Prediction of Interaction between Fish-derived Antifreeze Peptides and Fish Myosin by Molecular Docking
-
摘要: 冻藏期间蛋白质冷冻变性是引起解冻后鱼糜凝胶化能力下降的主要原因之一,而肌球蛋白重链(Myosin Heavy Chain,MHC)是鱼糜凝胶形成的主要贡献者。本研究使用不同生物酶酶解制备抗冻多肽,通过计算机模拟酶解技术筛选抗冻多肽。利用蛋白质同源建模构建海鲈鱼肌球蛋白空间结构,通过分子对接和分子动力学模拟解析抗冻多肽与海鲈鱼肌球蛋白重链的作用位点及可能的作用机制。结果表明,胰蛋白酶酶解物具有高抗冻活性,对菌体低温胁迫保护达到80.35%±4.39%,并具有良好的热滞活性及抑制重结晶作用。模拟胰蛋白酶酶解获得的肽段GPR与GPAGGK可以与肌球蛋白重链通过分子间作用力结合,其中GPAGGK的结合更为稳定。这种相互作用可以阻碍肌球蛋白在温度变化中的结构改变,其机理可能是抗冻多肽结合在肌球蛋白重链的结构空腔上,影响了结合水解离后引起的疏水键及二硫键等化学键的形成,阻碍蛋白侧链聚集、结构改变和冰晶的位移等,这有利于鱼糜及鱼糜制品在冷冻贮藏中的品质保持。本研究结果为抗冻多肽应用于鱼糜及其制品在冷冻贮藏过程中品质保持的应用提供科学依据。Abstract: Freeze denaturation during frozen storage is one of the main reasons for the decreased gelation ability of surimi after thawing. The myosin heavy chain (MHC) is the major contributor to the formation of surimi gel. In this study, the antifreeze peptides were prepared by hydrolysis of different enzymes and screened by computer simulated enzymatic hydrolysis technology. The spatial structure of sea bass myosin was constructed by protein homology modeling. The action sites and possible mechanism of sea bass myosin heavy chain with antifreeze peptides were analyzed by molecular docking and molecular dynamics simulation techniques. The results showed that the trypsin hydrolysates possessed high antifreeze activity, which the survival rate of bacteria was 80.35%±4.39% after freeze-thaw cycle, and had thermal hysteresis and ice recrystallization inhibition activity. The peptides GPR and GPAGGK obtained by simulated trypsin enzymolysis could combine with the myosin heavy chain by intermolecular force, and the binding of peptide GPAGGK to myosin was more stable. This binding could block the myosin structural changes in response to temperature change. The mechanism might be that the antifreeze peptides combine with the structural cavity of the myosin heavy chain, which affected the formation of hydrophobic bonds and disulfide bonds caused by the dissociation of the bound water. Thus the aggregation of protein side chains, structural changes and ice crystal displacement were impeded, which was conducive to the quality maintenance of surimi and surimi products in cryopreservation. This study would provide a theoretical basis for the application of antifreeze peptides in the quality maintenance of surimi and its products during frozen storage.
-
我国水域辽阔,生物资源丰富[1],是世界上渔业资源最丰富的国家[2]。海鲈鱼营养丰富,养殖产量高[3],常用于加工生产鱼糜制品,深受消费者的喜爱,产量和市场需求逐年增加。鱼糜及鱼糜制品在加工、储存、运输等过程中产生的冰晶生长和重结晶问题会导致鱼糜蛋白质的冷冻变性[4-6],使其分子内部原有的高度规律性的空间结构发生变化,导致蛋白质的理化性质和生物学性质都有所改变,是影响冻藏品质的关键。其中,鱼糜蛋白中的肌球蛋白是凝胶形成最主要的功能成分,影响着肉制品的品质[7-9]。肌球蛋白重链(Myosin Heavy Chain,MHC)具有与完整肌球蛋白形成凝胶的相同能力。而肌球蛋白轻链不能单独形成凝胶,只在肌球蛋白重链碱基凝胶形成中起辅助作用。因此,肌球蛋白重链在肌球蛋白形成凝胶三维网状结构的过程中起着非常重要的作用[10]。
抗冻多肽是一类小分子蛋白或蛋白质水解物[11],它是抗冻蛋白中局部具有抗冻活性的特异多肽链结构域[12-13]。抗冻多肽在结冰或亚结冰状态下能保护生物体免受伤害[14],它能非依数性降低溶液冰点[15]、有效降低冰晶生长率、抑制冰晶重结晶的发生,抑制冻结所造成的低温损伤[16-18]。实验室前期研究发现鱼源胶原蛋白制备的抗冻多肽,在低温冻融循环中对鱼糜表现出保护作用[19-20]。为研究鱼源胶原蛋白抗冻多肽对鱼糜的保护机制,本文使用不同蛋白酶酶解鱼源胶原蛋白,以嗜热链球菌的低温保护活性为指标筛选出能酶解产生高活性抗冻多肽的蛋白酶。为简化分离纯化及质谱等繁锁步骤,通过计算机模拟酶解鱼源胶原蛋白并预测活性肽性质,得到能与肌球蛋白直接作用的抗冻多肽序列,利用同源建模构建海鲈鱼肌球蛋白重链的空间结构。通过分子模拟技术探究抗冻多肽与海鲈鱼肌球蛋白的相互作用,以期为今后冷冻鱼糜产品开发应用提供理论基础。
1. 材料与方法
1.1 材料与仪器
鱼鳞胶原蛋白(Evynnis japonica) 安井食品集团股份有限公司;嗜热链球菌(Streptococcus thermophiles) 上海交通大学农业与生物学院;风味蛋白酶、碱性蛋白酶、中性蛋白酶、木瓜蛋白酶、胰蛋白酶 上海源叶生物科技有限公司;其他试剂 均为分析纯;海鲈鱼肌球蛋白重链(GenBank Accession No:AGT60847.1) 美国国立生物技术信息中心NCBI(https://www.ncbi.nlm.nih.gov/);鱼源胶原蛋白O93484 蛋白质数据库UniProt(https://www.uniprot.org/)。
SW-CJ-1F超净工作台 上海博迅公司;Genesys 10S紫外可见分光光度计 美国Thermo Fisher Scientific公司;214差示扫描量热仪 德国Netzsch公司;SMZ-745T体视显微镜、D-7500CCD高清相机 日本Nikon公司;RT4加热制冷循环器 德国VIVO公司。
1.2 实验方法
1.2.1 鱼源胶原蛋白的酶解
犁齿鲷鱼鳞清洗、烘干后粉碎成絮状。底物浓度3%(w/v),超声90 min(50 ℃,200 W),高温处理1 h(121 ℃,0.1 MPa),冷却后调节至酶最佳pH,分别加入5%(w/w)酶底比的碱性蛋白酶、中性蛋白酶、胰蛋白酶、风味蛋白酶和木瓜蛋白酶,在酶最适温度下酶解6 h,沸水浴灭酶后离心取上清液冻干。
1.2.2 抗冻多肽的低温保护活性测定
取50 μL二次活化的嗜热链球菌接种到4 mL M17液体培养基中,培养4 h(37 ℃,180 r/min),5000 r/min离心10 min,收集菌泥,用等体积的无菌水洗涤两次后重悬于两倍的无菌水中,获得嗜热链球菌菌液。分别从不同酶解液中取540 μL加60 μL菌液混匀,540 μL生理盐水加60 μL菌液做空白组。吸取50 μL到4 mL M17培养液中,培养7 h,检测600 nm处的吸光值A1,剩余的菌液于−20 ℃冷冻24 h且在起始时间内间隔2 h进行两次冻融循环。之后将菌液37 ℃水浴解冻10 min,再次取50 μL接种培养7 h后测吸光值A2。根据公式(1)计算存活率。
嗜热链球菌存活率(%)=A2A1×100 (1) 式中:A1表示冷冻前菌液OD600;A2表示冷冻后菌液OD600。
1.2.3 热滞活性测定
热滞活性测定参照Wu等[21]方法稍作变化。取5 μL 15 mg/mL样品或牛血清蛋白到铝样品盘,参比组为空白铝样品盘,将二者同时置入差示扫描量热仪中,以−2 ℃/min速率降温至−30 ℃,平衡5 min,再以2 ℃/min速率升温至保留温度(−0.5、−0.3、0 ℃),使样品处于部分熔融状态,平衡5 min。根据DSC热流曲线分析样品的抗冻活性。冰晶含量(
Φ )和THA分别按照公式(2)和(3)计算。Φ=1−ΔHfΔHm (2) THA=Th−T0 (3) 式中:Φ为样品中的冰晶含量(%);△Hf为保留温度停留后继续降温过程中体系的放热焓(J/g);△Hm为样品结晶的总放热焓(J/g);Th为保留温度(℃),即样品熔融峰所涵盖温度区间的某一温度;T0体系融化部分再次冻结时的起始温度(℃)。
1.2.4 重结晶抑制活性测定
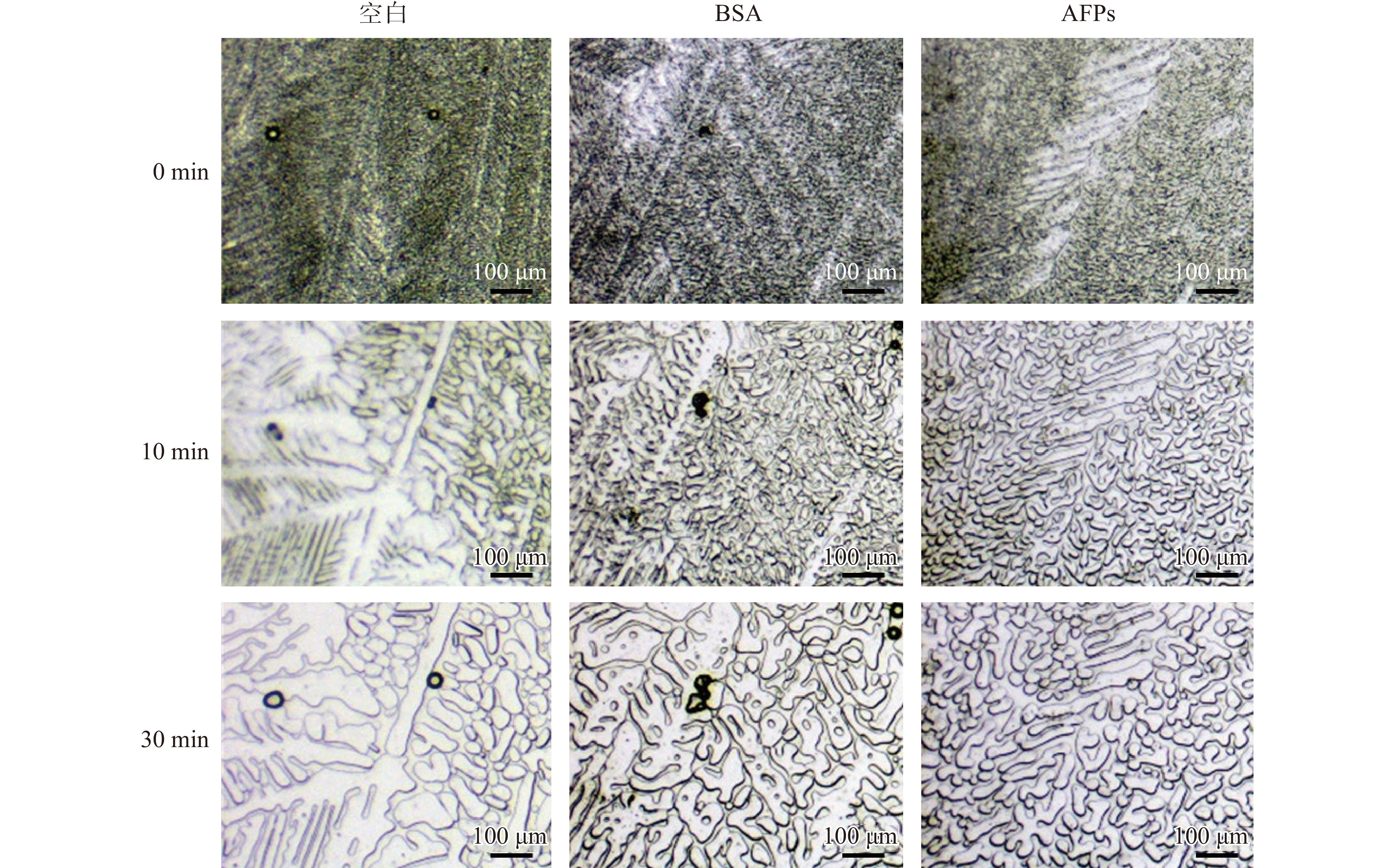
取3 μL AFPs(1.0 mg/mL,20%蔗糖溶液配制)于载玻片上,盖上盖玻片,置于冷台上。以−20 ℃/min速率快速冷却至−20 ℃并维持5 min,使其完全冻结;再以5 oC/min速率逐渐升温至−6 ℃,保持1 min;再以1 ℃/min让样品在−8~−6 ℃之间循环,模拟温度波动环境下的重结晶情况。并在−6 ℃分别保持0、10、30 min,通过CCD相机采集循环前后冰晶形态图像,通过OPLENIC软件分析冰晶尺寸变化。20%蔗糖溶液做空白组,BSA(1.0 mg/L,20%蔗糖溶液配制)做对照组。
1.2.5 计算机模拟酶解
在UniProt数据库中搜索鱼源胶原蛋白,选取O93484胶原蛋白序列,通过酶切工具Peptide Cutter(https://web.expasy.org/peptide_cutter/)选择胰蛋白酶进行模拟酶解[22]。
1.2.6 活性肽的性质预测
使用活性预测工具Peptide Ranker(http://distilldeep.ucd.ie/PeptideRanker/)对酶解获得的多肽片段活性进行预测,筛选高活性(>0.5)的肽片段。使用毒性预测工具ToxinPred(https://webs.iiitd.edu.in/raghava/toxinpred/multi_submit.php)筛选无毒的活性肽。使用抗冻蛋白/抗冻多肽数据库CryoProtect (http://codes.bio/cryoprotect/)筛选属于抗冻多肽的肽序列。
1.2.7 海鲈鱼肌球蛋白重链的同源建模
分别使用SWISS-MODEL(https://swissmodel.expasy.org/)、I-TASSER(https://zhanggroup.org/I-TASSER/)对肌球蛋白重链进行同源建模,选择准确度高的建模结果。
1.2.8 分子对接
使用Discovery studio 2019软件进行分子对接。进行''Prepare Protein''的前处理操作去除肌球蛋白重链晶体的水分子和配体并定义为受体。配体活性肽通过''Prepare Ligands''前处理并通过''Full Minimization''进行CHARMm力场最小化优化结构。处理后的肌球蛋白重链晶体以及抗冻多肽分别进行Dock Ligands(CDOCKER)分子对接。筛选结果中对接成功的化合物,并选取分值最高的对接结果。
1.2.9 分子动力学模拟
分子动力学模拟使用Discovery studio 2019软件进行。将分子对接的蛋白-多肽复合物导入,进行''Prepare Protein''前处理,添加力场charmm36,进行Solvation流程溶剂化复合物。打开动力学流程Standard Dynamics Cascade,设置Simulation Time (ps)为20、Simulation Time (ps)为200、Number of Processors为8,其它参数保持默认。
2. 结果与分析
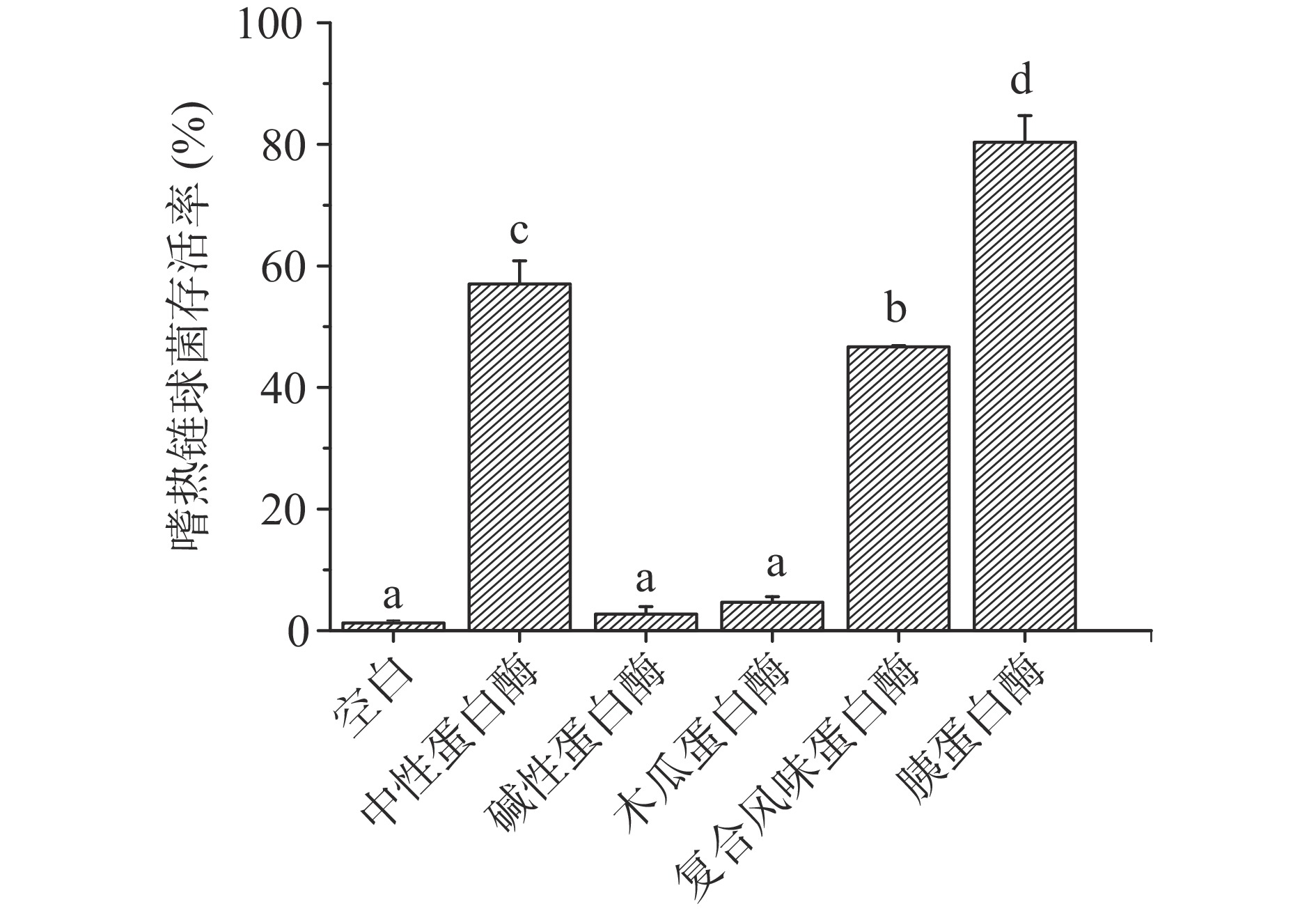
2.1 不同酶解产物的抗冻活性
选用五种蛋白酶分别测定不同酶解物的抗冻活性,以嗜热链球菌的低温保护活性为检测指标,结果如图1。由图可知,经过胰蛋白酶的水解后,鱼鳞酶解物对嗜热链球菌存活率显著提高(P<0.05),达到80.35%±4.39%。后续对其抗冻活性进行表征,并命名为AFPs。
2.2 热滞活性
在不同保留温度下测定了BSA和AFPs的部分融化过程DSC曲线,二者相应的DSC热流曲线结果如表1所示。随着保留温度Th的上升,BSA组的冰核百分含量(Φ)从91.8%下降到38.2%,而AFPs组的冰核百分含量(Φ)从44.7%下降到5.3%。在相同保留温度时,AFPs的冰核含量明显低于BSA,而热滞活性高于BSA。说明当Th越接近样品融点时,Φ含量越低,THA越高,THA与冰核百分含量(Φ)存在一定的负相关关系。
表 1 BSA和AFPs的冻结起始温度、保留温度、冰晶含量和热滞活性Table 1. Freezing initiation temperature, retention temperature, ice crystal content and thermal hysteresis activity of BSA and AFPs样品 △Hm(J/g) △Hf(J/g) Φ(%) Th(℃) T0(℃) THA −288.1 −23.6 91.8 −0.5 −0.7 0.2 BSA −288.1 −78.7 72.7 −0.3 −0.6 0.3 −288.1 −178.0 38.2 0.0 −0.2 0.2 −302.2 −167.0 44.7 −0.5 −0.7 0.2 AFPs −302.2 −231.9 23.3 −0.3 −0.6 0.3 −302.2 −286.1 5.3 0.0 −0.3 0.3 注:△Hm是样品冻结过程总放热焓;△Hf是样品从保留温度继续降温过程放热焓;Th是保留温度表示样品处于部分融融状态;T0是样品融化部分再次冻结时的起始温度;热滞活性是指Th与T0之间的差值。 2.3 重结晶抑制活性
大冰晶的表面能小于小冰晶的表面能,冻融过程中小冰晶不断聚合形成大冰晶,产生冰重结晶现象。而抗冻多肽可以使冷冻溶液中冰晶保持小尺寸状态,抑制冰晶的重结晶。如图2所示,在含有AFPs的溶液中,冰晶尺寸小于同浓度的BSA溶液和空白组。
2.4 计算机辅助挖掘鱼源抗冻多肽
选择PeptideCutter中的胰蛋白酶对鱼源胶原蛋白进行酶解切割,通过模拟酶解方法,省去分离纯化及质谱等步骤。经模拟酶解后1356个氨基酸的鱼源胶原蛋白序列被酶解为115条肽段,具体结果如表2所示。
表 2 模拟酶解肽段序列及切割位点Table 2. Mimic enzymatic hydrolysis peptide sequence and cleavage site肽段序列 切割位点 肽段序列 切割位点 MLSFVDNR 8 GGPGPSGPPGPSGANGEK 687 ILLLLAVTSLLASCQSGGLK 28 GESGSFGPAGPAGLR 702 GPR 31 GPSGER 708 GAK 34 GEGGPAGLPGFAGPPGSDGQSGPR 732 GPR 37 GEK 735 GDR 40 GPAGGK 741 GPQGPNGR 48 GDVGPAGPAGPSGQSGPSGASGPAGPPGGR 771 DGK 51 GDAGPSGLTGFPGAAGR 788 AGLPGIAGPPGPPGLGGNFAAQFDGGK 78 VGGPGPAGIAGPPGSAGPAGK 809 GSDPGPGPMGLMGSR 93 DGPR 813 GPNGPPGAPGPQGFTGHAGEPGEPGQTGSIGAR 126 GLR 816 GPTGSAGKPGEDGNNGRPGKPGDR 150 GDPGPGGPQGEQGVVGPAGISGDK 840 GGPGTQGAR 159 GPSGESGPPGAPGTAGPQGVLGPSGFVGLPGSR 873 GFPGTPGLPGMK 171 GDK 876 GHR 174 GLPGGPGAVGEPGR 890 GYNGLDGR 182 LGPAGASGPR 900 K 183 GPAGNIGMPGMTGTQGEAGR 920 GESGTAGAK 192 EGNSGNDGPPGRPGAAGFK 939 GETGAHGANGSPGPAGSR 210 GDR 942 GLNGER 216 GEPGSPGALGSSGQPGPNGPAGSAGRPGNR 972 GR 218 GESGPTGNGGPVGAVGAR 990 AGPAGPAGAR 228 GAPGPAGPR 999 GADGSTGPAGPAGPLGAAGPPGFPGAPGPK 258 GEK 1002 GEIGGAGSNGPSGPQGGR 276 GGAGEK 1008 GEPGINGAVGPVGPVGNPGNNGINGAK 303 GDR 1011 GAAGLPGVAGAPGFPGPR 321 GMK 1014 GGPGPQGPQGSTGAR 336 GLR 1017 GLGGDPGPSGQK 348 GHGGLQGMPGPNGPSGETGSAGITGPAGPR 1047 GDSGAK 354 GPAGPHGPPGK 1058 GEPGHSGVQGAAGPAGEEGK 374 DGR 1061 R 375 AGGHGAIGPVGHR 1074 GSTGEVGATGPAGLR 390 GSPGHLGPAGPPGSPGLPGPAGPAGGGYDQSGGYDEYR 1112 GAR 393 ADQPSFR 1119 GGAGTR 399 AK 1121 GLPGLEGR 407 DYEVDATIK 1130 GGPIGMPGAR 417 SLNSQIENLLTPEGSK 1146 GATGPGGIR 426 K 1147 GAPGDAGR 434 NPAR 1151 AGESGLTGAR 444 TCR 1154 GLPGNSGQGGPPGK 458 DIR 1157 EGPPGAAGLDGR 470 LSHPDWSSGFYWIDPNQGCIADAIK 1182 TGPPGPTGPR 480 AYCDFSTGHTCIHPHPESIAR 1203 GQPGNIGFPGPK 492 K 1204 GPGGEAGK 500 NWYR 1208 GGDK 504 SSENK 1213 GPTGATGLR 513 K 1214 GGPGADGNNGAPGPAGVVGNTGEK 537 HVWFGETINGGTEFAYNDETLSPQSMATQLAFMR 1248 GEQGPAGAPGFQGLPGPAGPAGEAGK 563 LLANQATQNITYHCK 1263 AGNQGMPGDQGLPGPAGVK 582 NSVAYMDGENGNLK 1277 GER 585 K 1278 GNSGPAGSAGSQGAIGAR 603 AVLLQGSNDVELR 1291 GPAGTPGPDGGK 615 AEGNSR 1297 GEPGSVGIVGAAGHQGPGGMPGER 639 FTFNVLEDGCTR 1309 GAGGTPGPK 648 HTGQWSK 1316 GEK 651 TVIEYR 1322 GEGGHR 657 TNKPSR 1328 GLEGNMGR 665 LPILDIAPLDIGEADQEFGLDIGPVCFK 1356 DGAR 669 2.5 鱼源抗冻多肽的筛选
将所有肽段进行Peptide Ranker活性预测,选择预测活性分数大于0.5的共47条肽段序列,并对其进行ToxinPred毒性预测,结果表明除GATGPGGIR序列外其余序列皆无毒,使用抗冻蛋白/抗冻多肽数据库CryoProtect筛选,结果表明除R序列外其余序列皆属于潜在的抗冻多肽。具体结果如表3。
表 3 肽段序列活性、毒性分析及抗冻活性预测Table 3. Peptide sequence activity, toxicity analysis and antifreeze activity prediction肽段序列 活性 毒性 是否属于抗冻多肽 GFPGTPGLPGMK 0.937367 无毒 是 GSDPGPGPMGLMGSR 0.936009 无毒 是 GPAGPHGPPGK 0.891256 无毒 是 GGPGPSGPPGPSGANGEK 0.882279 无毒 是 GQPGNIGFPGPK 0.870524 无毒 是 GPR 0.865974 无毒 是 GAAGLPGVAGAPGFPGPR 0.85897 无毒 是 GGPIGMPGAR 0.849845 无毒 是 GAPGPAGPR 0.836866 无毒 是 NWYR 0.819954 无毒 是 AGPAGPAGAR 0.768318 无毒 是 GR 0.766288 无毒 是 GLPGGPGAVGEPGR 0.759819 无毒 是 GPTGSAGKPGEDGNNGRPGKPGDR 0.759798 无毒 是 GESGSFGPAGPAGLR 0.749612 无毒 是 GPQGPNGR 0.745307 无毒 是 GGPGPQGPQGSTGAR 0.734252 无毒 是 GPAGTPGPDGGK 0.729162 无毒 是 EGNSGNDGPPGRPGAAGFK 0.729132 无毒 是 GDAGPSGLTGFPGAAGR 0.720995 无毒 是 GLR 0.714392 无毒 是 DGPR 0.676456 无毒 是 ADQPSFR 0.67453 无毒 是 GEPGINGAVGPVGPVGNPGNNGINGAK 0.654289 无毒 是 GMK 0.636573 无毒 是 EGPPGAAGLDGR 0.632804 无毒 是 AGGHGAIGPVGHR 0.629756 无毒 是 GGPGADGNNGAPGPAGVVGNTGEK 0.629674 无毒 是 AGNQGMPGDQGLPGPAGVK 0.615665 无毒 是 GLPGNSGQGGPPGK 0.607985 无毒 是 GEGGPAGLPGFAGPPGSDGQSGPR 0.607605 无毒 是 GEPGSPGALGSSGQPGPNGPAGSAGRPGNR 0.606938 无毒 是 TGPPGPTGPR 0.59095 无毒 是 GDPGPGGPQGEQGVVGPAGISGDK 0.589429 无毒 是 GAPGDAGR 0.574973 无毒 是 GNSGPAGSAGSQGAIGAR 0.566693 无毒 是 ILLLLAVTSLLASCQSGGLK 0.566648 无毒 是 GAGGTPGPK 0.557536 无毒 是 GAR 0.547871 无毒 是 GHR 0.546958 无毒 是 R 0.546236 无毒 否 LGPAGASGPR 0.54582 无毒 是 GPAGGK 0.538052 无毒 是 GEIGGAGSNGPSGPQGGR 0.526182 无毒 是 GESGPTGNGGPVGAVGAR 0.525565 无毒 是 GYNGLDGR 0.51965 无毒 是 GATGPGGIR 0.51466 有毒 是 2.6 鱼肌球蛋白的同源建模
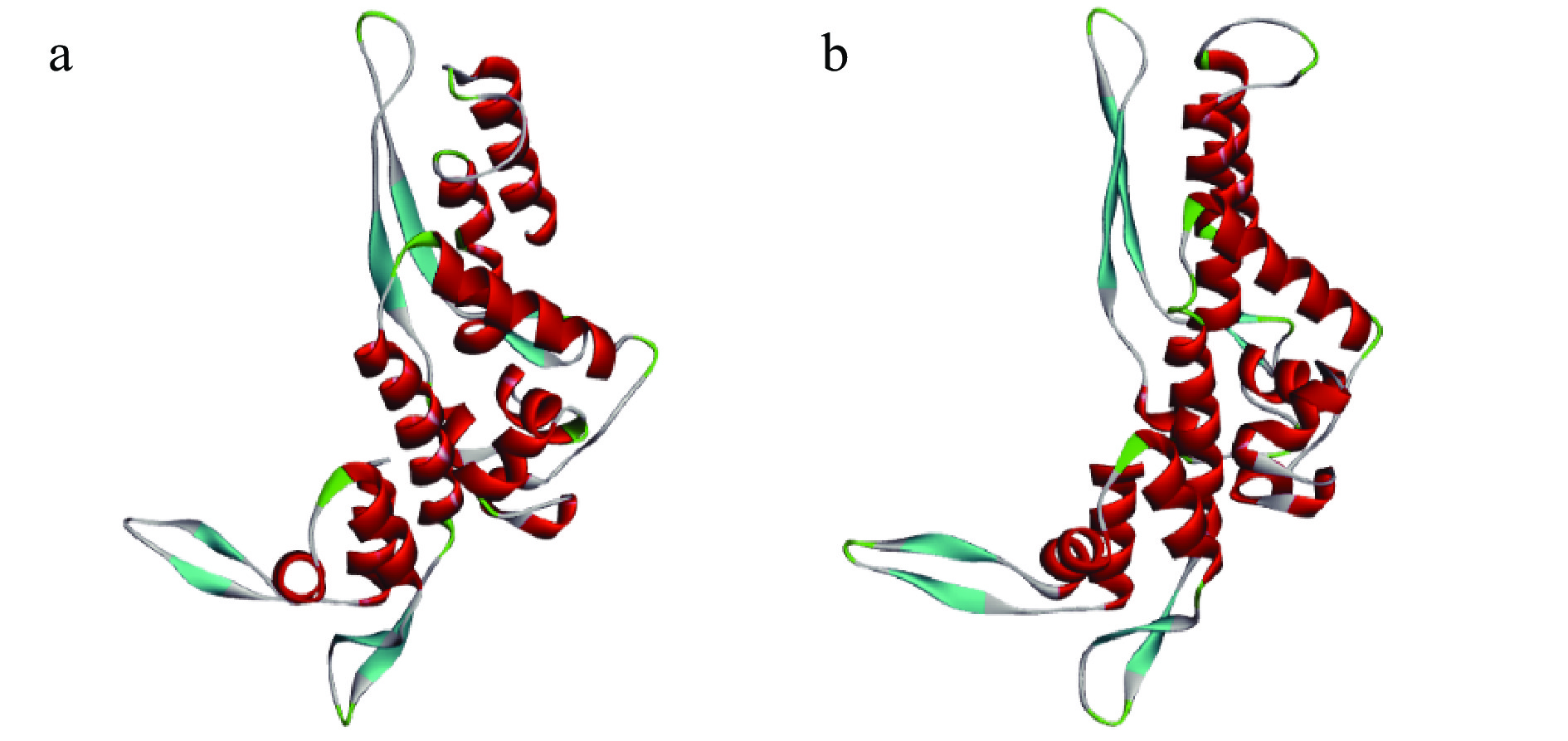
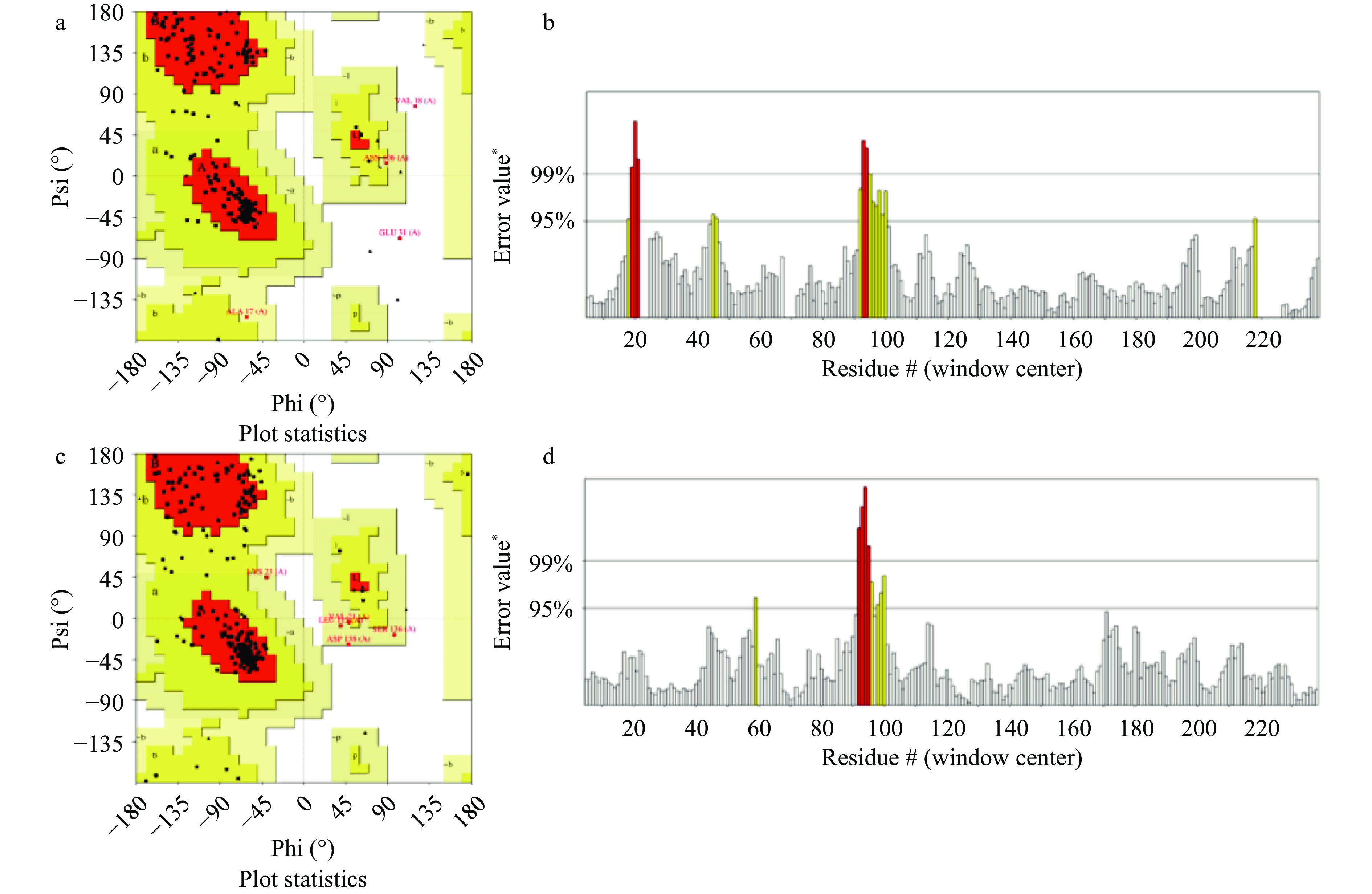
对海鲈鱼肌球蛋白重链进行同源建模,采用SWISS-MODEL构建的三维结构模型见图3a,I-TASSER构建的三维结构模型见图3b。为进一步说明同源建模结果的合理性,通过SAVESv6.0(https://saves.mbi.ucla.edu/)对建模结果进行评估。拉式构像图(Ramachandran plot)[23]是描述蛋白质结构中氨基酸残基二面角ψ和φ是否在合理区域的一种可视化方法,也可以反映出该蛋白质的构象是否合理[24-25]。由图4a、图4c可知,SWISS-MODEL构建的三维结构模型有氨基酸88.9%落在完全允许区,9.2%落在允许区,0.9%落在最大允许区,0.9%落在不允许区,99.1%落在合理的区域。I-TASSER构建的三维结构模型有氨基酸81.6%落在完全允许区,16.1%落在允许区,2.3%落在最大允许区,没有落在不允许区氨基酸,100%落在合理的区域。
蛋白三维结构准确性随着整体质量因素值的增加而增加。通常,高分辨率晶体结构的值可以达到95%,而分辨率较低的结构的值只能达到大约91%。由图4b、图4d可知,SWISS-MODEL构建的三维结构模型精确值为92.661%,I-TASSER构建的三维结构模型精确值为96.154%。与高分辨率晶体结构更接近,表明I-TASSER构建的三维结构模型的准确性相对较高。故I-TASSER构建的三维蛋白结构模型更可靠,用于后续的分子对接研究。
2.7 抗冻多肽与鱼肌球蛋白重链的分子对接
与肌球蛋白重链对接上的抗冻多肽序列共有GR、GMK、GAR、GPR、GPAGGK五条。Graham等[26]从雪蚤中纯化的抗冻蛋白氨基酸序列为GAAGAGSSGP。Cao等[27]通过序列分析推导出AP-3抗冻蛋白氨基酸组成为GLLGPLGPRGL。Wang等[28]纯化的鲨鱼皮胶原蛋白抗冻多肽氨基酸序列为GAIGPAGPLGP。Nikoo等[29]从黑龙江鲟鱼皮明胶中提取的抗冻多肽是PAGT,并验证出PAGT在肉糜模型系统中具有抗氧化和冷冻保护作用。Damodara等[30]分离的鱼明胶抗冻多肽氨基酸序列为KDGTPGQFGP(OH)PGAPGKGN(OH)H、NEGTPGTGPAGPP(OH)GFHTPK(OH)W,它们都含有GTPG-和GPP(OH)G-结构指纹。综上,选择更符合胶原蛋白抗冻多肽的序列GPR与GPAGGK进行进一步对接及动力学分析[31]。
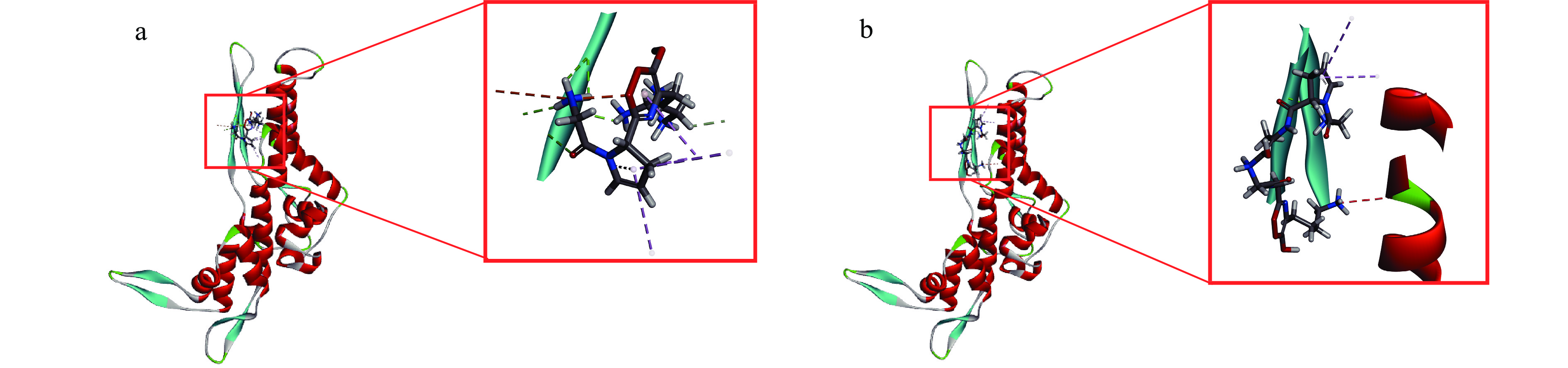
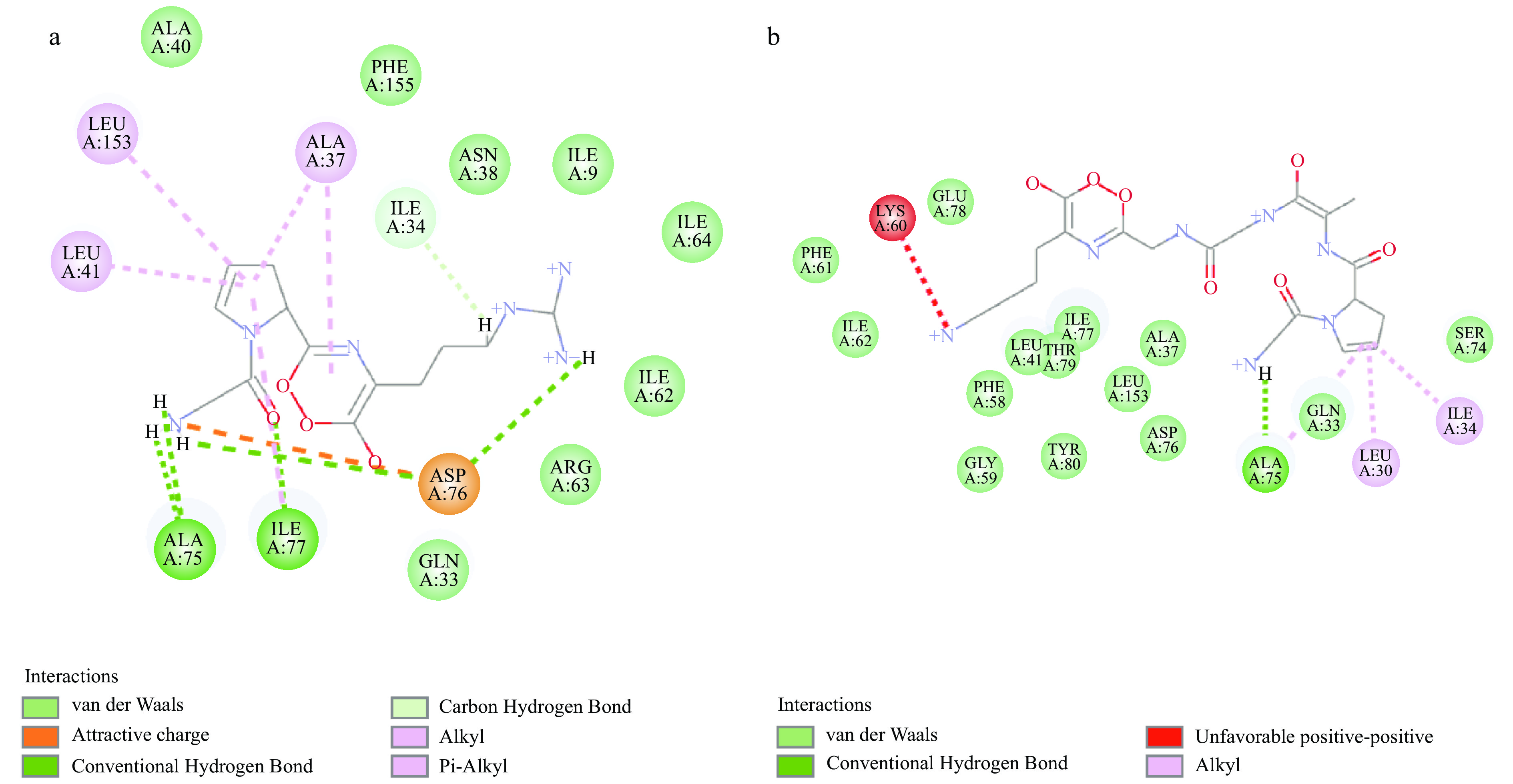
选择的对接结合空腔坐标为64.5359、85.5816、76.7437、半径为8,GPR和GPAGGK与肌球蛋白重链的对接结果如图5a、图5b所示,对接位点2D示意图如图6所示,模拟酶解肽段GPR可以与MHC中Ile34氨基酸形成碳氢键,与Asp76氨基酸形成静电作用,与Ala75、Asp76、Ile77氨基酸形成氢键,还能以范德华力与Ile9、Gln33、Asn38、Ala40、Ile62、Arg63、Ile64、Phe155氨基酸结合。模拟酶解肽段GPAGGK可以与MHC中Ala75氨基酸形成氢键,还能以范德华力与Gln33、Ala37、Leu41、Phe58、Gly59、Phe61、Ile62、Ser74、Asp76、Ile77、Glu78、Thr79、Tyr80、Leu153氨基酸结合。
在冻藏过程中,首先被冻结的为组织内的自由水。随着冻结时间的延长,自由水完全转化为冰后与蛋白质结合的结合水也开始发生冻结,脱离蛋白,从而导致疏水键及二硫键等化学键的形成并聚集,蛋白的各个侧链相互聚集,发生不可逆的变性[32]。抗冻多肽可以通过氢键、疏水相互作用和范德华力等分子之间作用力[33],吸附在液体冰晶表面,阻碍了冰晶在固、液界面位移及与水分子的结合[34-35]。通过抗冻多肽与肌球蛋白重链的分子对接发现,抗冻多肽还可以通过氢键、范德华力等分子间作用力与肌球蛋白直接作用,结合在肌球蛋白的结构空腔上,影响疏水键及二硫键等化学键的形成,阻碍蛋白侧链聚集与结构改变,对冰晶的位移也有一定影响作用。
2.8 抗冻多肽与海鲈鱼肌球蛋白重链的分子动力学模拟
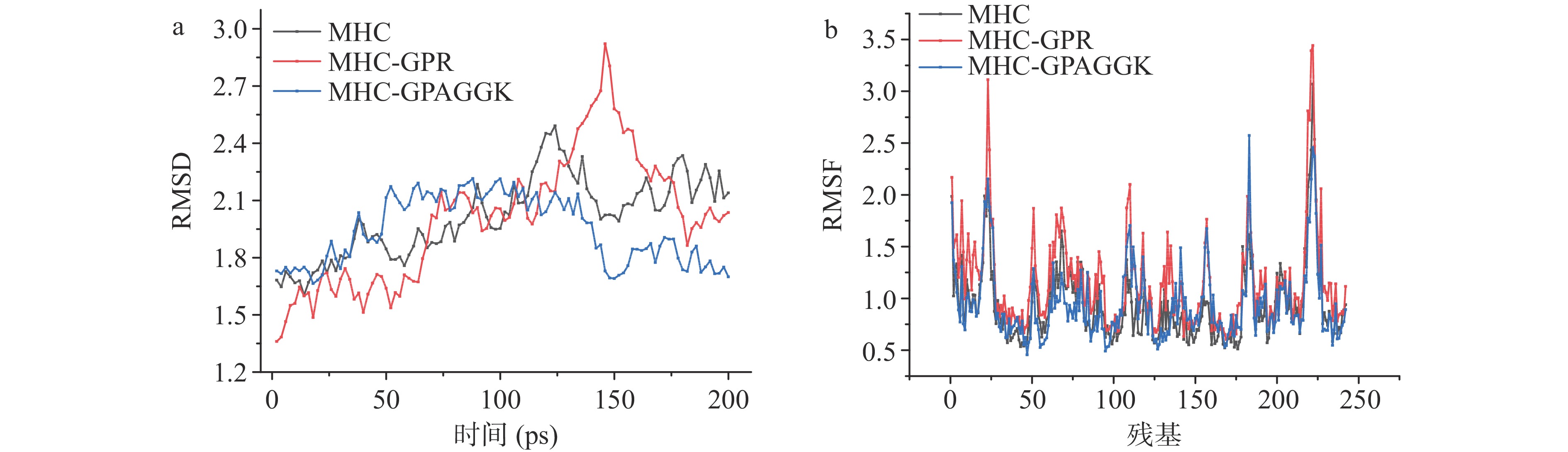
通过绘制蛋白质的RMSD作为构象变化的函数,可以可视化整体蛋白质稳定性。在分子动力学模拟中,RMSD用于评估与初始蛋白质结构的结构偏差(即估计蛋白质完整性)[36]。RMSD连续增加至高值表明构象不稳定[37]。MHC、MHC-GPR和MHC-GPAGGK计算出的RMSD值如图7a所示。RMSD值随着构象变化逐渐增加,MHC的RMSD值在构象60时达到最大,波动约为0.8 Å;MHC-GPR的RMSD值在构象73时达到最大,波动约为1.5 Å;MHC-GPAGGK的RMSD值相较其他较为稳定,波动约为0.5 Å,这些波动可能是因为肌球蛋白三级和二级结构的变化。与单独的MHC相比MHC-GPR初始稳定性会提高,但随着温度和时间变化,构象会变得更不稳定,这可能是因为GPR与MHC的结合并不牢固。而MHC-GPAGGK可以使蛋白在温度和时间的变化中变得构象更加稳定。
为了进一步表征肌球蛋白重链的构象灵活性,绘制了RMSF作为残基数的函数。RMSF值是残留灵活性的标准[38]。如图7b所示,残基17~32,173~187和214~231的RMSF值显著增加。这些具有较大RMSF波动的位置是肌球蛋白重链柔性较大的区域,推测是肌球蛋白重链与抗冻多肽作用的位置。通过RMSD与RMSF的结果,可以发现,抗冻多肽GPAGGK与肌球蛋白结合可以在温度变化的过程中,稳定构象,延缓蛋白变性。
3. 结论
本研究发现鱼源胶原蛋白的胰蛋白酶酶解物具有较高的抗冻活性,使用胰蛋白酶模拟酶解鱼源胶原蛋白,运用计算机筛选得到抗冻多肽,省去分离纯化及质谱等步骤。并与同源建模后的海鲈鱼肌球蛋白重链进行分子模拟,以研究鱼源胶原蛋白抗冻多肽对肌球蛋白的保护机制。分子对接结果表明,肽段GPR和GPAGGK能够通过碳氢键、氢键和范德华力等分子作用力对海鲈鱼肌球蛋白的结构及聚集行为产生影响,阻碍蛋白侧链聚集、结构改变和冰晶的位移等。分子动力学结果表明,肽段GPAGGK比肽段GPR结合的更稳定,由此结果可预测肽段GPAGGK对鱼糜及鱼糜制品在冷冻贮藏中的品质保持起到更明显的作用,GPAGGK可作为潜在的抗冻剂应用于后续抗冻鱼糜产品的研发。
-
表 1 BSA和AFPs的冻结起始温度、保留温度、冰晶含量和热滞活性
Table 1 Freezing initiation temperature, retention temperature, ice crystal content and thermal hysteresis activity of BSA and AFPs
样品 △Hm(J/g) △Hf(J/g) Φ(%) Th(℃) T0(℃) THA −288.1 −23.6 91.8 −0.5 −0.7 0.2 BSA −288.1 −78.7 72.7 −0.3 −0.6 0.3 −288.1 −178.0 38.2 0.0 −0.2 0.2 −302.2 −167.0 44.7 −0.5 −0.7 0.2 AFPs −302.2 −231.9 23.3 −0.3 −0.6 0.3 −302.2 −286.1 5.3 0.0 −0.3 0.3 注:△Hm是样品冻结过程总放热焓;△Hf是样品从保留温度继续降温过程放热焓;Th是保留温度表示样品处于部分融融状态;T0是样品融化部分再次冻结时的起始温度;热滞活性是指Th与T0之间的差值。 表 2 模拟酶解肽段序列及切割位点
Table 2 Mimic enzymatic hydrolysis peptide sequence and cleavage site
肽段序列 切割位点 肽段序列 切割位点 MLSFVDNR 8 GGPGPSGPPGPSGANGEK 687 ILLLLAVTSLLASCQSGGLK 28 GESGSFGPAGPAGLR 702 GPR 31 GPSGER 708 GAK 34 GEGGPAGLPGFAGPPGSDGQSGPR 732 GPR 37 GEK 735 GDR 40 GPAGGK 741 GPQGPNGR 48 GDVGPAGPAGPSGQSGPSGASGPAGPPGGR 771 DGK 51 GDAGPSGLTGFPGAAGR 788 AGLPGIAGPPGPPGLGGNFAAQFDGGK 78 VGGPGPAGIAGPPGSAGPAGK 809 GSDPGPGPMGLMGSR 93 DGPR 813 GPNGPPGAPGPQGFTGHAGEPGEPGQTGSIGAR 126 GLR 816 GPTGSAGKPGEDGNNGRPGKPGDR 150 GDPGPGGPQGEQGVVGPAGISGDK 840 GGPGTQGAR 159 GPSGESGPPGAPGTAGPQGVLGPSGFVGLPGSR 873 GFPGTPGLPGMK 171 GDK 876 GHR 174 GLPGGPGAVGEPGR 890 GYNGLDGR 182 LGPAGASGPR 900 K 183 GPAGNIGMPGMTGTQGEAGR 920 GESGTAGAK 192 EGNSGNDGPPGRPGAAGFK 939 GETGAHGANGSPGPAGSR 210 GDR 942 GLNGER 216 GEPGSPGALGSSGQPGPNGPAGSAGRPGNR 972 GR 218 GESGPTGNGGPVGAVGAR 990 AGPAGPAGAR 228 GAPGPAGPR 999 GADGSTGPAGPAGPLGAAGPPGFPGAPGPK 258 GEK 1002 GEIGGAGSNGPSGPQGGR 276 GGAGEK 1008 GEPGINGAVGPVGPVGNPGNNGINGAK 303 GDR 1011 GAAGLPGVAGAPGFPGPR 321 GMK 1014 GGPGPQGPQGSTGAR 336 GLR 1017 GLGGDPGPSGQK 348 GHGGLQGMPGPNGPSGETGSAGITGPAGPR 1047 GDSGAK 354 GPAGPHGPPGK 1058 GEPGHSGVQGAAGPAGEEGK 374 DGR 1061 R 375 AGGHGAIGPVGHR 1074 GSTGEVGATGPAGLR 390 GSPGHLGPAGPPGSPGLPGPAGPAGGGYDQSGGYDEYR 1112 GAR 393 ADQPSFR 1119 GGAGTR 399 AK 1121 GLPGLEGR 407 DYEVDATIK 1130 GGPIGMPGAR 417 SLNSQIENLLTPEGSK 1146 GATGPGGIR 426 K 1147 GAPGDAGR 434 NPAR 1151 AGESGLTGAR 444 TCR 1154 GLPGNSGQGGPPGK 458 DIR 1157 EGPPGAAGLDGR 470 LSHPDWSSGFYWIDPNQGCIADAIK 1182 TGPPGPTGPR 480 AYCDFSTGHTCIHPHPESIAR 1203 GQPGNIGFPGPK 492 K 1204 GPGGEAGK 500 NWYR 1208 GGDK 504 SSENK 1213 GPTGATGLR 513 K 1214 GGPGADGNNGAPGPAGVVGNTGEK 537 HVWFGETINGGTEFAYNDETLSPQSMATQLAFMR 1248 GEQGPAGAPGFQGLPGPAGPAGEAGK 563 LLANQATQNITYHCK 1263 AGNQGMPGDQGLPGPAGVK 582 NSVAYMDGENGNLK 1277 GER 585 K 1278 GNSGPAGSAGSQGAIGAR 603 AVLLQGSNDVELR 1291 GPAGTPGPDGGK 615 AEGNSR 1297 GEPGSVGIVGAAGHQGPGGMPGER 639 FTFNVLEDGCTR 1309 GAGGTPGPK 648 HTGQWSK 1316 GEK 651 TVIEYR 1322 GEGGHR 657 TNKPSR 1328 GLEGNMGR 665 LPILDIAPLDIGEADQEFGLDIGPVCFK 1356 DGAR 669 表 3 肽段序列活性、毒性分析及抗冻活性预测
Table 3 Peptide sequence activity, toxicity analysis and antifreeze activity prediction
肽段序列 活性 毒性 是否属于抗冻多肽 GFPGTPGLPGMK 0.937367 无毒 是 GSDPGPGPMGLMGSR 0.936009 无毒 是 GPAGPHGPPGK 0.891256 无毒 是 GGPGPSGPPGPSGANGEK 0.882279 无毒 是 GQPGNIGFPGPK 0.870524 无毒 是 GPR 0.865974 无毒 是 GAAGLPGVAGAPGFPGPR 0.85897 无毒 是 GGPIGMPGAR 0.849845 无毒 是 GAPGPAGPR 0.836866 无毒 是 NWYR 0.819954 无毒 是 AGPAGPAGAR 0.768318 无毒 是 GR 0.766288 无毒 是 GLPGGPGAVGEPGR 0.759819 无毒 是 GPTGSAGKPGEDGNNGRPGKPGDR 0.759798 无毒 是 GESGSFGPAGPAGLR 0.749612 无毒 是 GPQGPNGR 0.745307 无毒 是 GGPGPQGPQGSTGAR 0.734252 无毒 是 GPAGTPGPDGGK 0.729162 无毒 是 EGNSGNDGPPGRPGAAGFK 0.729132 无毒 是 GDAGPSGLTGFPGAAGR 0.720995 无毒 是 GLR 0.714392 无毒 是 DGPR 0.676456 无毒 是 ADQPSFR 0.67453 无毒 是 GEPGINGAVGPVGPVGNPGNNGINGAK 0.654289 无毒 是 GMK 0.636573 无毒 是 EGPPGAAGLDGR 0.632804 无毒 是 AGGHGAIGPVGHR 0.629756 无毒 是 GGPGADGNNGAPGPAGVVGNTGEK 0.629674 无毒 是 AGNQGMPGDQGLPGPAGVK 0.615665 无毒 是 GLPGNSGQGGPPGK 0.607985 无毒 是 GEGGPAGLPGFAGPPGSDGQSGPR 0.607605 无毒 是 GEPGSPGALGSSGQPGPNGPAGSAGRPGNR 0.606938 无毒 是 TGPPGPTGPR 0.59095 无毒 是 GDPGPGGPQGEQGVVGPAGISGDK 0.589429 无毒 是 GAPGDAGR 0.574973 无毒 是 GNSGPAGSAGSQGAIGAR 0.566693 无毒 是 ILLLLAVTSLLASCQSGGLK 0.566648 无毒 是 GAGGTPGPK 0.557536 无毒 是 GAR 0.547871 无毒 是 GHR 0.546958 无毒 是 R 0.546236 无毒 否 LGPAGASGPR 0.54582 无毒 是 GPAGGK 0.538052 无毒 是 GEIGGAGSNGPSGPQGGR 0.526182 无毒 是 GESGPTGNGGPVGAVGAR 0.525565 无毒 是 GYNGLDGR 0.51965 无毒 是 GATGPGGIR 0.51466 有毒 是 -
[1] 高荣, 吴茜婷, 张贝叶, 等. 我国食用渔业资源发展现状及未来展望[J]. 江西水产科技,2018(4):55−56. [GAO R, WU X T, ZHANG B Y, et al. Present situation and future prospect of edible fishery resources in China[J]. Jiangxi Fishery Science and Technology,2018(4):55−56. doi: 10.3969/j.issn.1006-3188.2018.04.026 [2] 裘乐芸, 邢倩, 邓泽元, 等. 植物多酚与鲢鱼肌球蛋白相互作用及其对肌原纤维蛋白结构和凝胶形成的影响[J]. 中国食品学报,2021,21(5):48−56. [QIU L Y, XING Q, DENG Z Y, et al. The interaction of plant polyphenols with silver carp myosin and its effects on the structure and gel formation of myofibrillar protein[J]. Journal of Chinese Institute of Food Science and Technology,2021,21(5):48−56. [3] 蔡路昀, 许晴, 曹爱玲. 不同超声辅助解冻方式对海鲈鱼品质的影响[J]. 食品工业科技,2020,41(24):264−271. [CAI L Y, XU Q, CAO A L. Effects of different ultrasound-assisted thawing methods on the quality of the sea bass[J]. Science and Technology of Food Industry,2020,41(24):264−271. [4] 余璐涵, 陈旭, 吴金鸿, 等. 不同低温冻融循环对鱼糜品质与加工特性的影响[J]. 食品工业科技,2022,43(7):9. [YU L H, CHEN X, WU J H, et al. Effects of freezing and thawing cycles on quality and processing characteristics of surimi[J]. Science and Technology of Food Industry,2022,43(7):9. doi: 10.13386/j.issn1002-0306.2021080165 [5] ZHAO Y, CHEN Z G, WU T. Cryogelation of alginate improved the freeze-thaw stability of oil-in-water emulsions[J]. Carbohydrate Polymers,2018,198:26−33. doi: 10.1016/j.carbpol.2018.06.013
[6] AN Y Q, YOU J, XIONG S B, et al. Short-term frozen storage enhances cross-linking that was induced by transglutaminase in surimi gels from silver carp (Hypophthalmichthys molitrix)[J]. Food Chemistry,2018,257:216−222. doi: 10.1016/j.foodchem.2018.02.140
[7] MORENO H M, HERRANZ B, PEREZ-MATEOS M, et al. New alternatives in seafood restructured products[J]. Critical Reviews in Food Science and Nutrition,2016,56(2):237−248. doi: 10.1080/10408398.2012.719942
[8] 高宇, 毕保良, 贾丹, 等. 青鱼和鲢鱼肌球蛋白热诱导凝胶特性的比较[J]. 食品工业科技,2021,42(3):1−5,12. [GAO Y, BI B L, JIA D, et al. Comparison of the properties of heat-induced gel of black carp and silver carp myosin[J]. Science and Technology of Food Industry,2021,42(3):1−5,12. [9] 梁雯雯, 郭建, 汪秋宽, 等. 不同解冻方式对鲢鱼肌球蛋白结构和性质的影响[J]. 食品工业科技,2019,40(21):7−12. [LIANG W W, GUO J, WANG Q K, et al. Effects of different thawing methods on the structure and properties of silver carp myosin[J]. Science and Technology of Food Industry,2019,40(21):7−12. [10] SIEGEL D G, SCHMIDT G R. Ionic, pH, and temperature effects on the binding ability of myosin[J]. Journal of Food Science,1979,44(6):1686−1689. doi: 10.1111/j.1365-2621.1979.tb09116.x
[11] 陈旭, 蔡茜茜, 汪少芸, 等. 抗冻肽的研究进展及其在食品工业的应用前景[J]. 食品科学,2019,40(17):331−337. [CHEN X, CAI X X, WANG S Y, et al. Recent progress and application prospects of antifreeze peptides in food industry[J]. Food Science,2019,40(17):331−337. doi: 10.7506/spkx1002-6630-20190303-025 [12] KUN H, MASTAI Y. Activity of short segments of type I antifreeze protein[J]. Biopolymers,2007,88(6):807−814. doi: 10.1002/bip.20844
[13] NOBEKAWA T, HAGIWARA Y. Interaction among the twelve-residue segment of antifreeze protein type I, or its mutants, water and a hexagonal ice crystal[J]. Molecular Simulation,2008,34(6):591−610. doi: 10.1080/08927020801986556
[14] CHEN X, WU J H, LI L, et al. Cryoprotective activity and action mechanism of antifreeze peptides obtained from tilapia scales on Streptococcus thermophilus during cold stress[J]. Journal of Agricultural and Food Chemistry,2019,67(7):1918−1926. doi: 10.1021/acs.jafc.8b06514
[15] KIM H J, LEE J H, HUR Y B, et al. Marine antifreeze proteins: Structure, function, and application to cryopreservation as a potential cryoprotectant[J]. Marine Drugs,2017,15(2):27. doi: 10.3390/md15020027
[16] DU L H, BETTI M. Identification and evaluation of cryoprotective peptides from chicken collagen: Ice-growth inhibition activity compared to that of type I antifreeze proteins in sucrose model systems[J]. Journal of Agricultural and Food Chemistry,2016,64(25):5232−5240. doi: 10.1021/acs.jafc.6b01911
[17] CLARKE C J, BUCKLEY S L, LINDNER N. Ice structuring proteins-a new name for antifreeze proteins[J]. CryoLetters,2002,23(2):89−92.
[18] 金泉, 张莉, 吴金鸿, 等. 丝胶抗冻肽在大肠杆菌中的重组表达及其抗冻活性初探[J]. 食品工业科技,2018,39(21):141−145,206. [JIN Q, ZHANG L, WU J H, et al. Recombinant expression of sericin antifreeze peptide in E. coli and its antifreeze activity[J]. Science and Technology of Food Industry,2018,39(21):141−145,206. [19] CHEN X, WU J H, LI X Z, et al. Investigation of the cryoprotective mechanism and effect on quality characteristics of surimi during freezing storage by antifreeze peptides[J]. Food Chemistry,2022,371:131054. doi: 10.1016/j.foodchem.2021.131054
[20] 李晓坤. 利用猪皮明胶制备抗冻多肽及其低温保护作用研究[D]. 福州: 福州大学, 2013. LI X K. Preparation of antifreeze polypeptide from pigskin gelatin and study on the cryoprotective activity[D]. Fuzhou: Fuzhou University, 2013.
[21] WU J H, RONG Y Z, WANG Z W, et al. Isolation and characterisation of sericin antifreeze peptides and molecular dynamics modelling of their ice-binding interaction[J]. Food Chemistry,2015,174:621−629. doi: 10.1016/j.foodchem.2014.11.100
[22] 孙晨松, 陈雯祺, 陈盈盈, 等. 基于分子对接虚拟筛选含酪氨酸残基的ACE抑制三肽[J]. 食品工业科技,2021,42(16):20−27. [SUN C S, CHEN W Q, CHEN Y Y, et al. Virtual screening of ACE inhibitory tripeptides containing tyrosine residues based on molecular docking[J]. Science and Technology of Food Industry,2021,42(16):20−27. [23] LASKOWSKI R A, MACARTHUR M W, MOSS D S, et al. Procheck: A program to check the stereochemical quality of protein structures[J]. J Appl Crystallogr, 1993, 26(Pt 2): 283−291.
[24] 权丽君. 蛋白质构效关系的计算方法研究[D]. 苏州: 苏州大学, 2017. QUAN L J. Study on applying computing techniques to protein structure-activity relationship[D]. Suzhou: Soochow University, 2017.
[25] 卫莺. 有机锡抗癌化合物与CYP3A4代谢酶的相互作用[D]. 太原: 山西医科大学, 2017. WEI Y. Investigation on the interaction between organotin antitumor compounds with human cytochrome P450 3A4 protease[D]. Taiyuan: Shanxi Medical University, 2017.
[26] GRAHAM L A, DAVIES P L. Glycine-rich antifreeze proteins from snow fleas[J]. Science,2005,310(5747):461. doi: 10.1126/science.1115145
[27] CAO H, ZHAO Y, ZHU Y B, et al. Antifreeze and cryoprotective activities of ice-binding collagen peptides from pig skin[J]. Food Chemistry,2016,194:1245−1253. doi: 10.1016/j.foodchem.2015.08.102
[28] WANG S Y, ZHAO J, CHEN L, et al. Preparation, isolation and hypothermia protection activity of antifreeze peptides from shark skin collagen[J]. LWT-Food Science and Technology,2014,55(1):210−217. doi: 10.1016/j.lwt.2013.07.019
[29] NIKOO M, BENJAKUL S, EHSANI A, et al. Antioxidant and cryoprotective effects of a tetrapeptide isolated from amur sturgeon skin gelatin[J]. Journal of Functional Foods,2014,7:609−620. doi: 10.1016/j.jff.2013.12.024
[30] DAMODARAN S, WANG S Y. Ice crystal growth inhibition by peptides from fish gelatin hydrolysate[J]. Food Hydrocolloids,2017,70:46−56. doi: 10.1016/j.foodhyd.2017.03.029
[31] CHEN X, WU J H, CAI X X, et al. Production, structure-function relationships, mechanisms, and applications of antifreeze peptides[J]. Comprehensive Reviews in Food Science and Food Safety,2021,20(1):542−562. doi: 10.1111/1541-4337.12655
[32] GRIFFITH M, EWART K V. Antifreeze proteins and their potential use in frozen foods[J]. Biotechnology Advances,1995,13(3):375−402. doi: 10.1016/0734-9750(95)02001-J
[33] DAVIES P L, JASON B, KUIPER M J, et al. Structure and function of antifreeze proteins[J]. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences,2002,357(1423):1781−1782.
[34] BAARDSNES J, KUIPER M J, DAVIES P L. Antifreeze protein dimer: When two ice-binding faces are better than one[J]. The Journal of Biological Chemistry,2003,278(40):38942−38947. doi: 10.1074/jbc.M306776200
[35] DALEY M E, SPYRACOPOULOS L, JIA Z, et al. Structure and dynamics of a β-helical antifreeze protein[J]. Biochemistry,2002,41(17):5515−5525. doi: 10.1021/bi0121252
[36] MONHEMI H, HOUSAINDOKHT M R, POUR A N. Effects of natural osmolytes on the protein structure in supercritical CO2: Molecular level evidence[J]. The Journal of Physical Chemistry B,2015,119(33):10406−10416. doi: 10.1021/acs.jpcb.5b03970
[37] CAMPBELL J D, BIGGIN P C, MARC B, et al. Extending the structure of an abc transporter to atomic resolution: Modeling and simulation studies of msba[J]. Biochemistry,2003,42(13):3666−3673. doi: 10.1021/bi027337t
[38] CERUSO M A, AMADEI A, DI N A. Mechanics and dynamics of b1 domain of protein g: Role of packing and surface hydrophobic residues[J]. Protein Science:A Publication of the Protein Society,1999,8(1):147−160.









 下载:
下载:
 下载:
下载:



