Determination of β-Nicotinamide Mononucleotide (NMN) in NMN Cross Border Products
-
摘要: 本文基于高效液相色谱法,通过优化色谱条件和样品前处理条件,建立了一种测定β-烟酰胺单核苷酸(NMN)胶囊和NMN片剂基质中NMN含量的检测方法,并进行方法学验证。结果表明,本方法在5~500 μg/mL范围内有较好的线性关系,方法检出限(LOD,S/N=3)为1.0 mg/kg,定量限(LOQ,S/N=10)为3.0 mg/kg,仪器精密度为0.3%。对于NMN片剂样品,方法稳定性为1.7%,重复性为1.8%;对于NMN胶囊样品,方法稳定性为0.6%,重复性RSD值0.6%。NMN片剂和NMN胶囊样品中NMN的检测回收率在90.9%~108.9%之间,相对标准偏差均不超过1.9%。综上,本方法具有较好的重现性、精密度、稳定性、重复性,结果准确可靠,可用于实际样品中NMN含量的准确测定。通过对6种市售的NMN产品进行检测,所有样品中均检出了NMN,但是部分产品的实际含量较其标称含量偏少,发现部分产品存在虚报NMN含量的问题。本文研究为制定NMN跨境产品中NMN含量的检测方法标准奠定了方法学基础,对于提高NMN相关产品质量,促进相关产业的便捷监管具有深远意义。Abstract: By optimizing the chromatographic conditions and sample pretreatment conditions, a method for determination of NMN content in NMN capsules and NMN tablets matrix was established based on high performance liquid chromatography, and the methodology was verified. The results showed that, the method with range of 5~500 μg/mL had a good linear relationship. The limit of detection (LOD, S/N=3) of NMN was 1.0 mg/kg, while the limit of quantitative (LOQ, S/N=10) was 3.0 mg/kg. The precision of the instrument was 0.3%. For NMN tablet samples, the stability of the method was 1.7% and the repeatability was 1.8%, while for NMN capsule samples, the stability of the method was 0.6%, and the repeatability RSD value was 0.6%. The recovery of NMN in NMN tablets and NMN capsules was between 90.9% and 108.9% with the RSD all no more than 1.9%. In conclusion, this method had good reproducibility, precision, stability and repeatability, could give accurate and reliable results, and could be used for the accurate determination of NMN in actual samples. Through the detection of 6 kinds of commercially available NMN products, NMN was detected in all samples, but the actual contents of some products were less than their nominal content. It was found that some products had the problem of false reporting of NMN content. This study would lay a methodological foundation for formulating the detection method standard of NMN content in NMN related dietary supplement products, and was of far-reaching significance for improving the quality of NMN related products and promote the convenient supervision of related industries.
-
Keywords:
- dietary supplement /
- β-nicotinamide mononucleotide (NMN) /
- HPLC
-
客座主编寄语:民以食为天,食以安为先。食品安全关系群众身体健康,关系中华民族未来。党的十八大以来,习近平总书记发表一系列重要论述,将食品安全作为重大政治任务,纳入国家战略统筹部署推进。进入新发展阶段,我国经济社会变革更加深刻,人民对美好生活的期待更加迫切,改革发展任务更加艰巨,对食品安全工作提出新的更高要求。现阶段,食品安全的定义已涵盖数量安全、质量安全和营养安全。为此本专栏特邀专家对目前国内外食品及相关产品质量安全控制新技术以及食品安全相关法规标准进行研究,旨在完善我国食品及相关产品标准和制度,促进食品产业高质量发展。
(客座主编:兰韬、田明)
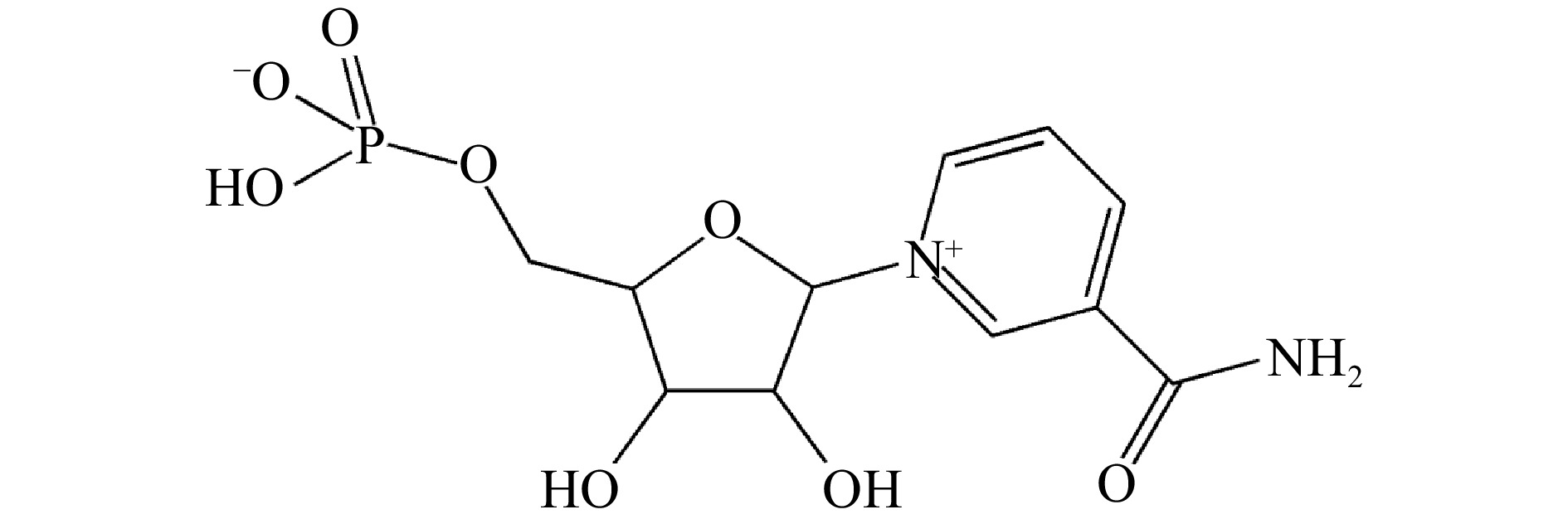
β-烟酰胺单核苷酸(β-nicotinamide mononucleotide,NMN)是一种具有生物活性的核苷酸,由磷酸基和含核苷的核糖与烟酰胺反应自然形成[1],分子结构如图1所示。在动物细胞中,NMN是一种细胞能量来源,是烟酰胺腺嘌呤二核苷酸(nicotinamide adenine dinucleotide,NAD+)生物合成的代谢物,在细胞增殖和功能中起着至关重要的作用[2]。研究表明补充NMN可以提高体内NAD+含量[3],从而延缓、改善、防止衰老诱导的代谢紊乱、老年疾病等[4-9],所以NMN近年来成为生理医学领域的研究热点,并成为医药、功能性食品、化妆品等行业的珍贵原料[10-11]。由于NMN在我国还未获得药品、保健食品、食品添加剂和新食品原料许可,所以主要作为跨境产品通过跨境电商平台进入国内。
人体可自身合成少量NMN[12],也会通过摄食西兰花、卷心菜等果蔬补充NMN[13]。但由于这些果蔬中NMN含量都较少,如果想通过食物来补充NMN非常困难。所以近年来以NMN为主要活性物质的膳食补充剂成为消费市场的宠儿,但是诸多乱象也如影随形,其中最严重的就是部分NMN产品根本不含有NMN或者NMN含量远少于其标称,达到以次充好的目的。目前国内可以在跨境电商平台上购买到NMN跨境产品,但这类产品售价普遍十分高昂,最便宜的也要1500元/瓶,贵的甚至有20000元/瓶[14],如果没有做好产品质量控制,将严重损害消费者权益。所以2021年2月国家市场监管总局食品经营司下发《关于排查违法经营“不老药”的函》(以下简称“排查函”),明确指出目前NMN在我国未获得药品、保健食品、食品添加剂和新食品原料许可,不能作为食品进行生产和经营,要求各个省级市场监管部门对相关经营者进行全面排查。
目前国际上还没有NMN跨境产品中NMN含量测定方法标准,无法对此类产品中NMN含量进行准确测定,就无法对相关食品进行监管。所以非常有必要研制NMN跨境产品中NMN含量的检测方法标准,可以有效解决NMN行业中以次充好问题,实现净化市场的目的,并满足监管需求。我国是NMN原料的主要生产国之一,开发相关标准方法对于促进我国NMN的综合利用具有重要意义。
NMN具有极性大、不易挥发、分子内成盐、易溶于水、难溶于有机溶剂的性质[15-18],前人研究[19]认为,该性质制约了很多常规定量分析方法的应用。常见的定量检测方法包括液相色谱-紫外法(HPLC-UV)[20]、液相色谱-质谱法(HPLC-MS)[21]、毛细管电泳法(CE)[22]、核磁共振波谱法(NMR)[23]。由于在NMN产品中NMN含量较高,通常都在10%以上,HPLC-MS、NMR方法灵敏度高、仪器昂贵,造成检测成本较高,而CE法装机量较少,应用还不够普遍。而HPLC-UV法以其操作简单、普适性好、应用广泛而成为NMN检测的利器。目前对NMN的定量检测主要集中于细胞提取物[24]、果蔬等基质[25],缺乏胶囊、片剂等剂型基质中NMN含量检测方法研究。有鉴于此,本文将通过优化样品的前处理方法和液相色谱方法,开发胶囊、片剂等剂型NMN跨境产品中NMN含量开发相应的检测方法,并通过方法学验证,为建立相应的检测方法标准奠定方法学基础。
1. 材料与方法
1.1 材料与仪器
NMN标准品:β-烟酰胺单核苷酸(C11H15N2O8P)(CAS 1094-61-7,纯度≥98%) 上海源叶生物科技有限公司;甲醇(CH4O) 色谱纯,德国Merck公司;甲酸(CH2O2) 色谱纯,国药集团化学试剂有限公司;6种市售NMN胶囊、NMN片剂产品(其中4种为片剂、2种为胶囊剂) 采购于天猫。
iChrom5100分析型国产液相色谱仪,配DAD检测器 大连依利特分析仪器有限公司;Venusil HILIC色谱柱(4.6 mm×250 mm,5 μm,100 Å) 天津博纳艾杰尔科技有限公司;Thmorgan-VM200型涡旋振荡器 托摩根生物科技有限公司;HITACHI-CF15RXII离心机 日立公司;Sartorius分析天平 赛多利斯科学仪器(北京)有限公司;ELGA纯水仪 北京诚驿恒仪科技有限公司;LMDTC-15F超声波清洗仪 北京绿棉科技有限公司。
1.2 实验方法
1.2.1 溶液配制
1.2.1.1 标准储备液
准确称取10 mg NMN(精确到0.01 mg)标准品置于10 mL容量瓶中,用50%甲醇水溶解并定容至10 mL,配成浓度为1000 μg/mL标准储备液,避光保存于4 ℃冰箱备用,有效期3个月。
1.2.1.2 标准工作溶液
以50%甲醇水溶液为溶剂,将NMN标准储备液稀释至500、250、100、50、25、10、5 μg/mL的系列标准工作液,此溶液需现用现配。
1.2.2 样品前处理
取去除胶囊的粉末状样品100 g于石英研钵中,研磨至碎,过0.18 mm筛备用。
准确称取已研细的去除胶囊的粉末状样品0.5 g(精确到0.001 g)于50 mL容量瓶中,加入25 mL 50%甲醇水溶解,超声20 min使其充分溶解,提取两次,合并提取液,以10000 r/min的速度离心5 min,取上清液经0.22 µm有机滤膜过滤,取250 μL样品溶液用50%甲醇水定容至10 mL后进样检测。
片剂样品前处理方法同上。
1.2.3 液相色谱条件
液相色谱柱为Venusil HILIC (4.6 mm×250 mm,5 μm,100 Å),进样体积为10 μL,流速1 mL/min,柱温:35 ℃,检测波长为235 nm,流动相A为0.1%甲酸水溶液,流动相B为0.1%甲酸甲醇溶液,采用等度洗脱,流动相A:流动相B=15:85(V/V)。
1.2.4 标准曲线的绘制
将配制好的5、10、25 、50 、100、250、500 μg/mL标准工作溶液按“1.2.3”的液相色谱条件进样分析,以NMN的浓度为横坐标,峰面积响应值为纵坐标,绘制系列标准曲线,进行线性回归,得到NMN的标准曲线线性回归方程。
1.2.5 精密度试验
在相同仪器条件下,取90 μg/mL浓度的标准工作溶液,按“1.2.3”色谱条件重复进样6次,测得各被检测组分的峰面积,代入标准曲线线性回归方程得出测定浓度值,再计算测定浓度的相对标准偏差RSD。
1.2.6 稳定性试验
在相同仪器条件下,精密称取0.5 g(精确到0.001g)已粉碎的片剂样品以及胶囊样品以“1.2.2”的前处理方法,制成两种基质的提取液,在同一天内,以“1.2.3”色谱条件进样分析,每2 h测定一次,总共测定6次,测得被检测组分的峰面积,代入标准曲线线性回归方程得出测定浓度值,再计算测定浓度的相对标准偏差RSD。
1.2.7 重复性试验
在相同的仪器条件下,精密称取0.5 g(精确到0.001 g)已粉碎的片剂和胶囊样品6份,按“1.2.2”前处理方法方法操作,将处理好的待测液按“1.2.3”色谱条件进样分析,测得样品中NMN的峰面积,代入标准曲线方程,计算测定浓度的标准偏差RSD。
1.2.8 定量限、检出限的测定
将“1.2.1.2”的标准工作溶液按“1.2.3”的液相色谱条件测定,测得NMN的峰面积,代入相应物质的标准曲线回归方程中。以最低水平标准溶液中目标组分的3倍平均信噪比为检出限(LOD),10倍平均信噪比为定量限(LOQ),计算得本方法的LOD和LOQ。
1.2.9 加标回收率试验
准确称取0.5 g(精确到0.001 g)已粉碎的片剂样品、胶囊样品各12份,编号1~12,其中1~3号作为本底(基质样品),加入一定量标准储备液,4~6号为加标量为5 μg/mL的待测液,7~9号为加标量为10 μg/mL的待测液,10~12号为加标量为50 μg/mL的待测液,按照“1.2.2”操作,将处理好的待测液以及低、中、高3种浓度的加标样品经HPLC分析,测得峰面积,计算回收率。
1.3 数据处理
本文标准曲线线性回归方程及相关系数由依利特iChrom5100 workstation处理所得,其他数据采用Origin 6.0和Excel 2010软件对数据进行处理并作图,实验数据取3次平行实验的平均值。
2. 结果与分析
2.1 液相色谱条件优化
经查阅文献[26-27],研究者们通常采用反相C18色谱柱来实现NMN的分离,但由于NMN的极性较大,几乎没有保留,出峰时间过早,容易与样品基质峰发生混淆。所以本文考虑使用对于极性样品分离效果较好的HILIC色谱柱[28-29]进行样品的分离。
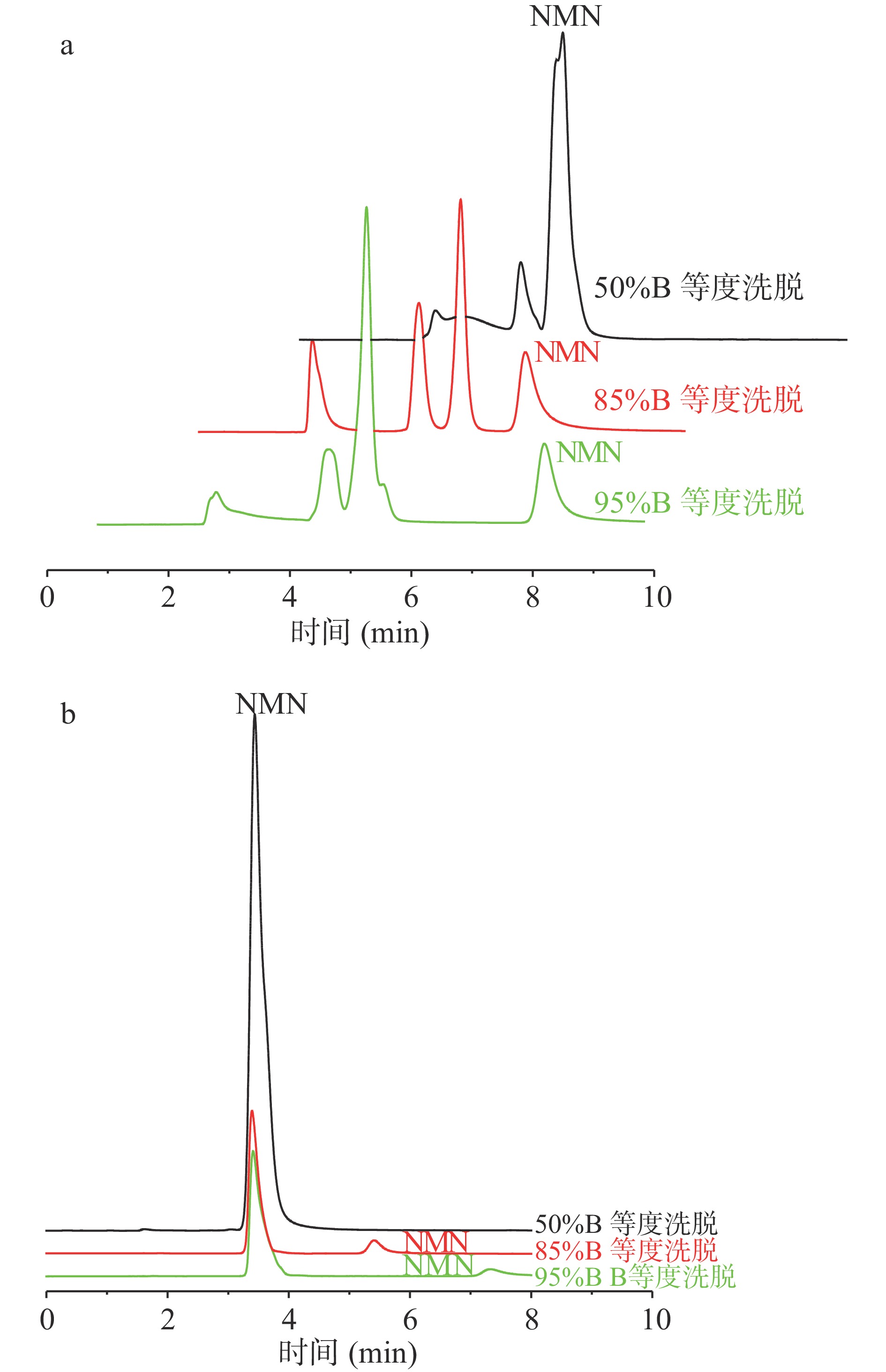
为尽可能地将待测的NMN与胶囊和片剂样品基质中其他成分分离开,首先对流动相溶液配比进行了优化。用50%甲醇水溶液作为提取液溶解胶囊和片剂样品配制成待测液,分别以95% B、85% B、50% B(V/V)的等度分离方式对NMN胶囊和片剂样品进行色谱分离,通过单标法对峰的归属进行了确证,由此判断检测时最佳的流动相比例。分离谱图如图2所示,其中图2a为NMN片剂样品的分离谱图,图2b为NMN胶囊的样品分离谱图。从图中可以看出,随着流动相中甲醇含量的增大,NMN的出峰时间逐渐后移。对于片剂样品以50%比例等度洗脱时,NMN与基质无法得到有效分离,而使用95% B比例等度洗脱时NMN的出峰时间较晚,影响分析方法的效率,而且也伴随着峰展宽。当使用85% B比例等度洗脱时分离效果最好,NMN的出峰时间较短,峰形也基本满足高斯分布,可以达到基质中杂质峰与被分析物色谱峰分离的效果,降低了杂质峰干扰的可能,从而省去对提取液进行净化处理的步骤,可以将分析方法的总体时间控制在8 min内完成,使得操作简便、耗时较短,提高了分析检测效率。对于基质较为简单的胶囊样品,可以看出同样使用85% B比例等度洗脱,具有最好的分离效果、峰形和分析效率。所以采用85% B比例等度洗脱进行后续实验。
对于可离子化的样品,甲酸具有改善峰形的作用[30]。因此,本文对流动相中甲酸的加入进行优化对比,在85%比例等度洗脱条件下,考察了0.1%甲酸加入前后NMN片剂和NMN胶囊剂样品基质中各被测组分分离效果,结果如图3所示。结果表明,不加甲酸,NMN的峰拖尾现象明显,而且出峰时间明显推迟了8 min。而加入甲酸使各被测组分的峰形得到了良好的改善,并将整个分析时间控制在8 min内,极大地提高了分析效率。这可能是由于甲酸的加入抑制了NMN的解离,降低了其在HILIC色谱填料上的保留,缩短了出峰时间。所以在流动相中加入甲酸进行后续实验。
综上,对于NMN片剂和NMN胶囊基质,采用添加甲酸的方式能够改善NMN峰形,因此以0.1%甲酸水溶液-0.1%甲酸甲醇溶液(15:85,V/V)为流动相进行分离。
2.2 提取溶剂优化
根据NMN的理化性质[10-12],NMN在水中溶解度较大,在甲醇中溶解度较小,在仪器及实验条件相同情况下,本实验选择了水溶液、50%甲醇水溶液、85%甲醇水溶液3种溶剂开展样品提取溶剂优化实验。由于NMN在甲醇中几乎不溶,故不选择用纯甲醇作为提取溶剂。取0.5 g粉碎后的片剂和胶囊样品,向其中各添加一定量的NMN标准储备液,使加标浓度为10 μg/mL,采用“1.2.2”的样品提取方式,分别以上述3种溶剂进行提取,并测定加标回收率,结果如下表1所示。从表1中可以看出,以50%甲醇水溶液作为提取溶剂,样品的回收率可控制在90%~110%之间,同时偏差最小,具有最佳的提取效果。因此,在后续实验中采用50%甲醇水提取溶剂进行提取。
表 1 不同溶剂对NMN提取效率的影响(n=3)Table 1. Effects of different solvents on the extraction efficiency of NMN (n=3)标准品 水溶液 85%甲醇水溶液 50%甲醇水溶液 回收率(%) RSD(%) 回收率(%) RSD(%) 回收率(%) RSD(%) NMN 70.5 1.1 65.3 7.6 95.7 0.4 2.3 标准曲线、检出限、定量限
以“1.2.3”中的色谱分析条件对NMN标准工作溶液进行测定,通过iChrom5100 workstation绘制标准曲线回归方程,其线性范围、相关系数、LOD及LOQ如表2所示。
表 2 NMN的线性范围、LOD和LOQTable 2. Standard curve, linear range, LOD and LOQ of NMN标准品 保留时间(min) 线性方程 r 线性范围(μg/mL) LOD(mg/kg) LOQ(mg/kg) NMN 5.53 Y=6.76X−13.44 0.999 5.0~500.0 1.0 3.0 由表2数据可知,本方法在5~500 μg/mL范围内有较好的线性关系,相关系数r为0.999。经计算,NMN的检出限(LOD,S/N=3)为1.0 mg/kg,定量限(LOQ,S/N=10)为3.0 mg/kg。
2.4 仪器精密度实验
按照“1.2.5”中实验步骤,对浓度为90 μg/mL的NMN标准溶液进行6次平行测定,将测得的NMN的响应值带入线性方程计算得到溶液中NMN的含量,并计算6次测定结果的RSD如表3所示。
表 3 精密度实验结果(μg/mL)Table 3. Precision test results (μg/mL)标准品 1 2 3 4 5 6 RSD(%) NMN 86.4 86.1 86.3 86.1 86.9 86.4 0.3 由表3数据可知,仪器精密度实验结果的RSD为0.3%,表明本文发展的液相色谱方法具有较好的重现性,方法精密度较好,可以用于后续实际样品中NMN含量的测定。
2.5 稳定性实验
以“1.2.6”中实验步骤进行操作,考察方法的稳定性,经过6次平行实验,测得片剂和胶囊样品中NMN的浓度与RSD如表4所示。
表 4 稳定性实验结果(mg/kg)Table 4. Results of stability experiments (mg/kg)基质 1 2 3 4 5 6 RSD (%) NMN(片剂) 40.7 40.9 41.8 41.9 41.8 40.3 1.7 NMN(胶囊) 141.0 142.3 142.0 142.4 142.9 143.4 0.6 由表4数据可知,对于NMN片剂和NMN胶囊,6次测定结果RSD均较小,说明结果都保持高度一致,可以保证方法的稳定性。
2.6 重复性实验
按照“1.2.7”中的实验步骤,将测得的NMN片剂、胶囊原液的6组平行样品中NMN的浓度及RSD计算汇总,结果如下表所示。
由表5数据可知,两种样品重复性实验的RSD在0.6%~1.8%之间,表明本方法重复性较好,表明本文开发的前处理方法结合HPLC方法可以用于NMN片剂和NMN胶囊中的NMN含量测定,并能使结果具有良好的重复性。
表 5 重复性实验结果(mg/kg)Table 5. Results of repeatability experiment (mg/kg)基质 1 2 3 4 5 6 RSD (%) 片剂 190.7 193.8 193.3 199.7 190.1 191.4 1.8 胶囊 264.4 262.3 262.7 263.3 260.2 263.7 0.6 2.7 加标回收率实验
按“1.2.9”中步骤进行操作,对制备的5、10、50 mg/kg的低、中、高三种加标水平的NMN片剂和NMN胶囊剂样品中NMN含量进行了测定,并计算回收率和RSD%,结果如表6所示。
表 6 加标回收率实验结果Table 6. Experimental results of standard recovery样品 本底值
(mg/kg)5 mg/kg 10 mg/kg 50 mg/kg 回收率
(%)RSD
(%)回收率
(%)RSD
(%)回收率
(%)RSD
(%)NMN片剂 31.8 95.7 0.8 95.7 0.6 108.9 0.2 NMN胶囊 41.2 90.9 0.2 93.1 0.2 94.4 1.9 由表6数据可知,NMN片剂和NMN胶囊样品中NMN的检测回收率在90.9%~108.9%之间,相对标准偏差均不超过1.9%,表明本方法回收率好,结果准确可靠,本方法可用于实际样品中NMN含量的准确测定。
2.8 实际样品测定
按照本文发展的NMN片剂和NMN胶囊剂样品基质中NMN含量的检测方法对市售6个品牌的NMN产品中的NMN含量进行分析测试,检测结果见表7。
表 7 6种不同NMN产品中NMN含量(g/kg,n=3)Table 7. NMN content in 6 different NMN products (g/kg, n=3)序号 NMN(测定值) 产品标称 1 65.3 75.0 2 268.0 520.3 3 68.2 188.3 4 486.0 500.0 5 197.0 未标称 6 493.0 500.0 实际样品分析结果表明,所有样品中都检出了NMN,但是部分产品的实际含量较其标称含量偏少,如2号样品中NMN含量只有其标称值的一半,3号样品中NMN含量只有其标称值的三分之一,涉及虚假宣称。
3. 结论
本文建立了一种NMN胶囊和NMN片剂基质中NMN含量的检测方法,方法通过调节色谱分离条件,使目标组分与基质成分完全分离,无需进行样品净化操作,同时控制流动相组成,整个分析时间控制在8 min内,具有操作简便、检测效率高、准确性好等优点。该方法为制定NMN跨境产品中NMN含量的检测方法标准奠定了方法学基础,对于提高NMN相关产品质量,促进相关产业的便捷监管具有深远意义。
-
表 1 不同溶剂对NMN提取效率的影响(n=3)
Table 1 Effects of different solvents on the extraction efficiency of NMN (n=3)
标准品 水溶液 85%甲醇水溶液 50%甲醇水溶液 回收率(%) RSD(%) 回收率(%) RSD(%) 回收率(%) RSD(%) NMN 70.5 1.1 65.3 7.6 95.7 0.4 表 2 NMN的线性范围、LOD和LOQ
Table 2 Standard curve, linear range, LOD and LOQ of NMN
标准品 保留时间(min) 线性方程 r 线性范围(μg/mL) LOD(mg/kg) LOQ(mg/kg) NMN 5.53 Y=6.76X−13.44 0.999 5.0~500.0 1.0 3.0 表 3 精密度实验结果(μg/mL)
Table 3 Precision test results (μg/mL)
标准品 1 2 3 4 5 6 RSD(%) NMN 86.4 86.1 86.3 86.1 86.9 86.4 0.3 表 4 稳定性实验结果(mg/kg)
Table 4 Results of stability experiments (mg/kg)
基质 1 2 3 4 5 6 RSD (%) NMN(片剂) 40.7 40.9 41.8 41.9 41.8 40.3 1.7 NMN(胶囊) 141.0 142.3 142.0 142.4 142.9 143.4 0.6 表 5 重复性实验结果(mg/kg)
Table 5 Results of repeatability experiment (mg/kg)
基质 1 2 3 4 5 6 RSD (%) 片剂 190.7 193.8 193.3 199.7 190.1 191.4 1.8 胶囊 264.4 262.3 262.7 263.3 260.2 263.7 0.6 表 6 加标回收率实验结果
Table 6 Experimental results of standard recovery
样品 本底值
(mg/kg)5 mg/kg 10 mg/kg 50 mg/kg 回收率
(%)RSD
(%)回收率
(%)RSD
(%)回收率
(%)RSD
(%)NMN片剂 31.8 95.7 0.8 95.7 0.6 108.9 0.2 NMN胶囊 41.2 90.9 0.2 93.1 0.2 94.4 1.9 表 7 6种不同NMN产品中NMN含量(g/kg,n=3)
Table 7 NMN content in 6 different NMN products (g/kg, n=3)
序号 NMN(测定值) 产品标称 1 65.3 75.0 2 268.0 520.3 3 68.2 188.3 4 486.0 500.0 5 197.0 未标称 6 493.0 500.0 -
[1] PAN F, KANG S F, ZHAO Y F, et al. Effect of β-nicotinamide mononucleotide on tumor formation and growth in a lung cancer mouse model[J]. Materials Chemistry Frontiers,2021,5:995−1002. doi: 10.1039/D0QM00897D
[2] TAMAS K, PRIYA B, STEFANO T, et al. Nicotinamide mononucleotide (NMN) treatment attenuates oxidative stress and rescues angiogenic capacity in aged cerebromicrovascular endothelial cells: A potential mechanism for the prevention of vascular cognitive impairment[J]. GeroScience,2019,41:619−630. doi: 10.1007/s11357-019-00074-2
[3] MARINESCU G C, POPESCU R G, STOIAN G, et al. β-Nicotinamide mononucleotide (NMN) production in Escherichia coli[J]. Scientific Reports,2018,8:12278. doi: 10.1038/s41598-018-30792-0
[4] RAJMAN L, CHWALEK K, SINCLAIR D A. Therapeutic potential of NAD-boosting molecules: The in vivo evidence[J]. Cell Metabolism,2018,27(3):529. doi: 10.1016/j.cmet.2018.02.011
[5] OKABE K, YAKU K, TOBE K, et al. Implications of altered NAD metabolism in metabolic disorders[J]. Journal of Biomedical Science,2019,26(1):1−2. doi: 10.1186/s12929-018-0495-4
[6] LI C, ZHOU Y N, RYCHAHOU P, et al. SIRT2 contributes to the regulation of intestinal cell proliferation and differentiation[J]. Cellular and Molecular Gastroenterology and Hepatology,2020,10(1):43−57. doi: 10.1016/j.jcmgh.2020.01.004
[7] ZHANG R, SHEN Y Y, ZHOU L, et al. Short-term administration of nicotinamide mononucleotide preserves cardiac mitochondrial homeostasis and prevents heart failure[J]. Journal of Molecular & Cellular Cardiology,2017,112:64.
[8] LI J, BONKOWSKI M S, MONIOT S, et al. A conserved NAD+ binding pocket that regulates protein-protein interactions during aging[J]. Science,2017,355(6331):1312−1317. doi: 10.1126/science.aad8242
[9] DAS A, HUANG G X, BONKOWSKI M S, et al. Impairment of an edothelial NAD+-H2S signaling network is a reversible cause of vascular Aging[J]. Cell,2018,173:74−89. doi: 10.1016/j.cell.2018.02.008
[10] 邓军, 罗统有, 刘道甫, 等, 烟酰胺核苷酸临床研究进展及化学制备方法[J]. 广东化工, 2021, 12: 98−100. DENG J, LUO T Y, LIU D F, et al. Clinical research and chemical preparation progress of nicotinamide nucleotide [J]. Guangdong Chemical Inustry, 2021, 12: 98−100.
[11] 任丽梅, 王晓茹, 祁永浩, 等. β-烟酰胺单核苷酸功能与合成研究进展[J]. 生物资源, 2021, 43(2): 127−132. REN L M, WANG X R, QI Y H, et al. Research progress on function and synthesis of β-nicotinamide mononucleotide[J]. Biotic Resources, 2021, 43(2): 127−132.
[12] 史海波, 赵海, 周春松, 等 β-烟酰胺单核苷酸制备研究进展[J]. 精细化工中间体, 2020, 50(4): 1−5. SHI H B, ZHAO H, ZHOU X S, et al. Progress in synthsis of β-nicotinamide mononucleotide[J]. Fine Chemical Intermediates, 2020, 50(4): 1−5.
[13] LIN J B, KUBOTA S, BAN N, et al. NAMPT-mediated NAD+ biosynthesis is essential for vision in mice[J]. Cell Reports,2016,17(1):69−85. doi: 10.1016/j.celrep.2016.08.073
[14] 王大宏, 关雪峰, 江斌, 等. NMN品类发展白皮书[EB/OL]. [2020-11-1]. http://www.shuzheng.com/2020%20NMN%E5%93%81%E7%B1%BB%E5%8F%91%E5%B1%95%E7%99%BD%E7%9A%AE%E4%B9%A6.pdf [15] UDDIN G M, YOUNGSON N A, DOYLE B M, et al. Nicotinamide mononucleotide (NMN) supplementation ameliorates the impact of maternal obesity in mice: Comparison with exercise[J]. Scientific Reports,2017,7(1):1−9. doi: 10.1038/s41598-016-0028-x
[16] YAMAMOTO T, BYUN J, ZHAI P, et al. Nicotinamide mononucleotide, an intermediate of NAD+ synthesis, protects the heart from ischemia and reperfusion[J]. PLoS One,2014,9(6):e98972. doi: 10.1371/journal.pone.0098972
[17] HOSSEINI L, FAROKHI-SISAKHT F, BADALZADEH R, et al. Nicotinamide mononucleotide and melatonin alleviate aging-induced cognitive impairment via modulation of mitochondrial function and apoptosis in the prefrontal cortex and hippocampus[J]. Neuroscience,2019,423:29−37. doi: 10.1016/j.neuroscience.2019.09.037
[18] SPINNLER R, GORSKI T, STOLZ K, et al. The adipocytokine nampt and its product NMN have no effect on beta-cell survival but potentiate glucose stimulated insulin secretion[J]. PLoS one,2013,8(1):e54106. doi: 10.1371/journal.pone.0054106
[19] YOSHINO J, IMAI S I. Accurate measurement of nicotinamide adenine dinucleotide (NAD+) with high-performance liquid chromatography[J]. Methods in Molecular Biology,2013,1077:203−215.
[20] LIU Y, YASAWONG M, YU B, Metabolic engineering of Escherichia coli for biosynthesis of β-nicotinamide mononucleotide from nicotinamide[J]. Microbial Biotechnology, 2021, 14(6): 2581−2591.
[21] MILLS K F, YOSHIDA S, STEIN L R, et al. Long-term administration of nicotinamide mononucleotide mitigates age-associated physiological decline in mice[J]. Cell Metabolism,2016,24:1−12. doi: 10.1016/j.cmet.2016.06.019
[22] LONG A N, OWENS K, SCHLAPPAL A E, et al. Effect of nicotinamide mononucleotide on brain mitochondrial respiratory deficits in an alzheime’s disease-relevant murine model[J]. BMC Neurology,2015,15(1):1−14. doi: 10.1186/s12883-014-0245-5
[23] 孟辰笑凝, 郭中原, 陈两绵, 等. 一种氢核磁定量分析技术测定NMN绝对含量的方法: 中国, 110658224A [P]. 2020-01-07. MENG C X N, GUO Z Y, CHEN L M, et al. A method for determining the absolute content of NMN by hydrogen nuclear magnetic quantitative analysis technology: China, 110658224A [P]. 2020-01-07.
[24] FORMENTINI L, MORONI F, CHIARUGI A. Detection and pharmacological modulation of nicotinamide mononucleotide (NMN) in vitro and in vivo[J]. Biochemical Pharmacology,2009,77(10):1612−1620. doi: 10.1016/j.bcp.2009.02.017
[25] 李东芹. 液质联用法测定蔬菜和水果中的烟酰胺单核苷酸[J]. 实验技术与管理[J]. 实验技术与管理, 2019, 36(9): 57-72. LI D Q. Determination of nicotinamide mononucleotide in vegetables and fruits by LC-MS[J]. Experimental Technology & Management, 2019, 36(9): 57-72.
[26] YAMADA K, HARA N, SHIBATA T. The simultaneous measurement of nicotinamide adenine dinucleotide and related compounds by liquid chromatography/electrospray ionization tandem mass spectrometry[J]. Analytical Biochemistry, 2006, 352(2):282-285.
[27] MARINESCU G C, POPESCU R G, DINISCHIOTU A. Size Exclusion chromatography method for purification of nicotinamide mononucleotide (NMN) from bacterial cells[J]. Scientific Reports,2018,8:4433. doi: 10.1038/s41598-018-22806-8
[28] 李欣, 姜燕, 郝旺青. 亲水作用色谱在中药极性成分分析中的应用进展[J]. 沈阳药科大学学报, 2020(8): 764-768. LI X, JIANG Y, HAO W Q. Application of hydrophilic interaction chromatography in the analysis of polar components of Chinese medicine[J]. Journal of Shenyang Pharmaceutical University, 2020(8): 764-768.
[29] GINER M P, CHRISTEN S, BARTOVA S, et al. A method to monitor the NAD+ metabolome from mechanistic to clinical applications[J]. International Journal of Molecular Sciences,2021,22(19):10598. doi: 10.3390/ijms221910598
[30] 黄芳, 黄晓兰, 吴惠勤, 等. 高效液相色谱-质谱法对饲料及食品添加剂中三聚氰胺的测定[J]. 分析测试学报,2008,27(3):313−315. [HUANG F, HUANG X L, WU H Q, et al. Determination of melamine in feed and food additives by high performance liquid chromatography-mass spectrometry[J]. Journal of Analysis and Testing,2008,27(3):313−315. doi: 10.3969/j.issn.1004-4957.2008.03.024 -
期刊类型引用(10)
1. 舒丽枝,时苗苗,张牧焓,卞欢,徐为民,王道营. 卟啉类化合物和游离铁对鸡胸肉肌原纤维蛋白理化特性的影响. 江苏农业学报. 2024(10): 1952-1961 .  百度学术
百度学术
2. 王晓芸,高霞,尤娟,尹涛,刘茹. 超声预处理对鲜湿鱼粉品质的影响及其作用机制. 食品科学. 2024(23): 213-220 .  百度学术
百度学术
3. 韩馨蕊,李颖,刘苗苗,范鑫,冯莉,曹云刚. 安石榴苷与焦磷酸钠对肌原纤维蛋白氧化稳定性及凝胶性能的影响. 食品科学. 2022(08): 15-21 .  百度学术
百度学术
4. 莫玲,香庆文,李晶晶,叶玉萍,赵超超. 孕哺期摄入氧化乳蛋白对子代小鼠机体氧化还原状态的影响. 食品科学技术学报. 2021(03): 122-128 .  百度学术
百度学术
5. 梁恽红,卢涵,张香美. 蛋白二、三级结构对鱼糜凝胶质构和持水力的影响及其测定方法研究进展. 东北农业大学学报. 2021(10): 87-96 .  百度学术
百度学术
6. 谢晨,熊泽语,李慧,金素莱曼,陈百科,包海蓉. 金针菇多糖对三文鱼片冻藏期间品质的影响. 食品与发酵工业. 2021(22): 178-183 .  百度学术
百度学术
7. 刘芳芳,林婉玲,李来好,吴燕燕,杨少玲,黄卉,杨贤庆,林织. 海鲈鱼糜加工及凝胶形成过程中蛋白质的变化机理. 食品科学. 2020(14): 15-22 .  百度学术
百度学术
8. 冯程,Manonose Tariro Upenyu,李志豪,王萍,余雄伟,付琴利,李述刚. 丙烯醛对籽瓜种仁蛋白质结构及凝胶特性影响研究. 食品科技. 2019(09): 66-71 .  百度学术
百度学术
9. 刁小琴,关海宁,李杨,刘丽美. 高压均质对肌原纤维蛋白乳化特性及结构的影响. 食品与发酵工业. 2019(18): 107-112 .  百度学术
百度学术
10. 郭兆斌,马纪兵,张丽,陈骋,陈立业,刘勇,韩玲,余群力. 传统风干牦牛肉加工过程中肌原纤维蛋白氧化对氨基酸的影响. 食品与发酵工业. 2019(22): 202-207+212 .  百度学术
百度学术
其他类型引用(4)





 下载:
下载:

 下载:
下载:
