Research Progress on the Biological Function and Bioavailability Improvement of Carotenoids
-
摘要: 类胡萝卜素是一种自然界中分布广泛的食品成分,具有多种生物学活性,受到诸多学者关注。文章主要从类胡萝卜素生物学功能、食品成分对类胡萝卜素吸收利用的影响及提高其生物学利用三方面综述其研究进展。类胡萝卜素具有特异性调控相关基因及蛋白的功能,进而具有多种生物活性。文章从机制的角度归纳总结类胡萝卜素的功能特性,整理了食品成分间相互作用对类胡萝卜素生物利用率的影响,总结了纳米载药系统技术、异构化处理技术以及通过食品加工方式三种提高类胡萝卜素生物利用率的方法,为类胡萝卜素功能性产品研发提供一定的理论参考。Abstract: Carotenoids are widely distributed food components in nature and have various biological activities, which have received much attention from scholars. The article reviewed the research progress of carotenoids in terms of their biological functions, the effects of food ingredients on carotenoid absorption and utilization, and the improvement of their biological utilization. The carotenoids specifically regulate the functions of related genes and proteins, which in turn have various biological activities. The article summarized the functional properties of carotenoids from a mechanistic perspective. After that, the effect of food component interactions on carotenoid bioavailability was organized. And three methods to improve the bioavailability of carotenoids are summarized (nano-drug delivery system technology, isomerization treatment technology, and food processing methods). The article would provide some theoretical references for the development of functional carotenoid products.
-
Keywords:
- carotenoids /
- structure /
- biological functions /
- bioavailability /
- food processing
-
类胡萝卜素是一种天然存在的脂溶性食品功能成分,自然界存在的类胡萝卜素可分为两类,一类仅含碳氢元素,如α-胡萝卜素、β-胡萝卜素、番茄红素等、另一类是其氧化衍生物,如叶黄素、玉米黄质、辣椒红素等。类胡萝卜素广泛存在于蔬果[1-3]、藻类[4]、水生生物[5-7]及微生物[8]中。从结构上看,类胡萝卜素存在多个共轭双键[9-10],常见的类胡萝卜素结构式如图1所示。
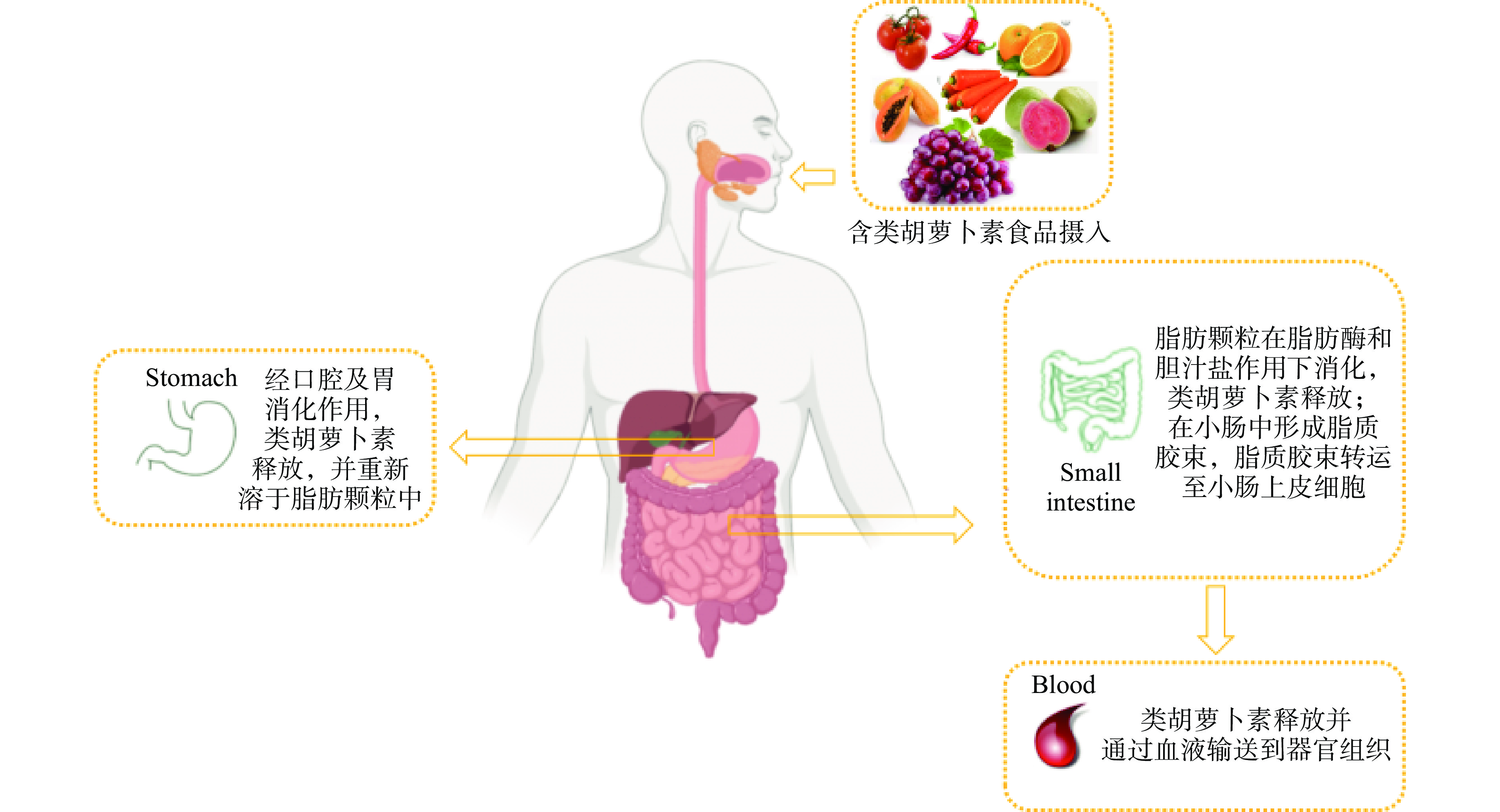
人体无法自身合成类胡萝卜素,所以需要从饮食中摄取[11-12]。富含类胡萝卜素的食品在口腔咀嚼及胃相消化的作用下,类胡萝卜素从食品基质中的释放,并重新溶解于脂肪微粒中。溶于脂肪微粒中的类胡萝卜素进入小肠后,在胆汁盐和胰脂肪酶的共同作用下,脂肪相被酶解,类胡萝卜素从脂肪微粒中释放,与胆汁盐等相互作用下形成胶束相,被小肠粘膜细胞吸收,之后经淋巴管通过血液输送到机体各组织器官中发挥生物学功能,类胡萝卜素在体内吸收与转运方式如图2所示[13-15]。
由于类胡萝卜素对机体具有诸多益处,部分学者在研究其生物活性已经进入分子营养学层面。同时,近年来对于类胡萝卜素功能食品逐渐进入大众视野。但此类产品产业化程度并不高,其主要原因可能由于其结构引起的较差的水溶性。目前,水溶性食品数量远高于脂溶性食品,采用适当的方式提高类胡萝卜素利用率是类胡萝卜素产业化急需解决的问题。另一方面,在产品生产中加入功能性协同作用的物质同时避免引入拮抗作用的物质也是在类胡萝卜素产品加工过程中需要注意的问题。文章由机制及应用层面,多角度阐述类胡萝卜素生物活性及提升生物利用率方法,以期为类胡萝卜素应用产业提供理论性参考。
1. 类胡萝卜素的功能特性
1.1 减少氧化应激
类胡萝卜素可猝灭机体病理条件下产生的活性氧簇(ROS),具有抗氧化作用[16]。Serpil等[17]在鲤鱼饲料中添加番茄红素(0.10 mg/kg,14 d),结果表明鲤鱼器官内还原型谷胱甘肽(GSH)、超氧化物歧化酶(SOD)、过氧化氢酶(CAT)和谷胱甘肽过氧化物酶(GSH-Px)相比未添加番茄红素组水平提升,同时丙二醛(MDA)含量下降,血液学参数指标上升。证明番茄红素可以通过减少氧化应激缓解三氯磷酸酯诱导的鲤鱼毒性。此外,Xie等[18]研究表明,虾青素也可通过激活核因子E2相关因子2(Nrf2),与抗氧化元件(ARE)结合形成Nrf-ARE信号通路,上调CAT、血红素氧化酶-1(HO-1)、GSH-Px等相关基因的表达,同时减少核转录因子(NF-κB)的表达,改善急性缺氧胁迫下鱼的生长性能与抗氧化能力,并减轻炎症反应。类胡萝卜素因其结构特点,其具有大量烯烃类双键,因此可以起到猝灭活性氧的作用,而大量疾病其产生原因之一即为机体过度氧化应激导致机体部分功能的异常,也为类胡萝卜素具有缓解多种病症的可能性。
1.2 调节脂质代谢
肥胖是一种与机体代谢密切相关的疾病,随着生活方式的转变,肥胖人群的比例持续升高,机体脂肪代谢的失调可能会导致高血脂、高血压、糖尿病等多种疾病。研究发现,肥胖可能与机体内类胡萝卜素含量降低存在一定的关联性[19]。Wang等[20]发现叶黄素可调控代谢紊乱大鼠肝脏中SIRT1信号通路,提高过氧化物酶体增殖物激活受体α(PPARα)、肉碱棕榈酰转移酶1A(CPT1A)、脂肪甘油三酯脂肪酶(ATGL)、激素敏感性甘油三酯脂肪酶等多种与脂肪动员相关的关键脂肪酶的表达,加速机体脂肪分解。连续5周饲喂叶黄素(25 mg/kg.d)后,高脂饮食诱导的脂质代谢紊乱大鼠体重显著减轻。Tian等[21]通过饲喂番茄红素(40 mg/kg)测定母鸡肝脏和肠道内关脂肪代谢的差异表达基因,通过基因表达图谱找到158种差异表达的基因,其中上调69种,下调89种,并发现番茄红素可显著增加在肝脏及肠道中过氧化物酶体增殖物激活受体γ共激活因子1(PGC1α)、过氧化物酶体增殖物激活受体(PPARα、PPARγ)、维甲酸X受体(RXRα、RXRγ)、维甲酸受体α(RARα),显著降低肝型脂肪酸结合蛋白(FABP1、FABP10)、长链脂肪酸转运蛋白(FATP4)的表达。研究发现番茄红素还可以改善线粒体功能,增强HO-1及醌氧还酶1(NQO1)的表达,加快脂肪分解,减少肝细胞脂质堆积,保持脂质代谢平衡,恢复非酒精性脂肪肝模型小鼠肝脏状态[22]。肥胖的产生主要由于脂肪细胞堆积及其含量增加引起的机体代谢异常,而类胡萝卜素具有调控体内相关基因的表达,促进机体产热过程并加速脂质溶解,进而促进机体脂肪代谢,减少脂肪细胞的堆积及机体脂质的沉积,具有调节肥胖的功能。
此外,类胡萝卜在降脂方面的作用可能也与调节肠道菌群有关,有研究表明,脂肪代谢失调和体内肠道菌群结构存在一定的联系,这可从肠道微生物代谢物氧化三甲胺(TMAO)中表现,在小鼠高脂模型小鼠饮食中添加辣椒红素后,小鼠血液中TMAO含量较未给予辣椒红素的小鼠下降,同时血清甘油三酯、总胆固醇、低密度脂蛋白胆固醇含量下降[23]。肠道菌群调控近年来一直是备受关注的方向之一,肥胖人群的肠道菌群与体内脂质代谢正常人群存在显著性差异。异常的肠道菌群会引起肠道微生物代谢物水平的变化,异常的肠道代谢因子会破坏肠道屏障进入血液,进而对机体器官起到损伤破坏的影响,引发脂肪肝、高血压等多种代谢疾病。类胡萝卜素可以通过调控相关影响因子,减少异常代谢物的产生,恢复受损的肠道屏障,起到缓解肥胖的作用。
同时,一些类胡萝卜素可以通过缓解氧化应激引起的炎症反应,达到降脂并改善机体病理状态[24]。动脉粥样硬化是一种由脂质沉积等原因引起的动脉管腔狭窄甚至阻塞的病理变化,其最早指示标志是粘附趋化因子的释放单核细胞内膜白细胞浸润,通过果糖诱导脐静脉内皮细胞及U937细胞测定α-胡萝卜素,β-胡萝卜素,β-隐黄素,叶黄素和番茄红素对单核细胞粘附的抑制作用,结果表明类胡萝卜素均以剂量依赖的方式下调粘附作用,降低炎性因子[25]。
1.3 视力保护
眼睛是人体十分重要的感觉器官,而现代人群视力减退的现象逐渐趋于年轻化,类胡萝卜素对于各年龄段的视力健康具有保护作用[26]。视神经病变是引起现代人群视力减退甚至致盲关键因素[27],引起病变的一个主要原因是代谢过程中过量的活性氧簇的产生引起损伤视神经细胞的蛋白质及DNA,导致细胞的损伤甚至凋亡,类胡萝卜素具有猝灭活性氧簇的作用,可减缓这一现象的发生。已有研究发现,类胡萝卜素如叶黄素和玉米黄质,具有增强视觉功能中的作用,但机制仍需进一步探究[28]。
Karakurt等[29]探究叶黄素对实验乙胺丁醇和异烟肼诱导的大鼠模型氧化性视神经病变的保护作用,结果表明叶黄素给药组的大鼠血清和组织中白细胞介素-1(IL-1β),肿瘤坏死因子-α(TNF-α)和丙二醛水平相比未给药组显著降低,大鼠眼部水肿和出血现象明显缓解。Ibrahim等[30]研究叶黄素及其脂质体对氧化应激引起的兔视网膜损伤的缓解作用,顺铂毒性诱导兔视网膜后,实验兔视网膜电图(ERG)反应光感受器细胞电位的a波及反映双极细胞对视觉冲动传导的b波波幅均降低,潜伏期均延长。注射叶黄素脂质体(1 mg/kg)后视网膜a、b波的波幅和潜伏期均基本得到恢复,并通过单细胞凝胶电泳实验测定发现叶黄素可以保护DNA免受损伤保护视网膜。Sahin等[31]发现叶黄素和玉米黄质异构体对视网膜感光细胞的保护作用,管饲叶黄素和玉米黄质异构体(100 mg/kg)的大鼠置于曝光条件下,视网膜mRNA中视紫红质(Rho)、生物活性肽(GNAT1)、神经细胞黏附分子(NCAM)、生长关联蛋白(GAP43)等水平提高,说明类胡萝卜素可以提高视网膜抗氧化能力及视神经再生作用,减少氧化应激,降低视网膜感光细胞的变性从而保护视力。此外,类胡萝卜素对糖尿病引起的视网膜病变[32]及年龄相关的黄斑变性[33-34],也有良好的治疗作用。近年来科技的发展,电子产品的广泛应用加速了日常工作学习的效率及质量,但是也在一定程度上对视力产生损伤,类胡萝卜素的特异性异戊二烯结构具有物理蓝光过滤特性,可能在蓝光到达光感受器等器官前与蓝光发生结合,减少因物理蓝光引起的视网膜血管及视神经细胞产生的炎症因子;同时类胡萝卜素也是良好的缓解氧化应激的功能因子,可以对活性氧簇产生猝灭作用。叶黄素、玉米黄质等类胡萝卜素也可以通过提高视觉感受器功能,上升关联蛋白表达量,起到缓解视力疲劳及视力保护的作用。
1.4 减缓神经退行性病变
类胡萝卜素具有恢复病理条件下神经系统的损伤的功能,研究表明,番茄红素可诱导肝细胞X受体(LXR)表达和激活LXR-PI3K-AKT信号通路,抑制淀粉样斑块沉积和神经炎症,改善神经血管的功能,提高在阿尔茨海默氏病小鼠模型的学习和记忆能力[35]。Zhu等[36]通过Morris水迷宫实验,发现给予番茄红素(100 mg/kg)的双侧颈动脉结扎的血管性痴呆大鼠逃避时间较模型组显著延长,对其海马区神经细胞进行免疫荧光组织化学和蛋白印迹分析结果表明,神经细胞排列趋向规则、胞浆颜色变浅、坏死细胞数量减少,同时SOD的活性提高。证明番茄红素可通过在抑制海马区的氧化应激,提高血管性痴呆大鼠的学习记忆能力。同时Zeni等[37]研究表明,叶黄素也可以提高机体抗氧化活性,减轻小脑部氧化应激,以缓解由谷氨酸和皮质酮诱导伴随氧化应激的神经抑郁疾病。神经退行性病变的主要产生原因极为复杂,其机制迄今尚未完全阐明。而目前为大众所熟知的产生机理为大脑及脊髓神经元细胞的逐渐损伤甚至坏死。产生此种现象的机制目前研究主要包括细胞的炎症反应、活性氧簇的产生引起的氧化应激等。类胡萝卜素具有的特异性烯烃类结构具有较好的缓解氧化应激以及缓解神经性炎症的作用,进而减轻神经退行性病变。
1.5 心血管系统的保护作用
机体心血管系统疾病的发生和氧化应激存在关联,病理状态下活性氧簇的过量产生并蓄积导致心脏发生病变,类胡萝卜素尤其番茄红素和虾青素可通过调控相关基因抑制氧化应激来保护心脏[38]。
Nrf2/HO-1是缓解氧化应激的一种信号通路,病理条件活性氧簇的过量产生,引起Nrf2表达,启动HO-1的转录,抑制氧化应激,Ouyang等[39]研究表明口服叶黄素(40 mg,28 d)后组织中Nrf2与HO-1表达增加,说明叶黄素可能通过调控的Nrf2/HO-1信号传导,同时可以显著上调CAT、SOD水平,对急性心肌缺血模型小鼠具有保护作用。Zeng等[40]研究发现,番茄红素可降低细胞外信号调节激酶(ERK1/2)、Jun氨基端激酶(JNK1/2)、p38丝裂原激活的蛋白激酶(p38)和磷酸化蛋白激酶B(p-AKT)、磷酸化糖原合酶激酶(GSK-3β)的磷酸化水平,抑制ROS依赖的丝裂原激活的蛋白激酶(MAPK)及(激活蛋白激酶B)Akt/GSK-3β信号通路,减少ROS的生成,同时上调心脏保护作用的抗氧化酶,达到抑制心脏肥大的作用。氧化应激的过度产生会引起心血管炎症反应,线粒体形态改变以及线粒体代谢异常,进而损伤机体心血管,引发一系列病理症状。类胡萝卜素可以通过调控PI3K/Akt、Nrf2/HO-1、Keap1-Nrf2-ARE等信号通路基因及蛋白的表达,达到缓解氧化应激,修复受损心血管细胞并产生保护机体心血管功能的作用。
1.6 抑制癌细胞生长与增殖
近年来,我国癌症发病率呈上升趋势,其发病率及死亡率仅次于心血管疾病[41],统计表明肺癌、胃癌、肝癌在我国的死亡率较高[42],严重威胁大众健康。癌症是一种或多种生物水平损伤的积累,引起细胞内的遗传物质改变或其他生理变化[41],这种损伤在一定程度上可以通过膳食调整得到改善。类胡萝卜素具有抑制癌细胞生长与增殖的功能,增强类胡萝卜素摄取可降低机体癌症发生的风险[43-45]。
Zhang等[46]研究5-氟尿嘧啶(5-FU)与β-胡萝卜素对食管磷癌细胞(Eca109)抑制作用,通过Western印迹法检测Eca109中的关键蛋白的变化,结果表明5-FU与β-胡萝卜素联用后,具有抗凋亡作用的关键蛋白B细胞淋巴瘤/白血病-2(Bcl-2)表达降低,而具有促凋亡作用的Bcl-2相关X蛋白(Bax)的表达升高,同时半胱氨酸天冬氨酸特异性蛋白酶(Caspase-3)达升高,表明5-FU与β-胡萝卜启动Eca109的凋亡,具有抑制癌细胞生长的作用。Kavalappa等[47]发现从海洋红藻中提取并纯化的β-胡萝卜素对肝癌HepG2细胞的抑制作用,Western印迹分析结果表明,HepG2细胞的Bcl-2表达降低、Bax蛋白表达增加,证明β-胡萝卜素可抑制HepG2细胞活力,同时研究表明可能呈剂量依赖性。研究还发现β-胡萝卜素可以下调多腺苷二磷酸核糖聚合酶(PARP)的表达,PARP失活会加速HepG2细胞的不稳定,而NF-κB的减少也标志着HepG2细胞的凋亡,所以β-胡萝卜素可有效抑制肝癌细胞的生长。Jiang等[48]在给予肺癌小鼠腹腔注射番茄红素后,肿瘤体积及重量相比未处理的小鼠明显降低,白细胞介素的水平(IL)-1和干扰素(IFN)γ表达升高,同时发现IFNγ能够调控蛋白激酶AKT信号传导,阻抑PD-L1的表达。证明了番茄红素能促进抗程序性细胞死亡基因(anti-PD-1)治疗肺癌。机体内癌细胞的过量产生由多种因素引起,常见的包括放射线、机械刺激等物理因素,长期暴露与有毒有害试剂、以及部分毒素如黄曲霉毒素等化学因素以及部分病毒引起的生物因素。细胞内癌基因的激活刺激了细胞的异常增殖与分化,类胡萝卜素具有提高细胞氧化还原能力并维持细胞内氧化还原平衡的作用,具有促进癌细胞凋亡、抑制癌细胞异常生长的功能。
1.7 提高免疫能力
类胡萝卜素可以通过提升机体的抗氧化能力,进而提高机体的免疫力。通过黄鳍鱼饲料中添加类胡萝卜素(150~200 mg/kg),发现类胡萝卜可以通过提升鱼的抗氧化能力提高血清内补体成分(C3、C4)、免疫球蛋白(Ig)及溶菌酶活性,提升肝脏内超氧化物歧化酶、肝脏抗菌多肽、免疫球蛋白(IgM)水平,提升了其抗菌及免疫能力,增加了黄鳍鱼的生长速率及体重[49]。
类胡萝卜素也可以提升红细胞的免疫能力,红细胞在人体起到运载氧气的作用,当红细胞数量因病理减少,运载氧气的能力下降,免疫力降低,机体将产生疾病。Zhang等[50]研究发现,番茄红素对黄曲霉毒素B1诱导的小鼠红细胞氧化应激具有保护作用,表现在番茄红素通过上调红细胞计数、血红蛋白及血细胞比容,降低红细胞体积分布宽度增加红细胞功能,同时免疫功能指标测定表明,番茄红素可通过增加红细胞C3b受体花环率(E-C3bRR)并降低红细胞免疫复合物花环率(E-ICRR)提升其免疫功能。机体免疫能力与遗传、年龄、饮食结构等多种因素有关,其中饮食是最易于调整同时也是最有效的方法之一,在饮食中添加类胡萝卜素的摄入可以通过提升细胞抗氧化能力,增强红细胞运载氧气效率提升机体免疫力。
2. 食品成分相互作用对类胡萝卜素生物利用率的影响
类胡萝卜素具有多种功能特性,研究表明,食品成分的相互作用可影响类胡萝卜素的生物利用度[51-52]。油脂、抗氧化剂、蛋白质、黄酮类等营养成分均会对类胡萝卜素在人体中的生物利用率产生相互作用。
2.1 油脂类
类胡萝卜素为脂溶性功能色素,日常饮食中将富含类胡萝卜素的物质与油脂同时摄入可以促进类胡萝卜素的吸收利用。研究表明,在类胡萝卜素与油脂同时摄入时,油脂经消化后产生游离脂肪酸,在小肠内形成胶束相,增强类胡萝卜素的生物利用率,而不同种类的油脂对类胡萝卜素生物利用率也存在影响。
以番茄红素为例,Zhao等[53]分别以芝麻油,亚麻籽油,核桃油为油相制备制备乳铁蛋白油包水型番茄红素纳米乳液,研究发现,芝麻油与亚麻籽油对番茄红素生物可及性比用核桃油制备的乳液高7 %,这可能是由于当油脂具有较低的粘度,较高的密度及较低的不饱和度时,对番茄红素的生物利用率提升。油脂对番茄红素生物利用率另一方面影响是不同油脂对番茄红素异构化率存在差异性,这在一定程度上也会影响番茄红素生物利用率。Masaki等[54]对比了10种植物油和2种动物油脂对番茄红素异构化率的影响,结果发现番茄红素的异构化范围从39.2%到50.7%不等。同时也证实了除不饱和度和粘度外,油脂中其他成分同样会影响类胡萝卜素生物利用率。
2.2 抗氧化剂类
抗氧化剂在食品加工中具有重要作用,其主要目的为防止食品成分发生氧化变质。有研究表明,具有抗氧化作用的功能成分,可通过减少类胡萝卜素的降解、褪色,改善其化学稳定性进而增强有效物质的保留,增加类胡萝卜素生物利用率。
以叶黄素为例,Frankjen等[55]发现添加抗坏血酸的叶黄素-玉米蛋白复合物颗粒相对稳定性增加约25%。Steiner等[56]发现加入白藜芦醇的叶黄素纳米复合物具有更强pH(2~8)以及钙离子(0~100 mmol/L)稳定性。Yan等[57]以牛血清白蛋白、绿原酸、葡聚糖共价制备的三元复合物作为乳化剂,以维生素E作为抗氧化剂制备负载叶黄素乳液,结果表明叶黄素的生物利用率相比于不引入抗氧化剂组由明显提高,添加抗氧化剂组叶黄素的生物利用率可达64.5%。而在虾青素的研究中,也有类似的研究结果,Chin等研究发现将α-生育酚和抗坏血酸引入虾青素纳米颗粒中,可以增强虾青素的稳定性,减少虾青素的降解,进而对其生物利用率存在积极作用[58]。
2.3 蛋白类
不同蛋白也会影响类胡萝卜的生物利用率,这可能与蛋白质可能影响类胡萝卜素的电子结构及成键方式有关。同时,蛋白类物质与类胡萝卜素存在一定的分子间作用力,也能够提升类胡萝卜素的稳定性。
Ling等[59]在体外模拟胃肠温育条件下分别研究乳清蛋白分离物(WPI),大豆分离蛋白(SPI)和酪蛋白酸钠(SC)制备的β-胡萝卜素纳米乳液生物利用率,结果表明,相比于其余两种蛋白类物质,大豆分离蛋白包封的β-胡萝卜素转化为胶束相比率最高(32.27%±1.41%)。蛋白的含量也会影响类胡萝卜素的生物利用率,Yi等[60]运用紫外-可见光谱及圆二色谱研究乳清蛋白分离物和酪蛋白酸钠对叶黄素生物利用率的影响,结果表明叶黄素与乳蛋白结合后的水溶性及稳定性提高,氧化及分解程度减少,且随乳蛋白引入量的增加,稳定性越高。
2.4 黄酮类
黄酮类成分对类胡萝卜素的生物利用率也表现出协同或拮抗作用,这可能是由于黄酮类物质影响脂溶性类胡萝卜素的相互聚集程度以及调控肠道细胞相关蛋白有关。Nie等[61]研究发现,在β-胡萝卜素中分别加入橙皮素和柚皮素(25μmol/L)后,加入橙皮素的β-胡萝卜素在Caco-2细胞模型及体内吸收实验中β-胡萝卜素的生物利用率提高,而加入柚皮素的β-胡萝卜素生物利用率出现抑制。原因可能是不同柑橘类黄烷酮影响脂溶性β-胡萝卜素的聚集,β-胡萝卜素聚集程度减小,与机体接触表面积增大,其生物利用率提高。Marques等[62]研究发现,黄酮类化合物可能提高肠道内高密度脂蛋白受体SR-BI的表达,进而对肠道内类胡萝卜素吸收利用存在正向调控作用。Meng等[63]制备了茶多酚-β-胡萝卜素水包油型复合纳米乳液,通过实验研究表明,添加茶多酚的纳米乳液的β-胡萝卜素生物利用率更高。
3. 提高类胡萝卜素生物利用率方法
根据类胡萝卜素与其他食品成分的相互作用,以及类胡萝卜素的自身结构特点,研究发现可以通过适当的技术对类胡萝卜素进行处理并提升类胡萝卜素生物利用率。常见的方法有制备纳米载药系统技术[64]、对全反式类胡萝卜素异构处理等[65],同时采用不同食品加工方式[66]均对类胡萝卜素的生物利用率也存影响。
3.1 纳米载药系统技术
纳米载药系统技术是一种具有纳米特征尺度的分散体系,将脂溶性或稳定性较差的食品成分选择相应的载体,制备纳米特征尺度的分散体系并运送的一种技术[67-68]。类胡萝卜素是一种脂溶性食品功能成分,极不稳定,限制其利用。研究表明,采用纳米载药系统技术处理的类胡萝卜素稳定性及生物利用率均得到提高[69]。Han等[70]将β-胡萝卜素嵌入扇贝性腺蛋白分离物及藻酸盐微球中并制备稳定化乳剂,乳剂在pH范围为3~8,温度37 ℃时均有良好的稳定性,同时β-胡萝卜素生物转化率也得到显著提高;Ba等[71]制备的β-胡萝卜素的玉米醇溶蛋白/羧甲基壳聚糖/茶多酚三元复合纳米颗粒,不仅可提高温度、酸性、离子强度以及紫外光等不同环境条件压力的能力,提高其储存稳定性,同时也可提高生物利用率。Zhong等[72]设计一种基于燕麦蛋白分离物及平菇β葡聚糖经美拉德反应形成的偶联物包封β-胡萝卜素的递送系统,模拟体外消化实验中,转化为胶束相的β-胡萝卜素约为36.29%,β-胡萝卜素体外生物利用度得到改善。Du等[73]通过Caco-2细胞模型研究发现,乳清蛋白包封的β-胡萝卜素纳米脂质载体,其细胞摄取相比未经处理的β-胡萝卜素增加3倍,同时跨膜渗透性也显著提高。
除β-胡萝卜素外,番茄红素、叶黄素、虾青素[74]等类胡萝卜素也可通过制备纳米载药系统的方式提高稳定性与利用率。Guo等[75]研究发现将同剂量番茄红素溶于橄榄油中直接口服或制备橄榄油负载的纳米级微乳等方式分别饲喂大鼠,研究表明番茄红素的生物利用度提高了2.1倍。文献[76-78]的研究也均表明,摄取纳米递送系统负载的叶黄素生物利用度提高。此外,新型的双载脂质体也可提升类胡萝卜素的持续有效释放[79]。
此外,在制备类胡萝卜素纳米载药系统时,选择适当的纳米载药系统配方对类胡萝卜素的吸收也存在影响,Gasa-Falcon等[80]采用不同乳化剂(吐温20,卵磷脂,酪蛋白酸钠,蔗糖棕榈酸酯)并改变其浓度(2%~8%),8%卵磷脂的纳米载药系统β-胡萝卜素生物利用率为最高(23.5%)。纳米载药系统技术在类胡萝卜素原始状态基础上,加入了蛋白、多糖、油脂等其他营养成分,所以在多元食品的应用上如高类胡萝卜素乳制品、蛋白类制品、富含油脂的涂层食品等;而此种技术在纯果汁饮料类食品相对具有一定的局限性。
3.2 异构化技术
自然界植物中存在的类胡萝卜素大多为全反式类胡萝卜素,从结构上看,由于多个共轭双键的存在,类胡萝卜素具有发生顺反异构并生成多种几何异构体的可能[81-82],顺式类胡萝卜的聚集性小于反式,异构化后的类胡萝卜素的生物利用率得到提升[83]。人体对顺式类胡萝卜素的利用效率高于反式,并且机体中反式类胡萝卜素的含量高于顺式,研究表明,类胡萝卜素可以通过加热[84]、超声[85]、碘单质催化[86]等方式发生异构。
Honda等[87]研究表明,小鼠饮食中添加番茄红素异构体,并与仅添加全反式番茄红素对比,4周后,小鼠肝脏中番茄红素的总浓度提高3倍以上,Yang等[88]通过制备番茄红素异构体纳米脂质载体,与相同方法制备的全反式番茄红素纳米脂质载体对比,发现番茄红素异构体纳米脂质载体的生物利用率约为是全反式番茄红素的2倍。Honda等[89]在给予母鸡饲喂番茄红素异构体,发现在蛋黄中检测到的番茄红素浓度为仅供给全反式番茄红素的约3倍,Yang等[90]碘掺杂的二氧化钛催化制备叶黄素异构体,表明异构体的生物利用率为14%~23%,高于全反式异构体。还有研究表明,异构化的类胡萝卜素相比于全反式胡萝卜素具有更强的功能特性,Weinrich等[91]发现β-胡萝卜素9-顺-异构体可通过调控线粒体的ATP生成,延长衰老果蝇的平均寿命。以上研究说明,类胡萝卜素的空间构型与吸收效率直接相关,而营养物质的吸收效率与其功能活性呈正相关性。
关于异构化可以提高类胡萝卜素生物利用率的另一原因是异构化后的类胡萝卜素物理性质,如溶解性等也会发生改变。研究发现,类胡萝卜素顺式异构体在机体胆汁酸微胶粒中更易溶,更易于被机体吸收[92]。Masaki等[93]研究发现通过差示扫描量热法(DSC)、X射线衍射(XRD)和扫描电子显微镜(SEM)证实异构化后的β-胡萝卜素和虾青素的溶解。然而,目前类胡萝卜素异构化的方法均会引起在反应过程中,类胡萝卜素会发生不同程度降解。同时,热促异构等方法引入的化学试剂难以去除,光致异构法的设备较难应用于实际生产等问题均导致类胡萝卜素异构化产业在短期内较难实现大规模推广,需要进一步研究。
3.3 食品加工方式
日常生活中,食品的加工方式也会影响类胡萝卜素的生物利用率,如在食品加工过程中添加未热烫洋葱,由于洋葱中存在的二烯丙基二硫化物有利于番茄红素的异构化,可提升番茄红素的利用率[94]。研究表明,在日常食品加工过程中,相比于煎炸和烤制,煮制(15~35 min)和蒸制(15~45 min)能较多的保留食品基质中的类胡萝卜素[95]。在饮料加工中,食品甜味剂、稳定剂的种类与添加量及体系中pH大小等也均可影响类胡萝卜素的生物利用率,如适量黄原胶的引入可以减少β-胡萝卜素在胃液酸性及酶类条件下降解与损失,使更多β-胡萝卜素进入肠道后被小肠细胞吸收并进入血液到达靶器官发挥生物学功能[96];在对一种胡萝卜汁中果胶、糖类及pH对β-胡萝卜素生物利用率影响的一项研究中发现,相比于柑橘果胶和苹果果胶,甜菜果胶的引入可以提高β-胡萝卜素生物利用率,这说明粘度越高、接枝度越高、分子量越大的多糖类物质具有更高的粘度,可以保护其中的类胡萝卜素防止其在经过胃肠道过程中降解损失;而在不同甜味剂的比较中,添加蔗糖的胡萝卜汁具有更高的生物利用率;在比较不同pH(1.5、3.8、6.8)对其生物利用率影响发现,pH为6.8时β-胡萝卜素生物利用率为最高[97]。在日常烹饪方式的选择上,适当时间的煮制和蒸制可以更多的保留类胡萝卜素并提升其生物利用率,在类胡萝卜素果汁类产品的加工生产过程中,控制体系环境为中性偏弱酸环境,并以蔗糖为甜味剂进行加工,可以提高类胡萝卜素的生物利用价值。
4. 结论与展望
类胡萝卜素是一种重要的脂溶性色素,具有调节脂质代谢、保护视力、减缓神经退行性病变、保护心血管、抑制癌细胞生长、提高机体免疫力等多种功能特性。类胡萝卜素生物利用率较低,在食品加工过程中应注意不同食品成分对类胡萝卜素的利用率存在促进或拮抗作用,同时可通过纳米载药系统技术、异构化技术等提升其利用率,另外还应注意食品加工方式对类胡萝卜素利用率的影响。
类胡萝卜素具有诸多功能特性,未来对于类胡萝卜素的研究可着眼于以下几方面,首先,在类胡萝卜素生物学功能上,已有学者在机理层面进行研究。目前,研究较多的是围绕类胡萝卜素可抵御机体氧化应激进而缓解或治疗相关疾病,随着研究的深入及一些组学概念的提出与发展,一些其他的机理也有待于探究。如类胡萝卜素通过“肠-肝轴”、“肠-脑轴”等方式,通过调控肠道代谢产物及肠道屏障的影响,具有缓解机体疾病的潜力。同时,以分子对接技术,利用类胡萝卜素的特异性结构,探究其与机体中器官表面特异性受体结合产生保护器官的作用,或者与病理条件下表达异常的受体结合达到使其活性降低的潜力。在功能食品的研发方面,可以将多种提升类胡萝卜素生物利用率方式的联用,如在新型纳米载药系统中包埋异构化的类胡萝卜素、在纳米载药系统中适当添加促进类胡萝卜素生物利用率的食品功能成分、以及靶向类胡萝卜素载体的研究与应用,提升类胡萝卜素的利用价值,为类胡萝卜素功能食品以更大规模进入市场提供更扎实的理论基础。
-
-
[1] HONG H, TAKAGI T, HARE T. An optimal saponification and extraction method to determine carotenoids in avocado[J]. Food Chemistry,2022,387:132923−132923. doi: 10.1016/j.foodchem.2022.132923
[2] GÓMEZ-MAQUEO A, BANDINO E, HORMAZA J I, et al. Characterization and the impact of in vitro simulated digestion on the stability and bioaccessibility of carotenoids and their esters in two Pouteria lucuma varieties[J]. Food Chemistry,2020,316:126369. doi: 10.1016/j.foodchem.2020.126369
[3] MELIZA L R, ISABELA S, PEDRO E D A. Ultrasound and ethanol pre-treatments to improve convective drying: Drying, rehydration and carotenoid content of pumpkin[J]. Food and Bioproducts Processing,2020,119:20−30. doi: 10.1016/j.fbp.2019.10.008
[4] LUCAS G C, JESSICA H D, BIANCA B A, et al. Spirulina sp. LEB 18 cultivation in outdoor pilot scale using aquaculture wastewater: High biomass, carotenoid, lipid and carbohydrate production[J]. Aquaculture,2020,525:735272. doi: 10.1016/j.aquaculture.2020.735272
[5] LARA Č, ŠEBOJKA K. Electrochemistry as a screening method in determination of carotenoids in crustacean samples used in everyday diet[J]. Food Chemistry,2020,309:125706. doi: 10.1016/j.foodchem.2019.125706
[6] TAN K, LIU H, ZHANG H, et al. Seasonal variation of total carotenoids content in the tissues of male and female golden noble scallops Chlamys nobilis[J]. Aquaculture,2020,518(C):734796.
[7] LINDA A, WANG Z, LI J, et al. Survival, retention rate and immunity of the black shell colored stocks of pearl oyster Pinctada fucata martensii after grafting operation[J]. Fish and Shellfish Immunology,2020,98:691−698. doi: 10.1016/j.fsi.2019.11.003
[8] LIU H, ZHANG C, ZHANG X, et al. A novel carotenoids-producing marine bacterium from noble scallop Chlamys nobilis and antioxidant activities of its carotenoid compositions[J]. Food Chemistry,2020,320:126629. doi: 10.1016/j.foodchem.2020.126629
[9] BRITTON G. Carotenoid research: History and new perspectives for chemistry in biological systems[J]. Biochimica et Biophysica Acta. Molecular and Cell Biology of Lipids,2020:158699.
[10] BONET M L, RIBOT J, GALMÉS S, et al. Carotenoids and carotenoid conversion products in adipose tissue biology and obesity: Pre-clinical and human studies[J]. Biochimica et Biophysica Acta. Molecular and Cell Biology of Lipids,2020,1865(11):158676. doi: 10.1016/j.bbalip.2020.158676
[11] 郑梦熳, 李文韵, 刘雨薇. 类胡萝卜素肠道吸收及生物利用度研究进展[J]. 食品工业科技,2021,42(15):403−411. [ZHENG M M, LI W Y, LIU Y W. Research progress on intestinal absorption and bioavailability of carotenoids[J]. Science and Technology of Food Industry,2021,42(15):403−411. doi: 10.13386/j.issn1002-0306.2020070335 [12] 陈叶, 戴竹青, 宋江峰, 等. 胶束化对Caco-2上皮细胞叶黄素吸收和转运的影响[J]. 食品工业科技, 2019, 40(20): 304-309. CHEN Y, DAI Z, SONG J, et al. , Effect of micellization on lutein absorption and transportation in Caco-2 epithelial cells[J]. Science and Technology of Food Industry, 2019, 40(20): 304-309. ] [13] BRAULIO C, JOSÉ de J O, SAUL R, et al. Effects of pectin on lipid digestion and possible implications for carotenoid bioavailability during pre-absorptive stages: A review[J]. Food Research International,2017,99(2):917−927.
[14] MÉLANIE C, ANDREIA A, ANA C, et al. Lycopene in human health[J]. LWT,2020,127:109323−109323. doi: 10.1016/j.lwt.2020.109323
[15] SUNA K, TAE Y H, IN K H. Analysis, bioavailability, and potential healthy effects of Capsanthin, natural red pigment from Capsicum spp.[J]. Food Reviews International,2009,25(3):198−213. doi: 10.1080/87559120902956141
[16] KRINSKY N I. Antioxidant functions of carotenoids[J]. Free Radical Biology and Medicine,1989,7(6):617−635. doi: 10.1016/0891-5849(89)90143-3
[17] YONAR S M, YONAR M E, PALA A, et al. Effect of trichlorfon on some haematological and biochemical changes in Cyprinus carpio: The ameliorative effect of lycopene[J]. Aquaculture Reports,2020,16:100246. doi: 10.1016/j.aqrep.2019.100246
[18] XIE J, FANG H, HE X, et al. Study on mechanism of synthetic astaxanthin and Haematococcus pluvialis improving the growth performance and antioxidant capacity under acute hypoxia stress of golden pompano (Trachinotus ovatus) and enhancing anti-inflammatory by activating Nrf2-ARE pathway to antagonize the NF-κB pathway[J]. Aquaculture,2020:518.
[19] KOJI S, TAKASHI I, RISA H, et al. Association of abdominal obesity with decreased serum levels of carotenoids in a healthy Japanese population[J]. Clinical Nutrition,2006,25(5):780−789. doi: 10.1016/j.clnu.2006.01.025
[20] WANG N, WANG D, ZHOU J, et al. Lutein prevents the excessive fat deposition in liver and abdominal tissues by activating SIRT1 and up-regulating ATGL and HSL in high fat diet rats (FS06-01-19)[J]. Current Developments in Nutrition, 2019, 3(1).
[21] TIAN H, LIU G, GUO Y, et al. Lycopene supplementation regulates the gene expression profile and fat metabolism of breeding hens[J]. Journal of Animal Physiology and Animal Nutrition,2020,104(3):936−945. doi: 10.1111/jpn.13344
[22] WANG J, GENG T, ZOU Q, et al. Lycopene prevents lipid accumulation in hepatocytes by stimulating PPARα and improving mitochondrial function[J]. Journal of Functional Foods,2020,67:103857. doi: 10.1016/j.jff.2020.103857
[23] WU T, GAO Y, HAO J, et al. Capsanthin extract prevents obesity, reduces serum TMAO levels and modulates the gut microbiota composition in high-fat-diet induced obese C57BL/6J mice[J]. Food Research International,2020,128:108774. doi: 10.1016/j.foodres.2019.108774
[24] FATEMEH H, ABDOLLAH H, BIZHAN H, et al. An energy-restricted high-protein diet supplemented with β-cryptoxanthin alleviated oxidative stress and inflammation in nonalcoholic fatty liver disease: A randomized controlled trial[J]. Nutrition Research,2020:73.
[25] LIN P, REN Q, WANG Q, et al. Carotenoids inhibit fructose-induced inflammatory response in human endothelial cells and monocytes[J]. Mediators of Inflammation,2020,2020:5373562.
[26] TOM L, ZHE L, MARINE N. Lutein and zeaxanthin supplement use is associated with increased macular pigment density over 15 years and greater contrast sensitivity in the carotenoids in age-related eye disease study of older-adult women[J]. Investigative Ophthalmology & Visual Science,2021,62(8):2950.
[27] ZHAO S, LAN X, WU J, et al. Protocol of global incidence and progression of age-related macular degeneration: A systematic review[J]. Medicine,2019,98(10):e14645. doi: 10.1097/MD.0000000000014645
[28] MA L, YAN S, HUANG Y, et al. Effect of lutein and zeaxanthin on macular pigment and visual function in patients with early age-related macular degeneration[J]. Ophthalmology,2012,119(11):2290−2297. doi: 10.1016/j.ophtha.2012.06.014
[29] KARAKURT Y, SÜLEYMAN H, KESKIN C F, et al. The effects of lutein on optic nerve injury induced by ethambutol and isoniazid: An experimental study[J]. Cutaneous and Ocular Toxicology,2019,38(2):136−140. doi: 10.1080/15569527.2018.1539010
[30] IBRAHIM A E, SHAFAA M W, KHEDR M H, et al. Comparative study between lutein and its liposomal form on cisplatin-induced retinal injury in rabbits[J]. Cutaneous and Ocular Toxicology,2019,38(3):279−285. doi: 10.1080/15569527.2019.1608227
[31] SAHIN K, GENCOGLU H, AKDEMIR F, et al. Lutein and zeaxanthin isomers may attenuate photo-oxidative retinal damage via modulation of G protein-coupled receptors and growth factors in rats[J]. Biochemical and Biophysical Research Communications,2019,516(1):163−170. doi: 10.1016/j.bbrc.2019.06.032
[32] REN Y, QI Y, SU X, et al. Therapeutic effect of lutein supplement on non-proliferative diabetic retinopathy: A retrospective study[J]. Medicine,2019,98(29):e15404. doi: 10.1097/MD.0000000000015404
[33] GE Y, ZHANG A, SUN R, et al. Penetratin modified lutein nanoemulsion in-situ gel for the treatment of age-related macular degeneration[J]. Expert Opinion on Drug Delivery,2020,17(4):603−619. doi: 10.1080/17425247.2020.1735348
[34] SAWA M, SHUNTO T, NISHIYAMA I, et al. Effects of lutein supplementation in Japanese patients with unilateral age-related macular degeneration: The sakai lutein study[J]. Scientific Reports,2020,10(1):5958. doi: 10.1038/s41598-020-62483-0
[35] XU X, TENG Y, ZOU J, et al. Effects of lycopene on vascular remodeling through the LXR-PI3K-AKT signaling pathway in APP/PS1 mice[J]. Biochemical and Biophysical Research Communications,2020,526(3):699−705. doi: 10.1016/j.bbrc.2020.02.063
[36] ZHU N, YIN X, LIN R, et al. Possible mechanisms of lycopene amelioration of learning and memory impairment in rats with vascular dementia[J]. Neural Regeneration Research,2020,15(2):332−341. doi: 10.4103/1673-5374.265565
[37] ZENI A L B, CAMARGO A, DALMAGRO A P. Lutein prevents corticosterone-induced depressive-like behavior in mice with the involvement of antioxidant and neuroprotective activities[J]. Pharmacology, Biochemistry, and Behavior,2019,179:63−72. doi: 10.1016/j.pbb.2019.02.004
[38] RICCIONI G. Carotenoids and cardiovascular disease[J]. Current Atherosclerosis Reports,2009,11(6):434−439. doi: 10.1007/s11883-009-0065-z
[39] OUYANG B, LI Z, JI X, et al. The protective role of lutein on isoproterenol-induced cardiac failure rat model through improving cardiac morphology, antioxidant status via positively regulating Nrf2/HO-1 signalling pathway[J]. Pharmaceutical Biology,2019,57(1):529−535. doi: 10.1080/13880209.2019.1649436
[40] ZENG J, ZHAO J, DONG B, et al. Lycopene protects against pressure overload-induced cardiac hypertrophy by attenuating oxidative stress[J]. The Journal of Nutritional Biochemistry,2019,66:70−78. doi: 10.1016/j.jnutbio.2019.01.002
[41] ROWLES J L, ERDMAN J W. Carotenoids and their role in cancer prevention[J]. Biochimica et Biophysica Acta. Molecular and Cell Biology of Lipids,2020,1865(11):158613. doi: 10.1016/j.bbalip.2020.158613
[42] 陈金东. 中国各类癌症的发病率和死亡率现状及发展趋势[J]. 遵义医学院学报,2018,41(6):653−662. [CHEN J. Trends of cancer incidence and mortality in China[J]. Journal of Zunyi Medical University,2018,41(6):653−662. doi: 10.3969/j.issn.1000-2715.2018.06.001 [43] WANG S, WU Y, WANG X, et al. Lycopene prevents carcinogen-induced cutaneous tumor by enhancing activation of the Nrf2 pathway through p62-triggered autophagic Keap1 degradation[J]. Aging,2020,12(9):8167−8190. doi: 10.18632/aging.103132
[44] MAZIDI M, FERNS G A, BANACH M. A high consumption of tomato and lycopene is associated with a lower risk of cancer mortality: Results from a multi-ethnic cohort[J]. Public Health Nutrition,2020,23(9):1569−1575. doi: 10.1017/S1368980019003227
[45] TAKUJI T, MASAHITO S, HISATAKA M. Cancer chemoprevention by carotenoids[J]. Molecules,2012,17(3):3202−3242. doi: 10.3390/molecules17033202
[46] ZHANG Y, ZHU X, HUANG T, et al. β-Carotene synergistically enhances the anti-tumor effect of 5-fluorouracil on esophageal squamous cell carcinoma in vivo and in vitro[J]. Toxicology Letters,2016,261:49−58. doi: 10.1016/j.toxlet.2016.08.010
[47] KAVALAPPA Y P, RUDRESH D U, GOPAL S S, et al. β-carotene isolated from the marine red alga, Gracillaria sp. potently attenuates the growth of human hepatocellular carcinoma (HepG2) cells by modulating multiple molecular pathways[J]. Journal of Functional Foods,2019,52:165−176. doi: 10.1016/j.jff.2018.11.015
[48] JIANG X, WU H, ZHAO W, et al. Lycopene improves the efficiency of anti-PD-1 therapy via activating IFN signaling of lung cancer cells[J]. Cancer Cell International,2020,12(9):8167−8190.
[49] LIU F, QU Y, WANG A, et al. Effects of carotenoids on the growth performance, biochemical parameters, immune responses and disease resistance of yellow catfish (Pelteobagrus fulvidraco) under high-temperature stress[J]. Aquaculture,2019,503:293−303. doi: 10.1016/j.aquaculture.2019.01.008
[50] ZHANG J, WANG P, XU F, et al. Protective effects of lycopene against AFB1-induced erythrocyte dysfunction and oxidative stress in mice[J]. Research in Veterinary Science,2020,129:103−108. doi: 10.1016/j.rvsc.2020.01.015
[51] MAIANI G, PERIAGO C M J, CATASTA G, et al. Carotenoids: Actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans[J]. Molecular Nutrition & Food Research,2009,53:S194−S218.
[52] CASTENMILLER J, WEST C, LINSSEN J, et al. The food matrix of spinach is a limiting factor in determining the bioavailability of β-carotene and to a lesser extent of lutein in humans[J]. The Journal of Nutrition,1999,129:349−355. doi: 10.1093/jn/129.2.349
[53] ZHAO C, WEI L, YIN B, et al. Encapsulation of lycopene within oil-in-water nanoemulsions using lactoferrin: Impact of carrier oils on physicochemical stability and bioaccessibility[J]. International Journal of Biological Macromolecules,2020,153:912−920. doi: 10.1016/j.ijbiomac.2020.03.063
[54] MASAKI H, KAZUYA M, YO W, et al. The E/Z isomer ratio of lycopene in foods and effect of heating with edible oils and fats on isomerization of (all-E)-lycopene[J]. European Journal of Lipid Science and Technology,2017,119(8).
[55] FRANKJEN Y de B, ARNOUT I, KRASSIMIR P V. Photo-stability of lutein in surfactant-free lutein-zein composite colloidal particles[J]. Food Chemistry:X,2020,5:100071. doi: 10.1016/j.fochx.2019.100071
[56] STEINER B M, SHUKLA V, MCCLEMENTS D J, et al. Encapsulation of lutein in nanoemulsions stabilized by resveratrol and Maillard conjugates[J]. Journal of Food Science,2019,84(9):2421−2431. doi: 10.1111/1750-3841.14751
[57] YAN Y, ZHU Q, DIAO J, et al. Enhanced physicochemical stability of lutein-enriched emulsions by polyphenol-protein-polysaccharide conjugates and fat-soluble antioxidant[J]. Food Hydrocolloids,2020,101(C).
[58] CHIN P T, IMEDEDDINE A N, NAVIDEH A. Protection of astaxanthin in astaxanthin nanodispersions using additional antioxidants[J]. Molecules,2013,18(7):.7699−7710. doi: 10.3390/molecules18077699
[59] LING C, WALLACE Y, RONG L, et al. Enzymatic degradation and bioaccessibility of protein encapsulated β-carotene nano-emulsions during in vitro gastro-intestinal digestion[J]. Food Hydrocolloids,2020,100(C):105177−105177.
[60] YI J, FAN Y, WALLACE Y, et al. Characterization of milk proteins–lutein complexes and the impact on lutein chemical stability[J]. Food Chemistry,2016,200:91−97. doi: 10.1016/j.foodchem.2016.01.035
[61] NIE M, ZHANG Z, LIU C, et al. Hesperetin and hesperidin improved β-carotene incorporation efficiency, intestinal cell uptake, and retinoid concentrations in tissues[J]. Journal of Agricultural and Food Chemistry,2019,67(12):3363−3371. doi: 10.1021/acs.jafc.9b00551
[62] MARQUES M C, HACKE A, NETO C A C, et al. Impact of phenolic compounds in the digestion and absorption of carotenoids[J]. Current Opinion in Food Science,2021,39:190−196. doi: 10.1016/j.cofs.2021.03.006
[63] MENG Q, LONG P, ZHOU J, et al. Improved absorption of β-carotene by encapsulation in an oil-in-water nanoemulsion containing tea polyphenols in the aqueous phase[J]. Food Research International,2018,116:731−736.
[64] LIU G, ZHOU Y, CHEN L. Intestinal uptake of barley protein-based nanoparticles for β-carotene delivery[J]. Acta Pharmaceutica Sinica B,2019,9(1):87−96. doi: 10.1016/j.apsb.2018.10.002
[65] GAZIANO J M, JOHNSON E J, RUSSELL R M, et al. Discrimination in absorption of transport of β-carotene isomers following oral supplemen tation with either all-trans or 9-cis β-carotene[J]. The American Journal of Clinical Nutrition,1995,61:1248−1254. doi: 10.1093/ajcn/61.6.1248
[66] MOSHA T, PACE R, ADEYEYE S, et al. Effect of traditional processing practices on the content of total carotenoid, β-carotene, α-carotene and vitamin A activity of selected Tanzanian vegetables[J]. Plant Foods for Human Nutrition,1997,50(3):189−201. doi: 10.1007/BF02436056
[67] ROHMAH M, RAHMADI A, RAHARJO S. Bioaccessibility and antioxidant activity of β-carotene loaded nanostructured lipid carrier (NLC) from binary mixtures of palm stearin and palm olein[J]. Heliyon,2022,8(2):e08913. doi: 10.1016/j.heliyon.2022.e08913
[68] NIU B, SHAO P, SUN P. Ultrasound-assisted emulsion electrosprayed particles for the stabilization of β-carotene and its nutritional supplement potential[J]. Food Hydrocolloids,2020,102(C):105634−105634.
[69] ABDUR R, TONG Q, SEID M, et al. Carotenoid-loaded nanocarriers: A comprehensive review[J]. Advances in Colloid and Interface Science,2020,275:102048. doi: 10.1016/j.cis.2019.102048
[70] HAN J, ZHANG Z, SHANG W, et al. Modulation of physicochemical stability and bioaccessibility of β-carotene using alginate beads and emulsion stabilized by scallop (Patinopecten yessoensis) gonad protein isolates[J]. Food Research International,2020,129(C):108875.
[71] BA C, FU Y, NIU F, et al. Effects of environmental stresses on physiochemical stability of β-carotene in zein-carboxymethyl chitosan-tea polyphenols ternary delivery system[J]. Food Chemistry,2020,311(C):125878.
[72] ZHONG L, MA N, WU Y, et al. Gastrointestinal fate and antioxidation of β-carotene emulsion prepared by oat protein isolate-Pleurotus ostreatus β-glucan conjugate[J]. Carbohydrate Polymers,2019,221:10−20. doi: 10.1016/j.carbpol.2019.05.085
[73] DU Y, BAO C, HUANG J, et al. Improved stability, epithelial permeability and cellular antioxidant activity of β-carotene via encapsulation by self-assembled α-lactalbumin micelles[J]. Food Chemistry,2019,271:707−714. doi: 10.1016/j.foodchem.2018.07.216
[74] HU Q, HU S, ERIKA F, et al. Chitosan-caseinate-dextran ternary complex nanoparticles for potential oral delivery of astaxanthin with significantly improved bioactivity[J]. International Journal of Biological Macromolecules,2020,151:747−756. doi: 10.1016/j.ijbiomac.2020.02.170
[75] GUO Y, MAO X, ZHANG J, et al. Oral delivery of lycopene-loaded microemulsion for brain-targeting: Preparation, characterization, pharmacokinetic evaluation and tissue distribution[J]. Drug Delivery,2019,26(1):1191−1205. doi: 10.1080/10717544.2019.1689312
[76] YUAN Y, LI H, LIU C, et al. Fabrication and characterization of lutein-loaded nanoparticles based on zein and sophorolipid: Enhancement of water solubility, stability, and bioaccessibility[J]. Journal of Agricultural & Food Chemistry,2019,67(43):11977−11985.
[77] YOSHIKI S, KODAI U, HIROKI S, et al. Development of novel lutein nanocrystal formulation with improved oral bioavailability and ocular distribution[J]. Journal of Functional Foods,2019,61:103499. doi: 10.1016/j.jff.2019.103499
[78] KOBAYASHI J, TOMINAGA E, OZEKI M, et al. Randomized controlled trial of a water-soluble formulation of lutein in humans[J]. Bioscience, Biotechnology, and Biochemistry,2019:2372−2374.
[79] JHAN S, PETHE A M. Double-loaded liposomes encapsulating lycopene β-cyclodextrin complexes: Preparation, optimization, and evaluation[J]. Journal of liposome research,2019:80−92.
[80] GASA-FALCON A, ODRIOZOLA-SERRANO I, OMS-OLIU G, et al. Impact of emulsifier nature and concentration on the stability of β-carotene enriched nanoemulsions during in vitro digestion[J]. Food & Function,2019,10(2):713−722.
[81] YU W, LIU J. Astaxanthin isomers: Selective distribution and isomerization in aquatic animals[J]. Aquaculture,2020,520:734915. doi: 10.1016/j.aquaculture.2019.734915
[82] HOCK-ENG K K, NAGENDRA P, KIN-WENG K, et al. Carotenoids and their isomers: Color pigments in fruits and vegetables[J]. Molecules,2011,16(2):1710. doi: 10.3390/molecules16021710
[83] HONDA M, TAKAHASHI N, KUWA T, et al. Spectral characterisation of Z-isomers of lycopene formed during heat treatment and solvent effects on the E/Z isomerisation process[J]. Food Chemistry,2015,171:323−329. doi: 10.1016/j.foodchem.2014.09.004
[84] MURAKAMI K, HONDA M, TAKEMURA R, et al. Effect of thermal treatment and light irradiation on the stability of lycopene with high Z-isomers content[J]. Food Chemistry,2018,250:253−258. doi: 10.1016/j.foodchem.2018.01.062
[85] MONICA A, FRANCESCA B, AGNESE P, et al. Effect of ultrasound treatment, oil addition and storage time on lycopene stability and in vitro bioaccessibility of tomato pulp[J]. Food Chemistry,2015,172:685−691. doi: 10.1016/j.foodchem.2014.09.140
[86] SUN Q, YANG C, LI J, et al. Lycopene: Heterogeneous catalytic E/Z isomerization and in vitro bioaccessibility assessment using a diffusion model[J]. Journal of Food Science,2016,81(10):C2381−C2389. doi: 10.1111/1750-3841.13419
[87] HONDA M, NAKAYAMA Y, NISHIKAWA S, et al. Z-Isomers of lycopene exhibit greater liver accumulation than the all- E-isomer in mice[J]. Bioscience, Biotechnology, and Biochemistry,2020,84(2):428−431. doi: 10.1080/09168451.2019.1677144
[88] YANG C, LIU H, SUN Q, et al. Enriched Z -isomers of lycopene-loaded nanostructured lipid carriers: Physicochemical characterization and in vitro bioaccessibility assessment using a diffusion model[J]. LWT,2019,111:767−773. doi: 10.1016/j.lwt.2019.05.106
[89] HONDA M, ISHIKAWA H, HAYASHI Y. Alterations in lycopene concentration and Z-isomer content in egg yolk of hens fed all-E-isomer-rich and Z-isomer-rich lycopene[J]. Animal Science Journal,2019,90(9):1261−1269. doi: 10.1111/asj.13276
[90] YANG C, FISCHER M, KIRBY C, et al. Bioaccessibility, cellular uptake and transport of luteins and assessment of their antioxidant activities[J]. Food Chemistry,2018,249:66−76. doi: 10.1016/j.foodchem.2017.12.055
[91] WEINRICH T, XU Y, WOSU C, et al. Mitochondrial function, mobility and lifespan are improved in drosophila melanogaster by extracts of 9- cis -β-carotene from Dunaliella salina[J]. Marine Drugs,2019,17(5):279−279. doi: 10.3390/md17050279
[92] DURING A, HUSSAIN M M, MOREL D W, et al. Carotenoid uptake and secretion by Caco-2 cells: β-carotene isomer selectivity and carotenoid interactions[J]. Journal of Lipid Research,2002,43(7):.1086−1095. doi: 10.1194/jlr.M200068-JLR200
[93] MASAKI H, TOMOHIKO K, HAKUTO K, et al. Enhanced solubility and reduced crystallinity of carotenoids, β-carotene and astaxanthin, by Z-isomerization[J]. European Journal of Lipid Science and Technology,2018,120(11):1800191.
[94] YU J, GLEIZE B, ZHANG L, et al. Heating tomato puree in the presence of lipids and onion: The impact of onion on lycopene isomerization[J]. Food chemistry,2019,296:9−16. doi: 10.1016/j.foodchem.2019.05.188
[95] VAMOUGNE K, MU T, ZHANG M, et al. Effects of cooking process on carotenoids and antioxidant activity of orange-fleshed sweet potato[J]. LWT,2019,104:134−141. doi: 10.1016/j.lwt.2019.01.011
[96] SHINJAE P, SAEHUN M, YONG-RO K. Effect of xanthan gum on lipid digestion and bioaccessibility of β-carotene-loaded rice starch-based filled hydrogels[J]. Food Research International,2018,105:440−445. doi: 10.1016/j.foodres.2017.11.039
[97] DING Y, LIU X, BI J, et al. Effects of pectin, sugar and pH on the β-carotene bioaccessibility in simulated juice systems[J]. LWT,2020,124(C):109125−109125.
-
期刊类型引用(1)
1. 代莹,刘双能,刘晋琦,邢莉那,朱童,周素梅,芦晶. 多重酶解协同制备绿豆基植物乳及其品质分析. 食品科技. 2024(09): 175-183 .  百度学术
百度学术
其他类型引用(0)





 下载:
下载:
 下载:
下载:
