Research Progress of Dietary Fiber Intervention in Inflammatory Bowel Disease
-
摘要: 炎症性肠病(Inflammatory bowel disease,IBD)是一种慢性、反复发作、久治难愈的胃肠道炎症性疾病,通常伴随着炎性细胞浸润及肠粘膜受损,具有一定的癌变风险。大量研究表明,膳食纤维(Dietary fiber,DF)作为一种益生元,在改善肠道菌群结构、强化肠道屏障功能、缓解肠道炎症等方面功效显著,具有多重营养及保健功能,在干预IBD方面表现出巨大的潜力,但其中的作用机制尚未阐明。此外,DFs由于水溶性不同可分为可溶性膳食纤维(Soluble dietary fiber,SDF)和不溶性膳食纤维(Insoluble dietary fiber,IDF),二者特性不同,对IBD的作用效果也不尽相同。因此,本文就SDF和IDF防治IBD的基本原理、现有证据及其作用机制进行综述,综合分析了IBD的DFs干预治疗策略,为DFs的深入研究和开发应用提供参考。Abstract: Inflammatory bowel disease (IBD) is a chronic, recurrent, and refractory inflammatory disease of the gastrointestinal tract, accompanied by inflammatory cell infiltration and damage to the intestinal mucosa, with a certain risk of cancer. A large number of studies have shown that dietary fiber, as a prebiotic, has significant effects in improving intestinal flora structure, strengthening intestinal barrier function, alleviating intestinal inflammation and other aspects, with multiple nutritional and health functions, showing great potential in the intervention of IBD, but the mechanism of action remains unclear. In addition, DFs can be classified into soluble dietary fiber (SDF) and insoluble dietary fiber (IDF) according to their water solubility, which have different characteristics and effects on IBD. Therefore, this paper reviews the basic principles, existing evidence and mechanism of SDF and IDF in the prevention and treatment of IBD, and comprehensively analyzes DFs intervention strategies for IBD, so as to provide reference for further research, development and application of DFs.
-
Keywords:
- dietary fiber /
- inflammatory bowel disease /
- pathogenic factor /
- action mechanism /
- intervention
-
炎症性肠病(Inflammatory bowel disease,IBD)是种病因尚不明确的肠道慢性炎症病变[1],患者常表现为持续性腹泻、腹痛、带血黏液脓便等胃肠道症状,严重者甚至会出现并发症或发展为结肠癌[1-3]。近年来,随着人们饮食结构逐渐西方化(即高糖、高脂、低纤维),IBD在世界范围内的发病率逐年上升,已成为全球性公共卫生难题[2]。IBD发病机制复杂,涉及多种因素,其中饮食是最重要的环境因素之一。国内外大量文献表明,植物来源的膳食纤维(Dietary fiber,DF)具有多重营养及保健功能,在干预IBD方面已表现出巨大的潜力。因此,本文就IBD的致病因素和DFs干预IBD的作用机制进行综述,为寻求IBD治疗新途径和功能性DFs的开发应用提供理论依据。
1. DFs概述
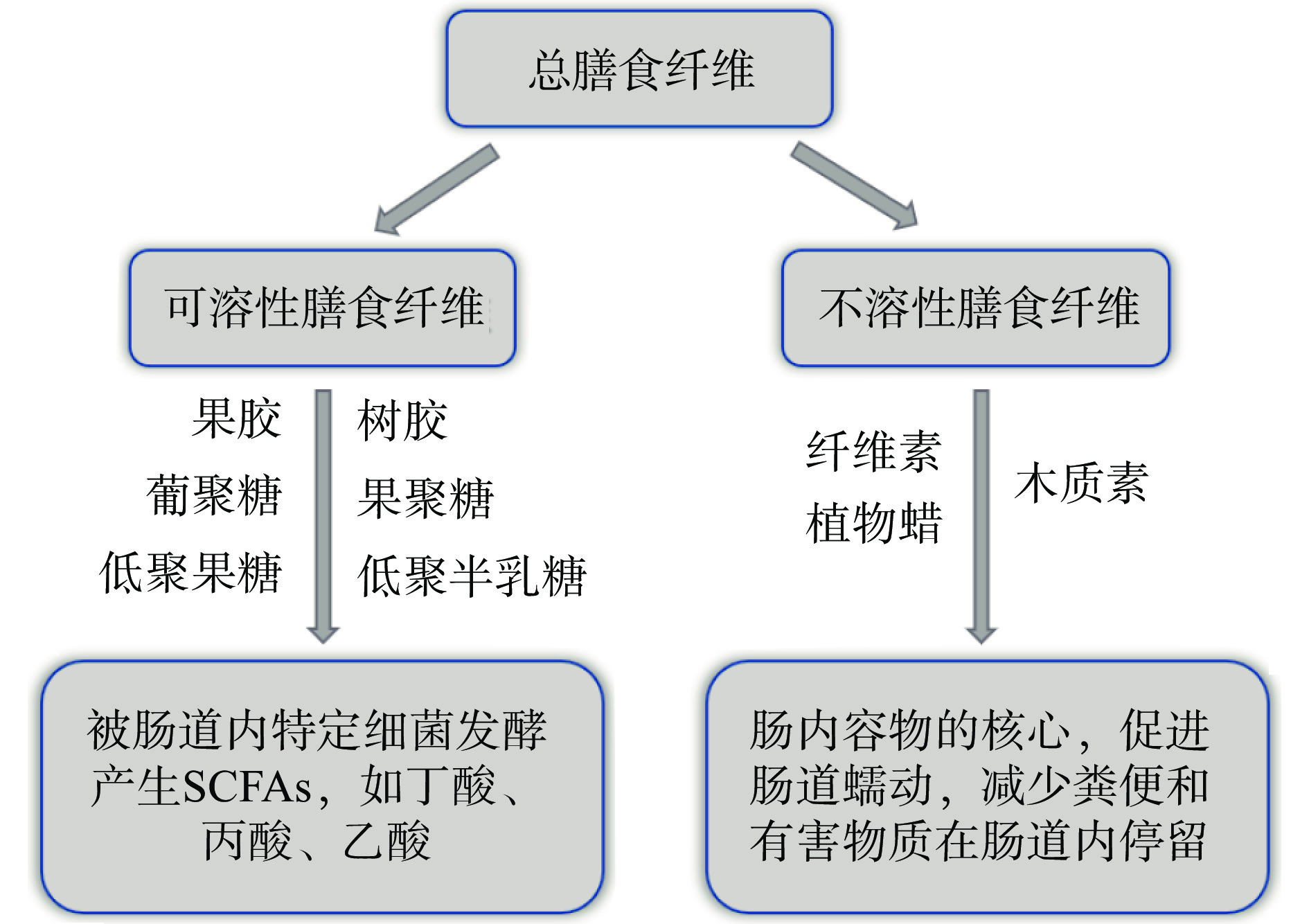
DFs是不能在小肠消化或吸收,但可部分或完全由后肠发酵,产生短链脂肪酸(Short-chain fatty acid,SCFA)等代谢物的可食用植物性成分[4-5]。既往研究表明,DFs在改善肠道菌群结构、强化肠道屏障功能、减轻肠道炎症症状[1, 6-7]等方面功效显著,是缓解及治疗肠道炎症性疾病的理想活性物质。根据水溶性,DFs可分为可溶性膳食纤维(Soluble dietary fiber,SDF)和不溶性膳食纤维(Insoluble dietary fiber,IDF)(图1),二者特性不同,呈现的功能也不尽相同[5]。科学合理地开发功能性DFs资源,不仅可以提高DFs的经济附加值,还能为IBD提供一种新的有效治疗模式,避免药物的毒副作用和残留问题,具有安全、疗效确切、不良反应少等诸多优点。
2. IBD概述
IBD以溃疡性结肠炎(Ulcerative colitis,UC)和克罗恩病(Crohn disease,CD)为代表,二者在临床表现、病理特征、病变范围、诊断治疗等方面存在重叠性和差异性(表1)。UC的病变部位局限于结肠、直肠的黏膜下层和盲肠端,一般从直肠发病,逆行蔓延至结肠,最后累及整个大肠[1, 8],以结肠黏膜和黏膜下层的弥漫性炎症为特征[3]。而CD涉及整个胃肠道,病变可位于口腔到肛门之间的任意胃肠道部位,炎症呈节段性分布,以肠道贯壁性破坏和增值性炎症改变为特征[9]。这两种亚型均可发生于任何年龄阶段,一般易发于20~40岁人群。21世纪以前,IBD高发于北美洲和欧洲等地[10-11]。近年来随着生活方式的西方化以及社会经济的快速发展,亚洲、非洲、南美、东欧等地IBD的发病率和流行率开始迅速攀升[2]。包括中国在内的众多发展中国家,IBD已较为常见,逐步成为消化系统疾病和慢性腹泻的主要原因,严重威胁人类健康,因此也越发受到全球医疗和科研工作者的关注。目前,治疗IBD尚无特效药,临床上主要依赖抗生素、激素等药物控制症状,但以药物治疗为靶点的研究结果喜忧参半,药物残留和细菌耐药性等问题严重威胁人类健康和生态环境的可持续发展[12],因此,寻求对IBD有缓解作用且副作用小的天然活性物质迫在眉睫。
表 1 UC和CD的异同点Table 1. Similarities and differences between UC and CDUC CD 病变范围 结肠、直肠的黏膜下层和盲肠端 口腔到肛门之间的任意胃肠道部位 病理特征 弥漫性、连续性 贯壁性、节段性 发病年龄 任何年龄阶段,一般易发于20~40岁的中、青年人群 症状 持续性腹泻、腹痛、带血黏液脓便、肛门内隐痛、发热、呕吐、体重减轻等 注:UC:Ulcerative colitis,溃疡性结肠炎;CD:Crohn disease,克罗恩病。 3. DFs干预IBD的机制分析
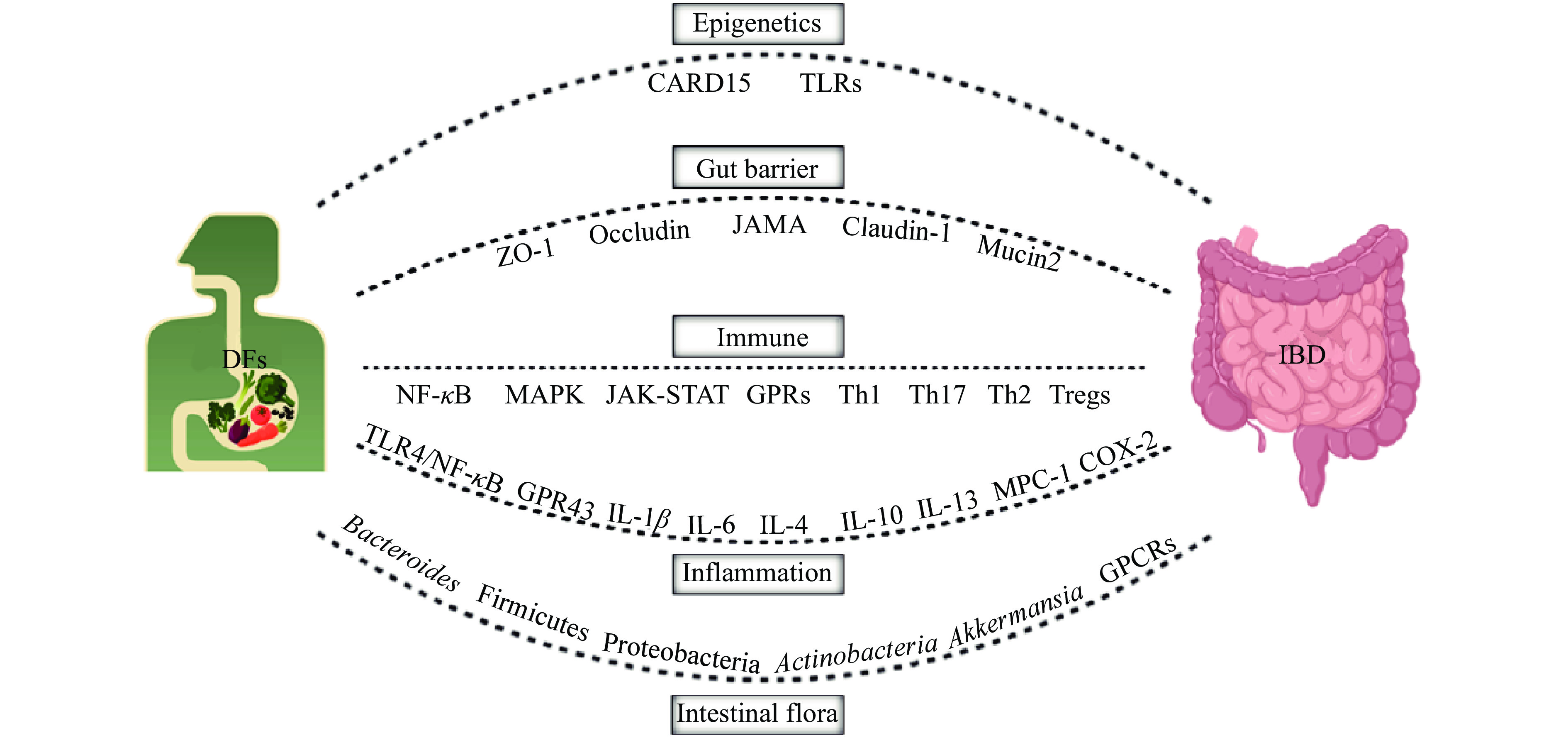
IBD的病因和发病机制尚未完全阐明,目前业界普遍认为是遗传、环境、免疫反应、肠道菌群等多个因素综合作用的结果,深入探究IBD的病因和发病机制将对寻求针对性干预治疗策略起到一定的指导作用。DFs在IBD防治方面虽已表现出巨大的潜力,但其中作用机制尚未十分明确,且SDF与IDF功能特性也不尽相同。已有研究提示,DFs对IBD的缓解作用与改善肠道菌群结构、修复肠黏膜屏障、抗炎、免疫调节等多途径有关(图2)。
3.1 调控表观遗传修饰
表观遗传是指在不改变DNA核苷酸序列的前提下,通过DNA甲基化、组蛋白修饰和RNA甲基化等形式引起基因表达的可遗传性改变,参与多种生命过程,在IBD发病中有重要作用[13]。此外,表观遗传可随地域发生改变,从基因型到表型的改变可能是由于一系列环境因素(营养等)所引起的[14]。最新流行病学资料[2, 10-11]显示,IBD的发病率和流行特点在不同地区、种族间存在较明显的差异。总体看来,英美等西方发达国家IBD的总体发病率远高于亚太地区国家,且IBD后代患CD或UC的危险性比正常人群高2~13倍[15]。表观遗传学相关基因的表达变化与肠黏膜免疫和防御反应密切相关,表明IBD的表观遗传变化和炎症存在直接的关系[14]。目前,与IBD进程相关联的易感基因位点尚未完全探明,多集中在:细胞凋亡募集结构域家族成员15(Caspase recruitment domain-containing protein 15,CARD15)、Toll样受体(Toll-like receptors,TLR)等[15],这些基因可能参与致病过程。营养供给决定了代谢产物的丰度,某些代谢产物是表观遗传酶的必要辅助因子,因此能影响基因表达的表观遗传调控[16]。有研究表明[13],DFs等营养的摄入可以修饰关键基因的表达,改变表观遗传现象,控制机体生理、病理等过程。此外,肠道菌群可能会将饮食因素和消化成分转化为调节炎症表观遗传学的代谢物。目前,DFs影响机体表观遗传修饰调控IBD的研究未见直接报道,但DFs经肠道菌群代谢所产生的SCFAs可提高机体结肠、肝脏、脂肪等组织组蛋白的乙酰化水平[17]。肠道菌群代谢产生的丁酸盐、乙酸盐等生物活性小分子物质,能够参与到宿主表观修饰和生理功能的调控中,从而影响宿主细胞发育、功能以及基因的表达[18]。DFs是否可直接调控或通过肠道菌群代谢间接影响宿主表观遗传过程继而缓解IBD有待深入研究。
3.2 修复肠道屏障
尽管遗传因素在IBD进程中的作用不容忽视,但仅有25%的IBD遗传关联性被阐明,且随着发展中国家物质生活条件的改善,本流行于西方国家的IBD在发展中国家也出现了流行趋势[2]。由此可见,除了遗传因素,饮食等环境因素也是刺激IBD易感人群发病的重要因素[19],可通过影响机体肠道通透性和黏液层等机制调控肠道炎症[20]。肠黏膜屏障是维持机体内环境稳态和肠道通透性稳定的重要结构,肠上皮细胞破坏、肠道紧密连接蛋白异常和肠道通透性增加是IBD的重要病理组织学变化[21]。当肠道不断接受外源性抗原刺激时肠道屏障可被破坏,肠道通透性增加,致使机体产生过度的抗原应激和氧化应激,引发反复炎性反应,最终导致或加重IBD等肠道炎症疾病。研究表明,富含饱和脂肪酸饮食和加工肉类的长期过多摄入会破坏肠道屏障的完整性,增加肠道通透性,提高IBD的患病风险[22]。摄入富含蛋白质食物的人,肠道中大肠杆菌等腐败菌数量明显增多,也会增加IBD的发病风险,这可能与硫化代谢物过多,影响肠上皮细胞的功能有关[23]。据统计,IBD患者总纤维摄入量显著低于健康群体,其中超过80%的IBD患者每日纤维摄入量达不到相应的国家推荐量(25~30 g/d)[24]。补充DFs对预防及缓解IBD具有积极作用。
3.2.1 SDF
SDF对机体肠道屏障的损伤修复作用已有大量报道。摄入10%(w/w)的瓜尔胶(Guar gum,GG)和部分水解瓜尔胶(Partially hydrolyzed guar gum,PHGG)可以缓解DSS诱导IBD大鼠的体重损失、IBD症状及肠黏膜屏障的损伤,抑制中性粒细胞的浸润和积累,上调紧密连接蛋白(ZO-1、Occludin、JAMA等)的表达,且粪便中有机酸含量增加,表明SDF在肠道发酵产生的SCFAs等有机酸是保护屏障功能的重要介质,可改善IBD小鼠的肠道屏障缺陷和炎症,抑制肠道中的炎症免疫反应[25]。SCFAs作为结肠黏膜上皮细胞的主要能量来源,可促进上皮细胞增殖,对维持肠黏膜屏障完整性,防止细菌移位起到重要作用[26]。IRAHA等[27]研究也发现了类似的结果,褐藻SDF可以剂量依赖方式阻止H2O2诱导的肠上皮屏障功能的破坏,显著提升了跨膜阻力,调控细胞通透性,同时上调肠上皮紧密连接蛋白Claudin-1等表达水平,并抑制TNF-α、IFN-γ、IL-13等炎性因子对肠道屏障的损伤,增强上皮的保护功能,促进上皮再生。
3.2.2 IDF
IDF对机体肠道屏障也有较好的保护作用。小鼠饲喂添加1.52%的大麦叶IDF日粮,能够上调DSS诱导结肠炎小鼠结肠紧密连接蛋白Occludin和Mucin 2的蛋白表达,下调Claudin-1的蛋白表达,保护结肠屏障功能[28],而Claudin-1与IBD患者症状严重程度呈正相关,且Claudin-1蛋白表达上调可抑制杯状细胞分化,并通过Notch依赖途径促进结肠癌的发生[29],因此,补充大麦叶IDF可通过降低Claudin-1蛋白表达达到缓解DSS诱导小鼠IBD症状的目的[30],其可能机制是IDF对肠道黏膜有直接的刺激作用,促进肠黏膜细胞的增长,防止肠道内有害菌过度生长及附着,维持肠道正常菌群,进而保护肠道屏障;同时,IDF也可刺激胆汁和胰液分泌,减少胆胰源性肠黏膜萎缩的发生[31-32]。由于木质纤维素在体内较难降解,因此,DFs成分和肠道菌群之间的互作对屏障完整性的调节也至关重要,在制定使用DFs化合物增强肠道屏障完整性的策略时,考虑肠道屏障不同组分间的互作十分必要[27]。
3.2.3 混合DFs
血浆D-乳酸浓度、二胺氧化酶(Dia-mine oxidase,DAO)活性、结肠形态等是评估肠粘膜屏障功能障碍的常用指标。在乙酸灌肠法诱导的大鼠IBD模型中,摄入SDF(菊粉:阿拉伯胶:果糖=1:1:1)和IDF(纤维素:抗性淀粉:大豆纤维=1:1:1)配制的复合DFs,可显著降低IBD大鼠血浆中D-乳酸浓度、DAO活性、结肠大体形态损伤以及结肠病理组织学评分[33],表明DFs的摄入可降低肠粘膜通透性,修复肠粘膜屏障,可能是DFs被肠道菌群酵解产生的SCAFs发挥了作用。AHL等[34]的研究也有类似结果,表明丁酸可显著上调结肠隐窝上皮紧密连接蛋白(ZO-1和Occludin)的蛋白表达,强化肠道屏障功能,缓解肠道炎症症状。综上,DSS诱导IBD小鼠摄入SDF和IDF后,均可通过酵解产生的SCFAs等代谢产物在一定程度上修复肠道屏障功能。通常SDF较IDF更易被菌群发酵产生SCFAs,表现出更多的生物活性,美国供能委员会推荐膳食纤维中IDF占70%~75%,SDF占25%~30%为宜,SDF与IDF的最佳比例受DFs来源、特性等诸多因素影响,还需更多实验进行进一步验证。
3.3 调节机体免疫
IBD是种非特异性肠道炎症疾病,与非特异性免疫关系密切[35]。非特异性免疫能够识别抗原、抑制致病微生物入侵、刺激并调节T/B细胞分化[36],在IBD的发生发展过程中发挥重要作用。初始CD4+T细胞接受抗原刺激后,在不同细胞因子诱导下可分化为不同功能的T细胞亚群,如辅助性T细胞1(T helper cell 1,Th1)、Th17、Th2、调节性T细胞(Regulatory T cells,Tregs)等。研究表明[37-38],UC患者肠黏膜中IL-13、IL-4等Th2相关细胞因子mRNA表达量上调,而CD患者肠粘膜中IL-23、IL-18、IL-12等Th1和Th17相关细胞因子mRNA表达量上调,提示Th2主要介导UC的黏膜炎症,Th1和Th17主要介导CD的透壁炎症。IBD大鼠受到外源性病原体或细胞损伤的刺激时,TLRs会与白细胞分化抗原14(CD-14)和髓样分化蛋白-2(MD-2)共同作用,激活NF-κB和丝裂原活化蛋白激酶(Mitogen-activated protein kinase,MAPK)信号通路,产生大量肿瘤坏死因子α(Tumor necrosis factor-α,TNF-α)、IL-1、IL-6等炎性细胞因子,加重炎症反应[39]。
3.3.1 SDF
SDF及其代谢物可通过受体介导的信号通路,调节机体免疫。海带和酿酒酵母来源的β-葡聚糖可在不改变Tregs相关靶点的情况下经JAK-STAT途径对Th17相关细胞因子(IL-17A、IL-17F和IL-22)及受体(IL-23R和IL-6)的表达均有免疫抑制作用,能够减轻IBD[40]。此外,阿拉伯木糖和菊粉饲喂能显著提升大鼠肠道中的黏蛋白水平,进而促进Akkermansia利用黏蛋白代谢产生丙酸,并通过Gpr43受体作用于肠道组织,从而引起一系列通路变化以达到免疫调节作用[41]。也有研究表明,SDF的代谢产物SCFAs可通过GPR途径激活NALP6蛋白,促进肠道黏膜中杯状细胞分泌黏液,使细菌和肠上皮细胞分离,放在细菌毒素入血引起过度免疫反应[42]。可见,不同来源的SDF调节肠道免疫途径不尽相同,可通过活化免疫细胞,调控炎性因子分泌,阻止有害菌黏附等途径缓解IBD。
3.3.2 IDF
IDF通过调节肠道免疫缓解IBD的报道相对较少,通常以肠道菌群代谢产物或调控菌群方式间接影响肠道免疫。野山杏果肉IDF在肠道内酵解产生的SCFAs可降低肠道内pH,起到抑制大肠杆菌、金黄色葡萄球菌,促进乳酸杆菌和双歧杆菌的作用[43]。乳酸杆菌、双歧杆菌等益生菌的益生效果归因于其代谢产物(SCFAs、细菌素、氢过氧化物、二级胆汁酸和乳酸等)能够促进肠道环境中细胞成分的释放,进而激活免疫反应,调节IBD中的肠道微生态失衡[44]。乳酸菌能够调节Treg细胞频率和树突状细胞(Dendriticceils,DCs)的激活,与DCs细胞之间的互作可能会影响随后对Th1、Th2、Th17或Treg细胞的抗原特异性T细胞应答[45]。此外,饲喂高纤维素饲料的小鼠显示其褪黑素受体(Melatonin receptors-1/2,Mt1和Mt2)基因表达增加,这些基因已被发现通过抗凋亡和免疫调节在结肠炎等验证模型中发挥保护作用[46]。总之,IDF调节肠道免疫的功能十分有限,与SDF联用也许能发挥更好的免疫调节作用。
3.3.3 混合DFs
DFs被肠道菌群酵解产生的SCFAs(及其盐类)可调节T细胞、B细胞、免疫细胞(巨噬细胞、Tregs等)以及多种信号通路(TLRs、NF-κB等)参与免疫调节,并抑制促炎因子(IL-1、IL-6、IL-17、TNF-α等)、促进抗炎因子(IL-4、IL-10、IL-13等)等的表达,进而修复损肠粘膜,减轻肠道炎症[47]。羊栖菜和薏苡的DFs能抑制NF-κB转录激活以及炎性细胞中髓过氧化物酶的活性,阻止中性粒细胞和巨噬细胞的浸润,最终实现DFs对肠道的保护[48]。此外,丁酸盐可抑制脂多糖诱导的单核细胞NF-κB等炎性因子表达及迁移,NF-κB的转录活性也随之降低,达到缓解肠道炎症的目的[47]。因此,DFs酵解产生的SCFAs(及其盐类)可介导NF-κB等信号通路在免疫和炎症反应中发挥积极作用,也可作用于免疫细胞,影响其分化、增殖和凋亡,进而影响IBD的发生发展进程。此外,SDF和IDF可通过调节肠道菌群结构,上调益生菌(乳酸菌、拟杆菌等)丰度,进而与免疫系统互作维持肠道健康,间接参与机体免疫调控[28, 49]。
3.4 缓解肠道炎症
炎症是机体对于刺激的一种防御反应,能够保护宿主免受非生物和生物因素引发的伤害,炎性因子是治疗肠道炎症的潜在靶点之一。炎性因子包括促炎因子(IL-1、IL-6、IL-17、TNF-α等)和抗炎因子(IL-4、IL-10、IL-13等),二者的动态表达是平衡宿主正常免疫的关键,也是导致IBD潜在病理学的重要原因。DFs(或肠道菌群代谢产物SCFAs)具有较好的抗炎作用,能促进抗炎因子的表达,抑制促炎因子的表达[50],从而缓解IBD等炎性疾病。
3.4.1 SDF
SDF能被肠道菌群代谢分解为乙酸、丙酸、丁酸等SCFAs,发挥抗炎效果。阿拉伯木聚糖可通过激活INF-γ依赖的Thp 1样免疫反应发挥抗炎作用[51],还可通过上调TLR-4刺激COX-2,并有助于减少结肠癌细胞系中的促炎细胞因子IL-8和TNF-α表达[52]。果胶在结肠内可被多种微生物(拟杆菌、芽孢杆菌、酵母等)完全酵解产生SCFAs及气体(CO2、H2、CH4等)[53],抑制toll样受体(TLR)1,2,4通路[54],减少促炎细胞因子IL-1β表达,降低小鼠的结肠炎症反应[55]。相比DSS模型组,IBD小鼠饲喂大豆皮SDF和IDF(每次100 mg/kg体重,每日三次)均可下调结肠NF-κB的蛋白表达和抑制TNF-α的激活,进而阻断TLR-4/NF-κB炎症信号通路缓解IBD,但SDF对IBD小鼠的治疗效果优于IDF,可能是由于SDF具有较强的发酵特性[49]。在IBD的多种动物模型中,饲粮添加低聚果糖、果胶等SDF已被证明可以缓解肠道炎症,其效果是增加了SCFAs产量或改变微生物菌群结构[22, 56]。在一些研究中,DFs补充剂只有结合特定饮食才有效,这也为了解饮食与DFs补充剂间的协同效应机制提供了线索[56-57]。
3.4.2 IDF
高DFs饮食通过改变小鼠肠道菌群和代谢物维持肠道内环境平衡,进而达到改善肠道炎症,预防IBD的效果[46],并且可以通过全身抗炎作用提高败血症小鼠的存活率[53]。饲喂添加1.52%的大麦叶IDF日粮,小鼠体内脱氧胆酸、熊去氧胆酸、石胆酸水平显著升高,与体重减轻、病理评分、DAI评分和促炎细胞因子(IL-6、TNF-α和IL-1β)表达呈高度负相关,显著减轻DSS诱导的急性IBD症状[28],其机制可能是微生物源胆汁酸可通过胆汁酸核受体增加结肠ROR γ+ Treg细胞的数量,减轻宿主对IBD的易感性,从而缓解IBD动物模型的肠道屏障损伤和炎症。此外,纤维素可被微生物(拟杆菌、梭菌、瘤胃球菌等)发酵生产SCFAs等产物,SCFAs进一步通过GPR43介导途径发挥抗炎作用,而GPR43在结肠上皮和免疫细胞均表达,GPR43的缺失可加重IBD[22]。
3.4.3 混合DFs
在DSS诱导的高脂饮食小鼠IBD模型中发现,摄入豌豆DFs可通过改善IBD小鼠的疾病活动指数评分,降低中性粒细胞浸润,抑制单核细胞趋化蛋白1(Monocyte chemoattractant protein,MPC-1)和炎症标志物(IL-6、IL-17、环氧合酶2(Cyclooxygenase-2,COX-2)等)的mRNA表达等途径发挥抗炎作用,缓解IBD症状[58]。此外,糙米DFs也可通过抑制IBD小鼠炎症因子(COX-2,TNF-α,IL-1β等)表达、上调调节性T细胞比例和盲肠细菌多样性(类杆菌丰度下调,肠球菌丰度上调,其中类杆菌可导致腹泻,与IBD和结直肠癌进程有关)等途径缓解小鼠结肠炎症[59],机制可能是肠内菌群酵解DFs释放的SCFAs和酚类化合物被肠细胞重吸收入血,进而发挥抗炎作用[60]。总的来说,DFs可被肠道菌群发酵,改善菌群多样性,维持肠道屏障功能,此外酵解产生具有抗炎特性的SCFAs,可抑制NF-κB等促炎因子的转录,缓解肠道炎症,降低患IBD的风险[61]。
3.5 调节肠道菌群
肠道菌群指寄居在肠道内的微生物群(包括细菌、真菌、病毒和原生动物),是连接外界环境和肠道黏膜的纽带,与肠道健康关系密切,也是IBD治疗药物的重要靶点[62]。肠道菌群失调,会激活T淋巴细胞,进而产生一系列细胞因子,促发不同特征的免疫病理损伤。研究表明[63],相比健康受试者,IBD患者肠道菌群多样性及丰度差异显著,其中拟杆菌门(Bacteroides)和厚壁菌门(Firmicutes)中的乳酸杆菌(Lactobacillus)、双歧杆菌(Bifidobacterium)等有益菌群丰度显著下调,变形菌门(Proteobacteria)和放线菌门(Actinobacteria)中的大肠杆菌(Escherichia coli,E. coli)等潜在致病菌的丰度则显著上调。其他研究也有类似结果[64-65]。据统计,健康人的胃肠道菌群以厚壁菌门和拟杆菌门成员为主,而IBD则表现为厚壁菌门比例较低、变形菌门丰度增加为主要特征的菌群失调有关[66]。饮食摄入的DFs主要借助肠道菌群的中介作用防治IBD等肠道炎症疾病,主要表现在对肠道菌群多样性、丰度及代谢产物的影响[22],还可为肠道菌群提供生长底物,改善有益菌的生存环境,维护肠道内环境稳态[67]。
3.5.1 SDF
SDFs由于其可发酵性,能较显著影响肠道菌群。大鼠经高脂饮食和注射低剂量链脲佐菌素(30 mg/kg体重)诱导肠道菌群紊乱模型,每天摄入菊粉(3 g/kg体重)可上调大鼠肠道内乳酸杆菌(Lactobacillus)、拟杆菌(Bacteroides)、考拉杆菌(Phascolarctobacterium)等有益菌丰度,下调大肠杆菌等有害菌丰度,改善大鼠肠道菌群失调,促进肠道菌群结构正常化[6]。通过对64项相关指标进行Meta分析也发现类似结果[68],表明SDFs,特别是果聚糖和低聚半乳糖的干预,会显著上调肠道乳酸杆菌(Lactobacillus)、双歧杆菌(Bifidobacterium)等益生菌的丰度。SDF发酵产生的SCFAs(乙酸酯、丁酸酯、丙酸酯等)通过多途径发挥益生作用,可为结肠黏膜细胞提供能量,减轻IBD炎症[69-70];也可以降低肠腔pH,使肠道呈酸性状态,有助于共生保护菌株的生长,抑制拟杆菌科等病原菌的定植[71]。乳酸菌、肠球菌、双歧杆菌等肠道菌群直接负责SCFAs的产生[72-73],SCFAs产生的数量和速率取决于肠道菌群的丰度和多样性[74],这也说明了可通过调控肠道菌群影响SCFAs的产生和吸收,缓解IBD。
3.5.2 IDF
IDF对机体肠道菌群结构也具有重要调节作用。小鼠饲喂添加1.52%的大麦叶IDF日粮28 d,可显著减轻DSS诱导的急性IBD症状,提高Parasutterella(参与胆汁酸代谢),Erysipelatoclostridium(丁酸产生菌)和Alistipes(与IBD症指数高度负相关)的丰度,显著降低Akkermansia丰度,其中,Akkermansia具有黏蛋白降解特性,过量Akkermansia会加剧DSS诱导IBD小鼠肠道屏障的破坏,加剧症状[75];当抗生素耗尽肠道菌群时,IDF的抗IBD作用随之消失,表明IDF的抗IBD作用高度依赖于肠道菌群[76];通过微生物代谢产物分析,发现IDF可增加DSS诱导小鼠粪便中SCFAs和二级胆汁酸的产量,表明大麦叶IDF可通过调节小鼠肠道菌群组成和增加菌群衍生代谢物,激活特异性G蛋白偶联受体(Gprotein-coupled receptors,GPCRs),抑制组蛋白去乙酰化酶,抑制促炎因子(IL-6、TNF-α、IL-1β)表达,强化屏障功能,发挥抗炎作用[28],类似研究结果在富含低聚果糖的菊粉、抗性淀粉和车前草DF中均有报道[28]。
3.5.3 混合DFs
DFs在调节机体肠道菌群结构、改善肠道健康方面已有诸多报道[77-78]。SHANG等[79]研究发现,妊娠后期母猪饲粮中添加麦麸DFs,可显著提升仔猪结肠中的乳杆菌科丰度,进而改善肠道健康。SDF和IDF虽然物化特性不同,但经肠道菌群酵解的主要代谢产物SCFAs(包括丁酸盐、丙酸盐等),可作为信号分子通过信号转导途径调节宿主体内多种代谢通路[80],维护肠道微生态平衡。研究发现[81],结肠癌患者粪便中丁酸盐、乙酸盐的水平显著低于健康群体,而DFs的摄入能够显著提高乙酸盐的水平,缓解肠道炎症,表明摄入高DFs食物,可以调节肠道SCFAs产量及组分。因此,高DFs饮食的保健功能可能很大程度上与SCFAs的益生作用有关。
3.6 抑制有害菌
活动期IBD患者肠道菌群紊乱,条件性致病菌(肠杆菌、变形杆菌等)丰度增加,可加重IBD肠道损伤症状[81]。桂玲等[82]通过体外研究发现,大豆DFs在酶解(50 ℃,pH4.8,半纤维素酶)初始阶段对致病菌(大肠杆菌、金黄色葡萄球菌、假单胞菌等)无抑菌效果,酶解160 min后出现抑菌效果,240 min后抑菌效果趋于稳定,抑菌圈直径可达9.7~11.3 mm,最低抑菌浓度为5.9%,可能是由于大豆DFs酶解到一定分子量,低聚半乳糖醛酸和半乳糖醛酸等产物可表现出明显的抑菌性。同样,山楂果胶本身也无抑菌活性,但被果胶酶水解到一定程度后,会产生小片段聚半乳糖醛酸等果胶寡糖,对大肠杆菌、金黄色葡萄球菌等均表现出较强的抑菌作用[83]。而草食动物肠道中枯草芽孢杆菌、黑曲霉等益生菌均可分泌半纤维素酶、果胶酶等。通常,IDF相比SDF更难降解,且酶解效率与酶活,酶解温度、pH,纤维来源、结构等因素均有关系。IBD患者也可适当补充益生菌促进DFs的酵解和代谢,预防或缓解IBD的发展。此外,DFs的另一酵解产物SCFAs,同样具有较好的抑菌效果[59]。
4. 结语与展望
综上所述,补充SDF和IDF均能起到一定的预防和辅助治疗IBD的效果,SDF由于更易被肠道菌群酵解产生SCFAs(及其盐类)、次级胆汁酸等代谢产物,因而能通过强化肠道屏障功能,抑制有害菌、促进有益菌生长,调节肠道不同类型细胞的免疫反应,抑制炎性因子表达,阻止炎性细胞浸润等多途径表现出更强的缓解肠道炎症的功效,发挥益生功能。DFs在IBD中的作用机制仍未十分明确,很难确认哪一种机制在DFs缓解IBD中占据主导地位,且不同来源及特性的DFs其物化、结构和功能特性也不尽相同,对IBD的治疗作用还需进一步研究。虽然目前多数文献指出DFs缓解IBD可能基于肠道菌群酵解产生的SCFAs,但随着代谢组学和超高效液相色谱技术的发展,更多菌群代谢物的结构和功能将被解析,有助于更深入挖掘DFs缓解IBD的机制。此外,目前多数研究数据仅限于得到最佳DFs添加水平和治疗效果,而饮食本身是复杂的,需要把饮食作为一个整体来评估,因而不同类型的高纤新型饮食模式对IBD的治疗效果也需更多的临床研究来证实。
-
表 1 UC和CD的异同点
Table 1 Similarities and differences between UC and CD
UC CD 病变范围 结肠、直肠的黏膜下层和盲肠端 口腔到肛门之间的任意胃肠道部位 病理特征 弥漫性、连续性 贯壁性、节段性 发病年龄 任何年龄阶段,一般易发于20~40岁的中、青年人群 症状 持续性腹泻、腹痛、带血黏液脓便、肛门内隐痛、发热、呕吐、体重减轻等 注:UC:Ulcerative colitis,溃疡性结肠炎;CD:Crohn disease,克罗恩病。 -
[1] NIU W, CHEN X, XU R, et al. Polysaccharides from natural resources exhibit great potential in the treatment of ulcerative colitis: A review[J]. Carbohydrate Polymers,2021,254:1−12.
[2] NG S C, SHI H Y, HAMIDI N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies[J]. The Lancet,2017,390(10114):2769−2778. doi: 10.1016/S0140-6736(17)32448-0
[3] NAKADA N, MIKAMI T, HORIE K, et al. Expression of CA2 and CA9 carbonic anhydrases in ulcerative colitis and ulcerative colitis-associated colorectal cancer[J]. Pathology International,2020,70(8):523−532. doi: 10.1111/pin.12949
[4] 张玲, 陈代文, 余冰, 等. 两种类型膳食纤维对BALB/c小鼠结肠细菌群落结构的影响[J]. 微生物学通报,2018,45(2):395−404. [ZHANG L, CHEN D W, YU B, et al. Two dietary fibers influence the bacterial community in the colon of BALB/c mice[J]. Microbiology China,2018,45(2):395−404. doi: 10.13344/j.microbiol.china.170320 ZHANG L, CHEN D W, YU B, et al. Two dietary fibers influence the bacterial community in the colon of BALB/c mice[J]. Microbiology China, 2018, 45(2): 395-404. doi: 10.13344/j.microbiol.china.170320
[5] QIAO H, SHAO H, ZHENG X, et al. Modification of sweet potato (Ipomoea batatas Lam.) residues soluble dietary fiber following twin-screw extrusion[J]. Food Chemistry,2021,335:1−10.
[6] ZHANG Q, YU H, XIAO X, et al. Inulin-type fructan improves diabetic phenotype and gut microbiota profiles in rats[J]. PeerJ,2018,6:1−24.
[7] WILMS E, JONKERS D, SAVELKOUL H, et al. The impact of pectin supplementation on intestinal barrier function in healthy young adults and healthy elderly[J]. Nutrients,2019,11(7):1−16.
[8] 宋亚芳, 裴丽霞, 赵婷婷, 等. 溃疡性结肠炎免疫因素发病机制的研究进展[J]. 医学研究生学报,2019,32(4):432−436. [SONG Y F, PEI L X, ZHAO T T, et al. Research progress on the pathogenesis of immune factors in ulcerative colitis[J]. Journal of Medical Postgraduates,2019,32(4):432−436. doi: 10.16571/j.cnki.1008-8199.2019.04.019 SONG Y F, PEI LX, ZHAO T T, et al. Research progress on the pathogenesis of immune factors in ulcerative colitis[J]. Journal of Medical Postgraduates, 2019, 32(4): 432-436. doi: 10.16571/j.cnki.1008-8199.2019.04.019
[9] CARUSO R, MATHES T, MARTENS E C, et al. A specific gene-microbe interaction drives the development of Crohn's disease-like colitis in mice[J]. Science Immunology,2019,4(34):1−15.
[10] KAPLAN G G, WINDSOR J W. The four epidemiological stages in the global evolution of inflammatory bowel disease[J]. Nature Reviews Gastroenterology & Hepatology,2020,18(1):56−66.
[11] KING D, REULEN R C, THOMAS T, et al. Changing patterns in the epidemiology and outcomes of inflammatory bowel disease in the United Kingdom: 2000-2018[J]. Alimentary Pharmacology & Therapeutics,2020,51(10):922−934.
[12] QIAO M, YING G, SINGER A C, et al. Review of antibiotic resistance in China and its environment[J]. Environment International,2018,110:160−172. doi: 10.1016/j.envint.2017.10.016
[13] JANG H, SERRA C. Nutrition, epigenetics, and diseases[J]. Clinical Nutrition Research,2014,3(1):1−8. doi: 10.7762/cnr.2014.3.1.1
[14] 黄艳, 窦传字, 刘慧荣, 等. 表观遗传修饰与溃疡性结肠炎[J]. 中国组织工程研究,2015,19(7):1099−1103. [HUANG Y, DOU C Z, LIU H R, et al. Ulcerative colitis and epigenetic modification[J]. Chinese Journal of Tissue Engineering Research,2015,19(7):1099−1103. doi: 10.3969/j.issn.2095-4344.2015.07.021 HUANG Y, DOU C Z, LIU H R, et al. Ulcerative colitis and epigenetic modification[J]. Chinese Journal of Tissue Engineering Research, 2015, 19(7): 1099-1103. doi: 10.3969/j.issn.2095-4344.2015.07.021
[15] 艾静, 王承党. 遗传与环境因素在炎症性肠病发病机制中的作用研究[J]. 国际消化病杂志,2014,34(2):110−113. [AI J, WANG C D. The role of genetic and environmental factors in the pathogenesis of inflammatory bowel disease[J]. International Journal of Digestive Diseases,2014,34(2):110−113. doi: 10.3969/j.issn.1673-534X.2014.02.012 AI J, WANG C D. The role of genetic and environmental factors in the pathogenesis of inflammatory bowel disease[J]. International Journal of Digestive Diseases, 2014, 34(2): 110-113. doi: 10.3969/j.issn.1673-534X.2014.02.012
[16] BULTMAN S J. Interplay between diet, gut microbiota, epigenetic events, and colorectal cancer[J]. Molecular Nutrition & Food Research,2017,61(1):1−12.
[17] KRAUTKRAMER K A, KREZNAR J H, ROMANO K A, et al. Diet-microbiota interactions mediate global epigenetic programming in multiple host tissues[J]. Molecular Cell,2016,64(5):982−992. doi: 10.1016/j.molcel.2016.10.025
[18] FRANK D N, ST AMAND A L, FELDMAN R A, et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases[J]. PNAS,2007,104(34):13780−13785. doi: 10.1073/pnas.0706625104
[19] ANANTHAKRISHNAN A N, BERNSTEIN C N, ILIOPOULOS D, et al. Environmental triggers in IBD: A review of progress and evidence[J]. Nature Reviews Gastroenterology & Hepatology,2018,15(1):39−49.
[20] ALEXA N, SASSON M F A N, RAMAN M M F. Diet in treatment of inflammatory bowel diseases[J]. Clinical Gastroenterology and Hepatology,2019,19(3):425−435.
[21] 李瑶, 黄金莉, 黄娟, 等. 肠道菌群与肠道屏障互作在炎症性肠病中的作用研究进展[J]. 胃肠病学和肝病学杂志,2021,30(1):10−15. [LI Y, HUANG J L, HUANG J, et al. Advance in study on interaction between gut microbiota and intestinal barrier ininflammatory bowel disease[J]. Chinese Journal of Gastroenterology and Hepatology,2021,30(1):10−15. doi: 10.3969/j.issn.1006-5709.2021.01.003 LI Y, HUANG J L, HUANG J, et al. Advance in study on interaction between gut microbiota and intestinal barrier ininflammatory bowel disease[J]. Chinese Journal of Gastroenterology and Hepatology, 2021, 30(1): 10-15. doi: 10.3969/j.issn.1006-5709.2021.01.003
[22] LEWIS J D, ABREU M T. Diet as a trigger or therapy for inflammatory bowel diseases[J]. Gastroenterology,2017,152(2):398−414. doi: 10.1053/j.gastro.2016.10.019
[23] 杨小冰, 金明玉, 吴小禾, 等. 膳食营养素与炎症性肠病关系研究进展[J]. 食品科学,2019,40(9):309−315. [YANG X B, JIN M Y, WU X H, et al. Progress in understanding the relationship between dietary nutrients and inflammatory bowel disease[J]. Food Science,2019,40(9):309−315. doi: 10.7506/spkx1002-6630-20180129-404 YANG X B, JIN M Y, WU X H, et al. Progress in understanding the relationship between dietary nutrients and inflammatory bowel disease[J]. Food Science, 2019, 40(9): 309-315. doi: 10.7506/spkx1002-6630-20180129-404
[24] DAY A S, DAVIS R, COSTELLO S P, et al. The adequacy of habitual dietary fiber intake in individuals with inflammatory bowel disease: A systematic review[J]. Journal of the Academy of Nutrition and Dietetics,2021,121(4):688−708. doi: 10.1016/j.jand.2020.12.001
[25] HUNG T V, SUZUKI T. Dietary fermentable fiber reduces intestinal barrier defects and inflammation in coliticmice[J]. The Journal of Nutrition,2016,146(10):1970−1979. doi: 10.3945/jn.116.232538
[26] HAMER H M, JONKERS D, VENEMA K, et al. Review article: The role of butyrate on colonic function[J]. Alimentary Pharmacology & Therapeutics,2008,27(2):104−119.
[27] IRAHA A. Fucoidan enhances intestinal barrier function by upregulating the expression of Claudin-1[J]. World Journal of Gastroenterology,2013,19(33):5500−5507. doi: 10.3748/wjg.v19.i33.5500
[28] TIAN M, LI D, MA C, et al. Barley leaf insoluble dietary fiber alleviated dextran sulfate sodium-induced mice colitis by modulating gut microbiota[J]. Nutrients,2021,13(3):846. doi: 10.3390/nu13030846
[29] WEBER C, NALLE S, TRETIAKOVA M, et al. Claudin-1 and Claudin-2 expression is elevated in inflammatory bowel disease and may contribute to early neoplastic transformation[J]. Laboratory Investigation,2008,10(88):1110−1120.
[30] GOWRIKUMAR S, AHMAD R, UPPADA S B, et al. Upregulated Claudin-1 expression promotes colitis-associated cancer by promoting β-catenin phosphorylation and activation in Notch/p-AKT-dependent manner[J]. Oncogene,2019,38(26):5321−5337. doi: 10.1038/s41388-019-0795-5
[31] LEI L, WALKER W A. Pathologic and physiologic interactions of bacteria with the gastrointestinal epithelium[J]. American Journal of Clinical Nutrition,2001,6(73):1124−1130.
[32] 陈德国, 武华. 复合膳食纤维对溃疡性结肠炎患者肠黏膜屏障功能的影响[J]. 中国医药导报,2013,10(4):34−38. [CHEN D G, WU H. Influence of dietary fiber complex on intestinal mucosa barrier in patients with ulcerative colitis[J]. China Medical Herald,2013,10(4):34−38. doi: 10.3969/j.issn.1673-7210.2013.04.013 CHEN D G, WU H. Influence of dietary fiber complex on intestinal mucosa barrier in patients with ulcerative colitis[J]. China Medical Herald, 2013, 10(4): 34-38. doi: 10.3969/j.issn.1673-7210.2013.04.013
[33] 张睿. 复合膳食纤维对实验性结肠炎大鼠肠黏膜屏障功能的保护作用[D]太原: 山西医科大学, 2010. ZHANG R. Effect of dietary fiber complex on intestinal mucosal barrier in rats with experimental colitis[D]. Taiyuan: Shanxi Medical University, 2010.
[34] AHL D, LIU H, SCHREIBER O, et al. Lactobacillus reuteri increases mucus thickness and ameliorates dextran sulphate sodium-induced colitis in mice[J]. Acta Physiologica,2016,217(4):300−310. doi: 10.1111/apha.12695
[35] 田亚针, 张晨曦, 杨涛, 等. 益生菌和粪菌移植调节炎症性肠病的研究进展[J]. 食品科学,2021,42(19):250−259. [TIAN Y Z, ZHANG C X, YANG T, et al. Research progress of probiotics and fecal microbiota transplantation in regulating inflammatory bowel disease[J]. Food Science,2021,42(19):250−259. doi: 10.7506/spkx1002-6630-20200914-166 TIAN Y Z, ZHANG C X, YANG T, et al. Research progress of probiotics and fecal microbiota transplantation in regulating inflammatory bowel disease[J]. Food Science, 2021, 42(19): 250-259. doi: 10.7506/spkx1002-6630-20200914-166
[36] RAMOS G P, PAPADAKIS K A. Mechanisms of disease: Inflammatory bowel diseases[J]. Mayo Clinic Proceedings,2019,94(1):155−165. doi: 10.1016/j.mayocp.2018.09.013
[37] DAVIS F P, KANNO Y, O'SHEA J J. A metabolic switch for Th17 pathogenicity[J]. Cell,2015,163(6):1308−1310. doi: 10.1016/j.cell.2015.11.033
[38] GONCALVES P, ARAUJO J R, DI SANTOJ P. A cross-talk between microbiota-derived short-chain fatty acids and the host mucosal immune system regulates intestinal homeostasis and inflammatory bowel disease[J]. Inflammatory Bowel Diseases,2018,24(3):558−572. doi: 10.1093/ibd/izx029
[39] CHAMANARA M, RASHIDIAN A, MEHR S E, et al. Melatonin ameliorates TNBS-induced colitis in rats through the melatonin receptors: Involvement of TLR4/MyD88/NF-κB signalling pathway[J]. Inflammopharmacology,2019,27(2):361−371. doi: 10.1007/s10787-018-0523-8
[40] RYAN M T, O'SHEA C J, COLLINS C B, et al. Effects of dietary supplementation with Laminaria hyperborea, Laminaria digitata, and Saccharomyces cerevisiae on the IL-17 pathway in the porcine colon[J]. Journal of Animal Science,2012,90(4):263−265.
[41] MASLOWSKI K M, VIEIRA A T, NG A, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43[J]. Nature,2009,461(7268):1282−1286. doi: 10.1038/nature08530
[42] 曹峻菡, 林鹏程, 王艳峰, 等. 海藻膳食纤维改善炎症性肠病(IBD)的作用机制研究进展[J]. 食品与机械,2021,37(6):1−7. [CAO J H, LIN P C, WANG Y F, et al. Research progress on the mechanism of seaweed dietary fiber in improving inflammatory bowel disease (IBD)[J]. Food & Machinery,2021,37(6):1−7. doi: 10.13652/j.issn.1003-5788.2021.06.001 CAO J H, LIN P C, WANG Y F, et al. Research progress on the mechanism of seaweed dietary fiber in improving inflammatory bowel disease (IBD) [J]. Food & Machinery, 2021, 37(6): 1-7. doi: 10.13652/j.issn.1003-5788.2021.06.001
[43] 彭禛菲, 阿依姑丽·艾合麦提, 王妙颖, 等. 野山杏果肉不溶性膳食纤维对小鼠肠道功能及肠道菌群的影响[J]. 食品工业科技,2020,41(8):307−310. [PENG Z F, AYGUL AHMAT, WANG M Y, et al. Effects of insoluble dietary fiber from wild apricot flesh on intestinal function and intestinal flora of mice[J]. Science and Technology of Food Industry,2020,41(8):307−310. doi: 10.13386/j.issn1002-0306.2020.08.049 PENG Z F, AYGUL AHMAT, WANG M Y, et al. Effects of insoluble dietary fiber from wild apricot flesh on intestinal function and intestinal flora of mice[J]. Science and Technology of Food Industry, 2020, 41(8): 307-310. doi: 10.13386/j.issn1002-0306.2020.08.049
[44] MARKOWIAK P, ŚLIŻEWSKA K. Effects of probiotics, prebiotics, and synbiotics on human health[J]. Nutrients,2017,9(9):1−12.
[45] BERMUDEZ-BRITO M, BORGHUIS T, DANIEL C, et al. L. plantarum WCFS1 enhances Treg frequencies by activating DCs even in absence of sampling of bacteria in the Peyer patches[J]. Scientific Reports,2018,8(1):1−10.
[46] SCHOELER M, CAESAR R. Dietary lipids, gut microbiota and lipid metabolism[J]. Reviews in Endocrine and Metabolic Disorders,2019,20(4):461−472. doi: 10.1007/s11154-019-09512-0
[47] SEGAIN J P, DE LA BLETIERE R, BOURREILLE A, et al. Butyrate inhibits inflammatory responses through NF-κB inhibition: Implications for Crohn's disease[J]. Gut,2000,47(3):397−403. doi: 10.1136/gut.47.3.397
[48] AZUMA K, OSAKI T, IFUKU S, et al. Suppressive effects of cellulose nanofibers-made from adlay and seaweed-on colon inflammation in an inflammatory bowel-disease model[J]. Bioactive Carbohydrates and Dietary Fibre,2013,2(1):65−72. doi: 10.1016/j.bcdf.2013.09.006
[49] YANG L, LIN Q, HAN L, et al. Soy hull dietary fiber alleviates inflammation in BALB/c mice by modulating the gut microbiota and suppressing the TLR-4/NF-κB signaling pathway[J]. Food & Function,2020,11(7):5965−5975.
[50] CAPITÁN-CAÑADAS F, ORTEGA-GONZÁLEZ M, GUADIX E, et al. Prebiotic oligosaccharides directly modulate proinflammatory cytokine production in monocytes via activation of TLR4[J]. Molecular Nutrition & Food Research,2014,58(5):1098−1110.
[51] OGAWA K, TAKEUCHI M, NAKAMURA N. Immunological effects of partially hydrolyzed arabinoxylan from corn husk in mice[J]. Bioscience, Biotechnology & Biochemistry,2005,69(1):19−25.
[52] MENDIS M, LECLERC E, SIMSEK S. Arabinoxylan hydrolyzates as immunomodulators in Caco-2 and HT-29 colon cancer cell lines[J]. Food & Function,2017,8(1):220−231.
[53] ARMSTRONG H, MANDER I, ZHANG Z, et al. Not all fibers are born equal; variable response to dietary fiber subtypes in IBD[J]. Frontiers in Pediatrics,2021,8:1−15.
[54] SALMAN H, BERGMAN M, DJALDETTI M, et al. Citrus pectin affects cytokine production by human peripheral blood mononuclear cells[J]. Biomedicine & Pharmacotherapy,2008,62(9):579−582.
[55] YE M B, LIM B O. Dietary pectin regulates the levels of inflammatory cytokines and immunoglobulins in interleukin-10 knockout mice[J]. Journal of Agricultural and Food Chemistry,2010,58(21):11281−11286. doi: 10.1021/jf103262s
[56] JOO E, YAMANE S, HAMASAKI A, et al. Enteral supplement enriched with glutamine, fiber, and oligosaccharide attenuates experimental colitis in mice[J]. Nutrition,2013,29(3):549−555. doi: 10.1016/j.nut.2012.09.007
[57] KOLEVA P, KETABI A, VALCHEVA R, et al. Chemically defined diet alters the protective properties of fructo-oligosaccharides and isomalto-oligosaccharides in HLA-B27 transgenic rats[J]. Plos One,2014,9(11):1−10.
[58] BIBI S, LEBOW N, ZHU M. Dietary green pea protects against DSS-induced colitis in mice challenged with high-fat diet[J]. Nutrients,2017,9(5):1−10.
[59] PRAENGAM K, SAHASAKUL Y, KUPRADINUN P, et al. Brown rice and retrograded brown rice alleviate inflammatory response in dextran sulfate sodium (DSS)-induced colitis mice[J]. Food & Function,2017,8(12):4630−4643.
[60] YING H, LE L R K, CHRISTOPHERSEN C T, et al. Manipulation of the gut microbiota using resistant starch is associated with protection against colitis-associated colorectal cancer in rats[J]. Carcinogenesis,2016,4(37):366−375.
[61] LYU J, ZHANG Y H, TIAN Z Q, et al. Astragalus polysaccharides protect against dextran sulfate sodium-induced colitis by inhibiting NF-κB activation[J]. International Journal of Biological Macromolecules,2017,98:723−729. doi: 10.1016/j.ijbiomac.2017.02.024
[62] ECKBURG P B, RELMAN D A. The role of microbes in Crohn's disease[J]. Clinical Infectious Diseases,2007,44(2):256−262. doi: 10.1086/510385
[63] PROSBERG M, BENDTSEN F, VIND I, et al. The association between the gut microbiota and the inflammatory bowel disease activity: A systematic review and meta-analysis[J]. Scandinavian Journal of Gastroenterology,2016,51(12):1407−1415. doi: 10.1080/00365521.2016.1216587
[64] YANG B, CHEN H, GAO H, et al. Bifidobacterium breve CCFM683 could ameliorate DSS-induced colitis in mice primarily via conjugated linoleic acid production and gut microbiota modulation[J]. Journal of Functional Foods,2018,49:61−72. doi: 10.1016/j.jff.2018.08.014
[65] HÅKANSSON Å, TORMO-BADIA N, BARIDI A, et al. Immunological alteration and changes of gut microbiota after dextran sulfate sodium (DSS) administration in mice[J]. Clinical and Experimental Medicine,2015,15(1):107−120. doi: 10.1007/s10238-013-0270-5
[66] MORGAN X C, TICKLE T L, SOKOL H, et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment[J]. Genome Biology,2012,13(9):1−18.
[67] JEFFERSON A, ADOLPHUS K. The effects of intact cereal grain fibers, including wheat bran on the gut microbiota composition of healthy adults: A systematic review[J]. Frontiers in Nutrition,2019,6:1−49. doi: 10.3389/fnut.2019.00001
[68] SO D, WHELAN K, ROSSI M, et al. Dietary fiber intervention on gut microbiota composition in healthy adults: A systematic review and meta-analysis[J]. The American Journal of Clinical Nutrition,2018,107(6):965−983. doi: 10.1093/ajcn/nqy041
[69] SOKOL H, LAY C, SEKSIK P, et al. Analysis of bacterial bowel communities of IBD patients: What has it revealed?[J]. Inflammatory Bowel Diseases,2008,14(6):858−867. doi: 10.1002/ibd.20392
[70] ARMSTRONG H, BORDING-JORGENSEN M, DIJK S, et al. The complex interplay between chronic inflammation, the microbiome, and cancer: Understanding disease progression and what we can do to prevent it[J]. Cancers,2018,10(83):1−29.
[71] SARTOR R B. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: Antibiotics, probiotics, and prebiotics[J]. Gastroenterology,2004,126(6):1620−1633. doi: 10.1053/j.gastro.2004.03.024
[72] LAM K, KEUNG H, KO K, et al. In vitro fermentation of beta-glucans and other selected carbohydrates by infant fecal inoculum: An evaluation of their potential as prebiotics in infant formula[J]. Bioactive Carbohydrates and Dietary Fibre,2018,14:20−24. doi: 10.1016/j.bcdf.2017.07.009
[73] ZHAO J, CHEUNG P C K. Fermentation of β-glucans derived from different sources by Bifidobacteria: Evaluation of their bifidogenic effect[J]. Journal of Agricultural and Food Chemistry,2011,59(11):5986−5992. doi: 10.1021/jf200621y
[74] WONG J M W, de SOUZA R, KENDALL C W C, et al. Colonic health: Fermentation and short chain fatty acids[J]. Journal of Clinical Gastroenterology,2006,40(3):235−243. doi: 10.1097/00004836-200603000-00015
[75] LI F, HAN Y, CAI X, et al. Dietary resveratrol attenuated colitis and modulated gut microbiota in dextran sulfate sodium-treated mice[J]. Food & Function,2020,11(1):1063−1073.
[76] KIM Y, HWANG S W, KIM S, et al. Dietary cellulose prevents gut inflammation by modulating lipid metabolism and gut microbiota[J]. Gut Microbes,2020,11(4):944−961. doi: 10.1080/19490976.2020.1730149
[77] WONG C, HARRIS P, FERGUSON L. Potential benefits of dietary fibre intervention in inflammatory bowel disease[J]. International Journal of Molecular Sciences,2016,17(6):1−22.
[78] YAO C K, STAUDACHER H M. The low-fibre diet: Contender in IBD, or has it had its time?[J]. Lancet Gastroenterol Hepatol,2019,4(5):339. doi: 10.1016/S2468-1253(19)30096-2
[79] SHANG Q H, LIU H S, LIU S J, et al. Effects of dietary fiber sources during late gestation and lactation on sow performance, milk quality, and intestinal health in piglets[J]. Journal of Animal Science,2019,12:4922−4933.
[80] KOH A, De VADDER F, KOVATCHEVA-DATCHARY P, et al. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites[J]. Cell,2016,165(6):1332−1345. doi: 10.1016/j.cell.2016.05.041
[81] LIU H, WALDEN T B, CAI D, et al. Dietary fiber in bilberry ameliorates pre-obesity events in rats by regulating lipid depot, cecal short-chain fatty acid formation and microbiota composition[J]. Nutrients,2019,11(6):1−17.
[82] 桂玲, 黄象男, 朱怀梅, 等. 大豆膳食纤维酶解液抑菌性的研究[J]. 华北农学报,2008,23(1):286−288. [GUI L, HUANG X N, ZHU H M, et al. Bacteriostatic action of the soybean dietary fiber hydrolysate[J]. Acta Agriculturae Boreali-Sinica,2008,23(1):286−288. doi: 10.7668/hbnxb.2008.S1.066 GUI L, HUANG X N, ZHU H M, et al. Bacteriostatic action of the soybean dietary fiber hydrolysate [J]. Acta AgriculturaeBoreali-Sinica, 2008, 23(1): 286-288. doi: 10.7668/hbnxb.2008.S1.066
[83] 刘田, 崔同, 高哲, 等. 山楂膳食纤维的研究进展[J]. 食品研究与开发,2020,41(6):199−204. [LIU T, CUI T, GAO Z, et al. Recent advances in dietary fiber of hawthorn[J]. Food Research and Development,2020,41(6):199−204. LIU T, CUI T, GAO Z, et al. Recent advances in dietary fiber of hawthorn[J]. Food Research and Development, 2020, 41(6): 199-204.
-
期刊类型引用(0)
其他类型引用(1)





 下载:
下载:
 下载:
下载:



