Detecting Donkey Meat Adulterated with Duck Meat by Portable Fluorescence Quantitative PCR
-
摘要: 为建立快速方便的驴肉制品分子鉴定方法,本文以驴肉和常见的掺假肉类(鸭肉)为研究对象,筛选特异性引物和TaqMan探针,利用便携式Mini8 Plus实时荧光定量PCR仪进行灵敏度和特异性实验,通过绘制扩增标准曲线及确定驴肉和鸭肉的质量与DNA比值常数,对不同掺入比例(加入定量的鸭肉制成含量分别为20%、40%、60%、80%)的模拟样品和实际驴肉样品进行检测。结果显示,该方法对驴、鸭肉均具有良好的特异性,可以与马、猪、山羊、梅花鹿、牛、鸡、狗肉明显区分;对驴源性DNA成分的检出限为0.01 ng/μL,鸭源性DNA成分的检出限为0.1 ng/μL,对驴肉与鸭肉混合物中鸭肉成分的灵敏度为0.1%(w/w);所建立的标准曲线线性关系良好,驴肉DNA扩增标准曲线:y=−3.584x+27.003,R2=0.9982;鸭肉DNA扩增标准曲线:y=−3.538x+30.907,R2=0.9991;采用已建立的方法对35份驴肉样本进行市场试点调查,发现6份(17.1%)驴肉样本中含有鸭肉成分。以上研究结果说明,该实时荧光定量PCR方法可用于驴肉产品中其他掺假肉类(鸭)的快速、准确检测,为驴肉及其制品的市场监管和相关执法提供有力的技术保障。Abstract: To develop a fast and convenient method for the molecular identification of donkey meat products, this study selected donkey meat and duck meat (a common meat used for adulteration) as the research objects, screened specific primers and TaqMan probes, and conducted sensitivity and specificity experiments with a portable Mini8 Plus real-time fluorescence quantitative PCR instrument. By drawing the amplification standard curve and determining the mass/DNA ratio constants of donkey meat and duck meat, it detected the real donkey meat samples and the simulated samples adulterated with different proportions of duck meat (20%, 40%, 60%, and 80%, respectively). The results showed that the method had good specificity for both donkey meat and duck meat, which could be clearly distinguished from meat from horses, pigs, goats, sika deer, cattle, chickens, and dogs. The detection limit of donkey-derived DNA components was 0.01 ng/μL, and that of duck-derived DNA components was 0.1 ng/μL. Its sensitivity to duck meat components in the mixture of donkey meat and duck meat was 0.1% (w/w). The established standard curve showed a sound linear relationship. The amplification standard curve of donkey DNA was: y=−3.584x+27.003, R2=0.9982. The amplification standard curve of duck DNA was: y=−3.538x+30.907, R2=0.9991. A market pilot survey was conducted on 35 donkey meat samples using the established method, and revealed that 6 donkey meat samples (17.1%) contained duck meat. These results indicated that the real-time PCR method could be used for the rapid and accurate detection of other meat (such as duck meat) adulterated in donkey meat products. The combination of a simple detection procedure with a portable real-time PCR system provides technical support for food adulteration monitoring and control at the retail or market level.
-
Keywords:
- real-time fluorescence PCR /
- donkey meat /
- duck meat /
- adulteration of meat products /
- TaqMan
-
民以食为天,食以安为先[1]。随着发展中国家肉类消费的增长,到2025年有可能超过1万亿美元[2],为了确保向人们提供高质量的产品,所有类型的肉类都必须进行严格的成分和质量分析[3]。另外许多重要的营养物质,如蛋白质、矿物质和维生素等,也都由肉类供应[4-6]。因此肉类中不同种类动物的混合是为最终消费者保证纯产品的一个主要问题[7]。2013年欧洲的马肉风波[8],超市出售的牛肉汉堡被发现含有未标记的马肉和其他肉类成分。这种掺假可能是无意的,因为工业设备、加工、储存和运输清洗不当[9];或有意的,目的是在产品中加入便宜的肉类,以降低成本[10],这不仅危害人体健康,侵犯了消费者的权利,而且对于那些因宗教习俗和民族选择而有饮食限制的消费者来说也是一种禁忌[11]。例如,伊斯兰教禁止食用猪肉[12]。对猪肉蛋白质过敏的现象在某些人群中也很常见[13],此外,在许多文化中都避免食用马肉[14]。肉类掺假导致消费者对卫生组织和食品安全监管机构失去信心和信任。因此,对肉制品进行掺假检测十分重要。
驴肉具有较高的营养价值,其脂肪含量与鸡肉相近,显著低于其他畜肉[15]。驴肉是一种高蛋白、低脂肪[16]的营养食品。驴生长周期长,驴肉供应不足,驴肉价格高,导致许多不法商家为了保证自身利益,在驴肉中掺入鸭肉,严重损害了消费者的权益[17]。
目前,基于蛋白质的肉类检测方法受到蛋白质变性、研发特异性抗体复杂等诸多要素的限制[18-19],未能得到普遍运用;基于光谱技术的检测办法特异性有限,只能为鉴别肉制品掺假提供间接的参考[20-21]。因此,这些技术目前并未普遍得到应用。便携式荧光定量PCR技术使用TaqMan探针法,该探针5’端标记有报告基团,3’端标记有荧光淬灭基团,在PCR过程中,其核心是利用Taq酶的3’→5’外切核酸酶活性,切断探针,产生荧光信号。因为探针和模板是特异性结合的,所以荧光信号的强弱就代表了模板的数量[22]。TaqMan实时荧光定量PCR已广泛应用于肉类的来源鉴定和定量检测[23]。其中,实时荧光定量PCR技术不依赖于抗原和抗体的特异性反应,不受代谢物的影响,可以实现多物种检测,其特异性好、灵敏度高、检测周期短、目标片段扩增小等优点,提高了食品中动物性成分定性鉴定的有效性,逐渐成为肉类物种检测鉴定[24]的核心方法。目前基于荧光定量PCR技术探针法应用广泛,例如,Fang等[25]建立了一种基于cytb设计TaqMan探针的鼠特异性实时PCR反应的检测方法,混合物中鼠的最低检出限为0.1%。Cesare等[26]建立的实时PCR反应方法的扩增效率在95%~100%之间。当加入其他物种时,该方法的最低检出限为1%。Raharjo等[27]开发了一种使用PCR技术检测牛肉丸中小鼠肉污染的特异性方法,设计的引物探针已被证明具有特异性,肉丸中老鼠肉的存在最低可检出5%(w/w)。
本文选用驴肉和鸭肉作为样本,确定驴鸭单拷贝特异性引物和探针,以9种动物基因组DNA为模板,应用便携式荧光PCR技术进行特异性验证、灵敏度分析和检出限试验,构建标准曲线并确定驴肉和鸭肉的质量与DNA比值常数,对驴肉中加入不同比例鸭肉(20%、40%、60%、80%)的模拟样品进行检测,同时对70份市售样品进行该方法的实际应用。为驴肉及其制品的市场监管和相关执法提供有力的技术保障。
1. 材料与方法
1.1 材料与仪器
来自9种动物物种的肉类样本均为标准物质,其中包括驴(NIM-RM4027)、鸭(GBW(E)091060)、猪(NIM-RM4032)、马(NIM-RM4028)、山羊(NIM-RM4031)、牛(NIM-RM4030)、梅花鹿(NIM-RM4026)、鸡(NIM-RM4025)、狗(NIM-RM4024) 购自中国计量科学院;生鲜驴肉和真空包装驴肉 购自保定超市及农贸市场;动物组织DNA中量试剂盒 杭州新景生物试剂开发有限公司;2×Phanta Max Master Mix (Dye Plus)、2×AceQ Universal U+Probe Master Mix V2* 南京诺唯赞生物科技股份有限公司;引物与荧光探针 均由上海生工生物工程技术服务有限公司合成。
NanoDrop 2000超微量分光光度计 Thermo Scientific;便携式Mini8 Plus实时荧光定量PCR仪 卡尤迪生物科技(北京)有限公司。
1.2 实验方法
1.2.1 肉类样品的制备
a.模拟掺假试验测灵敏度驴肉和鸭肉的比例:将驴肉和鸭肉绞成肉馅,按照99.99%:0.01%,99.9%:0.1%,99%:1%,90%:10%的比例充分混合;b.混合肉样中驴肉和鸭肉的比例:将驴肉和鸭肉绞成肉馅,按照20%:80%,40%:60%,60%:40%,80%:20%的比例充分混合。
1.2.2 DNA提取
采用动物组织DNA中量试剂盒说明书上所写步骤提取基因组DNA,提取的基因组DNA用NanoDrop 2000进行浓度检测,DNA提取物储存在−20 ℃冰箱。
1.2.3 引物和探针的筛选
从已经发表的文章和报告中,在NCBI(https://www.ncbi.nlm.nih.gov/)网站上通过BLAST将序列进行比对最终确定驴肉和鸭肉的特异性引物和探针(表1),交由上海生工生物工程技术服务有限公司合成。用6-FAM标记探针,探针3’将用BHQ-1作为淬灭剂,并用无菌水稀释至100 μmol/L作为引物储备液,取适量引物储备液稀释至浓度为10 μmol/L供使用[25]。
表 1 实验所用的引物和探针列表Table 1. The sequence of primers and probes in the experiment1.2.4 便携式荧光PCR扩增体系和参数
按照2×AceQ Universal U+Probe Master Mix V2*说明配制反应体系,体系总体积为20 μL:2×AceQ Universal U+Probe Master Mix V2* 10 μL,正向引物和反向引物各0.4 μL,探针0.2 μL,模板DNA 2 μL,其余用无菌水补齐,离心数秒后,放入便携式Mini8 Plus实时荧光定量PCR仪进行实时PCR扩增。反应条件为:37 ℃污染消化2 min;95 ℃预变性5 min;95 ℃循环反应10 s,60 ℃退火及延伸30 s;40个循环。反应完成后进行分析。
1.2.5 引物和探针的特异性
以山羊、梅花鹿、牛、鸡、狗、猪、马肉提取的DNA模板为阴性对照,驴肉、鸭肉中提取的DNA模板为阳性对照,以无菌水为空白对照,采用便携式Mini8 Plus实时荧光定量PCR仪进行PCR扩增,对筛选的引物和探针进行特异性检测[30]。
1.2.6 检出限
将驴肉和鸭肉DNA的浓度调整为10 ng/μL,用无菌水进行稀释,分别稀释至浓度为10、1、0.1、0.01、0.001 ng/μL,进行荧光定量PCR扩增,实验重复三次,每个梯度两个平行。
1.2.7 模拟掺假试验及灵敏度检测
将鸭肉与驴肉按质量以一定比例混合,制成鸭肉含量为0.01%、0.1%、1%、10%的混合肉样品,提取各组DNA,依照1.2.4项下的反应体系对该方法的灵敏度进行分析,实验重复3次进行,每次3个平行。
1.2.8 定量检测标准曲线
将驴和鸭源性DNA调整为10 ng/μL,进行2倍稀释即10、5、2.5、1.25、0.625 ng/μL。以模板DNA浓度的对数值lg(DNA)为横坐标,循环阈值(Cycle threshold valve,Ct)为纵坐标作图,分别绘制驴和鸭源性DNA扩增标准曲线。
1.2.9 相同质量的驴肉和鸭肉所提出的DNA浓度的差异
分别取驴、鸭肉各0.02 g和0.04 g按照1.2.2方法进行DNA提取,并用微量核酸蛋白仪测定DNA浓度。确定了驴肉和鸭肉质量与DNA浓度的比值常数。
1.2.10 混合肉样中驴肉和鸭肉的质量百分比
按照1.2.1(b)中肉样的比例关系进行混合肉样的制备,并提取总基因组DNA,在1.2.4的条件下进行实时荧光PCR试验,并通过驴肉和鸭肉各自的标准曲线计算出样品中所含有的相应物种的DNA含量:DNA驴,DNA鸭。
1.2.11 市售肉制品掺假的检测
为了验证所建立检测方法在实际驴肉样品检测中的应用价值,本文从超市、农贸市场随机购买了35份驴肉及其制品作为检测对象,包括25份生鲜驴肉和10份真空包装驴肉食品。准确称取经前处理后的200 mg待检样品进行DNA提取,采用现行定性检测标准“SN/T 3730.4-2013《食品及饲料中常见畜类品种的鉴定方法 第4部分:驴成分检测 实时荧光PCR法》[28]”、“NY/T 3309-2018《肉源性成分鉴定 实时荧光定性PCR法》[31]”进行检测,检测结果显示为阳性的样品再利用建立的定量方法进行定量检测,确定驴肉样品中含鸭源性成分的比例。
1.3 数据处理
利用便携式Mini8 Plus实时荧光定量PCR仪自带分析软件(Mini8 Plus),根据测定结果,利用Excel软件绘制线性关系曲线,建立线性关系式。
2. 结果与分析
2.1 基因组DNA的提取和质量表征
经测定,各肉类样品DNA浓度见表2,每个DNA提取物的DNA浓度在10~100 ng/μL之间。肉样DNA的A260 nm/A280 nm均在1.8~2.0之间,这表明所提取的DNA纯度可满足后续PCR反应实验。
表 2 标准样品制备的DNA效果Table 2. DNA effect of standard sample preparation样本名称 A260 nm/A280 nm DNA浓度(ng/μL) 驴 1.91 80.05 鸭 1.90 81.00 马 1.88 76.10 猪 1.89 73.55 山羊 1.80 75.45 牛 1.82 73.41 鸡 1.91 83.80 狗 1.86 85.30 梅花鹿 1.89 78.50 2.2 引物和探针的特异性验证
本研究选择山羊、梅花鹿、牛、鸡、狗、驴、猪、马、鸭的基因组DNA为模板,分别使用表1中所列的引物和探针对模板进行扩增。结果如表3所示,本研究所选用的引物和探针均具有较好的特异性。
表 3 引物与探针DNA扩增特异性验证Table 3. The DNA amplification results for testing the primers and probes驴 鸭 马 猪 山羊 梅花鹿 牛 鸡 狗 驴引物及
探针(Ct)25.40±0.33 N/A N/A N/A N/A N/A N/A N/A N/A 鸭引物及
探针(Ct)N/A 31.89±0.24 N/A N/A N/A N/A N/A N/A N/A 注:N/A:未检测到驴、鸭的DNA。 2.3 检出限及灵敏度检测
通过驴、鸭特异性引物和探针对5个不同质量浓度梯度的样本DNA进行实时荧光定量PCR扩增实验,结果如表4。稀释至0.01 ng/μL的驴基因组DNA样本Ct值为36.81±0.03。稀释至0.1 ng/μL的鸭基因组DNA样本Ct值为35.20±0.15。因此驴基因组DNA成分的检出限为0.01 ng/μL,鸭基因组DNA成分的检出限为0.1 ng/μL。
表 4 驴、鸭源性成分实时荧光PCR法检测限分析结果Table 4. Results of real-time fluorescence PCR for detection of donkey and duck componentsDNA浓度
(ng/μL)10 1 0.1 0.01 0.001 驴(Ct值) 20.78±0.11 24.42±0.01 30.09±0.13 36.81±0.03 N/A 鸭(Ct值) 26.35±0.02 29.91±0.08 35.20±0.15 N/A N/A 注:N/A:未检测到驴、鸭的DNA。 由于鸭肉的市场售价比驴肉低,所以市场上会出现驴肉中掺入鸭肉,从而获得不正当的利益。为了评价此次实验中检测方法的灵敏性,将驴肉和鸭肉按一定比例(10%、1%、0.1%、0.01%,其中百分比表示鸭肉的混合肉中所占比例)混合进行模拟掺假实验,检测结果如表5。当鸭肉质量含量为0.1%时也能成功检测出其源性,且Ct值为35.13±0.10。证明此引物和探针的灵敏度能达到0.1%。本研究建立的方法中驴基因组DNA成分的检出限为0.01 ng/μL,与“SN/T 3730.4-2013”[28](最低检出限为10 ng/μL)检出限相比更低;本研究建立的鸭肉灵敏度为0.1%与“NY/T 3309-2018”[31](灵敏度为1%)灵敏度相比更高。
表 5 模拟掺假样品的配制信息Table 5. Simulated adulterated sample preparation information样品 掺假比例(%) Ct平均值c 结果判断 驴a 鸭b 1 99.99 0.01 N/A 未检测到鸭肉成份 2 99.9 0.1 35.13±0.10 检测到鸭肉成份 3 99 1 33.07±0.22 检测到鸭肉成份 4 90 10 31.10±0.24 检测到鸭肉成份 注:a:驴肉在整个肉样本中的比例(w/w);b:鸭肉在整个肉样中的比例(w/w);c:Ct值是重复分析的平均值(n=3);N/A:未检测到鸭的DNA。 2.4 标准曲线
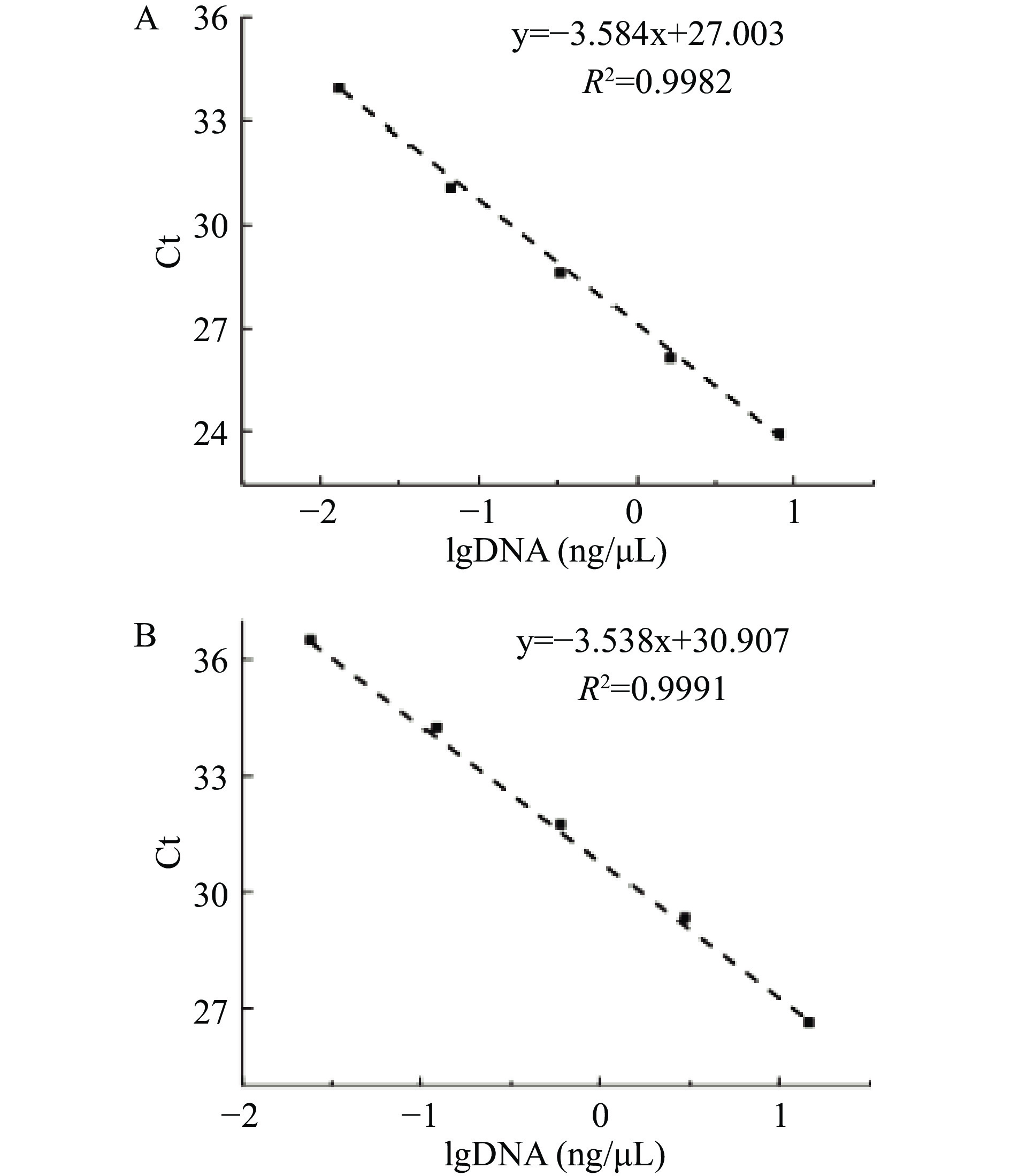
进行定量检测分析时,需要绘制标准曲线,按照1.2.8的方法绘制驴、鸭源性DNA扩增的标准曲线。结果如图1所示,驴肉DNA扩增标准曲线为:y=−3.584x+27.003,R2=0.9982;鸭肉DNA扩增标准曲线:y=−3.538x+30.907,R2=0.9991。建立的标准曲线线性关系良好,可以用于定量检测。
2.5 相同质量的驴肉和鸭肉所提出的DNA浓度的差异
在相同的提取和复溶条件下,来自同一物种、同一组织部位的样品质量和所提取DNA浓度之间的比例应为常数,本研究将其定义为d(106 μL)。为了对混合样本中不同物种的质量百分比进行分析,有必要确定驴肉和鸭肉质量与所提DNA浓度的比值常数d。分别取驴、鸭肉各0.02 g和0.04 g完成测试,按照1.2.2方法提取驴和鸭肉的DNA并测定浓度,按照公式d=M/DNA进行计算,结果如表6所示。当提取0.02 g驴肉样本时,M驴/DNA驴的比值为9.34×10−4;当提取0.04 g驴肉样本时,M驴/DNA驴的比值为9.28×10−4;当提取0.02 g鸭肉样本时,M鸭/DNA鸭的比值为0.40×10−4;当提取0.04 g鸭肉样本时,M鸭/DNA鸭的比值为1.89×10−4。样品质量与DNA浓度的比值,在充分破碎和相同提取方法的前提下,是由肉本身的特性决定的,与样品质量没有关系。当提取肉质量不同时,d值可能会存在操作误差或仪器检测误差。本研究中,d=M/DNA(d为两个不同质量试验的平均数)即d驴=9.31×10−4,d鸭=1.15×10−4。
表 6 驴和鸭肉质量与DNA比值常数确定Table 6. The mathematical conversion parameters about the mass of meat and DNA in donkey and duck样品类别 驴肉 鸭肉 质量(g) 0.02 0.04 0.02 0.04 DNA(ng/μL) 21.4 43.1 476.4 211.2 d(106 μL) 9.31×10−4 1.15×10−4 2.6 鉴定混合肉样中驴肉和鸭肉的质量百分比
按照1.2.1中肉样的比例关系进行混合肉样的制备,并提取总基因组DNA,在1.2.4的条件下进行实时荧光PCR试验,并通过驴肉和鸭肉各自的标准曲线计算出样品中所含有的相应物种的DNA含量:DNA驴,DNA鸭。根据公式d=M/DNA推算出样品中相应物种在样品质量中所占的百分比的公式即M驴/M鸭=(d驴×DNA驴)/(d鸭×DNA鸭),换算公式为M驴/M鸭=(93/11)×(DNA驴×DNA鸭)。结果如表7。
表 7 混合肉样中驴肉和鸭肉含量的定量检测Table 7. Quantitative determination of donkey meat and duck meat in mixed meat samples1 2 3 4 驴肉 鸭肉 驴肉 鸭肉 驴肉 鸭肉 驴肉 鸭肉 理论质
量(g)0.02 0.08 0.04 0.06 0.06 0.04 0.08 0.02 理论混合比例
(质量百分比%)20 80 40 60 60 40 80 20 扩增结果
(Ct)22.09 21.15 21.19 21.57 20.62 22.28 20.19 23.59 DNA
(ng/μL)23.44 575.44 41.69 436.52 60.26 275.42 79.43 117.49 检测混合百分
比例(质量
百分比%)25.62 74.38 44.67 55.33 64.91 35.09 85.11 14.89 绝对误差
(%)5.62 5.62 4.67 4.67 4.91 4.91 5.11 5.11 实验结果表明,检测质量百分比含量与理论混合百分比含量之间的绝对误差可控制在6%以内,满足驴肉中掺杂肉成分的检测要求。
2.7 市售肉制品检测
对于来源于农贸市场和超市的35份驴肉样品采用现行定性检测标准的方法进行检测,检测结果表明35份驴肉样品均检出驴肉成分,其中有6份也同时检出鸭肉成分。再利用建立的便携式荧光定量PCR法对样品进行定量检测。结果显示(表8),35个驴肉样品中有6个样品含有鸭源性成分,其中包括4份生鲜驴肉和2份真空包装驴肉,6份样品的驴肉含量不足100%,但是均大于95%,鸭肉含量很少,对商家无法产生利润。因此可能是样品局部位置受到污染,结合样品来源点为农贸市场,在加工,运输过程中有可能会受到鸭肉成分(鸭血)的污染,因此,判定6份驴肉样品为污染并非故意掺假。
表 8 市售驴肉制品的鸭肉成分荧光PCR检测结果Table 8. Fluorescent PCR detection results of duck meat components in donkey meat products样品种类 数量(份) 掺有鸭肉的样品数(份) 不合格率(份) 生鲜驴肉 25 4 17.1% 真空包装驴肉 10 2 3. 结论
本文建立的便携式荧光定量PCR法具有较强的特异性,驴肉和鸭肉的检出限分别为0.01 ng/μL和0.1 ng/μL,人工掺入鸭肉的驴肉提取DNA进行检测,灵敏度为0.1%(w/w)。建立了标准曲线,得到了良好的线性相关性。根据标准曲线,驴肉掺入鸭肉4种不同掺混比例在检测质量百分比的绝对误差在6%以内。
本实验使用的Mini8 Plus实时定量PCR仪体积小,携带方便,重仅2.1 kg。中文界面30 s完成程序设置,无需专业培训。可随意移动,移动后不用校正,LED光源寿命更长。适合现场检测,大大缩短样品交付时间,可快速检测驴肉掺假情况。本文建立的便携式荧光定量PCR检测方法相比其他肉类检测方法提高了检测效率,扩大了检测适应性位点。
-
表 1 实验所用的引物和探针列表
Table 1 The sequence of primers and probes in the experiment
表 2 标准样品制备的DNA效果
Table 2 DNA effect of standard sample preparation
样本名称 A260 nm/A280 nm DNA浓度(ng/μL) 驴 1.91 80.05 鸭 1.90 81.00 马 1.88 76.10 猪 1.89 73.55 山羊 1.80 75.45 牛 1.82 73.41 鸡 1.91 83.80 狗 1.86 85.30 梅花鹿 1.89 78.50 表 3 引物与探针DNA扩增特异性验证
Table 3 The DNA amplification results for testing the primers and probes
驴 鸭 马 猪 山羊 梅花鹿 牛 鸡 狗 驴引物及
探针(Ct)25.40±0.33 N/A N/A N/A N/A N/A N/A N/A N/A 鸭引物及
探针(Ct)N/A 31.89±0.24 N/A N/A N/A N/A N/A N/A N/A 注:N/A:未检测到驴、鸭的DNA。 表 4 驴、鸭源性成分实时荧光PCR法检测限分析结果
Table 4 Results of real-time fluorescence PCR for detection of donkey and duck components
DNA浓度
(ng/μL)10 1 0.1 0.01 0.001 驴(Ct值) 20.78±0.11 24.42±0.01 30.09±0.13 36.81±0.03 N/A 鸭(Ct值) 26.35±0.02 29.91±0.08 35.20±0.15 N/A N/A 注:N/A:未检测到驴、鸭的DNA。 表 5 模拟掺假样品的配制信息
Table 5 Simulated adulterated sample preparation information
样品 掺假比例(%) Ct平均值c 结果判断 驴a 鸭b 1 99.99 0.01 N/A 未检测到鸭肉成份 2 99.9 0.1 35.13±0.10 检测到鸭肉成份 3 99 1 33.07±0.22 检测到鸭肉成份 4 90 10 31.10±0.24 检测到鸭肉成份 注:a:驴肉在整个肉样本中的比例(w/w);b:鸭肉在整个肉样中的比例(w/w);c:Ct值是重复分析的平均值(n=3);N/A:未检测到鸭的DNA。 表 6 驴和鸭肉质量与DNA比值常数确定
Table 6 The mathematical conversion parameters about the mass of meat and DNA in donkey and duck
样品类别 驴肉 鸭肉 质量(g) 0.02 0.04 0.02 0.04 DNA(ng/μL) 21.4 43.1 476.4 211.2 d(106 μL) 9.31×10−4 1.15×10−4 表 7 混合肉样中驴肉和鸭肉含量的定量检测
Table 7 Quantitative determination of donkey meat and duck meat in mixed meat samples
1 2 3 4 驴肉 鸭肉 驴肉 鸭肉 驴肉 鸭肉 驴肉 鸭肉 理论质
量(g)0.02 0.08 0.04 0.06 0.06 0.04 0.08 0.02 理论混合比例
(质量百分比%)20 80 40 60 60 40 80 20 扩增结果
(Ct)22.09 21.15 21.19 21.57 20.62 22.28 20.19 23.59 DNA
(ng/μL)23.44 575.44 41.69 436.52 60.26 275.42 79.43 117.49 检测混合百分
比例(质量
百分比%)25.62 74.38 44.67 55.33 64.91 35.09 85.11 14.89 绝对误差
(%)5.62 5.62 4.67 4.67 4.91 4.91 5.11 5.11 表 8 市售驴肉制品的鸭肉成分荧光PCR检测结果
Table 8 Fluorescent PCR detection results of duck meat components in donkey meat products
样品种类 数量(份) 掺有鸭肉的样品数(份) 不合格率(份) 生鲜驴肉 25 4 17.1% 真空包装驴肉 10 2 -
[1] SAFDAR M, JUNEJO Y, ARMAN K, et al. A highly sensitive and specific tetraplex PCR assay for soybean, poultry, horse and pork species identification in sausages: Development and validation[J]. Meat Science,2014,98(2):296−300. doi: 10.1016/j.meatsci.2014.06.006
[2] ZHANG W J, XUE J H, et al. Economically motivated food fraud and adulteration in China: An analysis based on 1553 media reports[J]. Food Control,2016,67:192−198. doi: 10.1016/j.foodcont.2016.03.004
[3] ALI A A, ALTEMIMI A B, ALHELFI N, et al. Application of biosensors for detection of pathogenic food bacteria: A review[J]. Biosensors,2020,10(6):58. doi: 10.3390/bios10060058
[4] CASCELLA M, BIMONTE S, BARBIERI A, et al. Dissecting the mechanisms and molecules underlying the potential carcinogenicity of red and processed meat in colorectal cancer (CRC): An overview on the current state of knowledge[J]. Infectious Agents & Cancer,2018,13(1):3.
[5] KAMRUZZAMANM, MAKINOY, OSHITAS. Non-invasive analytical technology for the detection of contamination, adulteration, and authenticity of meat, poultry, and fish: A review[J]. Analytica Chimica Acta,2015,853:19−29. doi: 10.1016/j.aca.2014.08.043
[6] ZHENG X, LI Y, WEI W, et al. Detection of adulteration with duck meat in minced lamb meat by using visible near-infrared hyperspectral imaging[J]. Meat Science,2019,149:55−62. doi: 10.1016/j.meatsci.2018.11.005
[7] KANE D E, HELLBERG R S. Identification of species in ground meat products sold on the US commercial market using DNA-based methods[J]. Food Control,2015,59:158−163.
[8] 杜鹏. “马肉风波”与欧盟肉制品安全监管制度[J]. 世界农业,2015(4):82−86, 168. [DU P. Horsemeat incident and EU meat product safety supervision system[J]. World Agriculture,2015(4):82−86, 168. [9] SUPIAN K. Cross-contamination in processing, packaging, storage, and transport in halal supply chain[J]. 2018: 309−321.
[10] LIANOU A, PAPAKONSTANTINOU M, NYCHAS G, et al. Fraud in meat and poultry products[J]. Food Fraud,2021:85−108.
[11] 王建昌, 王金凤, 陈瑞春, 田振祥. 鸭肉冒充牛羊肉的分子生物学检测[J]. 肉类研究,2012,26(6):20−23. [WANG J C, WANG J F, CHEN R C, et al. Molecular biological detection of adulteration of mutton and beef with duck[J]. Meat Research,2012,26(6):20−23. [12] 彭媛媛, 武煊, 陶晓奇. 实时荧光PCR技术定量检测肉类掺假的研究进展[J]. 食品与发酵工业,2019,45(15):279−287. [PENG Y Y, WU X, TAO X Q. Quantitative detection of meat adulteration by real-time fluorescent PCR[J]. Food and Fermentation Industries,2019,45(15):279−287. doi: 10.13995/j.cnki.11-1802/ts.020130 [13] WILSON J M, PLATTS-MILLS T A E. Meat allergy and allergens[J]. Molecular Immunology,2018,100:107−112. doi: 10.1016/j.molimm.2018.03.018
[14] SMITH R, MCELWEE G. The “horse-meat” scandal: Illegal activity in the food supply chain[J]. Supply Chain Management,2020,26(5):565−578.
[15] 孙亚波, 刘正伟, 吕丹娜, 等. 不同蛋白水平日粮对辽西驴肉质营养成分的影响[J]. 饲料研究,2021,44(18):96−100. [SUN Y B, LIU Z W, LÜ D N, et al. Effect of different protein levels rations on meat nutrition composition of Liaoxi donkeys[J]. Feed Research,2021,44(18):96−100. doi: 10.13557/j.cnki.issn1002-2813.2021.18.022 [16] 尤娟, 罗永康, 张岩春, 等. 驴肉主要营养成分及与其它畜禽肉的分析比较[J]. 肉类研究,2008(7):20−22. [YOU J, LUO Y K, ZHANG Y C, et al. Nutrition composition of donkey meat and comparison with other livestock and poultry meat[J]. Meat Research,2008(7):20−22. doi: 10.3969/j.issn.1001-8123.2008.07.008 [17] 海小, 刘国强, 罗建兴, 等. 基于TaqMan实时荧光PCR检测鲜肉及加工肉制品中的驴源性成分[J]. 肉类研究,2018,32(4):62−67. [HAI X, LIU G Q, LUO J X, et al. Identification of donkey-derived ingredients in raw and processed meats using taqman probe based real-time polymerase chain reaction[J]. Meat Research,2018,32(4):62−67. [18] KREUZ G, ZAGON J, BROLL H, et al. Immunological detection of osteocalcin in meat and bone meal: A novel heat stable marker for the investigation of illegal feed adulteration[J]. Food Additives & Contaminants,2012,29(5):716−726.
[19] NURJULIANA M, CHE M Y, MAT H D. Rapid identification of pork for halal authentication using the electronicnose and gas chmmatography mass spectrometer with headspace analyzer[J]. Meat Science,2018,8(4):638−644.
[20] CHIC C, SHOWP L, KUOM L, et al. Fast differentiation of meats from fifteen animal species by liquid chromatography with electrochemical detection using copper nanoparticle plated electrodes[J]. Journal of Chromatography B,2007,846(1/2):230−239.
[21] KUMAR Y, KARNE S C. Spectral analysis: A rapid tool for species detection in meat products[J]. Trends in Food Science & Technology,2017,62:59−67.
[22] BAIIIN N Z, VOGENSEN F K, KARLSSON A H. Species determination: Can we detect and quantify meat adulteration?[J]. Meat Science,2009,83(2):165−174. doi: 10.1016/j.meatsci.2009.06.003
[23] MIGUEL A R, TERESA G, ISABEL G, et al. TaqMan real-time PCR for the detection and quantitation of pork in meat mixtures[J]. Meat Science,2005,70(1):113−120. doi: 10.1016/j.meatsci.2004.12.005
[24] KESMEN Z, GULLUCE A, SAHIN F, et al. Identification of meat species by TaqMan-based real-time PCR assay[J]. Meat Science,2009,82(4):444−449. doi: 10.1016/j.meatsci.2009.02.019
[25] FANG X, ZHANG C. Detection of adulterated murine components in meat products by TaqMan real-time PCR[J]. Food Chemistry,2016,192:485−490. doi: 10.1016/j.foodchem.2015.07.020
[26] CESARE C, MARCO D D, FEDERICA M. Development and validation of fast real-time PCR assays for species identification in raw and cooked meat mixtures[J]. Food Control,2012,23(2):400−404. doi: 10.1016/j.foodcont.2011.08.007
[27] RAHARJO T J, NURYANTI I, PATRIA F P, et al. Mitochondrial ND-1 gene-specific primer polymerase chain reaction to determine mice contamination in meatball[J]. International Food Research Journal,2018,25(2):638−642.
[28] 中华人民共和国国家质量监督检验检疫总局. SN/T 3730.4-2013. 食品及饲料中常见畜类品种的鉴定方法第4部分: 驴成分检测实时荧光PCR法[S]. 北京: 中国标准出版社, 2017. General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China. SN/T 3730.4-2013. Identification of domestic animal ingredient in food and feed-Part 4: Detection ofdonkey ingredient-real-time PCR method[S]. Beijing: China Standards Press, 2017.
[29] 陈笑云, 徐晓丽, 徐俊锋, 等. 鸭源性IL-2基因DNA标准物质及其用途, CN111088367A[P]. 2020 CHEN X Y, XU X L, XU J F, et al. Duck DERIVED IL-2 gene DNA standard material and its use, CN111088367A[P]. 2020.
[30] CHEN C, CHEN J, ZHANG Y, et al. Quantitative detection of beef and beef meat products adulteration by the addition of duck meat using micro drop digital polymerase chain reaction[J]. Journal of Food Quality,2020,2020(6):1−8.
[31] 中华人民共和国农业农村部. NY/T 3309-2018 肉类源性成分鉴定实时荧光定性PCR法[S]. 北京: 中国标准出版社, 2017 Ministry of Agriculture and Rural Affairs of the People's Republic of China. NY/T 3309-2018. Identification of animal-derived materials in meat-Qualitative realtime PCR method[S]. Beijing: China Standards Press, 2017.
-
期刊类型引用(6)
1. 马晓青,孙皓岩,魏宝红,胡淑曼,杨文哲,杨雪. 紫贻贝酶解工艺及抗炎活性研究. 食品科技. 2024(01): 116-120 .  百度学术
百度学术
2. 程钰,胡蓉,李强,蒋蕾,何跃辉,卢静,王淑军. 复合蛋白酶水解菲律宾蛤蜊及产物的生物学活性研究. 食品科技. 2024(06): 134-141 .  百度学术
百度学术
3. 付雪媛,杜芬,孙呈浩,王明丽,王长伟. 蛤蜊肽的制备工艺优化及其增强免疫活性. 食品工业科技. 2023(09): 244-253 .  本站查看
本站查看
4. 沈畅华,杨娟,张远红,曾晓房. 双酶酶解鸽胸肉工艺优化及抗氧化性评价. 食品与机械. 2023(04): 163-169 .  百度学术
百度学术
5. 吴伟东,马诗淳,陈锐,周广平,邓宇. 厌氧角蛋白降解菌KD-1粗酶和商业蛋白酶酶解猪肉的功能与评价. 中国沼气. 2022(02): 47-53 .  百度学术
百度学术
6. 李佳芸,王欣之,韦源青,卞慧敏,刘睿,吴皓. 马氏珍珠贝软体酶法制备降糖肽的工艺优化及肽段分析. 食品工业科技. 2021(22): 202-211 .  本站查看
本站查看
其他类型引用(0)





 下载:
下载:
 下载:
下载:
