Investigation of Active Fractions with Immune-enhancing Effects from the Stems and Leaves of Astragalus membranaceus and Its Chemical Components Identification
-
摘要: 目的:探讨黄芪茎叶增强免疫力的活性部位,并分析其化学成分。方法:采用环磷酰胺构建免疫低下小鼠模型,分别考察黄芪茎叶粗提物、石油醚部位、氯仿部位、乙酸乙酯部位、正丁醇部位和水部位对小鼠免疫力的影响,另设空白组、模型组和阳性组。连续给药28 d后,采用苏木素-伊红染色观察小鼠脾脏组织形态,并分析各组的免疫器官指数、免疫细胞能力及免疫因子水平,进而采用超高效液相色谱/离子淌度-四极杆飞行时间质谱对黄芪茎叶活性部位进行成分分析。结果:黄芪茎叶正丁醇部位表现出良好的增强免疫力活性,与模型组相比,该部位可显著提高小鼠的体重增长率、免疫器官指数、脾淋巴细胞增殖指数、巨噬细胞吞噬能力、迟发型变态反应程度和血清溶血素水平(P<0.05),并将小鼠血清中免疫球蛋白A、白细胞介素-6、干扰素-γ、肿瘤坏死因子-α水平分别显著提高了39.96%、15.87%、16.22%和47.68%(P<0.05)。经液相色谱-质谱联用分析鉴定,黄芪茎叶正丁醇部位的化学成分主要为黄酮类、皂苷类和酚酸类。结论:正丁醇部位是黄芪茎叶增强免疫力的活性部位,为后续深入研究黄芪茎叶的免疫调节作用奠定了基础。Abstract: Objective: Active fractions with immunomodulatory effects from the stems and leaves of Astragalus membranaceus (AMSL) were investigated and its chemical constituents were analyzed. Methods: The immunosuppression mice models were established by injection of cyclophosphamide, and the effects of several AMSL extracts on the immune response of mice were investigated, including crude extract, petroleum ether fraction, chloroform fraction, ethyl acetate fraction, n-butanol fraction and water fraction. Meanwhile, the control group, model group, and positive group were included. Hematoxylin-eosin staining was used to examine the spleen tissue morphology of mice after 28 d of continuous administration, and the immune organ indexes, immune cell capabilities, and levels of immune components in each group were analyzed. Furthermore, the components in the active fraction of AMSL were identified through ultra-performance liquid chromatography/ion mobility quadrupole time-of-flight mass spectrometry (UPLC/IM-QTOF-MS). Results: The n-butanol fraction from AMSL was proved to show significant immunomodulatory activity. In comparison to the model group, the growth rate of body weight, immune organ indexes, splenic lymphocyte proliferation index, macrophage phagocytic capacity, delayed allergic reaction severity, and serum hemolysin level in mice were significantly enhanced (P<0.05) in the group treated by this active fraction. Besides, the serum levels of immunoglobulin A, interleukin-6, interferon-γ and tumor necrosis factor-α were also significantly increased by 39.96%, 15.87%, 16.22% and 47.68% respectively after the intervention of this fraction (P<0.05), which was revealed to be primarily composed of such substances as flavonoids, saponins, and phenolic acids elucidated by UPLC/IM-QTOF-MS. Conclusion: The active fraction with immunomodulatory effects from AMSL was the n-butanol fraction, which established a basis for further study on the immunoregulatory activity of AMSL.
-
黄芪茎叶是豆科植物蒙古黄芪(Astragalus membranaceus (Fisch.) Bge. var. mongholicus (Bge.) Hsiao)或膜荚黄芪(Astragalus membranaceus (Fisch.) Bge.)的地上嫩茎和叶部分,其产量是地下根部的数倍[1]。由于黄芪茎叶是植物黄芪的非传统药用部位,每年伴随地下入药黄芪根的采挖,产生的近30万吨黄芪茎叶往往被直接丢弃或仅小部分作为低值饲料利用[2]。如何促进黄芪茎叶的精深开发与高值化应用成为一个亟待研究的重要课题。现代研究表明,黄芪茎叶含有与入药根部类似的多糖类、黄酮类、皂苷类等活性成分,被报道具有抗氧化[3]、抑菌[4]、抗病毒[5]等功效。毒理学研究表明黄芪茎叶的LD50>250 g/kg BW[6],具有极高的食用安全性,现已成为黑龙江省的地方食品[7],这为黄芪茎叶在功能食品领域的精深利用提供了坚实的理论和政策依据。然而,现有关于黄芪茎叶的功能研究主要集中于功效的整体评估,其功效成分尚不明确,这严重制约了黄芪茎叶在功能食品中的开发应用。
随着生活节奏的加快及学习工作压力的加大,特别是进入后疫情时代,免疫力低下逐渐成为民众普遍关注的健康问题,具有增强免疫力作用的功能食品或功效因子因此备受青睐[8]。植物黄芪入药根是一种补气固表的药食同源中药材,具有极强的增强免疫力作用,是增强免疫力产品开发的重要原料资源[9]。黄芪茎叶与入药黄芪根同属植物黄芪,且两者又含有相似类别的化学成分[10−11],这为以调节免疫作用为切入点深入开展黄芪茎叶的功效研究奠定了理论基础。既往研究表明,黄芪茎叶具有提高雏鸡生长性能与增强免疫功能的作用,可显著提高雏鸡细胞因子IL-2和IFN-γ含量[12],还可提高仔猪的免疫性能[13],然而其调节免疫的物质基础尚不明确。基于此,本研究采用环磷酰胺注射法构建小鼠免疫低下模型,通过比较黄芪茎叶粗提物及不同萃取部位的增强免疫力作用,探究黄芪茎叶发挥免疫调节功效的活性部位,并进一步明确活性部位的化学成分,以期为黄芪茎叶资源在增强免疫力功能食品领域的开发与精深利用提供科学基础。
1. 材料与方法
1.1 材料与仪器
SPF级5~8周龄ICR雄性小鼠 体重(20±2)g,购于斯贝福(北京)生物技术有限公司(许可证号:SCXK(京)2019-0010)(生产批号:No.110324230102006521),动物实验伦理审批号:AWE202309380。黄芪茎叶 采自山西省浑源县,经山西中医药大学中药资源与鉴定教研室刘计权教授鉴定;环磷酰胺 上海麦克林生化科技有限公司;盐酸左旋咪唑、刀豆蛋白A(ConA) 北京索莱宝科技有限公司;5%绵羊红细胞 广州鸿泉科技有限公司;RPMI-1640培养基 北京中生奥邦生物科技有限公司;印度墨汁、CCK-8(CellcountingKit-8)试剂 大连美仑生物技术有限公司;都氏试剂 厦门海标科技有限公司;ELISA试剂盒 江苏晶美生物科技有限公司;红细胞裂解液 北京博奥拓达科技有限公司;4%多聚甲醛组织固定液 武汉博士德生物工程有限公司;乙腈 质谱纯,购于赛默飞世尔科技(中国)有限公司。
AR223CN型电子分析天平 奥豪斯仪器有限公司;SB25-12D型超声波清洗机 宁波新芝生物科技股份有限公司;HC-2015高速离心机 安徽中科中佳科学仪器有限公司;Spectra Max 190酶标仪 北京生元诚业科技有限公司;BBS-V800SW-CJ型超净工作台 美国BIOBASE公司;Galaxy 170S CO2恒温细胞培养箱 德国Eppendorf公司;超高效液相色谱、VION离子淌度四极杆飞行时间质谱联用仪 上海沃特世科技有限公司。
1.2 实验方法
1.2.1 黄芪茎叶粗提物及不同萃取部位的制备
采用课题组前期建立的提取方法,称取黄芪茎叶粉末60 g,以料液比1:12加入65%乙醇,70 ℃超声提取40 min,合并提取2次的滤液旋蒸后浓缩至干得黄芪茎叶粗提物浸膏。取粗提物浸膏用20倍水复溶,依次用石油醚、氯仿、乙酸乙酯、正丁醇萃取三次,合并各萃取相并浓缩干燥,分别得石油醚部位、氯仿部位、乙酸乙酯部位、正丁醇部位和水部位。称取适量粗提物浸膏和各部位浸膏,用0.5% CMC-Na溶液稀释至一定浓度,备用。
1.2.2 动物分组与给药
小鼠适应一周后随机分为正常组(NC组)、模型组(MC组)、阳性组(PC组)、粗提物组(TE组)、石油醚部位组(PEF组)、氯仿部位组(CF组)、乙酸乙酯部位组(EAF组)、正丁醇部位组(BF组)和水部位组(WF组),每组12只,实验周期28 d。实验第1~3 d,除正常组外,其余各组小鼠按80 mg/(kg BW)剂量腹腔注射环磷酰胺造模,连续观察小鼠精神状态并记录体重变化。实验第4~28 d进行药物干预,其中阳性对照组按40 mg/(kg BW)剂量灌胃盐酸左旋咪唑溶液,不同部位组分别按320.64 mg/kg(粗提物组)、5.73 mg/kg(石油醚部位组)、14.61 mg/kg(氯仿部位组)、19.38 mg/kg(乙酸乙酯部位组)、66.61 mg/kg(正丁醇部位组)、155.61 mg/kg(水部位组)剂量给予灌胃,使不同给药组均具有相同的黄芪茎叶当量给予量(9 g)。以石油醚部位给药量的确定为例,黄芪茎叶每日用量设为9 g(参考《中国药典》2020年版黄芪每日临床用量),因石油醚部位得率为0.42%,故石油醚部位组的给药量为5.73 mg/kg(9×9.1×0.42%/60 kg)。正常组和模型组每日灌胃等量0.5% CMC-Na溶液。
1.2.3 免疫指标测定
参照《保健食品功能检验与评价方法(2023年版)》中方法进行免疫指标测定。其中,以迟发型变态反应与半数溶血值反映特异性细胞免疫与体液免疫功能,以碳廓清实验反映非特异性免疫功能。
1.2.3.1 体重及免疫器官指数测定
实验结束后称重,按下式(1)计算各组的体重增长率。摘眼球取血,待小鼠无生命体征后进行解刨,取胸腺、脾脏,置生理盐水中反复冲洗后去除筋膜,滤纸吸干脏器表面并称重,按下式(2)、(3)计算免疫器官指数。
体重增长率(%)=药物干预期体重变化量干预初始体重×100 (1) 胸腺指数=胸腺重量体重 (2) 脾脏指数=脾脏重量体重 (3) 1.2.3.2 细胞免疫功能测定(迟发型变态反应)
实验第21 d,对各组小鼠腹腔注射0.2 mL 5%绵羊红细胞(SRBC)进行致敏,之后5 d测量各组小鼠左后足趾厚度(即致敏前足趾厚度),随后在测量部位给每组小鼠皮下注射20 μL 20% SRBC,24 h后再次测量左后足趾厚度(即致敏后足趾厚度),致敏前后的足趾厚度差值即为足趾增厚度,以此反映迟发型变态反应的程度。
1.2.3.3 体液免疫功能测定(半数溶血值测定)
同1.2.3.2进行致敏,6 d后摘眼球取血,2500 r/min离心10 min后分离上层血清,取稀释200倍的血清样品1 mL于试管内,加入1 mL 10%豚鼠血清补体和0.5 mL 5% SRBC,37 ℃水浴30 min后冰浴终止反应,2500 r/min离心,15 min后取上清1 mL并加入3 mL都氏试剂即为样品管。以等体积生理盐水代替血清作为对照半数溶血管,测定540 nm处吸光度值并计算半数溶血值(HC50)。按下式(4)计算半数溶血值。
HC50=A样品ASRBC半数溶血×200 (4) 1.2.3.4 单核-巨噬细胞功能测定(碳廓清实验)
实验第28 d,小鼠尾静脉注射20%印度墨汁0.1 mL/10 g,分别于注射后2 min和10 min,从眼眶内眦静脉取血20 μL,分别加入2.0 mL 0.1%碳酸钠溶液中摇匀,以0.1%碳酸钠溶液作空白对照,测定600 nm处吸光度值,按下式(5)、(6)计算廓清指数K和吞噬指数。
K=lgOD1−lgOD2t2−t1 (5) 吞噬指数α=体重肝重+脾重×3√K (6) 式中:t1为注射后2 min;t2为注射后10 min;OD1为2 min所取血样的吸光度值;OD2为10 min所取血样的吸光度值。
1.2.3.5 脾淋巴细胞增殖试验(CCK-8法)
实验结束后,取无菌小鼠脾脏,于4层无菌纱布研磨后用无血清的RPMI-1640培养基冲洗,收集冲洗液并离心(1500 r/min,5 min),去除上清后加入1×红细胞裂解液反应10 min,再次离心去上清,加入PBS洗涤后获得脾细胞悬液[14]。将经细胞计数的脾细胞接种于96孔板(密度为5×105个),设试验孔(含5 μg/L ConA)与对照孔(不含ConA)在37 ℃、5% CO2条件下培养48 h,按照CCK-8试剂盒说明书检测每孔OD值,并按下式(7)计算脾淋巴细胞增殖指数[15]。
增殖指数=试验孔OD值对照孔OD值 (7) 1.2.3.6 血清免疫因子水平测定(ELISA试剂盒法)
严格参照ELISA试剂盒说明书,测定各组小鼠血清中免疫球蛋白A(IgA)、免疫球蛋白G(IgG)、白细胞介素2(IL-2)、白细胞介素6(IL-6)、干扰素γ(IFN-γ)和肿瘤坏死因子α(TNF-α)水平。
1.2.3.7 脾脏组织形态观察
解剖小鼠后分离脾脏组织,记录小鼠脾脏组织形态,经甲醛固定后进行常规HE染色,显微镜下观察小鼠脾脏病理学变化。
1.2.4 黄芪茎叶免疫活性部位的成分分析
采用超高效液相色谱/离子淌度-四极杆飞行时间质谱对所筛选出的黄芪茎叶免疫活性部位进行成分分析。色谱条件为:UPLC BEH C18色谱柱(2.1 mm×100 mm,1.7 μm),流速0.4 mL/min,检测波长254 nm和350 nm,进样量1 μL,流动相由0.1%甲酸水(A)-乙腈(B)组成,梯度洗脱(0~3 min,95%~80% A;3~10 min,80%~0% A;10~12 min,0% A;12~15 min,0%~95% A;15~20 min,95% A)。质谱条件为:采用正负离子分别扫描,质量扫描范围50~2000 m/z,雾化气温度:450 ℃,流量:900 L/h,毛细管电压:2 KV。
1.3 数据处理
使用SPSS 20. 0对实验结果进行统计学分析,结果以¯x±s表示,P<0.05表示差异显著。采用Graph Prism 8.0软件绘图。
2. 结果与分析
2.1 黄芪茎叶粗提物及不同萃取部位对小鼠体重增长率和免疫器官指数的影响
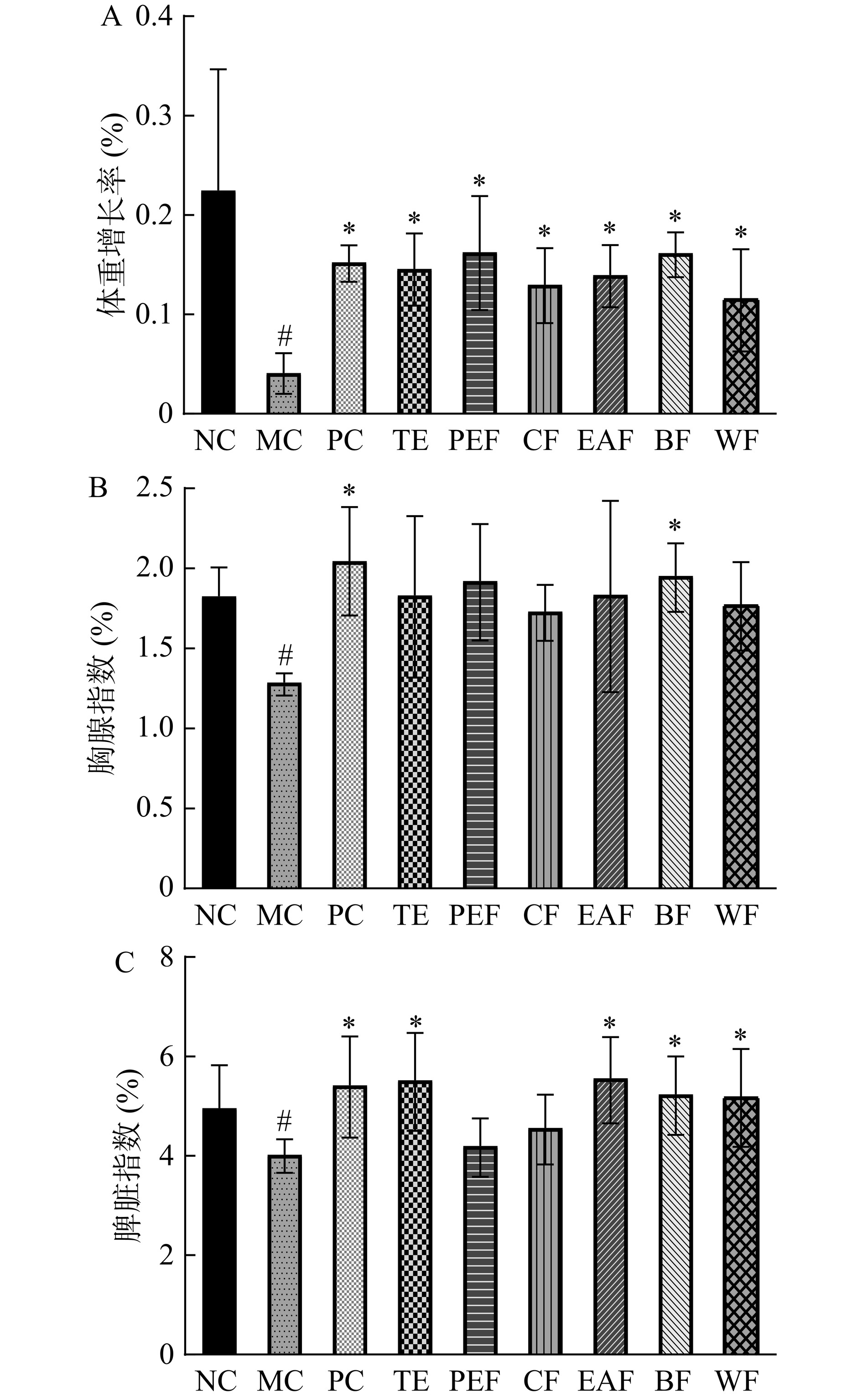
黄芪茎叶粗提物及不同萃取部位对小鼠体重增长率和免疫器官指数的影响结果如图1所示。体重变化是反映机体是否健康的首要指标,与正常组相比,模型组体重增长率显著降低(P<0.05);与模型组相比,粗提物及不同萃取部位组的体重增长率均显著提高(P<0.05)。
![]() 图 1 黄芪茎叶粗提物及不同萃取部位对小鼠体重增长率(A)、胸腺指数(B)和脾脏指数(C)的影响注:NC:正常组;MC:模型组;PC:阳性组;TE:粗提物组;PEF:石油醚部位组;CF:氯仿部位组;EAF:乙酸乙酯部位组;BF:正丁醇部位组;WF:水部位组。#表示模型组与正常组存在显著差异(P<0.05);*表示给药组与模型组相比存在显著差异(P<0.05),图2~图6同。Figure 1. Effects of the crude extract and different extraction fractions from Astragalus membranaceus stems and leaves on body weight growth rate (A), thymus index (B) and spleen index (C) of mice
图 1 黄芪茎叶粗提物及不同萃取部位对小鼠体重增长率(A)、胸腺指数(B)和脾脏指数(C)的影响注:NC:正常组;MC:模型组;PC:阳性组;TE:粗提物组;PEF:石油醚部位组;CF:氯仿部位组;EAF:乙酸乙酯部位组;BF:正丁醇部位组;WF:水部位组。#表示模型组与正常组存在显著差异(P<0.05);*表示给药组与模型组相比存在显著差异(P<0.05),图2~图6同。Figure 1. Effects of the crude extract and different extraction fractions from Astragalus membranaceus stems and leaves on body weight growth rate (A), thymus index (B) and spleen index (C) of mice胸腺和脾脏是免疫应答的重要场所,其指数能在一定程度上反映机体免疫功能的强弱[16−17]。与正常组相比,模型组胸腺指数显著减小(P<0.05),而阳性组、正丁醇部位组的胸腺指数较模型组显著增大(P<0.05),其余组别未能显著提高胸腺指数(P>0.05)。模型组脾脏指数较正常组显著减小(P<0.05),而阳性组、粗提物组、乙酸乙酯部位组、正丁醇部位组和水部位组的脾脏指数又较模型组显著升高(P<0.05)。由此可以推测出黄芪茎叶粗提物及不同萃取部位可维持免疫低下小鼠的体重,尤其是正丁醇部位还可显著促进免疫器官的发育,其作用效果与女贞子正丁醇部位一致[18]。
2.2 黄芪茎叶粗提物及不同萃取部位对小鼠迟发型变态反应的影响
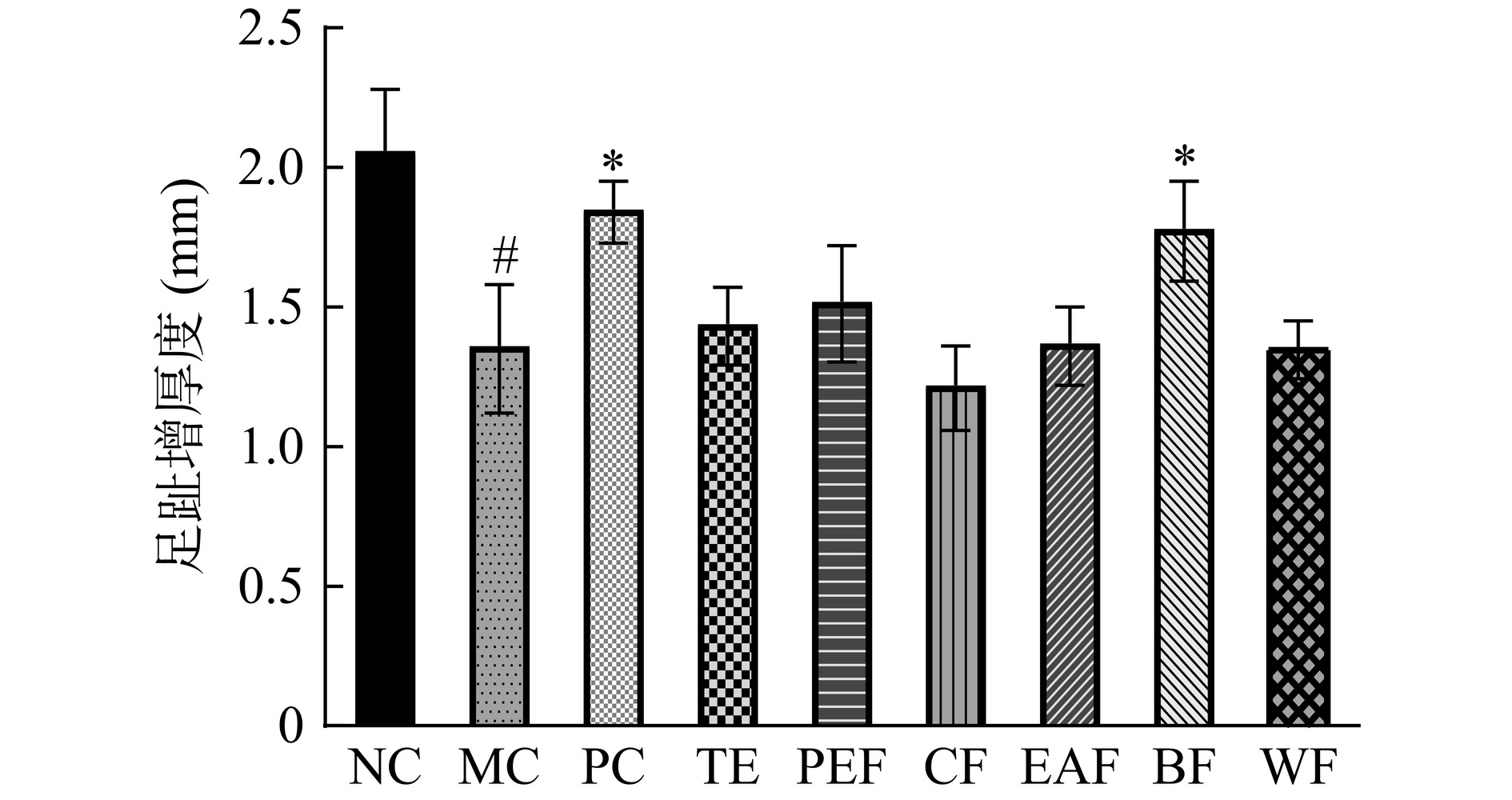
迟发型变态反应是由抗原诱导、效应T细胞介导的特异性免疫应答,是评价细胞免疫功能的常用方法之一,受抗原再次攻击时局部组织的肿胀程度可说明细胞免疫的强弱[19]。黄芪茎叶粗提物及不同萃取部位对小鼠迟发型变态反应的影响如图2所示。与正常组相比,模型组足趾增厚度显著降低(P<0.05),阳性组、正丁醇部位组的足趾增厚度又较模型组显著提高(P<0.05),说明黄芪茎叶正丁醇部位可提高免疫低下小鼠的细胞免疫功能。这一结果与黄芪甲苷显著抑制迟发型变态反应、恢复受损的细胞免疫系统的作用效果类似[20]。
2.3 黄芪茎叶粗提物及不同萃取部位对小鼠半数溶血值的影响
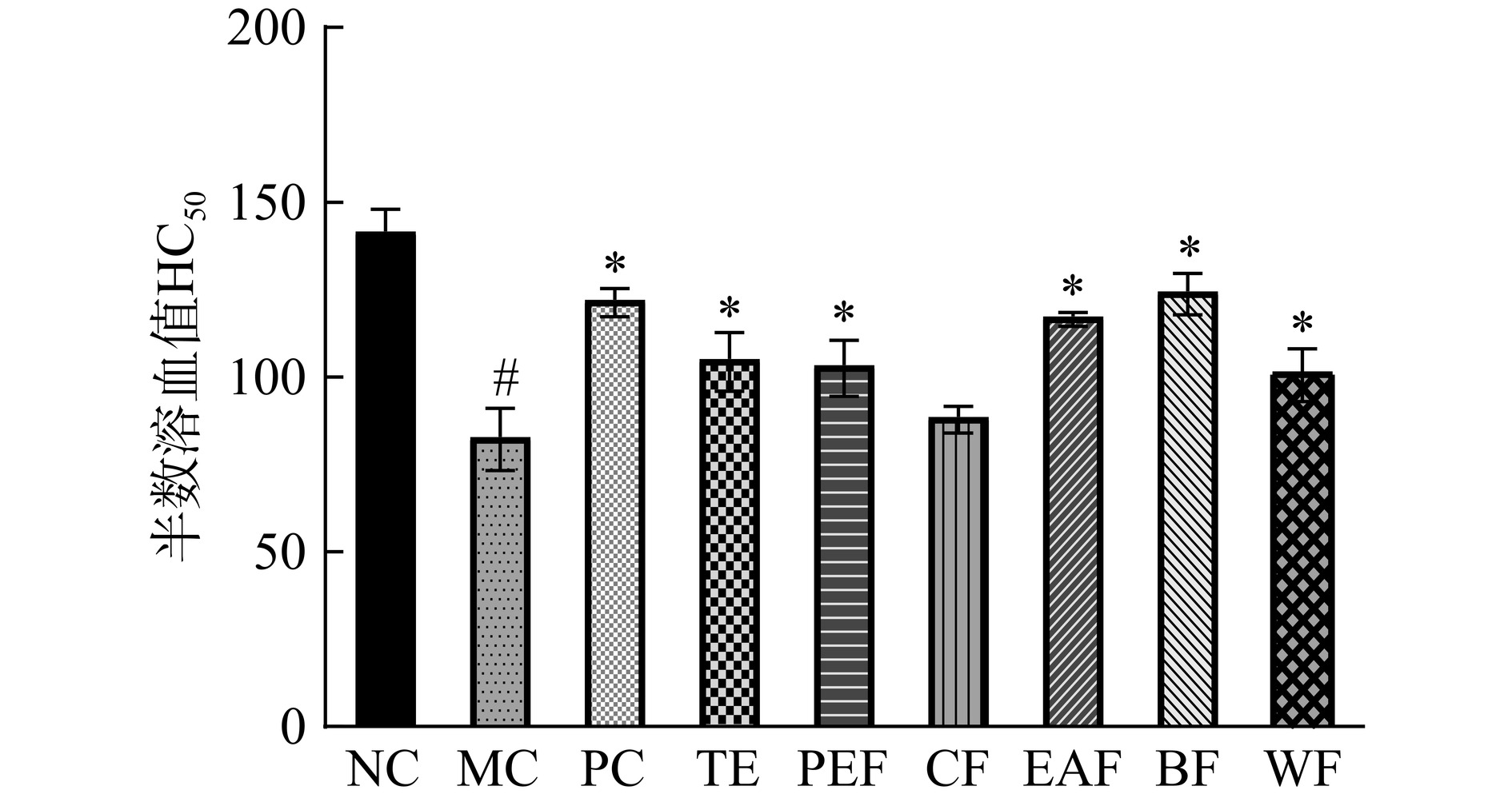
血清溶血素多在抗原攻击后产生,其生成水平表示产生特异性抗体的能力。血清溶血素含量常以半数溶血值(HC50)表示,半数溶血值HC50越高,表明体液免疫能力越强[21]。黄芪茎叶粗提物及不同萃取部位对小鼠半数溶血值的影响如图3所示。结果显示,与正常组相比,模型组HC50显著降低(P<0.05),除氯仿部位组外的其余给药组均具有较模型组更高的HC50(P<0.05),其中乙酸乙酯部位组(117.32±1.09)和正丁醇部位组(124.52±5.11)的HC50水平更接近于阳性组(122.08±3.25),说明乙酸乙酯部位和正丁醇部位可有效刺激免疫抑制小鼠产生溶血素抗体,改善体液免疫功能。
2.4 黄芪茎叶粗提物及不同萃取部位对小鼠巨噬细胞吞噬能力的影响
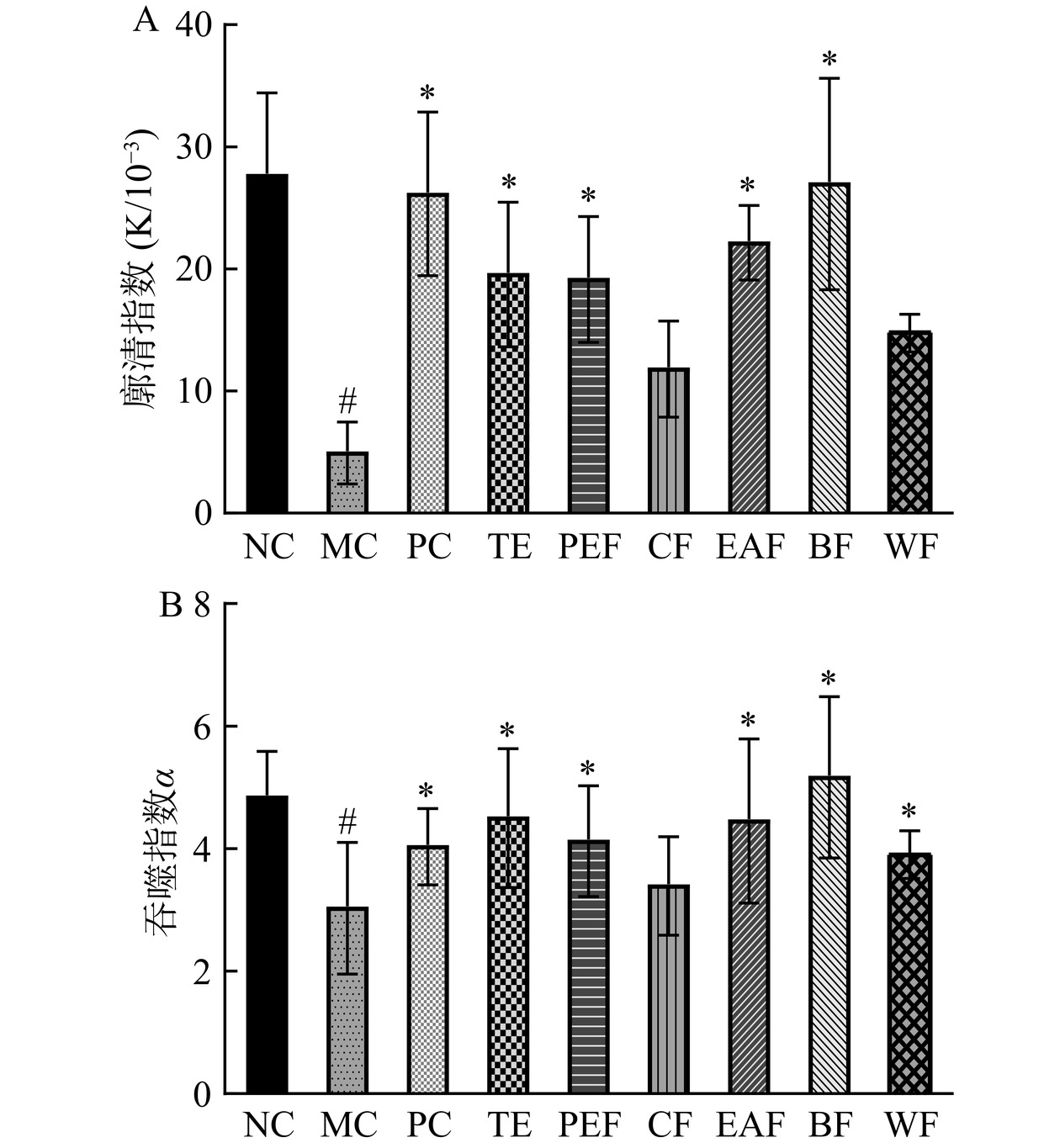
廓清指数和吞噬指数是反映巨噬细胞吞噬能力的关键指标,而巨噬细胞的吞噬能力又是评估非特异性免疫能力的重要依据[22−23]。黄芪茎叶粗提物及不同萃取部位对小鼠巨噬细胞吞噬能力的影响如图4所示。结果显示,与正常组相比,模型组廓清指数和吞噬指数显著降低(P<0.05),表明环磷酰胺明显抑制了小鼠的固有免疫功能。除氯仿部位组和水部位组外,其余给药组的廓清指数和吞噬指数均较模型组显著提高(P<0.05),说明黄芪茎叶粗提物、石油醚部位、乙酸乙酯部位和正丁醇部位均可不同程度地提高免疫低下小鼠的巨噬细胞吞噬能力,其中以正丁醇部位的作用更加突出。
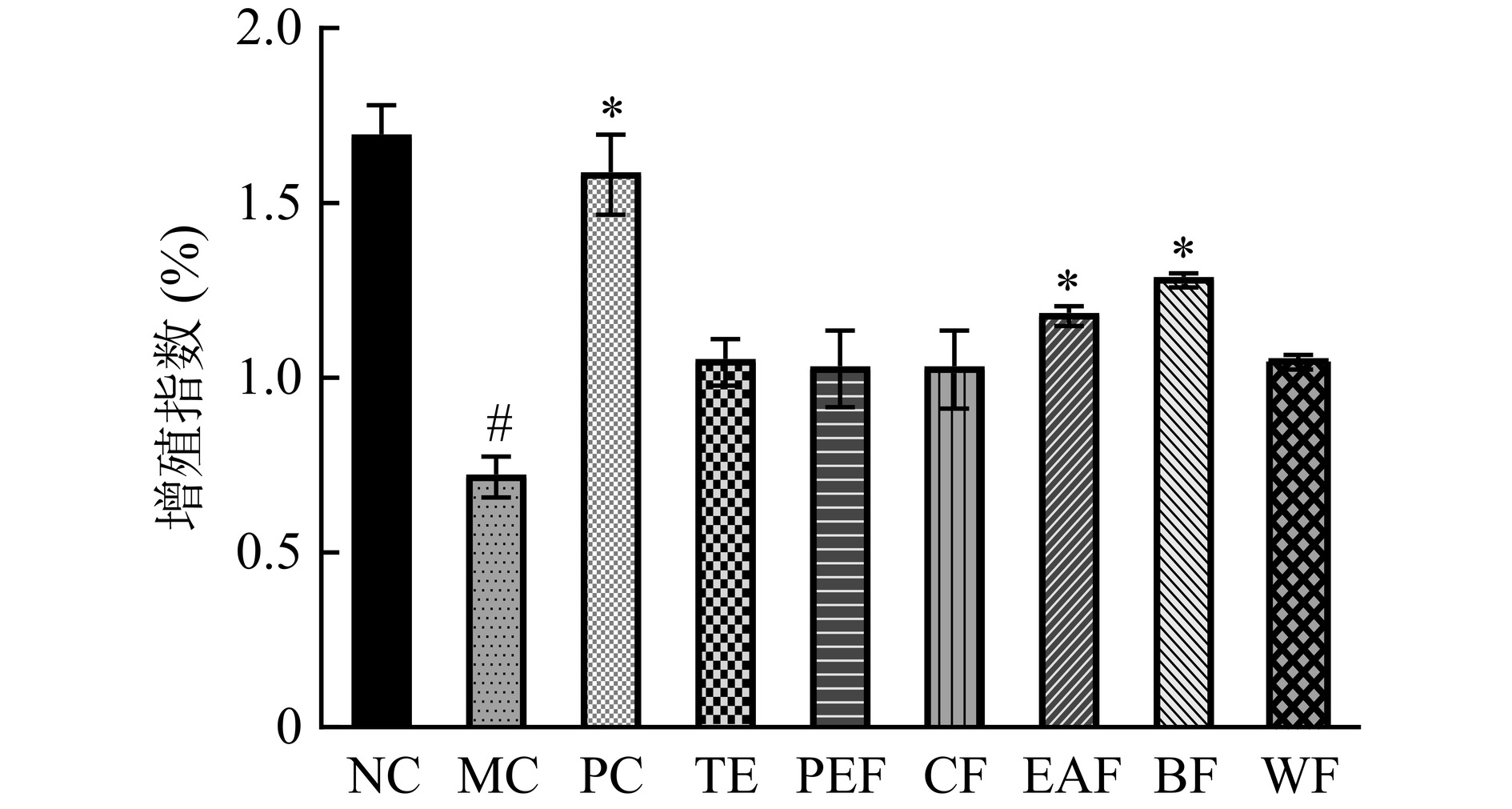
2.5 黄芪茎叶粗提物及不同萃取部位对脾淋巴细胞增殖的影响
黄芪茎叶粗提物及不同萃取部位对脾淋巴细胞增殖的影响如图5所示。脾淋巴细胞是机体特异性免疫应答中的关键细胞,其增殖分化程度可作为免疫功能的评价指标[24]。结果显示,与正常组相比,模型组脾淋巴细胞增殖指数显著降低(P<0.05);与模型组相比,阳性组、乙酸乙酯部位组和正丁醇部位组具有显著升高的脾淋巴细胞增殖指数(P<0.05),说明黄芪茎叶正丁醇部位和乙酸乙酯部位对增强免疫低下小鼠的脾淋巴细胞增殖能力有正向调节作用。杨婧妍等[25]发现板蓝根正丁醇部位同样具备促进小鼠脾淋巴细胞增殖的作用,与本研究结果相似,提示该部位中含有的极性物质可有效提高免疫细胞活性,进而影响免疫系统功能。
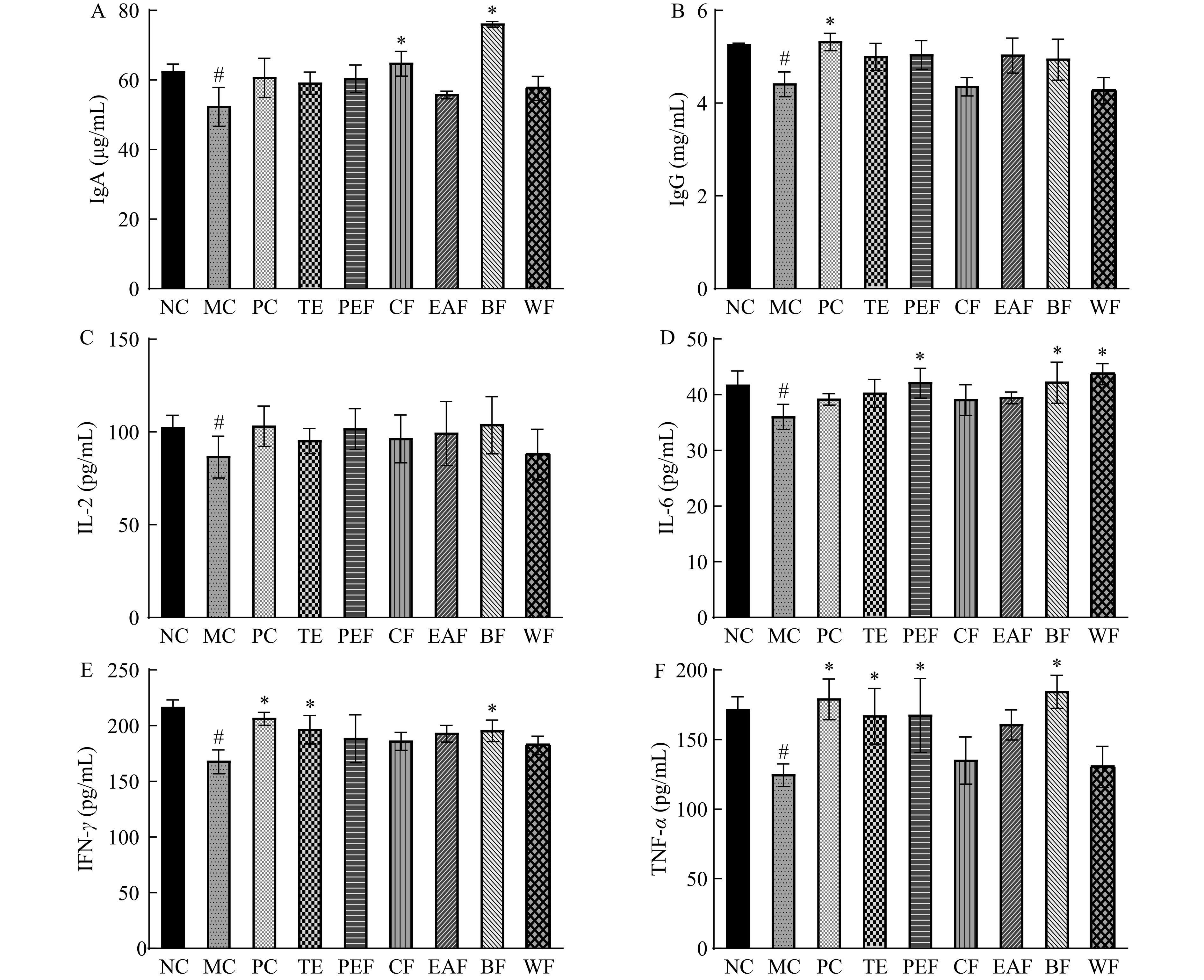
2.6 黄芪茎叶粗提物及不同萃取部位对血清免疫因子的影响
黄芪茎叶粗提物及不同萃取部位对血清免疫因子的影响如图6所示。免疫球蛋白、白细胞介素、干扰素和肿瘤坏死因子是调节免疫活性的关键物质,其中免疫球蛋白主要参与调控体液免疫功能,白细胞介素主要参与调节免疫细胞增殖过程,干扰素和肿瘤坏死因子主要负责调节细胞增殖分化与细胞效应功能[26−28]。结果显示,与正常组相比,模型组小鼠血清中IgA、IgG、IL-2、IL-6、IFN-γ、TNF-α水平均显著降低(P<0.05),表明经环磷酰胺造模后,模型小鼠的免疫水平得到了抑制;与模型组相比,除IL-2和IgG水平未受受试物的影响外(P>0.05),其余指标均在所给受试物的调节下表现出不同程度地显著变化。综合来看,正丁醇部位组小鼠血清中的IgA、IL-6、IFN-γ、TNF-α水平均较模型组显著提高(P<0.05),说明黄芪茎叶正丁醇部位可较好地恢复免疫低下小鼠血清免疫因子分泌减少的情况,提示着该部位拥有较强的免疫增强活性。JAK/STAT通路是一条参与免疫调节的重要信号通路,该通路的适度激活可促进免疫细胞的活化和增殖,IL-6和IFN-γ被报道可激活JAK/STAT通路,从而促进巨噬细胞表达和淋巴细胞产生抗体[29]。本研究所观察到的正丁醇部位对IL-6和IFN-γ分泌的促进提示着黄芪茎叶的增强免疫活性可能与JAK/STAT通路有关。
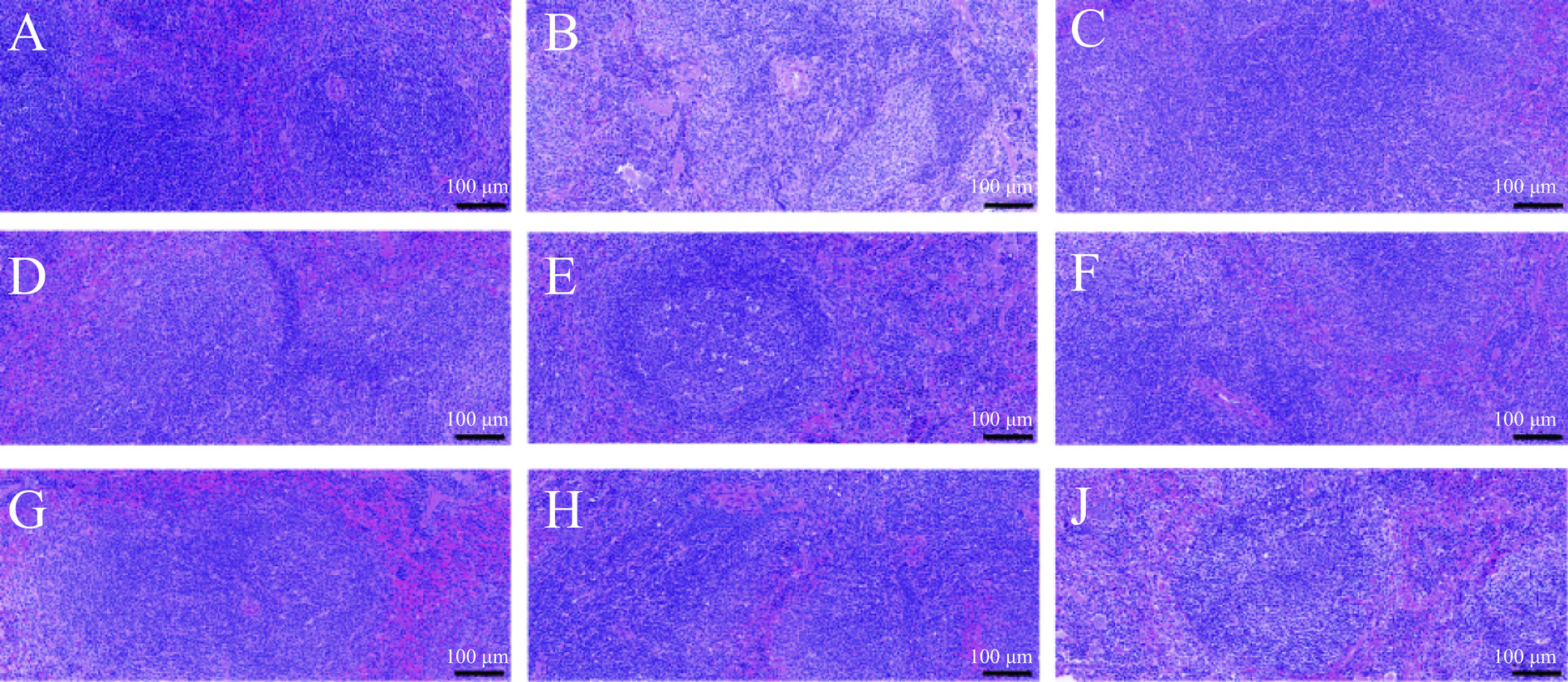
2.7 黄芪茎叶粗提物及不同萃取部位对小鼠脾脏组织形态的影响
小鼠脾脏HE染色结果如图7所示。脾脏是机体最重要的免疫器官,其组织结构也能从一定程度上反映机体免疫功能和损伤程度[30]。结果显示,正常组小鼠的脾脏组织结构完整,白髓与红髓之间分界清晰,白髓区呈现较深的蓝色,淋巴细胞紧密排列。与正常组相比,模型组小鼠的脾脏组织破损严重,发生严重病变;白髓与红髓结构紊乱、界限模糊,白髓区染色颜色浅,淋巴细胞数量减少,脾主动脉严重扭曲变形,脾小梁结构松散。
与模型组相比,阳性组小鼠的脾脏损伤得到缓解,白髓与红髓之间分界较清晰,白髓染色程度高,淋巴细胞数量多,整体结构较模型组有所改善,说明盐酸左旋咪唑可减轻环磷酰胺对脾脏的破坏。黄芪茎叶粗提物及不同萃取部位组对小鼠的脾脏损伤均有不同程度地改善,其中乙酸乙酯部位组与正丁醇部位组改善效果更为明显,主要表现为乙酸乙酯部位组脾脏白髓与红髓界限清晰、淋巴细胞数量增多;正丁醇部位组脾脏淋巴细胞数量增多、脾中央动脉形变程度低,提示黄芪茎叶乙酸乙酯部位和正丁醇部位可较好地恢复免疫低下小鼠的脾脏组织形态。
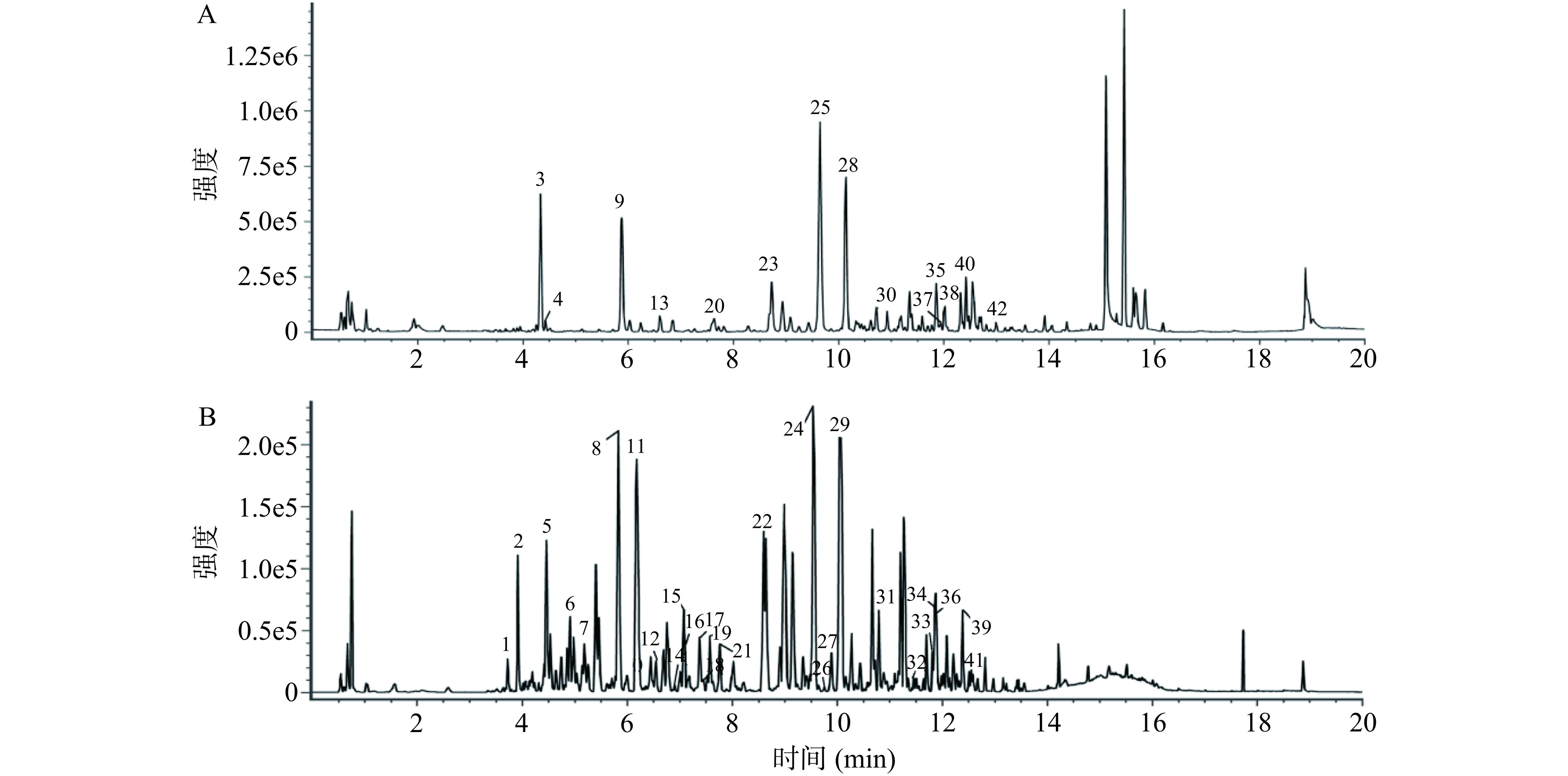
2.8 黄芪茎叶免疫活性部位成分分析
由前述可知,黄芪茎叶粗提物及不同萃取部位的免疫活性存在一定差异,其中正丁醇部位具有更为显著的多方面免疫活性。为了进一步明确黄芪茎叶增强免疫力功效的化学成分基础,对黄芪茎叶正丁醇部位的成分进行了分析,所得的BPI离子流图见图8。通过质荷比确定化合物的准分子离子峰,结合其二级质谱碎片离子,进行化学成分鉴定。从黄芪茎叶正丁醇部位中共鉴定出42种化合物,包括25种黄酮类、13种皂苷类和4种酚酸类,各化学成分信息详见表1。
表 1 黄芪茎叶正丁醇部位化学成分Table 1. Chemical constituents of n-butanol fraction from Astragalus membranaceus stems and leaves峰号 成分名称 保留时间(min) 分子式 分子量 质量偏差(ppm) 离子形式 1 Isovanillic acid 3.70 C8H8O4 167.03 −2.4 −H 2 2,3-Dihydroxybenzoic acid 3.92 C7H6O4 153.02 −4.1 −H 3 Vanillic acid 4.36 C8H8O4 151.04 −1.7 M-H2O+H 4 Daidzin 4.45 C21H20O9 417.12 0.5 +H 5 Taxifolin 3-O-rhamnoside 4.54 C21H22O11 449.11 −0.6 −H 6 Leucoside 4.86 C26H28O15 625.14 −1.2 +HCOO 7 Rutin 5.26 C27H30O16 609.15 −1.4 −H 8 Isoquercitrin 5.83 C21H20O12 463.09 −0.7 −H 9 Dihydromyricetin 5.88 C15H12O8 303.05 −1 M-H2O+H 10 Hesperidin 5.99 C28H34O15 609.18 −0.8 −H 11 Baicalin 6.20 C21H18O11 445.08 −1.6 −H 12 Astragalin 6.55 C21H20O11 447.09 −1.4 −H 13 Luteolin 6.61 C15H10O6 287.05 −0.9 +H 14 Calycosin 6.91 C16H12O5 283.06 −2.1 −H 15 Isorhamnetin 3,4'-diglucoside 7.01 C28H32O17 639.16 −1.6 −H 16 Odoratin 7.04 C17H14O6 313.07 −1.4 −H 17 Quercetin 3-O-β-D-(6''-p-coumaroyl)glucopyranosyl(1-2)-α-L-rhamnopyranoside 7.32 C36H36O18 801.19 −1.1 +HCOO 18 Isorhamnetin 7.45 C16H12O7 315.05 −1.4 −H 19 Quercetin 3-Caffeylrobinobioside 7.58 C36H36O19 771.18 −2 −H 20 Dihydromyricetin 7.63 C15H12O8 303.05 −0.9 M-H2O+H 21 Pratensein 7.74 C16H12O6 299.06 −1.7 −H 22 Tricin 7-O-glucoside 8.64 C21H20O10 477.10 −1.6 +HCOO 23 Isorhamnetin-3-O-β-D-glucoside 8.73 C16H12O7 317.07 −0.5 +H 24 Pratensein-7-O-β-D-glucoside 9.56 C22H22O11 461.11 −2.2 −H 25 Kaempferide 9.65 C16H12O6 301.07 −1.3 +H 26 Glycitin 9.76 C22H22O10 491.12 −0.8 +HCOO 27 Kaempferol-3-O-(6'''-trans-p-coumaroyl-2''-glucosyl)rhamnoside 9.88 C36H36O17 785.19 −1.6 +HCOO 28 Rhamnocitrin 10.14 C16H12O6 301.07 −0.4 +H 29 6''-O-Acetylglycitin 10.26 C24H24O11 533.13 −1.4 +HCOO 30 Astragaloside III 10.85 C41H68O14 785.47 0.7 +H 31 Cycloastragenol-6-O-β-D-glucoside 10.87 C36H60O10 697.42 −1.6 +HCOO 32 Astragaloside II 11.46 C43H70O15 871.47 −0.9 +HCOO, −H 33 Acetytastragaloside 11.82 C47H74O17 955.49 −1.2 +HCOO 34 Astragaloside VIII 11.85 C47H76O17 911.50 −1.2 −H 35 Cycloastragenol 11.87 C30H50O5 513.36 4.5 +Na 36 Soyasaponino 11.88 C48H78O18 941.51 −1.2 −H,+HCOO 37 Astragaloside Ⅰ 11.99 C45H72O16 891.47 −1.2 +Na,+H,
M-H2O+H38 Astragaloside IV 12.10 C41H68O14 767.46 −1.1 M-H2O+H 39 Neoastragaloside I 12.41 C45H72O16 913.48 −0.5 +HCOO 40 Isoastragaloside I 12.46 C45H72O16 869.49 −0.2 +H 41 Isoastragaloside Ⅳ 12.57 C41H68O14 783.45 −1.2 −H 42 Astragaloside 13.00 C41H68O14 785.47 −0.1 +H 研究表明,皂苷类成分是植物黄芪发挥免疫调节活性的主要物质,其可作用于多种免疫活性细胞,刺激细胞因子的分泌并促进正常机体生成抗体,如黄芪甲苷可上调环磷酰胺所致免疫抑制小鼠血清中IL-2和IFN-γ含量,使小鼠免疫功能有所改善[31]。黄酮类和酚酸类化合物也被报道可强化吞噬细胞的免疫活性,增强T、B淋巴细胞的免疫功能,影响机体免疫因子的表达[32−33]。这表明黄芪茎叶正丁醇部位的增强免疫力作用可能是其所含的黄酮类、皂苷类和酚酸类化合物协同作用的结果,但考虑到正丁醇部位中所含的丰富黄酮类成分,推测黄酮类成分是黄芪茎叶增强免疫力的潜在活性物质基础,具体还有待后续进一步深入研究。
3. 结论
本文以免疫低下模型小鼠为实验动物,从免疫器官、固有免疫、体液免疫、细胞免疫和免疫分子等多个维度探讨了黄芪茎叶的免疫调节作用,通过分析比较黄芪茎叶不同萃取部位的活性,发现正丁醇部位是黄芪茎叶发挥免疫增强功效的主要活性部位,为黄芪茎叶在增强免疫力功能食品领域的精深开发利用提供了一定的科学依据。后续可进一步对黄芪茎叶增强免疫力的具体功效成分及作用机制进行深入研究,以揭示黄芪茎叶作用于免疫系统的科学内涵。
-
图 1 黄芪茎叶粗提物及不同萃取部位对小鼠体重增长率(A)、胸腺指数(B)和脾脏指数(C)的影响
注:NC:正常组;MC:模型组;PC:阳性组;TE:粗提物组;PEF:石油醚部位组;CF:氯仿部位组;EAF:乙酸乙酯部位组;BF:正丁醇部位组;WF:水部位组。#表示模型组与正常组存在显著差异(P<0.05);*表示给药组与模型组相比存在显著差异(P<0.05),图2~图6同。
Figure 1. Effects of the crude extract and different extraction fractions from Astragalus membranaceus stems and leaves on body weight growth rate (A), thymus index (B) and spleen index (C) of mice
表 1 黄芪茎叶正丁醇部位化学成分
Table 1 Chemical constituents of n-butanol fraction from Astragalus membranaceus stems and leaves
峰号 成分名称 保留时间(min) 分子式 分子量 质量偏差(ppm) 离子形式 1 Isovanillic acid 3.70 C8H8O4 167.03 −2.4 −H 2 2,3-Dihydroxybenzoic acid 3.92 C7H6O4 153.02 −4.1 −H 3 Vanillic acid 4.36 C8H8O4 151.04 −1.7 M-H2O+H 4 Daidzin 4.45 C21H20O9 417.12 0.5 +H 5 Taxifolin 3-O-rhamnoside 4.54 C21H22O11 449.11 −0.6 −H 6 Leucoside 4.86 C26H28O15 625.14 −1.2 +HCOO 7 Rutin 5.26 C27H30O16 609.15 −1.4 −H 8 Isoquercitrin 5.83 C21H20O12 463.09 −0.7 −H 9 Dihydromyricetin 5.88 C15H12O8 303.05 −1 M-H2O+H 10 Hesperidin 5.99 C28H34O15 609.18 −0.8 −H 11 Baicalin 6.20 C21H18O11 445.08 −1.6 −H 12 Astragalin 6.55 C21H20O11 447.09 −1.4 −H 13 Luteolin 6.61 C15H10O6 287.05 −0.9 +H 14 Calycosin 6.91 C16H12O5 283.06 −2.1 −H 15 Isorhamnetin 3,4'-diglucoside 7.01 C28H32O17 639.16 −1.6 −H 16 Odoratin 7.04 C17H14O6 313.07 −1.4 −H 17 Quercetin 3-O-β-D-(6''-p-coumaroyl)glucopyranosyl(1-2)-α-L-rhamnopyranoside 7.32 C36H36O18 801.19 −1.1 +HCOO 18 Isorhamnetin 7.45 C16H12O7 315.05 −1.4 −H 19 Quercetin 3-Caffeylrobinobioside 7.58 C36H36O19 771.18 −2 −H 20 Dihydromyricetin 7.63 C15H12O8 303.05 −0.9 M-H2O+H 21 Pratensein 7.74 C16H12O6 299.06 −1.7 −H 22 Tricin 7-O-glucoside 8.64 C21H20O10 477.10 −1.6 +HCOO 23 Isorhamnetin-3-O-β-D-glucoside 8.73 C16H12O7 317.07 −0.5 +H 24 Pratensein-7-O-β-D-glucoside 9.56 C22H22O11 461.11 −2.2 −H 25 Kaempferide 9.65 C16H12O6 301.07 −1.3 +H 26 Glycitin 9.76 C22H22O10 491.12 −0.8 +HCOO 27 Kaempferol-3-O-(6'''-trans-p-coumaroyl-2''-glucosyl)rhamnoside 9.88 C36H36O17 785.19 −1.6 +HCOO 28 Rhamnocitrin 10.14 C16H12O6 301.07 −0.4 +H 29 6''-O-Acetylglycitin 10.26 C24H24O11 533.13 −1.4 +HCOO 30 Astragaloside III 10.85 C41H68O14 785.47 0.7 +H 31 Cycloastragenol-6-O-β-D-glucoside 10.87 C36H60O10 697.42 −1.6 +HCOO 32 Astragaloside II 11.46 C43H70O15 871.47 −0.9 +HCOO, −H 33 Acetytastragaloside 11.82 C47H74O17 955.49 −1.2 +HCOO 34 Astragaloside VIII 11.85 C47H76O17 911.50 −1.2 −H 35 Cycloastragenol 11.87 C30H50O5 513.36 4.5 +Na 36 Soyasaponino 11.88 C48H78O18 941.51 −1.2 −H,+HCOO 37 Astragaloside Ⅰ 11.99 C45H72O16 891.47 −1.2 +Na,+H,
M-H2O+H38 Astragaloside IV 12.10 C41H68O14 767.46 −1.1 M-H2O+H 39 Neoastragaloside I 12.41 C45H72O16 913.48 −0.5 +HCOO 40 Isoastragaloside I 12.46 C45H72O16 869.49 −0.2 +H 41 Isoastragaloside Ⅳ 12.57 C41H68O14 783.45 −1.2 −H 42 Astragaloside 13.00 C41H68O14 785.47 −0.1 +H -
[1] CUI L Y, MA Z N, WANG D F, et al. Ultrasound-assisted extraction, optimization, isolation, and antioxidant activity analysis of flavonoids from Astragalus membranaceus stems and leaves[J]. Ultrasonics Sonochemistry,2022,90:106190. doi: 10.1016/j.ultsonch.2022.106190
[2] 王强雄, 郭盛, 申柯欣, 等. 蒙古黄芪茎叶多类型资源性化学成分分析与价值评价[J]. 中国中药杂志,2023,48(24):6600−6612. [WANG Q X, GUO S, SHEN K X, et al. Chemical composition analysis and value evaluation of stems and leaves of Astragalus membranaceus var. mongholicus[J]. China Journal of Chinese Materia Medica,2023,48(24):6600−6612.] WANG Q X, GUO S, SHEN K X, et al. Chemical composition analysis and value evaluation of stems and leaves of Astragalus membranaceus var. mongholicus[J]. China Journal of Chinese Materia Medica, 2023, 48(24): 6600−6612.
[3] SAMUEL A O, HUANG B T, CHEN Y, et al. Antioxidant and antibacterial insights into the leaves, leaf tea and medicinal roots from Astragalus membranaceus (Fisch.) Bge[J]. Scientific Reports,2021,11(1):19625. doi: 10.1038/s41598-021-97109-6
[4] YAO T H, ZHANG T X, ZHAO Q H, et al. Effects of dietary supplementation with astragalus (Astragalus membranaceus) root or leaf meal on the hematology, serum biochemical parameters, histomorphology, oxidative status, and resistance of Phoxinus lagowskii against bacterial infection[J]. Aquaculture,2023,565:739135. doi: 10.1016/j.aquaculture.2022.739135
[5] ZHANG X Y, HAO J F, SUN C X, et al. Total astragalosides decrease apoptosis and pyroptosis by inhibiting enterovirus 71 replication in gastric epithelial cells[J]. Experimental and Therapeutic Medicine,2022,23(3):237. doi: 10.3892/etm.2022.11162
[6] 李慧, 李静辉, 崔莹, 等. 黄芪茎叶的安全性毒理学评价[J]. 现代食品科技,2021,37(10):332−339. [LI H, LI J H, CUI Y, et al. Evaluation of acute oral toxicity, genetic toxicity, and subchronic toxicity of Astragalus stem and leaf[J]. Modern Food Science and Technology,2021,37(10):332−339.] LI H, LI J H, CUI Y, et al. Evaluation of acute oral toxicity, genetic toxicity, and subchronic toxicity of Astragalus stem and leaf[J]. Modern Food Science and Technology, 2021, 37(10): 332−339.
[7] 黑龙江省卫生健康委员会. 食品安全地方标准干制黄芪茎叶:DBS 23/007-2019[S]. 黑龙江, 2019. [Heilongjiang Provincial Health Commission. Food safety local standard dried astragalus stems and leaves:DBS 23/007-2019[S]. Heilongjiang, 2019.] Heilongjiang Provincial Health Commission. Food safety local standard dried astragalus stems and leaves: DBS 23/007-2019[S]. Heilongjiang, 2019.
[8] 幸春容, 胡彦君, 李柏群, 等. 大健康产业背景下中药保健食品发展浅析[J]. 中国药业,2020,29(18):19−21. [XING C R, HU Y J, LI B Q, et al. Analysis on the development of Traditional Chinese Medicine health food under the background of enlarged health industry[J]. China Pharmaceuticals,2020,29(18):19−21.] doi: 10.3969/j.issn.1006-4931.2020.18.006 XING C R, HU Y J, LI B Q, et al. Analysis on the development of Traditional Chinese Medicine health food under the background of enlarged health industry[J]. China Pharmaceuticals, 2020, 29(18): 19−21. doi: 10.3969/j.issn.1006-4931.2020.18.006
[9] 奚佳玉, 苏圆锦, 赵鲲鹏, 等. 黄芪药食同源的研究进展[J]. 华西药学杂志,2023,38(6):718−724. [XI J Y, SU Y J, ZHAO K P, et al. Research progress in medicinal and food homologous of Astragali Radix[J]. West China Journal of Pharmaceutical Sciences,2023,38(6):718−724.] XI J Y, SU Y J, ZHAO K P, et al. Research progress in medicinal and food homologous of Astragali Radix[J]. West China Journal of Pharmaceutical Sciences, 2023, 38(6): 718−724.
[10] WANG E B, LIU T, LU X L, et al. Comparison of aerial parts of Astragalus membranaceus and Astragali Radix based on chemical constituents and pharmacological effects[J]. Food & Agricultural Immunology,2019,30(1):1046−1066.
[11] 施怀生, 毕小凤, 史宪海. UPLC-Q-Exactive四极杆-静电场轨道阱高分辨质谱联用分析黄芪根及其茎叶中黄酮和皂苷类成分[J]. 世界中西医结合杂志,2018,13(3):357−361,365. [SHI H S, BI X F, SHI X H, et al. Analysis on the chemical constituents of flavones and saponin in the root and stem leaf of astragalus membranaceus with ultra-high performance liquid chromatography coupled with hybridquadrupole-orbitrap mass spectrometry[J]. World Journal of Integrated Traditional and Western Medicine,2018,13(3):357−361,365.] SHI H S, BI X F, SHI X H, et al. Analysis on the chemical constituents of flavones and saponin in the root and stem leaf of astragalus membranaceus with ultra-high performance liquid chromatography coupled with hybridquadrupole-orbitrap mass spectrometry[J]. World Journal of Integrated Traditional and Western Medicine, 2018, 13(3): 357−361,365.
[12] XI N, KANG J, HAO L J, et al. Effects of ultrafine powder of the stem and leaf of Astragalus on immunity in chickens[J]. Italian Journal of Animal Science,2014,13(1):3022. doi: 10.4081/ijas.2014.3022
[13] LI Y, GUO S, ZHU Y, et al. Comparative analysis of twenty-five compounds in different parts of Astragalus membranaceus var. mongholicus and Astragalus membranaceus by UPLC-MS/MS[J]. Journal of Pharmaceutical Analysis,2019,9(6):392−399. doi: 10.1016/j.jpha.2019.06.002
[14] YU Y, SONG Q Q, HUANG L X, et al. Immunomodulatory activities of sulfated Cyclocarya paliurus polysaccharides with different degrees of substitution on mouse spleen lymphocytes[J]. Journal of Functional Foods,2020,64:103706. doi: 10.1016/j.jff.2019.103706
[15] 李丹, 王文千, 李明亮, 等. 蜂王浆对免疫功能低下小鼠免疫功能的影响[J]. 食品与发酵工业,2022,48(1):139−145. [LI D, WANG W Q, LI M L, et al. Effect of royal jelly on mice with immunodeficiency[J]. Food and Fermentation Industries,2022,48(1):139−145.] LI D, WANG W Q, LI M L, et al. Effect of royal jelly on mice with immunodeficiency[J]. Food and Fermentation Industries, 2022, 48(1): 139−145.
[16] TANG J, CHEN Z. The protective effect of γ-aminobutyric acid on the development of immune function in chickens under heat stress[J]. Journal of Animal Physiology and Animal Nutrition,2016,100(4):768−777. doi: 10.1111/jpn.12385
[17] CHEI S, OH H J, LEE K, et al. Dysfunction of B cell leading to failure of immunoglobulin response is ameliorated by dietary silk peptide in 14-month-old C57BL/6 mice[J]. Frontiers in Nutrition,2020,7:583186. doi: 10.3389/fnut.2020.583186
[18] 刘双时. 女贞子正丁醇部位提取物及其单体化合物对免疫抑制小鼠的免疫调节作用[D]. 杨凌:西北农林科技大学, 2023. [LIU S S. Effects of n-butanol extract from Fructus Ligustris L. and its monomer compound on immune regulation in immunosuppressed mice[D]. Yangling:Northwest A&F University, 2023.] LIU S S. Effects of n-butanol extract from Fructus Ligustris L. and its monomer compound on immune regulation in immunosuppressed mice[D]. Yangling: Northwest A&F University, 2023.
[19] GAO D, LIU Z J, LIU F, et al. Study of the immunoregulatory effect of Lactobacillus rhamnosus 1.0320 in immunosuppressed mice[J]. Journal of Functional Foods,2021,79:104423. doi: 10.1016/j.jff.2021.104423
[20] 蒋微, 蒋式骊, 刘平. 黄芪甲苷的药理作用研究进展[J]. 中华中医药学刊,2019,37(9):2121−2124. [JIANG W, JIANG S L, LIU P, et al. Research progress on pharmacologic effects of astragaloside IV[J]. Chinese Archives of Traditional Chinese Medicine,2019,37(9):2121−2124.] JIANG W, JIANG S L, LIU P, et al. Research progress on pharmacologic effects of astragaloside IV[J]. Chinese Archives of Traditional Chinese Medicine, 2019, 37(9): 2121−2124.
[21] NIU Y Y, DONG J, JIANG H M, et al. Effects of polysaccharide from Malus halliana Koehne flowers in cyclophosphamide-induced immunosuppression and oxidative stress on mice[J]. Oxidative Medicine and Cellular Longevity,2020,2020(7):1−10.
[22] CHEN J R, YANG Z Q, HU T J, et al. Immunomodulatory activity in vitro and in vivo of polysaccharide from Potentilla anserina[J]. Fitoterapia,2010,81(8):1117−1124. doi: 10.1016/j.fitote.2010.07.009
[23] 俞萍, 张庆贺, 姜虹延, 等. 不同分子质量的人参糖肽复合物增强小鼠免疫功能的研究[J]. 食品与发酵工业,2021,47(22):109−114. [YU P, ZHANG Q H, JlANG H Y, et al. lmmuno-enhancement of ginseng glycopeptide complex with different molecular weights on mice[J]. Food and Fermentation Industries,2021,47(22):109−114.] YU P, ZHANG Q H, JlANG H Y, et al. lmmuno-enhancement of ginseng glycopeptide complex with different molecular weights on mice[J]. Food and Fermentation Industries, 2021, 47(22): 109−114.
[24] 张妍, 林昌岫, 邵玉健, 等. 轮叶党参粗多糖对体外培养小鼠脾淋巴细胞及RAW 264.7细胞的免疫活性[J]. 食品工业科技,2018,39(12):311−315. [ZHANG Y, LIN C X, SHAO Y J, et al. Effect of polysaccharide from codonopsis lanceolata [Codonopsis lanceolata (Sieb. et Zucc.) Trauv. ] on immunomodulatory activity in mouse spleen lymphocytes and RAW 264.7[J]. Science and Technology of Food Industry,2018,39(12):311−315.] ZHANG Y, LIN C X, SHAO Y J, et al. Effect of polysaccharide from codonopsis lanceolata [Codonopsis lanceolata (Sieb. et Zucc.) Trauv. ] on immunomodulatory activity in mouse spleen lymphocytes and RAW 264.7[J]. Science and Technology of Food Industry, 2018, 39(12): 311−315.
[25] 杨婧妍, 何立巍, 董伟. 板蓝根正丁醇部位对免疫细胞增殖及体外抗氧化活性的研究[J]. 南京中医药大学学报,2015,31(2):160−164. [YANG J Y, HE L W, DONG W, et al. Immune cell proliferation function and in vitro antioxidant activity of n-butanol extract of Radix Isatidis[J]. Journal of Nanjing University of Traditional Chinese Medicine,2015,31(2):160−164.] YANG J Y, HE L W, DONG W, et al. Immune cell proliferation function and in vitro antioxidant activity of n-butanol extract of Radix Isatidis[J]. Journal of Nanjing University of Traditional Chinese Medicine, 2015, 31(2): 160−164.
[26] BELL C J M, SUN Y, NOWAK U M, et al. Sustained in vivo signaling by long-lived IL-2 induces prolonged increases of regulatory T cells[J]. Journal of Autoimmunity,2015,56:66−80. doi: 10.1016/j.jaut.2014.10.002
[27] 张燕丽, 孟凡佳, 田园, 等. 炙甘草的化学成分与药理作用研究进展[J]. 化学工程师,2019,33(8):60−63,66. [ZANG Y L, MENG F J, TlAN Y, et al. Study on chemical composition and pharmacological action of Licorice[J]. Chemical Engineer,2019,33(8):60−63,66.] ZANG Y L, MENG F J, TlAN Y, et al. Study on chemical composition and pharmacological action of Licorice[J]. Chemical Engineer, 2019, 33(8): 60−63,66.
[28] 甘思言, 衣伟萌, 乔石, 等. 太子参参须提取物对免疫抑制小鼠血清免疫指标和抗氧化指标的影响[J]. 畜牧与兽医,2020,52(8):121−124. [GAN S Y, YI W M, QlAO S, et al. Effect of extract from the fibrous root of Radix pseudostellariae on the serum immune andantioxidant indexes in immunosuppressed mice[J]. Animal Husbandry & Veterinary Medicine,2020,52(8):121−124.] GAN S Y, YI W M, QlAO S, et al. Effect of extract from the fibrous root of Radix pseudostellariae on the serum immune andantioxidant indexes in immunosuppressed mice[J]. Animal Husbandry & Veterinary Medicine, 2020, 52(8): 121−124.
[29] HU Q, BIAN Q H, RONG D C, et al. JAK/STAT pathway:Extracellular signals, diseases, immunity, and therapeutic regimens[J]. Frontiers in Bioengineering and Biotechnology,2023,11:1110765. doi: 10.3389/fbioe.2023.1110765
[30] 朱艳, 姜盛, 李明亮, 等. 紫苏籽蛋白对免疫力低下小鼠免疫调节功能的研究[J]. 食品工业科技,2020,41(21):322−326,332. [ZHU Y, JlANG S, LI M L, et al. Study on immune regulation of Perilla frutescens seed protein in mice with low immunity[J]. Science and Technology of Food Industry,2020,41(21):322−326,332.] ZHU Y, JlANG S, LI M L, et al. Study on immune regulation of Perilla frutescens seed protein in mice with low immunity[J]. Science and Technology of Food Industry, 2020, 41(21): 322−326,332.
[31] 胡逸中, 薛征, 胡炀. 黄芪甲苷对环磷酰胺所致免疫抑制小鼠免疫功能的影响[J]. 时珍国医国药,2021,32(8):1843−1844. [HU Y Z, XUE Z, HU Y. Effect of astragaloside on immune function of immunosuppressed mice induced by cyclophosphamide[J]. Lishizhen Medicine and Materia Medica,2021,32(8):1843−1844.] doi: 10.3969/j.issn.1008-0805.2021.08.14 HU Y Z, XUE Z, HU Y. Effect of astragaloside on immune function of immunosuppressed mice induced by cyclophosphamide[J]. Lishizhen Medicine and Materia Medica, 2021, 32(8): 1843−1844. doi: 10.3969/j.issn.1008-0805.2021.08.14
[32] 苏优拉, 陈贵林. 黄芪中黄酮类成分的研究进展[J]. 食品安全质量检测学报,2021,12(3):849−857. [SU Y L, CHEN G L. Research progress of flavonoids in Astragalus membranaceus (Fisch.) Bge[J]. Journal of Food Safety & Quality,2021,12(3):849−857.] SU Y L, CHEN G L. Research progress of flavonoids in Astragalus membranaceus (Fisch.) Bge[J]. Journal of Food Safety & Quality, 2021, 12(3): 849−857.
[33] SHAKOOR H, FEEHAN J, APOSTOLOPOULOS V, et al. Immunomodulatory effects of dietary polyphenols[J]. Nutrients,2021,13:728. doi: 10.3390/nu13030728





 下载:
下载:
 下载:
下载:



