The Properties of Probiotic Yoghurt with Wolfberry and Its Metabolic Changes of Polyphenolic Compounds
-
摘要: 为探究复合菌种发酵枸杞酸奶的生物活性物质含量、体外抗氧化能力的变化及引起变化的原因,对比研究了嗜热链球菌,嗜热链球菌和保加利亚乳杆菌(3:1),嗜热链球菌和植物乳植杆菌(1:1),嗜热链球菌和副干酪乳杆菌(1:1),嗜热链球菌、保加利亚乳杆菌和植物乳植杆菌(2:1:2),嗜热链球菌、保加利亚乳杆菌和副干酪乳杆菌(2:1:2)复配发酵的枸杞酸奶 。结果表明:复合菌种发酵提高了样品中多酚、黄酮的含量,降低了类胡萝卜素、多糖的含量。与其他样品相比,由嗜热链球菌、保加利亚乳杆菌和副干酪乳杆菌以2:1:2比例发酵的枸杞酸奶样品(SL-Lpc)的活菌数最高,类胡萝卜素、多糖含量无显著变化,多酚与黄酮含量分别提高了16.05%和14.97%,其DPPH自由基清除率、羟基自由基清除率和ABTS+自由基清除率分别可达96.33%、84.83%和98.37%,抗氧化能力显著高于其它样品(P<0.05)。相关性分析发现多酚类化合物(含黄酮)与体外抗氧化活性呈显著正相关。进一步探究发现发酵前后16种酚酸类差异代谢物(10种上调,6种下调)和12种黄酮类差异代谢物(9种上调,3种下调)发生了变化。多种酚酸类物质的上调和糖苷类黄酮的生成是发酵后抗氧化活性提升的主要原因。该研究为枸杞益生菌酸奶的开发提供参考。Abstract: In order to explore the effects of lactic acid bacteria on the bioactive substances and in vitro antioxidant capacity of wolfberry yoghurt, Streptococcus thermophilus, Streptococcus thermophilus and Lactobacillus bulgaricus (3:1), Streptococcus thermophilus and Lactobacillus plantarum (1:1), Streptococcus thermophilus and Lactobacillus paracasei (1:1), Streptococcus thermophilus, Lactobacillus bulgaricus and Lactobacillus plantarum (2:1:2), Streptococcus thermophilus, Lactobacillus bulgaricus and Lactobacillus paracasei (2:1:2) were used to prepare wolfberry yoghurt. The results showed that the fermentation could increased the contents of polyphenols and flavonoids and reduced the contents of the carotenoids and the polysaccharides. Compared to the other samples, the wolfberry yoghurt sample (SL-Lpc) fermented by the three strains of Streptococcus thermophilus, Lactobacillus bulgaricus and Lactobacillus paracasei, which was with the ratio of 2:1:2, showed the highest viable bacterial counts. In the SL-Lpc sample, the content of carotenoids and polysaccharides did not decrease significantly, while the content of polyphenols (0.094 mg/mL) and flavonoids (0.169 mg/mL) were increased by 16.05% and 14.97%, respectively. Its DPPH radical scavenging rate, hydroxyl radical scavenging rate, and ABTS+ radical scavenging rate could reach to 96.33%, 84.83%, and 98.37%, and the antioxidant capacity of sample SL-Lpc was significantly higher than other samples (P<0.05). Correlation analysis revealed that polyphenolic compounds (containing flavonoids) showed a significant positive correlation with in vitro antioxidant activity. Metabolomics analysis revealed that 16 phenolic acid differential metabolites (10 up-regulated and 6 down-regulated) and 12 flavonoid differential metabolites (9 up-regulated and 3 down-regulated) were changed during fermentation. The up-regulation of multiple phenolic acids and the production of glycosidic flavonoids were the main reasons for the increased antioxidant activity after fermentation. This study will provide a reference for the development of wolfberry probiotic yoghurt.
-
Keywords:
- wolfberry /
- probiotics /
- strain compound /
- yoghurt /
- polyphenol compound
-
枸杞是被国家卫生部首批列入《既是食品又是药品的物品名单》的食药同源浆果[1],其高水平的生物活性物质,如多糖、多酚、黄酮、类胡萝卜素等,具有保护肝脏、抗衰老、调节胃肠道、改善视网膜疾病等功效[2−5]。这些生物活性物质通过提供电子给自由基,使自由基稳定,从而减少自由基积累而导致的一系列氧化损伤[6]。近年来,随着人们对健康饮食的需求,功能性酸奶成为研究热点。研究者以浆果为基质[7−9],通过益生菌发酵提高酸奶生物活性物质的含量及抗氧化能力,从而起到改善肠道健康、提高免疫力等[10−11]功效,其具体功效的发挥与发酵菌种和浆果种类密切相关[6,12]。
目前,虽然有很多针对枸杞酸奶的研究,但主要侧重于工艺的优化、产品理化特性等方面,如Fan等[13]研究了二元益生菌(干酪乳杆菌和植物乳杆菌)与枸杞膳食纤维联合使用对酸奶的影响,发现益生菌和枸杞膳食纤维的添加可有效改善酸奶质构、活菌数及气味。目前,针对具体益生菌作用下发酵枸杞酸奶的主要生物活性物质变化及其抗氧化特性报道较少。
本文通过比较4种不同菌种复配制备的枸杞益生菌酸奶的主要生物活性物质含量及抗氧化能力变化,确定最佳发酵菌种及其配比。在此基础上,探究影响体外抗氧化活性最为重要的生物活性物质在发酵前后的变化,以期揭示多酚类差异代谢物对枸杞益生菌酸奶的影响。试验结果为后续枸杞益生菌酸奶的开发利用提供参考,同时也为功能性酸奶的开发提供借鉴。
1. 材料与方法
1.1 材料与仪器
嗜热链球菌(IMAU20149)、保加利亚乳杆菌(IMAU11954) 内蒙古农业大学乳品生物技术与工程教育部重点实验室。植物乳植杆菌(YHG-87)、副干酪乳杆菌(YHG-871) 宁夏大学食品科学与工程学院微生物实验室保藏菌种,所有菌种均由实验室自主分离,并经生理生化测定及16S rRNA测序进行了鉴定;枸杞原浆、牛奶 市售;1,1-二苯基-2-三硝基苯肼(1,1-diphenyl-2-picrylhydrazyl,DPPH)、2,2-联氮-二(3-乙基-苯并噻唑-6-磺酸)二铵盐(2,2'- Azinobis-(3-ethylbenzthiazoline-6-sulphonate),ABTS)、福林酚试剂、芦丁(纯度>99%)、过硫酸钾 分析纯,上海麦克林生化科技有限公司;没食子酸(纯度>99%) 德国默克集团有限公司;甲醇、硫酸亚铁、水杨酸、亚硝酸钠、硝酸铝、碳酸钠、氢氧化钠等均为市售分析纯。
WFJ72系列721型可见分光光度计 上海光谱仪器有限公司;PHSJ-3F pH计 上海仪电科学仪器股份有限公司;BSP-150 恒温培养箱 上海博迅实业有限公司医疗设备厂;EPOCH2NS-SN酶标仪 日本BIOTEK公司;日本岛津LC-20AT型高效液相色谱仪(配有紫外检测器和荧光检测器)、QTRAP 6500+LC-MS/MS 上海爱博才思分析仪器贸易有限公司。
1.2 实验方法
1.2.1 菌种的活化
将实验用菌种接种于无菌液体MRS培养基中,在37 ℃下恒温培养24 h。后取200 µL菌悬液,接种于无菌液体MRS培养基中进行二次传代,在37 ℃下恒温培养24 h。
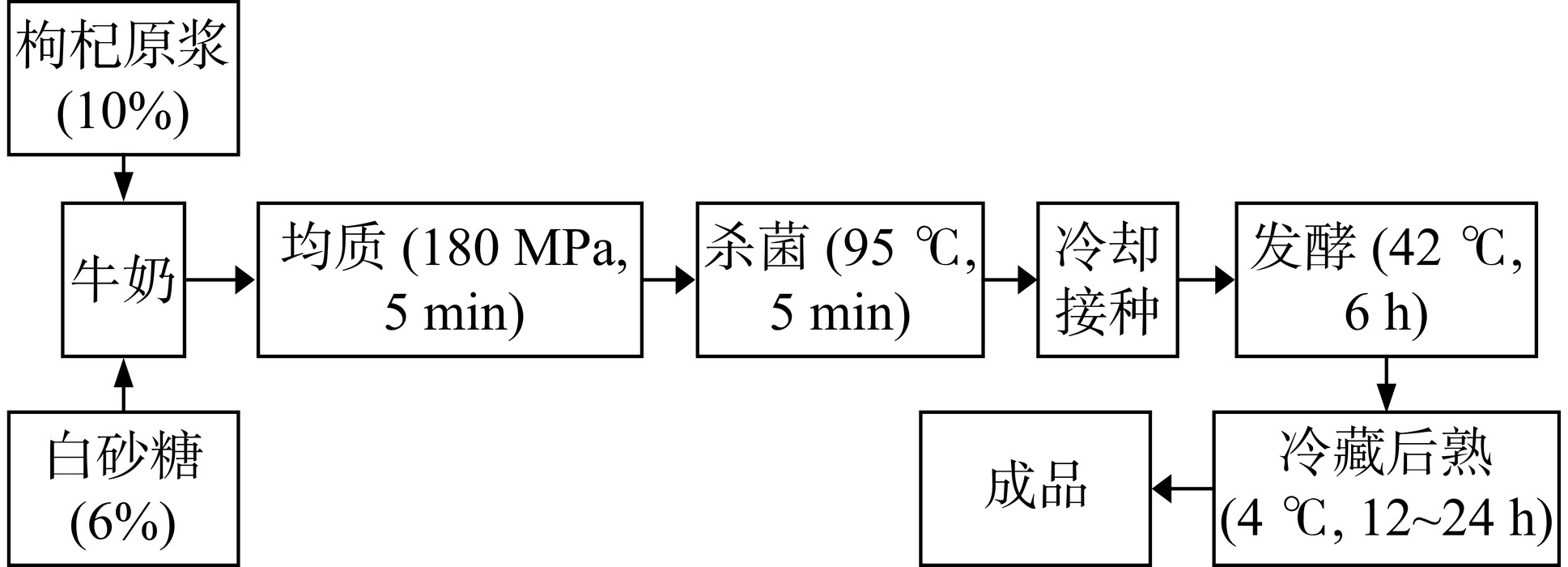
1.2.2 酸奶工艺流程及操作要点
枸杞益生菌酸奶的工艺流程见图1:
菌种具体复配情况见表1。
表 1 枸杞益生菌酸奶复配菌种Table 1. Wolfberry probiotic yoghurt compound strain酸奶样品编号 复配菌种 复配比例 PY 商业发酵剂(嗜热链球菌、保加利亚乳杆菌) 2:1 S 嗜热链球菌 − SL 嗜热链球菌、保加利亚乳杆菌 3:1 SLp 嗜热链球菌、植物乳植杆菌 1:1 SLpc 嗜热链球菌、副干酪乳杆菌 1:1 SL-Lp 嗜热链球菌、保加利亚乳杆菌、植物乳植杆菌 2:1:2 SL-Lpc 嗜热链球菌、保加利亚乳杆菌、副干酪乳杆菌 2:1:2 WM − − 注:表中PY为未添加枸杞原浆的普通酸奶;WM为未接种发酵剂的枸杞(10%枸杞原浆)牛奶;初始接种量统一为1×109 CFU/mL。 1.2.3 枸杞益生菌酸奶的活菌计数
采用选择性培养基测定酸奶中不同菌种活菌数。其中嗜热链球菌、保加利亚乳杆菌和植物乳杆菌的计数参考Veselá等[14]的方法略有改动。嗜热链球菌活菌计数,使用M17培养基于42 ℃有氧培养48 h;保加利亚乳杆菌活菌计数,使用MRS琼脂pH5.2,42 ℃厌氧培养48 h;植物乳植杆菌活菌计数,使用MRS琼脂加万古霉素(20 mg/L, pH5.6)的培养基,在37 ℃有氧培养48 h;副干酪乳杆菌活菌计数参考Demirci等[15]的方法略有改动,使用MRS琼脂加10 mg/L万古霉素的培养基,37 ℃厌氧培养48 h。
1.2.4 枸杞益生菌酸奶类胡萝卜素测定
参考Kan等[16]的方法,略有改动。分别测定发酵前后及不同菌种复配枸杞益生菌酸奶的类胡萝卜素含量,具体如下:
类胡萝卜素提取:准确称取5 g枸杞酸奶,用15 mL四氢呋喃超声提取10 min,过滤后收集滤液,滤渣用相同提取工艺提取至无色,合并滤液后,加20 g无水硫酸钠脱水过滤,于35 ℃浓缩蒸干然后用乙醇溶解并定容至25 mL,−80 ℃保存备用。过0.22 µm微孔滤膜,供液相上样用。所有操作均在避光条件下完成。设定色谱条件为:YMC C30色谱柱(150 mm×4.6 mm,3 μm);流动相A:甲醇:乙腈:水(81:14:5,v/v/v);流动相B:甲基叔丁基醚。梯度洗脱条件为:0 min,70%A,30%B;20 min,50%A,50%B;48 min,50%A,50%B;50 min,70%A,30%B;55 min,70%A,30%B。检测柱温:20 ℃;检测波长:450 nm;流速:1 mL/min进样量:20 μL。定量分析叶黄素、β-胡萝卜素和玉米黄质双棕榈酸酯的标准溶液。以峰面积为纵坐标,进样浓度为横坐标绘制工作曲线,得到线性回归方程,在此浓度范围内,浓度与峰面积呈良好的线性关系。根据回归方程计算枸杞中类胡萝卜素的含量。
1.2.5 枸杞酸奶样品测定液的提取
参考Trigueros等[17]的方法,略有改动。称取样品10 g,精确到0.01 g,加入15 mL含有50 µL浓盐酸的酸化甲醇,混匀,于12000 r/min,离心提取1 min,再于−20 ℃静置3 h,使蛋白充分沉淀后,再4 ℃,8000 r/min,离心10 min,取上清液用0.45 µm滤膜过滤,得到枸杞酸奶样品测定液,于4 ℃冷藏备用,用于多糖、多酚、黄酮及体外抗氧化活性测定。所有测定均重复3次。
1.2.6 枸杞益生菌酸奶中多糖的测定
采用苯酚-硫酸法[18]测定多糖含量。以无水葡萄糖为标准样制作标准曲线,结果以每毫升干基中葡萄糖的毫克数表示(简写为mg/mL)。
1.2.7 枸杞益生菌酸奶中多酚含量的测定
采用Folin-Ciocalteu比色法[17]进行多酚含量测定。以没食子酸为标样制作标准曲线,结果以毫升干基中所含没食子酸的毫克数表示(简写为mg/mL)。
1.2.8 枸杞益生菌酸奶中黄酮含量的测定
采用NaNO2-Al(NO3)3比色法[19]进行黄酮含量的测定。以芦丁为标样制作标准曲线,结果以毫升干基中所含芦丁的毫克数表示(简写为mg/mL)。
1.2.9 枸杞益生菌酸奶体外抗氧化活性的测定
参照Xu等[20]的方法测定DPPH自由基清除率。参照孙正霄等[21]的方法测定羟基自由基清除率。参照Chen等[22]的方法测定ABTS+自由基清除率。抗氧化能力计算见式(1):
清除率(%)=1−(A2−A1A0)×100 (1) 式中:A2为样品测定的吸光值,A1为空白对照的吸光值,A0为对照的吸光值。
1.2.10 枸杞益生菌酸奶多酚类化合物代谢组学测定
1.2.10.1 样品前处理
将−80 ℃样品取出解冻,涡旋混匀1 min,加入−20 ℃预冷的70%甲醇水内标提取液(每50 mg样本加入600 µL提取剂),经涡旋提取15 min,后于12000 r/min,4 ℃下离心3 min,取上清液,过0.22 µm微孔滤膜,保存至进样瓶中,用于LC-MS/MS广泛靶向代谢组学的测定。
1.2.10.2 LC-MS/MS条件
采用LC-MS/MS技术测定多酚类化合物代谢物。参考Liu等[23]的方法。①色谱条件:色谱柱Agilent SB-C18(1.8 µm,2.1 mm×100 mm),柱温40 ℃,流速0.35 mL/min,进样量2 µL;流动相:A相,超纯水(加入0.1%的甲酸),B相,乙腈(加入0.1%的甲酸);梯度洗脱程序:0 min,B相5%,9 min,B相95%,95% 维持1 min,10~11.1 min,B相5%,以5%平衡至14 min。②质谱条件:电喷雾离子源温度550 ℃;离子喷雾电压5500 V(正离子模式)/−4500 V(负离子模式);离子源气体I、气体II和气帘气分别设置为50、60和25 psi,碰撞诱导电离参数设置为高;三重四级杆扫描使用MRM模式,碰撞气体(氮气)设置为中等。
1.3 数据处理
数据用Excel 2019进行整理,并采用平均值±标准偏差表示,采用Origin 2024及SPSS软件进行显著性及相关性 分析并做图(P<0.05),采用Analyst 1.6.3处理质谱数据,MultiQuant软件进行色谱峰的积分和校正。根据UPLC-MS/MS检测平台和武汉迈维生物科技有限公司自建代谢数据库对样本的的代谢物质进行质谱分析。实验中各项指标均进行三次重复。
2. 结果与分析
2.1 不同菌种复配发酵枸杞酸奶的活菌数比较
不同菌种复配枸杞益生菌酸奶的乳酸菌数见表2。由表2可知,由嗜热链球菌、保加利亚乳杆菌和副干酪乳杆菌复配发酵的枸杞酸奶样品(即SL-Lpc)中的活菌数最高,分别达到了9.05×108、9.04×108、6.93×106 CFU/mL。与其余样品不同,经三种菌复合发酵的样品(SL-Lp和SL-Lpc)中的嗜热链球菌和保加利亚乳杆菌的活菌数均高于初始接种量。此外,无论是单一接种还是复合发酵,样品中的植物乳植杆菌和副干酪乳杆菌的活菌数均低于初始接种量。研究表明植物乳植杆菌和副干酪乳杆菌的加入会造成酪蛋白和部分多糖水解为氨基酸、甲酸、叶酸等物质,从而促进嗜热链球菌和保加利亚乳杆菌的生长[24]。而另一些研究表明,部分多酚类物质的生成或其含量的提高,会抑制乳酸菌的生长[25],而发酵后的枸杞酸奶中多酚类物质含量增加,这可能是本实验所使用的植物乳植杆菌和副干酪乳杆菌活菌数在发酵后降低的原因。
表 2 不同复配菌种枸杞益生菌酸奶活菌数(log10 CFU/mL)Table 2. Number of live bacteria in different compound strains of wolfberry probiotic yoghurt (log10 CFU/mL)2.2 不同菌种复配对发酵枸杞酸奶类胡萝卜素含量的影响
类胡萝卜素是人体维生素A的主要来源,具有抗氧化、延缓衰老、抗癌等功效[26−28]。枸杞鲜果中类胡萝卜素含量约为20~90 mg/100 g,主要是由玉米黄质双棕榈酸酸酯、叶黄素、β-胡萝卜素等组成[29],占枸杞果中类胡萝卜素含量的85%。有研究表明,类胡萝卜素易受酸性环境的影响产生不同程度的异构化[30],从而降低其稳定性及生物利用率[31]。不同菌种复配发酵枸杞酸奶的类胡萝卜素含量变化见表3。结果显示,发酵后类胡萝卜素含量呈现显著下降的趋势(P<0.05),其中玉米黄质双棕榈酸酯降低最为明显,发酵后平均降低了27.76%,其次是β-胡萝卜素、叶黄素。这可能由于发酵的酸性环境使得类胡萝卜素部分双键发生了顺式/反式异构化,破坏了该物质的稳定性。由嗜热链球菌单独发酵的S枸杞酸奶样品中玉米黄质双棕榈酸酯含量在所测样品中下降最少,降低了10.97%。样品SL-Lpc的类胡萝卜素变化幅度最小,发酵后SL-Lpc的叶黄素含量为1.12 µg/g,β-胡萝卜素为0.66 µg/g,玉米黄质双棕榈酸酯为51.14 µg/g。
表 3 不同菌种复配枸杞益生菌酸奶类胡萝卜素Table 3. Carotenoids of probiotic yogurt with different strains of wolfberry probiotic yoghurt酸奶样品编号 叶黄素(µg/g) β-胡萝卜素(µg/g) 玉米黄质双棕榈酸酯(µg/g) PY — 0.35±0.00d 0.48±0.01g S 1.07±0.00c 0.62±0.02b 57.17±1.73b SL 1.10±0.02ab 0.62±0.01b 49.18±1.07d SLp 1.00±0.01d 0.55±0.01c 44.07±0.05f SLpc 1.02±0.02d 0.56±0.02c 45.82±0.33e SL-Lp 1.09±0.03b 0.62±0.03b 50.55±1.07c SL-Lpc 1.12±0.01a 0.66±0.02a 51.14±0.30c WM 1.11±0.01ab 0.66±0.01a 63.44±0.26a 2.3 不同菌种复配对发酵枸杞酸奶中多糖及多酚类化合物含量的影响
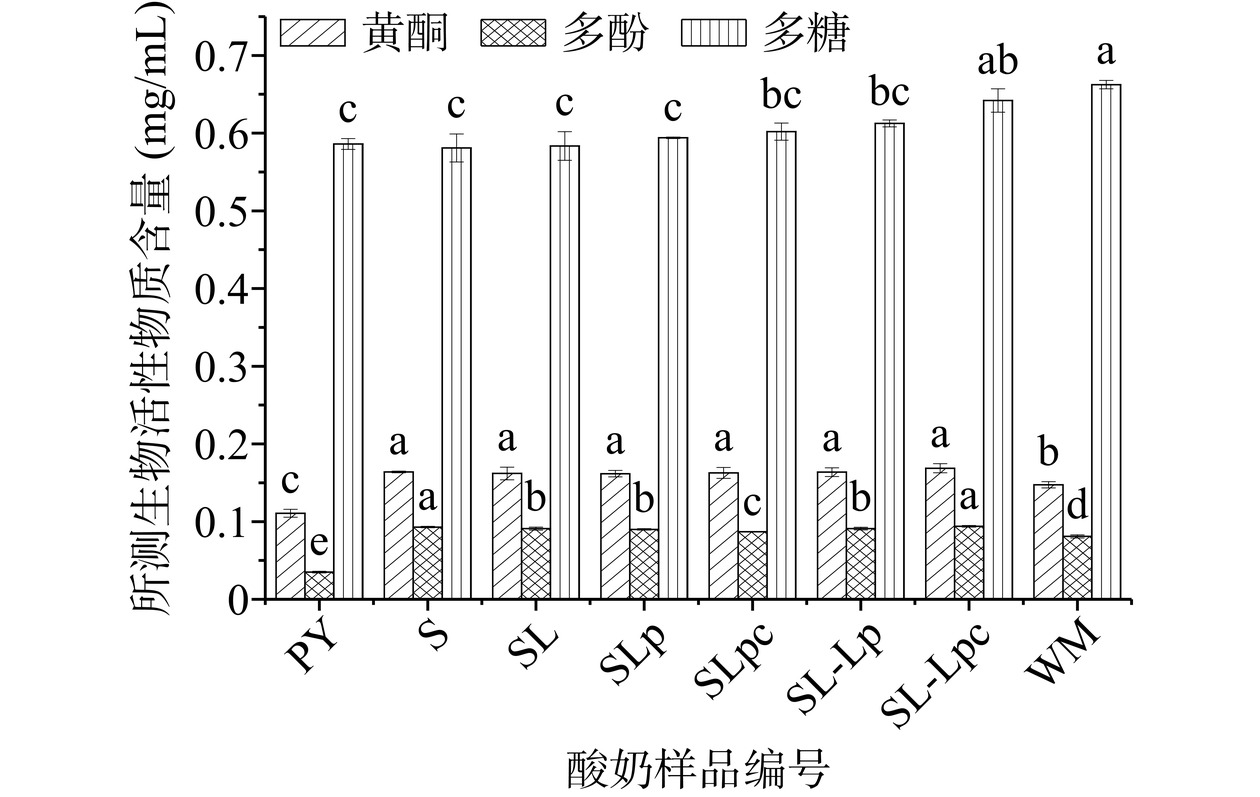
枸杞多糖是枸杞中主要的生物活性物质之一,占枸杞干果的4.18%~15.7%[32]。不同菌种复配发酵枸杞酸奶中多糖含量的比较见图2。实验结果显示发酵后酸奶中多糖含量整体下降。研究表明,枸杞多糖可作为乳酸菌的发酵底物,经乳酸菌代谢后枸杞多糖被分解,其糖苷键断裂生成可溶性小分子多糖,进而由不同的代谢途径生成各种副产物,如乳酸、乙酸等物质[33]。在所测样品中枸杞酸奶样品SL-Lpc中多糖含量降低最少,差异性不显著。嗜热链球菌单独发酵的枸杞酸奶样品S中多糖含量降低最为明显。可见,多糖含量的变化与乳酸菌种类及配比相关。
![]() 图 2 不同乳酸菌复配枸杞酸奶生物活性物质含量注:图中不同小写字母表示差异显著(P<0.05),图3同。Figure 2. Content of bioactive substances in wolfberry yoghurt compounded with different lactic acid bacteria
图 2 不同乳酸菌复配枸杞酸奶生物活性物质含量注:图中不同小写字母表示差异显著(P<0.05),图3同。Figure 2. Content of bioactive substances in wolfberry yoghurt compounded with different lactic acid bacteria多酚和黄酮是枸杞生物活性物质的另一重要来源。经不同菌种发酵后的枸杞酸奶中多酚和黄酮含量显著升高(P<0.05)(见图1)。其中SL-Lpc酸奶样品的多酚和黄酮含量升高最为明显,与未发酵样品(WM)相比多酚含量提高了16.05%,黄酮含量提高了14.97%。研究表明乳酸菌会产生β-葡萄糖苷酶使得多酚类化合物(含黄酮)发生去糖基化反应,从而转化为生物利用度更高的小分子量苷元类物质[34]。在我们随后抗氧化研究中也发现,复合酸奶抗氧化特性的提高与多酚类化合物(含黄酮)的提高密切相关(见表4)。
表 4 不同菌种复配枸杞益生菌酸奶生物活性物质含量和抗氧化能力的相关性分析Table 4. Correlation analysis of bioactives content and antioxidant capacity of probiotic yoghurt with different strains of compounded wolfberry项目 DPPH自由基
清除率羟基自由基
清除率ABTS+自由基
清除率黄酮 0.97*** 0.85** 0.83* 多酚 0.98*** 0.81* 0.76* 多糖 0.23 −0.044 0.059 叶黄素 0.99*** 0.69 0.70 β-胡萝卜素 0.91** 0.57 0.70 玉米黄质双棕榈酸 0.93*** 0.49 0.56 注:*代表在P<0.05水平上显著相关;**代表在P<0.01水平上极显著相关;***代表在P<0.001水平上极显著相关。 2.4 不同菌种复配发酵枸杞酸奶体外抗氧化能力的比较
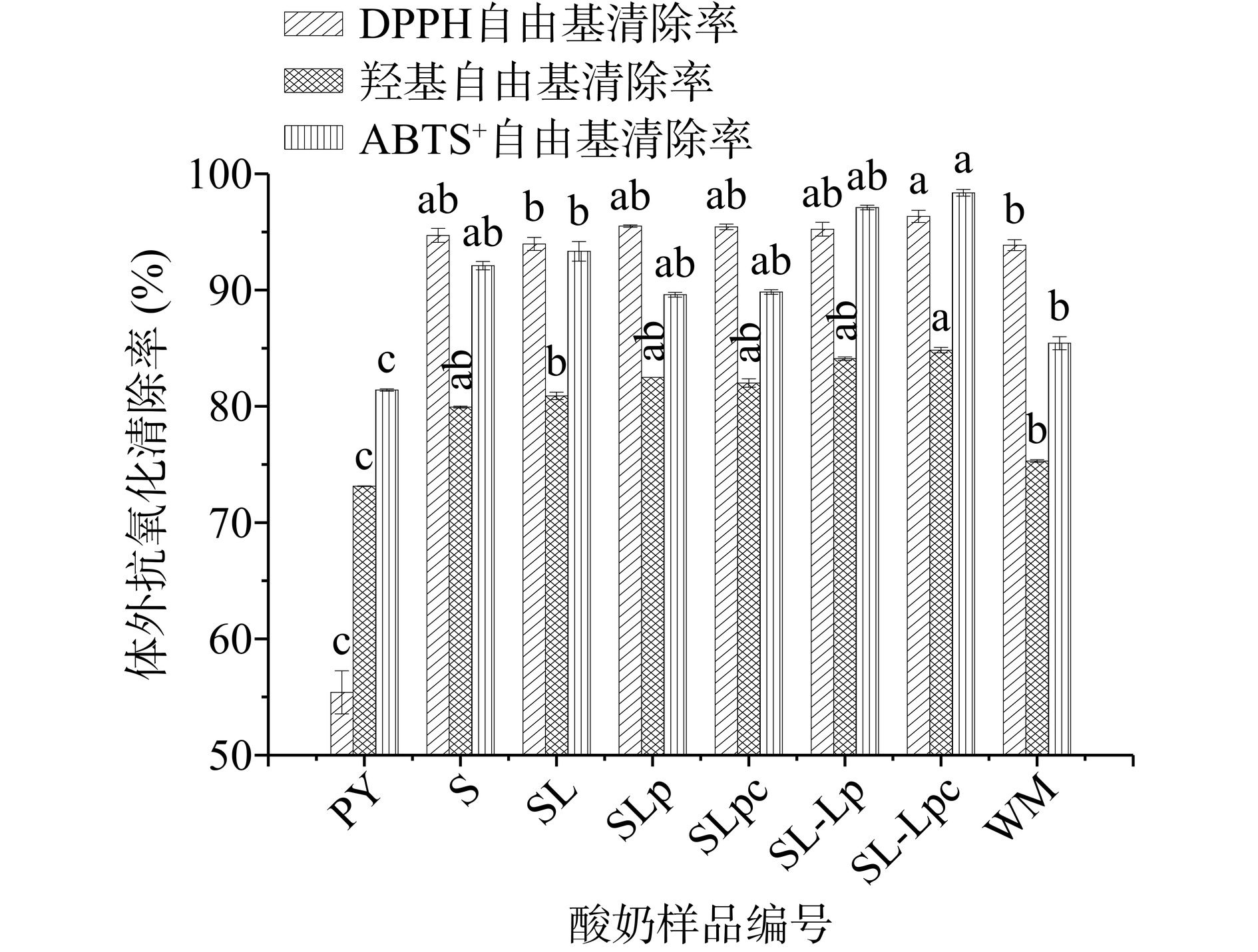
不同乳酸菌复配发酵枸杞酸奶的抗氧化能力见图3。与普通酸奶PY相比枸杞原浆的添加使得酸奶的抗氧化能力显著提高(P<0.05),DPPH自由基清除率平均提高了71.83%,羟基自由基清除率平均提高了12.64%,ABTS+自由基清除率提高了14.73%。这可能是由于乳酸菌分解枸杞酸奶中的多酚类化合物,使得大量供氢抗氧化剂产生,促进DPPH自由基中的肼和羟基自由基中铁离子的还原,减少ABTS+自由基的产生[35−37]。此外,与未发酵的枸杞牛奶(WM)相比,枸杞酸奶样品的抗氧化活性明显高于枸杞牛奶。其中,SL-Lpc抗氧化活性最高,其DPPH自由基清除率可达96.33%,羟基自由基清除率达84.83%,ABTS+自由基清除率达98.37%。可见,枸杞益生菌酸奶抗氧化活性的发挥不单单与枸杞相关,还与益生菌菌种种类及配比密切相关,这与Lizardo等[38]的研究结果相似。
2.5 不同菌种复配枸杞酸奶生物活性物质含量和抗氧化能力的相关性分析
采用皮尔逊相关系数表征枸杞酸奶中的主要生物活性物质与抗氧化指标的关系,结果见表4。除多糖外,所测定的其它5种指标均与体外抗氧化指标呈正相关,其中多酚、黄酮与三种抗氧化指标均呈显著正相关(P<0.05)。表明与多糖、类胡萝卜素类物质相比,多酚类物质(含黄酮)是影响枸杞益生菌酸奶抗氧化能力的主要因素,发酵后多酚、黄酮类物质含量显著增加,造成了酸奶的抗氧化能力也显著提高。
2.6 枸杞益生菌酸奶发酵前后多酚类化合物的代谢分析
为探究发酵对抗氧化能力呈显著正相关的多酚和黄酮的影响,采用UPLC-MS/MS广靶代谢分析进一步了解枸杞益生菌酸奶中多酚类化合物的变化情况。
2.6.1 主成分分析及正交偏最小二乘法判别分析
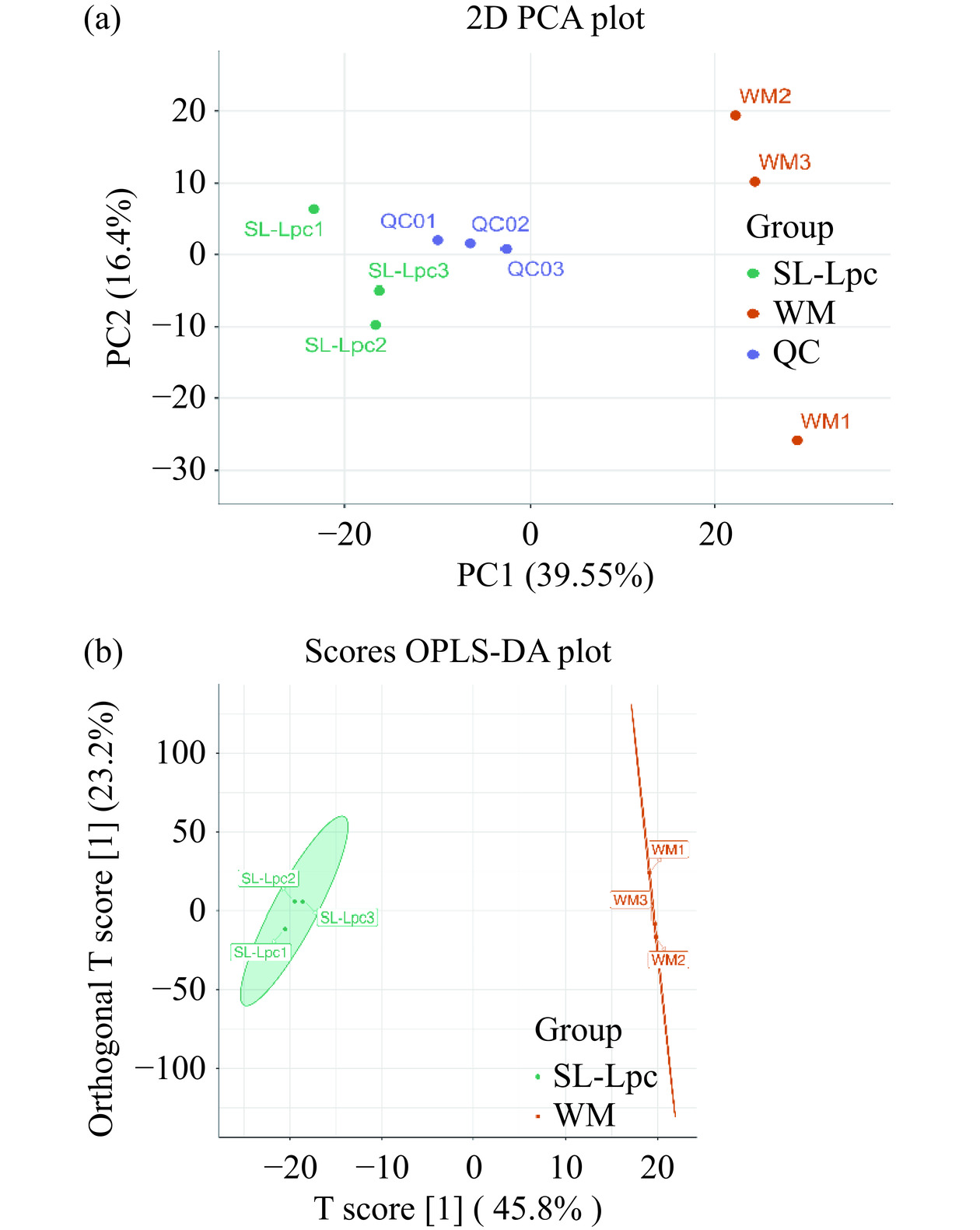
实验样本和质量控制样本(QC)的主成分分析见图4a,图中 QC样本聚集紧密,表明仪器稳定性高,实验数据可靠,且发酵前后两组实验样本在图中分离趋势明显。图4b为两组样品的正交偏最小二乘法(OPLS-DA)得分图。图中评价模型的预测指数(Q2)为0.94,变量的拟合指数(R2X)为0.69,因变量的拟合指数(R2Y)为0.999模型中Q2和R2Y均大于0.9,P值小于0.05,表明该模型为最佳模型,数据拟合程度高,能够很好的解释样本之间的差异。
2.6.2 枸杞益生菌酸奶发酵前后多酚类差异代谢物变化分析
多酚类化合物是具有多种化学结构的次生植物代谢产物。植物源食品中的多酚类化合物主要包括C6-C3-C6为碳骨架的黄酮类物质,C6-C1及C6-C3碳骨架的酚酸类物质,聚合多酚化合物单宁,吡喃酮核和苯环为骨架的香豆素,芳香醇氧化偶联合成的木脂素[39−40]。发酵前后枸杞酸奶中共检出230种多酚类代谢物,包括黄酮类物质99种,酚酸类物质96种,香豆素类物质28种,木脂素7种。以VIP>1,Fold Change≥2或Fold Change≤0.5为标准筛选差异代谢物,Fold Change≥2为上调差异代谢物,Fold Change≤0.5为下调差异代谢物,结果见表5。由表可知发酵前后多酚类差异代谢物包括酚酸及黄酮类物质,其中酚酸类差异代谢物16种(上调10种,下调6种)。上调的酚酸类差异代谢物包括苯甲酸、4-羟基苯甲酸、托品酸、2-羟基-(4-羟基苯基)丙酸、2-羟基-3-苯基丙酸、3-(4-羟基苯基)丙酸、4-羟苯基乳酸、2,4-二羟基苯乙酸甲酯、DL-3-苯基乳酸、2,4-二羟基苯乙酸甲酯。这些酚酸类物质可能经由乳酸菌的代谢催化、转化,使得多酚化合物发生裂变、水解、去甲基化、脱羧、脱羟基化等反应而生成[41]。Nurkenov等[42]、Lofft等[43]和Mu等[44]的研究表明乳酸菌发酵可提高植物样本中的4-羟基苯甲酸、3-(4-羟基苯基)丙酸、4-羟苯基乳酸的含量,从而显著提高体外抗氧化能力。而苯甲酸、2-羟基-3-苯基丙酸等与芳香环链接的酚酸类物质则能够改善枸杞酸奶风味[45],提高酸奶的可接受度。6种下调差异代谢物包括鱼腥草苷 A、对香豆酸甲酯、2-氨基-3-甲氧基苯甲酸、咖啡酸-4-O-葡萄糖苷、北升麻宁、阿魏酸甲酯。其中鱼腥草苷 A、咖啡酸-4-O-葡萄糖苷属糖苷类化合物,其下调可能由于乳酸菌产生的特定酶,将糖苷类物质分解为苷元类的结果[46];而对香豆酸甲酯、阿魏酸甲酯等酯化物的下调也可能是由于发酵造成的脂质降解所导致[47]。
表 5 多酚类代谢物表达变化Table 5. Changes in polyphenol metabolite expression分类 英文 中文 VIP Fold_Change P-value 上调/
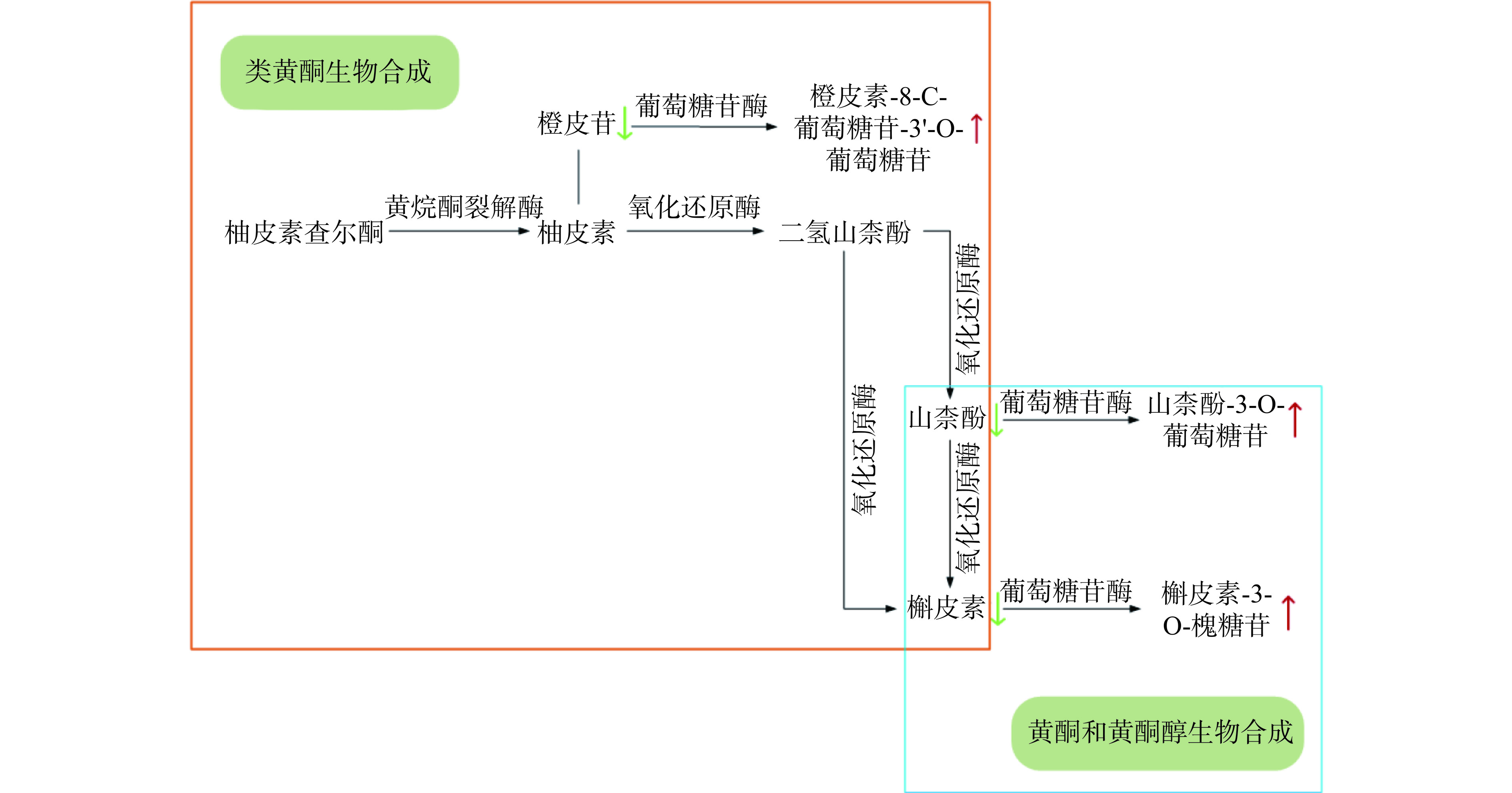
下调酚酸类 Tropic acid 托品酸 2.69103 43.96354 0.00032 ↑ 2-Hydroxy-3-(4-Hydroxyphenyl)Propanoic Acid 2-羟基-(4-羟基苯基)丙酸 2.46840 24.99070 0.00006 ↑ 2-Hydroxy-3-phenylpropanoic acid 2-羟基-3-苯基丙酸 2.05092 9.23805 0.00054 ↑ 3-(4-Hydroxyphenyl)-propionic acid 3-(4-羟基苯基)丙酸 1.95445 7.56910 0.00274 ↑ 4-Hydroxybenzoic acid 4-羟基苯甲酸 1.20774 2.21776 0.00165 ↑ 4-Hydroxyphenyllactic Acid 4-羟苯基乳酸 2.51511 27.99221 0.00006 ↑ 4-Nitrophenol 4-硝基苯酚 1.43096 2.97245 0.00197 ↑ Benzoic acid 苯甲酸 1.89792 6.74534 0.00379 ↑ DL-3-Phenyllactic acid DL-3-苯基乳酸 1.86208 6.27952 0.00014 ↑ Methyl 2,4-dihydroxyphenylacetate 2,4-二羟基苯乙酸甲酯 2.45850 23.48919 0.00010 ↑ houttuynoside A 鱼腥草苷A 1.24285 0.39984 0.06018 ↓ p-Coumaric acid methyl ester 对香豆酸甲酯 2.47922 0.03955 0.00940 ↓ 2-Amino-3-methoxybenzoic acid 2-氨基-3-甲氧基苯甲酸 1.13797 0.49974 0.00030 ↓ Caffeic Acid 4-O-Glucoside 咖啡酸-4-O-葡萄糖苷 2.92903 0.01004 0.04063 ↓ Cimidahurinine 北升麻宁 1.91912 0.13367 0.03541 ↓ Ferulic acid methyl ester 阿魏酸甲酯 2.47197 0.03921 0.01264 ↓ 黄酮类 Carpusin Carpusin 2.69103 43.96354 0.00032 ↑ Hesperetin-8-C-glucoside-3'-O-glucoside 橙皮素-8-C-葡萄糖苷-3'-O-葡萄糖苷 2.46840 24.99070 0.00006 ↑ Isorhamnetin-3-O-(2''-O-glucosyl)galactoside-7-O-glucoside 异鼠李素-3-O-(2''-O-葡萄糖基)半乳糖苷-7-O-葡萄糖苷 2.05092 9.23805 0.00054 ↑ Kaempferol-3-O-glucoside 山柰酚-3-O-葡萄糖苷 1.95445 7.56910 0.00274 ↑ Coniferin 松柏苷 1.80815 5.63445 0.00100 ↑ Kaempferol-3-O-sophoroside-7-O-rhamnoside 山柰酚-3-O-槐糖苷-7-O-鼠李糖苷 1.20774 2.21776 0.00165 ↑ Quercetin-3-O-sophoroside 槲皮素-3-O-槐糖苷 2.51511 27.99221 0.00006 ↑ Quercetin-4'-O-glucoside 槲皮素-4'-O-葡萄糖苷 1.43096 2.97245 0.00197 ↑ Quercetin-7-O-glucoside 槲皮素-7-O-葡萄糖苷 1.89792 6.74534 0.00379 ↑ Diosmetin-7-O-rutinoside (Diosmin) 香叶木苷 1.24285 0.39984 0.06018 ↓ Gossypetin-7-O-L-rhamnopyranoside 棉黄素-7-O-L-吡喃鼠李糖苷 2.47922 0.03955 0.00940 ↓ Hesperetin-7-O-rutinoside 橙皮苷 1.13797 0.49974 0.00030 ↓ 酸奶经发酵后共筛选出黄酮类差异代谢物有12种,其中上调差异代谢物9种,下调差异代谢物3种。上调差异代谢物包括:Carpusin、橙皮素-8-C-葡萄糖苷-3'-O-葡萄糖苷、异鼠李素-3-O-(2''-O-葡萄糖基)半乳糖苷-7-O-葡萄糖苷、山柰酚-3-O-葡萄糖苷、松柏苷、山柰酚-3-O-槐糖苷-7-O-鼠李糖苷、槲皮素-3-O-槐糖苷、槲皮素-4'-O-葡萄糖苷、槲皮素-7-O-葡萄糖苷。下调差异代谢物包括:香叶木苷、棉黄素-7-O-L-吡喃鼠李糖苷、橙皮苷。上调的差异代谢物多为糖苷类黄酮物质,可能是发酵过程中枸杞中的橙皮苷、槲皮素及山柰酚等物质,经类黄酮及黄酮和黄酮醇生物合成等途径在乳酸菌产生的葡萄糖苷酶的作用下生成了糖苷类物质[48],导致山柰酚-3-O-葡萄糖苷、槲皮素-3-O-槐糖苷、橙皮素-8-C-葡萄糖苷-3'-O-葡萄糖苷等衍生物的上调(见图4)。这些糖苷类物质抗氧化效果虽不及小分子的苷元类化合物,但却高于橙皮苷等糖基化黄酮的生物利用度[49]。研究显示,上调的橙皮素-8-C-葡萄糖苷-3'-O-葡萄糖苷具有抗氧化、抗炎、抗菌等益处[50]。山柰酚-3-O-葡萄糖苷及山柰酚-3-O-槐糖苷-7-O-鼠李糖苷两种山奈酚衍生物具有预防糖尿病的功效[51]。槲皮素-3-O-槐糖苷、槲皮素-4'-O-葡萄糖苷、槲皮素-7-O-葡萄糖苷三种槲皮素衍生物具有抗癌的功效[52]。因此可以推断,经益生菌发酵的枸杞酸奶,有利于枸杞中多酚向酚酸和苷元类物质的转化以及复杂的黄酮类化合物向小分子糖苷类化合物的转化,从而为酸奶功能性的提升提供了条件。这些数据为后期功能性酸奶的开发提供了理论依据。
3. 结论
不同乳酸菌复配发酵枸杞酸奶与枸杞牛奶及普通酸奶的比较发现,发酵普遍提高了复合酸奶中多酚、黄酮含量,降低了类胡萝卜素、多糖的含量。样品SL-Lpc的生物活性物质含量最高,且体外抗氧化活性显著高于其它样品。相关性分析发现多酚类化合物(含黄酮)与体外抗氧化活性显著正相关。酚酸和糖苷类黄酮是发酵前后多酚类化合物的主要差异代谢物,通过类黄酮生物合成与黄酮与黄酮醇生物合成途径发挥着作用。该研究结果为枸杞益生菌酸奶的开发利用提供了较为客观的理论依据,为后续枸杞益生菌酸奶的具体功能特性提供了参考。
-
图 2 不同乳酸菌复配枸杞酸奶生物活性物质含量
注:图中不同小写字母表示差异显著(P<0.05),图3同。
Figure 2. Content of bioactive substances in wolfberry yoghurt compounded with different lactic acid bacteria
表 1 枸杞益生菌酸奶复配菌种
Table 1 Wolfberry probiotic yoghurt compound strain
酸奶样品编号 复配菌种 复配比例 PY 商业发酵剂(嗜热链球菌、保加利亚乳杆菌) 2:1 S 嗜热链球菌 − SL 嗜热链球菌、保加利亚乳杆菌 3:1 SLp 嗜热链球菌、植物乳植杆菌 1:1 SLpc 嗜热链球菌、副干酪乳杆菌 1:1 SL-Lp 嗜热链球菌、保加利亚乳杆菌、植物乳植杆菌 2:1:2 SL-Lpc 嗜热链球菌、保加利亚乳杆菌、副干酪乳杆菌 2:1:2 WM − − 注:表中PY为未添加枸杞原浆的普通酸奶;WM为未接种发酵剂的枸杞(10%枸杞原浆)牛奶;初始接种量统一为1×109 CFU/mL。 表 2 不同复配菌种枸杞益生菌酸奶活菌数(log10 CFU/mL)
Table 2 Number of live bacteria in different compound strains of wolfberry probiotic yoghurt (log10 CFU/mL)
表 3 不同菌种复配枸杞益生菌酸奶类胡萝卜素
Table 3 Carotenoids of probiotic yogurt with different strains of wolfberry probiotic yoghurt
酸奶样品编号 叶黄素(µg/g) β-胡萝卜素(µg/g) 玉米黄质双棕榈酸酯(µg/g) PY — 0.35±0.00d 0.48±0.01g S 1.07±0.00c 0.62±0.02b 57.17±1.73b SL 1.10±0.02ab 0.62±0.01b 49.18±1.07d SLp 1.00±0.01d 0.55±0.01c 44.07±0.05f SLpc 1.02±0.02d 0.56±0.02c 45.82±0.33e SL-Lp 1.09±0.03b 0.62±0.03b 50.55±1.07c SL-Lpc 1.12±0.01a 0.66±0.02a 51.14±0.30c WM 1.11±0.01ab 0.66±0.01a 63.44±0.26a 表 4 不同菌种复配枸杞益生菌酸奶生物活性物质含量和抗氧化能力的相关性分析
Table 4 Correlation analysis of bioactives content and antioxidant capacity of probiotic yoghurt with different strains of compounded wolfberry
项目 DPPH自由基
清除率羟基自由基
清除率ABTS+自由基
清除率黄酮 0.97*** 0.85** 0.83* 多酚 0.98*** 0.81* 0.76* 多糖 0.23 −0.044 0.059 叶黄素 0.99*** 0.69 0.70 β-胡萝卜素 0.91** 0.57 0.70 玉米黄质双棕榈酸 0.93*** 0.49 0.56 注:*代表在P<0.05水平上显著相关;**代表在P<0.01水平上极显著相关;***代表在P<0.001水平上极显著相关。 表 5 多酚类代谢物表达变化
Table 5 Changes in polyphenol metabolite expression
分类 英文 中文 VIP Fold_Change P-value 上调/
下调酚酸类 Tropic acid 托品酸 2.69103 43.96354 0.00032 ↑ 2-Hydroxy-3-(4-Hydroxyphenyl)Propanoic Acid 2-羟基-(4-羟基苯基)丙酸 2.46840 24.99070 0.00006 ↑ 2-Hydroxy-3-phenylpropanoic acid 2-羟基-3-苯基丙酸 2.05092 9.23805 0.00054 ↑ 3-(4-Hydroxyphenyl)-propionic acid 3-(4-羟基苯基)丙酸 1.95445 7.56910 0.00274 ↑ 4-Hydroxybenzoic acid 4-羟基苯甲酸 1.20774 2.21776 0.00165 ↑ 4-Hydroxyphenyllactic Acid 4-羟苯基乳酸 2.51511 27.99221 0.00006 ↑ 4-Nitrophenol 4-硝基苯酚 1.43096 2.97245 0.00197 ↑ Benzoic acid 苯甲酸 1.89792 6.74534 0.00379 ↑ DL-3-Phenyllactic acid DL-3-苯基乳酸 1.86208 6.27952 0.00014 ↑ Methyl 2,4-dihydroxyphenylacetate 2,4-二羟基苯乙酸甲酯 2.45850 23.48919 0.00010 ↑ houttuynoside A 鱼腥草苷A 1.24285 0.39984 0.06018 ↓ p-Coumaric acid methyl ester 对香豆酸甲酯 2.47922 0.03955 0.00940 ↓ 2-Amino-3-methoxybenzoic acid 2-氨基-3-甲氧基苯甲酸 1.13797 0.49974 0.00030 ↓ Caffeic Acid 4-O-Glucoside 咖啡酸-4-O-葡萄糖苷 2.92903 0.01004 0.04063 ↓ Cimidahurinine 北升麻宁 1.91912 0.13367 0.03541 ↓ Ferulic acid methyl ester 阿魏酸甲酯 2.47197 0.03921 0.01264 ↓ 黄酮类 Carpusin Carpusin 2.69103 43.96354 0.00032 ↑ Hesperetin-8-C-glucoside-3'-O-glucoside 橙皮素-8-C-葡萄糖苷-3'-O-葡萄糖苷 2.46840 24.99070 0.00006 ↑ Isorhamnetin-3-O-(2''-O-glucosyl)galactoside-7-O-glucoside 异鼠李素-3-O-(2''-O-葡萄糖基)半乳糖苷-7-O-葡萄糖苷 2.05092 9.23805 0.00054 ↑ Kaempferol-3-O-glucoside 山柰酚-3-O-葡萄糖苷 1.95445 7.56910 0.00274 ↑ Coniferin 松柏苷 1.80815 5.63445 0.00100 ↑ Kaempferol-3-O-sophoroside-7-O-rhamnoside 山柰酚-3-O-槐糖苷-7-O-鼠李糖苷 1.20774 2.21776 0.00165 ↑ Quercetin-3-O-sophoroside 槲皮素-3-O-槐糖苷 2.51511 27.99221 0.00006 ↑ Quercetin-4'-O-glucoside 槲皮素-4'-O-葡萄糖苷 1.43096 2.97245 0.00197 ↑ Quercetin-7-O-glucoside 槲皮素-7-O-葡萄糖苷 1.89792 6.74534 0.00379 ↑ Diosmetin-7-O-rutinoside (Diosmin) 香叶木苷 1.24285 0.39984 0.06018 ↓ Gossypetin-7-O-L-rhamnopyranoside 棉黄素-7-O-L-吡喃鼠李糖苷 2.47922 0.03955 0.00940 ↓ Hesperetin-7-O-rutinoside 橙皮苷 1.13797 0.49974 0.00030 ↓ -
[1] 段昊, 刘改改, 松伟, 等. 枸杞子在我国保健食品中的应用[J]. 食品工业科技,2024,45(6):12−23. [DUAN H, LIU G G, SONG W, et al. Application of fruits of Lycium barharum L. in health food in China[J]. Science and Technology of Food Industry,2024,45(6):12−23.] DUAN H, LIU G G, SONG W, et al. Application of fruits of Lycium barharum L. in health food in China[J]. Science and Technology of Food Industry, 2024, 45(6): 12−23.
[2] LIANG J J, LI X H, LEI W Z, et al. Serum metabolomics combined with 16S rRNA sequencing to reveal the effects of Lycium barbarum polysaccharide on host metabolism and gut microbiota[J]. Food Research International,2023,165:112563. doi: 10.1016/j.foodres.2023.112563
[3] HUANG W Q, ZHAO M W, WANG X Y, et al. Revisiting the structure of arabinogalactan from Lycium barbarum and the impact of its side chain on anti-ageing activity[J]. Carbohydrate Polymers,2022,286:119282. doi: 10.1016/j.carbpol.2022.119282
[4] LIANG J L, YANG S, LIU Y Y, et al. Characterization and stability assessment of polyphenols bound to Lycium barbarum polysaccharide:Insights from gastrointestinal digestion and colon fermentation[J]. Food Research International,2024,179:114036. doi: 10.1016/j.foodres.2024.114036
[5] LIU F, LIU X B, ZHOU Y M, et al. Wolfberry-derived zeaxanthin dipalmitate delays retinal degeneration in a mouse model of retinitis pigmentosa through modulating STAT3, CCL2 and MAPK pathways[J]. Journal of Neurochemistry,2021,158(5):1131−1150. doi: 10.1111/jnc.15472
[6] SHORI A B, ALJOHANI G S, AL-ZAHRANI A J, et al. Viability of probiotics and antioxidant activity of cashew milk-based yogurt fermented with selected strains of probiotic Lactobacillus spp[J]. LWT,2022,153:112482. doi: 10.1016/j.lwt.2021.112482
[7] GUNENC A, KHOURY C, LEGAULT C, et al. Seabuckthorn as a novel prebiotic source improves probiotic viability in yogurt[J]. LWT-Food Science and Technology,2016,66:490−495. doi: 10.1016/j.lwt.2015.10.061
[8] ZYGMANTAITĖ G, KERŠIENĖ M, JASUTIENĖ I, et al. Extract isolated from cranberry pomace as functional ingredient in yoghurt production:Technological properties and digestibility studies[J]. LWT,2021,148:111751. doi: 10.1016/j.lwt.2021.111751
[9] SIGDEL A, OJHA P, KARKI T B. Phytochemicals and syneresis of osmo-dried mulberry incorporated yoghurt[J]. Food Science & Nutrition,2018,6(4):1045−1052.
[10] WANG Y, QU S J, CHEN M H, et al. Effects of buckwheat milk Co-fermented with two probiotics and two commercial yoghurt strains on gut microbiota and production of short-chain Fatty Acids[J]. Food Bioscience,2023,53:102537. doi: 10.1016/j.fbio.2023.102537
[11] GOUDA A S, ADBELRUHMAN F G, SABBAH ALENEZI H, et al. Theoretical benefits of yogurt-derived bioactive peptides and probiotics in COVID-19 patients–A narrative review and hypotheses[J]. Saudi Journal of Biological Sciences,2021,28(10):5897−905. doi: 10.1016/j.sjbs.2021.06.046
[12] AZEEZ KHALID ALBAYATI A, AĞÇAM E, KARACA O B, et al. Effects of prickly pear supplementation on physico-chemical, textural, microbiological and sensory characteristics of probiotic set yoghurts[J]. Food Bioscience,2024,60:104513. doi: 10.1016/j.fbio.2024.104513
[13] FAN X K, SHI Z H, XU J, et al. Characterization of the effects of binary probiotics and wolfberry dietary fiber on the quality of yogurt[J]. Food Chemistry,2023,406:135020. doi: 10.1016/j.foodchem.2022.135020
[14] VESELÁ K, KUMHEROVÁ M, KLOJDOVÁ I, et al. Selective culture medium for the enumeration of Lactobacillus plantarum in the presence of Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus[J]. LWT,2019,114:108365. doi: 10.1016/j.lwt.2019.108365
[15] DEMIRCI T, SERT D, AKTAŞ K, et al. Influence of hot and cold break tomato powders on survival of probiotic L. paracasei subsp. paracasei F19, texture profile and antioxidative activity in set-type yoghurts[J]. Lwt,2020,118:108855. doi: 10.1016/j.lwt.2019.108855
[16] KAN X H, YAN Y M, RAN L W, et al. Ultrasonic-assisted extraction and high-speed counter-current chromatography purification of zeaxanthin dipalmitate from the fruits of Lycium barbarum L[J]. Food Chemistry,2020,310:125854. doi: 10.1016/j.foodchem.2019.125854
[17] TRIGUEROS L, WOJDYŁO A, SENDRA E. Antioxidant activity and protein–polyphenol interactions in a pomegranate (Punica granatum L.) yogurt[J]. Journal of Agricultural and Food Chemistry,2014,62(27):6417−6425. doi: 10.1021/jf501503h
[18] WANG M Z, WANG J, FU L L, et al. Degradation of polysaccharides from Lycium barbarum L. leaves improves bioaccessibility and gastrointestinal transport of endogenous minerals[J]. International Journal of Biological Macromolecules,2020,143:76−84. doi: 10.1016/j.ijbiomac.2019.11.243
[19] 朱晓雪, 龚绵红, 杨秉坤, 等. 不同种类及加工方式对杂粮酸奶体外抗氧化活性的比较[J]. 农业工程学报,2023,39(8):268−275. [ZHU X X, GONG M H, YANG B K et al. Rative study on antioxidant activity of multigrain yoghurt bydifferent types and processing methods of coarse cereals[J]. Transactions of the Chinese Society of Agricultural Engineering,2023,39(8):268−275.] doi: 10.11975/j.issn.1002-6819.202301132 ZHU X X, GONG M H, YANG B K et al. Rative study on antioxidant activity of multigrain yoghurt bydifferent types and processing methods of coarse cereals[J]. Transactions of the Chinese Society of Agricultural Engineering, 2023, 39(8): 268−275. doi: 10.11975/j.issn.1002-6819.202301132
[20] XU K Q, FAN G J, WU C, et al. Preparation of anthocyanin-rich mulberry juice by microwave-ultrasonic combined pretreatment[J]. Food Science and Biotechnology,2022,31(12):1571−1581. doi: 10.1007/s10068-022-01147-3
[21] 孙正霄, 肖顺丽, 刘陆, 等. 枸杞咀嚼片免疫调节及抗氧化活性分析[J]. 中国实验方剂学杂志,2022,28(8):46−53. [SUN Z H, XIAO S L, LIU L, et al. Immunomodulatory and antioxidant activity of gouqi chewable tablets[J]. Chinese Journal of Experimental Traditional Medical Formulae,2022,28(8):46−53.] SUN Z H, XIAO S L, LIU L, et al. Immunomodulatory and antioxidant activity of gouqi chewable tablets[J]. Chinese Journal of Experimental Traditional Medical Formulae, 2022, 28(8): 46−53.
[22] CHEN G J, ZHANG S Q, RAN C X, et al. Extraction, characterization and antioxidant activity of water-soluble polysaccharides from Tuber huidongense[J]. International Journal of Biological Macromolecules,2016,91:431−442. doi: 10.1016/j.ijbiomac.2016.05.108
[23] LIU Y H, LÜ J H, LIU Z B, et al. Integrative analysis of metabolome and transcriptome reveals the mechanism of color formation in pepper fruit (Capsicum annuum L.)[J]. Food Chemistry,2020,306:125629. doi: 10.1016/j.foodchem.2019.125629
[24] FAN X K, ZHANG A, ZHANG T, et al. Effects of Semen Ziziphi Spinosae extract and binary probiotics co-fermentation on the quality of yogurt and their underlying molecular mechanisms[J]. Food Chemistry:X,2024,21:101191.
[25] DU H X, WANG X P, YANG H G, et al. Regulation on the quality of yogurt by phenolic fraction of mulberry pomace supplemented before and after fermentation[J]. Food Control,2023,144:109333. doi: 10.1016/j.foodcont.2022.109333
[26] ISLAM F, KHAN J, ZEHRAVI M, et al. Synergistic effects of carotenoids:Therapeutic benefits on human health[J]. Process Biochemistry,2024,136:254−272. doi: 10.1016/j.procbio.2023.11.033
[27] HONDA M. Z-Isomers of lycopene and β-carotene exhibit greater skin-quality improving action than their all-E-isomers[J]. Food Chemistry,2023,421:135954. doi: 10.1016/j.foodchem.2023.135954
[28] LINNEWIEL-HERMONI K, KHANIN M, DANILENKO M, et al. The anti-cancer effects of carotenoids and other phytonutrients resides in their combined activity[J]. Archives of Biochemistry and Biophysics,2015,572:28−35. doi: 10.1016/j.abb.2015.02.018
[29] 雷建武, 米佳, 罗青, 等. 枸杞中类胡萝卜素及体外抗氧化活性研究[J]. 食品工业,2015,36(12):5−8. [LEI J W, MI J, LUO Q, et al. Composition and antioxidant activity of different varieties of lycium[J]. The Food Industry,2015,36(12):5−8.] LEI J W, MI J, LUO Q, et al. Composition and antioxidant activity of different varieties of lycium[J]. The Food Industry, 2015, 36(12): 5−8.
[30] PEREIRA A L F, MACIEL T C, RODRIGUES S. Probiotic beverage from cashew apple juice fermented with Lactobacillus casei[J]. Food Research International,2011,44(5):1276−1283. doi: 10.1016/j.foodres.2010.11.035
[31] BOON C S, MCCLEMENTS D J, WEISS J, et al. Factors influencing the chemical stability of carotenoids in foods[J]. Critical Reviews in Food Science and Nutrition,2010,50(6):515−532. doi: 10.1080/10408390802565889
[32] 段昊, 刘改改, 松伟, 等. 枸杞子在我国保健食品中的应用[J/OL]. 食品工业科技, 1−20[2024-03-06]. https://doi.org/10.13386/j.issn1002-0306.2023060225. [DUAN H, LIU G G, SONG W, et al. Application of fruits of Lycium barharum L. in health food in China[J]. Science and Technology of Food Industry, 1−20[2024-03-06]. https://doi.org/10.13386/j.issn1002-0306.2023060225.] DUAN H, LIU G G, SONG W, et al. Application of fruits of Lycium barharum L. in health food in China[J]. Science and Technology of Food Industry, 1−20[2024-03-06]. https://doi.org/10.13386/j.issn1002-0306.2023060225.
[33] LIU S, HU J L, ZHONG Y D, et al. A review:Effects of microbial fermentation on the structure and bioactivity of polysaccharides in plant-based foods[J]. Food Chemistry,2024,440:137453. doi: 10.1016/j.foodchem.2023.137453
[34] QI J, HUANG H, WANG J, et al. Insights into the improvement of bioactive phytochemicals, antioxidant activities and flavor profiles in Chinese wolfberry juice by select lactic acid bacteria[J]. Food Bioscience,2021,43:101264. doi: 10.1016/j.fbio.2021.101264
[35] KWAW E, MA Y, TCHABO W, et al. Effect of lactobacillus strains on phenolic profile, color attributes and antioxidant activities of lactic-acid-fermented mulberry juice[J]. Food Chemistry,2018,250:148−154. doi: 10.1016/j.foodchem.2018.01.009
[36] CHENG Z Y, LI Y Z, CHANG W B. Kinetic deoxyribose degradation assay and its application in assessing the antioxidant activities of phenolic compounds in a Fenton-type reaction system[J]. Analytica Chimica Acta,2003,478(1):129−37. doi: 10.1016/S0003-2670(02)01435-6
[37] SIHAG S, PAL A, RAVIKANT, et al. Antioxidant properties and free radicals scavenging activities of pomegranate (Punica granatum L.) peels:An in vitro study[J]. Biocatalysis and Agricultural Biotechnology,2022,42:102368. doi: 10.1016/j.bcab.2022.102368
[38] LIZARDO R C M, CHO H D, WON Y S, et al. Fermentation with mono-and mixed cultures of Lactobacillus plantarum and L. casei enhances the phytochemical content and biological activities of cherry silverberry (Elaeagnus multiflora Thunb.) fruit[J]. Journal of the Science of Food and Agriculture,2020,100(9):3687−3696. doi: 10.1002/jsfa.10404
[39] LIU R Y, LAN H N, LIU Z H, et al. Microbial valorization of lignin toward coumarins:Challenges and perspectives[J]. Renewable and Sustainable Energy Reviews,2024,191:114205. doi: 10.1016/j.rser.2023.114205
[40] GAUR G, GÄNZLE M G. Conversion of (poly)phenolic compounds in food fermentations by lactic acid bacteria:Novel insights into metabolic pathways and functional metabolites[J]. Current Research in Food Science,2023,6:100448. doi: 10.1016/j.crfs.2023.100448
[41] XIAO Y, HE C, CHEN Y L, et al. UPLC–QQQ–MS/MS-based widely targeted metabolomic analysis reveals the effect of solid-state fermentation with Eurotium cristatum on the dynamic changes in the metabolite profile of dark tea[J]. Food Chemistry,2022,378:131999. doi: 10.1016/j.foodchem.2021.131999
[42] NURKENOV O A, FAZYLOV S D, SATPAEVA Z B, et al. Synthesis, structure and biological activity of hydrazones derived from 2- and 4-hydroxybenzoic acid hydrazides[J]. Chemical Data Collections,2023,48:101089. doi: 10.1016/j.cdc.2023.101089
[43] LOFFT Z, TAIBI A, MASSARA P, et al. Cranberry proanthocyanidin and its microbial metabolite 3, 4-dihydroxyphenylacetic acid, but Not 3-(4-Hydroxyphenyl)-propionic acid, partially reverse pro-inflammatory microRNA responses in human intestinal epithelial cells[J]. Molecular Nutrition & Food Research,2022,66(8):2100853.
[44] MU W M, YANG Y, JIA J H, et al. Production of 4-hydroxyphenyllactic acid by Lactobacillus sp. SK007 fermentation[J]. Journal of Bioscience and Bioengineering,2010,109(4):369−371. doi: 10.1016/j.jbiosc.2009.10.005
[45] LUO Z W, LEE S Y. Metabolic engineering of Escherichia coli for the production of benzoic acid from glucose[J]. Metabolic Engineering,2020,62:298−311. doi: 10.1016/j.ymben.2020.10.002
[46] YUAN K L, WU G K, LI X S, et al. Anthocyanins degradation mediated by β-glycosidase contributes to the color loss during alcoholic fermentation in a structure-dependent manner[J]. Food Research International,2024,175:113732. doi: 10.1016/j.foodres.2023.113732
[47] SI J Y, XIE J Y, ZHENG B, et al. Release characteristic of bound polyphenols from tea residues insoluble dietary fiber by mixed solid-state fermentation with cellulose degrading strains CZ-6 and CZ-7[J]. Food Research International,2023,173:113319. doi: 10.1016/j.foodres.2023.113319
[48] LEONARD W, ZHANG P, YING D, et al. Fermentation transforms the phenolic profiles and bioactivities of plant-based foods[J]. Biotechnology Advances,2021,49:107763. doi: 10.1016/j.biotechadv.2021.107763
[49] JI Z K, DENG W, CHEN D, et al. Recent understanding of the mechanisms of the biological activities of hesperidin and hesperetin and their therapeutic effects on diseases[J]. Heliyon,2024,10(5):e26862. doi: 10.1016/j.heliyon.2024.e26862
[50] LEE Y S, HUH J Y, NAM S H, et al. Enzymatic bioconversion of citrus hesperidin by Aspergillus sojae naringinase:Enhanced solubility of hesperetin-7-O-glucoside with in vitro inhibition of human intestinal maltase, HMG-CoA reductase, and growth of Helicobacter pylori[J]. Food Chemistry,2012,135(4):2253−2259. doi: 10.1016/j.foodchem.2012.07.007
[51] CHOI E H, CHUN Y S, KIM J, et al. Modulating lipid and glucose metabolism by glycosylated kaempferol rich roasted leaves of Lycium chinense via upregulating adiponectin and AMPK activation in obese mice-induced type 2 diabetes[J]. Journal of Functional Foods,2020,72:104072. doi: 10.1016/j.jff.2020.104072
[52] WU W X, LUO X M, WANG Y, et al. Combined metabolomics and transcriptomics analysis reveals the mechanism underlying blue light-mediated promotion of flavones and flavonols accumulation in Ligusticum chuanxiong Hort. microgreens[J]. Journal of Photochemistry and Photobiology B:Biology,2023,242:112692. doi: 10.1016/j.jphotobiol.2023.112692





 下载:
下载:
 下载:
下载:



