Research Progress of Bacteriocin Combined with Physical Technology in the Prevention and Control of Foodborne Pathogens and Spoilage Microorganisms
-
摘要: 由食源性致病菌和腐败微生物引发的食品安全问题已成为全球公共卫生的挑战之一。细菌素是一类抗菌肽,通常在某些细菌的核糖体内合成,被广泛用作生物防腐剂和食品添加剂。然而,单独使用细菌素存在一些局限性,比如抑菌谱窄、易被酶降解等。研究表明,将细菌素与物理技术结合可能会产生协同效应,可以扩大抑菌范围、增强抑菌效果并延长食品货架期,从而保障食品安全和人类健康。本文综述了细菌素与超声波处理、高压处理、热处理、脉冲电场以及纳米技术等物理技术协同的抑菌策略和机制,阐述了该策略对食品风味和品质的影响,并展望了其未来发展趋势和应用前景。旨在为细菌素与物理技术协同抑制食源性致病菌和腐败微生物的工业应用提供理论支持和参考依据。Abstract: Food safety issues caused by foodborne pathogens and spoilage microorganisms have become one of the public health challenges worldwide. Bacteriocins are antimicrobial peptides synthesized by some bacterial ribosomes and are widely used as bio-preservatives and food additives. However, bacteriocins may have some limitations when used individually, such as the narrow inhibitory spectrum and easy degradation by enzymes. Previous studies found that the combined use of bacteriocins and physical technologies may exhibit synergistic antimicrobial effects. This strategy could effectively expand the range of antimicrobial activity, enhance the antimicrobial effect, and prolong the shelf life of food, thereby ensuring food safety and human health. This paper reviews the synergistic antimicrobial strategies and mechanisms of bacteriocins in combination with physical technologies, including ultrasonic treatment, high-pressure treatment, heat treatment, pulsed electric field, and nanotechnology. Meanwhile, the influence of the combination of bacteriocins and physical technologies on food quality and safety is elaborated. Moreover, the development trend and application prospect of the antimicrobial strategy are discussed. The aim of this review is to provide the theoretical support and reference basis for the industrial application of bacteriocins combined with physical technologies to inhibit foodborne pathogens and spoilage microorganisms.
-
Keywords:
- bacteriocin /
- physical technology /
- synergistic effect /
- antimicrobial mechanism /
- food matrix
-
近年来,由食源性致病菌和腐败微生物所引起的食品安全事件频繁发生,严重危害了食品生产、公共健康和全球经济[1]。随着食源性致病菌和腐败微生物污染风险的提高和人们对健康食品需求的增加,寻找安全高效的新型抑菌技术和天然无毒的新型抑菌物质成为研究的热点[2]。传统抑菌方法虽可有效延长食品货架期和降低食品被致病菌污染的风险,但在实际应用中可能存在影响食品品质和降低营养价值等缺点[3]。市面上常用的化学防腐剂如山梨酸盐和苯甲酸盐等可有效抑制食源性致病菌和腐败微生物的生长繁殖并延长食品货架期[4]。然而,近年来一些研究表明,化学防腐剂可能对人类健康产生不利影响,如引起胃肠道疾病、过敏反应甚至存在致癌风险[5]。除化学防腐剂外,抗生素也具有良好抑菌能力,但抗生素的耐药性已成为威胁公共卫生和现代医学的全球性危机[6]。因此,找寻绿色、安全和高效的天然物质作为化学防腐剂和抗生素的替代品至关重要。
细菌素是一类由部分细菌的核糖体产生具有抑菌活性的蛋白类或多肽类物质,对革兰氏阳性菌和革兰氏阴性菌均具有良好抑制作用[7−8]。研究显示,目前已有多种细菌素被用作食品防腐剂,如乳酸链球菌素(Nisin)、片球菌素(Pediocin)和肠球菌素(Enterocin)等。其中,Nisin已获得美国食品药物管理局的批准,可作为食品添加剂用于食品中[9]。但一些细菌素存在着抑菌谱相对狭窄的局限性和抑菌效果易受到食品成分影响的缺点[10]。以上问题表明,细菌素在食品产业中的应用仍然存在部分问题亟待解决。
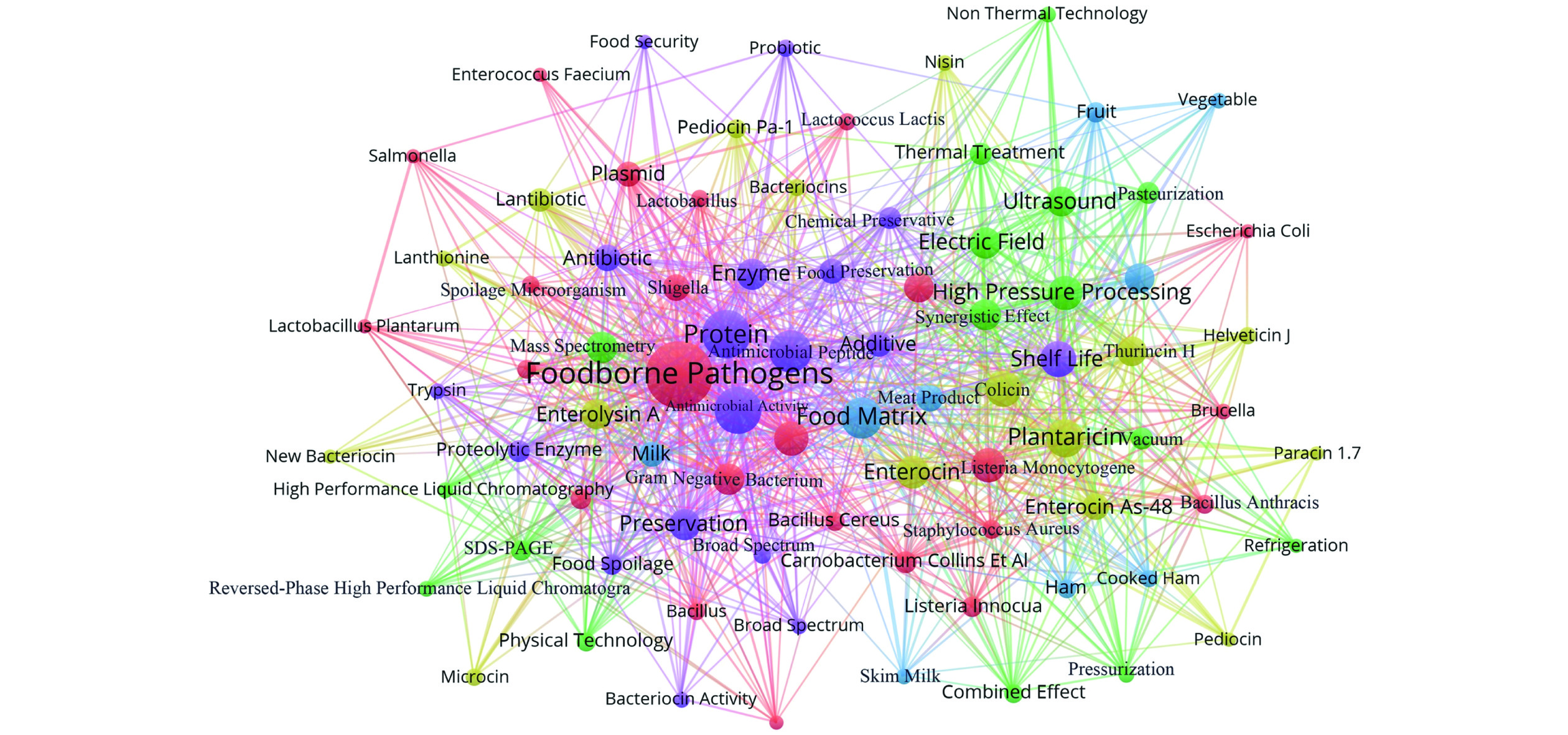
为了拓宽细菌素的抑菌谱、减少使用量并增强抑菌效果,将细菌素与超声波处理、高压处理、热处理、脉冲电场和纳米技术等物理技术协同使用引起了人们的广泛关注。为了从整体把握相关文献的数量和时序变化,以Web of Science为检索数据库,运用VOSviewer文献计量学软件对细菌素协同物理技术相关研究文献进行关键词提取并生成关键词共现图谱(图1)。总体来看,细菌素协同物理技术的研究热点主要涉及不同细菌素、物理技术、食源性致病菌和腐败微生物、食品基质、抑菌效果和抑菌机理等多个领域。此外,细菌素与多种物理技术结合使用也可能具有协同抑菌作用,如Mok等[11]研究了剪切应力、电场和Nisin三者结合使用对大肠杆菌K12和英诺克李斯特菌ATCC 33090的抑制效果,结果表明三者联合使用比单独使用的抑菌效果好且避免热处理对质量的负面影响。Çelen等[12]研究发现使用羧甲基纤维素封装Nisin形成纳米颗粒可以通过延长Nisin的释放时间和保持Nisin的抑菌活性,从而增强对牛奶中金黄色葡萄球菌的抑制作用。上述研究表明,细菌素与物理技术协同使用是抑制复杂食物环境中食源性致病菌和腐败微生物生长、拓宽抑菌谱和提高抑菌效果的有效策略[13]。本综述系统地阐述了细菌素协同超声波处理、高压处理、热处理、脉冲电场和纳米技术等物理技术的抑菌效果和抑菌机制,希望为细菌素现存问题提供解决方案和理论依据,同时为细菌素协同物理技术在食品领域中的产业化应用提供科学指导和参考。
1. 细菌素的概念、分类及抑菌作用机制
1.1 细菌素的概念
细菌素是一类由部分细菌的核糖体合成的、在细胞外释放的抗菌肽,对食源性致病菌和腐败微生物有良好的防控作用,且对食品品质影响较小[14−15]。研究表明,Nisin可以有效抑制鲈鱼片中产生生物胺的金黄色葡萄球菌ATCC 29213、粪肠球菌ATCC 29212和单核细胞增生李斯特菌ATCC 7677[16]。Zhang等[17]研究发现,凝结芽孢杆菌CGMCC 9951产生的粗细菌素可通过破坏细胞膜的完整性和产生孔隙使细胞内乳酸脱氢酶、核酸和蛋白质等可溶性物质泄漏和致病菌基因组DNA失活,从而影响单核细胞增生李斯特菌的正常生长和繁殖。因此,细菌素可以被认为是一种有效的生物抑菌剂。除上述抑菌作用外,某些细菌素还具有缓解炎症、预防肠道菌群失调[18]、抗肥胖和病毒[19−20]等生理活性和功能。
1.2 细菌素的分类
根据遗传特征、分子量和氨基酸序列,细菌素可被分为四类[21]。表1详细概括了四类细菌素的具体分类情况,并介绍了每类细菌素的主要特征及具有代表性的细菌素。其中,I类细菌素是指翻译后修饰的肽,包含19~50个氨基酸,其分子量通常<5 kDa,该类中最典型的例子是Nisin[22−23]。II类细菌素一般指热稳定、不含羊毛硫氨酸的肽,包含36~57个氨基酸,其分子量通常<10 kDa,典型代表有片球菌素PA-1(Pediocin PA-1)和肠球菌素AS-48(Enterocin AS-48)等。II类细菌素根据其特征可进一步分为四个亚类:IIa类(pediocin-like细菌素)、IIb类(双肽II类细菌素)、IIc类(环状细菌素)和IId类(未分类细菌素)[24]。III类细菌素由热不稳定的蛋白质组成,其分子量通常>30 kDa,如瑞士乳杆菌素J(Helveticin J)和肠溶素A(Enterolysin A)等[25]。IV类细菌素由携带脂质或碳水化合物的复合肽构成,具有较为复杂的结构。现有提纯手段未能将细菌素与复合大分子完全分离,所以把这类细菌素暂归为IV类细菌素[26]。除表1根据细菌素特征分类方法外,还可根据产生细菌素的细菌不同,把细菌素分为革兰氏阳性菌细菌素、革兰氏阴性菌细菌素和古细菌细菌素。
表 1 细菌素的分类Table 1. Classification of bacteriocins分类 特征 代表性细菌素 I类 翻译后修饰的肽,包含19~50多个氨基酸,分子量<5 kDa 乳酸链球菌素,美杀菌素,枯草菌素 II类 热稳定、不含羊毛硫氨酸的肽,包含36~57个氨基酸,分子量<10 kDa 片球菌素PA-1,肠球菌素AS-48 III类 热不稳定的蛋白质,分子量>30 kDa 瑞士乳杆菌素J,肠溶素A IV类 携带脂质或碳水化合物的复合肽 片球菌素SJ-1,格氏乳杆菌素A 1.3 细菌素的抑菌作用机制
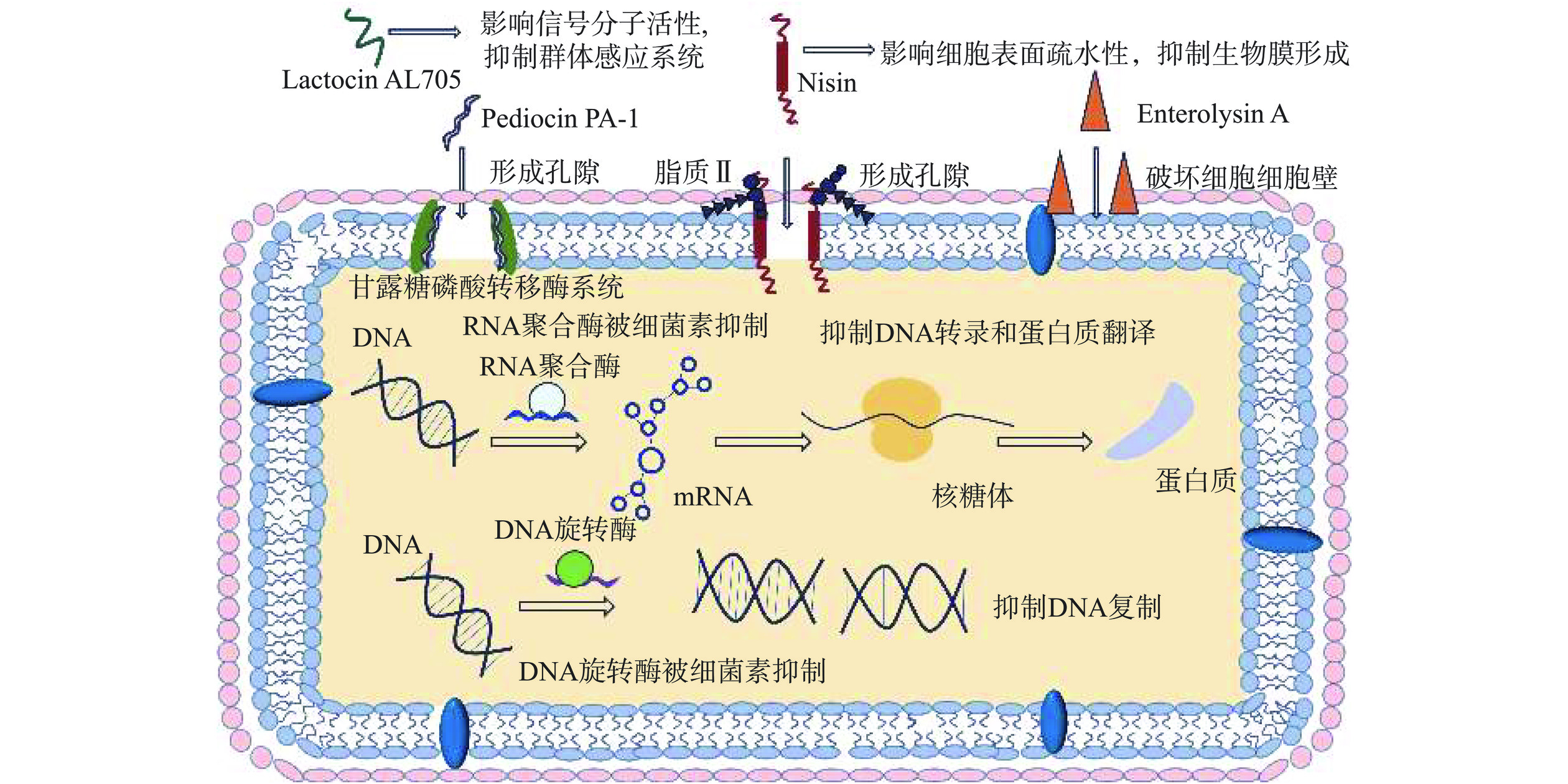
细菌素可通过破坏细胞膜和细胞壁完整性、抑制细菌基因表达、抑制生物膜形成和群体感应等方式抑制食源性致病菌和腐败微生物的生长繁殖[27],如图2所示。其具体抑菌机制主要分为三个方面:a.破坏细胞壁和细胞膜的完整性。细菌素可与脂质II或甘露糖磷酸转移酶系统结合使细胞膜形成孔隙,从而破坏细胞膜完整性和抑制致病菌生长[28−29]。此外,一些细菌素通过切割细胞壁中肽聚糖单元内的肽间键或使细胞壁中肽桥破裂,破坏细胞壁合成从而达到抑菌效果[30]。b.抑制细菌的基因表达。许多细菌素通过抑制致病菌DNA旋转酶和RNA聚合酶,从而干扰细胞DNA复制、转录和翻译过程,达到抑制致病菌的生长繁殖的作用[31]。c.其他抑菌机制。除上述抑菌机制外,细菌素也可通过影响细胞表面疏水性,调节生物膜和群体感应相关基因等方式,抑制食源性致病菌和腐败微生物的生长[32]。此外,细菌素还可通过影响群体感应信号分子的活性,抑制致病菌的群体感应系统,从而达到对食源性致病菌和腐败微生物的有效防控作用[33]。
2. 细菌素和物理技术对食品中食源性致病菌和腐败微生物的协同抑菌作用
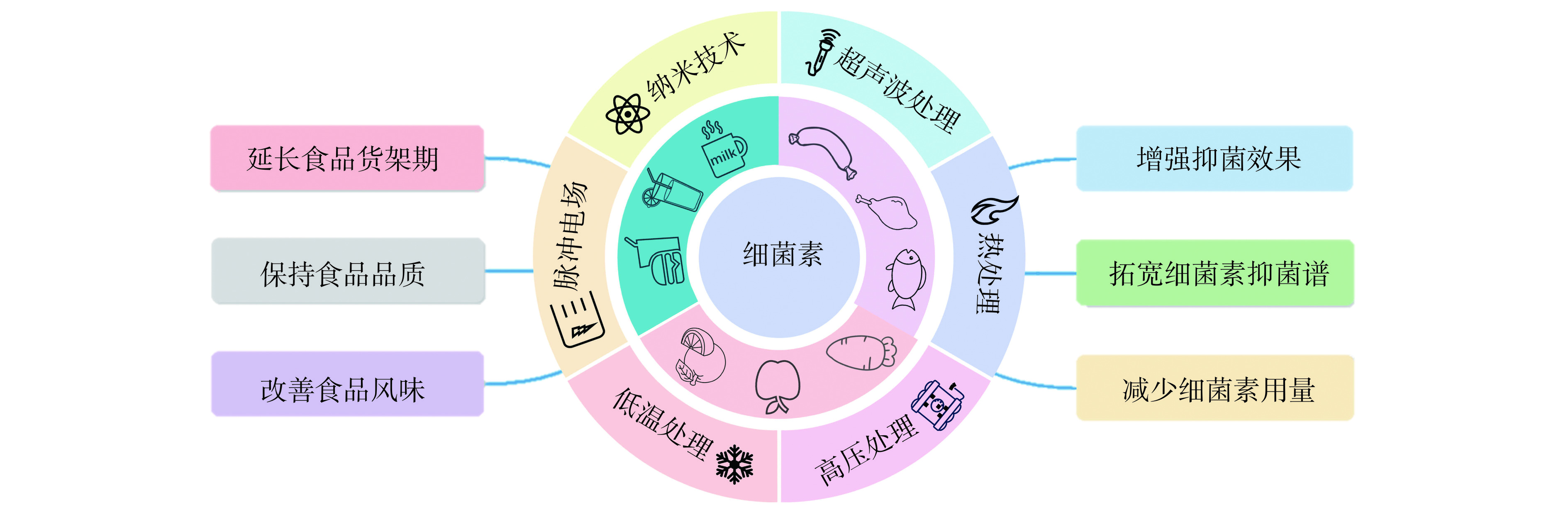
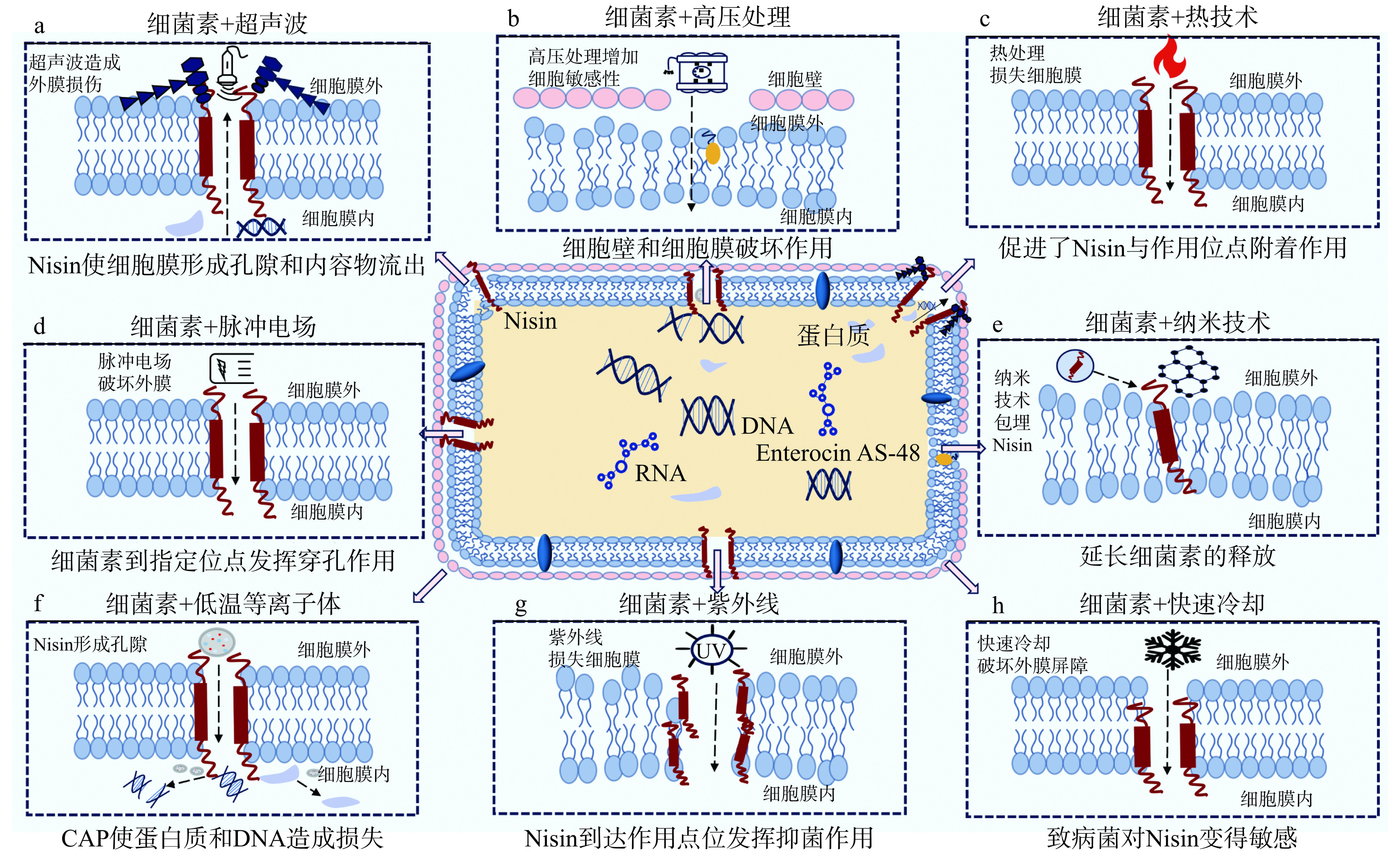
在细菌素的实际应用中,由于存在酶降解、pH的影响及细菌素与复杂食品成分的相互作用,可能导致细菌素的抑菌效果下降[34]。为了解决这些问题,细菌素与物理技术协同作用引起了广泛关注。如图3所示,不同的物理技术与细菌素协同使用具有增强对食品中食源性致病菌和腐败微生物的防控效果、延长食品货架期并保持食品品质等优点。研究发现,细菌素与各类物理技术可以通过增强孔隙形成、增强细胞敏感性、促进细菌素与作用位点附着作用和促进细菌素到达指定位点等方式增强抑菌效果,具体抑菌机制如图4所示。因此,下文对细菌素与超声波处理、高压处理、热处理、脉冲电场和纳米技术等物理技术协同作用的抑菌效果和抑菌机制进行了系统地总结,为细菌素与物理技术协同抑菌的工业应用提供参考。
2.1 细菌素和超声波处理的协同抑菌作用
超声波是指频率高于20 kHz的声波,该技术具有良好抑菌能力且具有处理时间短、处理温度低和营养价值提升等优点[35]。超声波抑菌原理主要为空化效应,即超声波在液体介质中形成了交替的压缩区和膨胀区并产生微小的空化泡。当空化泡迅速破裂时,产生了巨大的能量和压力,导致微生物死亡[36−37]。以上剧烈变化直接破坏食源性致病菌和腐败微生物的细胞壁并产生自由基反应,具有强氧化性的自由基破坏微生物细胞内的DNA并抑制酶活性[38]。Wang等[39]将绿芦笋在40 kHz超声波条件下处理10 min,然后在4 ℃条件下贮藏20 d,发现在第12 d和第16 d霉菌和酵母菌总数显著减少。
尽管超声波处理具有改善营养价值和加热温度低等优点,但单独超声波处理时抑菌效果可能有限[40−42]。研究发现,超声波和细菌素适当结合对食源性致病菌和腐败微生物具有有效防控作用,同时可能减少对食品品质和风味的破坏[43−44]。Liao等[45]将100 mg/L Nisin和20~25 kHz超声波结合处理苹果汁30 min,果汁中好氧菌、酵母菌和霉菌的活菌数显著减少,且褐变度、可滴定酸度和可溶性固形物含量在处理前后无显著变化。另外,有研究发现将20 kHz超声波和0.175 mol/L Nisin联合处理10 min,福氏志贺氏菌减少了1.48 lg CFU/mL,而单独使用超声波或Nisin对福氏志贺氏菌基本没有抑制作用[46]。由于细菌素可以通过在细胞膜上形成孔隙来防控食源性致病菌和腐败微生物,因此细菌素与超声波联合使用也可诱导孔隙的形成,从而增强其抗菌活性。Ruiz-De等[47]采用苏云金芽孢杆菌产生的细菌素苏云金菌素H(thurincin H)和20~25 kHz超声波联合处理,超声波先破坏英诺克李斯特菌ATCC 33090和大肠杆菌K12的外膜并促使thurincin H进一步渗透到细胞中,从而促进细菌素与英诺克李斯特菌ATCC 33090和大肠杆菌K12细胞膜的相互作用。此外,曾有研究发现,由于大肠杆菌具有细胞外膜,使Nisin单独到达脂质II困难。然而,当Nisin与超声波协同使用时,超声波可使大肠杆菌外膜短暂损伤,允许Nisin进入细胞膜并与脂质II结合,从而使致病菌形成孔隙和内容物流出[48−49]。以上研究表明,细菌素与超声波处理联合使用可能具有协同抑菌作用,并可能通过超声波技术先导致细胞外膜损伤,然后使细菌素进入指定位置形成孔隙从而达到抑菌作用(图4a)。另外,现有研究虽暂未发现细菌素协同超声波对食品的风味和品质不利影响的报道,但仍需进一步研究和试验。综上所述,细菌素协同超声波处理对拓宽抑菌谱、改善食品品质和延长食品货架期有良好的研究价值和应用前景。
2.2 细菌素和高压处理的协同抑菌作用
高压处理(High pressure processing,HPP),指利用液体为压力传输介质,在100~1000 MPa的压力下对食品进行物理处理[50−51]。HPP技术抑菌机制为HPP使微生物内蛋白质变性,导致酶失活、生化功能和遗传机制受到影响,从而破坏细胞稳定性[52−53]。研究发现,在250 MPa高压处理3 min可以有效抑制冷冻鲑鱼片中的沙门氏菌和单核细胞增生李斯特菌[54]。Monteiro等[55]报道了在300 MPa条件下处理10 min可使空气中和真空中冷藏鱼片的货架期延长7 d,且减少脂质氧化和颜色变化。因此,高压处理可以被认为是一种更有效防控食源性致病菌和腐败微生物的技术。然而,HPP技术虽具有延长货架期、保留食物原始颜色和风味等优点,但对设备要求较高且价格昂贵[56−58]。
近年来,细菌素协同高压处理在防控食源性致病菌和腐败微生物方面表现出巨大潜力,许多研究探讨了其在食品保鲜中的发展趋势和应用[59−60]。一方面,细菌素协同高压处理对致病菌具有良好的防控能力,如细菌素 Pediocin PA-1 和 HPP 以及噬菌体 P100 联合使用对牛奶中单核细胞增生李斯特菌具有协同增效抑制作用[61]。另一方面,细菌素协同高压处理对食品品质可能存在有利作用。在500 IU/mL Nisin和450 MPa HPP条件下,液态胶束酪蛋白浓缩物中微生物显著减少,同时酸度和颜色变化减小[62]。此外,细菌素与HPP技术协同抑菌的作用机制也在不断被探索[63−64]。Pokhrel等[65]证实了HPP技术与Nisin联合使用通过HPP技术先破坏了细胞外膜,然后Nisin破坏细胞质膜的电势差,抑制胡萝卜汁中的英诺克李斯特菌ATCC 51742和大肠杆菌ATCC 11755。把HPP和Enterocin AS-48联合使用可通过增加细胞对细菌素的敏感性,加强细菌素对敏感细菌的细胞壁和细胞膜破坏作用,从而抑制细菌生长繁殖[66]。另外,细菌素与HPP技术的协同增效作用可能减少耐高压食源性致病菌和腐败微生物的数量。单独使用HPP对单核细胞增生李斯特菌的抑菌效果不显著,联合使用时,单核细胞增生李斯特菌的数量显著减少[67]。综上所述,一方面,HHP处理与细菌素联合作用通过HPP技术增强了致病菌细胞对细菌素的敏感性或破坏细胞外膜,从而加速了细菌素对致病菌细胞膜的破坏(图4b)。另一方面,细菌素与HPP技术组合处理后使耐压致病菌数量减少。
2.3 细菌素和热处理的协同抑菌作用
为了延长食品货架期,通常使用热处理的方法保存食品以抑制食品中存在的食源性致病菌和腐败微生物[68]。然而,传统热处理由于存在更高的传热阻力导致处理时间延长、营养损失和效率降低,新型热处理如射频加热虽可抑制大部分致病菌,但芽孢杆菌芽孢数量无法完全被抑制[69−70]。曾有研究发现,将加热温度从90.5 ℃升至99.0 ℃,蜡样芽孢杆菌芽孢数量仅降低2.53 lg CFU/mL[71]。
一些研究证实了细菌素与热处理具有协同作用,可有效防控食源性致病菌和腐败微生物的生长繁殖、延长食品的货架期和改变致病菌芽孢的耐热性[72−73]。如将60 ℃热处理联合25 μg/mL Enterocin AS-48,可延缓金黄色葡萄球菌在酱汁中的生长,而仅使用细菌素仍可检测到2 lg CFU/mL的活细胞[74]。另外,用0.40 g/kg的Nisin和射频加热联合处理预制胡萝卜,不仅使其中金黄色葡萄球菌、枯草芽孢杆菌和大肠杆菌被有效抑制,而且使其货架期从6 d延长至42 d[75]。在抑制食源性致病菌和腐败微生物的过程中,热处理导致细胞膜损伤,促进了Nisin与作用位点附着作用并增强了穿孔作用,从而抑制了细胞的代谢和增殖[76]。Liu等[77]也证实了热处理导致细胞疏水性和渗透性增加以及脂多糖释放增多,对细胞外膜渗透屏障产生瞬时损伤,使Nisin可以暂时进入细胞质膜抑制细胞代谢增殖。除此之外,致病菌芽孢生长也会在此过程中受到影响。将80 ℃射频加热和0.312 mg/g Nisin协同使用有效抑制了豆腐中芽孢杆菌芽孢的生长[78]。以上研究表明,细菌素与热处理的结合通过增加细菌素与作用位点的附着作用和穿孔作用,抑制致病菌的生长繁殖,还可以通过损伤细胞的渗透屏障来抑制细胞的代谢增殖(图4c)。
2.4 细菌素和脉冲电场的协同抑菌作用
脉冲电场(Pulsed Electric Field,PEF)是一种新型非热技术,由于其能够减少微生物负荷,同时保持食品感官和营养品质,已被广泛应用于食品中[79]。脉冲电场的抑菌机理主要是通过使微生物细胞的细胞质膜形成新孔隙或破坏现有孔隙使细胞膜破裂,从而抑制细胞生长[80]。此外,脉冲电场对食品品质可能存在有利影响。如用脉冲电场处理母乳不仅可使细菌总数减少4 lg CFU/mL,且很好保留了母乳中生物活性成分[81]。近年来,越来越多研究发现细菌素与脉冲电场具有协同抑菌作用,将30 kV/cm脉冲电场和Nisin协同使用可以使苹果汁中的大肠杆菌K12减少4 lg CFU/mL[82]。Novickij等[83]发现负载Nisin的纳米颗粒和30 kV/cm脉冲电场联合使用增强了对鼠伤寒沙门氏菌和英诺克李斯特菌的防控作用。该实验进一步探究发现PEF和负载Nisin的纳米颗粒协同使用通过提高鼠伤寒沙门氏菌和英诺克李斯特菌对Nisin的敏感性,从而达到抑制食源性致病菌和腐败微生物生长的作用。另外,研究发现,在苹果汁中将35 kV/cm PEF与2.0 μg/mL Enterocin AS-48联合使用可抑制4.87 lg CFU/mL食二酸乳杆菌的生长,且其抑菌机制与细胞膜中孔隙的形成密切相关[84]。综上所述,细菌素与脉冲电场协同使用可通过增强致病菌对细菌素的敏感性或促进细胞膜孔隙的形成(图4d),从而达到防控食源性致病菌和腐败微生物细胞的生长增殖的作用。
2.5 细菌素和纳米技术的协同抑菌作用
纳米技术是在纳米尺度(直径范围为1~100 nm)上研究物质的特性和相互作用,在食品加工、食品保鲜、智能包装、质量控制和延长食品货架期等领域具有很好的应用[85−86]。大量研究表明,Nisin与纳米技术协同使用具有缓释和提高食品储存稳定性等优点,且在食品保鲜和抑菌方面有巨大的发展潜力和应用前景[87]。此外,纳米技术和细菌素协同使用可通过增强细菌素抗菌效果以延长货架期。将Nisin包装在可溶性大豆多糖纳米颗粒中可以抑制番茄汁中单核细胞增生李斯特菌、枯草芽孢杆菌和金黄色葡萄球菌的生长,从而延长番茄汁货架期[88]。Radaic等[89]的研究证实了纳米技术协同细菌素不仅通过延长细菌素的释放抑制致病菌,还可以改善细菌素的溶解性和稳定性,从而有效地将细菌素递送至特定的作用部位来增强细菌素的抑菌效果。以上研究表明,细菌素与纳米技术联合使用可能具有协同抑菌作用,能通过延长细菌素释放和改善细菌素稳定性和溶解度防控食源性致病菌和腐败微生物生长繁殖(图4e)。
2.6 细菌素和其他物理技术的协同抑菌作用
随着研究的不断深入,其他类型的物理技术如低温等离子体、紫外线、冷却处理和脉冲光等也表现出与细菌素的协同抑菌作用。表2对近些年细菌素协同物理技术在防控食品中食源性致病菌和腐败微生物的部分研究成果进行了总结,由此可见,细菌素协同物理技术对抑制食源性致病菌和腐败微生物具有巨大贡献,对高品质食品的加工和贮藏也有着重要的指导意义和作用。越来越多研究发现,Nisin和低温等离子体(Cold atmospheric plasma,CAP)联合使用对食源性致病菌和腐败微生物具有抑制作用。将Nisin和CAP联合使用处理3 min,苹果上单核细胞增生李斯特菌减少4 lg CFU/mL[90]。此外,Costello等[91]发现CAP联合Nisin对基于含酵母提取物胰蛋白酶大豆肉汤和黄原胶的食品模型中英诺克李斯特菌ATCC 33090的抑菌效果比单独使用Nisin效果好。同时,该研究也证实了其作用机理为Nisin首先在细胞膜上形成孔隙使CAP更容易进入细胞,CAP作用于活性氧等细胞成分,对细胞内蛋白质造成损伤并损失细胞内的DNA或抑制其复制,从而抑制细胞增殖(图4f)。如上所述,细菌素与低温等离子体技术联合使用具有良好的抑菌效果,有望成为食品行业新型抑菌方式之一。然而,现有对细菌素与CAP联合使用的研究较少且缺乏对其抑菌机理和量效关系的深层次探索,对其抑菌作用有待进一步研究突破。
表 2 细菌素协同物理技术在不同食品基质中的抑菌作用效果Table 2. Antimicrobial effect of bacteriocin synergistic physical technology in different food matrices物理技术 细菌素 目标菌 食品基质 作用效果 参考文献 技术名称 技术参数 超声波 20~25 kHz,950 W,
15 min,37~52 ℃Nisin 好氧菌、酵母菌、霉菌 苹果汁 活菌数减少,褐变度、可滴定酸度和可溶性固形物含量无明显变化 [45] 20~25 kHz,150 W,
16 min,30±5 ℃Thurincin H 英诺克李斯特菌ATCC 33090、大肠杆菌K12 牛奶和橙汁 细胞膜损伤,核酸、蛋白质渗漏,抑制致病菌生长繁殖 [47] 20 kHz,130 W,
20 min,7 ℃Nisin 福氏志贺氏菌 − 福氏志贺氏菌减少了1.48 lg CFU/mL [46] 44、500和1000 kHz,
30 W,15 min,37 ℃Nisin 大肠杆菌 ATCC 25922 − 大肠杆菌外膜造成短暂损伤,Nisin进入细胞膜并与脂质II结合,使致病菌形成孔隙 [48] 高压 450 MPa,5 min Nisin 单核细胞增生李斯特菌CTC 1034 液态胶束酪蛋
白浓缩物微生物显著减少,酸度和颜色变化较小 [62] 300 MPa,5 min Pediocin PA-1 单核细胞增生李斯特菌ATCC 49594 牛奶 致病菌的生长受到抑制 [61] 400 MPa,20 min Enterocin AS-48 细菌和真菌活菌数 番荔枝浆 增加受伤细胞对细菌素的敏感性,抑制致病菌 [66] 200 MPa,2 min Nisin 英诺克李斯特菌ATCC 51742、大肠杆菌ATCC 11755 胡萝卜汁 HPP先破坏细胞外膜,然后Nisin破坏细胞质膜的电势差抑制致病菌 [65] 热处理 60 °C,15 min Nisin 金黄色葡萄球菌ATCC 23587、枯草芽孢杆菌ATCC 23587和大肠杆菌ATCC 23716 预制胡萝卜 货架期从6 d延长至42 d [75] 80 ℃,10 min Nisin 枯草芽孢杆菌ATCC6633 豆腐 抑制了豆腐中芽孢杆菌芽孢的生长 [78] 60 ℃,5 min Enterocin AS-48 金黄色葡萄球菌
CECT 976酱汁 延缓了金黄色葡萄球菌在酱汁中的生长 [74] 脉冲电场 30 kV/cm Nisin 鼠伤寒沙门氏菌LT2、
英诺克李斯特菌CECT 910T− 致病菌减少2~2.5 lg CFU/mL [83] 30 kV/cm Nisin 大肠杆菌K12 苹果汁 大肠杆菌K12减少4 lg CFU/mL [82] 纳米技术 − Nisin 单核细胞增生李斯特菌CICC 21633、枯草芽孢杆菌CICC 10275和金黄色葡萄球菌CICC 10384 番茄汁 延长货架期 [88] 紫外线 254 nm Nisin 酸性脂环芽孢杆菌 橙汁 消除了酸曲霉孢子,未降解果汁中维生素 [92] 低温等离子体 3 min Nisin 单核细胞增生李斯特菌F8027 苹果 单核细胞增生李斯特菌减少了4 lg CFU/mL [90] 快速冷却 5 ℃ Nisin 大肠杆菌O157:H7 NCTC 12900 − 破坏细胞膜的外膜屏障,使大肠杆菌对Nisin敏感 [93] 脉冲光 10 s Nisin 大肠杆菌O157:H7 C9490、E02128和F00475 长叶莴苣 抑制了5 lg CFU/mL大肠杆菌 O157:H7 [95] 注:−文献未报道。 紫外线技术因其抑菌能力、去污效果和对食品质量影响较小而被广泛应用于食品工业。一些学者研究发现紫外线与细菌素联合使用也可能具有协同抑菌能力。将254 nm紫外线和15.62 μg/mL Nisin结合使用可通过改变橙汁中酸性脂环芽孢杆菌芽孢结构的完整性,抑制其生长繁殖且保留橙汁中维生素C含量[92](图4g)。此外,研究表明,冷却与Nisin可能对食源性致病菌和腐败微生物也存在防控作用,在5 ℃条件下快速冷却,通过破坏细胞膜的外膜屏障使大肠杆菌对Nisin变得敏感(图4h),从而抑制大肠杆菌O157:H7 NCTC 12900生长繁殖[93]。另一方面,细菌素协同冷却处理不仅能够抑制致病菌,对食品品质可能存在有利影响。Bermudez-Aguirre等[94]研究发现冷却和Nisin协同使用不仅能抑制鸡蛋液中沙门氏菌的生长,还能够使鸡蛋液pH和颜色变化微小。除上述物理技术以外,研究发现Nisin协同10 s脉冲光处理抑制了5 lg CFU/mL长叶莴苣中的大肠杆菌O157:H7[95]。由上述内容可知,细菌素与不同类型物理技术协同使用可以通过增强穿孔作用和致病菌对细菌素的敏感性等方式防控食源性致病菌和腐败微生物的生长。然而,细菌素联合物理技术在细胞层面和分子层面的抑菌机制缺乏更深层次研究。此外,细菌素和物理技术协同使用对食品理化、感官、营养和功能特性的影响仍需进一步试验和研究。随着科技创新的不断进步和研究的持续深入,相信细菌素与物理技术精准协同抑菌策略将有望成为食品工业防控食源性致病菌和腐败微生物的重要技术手段,并更好地为食品安全与人类健康保驾护航。
3. 结论与展望
细菌素作为一种天然防腐剂具有良好的抑菌效果,然而其单独使用时可能存在抑菌谱较窄、易受食品基质影响等问题。目前越来越多研究表明,细菌素与超声波处理、高压处理、热处理、脉冲电场和纳米技术等物理技术协同使用在微生物防控方面有巨大的应用前景和研究潜力。然而,目前大多数研究多聚焦细菌素与物理技术协同使用在乳、肉和蔬果等制品中的抑菌效果,对于其对食品理化、感官和营养特性的影响以及在分子层面的抑菌机理仍缺乏系统研究,未来可结合基因组学、转录组学、蛋白组学等多组学对细菌素协同物理技术的分子机制进行整合分析。其次,对细菌素构效关系、物理技术参数优化以及细菌素与物理技术组合机制的深入研究有助于实现抑菌效果的精确调控。除此之外,一些新型细菌素的抑菌能力和安全性问题还需进一步探索,今后可利用生物传感技术和人工智能等现代技术高速有效地检测经过物理技术和细菌素联合处理后的食品样品中的危害因子。另一方面,细菌素协同物理技术抑制致病菌的实验大多仅限于实验室研究,有必要开展在实际食品生产中的应用试验,明确食品基质对其协同抑菌效果的影响,为实现食品行业工业化应用提供依据。相信随着物理技术和细菌素协同抑菌研究的逐渐深入,细菌素协同物理技术的抑菌策略有望成为对抗食源性致病菌和腐败微生物污染及保障食品安全的新型防控手段。
-
表 1 细菌素的分类
Table 1 Classification of bacteriocins
分类 特征 代表性细菌素 I类 翻译后修饰的肽,包含19~50多个氨基酸,分子量<5 kDa 乳酸链球菌素,美杀菌素,枯草菌素 II类 热稳定、不含羊毛硫氨酸的肽,包含36~57个氨基酸,分子量<10 kDa 片球菌素PA-1,肠球菌素AS-48 III类 热不稳定的蛋白质,分子量>30 kDa 瑞士乳杆菌素J,肠溶素A IV类 携带脂质或碳水化合物的复合肽 片球菌素SJ-1,格氏乳杆菌素A 表 2 细菌素协同物理技术在不同食品基质中的抑菌作用效果
Table 2 Antimicrobial effect of bacteriocin synergistic physical technology in different food matrices
物理技术 细菌素 目标菌 食品基质 作用效果 参考文献 技术名称 技术参数 超声波 20~25 kHz,950 W,
15 min,37~52 ℃Nisin 好氧菌、酵母菌、霉菌 苹果汁 活菌数减少,褐变度、可滴定酸度和可溶性固形物含量无明显变化 [45] 20~25 kHz,150 W,
16 min,30±5 ℃Thurincin H 英诺克李斯特菌ATCC 33090、大肠杆菌K12 牛奶和橙汁 细胞膜损伤,核酸、蛋白质渗漏,抑制致病菌生长繁殖 [47] 20 kHz,130 W,
20 min,7 ℃Nisin 福氏志贺氏菌 − 福氏志贺氏菌减少了1.48 lg CFU/mL [46] 44、500和1000 kHz,
30 W,15 min,37 ℃Nisin 大肠杆菌 ATCC 25922 − 大肠杆菌外膜造成短暂损伤,Nisin进入细胞膜并与脂质II结合,使致病菌形成孔隙 [48] 高压 450 MPa,5 min Nisin 单核细胞增生李斯特菌CTC 1034 液态胶束酪蛋
白浓缩物微生物显著减少,酸度和颜色变化较小 [62] 300 MPa,5 min Pediocin PA-1 单核细胞增生李斯特菌ATCC 49594 牛奶 致病菌的生长受到抑制 [61] 400 MPa,20 min Enterocin AS-48 细菌和真菌活菌数 番荔枝浆 增加受伤细胞对细菌素的敏感性,抑制致病菌 [66] 200 MPa,2 min Nisin 英诺克李斯特菌ATCC 51742、大肠杆菌ATCC 11755 胡萝卜汁 HPP先破坏细胞外膜,然后Nisin破坏细胞质膜的电势差抑制致病菌 [65] 热处理 60 °C,15 min Nisin 金黄色葡萄球菌ATCC 23587、枯草芽孢杆菌ATCC 23587和大肠杆菌ATCC 23716 预制胡萝卜 货架期从6 d延长至42 d [75] 80 ℃,10 min Nisin 枯草芽孢杆菌ATCC6633 豆腐 抑制了豆腐中芽孢杆菌芽孢的生长 [78] 60 ℃,5 min Enterocin AS-48 金黄色葡萄球菌
CECT 976酱汁 延缓了金黄色葡萄球菌在酱汁中的生长 [74] 脉冲电场 30 kV/cm Nisin 鼠伤寒沙门氏菌LT2、
英诺克李斯特菌CECT 910T− 致病菌减少2~2.5 lg CFU/mL [83] 30 kV/cm Nisin 大肠杆菌K12 苹果汁 大肠杆菌K12减少4 lg CFU/mL [82] 纳米技术 − Nisin 单核细胞增生李斯特菌CICC 21633、枯草芽孢杆菌CICC 10275和金黄色葡萄球菌CICC 10384 番茄汁 延长货架期 [88] 紫外线 254 nm Nisin 酸性脂环芽孢杆菌 橙汁 消除了酸曲霉孢子,未降解果汁中维生素 [92] 低温等离子体 3 min Nisin 单核细胞增生李斯特菌F8027 苹果 单核细胞增生李斯特菌减少了4 lg CFU/mL [90] 快速冷却 5 ℃ Nisin 大肠杆菌O157:H7 NCTC 12900 − 破坏细胞膜的外膜屏障,使大肠杆菌对Nisin敏感 [93] 脉冲光 10 s Nisin 大肠杆菌O157:H7 C9490、E02128和F00475 长叶莴苣 抑制了5 lg CFU/mL大肠杆菌 O157:H7 [95] 注:−文献未报道。 -
[1] MUKURUMBIRA A R, SHELLIE R A, KEAST R, et al. The antimicrobial efficacy of native Australian essential oils in liquid and vapour phase against foodborne pathogens and spoilage microorganisms[J]. Food Control,2023,151:109774. doi: 10.1016/j.foodcont.2023.109774
[2] THOMAS G A, GIL T P, MÜLLER C T, et al. From field to plate:How do bacterial enteric pathogens interact with ready-to-eat fruit and vegetables, causing disease outbreaks[J]. Food Microbiology,2024,117:104389. doi: 10.1016/j.fm.2023.104389
[3] WANG J, CHEN J Y, SUN Y Y, et al. Ultraviolet-radiation technology for preservation of meat and meat products:Recent advances and future trends[J]. Food Control,2023,148:109684. doi: 10.1016/j.foodcont.2023.109684
[4] SALLAM K I, RASLAN M T, SABALA R F, et al. Antimicrobial effect of garlic against foodborne pathogens in ground mutton[J]. Food Microbiology,2024,120:104462. doi: 10.1016/j.fm.2023.104462
[5] WANG J, REN B J, BAK J H, et al. Preservative effects of composite biopreservatives on goat meat during chilled storage:Insights into meat quality, high-throughput sequencing and molecular docking[J]. LWT–Food Science and Technology,2023,184:115033.
[6] ZARZECKA U, ZADERNOWSKA A, CHAJECKA-WIERZCHOWSKA W, et al. High-pressure processing effect on conjugal antibiotic resistance genes transfer in vitro and in the food matrix among strains from starter cultures[J]. International Journal of Food Microbiology,2023,388:110104. doi: 10.1016/j.ijfoodmicro.2023.110104
[7] LI Y J, YU S, WENG P F, et al. Purification and antimicrobial mechanism of a novel bacteriocin produced by Lactiplantibacillus plantarum FB-2[J]. LWT–Food Science and Technology,2023,185:115123.
[8] O’CONNOR P M, KUNIYOSHI T M, OLIVEIRA R P S, et al. Antimicrobials for food and feed:A bacteriocin perspective[J]. Current Opinion in Biotechnology,2020,61:160−167. doi: 10.1016/j.copbio.2019.12.023
[9] GRUSKIENE R, GALINSKAITE A, KAVLEISKAJA T, et al. Fucoidan as a carrier of antimicrobial peptide:Preparation and characterization of nisin-loaded particles[J]. LWT–Food Science and Technology,2024,191:115598.
[10] RENDUELES C, DUARTE A C, ESCOBEDO S, et al. Combined use of bacteriocins and bacteriophages as food biopreservatives. A review[J]. International Journal of Food Microbiology,2022,368:109611. doi: 10.1016/j.ijfoodmicro.2022.109611
[11] MOK J H, PYATKOVSKYY T, YOUSEF A, et al. Synergistic effects of shear stress, moderate electric field, and nisin for the inactivation of Escherichia coli K12 and Listeria innocua in clear apple juice[J]. Food Control,2020,113:107209. doi: 10.1016/j.foodcont.2020.107209
[12] ÇELEN T, ANUMUDU C, MIRI T, et al. Nisin:Carboxymethylcellulose polyion complex (PIC) nanoparticles. Preparation and antimicrobial activity[J]. Carbohydrate Polymers,2023,317:121032. doi: 10.1016/j.carbpol.2023.121032
[13] YOON J H, JEONG D Y, LEE S B, et al. Decontamination of Listeria monocytogenes in king oyster mushrooms (Pleurotus eryngii) by combined treatments with organic acids, nisin, and ultrasound[J]. LWT–Food Science and Technology,2021,144:111207.
[14] YING J P, FU C M, WU Y C, et al. Combined analysis of transcriptomics and metabolomics provide insights into the antibacterial mechanism of bacteriocin XJS01 against multidrug-resistant Staphylococcus aureus[J]. Science of the Total Environment,2024,917:170412. doi: 10.1016/j.scitotenv.2024.170412
[15] YING J P, WU G, ZHANG Y M, et al. Proteomic analysis of Staphylococcus aureus exposed to bacteriocin XJS01 and its bio-preservative effect on raw pork loins[J]. Meat Science,2023,204:109258. doi: 10.1016/j.meatsci.2023.109258
[16] UCAR Y, OZOGUL Y, DURMUS M, et al. The effects of nisin on the growth of foodborne pathogens and biogenic amine formation: In vivo and in vitro studies[J]. Food Bioscience,2021,43:101266. doi: 10.1016/j.fbio.2021.101266
[17] ZHANG J, GU S B, ZHANG T R, et al. Characterization and antibacterial modes of action of bacteriocins from Bacillus coagulans CGMCC 9951 against Listeria monocytogenes[J]. LWT–Food Science and Technology,2022,160:113272.
[18] ISMAEL M, QAYYUM N, GU Y X, et al. Protective effect of plantaricin bio-LP1 bacteriocin on multidrug-resistance Escherichia coli infection by alleviate the inflammation and modulate of gut-microbiota in BALB/c mice model[J]. International Journal of Biological Macromolecules,2023,246:125700. doi: 10.1016/j.ijbiomac.2023.125700
[19] AL-EMARAH M K, KAZERANI H R, TAGHIZAD F, et al. Anti-obesity effect of the bacterial product nisin in an NIH Swiss mouse model[J]. Lipids in Health and Disease,2023,22(1):23. doi: 10.1186/s12944-023-01788-1
[20] BAHY R, EMARA M, ELALEM N, et al. A new bacteriocin-like-inhibitory substance from Egyptian dairy products isolated from Enterococcus faecium with anti-SARS-CoV-2 activity[J]. Process Biochemistry,2023,134(P2):47−54.
[21] EGHBAL N, VITON C, GHARSALLAOUI A. Nano and microencapsulation of bacteriocins for food applications:A review[J]. Food Bioscience,2022,50(PB):102173.
[22] BANGAR S P, CHAUDHARY V P, SINGH T P, et al. Retrospecting the concept and industrial significance of LAB bacteriocins[J]. Food Bioscience,2022,46:101607. doi: 10.1016/j.fbio.2022.101607
[23] WORAPRAYOTE W, JANYAPHISAN T, ADUNPHATCHARAPHON S, et al. Bacteriocinogenic lactic acid bacteria from Thai fermented foods:Potential food applications[J]. Food Bioscience,2023,52:102385. doi: 10.1016/j.fbio.2023.102385
[24] YI Y L, LI P, ZHAO P, et al. Current status and potentiality of class II bacteriocins from lactic acid bacteria:Structure, mode of action and applications in the food industry[J]. Trends in Food Science & Technology,2022,120:387−401.
[25] YU W, GUO J Q, LIU Y Y, et al. Potential impact of combined inhibition by bacteriocins and chemical substances of foodborne pathogenic and spoilage bacteria:A review[J]. Foods,2023,12(16):3128. doi: 10.3390/foods12163128
[26] DABA G M, ELKHATEEB W A. Ribosomally synthesized bacteriocins of lactic acid bacteria:Simplicity yet having wide potentials - A review[J]. International Journal of Biological Macromolecules,2024,256(P1):128325.
[27] NISA M, DAR R A, FOMDA B A, et al. Combating food spoilage and pathogenic microbes via bacteriocins:A natural and eco-friendly substitute to antibiotics[J]. Food Control,2023,149:109710. doi: 10.1016/j.foodcont.2023.109710
[28] WU J J, ZANG M W, WANG S W, et al. Nisin:From a structural and meat preservation perspective[J]. Food Microbiology,2023,111:104207. doi: 10.1016/j.fm.2022.104207
[29] CUI Y L, LUO L L, WANG X, et al. Mining, heterologous expression, purification, antibactericidal mechanism, and application of bacteriocins:A review[J]. Comprehensive Reviews in Food Science and Food Safety,2020,20(1):863−899.
[30] MENG F Q, ZHU X Y, ZHAO H Z, et al. A class III bacteriocin with broad-spectrum antibacterial activity from Lactobacillus acidophilus NX2-6 and its preservation in milk and cheese[J]. Food Control,2021,121:107597. doi: 10.1016/j.foodcont.2020.107597
[31] TELHIG S, BEN SAID L, ZIRAH S, et al. Bacteriocins to thwart bacterial resistance in gram negative bacteria[J]. Frontiers In Microbiology,2020,11:586433. doi: 10.3389/fmicb.2020.586433
[32] PANG X Y, SONG X Y, CHEN M J, et al. Combating biofilms of foodborne pathogens with bacteriocins by lactic acid bacteria in the food industry[J]. Comprehensive Reviews in Food Science and Food Safety,2022,21(2):1657−1676. doi: 10.1111/1541-4337.12922
[33] MELIAN C, SEGLI F, GONZALEZ R, et al. Lactocin AL705 as quorum sensing inhibitor to control Listeria monocytogenes biofilm formation[J]. Journal of Applied Microbiology,2019,127(3):911−920. doi: 10.1111/jam.14348
[34] MERAL R, ALAV A, KARAKAS C, et al. Effect of electrospun nisin and curcumin loaded nanomats on the microbial quality, hardness and sensory characteristics of rainbow trout fillet[J]. LWT–Food Science and Technology,2019,113:108292.
[35] LI B, ZHONG M M, SUN Y F, et al. Recent advancements in the utilization of ultrasonic technology for the curing of processed meat products:A comprehensive review[J]. Ultrasonics Sonochemistry,2024,103:106796. doi: 10.1016/j.ultsonch.2024.106796
[36] WANG Y, YANG Y Q, LI Z, et al. Research advances on the effects of thermal and non-thermal processing techniques on the physicochemical properties and microbiological control of liquid eggs[J]. Food Control,2024,155:110106. doi: 10.1016/j.foodcont.2023.110106
[37] YANG F, SHI C H, YAN L C, et al. Low-frequency ultrasonic treatment:A potential strategy to improve the flavor of fresh watermelon juice[J]. Ultrasonics Sonochemistry,2022,91:106238. doi: 10.1016/j.ultsonch.2022.106238
[38] CHEN F Y, ZHANG M, YANG C H. Application of ultrasound technology in processing of ready-to-eat fresh food:A review[J]. Ultrasonics Sonochemistry,2020,63:104953. doi: 10.1016/j.ultsonch.2019.104953
[39] WANG J, FAN L P. Effect of ultrasound treatment on microbial inhibition and quality maintenance of green asparagus during cold storage[J]. Ultrasonics Sonochemistry,2019,58:104631. doi: 10.1016/j.ultsonch.2019.104631
[40] TANG T T, ZHANG M, LAW C L, et al. Effects of ultrasound combined technology on quality and volatile compound properties of chili sauce[J]. Food Bioscience,2023,53:102771. doi: 10.1016/j.fbio.2023.102771
[41] KITSIOU M, PURK L, IOANNOU C, et al. On the evaluation of the antimicrobial effect of grape seed extract and cold atmospheric plasma on the dynamics of Listeria monocytogenes in novel multiphase 3D viscoelastic models[J]. International Journal of Food Microbiology,2023,406:110395. doi: 10.1016/j.ijfoodmicro.2023.110395
[42] YANG H, ZHAO X J, SONG L Y, et al. Synergistic antibacterial and anti-biofilm mechanisms of ultrasound combined with citral nanoemulsion against Staphylococcus aureus 29213[J]. International Journal of Food Microbiology,2023,391-393:110150. doi: 10.1016/j.ijfoodmicro.2023.110150
[43] YOON J H, JEONG D Y, LEE S B, et al. Control of Listeria monocytogenes and Escherichia coli O157:H7 in enoki mushrooms (Flammulina velutipes) by combined treatments with organic acids, nisin, and ultrasound[J]. Food Control,2022,129:108204.
[44] CASCO M A, JAGUS R J, AGUERO M V, et al. Ultrasound and its combination with natural antimicrobials:Effects on shelf life and quality stability of a fruit and vegetable smoothie[J]. Food and Bioprocess Technology,2022,15(1):203−218. doi: 10.1007/s11947-021-02745-5
[45] LIAO H M, JIANG L F, CHENG Y L, et al. Application of nisin-assisted thermosonication processing for preservation and quality retention of fresh apple juice[J]. Ultrasonics Sonochemistry,2018,42:244−249. doi: 10.1016/j.ultsonch.2017.11.020
[46] DE FREITAS L L, PRUDÊNCIO C V, PEÑA W E L, et al. Modeling of Shigella flexneri inactivation by combination of ultrasound, pH and nisin[J]. LWT–Food Science and Technology,2019,109:40−46.
[47] RUIZ-DE A D, CASADOS-VÁZQUEZ L E, OZUNA C. The synergistic effect of thurincin H and power ultrasound:An alternative for the inactivation of Listeria innocua ATCC 33090 and Escherichia coli K-12 in liquid food matrices[J]. Food Control,2022,135:108778. doi: 10.1016/j.foodcont.2021.108778
[48] COSTELLO K M, VELLIOU E, GUTIERREZ-MERINO J, et al. The effect of ultrasound treatment in combination with nisin on the inactivation of Listeria innocua and Escherichia coli[J]. Ultrasonics Sonochemistry,2021,79:105776. doi: 10.1016/j.ultsonch.2021.105776
[49] ZHAO G, KEMPEN P J, ZHENG T, et al. Synergistic bactericidal effect of nisin and phytic acid against Escherichia coli O157:H7[J]. Food Control,2023,144:109324. doi: 10.1016/j.foodcont.2022.109324
[50] DHENGE R, RINALDI M, RODOLFI M, et al. Modification of structural characteristics of vegetables by high-pressure processing:A review[J]. Food Bioscience,2023,56:103407. doi: 10.1016/j.fbio.2023.103407
[51] ZHAO S L, PAN Z T, AZARAKHSH N, et al. Effects of high-pressure processing on the physicochemical and adsorption properties, structural characteristics, and dietary fiber content of kelp (Laminaria japonica)[J]. Current Research in Food Science,2024,8:100671. doi: 10.1016/j.crfs.2023.100671
[52] BIGI F, MAURIZZI E, QUARTIERI A, et al. Non-thermal techniques and the “hurdle” approach:How is food technology evolving?[J]. Trends in Food Science & Technology,2023,132:11−39.
[53] FERREIRA N B M, RODRIGUES M I, CRISTIANINI M. Effect of high pressure processing and water activity on pressure resistant spoilage lactic acid bacteria (Latilactobacillus sakei) in a ready-to-eat meat emulsion model[J]. International Journal of Food Microbiology,2023,401:110293. doi: 10.1016/j.ijfoodmicro.2023.110293
[54] BOZIARIS I S, PARLAPANI F F, DEWITT C A M. High pressure processing at ultra-low temperatures:Inactivation of foodborne bacterial pathogens and quality changes in frozen fish fillets[J]. Innovative Food Science & Emerging Technologies,2021,74:102811.
[55] MONTEIRO M L G, ROSÁRIO D K A, NETO L T, et al. Exploring high hydrostatic pressure for enhancing the preservation of white and dark muscle fish fillets stored at different packaging systems under refrigeration[J]. Food Control,2024,155:110038. doi: 10.1016/j.foodcont.2023.110038
[56] LIU H K, LIN Y, SUN M Y, et al. The effects of high-pressure processing on the nutritional quality of sprouts:A review[J]. Food Bioscience,2023,56:103384. doi: 10.1016/j.fbio.2023.103384
[57] ALI S, REZENDE V T, REZENDE V T, et al. Food processing and challenges in the food production and quality:The foodomics approach[J]. Food Bioscience,2023,56:103217. doi: 10.1016/j.fbio.2023.103217
[58] RIFNA E J, SINGH S K, CHAKRABORTY S, et al. Effect of thermal and non-thermal techniques for microbial safety in food powder:Recent advances[J]. Food Research International,2019,126:108654. doi: 10.1016/j.foodres.2019.108654
[59] DALLAGNOL A M, BARRIO Y, CAP M, et al. Listeria inactivation by the combination of high hydrostatic pressure and lactocin AL705 on cured-cooked pork loin slices[J]. Food and Bioprocess Technology,2017,10(10):1824−1833. doi: 10.1007/s11947-017-1956-6
[60] MODUGNO C, KMIHA S, SIMONI H, et al. High pressure sensitization of heat-resistant and pathogenic foodborne spores to nisin[J]. Food Microbiology,2019,84:103244. doi: 10.1016/j.fm.2019.103244
[61] KOMORA N, MACIEL C, PINTO C A, et al. Non-thermal approach to Listeria monocytogenes inactivation in milk:The combined effect of high pressure, pediocin PA-1 and bacteriophage P100[J]. Food Microbiology,2020,86:103315. doi: 10.1016/j.fm.2019.103315
[62] GARCÍA A, ITURMENDI N, MATE J I, et al. Combined effect of nisin addition and high pressure processing on the stability of liquid micellar casein concentrates[J]. International Dairy Journal,2022,130:105361. doi: 10.1016/j.idairyj.2022.105361
[63] LIU L, DENG X, HUANG L, et al. Comparative effects of high hydrostatic pressure, pasteurization and nisin processing treatments on the quality of pickled radish[J]. LWT–Food Science and Technology,2022,167:113833.
[64] BURGOS M J G, AGUAYO M D L, PULIDO R P, et al. Analysis of the microbiota of refrigerated chopped parsley after treatments with a coating containing enterocin AS-48 or by high-hydrostatic pressure[J]. Food Research International,2017,99(P1):91−97.
[65] POKHREL P R, TONIAZZO T, BOULET C, et al. Inactivation of Listeria innocua and Escherichia coli in carrot juice by combining high pressure processing, nisin, and mild thermal treatments[J]. Innovative Food Science and Emerging Technologies,2019,54:93−102. doi: 10.1016/j.ifset.2019.03.007
[66] PULIDO R P, TOLEDO J, GRANDE M J, et al. Analysis of the effect of high hydrostatic pressure treatment and enterocin AS-48 addition on the bacterial communities of cherimoya pulp[J]. International Journal of Food Microbiology,2015,196:62−69. doi: 10.1016/j.ijfoodmicro.2014.11.033
[67] MARTILLANES S, ROCHA-PIMIENTA J, LLERA-OYOLA J, et al. Control of Listeria monocytogenes in sliced dry-cured Iberian ham by high pressure processing in combination with an eco-friendly packaging based on chitosan, nisin and phytochemicals from rice bran[J]. Food Control,2021,124:107933. doi: 10.1016/j.foodcont.2021.107933
[68] LIU F F, CHEN Y, CHEN J L, et al. Characteristic aroma improvement mechanisms of heat-sterilized bayberry juice regulated by exogenous polyphenols[J]. Food Chemistry,2023,427:136644. doi: 10.1016/j.foodchem.2023.136644
[69] ZHANG Y, PANDISELVAM R, ZHU H K, et al. Impact of radio frequency treatment on textural properties of food products:An updated review[J]. Trends in Food Science & Technology,2022,124:154−166.
[70] JIAO S S, ZHANG H J, HU S H, et al. Radio frequency inactivation kinetics of Bacillus cereus spores in red pepper powder with different initial water activity[J]. Food Control,2019,105:174−179. doi: 10.1016/j.foodcont.2019.05.038
[71] VIJAY K, MARANGELI O, HWANG C, et al. Thermal inactivation of Bacillus cereus spores during cooking of rice to ensure later safety of boudin[J]. LWT–Food Science and Technology,2020,122:108955.
[72] WU M J, MA Y, DOU X, et al. A review of potential antibacterial activities of nisin against Listeria monocytogenes:The combined use of nisin shows more advantages than single use[J]. Food Research International,2023,164:112363. doi: 10.1016/j.foodres.2022.112363
[73] XU Y M, GUAN X Y, WANG S J. Synergistic bactericidal mechanisms of RF energy simultaneously combined with cinnamon essential oil or epsilon-polylysine against Salmonella revealed at cellular and metabolic levels[J]. International Journal of Food Microbiology,2024,408:110447. doi: 10.1016/j.ijfoodmicro.2023.110447
[74] BURGOS M J G, ABRIOUEL H, LUCAS R, et al. Increasing the microbial inactivation of Staphylococcus aureus in sauces by a combination of enterocin AS-48 and 2-nitropropanol, and mild heat treatments[J]. Food Control,2012,25(2):740−744. doi: 10.1016/j.foodcont.2011.12.018
[75] DU T Y, JIANG J X, SUO M Z, et al. Nisin combined with radio frequency:Effectiveness on microbial growth, physiological quality, and structure of pre-made carrots[J]. Food Control,2024,160:110355. doi: 10.1016/j.foodcont.2024.110355
[76] ZHANG H C, TIKEKAR R V, DING Q, et al. Inactivation of foodborne pathogens by the synergistic combinations of food processing technologies and food‐grade compounds[J]. Comprehensive Reviews in Food Science and Food Safety,2020,19(4):2110−2138. doi: 10.1111/1541-4337.12582
[77] LIU G R, NIE R, LIU Y S, et al. Combined antimicrobial effect of bacteriocins with other hurdles of physicochemic and microbiome to prolong shelf life of food:A review[J]. The Science of the Total Environment,2022,825:154058. doi: 10.1016/j.scitotenv.2022.154058
[78] CUI B Z, WANG K, HU N, et al. Combination of radio frequency heating and antibacterial agents for microorganism control in the production of packaged tofu[J]. Food Control,2023,154:110015. doi: 10.1016/j.foodcont.2023.110015
[79] LI Y Y, PADILLA-ZAKOUR O I. Evaluation of pulsed electric field and high-pressure processing on the overall quality of refrigerated concord grape juice[J]. LWT–Food Science and Technology,2024,198(15):116002.
[80] AALIYA B, SUNOOJ K V, NAVAF M, et al. Recent trends in bacterial decontamination of food products by hurdle technology:A synergistic approach using thermal and non-thermal processing techniques[J]. Food Research International,2021,147:110514. doi: 10.1016/j.foodres.2021.110514
[81] ZHANG J, GHASEMI N, ZARE F, et al. Nanosecond pulsed electric field treatment of human milk:Effects on microbiological inactivation, whey proteome and bioactive protein[J]. Food Chemistry,2022,406:135073.
[82] JIN T Z, ABOELHAGGAG R M, GUO M M. Apple juice preservation using combined nonthermal processing and antimicrobial packaging[J]. Journal of Food Protection,2021,84(9):1528−1538. doi: 10.4315/JFP-21-035
[83] NOVICKIJ V, STANEVICIENE R, STAIGVILA G, et al. Effects of pulsed electric fields and mild thermal treatment on antimicrobial efficacy of nisin-loaded pectin nanoparticles for food preservation[J]. LWT–Food Science and Technology,2020,120:108915.
[84] VIEDMA P M, ABRIOUEL H, LÓPEZ A S, et al. Effect of enterocin AS-48 in combination with high-intensity pulsed-electric field treatment against the spoilage bacterium Lactobacillus diolivorans in apple juice[J]. Food Microbiology,2009,26(5):491−496. doi: 10.1016/j.fm.2009.03.001
[85] HARIS M, HUSSAIN T, MOHAMED H I, et al. Nanotechnology – A new frontier of nano-farming in agricultural and food production and its development[J]. Science of the Total Environment,2023,857(3):159639.
[86] SHAH M A, SHAHNAZ T, ZEHAB-UD-DIN, et al. Application of nanotechnology in the agricultural and food processing industries:A review[J]. Sustainable Materials and Technologies,2024,39:e00809. doi: 10.1016/j.susmat.2023.e00809
[87] BAHRAMI A, DELSHADI R, JAFARI S M, et al. Nanoencapsulated nisin:An engineered natural antimicrobial system for the food industry[J]. Trends in Food Science & Technology,2019,94:20−31.
[88] LUO L J, WU Y, LIU C, et al. Designing soluble soybean polysaccharides-based nanoparticles to improve sustained antimicrobial activity of nisin[J]. Carbohydrate Polymers,2019,225:115251. doi: 10.1016/j.carbpol.2019.115251
[89] RADAIC A, DE JESUS M B, KAPILA Y L. Bacterial anti-microbial peptides and nano-sized drug delivery systems:The state of the art toward improved bacteriocins[J]. Journal of Controlled Release,2020,321:100−118. doi: 10.1016/j.jconrel.2020.02.001
[90] UKUKU D O, NIEMIRA B A, UKANALIS J. Nisin-based antimircobial combination with cold plasma treatment inactivate Listeria monocytogenes on Granny Smith apples[J]. LWT–Food Science and Technology,2019,104:120−127.
[91] COSTELLO K M, SMET C, GUTIERREZ-MERINO J, et al. The impact of food model system structure on the inactivation of Listeria innocua by cold atmospheric plasma and nisin combined treatments[J]. International Journal of Food Microbiology,2021,337:108948. doi: 10.1016/j.ijfoodmicro.2020.108948
[92] FERREIRA T V, MIZUTA A G, DE MENEZES J L, et al. Effect of ultraviolet treatment(UV–C) combined with nisin on industrialized orange juice in Alicyclobacillus acidoterrestris spores[J]. LWT–Food Science and Technology,2020,133:109911.
[93] BI X F, WANG Y T, HU X S, et al. Decreased resistance of sublethally injured Escherichia coil O157:H7 to salt, mild heat, nisin and acids induced by high pressure carbon dioxide[J]. International Journal of Food Microbiology,2018,269:137−143. doi: 10.1016/j.ijfoodmicro.2018.01.027
[94] BERMUDEZ-AGUIRRE D, NIEMIRA B A. Effect of nisin, EDTA, and abuse temperature on the growth of Salmonella Typhimurium in liquid whole egg during refrigerated storage[J]. Food Research International,2023,174(1):113568.
[95] MUKHOPADHYAY S, UKUKU D O, OLANYA O M, et al. Combined treatment of pulsed light and nisin-organic acid based antimicrobial wash for inactivation of Escherichia coli O157:H7 in romaine lettuce, reduction of microbial loads, and retention of quality[J]. Food Microbiology,2024,118:104402. doi: 10.1016/j.fm.2023.104402





 下载:
下载:
 下载:
下载:
