Improvement in the Glycolipid Metabolism of Diabetic Mice Induced by High-fat Diet with Streptozotocin Supplemented with Ganoderma lucidum Polysaccharide Beverage
-
摘要: 为探究长白山赤灵芝子实体多糖饮料对糖尿病小鼠的糖脂代谢的影响及保护作用。本研究利用高脂饮食结合腹腔注射链脲佐菌素诱导法建立糖尿病小鼠模型,设立正常对照组、模型对照组,并以盐酸二甲双胍为阳性对照,灌胃低、中、高不同剂量的灵芝多糖饮料探究对糖尿病小鼠的糖脂代谢改善作用,测定经灵芝多糖饮料作用后小鼠的空腹血糖值、胰岛素抵抗指数以及甘油三酯、高密度脂蛋白胆固醇、低密度脂蛋白胆固醇、超氧化物歧化酶活力、丙二醛等相关指标的变化情况。结果表明:与模型组相比,灵芝多糖饮料低、中、高剂量组的小鼠空腹血糖值分别下降了35.46%、39.05%、42.28%,胰岛素抵抗指数分别极显著(P<0.01)下降43.10%、50.25%、54.18%;高剂量组小鼠甘油三酯、低密度脂蛋白胆固醇含量分别下降46.83%、50.79%,高密度脂蛋白胆固醇上升107.81%;此外,灵芝多糖饮料能够提高糖尿病小鼠血清中超氧化物歧化酶活力,极显著降低丙二醛含量(P<0.01)其中L-GLB组、M-GLB组、H-GLB组分别下降18.66%、30.15%、44.54%,因此,灵芝多糖饮料可以缓解糖尿病引发的氧化应激,改善糖尿病引起的脏器肿大,保护DM小鼠的肝脏、肾脏和脾脏,在一定程度上能够改善由长期高血糖导致的肝脏损伤。Abstract: This study aimed to investigate the effects of Changbaishan Ganoderma lucidum seed body polysaccharide beverage on the glucose and lipid metabolism of diabetic mice. High-fat diet combined with intraperitoneal injection of streptozotocin was used to establish a diabetic mouse model. A normal control group and a model control group were also set up. Metformin hydrochloride was used as a positive control. G. lucidum polysaccharide beverage was administered at different doses of low (L-GLB), medium (M-GLB), and high (H-GLB) to investigate its improvement effects on the glucose and lipid metabolism of diabetic mice. Fasting blood glucose, insulin resistance index, triglyceride, high- and low-density lipoprotein cholesterol, superoxide dismutase activity, and malondialdehyde were measured in the mice after receiving the G. lucidum polysaccharide beverage. Compared with those of the model group, the fasting blood glucose levels of the mice in the L-GLB, M-GLB, and H-GLB groups decreased by 35.46%, 39.05%, and 42.28%, respectively, and their homeostatic model assessment of insulin resistance decreased by 43.10%, 50.25%, and 54.18%, respectively (P<0.01). The triglyceride levels and LDL cholesterol of the mice in the high-dose group decreased by 46.83% and 50.79%, respectively, and their HDL cholesterol increased by 107.81%. G. lucidum polysaccharide beverage improved the superoxide dismutase activity in the serum of diabetic mice and significantly reduced the malondialdehyde content (P<0.01) by 18.66%, 30.15%, and 44.54% in the L-GLB, M-GLB, and H-GLB groups, respectively. Therefore, G. lucidum polysaccharide beverage can relieve diabetes-induced oxidative stress and organ enlargement, protect the liver, kidney and spleen of diabetic mice, and improve the liver damage caused by long-term hyperglycemia to a certain extent.
-
糖尿病(diabetes mellitus,DM)是一种由胰岛素抵抗或分泌不足引起的慢性代谢疾病,主要分为1型、2型、妊娠期和特殊类型[1]。DM患者机体长期处于高血糖状态会引发多种慢性代谢类疾病,还会造成肝脏、肾脏等多种器官损伤[2]。胰岛素不仅影响着血糖的高低,还在调控脂质代谢中起着重要作用[3],胰岛功能不足会导致脂质代谢酶活性降低,造成脂代谢紊乱[4]。近年来,以“药食同源”物质及其提取物用于预防和辅助治疗DM已成当下研究热点。
灵芝是我国一种珍贵的药食两用真菌。灵芝多糖类具有免疫调节[5]、抗肿瘤[6]、降血糖[7]及抗氧化[8]等作用吸引了众多消费者的关注,目前已报道的灵芝中主要活性成分多糖及三萜类化合物均表现出了良好的降血糖和降血脂活性[9−10]。随着生活水平的提高及健康意识的增强,人们对于饮料产品有了天然健康、低糖保健的新需求,美味可口又富含营养的饮品受到了消费者的广泛青睐,但对于灵芝尤其是饮料类型的产品研发较少,本研究基于灵芝多糖的降血糖作用研发具有辅助降血糖作用的功能性饮料,将木质及纤维化程度高、苦涩味较重且难以直接食用的灵芝子实体加工成便捷美味、营养丰富且质量稳定的功能性饮品,探究该饮品对高脂联合链脲佐菌素(streptozotocin,STZ)诱导的DM小鼠的糖脂代谢调节作用,拓展灵芝在功能性食品中的开发,不仅能提升灵芝产品的附加值,推动我省灵芝产业的发展,扩充灵芝产业链,同时可以丰富DM等特殊人群的饮食需求,为DM营养干预治疗提供新选择。
1. 材料与方法
1.1 材料与仪器
SPF级雄性昆明小鼠(4周龄,体重18~22 g) 50只小鼠(本动物试验来源于吉林农业大学实验动物中心,使用许可证号:SYXK(吉)2018-0023;伦理审批号:2022 09 22 001 )日常维持饲料由吉林农业大学实验动物中心提供;高脂高糖饲料[11] 实验室自制;长白山赤灵芝子实体 吉林省铭锡堂参茸特产有限公司;纤维素酶(100000 U/g) 、半纤维素酶(50000 U/g)、中性蛋白酶(100000 U/g) 河南佰隆生物科技有限公司;盐酸二甲双胍 拜耳医药保健有限公司;总胆固醇(total cholesterol,TC)测试盒、甘油三酯(triglyceride,TG)测试盒、高密度脂蛋白胆固醇(high-density lipoprotein cholesterol,HDL-C)测试盒、低密度脂蛋白胆固醇(low-density lipoprotein cholesterol,LDL-C)测试盒、超氧化物歧化酶(SOD)测定试剂盒、丙二醛(MDA)测试盒 南京建成生物工程研究所;小鼠胰岛素(insulin,INS)酶联免疫分析试剂盒 上海酶联生物科技有限公司。
GA-3型血糖测试仪 三诺生物传感股份有限公司;Centrifuge 5804R型高速冷冻离心机 德国 Eppendorf公司;30086376 Spark 型酶标仪 瑞士TECAN集团;UV-2600i型紫外可见分光光度计 岛津仪器(苏州)有限公司;FDU-1200 EYELA 型冷冻干燥机 东京理化Eyela公司。
1.2 实验方法
1.2.1 灵芝子实体水提物的制备
将灵芝子实体清洗干净,烘干至恒重,超微粉碎后过100目筛,储存备用。灵芝子实体超微粉与磷酸盐缓冲液(pH6.0)按料液比为1:30(g/mL)加入复合酶(3%纤维素酶、2%半纤维素酶、3%中性蛋白酶),56 ℃酶解82 min,90 ℃灭酶活10 min,趁热过滤,滤液减压浓缩,浓缩液在−80 ℃冰箱中冷冻过夜,冻干得灵芝子实体水提物[12]。
1.2.2 灵芝多糖饮料的制备
以灵芝子实体超微粉为原料,复合酶法制备灵芝水提液。将灵芝水提液真空浓缩至1/8体积后作为原料,赤藓糖醇和柠檬酸为调味剂,β-环状糊精为矫味剂[13],纯净水为溶剂,灵芝水提浓缩液添加量58.32%、赤藓糖醇添加量6.83%、柠檬酸添加量0.04%、β-环状糊精添加量1.39%,纯净水添加量33.42%,按此比例进行调配。将调配好的混合液置于高压均质机中,在20 MPa的均质压力下均质处理2次,每次均质3 min。100 ℃下杀菌20 min。制得的灵芝多糖饮料每100 mL中多糖含量达257.23 mg(参照 NY/T 1676-2008 食用菌中粗多糖含量的测定方法,以葡萄糖为标准品对灵芝多糖饮料中粗多糖含量进行定量分析),各项微生物指标均符合国家标准规定的限量要求。经旋转蒸发仪浓缩10倍后,分装至5 mL离心管中,4 ℃冰箱保存备用[14]。
1.2.3 DM小鼠模型的建立
利用高脂饮食联合STZ诱导法建立DM小鼠模型[15]。小鼠适应性喂养一周后,将小鼠随机分为2组,正常组(n=8),继续喂养基础饲料;造模组(n=42),喂养高脂饲料。4周后,造模组小鼠连续3 d腹腔注射40 mg/(kg·bw) STZ注射液,正常组小鼠注射同等剂量柠檬酸-柠檬酸钠缓冲溶液[16],72 h后,造模组小鼠禁食不禁水12 h,次日早上连续两次测定小鼠的空腹血糖水平,小鼠血糖值≥11.1 mmol/L的即为造模成功[17−18]。对不成模的小鼠进行记录,3 d后按20 mg/(kg·bw)剂量再次补注STZ注射液。
1.2.4 动物实验设计及分组
造模成功的小鼠随机分为5组(每组8只),各组小鼠每日固定时间灌胃1次,连续灌胃4周,具体实验分组、饲养饲料、灌胃药物及剂量见表1[19]。
表 1 实验动物分组及处理Table 1. Grouping and treatment of experimental animals组别 饲料 药物 剂量 正常对照组(NC) 基础饲料 生理盐水 0.1 mL/(10 g·bw) 模型对照组(MC) 高糖高脂饲料 生理盐水 0.1 mL/(10 g·bw) 阳性对照组(PC) 高糖高脂饲料 盐酸二甲双胍溶液 0.1 mL/(10 g·bw) 饮料低剂量组(L-GLB) 高糖高脂饲料 灵芝多糖饮料 0.025 mL/(10 g·bw) 饮料中剂量组(M-GLB) 高糖高脂饲料 灵芝多糖饮料 0.05 mL/(10 g·bw) 饮料高剂量组(H-GLB) 高糖高脂饲料 灵芝多糖饮料 0.1 mL/(10 g·bw) 注:盐酸二甲双胍溶液的浓度为15 mg/mL,相当于灌胃150 mg/(kg·bw) 剂量。 1.2.5 指标检测
1.2.5.1 小鼠体质量、进食量和饮水量测定
试验期间,定时记录小鼠每日的进食和饮水量;每周固定时间称量小鼠体重。
1.2.5.2 脏器指数的测定
试验结束后,各组小鼠禁食不禁水12 h,脱颈处死小鼠,解剖其肝脏、肾脏和脾脏组织,用生理盐水洗净,滤纸吸干表面水分后称重并记录。各脏器指数按下式计算。
脏器指数(%)=脏器质量(g)体质量(g)×100 1.2.5.3 空腹血糖值(fasting blood glucose,FBG)的测定
灌胃结束后第0、1、2、3、4周的固定时间[20],各组小鼠禁食不禁水6 h,取小鼠尾静脉血测定FBG。
1.2.5.4 口服葡萄糖耐量(oral glucose tolerance test,OGTT)的测定
最后一次灌胃结束,各组小鼠禁食不禁水12 h后,按2 g/(kg·bw)剂量灌胃葡萄糖溶液,分别于灌胃后0、0.5、1、2 h对小鼠的血糖值进行测量,比较各组小鼠在灌胃葡萄糖溶液后各时间点血糖值的变化情况及曲线下面积(area under curve,AUC)[21],AUC计算公式如下:
AUC(mmol/L⋅h)=0.5A+B+C+0.5D2 式中:A、B、C和D分别为灌胃葡萄糖溶液后0、0.5、1、2 h的血糖值(mmol/L)。
1.2.5.5 小鼠血清指标检测
小鼠通过眼眶静脉取血于离心管中,室温静置2 h,4 ℃、3500 r/min离心15 min,收集血清于离心管中,−80 ℃冰箱中冻存。按照试剂盒说明书测定INS、TC、TG、HDL-C、LDL-C、SOD和MDA水平,按下式计算小鼠空腹血清中的胰岛素抵抗指数(homeostatic model assessment of insulin resistance,HOMA-IR)。
HOMA−IR=FBG×INS22.5 1.2.5.6 肝脏组织病理学切片的制备
小鼠肝脏组织经4%多聚甲醛溶液固定处理24 h,脱水后,将肝脏组织包埋于石蜡中切片(厚度约4 μm),并采用苏木精-伊红(hematoxylin-eosin staining,HE)染色法进行染色借助光学显微镜对肝脏组织的病理学变化进行观察,保存图片进行分析。
1.3 数据处理
研究结果采用平均值±标准偏差表示。使用GraphPad Prism 8.0和IBS SPSS 25.0软件进行试验数据的统计分析及图表的绘制,各组数据采用单因素方差分析。
2. 结果与分析
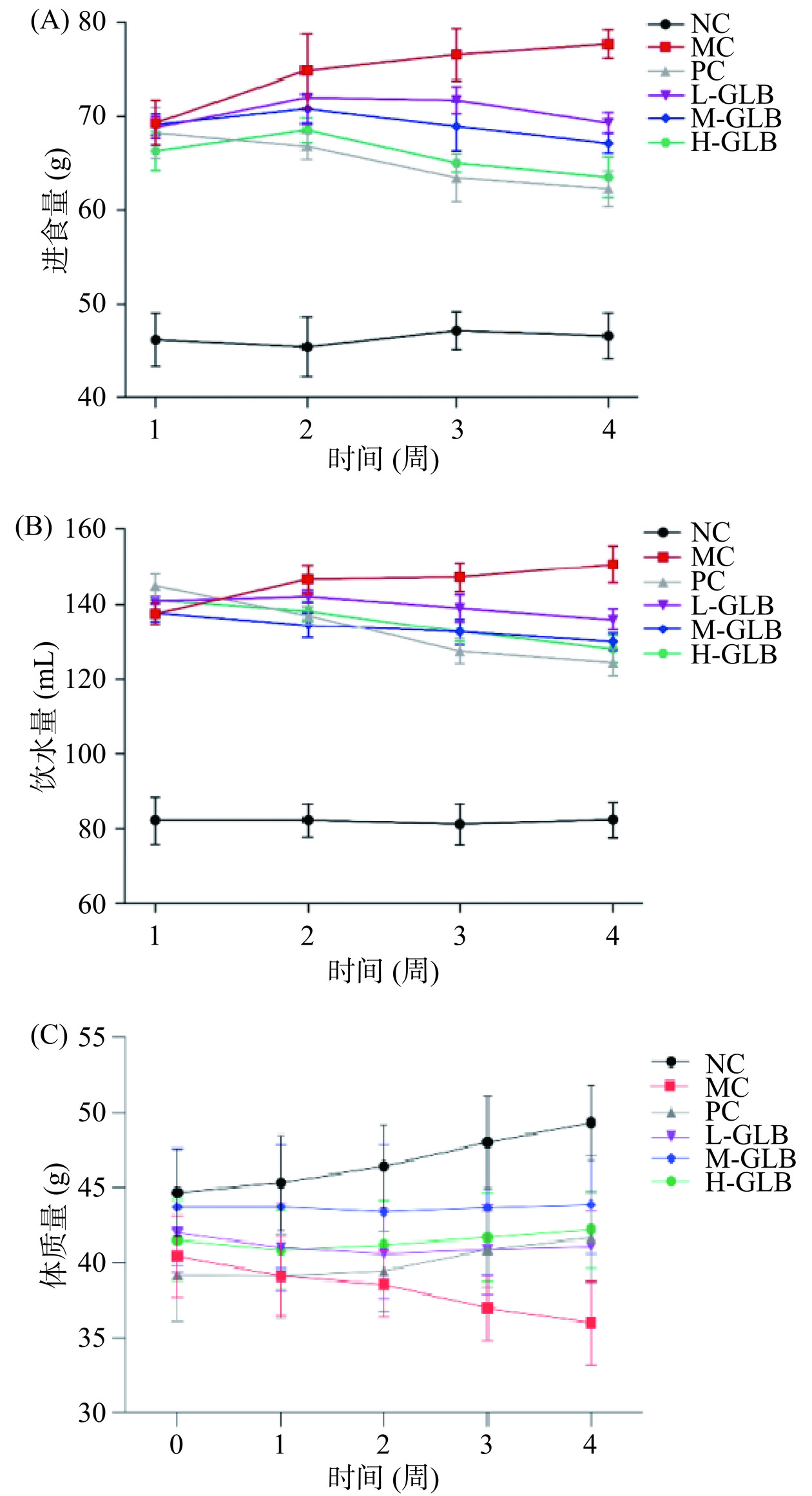
2.1 灵芝多糖饮料对小鼠进食饮水量及体质量的影响
各组小鼠的进食、饮水量、体质量变化情况如图1所示,与NC组相比,MC组小鼠的进食和饮水量呈现上升趋势,体质量呈现下降趋势,DM小鼠多饮多食及体重减轻症状明显。经灌服28 d阳性药物和灵芝多糖饮料后,与MC组相比,PC组及饮料低、中、高剂量组小鼠的进食和饮水量均有不同程度的下降,其中L-GLB组、M-GLB组、H-GLB组的饮食量分别下降10.69%、13.52%、18.24%,饮水量分别下降9.80%、13.75%、15.05%。PC组、M-GLB组和H-GLB组小鼠的体质量较初始体重有所上升,与MC组相比差异显著(P<0.05),其较初始体重增长率分别为6.38%、0.41%和1.75%,而L-GLB组小鼠体重较初始体重下降了2.21%,但显著高于MC组体重(P<0.05),表明灵芝多糖饮料能够改善DM小鼠多饮多食状况,抑制体质量减轻。
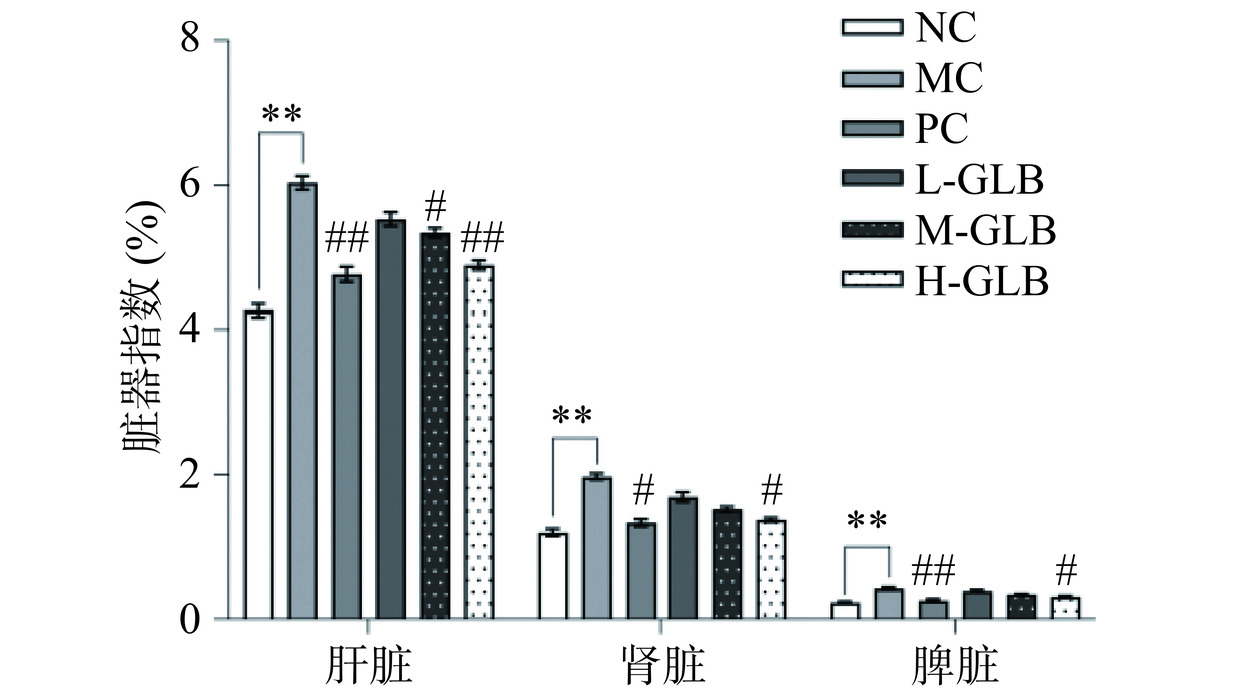
2.2 灵芝多糖饮料对小鼠脏器指数的影响
DM会诱导脏器肿大及肝脏组织病理学损伤。灵芝多糖饮料对DM小鼠脏器指数的影响如图2所示,MC组的肝脏指数、肾脏指数和脾脏指数均极显著高于NC组(P<0.01),与MC组相比,L-GLB组、M-GLB组、H-GLB组的肝脏指数分别降低了8.40%、11.49%、18.91%。与NC组相比,MC组的肾脏指数极显著上升64.25%(P<0.01),表明长期的慢性高血糖使得DM小鼠肾脏受到损伤,产生DM肾病[22],经二甲双胍和灵芝多糖饮料干预后,L-GLB组、M-GLB组、H-GLB组的肾脏指数分别降低23.50%、37.42%、49.67%。与MC组相比,L-GLB组、M-GLB组、H-GLB组的脾脏指数分别降低2.67%、6.67%、9.17%。H-GLB组与MC组比较显著下降(P<0.05)。结果表明,灵芝多糖饮料能够改善DM小鼠脏器肿大,保护DM小鼠的肝脏、肾脏和脾脏。
2.3 灵芝多糖饮料对小鼠FBG的影响
FBG是DM最常用的检测指标。各组小鼠FBG的变化情况如图3所示,造模成功的小鼠FBG均>11.1 mmol/L,且极显著高于NC组(P<0.01)。试验期间,NC组血糖值稳定于正常范围内(3.9~6.1 mmol/L),而MC 组血糖值持续增高至21.97 mmol/L,经4周灌胃治疗后,PC组及灵芝饮料低、中、高剂量组的血糖值均显著低于MC组(P<0.05),其中L-GLB组、M-GLB组、H-GLB 组的FBG与MC组相比分别下降了35.46%、39.05%和42.28%,表明灵芝饮料有效减缓了DM小鼠空腹血糖的上升。Shao等[23]测定了灵芝多糖对2型DM小鼠的降血糖作用,发现高剂量组(180 mg/kg)小鼠的FBG下降42.25%,本实验中灵芝多糖饮料减缓小鼠FBG能力与之效果相近,说明研发的灵芝多糖饮料并未降低灵芝多糖的降糖效果。
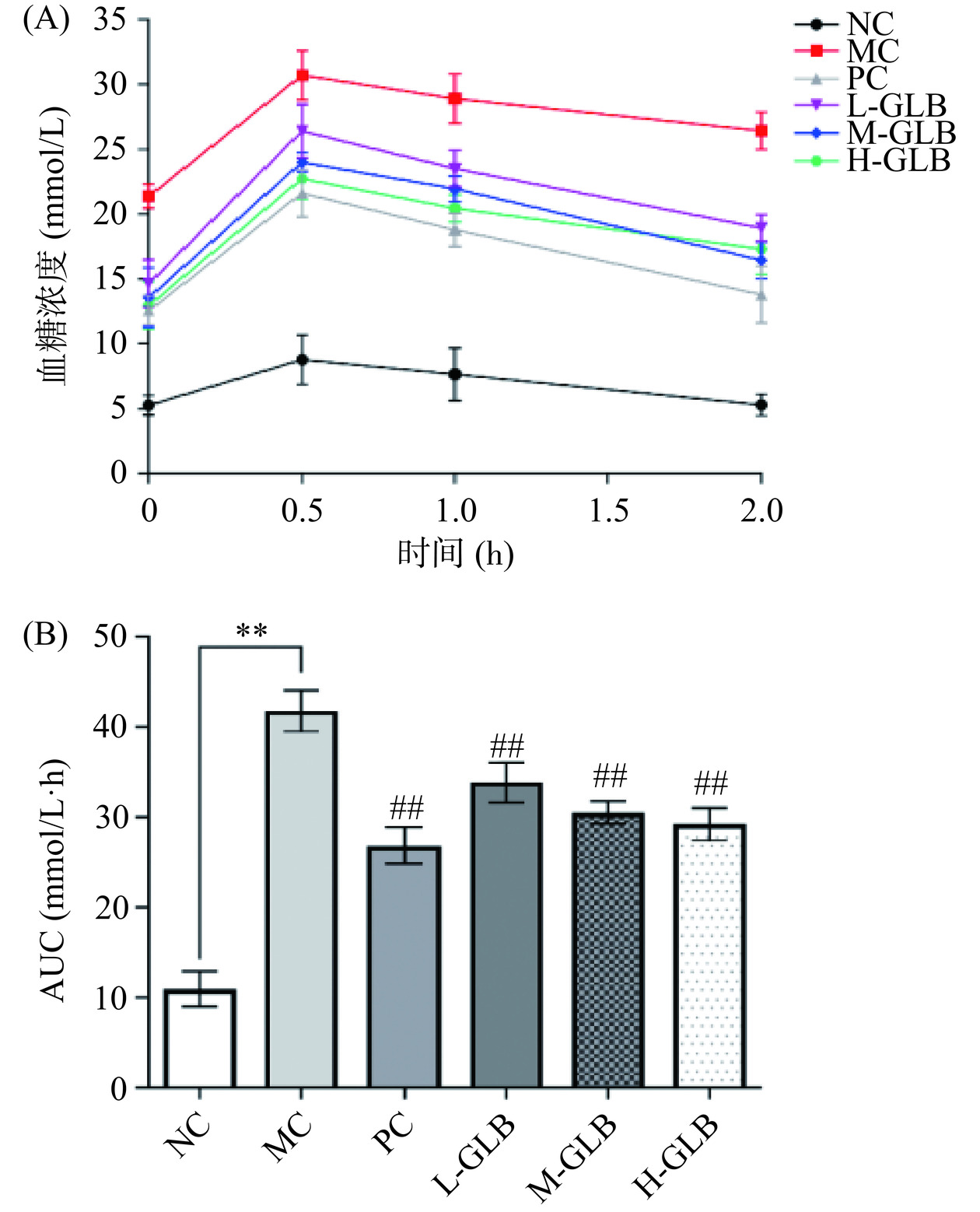
2.4 灵芝多糖饮料对小鼠OGTT及AUC的影响
OGTT是指机体对血糖浓度的调节能力,是评价小鼠血糖代谢调节能力的标准方法[24],通过测定小鼠OGTT及AUC的变化情况,判断灌服灵芝多糖饮料对DM小鼠的血糖代谢调节能力的影响。由图4(A)可知,NC组小鼠的血糖值在2 h后恢复至正常水平5.30 mmol/L,而MC组小鼠的血糖仍保持在较高值26.62 mmol/L,是NC组的5.02倍,与MC组相比,PC组及饮料低、中、高剂量组的血糖值于1.0、2.0 h快速下降,2.0 h后的血糖值与初始水平相近,但未达到正常值范围;由图4(B)可知,MC组的AUC较NC组极显著上升(P<0.01),与MC组相比,PC组及灵芝饮料干预组均能极显著降低DM小鼠的AUC(P<0.01),其中,L-GLB组、M-GLB组和H-GLB组的AUC分别下降了20.12%、26.99%、30.18%。表明灵芝多糖饮料能提高DM小鼠血糖代谢调节能力,稳定其血糖水平。
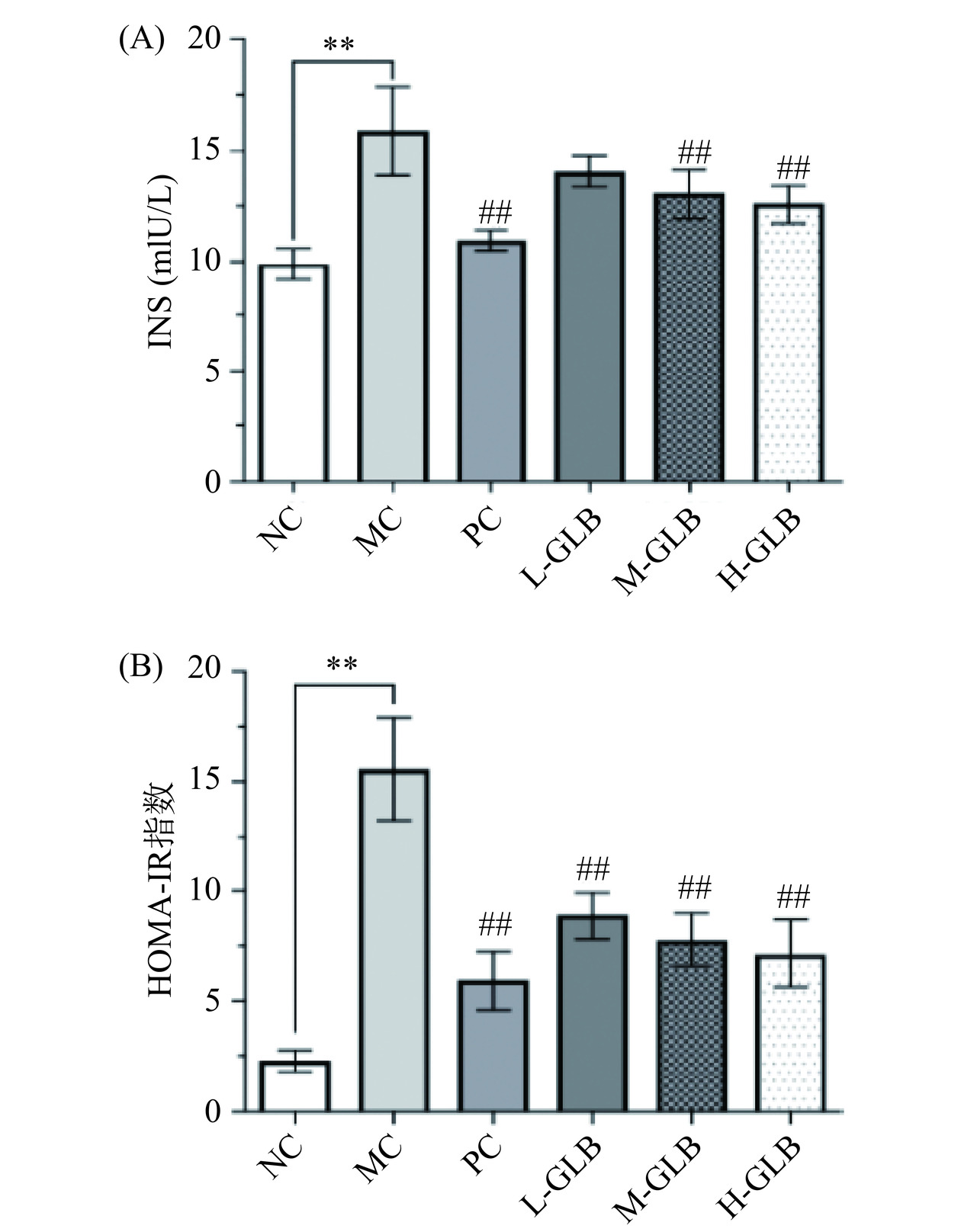
2.5 灵芝多糖饮料对小鼠INS及HOMA-IR的影响
INS为胰岛β细胞分泌的双链多肽激素,是唯一降低血糖的激素。有研究发现灵芝多糖能够改善胰岛β细胞功能障碍,增加胰岛素的分泌量,促进葡萄糖转运和改善葡萄糖氧化作用以降低血糖水平,另可通过增强葡萄糖代谢相关酶的活性来提高胰岛素敏感性,降低胰岛素抵抗[25]。因此测定小鼠血清INS及HOMA-IR的变化情况是考察灵芝多糖饮料降血糖能力的重要指标。如图5所示,MC组小鼠的INS及HOMA-IR极显著高于NC组(P<0.01),说明DM小鼠胰岛素敏感性下降,存在胰岛素抵抗,经阳性药物与灵芝多糖饮料干预后,PC组、M-GLB组、H-GLB组的INS水平与MC组相比极显著降低(P<0.01),且L-GLB组、M-GLB组和H-GLB组的HOMA-IR指数也分别极显著下降43.10%、50.25%、54.18%(P<0.01),结果表明灵芝多糖饮料可以减少DM小鼠胰岛素抵抗,提高胰岛素敏感性。
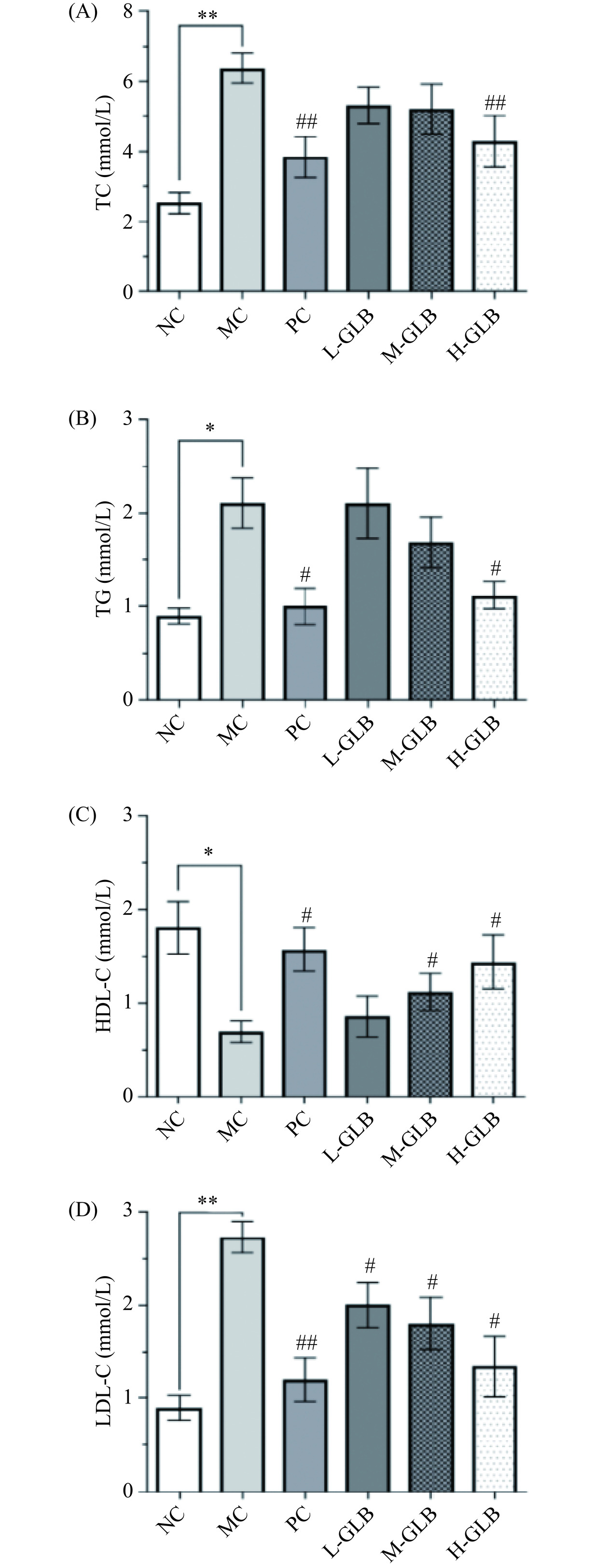
2.6 灵芝多糖饮料对小鼠血脂水平的影响
糖脂代谢间具有密切的关联性,DM患者通常伴有脂代谢异常的情况,而机体中血脂水平的变化可以用来衡量脂代谢情况,因此控制血脂水平被认为和控制血糖同等重要[26]。如图6所示,与NC组相比,MC组小鼠TC、TG和LDL-C水平均显著或极显著上升(P<0.05或P<0.01),HDL-C水平显著下降(P<0.05),表明DM小鼠的血脂代谢紊乱;经灌服阳性药物和灵芝多糖饮料后,H-GLB组小鼠的TC水平较MC组比较极显著降低(P<0.01),L-GLB组与M-GLB组降低效果不显著(P>0.05);L-GLB组的TG水平较MC组差异不大,M-GLB组TG水平降低19.63%,H-GLB组TG水平显著降低46.83%(P<0.05);L-GLB组、M-GLB组及H-GLB组小鼠LDL-C水平与MC组相比分别显著下降了26.37%、33.83%、50.79%(P<0.05);与MC组相比,M-GLB组和H-GLB组HDL-C水平分别显著上升61.30%、107.81%(P<0.05)。表明灵芝多糖饮料具有改善DM小鼠血脂代谢紊乱,降低血脂水平的作用。Yang等[20]研究了党参粗多糖对高脂饮食和STZ诱导的2型DM小鼠降血糖作用,研究发现党参粗多糖干预能降低血清TG、TC和LDL-C浓度,同时提高HDL-C浓度,改善脂代谢紊乱,与本研究结果一致。
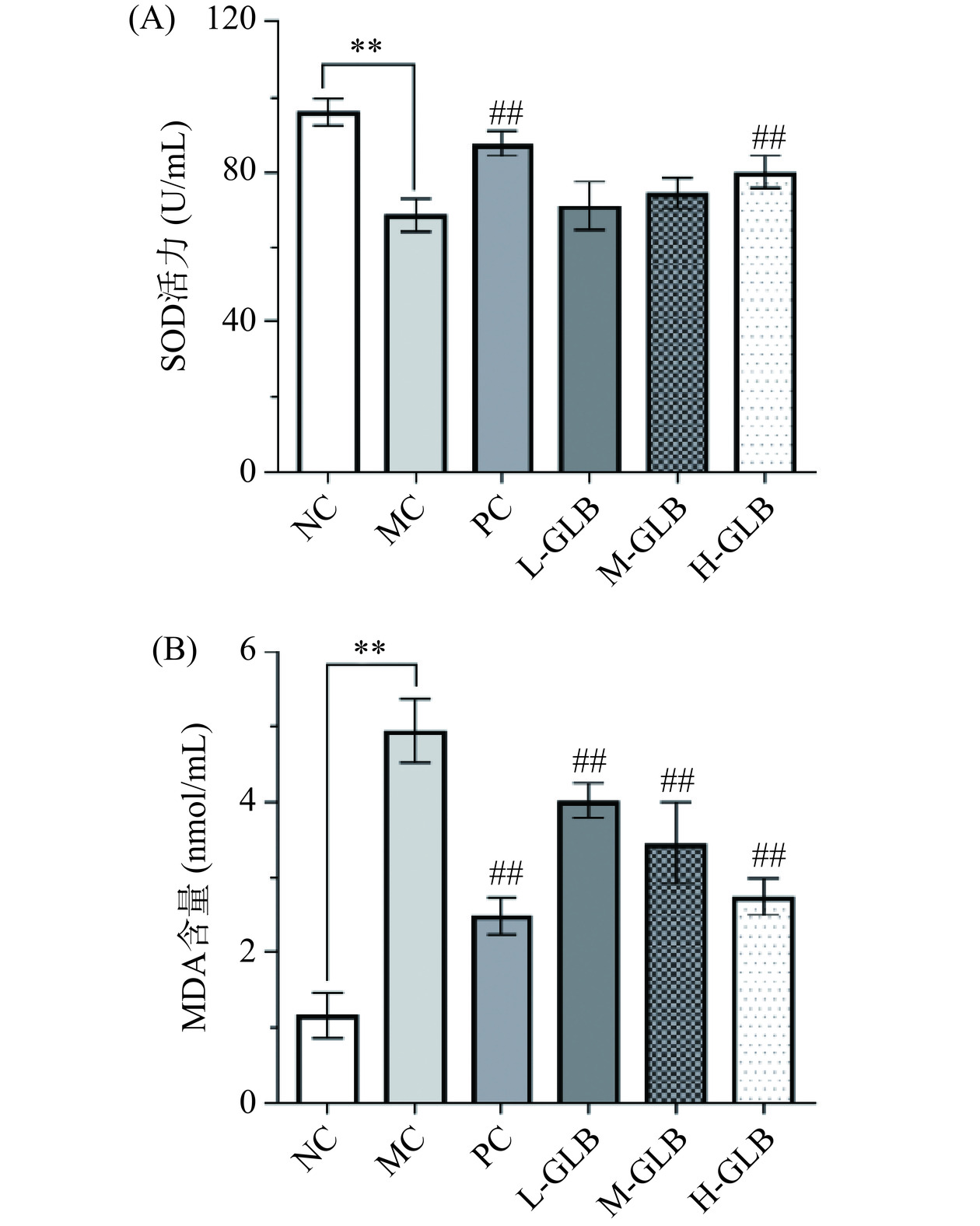
2.7 灵芝多糖饮料对小鼠氧化应激水平的影响
DM引起的血糖浓度长期升高会导致机体内活性氧形成过量或抗氧化能力不足,即发生氧化应激[27],引起DM患者胰岛β细胞及胰岛素抗体损伤的进一步加剧,这也是DM发生的原因之一[28]。因此本研究测定了小鼠模型中SOD活力与MDA含量的变化情况,由图7可知,MC组的SOD活力较NC组极显著下降(P<0.01),MDA含量极显著增高(P<0.01),说明本实验小鼠模型发生氧化损伤。经灌服阳性药物与灵芝多糖饮料后,各组的SOD活力均升高,其中PC组和H-GLB组分别极显著增加27.35%、16.54%(P<0.01);L-GLB组、M-GLB组、H-GLB组分别极显著下降18.66%、30.15%、44.54%(P<0.01),其中高剂量组与PC组缓解氧化应激能力相近,证明灵芝多糖饮料可提高抗氧化酶系统活力,降低脂质过氧化损伤水平,调节DM引发的氧化应激。与本研究结果类似,毛美林等[29]探究榆黄蘑菌丝体多糖的降血糖作用时测定相关的抗氧化指标,结果表明其能提高抗氧化酶活性,降低DM小鼠血清和肝脏中的活性氧水平,减轻机体细胞的活性氧损伤,加速糖代谢并增强胰岛素敏感性。
2.8 灵芝多糖饮料对小鼠肝脏病理学形态的影响
肝脏是胰岛素的靶器官,是维持血糖的重要器官[30],DM引发的氧化应激会造成肝损伤。因此通过对小鼠的肝组织进行病理分析,判断灵芝多糖饮料对小鼠肝损伤的作用效果。如图8所示,NC组小鼠肝脏组织细胞排列整齐,组织结构正常;MC组小鼠肝细胞组织排列紊乱,胞质中可见大小不一的脂肪空泡,脂肪面积较大,出现大量脂肪滴,轻微发炎;与MC组相比,L-GLB组和M-GLB组小鼠肝脏中脂肪空泡体积及数量均减小,脂肪变性得到了一定程度的抑制;PC组和H-GLB组小鼠肝脏脂质积累明显改善,组织结构趋近于正常,表明灵芝多糖饮料可在一定程度上改善由长期高血糖导致的DM小鼠肝脏损伤。Zhou等[31]在探究黑洋葱多糖能在治疗DM的同时对肝脏和肾脏产生协同的保护作用时,通过观察肝脏组织切片发现黑洋葱多糖治疗可改善病理性肝组织的变化,证实黑洋葱多糖能对DM引起的肝损伤起到保护作用,与本研究结果一致。
3. 结论
本研究实验表明灵芝多糖饮料可改善DM小鼠多饮多食及体质量负增长;灌服灵芝多糖饮料可有效减缓DM小鼠FBG的上升,改善糖耐量,降低AUC,并使DM小鼠血清中 INS水平及HOMA-IR指数下降,能较好地改善DM引发的糖代谢紊乱,减少胰岛素抵抗;不同剂量的灵芝多糖饮料均可降低DM小鼠血清中TC、TG、LDL-C水平,提升HDL-C含量,改善血脂代谢紊乱,下调过高的血脂水平;灵芝多糖饮料可上调DM小鼠血清中SOD活力,下调MDA含量,提高抗氧化酶系统活力,降低脂质过氧化损伤,调节DM引发的氧化应激,缓解DM诱发的脏器肿大的状况,对DM小鼠的肝脏、肾脏和脾脏的损伤均起到一定的修复作用,此研究为灵芝功能性食品的深度开发提供了理论基础,丰富了辅助降糖产品的种类。
-
表 1 实验动物分组及处理
Table 1 Grouping and treatment of experimental animals
组别 饲料 药物 剂量 正常对照组(NC) 基础饲料 生理盐水 0.1 mL/(10 g·bw) 模型对照组(MC) 高糖高脂饲料 生理盐水 0.1 mL/(10 g·bw) 阳性对照组(PC) 高糖高脂饲料 盐酸二甲双胍溶液 0.1 mL/(10 g·bw) 饮料低剂量组(L-GLB) 高糖高脂饲料 灵芝多糖饮料 0.025 mL/(10 g·bw) 饮料中剂量组(M-GLB) 高糖高脂饲料 灵芝多糖饮料 0.05 mL/(10 g·bw) 饮料高剂量组(H-GLB) 高糖高脂饲料 灵芝多糖饮料 0.1 mL/(10 g·bw) 注:盐酸二甲双胍溶液的浓度为15 mg/mL,相当于灌胃150 mg/(kg·bw) 剂量。 -
[1] MA Quantao, LI Yaqi, LI Pengfei, et al. Research progress in the relationship between type 2 diabetes mellitus and intestinal flora[J]. Biomedicine & Pharmacotherapy,2019,117:109−138.
[2] 马永强, 张凯, 王鑫, 等. 甜玉米芯多糖对糖尿病大鼠的降血糖作用[J]. 食品科学,2020,41(13):169−173. [MA Yongqiang, ZHANG Kai, WANG Xin, et al. Hypoglycaemic effects of sweet corn kernel polysaccharides in diabetic rats[J]. Food Science,2020,41(13):169−173.] doi: 10.7506/spkx1002-6630-20190702-030 MA Yongqiang, ZHANG Kai, WANG Xin, et al. Hypoglycaemic effects of sweet corn kernel polysaccharides in diabetic rats[J]. Food Science, 2020, 41(13): 169−173. doi: 10.7506/spkx1002-6630-20190702-030
[3] HAN Xiao, YANG Fei, ZHANG Zhengyi, et al. 4EBP2-regulated protein translation has a critical role in high-fat diet–induced insulin resistance in hepatocytes[J]. Journal of Biological Chemistry,2023,299(11):105315−105315. doi: 10.1016/j.jbc.2023.105315
[4] 刘芬芬, 蒲首丞, 赵雯靓, 等. 金花茶花对2型糖尿病小鼠的降糖及抗氧化作用[J]. 食品科学, 2024, 45(3): 94-101. [LIU Fenfen, PU Shousheng, ZHAO Wenliang, et al. Hypoglycaemic and antioxidant effects of Camellia sinensis on type 2 diabetic mice[J]. Food Science, 2024, 45(3): 94-101.] LIU Fenfen, PU Shousheng, ZHAO Wenliang, et al. Hypoglycaemic and antioxidant effects of Camellia sinensis on type 2 diabetic mice[J]. Food Science, 2024, 45(3): 94-101.
[5] LI Jia, GU Feifei, CAI Chao, et al. Purification, structural characterization, and immunomodulatory activity of the polysaccharides from Ganoderma lucidum[J]. International Journal of Biological Macromolecules,2020,143(C):806−813.
[6] MARCELA R D C, TALITA F F, KELLY K I, et al. Ganoderma lucidum polysaccharides inhibit in vitro tumorigenesis, cancer stem cell properties and epithelial-mesenchymal transition in oral squamous cell carcinoma[J]. Journal of Ethnopharmacology,2021,286(25):114891−114905.
[7] 吴睿婷, 付王威, 万敏, 等. 黑灵芝多糖对糖尿病大鼠血糖血脂调节及肠道菌群的影响[J]. 食品科学,2022,43(5):91−102. [WU Ruiting, FU Wangwei, WAN Min, et al. Effects of black Ganoderma lucidum polysaccharides on the regulation of blood glucose, blood lipids and intestinal flora in diabetic rats[J]. Food Science,2022,43(5):91−102.] doi: 10.7506/spkx1002-6630-20201203-046 WU Ruiting, FU Wangwei, WAN Min, et al. Effects of black Ganoderma lucidum polysaccharides on the regulation of blood glucose, blood lipids and intestinal flora in diabetic rats[J]. Food Science, 2022, 43(5): 91−102. doi: 10.7506/spkx1002-6630-20201203-046
[8] KANG Qiaozhen, CHEN Sisi, LI Shufang, et al. Comparison on characterization and antioxidant activity of polysaccharides from Ganoderma lucidum by ultrasound and conventional extraction[J]. International Journal of Biological Macromolecules,2018,124(C):1137−1144.
[9] LI Lu, XU Jiaxin, CAO Yingjia, et al. Preparation of Ganoderma lucidum polysaccharide chromium (III) complex and its hypoglycemic and hypolipidemic activities in high-fat and high-fructose diet-induced pre-diabetic mice[J]. International Journal of Biological Macromolecules,2019,140:782−793. doi: 10.1016/j.ijbiomac.2019.08.072
[10] REN Lei. Protective effect of ganoderic acid against the streptozotocin induced diabetes, inflammation, hyperlipidemia and microbiota imbalance in diabetic rats[J]. Saudi Journal of Biological Sciences,2019,26(8):1961−1972. doi: 10.1016/j.sjbs.2019.07.005
[11] 陈彦君, 刘家宏, 张翔, 等. 多元复合抗性淀粉对高糖高脂模型小鼠代谢调节作用及机制[J]. 食品工业科技,2021,42(19):357−362. [CHEN Yanjun, LIU Jiahong, ZHANG Xiang, et al. Metabolic regulation and mechanism of multivariate composite resistant starch in high glucose and high fat model mice[J]. Food Industry Science and Technology,2021,42(19):357−362.] CHEN Yanjun, LIU Jiahong, ZHANG Xiang, et al. Metabolic regulation and mechanism of multivariate composite resistant starch in high glucose and high fat model mice[J]. Food Industry Science and Technology, 2021, 42(19): 357−362.
[12] 丁霄霄. 灵芝活性成分的制备及其残渣利用[D]. 镇江:江苏大学, 2019:22-27. [Ding Xiaoxiao. Preparation of active ingredients of Ganoderma lucidum and its residue utilisation[D]. Zhenjiang:Jiangsu University, 2019:22-27.] Ding Xiaoxiao. Preparation of active ingredients of Ganoderma lucidum and its residue utilisation[D]. Zhenjiang: Jiangsu University, 2019: 22-27.
[13] SHWETA D, SUMIT G, REKHA S S, et al. Debittering of bitter gourd juice using β-cyclodextrin:Mechanism and effect on antidiabetic potential[J]. Food Chemistry,2018,262(1):78−85.
[14] 王佳瑶, 李源, 李一鸣, 等. 玉米副产物发酵饮料对链脲佐菌素诱导的糖尿病小鼠糖脂代谢的改善作用[J]. 食品工业科技,2023,44(4):395−402. [WANG Jiayao, LI Yuan, LI Yiming, et al. Improvement of glycolipid metabolism in streptozotocin-induced diabetic mice by corn by-product fermented beverage[J]. Food Industry Science and Technology,2023,44(4):395−402.] WANG Jiayao, LI Yuan, LI Yiming, et al. Improvement of glycolipid metabolism in streptozotocin-induced diabetic mice by corn by-product fermented beverage[J]. Food Industry Science and Technology, 2023, 44(4): 395−402.
[15] 常松林, 高晓余, 柳双凤, 等. α-亚麻酸对高脂饮食和链脲佐菌素诱导Ⅱ型糖尿病模型小鼠的降血糖作用[J]. 中国食品学报,2021,21(10):86−94. [CHANG Songlin, GAO Xiaoyu, LIU Shuangfeng, et al. Hypoglycaemic effects of α-linolenic acid in mice with high-fat diet and streptozotocin-induced type II diabetes[J]. Chinese Journal of Food,2021,21(10):86−94.] CHANG Songlin, GAO Xiaoyu, LIU Shuangfeng, et al. Hypoglycaemic effects of α-linolenic acid in mice with high-fat diet and streptozotocin-induced type II diabetes[J]. Chinese Journal of Food, 2021, 21(10): 86−94.
[16] WEI Xiaoyan, TAO Jinhua, SHEN Yumeng, et al. Sanhuang Xiexin Tang ameliorates type 2 diabetic rats via modulation of the metabolic profiles and NF-κB/PI-3K/Akt signaling pathways[J]. Frontiers in Pharmacology,2018,9:955−971. doi: 10.3389/fphar.2018.00955
[17] GUO Xiaoxuan, WANG Yong, WANG Kai, et al. Stability of a type 2 diabetes rat model induced by high-fat diet feeding with low-dose streptozotocin injection[J]. Journal of Zhejiang University-Science B,2018,19(7):559−569. doi: 10.1631/jzus.B1700254
[18] MARC A B, ABDALLAH D, KHALIL H, et al. Microvascular vasodilator properties of the angiotensin II type 2 receptor in a mouse model of type 1 diabetes[J]. Scientific Reports,2017,7(1):425−456. doi: 10.1038/s41598-017-00545-6
[19] 何静, 高婉婷, 明亮, 等. 驼乳对Ⅱ型糖尿病小鼠的糖脂代谢及胰岛素抵抗的影响[J]. 中国食品学报,2019,19(3):115−120. [HE Jing, GAO Wanting, MING Liang, et al. Effects of camel's milk on glucose-lipid metabolism and insulin resistance in type II diabetic mice[J]. Chinese Journal of Food,2019,19(3):115−120.] HE Jing, GAO Wanting, MING Liang, et al. Effects of camel's milk on glucose-lipid metabolism and insulin resistance in type II diabetic mice[J]. Chinese Journal of Food, 2019, 19(3): 115−120.
[20] YANG Mingjun, LÜ Jinhui, YANG Jumei, et al. Effects of Codonopsis pilosula crude polysaccharides by hypoglycemic and modulating gut microbiome in a high-fat diet and streptozotocin-induced mouse model of T2DM[J]. Journal of Functional Foods,2023,111:105893. doi: 10.1016/j.jff.2023.105893
[21] XU Tanye, LI Guodao, WANG Xiaobo, et al. Inonotus obliquus polysaccharide ameliorates serum profiling in STZ-induced diabetic mice model[J]. Biomedcentral Chemistry,2021,15(1):64.
[22] 万凤奇. 枸杞多糖调节肝脏葡萄糖产生和抗糖尿病肾炎的作用研究[D]. 兰州:中国科学院大学(中国科学院近代物理研究所), 2023:61−73. [WAN Fengqi. Regulation of hepatic glucose production and anti-diabetic nephritis by polysaccharides of Lycium barbarum[D]. Lanzhou:University of Chinese Academy of Sciences (Institute of Modern Physics, Chinese Academy of Sciences), 2023:61−73.] WAN Fengqi. Regulation of hepatic glucose production and anti-diabetic nephritis by polysaccharides of Lycium barbarum[D]. Lanzhou: University of Chinese Academy of Sciences (Institute of Modern Physics, Chinese Academy of Sciences), 2023: 61−73.
[23] SHAO Weimimg, XIAO Chun, YONG Tianqiao, et al. A polysaccharide isolated from Ganoderma lucidum ameliorates hyperglycemia through modulating gut microbiota in type 2 diabetic mice[J]. International Journal of Biological Macromolecules,2021,197(1):23−38.
[24] ARMIN A, M N H, BRIAN J B, et al. Chronic kidney disease is associated with attenuated plasma metabolome response to oral glucose tolerance testing[J]. Journal of Renal Nutrition:the Official Journal of the Council on Renal Nutrition of the National Kidney Foundation,2022,33(2):316−325.
[25] 陈嘉骏, 王颖, 桑婷婷, 等. 灵芝多糖在糖尿病及其并发症防治中的研究进展[J]. 中草药,2022,53(3):937−947. [CHEN Jiajun, WANG Ying, SAN Tingting, et al. Research progress of Ganoderma lucidum polysaccharides in the prevention and treatment of diabetes mellitus and its complications[J]. Chinese Herbal Medicine,2022,53(3):937−947.] CHEN Jiajun, WANG Ying, SAN Tingting, et al. Research progress of Ganoderma lucidum polysaccharides in the prevention and treatment of diabetes mellitus and its complications[J]. Chinese Herbal Medicine, 2022, 53(3): 937−947.
[26] WANG Wenshuai, ZHANG Yaohan, WANG Zhiying, et al. Ganoderma lucidum polysaccharides improve lipid metabolism against high-fat diet-induced dyslipidemia[J]. Journal of Ethnopharmacology,2023,309(12):116321−116332.
[27] WANG Xutao, GONG Yan, ZHOU Bin, et al. Ursolic acid ameliorates oxidative stress, inflammation and fibrosis in diabetic cardiomyopathy rats[J]. Biomedicine & Pharmacotherapy,2018,97:1461−1467.
[28] LI Xiaopeng, WU Qian, SUI Yong, et al. Dietary supplementation of A-type procyanidins from litchi pericarp improves glucose homeostasis by modulating mTOR signaling and oxidative stress in diabetic ICR mice[J]. Journal of Functional Foods,2018,44:155−165. doi: 10.1016/j.jff.2017.12.024
[29] 毛美林, 邓子言, 李万晴, 等. 一种榆黄蘑菌丝体多糖的结构表征及其降血糖作用[J]. 食品科学,2023,44(11):115−123. [MAO Meilin, Deng Ziyan, LI Wanqing, et al. Structural characterisation of a mycelial polysaccharide from Elm mushrooms and its hypoglycemic effect[J]. Food Science,2023,44(11):115−123.] doi: 10.7506/spkx1002-6630-20220715-178 MAO Meilin, Deng Ziyan, LI Wanqing, et al. Structural characterisation of a mycelial polysaccharide from Elm mushrooms and its hypoglycemic effect[J]. Food Science, 2023, 44(11): 115−123. doi: 10.7506/spkx1002-6630-20220715-178
[30] DONG Xuan, ZHAO Shuxiang, YIN Xiaolu, et al. Silk sericin has significantly hypoglycaemic effect in type 2 diabetic mice via anti-oxidation and anti-inflammation[J]. International Journal of Biological Macromolecules,2020,150(1):1061−1071.
[31] ZHOU Ning, ZHAO Ye, ZHANG Lingang , et al. Protective effects of black onion polysaccharide on liver and kidney injury in T2DM rats through the synergistic impact of hypolipidemic and antioxidant abilities[J]. International Journal of Biological Macromolecules,2022,223(Pt A):378−390.





 下载:
下载:


 下载:
下载:



