Effect of Enteromorpha Polysaccharide on Intestinal Bacteria and Short Chain Fatty Acids in Obese Golden Hamsters
-
摘要: 目的:探讨浒苔多糖对肥胖金黄地鼠血脂和肠道菌群构成的影响。方法:将40只雄性金黄地鼠按体重随机分为正常对照组(ND组)、高脂模型组(HFD组)、低剂量浒苔多糖组(LEP组,300 mg/kg·BW)和高剂量浒苔多糖组(HEP组,450 mg/kg·BW)。除对照组(ND 组)以普通饲料喂养外,其余组均以高脂饲料喂养。其中低、高剂量浒苔多糖组连续灌胃浒苔多糖水溶液。干预12周后,检测血脂水平,并采用16S rDNA高通量测序和气相色谱法分别比较各组间肠道菌群的多样性以及粪便中短链脂肪酸含量差异。结果:干预12周后,HFD组地鼠的体重、血清总胆固醇(Cholesterol,TC)、甘油三酯(Triglyceride,TG)和低密度脂蛋白胆固醇(Low density lipoprotein- cholesterol,LDL-C)显著高于ND组(P<0.05)。高剂量浒苔多糖可显著降低血清TC、TG、LDL-C和谷丙转氨酶(Alanine aminotransferase,ALT)水平(P<0.05)。16S rDNA高通量测序结果表明,在门水平上,HFD组厚壁菌门/拟杆菌门比例显著高于ND组(P<0.05)。与HFD组相比,HEP组的厚壁菌门/拟杆菌门比例显著降低(P<0.05);在属水平上,HFD组的Eubacterium_coprostanoligenes_group(真杆菌属)、Lachnospiraceae_UCG-006(毛螺菌科 UCG-006)的相对丰度显著高于ND组(P<0.05),经高剂量浒苔多糖干预后,Eubacterium_coprostanoligenes_group(真杆菌属)、Lachnospiraceae_UCG-006(毛螺菌科 UCG-006)的相对丰度相对于HFD组显著降低(P<0.05)。此外,高脂饮食导致粪便中短链脂肪酸含量减少,高剂量浒苔多糖干预可显著增加粪便中短链脂肪酸含量(P<0.05)。结论:浒苔多糖可以通过调节高脂饲料喂养金黄地鼠肠道菌群构成以及短链脂肪酸生成,从而改善肥胖金黄地鼠的脂质代谢紊乱。Abstract: Objective: To investigate the effect of Enteromorpha polysaccharide (EP) on blood lipids and intestinal bacteria in obese golden hamsters. Method: Forty male golden hamsters were randomly divided into four groups, including control group (ND), model group (HFD), low-dose EP group (LEP, 300 mg/kg·BW), and high-dose EP group (HEP, 450 mg/kg·BW). The ND group was fed an ordinary diet, while the other three groups were given a high-fat diet. Among the high-fat diet groups, the LEP and HEP groups were continuously administered EP aqueous solution intragastrically. Twelve weeks later, the serum lipid levels were evaluated, the diversity and structural changes in the gut bacteria were examined using 16S rDNA sequencing, and the short-chain fatty acid concentration in faeces was examined using a gas chromatography flame ionisation detector (GC-FID). Results: After 12 weeks, the body weight, serum total cholesterol (TC), triglyceride (TG), and low-density lipoprotein cholesterol (LDL-C) of hamsters in the HFD group were considerably higher than those in the ND group (P<0.05). In contrast, high-dose EP treatment led to a significant decrease in serum TC, TG, LDL-C, and alanine aminotransferase (ALT) levels (P<0.05). Results of 16S rDNA sequencing revealed that at the phylum level the proportion of Firmicutes/Bacteroidetes was substantially higher in the HFD group than in the ND group (P<0.05). However, the level of Firmicutes/Bacteroidetes was much lower in the HEP group, compared to the HFD group (P<0.05). At the genus level, Eubacterium_coprostanoligenes_group and Lachnospiraceae UCG-006 were more abundant in the HFD group than in the ND group (P<0.05). Following high-dose EP intervention, the relative abundance of Eubacterium_coprostanoligenes_group and Lachnospiraceae_UCG-006 fell significantly, compared to the HFD group (P<0.05). Additionally, a high-fat diet feeding resulted in a decrease in the content of short-chain fatty acids in faeces, while high-dose EP intervention significantly increased the short-chain fatty acid content in faeces (P<0.05). Conclusion: The administration of EP alleviates the metabolic disorders of obese golden hamsters fed with a high-fat diet by moderating the composition of intestinal bacteria and enhancing the production of short-chain fatty acids.
-
随着人们生活水平的提高和膳食结构的改变,超重/肥胖人口占比不断增加,并且逐渐向年轻化发展,现阶段超重/肥胖已成为严重影响国人身心健康的主要公共卫生问题。《中国居民营养与慢性病状况报告(2020年)》显示,我国超过一半的成人出现超重/肥胖,其中6~17岁青少年、6岁以下儿童超重/肥胖率分别达到19.0%和10.4%[1-2]。
研究发现,肠道菌群与高脂血症、肥胖、2型糖尿病、心血管疾病等有着紧密的关联[3]。健康者与肥胖者在肠道菌群组成上有明显差别,提示肠道菌群可能在肥胖发生发展中发挥着重要作用。肥胖患者的肠道菌群可以促进能量的吸收,导致能量过度积累,体重增加[4]。Turnbaugh等[5]发现高脂诱导的肥胖小鼠厚壁菌门丰度显著升高,而这种改变在恢复正常饮食一段时间后能完全逆转肥胖的发生发展,说明调节肠道菌群失调可以改善由高脂饮食引起的肥胖。
浒苔是绿藻纲石莼科的一属,含有丰富的碳水化合物、蛋白质、粗纤维和维生素,其中微量元素特别是铁、铜、锌含量均高于同海域生长的其他藻类[6]。浒苔多糖是从浒苔中提取得到的一种含有大量硫酸基与糖醛酸的硫酸杂多糖,主要由鼠李糖、葡萄糖醛酸、阿拉伯糖、岩藻糖、木糖和葡萄糖构成,具有调节血糖和血脂代谢、抗氧化、抗肿瘤等作用[7]。研究表明,补充绿藻多糖可以增加短链脂肪酸(SCFAs)的产量,刺激Akkermansia muciniphila(嗜黏蛋白阿克曼菌)等有益菌的生长,抑制潜在病原菌的生长[8]。Ren等[9]发现浒苔多糖可以改善便秘引起的肠道菌群变化,同时浒苔多糖能够减轻化疗药物顺铂引起的肠道菌群紊乱,并能够减轻顺铂造成的多种肠道屏障损伤。Kong等[10]将浒苔多糖以及海带多糖同未食用膳食纤维的健康人类粪便在体外共同发酵培养48 h发现多糖可显著降低粪便pH并增加粪便中SCFAs(如乙酸、丁酸)的含量,同时对肠道菌群发挥有益调节作用(增加乳酸菌和双歧杆菌的含量)。但是有关浒苔多糖的抗肥胖作用是否与其调节高脂饮食导致的肠道菌群失衡有关,目前还未见报道。
本研究利用高脂饲料诱导建立肥胖模型,以浒苔多糖作为受试物,利用16S rDNA高通量测序技术测定肠道菌群的变化情况,并检测粪便SCFAs含量,探讨浒苔多糖对肥胖金黄地鼠肠道菌群构成与其代谢产物SCFAs的影响,同时也为多糖类物质生物学活性的机制研究提供理论依据。
1. 材料与方法
1.1 材料与仪器
6周龄雄性LVG叙利亚金黄地鼠(120±10 g) 购自北京维通利华实验动物技术有限公司,实验动物许可证号为SCXK(京)2016-0011,伦理号为2018-037;对照饲料与高脂饲料 购自南通特洛菲饲料科技公司,其中对照饲料脂肪供能比为10%,高脂饲料脂肪供能比为35%,以上饲料均经辐照灭菌处理并储存于4 ℃冰箱;浒苔粉 福建省海兴保健食品有限公司;总胆固醇(TC)测定试剂盒、甘油三酯(TG)测定试剂盒、高密度脂蛋白胆固醇(HDL-C)测定试剂盒、低密度脂蛋白胆固醇(LDL-C)测定试剂盒、丙氨酸氨基转移酶(ALT)测定试剂盒、天门冬氨酸氨基转移酶(AST)测定试剂盒 南京建成有限公司;乙酸标准品、丙酸标准品、异丁酸标准品、丁酸标准品、异戊酸标准品、戊酸标准品、庚酸标准品、2-乙基丁酸标准品(内标) 美国sigma公司;己酸标准品 德国Dr.Ehrenstorfer公司。
DK-S26水浴锅、DHG-9070A电热恒温鼓风干燥箱 上海精宏实验设备有限公司;FD-2A冷冻干燥机 北京博医康实验仪器有限公司;N-1100旋转蒸发仪、OBS-2000恒温水浴锅 东京理化公司;SHB-Ⅲ循环水式真空泵 郑州长城科工贸有限公司;TDL-5-A低速大容量离心机 上海安亭科学仪器厂;7890A气相色谱仪(GC)配FID检测器、毛细管柱Agilent DB-FFAP(30 m×0.25 mm×0.25 μm) 美国安捷伦公司。
1.2 实验方法
1.2.1 浒苔多糖制备方法
取浒苔粉60 g溶于3 L蒸馏水中,80 ℃水浴2 h,纱布过滤,舍弃滤渣,滤液中加入浒苔粉60 g,80 ℃水浴2 h,纱布过滤,舍弃滤渣,使用旋转蒸发仪将滤液浓缩至400 mL左右,3000 r/min离心10 min取上清液,加入4倍体积食用酒精(纯度95%),静置醇沉过夜,沉淀物沥去酒精,冷冻干燥,研磨成粉,即得到本次实验所用的浒苔多糖。经红外光谱和气相色谱检测表明浒苔多糖是由鼠李糖、葡萄糖醛酸、阿拉伯糖、岩藻糖、木糖和葡萄糖组成,其单糖比例分别为5.12:1.32:3.38:1.62:1:1.03。
1.2.2 动物实验方案
40只雄性叙利亚金黄地鼠饲养于福建医科大学动物实验中心SPF级屏障环境内,饲养温度在18~22 ℃,湿度在50~60%之间,保持12 h:12 h的明暗交替时间。适应性喂养1周后,按体重随机分为4组,每组10只,分别为正常对照组(ND组)、高脂模型组(HFD组)、低剂量浒苔多糖组(LEP组,300 mg/kg·BW)和高剂量浒苔多糖组(HEP组,450 mg/kg·BW)。正常对照组给予普通对照饲料喂养,其余3组给予高脂饲料喂养。饲料干预同时,LEP组和HEP组分别给予300 mg/kg·BW和450 mg/kg·BW浒苔多糖溶液灌胃,ND组和HFD组给予等体积蒸馏水灌胃,每日灌胃一次,连续干预12周。饲养期间自由饮食,每周称量并记录体重,垫料每周更换1~2次。
1.2.3 动物血清及器官组织的获取
金黄地鼠干预12周后,禁食不禁水过夜12 h,采用二氧化碳窒息麻醉,摘眼球取血,收集后的血液在室温静置2 h,后4 ℃离心(4000 r/min,10 min),收集上清保存于−80 ℃冰箱。取血后,解剖地鼠,分离取肝、附睾脂肪、肾周脂肪,生理盐水漂洗去除积血,拭净后电子天平称重并记录。脏器系数(g/100 g BW)=100×(脏器重量/体重)。
1.2.4 生化指标检测
血清总胆固醇(TC)、甘油三酯(TG)、低密度脂蛋白胆固醇(LDL-C)、高密度脂蛋白胆固醇(HDL-C)、谷丙转氨酶(ALT)、谷草转氨酶(AST)按照试剂盒说明书进行测定。
1.2.5 粪便样本的收集与处理
在实验最后一周,收集金黄地鼠粪便于−80 ℃保存,用于肠道微生物分析及短链脂肪酸含量的测定。采集的粪便样本委托北京诺禾致源科技股份有限公司进行粪便微生物16S rDNA测序。测序分析过程如下:对送检样本进行DNA提取与检测,然后进行PCR扩增、产物纯化、文库制备及库检,基于IonS5TMXL测序平台,使用Cutadapt软件过滤和按barcode拆分样本后,进行OTUs(Operational taxonomic units)聚类和物种注释。用Mothur方法与SILVA132的SSUrRNA数据库进行物种注释分析,获得分类学信息并分别在各个分类水平:kingdom(界)、phylum(门)、class(纲)、order(目)、family(科)、genus(属)、species(种)统计各样本的群落组成。
1.2.6 短链脂肪酸的测定
短链脂肪酸的定性定量分析委托武汉安隆科讯技术有限公司进行。样品制备过程如下:准确称量冷冻粪便样本100 mg,加入500 μL超纯水,涡旋5 min,4 ℃,13000 r/min离心5 min,取上清液备用;准确移取预处理好的样本溶液200 μL,加入8 μL 2-乙基丁酸(内标),加入氯化钠固体约0.1 g,混合均匀;加入50%的H2SO4溶液20 μL,涡旋混匀;加入无水乙醚200 μL涡旋1 min,上下振摇1 min,随后经13000 r/min离心5 min,取上清,再加入无水乙醚100 μL,重复提取2次,合并上层清液;取上清液100 μL于进样瓶中进行色谱分析。标准溶液制备过程如下:配制5、10、20、50、100、200、500、1000、2000 μmol/L不同浓度梯度的混合标准溶液,并且固定内标浓度为200 μmol/L。采用气相色谱-氢焰离子化检测器联用法(GC-FID)检测短链脂肪酸的含量和组成,绘制横坐标为保留时间,纵坐标为色谱峰强度的色谱图。
色谱分析条件如下:色谱柱为Agilent DB-FFAP(30 m×0.25 mm×0.25 μm);设置进样口温度200 ℃,检测器温度240 ℃,进样量为2 μL;升温程序:第1阶段设定速率为30 ℃/min,温度为95~200 ℃,时间为3.5 min,第2阶段速率不变,温度调整为200 ℃,时间2 min;设定分流比为5:1;柱流量为3.0 mL/min。
1.3 数据处理
所有数据采用IBM SPSS 20.0软件进行统计分析。计量资料用均数±标准差(
¯X ±S)表示,各指标数据经过正态性检验和方差齐性检验,进行单因素方差分析(One-Way ANOVA),若方差齐性则采用LSD法进行两两比较,若方差不齐则采用Dunnet’T3进行两两比较,检验水准α=0.05。2. 结果与分析
2.1 浒苔多糖对金黄地鼠体重、脏器系数、血脂及肝功能的影响
如表1所示,高脂喂养12周后,与ND组相比,HFD组最终体重、睾周脂肪系数和肾周脂肪系数显著升高(P<0.05)。与HFD组相比,LEP组初始以及最终体重、睾周脂肪系数和肾周脂肪系数均无显著差异(P>0.05);而HEP组显著降低了金黄地鼠最终体重、睾周脂肪系数和肾周脂肪系数(P<0.05),说明高剂量浒苔多糖干预可逆转HFD引起的体重、体脂增加。血脂检测结果表明,相较于ND组,HFD组金黄地鼠血清TC、TG、LDL-C水平显著升高(P<0.05),血清HDL-C水平显著降低(P<0.05),提示肥胖模型建立成功。与HFD组相比,LEP组金黄地鼠仅TC及LDL-C水平显著降低(P<0.05);而HEP组金黄地鼠血清TC、TG、LDL-C水平均显著降低(P<0.05)。此外,高剂量浒苔多糖干预可显著逆转由高脂饮食干预引起的血清ALT水平升高(P<0.05)。以上结果说明高剂量浒苔多糖对高脂饮食引起的血脂异常改善效果更为显著,因此后续肠道菌群及短链脂肪酸分析选择ND组、HFD组和HEP组进行。
表 1 浒苔多糖对金黄地鼠体重、脏器系数、血脂和肝功能的影响Table 1. Effect of Enteromorpha polysaccharide on body weight, organ coefficient, blood lipid and liver function in golden hamster分组 ND组 HFD组 LEP组 HEP组 体重(BW,g) 初始体重 129.28±6.12a 126.69±9.43a 128.07±6.53a 123.12±6.68a 最终体重 156.12±12.19b 174.71±13.96a 168.08±8.35ab 159.52±12.35b 脏器系数(g/100 g BW) 睾周脂肪系数 2.20±0.29b 3.07±0.36a 2.97±0.35a 2.47±0.38b 肾周脂肪系数 1.48±0.27b 2.35±0.39a 2.04±0.43a 1.69±0.26b 血脂参数(mmol/L) TC 4.55±0.37c 10.53±1.05a 8.91±1.20b 7.97±0.57b TG 1.10±0.21c 5.45±2.09a 5.10±1.02a 3.78±0.74b LDL-C 1.14±0.39c 4.60±1.05a 3.48±0.72b 2.84±0.37b HDL-C 3.61±0.49a 2.60±0.49b 2.79±0.53b 2.06±0.29c 肝功能(U/L) 血清ALT 19.23±6.12c 41.99±13.34a 28.30±10.45b 17.82±6.13c 血清AST 16.40±4.49a 15.16±4.84a 13.10±2.61a 12.77±1.41a 注:同行不同小写字母表示组间差异有统计学意义(P<0.05);表2同。 2.2 浒苔多糖对高脂饲料喂养金黄地鼠肠道菌群的影响
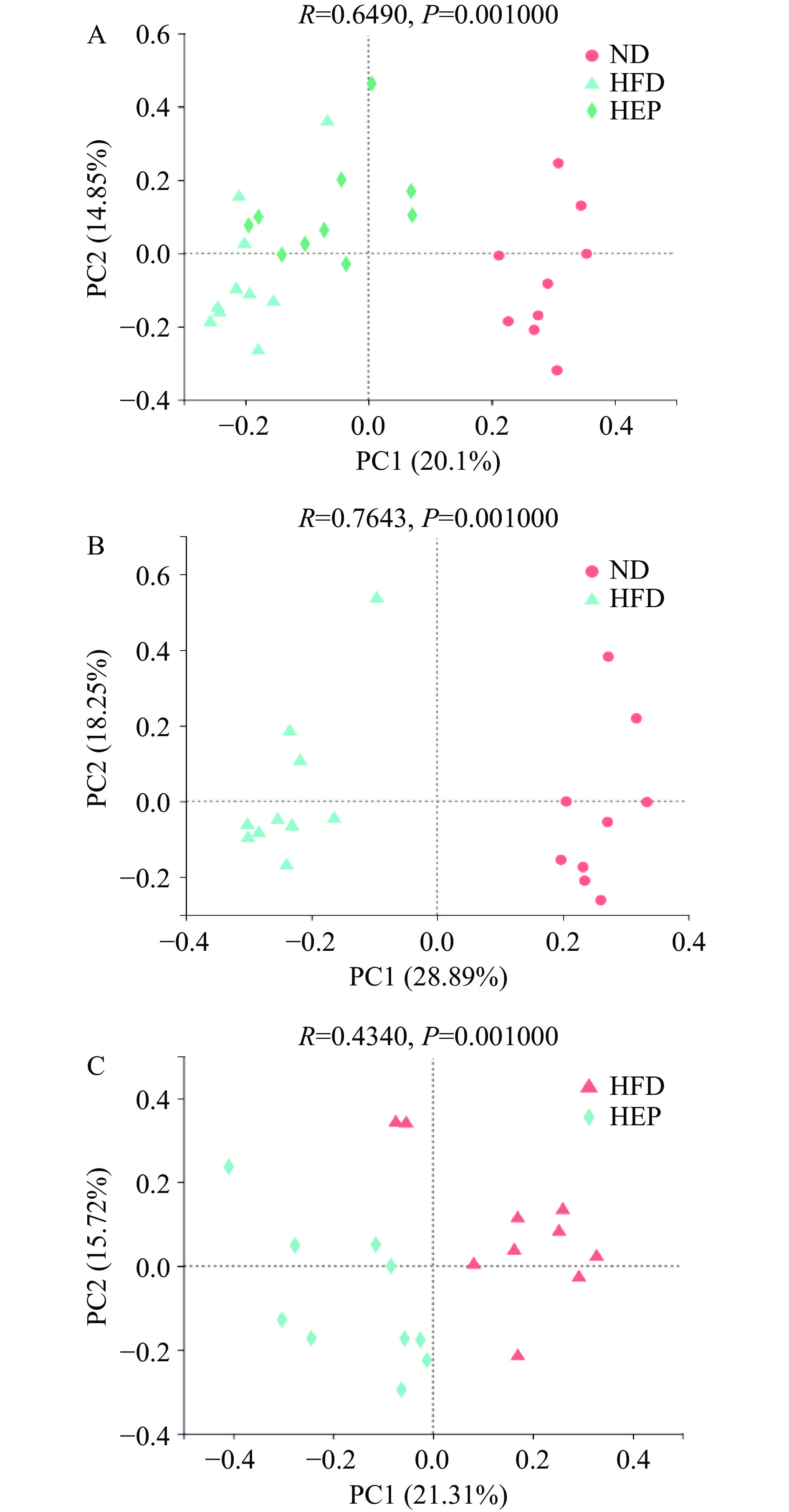
2.2.1 主坐标分析
如图1A所示,多组间的肠道菌群的组成具有统计学差异(P<0.05)。图1B中,HFD组与ND组样本沿第一主坐标(PC1)明显分离,说明高脂饲料喂养可导致肠道微生物群落构成发生变化(P<0.05)。如图1C所示,HEP组和HFD组肠道菌群组成出现显著差异(P<0.05),说明浒苔多糖的干预会影响肠道微生物群落构成。
2.2.2 门分类水平的微生物群落物种组成变化
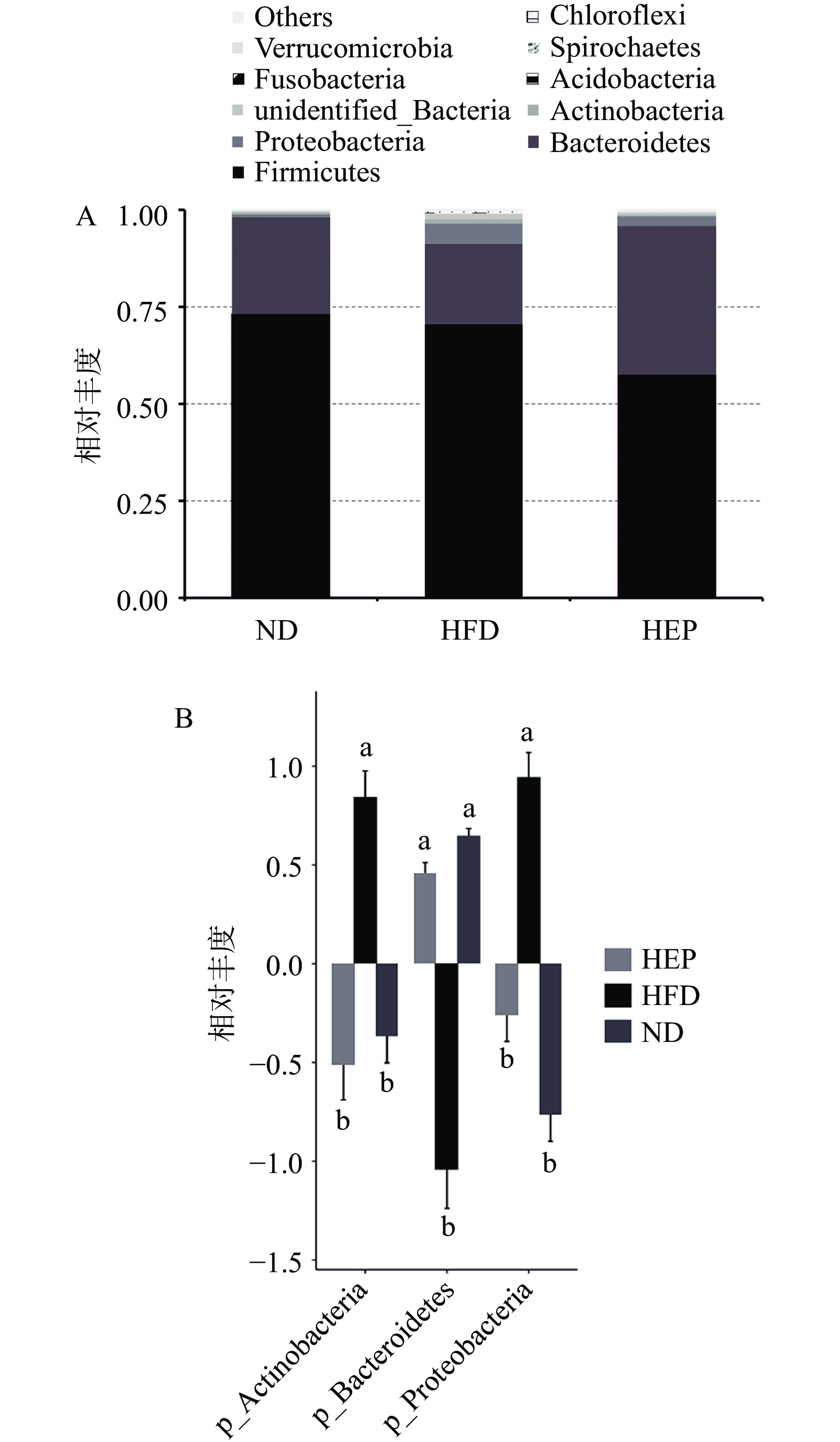
为了解浒苔多糖干预对高脂饲料喂养金黄地鼠肠道微生物群落构成变化的影响,在门水平上对不同组金黄地鼠的肠道微生物群落组成进行比较。如图2A所示,不同组间的Firmicutes(厚壁菌门)、Bacteroidetes(拟杆菌门)和Proteobacteria(变形菌门)总占比超过90%。其中 Firmicutes、Bacteroidetes是肠道中的优势菌群。与HFD组相比,HEP干预降低了厚壁菌门的相对丰度。研究发现,厚壁菌能将多糖转化为易吸收的单糖类和短链脂肪酸,过多的短链脂肪酸会为宿主提供额外的能量,从而促进肥胖[11]。
图2B通过对不同组间具有统计学差异的菌门进行多重比较发现,相比于HFD组,HEP组Bacteroidetes的相对丰度组显著增加,而Proteobacteria和Actinobacteria相对丰度显著降低(P<0.05)。Bacteroidetes含有丰富的碳水化合物活性酶(CAzyme),可以生成SCFAs,调节机体的能量平衡[12]。而Proteobacteria是一种兼性厌氧菌,其水平的增加可以导致肠道上皮细胞功能障碍和肠道屏障的破坏,促进炎症发生[13]。以上结果说明浒苔多糖干预后可逆转高脂饮食导致的金黄地鼠肠道菌群比例失衡。
2.2.3 属分类水平的微生物群落物种组成变化
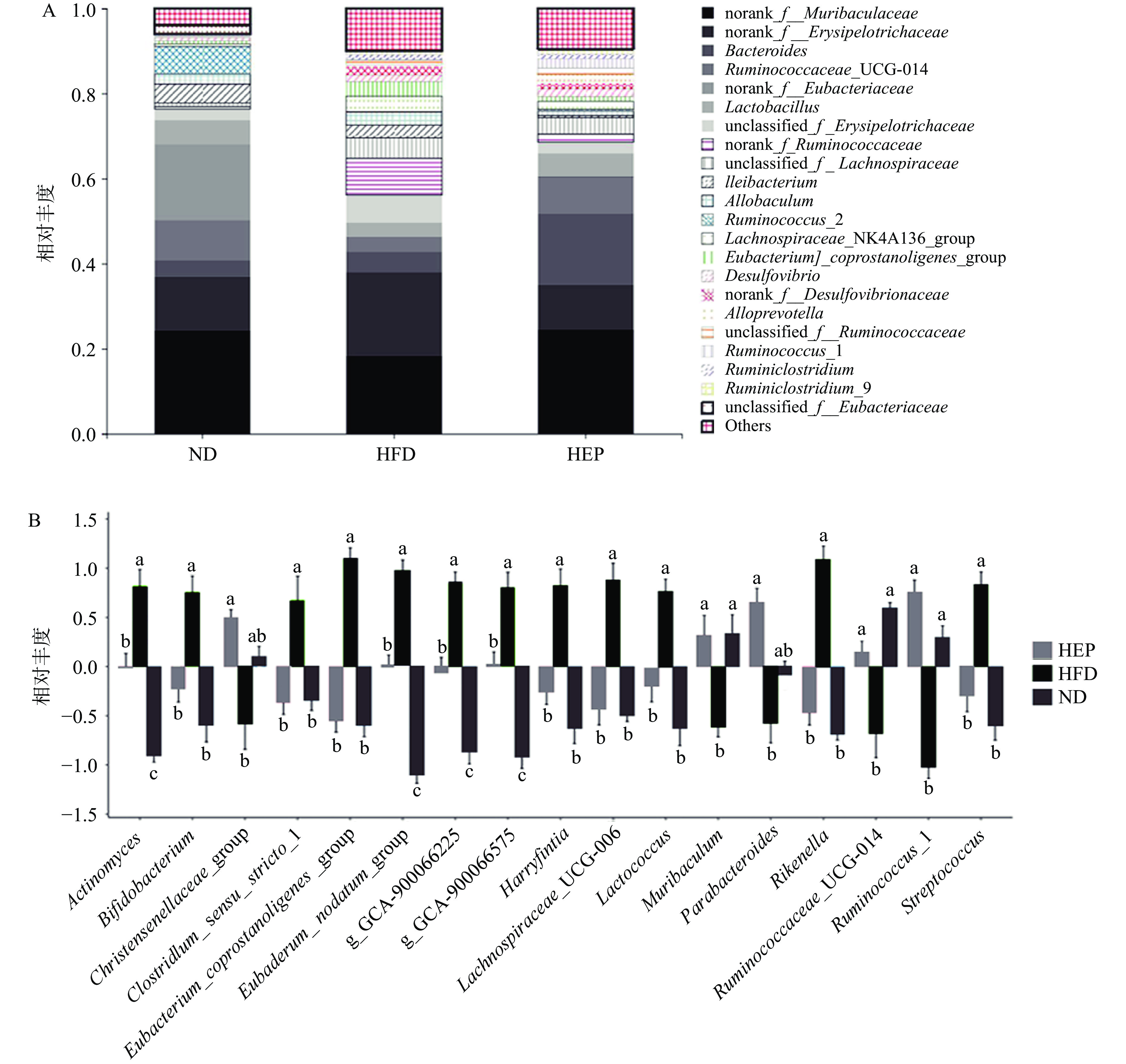
如图3A和图3B所示,在属水平上,与ND组相比,HFD组中Eubacterium_coprostanoligenes_group(真杆菌属)、Lachnospiraceae_UCG-006(毛螺菌科_UCG-006)和Rikenella(理研菌属)等显著增加(P<0.05),而Ruminococcaceae_UCG-014(瘤胃球菌_UCG-014)和Ruminococcus_1(瘤胃球菌_1)等相对丰度显著减少(P<0.05)。经高剂量浒苔多糖干预后(与HFD组相比),显著增加了Ruminococcaceae_UCG-014(瘤胃球菌_UCG-014)和Ruminococcus_1(瘤胃球菌_1)的相对丰度(P<0.05),减少了Eubacterium_coprostanoligenes_group(真杆菌属)、Lachnospiraceae_UCG-006(毛螺菌科_UCG-006)和Rikenella(理研菌属)的相对丰度(P<0.05)。研究表明,瘤胃球菌具有降解抗性淀粉和发酵膳食纤维的功能,其相对丰度与肥胖和超重女性中脂肪因子水平呈显著负相关[14],而与短链脂肪酸(乙酸和丁酸等)含量呈显著正相关[15]。另有研究发现,HFD组中Lachnospiraceae_UCG-006(毛螺菌科UCG-006)相对丰度的增加与机体的超重和肥胖密切正相关[16-17]。此外,HFD组中显著富集的Eubacterium_coprostanoligenes_group(真杆菌属)可通过发酵葡萄糖而产生大量丁酸或乙酸等短链脂肪酸,但过量的丁酸盐可能会降低益生菌的比例并对脂质代谢产生有害影响[18]。因此,浒苔多糖干预可能通过增加瘤胃球菌等有益菌的丰度,减少毛螺菌科和真杆菌属等有害菌的丰度,从而调节机体代谢。
2.2.4 LEfSe分析
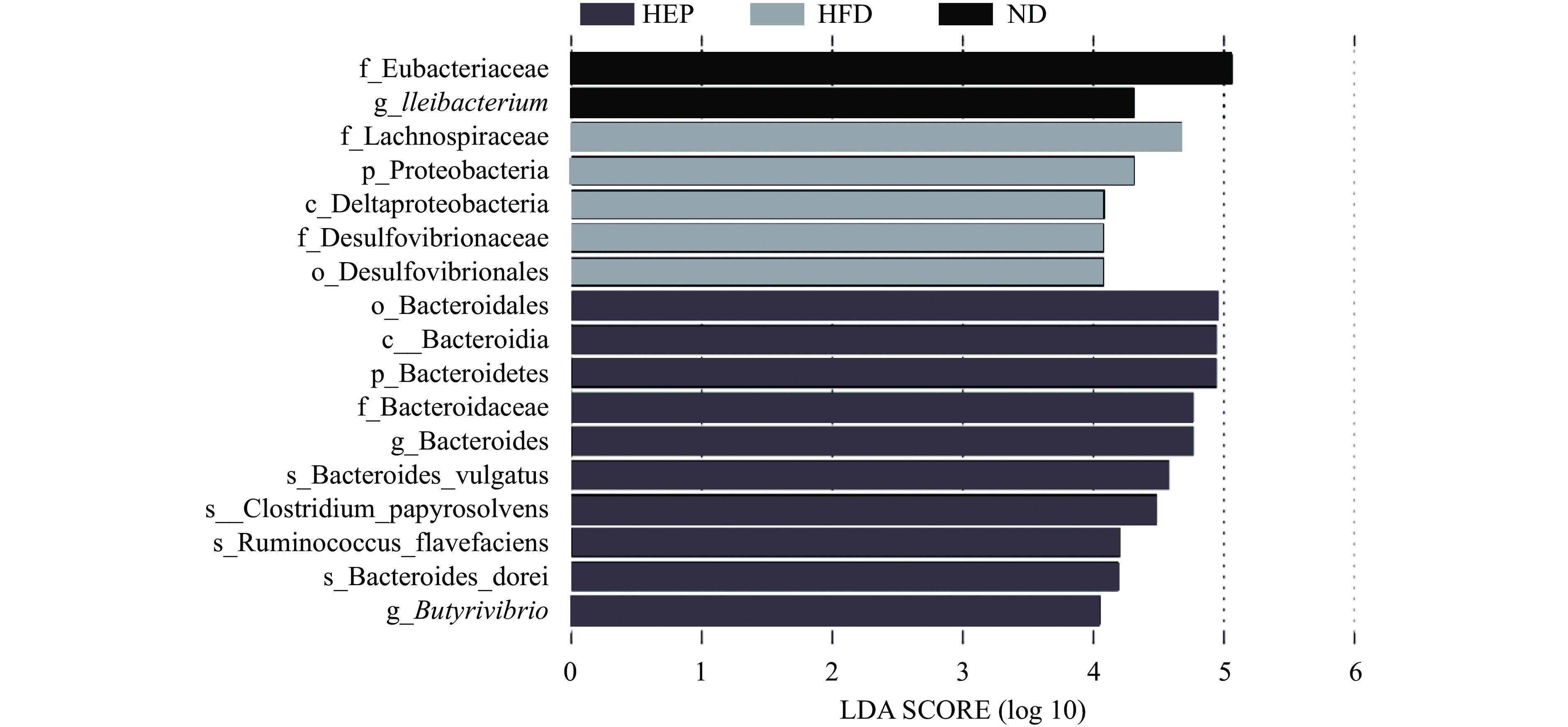
图4为LDA值柱状图,列出了LDA得分大于阈值的有差异的微生物物种,柱体长度越长,显著差异物种的影响越大,设定阈值为LDA>4.0。实验结果显示,Eubacteriaceae(真杆菌科)、Ileibacterium在ND组中显著富集,Lachnospiraceae(毛螺菌科)、Proteobacteria(变形菌门)、Deltaproteobacteria(变形菌纲)、Desulfovibrionaceae(脱硫弧菌科)、Desulfovibrionales(脱硫弧菌目)在HFD组显著富集。研究发现,肥胖小鼠的肠道菌群中脱硫弧菌相对丰度显著高于对照小鼠,其相对丰度增加可能影响肠道中上皮细胞脂质吸收,从而导致肥胖发生发展[19]。而HEP组中显著富集的Bacteroidales(拟杆菌目)、Bacteroidia(拟杆菌纲)、Bacteroidetes(拟杆菌门)、Butyrivibrio(丁酸弧菌属)与短链脂肪酸的产生密切相关。有研究表明,拟杆菌门和丁酸弧菌相对丰度的增加可以通过增加粪便中短链脂肪酸的含量,调节机体的能量代谢,缓解肥胖的发生发展[20-21]。
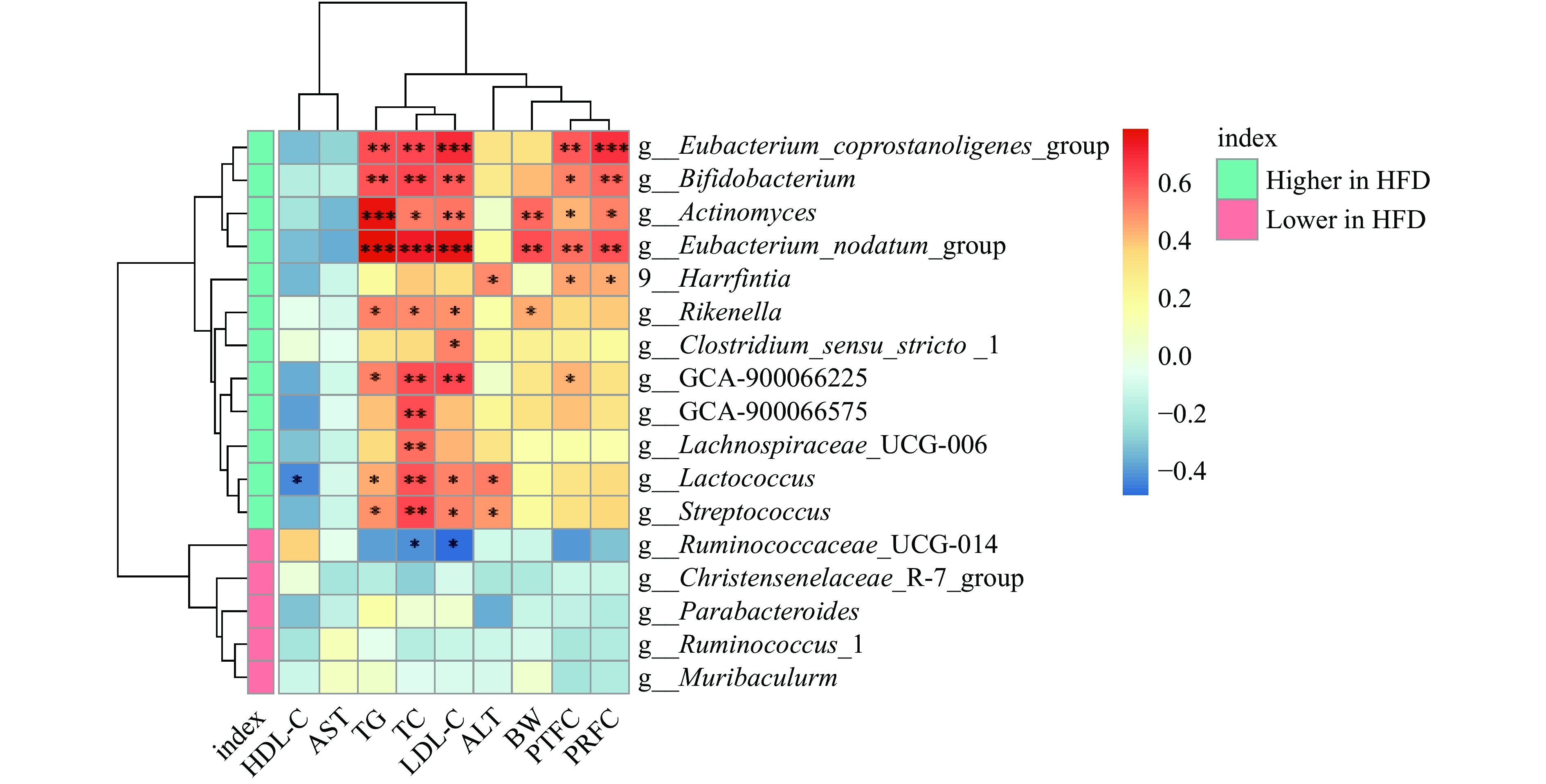
2.2.5 肠道菌群与肥胖相关指标的相关性分析
研究表明,肠道微生物中益生菌(嗜酸乳杆菌和双歧杆菌)相对丰度的增加可以改善异常血脂水平,而有害菌群(粪链球菌和真杆菌属)会对机体脂代谢造成不利影响[22]。本研究通过相关性分析,探究了肥胖相关指标与肠道微生物之间的关系。如图5所示,与HEP组相比,在HFD组显著增加的肠道菌群与体重、脂肪脏器系数和血脂指标呈正相关,如Lachnospiraceae_UCG-006(毛螺菌科UCG-006)与TC水平呈显著正相关(P<0.01),Streptococcus(链球菌属)与TC、TG和LDL-C呈显著正相关(P<0.05);而在HFD组显著减少的肠道菌群Ruminococcaceae_UCG-014与LDL-C和TC呈显著负相关(P<0.05)。与相关文献报道,血脂异常和脂肪沉积个体的粪便样本中检出链球菌的丰度与LDL-C水平呈显著正相关结论一致。另有研究表明,地中海饮食可通过增加瘤胃球菌的丰度改善肥胖受试者的TC和LDL-C水平[23-25],说明浒苔多糖可能通过调节菌群丰度从而改善血脂水平。
2.3 浒苔多糖对高脂饲料喂养金黄地鼠短链脂肪酸组成的影响
2.3.1 浒苔多糖对金黄地鼠粪便短链脂肪酸含量的影响
膳食纤维或多糖可能通过恢复肠道代谢而起到减肥作用[26]。此外,作为膳食纤维的微生物代谢物的短链脂肪酸(SCFAs)在饲喂HFD后明显减少[27]。本研究中,金黄地鼠粪便SCFAs含量如表2所示,与ND组相比,HFD组金黄地鼠粪便各种SCFA含量均显著降低(P<0.05);与HFD组相比,HEP组金黄地鼠粪便propanoic acid(丙酸)、isobutyric acid(异丁酸)、isovaleric acid(异戊酸)含量显著升高(P<0.05)。以上结果表明,浒苔多糖可通过增加粪便中短链脂肪酸含量改善肥胖所引起的代谢紊乱。
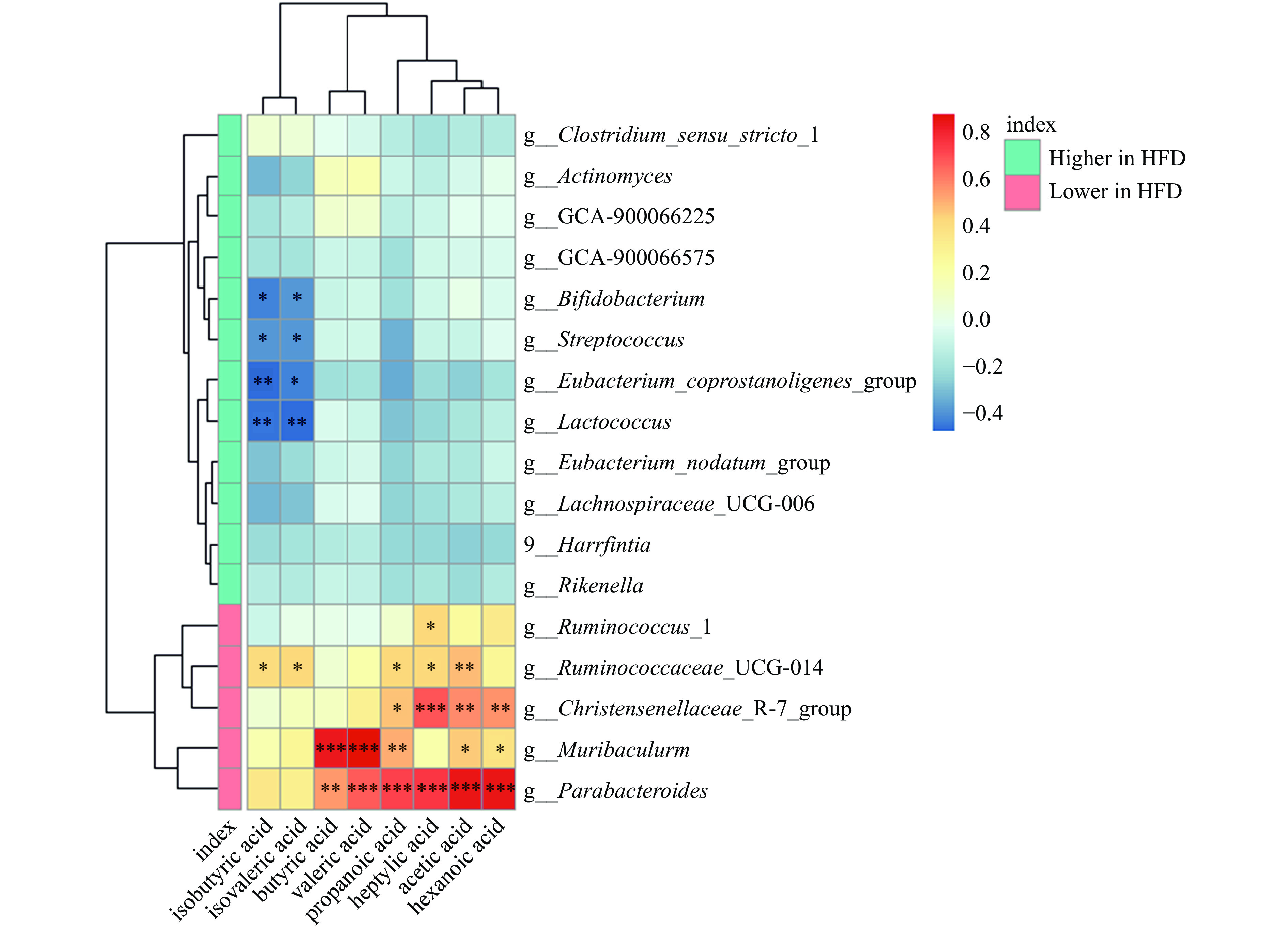
表 2 浒苔多糖对金黄地鼠粪便短链脂肪酸含量的影响(mg/g)Table 2. Effect of Enteromorpha polysaccharide on short-chain fatty acid content in feces of golden hamsters (mg/g)分组 ND组 HFD组 HEP组 Acetic acid 1.433±0.440a 0.507±0.074b 0.594±0.136b Propanoic acid 0.275±0.149a 0.063±0.021b 0.143±0.067a Isobutyric acid 0.016±0.007a 0.011±0.006b 0.019±0.006a Butyric acid 0.363±0.178a 0.103±0.075b 0.214±0.138ab Isovaleric acid 0.034±0.013a 0.019±0.011b 0.036±0.011a Valeric acid 0.143±0.068a 0.020±0.008c 0.048±0.020bc Hexanoic acid 0.234±0.132a 0.017±0.002b 0b Heptylic acid 0.013±0.006a 0b 0b 2.3.2 菌群与SCFAs的Spearman相关性分析
肠道菌群可以将多糖降解为单糖或低聚糖,促进机体对多糖的消化和吸收,并产生SCFAs[12],因此采用相关性分析进一步探索菌群与粪便SCFAs含量的关系。如图6所示,Bifidobacterium(双歧杆菌属)、Streptococcus(链球菌属)、Eubacterium_coprostanoligenes_group(真杆菌属)和Lactococcus(乳球菌属)的相对丰度与isobutyric acid(异丁酸)和isovaleric acid(异戊酸)的浓度呈显著负相关(P<0.05或P<0.01),而Parabacteroides(狄氏副拟杆菌)与acetic acid(乙酸)、propanoic acid(丙酸)、butyric acid(丁酸)、valeric acid(戊酸)、hexanoic acid(己酸)和heptylic acid(庚酸)呈显著正相关(P<0.01)。此外Ruminococcaceae_UCG-014(瘤胃球菌_UCG-014)与acetic acid(乙酸)、propanoic acid(丙酸)、isobutyric acid(异丁酸)、isovaleric acid(异戊酸)和heptylic acid(庚酸)呈显著正相关(P<0.05或P<0.01)。研究发现,从海带中提取的不溶性膳食纤维通过调节肠道菌群失调,促进SCFAs产生菌的丰度增加来减轻高脂饮食诱导的肥胖[28]。另有研究发现坛紫菜多糖可作为肠道菌群的唯一碳源,增加SCFAs的生成,从而促进益生菌(双歧杆菌)的生长[29]。以上结果表明,浒苔多糖可能通过被Parabacteroides(狄氏副拟杆菌)与Ruminococcaceae_UCG-014(瘤胃球菌科_UCG-014)酵解生成SCFAs进而调节机体代谢。
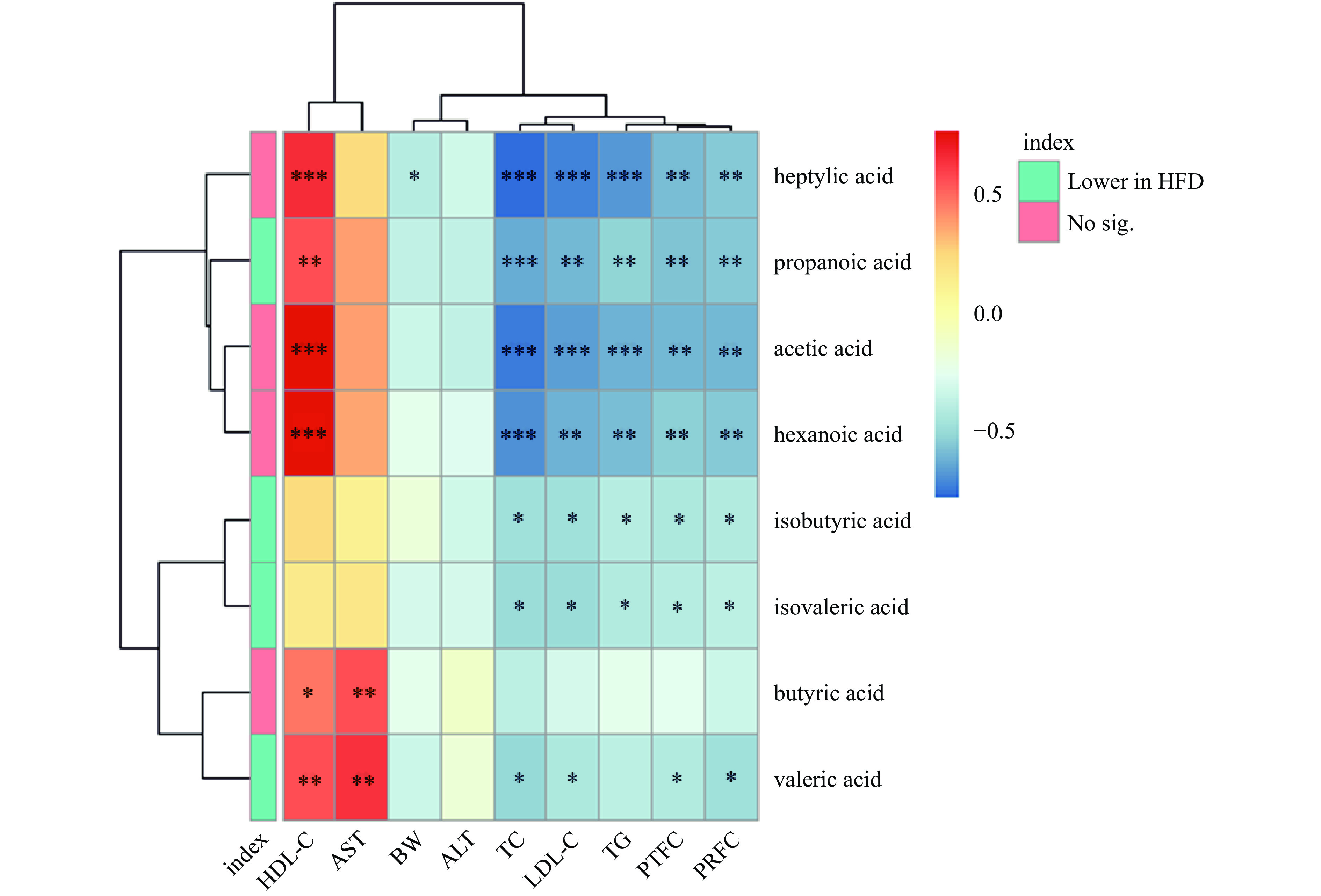
2.3.3 短链脂肪酸与肥胖相关指标的Spearman相关性分析
如图7所示,在HFD组中显著减少的propanoic acid(丙酸)、isobutyric acid(异丁酸)、isovaleric acid(异戊酸)和valeric acid(戊酸)均与TC、LDL-C、睾周脂肪脏器系数及肾周脂肪脏器系数呈显著的负相关(P<0.05),其中propanoic acid(丙酸)和valeric acid(戊酸)与HDL-C呈显著的正相关(P<0.01)。因此,SCFAs的含量减少可能与肥胖引发的机体代谢紊乱有关。
3. 讨论与结论
在本研究中,高剂量浒苔多糖干预可显著降低高脂饲料喂养的金黄地鼠体重、肾周及睾周脂肪系数(P<0.05),同时降低金黄地鼠血清TC、TG及LDL-C水平,说明高剂量浒苔多糖干预可有效预防高脂饲料诱导的脂代谢紊乱。此外,肠道菌群的测序结果显示高剂量浒苔多糖可以逆转由高脂饲料导致的肠道菌群紊乱,主要表现在改变肠道菌群的构成比例及组成,显著增加可调节脂质代谢的Muribaculum(肠鼠杆菌)、Parabacteroides(狄氏副拟杆菌)和瘤胃球菌等有益菌属的相对丰度。
天然多糖是改善肠道菌群的有利底物,可促进有益菌的生长,减少条件致病菌的丰度[30]。在本研究中,补充浒苔多糖可增加拟杆菌门的相对丰度,降低厚壁菌门的相对丰度,从而逆转由高脂饲料诱导引起的肠道厚壁菌门/拟杆菌门比例升高。Nguyen等[31]的研究表明,补充昆布多糖可以改变高脂饮食小鼠的肠道菌群组成和比例,摄入昆布多糖的小鼠,其肠道菌群中有益菌狄氏副杆菌(Parabacteroides)等的相对丰度显著增加。有益菌的生长可促进肠道健康,进而改善系统炎症,缓解代谢综合征。本研究还显示与HFD组相比,HEP组瘤胃球菌和丁酸弧菌等有益菌的丰度显著增加(P<0.05),脱硫弧菌的丰度显著减少(P<0.05)。Chen等[32]研究也发现,补充竹屑多糖的小鼠通过降低有害菌(肠杆菌和脱硫弧菌)相对丰度,增加有益菌(阿克曼菌属和乳球菌属)相对丰度,从而减轻肥胖所引起的机体炎症。
另一方面,天然多糖可以通过促进SCFAs生成来改善炎症反应。本研究发现HEP组金黄地鼠粪便丙酸、异丁酸、异戊酸、戊酸等SCFAs含量较HFD组显著增多。与Li等[33]研究中,通过补充凝胶多糖,从而增加高脂饮食诱导的肥胖小鼠SCFAs生成并刺激有益菌的生长结果一致。此外,本研究显示丁酸、戊酸及丙酸含量与Muribaculum(肠鼠杆菌)和Parabacteroides distasonis(狄氏副拟杆菌)水平呈正相关。LAGKOUVARDOS等[34]采用基因组分析表明,Muribaculum(肠鼠杆菌)具有降解多种复杂碳水化合物的能力,可促进SCFAs的生成。因此,浒苔多糖可能通过促进肥胖金黄地鼠肠道菌群产生更多的SCFAs,从而缓解肥胖的发生发展。
综上所述,浒苔多糖能显著降低金黄地鼠体重及内脏脂肪系数,并改善高脂饮食造成的异常血脂水平,其中高剂量浒苔多糖效果更为显著。此外,相关性分析表明在HFD组显著降低的菌属与肥胖相关指标呈正相关,推测高剂量浒苔多糖发挥抑制肥胖的功效可能与调节肠道菌群组成,进而增加肠道中SCFAs含量生成有关。
-
表 1 浒苔多糖对金黄地鼠体重、脏器系数、血脂和肝功能的影响
Table 1 Effect of Enteromorpha polysaccharide on body weight, organ coefficient, blood lipid and liver function in golden hamster
分组 ND组 HFD组 LEP组 HEP组 体重(BW,g) 初始体重 129.28±6.12a 126.69±9.43a 128.07±6.53a 123.12±6.68a 最终体重 156.12±12.19b 174.71±13.96a 168.08±8.35ab 159.52±12.35b 脏器系数(g/100 g BW) 睾周脂肪系数 2.20±0.29b 3.07±0.36a 2.97±0.35a 2.47±0.38b 肾周脂肪系数 1.48±0.27b 2.35±0.39a 2.04±0.43a 1.69±0.26b 血脂参数(mmol/L) TC 4.55±0.37c 10.53±1.05a 8.91±1.20b 7.97±0.57b TG 1.10±0.21c 5.45±2.09a 5.10±1.02a 3.78±0.74b LDL-C 1.14±0.39c 4.60±1.05a 3.48±0.72b 2.84±0.37b HDL-C 3.61±0.49a 2.60±0.49b 2.79±0.53b 2.06±0.29c 肝功能(U/L) 血清ALT 19.23±6.12c 41.99±13.34a 28.30±10.45b 17.82±6.13c 血清AST 16.40±4.49a 15.16±4.84a 13.10±2.61a 12.77±1.41a 注:同行不同小写字母表示组间差异有统计学意义(P<0.05);表2同。 表 2 浒苔多糖对金黄地鼠粪便短链脂肪酸含量的影响(mg/g)
Table 2 Effect of Enteromorpha polysaccharide on short-chain fatty acid content in feces of golden hamsters (mg/g)
分组 ND组 HFD组 HEP组 Acetic acid 1.433±0.440a 0.507±0.074b 0.594±0.136b Propanoic acid 0.275±0.149a 0.063±0.021b 0.143±0.067a Isobutyric acid 0.016±0.007a 0.011±0.006b 0.019±0.006a Butyric acid 0.363±0.178a 0.103±0.075b 0.214±0.138ab Isovaleric acid 0.034±0.013a 0.019±0.011b 0.036±0.011a Valeric acid 0.143±0.068a 0.020±0.008c 0.048±0.020bc Hexanoic acid 0.234±0.132a 0.017±0.002b 0b Heptylic acid 0.013±0.006a 0b 0b -
[1] NITTARI G, SCURI S, PETRELLI F, et al. Fighting obesity in children from European World Health Organization Member States. Epidemiological data, medical-social aspects, and prevention programs[J]. Clin Ter,2019,170(3):e223−e230.
[2] PAN X F, WANG L, PAN A. Epidemiology and determinants of obesity in China[J]. Lancet Diabetes Endocrinol,2021,9(6):373−392. doi: 10.1016/S2213-8587(21)00045-0
[3] LEE S J, SHIN S W. Mechanisms, pathophysiology, and management of obesity[J]. N Engl J Med,2017,376(15):1491−1492.
[4] LIU B N, LIU X T, LIANG Z H, et al. Gut microbiota in obesity[J]. World Journal of Gastroenterology,2021,27(25):3837−3850. doi: 10.3748/wjg.v27.i25.3837
[5] TURNBAUGH P J, LEY R E, MAHOWALD M A, et al. An obesity-associated gut microbiome with increased capacity for energy harvest[J]. Nature,2006,444(7122):1027−1031. doi: 10.1038/nature05414
[6] 高鑫, 山珊, 曾德永, 等. 石莼属绿藻多糖的生物活性研究进展[J]. 食品工业科技,2021,42(2):364−369. [GAO X, SHAN S, ZENG D Y, et al. Research progress on biological activity of ulvan[J]. Science and Technology of Food Industry,2021,42(2):364−369. doi: 10.13386/j.issn1002-0306.2020040007 [7] TENG Z, QIAN L, ZHOU Y. Hypolipidemic activity of the polysaccharides from Enteromorpha prolifera[J]. Int J Biol Macromol,2013,62:254−256. doi: 10.1016/j.ijbiomac.2013.09.010
[8] SHANG Q, WANG Y, PAN L, et al. Dietary polysaccharide from Enteromorpha clathrata modulates gut microbiota and promotes the growth of Akkermansia muciniphila, Bifidobacterium spp. and Lactobacillus spp.[J]. Mar Drugs,2018,16(5):167. doi: 10.3390/md16050167
[9] REN X, LIU L, GAMALLAT Y, et al. Enteromorpha and polysaccharides from Enteromorpha ameliorate loperamide-induced constipation in mice[J]. Biomed Pharmacother,2017,96:1075−1081. doi: 10.1016/j.biopha.2017.11.119
[10] KONG Q, DONG S Y, GAO J, et al. In vitro fermentation of sulfated polysaccharides from E. prolifera and L. japonica by human fecal microbiota[J]. Int J Biol Macromol,2016,91:867−871. doi: 10.1016/j.ijbiomac.2016.06.036
[11] 张宵, 刘杨, 滕博, 等. 基于肠道菌群的海藻多糖对部分疾病影响的研究进展[J]. 食品工业科技,2021,42(18):421−426. [ZHANG X, LIU Y, TENG B, et al. Research progress of the effects of seaweed polysaccharides on some diseases based on intestinal flora[J]. Science and Technology of Food Industry,2021,42(18):421−426. doi: 10.13386/j.issn1002-0306.2020080239 [12] CABRAL L, PERSINOTI G F, PAIXAO D A A, et al. Gut microbiome of the largest living rodent harbors unprecedented enzymatic systems to degrade plant polysaccharides[J]. Nature Communications, 2022, 13(1): 629-629.
[13] LITVAK Y, BYNDLOSS M X, TSOLIS R M, et al. Dysbiotic Proteobacteria expansion: A microbial signature of epithelial dysfunction[J]. Curr Opin Microbiol,2017,39:1−6. doi: 10.1016/j.mib.2017.07.003
[14] GOMEZ-ARANGO L F, BARRETT H L, MCINTYRE H D, et al. Connections between the gut microbiome and metabolic hormones in early pregnancy in overweight and obese women[J]. Diabetes,2016,65(8):2214−2223. doi: 10.2337/db16-0278
[15] VOJINOVIC D, RADJABZADEH D, KURILSHIKOV A, et al. Relationship between gut microbiota and circulating metabolites in population-based cohorts[J]. Nature Communications,2019,10(1):5813. doi: 10.1038/s41467-019-13721-1
[16] TUN H M, BRIDGMAN S L, CHARI R, et al. Roles of birth mode and infant gut microbiota in intergenerational transmission of overweight and obesity from mother to offspring[J]. Jama Pediatr,2018,172(4):368−377. doi: 10.1001/jamapediatrics.2017.5535
[17] ZHAO L, ZHANG Q, MA W N, et al. A combination of quercetin and resveratrol reduces obesity in high-fat diet-fed rats by modulation of gut microbiota[J]. Food Funct,2017,8(12):4644−4656. doi: 10.1039/C7FO01383C
[18] WEI W, JIANG W B, TIAN Z, et al. Fecal g. Streptococcus and g. Eubacterium_coprostanoligenes_group combined with sphingosine to modulate the serum dyslipidemia in high-fat diet mice[J]. Clin Nutr,2021,40(6):4234−4245. doi: 10.1016/j.clnu.2021.01.031
[19] PETERSEN C, BELL R, KIAG K A, et al. T cell-mediated regulation of the microbiota protects against obesity[J]. Science,2019,365:340.
[20] PIDCOCK S E, SKVORTSOV T, SANTOS F G, et al. Phylogenetic systematics of Butyrivibrio and Pseudobutyrivibrio genomes illustrate vast taxonomic diversity, open genomes and an abundance of carbohydrate-active enzyme family isoforms[J]. Microb Genomics,2021,7(10):000638.
[21] CANI P D. Microbiota and metabolites in metabolic diseases[J]. Nat Rev Endocrinol,2019,15(2):69−70. doi: 10.1038/s41574-018-0143-9
[22] DENG X L, MA J, SONG M T, et al. Effects of products designed to modulate the gut microbiota on hyperlipidaemia[J]. Eur J Nutr,2019,58(7):2713−2729. doi: 10.1007/s00394-018-1821-z
[23] SCHOELER M, CAESAR R J R I E, DISORDERS M. Dietary lipids, gut microbiota and lipid metabolism[J]. 2019, 20(4): 461-472.
[24] FU J, BONDER M J, CENIT M C, et al. The gut microbiome contributes to a substantial proportion of the variation in blood lipids[J]. Circulation Research,2015,117(9):817−824. doi: 10.1161/CIRCRESAHA.115.306807
[25] MESLIER V, LAIOLA M, ROAGER H M, et al. Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake[J]. Gut,2020,69(7):1258−1268. doi: 10.1136/gutjnl-2019-320438
[26] MAKKI K, DEEHAN E C, WALTER J, et al. The impact of dietary fiber on gut microbiota in host health and disease[J]. Cell Host Microbe,2018,23(6):705−715. doi: 10.1016/j.chom.2018.05.012
[27] KLANCIC T, REIMER R A. Gut microbiota and obesity: Impact of antibiotics and prebiotics and potential for musculoskeletal health[J]. J Sport Health Sci,2020,9(2):110−118. doi: 10.1016/j.jshs.2019.04.004
[28] MO X, SUN Y, LIANG X, et al. Insoluble yeast β-glucan attenuates high-fat diet-induced obesity by regulating gut microbiota and its metabolites[J]. 2022, 281: 119046.
[29] XU S, AWEYA J, LI N, et al. Microbial catabolism of porphyra haitanensis polysaccharides by human gut microbiota[J]. 2019, 289: 177-186.
[30] TANG C, DING R, SUN J, et al. The impacts of natural polysaccharides on intestinal microbiota and immune response-A review[J]. 2019, 10(5): 2290-2312.
[31] NGUYEN S, KIM J, GUEVARRA R, et al. Laminarin favorably modulates gut microbiota in mice fed a high-fat diet[J]. Food & Function,2016,7(10):4193−4201.
[32] CHEN Y F, JIN L, LI Y H, et al. Bamboo-shaving polysaccharide protects against high-diet induced obesity and modulates the gut microbiota of mice[J]. Journal of Functional Foods,2018,49:20−31. doi: 10.1016/j.jff.2018.08.015
[33] LI S Y, WANG L N, LIU B, et al. Unsaturated alginate oligosaccharides attenuated obesity-related metabolic abnormalities by modulating gut microbiota in high-fat-diet mice[J]. Food Funct,2020,11(5):4773−4784. doi: 10.1039/C9FO02857A
[34] LAGKOUVARDOS I, LESKER T R, HITCH T C A, et al. Sequence and cultivation study of Muribaculaceae reveals novel species, host preference, and functional potential of this yet undescribed family[J]. Microbiome,2019,7(1):28. doi: 10.1186/s40168-019-0637-2
-
期刊类型引用(8)
1. 张全通,郑尧,杨柳,张帅帅,郭全友. 计算机视觉结合卷积神经网络快速检测南极磷虾粉中的虾青素含量. 食品工业科技. 2025(03): 11-18 .  本站查看
本站查看
2. 刘鑫,马本学,李玉洁,陈金成,喻国威. 基于改进YOLOv7-ByteTrack的干制哈密大枣缺陷检测与计数系统. 农业工程学报. 2024(03): 303-312 .  百度学术
百度学术
3. 贾雅欣,李传峰,罗华平,吴明清. 基于边缘轮廓定积分测量红枣体积的研究. 塔里木大学学报. 2024(01): 75-83 .  百度学术
百度学术
4. 朱丽娟. 基于机器视觉的红枣裂纹特征提取. 科技风. 2024(10): 17-19 .  百度学术
百度学术
5. 吴思,诸定莲,鲁梦瑶,万以磊,高亮亮,陈龙,汤卫荣,吴文彪. 基于机器视觉的大闸蟹自动分级分选设备研究与开发. 扬州大学学报(农业与生命科学版). 2024(04): 137-146 .  百度学术
百度学术
6. 汤文祺,曹玉华,李应果. 基于机器视觉的一品红自动分级方法研究. 现代农业装备. 2024(05): 53-58 .  百度学术
百度学术
7. 赵晓梅,李洪港. 基于X射线图像的金属腐蚀深度估计方法. 山东冶金. 2023(05): 25-27 .  百度学术
百度学术
8. 蒋平. 一种快速红枣表面缺陷识别方法. 大众标准化. 2023(23): 55-57 .  百度学术
百度学术
其他类型引用(13)






 下载:
下载:
 下载:
下载:



