Effects of Processing on Structural Properties and Biological Activities of Polysaccharides from Polygonatum spp.: A Review
-
摘要: 黄精作为药食同源品种,包括黄精(Polygonatum sibiricum Red.)、滇黄精(Polygonatum kingianum Coll. et Hemsl)及多花黄精(Polygonatum cyrtonema Hua)3种,具有多种生理活性,其中多糖发挥着重要作用。由于生黄精辛辣,其生用对人咽喉有强烈的刺激性。因此,药用或食用前需经过炮制加工处理,旨在减毒和提质增效。传统及现代加工方式均会引起黄精多糖含量下降,进而改变其结构和生理活性。基于文献研究,综述了加工方式(包括蒸、炖、辅以黄酒、晒制等)对黄精多糖含量、结构(单糖组成、分子量等)及其生理活性(抗氧化、调节糖脂代谢、增强免疫力、抗疲劳、抑菌等)的影响,为黄精资源在加工中的高效利用和精准控制提供参考。Abstract: Rhizomes from Polygonatum spp. have multi-functions of both medicine and foods. Three major species are Polygonatum sibiricum Red., Polygonatum kingianum Coll. et Hemsl and Polygonatum cyrtonema Hua. As the key component, the polysaccharides from Polygonatum spp. have various biological activities. The astringency of fresh rhizomes of Polygonatum spp. can cause throat irritation. Thus, the rhizomes of Polygonatum spp. must be processed before consumption as foods or medicine to reduce toxicity and enhance efficacy. However, both of traditional and modern processing methods lead to significant reduction in polysaccharide content, which also change its structure and biological value. Thus, based on literature research, this article summarizes common processing methods of Polygonatum spp., including steaming, stewing, with wine, and shining. In addition, effects of processing methods on polysaccharide content, its structural properties (including monosaccharide composition, molecular weight, etc.), and its physiological activities (including antioxidant activity, regulation of glucose and lipid metabolism, immune-enhancing activity, anti-fatigue activity, antibacterial activity, etc.) are reviewed. It is meaningful to provide references for efficiently industrial use and accurate control during processing of Polygonatum spp..
-
Keywords:
- processing /
- Polygonatum spp. /
- polysaccharide /
- structure /
- biological activities
-
黄精为百合科植物,具有良好的保健功能。药食同源目录中收录的黄精有3种,分别为黄精(Polygonatum sibiricum Red.)、滇黄精(Polygonatum kingianum Coll. et Hemsl)、多花黄精(Polygonatum cyrtonema Hua)。按形状不同,习称“鸡头黄精”、“大黄精”、“姜形黄精”。黄精的活性成分包括多糖[1-4]、皂苷[5]、黄酮[1, 6]、多酚[7-10]等。生黄精辛辣,其生用对人咽喉有强烈的刺激性,易引发过敏反应,且生用补性较差,故黄精需要炮制后入药或食用,以达到减毒增效和提质增效,且炮制后的黄精口感和质地均佳。但是熟黄精的活性成分发生显著变化[11]。多糖是黄精中核心功能性成分,中国药典仅对于多糖进行定量评价:生黄精多糖含量要求≥7%,炮制后的黄精多糖含量要求≥4%[12]。然而加工引起的黄精多糖含量的降低可能进一步引起多糖结构及生理活性的变化,但该问题并未引起重视,这也是黄精产业的瓶颈所在。因此,本文综述黄精常用的加工方式以及加工对三个品种黄精中多糖的含量、结构及生理活性的影响,为黄精资源在加工中的高效利用和精准控制提供参考。
1. 黄精的加工方式
黄精加工的初衷是去除“刺人咽喉”的不良反应。有研究说明,与生黄精相比,加工后的黄精可显著降低溶血率,因此刺激性降低[13]。从古至今,黄精的炮制方法及工艺多样,其炮制工艺经历生用→单蒸(出于《雷公炮制论》)→重蒸(出于《千金翼方》)→九蒸九晒(出于《食疗本草》)的演变[11, 14]。以“九蒸九制”为代表的反复蒸晒的方法是黄精沿用已久方法,也是现阶段黄精研究采用的最普遍的加工方式。但《中国药典》及各地的炮制规范中未收录该方法。
如表1所示,黄精的古今炮制法存在一脉相承的相似性,即蒸制是黄精的基本炮制方法[14],单次蒸制时间一般为4~6 h。也有研究采用高压蒸制来提高效率[15-18]。另外,还可采用炖煮的方式[17, 19-20]。除此之外,《中国药典》中黄精饮片经典的炮制方法将黄精与黄酒拌匀后共同蒸制,俗称酒制[12, 17, 20-24]。其他辅料还包括姜、黑豆、白酒、蜂蜜等[14, 25]。为了保证黄精能长期保存,传统黄精加工通过晒制来降低水分活度。但现代加工常采用烘箱烘干的方式。
表 1 不同加工方式对黄精中多糖含量的影响Table 1. Effects of different processing methods on polysaccharides content in Polygonatum spp.黄精品种 蒸制工艺 晒制/烘干工艺 重复次数 多糖含量变化(基于干重) 参考文献 多花黄精 清蒸
一蒸为1 h,随后在上一次基础上增加0.5 h60 ℃,2 h 9次 19.87%~23.71%(一次)→20.96%~23.10%(三次)→
11.90%~15.52%(七次)→5.49%~11.60%(九次)[30] 热河黄精
(多花玉竹)清蒸5 h后闷一夜 阳光下晒半干 9次 17.02%(生品)→10.04(一次)→5.62%(二次)
→5.80%(九次)[31] 多花黄精 清蒸6 h 60 ℃ 9次 15.0%(生品)→5.8%(二次)→4.5%(九次) [22] 鸡头黄精 清蒸6 h 45 ℃至衡重 9次 12%(生品)→ 8.89%(一次)→1%(三~九次) [5] 鸡头黄精 清蒸6 h 60 ℃ 9次 14.36%(生品)→≈4%(三次)→随后稳定 [32] 滇黄精 清蒸6 h 55 ℃,12 h 9次 13.034%(生品)→9.491%(二次)→8.203%(六次)→
6.988%(九次)[13] 多花黄精 − − 9次 9.602%(二次)→3.185%(四次)→2.043%(九次) [33] 多花黄精 − − 9次 ≈6~7%(生品)→≈3%(三次)→随后稳定 [34] 鸡头黄精 − − 9次 7.973%(二次)→3.584%(四次)→2.19%(九次) [33] 多花黄精 0.12 MPa高压蒸0.5 h − 9次 ≈20%、18%、6%(生品)→≈10%、8%、2%(九次) [18] 多花黄精 0.15 MPa高压蒸1.5 h 70 ℃,24 h 10次 17.56%(生品)→13.39%(一次)→10.95%(三次)→
8.03%(十次)[15, 17] 多花黄精 黄精加水(1:1),
蒸4 h60 ℃,12 h 9次 11.60%(生品)→6.98(一次)→2.99%(二次)
→3.99%(九次)[19] 滇黄精 黄精加水(1:2),
蒸12~96 h105 ℃ 1次 7.34%(生品)→9.73%(12 h)→4.26%(24 h)
→4.80%(96 h)[26] 多花黄精 酒制
与黄酒拌匀,蒸6 h60 ℃ 9次 15%(生品)→4.7%(二次)→3.8%(九次) [22] 多花黄精 酒制
与黄酒拌匀,蒸制6 h− 1次 平均为8.50% [35] 滇黄精 酒制
与黄酒浸润12 h,蒸制6 h45 ℃,12 h 9次 12.66%(生品)→7.76%(三次)→4.42%(九次) [36] 多花黄精 酒制
与黄酒拌匀闷润,蒸8 h太阳晒至皮微干 9次 野生:22.18%(一次)→32.81%(五次)→12.67%(九次);
栽培:18.68%(一次)→31.51%(四次)→10.84%(九次)[21, 37] 多花黄精 酒制
与黄酒浸泡后蒸制− 9次 7.31%(生品)→1.51%(四次)→1.52%(九次);
市售1.99%[23] 鸡头黄精 发酵:与小麦麦麸混匀接种灵芝液体种子,发酵12天;
蒸制:黄精加水浸润后取出蒸6 h,停火焖6 h;
酒制:黄精与黄酒闷润,蒸10 h。60 ℃,12 h 1次 生品:≈14%;
发酵:≈12.5%;
蒸制:≈8%;
酒制:≈6.5%[38] 注:−表示未提及。 目前黄精的加工工艺研究未成体系,但其原因是无可控的炮制工艺参数。另外,黄精炮制终点的判断依靠主观经验判断。例如,药典中黄精炮制终点描述为炖透或蒸透[12],指标过于宽泛。因此,通过深入研究加工过程中黄精活性成分变化规律从而规范其工艺参数显得尤为必要。
2. 不同加工方式对黄精多糖含量的影响
目前加工方式对黄精中多糖含量的影响的研究多基于“九蒸九制”的方法。基于不同方式(清蒸、炖煮、酒蒸等)的反复处理均能引起黄精中多糖含量的急剧降低。多数研究中,熟黄精中多糖含量均降低近一半以上(表1)。其次,加以辅料对多糖的下降无影响,清蒸和酒制均可引起多糖含量随着蒸制次数增加而下降[22],并且最终多糖含量相近(清蒸:5.5%~6.9%,酒蒸:5.6%~6.5%)[24]。三类黄精中的多糖显著下降多发生在前三蒸,随后多糖含量稳定。蒸制引起多糖含量下降可能与时间有关而非次数。以滇黄精为例,多糖下降发生在前48 h,随后增加时间对多糖含量无影响[26]。因此,减少蒸制时间可有效减少多糖损失[27]。炮制引起的多糖损失与传统认为的炮制补益且增强药效存在差距[14]。相对于蒸制,黄精加工耗时最长的干燥对多糖的影响研究较少。如表1所示,黄精干燥通常采用热风干燥,温度为45~70 ℃,烘干时间大多≥12 h。也有研究采用105 ℃烘干黄精[26],但黄精多糖在高温烘干中是否受到影响值得进一步研究。相比于热风干燥,微波真空干燥更有利于保存多花黄精中多糖含量[28-29],说明烘干的温度对于黄精多糖的保留也很重要。
综上,不同加工方式均能引起黄精中多糖含量显著下降,而未来的研究可以多糖含量为质量控制标准,明晰引起多糖下降的关键控制因素(温度、水分、时间等)。同时,以水分活度为标准,控制烘干条件(方式、温度、时间),以提高黄精的加工效率。
3. 黄精多糖结构及其在加工中的变化
三类黄精(生品)多糖的结构有所不同(表2)。生多花黄精多糖以中性多糖为主的果聚糖[3, 39-45]。也有报道多花黄精多糖的单糖组成以葡萄糖为主[45],这与单糖检测方法有关。果糖在检测前处理(强酸水解)下,可以转变为甘露糖及葡萄糖,因此果糖无法被识别[46]。生鸡头黄精多糖是以半乳糖、甘露糖为主形成的中性多糖[22, 47-54]。生滇黄精多糖是以葡萄糖为主形成的中性多糖[2]。三种不同来源黄精多糖的单糖组成有较大不同,因而三种黄精多糖指纹图谱上存在差异[36, 55],可能对其活性功能有一定的影响。
表 2 黄精多糖结构、活性及其在加工中的变化Table 2. Structure and bioactivity of polysaccharides from Polygonatum spp. and their changes during processing黄精品种
(或产地)加工工艺 多糖提取、
分离纯化方法多糖结构信息 活性 参考文献 多花黄精 生品 80 ℃热水提取
→离子柱,取中性组分
→凝胶柱中性多糖,分子量:8.5 kDa,单糖组成:果糖、葡萄糖(28:1),β-呋喃果糖构型 降血糖(大鼠及NCI-H716细胞模型):通过T1R2/T1R3介导的cAMP促进肠内分泌细胞释放GLP-1。
抗疲劳(小鼠模型):通过osteocalcin 信号通路[39-40]
[43]多花黄精 生品 常温、辅以超声提取
→离子柱,取中性组分
→凝胶柱PCP-1,中性多糖(占55.3%),分子量:2.966 kDa,单糖组成:甘露糖、葡萄糖、半乳糖、木糖、阿拉伯糖(1:17.53:7.02:0.27:0.59),以β糖苷键为主,含有6糖残基 抗氧化(DPPH清除率、O2-清除率,Fe2+螯合能力)
免疫刺激活性(RAW264.7细胞模型):促进细胞增殖、增强吞噬活性、促进NO释放[45] 多花黄精 生品 80 ℃热水提取
→离子柱,取中性组分→凝胶柱中性多糖,分子量:约4.8 kDa,单糖组成:果糖、葡萄糖(28:1),含有(2→1)β果聚糖为主链及(2→6)果聚糖为侧链(中间含有以新蔗果三糖形式存在的α-D葡萄糖)果聚糖 免疫刺激活性(RAW264.7细胞模型):促进细胞增殖、增强吞噬活性、促进TNF-α释放 [3] 多花黄精 生品 50%乙醇提取
→Bio-Gel P4柱中性多糖,聚合度28,分支果聚糖:每3个(2→1)β-D果糖键主链上,连接(2→6)果聚糖β-D果糖键分支;葡萄糖位于新蔗果三糖内 抗疱疹病毒活性 [41-42] 多花黄精
(九华)生品 沸水提取 分子量:5.34 kDa,单糖组成a:半乳糖醛酸(19.48%)、鼠李糖(17.92%)、甘露糖(18.56%)、葡萄糖(8.45%)、半乳糖(35.59%) − [61] 多花黄精 生品 分级提取:
PCP1(80 ℃热水))
→PCP2(0.1% NaOH)
→PCP3(0.5% NaOH)
→PCP4(1% NaOH)
→PCP5(2% NaOH)PCP1,得率40.3%,分子量:2.09 kDa,单糖组成a:以甘露糖(33.5%)、半乳糖(24.0%)、葡萄糖(20.7%)、半乳糖醛酸(19.3%)为主,以β构型为主,含有6种糖残基
PCP2,得率14.2%,分子量:38.6 kDa,单糖组成a:以半乳糖(59.8%)、阿拉伯糖(18.5%)为主
PCP3,得率20.1%,分子量:42.6 kDa,单糖组成a:以半乳糖(58.7%)、阿拉伯糖(22.2%)为主,以α构型为主,含有4种糖残基
PCP4,得率13.5%,分子量:34.3 kDa,单糖组成a:以半乳糖(61.3%)、阿拉伯糖(21.0%)为主
PCP5,得率6.4%,分子量:24.1 kDa,单糖未检测,以α构型为主,含有2种糖残基− [60] 多花黄精 生品 分级提取:
HBSS(醋酸缓冲液、pH5.2、70 ℃)→
CHSS(EDTA、草酸铵、醋酸缓冲液、pH5.2、
70 ℃)→
DASS(0.05 mol/L NaOH、NaB4、4 ℃)→
CASS(6 mol/L NaOH、NaB4、4 ℃)HBSS,总糖含量78.39%,单糖组成a:甘露糖(54.55%)、半乳糖(15.27%)、葡萄糖(14.91%)、阿拉伯糖(12.16%)为主
CHSS,总糖含量71.86%,糖醛酸17.73%,单糖组成a:阿拉伯糖(36.97%)、半乳糖(24.85%)、甘露糖(22.25)为主
DASS,总糖含量69.99%,糖醛酸28.34%,单糖组成a:半乳糖(54.91%)、阿拉伯糖(26.61%)为主
CASS,总糖含量73.73%,单糖组成a:葡萄糖(26.1%)、木糖(24.05%)、半乳糖(20.06%)抗氧化(DPPH、羟基自由基、ABTS自由基清除率、Fe2+螯合能力、还原力等):DASS最佳
抗菌活性:DASS最强
抗凝血:CASS最强
抗炎(小鼠脾淋巴细胞):抑制TNF-α产生:CASS最佳
免疫刺激活性(小鼠脾淋巴细胞):产生IFN-γ,HBSS最佳
抗肿瘤(Hela细胞):CASS最佳[85-88] 多花黄精 蒸制
6 h沸水提取 分子量:1200~4400 kDa(峰1),3.4~4.2 kDa(峰2),单糖组成:果糖(24.6%~70.3%)、鼠李糖(1.1%~4.8%)、半乳糖醛酸(11.2%~41.4%)、葡萄糖(1.0%~1.9%)、半乳糖(10.5%~20.5%)、阿拉伯糖及木糖(1.2%~7.7%) − [44] 多花黄精
(九华)酒制
(黄酒浸润
→蒸5 h后
干燥)沸水提取 分子量:76.4 kDa,单糖组成a:半乳糖醛酸(8.38%)、鼠李糖(23.23%)、阿拉伯糖(8.47%)、甘露糖(20.07%)、葡萄糖(6.29%)、半乳糖(35.56%) − [61] 多花黄精
(九华)九蒸九制
(蒸制7 h→
65 ℃烘干
6 h,重复
9次)沸水提取 分子量: 5.53 kDa(峰1)及75.8 kDa(峰2),单糖组成a:半乳糖醛酸(17.29%)、鼠李糖(21.50%)、甘露糖(19.91%)、葡萄糖(5.19%)、半乳糖(36.12%) − [61] 鸡头黄精 生品 80 ℃热水提取 PSPC,总糖含量83.28%,蛋白含量3.41%,糖醛酸含量9.41%,多酚含量0.27%,分子量:4.01 kDa,单糖组成:半乳糖(29.63%),甘露糖(36.10%),葡萄糖(15.09%),半乳糖醛酸(10.20%) 免疫刺激活性(RAW264.7细胞模型):促进细胞增殖、增强吞噬活性及酸性磷酸酶活性、促进NO释放;(脾虚小鼠模型):加强免疫功能,提高IL-2、IL-6、TNF-α、IFN-γ等 [48] 鸡头黄精 生品 80 ℃热水提取
→离子柱,得4个组分P-1,得率71.40%,中性多糖,总糖含量72.52%,分子量:1.18~500 kDa
P-2,得率1.95%,总糖含量73.35%;糖醛酸含量5.00%,分子量:5.01~86.2 kDa
P-3,得率1.14%,总含量75.86%;糖醛酸含量9.21%,分子量:4.00~105 kDa
P-4,得率1.64%,总糖含量72.36%;糖醛酸含量29.32%,分子量:2.30~105 kDa
单糖组成a:均含有核糖、鼠李糖、半乳糖、葡萄糖、木糖、阿拉伯糖,但比例不同抗氧化(DPPH、ABTS清除率等):P-4>P-3>P-2>PSP>P-1 [52] 鸡头黄精 生品 90 ℃热水提取 粘均分子量:300 kDa,具有三股螺旋结构、网孔片状和网链形貌,较强热稳定性 抗氧化(ABTS、羟自由基、DPPH清除率)
体外降血糖(抑制α-葡萄糖苷酶):IC50=2.63 mg/mL
体内降血糖(糖尿病小鼠模型):促进胰岛素分泌、促进肝糖原合成、缓解自由基对组织损伤[96] 鸡头黄精
(河北)生品 沸水提取(PSP)→
离子柱子,得4个组分PSP,单糖组成a:半乳糖(63.50%),鼠李糖(5.14%),甘露糖(8.04%),葡萄糖(1.75%),木糖(1.57%)
PSP1,分子量:4.415 kDa,单糖组成:以半乳糖(82.91%)、甘露糖(14.96%)为主
PSP2,分子量:2.235 kDa,单糖组成:以半乳糖(74.27%)、鼠李糖(20.54%)为主
PSP3,分子量:7.743 kDa,单糖组成:以鼠李糖为主(57.69%)、半乳糖(37.17%)为主
PSP4,分子量:6.467 kDa,单糖组成:以鼠李糖(72.63%)、半乳糖(20.74%)为主抗氧化(DPPH、ABTS);
免疫刺激活性(RAW 264.7细胞):促进细胞增殖、增强吞噬活性,释放NO及细胞因子(TNF-α、IL-6),促进TNF-α和IL-6 mRNA表达,促进IkB-α降解,促进p38、MAPK、NF-κB、iNOS及COX-2的表达,同时促进NF-κB核转移过程;
免疫刺激活性(环磷酰胺免疫抑制小鼠模型):激活体内特异性免疫应答反应,对细胞免疫和体液免疫具有积极调节作用[47, 51] 鸡头黄精 生品 沸水结合0.1 mol/L NaOH提取
→离心柱,取中性组分
→凝胶柱PSPJWA,分子量:141 kDa,单糖组成:半乳糖、阿拉伯糖、鼠李糖(14:4:1),含有4种糖苷键,为新型阿拉伯半乳聚糖结构 抗氧化(DPPH、ATBS、羟自由基清除率、Fe2 +还原性) [62] 鸡头黄精 生品 40 ℃温水辅以超声提取→离子柱,得2个组分→凝胶柱,取第1组分 PSP-1A,分子量:数均分子量(Mn)为12.83 kDa,重均分子量(Mw)为21.58 kDa,分布宽度指数1.68 抗氧化(DPPH、ABTS、羟基自由基、还原力) [53] 鸡头黄精
(湖北)− 常温、超高压
(255 MPa)提取平均粒径6.151,粘均分子量326.713 kDa(中等聚多糖),单糖组成:鼠李糖、木糖、果糖、葡萄糖、核糖以及半乳糖(1:1.24:2.45:5.14:4.49:0.81),含有α、β两种糖苷键 抗氧化(DPPH、羟基自由基、SAFR自由基清除率) [97-98] 鸡头黄精 酒制
(黄酒浸润
→蒸制4 h)80 ℃热水提取 PSPW,总糖含量41.85%,蛋白含量29.58%,糖醛酸含量20.12%,多酚含量3.03%,分子量14.2 kDa,单糖组成:半乳糖(78.77%)、甘露糖(5.5%)、半乳糖醛酸(13.84%) 免疫刺激活性(RAW264.7细胞模型及脾虚小鼠模型):酒制黄精多糖优于生黄精多糖 [48] 鸡头黄精 蒸制6 h 沸水提取 分子量:740~2500 kDa(峰1),2.8~5.4 kDa(峰2),单糖组成:果糖(29.8%~84.7%)、鼠李糖(1.6%~4.6%)、半乳糖醛酸(6.1%~28.6%)、葡萄糖(0.8%~1.9%)、半乳糖(4.7%~30.7%)、阿拉伯糖及木糖(1.1%~9.7%) − [44] 鸡头黄精 蒸制12 h 沸水提取
→离子柱,取中性组分
→凝胶柱PSW1a,中性多糖,高度分支半乳甘露聚糖(1,4,β甘露糖为主链,每9个的O-6替换成β半乳糖)
PSW1b-2,中性多糖,分支半乳聚糖(1,4 β吡喃半乳糖为主链,每7个O-6替换成β半乳糖)− [50] 鸡头黄精 − 沸水提取
→离子柱,得2个组分F1,分子量:132.6 kDa,单糖组成a:甘露糖(75.4%),葡萄糖(15.2%),半乳糖(4.75%),阿拉伯糖(4.15%),以1→4甘露吡喃糖苷为主链,葡萄吡喃糖苷接在O-6分支 免疫刺激活性(RAW 264.7细胞模型):调控MR及TLR4信号通路;
免疫刺激活性(NK细胞模型):调控CR3及TRL2信号通路[49] 滇黄精 生品 80 ℃热水提取 总糖67.2%,蛋白含量17.1%,糖醛酸21.4%,单糖组成:甘露糖、核糖、鼠李糖、半乳糖醛酸、盐酸氨基葡萄糖、葡萄糖醛酸、半乳糖、葡萄糖、木糖、阿拉伯糖、岩藻糖(69.6:1.53:4.14:16.14:4.15:9.19:6.5:6.24:5.63:56.38:10.44) 抗氧化(DPPH、羟自由基清除率)
体外降血糖(HepG2细胞模型):促进葡萄糖消耗[36] 滇黄精 生品 80 ℃热水提取
→离子柱,得中性组分→凝胶柱PKPS-1,分子量:14.05 kDa,单糖组成:葡萄糖、甘露糖、半乳糖醛酸、半乳糖、葡萄糖醛酸、阿拉伯糖(7.22:1.0:0.16:0.11:0.05:0.02),无三股螺旋结构 降血糖(HepG2细胞、糖尿病小鼠):通过PI3K/AKT调控血糖 [2] 滇黄精 生品 90 ℃提取 粘均分子量:160 kDa,具有三股螺旋结构,网孔片状结构及多分支链聚集 抗氧化(ABTS、羟自由基、DPPH清除率)
体外降血糖(抑制α-葡萄糖苷酶):IC50=4.57 mg/mL[96] 滇黄精 生品 沸水提取
→50%、70%乙醇沉
→离子柱
→凝胶柱,得3个组分PKS-1,分子量:850.018 kDa;
PKS-2,分子量:266.085 kDa;
PKS-3,分子量:474.799 kDa。
均为酸性糖,含有β-吡喃糖苷键,均含有甘露糖、半乳糖醛酸、葡萄糖醛酸、半乳糖、阿拉伯糖和岩藻糖抗氧化(总还原力、DPPH、羟自由基清除率):PKS> PKS-2>PKS-1 [36, 57] 滇黄精 饮片,
可能为酒制沸水提取
→50%乙醇沉淀(F1)→75%乙醇沉淀(F2)→90%乙醇沉淀(F3)F1,总糖78.0%,糖醛量28.3%,分子量:149.4 kDa
F2,总糖66.9%,糖醛酸16.2%,分子量:40.3 kDa
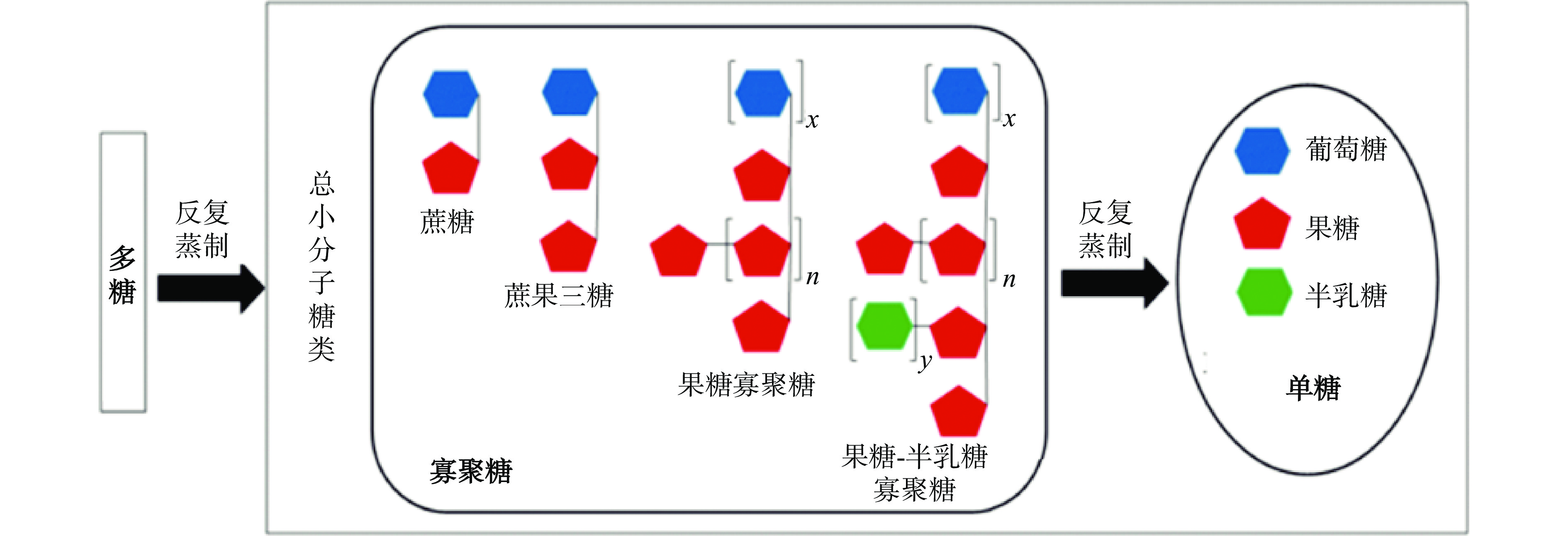
F3,总糖64.5%,糖醛酸4.93%,分子量:1.47~3.76 kDa− [58] 滇黄精 蒸制6 h 沸水提取 分子量:890~1400 kDa(峰1),4.0~5.3 kDa(峰2),单糖组成:果糖(31.7%~72.8%)、鼠李糖(2.7%~4.8%)、半乳糖醛酸(10.1%~36.0%)、葡萄糖(0.9%~2.7%)、半乳糖(8.1%~20.7%)、阿拉伯糖及木糖(3.5%~6.0%) − [44] 注:−表示未测定或者方法中未提及;a表示单糖组成基于质量比,而未标明a则表明单糖组成基于摩尔质量比。 熟黄精不仅多糖含量显著降低,而还原糖、总体单糖含量显著上升[23, 26, 56],说明黄精多糖发生降解。有研究发现(如图1所示),在反复蒸制过程中,多花黄精多糖可先降解为寡聚糖,随后进一步降解成单糖[18]。与此同时,黄精多糖中单糖组成结构上发生变化。首先,蒸制使得三类黄精的多糖从以中性多糖为主转为含有酸性多糖。熟黄精多糖中半乳糖醛酸摩尔质量比增加至6.1%~41.4%[44, 48, 57-58]。其次,黄精多糖的单糖组成在蒸制中发生变化,生多花黄精多糖以果糖为主,占96.56%mol[39-40],但熟多花黄精多糖中果糖摩尔质量占比降低至24.6%,半乳糖醛酸及半乳糖的摩尔质量比也分别增加至20.5%及41.4%[44]。鸡头黄精多糖随着蒸制次数增加,甘露糖含量不断下降(52.47%→18.86%),葡萄糖含量先降低后增加(36.84%→32.17%→52.20%),半乳糖及阿拉伯糖含量均增加(7.32%→21.77%及3.37%→7.58%)[32]。也有研究说明鸡头黄精多糖随着蒸制次数增加,甘露糖的摩尔质量占比不断下降(51.2%→17.5%),葡萄糖摩尔质量占比先增加后降低(21.4%→65.5%→46.5%),半乳糖的摩尔质量占比先降低后增加(26.9%→8.97%→30.1%),阿拉伯糖的摩尔质量占比在蒸制过程中有增有减,但熟鸡头黄精多糖中阿拉伯糖摩尔质量比例显著高于生鸡头黄精多糖(0.46%(生黄精),5.89%(九蒸九制))[54]。
但是,黄精多糖蒸制过程中降解的同时还发生了聚集。蒸制可导致三种黄精多糖分子量显著增加[36, 44, 48, 59]。生多花黄精多糖的分子量为2.09~8.5 kDa[3, 39-40, 45, 60-61]。而蒸制6 h后的多花黄精多糖中除了低分子量的组分(3.4~4.0 kDa)之外,还含有高分子量组分(1200~4400 kDa)[44]。另外,“九蒸九制”的多花黄精的多糖有2个片段,分别为5.53 kDa及75.8 kDa,酒黄精多糖中有一个高分子片段76.4 kDa[61]。蒸制后的鸡头黄精分子量增加[44, 48, 54]。例如,酒蒸6 h后的鸡头黄精多糖的分子量也从4.01 kDa增加至14.2 kDa[48]。也有研究发现在“九蒸九制”过程中,鸡头黄精分子量从6.06 kDa先增至10.3 kDa(一蒸),随后降至4.63~5.10 kDa(二~四蒸),随后分子量显著增至44.3~75.3 kDa(五蒸~九蒸)[54]。与此同时,通过PCA分类可得出,鸡头黄精(生品)多糖、一至四蒸的鸡头黄精多糖、五至九蒸的鸡头黄精多糖分别为三个品类的多糖[54]。滇黄精(生品)多糖分子量为14.05 kDa[2],而滇黄精(饮片)多糖分子量为149.4 kDa[58]。有研究说明,滇黄精(生品)中多糖及八至九蒸的熟黄精中多糖分别为两类多糖,而中间一至七蒸的熟黄精多糖差异较大,较为分散[36]。黄精多糖在蒸制过程分子量增加可能是由于多糖分子含有大量的羟基,由于分子间相互作用力,尤其是酸性多糖容易形成不同程度的聚集体,导致多糖分子量增加。
综上,由于三种生黄精多糖结构上有较大区别,因而在研究中需要区别不同黄精品种。进一步,在加工过程中,三种熟黄精中多糖的结构与生黄精中多糖具有显著差异。但目前仍然缺乏对加工过程黄精多糖的结构动态变化规律的系统解析,例如糖苷键断裂的方式,多糖链组成及高级溶液构象等。
4. 黄精多糖活性及其在加工中的变化
多糖的结构与功能有密切关系,黄精多糖在加工过程中结构的变化会影响其活性,如表2所示。
4.1 抗氧化活性
研究发现,三类的生黄精多糖均具有抗氧化活性[45, 52, 57],但与通用的阳性对照维生素C相比,黄精多糖抗氧化活性较低[53, 62]。鸡头黄精蒸制后,其多糖体外抗氧化活性随着蒸制次数的增加呈现增加的趋势[32, 54],尤其在第四蒸后显著增加[54]。“九蒸九制”过程中的鸡头黄精多糖的抗氧化活性与其单糖组成相关,即鸡头黄精多糖的ABTS自由基清除及羟自由基清除率与甘露糖摩尔质量占比成反比,与阿拉伯糖摩尔质量占比成正比,ABTS自由基清除率与葡萄糖摩尔质量占比成正比[54]。不同干燥方法可改变黄精多糖的抗氧化活性,微波真空干燥法获得的黄精的抗氧化活性显著高于热风干燥[28]。经过不同处理(发酵、蒸、酒制)后的水提液体,发酵和蒸制后的样品的DPPH自由基清除率较生黄精样品均提高,发酵和酒制后的样品铁离子还原能力显著提高[38]。由于该研究采用水提液,而水提液中的其他物质也可能引起抗氧化活性的变化。另外,体外细胞研究表明黄精多糖能通过增强细胞内抗氧化活性,提高HT22细胞的存活率,改善线粒体功能,从而保护细胞因H2O2引起的氧化损伤,其作用机制与激活SIRT1/AMPK/PGC-1α信号通路有关[63]。
4.2 降血糖
三类生黄精多糖均具有降血糖功效。市售鸡头黄精多糖不仅可降低STZ诱导糖尿病大鼠的血糖,而且可限制视网膜病理性血管生成[64]。通过下调信号通路(Bax、EGF、p38、VEGF和TGF-β)及上调Bcl-2来抑制视网膜组织中细胞凋亡,进而减缓糖尿病引起的视网膜受损[64]。滇黄精多糖可以降低二型糖尿病大鼠的空腹血糖,调节肠道菌群,促进短链脂肪酸产生[65]。鸡头黄精多糖在体外模拟酵解过程中能够显著提高来源于二型糖尿病小鼠的肠道菌群的丰富度和多样性[66]。多花黄精多糖能够提高一型糖尿病大鼠生存率、降低空腹血糖,具有肝保护作用(降低炎症因子)[67]。同时,多花黄精多糖能促进GLP-1生成,而GLP-1与胰岛功能、饮食控制、血糖稳态、炎症及心血管保护作用有关[39-40]。王艳芳[68]研究发现,高压蒸制结合酒制后的滇黄精多糖也具有降低高脂小鼠血糖、空腹糖耐量、血清胰岛素及胰岛素抵抗的作用。总之,加工前后的黄精多糖均具有降血糖作用,其降血糖机制主要通过调节肠道菌群及改善胰岛素功能。但是,目前尚缺乏生、熟黄精多糖之间的降血糖效果及机制差异上比较的研究。
4.3 改善脂肪代谢异常
生鸡头黄精多糖可通过AMPK通路,调节脂肪代谢及炎症反应从而改善高脂诱导的小鼠肥胖[69]。滇黄精多糖也可改善脂肪代谢异常[68, 70-72]。生品及酒制滇黄精多糖均能通过改善线粒体功能而缓解高脂饮食诱导的非酒精性脂肪肝,其机制可能与增强线粒体的抗氧化应激、能量代谢、脂肪酸β-氧化以及抑制肝细胞凋亡有关。但酒制的滇黄精多糖在降低血清与肝脏脂质,提高线粒体能量代谢及抑制线粒体介导的肝细胞凋亡方面效果优于生滇黄精多糖,而生滇黄精多糖抗氧化应激效果优于加工后的滇黄精多糖的效果[71]。因而,生、熟黄精的多糖具有改善脂肪代谢异常的作用,二者在降血脂的机制上各有优势。
4.4 增强免疫力
生多花黄精多糖[3]、生、熟鸡头黄精多糖[47, 49, 73]在体内、体外实验中具有显著的提高免疫力的活性,但滇黄精的多糖研究较少。鸡头黄精(生品)的多糖可以通过多条通路(MR及TLR4[49]、NF-κB/MAPK[47])刺激RAW264.7细胞的免疫活性,也可通过CR3及TRL2通路刺激NK细胞活性[49]。另外,黄精(湖北)多糖可以提高环磷酰胺免疫抑制小鼠模型的脾脏指数、胸腺指数、促进溶血素形成、提高腹腔巨噬细胞吞噬功能[74]。黄精(延边)多糖可以促进小鼠脾淋巴细胞产生IL-2及IFN-γ;促进RAW264.7巨噬细胞增殖、产生IL-6(白细胞介素-6,Interleukin-6)及TNF-α(肿瘤坏死因子-α,Tumor necrosis factor-α)、NO(一氧化氮,Nitric oxide)、提高iNOS mRNA水平,从而提高免疫活性[75]。
加工可改变黄精多糖的免疫活性。研究表明,基于RAW264.7细胞模型及动物模型,酒制鸡头黄精多糖的免疫刺激活性优于生黄精多糖[48]。另外有研究表明,鸡头黄精(生品)多糖及鸡头黄精(酒制)多糖均具有免疫调节作用,分子量<50 kDa的组分是多糖的有效部位,且酒制后的黄精多糖抑制炎症反应作用加强[27]。因此,虽然研究已报道酒制后黄精多糖具有比生黄精多糖更高的免疫刺激能力[48],但是由于酒制黄精加入了黄酒,黄酒的作用值得单独探究。不同炮制方式(四制及九制)黄精水提物对气阴两虚大鼠模型具有不同优势,四制黄精水提物调节血脂代谢改善体重方面最佳,而九制黄精水提物在改善肝功能,提高免疫球蛋白效果较为突出[23]。然而该研究采用水提物中可能还有其他的活性组分,需进一步明晰其多糖的实际贡献。综上,黄精中核心的多糖的免疫刺激活性在加工中的变化值得进一步明晰。
4.5 抗疲劳
三类生黄精多糖在动物模型上均具有显著的抗疲劳效果,包括延长小鼠负重游泳时间、降低运动后的血清尿素氮、提高肝糖原[20, 76-80]。其中,多花黄精多糖还能显著降低负重游泳后的血乳酸含量[76]。但是,三种黄精(生品)多糖在抗疲劳效果无显著差异。多花黄精的中性多糖组分PCP-1的抗疲劳机制是通过osteocalcin信号通路[43]。通过超高压(293 MPa)提取的鸡头黄精(生品)多糖通过提高机体代谢与抗氧化能力,减少腓肠肌线粒体应激损伤实现抗疲劳功效[81]。
通过不同炮制方法(纹制、“九蒸九晒”、酒制)获得的黄精粗多糖能够提高小鼠负重游泳时间[82-84]。生黄精粗多糖比炮制后的样品能显著提高SOD及降低MDA[82]。但也有研究表明多花黄精炮制品中多糖优于生黄精中多糖,包括抗疲劳效果及组织中SOD活力的提高,MDA含量的降低,从而抗氧化程度增加[84]。但该研究采用水煎液体,未对多糖进行进一步纯化分离,水提液中存在较多的其他物质,例如,多酚等可能对结果产生干扰。有研究表明,古法炮制后的黄精具有良好的抗疲劳效果[20],但该研究未与生黄精进行比较,因此炮制方法对于黄精多糖的影响尚未明确。虽然研究表明生、熟黄精多糖在同等剂量下均具有抗疲劳效果[82-83]。但是,由于加工降低黄精多糖含量,多糖的降低对于其实际抗疲劳效果可能产生不利影响。
4.6 抑菌活性
采用分级提取的生多花黄精获得的不同多糖均具有抑菌活性[85-88]。加工对于黄精的抑菌活性有一定的改变[16]。例如,高压2 h获得熟黄精多糖对大肠杆菌、金黄色葡萄球菌的抑制效果较好,生黄精多糖则对枯草芽孢杆菌的抑制作用相对较强[16]。
4.7 其他活性
除上所述,黄精多糖还拥有多种生理活性,其中鸡头黄精多糖的研究较多。鸡头黄精多糖,通过增强免疫力(TLR4-MAPK/NF-κB信号通路)从而抗肺肿瘤[73]。多花黄精粗多糖能够体外抑制S180癌细胞及人乳腺癌细胞MCF-7,在体内具有抑制S180肉瘤的作用,其机制也是通过增强免疫力[89]。鸡头黄精多糖还可以提高D-半乳糖诱导衰老大鼠的学习和记忆能力,改善其肾脏组织的病变,通过Klotho-FGF23内分泌轴,改善抗氧化,平衡钙、磷代谢[90]。黄精多糖还具有提高阿尔兹海默病症模型的学习记忆能力[91-92]。鸡头黄精多糖能够通过抑制NGAL或者KIM-1 mRNA的表达,抑制p38MAPK/ATF2信号通路从而抑制TNF-α、IL-1β及IL-6进而修复庆大霉素诱导的急性肾损伤[93]。鸡头黄精多糖对椎间盘退变大鼠髓核细胞凋亡、炎症和氧化应激的保护作用[94]。鸡头黄精多糖还可以通过促进VEGF、骨钙素表达抑制Col1a1、ACP5、CTSK蛋白表达,促进大鼠胫骨骨折愈合[95]。
综上,目前生、熟黄精多糖在不同的生物活性上各有优势。生黄精的活性研究较为充分,需要加强对熟黄精多糖的活性及机制研究,这对于黄精功能食品的开发利用尤为重要。进一步,系统比较加工对黄精多糖的生物活性的影响对于黄精加工的精准控制具有重要作用。
5. 展望
综上,基于文献检索,本文对黄精常用的加工方式进行综述,分析目前加工存在的问题。进一步阐述加工方式对黄精中多糖含量、结构及活性的影响。已有研究已明确黄精在加工过程中发生多糖含量显著下降,并且黄精多糖结构降解同时发生聚集,但引起多糖含量及结构变化的关键控制点尚不明确。现有研究发现加工可一定程度改变黄精多糖的生物活性,因此不可忽视加工对于黄精多糖构效关系的影响,但目前尚未开展深入研究。因此,解析加工过程中黄精多糖含量及多糖结构和活性的变化可帮助控制过度加工引起的黄精多糖含量的下降等不良影响。这些拓展研究将为黄精食品生产中精准控制及开发利用提供重要理论基础和科学依据,对黄精产业规范化加工及其健康持续发展具有重要意义。
-
表 1 不同加工方式对黄精中多糖含量的影响
Table 1 Effects of different processing methods on polysaccharides content in Polygonatum spp.
黄精品种 蒸制工艺 晒制/烘干工艺 重复次数 多糖含量变化(基于干重) 参考文献 多花黄精 清蒸
一蒸为1 h,随后在上一次基础上增加0.5 h60 ℃,2 h 9次 19.87%~23.71%(一次)→20.96%~23.10%(三次)→
11.90%~15.52%(七次)→5.49%~11.60%(九次)[30] 热河黄精
(多花玉竹)清蒸5 h后闷一夜 阳光下晒半干 9次 17.02%(生品)→10.04(一次)→5.62%(二次)
→5.80%(九次)[31] 多花黄精 清蒸6 h 60 ℃ 9次 15.0%(生品)→5.8%(二次)→4.5%(九次) [22] 鸡头黄精 清蒸6 h 45 ℃至衡重 9次 12%(生品)→ 8.89%(一次)→1%(三~九次) [5] 鸡头黄精 清蒸6 h 60 ℃ 9次 14.36%(生品)→≈4%(三次)→随后稳定 [32] 滇黄精 清蒸6 h 55 ℃,12 h 9次 13.034%(生品)→9.491%(二次)→8.203%(六次)→
6.988%(九次)[13] 多花黄精 − − 9次 9.602%(二次)→3.185%(四次)→2.043%(九次) [33] 多花黄精 − − 9次 ≈6~7%(生品)→≈3%(三次)→随后稳定 [34] 鸡头黄精 − − 9次 7.973%(二次)→3.584%(四次)→2.19%(九次) [33] 多花黄精 0.12 MPa高压蒸0.5 h − 9次 ≈20%、18%、6%(生品)→≈10%、8%、2%(九次) [18] 多花黄精 0.15 MPa高压蒸1.5 h 70 ℃,24 h 10次 17.56%(生品)→13.39%(一次)→10.95%(三次)→
8.03%(十次)[15, 17] 多花黄精 黄精加水(1:1),
蒸4 h60 ℃,12 h 9次 11.60%(生品)→6.98(一次)→2.99%(二次)
→3.99%(九次)[19] 滇黄精 黄精加水(1:2),
蒸12~96 h105 ℃ 1次 7.34%(生品)→9.73%(12 h)→4.26%(24 h)
→4.80%(96 h)[26] 多花黄精 酒制
与黄酒拌匀,蒸6 h60 ℃ 9次 15%(生品)→4.7%(二次)→3.8%(九次) [22] 多花黄精 酒制
与黄酒拌匀,蒸制6 h− 1次 平均为8.50% [35] 滇黄精 酒制
与黄酒浸润12 h,蒸制6 h45 ℃,12 h 9次 12.66%(生品)→7.76%(三次)→4.42%(九次) [36] 多花黄精 酒制
与黄酒拌匀闷润,蒸8 h太阳晒至皮微干 9次 野生:22.18%(一次)→32.81%(五次)→12.67%(九次);
栽培:18.68%(一次)→31.51%(四次)→10.84%(九次)[21, 37] 多花黄精 酒制
与黄酒浸泡后蒸制− 9次 7.31%(生品)→1.51%(四次)→1.52%(九次);
市售1.99%[23] 鸡头黄精 发酵:与小麦麦麸混匀接种灵芝液体种子,发酵12天;
蒸制:黄精加水浸润后取出蒸6 h,停火焖6 h;
酒制:黄精与黄酒闷润,蒸10 h。60 ℃,12 h 1次 生品:≈14%;
发酵:≈12.5%;
蒸制:≈8%;
酒制:≈6.5%[38] 注:−表示未提及。 表 2 黄精多糖结构、活性及其在加工中的变化
Table 2 Structure and bioactivity of polysaccharides from Polygonatum spp. and their changes during processing
黄精品种
(或产地)加工工艺 多糖提取、
分离纯化方法多糖结构信息 活性 参考文献 多花黄精 生品 80 ℃热水提取
→离子柱,取中性组分
→凝胶柱中性多糖,分子量:8.5 kDa,单糖组成:果糖、葡萄糖(28:1),β-呋喃果糖构型 降血糖(大鼠及NCI-H716细胞模型):通过T1R2/T1R3介导的cAMP促进肠内分泌细胞释放GLP-1。
抗疲劳(小鼠模型):通过osteocalcin 信号通路[39-40]
[43]多花黄精 生品 常温、辅以超声提取
→离子柱,取中性组分
→凝胶柱PCP-1,中性多糖(占55.3%),分子量:2.966 kDa,单糖组成:甘露糖、葡萄糖、半乳糖、木糖、阿拉伯糖(1:17.53:7.02:0.27:0.59),以β糖苷键为主,含有6糖残基 抗氧化(DPPH清除率、O2-清除率,Fe2+螯合能力)
免疫刺激活性(RAW264.7细胞模型):促进细胞增殖、增强吞噬活性、促进NO释放[45] 多花黄精 生品 80 ℃热水提取
→离子柱,取中性组分→凝胶柱中性多糖,分子量:约4.8 kDa,单糖组成:果糖、葡萄糖(28:1),含有(2→1)β果聚糖为主链及(2→6)果聚糖为侧链(中间含有以新蔗果三糖形式存在的α-D葡萄糖)果聚糖 免疫刺激活性(RAW264.7细胞模型):促进细胞增殖、增强吞噬活性、促进TNF-α释放 [3] 多花黄精 生品 50%乙醇提取
→Bio-Gel P4柱中性多糖,聚合度28,分支果聚糖:每3个(2→1)β-D果糖键主链上,连接(2→6)果聚糖β-D果糖键分支;葡萄糖位于新蔗果三糖内 抗疱疹病毒活性 [41-42] 多花黄精
(九华)生品 沸水提取 分子量:5.34 kDa,单糖组成a:半乳糖醛酸(19.48%)、鼠李糖(17.92%)、甘露糖(18.56%)、葡萄糖(8.45%)、半乳糖(35.59%) − [61] 多花黄精 生品 分级提取:
PCP1(80 ℃热水))
→PCP2(0.1% NaOH)
→PCP3(0.5% NaOH)
→PCP4(1% NaOH)
→PCP5(2% NaOH)PCP1,得率40.3%,分子量:2.09 kDa,单糖组成a:以甘露糖(33.5%)、半乳糖(24.0%)、葡萄糖(20.7%)、半乳糖醛酸(19.3%)为主,以β构型为主,含有6种糖残基
PCP2,得率14.2%,分子量:38.6 kDa,单糖组成a:以半乳糖(59.8%)、阿拉伯糖(18.5%)为主
PCP3,得率20.1%,分子量:42.6 kDa,单糖组成a:以半乳糖(58.7%)、阿拉伯糖(22.2%)为主,以α构型为主,含有4种糖残基
PCP4,得率13.5%,分子量:34.3 kDa,单糖组成a:以半乳糖(61.3%)、阿拉伯糖(21.0%)为主
PCP5,得率6.4%,分子量:24.1 kDa,单糖未检测,以α构型为主,含有2种糖残基− [60] 多花黄精 生品 分级提取:
HBSS(醋酸缓冲液、pH5.2、70 ℃)→
CHSS(EDTA、草酸铵、醋酸缓冲液、pH5.2、
70 ℃)→
DASS(0.05 mol/L NaOH、NaB4、4 ℃)→
CASS(6 mol/L NaOH、NaB4、4 ℃)HBSS,总糖含量78.39%,单糖组成a:甘露糖(54.55%)、半乳糖(15.27%)、葡萄糖(14.91%)、阿拉伯糖(12.16%)为主
CHSS,总糖含量71.86%,糖醛酸17.73%,单糖组成a:阿拉伯糖(36.97%)、半乳糖(24.85%)、甘露糖(22.25)为主
DASS,总糖含量69.99%,糖醛酸28.34%,单糖组成a:半乳糖(54.91%)、阿拉伯糖(26.61%)为主
CASS,总糖含量73.73%,单糖组成a:葡萄糖(26.1%)、木糖(24.05%)、半乳糖(20.06%)抗氧化(DPPH、羟基自由基、ABTS自由基清除率、Fe2+螯合能力、还原力等):DASS最佳
抗菌活性:DASS最强
抗凝血:CASS最强
抗炎(小鼠脾淋巴细胞):抑制TNF-α产生:CASS最佳
免疫刺激活性(小鼠脾淋巴细胞):产生IFN-γ,HBSS最佳
抗肿瘤(Hela细胞):CASS最佳[85-88] 多花黄精 蒸制
6 h沸水提取 分子量:1200~4400 kDa(峰1),3.4~4.2 kDa(峰2),单糖组成:果糖(24.6%~70.3%)、鼠李糖(1.1%~4.8%)、半乳糖醛酸(11.2%~41.4%)、葡萄糖(1.0%~1.9%)、半乳糖(10.5%~20.5%)、阿拉伯糖及木糖(1.2%~7.7%) − [44] 多花黄精
(九华)酒制
(黄酒浸润
→蒸5 h后
干燥)沸水提取 分子量:76.4 kDa,单糖组成a:半乳糖醛酸(8.38%)、鼠李糖(23.23%)、阿拉伯糖(8.47%)、甘露糖(20.07%)、葡萄糖(6.29%)、半乳糖(35.56%) − [61] 多花黄精
(九华)九蒸九制
(蒸制7 h→
65 ℃烘干
6 h,重复
9次)沸水提取 分子量: 5.53 kDa(峰1)及75.8 kDa(峰2),单糖组成a:半乳糖醛酸(17.29%)、鼠李糖(21.50%)、甘露糖(19.91%)、葡萄糖(5.19%)、半乳糖(36.12%) − [61] 鸡头黄精 生品 80 ℃热水提取 PSPC,总糖含量83.28%,蛋白含量3.41%,糖醛酸含量9.41%,多酚含量0.27%,分子量:4.01 kDa,单糖组成:半乳糖(29.63%),甘露糖(36.10%),葡萄糖(15.09%),半乳糖醛酸(10.20%) 免疫刺激活性(RAW264.7细胞模型):促进细胞增殖、增强吞噬活性及酸性磷酸酶活性、促进NO释放;(脾虚小鼠模型):加强免疫功能,提高IL-2、IL-6、TNF-α、IFN-γ等 [48] 鸡头黄精 生品 80 ℃热水提取
→离子柱,得4个组分P-1,得率71.40%,中性多糖,总糖含量72.52%,分子量:1.18~500 kDa
P-2,得率1.95%,总糖含量73.35%;糖醛酸含量5.00%,分子量:5.01~86.2 kDa
P-3,得率1.14%,总含量75.86%;糖醛酸含量9.21%,分子量:4.00~105 kDa
P-4,得率1.64%,总糖含量72.36%;糖醛酸含量29.32%,分子量:2.30~105 kDa
单糖组成a:均含有核糖、鼠李糖、半乳糖、葡萄糖、木糖、阿拉伯糖,但比例不同抗氧化(DPPH、ABTS清除率等):P-4>P-3>P-2>PSP>P-1 [52] 鸡头黄精 生品 90 ℃热水提取 粘均分子量:300 kDa,具有三股螺旋结构、网孔片状和网链形貌,较强热稳定性 抗氧化(ABTS、羟自由基、DPPH清除率)
体外降血糖(抑制α-葡萄糖苷酶):IC50=2.63 mg/mL
体内降血糖(糖尿病小鼠模型):促进胰岛素分泌、促进肝糖原合成、缓解自由基对组织损伤[96] 鸡头黄精
(河北)生品 沸水提取(PSP)→
离子柱子,得4个组分PSP,单糖组成a:半乳糖(63.50%),鼠李糖(5.14%),甘露糖(8.04%),葡萄糖(1.75%),木糖(1.57%)
PSP1,分子量:4.415 kDa,单糖组成:以半乳糖(82.91%)、甘露糖(14.96%)为主
PSP2,分子量:2.235 kDa,单糖组成:以半乳糖(74.27%)、鼠李糖(20.54%)为主
PSP3,分子量:7.743 kDa,单糖组成:以鼠李糖为主(57.69%)、半乳糖(37.17%)为主
PSP4,分子量:6.467 kDa,单糖组成:以鼠李糖(72.63%)、半乳糖(20.74%)为主抗氧化(DPPH、ABTS);
免疫刺激活性(RAW 264.7细胞):促进细胞增殖、增强吞噬活性,释放NO及细胞因子(TNF-α、IL-6),促进TNF-α和IL-6 mRNA表达,促进IkB-α降解,促进p38、MAPK、NF-κB、iNOS及COX-2的表达,同时促进NF-κB核转移过程;
免疫刺激活性(环磷酰胺免疫抑制小鼠模型):激活体内特异性免疫应答反应,对细胞免疫和体液免疫具有积极调节作用[47, 51] 鸡头黄精 生品 沸水结合0.1 mol/L NaOH提取
→离心柱,取中性组分
→凝胶柱PSPJWA,分子量:141 kDa,单糖组成:半乳糖、阿拉伯糖、鼠李糖(14:4:1),含有4种糖苷键,为新型阿拉伯半乳聚糖结构 抗氧化(DPPH、ATBS、羟自由基清除率、Fe2 +还原性) [62] 鸡头黄精 生品 40 ℃温水辅以超声提取→离子柱,得2个组分→凝胶柱,取第1组分 PSP-1A,分子量:数均分子量(Mn)为12.83 kDa,重均分子量(Mw)为21.58 kDa,分布宽度指数1.68 抗氧化(DPPH、ABTS、羟基自由基、还原力) [53] 鸡头黄精
(湖北)− 常温、超高压
(255 MPa)提取平均粒径6.151,粘均分子量326.713 kDa(中等聚多糖),单糖组成:鼠李糖、木糖、果糖、葡萄糖、核糖以及半乳糖(1:1.24:2.45:5.14:4.49:0.81),含有α、β两种糖苷键 抗氧化(DPPH、羟基自由基、SAFR自由基清除率) [97-98] 鸡头黄精 酒制
(黄酒浸润
→蒸制4 h)80 ℃热水提取 PSPW,总糖含量41.85%,蛋白含量29.58%,糖醛酸含量20.12%,多酚含量3.03%,分子量14.2 kDa,单糖组成:半乳糖(78.77%)、甘露糖(5.5%)、半乳糖醛酸(13.84%) 免疫刺激活性(RAW264.7细胞模型及脾虚小鼠模型):酒制黄精多糖优于生黄精多糖 [48] 鸡头黄精 蒸制6 h 沸水提取 分子量:740~2500 kDa(峰1),2.8~5.4 kDa(峰2),单糖组成:果糖(29.8%~84.7%)、鼠李糖(1.6%~4.6%)、半乳糖醛酸(6.1%~28.6%)、葡萄糖(0.8%~1.9%)、半乳糖(4.7%~30.7%)、阿拉伯糖及木糖(1.1%~9.7%) − [44] 鸡头黄精 蒸制12 h 沸水提取
→离子柱,取中性组分
→凝胶柱PSW1a,中性多糖,高度分支半乳甘露聚糖(1,4,β甘露糖为主链,每9个的O-6替换成β半乳糖)
PSW1b-2,中性多糖,分支半乳聚糖(1,4 β吡喃半乳糖为主链,每7个O-6替换成β半乳糖)− [50] 鸡头黄精 − 沸水提取
→离子柱,得2个组分F1,分子量:132.6 kDa,单糖组成a:甘露糖(75.4%),葡萄糖(15.2%),半乳糖(4.75%),阿拉伯糖(4.15%),以1→4甘露吡喃糖苷为主链,葡萄吡喃糖苷接在O-6分支 免疫刺激活性(RAW 264.7细胞模型):调控MR及TLR4信号通路;
免疫刺激活性(NK细胞模型):调控CR3及TRL2信号通路[49] 滇黄精 生品 80 ℃热水提取 总糖67.2%,蛋白含量17.1%,糖醛酸21.4%,单糖组成:甘露糖、核糖、鼠李糖、半乳糖醛酸、盐酸氨基葡萄糖、葡萄糖醛酸、半乳糖、葡萄糖、木糖、阿拉伯糖、岩藻糖(69.6:1.53:4.14:16.14:4.15:9.19:6.5:6.24:5.63:56.38:10.44) 抗氧化(DPPH、羟自由基清除率)
体外降血糖(HepG2细胞模型):促进葡萄糖消耗[36] 滇黄精 生品 80 ℃热水提取
→离子柱,得中性组分→凝胶柱PKPS-1,分子量:14.05 kDa,单糖组成:葡萄糖、甘露糖、半乳糖醛酸、半乳糖、葡萄糖醛酸、阿拉伯糖(7.22:1.0:0.16:0.11:0.05:0.02),无三股螺旋结构 降血糖(HepG2细胞、糖尿病小鼠):通过PI3K/AKT调控血糖 [2] 滇黄精 生品 90 ℃提取 粘均分子量:160 kDa,具有三股螺旋结构,网孔片状结构及多分支链聚集 抗氧化(ABTS、羟自由基、DPPH清除率)
体外降血糖(抑制α-葡萄糖苷酶):IC50=4.57 mg/mL[96] 滇黄精 生品 沸水提取
→50%、70%乙醇沉
→离子柱
→凝胶柱,得3个组分PKS-1,分子量:850.018 kDa;
PKS-2,分子量:266.085 kDa;
PKS-3,分子量:474.799 kDa。
均为酸性糖,含有β-吡喃糖苷键,均含有甘露糖、半乳糖醛酸、葡萄糖醛酸、半乳糖、阿拉伯糖和岩藻糖抗氧化(总还原力、DPPH、羟自由基清除率):PKS> PKS-2>PKS-1 [36, 57] 滇黄精 饮片,
可能为酒制沸水提取
→50%乙醇沉淀(F1)→75%乙醇沉淀(F2)→90%乙醇沉淀(F3)F1,总糖78.0%,糖醛量28.3%,分子量:149.4 kDa
F2,总糖66.9%,糖醛酸16.2%,分子量:40.3 kDa
F3,总糖64.5%,糖醛酸4.93%,分子量:1.47~3.76 kDa− [58] 滇黄精 蒸制6 h 沸水提取 分子量:890~1400 kDa(峰1),4.0~5.3 kDa(峰2),单糖组成:果糖(31.7%~72.8%)、鼠李糖(2.7%~4.8%)、半乳糖醛酸(10.1%~36.0%)、葡萄糖(0.9%~2.7%)、半乳糖(8.1%~20.7%)、阿拉伯糖及木糖(3.5%~6.0%) − [44] 注:−表示未测定或者方法中未提及;a表示单糖组成基于质量比,而未标明a则表明单糖组成基于摩尔质量比。 -
[1] 王丹, 张鸿, 刘嘉丽, 等. 不同产地多花黄精生物活性成分含量比较[J]. 湖南农业科学,2020,7:89−92, 96. [WANG Dan, ZHANG Hong, LIU Jiali, et al. Comparative analysis of bioactive components of Polygonatum cyrtonema Hua from different habitats[J]. Hunan Agricultural Sciences,2020,7:89−92, 96. doi: 10.16498/j.cnki.hnnykx.2020.007.023 WANG Dan, ZHANG Hong, LIU Jiali, et al. Comparative analysis of bioactive components of Polygonatum cyrtonema Hua from different habitats[J]. Hunan Agricultural Sciences, 2020, 7: 89-92, 96 doi: 10.16498/j.cnki.hnnykx.2020.007.023
[2] LI Ruoshi, TAO Aien, YANG Runmei, et al. Structural characterization, hypoglycemic effects and antidiabetic mechanism of a novel polysaccharides from Polygonatum kingianum Coll. et Hemsl[J]. Biomedicine Pharmacotherapy,2020,131:110687. doi: 10.1016/j.biopha.2020.110687
[3] ZHAO Ping, ZHOU Huifang, ZHAO Chengcheng, et al. Purification, characterization and immunomodulatory activity of fructans from Polygonatum odoratum and P. cyrtonema[J]. Carbohydrate Polymers,2019,214:44−52. doi: 10.1016/j.carbpol.2019.03.014
[4] WANG Yongjie, LIU Na, XUE Xia, et al. Purification, structural characterization and in vivo immunoregulatory activity of a novel polysaccharide from Polygonatum sibiricum[J]. International Journal of Biological Macromolecules,2020,160:688−694. doi: 10.1016/j.ijbiomac.2020.05.245
[5] 杨圣贤, 杨正明, 陈奕军, 等. 黄精"九蒸九制"炮制过程中多糖及皂苷的含量变化[J]. 湖南师范大学学报,2015,5:141−144. [YANG Shengxian, YANG Zhengming, CHEN Yijun, et al. Study on the polysaccharides and saoonins constituents of Polygonatum sibiricum Red. in "Nine-steam-nine-bask" processing[J]. Journal of Medical Science of Huan Normal University,2015,5:141−144. YANG Shengxian, YANG Zhengming, CHEN Yijun, et al. Study on the polysaccharides and saoonins constituents of Polygonatum sibiricum Red. in "Nine-steam-nine-bask" processing[J]. Journal of Medical Science of Huan Normal University, 2015, 5: 141-144.
[6] 何兰香, 丁科, 谢明华, 等. 酶法-超声提取黄精总黄酮及其抗氧化活性研究[J]. 中国现代应用药学,2019,36(9):1075−1080. [HE Lanxiang, DING Ke, XIE Minghua, et al. Study on enzymatic-ultrasonic assisted extraction of total flavonoids from Polygonatum Sibirici and its antioxidant activities[J]. Chinese Journal of Modern Drug Application,2019,36(9):1075−1080. doi: 10.13748/j.cnki.issn1007-7693.2019.09.009 HE Lanxiang, DING Ke, XIE Minghua, et al. Study on enzymatic-ultrasonic assisted extraction of total flavonoids from Polygonatum Sibirici and its antioxidant activities[J]. Chinese Journal of Modern Drug Application, 2019, 36(9): 1075-1080. doi: 10.13748/j.cnki.issn1007-7693.2019.09.009
[7] 巫永华, 刘恩岐, 张建萍, 等. 黄精多酚的闪式提取及抗氧化活性研究[J]. 食品科技,2017,42(8):231−236. [WU Yonghua, LIU Enqi, ZHANG Jianping, et al. Extraction and antioxidant activities of polyphenols from Polygonatum[J]. Food Science and Technology,2017,42(8):231−236. doi: 10.13684/j.cnki.spkj.2017.08.042 WU Yonghua , LIU Enqi, ZHANG Jianping, et al. Extraction and antioxidant activities of polyphenols from Polygonatum[J]. Food Science and Technology, 2017, 42 (8): 231-236. doi: 10.13684/j.cnki.spkj.2017.08.042
[8] 陈克克, 强毅. 响应面法优化超声波辅助黄精多酚的提取及其抗菌活性[J]. 陕西师范大学学报,2018,46(1):91−96. [CHEN Keke, QIANG Yi. Optimization of ultrasonic assisted extraction of polyphenols from Polygonatum sibiricum Red. using resopnse surface methodology and its antibacterial activity[J]. Journal of Shanxi Normal University(Natural Science Edition),2018,46(1):91−96. CHEN Keke, Qiang Yi. Optimization of ultrasonic assisted extraction of polyphenols from Polygonatum sibiricum Red. using resopnse surface methodology and its antibacterial activity[J]. Journal of Shanxi Normal University(Natural Science Edition), 2018, 46(1): 91-96.
[9] 张国强, 郭晓东, 薛文华, 等. 西藏野生卷叶黄精多酚的提取及其抗氧化活性分析[J]. 食品科学,2017,38(6):236−241. [ZHANG Guoqiang, GUO Xiaodong, XUE Wenhua, et al. Extraction and antioxidant activities of polyphenols from Tibetan wild Polygonatum cirrhifolium[J]. Food Science,2017,38(6):236−241. doi: 10.7506/spkx1002-6630-201706037 ZHANG Guoqiang, GUO Xiaodong, XUE Wenhua, et al. Extraction and antioxidant activities of polyphenols from Tibetan wild Polygonatum cirrhifolium[J]. Food Science, 2017, 38(6): 236-241. doi: 10.7506/spkx1002-6630-201706037
[10] 巫永华, 张建萍, 赵节昌, 等. 大孔树脂纯化黄精多酚及其抗氧化性与组成分析[J]. 农业工程学报,2020,36(1):318−326. [WU Yonghua, ZHANG Jianping, ZHAO Jiechang, et al. Antioxidant and purification of Polygonatum sibiricum polyphenols using macroporous resin[J]. Transactions of the Chinese Society of Agricultural Engineering,2020,36(1):318−326. doi: 10.11975/j.issn.1002-6819.2020.01.038 WU Yonghua, ZHANG Jianping, ZHAO Jiechang, et al. Antioxidant and purification of Polygonatum sibiricum polyphenols using macroporous resin[J]. Transactions of the Chinese Society of Agricultural Engineering. 2020, 36(1): 318-326. doi: 10.11975/j.issn.1002-6819.2020.01.038
[11] 秦宇雯, 张丽萍, 赵祺, 等. 九蒸九晒黄精炮制工艺的研究进展[J]. 中草药,2020,51(21):5631−5637. [QIN Yuwen, ZHANG Liping, ZHAO Qi, et al. Research progress on Polygonatum cyrtonema processed by nine times steaming and nine times shining[J]. Chinese Traditional and Herbal Drugs,2020,51(21):5631−5637. QIN Yuwen, ZHANG Liping ZHAO Qi, et al. Research progress on Polygonatum cyrtonema processed by nine times steaming and nine times shining[J]. Chinese Traditional and Herbal Drugs, 51(21): 5631-5637.
[12] 国家药典委员会. 中华人民共和国药典[M]. 北京: 中国医药科技出版社, 2020. Chinese Pharmacopoeia Commission. Pharmacopoeia of the People's Republic of China[M]. Beijing: China Medical and Technology Press, 2020.
[13] 林雨, 佘亮, 魏馨瑶, 等. 黄精炮制前后的化学成分变化及其减毒增效研究[J]. 中药材,2021,6:1353−1359. [LIN Yu, SHE Liang, WEI Xinyao, et al. Study on chemical constituents, detoxification and synergism of Polygonati Rhizoma before and after processing[J]. Journal of Chinese Medicinal Materials,2021,6:1353−1359. doi: 10.13863/j.issn1001-4454.2021.06.012 LIN Yu, SHE Liang, WEI Xinyao, et al. Study on chemical constituents, detoxification and synergism of Polygonati Rhizoma before and after processing[J]. Journal of Chinese Medicinal Materials, 2021, 6: 1353-1359. doi: 10.13863/j.issn1001-4454.2021.06.012
[14] 任洪民, 邓亚羚, 张金莲, 等. 药用黄精炮制的历史沿革, 化学成分及药理作用研究进展[J]. 中国中药杂志,2020,45(17):153−172. [REN Hongmin, DENG Yaling, ZHANG Jinlian, et al. Research progress on processing history evolution, chemical components and pharmacological effects of Polygonati Rhizoma[J]. China Journal of Chinese Materia Medica,2020,45(17):153−172. REN Hongmin, DENG Yaling, ZHANG Jinlian, et al. Research progress on processing history evolution, chemical components and pharmacological effects of Polygonati Rhizoma[J]. China Journal of Chinese Materia Medica, 2020, 45(17): 153-172.
[15] 李瑞, 廖念, 周逸群, 等. 基于功效成分优选多蒸黄精炮制工艺[J]. 时珍国医国药,2019,30(2):331−333. [LI Rui, LIAO Nian, ZHOU Yiqun, et al. Optimization of processing technology of multi-steamed Polygonatum based on efficacy components[J]. Lishizhen Medicine and Materia Medica Research,2019,30(2):331−333. LI Rui, LIAO Nian, ZHOU Yiqun, et al. Optimization of processing technology of multi-steamed Polygonatum based on efficacy components[J]. Lishizhen Medicine and Materia Medica Research, 2019, 30(2): 331-333.
[16] 曹冠华, 李泽东, 赵荣华, 等. 生黄精多糖与制黄精多糖抑菌效果比较研究[J]. 食品科技,2017(9):202−206. [CAO Guanhua, LI Zedong, ZHAO Ronghua, et al. Compare of antibacterial effect produced by polysaccharides between raw materials and processing Polygonatum sibiricum[J]. Food Science and Technology,2017(9):202−206. doi: 10.13684/j.cnki.spkj.2017.09.038 CAO Guanhua, LI Zedong, ZHAO Ronghua, et al. Compare of antibacterial effect produced by polysaccharides between raw materials and processing Polygonatum sibiricum[J]. Food Science and Technology, 2017(9): 202-206. doi: 10.13684/j.cnki.spkj.2017.09.038
[17] 廖念. 多花黄精产地加工炮制及其质量标准的研究[D]. 长沙: 湖南中医药大学, 2018. LIAO Nian. Study on processing and quality standard of Polygonum cyrtonema from producing area[J]. Changsha: Hunan University of Chinese Medicine, 2018.
[18] FAN Baolei, WEI Guoliang, GAN Xiaofeng, et al. Study on the varied content of Polygonatum cyrtonema polysaccharides in the processing of steaming and shining for nine times based on HPLC-MS/MS and chemometrics[J]. Microchemical Journal,2020,159:105352. doi: 10.1016/j.microc.2020.105352
[19] 马佳丽, 蒋殷盈, 蒋福升, 等. 九蒸九制多花黄精炮制过程变化研究[J]. 浙江中医药大学学报,2020,44(5):480−485. [MA Jiali, JIANG Yinying, JIANG Fusheng, et al. Study on the changes of “Nine-steam-nine-bask” of Polygonatum cyrtonema[J]. Journal of Zhejiang Chinese Medical University,2020,44(5):480−485. doi: 10.16466/j.issn1005-5509.2020.05.018 MA Jiali, JIANG Yinying, JIANG Fusheng, et al. Study on the changes of “Nine-steam-nine-bask” of Polygonatum cyrtonema[J]. Journal of Zhejiang Chinese Medical University, 2020, 44(5): 480-485. doi: 10.16466/j.issn1005-5509.2020.05.018
[20] 陈靓雯, 柯晓燕. 古法炮制多花黄精提取物抗疲劳作用研究及其机制探讨[J]. 科学技术创新,2019(4):3−4. [CHEN Liangwen, KE Xiaoyan. Study on anti-fatigue effect and mechanism of extract of Polygonum cyrtonema processed by ancient method[J]. Scientific and Technological Innovation,2019(4):3−4. doi: 10.3969/j.issn.1673-1328.2019.04.003 CHEN Liangwen, KE Xiaoyan. Study on anti-fatigue effect and mechanism of extract of Polygonum cyrtonema processed by ancient method[J]. Ke Xue Ji Shu Chuang Xin, 2019, (4): 3-4. doi: 10.3969/j.issn.1673-1328.2019.04.003
[21] 胡叶青, 胡云飞, 吴其国. 九华黄精九蒸九晒炮制过程中总多糖的含量变化研究[J]. 佛山科学技术学院学报(自然科学版),2019,37(6):58−62. [HU Yeqing, HU Yunfei, WU Qiguo. Study on the total polysaccharides content of Jiuhua Rhizoma Polygnoati in nine-steam-nine-bask processing[J]. Journal of Foshan University(Natural Science Edition),2019,37(6):58−62. doi: 10.13797/j.cnki.jfosu.1008-0171.2019.0085 HU Yeqing, HU Yunfei, WU Qiguo. Study on the total polysaccharides content of Jiuhua Rhizoma Polygnoati in nine-steam-nine-bask processing[J]. Journal of Foshan University(Natural Science Edition), 2019, 37(6): 58-62. doi: 10.13797/j.cnki.jfosu.1008-0171.2019.0085
[22] 张洪坤, 吴桂芳, 黄玉瑶, 等. 黄精不同九制炮制的过程研究[J]. 时珍国医国药,2019,30(3):602−605. [ZHANG Hongkun, WU Guifang, HUANG Yuyao, et al. Study on the process of Polygonatum sibiricum Red. in different nine fold processing[J]. Lishizhen Medicine and Materia Medica Research,2019,30(3):602−605. ZHANG Hongkun, WU Guifang, HUANG Yuyao, et al. Study on the process of Polygonatum sibiricum Red. in different nine fold processing[J]. Lishizhen Medicine and Materia Medica Research, 2019, 30(3): 602-605.
[23] 马慕秾. 炮制方法对黄精多糖成分和“补气养阴”功效相关药理作用的影响[D]. 杭州: 浙江中医药大学, 2019. MA Munong. Effects of processing methods on the components of Polygonatum polysaccharide and the related pharmacological effects of "Invigorating Qi and Nourishing Yin"[D]. Hangzhou: Zhejiang Chinese Medical University, 2019.
[24] 宋艺君, 郭涛, 周晓程. 不同产地黄精经不同方法炮制后多糖, 5-羟甲基糠醛的含量变化[J]. 中国药房,2017,28(16):2256−2258. [SONG Yijun, GUO Tao, ZHOU Xiaocheng. Contents changes of polysaccharide and 5-HMF in Polygonati Rhizoma from different producing areas after different processing[J]. China Pharmacy,2017,28(16):2256−2258. doi: 10.6039/j.issn.1001-0408.2017.16.26 SONG Yijun, GUO Tao, ZHOU Xiaocheng. Contents changes of polysaccharide and 5-HMF in Polygonati Rhizoma from different producing areas after different processing[J]. China Pharmacy, 2017, 28(16): 2256-2258. doi: 10.6039/j.issn.1001-0408.2017.16.26
[25] 周改莲, 李健, 徐梦云, 等. 广西产多花黄精不同炮制工艺研究[J]. 中国民族民间医药,2018,27(327):38−41. [ZHOU Gailian, LI Jian, XU Mengyun, et al. Study on different processing techniques of Guangxi Polygonatum cyrtonema[J]. Chinese Journal of Ethnomedicine and Ethnopharmacy,2018,27(327):38−41. ZHOU Gailian, LI Jian, XU Mengyun, et al. Study on different processing techniques of Guangxi Polygonatum cyrtonema[J]. Chinese Journal of Ethnomedicine and Ethnopharmacy, 2018, 27(327): 38-41.
[26] 刘明研, 冯亚娟, 黄秋正, 等. 蒸制时间对滇黄精色泽、可溶性成分及糖含量的影响[J]. 食品工业科技,2019,40(5):43−47, 53. [LIU Mingyan, FENG Yajuan, HUANG Qiuzheng, et al. Influence of steaming time on color, the content of soluble substance and sugar in Polygonatum kingianum[J]. Science and Technology of Food Industry,2019,40(5):43−47, 53. doi: 10.13386/j.issn1002-0306.2019.05.007 LIU Mingyan, FENG Yajuan, HUANG Qiuzheng, et al. Influence of steaming time on color, the content of soluble substance and sugar in Polygonatum kingianum[J]. Science and Technology of Food Industry, 2019, 40(5): 43-47, 53. doi: 10.13386/j.issn1002-0306.2019.05.007
[27] 金鹏程, 吴丽华, 吴昕怡, 等. Box-Behnken响应面法优化滇黄精产地加工炮制一体化工艺对黄精多糖的影响[J]. 中国现代中药,2021,23(4):674−679. [JIN Pengcheng, WU Lihua, WU Xinyi, et al. Effect of integrative technology of primary processing of Polygonatum kingianum optimization by Box-Benhken response surface method on polysaccharide[J]. Modern Chinese Medicine,2021,23(4):674−679. doi: 10.13313/j.issn.1673-4890.20200410001 JIN Pengcheng, WU Lihua, WU Xinyi, et al. Effect of integrative technology of primary processing of Polygonatum kingianum optimization by Box-Benhken response surface method on polysaccharide[J]. Modern Chinese Medicine, 2021, 23(4): 674-679. doi: 10.13313/j.issn.1673-4890.20200410001
[28] 衡银雪, 郑旭煦, 殷钟意, 等. 不同干燥方法对黄精干燥特性和品质的影响[J]. 食品工业科技,2018,39(7):158−161. [HENG Yinxue, ZHENG Xuxu, YIN Zhongyi, et al. The effects of different drying methods on the drying characteristics and quality of Polygonatum odoratum[J]. Science and Technology of Food Industry,2018,39(7):158−161. doi: 10.13386/j.issn1002-0306.2018.07.029 HENG Yinxue, ZHENG Xuxu, YIN Zhongyi, et al. The effects of different drying methods on the drying characteristics and quality of Polygonatum odoratum[J]. Science and Technology of Food Industry, 2018, 39(7): 158-161. doi: 10.13386/j.issn1002-0306.2018.07.029
[29] 朱新焰, 丛琨, 石亚娜, 等. 不同初加工方法对黄精品质的影响研究[J]. 中国药房,2019,30(18):2537−2541. [[ZHU Xinyan, CONG Kun, SHI Yana, et al. Study on the effects of different primary processing methods on the quality of Polygonatum sibiricum[J]. China Pharmacy,2019,30(18):2537−2541. [ZHU Xinyan, CONG Kun, SHI Yana, et al. Study on the effects of different primary processing methods on the quality of Polygonatum sibiricum[J]. China Pharmacy, 2019, 30(18): 2537-2541.
[30] 潘克琴, 李丹丹, 王华磊, 等. 九蒸九制对不同龄节多花黄精品质的影响[J]. 特产研究,2021,43(3):23−27. [PAN Keqin, LI Dandan, WANG Hualei, et al. Effects of nine steaming and nine processing systems on the quality of Polygonatum cyrtonema Hua in different ages[J]. Special Wild Economic Animal and Plant Research,2021,43(3):23−27. doi: 10.16720/j.cnki.tcyj.2021.058 PAN Keqin, LI Dandan, WANG Hualei, et al. Effects of nine steaming and nine processing systems on the quality of Polygonatum cyrtonema Hua in different ages[J]. Special Wild Economic Animal and Plant Research , 2021, 43(3): 23-27. doi: 10.16720/j.cnki.tcyj.2021.058
[31] 陈文华, 谭会颖, 邴帅, 等. 传统炮制工艺对热河黄精多糖含量的影响[J]. 时珍国医国药,2018,29(12):2940−2942. [CHEN Wenhua, TAN Huiying, BING Shuai, et al. Effects of traditional processing technology on polysaccharide content of JeholZeolium[J]. Lishizhen Medicine and Materia Medica Research,2018,29(12):2940−2942. CHEN Wenhua, TAN Huiying, BING Shuai, et al. Effects of traditional processing technology on polysaccharide content of JeholZeolium[J]. Lishizhen Medicine and Materia Medica Research, 2018, 29(12): 2940-2942.
[32] 吴丰鹏, 李芹英, 吴彦超, 等. 九蒸九制对黄精多糖单糖组成及其抗氧化性的影响[J]. 食品工业科技,2021,42(2):42−46. [WU Fengpeng, LI Qinying, WU Yanchao, et al. Effects of nine-steam-nine-bask on the monosaccharide composition and antioxidant activities of Polygonatum sibiricum polysaccharide[J]. Science and Technology of Food Industry,2021,42(2):42−46. doi: 10.13386/j.issn1002-0306.2020030160 WU Fengpeng, LI Qinying, WU Yanchao, et al. Effects of nine-steam-nine-bask on the monosaccharide composition and antioxidant activities of Polygonatum sibiricum polysaccharide[J]. Science and Technology of Food Industry, 2021, 42(2): 42-46. doi: 10.13386/j.issn1002-0306.2020030160
[33] 瞿昊宇, 冯楚雄, 谢梦洲, 等. 不同炮制方法对黄精多糖含量的影响[J]. 湖南中医药大学学报,2015,35(12):53−55. [QU Haoyu, FENG Chuxiong, XIE Mengzhou, et al. Effcet of different processing methods on the content of polygahatous polysaccharides[J]. Journal of Traditional Chinese Medicine University of Hunan,2015,35(12):53−55. doi: 10.3969/j.issn.1674-070X.2015.12.015 QU Haoyu, FENG Chuxiong, XIE Mengzhou, et al. Effcet of different processing methods on the content of Polygahatous polysaccharides[J]. Journal of Traditional Chinese Medicine University of Hunan, 2015, 35(12): 53-55. doi: 10.3969/j.issn.1674-070X.2015.12.015
[34] 陈瑞瑞, 祖艳红, 石丁夫, 等. 多花黄精从生粉到九蒸九晒过程中多糖的变化[J]. 安徽农业科学,2019,47(18):181−182. [CHEN Ruirui, ZU Yanhong, SHI Dingfu, et al. Changes of polysaccharides in the process of Polygonatum cyrtonema from raw flour to nine steaming and nine drying[J]. Journal of Anhui Agricultural Sciences,2019,47(18):181−182. doi: 10.3969/j.issn.0517-6611.2019.18.049 CHEN Ruirui, ZU Yanhong, SHI Dingfu, et al. Changes of polysaccharides in the process of Polygonatum cyrtonema from raw flour to nine steaming and nine drying[J]. Journal of Anhui Agricultural Sciences, 2019, 47(18): 181-182. doi: 10.3969/j.issn.0517-6611.2019.18.049
[35] 王迎香, 唐子惟, 彭腾, 等. 苯酚-硫酸法测定酒蒸多花黄精多糖含量的优化[J]. 食品工业科技,2021,42(18):300−308. [WANG Yingxiang, TANG Ziwei, PENG Teng, et al. Optimization of phenol sulfuric acid method for the polysaccharide content of wine-steamed Polygonatum cyrtonema Hua[J]. Science and Technology of Food Industry,2021,42(18):300−308. doi: 10.13386/j.issn1002-0306.2021010069 WANG Yingxiang, TANG Ziwei, PENG Teng, et al. Optimization of phenol sulfuric acid method for the polysaccharide content of wine-steamed Polygonatum cyrtonema Hua[J]. Science and Technology of Food Industry, 2021, 42(18): 300-308. doi: 10.13386/j.issn1002-0306.2021010069
[36] 陶爱恩. 滇黄精多糖的理化性质、活性筛选与质量评价研究[D]. 大理: 大理大学, 2019. TAO Aien. Study on physicochemical properties, activity screening and quality evaluation of polysaccharides from Polygonatum kingianum[D]. Dali: Dali University, 2019.
[37] 胡叶青, 胡云飞, 祝凌丽, 等. 九华黄精"九蒸九晒"炮制过程中5-羟甲基糠醛的含量变化[J]. 德州学院学报,2019,35(4):29−32. [HU Yeqing, HU Yunfei, ZHU Lingli, et. al. Study on the 5-hydroxymethylfurfural constituents of Jiuhua Rhizoma Polygnoatiin "Nine-steam-nine-bask" processing[J]. Journal of Dezhou University,2019,35(4):29−32. doi: 10.3969/j.issn.1004-9444.2019.04.008 HU Yeqing, HU Yunfei, ZHU Lingli, et. al. Study on the 5-hydroxymethylfurfural constituents of Jiuhua Rhizoma Polygnoatiin "Nine-steam-nine-bask" processing[J]. Journal of Dezhou University, 2019, 35(4): 29-32. doi: 10.3969/j.issn.1004-9444.2019.04.008
[38] 杨婧娟, 张希, 马雅鸽. 发酵对黄精主要活性成分及其抗氧化活性和刺激性的影响[J]. 食品工业科技,2020,41(2):52−58. [YANG Jingjuan, ZHANG Xi, MA Yage. Effects of fermentation on main bioactive components, antioxidant activities and irritation of Polygonatum sibiricum[J]. Science and Technology of Food Industry,2020,41(2):52−58. doi: 10.13386/j.issn1002-0306.2020.02.009 YANG Jingjuan, ZHANG Xi, MA Yage. Effects of fermentation on main bioactive components, antioxidant activities and irritation of Polygonatum sibiricum[J]. Science and Technology of Food Industry, 2020, 41(2): 52-58. doi: 10.13386/j.issn1002-0306.2020.02.009
[39] XIE Songzi, YANG Guang, JIANG Xianmin, et al. Polygonatum cyrtonema Hua polysaccharide promotes GLP-1 secretion from enteroendocrine L-cells through sweet taste receptor-mediated cAMP signaling[J]. Journal of Agricultural and Food Chemistry,2020,68(25):6864−6872. doi: 10.1021/acs.jafc.0c02058
[40] 杨光. 多花黄精多糖对GLP-1分泌与表达的调节及其分子机制研究[D]. 合肥: 合肥工业大学, 2018. YANG Guang. The effect of Polygonatum cyrtonema Hua polysaccharides (PCP) on the expression and secretion of GLP-1 and its molecular mechanism[D]. Hefei: Hefei University of Technology, 2018.
[41] LIU Fen, LIU Yinghua, MENG Yiwen, et al. Structure of polysaccharide from Polygonatum cyrtonema Hua and the antiherpetic activity of its hydrolyzed fragments[J]. Antiviral Research,2004,63(3):183−189. doi: 10.1016/j.antiviral.2004.04.006
[42] LIU Xiaoxiao, WAN Zhenjiang, SHI Lin, et al. Preparation and antiherpetic activities of chemically modified polysaccharides from Polygonatum cyrtonema Hua[J]. Carbohydrate Polymers,2011,83(2):737−742. doi: 10.1016/j.carbpol.2010.08.044
[43] SHEN Wendi, LI Xueying, DENG Yuanyuan, et al. Polygonatum cyrtonema Hua polysaccharide exhibits anti-fatigue activity via regulating osteocalcin signaling[J]. International Journal of Biological Macromolecules,2021,175:235−241. doi: 10.1016/j.ijbiomac.2021.01.200
[44] ZHAO Ping, LI Xia, WANG Ying, et al. Characterisation and saccharide mapping of polysaccharides from four common Polygonatum spp[J]. Carbohydrate Polymers,2020,233:115836. doi: 10.1016/j.carbpol.2020.115836
[45] 雍潘. 多花黄精的多糖提取、纯化、结构解析及活性研究[D]. 成都: 西南民族大学, 2019. YONG Pan. Isolation, purification, structural characterization and biological activity of polysaccharides from Polygonatum cyrtonema Hua[D]. Chengdu: Southwest Minzu University, 2019.
[46] LI Ning, SHI Chenchen, SHI Songshan, et al. An inulin-type fructan isolated from Artemisia japonica and its anti-arthritic effects[J]. Journal of Functional Foods,2017,29:29−36. doi: 10.1016/j.jff.2016.11.033
[47] ZHANG Jiaozhen, LIU Na, SUN Chao, et al. Polysaccharides from Polygonatum sibiricum Delar. ex Redoute induce an immune response in the RAW264.7 cell line via an NF-κB/MAPK pathway[J]. RSC Advances,2019,9(31):17988−17994. doi: 10.1039/C9RA03023A
[48] SUN Tingting, ZHANG Hong, LI Ye, et al. Physicochemical properties and immunological activities of polysaccharides from both crude and wine-processed Polygonatum sibiricum[J]. International Journal of Biological Macromolecules,2020,143:255−264. doi: 10.1016/j.ijbiomac.2019.11.166
[49] YELITHAO K, SURAYOT U, PARK W, et al. Effect of sulfation and partial hydrolysis of polysaccharides from Polygonatum sibiricum on immune-enhancement[J]. International Journal of Biological Macromolecules,2019,122:10−18. doi: 10.1016/j.ijbiomac.2018.10.119
[50] LIU Liu, DONG Qun, DONG Xiaotang, et al. Structural investigation of two neutral polysaccharides isolated from rhizome of Polygonatum sibiricum[J]. Carbohydrate Polymers,2007,70(3):304−309. doi: 10.1016/j.carbpol.2007.04.012
[51] 刘娜. 黄精多糖的分离、鉴定及免疫调节功效研究[D]. 济南: 山东大学, 2017. LIU Na. Isolation, identification and study on the immunomodulatory effect of polysaccharide from Polygonatum sibiricum[D]. Jinan: Shangdong University, 2017.
[52] ZHANG Hui, CAI Xiuting, TIAN Qinghua, et al. Microwave-assisted degradation of polysaccharide from Polygonatum sibiricum and antioxidant activity[J]. Journal of Food Science and Technology (Mysore),2019,84(4):754−761.
[53] 张遥遥, 张梦, 胡悦, 等. 黄精多糖的提纯, 硫酸化和羧甲基化修饰及其抗氧化活性研究[J]. 食品工业科技,2019,40(21):45−51. [ZHANG Yaoyao, ZHANG Meng, HU Yue, et al. Purification, sulfation modification and carboxymethylation modification of polysaccharides from Polygonatum sibiricum and its antioxidant activity[J]. Science and Technology of Food Industry,2019,40(21):45−51. ZHANG Yaoyao, ZHANG Meng, HU Yue, et al. Purification, sulfation modification and carboxymethylation modification of polysaccharides from Polygonatum sibiricum and its antioxidant activity[J]. Science and Technology of Food Industry, 2019, 40(21): 45-51.
[54] LI Qinying, ZENG Jun, GONG Pixian, et al. Effect of steaming process on the structural characteristics and antioxidant activities of polysaccharides from Polygonatum sibiricum rhizomes[J]. Glycoconjugate Journal,2021,38(5):561−572. doi: 10.1007/s10719-021-10013-z
[55] 杜泽飞. 黄精的化学成分、炮制与结构修饰研究及黄精属植物系统发育分析[D]. 大理: 大理大学, 2020. DU Zefei. Studies on the chemical constituents, processing and structural modification from the Polygonati Rhizoma and phylogenetic analysis in plants of Polygonatum species[D]. Dali: Dali University, 2020.
[56] JIN Jian, LAO Jia, ZHOU Rongrong, et al. Simultaneous identification and dynamic analysis of saccharides during steam processing of rhizomes of Polygonatum cyrtonema by HPLC-QTOF-MS/MS[J]. Molecules,2018,23(11):2855. doi: 10.3390/molecules23112855
[57] 王婧, 陶爱恩, 杨燕, 等. 滇黄精中多糖的分离与抗氧化活性研究[J]. 中草药,2021,52(16):4789−4796. [WANG Jing, TAO Aien, YANG Yan, et al. Separation, physicochemical properties and anti-oxidant activities of three polysaccharides from Polygonatum kingianum[J]. Chinese Traditional and Herbal Drugs,2021,52(16):4789−4796. WANG Jing, TAO Aien, YANG Yan, et al. Separation, physicochemical properties and anti-oxidant activities of three polysaccharides from Polygonatum kingianum[J]. Chinese Traditional and Herbal Drugs, 52(16): 4789-4796.
[58] 顾健. 滇黄精多糖的制备与质量分析[D]. 苏州: 苏州大学, 2018. GU Jian. Preparation and determination of polysaccharide in Polygonatum kingianum[D]. Suzhou: Soochow University, 2018.
[59] 万晓莹, 刘振丽, 宋志前, 等. 黄精炮制前后多糖的相对分子质量分布和免疫活性比较[J]. 中国实验方剂学杂志,2021,27(15):83−90. [WAN Xiaoying, LIU Zhenli, SONG Zhiqian, et al. Comparison of relative molecular weight distribution and immune activity of polysaccharides in Polygonati Rhizoma before and after processing[J]. Chinese Journal of Experimental Traditional Medical Formulae,2021,27(15):83−90. doi: 10.13422/j.cnki.syfjx.20210446 WAN Xiaoying, LIU Zhenli, SONG Zhiqian. Comparison of relative molecular weight distribution and immune activity of polysaccharides in Polygonati Rhizoma before and after processing[J]. Chinese Journal of Experimental Traditional Medical Formulae, 2021, 27(15): 83-90. doi: 10.13422/j.cnki.syfjx.20210446
[60] 王坤, 岳永德, 汤锋, 等. 多花黄精多糖的分级提取及结构初步分析[J]. 天然产物研究与开发,2014,26(3):364−369. [WANG Kun, YUE Yongde, TANG Feng, et al. Sequential extraction and structural analysis of polysaccharides from Polygonatum cyrtonema Hua[J]. Natural Product Research and Development,2014,26(3):364−369. doi: 10.16333/j.1001-6880.2014.03.015 WANG Kun, YUE Yongde, TANG Feng, et al. Sequential extraction and structural analysis of polysaccharides from Polygonatum cyrtonema Hua[J]. Natural Product Research and Development, 2014, 26(3): 364-369. doi: 10.16333/j.1001-6880.2014.03.015
[61] 徐如静, 梁娟, 俞年军, 等. 九华黄精炮制前后多糖类成分结构变化研究[J]. 安徽中医药大学学报,2021,40(2):91−96. [XU Rujing, LIANG Juan, YU Nianjun, et al. Structural change of polysaccharide constituents from Polygonatum cyrtonema in Jiuhua Mountain after processing[J]. Journal of Anhui University of Chinese Medicine,2021,40(2):91−96. doi: 10.3969/j.issn.2095-7246.2021.02.022 XU Rujing, LIANG Juan, YU Nianjun, et al. Structural change of polysaccharide constituents from Polygonatum cyrtonema in Jiuhua Mountain after processing[J]. Journal of Anhui University of Chinese Medicine, 2021, 40(2): 91-96. doi: 10.3969/j.issn.2095-7246.2021.02.022
[62] LI Xiaojun, CHEN Qi, LIU Guoku, et al. Chemical elucidation of an arabinogalactan from rhizome of Polygonatum sibiricum with antioxidant activities[J]. International Journal of Biological Macromolecules,2021,190:730−738. doi: 10.1016/j.ijbiomac.2021.09.038
[63] 张玉琴, 刘垚君, 王欣垚, 等. 黄精多糖对H2O2诱导HT22细胞氧化损伤的保护作用[J]. 江西中医药大学学报,2021,33(2):4. [ZHANG Yuqin, LIU Yaojun, WANG Xinyao, et al. Protective effect of Polygonatum polysaccharides on oxidative injury induced by hydrogen peroxide in HT22 cells[J]. Journal of Jiangxi University of Traditional Chinese Medicine,2021,33(2):4. ZHANG Yuqin, LIU Yaojun, WANG Xinyao. Protective effect of Polygonatum polysaccharides on oxidative injury induced by hydrogen peroxide in HT22 cells[J]. Journal of Jiangxi University of Traditional Chinese Medicine, 2021, 33(2): 4.
[64] WANG Yi, LAN Changjun, LIAO Xuan, et al. Polygonatum sibiricum polysaccharide potentially attenuate diabetic retinal injury in a diabetic rat model[J]. Journal of Diabetes Investigation,2019,10(4):915−924. doi: 10.1111/jdi.12976
[65] YAN Hongli, LU Jianmei, WANG Yanfang, et al. Intake of total saponins and polysaccharides from Polygonatum kingianum affects the gut microbiota in diabetic rats[J]. Phytomedicine,2017,26:45−54. doi: 10.1016/j.phymed.2017.01.007
[66] 杨明琛, 袁梦欣, 陆维, 等. 黄精多糖体外消化特性及对Ⅱ型糖尿病小鼠肠道菌群的调节作用[J]. 现代食品科技,2021,37(8):14−21. [YANG Mingchen, YUAN Mengxin, LU Wei, et al. In vitro digestion properties of Polygonatum sibiricum polysaccharide and its regulatory action on the gut microbiota in T2DM mice[J]. Modern Food Science & Technology,2021,37(8):14−21. doi: 10.13982/j.mfst.1673-9078.2021.8.1181 YANG Mingchen, YUAN Mengxin, LU Wei, et al. In vitro digestion properties of Polygonatum sibiricum polysaccharide and its regulatory action on the gut microbiota in T2DM mice[J]. Modern Food Science & Technology, 2021, 37(8): 14-21. doi: 10.13982/j.mfst.1673-9078.2021.8.1181
[67] LU Yi, SHI Zhenfeng, LI Lei. Effects of polysaccharide from Polygonatum cyrtonema Hua on blood glucose, survival rate and liver cell protection in type I diabetic mice[J]. Acta Microscopica,2020,29(5):2711−2717.
[68] 王艳芳. 滇黄精多糖改善大鼠脂代谢紊乱的作用研究[D]. 昆明: 云南中医学院, 2017. WANG Yanfang. Effects and mechanisms of polysaccharide from Polygonatum kingianum on lipid metabolism disorder in rats[D]. Kunming: Yunnan University of Chinese Medicine, 2017.
[69] LIU Bo, TANG Yuan, SONG Zhenyan, et al. Polygonatum sibiricum F. Delaroche polysaccharide ameliorates HFD-induced mouse obesity via regulation of lipid metabolism and inflammatory response[J]. Molecular Medicine Reports,2021,24(1):1−10.
[70] GU Wen, WANG Yanfang, ZENG Linxi, et al. Polysaccharides from Polygonatum kingianum improve glucose and lipid metabolism in rats fed a high fat diet[J]. Biomedicine and Pharmacotherapy,2020,125:109910. doi: 10.1016/j.biopha.2020.109910
[71] 王曦. 基于线粒体功能调节的滇黄精缓解非酒精脂肪肝药效及机制探究[D]. 昆明: 云南中医药大学, 2019. WANG Xi. Study on the efficacy and mechanism of Polygonatum kingianum on relieving nonalcoholic fatty liver based on mitochondrial function regulation[D]. Kunming: Yunnan University of Chinese Medicine, 2019.
[72] YANG Xingxin, WEI Jiadi, MU Jiankang, et al. Integrated metabolomic profiling for analysis of antilipidemic effects of Polygonatum kingianum extract on dyslipidemia in rats[J]. World Journal of Gastroenterology,2018,24(48):5505−5524. doi: 10.3748/wjg.v24.i48.5505
[73] LONG Tingting, LIU Zijing, SHANG Jingchuan, et al. Polygonatum sibiricum polysaccharides play anti-cancer effect through TLR4-MAPK/NF-κB signaling pathways[J]. International Journal of Biological Macromolecules,2018,111:813−821. doi: 10.1016/j.ijbiomac.2018.01.070
[74] 傅圣斌, 钱建鸿, 陈乐意, 等. 黄精多糖的提取及其对小鼠免疫活性的影响[J]. 中国食品学报,2013,13(1):68−72. [FU Shengbin, QIAN Jianhong, CHEN Leyi, et al. Extracting of Polygonatum polysaccharides and effecting on the immunological activity in immunosuppressed mice[J]. Journal of Chinese Institute of Food Science and Technology,2013,13(1):68−72. doi: 10.16429/j.1009-7848.2013.01.002 FU Shengbin, QIAN Jianhong, CHEN Leyi, et al. Extracting of Polygonatum polysaccharides and effecting on the immunological activity in immunosuppressed mice[J]. Journal of Chinese Institute of Food Science and Technology, 2013, 13(1): 68-72. doi: 10.16429/j.1009-7848.2013.01.002
[75] 于思文, 张妍, 田海玲, 等. 黄精粗多糖对体外培养小鼠脾淋巴细胞及巨噬细胞免疫活性的影响[J]. 延边大学医学学报,2019,42(2):33−36. [YU Siwen, ZHANG Yan, TIAN Hailing, et al. Effects of crude polysaccharide from Polygonatum sibiricum on the immune activity of mouse spleen lymphocytes and macrophages cultured in vitro[J]. Journal of Medical Science Yanbian University,2019,42(2):33−36. YU Siwen, ZHANG Yan, TIAN Hailing, et al. Effects of crude polysaccharide from Polygonatum sibiricum on the immune activity of mouse spleen lymphocytes and macrophages cultured in vitro[J]. Journal of Medical Science Yanbian University, 2019, 42(2): 33-36.
[76] 付莉慧. 滇黄精粗多糖含片制备工艺及其抗疲劳作用的初步研究[D]. 昆明: 云南中医药大学, 2019. FU Lihui. Preliminary study on preparation process and anti- fatigue effect of Polygonatum kingianum crude polysaccharide tablets[D]. Kuming: Yunnan University of Chinese Medicine, 2019.
[77] 刘诗琼, 秦晓群, 李世胜. 黄精多糖对小鼠抗疲劳作用的实验研究[J]. 中国当代医药,2009,16(10):31−32. [LIU Shiqiong, QIN Xiaoqun, LI Shisheng. Effects of polysaccharides from Polygonatum on fatigue in mice[J]. China Modern Medicine,2009,16(10):31−32. doi: 10.3969/j.issn.1674-4721.2009.10.017 LIU Shiqiong, QIN Xiaoqun, LI Shisheng. Effects of polysaccharides from Polygonatum on fatigue in mice[J]. China Modern Medicine, 2009, 16(10): 31-32. doi: 10.3969/j.issn.1674-4721.2009.10.017
[78] 王玉勤, 于晓婷, 吴晓岚, 等. 黄精多糖对力竭小鼠脑组织自由基代谢影响[J]. 中国公共卫生,2014,30(9):1165−1167. [WANG Yuqin, YU Xiaoting, WU Xiaolan, et al. Effects of Polygonatum sibiricum polysaccharides on free radical metabolism in brain tissue of mice at exhaustion and recovery[J]. Chinese Journal of Public Health,2014,30(9):1165−1167. doi: 10.11847/zgggws2014-30-09-18 WANG Yuqin, YU Xiaoting, WU Xiaolan, et al. Effects of Polygonatum sibiricum polysaccharides on free radical metabolism in brain tissue of mice at exhaustion and recovery[J]. Chinese Journal of Public Health, 2014, 30(9): 1165-1167. doi: 10.11847/zgggws2014-30-09-18
[79] 卢焕俊, 刘思源, 李香兰. 黄精提取液对正常小鼠抗疲劳能力的影响及机制探讨[J]. 山东医药,2014,54(27):39−41. [LU Huanjun, LIU Siyuan, LI Xianglan. Study on the efficacy and mechanism of antifatigue ability of Polygonatum extract on normal mice[J]. Shandong Medical Journal,2014,54(27):39−41. doi: 10.3969/j.issn.1002-266X.2014.27.013 LU Huanjun, LIU Siyuan, LI Xianglan. Study on the efficacy and mechanism of antifatigue ability of Polygonatum extract on normal mice[J]. Shandong Medical Journal, 2014, 54(27): 39-41. doi: 10.3969/j.issn.1002-266X.2014.27.013
[80] 石娟, 赵煜, 雷杨, 等. 黄精粗多糖抗疲劳抗氧化作用的研究[J]. 时珍国医国药,2011,22(6):1409−1410. [SHI Juan, ZHAO Yu, LEI Yang, et al. Study on the anti-fatigue and anti-oxidation effect of crude polysaccharide from Polygonatum sibiricum[J]. Lishizhen Medicine and Materia Medica Research,2011,22(6):1409−1410. SHI Juan, ZHAO Yu, LEI Yang, et al. Study on the anti-fatigue and anti-oxidation effect of crude polysaccharide from Polygonatum sibiricum[J]. Lishizhen Medicine and Materia Medica Research, 2011, 22(6): 1409-1410.
[81] 张士凯, 王敏, 程欣欣, 等. 超高压提取黄精多糖及提高运动耐力机制[J]. 核农学报,2021,35(9):2094−2101. [ZHANG Shikai, WANG Min, CHENG Xinxin, et al. Ultrahigh pressure extraction of Polygonatum sibiricum polysaccharide and mechanism of improving exercise endurance[J]. Journal of Nuclear Agricultural Sciences,2021,35(9):2094−2101. doi: 10.11869/j.issn.100-8551.2021.09.2094 ZHANG Shikai, WANG Min, CHENG Xinxin, et al. Ultrahigh pressure extraction of Polygonatum sibiricum polysaccharide and mechanism of improving exercise endurance[J]. Journal of Nuclear Agricultural Sciences, 2021, 35(9): 2094-2101. doi: 10.11869/j.issn.100-8551.2021.09.2094
[82] 杨华杰, 龚千锋, 于欢, 等. 黄精不同炮制品抗疲劳及抗氧化作用比较研究[J]. 江西中医药,2018,49(2):64−67. [YANG Huajie, GONG Qianfeng, YU Huan, et al. Study on anti-fatigue and anti-oxidation effect of different processed products of Polygonatum sibiricum[J]. Jiangxi Journal of Traditional Chinese Medicine,2018,49(2):64−67. YANG Huajie, GONG Qianfeng, YU Huan, et al. Study on anti-fatigue and anti-oxidation effect of different processed products of Polygonatum sibiricum[J]. Jiangxi Journal of Traditional Chinese Medicine, 2018, 49(2): 64-67.
[83] 吴晓岚, 王玉勤, 车光异, 等. 黄精和玉竹抗疲劳作用的实验研究[J]. 中国冶金工业医学杂志,2009,26(3):271−272. [WU Xiaolan, WANG Yuqin, CHE Guangyi, et al. Study on anti-fatigue effect of Polygonatum sibiricum and Polygonatum odoratum[J]. Chinese Medical Journal of Metallurgical Industry,2009,26(3):271−272. doi: 10.13586/j.cnki.yjyx1984.2009.03.011 WU Xiaolan, WANG Yuqin, CHE Guangyi, et al. Study on anti-fatigue effect of Polygonatum sibiricum and Polygonatum odoratum[J]. Chinese Medical Journal of Metallurgical Industry, 2009, 26(3): 271-272. doi: 10.13586/j.cnki.yjyx1984.2009.03.011
[84] 陈杨杨, 胡慧玲, 奉关妹, 等. 多花黄精炮制前后对游泳力竭小鼠抗疲劳抗氧化的影响[J]. 中药药理与临床,2021,37(2):92−97. [CHEN Yangyang, HU Huiling, FENG Guanmei, et al. Anti-fatigue and anti-oxidant effects of crude and processed Polygonatum cyrtonema on exhaustive swimming mice[J]. Pharmacology and Clinics of Chinese Materia Medica,2021,37(2):92−97. doi: 10.13412/j.cnki.zyyl.2021.02.011 CHEN Yangyang, HU Huiling, FENG Guanmei, et al. Anti-fatigue and anti-oxidant effects of crude and processed Polygonatum cyrtonema on exhaustive swimming mice[J]. Pharmacology and Clinics of Chinese Materia Medica, 2021, 37(2): 92-97. doi: 10.13412/j.cnki.zyyl.2021.02.011
[85] LI Ling, LIAO Buyan, THAKUR Kiran, et al. The rheological behavior of polysaccharides sequential extracted from Polygonatum cyrtonema Hua[J]. International Journal of Biological Macromolecules,2018,109:761−771. doi: 10.1016/j.ijbiomac.2017.11.063
[86] LI Ling, THAKUR Kiran, LIAO Buyan, et al. Antioxidant and antimicrobial potential of polysaccharides sequentially extracted from Polygonatum cyrtonema Hua[J]. International Journal of Biological Macromolecules,2018,114:317−323. doi: 10.1016/j.ijbiomac.2018.03.121
[87] LI Ling, THAKUR Kiran, CAO Yuyao, et al. Anticancerous potential of polysaccharides sequentially extracted from Polygonatum cyrtonema Hua in human cervical cancer Hela cells[J]. International Journal of Biological Macromolecules,2020,148:843−850. doi: 10.1016/j.ijbiomac.2020.01.223
[88] 李玲. 连续制备的多花黄精多糖的理化性质及活性研究[D]. 合肥: 合肥工业大学, 2018. LI Ling. Studies on the physicochemical properties and activities of polysaccharides sequentially extracted from Polygonatum cyrtonema Hua[D]. Hefei: Hefei University of Technology, 2018.
[89] 叶红翠, 张小平, 余红, 等. 多花黄精粗多糖抗肿瘤活性研究[J]. 中国实验方剂学杂志,2008(6):34−36. [YE Hongcui, ZHANG Xiaoping, YU Hong, et al. Study on anti-tumor function of polysaccharide from Polygonatum cyrtonema Hua[J]. Chinese Journal of Experimental Traditional Medical Formulae,2008(6):34−36. doi: 10.3969/j.issn.1005-9903.2008.06.014 YE Hongcui, ZHANG Xiaoping, YU Hong, et al. Study on anti-tumor function of polysaccharide from Polygonatum cyrtonema Hua[J]. Chinese Journal of Experimental Traditional Medical Formulae, 2008(6): 34-36. doi: 10.3969/j.issn.1005-9903.2008.06.014
[90] ZHENG Shaoyang. Protective effect of Polygonatum sibiricum polysaccharide on D-galactose-induced aging rats model[J]. Scientific Reports,2020,10:2246. doi: 10.1038/s41598-020-59055-7
[91] 陈毅飞, 刘凯菲, 吴世敏, 等. 黄精多糖对阿尔茨海默病模型斑马鱼p38MAPK/N-cadherin的影响[J]. 中国药理学与毒理学杂志,2021,35(9):659−660. [CHEN Yifei, LIU Kaifei, WU Shimi, et al. Effect of rhynchophylline on p38MAPK/N-cadherin in zebrafish with Alzheimer disease[J]. Chinese Journal of Pharmacology and Toxicology,2021,35(9):659−660. CHEN Yifei, LIU Kaifei, WU Shimi, et al. Effect of rhynchophylline on p38MAPK/N-cadherin in zebrafish with Alzheimer disease[J]. Chinese Journal of Pharmacology and Toxicology, 2021, 35(9): 659-660.
[92] 吴燊荣, 李友元, 吴曦, 等. 黄精多糖对阿尔茨海默病模型小鼠学习记忆力影响及作用机制研究[J]. 中华老年医学杂志,2008(4):291−295. [WU Shenrong, LI Youyuan, WU Xi, et al. Effect of polygona-polysaccharose on learning and memorizing ability and its possible mechanism in Alzheimer disease mice[J]. Chinese Journal of Geriatrics,2008(4):291−295. WU Shenrong, LI Youyuan, WU Xi, et al. Effect of polygona-polysaccharose on learning and memorizing ability and its possible mechanism in Alzheimer disease mice[J]. Chinese Journal of Geriatrics, 2008(4): 291-295.
[93] HAN Chunyang, SUN Taotao, LIU Yawei, et al. Protective effect of Polygonatum sibiricum polysaccharides on gentamicin-induced acute kidney injury in rats via inhibiting p38 MAPK/ATF2 pathway[J]. International Journal of Biological Macromolecules,2020,151:595−601. doi: 10.1016/j.ijbiomac.2020.02.049
[94] ZHAI Zhaohui, LI Zhaoxin, JI Zhonglei, et al. Protective effect of Polygonatum sibiricum polysaccharides on apoptosis, inflammation, and oxidative stress in nucleus pulposus cells of rats with the degeneration of the Intervertebral disc[J]. International Journal of Polymer Science,2019,2019:8925807.
[95] 王一飞, 薛锋. 黄精多糖对胫骨骨折大鼠骨折愈合的作用机制[J]. 中国老年学杂志,2021,41(17):3803−3807. [WANG Yifei, XUE Feng. Mechanism of Polygonatum polysaccharide on fracture healing of tibia fracture in rats[J]. Chinese Journal of Gerontology,2021,41(17):3803−3807. doi: 10.3969/j.issn.1005-9202.2021.17.046 WANG Yifei, XUE Feng. Mechanism of Polygonatum polysaccharide on fracture healing of tibia fracture in rats[J]. Chinese Journal of Gerontology, 2021, 41(17): 3803-3807. doi: 10.3969/j.issn.1005-9202.2021.17.046
[96] 王艺. 黄精, 滇黄精多糖的结构表征与降血糖活性分析[D]. 西安: 陕西师范大学, 2019. WANG Yi. Structural characterization and hypoglycemic activity analysis of polysaccharides from Polygonatum dioscorea[D]. Xi'an: Shaanxi Normal University, 2019.
[97] 魏炜, 李彦伟, 刘凤霞, 等. 响应面法优化超高压提取黄精多糖工艺[J]. 精细化工,2019,36(5):875−881. [WEI Wei, LI Yanwei, LIU Fengxia, et al. Optimization of ultrahigh pressure extraction of polysaccharides from Polygonatum cyrtonema Hua by response surface methodology[J]. Fine Chemicals,2019,36(5):875−881. doi: 10.13550/j.jxhg.20180756 WEI Wei, LI Yanwei, LIU Fengxia, et al. Optimization of ultrahigh pressure extraction of polysaccharides from Polygonatum cyrtonema Hua by response surface methodology[J]. Fine Chemicals, 2019, 36(5): 875-881. doi: 10.13550/j.jxhg.20180756
[98] 李彦伟. 超高压提取黄精多糖工艺优化、结构分析及抗氧化性研究[D]. 大连: 大连理工大学, 2020. LI Yanwei. Study on the process optimization, structure and antioxidant activity of Polygonatum polysaccharides by ultra-high pressure extraction[D]. Dalian: Dalian University of Technology, 2020.
-
期刊类型引用(9)
1. 贾娟,杨雯雯. 响应面法优化猴头菇挤压膨化食品配方及工艺的研究. 粮油科学与工程. 2024(03): 12-19 .  百度学术
百度学术
2. 马传贵,沈亮,张志秀. 猴头菇多糖的提取、结构特性及药理作用研究进展. 食药用菌. 2024(04): 239-245 .  百度学术
百度学术
3. 王艳菊,刘晓兰,李冠龙,郑喜群. 玉米浆水解液对猴头菇菌丝生长及子实体相关性状的影响. 食品研究与开发. 2024(22): 54-61 .  百度学术
百度学术
4. 张帅,高媛,杨杨,马春敏,许馨予,边鑫,张娜. 多糖的生物活性及其对胃肠道功能的影响. 中国食品学报. 2024(10): 438-448 .  百度学术
百度学术
5. 王雨阳,杨焱,吴迪,李文,陈万超,张忠. 几种药食两用类物质对酒精性胃黏膜损伤的保护及其抑制幽门螺旋杆菌作用. 安徽农业科学. 2023(06): 157-162 .  百度学术
百度学术
6. 刘世柱,吴欣灿,胡丽玲,王家俊,吴志君,周晓云,吴经伟. 猴头菇的多糖提取工艺及应用. 中国食用菌. 2022(06): 90-93 .  百度学术
百度学术
7. 庄伟鹏,傅金奕,林金福,张维瑞,张承康,魏奇. 响应面法优化蛹虫草猴头菇复合鱼肉香肠加工工艺. 食品工业. 2022(07): 92-96 .  百度学术
百度学术
8. 夏蕴实,孙印石,刘畅,李志满,姜辉,王梓. 三种动物油脂对大鼠急性胃粘膜损伤的影响. 食品工业科技. 2021(19): 369-375 .  本站查看
本站查看
9. 黄福东,何华奇. 猴头菇外泌体和浸膏对乙醇性胃损伤的抗氧化作用. 安徽科技学院学报. 2020(03): 22-29 .  百度学术
百度学术
其他类型引用(1)





 下载:
下载:
 下载:
下载:



