Research Progress on the Preparation, Purification and Application of Chitooligosaccharides
-
摘要: 壳寡糖(Chitooligosaccharide,COS)是几丁质或壳聚糖降解后获得的N-乙酰-D-葡萄糖胺和D-葡萄糖胺的同聚或异聚物,具有抗菌、抗氧化、抗肿瘤、免疫调节等多种生物活性,已被广泛应用于医药、食品、农业等领域。目前,酶法、物理法和化学法均已被报道用于制备COS。然而,单一法制备COS有一定的局限性,很难高效、绿色地获得具有特定聚合度和乙酰度的目标产物。因此,COS的制备已经从单一法转向至协同催化体系的探索。此外,借助超滤法、活性炭吸附法、色谱法等分离纯化技术也可以提高COS的纯度。本文对近年来COS的制备和纯化方法以及应用研究进展进行了综述,以期为高品质COS的生产以及应用领域的拓展提供理论基础。Abstract: Chitooligosaccharides (COS) is homo- or heterooligomers of N-acetylglucosamine and D-glucosamine obtained via degradation of chitin or chitosan. COS is useful for remarkably wide spectrum of applications in the pharmaceutical, food, and agricultural industries due in part to their antimicrobial, antioxidant, antitumor, and immunomodulatory activities. Enzymatic, physical, and chemical methods for the preparation of COS has been reported. At present, the preparation of COS by any single method faces limitations, including difficulty in obtaining target products with specific degrees of polymerization and acetylation. Therefore, COS preparation protocols have shifted from single method strategies to explorations of cooperative catalytic systems. COS purity can also be improved by incorporating separation and purification techniques including ultrafiltration, activated carbon adsorption, and chromatography. In this paper, progress in COS preparation and purification methods and COS applications are reviewed, with the objective of providing a theoretical basis for improved production and expansion of applications for high quality COS.
-
Keywords:
- chitin /
- chitosan /
- chitooligosaccharides /
- preparation /
- separation and purification /
- application
-
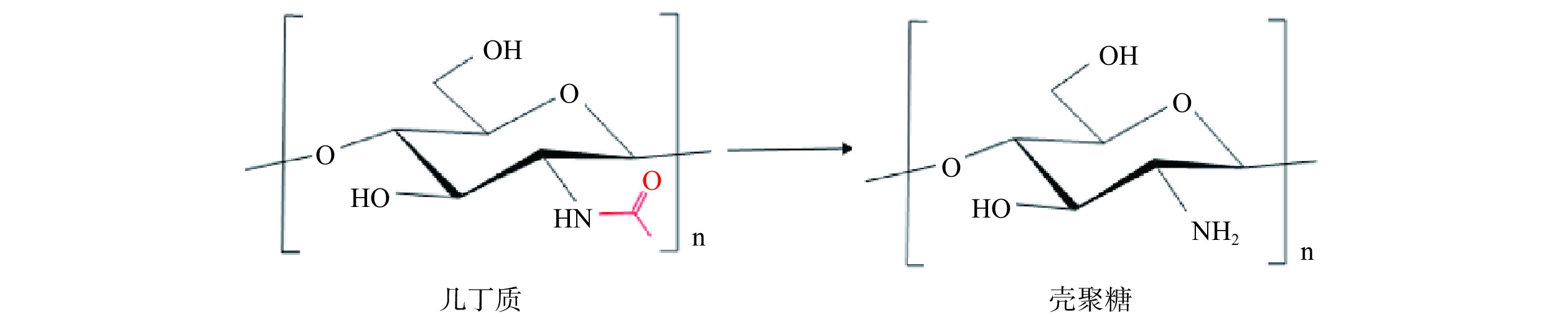
几丁质(Chitin,CI)又名甲壳素,是一种天然多糖,是由N-乙酰-D-葡萄糖胺(N-acetyl-D-glucosamine,GlcNAc)通过β-1,4-糖苷键连接而成的高分子聚合物,是世界上仅次于纤维素的第二丰富的多糖[1−2],广泛存在真菌和藻类的细胞壁以及节肢动物(如昆虫、虾和蟹)的外壳中[3]。CI经脱乙酰化作用后得到壳聚糖(Chitosan,CS)(图1),由于质子化作用,CS可以溶于酸性介质,如稀乙酸和甲酸[4−5]。但是,CI和CS的分子量和聚合度(Degree of Polymerization,DP)较高,结构致密,水溶性差,因而限制了其在工业上的应用。壳寡糖(Chitooligosaccharide,COS)是CI或CS通过酶法或化学法降解后获得的GlcNAc和D-葡萄糖胺(D-glucosamine,GlcN)的同源或异源低聚物,DP为2~20。COS是目前已知的少数碱性寡糖之一,具有抗菌、抗氧化、抗病毒、免疫调节等生物活性[6−8],在食品、医疗保健和农业等领域有巨大的应用价值。基于此,将CI转化为溶解度高、生物活性广泛的COS更有利于推动其在商业中的应用[4,9−10]。
COS的制备方法包括物理法、传统化学法、电化学法、酶法以及化学-酶法。传统化学法利用酸或碱脱去CI的乙酰基提高底物的溶解度,随后发生解聚作用生成水溶性的COS。该法操作简单,更容易实现工业化生产。COS的生物功能与其DP和乙酰化程度(Degree of Acetylation,DA)密切相关。然而,化学反应条件剧烈,很难获得具有特定DP和DA的COS产物。因此,研究者将目光转向了反应过程温和、可控、对环境更加友好的酶法。近些年来,酶法水解CI制备COS一直是研究的热点。为了解决CI结晶度高、酶法水解效率低的问题,更多的反应工程策略被提出,如多酶联合法以及化学-酶法。同时,为了进一步获得高纯度、高品质的COS以满足生物医学和食品加工业的需求,研究者们探索了不同的分离纯化方法,如超滤法、色谱法和活性炭吸附法等。因此,本文对近些年COS的制备和纯化方法进行了总结,同时对COS的应用研究进展进行了综述,以期为高品质COS的制备以及应用领域的拓展提供理论基础。
1. 壳寡糖的制备
1.1 酶解法
酶法是一种反应条件较为温和的方法,通常在常温、常压和中性pH环境下催化反应的进行,很少使用对环境有害的试剂,生成的产物供人们使用安全,因而产物可被用于农业、生物技术和生物医学等多个领域[11]。与此同时,随着遗传学、蛋白质工程和生物信息学等生物技术的发展,酶在许多工业过程中的应用开启了新时代。在过去的几十年里,酶法降解CI和CS已被认为是一种十分具有发展前景的方法。目前,用于水解CI和CS的酶分为专一性酶和非专一性酶。专一性酶包括几丁质酶和壳聚糖酶等,而非专一性酶包括纤维素酶、溶菌酶、果胶酶、蛋白酶、脂肪酶和胃蛋白酶等。
1.1.1 几丁质酶
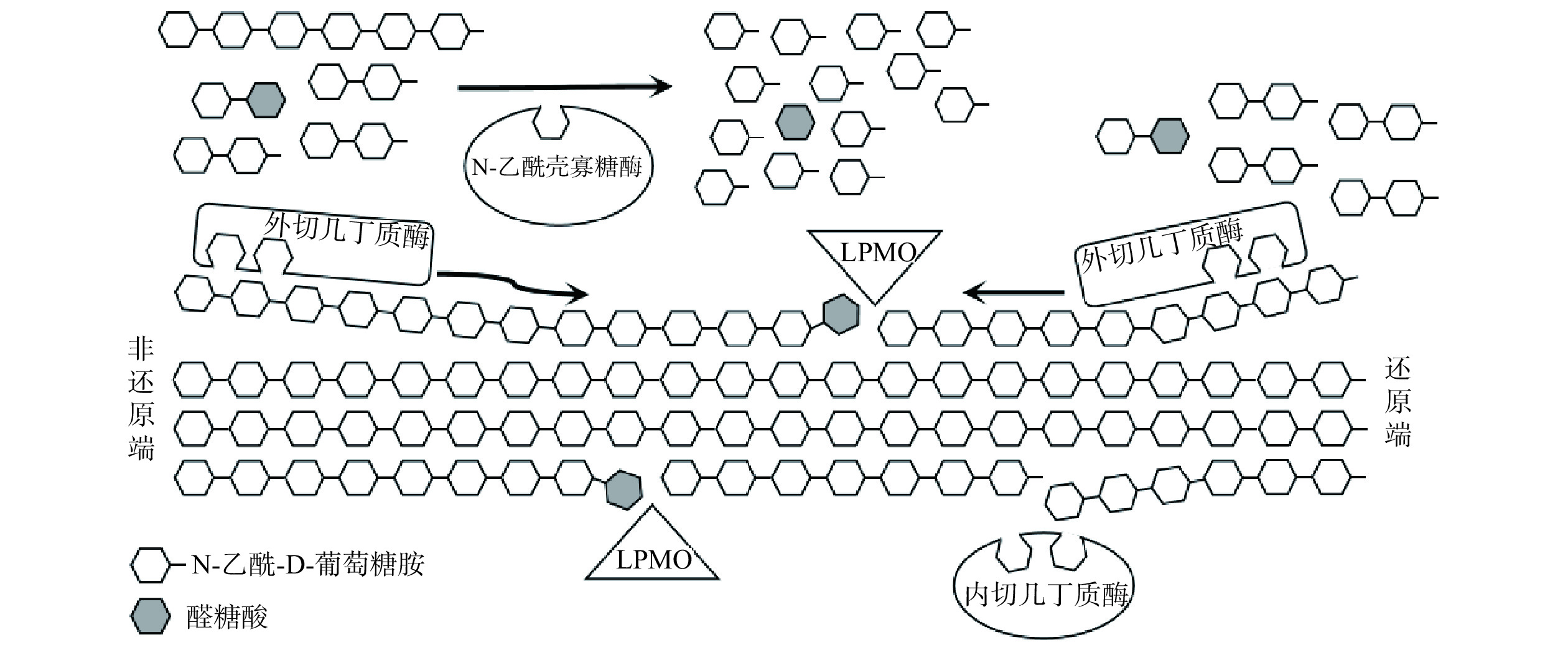
几丁质酶是专一性降解CI生成N-乙酰壳寡糖或单糖的一类酶的总称,由多种微生物(包括病毒、细菌和真菌)、昆虫或高等动植物合成[11−12]。根据水解作用模式,可以将几丁质酶分为内切几丁质酶(EC 3.2.1.14)、外切几丁质酶(EC 3.2.1.29)和N-乙酰氨基葡萄糖苷酶(EC 3.2.1.52)。内切几丁质酶在多糖链内部随机水解CI的糖苷键,生成GlcNAc或其可溶性低聚物。外切几丁质酶在CI的还原端或非还原端水解多糖链生成(GlcNAc)2,而N-乙酰氨基葡萄糖苷酶可以将(GlcNAc)2水解为GlcNAc或从N-乙酰壳寡糖的非还原端释放GlcNAc[13](图2)。
在自然界中,CI资源十分丰富,在CI降解微生物的作用下将其转化为可被利用的碳源和氮源以维持生态系统的稳定。因而,研究者们一直致力于从富含几丁质的环境中分离、纯化几丁质降解微生物,进而获得几丁质酶、壳聚糖酶或溶解性多糖单加氧酶等参与几丁质降解的酶系。Le等[15]从盐虾样品中筛选到了能够降解几丁质的微生物Salinivibrio sp. BAO-1801,并从其发酵液中纯化到了几丁质酶BAO-1801。该几丁质酶水解胶体几丁质后生成的主要产物为(GlcNAc)2,反应8 h后产率为71.5%。Wang等[16]从虾壳废弃物中筛选到了能够产低温几丁质酶的菌株Trichoderma gamsii R1,当以纯化的几丁质酶水解胶体几丁质时,生成的产物为GlcNAc、(GlcNAc)2和(GlcNAc)3,产率分别为1.73、11.62和1.92 mg/mL。Fu等[17]从海洋沉积物中分离到了一株海洋细菌Exiguobacterium antarcticum DW2,该菌株产生的几丁质酶EaChi39能够将胶体几丁质水解为GlcNAc、(GlcNAc)2和(GlcNAc)3,产率分别为9.9、14.8和5.0 mg/mL。
为了解决野生酶产量低、分离纯化过程复杂等问题,可以借助基因工程技术对几丁质酶基因进行异源表达。Thomas等[18]在Escherichia coli M15中克隆表达了Vibrio campbellii几丁质酶基因,得到的重组几丁质酶VhChiA能够以鱿鱼软骨、虾壳和蟹壳来源的胶体几丁质作为底物,主要产物均是(GlcNAc)2,产率分别为0.60、0.85和0.34 mmol/L。Gao等[19]在E. coli BL21克隆、表达了来自Streptomyces albolongus ATCC 27414的几丁质酶基因,获得的重组几丁质酶SaChiA4能够将胶体几丁质水解为GlcNAc和(GlcNAc)2,产率分别为0.87和2.17 mg/mL。大多数能够分解CI的微生物细胞内含有多个几丁质降解酶,而几丁质的高效降解可能是由这些酶联合作用完成[20]。Suzuki等[21]报道了来自Serratia marcescens 2170的三个重组几丁质酶Chi A、Chi B和Chi C1,当Chi A与Chi B或Chi A与Chi C1共同作用降解CI粉末时,(GlcNAc)1-3的总产量分别提高了80%和45%,而当三种几丁质酶同时作用于几丁质粉末时,(GlcNAc)1-3的总产量约提高了100%。因此,多种几丁质酶联合使用是实现几丁质高效降解的有效手段,并且已在多篇报道中被证实[22−24]。
1.1.2 壳聚糖酶
CI降解的另一种途径是将其去乙酰化为CS,之后在壳聚糖酶(EC 3.2.1.132)的作用下转化为COS。壳聚糖酶是一种糖苷水解酶,能够水解部分乙酰化CS中的β-1,4-糖苷键[25−26]。根据其对CS链中四种结构单元即GlcNAc-GlcN(A-D)、GlcN-GlcNAc(D-A)、GlcN-GlcN(D-D)和GlcNAc-GlcNAc(A-A)的水解特异性,可分为四种类型(CLASS Ⅰ-Ⅳ)(表1)[27]。而根据对底物的水解作用位点,壳聚糖酶又可分为内切壳聚糖酶和外切壳聚糖酶两种类型。内切壳聚糖酶的酶解产物主要为二聚体到六聚体,而外切壳聚糖酶的酶解产物只有单糖[28]。Doan等[29]从Paenibacillus sp. TKU047的发酵液中纯化到了内切壳聚糖酶TKU047,随后该课题组以粗酶液酶解脱乙酰度为98%的壳聚糖,产物为DP 2~9的COS,产率为68.44%。赵华等[30]通过硫酸铵沉淀分离纯化Bacillus cereus发酵上清液中的壳聚糖酶,利用响应面法获得了最佳酶解条件,最终COS的产物浓度可达到35.73 μmol/mL。罗洒等[31]克隆、表达了来自Bacillus amyloliquefaciens ECU08的内切壳聚糖酶,该酶酶解脱乙酰度为87.53%的CS后主要产生DP为2~3的COS,并且在30 L规模的扩大试验研究中总产物的生产收率和原料收率分别为75.8%和85.1%。物料衡算和经济核算表明,该工艺收益较高,适用于工业化生产。
表 1 四种不同裂解结构单元的壳聚糖酶Table 1. Chitosanase with four different cleavage structural units分类 裂解结构单元 CLASS Ⅰ D-D,A-D CLASS Ⅱ D-D CLASS Ⅲ D-D,D-A CLASS Ⅳ D-D,D-A,A-D 1.1.3 溶解性多糖单加氧酶
在自然界中,除了几丁质酶和壳聚糖酶等糖苷水解酶外,溶解性多糖单加氧酶(EC 1.14.99.54,Lytic Polysaccharide Monooxygenase,LPMO)也是参与几丁质降解的关键酶之一。LPMO是一种铜依赖性酶[32],铜离子结合在特殊的组氨酸支架中,使LPMO具有显著的氧化能力,在电子供体(如抗坏血酸)存在的情况下,可以催化多糖结晶区域的糖苷键氧化裂解,使氧化位点附近区域的结晶度降低,并且产生新的链端供糖苷水解酶识别,进而提高水解效率(图2)[14,33−34]。LPMO与水解酶协同作用,在结晶性多糖如纤维素和CI的生物转化方面发挥了重要作用。Zhang等[35]将来源于Bacillus amyloliquefaciens的BtLPMO10A和BtLPMO10B分别与S. marcescens来源的几丁质酶SmChiB协同作用降解α-几丁质,与SmChiB单独酶解组相比,(GlcNAc)2的产量由0.21 mg/mL分别提高至1.35和1.17 mg/mL。Vaaje-Kolstad等[36]克隆、表达了来源于Lactococcus lactis ssp. lactis IL1403的几丁质酶LlChi18A和LPMO(LlCBP33A),并用于降解α-几丁质和β-几丁质。以α-几丁质作为底物,相同反应时间下,仅以LlChi18A作为催化剂时(GlcNAc)2的产率约为130 μmol/L,在LlCBP33A的协同作用下(GlcNAc)2的产率提高至大约165 μmol/L。以β-几丁质作为底物,仅以LlChi18A作为催化剂反应350 h后(GlcNAc)2的产率约为160 μmol/L,但是在LlCBP33A的协同作用下仅需50 h即可达到相当的产率。之后,该课题组克隆、表达了来源于Enterococcus faecalis V583的几丁质酶EfChi18A和LPMO(EfCBM33A)降解α-几丁质和β-几丁质。仅以EfChi18A作为催化剂时(GlcNAc)2的产率分别约为60和300 μmol/L,在EfCBM33A的协同作用下(GlcNAc)2的产率分别提高至大约85和1050 μmol/L[37]。LPMO在与几丁质酶协同作用降解CI方面展现了优越的催化性能,从其被发现能够氧化裂解结晶多糖后一直是研究的热点,并且从工业和科学的角度来说都具有重要的研究意义。然而,LPMO在催化反应过程中需要加入电子供体,在工业大规模应用中额外添加电子供体生产成本较高。因此,寻找价格低廉的电子供体,构建高效降解几丁质的生物催化体系,并揭示其协同作用机制是未来研究的重要方向。
1.1.4 非专一性酶
除了上述特异性酶类外,还有一些非特异性酶类对CI和CS也展现了较高的水解活性。黄晓月等[38]以被广泛用于食品工业的木瓜蛋白酶作为催化剂水解CS,通过单因素及响应面设计优化工艺条件最终获得DP为6~10的COS,产率为45.07%。Dong等[39]对比研究了纤维素酶、木瓜蛋白酶、α-淀粉酶、溶菌酶、木聚糖酶和β-糖苷酶对CS的水解活性,其中纤维素酶和木瓜蛋白酶展现了较高的水解活力。随后,该课题组构建了包括纤维素酶、木瓜蛋白酶和壳聚糖酶的联合酶系统水解CS,制备的COS DP为6~8,目标产物的产率为79.84%。另有报道采用α-淀粉酶水解经H2O2预处理后的CS制备出DP为2~8的COS,在最适反应条件下,COS的产量约为1.05 mmol/g[40]。
1.2 物理法
用于制备COS的物理法包括紫外辐射、超声破碎和微波处理等。Xing等[41]采用微波辐射法(80 ℃,800 W,辐射时间25 min)制备的COS聚合度为2~6,并且具有免疫调节活性。Wu等[42]利用涡轮空化装置进行旋流空化降解CS,反应条件经优化后CS的结晶度降低了83.65%,溶解性得到极大的增强。Margoutidis等[43]利用球磨机使CI的结晶度降低50%,并且添加天然粘土高岭石可以使CI的溶解度增加1倍,获得的产物为GlcNAc和(GlcNAc)2。物理法操作相对简单,但是获得的产物产量较低,且降解程度有限,进而限制了其大规模生产,无法产生较大的社会经济效益[44−46]。
1.3 化学法
目前,用于水解CS制备COS的化学试剂有酸(如盐酸、亚硝酸和磷酸)和氧化还原剂(如过氧化氢、臭氧和次氯酸)[47−49]。酸性溶液中的氢离子能够与CS分子中的游离氨基结合,使得CS的分子间氢键断裂,从而获得DP不等的COS[50]。如季者等[51]研究了盐酸对CS的降解作用,在60 ℃下、以9 mol/L的盐酸降解CS后获得的主要产物为壳五糖和壳六糖,其总产量为16.2%。氧化剂在水溶液中形成的游离自由基可以断裂CS的糖苷键,进而形成DP不等的COS[50]。如焦富颖[52]利用H2O2降解CS制备COS,在最优反应条件下,即0.5wt%壳聚糖,8vol% H2O2,反应时间5 h,反应温度50 ℃,产物的分子量在2000 Da左右,收率为85%。化学法制备COS操作简单,但是降解产物的DP范围分布广泛,产量低。使用酸性溶液制备COS时,生成的产物安全性低,生成的二级产物难以分离,残留酸和由此产生的有毒废弃物后续处理复杂,易造成环境污染[53]。使用氧化剂降解CS时,若氧化剂浓度或反应温度过高,会导致CS水解过度,产物的氨基损失较多,颜色也会由于褐变而加深,使得品质下降。因此,CS经氧化剂部分降解后,需结合后续的分离纯化过程以得到高品质的COS。
此外,电化学法也被报道用于制备COS。通常来讲,电极材料基本分为两种类型,一种为活性电极(如Ti/TiO2-RuO2电极),另一种为非活性电极(如Ti/Sb-SnO2电极)[54]。Cai等[55]利用Ti/TiO2-RuO2电极降解CS,CS的分子量随着电流密度的增加而降低。当电流密度增大至160 mA/cm2时,处理1 h后CS的黏均分子量由491 kDa降低至33 kDa。之后,该课题组分别利用Ti/Sb-SnO2和Ti/TiO2-RuO2电极降解CS,在60 mA/cm2的电流密度条件下处理1 h后,CS的黏均分子量由479 kDa分别降低至46 kDa和158 kDa[54]。电化学法操作简单,无污染,具有潜在的应用价值。但是,该方法存在电极寿命短、易失效等问题。因此,开发出具有更高电流效率和更低成本的制备装置是该领域未来研究的重点。
1.4 化学-酶法
单一法制备COS会存在一定的不足,如酶解法无法高效水解CI致密的结晶结构,多数情况下需要将CI制备成胶体CI。通过化学法预处理CI能够提高酶解效率,起到“1+1>2”的效果。郑必胜等[56]在60 ℃下用过氧化氢(4%,w/v)、乙酸(4%,w/v)预处理脱乙酰度为96.7%的CS,使得CS的表面结构被破坏,孔隙增多,获得DP<10的COS,产率约为62%。Sivaramakrishna等[57−58]利用氢氧化钾(11.2%~20%)水溶液或氢氧化钾(11.2%~20%)-尿素(4%)水溶液预处理CI,结果表明CI经预处理后酶解效率显著提高,COS产率约为70%。
上述用于预处理CI的化学试剂为强酸或强碱试剂,反应条件苛刻,对环境不友好。为了满足绿色化工的生产理念,亟需绿色、温和的溶剂来替代上述化学试剂。离子液体(Ionic liquids,ILs)是一种在室温下以液体形式存在的离子化合物,具有熔点低、稳定性高、无挥发性、可回收等特点,因而对环境的化学污染较小。ILs能够溶解天然聚合物,如CI[59]或纤维素[60]。几丁质经ILs预处理并再生后,氢键网络发生重排,表面结构被破坏,结晶度降低[23,61]。因此,研究者们构建了基于ILs和几丁质酶的化学-酶法降解几丁质制备单糖和寡糖(表2)。尽管ILs被称为“绿色溶剂”,但是其对水生和陆地生态系统的影响还有待评估[62]。
表 2 ILs-酶法制备COSTable 2. Preparation of COS by ILs-enzymatic process底物 离子液体及预处理条件 酶 产物产量
(mg/g几丁质)提高倍数a
(GlcNAc、(GlcNAc)2)文献 GlcNAc (GlcNAc)2 α-几丁质 1-乙基-3-甲基咪唑鎓,110 ℃孵育40 min 来自Trichoderma viride的几丁质酶 185.0 <4 15、无明显变化 [61] α-几丁质 1-乙基-3-甲基咪唑鎓,110 ℃孵育40 min 来自Streptomyces griseus的几丁质酶 < 1 705.0 无明显变化、1.5 [61] α-几丁质 1-乙基-3-甲基咪唑鎓乙酸酯,
105 ℃孵育30 min来自Streptomyces albolongus的
几丁质酶175.62 341.70 9.10、6.43 [63] 蟹壳粉
(粒径<100 目)1-丁基-3-甲基咪唑醋酸盐,
100 ℃孵育60 min来自Paenibacillus pasadenensis的
几丁质酶chip1178.6 368.8 3.71、无明显变化 [23] 蟹壳粉
(粒径<100 目)1-丁基-3-甲基咪唑醋酸盐,
100 ℃孵育60 min来自Paenibacillus pasadenensis的
几丁质酶chip2约25 561.3 2、2 [23] 虾壳粉
(粒径<90 μm)1-乙基-3-甲基咪唑鎓,虾壳粉与ILs以1:49(g/ml)混合后,微波脉冲加热2.5 min,
用蒸馏水再生提取生成的CI,之后风干
研磨至粒径< 125 μm来自Streptomyces griseus的几丁质酶 − 200.8 1.41b [64] 注:a表示相同酶解条件下,与未经ILs预处理的底物相比;b表示相同酶解条件下,与市售CI相比。 2. 壳寡糖的纯化
通过酶法、化学法或协同法降解CI或CS后获得的产物通常为单体、低聚物或同分异构体的混合物,产物的分子量和DP分布较宽。为了更好地揭示COS的构效关系以及满足生物医学和食品加工业对其纯度和质量的要求,选择合适的方法从混合物中分离和鉴定所需要的COS至关重要。由于COS分子中含有较多的氨基和羟基,并且存在较强的分子间或分子内作用力,因而增加了分离纯化的难度。
2.1 超滤法
利用膜生物反应器超滤提纯COS是较为常见的方法,具有操作简便、绿色环保、成本低等优点。影响COS回收率的因素包括膜的类型、操作温度、进料溶液的pH和溶液的浓度[65]。Yu等[66]采用截留分子量为3 kDa的超滤膜制备膜生物反应器,当停留时间为50 min时,高DP的COS(DP≥5)产物纯度约为28%,当停留时间为100 min时,高DP的COS(DP≥5)产物纯度约为48%。Aider等[67]利用电渗析和超滤联合法分离纯化DP为2~4的COS混合物,研究发现二聚体在不同的pH下均能达到最佳纯化效果,其次是三聚体,最后是四聚体。根据处理时间的不同,在pH6的条件下可以将二聚体和三聚体分离出来,或者在pH7条件下仅将二聚体分离出来。仲伟伟等[68]选用截留分子量为10 kDa的卷式超滤膜对COS粗品进行超滤,超滤所得样品纯度为78.58%。吴健锋[69]在35 ℃下采用2.5 kDa的超滤膜截留DP 为2~6的COS,最终目标产物的纯度高达93.88%。膜生物反应器易于操作,通过截留分子大小的方式可以有效地提高产物纯度,更好地达到分离纯化的目的。
2.2 色谱法
色谱法纯化COS包括离子交换色谱(Ion Exchange,IEC)法、薄层色谱(Thin Layer Chromatography,TLC)法和高效液相色谱(High Performance Liquid Chromatography,HPLC)法等。IEC法是利用被分离组分与固定相之间发生离子交换的能力差异来实现分离的方法,一般选择离子交换树脂作为固定相。Wei[70]及Li等[71]利用CM-葡聚糖凝胶C-25柱对不同DA的壳六糖成功进行了分离纯化,可得到纯度高达93%的壳六糖。TLC法是利用各组分对同一吸附剂吸附能力不同,在流动相(溶剂)流过固定相(吸附剂)不断发生吸附—解吸过程中将各组分分离。该法以相对较低的成本,基于DP对低聚物混合物进行分析。Chen等[72]在硅胶板上以甲醛:甲醇:25%氨水:水=5:10:1.5:1(v/v/v/v)混合试剂作为展开剂成功分离出GlcN,Le等[15]以正丁醇:乙酸:水=2:1:1(v/v/v)混合试剂作为展开剂在硅胶板上成功分离出DP为1~6的COS。除此之外,已被报道用于分离COS的展开剂还有正丁醇:甲醇:25%氨水:水=5:4:2:1(v/v/v)[56]、正丙醇:水:28%氨水=70:15:15(v/v/v)[73]和正丁醇:水:乙酸:氨=10:5:5:1(v/v/v/v)等[74]。相较于TLC法,HPLC法可以结合质谱法基于DP和DA进行精确分析。由于含有乙酰氨基,GlcNAc在204 nm处有最大紫外吸收。因此,配备紫外检测器的HPLC只能检测到部分乙酰化或完全乙酰化的COS。除了紫外检测器外,也可以配备示差折光检测器对COS进行分析。严佳佳等[75]利用色谱柱SB-C18(4.6 mm×250 mm,5 μm)分离纯化到了酶解产物中的GlcN和GlcNAc,Li等[63]利用色谱柱Sugar Pak I(6.5×300 mm)对酶解产物中的GlcNAc和(GlcNAc)2进行分离纯化。此外,Aminex HPX-87H(300×7.8 mm)[23]、Shodex Asahipak NH2P-50 4E(4.6×250 mm)[68]和Shodex Asahipak NH2P-50E(4.6×250 mm)[7]等色谱柱均被用于COS的分离纯化。
2.3 其他分离纯化方法
活性炭因结构疏松多孔、表面积大而具有较强的吸附性,同时由于成本较低而被广泛应用。Yu等[76]通过间歇模式实验,探究了活性炭对COS吸附效率的影响因素。结果表明,活性炭颗粒越小对COS的吸附能力越强,pH为8~9时吸附量最大,在接触时间小于60 min时随着接触时间的延长吸附量增大,而后达到吸附平衡状态,温度对活性炭吸附COS的能力无显著影响。毛细管电泳(Capillary Electrophoresis,CE)法也用于分离纯化COS,仅需要少量的溶质和溶剂,分离时间短,分辨率高。然而,复杂的衍生化过程以及昂贵材料的使用使得该法经济成本较高[77]。此外,基质辅助激光解吸电离飞行时间质谱(Matrix Assisted Laser Desorption Ionization Time of Flight Mass Spectrometry,MALDI-TOF-MS)技术是分析生物分子,如脂类、糖类、多肽和其他有机大分子的最合适的技术。但是,该方法不适合检测分子量低于500 Da的样品[78]。
3. 壳寡糖的应用
3.1 壳寡糖在食品加工贮藏领域的应用
COS具有广泛的抗菌活性,可以抑制多种致病菌和腐败微生物的生长[79−80],因而已被用于食品防腐和果蔬保鲜。在酿酒过程中加入500 mg/L的COS(平均分子量<2000 Da,DP 2~10)可以抑制腐败微生物的生长,但是对酿酒酵母的生长无影响[81]。向生牛乳中添加0.24% COS并置于4 ℃下保存12 d,与未添加COS的对照组相比,其嗜热菌和嗜冷菌至少降低3个数量级[82]。在面包中加入1%的COS,面包中食源性病原菌及根霉菌的生长均受到抑制[83]。COS还具有良好的成膜特性,以浓度为1.5 g/100 mL的COS(分子量700 Da左右)溶液对鲜切苹果进行涂膜处理,菌落总数、霉菌、酵母菌和大肠菌群数明显低于对照组。COS涂膜处理还能调控抑制鲜切苹果呼吸强度的增强,保持可溶性固形物和可滴定酸含量的稳定,防止失重率增加,减缓软化[84]。
COS具有抗氧化活性,可以改善食品品质,延长食品的货架期。向低筋小麦粉中加入1%的COS后,不仅能够改善饼干制品的组织结构,使酥性饼干断面结构气孔细密均匀,同时降低饼干的硬度和咀嚼性,提高饼干的酥松度,改善口感滋味。与此同时,COS的加入使得饼干样品的酸价、过氧化值和TBA值降低,有效延缓了储藏期饼干的氧化酸败,延长了酥性饼干的保质期[85]。以4 mg/mL的COS溶液(DP 2~4)对湘派休闲豆干进行涂膜处理,能够有效延缓样品在常温贮藏过程中微生物的生长繁殖及品质劣变,使样品货架期延长20 d以上[86]。此外,COS对食物在预处理过程中引起的多不饱和脂肪酸氧化有抑制作用,进而延长食物的货架期[87]。
COS可以改善贮藏过程中果皮活性氧的代谢情况,进而降低果皮褐变发病率。刘丽丹等[88]用0.5%的COS溶液浸泡枇杷果实,使果实在冷藏期间保持较高的可溶性固形物、还原型抗坏血酸及还原糖含量,同时多酚氧化酶活性受到一定抑制,枇杷果实失重率、褐变指数和腐烂指数均明显降低。赵韩栋等[89]用0.5%的COS(平均分子量1500~2000 Da,脱乙酰度95%)水溶液对皇冠梨进行贮前浸泡处理,在低温贮藏过程中,果皮中多酚氧化酶、脂氧合酶活性的上升,过氧化氢酶、抗坏血酸过氧化物酶、超氧化物歧化酶和苯丙氨酸解氨酶的活性维持在较高水平,果皮中总酚、抗坏血酸维持在较高水平,过氧化氢和丙二醛的积累减少,果实褐变发病率和发病指数分别下降了89%和32%。段树华等[90]用浓度为1%的COS溶液浸泡处理甜樱桃,使得低温贮藏过程中果实的可滴定酸、维生素C、总酚的含量下降,多酚氧化酶、过氧化物酶、过氧化氢酶、超氧化物歧化酶的活性升高,丙二醛含量降低,膜脂质过氧化水平降低,有效防止了甜樱桃果实在贮藏期的的衰老褐变,保持了果实的风味品质。
COS具有良好的吸湿性、持水性和热稳定性,并且能够防止淀粉老化。制作海绵蛋糕时,向低筋小麦粉中加入1wt%的COS(分子质量<3000 Da)有助于增加面糊的黏弹性以及降低面糊密度,可使蛋糕成品表面色泽均匀、组织细腻、有弹性、气孔均匀、滋味与口感良好[91]。制作挤压面粉制品时,向小麦粉中加入3.2wt%的COS(纯度>80%)可显著增强面团的面筋蛋白网络,减缓淀粉的降解过程,面制品的含水率、膨胀率及吸油率增加,硬度降低,品质得到提升[92]。
3.2 壳寡糖在医疗保健领域的应用
COS具有多种生物活性,被肠上皮细胞吸收后可以到达身体的各个部位,进而发挥其生理功能[93]。王胜田等[94]研制了一种含COS、银杏叶提取物、丹参提取物及淀粉的COS胶囊,具有辅助降血脂作用,并且对化学性肝损伤有辅助保护功能。郝桂娟等[95]研究发现,COS-Zn2+配合物对氧化衰老模型小鼠的机体脏器恢复有一定的帮助作用,能够显著提高机体血清、肾脏和肝脏中的超氧化物歧化酶、谷胱甘肽过氧化物酶、过氧化氢酶活性和总抗氧化能力,展现了较好的抗衰老及增强机体免疫功能作用。苏政权等[96]研制了一种COS肠溶胶囊,内容物包括肠溶辅料、壳寡糖和释放调节剂,三者的质量比为(3~5):1:1。在COS的干预下,小鼠肠道菌群能减少高脂高糖饮食中膳食脂肪的摄入,从而实现抗肥胖的效果;血糖水平也显著降低,即该COS肠溶胶囊表现出良好的辅助调节血糖作用。COS(分子量约为2000 Da)可以打开小肠微绒毛上皮细胞间的紧密连接,从而提高肠道的通透性。基于此,COS可作为BCSⅢ类药物的口服吸收促进剂[97]。
COS已被批准为“新食品原料”[98],因此,诸多含有COS成分的功能性食品已被开发,以期使得饮食干预成为治疗某些疾病的有效手段。庄林等[99]研究发现以COS、海洋鱼低聚肽、樱桃粉、茯苓粉为主要成分的复合固体饮料能够通过调节小鼠肠道菌群组成和短链脂肪酸水平改善高尿酸血症。姜雅杰等[100]研究了含有COS、白芸豆提取物、水苏糖、葡聚糖及亚麻籽油的复合固体饮料对Ⅱ型糖尿病小鼠肠道菌群结构的影响,结果表明该复合固体饮料可以促进肠道内有益菌的生长与定植,降低条件致病菌的相对丰度,在提高肠道免疫力同时具有辅助治疗Ⅱ型糖尿病的功效。喻凯[101]研发了一种能够增强肠胃功能及免疫力的几丁寡糖食品,其配方主要包含几丁寡糖、膳食纤维及益生菌类。王孝文等[102]开发了一种以COS、浒苔多糖、药用淀粉、硬脂酸镁为主要原料的保健食品,该产品有益于提高机体免疫力,增强免疫功能。总之,COS呈现出的多元化功能使其在保健品的开发中具有很高的应用价值。
3.3 壳寡糖在农业领域的应用
在农田中施加一定量的COS不仅可以起到抗病毒、抑菌的功效,还能在一定程度上调节植物的生长发育。在抗烟草花叶病毒的研究中发现,COS可以提高叶片中多种防御酶的活性,不仅可以有效减少烟叶感染烟草花叶病毒的枯斑数,也可以降低已染病毒烟草中叶绿素的下降幅度[103]。高雨萌[104]等探究了COS(平均分子量3000 Da,脱乙酰度为90%)对花椒干腐病的防治效果,发现浓度为0.5 mg/mL的COS溶液对花椒盆栽苗干腐病的防治效果可达到65.02%。COS在低温下可以使水稻的相对电导率显著降低,降低丙二醛对水稻的危害,进而减少低温对水稻幼苗的伤害。COS也可以提高幼苗的抗旱能力,对提高作物的耐盐能力有一定的效果[105]。金国强等[106]分别用分子量为1500和2500 Da的COS溶液对宫川温州蜜柑进行1000倍树冠叶面喷雾处理后,果实中可溶性固形物含量升高,可滴定酸浓度降低,果实的品质得到了改善。黄雪燕等[107]分别用分子量为1000和2000 Da的COS溶液对温岭高橙进行200、500和1000倍的叶片喷雾和根部浇灌处理,收获的单果质量均高于对照组。此外,COS在防治果蔬的采后病害[108]、诱导植物先天免疫[109]、增强植物生理反应[110]等方面的应用也被广泛报道。
4. 结论
CI在自然界中含量丰富,生物相容性好,无毒性作用,降解后获得的COS分子量低,溶解度高,具有多种生物活性。迄今为止,尽管关于COS的制备、纯化和应用研究已取得了巨大进展,但是仍存在一定的不足。对于如何绿色、大规模地获取高纯度的COS仍是当下面临的主要挑战。传统化学法较易于实现工业化生产,但是由于反应过程不可控制而无法保持产物品质的稳定性,进而限制了其在下游特定领域的应用。此外,反应过程中使用的化学试剂对环境有害。酶法反应过程温和,反应过程可控,尤其是采用基因工程技术对酶进行改造以及采用协同催化体系(如多酶联合法或化学-酶法等)后,CI的降解效率显著提高。因此,优质酶种的挖掘、制备工艺的优化仍是未来努力的方向。物理法和电化学法的出现也为COS的制备提供了新的技术路径,值得进一步探索。在COS的高效制备过程中,可能存在目标产物纯度低、杂质过多等问题,因此如何选择合适的分离纯化方法、并以较低的经济成本建立大规模的纯化体系仍需继续深入研究。此外,COS在食品加工贮藏领域、医疗保健领域和农业领域的应用已被广泛报道,但是对于COS生物活性背后的作用机制仍不明确。因此,对于COS的构效关系仍需不断探索,这对于实现其在食品及其他领域的应用具有重要意义。
-
表 1 四种不同裂解结构单元的壳聚糖酶
Table 1 Chitosanase with four different cleavage structural units
分类 裂解结构单元 CLASS Ⅰ D-D,A-D CLASS Ⅱ D-D CLASS Ⅲ D-D,D-A CLASS Ⅳ D-D,D-A,A-D 表 2 ILs-酶法制备COS
Table 2 Preparation of COS by ILs-enzymatic process
底物 离子液体及预处理条件 酶 产物产量
(mg/g几丁质)提高倍数a
(GlcNAc、(GlcNAc)2)文献 GlcNAc (GlcNAc)2 α-几丁质 1-乙基-3-甲基咪唑鎓,110 ℃孵育40 min 来自Trichoderma viride的几丁质酶 185.0 <4 15、无明显变化 [61] α-几丁质 1-乙基-3-甲基咪唑鎓,110 ℃孵育40 min 来自Streptomyces griseus的几丁质酶 < 1 705.0 无明显变化、1.5 [61] α-几丁质 1-乙基-3-甲基咪唑鎓乙酸酯,
105 ℃孵育30 min来自Streptomyces albolongus的
几丁质酶175.62 341.70 9.10、6.43 [63] 蟹壳粉
(粒径<100 目)1-丁基-3-甲基咪唑醋酸盐,
100 ℃孵育60 min来自Paenibacillus pasadenensis的
几丁质酶chip1178.6 368.8 3.71、无明显变化 [23] 蟹壳粉
(粒径<100 目)1-丁基-3-甲基咪唑醋酸盐,
100 ℃孵育60 min来自Paenibacillus pasadenensis的
几丁质酶chip2约25 561.3 2、2 [23] 虾壳粉
(粒径<90 μm)1-乙基-3-甲基咪唑鎓,虾壳粉与ILs以1:49(g/ml)混合后,微波脉冲加热2.5 min,
用蒸馏水再生提取生成的CI,之后风干
研磨至粒径< 125 μm来自Streptomyces griseus的几丁质酶 − 200.8 1.41b [64] 注:a表示相同酶解条件下,与未经ILs预处理的底物相比;b表示相同酶解条件下,与市售CI相比。 -
[1] SCHMITZ C, AUZA L G, KOBERIDZE D, et al. Conversion of chitin to defined chitosan oligomers:Current status and future prospects[J]. Marine Drugs,2019,17(8):452. doi: 10.3390/md17080452
[2] NOVIKOV V Y, RYSAKOVA K S, SHUMSKAYA N V, et al. King crab gills as a new source of chitin/chitosan and protein hydrolysates[J]. International Journal of Biological Macromolecules,2023,232:123346. doi: 10.1016/j.ijbiomac.2023.123346
[3] KOU S G, PETERS L M, MUCALO M R. Chitosan:A review of sources and preparation methods[J]. International Journal of Biological Macromolecules,2021,169:85−94. doi: 10.1016/j.ijbiomac.2020.12.005
[4] KUMAR M, BRAR A, VIVEKANAND V, et al. Bioconversion of chitin to bioactive chitooligosaccharides:Amelioration and coastal pollution reduction by microbial resources[J]. Marine Biotechnology,2018,20(3):269−281. doi: 10.1007/s10126-018-9812-x
[5] 刘梦琪, 吕瑞, 陈菊, 等. 壳聚糖的抗菌作用及在抑菌活性包装中的应用进展[J]. 食品科学,2024,45(1):261−271. [LIU M Q, LÜ R, CHEN J, et al. Antibacterial action of chitosan and its application in antibacterial active packaging[J]. Food Science,2024,45(1):261−271.] doi: 10.7506/spkx1002-6630-20230119-146 LIU M Q, LÜ R, CHEN J, et al. Antibacterial action of chitosan and its application in antibacterial active packaging[J]. Food Science, 2024, 45(1): 261−271. doi: 10.7506/spkx1002-6630-20230119-146
[6] 周勇. 酶法脱乙酰化制备壳寡糖的研究进展[J]. 安徽农学通报, 2021, 27(11):27-31, 118. [ZHOU Y. Research progress of enzymatic deacetylation to make chitosan oligosaccharides[J]. Anhui Agricultural Science Bulletin, 2021, 27(11):27-31, 118.] ZHOU Y. Research progress of enzymatic deacetylation to make chitosan oligosaccharides[J]. Anhui Agricultural Science Bulletin, 2021, 27(11): 27-31, 118.
[7] KUMAR M, MADHUPRAKASH J, BALAN V, et al. Chemoenzymatic production of chitooligosaccharides employing ionic liquids and Thermomyces lanuginosus chitinase[J]. Bioresource Technology,2021,337:125399. doi: 10.1016/j.biortech.2021.125399
[8] WANG Y Y, ZHAO K, LI L, et al. A review of the immune activity of chitooligosaccharides[J]. Food Science and Technology,2023,43:e97822. doi: 10.1590/fst.97822
[9] LÜ J R, LÜ X H, MA M H, et al. Chitin and chitin-based biomaterials:A review of advances in processing and food applications[J]. Carbohydrate Polymers,2023,299:120142. doi: 10.1016/j.carbpol.2022.120142
[10] KASPRZAK D, GALIŃSKI M. Chitin and chitin-cellulose composite hydrogels prepared by ionic liquid-based process as the novel electrolytes for electrochemical capacitors[J]. Journal of Solid State Electrochemistry,2021,25(10-11):2549−2563. doi: 10.1007/s10008-021-05036-3
[11] TAOKAEW S, KRIANGKRAI W. Chitinase-assisted bioconversion of chitinous waste for development of value-added chito-oligosaccharides products[J]. Biology,2023,12(1):87. doi: 10.3390/biology12010087
[12] LE B, YANG S H. Microbial chitinases:Properties, current state and biotechnological applications[J]. World Journal of Microbiology and Biotechnology,2019,35(9):144. doi: 10.1007/s11274-019-2721-y
[13] CHAVAN S B, DESHPANDE M V. Chitinolytic enzymes:an appraisal as a product of commercial potential[J]. Biotechnology Progress,2013,29(4):833−846. doi: 10.1002/btpr.1732
[14] COURTADE G, AACHMANN F L. Chitin-active lytic polysaccharide monooxygenases[J]. Advances in Experimental Medicine and Biology,2019,1142:115−129.
[15] LE B, YANG S H. Characterization of a chitinase from Salinivibrio sp. BAO-1801 as an antifungal activity and a biocatalyst for producing chitobiose[J]. Journal of Basic Microbiology,2018,58(10):848−856. doi: 10.1002/jobm.201800256
[16] WANG J R, ZHU M J, WANG P, et al. Biochemical properties of a cold-active chitinase from marine Trichoderma gamsii R1 and its application to preparation of chitin oligosaccharides[J]. Marine Drugs,2023,21(6):332. doi: 10.3390/md21060332
[17] FU X, GUO X X, JIN Y G, et al. Bioconversion of chitin waste using a cold-adapted chitinase to produce chitin oligosaccharides[J]. LWT-Food Science and Technology,2020,133:109863. doi: 10.1016/j.lwt.2020.109863
[18] THOMAS R, FUKAMIZO T, SUGINTA W. Bioeconomic production of high-quality chitobiose from chitin food wastes using an in-house chitinase from Vibrio campbellii[J]. Bioresources and Bioprocessing,2022,9(1):86. doi: 10.1186/s40643-022-00574-8
[19] GAO L, SUN J A, SECUNDO F, et al. Cloning, characterization and substrate degradation mode of a novel chitinase from Streptomyces albolongus ATCC 27414[J]. Food Chemistry,2018,261:329−336. doi: 10.1016/j.foodchem.2018.04.068
[20] DAHIYA N, TEWARI R, HOONDAL G S. Biotechnological aspects of chitinolytic enzymes:A review[J]. Applied Microbiology and Biotechnology,2006,71(6):773−782. doi: 10.1007/s00253-005-0183-7
[21] SUZUKI K, SUGAWARA N, SUZUKI M, et al. Chitinases A, B, and C1 of Serratia marcescens 2170 produced by recombinant Escherichia coli:enzymatic properties and synergism on chitin degradation[J]. Bioscience, Biotechnology, and Biochemistry,2002,66(5):1075−1083. doi: 10.1271/bbb.66.1075
[22] ZHANG Q, ZHANG X Y, HE Y C, et al. The synergistic action of two chitinases from Vibrio harveyi on chitin degradation[J]. Carbohydrate Polymers,2023,307:120640. doi: 10.1016/j.carbpol.2023.120640
[23] XU P, WU X L, GUO X X, et al. Double-chitinase hydrolysis of crab shell chitin pretreated by ionic liquid to generate chito-oligosaccharide[J]. ACS Sustainable Chemistry and Engineering,2019,7(1):1683−1691. doi: 10.1021/acssuschemeng.8b05447
[24] MUKHERJEE S, BEHERA P K, MADHUPRAKASH J. Efficient conversion of crystalline chitin to N-acetylglucosamine and N, N’-diacetylchitobiose by the enzyme cocktail produced by Paenibacillus sp. LS1[J]. Carbohydrate Polymers,2020,250:116889. doi: 10.1016/j.carbpol.2020.116889
[25] 谢杰, 李玉斌, 刘京伟, 等. 壳聚糖酶结构与功能的研究进展[J]. 生物工程学报,2023,39(3):912−929. [XIE J, LI Y B, LIU J W, et al. Advances in the structure and function of chitosanase[J]. Chinese Journal of Biotechnology,2023,39(3):912−929.] XIE J, LI Y B, LIU J W, et al. Advances in the structure and function of chitosanase[J]. Chinese Journal of Biotechnology, 2023, 39(3): 912−929.
[26] SUN H H, ZHAO L, MAO X Z, et al. Identification of a key loop for tuning transglycosylation activity in the substrate-binding region of a chitosanase[J]. Journal of Agricultural and Food Chemistry,2023,71(14):5585−5591. doi: 10.1021/acs.jafc.3c00110
[27] 曹世宁. 草酸青霉壳聚糖酶的酶学性质研究、分子克隆及食品级表达[D]. 无锡:江南大学, 2022. [CAO S N. Study on enzymatic properties, molecular cloning and food-grade expression of chitosanase from penicillium oxalicum[D]. Wuxi:Jiangnan University, 2022.] CAO S N. Study on enzymatic properties, molecular cloning and food-grade expression of chitosanase from penicillium oxalicum[D]. Wuxi: Jiangnan University, 2022.
[28] PATANTIS G, ZILDA D S, FAWZYA Y N, et al. Purification of chitosanase from Stenotrophomonas maltophilia KPU 2123 and Micromonospora sp. T5a1 for chitooligosacharide production[J]. Iop Conference Series:Earth and Environmental Science,2020,404(1):12078. doi: 10.1088/1755-1315/404/1/012078
[29] DOAN C T, TRAN T N, NGUYEN V B, et al. Bioprocessing of squid pens waste into chitosanase by Paenibacillus sp. TKU047 and its application in low-molecular weight chitosan oligosaccharides production[J]. Ploymers,2020,12(5):1163. doi: 10.3390/polym12051163
[30] 赵华, 樊龙星, 张朝正. 响应面法优化组成型壳聚糖酶酶解条件[J]. 中国酿造,2020,39(1):165−169. [ZHAO H, FAN L X, ZHANG C Z. Optimization of enzymatic hydrolysis conditions of compositional chitosanase by response surface methodology[J]. China Brewing,2020,39(1):165−169.] doi: 10.11882/j.issn.0254-5071.2020.01.032 ZHAO H, FAN L X, ZHANG C Z. Optimization of enzymatic hydrolysis conditions of compositional chitosanase by response surface methodology[J]. China Brewing, 2020, 39(1): 165−169. doi: 10.11882/j.issn.0254-5071.2020.01.032
[31] 罗洒. 新型壳聚糖酶的髙效表达及壳寡糖制备工艺研究[D]. 上海:华东理工大学, 2019. [LUO S. High-level expression of novel chitosanase and preparation of chitooligosaccharides[D]. Shanghai:East China University of Science and Technology, 2019.] LUO S. High-level expression of novel chitosanase and preparation of chitooligosaccharides[D]. Shanghai: East China University of Science and Technology, 2019.
[32] GUO X, AN Y J, JIANG L Y, et al. The discovery and enzymatic characterization of a novel AA10 LPMO from Bacillus amyloliquefaciens with dual substrate specificity[J]. International Journal of Biological Macromolecules,2022,203:457−465. doi: 10.1016/j.ijbiomac.2022.01.110
[33] KUMAR M, RAJPUT M, SONI T, et al. Chemoenzymatic production and engineering of chitooligosaccharides and N-acetyl glucosamine for refining biological activities[J]. Frontiers in Chemistry,2020,8:469. doi: 10.3389/fchem.2020.00469
[34] CIANO L, DAVIES G J, TOLMAN W B, et al. Bracing copper for the catalytic oxidation of C-H bonds[J]. Nature Catalysis,2018,1(8):571−577. doi: 10.1038/s41929-018-0110-9
[35] ZHANG H Y, ZHOU H C, ZHAO Y, et al. Comparative studies of two AA10 family lytic polysaccharide monooxygenases from Bacillus thuringiensis[J]. Peer J,2023,11:e14670. doi: 10.7717/peerj.14670
[36] VAAJE-KOLSTAD G, BUNAES A C, MATHIESEN G, et al. The chitinolytic system of Lactococcus lactis ssp. lactis comprises a nonprocessive chitinase and a chitin-binding protein that promotes the degradation of α- and β-chitin[J]. The FEBS Journal,2009,276(8):2402−2415. doi: 10.1111/j.1742-4658.2009.06972.x
[37] VAAJE-KOLSTAD G, BOHLE L A, GASEIDNES S, et al. Characterization of the chitinolytic machinery of Enterococcus faecalis V583 and high-resolution structure of its oxidative CBM33 enzyme[J]. Journal of Molecular Biology,2012,416(2):239−254. doi: 10.1016/j.jmb.2011.12.033
[38] 黄晓月, 毕思远, 区家豪, 等. 木瓜蛋白酶法制备抗氧化活性壳寡糖的工艺优化[J]. 生物学杂志,2022,39(1):104−109. [HUANG X Y, BI S Y, OU J H, et al. Process optimization of preparation of antioxidant chitooligosaccharides by papain[J]. Journal of Biology,2022,39(1):104−109.] doi: 10.3969/j.issn.2095-1736.2022.01.104 HUANG X Y, BI S Y, OU J H, et al. Process optimization of preparation of antioxidant chitooligosaccharides by papain[J]. Journal of Biology, 2022, 39(1): 104−109. doi: 10.3969/j.issn.2095-1736.2022.01.104
[39] DONG H Z, WANG Y S, ZHAO L M, et al. Key technologies of enzymatic preparation for DP 6-8 chitooligosaccharides[J]. Journal of Food Process Engineering,2015,38(4):336−344. doi: 10.1111/jfpe.12159
[40] QIAN J Q, SHI B B, MO L Y et al. Preparation of chitooligosaccharides by α-amylase from chitosan with oxidative pretreatment[J]. Journal of Chemical Technology and Biotechnology,2021,96(12):3408−3413. doi: 10.1002/jctb.6901
[41] XING R, LIU Y L, LI K C, et al. Monomer composition of chitooligosaccharides obtained by different degradation methods and their effects on immunomodulatory activities[J]. Carbohydrate Polymers,2017,157:1288−1297. doi: 10.1016/j.carbpol.2016.11.001
[42] WU Y, HUANG Y C, ZHOU Y, et al. Degradation of chitosan by swirling cavitation[J]. Innovative Food Science and Emerging Technologies,2014,23:188−193. doi: 10.1016/j.ifset.2014.02.001
[43] MARGOUTIDIS G, PARSONS V H, BOTTARO C S, et al. Mechanochemical amorphization of α-chitin and conversion into oligomers of N-acetyl-D-glucosamine[J]. ACS Sustainable Chemistry and Engineering,2018,6(2):1662−1669. doi: 10.1021/acssuschemeng.7b02870
[44] 郭蔓, 赵华, 张朝正. 壳寡糖的制备及应用研究[J]. 中国食品添加剂,2022,33(10):267−271. [GUO M, ZHAO H, ZHANG C Z. Preparation and application of chitosan oligosaccharides[J]. China Food Additives,2022,33(10):267−271.] GUO M, ZHAO H, ZHANG C Z. Preparation and application of chitosan oligosaccharides[J]. China Food Additives, 2022, 33(10): 267−271.
[45] LIANG S, SUN Y X, DAI X L. A review of the preparation, analysis and biological functions of chitooligosaccharide[J]. International Journal of Molecular Sciences,2018,19(8):2197. doi: 10.3390/ijms19082197
[46] BENCHAMAS G, HUANG G L, HUANG S Y, et al. Preparation and biological activities of chitosan oligosaccharides[J]. Trends in Food Science and Technology,2021,107:38−44. doi: 10.1016/j.jpgs.2020.11.027
[47] 卢春兰, 王蓓. 壳寡糖和几丁寡糖的制备方法及其在水产上的应用[J]. 广东农业科学,2023,50(2):136−146. [LU C L, WANG B. Preparation method of chitosan oligosaccharide and chitin oligosaccharide and their application in aquaculture[J]. Guangdong Agricultural Sciences,2023,50(2):136−146.] LU C L, WANG B. Preparation method of chitosan oligosaccharide and chitin oligosaccharide and their application in aquaculture[J]. Guangdong Agricultural Sciences, 2023, 50(2): 136−146.
[48] MITTAL A, SINGH A, HONG H, et al. Chitooligosaccharides from shrimp shell chitosan prepared using H2O2 or ascorbic acid/H2O2 redox pair hydrolysis:Characteristics, antioxidant and antimicrobial activities[J]. International Journal of Food Science and Technology,2023,58(5):2645−2660. doi: 10.1111/ijfs.15696
[49] ALJBOUR N D, BEG M D H, GIMBUN, J. Acid hydrolysis of chitosan to oligomers using hydrochloric acid[J]. Chemical Engineering and Technology,2019,42(9):1741−1746. doi: 10.1002/ceat.201800527
[50] 原佳琪, 梁爽, 孙雅煊, 等. 壳寡糖的制备及生物学活性研究进展[J]. 生命的化学,2019,39(4):759−765. [YUAN J Q, LIANG S, SUN Y X, et al. Research progresses on the preparation and biological activities of chitooligosaccharides[J]. Chemistry of Life,2019,39(4):759−765.] YUAN J Q, LIANG S, SUN Y X, et al. Research progresses on the preparation and biological activities of chitooligosaccharides[J]. Chemistry of Life, 2019, 39(4): 759−765.
[51] 季者, 蒋霞云, 李小倩, 等. 盐酸法降解壳聚糖制备特定聚合度壳寡糖(DP=5~7)的研究[J]. 上海海洋大学学报,2013,22(4):634−640. [JI Z, JIANG X Y, LI X Q, et al. Preparation of pentamer-to-heptamer chitooligosaccharides by hydrochloric acidic degradation of chitosan[J]. Journal of Shanghai Ocean University,2013,22(4):634−640.] JI Z, JIANG X Y, LI X Q, et al. Preparation of pentamer-to-heptamer chitooligosaccharides by hydrochloric acidic degradation of chitosan[J]. Journal of Shanghai Ocean University, 2013, 22(4): 634−640.
[52] 焦富颖. 黄粉虫蜕制备壳寡糖及其应用[D]. 重庆:重庆工商大学, 2018. [JIAO F Y. Preparation of chito oligosaccharide from Tenebrio molitor slough and its application[D]. Chongqing:Chongqing Technology and Business University, 2018.] JIAO F Y. Preparation of chito oligosaccharide from Tenebrio molitor slough and its application[D]. Chongqing: Chongqing Technology and Business University, 2018.
[53] OKORO O V, NIE L, GUNDUZ O, et al. Technoeconomic assessment of biopolymer production from crustacean waste with the UK as a case study[J]. Sustainability,2023,15(3):2280. doi: 10.3390/su15032280
[54] GU Z M, CAI Q Y, LIU Y, et al. Electrochemical degradation of chitosan using Ti/Sb-SnO2 electrode[J]. Journal of Polymers and the Environment,2013,21(2):479−486. doi: 10.1007/s10924-012-0532-4
[55] CAI Q Y, GU Z M, CHEN Y, et al. Degradation of chitosan by an electrochemical process[J]. Carbohydrate Polymers,2010,79(3):783−785. doi: 10.1016/j.carbpol.2009.08.022
[56] 郑必胜, 周萌. 壳聚糖氧化降解制备壳寡糖的研究[J]. 现代食品科技,2012,28(8):959−963. [ZHENG B S, ZHOU M. Oxidation degradation of chitosan for preparation of oligochitosan[J]. Modern Food Science and Technology,2012,28(8):959−963.] ZHENG B S, ZHOU M. Oxidation degradation of chitosan for preparation of oligochitosan[J]. Modern Food Science and Technology, 2012, 28(8): 959−963.
[57] SIVARAMAKRISHNA D, BHUVANACHANDRA B, MALLAKUNTLA M K, et al. Pretreatment with KOH and KOH-urea enhanced hydrolysis of α-chitin by an endo-chitinase from Enterobacter cloacae subsp. cloacae[J]. Carbohydrate Polymers,2020,235:115952. doi: 10.1016/j.carbpol.2020.115952
[58] SIVARAMAKRISHNA D, BHUVANACHANDRA B, NADENDLA S R, et al. Efficient conversion of α-chitin by multi-modular chitinase from Chitiniphilus shinanonensis with KOH and KOH-urea pretreatment[J]. Carbohydrate Polymers,2020,250:116923. doi: 10.1016/j.carbpol.2020.116923
[59] SULTHAN R, REGHUNADHAN A, SAMBHUDEVAN S. A new era of chitin synthesis and dissolution using deep eutectic solvents-comparison with ionic liquids[J]. Journal of Molecular Liquids,2023,380:121794. doi: 10.1016/j.molliq.2023.121794
[60] GAO X L, LIU H, SHUAI J B, et al. Rapid transesterification of cellulose in a novel DBU-derived ionic liquid:Efficient synthesis of highly substituted cellulose acetate[J]. International Journal of Biological Macromolecules,2023,242:125133. doi: 10.1016/j.ijbiomac.2023.125133
[61] HUSSON E, HADAD C, HUET G, et al. Effect of room temperature ionic liquids on selective biocatalytic hydrolysis of chitin via sequential or simultaneous strategies[J]. Green Chemistry,2017,19(17):4122−4131. doi: 10.1039/C7GC01471F
[62] STEUDTE S. Investigations on the stability and ecotoxicity of selected ionic liquid cations and anions[D]. Bremen:Universitaet Bremen, 2014:10698870.
[63] LI J, HUANG W C, GAO L, et al. Efficient enzymatic hydrolysis of ionic liquid pretreated chitin and its dissolution mechanism[J]. Carbohydrate Polymers,2019,211:329−335. doi: 10.1016/j.carbpol.2019.02.027
[64] BERTON P, SHAMSHINA J L, OSTADJOO S, et al. Enzymatic hydrolysis of ionic liquid-extracted chitin[J]. Carbohydrate Polymers,2018,199:228−235. doi: 10.1016/j.carbpol.2018.07.014
[65] SINHA S, CHAND S, TRIPATHI P. Recent progress in chitosanase production of monomer-free chitooligosaccharides:Bioprocess strategies and future applications[J]. Applied Biochemistry and Biotechnology,2016,180(5):883−899. doi: 10.1007/s12010-016-2140-6
[66] YU W L, HSIAO Y C, CHIANG B. Production of high degree polymerized chitooligosaccharides in a membrane reactor using purified chitosanase from Bacillus cereus[J]. Food Research International,2009,42(9):1355−1361. doi: 10.1016/j.foodres.2009.06.008
[67] AIDER M, BRUNET S, BAZINET L. Effect of pH and cell configuration on the selective and specific electrodialytic separation of chitosan oligomers[J]. Separation and Purification Technology,2008,63:612−619. doi: 10.1016/j.seppur.2008.07.018
[68] 仲伟伟. 壳寡糖的分离纯化及稳定性研究[D]. 无锡:江南大学, 2018. [ZHONG W W. Study on isolation, purification and stability of chitooligosaccharides[D]. Wuxi:Jiangnan University, 2018.] ZHONG W W. Study on isolation, purification and stability of chitooligosaccharides[D]. Wuxi: Jiangnan University, 2018.
[69] 吴健锋. 专一酶水解壳聚糖高效制备壳寡糖的研究[D]. 广东:华南理工大学, 2018. [WU J F. Study on efficient preparation of chitosan oligosaccharide by specific enzyme hydrolysis[D]. Guangdong:South China University of Technology, 2018.] WU J F. Study on efficient preparation of chitosan oligosaccharide by specific enzyme hydrolysis[D]. Guangdong: South China University of Technology, 2018.
[70] WEI X L, WANG Y F, XIAO J B, et al. Separation of chitooligosaccharides and the potent effects on gene expression of cell surface receptor CR3[J]. International Journal of Biological Macromolecules,2009,45(4):432−436. doi: 10.1016/j.ijbiomac.2009.07.003
[71] LI K C, XING R G, LIU S, et al. Access to N-acetylated chitohexaose with well-defined degrees of acetylation[J]. Biomed Research International,2017,2017:2486515.
[72] CHEN D, CHEN C C, ZHENG X H, et al. Chitosan oligosaccharide production potential of Mitsuaria sp. C4 and its whole-genome sequencing[J]. Frontiers in Microbiology,2021,12:695571. doi: 10.3389/fmicb.2021.695571
[73] SEKI K, NISHIYAMA Y, MITSUTOMI M. Characterization of a novel exo-chitosanase, an exo-chitobiohydrolase, from Gongronella butleri[J]. Journal of Bioscience and Bioengineering,2019,127(4):425−429. doi: 10.1016/j.jbiosc.2018.09.009
[74] XU Y X, WANG H, ZHU B W, et al. Purification and biochemical characterization of a novel chitosanase cloned from the gene of Kitasatospora setae KM-6054 and its application in the production of chitooligosaccharides[J]. World Journal of Microbiology and Biotechnology,2023,39(5):111. doi: 10.1007/s11274-023-03561-z
[75] 严佳佳, 尤田, 张雪梅, 等. 一种海洋几丁质酶产生菌的筛选及酶学性质研究[J]. 食品与发酵工业,2023,49(13):40−48. [YAN J J, YOU T, ZHANG X M, et al. Screening of a marine chitinase-producing bacterium and its enzymatic properties[J]. Food and Fermentation Industries,2023,49(13):40−48.] YAN J J, YOU T, ZHANG X M, et al. Screening of a marine chitinase-producing bacterium and its enzymatic properties[J]. Food and Fermentation Industries, 2023, 49(13): 40−48.
[76] YU Y, LI K C. Adsorption characteristics of chitooligosaccharides onto activated charcoal in aqueous solutions[J]. Journal of Oceanology and Limnology,2020,38(2):342−350. doi: 10.1007/s00343-019-8327-2
[77] HATTORI T, SAKABE Y, OGATA M, et al. Enzymatic synthesis of an α-chitin-like substance via lysozyme-mediated transglycosylation[J]. Carbohydrate Research,2012,347(1):16−22. doi: 10.1016/j.carres.2011.09.025
[78] LI K C, XING R E, LIU S, et al. Advances in preparation, analysis and biological activities of single chitooligosaccharides[J]. Carbohydrate Polymers,2016,139:178−190. doi: 10.1016/j.carbpol.2015.12.016
[79] ALLAN C P, HADWIGEI L A. The fungicidal effect of chitosan on fungi of varying cell wall composition[J]. Experimental Mycology,1979,3:285−287. doi: 10.1016/S0147-5975(79)80054-7
[80] JEON Y, PARK P, KIM S. Antimicrobial effect of chitooligosaccharides produced by bioreactor[J]. Carbohydrate Polymers,2001,44(1):71−76. doi: 10.1016/S0144-8617(00)00200-9
[81] HAO Z M, ZHANG Y R, SUN Z, et al. Chitooligosaccharide as a possible replacement for sulfur dioxide in winemaking[J]. Applied Sciences,2020,10(2):578. doi: 10.3390/app10020578
[82] TSAI G J, WU Z Y, SU W H. Antibacterial activity of a chitooligosaccharide mixture prepared by cellulase digestion of shrimp chitosan and its application to milk preservation[J]. Journal of Food Protection,2000,63(6):747−752. doi: 10.4315/0362-028X-63.6.747
[83] RAKKHUMKAEW N, PENGSUK C. Chitosan and chitooligosaccharides from shrimp shell waste:Characterization, antimicrobial and shelf life extension in bread[J]. Food Science and Biotechnology,2018,27(4):1201−1208. doi: 10.1007/s10068-018-0332-2
[84] 陈颖, 刘程惠, 白雯睿, 等. 壳寡糖涂膜对鲜切苹果的保鲜作用[J]. 食品工业科技,2019,40(9):269−274. [CHEN Y, LIU C H, BAI W R, et al. Effects of chitosan oligosaccharide on preservation of fresh-cut apples[J]. Science and Technology of Food Industry,2019,40(9):269−274.] CHEN Y, LIU C H, BAI W R, et al. Effects of chitosan oligosaccharide on preservation of fresh-cut apples[J]. Science and Technology of Food Industry, 2019, 40(9): 269−274.
[85] 陈丹, 王月慧, 朱玥, 等. 壳寡糖对面团、酥性饼干品质及抗氧化性的影响[J]. 食品工业科技,2023,44(18):84−90. [CHEN D, WANG Y H, ZHU Y, et al. Effect of chito-oligosaccharide on the quality and antioxidation of dough and crispy biscuits[J]. Science and Technology of Food Industry,2023,44(18):84−90.] CHEN D, WANG Y H, ZHU Y, et al. Effect of chito-oligosaccharide on the quality and antioxidation of dough and crispy biscuits[J]. Science and Technology of Food Industry, 2023, 44(18): 84−90.
[86] 龚周亮, 赵良忠, 刘汁琪, 等. 壳寡糖在湘派休闲豆干保鲜中的应用研究[J]. 食品安全导刊,2023(10):62−65. [GONG Z L, ZHAO L Z, LIU Z Q, et al. Study on application of chito-oligosaccharide in preservation of leisure dried bean in hunan pie[J]. China Food Safety Magazine,2023(10):62−65.] GONG Z L, ZHAO L Z, LIU Z Q, et al. Study on application of chito-oligosaccharide in preservation of leisure dried bean in hunan pie[J]. China Food Safety Magazine, 2023(10): 62−65.
[87] SINGH A, BENJAKUL S. The combined effect of squid pen chitooligosaccharides and high voltage cold atmospheric plasma on the shelf-life extension of Asian sea bass slices stored at 4 ℃[J]. Innovative Food Science and Emerging Technologies,2020,64:102339. doi: 10.1016/j.ifset.2020.102339
[88] 刘丽丹, 王帅, 金臻岚. 壳寡糖处理对冷藏期间大五星枇杷贮藏品质的影响[J]. 保鲜与加工,2018,18(2):39−43,49. [LIU L D, WANG S, JIN Z L. Effects of chito-oligosaccharide treatment on storage quality of big five-pointed star loquat fruit during storage at low temperature[J]. Storage and Process,2018,18(2):39−43,49.] LIU L D, WANG S, JIN Z L. Effects of chito-oligosaccharide treatment on storage quality of big five-pointed star loquat fruit during storage at low temperature[J]. Storage and Process, 2018, 18(2): 39−43,49.
[89] 赵韩栋, 秦畅, 郭风军, 等. 采后壳寡糖处理减轻皇冠梨果皮褐变的研究[J]. 中国食品学报,2022,22(4):276−284. [ZHAO H D, QIN C, GUO F J, et al. Study on chitosan oligosaccharides treatment alleviating peel browning of Huanggaun pear[J]. Journal of Chinese Institute of Food Science and Technology,2022,22(4):276−284.] ZHAO H D, QIN C, GUO F J, et al. Study on chitosan oligosaccharides treatment alleviating peel browning of Huanggaun pear[J]. Journal of Chinese Institute of Food Science and Technology, 2022, 22(4): 276−284.
[90] 段树华, 罗皓, 兰林, 等. 壳寡糖处理对甜樱桃采后贮藏品质的影响[J]. 北方园艺,2023(6):87−95. [DUAN S H, LUO H, LAN L, et al. Effects of chitosan oligosaccharide treatment on the quality of sweet cherries after harvest storage[J]. Northern Horticulture,2023(6):87−95.] DUAN S H, LUO H, LAN L, et al. Effects of chitosan oligosaccharide treatment on the quality of sweet cherries after harvest storage[J]. Northern Horticulture, 2023(6): 87−95.
[91] 吴会敏, 王月慧, 朱玥, 等. 壳寡糖对海绵蛋糕物理及感官特性的影响[J]. 食品科技,2022,47(6):270−277. [WU H M, WANG Y H, ZHU Y, et al. Effect of chitooligosaccharide on sponge cake batter properties and cake quality[J]. Food Science and Technology,2022,47(6):270−277.] WU H M, WANG Y H, ZHU Y, et al. Effect of chitooligosaccharide on sponge cake batter properties and cake quality[J]. Food Science and Technology, 2022, 47(6): 270−277.
[92] KE Y, DING B B, FU Y, et al. Effects of chitosan oligosaccharide and Hyriopsis cumingii polysaccharide on the quality of wheat flour and extruded flour products[J]. Food Science and Biotechnology,2021,30(7):911−919. doi: 10.1007/s10068-021-00933-9
[93] NAVEED M, PHIL L, SOHAIL M, et al. Chitosan oligosaccharide (COS):An overview[J]. International Journal of Biological Macromolecules,2019,129:827−843. doi: 10.1016/j.ijbiomac.2019.01.192
[94] 王胜田, 姚乾元. 一种用于保肝护肝的壳寡糖胶囊:中国, 113304188A[P]. 2021-08-27. [WANG S T, YAO Q Y. A chitooligosaccharide capsule for liver protection:China, 113304188A[P]. 2021-08-27.] WANG S T, YAO Q Y. A chitooligosaccharide capsule for liver protection: China, 113304188A[P]. 2021-08-27.
[95] 郝桂娟, 张宾, 章样扬, 等. 壳寡糖锌配合物对氧化衰老模型小鼠的抗氧化作用[J]. 核农学报,2019,33(6):1156−1164. [HAO G J, ZHANG B, ZHANG Y Y, et al. Antioxidant effect of chitooligosaccharide-zinc complex on the oxidative aging mice model[J]. Journal of Nuclear Agricultural Sciences,2019,33(6):1156−1164.] HAO G J, ZHANG B, ZHANG Y Y, et al. Antioxidant effect of chitooligosaccharide-zinc complex on the oxidative aging mice model[J]. Journal of Nuclear Agricultural Sciences, 2019, 33(6): 1156−1164.
[96] 苏政权, 曹华, 白研, 等. 一种壳寡糖肠溶胶囊及其制备方法和应用:中国, 114376982B[P]. 2023-06-30. [SU Z Q, CAO H, BAI Y, et al. A chitooligosaccharide enteric-soluble capsule and its preparation process and application:China, 114376982B[P]. 2023-06-30.] SU Z Q, CAO H, BAI Y, et al. A chitooligosaccharide enteric-soluble capsule and its preparation process and application: China, 114376982B[P]. 2023-06-30.
[97] 罗雷, 祁一鸣, 任琼赭, 等. 壳寡糖在提升肠道通透性和作为BCSⅢ类药物口服吸收促进剂方面的应用:中国, 113813390B[P]. 2023-03-31. [LUO L, QI Y M, REN Q Z, et al. Application of chitooligosaccharide in enhancing intestinal permeability and serving as oral absorption promoter for BCSIII drugs:China, 113813390B[P]. 2023-03-31.] LUO L, QI Y M, REN Q Z, et al. Application of chitooligosaccharide in enhancing intestinal permeability and serving as oral absorption promoter for BCSIII drugs: China, 113813390B[P]. 2023-03-31.
[98] 徐然, 左华江, 唐春怡, 等. 壳聚糖类吸附材料的制备及应用研究进展[J]. 现代化工,2020,40(9):25−29. [XU R, ZUO H J, TANG C Y, et al. Advances in preparation and application of chitosan-based adsorption materials[J]. Modern Chemical Industry,2020,40(9):25−29.] XU R, ZUO H J, TANG C Y, et al. Advances in preparation and application of chitosan-based adsorption materials[J]. Modern Chemical Industry, 2020, 40(9): 25−29.
[99] 庄林, 操俊, 李月婵, 等. 壳寡糖复合固体饮料对高尿酸血症小鼠肠道微生态的调节作用研究[J]. 食品研究与开发,2022,43(11):56−62. [ZHUANG L, CAO J, LI Y C, et al. Effect of chitooligosaccharide compound solid beverage on the intestinal microecology of hyperuricemia mice[J]. Food Research and Development,2022,43(11):56−62.] ZHUANG L, CAO J, LI Y C, et al. Effect of chitooligosaccharide compound solid beverage on the intestinal microecology of hyperuricemia mice[J]. Food Research and Development, 2022, 43(11): 56−62.
[100] 姜雅杰, 王畅, 席茂盛, 等. 壳寡糖复合固体饮料对Ⅱ型糖尿病小鼠肠道菌群结构的影响[J]. 食品工业科技,2020,41(8):301−306. [JIANG Y J, WANG C, XI M S, et al. Effect of chitooligosaccharide compound solid beverage on intestinal flora of type II diabetic mice[J]. Science and Technology of Food Industry,2020,41(8):301−306.] JIANG Y J, WANG C, XI M S, et al. Effect of chitooligosaccharide compound solid beverage on intestinal flora of type II diabetic mice[J]. Science and Technology of Food Industry, 2020, 41(8): 301−306.
[101] 喻凯. 一种增强肠胃功能及免疫力几丁寡糖食品及其制备方法:中国, 115428851A[P]. 2022-12-06. [YU K. A chitin oligosaccharide food that enhances gastrointestinal function and immunity and its preparation process:China, 115428851A[P]. 2022-12-06.] YU K. A chitin oligosaccharide food that enhances gastrointestinal function and immunity and its preparation process: China, 115428851A[P]. 2022-12-06.
[102] 王孝文, 台文静, 王海华, 等. 一种具有增强免疫功能的浒苔多糖保健食品及制备方法:中国, 114376233A[P]. 2022-04-22. [WANG X W, TAI W J, WANG H H, et al. A health food containing polysaccharides from Enteromorpha prolifera with enhanced immune function and its preparation process:China, 114376233A[P]. 2022-04-22.] WANG X W, TAI W J, WANG H H, et al. A health food containing polysaccharides from Enteromorpha prolifera with enhanced immune function and its preparation process: China, 114376233A[P]. 2022-04-22.
[103] 孙翠红, 徐翠莲, 赵铭钦, 等. 壳寡糖及其衍生物抗烟草花叶病毒机理的初步研究[J]. 中国烟草科学,2015,36(2):87−92. [SUN C H, XU C L, ZHAO M Q, et al. The inhibitory effects of chitooligosaccharides and its derivatives on Tobacco Mosaic Virus (TMV)[J]. Chinese Tobacco Science,2015,36(2):87−92.] SUN C H, XU C L, ZHAO M Q, et al. The inhibitory effects of chitooligosaccharides and its derivatives on Tobacco Mosaic Virus (TMV)[J]. Chinese Tobacco Science, 2015, 36(2): 87−92.
[104] 高雨萌, 费昭雪, 史健飞, 等. 壳寡糖对花椒干腐病菌的抑菌活性研究[J]. 中国森林病虫,2021,40(3):1−8. [GAO Y M, FEI Z X, SHI J F, et al. Antifungal activities of oligochitosan against the pathogen of stem canker of Zanthoxylum bungeanum[J]. Forest Pest and Disease,2021,40(3):1−8.] GAO Y M, FEI Z X, SHI J F, et al. Antifungal activities of oligochitosan against the pathogen of stem canker of Zanthoxylum bungeanum[J]. Forest Pest and Disease, 2021, 40(3): 1−8.
[105] 尹雅洁, 张宗杰, 夏险, 等. 壳寡糖对水稻幼苗生长及抗逆性影响[J]. 生物学杂志,2021,38(1):77−80. [YI Y J, ZHANG Z J, XIA X, et al. Effects of oligochitosan on the growth and stress resistance of rice seedlings under abiotic stress[J]. Journal of Biology,2021,38(1):77−80.] YI Y J, ZHANG Z J, XIA X, et al. Effects of oligochitosan on the growth and stress resistance of rice seedlings under abiotic stress[J]. Journal of Biology, 2021, 38(1): 77−80.
[106] 金国强, 李永杰, 高恒锦, 等. 壳寡糖对早熟温州蜜柑果实品质的影响[J]. 浙江柑橘,2020,37(2):20−24. [JIN G Q, LI Y J, GAO H J, et al. Effect of chitooligosaccharides on the quality of early maturing wenzhou honey citrus fruit[J]. Zhejiang Ganju,2020,37(2):20−24.] JIN G Q, LI Y J, GAO H J, et al. Effect of chitooligosaccharides on the quality of early maturing wenzhou honey citrus fruit[J]. Zhejiang Ganju, 2020, 37(2): 20−24.
[107] 黄雪燕, 金伟, 赵灵云. 壳寡糖对温岭高橙果实膨大和品质的影响[J]. 农业科技通讯,2018(12):186−188. [HUANG X Y, JIN W, ZHAO L Y. Effect of chitooligosaccharides on the expansion and quality of wenling high orange fruit[J]. Bulletin of Agricultural Science and Technology,2018(12):186−188.] HUANG X Y, JIN W, ZHAO L Y. Effect of chitooligosaccharides on the expansion and quality of wenling high orange fruit[J]. Bulletin of Agricultural Science and Technology, 2018(12): 186−188.
[108] BADAWY M E I, RABEA E I. Potential of the biopolymer chitosan with different molecular weights to control postharvest gray mold of tomato fruit[J]. Postharvest Biology and Technology,2009,51(1):110−117. doi: 10.1016/j.postharvbio.2008.05.018
[109] DAS S N, MADHUPRAKASH J, SARMA P V, et al. Biotechnological approaches for field applications of chitooligosaccharides (COS) to induce innate immunity in plants[J]. Critical Reviews in Biotechnology,2015,35(1):29−43. doi: 10.3109/07388551.2013.798255
[110] KATIYAR D, HEMANTARANJAN A, SINGH B. Chitosan as a promising natural compound to enhance potential physiological responses in plant:A review[J]. Indian Journal of Plant Physiology,2015,20(1):1−9. doi: 10.1007/s40502-015-0139-6
-
期刊类型引用(3)
1. 徐田田,王咪,董佳彤,许馨,许云舒,许青松. 壳寡糖偶联链霉素衍生物的合成及其抗菌活性研究. 现代农业研究. 2025(01): 83-86 .  百度学术
百度学术
2. 贾飞鸿,江宁,杨慧晶,刘钱媛,孙荣雪,王成,纪倩倩,马艳弘,王愈. 超声-微波预处理协同复合酶法制备壳寡糖的工艺优化及其抗氧化活性. 食品工业科技. 2024(17): 190-199 .  本站查看
本站查看
3. 庄钰鑫,刘慧泉,许铭. 真菌几丁质酶研究进展. 微生物学报. 2024(11): 4022-4035 .  百度学术
百度学术
其他类型引用(2)





 下载:
下载:
 下载:
下载:



