Transcriptome Analysis of Effect of Cordyceps militaris Aqueous Extract in Human Gastric Cancer Cell BGC-823 on Different RCD Pathways
-
摘要: 目的:基于转录组测序技术,分析预测蛹虫草(XG)水提物影响人胃癌细胞BGC-823生长活性的主要调节性细胞死亡(Regulated Cell Death,RCD)信号通路。方法:MTT法测定XG水提物对胃癌细胞增殖能力的影响,转录组测序分析显著差异基因及其京都基因与基因组百科全书(Kyoto Encyclopedia of Genes and Genomes,KEGG)通路富集情况,获得凋亡、自噬、坏死性凋亡及铁死亡信号通路的hub基因(10个)。通过激光共聚焦、流式细胞术、实时荧光定量PCR(qRT-PCR)进一步验证XG水提物对胃癌细胞凋亡的诱导情况。结果:XG水提物对人胃癌细胞BGC-823生长活性的抑制作用具有剂量依赖特征。转录组测序共筛选到5885个差异表达基因,并在凋亡(P=0.001)及自噬(P=0.008)信号通路存在显著性富集,除铁死亡外,其它信号通路的hub基因的表达量均被显著性抑制,包括caspase依赖性凋亡信号通路的关键因子CASP3、CASP9、CASP7和APAF1,自噬小体形成相关的基因ULK1、ULK2、UVRAG、Atg14及Atg5。细胞学检测结果显示,胃癌细胞经XG水提物处理后呈典型的凋亡形态学特征,早期凋亡率为24.8%,细胞周期阻滞于G2/M期。qRT-PCR检测显示,BCL2、BCL2L1、CASP3、CASP9及XIAP的表达受到显著抑制(P<0.01),ENDOG和AIF表达量显著上调(P<0.01),与转录组测序结果一致。结论:蛹虫草主要通过caspase非依赖性线粒体凋亡途径抑制人胃癌细胞BGC-823的生长活性,同时可以影响自噬等信号通路的激活,为蛹虫草在功能性食品的开发提供理论支持。Abstract: Objective: The main regulated cell death (RCD) signaling pathway of Cordyceps militaris (XG) water extract that affects the growth activity of the human gastric cancer cell line BGC-823 was analyzed and predicted using transcriptome sequencing technology. Methods: The effect of XG aqueous extract on the proliferation of gastric cancer cells was determined using the MTT method. Transcriptome analysis revealed significant differentially expressed genes (DEGs) and their Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment, resulting in the identification of 10 hub genes involved in the apoptosis, autophagy, necroptosis, and ferroptosis signaling pathways. Laser confocal microscopy, flow cytometry, and quantitative real time polymerase chain reaction (qRT-PCR) were employed to further validate the induction of apoptosis in gastric cancer cells by the XG aqueous extract. Results: The inhibitory effect of XG water extract on the growth of the human gastric cancer cell line BGC-823 was dose-dependent. A total of 5885 differentially expressed genes were identified through transcriptome sequencing analysis, with significant enrichment in the apoptosis (P=0.001) and autophagy (P=0.008) signaling pathways. In addition to ferroptosis, the expression levels of hub genes associated with other signaling pathways were significantly inhibited, including the key factor of caspase-dependent apoptosis (CASP3, CASP9, CASP7, APAF1) as well as ULK1, ULK2, UVRAG, Atg14, and Atg5 related to autophagosome formation. Cytological examination revealed typical apoptotic morphological characteristics in gastric cancer cells treated with the XG water extract, with an early apoptotic rate reaching 24.8%, while cell cycle arrest occurred at the G2/M stage. qRT-PCR showed that the expression of BCL2, BCL2L1, CASP3, CASP9, and XIAP was significantly inhibited (P<0.01), whereas that of ENDOG and AIF was significantly upregulated (P<0.01), consistent with the results of transcriptome sequencing. Conclusion: C. militaris primarily inhibits the growth of the human gastric cancer cell line BGC-823 through a caspase-independent mitochondrial apoptosis pathway while also influencing the activation of autophagy and other signaling pathways. These findings provide theoretical support for using C. militaris in functional foods.
-
Keywords:
- Cordyceps militaris /
- apoptosis /
- autophagy /
- necroptosis /
- ferroptosis
-
蛹虫草(Cordyceps militaris)作为新资源食品[1],不仅具有传统食物的安全性,同时由于富含虫草素、喷司他丁、多糖等生物活性成分,具有抗肿瘤、抗氧化、调节免疫、降血糖、降血脂等多种生物学功能[2−3],在东南亚具有十分悠久的药用使用历史。研究者们先后利用肺癌[4]、肝癌[5]、肠癌[6]、胰腺癌[7]、乳腺癌[8]、宫颈癌[9]等人类细胞株,证明蛹虫草及其多种生物活性成分可以通过诱导凋亡的方式发挥抗肿瘤活性。蛹虫草醇提物可以通过影响TNFR表达量,反向调控NF-κB活化,下调Bcl-xL和BCL2的表达,激活卵巢癌细胞的外源性凋亡途径[10]。通过抑制SMO/PTCH1信号通路,诱导非小细胞肺癌A549发生凋亡[11]。同时,虫草素作为蛹虫草抗肿瘤活性的核心成分,可以通过诱导细胞凋亡[12−13]、抑制细胞转移[14−15]、影响信号传导[16−17]等多种途径发挥抗肿瘤作用。蛹虫草多糖对人肿瘤细胞SMMC-7721,BGC-823和MCF-7的生长活性具有明显的抑制作用,主要通过阻滞G0/G1细胞周期、减少DNA合成等方式诱导细胞凋亡[18]。蛹虫草已经成为天然抗肿瘤功能性食品筛选的重要真菌资源。
调节性细胞死亡(Regulated Cell Death,RCD)是受特定信号转导途径控制,可通过遗传信号或药物干预进行调控的一类死亡方式。作为RCD途径中研究最深入的细胞死亡方式,诱导凋亡被广泛应用于临床的抗肿瘤策略[19]。通过某些药物或基因同时调控多个RCD信号通路,可避免肿瘤细胞对某一特定类型RCD耐药性的发生[20],已成为未来癌症药物研发的新方向。研究显示,蛹虫草除了可以激活多种肿瘤细胞凋亡信号通路外[21],对RCD途径亦具有复杂的调节作用,Hu等[22]发现200 μg/mL蛹虫草提取物能够通过CASP3/PARP/GSDME通路诱导人肺癌细胞A549发生凋亡和焦亡。虫草素可以通过调节Dkk1/β-catenin信号通路,上调自噬相关基因Atg8、beclin、LC3的表达,抑制卵巢癌细胞的生长[23]。0.75 mg/kg蛹虫草能够提高正常心脏细胞的GPX4酶活性,抑制阿霉素诱导的细胞铁死亡,有效率达90%,远远高于临床药物右丙亚胺(50%)[24]。坏死性凋亡、自噬和铁死亡是近年来关注度较高的3种RCD形式,同时与凋亡信号通路存在着密切的关联关系。本课题组前期研究结果证实,蛹虫草水提物中富含虫草素、腺苷、喷司他丁、麦角甾醇、多糖以及无机元素等多种生物活性成分,并能有效抑制人肺癌细胞A549、人肝癌细胞HepG2、人乳腺癌细胞MCF-7、小鼠黑色素瘤细胞B16的增殖活性[25],但是具体机制尚不明晰。因此,在此基础之上,本论文依托转录组测序技术,分析预测蛹虫草的抗肿瘤活性是否存在多靶向调控RCD信号通路的情况,相关研究尚无报道,本研究结果将为蛹虫草在抗肿瘤功能性食品方面的深入开发提供新思路。
1. 材料与方法
1.1 材料与仪器
人胃癌细胞BGC-823 购自武汉普诺赛生命科技有限公司,实验室液氮长期保藏;蛹虫草子实体为功能性蛹虫草品系 由辽宁省功能性蛹虫草重点实验室栽培提供;培养基RPMI-1640 购自美国Hyclone公司;胎牛血清 购自杭州四季青公司;DMSO(二甲基亚砜) 购自Sigma公司;TRIzol 购自美国Invitrogen公司;Prime Script RT reagent Kit with gDNA Eraser逆转录试剂盒 购自日本TaKaRa公司;Annexin V-FITC/PI凋亡检测试剂盒 购自南京诺唯赞生物科技股份有限公司;MTT、4%多聚甲醛 购自上海碧云天公司。
D50-UVIPURE型凝胶成像仪 英国UVItec Limited公司;JC-6000超净工作台 上海博迅医疗生物仪器股份有限公司;ETC811型PCR扩增仪 北京东胜创新生物科技有限公司;QuantShudio™ 3实时荧光定量PCR仪 美国Applied Biosystems公司;IMPLEN-N50超微量紫外分光光度计 美国Thermo公司;EPS300电泳仪 上海天能公司;Infinite F50酶标仪 上海帝肯实验器材有限公司;BD CantoⅡ流式细胞分析仪 美国BD公司;A1R型激光共聚焦扫描显微镜 上海尼康仪器有限公司;HHCP-7型恒温CO2细胞培养箱 上海博迅医疗生物仪器股份有限公司。
1.2 实验方法
1.2.1 蛹虫草水提物的制备
蛹虫草子实体(XG)60 ℃烘干至恒重,机械粉碎后过80目筛网,获得蛹虫草干粉。取2.5 g蛹虫草干粉,80 mL去离子水,85 ℃水浴浸提3.5 h,4000 r/min离心10 min,上清液真空冷冻干燥过夜,获得冻干粉。使用前以RPMI-1640完全培养基溶解,0.22 μm微孔滤膜过滤,4 ℃密封保存。
1.2.2 MTT法检测细胞抑制率
人胃癌细胞BGC-823以含10%胎牛血清的RPMI-1640完全培养液,37 ℃、5%CO2常规培养。调整细胞密度为5×105个/mL接种于96孔板,培养8 h。实验孔分别加入200 μL XG水提物(0.05、0.1、0.2、0.5、1、2和5 mg/mL),对照孔加入等体积的完全培养基,n=5,常规培养24 h。加入5 mg/mL的MTT溶液20 μL/孔,孵育4 h,加入DMSO 150 μL/孔,酶标仪检测各孔的OD492值,计算XG水提物对人胃癌细胞BGC-823生长活性的抑制率[26],计算公式如下:抑制率(%)=(OD对照孔−OD实验孔)/OD对照孔×100。
1.2.3 转录组测序与分析
以5×105 个/mL的细胞密度将细胞接种于6孔板中,培养8 h后,去上清,实验组(XG组)加入4 mg/mL XG水溶液,2 mL/孔,对照组(CK组)加入等体积的完全培养基,培养24 h后分别收集XG组和CK组细胞,责成广州基迪奥生物科技有限公司通过Illumina HiSeqTM 4000测序平台进行RNA-seq测序分析,获取转录组原始数据。从原始数据中去除适配器序列和低质量的reads后,应用HISAT2软件将Clean Reads与参考基因组(OryzasativaIRGSP1.0.51genomefa)进行快速精确的比对,获取Reads在参考基因组上的定位信息。随后使用String Tie软件获得反映基因表达水平的每千碱基片段(Fragments Per Kilobase Million,FPKM)。使用DESeq2软件对RNA-seq进行了差异表达分析。参照CK组基因表达情况,以P<0.05且|log2(fold-change)|>1作为筛选标准,筛选XG组的显著差异表达基因(Differentially Expressed Genes,DEGs),进行表达量分析和KEGG富集(https://www.kegg.jp/)分析。
1.2.4 蛋白互作网络构建及hub基因的筛选
根据京都基因与基因组百科全书(Kyoto Encyclopedia of Genes and Genomes,KEGG)数据库信息,分别获得凋亡(ko04210)、坏死性凋亡(ko04217)、自噬(ko04140)和铁死亡(ko04216)信号通路的DEGs。通过STRING数据库(https://cn.string-db.org/,V.12.0)分别构建上述信号通路DEGs的蛋白质相互作用(Protein-Protein Interaction,PPI)网络图,置信度>4被认为具有统计学意义。经Cytoscape(V.3.9.1)软件的MCODE插件对PPI得分最高模块的DEGs进行标记,基于CytoHubba插件MCC算法将排名前10的DEGs确定为该信号通路的核心基因(hub gene)。
1.2.5 细胞凋亡形态的共聚焦显微镜观察
分别收集XG组和CK组细胞,依次加入500 μL 1×Binding Buffer、5 μL Annexin V-FITC和5 μL PI染液,室温避光孵育10 min。4%多聚甲醛固定10 min,加入适量PBS,激光共聚焦扫描显微镜进行观察,并采集图像。
1.2.6 细胞凋亡率的流式细胞术检测
分别收集XG组和CK组细胞,经1×Binding Buffer重悬,根据Annexin V-FITC/PI凋亡检测试剂盒操作说明,加入5 μL Annexin V-FITC和5 μL PI溶液,避光染色15 min,流式仪检测细胞凋亡率[27]。
1.2.7 细胞周期的流式细胞术检测
分别收集XG组和CK组细胞,加入4 ℃预冷的70%乙醇1 mL,4 ℃过夜。PBS清洗,PI染液重悬细胞,37 ℃避光孵育30 min,流式细胞仪检测细胞周期。
1.2.8 凋亡相关基因的实时荧光定量PCR(qRT-PCR)检测
根据转录组数据分析结果,选取8个凋亡信号通路的DEGs(表1),以β-actin为内参基因,进行qRT-PCR检测。具体操作如下:转录组测序同批次样本的总RNA,按试剂盒说明逆转录成cDNA后测定DNA浓度。使用Primer Premier(V.5.0)设计特异性扩增引物。采用QuantShudio™ 3荧光定量PCR仪进行qRT-PCR扩增(n=3):预变性95 ℃ 10 min,解链95 ℃ 15 s,退火60 ℃ 1 min,共40个循环。采用2−△△Ct计算各基因的表达量,并与转录组数据进行比较。
表 1 qRT-PCR 引物Table 1. Primers for qRT-PCR引物名称 引物序列(5'-3') BCL2-F GTGAAGTCAACATGCCTGCC BCL2-R ACAGCCTGCAGCTTTGTTTC BCL2L1-F TGGTTCCTGAGCTTCGCAAT BCL2L1-R TATCACAGGTCGGGAGAGGA CASP-9-F GCCCCATATGATCGAGGACA CASP-9-R GTTCGCAGAAACGAAGCCAG CASP-3-F TACCTGTGGCTGTGTATCCG CASP-3-R TCAGTGTTCTCCATGGATACCT XIAP-F ATATACCCGAGGAACCCTGCC XIAP-R TTCCGGCCCAAAACAAAGA BAK-F TCTCTGGGACCTCCTTAGCC BAK-R CAGTCTCTTGCCTCCCCAAG AIF-F CCTCTACCCTCTATGCCAGGA AIF-R GCAGCAGTTCAAAGACACCC ENDOG-F GCCACCAACGCCGACTAC ENDOG-R AGGTAGAACGTGTCGTCCAT actin-F TCCAGCCTTCCTTCTTGGGT actin-R GCACTGTGTTGGCATAGAGGT 1.3 数据处理
使用IBM SPSS(V.23.0)软件进行数据统计分析,P<0.05被认为具有统计学意义,采用Grahph prism(V.8.4.3.686)软件进行绘图。
2. 结果与分析
2.1 XG水提物对人胃癌细胞生长活性的影响
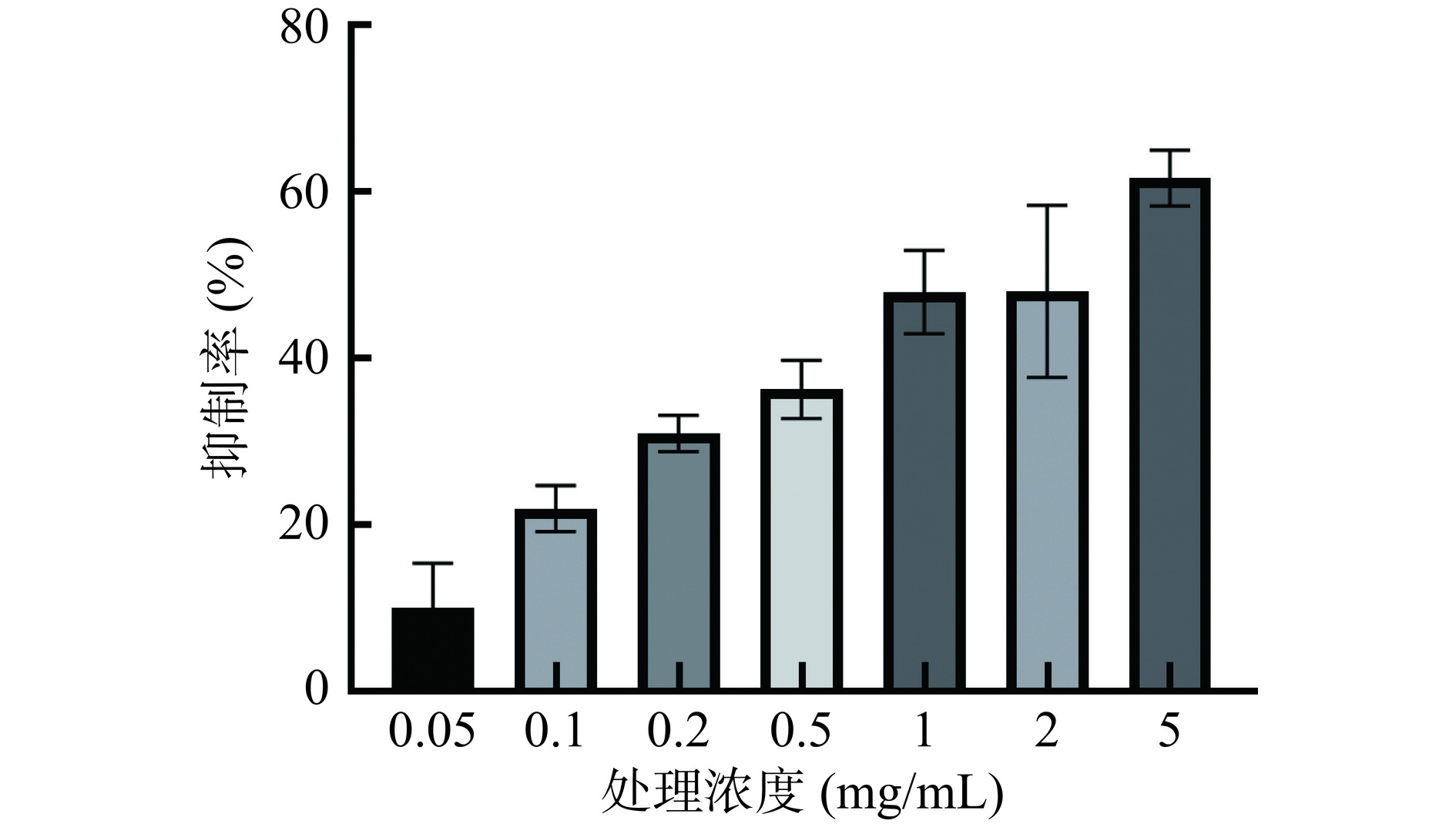
MTT检测结果显示,经过24 h的处理,0.05~5 mg/mL XG水溶液对人胃癌细胞BGC-823的生长活性均具有抑制作用(P<0.05),并呈剂量依赖特征(图1)。通过线性回归计算,当XG水提物处理浓度为4 mg/mL时,XG水提物对细胞的生长抑制率达到50%,因此,选取4 mg/mL处理浓度进行后续实验。
2.2 XG水提物对人胃癌细胞转录组的影响
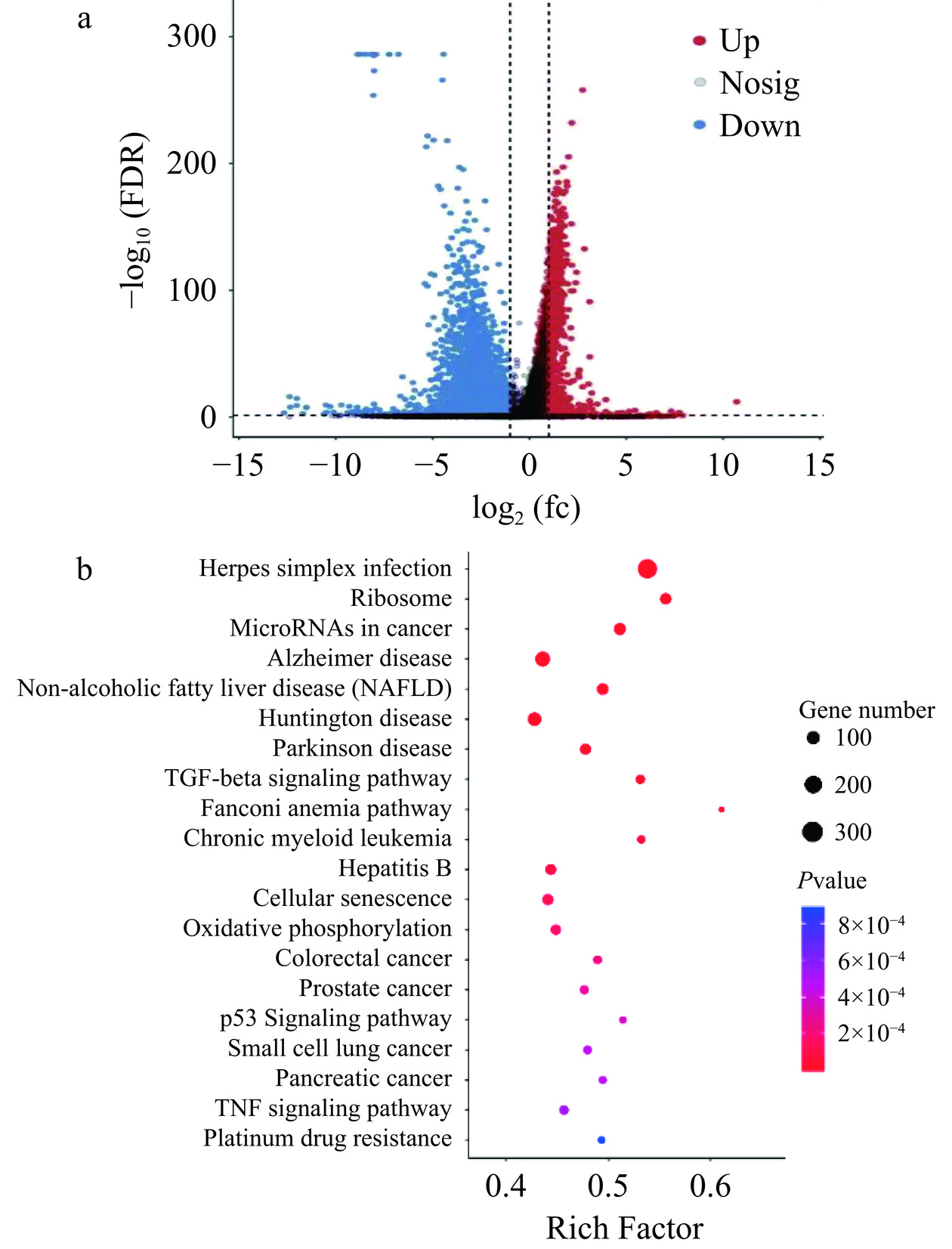
基于CK组基因表达量,XG组共筛选到5885个DEGs,其中有943个DEGs上调表达,4942个DEGs下调表达。火山图显示了差异表达基因的分布情况,表达量差异倍数主要集中在−10~10之间,且下调表达DEGs多于上调表达DEGs。KEGG富集结果显示,DEGs主要被注释到339条信号通路,以错误检出率(False discovery rate,FDR)<0.05为标准,前20条显著富集的通路见图2b,除疾病相关通路如单纯疱疹感染(Herps simplex infection)、阿尔茨海默病(Alzheimer disease)、非酒精性脂肪性肝病(Non-alcoholic Fatty Liver Disease,NAFLD)外,主要为与细胞生长、增殖调控密切相关的信号通路,如转化生长因子β信号通路(TGF-beta signaling pathway)、P53信号通路(P53 signaling pathway)、肿瘤坏死因子信号通路(TNF signaling pathway)。同时,凋亡(P=0.001)、自噬(P=0.008)信号通路存在显著富集,坏死性凋亡(P=0.178)和铁死亡(P=0.111)信号通路富集不显著。
2.3 4个RCD信号通路DEGs的分析
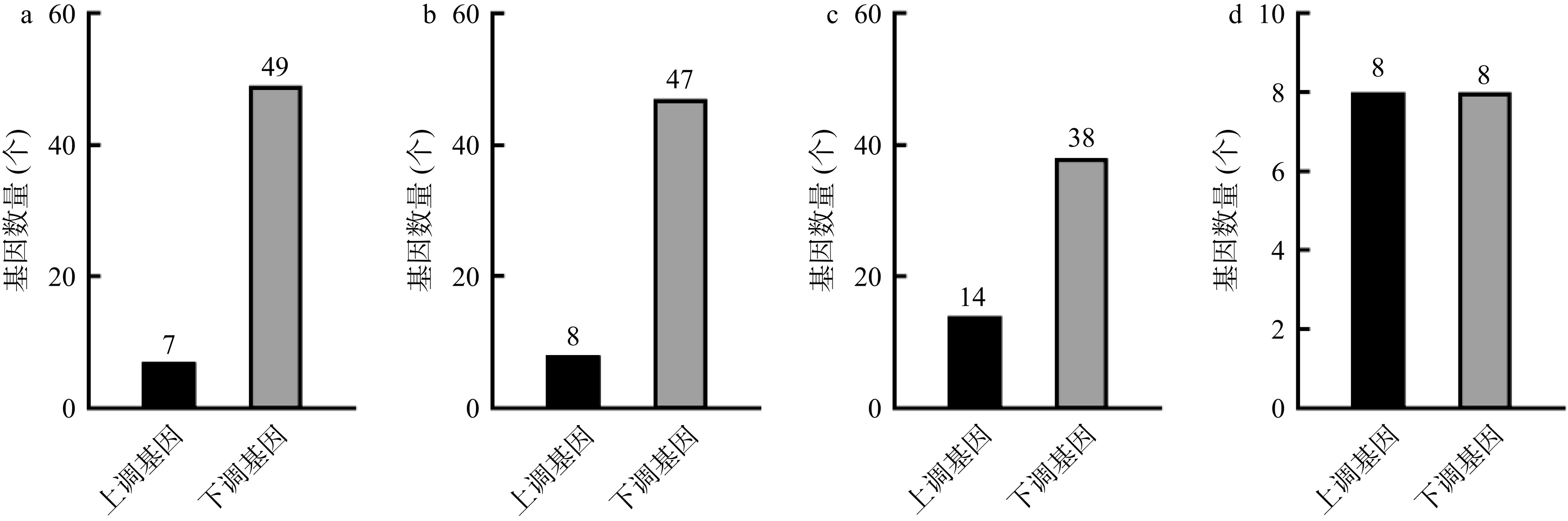
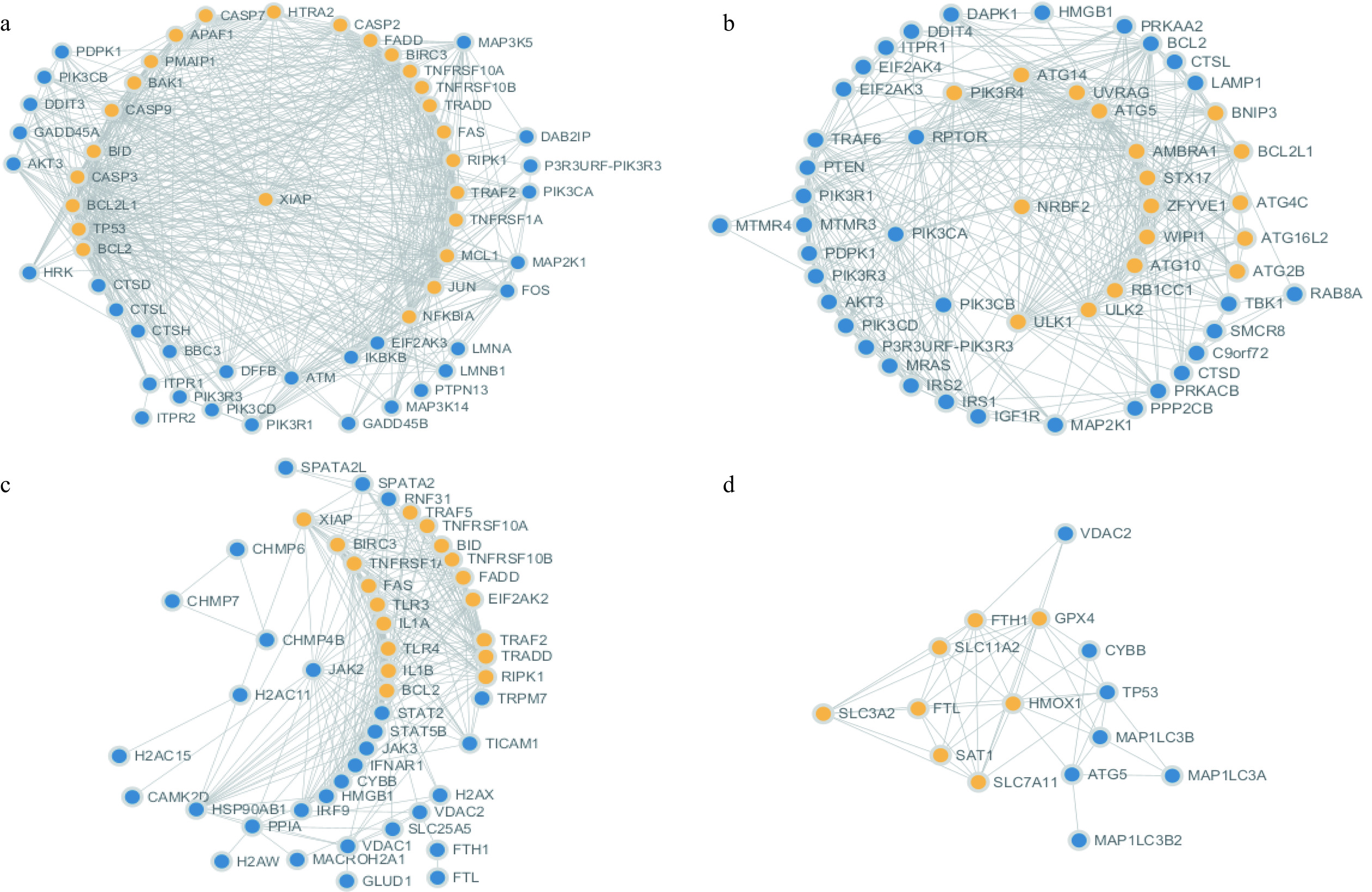
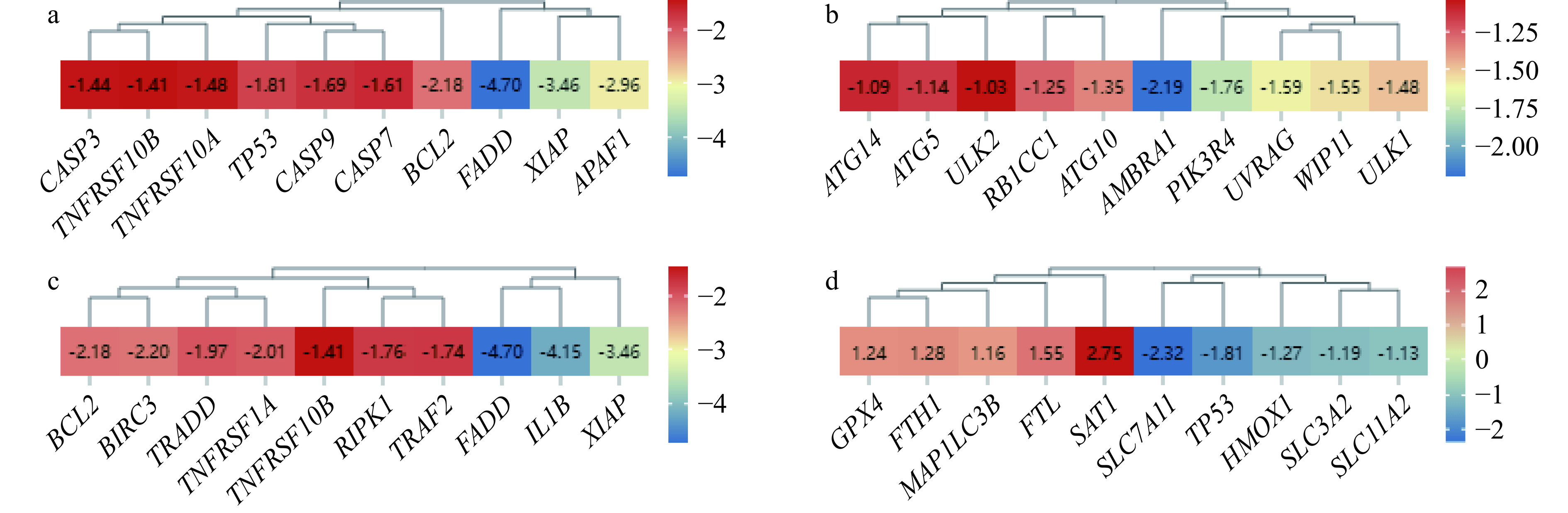
为了进一步探讨XG水提物抑制人胃癌细胞生长活性的机制,对凋亡、自噬、坏死性凋亡和铁死亡4个RCD信号通路的DEGs进行了分析及验证。凋亡、自噬及坏死性凋亡信号通路分别筛选到DEGs 56个、55个和52个,且下调DEGs数量均高于上调DEGs。铁死亡信号通路的DEGs数量最少,仅有16个,上调DEGs与下调DEGs均为8个(图3)。将4个RCD信号途径的DEGs分别导入STRING数据库,构建其PPI网络图,得分最高模块中的DEGs以黄色标记(图4)。凋亡信号通路DEGs的PPI网络最为复杂,包括55个节点和588条边,铁死亡信号通路最为简单,包括15个节点和49条边。各信号通路分别筛选到10个hub基因(图5),其中,凋亡、自噬及坏死性凋亡信号通路的hub基因均位于得分最高的PPI模块中,且各hub基因表达量受到显著抑制。hub基因如CASP3、CASP9、CASP7和APAF1是caspase依赖性凋亡启动和执行的关键蛋白。ULK1、ULK2、UVRAG、Atg14及Atg5主要参与了自噬小体的形成过程。同时,XG水提物促进了铁死亡信号通路hub基因GPX4、MAP1LC3B、FTH1、FTL和SAT1的表达。
2.4 XG水提物对人胃癌细胞凋亡的诱导
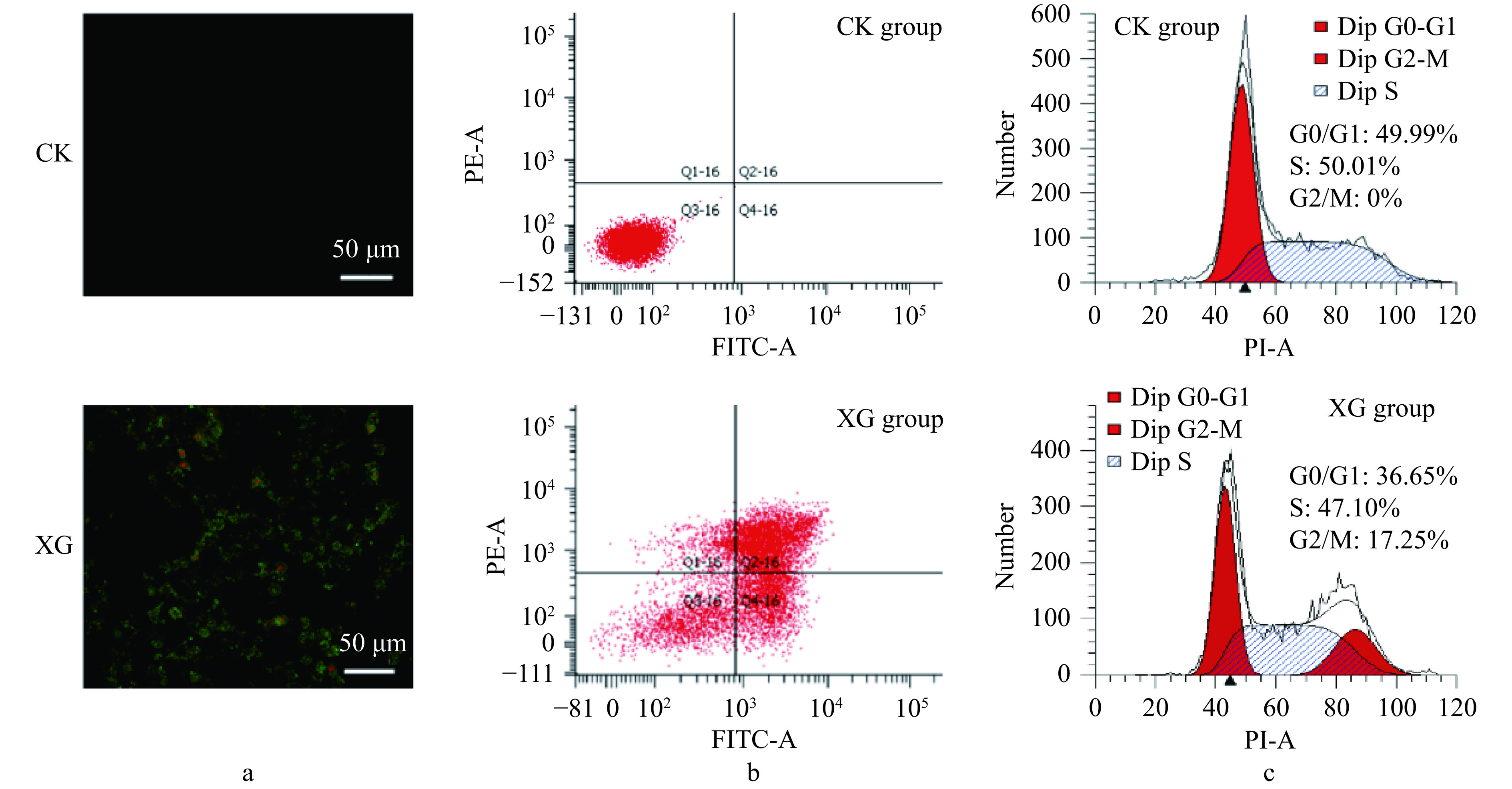
采用激光共聚焦显微镜记录了XG水提物对人胃癌细胞BGC-823细胞形态的影响(图6a)。CK组细胞Annexin V-FITC和PI无荧光信号,均为低染。XG水提物处理可以引起人胃癌细胞出现典型的细胞凋亡形态特征,凋亡早期仅细胞膜呈绿色荧光,同时出现细胞核红色荧光则为晚期凋亡细胞。流式细胞术检测结果显示,4 mg/mL XG水提物处理人胃癌细胞24 h,诱导的总凋亡率达54.6%,其中,早期凋亡率为24.8%,晚期凋亡细胞略多于早期凋亡(图6b)。同时,相对于CK组,XG组S期细胞群比例由50.01%下降至47.10%,G2/M上升至17.25%,出现G2/M期阻滞的状况(图6c),具有典型的细胞凋亡特征。
2.5 凋亡信号通路DEGs表达量变化
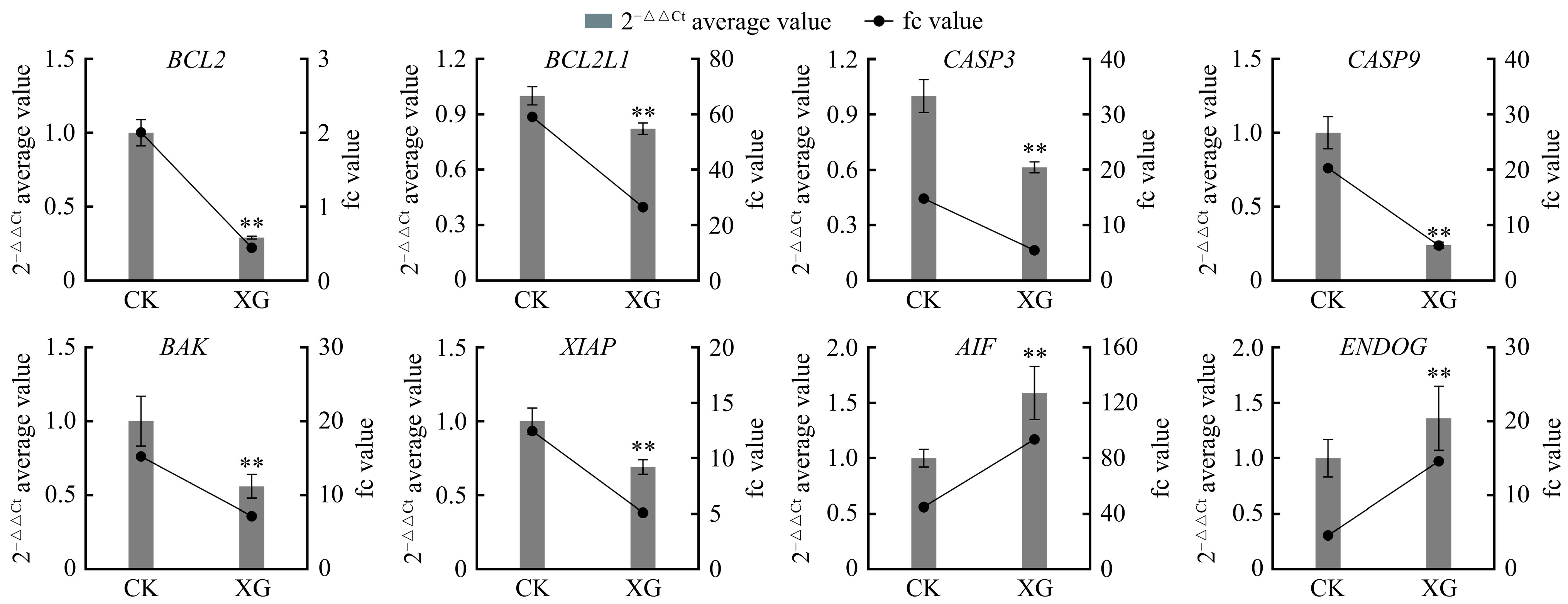
根据转录组数据分析结果及文献调研,选取了凋亡信号通路上的CASP3、CASP9及BCL2等8个关键基因,对其相对表达量进行了检测。结果显示,相对于CK组,蛹虫草显著抑制了hub基因BCL2、BCL2L1、CASP3、CASP9及XIAP的表达(P<0.01),非hub基因ENDOG和AIF表达量显著上调(P<0.01),BAK基因的表达量被显著抑制(P<0.01)(图7)。同时,上述基因相对表达量变化趋势与RNA-seq测序分析结果基本一致,说明本转录组数据具有良好的准确性和可重复性。
3. 讨论与结论
凋亡作为细胞调节性死亡的主要机制之一,被广泛用于癌症靶向治疗的开发和临床应用。转录组测序结果显示,凋亡是蛹虫草水提物影响人胃癌细胞生长活性最显著的RCD信号通路。作为一种具有特殊形态的食药两用真菌,诱导肿瘤细胞凋亡一直是蛹虫草抗肿瘤活性研究的热点。本研究转录组结果显示,BCL2与XIAP是凋亡通路的hub基因,其表达量受到XG水提物的显著抑制,且XG组BAX/BCL2比值从8.49上升至24.62。线粒体凋亡途径受到Bcl-2家族蛋白互作网络的严格控制。BAX/BCL2比值的上调将促进细胞凋亡的发生[28],当BCL2被抑制时,细胞进入凋亡效应期[29];XIAP是IAP家族中最强的凋亡抑制因子,主要通过阻遏线粒体途径抑制细胞凋亡的发生[30]。蛹虫草提取物(IC50=73.48 µg/mL)和虫草素(IC50=9.58 µmol/L)能通过增加BAX/BCL2蛋白的比例,下调XIAP的表达,有效诱导人乳腺癌细胞MCF-7凋亡的发生[31]。抗凋亡蛋白XIAP和BCL2的双重下调,会强化细胞凋亡及化疗敏感性,并克服癌细胞的耐药性[32]。蛹虫草水提物可引起MCF-7和HepG2细胞CASP3表达量的增加,通过caspase酶依赖性线粒体凋亡通路诱导凋亡[21]。蛹虫草可通过抑制CASP3、CASP8表达,下调bcl-2表达量来诱导耐卡铂SKOV-3细胞凋亡[33]。但是,本实验中hub基因CASP3、CASP7、CASP9及APAF1的表达均被显著抑制,非hub基因AIFM1、AIFM2和ENDOG表达量显著上调(P<0.01)。ENDOG和AIF是caspase酶非依赖性凋亡途径中的重要蛋白分子[34],线粒体外膜通透化发生的前提下,两者均可以通过细胞核转位,引起细胞核染色质聚集和DNA片断化,进而诱导Caspases非依赖性凋亡[35]。进一步的细胞学检测结果显示,0.05~5 mg/mL XG水溶液对人胃癌细胞BGC-823的增殖能力具有剂量依赖性抑制作用,24 h处理后,出现典型的细胞凋亡形态学特征及G2/M细胞周期阻滞的状态。G2/M期检查点是肿瘤治疗的重要切入点,绝大部分肿瘤细胞DNA损伤后,会指向性的滞留于G2/M期,进一步触发凋亡[36]。qRT-PCR检测进一步证实,上述凋亡相关DEGs的相对表达量变化与RNA-seq测序分析结果基本一致。因此,XG水提物诱导人胃癌细胞BGC-823的凋亡主要与线粒体途径有关,且不依赖caspases酶的激活。
此外,自噬信号通路也存在显著富集的情况。研究显示,肿瘤中心缺氧部位具有更高的自噬活性,为肿瘤细胞生长提供能量,从而促进肿瘤存活[37]。“离巢”肿瘤细胞的自噬作用可以促进转移细胞的存活和扩增[38−40]。自噬小体的形成是启动自噬的第一步骤,涉及多蛋白复合物的顺序激活,该过程始于ULK1激酶复合物(ULK1、ULK2、G13、FIP200和Atg101组成)激活III型PI3KC3复合物,Atg14是PI3K-CI的核心成分,启动自噬体形成,UVRAG是PI3KC3复合物招募的可变组分[41],该基因可以通过UVRAG-Beclin1或UVRAG-BAX两种复合体分别促发细胞自噬或凋亡[42]。UVRAG的抑制表达可以促进肿瘤细胞发生BAX诱导的线粒体凋亡[43],UVRAG通过结合和激活Beclin1-PI3KC3复合物,促进自噬体的形成,进而诱导细胞自噬[44]。而Atg8-II复合物(Atg12、Atg5及Atg16等组成)可以促进早期吞噬膜的构建和其曲率的形成,Atg5则是吞噬膜延伸的关键蛋白[45]。ULK1、ULK2、UVRAG、Atg14及Atg5均为自噬信号通路的hub基因,表达量显著下调,说明XG水提物对人胃癌细胞BGC-823细胞自噬小体的形成过程可能具有一定的抑制作用。
为了更加全面的了解蛹虫草抑制人胃癌细胞生长活性的机制,本研究通过转录组对铁死亡及坏死性凋亡信号通路的hub基因进行了筛选。GPx4蛋白是经典铁死亡模型的核心。GPx4以GSH为还原剂,催化脂质过氧化物还原成对应的醇,被认为是保护细胞免受脂质过氧化损伤的重要机制。抗氧化体系System Xc-是由SLC7A11和SLC3A2两个亚基组成的异二聚体,大多数肿瘤细胞主要通过该体系获取足够的胱氨酸和谷氨酸以合成还原性GSH[46−47]。虽然XG水提物激活了GPx4的表达,但是由于hub基因SLC7A11和SLC3A2表达量的显著抑制,将直接形成GSH的合成障碍,进而抑制GPx4形成的抗氧化防御,因此,XG水提物可能对胃癌细胞内的脂质过氧化物堆积及内源性细胞铁死亡具有诱导作用。
坏死性凋亡是在凋亡不足条件下激活的细胞防御途径。TNF家族成员及其配体结合后,RIPK1/RIPK3介导了典型的坏死性凋亡信号转导。本研究中,该信号通路共获得包括FADD、TRADD、BIRC3、TNFRSF1A、TNFRSF10B、TRAF2在内的6个hub基因,均为死亡受体及其相关配体基因[48−49],同时表达量被显著抑制。同时,hub基因RIPK1是凋亡和坏死性凋亡途径关键的上游调节因子[50],遗传和临床研究表明,CASP8对RIPK1的切割在防止RIPK1激酶介导的细胞凋亡和坏死中具有重要作用,若CASP8活化,启动细胞凋亡;若CASP8被抑制,RIPK1募集RIPK3通过磷酸化形成“坏死小体”复合物[51]。人胃癌细胞BGC-823的RIPK1及CASP8的表达量被XG水提物显著抑制(P<0.01)。因此,XG水提物对TNF家族介导的坏死性凋亡信号途径存在抑制的可能性。
综上所述,本研究通过细胞生物学手段和转录组测序分析,验证了蛹虫草主要通过诱导人胃癌细胞BGC-823凋亡发挥抗肿瘤活性,主要激活了人胃癌细胞BGC-823的Caspase酶非依赖性线粒体凋亡信号通路,同时,可以影响自噬信号通路的激活。鉴于RCD信号通路的多靶向性和抗肿瘤机制的复杂性,某条信号通路的激活或抑制是否实质性影响了肿瘤细胞的生长活性,还有待深入的实验研究加以研究和证实。
-
表 1 qRT-PCR 引物
Table 1 Primers for qRT-PCR
引物名称 引物序列(5'-3') BCL2-F GTGAAGTCAACATGCCTGCC BCL2-R ACAGCCTGCAGCTTTGTTTC BCL2L1-F TGGTTCCTGAGCTTCGCAAT BCL2L1-R TATCACAGGTCGGGAGAGGA CASP-9-F GCCCCATATGATCGAGGACA CASP-9-R GTTCGCAGAAACGAAGCCAG CASP-3-F TACCTGTGGCTGTGTATCCG CASP-3-R TCAGTGTTCTCCATGGATACCT XIAP-F ATATACCCGAGGAACCCTGCC XIAP-R TTCCGGCCCAAAACAAAGA BAK-F TCTCTGGGACCTCCTTAGCC BAK-R CAGTCTCTTGCCTCCCCAAG AIF-F CCTCTACCCTCTATGCCAGGA AIF-R GCAGCAGTTCAAAGACACCC ENDOG-F GCCACCAACGCCGACTAC ENDOG-R AGGTAGAACGTGTCGTCCAT actin-F TCCAGCCTTCCTTCTTGGGT actin-R GCACTGTGTTGGCATAGAGGT -
[1] 多个食药用菌品种入选农业植物新品种保护名录[J]. 中国食品学报, 2019, 19(3):92. [Several edible and medicinal fungi were included in the protection list of new varieties of agricultural plants[J]. J Chin Inst Food Sci Technol, 2019, 19(3):92.] Several edible and medicinal fungi were included in the protection list of new varieties of agricultural plants[J]. J Chin Inst Food Sci Technol, 2019, 19(3): 92.
[2] KONTOGIANNATOS D, KOUTROTSIOS G, XEKALAKI S, et al. Biomass and Cordycepin production by the medicinal mushroom Cordyceps militaris-A review of various aspects and recent trends towards the exploitation of a valuable fungus[J]. J Fungi (Basel),2021,7(11):986−1003. doi: 10.3390/jof7110986
[3] LIU Y, GUO Z J, ZHOU X W. Chinese Cordyceps:Bioactive components, antitumor effects and underlying mechanism-A review[J]. Molecules,2022,27(19):6576−6593. doi: 10.3390/molecules27196576
[4] JEONG M K, YOO H S, KANG I C. The extract of Cordyceps militaris inhibited the proliferation of cisplatin-resistant A549 lung cancer cells by downregulation of H-Ras[J]. J Med Food,2019,22(8):823−832. doi: 10.1089/jmf.2018.4232
[5] LEE S, LEE H H, KIM J, et al. Anti-tumor effect of Cordyceps militaris in HCV-infected human hepatocarcinoma 7.5 cells[J]. J Microbiol,2015,53(7):468−474. doi: 10.1007/s12275-015-5198-x
[6] SEO H, SONG J, KIM M, et al. Cordyceps militaris grown on germinated soybean suppresses KRAS-driven colorectal cancer by inhibiting the RAS/ERK pathway[J]. Nutrients,2018,11(1):20−33. doi: 10.3390/nu11010020
[7] LI X Y, TAO H, JIN C, et al. Cordycepin inhibits pancreatic cancer cell growth in vitro and in vivo via targeting FGFR2 and blocking ERK signaling[J]. Chin J Nat Med,2020,18(5):345−355.
[8] SUKSIRIWORAPONG J, PONGPRASERT N, BUNSUPA S, et al. CD44-Targeted lipid polymer hybrid nanoparticles enhance anti-breast cancer effect of Cordyceps militaris extracts[J]. Pharmaceutics,2023,15(6):1771−1790. doi: 10.3390/pharmaceutics15061771
[9] TANIA M, SHAWON J, SAIF K, et al. Cordycepin downregulates Cdk-2 to interfere with cell cycle and increases apoptosis by generating ROS in cervical cancer cells:In vitro and in silico study[J]. Curr Cancer Drug Targets,2019,19(2):152−159. doi: 10.2174/1568009618666180905095356
[10] JO E, JANG H J, YANG K E, et al. Cordyceps militaris induces apoptosis in ovarian cancer cells through TNF-α/TNFR1-mediated inhibition of NF-κB phosphorylation[J]. BMC Complement Med Ther,2020,20(1):1−12. doi: 10.1186/s12906-019-2780-5
[11] JO E, JANG H J, SHEN L, et al. Cordyceps militaris exerts anticancer effect on non–small cell lung cancer by inhibiting hedgehog signaling via suppression of TCTN3[J]. Integr Cancer Ther,2020,19:1−14. doi: 10.1158/1535-7163.1.19.1
[12] TUNG K L, WU S Z, YANG C C, et al. Cordycepin induces apoptosis through JNK-mediated caspase activation in human OEC-M1 oral cancer cells[J]. Evid Based Complement Alternat Med,2022,2022:1842363.
[13] SHI L, CAO H, FU S, et al. Cordycepin enhances hyperthermia-induced apoptosis and cell cycle arrest by modulating the MAPK pathway in human lymphoma U937 cells[J]. Mol Biol Rep,2022,49(9):8673−8683. doi: 10.1007/s11033-022-07705-6
[14] GUO Z, CHEN W, DAI G, et al. Cordycepin suppresses the migration and invasion of human liver cancer cells by downregulating the expression of CXCR4[J]. Int J Mol Med,2020,45(1):141−150.
[15] WANG Y, LÜ Y, LIU T S, et al. Cordycepin suppresses cell proliferation and migration by targeting CLEC2 in human gastric cancer cells via Akt signaling pathway[J]. Life Sci,2019,223:110−119. doi: 10.1016/j.lfs.2019.03.025
[16] BINLATEH T, UPPATCHA N, THEPCHAI J, et al. Cordycepin attenuates migration and invasion of HSC-4 oral squamous carcinoma cells through autophagy-dependent FAK/Akt and MMP2/MMP9 suppression[J]. J Dent Sci,2022,17(4):1677−1688. doi: 10.1016/j.jds.2022.03.002
[17] LIU T, ZHU G, YAN W, et al. Cordycepin inhibits cancer cell proliferation and angiogenesis through a DEK interaction via ERK signaling in cholangiocarcinoma[J]. J Pharmacol Exp Ther,2020,373(2):279−289. doi: 10.1124/jpet.119.263202
[18] CHEN C, WANG M L, JIN C, et al. Cordyceps militaris polysaccharide triggers apoptosis and G0/G1 cell arrest in cancer cells[J]. J Asia-Paci Ent,2015,18(3):433−438. doi: 10.1016/j.aspen.2015.04.015
[19] PENG F, LIAO M, QIN R, et al. Regulated cell death (RCD) in cancer:Key pathways and targeted therapies[J]. Signal Transduct Target Ther,2022,7(1):286−351. doi: 10.1038/s41392-022-01110-y
[20] 龚琳婧, 石毓君. 癌症中调节性细胞死亡方式的研究进展与未来展望[J]. 中国普外基础与临床杂志,2022,29(5):582−584. [GONG L J, SHI L J. Progress and prospects:regulated cell death in cancer[J]. Chin J Bases and Clinics in General Surgery,2022,29(5):582−584.] GONG L J, SHI L J. Progress and prospects: regulated cell death in cancer[J]. Chin J Bases and Clinics in General Surgery, 2022, 29(5): 582−584.
[21] SONG J, WANG Y, TENG M, et al. Cordyceps militaris induces tumor cell death via the caspase-dependent mitochondrial pathway in HepG2 and MCF-7 cells[J]. Mol Med Rep,2016,13(6):5132−5140. doi: 10.3892/mmr.2016.5175
[22] HU Z, LAI Y, MA C, et al. Cordyceps militaris extract induces apoptosis and pyroptosis via caspase-3/PARP/GSDME pathways in A549 cell line[J]. Food Sci Nutr,2022,10(1):21−38. doi: 10.1002/fsn3.2636
[23] JANG H J, YANG K E, HWANG I H, et al. Cordycepin inhibits human ovarian cancer by inducing autophagy and apoptosis through Dickkopf-related protein 1/β-catenin signaling[J]. Am J Transl Res,2019,11(11):6890−6906.
[24] 恩施土家族苗族自治州中心医院. 激活细胞GXP4酶的铁死亡抑制剂药物及其应用的制作方法:CN202110692940.1[P]. 2021-10-12. [En Shi Tujia and Miao Autonomous Prefecture Central Hospital. Ferroptosis inhibitor drugs that activate GXP4 enzyme in cells and methods for application:CN202110692940.1[P]. 2021-10-12.] En Shi Tujia and Miao Autonomous Prefecture Central Hospital. Ferroptosis inhibitor drugs that activate GXP4 enzyme in cells and methods for application: CN202110692940.1[P]. 2021-10-12.
[25] 张鑫喆. 不同虫草抗肿瘤活性的细胞学比较研究[D]. 沈阳:沈阳师范大学, 2019. [ZHANG X Z. Comparative study on the antitumor activity of different Cordyceps[D]. Shenyang:Shenyang Normal University, 2019.] ZHANG X Z. Comparative study on the antitumor activity of different Cordyceps[D]. Shenyang: Shenyang Normal University, 2019.
[26] 高川, 马敏俊, 何志刚, 等. 二氢青蒿素对体外培养胃癌细胞株BGC-823周期蛋白D1 P16蛋白表达的影响[J]. 中国药物与临床,2020,20(4):508−511. [GAO C, MA M J, HE Z G. Effect of dihydroartemisinin on protein expression of cyclin D1 and P16 in gastric cancer cell line BGC-823 in vitro[J]. Chin Rem & Clinics,2020,20(4):508−511.] GAO C, MA M J, HE Z G. Effect of dihydroartemisinin on protein expression of cyclin D1 and P16 in gastric cancer cell line BGC-823 in vitro[J]. Chin Rem & Clinics, 2020, 20(4): 508−511.
[27] 张健, 张吉炯. LncRNA UNC5B-AS1靶向miR-339-5p调控人肺腺癌细胞系A549增殖和凋亡[J]. 基础医学与临床,2022,42(3):454−460. [ZHANG J, ZHANG J J. LncRNA UNC5B-AS1 targeting miR-339-5p regulates the proliferation and apoptosis of human lung adenocarcinoma cell line A549[J]. Basic Clinic Med,2022,42(3):454−460.] doi: 10.3969/j.issn.1001-6325.2022.03.018 ZHANG J, ZHANG J J. LncRNA UNC5B-AS1 targeting miR-339-5p regulates the proliferation and apoptosis of human lung adenocarcinoma cell line A549[J]. Basic Clinic Med, 2022, 42(3): 454−460. doi: 10.3969/j.issn.1001-6325.2022.03.018
[28] ZHANG D, LI X, SONG D, et al. Atractylenolide III induces apoptosis by regulating the Bax/Bcl-2 signaling pathway in human colorectal cancer HCT-116 cells in vitro and in vivo[J]. Anticancer Drugs,2022,33(1):30−47. doi: 10.1097/CAD.0000000000001136
[29] 段小娴. bcl-2基因家族对细胞凋亡的调控[J]. 中国医学文摘(肿瘤学),2001,15(1):80−81. [DUAN X X. Regulation of apoptosis by bcl-2 gene family[J]. Chin Med Abstracts,2001,15(1):80−81.] DUAN X X. Regulation of apoptosis by bcl-2 gene family[J]. Chin Med Abstracts, 2001, 15(1): 80−81.
[30] JOST P J, VUCIC D. Regulation of cell death and Immunity by XIAP[J]. Cold Spring Harb Perspect Biol,2020,12(8):36−26.
[31] LEE D, LEE W Y, JUNG K, et al. The inhibitory effect of cordycepin on the proliferation of MCF-7 breast cancer cells, and its mechanism:An investigation using network pharmacology-based analysis[J]. Biomolecules,2019,9(9):407−421. doi: 10.3390/biom9090407
[32] MAMRIEV D, ABBAS R, KLINGLER F M, et al. A small-molecule ARTS mimetic promotes apoptosis through degradation of both XIAP and Bcl-2[J]. Cell Death Dis,2020,11(6):483−98. doi: 10.1038/s41419-020-2670-2
[33] JO E, JANG H J, YANG K E, et al. Cordyceps militaris exerts antitumor effect on carboplatin-resistant ovarian cancer via activation of ATF3/TP53 signaling in vitro and in vivo[J]. Nat Prod Commun,2020,15(1):1−14.
[34] LIN C H, LIN K H, KU H J, et al. Amentoflavone induces caspase-dependent/-independent apoptosis and dysregulates cyclin-dependent kinase-mediated cell cycle in colorectal cancer in vitro and in vivo[J]. Environ Toxicol,2023,38(5):1078−1089. doi: 10.1002/tox.23749
[35] CHEN C J, SHIH Y L, YEH M Y, et al. Ursolic acid induces apoptotic cell death through AIF and Endo G release through a mitochondria-dependent pathway in NCI-H292 human lung cancer cells in vitro[J]. In Vivo,2019,33(2):383−391. doi: 10.21873/invivo.11485
[36] LIM S, KALDIS P. Cdks, cyclins and CKIs:Roles beyond cell cycle regulation[J]. Development,2013,140(15):3079−3093. doi: 10.1242/dev.091744
[37] KIMMELMAN A C, WHITE E. Autophagy and tumor metabolism[J]. Cell Metab,2017,25(5):1037−1043. doi: 10.1016/j.cmet.2017.04.004
[38] JEON S J, AHN J H, HALDER D, et al. TIPRL potentiates survival of lung cancer by inducing autophagy through the eIF2α-ATF4 pathway[J]. Cell Death Dis,2019,10(12):959−975. doi: 10.1038/s41419-019-2190-0
[39] VERA-RAMIREZ L, VODNALA S K, NINI R, et al. Autophagy promotes the survival of dormant breast cancer cells and metastatic tumor recurrence[J]. Nat Commun,2018,9(1):1944−1955. doi: 10.1038/s41467-018-04070-6
[40] SIMPSON J E, GAMMOH N. The impact of autophagy during the development and survival of glioblastoma[J]. Open Biol,2020,10(9):200184. doi: 10.1098/rsob.200184
[41] CHANG C, YOUNG L N, MORRIS K L, et al. Bidirectional control of autophagy by BECN1 BARA domain dynamics[J]. Mol Cell,2019,73(2):339−353. doi: 10.1016/j.molcel.2018.10.035
[42] HUANGFU L, LIANG H, WANG G, et al. miR-183 regulates autophagy and apoptosis in colorectal cancer through targeting of UVRAG[J]. Oncotarget,2016,7(4):4735−4745. doi: 10.18632/oncotarget.6732
[43] YIN X, CAO L, KANG R, et al. UV irradiation resistance-associated gene suppresses apoptosis by interfering with BAX activation[J]. EMBO Rep,2011,12(7):727−734. doi: 10.1038/embor.2011.79
[44] KANG R, ZEH H J, LOTZE M T, et al. The Beclin 1 network regulates autophagy and apoptosis[J]. Cell Death Differ,2011,18(4):571−580. doi: 10.1038/cdd.2010.191
[45] RANGEL M, KONG J, BHATT V, et al. Autophagy and tumorigenesis[J]. Febs J,2022,289(22):7177−7198. doi: 10.1111/febs.16125
[46] KOPPULA P, ZHUANG L, GAN B. Cystine transporter SLC7A11/xCT in cancer:Ferroptosis, nutrient dependency, and cancer therapy[J]. Protein Cell,2021,12(8):599−620. doi: 10.1007/s13238-020-00789-5
[47] YANG W S, SRIRAMARATNAM R, WELSCH M E, et al. Regulation of ferroptotic cancer cell death by GPX4[J]. Cell,2014,156(2):317−331.
[48] FISCHER R, KONTERMANN R E, PFIZENMAIER K. Selective targeting of TNF receptors as a novel therapeutic approach[J]. Front Cell Dev Biol,2020,8:401−421. doi: 10.3389/fcell.2020.00401
[49] VAN LOO G, BERTRAND M J M. Death by TNF:A road to inflammation[J]. Nat Rev Immunol,2023,23(5):289−303. doi: 10.1038/s41577-022-00792-3
[50] XU D, ZOU C, YUAN J. Genetic regulation of RIPK1 and necroptosis[J]. Annu Rev Genet,2021,55(1):235−263. doi: 10.1146/annurev-genet-071719-022748
[51] SEO J, NAM Y W, KIM S, et al. Necroptosis molecular mechanisms:Recent findings regarding novel necroptosis regulators[J]. Exp Mol Med,2021,53(6):1007−1017. doi: 10.1038/s12276-021-00634-7





 下载:
下载:
 下载:
下载:



