Protective Effect of Extract from America Ginseng and Hovenia dulcis Thunb against Acute Alcohol-induced Liver Injury in Mice
-
摘要: 目的:研究西洋参枳椇子组合物对酒精所致小鼠急性肝损伤的预防保护作用。方法:ICR小鼠适应饲养1周后,随机分为正常组、模型组、西洋参枳椇子组合物(Ⅰ,Ⅱ,Ⅲ)组。对小鼠连续灌胃西洋参枳椇子组合物(100 mg/kg∙bw)14 d后,除正常组外,其余各组均在24 h内灌胃3次酒精(5 g/kg)诱导肝损伤。末次给予酒精6 h后,处死小鼠,检测各组小鼠血清中AST、ALT活力及TG含量,检测肝脏中GSH-Px、SOD活力及GSH和MDA含量,通过H & E染色观察肝脏病理组织变化,利用蛋白印记方法检测肝组织中p-ERK、p-JNK和p-P38蛋白的表达。结果:与模型组小鼠比较,西洋参枳椇子组合物能够明显降低由急性酒精摄入引起的血清中AST、ALT活力升高(P<0.05),降低血清中TG水平(P<0.05)和肝组织中MDA含量(P<0.01),提高肝组织中抗氧化酶SOD和GSH-Px活力(P<0.01),且升高肝组织中GSH水平(P<0.01)。病理切片观察表明,模型组以出现脂肪滴为变性为主,部分出现炎性细胞浸润,各给药组对小鼠肝脏病理变化有不同程度改善作用,西洋参枳椇子组合物极显著抑制p-ERK、p-JNK和p-P38表达(P<0.01),抑制肝细胞凋亡。结论:西洋参枳椇子组合物对急性酒精诱导的小鼠肝损伤具有较好的预防保护作用。Abstract: Objective: To study the preventive effects of American ginseng and Hovenia dulcis Thunb (AGH) on alcohol-induced acute liver injury in mice. Methods: After one week of adaptation, ICR mice were randomly divided into normal group, model group and AGH (Ⅰ,Ⅱ,Ⅲ) groups. After giving the mice a continuous intragastric administration of AGH (100 mg/kg∙bw) for 14 days, all groups except the normal group were given three times of alcohol (5 g/kg) within 24 hours to induce liver damage. After the third alcohol administration for 6 hours, the mice were sacrificed. The activities of Aspartate aminotransferase (AST), Alanine aminotransferase (ALT) and the content of Triglycerides (TG) in serum were tested. The activity of Glutathione peroxidase (GSH-Px), Superoxide dismutase (SOD) and the contents of Glutathione (GSH) and Malondialdehyde (MDA) were detected. Pathological changes of liver were observed by H & E staining. The expression of p-ERK, p-JNK and p-P38 protein in liver tissue was detected by WB method. Results: Compared with model group, AGH could significantly reduce the activities of AST and ALT in serum (P<0.05), decreased the level of TG in serum (P<0.05) and MDA content in liver tissue (P<0.01). Meanwhile, AGH could also increase the activities of SOD and GSH-Px in liver tissue (P<0.01) and improve the level of GSH in liver tissue (P<0.01). Pathological observation showed that the main degeneration was fat drops, and some inflammatory cells were infiltrated in model group. AGH group had different degree of improvement on the pathological changes of the liver in mice. AGH significantly inhibited the expression of p-ERK, p-JNK and p-P38 (P<0.01) and inhibited the apoptosis of hepatocytes. Conclusion: AGH has obvious protective effect on acute alcohol-induced liver injury in mice.
-
Keywords:
- Amercia ginseng /
- Hovenia dulcis Thunb /
- alcohol /
- liver injury /
- protective effect /
- compatibility
-
西洋参(American ginseng,AG)为五加科植物西洋参(Panax quinquefolium Linn.)的干燥根,具有补气养阴、清热生津的功效[1]。西洋参主要活性成分为人参皂苷和多糖成分[2-3],已被证明具有抗抑郁[4]、抗肿瘤[5]、提高机体免疫力[6]、降血糖[7]和抗肥胖[8]等多种生物活性。有研究证明西洋参可消除小鼠脂肪肝、减少肝脏和肠道脂蛋白分泌,降低脂质循环水平,逆转代谢综合征[9-10]。枳椇子(Hovenia dulcis Thunb)为鼠李科拐枣属植物枳椇的种子,具有解酒毒、止呕、清热利尿等功效。近年来,中药配伍类解酒制品纷纷涌向市场,主要成分以葛根、葛花和枳椇子为主,如食源复方解酒口服液、葛根解酒方、葛根-枳椇子解酒组合物等复方解酒口服液[11]。其中枳椇子常被用于解酒护肝产品开发中[12-14]。在2018年西洋参被卫健委批准为既是药品又是食品,引起了大家对西洋参的广泛关注。西洋参的特别之处在于补而无燥,常作为滋阴补肾中药出现在复方当中。基于西洋参与枳椇子的药效作用,本研究将西洋参与枳椇子进行配伍组合治疗酒精性肝损伤。
几十年来,医治酒精性肝损伤(ALD)仍未找到特效药物,因此,开展中药配伍理论的研究思路可能是治疗疾病的新方向。本研究通过预防灌胃小鼠组合物后建立急性酒精性肝损伤模型,检测小鼠血清中生化指标变化和抗氧化应激能力,观察肝脏病理组织病变程度和MAPK信号通路的变化。首次将西洋参与枳椇子配伍后探讨其对酒精性肝损伤的作用,为西洋参的开发应用提供依据。
1. 材料与方法
1.1 材料与仪器
清洁级ICR健康雄性小鼠50只,体重为20~22 g 辽宁长生生物技术股份有限公司,动物生产许可证SCXK(辽)2015-0001,合格编号211002300023526;西洋参 吉林省抚松县;枳椇子 山东济南秦越人农业发展有限公司;天冬氨酸氨基转移酶(Aspartateaminotransferase,AST)试剂盒、丙氨酸氨基转移酶(Alanine aminotransferase,ALT)试剂盒、甘油三酯(Triglyceride,TG)、丙二醛(Malondialdehyde,MDA)试剂盒、谷胱甘肽(Glutathione,GSH)试剂盒、超氧化物歧化酶(Superoxide dismutase,SOD)、谷胱甘肽过氧化酶(Glutathione peroxidase,GSH-Px)试剂盒、BCA法总蛋白测定试剂盒 南京建成生物工程研究所;苏木素染液和伊红染液 Sigma-Aldrich公司;中性树胶 索莱宝生物科技有限公司,RIPA(Radio-Immunoprecipitation assay)裂解缓冲液 Bio-world公司,BCA试剂盒 碧云天生物技术有限公司;化学发光试剂盒 美国密理博公司;phospho-ERK(p-ERK)、phospho-JNK(p-JNK)、phospho-P38(p-P38) 美国Santa cruz生物技术公司;GAPDH和二抗(鼠抗lgG) 美国Abcam公司。
Heraeus Megafuge 8R型超高速冷冻离心机 赛默飞世尔科技有限公司;IX51型显微镜 奥林巴斯公司;Epoch2型酶标仪 美国博腾仪器有限公司;MS204S型电子分析天平 瑞士Mettler Toledo公司;EG1150型生物组织包埋机、RM2265型切片机、HI1210型水浴锅、HI1220型烘片仪 徕卡显微系统(上海)贸易有限公司;Alpha2-4LD plus真空冷冻干燥机 德国Christ公司;ChemiDocTM MP型全能成像仪 美国Bio-Rad公司。
1.2 实验方法
1.2.1 西洋参枳椇子提取物的制备
将西洋参和枳椇子放置于电热恒温鼓风干燥箱中,在60 ℃条件下干燥12 h,分别按质量比1:1、2:1和1:2混合,将干燥的混合物粉碎,过60目筛网,向混合物中加入重量比为8倍量蒸馏水,浸泡1 h,回流提取1 h,然后以5000 r/min,离心10 min,获得上清液;再向残渣中加入按重量比为5倍量蒸馏水,回流提取0.5 h,离心,获得上清液[11],合并两次上清液,将三种水煎液放置于−80 ℃条件下冷冻4 h后,真空冷冻干燥,即为组合物Ⅰ、组合物Ⅱ和组合物Ⅲ,产率分别为16.7%、19.3%和16.75%,产率(%)=提取物干燥后重量(g)/生药重量(g)。
1.2.2 动物分组与给药方法
ICR小鼠在控温(22±3)℃,相对湿度为(55%±15%)环境中饲养,自由进食、饮水适应1周后,随机分为五组,即为正常组、模型组、组合物Ⅰ组、组合物Ⅱ组和组合物Ⅲ组。西洋参枳椇子组合物组以100 mg/kg∙bw剂量连续灌胃小鼠14 d,正常组及模型组给予生理盐水。各组在最后一次给予受试物30 min后,除正常组外,24 h内灌胃小鼠3次酒精(5 g/kg),酒精灌胃结束间隔6 h后,取血,麻醉处死小鼠,分离肝脏[15]。
1.2.3 血液和组织样本采集
末次给予酒精6 h后,取血后,麻醉处死,分离肝脏,选取左叶常温保存于福尔马林中,其余肝脏保存于−80 ℃。将血液室温静置30 min,在4 ℃,以1000 r/min离心30 min,分离血清,保存于−80 ℃。
1.2.4 生化指标检测
血清中AST、ALT和TG水平与肝组织中GSH-Px、SOD、GSH试剂盒和MDA试剂盒指标检测按照试剂盒提供的说明书进行规范操作。
1.2.5 肝脏病理学检测
将固定肝组织标本切取厚度不超过0.5 cm小块置于包埋盒中,用自来水冲洗24 h,经由低浓度到高浓度酒精脱水,用溶于石蜡的透明剂二甲苯透明后,浸蜡包埋。用蜡块固定在旋转切片机上,切成5 μm厚度薄片,温水展开贴在载玻片上。将切片进行苏木精和伊红(H&E)染色,中性树胶封片,显微镜下观察肝组织病理学变化。
1.2.6 蛋白印记检测肝组织中蛋白的表达
运用BCA法测定细胞蛋白浓度,将SDS-PAGE(10%~12%)分离凝胶分离等量的蛋白样品,转移到PVDF膜上,然后封闭1 h,经p-ERK、p-JNK、p-P38和GAPDH一抗孵育,4 ℃条件下放置过夜,然后使用酶标二抗室温孵育1 h。将A和B试剂等体积混合,PVDF膜与混合液充分接触,在全能型成像系统中曝光。
1.3 数据处理
实验结果以平均值±标准差(
ˉx ±S)表示,采用SAS 9.2软件GLM过程进行单因素方差分析,利用Duncan法进行显著性分析,P<0.05为差异显著,P<0.01为差异极显著。2. 结果与分析
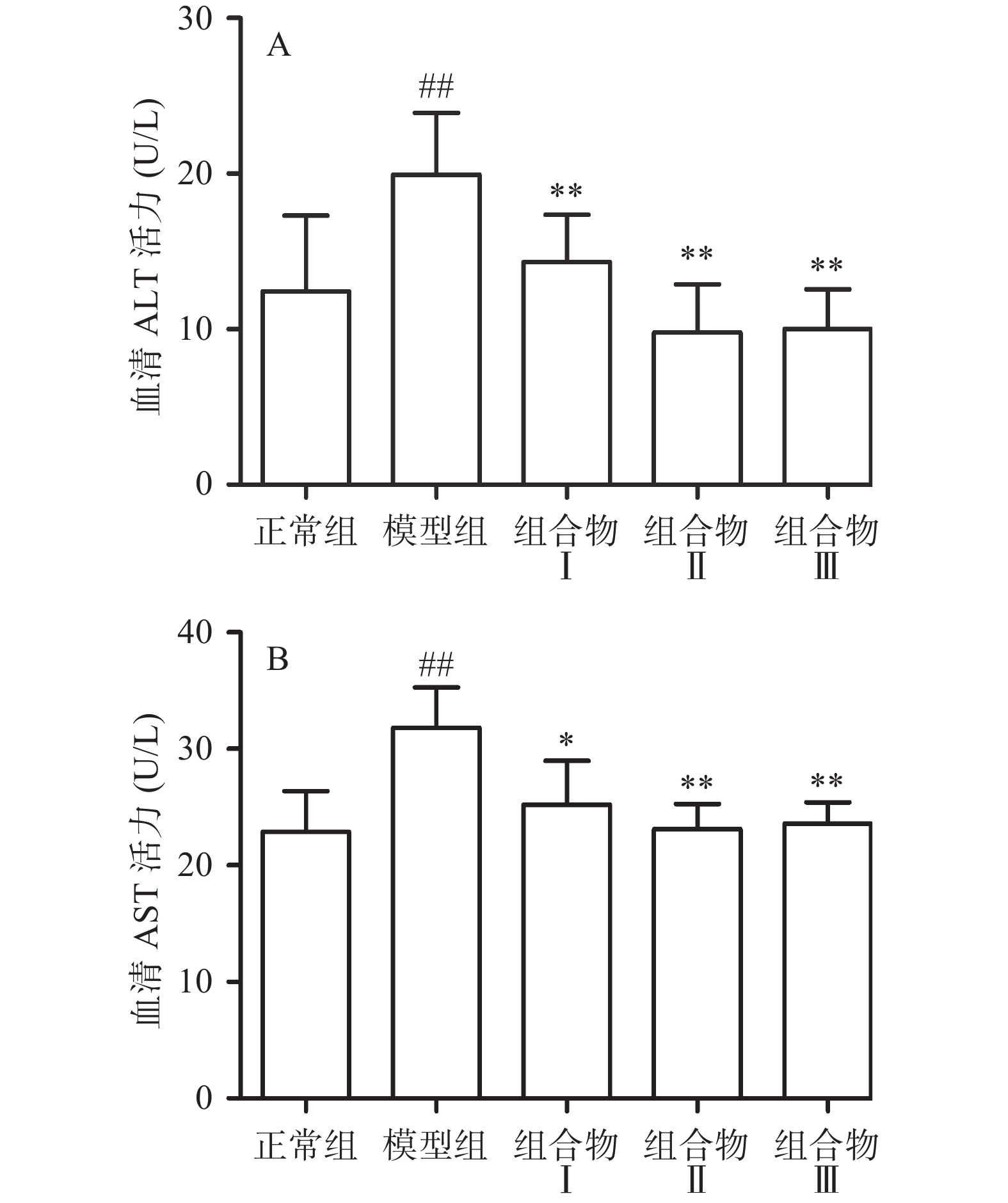
2.1 西洋参枳椇子组合物对小鼠血清转氨酶的影响
AST主要分布在肝细胞细胞质和线粒体中,ALT主要分布在细胞质中,肝细胞损伤后释放到血液,两种转氨酶活性增加,血液中AST和ALT酶水平可以作为肝损伤信号,反映肝脏受损情况[16]。如图1所示,与正常组相比,模型组中ALT和AST活力极显著升高(P<0.01),表明酒精引起小鼠肝细胞损伤,转氨酶活力升高;与模型组相比,西洋参枳椇子组合物Ⅰ极显著降低ALT活力(P<0.01)且显著降低AST水平(P<0.05),西洋参枳椇子组合物Ⅱ、Ⅲ极显著降低ALT和AST活力(P<0.01),西洋参给药组间无显著性差异。血清中转氨酶的活力下降,说明西洋参枳椇子组合物具有保护肝细胞的作用。
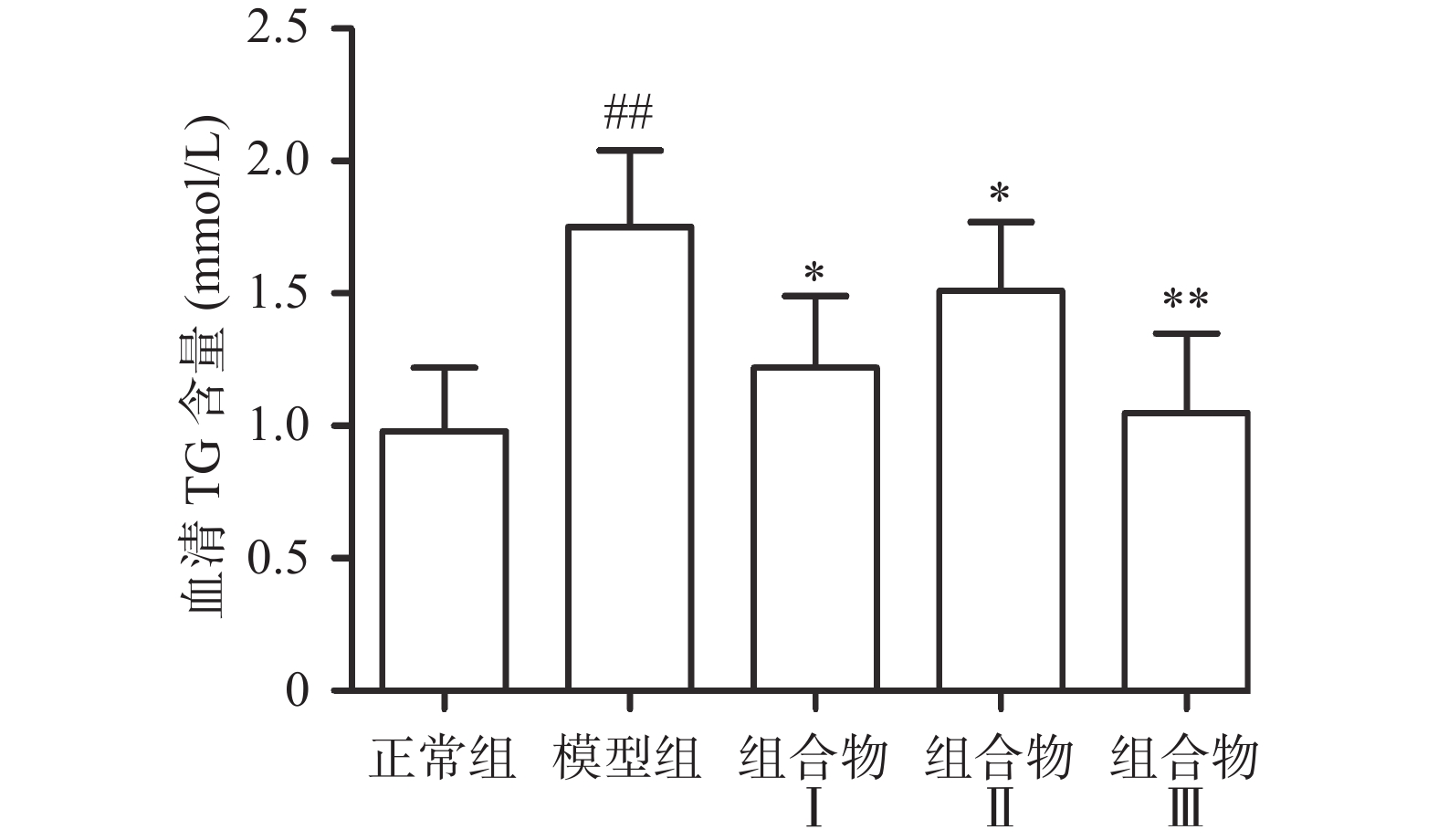
2.2 西洋参枳椇子组合物对血清中甘油三酯的影响
甘油三酯由肝脏合成,是脂肪酸在人体内储存和运输的主要形式,过量摄入酒精可以使肝脏中甘油三酯积累,引起肝脏脂质代谢异常[17]。与正常组相比,模型组小鼠血清中TG含量极显著升高(P<0.01);与模型组比较,组合物Ⅰ、Ⅱ组显著降低小鼠血清中TG含量(P<0.05),组合物Ⅲ极显著降低TG含量(P<0.01),各组间无显著性差异。结果表明组合物能够降低小鼠血清脂类物质水平(图2)。
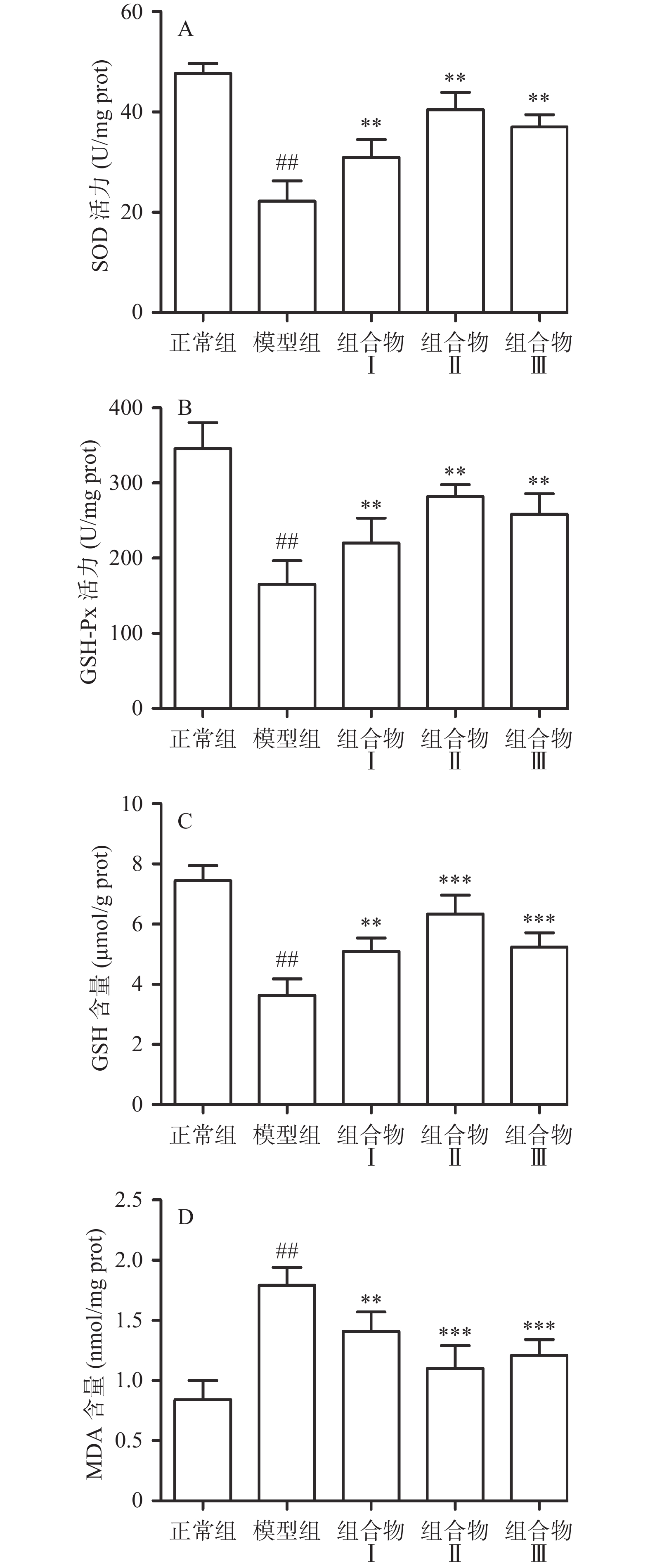
2.3 西洋参枳椇子组合物对小鼠肝组织抗氧化能力的影响
酒精在肝脏被酒精脱氢酶转化为有害物质乙醛,乙醛随后被醛脱氢酶代谢为乙酸,在代谢过程中可以促进自由基如活性氧(ROS)产生,进而引起脂质过氧化[18]。此外,过量ROS导致脂质过氧化产物MDA产生,使抗氧化剂水平降低,导致氧化还原平衡失调[19]。肝细胞内含有内源性抗氧化酶,SOD和GSH-Px可以维持氧化应激稳态,但酒精摄入增加活性氧水平,影响抗氧化酶水平,从而导致肝脏损伤[20]。实验结果显示,与正常组相比,模型组小鼠肝组织内抗氧化酶SOD、GSH-Px活力极显著降低(P<0.01),还原型GSH含量极显著降低(P<0.01)和MDA含量极显著升高(P<0.01);与模型组相比,组合物Ⅰ、Ⅱ、Ⅲ极显著升高了SOD、GSH-Px活力(P<0.01)和GSH水平(P<0.01),极显著降低MDA含量(P<0.01)。组合物Ⅱ中GSH-Px、SOD活力和GSH含量极显著高于组合物Ⅰ,具有显著性差异(P<0.01),同时GSH-Px和SOD活力也高于组合物Ⅲ,但无统计学差异;组合物Ⅱ中MDA含量极显著低于组合物Ⅰ(P<0.05),低于组合物Ⅲ,但无统计学差异。由此可说明组合物Ⅱ抗氧化能力高于组合物Ⅰ和组合物Ⅲ,结果提示该组合物明显改善小鼠肝组织中酒精引起的氧化应激情况(图3)。
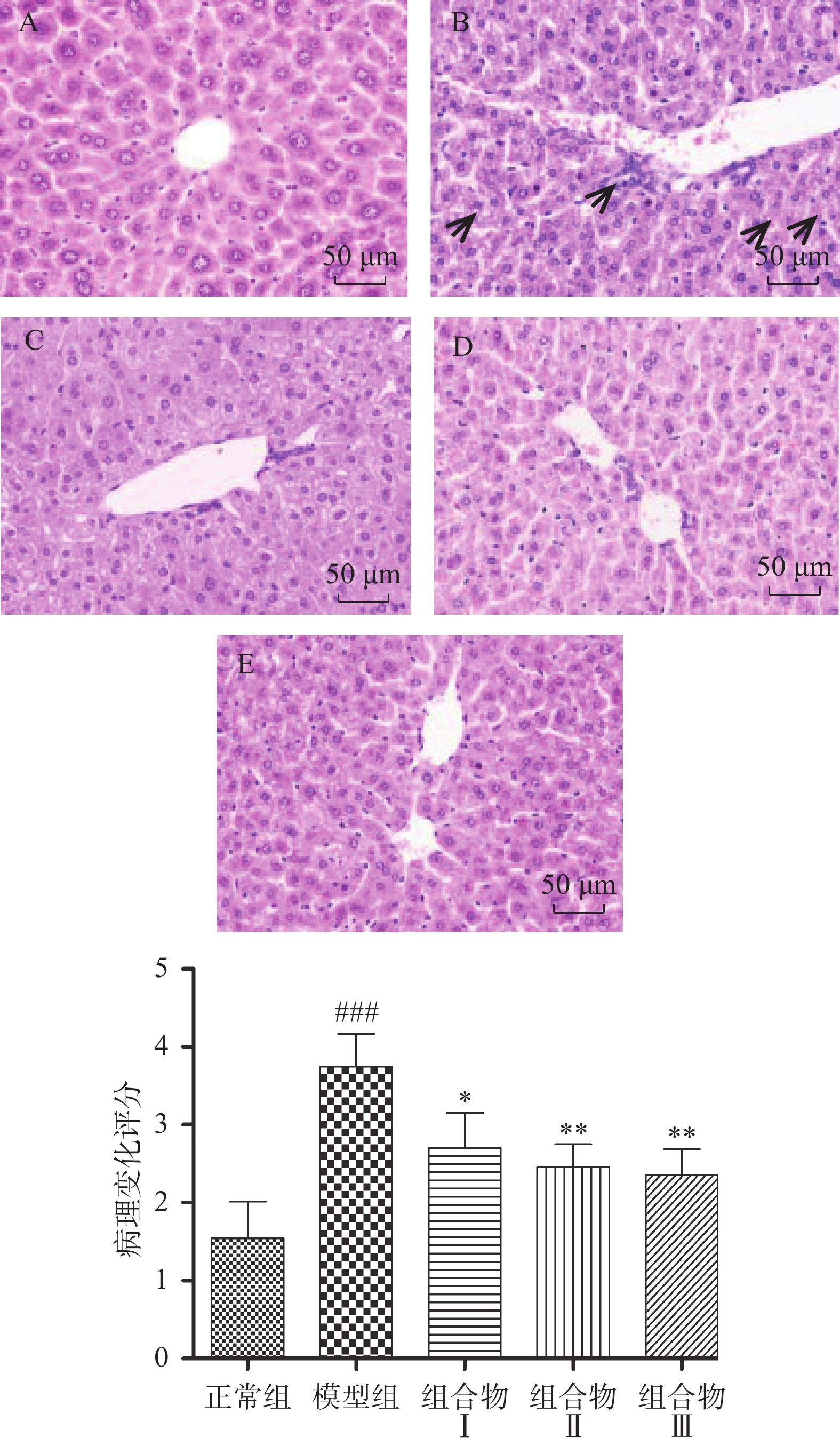
2.4 西洋参枳椇子组合物对小鼠肝脏病理的影响
肝脏病理组织H&E染色结果显示,正常组小鼠肝细胞排列整齐,形态正常,未出现明显变性、坏死;模型组小鼠肝组织中细胞间出现大量白色脂滴和炎性细胞浸润情况,说明酒精引起肝细胞中脂质代谢异常;西洋参枳椇子组中小鼠肝细胞排列逐渐紧密、脂滴数量逐渐减少并且炎性浸润程度减轻,说明组合物具有改善脂质代谢和抗炎的作用(图4)。
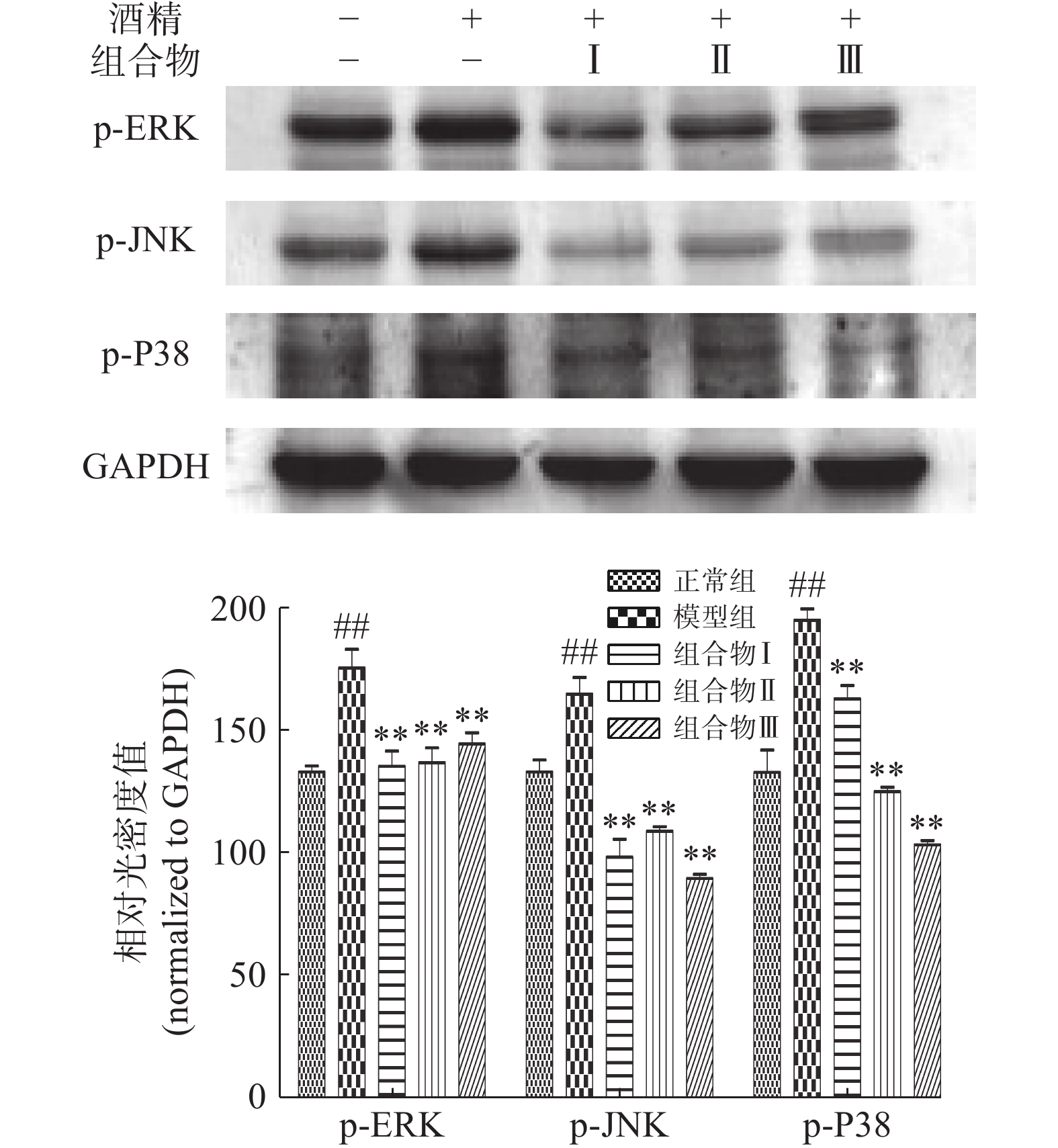
2.5 西洋参枳椇子组合物对小鼠肝脏MAPK信号通路的影响
与正常组相比,模型组中p-ERK,p-JNK和p-P38的水平明显增加(P<0.01),组合物,组合物Ⅰ、Ⅱ、Ⅲ极显著降低p-ERK、p-JNK和p-P38的表达(P<0.01)。结果表明,西洋参枳椇子组合物可能抑制ERK、JNK和P38的磷酸化从而减轻肝细胞凋亡(图5)。
3. 结论
肝脏是药物代谢和解毒功能的重要器官,日常生活中过量饮酒或服用药物都会引发肝脏的损伤[21-22]。肝细胞中储存了大量血清转氨酶AST和ALT,当细胞膜受损时,膜通透性增加,胞内大量AST、ALT扩散到血液中,以AST、ALT水平高低判断肝细胞是否损伤[16]。而且在ALD早期发病机理研究表明,酒精进入机体后,乙醇可影响脂肪代谢,导致TG在肝细胞内沉积,大量蓄积导致病变[23-24]。酒精经酶代谢时,能够诱导细胞内产生ROS,消耗大量还原性物质如GSH,从而无法清除过多自由基,引起氧化应激,导致细胞脂质过氧化产物迅速增加,肝细胞膜受损[25]。因此GSH多少是衡量抗氧化能力重要因素[26],MDA含量可以反映过氧化损伤和细胞受损程度[18]。
ALD早期损伤多可逆转,如及时进行针对性治疗可防止发展到肝硬化、肝癌等疾病,有效预防与治疗ALD已成为医药与功能性食品领域研究热点,并且研究证明食药同源天然产物具有较好预防保护酒精性肝损伤的作用[27]。因此,本实验采用食药同源西洋参和枳椇子组合物,通过西洋参与枳椇子配伍后提取出有效成分,并发现其对酒精性肝损伤具有保护作用,其作用机制可能与改善脂质代谢、提高抗氧化能力和抑制MAPK信号通路活化有关,这一结果为西洋参与枳椇子配伍应用提供了理论基础。
-
-
[1] 国家药典委员会. 中华人民共和国药典[M]. 一部. 2020年版, 北京: 中国医药科技出版社, 2020: 136. National Pharmacopoeia Commission. Pharmacopoeia of the People's Republic of China[M]. Part I. 2020 Edition, Beijing: China Medical Science and Technology Press, 2020: 136.
[2] 李珊珊, 孙印石. 西洋参多糖结构与药理活性研究进展[J]. 特产研究,2017,39(3):68−71. [LI S S, SUN Y S. Research achievements on structures and activities of polysaccharide from Panax quinquefolius[J]. Special Wild Economic Animal and Plant Research,2017,39(3):68−71. [3] 王蕾, 王英平, 许世泉, 等. 西洋参化学成分及药理活性研究进展[J]. 特产研究,2007(3):73−77. [WANG L, WANG Y P, XU S Q, et al. A review on studies of the components and pharmacological activity of Panax quinquefolium L doi: 10.3969/j.issn.1001-4721.2007.03.023 J]. Special Wild Economic Animal and Plant Research,2007(3):73−77. doi: 10.3969/j.issn.1001-4721.2007.03.023
[4] LI Z M, ZHAO L J, CHEN J B, et al. Ginsenoside Rk1 alleviates LPS-induced depression-like behavior in mice by promoting BDNF and suppressing the neuroinflammatory response[J]. Biochemical and Biophysical Research Communications,2020,530(4):658−664. doi: 10.1016/j.bbrc.2020.07.098
[5] WANG C Z, HUANG W H, ZHANG C F, et al. Role of intestinal microbiome in American ginseng-mediated colon cancer prevention in high fat diet-fed AOM/DSS mice[J]. Clinical & Translational Oncology,2018,20(3):302−312.
[6] GHOSH R, BRYANT D L, FARONE A L. Panax quinquefolius (North American Ginseng) polysaccharides as immunomodulators: Current research status and future directions[J]. Molecules,2020,25(24):5854−5877. doi: 10.3390/molecules25245854
[7] VUKSAN V, XU Z Z, JOVANOVSKI E, et al. Efficacy and safety of American ginseng (Panax quinquefolius L.) extract on glycemic control and cardiovascular risk factors in individuals with type 2 diabetes: A double-blind, randomized, cross-over clinical trial[J]. European Journal of Nutrition,2019,58(3):1237−1245. doi: 10.1007/s00394-018-1642-0
[8] RUI L, JING Z Z, WEN C L, et al. Anti-obesity effects of protopanaxdiol types of Ginsenosides isolated from the leaves of American ginseng (Panax quinquefolius L.) in mice fed with a high-fat diet[J]. Fitoterapia,2010,81(8):1079−1087. doi: 10.1016/j.fitote.2010.07.002
[9] SINGH R K, LUI E, WRIGHT D, et al. Alcohol extract of North American ginseng (Panax quinquefolius) reduces fatty liver, dyslipidemia, and other complications of metabolic syndrome in a mouse model[J]. Canadian Journal of Physiology Pharmacology,2017,95(9):1046−1057. doi: 10.1139/cjpp-2016-0510
[10] YU C, WEN X D, ZHANG Z, et al. American ginseng significantly reduced the progression of high-fat-diet-enhanced colon carcinogenesis in Apc(Min/+) mice[J]. Journal of Ginseng Research,2015,39(3):230−237. doi: 10.1016/j.jgr.2014.12.004
[11] 张鸿志, 李璐, 刘永明, 等. 解酒制品研究进展[J]. 酿酒科技,2021(9):65−73. [ZHANG H Z, LI L, LIU Y M, et al. Research progress in anti-Hangover products[J]. Liquor-Making Science & Technology,2021(9):65−73. [12] 李志满, 邵紫君, 李珊珊, 等. 人参枳椇子提取物对小鼠酒精性肝损伤的保护作用[J]. 食品工业科技,2019,40(14):302−306, 313. [LI Z M, SHAO Z J, LI S S, et al. Protective effect of extract from Panax ginsen and Hovenia dulcis Thunb against alcohol-induced liver injury in mice[J]. Science and Technology of Food Industry,2019,40(14):302−306, 313. [13] 柳海艳, 钟赣生, 李怡文, 等. 醇提和水提葛花枳椇子及其配伍对酒精性肝损伤大鼠肝脏抗氧化功能的影响[J]. 中华中医药杂志,2012,27(4):1181−1184. [LIU H Y, ZHONG G S, LI Y W, et al. Effect of different proportion of Flos Puerariae and Semen Hoveniae extracted by alcohol or water on the livers antioxidant function of alcoholic liver injury rats[J]. China Journal of Traditional Chinese Medicine and Pharmacy,2012,27(4):1181−1184. [14] 唐晖慧, 金美东. 枳椇子、桑椹、青果复方组合物对小鼠肝脏的保护作用[J]. 食品工业科技,2014,35(12):354−358. [TANG H H, JIN M D. Hepatoprotective effect of mixture of Hovenia duleis, Morus alba, and Canarium album on liver of mice[J]. Science and Technology of Food Industry,2014,35(12):354−358. [15] 王瑞婕, 杨勇, 白婷. 大黄素甲醚对酒精性肝损伤中SIRT1-AMPK通路的影响[J]. 中国药理学通报,2020,36(11):1557−1562. [WANG R J, YANG Y, BAI T. Effect of physcion on SIRT1-AMPK pathway in alcoholic liver injury[J]. Chinese Pharmacological Bulletin,2020,36(11):1557−1562. doi: 10.3969/j.issn.1001-1978.2020.11.015 [16] YANG M H, CHEN M, MO H H, et al. Utilizing experimental mouse model to identify effectors of hepatocellular carcinoma induced by HBx antigen[J]. Cancers (Basel),2020,12(2):409−441. doi: 10.3390/cancers12020409
[17] ALVES-BEZERRA M, COHEN D E. Triglyceride metabolism in the liver[J]. Comprehensive Physiology,2017,8(1):1−8.
[18] LIANG H W, YANG T Y, TENG C S, et al. Mulberry leaves extract ameliorates alcohol-induced liver damages through reduction of acetaldehyde toxicity and inhibition of apoptosis caused by oxidative stress signals[J]. International Journal of Medical Sciences,2021,18(1):53−64. doi: 10.7150/ijms.50174
[19] XING H, JIA K, HE J, et al. Establishment of the tree shrew as an alcohol-induced fatty liver model for the study of alcoholic liver diseases[J]. PLoS One,2015,10(6):e0128253. doi: 10.1371/journal.pone.0128253
[20] KOURKOUMPETIS T, SOOD G. Pathogenesis of alcoholic liver disease: An update[J]. Clinics in Liver Disease,2019,23(1):71−80. doi: 10.1016/j.cld.2018.09.006
[21] WANG Z, HAO W, HU J, et al. Maltol improves APAP-induced hepatotoxicity by inhibiting oxidative stress and inflammation eesponse via NF-κB and PI3K/Akt signal pathways[J]. Antioxidants (Basel),2019,8(9):395−410. doi: 10.3390/antiox8090395
[22] TESCHKE R. Alcoholic liver disease: Alcohol metabolism, cascade of molecular mechanisms, cellular targets, and clinical aspects[J]. Biomedicines,2018,6(4):106−163. doi: 10.3390/biomedicines6040106
[23] LI M, WU C, GUO H, et al. Mangiferin improves hepatic damage-associated molecular patterns, lipid metabolic disorder and mitochondrial dysfunction in alcohol hepatitis rats[J]. Food & Function,2019,10(6):3514−3534.
[24] ZHANG Y, JIANG M, CUI B W, et al. P2X7R receptor-targeted regulation by tetrahydroxystilbene glucoside in alcoholic hepatosteatosis: A new strategy towards macrophage-hepatocyte crosstalk[J]. British Journal of Pharmacology,2020,177(12):2793−811. doi: 10.1111/bph.15007
[25] ZHANG W, YANG J, LIU J, et al. Red yeast rice prevents chronic alcohol-induced liver disease by attenuating oxidative stress and inflammatory response in mice[J]. Journal of Food Biochemistry,2021,00:e13672.
[26] VAIRETTI M, DI PASQUA L G, CAGNA M, et al. Changes in glutathione content in liver diseases: An update[J]. Antioxidants (Basel),2021,10(3):364−403. doi: 10.3390/antiox10030364
[27] 曲航, 高鑫, 伊娟娟, 等. 食源性天然产物对酒精性肝损伤的防护作用研究进展[J]. 食品科学,2020,41(17):283−290. [QU H, GAO X, YI J J, et al. Review on the protective effects of food-derived natural compounds on alcohol-induced liver injury[J]. Food Science,2020,41(17):283−290. doi: 10.7506/spkx1002-6630-20190920-262






 下载:
下载:
 下载:
下载:
