Purification, Characterization and Antibacterial Mechanism of Plantaricin 2-1
-
摘要: 为了探究植物乳植杆菌L3(Lactiplantibacillus plantarum L3,L. plantarum L3)所产细菌素的基本性质及其抑菌机制,本研究利用MRS培养L. plantarum L3,采用乙酸乙酯萃取L. plantarum L3发酵液,然后通过葡聚糖凝胶层析、阳离子交换层析、反相高效液相色谱(RP-HPLC)分离纯化,再利用液相色谱串联质谱(LC-MS/MS)进行鉴定;以单核增生李斯特菌和大肠杆菌作为指示菌,通过结晶紫染色,扫描电镜、红外光谱、荧光光谱分析,胞外碱性磷酸酶(AKP)、ATP、乳酸脱氢酶(LDH)含量测定等方法分析了该细菌素的抑菌机制。结果表明:植物乳植杆菌素2-1(P2-1)为一种新型细菌素,分子量为983.564 Da。P2-1对两种指示菌最小抑菌浓度均为28.5 μg/mL。P2-1的抑菌过程包括抑制/清除指示菌生物膜;破坏细胞壁,导致AKP外泄;破坏细胞膜,引发细胞内容物(如ATP、LDH)的流出;结合指示菌的DNA并破坏其结构。综上,P2-1对指示菌能够造成多重损伤,并最终导致菌体死亡。上述结果为P2-1作为绿色生物防腐剂的开发应用奠定了理论基础。
-
关键词:
- 植物乳植杆菌素2-1 /
- 纯化 /
- 鉴定 /
- 抑菌机理
Abstract: To explore the basic properties and antibacterial mechanism of bacteriocin produced by Lactobacillus plantarum L3, the strain was sub-cultured in MRS medium, and the broth was extracted by ethyl acetate extraction, followed by purification serially through dextran gel chromatography, cation exchange chromatography and reverse phase high performance liquid chromatography (RP-HPLC), and finally identified by liquid chromatography tandem mass spectrometry (LC-MS/MS). Listeria monocytogenes and Escherichia coli were used as indicator strain and the antibacterial mechanism of the plantaricin was determined by crystal violet staining, scanning electron microscopy, infrared spectroscopy, fluorescence spectroscopy, extracellular alkaline phosphatase (AKP), ATP, and lactate dehydrogenase (LDH) content analysis. Results showed that plantaricin P2-1 was a novel bacteriocin with the molecular weight of 983.564 Da. The minimum inhibitory concentration (MIC) of P2-1 against two indicator strains was 28.5 μg/mL. Plantaricin P2-1 inhibited and/or eradicate the biofilms of two indicator strains. Additionally, plantaricin P2-1 could cause the death of indicator strains by destroying cell integrity, breaking down cytoderm and cytomembrane, as indicated by leakage of AKP, ATP and LDH. Additionally, P2-1 demonstrated the ability to bind to the genomic DNA of the indicator strains, thereby disrupting their DNA structure. These results suggested that P2-1 can cause multiple damages to indicator bacteria and ultimately lead to bacterial death. The above results lay a theoretical foundation for the development and application of P2-1 as a green biological preservative.-
Keywords:
- plartanicin 2-1 /
- purification /
- characterization /
- antibacterial mechanism
-
细菌素是一种由细菌在核糖体内合成并释放到胞外,具有抑菌或杀菌效果的肽或蛋白质[1]。细菌素对多种消化酶敏感,进入消化道后可被降解。以细菌素作为食品防腐剂不会改变食品本身的品质,同时也不会对消费者的身体健康产生不利影响[2]。因此近年来,利用细菌素对食品进行防腐[3]、疾病治疗等研究越来越多[4]。乳酸菌作为益生菌能产生多种细菌素[5],乳酸菌也因其广谱的抑菌范围、较大的pH耐受范围、较好的耐热性等特性而备受关注[6]。
细菌素对微生物的抑制机制目前尚未完全阐明。最初有人提出,这些肽或蛋白质可导致细胞膜出现孔隙甚至细胞崩塌[7]。然而,最近的研究发现了其他可能的作用模式,如与特定细胞内靶点的相互作用、抑制核酸和蛋白质的合成、干扰细胞成分(如细胞壁)的形成,以及抑制酶的活性等[8]。不同的细菌素,对致病菌的作用方式也不尽相同。
本研究预实验发现植物乳植杆菌L3(L. plantarum L3)发酵上清液具有良好的抑菌效果,经初步鉴定该抑菌物质为肽或蛋白质,因此对植物乳植杆菌L3所产细菌素进行纯化、鉴定,并以致病菌生物膜、细胞壁、细胞膜、DNA作为潜在作用靶点,探究植物乳植杆菌L3所产细菌素的抑菌机理,以期为开发天然新型生物防腐剂提供理论依据。
1. 材料与方法
1.1 材料与仪器
植物乳植杆菌L3(Lactobacillus plantarum L3,NCBI序列号为 MT781360) 分离自东北长保质期酸菜;单核细胞增生李斯特菌(Listeria monocytogenes)CMCC 54001、大肠杆菌(Escherichia coli)ATCC 25922 均为黑龙江大学微生物重点实验室保藏菌株(−20 ℃);MRS肉汤 北京索莱宝科技有限公司;琼脂粉、蛋白胨、酵母浸粉为生物试剂、氯化钠(分析纯)、BCA蛋白浓度测定试剂盒 北京奥博星生物技术有限公司;氢氧化钠(分析纯) 天津市大陆化学试剂厂;盐酸(分析纯) 天津市科密欧化学试剂有限公司;乙酸乙酯(分析纯) 天津市天力化学试剂有限公司;甲醇、乙酸(分析纯) 天津市富宇精细化工有限公司;乳酸脱氢酶(Lactate Dehydrogenase,LDH)试剂盒、ATP含量(磷钼酸比色法)测定试剂盒、碱性磷酸酶(Alkaline Phosphatase,AKP)含量测定试剂盒 苏州格瑞斯生物科技有限公司;Sephadex G-50、SP Sepharose Fast Flow填料 上海源叶生物科技有限公司;胃蛋白酶(3000 U/mg)、胰蛋白酶(≥250 U/mg)、木瓜蛋白酶(800000 U/g) 高级纯,北京博奥拓达科技有限公司;细菌基因组提取试剂盒(离心柱型) 北京天根生化科技有限公司。
HZQ-X100振荡培养箱 哈尔滨市东联电子技术开发有限公司;VersaMAX型酶标仪 上海美谷分子仪器有限公司;Allegra X-64R高速台式离心机 美国Backman Coulter公司;DYCZ-24DN垂直蛋白电泳槽 北京六一生物有限公司;JP-3000核酸蛋白检测仪 上海嘉鹏有限公司;FN-2004 N型电子天平 上海力辰仪器有限公司;BTP真空冷冻干燥机 美国SP Virtis公司;Quiksep-50D半制备高压液相色谱仪 北京慧德易科技有限公司;FE28型pH计 上海叶拓科技有限公司;JSM-IT800扫描电子显微镜 日本电子株式会社。
1.2 实验方法
1.2.1 L. plantarum L3菌种培养及无细胞上清液(CFS)制备
将−20 ℃甘油保藏的L. plantarum L3活化二代,以菌浓度>1010 CFU/mL、2%接种量(V/V)接种至MRS培养基中,37 ℃、120 r/min振荡培养,培养24 h,6797×g离心10 min,收集上清液,获得CFS。
1.2.2 CFS抑菌效价测定
以单核细胞增生李斯特菌为指示菌株,采用牛津杯法测定CFS的抑菌活性。将菌浓度为108 CFU/mL的单核细胞增生李斯特菌100 μL涂布于琼脂培养基上。在8 mm孔中加入200 μL CFS,37 ℃培养24 h。抑菌效价单位用AU/mL表示,将CFS用蒸馏水2倍稀释,测定每个稀释度细菌素抑菌活性,以能够出现抑菌圈的最高稀释度定义为一个活力单位(AU)[9]。
抑菌活性(AU/mL)=2n1000x 式中:n:对指示菌显示抑菌圈的孔数,x:每个孔中添加细菌素的体积。
1.2.3 CFS抑菌物排酸、排过氧化氢试验
排酸实验研究过程如下:用5 mol/L的NaOH将CFS pH调节至6,以未调pH的CFS做对照,以单核细胞增生李斯特菌为指示菌,采用牛津杯法检测抑菌活性。排除过氧化氢影响研究:取6 mL CFS,将其pH调至7,加入60 mg过氧化氢酶,37 ℃水浴2 h后,将pH调至6。采用牛津杯法检测抑菌活性,指示菌为单核细胞增生李斯特菌[10]。
1.2.4 CFS抑菌物的蛋白酶敏感性测定
将胃蛋白酶、胰蛋白酶、木瓜蛋白酶分别添加到已调至各酶最适pH的CFS至终浓度为1 mg/mL,37 ℃水浴2 h后,调pH至CFS初始pH,同时以未经酶处理的CFS为对照,采用牛津杯法检测抑菌活性,指示菌为单核细胞增生李斯特菌。
1.2.5 植物乳植杆菌素的分离纯化
1.2.5.1 乙酸乙酯萃取
将CFS与乙酸乙酯以7:5的比例(V/V)进行混合,充分搅拌4 h,然后放在4 ℃萃取过夜,收集上层有机相,于42 ℃下对有机相进行旋转蒸发完全,采用蒸馏水溶解残留物即为植物乳植杆菌素粗提物,冷冻干燥备用。以单核细胞增生李斯特菌为指示菌,采用牛津杯法对其粗提物进行抑菌性分析[11]。
1.2.5.2 葡聚糖凝胶层析
采用葡聚糖凝胶G-50对植物乳植杆菌素粗提物进行纯化。将填料装柱后,采用纯水将基线跑至平稳,将10 mg植物乳植杆菌素粗提物加入1 mL蒸馏水进行溶解,经0.22 μm滤膜过滤上样,上样量为2 mL,洗脱液为纯水,流速为0.5 mL/min。检测280 nm下洗脱液的吸光值,收集洗脱峰组分,分别测定其对单核细胞增生李斯特菌的抑菌活性,将有抑菌活性的组分真空冷冻干燥备用[12]。
1.2.5.3 阳离子交换层析
采用SP Sepharose Fast Flow层析对上一步得到的植物乳植杆菌素继续纯化。将填料装柱后,采用pH3柠檬酸-磷酸盐缓冲液将基线跑至平稳,将10 mg植物乳植杆菌素加入1 mL蒸馏水进行溶解,经0.22 μm滤膜过滤上样,洗脱液为1 mol/L NaCl溶液,上样量为1 mL,流速为0.3 mL/min。检测280 nm下洗脱液的吸光度值,收集洗脱峰组分。采用透析袋脱盐处理,分别测定其对单核细胞增生李斯特菌的抑菌活性,将有抑菌活性的组分真空冷冻干燥备用[13]。
1.2.5.4 半制备反向高效液相色谱(RP-HPLC)
将经过阳离子交换层析得到的植物乳植杆菌素采用RP-HPLC进行进一步纯化。HPLC检测条件:色谱柱为C18柱(4.6×250 mm,5 μm),流动相:A:纯水,B:色谱级甲醇,流速为2 mL/min,进样量100 μL,梯度洗脱:0~4 min,90%流动相A+10%流动相B;4~31 min,50%流动相A+50%流动相B;50~65 min,100%流动相B,在OD280 nm处检测洗脱液的吸光度值,收集各个洗脱峰组分,分别测定其对单核细胞增生李斯特菌的抑菌活性,将有抑菌活性的洗脱液真空冷冻干燥浓缩[14]。
1.2.6 采用LC-MS/MS鉴定
预柱为300 μm i.d.×5 mm,packing:PepMapm Neo C18 5 μm,100A;分析柱为100 μm i.d.×180 mm,packing:Reprosil-Pur 120 C18-AQ 3 μm ;流动相A:0.1%甲酸,B:0.1%甲酸+80% CAN;上样量为400 ng,流速为600 nL/min,梯度洗脱:0~2 min,4%流动相B;2~35 min,8%流动相B;35~55 min,40%流动相B;55~66 min,95%流动相B,每个组分分析时间:66 min。
1.2.7 P2-1对指示菌最小抑菌浓度(MIC)的测定
分别吸取100 μL培养至对数生长期的单核细胞增生李斯特菌和大肠杆菌菌液(108 CFU/mL)于96孔板中备用,采用二倍倍比稀释法将细菌素P2-1分别稀释至228、114、57、28.5、14.25、7.125、3.562 μg/mL,随后吸取100 μL稀释后的各个浓度P2-1加入带有菌液的96孔板中,37 ℃,孵育24 h。采用酶标仪测定590 nm处的吸光度值。阴性对照为LB培养基,阳性对照为不添加P2-1的菌悬液,测定P2-1完全抑制指示菌生长的浓度[15]。
1.2.8 P2-1对指示菌生物膜的影响
1.2.8.1 P2-1抑制指示菌生物膜形成能力的测定
参考Qu等[16]的方法,将培养至对数生长期的单核细胞增生李斯特菌、大肠杆菌菌液(108 CFU/mL)按2%接种量接种至新鲜LB培养基中,在96孔板中加入菌液以及等体积浓度为1×MIC、2×MIC的P2-1,37 ℃分别培养12、24、36、48和60 h。弃去孔中菌液,洗去未黏附的细菌,甲醇固定后烘干。每个孔中加入1% 200 μL的结晶紫染液,染色15 min。然后用PBS清洗去除未结合的染料,随后加入乙醇,充分溶解染料,用酶标仪检测 590 nm处的吸光度。对照组为未经P2-1处理的单核细胞增生李斯特菌和大肠杆菌。
1.2.8.2 P2-1对指示菌生物膜清除能力的测定
采用结晶紫染色法测定P2-1对指示菌已形成生物膜的清除能力。参照Niaz等[17]的方法,分别取单核细胞增生李斯特菌、大肠杆菌菌液(108 CFU/mL)置于6孔板中,将盖载片放入6孔板底部,37 ℃培养24 h。取出盖玻片,用PBS洗涤,放入另一个无菌的6孔板中,分别加入等体积浓度为1×MIC、2×MIC的P2-1溶液,37 ℃培养24 h后取出盖玻片,用PBS洗涤、无水甲醇固定,1%结晶紫染色15 min,去掉染液后用蒸馏水洗涤,干燥后在显微镜下观察。将盖玻片放回6孔板,乙醇溶解后测量590 nm处的吸光度值,对照组为未经P2-1处理的指示菌。
清除率(%)=A0−A1A0×100 式中:A0:对照组吸光度值;A1:实验组吸光度值。
1.2.9 指示菌细胞形态完整性鉴定
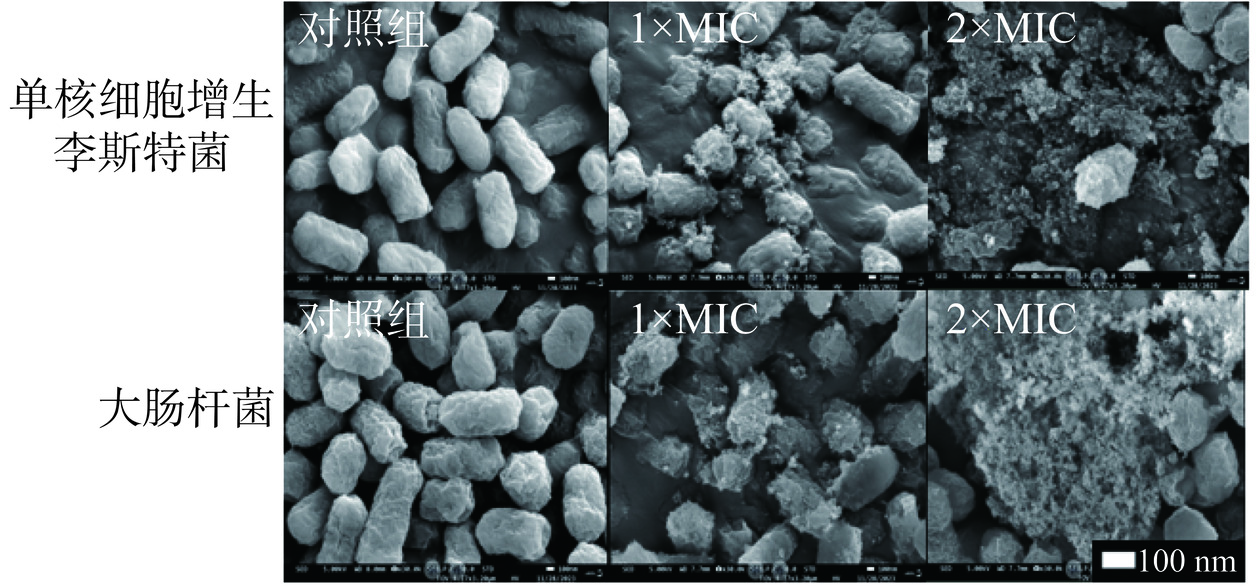
在指示菌(108 CFU/mL)菌悬液中分别加入等体积浓度为1×MIC、2×MIC的P2-1,以未添加P2-1为对照组,置于37 ℃培养24 h,6797×g离心10 min,收集沉淀。沉淀在2.5%戊二醛溶液中固定过夜,用1 mol/L磷酸盐缓冲溶液(pH7.0)洗涤沉淀3次,乙醇脱水后将菌体涂于载玻片上,自然晾干,喷金,利用SEM观察细胞形态[18]。
1.2.10 P2-1对指示菌细胞壁完整性的影响
1.2.10.1 指示菌胞外碱性磷酸酶(AKP)含量测定
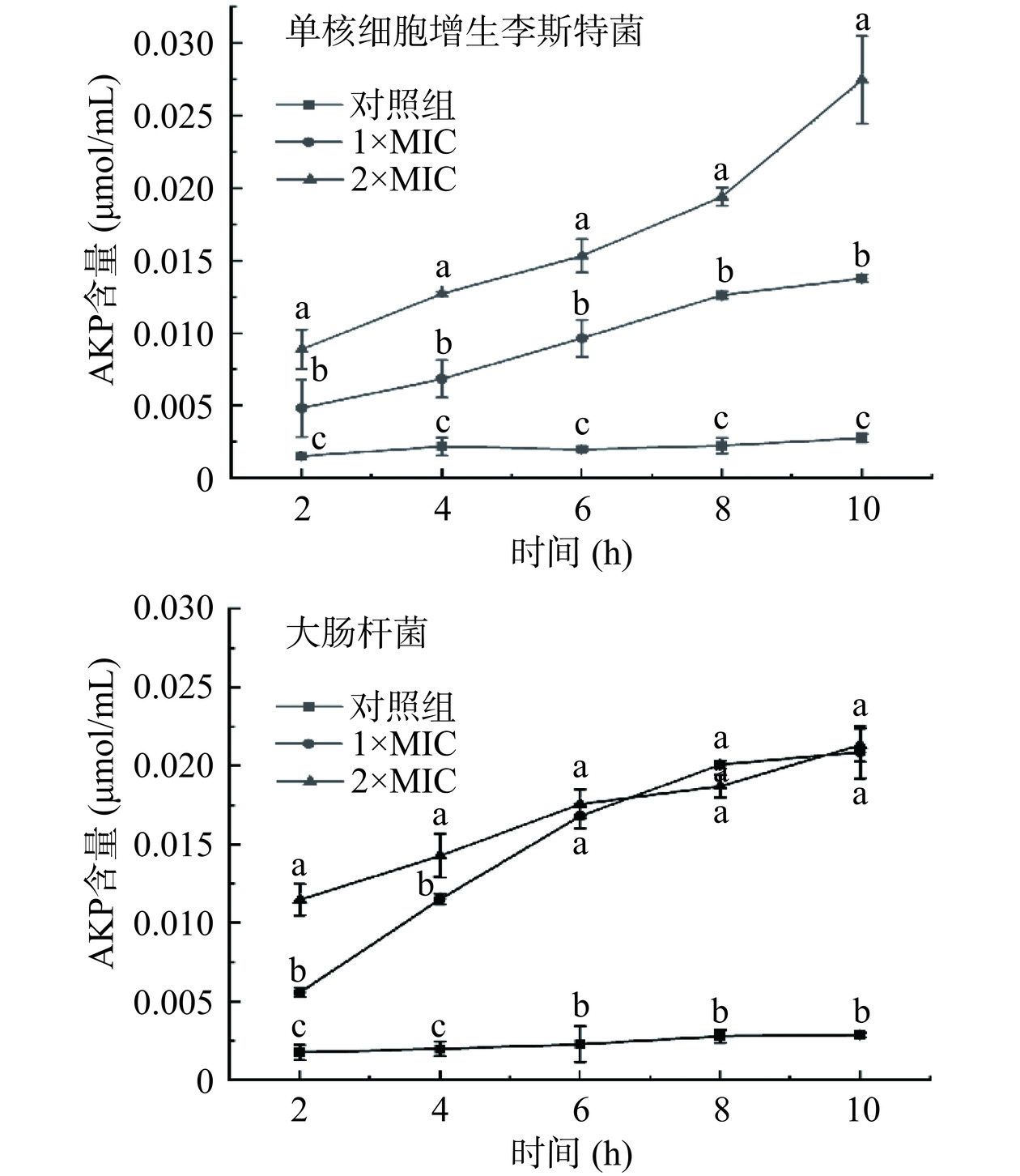
将培养至对数生长期的单核细胞增生李斯特菌、大肠杆菌菌液(108 CFU/mL)按2%接种量接种至新鲜LB培养基中,分别添加等体积浓度为1×MIC、2×MIC的P2-1,37 ℃分别培养2、4、6、8、10 h后取菌悬液,离心后测定上清液中AKP含量(按AKP含量测定试剂盒说明书操作),对照组为未添加P2-1的指示菌菌悬液[19]。
1.2.10.2 P2-1对指示菌细胞壁作用位点测定
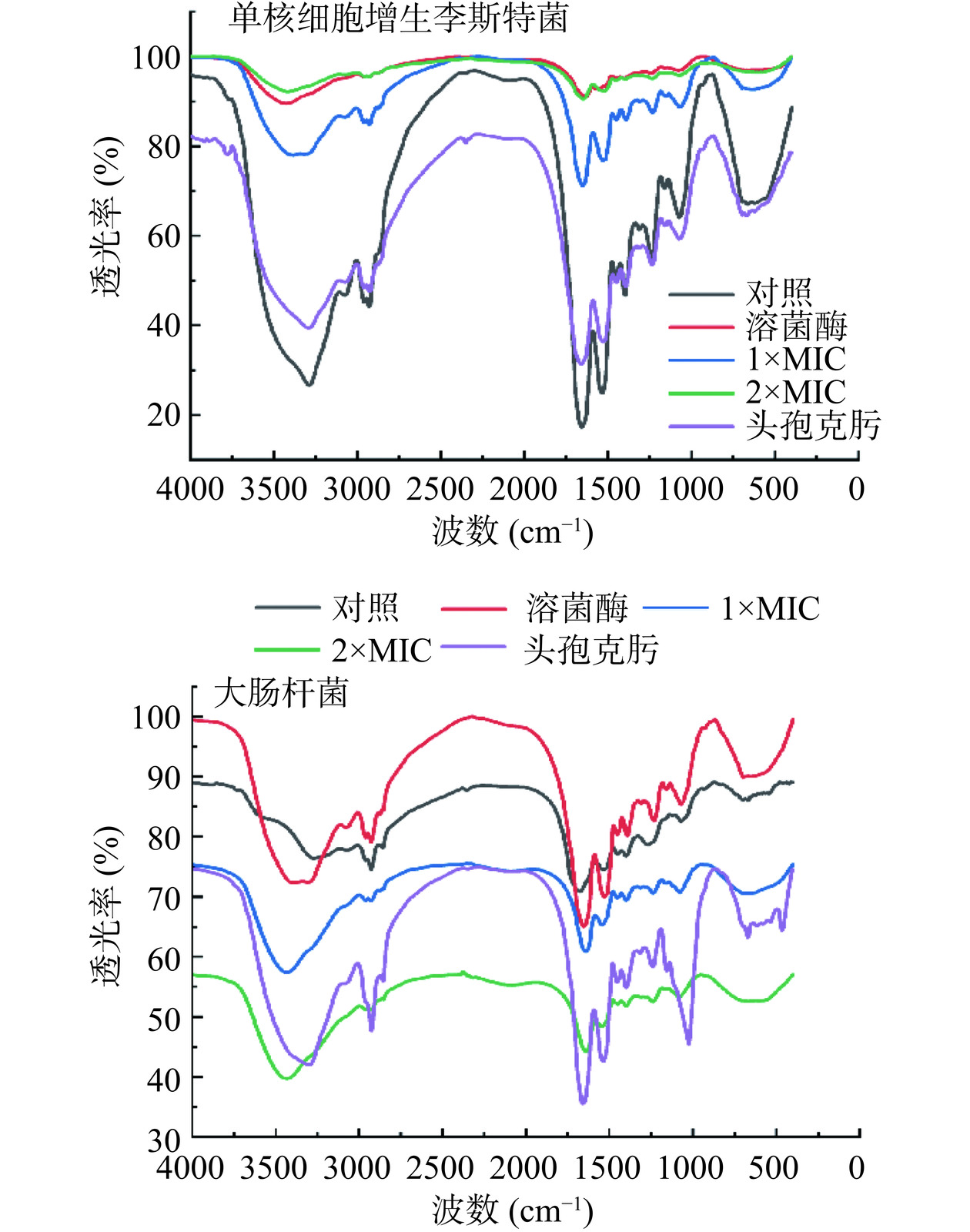
将培养至对数生长期的单核细胞增生李斯特菌、大肠杆菌菌液(108 CFU/mL)与等体积的溶菌酶(0.5 mg/mL)、浓度为1×MIC、2×MIC的P2-1或头孢克肟(0.5 mg/mL)混合,37 ℃孵育24 h,对照组为原始指示菌菌液。处理结束后,6797×g离心10 min,收集菌体沉淀,PBS洗涤沉淀3次后于100 ℃沸水浴中灭活处理20 min。将灭活菌体于冰浴中超声处理12 min(功率400 W、间隔2 s、破碎1 min),85×g离心15 min,去除沉淀,上清液再以8512×g离心15 min,将沉淀于烘箱中干燥、溴化钾压片后进行红外光谱分析[20]。
1.2.11 P2-1对指示菌细胞膜完整性的影响
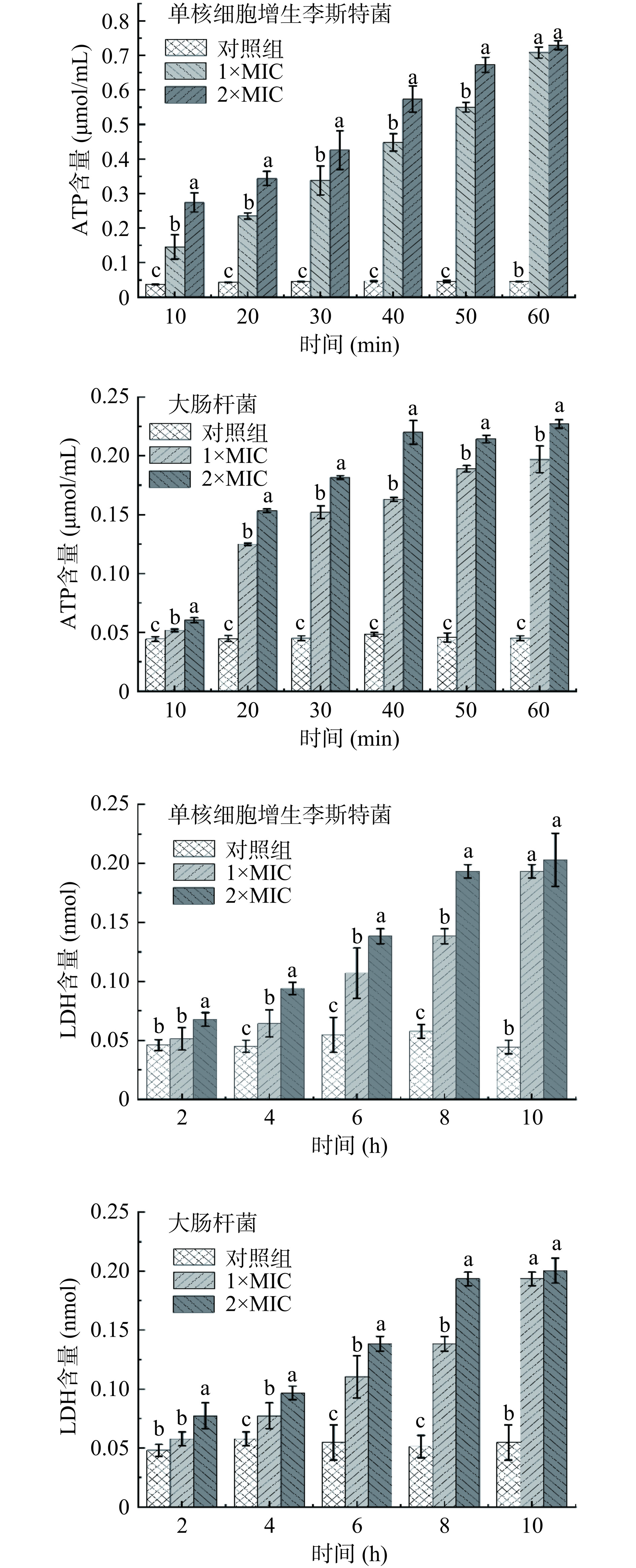
将培养至对数生长期的单核细胞增生李斯特菌、大肠杆菌菌液(108 CFU/mL)按2%接种量接种至新鲜LB培养基中,分别添加等体积浓度为1×MIC、2×MIC的P2-1,以未添加P2-1的指示菌菌液为对照组。于37℃分别培养10、20、30、40、50、60 min后取菌悬液,离心后测定上清液中ATP含量(按ATP含量测定试剂盒说明书操作)[21]。于37 ℃分别培养2、4、6、8、10 h后取菌悬液,离心后测定上清液中LDH含量(按LDH含量测定试剂盒说明书操作)[22]。
1.2.12 P2-1对指示菌基因组DNA的破坏能力测定
1.2.12.1 指示菌与P2-1共培养后的基因组DNA损伤分析
将浓度为1×MIC、2×MIC的P2-1溶液与等量指示菌菌悬液(108 CFU/mL)分别共孵育4、8、12 h后,提取基因组DNA(按细菌基因组提取试剂盒说明书操作)并进行琼脂糖凝胶电泳,以未添加P2-1的指示菌DNA为对照[23]。
1.2.12.2 指示菌基因组DNA与P2-1直接作用后的损伤分析
提取对数生长期指示菌的基因组DNA,加入等体积浓度为1×MIC、2×MIC的P2-1分别孵育30 min、60 min,进行琼脂糖凝胶电泳,以未添加P2-1的指示菌基因组DNA为对照[9,24]。
1.2.12.3 P2-1与指示菌基因组DNA结合方式分析
将指示菌基因组DNA溶液3 mL与1%溴化乙锭(EB)溶液15 µL混合,然后再分别加入1 mL浓度为0.5×MIC,1×MIC,2.5×MIC的P2-1溶液,以未添加P2-1的EB-DNA混合液为对照,37 ℃水浴避光孵育30 min,在550~800 nm波长范围进行光谱扫描。荧光光谱仪的狭缝设置为Ex=Em=5 nm,荧光激发波长λex=490 nm,样品池为1 cm石英池[25]。
1.3 数据处理
每组实验重复3次。使用Origin 2021计算平均值和标准误差值,数据采用平均值±标准差表示。单因素方差分析采用Fisher检验来确定是否存在统计学显著性差异(P<0.05)。
2. 结果与分析
2.1 CFS抑菌活性测定
本实验以单核细胞增生李斯特菌为指示菌株,采用琼脂孔扩散法测定L. plantarum L3发酵上清液(CFS)的抗菌活性(表1),结果表明,CFS对单核细胞增生李斯特菌抑菌圈直径为18.33±1.15 mm,抑菌效价为400 AU/mL。有研究表明,从四川泡菜中分离到的植物乳植杆菌IMAU80043所产CFS对大肠杆菌抑菌圈直径为34.35±2.31 mm[26],而植物乳植杆菌DLP1所产CFS对荧光假单胞菌抑菌效价为35 AU/mL[9]。这些结果表明,CFS的抑菌活性与植物乳植杆菌的来源以及测试的菌株有关。
表 1 不同处理对CFS抑菌活性的影响Table 1. Effect of different treatments on antibacterial activity of CFS处理方式 抑菌圈直径(mm) 对照组(pH3.213±0.005) 18.33±1.15 调至pH6 10.33±0.57 过氧化氢酶 9.33±0.57 胰蛋白酶 8.00±1.00 木瓜蛋白酶 − 胃蛋白酶 − 2.2 CFS抑菌物的性质确定
如表1所示,CFS经排酸、排过氧化氢后抑菌活性有所下降,但没有完全消失,表明CFS中除有机酸和过氧化氢以外,还有其他的抑菌物质。为此,本实验进一步在CFS中加入胰蛋白酶、木瓜蛋白酶及胃蛋白酶,结果显示,除了加入胰蛋白酶的CFS仍有少量抑菌活性外,其余上清液均无抑菌性,说明该抑菌成分对蛋白酶敏感,具有蛋白质性质[1]。
2.3 细菌素的分离纯化
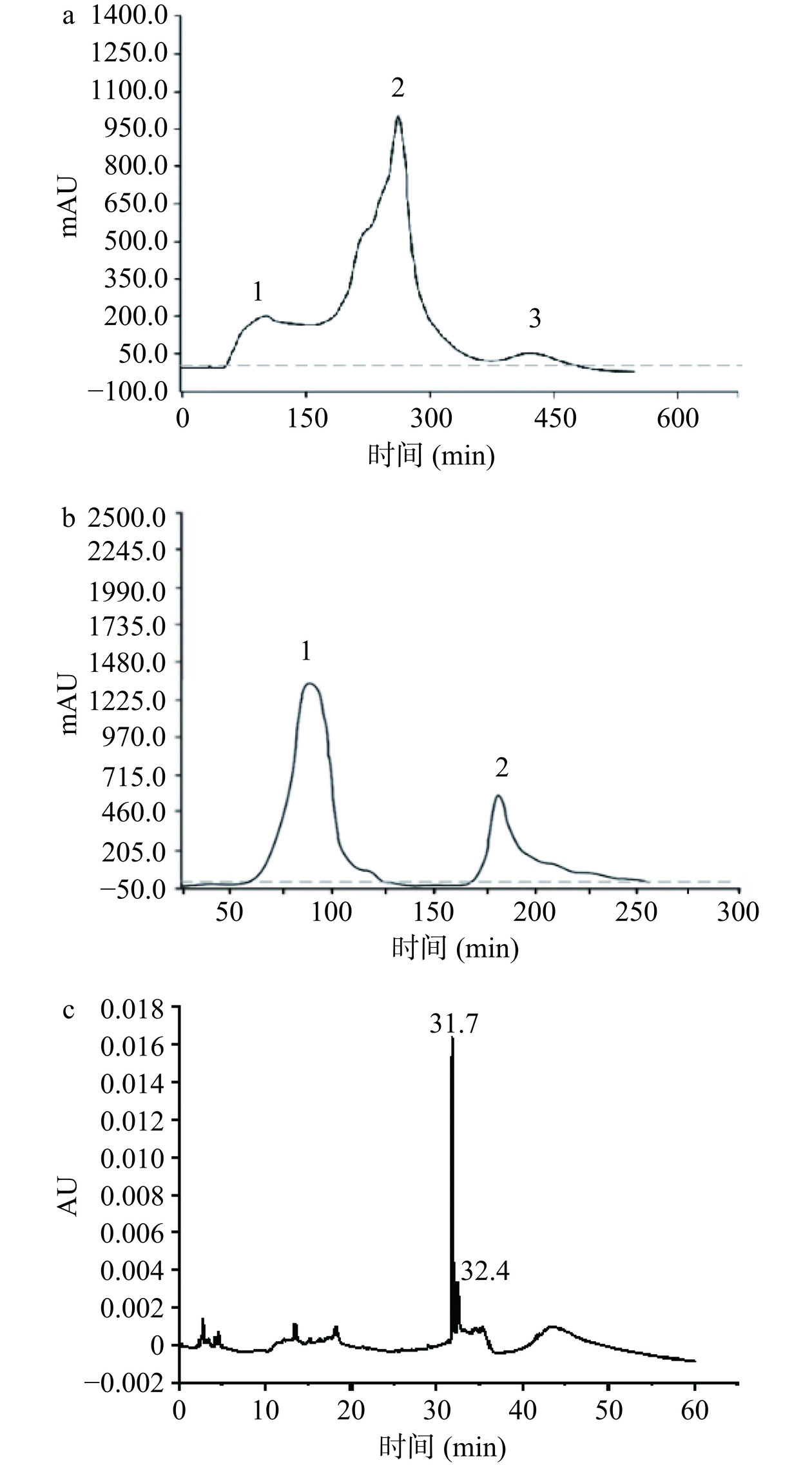
如图1所示,本研究首先对细菌素分别采用乙酸乙酯萃取法粗提、Sephadex G-50洗脱(如图1a),分别对3个峰对应的洗脱物进行抑菌性检测(指示菌为单核细胞增生李斯特菌),收集有抑菌效果峰2进行下一步阳离子交换色谱柱纯化(图1b),抑菌性检测结果显示只有峰1样品具有抑菌效果,收集峰1冷冻干燥。采用RP-HPLC对细菌素进一步纯化,结果如图1c所示,在31.7 min和32.4 min时出现两个特征峰,对两个特征峰洗脱液进行抑菌性检测,只有31.7 min峰具有抑菌效果,将该峰对应的物质命名为植物乳植杆菌素2-1(P2-1),冷冻干燥P2-1备用(表2)。
表 2 细菌素的纯化过程及效价Table 2. Procedures of bacteriocin purification and the corresponding titers纯化方法 乙酸乙酯
萃取Sephadex G-50柱纯化 阳离子交换色谱柱纯化 峰1 峰2 峰3 峰1 峰2 抑菌效价
(AU/mL)3200.0 − 12800.0 − 2666.6 − 注:“−”表示无抑菌活性。 2.4 P2-1的LC-MS/MS鉴定
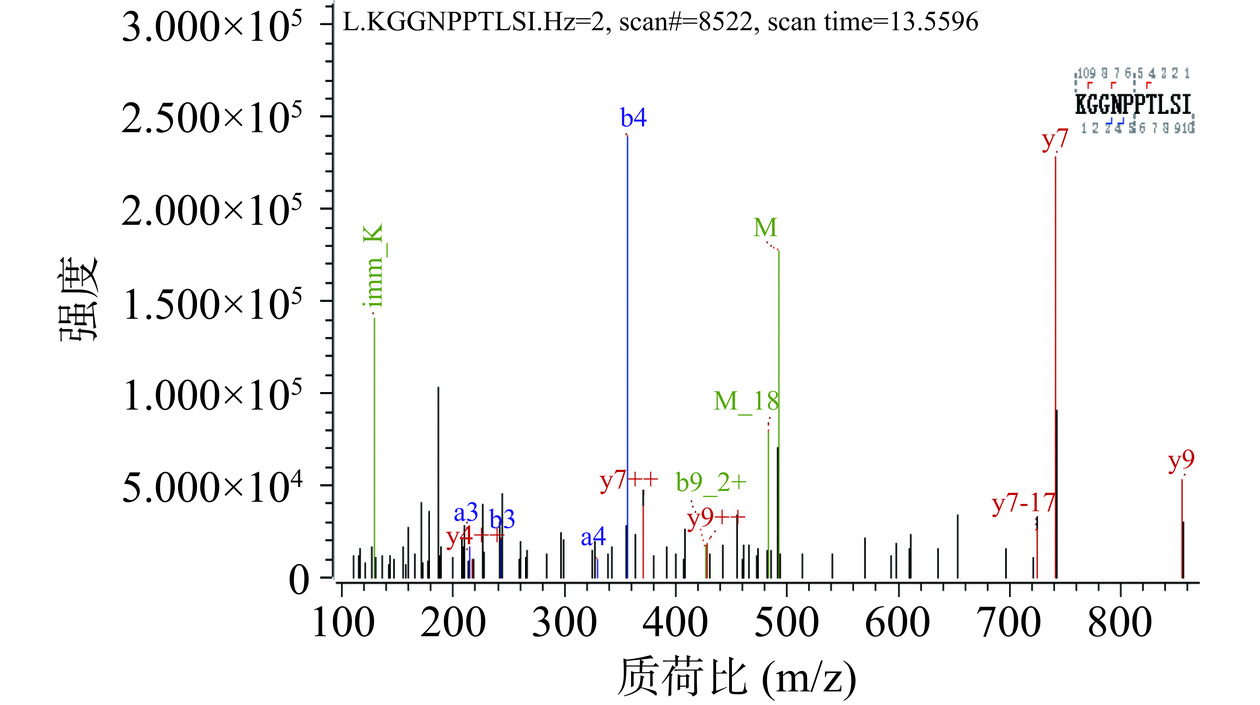
经RP-HPLC纯化后的P2-1进行LC-MS/MS质谱分析结果如图2,测得部分植物乳植杆菌素2-1肽段的分子量为983.564 Da,该肽段氨基酸序列为KGGNPPTLSI。检索通用蛋白数据库(UniProt)中细菌素数据库未发现有同源性序列,表明P2-1为潜在的新型细菌素。
2.5 P2-1对单核细胞增生李斯特菌及大肠杆菌抑菌机制研究
2.5.1 P2-1对指示菌的最小抑菌浓度
细菌素的抑菌性能与其浓度有关,最小抑菌浓度(MIC)越小说明细菌素抑菌性越强。以单核细胞增生李斯特菌和大肠杆菌为指示菌,P2-1对两种指示菌的MIC均为28.5 μg/mL。
2.5.2 P2-1对指示菌生物膜的影响
2.5.2.1 P2-1对指示菌生物膜形成的影响
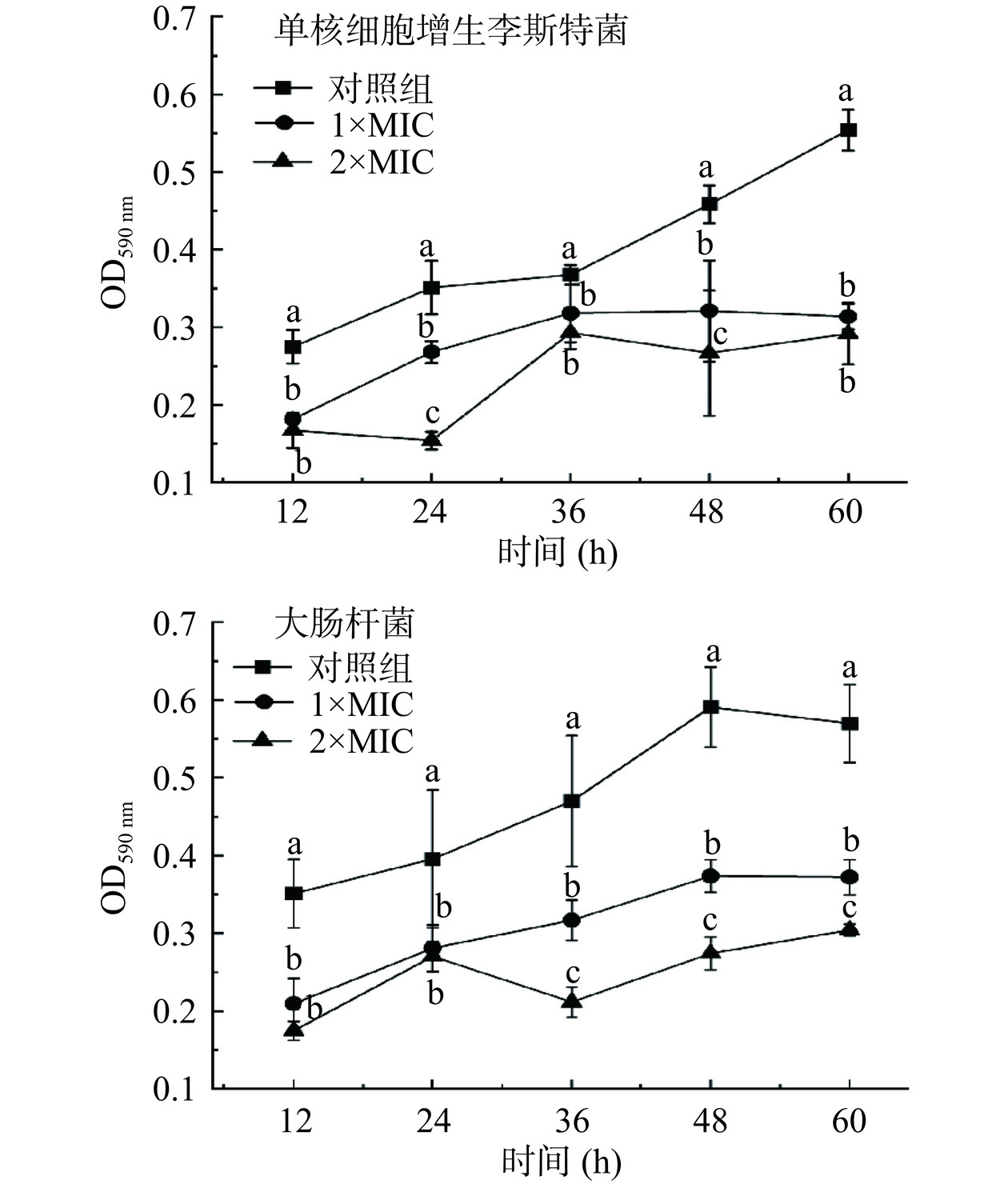
生物膜是微生物在细胞外形成的一种含有多糖的粘性物质,能够在菌体表面形成保护层,提高菌体对胞外环境的抵抗能力[27]。破坏致病菌生物膜可以对致病菌生长起到抑制作用。通过测定黏附在孔板底部的生物膜内细菌浓度,可以进一步反应生物膜的生成量。从图3可以看出,随着培养时间的延长,对照组吸光值呈上升趋势,说明培养过程中指示菌的生物膜逐渐形成。与对照组相比,1×MIC和2×MIC P2-1处理后指示菌吸光值均显著低于对照组(P<0.05),说明P2-1可以抑制单核细胞增生李斯特菌和大肠杆菌的生物膜形成。
2.5.2.2 P2-1对指示菌生物膜的清除作用
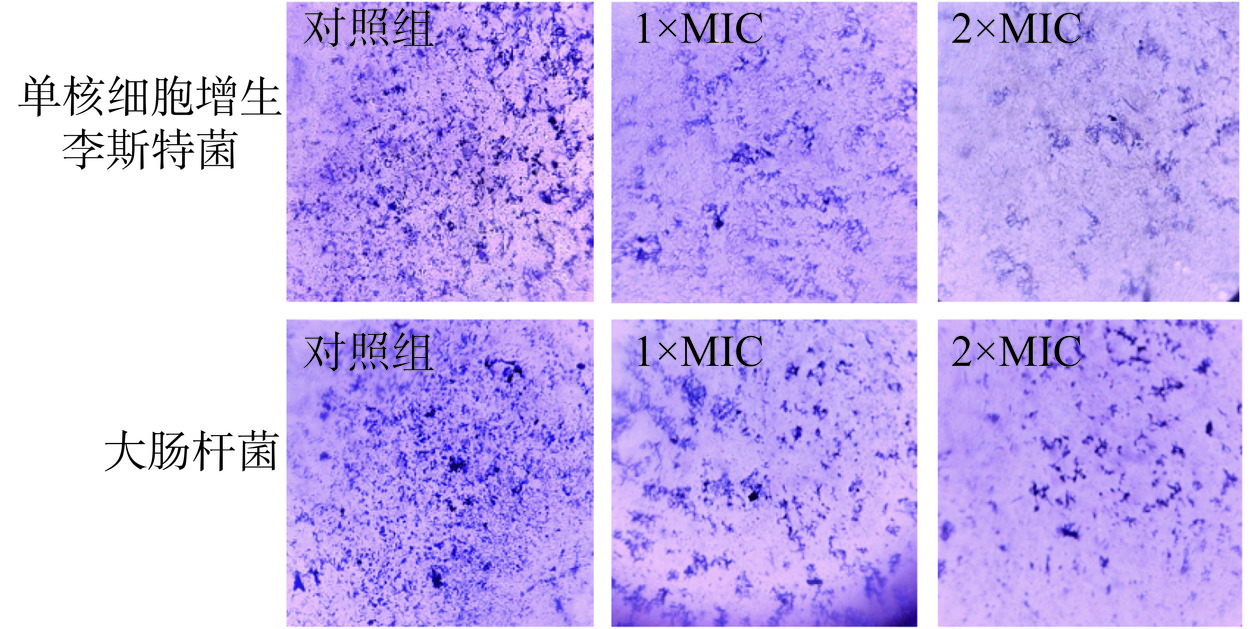
以1×MIC、2×MIC的P2-1处理已形成的单核细胞增生李斯特菌和大肠杆菌生物膜。从图4中也可以看出,经P2-1处理后,盖玻片上的生物膜从致密变的稀疏,且经2×MIC P2-1处理24 h后,对单核细胞增生李斯特菌生物膜清除率达到61.5%,对大肠杆菌生物膜清除率达到46.9%。说明P2-1对已形成的致病菌生物膜具有良好的清除作用。
2.5.3 P2-1对指示菌细胞形态完整性的影响
经P2-1处理后的指示菌菌体扫描电镜观察结果如图5所示,未处理的指示菌细胞形态完整,细胞呈短杆状,细胞边缘整齐且清晰。经P2-1处理后,细胞表面开始出现皱缩和凹陷,细胞形态明显被破坏,2×MIC处理组几乎观察不到完整的细胞形态。结果表明,P2-1可破坏指示菌细胞形态完整,且随着P2-1浓度的增加,破坏程度也呈现加剧趋势。
2.5.4 P2-1对指示菌细胞壁的影响
2.5.4.1 P2-1对指示菌胞外AKP含量的影响
碱性磷酸酶(AKP)存在于细菌细胞壁和细胞膜之间,当细胞壁的通透性增大或者被破坏时,AKP会释放至胞外,因此指示菌菌液中AKP含量可以反映其细胞壁的损伤情况[19]。由图6可知,与对照组相比,不同浓度P2-1处理后的指示菌上清液中AKP的含量均有所增加。培养时间和P2-1浓度均与经P2-1处理的单核细胞增生李斯特菌菌悬液AKP含量呈正相关;大肠杆菌菌悬液经P2-1处理后,0~6 h内AKP含量随培养时间及细菌素浓度的增加而逐渐增加,6 h后AKP含量增加缓慢,最终在10 h时趋于稳定。2×MIC P2-1孵育10 h后的单核细胞增生李斯特菌和大肠杆菌胞外AKP含量分别为对照组的10倍和7.6倍。
2.5.4.2 P2-1对指示菌细胞壁肽聚糖的作用方式分析
参考范为群[28]对红外光谱各峰进行归属,890 cm−1归属于β-1,4糖苷键的特征吸收峰,溶菌酶可水解革兰氏阳性菌和革兰氏阴性菌细胞壁肽聚糖主链上N-乙酰胞壁酸和N-乙酰葡萄糖胺之间的β-1,4糖苷键而导致细菌溶解[29]。由于细菌细胞壁的结构和性质不同,革兰氏阳性菌对溶菌酶更敏感[30]。所以细胞壁肽聚糖β-1,4糖苷键被水解的程度越大,则890 cm−1处的透光率就越大,表明细胞壁损伤越大。如图7所示,单核细胞增生李斯特菌经溶菌酶、1×MIC和2×MIC处理后的细胞壁在890 cm−1透光率均高于对照组,表明P2-1对单核细胞增生李斯特菌细胞壁的作用方式可能与溶菌酶相似。大肠杆菌细胞壁对照组890 cm−1处的透光率小于溶菌酶组但大于1×MIC和2×MIC组,说明P2-1破坏大肠杆菌细胞壁的方式可能与溶菌酶不同。
伯酰胺和仲酰胺的表征位点位于3400~3500 cm−1和1620~1650 cm−1处[28]。头孢类抗生素能够抑制革兰氏阳性菌细胞壁肽聚糖四肽侧链和五肽桥的交联导致肽聚糖合成受阻,此时伯酰胺和仲酰胺表征位点的透光率会上升。单核细胞增生李斯特菌头孢克肟组、1×MIC组和2×MIC组细胞壁在3400~3500 cm−1和1620~1650 cm−1的透光率均高于对照组,说明P2-1也可能以与头孢克肟相似的方式抑制单核细胞增生李斯特菌(革兰氏阳性菌)细胞壁合成。青霉素结合蛋白(Penicillin-binding proteins,PBPs)参与革兰氏阴性细菌胞壁肽聚糖合成的最后阶段[31],对细菌胞壁肽聚糖的形成、成熟和功能发挥决定性作用[32]。头孢类抗生素是革兰氏阴性菌细胞壁肽聚糖四肽末尾的D-丙氨酰-D-丙氨酸前体小肽的结构类似物,能与E. coli的PBPs竞争性结合形成酰基酶复合物,使PBPs失活,从而抑制细胞壁肽聚糖合成,达到抑菌作用,酰基酶复合物的形成则会导致伯酰胺和仲酰胺表征位点的透光率下降。本研究中大肠杆菌头孢克肟组、1×MIC和2×MIC组细胞壁在1620~1650 cm−1和3400~3500 cm−1的透过率均低于对照组,此结果说明P2-1对大肠杆菌细胞壁的作用方式可能与头孢克肟相似。
2.5.5 P2-1对指示菌细胞膜的影响
细胞膜不仅是细胞的结构组分,也是细胞的重要保护屏障。当细胞膜遭到破坏时,细胞内的DNA、ATP和蛋白质等大分子物质会泄漏至胞外,因此胞内物质泄漏量可用作评价细胞膜完整性的指标[33]。
P2-1使指示菌细胞膜内ATP和LDH泄露结果如图8所示,与对照组相比,P2-1处理可使指示菌上清液中ATP含量均显著上升(P<0.05),LDH含量除了2 h时,其余时间均显著上升(P<0.05)。2×MIC的P2-1孵育60 min后的单核细胞增生李斯特菌和大肠杆菌胞外ATP含量分别为对照组的16.2倍和5.04倍,2×MIC的P2-1孵育10 h后单核细胞增生李斯特菌和大肠杆菌胞外LDH含量分别为对照组的4.57倍和3.7倍。此结果表明P2-1可通过破坏单核细胞增生李斯特菌和大肠杆菌细胞膜的完整性,导致胞内的大分子物质泄漏。
2.5.5.1 P2-1对指示菌胞内DNA的损伤分析
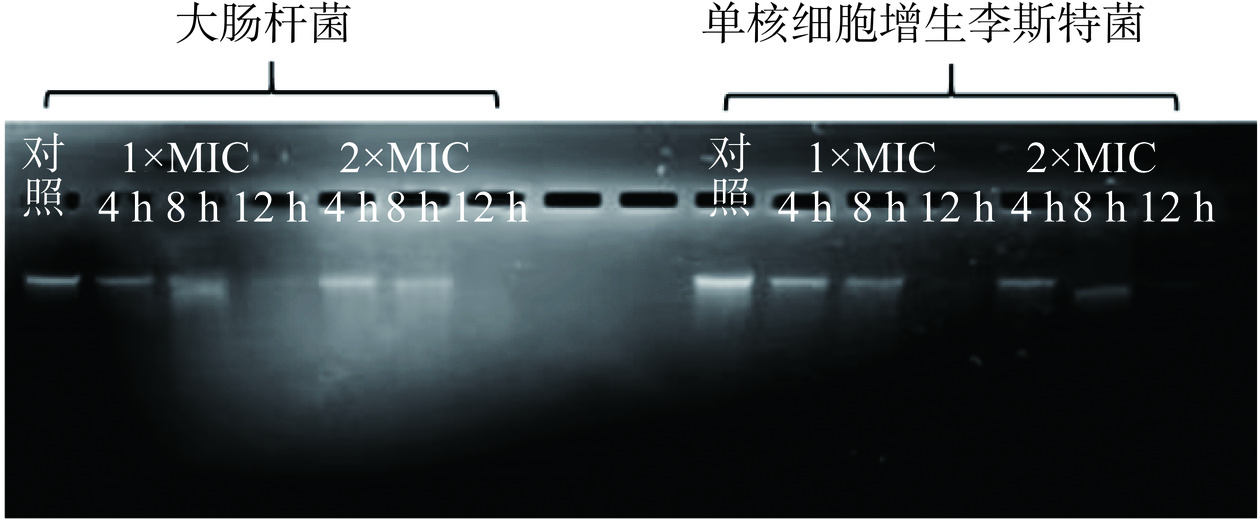
将P2-1与指示菌共培养4、8、12 h后的基因组DNA损伤情况结果如图9所示。对照组基因组DNA电泳后呈现单一的条带,表明胞内的基因组DNA结构完整。而经过P2-1作用后提取的菌体基因组DNA电泳条带随着处理时间增加而逐渐变暗,至12 h左右几乎完全消失。以上结果说明P2-1可导致活细菌胞内基因组DNA发生断裂。
2.5.5.2 P2-1对指示菌游离DNA的损伤分析
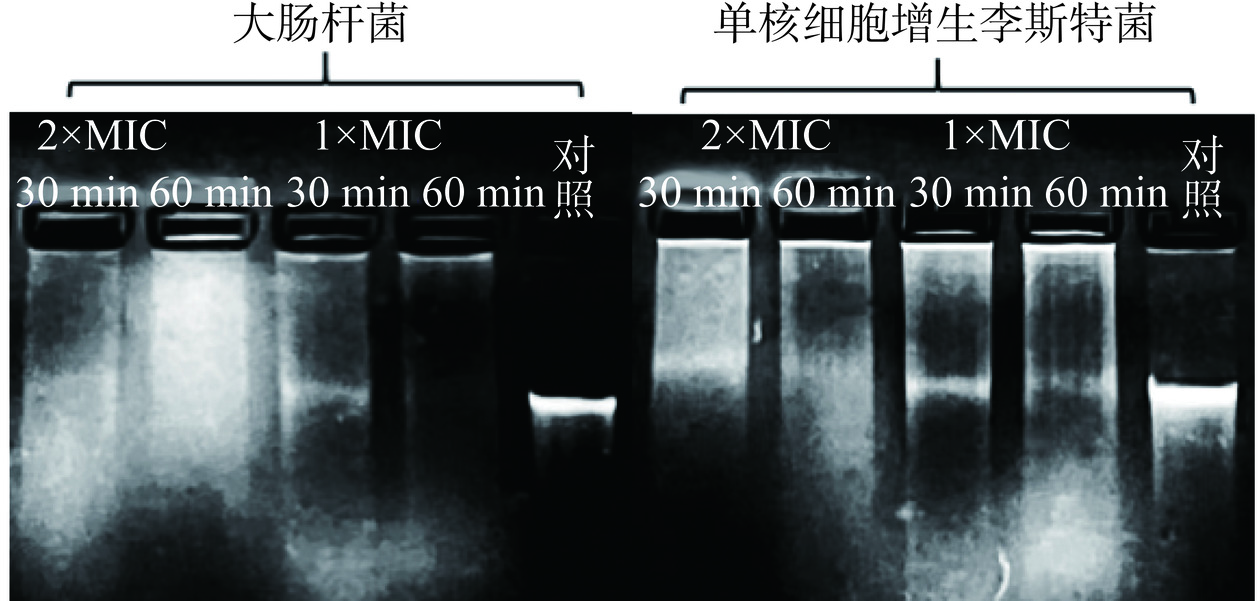
将P2-1直接与指示菌基因组DNA溶液混合并孵育30 min和60 min,进一步观察其对DNA的直接损伤情况。如图10所示,与对照组相比,经P2-1作用后的基因组DNA电泳带呈现明显的呈弥散状,进一步说明了P2-1可以直接破坏指示菌基因组DNA结构的完整性。
2.5.5.3 P2-1与DNA的结合方式分析
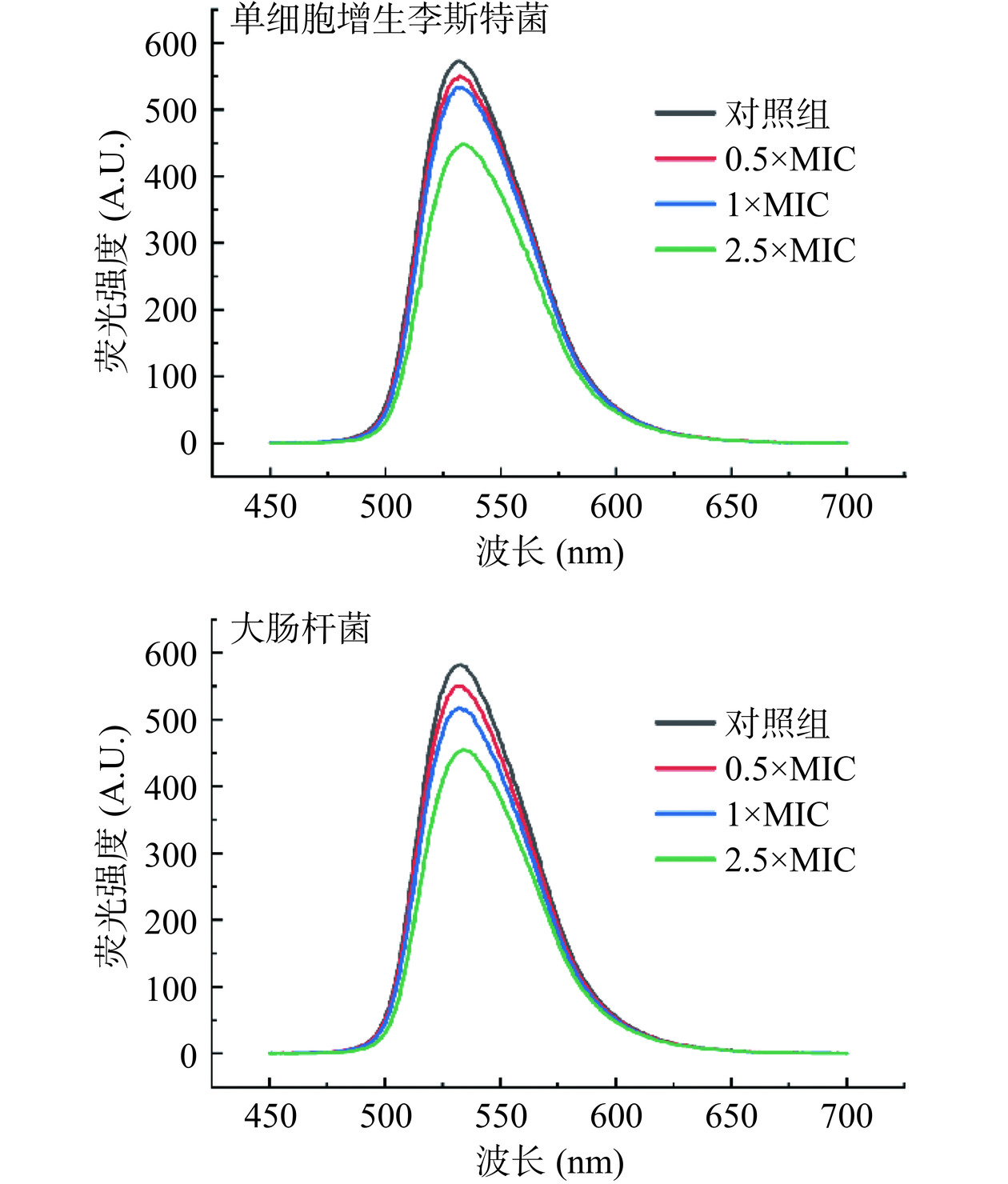
溴化乙锭(Ethidium Bromide,EB)可以通过静电作用与双链DNA的磷酸骨架结合,并可在紫外线照射下发出荧光。当另一种分子与DNA发生类似结合作用时,两种物质便会相互竞争DNA磷酸骨架的可结合位点,从而导致EB荧光强度减弱[34−35]。因此可通过测定DNA-EB体系的荧光光谱和荧光强度的变化,分析P2-1与DNA的结合方式。结果如图11所示,在535 nm左右EB-DNA体系荧光强度随着P2-1浓度的不断增加而逐渐降低,说明P2-1可与EB竞争DNA的结合位点,此结果表明P2-1与细菌DNA结合方式应与EB类似。
3. 讨论
目前大多数细菌素都由革兰氏阳性菌产生,它们的性质和纯化方法却不尽相同。细菌素Z057的纯化包括乙酸乙酯萃取、葡聚凝胶层析和RP-HPLC[36],纯化植物乳植杆菌SHY21-2细菌素的方法则更为复杂,包括硫酸铵沉淀、阳离子交换层析、凝胶过滤层析和RP-HPLC[13]。过去5年发现的新细菌素大多数是通过硫酸铵沉淀、有机溶剂萃取、离子交换色谱或RP-HPLC进行纯化[37]。然而,由于最合适的纯化方法取决于细菌素的特性,因此没有一种方法适用于所有细菌素。本研究采用乙酸乙酯萃取、葡聚糖凝胶层析、阳离子交换层析、RP-HPLC对L. plantarum L3所产细菌素(P2-1)进行了提取和纯化,经LC-MS/MS测定出了部分P2-1肽段,分子量为983.564 Da,氨基酸序列为KGGNPPTLSI,与通用蛋白数据库(UniProt)中细菌素数据库已有序列均无同源性。
细菌素BMP11对大肠杆菌和单核细胞增生李斯特菌的MIC为0.6和38.4 mg/mL[38],细菌素A32对大肠杆菌的MIC为1.25 mg/mL[39],细菌素Plantaricin-fzu 122对大肠埃希氏菌ATCC 25922最小抑菌浓度为32 μg/mL[14],细菌素ZFM225对黄色微球菌CICC 10209的最小抑制浓度为125 μg/mL[40]。本研究选取单核细胞增生李斯特菌和大肠杆菌做为指示菌,P2-1对两种指示菌的MIC为28.5 μg/mL。与以上细菌素对比,P2-1对大肠杆菌和单核细胞增生李斯特菌具有高效的抗菌活性,可进一步开发应用。
到目前为止,许多乳酸菌细菌素已被证明可以阻止食源性病原菌形成生物膜。例如,细菌素XJS01处理前后的生物膜定量分析证实XJS01是金黄色葡萄球菌生物膜形成的有效抑制剂[37],来自L. brevis DF01的细菌素可以抑制大肠杆菌和鼠伤寒杆菌生物膜的形成[41]。本试验中的P2-1可以有效地抑制两种指示菌生物膜生成和清除已生成生物膜。
目前,细菌素对致病菌产生损伤的作用机理多不明确。先前有研究表明,细菌素A32可以破坏大肠杆菌和金黄色葡萄球菌细胞壁,但对金黄色葡萄球菌细胞壁的破坏作用更强,其原因可能与2种细菌在细胞壁结构组成上的差异有关[39]。所以本研究中两种指示菌之间的胞外AKP泄漏量的差异,也可能与革兰氏阳性菌和革兰氏阴性菌细胞壁结构不同有关。李玉珍等[42]采用红外光谱研究金抗肽SIF4对大肠杆菌细胞壁作用位点时发现,1×MIC SIF4处理后的细胞壁多糖信息区、蛋白质与脂肪酸混合信息区、脂肪酸信息区峰值变化明显,且透光率下降,说明细胞壁是金抗肽SIF4的潜在抑菌效应靶点。朱雨龙等[20]研究硝酸镧对枯草芽孢杆菌细胞壁的影响时,发现经溶菌酶处理后,枯草芽孢杆菌细胞壁红外光谱在890 cm−1透光率上升,说明细胞壁被溶菌酶水解。本研究扫描电镜结果显示P2-1可以破坏指示菌的细胞形态完整,导致内容物释放。经过进一步的探究发现,P2-1处理的两种指示菌胞外AKP含量均随着浓度和处理时间的增加而上升,且在相同浓度和处理时间的前提下,单核细胞增生李斯特菌菌悬液中的AKP含量显著高于大肠杆菌组。这种差异可能是因为二者细胞壁结构不同导致的。经P2-1处理的指示菌细胞壁的红外光谱分析结果表明,P2-1可以水解单核细胞增生李斯特菌细胞壁肽聚糖的β-1,4糖苷键,并可能以与头孢克肟类似的方式抑制指示菌细胞壁肽聚糖的合成。
细菌素能使致病菌细胞质泄漏、导致关键激酶活性降低[33],这些变化最终会导致细胞死亡。细菌素LFX01可破坏金黄色葡萄球菌和大肠杆菌细胞膜,从而导致细胞内无机磷离子、ATP泄露[43],最终细胞破碎而死亡。经细菌素CHQS处理后的伊氏李斯特菌表面出现了形态和结构的改变,细胞膜完整性被破坏,导致胞内ATP等内容物外泄,最终死亡[44]。植物乳杆菌素L-1对单核细胞增生李斯特氏菌的作用方式主要是通过细胞膜形成非选择性孔洞使得离子和小分子生命物质外泄,导致细胞死亡[22]。本研究中P2-1可以使指示菌胞外AKP、ATP和LDH含量显著增加,表明其抑制和杀死单核细胞增生李斯特氏菌和大肠杆菌的方式很可能为先破坏指示菌细胞壁完整,之后进一步破坏指示菌的细胞膜,导致细胞内大分子内容物泄露,最终导致细胞死亡。
DNA作为生命信息的载体,在细胞生理功能发挥过程中起着至关重要的作用。对于细菌素与细菌胞内DNA的相互作用方式,也越来越引起人们的广泛关注。研究发现部分细菌素能够与细菌DNA结合,从而影响细菌生理功能。Miao等[45]研究发现细菌素F1能够破坏金黄色葡萄球菌的细胞膜,并与其基因组DNA结合,进而导致菌体死亡。细菌素LAX通过抑制大肠杆菌和金黄色葡萄球菌的DNA合成来实现其抑菌效果[46]。本研究中P2-1可以与EB类似方式嵌入DNA磷酸骨架,结合后可进一步破坏DNA结构,最终使指示菌胞内DNA和游离DNA发生断裂,这一结果与家蝇抗菌肽对大肠杆菌DNA的作用方式一致[38]。
4. 结论
本研究分离纯化得到的P2-1为一种新型细菌素,它可以通过多重机制来协同破坏单核细胞增生李斯特菌和大肠杆菌菌体。但P2-1对指示菌的抑制和杀灭机制所涉及到的转录组学和蛋白质组学研究仍有待进一步深入,同时提高P2-1的产量也是影响其未来能否商业化生产的重要内容。综上,P2-1具有优良的抑菌特性,其对指示菌菌体破坏方式是多重的,本研究为植物乳植杆菌素2-1作为绿色生物防腐剂的开发应用奠定了理论基础。
-
表 1 不同处理对CFS抑菌活性的影响
Table 1 Effect of different treatments on antibacterial activity of CFS
处理方式 抑菌圈直径(mm) 对照组(pH3.213±0.005) 18.33±1.15 调至pH6 10.33±0.57 过氧化氢酶 9.33±0.57 胰蛋白酶 8.00±1.00 木瓜蛋白酶 − 胃蛋白酶 − 表 2 细菌素的纯化过程及效价
Table 2 Procedures of bacteriocin purification and the corresponding titers
纯化方法 乙酸乙酯
萃取Sephadex G-50柱纯化 阳离子交换色谱柱纯化 峰1 峰2 峰3 峰1 峰2 抑菌效价
(AU/mL)3200.0 − 12800.0 − 2666.6 − 注:“−”表示无抑菌活性。 -
[1] DU H C, YANG J, LU X H, et al. Purification, characterization, and mode of action of plantaricin GZ1-27, a novel bacteriocin against Bacillus cereus[J]. Journal of Agricultural and Food Chemistry,2018,66(18):4716−4724. doi: 10.1021/acs.jafc.8b01124
[2] 彭书东, 李键, 刘士健, 等. 乳酸菌细菌素生物合成机制、抑菌机制及应用研究进展[J]. 食品与发酵工业,2019,45(6):236−242. [PENG S D, LI J, LIU S J, et. al. Research progress on biosynthesis antibacterial mechanism and application of lactic acid bacteria bacteriocin[J]. Food and Fermentation Industries,2019,45(6):236−242.] PENG S D, LI J, LIU S J, et. al. Research progress on biosynthesis antibacterial mechanism and application of lactic acid bacteria bacteriocin[J]. Food and Fermentation Industries, 2019, 45(6): 236−242.
[3] WANG Y, QIN Y X, ZHANG Y, et al. Antibacterial mechanism of plantaricin LPL-1, a novel class IIa bacteriocin against Listeria monocytogenes[J]. Food Control,2019,97:87−93. doi: 10.1016/j.foodcont.2018.10.025
[4] MICHAEL C A, DOMINEY-HOWES D, LABBATE M. The antimicrobial resistance crisis:Causes, consequences, and management[J]. Frontiers in Public Health,2014,2:145−153.
[5] DARBANDI A, ASADI A, MAHDIZADE M A, et al. Bacteriocins:Properties and potential use as antimicrobials[J]. Journal of Clinical Laboratory Analysis,2022,36(1):e24093. doi: 10.1002/jcla.24093
[6] ZENDO T. Screening and characterization of novel bacteriocins from lactic acid bacteria[J]. Bioscience, Biotechnology, and Biochemistry,2013,77(5):893−899. doi: 10.1271/bbb.130014
[7] SANTOS J C P, SOURSA R C S, OTONI C G, et al. Nisin and other antimicrobial peptides:Production, mechanisms of action, and application in active food packaging[J]. Innovative Food Science & Emerging Technologies,2018,48:179−194.
[8] HAVARD J, PAMELA H, HANCOCK R E W. Peptide antimicrobial agents[J]. Clinical Microbiology Reviews,2006,19(3):491−511. doi: 10.1128/CMR.00056-05
[9] 吕欣然. 植物乳杆菌素 DLP1 对荧光假单胞菌的抑制作用及机制研究[D]. 北京:北京林业大学, 2019. [LÜ X R. Antibacterical effects and mechanism of plantaricin DLP1 on Pseudomonas fluorescens[D]. Beijing:Beijing Forestry University, 2019.] LÜ X R. Antibacterical effects and mechanism of plantaricin DLP1 on Pseudomonas fluorescens[D]. Beijing: Beijing Forestry University, 2019.
[10] 吴小艳, 刘文星, 刘忠义, 等. 芒果酸奶发酵及后熟过程中乳酸菌素的产生及其抑菌作用[J]. 食品与发酵工业,2021,47(7):183−188. [WU X Y, LIU W X, LIU Z Y, et al. Production and bacteriostasis of lactobacillin during the fermentation and ripening of mango yoghurt[J]. Food and Fermentation Industries,2021,47(7):183−188.] WU X Y, LIU W X, LIU Z Y, et al. Production and bacteriostasis of lactobacillin during the fermentation and ripening of mango yoghurt[J]. Food and Fermentation Industries, 2021, 47(7): 183−188.
[11] LÜ X R, MIAN L H, MA H H, et al. Purification, characterization and action mechanism of plantaricin JY22, a novel bacteriocin against Bacillus cereus produced by Lactobacillus plantarum JY22 from golden carp intestine[J]. Food Science and Biotechnology,2017,27:695−703.
[12] GAO Y R, LI B L, LI D P, et al. Purification and characteristics of a novel bacteriocin produced by Enterococcus faecalis L11 isolated from Chinese traditional fermented cucumber[J]. Biotechnol Letters,2016,38(5):871−877. doi: 10.1007/s10529-016-2055-x
[13] PENG S D, SONG J J, ZENG W Y, et al. A broad-spectrum novel bacteriocin produced by Lactobacillus Plantarum SHY 21–2 from yak yogurt:Purification, antimicrobial characteristics and antibacterial mechanism[J]. LWT-Food Science and Technology,2021,142:110955. doi: 10.1016/j.lwt.2021.110955
[14] 韩金志, 姚思羽, 沈昊, 等. 植物乳杆菌FZU122产细菌素的分离鉴定及其抑菌活性[J]. 福州大学学报(自然科学版),2021,49(1):129−134. [HAN J Z, YAO S Y, SHEN H, et al. Purification, molecular characterization of a novel bacteriocin produced by Lactobacillus plantarum FZU122 and its antibacterial activity[J]. Journal of Fuzhou University (Natural Science Edition),2021,49(1):129−134.] doi: 10.7631/issn.1000-2243.20224 HAN J Z, YAO S Y, SHEN H, et al. Purification, molecular characterization of a novel bacteriocin produced by Lactobacillus plantarum FZU122 and its antibacterial activity[J]. Journal of Fuzhou University (Natural Science Edition), 2021, 49(1): 129−134. doi: 10.7631/issn.1000-2243.20224
[15] XIANG Y Z, LI X Y, ZHENG H L, et al. Purification and antibacterial properties of a novel bacteriocin against Escherichia coli from Bacillus subtilis isolated from blueberry ferments[J]. LWT-Food Science and Technology,2021,146:111456. doi: 10.1016/j.lwt.2021.111456
[16] QU L, SHE P F, WANG Y X, et al. Effects of norspermidine on Pseudomonas aeruginosa biofilm formation and eradication[J]. Microbiologyopen,2016,5(3):402−412. doi: 10.1002/mbo3.338
[17] NIAZ T, SHABBIR S, NOOR T, et al. Antimicrobial and antibiofilm potential of bacteriocin loaded nano-vesicles functionalized with rhamnolipids against foodborne pathogens[J]. LWT-Food Science and Technology,2019,116:108583. doi: 10.1016/j.lwt.2019.108583
[18] YI L H, LUO L L, LÜ X. Efficient exploitation of multiple novel bacteriocins by combination of complete genome and peptidome[J]. Frontiers in Microbiology,2018,9:370304.
[19] 刘昊, 赵自冰, 吴丹丹, 等. 黄芩苷对大肠杆菌细胞通透性的影响[J]. 中国畜牧兽医,2017,44(6):1890−1894. [LIU H, ZHAO Z B, WU D D, et al. Effect of baicalin on cell permeability of Escherichia coli[J]. China Animal Husbandry & Veterinary Medicine,2017,44(6):1890−1894.] LIU H, ZHAO Z B, WU D D, et al. Effect of baicalin on cell permeability of Escherichia coli[J]. China Animal Husbandry & Veterinary Medicine, 2017, 44(6): 1890−1894.
[20] 朱雨龙, 邵佳雯, 焦子颖, 等. 硝酸镧影响枯草芽孢杆菌生长及细胞壁结构的机理[J]. 稀土,2021,42(4):84−90. [ZHU Y L, SHAO J W, JIAO Z Y, et al. Effects mechanism of lanthanum nitrate on the growth ang cell wall structure of Bacillus stubtilis[J]. Chinese Rare Earths,2021,42(4):84−90.] ZHU Y L, SHAO J W, JIAO Z Y, et al. Effects mechanism of lanthanum nitrate on the growth ang cell wall structure of Bacillus stubtilis[J]. Chinese Rare Earths, 2021, 42(4): 84−90.
[21] BENDALI F, GAILLARD-MARTINIE B, HEBRAUD M, et al. Kinetic of production and mode of action of the Lactobacillus paracasei subsp. paracasei anti-listerial bacteriocin, an Algerian isolate[J]. LWT-Food Science and Technology,2008,41(10):1784−1792. doi: 10.1016/j.lwt.2008.02.010
[22] 周伟, 刘国荣, 李平兰, 等. 植物乳杆菌素L-1对单核细胞增生李斯特氏菌作用机理研究[J]. 微生物学报,2007,47(2):260−264. [ZHOU W, LIU G R, LI P L, et al. Mode of action of plantaricin L-1, an antilisteria bacteriocin produced by Lactobacillus plantarum[J]. Acta Microbiologica Sinica,2007,47(2):260−264.] doi: 10.3321/j.issn:0001-6209.2007.02.015 ZHOU W, LIU G R, LI P L, et al. Mode of action of plantaricin L-1, an antilisteria bacteriocin produced by Lactobacillus plantarum[J]. Acta Microbiologica Sinica, 2007, 47(2): 260−264. doi: 10.3321/j.issn:0001-6209.2007.02.015
[23] 唐亚丽. 家蝇抗菌肽的分离及其对细胞壁膜和DNA的作用[D]. 无锡:江南大学, 2009. [TANG Y L. Purification of antimicrobial peptides from housefly larvae and their effects onbacterial membranes and DNA[D]. Wuxin:Jiangnan University. 2009.] TANG Y L. Purification of antimicrobial peptides from housefly larvae and their effects onbacterial membranes and DNA[D]. Wuxin: Jiangnan University. 2009.
[24] 王鸿博. 屎肠球菌BZ2产细菌素对单增李斯特氏菌的作用机制及应用研究[D]. 呼和浩特:内蒙古农业大学, 2022. [WANG H B. Mechanism and application of bacteriocin producing by Enterocccus faecium BZ2 against Listeria monocytogenes[D]. Hohhot:Inner Mongolia Agricultural University, 2022.] WANG H B. Mechanism and application of bacteriocin producing by Enterocccus faecium BZ2 against Listeria monocytogenes[D]. Hohhot: Inner Mongolia Agricultural University, 2022.
[25] TANG Y L, SHI Y H, ZHAO W, et al. Interaction of MDpep9, a novel antimicrobial peptide from Chinese traditional edible larvae of housefly, with Escherichia coli genomic DNA[J]. Food Chemistry,2009,115(3):867−872. doi: 10.1016/j.foodchem.2008.12.102
[26] 翟佳琳, 赵景娜, 王丹丹, 等. 具有抑菌活性植物乳杆菌的筛选及抑菌物质特性的研究[J]. 食品与发酵工业,2022,48(13):84−90. [ZHAI J L, ZHAO J N, WANG D D, et. al. Screening of Lactiplantibacillus plantarum with bacteriostatic activity and study on properties of bacteriostatic substances[J]. Food and Fermentation Industries,2022,48(13):84−90.] ZHAI J L, ZHAO J N, WANG D D, et. al. Screening of Lactiplantibacillus plantarum with bacteriostatic activity and study on properties of bacteriostatic substances[J]. Food and Fermentation Industries, 2022, 48(13): 84−90.
[27] SHAHIN K, BOUZARI M, WANG R. Complete genome sequence analysis of a lytic Shigella flexneri vB_SflS-ISF001 bacteriophage[J]. Turkish Journal of Biology, 2019:99−112.
[28] 范为群. 分析化学[M]宁夏:宁夏人民教育出版社, 2018:157. [FAN W Q. Analytical chemistry[M] Ningxia:Ningxia people's Publishing House, 2018:157.] FAN W Q. Analytical chemistry[M] Ningxia: Ningxia people's Publishing House, 2018: 157.
[29] HAO X P, CHEN S G, ZHU H Z, et al. The synergy of graphene oxide and polydopamine assisted immobilization of lysozyme to improve antibacterial properties[J]. Chemistry Select,2017,2(6):2174−2182.
[30] LEŚNIEROWSKI G, YANG T. Lysozyme and its modified forms:A critical appraisal of selected properties and potential[J]. Trends in Food Science & Technology,2021,107:333−342.
[31] LOVERING A L, DE CASTRO L, LIM D, et al. Structural analysis of an “open” form of PBP1B from Streptococcus pneumoniae[J]. Protein Science,2006,15(7):1701−1709. doi: 10.1110/ps.062112106
[32] STRAUME D, PIECHOWIAK K W, KJOS M, et al. Class a PBPs:It is time to rethink traditional paradigms[J]. Molecular Microbiology,2021,116(1):41−52. doi: 10.1111/mmi.14714
[33] YI L H, LUO L L, CHEN J X, et al. Cell wall and DNA damage of Staphylococcus aureus by bacteriocin BM1157[J]. LWT-Food Science and Technology,2020,134:109842. doi: 10.1016/j.lwt.2020.109842
[34] 刘雪平, 冯素玲, 潘自红, 等. 溴化乙锭探针研究劳氏青莲与脱氧核糖核酸的相互作用[J]. 光谱学与光谱分析,2006(10):1895−1898. [LIU X P, FENG S L, PAN Z H, et, al. Sturies on the interaction between thionien and deoxyribo-nuclei acid by ethidium bromide probe[J]. Spectroscopy and Spectral Analysis,2006(10):1895−1898.] doi: 10.3321/j.issn:1000-0593.2006.10.030 LIU X P, FENG S L, PAN Z H, et, al. Sturies on the interaction between thionien and deoxyribo-nuclei acid by ethidium bromide probe[J]. Spectroscopy and Spectral Analysis, 2006(10): 1895−1898. doi: 10.3321/j.issn:1000-0593.2006.10.030
[35] 刘忠渊, 徐涛, 郑树涛, 等. 新疆家蚕抗菌肽Cecropin-XJ与细菌DNA相互作用的光谱研究[J]. 光谱学与光谱分析,2008(3):612−616. [LIU Z Y, XU T, ZHENG S T, et, al. Study on the interaction mechanism of antimicrobial petide Cecropin-XJ in Xinjiang silkworm and Staphylococcus aureus DNA by spectra[J]. Spectroscopy and Spectral Analysis,2008(3):612−616.] doi: 10.3964/j.issn.1000-0593.2008.03.032 LIU Z Y, XU T, ZHENG S T, et, al. Study on the interaction mechanism of antimicrobial petide Cecropin-XJ in Xinjiang silkworm and Staphylococcus aureus DNA by spectra[J]. Spectroscopy and Spectral Analysis, 2008(3): 612−616. doi: 10.3964/j.issn.1000-0593.2008.03.032
[36] HAN X P, ZHANG M Y, PENG J Y, et al. Purification and characterization of a novel bacteriocin from Lactiplantibacillus plantarum Z057, and its antibacterial and antibiofilm activities against Vibrio parahaemolyticus[J]. LWT-Food Science and Technology,2023,173:114358. doi: 10.1016/j.lwt.2022.114358
[37] LI H W, XIANG Y Z, ZHANG M, el al. A novel bacteriocin from Lactobacillus salivarius against Staphylococcus aureus:Isolation, purification, identification, antibacterial and antibiofilm activity[J]. LWT-Food Science and Technology,2021,140:110821.
[38] YI L H, LI X, LUO L L, et al. A novel bacteriocin BMP11 and its antibacterial mechanism on cell envelope of Listeria monocytogenes and Cronobacter sakazakii[J]. Food Control,2018,91:160−169. doi: 10.1016/j.foodcont.2018.03.038
[39] 高娟娟, 贾丽艳, 畅盼盼, 等. 枯草芽孢杆菌细菌素A32的抑菌机理研究[J]. 中国食品学报,2021,21(19):56−64. [GAO J J, JIA L Y, CHANG P P, et al. Study on the inhibition mechanism of bacteriocin A32 producing by Bacillus subtilis[J]. Journal of Chinese Institute of Food Science and Technology,2021,21(19):56−64.] GAO J J, JIA L Y, CHANG P P, et al. Study on the inhibition mechanism of bacteriocin A32 producing by Bacillus subtilis[J]. Journal of Chinese Institute of Food Science and Technology, 2021, 21(19): 56−64.
[40] SHENTU H F, YE P X, ZHOU Q Q, et al. Purification, characterization, and mode of action of Sakacin ZFM225, a novel bacteriocin from Lactobacillus sakei ZFM225[J]. Biochemistry and Biophysics Reports,2023,35:101494. doi: 10.1016/j.bbrep.2023.101494
[41] KIM N N, KIM W J, KANG S S. Anti-biofilm effect of crude bacteriocin derived from Lactobacillus brevis DF01 on Escherichia coli and Salmonella Typhimurium[J]. Food Control,2019,98:274−280. doi: 10.1016/j.foodcont.2018.11.004
[42] 李玉珍, 肖怀秋, 刘淼, 等. 金抗肽SIF4对大肠杆菌基于细胞壁靶点的非细胞质膜损伤抑菌机理[J]. 食品与生物技术学报,2023,42(6):78−83. [LI Y Z, XIAO H Q, LIU M, et al. Non-cytoplasmic membrane damage antimicrobial mechanism of metai antimicrobial peptide SIF4 against Escherichia coil based on cell wall target[J]. Journal of Food Science and Biotechnology,2023,42(6):78−83.] doi: 10.3969/j.issn.1673-1689.2023.06.010 LI Y Z, XIAO H Q, LIU M, et al. Non-cytoplasmic membrane damage antimicrobial mechanism of metai antimicrobial peptide SIF4 against Escherichia coil based on cell wall target[J]. Journal of Food Science and Biotechnology, 2023, 42(6): 78−83. doi: 10.3969/j.issn.1673-1689.2023.06.010
[43] XIN W G, WU G, YING J P, el al. Antibacterial activity and mechanism of action of bacteriocin LFX01 against Staphylococcus aureus and Escherichia coli and its application on pork model[J]. Meat Science,2023,196:109045. doi: 10.1016/j.meatsci.2022.109045
[44] CAO S, DU R P, ZHAO F K, et al. The mode of action of bacteriocin CHQS, a high antibacterial activity bacteriocin produced by Enterococcus faecalis TG2[J]. Food Control,2019,96(2019):470−478.
[45] MIAO J Y, ZHOU J L, LIU G, et al. Membrane disruption and DNA binding of Staphylococcus aureus cell induced by a novel antimicrobial peptide produced by Lactobacillus paracasei subsp. tolerans FX-6[J]. Food Control,2016,59:609−613. doi: 10.1016/j.foodcont.2015.06.044
[46] YI L H, DANG J, ZHANG L H, et al. Purification, characterization and bactericidal mechanism of a broad spectrum bacteriocin with antimicrobial activity against multidrug-resistant strains produced by Lactobacillus coryniformis XN8[J]. Food Control,2016,67:53−62. doi: 10.1016/j.foodcont.2016.02.008





 下载:
下载:
 下载:
下载:



