolomics analysis[J]. Food Chem X, 2022(Aug 9),15: 100415.
[6] HASEGAWA H, SUNG J H, MATSUMIYAS S, et al. Main ginseng saponin metabolites formed by intestinal bacteria[J]. Planta Medica,1998,62(5):453−457.
[7] HASEGAWA H, SUNG J H, BENNO Y. Role of human intestinal prevotella oris in hydrolyzing ginseng saponins[J]. Planta Medica,1997,63(5):436−440. doi: 10.1055/s-2006-957729
[8] 上官棣华, 刘国诠. 人参成分的代谢研究进展[J]. 中草药,1999(11):865−870. [SHANGGUAN L H, LIU G Q. Research progress in the metabolism of ginseng components[J]. Chinese Traditional and Herbal Drugs,1999(11):865−870. doi: 10.3321/j.issn:0253-2670.1999.11.028 SHANGGUAN L H, LIU G Q. Research progress in the metabolism of ginseng components [J]. Chinese Traditional and Herbal Drugs, 1999(11): 865-870. doi: 10.3321/j.issn:0253-2670.1999.11.028
[9] LEE BYUNG-HOON, LEE, SANG-JUN HUI, et al. In vitro antigenotoxic activity of novel ginseng saponin metabolites formed by intestinal bacteria[J]. Planta Medica,1998,64(6):500−503. doi: 10.1055/s-2006-957501
[10] YOSHIMASA Y, MASAYA H, HIDESHI K. Effects of ginsenosides on impaired performance caused by scopolamine in rats[J]. European Journal of Pharmacology,1996,312(2):149−151. doi: 10.1016/0014-2999(96)00597-3
[11] KIM W Y, KIM J M, HAN S B, et al. Steaming of ginseng at hightemperature enhances biological activity[J]. J Nat Prod,2000,63(12):1702−1704. doi: 10.1021/np990152b
[12] GUO H Y, XING Y, SUN Y Q, et al. Ginsengenin derivatives synthesized from 20(R)-panaxotriol: Synthesis, characterization, and antitumor activity targeting HIF-1 pathway[J]. Ginseng Res,2022,Nov;46(6):738−749.
[13] KIM GO, KIM N, SONG GY, et al. Inhibitory activities of rare ginsenoside Rg4 on cecal ligation and puncture-induced sepsis[J]. Int J Mol Sci, 2022 16; 23(18): 10836. doi: 10.3390/ijms231810836.
[14] CHEN Z, WANG G, XIE X, et al. Ginsenoside Rg5 allosterically interacts with P2RY12 and ameliorates deep venous thrombosis by counteracting neutrophil NETosis and inflammatory response[J]. Front Immunol, 2022 Aug 12;13: 918476.
[15] 刘彦楠. 人参皂苷Rg5的制备及其抗胃癌和乳腺癌活性研究[D]. 西安: 西北大学, 2019 LIU Y N. Preparation of ginsenoside Rg5 and its anti gastric and breast cancer activity[D].Xi’an: Northwest University, 2019.
[16] DENG J J, LIU Y, DUAN Z G, et al. Protopanaxadiol and protopanaxatriol-type saponins ameliorate glucose and lipid metabolism in type 2 diabetes mellitus in high-fat diet /streptozocin-induced mice[J]. Front Pharmacol,2017,8:506. doi: 10.3389/fphar.2017.00506
[17] MAENG Y S, MAHARJAN S, KIM J H, et al. Rk1, a ginsenoside, is a new blocker of vascular leakage acting through actin structure remodeling[J], PLoS One, 2013, 8(7) : e68659.
[18] RYOO N, RAHMAN M A, HWANG H, et al. Ginsenoside Rk1 is a novel inhibitor of NMDA receptors in cultured rat hippocampal neurons[J]. J Ginseng Res,2020,44(3):490−495. doi: 10.1016/j.jgr.2019.04.002
[19] OH J M, LEE J, IM W T, et al. Ginsenoside Rk1 induces apoptosis in neuroblastoma cells through loss of mitochondrial membrane potential and activation of caspases[J]. Int J Mol Sci,2019,20(5):1213. doi: 10.3390/ijms20051213
[20] SIDDIQI M H, SIDDIQI M Z, AHN S, et al. Stimulative effect of ginsenosides Rg5: Rk1 on murine osteoblastic MC3T3-E1 cells[J]. Phytother Res,2014,28(10):1447−1455. doi: 10.1002/ptr.5146
[21] KRISHIKA S, RAHUL VIKRAM S. Production aspects of testosterone by microbial biotransformation and future prospects[J]. Steroids, 2020, 159(C).
[22] 南博, 游颖, 王雨珊, 等. 微生物法转化人参皂苷的研究进展[J]. 食品研究与开发,2017,38(14):196−199. [NAN B, YOU Y, WANG Y S, et al. Research progress on microbial transformation of ginsenosides[J]. Food Research and Development,2017,38(14):196−199. doi: 10.3969/j.issn.1005-6521.2017.14.042 NAN B, YOU Y, WANG Y S, et al. Research Progress on Microbial Transformation of Ginsenosides[J]. Food Research And Development, 2017, 38(14): 196-199 doi: 10.3969/j.issn.1005-6521.2017.14.042
[23] 周中流, 李春燕, 陈林浩, 等. 天然产物皂苷类化合物生物转化的研究进展[J]. 中国实验方剂学杂志,2019,25(16):173−192. [ZHOU Z L, LI C Y, CHEN L H, et al. Biotransformation of natural saponins[J]. Chinese Journal of Experimental Traditional Medical Formulae,2019,25(16):173−192. doi: 10.13422/j.cnki.syfjx.20190815 ZHOU Z L, LI C Y, CHEN L H, et al. Biotransformation of Natural Saponins[J]. Chinese Journal of Experimental Traditional Medical Formulae, 2019, 25(16): 173-192. doi: 10.13422/j.cnki.syfjx.20190815
[24] BAE E A , HAN M J,CHOO M K, et al. Metabolism of 20(S)- and 20(R)-Ginsenoside Rg3 by human intestinal bacteria and its relation to in vitro biological activities[J]. Biol Pharm Bull,2002,25(1):58−63. doi: 10.1248/bpb.25.58
[25] 韩铭鑫, 李方彤, 张琰, 等. 稀有原人参二醇型皂苷的人肠道菌群生物转化[J]. 高等学校化学学报,2019,40(7):1390−1396. [HAN M X, LI F T, ZHANG Y, et al. Biotransformation of rare protopanaxadiol saponinby human intestinal microflora[J]. Chemical Journal of Chinese Universities,2019,40(7):1390−1396. doi: 10.7503/cjcu20180812 HAN M X, LI F T, ZHANG Y, et al. Biotransformation of Rare Protopanaxadiol Saponinby Human Intestinal Microflora[J]. Chemical Journal of Chinese Universities, 2019, 40(07): 1390-1396. doi: 10.7503/cjcu20180812
[26] 张琰, 李方彤, 韩铭鑫, 等. 通过RRLC-Q-TOF MS和UPLC-QQQ MS分析原人参三醇型皂苷在人肠道菌群中的代谢产物[J]. 质谱学报,2020,41(1):66−75. [ZHANG Y, LI F T, HAN M X, et al. Analysis of metabolites of protopanaxatriol saponins in human intestinal flora by RRLC-Q-TOF MS and UPLC-QQQ MS[J]. Journal of Chinese Mass Spectrometry Society,2020,41(1):66−75. doi: 10.7538/zpxb.2019.0046 ZHANG Y, LI F T, HAN M X, et al. Analysis of Metabolites of Protopanaxatriol Saponins in Human Intestinal Flora by RRLC-Q-TOF MS and UPLC-QQQ MS[J]. Journal of Chinese Mass Spectrometry Society, 2020, 41(01): 66-75. doi: 10.7538/zpxb.2019.0046
[27] 唐岚, 傅璐璐, 沈丽婷, 等. 大鼠肠道菌群对三七总皂苷体外降解的研究[J]. 中草药,2018,49(2):396−399. [TANG L, FU L L, SHEN L T, et al. Degradation of total saponins of Panax notoginseng by intestinal flora of ratsi n vitro[J]. Chinese Traditional and Herbal Drugs,2018,49(2):396−399. doi: 10.7501/j.issn.0253-2670.2018.02.020 TANG L, FU L L, SHEN L T, et al. Degradation of total saponins of Panax notoginseng by intestinal flora of rats in vitro[J]. Chinese Traditional and Herbal Drugs, 2018, 49(02): 396-399. doi: 10.7501/j.issn.0253-2670.2018.02.020
[28] GUO Y P, CHEN M Y, SHAO L, et al. Quantification of panax notoginseng saponins metabolites in rat plasma with in vivo gut microbiota-mediated biotransformation by HPLC-MS/MS[J]. Chinese Journal of Natural Medicines,2019,17(3):231−240. doi: 10.1016/S1875-5364(19)30026-3
[29] 陈思键, 吴冬雪, 刘淑莹, 等. 人参皂苷化学转化与生物转化研究进展[J]. 中成药,2022,44(5):1539−1545. [CHEN S J, WU D X, LIU S Y, et al. Advances in chemical and biological transformation of ginsenoside[J]. Chinese Traditional Patent Medicine,2022,44(5):1539−1545. doi: 10.3969/j.issn.1001-1528.2022.05.031 CHEN S J, WU D X, LIU S Y, et al. Advances in chemical and biological transformation of ginsenoside[J]. Chinese Traditional Patent Medicine, 2022, 44(5): 1539-1545. doi: 10.3969/j.issn.1001-1528.2022.05.031
[30] 王珊珊, 胡萍, 余少文. 天然产物微生物转化的研究进展[J]. 中国新药杂志,2016,25(1):71−75. [WANG S S, HU P, YU S W. Progress in research of biotransformation of natural products[J]. Chinese Journal of New Drugs,2016,25(1):71−75. WANG S S, HU P, YU S W. Progress in research of biotransformation of natural products [J]. Chinese Journal of New Drugs, 2016, 25(1): 71-75.
[31] 高娟, 周安东, 原野, 等. 黑曲霉降解人参皂苷Rb1制备稀有皂苷Compound K[J]. 生物技术进展,2016,6(2):98−104. [GAO J, ZHOU A D, YUAN Y, et al. Enzymatic degradation of ginsenoside Rb1 for preparation of compound K by Aspergillus niger sp. J7[J]. Current Biotechnology,2016,6(2):98−104. doi: 10.3969/j.issn.2095-2341.2016.02.04 GAO J, ZHOU A D, YUAN Y, et al. Enzymatic Degradation of Ginsenoside Rb1 for Preparation of Compound K by Aspergillus niger sp. J7[J]. Current Biotechnology, 2016, 6(02): 98-104. doi: 10.3969/j.issn.2095-2341.2016.02.04
[32] LIU C Y, ZUO K Z, YU H S, et al. Preparation of minor ginsenosides C-Mx and C-K from notoginseng leaf ginsenosides by a special ginsenosidase type-I[J]. Process Biochemistry,2015,50(12):2158−2167. doi: 10.1016/j.procbio.2015.10.011
[33] SONG X L, WU H, PIAO X C, et al. Microbial transformation of ginsenosides extracted from Panax ginseng adventitious roots in an airlift bioreactor[J]. Electronic Journal of Biotechnology,2017,26:20−26. doi: 10.1016/j.ejbt.2016.12.005
[34] YAN Q, ZHOU W, SHI X L, et al. Biotransformation pathways of ginsenoside Rb1 to compound K by β-glucosidases in fungus Paecilomyces bainier sp. 229[J]. Process Biochemistry, 2010, 45(9): 1550-1556.
[35] YAN Q, ZHOU W, SHI X L, et al. Biotransformation pathways of ginsenoside Rb1 to compound K by β-glucosidases in fungus Paecilomyces bainier sp. 229[J]. Process Biochemistry,2010,45(9):1550−1556. doi: 10.1016/j.procbio.2010.06.007
[36] 陈旸, 张美萍, 王义, 等. 枯草芽孢杆菌转化人参总苷为Rg3的研究[J]. 时珍国医国药,2014,25(11):2676−2678. [CHEN Y, ZHANG M P, WANG Y, et al. Microbial transformed ginsenoside Rg3 from total saponins of Panax ginseng by Bacillus subtilis[J]. Lishizhen Medicine and Materia Medica Research,2014,25(11):2676−2678. CHEN Y, ZHANG M P, WANG Y, et al. Microbial transformed ginsenoside Rg3 from total saponins of Panax ginseng by Bacillus subtilis [J]. Lishizhen Medicine and Materia Medica Research, 2014, 25(11): 2676-2678.
[37] ZHANG R, HUANG X M, YAN H J, et al. Highly selective production of compound k from ginsenoside Rd by hydrolyzing glucose at C-3 glycoside using β-glucosidase of bfidobacterium breve ATCC 15700[J]. Journal of Microbiology and Biotechnology,2019,29(3):410−418. doi: 10.4014/jmb.1808.08059
[38] ALMANDO G, NI M, FATIMAHAB, al. Enzymatic biotransformation of ginsenoside Rb1 by recombinant β-glucosidase of bacterial isolates from Indonesia[J]. Biocatalysis and Agricultural Biotechnology,2020,23(C):101449−101449.
[39] LI L, LEE SOO JIN, YUAN Q P, et al. Production of bioactive ginsenoside Rg3(S) and compound K using recombinant Lactococcus lactis[J]. Journal of Ginseng Research,2017,42(4):412−418.
[40] PEI J J, XIE J C, YIN R, et al. Enzymatic transformation of ginsenoside Rb1 to ginsenoside 20(S)-Rg3 by GH3 β-glucosidase from Thermotoga thermarum DSM 5069 T[J]. Journal of Molecular Catalysis B:Enzymatic,2015,113:104−109. doi: 10.1016/j.molcatb.2014.12.012
[41] ZHANG S H, XIE J C, ZHAO L G. Cloning, overexpression and characterization of a thermostable β-xylosidase fromThermotoga petrophila and cooperated transformation of ginsenoside extract to ginsenoside 20(S)-Rg3 with a β-glucosidase[J]. Bioorganic Chemistry,2019,85:159−167. doi: 10.1016/j.bioorg.2018.12.026
[42] 李琦, 童欣怡, 蒋玉洁, 等. 全细胞催化剂pelB-Xln-DT构建及其在水解三七皂苷R1中的应用[J]. 林业工程学报,2020,5(4):114−120. [LI Q, TONG X Y, JIANG Y J, et al. Construction of whole cell catalyst pelB-Xln-DT and its application in biotransformation of Panax notoginsenoside R1[J]. Journal of Forestry Engineering,2020,5(4):114−120. LI Q, TONG X Y, JIANG Y J, et al. Construction of whole cell catalyst pelB-Xln-DT and its application in biotransformation of Panax notoginsenoside R1[J]. Journal of Forestry Engineering, 2020, 5(04): 114-120.
[43] MIN-JI KIM, JITENDRA UPADH A Y, MIN-SUNYOON, et al. Highly regioselective biotransformation of ginsenoside Rb2 into compound Y and compound K by β-glycosidase purified from armillaria mellea mycelia[J]. Journal of Ginseng Research,2017,42(4):504−511.
[44] 钟雅婷. 人参皂苷转化菌株 GsBt3 的筛选及其转化西洋参总皂苷的研究[D]. 上海: 上海师范大学, 2012 ZHONG Y T. Screening of ginseng saponin transforming strain GsBt3 and its transformation into total saponins of Panax quinquefolium[D]. Shanghai: Shanghai Normal University, 2012.
[45] 丛悦怡, 孙佳, 于恩, 等. 红曲霉发酵转化人参皂苷Rg3的研究[J]. 中草药,2018,49(6):1298−1303. [CONG Y Y, SUN J, YU E, et al. Study on transformation of ginsenoside Rg3 fermented by Monascus purpureus[J]. Chinese Traditional and Herbal Drugs,2018,49(6):1298−1303. doi: 10.7501/j.issn.0253-2670.2018.06.010 CONG Y Y, SUN J, YU en, et al. Study on transformation of ginsenoside Rg3 fermented by Monascus purpureus[J]. Chinese Traditional and Herbal Drugs, 2018, 49(06): 1298-1303. doi: 10.7501/j.issn.0253-2670.2018.06.010
[46] 蒋磊, 赵寿经, 李然, 等. 酶法转化人参皂苷Re为Rg1的研究[J]. 特产研究,2006(2):28−31. [JIANG L, ZAO S J, LI R, et al. A research of converting ginsenoside Re to Rg1 in enzyme reaction[J]. Special Wild Economic Animal and Plant,2006(2):28−31. JIANG L, ZAO S J, LI R, et al. A research of converting ginsenoside Re to Rg1 in enzyme reaction[J] Special Wild Economic Animal and Plant, 2006(2):28-31
[47] ZHUANG Y, YANG G Y, CHEN X H, et al. Biosynthesis of plant-derived ginsenoside Rh2 in yeast via repurposing a key promiscuous microbial enzyme[J]. Metabolic Engineering,2017,42:25−32. doi: 10.1016/j.ymben.2017.04.009
[48] BYEONG-MIN JEON, JONG-IN BAEK, MIN-SUNG KIM, et al. Characterization of a novel ginsenoside MT1 produced by an enzymatic transrhamnosylation of protopanaxatriol-type ginsenosides Re[J]. Biomolecules,2020,10(4):525−525. doi: 10.3390/biom10040525
[49] MUHAMMAD ZUBAIR SIDDIQI, HIPOLITO AMARAL XIMENES, BONG-KYU SONG, et al. Enhanced production of ginsenoside Rh2(S) from PPD-type major ginsenosides using BglSk cloned from Saccharibacillus kuerlensis together with two glycosidase in series[J]. Saudi Journal of Biological Sciences,2021,04:079.
[50] JITENDRA UPADHYAYA, MIN-JI KIM, YOUNG-HOI KIM, et al. Enzymatic formation of compound-K from ginsenoside Rb1 by enzyme preparation from cultured mycelia of Armillaria mellea[J]. Journal of Ginseng Research,2016,40(2):105−112. doi: 10.1016/j.jgr.2015.05.007
[51] HYOJIN L, SEUNG I A, BYUNG WOOK YANG, et al. Biotransformation of ginsenosides by eoyukjang-derived lactic acid bacteria in mountain-cultivated ginseng[J]. Microbiology and Biotechnology Letters,2019,47(2):201−210. doi: 10.4014/mbl.1810.10003
[52] YE L, ZHOU C Q, ZHOU W, et al. Biotransformation of ginsenoside Rb1 to ginsenoside Rd by highly substrate-tolerant Paecilomyces bainier 229-7[J]. Bioresource Technology,2010,101(20):7872−7876. doi: 10.1016/j.biortech.2010.04.102
[53] 杨元超, 王英平, 闫梅霞, 等. 人参皂苷compound K转化菌株的筛选[J]. 中国中药杂志,2011,36(12):1596−1598. [YANG Y C, WANGY P, YAN M X, et al. Screening of plant pathogenic fungi by ginsenoside compound K production[J]. China Journal of Chinese Materia Medica,2011,36(12):1596−1598. YANG Y C, WANGY P, YAN M X, et al. Screening of plant pathogenic fungi by ginsenoside compound K production[J]. China Journal of Chinese Materia Medica, 2011, 36(12): 1596-1598.
[54] HU Y B, WANG N, YAN X C, et al. Ginsenoside Re impacts on biotransformation products of ginsenoside Rb1 by Cellulosimicrobium cellulans sp. 21 and its mechanisms[J]. Process Biochemistry, 2019, 77: 57-62.
[55] 金艳, 金香梅, 尹成日. 鞘氨醇单胞菌2-F2将人参主皂苷Re转化为人参稀有皂苷Rh1[J]. 延边大学农学学报,2011,33(2):103−107. [JIN Y, JIN X M, YIN C R. Biotransformation of major ginsenoside Re to minor ginsenoside Rh1 by Sphingomonas sp. 2-F2[J]. Agricultural Science Journal of Yanbian University,2011,33(2):103−107. doi: 10.3969/j.issn.1004-7999.2011.02.006 JIN Y, JIN X M, YIN C R. Biotransformation of major ginsenoside Re to minor ginsenoside Rh1by Sphingomonas sp. 2-F2[J]. Agricultural Science Journal of Yanbian University, 2011, 33(02): 103-107. doi: 10.3969/j.issn.1004-7999.2011.02.006
[56] 梁志齐, 张京楼, 金海珠, 等. 人参皂苷Rg3生物转化法制备Rh2[J]. 人参研究,2018,30(3):6−10. [LIANG Z Q, ZHANG J L, JING H Z, et al. Microbiological transformation of ginsenoside Rg3 into Rh2[J]. Ginseng Research,2018,30(3):6−10. doi: 10.19403/j.cnki.1671-1521.2018.03.002 LIANG Z Q, ZHANG J L, JING H Z, et al. Microbiological Transformation of Ginsenoside Rg3 into Rh2[J]. Ginseng Research, 2018, 30(03): 6-10. doi: 10.19403/j.cnki.1671-1521.2018.03.002
[57] SU J H, XU J H, LU W Y, et al. Enzymatic transformation of ginsenoside Rg3 to Rh2 using newly isolatedFusarium proliferatum ECU2042[J]. Journal of Molecular Catalysis B Enzymatic,2006,38(2):113−118. doi: 10.1016/j.molcatb.2005.12.004
[58] 陈小春, 戴柱, 傅荣昭. 生物转化法制备稀有人参皂苷Rh2[J]. 江西化工,2019(2):55−57. [CHEN X C, DAI Z, FU R Z. Biocatalytic synthesis of rare ginsenoside Rh2[J]. Jiangxi Chemical Industry,2019(2):55−57. doi: 10.3969/j.issn.1008-3103.2019.02.016 CHEN X C, DAI Z, FU R Z. Biocatalytic Synthesis of rare ginsenoside Rh2[J]. Jiangxi Chemical Industry, 2019(02): 55-57. doi: 10.3969/j.issn.1008-3103.2019.02.016
[59] SU J H, XU J H, YU H L, et al. Properties of a novel β-glucosidase from Fusarium proliferatum ECU2042 that converts ginsenoside Rg3 into Rh2[J]. Journal of Molecular Catalysis B Enzymatic,2009,57(1-4):278−283. doi: 10.1016/j.molcatb.2008.09.017
[60] 吴秀丽, 王艳, 赵文倩, 等. 一种真菌对人参皂苷Rg3的转化[J]. 微生物学报,2008(9):1181−1185. [WU X L, WANG Y, ZHAO W Q, et al. Fungal biotransformation of ginsenoside Rg3[J]. Acta Microbiologica Sinica,2008(9):1181−1185. doi: 10.3321/j.issn:0001-6209.2008.09.008 WU X L, WANG Y, ZHAO W Q, et al. Fungal biotransformation of ginsenoside Rg3[J]. Acta Microbiologica Sinica, 2008(09): 1181-1185. doi: 10.3321/j.issn:0001-6209.2008.09.008
[61] CHEN H, DONG, ZHI F, et al. Discovery, synthesis, and structure-activity relationships of 20S-dammar-24-en-2α, 3β, 12β, 20-tetrol (GP) derivatives as a new class of AMPKα2β1γ1 activators[J]. Bioorganic & medicinal chemistry,2016,24(12):2688−96.
[62] XIN S, JL A, YU X A, et al. Highly regioselective biotransformation of ginsenoside Rg1 to 25-OH derivatives of 20(S/R)-Rh1 by cordyceps sinensis-science direct[J]. Bioorganic & Medicinal Chemistry Letters,2020,30(21):127−504.
[63] LIU J S, YU X N, QIU Z D, et al. Cordyceps sinensis-mediated biotransformation of notoginsenoside R1 into 25-OH-20(S/R)-R2 with elevated cardioprotective effect against DOX induced cell injury[J]. RSC Advances,2022,12:129−38. doi: 10.1039/D1RA08249C
[64] CHEN G T, GE H J, SONG Y, et al. Biotransformation of 20(S)-protopanaxatriol by Mucor racemosus and the anti-cancer activities of some products[J]. Biotechnology Letters,2015,37(10):2005−2009. doi: 10.1007/s10529-015-1877-2
[65] KIM M Y, CHO J Y. 20S-dihydroprotopanaxadiol, a ginsenoside derivative, boosts innate immune responses of monocytes and macrophages[J]. Journal of Ginseng Research,2013,Jul,37(3):293−9.
[66] AKANAKA M, ZHU P, BO Z, et al. Intravenous infusion of dihydroginsenoside Rb1 prevents compressive spinal cord injury and ischemic brain damage through upregulation of VEGF and Bcl-XL[J]. J Neurotrauma,2007,24(6):1037−1054. doi: 10.1089/neu.2006.0182
[67] CHEN F, ZHENG S L, HU J N, et al. Octyl ester of ginsenoside Rh2 induces apoptosis and G1 cell cycle arrest in human HepG2 cells by activating the extrinsic apoptotic pathway and modulating the Akt/p38 MAPK signaling pathway[J]. Journal of Agricultural & Food Chemistry,2016,acs.jafc.:6b03519.
[68] DU G J, DAI Q, WILLIAMS S, et al. Synthesis of protopanaxadiol derivatives and evaluation of their anticancer activities[J]. Anti-cancer Drugs,2011,22(1):35. doi: 10.1097/CAD.0b013e32833fde29
[69] XU D, TAO L, YAN L, et al. 2-Pyrazine-PPD, a novel dammarane derivative, showed anticancer activity by reactive oxygen species-mediate apoptosis and endoplasmic reticulum stress in gastric cancer cells[J]. European Journal of Pharmacology, 2020, 881.
[70] XU D, YUAN Y, FAN Z, et al. 4-XL-PPD, a novel ginsenoside derivative, as potential therapeutic agents for gastric cancer shows anti-cancer activity via inducing cell apoptosis medicated generation of reactive oxygen species and inhibiting migratory and invasive[J]. Biomedicine & Pharmacotherapy,2019(118):108.
[71] LI Y, BALDAUF S, LIM E K, et al. Phylogenetic analysis of the UDP-glycosyltransferase multigene family of Arabidopsis thaliana[J]. Journal of Biological Chemistry,2001,276(6):4338. doi: 10.1074/jbc.M007447200
[72] CHRISTENSEN L P. Ginsenosides: Chemistry, biosynthesis, analysis and potential health effects (Chapter 1)[J]. Adv Food Nutr Res,2008,55(55):1−99.
[73] WANG D D, YEON-JU KIM, NAM BAEK, et al. Glycosyltransformation of ginsenoside Rh2 into two novel ginsenosides using recombinant glycosyltransferase from Lactobacillus rhamnosus and its in vitro applications[J]. Journal of Ginseng Research,2021,45(1):48−57. doi: 10.1016/j.jgr.2019.11.004
[74] HU Y, XUE J, MIN J, et al. Biocatalytic synthesis of ginsenoside Rh2 using Arabidopsis thaliana glucosyltransferase-catalyzed coupled reactions[J]. Journal of Biotechnology,2020,309:107−112. doi: 10.1016/j.jbiotec.2020.01.003
[75] JUNG S C, KIM W, PARK S C, et al. Two ginseng UDP-glycosyltransferases synthesize ginsenoside Rg3 and Rd[J]. Plant & cell physiology,2014,55(12):2177−88.
[76] KHOROLRAGCHAA A, KIM Y J, Rahimi Y J, et al. Grouping and characterization of putative glycosyltransferase genes from Panax ginseng Meyer[J]. Gene, 536(1): 186–192.
[77] WARNECKE D, ERDMANN R, FAHL A, et al. Cloning and functional expression of UGT genes encoding sterol glucosyltransferases from Saccharomyces cerevisiae, Candida albicans, Pichia pastorisand dictyostelium discoideum[J]. J Biol Chem,1999,274(19):13048−13059. doi: 10.1074/jbc.274.19.13048
[78] ZHAO J N, WANG R F, ZHAO S J, et al. Advance in glycosyltransferases, the important bioparts for production of diversified ginsenosides[J]. Chinese Journal of Natural Medicines,2020,18(9):643−658. doi: 10.1016/S1875-5364(20)60003-6
-
期刊类型引用(2)
1. 汪吉鹏,朱滕滕,刘璐,马铖,魏晓博,刘慧燕,方海田. λ-Red重组技术结合复合诱变提高大肠杆菌L-异亮氨酸合成能力. 食品工业科技. 2025(02): 167-174 .  本站查看
本站查看
2. 戴一佳,赵亮. 益生菌对发酵乳品质影响的研究进展. 食品工业科技. 2024(08): 388-396 .  本站查看
本站查看
其他类型引用(3)





 下载:
下载:
 下载:
下载:



