Degradation Kinetics of Ascorbic Acid in Xanthan Gum Solution
-
摘要: 抗坏血酸(Ascorbic acid,AA)会与多糖反应导致多糖的流变及结构特性发生变化,然而这一反应过程中AA的降解特征及其降解动力学尚待进一步研究。本文以具有高黏性及良好稳定性的黄原胶(Xanthan gum,XG)作为多糖基构建模拟体系,探究了不同底物浓度、反应温度以及添加H2O2或金属离子(Fe2+与Cu2+)等反应条件下AA在XG溶液中的变化过程。结果表明,相比于纯水体系,AA在XG溶液中的降解程度更为显著,1 mmol/L的AA降解率在体系中存在0.2%XG(w/v)时由初始的7.03%增加至11.72%;提高反应温度也会加速AA与XG的反应,90 ℃加热处理1 h会导致AA降解率增加至45.59%;AA在该体系下的降解过程符合二级反应动力学规律。添加H2O2以及金属离子(Fe2+与Cu2+)会明显加速AA的降解,而XG溶液体系会减弱金属离子对AA的降解效果,故而金属离子与XG对AA的降解具有拮抗作用。因此,AA在纯水中加热会发生降解,而添加XG以及H2O2与金属离子(Fe2+与Cu2+)均会改变AA的降解速率,以上结果为控制食品加工过程中AA的降解提供了重要的数据支持。Abstract: Ascorbic acid (AA) reacts with polysaccharides, resulting in changes in the rheological and structural properties of the polysaccharides. However, the degradation characteristics of AA and its degradation kinetics during this reaction require further investigation. In this study, xanthan gum (XG), a highly viscous and stable polysaccharide, was employed as the polysaccharide base for the construction of a simulation system. The changes of AA in XG solutions were investigated under varying substrate concentrations, reaction temperatures, and reaction conditions, including the addition of H2O2 or metal ions (Fe2+ and Cu2+). The results showed that the degradation of AA within XG solution surpassed that in pure water. Particularly, the degradation rate of AA, which was originally 7.03% in the absence of XG, increased to 11.72% when 0.2%XG (w/v) was present in the system at a concentration of 1 mmol/L. In addition, elevated reaction temperature accelerated the reaction between AA and XG, leading to a degradation rate of AA increased to 45.59% after heating at 90 ℃for 1 h. The degradation process of AA in this system followed the second-order reaction kinetics equation. The presence of H2O2 and metal ions (Fe2+ and Cu2+) notably accelerated AA degradation, whereas the XG solution system attenuated the degradation impact of metal ions on AA. It was found that the degradation of AA by metal ions and XG was antagonistic. In conclusion, thermal treatment of AA in pure water induced its degradation, with the addition of XG, H2O2, and metal ions (Fe2+ and Cu2+) significantly affected the degradation rate of AA. These findings hold significance for the regulation of AA degradation in food processing contexts.
-
抗坏血酸(Ascorbic acid,AA)即维生素C,属于人体必需的营养素之一,参与体内多种重要的生命活动[1],如胶原蛋白的合成、多巴胺的转化、亚铁离子的氧化等[2−3]。同时AA也是一种常见的食品添加剂,在果蔬汁饮料、葡萄酒[4]等产品中发挥抗氧化作用[5−6]。例如,AA在苹果汁中的添加能够抑制多酚氧化酶引起的褐变[7]。AA因其较高的反应活性,在有氧与无氧的反应过程中均会发生降解[8−9],其降解过程涉及多种氧化、还原和分子间重排的反应[10],并且受到底物浓度、氧分压与温度等环境因素的影响[11]。
有研究表明AA会与多糖发生相互作用[12−13],AA在水溶液中与溶解氧反应生成H2O2[14−15],同时被活化为抗坏血酸自由基[16],体系内的自由基进攻糖链,进而导致了多糖的氧化降解[17]。与多糖黏度降低相比,AA与多糖相互作用对AA降解的影响目前关注较少。研究发现,多糖与AA相互作用可能促进AA的降解[18],也可能抑制AA的降解[19],这与具体的反应体系密切相关。黄原胶(Xanthan gum,XG)因其高黏性与良好的稳定性[20],在食品[21]及其他工业产品中被用作稳定[22−23]、凝胶[24]和增稠剂[25]。研究表明XG的高黏性能够在一定程度上抑制番茄冰糕中AA的降解反应[26]。然而,在不同的水溶液体系下,XG与AA相互作用如何影响AA的氧化降解鲜见报道。
因此,为了探究溶液体系内XG与AA相互作用过程中AA的降解机制及其影响因素,本文采用高效液相色谱定量测定了不同反应条件下AA降解过程的变化,探究不同因素对AA在XG溶液中降解动力学的影响,旨在为AA在富含XG食品体系中的应用提供参考。
1. 材料与方法
1.1 材料与仪器
黄原胶、抗坏血酸(99%)、甲醇 色谱级,美国Sigma-Aldrich公司;偏磷酸、无水硫酸铜(99.95%)、硫酸亚铁七水合物(99.95%) 分析纯,上海阿拉丁生化科技股份有限公司;过氧化氢 分析纯,天津玉福泰化学试剂有限公司。
1260 Infinity Ⅱ高效液相色谱仪 美国Agilent公司;IKA-RCT基本型磁力加热搅拌器 德国IKA公司;Milli-Q超纯水仪 美国Millipore公司;AL104电子天平 梅特勒-托利多仪器上海有限公司。
1.2 实验方法
1.2.1 AA与XG反应体系的构建
称取4 mg黄原胶粉末,加入超纯水磁力搅拌充分溶解(XG终浓度为0.2%,w/v),而后添加100 μL AA(终浓度为1 mmol/L)并搅拌直至体系均匀,于60 ℃水浴加热1 h后将样品静置于23 ℃环境中。探究不同因素时均在同一实验条件下增加了AA在水溶液降解过程的测定作为对照。
AA浓度因素:分别在XG溶液(终浓度0.2%,w/v)和纯水中加入AA并于60 ℃下反应,体系中AA初始浓度为0.1、1与5 mmol/L,分别在反应0、2、4、6、8与24 h取样。
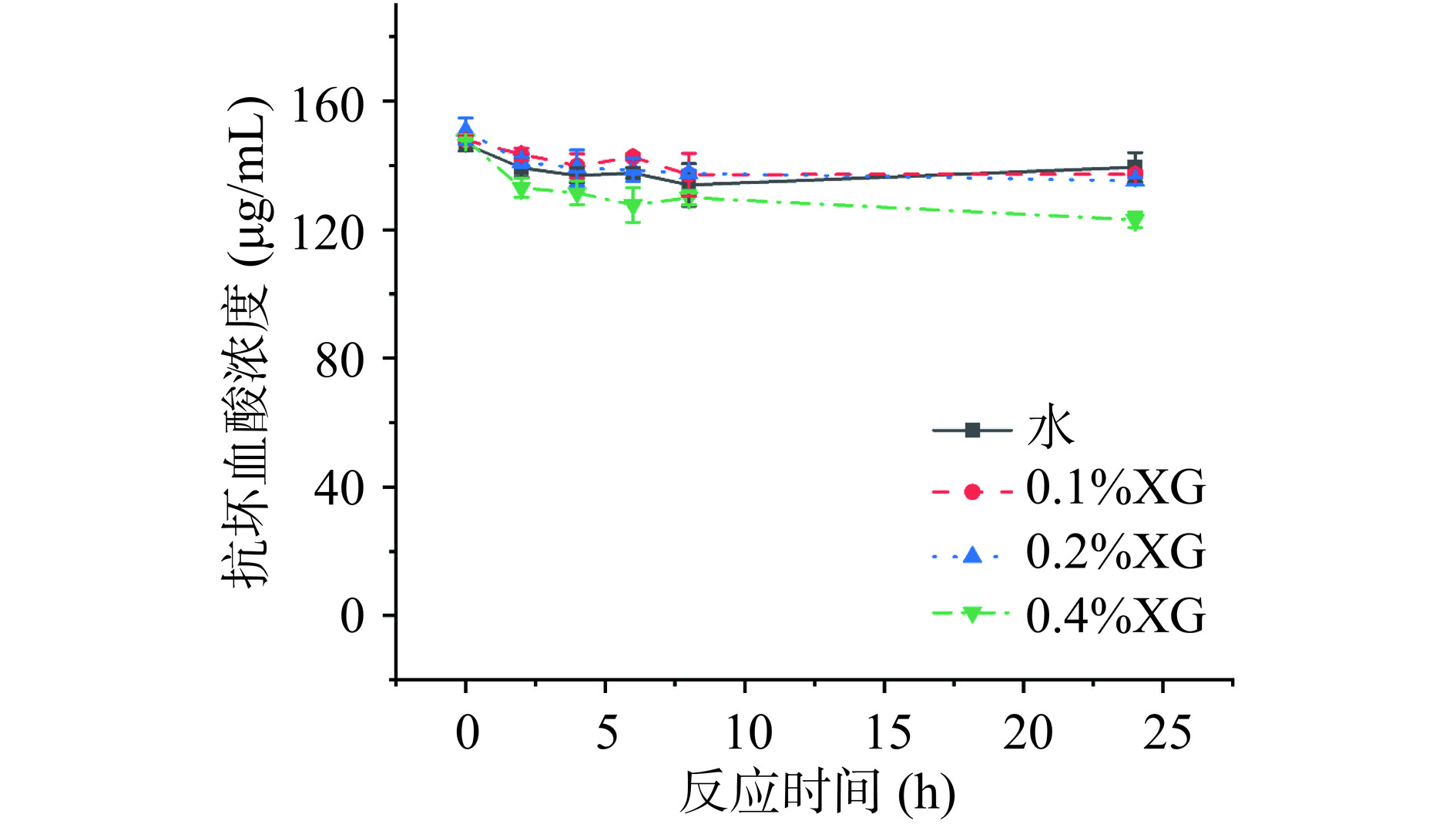
XG浓度因素:在不同XG溶液和纯水中加入AA(终浓度为1 mmol/L)并于60 ℃下反应,XG浓度分别为0.1%、0.2%与0.4%(w/v),在反应 0、2、4、6、8与24 h取样。
反应温度因素:在XG溶液(终浓度0.2%,w/v)和纯水中加入AA(终浓度为1 mmol/L),分别于30、60与90 ℃下反应,在反应0、2、4、6、8与24 h取样。
添加H2O2因素:在XG溶液(终浓度0.2%,w/v)和纯水中加入AA(终浓度为1 mmol/L)与H2O2并于60 ℃下反应,使得H2O2浓度为25、50与100 mmol/L,在反应0、0.17、0.33、0.50、0.67、0.83与1 h取样。
添加Fe2+因素:在XG溶液(终浓度0.2%,w/v)和纯水中加入AA(终浓度为1 mmol/L)与FeSO4并于60 ℃下反应,使得Fe2+浓度为0.01、0.1与1 mmol/L,在反应0、0.5、1.0、2.0、3.0与4.0 h取样。
添加Cu2+因素:在XG溶液(终浓度0.2%,w/v)和纯水中加入AA(终浓度为1 mmol/L)与CuSO4并于60 ℃下反应,使得Cu2+浓度为0.01、0.1与1 mmol/L,在反应0、0.08、0.17、0.25与0.33 h取样。
1.2.2 AA含量的测定
采用HPLC检测反应体系中一定反应时间内AA的含量,方法参考胡志群等[27]稍加修改:反应体系中取200 μL,用0.2%(w/v)偏磷酸溶液定容至5 mL,过0.22 µm的水系滤膜进样分析。采用外标法求得AA浓度,并计算出AA降解率。
降解率(%)=初始AA浓度−最终AA浓度初始AA浓度×100 (1) 仪器参数:采用Diamonsil-C18色谱柱(5 µm,4.6 mm×250 mm),柱温25 ℃,检测波长245 nm。流动相A为纯甲醇,B为0.2%偏磷酸溶液,以10%A与90%B等度洗脱5 min,流速为0.7 mL/min,进样量为20 μL。
1.2.3 AA降解动力学分析
根据1.2.2测得不同反应条件下AA随反应时间的含量变化,采用线性回归分析AA的降解速率,按照公式(2)~(4)计算并拟合得到零级方程、一级方程和二级方程对应的AA降解速率常数以及相应的线性回归系数R2,从而判断不同反应条件下AA的降解动力学[10]。
Ct=C0−k1t (2) lnCtC0=−k2t (3) 1Ct−1C0=k3t (4) 式中:k1表示零级降解速率常数;k2表示一级降解速率常数;k3表示二级降解速率常数;t表示时间,min;Ct表示反应物质量浓度,mg/mL;C0表示初始反应物质量浓度,mg/mL。
1.3 数据处理
实验平行测定3次,结果以平均值±标准差表示,使用IBM SPSS Statistics 26.0软件进行正态性检验(Shapiro-Wilk检验)与方差齐性分析(Levene法),而后进行单因素方差分析,采用Duncan多重比较,分析差异显著性(P<0.05差异显著),采用Origin 2021作图。
2. 结果与分析
2.1 不同反应条件下AA降解过程
2.1.1 不同AA浓度的反应体系中AA降解过程
由于AA在不同浓度条件下表现出不同的化学活性,较低浓度的AA表现为氧化性而高浓度的AA表现出还原性[28]。结合前期实验结果,选取3种AA浓度测定其在XG溶液中的变化过程。图1展示了不同起始浓度下(0.1、1和5 mmol/L),AA在24 h内的含量变化规律。结果可见随着反应时间的增加AA含量均会出现下降,纯水中1 mmol/L AA在24 h内浓度自159.92 μg/mL减少至148.70 μg/mL,而XG溶液中AA的降解率明显高于纯水中相同浓度的AA(表1),并且低浓度的AA降解程度更大,其中0.1 mmol/L AA的降解率最为明显,24 h达到了23.54%,而5 mmol/L AA只有3.38%(但其绝对差值仍显示5 mmol/L浓度体系下降解的AA更多)。因此低浓度AA的反应性更强,更容易发生降解,而高浓度的AA因具有抗氧化性而更为稳定。
表 1 不同反应体系中的AA降解率分析Table 1. Analysis of the degradation rate of AA in different reaction systems反应条件 初始浓度(μg/mL)* 最终浓度(μg/mL)* 浓度变化(μg/mL) 降解率(%)** 不同AA浓度 水+0.1 mmol/L AA 17.10 14.96 2.14±0.64c 12.52±3.80b XG+0.1 mmol/L AA 16.84 12.88 3.97±0.28c 23.54±1.32a 水+1 mmol/L AA 159.92 148.70 11.22±3.90bc 7.03±2.49c XG+1 mmol/L AA 162.67 143.61 19.06±1.38ab 11.72±0.86b 水+5 mmol/L AA 867.65 863.76 3.89±10.28c 0.45±1.18d XG+5 mmol/L AA 871.94 842.43 29.51±11.29a 3.38±1.26cd 不同XG浓度 水+1 mmol/L AA 146.64 139.45 7.19±4.10c 4.91±2.79c 0.1% XG+1 mmol/L AA 147.90 137.23 10.67±2.19bc 7.21±1.42bc 0.2% XG+1 mmol/L AA 150.70 135.20 15.50±4.92b 10.23±2.98b 0.4% XG+1 mmol/L AA 148.09 123.08 25.01±2.13a 16.89±1.47a 不同反应温度 水+1 mmol/L AA-30 ℃ 158.25 150.86 7.38±4.78d 4.64±2.95d XG+1 mmol/L AA-30 ℃ 157.26 153.09 4.16±2.70d 2.64±1.69d 水+1 mmol/L AA-60 ℃ 159.92 148.70 11.22±3.90cd 7.03±2.49cd 不同反应温度 XG+1 mmol/L AA-60 ℃ 162.67 143.61 19.06±1.38c 11.72±0.86c 水+1 mmol/L AA-90 ℃ 158.91 127.54 31.36±8.27b 19.72±5.06b XG+1 mmol/L AA-90 ℃ 157.12 85.50 71.62±1.28a 45.59±0.92a 添加H2O2 水+1 mmol/L AA 181.97 175.92 6.05±0.75d 3.32±0.32c XG+1 mmol/L AA 183.09 170.59 12.49±0.36d 6.83±0.25c 水+1 mmol/L AA+25 mmol/L H2O2 160.16 15.34 144.82±7.24b 90.65±3.79b XG+1 mmol/L AA+25 mmol/L H2O2 148.30 15.45 132.86±8.01c 89.54±3.16b 水+1 mmol/L AA+50 mmol/L H2O2 153.23 0.00 153.23±2.02a 100.00±0.00a XG+1 mmol/L AA+50 mmol/L H2O2 153.85 0.00 153.85±3.22a 100.00±0.00a 水+1 mmol/L AA+100 mmol/L H2O2 150.38 0.00 150.38±1.02ab 100.00±0.00a XG+1 mmol/L AA+100 mmol/L H2O2 146.39 0.00 146.38±2.37ab 100.00±0.00a 添加Fe2+ 水+1 mmol/L AA 168.04 165.09 2.95±4.48g 1.74±2.65f XG+1 mmol/L AA 168.46 163.46 5.00±6.26fg 2.96±3.71f 水+1 mmol/L AA+0.01 mmol/L Fe2+ 158.43 120.77 37.67±10.00c 23.57±5.05c XG+1 mmol/L AA+0.01 mmol/L Fe2+ 158.15 142.91 15.25±3.10de 9.62±1.83e 水+1 mmol/L AA+0.1 mmol/L Fe2+ 138.47 114.39 24.08±2.35d 17.39±1.70d XG+1 mmol/L AA+0.1 mmol/L Fe2+ 137.52 123.80 13.72±5.45ef 9.90±3.74e 水+1 mmol/L AA+1 mmol/L Fe2+ 121.66 0.00 121.66±3.65a 100.00±0.00a XG+1 mmol/L AA+1 mmol/L Fe2+ 118.87 64.48 54.40±4.21b 45.72±2.35b 添加Cu2+ 水+1 mmol/L AA 169.89 146.46 23.43±17.83d 13.73±10.42d XG+1 mmol/L AA 167.06 157.55 9.50±2.30e 5.68±1.35e 水+1 mmol/L AA+0.01 mmol/L Cu2+ 164.37 12.93 151.44±3.22b 92.13±1.01b XG+1 mmol/L AA+0.01 mmol/L Cu2+ 168.10 65.57 102.53±3.46c 61.01±2.57c 水+1 mmol/L AA+0.1 mmol/L Cu2+ 161.56 0.00 161.56±9.56ab 100.00±0.00a XG+1 mmol/L AA+0.1 mmol/L Cu2+ 168.66 0.00 168.66±0.95a 100.00±0.00a 水+1 mmol/L AA+1 mmol/L Cu2+ 168.77 0.00 168.77±0.92a 100.00±0.00a XG+1 mmol/L AA+1 mmol/L Cu2+ 168.97 0.00 168.97±1.13a 100.00±0.00a 注:*,初始浓度与最终浓度均为3次实验结果的平均值;**,同一反应体系且同一指标下不同字母之间表示存在显著性差异(P<0.05);若无明确标注,表中XG浓度为0.2%(w/v),反应体系为60 ℃反应1 h后静置于23 ℃,最终浓度为24 h测定的AA含量。 2.1.2 不同XG浓度的反应体系中AA降解过程
图2显示了不同XG浓度的反应体系中,1 mmol/L AA在反应24 h内的含量变化。随着XG浓度的增加,AA的降解逐渐加剧,0.4% XG体系中AA降解最为明显,在24 h内浓度由148.09 μg/mL下降为123.08 μg/mL,降解率从纯水体系的4.91%提高到16.89%。由于多糖会与AA发生反应[28],AA首先生成抗坏血酸自由基中间体,而后向脱氢抗坏血酸以及其他降解产物转换,当这一降解体系中的XG浓度增加时,XG会消耗一部分抗坏血酸自由基,进而加速了AA的降解。
2.1.3 不同反应温度下体系中AA降解过程
有研究表明,AA与多糖的反应涉及自由基反应[3,29]。由于温度是自由基产生的一个重要影响因素,因此继续探究不同温度下AA在XG中的浓度变化规律。图3为不同反应温度下AA的降解过程,在AA水溶液中,30 ℃和60 ℃时降解程度较小,反应24 h时降解率仅为4.64%和7.03%,而90 ℃时明显上升,降解率达到19.72%。这与其他文献[11,30]有关AA降解动力学研究相一致,温度的升高会加剧AA的降解,并且随着温度的升高会有更大的氧依赖性。而相较于水中,XG溶液体系中温度的升高对AA降解的促进更为明显,随着温度的上升,AA在水溶液中的降解率从4.64增加到19.72%,而AA在XG溶液中的降解率则从2.64%增加至45.59%,明显比水溶液体系中更为剧烈。这表明温度与XG对AA的降解具有协同作用,推测温度的升高可能加快了自由基的产生,促进了XG与AA的相互作用[31],进而加速AA的降解。
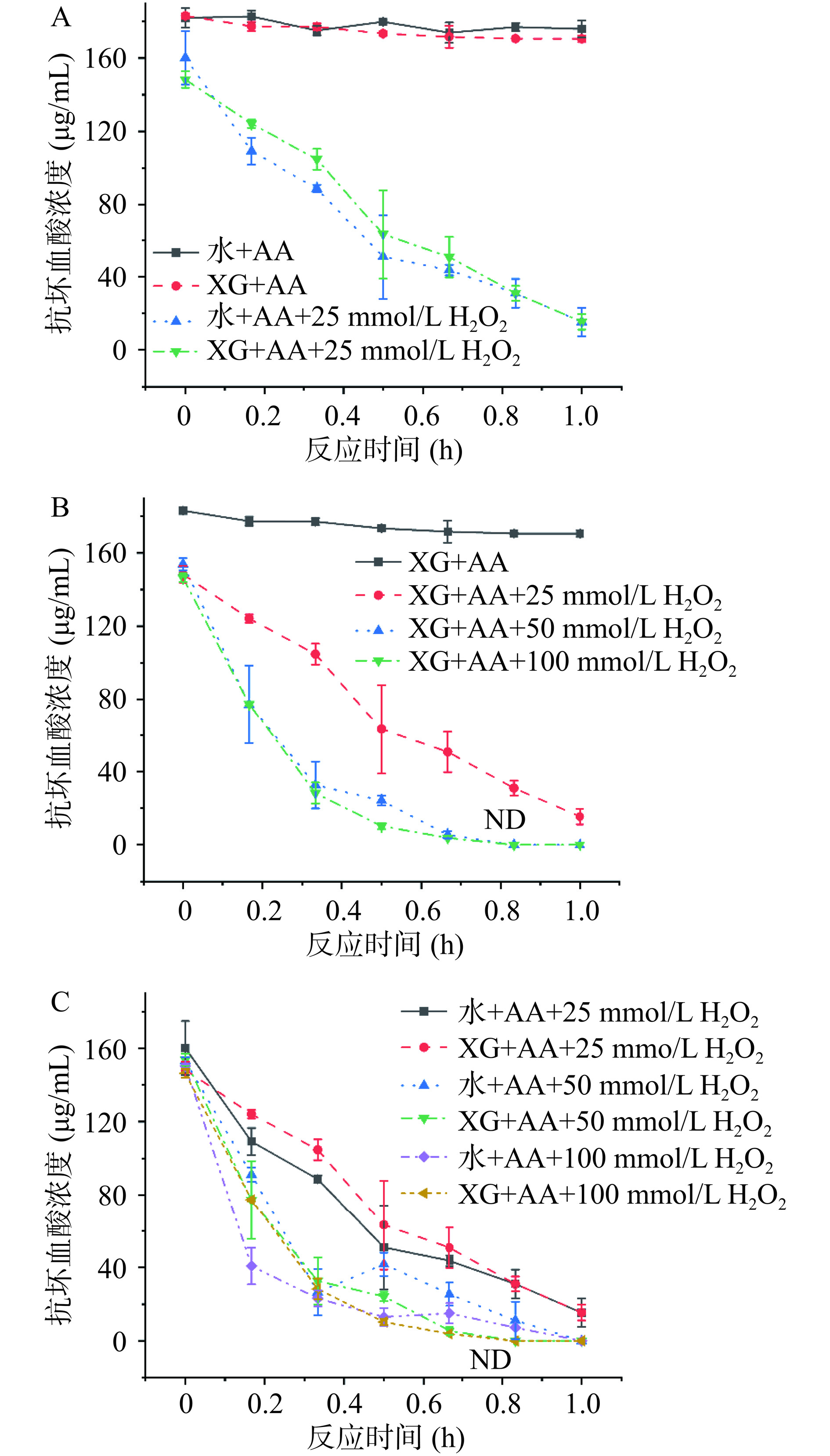
2.1.4 添加H2O2体系中AA降解过程
有研究表明AA的氧化降解,受H2O2等因素影响[31−33]。为进一步明确H2O2对AA与XG反应的影响,选择3种浓度的H2O2添加在XG-AA体系中,测定了60 min内AA的含量变化(图4)。图4A显示,与未添加H2O2条件相比,添加H2O2的体系中AA迅速下降,在60 min即降解了90.65%。对比3种H2O2的浓度(图4B)能够发现,H2O2浓度越高,AA降解越剧烈,甚至50 mmol/L与100 mmol/L H2O2处理时在反应40 min时降解率即达到100%。图4C可知添加H2O2的体系中AA在水中与XG中降解程度基本一致,甚至XG溶液中AA的降解稍缓。因此H2O2与XG对AA的降解具有拮抗作用,这可能由于H2O2与AA的反应较为剧烈,AA能够作为催化剂引发H2O2参与的芬顿反应,因此H2O2存在的体系中AA会大量被消耗,而XG的存在会增加体系黏度[17],减缓AA与H2O2的反应。
2.1.5 添加金属离子体系中AA降解过程
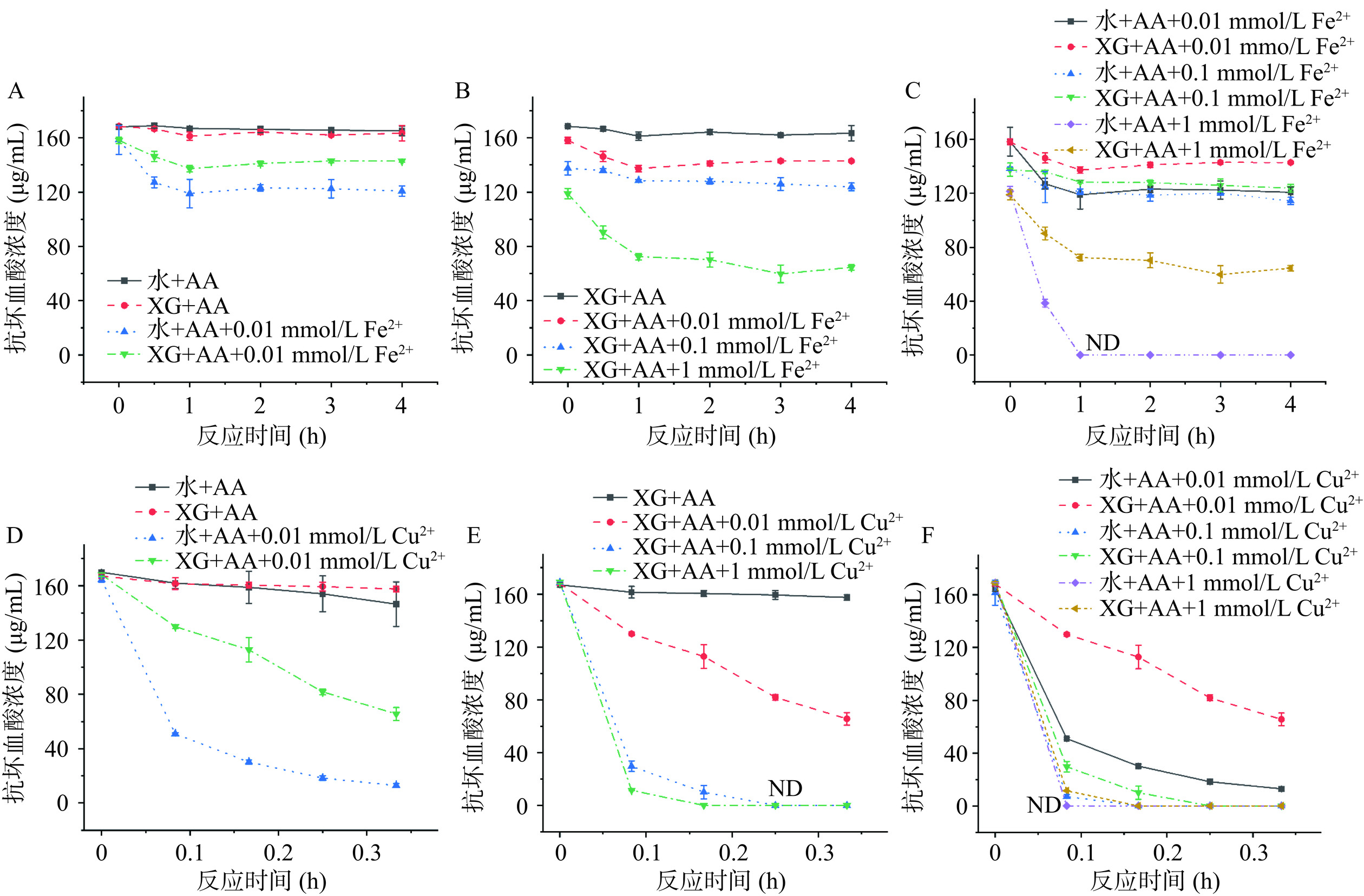
图5为添加Fe2+与Cu2+体系中AA降解过程。在添加Cu2+的条件下,AA在20 min内就会完全降解,而Fe2+的添加只有在最高浓度且加热到60 min情况下AA才会完全降解。这可能由于Cu2+氧化性更强,更容易与AA反应从而消耗了反应体系中的AA。在添加Fe2+或Cu2+体系下,AA在XG溶液中的降解程度明显弱于纯水中(表1),当添加1 mmol/L Fe2+时,AA在纯水中的降解率为100%,而AA在XG溶液中只为45.72%,因此Fe2+或Cu2+与XG对AA的降解为拮抗作用。金属离子(Fe2+或Cu2+)为芬顿反应常见的反应底物,因此会消耗AA,但由于XG的存在会增加体系的黏度[17],这也将阻碍金属离子与AA快速反应,因此产生了延缓的效果。
![]() 图 5 添加金属离子体系中AA降解过程注:(A)添加0.01 mmol/L Fe2+的纯水与0.2%XG(w/v)溶液中AA含量变化;(B)添加0、0.01、0.1和1 mmol/L Fe2+的0.2%XG(w/v)溶液中AA含量变化;(C)添加0.01、0.1和1 mmol/L Fe2+的纯水与0.2%XG(w/v)溶液中AA含量变化;(D)添加0.01 mmol/L Cu2+的纯水与0.2%XG(w/v)溶液中AA含量变化;(E)添加0、0.01、0.1和1 mmol/L Cu2+的0.2%XG(w/v)溶液中AA含量变化;(F)添加0.01、0.1和1 mmol/L Cu2+的纯水与0.2%XG(w/v)溶液中AA含量变化;反应体系内含1 mmol/L AA,在60 ℃加热1 h后静置于23 ℃;图中ND表示AA含量未检出。Figure 5. Degradation process of AA in systems with added metal ions
图 5 添加金属离子体系中AA降解过程注:(A)添加0.01 mmol/L Fe2+的纯水与0.2%XG(w/v)溶液中AA含量变化;(B)添加0、0.01、0.1和1 mmol/L Fe2+的0.2%XG(w/v)溶液中AA含量变化;(C)添加0.01、0.1和1 mmol/L Fe2+的纯水与0.2%XG(w/v)溶液中AA含量变化;(D)添加0.01 mmol/L Cu2+的纯水与0.2%XG(w/v)溶液中AA含量变化;(E)添加0、0.01、0.1和1 mmol/L Cu2+的0.2%XG(w/v)溶液中AA含量变化;(F)添加0.01、0.1和1 mmol/L Cu2+的纯水与0.2%XG(w/v)溶液中AA含量变化;反应体系内含1 mmol/L AA,在60 ℃加热1 h后静置于23 ℃;图中ND表示AA含量未检出。Figure 5. Degradation process of AA in systems with added metal ions2.2 AA降解动力学分析
为了进一步探究AA与XG的反应过程,根据3种反应动力学方程对AA的降解过程进行拟合。表2显示了不同AA浓度的反应体系中AA降解动力学方程拟合参数,其中k值为线性拟合方程的斜率,其大小在一定程度上反映AA的降解速率。与AA水溶液对照相比,XG中的AA降解速率更大,这也印证了XG的存在会加速AA的降解。而5 mmol/L AA拟合出的相关系数较小,这是由于其降解率较低,没有明显的降低趋势。由不同XG浓度的体系中AA降解动力学方程拟合参数可知,随着XG浓度的增加,AA的降解速率升高。
表 2 不同反应体系中AA降解动力学方程拟合参数Table 2. Parameters for fitting the kinetic equation of AA degradation with different reaction systems反应条件 零级方程 一级方程 二级方程 k1 调整后R2 k2 调整后R2 k3 调整后R2 水+0.1 mmol/L AA 2.77E-02 0.43 1.81E-03 0.43 1.19E-04 0.44 XG+0.1 mmol/L AA 5.91E-02 0.87 4.38E-03 0.88 3.26E-04 0.89 水+1 mmol/L AA 4.36E-01 0.87 2.85E-03 0.88 1.86E-05 0.89 XG+1 mmol/L AA 4.40E-01 0.99 2.97E-03 0.99 2.01E-05 0.99 水+5 mmol/L AA 4.40E-02 −0.30 5.07E-05 −0.30 5.85E-08 −0.30 XG+5 mmol/L AA 5.08E-01 0.47 5.99E-04 0.48 7.07E-07 0.48 水+1 mmol/L AA −7.63E-02 −0.21 −5.52E-04 −0.22 −4.00E-06 −0.22 0.1% XG+1 mmol/L AA 2.27E-01 0.27 1.62E-03 0.28 1.16E-05 0.28 0.2% XG+1 mmol/L AA 2.29E-01 0.81 1.66E-03 0.82 1.21E-05 0.82 0.4% XG+1 mmol/L AA 4.09E-01 0.82 3.21E-03 0.82 2.52E-05 0.83 水+1 mmol/L AA-30 ℃ 2.75E-01 0.64 1.79E-03 0.64 1.16E-05 0.65 XG+1 mmol/L AA-30 ℃ 1.02E-01 0.52 6.62E-04 0.52 4.29E-06 0.53 水+1 mmol/L AA-60 ℃ 4.36E-01 0.87 2.85E-03 0.88 1.86E-05 0.89 XG+1 mmol/L AA-60 ℃ 4.40E-01 0.99 2.97E-03 0.99 2.01E-05 0.99 水+1 mmol/L AA-90 ℃ 4.16E-01 0.63 3.16E-03 0.64 2.39E-05 0.64 XG+1 mmol/L AA-90 ℃ 1.79E-01 0.37 2.03E-03 0.38 2.32E-05 0.39 注:若无明确标注,表中XG浓度为0.2%(w/v),反应体系为60 ℃反应1 h后静置于23 ℃。 分析不同反应温度下体系中AA降解动力学方程拟合参数,结果发现3种反应温度的降解速率变化不明显,对比2.1.3中AA降解程度的明显差异,可能的原因是3种反应温度的区别在于反应初期的1 h内环境温度的不同,而2 h起它们的条件相同,90 ℃明显的降解主要发生在加热过程中。综合对比表2中反应动力学方程的拟合相关系数,AA在水中与XG中的降解均更符合二级反应动力学方程。
3. 结论
本文以具有高黏性及良好稳定性的XG作为多糖基构建了模拟体系,采用液相色谱测定了不同底物浓度、反应温度以及添加H2O2或金属离子(Fe2+与Cu2+)等反应条件下AA在XG溶液中的含量变化,结果表明相比于纯水体系,AA在XG溶液中的降解程度更为显著,1 mmol/L的AA降解率在体系中存在0.2%XG(w/v)时由初始的7.03%增加至11.72%。并且提高反应温度也会加速AA与XG的反应,90 ℃加热处理1 h会导致AA降解率增加至45.59%。而添加H2O2以及金属离子(Fe2+与Cu2+)会明显加速AA的降解,但XG的存在会减弱它们对AA剧烈的降解效果,因此其与XG对AA的降解为拮抗作用。进一步采用3种反应动力学的方程拟合不同反应条件下AA降解过程,通过降解速率的变化更加精准的评估不同条件下AA降解剧烈程度,并且AA在该体系下的降解过程更符合二级反应动力学的规律。以上结果有望为研究AA与多糖互作机制,控制食品加工过程中AA的降解提供数据支持。
-
图 5 添加金属离子体系中AA降解过程
注:(A)添加0.01 mmol/L Fe2+的纯水与0.2%XG(w/v)溶液中AA含量变化;(B)添加0、0.01、0.1和1 mmol/L Fe2+的0.2%XG(w/v)溶液中AA含量变化;(C)添加0.01、0.1和1 mmol/L Fe2+的纯水与0.2%XG(w/v)溶液中AA含量变化;(D)添加0.01 mmol/L Cu2+的纯水与0.2%XG(w/v)溶液中AA含量变化;(E)添加0、0.01、0.1和1 mmol/L Cu2+的0.2%XG(w/v)溶液中AA含量变化;(F)添加0.01、0.1和1 mmol/L Cu2+的纯水与0.2%XG(w/v)溶液中AA含量变化;反应体系内含1 mmol/L AA,在60 ℃加热1 h后静置于23 ℃;图中ND表示AA含量未检出。
Figure 5. Degradation process of AA in systems with added metal ions
表 1 不同反应体系中的AA降解率分析
Table 1 Analysis of the degradation rate of AA in different reaction systems
反应条件 初始浓度(μg/mL)* 最终浓度(μg/mL)* 浓度变化(μg/mL) 降解率(%)** 不同AA浓度 水+0.1 mmol/L AA 17.10 14.96 2.14±0.64c 12.52±3.80b XG+0.1 mmol/L AA 16.84 12.88 3.97±0.28c 23.54±1.32a 水+1 mmol/L AA 159.92 148.70 11.22±3.90bc 7.03±2.49c XG+1 mmol/L AA 162.67 143.61 19.06±1.38ab 11.72±0.86b 水+5 mmol/L AA 867.65 863.76 3.89±10.28c 0.45±1.18d XG+5 mmol/L AA 871.94 842.43 29.51±11.29a 3.38±1.26cd 不同XG浓度 水+1 mmol/L AA 146.64 139.45 7.19±4.10c 4.91±2.79c 0.1% XG+1 mmol/L AA 147.90 137.23 10.67±2.19bc 7.21±1.42bc 0.2% XG+1 mmol/L AA 150.70 135.20 15.50±4.92b 10.23±2.98b 0.4% XG+1 mmol/L AA 148.09 123.08 25.01±2.13a 16.89±1.47a 不同反应温度 水+1 mmol/L AA-30 ℃ 158.25 150.86 7.38±4.78d 4.64±2.95d XG+1 mmol/L AA-30 ℃ 157.26 153.09 4.16±2.70d 2.64±1.69d 水+1 mmol/L AA-60 ℃ 159.92 148.70 11.22±3.90cd 7.03±2.49cd 不同反应温度 XG+1 mmol/L AA-60 ℃ 162.67 143.61 19.06±1.38c 11.72±0.86c 水+1 mmol/L AA-90 ℃ 158.91 127.54 31.36±8.27b 19.72±5.06b XG+1 mmol/L AA-90 ℃ 157.12 85.50 71.62±1.28a 45.59±0.92a 添加H2O2 水+1 mmol/L AA 181.97 175.92 6.05±0.75d 3.32±0.32c XG+1 mmol/L AA 183.09 170.59 12.49±0.36d 6.83±0.25c 水+1 mmol/L AA+25 mmol/L H2O2 160.16 15.34 144.82±7.24b 90.65±3.79b XG+1 mmol/L AA+25 mmol/L H2O2 148.30 15.45 132.86±8.01c 89.54±3.16b 水+1 mmol/L AA+50 mmol/L H2O2 153.23 0.00 153.23±2.02a 100.00±0.00a XG+1 mmol/L AA+50 mmol/L H2O2 153.85 0.00 153.85±3.22a 100.00±0.00a 水+1 mmol/L AA+100 mmol/L H2O2 150.38 0.00 150.38±1.02ab 100.00±0.00a XG+1 mmol/L AA+100 mmol/L H2O2 146.39 0.00 146.38±2.37ab 100.00±0.00a 添加Fe2+ 水+1 mmol/L AA 168.04 165.09 2.95±4.48g 1.74±2.65f XG+1 mmol/L AA 168.46 163.46 5.00±6.26fg 2.96±3.71f 水+1 mmol/L AA+0.01 mmol/L Fe2+ 158.43 120.77 37.67±10.00c 23.57±5.05c XG+1 mmol/L AA+0.01 mmol/L Fe2+ 158.15 142.91 15.25±3.10de 9.62±1.83e 水+1 mmol/L AA+0.1 mmol/L Fe2+ 138.47 114.39 24.08±2.35d 17.39±1.70d XG+1 mmol/L AA+0.1 mmol/L Fe2+ 137.52 123.80 13.72±5.45ef 9.90±3.74e 水+1 mmol/L AA+1 mmol/L Fe2+ 121.66 0.00 121.66±3.65a 100.00±0.00a XG+1 mmol/L AA+1 mmol/L Fe2+ 118.87 64.48 54.40±4.21b 45.72±2.35b 添加Cu2+ 水+1 mmol/L AA 169.89 146.46 23.43±17.83d 13.73±10.42d XG+1 mmol/L AA 167.06 157.55 9.50±2.30e 5.68±1.35e 水+1 mmol/L AA+0.01 mmol/L Cu2+ 164.37 12.93 151.44±3.22b 92.13±1.01b XG+1 mmol/L AA+0.01 mmol/L Cu2+ 168.10 65.57 102.53±3.46c 61.01±2.57c 水+1 mmol/L AA+0.1 mmol/L Cu2+ 161.56 0.00 161.56±9.56ab 100.00±0.00a XG+1 mmol/L AA+0.1 mmol/L Cu2+ 168.66 0.00 168.66±0.95a 100.00±0.00a 水+1 mmol/L AA+1 mmol/L Cu2+ 168.77 0.00 168.77±0.92a 100.00±0.00a XG+1 mmol/L AA+1 mmol/L Cu2+ 168.97 0.00 168.97±1.13a 100.00±0.00a 注:*,初始浓度与最终浓度均为3次实验结果的平均值;**,同一反应体系且同一指标下不同字母之间表示存在显著性差异(P<0.05);若无明确标注,表中XG浓度为0.2%(w/v),反应体系为60 ℃反应1 h后静置于23 ℃,最终浓度为24 h测定的AA含量。 表 2 不同反应体系中AA降解动力学方程拟合参数
Table 2 Parameters for fitting the kinetic equation of AA degradation with different reaction systems
反应条件 零级方程 一级方程 二级方程 k1 调整后R2 k2 调整后R2 k3 调整后R2 水+0.1 mmol/L AA 2.77E-02 0.43 1.81E-03 0.43 1.19E-04 0.44 XG+0.1 mmol/L AA 5.91E-02 0.87 4.38E-03 0.88 3.26E-04 0.89 水+1 mmol/L AA 4.36E-01 0.87 2.85E-03 0.88 1.86E-05 0.89 XG+1 mmol/L AA 4.40E-01 0.99 2.97E-03 0.99 2.01E-05 0.99 水+5 mmol/L AA 4.40E-02 −0.30 5.07E-05 −0.30 5.85E-08 −0.30 XG+5 mmol/L AA 5.08E-01 0.47 5.99E-04 0.48 7.07E-07 0.48 水+1 mmol/L AA −7.63E-02 −0.21 −5.52E-04 −0.22 −4.00E-06 −0.22 0.1% XG+1 mmol/L AA 2.27E-01 0.27 1.62E-03 0.28 1.16E-05 0.28 0.2% XG+1 mmol/L AA 2.29E-01 0.81 1.66E-03 0.82 1.21E-05 0.82 0.4% XG+1 mmol/L AA 4.09E-01 0.82 3.21E-03 0.82 2.52E-05 0.83 水+1 mmol/L AA-30 ℃ 2.75E-01 0.64 1.79E-03 0.64 1.16E-05 0.65 XG+1 mmol/L AA-30 ℃ 1.02E-01 0.52 6.62E-04 0.52 4.29E-06 0.53 水+1 mmol/L AA-60 ℃ 4.36E-01 0.87 2.85E-03 0.88 1.86E-05 0.89 XG+1 mmol/L AA-60 ℃ 4.40E-01 0.99 2.97E-03 0.99 2.01E-05 0.99 水+1 mmol/L AA-90 ℃ 4.16E-01 0.63 3.16E-03 0.64 2.39E-05 0.64 XG+1 mmol/L AA-90 ℃ 1.79E-01 0.37 2.03E-03 0.38 2.32E-05 0.39 注:若无明确标注,表中XG浓度为0.2%(w/v),反应体系为60 ℃反应1 h后静置于23 ℃。 -
[1] DU J, CULLEN J J, BUETTNER G R. Ascorbic acid:Chemistry, biology and the treatment of cancer[J]. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer,2012,1826(2):443−457. doi: 10.1016/j.bbcan.2012.06.003
[2] CHAUDHRY A, BASHIR F, ADIL S F, et al. Ascorbic acid-mediated Fe/Cu nanoparticles and their application for removal of COD and phenols from industrial wastewater[J]. Journal of King Saud University-Science,2022,34(4):101927. doi: 10.1016/j.jksus.2022.101927
[3] NJUS D, KELLEY P M, TU Y J, et al. Ascorbic acid:The chemistry underlying its antioxidant properties[J]. Free Radical Biology and Medicine,2020,159:37−43. doi: 10.1016/j.freeradbiomed.2020.07.013
[4] ZHANG X, BLACKMAN J W, CLARK A C. Ascorbic acid addition to rosé:Impact on the oxidative and reductive development of bottled wine[J]. Food Chemistry,2023,424:136418. doi: 10.1016/j.foodchem.2023.136418
[5] 郑伟, 张相钊, 邓鑫峰, 等. 枇杷原浆护色配方优化及贮藏期间品质变化[J]. 食品工业科技,2024,45(14):184−193. [ZHENG W, ZHANG X Z, DENG X F, et al. The optimization of loquat puree color protection formula and quality change during storage[J]. Science and Technology of Food Industry,2024,45(14):184−193.] ZHENG W, ZHANG X Z, DENG X F, et al. The optimization of loquat puree color protection formula and quality change during storage[J]. Science and Technology of Food Industry, 2024, 45(14): 184−193.
[6] 杨晓聪, 陈晖, 陈顺心, 等. 不同抑制剂对鲜榨香蕉汁的防褐变影响[J]. 农产品加工,2023(23):12−15,19. [YANG X C, CHEN H, CHEN S X, et al. Effects of different inhibitors on anti-browning of fresh banana juice[J]. Farm Products Processing,2023(23):12−15,19.] YANG X C, CHEN H, CHEN S X, et al. Effects of different inhibitors on anti-browning of fresh banana juice[J]. Farm Products Processing, 2023(23): 12−15,19.
[7] 成晨亚琼, 赵鹏涛, 王晓宇, 等. 苹果汁褐变及抗氧化剂护色机理研究进展[J]. 食品工业科技,2022,43(18):447−455. [CHENG C Y Q, ZHAO P T, WANG X Y, et al. Research progress in the browning mechanism of apple juice and their color protection mechanism by antioxidants[J]. Science and Technology of Food Industry,2022,43(18):447−455.] CHENG C Y Q, ZHAO P T, WANG X Y, et al. Research progress in the browning mechanism of apple juice and their color protection mechanism by antioxidants[J]. Science and Technology of Food Industry, 2022, 43(18): 447−455.
[8] TU Y J, NJUS D, SCHLEGEL H B. A theoretical study of ascorbic acid oxidation and HOO•/O2•− radical scavenging[J]. Organic & Biomolecular Chemistry,2017,15(20):4417−4431.
[9] ROIG M G, RIVERA Z S, KENNEDY J F. L-ascorbic acid:An overview[J]. International Journal of Food Sciences and Nutrition,1993,44(1):59−72. doi: 10.3109/09637489309017424
[10] 李洁, 李彩云, 舒佳欣, 等. 抗坏血酸降解机理以及脱氢抗坏血酸对食品的影响研究进展[J]. 现代食品科技,2022,38(10):329−336. [LI J, LI C Y, SHU J X, et al. Research progress on degradation mechanism of ascorbic acid and effects of dehydroascorbic acid on food properties[J]. Modern Food Science and Technology,2022,38(10):329−336.] LI J, LI C Y, SHU J X, et al. Research progress on degradation mechanism of ascorbic acid and effects of dehydroascorbic acid on food properties[J]. Modern Food Science and Technology, 2022, 38(10): 329−336.
[11] GÓMEZ RUIZ B, ROUX S, COURTOIS F, et al. Kinetic modelling of ascorbic and dehydroascorbic acids concentrations in a model solution at different temperatures and oxygen contents[J]. Food Research International,2018,106:901−908. doi: 10.1016/j.foodres.2018.01.051
[12] KIVELÄ R, NYSTRÖM L, SALOVAARA H, et al. Role of oxidative cleavage and acid hydrolysis of oat beta-glucan in modelled beverage conditions[J]. Journal of Cereal Science,2009,50(2):190−197. doi: 10.1016/j.jcs.2009.04.012
[13] ZHU X, CHEN J, WANG H, et al. Mechanism of viscosity reduction of okra pectic polysaccharide by ascorbic acid[J]. Carbohydrate Polymers,2022,284:119196. doi: 10.1016/j.carbpol.2022.119196
[14] FAURE A M, ANDERSEN M L, NYSTRÖM L. Ascorbic acid induced degradation of beta-glucan:Hydroxyl radicals as intermediates studied by spin trapping and electron spin resonance spectroscopy[J]. Carbohydrate Polymers,2012,87(3):2160−2168. doi: 10.1016/j.carbpol.2011.10.045
[15] MÄKELÄ N, SONTAG-STROHM T, MAINA N H. The oxidative degradation of barley β-glucan in the presence of ascorbic acid or hydrogen peroxide[J]. Carbohydrate Polymers,2015,123:390−395. doi: 10.1016/j.carbpol.2015.01.037
[16] JIN X, DU X, LIU G, et al. Efficient destruction of basic organo-nitrogenous compounds in liquid hydrocarbon fuel using ascorbic acid/H2O2 system under ambient condition[J]. Journal of Hazardous Materials,2023,459:132242. doi: 10.1016/j.jhazmat.2023.132242
[17] PILLAY NARRAINEN A, LOVELL P A. Mechanism and kinetics of free-radical degradation of xyloglucan in aqueous solution[J]. Polymer,2010,51(26):6115−6122. doi: 10.1016/j.polymer.2010.10.049
[18] HUNG L, HORAGAI Y, KIMURA Y, et al. Decomposition and discoloration of L-ascorbic acid freeze-dried with saccharides[J]. Innovative Food Science & Emerging Technologies,2007,8(4):500−506.
[19] SUN-WATERHOUSE D, SMITH B G, O’CONNOR C J, et al. Effect of raw and cooked onion dietary fibre on the antioxidant activity of ascorbic acid and quercetin[J]. Food Chemistry,2008,111(3):580−585. doi: 10.1016/j.foodchem.2008.04.023
[20] BHAT I M, WANI S M, MIR S A, et al. Advances in xanthan gum production, modifications and its applications[J]. Biocatalysis and Agricultural Biotechnology,2022,42:102328. doi: 10.1016/j.bcab.2022.102328
[21] 杨作乾, 陈学亭, 王曼, 等. 亲水胶体对马铃薯冷冻面团及烘焙特性的作用[J]. 食品与发酵工业,2024,50(19):244−250. [YANG Z Q, CHEN X T, WANG M, et al. Effects of hydrocolloids on quality of frozen potato dough and its baking characteristics[J]. Food and Fermentation Industries,2024,50(19):244−250.] YANG Z Q, CHEN X T, WANG M, et al. Effects of hydrocolloids on quality of frozen potato dough and its baking characteristics[J]. Food and Fermentation Industries, 2024, 50(19): 244−250.
[22] 姚欣鹏, 曹传爱, 孔保华, 等. 黄原胶浓度对猪血浆蛋白-黄原胶基油凝胶结构及功能性质的影响[J]. 食品工业科技,2024,45(17):95−104. [YAO X P, CAO C A, KONG B H, et al. Effect of xanthan gum concentration on structure and functional properties of porcine plasma protein-xanthan gum oleogel[J]. Science and Technology of Food Industry,2024,45(17):95−104.] YAO X P, CAO C A, KONG B H, et al. Effect of xanthan gum concentration on structure and functional properties of porcine plasma protein-xanthan gum oleogel[J]. Science and Technology of Food Industry, 2024, 45(17): 95−104.
[23] 朱俊坤, 韩四海, 刘建学, 等. 不同亲水胶体对百合浊汁饮料稳定性的影响[J]. 食品与发酵工业,2024,50(19):236−243. [ZHU J K, HAN S H, LIU J X, et al. Effect of different hydrocolloids on stability of lily cloudy beverage[J]. Food and Fermentation Industries,2024,50(19):236−243.] ZHU J K, HAN S H, LIU J X, et al. Effect of different hydrocolloids on stability of lily cloudy beverage[J]. Food and Fermentation Industries, 2024, 50(19): 236−243.
[24] AN Z, LIU Z, MO H, et al. Preparation of Pickering emulsion gel stabilized by tea residue protein/xanthan gum particles and its application in 3D printing[J]. Journal of Food Engineering,2023,343:111378. doi: 10.1016/j.jfoodeng.2022.111378
[25] NSENGIYUMVA E M, ALEXANDRIDIS P. Xanthan gum in aqueous solutions:Fundamentals and applications[J]. International Journal of Biological Macromolecules,2022,216:583−604. doi: 10.1016/j.ijbiomac.2022.06.189
[26] RAMADHANY P, IRAWAN G. The influence of xanthan gum and lemon juice on the quality of tomato sorbet[J]. Journal of Food Technology and Industry,2022,33(2):148−156.
[27] 胡志群, 王惠聪, 胡桂兵. 高效液相色谱测定荔枝果肉中的糖、酸和维生素C[J]. 果树学报,2005,22(5):582−585. [HU Z Q, WANG H C, HU G B. Measurement of sugars, organic acids and vitamin C in litchi fruit by high performance liquid chromatography[J]. Journal of Fruit Science,2005,22(5):582−585.] doi: 10.3969/j.issn.1009-9980.2005.05.035 HU Z Q, WANG H C, HU G B. Measurement of sugars, organic acids and vitamin C in litchi fruit by high performance liquid chromatography[J]. Journal of Fruit Science, 2005, 22(5): 582−585. doi: 10.3969/j.issn.1009-9980.2005.05.035
[28] ZOU M Y, NIE S P, YIN J Y, et al. Ascorbic acid induced degradation of polysaccharide from natural products:A review[J]. International Journal of Biological Macromolecules,2020,151:483−491. doi: 10.1016/j.ijbiomac.2020.02.193
[29] KIVELÄ R, GATES F, SONTAG-STROHM T. Degradation of cereal beta-glucan by ascorbic acid induced oxygen radicals[J]. Journal of Cereal Science,2009,49(1):1−3. doi: 10.1016/j.jcs.2008.09.003
[30] MONTAÑO A, CASADO F J, REJANO L, et al. Degradation kinetics of the antioxidant additive ascorbic acid in packed table olives during storage at different temperatures[J]. Journal of Agricultural and Food Chemistry,2006,54(6):2206−2210. doi: 10.1021/jf058159o
[31] LI J, LI S, ZHENG Y, et al. Fast preparation of rhamnogalacturonan I enriched low molecular weight pectic polysaccharide by ultrasonically accelerated metal-free Fenton reaction[J]. Food Hydrocolloids,2019,95:551−561. doi: 10.1016/j.foodhyd.2018.05.025
[32] MÄKELÄ N, SONTAG-STROHM T, SCHIEHSER S, et al. Reaction pathways during oxidation of cereal β-glucans[J]. Carbohydrate Polymers,2017,157:1769−1776. doi: 10.1016/j.carbpol.2016.11.060
[33] IURLARO A, DALESSANDRO G, PIRO G, et al. Evaluation of glycosidic bond cleavage and formation of oxo groups in oxidized barley mixed-linkage β-glucans using tritium labelling[J]. Food Research International,2014,66:115−122. doi: 10.1016/j.foodres.2014.09.008






 下载:
下载:

 下载:
下载:



