Research Progress on Mitochondrial Protective Effects of Plant Polyphenols
-
摘要: 植物多酚是一类广泛存在于自然界的天然酚类化合物,具有抗氧化、抗炎、抗肿瘤、保护心脑血管等生物活性。线粒体作为细胞的能量工厂,其功能障碍与人体代谢异常及疾病的发展密切相关。近年来,植物多酚在保护线粒体功能方面的作用受到广泛关注。本文总结了植物多酚的分类和来源,重点讨论了植物多酚通过调控线粒体活性氧自由基以及钙离子水平、提高线粒体呼吸链酶活性、调节线粒体的分裂融合和自噬的方式,发挥线粒体保护作用;并且在治疗与线粒体功能障碍相关的糖尿病、肺部疾病、脂肪性肝病、心血管疾病以及神经退行性疾病中具有潜在作用。通过总结多酚对线粒体的保护作用,有利于科研人员深入探索多酚与线粒体功能调控之间的构效关系以及解析其之间存在的分子机制。这项工作不仅可以为开发新型植物源功能性成分提供科学基础,也为人类健康的饮食干预提供了新的视角。Abstract: Plant polyphenols are a class of natural phenolic compounds widely found in nature, known for their significant biological activities such as antioxidant, anti-inflammatory, anti-tumor and cardiovascular and cerebrovascular protective effects. Mitochondria are the energy factories of cells, and their dysfunctions are closely related to human metabolic abnormalities and the development of diseases. In recent years, the role of plant polyphenols in protecting mitochondrial function has received widespread attention. This review summarizes the classification and sources of plant polyphenols, and focuses on their mitochondrial protective effects, which include regulating the levels of mitochondrial reactive oxygen species as well as calcium ions, increasing the activity of mitochondrial respiratory chain enzymes, and regulating mitochondrial fission, fusion and autophagy. These properties with the potential roles contribute to treat diabetes mellitus, lung diseases, fatty liver disease, cardiovascular diseases, and neurodegenerative diseases associated with mitochondrial dysfunction. By summarizing the protective effects of polyphenols on mitochondria, this review will be beneficial for researchers to explore the conformational relationship between polyphenols and the regulation of mitochondrial function and to analyze the molecular mechanisms involved. The review not only provides a scientific basis for the development of novel plant-derived functional ingredients, but also provides a new perspective for dietary intervention on human diseases.
-
Keywords:
- plant polyphenols /
- mitochondria /
- dysfunction /
- diseases /
- mechanisms of protective effect
-
植物多酚是一种以苯酚为基本骨架,具有多个酚羟基基团的植物次生代谢物的总称[1],目前自然界已发现的天然多酚超过8000种。许多研究表明,没食子酸、白藜芦醇、槲皮素、姜黄素等植物多酚具有广泛的功能活性[2]。表没食子儿茶素没食子酸酯(Epigallocatechin gallate,EGCG)、竹叶黄酮,二氢槲皮素、甘蔗多酚先后被批准为我国新资源食品[3−6]。2024年多酚的全球市场容量达到1247万吨,预计2030年达到1400万吨,市场前景十分广阔[7]。
线粒体是动态的双膜结合细胞器,在各种细胞中发挥关键作用。线粒体内活性氧自由基水平、钙离子水平的失调、呼吸链酶活性的降低、分裂融合的失调已被证明与糖尿病、肺部疾病、脂肪性肝病、心血管疾病和神经退行性疾病等疾病的发生和发展有关[8]。因此,在探索对线粒体功能障碍相关疾病有潜在治疗作用的天然植物化学物中,植物多酚由于具有降血糖、抗氧化、降血脂、抗肿瘤、调节代谢等多种多样的生物活性而被广泛研究[9]。多酚中苯环上的羟基能够通过清除线粒体内过量的活性氧自由基并提高抗氧化酶的活性,抑制氧化应激,从而发挥对线粒体的保护作用,缓解糖尿病、心血管疾病、神经性退行性疾病、脂肪性肝病等[10−11]。EGCG是从绿茶中分离出的主要多酚类化合物,可调节线粒体代谢,包括线粒体生物能量学以及线粒体介导的细胞凋亡,有利于预防肝脂肪变性[12−13]。从姜黄根茎中提取的姜黄素,通过调节多种线粒体相关的信号通路,对急性肺损伤、哮喘、慢性阻塞性肺疾病等肺部疾病具有治疗潜力[14]。
目前已有大量研究探索了多酚在线粒体中发挥生物活性的分子机制及其构效关系。因此,本文对国内外近年来有关植物多酚对线粒体保护作用的研究报道进行了综合整理,总结了植物多酚中类黄酮、酚酸、木脂素和芪类化合物的结构特征及相关来源,不同结构的多酚对线粒体的保护作用,以及对线粒体功能障碍相关疾病的潜在治疗作用,以期为多酚化合物对线粒体保护的分子机制的解析提供一定的参考。
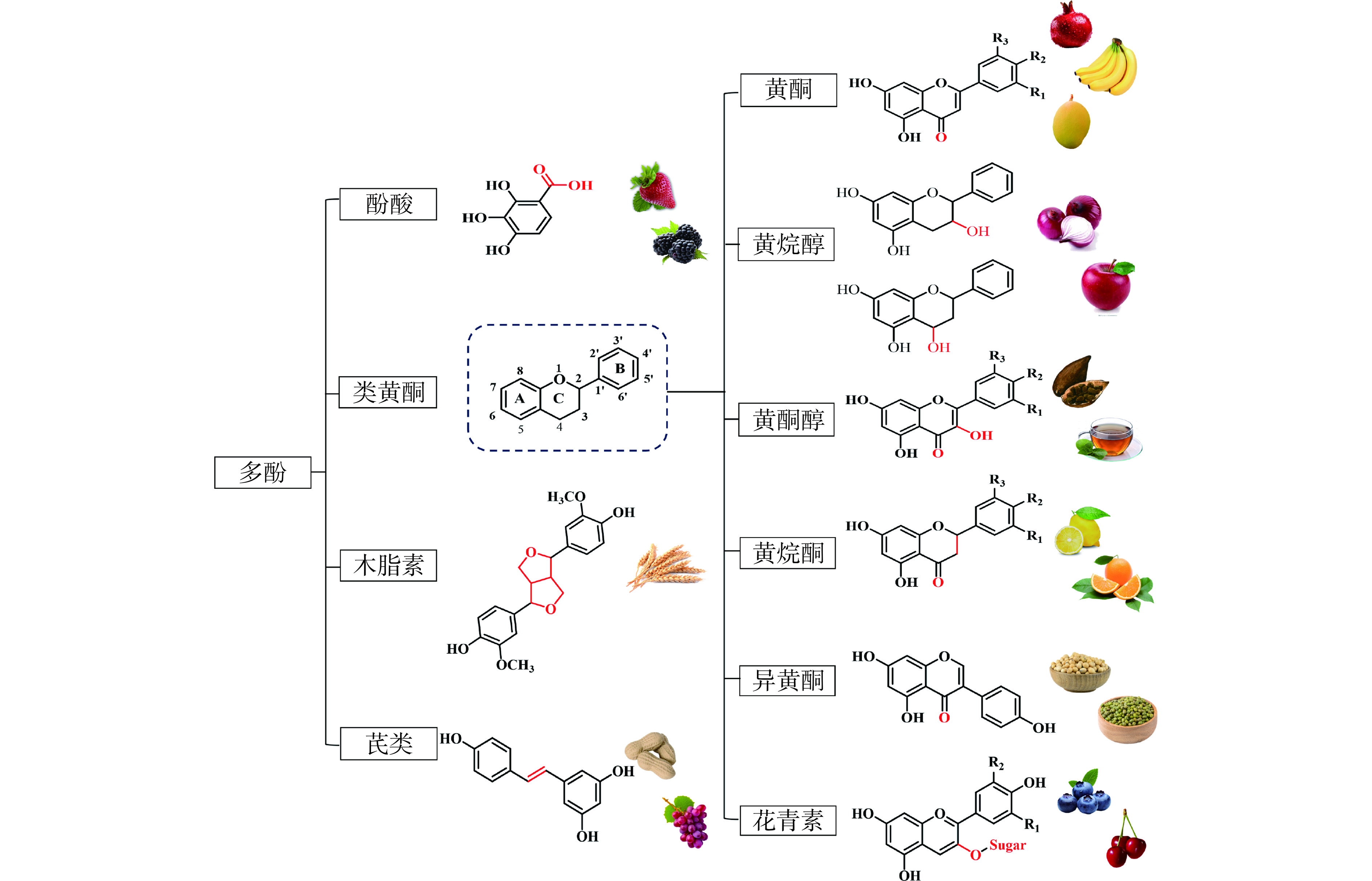
1. 植物多酚的分类和来源
1.1 植物多酚的分类
植物多酚具有独特的分子构象,根据其化学结构特点可以分为四类:分别是类黄酮、酚酸、木脂素和芪类化合物。类黄酮化合物具有常见的C6-C3-C6结构,其中酚环(环A和环B)由杂环(环C)连接。这个杂环(环C)通常是一个闭合的吡喃,如图1所示。类黄酮物质分子的结构多样性源于中心吡喃环的羟基化形式和氧化态的变化,包含黄酮、异黄酮、黄酮醇、黄烷醇、黄烷酮、花青素等多个不同的亚类[15]。木犀草素和芹菜素是常见的黄酮类化合物。异黄酮的基本结构与黄酮的区别在于异黄酮的环B位于C3的位置上,主要包括黄豆苷元、染料木黄酮等;黄酮醇具有3-羟基黄酮骨架,根据酚羟基的位置,存在数百种不同的黄酮醇,包括槲皮素、异槲皮素、山奈酚、漆黄素等[16−17]。黄烷酮的结构特点为在类黄酮的C4上连接了羰基,其代表性物质为柚皮素和橙皮素;黄烷醇是一类多元酚类化合物,主要有黄烷-3-醇和黄烷-4-醇,代表性物质为儿茶素、表儿茶素、没食子儿茶素、表没食子儿茶素、EGCG等[18]。而花青素是多羟基和多甲氧基2-苯基-苯并吡喃的衍生物,构成了最大的水溶性植物色素。目前在自然界中发现的花青素种类已超过600种,如矢车菊素、天竺葵素、飞燕草素等[19−21]。酚酸类化合物根据其所含羧基的链长不同,可以分为羟基苯甲酸和羟基肉桂酸两类,其基本骨架分别为C6-C1和C6-C3,其中包括没食子酸、绿原酸、阿魏酸、咖啡酸、对羟基苯甲酸、香草酸等[22]。木脂素类化合物由两个苯丙酮单元组成,其基本骨架为C6-C3-C3-C6,包括松脂素、表松脂醇、丁香树脂酚等[23−24]。由两个芳香环通过碳碳双键相连组成的芪类化合物具有常见的C6-C2-C6结构,包括白藜芦醇、白藜芦醇苷、白皮杉醇等[25]。
1.2 植物多酚的来源
自然界的天然多酚多数存在于木本植物,在植物的根、茎、叶中都有所分布,并且作为重要的天然植物活性成分,被广泛应用于国内外功能性食品和膳食补充剂中[26]。在类黄酮化合物中,不同的亚类来源具有明显的差异性。Tsao[15]研究表明黄酮类物质在石榴、香蕉、芒果等果蔬中较为常见;并且黄酮醇类物质作为食品中最普遍的类黄酮化合物,其最丰富的来源是洋葱、羽衣甘蓝、韭菜、西兰花和蓝莓等[27]。黄烷酮类物质在柑橘属的水果中含量丰富,包括葡萄、橙子、血橙、柠檬和葡萄柚等[28]。黄烷醇类物质多存在于谷物、豆类、水果、啤酒、红酒、茶、可可和苹果等常见的食物中[27]。异黄酮类物质主要存在于豆科植物及其制品中,例如大豆、红车轴草和苜蓿等[29]。花青素属于水溶性色素,分布在许多植物组织的液泡中,浆果类含量较高[21]。而非类黄酮化合物中,酚酸类物质在中草药中的含量很高,其中最常见的咖啡酸、鼠尾草酸、阿魏酸、没食子酸、对香豆酸、迷迭香、香草酸已被确定为中药物质牛至、花椒、迷迭香、鼠尾草、薄荷等的成分[30];Berenshtein等[31]研究表明亚麻籽、芝麻等种子是木脂素类化合物中最丰富的膳食来源,但在其他谷物、水果、某些蔬菜和饮料中含量较低,例如荞麦、葡萄柚、西兰花、红酒等。芪类化合物天然存在于葡萄、蓝莓、红酒中,其中白藜芦醇已被广泛研究,多存在于葡萄皮、浆果、花生和一些药用植物中[32]。
此外,许多农产品的副产物中富含活性多酚化合物,板栗壳中含有类黄酮、酚酸等多酚类物质,如槲皮素、山奈酚、没食子酸以及大量单宁类物质[33];牡丹籽粕、牡丹叶、牡丹籽种皮中都含有白藜芦醇、对羟基苯甲酸、咖啡酸等[34−35]。Rojas-García等[36]研究表明葡萄籽和葡萄皮提取物中富含白藜芦醇、儿茶素和没食子酸。荔枝籽提取物中富含儿茶素和原花青素。多酚类物质广泛分布于水果、蔬菜以及农产品副产物中,拥有巨大的开发潜力。本文关于多酚类物质对线粒体保护作用的综述,将总结植物多酚作为食源性成分治疗线粒体功能障碍及线粒体功能障碍相关疾病的潜力。
2. 多酚的线粒体保护作用
多酚具有独特的分子构象和生物活性,随着研究的深入,多酚对机体的保护作用机制逐渐被揭示。多酚可以通过调节线粒体活性氧自由基水平、调节线粒体钙离子水平、提高线粒体呼吸链酶活性、调节线粒体分裂融合的失衡及自噬的异常缓解线粒体功能障碍,从而干预糖尿病、肺部疾病、脂肪性肝病、心血管疾病、神经退行性疾病的发生发展,如图2所示。多酚的这些调控作用可能通过活性氧自由基的直接清除,也可以通过对上游基因通路的调控,以及直接与线粒体蛋白质发生相互作用,影响线粒体功能的发挥等。
2.1 调节线粒体ROS的水平
多酚类化合物的多羟基结构赋予其还原性,能够清除细胞内过量的活性氧自由基(Reactive oxygen species,ROS),降低氧化应激损伤;多酚除了直接清除ROS外,还可以通过提高一些内源性抗氧化酶活性,降低ROS的含量,进而降低线粒体的氧化应激损伤。重要的是,线粒体作为细胞内ROS的主要产生部位,维持其内部ROS水平的平衡对于细胞的正常生理功能具有重要作用[37]。ROS的过载会导致线粒体中氧化产物积聚,线粒体DNA和线粒体蛋白发生氧化损伤,过氧化物含量增加,线粒体膜电位降低,抗氧化作用被抑制,以及线粒体的正常生理功能受阻等一系列不良反应,甚至与糖尿病、肺部疾病、脂肪性肝病、心血管疾病的发生有关,最终对机体造成不可逆的影响[38−46]。首先,多酚类物质直接清除ROS的机制为多酚结构中与苯环结合的羟基向ROS提供一个氢原子或单个电子,生成苯氧基自由基;之后与第二个自由基反应后,形成稳定的醌结构[47]。Sarkar等[48]研究表明在类黄酮化合物中环B中的邻二羟基(邻苯二酚)结构、与环C中的2,3-双键、4-氧代基团、3-和5-羟基三个结构对于自由基的清除作用十分重要。并且儿茶酚以及邻苯三酚的存在可增强类黄酮和其他酚类物质的自由基清除活性。但是多酚的直接抗氧化活性在体内似乎是无效的,更多可能是多酚通过调节基因表达和诱导内源性抗氧化酶防御系统发挥间接抗氧化作用。
内源性抗氧化酶主要包括超氧化物歧化酶(Superoxide dismutase,SOD)、谷胱甘肽过氧化物酶(Glutathione peroxidase,GSH-Px)和过氧化氢酶(Catalase,CAT)共同协作,将活性氧物质转化为无害的物质,从而保护线粒体免受氧化损伤[49]。石榴中的安石榴甙通过提高II型糖尿病小鼠体内的SOD和CAT等抗氧化酶的水平,提高线粒体膜电位,降低线粒体氧化应激的损伤[50]。Palsamy等[51]研究表明白藜芦醇可以通过提高受损CAT、GSH-Px 和SOD的酶活性,增加胰岛活力,进而恢复Ⅱ型糖尿病大鼠胰岛细胞中的线粒体损伤。单宁酸在体内可以通过靶向受损线粒体,促进级联反应清除ROS,改善线粒体膜电位,进而恢复线粒体功能[52]。
Calabrese[53]发现大多数多酚在高浓度下显示出细胞毒性,而在低剂量下触发轻度化学应激,导致保护基因的表达增加。通过进一步研究发现,多酚可以通过激活NF-E2相关因子2 (NF-E2-related factor 2, Nrf2)/抗氧化反应元件(Antioxidant response elements,ARE)信号通路上调抗氧化酶的表达,进而在体内发挥抗氧化作用[54]。在正常细胞中Nrf2主要存在于细胞质中处于非活性状态,受到外来刺激后Nrf2转运至细胞核中与ARE结合。付凯等[55]研究表明茶多酚通过激活Nrf2/HO-1(HO-1是最易被Nrf2激活的抗氧化酶)抗氧化通路,减轻由阿司匹林诱导的胃黏膜上皮细胞(GES-1)的氧化应激损伤。此外,多酚还通过调控沉默信息调节因子(Silent information regulator two,SIRT)、核因子κB(Nuclear factor kappa-B,NF-κB)等信号通路参与机体的抗氧化过程。白藜芦醇可以激活存在于细胞核和线粒体中的沉默蛋白(Sir2-related enzymes,Sirtuin)中SIRT1和SIRT3,进一步改善线粒体代谢,减少ROS的产生,降低氧化应激损伤,调节糖尿病等代谢性疾病[56]。石榴皮多酚以抑制脂多糖诱导的核因子κB的抑制蛋白(Inhibitor of NF-κB,IκB-α)表达,调控NF-κB的活性,从而阻碍Caspase级联反应诱发的结肠细胞损伤,显著降低ROS水平,恢复线粒体膜电位,抑线粒体通透转换孔的开放程度[57]。虽然多酚对于ROS的调节机制已有研究,但是具体的构效关系及相关通路的联系仍需要进一步解析。
2.2 调节线粒体钙离子水平
多酚不仅能够维持正常细胞内钙离子稳态,还能通过增加细胞内钙离子的含量促进癌细胞的死亡。Cherubini等[58]研究表明钙离子水平的失调是神经退行性疾病的重要标志。多酚类物质可以通过降低钙离子通道的通透性,使线粒体内部钙离子处于平稳的水平。线粒体作为调节细胞质钙离子水平的关键细胞器,可以通过线粒体通透性转换孔(Mitochondrial permeability transition pore,mPTP)、瞬时受体电位M2(Transient receptor potential melastatin 2,TRPM2)通道及其相关调节因子将钙离子跨线粒体内膜转运到线粒体基质[59]。钙离子在线粒体中不仅可以控制三磷酸腺苷(Adenosine triphosphate,ATP)的产生,也会影响细胞对代谢应激的反应[60]。mPTP是依赖于钙离子浓度的非特异性通道,存在正常的低电导状态和异常的高电导状态。当线粒体基质中钙离子过载会使mPTP处于高电导状态,释放细胞色素C,最终导致细胞凋亡[61]。在中枢神经系统的神经元兴奋性毒性损伤中发现线粒体钙离子过载会引发mPTP打开以及线粒体肿胀。亲环蛋白D(Cyclophilin D,CypD)存在于线粒体基质中,是目前唯一在基因上证实与mPTP开放有关的线粒体蛋白。Wu等[62]研究发现EGCG可以与CypD相互作用,可以抑制CypD的激活。通过抑制CypD的激活可以进一步阻止mPTP的打开,对于预防心血管疾病、神经退行性疾病、脂肪性肝病具有重要作用[63−64]。TRPM2通道是可渗透钙离子的非选择性阳离子通道,在神经退行性疾病中发挥重要作用[65]。姜黄素还可以通过抑制TRPM2通道,抑制线粒体钙离子水平过载,对脂肪性肝损伤有治疗的潜力[66]。另外,丹参多酚酸可以显著降低缺血性中风恢复期内细胞中钙离子浓度、ROS的释放量,提高线粒体的膜电位,抑制细胞的凋亡[67]。辛庆峰等[68]发现异鼠李素可以通过改善细胞内钙离子稳态、降低线粒体的功能损伤以及线粒体凋亡通路中细胞色素C和Caspase-9蛋白表达,有效减轻阿霉素对于大鼠心肌的损伤。在癌细胞中,一些多酚可以进一步诱发钙离子失调,从而促进细胞凋亡。白藜芦醇通过增强线粒体对钙离子的摄取,造成钙离子过载以及mPTP打开,最终导致癌细胞的死亡[69]。姜黄素可以提高人肝细胞癌细胞系(HepG2)的钙离子水平,破坏线粒体膜电位,导致线粒体膜凋亡素的释放和细胞的凋亡[70]。植物多酚对于钙离子进入线粒体的机制仍不清晰,更多结果只能显示出多酚对于钙离子水平调控的结果,对于调控的过程缺乏深入的研究。
2.3 影响线粒体呼吸链的酶活性
多酚通过促进线粒体呼吸链酶的活性,进而提高线粒体ATP的合成效率。线粒体通过完整的呼吸链合成高能磷酸化合物—ATP,为细胞乃至机体的生命活动提供能量。该呼吸链是按序排列的一系列反应体系,主要由复合物I(NADH泛醌氧化还原酶)、复合物Ⅱ(琥珀酸脱氢酶)、复合物Ⅲ(细胞色素C还原酶)、复合物Ⅳ(细胞色素C氧化酶)、复合物Ⅴ(ATP合酶)这五种酶复合物、辅酶Q以及细胞色素C构成,对于细胞的生命活动具有非常重要的作用[71]。复合物V通过利用复合物I~IV产生的质子梯度将ADP磷酸化生成ATP。因此,呼吸链复合物的酶活性对于ATP的产生效率有很关键的作用。低剂量的白藜芦醇通过与烟酰胺腺嘌呤二核苷酸(NAD+)竞争的方式结合在线粒体复合物Ⅰ上,增强其活性[72]。除此之外,受白藜芦醇影响的另一个靶点是ATP合酶。研究表明,微摩尔至纳摩尔浓度的白藜芦醇能够提高大鼠肝线粒体ATP合酶的活性[73]。葛根素通过提高呼吸链复合物Ⅰ、Ⅱ的活性,改善线粒体的呼吸,调节NLRP3-caspase-1-GSDMD介导的细胞焦亡通路,对心血管疾病具有治疗潜力[74]。Yoshida等[75]发现苹果多酚可以提高呼吸链复合物Ⅱ、Ⅲ和Ⅳ的活性,从而提高线粒体的ATP合成效率,增强肌肉耐力。在脾淋巴细胞中,核桃多酚可以抑制马拉硫磷对线粒体呼吸链的氧化毒性,恢复线粒体呼吸链复合物Ⅰ、Ⅱ的活性,保护脾淋巴细胞[76]。衔接蛋白p66Shc是一种氧化还原酶,以线粒体依赖性方式产生ROS,诱导心血管疾病、糖尿病等疾病的发生[77−78]。目前,已有研究表明,沉默或抑制p66Shc可以减轻线粒体功能损伤,进而缓解对于细胞的损害。并且该蛋白在心血管疾病、糖尿病等疾病中具有重要作用[79]。Song等[80]利用p66Shc作为靶点,发现原花青素通过下调p66Shc的表达,使线粒体呼吸链复合物的酶活性提高,进而保护线粒体的功能,抑制细胞的凋亡。
但是某些多酚类化合物对线粒体呼吸链具有不良的影响,例如法国海洋松树皮多酚通过NADH-泛醌、琥珀酸-泛醌和泛醇-细胞色素C还原酶抑制线粒体呼吸链酶活性[81]。黄酮的核心结构中C4-酮基和C2,3双键主要作用位点为呼吸链复合物Ⅰ和Ⅲ,通过相互作用表现出一定的细胞毒性[82]。因此,多酚类物质对于线粒体的呼吸链酶活性的作用随着细胞生理状态以及多酚的化学结构的不同而有所不同。
2.4 调节线粒体的分裂融合
多酚通过促进线粒体的分裂融合,提高线粒体的质量。线粒体为了维持其数量和形态的稳定性,维持细胞内的氧化磷酸化(Oxidative phosphorylation,OXPHOS),不断进行分裂与融合[83]。线粒体的分裂融合通过维持线粒体的含量和质量干预心血管疾病的发生发展[84]。GTP酶家族可介导线粒体的融合与分裂,对哺乳动物的线粒体融合起作用的已知主要蛋白分别是线粒体融合蛋白1(Mitochondrial fusion protein 1,Mfn1)、线粒体融合蛋白2(Mitochondrial fusion protein 2,Mfn2)和视神经萎缩相关蛋白1(Optic atrophy,OPA1),对分裂起作用的已知主要蛋白分别是动力相关蛋白1(Dynamin-related protein 1,Drp1)、线粒体裂解因子(Mitochondrial fission factor,Mff)和线粒体分裂蛋白(Mitochondrial fission protein,Fis1)[85]。线粒体融合和分裂蛋白可以作为干预心血管疾病的靶点[86]。
在心肌祖细胞分化的过程中,黄芪总甙通过BCL2相互作用蛋白3(BCL2 interacting protein 3,BNIP3L)和FUN14结构域蛋白1(FUN14 domain-containing protein 1,FUNDC1)恢复了Mfn2和OPA1的表达,降低了Drp1的表达,进而促进了线粒体的融合,在心肌保护中发挥重要作用[87]。芹菜素可以缓解大鼠体内由3-氯-1,2-丙二醇(3-Chloro 1,2-propanediol,3-MCPD)诱导的Fis1、Drp1表达的上调和Mfn1、Mfn2表达的下调,促进了线粒体的融合,有效缓解3-MCPD诱导的肾损伤[88]。牡荆素通过环腺苷酸激活交换蛋白1/RAS相关蛋白1(Exchange protein directly activated by cyclic adenosine monophosphate 1/Ras-proximate-1,Epac1/Rap1)信号上调Mfn2表达,并抑制Drp1的表达,改善线粒体的分裂融合状态,减轻大鼠的心肌缺血/再灌注损伤[89]。Cao等[90]从石胆草中提取出两种多酚,3,4-二羟基苯乙醇-8-氧-[4-氧-反式-咖啡酰-β-D-呋喃硫糖基-(1→3)-β-D-吡喃葡萄糖基(1→6)][1]-β-D-吡喃葡萄糖苷(SDA-1-8)和羟基酪醇(Hydroxytyrosol,HT),对Drp1具有较高的结合亲和力,并通过平衡线粒体分裂和融合,改善阿尔茨海默症(Alzheimer’s disease,AD)的神经损伤。针对调控线粒体融合裂变的分子机制以及多酚类小分子调节剂与相应蛋白之间的构效关系仍然存在不足。未来可以将GTP酶家族作为靶点蛋白,进一步探索多酚类物质对该家族的分子机制。
2.5 调节线粒体自噬
多酚通过促进线粒体的自噬,清除受损线粒体,进而降低机体的损伤。线粒体自噬是通过识别受损线粒体发出的信号,将其降解,对维持线粒体数量的平衡和正常生理功能至关重要。但是如果线粒体太少会降低ATP水平,而线粒体过多会产生过量的ROS并导致细胞色素C释放出来。并且自噬失调会导致线粒体功能障碍和神经炎症,从而导致神经退行性疾病的发生[91−92]。Ranjbarvaziri等[93]对肥厚型心肌病患者的研究发现,患者体内确实发生线粒体自噬清除功能失调。目前,调控线粒体自噬的机制主要包括由,E3泛素连接酶(E3 ubiquitin-protein ligase,Parkin)与线粒体外膜蛋白张力蛋白同源物诱导的蛋白激酶1(PTEN-induced putative kinase 1,PINK1)和腺苷酸活化蛋白激酶(Adenosine monophos phate-activated protein kinase,AMPK)/哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin,mTOR)介导的线粒体自噬。而PINK1和parkin是调控线粒体自噬的重要组分,有助于维持线粒体正常功能[94−95]。
已有研究表明,EGCG、姜黄素、槲皮素、白藜芦醇等天然植物多酚可以调控线粒体的自噬[96]。EGCG通过增加 PINK1 和 parkin 的水平,增强线粒体自噬,减少细胞凋亡,改善老年II型糖尿病大鼠的记忆力[97]。姜黄素通过细胞中的PINK1-Parkin通路以线粒体自噬依赖性的方式缓解线粒体损伤[98]。槲皮素通过促进线粒体自噬来抑制线粒体RNA(mitochondrial RNA,mtRNA)介导的小胶质细胞含有NACHT、LRR和PYD结构域的蛋白3(NACHT, LRR, and PYD domains-containing protein 3,NLRP3)的激活,从而预防神经元损伤,除此之外还通过增加Forkhead box O3(FOXO3)、AMPK、细胞外调节蛋白激酶(Extracellular regulated protein kinases 2,ERK2)和parkin蛋白的活性诱导小鼠肝细胞的线粒体自噬,对治疗非酒精性脂肪肝具有重要作用[99−101]。白藜芦醇通过激活AMPK/mTOR,并且以ATP竞争性方式直接抑制mTOR调控的线粒体自噬。除此之外,白藜芦醇可以通过增加SIRT3、FOXO3、PINK1和parkin活性来诱导大鼠心肌的线粒体自噬[91,102−104]。关于线粒体自噬的机制已有众多研究,但是针对多酚对于线粒体自噬相关蛋白的确切相互作用,以及后续涉及的分子机制仍需要进一步探索与验证。
3. 结论
植物多酚可以通过调节ROS和钙离子水平、提高线粒体呼吸链酶活性、调节线粒体分裂融合和自噬等方式保护线粒体功能,从而改善与线粒体功能障碍相关的糖尿病、肺部疾病、心血管疾病、脂肪性肝病以及神经退行性疾病的发生与发展。虽然在细胞层面上,多酚对于线粒体的保护作用取得了一些进展,但是目前针对线粒体功能障碍中各通路的研究以及通路之间的联系仍不全面,这导致后续研究多酚对线粒体在不同生理状态下的分子机制存在很多不足;并且关于多酚类物质对于线粒体作用的研究方法面临众多困难,如由于疾病本身的复杂性与多变性,导致始终无法真实模拟相应的疾病模型以及针对部分指标仍然无法即时检测到线粒体内部的变化。未来希望随着研究体系的不断完善,可以进一步阐明不同结构的植物多酚与线粒体保护的构效关系,探究清晰多酚对线粒体保护的分子机制,为利用植物多酚精准干预或治疗线粒体功能障碍相关疾病提供更多的科学参考。
-
[1] FRAGA C G, GALLEANO M, VERSTRAETEN S V, et al. Basic biochemical mechanisms behind the health benefits of polyphenols[J]. Molecular Aspects of Medicine,2010,31(6):435−445. doi: 10.1016/j.mam.2010.09.006
[2] 易莹, 樊敏, 李权. 常见植物多酚化合物的介绍[J]. 化学教育(中英文),2022,43(11):1−6. [YI Ying, FAN Min, LI Quan. Introduction of common plant polyphenols compounds[J]. Chinese Journal of Chemical Education,2022,43(11):1−6.] YI Ying, FAN Min, LI Quan. Introduction of common plant polyphenols compounds[J]. Chinese Journal of Chemical Education, 2022, 43(11): 1−6.
[3] 中华人民共和国国家卫生健康委员会. 关于巴拉圭冬青叶(马黛茶叶)等9种“三新食品”的公告[EB/OL]. (2023-11-23) [2024-7-18]. http://www.nhc.gov.cn/sps/s7892/202311/734de9d26bd441d9b15131dfacd3f253.shtml. [National Health Commission of the People's Republic of China. Announcement on 9 “Three New Foods”, including Yerba mate[EB/OL]. (2023-11-23) [2024-7-18]. http://www.nhc.gov.cn/sps/s7892/202311/734de9d26bd441d9b15131dfacd3f253.shtml.] National Health Commission of the People's Republic of China. Announcement on 9 “Three New Foods”, including Yerba mate[EB/OL]. (2023-11-23) [2024-7-18]. http://www.nhc.gov.cn/sps/s7892/202311/734de9d26bd441d9b15131dfacd3f253.shtml.
[4] 中华人民共和国国家卫生和计划生育委员会. 关于批准番茄籽油等9种新食品原料的公告[EB/OL]. (2014-12-19) [2024-07-18]. http://www.nhc.gov.cn/sps/s3585/201412/5ecadb810d5d4f1eac12a3d6c9f7f6bc.shtml. [National Health and Family Planning Commission of the People's Republic of China. Announcement on approval of 9 new food ingredients including tomato seed oil[EB/OL]. (2014-12-19) [2024-07-18]. http://www.nhc.gov.cn/sps/s3585/201412/5ecadb810d5d4f1eac12a3d6c9f7f6bc.shtml.] National Health and Family Planning Commission of the People's Republic of China. Announcement on approval of 9 new food ingredients including tomato seed oil[EB/OL]. (2014-12-19) [2024-07-18]. http://www.nhc.gov.cn/sps/s3585/201412/5ecadb810d5d4f1eac12a3d6c9f7f6bc.shtml.
[5] 中华人民共和国国家卫生健康委员会. 关于β-1, 3/α-1, 3-葡聚糖等6种“三新食品”的公告[EB/OL]. (2021-04-15) [2023-07-18]. http://www.nhc.gov.cn/sps/s3585/201412/5ecadb810d5d4f1eac12a3d6c9f7f6bc.shtml. [National Health Commission of the People's Republic of China. Announcement on 6 types of “Three New Foods” including β-1, 3/α-1, 3-glucan[EB/OL]. (2021-04-15) [2023-07-18]. http://www.nhc.gov.cn/sps/s3585/201412/5ecadb810d5d4f1eac12a3d6c9f7f6bc.shtml.] National Health Commission of the People's Republic of China. Announcement on 6 types of “Three New Foods” including β-1, 3/α-1, 3-glucan[EB/OL]. (2021-04-15) [2023-07-18]. http://www.nhc.gov.cn/sps/s3585/201412/5ecadb810d5d4f1eac12a3d6c9f7f6bc.shtml.
[6] 中华人民共和国国家卫生健康委员会. 关于莱茵衣藻等36种“三新食品”的公告[EB/OL]. (2022-05-05) [2024-7-18]. http://www.nhc.gov.cn/sps/s7892/202311/734de9d26bd441d9b15131dfacd3f253.shtml. [National Health Commission of the People's Republic of China. Announcement on 36 “Three New Foods”, including Chlamydomonas reinhardtii[EB/OL]. (2022-05-05) [2024-7-18]. http://www.nhc.gov.cn/sps/s7892/202311/734de9d26bd441d9b15131dfacd3f253.shtml.] National Health Commission of the People's Republic of China. Announcement on 36 “Three New Foods”, including Chlamydomonas reinhardtii[EB/OL]. (2022-05-05) [2024-7-18]. http://www.nhc.gov.cn/sps/s7892/202311/734de9d26bd441d9b15131dfacd3f253.shtml.
[7] STATISTA RESEARCH DEPARTMENT. Phenol market volume worldwide 2015-2030[R]. New York:Statista Research Department, 2023.
[8] ANGELOVA P R, ABRAMOV A Y. Role of mitochondrial ROS in the brain:From physiology to neurodegeneration[J]. Federation of European Biochemical Societies Letters,2018,592(5):692−702. doi: 10.1002/1873-3468.12964
[9] 张锦荣, 王彦阳. 植物多酚类化合物提取技术概况[J]. 种子科技,2019,37(9):24−26. [ZHANG Jinrong, WANG Yanyang. Overview of extraction technology of plant polyphenols[J]. Seed Science& Technology,2019,37(9):24−26.] doi: 10.3969/j.issn.1005-2690.2019.09.013 ZHANG Jinrong, WANG Yanyang. Overview of extraction technology of plant polyphenols[J]. Seed Science& Technology, 2019, 37(9): 24−26. doi: 10.3969/j.issn.1005-2690.2019.09.013
[10] 林安贵, 杨灵灵. 植物提取物槲皮素调节小鼠的能量代谢和氧化应激[J]. 基因组学与应用生物学,2020,39(1):320−325. [LIN Angui, YANG Lingling. Plant extract quercetin regulates energy metabolism and oxidative stress in mice[J]. Genomics and Applied Biology,2020,39(1):320−325.] LIN Angui, YANG Lingling. Plant extract quercetin regulates energy metabolism and oxidative stress in mice[J]. Genomics and Applied Biology, 2020, 39(1): 320−325.
[11] 康萌, 李雪梅, 万静之, 等. 二氢杨梅素肝脏疾病保护作用及机制研究进展[J]. 中南药学,2024,22(5):1300−1304. [KANG Meng, LI Xuemei, WAN Jingzhi, et al. Research progress in the effect of dihydromyricetin on the prevention and treatment of liver diseases and related mechanism[J]. Central South Pharmacy,2024,22(5):1300−1304.] KANG Meng, LI Xuemei, WAN Jingzhi, et al. Research progress in the effect of dihydromyricetin on the prevention and treatment of liver diseases and related mechanism[J]. Central South Pharmacy, 2024, 22(5): 1300−1304.
[12] SHI W M, LI L F, DING Y C, et al. The critical role of epigallocatechin gallate in regulating mitochondrial metabolism[J]. Future Medicinal Chemistry,2018,10(7):795−809. doi: 10.4155/fmc-2017-0204
[13] ZHOU J, FARAH B L, SINHA R A, et al. Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, stimulates hepatic autophagy and lipid clearance[J]. PloS One,2014,9(1):e87161. doi: 10.1371/journal.pone.0087161
[14] 顾艳利, 宋勇, 张方. 姜黄素在肺部炎症性疾病中的免疫调节作用[J]. 中华肺部疾病杂志(电子版),2021,14(4):539−542. [GU Yanli, SONG Yong, ZHANG Fang. Lmmunomodulatory effects of curcumin in pulmonary inflammatory diseases[J]. Chinese Journal of Lung Diseases(Electronic Edition),2021,14(4):539−542.] doi: 10.3877/cma.j.issn.1674-6902.2021.04.040 GU Yanli, SONG Yong, ZHANG Fang. Lmmunomodulatory effects of curcumin in pulmonary inflammatory diseases[J]. Chinese Journal of Lung Diseases(Electronic Edition), 2021, 14(4): 539−542. doi: 10.3877/cma.j.issn.1674-6902.2021.04.040
[15] TSAO R. Chemistry and biochemistry of dietary polyphenols[J]. Nutrients,2010,2(12):1231−1246. doi: 10.3390/nu2121231
[16] MARTIN M Á, RAMOS S. Impact of cocoa flavanols on human health[J]. Food and Chemical Toxicology,2021,151:112121. doi: 10.1016/j.fct.2021.112121
[17] KŘÍŽOVÁ L, DADÁKOVÁ K, KAŠPAROVSKÁ J, et al. Isoflavones[J]. Molecules,2019,24(6):1076. doi: 10.3390/molecules24061076
[18] LUO Y, JIAN Y Q, LIU Y K, et al. Flavanols from nature:A phytochemistry and biological activity review[J]. Molecules,2022,27(3):719. doi: 10.3390/molecules27030719
[19] ZAA C A, MARCELO Á J, AN Z, et al. Anthocyanins:Molecular aspects on their neuroprotective activity[J]. Biomolecules,2023,13(11):1598. doi: 10.3390/biom13111598
[20] KONG J M, CHIA L S, GOH N K, et al. Analysis and biological activities of anthocyanins[J]. Phytochemistry,2003,64(5):923−933. doi: 10.1016/S0031-9422(03)00438-2
[21] CHEN B H, STEPHEN INBARAJ B. Nanoemulsion and nanoliposome based strategies for improving anthocyanin stability and bioavailability[J]. Nutrients,2019,11(5):1052. doi: 10.3390/nu11051052
[22] 黄永健, 荀航, 张保, 等. HPLC同时测定竹笋中8种酚酸类物质含量的方法研究及其应用[J]. 南京林业大学学报(自然科学版),2024,48(3):237−244. [HUANG Yongjian, XUN Hang, ZHANG Bao, et al. Simultaneous determination of eight phenolic acids in bamboo shoots by HPLC and its applications[J]. Journal of Nanjing Forestry University(Natural Sciences Edition),2024,48(3):237−244.] doi: 10.12302/j.issn.1000-2006.202205047 HUANG Yongjian, XUN Hang, ZHANG Bao, et al. Simultaneous determination of eight phenolic acids in bamboo shoots by HPLC and its applications[J]. Journal of Nanjing Forestry University(Natural Sciences Edition), 2024, 48(3): 237−244. doi: 10.12302/j.issn.1000-2006.202205047
[23] 侯萍, 任晨阳, 黄艳, 等. 红叶野桐叶中的木脂素类化合物[J]. 广西植物,2024,44(6):1151−1158. [HOU Ping, REN Chenyang, HUANG Yan, et al. Lignans from the leaves of Mallotus paxii[J]. Guihaia,2024,44(6):1151−1158.] HOU Ping, REN Chenyang, HUANG Yan, et al. Lignans from the leaves of Mallotus paxii[J]. Guihaia, 2024, 44(6): 1151−1158.
[24] 黄樱华, 张林, 李锦妍, 等. 流苏石斛中3种木脂素类成分的含量测定研究[J]. 广州中医药大学学报,2024,41(1):207−212. [HUANG Yinghua, ZHANG Lin, LI Jinyan, et al. Determination of the contents of three lignans in Dendrobium fimbriatum Hook[J]. Journal of Guangzhou University of Traditional Chinese Medicine,2024,41(1):207−212.] HUANG Yinghua, ZHANG Lin, LI Jinyan, et al. Determination of the contents of three lignans in Dendrobium fimbriatum Hook[J]. Journal of Guangzhou University of Traditional Chinese Medicine, 2024, 41(1): 207−212.
[25] 姜丽丽, 尹航, 闫明睿, 等. 天然芪类化合物及其抗α-葡萄糖苷酶活性的研究进展[J]. 上海中医药大学学报,2021,35(2):116−124. [JIANG Lili, YIN Hang, YAN Mingrui, et al. Research progress on natural stilbenes and its inhibitory activity on α-glucosidase[J]. Academic Journal of Shanghai University of Traditional Chinese Medicine,2021,35(2):116−124.] JIANG Lili, YIN Hang, YAN Mingrui, et al. Research progress on natural stilbenes and its inhibitory activity on α-glucosidase[J]. Academic Journal of Shanghai University of Traditional Chinese Medicine, 2021, 35(2): 116−124.
[26] 王永涛. 白皮松植物多酚的地理变异及其与环境因子的耦合关系[D]. 咸阳:西北农林科技大学, 2016. [WANG Yongtao. The geographic variation of plant polyphenols and coupling relationship with environmental factors of Pinus bungeana[D]. Xianyang:Northwest A&F University, 2016.] WANG Yongtao. The geographic variation of plant polyphenols and coupling relationship with environmental factors of Pinus bungeana[D]. Xianyang: Northwest A&F University, 2016.
[27] MANACH C, SCALBERT A, MORAND C, et al. Polyphenols:Food sources and bioavailability[J]. The American Journal of Clinical Nutrition,2004,79(5):727−747. doi: 10.1093/ajcn/79.5.727
[28] KANAZE F I, BOUNARTZI M I, GEORGARAKIS M, et al. Pharmacokinetics of the citrus flavanone aglycones hesperetin and naringenin after single oral administration in human subjects[J]. European Journal of Clinical Nutrition,2007,61(4):472−477. doi: 10.1038/sj.ejcn.1602543
[29] CHEN L R, KO N Y, CHEN K H. Isoflavone supplements for menopausal women:A systematic review[J]. Nutrients,2019,11(11):2649. doi: 10.3390/nu11112649
[30] KIOKIAS S, OREOPOULOU V. A review of the health protective effects of phenolic acids against a range of severe pathologic conditions (Including coronavirus-based infections)[J]. Molecules,2021,26(17):5405. doi: 10.3390/molecules26175405
[31] BERENSHTEIN L, OKUN Z, SHPIGELMAN A. Stability and bioaccessibility of lignans in food products[J]. American Chemical Society Omega,2024,9(2):2022−2031.
[32] GÜLÇIN İ. Antioxidant properties of resveratrol:A structure-activity insight[J]. Innovative Food Science & Emerging Technologies,2010,11(1):210−218.
[33] 张琳. 板栗总苞抗糖尿病活性成分及Seseli hartvigii的化学成分研究[D]. 沈阳:沈阳药科大学, 2015. [ZHANG Lin. Studies on anti-diabetic constituents of involucre of Castanea mollissima Blume and chemical substants of Seseli hartvigii[D]. Shenyang:Shenyang Pharmaceutical University, 2015.] ZHANG Lin. Studies on anti-diabetic constituents of involucre of Castanea mollissima Blume and chemical substants of Seseli hartvigii[D]. Shenyang: Shenyang Pharmaceutical University, 2015.
[34] 陈凤真, 王波, 赵贵红, 等. 提高牡丹副产物附加值对策研究[J]. 中国果菜,2023,43(5):50−54. [CHEN Fengzhen, WANG Bo, ZHAO Guihong, et al. Study on countermeasures for increase the additional value of peony by-products[J]. China Fruit Vegetable,2023,43(5):50−54.] CHEN Fengzhen, WANG Bo, ZHAO Guihong, et al. Study on countermeasures for increase the additional value of peony by-products[J]. China Fruit Vegetable, 2023, 43(5): 50−54.
[35] 陈怡琳, 张峰玮, 王健英, 等. 牡丹非药用部位化学成分和药理作用研究进展[J]. 基层中医药,2023,2(5):115−124. [CHEN Yilin, ZHANG Fengwei, WANG Jianying, et al. Research progress on chemical components and pharmacological effects of non-medicinal parts of Paeonia suffruticosa Andr[J]. Basic Traditional Chinese Medicine,2023,2(5):115−124.] CHEN Yilin, ZHANG Fengwei, WANG Jianying, et al. Research progress on chemical components and pharmacological effects of non-medicinal parts of Paeonia suffruticosa Andr[J]. Basic Traditional Chinese Medicine, 2023, 2(5): 115−124.
[36] ROJAS-GARCÍA A, FERNÁNDEZ-OCHOA Á, CÁDIZ-GURREA M de la L, et al. Neuroprotective effects of agri-food by-products rich in phenolic compounds[J]. Nutrients,2023,15(2):449. doi: 10.3390/nu15020449
[37] LIU X L, WANG Y D, YU X M, et al. Mitochondria-mediated damage to dopaminergic neurons in parkinson’s disease (review)[J]. International Journal of Molecular Medicine,2018,41(2):615−623.
[38] 朱潇旭, 段小花, 李瑞霞, 等. 线粒体功能障碍与非酒精性脂肪肝发病关系的研究进展[J]. 山东医药,2018,58(29):108−111. [ZHU Xiaoxu, DUAN Xiaohua, LI Ruixia, et al. Research progress on the relationship between mitochondrial dysfunction and nonalcoholic fatty liver disease[J]. Shandong Medical Journal,2018,58(29):108−111.] doi: 10.3969/j.issn.1002-266X.2018.29.033 ZHU Xiaoxu, DUAN Xiaohua, LI Ruixia, et al. Research progress on the relationship between mitochondrial dysfunction and nonalcoholic fatty liver disease[J]. Shandong Medical Journal, 2018, 58(29): 108−111. doi: 10.3969/j.issn.1002-266X.2018.29.033
[39] YANG R, TAN C, NAJAFI M. Cardiac inflammation and fibrosis following chemo/radiation therapy:Mechanisms and therapeutic agents[J]. Inflammopharmacology,2022,30(1):73−89. doi: 10.1007/s10787-021-00894-9
[40] VARUGHESE M, PATOLE S, SHAMA A, et al. Permissive hypercapnia in neonates:The case of the good, the bad, and the ugly[J]. Pediatric Pulmonology,2002,33(1):56−64. doi: 10.1002/ppul.10032
[41] OTT M, GOGVADZE V, ORRENIUS S, et al. Mitochondria, oxidative stress and cell death[J]. Apoptosis:An International Journal on Programmed Cell Death,2007,12(5):913−922. doi: 10.1007/s10495-007-0756-2
[42] IPSEN D H, LYKKESFELDT J, TVEDEN-NYBORG P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease[J]. Cellular and Molecular Life Sciences,2018,75(18):3313−3327. doi: 10.1007/s00018-018-2860-6
[43] ZHOU B, TIAN R. Mitochondrial dysfunction in pathophysiology of heart failure[J]. The Journal of Clinical Investigation,2018,128(9):3716−3726. doi: 10.1172/JCI120849
[44] SANKAR V, PANGAYARSELVI B, PRATHAPAN A, et al. Desmodium gangeticum (Linn.) DC. exhibits antihypertrophic effect in isoproterenol-induced cardiomyoblasts via amelioration of oxidative stress and mitochondrial alterations[J]. Journal of Cardiovascular Pharmacology,2013,61(1):23−34. doi: 10.1097/FJC.0b013e3182756ad3
[45] MISRA M K, SARWAT M, BHAKUNI P, et al. Oxidative stress and ischemic myocardial syndromes[J]. Medical Science Monitor:International Medical Journal of Experimental and Clinical Research,2009,15(10):RA209−219.
[46] LI C J, ZHANG Q M, LI M Z, et al. Attenuation of myocardial apoptosis by alpha-lipoic acid through suppression of mitochondrial oxidative stress to reduce diabetic cardiomyopathy[J]. Chinese Medical Journal,2009,122(21):2580−2586.
[47] SARKAR A, MIDDYA T R, JANA A D. A QSAR study of radical scavenging antioxidant activity of a series of flavonoids using DFT based quantum chemical descriptors--the importance of group frontier electron density[J]. Journal of Molecular Modeling, 2012, 18(6):2621-2631.
[48] SARKAR A, MIDDYA T R, JANA A D. A QSAR study of radical scavenging antioxidant activity of a series of flavonoids using DFT based quantum chemical descriptors-the importance of group frontier electron density[J]. Journal of Molecular Modeling,2012,18(6):2621−2631. doi: 10.1007/s00894-011-1274-2
[49] 徐敏, 廉法英, 邵欢. 栀子苷调节AMPK/SIRT1/NF-κB信号通路对子痫前期大鼠氧化应激和炎症损伤的影响[J]. 中国优生与遗传杂志,2023,31(8):1593−1598. [XU Min, LIAN Faying, SHAO Huan. Influence of geniposide on oxidative stress and inflammatory injury in preeclamptic rats by regulating AMPK/SIRT1/NF-κB signaling pathway[J]. Chinese Journal of Birth Health & Heredity,2023,31(8):1593−1598.] XU Min, LIAN Faying, SHAO Huan. Influence of geniposide on oxidative stress and inflammatory injury in preeclamptic rats by regulating AMPK/SIRT1/NF-κB signaling pathway[J]. Chinese Journal of Birth Health & Heredity, 2023, 31(8): 1593−1598.
[50] ZHANG Y H, TAN X Y, CAO Y, et al. Punicalagin protects against diabetic liver injury by upregulating mitophagy and antioxidant enzyme activities[J]. Nutrients,2022,14(14):2782. doi: 10.3390/nu14142782
[51] PALSAMY P, SUBRAMANIAN S. Ameliorative potential of resveratrol on proinflammatory cytokines, hyperglycemia mediated oxidative stress, and pancreatic β-cell dysfunction in streptozotocin-nicotinamide-induced diabetic rats[J]. Journal of Cellular Physiology,2010,224(2):423−432. doi: 10.1002/jcp.22138
[52] ZHANG J J, GAO B L, YE B L, et al. Mitochondrial-targeted delivery of polyphenol-mediated antioxidases complexes against pyroptosis and inflammatory diseases[J]. Advanced Materials,2023,35(11):2208571. doi: 10.1002/adma.202208571
[53] CALABRESE E J. Neuroscience and hormesis:Overview and general findings[J]. Critical Reviews in Toxicology,2008,38(4):249−252. doi: 10.1080/10408440801981957
[54] TSUJI P A, STEPHENSON K K, WADE K L, et al. Structure-activity analysis of flavonoids:Direct and indirect antioxidant, and antiinflammatory potencies and toxicities[J]. Nutrition and Cancer,2013,65(7):1014−1025. doi: 10.1080/01635581.2013.809127
[55] 付凯, 王永辉, 贠洁, 等. 茶多酚激活Nrf2/HO-1通路减轻阿司匹林诱导的GES-1细胞损伤的机制研究[J]. 中国药学杂志,2024,59(1):45−51. [FU Kai, WANG Yonghui, YUN Jie, et al. Mechanism study of tea polyphenols alleviating aspirin-induced injury in GES-1 cells through activating Nrf2/HO-1 pathway[J]. Chinese Pharmaceutical Journal,2024,59(1):45−51.] doi: 10.11669/cpj.2024.01.006 FU Kai, WANG Yonghui, YUN Jie, et al. Mechanism study of tea polyphenols alleviating aspirin-induced injury in GES-1 cells through activating Nrf2/HO-1 pathway[J]. Chinese Pharmaceutical Journal, 2024, 59(1): 45−51. doi: 10.11669/cpj.2024.01.006
[56] HUANG D D, SHI G, JIANG Y, et al. A review on the potential of resveratrol in prevention and therapy of diabetes and diabetic complications[J]. Biomedicine & Pharmacotherapy,2020,125:109767.
[57] 张恒恺. 石榴皮多酚与短链脂肪酸对结肠细胞功能损伤保护机制研究[D]. 西安:陕西师范大学, 2022. [ZHANG Hengkai. Study on the protective mechanism of pomegranate peel polyphenols and short-chain fatty acids on colonic cell function damage[D]. Xi’an:Shaanxi Normal University, 2022.] ZHANG Hengkai. Study on the protective mechanism of pomegranate peel polyphenols and short-chain fatty acids on colonic cell function damage[D]. Xi’an: Shaanxi Normal University, 2022.
[58] CHERUBINI M, LOPEZ-MOLINA L, GINES S. Mitochondrial fission in Huntington’s disease mouse striatum disrupts ER-mitochondria contacts leading to disturbances in Ca2+ efflux and reactive oxygen species (ROS) homeostasis[J]. Neurobiology of Disease,2020,136:104741. doi: 10.1016/j.nbd.2020.104741
[59] VULTUR A, GIBHARDT C S, STANISZ H, et al. The role of the mitochondrial calcium uniporter (MCU) complex in cancer[J]. Pflügers Archiv-European Journal of Physiology,2018,470(8):1149−1163.
[60] ELUSTONDO P A, NICHOLS M, ROBERTSON G S, et al. Mitochondrial Ca2+ uptake pathways[J]. Journal of Bioenergetics and Biomembranes,2017,49(1):113−119. doi: 10.1007/s10863-016-9676-6
[61] BROOKES P S, YOON Y, ROBOTHAM J L, et al. Calcium, ATP, and ROS:A mitochondrial love-hate triangle[J]. American Journal of Physiology. Cell Physiology,2004,287(4):C817−833. doi: 10.1152/ajpcell.00139.2004
[62] WU A, ZHANG J, LI Q, et al. (-)-Epigallocatechin-3-gallate directly binds cyclophilin D:A potential mechanism for mitochondrial protection[J]. Molecules,2022,27(24):8661. doi: 10.3390/molecules27248661
[63] BERNARDI P, GERLE C, HALESTRAP A P, et al. Identity, structure, and function of the mitochondrial permeability transition pore:Controversies, consensus, recent advances, and future directions[J]. Cell Death & Differentiation,2023,30(8):1869−1885.
[64] LI Y, WU J, YANG M, et al. Physiological evidence of mitochondrial permeability transition pore opening caused by lipid deposition leading to hepatic steatosis in db/db mice[J]. Free Radical Biology & Medicine,2021,162:523−532.
[65] YING Y, JIANG P. Research progress on transient receptor potential melastatin 2 channel in nervous system diseases[J]. Journal of Zhejiang University (Medical Sciences),2021,50(2):267−276. doi: 10.3724/zdxbyxb-2021-0110
[66] KHERADPEZHOUH E, BARRITT G J, RYCHKOV G Y. Curcumin inhibits activation of TRPM2 channels in rat hepatocytes[J]. Redox Biology,2016,7:1−7. doi: 10.1016/j.redox.2015.11.001
[67] 曹玉爽, 徐耀, 杨娟, 等. 丹参多酚酸与三七总皂苷合用保护OGD/R损伤胶质细胞线粒体、促进神经因子表达作用研究[J]. 云南中医学院学报,2021,44(4):1−8,21. [CAO Yushuang, XU Yao, YANG Juan, et al. Study on the effects of SAL and PNS on protecting glial mitochondria and promoting the expression of neural factors[J]. Journal of Yunnan University of Chinese Medicine,2021,44(4):1−8,21.] CAO Yushuang, XU Yao, YANG Juan, et al. Study on the effects of SAL and PNS on protecting glial mitochondria and promoting the expression of neural factors[J]. Journal of Yunnan University of Chinese Medicine, 2021, 44(4): 1−8,21.
[68] 辛庆锋, 孙有利, 李超彦, 等. 异鼠李素通过抑制钙超载和线粒体功能损伤减轻阿霉素所致大鼠心肌细胞凋亡[J]. 中南药学,2017,15(7):915−918. [XIN Qingfeng, SUN Youli, LI Chaoyan, et al. Isorhamnetin reduces the injury of myocardial cell apoptosis induced by adriamycin by inhibiting calcium overload and mitochondrial function[J]. Central South Pharmacy,2017,15(7):915−918.] doi: 10.7539/j.issn.1672-2981.2017.07.012 XIN Qingfeng, SUN Youli, LI Chaoyan, et al. Isorhamnetin reduces the injury of myocardial cell apoptosis induced by adriamycin by inhibiting calcium overload and mitochondrial function[J]. Central South Pharmacy, 2017, 15(7): 915−918. doi: 10.7539/j.issn.1672-2981.2017.07.012
[69] ASHRAFIZADEH M, JAVANMARDI S, MORADI-OZARLOU M, et al. Natural products and phytochemical nanoformulations targeting mitochondria in oncotherapy:An updated review on resveratrol[J]. Bioscience Reports,2020,40(4):BSR20200257. doi: 10.1042/BSR20200257
[70] WANG M, RUAN Y X, CHEN Q, et al. Curcumin induced HepG2 cell apoptosis-associated mitochondrial membrane potential and intracellular free Ca2+ concentration[J]. European Journal of Pharmacology,2011,650(1):41−47. doi: 10.1016/j.ejphar.2010.09.049
[71] NOLFI-DONEGAN D, BRAGANZA A, SHIVA S. Mitochondrial electron transport chain:Oxidative phosphorylation, oxidant production, and methods of measurement[J]. Redox Biology,2020,37:101674. doi: 10.1016/j.redox.2020.101674
[72] GUEGUEN N, DESQUIRET-DUMAS V, LEMAN G, et al. Resveratrol directly binds to mitochondrial complex I and increases oxidative stress in brain mitochondria of aged mice[J]. PLoS ONE,2015,10(12):e0144290. doi: 10.1371/journal.pone.0144290
[73] KIPP J L, RAMIREZ V D. Effect of estradiol, diethylstilbestrol, and resveratrol on F0F1-ATPase activity from mitochondrial preparations of rat heart, liver, and brain[J]. Endocrine,2001,15(2):165−175. doi: 10.1385/ENDO:15:2:165
[74] 孙姝婵, 龚迪菲, 袁天翊, 等. 葛根素通过改善线粒体呼吸功能减轻血管内皮细胞氧化损伤[J]. 药学学报,2022,57(5):1352−1360. [SUN Shuchan, GONG Difei, YUAN Tianyi, et al. Puerarin reduces oxidative damage to vascular endothelial cells by improving mitochondrial respiratory function[J]. Acta Pharmaceutica Sinica,2022,57(5):1352−1360.] SUN Shuchan, GONG Difei, YUAN Tianyi, et al. Puerarin reduces oxidative damage to vascular endothelial cells by improving mitochondrial respiratory function[J]. Acta Pharmaceutica Sinica, 2022, 57(5): 1352−1360.
[75] YOSHIDA Y, TAMURA Y, KOUZAKI K, et al. Dietary apple polyphenols enhance mitochondrial turnover and respiratory chain enzymes[J]. Experimental Physiology,2023,108(10):1295−1307. doi: 10.1113/EP091154
[76] 万一方. 核桃多酚缓解马拉硫磷对小鼠脾淋巴细胞所致氧化毒性[D]. 北京:北京林业大学, 2020. [WAN Yifang. Walnut polyphenol attenuates oxidative toxicity induced by malathion in murine splenic lymphocyte[D]. Beijing:Beijing Forestry University, 2020.] WAN Yifang. Walnut polyphenol attenuates oxidative toxicity induced by malathion in murine splenic lymphocyte[D]. Beijing: Beijing Forestry University, 2020.
[77] HASLEM L, HAYS J M, HAYS F A. p66Shc in cardiovascular pathology[J]. Cells,2022,11(11):1855. doi: 10.3390/cells11111855
[78] ALBIERO M, D’ANNA M, BONORA B M, et al. Hematopoietic and nonhematopoietic p66Shc differentially regulates stem cell traffic and vascular response to ischemia in diabetes[J]. Antioxidants & Redox Signaling,2022,36(10-12):593−607.
[79] 王沛, 剡冬冬, 彭瑜, 等. 衔接蛋白p66Shc在心肌缺血再灌注损伤中的作用研究进展[J]. 解放军医学杂志,2023,48(4):456−460. [WANG Pei, YAN Dongdong, PENG Yu, et al. Research progress on the role of adaptor protein p66Shc in myocardial ischemia-reperfusion injury[J]. Medical Journal of Chinese People’s Liberation Army,2023,48(4):456−460.] doi: 10.11855/j.issn.0577-7402.2022.04.0456 WANG Pei, YAN Dongdong, PENG Yu, et al. Research progress on the role of adaptor protein p66Shc in myocardial ischemia-reperfusion injury[J]. Medical Journal of Chinese People’s Liberation Army, 2023, 48(4): 456−460. doi: 10.11855/j.issn.0577-7402.2022.04.0456
[80] SONG Y, YU H, SUN Q, et al. Grape seed proanthocyanidin extract targets p66Shc to regulate mitochondrial biogenesis and dynamics in diabetic kidney disease[J]. Frontiers in Pharmacology,2023,13:1035755. doi: 10.3389/fphar.2022.1035755
[81] MOINI H, ARROYO A, VAYA J, et al. Bioflavonoid effects on the mitochondrial respiratory electron transport chain and cytochrome c redox state[J]. Redox Report,1999,4(1-2):35−41. doi: 10.1179/135100099101534729
[82] VALDAMERI G, HERRERIAS T, CARNIERI E G S, et al. Importance of the core structure of flavones in promoting inhibition of the mitochondrial respiratory chain[J]. Chemico-Biological Interactions,2010,188(1):52−58. doi: 10.1016/j.cbi.2010.07.016
[83] MA Y W, WANG L H, JIA R B. The role of mitochondrial dynamics in human cancers[J]. American Journal of Cancer Research,2020,10(5):1278−1293.
[84] NI Y, DENG J, LIU X, et al. Echinacoside reverses myocardial remodeling and improves heart function via regulating SIRT1/FOXO3a/MnSOD axis in HF rats induced by isoproterenol[J]. Journal of Cellular and Molecular Medicine,2021,25(1):203−216. doi: 10.1111/jcmm.15904
[85] 郑凯, 杨梅桂, 闫朝君, 等. 线粒体动力学与细胞凋亡[J]. 中国细胞生物学学报,2019,41(8):1467−1476. [ZHENG Kai, YANG Meigui, YAN Chaojun, et al. Mitochondrial dynamics and apoptosis[J]. Chinese Journal of Cell Biology,2019,41(8):1467−1476.] ZHENG Kai, YANG Meigui, YAN Chaojun, et al. Mitochondrial dynamics and apoptosis[J]. Chinese Journal of Cell Biology, 2019, 41(8): 1467−1476.
[86] ONG S B, KALKHORAN S B, CABRERA-FUENTES H A, et al. Mitochondrial fusion and fission proteins as novel therapeutic targets for treating cardiovascular disease[J]. European Journal of Pharmacology,2015,763:104−114. doi: 10.1016/j.ejphar.2015.04.056
[87] 黄海军. 黄芪总甙通过干预BNIP3LNIX和FUNDC1介导的线粒体分裂和融合影响心肌重构的机制[D]. 杭州:江中医药大学, 2023. [HUANG Haijun. The mechanism of astragalosides affecting myocardial remodeling by interfering with BNIP3LNIX and FUNDC1-mediated mitochondrial fission and fusion[D]. Hangzhou:Zhejiang Chinese Medical University, 2023.] HUANG Haijun. The mechanism of astragalosides affecting myocardial remodeling by interfering with BNIP3LNIX and FUNDC1-mediated mitochondrial fission and fusion[D]. Hangzhou: Zhejiang Chinese Medical University, 2023.
[88] 钟玉杰, 师振强, 晋程妮, 等. 芹菜素对3-氯-1, 2-丙二醇诱导的大鼠肾损伤及线粒体分裂融合的影响[J]. 食品科学,2019,40(9):107−114. [ZHONG Yujie, SHI Zhenqiang, JIN Chengni, et al. Effect of apigenin on 3-chloro-1, 2-propanediol induced renal injury and mitochondrial fission and fusion in rats[J]. Food Science,2019,40(9):107−114.] doi: 10.7506/spkx1002-6630-20171121-268 ZHONG Yujie, SHI Zhenqiang, JIN Chengni, et al. Effect of apigenin on 3-chloro-1, 2-propanediol induced renal injury and mitochondrial fission and fusion in rats[J]. Food Science, 2019, 40(9): 107−114. doi: 10.7506/spkx1002-6630-20171121-268
[89] YANG H H, XUE W, DING C J, et al. Vitexin mitigates myocardial ischemia/reperfusion injury in rats by regulating mitochondrial dysfunction via epac1-rap1 signaling[J]. Oxidative Medicine and Cellular Longevity,2021,2021:e9921982. doi: 10.1155/2021/9921982
[90] CAO B, ZENG M N, HAO F X, et al. Two polyphenols isolated from Corallodiscus flabellata B. L. Burtt ameliorate amyloid β-protein induced Alzheimer’s disease neuronal injury by improving mitochondrial homeostasis[J]. Behavioural Brain Research,2023,440:114264. doi: 10.1016/j.bbr.2022.114264
[91] 罗云彦. 白藜芦醇通过激活parkin介导的线粒体自噬提高猪肠道抗氧化能力的作用及其机制[D]. 南宁:广西大学, 2023. [LUO Yunyan. Effects of resveratrol on enhancing antioxidant capacity of porcine intestinal tract by activating parkin-mediated mitochondrial autophagy and its mechanism[D]. Nanning:Guangxi University, 2023.] LUO Yunyan. Effects of resveratrol on enhancing antioxidant capacity of porcine intestinal tract by activating parkin-mediated mitochondrial autophagy and its mechanism[D]. Nanning: Guangxi University, 2023.
[92] KAMAT P K, KALANI A, KYLES P, et al. Autophagy of mitochondria:A promising therapeutic target for neurodegenerative disease[J]. Cell Biochemistry and Biophysics,2014,70(2):707−719. doi: 10.1007/s12013-014-0006-5
[93] RANJBARVAZIRI S, KOOIKER K B, ELLENBERGER M, et al. Altered cardiac energetics and mitochondrial dysfunction in hypertrophic cardiomyopathy[J]. Circulation,2021,144(21):1714−1731. doi: 10.1161/CIRCULATIONAHA.121.053575
[94] CHANDRASEKARAN V, HEDIYAL T A, ANAND N, et al. Polyphenols, autophagy and neurodegenerative diseases:A review[J]. Biomolecules,2023,13(8):1196. doi: 10.3390/biom13081196
[95] SPRINGER M Z, MACLEOD K F. In brief:Mitophagy:Mechanisms and role in human disease[J]. The Journal of Pathology,2016,240(3):253−255. doi: 10.1002/path.4774
[96] 曹雨欣, 张彦青, 戚务勤, 等. 食源性天然产物调控线粒体自噬预防神经退行性疾病的研究进展[J]. 食品科学,2024,45(1):301−312. [CAO Yuxin, ZHANG Yanqing, QI Wuqin, et al. Food-derived natural products prevent neurodegenerative diseases by regulating mitophagy:A review of research progress[J]. Food Science,2024,45(1):301−312.] doi: 10.7506/spkx1002-6630-20230113-104 CAO Yuxin, ZHANG Yanqing, QI Wuqin, et al. Food-derived natural products prevent neurodegenerative diseases by regulating mitophagy: A review of research progress[J]. Food Science, 2024, 45(1): 301−312. doi: 10.7506/spkx1002-6630-20230113-104
[97] FENG W J, LV C H, CHENG L, et al. Targeting ERS-mitophagy in hippocampal neurons to explore the improvement of memory by tea polyphenols in aged type 2 diabetic rats[J]. Free Radical Biology and Medicine,2024,213:293−308. doi: 10.1016/j.freeradbiomed.2024.01.044
[98] JIN Z Z, CHANG B H, WEI Y L, et al. Curcumin exerts chondroprotective effects against osteoarthritis by promoting AMPK/PINK1/Parkin-mediated mitophagy[J]. Biomedicine & Pharmacotherapy,2022,151:113092.
[99] HAN X J, XU T S, FANG Q J, et al. Quercetin hinders microglial activation to alleviate neurotoxicity via the interplay between NLRP3 inflammasome and mitophagy[J]. Redox Biology,2021,44:102010. doi: 10.1016/j.redox.2021.102010
[100] YU X, XU Y Y, ZHANG S S, et al. Quercetin attenuates chronic ethanol-induced hepatic mitochondrial damage through enhanced mitophagy[J]. Nutrients,2016,8(1):27. doi: 10.3390/nu8010027
[101] CAO P, WANG Y, ZHANG C, et al. Quercetin ameliorates nonalcoholic fatty liver disease (NAFLD) via the promotion of AMPK-mediated hepatic mitophagy[J]. The Journal of Nutritional Biochemistry,2023,120:109414. doi: 10.1016/j.jnutbio.2023.109414
[102] 石拴霞, 王纪田, 宋诚, 等. 白藜芦醇介导AMPK/mTOR信号通路调控线粒体自噬缓解小鼠精原细胞氧化应激损伤和凋亡[J]. 中国药学杂志,2024,59(5):416−424. [SHI Shuanxia, WANG Jitian, SONG Cheng, et al. Investigation on resveratrol regulating mitophagy to alleviate oxidative stress injury and apoptosis of Gc-1 spg cells via AMPK/mTOR signaling pathway[J]. Chinese Pharmaceutical Journal,2024,59(5):416−424.] SHI Shuanxia, WANG Jitian, SONG Cheng, et al. Investigation on resveratrol regulating mitophagy to alleviate oxidative stress injury and apoptosis of Gc-1 spg cells via AMPK/mTOR signaling pathway[J]. Chinese Pharmaceutical Journal, 2024, 59(5): 416−424.
[103] PARK D, JEONG H, LEE M N, et al. Resveratrol induces autophagy by directly inhibiting mTOR through ATP competition[J]. Scientific Reports,2016,6(1):21772. doi: 10.1038/srep21772
[104] CHUNG S, YAO H W, CAITO S, et al. Regulation of SIRT1 in cellular functions:Role of polyphenols SIRT1[J]. Archives of Biochemistry and Biophysics,2010,501(1):79−90. doi: 10.1016/j.abb.2010.05.003





 下载:
下载:

 下载:
下载:



