Enzymatic Characterization of Xylanase TgXyn2
-
摘要: 目的:本研究旨在发掘和研究一种新的低温酸性内切- 1,4 -β-木聚糖酶TgXyn2并研究其酶学特性,为其在食品等轻工业生产中的实际应用提供理论依据。方法:利用pCold-TF表达质粒在大肠杆菌中异源表达,纯化后检测其酶学特性,并通过同源建模分析其三维结构。结果:该酶的最适温度为35 ℃,最适pH为5.0,Km为1.287 μmol L−1,Vmax为2.083 μmol min−1 mg−1;同源建模结果表明,TgXyn2由2个反向平行的β-折叠和1个α-螺旋构成,是典型的糖苷水解酶GH11家族木聚糖酶。结论:本文研究的TgXyn2具有良好的酶学特性,在食品等行业具有较好的应用潜力。Abstract: Objectives: This study aimed to discover a acidic xylanase TgXyn2 with high enzymatic activities at low temperatures, which might have potential application in food industry. Method: TgXyn2 was heterologously expressed in E.coli with the expression vector pCold-TF. Results: The optimum reaction condition of TgXyn2 was 35 ℃ and pH5.0, respectively. Its Km was 1.287 μmol L−1 and Vmax was 2.083 μmol min−1 mg−1. Homology modeling showed that TgXyn2 had two antiparallel β-sheets and an α-helix, which was typical for the GH11 family. Conclusions: TgXyn2 had good enzymatic activities, which has potential application in food industry.
-
Keywords:
- Trichoderma guizhouense /
- xylanase /
- enzymatic activity /
- GH11 /
- food industry
-
半纤维素是地球上第二丰富的可再生生物资源。木聚糖是半纤维素的主要骨架,由β-(1,4)糖苷键连接的木糖亚基组成,并在主链木糖2号和3号位存在不同的取代基[1]。半纤维素对维持植物细胞壁的完整性和细胞纤维的凝聚力起到重要的作用,广泛分布于植物的细胞壁中,约占植物细胞干重的15%~35%[2]。木聚糖作为分子量大的高聚物,需要先降解成小分子的低聚木糖,才可以被有效地利用[3]。
木聚糖酶在自然界分布广泛,可从动物、植物和微生物中获得。木聚糖水解酶系(Xylanolytic enzyme systems)是一类降解木聚糖的酶系,包括内切-1,4-β-木聚糖酶、β-D-木糖苷酶、α-L-阿拉伯糖苷酶、α-D-葡糖苷酸酶、乙酰基木聚糖酶和酚酸酯酶[4],多应用在烘焙、酿造、饲料等食品工业中[5]。在烘焙食品生产中,木聚糖酶可以通过改变面筋网络结构增大面团体积,使面团的弹性和延展性增加,干燥度、硬度及持水性降低,咀嚼性和黏性减少,从而改善面团的加工及稳定性能[6];在葡萄酒酿造工业中,木聚糖酶有利于葡萄皮浸渍和颜色的提取,易于澄清和过滤,改善葡萄酒质量和稳定性[7];在饲料行业中,木聚糖酶能够降低谷物在动物肠道中的食糜黏度,提高饲料消化率[8]。
本文研究一种从贵州木霉菌株(Trichoderma guizhouense)中发现的内切-1,4-β-木聚糖酶TgXyn2(OPB43840.1,Endo-1,4-beta-xylanase 2),属于第11家族的木聚糖酶[9],可以有效地降解木聚糖主链的β-1,4木糖苷键。本文通过大肠杆菌异源表达获得TgXyn2,以木聚糖为底物,在不同的温度、pH、底物来源、金属离子及变性剂条件下研究其酶学特征,以期发掘一种新的木聚糖降解酶,为其在食品等轻工业生产中的实际应用提供理论依据。
1. 材料与方法
1.1 材料与仪器
大肠杆菌E.coli BL21-Gold(DE3)菌株、表达质粒pCold-TF 实验室保存;LB液体培养基:蛋白胨10 g/L、酵母提取物5 g/L、氯化钠10 g/L;LB固体培养基:蛋白胨10 g/L,酵母提取物5 g/L,氯化钠10 g/L,1.5%琼脂;基本培养基:十二水合磷酸氢二钠13.24 g/L、无水硫酸镁0.12 g/L、磷酸二氢钾1.6 g/L、硫酸铵7 g/L、柠檬酸氢二胺0.5 g/L、4%甘油。
G180TW高压灭菌锅 美国致微ZWALWAY公司;IS-RDS3叠加式恒温摇床 美国CRYSTAL公司;ELITIST 22K-R立式高速冷冻离心机 湖南吉尔森科技发展股份有限公司;SW-CJ-IFD超净工作台 江苏净化设备有限公司;UH-03低温高压细胞破碎仪 永联生物科技有限公司;PTT-A1000电子天平 福州华志科学仪器有限公司;B-500超微量核酸蛋白检测仪 上海元析仪器有限公司;MDF-382E超低温保存箱 松下冷链大连有限公司;VELOCITY 18R高速冷冻离心机 南京基天生物技术有限公司;JY300C电泳仪 君意电泳;NAS8000蛋白纯化系统 苏州利穗。
1.2 实验方法
1.2.1 构建质粒与转化菌种
木聚糖酶TgXyn2的氨基酸序列由南京农业大学理学院万群课题组提供,来自贵州。TgXyn2氨基酸序列总长度为185,由通用生物系统(安徽)有限公司合成并克隆至pCold-TF表达载体上。上游引物:ACGCCATATCGCCGAAAGG;下游引物:GGCAGGGATCTTAGATTCTG;过程:以第一链cDNA为模板,用上游引物和下游引物进行PCR扩增。扩增程序如下:95 ℃,3 min;95 ℃,30 s,60 ℃,30 s,72 ℃,1 min,30个循环;72 ℃延伸7 min,即可获得PCR扩增产物。利用碱裂解法大量提取和纯化表达质粒,再用热休克的方法,将质粒转入表达菌株,具体过程如下:取50 µL表达感受态Rosetta冰上解冻后,取2 µL重组质粒缓慢加入并冰置25 min,42 ℃水浴热激45 s,冰浴2 min;加入500 µL无抗LB溶液,37 ℃ 200 r/min下培养1 h。取适量菌液涂布到含有1 µL/mL氨苄青霉素的LB固体培养皿中,倒置在37 ℃保温箱里培养12 h。
1.2.2 酶的表达与纯化
挑取固体培养皿上生长出来的单菌落接种到含50 mg/L氨苄青霉素的LB液体培养基中,在37 ℃,200 r/min培养至OD600为0.6,降温到15 ℃,加入诱导剂0.5 mmol/L IPTG,继续培养24 h。通过离心收集细胞,用裂解液溶解沉淀细胞,低温高压细胞破碎仪破碎后,离心取上清,通过Ni亲和层析柱纯化TgXyn2,获得粗酶液然后加入1%(质量比)的TEV;混合物透析过夜后,再次纯化透析,在280 nm的吸光度下测量确定蛋白质浓度,分装保存。
采用SDS-PAGE电泳分析方法[10],配制10%分离胶与4 %浓缩胶,将蛋白按照1:1的比例加入到含有5%的β-巯基乙醇样品缓冲溶液中,95 ℃加热4 min,12000×g离心1 min上清液电泳,上样跑胶,煮沸水洗染色后进行胶图分析,鉴定蛋白表达情况。
1.2.3 TgXyn2酶学特性
1.2.3.1 酶活测定
木聚糖酶能够将木聚糖降解为木糖等还原性糖,因此可以通过比色法DNS法[11]测定产生的还原糖,进而确定木聚糖酶的酶活[12]。本文以0.2%(m/v)木聚糖为底物,每组处理3个重复,测定时每个试管加入100 μL的0.5 mg/mL TgXyn2酶液进行反应,反应时间10 min。酶活力(U)单位定义:在一定条件下,1 mg木聚糖在1 min内催化底物产生1 μmol还原糖为1个酶活力单位(U)。通过使用Origin 9.0(OriginLab,USA)Hill函数,当n=1时可以模拟米氏方程,拟合得到Vmax和Km值。
1.2.3.2 温度对酶活性的影响
酶反应的温度梯度设定为25、30、35、40、45、50和55 ℃,各组pH保持在5.0。
1.2.3.3 pH对酶活性的影响
各组反应体系温度为35 ℃,考察pH2.0、3.0、4.0、5.0、6.0、7.0、8.0的影响,pH条件2.0和3.0使用的是50 mmol/L甘氨酸-盐酸缓冲液,pH条件4.0、5.0、6.0使用的是50 mmol/L乙酸-乙酸钠缓冲液,pH7.0、8.0条件使用的是50 mmol/L柠檬酸-磷酸氢二钠缓冲液。
1.2.3.4 酶动力学参数
在最适条件下,底物梯度浓度分别设置0.1~5.0 mg mL−1(从第二组开始,每0.2 mg mL−1设置一组),并以此计算酶动力学参数。
对测得的反应速度与相应底物浓度进行回归分析,求得Km值。计算公式为米氏方程(Michaelis-Menten equation):v=Vmax×[S]/(Km+[S]),v代表反应初速度,Vmax代表最大反应速度,[S]代表底物浓度。此运算可同时得到Vmax。同理,根据公式Vmax=Kcat×[E],计算Kcat。
1.2.3.5 不同化学试剂对酶活性的影响
分别在酶反应体系中加入0.1 mmol/L的盐酸胍、EDTA、DTT、SDS等化学试剂,在最适条件下与0.2%(m/v)的木聚糖底物进行反应。以未加化学试剂的情况下测得的TgXyn2活性为100%,比较不同化学试剂对酶活性的影响。
1.2.3.6 金属离子对酶活性的影响
分别在酶反应体系中加入0.1 mmol/L的Na+、K+、Ca2+、Fe3+、Cu2+、Mg2+、Zn2+、Co2+、Cd2+、Mn2+、Li+等金属离子,在最适条件下与底物进行反应。以未加金属离子的情况下测得的TgXyn2活性为100%,比较不同金属离子对酶活性的影响。
1.2.3.7 酶的温度耐受性
将TgXyn2在20~55 ℃(每隔5 ℃设置一组)条件下分别保温1 h后,在最适条件下与底物进行反应。以未保温处理的TgXyn2活性为100%,比较经过不同温度处理后TgXyn2的酶活。
1.2.3.8 酶的酸碱耐受性
选用pH范围为2.0~8.0的缓冲液稀释酶液,并将处理后的酶液于4 ℃条件下存放1 h后,在最适条件下与底物进行反应。以未经缓冲液处理的TgXyn2活性为100%,比较经过不同酸碱度处理后TgXyn2的酶活。
1.2.3.9 酶分解不同底物的能力
分别以(甘蔗渣来源)木聚糖、(小麦麸皮来源)木聚糖、(燕麦浆来源)木聚糖、微晶纤维素(MCC)作为酶反应体系中的底物,底物浓度为0.2%,在最适条件下与木聚糖酶TgXyn2进行反应。以(甘蔗渣来源)木聚糖为底物时的TgXyn2活性为100%,测定反应后体系的旋光度,比较TgXyn2对不同底物的分解能力。
1.2.4 同源结构建模
将TgXyn2的氨基酸序列上传至I-TASSER在线网站(https://zhanglab.ccmb.med.umich.edu/I-TASSER/)进行同源三维结构建模[13],利用PyMOL软件画出蛋白三维结构图[14]。
1.3 数据处理
采用GraphPad Prism7处理软件进行数据处理和统计分析,根据实验数据绘制折线图和柱状图,总结现象趋势,分析实验结论以评价模型的统计意义。
2. 结果与分析
2.1 内切-1,4-β-木聚糖酶的表达、纯化与电泳鉴定
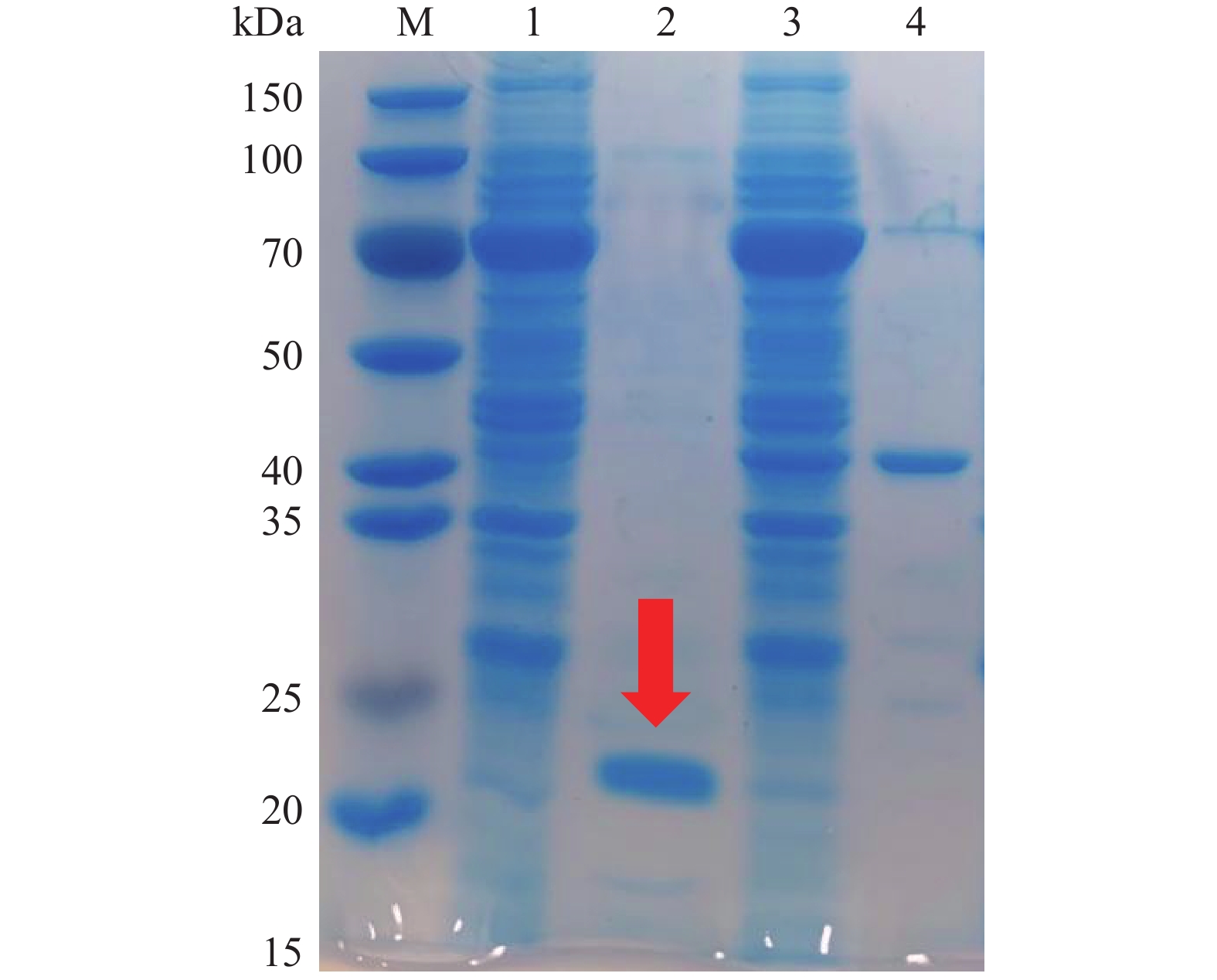
利用ExPASy在线网站预测并结合已知基因序列,通过氨基酸序列计算得出TgXyn2的分子量大约是20.19 kDa。根据上述的实验操作,用碱裂解法大量提取和纯化表达质粒,用热休克法将质粒转入E.coli BL21-Gold(DE3)表达宿主细胞,对TgXyn2成功诱导表达,SDS-PAGE胶图结果如图1所示。
在标准蛋白M条带的相对分子质量20 kDa的位置附近出现清晰可见的一条较浓的条带,与预测的木聚糖酶TgXyn2分子量20.19 kDa十分接近,说明TgXyn2得到了有效表达和纯化。上样剩余条带1与上样流出条带3,均在70 kDa附近存在浓度较高的蛋白,经推断可知为含有融合蛋白的目的蛋白;杂蛋白条带4主要集中在40~50 kDa范围内,经推断可知此为酶切后的融合蛋白。
2.2 内切-1,4-β-木聚糖酶酶学性质分析
2.2.1 温度对酶活性的影响
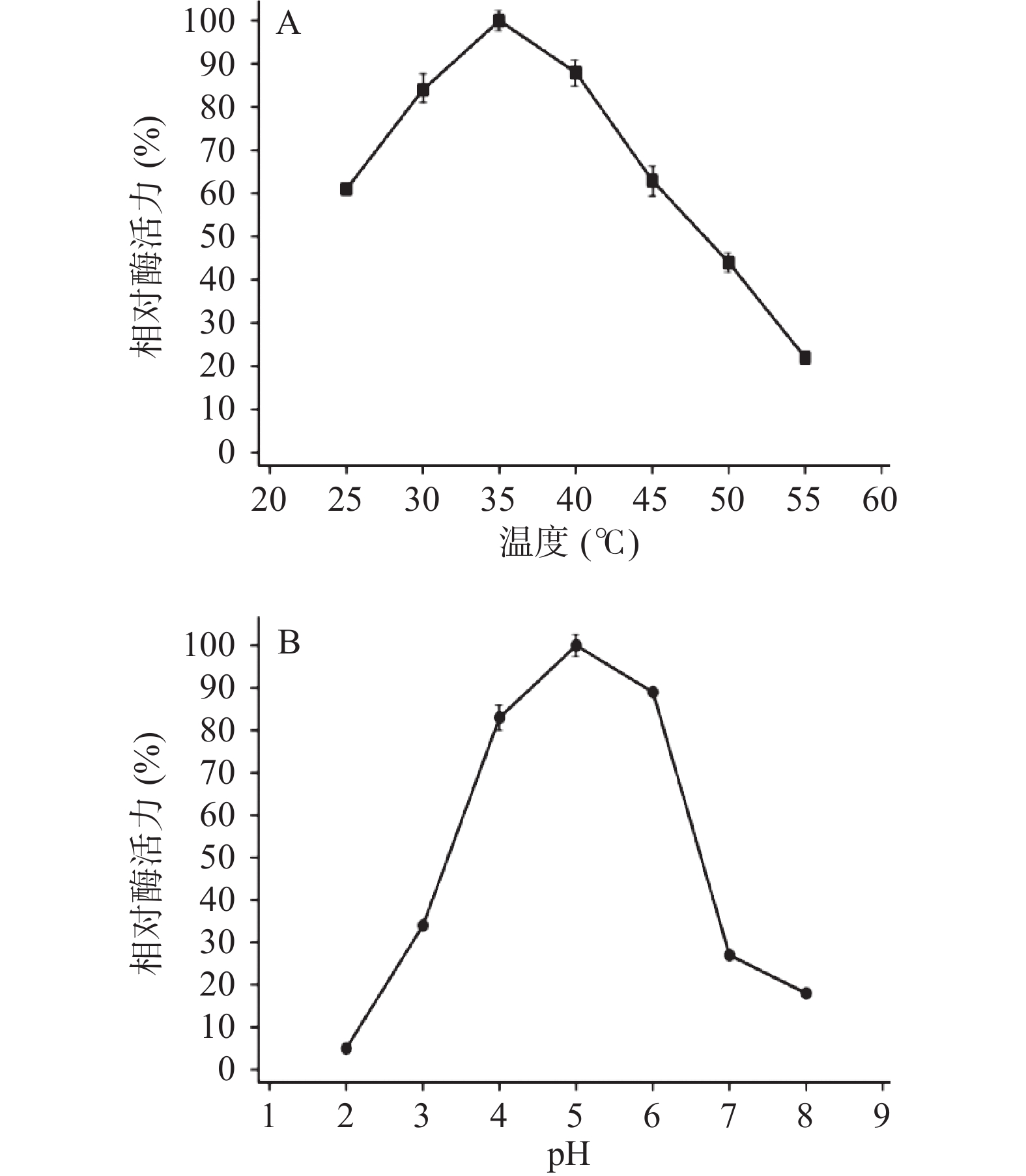
TgXyn2在35 ℃时酶活性达到最大,而在低温下(25 ℃左右)依旧能保持60%以上的活性,温度达到50 ℃以上时酶活性明显降低,温度继续升高后酶活性降低至20%以下(图2A),原因可能是在高温条件下蛋白质结构发生改变,不能形成与底物结合的空间结构,从而失去降解底物的能力。
2.2.2 pH对酶活性的影响
TgXyn2活性在pH5.0时达到最大值,在pH6.0~7.0过程中活性明显下降,pH8.0时活性低于20%;而在pH3.0时仍保留了35%左右的酶活,可以推断出TgXyn2更适应偏酸性环境(图2B)。因此,TgXyn2酶在最适pH范围4.0~6.0之间表现出较高活性,大于或小于最适pH,都会降低酶活性。主要是因为过酸、过碱影响酶的稳定性,进而使酶遭受不可逆破坏。此外也存在改变底物分子和酶分子的带电状态和天然构象,从而影响酶和底物的结合的情况。
2.2.3 酶动力学参数的计算
结合图2A、图2B实验数据,通过使用Origin 9.0(OriginLab,USA)Hill函数,当n=1时可以模拟米氏方程,拟合计算出TgXyn2酶催化(甘蔗渣来源)木聚糖底物的Km、Vmax值。得到在pH5.0、温度35 ℃的最适条件下TgXyn2的比活力为84.47 U/mg,Km=1.287 mg/mL,Vmax=2.083 μmol·min−1·mg−1;由Vmax=Kcat×[E0]得Kcat=57.47 s−1。
当酶有多个可利用的底物时,其对不同底物的催化效率可能差别很大。可以用Kcat/Km来确定酶的最适底物,当利用蛋白质工程对酶进行改造时,不同突变型的Kcat/Km也是需要要测定的参数,用来表征酶催化效率的变化情况。
2.2.4 不同化学试剂对酶活性的影响
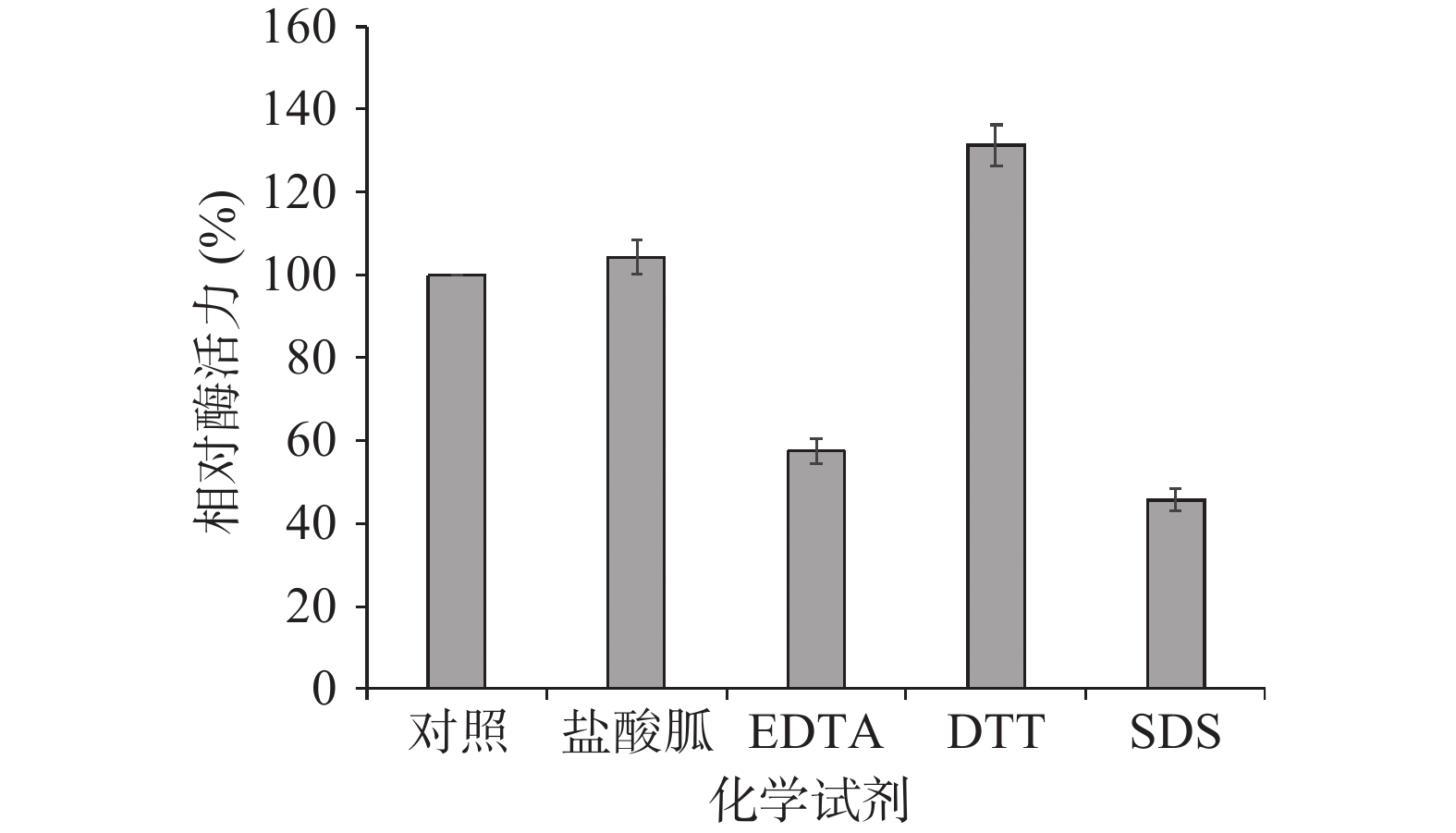
据方差分析,1 mmol/L的DTT对TgXyn2的酶活性具有明显提高作用,能够达到未经处理的TgXyn2活性的137.31%;1 mmol/L的盐酸胍对酶活性的影响效果不明显,只达到103.69%;而1 mmol/L的EDTA、SDS对TgXyn2具有明显抑制作用,加入酶反应体系后,酶活分别下降到未经处理的TgXyn2活性的57.11%和45.12%(图3)。综上所述,DTT对TgXyn2的活性具有促进作用,EDTA和SDS对酶活均有较大抑制作用。
2.2.5 金属离子对酶活性的影响
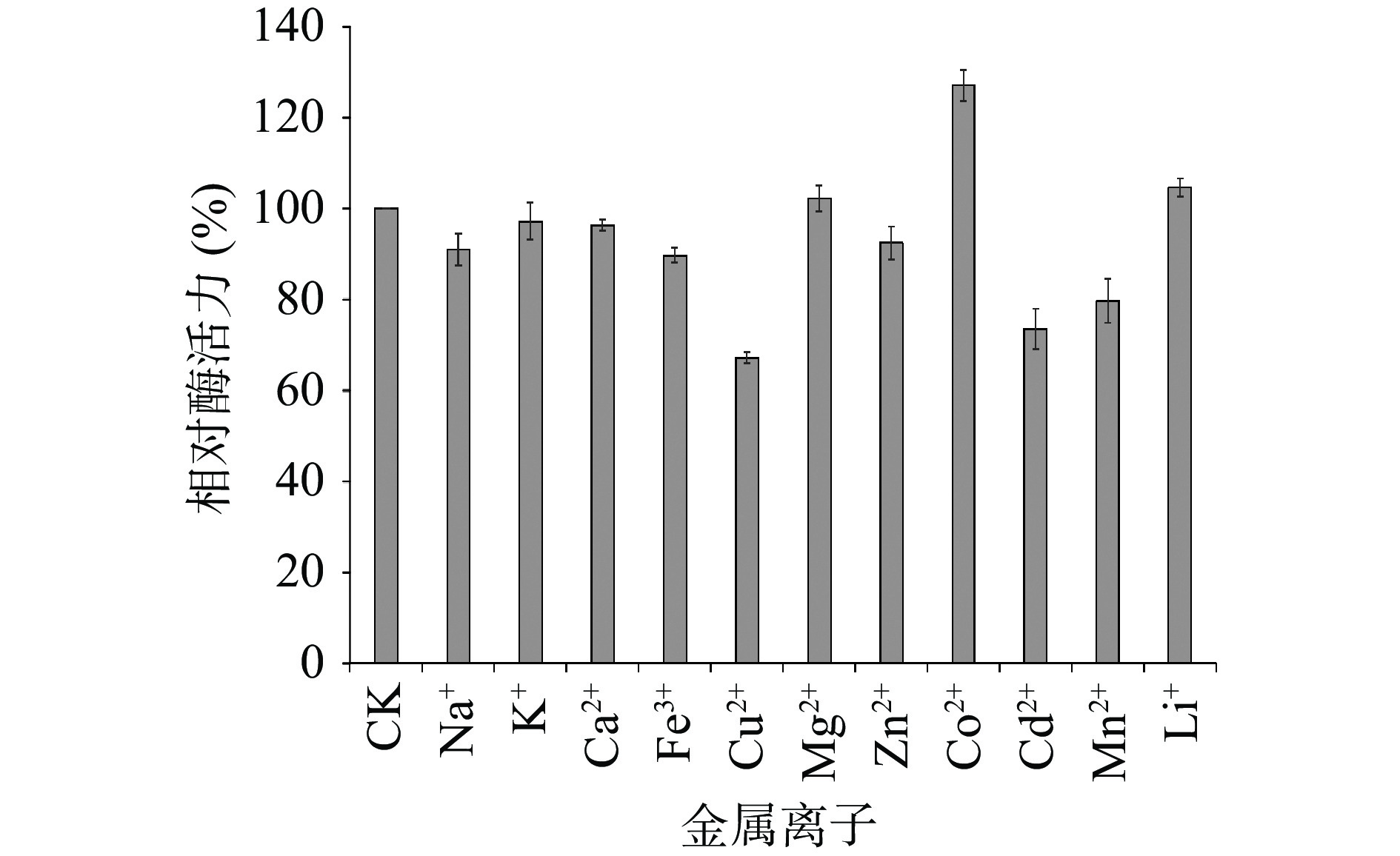
1 mmol/L的Co2+可以明显提高TgXyn2的酶活性,能够达到未经处理的TgXyn2活性的127.13%;而1 mmol/L的Cu2+、Cd2+、Mn2+和Fe3+的环境下TgXyn2活性下降明显,分别下降到未经处理的TgXyn2活性的67.22%、73.64%、79.73%和89.70%(图4)。综上所述,Co2+对TgXyn2活性的促进最明显,Cu2+等对酶活有较大抑制作用。马腾飞等[15]研究表明,在毕赤酵母中表达的木聚糖酶XynB,同样是Co2+对木聚糖酶活性有较明显的促进作用, Cu2+对其有抑制作用,不同的是Fe2+对该酶的活性有微弱的促进作用。由于金属离子可以直接与酶的过渡态功能基团配位,稳定过渡态的几何结构和电荷,或者通过长程的静电作用稳定过渡态电荷,也可以通过引起pKa的改变,从而活化碱基或糖基,因此金属离子对于酶的活性具有一定的促进或抑制作用。
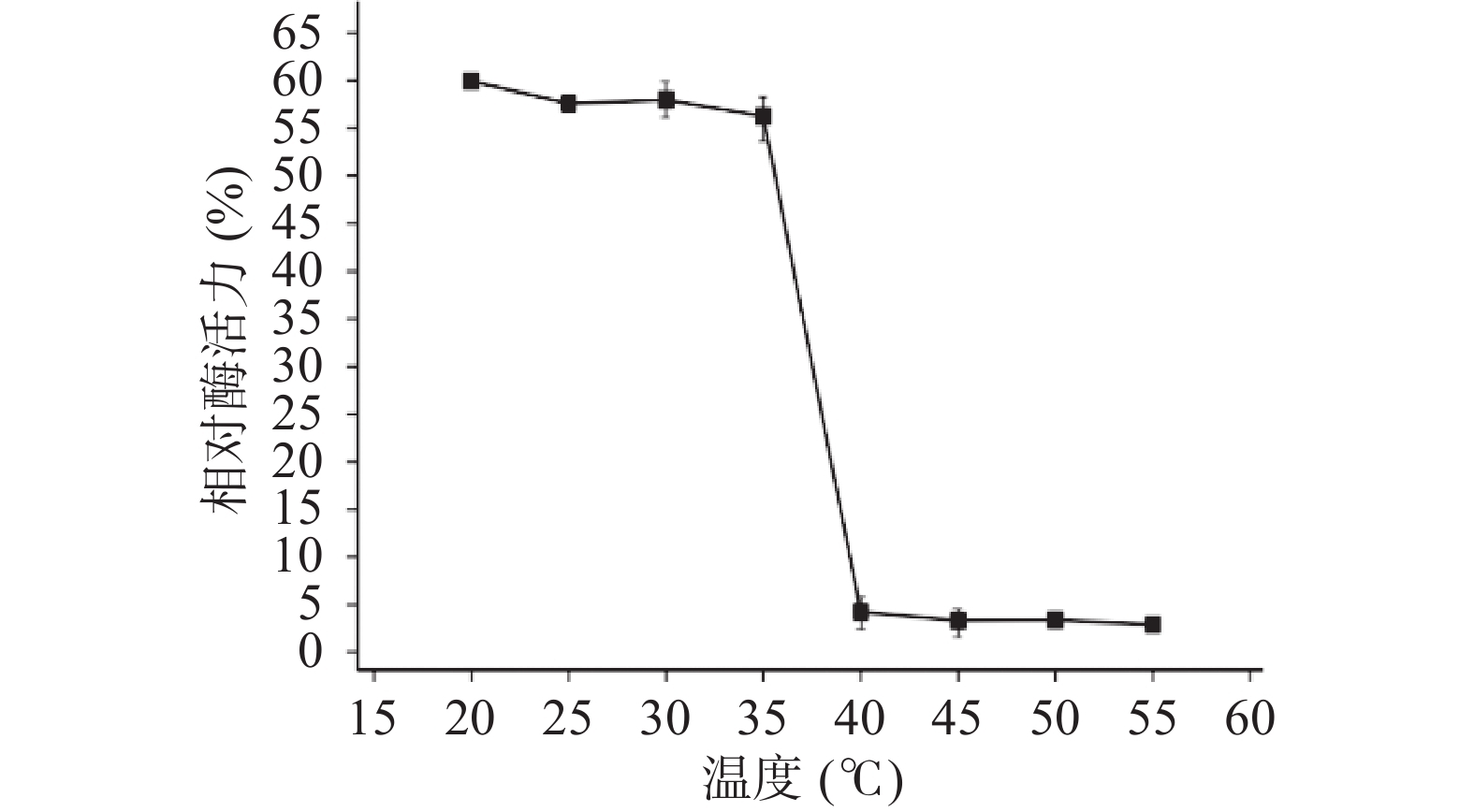
2.2.6 酶的温度耐受性
木聚糖酶TgXyn2在20~35 ℃温度范围内处理后的残余酶活较为稳定,能保持在原酶活性的55%以上。超过40 ℃时,处理后的残余酶活显著(P<0.05)降低至5%以下,说明温度对木聚糖酶TgXyn2有明显的影响(图5),超过40 ℃时酶活较低的原因,主要是酶的构象被破坏,底物不能和活性中心有效结合。该酶的温度耐受性不高,热稳定性具有很大的改造空间[16]。相比于从高寒草甸土壤样品中筛选出低温木聚糖酶FY8[17],其最适反应温度为40 ℃,20~70 ℃时酶活力均随温度升高先增后降,在40 ℃时酶活力为最大值,与本文的TgXyn2最适温度接近,所以也属于低温酶,但其温度耐受范围更广,热稳定性更好,耐酸性则稍差。
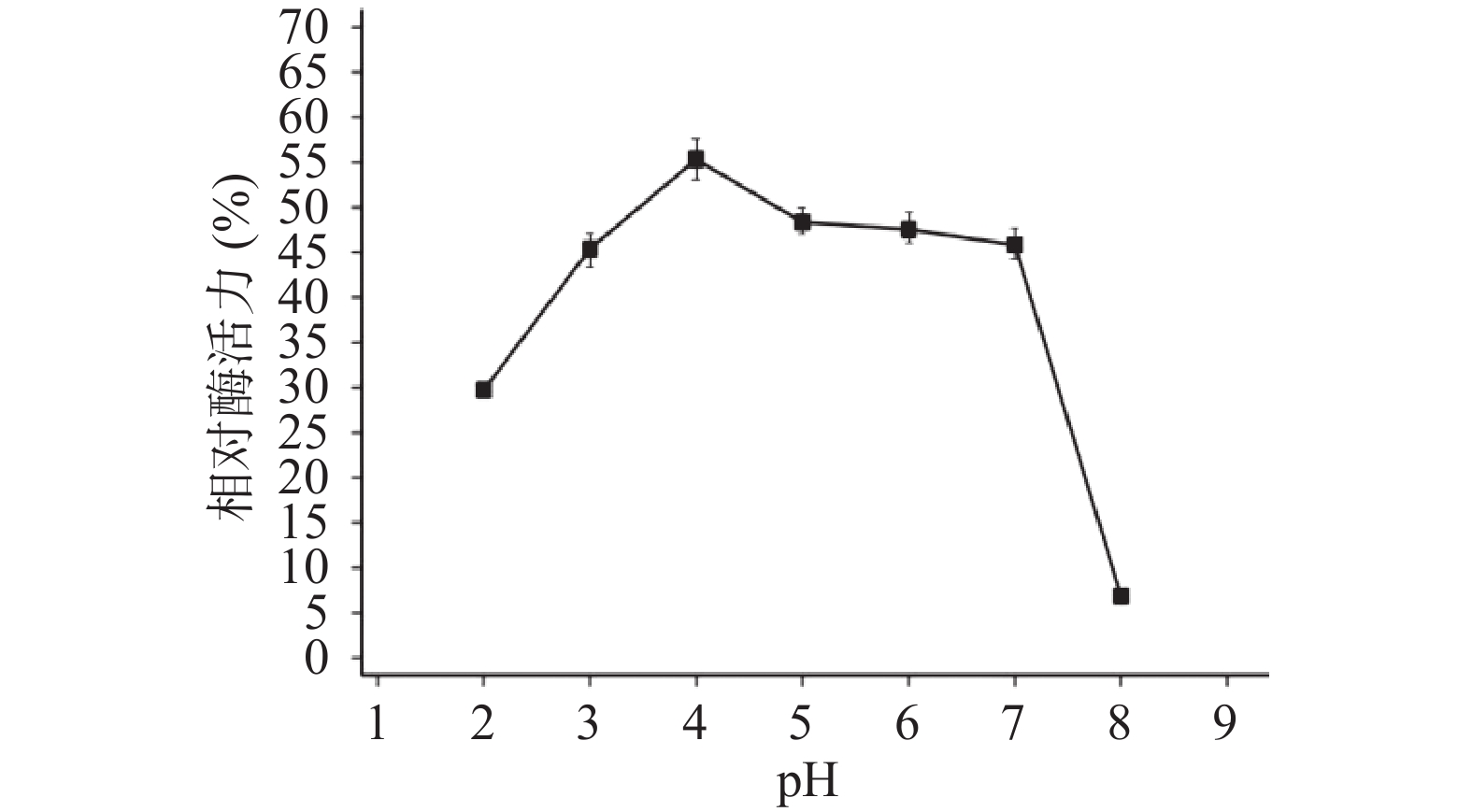
2.2.7 酶的酸碱耐受性
木聚糖酶TgXyn2在pH3.0~7.0范围内处理后的残余酶活较为稳定,能保持在45%以上,在pH4左右酶活残留最高,超过55%;在pH低于2.0时残余酶活略有下降,在30%左右;而pH高于7.0时,处理后的残余酶活降低至5%左右,由此可见该酶更适应酸性环境[18](图6)。
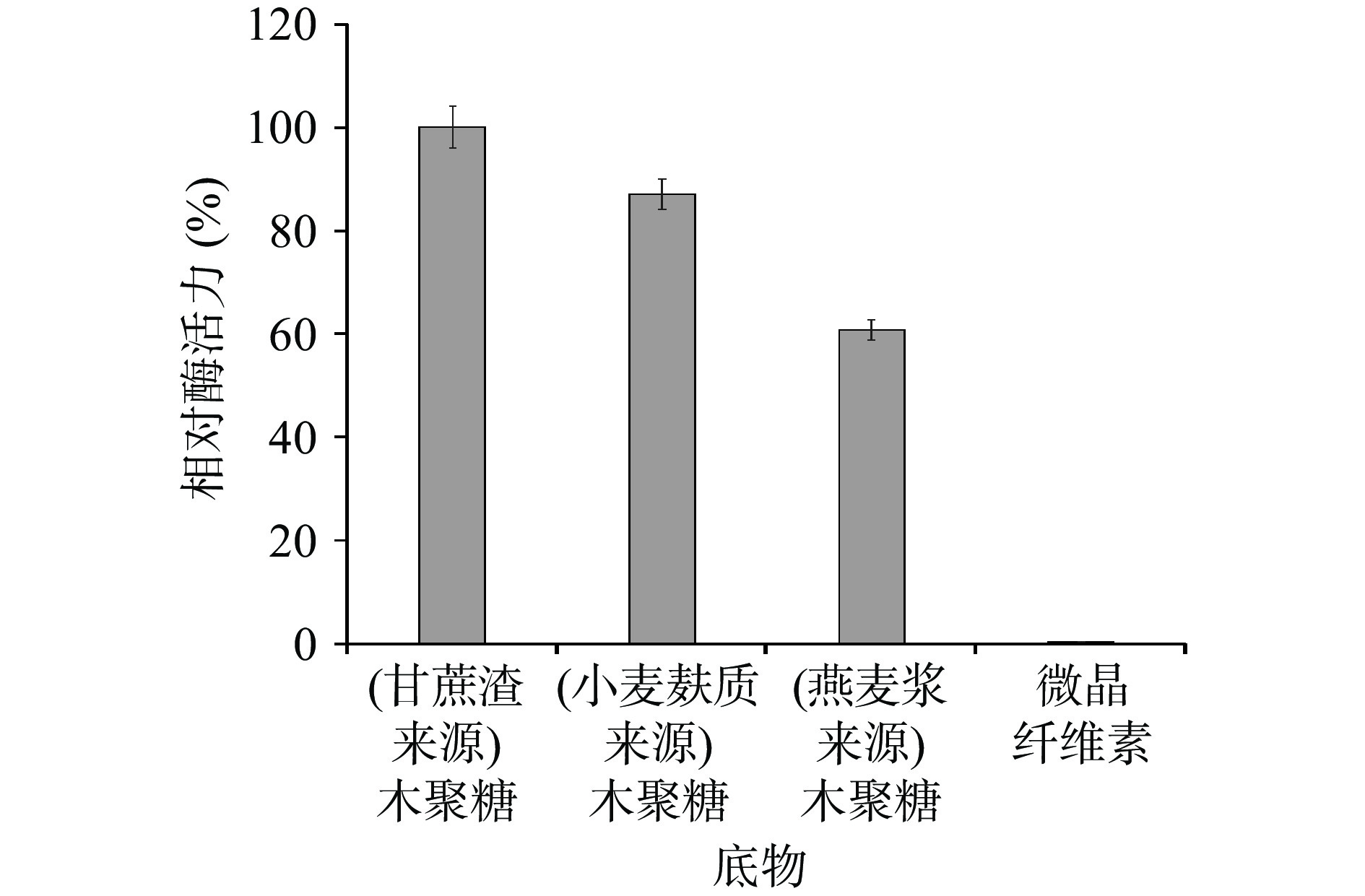
2.2.8 酶降解不同底物的能力
在测定的4种底物中,TgXyn2只能降解木聚糖类底物,水解甘蔗渣来源的木聚糖能力最强,以水解甘蔗渣来源的木聚糖时测得的TgXyn2酶活性为100%,水解小麦麸质来源和燕麦浆来源的木聚糖分别达到86.92%和59.66%;TgXyn2对微晶纤维素MCC这类非木聚糖底物基本没有表现出水解活性(图7)。类似的还有马腾飞等[15]研究的在毕赤酵母中表达的木聚糖酶XynB,该木聚糖酶同样特异性较强,对可溶性淀粉、微晶纤维素、魔芋苷聚糖、低聚果糖完全不能水解。说明这两类木聚糖酶在水解方面有很强的特异性,其活性部位的构象只与特定的底物发生互补结合,能够专一地水解木聚糖。
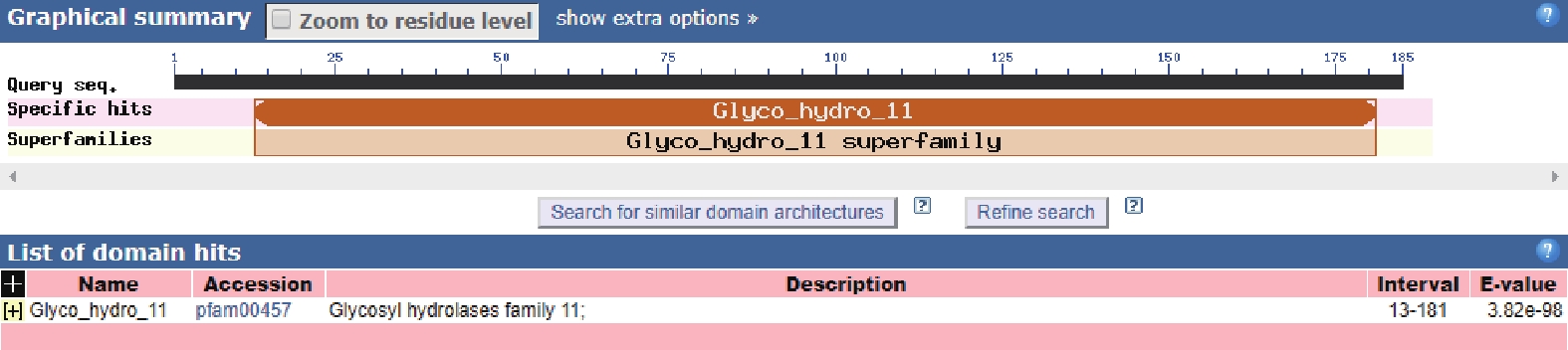
2.3 TgXyn2的同源蛋白结构预测及序列分析
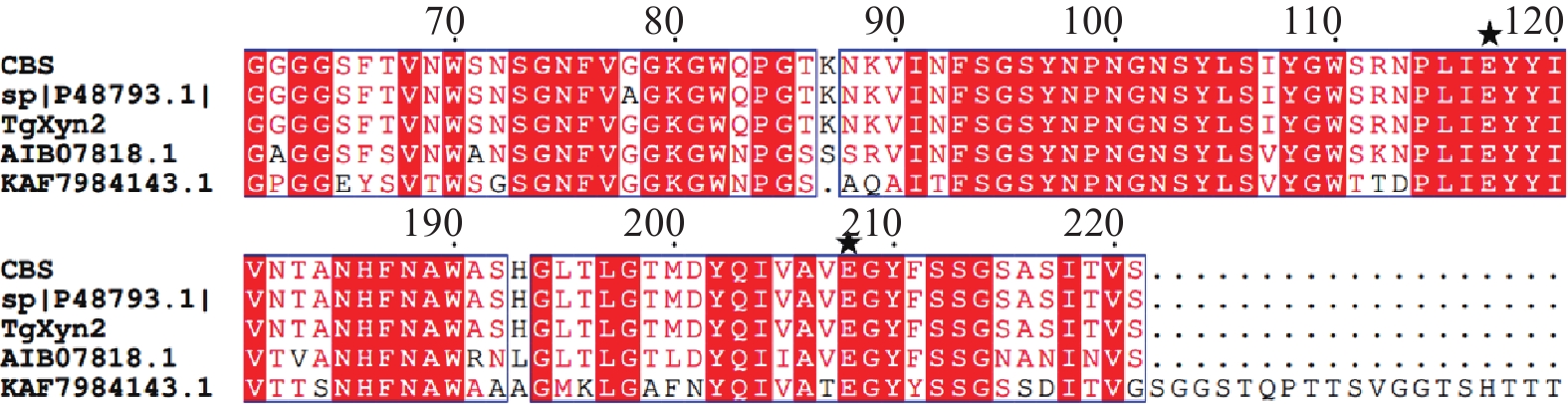
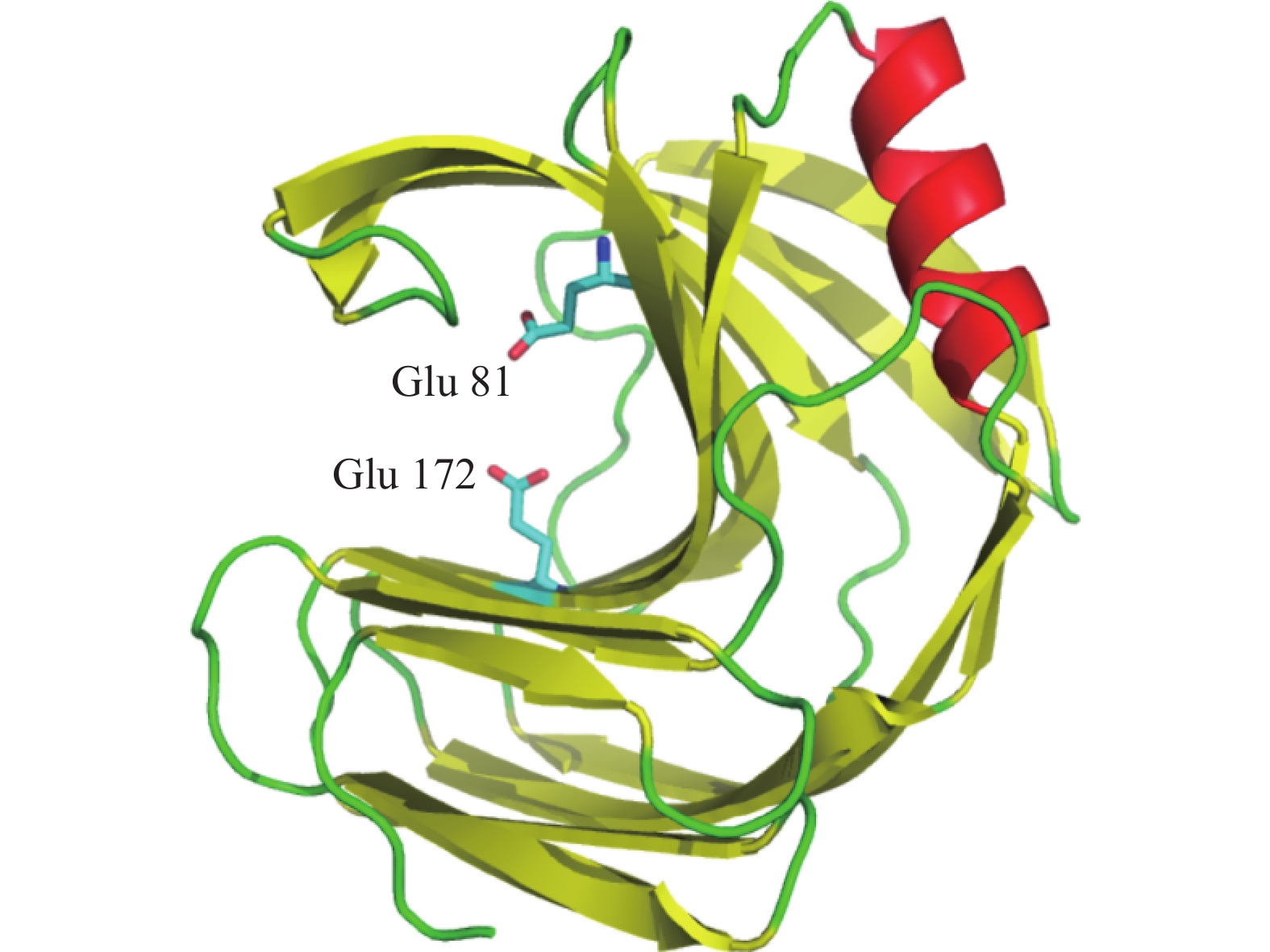
通过在NCBI网站(https://blast.ncbi.nlm.nih.gov/Blast.cgi)上将TgXyn2的序列进行序列对比,我们发现TgXyn2与哈茨木霉CBS 226.95的序列相似度为100%,目前并没有关于哈茨木霉CBS 226.95的相关研究报道。TgXyn2的结构域和同源蛋白结构分析结果(图8)和TgXyn2的序列比对分析结果(图9)表明,TgXyn2与GH11家族关系密切,分析TgXyn2中所有可能作为催化残基的保守氨基酸,只有Glu 172和Glu 81位于酶与底物作用的活性中心[19],这些位点可能为该蛋白的活性中心。
对TgXyn2的三维结构进行建模,由三维结构(图10)可知,木聚糖酶TgXyn2整个酶分子呈右手型结构,由1个α-螺旋和2个反向平行的β-折叠构成。GH11家族木聚糖酶的催化机制是经典的酸碱催化机制[20],由图可知该酶的活性中心Glu 172和Glu 81在反应隧道内,因此具有极大的改造空间,例如可通过导入二硫键等突变手段使得反应隧道打开,增大活性中心的接触面,提高该酶的活性。
3. 讨论与结论
当前关于木聚糖酶的研究颇多,但由于木聚糖酶的多样性和复杂性,以及自然界发现的木聚糖酶多为碱性木聚糖酶等因素,使得木聚糖酶在食品等行业中的应用有待提高。对木聚糖酶的热稳定性和酸碱稳定性等方面进行研究和改造,不仅对理解蛋白结构与功能的关系有重要意义,而且能为生产应用带来高效性和经济效益。例如目前木聚糖酶领域的一项研究热点:运用蛋白质工程技术手段对木聚糖酶进行分子改造[21],让位于N末端“手掌”区域的取代残基和突变体中新生成的氢键来帮助提高酶的热稳定性。稳定性的显著提高将为木聚糖酶在不同生产领域的应用铺平道路[22]。
本文所研究的TgXyn2是从贵州木霉菌株(Trichoderma guizhouense)中发现的低温耐酸性木聚糖酶,分子量为20.19 kDa,可以有效降解木聚糖主链的β-1,4木糖苷键。TgXyn2的酶促反应最适作用温度与宋文芳等[23]从若尔盖沼泽的草甸淤泥中分离筛选出能同步产生低温纤维素酶和低温木聚糖酶的低温厌氧纤维素菌CD-2所产木聚糖酶接近,均为35 ℃;最适pH与郑亚伦等[24]对重组菌株BA-TB-1进行诱导表达获得的木聚糖酶接近;与已报道[25-26]的木霉(Trichoderma)及黑曲霉(Aspergillus niger)所产木聚糖酶相比,本实验所研究的木聚糖酶TgXyn2的最适pH更接近中性。
在pH3~7的范围内、尤其是在pH4~6的范围内TgXyn2能够保持较高的酶活力,即使是在鸡的肌胃即pH2.5~3的酸性环境内,该酶的酶活力下降依旧不显著,说明家禽的消化道内酸碱度环境对酶TgXyn2的活性影响较小[27-29]。然而木聚糖酶TgXyn2的最适温度在35 ℃,在超过40 ℃的环境中,酶活力下降很快,因此不能完全适应家禽的胃肠道温度。如将酶TgXyn2用作肉鸡的基础饲粮食品等行业,还需对TgXyn2进行必要的热稳定性改造。
木聚糖酶TgXyn2具备一定的耐酸性,但其热稳定性还需进一步改良。综合来看,酶TgXyn2在食品、造纸、纺织等行业有一定的应用价值。例如Liu等[30]研究了巴氏拟杆菌(Paenibacillus barengoltzii)产的新型木聚糖酶的生化特性,并以此指导其在玉米芯和甘蔗渣生产低聚木糖中的应用,本研究发现TgXyn2对于甘蔗渣来源的木聚糖分解能力较强,在食品加工活动中有发挥作用的潜力。木聚糖酶家族资源丰富,应用广泛,未来发展潜力巨大,除上述食品饲料等行业外,它还非常广泛地应用于环境保护[31]和农产品加工等诸多行业中。随着木聚糖酶理论基础的继续发展,应用研究也越来越受到重视,木聚糖酶的研究已成为糖化学催化领域中十分重要的分支,在食品、农业、新能源、医药等领域[32]具有优秀的研究潜力和广阔的应用前景。
-
-
[1] 黄坤龙, 苏小运, 姚斌. 生长抑素和耐热木聚糖酶的融合表达及性质研究[J]. 生物技术通报,2020,36(9):235−243. [HUANG K L, SU X Y, YAO B. Fusion expression and characterization of somatostatin and thermostable xylanase[J]. Biotechnology Bulletin,2020,36(9):235−243. [2] 王梦竹, 方颖, 李勍, 等. 植物细胞壁纳米结构与纳米纤维素的化学纯化处理结合机械解纤法制备[J]. 高分子学报,2020,51(6):586−597. [WANG M Z, FANG Y, LI Q, et al. Preparation of plant cell wall nanostructures and nano cellulose by chemical purification combined with mechanical fibrillation[J]. Acta Polymer Sinica,2020,51(6):586−597. doi: 10.11777/j.issn1000-3304.2020.20037 [3] 汤勇, 蔡俊. β-木糖苷酶的研究进展[J]. 中国酿造,2018,37(10):14−19. [TANG Y, CAI J. Research progress of β-xylosidase[J]. Brewing in China,2018,37(10):14−19. doi: 10.11882/j.issn.0254-5071.2018.10.004 [4] CRISTINA V, JAVIER PASTOR F I, TERESA V, et al. Antioxidant activity of xylooligosaccharides produced from glucuronoxylan by Xyn10A and Xyn30D xylanases and eucalyptus autohydrolysates[J]. Carbohydrate Polymers,2018,194:43−50. doi: 10.1016/j.carbpol.2018.04.028
[5] 温博婷, 孙丽超, 王凤忠, 等. 微生物木聚糖酶的研究进展及其在食品领域的应用[J]. 生物产业技术,2017(5):81−86. [WEN B T, SUN L C, WANG F Z, et al. Research progress of microbial xylanase and its application in food field[J]. Biotechnology,2017(5):81−86. [6] 刘凯, 肖付才. 木聚糖酶对面团特性及全麦面包品质影响[J]. 粮食与油脂,2021,34(4):23−26. [LIU K, XIAO F C. Effect of xylanase on dough characteristics and whole wheat bread quality[J]. Food and Oil,2021,34(4):23−26. doi: 10.3969/j.issn.1008-9578.2021.04.007 [7] LOUW C, GRANGE D L, PRETORIUS I S, et al. The effect of polysaccharide-degrading wine yeast transformants on the efficiency of wine processing and wine flavour[J]. Journal of Biotechnology,2006,125(4):447−461. doi: 10.1016/j.jbiotec.2006.03.029
[8] 尚庆辉, 朴香淑, 王玉璘. 木聚糖酶在畜禽饲料中应用效果及其机理的研究进展[J]. 饲料工业,2017,38(2):17−24. [SHANG Q H, PU X S, WANG Y L. Research progress on application effect and mechanism of xylanase in animal feed[J]. Feed Industry,2017,38(2):17−24. [9] VAN Gool M P, VAN Muiswinkel G C J, HINZ S W A, et al. Two GH10 endo-xylanases from Myceliophthora thermophila C1 with and without cellulose binding module act differently towards soluble and insoluble xylans[J]. Bioresource Technology,2012,119(1):123−132.
[10] BRADFORD MARION M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding[J]. Academic Press,1976,72(1-2):248−254.
[11] 李治宏. 11家族木聚糖酶的热稳定性分子改造[D]. 南京: 南京农业大学, 2018: 3−34. LI Z H. Molecular modification of thermostability of 11 family xylanases[D]. Nanjing: Nanjing Agricultural University, 2018: 3−34.
[12] BAILEY M J, BIELY P POUTANEN K. Interlaboratory testing of methods for assay of xylanase activity[J]. Elsevier,1992,23(3):257−270.
[13] BIASINI M, BIENERT S, WATERHOUSE A, et al. SWISS-MODEL: Modelling protein tertiary and quaternary structure using evolutionary information[J]. Nucleic Acids Research, 2014, 42(Web Server issue).
[14] 李宁. 基于噬菌体展示的多肽预处理以及表位预测研究[D]. 成都: 电子科技大学, 2019: 13−75. LI N. Peptide pretreatment and epitope prediction based on phage display[D]. Chengdu: University of Electronic Science and Technology, 2019: 13−75.
[15] 马腾飞, 杨江科, 韩正刚, 等. 木聚糖酶基因XynB在毕赤酵母中的表达及酶学性质研究[J]. 食品科技,2018,43(5):1−9. [MA T F, YANG J K, HAN Z G, et al. Expression and characterization of xylanase gene XynB in Pichia pastoris[J]. Food science and technology,2018,43(5):1−9. [16] 张伟, 刘秀霞, 杨艳坤, 等. 利用内源元件在谷氨酸棒杆菌中分泌表达木聚糖酶[J]. 生物工程学报,2019,35(3):425−434. [ZHANG W, LIU X X, YANG Y K, et al. Secretory expression of xylanase in Corynebacterium glutamicum using endogenous elements[J]. Acta Bioengineering,2019,35(3):425−434. [17] 曹慧, 张会会, 张腾月, 等. 产低温纤维素酶和木聚糖酶真菌的筛选鉴定及酶学性质[J/OL]. 饲料研究: 1−8[2021-07-11]. http://kns.cnki.net/kcms/detail/11.2114.S.20201224.1754.002.html. CAO H, ZHANG H H, ZHANG T Y, et al. Screening, identification and enzymatic properties of fungi producing low temperature cellulase and xylanase[J/OL]. Feed Research: 1−8[2021-07-11]. http://kns.cnki.net/kcms/detail/11.2114.S.20201224.1754.002.html.
[18] 杨颖, 玉王宁, 金一, 等. 常压室温等离子体快速诱变绿色糖单孢菌筛选木聚糖酶高产菌株及其酶学性质研究[J]. 微生物学通报,2013,40(5):905−915. [YANG Y, YU W N, JIN Y, et al. Screening of high xylanase producing strain by atmospheric pressure and room temperature plasma rapid mutagenesis and its enzymatic properties[J]. Microbiology Bulletin,2013,40(5):905−915. [19] 胡沂淮, 邵蔚蓝. 木聚糖酶[J]. 生命的化学, 2002, 22(3): 281−285. HU Y H, SHAO W L. Chemistry of xylanase[J]. Life, 2002, 22(3): 281−285.
[20] WANG H, WANG K, GUAN Z, et al. Computational study of non-catalytic T-loop pocket on CDK proteins for drug development[J]. 中国物理B,2017,26(12):128702−128702. [21] 冼亮, 秦艳, 朱婧, 等. 内切木聚糖酶的应用及其酶制剂开发技术[J]. 广西科学院学报,2020,36(3):300−308. [XIAN L, QIN Y, ZHU J, et al. Application of endoxylanase and development technology of enzyme preparation[J]. Journal of Guangxi Academy of Sciences,2020,36(3):300−308. [22] XING Hongguan, ZOU Gen, LIU Chunyan, et al. Improving the thermostability of a GH11 xylanase by directed evolution and rational design guided by B-factor analysis[J]. Enzyme and Microbial Technology,2021,143:109720−109720. doi: 10.1016/j.enzmictec.2020.109720
[23] 宋文芳, 胡国全, 徐彦胜. 一株低温厌氧纤维素降解细菌的分离、鉴定及其酶学性质的研究[J]. 西南农业学报,2011,24(4):1317−1322. [SONG W F, HU G Q, XU Y S. Isolation, identification and enzymatic properties of a low temperature anaerobic cellulose degrading bacterium[J]. Journal of Southwest Agriculture,2011,24(4):1317−1322. doi: 10.3969/j.issn.1001-4829.2011.04.018 [24] 郑亚伦, 夏瑛, 李良, 等. 源于解淀粉芽孢杆菌酸性木聚糖酶酶学性质的研究[J]. 食品与发酵工业,2020,46(24):58−65. [ZHENG Y L, XIA Y, LI L, et al. Study on the enzymatic properties of acid xylanase from Bacillus amylopectin[J]. Food and Fermentation Industry,2020,46(24):58−65. [25] 怀文辉, 何秀萍, 郭文洁, 等. 微生物木聚糖降解酶研究进展及应用前景[J]. 微生物学通报,2000(2):137−139. [HUAI W H, HE X P, GUO T J, et al. Research progress and application prospect of microbial xylanase[J]. Bulletin of Microbiology,2000(2):137−139. doi: 10.3969/j.issn.0253-2654.2000.02.017 [26] 陈红歌, 朱静, 梁改芹, 等. 酸性木聚糖酶产生菌的筛选及产酶条件[J]. 微生物学报,1999(4):66−70. [CHEN H G, ZHU J, LIANG G Q, et al. Screening and enzyme producing conditions of acid xylanase producing strain[J]. Acta microbiologica Sinica,1999(4):66−70. [27] 王剑, 尚庆辉, 张连华, 等. 木聚糖酶对肉鸡生长性能和肠道健康的影响[J/OL]. 动物营养学报: 1−13[2021-06-09]. http://kns.cnki.net/kcms/detail/11.5461.S.20210419.1452.092.html. WANG J, SHANG Q H, ZHANG L H, et al. . Effects of xylanase on growth performance and intestinal health of broilers[J/OL]. Acta Zoologica Sinica: 1−13[2021-06-09]. http://kns.cnki.net/kcms/detail/11.5461.S.20210419.1452.092.html.
[28] ERI T, AKINORI K, SATOSHI W, et al. Gastric and intestinal proteases resistance of chicken acidic chitinase nominates chitin-containing organisms for alternative whole edible diets for poultry[J]. Scientific Reports,2017,7(1):20878−20881.
[29] 朱崇淼, 毛胜勇, 孙云章, 等. 厌氧真菌发酵液中木聚糖酶活力的初步研究[J]. 南京农业大学学报,2004(1):120−123. [ZHU C M, MAO S Y, SUN Y Z, et al. Preliminary study on xylanase activity in anaerobic fungal fermentation broth[J]. Journal of Nanjing Agricultural University,2004(1):120−123. [30] LIU Xueqiang, LIU Yu, JIANG Zhengqiang, et al. Biochemical characterization of a novel xylanase from Paenibacillus barengoltzii and its application in xylooligosaccharides production from corncobs[J]. Food Chemistry,2018,264:310−318. doi: 10.1016/j.foodchem.2018.05.023
[31] 关莹, 薛敏, 王伟. 饲用酶制剂在水产动物中应用的最新研究进展[J]. 中国渔业质量与标准,2021,11(1):61−67. [GUAN Y, XUE M, WANG W. The latest research progress on the application of feed enzymes in aquatic animals[J]. China Fisheries Quality and Standard,2021,11(1):61−67. [32] ZHOU Yan, YUAN Zhongyao, HAO Weiwei, et al. Advances in alfalfa transgenic technology and its application[J]. Agricultural Biotechnology,2013,2(Z1):7−12.
-
期刊类型引用(2)
1. 汤龙章,邱清华,刘婵娟,付戴波,欧阳克蕙,赵向辉. 一种瘤胃木聚糖酶的酶学特性分析及其对农作物秸秆的水解作用. 微生物学报. 2024(10): 3840-3852 .  百度学术
百度学术
2. 陈康太,杨思文,刁梦月,王宵宵,郭双,李恩中,李同彪. Loop结构引入半胱氨酸对木聚糖酶XynASP热稳定性的影响. 中国酿造. 2022(10): 106-112 .  百度学术
百度学术
其他类型引用(2)





 下载:
下载:
 下载:
下载:



