Simultaneous Determination of Nine Chlorogenic Acids and Flavonoids in Mori Folium by QAMS
-
摘要: 目的:建立一测多评法同时测定桑叶中新绿原酸、绿原酸、隐绿原酸、芦丁、异槲皮苷、异绿原酸B、紫云英苷、异绿原酸A和异绿原酸C的含量。方法:采用Agilent TC-C18色谱柱(250 mm×4.6 mm,5 μm),以乙腈(A)-0.1%磷酸水溶液(B)为流动相,梯度洗脱,流速1.0 mL/min,检测波长260、320 nm,柱温30 ℃。以绿原酸为内参物,分别采用外标法和一测多评(多点校正、斜率校正和单点校正)测定桑叶中9个成分的含量并比较4种方法之间的差异,另外采用多点校正和两点校正法进行色谱峰定位,验证该方法的准确性和可行性。结果:新绿原酸、隐绿原酸、芦丁、异槲皮苷、异绿原酸B、紫云英苷、异绿原酸A和异绿原酸C相对于绿原酸的校正因子分别为0.9072、0.8736、0.6207、0.8547、1.1936、0.5501、1.4369和1.2244(多点校正),且在不同条件下相对校正因子耐用性(RSD<1.5%)和重现性(RSD<5%)良好。外标法和一测多评(多点校正、斜率校正和单点校正)测得桑叶中9个成分含量的结果之间无差异(RSD<1.5%)。相较于相对保留时间,多点校正和两点校正可改善色谱峰的准确定位(相对误差|RE|<3%)。结论:建立了桑叶中9种成分的一测多评法,该法准确可行,可用于桑叶的质量控制。Abstract: Objective: To establish a HPLC method for the determination of neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid, rutin, isoquercitrin, isochlorogenic acid B, astragalin, isochlorogenic acid A and isochlorogenic acid C in Mori Folium using the quantitative analysis of multi-components by single marker (QAMS). Methods: The analysis was performed on an Agilent TC-C18 column (250 mm×4.6 mm, 5 μm), with acetonitrile (A)-0.1% phosphoric acid (B) as mobile phase at the flow rate of 1.0 mL/min for gradient elution, as well as the wavelengths were 260 and 320 nm and the column temperature was 30 ℃. The chlorogenic acid was used as the internal reference. The contents of nine components in Mori Folium were calculated by the external standard method and QAMS (multi-point correction, gradient correction and single-point correction) respectively, and the differences among the four methods were compared. In addition, the accuracy and feasibility of the method were verified by using multi-point correction and two-point correction to locate chromatographic peaks of the components. Results: The relative correction factors of neochlorogenic acid, cryptochlorogenic acid, rutin, isoquercitrin, isochlorogenic acid B, asiaticoside, isochlorogenic acid A and isochlorogenic acid C to chlorogenic acid were 0.9072, 0.8736, 0.6207, 0.8547, 1.1936, 0.5501, 1.4369 and 1.2244, respectively (multi-point correction). The durability (RSD<1.5%) and the reproducibility (RSD<5%) of the relative correction factors were positive under different conditions. The content of nine components in Mori Folium was determined simultaneously by the external standard method and QAMS (multi-point correction, gradient correction and single-point correction), and there was no difference between the results obtained from the four calculation methods (RSD<1.5%). Compared to the relative retention time, multi-point correction and two-point correction could improve the accurate localization of the chromatographic peaks (relative error |RE|<3%). Conclusion: The method of QAMS for nine components determination in Mori Folium was established, which was accurate and feasible and could be applied for the quality control of Mori Folium.
-
桑叶为桑科植物桑(Morus alba L.)的干燥叶片,于2005年被中国药典收录,具有疏散风热,清肺润燥,清肝明目的功效[1]。现有研究表明,桑叶中主要活性成分为多酚类以及生物碱类、多糖类等[2−5]。课题组前期建立桑叶HPLC指纹图谱时指认出新绿原酸、隐绿原酸、芦丁、异槲皮苷、异绿原酸B、紫云英苷、异绿原酸A和异绿原酸C 9种成分,而绿原酸类和芦丁类黄酮均与桑叶的抗炎、抗氧化、抗菌等药理活性密切相关[6−10]。目前,2020年版中国药典仅对桑叶中芦丁含量进行了下限规定[1],然而单指标成分含量评价其质量具有一定局限性。已有学者通过建立绿原酸、芦丁等多成分含量测定方法评价桑叶质量[11−13],但个别成分含量极低不具通用性,且外标法测定对标准品的需求较大,限制了桑叶多指标质量控制的建立和应用推广。王智民教授等[14]于2006年首次提出的一测多评法(Quantitative Analysis of Multi-components by Single marker,QAMS)已被广泛应用于中药、食品等领域的多成分质量评价方法的建立[15−16],并获得《中国药典》《美国药典》等国内、国际法定检验机构的认可[17−19]。本研究借鉴“以一评多”的特点,以桑叶中含量最高的绿原酸为内参物,通过建立QAMS法对上述另外8种化学成分进行同步测定,并与外标法测定结果进行比较,评价该技术的可行性与适用性,以期为桑叶的多指标质量控制提供参考。
1. 材料与方法
1.1 材料与仪器
桑叶 10批样品信息见表1,经新疆医科大学肖辉教授鉴定均为桑(Morus alba L.)干燥叶;乙腈、甲醇 色谱纯,德国Meker公司;磷酸 分析纯,天津市光复精细化工研究所;水 超纯水;芦丁(批号:DSTDL001701)、紫云英苷(批号:DSTDZ000102)、异槲皮苷(批号:DSTDY000603)、新绿原酸(批号:DSTDX001504)、隐绿原酸(批号:DST221220-035)、绿原酸(批号:DSTDL002103)、异绿原酸A(批号:DSTDY003603)、异绿原酸B(批号:DSTDY003703)、异绿原酸C(批号:DSTDY003804)质量分数均≥98% 成都德思特生物技术有限公司。
表 1 桑叶样品信息Table 1. Information table of Mori Folium samples样品编号 采收日期/批次 产地 来源 S1 2022.7 新疆托克逊 采收 S2 2212079 广西 购买 S3 20221122 陕西商洛 购买 S4 2022.7 新疆吐鲁番 采收 S5 2022.7 四川宜宾 采收 S6 2022.7 四川宜宾 采收 S7 2022.6 新疆喀什 采收 S8 200901 河南南阳 购买 S9 121123-201806 国标物质 购买 S10 20200417 安徽亳州 购买 Waters ACQUITY UPLC H-CLASS型高效液相色谱仪 沃特世科技有限公司;UltiMate-3000型高效液相色谱仪 赛默飞世尔科技公司;色谱柱(规格均为250 mm×4.6 mm,5 μm)Agilent TC-C18、Agilent-Eclipse Plus C18 安捷伦科技有限公司;SHIMADZU Inertsil ODS-3色谱柱 日本岛津公司;MS205DU型十万分之一电子分析天平、ME802型电子天平 梅特勒托利多仪器有限公司;KQ-600 KED型数控超声波清洗器 昆山市超声仪器有限公司;3-30K型离心机 德国Sigma公司;U2型超纯水仪 四川优普超纯科技有限公司。
1.2 实验方法
1.2.1 供试品溶液的制备
10批桑叶样品粉碎过60目筛。精密称取桑叶粉末1.00 g置具塞锥形瓶中,1:50料液比加入50%甲醇溶液适量,超声提取30 min(功率250 W,频率40 kHz),取出,7000 r/min离心10 min,上清液转至50 mL棕色容量瓶中,用50%甲醇定容至刻度,以0.22 μm微孔滤膜过滤至样品小瓶,待测。
1.2.2 对照品溶液的制备
分别精密称定各对照品适量,置于20 mL棕色容量瓶中,以甲醇溶解制成含有新绿原酸1.3700 mg/mL、绿原酸2.5290 mg/mL、隐绿原酸1.1345 mg/mL、芦丁1.0430 mg/mL、异槲皮苷1.2000 mg/mL、异绿原酸B 1.9225 mg/mL、紫云英苷0.5620 mg/mL、异绿原酸A 0.5945 mg/mL和异绿原酸C 0.5085 mg/mL的混合对照品储备液,置−20 ℃冰箱避光保存备用。使用前用初始流动相稀释至所需质量浓度,即得。
1.2.3 液相色谱条件
色谱柱:Agilent TC-C18(4.6×250 mm,5 μm);流动相:乙腈(A)-0.1%磷酸水(B);梯度洗脱(0~6 min,8%~12%A;6~15 min,12%~30%A;15~25 min,30%~40%A;25~26 min,40%~8%A);波长切换(0~13 min,320 nm;13~17.4 min,260 nm;17.4~26 min,320 nm);流速:1.0 mL/min;柱温:30 ℃;进样量:10 μL。
1.2.4 QAMS方法学考察
1.2.4.1 系统适应性
精密吸取混合对照品溶液和桑叶供试品溶液各10 μL,根据1.2.3色谱条件进样检测。
1.2.4.2 线性关系
分别依次精密吸取1.2.2项混合对照品储备液5、20、50、100、200、400 μL置5 mL棕色容量瓶中,用初始流动相稀释定容至刻度,混匀,制得系列不同质量浓度的混合对照品溶液I~VI,按1.2.3项色谱条件依次进样测定,记录峰面积。
1.2.4.3 精密度、重复性和稳定性
精密取1.2.2项下混合对照品溶液适量,连续进样测定6次,进行精密度试验;精密称定同一批次桑叶样品6次,按1.2.1项方法平行制备6份供试品溶液,进样测定,以外标法计算各成分含量,进行重复性试验;取同一桑叶供试品溶液,分别于室温下0、2、4、6、14、26 h进样测定,进行稳定性试验。
1.2.4.4 加样回收率
取同一桑叶样品12份,每份0.50 g,精密称定,将其分成4组,每组3份,随机选择3组分别精密添加含新绿原酸1.2121 mg/mL,绿原酸2.2374 mg/mL,隐绿原酸1.0109 mg/mL,芦丁0.9210 mg/mL,异槲皮苷1.0605 mg/mL,异绿原酸B 1.6938 mg/mL,紫云英苷0.5159 mg/mL,异绿原酸A 0.4969 mg/mL,异绿原酸C 0.4430 mg/mL的混合对照品溶液250、375、500 μL,4组均按照1.2.1项方法制备供试溶液,按1.2.3项色谱条件进样测定,进行加样回收率试验。
1.2.4.5 相对校正因子的3种计算方法
a. 多点校正法:在一定的线性范围,成分的量与检测器响应成正比,以多个浓度下fi/s的平均值作为相对校正因子(Relative Correction Factor,RCF)[17]。本研究采用1.2.2项系列不同质量浓度的混合对照品溶液I~VI的质量浓度和色谱峰峰面积,以桑叶中含量较高且对照品价廉易得的绿原酸为内参物,运用公式fi/s=fi/fs=(Ai/Ci)/(As/Cs)=AiCs/AsCi,式中,f、C和A分别代表RCF、质量浓度和峰面积,i和s分别代表待测成分和内参物,计算各成分RCF。
b. 斜率校正法:根据文献[20],标准曲线公式A=a·C+b(A为色谱峰面积,C为质量浓度,a为斜率,b为截距),当a/b>100时,b/a可忽略不计,得C=A/a,可以根据标准曲线斜率之比快速计算相对校正因子fi/s=ai/as,待测成分浓度为Ci=Ai/ai=Ai/(as·fi/s),式中,ai为待测成分标准曲线斜率,as为参照物标准曲线斜率,Ai为待测成分峰面积。
c.单点校正法:根据文献[21−22],以外标法浓度点计算相对校正因子。外标法公式为Ai′/Ci′=Ai/Ci,Ci′=Ai′/(Ai/Ci)=Ai′/ai。式中,Ai′和Ci′为待测成分样品的峰面积和浓度,Ai和Ci为待测成分对照品的峰面积和浓度,ai为单点斜率(外标法浓度点过原点的曲线斜率)。同理得校正因子fi/s=fi/fs=(Ai/Ci)/(As/Cs)=ai/as,即外标法浓度点的单点斜率之比。Ci′=Ai′/ai= Ai/(as·fi/s)。其公式同斜率校正类似,斜率校正因子为标准曲线斜率之比,而单点校正为外标法浓度点的单点斜率之比。
1.2.4.6 耐用性和重现性
在一定范围内适当改变色谱条件,包括水相磷酸比例(0.05%、0.1%、0.2%)、流动相各梯度点比例(1.2.3色谱条件中水相各梯度比例减少2%)、柱温(25、28、30、32、34 ℃)、流速(0.8、0.9、1.0、1.05 mL/min)和检测波长(1.2.3色谱条件中各点波长±2 nm),以及使用2套液相色谱系统Waters ACQUITY UPLC H-CLASS、UltiMate-3000和3种不同厂家或型号的色谱柱(250 mm×4.6 mm,5 μm)Agilent TC-C18、Agilent-Eclipse Plus C18、SHIMADZU Inertsil ODS-3,进行RCF和相对保留时间(rx/s=tRx/tRs,其中tRx、tRs分别为待测成分、内参物的保留时间,rx/s为二者的比值即待测成分的相对保留时间)的耐用性和重现性考察。
1.2.4.7 待测组分色谱峰定位的3种方法
a. 相对保留时间:在一测多评技术中,文献普遍采用相对保留时间进行待测成分的色谱峰定位[17,23−25],实际应用推广时操作较为繁琐。参考文献[21],利用标准相对保留时间(建立标准所用Agilent TC-C18下的待测成分保留时间/内参物保留时间)预测理论保留时间,则可简化其过程,即:每根色谱柱各峰的预测保留时间(y)=每根色谱柱内参物(绿原酸)的实测保留时间×标准相对保留时间(Agilent TC-C18)(x)。
b. 多点校正法:以建标准所用的Agilent TC-C18的保留时间为横坐标X,其他验证所用色谱柱的保留时间为纵坐标Y(以SHIMADZU Inertsil ODS-3为例),绘制标准曲线并计算校正方程[26]。
c. 两点校正法:本研究中的内参物绿原酸和另1个易辨认且对照品易获得的芦丁进行两点校正,即采用建标准所用的Agilent TC-C18时绿原酸和芦丁的保留时间为横坐标X,以其他验证所用色谱柱时绿原酸和芦丁的保留时间为纵坐标Y(以SHIMADZU Inertsil ODS-3为例),绘制标准曲线并计算校正方程[21]。
1.2.5 含量测定
取10批次桑叶样品,按1.2.1项下方法每批次制备2份平行供试品溶液,按1.2.3项下色谱条件进样测定,记录峰面积,分别用外标法、一测多评法(多点校正、斜率校正、单点校正)计算各成分的含量。
1.3 数据处理
利用 Microsoft Excel 2019 软件进行数据汇总和分析,应用 IBM SPSS Statistics 25.0 软件进行方差分析和t检验分析。
2. 结果与分析
2.1 QAMS方法学考察结果
2.1.1 系统适应性
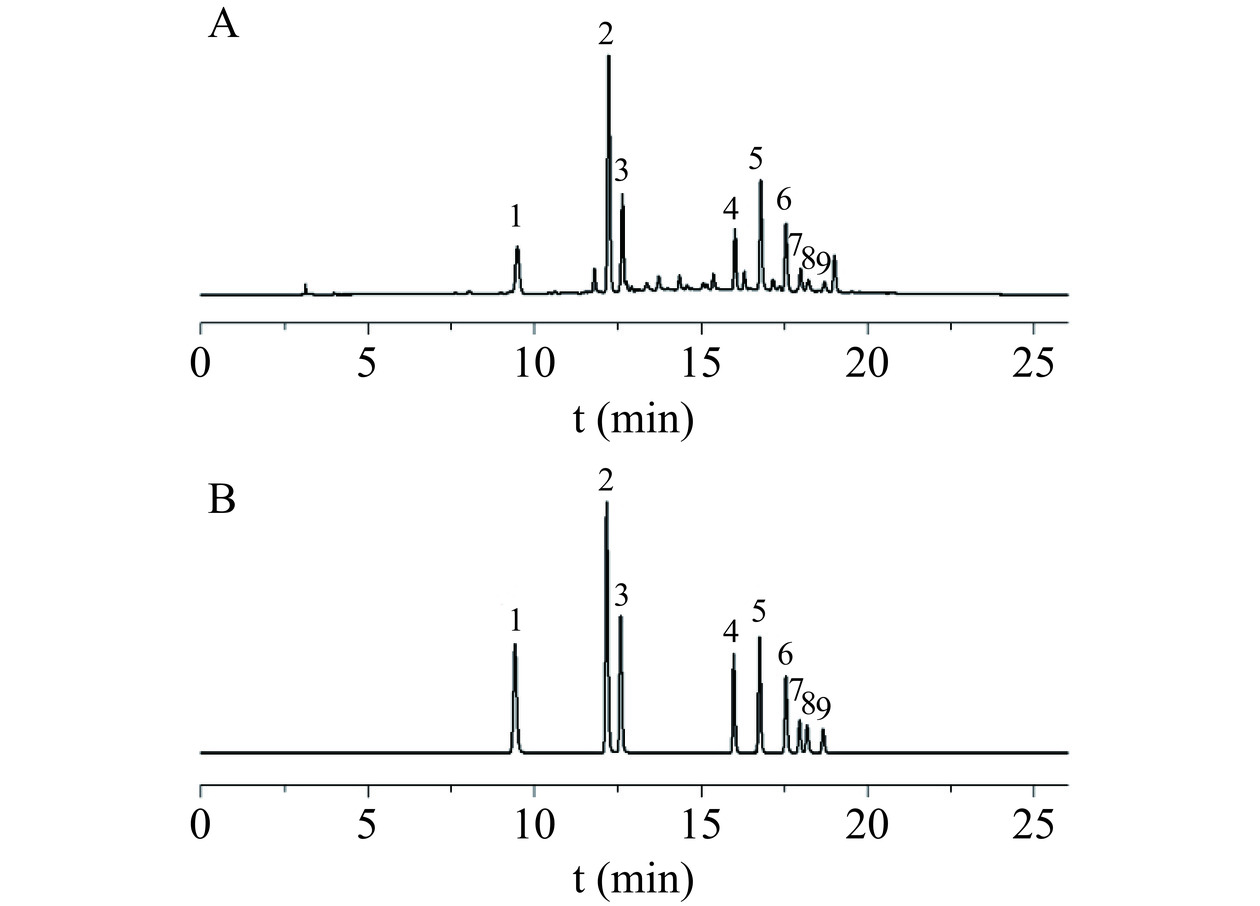
记录混合对照品溶液和桑叶供试品溶液色谱图见图1,由Waters Empower工作站计算得出新绿原酸、绿原酸、隐绿原酸、芦丁、异槲皮苷、异绿原酸B、紫云英苷、异绿原酸A和异绿原酸C与其相邻色谱峰的分离度分别为17.62、3.26、28.49、6.58、6.40、3.27、1.77、3.54,理论塔板均在10000以上。
2.1.2 线性关系
以峰面积(Y)为纵坐标,各成分的质量浓度(X)为横坐标,绘制标准曲线,结果见表2,9个成分的质量浓度均与色谱峰面积线性关系良好,R2≥0.9997。
表 2 9种成分的线性关系Table 2. Results of linear relationship of nine kinds of components编号 成分 回归方程 线性范围(μg/mL) R2 1 新绿原酸 Y=27563.61X+1390.943 1.3700~109.6000 0.9998 2 绿原酸 Y=30052.85X+10255.98 2.5290~202.3200 0.9998 3 隐绿原酸 Y=26499.33X+1865.864 1.1345~90.7600 0.9998 4 芦丁 Y=18799.6X+254.8852 1.0430~83.4400 0.9998 5 异槲皮苷 Y=25864.39X+1262.257 1.2000~96.0000 0.9998 6 异绿原酸B Y=36055.92X+4077.8 1.9225~153.8000 0.9998 7 紫云英苷 Y=16894.28X+246.8369 0.5620~44.9600 0.9997 8 异绿原酸A Y=43818.15X−1095.45 0.5945~47.5600 0.9998 9 异绿原酸C Y=37590.87X−1801.718 0.5085~40.6800 0.9998 2.1.3 精密度、重复性和稳定性
如表3所示,按照1.2.4.3项下进行试验,9个成分的精密度、重复性和稳定性RSD均小于2.5%,表明该方法的精密度、重复性和稳定性良好。
表 3 9种成分的精密度、重复性和稳定性Table 3. Precision, repeatability and stability of nine kinds of components成分 精密度 重复性 稳定性 RSD(%) RSD(%) RSD(%) 新绿原酸 0.05 0.83 2.16 绿原酸 0.05 1.11 2.18 隐绿原酸 0.14 1.56 0.53 芦丁 0.07 0.08 0.28 异槲皮苷 0.18 2.35 1.28 异绿原酸B 0.13 2.39 1.23 紫云英苷 0.11 2.00 0.51 异绿原酸A 0.32 1.86 0.51 异绿原酸C 0.39 2.33 0.28 2.1.4 加样回收率
样品中新绿原酸、绿原酸、隐绿原酸、芦丁、异槲皮苷、异绿原酸B、紫云英苷、异绿原酸A和异绿原酸C的平均含量分别为0.5029、1.5404、0.7360、0.5062、0.7143、0.3381、0.2267、0.0810、0.0761 mg/g,加样回收率(n=9)分别为100.04%、99.36%、99.89%、99.54%、99.86%、98.59%、99.69%、99.63%、100.83%,RSD分别为0.69%、1.06%、1.96%、0.74%、0.17%、0.25%、0.31%、0.30%、0.31%,表明该方法具有良好的准确度,方法可行。
2.1.5 相对校正因子
多点校正法计算不同浓度间新绿原酸、隐绿原酸、芦丁、异槲皮苷、异绿原酸B、紫云英苷、异绿原酸A和异绿原酸C的相对校正因子RSD均<3%,三种方法计算得到的RCF结果见表4,RSD均<1.50%。
表 4 桑叶各成分相对校正因子Table 4. Relative correction factors of various components of Mori Folium校正方法 f1/2 f3/2 f4/2 f5/2 f6/2 f7/2 f8/2 f9/2 多点校正 0.9072 0.8736 0.6207 0.8547 1.1936 0.5501 1.4369 1.2244 斜率校正 0.9172 0.8818 0.6256 0.8606 1.1998 0.5622 1.4580 1.2508 单点校正 0.9073 0.8737 0.6207 0.8547 1.1937 0.5504 1.4372 1.2247 平均值 0.9106 0.8764 0.6223 0.8567 1.1957 0.5542 1.4440 1.2333 RSD(%) 0.63 0.53 0.45 0.40 0.29 1.24 0.84 1.23 2.1.6 耐用性和重现性
2.1.6.1 耐用性
按照1.2.4.6项下调整色谱条件,各待测成分RCF的RSD均<1.5%,表明色谱条件的变化对各待测成分RCF的影响较小,耐用性良好。在色谱峰定位上,以流动相梯度比例变化时的影响较大,各待测成分的相对保留时间RSD>5%,其他色谱条件改变时影响较小,相对保留时间RSD≤3.40%。
2.1.6.2 重现性
按照1.2.4.6项下进行试验,结果表明除紫云英苷RCF的RSD为4.27%外,其余待测组分RCF的RSD均<1.5%,各待测成分相对保留时间的RSD均<5%,提示RCF在不同仪器和不同色谱柱下具有良好的重现性,结果见表5、表6。
表 5 RCF重现性Table 5. Reproducibility of relative correction factors液相仪器 色谱柱 f1/2 f3/2 f4/2 f5/2 f6/2 f7/2 f8/2 f9/2 沃特世 Agilent TC-C18 0.9072 0.8736 0.6207 0.8547 1.1936 0.5501 1.4369 1.2244 Agilent-Eclipse Plus C18 0.9160 0.8769 0.6225 0.8557 1.1903 0.5925 1.4325 1.1987 SHIMADZU Inertsil ODS-3 0.9168 0.8778 0.6274 0.8619 1.1933 0.5910 1.4460 1.1803 赛默飞 Agilent TC-C18 0.9254 0.8738 0.6327 0.8731 1.1698 0.5393 1.4455 1.1887 Agilent-Eclipse Plus C18 0.9167 0.8804 0.6287 0.8669 1.1729 0.5414 1.3896 1.2031 SHIMADZU Inertsil ODS-3 0.9226 0.8841 0.6319 0.8701 1.1701 0.5715 1.4248 1.1885 平均值 0.9174 0.8778 0.6273 0.8637 1.1817 0.5643 1.4292 1.1973 RSD(%) 0.69 0.46 0.78 0.88 1.00 4.27 1.47 1.30 表 6 相对保留时间重现性Table 6. Reproducibility of relative retention time液相仪器 色谱柱 r1/2 r3/2 r4/2 r5/2 r6/2 r7/2 r8/2 r9/2 沃特世 Agilent TC-C18 0.7849 1.0326 1.3039 1.3668 1.4310 1.4632 1.4832 1.5212 Agilent-Eclipse Plus C18 0.7251 1.0372 1.3599 1.4269 1.5024 1.5341 1.5630 1.6059 SHIMADZU Inertsil ODS-3 0.7609 1.0359 1.3207 1.3857 1.4547 1.4870 1.5086 1.5493 赛默飞 Agilent TC-C18 0.7792 1.0302 1.2881 1.3497 1.4100 1.4421 1.4610 1.4968 Agilent-Eclipse Plus C18 0.7060 1.0445 1.3908 1.4587 1.5359 1.5679 1.5976 1.6420 SHIMADZU Inertsil ODS-3 0.7423 1.0392 1.3334 1.3985 1.4680 1.5000 1.5217 1.5637 平均值 0.7497 1.0366 1.3328 1.3977 1.4670 1.4991 1.5225 1.5632 RSD(%) 4.13 0.49 2.82 2.86 3.15 3.08 3.32 3.43 2.1.7 色谱峰定位
相对保留时间:利用标准相对保留时间推算理论保留时间,见表7,结果表明待测成分的保留时间越靠近内参物,其实测和预测保留时间的相对误差RE [RE(%)=[(实测值-预测值)/实测值]×100)]相对较小,定位较准确,反之则RE越大,部分色谱峰甚至超过±8.00%。这种情况在色谱峰众多,紫外吸收相近或无紫外吸收参照时,将很难准确定位待测成分,一测多评技术也就难以推广应用。
表 7 3种方法预测保留时间进行色谱峰定位Table 7. Predicting retention time to locate chromatographic peaks by three ways仪器 色谱柱 保留时间(tR) 新绿原酸 绿原酸 隐绿原酸 芦丁 异槲皮苷 异绿原酸B 紫云英苷 异绿原酸A 异绿原酸C 沃特世 Agilent TC-C18 标准tR(X) 9.6877 12.3425 12.7453 16.0932 16.8698 17.6618 18.0590 18.3065 18.7753 标准相对保留时间(x) 0.7849 1.0000 1.0326 1.30390 1.3668 1.4310 1.4632 1.4832 1.5212 Agilent-Eclipse
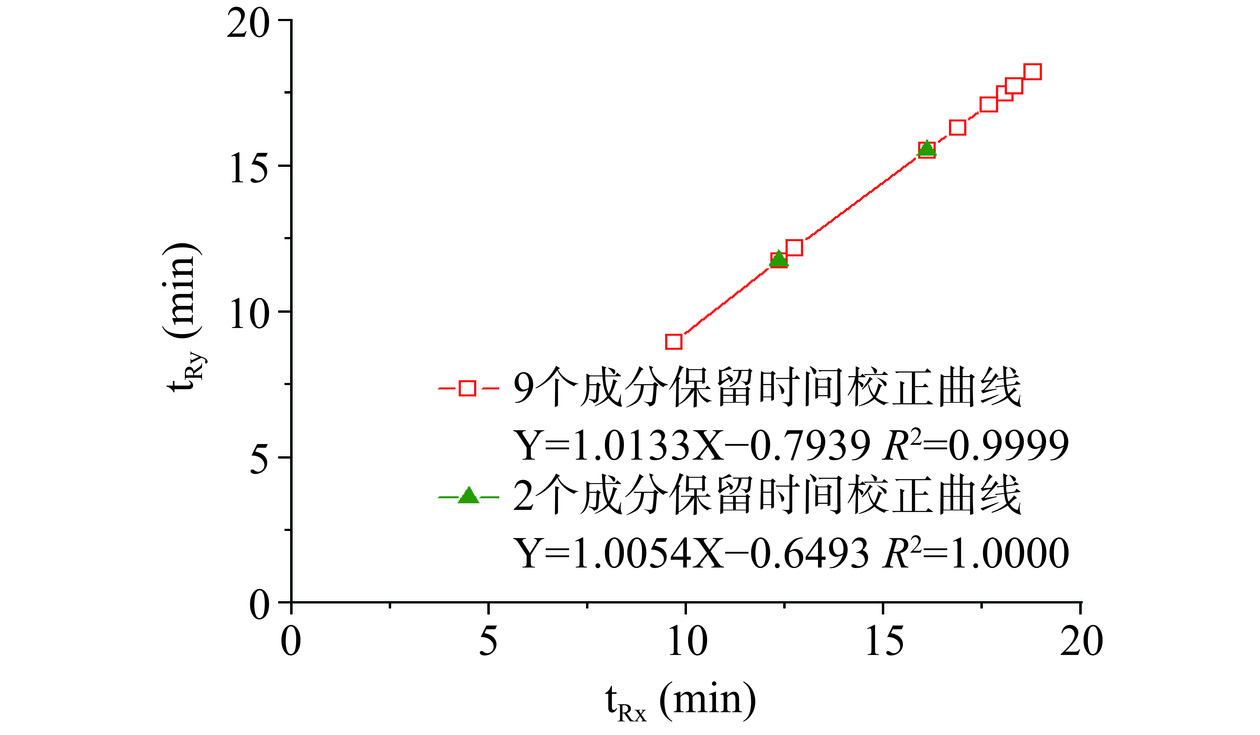
Plus C18实测tR 7.7900 10.7430 11.1430 14.6090 15.3290 16.1400 16.4810 16.7910 17.2520 预测tR(y=10.743x) 8.4322 10.7430 11.0932 14.0078 14.6835 15.3732 15.7192 15.9340 16.3423 RE(%) −8.24 0.00 0.45 4.12 4.21 4.75 4.62 5.10 5.27 预测tR(Y=1.0310X−2.0691)(多点法) 7.9189 10.6560 11.0713 14.5230 15.3237 16.1402 16.5497 16.8049 17.2882 RE(%) −1.65 0.81 0.64 0.59 0.03 0.00 −0.42 −0.08 −0.21 预测tR(Y=1.0307X−1.9789)(两点法) 8.0062 10.7425 11.1577 14.6084 15.4088 16.2251 16.6345 16.8896 17.3728 RE(%) −2.78 0.00 −0.13 0.00 −0.52 −0.53 −0.93 −0.59 −0.70 SHIMADZU
Inertsil ODS-3实测tR 8.9480 11.7600 12.1820 15.5310 16.2960 17.1070 17.4870 17.7410 18.2200 预测tR(y=11.760x) 9.2304 11.7600 12.1434 15.3339 16.0736 16.8286 17.2072 17.4424 17.8893 RE(%) −3.16 0.00 0.32 1.27 1.36 1.63 1.60 1.68 1.81 预测tR(Y=1.0133X−0.7939)(多点法) 9.0226 11.7128 12.1209 15.5133 16.3003 17.1028 17.5053 17.7561 18.2311 RE(%) −0.83 0.40 0.50 0.11 −0.03 0.02 −0.10 −0.08 −0.06 预测tR(Y=1.0054X−0.6493)(两点法) 9.0907 11.7598 12.1648 15.5308 16.3116 17.1079 17.5072 17.7561 18.2274 RE(%) −1.59 0.00 0.14 0.00 −0.10 −0.01 −0.12 −0.08 −0.04 赛默飞 Agilent TC-C18 实测tR 10.3250 13.2500 13.6500 17.0670 17.8830 18.6830 19.1080 19.3580 19.8330 预测tR(y=13.250x) 10.3999 13.2500 13.6820 17.2767 18.1101 18.9608 19.3874 19.6524 20.1559 RE(%) −0.73 0.00 −0.23 −1.23 −1.27 −1.49 −1.46 −1.52 −1.63 预测tR(Y=1.0386X+0.3546)(多点法) 10.4162 13.1735 13.5919 17.0690 17.8756 18.6981 19.1107 19.3677 19.8546 RE(%) −0.88 0.58 0.43 −0.01 0.04 −0.08 −0.01 −0.05 −0.11 预测tR(Y=1.0177X+0.6893)(两点法) 10.5485 13.2503 13.6602 17.0673 17.8577 18.6637 19.0679 19.3198 19.7969 RE(%) −2.16 0.00 −0.07 0.00 0.14 0.10 0.21 0.20 0.18 Agilent-Eclipse
Plus C18实测tR 7.5420 10.6830 11.1580 14.8580 15.5830 16.4080 16.7500 17.0670 17.5420 预测tR(y=10.683x) 8.3851 10.6830 11.0313 13.9296 14.6015 15.2874 15.6314 15.8450 16.2510 RE(%) −11.18 0.00 1.14 6.25 6.30 6.83 6.68 7.16 7.36 预测tR(Y=1.0902X−2.8468)(多点法) 7.7147 10.6090 11.0481 14.6980 15.5447 16.4081 16.8411 17.1109 17.6220 RE(%) −2.29 0.69 0.98 1.08 0.25 0.00 −0.54 −0.26 −0.46 预测tR(Y=1.1131X−3.0558)(两点法) 7.7276 10.6826 11.1310 14.8575 15.7220 16.6035 17.0457 17.3212 17.8430 RE(%) −2.46 0.00 0.24 0.00 −0.89 −1.19 −1.77 −1.49 −1.72 SHIMADZU
Inertsil ODS-3实测tR 8.8330 11.9000 12.3670 15.8670 16.6420 17.4690 17.8500 18.1080 18.6080 预测tR(y=11.900x) 9.3403 11.9000 12.2879 15.5164 16.2649 17.0289 17.4121 17.6501 18.1023 RE/% −5.74 0.00 0.64 2.21 2.27 2.52 2.45 2.53 2.72 预测tR(Y=1.0629X−1.3044)(多点法) 8.9927 11.8144 12.2426 15.8011 16.6265 17.4683 17.8905 18.1536 18.6519 RE(%) −1.81 0.72 1.01 0.42 0.09 0.00 −0.23 −0.25 −0.24 预测tR(Y=1.0577X−1.1543)(两点法) 9.0924 11.9004 12.3264 15.8675 16.6889 17.5266 17.9467 18.2085 18.7043 RE(%) −2.94 0.00 0.33 0.00 −0.28 −0.33 −0.54 −0.55 −0.52 多点校正法:标准曲线及校正方程见图2(红色空心方框),结果表明在相同仪器不同色谱柱(Agilent TC-C18和SHIMADZU Inertsil ODS-3)下9种成分的保留时间相关性较好(R2=0.9999)。即使采用不同的仪器,不同的色谱柱,其R2依然可以达到0.9990以上。
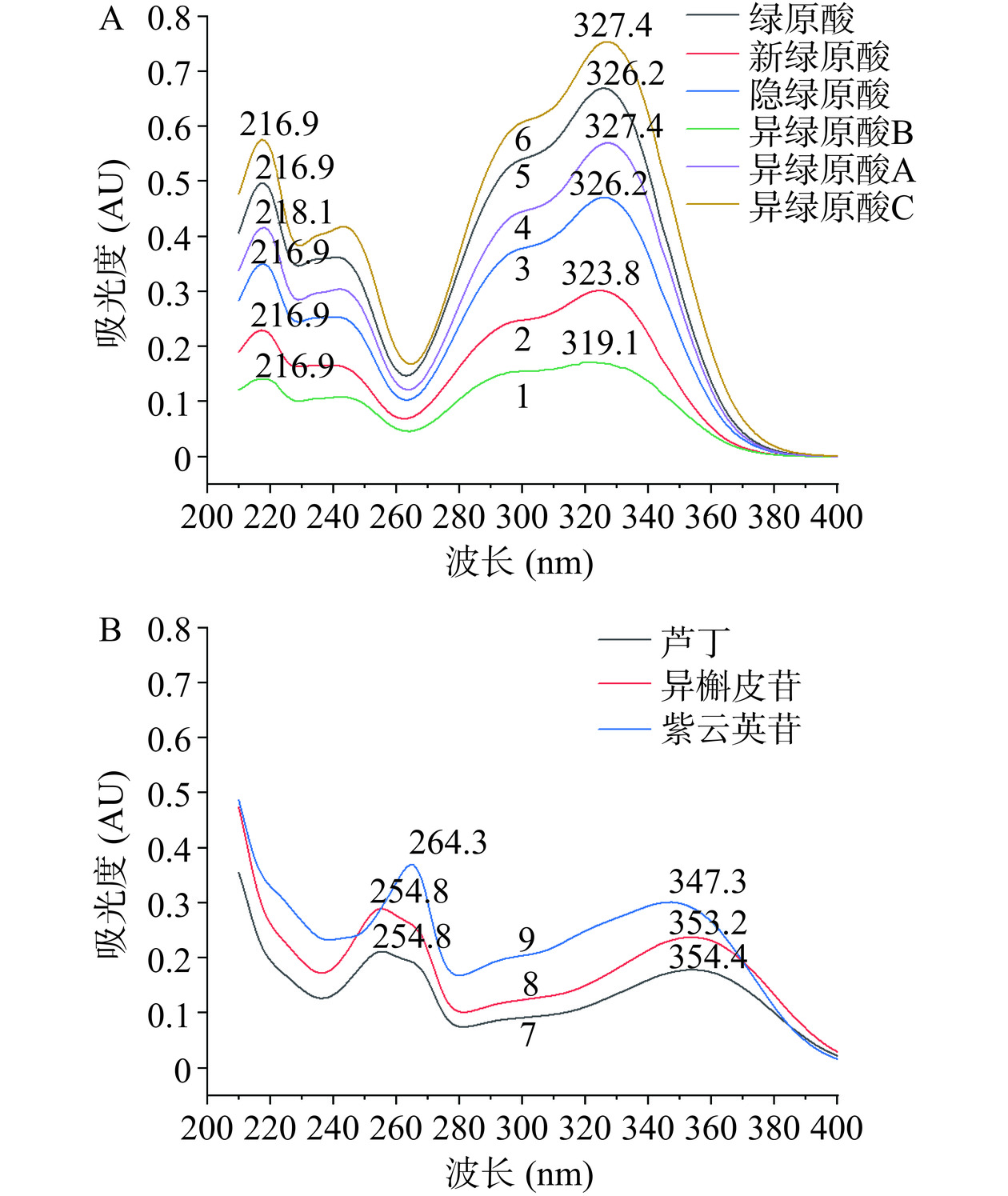
两点校正法:标准曲线及校正方程见图2(绿色实心三角形)。由图2可见,两点校正的校正曲线与9个点绘制的标准曲线基本重合,用以预测保留时间足够。采用Agilent TC-C18色谱柱时各成分的保留时间分别带入校正方程,即可计算多点和两点校正情况下相应色谱柱各成分的预测出峰时间,见表7。由表7可见,相较于相对保留时间,采用多点或两点校正预测出峰时间时,无论色谱峰保留时间靠前或者靠后或采用不同的色谱柱,其RE较低,均<3%,预测值与实际值非常接近,本研究中9个待测成分色谱峰均可获得准确的预测值。另外,在一测多评应用推广时,若该色谱峰附近还有其他色谱峰干扰定位,可以各成分的紫外吸收光谱(见图3)、整体峰形或峰面积百分比作为补充参考条件,即可在一定程度上准确定位色谱峰。
2.2 含量测定
为验证一测多评的准确性,分析发现4种计算方法所得结果之间的RSD均<1.50%,同时采用方差分析检验4种计算方法间结果的差异性,P值接近1.000,远大于0.05,说明4种计算方法结果间差异无显著性。另将3种校正方法的结果分别与外标法间进行比较,计算得|RE|<3.00%,同时采用t检验分析其间差异性,结果表明3种校正方式与外标法间结果差异无显著性,具有良好的一致性,见表8。
表 8 桑叶各成分含量测定结果(mg/g)Table 8. Determination results of various components of Mori Folium (mg/g)样品 定量方法 新绿原酸 隐绿原酸 芦丁 异槲皮苷 异绿原酸B 紫云英苷 异绿原酸A 异绿原酸C S1 外标法 1.0090 1.4603 1.0119 1.4298 0.6746 0.4521 0.1622 0.1518 多点校正 1.0171 1.4695 1.0149 1.4342 0.6799 0.4605 0.1624 0.1518 斜率校正 1.0059 1.4558 1.0070 1.4243 0.6765 0.4504 0.1601 0.1486 单点校正 1.0149 1.4633 1.0142 1.4342 0.6817 0.4557 0.1610 0.1506 RSD(%) 0.51 0.39 0.35 0.33 0.47 0.98 0.67 1.00 S2 外标法 0.6607 1.3804 1.5536 1.8416 0.6035 0.9452 0.1846 0.1390 多点校正 0.6680 1.3916 1.5606 1.8497 0.6100 0.9636 0.1853 0.1390 斜率校正 0.6607 1.3786 1.5484 1.8369 0.6068 0.9423 0.1826 0.1361 单点校正 0.6666 1.3858 1.5594 1.8497 0.6116 0.9535 0.1837 0.1379 RSD(%) 0.58 0.42 0.36 0.34 0.59 1.01 0.63 0.99 S3 外标法 0.5447 1.0155 0.9947 1.0578 0.3990 0.3577 0.1388 0.1113 多点校正 0.5495 1.0215 0.9963 1.0603 0.4039 0.3640 0.1386 0.1105 斜率校正 0.5434 1.0120 0.9885 1.0529 0.4019 0.3560 0.1366 0.1082 单点校正 0.5483 1.0173 0.9956 1.0603 0.4050 0.3602 0.1374 0.1096 RSD(%) 0.53 0.39 0.36 0.33 0.65 0.97 0.75 1.21 S4 外标法 1.1633 1.6984 1.3429 1.8122 0.7758 0.7120 0.1676 0.1355 多点校正 1.1711 1.7068 1.3453 1.8154 0.7803 0.7241 0.1677 0.1352 斜率校正 1.1583 1.6908 1.3348 1.8028 0.7763 0.7081 0.1653 0.1323 单点校正 1.1686 1.6996 1.3443 1.8154 0.7824 0.7165 0.1663 0.1340 RSD(%) 0.49 0.39 0.36 0.33 0.41 0.96 0.69 1.08 S5 外标法 0.9335 1.6615 1.2327 0.9481 1.1179 0.3483 0.3350 0.1691 多点校正 0.9435 1.6756 1.2393 0.9542 1.1259 0.3558 0.3377 0.1698 斜率校正 0.9332 1.6599 1.2296 0.9476 1.1201 0.3480 0.3328 0.1662 单点校正 0.9415 1.6685 1.2384 0.9542 1.1288 0.3521 0.3348 0.1684 RSD(%) 0.57 0.43 0.38 0.39 0.45 1.05 0.60 0.93 S6 外标法 0.8631 1.8627 1.0158 0.4069 0.8688 0.1927 0.4092 0.1394 多点校正 0.8722 1.8774 1.0210 0.4108 0.8760 0.1972 0.4125 0.1395 斜率校正 0.8627 1.8599 1.0130 0.4080 0.8715 0.1928 0.4066 0.1365 单点校正 0.8703 1.8696 1.0202 0.4108 0.8783 0.1951 0.4090 0.1383 RSD(%) 0.56 0.42 0.37 0.49 0.49 1.10 0.59 1.01 S7 外标法 0.9936 1.5280 1.4353 1.2779 1.0044 0.3269 0.1799 0.1498 多点校正 1.0023 1.5384 1.4402 1.2829 1.0103 0.3334 0.1804 0.1499 斜率校正 0.9913 1.5240 1.4289 1.2740 1.0051 0.3260 0.1778 0.1467 单点校正 1.0001 1.5320 1.4391 1.2829 1.0129 0.3299 0.1788 0.1486 RSD(%) 0.52 0.40 0.36 0.34 0.41 1.02 0.65 1.00 S8 外标法 0.5004 1.0204 0.6621 0.7123 0.3074 0.2405 0.1382 0.1243 多点校正 0.5058 1.0280 0.6644 0.7158 0.3129 0.2454 0.1382 0.1239 斜率校正 0.5002 1.0184 0.6592 0.7108 0.3113 0.2399 0.1362 0.1212 单点校正 0.5047 1.0237 0.6639 0.7158 0.3138 0.2428 0.1370 0.1228 RSD(%) 0.58 0.41 0.35 0.35 0.91 1.03 0.71 1.13 S9 外标法 0.3712 0.9924 0.7505 0.8073 0.5302 0.3158 0.1578 0.1149 多点校正 0.3755 0.9991 0.7524 0.8103 0.5353 0.3217 0.1579 0.1142 斜率校正 0.3714 0.9898 0.7465 0.8047 0.5326 0.3146 0.1556 0.1118 单点校正 0.3747 0.9949 0.7519 0.8103 0.5367 0.3183 0.1565 0.1133 RSD(%) 0.59 0.40 0.36 0.33 0.54 0.99 0.70 1.18 S10 外标法 0.2984 0.9812 1.7469 1.4196 1.0218 0.4567 0.2296 0.1214 多点校正 0.3032 0.9904 1.7551 1.4268 1.0291 0.4661 0.2309 0.1212 斜率校正 0.2999 0.9812 1.7414 1.4169 1.0238 0.4558 0.2276 0.1186 单点校正 0.3025 0.9863 1.7538 1.4268 1.0318 0.4612 0.2289 0.1202 RSD(%) 0.74 0.45 0.36 0.36 0.45 1.03 0.60 1.06 3. 结论
本研究采用高效液相色谱联合QAMS对桑叶中9种成分(新绿原酸、绿原酸、隐绿原酸、芦丁、异槲皮苷、异绿原酸A、紫云英苷、异绿原酸A和异绿原酸C)进行含量测定,经考察精密度、重复性及稳定性的RSD值小于2.5%,平均回收率在98.59%~100.83%之间,均符合要求。由于绿原酸在桑叶中含量较高,其对照品价廉,稳定性良好,故选择其作为内参物,建立与其他成分之间的RCFs。所建立的RCFs在不同水相磷酸比例、不同流速、不同柱温和不同检测波长下相对保留时间的RSD值≤3.40%,提示RCFs耐用性良好。在不同品牌仪器和色谱柱下,紫云英苷的RCF变化较大(RSD为4.27%),其余待测组分RCF的RSD均<1.5%,提示RCFs重现性良好。在色谱峰定位中,本研究对比了相对保留时间、多点校正和两点校正之间的差异,相对保留时间对远离内参物的待测成分定位效果不理想,经校正后,预测保留时间和实测保留时间无明显差异,色谱峰定位的准确性得到改善。为进一步验证一测多评的准确性,本研究比较了外标法测定值和QAMS(多点校正、斜率校正和单点校正)计算值间的差异,结果表明QAMS的3种校正方法结果与外标法间|RE|<3.00%, t检验分析其间结果差异无显著性,具有良好的一致性,验证了该QAMS法的准确性和可行性。本研究在药典规定成分的基础上,从芦丁单一指标增加了绿原酸类和黄酮类等8个指标,为进一步完善和提高桑叶的质量控制标准提供科学依据,也将有利于QAMS在中药质量控制中的推广。
-
表 1 桑叶样品信息
Table 1 Information table of Mori Folium samples
样品编号 采收日期/批次 产地 来源 S1 2022.7 新疆托克逊 采收 S2 2212079 广西 购买 S3 20221122 陕西商洛 购买 S4 2022.7 新疆吐鲁番 采收 S5 2022.7 四川宜宾 采收 S6 2022.7 四川宜宾 采收 S7 2022.6 新疆喀什 采收 S8 200901 河南南阳 购买 S9 121123-201806 国标物质 购买 S10 20200417 安徽亳州 购买 表 2 9种成分的线性关系
Table 2 Results of linear relationship of nine kinds of components
编号 成分 回归方程 线性范围(μg/mL) R2 1 新绿原酸 Y=27563.61X+1390.943 1.3700~109.6000 0.9998 2 绿原酸 Y=30052.85X+10255.98 2.5290~202.3200 0.9998 3 隐绿原酸 Y=26499.33X+1865.864 1.1345~90.7600 0.9998 4 芦丁 Y=18799.6X+254.8852 1.0430~83.4400 0.9998 5 异槲皮苷 Y=25864.39X+1262.257 1.2000~96.0000 0.9998 6 异绿原酸B Y=36055.92X+4077.8 1.9225~153.8000 0.9998 7 紫云英苷 Y=16894.28X+246.8369 0.5620~44.9600 0.9997 8 异绿原酸A Y=43818.15X−1095.45 0.5945~47.5600 0.9998 9 异绿原酸C Y=37590.87X−1801.718 0.5085~40.6800 0.9998 表 3 9种成分的精密度、重复性和稳定性
Table 3 Precision, repeatability and stability of nine kinds of components
成分 精密度 重复性 稳定性 RSD(%) RSD(%) RSD(%) 新绿原酸 0.05 0.83 2.16 绿原酸 0.05 1.11 2.18 隐绿原酸 0.14 1.56 0.53 芦丁 0.07 0.08 0.28 异槲皮苷 0.18 2.35 1.28 异绿原酸B 0.13 2.39 1.23 紫云英苷 0.11 2.00 0.51 异绿原酸A 0.32 1.86 0.51 异绿原酸C 0.39 2.33 0.28 表 4 桑叶各成分相对校正因子
Table 4 Relative correction factors of various components of Mori Folium
校正方法 f1/2 f3/2 f4/2 f5/2 f6/2 f7/2 f8/2 f9/2 多点校正 0.9072 0.8736 0.6207 0.8547 1.1936 0.5501 1.4369 1.2244 斜率校正 0.9172 0.8818 0.6256 0.8606 1.1998 0.5622 1.4580 1.2508 单点校正 0.9073 0.8737 0.6207 0.8547 1.1937 0.5504 1.4372 1.2247 平均值 0.9106 0.8764 0.6223 0.8567 1.1957 0.5542 1.4440 1.2333 RSD(%) 0.63 0.53 0.45 0.40 0.29 1.24 0.84 1.23 表 5 RCF重现性
Table 5 Reproducibility of relative correction factors
液相仪器 色谱柱 f1/2 f3/2 f4/2 f5/2 f6/2 f7/2 f8/2 f9/2 沃特世 Agilent TC-C18 0.9072 0.8736 0.6207 0.8547 1.1936 0.5501 1.4369 1.2244 Agilent-Eclipse Plus C18 0.9160 0.8769 0.6225 0.8557 1.1903 0.5925 1.4325 1.1987 SHIMADZU Inertsil ODS-3 0.9168 0.8778 0.6274 0.8619 1.1933 0.5910 1.4460 1.1803 赛默飞 Agilent TC-C18 0.9254 0.8738 0.6327 0.8731 1.1698 0.5393 1.4455 1.1887 Agilent-Eclipse Plus C18 0.9167 0.8804 0.6287 0.8669 1.1729 0.5414 1.3896 1.2031 SHIMADZU Inertsil ODS-3 0.9226 0.8841 0.6319 0.8701 1.1701 0.5715 1.4248 1.1885 平均值 0.9174 0.8778 0.6273 0.8637 1.1817 0.5643 1.4292 1.1973 RSD(%) 0.69 0.46 0.78 0.88 1.00 4.27 1.47 1.30 表 6 相对保留时间重现性
Table 6 Reproducibility of relative retention time
液相仪器 色谱柱 r1/2 r3/2 r4/2 r5/2 r6/2 r7/2 r8/2 r9/2 沃特世 Agilent TC-C18 0.7849 1.0326 1.3039 1.3668 1.4310 1.4632 1.4832 1.5212 Agilent-Eclipse Plus C18 0.7251 1.0372 1.3599 1.4269 1.5024 1.5341 1.5630 1.6059 SHIMADZU Inertsil ODS-3 0.7609 1.0359 1.3207 1.3857 1.4547 1.4870 1.5086 1.5493 赛默飞 Agilent TC-C18 0.7792 1.0302 1.2881 1.3497 1.4100 1.4421 1.4610 1.4968 Agilent-Eclipse Plus C18 0.7060 1.0445 1.3908 1.4587 1.5359 1.5679 1.5976 1.6420 SHIMADZU Inertsil ODS-3 0.7423 1.0392 1.3334 1.3985 1.4680 1.5000 1.5217 1.5637 平均值 0.7497 1.0366 1.3328 1.3977 1.4670 1.4991 1.5225 1.5632 RSD(%) 4.13 0.49 2.82 2.86 3.15 3.08 3.32 3.43 表 7 3种方法预测保留时间进行色谱峰定位
Table 7 Predicting retention time to locate chromatographic peaks by three ways
仪器 色谱柱 保留时间(tR) 新绿原酸 绿原酸 隐绿原酸 芦丁 异槲皮苷 异绿原酸B 紫云英苷 异绿原酸A 异绿原酸C 沃特世 Agilent TC-C18 标准tR(X) 9.6877 12.3425 12.7453 16.0932 16.8698 17.6618 18.0590 18.3065 18.7753 标准相对保留时间(x) 0.7849 1.0000 1.0326 1.30390 1.3668 1.4310 1.4632 1.4832 1.5212 Agilent-Eclipse
Plus C18实测tR 7.7900 10.7430 11.1430 14.6090 15.3290 16.1400 16.4810 16.7910 17.2520 预测tR(y=10.743x) 8.4322 10.7430 11.0932 14.0078 14.6835 15.3732 15.7192 15.9340 16.3423 RE(%) −8.24 0.00 0.45 4.12 4.21 4.75 4.62 5.10 5.27 预测tR(Y=1.0310X−2.0691)(多点法) 7.9189 10.6560 11.0713 14.5230 15.3237 16.1402 16.5497 16.8049 17.2882 RE(%) −1.65 0.81 0.64 0.59 0.03 0.00 −0.42 −0.08 −0.21 预测tR(Y=1.0307X−1.9789)(两点法) 8.0062 10.7425 11.1577 14.6084 15.4088 16.2251 16.6345 16.8896 17.3728 RE(%) −2.78 0.00 −0.13 0.00 −0.52 −0.53 −0.93 −0.59 −0.70 SHIMADZU
Inertsil ODS-3实测tR 8.9480 11.7600 12.1820 15.5310 16.2960 17.1070 17.4870 17.7410 18.2200 预测tR(y=11.760x) 9.2304 11.7600 12.1434 15.3339 16.0736 16.8286 17.2072 17.4424 17.8893 RE(%) −3.16 0.00 0.32 1.27 1.36 1.63 1.60 1.68 1.81 预测tR(Y=1.0133X−0.7939)(多点法) 9.0226 11.7128 12.1209 15.5133 16.3003 17.1028 17.5053 17.7561 18.2311 RE(%) −0.83 0.40 0.50 0.11 −0.03 0.02 −0.10 −0.08 −0.06 预测tR(Y=1.0054X−0.6493)(两点法) 9.0907 11.7598 12.1648 15.5308 16.3116 17.1079 17.5072 17.7561 18.2274 RE(%) −1.59 0.00 0.14 0.00 −0.10 −0.01 −0.12 −0.08 −0.04 赛默飞 Agilent TC-C18 实测tR 10.3250 13.2500 13.6500 17.0670 17.8830 18.6830 19.1080 19.3580 19.8330 预测tR(y=13.250x) 10.3999 13.2500 13.6820 17.2767 18.1101 18.9608 19.3874 19.6524 20.1559 RE(%) −0.73 0.00 −0.23 −1.23 −1.27 −1.49 −1.46 −1.52 −1.63 预测tR(Y=1.0386X+0.3546)(多点法) 10.4162 13.1735 13.5919 17.0690 17.8756 18.6981 19.1107 19.3677 19.8546 RE(%) −0.88 0.58 0.43 −0.01 0.04 −0.08 −0.01 −0.05 −0.11 预测tR(Y=1.0177X+0.6893)(两点法) 10.5485 13.2503 13.6602 17.0673 17.8577 18.6637 19.0679 19.3198 19.7969 RE(%) −2.16 0.00 −0.07 0.00 0.14 0.10 0.21 0.20 0.18 Agilent-Eclipse
Plus C18实测tR 7.5420 10.6830 11.1580 14.8580 15.5830 16.4080 16.7500 17.0670 17.5420 预测tR(y=10.683x) 8.3851 10.6830 11.0313 13.9296 14.6015 15.2874 15.6314 15.8450 16.2510 RE(%) −11.18 0.00 1.14 6.25 6.30 6.83 6.68 7.16 7.36 预测tR(Y=1.0902X−2.8468)(多点法) 7.7147 10.6090 11.0481 14.6980 15.5447 16.4081 16.8411 17.1109 17.6220 RE(%) −2.29 0.69 0.98 1.08 0.25 0.00 −0.54 −0.26 −0.46 预测tR(Y=1.1131X−3.0558)(两点法) 7.7276 10.6826 11.1310 14.8575 15.7220 16.6035 17.0457 17.3212 17.8430 RE(%) −2.46 0.00 0.24 0.00 −0.89 −1.19 −1.77 −1.49 −1.72 SHIMADZU
Inertsil ODS-3实测tR 8.8330 11.9000 12.3670 15.8670 16.6420 17.4690 17.8500 18.1080 18.6080 预测tR(y=11.900x) 9.3403 11.9000 12.2879 15.5164 16.2649 17.0289 17.4121 17.6501 18.1023 RE/% −5.74 0.00 0.64 2.21 2.27 2.52 2.45 2.53 2.72 预测tR(Y=1.0629X−1.3044)(多点法) 8.9927 11.8144 12.2426 15.8011 16.6265 17.4683 17.8905 18.1536 18.6519 RE(%) −1.81 0.72 1.01 0.42 0.09 0.00 −0.23 −0.25 −0.24 预测tR(Y=1.0577X−1.1543)(两点法) 9.0924 11.9004 12.3264 15.8675 16.6889 17.5266 17.9467 18.2085 18.7043 RE(%) −2.94 0.00 0.33 0.00 −0.28 −0.33 −0.54 −0.55 −0.52 表 8 桑叶各成分含量测定结果(mg/g)
Table 8 Determination results of various components of Mori Folium (mg/g)
样品 定量方法 新绿原酸 隐绿原酸 芦丁 异槲皮苷 异绿原酸B 紫云英苷 异绿原酸A 异绿原酸C S1 外标法 1.0090 1.4603 1.0119 1.4298 0.6746 0.4521 0.1622 0.1518 多点校正 1.0171 1.4695 1.0149 1.4342 0.6799 0.4605 0.1624 0.1518 斜率校正 1.0059 1.4558 1.0070 1.4243 0.6765 0.4504 0.1601 0.1486 单点校正 1.0149 1.4633 1.0142 1.4342 0.6817 0.4557 0.1610 0.1506 RSD(%) 0.51 0.39 0.35 0.33 0.47 0.98 0.67 1.00 S2 外标法 0.6607 1.3804 1.5536 1.8416 0.6035 0.9452 0.1846 0.1390 多点校正 0.6680 1.3916 1.5606 1.8497 0.6100 0.9636 0.1853 0.1390 斜率校正 0.6607 1.3786 1.5484 1.8369 0.6068 0.9423 0.1826 0.1361 单点校正 0.6666 1.3858 1.5594 1.8497 0.6116 0.9535 0.1837 0.1379 RSD(%) 0.58 0.42 0.36 0.34 0.59 1.01 0.63 0.99 S3 外标法 0.5447 1.0155 0.9947 1.0578 0.3990 0.3577 0.1388 0.1113 多点校正 0.5495 1.0215 0.9963 1.0603 0.4039 0.3640 0.1386 0.1105 斜率校正 0.5434 1.0120 0.9885 1.0529 0.4019 0.3560 0.1366 0.1082 单点校正 0.5483 1.0173 0.9956 1.0603 0.4050 0.3602 0.1374 0.1096 RSD(%) 0.53 0.39 0.36 0.33 0.65 0.97 0.75 1.21 S4 外标法 1.1633 1.6984 1.3429 1.8122 0.7758 0.7120 0.1676 0.1355 多点校正 1.1711 1.7068 1.3453 1.8154 0.7803 0.7241 0.1677 0.1352 斜率校正 1.1583 1.6908 1.3348 1.8028 0.7763 0.7081 0.1653 0.1323 单点校正 1.1686 1.6996 1.3443 1.8154 0.7824 0.7165 0.1663 0.1340 RSD(%) 0.49 0.39 0.36 0.33 0.41 0.96 0.69 1.08 S5 外标法 0.9335 1.6615 1.2327 0.9481 1.1179 0.3483 0.3350 0.1691 多点校正 0.9435 1.6756 1.2393 0.9542 1.1259 0.3558 0.3377 0.1698 斜率校正 0.9332 1.6599 1.2296 0.9476 1.1201 0.3480 0.3328 0.1662 单点校正 0.9415 1.6685 1.2384 0.9542 1.1288 0.3521 0.3348 0.1684 RSD(%) 0.57 0.43 0.38 0.39 0.45 1.05 0.60 0.93 S6 外标法 0.8631 1.8627 1.0158 0.4069 0.8688 0.1927 0.4092 0.1394 多点校正 0.8722 1.8774 1.0210 0.4108 0.8760 0.1972 0.4125 0.1395 斜率校正 0.8627 1.8599 1.0130 0.4080 0.8715 0.1928 0.4066 0.1365 单点校正 0.8703 1.8696 1.0202 0.4108 0.8783 0.1951 0.4090 0.1383 RSD(%) 0.56 0.42 0.37 0.49 0.49 1.10 0.59 1.01 S7 外标法 0.9936 1.5280 1.4353 1.2779 1.0044 0.3269 0.1799 0.1498 多点校正 1.0023 1.5384 1.4402 1.2829 1.0103 0.3334 0.1804 0.1499 斜率校正 0.9913 1.5240 1.4289 1.2740 1.0051 0.3260 0.1778 0.1467 单点校正 1.0001 1.5320 1.4391 1.2829 1.0129 0.3299 0.1788 0.1486 RSD(%) 0.52 0.40 0.36 0.34 0.41 1.02 0.65 1.00 S8 外标法 0.5004 1.0204 0.6621 0.7123 0.3074 0.2405 0.1382 0.1243 多点校正 0.5058 1.0280 0.6644 0.7158 0.3129 0.2454 0.1382 0.1239 斜率校正 0.5002 1.0184 0.6592 0.7108 0.3113 0.2399 0.1362 0.1212 单点校正 0.5047 1.0237 0.6639 0.7158 0.3138 0.2428 0.1370 0.1228 RSD(%) 0.58 0.41 0.35 0.35 0.91 1.03 0.71 1.13 S9 外标法 0.3712 0.9924 0.7505 0.8073 0.5302 0.3158 0.1578 0.1149 多点校正 0.3755 0.9991 0.7524 0.8103 0.5353 0.3217 0.1579 0.1142 斜率校正 0.3714 0.9898 0.7465 0.8047 0.5326 0.3146 0.1556 0.1118 单点校正 0.3747 0.9949 0.7519 0.8103 0.5367 0.3183 0.1565 0.1133 RSD(%) 0.59 0.40 0.36 0.33 0.54 0.99 0.70 1.18 S10 外标法 0.2984 0.9812 1.7469 1.4196 1.0218 0.4567 0.2296 0.1214 多点校正 0.3032 0.9904 1.7551 1.4268 1.0291 0.4661 0.2309 0.1212 斜率校正 0.2999 0.9812 1.7414 1.4169 1.0238 0.4558 0.2276 0.1186 单点校正 0.3025 0.9863 1.7538 1.4268 1.0318 0.4612 0.2289 0.1202 RSD(%) 0.74 0.45 0.36 0.36 0.45 1.03 0.60 1.06 -
[1] 国家药典委员会. 中华人民共和国药典2020年版(一部)[S]. 北京:中国医药科技出版社, 2020:310. [Chinese Pharmacopoeia Commission. Pharmacopoeia of the People's Republic of China 2020 (part I)[S]. Beijing:China Medical Science Press, 2020:310.] Chinese Pharmacopoeia Commission. Pharmacopoeia of the People's Republic of China 2020 (part I)[S]. Beijing: China Medical Science Press, 2020: 310.
[2] BATIHA G E, AL-SNAFI A E, THUWAINI M M, et al. Morus alba:A comprehensive phytochemical and pharmacological review[J]. Naunyn-schmiedebergs Archives of Pharmacology,2023,396(7):1399−1413. doi: 10.1007/s00210-023-02434-4
[3] 刘有志, 朱志飞, 吴月峰, 等. 桑源药材的历史沿革及现代研究进展[J]. 中成药,2023,45(1):175−184. [LIU Y Z, ZHU Z F, WU Y F, et al. Historical evolution and modern research progress of medicinal herbs of mulberry origin[J]. Chinese Traditional Patent Medicin,2023,45(1):175−184.] doi: 10.3969/j.issn.1001-1528.2023.01.032 LIU Y Z, ZHU Z F, WU Y F, et al. Historical evolution and modern research progress of medicinal herbs of mulberry origin[J]. Chinese Traditional Patent Medicin, 2023, 45(1): 175−184. doi: 10.3969/j.issn.1001-1528.2023.01.032
[4] THAIPITAKWONG T, NUMHOM S, ARAMWIT P. Mulberry leaves and their potential effects against cardiometabolic risks:A review of chemical compositions, biological properties and clinical efficacy[J]. Pharmaceutical Biology,2018,56(1):109−118. doi: 10.1080/13880209.2018.1424210
[5] LÜ Q, LIN J, WU X, et al. Novel active compounds and the anti-diabetic mechanism of mulberry leaves[J]. Frontiers in Pharmacology,2022,13:986931. doi: 10.3389/fphar.2022.986931
[6] 张宏琛, 余琳, 石慧. 桑叶多酚对鲜切生菜的保鲜效果[J]. 食品与发酵工业,2022,48(7):195−200. [ZHANG H C, YU L, SHI H. Effect of mulberry (Morus alba L.) leaf polyphenols on the preservation of fresh-cut lettuce[J]. Food and Fermentation Industries,2022,48(7):195−200.] ZHANG H C, YU L, SHI H. Effect of mulberry (Morus alba L.) leaf polyphenols on the preservation of fresh-cut lettuce[J]. Food and Fermentation Industries, 2022, 48(7): 195−200.
[7] 王春莉, 陈忠琴, 徐蕾蕾, 等. 绿色合成桑叶银纳米粒及其抗菌抗癌活性[J]. 精细化工,2021,38(1):130−137. [WANG C L, CHEN Z Q, XU L L, et al. Green synthesis of silver nanoparticles with aqueous Folium Mori extracts and their antimicrobial and anticancer activities[J]. Fine Chemicals,2021,38(1):130−137.] WANG C L, CHEN Z Q, XU L L, et al. Green synthesis of silver nanoparticles with aqueous Folium Mori extracts and their antimicrobial and anticancer activities[J]. Fine Chemicals, 2021, 38(1): 130−137.
[8] GAO T, CHEN J, XU F, et al. Mixed mulberry fruit and mulberry leaf fermented alcoholic beverages:Assessment of chemical composition, antioxidant capacity in vitro and sensory evaluation[J]. Foods,2022,11(19):3125. doi: 10.3390/foods11193125
[9] CHEN S, XI M, GAO F, et al. Evaluation of mulberry leaves hypoglycemic properties and hypoglycemic mechanisms[J]. Frontiers in Pharmacology,2023,14:1045309. doi: 10.3389/fphar.2023.1045309
[10] MARCHETTI L, TRUZZI E, FROSI I, et al. In vitro bioactivity evaluation of mulberry leaf extracts as nutraceuticals for the management of diabetes mellitus[J]. Food & Function,2022,13(8):4344−4359.
[11] 白娟, 朱倩云, 白华, 等. 高效液相色谱法同时测定桑叶中绿原酸及4种黄酮类成分的含量[J]. 中药新药与临床药理,2020,31(4):469−472. [BAI J, ZHU Q Y, BAI H, et al. Simultaneous determination of chlorogenic acid and four flavonoids in Mori Folium by HPLC[J]. Traditional Chinese Drug Research & Clinical Pharmacology,2020,31(4):469−472.] BAI J, ZHU Q Y, BAI H, et al. Simultaneous determination of chlorogenic acid and four flavonoids in Mori Folium by HPLC[J]. Traditional Chinese Drug Research & Clinical Pharmacology, 2020, 31(4): 469−472.
[12] 邓明慧, 冯玉, 路娟, 等. UPLC波长切换法同时测定桑叶中6种指标成分的含量[J]. 食品与药品,2020,22(1):25−29. [DENG M H, FENG Y, LU J, et al. Simultaneous determination of six index components in Mori Folium by UPLC wavelength switching method[J]. Food and Drug,2020,22(1):25−29.] doi: 10.3969/j.issn.1672-979X.2020.01.006 DENG M H, FENG Y, LU J, et al. Simultaneous determination of six index components in Mori Folium by UPLC wavelength switching method[J]. Food and Drug, 2020, 22(1): 25−29. doi: 10.3969/j.issn.1672-979X.2020.01.006
[13] 孙莲, 严雷, 勉强辉, 等. HPLC法同时测定新疆药桑叶中6个活性成分[J]. 中成药,2013,35(9):1954−1957. [SUN L, YAN L, MIAN Q H, et al. Simultaneous determination of six active constituents in Mori ningrae Folium by HPLC[J]. Chinese Traditional Patent Medicine,2013,35(9):1954−1957.] doi: 10.3969/j.issn.1001-1528.2013.09.028 SUN L, YAN L, MIAN Q H, et al. Simultaneous determination of six active constituents in Mori ningrae Folium by HPLC[J]. Chinese Traditional Patent Medicine, 2013, 35(9): 1954−1957. doi: 10.3969/j.issn.1001-1528.2013.09.028
[14] 王智民, 高慧敏, 付雪涛, 等. “一测多评”法中药质量评价模式方法学研究[J]. 中国中药杂志,2006(23):1925−1928. [WANG Z M, GAO H M, FU X T, et al. Multi-components quantitation by one marker new method for quality evaluation of Chinese herbal medicine[J]. China Journal of Chinese Materia Medica,2006(23):1925−1928.] doi: 10.3321/j.issn:1001-5302.2006.23.001 WANG Z M, GAO H M, FU X T, et al. Multi-components quantitation by one marker new method for quality evaluation of Chinese herbal medicine[J]. China Journal of Chinese Materia Medica, 2006(23): 1925−1928. doi: 10.3321/j.issn:1001-5302.2006.23.001
[15] SU C, LI C, SUN K, et al. Quantitative analysis of bioactive components in walnut leaves by UHPLC-Q-Orbitrap HRMS combined with QAMS[J]. Food Chemistry,2020,331:127180. doi: 10.1016/j.foodchem.2020.127180
[16] CHEN Q, WANG Z, YANG B, et al. Determination of main alkylamides responsible for Zanthoxylum bungeanum pungency through quantitative analysis of multi-components by a single marker[J]. Food Chemistry,2022,396:133645. doi: 10.1016/j.foodchem.2022.133645
[17] 王智民, 钱忠直, 张启伟, 等. 一测多评法建立的技术指南[J]. 中国中药杂志,2011,36(6):657−658. [WANG Z M, QIAN Z Z, ZHANG Q W, et al. Technical guidelines for the establishment of quantitative analysis of multi-components by single marker[J]. China Journal of Chinese Materia Medica,2011,36(6):657−658.] WANG Z M, QIAN Z Z, ZHANG Q W, et al. Technical guidelines for the establishment of quantitative analysis of multi-components by single marker[J]. China Journal of Chinese Materia Medica, 2011, 36(6): 657−658.
[18] 朱晶晶, 王智民, 高慧敏, 等. 一测多评法在中药质量评价中的应用研究进展[J]. 中国实验方剂学杂志,2016,22(16):220−228. [ZHU J J, WANG Z M, GAO H M, et al. Advances on quality evaluation of Chinese materia medica by QAMS[J]. Chinese Journal of Experimental Traditional Medical Formulae,2016,22(16):220−228.] ZHU J J, WANG Z M, GAO H M, et al. Advances on quality evaluation of Chinese materia medica by QAMS[J]. Chinese Journal of Experimental Traditional Medical Formulae, 2016, 22(16): 220−228.
[19] 熊静, 龚易昕悦, 王润月, 等. 一测多评法在食品研究中的应用进展[J]. 食品工业科技,2020,41(22):351−357. [XIONG J, GONG Y X Y, WANG R Y, et al. Research progress on quantitative analysis of multi-components by single marker for food[J]. Science and Technology of Food Industry,2020,41(22):351−357.] XIONG J, GONG Y X Y, WANG R Y, et al. Research progress on quantitative analysis of multi-components by single marker for food[J]. Science and Technology of Food Industry, 2020, 41(22): 351−357.
[20] 何兵, 杨世艳, 张燕. 一测多评中待测成分校正和定位的新方法研究[J]. 药学学报,2012,47(12):1653−1659. [HE B, YANG S Y, ZHANG Y. A new method of calibration and positioning in quantitative analysis of multi-components by single marker[J]. Acta Pharmaceutica Sinica,2012,47(12):1653−1659.] HE B, YANG S Y, ZHANG Y. A new method of calibration and positioning in quantitative analysis of multi-components by single marker[J]. Acta Pharmaceutica Sinica, 2012, 47(12): 1653−1659.
[21] 何兵, 田吉, 杨世艳. 一种新的校正方式单点校正在桑叶一测多评中的应用[J]. 药物分析杂志,2021,41(7):1133−1147. [HE B, TIAN J, YANG S Y. Application of a new single-point correction method in QAMS of Mori Folium[J]. Journal of Pharmaceutical Analysis,2021,41(7):1133−1147.] HE B, TIAN J, YANG S Y. Application of a new single-point correction method in QAMS of Mori Folium[J]. Journal of Pharmaceutical Analysis, 2021, 41(7): 1133−1147.
[22] 何兵, 吴建明, 梁思成, 等. 宜宾黄柏和四川其他产区黄柏一测多评和指纹图谱对比研究[J]. 药物分析杂志,2023,43(3):494−508. [HE B, WU J M, LIANG S C, et al. Comparative study on QAMS and fingerprint of Phellodendron chinense Schneid. from Yibin and other Sichuan producing areas[J]. Journal of Pharmaceutical Analysis,2023,43(3):494−508.] HE B, WU J M, LIANG S C, et al. Comparative study on QAMS and fingerprint of Phellodendron chinense Schneid. from Yibin and other Sichuan producing areas[J]. Journal of Pharmaceutical Analysis, 2023, 43(3): 494−508.
[23] 姬翔宇, 张子雯, 陈姿伊, 等. 一测多评法同时测定雷公藤药材及制剂雷公藤多苷片中7个质控成分[J]. 中草药,2022,53(17):5338−5347. [JI X Y, ZHANG Z W, CHEN Z Y, et al. Simultaneous quantitative determination of seven components in Tripterygium wilfordii herbs and preparations of Tripterygium wilfordii polyglycosides tablets by QAMS method[J]. Chinese Traditional and Herbal Drugs,2022,53(17):5338−5347.] JI X Y, ZHANG Z W, CHEN Z Y, et al. Simultaneous quantitative determination of seven components in Tripterygium wilfordii herbs and preparations of Tripterygium wilfordii polyglycosides tablets by QAMS method[J]. Chinese Traditional and Herbal Drugs, 2022, 53(17): 5338−5347.
[24] 张军鹏, 庄辉, 刘斌, 等. 一测多评法测定防风中6个有效成分的含量[J]. 药物分析杂志,2023,43(7):1163−1171. [ZHANG J P, ZHUANG H, LIU B, et al. Simultaneous determination of six kinds of components in Saposhnikoviae Radix by QAMS[J]. Journal of Pharmaceutical Analysis,2023,43(7):1163−1171.] ZHANG J P, ZHUANG H, LIU B, et al. Simultaneous determination of six kinds of components in Saposhnikoviae Radix by QAMS[J]. Journal of Pharmaceutical Analysis, 2023, 43(7): 1163−1171.
[25] 秦迎丹, 宋璇, 孙晨, 等. 一测多评法测定醋延胡索中5种生物碱的含量[J]. 食品工业科技,2023,44(21):302−308. [QIN Y D, SONG X, SUN C, et al. Determination of five alkaloids in vinegar corydalis yanhusuo by quantitative analysis of multi-components by single marker method[J]. Science and Technology of Food Industry,2023,44(21):302−308.] QIN Y D, SONG X, SUN C, et al. Determination of five alkaloids in vinegar corydalis yanhusuo by quantitative analysis of multi-components by single marker method[J]. Science and Technology of Food Industry, 2023, 44(21): 302−308.
[26] 王龙星, 肖红斌, 梁鑫淼. 一种提高色谱指纹谱保留时间重现性的新方法[J]. 分析化学,2003(10):1232−1236. [WANG L X, XIAO H B, LAING X M. A new method to improve the reproducibility of retention time on reversed phase C18 columns in different laboratories[J]. Chinese Journal of Analytical Chemistry,2003(10):1232−1236.] WANG L X, XIAO H B, LAING X M. A new method to improve the reproducibility of retention time on reversed phase C18 columns in different laboratories[J]. Chinese Journal of Analytical Chemistry, 2003(10): 1232−1236.





 下载:
下载:
 下载:
下载:



