Enhancing the Ability of Escherichia coli to Synthesise L-Isoleucine Using λ-Red Recombinant Technology Combined with Complex Mutagenesis
-
摘要: 本研究旨在通过λ-Red重组技术结合复合诱变方法提高大肠杆菌L-异亮氨酸合成能力。以E. coli NXA为出发菌株,首先采用λ-Red同源重组敲除编码支链氨基酸转运蛋白基因brnQ,获得突变菌株E. coli NXA1。然后将E. coli NXA1经常温常压等离子体(ARTP)、紫外(UV)与亚硝基胍(NTG)多轮复合诱变,以α-氨基丁酸(α-AB)为结构类似物进行筛选,筛选得到突变菌株E. coli NXA2。摇瓶发酵结果表明,在37 ℃、200 r/min条件下发酵40 h后,E. coli NXA1的L-异亮氨酸滴度为2.76 g/L,较E. coli NXA提高了33.98%;E. coli NXA2的L-异亮氨酸滴度为3.22 g/L,较E. coli NXA1提高了16.67%,较E. coli NXA提高了56.31%。对菌株E. coli NXA2经连续传代20代后,表现出较好的遗传稳定性。λ-Red重组技术结合复合诱变对大肠杆菌提高L-异亮氨酸合成能力有明显效果,为选育L-异亮氨酸高产菌株奠定理论基础。Abstract: To improve the synthetic ability of E. coli L-isoleucine by combining λ-Red recombination with complex mutagenesis. Firstly, taking E. coli NXA as the original strain, λ-Red homologous recombination was used to knock out the coding gene brnQ of branched chain amino acid transport protein to obtain mutant strain E. coli NXA1. Secondly, E. coli NXA1 was subjected to multiple rounds of complex mutagenesis with atmospheric room temperature plasma (ARTP), ultraviolet (UV), and nitrosoguanidine (NTG), which was screened to obtain the mutant strain E. coli NXA2 with α-AB being structural analogue. The fermentation results showed that L-isoleucine titer of E. coli NXA1 was 2.76 g/L, which was 33.98% higher than E. coli NXA after fermentation for 40 hours at 37 ℃ and 200 r/min. The L-isoleucine titer of E. coli NXA2 was 3.22 g/L, which was 16.67% higher than E. coli NXA1 and 56.31% higher than E. coli NXA. After 20 continuous passages of E. coli NXA2, the good genetic stability could be reflected. The combination of λ-Red recombination and complex mutagenesis has a significant effect on improving the synthetic ability of E. coli L-isoleucine, laying a theoretical basis for the breeding of L-isoleucine high-producing strains.
-
Keywords:
- Escherichia coli /
- λ-Red recombinant technology /
- brnQ gene /
- complex mutagenesis /
- fermentation /
- L-isoleucine
-
L-异亮氨酸是一种重要的支链氨基酸[1],在食品[2]、化工及饲料[3]等多个行业中都广泛应用[4],主要通过微生物发酵法生产。支链氨基酸转运蛋白关键基因brnQ可以促进L-异亮氨酸的摄入,使细胞内L-异亮氨酸增加,增强L-异亮氨酸对苏氨酸脱水酶与乙酰羟基酸合成酶等限速酶的反馈抑制,所以支链氨基酸转运蛋白基因brnQ的敲除对L-异亮氨酸产量的合成有重要影响[5]。Leyval等[6]通过控制发酵培养条件提高合成途径酶活、分析代谢通路关键酶与其底物的亲和力及支链氨基酸的反馈抑制;Xie等[7]通过敲除谷氨酸棒杆菌brnQ基因使L-异亮氨酸产量为22.3 g/L,较出发菌株(20.2 g/L)提高了10.4%。
λ-Red同源重组系统是大肠杆菌中最常用的基因重组系统[8],该系统整个操作过程简单、高效[9],这种仅依靠局部单基因敲除不足以最大限度地提高目标氨基酸生物合成的碳通量[10−11],传统诱变可以诱导DNA损伤,同时在多个基因中产生更多样化的突变,提高基因编辑的速度和效率,从而提高L-异亮氨酸生物合成碳通量。因此,需要将代谢工程与诱变技术相结合。常压室温等离子体(Atmosphe Reicandroom Temperature Plasma,ARTP)是一种新开发的诱变工具[12],该技术操作简便[13−14]、效率高[15]。紫外诱变最有效的波长为253~265 nm[16−17]。亚硝基胍(Nitrosoguanidine,NTG)具有突变效率高、易于实施的优点[18]。诸多策略独立运用能够达成既定的变异特征,然而迭代施用单一技术可能会减缓变异频率。相较之下,综合诱变技术有效缓解菌株抗性形成[19−20]。因此,基于λ-Red同源重组技术和复合诱变构建和筛选出更理想的高产菌株,有着重要的意义。苏氨酸脱水酶是L-异亮氨酸合成途径中的关键限速酶,L-缬氨酸对其有反馈抑制作用,α-氨基丁酸(α-AB)是L-缬氨酸的结构类似物,选育α-AB的抗性突变菌,可解除L-缬氨酸对苏氨酸脱水酶的反馈抑制作用[21],从而提高L-异亮氨酸的产量。
本研究以E. coli NXA作为出发菌株,采用λ-Red基因重组技术敲除编码转运蛋白基因brnQ,并结合ARTP、紫外与NTG进行复合诱变处理菌株,α-AB作为结构类似物进行筛选,以L-异亮氨酸浓度为主要筛选指标,旨在获得高产L-异亮氨酸的大肠杆菌并为其后续改造及提高L-异亮氨酸产量奠定基础。
1. 材料与方法
1.1 材料与仪器
Escherichia coli NXA 宁夏大学食品与科学工程学院重点实验室保存;氨苄青霉素(Amp)、氯霉素(Cm)、L-阿拉伯糖 北京索莱宝科技有限公司;酵母粉、琼脂粉 范德生物科技有限公司;蛋白胨、NaCl、葡萄糖、硫酸铵、磷酸二氢钾、磷酸氢二钾、碳酸钙、硫酸镁、硫酸亚铁 大茂化学试剂有限公司;LB培养基 上海阿拉丁科技股份有限公司;L-异亮氨酸标准品、SOC培养基、苏氨酸、蛋氨酸、赖氨酸 美国赛默飞世尔科技公司;玉米浆、维生素B1、邻苯二甲醛 上海麦克林生化科技股份有限公司;生物素 碧云天生物技术有限公司;上述试剂均为分析纯。
SBA生物传感分析仪 山东省科学院生物研究所;LC-16高效液相色谱仪 通微分析技术有限公司;安捷伦C18(25 mm×4.6 mm,3.5 µm) 安捷伦科技有限公司;LRH-150-B生化恒温培养箱 上海一恒科技有限公司;UNano-2000核酸纯度测定仪 德国伯赫公司;BioRad T100 常规PCR仪 德国艾本德股份公司;OI-BlueBox凝胶成像仪、Celetrix SP100细胞电转仪 台州赛瑞崔克生物技术有限公司;TGL-16M台式高速冷冻离心机 湖南湘仪实验室仪器开发有限公司。
1.2 实验方法
1.2.1 培养基的制备
大肠杆菌生长培养基为LB培养基,发酵培养基为葡萄糖30.0 g/L、硫酸铵20.5 g/L、玉米浆15 ml/L、磷酸二氢钾2.0 g/L、磷酸氢二钾3.0 g/L、碳酸钙2.5 g/L、硫酸镁2.0 g/L、硫酸亚铁0.01 g/L、苏氨酸0.1g/L、蛋氨酸0.1 g/L、赖氨酸0.1 g/L、生物素0.5 g/L、维生素B11.0 g/L、氯化钠2.0 g/L。调节pH为7.0,121 ℃条件下灭菌15 min。固体培养基在LB液体培养基基础上添加2%琼脂粉。
1.2.2 菌株的活化与菌悬液的制备
将−80 ℃甘油管中保存的菌株解冻后,取100 μL接种于含10 mL LB肉汤培养基的试管中,于37 ℃静置培养12 h,活化3代后,用于后续的发酵实验。接种LB培养基中,培养温度37 ℃、摇床转速220 r/min,培养至对数期。用生理盐水稀释菌液将菌密度控制在107 CFU/mL。制得菌悬液,备用。
1.2.3 brnQ基因敲除
从E. coli DH5α中分别提取质粒pKD3、pKD46和pCP20用于后续基因敲除实验。
brnQ基因敲除参考Yamamoto等[22]的方法:通过PCR扩增融合含靶基因上下游各50 bp同源臂和质粒pKD3中氯霉素抗性基因(Cm)的打靶片段。将携带温敏型质粒pKD46的E. coli NXA单菌落接种至含有50 µg/mL氨苄抗性(Amp)的LB中,过夜培养。以1%(v/v)接种量转接至含有50 µg/mL AmpLB中,在OD600 nm为0.2时加入终浓度为10 mmol/L阿拉伯糖,继续培养至OD600 nm为0.5时,离心收集菌体并制备感受态。将5 μg打靶片段与感受态混匀,在电压2.5 kV下进行电转化,加入900 μL SOC培养基复苏3 h,涂布于LB平板上,经筛选验证重组成功的菌株在42 ℃条件下培养来消除pKD46。再导入pCP20质粒,涂布于Cm抗性平板中,30 ℃过夜培养,采用brnQ-outF、brnQ-outR 引物对单菌落进行验证并测序,经42 ℃培养消除pCP20质粒后最终得到菌株E. coli NXA1。引物如表1所示。
表 1 引物序列Table 1. Sequence of primers引物名称 序列(5’-3’) F-FRT AAGCAGCTCCAGCCTACACACTGTTGATTTGTACAGTGTC R-FRT CTAAGGAGGATATTCATATGAATGTAGCACCAAGTGAATA brnQ-F CATATGAATATCCTCCTTAGTTCCTATTC brnQ-R GAGCTGCTTCGAAGTTCCTA brnQ-upF TCCACAGGCAGTATGATTTATGACCCATCAATTAAGATCGCGCGATATC brnQ-upR CGCAGCCCCGTGGTTAAAACAAATGTTCAGTGATTTAGTGAGCGCTGG brnQ-outF AATATACCGCTGTATCATCCCCAGG brnQ-outR AGTGCCATAAAGCGTATGTGGC 1.2.4 菌株生长曲线的绘制
将大肠杆菌接种于LB液体培养基,于37 ℃过夜培养14~16 h,再以10%(v/v)的接种量接种到发酵培养基中,于37 ℃摇床培养。从0 h开始,每隔2 h,取适量菌悬液测其600 nm处的吸光值,以培养时间为横坐标,OD600 nm值为纵坐标,绘制菌株的生长曲线。
1.2.5 ARTP诱变
将敲除基因的大肠杆菌活化两次并将其转移至装液量为20 mL的100 mL锥形瓶中,置于恒温摇床中37 ℃、200 r/min过夜培养,培养至对数期,菌液离心后重悬液体,控制OD600 nm为0.6~0.7,取10 μL菌悬液均匀涂到经过灭菌的ARTP专用载片上[23],设置仪器功率110 W,气流量参数10 SML,诱变时间设置为5、10、15、20、25、30、35 s[24]。诱变结束后,充分洗脱载片,稀释一定倍数后取100 μL涂布于LB固体培养基平板上,温度37 ℃,过夜培养。采用菌落计数法计算致死率,并绘制致死率曲线,通过产量变化计算正突变率,并绘制致死率曲线与正突变率曲线图,致死率与正突变率计算公式如下。最后,将选定的菌落在新鲜培养基上纯化,并在α-AB抗性平板上测试最终耐受性,步骤如1.2.6。选择最耐受的菌株进行紫外诱变。每个实验均采取三个平行,测试结果以平均值表示。
致死率(%)=对照组菌落总数−ARTP诱变处理菌落总数对照组菌落总数×100 正突变率(%)=产量增加超过5%的突变菌落总数突变菌落数总数(产量变化5%以上)×100 1.2.6 突变菌株的筛选
将配制好的17 g/L的α-AB按梯度稀释,分别稀释制成终浓度为 17、16、15、14、13 g/L抗性平板,将每一次诱变得到的菌株分别涂布于不同浓度的抗性平板上。37 ℃培养12 h,筛选菌落较大的为目标菌。
1.2.7 UV诱变
将诱变后的大肠杆菌活化5次,培养至对数期,取10 mL菌液于灭过菌的平皿中[25],平皿置于磁力搅拌器上,垂直摆放在波长为254 nm的紫外灯下[26],在25 cm距离处进行紫外线照射,照射时间分别为0、2、4、6、8、10、12、14、16、18、20 s[27]。照射完毕后,在红光条件下进行后续操作,同时将诱变菌悬液置于黑暗冰箱1 h,抑制突变菌株修复酶活,提高突变效率[28],移取0.1 mL稀释至10−4、10−5、10−6的菌悬液涂布于LB固体培养基上,于30 ℃避光培养12 h,采用菌落计数法计算致死率,并绘制致死率曲线,通过产量变化计算正突变率,并绘制致死率曲线与正突变率曲线图,致死率计算公式如下,正突变率计算公式如1.2.5。最后,将选定的菌落在新鲜培养基上纯化,并在α-AB抗性平板上测试最终耐受性,步骤如1.2.6。选择最耐受的菌株进行NTG诱变。每个实验均采取三个平行,测试结果以平均值表示。
致死率(%)=未照射平板的菌落数−照射平板的菌落数未照射平板的菌落数×100 1.2.8 NTG 诱变
1.2.8.1 诱变处理
称取50 mg NTG于5 mL棕色容量瓶中,用丙酮溶液定容,配制成NTG母液,逐级稀释,用丙酮溶液定容,得NTG质量浓度为100、500、1000 μg/mL的NTG溶液,备用。
1.2.8.2 致死率计算
分别用100、500、1000 μg/mL的NTG溶液浓度去进行化学诱变,诱变时间为10、20、30、40、50、60 min[29]。将不同时间诱变菌株取样后立即稀释1000倍终止反应[30];梯度稀释后的菌悬液涂布于LB固体培养基上,于37 ℃培养12 h,采用菌落计数法计算致死率,并绘制致死率曲线,通过产量变化计算正突变率,并绘制致死率曲线与正突变率曲线图,致死率计算公式如下,正突变率计算公式如1.2.5。最后,将选定的菌落在新鲜培养基上纯化,并在α-AB抗性平板上测试最终耐受性,步骤如1.2.6。选择最耐受的菌株进行下一步试验。每个实验均采取三个平行,测试结果以平均值表示。
致死率(%)=未经化学诱变的菌落数−化学诱变的菌落数未经化学诱变的菌落数×100 1.2.9 遗传稳定性验证
将复合诱变后的菌株接种到LB液体培养基进行富集培养24 h,为I代菌株;I代菌株移植,平板培养,即II代菌株;转移II代菌株,平板培养,即III代菌株。以此类推,这些菌株接种了20代。比较每一代菌株的生长活性与L-异亮氨酸产量,以验证遗传稳定性。
1.2.10 柱前衍生HPLC法测定发酵液中L-异亮氨酸浓度
配制标准溶液:准确称取0.05 g的L-异亮氨酸标品,将其溶于10 mL的ddH2O中,制成5 g/L的L-异亮氨酸标准液,将其稀释为浓度为1~5 g/L的标准液。以外标法计算发酵液中L-异亮氨酸的含量。
将出发菌株E. coli NXA、敲除brnQ基因的菌株E. coli NXA1及诱变筛选得到的菌株E. coli NXA2分别接种到20 mL液体培养基中,37 ℃,200 r/min培养16 h得到发酵种子液,将该种子接种至总体积为500 mL的摇瓶(内含50 mL培养基)中进行发酵培养。发酵条件为摇床转速200 r/min,温度37 ℃,用50%(v/v)氨水溶液控制发酵过程pH为7.0。发酵40 h,每隔4 h取一次样,将菌液进行过膜处理,采用HPLC检测异亮氨酸含量,2,4-二硝基氟苯衍生法对样品进行柱前衍生化处理[31]。
衍生程序:取0.1 mL标品溶液,加入到1 mL棕色EP管中,再分别加入硼酸缓冲溶液和2,4-二硝基氟苯各0.1 mL,放入60 ℃水浴中,暗处反应60 min。取出,冷却至室温,用平衡缓冲溶液(pH7.0磷酸缓冲溶液)定容至1.0 mL,静置20 min,取20 µL进样分析。
色谱条件:流速1 mL/min;检测波长338 nm;柱温28 ℃;进样量20 µL;梯度洗脱程序如表2所示。
表 2 高效液相梯度洗脱程序Table 2. High performance liquid phase gradient elution program时间(min) 流速(mL/min) 流动相体积分数(%) A B 0 1.0 70 30 15 1.0 63 37 32 1.0 43 57 41 1.0 35 65 46 1.0 10 90 1.3 数据处理
采用Excel 2019与Origin对数据进行整理和作图。每组实验平行测定3次,结果以“平均值±标准差”表示。
2. 结果与分析
2.1 大肠杆菌brnQ基因的敲除
以pKD3质粒为模板进行打靶片段制备,如图1A所示,PCR扩增出1106 bp左右大小的条带,大小正确;基因工程菌E. coli NXA用引物brnQ-upF/brnQ-upR进行PCR验证,如图1B泳道4所示,扩增出1535 bp左右大小的条带,大小正确;E. coli NXA1用引物brnQ-outF/brnQ-outR进行PCR验证,如图1B泳道3所示,扩增出1266 bp左右大小的条带,电泳结果显示敲除了正确大小的条带,brnQ基因敲除成功。用brnQ-outF和brnQ-outR这对引物再次进行PCR扩增鉴定,如图1B泳道2所示,氯霉素抗性基因消除的克隆产物长度缩短为334 bp。从PCR产度判断,此克隆的氯霉素抗性基因已被删除,PCR产物结果与设计一致。通过λ-Red同源重组基因编辑技术敲除brnQ基因,得到了菌株E. coli NXA1,L-异亮氨酸滴度较出发菌株E. coli NXA提高了33.98%。
2.2 复合诱变条件确定
选用培养14~16 h对数期生长菌株进行复合诱变[32],如图2、图3所示,当ARTP处理时间25 s,致死率为90.37%,此时,正突变率最高,达到20.14%;当紫外照射时间为10 s时,致死率为92.39%,正突变率最高,为11.72%。随着处理时间的延长及NTG诱变剂量的增大,菌株致死率在不断增大。NTG 浓度较低时,诱变效果不佳,浓度过高会影响菌落再生,不利于筛选,选择NTG浓度500 µg/mL,处理时间为40 min正突变率最大,为26.24%,致死率为91.55%。每次诱变后都用α-AB筛选高耐受性菌株,达到能够解除L-异亮氨酸反馈抑制的浓度;ARTP-UV-NTG复合诱变后,进行致死率和正突变率的计算,三次重复实验结果见图2、图3及表3。Lu等[33]通过UV、LA、UV-LA的阳性突变率结果表明复合突变的阳性突变率高于单独突变。综上所述,为提高微生物发生突变的概率并降低回复突变率,复合诱变最终选择ARTP处理时间25 s;紫外照射时8 s;NTG 浓度500 µg/mL,处理时间 40 min的条件筛选得到突变菌株E. coli NXA2。
表 3 ARTP-UV-NTG复合诱变对大肠杆菌的影响Table 3. Effects of ARTP-UV-NTG complex mutagenesis on E. coli重复 致死率(%) 正突变率(%) 1 91.64±1.71 25.72±1.13 2 90.45±1.21 26.24±0.92 3 90.27±1.53 25.92±0.84 平均值 90.79 25.96 2.3 抗α-AB突变菌株的筛选
在L-异亮氨酸的生物合成途径中,苏氨酸脱水反应是第一个限速步骤,苏氨酸脱水酶受L-缬氨酸的反馈抑制。因此,选育L-缬氨酸结构类似物抗性的突变株可以从遗传上减弱或解除这些反馈抑制作用,增强L-异亮氨酸合成途径的代谢流,提高L-异亮氨酸的产出, 张伟国[34]通过DES、UV和NTG复合诱变结合结构类似物抗性筛选,使L-异亮氨酸积累达到3%。本研究利用平板进行初筛,初筛实验结果如图4所示,菌株E. coli NXA1 α-AB最高使用浓度为12 g/L,E. coli NXA2 α-AB最高使用浓度为16 g/L。α-AB通过遗传性地解除支链氨基酸对乙酰羟基酸合成酶的协同反馈阻遏以及L-缬氨酸对乙酰羟基酸合成酶的反馈抑制作用[21],提高菌株E. coli NXA2 L-异亮氨酸的合成能力。孔帅[35]通过添加临界致死浓度的α-氨基丁酸筛选得到L-异亮氨酸产量提高16.2%的优势菌株。
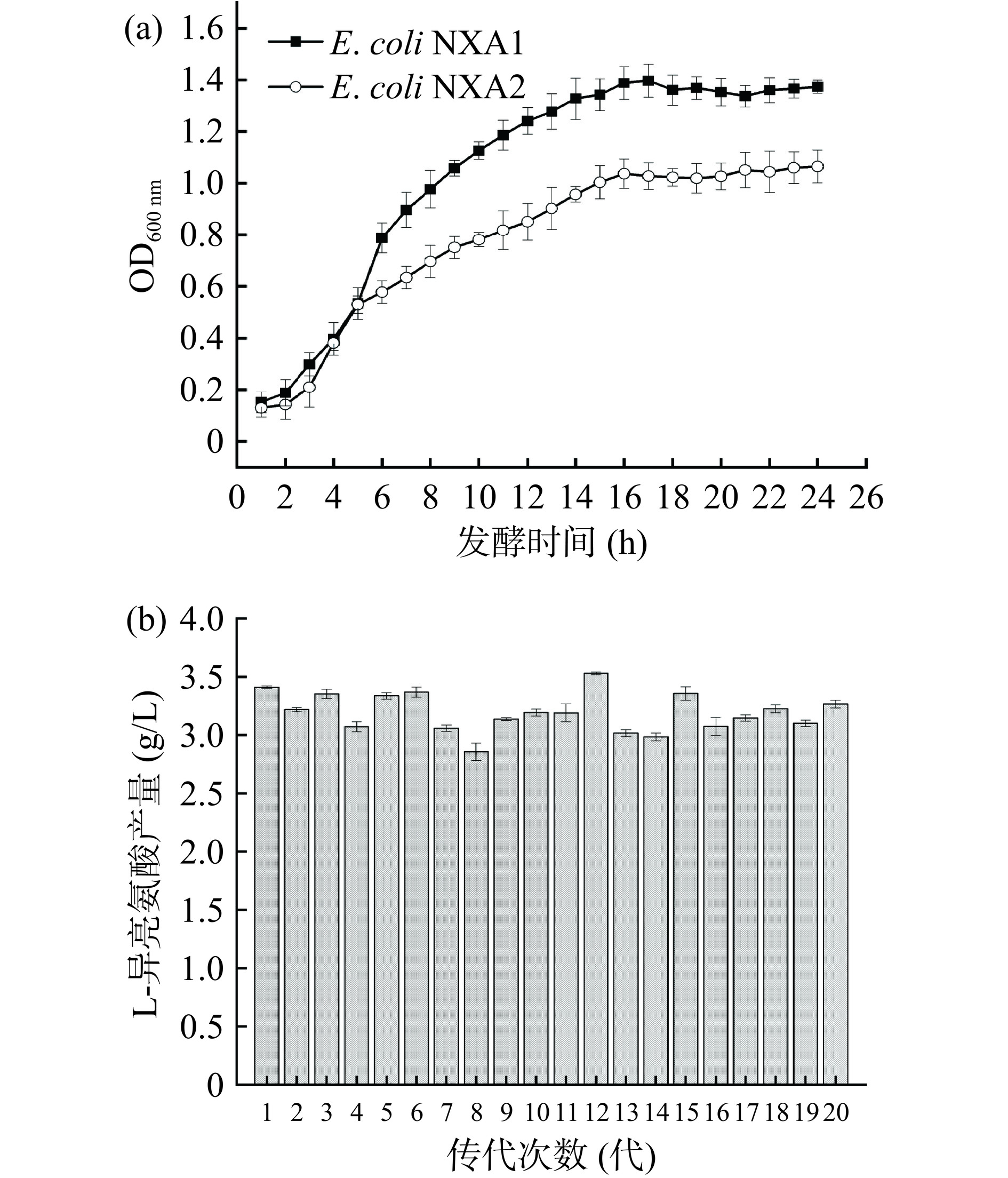
2.4 E. coli NXA2遗传稳定性测定
将筛选出的突变菌株E. coli NXA2连续传代培养20次后与E. coli NXA1进行生长曲线测定,E. coli NXA2每一次传代后培养40 h后摇瓶发酵测定L-异亮氨酸产量,结果见图5。根据图5(a)可以看出E. coli NXA1在6 h菌体含量出现了快速增长,7~22 h菌体浓度逐渐增长,达到生长对数期后趋于稳定;E. coli NXA2在3~5 h菌体含量快速增长,6~17 h菌体含量缓慢增长至对数期,之后趋于稳定;筛选出的菌株E. coli NXA2经过传代培养 20 次后仍能正常生长,将该突变菌株每一次传代后都进行摇瓶发酵,根据图5(b)看出连续传代发酵液中 L-异亮氨酸浓度相对稳定,说明突变菌株E. coli NXA2遗传稳定性良好。
2.5 突变菌株的L-异亮氨酸摇瓶发酵结果分析
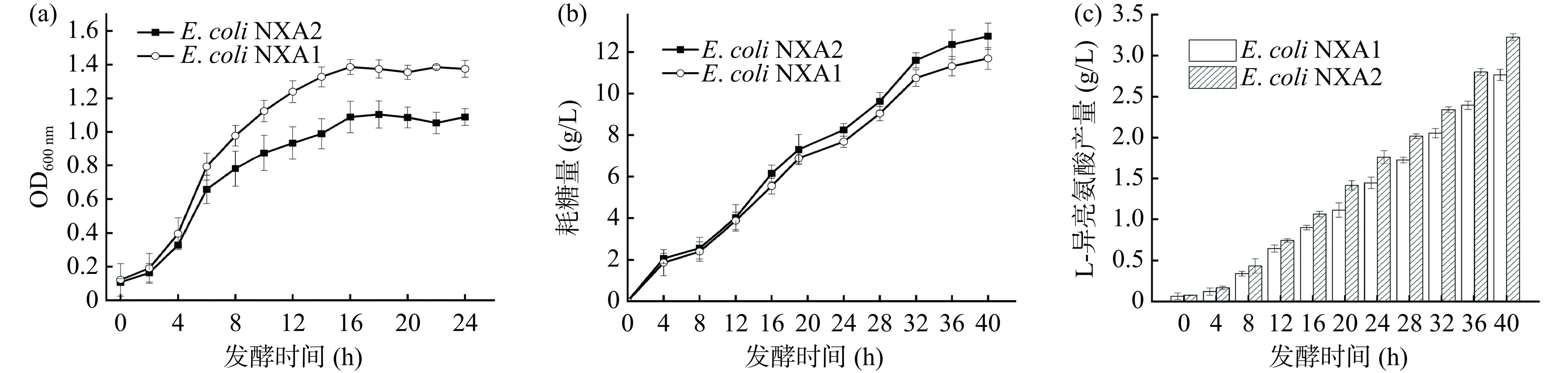
首先将菌株 E. coli NXA和E. coli NXA1同时摇瓶发酵40 h,每隔4 h采集一次样品,在600 nm处测定发酵液的吸光值,取上清液用生物传感分析仪检测残糖含量,同时用HPLC检测L-异亮氨酸含量。如图6(a)和图6(b)所示,在发酵前16 h,菌株E. coli NXA1的生长速率快于菌株E. coli NXA,菌体浓度也高于菌株E. coli NXA,其耗糖量也高于菌株E. coli NXA;20 h后菌株生长趋于平缓,但是耗糖量明显高于E. coli NXA,L-异亮氨酸产量只是在缓缓积累,主要能量还是提供给菌株的生长;当菌株进入稳定期后,L-异亮氨酸累积加快;发酵40 h后,由图6(c)可知,菌株E. coli NXA L-异亮氨酸产量为(2.06±0.06)g/L;菌株E. coli NXA1 L-异亮氨酸产量为(2.76±0.02)g/L,较E. coli NXA提高了33.98%。E. coli NXA1菌株生长的更快,消耗更多的葡萄糖,产生更多的L-异亮氨酸,可能是通过阻断L-异亮氨酸的摄入,使细胞内L-异亮氨酸减少,可能削弱了L-异亮氨酸对苏氨酸脱水酶与乙酰羟基酸合成酶等限速酶的反馈抑制。
将菌株 E. coli NXA1和E. coli NXA2同时摇瓶发酵,发酵条件同上。如图7(a)和图7(b)所示,在发酵前6 h,菌株E. coli NXA1的生长速率快于菌株E. coli NXA2,菌体浓度也高于菌株E. coli NXA2;发酵16~24 h,菌株E. coli NXA1持续生长,而E. coli NXA2已经趋于平缓;24 h后菌株E. coli NXA1生长趋于平缓;菌株E. coli NXA2的耗糖量始终高于E. coli NXA1,碳源可能更多的流向L-异亮氨酸合成途径;当发酵40 h后,由图7(c)可知,菌株E. coli NXA1 L-异亮氨酸产量为(2.72±0.04)g/L;菌株E. coli NXA2 L-异亮氨酸产量为(3.22±0.06)g/L,较E. coli NXA1 L-异亮氨酸产量增加了18.38%,由于α-AB通过遗传性地削弱L-缬氨酸对乙酰羟基酸合成酶的反馈抑制作用,增强了主代谢通路,减少支路代谢,从而提升大肠杆菌生产L-异亮氨酸的能力。
3. 结论
本研究在敲除L-异亮氨酸胞内转运关键蛋白的合成基因brnQ后,明显提高了大肠杆菌生产异亮氨酸的能力,工程菌E. coli NXA1 L-异亮氨酸产量比出发菌株提高了33.98%,之后针对工程菌E. coli NXA1 进行复合诱变选育,当ARTP处理时间25 s;紫外照射时间8 s;NTG 浓度500 µg/mL,处理时间40 min时,大肠杆菌E. coli NXA2 L-异亮氨酸的产量可以达到3.22 g/L,是出发菌株的1.56倍。本实验为通过基因敲除结合复合诱变育种提高大肠杆菌L-异亮氨酸合成能力提供一定的理论基础。
-
表 1 引物序列
Table 1 Sequence of primers
引物名称 序列(5’-3’) F-FRT AAGCAGCTCCAGCCTACACACTGTTGATTTGTACAGTGTC R-FRT CTAAGGAGGATATTCATATGAATGTAGCACCAAGTGAATA brnQ-F CATATGAATATCCTCCTTAGTTCCTATTC brnQ-R GAGCTGCTTCGAAGTTCCTA brnQ-upF TCCACAGGCAGTATGATTTATGACCCATCAATTAAGATCGCGCGATATC brnQ-upR CGCAGCCCCGTGGTTAAAACAAATGTTCAGTGATTTAGTGAGCGCTGG brnQ-outF AATATACCGCTGTATCATCCCCAGG brnQ-outR AGTGCCATAAAGCGTATGTGGC 表 2 高效液相梯度洗脱程序
Table 2 High performance liquid phase gradient elution program
时间(min) 流速(mL/min) 流动相体积分数(%) A B 0 1.0 70 30 15 1.0 63 37 32 1.0 43 57 41 1.0 35 65 46 1.0 10 90 表 3 ARTP-UV-NTG复合诱变对大肠杆菌的影响
Table 3 Effects of ARTP-UV-NTG complex mutagenesis on E. coli
重复 致死率(%) 正突变率(%) 1 91.64±1.71 25.72±1.13 2 90.45±1.21 26.24±0.92 3 90.27±1.53 25.92±0.84 平均值 90.79 25.96 -
[1] REN M C, SHAO M, LIANG H L, et al. Role of dietary isoleucine supplementation in facilitating growth performance and muscle growth in juvenile largemouth bass (Micropterus salmoides)[J]. Aquaculture Reports,2023,33:101783. doi: 10.1016/j.aqrep.2023.101783
[2] HAO Y N, PAN X W, YOU J J, et al. Microbial production of branched chain amino acids:Advances and perspectives[J]. Bioresource Technology,2024,397:130502. doi: 10.1016/j.biortech.2024.130502
[3] WENDISCH V F. Metabolic engineering advances and prospects for amino acid production[J]. Metabolic Engineering,2020,58:17−34. doi: 10.1016/j.ymben.2019.03.008
[4] 王壮壮, 魏佳, 于海波, 等. L-异亮氨酸高产菌选育及其培养基优化[J]. 生物技术通报,2019,35(1):82−89. [WANG Z Z, WEI J, YU H B, et al. Breeding of L-isoleucine producing bacteria and optimization of culture medium[J]. Biotechnology Bulletalin,2019,35(1):82−89.] WANG Z Z, WEI J, YU H B, et al. Breeding of L-isoleucine producing bacteria and optimization of culture medium[J]. Biotechnology Bulletalin, 2019, 35(1): 82−89.
[5] ZHANG Y C, LIU Y D, ZHANG S Y, et al. Metabolic engineering of Corynebacterium glutamicum WM001 to improve L-isoleucine production[J]. Biotechnology and Applied Biochemistry,2021,68(3):568−584. doi: 10.1002/bab.1963
[6] LEYVAL D, UY D, DELAUNAY S, et al. Characterisation of the enzyme activities involved in the valine biosynthetic pathway in a valine-producing strain of Corynebacterium glutamicum[J]. Journal of Biotechnology,2003,104(1):241−252.
[7] XIE X X, XU L L, SHI J M, et al. Effect of transport proteins on l-isoleucine production with the l-isoleucine-producing strain Corynebacterium glutamicum YILW[J]. Journal of Industrial Microbiology and Biotechnology,2012,39(10):1549−1556. doi: 10.1007/s10295-012-1155-4
[8] 李文静. 构建大肠杆菌双重选择系统的研究[D]. 合肥:合肥工业大学, 2023. [LI W J. Construction of a dual selection system for Escherichia coli[D]. Hefei:Hefei University of Technology, 2023.] LI W J. Construction of a dual selection system for Escherichia coli[D]. Hefei: Hefei University of Technology, 2023.
[9] 张忠喜, 顾金杰, 林汉标, 等. 基于Red重组系统的阴沟肠杆菌基因重组技术[J]. 食品工业科技,2018,39(21):134−140,151. [ZHANG Z X, GU J J, LIN H B, et al. Gene recombination technology of Enterobacterium cloacae based on red recombinant system[J]. Food Industry Technology,2018,39(21):134−140,151.] ZHANG Z X, GU J J, LIN H B, et al. Gene recombination technology of Enterobacterium cloacae based on red recombinant system[J]. Food Industry Technology, 2018, 39(21): 134−140,151.
[10] AKANIRO I R, OLADIPO A A, ONWUJEKWE E C. Metabolic engineering approaches for scale-up of fermentative biohydrogen production-A review[J]. International Journal of Hydrogen Energy,2024,52:240−264. doi: 10.1016/j.ijhydene.2023.04.328
[11] ZHOU Y P, SONG F, YANG H R, et al. Construction of a food-grade gene editing system based on CRISPR-Cas9 and its application in Lactococcus lactis NZ9000[J]. Biotechnology Letters,2023,45(8):955−966. doi: 10.1007/s10529-023-03398-4
[12] 潘丽娜, 唐溶雪, 康文丽, 等. 物理诱变和高通量筛选在益生菌选育中的应用[J]. 食品工业科技,2023,44(13):458−465. [PAN L N, TANG R X, KANG W L, et al. Application of physical mutagenesis and high-throughput screening in the selection and breeding of probiotics[J]. Food Industry Science and Technology,2023,44(13):458−465.] PAN L N, TANG R X, KANG W L, et al. Application of physical mutagenesis and high-throughput screening in the selection and breeding of probiotics[J]. Food Industry Science and Technology, 2023, 44(13): 458−465.
[13] HUANG W W, GE X Y, HUANG Y, et al. High‐yield strain of fusidic acid obtained by atmospheric and room temperature plasma mutagenesis and the transcriptional changes involved in improving its production in fungus Fusidium coccineum[J]. Journal of Applied Microbiology,2021,130(2):405−415. doi: 10.1111/jam.14797
[14] WANG Q, CHEN Y L, FU J J, et al. High-throughput screening of lycopene-overproducing mutants of Blakeslea trispora by combining ARTP mutation with microtiter plate cultivation and transcriptional changes revealed by RNA-seq[J]. Biochemical Engineering Journal,2020,161:107664. doi: 10.1016/j.bej.2020.107664
[15] SUN J R, LI J L, YAO L L, et al. UV-ARTP-DES compound mutagenesis breeding improves natamycin production of Streptomyces natalensis HW-2 and reveals transcriptional changes by RNA-seq[J]. Food Science and Biotechnology,2023,32(3):341−352. doi: 10.1007/s10068-022-01191-z
[16] YU L, LI F, NI J, et al. UV-ARTP compound mutagenesis breeding improves macrolactins production of Bacillus siamensis and reveals metabolism changes by proteomic[J]. Journal of Biotechnology,2024,381:36−48. doi: 10.1016/j.jbiotec.2023.12.011
[17] SIVARAMAKRISHNAN R, INCHAROENSAKDI A. Enhancement of lipid production in Scenedesmus sp. by UV mutagenesis and hydrogen peroxide treatment[J]. Bioresource Technology,2017,235:366−370. doi: 10.1016/j.biortech.2017.03.102
[18] WANG S, ZHANG L, YANG G P, et al. Breeding 3 elite strains of Nannochloropsis oceanica by nitrosoguanidine mutagenesis and robust screening[J]. Algal Research,2016,19:104−108. doi: 10.1016/j.algal.2016.07.021
[19] NING Z F, MA C Y, ZHONG W Z, et al. Compound mutation by ultraviolet and diethyl sulfate of protease producing thermophilic bacteria to hydrolyze excess sludge[J]. Bioresource Technology, 2024:130330.
[20] 赵志军, 赵婷, 刘延波, 等. 酯化型红曲菌复合诱变选育及其固态发酵条件优化[J]. 食品工业科技,2021,42(2):76−82. [ZHAO Z J, ZHAO T, LIU Y B, et al. Complex mutagenesis selection of esterifying Rhodococcus erythropolis and optimisation of its solid-state fermentation conditions[J]. Food Industry Science and Technology,2021,42(2):76−82.] ZHAO Z J, ZHAO T, LIU Y B, et al. Complex mutagenesis selection of esterifying Rhodococcus erythropolis and optimisation of its solid-state fermentation conditions[J]. Food Industry Science and Technology, 2021, 42(2): 76−82.
[21] 沈加彬, 罗磊, 施碧红, 等. L-异亮氨酸产生菌黄色短杆菌的选育[J]. 氨基酸和生物资源,2011,33(4):38−41. [SHEN J B, LUO L, SHI B H, et al. Breeding of L-isoleucine producing bacterium geltonoji bacila[J]. Amino acids and biological resources,2011,33(4):38−41.] SHEN J B, LUO L, SHI B H, et al. Breeding of L-isoleucine producing bacterium geltonoji bacila[J]. Amino acids and biological resources, 2011, 33(4): 38−41.
[22] YAMAMOTO S, IZUMIYA H, MORITA M, et al. Application of λ Red recombination system to Vibrio cholerae genetics:Simple methods for inactivation and modification of chromosomal genes[J]. Gene,2009,438(1):57−64.
[23] 陈尚里, 于福田, 沈圆圆, 等. 高产抗菌脂肽Fengycin芽孢杆菌的诱变育种和发酵条件优化[J]. 食品工业科技,2023,44(23):134−143. [CHEN S L, YU F T, SHEN Y Y, et al. Mutation breeding and fermentation condition optimization of Bacillus subtilis with high production of antibacterial peptide Fengxin[J]. Food Industry Technology,2023,44(23):134−143.] CHEN S L, YU F T, SHEN Y Y, et al. Mutation breeding and fermentation condition optimization of Bacillus subtilis with high production of antibacterial peptide Fengxin[J]. Food Industry Technology, 2023, 44(23): 134−143.
[24] 邹宗胜, 王婧雅, 赵运英, 等. 高产纤维素酶突变株的筛选及其产酶条件优化[J]. 食品科学,2019,40(6):48−54. [ZOU Z S, WANG J Y, ZHAO Y Y, et al. Screening of high-yield cellulase mutant strains and optimization of enzyme production conditions[J]. Food Science,2019,40(6):48−54.] ZOU Z S, WANG J Y, ZHAO Y Y, et al. Screening of high-yield cellulase mutant strains and optimization of enzyme production conditions[J]. Food Science, 2019, 40(6): 48−54.
[25] 夏俊芳, 王小灵, 古丽娜孜, 等. 递推式ARTP-UV复合诱变筛选高产β-葡萄糖苷酶菌株[J]. 食品工业科技,2020,41(15):129−134,142. [XIA J F, WANG X L, GURINAZ, et al. Recursive ARTP-UV complex mutagenesis screening of high-yielding β-glucosidase strains[J]. Food Industry Science and Technology,2020,41(15):129−134,142.] XIA J F, WANG X L, GURINAZ, et al. Recursive ARTP-UV complex mutagenesis screening of high-yielding β-glucosidase strains[J]. Food Industry Science and Technology, 2020, 41(15): 129−134,142.
[26] 黄玉, 尼玛扎西, 薛正莲, 等. ARTP与紫外线复合诱变选育高性能绿僵菌菌株[J]. 食品工业科技, 2020, 42(4):60−64,70. [HUANG Y, NI M Z X, XUE Z L, et al. Selection of high-performance Green Streptomyces strains by combined mutagenesis of ARTP and UV light[J]. Food Industry Technology, 42(4):60−64,70.] HUANG Y, NI M Z X, XUE Z L, et al. Selection of high-performance Green Streptomyces strains by combined mutagenesis of ARTP and UV light[J]. Food Industry Technology, 42(4): 60−64,70.
[27] 张昳. 复合诱变选育多杀菌素生产菌及性能研究[D]. 苏州:苏州科技大学, 2023. [ZHANG Y. Complex mutagenesis to select polymyxin-producing bacteria and its performance[D]. Suzhou:Suzhou University of Science and Technology, 2023.] ZHANG Y. Complex mutagenesis to select polymyxin-producing bacteria and its performance[D]. Suzhou: Suzhou University of Science and Technology, 2023.
[28] 杜敬彩, 赵刚, 李自云, 等. 亚硝基胍-紫外复合诱变选育高产衣康酸菌株[J]. 生物化工,2023,9(3):30−34. [DU J C, ZHAO G, LI Z Y, et al. Breeding of high-yield Itaconic acid strains through nitroso guanidine ultraviolet al composite mutagenesis[J]. Biochemical,2023,9(3):30−34.] DU J C, ZHAO G, LI Z Y, et al. Breeding of high-yield Itaconic acid strains through nitroso guanidine ultraviolet al composite mutagenesis[J]. Biochemical, 2023, 9(3): 30−34.
[29] XIE M, LI Q, HU X P, et al. Effects of a NTG-based chemical mutagenesis on the propamocarb-tolerance of the Nematophagous fungus Lecanicillium attenuatum[J]. Pesticide Biochemistry and Physiology,2017,141:71−75. doi: 10.1016/j.pestbp.2016.11.011
[30] 刘晨, 张丽萍. 亚硝基胍-紫外复合诱变筛选高产苯乳酸菌株[J]. 中国食品学报,2015,15(9):41−46. [LIU C, ZHANG L P. Nitrosoguanidine-ultraviolet compound mutagenesis screening of high-yielding phenyl lactic acid strains[J]. Chinese Journal of Food,2015,15(9):41−46.] LIU C, ZHANG L P. Nitrosoguanidine-ultraviolet compound mutagenesis screening of high-yielding phenyl lactic acid strains[J]. Chinese Journal of Food, 2015, 15(9): 41−46.
[31] 赵英莲, 牟德华, 李艳. 2,4-二硝基氟苯柱前衍生HPLC检测树莓中游离氨基酸[J]. 食品科学,2015,36(6):178−182. [ZHAO Y L, MOU D H, LI Y. HPLC det alection of free amino acids in raspberry using 2,4-dinitrofluorobenzene pre column derivatization[J]. Food Science,2015,36(6):178−182.] ZHAO Y L, MOU D H, LI Y. HPLC det alection of free amino acids in raspberry using 2,4-dinitrofluorobenzene pre column derivatization[J]. Food Science, 2015, 36(6): 178−182.
[32] 凌思雨, 王洲, 张会敏, 等. 常压室温等离子体诱变与微生物微液滴培养选育谷胱甘肽高产菌株[J]. 食品科学,2023,44(4):200−208. [LING S Y, WANG Z, ZHANG H M, et al. Breeding of high glutathione producing strains through atmospheric pressure room temperature plasma mutagenesis and microbial droplet al culture[J]. Food Science,2023,44(4):200−208.] LING S Y, WANG Z, ZHANG H M, et al. Breeding of high glutathione producing strains through atmospheric pressure room temperature plasma mutagenesis and microbial droplet al culture[J]. Food Science, 2023, 44(4): 200−208.
[33] LU F, CHAO J P, ZHAO X L, et al. Enhancing protease activity of Bacillus subtilis using UV-laser random mutagenesis and high-throughput screening[J]. Process Biochemistry,2022,119:119−127. doi: 10.1016/j.procbio.2022.05.018
[34] 张伟国. L-异亮氨酸产生菌选育的研究[J]. 氨基酸和生物资源,1996(3):1−5. [ZHANG W G. A study on the breeding of L-isoleucine producing bacteria[J]. Amino Acids and Biological Resources,1996(3):1−5.] ZHANG W G. A study on the breeding of L-isoleucine producing bacteria[J]. Amino Acids and Biological Resources, 1996(3): 1−5.
[35] 孔帅. 高产L-异亮氨酸菌种选育及其发酵特性研究[D]. 宜昌:三峡大学, 2022. [KONG S. Selection of high-yielding L-isoleucine strains and their fermentation characteristics[D]. Yichang:Three Gorges University, 2022.] KONG S. Selection of high-yielding L-isoleucine strains and their fermentation characteristics[D]. Yichang: Three Gorges University, 2022.





 下载:
下载:





 下载:
下载:



