Effects of Lacidophilin on Inflammatory and Intestinal Microflora in Mice with Helicobacter pylori Induced Gastritis
-
摘要: 目的:探讨乳酸菌素对幽门螺杆菌(Helicobacter pylori,H. pylori)感染胃炎小鼠炎症和肠道菌群的影响。方法:通过口服H. pylori混悬液制备H. pylori感染小鼠模型。将H. pylori感染成功的小鼠随机分为模型组和乳酸菌素组,进行干预4周。革兰氏染色、Warthin-Starry银染色和H. pylori免疫组化染色验证小鼠胃黏膜H. pylori的定植,HE染色观察小鼠胃黏膜组织病理学改变,免疫组化法测定小鼠胃黏膜iNOS、IL-1β和3-Nitrotyrosine的表达,16S rRNA测序分析肠道菌群结构。结果:造模组小鼠H. pylori定植成功;乳酸菌素能改善H. pylori感染性胃炎小鼠胃组织病理形态;抑制胃黏膜中iNOS、IL-1β和3-Nitrotyrosine的表达。相较于H. pylori感染组,乳酸菌素组小鼠的肠道菌群组成结构具有明显差异。乳酸菌素可通过调节厚壁菌门、拟杆菌门、放线菌门和疣微菌门的组成,增加肠道菌群的多样性。在属水平上,乳酸菌素可促进g_norank_f_Muribaculaceae、阿克曼氏菌属(Akkermansia)和另枝菌属(Alistipes)等产生短链脂肪酸细菌的生长,抑制杜氏杆菌属(Dubosiella)等炎症相关细菌的增殖,改善肠道菌群组成结构。结论:乳酸菌素能够恢复由H. pylori感染破坏的肠道菌群结构,促进有益菌的增殖,降低炎症反应并缓解胃黏膜氧化损伤,对H. pylori感染性胃炎小鼠发挥保护作用。Abstract: Objective: The aim of this study was to investigate the effect of lacidophilin on inflammation and intestinal microflora in H. pylori-induced gastritis mice. Methods: Established an H. pylori-infected mice model by orally administering an H. pylori suspension. The mice were randomly divided into a model group and a lacidophilin treatment group, which received treatment for four weeks. Gram staining, Warthin-Starry silver staining, and H. pylori immunohistochemical staining were conducted to confirm the colonization of H. pylori in the gastric mucosa of mice. Hematoxylin and eosin (HE) staining was performed to observe any histopathological changes in the gastric mucosa of mice. Immunohistochemistry was carried out to observe the expression of iNOS, IL-1β, and 3-Nitrotyrosine in the gastric mucosa of mice. Additionally, the structure of intestinal microflora was analyzed using 16S rRNA sequencing. Results: The successful establishment of H. pylori-infected mice. Lacidophilin improved the stomach histopathological morphology in mice with H. pylori-induced gastritis and inhibited iNOS, IL-1β, and 3-Nitrotyrosine expression in the gastric mucosa. The composition and structure of the gut microbiota among the lacidophilin group showed noticeable differences in comparison to the H. pylori-infected group. Lacidophilin augmented the diversity of intestinal microflora in H. pylori-infected mice by regulating the abundance of Firmicutes, Actinobacteriota, Bacteroidete, and Verrucomicrobiota. At the genus level, Lacidophilin resulted in significant growth stimulation of beneficial bacteria such as g_norank_f_Muribaculaceae, Akkermansia, and Alistipes, while suppressing the proliferation of inflammation-related bacteria such as Dubosiella, thereby improving the composition and structure of the gut microbiota. Conclusion: Lacidophilin has the potential to rebalance the composition and structure of gut microbiota that has been compromised by H. pylori infection. It may have a protective effect on mice with H. pylori-induced gastritis by promoting the growth of beneficial bacteria, reducing inflammatory reactions, and alleviating oxidative damage to the gastric mucosa.
-
Keywords:
- lacidophilin /
- Helicobacter pylori /
- gastritis /
- intestinal microflora
-
幽门螺杆菌(Helicobacter pylori,H. pylori)是一种存在于胃黏膜上皮的微需氧性革兰氏阴性菌。据统计,我国已感染H. pylori的人群超过50%[1]。H. pylori患者几乎均处于慢性胃炎状态,并会进一步发展为消化性溃疡、胃黏膜相关淋巴组织淋巴瘤,甚至胃癌[2−3]。目前,H. pylori感染的一线临床治疗主要是四联疗法,包括质子泵抑制剂、2种抗生素(如克拉霉素和阿莫西林)和铋剂[4−5]。然而,由于抗生素耐药性的产生、患者依从性差以及肠道菌群紊乱引起的不良反应等原因,抗生素治疗失败的风险增加[6]。此外,尽管抗生素可以消灭H. pylori,但对炎症反应和胃粘膜受损的作用有限。
肠道菌群在H. pylori引发的疾病进展中起着重要作用。研究表明,H. pylori通过分泌毒力因子细胞毒素相关基因A(Cag A)以及激活宿主免疫反应,导致肠道菌群失衡,从而促进炎症和细胞增殖,进而引发癌症等人类疾病[7−8]。此外,H. pylori感染引起的肠道菌群失衡也可能与一系列其他全身性疾病有关,比如阿尔兹海默症[9]和动脉粥样硬化[6]。在炎症反应和免疫应答过程中,肠道中的有益菌发挥着重要作用[10]。例如,拟杆菌属、梭状芽孢杆菌属和粪杆菌属等微生物可增加T调节细胞或刺激抗炎细胞因子的产生[11]。婴儿型双歧杆菌通过产生短链脂肪酸(short-chain fatty acids,SCFAs),可以通过微生物群-肠道-脑轴减少炎性细胞因子并增强免疫调节反应[12]。
嗜酸乳杆菌具有调节免疫[13]、抑制炎症反应[14]、促进黏膜修复[15]等功能。乳酸菌素是一种由纯乳酸菌代谢产生的后生元制剂,具有抑制致病菌的生长,促进有益菌繁殖并改善胃肠道生态环境的作用[16]。前期研究表明,乳酸菌素片联合抗生素疗法可提高H. pylori感染溃疡患者的根除率,促进溃疡愈合,并降低不良反应发生率[17−19]。然而,尚未有关于乳酸菌素在H. pylori诱导慢性胃炎中炎症反应和肠道菌群的作用机制报道。因此,本研究旨在探讨乳酸菌素对H. pylori感染性胃炎小鼠炎症和肠道菌群的影响,为后生元治疗H. pylori感染性胃炎提供新的研究方向。
1. 材料与方法
1.1 材料与仪器
幽门螺杆菌PMSS1 由南昌大学第一附属医院消化科吕农华教授惠赠;6周龄SPF级雄性C57BL/6小鼠,体重(20±2)g,许可证号:SCXK(苏)2018-0008 购自江苏集萃药康生物科技股份有限公司;乳酸菌素 江中药业股份有限公司;脑心浸液 美国BD公司;布氏琼脂、革兰氏染色液 索莱宝生物科技公司;羊血 温州市康泰生物公司;胎牛血清(FBS) 美国Thermo Fisher Scientific公司;幽门螺旋杆菌添加剂 青岛海博生物技术有限公司;Helicobacter pylori抗体试剂(免疫组织化学) 北京中杉金桥生物有限公司;Warthin-Starry银染色液 南京森贝伽生物科技有限公司;iNOS抗体、IL-1β抗体、3-Nitrotyrosine抗体 美国Abcam公司。
MCO-170MUVHL-PC型三气培养箱 上海普和希健康医疗器械有限公司;DM3000正置荧光显微镜 徕卡显微系统(上海)贸易有限公司;HFsafe-1200LC型生物安全柜 上海力康科学仪器有限公司。
1.2 实验方法
1.2.1 H. pylori的培养
将−80 ℃冻存的H. pylori菌株取出后,37 ℃解冻,取少量混悬菌液接种于含有1%幽门螺旋杆菌添加剂和5%羊血的固体平板中,置于微需氧培养箱(5%O2,10%CO2,85%N2)中培养。72 h后,将平板中的菌落混悬于脑心浸液肉汤中,并于600 nm下测定OD值,依据1 OD=1×109 CFU/mL将菌液稀释至2×109 CFU/mL。
1.2.2 H. pylori感染胃炎模型构建、分组及给药
C57BL/6小鼠适应性饲养1周后,建立H. pylori感染小鼠模型[20]:禁食禁水12 h,灌胃给予小鼠H. pylori混悬液0.3 mL,2 h后恢复饮食,隔日灌胃1次,共7次。在末次灌胃之后,从感染组和未感染组各取3只小鼠,解剖取胃组织,采用革兰氏染色,H. pylori免疫组化染色,Warthin-Starry银染色观察评价H. pylori在小鼠胃内定植情况。所有动物实验程序均经江中药业股份有限公司动物护理与使用委员会批准,并根据国家实验动物伦理审查指南进行。
H. pylori感染C57BL/6小鼠在4周后观察到中性粒细胞浸润固有层和上皮细胞[21]。造模结束一个月后,将制备成功的H. pylori感染小鼠随机分为模型组(MC)和乳酸菌素组(LB),每组8只,分别给予生理盐水和1.2 g·kg−1的乳酸菌素,阴性对照组(n=8)小鼠灌胃给予生理盐水,每日一次,持续4周。末次给药24 h后,处死小鼠,剖腹取胃组织沿大弯侧剪开,用生理盐水洗净后分为多部份,用于后续检测。
1.2.3 银染色
Warthin-Starry银染色鉴定H. pylori定植的准确性高[22],其具体操作步骤如下:将固定好的胃组织依次进行脱水、石蜡包埋、切片和脱蜡处理。然后,使用梯度乙醇溶液进行水化,再将切片依次浸入酸性硝酸银溶液和Warthin-Starry染色液中,于56 ℃水浴染色至切片呈淡黄棕色。最后,进行常规脱水,二甲苯透明,中性树脂封固和拍片。
1.2.4 免疫组化检测胃组织iNOS、3-Nitrotyrosine和IL-1β的表达
将上述制好的切片脱蜡后,进行高温抗原修复,血清封闭,再分别加入一抗(iNOS按1:2000稀释;IL-1β按1:500稀释;3-Nitrotyrosine按1:100稀释;Helicobacter pylori抗体按1:100稀释),4 ℃孵育过夜,再滴加二抗37 ℃孵育30 min。DAB显色后,于苏木素染液中复染,脱水,透明,中性树脂封片,于光学显微镜下观察小鼠胃组织中iNOS、3-Nitrotyrosine和IL-1β的表达情况,以及H. pylori在胃黏膜上的定植情况。每组取4张切片于200倍光镜下随机选取3个不连续的阳性表达视野,采用Image-Pro Plus 6.0图像分析系统测定iNOS、3-Nitrotyrosine和IL-1β的平均光密度[23]。
1.2.5 胃组织HE染色
胃组织切片脱蜡后,进行苏木素染液染色4 min,然后经伊红染液染色5 min,脱水,透明,中性树脂封片,拍片,观察小鼠胃黏膜组织形态学变化。
1.2.6 肠道菌群16S rRNA基因高通量测序
取约0.2 g小鼠盲肠内容物样本,使用E.Z.N.A.® Soil DNA Kit进行DNA抽提,利用引物338F和806R对提取出来的肠道菌群16S rRNA基因的V3~V4可变区进行扩增[24]。扩增程序如下:95 ℃预变性3 min;然后进行30个循环,每个循环包括95 ℃变性30 s,55 ℃退火30 s,72 ℃延伸45 s;最后进行72 ℃延伸10 min[25]。采用AxyPrep DNA Gel Extraction Kit对PCR产物进行纯化,并使用微型荧光计Quantus™ Fluorometer进行定量检测。利用NEXTFLEX Rapid DNA-Seq Kit构建测序文库,并使用Illumina Miseq平台进行测序。对获得的原始数据使用Fastp(v0.19.6)软件进行质控,然后使用Flash(v1.2.11)软件进行拼接,再利用Uparse算法(v11)对优化序列进行提取,按照97%的相似度进行OTU聚类,同时在聚类过程中去除嵌合体,以获得OUT代表序列。采用RDP classifier(v2.13)贝叶斯算法对97%相似度的OTU代表序列进行分类学分析。使用Mothur(v1.30.2)和Qiime(v1.9.1)软件计算α和β多样性,并生成各分类学水平丰度表。使用偏最小二乘法判别分析和LEfSe分析来进行菌群比较和组间差异分析。
1.3 数据处理
采用SPSS 20.0统计软件对数据进行分析,各组实验结果以均值±标准差(±s)表示,多组间比较采用单因素方差分析,P<0.05表示具有显著性差异。
2. 结果与分析
2.1 H. pylori在小鼠胃黏膜组织定植情况
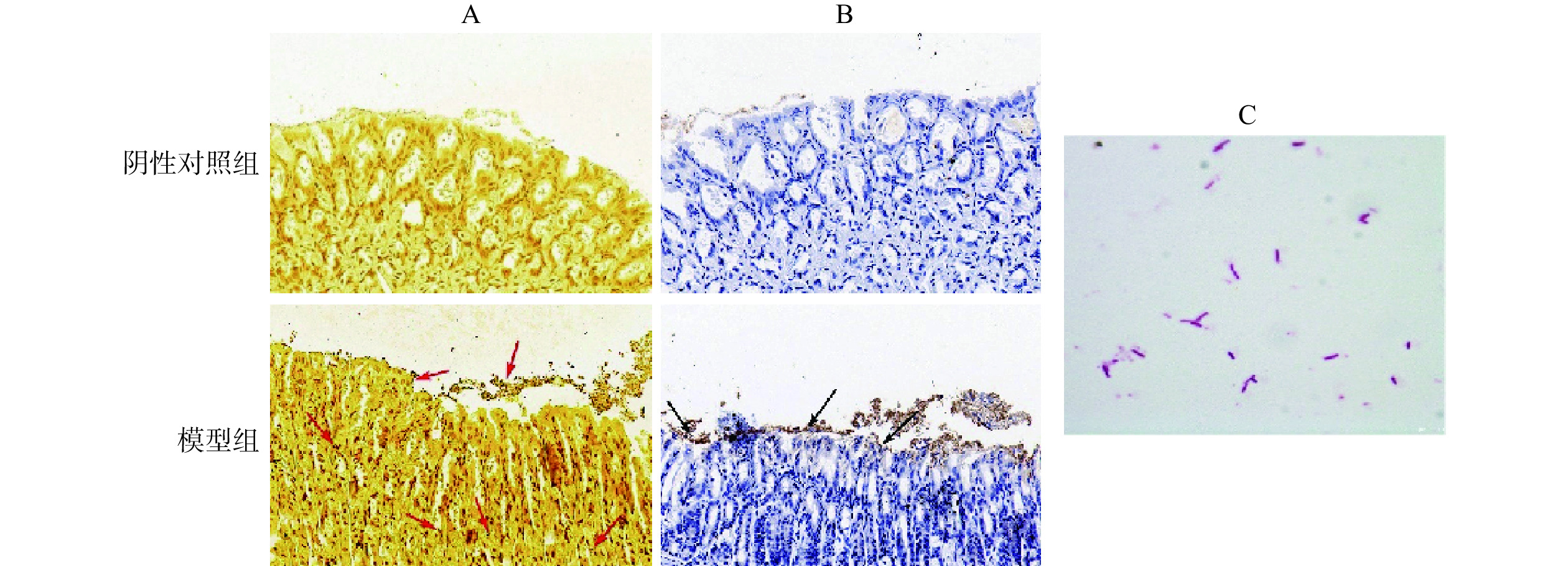
胃组织经Warthin-Starry银染色后(图1A),H. pylori呈现棕黑色,胞内黏液及胞质显示浅黄色。经免疫组化染色后(图1B),H. pylori呈黄褐色,细胞核呈蓝色。与阴性对照组相比,Warthin-Starry银染色结果显示感染组胃组织黏液层、上皮表面和细胞间均存在棕黑色棒状或微螺旋形菌体(箭头处),而免疫组化染色显示感染组胃黏膜上皮中存在棕黄色菌体(箭头处)。图1C为革兰氏染色镜检结果,感染组胃组织匀浆后于布氏琼脂平板上培养3 d,取菌落进行革兰氏染色,可见呈红色的杆状、微螺旋形或S形菌体,为革兰氏阴性菌。鉴定结果证实感染组已定植H. pylori。
2.2 各组胃黏膜组织病理学观察
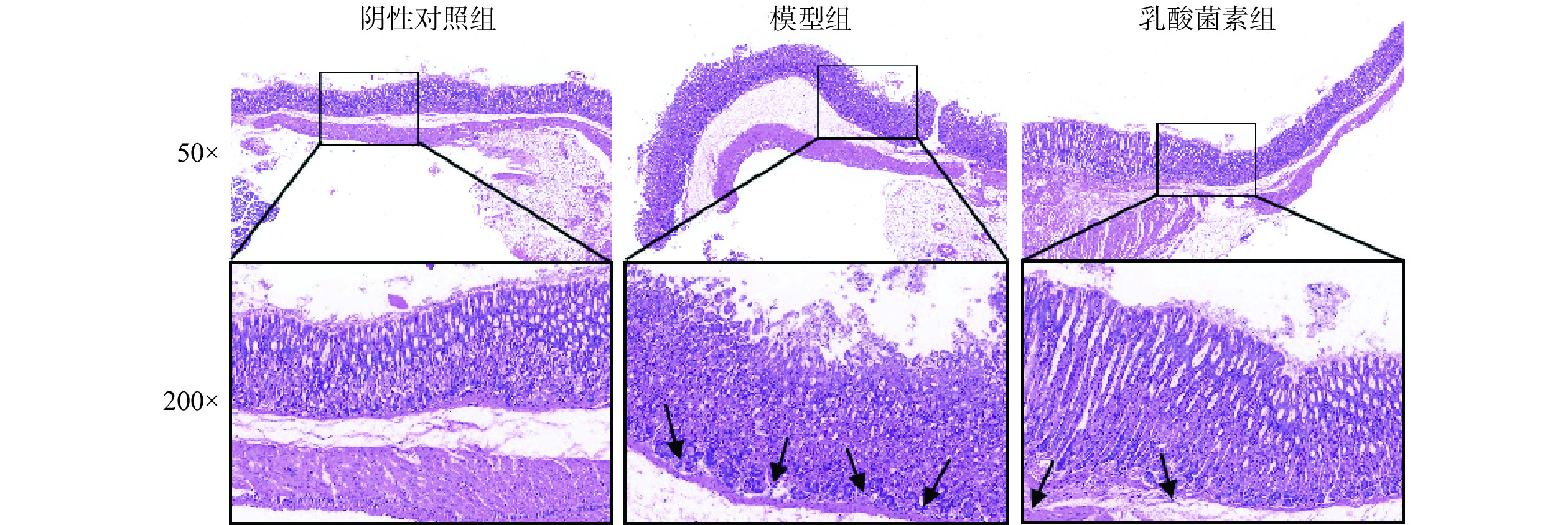
胃组织HE染色结果显示(图2),阴性对照组胃黏膜上皮结构完整,细胞排列规整,腺管排列紧密有序,壁细胞及主细胞界限清晰,无明显炎症反应。模型组胃黏膜上皮细胞坏死脱落,腺管排列紊乱松散,腺体腔减小且数目减少,黏膜层有炎症细胞(箭头处)浸润。与模型组相比,乳酸菌素组胃黏膜上皮结构趋于清晰完整,腺管排列更有序,腺体数量增加,炎症细胞浸润减少。
2.3 各组小鼠免疫组化结果
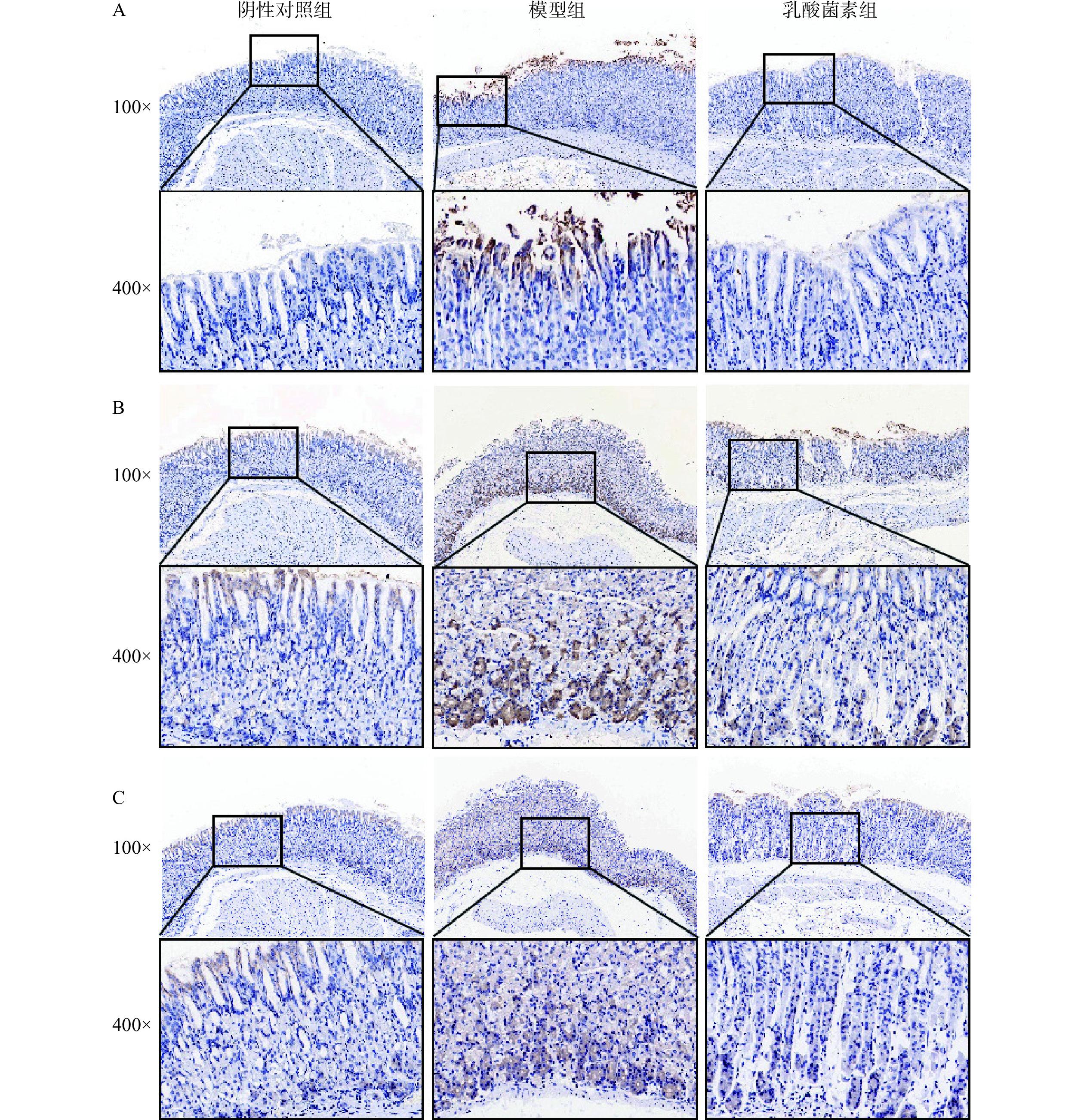
如图3所示,显微镜下观察胃组织中iNOS、3-Nitrotyrosine和IL-1β的表达情况,呈棕黄色为阳性表达,iNOS主要在胃上皮细胞胞质中表达,3-Nitrotyrosine和IL-1β主要在胃上皮细胞和腺体细胞胞质中表达。结果如表1所示,与阴性对照组相比,感染H. pylori小鼠胃组织中iNOS、3-Nitrotyrosine和IL-1β的平均光密度显著性升高(P<0.01,P<0.001),乳酸菌素给药后,iNOS、3-Nitrotyrosine和IL-1β的平均光密度显著降低(P<0.05,P<0.01)。
表 1 各组小鼠胃组织中iNOS、3-Nitrotyrosine和IL-1β的平均光密度统计结果(n=4,±s)Table 1. Mean optical density statistics of iNOS, 3-Nitrotyrosine, and IL-1β in the gastric tissue of mice in each group (n=4, ±s)组别 iNOS 3-Nitrotyrosine IL-1β 阴性对照组 0.133±0.020 0.117±0.012 0.122±0.025 模型组 0.383±0.120## 0.213±0.024### 0.185±0.024## 乳酸菌素组 0.141±0.042** 0.149±0.021** 0.131±0.028* 注:与阴性对照组相比,##表示P<0.01,###表示P<0.001;与模型组相比,*表示P<0.05,**表示P<0.01。 2.4 乳酸菌素对H. pylori小鼠肠道菌群的影响
2.4.1 测序质量评估
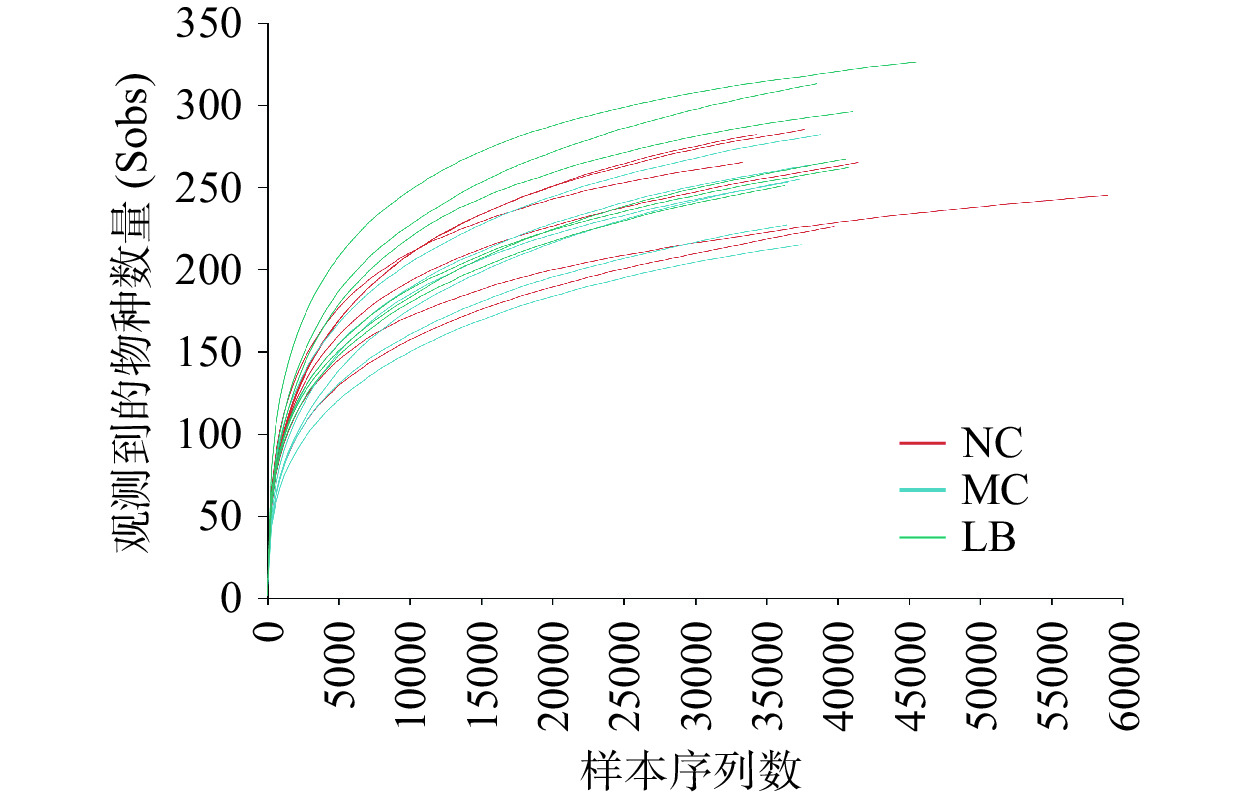
为了评估H. pylori小鼠盲肠内容物中16S rRNA基因的测序质量和深度,绘制了Sob指数稀释曲线。如图4所示,Sob指数曲线随样本测序深度的增加而逐渐增大,最终趋于平稳,表明当前测序数据量足以检测到各样本中绝大多数的菌群。
2.4.2 α多样性
α多样性采用ACE指数、Chao指数、Shannon指数和Simpson指数分析。ACE指数和Chao指数反映各组小鼠肠道菌群的丰度;Shannon指数和Simpson指数是衡量各组小鼠肠道菌群多样性的指标。结果如表2所示,与阴性对照组相比,模型组的ACE指数、Chao指数、Shannon指数和Simpson指数均无显著性差异(P>0.05),说明感染H. pylori对小鼠肠道菌群α多样性无显著性影响。与模型组相比,乳酸菌素组的ACE指数、Chao指数和Shannon指数均显著性升高(P<0.05或P<0.01),Simpson指数显著性降低(P<0.01),说明乳酸菌素能显著提高感染H. pylori小鼠的肠道菌群α多样性。
表 2 小鼠肠道菌群α多样性指数统计(n=6,±s)Table 2. α Diversity index of mice intestinal microflora (n=6, ±s)组别 Shannon Simpson ACE Chao 阴性对照组 3.16±0.34 0.13±0.07 307.54±16.87 312.82±18.86 模型组 3.04±0.24 0.11±0.02 297.62±20.54 297.08±21.38 乳酸菌素组 3.32±0.44## 0.09±0.05## 309.32±39.72# 314.75±40.30e 注: 与模型组相比,#表示P<0.05;##表示P<0.01。 2.4.3 β多样性
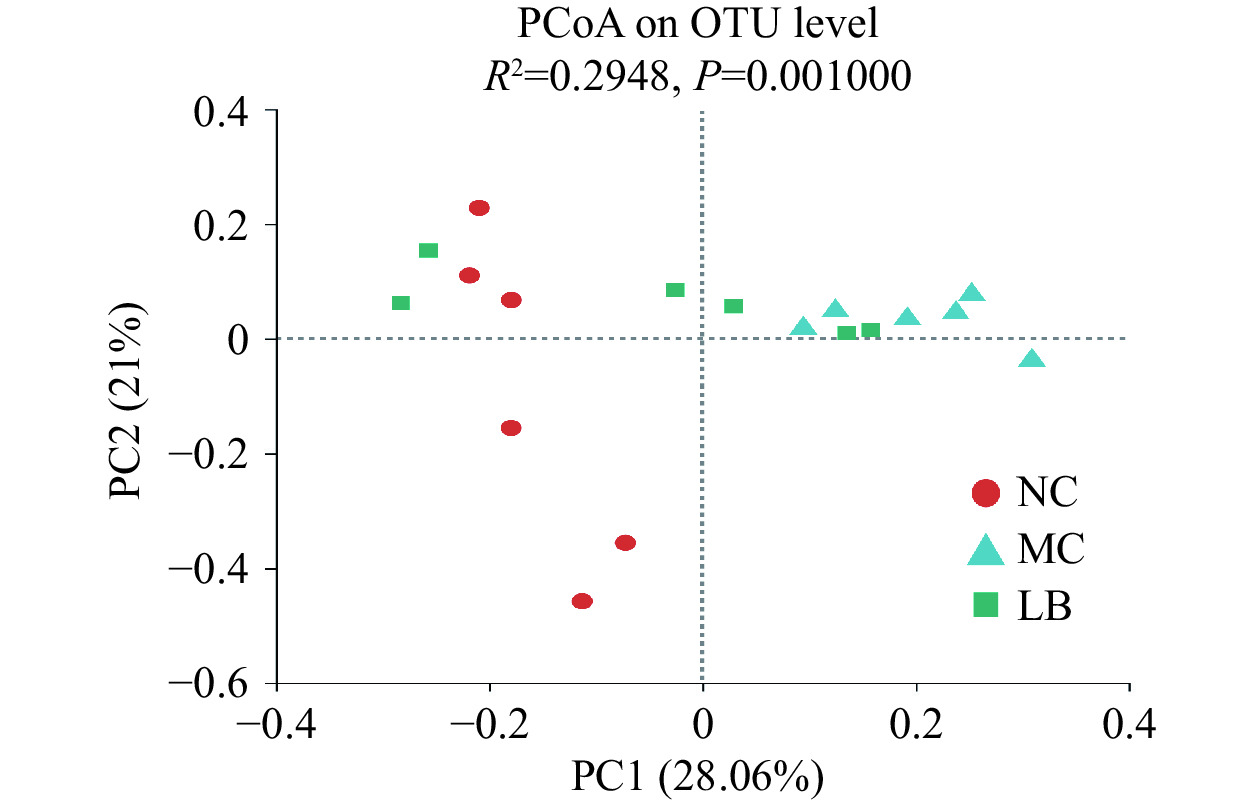
β多样性评价采用基于Bray_Curtis距离的主坐标分析(Principal coordinate analysis,PCoA)。肠道菌群群落组成差异较小的样本在图中分布得更近。如图5所示,三组间样本有明显的分离(R2=0.2948,P=0.001)。其中,阴性对照组和模型组的样本点完全分开(R2=0.2994,P=0.003),表明感染H.pylori的小鼠肠道菌群结构组成发生了显著性改变。乳酸菌素组样本点与模型组趋于分开(R2=0.2298,P=0.015),且乳酸菌素组样本点与阴性对照组更接近,说明乳酸菌素干预恢复了感染H. pylori小鼠的肠道菌群结构组成。
2.4.4 门分类水平菌群相对丰度变化
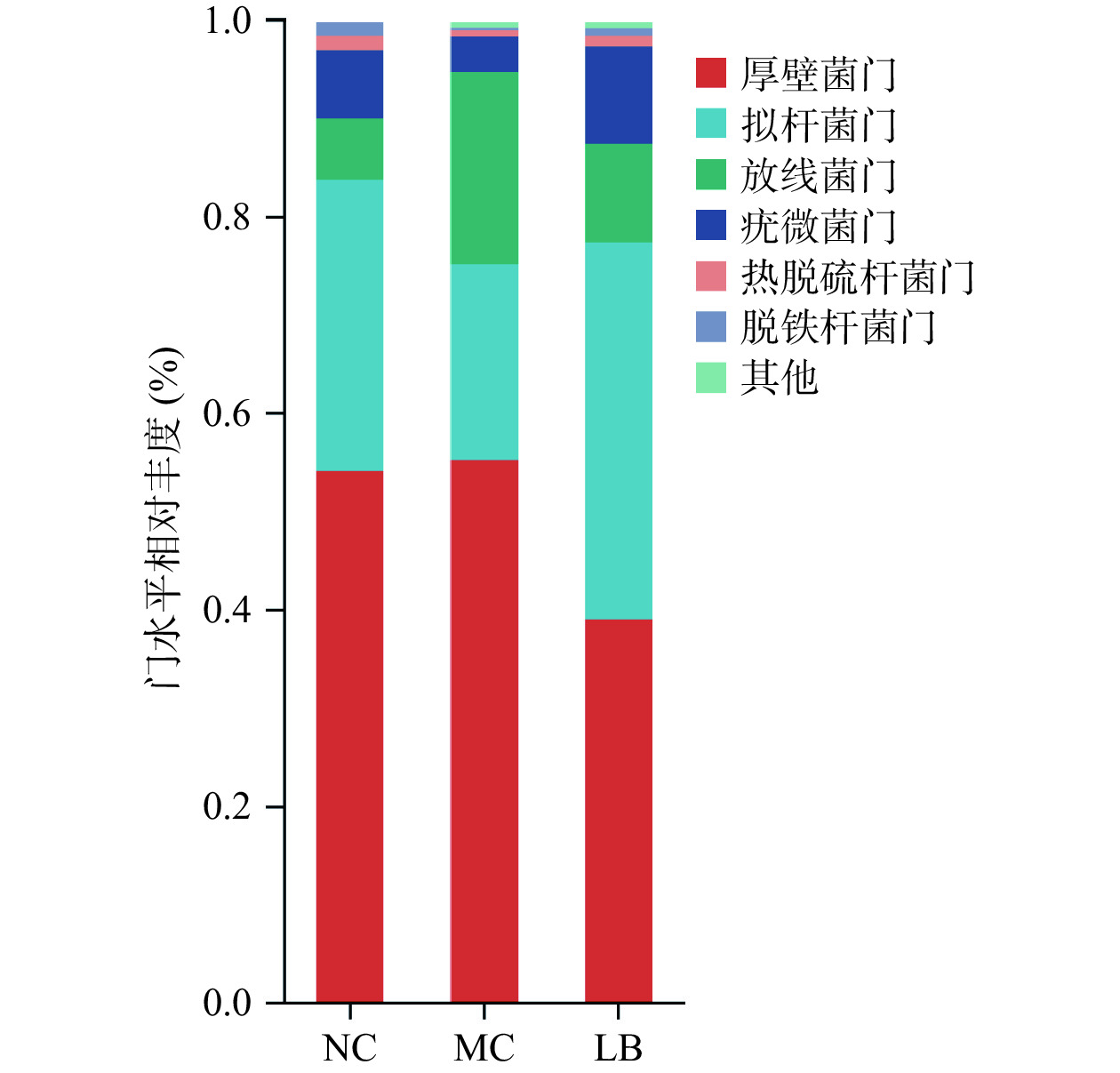
如图6所示,在门水平上,三组小鼠的肠道菌群以厚壁菌门、拟杆菌门、放线菌门和疣微菌门为主。如表3所示,与阴性对照组相比,模型组放线菌门丰度显著性升高(P<0.01),疣微菌门丰度显著性降低(P<0.05),拟杆菌门丰度降低但无显著性(P>0.05),厚壁菌门/拟杆菌门比值(Firmicutes/Bacteroidete,F/B)略高(P>0.05);与模型组相比,乳酸菌素组的厚壁菌门和放线菌门丰度显著性降低(P<0.05),拟杆菌门和疣微菌门丰度显著性升高(P<0.05),F/B比值降低(P<0.05)。
表 3 小鼠肠道菌群门水平相对丰度(n=6,±s)Table 3. Relative abundance of mice intestinal flora phylum levels (n=6, ±s)组别 厚壁菌门(%) 拟杆菌门(%) 放线菌门(%) 疣微菌门(%) 厚壁菌门/拟杆菌门(F/B) 阴性对照组 53.94±10.61 29.70±9.71 6.21±2.52 6.93±4.08 2.26±1.36 模型组 55.11±12.91 19.88±12.82 19.58±3.90## 3.59±5.43# 3.37±1.74 乳酸菌素组 38.88±11.56* 38.41±6.90** 9.98±8.09* 9.93±2.45* 1.27±0.78* 注: 与阴性对照组比较,#表示P<0.05,##表示P<0.01;与模型组比较,*表示P<0.05,**表示P<0.01。 2.4.5 属分类水平菌群相对丰度变化
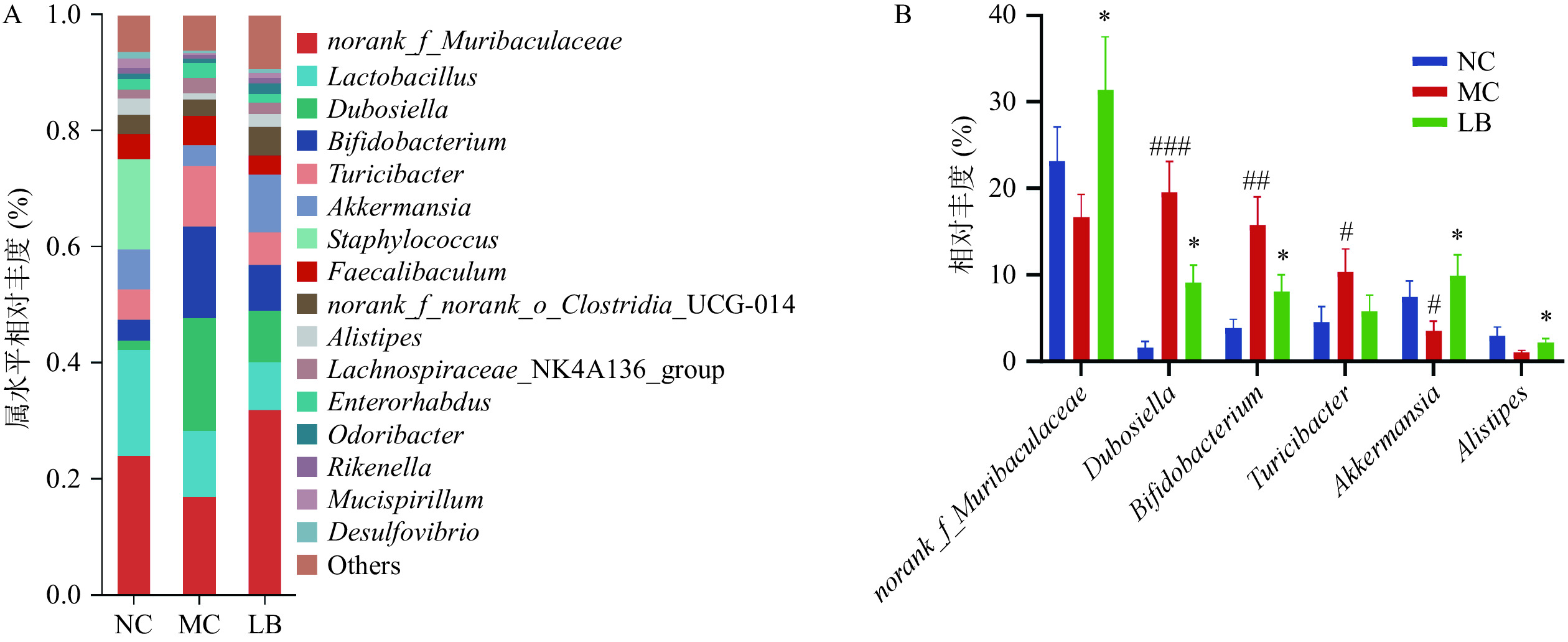
在属水平上,各组小鼠肠道菌群组成如图7A所示。各组菌属排名前10中具有显著性差异的菌属如图7B所示,与阴性对照组比较,模型组的杜氏杆菌属(Dubosiella),双歧杆菌属(Bifidobacterium),苏黎世杆菌属(Turicibacter)的相对丰度显著增加(P<0.05),阿克曼氏菌属(Akkermansia)的相对丰度显著降低(P<0.05),g_norank_f_Muribaculaceae和另枝菌属(Alistipes)的相对丰度呈下降趋势,但差异无统计学意义(P>0.05)。与模型组相比,乳酸菌素组的g_norank_f_Muribaculaceae、阿克曼氏菌属和另枝菌属的相对丰度均显著升高(P<0.05),杜氏杆菌属和双歧杆菌属的相对丰度均显著降低(P<0.05)。
2.4.6 差异菌群筛选
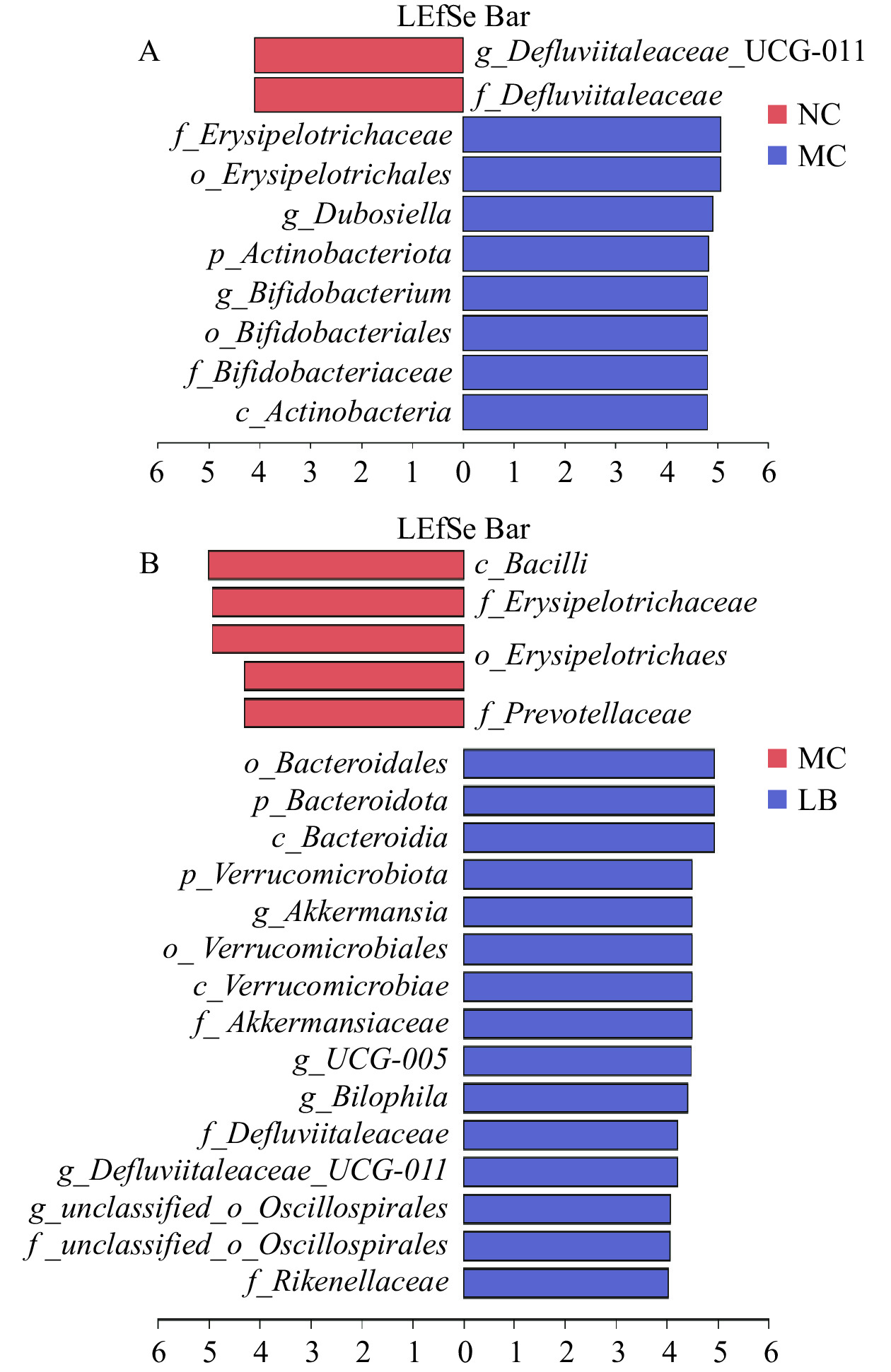
采用LEfSe分析检测组间在丰度上具有显著性差异的菌群,以LDA>4.0且P<0.05为标准绘制LDA判别柱形图(图8)。阴性对照组与模型组中具有显著性差异的分类群主要属于放线菌门,模型组与乳酸菌素组中差异显著的分类群主要属于拟杆菌门和疣微菌门。与阴性对照组比较,模型组中有8种显著差异分类群,即丹毒丝菌目(Erysipelotrichales)、丹毒丝菌科(Erysipelotrichaceae)、杜氏杆菌属,以及放线菌门、放线菌纲(Actinobacteria)、双歧杆菌目(Bifidobacteriales)、双歧杆菌科(Bifidobacteriaceae)和双歧杆菌属。与模型组比较,乳酸菌素组具有15种显著差异分类群,主要包括疣微菌门、疣微菌纲(Verrucomicrobiae)、疣微菌目(Verrucomicrobiales)、阿克曼氏菌科(Akkermansiaceae)、阿克曼氏菌属,以及拟杆菌门、拟杆菌纲(Bacteroidia)、拟杆菌目(Bacteroidales)和理研菌科(Rikenellaceae)等。
3. 讨论与结论
H. pylori感染与慢性胃炎的发生密切相关[26]。研究表明,H. pylori感染能够通过多种毒力因子诱导NF-κB信号通路的活化,从而促进iNOS和IL-1β等多种细胞因子的上调[27]。IL-1β是一种促炎细胞因子,具有抑制胃酸分泌和增强炎症的作用,其过表达与H. pylori感染期间炎症反应的启动密切相关[28]。iNOS主要由中性粒细胞和单核细胞分泌,可通过催化NO的合成,并在超氧化物和氧气的作用下生成过氧亚硝酸盐,进而引起蛋白质和核酸的硝化和氧化[29−30]。3-Nitrotyrosine作为蛋白质硝化产物之一,是过氧亚硝酸盐和其他活性氧或氮诱导的蛋白质损伤的生物标志物[29]。已有研究报道,H. pylori感染胃炎患者的胃黏膜炎性细胞浸润增加,且iNOS、IL-1β和3-Nitrotyrosine的表达显著上调[31−33]。在本研究中,通过乳酸菌素干预后发现,H. pylori感染诱导的小鼠胃黏膜中iNOS、IL-1β和3-Nitrotyrosine的表达显著下降,同时乳酸菌素也显著减少了侵入性炎症细胞的数量,这些结果表明乳酸菌素可缓解H. pylori感染小鼠胃黏膜的炎症反应和氧化损伤。
肠道菌群在人体的免疫系统中发挥重要作用,其动态平衡的维持与身体健康密不可分[34−35]。厚壁菌门和拟杆菌门是肠道菌群中主要的两个优势菌群,其组成比值的增加与免疫系统的异常激活和炎症的产生密切相关[36−37]。据报道,H. pylori感染会增加小鼠体内厚壁菌门/拟杆菌门的比值和放线菌门的相对丰度,并降低疣微菌门的相对丰度,而补充益生菌可以恢复肠道菌群的组成[38]。与先前的研究结果一致[16],乳酸菌素可以调节厚壁菌门和拟杆菌门的相对丰度,并显著提高肠道菌群的多样性。本研究表明,乳酸菌素可以调节肠道菌群组成,有助于维持肠道正常生态,恢复H. pylori感染引起的肠道菌群破坏。
另外,16S rRNA基因测序结果显示,相较于模型组,乳酸菌素组小鼠的肠道菌群中归属于丹毒丝菌科的杜氏杆菌属丰度降低,阿克曼氏菌属、g_norank_f_Muribaculaceae和另枝菌属丰度升高。研究发现,杜氏杆菌属丰度的增加被认为与肠道炎症和肠道菌群失调密切相关[39]。此外,有研究报道丹毒丝菌科与胃肠道炎症相关疾病有关,在炎症性肠病、结直肠癌和其他炎症性疾病患者中发现丹毒丝菌科丰度显著增加[40]。g_norank_f_Muribaculaceae和另枝菌属均属于拟杆菌门拟杆菌目。另枝菌属可以产生乙酸盐,一种具有抗炎作用的SCFAs[41]。SCFAs是由肠道有益菌代谢而产生的有益代谢产物,包括乙酸、丙酸和丁酸等,具有调节免疫、炎症、氧化应激以及肠屏障功能等多种作用。g_norank_f_Muribaculaceae可通过产生SCFAs和O-聚糖,促进有益菌定植于肠道并维持肠道菌群的动态平衡[42]。阿克曼氏菌属属于疣微菌门,能够产生乙酸盐和丙酸盐,与肠道黏膜的通透性密切相关[43]。Deng等[44]研究发现阿克曼氏菌属与炎症指标(如IL-6和TNF-α)呈负相关,可直接黏附于肠道细胞,增强肠道黏液层的完整性,并减少在炎症中起关键作用的基因的表达,从而发挥抗炎活性。
综上所述,乳酸菌素可以改善H. pylori感染小鼠胃黏膜的炎症反应和氧化损伤,并可恢复肠道菌群的相对丰度,促进有益菌的生长并抑制有害菌的繁殖,发挥调节肠道菌群结构的作用。本研究探讨了乳酸菌素对H. pylori感染小鼠炎症及肠道菌群的调节作用,为其临床用药提供有力支撑。
-
表 1 各组小鼠胃组织中iNOS、3-Nitrotyrosine和IL-1β的平均光密度统计结果(n=4,±s)
Table 1 Mean optical density statistics of iNOS, 3-Nitrotyrosine, and IL-1β in the gastric tissue of mice in each group (n=4, ±s)
组别 iNOS 3-Nitrotyrosine IL-1β 阴性对照组 0.133±0.020 0.117±0.012 0.122±0.025 模型组 0.383±0.120## 0.213±0.024### 0.185±0.024## 乳酸菌素组 0.141±0.042** 0.149±0.021** 0.131±0.028* 注:与阴性对照组相比,##表示P<0.01,###表示P<0.001;与模型组相比,*表示P<0.05,**表示P<0.01。 表 2 小鼠肠道菌群α多样性指数统计(n=6,±s)
Table 2 α Diversity index of mice intestinal microflora (n=6, ±s)
组别 Shannon Simpson ACE Chao 阴性对照组 3.16±0.34 0.13±0.07 307.54±16.87 312.82±18.86 模型组 3.04±0.24 0.11±0.02 297.62±20.54 297.08±21.38 乳酸菌素组 3.32±0.44## 0.09±0.05## 309.32±39.72# 314.75±40.30e 注: 与模型组相比,#表示P<0.05;##表示P<0.01。 表 3 小鼠肠道菌群门水平相对丰度(n=6,±s)
Table 3 Relative abundance of mice intestinal flora phylum levels (n=6, ±s)
组别 厚壁菌门(%) 拟杆菌门(%) 放线菌门(%) 疣微菌门(%) 厚壁菌门/拟杆菌门(F/B) 阴性对照组 53.94±10.61 29.70±9.71 6.21±2.52 6.93±4.08 2.26±1.36 模型组 55.11±12.91 19.88±12.82 19.58±3.90## 3.59±5.43# 3.37±1.74 乳酸菌素组 38.88±11.56* 38.41±6.90** 9.98±8.09* 9.93±2.45* 1.27±0.78* 注: 与阴性对照组比较,#表示P<0.05,##表示P<0.01;与模型组比较,*表示P<0.05,**表示P<0.01。 -
[1] 梁雪, 徐洪远, 王伟, 等. 胃复康颗粒通过NLRP3炎性小体通路改善Hp胃炎的作用[J]. 时珍国医国药,2022,33(10):2369−2374. [LIANG X, XU H Y, WANG W, et al. Weifukang granules attenuates Hp induced gastritis through NLRP3 inflammasome pathway[J]. Lishizhen Medicine and Materia Medica Research,2022,33(10):2369−2374.] doi: 10.3969/j.issn.1008-0805.2022.10.17 LIANG X, XU H Y, WANG W, et al. Weifukang granules attenuates Hp induced gastritis through NLRP3 inflammasome pathway[J]. Lishizhen Medicine and Materia Medica Research, 2022, 33(10): 2369−2374. doi: 10.3969/j.issn.1008-0805.2022.10.17
[2] 赵峰, 刘露, 孙颖, 等. Hp感染对慢性胃炎患者胃黏膜病变和miR-22与NLRP3炎症小体和肠道菌群的影响[J]. 中华医院感染学杂志,2022,32(19):2940−2944. [ZHAO F, LIU L, SUN Y, et al. Effects of Hp in fection on gastric mucosal lesions, miR-22 and NLRP3 inflammasomes and intestinal flora in patients with chronic gastritis[J]. Chinese Journal of Nosocomiology,2022,32(19):2940−2944.] ZHAO F, LIU L, SUN Y, et al. Effects of Hp in fection on gastric mucosal lesions, miR-22 and NLRP3 inflammasomes and intestinal flora in patients with chronic gastritis[J]. Chinese Journal of Nosocomiology, 2022, 32(19): 2940−2944.
[3] 刘培文, 陆敏强, 何占坤, 等. 百秋李醇对幽门螺杆菌致小鼠胃炎的保护作用[J]. 中国医院药学杂志,2022,42(1):19−23,28. [LIU P W, LU M Q, HE Z K, et al. Protective effect of patchouli alcohol on Helicobacter pylori-induced gastritis in mice[J]. Chinese Journal of Hospital Pharmacy,2022,42(1):19−23,28.] LIU P W, LU M Q, HE Z K, et al. Protective effect of patchouli alcohol on Helicobacter pylori-induced gastritis in mice[J]. Chinese Journal of Hospital Pharmacy, 2022, 42(1): 19−23,28.
[4] 刘文忠, 谢勇, 陆红, 等. 第五次全国幽门螺杆菌感染处理共识报告[J]. 中华内科杂志,2017,56(7):532−545. [LIU W Z, XIE Y, LU H, et al. The fifth national consensus report on the management of Helicobacter pylori infection[J]. Chinese Journal of Internal Medicine,2017,56(7):532−545.] LIU W Z, XIE Y, LU H, et al. The fifth national consensus report on the management of Helicobacter pylori infection[J]. Chinese Journal of Internal Medicine, 2017, 56(7): 532−545.
[5] NYSSEN O P, BORDIN D, TEPES B, et al. European Registry on Helicobacter pylori management (Hp-EuReg):Patterns and trends in first-line empirical eradication prescription and outcomes of 5 years and 21533 patients[J]. Gut,2021,70(1):40−54. doi: 10.1136/gutjnl-2020-321372
[6] FRANCISCO A J. Helicobacter pylori infection induces intestinal dysbiosis that could be related to the onset of atherosclerosis[J]. Biomed Research International,2022,2022:9943158.
[7] CUI M Y, CUI Z Y, ZHAO M Q, et al. The impact of Helicobacter pylori infection and eradication therapy containing minocycline and metronidazole on intestinal microbiota[J]. BMC Microbiol,2022,22(1):321. doi: 10.1186/s12866-022-02732-6
[8] JONES T A, HERNANDEZ D Z, WONG Z C, et al. The bacterial virulence factor CagA induces microbial dysbiosis that contributes to excessive epithelial cell proliferation in the drosophila gut[J]. PLoS Pathogens,2017,13(10):e1006631. doi: 10.1371/journal.ppat.1006631
[9] BAJ J, FORMA A, FLIEGER W, et al. Helicobacter pylori infection and extragastric diseases-a focus on the central nervous system[J]. Cells,2021,10(9):2191. doi: 10.3390/cells10092191
[10] ZHENG D P, LIWINSKI T, ELINAV E. Interaction between microbiota and immunity in health and disease[J]. Cell Research,2020,30(6):492−506. doi: 10.1038/s41422-020-0332-7
[11] DUBIN K, CALLAHAN M K, REN B Y, et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis[J]. Nature Communications,2016,7(1):10391. doi: 10.1038/ncomms10391
[12] ALSHARAIRI N A. Therapeutic potential of gut microbiota and its metabolite short-chain fatty acids in neonatal necrotizing enterocolitis[J]. Life (Basel),2023,13(2):561. doi: 10.3390/life13020561
[13] HRDý J, COUTURIER-MAILLARD A, BOUTILLIER D, et al. Oral supplementation with selected Lactobacillus acidophilus triggers IL-17-dependent innate defense response, activation of innate lymphoid cells type 3 and improves colitis[J]. Scientific Reports,2022,12(1):17591. doi: 10.1038/s41598-022-21643-0
[14] 张丽娟, 田欣圆, 赵璟, 等. 嗜酸乳杆菌LA-GHB1756对阿司匹林引起的小鼠肠道炎症的影响[J]. 解放军医学杂志,2022,47(10):976−983. [ZHANG L J, TIAN X Y, ZHAO J, et al. Effect of Lactobacillus acidophilus LA-GHB1756 on alleviating inflammatory bowel disease in mice caused by aspirin[J]. Medical Journal of Chinese People's Liberation Army,2022,47(10):976−983.] ZHANG L J, TIAN X Y, ZHAO J, et al. Effect of Lactobacillus acidophilus LA-GHB1756 on alleviating inflammatory bowel disease in mice caused by aspirin[J]. Medical Journal of Chinese People's Liberation Army, 2022, 47(10): 976−983.
[15] 蔡丽君, 王晓丽, 远文瑛. 复方嗜酸乳杆菌对腹泻型肠易激综合征模型大鼠肠黏膜屏障功能的保护作用[J]. 中国临床药理学杂志,2021,37(3):246−249. [CAI L J, WANG X L, YAN W Y. Protective effect of compound Lactobacillus acidophilus on intestinal mucosal barrier function in rats with diarrhea-predominant irritable bowel syndrome[J]. The Chinese Journal of Clinical Pharmacology,2021,37(3):246−249.] CAI L J, WANG X L, YAN W Y. Protective effect of compound Lactobacillus acidophilus on intestinal mucosal barrier function in rats with diarrhea-predominant irritable bowel syndrome[J]. The Chinese Journal of Clinical Pharmacology, 2021, 37(3): 246−249.
[16] 詹扬, 万建华, 李颖萌, 等. 乳酸菌素片对抗生素相关性腹泻小鼠肠道菌群的影响[J]. 中国乳品工业,2021,49(8):4−9,36. [ZHAN Y, WAN J H, LI Y M, et al. Effects of Lacidophilin tablets on gut microbiota in antibiotic-associated diarrhea model mice[J]. China Dairy Industry,2021,49(8):4−9,36.] ZHAN Y, WAN J H, LI Y M, et al. Effects of Lacidophilin tablets on gut microbiota in antibiotic-associated diarrhea model mice[J]. China Dairy Industry, 2021, 49(8): 4−9,36.
[17] 常菲, 奚宇杰, 杨青, 等. 乳酸菌素片联合标准四联疗法对社区消化性溃疡患者胃电图及肠道菌群的影响[J]. 中国微生态学杂志,2021,33(9):1049−1054. [CHANG F, XI Y J, YANG Q, et al. Effects of Lacidophilin tablets combined with standard quadruple therapy on electrogastrogram and intestinal flora in patients with peptic ulcer in community[J]. Chinese Journal of Microecology,2021,33(9):1049−1054.] CHANG F, XI Y J, YANG Q, et al. Effects of Lacidophilin tablets combined with standard quadruple therapy on electrogastrogram and intestinal flora in patients with peptic ulcer in community[J]. Chinese Journal of Microecology, 2021, 33(9): 1049−1054.
[18] 王为, 徐爱蕾, 黄毅, 等. 微生态制剂在根除幽门螺杆菌治疗中的价值[J]. 临床消化病杂志,2015,27(3):150−153. [WANG W, XU A L, HUANG Y, et al. Effects of microbial ecological agent in the Helicobacter pylori eradicating treatment[J]. Chinese Journal of Clinical Gastroenterology,2015,27(3):150−153.] WANG W, XU A L, HUANG Y, et al. Effects of microbial ecological agent in the Helicobacter pylori eradicating treatment[J]. Chinese Journal of Clinical Gastroenterology, 2015, 27(3): 150−153.
[19] 宋远昌. 幽门螺杆菌感染合并消化性溃疡的治疗分析[J]. 临床合理用药杂志,2014,7(12):73−74. [SONG Y C. Diagnostic analysis of Helicobacter pylori complicated with peptic ulcer infection[J]. Chinese Journal of Clinical Rational Drug Use,2014,7(12):73−74.] SONG Y C. Diagnostic analysis of Helicobacter pylori complicated with peptic ulcer infection[J]. Chinese Journal of Clinical Rational Drug Use, 2014, 7(12): 73−74.
[20] ZHANG W, ZHOU Y, FAN Y, et al. Metal-organic-framework-based hydrogen-release platform for multieffective Helicobacter pylori targeting therapy and intestinal flora protective capabilities[J]. Advanced Materials,2022,34(2):e2105738. doi: 10.1002/adma.202105738
[21] KIM D H, KIM S W, SONG Y J, et al. Long-term evaluation of mice model infected with Helicobacter pylori:Focus on gastric pathology including gastric cancer[J]. Alimentary Pharmacology and Therapeutics,2003,18:14−23.
[22] 沙莉, 朱东兵, 沈小建, 等. 银染与免疫组化染色检测幽门螺杆菌的效果对比[J]. 诊断病理学杂志,2013,20(8):509−510. [SHA L, ZHU D B, SHEN X J, et al. Comparison of the effect of silver staining and immunohistochemical staining in the detection of Helicobacter pylori[J]. Chinese Journal of Diagnostic Pathology,2013,20(8):509−510.] SHA L, ZHU D B, SHEN X J, et al. Comparison of the effect of silver staining and immunohistochemical staining in the detection of Helicobacter pylori[J]. Chinese Journal of Diagnostic Pathology, 2013, 20(8): 509−510.
[23] 王嘉文, 宋璐, 詹华奎. 补肾活血祛风方对阿霉素肾病大鼠肾组织NF-κB及IL-18水平的干预研究[J]. 北京中医药大学学报,2021,44(11):1011−1019. [WANG J W, SONG L, ZHAN H K. Effect of Bushen Huoxue Qufeng formucl on the levels of NF-κB and IL-18 in renal tissue of rats with adriamycin nephrosis[J]. Journal of Beijing University of Traditional Chinese Medicine,2021,44(11):1011−1019.] doi: 10.3969/j.issn.1006-2157.2021.11.009 WANG J W, SONG L, ZHAN H K. Effect of Bushen Huoxue Qufeng formucl on the levels of NF-κB and IL-18 in renal tissue of rats with adriamycin nephrosis[J]. Journal of Beijing University of Traditional Chinese Medicine, 2021, 44(11): 1011−1019. doi: 10.3969/j.issn.1006-2157.2021.11.009
[24] LIU J H, ZHANG M L, ZHANG R Y, et al. Comparative studies of the composition of bacterial microbiota associated with the ruminal content, ruminal epithelium and in the faeces of lactating dairy cows[J]. Microbial Biotechnology,2016,9(2):257−68. doi: 10.1111/1751-7915.12345
[25] LU C Y, SUN T T, LI Y Y, et al. Modulation of the gut microbiota by krill oil in mice fed a high-sugar high-fat diet[J]. Frontiers in Microbiology,2017,8:905. doi: 10.3389/fmicb.2017.00905
[26] 郭殿华, 程芃, 陈卿奇, 等. Hp感染及胃黏膜TFF1/GKN2表达与慢性胃炎 OLGA/OLGIM分期的关系[J]. 中华医院感染学杂志,2023,33(4):527−531. [GUO D H, CHENG P, CHEN Q Q, et al. Relationship between Hp infection, TFF1/GKN2 expression in gastric mucosa and OLGA/OLGIM staging of chronic gastritis[J]. Chinese Journal of Nosocomiology,2023,33(4):527−531.] GUO D H, CHENG P, CHEN Q Q, et al. Relationship between Hp infection, TFF1/GKN2 expression in gastric mucosa and OLGA/OLGIM staging of chronic gastritis[J]. Chinese Journal of Nosocomiology, 2023, 33(4): 527−531.
[27] BAJ J, FORMA A, SITARZ M, et al. Helicobacter pylori virulence factors-mechanisms of bacterial pathogenicity in the gastric microenvironment[J]. Cells, 2020, 10(1).
[28] EL FILALY H, OUTLIOUA A, DESTERKE C, et al. IL-1 polymorphism and Helicobacter pylori infection features:highlighting VNTR's potential in predicting the susceptibility to infection-associated disease development[J]. Microorganisms,2023,11(2):353. doi: 10.3390/microorganisms11020353
[29] NAM K T, OH S Y, AHN B, et al. Decreased Helicobacter pylori associated gastric carcinogenesis in mice lacking inducible nitric oxide synthase[J]. Gut,2004,53(9):1250−5. doi: 10.1136/gut.2003.030684
[30] LI C Q, PIGNATELLI B, OHSHIMA H. Increased oxidative and nitrative stress in human stomach associated with cagA+ Helicobacter pylori infection and inflammation[J]. Digestive Diseases and Sciences,2001,46(4):836−844. doi: 10.1023/A:1010764720524
[31] CHERDANTSEVA L A, POTAPOVA O V, SHARKOVA T V, et al. Association of Helicobacter pylori and iNOS production by macrophages and lymphocytes in the gastric mucosa in chronic gastritis[J]. Journal of Immunology Research,2014,2014:762514.
[32] FEHLINGS M, DROBBE L, MOOS V, et al. Comparative analysis of the interaction of Helicobacter pylori with human dendritic cells, macrophages, and monocytes[J]. Infection and Immunity,2012,80(8):2724−34. doi: 10.1128/IAI.00381-12
[33] SAKAGUCHI A A, MIURA S, TAKEUCHI T, et al. Increased expression of inducible nitric oxide synthase and peroxynitrite in Helicobacter pylori gastric ulcer[J]. Free Radical Biology and Medicine,1999,27(7-8):781−789. doi: 10.1016/S0891-5849(99)00124-0
[34] CARDING S, VERBEKE K, VIPOND D T, et al. Dysbiosis of the gut microbiota in disease[J]. Microbial Ecology in Health and Disease,2015,26:26191.
[35] HILLS R D J R, PONTEFRACT B A, MISHCON H R, et al. Gut microbiome:Profound implications for diet and disease[J]. Nutrients,2019,11(7):1613. doi: 10.3390/nu11071613
[36] STRATI F, CAVALIERI D, ALBANESE D, et al. New evidences on the altered gut microbiota in autism spectrum disorders[J]. Microbiome,2017,5(1):24. doi: 10.1186/s40168-017-0242-1
[37] STOJANOV S, BERLEC A, ŠTRUKELJ B. The influence of probiotics on the Firmicutes/Bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease[J]. Microorganisms,2020,8(11):1715. doi: 10.3390/microorganisms8111715
[38] LAI C H, LIN T L, HUANG M Z, et al. Gut commensal parabacteroides goldsteinii MTS01 alters gut microbiota composition and reduces cholesterol to mitigate Helicobacter pylori-induced pathogenesis[J]. Frontiers in Immunology,2022,13:916848. doi: 10.3389/fimmu.2022.916848
[39] SHANG J, GUO H, LI J, et al. Exploring the mechanism of action of Sanzi formula in intervening colorectal adenoma by targeting intestinal flora and intestinal metabolism[J]. Frontiers in Microbiology,2022,13:1001372. doi: 10.3389/fmicb.2022.1001372
[40] LI J C, ZHANG J, ZHANG Y L, et al. Effect and correlation of Rosa roxburghii tratt fruit vinegar on obesity, dyslipidemia and intestinal microbiota disorder in high-fat diet mice[J]. Foods,2022,11(24):4108. doi: 10.3390/foods11244108
[41] LI H, WANG Q L, CHEN P Z, et al. Ursodeoxycholic acid treatment restores gut microbiota and alleviates liver inflammation in non-alcoholic steatohepatitic mouse model[J]. Frontiers in Pharmacology,2021,12:788558. doi: 10.3389/fphar.2021.788558
[42] LUO X M, ZHANG B Y, PAN Y H, et al. Phyllanthus emblica aqueous extract retards hepatic steatosis and fibrosis in NAFLD mice in association with the reshaping of intestinal microecology[J]. Frontiers in Pharmacology,2022,13:893561. doi: 10.3389/fphar.2022.893561
[43] YAN J L, PAN Y B, SHAO W M, et al. Beneficial effect of the short-chain fatty acid propionate on vascular calcification through intestinal microbiota remodelling[J]. Microbiome,2022,10(1):195. doi: 10.1186/s40168-022-01390-0
[44] DENG Q H, WANG W J, ZHANG L Y, et al. Gougunao tea polysaccharides ameliorate high-fat diet-induced hyperlipidemia and modulate gut microbiota[J]. Food & Function,2022,14(2):703−719.
-
期刊类型引用(1)
1. 陈孜旋,佟苗苗,吴庆林,孙佳婷,尹小溪,吕添熠,张睦清,李丽. 薏苡仁对葡聚糖硫酸钠诱导的溃疡性结肠炎小鼠肠道菌群的影响. 食品工业科技. 2025(06): 369-376 .  本站查看
本站查看
其他类型引用(2)





 下载:
下载:
 下载:
下载:



