Research Progress on the Probiotic Characteristics and Mechanism of Bacillus coagulans
-
摘要: 凝结芽孢杆菌作为益生菌,近年来因其特有的益生特性备受关注。鉴于此,在深度解析凝结芽孢杆菌益生特性的基础上,结合目前国内外的研究现状和未来发展趋势,从消化道微生态、营养物质的消化吸收和免疫系统三方面揭示了凝结芽孢杆菌在食品领域的应用机制,并展望了未来在本领域的研究热点,旨在为凝结芽孢杆菌在食品工业中的广泛应用提供支撑,助力益生菌产业的可持续发展。Abstract: Bacillus coagulans, as a probiotic, has attracted much attention in recent years for its unique probiotic characteristics. In view of this, the current research status and future development trend at home and abroad are reviewed, based on the in-depth analysis of the probiotic properties of Bacillus coagulans. This paper reveals the application mechanism of Bacillus coagulans in the food field from three aspects: Digestive tract microecology, digestion and absorption of nutrients and immune system. Meanwhile, this paper foresees the future research hotspots in this field, aiming to support the wide application of Bacillus coagulans in food industry and help the sustainable development of probiotic industry.
-
Keywords:
- Bacillus coagulans /
- probiotic characteristics /
- probiotic /
- mechanism
-
凝结芽孢杆菌(Bacillus coagulans)是一种产芽孢的乳酸菌,因显著的益生特性而备受关注,成为研究热点并被逐渐应用[1]。目前,凝结芽孢杆菌已经被广泛运用于保健食品中,可以促进肠道消化。但其益生特性还没有得到很好地应用。研究发现,凝结芽孢杆菌主要通过提高免疫力、调节消化道微生态平衡和促进营养物质的消化吸收等三种途径改善机体的健康,在大健康背景下具有重要的研发意义。由于具有以上益生特性,凝结芽孢杆菌可以改善人类健康,缓解人类疾病,而在临床应用中发挥作用,从而应用于更多的保健食品和益生菌食品中。凝结芽孢杆菌有助于治疗由不同炎症因子引起的多种免疫疾病,并且缓解各种消化道疾病,发挥益生作用。但是,凝结芽孢杆菌益生特性相关的机制并没有得到很好的剖析和总结。鉴于此,在详细剖析凝结芽孢杆菌益生特性的基础上,借助于本领域的相关研究,深入探究和讨论其在食品领域的应用机制,并探讨了凝结芽孢杆菌未来发展趋势,旨在为健康中国和食品安全国家战略的实施助力。
1. 凝结芽孢杆菌调节消化道微生态平衡的作用及机制
1.1 凝结芽孢杆菌调节消化道微生态平衡作用
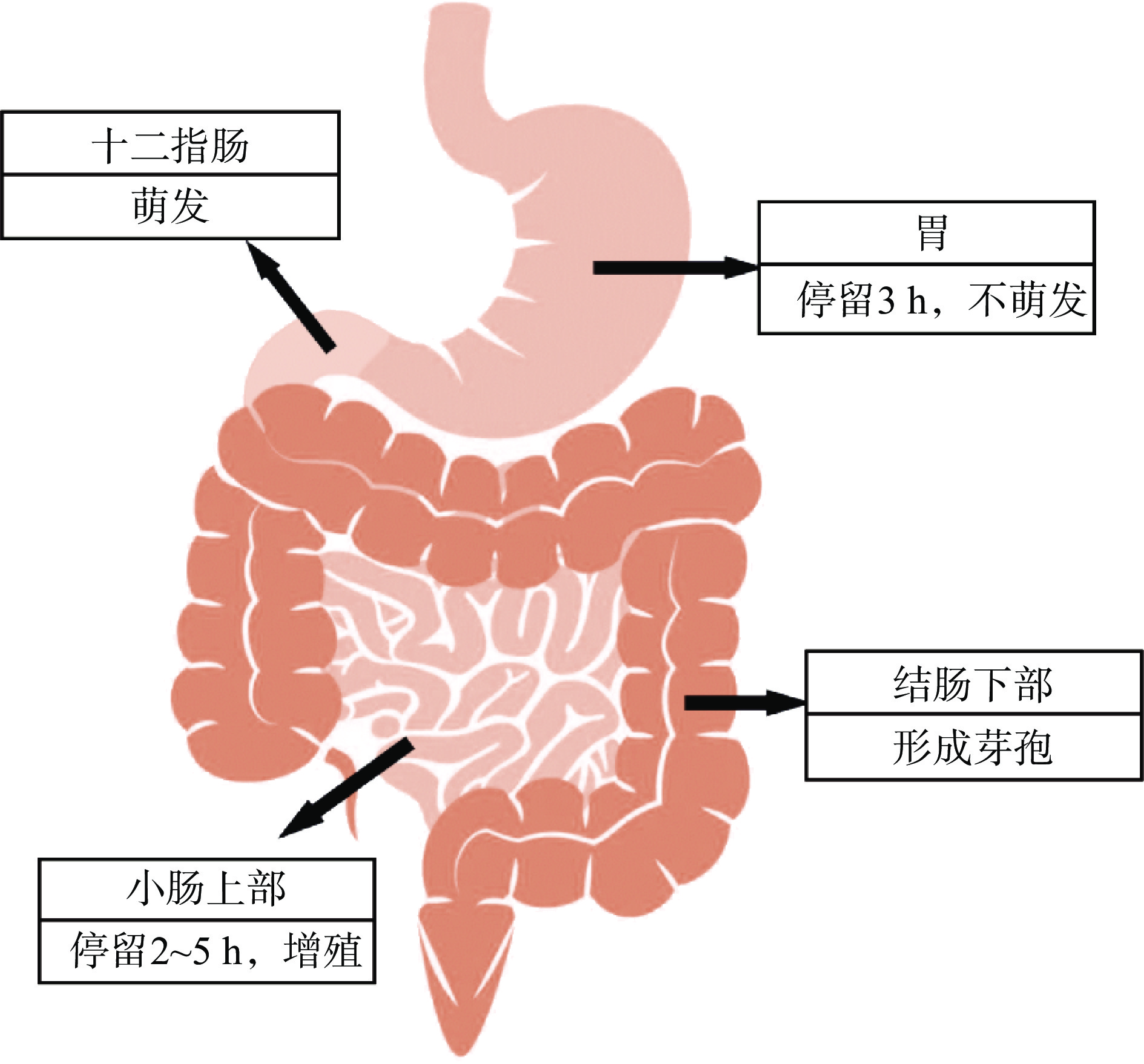
调节消化道微生态平衡是凝结芽孢杆菌的益生特性。凝结芽孢杆菌芽孢以食用口服方式进入人体,在口腔中进行物理消化和酶降解之后,经过蠕动通过食道运输到胃,胃液的存在使凝结芽孢杆菌芽孢难以萌发。对于成年人来说,这一阶段大约需要3 h,然后芽孢通过胃部消化环境后,开始在十二指肠内萌发,并在小肠上部增殖[2]。通常,凝结杆菌在小肠的停留时间为2~5 h。最后,存活的凝结芽孢杆菌将向下运动到大肠,并在结肠下部形成芽孢[3],参见图1。凝结芽孢杆菌的产芽孢特性使其拥有极高的抗逆性,保障该菌能通过消化道的复杂环境,最终在肠道定植,进而增加益生菌的数量,抑制致病菌,从而改善肠道菌群结构,调节消化道微生态平衡[2]。
1.2 凝结芽孢杆菌调节消化道微生态平衡机制
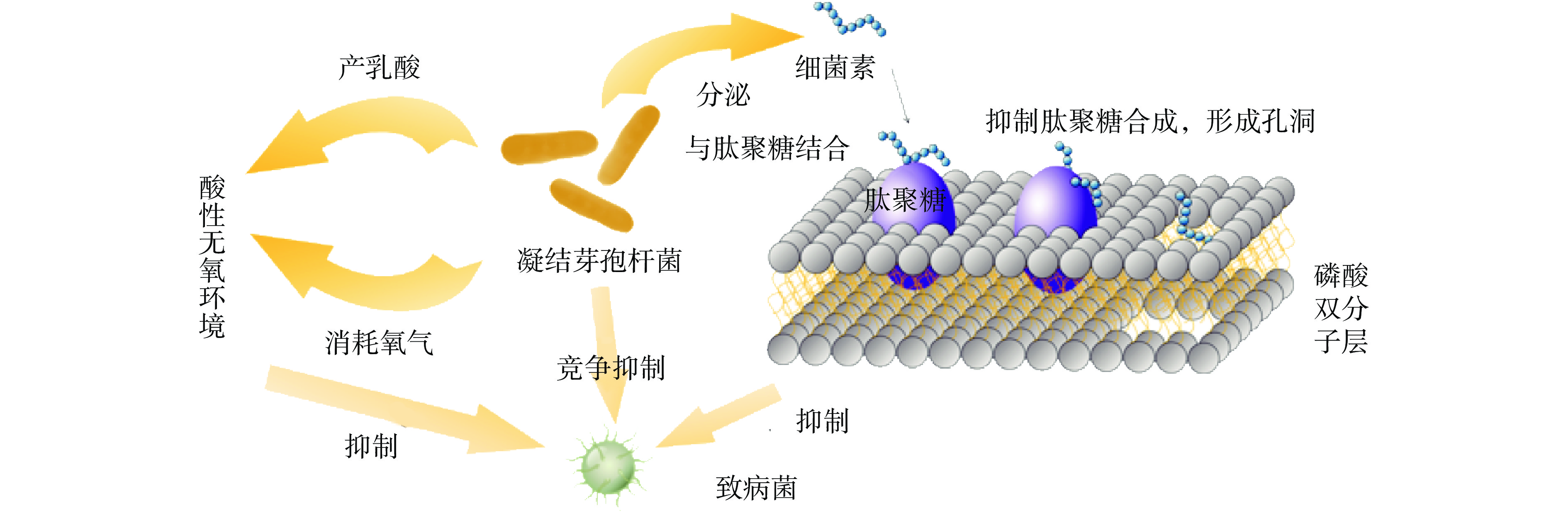
凝结芽孢杆菌可以分泌细菌素等抑菌物质来抑制致病微生物的生长,从而改善消化道微生态[3]。Abada等[4]从一株凝结芽孢杆菌中表征获得一种细菌素,具有耐热性和广谱抗菌谱,可以抑制致病菌。凝结芽孢杆菌L1208可以分泌一种抑制腐败菌的生长的新型细菌素——凝固素L1208[5]。细菌素的杀菌机制主要分为破坏细胞膜和抑制基因表达两方面[6]。带正电的多肽通过静电吸引结合到带负电的细菌细胞膜上,其C-端具有α-螺旋结构,通过疏水作用与磷脂结合,磷脂插入细胞膜,破坏细胞膜结构,导致孔的形成[7]。细菌素和肽聚糖结合,抑制肽聚糖合成,形成孔洞,参见图2。Zhang等[8]研究发现,凝结芽孢杆菌CGMCC 9951产生的细菌素可以诱导单增李斯特菌细胞膜损伤,导致酶、核酸、蛋白质、三磷酸腺苷和钾离子等[9-10]细胞内容物发生泄漏。细菌素还可以通过抑制基因表达来抑制致病微生物的生长。有些凝结芽孢杆菌产生的细菌素还可以通过跨膜作用进入细胞,干扰细胞的正常生命活动。凝结芽孢杆菌CGMCC 9951产生的细菌素还可以插入DNA螺旋槽和DNA相结合,从而影响DNA的结构和功能,导致基因组DNA的失活,进而抑制致病微生物[8]。凝结芽孢杆菌CGMCC 9951可以产生一种环状结构的细菌素,环状结构可维持细菌素结构完整性并增强生物活性,有利于其加工、储存和应用。
此外,凝结芽孢杆菌还可以通过调节消化道环境来促进益生菌生长并抑制致病菌,进而改善机体消化道微生态。凝结芽孢杆菌有利于形成一个厌氧和酸性的消化道环境,这种环境不适合致病菌生长,从而促进了益生菌的生长。凝结芽孢杆菌可以产生L型乳酸,降低消化道环境pH,抑制致病菌,并为乳酸菌等益生菌提供更有利的生长环境。凝结芽孢杆菌作为兼性厌氧菌,可以消耗消化道中的游离氧,减少氧化还原反应。凝聚芽孢杆菌RK-02产生的细胞外多糖(Extracellular Polysaccharides,EPS)是由四种不同的单糖(葡萄糖、岩藻糖、半乳糖和甘露糖)组成的单一杂聚体,这类生物分子具有清除消化道中的通过各种代谢反应形成的活性氧(Reactive Oxygen Species,ROS)的能力,因此它们具有抗氧化和自由基清除活性[11],从而消耗消化道中氧气,形成厌氧环境。Ara等[12]研究表明,食用凝结芽孢杆菌能够增加消化道中特定益生菌的数量,包括乳酸杆菌和双歧杆菌。凝结芽孢杆菌可以提高肠道菌群中的物种丰度[13],如通过暂时性地增殖乳酸菌、总需氧菌和总厌氧菌以及降低肠道中的肠杆菌科的细菌数量[14],以此来改善消化道微生态和调节肠道菌群结构。此外,凝结芽孢杆菌还通过增加拟杆菌门细菌和减少厚壁菌门微生物,缓解了过敏原诱导的肠道菌群失调[15]。Wang等[16]研究使用了包含凝结芽孢杆菌的益生菌合剂,通过恢复肠道粘膜微生物生态,促进短链脂肪酸产生,增加IL-10的表达和肠屏障功能,从而减少了细菌对肠道深部组织的侵袭,维持了宿主微生物的平衡进而减轻结肠炎症状[17]。
另一方面,凝结芽孢杆菌可以竞争性地抑制部分消化道中的致病菌,例如耐万古霉素肠球菌[18],从而维持消化道微生态的稳定。凝结芽孢杆菌通过种群竞争[19]来抑制致病菌在消化道中定植和侵袭来降低致病菌感染风险[20]。一项研究表明,凝结芽孢杆菌可以被用于预防沙门氏菌感染,它们会在肠道内与沙门氏菌竞争,并增强宿主粘膜免疫系统,延迟沙门氏菌向淋巴结、脾脏和肝脏的渗透,减少炎症介质,减少氧化应激,并减少血液和生化变化,提升宿主对沙门氏菌的免疫能力[21]。通过调节消化道微生物区系丰度,凝结芽孢杆菌还可以减轻铅对肠道绒毛的损伤[22]。
凝结芽孢杆菌通过改善消化道微生态对消化道疾病具有防控作用[23],例如急性腹泻、肠易激综合征、便秘和结肠炎,因此食用凝结芽孢杆菌逐步应用于大健康产业[24],参见表1。
表 1 凝结芽孢杆菌在消化道上的益生应用Table 1. Probiotic application of Bacillus coagulans in the gastrointestinal tract症状 菌株 受试人群 受试时间 结果 文献 急性腹泻 \ 足月婴儿 1年 预防包括轮状病毒在内引发的急性腹泻 [25] \ 4~24月 5 d 腹泻发生率和持续时间降低25% [26] 抗生素相关性腹泻 \ \ 10 d 治疗组只有29%的儿童腹泻,腹泻的平均持续时间也显著缩短,而安慰剂组有62%的儿童腹泻 [27] \ 55.6±5.9岁 4周 显著降低AAD的发生率和与使用抗生素相关的不良反应,患者腹泻率从32.2%降低至9.9% [28-29] 肠易激综合征 Unique IS2 18~60岁 8周 有效缓解成年人症状,减轻腹部不适和完全自发排便,18.87%患者完全缓解,64.15%患者症状明显缓解 [30] Unique IS2 4~12岁 8周 有效缓解儿童症状,缓解腹部疼痛和腹泻,益生菌组的患者症状评分显著低于安慰剂组,分别为3.5±2.28和8.6±2.60 [31] MTCC 5856 18~55岁 90 d 临床症状(如腹胀、呕吐、腹泻、腹痛和大便频率)都有显著减少 [32] 便秘 SNZ 1969 20~65岁 8周 排便次数提高,完全自发排便 [33] 炎症性肠病 Unique IS2 18~60岁 4周 减少疼痛,缓解症状,益生菌治疗组中43.75%的患者症状评分降低,安慰剂组中28.57%的患者症状评分降低 [34] 2. 凝结芽孢杆菌的促进营养物质消化吸收作用及机制
2.1 凝结芽孢杆菌促进营养物质消化吸收作用
凝结芽孢杆菌的益生特性还包括促进机体对营养物质的消化吸收。Stecker等[35]研究发现,在浓缩牛奶等蛋白质中添加凝结芽孢杆菌GBI-30,6086可以促进氨基酸吸收并显著促进精氨酸和异亮氨酸吸收,进而提升蛋白质消化吸收速率。这对于那些由于衰老或消化道损害等因素而无法吸收较大剂量蛋白质的人具有一定意义。凝结芽孢杆菌还可以促进机体消化吸收乳糖,缓解乳糖不耐受症。凝结芽孢杆菌还有利于淀粉和脂肪在机体内的分解。
2.2 凝结芽孢杆菌促进营养物质消化吸收机制
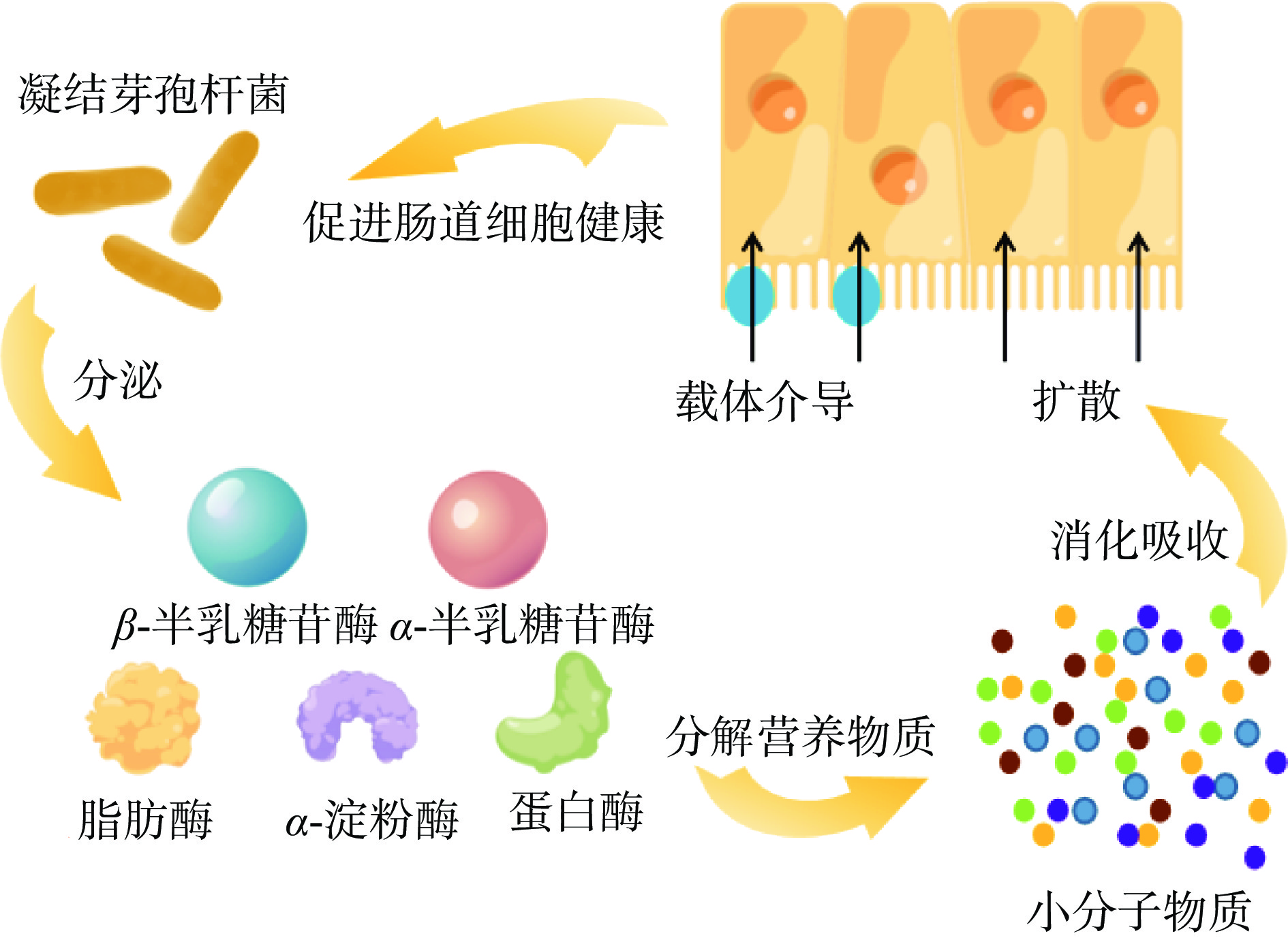
凝结芽孢杆菌可以通过酶的产生影响消化道中参与消化吸收的物质产生以及改善肠道细胞健康,来促进机体对营养物质的消化吸收,参见图3。Aulitto等[36]研究显示,凝结芽孢杆菌MA-13可以产生β-半乳糖苷酶和α-半乳糖苷酶用于分解乳糖。凝结芽孢杆菌产生的半乳糖苷酶可以分解乳糖进而有效缓解乳糖不耐受症。凝结芽孢杆菌不仅可以产生α-淀粉酶和脂肪酶,还可以通过分解葡萄糖等糖类产生乳酸[37],其中混合糖可以提高产乳酸效率[38],该菌还可以分解木质纤维素水解物[39]。凝结芽孢杆菌IPE22能够分泌足够活性的淀粉酶,充分水解淀粉后并进一步产生乳酸[40]。凝结芽孢杆菌GBI-30,6086菌株已被证明能产生多种酶,将蛋白质和碳水化合物降解成小分子肽和游离氨基酸,从而促进小肠上部的新陈代谢,提高了蛋白质利用率,改善结肠的肠道环境,减少有毒代谢物[41-42]。此外,凝结芽孢杆菌还可以改善肠道细胞健康,从而促进机体对营养物质的消化吸收。凝结芽孢杆菌可以通过减少炎症,显著改善肠道细胞健康,从而通过改善绒毛吸收区域的肠道细胞发育来增强机体对营养物质的消化吸收。凝结芽孢杆菌还可以刺激肠道蠕动,减少胺等有害物质的产生,改善消化道代谢环境,从而促进对营养物质的消化吸收。
3. 凝结芽孢杆菌的免疫调节作用及机制
3.1 凝结芽孢杆菌免疫调节作用
改善机体免疫能力同样也是凝结芽孢杆菌的益生特性。凝结芽孢杆菌食用后可以显著调节人体免疫系统平衡,调节人体免疫功能。Shinde等[43]研究发现,在正常或炎症条件下,凝结芽孢杆菌MTCC 5856芽孢都可以通过减少促炎细胞因子IL-8的分泌和增加抗炎细胞因子IL-10的分泌,对HT-29细胞产生显著的免疫调节作用。灭活的凝结芽孢杆菌GBI-30,6086还可以提高运动后人体的中性粒细胞和单核细胞水平,影响促炎和抗炎细胞因子,从而提高人体免疫能力[44]。
3.2 凝结芽孢杆菌免疫调节机制
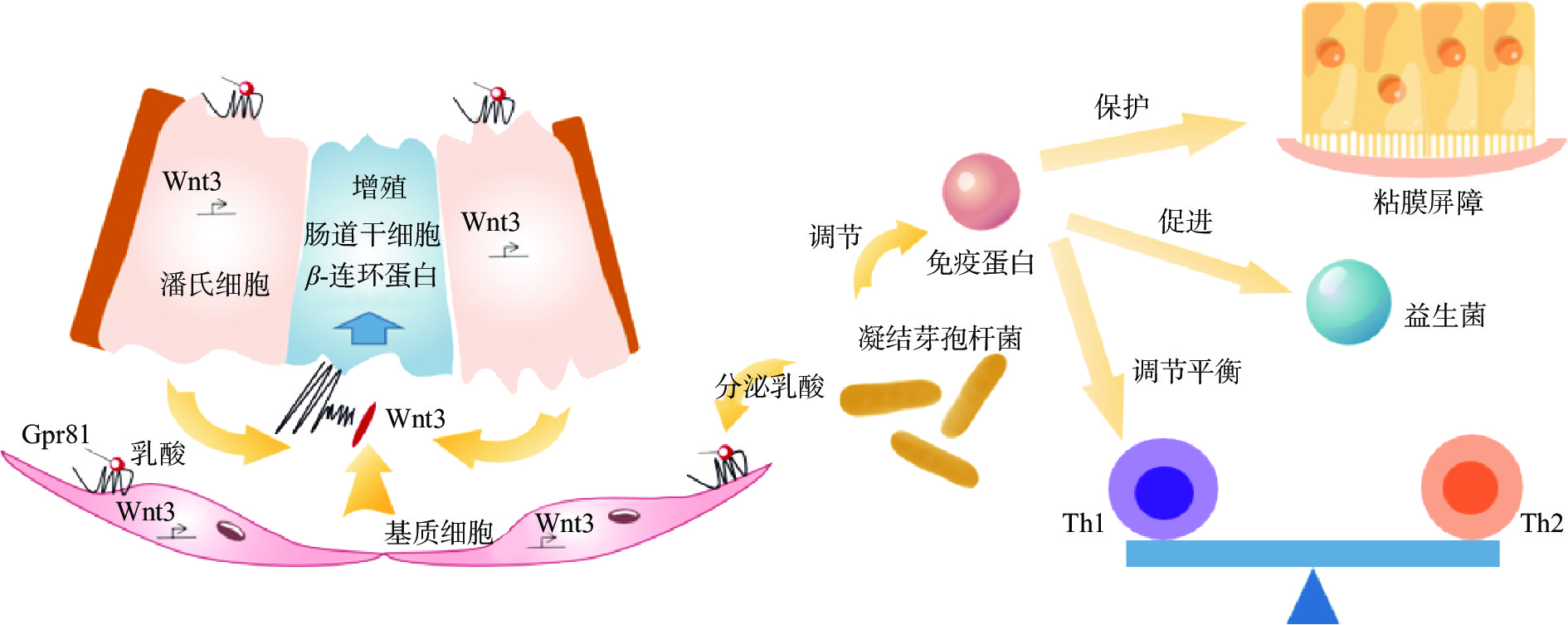
首先,凝结芽孢杆菌可以直接调节免疫细胞。Jensen等[45]研究发现凝结芽孢杆菌GBI-30,6086能够激活人体免疫细胞,以此改变了免疫激活和抗炎细胞因子及趋化因子的产生。凝结芽孢杆菌可以通过产生乳酸来影响免疫系统,乳酸通过与潘氏细胞和肠道基质细胞产生的乳酸受体Gpr81结合,以Wnt/β-连环蛋白信号通路刺激肠道干细胞增殖[46]。凝结芽孢杆菌直接作用于癌细胞,诱导癌细胞死亡,从而可以应用于癌症的治疗。凝结芽孢杆菌的热灭活培养上清液能更有效地诱导结肠癌细胞凋亡,可考虑用于结肠癌的辅助治疗。与非癌性HEK 293T细胞相比,凝结芽孢杆菌Unique IS2使人体结肠癌、宫颈癌和慢性髓性白血病细胞系的细胞增殖分别降低了22%、31.7%和19.5%,促凋亡蛋白BAX增加,抗凋亡蛋白Bcl2减少,线粒体膜电位降低,细胞色素C释放增加[47]。
其次,凝结芽孢杆菌还可以通过调节免疫蛋白来提高机体免疫能力。肠道碱性磷酸酶(Intestinal Alkaline Phosphatase,IAP)是一种由肠道上皮细胞表达的内源性蛋白,IAP的表达功能丧失与肠道炎症增加、生物失调、肠屏障结构受损、细菌移位以及随后的系统性炎症有关[48]。凝结芽孢杆菌可以通过刺激肠道IAP活性来缓解肠道炎症[49]。凝结芽孢杆菌13002可以提高血清免疫球蛋白水平、上调IFN-γ和IL-4来保护粘膜屏障和维持Th1/Th2的平衡以及促进益生菌生长,以此减少烷化剂环磷酰胺对人体的损伤,进而提高人体免疫力[50]。Anaya-Loyola等[51]研究发现,凝结芽孢杆菌GBI-30,6068可以调节免疫蛋白TNF-α、CD163、G-CSF、ICAM-1、IL-6、IL-8、MCP-2、RAGE、uPAR和PF4等,从而提高免疫能力,缓解上呼吸道和消化道症状。凝结芽孢杆菌还可以通过促进半胱氨酸-天冬氨酸蛋白酶基因等凋亡基因的表达来诱导癌细胞凋亡[52]。凝结芽孢杆菌通过调节免疫蛋白来缓解炎症,因此运用于临床。包含凝结芽孢杆菌的益生菌混合物作为类风湿性关节炎治疗的辅助补充剂,有利于缓解疾病症状[53],使红细胞沉降率(Erythrocyte Sedimentation Rate,ESR)[54]和C-反应蛋白[55]水平显著降低。凝结芽孢杆菌通过刺激抗炎细胞因子、诱导调节性T细胞、调节Th1/Th2平衡和抗菌物质的分泌而进行炎症的免疫调节。凝结芽孢杆菌的免疫调节作用机制见图4。
近年来,食物过敏受到人们广泛关注[56],部分研究揭示了凝结芽孢杆菌的抗过敏机制,为未来凝结芽孢杆菌在抗过敏方面的应用提供了理论支撑。凝结芽孢杆菌09.712能显著改善上皮屏障功能,促进淋巴细胞增殖。凝结芽孢杆菌通过诱导CD4+Foxp3+Tregs和IL-10的产生而改变Th1/Th2/Treg平衡,从而抑制对虾原肌球蛋白致敏。包含凝结芽孢杆菌09.712的益生菌混合剂还可减轻食物过敏引起的粘膜疾病[57]。食物过敏原(如虾原肌球蛋白)的摄入诱导了脾脏中过敏T细胞的分化,随后小肠中的炎性细胞浸润,最终导致过敏细胞因子的产生,杯状细胞过度增殖和隐窝细胞凋亡。而益生菌混合剂的摄入可能通过mTOR信号通路调节消化道代谢,特别是氨基酸代谢途径,诱导抗过敏T细胞分化,抑制炎性细胞浸润[5]。凝结芽孢杆菌可以分解食物中的过敏原,从而降低过敏风险[58]。
4. 凝结芽孢杆菌的其他益生特性及机制
凝结芽孢杆菌还可以直接影响人体消化道中其他物质的产生,如胆汁酸、胆固醇和短链脂肪酸等,从而对人体产生益生作用。胆汁酸是胆汁的重要组分,由肝脏分泌至消化道中,帮助消化吸收饮食中的脂质,但食用豆浆会显著增加次级胆汁酸的产生,如脱氧胆酸和ω-鼠李酸,这会影响消化道环境,不利于机体的消化吸收,并提高肝脏和结肠的癌症风险[59]。但是,凝结芽孢杆菌和豆浆的一起服用可以抑制次生胆汁酸的产生,改善了消化道中胆汁酸的代谢,改善了消化道环境,进而促进机体对营养物质的消化吸收[60]。凝结芽孢杆菌还可以降低人体消化道中胆固醇的含量。凝结芽孢杆菌MTCC 5856通过多种机制,包括胆盐去结合、吸收胆固醇、促进胆固醇与静息细胞和死亡细胞结合等,在消化道中帮助去除富含胆固醇的食物(蛋黄、肝脏和黄油)中的胆固醇,从而降低胆固醇[61]。除此之外,凝结芽孢杆菌还可以产生短链脂肪酸,如丁酸和丙酸,丁酸能够抑制肝脏中胆固醇的生物合成,丙酸可以抑制脂肪酸的合成,最终降低胆固醇的生物合成速率,从而降低胆固醇水平[62],改善了消化道中的胆固醇代谢[63]。
5. 结论与展望
鉴于有效的益生作用和产芽孢赋予的极强抗逆性,凝结芽孢杆菌被认为是一种具有广阔开发前景的益生菌。研究表明,凝结芽孢杆菌的益生特性是多种机制综合协调过程的结果,涉及机体消化道微生态、代谢和免疫系统。通过对机制的深入探究,逐渐形成机制网络,有利于为未来凝结芽孢杆菌的广泛应用提供全面的理论支持。但是,凝结芽孢杆菌的益生特性机制探究的还不够深入,未来可以在小分子代谢产物、影响免疫功能的代谢产物、细菌素等互作机制方面进一步探究。评估菌株的安全性和益生特性是在食品行业应用的前提条件,因此作为益生菌,不同来源的凝结芽孢杆菌安全性的评估仍需要受到关注,尽管有部分关于凝结芽孢杆菌安全性的研究,但相关研究还不够全面。后续研究中,可以通过对基因组中活性抗生素耐药基因、毒力因子基因和毒素编码基因的检测,进一步探究凝结芽孢杆菌的安全性,以满足不同应用场景的需要。
-
表 1 凝结芽孢杆菌在消化道上的益生应用
Table 1 Probiotic application of Bacillus coagulans in the gastrointestinal tract
症状 菌株 受试人群 受试时间 结果 文献 急性腹泻 \ 足月婴儿 1年 预防包括轮状病毒在内引发的急性腹泻 [25] \ 4~24月 5 d 腹泻发生率和持续时间降低25% [26] 抗生素相关性腹泻 \ \ 10 d 治疗组只有29%的儿童腹泻,腹泻的平均持续时间也显著缩短,而安慰剂组有62%的儿童腹泻 [27] \ 55.6±5.9岁 4周 显著降低AAD的发生率和与使用抗生素相关的不良反应,患者腹泻率从32.2%降低至9.9% [28-29] 肠易激综合征 Unique IS2 18~60岁 8周 有效缓解成年人症状,减轻腹部不适和完全自发排便,18.87%患者完全缓解,64.15%患者症状明显缓解 [30] Unique IS2 4~12岁 8周 有效缓解儿童症状,缓解腹部疼痛和腹泻,益生菌组的患者症状评分显著低于安慰剂组,分别为3.5±2.28和8.6±2.60 [31] MTCC 5856 18~55岁 90 d 临床症状(如腹胀、呕吐、腹泻、腹痛和大便频率)都有显著减少 [32] 便秘 SNZ 1969 20~65岁 8周 排便次数提高,完全自发排便 [33] 炎症性肠病 Unique IS2 18~60岁 4周 减少疼痛,缓解症状,益生菌治疗组中43.75%的患者症状评分降低,安慰剂组中28.57%的患者症状评分降低 [34] -
[1] HYRONIMUS B, LE M C, SASSI A H, et al. Acid and bile tolerance of spore-forming lactic acid bacteria[J]. International Journal of Food Microbiology,2000,61(2-3):193−197. doi: 10.1016/S0168-1605(00)00366-4
[2] CASULA G, CUTTING S M. Bacillus probiotics: Spore germination in the gastrointestinal tract[J]. Applied and Environmental Microbiology,2002,68(5):2344−2352. doi: 10.1128/AEM.68.5.2344-2352.2002
[3] CAO J, YU Z, LIU W, et al. Probiotic characteristics of Bacillus coagulans and associated implications for human health and diseases[J]. Journal of Functional Foods,2020,64:103643. doi: 10.1016/j.jff.2019.103643
[4] ABADA E A E M. Isolation and characterization of a antimicrobial compound from Bacillus coagulans[J]. Animal Cells and Systems,2008,12(1):41−46. doi: 10.1080/19768354.2008.9647152
[5] FU L, WANG C, RUAN X, et al. Preservation of large yellow croaker (Pseudosciaena crocea) by Coagulin L1208, a novel bacteriocin produced by Bacillus coagulans L1208[J]. International Journal of Food Microbiology,2018,266:60−68. doi: 10.1016/j.ijfoodmicro.2017.11.012
[6] CUI Y, LUO L, WANG X, et al. Mining, heterologous expression, purification, antibactericidal mechanism, and application of bacteriocins: A review[J]. Comprehensive Reviews in Food Science,2021,20(1):863−899. doi: 10.1111/1541-4337.12658
[7] TSENG T S, TSAI K C, CHEN C. Characterizing the structure-function relationship reveals the mode of action of a novel antimicrobial peptide, P1, from jumper ant Myrmecia pilosula[J]. Molecular Bio Systems,2017,13(6):1193−1201.
[8] ZHANG J, GU S, ZHANG T, et al. Characterization and antibacterial modes of action of bacteriocins from Bacillus coagulans CGMCC 9951 against Listeria monocytogenes[J]. LWT,2022:113272.
[9] LV X, MIAO L, MA H, et al. Purification, characterization and action mechanism of plantaricin JY22, a novel bacteriocin against Bacillus cereus produced by Lactobacillus plantarum JY22 from golden carp intestine[J]. Food Science Biotechnology,2018,27(3):695−703. doi: 10.1007/s10068-017-0280-2
[10] YI L H, LI X, LUO L L, et al. A novel Bacteriocin BMP11 and its antibacterial mechanism on cell envelope of Listeria monocytogenes and Cronobacter sakazakii[J]. Food Control,2018,91:160−169. doi: 10.1016/j.foodcont.2018.03.038
[11] KODALI V P, SEN R. Antioxidant and free radical scavenging activities of an exopolysaccharide from a probiotic bacterium[J]. Biotechnology Journal,2008,3(2):245−251. doi: 10.1002/biot.200700208
[12] ARA K, MEGURO S, HASE T, et al. Effect of spore-bearing lactic acid-forming bacteria (Bacillus coagulans SANK 70258) administration on the intestinal environment, defecation frequency, fecal characteristics and dermal characteristics in humans and rats[J]. Microbial Ecology in Health and Disease,2009,14(1):4−13.
[13] MAITY C, GUPTA P A K, SAROJ D B, et al. Impact of a gastrointestinal stable probiotic supplement Bacillus coagulans LBSC on human gut microbiome modulation[J]. Journal of Dietary Supplements,2021,18(6):577−596. doi: 10.1080/19390211.2020.1814931
[14] ABHARI K, SHEKARFOROUSH S, SAJEDIANFARD J, et al. The effects of probiotic, prebiotic and synbiotic diets containing Bacillus coagulans and inulin on rat intestinal microbiota[J]. Iranian Journal of Veterinary Research,2015,16(3):267.
[15] FU L, FU S, WANG C, et al. Yogurt-sourced probiotic bacteria alleviate shrimp tropomyosin-induced allergic mucosal disorders, potentially through microbiota and metabolism modifications[J]. Allergology International,2019,68(4):506−514. doi: 10.1016/j.alit.2019.05.013
[16] WANG Y, XIE Q, ZHANG Y, et al. Combination of probiotics with different functions alleviate DSS-induced colitis by regulating intestinal microbiota, IL-10, and barrier function[J]. Appl Microbiol Biotechnol,2020,104(1):335−349. doi: 10.1007/s00253-019-10259-6
[17] SHINDE T, PERERA A P, VEMURI R, et al. Synbiotic supplementation with prebiotic green banana resistant starch and probiotic Bacillus coagulans spores ameliorates gut inflammation in mouse model of inflammatory bowel diseases[J]. European Journal of Nutrition,2020,59(8):3669−3689. doi: 10.1007/s00394-020-02200-9
[18] DONSKEY C, HOYEN C, DAS S, et al. Effect of oral Bacillus coagulans administration on the density of vancomycin-Resistant enterococci in the stool of colonized mice[J]. Letters in Applied Microbiology,2001,33(1):84−88. doi: 10.1046/j.1472-765X.2001.00948.x
[19] AMOAH K, HUANG Q C, TAN B P, et al. Dietary supplementation of probiotic Bacillus coagulans ATCC 7050, improves the growth performance, intestinal morphology, microflora, immune response, and disease confrontation of Pacific white shrimp, Litopenaeus vannamei[J]. Fish Shellfish Immunol,2019,87:796−808. doi: 10.1016/j.fsi.2019.02.029
[20] ZHEN W, SHAO Y, GONG X, et al. Effect of dietary Bacillus coagulans supplementation on growth performance and immune responses of broiler chickens challenged by Salmonella enteritidis[J]. Poultry Science,2018,97(8):2654−2666. doi: 10.3382/ps/pey119
[21] MAZKOUR S, SHEKARFOROUSH S S, BASIRI S, et al. Effects of two probiotic spores of Bacillus species on hematological, biochemical, and inflammatory parameters in Salmonella typhimurium infected rats[J]. Scientific Report,2020,10(1):8035. doi: 10.1038/s41598-020-64559-3
[22] XING S C, HUANG C B, MI J D, et al. Bacillus coagulans R11 maintained intestinal villus health and decreased intestinal injury in lead-exposed mice by regulating the intestinal microbiota and influenced the function of faecal microRNAs[J]. Environmental Pollution, 2019, 255(Pt 2): 113139.
[23] SALMINEN S, COLLADO M C, ENDO A, et al. The international scientific association of probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics[J]. Nature Review Gastroenterol Hepatol,2021,18(9):649−667. doi: 10.1038/s41575-021-00440-6
[24] MU Y, CONG Y. Bacillus coagulans and its applications in medicine[J]. Beneficial Microbes,2019,10(6):679−688. doi: 10.3920/BM2019.0016
[25] CHANDRA R J N R. Effect of Lactobacillus on the incidence and severity of acute rotavirus diarrhoea in infants: A prospective placebo-controlled double-blind study[J]. Nutrition Research,2002,22(1−2):65−69. doi: 10.1016/S0271-5317(01)00367-0
[26] DUTTA P, MITRA U, DUTTA S, et al. Randomised controlled clinical trial of Lactobacillus sporogenes (Bacillus coagulans), used as probiotic in clinical practice, on acute watery diarrhoea in children[J]. Tropical Medicine and International Health,2011,16(5):555−561. doi: 10.1111/j.1365-3156.2011.02745.x
[27] LA R M, BOTTARO G, GULINO N, et al. Prevention of antibiotic-associated diarrhea with Lactobacillus sporogens and fructo-oligosaccharides in children: A multicentric double-blind vs placebo study[J]. Minerva Pediatrica,2003,55(5):447−452.
[28] HOROSHEVA T V, VODYANOY V, SOROKULOVA I. Efficacy of Bacillus probiotics in prevention of antibiotic-associated diarrhoea: A randomized, double-blind, placebo-controlled clinical trial[J]. JMM Case Reports,2014,1(3):e004036.
[29] JOHNSTON B C, GOLDENBERG J Z, VANDVIK P O, et al. Probiotics for the prevention of pediatric antibiotic-associated diarrhea[J]. Cochrane Database of Systematic Reviews,2011(11):CD004827.
[30] MADEMPUDI R S, AHIRE J J, NEELAMRAJU J, et al. Randomized clinical trial: The effect of probiotic Bacillus coagulans Unique IS2 vs. placebo on the symptoms management of irritable bowel syndrome in adults[J]. Scientific Report,2019,9(1):12210. doi: 10.1038/s41598-019-48554-x
[31] SUDHA M R, JAYANTHI N, AASIN M, et al. Efficacy of Bacillus coagulans Unique IS2 in treatment of irritable bowel syndrome in children: A double blind, randomised placebo controlled study[J]. Beneficial Microbes,2018,9(4):563−572. doi: 10.3920/BM2017.0129
[32] MAJEED M, NAGABHUSHANAM K, NATARAJAN S, et al. Bacillus coagulans MTCC 5856 supplementation in the management of diarrhea predominant irritable bowel syndrome: A double blind randomized placebo controlled pilot clinical study[J]. Nutrition Journal,2016,15(1):21.
[33] KANG S, PARK M Y, BROOKS I, et al. Spore-forming Bacillus coagulans SNZ 1969 improved intestinal motility and constipation perception mediated by microbial alterations in healthy adults with mild intermittent constipation: A randomized controlled trial[J]. Food Research International,2021,146:110428. doi: 10.1016/j.foodres.2021.110428
[34] BAMOLA V D, DUBEY D, SAMANTA P, et al. Effect of Bacillus coagulans Unique IS-2 in inflammatory bowel disease (IBD): A randomized controlled trial[J]. Cold Spring Harbor Laboratory Press,2021:1.
[35] STECKER R A, MOON J M, RUSSO T J, et al. Bacillus coagulans GBI-30, 6086 improves amino acid absorption from milk protein[J]. Nutrition & Metabolism,2020,17(1):93.
[36] AULITTO M, STRAZZULLI A, SANSONE F, et al. Prebiotic properties of Bacillus coagulans MA-13: Production of galactoside hydrolyzing enzymes and characterization of the transglycosylation properties of a GH42 beta-galactosidase[J]. Microbial Cell Factories,2021,20(1):71. doi: 10.1186/s12934-021-01553-y
[37] YAO K, LIU D M, BRENNAN C S. Gelatinised and hydrolysed corn starch is a cost-effective carbon source with higher production of L-lactic acid by Bacillus coagulans compared with glucose[J]. International Journal of Food Science & Technology,2020,56(5):2384−2394.
[38] ABDEL-RAHMAN M A, HASSAN S E-D, ALREFAEY H M A, et al. Efficient co-utilization of biomass-derived mixed sugars for lactic acid production by Bacillus coagulans Azu-10[J]. Fermentation,2021,7(1):28. doi: 10.3390/fermentation7010028
[39] JIANG T, QIAO H, ZHENG Z, et al. Lactic acid production from pretreated hydrolysates of corn stover by a newly developed Bacillus coagulans strain[J]. PLoS One,2016,11(2):149101.
[40] WANG Y, CAO W, LUO J, et al. One step open fermentation for lactic acid production from inedible starchy biomass by thermophilic Bacillus coagulans IPE22[J]. Bioresource Technology,2019,272:398−406. doi: 10.1016/j.biortech.2018.10.043
[41] JAGER R, PURPURA M, FARMER S, et al. Probiotic Bacillus coagulans GBI-30, 6086 improves protein absorption and utilization[J]. Probiotics Antimicrob Proteins,2018,10(4):611−615. doi: 10.1007/s12602-017-9354-y
[42] KELLER D, VAN DINTER R, CASH H, et al. Bacillus coagulans GBI-30, 6086 increases plant protein digestion in a dynamic, computer-controlled in vitro model of the small intestine (TIM-1)[J]. Beneficial Microbes,2017,8(3):491−496. doi: 10.3920/BM2016.0196
[43] SHINDE T, VEMURI R, SHASTRI M D, et al. Probiotic Bacillus coagulans MTCC 5856 spores exhibit excellentin-vitro functional efficacy in simulated gastric survival, mucosal adhesion and immunomodulation[J]. Journal of Functional Foods,2019,52:100−108. doi: 10.1016/j.jff.2018.10.031
[44] KALMAN D S, HEWLINGS S. Inactivated probiotic Bacillus coagulans GBI-30 demonstrates immunosupportive properties in healthy adults following stressful exercise[J]. Journal of Probiotics & Health,2018,6(1):1000190.
[45] JENSEN G S, CASH H A, FARMER S, et al. Inactivated probiotic Bacillus coagulans GBI-30 induces complex immune activating, anti-inflammatory, and regenerative markers in vitro[J]. Journal of Inflammation Research,2017,10:107−117. doi: 10.2147/JIR.S141660
[46] LEE Y S, KIM T Y, KIM Y, et al. Microbiota-derived lactate accelerates intestinal stem-cell-mediated epithelial development[J]. Cell Host Microbe,2018,24(6):833−846. doi: 10.1016/j.chom.2018.11.002
[47] MADEMPUDI R S, KALLE A M. Antiproliferative effects of Bacillus coagulans Unique IS2 in colon cancer cells[J]. Nutrition and Cancer,2017,69(7):1062−1068. doi: 10.1080/01635581.2017.1359317
[48] FAWLEY J, GOURLAY D M. Intestinal alkaline phosphatase: A summary of its role in clinical disease[J]. Journal of Surgical Research,2016,202(1):225−234. doi: 10.1016/j.jss.2015.12.008
[49] MALO M S, ALAM S N, MOSTAFA G, et al. Intestinal alkaline phosphatase preserves the normal homeostasis of gut microbiota[J]. Gut,2010,59(11):1476−1484. doi: 10.1136/gut.2010.211706
[50] ZHAO S, PENG X, ZHOU Q Y, et al. Bacillus coagulans 13002 and fructo-oligosaccharides improve the immunity of mice with immunosuppression induced by cyclophosphamide through modulating intestinal-derived and fecal microbiota[J]. Food Research International,2021,140:109793. doi: 10.1016/j.foodres.2020.109793
[51] ANAYA-LOYOLA M A, ENCISO-MORENO J A, LOPEZ-RAMOS J E, et al. Bacillus coagulans GBI-30, 6068 decreases upper respiratory and gastrointestinal tract symptoms in healthy Mexican scholar-aged children by modulating immune-related proteins[J]. Food Research International,2019,125:108567. doi: 10.1016/j.foodres.2019.108567
[52] DOLATI M, TAFVIZI F, SALEHIPOUR M, et al. Inhibitory effects of probiotic Bacillus coagulans against MCF7 breast cancer cells[J]. Iranian Journal of Microbiology,2021,13(6):839−847.
[53] SKOCZYNSKA M, SWIERKOT J. The role of diet in rheumatoid arthritis[J]. Reumatologia,2018,56(4):259−267. doi: 10.5114/reum.2018.77979
[54] ROY A, KUMAR Y, FATIMA S. A prospective, randomized, single-center, two-arm, open-label study to evaluate the efficacy of biotherapi®, a two-strain bacillus probiotic blend, as an adjunctive therapy in the treatment of rheumatoid arthritis[J]. Indian Journal of Rheumatology,2021,16(3):254. doi: 10.4103/injr.injr_281_20
[55] SANCHEZ P, LETAROUILLY J G, NGUYEN Y, et al. Efficacy of probiotics in rheumatoid arthritis and spondyloarthritis: A systematic review and meta-analysis of randomized controlled trials[J]. Nutrients,2022,14(2):354. doi: 10.3390/nu14020354
[56] LOH W, TANG M L K. The epidemiology of food allergy in the global context[J]. Environmental Research and Public Health,2018,15(9):2043. doi: 10.3390/ijerph15092043
[57] FU L, PENG J, ZHAO S, et al. Lactic acid bacteria-specific induction of CD4+Foxp3+T cells ameliorates shrimp tropomyosin-induced allergic response in mice via suppression of mTOR signaling[J]. Scientific Report,2017,7(1):1987. doi: 10.1038/s41598-017-02260-8
[58] SCHUPACK D A, MARS R A T, VOELKER D H, et al. The promise of the gut microbiome as part of individualized treatment strategies[J]. Nature Review Gastroenterol Hepatol,2022,19(1):7−25. doi: 10.1038/s41575-021-00499-1
[59] DEGIROLAMO C, MODICA S, PALASCIANO G, et al. Bile acids and colon cancer: Solving the puzzle with nuclear receptors[J]. Trends in Molecular Medicine,2011,17(10):564−572. doi: 10.1016/j.molmed.2011.05.010
[60] LEE Y, YOSHITSUGU R, KIKUCHI K, et al. Combination of soya pulp and Bacillus coagulans lilac-01 improves intestinal bile acid metabolism without impairing the effects of prebiotics in rats fed a cholic acid-supplemented diet[J]. British Journal of Nutrition,2016,116(4):603−610.
[61] MAJEED M, MAJEED S, NAGABHUSHANAM K, et al. Evaluation of thein vitrocholesterol-lowering activity of the probiotic strain Bacillus coagulans MTCC 5856[J]. International Journal of Food Science & Technology,2019,54(1):212−220.
[62] DEN BESTEN G, VAN EUNEN K, GROEN A K, et al. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism[J]. Journal of Lipid Research,2013,54(9):2325−2340. doi: 10.1194/jlr.R036012
[63] BO T, SHAO S, WU D, et al. Relative variations of gut microbiota in disordered cholesterol metabolism caused by high-cholesterol diet and host genetics[J]. Microbiologyopen,2017,6(4):e00491. doi: 10.1002/mbo3.491





 下载:
下载:
 下载:
下载:



