Preparation, Structure Identification of ACE Inhibitory Glycopeptide from Quinoa and Its Stability in Vitro
-
摘要: 利用毛霉和米根霉混合发酵藜麦制备藜麦源血管紧张素转化酶(angiotensin-I converting enzyme,ACE)抑制糖肽。通过单因素实验和响应面试验优化发酵工艺条件,发酵液经真空浓缩、醇沉、葡聚糖凝胶G-15和反相高效液相色谱分离纯化,利用傅里叶红外光谱法判断官能团构成,β-消除法判断糖肽键链接方式,并分析温度、pH、金属离子以及体外模拟消化对活性的影响。结果表明:在料液比1:18 g/mL、毛霉与米根霉接种量比1:1、总接种量2.8%(φ)、发酵时间8.7 d时,得到ACE抑制率为64.22%±4.57%的藜麦发酵液,分离纯化后获得单一组分F3b。利用傅里叶红外光谱检测,发现F3b具有多肽和多糖的特征吸收,β-消除法确定其存在O-糖肽键。体外稳定性研究结果表明,温度(25~55 °C)对F3b活性影响较小,100 ℃处理后ACE抑制率为25 ℃时的86.23%±3.47%;不同pH条件(pH2~12)对其活性无显著影响(P>0.05);在Zn2+和Fe3+浓度为4 mmol/L时,ACE抑制率分别为对照的109.91%±8.12%和117.43%±6.78%,增强其活性;相同浓度的K+和Ca2+减弱其活性,ACE抑制率分别为对照的78.94%±2.18%和85.31%±4.99%;模拟胃肠消化过程中,F3b活性略有降低,ACE抑制率为对照的70.00%±3.30%。该研究丰富了ACE抑制肽的种类,为藜麦资源高值化利用提供理论和数据技术参考。Abstract: The aim of this study was to purify angiotensin I-converting enzyme (ACE) inhibitory glycopeptide from quinoa fermented by Mucor wutungkiao and Rhizopus oryzae. The fermentation process conditions were optimized by a single factor experiment and a response surface experiment. The fermentation liquid was separated and purified by vacuum concentration, alcohol precipitation, Sephadex G-15 and reverse-phase high performance liquid chromatography. Fourier infrared spectroscopy was used to determine the composition of functional groups and β-elimination method to determine the glycopeptide bonding type. The effects of temperature, pH, metal ions, and in vitro simulated digestion on the activity were analyzed. The results showed that the optimal fermentation parameters were as follows: 8.7 d of fermentation time, 2.8% (φ) of total inoculum size containing Mucor wutungkiao to Rhizopus oryzae in a volume ratio of 1:1, and 1:18 g/mL of a solid-liquid ratio. The ACE inhibition rate was 64.22%±4.57%. A novel glycopeptides F3b, was isolated and purified by ethanol precipitation, Sephadex G-15, and reverse-phase high performance liquid chromatography. Fourier-transform infrared spectroscopy proved the presence of polypeptides and polysaccharides in F3b, and β-elimination reaction further demonstrated that the glycoprotein was an O-linked type. In vitro stability results indicate that the ACE inhibitory activity of glycopeptides was hardly changed under heating conditions from 25 to 55 ℃, and was 86.23%±3.47% of the original activity at 100 °C. Conversely, pH (from 2 to 12) had no significant effect (P>0.05) on the ACE inhibitory activity of glycopeptides. 4 mmol/L of Zn2+ and Fe3+ increased the ACE inhibitory activity of F3b to 109.91%±8.12% and 117.43%±6.78% of the control, respectively. While same concentration of K+ and Ca2+ reduced the ACE inhibitory activity to 78.94%±2.18% and 85.31%±4.99% of the control, respectively. After simulated gastrointestinal digestion, the ACE inhibitory activity of F3b decreased to 70.00%±3.30% of the control. In summary, this study could enrich the variety of ACE inhibitory peptides and provide technical reference for high-value utilization of quinoa.
-
高血压是普遍存在于中老年人群中的一种疾病,能够对机体其它器官的正常运行造成不良影响,是导致心血管疾病形成的重要因素。我国高血压患者约2.7亿人,是患病人数最高的慢性病之一且逐渐年轻化[1]。血管紧张素转化酶(angiotensin-I converting enzyme,ACE)是一种促使非活性激素血管紧张素原I生成活性高血压激素血管紧张素II的催化剂,其活性强弱能够影响血压的高低[2]。通过化学合成法制备的卡托普利、赖诺普利是能够抑制ACE的活性的药物,在临床上普遍使用[3]。此类通过化学合成的药物在一定程度上能够缓解血压升高,但患者长期服用该类药物后会引起皮疹、头痛、干咳等不良症状[4-5]。糖肽是通过共价键连接糖基和多肽而形成的复合物,广泛存在于自然界的动植物以及微生物中。研究发现,糖肽具有降血压、降血糖、抑菌、抗氧化等生物功能[6-8]。近些年,研究人员陆续从植物根茎[9]、谷物[10]、肉类[11]以及油料种子[12]中分离出具有ACE抑制效果的糖肽,并在ACE抑制糖肽的制备、结构表征以及抑制机制方面进行研究。
藜麦是一种原产于南美印地斯山脉的一种古老的农作物,可在高盐、干旱、营养贫瘠的土壤中种植。我国于1988年首次引进,目前藜麦在我国西部和东北部地区均有种植[13]。藜麦中含有丰富的蛋白质,蛋白质平均含量在12%~23%之间,其必需氨基酸的组成和含量显著高于小麦、水稻、大豆和玉米等常见禾谷类作物,是优质的生物活性肽来源[14-15]。
微生物发酵是常用的从食物中获得生物活性肽的方式,能够有效地分解和转化生物大分子并产生酶和各种次级代谢产物[16]。霉菌具有极强的糖化酶和蛋白酶生成能力,能够将淀粉和蛋白质分解为多糖和多肽,被广泛用于干腌火腿、奶酪、酱油等食品加工与发酵过程[17-19]。Anna等[20]用少孢米根霉、米曲霉和间型脉孢菌发酵藜麦,发现采用少孢米根霉发酵后,游离酚类、总肽和单个游离氨基酸水平均显著增加,抗氧化能力显著提升。Wei等[21]利用毛霉和植物乳杆菌发酵腐乳,并从中鉴定出具有ACE抑制活性和抗氧化活性的肽段。所以可用霉菌生产藜麦源ACE抑制糖肽。
本研究以藜麦为原料,利用毛霉和米根霉发酵生产ACE抑制糖肽。以单因素实验和响应面试验优化藜麦源ACE抑制糖肽的生产工艺,通过乙醇沉淀、葡聚糖凝胶G-15和反相高效液相色谱(Reverse-phase High Performance Liquid Chromatography, RP-HPLC)分离出高活性的ACE抑制糖肽,并结合傅里叶红外光谱(Fourier-transform Infrared Spectroscopy, FTIR)和紫外光谱分析糖肽结构,同时考察了ACE抑制糖肽的物理、化学稳定性以及消化稳定性,为ACE抑制肽的种类,为藜麦资源高值化利用提供理论和数据技术参考。
1. 材料与方法
1.1 材料与仪器
藜麦 格尔木纳木蓝商贸有限公司;毛霉(Mucor wutungkiao,CICC 3109)、米根霉(Rhizopus oryzae,CICC 41214) 中国工业微生物菌种保藏中心;ACE ≥2.0 units/mg,德国Sigma公司;茚三酮 98%,上海源叶生物科技有限公司;Gly-Gly-Tyr-Arg(95%)、马尿酸-组氨酸-亮氨酸(N-Hippuryl-His-Leu hydrate, HHL)(98%) 上海麦克林生物有限公司;马铃薯葡萄糖琼脂培养基 杭州微生物试剂有限公司;ɑ-淀粉酶 1~2万U/g,南宁庞博生物工程有限公司;葡聚糖凝胶G-15 台州四甲生化塑料厂;其它试剂 均为国产分析纯。
infinite 200 Pro多功能酶标仪 瑞士Tecan公司;spectraMax Plus 38型酶标仪 美谷分子;Nicolet型傅里叶红外光谱仪 美国尼高力仪器公司;Waters 2695高效液相色谱仪 美国Waters公司;HD-5型电脑紫外检测仪、SBS-100型自动部分收集器 上海青浦沪西仪器厂;UV-2600型紫外分光光度计 日本岛津公司;FreeZone2.5冷冻干燥机 宁波新芝生物科技股份有限公司;ZY98-01型旋转蒸发仪 上海豫康科教仪器设备有限公司。
1.2 实验方法
1.2.1 发酵菌种制备
毛霉和米根霉分别使用马铃薯葡萄糖琼脂斜面活化两次,再用少量无菌生理盐水反复冲洗菌丝表面,使孢子分散在生理盐水中,之后调整孢子浓度至105~106 CFU/mL。
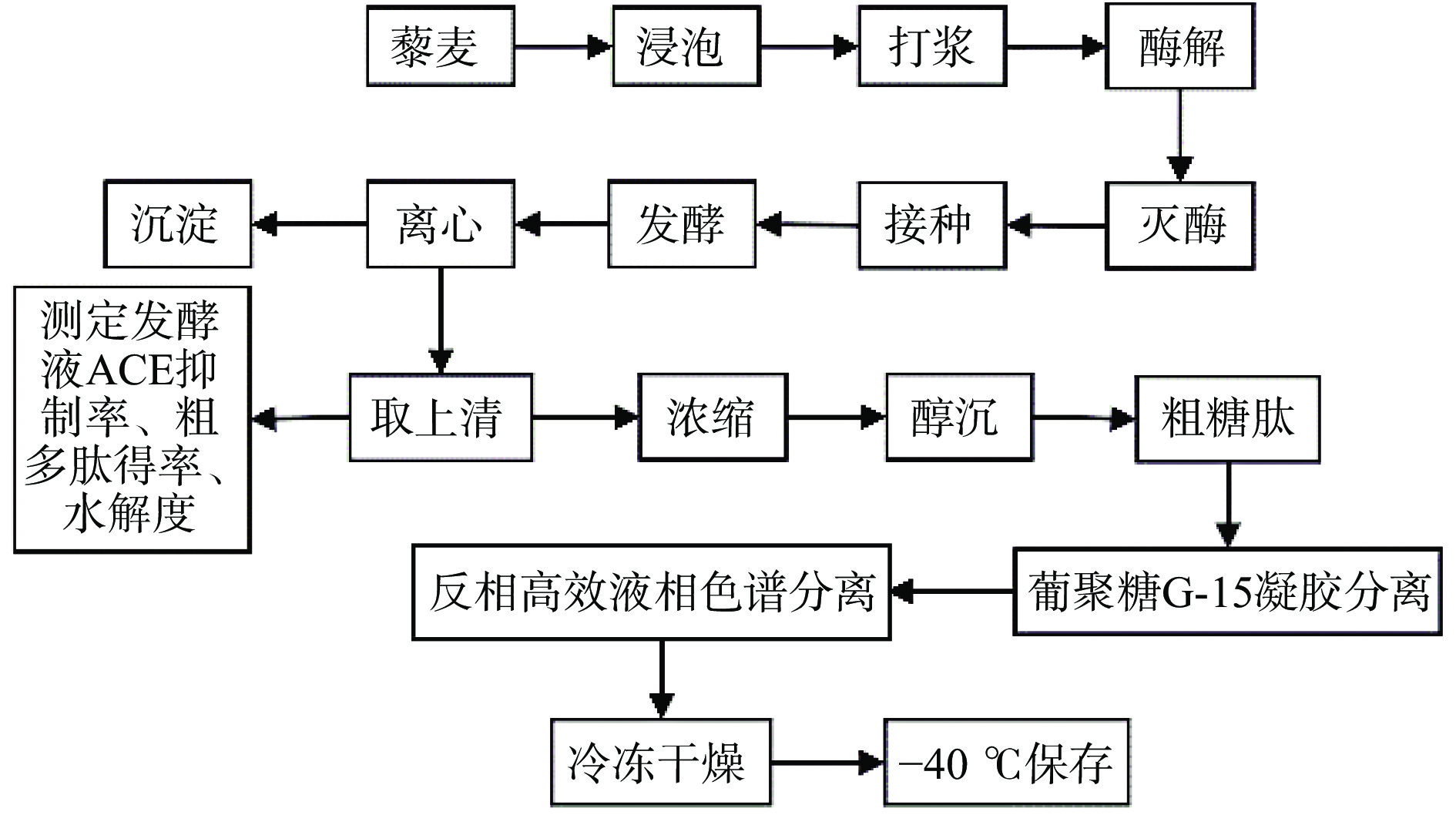
1.2.2 藜麦ACE抑制糖肽制备工艺流程
具体步骤见图1:藜麦和水以一定比例在25 ℃下浸泡12 h后用打浆机打成匀浆,全部转入烧杯中,加入藜麦质量分数1%的ɑ-淀粉酶于80 ℃水浴酶解40 min后升温至100 ℃,灭酶10 min。冷却至25 ℃后接种菌种发酵,发酵液于10000 r/min离心10 min,取上清,真空浓缩至原先的1/10,再加入4倍体积的无水乙醇于4 ℃静置12 h,取沉淀,得粗糖肽。粗糖肽再经葡聚糖凝胶G-15分离和RP-HPLC分离纯化得到纯的藜麦糖肽,冷冻干燥后于−40 ℃保存。
1.2.3 单因素实验设计
以发酵时间9 d,总接种量3%,毛霉和米根霉接种量比1:1,料液比1:10 g/mL为固定条件,探究发酵时间(3、6、9、12、15 d)、总接种量(1%、3%、5%、7%、9%)、毛霉和米根霉接种量比(4:1、3:2、1:1、2:3、1:4)、料液比(1:5、1:10、1:20、1:30、1:40 g/mL)4个因素对ACE抑制率、粗多肽得率和水解度的影响。
1.2.4 响应面试验设计
如表1,根据单因素实验,选取发酵时间、总接种量、毛霉和米根霉接种量比、料液比进行4因素3水平响应面优化试验,以ACE抑制率为响应值,优化ACE抑制糖肽发酵工艺。
表 1 响应面试验设计因素与水平Table 1. Factors and levels of response surface experiment因素 水平 −1 0 1 A发酵时间(d) 6 9 12 B总接种量(%) 1 3 5 C毛霉和米根霉接种量比 3:2 1:1 2:3 D料液比(g/mL) 1:10 1:20 1:30 1.2.5 蛋白质水解度、多肽含量及ACE抑制率的测定
蛋白质水解度测定参考赵新淮等[22]的方法,采用茚三酮比色法测定。多肽含量测定参考刘璇璇等[23]的方法,采用双缩脲法测定。
ACE抑制率测定参考Li等[24]的方法,将样品用pH8.3含NaCl(0.3 mol/L)的硼酸盐缓冲液稀释10倍,取40 μL稀释后样品与5 μL 5 munits/mL ACE溶液和20 μL 5 mmol/L HHL溶液混匀,37 ℃培养箱中反应1 h,之后加入150 μL 1.0 mol/L NaOH溶液终止酶反应。再加入40 μL 2% OPA甲醇溶液,混匀,室温放置20 min,最后加入40 μL 6 mol/L HCl终止衍生反应。将反应后溶液用蒸馏水稀释10倍后用荧光分光光度计测定荧光强度。测定条件为激发波长为340 nm,发射波长455 nm,狭缝宽度5 nm。每组实验平行测定3次。实验各组物质加入量如表2所示。ACE抑制率(%)的计算公式如下:
表 2 OPA法测定ACE抑制率加样方法Table 2. Preparation of reaction system used for measurement of ACE inhibition rate by OPA method添加量(μL) 样品 对照 实验组 空白组 实验组 空白组 硼酸盐缓冲液 0 5 40 45 稀释后样品 40 40 0 0 ACE 5 0 5 0 HHL 20 20 20 20 NaOH 150 150 150 150 OPA甲醇 40 40 40 40 HCl 40 40 40 40 蒸馏水 2950 2950 2950 2950 ACE抑制率(%)=(1−A2−A1B2−B1)×100 式中:A2为样品实验组的荧光强度;A1为样品空白组的荧光强度;B2为对照实验组的荧光强度;B1为对照空白组的荧光强度。
1.2.6 糖肽的葡聚糖凝胶G-15分离
参考向媛嫄等[25]的方法,将粗糖肽用去离子水溶解,用0.22 μm的滤膜过滤,再用葡聚糖凝胶G-15分离。葡聚糖凝胶G-15用去离子水充分溶胀,装入16 mm×600 mm的玻璃层析柱中,用去离子水平衡,待洗脱曲线为直线后上样。用移液枪吸取1 mL过滤后的样品缓慢加到柱床上,控制流速为12滴/min,每0.5 min收集1管。用紫外检测仪检测280 nm处紫外吸收值,绘制多肽洗脱曲线。用苯酚-硫酸法测定每一管洗脱液中多糖的紫外吸收值,绘制多糖洗脱曲线。收集两条洗脱曲线峰重合部分对应的组分,浓缩、冻干,配制成2 mg/mL的溶液,分别测定ACE抑制率。选择ACE抑制率较高的组分进行下一步分析。
1.2.7 糖肽的RP-HPLC分离及纯度分析
1.2.7.1 糖肽的RP-HPLC分离
将1.2.6葡聚糖凝胶G-15分离得到的组分F3用RP-HPLC进行梯度洗脱,参考Wen等[26]的方法并稍作修改。RP-HPLC色谱柱(BioBasic-18 250 mm×4.6 mm,300 Å,5 μm),流动相A为0.1%三氟乙酸-水溶液,流动相B为0.1%三氟乙酸-乙腈溶液。梯度洗脱程序:0~25 min,90%~70% A;25~30 min,70%~50% A;30~40 min,50%~10% A,流速0.8 mL/min,样品质量浓度1 mg/mL,柱温25 ℃,检测波长:220 nm,进样量50 μL。重复收集各洗脱峰,浓缩、冻干,配制成1 mg/mL的溶液,测定ACE抑制率。选择ACE抑制率较高的组分进行纯度分析及结构表征和稳定性分析。
1.2.7.2 纯度分析
参考Yang等[27]的方法并稍作修改。RP-HPLC色谱柱(BioBasic-18 250 mm×4.6 mm,300 Å,5 μm),流动相A为0.1%三氟乙酸-水溶液,流动相B为0.1%三氟乙酸-乙腈溶液。梯度洗脱程序:0~5 min,90%A;5~20 min,90%~70% A;20~30 min,70%~50%A;30~35 min,35%~40% A,流速0.8 mL/min,样品质量浓度0.5 mg/mL,柱温25 ℃,检测波长:220 nm,进样量10 μL。根据峰面积占比计算纯度。
1.2.8 结构分析
1.2.8.1 FTIR分析
参考曲金萍等[28]的方法,将ACE抑制糖肽冻干粉和干燥的溴化钾按质量比范围为1:50~1:200加入研钵中,在红外灯下充分研磨成粉末。取少量样品-溴化钾粉末放入模具中,用压片机制成薄而透明的小圆片,扫描4000~500 cm−1范围红外光谱,并用OMNIC软件分析图谱。
1.2.8.2 糖肽键分析
参考沈柱英等[29]的方法,取2 mg ACE抑制糖肽冻干粉,分别用蒸馏水和0.2 mol/L NaOH溶液配制成1 mg/mL的样品溶液。分别将样品溶液放入45 ℃水浴锅中反应2 h,然后在200~400 nm范围扫描紫外光谱。
1.2.9 ACE抑制糖肽体外稳定性研究
1.2.9.1 温度对ACE抑制糖肽活性的影响
参考姬中伟[30]的方法,将ACE抑制糖肽冻干粉用蒸馏水配制成1 mg/mL的样品溶液,分别放置于25、40、55、70、85、100 ℃环境下保温3 h,取出后放入25 ℃水浴冷却并测定ACE抑制率。
1.2.9.2 pH对ACE抑制糖肽活性的影响
参考姬中伟[30]的方法并稍作修改,将ACE抑制糖肽冻干粉分别用pH为2、4、6、8、10、12的磷酸盐缓冲液配制成1 mg/mL的样品溶液,在25 ℃静置3 h后测定ACE抑制率。
1.2.9.3 金属离子对ACE抑制糖肽活性的影响
参考Zheng等[31]的方法并稍作修改,将ACE抑制糖肽冻干粉用蒸馏水配制成2 mg/mL的样品溶液,分别取50 μL的样品溶液和50 μL不同浓度(2、4、6、8、10 mmol/L)的K+、Ca2+、Na+、Mg2+、Zn2+、Fe3+溶液混合,在25 ℃放置2 h后测定ACE抑制率。
1.2.9.4 体外模拟胃肠消化对ACE抑制糖肽活性的影响
a.胃蛋白酶消化对ACE抑制糖肽活性的影响:参考Chai等[32]的方法并稍作修改,用pH为2.0的HCl-KCl缓冲液配制质量分数1%的胃蛋白酶溶液,加入ACE抑制糖肽冻干粉,配制成1 mg/mL的样品溶液,放置于37 ℃恒温水浴槽3 h,期间每隔1 h取样,经90 ℃灭酶10 min后放入25 ℃水浴冷却并测定ACE抑制率。
b.胰蛋白酶消化对ACE抑制糖肽活性的影响:参考Chai等[32]的方法并稍作修改,将经过模拟胃消化后的样品用0.2 mol/L的NaOH调节pH至8.0,加入质量分数1%的胰蛋白酶,在37 ℃水浴反应3 h。每隔1 h取样,在90 ℃灭酶10 min后放入25 ℃水浴冷却并测定ACE抑制率。
1.3 数据处理
每组试验数据重复测定三次,采用Design Expert12进行响应面设计分析,采用SPSS Statistics 26.0软件进行统计学分析,设定差异水平判别标准为P<0.05,用Origin 2018软件绘制相关图表。
2. 结果与分析
2.1 单因素实验
2.1.1 发酵时间对ACE抑制率、粗多肽得率和水解度的影响
如图2所示,发酵时间从3~9 d时ACE抑制率、粗多肽得率和水解度均增长较快,9 d后ACE抑制率几乎不变,水解度增长平缓,但粗多肽得率则与之相反,呈下降趋势,在9 d时ACE抑制率和粗多肽得率分别为64.31%±1.87%和5.40%±0.06%。这与Pebrianti等[33]的研究结果相似,随着发酵时间的延长,水解度逐渐升高,ACE抑制率到达峰值之后不再增加。可能因为随着发酵的进行,菌生长较快,蛋白酶分泌较多,蛋白质被快速分解多肽,在9 d时达到最高;发酵至9 d以后,菌逐渐进入衰亡期,产酶较少,营养物质被不断利用,使得多肽被进一步水解成氨基酸,使粗多肽得率下降,水解度逐渐升高[34]。因此,发酵时间选择为9 d。
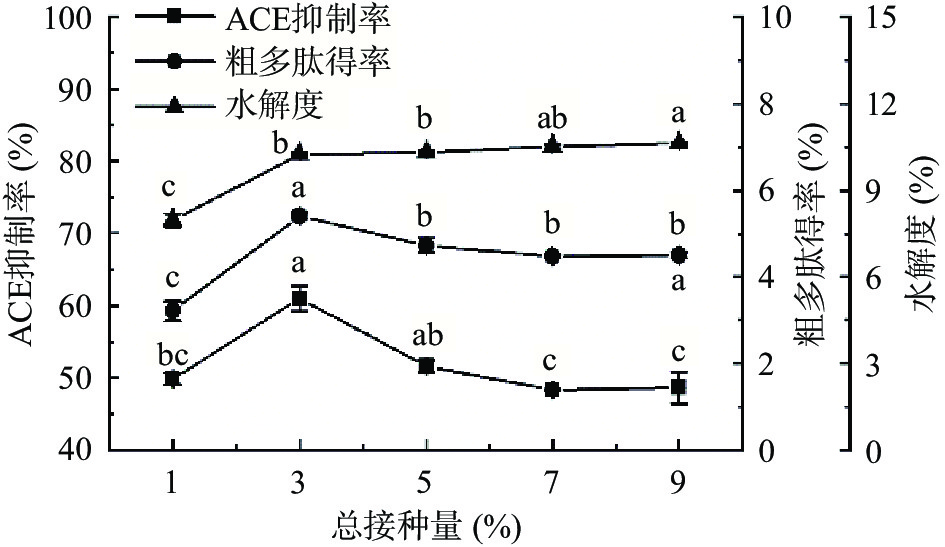
2.1.2 总接种量对ACE抑制率、粗多肽得率和水解度的影响
如图3所示,随总接种量增加多肽得率和ACE抑制率先增加后降低,水解度则持续增加。总接种量为3%时ACE抑制率和粗多肽得率均达到最大值,分别为60.95%±1.72%和5.41%±0.06%,增加菌接种量反而使粗多肽得率和ACE抑制率降低。这与李景等[35]的研究结果相似,其用米曲霉发酵豆粕,ACE抑制率和粗多肽得率随接种量的增加而先增后减。可能是因为菌接种量增加,缩短了菌生长的延滞期,促进蛋白质的分解,使粗多肽含量和水解度增加以及ACE抑制率增加;但菌接种量过大时,会使得菌体大量繁殖,使营养物质被快速分解利用,使粗多肽含量下降,具有ACE活性的肽段被进一步水解,水解度提升,ACE抑制率下降[36]。因此,总接种量选择为3%。
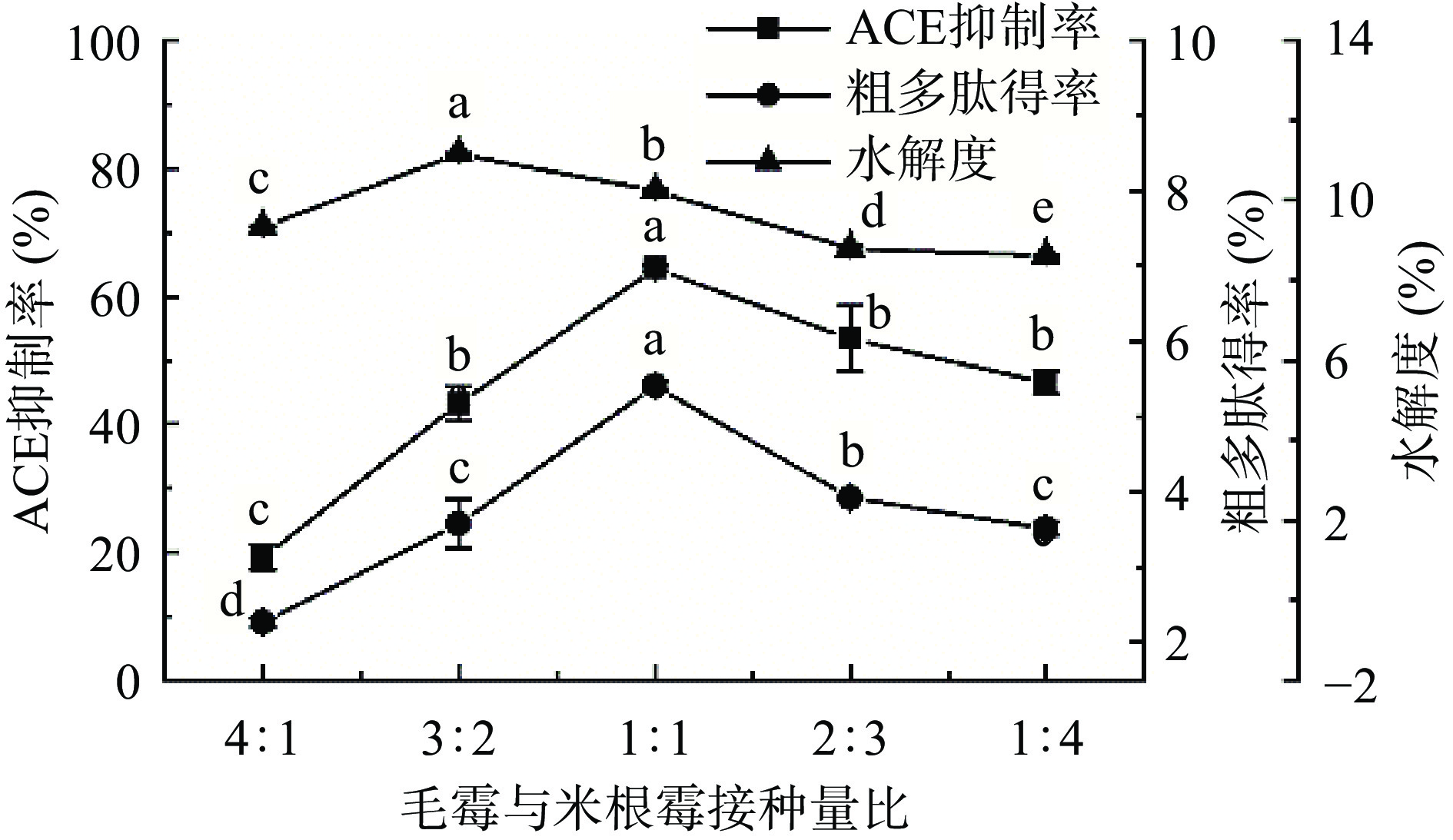
2.1.3 毛霉与米根霉接种量比对ACE抑制率、粗多肽得率和水解度的影响
如图4所示,当毛霉与米根霉接种量比过大或过小时各项评价指标均较低。接种量比在4:1时,ACE抑制率和粗多肽得率最低,分别为19.12%±1.96%和2.26%±0.05%;当接种量比为1:1时ACE抑制率和粗多肽得率达到最高,分别为64.31%±0.36%和5.41%±0.06%;当接种量比继续增加时ACE抑制率和粗多肽得率逐渐降低。可能因为在毛霉与米根霉接种量比为4:1~1:1时,随着米根霉比例的增加,发酵液中糖化酶含量增加,使碳源更易被毛霉利用,促进毛霉的生长繁殖,分泌蛋白酶,使粗多肽含量提高[37]。当接种量比在1:1~1:4时,米根霉比例过大,迅速成为优势菌种,进而抑制毛霉生长繁殖,导致ACE抑制率、粗多肽含量以及水解度降低[38]。因此,毛霉与米根霉接种量比选择为1:1。
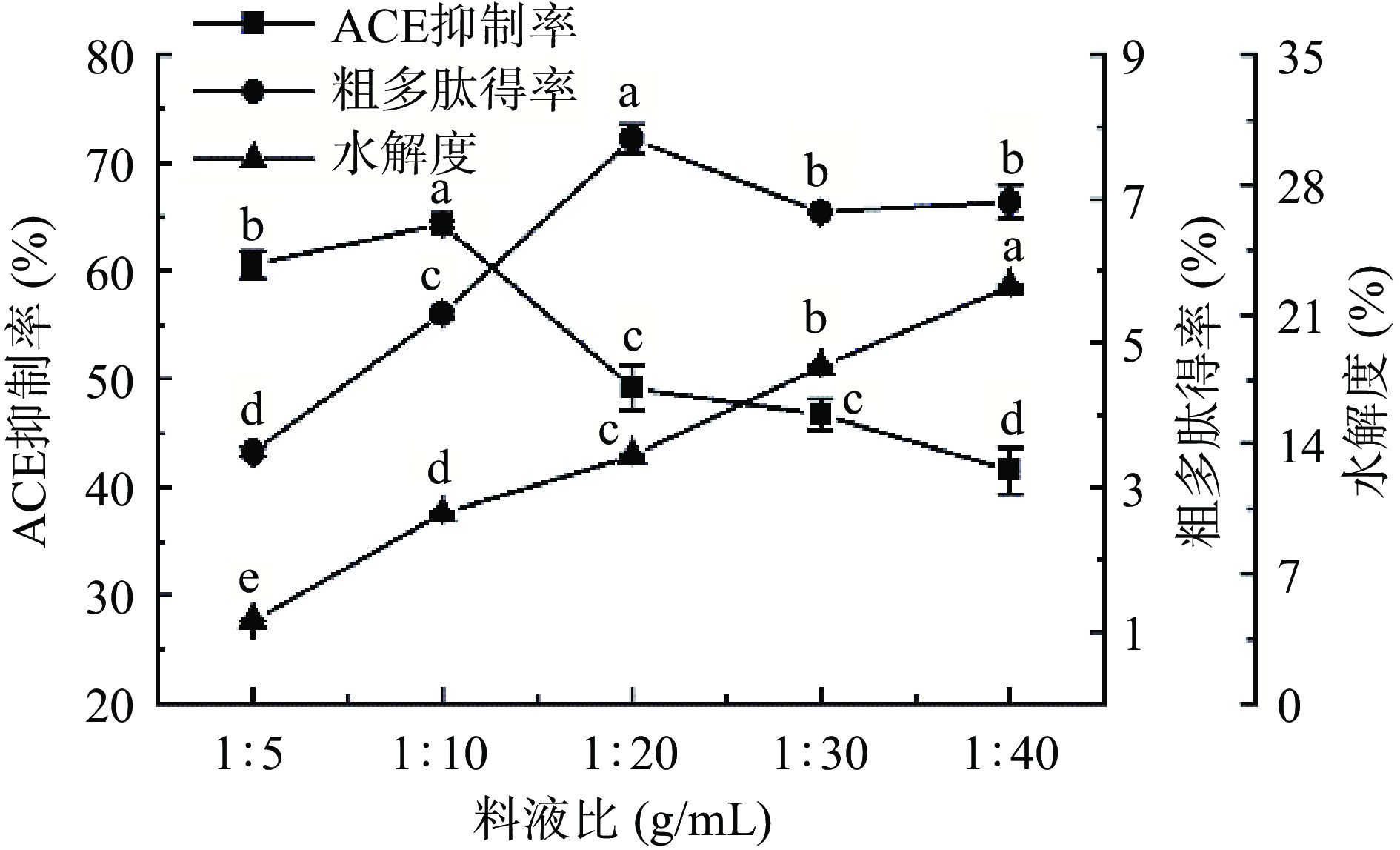
2.1.4 料液比对ACE抑制率、粗多肽得率和水解度的影响
如图5所示,当料液比降低,ACE抑制率、粗多肽得率随料液比减小而先增大后减小,水解度逐渐增加。当料液比为1:20 g/mL时粗多肽得率最佳,ACE抑制率较高,分别为7.83%±0.20%和49.22%±2.03%。可能因为当料液比较高时,发酵基质含水量较少, 不利于菌的生长繁殖,产酶量较少,导致ACE抑制率和粗多肽含量较低。当料液比较过低时,水添加量较多,使营养物质浓度较低,从而使ACE抑制率、粗多肽得率降低[39]。因此,料液比选择为1:20 g/mL。
2.2 响应面试验优化
2.2.1 响应面试验优化结果
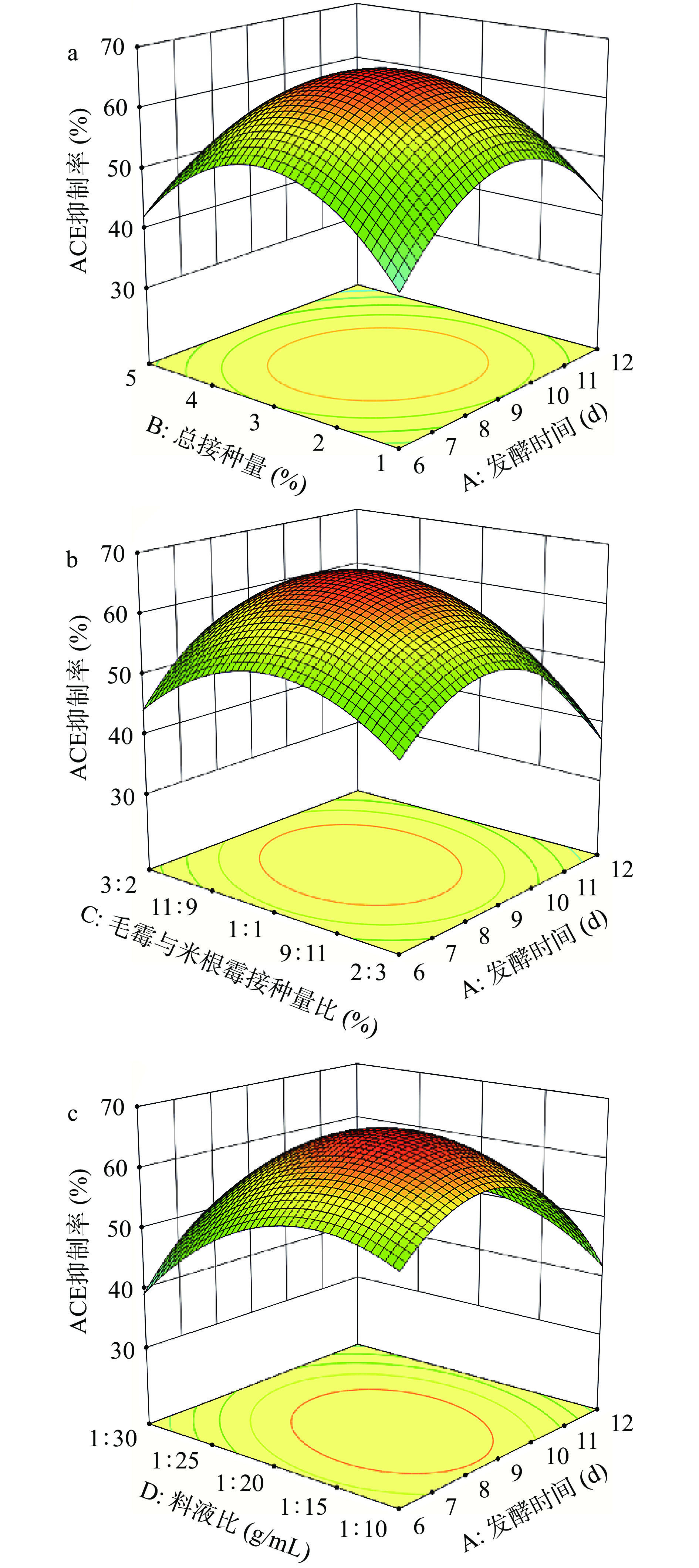
由图2~图5可知,不同发酵时间、总接种量、毛霉与米根霉接种量比、料液比总体上对ACE抑制率和粗多肽得率呈先升高后下降的趋势,所以在单因素设计的基础上,选择发酵时间(A)、总接种量(B)、毛霉与米根霉接种量比(C)、料液比(D)进行4因素3水平响应面设计,以ACE抑制率作为响应值,试验方案及结果见表3。
表 3 响应面试验设计与结果Table 3. Experimental design and results for response surface analysis实验号 A B C D ACE抑制率(%) 1 0 0 1 1 43.29 2 1 −1 0 0 37.62 3 −1 0 −1 0 45.90 4 0 0 0 0 65.40 5 1 0 0 1 43.91 6 0 0 0 0 66.75 7 1 0 0 −1 43.08 8 0 −1 −1 0 47.53 9 0 0 −1 −1 46.05 10 0 0 1 −1 52.57 11 −1 0 0 −1 53.49 12 0 −1 1 0 48.27 13 1 0 −1 0 39.45 14 −1 −1 0 0 39.42 15 0 0 0 0 65.89 16 1 0 1 0 48.23 17 0 0 0 0 67.82 18 −1 0 1 0 43.46 19 0 0 0 0 63.18 20 −1 1 0 0 43.86 21 0 −1 0 −1 51.16 22 −1 0 0 1 40.08 23 0 1 0 −1 44.04 24 0 1 0 1 40.63 25 1 1 0 0 30.80 26 0 −1 0 1 41.53 27 0 1 −1 0 37.43 28 0 1 1 0 41.98 29 0 0 −1 1 41.63 运用Design-Expert 12软件对实验结果进行回归拟合,得到的回归方程如下:
Y=65.81−1.93A−2.23B+1.65C−3.28D−2.81AB+2.80AC+3.56AD+0.9542BC+1.56BD−1.22CD−12.80A2−13.43B2−9.49C2−8.78D2
由表4可知,该模型P<0.0001,显著;失拟项P>0.05,不显著;模型决定系数R2=0.9652,校正决定系数RAdj2=0.9303,表明该模型拟合度较高。经过回归分析可知,总接种量和料液比对ACE抑制率极显著(P<0.01),毛霉与米根霉接种量比和发酵时间ACE抑制率显著(P<0.05)。各影响因素影响强度的强弱顺序依次为:料液比>总接种量>发酵时间>毛霉与米根霉接种量比。发酵时间与其它三个因素的交互作用显著,表明发酵时间与总接种量、发酵时间与毛霉与米根霉接种量比、发酵时间与料液比的交互作用对ACE抑制率均有显著影响,各因素交互作用响应面图如图6所示。
表 4 回归方程的方差分析Table 4. Analysis of variance (ANOVA) of regression equations响应值 方差来源 自由度 均方 F值 P值 显著性 模型 2578.97 14 184.21 27.70 0.000 ** A 44.50 1 44.50 6.69 0.022 * B 59.87 1 59.87 9.00 0.010 ** C 32.69 1 32.69 4.92 0.044 * D 128.82 1 128.82 19.37 0.001 ** AB 31.69 1 31.69 4.76 0.047 * AC 31.47 1 31.47 4.73 0.047 * AD 50.74 1 50.74 7.63 0.015 * BC 3.64 1 3.64 0.5476 0.472 BD 9.68 1 9.68 1.46 0.248 CD 5.92 1 5.92 0.8899 0.362 A² 1063.00 1 1063.00 159.84 0.000 ** B² 1170.55 1 1170.55 176.01 0.000 ** C² 584.60 1 584.60 87.90 0.000 ** D² 500.50 1 500.50 75.26 0.000 ** 残差 93.11 14 6.65 失拟项 81.13 10 8.11 2.71 0.175 不显著 纯误差 11.98 4 2.99 总离差 2672.08 28 注:“**”表示影响极显著(P<0.01);“*”表示影响显著(P<0.05)。 图6a中,随总接种量和发酵时间增加,ACE抑制率先增加后减小。等高线呈椭圆形,表明总接种量和发酵时间交互作用显著。图6b中,以总接种量和料液比为中心水平值时,ACE抑制率随发酵时间延长呈先上升而后降低的趋势。在图6c中,ACE抑制率随发酵时间变化趋势的与图6b相同,随料液比增加呈上升后平缓的趋势。等高线呈椭圆形,表明料液比和发酵时间交互作用显著。
2.2.2 验证实验
利用Design Expert 12软件进行工艺参数的优化,得到藜麦源ACE抑制糖肽的最佳发酵工艺:发酵时间为8.74 d,总接种量为2.83%,毛霉与米根霉接种量比为127:123,料液比为1:17.83 g/mL,在此条件下发酵液的ACE抑制率可达到66.42%±3.63%。根据实验的可操作性,调整发酵条件为:发酵时间为8.7 d,总接种量为2.8%,毛霉与米根霉接种量比为1:1,料液比为1:18 g/mL,三次试验,得到的验证结果为64.22%±4.57%,与理论值接近,说明该模型能够较好地优化藜麦ACE抑制糖肽的生产工艺。
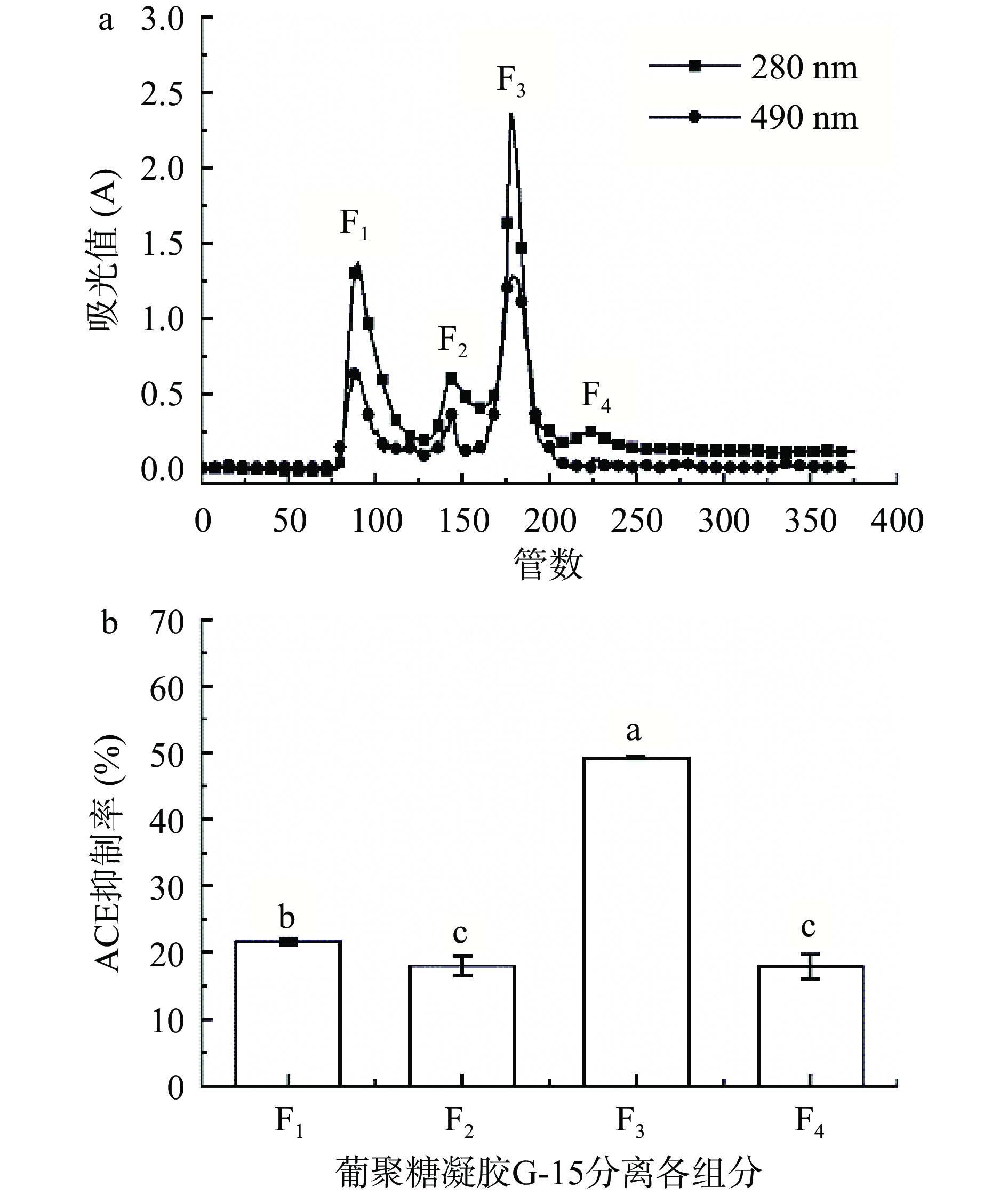
2.3 葡聚糖凝胶G-15分离
由图7a可知,粗糖肽的葡聚糖凝胶G-15在280 nm处的洗脱曲线出现四个多肽吸收峰,分别计为F1、F2、F3、F4,在490 nm处的洗脱曲线出现四个多糖吸收峰且多肽吸收峰重合。分别收集4个组分,浓缩、冻干。配制成2 mg/mL糖肽溶液,测定ACE抑制率,结果如图7b。由图7可知,组分F1、F2、F4对ACE的抑制率显著低于组分F3(P<0.05),且组分F3含量较高,因此选择组分F3进行下一步分析。
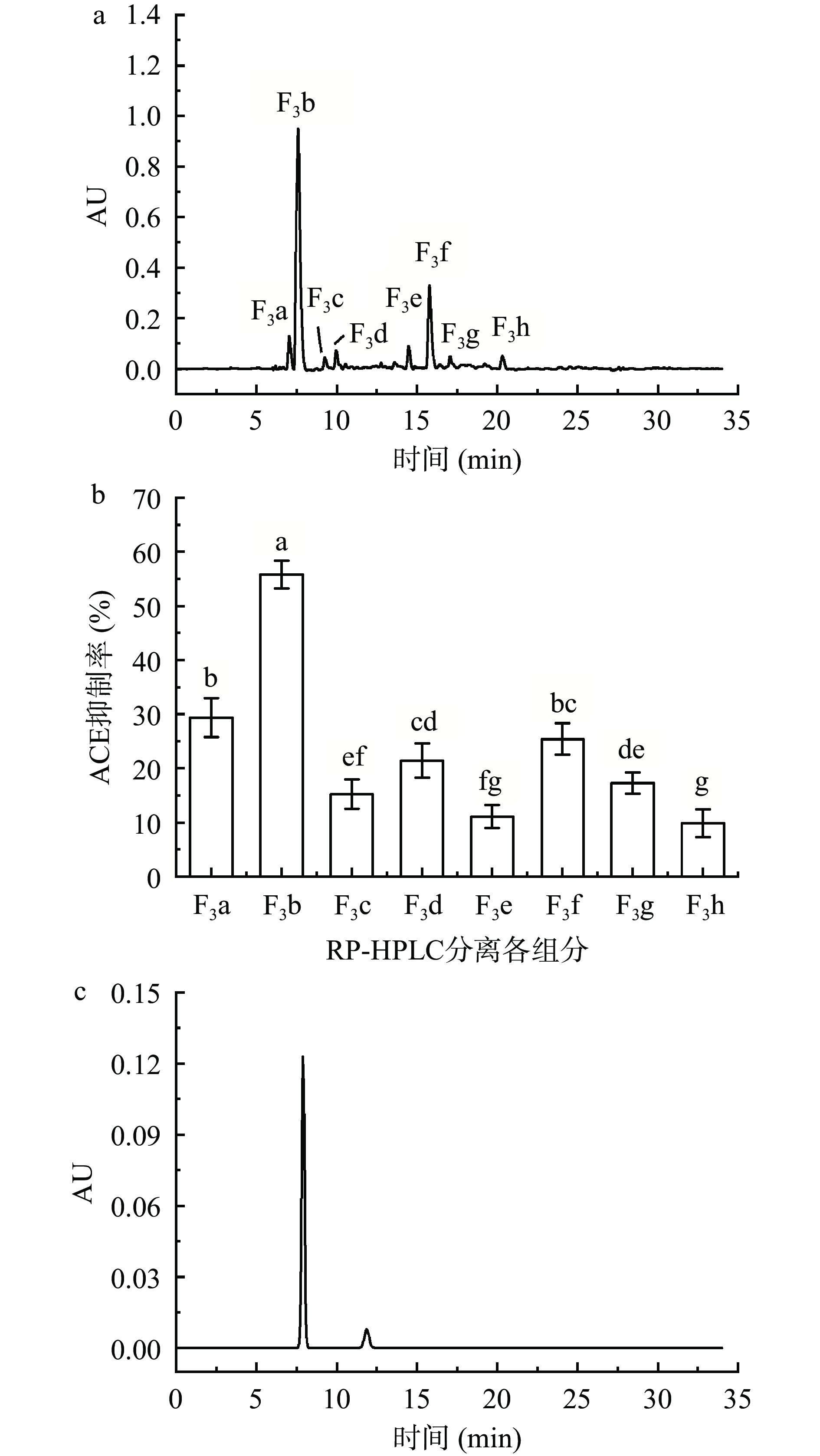
2.4 RP-HPLC分离纯化和纯度分析
如图8a所示,将葡聚糖凝胶G-15分离得到的组分F3用RP-HPLC进行梯度洗脱,得到8个吸收峰,分别记为F3a~F3h。分别重复收集各组分,冻干,配制成1 mg/mL糖肽溶液,测定ACE抑制率,结果如图8b。由图8可知,组分F3b的ACE抑制率显著高于其他组分(P<0.05),且组分F3b的糖肽含量最高,纯度为93.73%,IC50为0.94 mg/mL。所以重复收集组分F3b、冻干,进行后续分析。
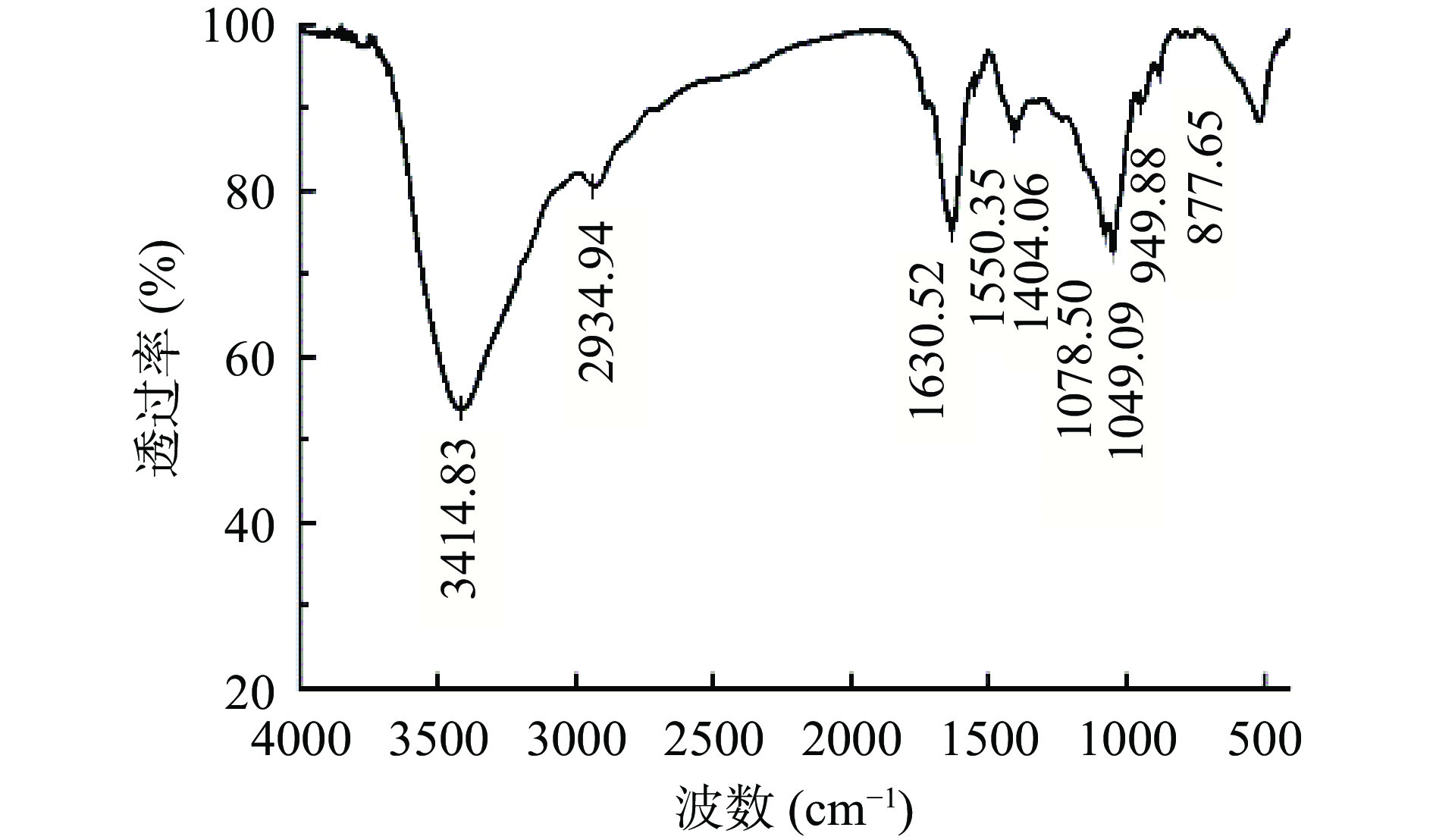
2.5 FTIR扫描分析
由图9分析可知,F3b在1630 cm−1附近处出现吸收峰,是肽键上C=O键伸缩振动与N-H的变角振动产生;在1550.35 cm−1附近出现吸收峰是肽键上C=O键伸缩振动产生,是肽键的特征吸收峰[40]。在3414.83 cm−1附近出现大而强的吸收峰,表明此处有O-H键和N-H键的伸缩振动[41]。2934.94 cm−1处的吸收是由饱和烃基上C-H键伸缩振动产生,在1404.06 cm−1的吸收峰主要是由肽键上C-H变角振动产生[42]。在1078.50和1049.09 cm−1两处的吸收峰可能由C-O-C的振动、O-H的变形振动产生[43]。949.88和877.65 cm−1两处的吸收峰可能分别由β、α-型糖苷键产生[44]。
2.6 糖肽键分析
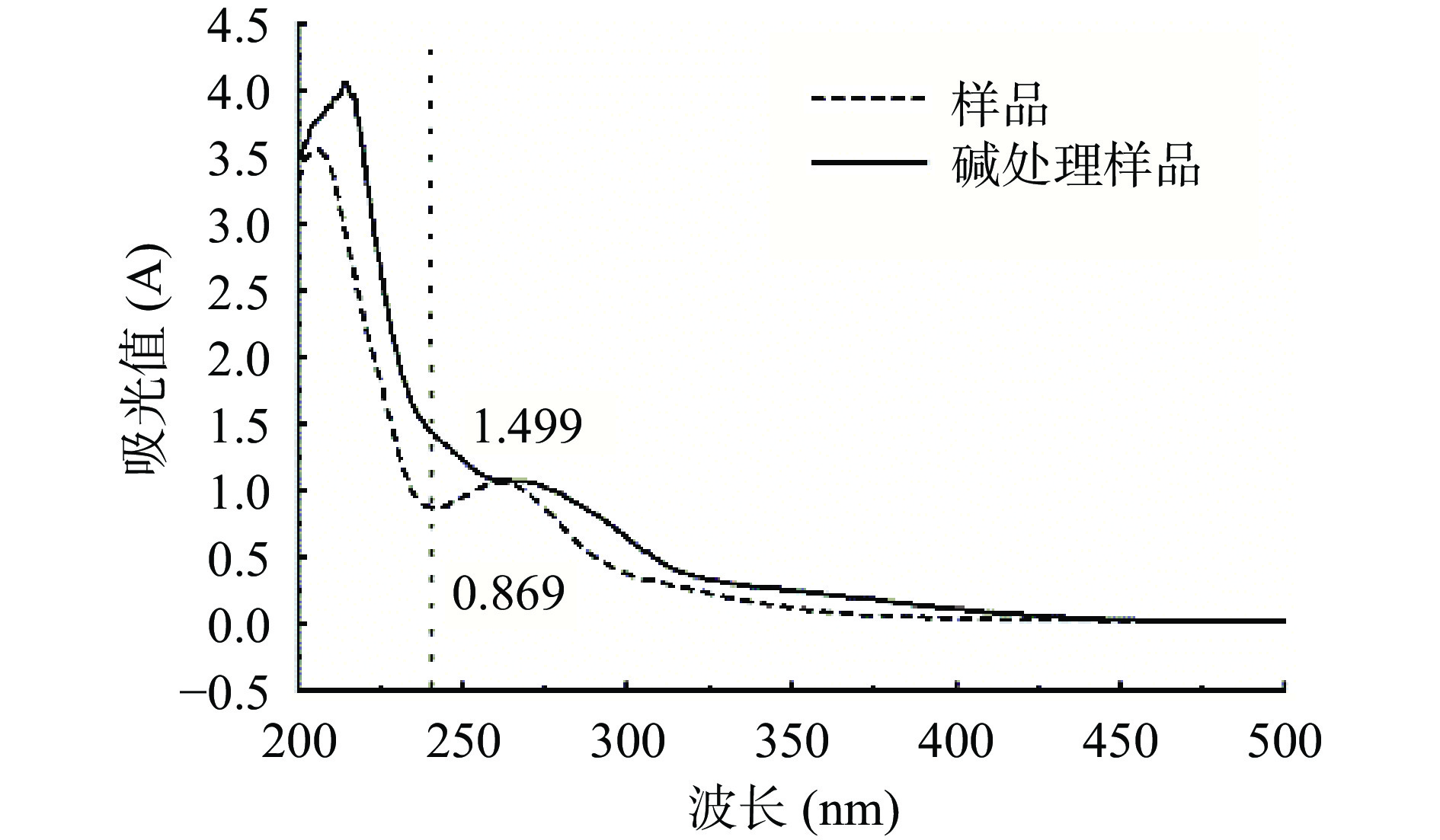
由图10分析可知,F3b经过碱处理后,在240 nm处吸光值较未经过碱处理的样品明显增大,从0.869增加至1.499。因糖链可以和含羟基的氨基酸以O-糖肽键相连接,样品经过稀碱处理后,O-糖肽键断裂,从而生成在240 nm处有明显吸收的α-氨基丙烯酸、α-氨基丁酸。所以,通过对比碱处理前、后该波长处的样品吸光值,能够证明其含有O-糖肽键[45-46]。
2.7 F3b体外稳定性研究
2.7.1 温度对F3b活性影响
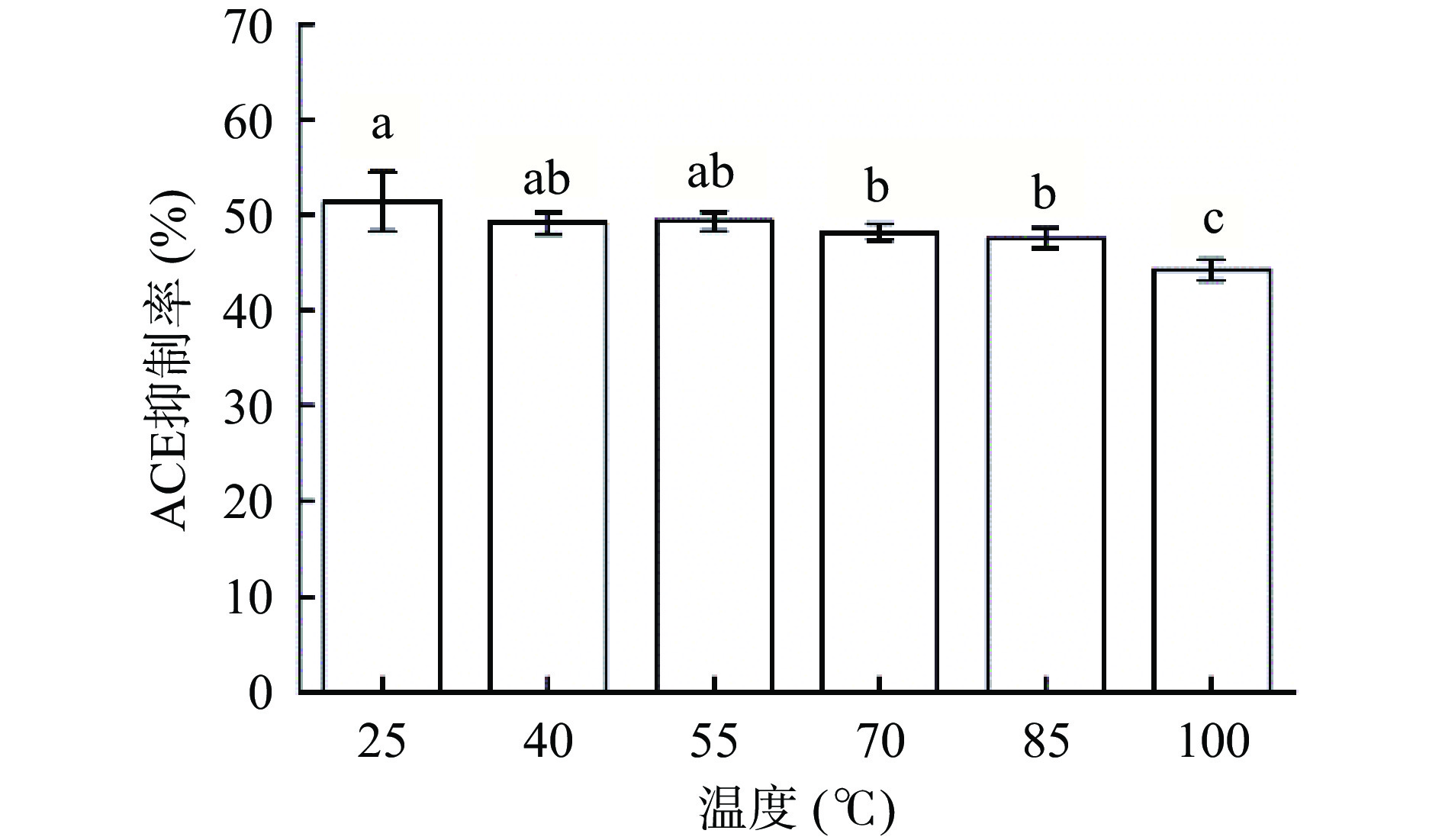
热处理会对蛋白质的结构造成不良影响,高温下形成二硫键而使蛋白质结构发生改变[47]。如图11所示,温度对F3b的活性造成一些不良影响,随温度升高,ACE抑制糖肽的活性逐渐降低,当温度在25~55 ℃之间变化时,温度对F3b的活性影响不显著;当温度继续升高,会对其活性造成影响,当温度升高至100 ℃时,ACE抑制率约为25 ℃时的86.23%±3.47%。该结果和Li等[48]的研究结果类似,在40 ℃时对黑大豆肽的ACE抑制活性没有显著影响(P>0.05),较高温度能够降低黑大豆肽的ACE抑制活性。可能是因为ACE抑制糖肽分子量较小,升高温度对多肽二级结构影响较小,所以肽的活性得到保留[49]。
2.7.2 pH处理对F3b活性影响
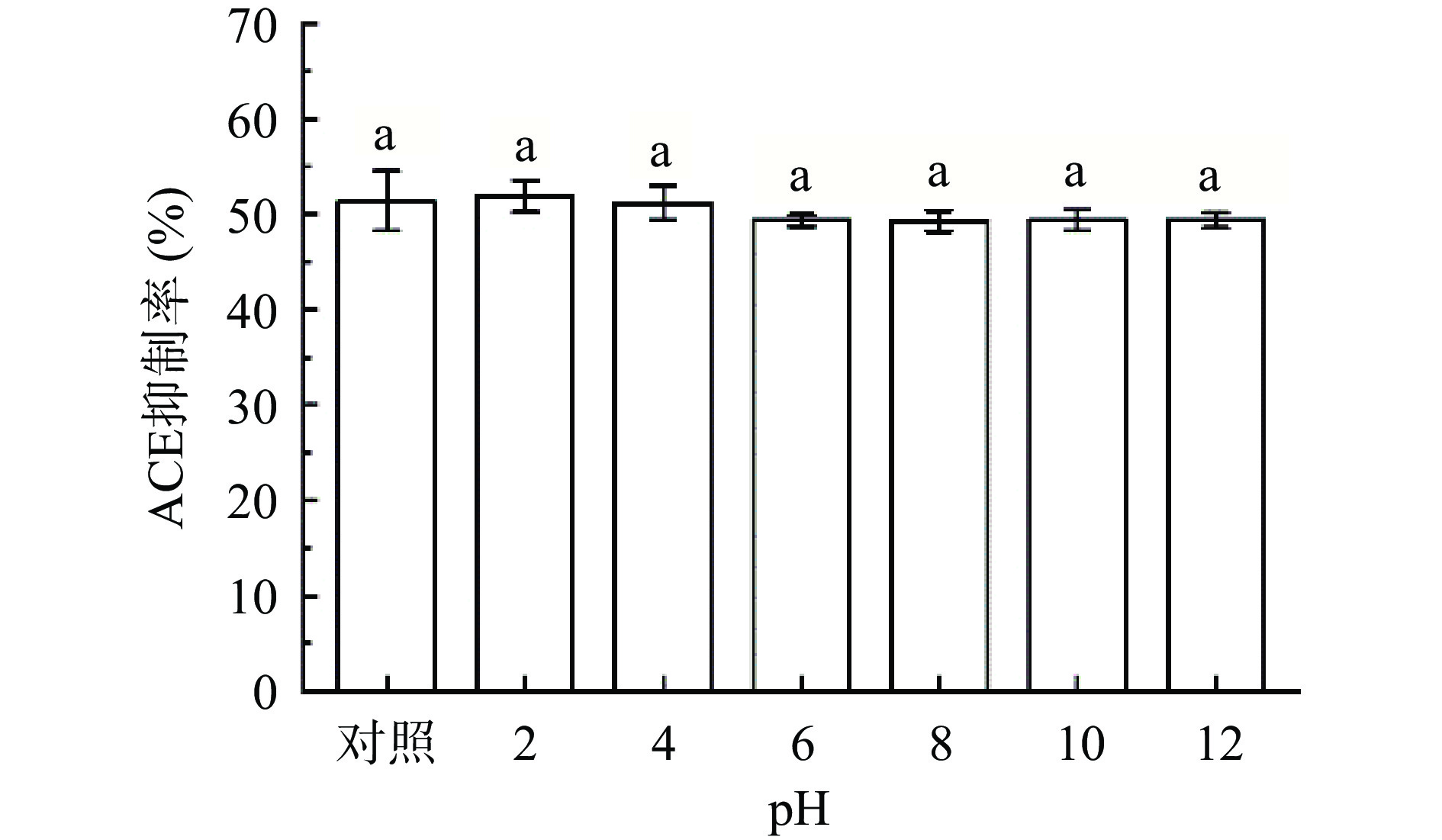
如图12所示,pH变化对F3b的活性无显著影响。表明分离得到的F3b具有良好的pH稳定性,在消化时胃酸可能不会破坏其活性[50]。Wang等[51]从高原节肢螺旋菌蛋白的水解物中分离鉴定出一种新的ACE抑制肽Pro-Thr-Gly-Asn-Pro-Leu-Ser-Pro,在pH为3~11的条件下具有很强的稳定性。
2.7.3 不同金属离子处理对F3b活性影响
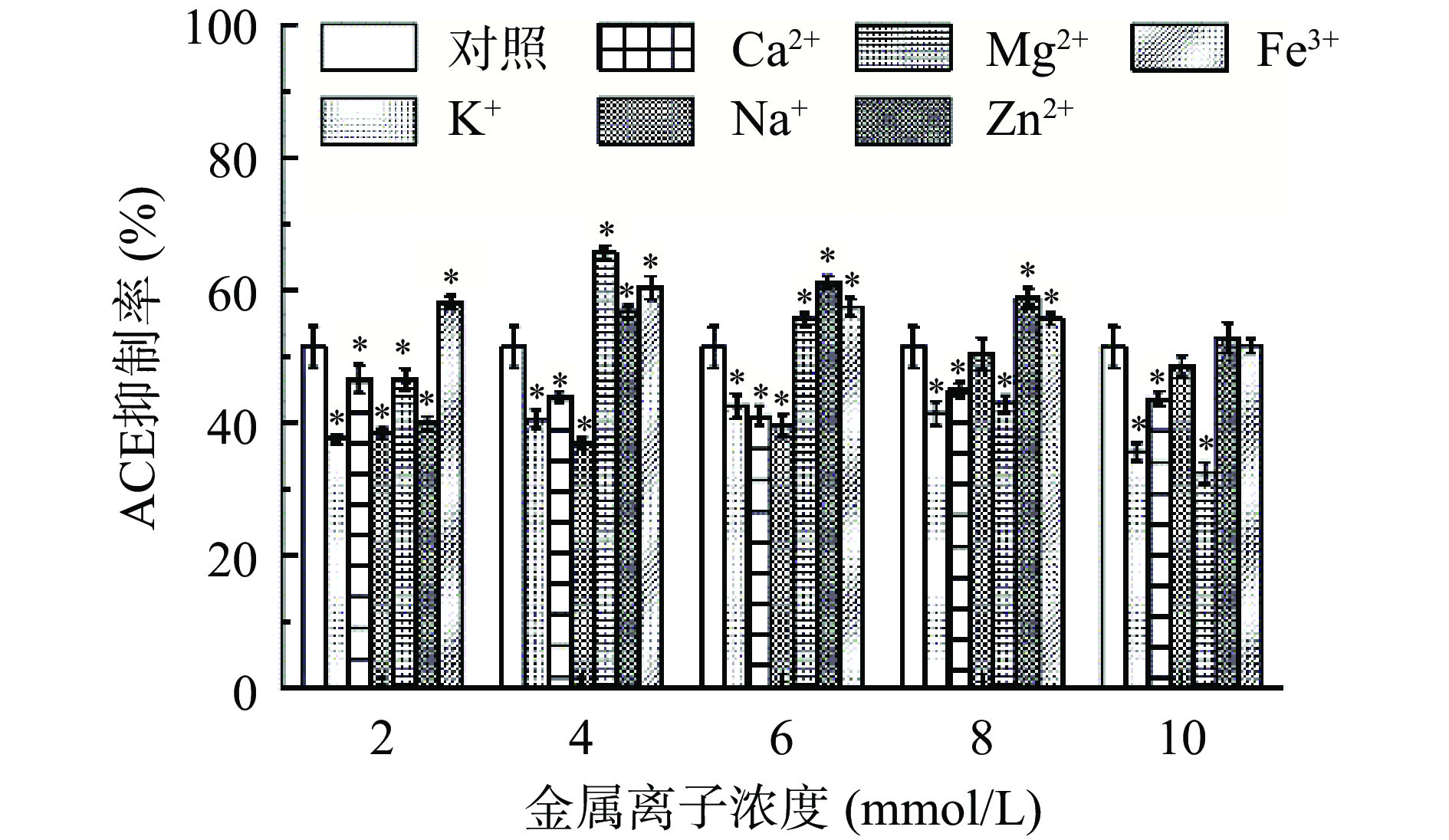
金属离子和多肽的氨基酸残基之间可以形成配位键,使多肽的二级结构发生改变,从而影响其活性[52]。如图13所示,经过金属离子处理后ACE抑制糖肽的活性在一定程度上受到影响。K+、Ca2+和Mg2+在2~10 mmol/L浓度范围内对ACE抑制糖肽的活性均有显著影响(P<0.05),对ACE抑制糖肽的活性有抑制作用,在K+和Ca2+浓度为4 mmol/L时,ACE抑制率分别为对照的78.94%±2.18%和85.31%±4.99%。Lai等[53]研究发现,绿茶源ACE抑制肽对Ca2+和Mg2+敏感,在离子浓度为1 mmol/L时ACE抑制率仅为26.21%。Na+浓度在低于6 mmol/L时对ACE抑制糖肽的活性有抑制效果显著(P<0.05),当浓度提高至8 mmol/L时对ACE抑制糖肽的活性影响不显著(P>0.05)。高泽汝[54]研究发现玉米胚芽ACE抑制肽Leu-Pro-Gly-Pro和Val-Thr-Leu-Leu 在2%~10%的NaCl浓度下能够维持活性。Zn2+浓度在2 mmol/L时对ACE抑制糖肽的活性有抑制作用;当浓度继续增加,ACE抑制糖肽的活性先增加后降低,在6 mmol/L时最大。Fe3+在2~8 mmol/L浓度范围内对ACE抑制糖肽的活性影响显著,能够提高ACE抑制糖肽的活性。在Zn2+和Fe3+浓度为4 mmol/L时,ACE抑制率分别为对照的109.91%±8.12%和117.43%±6.78%。沈嘉森等[55]研究发现龙须菜源ACE抑制肽暴露于5 mmol/L的Zn2+时能够提高ACE抑制肽的活性,暴露于5 mmol/L的K+和Ca2+时对ACE抑制肽有抑制作用,与本文的研究结果相似。
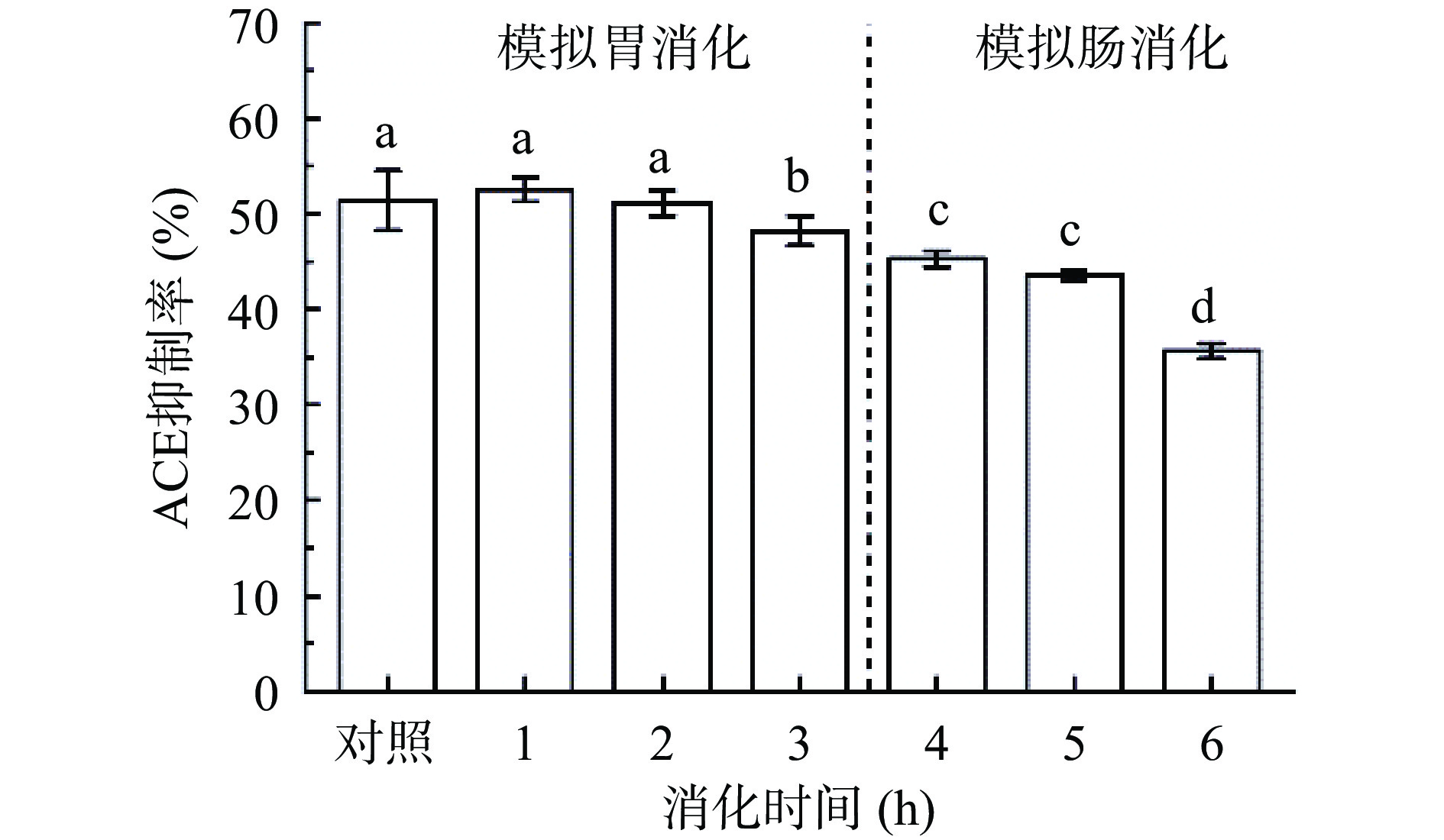
2.7.4 模拟胃肠消化对F3b活性影响
肽胃肠道消化稳定性之间的差异可能是由于肽的特性所引起的,包括疏水性、酸碱性质和C或N末端氨基酸的类型[56]。如图14所示,经胃蛋白酶酶解2 h时F3b的活性变化不显著(P>0.05),酶解至第3 h时ACE抑制糖肽活性显著降低(P<0.05)。可能因为胃蛋白酶的作下F3b上的部分肽键断裂,使活性降低。在胰蛋白酶酶解阶段,F3b活性随酶解时间延长活性逐渐下降,酶解至6 h时,活性为原先的70.00%±3.30%。Mirzaei等[57]对合成肽段Val-Leu-Ser-Thr-Ser-Phe-Pro-Pro-Lys(VLSTSFPPK)、Val-Leu-Ser-Thr-Ser-Phe-Pro-Pro-Trp(VLSTSFPPW)、Val-Leu-Ser-Thr-Ser-Phe-Pro-Pro-Tyr(VLSTSFPPY)、Val-Leu-Ser-Thr-Ser-Phe-Pro-Pro-Phe(VLSTSFPPF)进行类似的研究,经胃蛋白酶水解后VLSTSFPPW、VLSTSFPPY、VLSTSFPPF的ACE抑制率显著下降(P<0.05),经胰蛋白酶消化后,肽段被进一步水解,消化4.5 h后四种肽的ACE抑制活性分别下降2.75、2.51、2.66和2.15倍。在胰蛋白酶的作用下,多肽被进一步水解,使得活性降低[58]。可能是与多肽的疏水性有关,Mirzaei等[57]结果表明疏水性降低了肽的胃稳定性。
3. 结论
本文采用毛霉和米根霉混合发酵藜麦,通过响应面试验优化出最优的发酵工艺。在此基础上通过乙醇沉淀、真空浓缩、葡聚糖凝胶G-15分离和RP-HPLC分离制得ACE抑制糖肽(F3b),并对其ACE抑制活性、结构和稳定性做了初步的研究。通过FTIR分析证明其存在多糖和多肽的结构,通过β-消除法判断出糖链与肽链以O-糖肽键连接。分析其体外稳定性发现,在25~55 ℃环境处理3 h时活性基本得到保留,pH变化对其活性无显著影响(P>0.05);金属离子Fe3+、Zn2+和Na+在一定浓度下均能增强其ACE抑制活性,K+和Ca2+则对其活性有一定抑制作用;通过体外模拟胃肠消化表明,在胃消化阶段其活性能够较好保留,肠消化阶段F3b可能被胰蛋白酶分解,使活性下降,但在消化6 h后ACE抑制活性能够保留70.00%±3.30%以上。总体而言,该F3b在热、酸碱、金属离子以及胃肠环境有一定的稳定性。因此,本研究可丰富ACE抑制肽的种类,为藜麦资源的高值化利用提供参考。后续将深入研究其氨基酸、单糖组成及对ACE的抑制机理。
-
表 1 响应面试验设计因素与水平
Table 1 Factors and levels of response surface experiment
因素 水平 −1 0 1 A发酵时间(d) 6 9 12 B总接种量(%) 1 3 5 C毛霉和米根霉接种量比 3:2 1:1 2:3 D料液比(g/mL) 1:10 1:20 1:30 表 2 OPA法测定ACE抑制率加样方法
Table 2 Preparation of reaction system used for measurement of ACE inhibition rate by OPA method
添加量(μL) 样品 对照 实验组 空白组 实验组 空白组 硼酸盐缓冲液 0 5 40 45 稀释后样品 40 40 0 0 ACE 5 0 5 0 HHL 20 20 20 20 NaOH 150 150 150 150 OPA甲醇 40 40 40 40 HCl 40 40 40 40 蒸馏水 2950 2950 2950 2950 表 3 响应面试验设计与结果
Table 3 Experimental design and results for response surface analysis
实验号 A B C D ACE抑制率(%) 1 0 0 1 1 43.29 2 1 −1 0 0 37.62 3 −1 0 −1 0 45.90 4 0 0 0 0 65.40 5 1 0 0 1 43.91 6 0 0 0 0 66.75 7 1 0 0 −1 43.08 8 0 −1 −1 0 47.53 9 0 0 −1 −1 46.05 10 0 0 1 −1 52.57 11 −1 0 0 −1 53.49 12 0 −1 1 0 48.27 13 1 0 −1 0 39.45 14 −1 −1 0 0 39.42 15 0 0 0 0 65.89 16 1 0 1 0 48.23 17 0 0 0 0 67.82 18 −1 0 1 0 43.46 19 0 0 0 0 63.18 20 −1 1 0 0 43.86 21 0 −1 0 −1 51.16 22 −1 0 0 1 40.08 23 0 1 0 −1 44.04 24 0 1 0 1 40.63 25 1 1 0 0 30.80 26 0 −1 0 1 41.53 27 0 1 −1 0 37.43 28 0 1 1 0 41.98 29 0 0 −1 1 41.63 表 4 回归方程的方差分析
Table 4 Analysis of variance (ANOVA) of regression equations
响应值 方差来源 自由度 均方 F值 P值 显著性 模型 2578.97 14 184.21 27.70 0.000 ** A 44.50 1 44.50 6.69 0.022 * B 59.87 1 59.87 9.00 0.010 ** C 32.69 1 32.69 4.92 0.044 * D 128.82 1 128.82 19.37 0.001 ** AB 31.69 1 31.69 4.76 0.047 * AC 31.47 1 31.47 4.73 0.047 * AD 50.74 1 50.74 7.63 0.015 * BC 3.64 1 3.64 0.5476 0.472 BD 9.68 1 9.68 1.46 0.248 CD 5.92 1 5.92 0.8899 0.362 A² 1063.00 1 1063.00 159.84 0.000 ** B² 1170.55 1 1170.55 176.01 0.000 ** C² 584.60 1 584.60 87.90 0.000 ** D² 500.50 1 500.50 75.26 0.000 ** 残差 93.11 14 6.65 失拟项 81.13 10 8.11 2.71 0.175 不显著 纯误差 11.98 4 2.99 总离差 2672.08 28 注:“**”表示影响极显著(P<0.01);“*”表示影响显著(P<0.05)。 -
[1] 马丽媛, 吴亚哲, 陈伟伟. 《中国心血管病报告2018》要点介绍[J]. 中华高血压杂志,2019,27(83):712−716. [MA Liyuan, WU Yazhe, CHEN Weiwei. Key points of China Cardiovascular Disease Report 2018[J]. Chin J Hypertension,2019,27(83):712−716. MA Liyuan, WU Yazhe, CHEN Weiwei. Key points of China Cardiovascular Disease Report 2018[J]. Chin J Hypertension, 2019, 27(83): 712-716.
[2] ZHANG Biying, LIU Jingbo, WEN Hedi, et al. Structural requirements and interaction mechanisms of ACE inhibitory peptides: Molecular simulation and thermodynamics studies on LAPYK and its modified peptides[J]. Food Science and Human Wellness,2022,11(6):1623−1630. doi: 10.1016/j.fshw.2022.06.021
[3] NINGRUM S, SUTRISNO A, HSU J L. An exploration of angiotensin-converting enzyme (ACE) inhibitory peptides derived from gastrointestinal protease hydrolysate of milk using a modified bioassay-guided fractionation approach coupled with in silico analysis[J]. Journal of Dairy Science, 2022, 105(3): 1913-1928.
[4] GU Yuchen, WU Jianping. LC-MS/MS coupled with QSAR modeling in characterising of angiotensin I-converting enzyme inhibitory peptides from soybean proteins[J]. Food Chemistry,2013,141(3):2682−2690. doi: 10.1016/j.foodchem.2013.04.064
[5] ABEDIN M M, CHOURASIA R, PHUKON L C, et al. Characterization of ACE inhibitory and antioxidant peptides in yak and cow milk hard chhurpi cheese of the Sikkim Himalayan region[J]. Food Chemistry,2022,13:100231.
[6] 薛张芝, 张洪超, 丁源, 等. 墨鱼缠卵腺糖蛋白的抗氧化及抑菌活性研究[J]. 核农学报,2019,33(97):1544−1550. [XUE Zhangzhi, ZHANG Hongchao, DING Yuan, et al. Antioxidative and antibacterial activities of glycoprotein from cuttlefish oviposita[J]. Chinese Journal of Nuclear Agronomy,2019,33(97):1544−1550. doi: 10.11869/j.issn.100-8551.2019.08.1544 XUE Zhangzhi, ZHANG Hongchao, DING Yuan, et al. Antioxidative and antibacterial activities of glycoprotein from cuttlefish oviposita[J]. Chinese Journal of Nuclear Agronomy, 2019, 33(97): 1544-1550. doi: 10.11869/j.issn.100-8551.2019.08.1544
[7] QIN Gaoyixin, XU Wu, LIU Junping, et al. Purification, characterization and hypoglycemic activity of glycoproteins obtained from pea (Pisum sativum L.)[J]. Food Science and Human Wellness,2021,10(3):297−307. doi: 10.1016/j.fshw.2021.02.021
[8] LI Shanshan, TAO Lingchen, YU Xinyu, et al. Royal jelly proteins and their derived peptides: Preparation, properties, and biological activities[J]. Journal of Agricultural and Food Chemistry,2021,69(48):14415−14427. doi: 10.1021/acs.jafc.1c05942
[9] 张宏玲. 生姜糖蛋白提取纯化及活性研究[D]. 锦州: 渤海大学, 2019 ZHANG Hongling. Extraction, purification and activity of ginger glycoprotein[D]. Jinzhou: Bohai University, 2019.
[10] KARAMI Z, PEIGHAMBARDOUST S H, HESARI J, et al. Antioxidant, anticancer and ACE-inhibitory activities of bioactive peptides from wheat germ protein hydrolysates[J]. Food Bioscience,2019,32:100450.
[11] 刘文. 牡蛎加工下脚料综合利用的关键技术研究[D]. 宁波: 宁波大学, 2013 LIU Wen. Research on the key technology of comprehensive utilization of oyster processing waste[D]. Ningbo: Ningbo University, 2013.
[12] AONDONA M M, IKYA J K, UKEYIMA M T, et al. In vitro antioxidant and antihypertensive properties of sesame seed enzymatic protein hydrolysate and ultrafiltration peptide fractions[J]. Journal of Food Biochemistry,2021,45(1):e13587.
[13] 齐天明, 李志坚, 秦培友, 等. 藜麦栽培技术研究与应用展望[J]. 中国农业科技导报,2022,24(84):157−165. [QI Tianming, LI Zhijian, QIN Peiyou, et al. Research and application prospects of quinoa cultivation technology[J]. China Agricultural Science and Technology Herald,2022,24(84):157−165. doi: 10.13304/j.nykjdb.2021.0190 QI Tianming, LI Zhijian, QIN Peiyou, et al. Research and application prospects of quinoa cultivation technology[J]. China Agricultural Science and Technology Herald, 2022, 24(84): 157-165. doi: 10.13304/j.nykjdb.2021.0190
[14] DAKHILI S, ABDOLALIZADEH L, HOSSEINI S M, et al. Quinoa protein: Composition, structure and functional properties[J]. Food Chemistry,2019,299:125161.
[15] MIR N A, RIAR C S, SINGH S. Effect of pH and holding time on the characteristics of protein isolates from Chenopodium seeds and study of their amino acid profile and scoring[J]. Food Chemistry,2018,272(Jan.30):165−173.
[16] SHARMA R, GARG P, KUMAR P, et al. Microbial fermentation and its role in quality improvement of fermented foods[J]. Fermentation,2020,6(4):106. doi: 10.3390/fermentation6040106
[17] CHANGYU Zhou, QIANG Xia, LIHUI Du, et al. Recent developments in off-odor formation mechanism and the potential regulation by starter cultures in dry-cured ham[J]. Critical Reviews in Food Science and Nutrition, 2022: 1−15.
[18] SUZUKI S, OHMORI H, HAYASHIDA S, et al. Lipase and protease activities in Koji cheeses surface-ripened with Aspergillus strains[J]. Food Science and Technology Research,2021,27(3):543−549. doi: 10.3136/fstr.27.543
[19] LAI C Y, HOU C Y, CHUANG P T, et al. Microbiota and mycobiota of soy sauce-supplied lactic acid bacteria treated with high pressure[J]. Fermentation,2022,8(7):338. doi: 10.3390/fermentation8070338
[20] ANNA S J, BOŻENA S, ANA M C, et al. Mould starter selection for extended solid-state fermentation of quinoa[J]. LWT,2019,99:231−237. doi: 10.1016/j.lwt.2018.09.055
[21] WEI Guanmian, REGENSTEIN J M, LIU Xiaoming, et al. Comparative aroma and taste profiles of oil furu (soybean curd) fermented with different mucor strains[J]. Journal of Food Science,2020,85(11):1−9.
[22] 赵新淮, 冯志彪. 蛋白质水解物水解度的测定[J]. 食品科学,1994,11(23):65−67. [ZHAO Xinhuai, FENG Zhibiao. Determination of the degree of hydrolysis of protein hydrolyzates[J]. Food Science,1994,11(23):65−67. ZHAO Xinhuai, FENG Zhibiao. Determination of the degree of hydrolysis of protein hydrolyzates[J]. Food Science, 1994, 11(23): 65-67.
[23] 刘璇璇, 武莹敏, 朱振宝, 等. 水酶法联产核桃油和核桃多肽工艺优化及油脂脂肪酸分析[J]. 食品工业科技,2022,43(18):217−224. [LIU Xuanxuan, WU Yingmin, ZHU Zhenbao, et al. Optimization of synchronous extraction process of oil and polypeptide from walnut by aqueous enzymatic method and the fatty acid composition analysis of its oil[J]. Science and Technology of Food Industry,2022,43(18):217−224. LIU Xuanxuan, WU Yingmin, ZHU Zhenbao, et al. Optimization of synchronous extraction process of oil and polypeptide from walnut by aqueous enzymatic method and the fatty acid composition analysis of its oil[J]. Science and Technology of Food Industry, 2022, 43(18): 217−224.
[24] LI Fengjuan, YIN Lijun, XIN Xin, et al. Changes in angiotensin I-converting enzyme inhibitory activities during the ripening of douchi (a Chinese traditional soybean product) fermented by various starter cultures[J]. International Journal of Food Properties,2010,13(3):512−524. doi: 10.1080/10942910802688176
[25] 向媛嫄, 王文林, 宋海云, 等. 去糖基化对水溶澳洲坚果糖肽结构和抗氧化性的影响[J]. 食品与发酵工业,2021,47(11):98−103. [XIANG Yuanyuan, WANG Wenlin, SONG Haiyun, et al. Effect of deglycosylation on the structure and antioxidant activity of water-soluble macadamia glycopeptide[J]. Food and Fermentation Industries,2021,47(11):98−103. XIANG Yuanyuan, WANG Wenlin, SONG Haiyun, et al. Effect of deglycosylation on the structure and antioxidant activity of water-soluble macadamia glycopeptide[J]. Food and Fermentation Industries, 2021, 47(11): 98-103.
[26] WEN Chaoting, ZHANG Jixian, FENG Yuqin, et al. Purification and identification of novel antioxidant peptides from watermelon seed protein hydrolysates and their cytoprotective effects on H2O2-induced oxidative stress[J]. Food Chemistry,2020,327:127059. doi: 10.1016/j.foodchem.2020.127059
[27] YANG Jing, HUANG Jichao, DONG Xiaoli, et al. Purification and identification of antioxidant peptides from duck plasma proteins[J]. Food Chemistry,2020,319:126534. doi: 10.1016/j.foodchem.2020.126534
[28] 曲金萍, 陈金玉, 张坤生, 等. 响应面法优化鸡胸肉肌原纤维蛋白抗氧化肽的制备及其二级结构研究[J]. 食品研究与开发,2020,41(20):1−8. [QU Jingping, CHEN Jingyu, ZHANG Kunsheng, et al. Response surface methodology to optimize the preparation of chicken breast myofibrillar protein antioxidant peptide and its secondary structure research[J]. Food Research and Development,2020,41(20):1−8. QU Jingping, CHEN Jingyu, ZHANG Kunsheng, et al. Response surface methodology to optimize the preparation of chicken breast myofibrillar protein antioxidant peptide and its secondary structure research[J]. Food Research and Development, 2020, 41(20): 1- 8.
[29] 沈柱英, 黄占旺, 肖建辉, 等. 纳豆糖蛋白的分离纯化、结构表征及其免疫活性研究[J]. 食品科学,2015,36(82):215−222. [SHEN Zhuying, HUANG Zhanwang, XIAO Jianhui, et al. Isolation, purification, structural characterization and immune activity of Natto glycoprotein[J]. Food Science,2015,36(82):215−222. SHEN Zhuying, HUANG Zhanwang, XIAO Jianhui, et al. Isolation, purification, structural characterization and immune activity of Natto glycoprotein [J]. Food Science, 2015, 36(82): 215-222.
[30] 姬中伟. 小米醇溶蛋白肽的制备及其抗氧化与抗炎活性研究[D]. 无锡: 江南大学, 2020 Ji Zhongwei. Preparation and antioxidant and anti-inflammatory activities of millet gliadin peptide[D]. Wuxi: Jiangnan University, 2020.
[31] ZHENG Yajun, SHI Panqi, LI Yan, et al. A novel ACE-inhibitory hexapeptide from camellia glutelin-2 hydrolysates: Identification, characterization and stability profiles under different food processing conditions[J]. LWT,2021,147:111682. doi: 10.1016/j.lwt.2021.111682
[32] CHAI T T, XIAO J B, DASS S M, et al. Identification of antioxidant peptides derived from tropical jackfruit seed and investigation of the stability profiles[J]. Food Chemistry,2021,340:127876. doi: 10.1016/j.foodchem.2020.127876
[33] PEBRIANTI S A, CAHYANTO M N, INDRATI R. Angiotensin I-converting enzyme (ACE) inhibitory activity of ace inhibitory peptides produced during the fermentation of pigeon pea (Cajanus cajan) tempe[J]. Indonesian Food and Nutrition Progress,2020,16(2):47. doi: 10.22146/ifnp.46921
[34] 王婕娉, 刘睿, 丁士勇, 等. 响应面优化混菌发酵蜂王幼虫制备抗氧化肽及其结构鉴定[J]. 食品与发酵工业,2022,48(6):210−217. [WANG Jieping, LIU Rui, DING Shiyong, et al. Preparation of antioxidant peptides by response surface optimization of mixed fermentation from queen bee larvae and its structure identification[J]. Food and Fermentation Industries,2022,48(6):210−217. WANG Jieping, LIU Rui, DING Shiyong, et al. Preparation of antioxidant peptides by response surface optimization of mixed fermentation from queen bee larvae and its structure identification[J]. Food and Fermentation Industries, 2022, 48(6): 210-217.
[35] 李景, 陈定刚. 响应面优化米曲霉固态发酵豆粕制备ACE抑制肽条件[J]. 食品研究与开发,2018,39(196):120−125. [LI Jing, CHEN Dinggang. Preparation of ACE inhibitory peptide from soybean meal fermented by Aspergillus oryzae by response surface optimization[J]. Food Research and Development,2018,39(196):120−125. LI Jing, CHEN Dinggang. Preparation of ACE inhibitory peptide from soybean meal fermented by Aspergillus oryzae by response surface optimization [J]. Food Research and Development, 2018, 39(196): 120-125.
[36] 李雨霖, 倪婕, 薛璐瑜, 等. 解淀粉芽孢杆菌YA289降解虾头蛋白的发酵工艺优化[J]. 现代食品科技,2022,165(165):1−10. [LI Yulin, NI Jie, XUE Luyu, et al. Optimization of fermentation technology for degradation of shrimp head protein by Bacillus amyloliticus YA289[J]. Modern Food Science and Technology,2022,165(165):1−10. doi: 10.13982/j.mfst.1673-9078.2022.10.1399 LI Yulin, NI Jie, XUE Luyu, et al. Optimization of fermentation technology for degradation of shrimp head protein by Bacillus amyloliticus YA289[J]. Modern Food Science and Technology, 2022, 165 (165): 1-10. doi: 10.13982/j.mfst.1673-9078.2022.10.1399
[37] 李顺. 总状毛霉和米根霉混合发酵腐乳研究[D]. 合肥: 合肥工业大学, 2017 LI Shun. Study on fermented beancurd mixed with Mucor racemose and Rhizopus oryzae[D]. Hefei: Hefei University of Technology, 2017.
[38] 王銮, 包怡红, 康宁. 混菌固态发酵榛仁粕制备降血压肽工艺优化研究[J]. 中国粮油学报,2018,33(164):35−41. [Wang Luan, BAO Yihong, Kang Ning. Optimization of preparation of blood pressure peptide from hazelnut meal by solid-state fermentation with mixed bacteria[J]. Journal of the China Cereals and Oils Society,2018,33(164):35−41. doi: 10.3969/j.issn.1003-0174.2018.12.007 Wang Luan, BAO Yihong, Kang Ning. Optimization of preparation of blood pressure peptide from hazelnut meal by solid-state fermentation with mixed bacteria[J]. Journal of the China Cereals and Oils Society, 2018, 33(164): 35-41 doi: 10.3969/j.issn.1003-0174.2018.12.007
[39] 高洁, 高翔, 于佳, 等. 纳豆菌发酵扇贝裙边制备ACE抑制肽的工艺优化[J]. 现代食品科技,2018,34(168):165−175. [GAO Jie, GAO Xiang, YU Jia, et al. Optimization of process for preparation of ACE inhibitory peptide from scallop skirt fermented by natto[J]. Modern Food Science and Technology,2018,34(168):165−175. doi: 10.13982/j.mfst.1673-9078.2018.11.026 GAO Jie, GAO Xiang, YU Jia, et al. Optimization of process for preparation of ACE inhibitory peptide from scallop skirt fermented by natto[J]. Modern Food Science and Technology, 2018, 34(168): 165-175. doi: 10.13982/j.mfst.1673-9078.2018.11.026
[40] SUN Runan, LIU Xiaofang, YU Yuan, et al. Preparation process optimization, structural characterization and in vitro digestion stability analysis of Antarctic krill (Euphausia superba) peptides-zinc chelate[J]. Food Chemistry,2021,340:128056. doi: 10.1016/j.foodchem.2020.128056
[41] VIDAL A R, DUARTE L P, SCHMIDT M M, et al. Extraction and characterization of collagen from sheep slaughter by-products[J]. Waste Management,2020,102(1):838−846.
[42] KARAMI M, SHAHRAKY M K, RANJBAR M, et al. Preparation, purification, and characterization of low-molecular-weight hyaluronic acid[J]. Biotechnology Letters,2021,43(1):133−142. doi: 10.1007/s10529-020-03035-4
[43] TUVAANJAV S, KOMATA M, MA C, et al. Isolation and antiviral activity of water-soluble Cynomorium songaricum Rupr. polysaccharides[J]. Journal of Asian Natural Products Research,2016,18(2):159−171. doi: 10.1080/10286020.2015.1082547
[44] ZENG Fanke, CHEN Wenbo, HE Ping, et al. Structural characterization of polysaccharides with potential antioxidant and immunomodulatory activities from Chinese water chestnut peels[J]. Carbohydrate Polymers,2020,246:116551. doi: 10.1016/j.carbpol.2020.116551
[45] ALBERT S B, ROBERT G. Structure of the O-linked oligosaccharides from a major thyroid cell surface glycoprotein[J]. Archives of Biochemistry & Biophysics,1997,343(1):73−80.
[46] ZHANG Haiqiang, HAN Luanwei, SUN Xiaomei, et al. A glycoprotein from mountain cultivated ginseng: Insights into their chemical characteristics and intracellular antioxidant activity[J]. International Journal of Biological Macromolecules,2022,217:761−774. doi: 10.1016/j.ijbiomac.2022.07.023
[47] LIU K, DU R, CHEN F. Stability of the antioxidant peptide SeMet-Pro-Ser identified from selenized brown rice protein hydrolysates[J]. Food Chem,2020,319:126540. doi: 10.1016/j.foodchem.2020.126540
[48] LI Meiqing, XIA Shanwei, ZHANG Yijun, et al. Optimization of ACE inhibitory peptides from black soybean by microwave-assisted enzymatic method and study on its stability[J]. LWT,2018,98:358−365. doi: 10.1016/j.lwt.2018.08.045
[49] UNNIKRISHNAN P, KIZHAKKETHIL B P, GEORGE J C, et al. Antioxidant peptides from dark meat of yellowfin tuna (Thunnus albacares): Process optimization and characterization[J]. Waste and Biomass Valorization,2021,12(4):1845−1860. doi: 10.1007/s12649-020-01129-8
[50] WANG Rui, YUN Jianmin, WU Shujuan, et al. Optimisation and characterisation of novel angiotensin-converting enzyme inhibitory peptides prepared by double enzymatic hydrolysis from Agaricus bisporus scraps[J]. Foods,2022,11(3):394. doi: 10.3390/foods11030394
[51] WANG Kai, LUO Qinwen, HONG Hui, et al. Novel antioxidant and ACE inhibitory peptide identified from Arthrospira platensis protein and stability against thermal/pH treatments and simulated gastrointestinal digestion[J]. Food Research International,2020,139(1):109908.
[52] SOVAGO I, KALLAY C, VARNAGY K. Peptides as complexing agents: Factors influencing the structure and thermodynamic stability of peptide complexes[J]. Coordination Chemistry Reviews,2012,256(19−20):2225−2233. doi: 10.1016/j.ccr.2012.02.026
[53] LAI Xingfei, PAN Shunshun, ZHANG Wenji, et al. Properties of ACE inhibitory peptide prepared from protein in green tea residue and evaluation of its anti-hypertensive activity[J]. Process Biochemistry,2020,92:277−287. doi: 10.1016/j.procbio.2020.01.021
[54] 高泽汝. 玉米胚芽ACE抑制肽的制备及作用机制和稳定性研究[D]. 郑州: 河南工业大学, 2021 GAO Zheru. Preparation, mechanism and stability of ACE-inhibiting peptide from maize germ[D]. Zhengzhou: Henan University of Technology, 2021.
[55] 沈嘉森, 苏永昌, 陈晓婷, 等. 龙须菜ACE抑制肽的体外稳定性和抗氧化活性研究[J]. 食品工业科技,2022,43(7):384−392. [SHEN Jiasen, SU Yongchang, CHEN Xiaoting, et al. Study on in vitro stability and antioxidant activity of ACE inhibitory peptide from Gracilaria lemaneiformis[J]. Science and Technology of Food Industry,2022,43(7):384−392. doi: 10.13386/j.issn1002-0306.2021080345 SHEN Jiasen, SU Yongchang, CHEN Xiaoting, et al. Study on in vitro stability and antioxidant activity of ACE inhibitory peptide from Gracilaria lemaneiformis[J]. Science and Technology of Food Industry, 2022, 43(7): 384−392. doi: 10.13386/j.issn1002-0306.2021080345
[56] WONG F C, JIANBO X, ONG M G L, et al. Identification and characterization of antioxidant peptides from hydrolysate of blue-spotted stingray and their stability against thermal, pH and simulated gastrointestinal digestion treatments[J]. Food Chemistry,2019,271:614−622. doi: 10.1016/j.foodchem.2018.07.206
[57] MIRZAEI M, MIRDAMADI S, SAFAVI M, et al. The stability of antioxidant and ACE-inhibitory peptides as influenced by peptide sequences[J]. LWT- Food Science and Technology,2020,130:109710. doi: 10.1016/j.lwt.2020.109710
[58] 李烨彤, 苏志光, 张惠, 等. 糖基化大豆蛋白酶解物的分离纯化及耐消化稳定性的研究[J]. 大豆科学,2015,34(61):474−479. [LI Yatong, SU Zhiguang, ZHANG Hui, et al. Isolation and purification of glycated soybean protease hydrolysate and its stability in digestion[J]. Soybean Science,2015,34(61):474−479. LI Yatong, SU Zhiguang, ZHANG Hui, et al. Isolation and purification of glycated soybean protease hydrolysate and its stability in digestion[J]. Soybean Science, 2015, 34(61): 474-479.
-
期刊类型引用(2)
1. 高玮,李若兰,曾婧辉,力娜,宋凯,韩世范,朱瑞芳. 药食同源物质及其非营养素在肺癌防治中的研究进展. 护理研究. 2024(10): 1741-1746 .  百度学术
百度学术
2. 孙佳慧,周援,孙月娥,李春阳,王卫东,张军. 黄芪生物活性成分及在食品中的应用研究进展. 中国食品添加剂. 2023(12): 272-276 .  百度学术
百度学术
其他类型引用(1)





 下载:
下载:


 下载:
下载:



