| [1] |
|
| [2] |
Pereira P R, Silva J T, Verícimo M A, et al. Crude extract from taro ( Colocasia esculenta) as a natural source of bioactive proteins able to stimulate haematopoietic cells in two murine models[J]. Journal of Functional Foods,2015,18:333−343. doi: 10.1016/j.jff.2015.07.014
|
| [3] |
Wu C, Lin K. The Antioxidative Characteristics of taro and sweet potato protein hydrolysates and their inhibitory capability on angiotensin converting enzyme[J]. Food Science and Technology Research,2017,23(6):845−853. doi: 10.3136/fstr.23.845
|
| [4] |
Pereira P R, Corrêa A C N T, Vericimo M A, et al. Tarin, a potential immunomodulator and cox-inhibitor lectin found in taro ( Colocasia esculenta)[J]. Comprehensive Reviews in Food Science and Food Safety,2018,17(4):878−891. doi: 10.1111/1541-4337.12358
|
| [5] |
Yu J, Liu P, Duan J, et al. Itches-stimulating compounds from Colocasia esculenta (taro): Bioactive-guided screening and LC-MS/MS identification[J]. Bioorganic & Medicinal Chemistry Letters,2015,25(20):4382−4386.
|
| [6] |
Ribeiro P P, Mere D A E, Afonso V M, et al. Purification and characterization of the lectin from taro (Colocasia esculenta) and its effect on mouse splenocyte proliferation in vitro and in vivo[J]. The Protein Journal, 2014, 33(1): 92-99.
|
| [7] |
童晶晶, 张晓元, 赖富饶, 等. 不同溶剂对香芋蛋白提取及活性的影响[J]. 现代食品科技,2016,32(1):194−202.
|
| [8] |
|
| [9] |
罗丽平, 李冰晶, 赵景芳, 等. 三种食用菌提取物体外抗氧化与降血糖活性研究[J]. 食品工业科技,2020,41(20):324−329.
|
| [10] |
李波, 包怡红, 高锋, 等. 红松松球鳞片多酚对α-淀粉酶和α-葡萄糖苷酶的抑制作用[J]. 食品工业科技,2015,36(1):63−65.
|
| [11] |
Kumari B, Sharma P, Nath A K. α-Amylase inhibitor in local Himalyan collections of Colocasia: Isolation, purification, characterization and selectivity towards α-amylases from various sources[J]. Pesticide Biochemistry and Physiology,2012,103(1):49−55. doi: 10.1016/j.pestbp.2012.03.003
|
| [12] |
Tiengburanatam N, Boonmee A, Sangvanich P, et al. A novel alpha-glucosidase inhibitor protein from the rhizomes of Zingiber ottensii Valeton[J]. Appl Biochem Biotechnol,2010,162(7):1938−1951. doi: 10.1007/s12010-010-8971-7
|
| [13] |
Mcewan R , Madivha R P , Djarova T , et al. Alpha-amylase inhibitor of amadumbe (Colocasia esculenta): Isolation, purification and selectivity toward alpha-amylases from various sources[J]. African Journal of Biochemistry Research,2010,4(9):220−224.
|
| [14] |
Sharma K K, Pattabiraman T N. Natural plant enzyme inhibitors. Isolation and characterisation of two α-amylase inhibitors from Colocasia antiquorum tubers[J]. Journal of the Science of Food and Agriculture,1980,31(10):981−991. doi: 10.1002/jsfa.2740311003
|
| [15] |
童晶晶. 香芋蛋白的提取及其胰蛋白酶抑制剂的研究[D]. 广州: 华南理工大学, 2016.
|
| [16] |
|
| [17] |
|
| [18] |
黄文. 白果活性蛋白的分离、纯化、结构及其生物活性研究[D]. 武汉: 华中农业大学, 2002.
|
| [19] |
|
| [20] |
周晓薇, 王静, 段浩, 等. 铁棍山药蛋白质的分离纯化及体外抗氧化活性[J]. 食品科学,2011,32(9):31−35.
|
| [21] |
李雨哲. 马氏珍珠贝糖蛋白提取纯化及其抗氧化活性的研究[D]. 广州: 华南理工大学, 2011.
|
| [22] |
崔竹梅, 王红玲, 张占琴, 等. 鹰嘴豆籽粒清蛋白和球蛋白等电点、相对分子量的测定[J]. 干旱地区农业研究,2007(6):89−95.
|
| [23] |
周仲驹等夏其昌张祥明. 蛋白质电泳技术指南[M]. 北京: 化学工业出版社, 2007: 23−30.
|
| [24] |
刘睿, 潘思轶, 刘亮, 等. 高粱原花青素对α-淀粉酶活力抑制动力学的研究[J]. 食品科学,2005(9):171−174.
|
| [25] |
|
| [26] |
Wu Y, Zhou Q, Chen X, et al. Comparison and screening of bioactive phenolic compounds in different blueberry cultivars: Evaluation of anti-oxidation and α-glucosidase inhibition effect[J]. Food Research International,2017,100:312−324. doi: 10.1016/j.foodres.2017.07.004
|
| [27] |
张永军, 黄惠华. 茶多酚对胰α-淀粉酶的抑制动力学研究[J]. 食品工业,2010,31(1):7−9.
|
| [28] |
|
| [29] |
|
| [30] |
Damares C. Monte-Neshich T L R R. Characterization and spatial localization of the major globulin families of taro (Colocasia esculenta L. Schott) tubers[J]. Plant Science, 1995, 112: 149-159.
|
| [31] |
伍城颖, 吴启南, 王红, 等. 芡种皮多酚提取物体外抑制α-葡萄糖苷酶和α-淀粉酶活性研究[J]. 食品工业科技,2015,36(16):91−94.
|






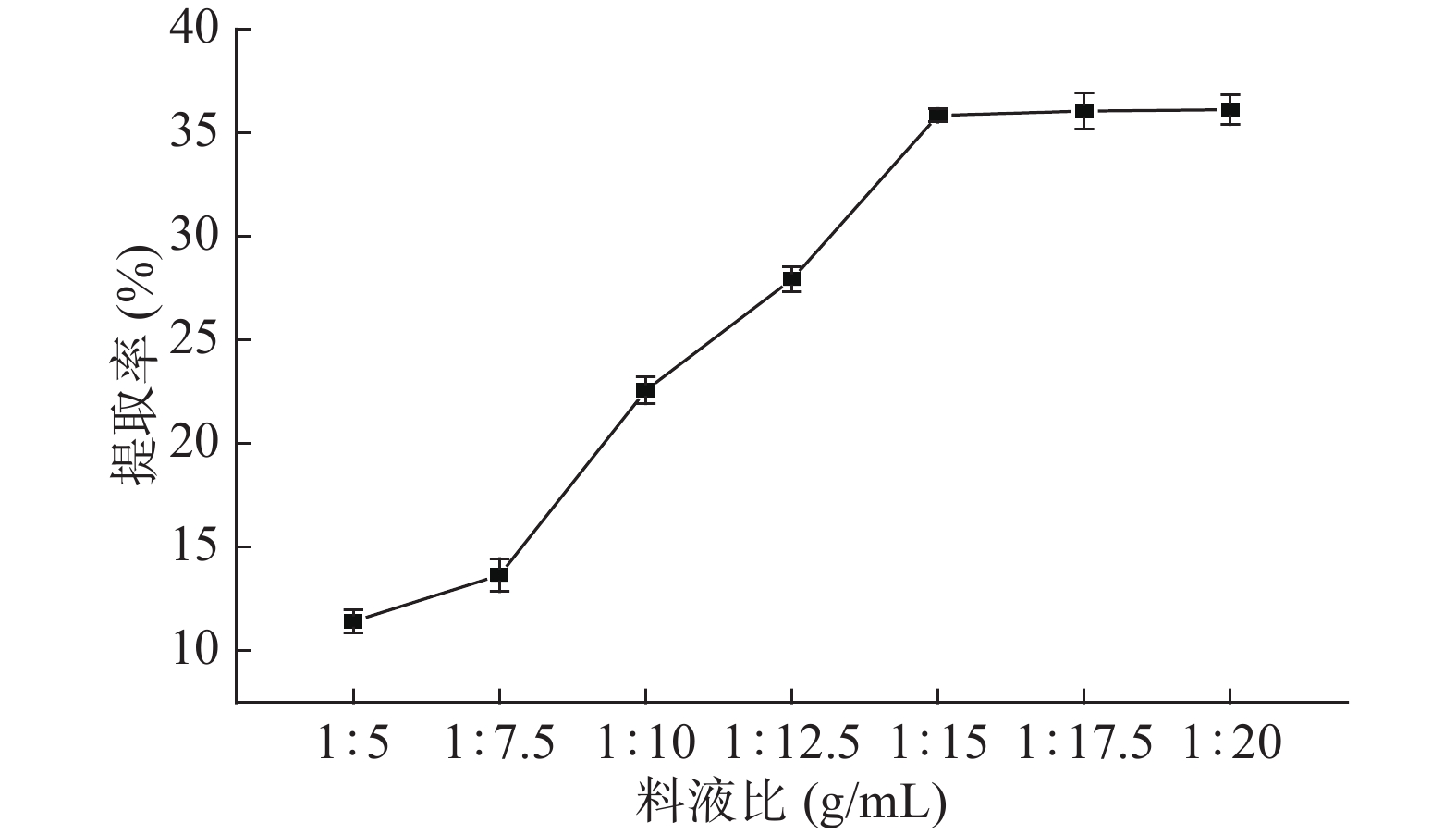




 DownLoad:
DownLoad: