Research Progress in Extraction, Purification and Anticancer Function of Sulforaphane
-
摘要: 硫代葡萄糖苷是十字花科蔬菜中一类重要的含硫阴离子亲水性天然产物,其在内源黑芥子酶的催化下,可以水解生成具有抗癌、抗氧化、抑菌等多种功效的活性物质,引起研究者们的广泛关注。萝卜硫素是目前发现的抗癌效果最好、效力最强的一种硫代葡萄糖单体,作为药品或功能食品的有效成分,具有较高的研究价值和广阔的应用前景。本研究概述了萝卜硫素的来源、结构性质、功能活性,系统介绍了其提取工艺、分离纯化方法和抗癌应用等相关研究进展,并对其研究前景进行了探讨与展望,以期为萝卜硫素功能成分的研发和高附加值利用提供研究基础和技术支撑。Abstract: Glucosinolates are a kind of important sulfur-containing anionic hydrophilic natural products in cruciferous vegetables. Under the catalysis of endogenous myrosinase, glucosinolates can be hydrolyzed to produce active substances with anti-cancer, anti-oxidation, antimicrobial and other effects, which have attracted wide attention of researchers. Sulforaphane is a thioglucose monomer with the best anticancer effect and the strongest efficacy. As an effective component of drugs or functional foods, it has high research value and broad application prospects. In this study, the source, structure, properties, functional activities of sulforaphane are summarized, and its extraction technology, separation and purification methods and anticancer applications are systematically introduced. The research prospect is discussed and anticipated, in order to provide research basis and technical support for the research and development of sulforaphane functional components and high value-added utilization.
-
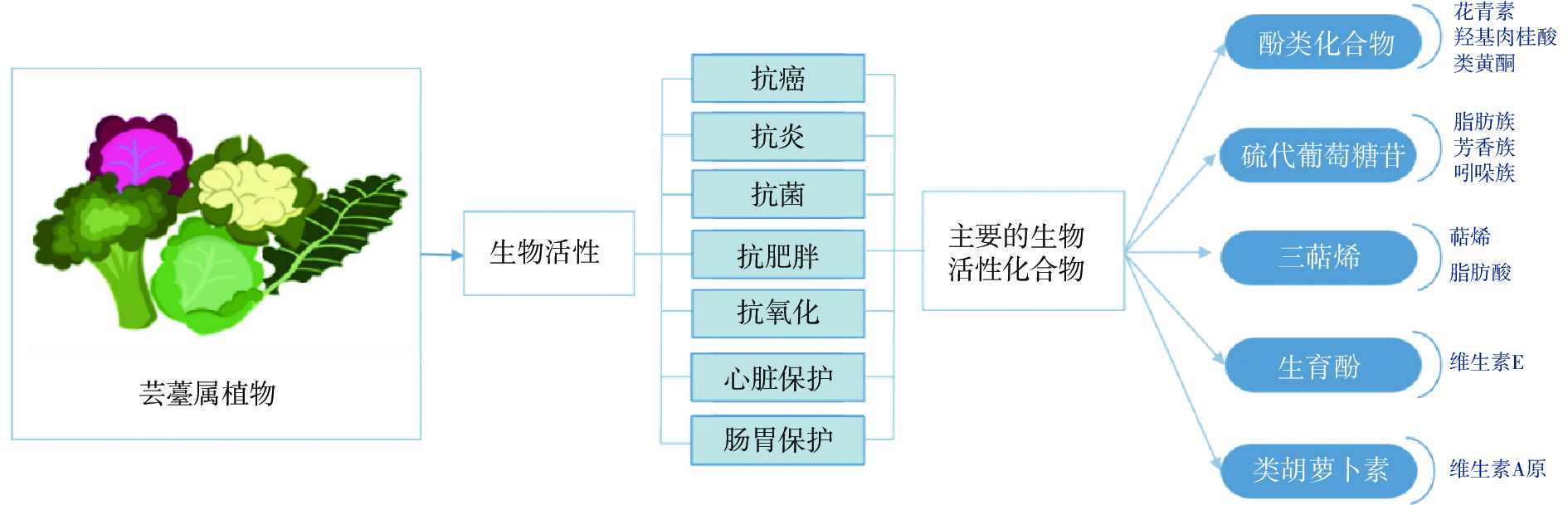
十字花科植物属于双子叶被子植物,有330多个属,约3700多种,是世界范围内具有重要经济价值和营养价值的物种。在我国,芸薹属和萝卜属是主要的蔬菜和油料作物。芸薹属植物是十字花科植物中最重要的一个属[1],包括卷心菜、西兰花、花椰菜、芥菜、甘蓝等,其中含有多种生物活性物质,如多酚类、黄酮类、萜类、类胡萝卜素以及芥子油苷等,可产生抗癌、抗炎、抗菌等多种功效(图1),因此受到广泛关注。在十字花科蔬菜的众多生物活性物质当中,硫代葡萄糖苷具有十分重要的作用,它是植物体内合成的一类含氮次级化合物[2],包括120多种结构[3],根据侧链基团的不同可分为芳香族、脂肪族和吲哚族三类[4]。当十字花科蔬菜因收割、加工、咀嚼或植物降解而使植物细胞破碎时[5],硫葡糖苷葡糖水解酶(黑芥子硫酸苷酶,简称黑芥子酶)释放出来,脂肪族中的4-甲基亚磺酰基丁基硫代葡萄糖苷经水解可产生萝卜硫素[6]。
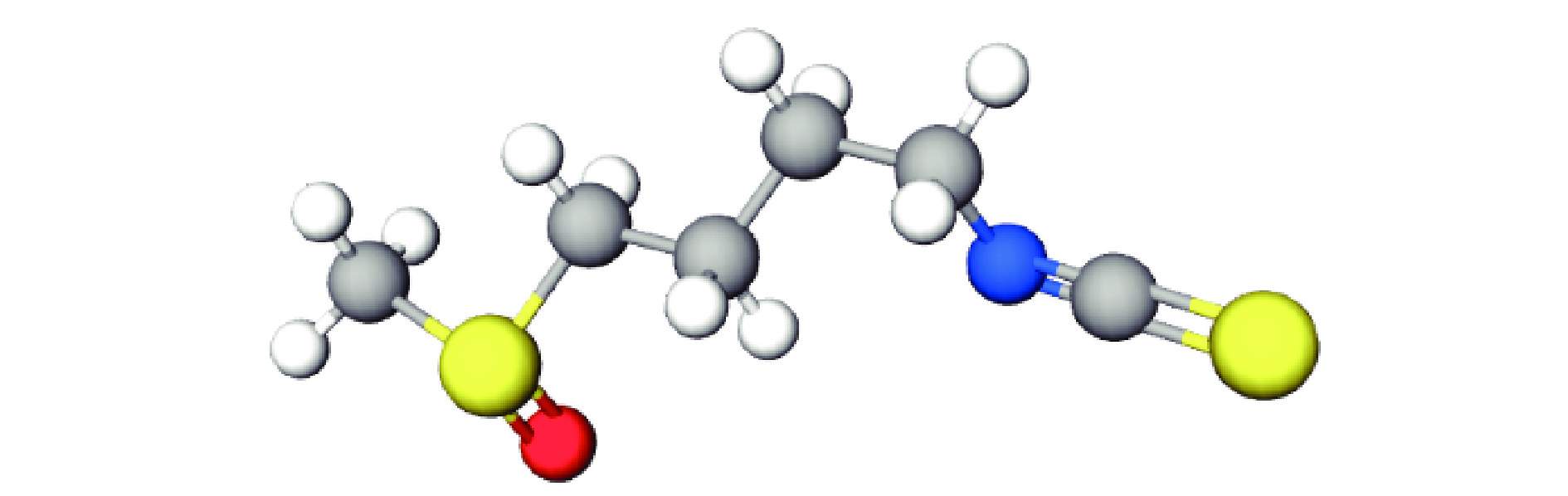
萝卜硫素(1-异硫氰酸酯-4-甲基亚磺酰基丁烷,Sulforaphane)[7],又被称为“莱菔硫烷”,属于一种异硫氰酸盐,常温条件下为黄色或无色的液体,极易溶于有机溶剂,在水中也有一定的溶解性[8],在高温和碱性条件下易被分解[9-10],其分子式为C6H11S2NO,相对分子质量为177.3,分子模型见图2。萝卜硫素不仅具有代谢解毒[11]、抗氧化[12]、抗炎[13]、抗菌[14]、免疫调节[15]的作用,还是目前蔬菜中发现的抗癌效果最强的天然活性物质。试验证明,萝卜硫素可以诱导PhaseⅡ(致癌因子解毒)酶类的产生[16-17],如谷胱甘肽-S-转移酶、环氧化物酶、醌还原酶等,这些酶类可以摧毁致癌因子的活性中心或将它们与内源配基结合起来,加速将其排出体外,防止致癌物质破坏健康细胞内的遗传因子[18]。目前,已有许多萝卜硫素相关报道,但系统全面的概述较少,需要进行综述研究。该综述即对萝卜硫素提取、纯化方法以及抗癌应用等方面的研究进展进行了系统地介绍,并对研究前景进行了展望。
1. 萝卜硫素的提取
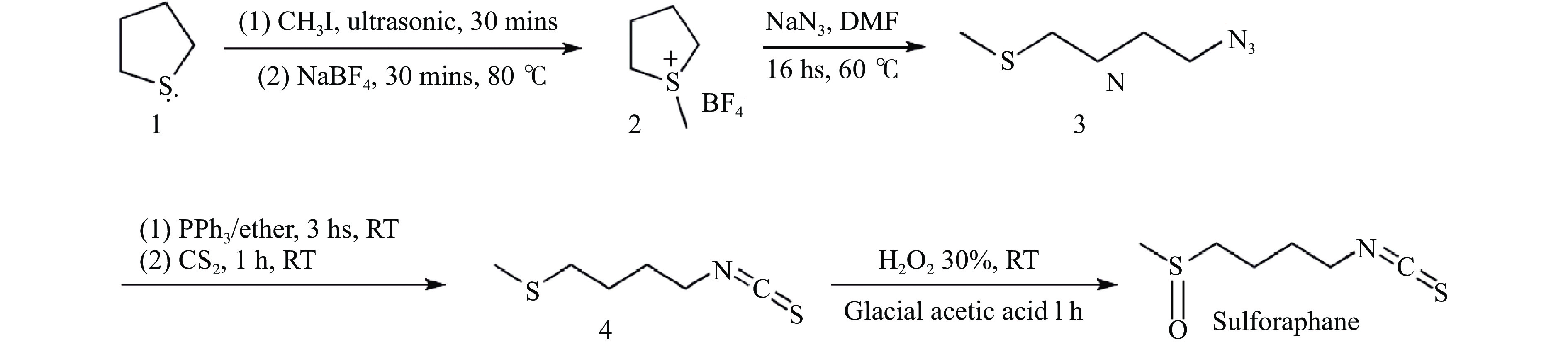
萝卜硫素制备方法主要有化学合成法[19]、酶法[20-21]以及半合成法[22]。化学合成法即通过各种化学反应生成萝卜硫素,一种萝卜硫素的化学合成法是以四氢噻吩、甲基碘、四氟硼酸钠为原料,合成S-甲基四氢噻吩鎓四氟硼酸盐,再与叠氮化钠反应生成1-叠氮-(4-甲基磺酰基)丁烷,经施陶丁格反应得到异硫氰酸酯,最后经H2O2氧化得到萝卜硫素(图3)。萝卜硫素具有旋光异构体,并且基团活性强,化学反应条件难以控制,步骤繁琐并且成本高昂,故化学合成法在实际生产中较少应用[23]。酶法是以十字花科蔬菜及种子为原料,原料中萝卜硫苷经黑芥子酶水解,最终得到萝卜硫素。半合成法是将化学合成法与酶法结合起来,以萝卜硫苷结构类似物为原料,通过生物或化学转化法得到萝卜硫苷,再水解得到萝卜硫素。该方法优点是原料廉价易得,缺点是在提取硫代葡萄糖苷前需经过灭酶处理,生成的萝卜硫苷还需加入外源硫代葡萄糖苷酶水解得到萝卜硫素[24]。化学合成法和半合成法均需使用大量有机溶剂,不利于萝卜硫素的实际应用,因此,目前多数研究仍采用酶法获取萝卜硫素。
1.1 植物种子中萝卜硫素的提取
研究表明,十字花科芸薹属植物花椰菜、青花菜和甘蓝等的硫代葡萄糖苷含量较高,尤其在其种子中的含量最为丰富。吴元锋等[25]以西兰花、甘蓝、芥蓝、羽衣甘蓝、球茎甘蓝、大白菜、花菜7个种类的芸薹属蔬菜共28个品种的种子为原料,比较了它们的萝卜硫素提取率,结果发现西兰花种子中萝卜硫素的提取率要比其它种子高很多,其中绿龙1号提取率最高,达1575.5 mg/kg。西兰花属于芸薹属甘蓝种的一个变种,研究发现,西兰花虽然不是总硫代葡萄糖苷含量最高的蔬菜,但西兰花中硫代葡萄糖苷均具有较高的活性,酶解产物最高的是萝卜硫素。因此,人们常以西兰花种子为原料提取萝卜硫素,制备过程一般包括脱脂、酶解、提取三步。
1.1.1 脱脂
西兰花种子含有大量的油脂,阻碍芥子酶与硫代葡萄糖苷的接触[26],因此在酶解前要对种子进行脱脂处理。也有研究者认为脱脂可能会使芥子酶失活而减少异硫氰酸盐的形成,探究了原料脱脂对萝卜硫素提取率的影响,结果表明仍然是先脱脂再提取的提取率更高[25],因此在酶解之前要对种子进行脱脂处理,常使用有机溶剂如正己烷、石油醚等脱脂。黄忆真[27]将超临界CO2萃取法应用于西兰花种子脱脂过程,并将其与传统的石油醚脱脂进行了对比,结果发现采用石油醚脱脂时,提取液较为浑浊,而超临界脱脂法的出油率更高,萝卜硫素得率也更高。在对超临界CO2萃取工艺进行优化后可除去西兰花种子中94%的油脂,优化结果为:萃取压力30 MPa、CO2流量7 BV/h、粉碎粒径40目、萃取时间4 h、萃取温度40 ℃,有利于后期萝卜硫素的提取纯化。
1.1.2 酶解
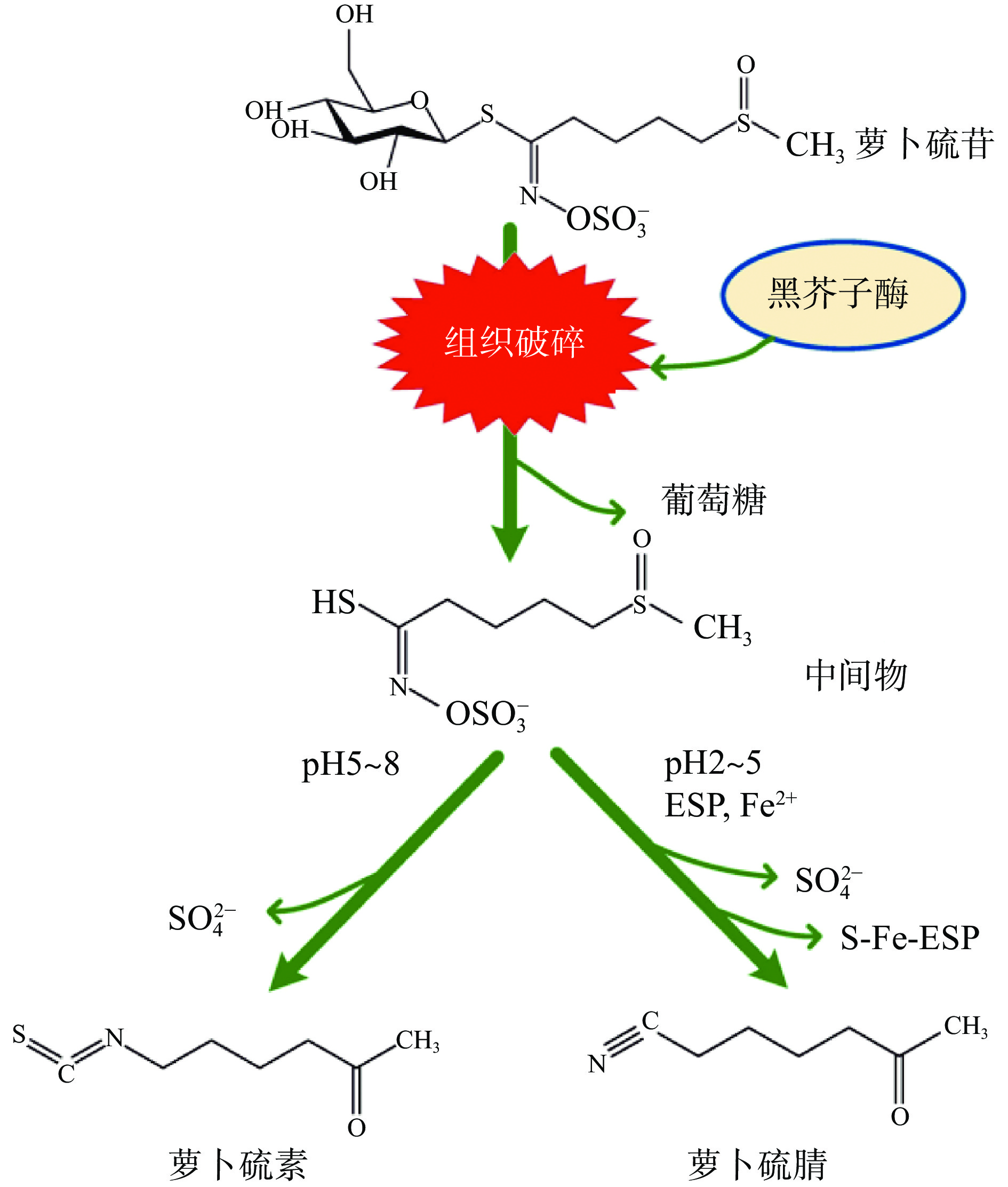
酶解是萝卜硫素获取过程中至关重要的一步,在不经酶解的情况下,萝卜硫素的含量几乎为0,而酶解条件对于萝卜硫素提取率则有着重要的影响。萝卜硫苷水解产物有4种,最常见的是萝卜硫素与萝卜硫腈(图4)。研究表明,萝卜硫素生成的最适pH为5.0~8.0,酶解时间为8~12 h。考虑成本因素,酶解过程一般采用内源性酶。谢述琼等[28]通过单因素梯度实验研究,采用自身芥子苷酶酶解方式得到西兰花种子中萝卜硫素,确定其最佳的酶解时间为8 h,酶解料液比为1:2,酶解pH为7.0,萝卜硫素含量最大为6.682 mg/g。吴华彰等[29]采用正交试验对西兰花种子中萝卜硫素提取条件进行了优化,以pH为5.0的0.1 mol/L醋酸-醋酸钠萝卜汁缓冲液为酶解缓冲体系时萝卜硫素提取率高,这是由于萝卜中含有大量的硫代葡萄糖苷水解酶,在酶解缓冲液中添加萝卜汁可促进酶解反应的进行,同时该试验还添加了Zn2+和VC以激活硫代葡萄糖苷水解酶的活性,且VC还有稳定产物的作用,最终确定了Zn2+浓度0.1 mol/L、VC浓度0.5 mg/mL为最佳条件,在此酶解条件下西兰花种子中萝卜硫素得率达到17.066 mg/g。
1.1.3 提取
硫代葡萄糖苷酶解后,一般用丙酮[31]、乙酸乙酯[32]、二氯甲烷[33-34]等提取萝卜硫素,也有少数研究者以水[35]为提取溶剂。研究表明,二氯甲烷作萃取剂时,萝卜硫素的提取率最高,而且容易挥发,在旋转蒸发时容易除去。但是二氯甲烷毒性较大,当提取的萝卜硫素用作食品或药品时,应采用乙酸乙酯作为萃取剂。近年来,超声辅助萃取法在植物活性成分的提取中得到了广泛的应用。与传统的溶剂萃取法相比,超声辅助萃取法具有溶剂用量较少,萃取时间较短等特点。超声波辅助酶解代替室温下静置酶解,可以借助超声波的涡旋和空化作用,使萝卜硫苷与黑芥子酶的接触面积和频率增加,从而缩短酶解时间,提高提取效率,超声时间60 min即可达到室温下静置酶解10 h的萝卜硫素提取量[31]。谢述琼等[28]借助超声辅助提取西兰花种子中的萝卜硫素,结果表明,最佳条件为:超声浸提溶剂为80%乙醇,超声浸提时间为30 min,超声浸提的次数为2次。超声浸提溶剂选择乙醇是因该溶剂相对更易采购,且在生产中更无毒更安全。萝卜硫素提取出后,将提取液旋转蒸发浓缩,冷冻干燥后即得到萝卜硫素粗品。
1.2 植物体中萝卜硫素的提取
各种蔬菜中,西兰花种子中萝卜硫素的前体含量高,容易得到,研究者多以西兰花种子为原料提取萝卜硫素,但由于种子价格高,提取成本高,难以实现产业化,故采用此原料不能产生较好的经济效益。以苗或蔬菜为原料提取萝卜硫素时,只需酶解、提取两步,方法与以种子为原料基本相同,且蔬菜易获取,适合大规模生产。因此,有些研究者以植物体本身为原料提取萝卜硫素,并不断探索和优化其提取工艺。
1.2.1 西兰花中萝卜硫素的提取
西兰花作为自然界中萝卜硫苷含量最丰富的蔬菜之一[36],常被用于萝卜硫素的提取。胡翠珍等[23]以西兰花鲜食部分绿色幼嫩花茎和花蕾为原料,利用响应面法对添加剂和超声波辅助酶解西兰花提取萝卜硫素工艺进行优化,最终得到:添加外源芥子酶提取萝卜硫素的优化条件为抗坏血酸添加量为4.659 mmol/L,EDTA为6 mmol/L,MgSO4为8.5 mmol/L,CaCl2为4.0 mmol/L,在30 ℃下酶解60 min,萝卜硫素提取率为1813.26 μg/g。唐斌等[37]同时以西兰花鲜食部分幼嫩花茎和花蕾为原料,添加外源芥子酶,对西兰花进行酶解并超声波辅助乙醇提取萝卜硫素,利用单因素及响应面法对工艺参数进行筛选及优化,确定了西兰花中萝卜硫素的最佳提取参数为酶解时间0.95 h,料液比1:41.8,提取时间1.1 h,此条件下西兰花中萝卜硫素提取率达1814.59 μg/g。
西兰花中纤维素含量较高,因此可以添加纤维素酶水解植物细胞壁上的纤维素,从而提高细胞质中萝卜硫苷以及黑芥子酶的释放,促进萝卜硫素的生成。张静等[38]以西兰花为原料,采用纤维素酶辅助提取法并优化酶解工艺,结果显示,酶解时间7.1 h,酶解pH6.0,酶解温度36.8 ℃,纤维素酶添加量2.5%时,萝卜硫素提取量为(408.74±0.83)μg/g。
对于萝卜硫素的提取,较少学者以西兰花采收后副产物(丢弃的根茎叶)为原料,因为硫苷在未发芽的种子中含量最高,随着芽苗的生长而逐渐下降,含量由高到低依次为:种子、芽苗、子叶、下胚轴、根[39]。在实际生活中,西兰花采收后丢弃大量的根茎叶,既造成浪费又污染环境,因此对其进行萝卜硫素等生物活性成分的提取和利用具有重要的实际意义。赵登奇等[40]对西兰花叶中的生物活性成分含量进行了探究,测定西兰花废弃叶中萝卜硫素含量为92.50 μg/g DW,为西兰花副产物的回收利用提供了数据支持。张锦华等[31]以西兰花副产物为原料,利用外源酶和超声波辅助酶解提取萝卜硫素,酶解浸提工艺经优化后得到的萝卜硫素最佳提取条件为:酶解pH5.0、酶解温度35 ℃、酶解时间67 min,萝卜硫素提取量为151.39 μg/g。除酶解过程中使用超声波进行辅助,该试验还将超声波辅助应用于提取过程中:经超声酶解后的样品进行真空冷冻干燥,再用丙酮溶解,超声提取90 min,抽滤,即可得到萝卜硫素粗提取液。在西兰花副产物中提取萝卜硫素不仅可以提高西兰花的综合利用率,还为萝卜硫素的工业化生产提供了发展方向。
1.2.2 萝卜中萝卜硫素的提取
胭脂萝卜是提取食用天然色素萝卜红素的理想原料之一,然而在红色素生产过程中会产生的大量萝卜废渣,这些废渣几乎全部被废弃,这不仅造成资源的浪费,还容易污染环境。阳晖等[41]发现这些萝卜废渣中不仅含有大量萝卜硫素前体物质-萝卜硫苷,而且还含有可以降解萝卜硫苷生成萝卜硫素的葡萄糖硫苷酶,因此,可以利用胭脂萝卜废渣来制备萝卜硫素,不仅降低生产萝卜硫素的成本,而且还有利于胭脂萝卜废渣的循环利用,同时为大规模开发萝卜硫素系列产品提供实践基础。根据其在胭脂萝卜废渣中提取萝卜硫素的酶解工艺优化试验得到:在酶解温度为31 ℃,酶解时间为7.7 h,pH5.6,VC添加量为0.24 mg/kg条件下,萝卜硫素的得率最高为0.05613%。阳晖等[42]还以胭脂萝卜废渣为原料,以乙酸乙酯为提取剂,利用响应面分析法对超声辅助提取萝卜硫素的工艺条件进行优化,结果表明,当提取温度为26 ℃、液固比例为31:1、提取时间为2.9 h、超声时间为29 min时提取效果最佳,提取率为0.04973%。
红心萝卜(心里美萝卜)也是提取萝卜硫素常用的原料之一,该物易得且价格便宜,萝卜硫苷的含量也相对较高。张国良等[43]以红心萝卜为原料,对水解条件进行正交试验,以乙酸乙酯萃取3次,然后进行分离纯化,最终得到纯度为90.67%的样品,平均回收率为99.03%。红心萝卜是北方较为常见的蔬菜种植品种,该试验不仅为萝卜硫素的工业化生产、成本控制提供了方向,同时也为北方红心萝卜的开发利用、经济效益的提高提供了可行性指导意见。
综上,针对萝卜硫素的提取,一般采用液-液萃取法,包括水提法、有机溶剂萃取法、超声波辅助萃取法等。在Tanongkankit等[44]的试验中,还采取了微波辅助萃取法提取甘蓝中的萝卜硫素,并与水提法、有机溶剂萃取法进行比较,结果表明,微波辅助萃取比传统萃取效率更高,在微波功率为12 W,萃取温度为(37.5±0.5)℃时萃取1.5 min,提取率可达(0.028±0.011)mg/g。
2. 萝卜硫素的纯化
液-液萃取后得到的萝卜硫素纯度较低,为了得到更高纯度的萝卜硫素,以便后续相关产品的开发,通常需要对其进行纯化。对于萝卜硫素的纯化工艺,目前已有多种方法,主要包括大孔树脂吸附法、柱层析法、反相高效制备液相色谱法、高速逆流色谱法、半制备高效液相色谱法等。
2.1 大孔树脂吸附法
大孔吸附树脂是具有多孔性和较大比表面积的一类新型非离子高分子化合物,通过物理吸附从溶液中有选择地吸附有机物质达到分离提纯的目的。大孔树脂在天然活性成分如皂苷、黄酮、内酯、生物碱、多酚等大分子化合物的提取分离中已被广泛应用,对中药成分、抗生素、维生素等物质也有良好的吸附效果[45]。Li等[46]采用大孔树脂吸附法,实现了萝卜硫素的分离纯化,并对萝卜硫素在HP20、SP207、SP850和HP2MGL这几种大孔树脂上的吸附解吸性能进行了对比,结果发现SP850树脂对萝卜硫素的分离性能最好,最终得到了纯度为85.9%的萝卜硫素。刘锡建等[47]也对SP850树脂分离萝卜硫素进行了报道,结果显示,通过SP850树脂吸附解吸,最终得到的萝卜硫素纯度为88.7%,高于Li等的结果。所确定的SP850树脂纯化萝卜硫素的最适条件为:上柱液体积20 BV、上柱流速5 BV/h,洗脱液为6 BV的50%乙醇,洗脱速度3 BV/h。研究表明,SP850树脂吸附性能好、洗脱条件温和、洗脱率高,成本相对较低,适合工业化大规模生产。
2.2 柱层析法
柱层析法又称柱色谱法,主要原理是根据样品混合物中各组分在固定相和流动相中分配系数不同,经多次反复分配将组分分离开来。在萝卜硫素的纯化中,主要有硅胶柱层析法和凝胶柱层析法。
2.2.1 硅胶柱层析法
硅胶机械强度优良、表面易改性,是应用最广泛的色谱柱填料[48]。硅胶柱层析法是根据物质在硅胶上的吸附力不同而将其分离,极性较大的物质与硅胶作用强,保留时间长;相反,极性弱的物质与硅胶作用弱,保留时间短,物质在固定相与流动相间通过反复的吸附、解吸过程,得以分离。张国良等[43]采用硅胶柱层析法纯化萝卜硫素,2次上柱后得到纯度为90.67%的纯品;Liang等[49]以硅胶为固定相,三氯甲烷/甲醇为流动相,以正己烷和乙醇为洗脱剂梯度洗脱,得到90%以上纯度的萝卜硫素。Liang等认为,该方法操作压力低且成本相对较低。
2.2.2 凝胶柱层析法
凝胶柱层析选择性高、干扰因素少且可以循环利用,是经典的分离纯化技术,在化工、生物、制药、环境等领域中被广泛应用[50-55]。葡聚糖凝胶中最常见的是Sephadex系列,对于萝卜硫素的纯化,常使用的型号为Sephadex LH-20。Sephadex LH-20的分离原理主要有两方面:一是凝胶的过滤作用,二是在反相溶剂中的反向分配作用。Sephadex LH-20洗脱溶剂分为反相和正相两类,样品极性大,选用反相溶剂洗脱(甲醇-水),样品极性小,则选用正相溶剂洗脱(氯仿-甲醇)。萝卜硫素极性小,一般采用正相系统,以氯仿-甲醇为洗脱剂[56]。
为了获得更高纯度的萝卜硫素,有些学者在试验过程中先后采用硅胶柱和凝胶柱两种层析法进行纯化。李扬[56]、苏光耀[57]均采用了硅胶柱层析法初步纯化,Sephadex LH-20凝胶柱层析法进一步纯化的方法,得到萝卜硫素精制品。萝卜硫素粗提物上硅胶层析柱,以正己烷和丙酮为洗脱剂进行梯度洗脱,初步分离后上Sephadex LH-20凝胶层析柱再进行洗脱。苏光耀和李扬得到的萝卜硫素纯度分别为80%±2.8%和90.4%,这可能是由于硅胶层析时梯度洗脱的浓度及Sephadex LH-20凝胶层析时选用的洗脱剂不同所致。苏光耀硅胶层析梯度洗脱时正己烷:丙酮的浓度为:5:5、4:6、3:7、2:8,Sephadex LH-20凝胶柱层析的洗脱剂为丙酮;而李扬硅胶层析梯度洗脱时正己烷:丙酮的浓度为:1:1、1:3和纯丙酮,Sephadex LH-20凝胶柱层析的洗脱剂为氯仿-甲醇。李扬还对Sephadex LH-20凝胶柱层析的工艺参数进行了优化,确定的最佳分离条件为:流速30 s/滴、填料高度70 cm、进样浓度40 mg/mL。
2.3 反相高效制备液相色谱法(RP-HPLC)
高效液相色谱法(HPLC)是在经典液相色谱法和气相色谱法的基础上发展起来的新型分离分析技术 [58]。根据固定相和流动相极性的强弱,可分为正相高效液相色谱法和反相高效液相色谱法,在正相高效液相色谱中,固定相的极性大于流动相的极性,而反相液相色谱正好与之相反,它的固定相极性小于流动相极性[59]。反相高效液相色谱法的应用日益广泛,在高效液相色谱法中占重要地位。学者常以C18为固定相[60-62],以丙酮/水、乙腈/水或甲醇/水等为流动相,利用反相高效液相色谱法制备萝卜硫素纯品。
2.4 高速逆流色谱法(HSCCC)
高速逆流色谱法是在逆流色谱法(CCC)的基础上开发的一种新型的、连续高效的液—液色谱技术,广泛应用于各种天然产物和合成产物的分离和纯化[63]。HSCCC的原理是利用两相不互溶的两相溶剂体系在高速旋转的螺旋管内建立起一种特殊的单向性流体动力学平衡,一相在离心力的作用下作为固定相保留在柱中,而另一相携带样品作为流动相由恒流泵驱动在柱中流动[64]。Liang等[65]和李椿方[66]均对HSCCC分离纯化萝卜硫素进行了报道,他们均以西兰花种子为原料,在高速逆流色谱法中通过正己烷/乙酸乙酯/甲醇/水(1:5:1:5,v/v/v/v)体系对萝卜硫素进行纯化,而Liang等得到纯度为97%的萝卜硫素,李椿方得到纯度为91%的萝卜硫素,这可能是由于Liang等将西兰花种子水解后用乙酸乙酯提取再纯化,李椿方没有用乙酸乙酯提取,而是直接将水解液高速离心得到的萝卜硫素粗提物进行纯化。
2.5 半制备高效液相色谱法
半制备高效液相色谱是制备型液相色谱的一种,区别主要是流速以及色谱柱内径等的不同。张锦华等[31]认为半制备HPLC法纯化和富集萝卜硫素具有灵敏度高、分离效果好和回收率高等优点,所得提取物虽纯度略低但可通过优化色谱条件和精确萝卜硫素的收集时间来提高,较回收率低更易改进。郭楠[67]对萝卜硫素粗提取液过滤后采用半制备HPLC分离纯化。以乙腈和水为流动相,进行梯度洗脱,依据萝卜硫素标准品的出峰时间对应收集萝卜硫素洗脱液,并以此为单元富集萝卜硫素,洗脱液旋蒸至干得到萝卜硫素纯品。该法操作方便、高效快速,在生物、医药以及食品等领域都具有广阔的应用前景。
目前,以上几种方法均已应用在萝卜硫素的纯化中。大孔树脂吸附法不使用有机溶剂,成本低,适合工业化生产,但缺点是纯化的萝卜硫素纯度略低;硅胶柱层析法操作压力低,成本较低,但一般需经过多方法、反复多次操作才能获得纯度较高的产品;凝胶柱层析法选择性高、干扰因素少、凝胶柱可循环利用,然而其分离时间较长;反相高效制备液相色谱法具有高速、高效、高灵敏度、色谱柱可反复使用、样品易回收、纯度高等的优点,但相比于其他方法成本较高,不适合大规模生产;高速逆流色谱法不耗费色谱填料,样品无需预处理且纯度、回收率高,但同样分离时间较长,且存在放大困难的问题;半制备高效液相色谱法操作方便、高效快速,具有灵敏度高、分离效果好、回收率高的优势,且工业型制备色谱可用于工业生产,但纯化后样品的纯度相对较低。由此可见,每种方法都各有利弊,研究者可根据实际情况加以选择,并通过试验不断优化纯化工艺,为萝卜硫素的生产及应用提供理论依据。
3. 抗癌功能研究
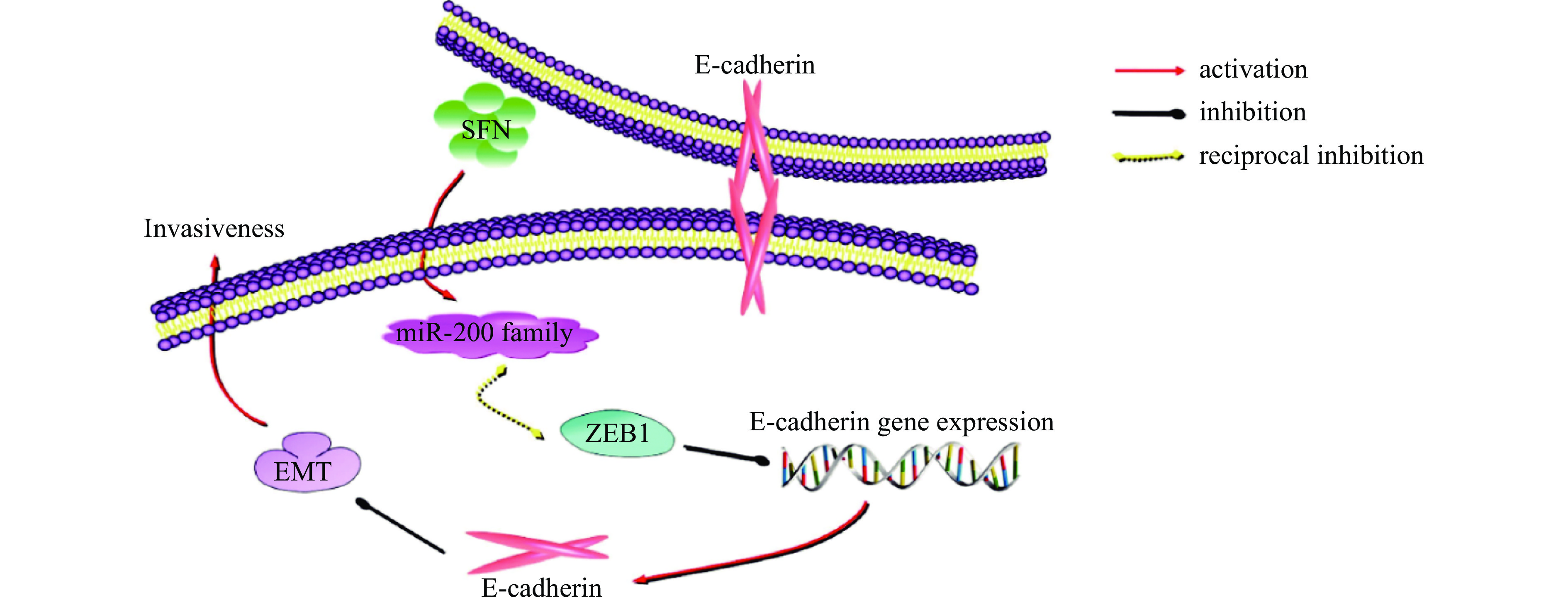
癌症是导致人类死亡的主要疾病之一,对人类的生命健康造成了严重的威胁,所以人们十分重视防癌抗癌的应用研究。根据目前已有报道,经常食用十字花科蔬菜可降低癌症的发病率[68]。流行病研究也表明,经常摄入十字花科蔬菜与患胰腺癌[69-73]、乳腺癌[74]、肾癌[75-76]、膀胱癌[77]、前列腺癌[78]、卵巢癌[79]的风险成反比,这是因为十字花科蔬菜中富含硫代葡萄糖苷,经黑芥子酶水解或肠道菌群水解后产生萝卜硫素。萝卜硫素是蔬菜中具有抗癌能力的天然活性物质之一,具有抑制癌细胞增殖、诱导解毒酶、细胞周期停滞和凋亡的能力[80-81],如在Huang等[82]发现萝卜硫素可以通过靶向miR200c/ZEB1轴逆转上皮间质转化来抑制膀胱癌细胞的侵袭(图5);Huang等[83]报道了萝卜硫素对肝癌细胞的作用,研究表示萝卜硫素可以阻断肝癌细胞中MAKP7这一信号通路,触发HepG2细胞的G2/M阻滞,降低肝癌HepG2细胞的生存能力,且以剂量依赖的方式抑制肝癌细胞的迁移和侵袭(图6)。
多个研究表明,萝卜硫素可通过不同机制对多种类型癌症发挥治疗作用。Zhu等[84]在靶向GSK3β的miR-19(一种关键的致癌miRNA)介导萝卜硫素抑制肺癌干细胞的研究中报道了萝卜硫素可以通过抑制miR-19和Wnt/β-连环蛋白途径对肺癌干细胞有抑制作用;Kiani等[85]对花椰菜芽中提取的不同浓度的萝卜硫素对正常和胃癌细胞系中CDX1/2以及miRNA-9和miRNA-326的生存力、死亡模式和表达变化的影响进行了评估,结果显示,在不同浓度的萝卜硫素下,观察到胃癌细胞系(AGS和MKN45)中CDX1、CDX2、miR-9和miR-326的表达发生显著变化,这可能是萝卜硫素在特定剂量下影响胃癌细胞系,并通过改变CDX1、CDX2、miR-9和miR-326的表达来改变其增值率;对于结直肠癌,萝卜硫素也有较好的抑制作用,Gwon等[86]报道了相关研究,结果表明,萝卜硫素可以通过Nrf2和p53轴之间的串扰影响结直肠癌细胞增殖和线粒体功能;Burnett等[87]还将萝卜硫素与紫杉烷联合使用,发现萝卜硫素可以抑制乳腺癌细胞的增殖,紫杉烷或多西他赛治疗在增加TNBC细胞系中IL-6的同时也丰富了乳腺癌干细胞,而加入萝卜硫素不仅显著增强了对大块肿瘤细胞的细胞毒性,还大大抑制了乳腺癌干细胞的扩张。除此之外,萝卜硫素还被证实对胰腺癌[88]、宫颈癌[89]、前列腺癌[90]等癌细胞均有抑制作用。
4. 结语与展望
萝卜硫素已被证明可以抑制癌细胞增殖、引起癌细胞凋亡,对于多种癌症细胞的抑制作用都有了相关报道,这引起了国内外学者的广泛关注,而萝卜硫素的提取纯化工艺就显得尤为重要,目前仍存在一些问题有待进一步探究:抑制西兰花中上皮硫特异蛋白(ESP)活性可以提高萝卜硫素得率,如何保证在抑制ESP活性的同时保持自身黑芥子酶活性不受影响;针对添加外源芥子酶提取萝卜硫素的研究,如何准确确定酶的添加量,使酶解理化环境保持在萝卜硫素最佳生成条件下;提取萝卜硫素时,提取效果较好的二氯甲烷和乙酸乙酯对人体有毒性作用;要想得到更高纯度的萝卜硫素,还需将不同的纯化方法相结合,但步骤过多可能会导致萝卜硫素的损失。虽然萝卜硫素对癌细胞有抑制作用,但在实际应用中仍存在一些困难,如对于胰腺癌患者,伴随着胰腺癌病情的发展,会产生食欲不振、恶心呕吐等问题,在服用了西兰花芽粉胶囊后会增加消化负担[91],一些患者摄入胶囊后可能会因为西兰花味而恶心呕吐,影响患者的身体健康,不利于癌症的治疗。
未来仍需不断探索和优化萝卜硫素提取纯化工艺,在萝卜硫素防癌保健品及抗癌药品上研发新的种类,以满足市场多样化的需求。今后可对不同环境中ESP和黑芥子酶的活性进行深入研究,在外源酶酶液的制备方法、浓度大小以及酶活性强弱等方面开展相关试验,为硫代葡萄糖苷水解为萝卜硫素提供最佳条件。乙醇成本较低,且毒性低,更利于萝卜硫素在人体的应用,今后可重点选择以乙醇为萃取溶剂进行研究,并不断优化工艺以提高提取率。在萝卜硫素完整的生产工艺中,可对如何精简步骤及减少损失进行探索研究,以便投入工业化生产。对于以西兰花为原料,因西兰花味而影响治疗效果的药品,可在制备过程中去除西兰花味或添加风味物质掩盖西兰花味。总之,在制备萝卜硫素保健品或药品时,需做到绿色、环保、无毒,并且满足萝卜硫素纯度高、副作用小、易被消费者接受的要求,从而更好地应用于人体的防癌抗癌当中。
-
-
[1] GONZÁLEZ K M F, ALMANZA A Y H, SALCIDO N M D F. The value of bioactive compounds of cruciferous vegetables (Brassica) as antimicrobials and antioxidants: A review[J]. Journal of Food Biochemistry,2020,44(10):e13414.
[2] TORTORELLA S M, ROYCE S G, LICCIARDI P V, et al. Dietary sulforaphane in cancer chemoprevention: The role of epigenetic regulation and HDAC inhibition[J]. Antioxidants & Redox Signaling,2015,22(16):1382−1424.
[3] LI Z, ZHENG S, LIU Y, et al. Characterization of glucosinolates in 80 broccoli genotypes and different organs using UHPLC-Triple-TOF-MS method[J]. Food Chemistry,2020,334:127519.
[4] 郑姝宁, 张延国, 郭宁, 等. 羽衣甘蓝和白菜型油菜硫代葡萄糖苷超高效液相色谱—飞行时间质谱分析方法[J]. 园艺学报,2016,43(12):2481−2490. [ZHENG S N, ZHANG Y G, GUO N, et al. Glucosinolate analysis in kale and yellow sarson based on UHPLC-Triple-TOF-MS[J]. Acta Horticulturae Sinica,2016,43(12):2481−2490. doi: 10.16420/j.issn.0513-353x.2016-0479 ZHENG S N, ZHANG Y G, GUO N, et al. Glucosinolate analysis in kale and yellow sarson based on UHPLC-Triple-TOF-MS[J]. Acta Horticulturae Sinica, 2016, 43(12): 2481-2490. doi: 10.16420/j.issn.0513-353x.2016-0479
[5] ABUKHABTA S, GHAWI S K, KARATZAS K A, et al. Sulforaphane-enriched extracts from glucoraphanin-rich broccoli exert antimicrobial activity against gut pathogens in vitro and innovative cooking methods increase in vivo intestinal delivery of sulforaphane[J]. European Journal of Nutrition,2020,60(prepublish):1−14.
[6] SUN J, WANG Y, PANG X, et al. The effect of processing and cooking on glucoraphanin and sulforaphane in brassica vegetables[J]. Food Chemistry,2021,360:130007. doi: 10.1016/j.foodchem.2021.130007
[7] LIU H R, XIA Z Y, WANG N L. Sulforaphane modulates TGFbeta2-induced conjunctival fibroblasts activation and fibrosis by inhibiting PI3K/Akt signaling[J]. Int J Ophthalmol,2020,13(10):1505−1511. doi: 10.18240/ijo.2020.10.01
[8] 张胜智. 西兰花种子中硫代葡萄糖苷的提取及高纯度莱菔硫烷的制备工艺研究[D]. 北京: 北京化工大学, 2012. ZHANG S Z. Separation of glucosinolates in broccoli seeds and the preparation of high purity sulforaphane[D]. Beijing: Beijing University of Chemical Technology, 2012.
[9] MAHN A, SAAVEDRA A, RUBIO M P. Kinetic study of sulforaphane stability in blanched and un-blanched broccoli (Brassica oleracea var. italica) florets during storage at low temperatures[J]. Journal of Food Science and Technology,2018,55(11):4687−4693. doi: 10.1007/s13197-018-3395-4
[10] 董俊杰, 施琪浩, 张英杰, 等. 拓展实验萝卜硫素质谱分析与指认[J]. 当代化工,2020,49(8):1643−1646, 1663. [DONG J J, SHI Q H, ZHANG Y J, et al. Mass spectroscopic analysis and identification of sulforaphane for materials and pharmaceutical professional expansion experiments[J]. Contemporary Chemical Industry,2020,49(8):1643−1646, 1663. doi: 10.3969/j.issn.1671-0460.2020.08.020 DONG J J, SHI Q H, ZHANG Y J, et al. Mass spectroscopic analysis and identification of sulforaphane for materials and pharmaceutical professional expansion experiments[J]. Contemporary Chemical Industry, 2020, 49(8): 1643-1646, 1663. doi: 10.3969/j.issn.1671-0460.2020.08.020
[11] ALKHARASHI N A O, PERIASAMY V S, ATHINARAYANAN J, et al. Sulforaphane alleviates cadmium-induced toxicity in human mesenchymal stem cells through POR and TNFSF10 genes expression[J]. Biomedicine & Pharmacotherapy,2019,115:108896.
[12] AKBARI E, NAMAZIAN M. Sulforaphane: A natural product against reactive oxygen species[J]. Computational and Theoretical Chemistry,2020:1183.
[13] ALA’A A B, LUAY A Q. Sulforaphane from broccoli attenuates inflammatory hepcidin by reducing IL-6 secretion in human HepG2 cells[J]. Journal of Functional Foods,2020,75:104210. doi: 10.1016/j.jff.2020.104210
[14] DERAMAUDT T B, ALI M, VINIT S, et al. Sulforaphane reduces intracellular survival of Staphylococcus aureus in macrophages through inhibition of JNK and p38 MAPK-induced inflammation[J]. International Journal of Molecular Medicine,2020,45(6):1927−1941.
[15] ANDREA M, ANTONIO C. Potential of sulforaphane as a natural immune system enhancer: A Review[J]. Molecules,2021,26(3):752. doi: 10.3390/molecules26030752
[16] ELKASHTY O A, TRAN S D. Sulforaphane as a promising natural molecule for cancer prevention and treatment[J]. Current Medical Science,2021,41(2):250−269. doi: 10.1007/s11596-021-2341-2
[17] CEDROWSKI J, DĄBROWA K, KROGULSOBCZAK A, et al. A lesson learnt from food chemistry—Elevated temperature triggers the antioxidant action of two edible isothiocyanates: Erucin and sulforaphane[J]. Antioxidants,2020,9(11):1090. doi: 10.3390/antiox9111090
[18] 季宇彬, 池文杰, 邹翔, 等. 西兰花中萝卜硫素提取、分离与抗癌活性研究[J]. 哈尔滨商业大学学报(自然科学版),2005(3):270−273. [JI Y B, CHI W J, ZHOU X, et al. Study on extraction, isolation and anticarcinogenic activity of sulforaphane in broccoli[J]. Journal of Harbin University of Commerce (Natural Sciences Edition),2005(3):270−273. doi: 10.19492/j.cnki.1672-0946.2005.03.002 JI Y B, CHI W J, ZHOU X, et al. Study on extraction, isolation and anticarcinogenic activity of sulforaphane in broccoli[J]. Journal of Harbin University of Commerce (Natural Sciences Edition), 2005(3): 270-273. doi: 10.19492/j.cnki.1672-0946.2005.03.002
[19] 蒋秀芹. 抗癌药物莱菔硫烷类化合物的合成及其性能研究[D]. 北京: 北京化工大学, 2016. JIANG X Q. Study on synthesis and properties of anti-cancer drugs sulforaphane compounds[D]. Beijing: Beijing University of Chemical Technology, 2016.
[20] LÓPEZ-CERVANTES J, TIRADO-NORIEGA L G, SÁNCHEZ-MACHADO D I, et al. Biochemical composition of broccoli seeds and sprouts at different stages of seedling development[J]. International Journal of Food Science & Technology,2013,48(11):2267−2275.
[21] G W L, A M K, A B B, et al. Glucoraphanin and 4-hydroxyglucobrassicin contents in seeds of 59 cultivars of broccoli, raab, kohlrabi, radish, cauliflower, brussels sprouts, kale, and cabbage[J]. Journal of Agricultural and Food Chemistry,2004,52(4):916−926. doi: 10.1021/jf0307189
[22] VO D V, TRUONG V D, TRAN T D, et al. A new and effective approach to the synthesis of sulforaphane[J]. Letters in Organic Chemistry,2016,13(1):7−10.
[23] 胡翠珍, 李胜, 马绍英, 等. 响应面优化西兰花中萝卜硫素复合提取工艺[J]. 食品工业科技,2016,37(4):271−277. [HU C J, LI S, MA S Y, et al. Response surface optimizes the compound extraction process of sulforaphane from broccoli[J]. Science and Technology of Food Industry,2016,37(4):271−277. doi: 10.13386/j.issn1002-0306.2016.04.046 HU C J, LI S, MA S Y, et al. Response surface optimizes the compound extraction process of sulforaphane from broccoli[J]. Science and Technology of Food Industry, 2016, 37(4): 271-277. doi: 10.13386/j.issn1002-0306.2016.04.046
[24] 吴元锋, 徐维亮, 申雨珂, 等. 萝卜硫素制备及纯化工艺研究进展[J]. 食品工业科技,2016,37(19):381−386. [WU Y F, XU W L, SHEN Y K, et al. Preparation and purification of sulforaphane-an overview[J]. Science and Technology of Food Industry,2016,37(19):381−386. doi: 10.13386/j.issn1002-0306.2016.19.066 WU Y F, XU W L, SHEN Y K, et al. Preparation and purification of sulforaphane-an overview[J]. Science and Technology of Food Industry, 2016, 37(19): 381-386. doi: 10.13386/j.issn1002-0306.2016.19.066
[25] 吴元锋, 沈莲清, 毛建卫, 等. 芸苔属植物种子中萝卜硫素的提取工艺研究[J]. 食品与生物技术学报,2009,28(5):647−651. [WU Y F, SHEN L Q, MAO J W, et al. Study on extraction of sulforaphane from broccoli[J]. Journal of Food Science and Biotechnology,2009,28(5):647−651. doi: 10.3321/j.issn:1673-1689.2009.05.013 WU Y F, SHEN L Q, MAO J W, et al. Study on extraction of sulforaphane from broccoli[J]. Journal of Food Science and Biotechnology, 2009, 28(5): 647-651. doi: 10.3321/j.issn:1673-1689.2009.05.013
[26] 林毅, 张金娟, 李晓露, 等. 西兰花种子中萝卜硫素的提取工艺研究[J]. 化学与生物工程,2014,31(12):48−50. [LIN Y, ZHANG J J, LI X X, et al. Study on extraction process of sulforaphane from broccoli seeds[J]. Chemistry & Bioengineering,2014,31(12):48−50. doi: 10.3969/j.issn.1672-5425.2014.12.014 LIN Y, ZHANG J J, LI X X, et al. Study on extraction process of sulforaphane from broccoli seeds[J]. Chemistry & Bioengineering, 2014, 31(12): 48-50. doi: 10.3969/j.issn.1672-5425.2014.12.014
[27] 黄忆真. 西兰花种子中萝卜硫素的提取分离与微胶囊化工艺研究[D]. 杭州: 浙江大学, 2019. HUANG Y Z. Study on extraction separation and microencapsulation of sulforaphane from broccoli seed[D]. Hangzhou: Zhejiang University, 2019.
[28] 谢述琼, 何珺, 舒华. 西兰花种子中萝卜硫素酶解浸提工艺研究[J]. 广州化工,2016,44(8):73−75, 99. [XIE S Q, HE J, SHU H. Leaching process research of sulforaphane with broccoli seeds[J]. Guangzhou Chemical Industry,2016,44(8):73−75, 99. doi: 10.3969/j.issn.1001-9677.2016.08.027 XIE S Q, HE J, SHU H. Leaching process research of sulforaphane with broccoli seeds[J]. Guangzhou Chemical Industry, 2016, 44(8): 73-75, 99. doi: 10.3969/j.issn.1001-9677.2016.08.027
[29] 吴华彰, 赵云利. 西兰花种子提取萝卜硫素的酶解体系[J]. 光谱实验室,2012,29(1):431−434. [WU H Z, ZHAO Y L. Enzymatic degradation system for sulforaphane extraction from broccoli seeds[J]. Chinese Journal of Spectroscopy Laboratory,2012,29(1):431−434. doi: 10.3969/j.issn.1004-8138.2012.01.106 WU H Z, ZHAO Y L. Enzymatic degradation system for sulforaphane extraction from broccoli seeds[J]. Chinese Journal of Spectroscopy Laboratory, 2012, 29(1): 431-434. doi: 10.3969/j.issn.1004-8138.2012.01.106
[30] WU Y F, LÜ C Z, ZOU L G, et al. Approaches for enhancing the stability and formation of sulforaphane[J]. Food Chemistry,2021,345:128771. doi: 10.1016/j.foodchem.2020.128771
[31] 张锦华, 郭楠, 杨妍, 等. 西兰花副产物中萝卜硫素提取、纯化及鉴定[J]. 食品科学,2019,40(8):248−255. [ZHANG J H, GUO N, YANG Y, et al. Extraction, purification and identification of sulforaphane from broccoli byproducts[J]. Food Science,2019,40(8):248−255. doi: 10.7506/spkx1002-6630-20180907-077 ZHANG J H, GUO N, YANG Y, et al. Extraction, purification and identification of sulforaphane from broccoli byproducts[J]. Food Science, 2019, 40(8): 248-255. doi: 10.7506/spkx1002-6630-20180907-077
[32] ZHANG S, YING D Y, CHENG L J, et al. Sulforaphane in broccoli-based matrices: Effects of heat treatment and addition of oil[J]. LWT,2020,128:109443. doi: 10.1016/j.lwt.2020.109443
[33] LIANG H, YUAN Q P, DONG H R, et al. Determination of sulforaphane in broccoli and cabbage by high-performance liquid chromatography[J]. Journal of Food Composition and Analysis,2006,19(5):473−476. doi: 10.1016/j.jfca.2005.11.005
[34] CAMPAS-BAYPOLI O N, SANCHEZ-MACHADO D I, BUENO-SOLANO C, et al. HPLC method validation for measurement of sulforaphane level in broccoli by-products[J]. Biomed Chromatogr,2010,24(4):387−392.
[35] PONGMALAI P, DEVAHASTIN S, CHIEWCHAN N, et al. Enhancing the recovery of cabbage glucoraphanin through the monitoring of sulforaphane content and myrosinase activity during extraction by different methods[J]. Separation and Purification Technology,2017,174:338−344. doi: 10.1016/j.seppur.2016.11.003
[36] GU Z, GUO Q, GU Y. Factors influencing glucoraphanin and sulforaphane formation in brassica plants: A review[J]. Journal of Integrative Agriculture,2012,11(11):1804−1816. doi: 10.1016/S2095-3119(12)60185-3
[37] 唐斌, 马绍英, 李胜, 等. 响应面优化西兰花中萝卜硫素的超声辅助提取工艺[J]. 甘肃农业大学学报,2015,50(3):171−177. [TANG B, MA S Y, LI S, et al. Optimization on extraction techniques of sulforaphane from broccoli with ultrasound-assisted by response surface methodology[J]. Journal of Gansu Agricultural University,2015,50(3):171−177. doi: 10.3969/j.issn.1003-4315.2015.03.029 TANG B, MA S Y, LI S, et al. Optimization on extraction techniques of sulforaphane from broccoli with ultrasound-assisted by response surface methodology[J]. Journal of Gansu Agricultural University, 2015, 50(3): 171-177. doi: 10.3969/j.issn.1003-4315.2015.03.029
[38] 张静, 马永强, 冯进, 等. 响应面法优化纤维素酶辅助提取西蓝花萝卜硫素工艺研究[J]. 食品科技,2020,45(12):188−195. [ZHANG J, MA Y Q, FENG J, et al. Optimization of the cellulose-assisted extraction technique of sulforaphane from broccoli by response surface method[J]. Food Science and Technology,2020,45(12):188−195. doi: 10.13684/j.cnki.spkj.2020.12.029 ZHANG J, MA Y Q, FENG J, et al. Optimization of the cellulose-assisted extraction technique of sulforaphane from broccoli by response surface method[J]. Food Science and Technology, 2020, 45(12): 188-195. doi: 10.13684/j.cnki.spkj.2020.12.029
[39] GUO L, YANG R, WANG Z, et al. Glucoraphanin, sulforaphane and myrosinase activity in germinating broccoli sprouts as affected by growth temperature and plant organs[J]. Journal of Functional Foods,2014,9:70−77. doi: 10.1016/j.jff.2014.04.015
[40] 赵登奇, 孙亚天, 黄建颖, 等. 西兰花叶中生物活性成分的测定[J]. 核农学报,2020,34(6):1266−1271. [ZHAO D Q, SUN Y T, HUAN J Y, et al. Determination of bioactive ingredients in broccoli leaves[J]. Journal of Nuclear Agricultural Sciences,2020,34(6):1266−1271. doi: 10.11869/j.issn.100-8551.2020.06.1266 ZHAO D Q, SUN Y T, HUAN J Y, et al. Determination of bioactive ingredients in broccoli leaves[J]. Journal of Nuclear Agricultural Sciences, 2020, 34(6): 1266-1271. doi: 10.11869/j.issn.100-8551.2020.06.1266
[41] 阳晖, 赵学勤, 李昌满, 等. 胭脂萝卜废渣中提取萝卜硫素的酶解工艺优化[J]. 食品工业科技,2016,37(5):207−211. [YANG H, ZHAO X Q, LI C M, et al. Optimization of enzymatic hydrolysis conditions for extracting sulforaphane from waste of carmine radish[J]. Science and Technology of Food Industry,2016,37(5):207−211. doi: 10.13386/j.issn1002-0306.2016.05.032 YANG H, ZHAO X Q, LI C M, et al. Optimization of enzymatic hydrolysis conditions for extracting sulforaphane from waste of carmine radish[J]. Science and Technology of Food Industry, 2016, 37(5): 207-211. doi: 10.13386/j.issn1002-0306.2016.05.032
[42] 阳晖, 杨呈凤, 李昌满, 等. 胭脂萝卜废渣中萝卜硫素的提取工艺研究[J]. 食品科技,2016,41(2):259−264. [YANG H, YANG C F, LI C M, et al. Process of sulforaphane extracted from waste of carmine radish[J]. Food Science and Technology,2016,41(2):259−264. doi: 10.13684/j.cnki.spkj.2016.02.049 YANG H, YANG C F, LI C M, et al. Process of sulforaphane extracted from waste of carmine radish[J]. Food Science and Technology, 2016, 41(2): 259-264. doi: 10.13684/j.cnki.spkj.2016.02.049
[43] 张国良, 赵秀娟, 张宇秋, 等. 红心萝卜中莱菔烷提取及纯化方法建立[J]. 中国公卫生,2009,25(10):1256−1257. [ZHANG G L, ZHAO X J, ZHANG Y Q, et al. Establishment of extraction and purification method of sulforaphane from radish[J]. Chinese Journal of Public Health,2009,25(10):1256−1257. ZHANG G L, ZHAO X J, ZHANG Y Q, et al. Establishment of extraction and purification method of sulforaphane from radish[J]. Chinese Journal of Public Health, 2009, 25(10): 1256-1257.
[44] TANONGKANKIT Y, SABLANI S S, CHIEWCHAN N, et al. Microwave-assisted extraction of sulforaphane from white cabbages: Effects of extraction condition, solvent and sample pretreatment[J]. Journal of Food Engineering,2013,117(1):151−157. doi: 10.1016/j.jfoodeng.2013.02.011
[45] BARBOZA M, ALMEIDA R M R G, HOKKA C O. Influence of temperature on the kinetics of adsorption and desorption of clavulanic acid by ionic exchange[J]. Biochemical Engineering Journal,2003,14(1):19−26. doi: 10.1016/S1369-703X(02)00103-1
[46] LI C, LIANG H, YUAN Q, et al. Optimization of sulforaphane separation from broccoli seeds by macroporous resins[J]. Separation Science and Technology,2008,43(3):609−623. doi: 10.1080/01496390701787222
[47] 刘锡建, 肖稳发, 曹俭, 等. SP850树脂分离萝卜硫素[J]. 食品与发酵工业,2011,37(7):197−200. [LIU X J, XIAO W F, CAO J, et al. Separation of sulforaphen by SP850 macroporous resin adsorbent[J]. Food and Fermentation Industries,2011,37(7):197−200. doi: 10.13995/j.cnki.11-1802/ts.2011.07.046 LIU X J, XIAO W F, CAO J, et al. Separation of sulforaphen by SP850 macroporous resin adsorbent[J]. Food and Fermentation Industries, 2011, 37(7): 197-200. doi: 10.13995/j.cnki.11-1802/ts.2011.07.046
[48] 杨俊佼, 张硕. 单分散高纯硅胶色谱柱填料的制备[J]. 高等学校化学学报,2012,33(4):689−694. [YANG J J, ZHANG S. Preparation of monodisperse and high-purity silica packing materials[J]. Chemical Journal of Chinese Universities,2012,33(4):689−694. doi: 10.3969/j.issn.0251-0790.2012.04.009 YANG J J, ZHANG S. Preparation of monodisperse and high-purity silica packing materials[J]. Chemical Journal of Chinese Universities, 2012, 33(4): 689-694. doi: 10.3969/j.issn.0251-0790.2012.04.009
[49] LIANG H, YUAN Q, XIAO Q. Purification of sulforaphane from Brassica oleracea seed meal using low-pressure column chromatography[J]. Journal of Chromatography B,2005,828(1−2):91−96. doi: 10.1016/j.jchromb.2005.09.041
[50] BOI V N, TRANG N T M, CUONG D X, et al. Antioxidant phlorotannin from brown algae sargassum dupplicatum: Enzyme-assissted extraction and purification[J]. World Journal of Food Science and Technology,2020,4(2):62−68. doi: 10.11648/j.wjfst.20200402.17
[51] MAMYLOV S G, SKRIPKINA T S, TIKHOVA V D, et al. Thermal analysis of mechanochemically activated humic acids of brown coal[J]. Journal of Physics:Conference Series,2020,1675(1):12093. doi: 10.1088/1742-6596/1675/1/012093
[52] EBE H, CHIBA T, OHISA S, et al. Gel permeation chromatography purification process for highly efficient perovskite nanocrystal light-emitting devices[J]. Journal of Photopolymer Science and Technology,2020,33(4):393−397. doi: 10.2494/photopolymer.33.393
[53] ZHANG Y S, ZHANG Q Y, WANG B, et al. Chemical constituents from Ampelopsis grossedentata[J]. Journal of Chinese Pharmaceutical Sciences,2006(4):211−214.
[54] ANDERSEN O M, FOSSEN T, TORSKANGERPOLL K, et al. Anthocyanin from strawberry (Fragaria ananassa) with the novel aglycone, 5-carboxypyranopelargonidin[J]. Phytochemistry,2003,65(4):405−410.
[55] WANG B, QUAN Y, GUO H. Discrimination of car headlight plastic by gel permeation chromatography[J]. Journal of Forensic Science and Medicine,2015,1(1):43−47. doi: 10.4103/2349-5014.157909
[56] 李扬. 西兰花芽苗菜中莱菔硫烷的分离纯化及抗癌活性的测定[D]. 大庆: 黑龙江八一农垦大学, 2009. LI Y. Separation and purification of sulforaphane from broccoli sprouts and determination of anticancer activity[D]. Daqing: Heilongjiang Bayi Agricultural University, 2009.
[57] 苏光耀. 西兰花种子中硫苷酶解产物萝卜硫素的提取分离与结构鉴定[D]. 杭州: 浙江工商大学, 2007. SU G Y. Sulforaphane hydrolyzed from glucosinolate in broccoli seed[D]. Hangzhou: Zhejiang Gongshang University, 2007.
[58] REKHI H, RANI S, SHARMA N, et al. A review on recent applications of high-performance liquid chromatography in metal determination and speciation analysis[J]. Critical Reviews in Analytical Chemistry,2017,47(6):524−537. doi: 10.1080/10408347.2017.1343659
[59] MALVIYA R, BANSAL V, PAL O P, et al. High performance liquid chromatography: A short review[J]. Journal of Global Pharma Technology,2010,2(5):22−26.
[60] MATUSHESKI N V, WALLING M A, JUVIK J A, et al. Preparative HPLC method for the purification of sulforaphane and sulforaphane nitrile from Brassica oleracea[J]. Journal of Agricultural and Food Chemistry,2001,49(4):1867−1872. doi: 10.1021/jf0013860
[61] KORE A M, SPENCER G F, WALLIG M A. Purification of the ω-(methylsulfinyl)alkyl glucosinolate hydrolysis products: 1-isothiocyanato-3-(methylsulfinyl)propane, 1-isothiocyanato-4-(methylsulfinyl)butane, 4-(methylsulfinyl)butanenitrile, and 5-(methylsulfinyl)pentanenitrile from broccoli and Lesquerella fendleri[J]. Journal of Agricultural and Food Chemistry,1993,41(1):89−95. doi: 10.1021/jf00025a019
[62] HAO L, CHUNFANG L, QIPENG Y, et al. Separation and purification of sulforaphane from broccoli seeds by solid phase extraction and preparative high-performance liquid chromatography[J]. Journal of Agricultural and Food Chemistry,2007,55(20):8047−8053. doi: 10.1021/jf0706833
[63] ITO Y. Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography[J]. Journal of Chromatogr A,2004,1065(2):145−168.
[64] YANG Y, KHAN B M, ZHANG X, et al. Advances in separation and purification of bioactive polysaccharides through high-speed counter-current chromatography[J]. Journal of Chromatographic Science,2020,58(10):992−1000. doi: 10.1093/chromsci/bmaa063
[65] LIANG H, LI C, YUAN Q, et al. Application of high-speed countercurrent chromatography for the isolation of sulforaphane from broccoli seed meal[J]. Journal of Agricultural and Food Chemistry,2008,56(17):7746−7749. doi: 10.1021/jf801318v
[66] 李椿方. 大孔吸附树脂和逆流色谱分离纯化莱菔硫烷的研究[D]. 北京: 北京化工大学, 2008. LI C F. Separation and purification of sulforaphane by macroporous resins and counter-current chromatography[D]. Beijing: Beijing University of Chemical Technology, 2008.
[67] 郭楠. 西兰花副产物中萝卜硫素提取及其对癌症转移的影响[D]. 太原: 山西大学, 2019. GUO N. Extraction of sulforaphane from broccoli by-products and its effect on cancer metastasis[D]. Taiyuan: Shanxi University, 2019.
[68] HAYES J D, KELLEHER M O, EGGLESTON I M. The cancer chemopreventive actions of phytochemicals derived from glucosinolates[J]. European Journal of Nutrition,2008,47(Suppl 2):73−88.
[69] LI L Y, LUO Y, LU M D, et al. Cruciferous vegetable consumption and the risk of pancreatic cancer: A meta-analysis[J]. World Journal of Surgical Oncology,2015,13:44. doi: 10.1186/s12957-015-0454-4
[70] LARSSON S C, HAKANSSON N, NASLUND I, et al. Fruit and vegetable consumption in relation to pancreatic cancer risk: A prospective study[J]. Cancer Epidemiol Biomarkers & Prevention,2006,15(2):301−305.
[71] HEINEN M M, VERHAGE B A, GOLDBOHM R A, et al. Intake of vegetables, fruits, carotenoids and vitamins C and E and pancreatic cancer risk in The Netherlands cohort study[J]. International Journal of Cancer,2012,130(1):147−158. doi: 10.1002/ijc.25989
[72] CHAN J M, WANG F, HOLLY E A. Vegetable and fruit intake and pancreatic cancer in a population-based case-control study in the San Francisco bay area[J]. Cancer Epidemiol Biomarkers & Prevention,2005,14(9):2093−2097.
[73] NOTHLINGS U, WILKENS L R, MURPHY S P, et al. Vegetable intake and pancreatic cancer risk: The multiethnic cohort study[J]. American Journal of Epidemiology,2007,165(2):138−147.
[74] LIU X, LÜ K. Cruciferous vegetables intake is inversely associated with risk of breast cancer: A meta-analysis[J]. The Breast,2013,22(3):309−313. doi: 10.1016/j.breast.2012.07.013
[75] LIU B, MAO Q, WANG X, et al. Cruciferous vegetables consumption and risk of renal cell carcinoma: A meta-analysis[J]. Nutrition and Cancer,2013,65(5):668−676. doi: 10.1080/01635581.2013.795980
[76] ZHAO J, ZHAO L. Cruciferous vegetables intake is associated with lower risk of renal cell carcinoma: Evidence from a meta-analysis of observational studies[J]. PLoS One,2013,8(10):e75732. doi: 10.1371/journal.pone.0075732
[77] LIU B, MAO Q, LIN Y, et al. The association of cruciferous vegetables intake and risk of bladder cancer: A meta-analysis[J]. World Journal of Urology,2013,31(1):127−133. doi: 10.1007/s00345-012-0850-0
[78] KIRSH V A, PETERS U, MAYNE S T, et al. Prospective study of fruit and vegetable intake and risk of prostate cancer[J]. Journal of the National Cancer Institut,2007,99(15):1200−1209. doi: 10.1093/jnci/djm065
[79] HAN B, LI X, YU T. Cruciferous vegetables consumption and the risk of ovarian cancer: A meta-analysis of observational studies[J]. Diagnostic Pathology,2014,9(1):7. doi: 10.1186/1746-1596-9-7
[80] GU H F, MAO X Y, DU M. Metabolism, absorption, and anti-cancer effects of sulforaphane: An update[J]. Critical Reviews in Food Science and Nutrition,2021:1−17.
[81] MOHAMMAD M K, SHARMIN A, CHIN-NU L, et al. Sulforaphane as an anticancer molecule: Mechanisms of action, synergistic effects, enhancement of drug safety, and delivery systems[J]. Archives of Pharmacal Research,2020,43(9):371−384.
[82] HUANG L, LI B L, HE C X, et al. Sulforaphane inhibits human bladder cancer cell invasion by reversing epithelial-to-mesenchymal transition via directly targeting microRNA-200c/ZEB1 axis[J]. Journal of Functional Foods,2018,41:118−126. doi: 10.1016/j.jff.2017.12.034
[83] HUANG B, LEI S, WANG D, et al. Sulforaphane exerts anticancer effects on human liver cancer cells via induction of apoptosis and inhibition of migration and invasion by targeting MAPK7 signalling pathway[J]. Journal of B U ON:Official Journal of the Balkan Union of Oncology,2020,25(2):959−964.
[84] ZHU J, WANG S, CHEN Y, et al. MiR-19 targeting of GSK3beta mediates sulforaphane suppression of lung cancer stem cells[J]. The Journal of Nutritional Biochemistry,2017,44:80−91. doi: 10.1016/j.jnutbio.2017.02.020
[85] KIANI S, AKHAVAN-NIAKI H, FATTAHI S, et al. Purified sulforaphane from broccoli (Brassica oleracea var. italica) leads to alterations of CDX1 and CDX2 expression and changes in miR-9 and miR-326 levels in human gastric cancer cells[J]. Gene,2018,678:115−123. doi: 10.1016/j.gene.2018.08.026
[86] GWON Y, OH J, KIM J S. Sulforaphane induces colorectal cancer cell proliferation through Nrf2 activation in a p53-dependent manner[J]. Applied Biological Chemistry,2020,63(1):1−11. doi: 10.1186/s13765-019-0484-7
[87] BURNETT J P, LIIM G, LI Y, et al. Sulforaphane enhances the anticancer activity of taxanes against triple negative breast cancer by killing cancer stem cells[J]. Cancer Letters,2017,394:52−64. doi: 10.1016/j.canlet.2017.02.023
[88] GEROGIKOU C, YIN L, GLADKICH J, et al. Inhibition of miR30a-3p by sulforaphane enhances gap junction intercellular communication in pancreatic cancer[J]. Cancer Letters,2020,469:238−245. doi: 10.1016/j.canlet.2019.10.042
[89] SHARMA C, SADRIEH L, PRIYANI A, et al. Anti-carcinogenic effects of sulforaphane in association with its apoptosis-inducing and anti-inflammatory properties in human cervical cancer cells[J]. Cancer Epidemiology,2011,35(3):272−278. doi: 10.1016/j.canep.2010.09.008
[90] ALUMKAL J J, SLOTTKE R, SCHWARTZMAN J, et al. A phase II study of sulforaphane-rich broccoli sprout extracts in men with recurrent prostate cancer[J]. Invest New Drugs,2015,33(2):480−489. doi: 10.1007/s10637-014-0189-z
[91] LOZANOVSKI V J, POLYCHRONIDIS G, GROSS W, et al. Broccoli sprout supplementation in patients with advanced pancreatic cancer is difficult despite positive effects-results from the POUDER pilot study[J]. Invest New Drugs,2020,38(1):776−784.





 下载:
下载:

 下载:
下载:



