Purification and Composition of Polysaccharides from Pseudostellaria heterophylla and Protective Effect on Raw 264.7 Cells Induced by LPS
-
摘要: 太子参是我国传统中药材,为明确其多糖的组成与对Raw 264.7巨噬细胞的保护作用,本研究采用水提醇沉法提取其主要活性成分多糖,并通过阴离子交换柱层析纯化得太子参多糖(Pseudostellaria heterophylla polysaccharide,PHP);利用凝胶渗透色谱(GPC)法、高效液相色谱(HPLC)法测定其分子量、单糖组成,紫外光谱扫描、傅里叶红外光谱扫描初步解析其结构特征。进一步通过小鼠单核巨噬细胞白血病细胞(Raw 264.7)模型探究了PHP对LPS诱导损伤的保护作用。结果表明,太子参粗多糖的得率为8.23%,纯度为51.47%,而经DEAE-52柱层析分离纯化后得的PHP纯度达到了80.52%;PHP的分子量在500~92×103 Da之间,单糖组成为葡萄糖、半乳糖、阿拉伯糖、甘露糖及葡萄糖醛酸。细胞实验确定80~640 µg/mL为PHP的安全剂量,此浓度下可以提高脂多糖(Lipopolysaccharide,LPS)诱导损伤下的细胞存活率,640 µg/mL的PHP剂量下存活率最大提升了7.21%,并且PHP可改善Raw 264.7巨噬细胞形态。综上,本研究提纯了太子参中多糖,对PHP进行了结构表征,并初步证实了PHP对LPS诱导损伤的Raw 264.7巨噬细胞有保护作用,这为太子参多糖的功能活性探索及构效、量效等深入研究奠定了基础。
-
关键词:
- 太子参多糖 /
- 单糖组成 /
- Raw 264.7巨噬细胞 /
- 保护作用
Abstract: To determine the composition and protective effect on Raw 264.7 cells of polysaccharide from Pseudostellaria heterophylla as a traditional Chinese medicinal, the main active ingredient-Pseudostellaria heterophylla polysaccharide (PHP) was extracted by water, precipitated by ethanol, and purified by anion exchange column chromatography. Gel permeation chromatography (GPC) and high performance liquid chromatography (HPLC) were used to determine molecular weight and monosaccharide composition of PHP. Ultraviolet spectroscopy (UV) and Fourier transform infrared spectroscopy (FT-IR) were used for primary structural characterization of PHP. Furthermore, the Raw 264.7 cell model was used to explore protective effect of PHP on Raw 264.7 cells induced by LPS. The results showed that the yield of crude polysaccharide of Pseudostellaria heterophylla was 8.23%, and the polysaccharide content was 51.47%. After purification by DEAE-52 column chromatography, the polysaccharide content increased to 80.52%, and the molecular weight distribution was between 500 to 92×103 Da. The monosaccharide components of PHP include glucose, galactose, arabinose, mannose and glucuronic acid. Cell experiments showed that the safe dose of PHP was 80~640 µg/mL and could increase the survival rate of cells induced by lipopolysaccharide (LPS). At a dose of 640 µg/mL, the survival rate increased by 7.21%, and it was found that PHP could improve the morphology of Raw 264.7 cells. In summary, in this study the polysaccharides from Pseudostellaria heterophylla were purified, the structure of PHP was characterized, and it was preliminarily confirmed that PHP had potential protective effect on LPS-induced damage to Raw 264.7 cells. This laid the foundation for the follow-up exploration of functional activity, structure-effect and dose-effect of PHP. -
太子参是石竹科植物孩儿参Pseudostellaria heterophylla (Miq.) Pax ex Pax et Hoffm.干燥后的块根,具有益气、健脾润肺之功效[1]。近30年,太子参的年需求量表现出强劲的增长趋势,支撑其市场需求不断攀升的背后,是太子参较为稳定、温和的功能性。现有研究表明,太子参有抗肿瘤[2-3]、免疫调节[4-5]、降血糖[6-7]、抗氧化应激[8]、保护心肌[9]等保健功效。目前公认多糖是太子参中最主要的活性物质之一,然而有关太子参多糖的研究大多集中在粗多糖的功能活性评价上,缺乏前期的纯化工艺、理化性质研究基础,其功能、药理活性的物质基础和指标性成分不够明确[10-11],这给太子参多糖资源的开发和利用带来了局限。
免疫系统是一个错综复杂又重要的生理体系,伴随着免疫系统的钝化和免疫应答的失衡,各种疾病接踵而至[12],通过调节免疫来预防或治疗疾病已成为研究热点之一。已有研究表明太子参在免疫调节方面表现出潜力,陈耀金等[4]利用太子参胶囊粉灌胃小鼠,结果表明其对免疫功能低下小鼠的免疫力有增强效果;并且张炎达等[5]从太子参须获得的粗提物也表现出显著改善小鼠免疫功能。由于小鼠单核巨噬细胞白血病细胞(Raw 264.7)体外培养过程中也具备对抗原的较强吸附与吞噬能力,并释放相关的免疫调节因子,在一定程度上能够模拟体内巨噬细胞有关的免疫调节作用,是该类研究中常用的模型细胞[13]。因此,本研究提纯了太子参多糖,并测定了太子参多糖的分子量与组成以及初步表征其结构,以此基础利用Raw 264.7细胞模型初探了太子参多糖对脂多糖(Lipopolysaccharide, LPS)损伤Raw264.7巨噬细胞的保护作用,以期促进太子参资源的开发应用。
1. 材料与方法
1.1 材料与仪器
太子参 安徽皖南烟叶公司太子参种植基地提供,采收年份为2019年,经晒干后直接运回实验室,于−20 ℃保存留用;小鼠单核巨噬细胞系Raw 264.7 中国科学院细胞库提供;DEAE Cellulose-52 北京拜尔迪生物技术有限公司;单糖标准品(葡萄糖、半乳糖、木糖、阿拉伯糖、岩藻糖、甘露糖、鼠李糖、葡萄糖醛酸、半乳糖醛酸、核糖、N-乙酰-氨基葡萄糖、N-乙酰-氨基半乳糖) 上海源叶生物科技有限公司;葡聚糖分子量标准品(1、3、12、70、126、287 kDa)、青霉素-链霉素双抗(100×)、噻唑蓝(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide,MTT)、二甲基亚砜(dimethyl sulfoxide,DMSO) 北京索莱宝科技有限公司;脂多糖(lipopolysaccharide,LPS) Sigma公司;DMEM高糖培养基 美国Gibco公司;类胎牛血清 美国HyClone公司。
紫外可见分光光度计 北京普析通用仪器有限责任公司;IR Tracer-10傅里叶变换红外光谱仪 岛津企业管理(中国)有限公司;U3000高效液相色谱仪 赛默飞世尔科技公司;Agilent 1206高效液相色谱仪 安捷伦科技有限公司;XSP-C204光学显微镜 CIC公司;RT-6100酶标仪 雷杜生命科学股份有限公司。
1.2 实验方法
1.2.1 太子参粗多糖的提取
参考Hu等[14]的方法,并稍作修改:太子参破碎成粉,过40目筛,以蒸馏水为溶剂,100 ℃水浴提取120 min(1:8,m/v),8000 r/min离心15 min,取上清液备用,残渣按照相同方法反复提取共3次,合并上清液。旋蒸浓缩、Sevag法除蛋白后加入无水乙醇配成终浓度为80%的乙醇溶液,于4 ℃条件下沉淀过夜12 h,8000 r/min离心5 min后,收集沉淀,冷冻干燥,得太子参粗多糖。
1.2.2 太子参粗多糖的纯化工艺优化
DEAE Cellulose-52是一种弱碱性阴离子填料,当粗多糖溶液通过时,中性多糖可以流出,酸性多糖吸附在色谱物质中,从而实现对中性与酸性多糖的有效分离[15]。因此,本研究使用DEAE Cellulose-52填料进行粗多糖的纯化,参考雷思敏等[16]的方法,并稍作修改:以DEAE Cellulose-52为柱填料,Tris-HCl缓冲液溶解粗多糖上样,上样粗多糖浓度为1 mg/mL,采用NaCl盐溶液进行洗脱,流速为2.0 mL/min,10 mL/tube收集,苯酚-硫酸法跟踪检测洗脱液,绘制洗脱曲线。
1.2.2.1 pH的选择
预实验测定了缓冲液环境为pH7.2~7.8的洗脱效果,其中pH7.6的效果较佳。在预实验结果的基础上,准确称取太子参粗多糖样品25.0 mg,分别溶于25 mL pH 7.4、7.6、7.8的Tris-HCl缓冲液中,0.45 µm微孔滤膜过滤后缓慢上样,吸附15 min,用0~0.3 mol/L NaCl的Tris-HCl缓冲液洗脱。
1.2.2.2 洗脱盐浓度的选择
参考1.2.2.1中步骤,参数加以修改:pH7.6缓冲液条件下,分别含0~0.1(0、0.1)、0~0.3(0、0.1、0.2、0.3)、0~0.5(0、0.1、0.2、0.3、0.4、0.5)mol/L NaCl的Tris-HCl缓冲液梯度洗脱,浓度梯度为0.1 mol/L。
1.2.3 太子参多糖(PHP)的理化特征及结构表征
1.2.3.1 紫外光谱扫描
称取适量冷冻干燥后的PHP,用去离子水配制成100 µg/mL的PHP溶液,在25 ℃,波长190~400 nm范围的紫外扫描仪中进行扫描。
1.2.3.2 红外光谱扫描
称取2 mg冷冻干燥后的PHP,与100 mg经120 ℃烘干4 h的溴化钾研磨混合后压片,在4000~400 cm−1的范围进行红外光谱扫描[17]。
1.2.3.3 分子量检测
采用凝胶渗透色谱(Gel permeation chromatography,GPC)测定PHP的分子量分布[18]。具体如下:色谱仪为Agilent 1206,选用Agilent PL aquageL-OH MIXED-M色谱柱(7.5 mm×300 mm, 8 μm),检测器为示差折光检测器,进样量为20 μL,流动相为0.1 mol/L NaNO3溶液,流速为1.0 mL/min,柱温为30 ℃。
1.2.3.4 单糖组成
采用1-苯基-3-甲基-5-吡唑啉酮(1-phenyl-3-methyl-5-pyrazolone,PMP)柱前衍生化结合U3000高效液相色谱(HPLC)法检测PHP的单糖组成。参考戴军等的方法[19],并稍作修改。PMP衍生:准确吸取250 µL混合对照溶液到5 mL EP管中,加入250 µL 0.6 mol/L NaOH,500 µL 0.4 mol/L PMP-甲醇,70 ℃反应1 h。冷水中冷却10 min;加入500 µL 0.3 mol/L HCl中和,再加入1 mL氯仿漩涡1 min,3000 r/min离心10 min,小心取上清,萃取3次。上清用HPLC进行检测。检测条件:色谱仪为赛默飞U3000,选用Xtimate C18 色谱柱(4.6 mm×200 mm,5 µm),进样量为20 µL,流动相为0.05 mol/L的磷酸二氢钾溶液:乙腈(83:17),流速为1.0 mL/min,柱温30 ℃,检测波长为250 nm。
1.2.4 PHP对Raw 264.7巨噬细胞的保护作用
1.2.4.1 PHP对Raw264.7巨噬细胞的毒性作用浓度
细胞种板、培养参考Lee等[20]的方法。取对数生长期细胞以1.0×105个/孔接种于96孔培养板,每孔体积100 μL,37 ℃、5% CO2培养箱中培养12 h,待细胞贴壁后,弃去上清液,分别加入含80、160、320、640、960 μg/mL PHP的饥饿培养基(含2% 胎牛血清)100 μL,空白对照组为相同体积的饥饿培养基,同一处理设置6个平行复孔。24 h后采用MTT法检测细胞存活率,用酶标仪检测各孔在492 nm下的吸光值。
1.2.4.2 PHP对LPS诱导伤害的细胞存活率影响
LPS诱导的免疫损伤是研究免疫调节作用的常用模型。参考Jin等[21]的方法,并稍作修改。取对数生长期细胞1.0×105个/孔接种于96孔培养板,每孔体积100 μL,37 ℃、5% CO2培养箱中培养24 h,待细胞贴壁后,弃去上清液,分别加入含80、160、320、640 μg/mL PHP的饥饿培养基100 μL,空白对照组和阳性对照组均加入相同体积饥饿培养基。处理4 h后,更换培养液,除空白对照组外均加入含1 μg/mL LPS的饥饿培养基100 μL,继续培养20 h。同一处理设置6个平行复孔,随后采用MTT法检测各孔在492 nm下的吸光值,计算细胞存活率,并采用光学显微镜对培养结束后的细胞形态进行观察。
式中:A2表示实验组的吸光值;A1表示对照组吸光值;A0表示空白组吸光值。
1.2.5 太子参多糖的测定
采用苯酚-硫酸法测定太子参多糖[22]。具体如下:准确称取无水葡萄糖10 mg,用蒸馏水定容至100 mL,摇匀后即得100 mg/L的葡萄糖标准溶液。分别量取0.1、0.3、0.5、0.7、0.9、1.1、1.3 mL于试管中,加蒸馏水定容至2 mL。先加入1 mL 5%苯酚,振荡摇匀后再加入5 mL浓硫酸,振荡摇匀后于100 ℃沸水浴中先加热15 min,后冰浴5 min,于490 nm波长处测定吸光度。以2 mL蒸馏水按同样显色操作为空白对照。分别以葡萄糖含量(μg)为横坐标,吸光度为纵坐标,绘制标准曲线。太子参粗多糖得率及纯度计算公式如下:
式中:m表示由标准曲线计算出的多糖质量,mg;M1表示提取时太子参粉的质量,mg;M2指太子参粗多糖的质量,mg;n表示稀释倍数。
1.3 数据处理
测定结果采用平均值±标准差表示,数据采用SPSS Statistics 23软件进行单因素方差分析,采用Excel、Origin 2017软件作图。
2. 结果与分析
2.1 太子参多糖的测定
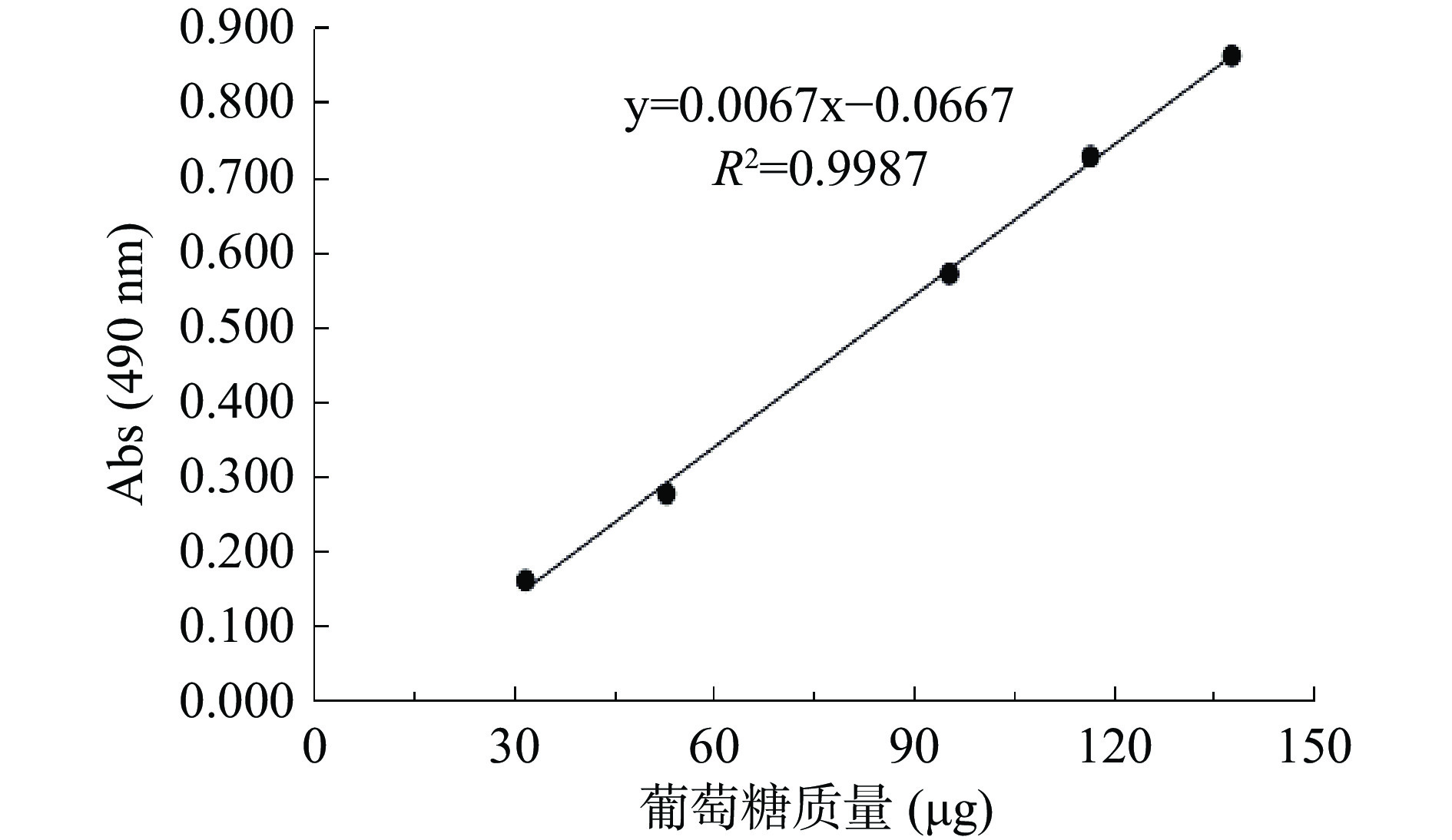
由图1可知,葡萄糖标准曲线方程为y=0.0067x−0.0667,其R2=0.9987,表明在32~138 µg的质量范围内具备良好的线性关系。通过水提醇沉法对太子参粗多糖进行提取,由苯酚-硫酸法检测得知太子参多糖得率为8.23%±0.78%,多糖纯度为51.47%±2.59%。与Hu等的文献相比,多糖纯度提高了7.07%[14]。
2.2 太子参多糖的纯化工艺优化
2.2.1 pH条件优化
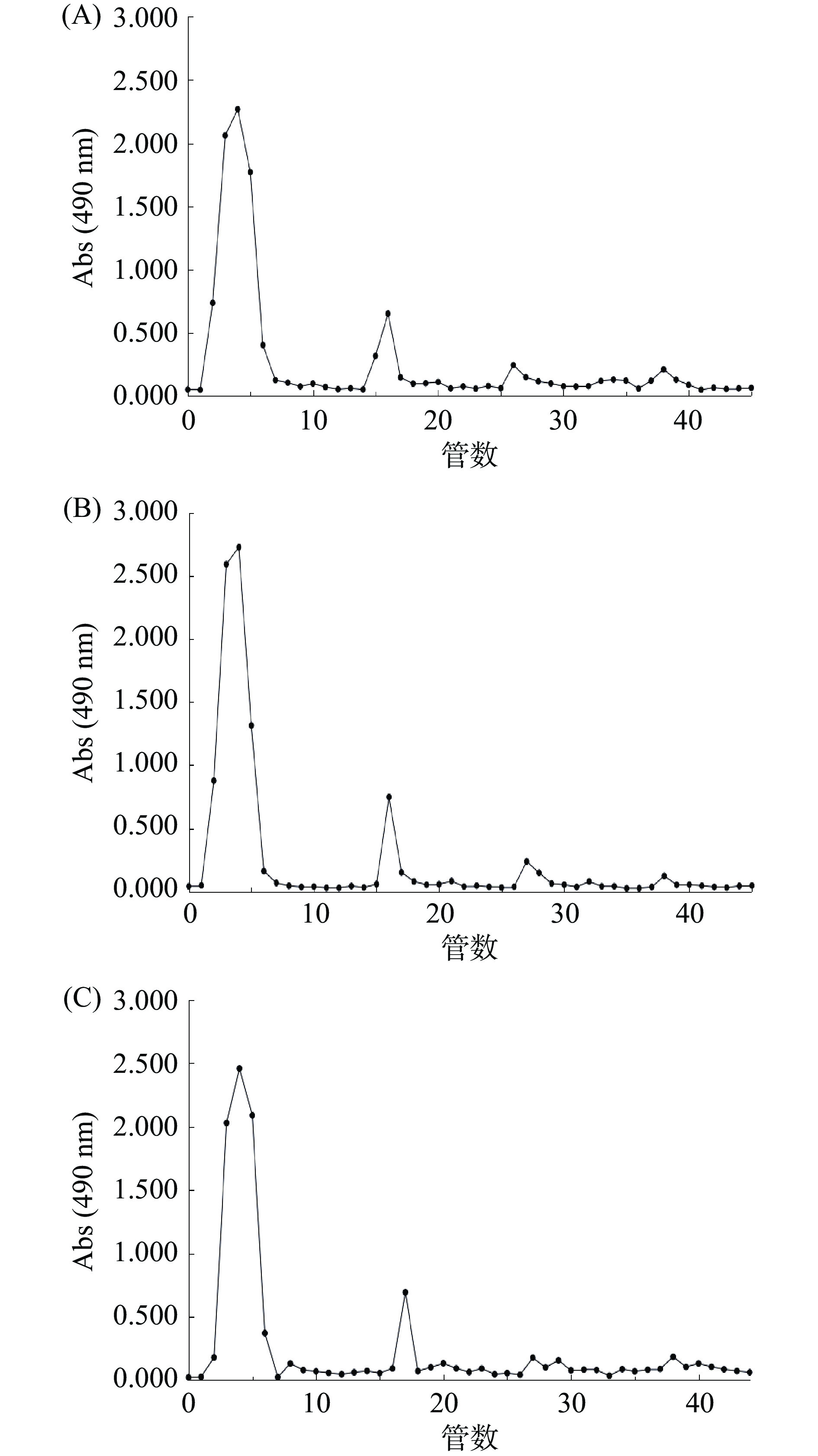
DEAE Cellulose-52 是一种阴离子交换树脂,在碱性条件下有利于交换发生。基于此,本研究首先对比了太子参粗多糖在不同弱碱性(pH为7.4、7.6、7.8)环境下的洗脱效果,以确定洗脱液的最佳pH。由图2可知,该粗提物中性多糖含量高,酸性多糖含量相对较低。并且不同pH的洗脱液处理时,表现出不同分离效果;当pH为7.4(图A)时,前半段分离效果比较好,而后三个峰存在连续拖尾现象;当pH为7.6(图B)时,洗脱峰分离度高,无拖尾现象,主要峰的吸光值最高,洗脱效果较好;当pH为7.8(图C)时,虽然峰形明显,但峰形不够尖锐。因此,选择pH为7.6作为太子参多糖纯化的pH条件。
2.2.2 梯度洗脱盐浓度的选择
进一步对比了不同盐浓度(0~0.1、0~0.3、0~0.5 mol/L,NaCl)的洗脱液对太子参粗多糖的洗脱效果。由图3可知,在0~0.1 mol/L的NaCl条件下,峰分离度高,但峰数少,具有一定分离效果;在0~0.3 mol/L的NaCl条件下,多糖被主要分成5个峰,其中前3个分离度好,且吸光值高。在0~0.5 mol/L的NaCl条件下,多糖被分成更多级分,但分离度差,拖尾现象严重,不利于选择组分进行收集。因此本实验选择0~0.3 mol/L的NaCl作为太子参多糖纯化的盐浓度洗脱条件。
综上,以pH7.6的Tris-HCl缓冲液上样,选择0~0.3 mol/L NaCl的盐溶液进行洗脱,收集第1个主峰,旋蒸浓缩,透析除盐,冷冻干燥,即得太子参多糖。采用苯酚-硫酸法检测其纯度为80.52%±1.84%,相比于现有文献,用于功能研究的太子参的多糖提取物,纯度提高了17.02%[23]。
2.3 PHP的结构表征
2.3.1 紫外光谱扫描
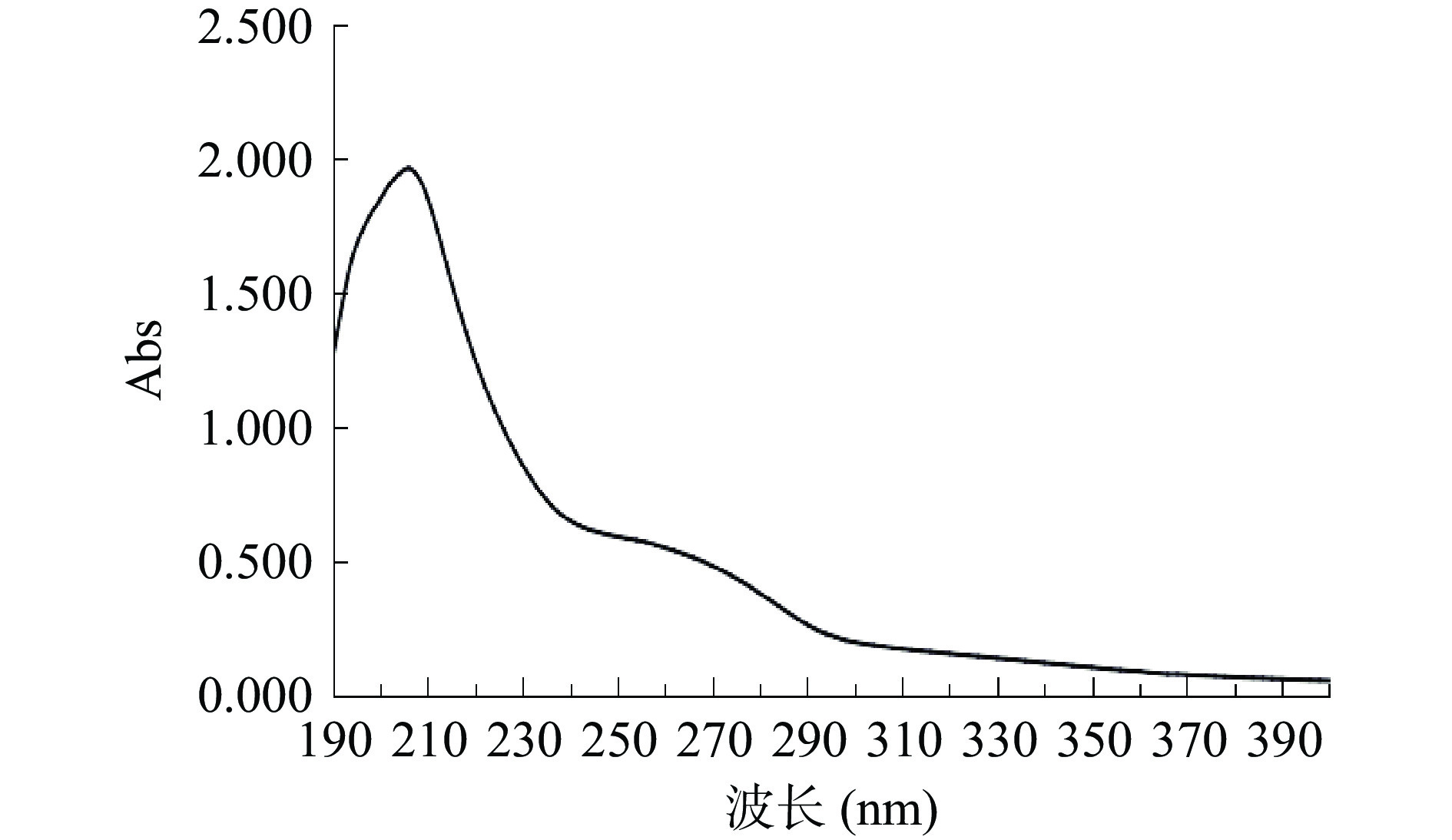
如图4所示,曲线整体呈先上升再下降的趋势,在210 nm左右有最大吸收,且吸光值高达2.000,而210 nm为多糖的特征吸收峰[24],此处吸光值高说明PHP的主要成分为多糖。图中在240~290 nm处有一个较宽的小吸收峰,吸光值低于0.500。260和280 nm分别是核酸和蛋白质的特征吸收波长[25],这说明纯度为80.52%的PHP的杂质主要可能为蛋白质和核酸。
2.3.2 红外光谱扫描
红外图谱常常用来鉴定多糖的初级结构。在中红外图谱解析中,一般将波数4000~1300 cm−1称为特征区,可以根据分子官能团和基团吸收峰来判断物质类别。1300~400 cm−1称为指纹区,可以判断糖苷键类型和构型[26]。
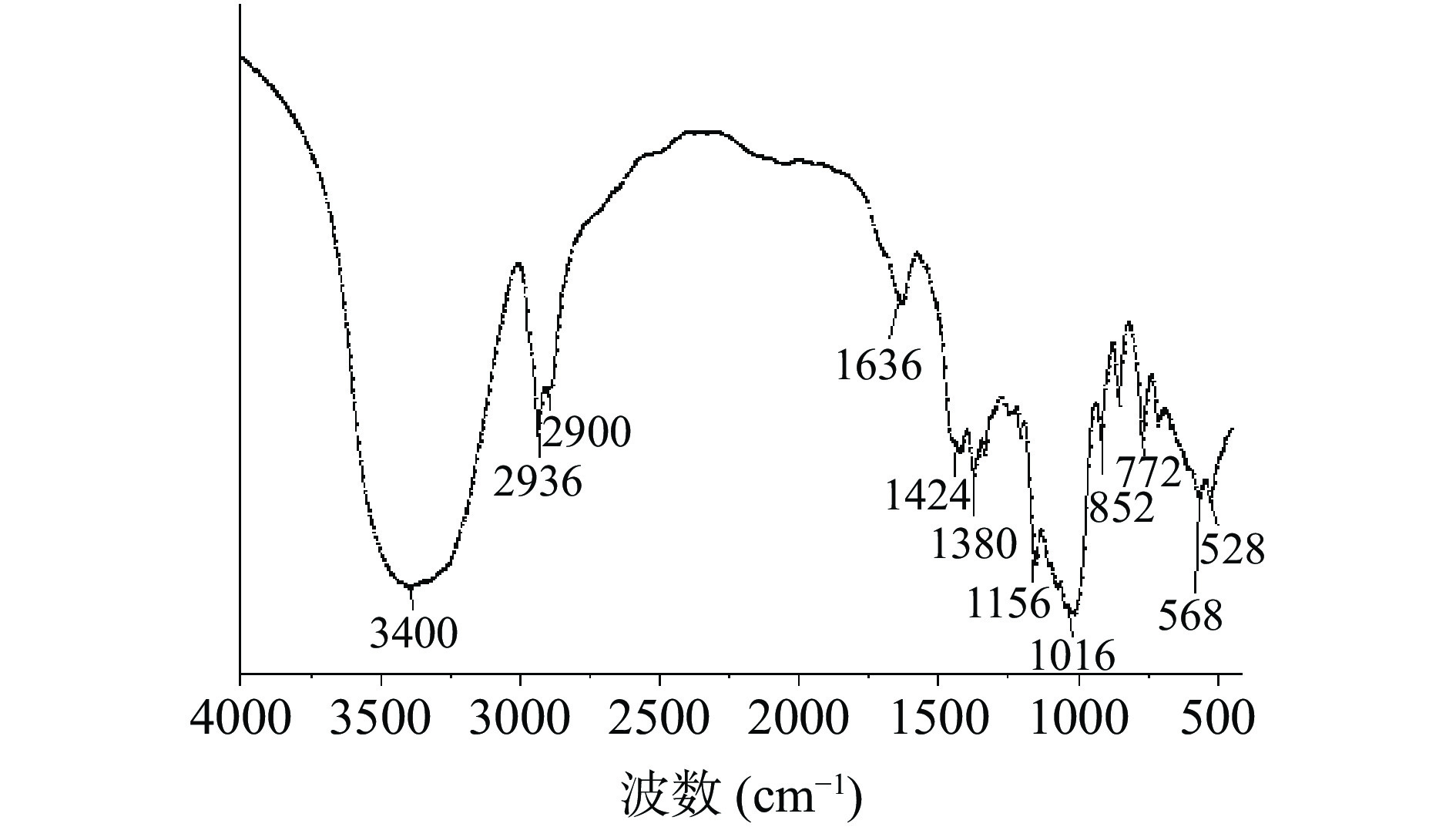
PHP的红外扫描图谱见图5。图中3400 cm−1的-OH伸缩振动峰、2936 cm−1的C-H键的伸缩振动峰和1380 cm−1的C-H键的弯曲振动峰,联合证明所检测化合物为糖类化合物[27-29]。在1636 和1424 cm−1分别为-COOH的非对称及对称伸缩振动峰。图谱在1000~1200 cm−1的三个吸收峰是吡喃环吸收振动引起的,具有C-O-C骨架[30],α型的C-H在844 cm−1会有一个吸收峰,而β型的在891 cm−1处有一个吸收峰[31],图中在852 cm−1有一个吸收峰,说明含有α-型糖苷键。综上说明PHP是一种α-吡喃糖。
2.3.3 分子量检测
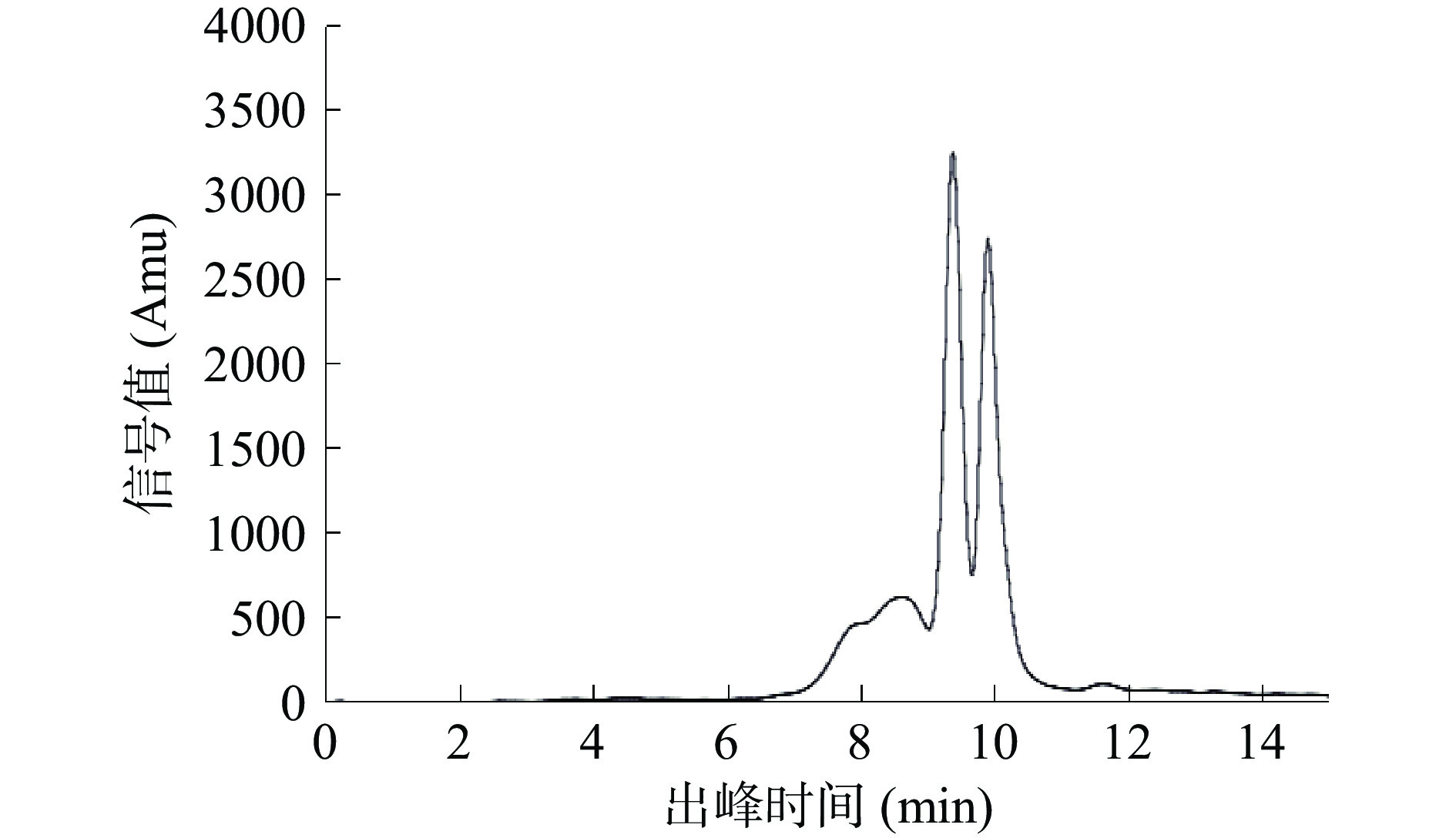
多糖的溶解度和粘度受其分子量大小的影响,并且多糖链的大小与结构与其生物活性密切相关[30]。以出峰时间(t)为横坐标,分子量的对数值(lg Mw)为纵坐标,绘制标准曲线,得回归方程为lg Mw=−1.2807 t+15.374,R2=0.9981。
如图6所示,PHP主要被分成3个峰,其中2个强吸收峰的出峰时间在9.38与9.90 min,代入标准曲线计算分子量分别为2.3×103和500 Da。此外,8.13~9.10 min有1个较大连续峰,代入标曲后分子量在5.2×103~92×103 Da之间。结合文献,太子参中多糖的分子量基本在3×103~2.2×105 Da之间[14,32-33]。有研究报道,醇沉的乙醇终浓度不同,所得多糖的分子量会不同[34],李松法等[35]采用不同浓度的乙醇分级醇沉了黄芪多糖,发现醇沉所得黄芪多糖的分子量随乙醇浓度的升高而降低。本研究中太子参多糖分子量在500~92×103 Da之间,与现有的太子参多糖的分子量存在差异,这可能是使用80%乙醇浓度醇沉导致的。
2.3.4 单糖组成检测
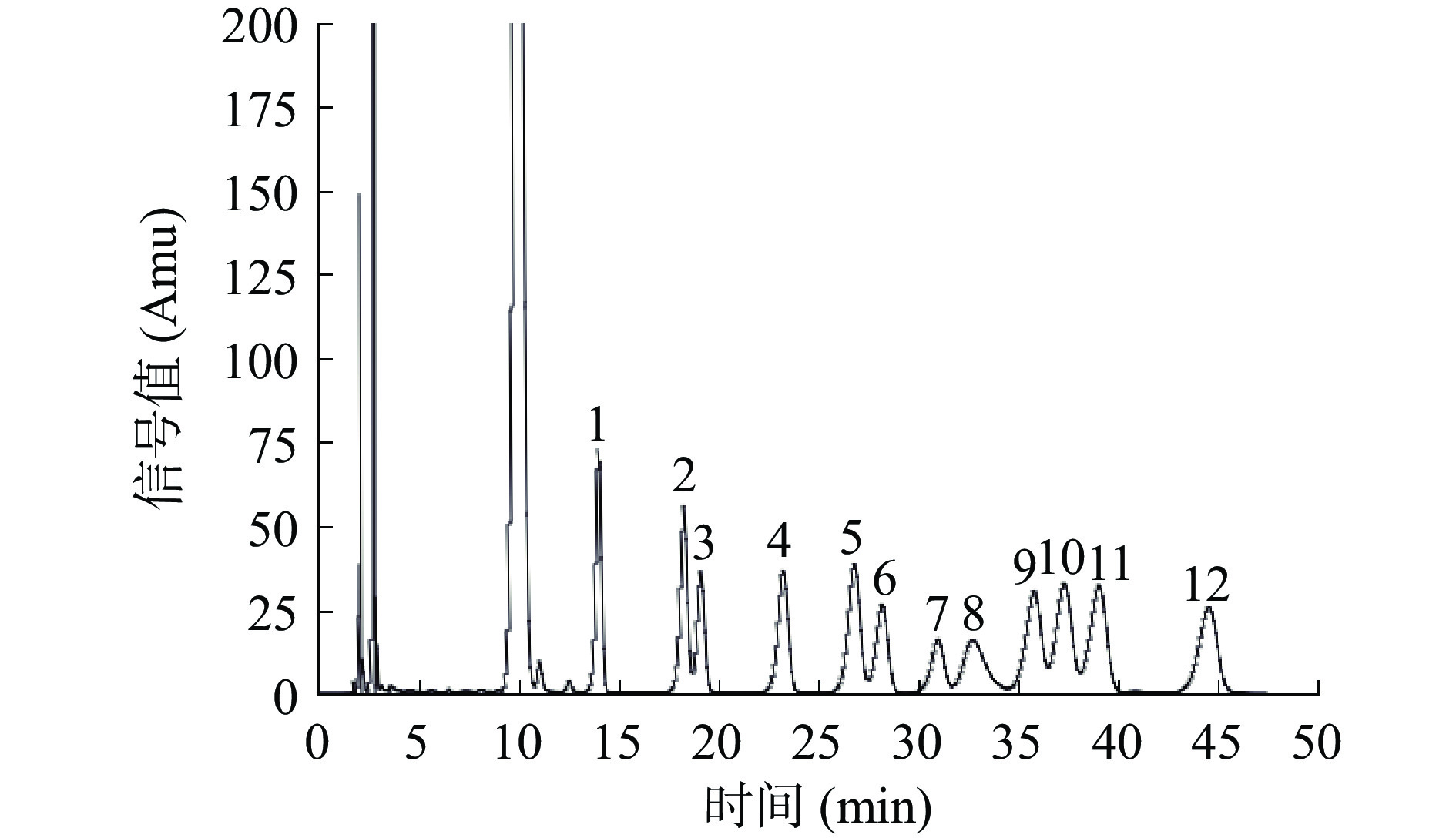
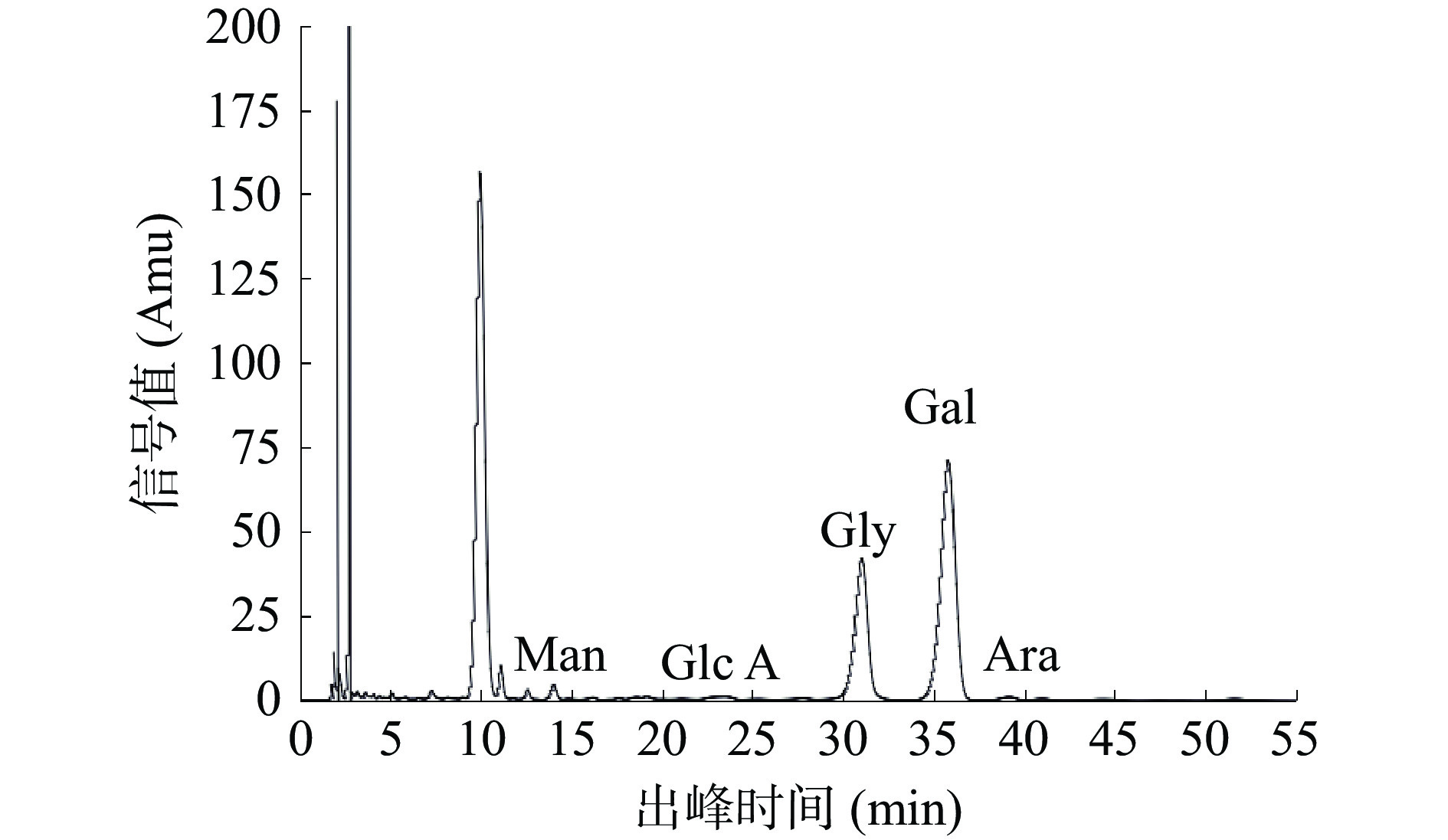
标准单糖出峰时间及顺序见图7。图8为PHP的HPLC色谱图,PHP主要是由葡萄糖、半乳糖、阿拉伯糖、甘露糖和葡萄糖醛酸组成。郭守斌测定了10种不同产地太子参的单糖,总结出太子参的单糖组成为半乳糖、D-甘露糖、鼠李糖、阿拉伯糖、D-无水葡萄糖、D-葡萄糖醛酸、D-半乳糖醛酸,且不同产地太子参多糖中单糖组成略有差异[36],这与本实验结果是基本一致的。夏和先等比较了组培太子参和野生太子参的单糖组成,发现其单糖组成基本相同,但单糖的比例明显不同[37]。葡萄糖与半乳糖是PHP中最主要的两种单糖,这与金针菇、杏鲍菇、猴头菇等食用菌多糖的单糖组成相类似[38],可能具有与其类似的免疫调节、降血糖血脂、抗疲劳等生理活性[39]。
2.4 PHP对Raw 264.7巨噬细胞的保护作用
2.4.1 PHP对Raw 264.7巨噬细胞的毒性作用浓度
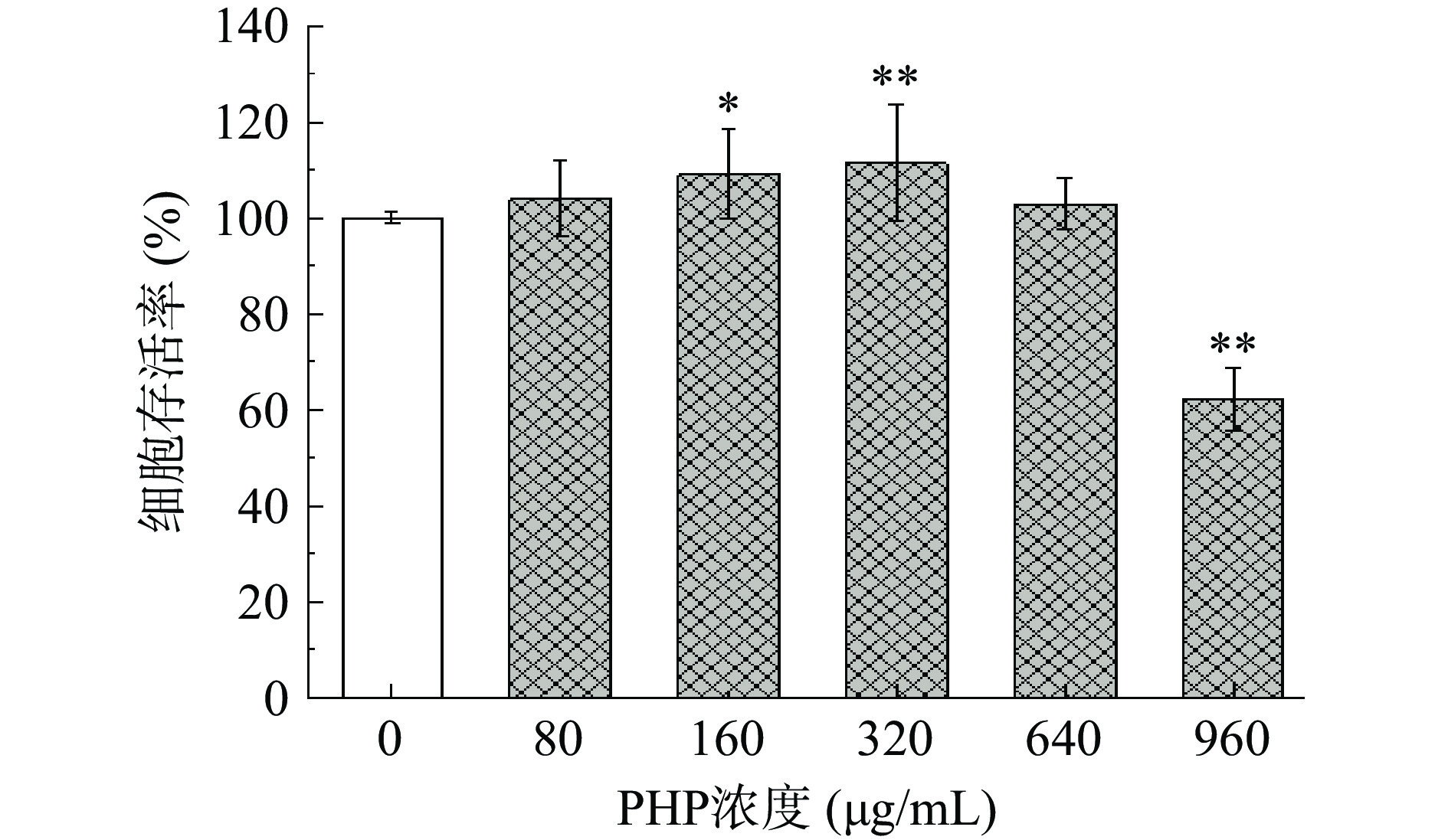
PHP 对 Raw 264.7 巨噬细胞毒性作用结果如图9所示。PHP在80~640 µg/mL范围内,对Raw 264.7巨噬细胞的存活率具有提升作用,且呈现剂量-反应关系,在添加量为320 µg/mL时,细胞存活率最大,为111.35%。但是当浓度达到960 µg/mL后,PHP对细胞表现出毒害作用,因此,选择PHP浓度为80~640 µg/mL进行下一步实验。张丽娟等[40]探究了太子参多糖对Raw 264.7巨噬细胞免疫调节作用的影响,结果表明太子参多糖的安全浓度为600 μg/mL,这与本文研究结果相近。研究表明多糖可以通过促进巨噬细胞的增殖,从而增强巨噬细胞的吞噬作用[41]。此外,铁皮石斛多糖、杏鲍菇等多糖的单糖组成均以葡萄糖、半乳糖、甘露糖为主[38, 42],与本研究中PHP的单糖组成较为一致,且此类多糖均表现出免疫调节活性[42-43],进一步说明PHP具备潜在的免疫调节活性。
2.4.2 PHP对LPS诱导免疫损伤的Raw 264.7巨噬细胞存活的影响
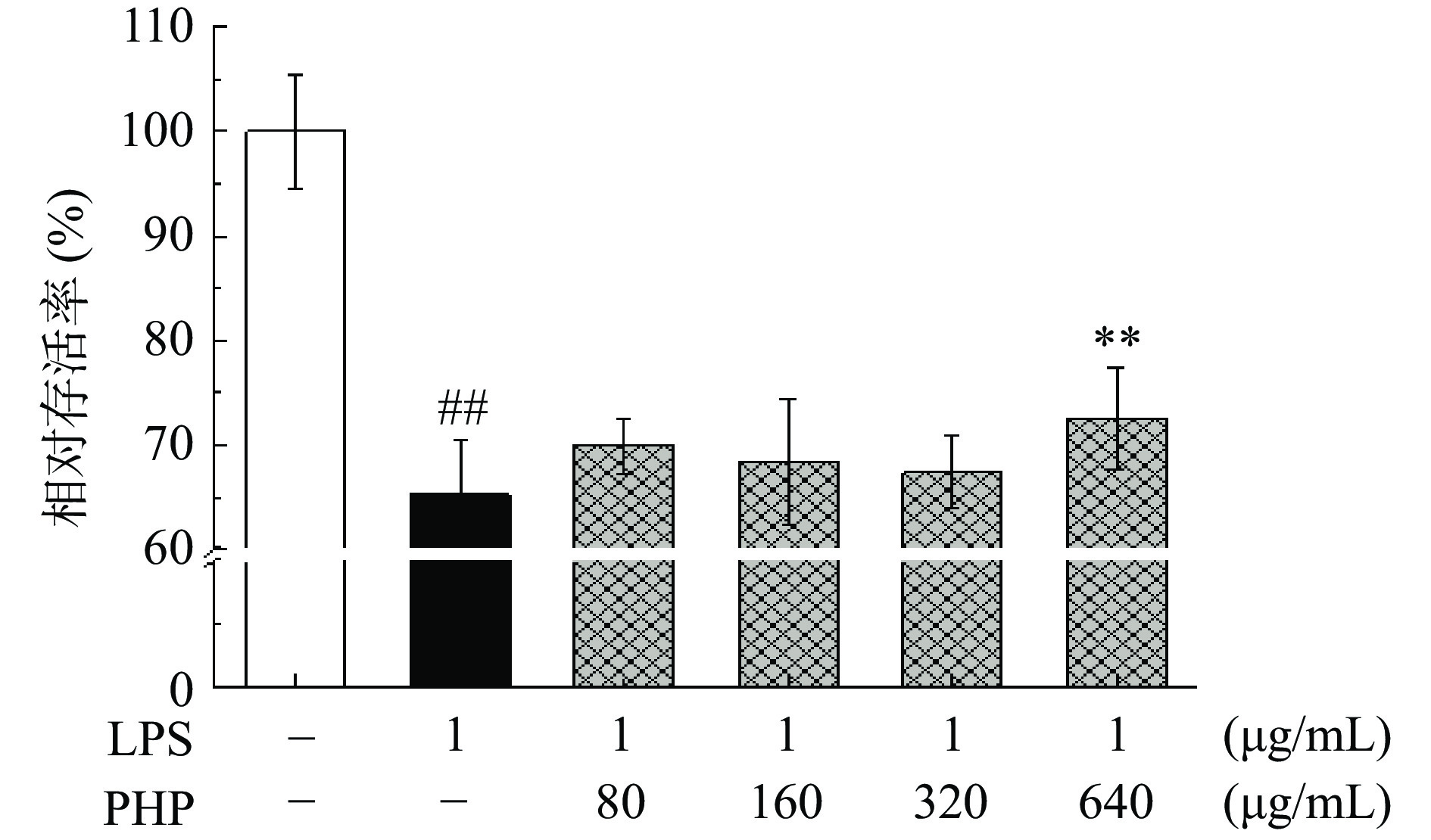
PHP对LPS诱导的免疫损伤下细胞存活检测结果如图10所示。与空白组相比,在1 μg/mL的LPS的诱导损伤下,细胞相对存活率极显著降低(P<0.01),说明本实验成功建立了免疫损伤模型。不同浓度的PHP处理后,Raw 264.7巨噬细胞存活率有一定提升效果;当剂量为640 μg/mL时,细胞存活率极显著提高了7.21%(P<0.01)。这表明太子参多糖对Raw 264.7巨噬细胞的免疫损伤具有一定缓解作用。
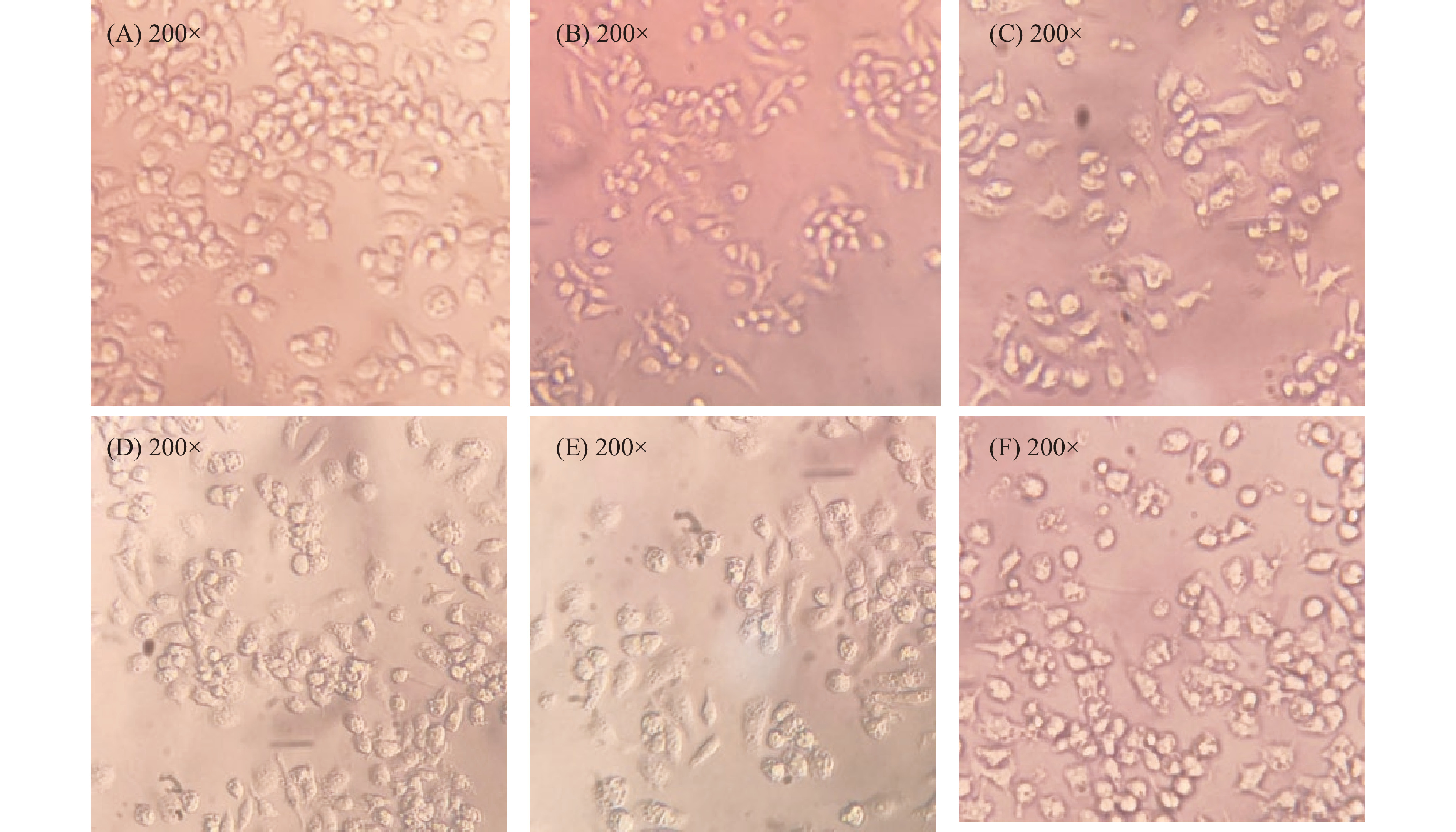
Raw 264.7巨噬细胞最好的状态呈透亮的圆形,细胞吞噬抗原过多、培养环境恶劣都会促使该细胞呈现梭形、长梭形[44]。由图11可知,空白对照组的Raw 264.7巨噬细胞呈椭圆或圆形。模型组在LPS刺激下,细胞发生形态变化,呈菱形、梭形,细胞密度明显少于空白对照组。陈光勇[45]、郑胜眉[46]等显微镜下观察Raw 264.7巨噬细胞时,LPS处理组同样出现了数量降低、长梭状的细胞形变等。加入PHP的处理组中,细胞形态出现明显改善,大多细胞呈标准圆形、类椭圆形、纺锤形等,这表明PHP具有改善Raw 264.7巨噬细胞形态的作用。
3. 结论
本实验通过水提醇沉法从太子参中提取了太子参粗多糖,得率为8.23%,纯度为51.47%。并采用DEAE Cellulose-52离子交换柱法纯化得太子参多糖(PHP),其纯度为80.52%。PHP是一种α-吡喃糖,分子量在500~92×103 Da,主要由葡萄糖、半乳糖、阿拉伯糖、甘露糖及葡萄糖醛酸组成。以LPS诱导的Raw 264.7小鼠巨噬细胞为模型,PHP可以提高免疫损伤后的细胞存活率,改善细胞形态,说明了PHP对由LPS诱导损伤的Raw 264.7巨噬细胞有保护作用,初步表明具备体外调节免疫的潜力。
-
-
[1] 刘训红, 阚毓铭. 太子参研究概述[J]. 时珍国医国药,2000,11(12):1131−1132. [LIU Xunhong, KAN Yuming. Overview of the research on Pesudostellaria heterophylla[J]. Lishizhen Medicine and Materia Medica Research,2000,11(12):1131−1132. doi: 10.3969/j.issn.1008-0805.2000.12.078 LIU Xunhong, KAN Yuming. Overview of the research on Pesudostellaria heterophylla[J]. Lishizhen Medicine and Materia Medica Research, 2000, 11(12): 1131-1132. doi: 10.3969/j.issn.1008-0805.2000.12.078
[2] SUN H W, SHI K Q, QI K, et al. Pseudostellaria heterophylla extract polysaccharide H-1-2 suppresses pancreatic cancer by inhibiting hypoxia-induced AG2[J]. Molecular Therapy-Oncolytics,2020,17:61−69. doi: 10.1016/j.omto.2020.03.007
[3] YOU S Y, LIU X W, XU G T, et al. Identification of bioactive polysaccharide from Pseudostellaria heterophylla with its anti-inflammatory effects[J]. Journal of Functional Foods,2021,78(150):104353.
[4] 陈耀金, 陈建洪, 刘伟招, 等. 太子参胶囊增强免疫作用实验研究[J]. 中医药临床杂志,2015,27(8):1172−1174. [CHEN Yaojin, WANG Gong, CHEN Jianhong, et al. Study on Taizishen capsule’s functions in enhancing immunity[J]. Clinical Journal of Traditional Chinese Medicine,2015,27(8):1172−1174. CHEN Yaojin, WANG Gong, CHEN Jianhong, et al. Study on Taizishen capsule’s functions in enhancing immunity[J]. Clinical Journal of Traditional Chinese Medicine, 2015, 27(8): 1172-1174.
[5] 张炎达, 潘慧青, 衣伟萌, 等. 太子参须提取物对小鼠免疫功能的影响[J]. 现代畜牧兽医,2021(10):46−49. [ZHANG Yanda, PAN Huiqing, YI Weimeng, et al. Effect of Pseudostellaria heterophylla fibrous root extraction on immunologic functions of mice[J]. Graziery Veterinary Sciences,2021(10):46−49. ZHANG Yanda, PAN Huiqing, YI Weimeng, et al. Effect of Pseudostellaria heterophylla fibrous root extraction on immunologic functions of mice[J]. Graziery Veterinary Sciences, 2021(10): 46-49
[6] CHEN J L, PANG W S, KAN Y J, et al. Structure of a pectic polysaccharide from Pseudostellaria heterophylla and stimulating insulin secretion of INS-1 cell and distributing in rats by oral[J]. International Journal of Biological Macromolecules,2018,106:456−463. doi: 10.1016/j.ijbiomac.2017.08.034
[7] FANG Z H, DUAN X C, ZHAO J D, et al. Novel polysaccharide H-1-2 from Pseudostellaria heterophylla alleviates type 2 diabetes mellitus[J]. Cellular Physiology and Biochemistry,2018,49(3):996−1006.
[8] YANG C J, YOU L T, YIN X B, et al. Heterophyllin B ameliorates lipopolysaccharide-induced inflammation and oxidative stress in Raw264.7 macrophages by suppressing the PI3K/Akt pathways[J]. Molecules,2018,23(4):717. doi: 10.3390/molecules23040717
[9] WANG Z, LIAO S G, HE Y, et al. Protective effects of fractions from Pseudostellaria heterophylla against cobalt chloride-induced hypoxic injury in H9c2 cell[J]. Journal of Ethnopharmacology,2013,147(2):540−545. doi: 10.1016/j.jep.2013.03.053
[10] 陈小英, 曾晏萍, 刘汉儒, 等. 太子参须多糖粗提物对小鼠免疫功能的调节作用[J]. 西南大学学报(自然科学版),2020,42(4):56−64. [CHEN Xiaoying, ZENG Yanping, LIU Hanru, et al. Effects of crude polysaccharides from Radix pseudostellariae on immunoregulation function in mice[J]. Journal of Southwest University (Natural Science Edition),2020,42(4):56−64. CHEN Xiaoying, ZENG Yanping, LIU Hanru, et al. Regulating effect of crude extract of Polysaccharides from Radix Pseudoginsengensis on the immune function of mice[J]. Journal of Southwest University (Natural Science Edition), 2020, 42(4): 56-64.
[11] 冯丹, 郝思钰, 付杨, 等. 太子参多糖注射液对免疫抑制小鼠免疫功能的影响[J]. 中兽医医药杂志,2020,39(3):74−77. [FENG Dan, HAO Siyu, FU Yang, et al. Effect of Taizishen polysaccharide injection on the immune function of immunosuppressive mice[J]. Journal of Traditional Chinese Veterinary Medicine,2020,39(3):74−77. FENG Dan, HAO Siyu, FU Yang, et al. Effect of Taizishen polysaccharide injection on the immune function of immunosuppressive mice[J]. Journal of Traditional Chinese Veterinary Medicine, 2020, 39(3): 74-77.
[12] SARAH J, CLEMENTS, SIMON R CARDING. Diet, the intestinal microbiota, and immune health in aging[J]. Critical Reviews in Food Science and Nutrition,2018,58(4):651−661. doi: 10.1080/10408398.2016.1211086
[13] 崔石阳, 姜帆, 韩建春, 等. 北五味子多糖对RAW 264.7巨噬细胞的免疫调节作用[J]. 食品科学,2017,38(19):201−205. [CUI Shiyang, JIANG Fan, HAN Jianchun, et al. The immunomodulatory effect of Schisandra chinensis polysaccharide on Raw 264.7 cells[J]. Food Science,2017,38(19):201−205. doi: 10.7506/spkx1002-6630-201719032 CUI Shiyang, JIANG Fan, HAN Jianchun, et al. The immunomodulatory effect of Schisandra chinensis polysaccharide on Raw 264.7 cells[J]. Food Science, 2017, 38 (19): 201-205. doi: 10.7506/spkx1002-6630-201719032
[14] HU J, PANG W S, CHEN J L, et al. Hypoglycemic effect of polysaccharides with different molecular weight of Pseudostellaria heterophylla[J]. BMC Complementary and Alternative Medicine,2013,13(1):1−9. doi: 10.1186/1472-6882-13-1
[15] 野津, 张文森, 王知斌, 等. DEAE-52在中药多糖分离纯化中的应用[J]. 化学工程师,2019,33(11):43−45,22. [YE Jin, ZHANG Wensen, WANG Zhibin, et al. Application of DEAE-52 in the separation and purification of polysaccharides from traditional Chinese medicine[J]. Chemical Engineers,2019,33(11):43−45,22. YE Jin, ZHANG Wensen, WANG Zhibin, et al. Application of DEAE-52 in the separation and purification of polysaccharides from traditional Chinese medicine[J]. Chemical Engineers, 2019, 33(11): 43-45, 22.
[16] 雷思敏, 肖榕, 章莹, 等. 铁皮石斛中性多糖分离纯化及其体内免疫调节作用研究[J]. 中药新药与临床药理,2018,29(6):748−753. [LEI Simin, XIAO Rong, ZHANG Ying, et al. Isolation and purification of neutral polysaccharides from Dendrobium candidum and its immune regulation in vivo[J]. New Chinese Medicines and Clinical Pharmacology,2018,29(6):748−753. LEI Simin, XIAO Rong, ZHANG Ying, et al. Isolation and purification of neutral polysaccharides from Dendrobium candidum and its immune regulation in vivo[J]. New Chinese Medicines and Clinical Pharmacology, 2018 , 29(6): 748-753.
[17] 陈杨扬, 李娇, 王向红, 等. 滑子菇多糖的结构及体外生物活性探究[J]. 中国食品学报,2019,19(6):68−73. [CHEN Yangyang, LI Jiao, WANG Xianghong, et al. Structural characterization and bioactivities of polysaccharides from Pholiota nameko in vitro[J]. Journal of Chinese Institute of Food Science and Technology,2019,19(6):68−73. CHEN Yangyang, LI Jiao, WANG Xianghong, et al. Structural characterization and bioactivities of polysaccharides from Pholiota nameko in vitro[J]. Journal of Chinese Institute of Food Science and Technology, 2019, 19(6): 68-73.
[18] 宁慧娟. 灵芝子实体多糖碱性电解水提取工艺优化及其性质分析[D]. 北京: 中国农业大学, 2018. NING Huijuan. Optimization of alkaline electrolyzed water extraction and characterization of polysaccharide from Ganoderma lucidum fruiting bodies[D]. Beijing: China Agricultural University, 2018.
[19] 戴军, 朱松, 汤坚, 等. PMP柱前衍生高效液相色谱法分析杜氏盐藻多糖的单糖组成[J]. 分析测试学报,2007(2):206−210. [DAI Jun, ZHU Song, TANG Jian, et al. Analysis of monosaccharide compositions in polysaccharides from D. salina by precolumn derivatization high performance liquid chromatography[J]. Journal of Instrumental Analysis,2007(2):206−210. doi: 10.3969/j.issn.1004-4957.2007.02.015 DAI Jun, ZHU Song, TANG Jian, et al. Analysis of monosaccharide compositions in polysaccharides from D. Salina by precolumn derivatization high performance liquid chromatography[J]. Journal of Instrumental Analysis, 2007(2): 206-210. doi: 10.3969/j.issn.1004-4957.2007.02.015
[20] LEE S M, Y M, P H. Protective effects of Paeonia lactiflora pall on hydrogen peroxide-inducedapoptosis in PC12 cells[J]. Bioscience, Biotechnology and Biochemistry,2008,5(72):1272−1277.
[21] JIN S E, KIM O S, YOO S, et al. Anti-inflammatory effect and action mechanisms of traditional herbal formula Gamisoyo-san in Raw264.7 macrophages[J]. BMC Complementary and Alternative Medicine,2016,16(1):1−11.
[22] 林巧美, 庞文生, 曾洁, 等. 福建柘荣不同品种太子参浸出物及多糖含量测定[J]. 中国民族民间医药,2019,28(3):21−23. [LIN Qiaomei, PANG Wensheng, ZENG Jie, et al. Experimental study on the extracts and polysaccharides of different sarieties of Pseudostellaria heterophylla from Zhelong Fujian province[J]. Chinese Journal of Ethnomedicine and Ethnopharmacy,2019,28(3):21−23. LIN Qiaomei, PANG Wensheng, ZENG Jie, et al. Experimental study on the extracts and polysaccharides of different sarieties of Pseudostellaria heterophylla from Zhelong Fujian province[J]. Chinese Journal of Ethnomedicine and Ethnopharmacy, 2019, 28(3): 21-23.
[23] 闵思明, 赵晓瑶, 陈赛红, 等. 太子参参须多糖对免疫抑制小鼠的免疫调节作用研究[J]. 动物医学进展,2020,41(8):23−28. [MIN Siming, ZHAO Xiaoyao, CHEN Saihong, et al. Immunomodulatory effects of radix pseudostellariae fibrous root polysaccharides on cyclophosphamide-induced immunosuppressed mice[J]. Progress in Veterinary Medicine,2020,41(8):23−28. doi: 10.3969/j.issn.1007-5038.2020.08.005 MIN Siming, ZHAO Xiaoyao, CHEN Saihong, et al. Immunomodulatory effects of radix Pseudostellariae fibrous root polysaccharides on cyclophosphamide-induced immunosuppressed mice[J]. Progress in Veterinary Medicine, 2020, 41(8): 23-28. doi: 10.3969/j.issn.1007-5038.2020.08.005
[24] 陶俊, 文汉. 油茶籽多糖分离纯化和结构分析[J]. 食品工业科技,2011,32(6):132−135. [TAO Jun, WEN Han. Isolation, purification and structural analysis of Camellia seed polysaccharides[J]. Science and Technology of Food Industry,2011,32(6):132−135. TAO Jun, WEN Han. Isolation, Purification and structural analysis of Camellia seed polysaccharides[J]. Science and Technology of Food Industry, 2011, 32(6): 132-135.
[25] 张发宇, 余金卫, 张浏, 等. 巢湖蓝藻藻蓝蛋白纯化过程中紫外-可见吸收光谱特征分析[J]. 光谱学与光谱分析,2017,37(3):806−810. [ZHANG Fayu, YU Jinwei, ZHANG Liu, et al. Characteristic analysis of ultraviolet-visible absorption spectrum during purification of cyanobacterial cyanophytein in Chaohu Lake[J]. Spectroscopy and Spectral Analysis,2017,37(3):806−810. ZHANG Fayu, YU Jinwei, ZHANG Liu, et al. Characteristic analysis of ultraviolet-visible absorption spectrum during purification of cyanobacterial cyanophytein in Chaohu Lake[J]. Spectroscopy and Spectral Analysis, 2017, 37(3): 806-810.
[26] 董洲. 野葛根多糖的提取、分离纯化、结构鉴定及对小鼠巨噬细胞RAW264.7的免疫调节活性研究[D]. 广州: 华南理工大学, 2018. DONG Zhou. Extraction, isolation, purification, structural characterization and immunomodulatory activity in RAW264.7 cells of the polysaccharides from Pueraria lobate (willd. ) Ohwi root[D]. Guangzhou: South China University of Technology, 2018.
[27] 黄娜. 血清色素上皮衍生因子(PEDF)与糖尿病肾病蛋白尿的相关性及其机制研究[D]. 上海: 中国人民解放军陆军军医大学, 2019. HUANG Na. The effect and mechanism of increased levels of serum pigment epithelium-derived factor in proteinuria in diabetic kidney disease[D]. Shanghai: Army Medical University, 2019.
[28] HAN L, SUO Y, YANG Y, et al. Optimization, characterization, and biological activity of polysaccharides from Berberis dasystachya Maxim[J]. International Journal of Biological Macromolecules,2016,85:655−666. doi: 10.1016/j.ijbiomac.2015.10.038
[29] ZHANG L, ZHANG W, WANG Q, et al. Purification, antioxidant and immunological activities of polysaccharides from Actinidia chinensis roots[J]. International Journal of Biological Macromolecules,2015,72:975−983. doi: 10.1016/j.ijbiomac.2014.09.056
[30] LÜ X, CHEN D, YANG L, et al. Comparative studies on the immunoregulatory effects of three polysaccharides using high content imaging system[J]. International Journal of Biological Macromolecules,2016,86:28−42. doi: 10.1016/j.ijbiomac.2016.01.048
[31] 王银平. 玉木耳多糖的制备、结构表征及其与乳清蛋白相互作用研究[D]. 长春: 吉林大学, 2020. WANG Yinping. Preparation and structural characterization of polysaccharides from Auricularia cornea var. Li. and their interactions with whey protein[D]. Changchun: Jilin University, 2020.
[32] 史文涛, 庞文生, 胡娟. 高效凝胶色谱法测定太子参均一多糖分子量[J]. 中国民族民间医药,2015,24(2):20−30. [SHI Wentao, PANG Wensheng, HU Juan. Determination of the molecular weight of uniform polysaccharides of radix pseudostellariae by high performance gel chromatography[J]. Chinese National Folk Medicine,2015,24(2):20−30. SHI Wentao, PANG Wensheng, HU Juan. Determination of the molecular weight of uniform polysaccharides of Radix Pseudostellariae by high performance gel chromatography[J]. Chinese National Folk Medicine, 2015, 24(2): 20-30.
[33] 杨斌, 庞文生, 胡娟. 太子参均一多糖的分离与表征[J]. 中国民族民间医药,2017,26(4):11−16. [YANG Bin, PANG Wensheng, HU Juan. Isolation and characterization of homogeneous polysaccharides from radix pseudostellariae[J]. Chinese National Folk Medicine,2017,26(4):11−16. YANG Bin, PANG Wensheng, HU Juan. Isolation and characterization of homogeneous polysaccharides from Radix Pseudostellariae[J]. Chinese National Folk Medicine, 2017, 26(4): 11-16.
[34] 刘艳红, 唐祥, 李娅琳, 等. 活性多糖提取纯化及结构解析的研究进展[J]. 中国民族民间医药,2020,29(3):67−73. [LIU Yanhong, TANG Xiang, LI Yalin, et al. Research progress on the extraction and purification and structure analysis of active polysaccharides[J]. Chinese Ethnic Folk Medicine,2020,29(3):67−73. LIU Yanhong, TANG Xiang, LI Yalin, et al. Research progress on the extraction and purification and structure analysis of active polysaccharides[J]. Chinese Ethnic Folk Medicine, 2020, 29(3): 67-73.
[35] 李红法, 郭松波, 满淑丽, 等. 乙醇分级沉淀提取黄芪多糖及其理化性质和抗氧化活性研究[J]. 中国中药杂志,2015,40(11):2111−2116. [LI Hongfa, GUO Songbo, MAN Shuli, et al. Extraction of Astragalus polysaccharide by ethanol fractional precipitation and its physical and chemical properties and antioxidant activity[J]. China Journal of Chinese Materia Medica,2015,40(11):2111−2116. LI Hongfa, GUO Songbo, MAN Shuli, et al. Extraction of Astragalus polysaccharide by ethanol fractional precipitation and its physical and chemical properties and antioxidant activity[J]. China Journal of Chinese Materia Medica, 2015, 40(11): 2111-2116.
[36] 郭守斌. 柱前衍生超高效液相色谱法分析太子参多糖中单糖的组成[J]. 中国现代医学杂志,2016,26(23):37−41. [GUO Shoubin. Analysis of monosaccharide composition of Pseudostellaria heterophylla polysaccharides by pre-column derivatization ultra performance liquid chromatography[J]. China Journal of Modern Medicine,2016,26(23):37−41. doi: 10.3969/j.issn.1005-8982.2016.23.008 GUO Shoubin. Analysis of monosaccharide composition of Pseudostellaria heterophylla polysaccharides by pre-column derivatization ultra performance liquid chromatography[J]. China Journal of Modern Medicine, 2016, 26(23): 37-41. doi: 10.3969/j.issn.1005-8982.2016.23.008
[37] 夏和先, 陈乃东, 姚厚军, 等. 不同种源的太子参多糖含量及其单糖组成GC-MS研究[J]. 天然产物研究与开发,2016,28(4):542−546. [XIA Hexian, CHEN Naidong, YAO Houjun, et al. Study on the polysaccharide content and monosaccharide composition of Pseudostellaria heterophylla from different provenances by GC-MS[J]. Natural Products Research and Development,2016,28(4):542−546. XIA Hexian, CHEN Naidong, YAO Houjun, et al. Study on the polysaccharide content and monosaccharide composition of Pseudostellaria heterophylla from different provenances by GC-MS[J]. Natural Products Research and Development, 2016, 28(4): 542-546.
[38] 商佳琦, 邹丹阳, 滕翔宇, 等. 5种食用菌多糖的结构特征及抗氧化活性对比[J]. 食品工业科技,2020,41(15):77−83. [SHANG Jiaqi, ZOU Danyang, TENG Xiangyu, et al. Structural characterization and antioxidant activity of five kinds of edible fungus polysaccharides[J]. Science and Technology of Food Industry,2020,41(15):77−83. SHANG Jiaqi, ZOU Danyang, TENG Xiangyu, et al. Structural characterization and antioxidant activity of five kinds of edible fungus polysaccharides[J]. Science and Technology of Food Industry, 2020, 41(15): 77-83.
[39] 宋立立, 张兆英. 食用菌多糖的生物活性研究现状[J]. 中国食用菌,2019,38(2):14−16. [SONG Lili, ZHANG Zhaoying. The status of biological activity of mushroom polysaccharides[J]. Edible Fungi of China,2019,38(2):14−16. SONG Lili, ZHANG Zhaoying. The status of biological activity of mushroom polysaccharides[J]. Edible Fungi of China, 2019, 38(2): 14-16.
[40] 张丽娟, 王珍, 廖尚高. 太子参多糖对RAW 264.7巨噬细胞免疫调节作用的初步研究[J]. 中国野生植物资源,2018,37(4):14−17. [ZHANG Lijuan, WANG Zhen, LIAO Shanggao. Preliminary study on the immunomodulatory activity of Pseudostellaria polysaccharides on macrophage cell line RAW 264.7[J]. Chinese Wild Plant Resources,2018,37(4):14−17. doi: 10.3969/j.issn.1006-9690.2018.04.003 ZHANG Lijuan, WANG Zhen, LIAO Shanggao. Preliminary study on the immunomodulatory activity of Pseudostellaria polysaccharides on macrophage cell line RAW 264.7[J]. Chinese Wild Plant Resources, 2018, 37(4): 14-17. doi: 10.3969/j.issn.1006-9690.2018.04.003
[41] 方瑶, 毛旭虎. 小鼠巨噬细胞RAW264.7的培养技巧及经验总结[J]. 现代生物医学进展,2012,12(22):4358−4359. [FANG Yao, MAO Xuhu. Cultivation skills and experience summary of mouse macrophage RAW264.7[J]. Progress in Modern Biomedicine,2012,12(22):4358−4359. FANG Yao, MAO Xuhu. Cultivation skills and experience summary of mouse macrophage RAW264.7[J]. Progress in Modern Biomedicine, 2012, 12(22): 4358-4359.
[42] 张勇. 铁皮石斛茎、叶、花多糖理化性质及抗氧化、免疫调节活性研究[D]. 杭州: 浙江大学, 2016. ZHANG Yong. Study on the physicochemical properties and antioxidant and immunomodulatory activities of polysaccharides from stems, leaves and flowers of Dendrobium candidum[D]. Hangzhou: Zhejiang University, 2016.
[43] 亢玉莹. 七种真菌多糖免疫活性的筛选及杏鲍菇多糖免疫机制的研究[D]. 长春: 东北师范大学, 2018. KANG Yuying. Screening of seven fungal polysaccharides for immune activity and study on immune mechanism of Pleurotus eryngii polysaccharide[D]. Changchun: Northeast Normal University, 2018.
[44] 王成蓉, 吴金华, 涂丽蓉, 等. 肉苁蓉多糖对鸡免疫ND苗后血液中T淋巴细胞及IFN-γ活性的影响[J]. 四川畜牧兽医,2008(8):29−30,32. [WANG Chengrong, WU Jinhua, TU Lirong, et al. Effects of Cistanche deserticola polysaccharides on T lymphocytes and IFN-γ activity in the blood of chickens immunized with ND vaccine[J]. Sichuan Animal and Veterinary Sciences,2008(8):29−30,32. doi: 10.3969/j.issn.1001-8964.2008.08.013 WANG Chengrong, WU Jinhua, TU Lirong, et al. Effects of Cistanche deserticola polysaccharides on T lymphocytes and IFN-γ activity in the blood of chickens immunized with ND vaccine[J]. Sichuan Animal and Veterinary Sciences, 2008(8): 29-30, 32. doi: 10.3969/j.issn.1001-8964.2008.08.013
[45] 郑胜眉, 周兴, 黄文涛, 等. 岩白菜素对LPS诱导RAW264.7巨噬细胞炎性因子产生及细胞形态变化的影响[J]. 中药材,2020,43(1):206−210. [ZHENG Shengmei, ZHOU Xing, HUANG Wentao, et al. Effects of Bergenin on LPS-induced inflammatory factor production and cell morphological changes in Raw264.7 cells[J]. Chinese Medicinal Materials,2020,43(1):206−210. ZHENG Shengmei, ZHOU Xing, HUANG Wentao, et al. Effects of Bergenin on LPS-induced inflammatory factor production and cell morphological changes in Raw264.7 cells[J]. Chinese Medicinal Materials, 2020, 43(1): 206-210.
[46] 陈广勇, 韩乾杰, 张玲玲, 等. 人参多糖对脂多糖刺激小鼠巨噬细胞的免疫调控作用[J]. 动物健康,2021,57(2):182−186. [CHEN Guangyong, HAN Ganjie, ZHANG Lingling, et al. The immune regulation effect of ginseng polysaccharide on lipopolysaccharide-stimulated mouse macrophages[J]. Animal Health,2021,57(2):182−186. CHEN Guangyong, HAN Ganjie, ZHANG Lingling, et al. The immune regulation effect of ginseng polysaccharide on lipopolysaccharide-stimulated mouse macrophages[J]. Animal Health, 2021, 57(2): 182-186.








 下载:
下载:

 下载:
下载:



