Research Progress on Immune Regulation Activities of Marine Sulfate Polysaccharides
-
摘要: 随着现代生活方式的改变,免疫力低下成为了越来越多人面临的健康问题,开发具有免疫调节活性的天然活性产物成为当前研究的热点问题之一。海洋硫酸多糖是从海洋生物中提取分离的一种富含硫酸基团的天然活性产物,其结构独特,在免疫调节活性方面展现了巨大潜力。为了更好地研究海洋硫酸多糖对免疫调节活性的影响及机制,本文对目前有关海洋硫酸多糖的研究文献进行分析和总结,根据结构及来源对海洋硫酸多糖的种类进行概述,重点从调节巨噬细胞、自然杀伤细胞、T/B淋巴细胞、补体系统和肠道微生物功能五个方面,综述海洋硫酸多糖发挥免疫调节活性的作用及机制。本文为海洋硫酸多糖的免疫调节活性及作用机制等后续研究提供理论基础,为开发新型海洋硫酸多糖免疫增强剂提供新思路,未来有望开发基于海洋硫酸多糖的针对免疫力低下、肿瘤和自身免疫性疾病的功能性食品或特殊医学用途食品,对食品行业的发展和人类的健康起到积极推动作用。Abstract: With changes in modern lifestyles, weakened immunity becomes a health problem for an increasing number of people. The development of natural active products with immunomodulatory activity becomes a popular research topic. Marine sulfated polysaccharides are natural active products that are rich in sulfate groups and are extracted and isolated from marine organisms. They have a unique structure and multiple biological activities and have shown great potential with regard to having immune regulatory activity. To better understand the effects of marine sulfated polysaccharides on immune regulatory activity and the associated underlying mechanisms, this article was designed to analyse and summarize the current research literature on marine sulfated polysaccharides. The types of marine sulfated polysaccharides are summarized based on their structure and source, with a focus on functions involving regulating macrophages, natural killer cells, T/B lymphocytes, complement systems, and gut microbiota. The immune regulatory activity of marine sulfated polysaccharides and the underlying mechanisms are summarized. This article provides a theoretical basis for subsequent research on the immunomodulatory activity and structure-activity relationships of marine sulfated polysaccharides and provides novel ideas for the development of new marine sulfated polysaccharide immunoenhancers. In the future, functional foods or special medical foods based on marine sulfated polysaccharides designed to treat immune deficiency, tumours, and autoimmune diseases may be developed, and these foods will play a positive role in promoting the development of the food industry and human health.
-
Keywords:
- marine sulfate polysaccharide /
- immune regulation /
- mechanism /
- gut microbiota
-
海洋是新型天然活性产物的宝库,海洋天然产物具有陆地天然产物鲜少具备的化学结构和生物特性,来自于海洋的多糖、皂苷和多肽等天然产物均由于其独特的生物活性而备受关注。海洋多糖是一类来源于海洋的,由1个以上单糖组成的多羟基醛或多羰基酮高分子化合物,与陆地多糖相比,海洋多糖分子链中单糖分子的部分羟基通常被硫酸基团取代,比陆地多糖具有更高的硫酸根含量[1]。大量研究对海洋硫酸多糖的结构及生物活性进行了表征和评估,其具有抗病毒、抗寄生虫、抗过敏、抗肿瘤、抗代谢综合征以及调节肠道菌群等多种作用[2],且硫酸多糖比未硫酸化多糖具有更好的生物学特性[3]。发挥这些生理功能都与其免疫调节活性息息相关,因此海洋硫酸多糖在免疫调节方面展现出了巨大潜力。
免疫是机体的一种自我保护行为,免疫反应能够清除外部细菌、病毒和其他有害物质,因此免疫调节对机体的健康至关重要。在生活节奏日益加快的现代社会,免疫力低下已成为普遍公共健康问题,逐渐引起了人们的重视。海洋硫酸多糖具有良好的免疫调节活性,有望作为免疫增强剂应用于功能性食品领域,因此,海洋硫酸多糖的免疫调节活性及机制是当前的研究热点之一。有研究对免疫活性多糖的构效关系进行了系统总结[4],也有研究对海洋多糖的免疫调节活性和硫酸多糖的免疫调节机制进行了系统总结[5−6]。本文系统归纳不同种类的海洋硫酸多糖结构特征,着重从调节巨噬细胞、T/B淋巴细胞、自然杀伤细胞、补体系统和肠道微生物等方面,总结海洋硫酸多糖的免疫调节活性及作用机制,以期为海洋硫酸多糖的免疫调节活性及作用机制的后续研究提供理论基础,为开发新型海洋硫酸多糖免疫增强剂提供新思路。
1. 海洋硫酸多糖的分类及结构特征
海洋硫酸多糖是一类含有硫酸基团的复杂多糖,具有独特的结构特征,海洋硫酸多糖主要存在于藻类植物、鱼类、海洋无脊椎动物以及海洋微生物中[7]。根据结构和来源,海洋硫酸多糖可分为岩藻聚糖硫酸酯(Fucoidan sulfate,FUC)、卡拉胶(Carrageenan,CGN)、石莼聚糖(Ulva lactuca,ULV)、硫酸软骨素(Chondroitin sulfate,CS)、岩藻糖基化硫酸软骨素(Fucoidanosylated chondroitin sulfate,FCS)和硫酸可拉坦(Keratan sulfate,KS)。表1总结了海洋硫酸多糖的主要来源,以及相对分子质量、硫酸基团含量和糖苷键连接方式等结构特征。陆地来源的免疫活性多糖主要有葡聚糖、甘露聚糖、果胶多糖、阿拉伯半乳聚糖、半乳聚糖、透明质酸、果聚糖和木聚糖,多为中性多糖或是含有糖醛酸的酸性多糖[4]。海洋硫酸多糖通常含有较高的硫酸基团,个别含量可以高达近50%,硫酸基团含量和取代模式对其免疫调节活性具有重要影响,这也是海洋硫酸多糖的主要结构特征。海洋植物硫酸多糖结构往往更加复杂,支链取代和硫酸基团取代方式多样,这对功能因子的活性及机制研究和质量控制带来了巨大的困难,而海洋动物硫酸多糖通常具有重复序列,结构相对简单,且作用靶点相对明确,这为其活性及机制研究提供了重要保证[1]。
表 1 海洋硫酸多糖的主要来源及结构特征Table 1. Main sources and structural characteristics of marine sulfated polysaccharides来源 种类 相对分子质量
(106 Da)硫酸基团含量
(%)糖苷键连接 刺参[8]
(Apostichopus japonicus)FUC 1.970 22.3 α-L-Fucp2(OSO3−)-1→3,(α-L-Fucp-1→4-α-L-Fucp-1→)4-α-L-Fucp2
(OSO3−)-1→3-α-L-Fucp2(OSO3−)褐藻[9]
(Algae brunas)FCS 0.095~0.418 35.0 →4)-β-D-GlcA-(1→3)-β-D-GalNAc-(1→ 红藻[10]
(Algae rubri)CGN 0.500~1.000 26.5 α-(1→4)-D-DD; 3,6-DA; β-(1→3)-D-DD 绿藻[11]
(Conglobata kjellman)ULV 0.068 30.3 →4)-α/β-L-Rhap-(1→; →4)-β-D-Xylp-(1→; →4)-β-D-GlcAp-(1→ 施氏鲟鱼[12]
(Acipenser schrenckii)CS 0.300 48.9 →4)-β-D-GlcA-(1→3)-β-D-GalNAcp4(OSO3−)-(1→; →4)-β-D-GlcA-(1→3)-β-D-GalNAc-(1→ 鲨鱼[13]
(Shark cartilage)KS 0.045 26.1 →3)-β-D-Gal-(1→4)-β-D-GlcNAc-(1→ 注:FUC:岩藻聚糖硫酸酯;CGN:卡拉胶;ULV:石莼聚糖;CS:硫酸软骨素;FCS:岩藻糖基化硫酸软骨素;KS:硫酸可拉坦;Fuc:岩藻糖;Xyl:木糖;Gal:半乳糖;GlcA:葡萄糖醛酸;GalNAc:N-乙酰半乳糖胺;Rha:鼠李糖;DD:吡喃半乳糖;DA:脱水半乳糖。 1.1 岩藻聚糖硫酸酯
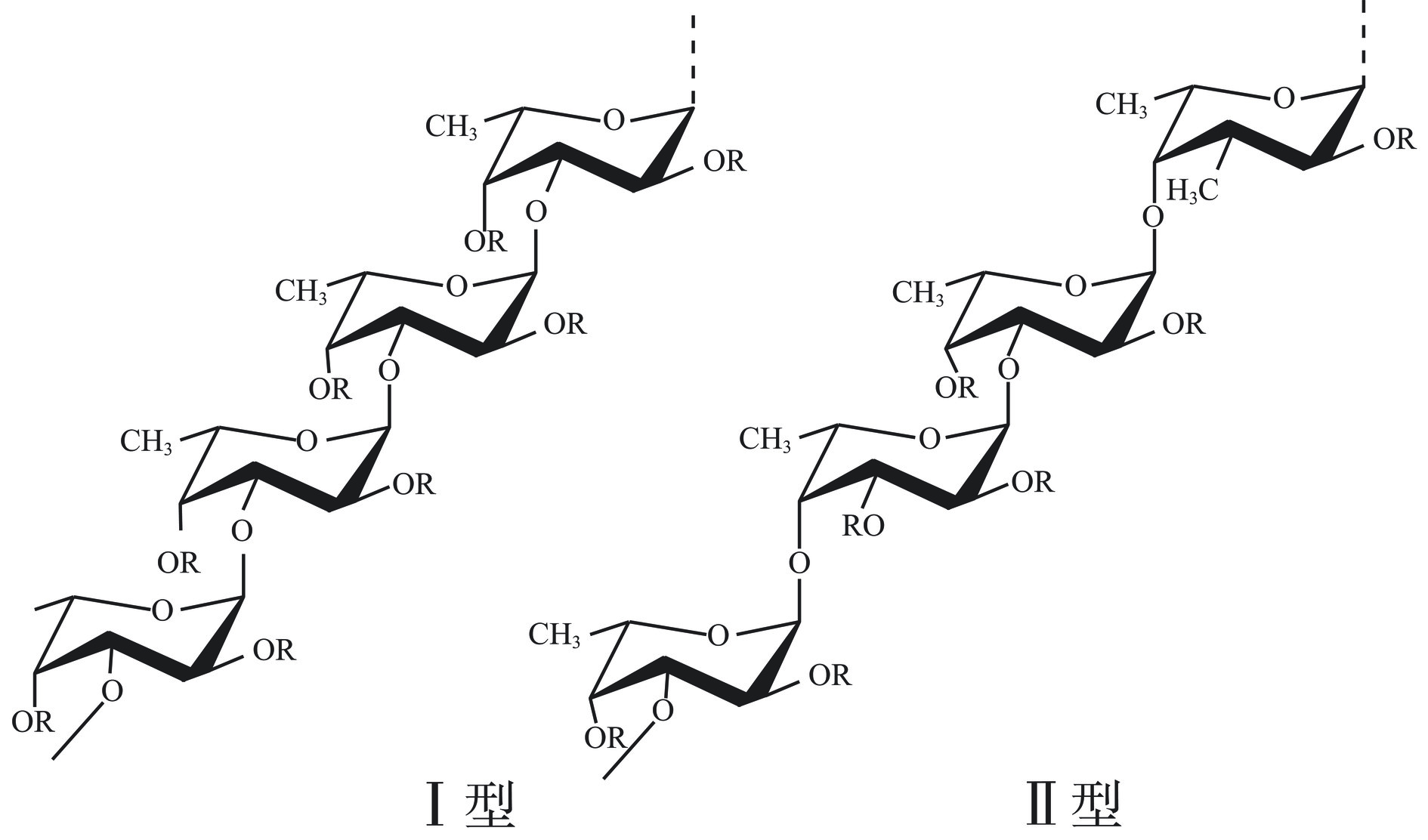
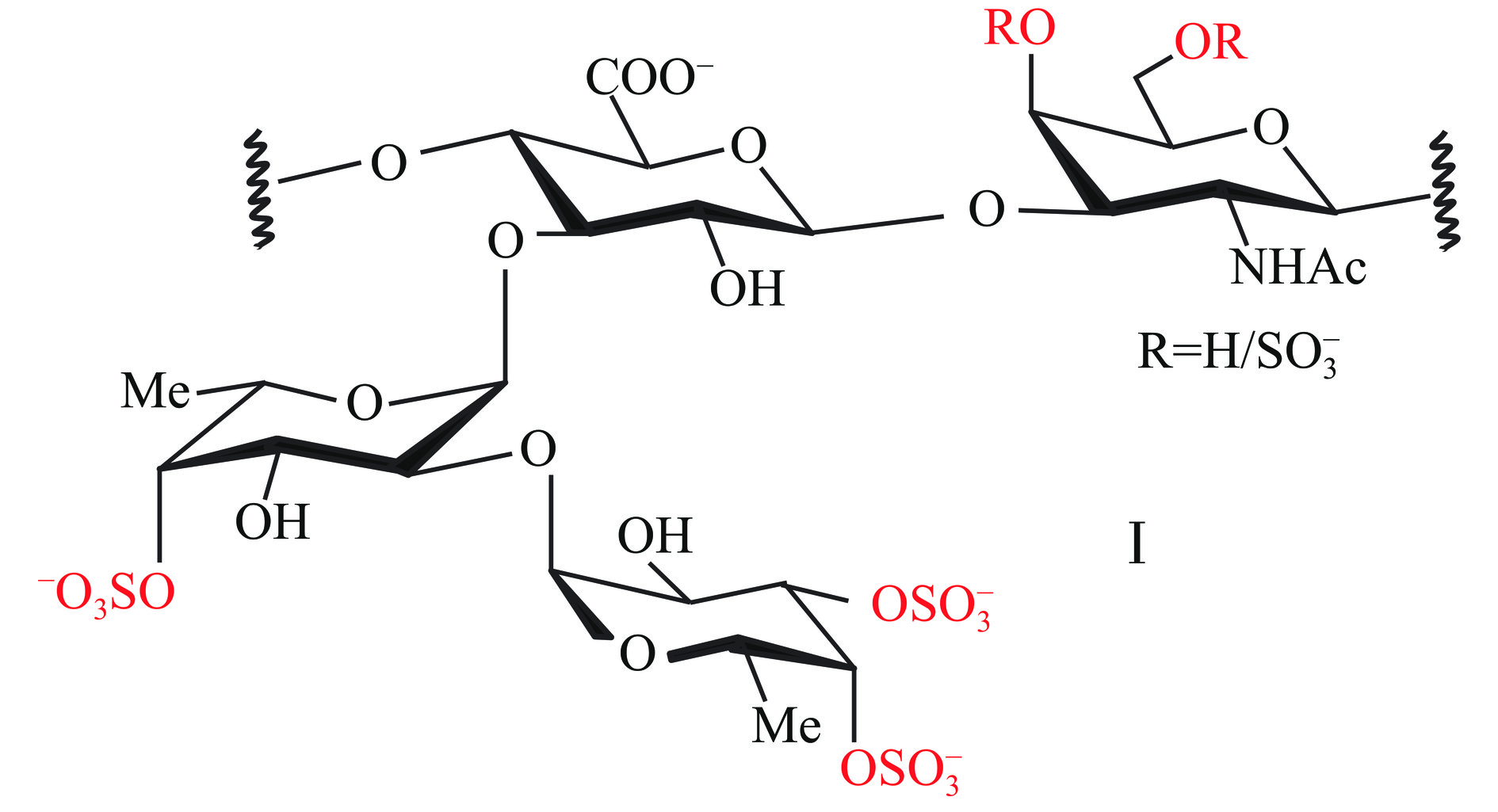
岩藻聚糖硫酸酯又名岩藻多糖,主要存在于各种海参及褐藻类海洋生物中。不同来源的岩藻聚糖硫酸酯化学结构不尽相同,除了主要成分L-岩藻糖外,还有少部分D-甘露糖、D-半乳糖、葡萄糖、阿拉伯糖、葡萄糖醛酸以及大量的硫酸基团(含量约为20%~45%),有些还具有乙酰基和蛋白质[14]。岩藻聚糖硫酸酯的分子量分布广泛[15](低分子量岩藻聚糖硫酸酯分子量小于104 Da,中等分子量岩藻聚糖硫酸酯分子量介于104~107 Da,高分子量岩藻聚糖硫酸酯分子量大于107 Da),其结构研究进展较为缓慢。褐藻中的岩藻聚糖硫酸酯通常具有支链结构,厚叶马尾藻(Sargassum crassifolium)岩藻聚糖硫酸酯侧链中含有半乳吡喃糖,通过(1→4)连接于主链L-岩藻吡喃糖的C4位置,而南方团扇藻(Padina australis)岩藻聚糖硫酸酯的侧链多是硫酸化的半乳糖-岩藻糖二糖或硫酸化的半乳糖吡喃糖通过(1→3)及(1→4)连接于主链结构[16]。海参中的岩藻聚糖硫酸酯的组成以L-岩藻糖为主,含有极少量的其他单糖,一般是直链结构,由α(1→3)或α(1→4)连接的岩藻糖构成,硫酸基团位于岩藻糖的C-2或C-2,4位,少数海参岩藻聚糖硫酸酯也存在支链[17]。刺参岩藻聚糖硫酸酯α(1→3)糖苷键连接的岩藻糖主链的C-2位上存在岩藻糖支链,硫酸根位于主链和支链岩藻糖的C-2或C-4位上[18]。另一种刺参岩藻聚糖硫酸酯主链则是由α(1→3)糖苷键连接的岩藻糖构成三糖重复单元,并且主链的C-4位上存在非硫酸化岩藻糖二糖支链,硫酸根位于主链岩藻糖的C-2位上[8]。大西洋海参(Holothuria coluber)主链由α(1→4)糖苷键连接的岩藻糖构成四糖重复单元,并且主链的C-3位上存在岩藻糖支链,硫酸根位于主链岩藻糖的C-3位和支链岩藻糖的C-4位上[19]。作为一种聚阴离子硫酸多糖,岩藻聚糖硫酸酯中的多糖主链可分为Ⅰ型链和Ⅱ型链两种类型(图1),Ⅰ型链包含重复的(1→3)连接α-L-吡喃岩藻糖残基,而Ⅱ型链包含交替的(1→3)和(1→4)连接的α-L-吡喃岩藻糖残基[20]。岩藻聚糖硫酸酯具有调节免疫、抗氧化、保护肝脏、抗肿瘤、降血压、抗菌、治疗肾衰竭和泌尿系统感染等功能,除了在医药和功能性食品领域的应用外,岩藻聚糖硫酸酯还可以作为基质材料应用于药物递送系统中,如岩藻聚糖硫酸酯-壳聚糖纳米材料等[21]。
1.2 卡拉胶
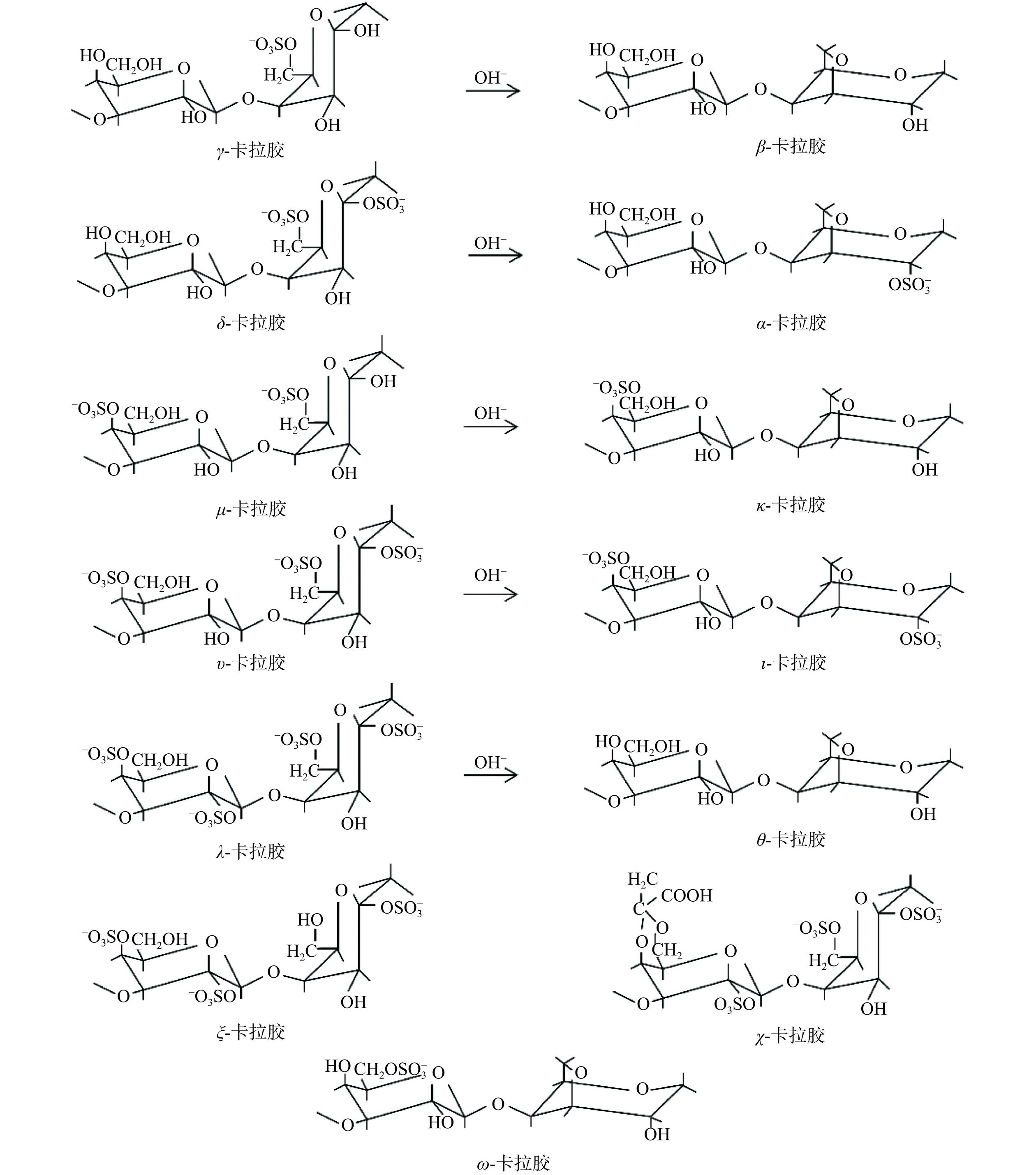
卡拉胶主要存在于红藻中,是一类含有15%~40%硫酸基团的线性高分子多糖,通常以重复的(1→4)-α-D-半乳吡喃糖-(1→3)-β-D-半乳吡喃糖(或3,6内醚-D-半乳吡喃糖二糖)单元作为骨架结构,并且具有大于105 Da的相对分子量[22]。如图2所示,根据卡拉胶的重复二糖单元上硫酸基团的分布,以及(1→4)-α-D-吡喃半乳糖残基上3,6-内醚键的形成情况,可以将卡拉胶分为κ-、λ-和α-卡拉胶三大类,又可根据1,3连接的D-半乳糖单元上的硫酸基团连接位点,细分为κ-族的κ-、ι-、μ-和υ-卡拉胶,λ-族的λ-、θ-和ξ-卡拉胶,以及β-族的β-、δ-、γ-和α-卡拉胶,此外还有不属于以上三族的ω-卡拉胶[23−24]。卡拉胶主要应用于食品加工业,作为稳定剂、增稠剂和凝胶剂,改善食品的硬度、黏性及咀嚼性等食用品质[25−26]。与此同时,卡拉胶也具有一定的生物活性,如抗病毒、抗癌和免疫调节活性等。
1.3 石莼聚糖
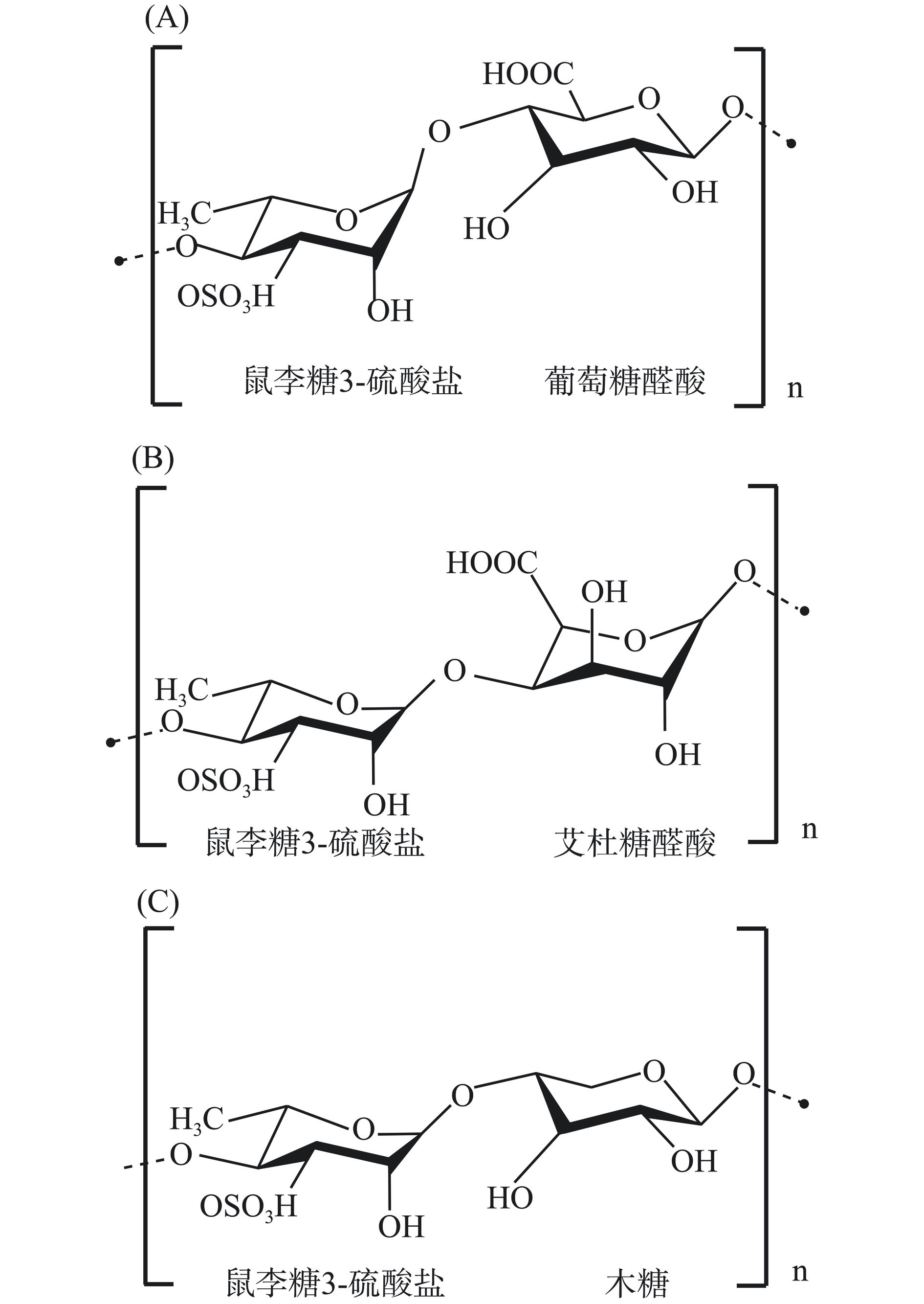
石莼聚糖是一种复杂的阴离子硫酸化多糖,主要来源于海洋绿藻的细胞壁,一般占石莼属绿藻干重的8%~29%,主要由鼠李糖、葡萄糖醛酸、艾杜糖醛酸和木糖组成,葡糖糖醛酸或是艾杜糖醛酸与鼠李糖以醛二糖酸的形式存在,形成特征性的重复双糖单元[27],硫酸基团含量介于14.3%~23.2%,分子量分布范围介于5.3×107~3.2×108 Da[28]。糖残基主要通过α-(1→4)和β-(1→4)连接构成主链,分别为α-1,4-和α-1,2,4-L-鼠李糖、β-1,4-D-葡萄糖醛酸和β-1,4-L-木糖,分支结构主要在鼠李糖或是糖醛酸的O-2位,硫酸化位点主要在鼠李糖的C-3或者是C-2和C-3位,MOU等[29]研究发现,部分硫酸基团也存在于C-4位置上。如图3所示,根据重复双糖单元的不同类型,石莼聚糖可统分为A类硫酸三丁酸酯和B类硫酸三丁酸酯两类,A类硫酸三丁酸酯由葡萄糖醛酸和鼠李糖3-硫酸盐共同组成双糖单元,B类硫酸三丁酸酯则是由艾杜糖醛酸和鼠李糖3-硫酸盐共同组成双糖单元[30]。然而在某些情况下,木糖或硫酸木糖残留物会代替糖醛酸,此时的重复双糖单元被称为硫钒二糖,以硫钒二糖3-硫酸盐和硫钒二糖2',3-二硫酸盐来表示。石莼聚糖具有生物降解性、细胞相容性和广泛的生物活性,可作为替代塑料的可降解薄膜用于食品包装领域,也可以作为具有抗氧化性的包装涂料,用于食品保鲜领域[31]。
1.4 硫酸软骨素
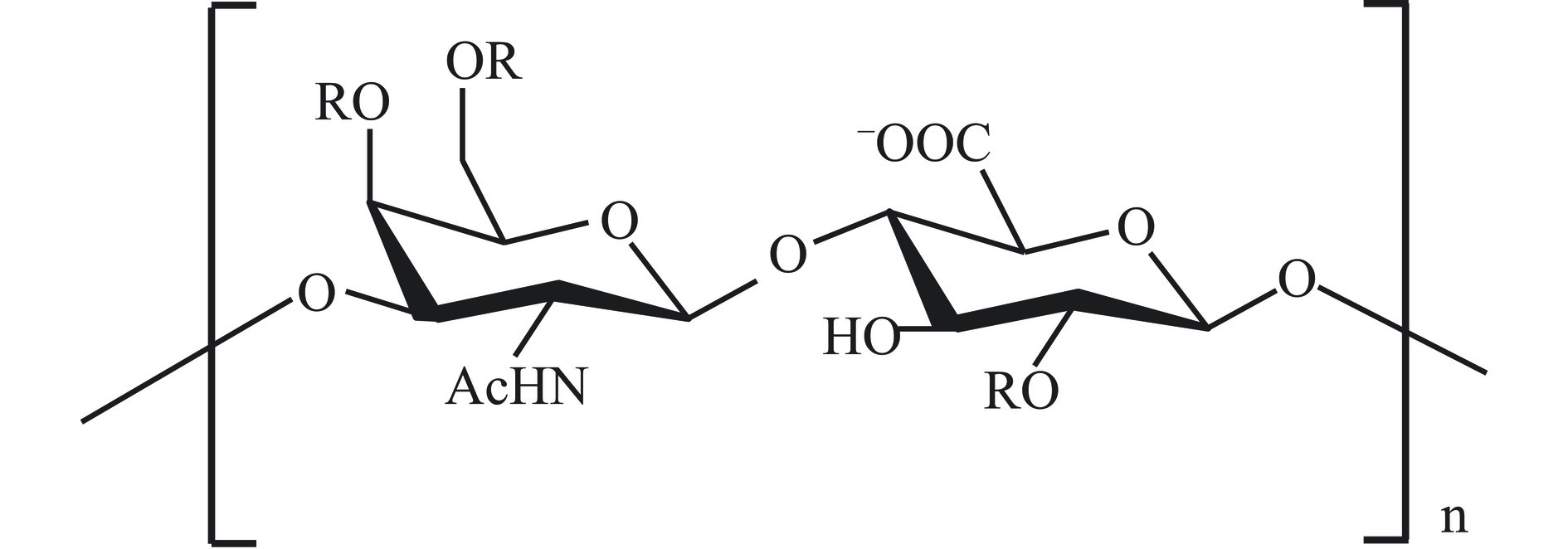
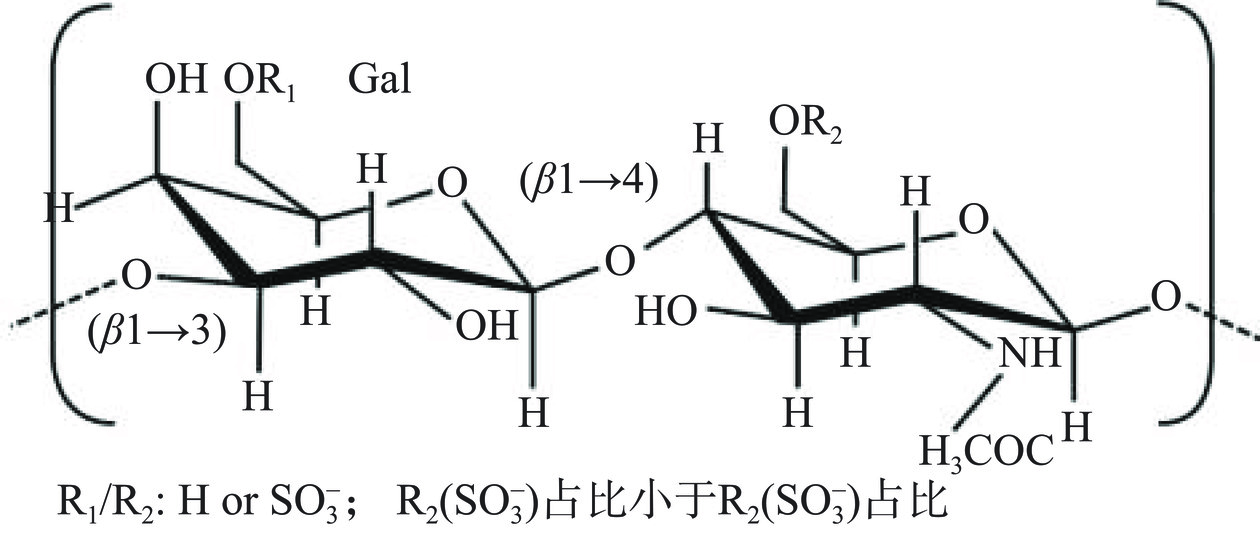
硫酸软骨素是哺乳动物软组织中的代表物质,在海洋生物中广泛存在于鱼类中,是一类由D-葡萄糖醛酸和N-乙酰-D-半乳糖胺组成的重复双糖单元连接成的线性硫酸化多糖(图4),通常在2-乙酰氨基-2-去氧-半乳糖残基的4位和6位或是4,6位同时被硫酸化,分子量介于5×104~105 Da之间[32]。由于N-乙酰-D-半乳糖胺和D-葡萄糖醛酸的硫酸基团取代程度不同,以及硫酸基团在己糖胺上连接位点不同,硫酸软骨素可分为O、A、C、D和E等亚型。A型硫酸软骨素和C型硫酸软骨素均含有D-葡萄糖醛酸和2-氢过氧基脱氧-D-半乳糖,并含有等量的乙酰基和硫酸基团,其应用范围较其他亚型硫酸软骨素也更为广泛[33]。左格格等[34]利用紫外、醋酸纤维电泳、红外光谱和高效液相色谱,对罗非鱼不同部位的硫酸软骨素理化性质和结构特征进行分析,测得鱼头和鱼尾所含硫酸软骨素主要为C型,含量分别为46.10%和41.11%,鱼脊骨和鱼鳍所含硫酸软骨素主要为A型,含量分别为71.86%和69.59%。硫酸软骨素药用效果良好且毒副作用小,常被用于防治关节炎等疾病的治疗[35],关于硫酸软骨素调节免疫功能的报道,来源多为哺乳动物软组织中的提取物,然而哺乳动物软组织硫酸软骨素与海洋来源的硫酸软骨素结构差异较大,自然界中存在的硫酸软骨素主要以4-硫酸化基团(4S)和6-硫酸化基团(6S)为主,海洋来源的硫酸软骨素通常含有更高的6S,4S/6S比值通常小于1[36]。硫酸软骨素有着人体“软黄金”之称,在医学领域有着极高的应用价值,是目前市场上应用较为成熟的治疗关节疾病药物。与陆生动物相比,海洋来源的硫酸软骨素不存在生物毒素和污染物,海洋生物被认为是硫酸软骨素的良好来源[37]。此外,硫酸软骨素还可以作为膳食补充剂和保湿剂应用于功能性食品和化妆品领域。
1.5 岩藻糖基化硫酸软骨素
岩藻糖基化硫酸软骨素是发现于海参体壁中的一种结构较为特殊的硫酸软骨素,除了具有传统的硫酸软骨素骨架[→4-β-D-GlcA-(1→3)-β-D-GalNAc-(1→]n,葡萄糖醛酸残基的O-3上还附着独特的硫酸化岩藻糖分支,硫酸基团可能位于岩藻糖的C-2、C-3和C-4处[38],其糖链上同样有部分羟基发生硫酸基团取代(图5)。从不同的海参中均能够分离纯化出岩藻糖基化硫酸软骨素,例如,革皮氏海参(Pearsonothuria graeffei)、挪威拟刺参(Stichopus tremulus)、荡皮海参(Holothuria vagabunda)、美国肉参(Isostichopus badionotus)和墨西哥海参等,不同海参的岩藻糖基化硫酸软骨素均具有相似的硫酸软骨素骨架结构,但其岩藻糖的分支则会呈现出不同的硫酸化模式[39]。
Gong等[38]对从刺参体壁中提取到的岩藻糖基化硫酸软骨素进行结构解析,证明其主链含有GalNAc、GalNAc4S、GalNAc6S和GalNAc4,6S四种N-乙酰氨基半乳糖硫酸化取代模式,支链含有Fuc2,4S、Fuc4S、Fuc3,4S三种岩藻糖硫酸化取代模式。Ustyuzhanina等[40]从海棒槌(Paracaudina chilensis)和黄疣海参(Holothuria hilla)中获得两种岩藻糖基化硫酸软骨素,除岩藻糖基残基的硫酸化模式的不同,其岩藻糖基的比例也存在一定差异。此外,还发现在O-6连接的N-乙酰氨基半乳糖残基上含有不同寻常的二糖分支Fuc4S-(1→2)-Fuc3S4S。岩藻糖基化硫酸软骨素结构差异不仅体现在N-乙酰氨基半乳糖和岩藻糖残基的硫酸化模式,还体现在葡萄糖醛酸残基的硫酸化、N-乙酰氨基半乳糖残基的岩藻糖基化以及更复杂的二岩藻糖基分支方面的差异性。岩藻糖基化硫酸软骨素是一种重要的生物活性多糖,尤其在抗凝血和抗血栓药物研发方面展现出了巨大的应用潜力,但是仍需进一步的临床研究和开发。
1.6 硫酸可拉坦
硫酸可拉坦是众多糖胺聚糖中唯一不含糖醛酸的糖胺聚糖,主链由3-α-半乳糖和4-N-乙酰基-β-葡糖胺交替连接的重复二糖单元构成(图6),并且两个单糖的6-O键被硫酸化,从而产生硫酸可拉坦的高硫酸化、单硫酸化以及非硫酸化区域。硫酸可拉坦以硫酸化聚乙酰乳糖胺作为主链,与蛋白质核心相连形成蛋白聚糖,广泛存在于海洋鲨鱼软骨、斑马鱼和鱼类皮肤中[41]。少量L-岩藻糖和N-乙酰神经氨酸的存在进一步区分出了硫酸可拉坦的I、II和III型,这些残基赋予了多糖链对果胶酶-I、II和末端-β-半乳糖苷酶的降解抗性特征[42]。硫酸软骨素在大量应用于骨关节保护和治疗类的膳食补充剂和药物,同样作为糖胺聚糖的硫酸可拉坦是商品化硫酸软骨素的一种共纯化杂质,但是与药典中有限量要求的杂质不同的是,硫酸可拉坦是一种非限制性杂质[43]。
2. 海洋硫酸多糖的免疫调节作用机制
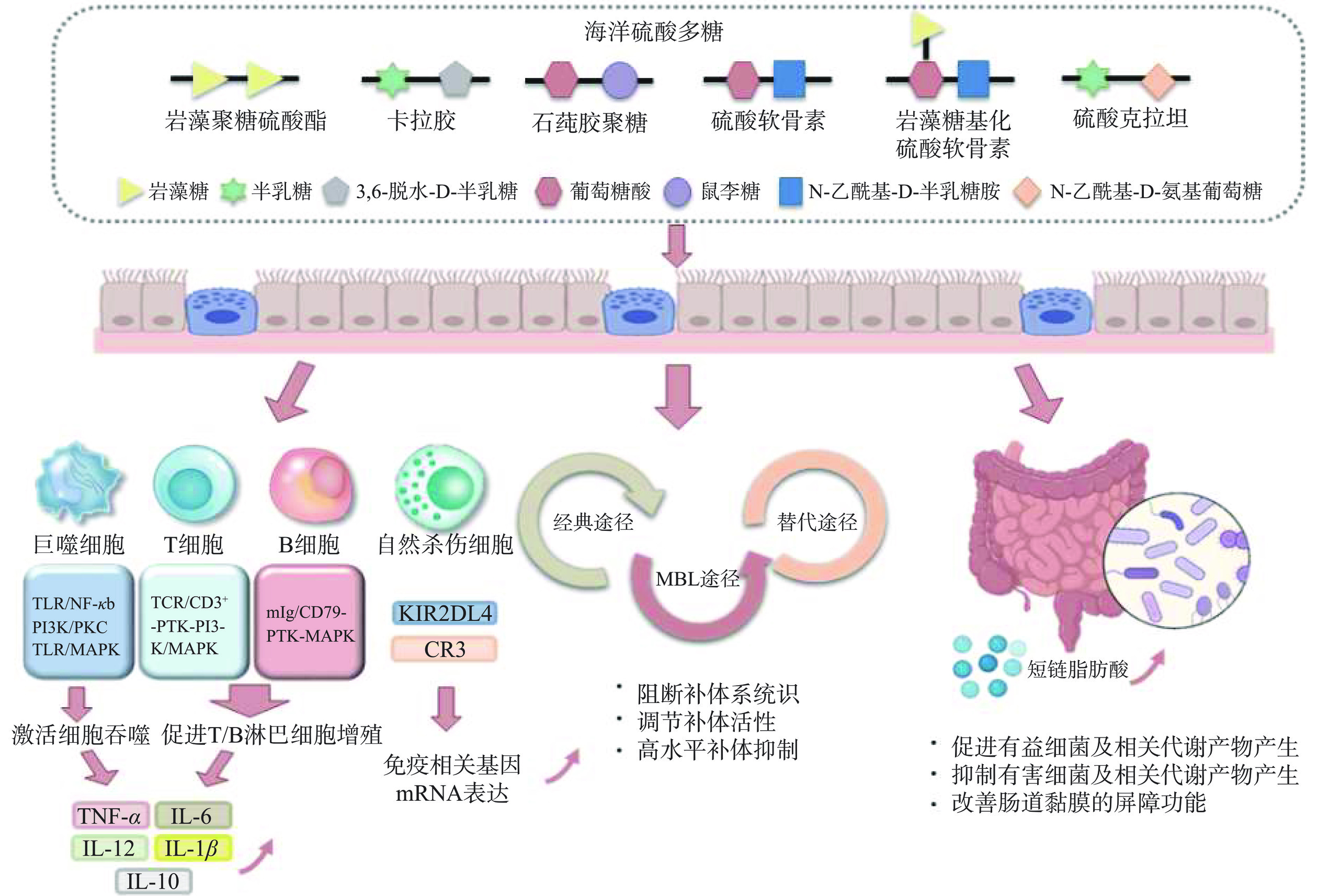
近年来海洋硫酸多糖的免疫调节活性受到了广泛关注,具有良好安全性和免疫调节活性的海洋硫酸多糖是开发免疫调节功能性食品的理想原料[44]。目前的研究表明,海洋硫酸多糖能够通过调节免疫系统从而发挥其免疫调节作用,在增强巨噬细胞吞噬功能、促进抗体合成、促进淋巴细胞增殖、增强自然杀伤细胞活性、提高细胞因子、白介素因子(IL)和肿瘤坏死因子-α(TNF-α)血清水平,以及调节肠道微生物等方面具有显著效果(图7)[10]。海洋硫酸多糖的免疫调节活性具有多途径、多环节、多靶点的特点,并且海洋硫酸多糖结构会因来源、制备方法、季节和环境条件而发生变化,结构和组成上的差异导致发挥免疫调节活性的作用机制不同。因此有必要对不同结构的海洋硫酸多糖免疫调节活性及机制研究进行总结,以便更好地理解海洋硫酸多糖调节免疫应答的过程及相关的信号转导途径。
2.1 海洋硫酸多糖对巨噬细胞的调节作用
巨噬细胞作为免疫系统抵御病原微生物的首道防线,通过识别和吞噬病原微生物,递呈抗原,释放细胞因子来调节免疫系统[6]。海洋硫酸多糖可以有效激活巨噬细胞的吞噬功能,并通过调节产生NO、活性氧以及不同类型和数量的细胞炎症因子引起局部免疫应答,发挥多种免疫调节功能[45]。海地瓜(Acaudina molpadioides)岩藻聚糖硫酸酯可以有效增强巨噬细胞的吞噬能力,且在作用前期(6 h和12 h)效果显著,具有较好的免疫调节活性[46]。Kidgell等[47]通过测定巨噬细胞RAW 264.7释放的炎症介导细胞因子水平,量化了石莼聚糖组分对巨噬细胞的免疫调节作用,在浓度为100 μg/mL时,高分子量的石纯聚糖组分通过分泌IL-10显示出抗炎作用,还能通过少量增加IL-1β和IL-6来调节LPS诱导的炎症。红刺参岩藻糖基化硫酸软骨素能刺激巨噬细胞产生NO、TNF-α、IL-1β和IL-6[48],海带岩藻聚糖硫酸酯则能刺激巨噬细胞产生NO和IL-1[49]。巨噬细胞吞噬活性和细胞因子的释放所产生的免疫调节效果,可能是由于海洋硫酸多糖调控相关免疫信号通路引起的。Jayawardena等[50]的研究表明,马尾藻硫酸多糖通过阻断巨噬细胞TLR/NF-κb信号转导通路,从而起到抗炎作用。Xu等[51]研究发现,硫酸可拉坦的二糖片段能够抑制LPS和干扰素γ刺激的巨噬细胞中IL-12的产生,这是由于硫酸可拉坦的二糖片段能通过与巨噬细胞表面的TLR-4受体结合,抑制磷酸蛋白激酶C(P-PKC)及磷酸肌醇-3激酶(PI3K)通路,从而抑制IL-12的分泌起到抗炎作用。海洋微藻硫酸多糖促进巨噬细胞活化,增加NO的释放则是通过丝裂原活化蛋白激酶(MAPK)信号通路中的应激活化蛋白激酶-1(JNK-1)实现的[52]。
另外,海洋硫酸多糖对巨噬细胞的调节作用与硫酸基团的含量和相对分子质量相关。高硫酸基团含量的褐藻多糖对巨噬细胞具有较好的免疫调节活性,去除硫酸基团后免疫调节活性降低[53]。一般来说,多糖的硫酸化不仅可以改变硫酸基团的空间位阻和静电斥力效应,还可以改变链的水溶性和弯曲性[54]。因此,硫酸化可以改善多糖的结构特征并增强其免疫能力。江蓠(Gracilaria rubra)硫酸多糖通过促进巨噬细胞增殖发挥其免疫活性,且活性随着分子量的降低而逐渐增加[55]。综上,海洋硫酸多糖能够通过调控TLR/NF-κb、PI3K/PKC和TLR/MAPK信号转导途径,激活巨噬细胞的吞噬功能、促进NO、活性氧以及细胞炎症因子的释放,发挥免疫调节活性,高硫酸基团含量和低分子量的海洋硫酸多糖具有更好的免疫调节活性。
2.2 海洋硫酸多糖对T/B淋巴细胞的调节作用
淋巴细胞是机体的主要免疫细胞,包括T淋巴细胞和B淋巴细胞。T淋巴细胞主要参与细胞免疫反应,硫酸多糖通过结合T细胞受体(TCR)和CD3+形成的复合物介导T细胞活化,随后蛋白酪氨酸激酶(PTK)被激活,从而通过PI3-K或MAPK途径激活T淋巴细胞免疫反应[56]。B淋巴细胞主要参与体液免疫反应,表面存在大量膜免疫球蛋白受体(mIg),硫酸多糖同样能够结合mIg和CD79形成的复合物,激活PTK,在PTK的催化作用下,丝/苏氨酸蛋白激酶(MAPK)被进一步激活并产生激活蛋白-1(AP-1),从而调控B淋巴细胞相关基因的表达[57]。海洋硫酸多糖对淋巴细胞的免疫调节作用体现在对淋巴细胞增殖和细胞因子分泌的正向影响上。褐藻苷苔(Ecklonia cava)硫酸多糖能够剂量依赖性地促进淋巴细胞增殖,且无细胞毒性,特别是显著增强CD3+成熟T细胞和CD45R/B220+ Pan B细胞的增殖和分化[58],从而发挥免疫调节活性(作用)。坛紫菜硫酸多糖则可以诱导淋巴细胞产生TNF-α和IL-10发挥免疫调节活性[59],苔浒硫酸多糖则是通过显著增加IFN-γ和IL-2的分泌,而不改变IL-4和IL-5的释放,来发挥免疫调节活性[53]。
研究表明,海洋硫酸多糖对淋巴细胞的免疫调节作用与硫酸基团的含量有关,来自于糖海带(Saccharina latissima)的2个岩藻聚糖硫酸酯组分对B淋巴细胞均具有免疫调节活性,且硫酸基团含量是影响免疫效应的关键,脱硫后岩藻聚糖硫酸酯对B淋巴细胞的活化能力显著减弱[60]。综上,海洋硫酸多糖通过TCR/CD3+-PTK-PI3-K/MAPK信号转导途径激活T淋巴细胞,通过mIg/CD79-PTK-MAPK信号转导途径激活B淋巴细胞,促进T/B淋巴细胞增殖和细胞因子分泌,高硫酸基团含量的海洋硫酸多糖具有更好的免疫调节活性。
2.3 海洋硫酸多糖对自然杀伤细胞的调节作用
作为一种溶解性淋巴细胞,自然杀伤细胞在免疫系统中同样起到十分关键的作用,能够识别靶细胞、杀伤介质,在某些情况下还能参与超敏反应和自身免疫性疾病的发生[5]。大量研究证实海洋硫酸多糖对自然杀伤细胞具有调节作用,多管藻(Polysiphonia senticulosa)的硫酸多糖可以增强自然杀伤细胞活性,促进T淋巴细胞增殖,提高巨噬细胞的吞噬活性并产生NO[61]。刺松藻(Codium fragile)硫酸多糖上调了自然杀伤细胞活化相关的标志物CD69的表达,显著增加了IFN-γ、细胞毒性介质穿孔素和颗粒酶B的释放,是一种潜在的自然杀伤细胞促进剂[62]。不同结构的海洋硫酸多糖与自然杀伤细胞的受体结合能力不同,有研究表明,岩藻聚糖硫酸酯最有可能通过自然杀伤细胞表面的III型补体受体(CR3)实现自然杀伤细胞的活化[63],而硫酸乙酰肝素则能够与NK细胞抑制性受体2DL4(KIR2DL4)相互作用,激活自然杀伤细胞并诱导细胞因子的产生[64]。不同来源的海洋硫酸多糖对自然杀伤细胞的激活作用也存在差异,Zhang等[65]对比了泡叶藻(Ascophyllum nodosum)、裙带菜(Undaria pinnatifida)、巨藻(Macrocystis pyrifera)、墨角藻(Fucus vesiculosus)和腔昆布(Ecklonia cava)中硫酸多糖刺激体内自然杀伤细胞的潜力。结果表明,腔昆布硫酸多糖ECF能够显著促进脾脏中自然杀伤细胞数量的增加,对自然杀伤细胞的活化作用最强,并且ECF以自然杀伤细胞依赖的方式有效地阻止了CT-26癌细胞在肺中的浸润,可能是自然杀伤细胞介导的抗癌免疫的合适候选物。
海洋硫酸多糖对自然杀伤细胞的激活作用与其硫酸化程度有关,Surayot等[63]研究了刺参岩藻聚糖硫酸酯及其脱硫产物对自然杀伤细胞的活化和细胞毒性作用,脱硫处理显著降低了自然杀伤细胞的细胞毒性水平和激活因子的mRNA表达。综上,海洋硫酸多糖通过KIR2DL4和CR3受体激活自然杀伤细胞,促进自然杀伤细胞增殖,上调免疫相关基因的mRNA表达,高硫酸基团含量和多糖-受体强亲和力对自然杀伤细胞的激活有促进作用。
2.4 海洋硫酸多糖对补体系统的调节作用
补体系统是一组存在于机体血清中的非特异性球蛋白,主要由肝脏产生的血浆蛋白或细胞受体表达的膜蛋白组成,包括3种常见的激活途径,分别是经典补体途径、甘露糖结合凝集素途径和替代补体途径[66]。海洋硫酸多糖能够通过调节经典补体途径和替代补体途径参与免疫反应,甘露糖结合凝集素途径可以被特定微生物多糖激活[67],然而很少有报道研究海洋硫酸多糖对这一途径的影响。现有研究证实了海洋硫酸多糖调节补体系统活性的有效性,揭示了海洋硫酸多糖作为补体抑制剂在治疗免疫相关疾病方面的潜在价值。Zvyagintseva等[68]研究了几种褐藻中水溶性硫酸多糖对补体系统的影响,结果表明,所有褐藻硫酸多糖都具有结合补体的能力。Blondin等[69]同样证实了从褐藻中提取的岩藻聚糖硫酸酯是补体系统激活的有效抑制剂,且以剂量依赖的方式抑制整个血清中的经典和替代途径激活。褐藻硫酸多糖可以与经典补体途径识别阶段的C1q蛋白结合,阻断补体系统的识别以调节补体系统的活性。褐藻硫酸多糖还能与经典补体途径激活阶段的C4蛋白互作形成复合物,阻断补体系统的激活作用来调节补体系统的活性[70]。
海洋硫酸多糖对补体系统的调节作用与其硫酸化程度和分子量有关。来自于褐藻(Laminaria cichorioides)的高度硫酸化α-L-葡聚糖对替代补体途径具有最大的活性[68]。硫酸乙酰肝素的O-硫酸化程度越高,分子量越大,其补体抑制能力越强[71−73]。岩藻聚糖硫酸酯则与之相反,低分子量的岩藻聚糖硫酸酯表现出了高水平的补体抑制活性。Tissot等[74]的研究同样表明,褐藻中低分子量硫酸多糖可以实现高水平的补体抑制活性。
2.5 海洋硫酸多糖基于肠道微生物对免疫活性的调节作用
海洋硫酸多糖通常相对分子质量较大,人体缺乏能够消化海洋硫酸多糖的酶,且难以直接吸收[75]。在人体肠道中存在数以万亿的肠道微生物,种类繁多,数量惊人,是人体细胞总量的10倍以上,被认为是人体的“第二套基因库”[76]。海洋硫酸多糖只有通过肠道微生物的降解作用,才能被人体利用,同时,多糖也会对肠道菌群的结构产生一定影响[2]。因此,部分研究人员认为肠道微生物是海洋硫酸多糖发挥健康益处的关键靶点。大量研究证明,肠道微生物降解海洋硫酸多糖产生的代谢产物(如乙酸、丙酸和丁酸等短链脂肪酸),可作为肠上皮细胞的能量来源,具有调节肠道微生物群失调和抵抗病原体入侵等功能[77],进一步增强肠道屏障,防止肠道中炎症诱导因子转移到体内,抑制结肠炎症,发挥增强免疫活性[78]。不同类型的海洋硫酸多糖均能够选择性地增加短链脂肪酸产生菌的丰度。例如,刺参中的硫酸多糖显著增加了BALB/c小鼠中拟杆菌、异杆菌、异普氏菌和罗氏菌的丰度[79],海地瓜(Apostichopus. molpadioides)中的岩藻糖基硫酸软骨素增加了肥胖小鼠中乳酸杆菌和双歧杆菌的丰度[80]。有研究表明,致病菌或条件致病菌丰度的增加与炎症引起的免疫相关疾病有关,这些革兰氏阴性菌含有的脂多糖(LPS)是产生炎症的最关键介质之一[81]。海洋硫酸多糖的摄入可以降低动物模型中LPS的含量,这可能与肠道中大肠杆菌和脱硫弧菌的比例降低有关[82]。综上,海洋硫酸多糖通过促进有益细菌及其相关代谢产物的产生,抑制有害细菌及其相关代谢产物的产生,以及改善肠道粘膜的屏障功能发挥增强免疫活性。
3. 结论与展望
海洋硫酸多糖根据来源和典型结构特征可分为不同种类,在调节人体免疫系统中起着至关重要的作用,能够从调节巨噬细胞、自然杀伤细胞、T/B淋巴细胞、补体系统和肠道微生物功能五个方面,发挥免疫调节活性。海洋硫酸多糖的免疫活性不仅取决于来源和制备手段,还取决于其结构特征(如相对分子质量、硫酸基团取代度和取代模式等),其作用机制受各种相关因素的组合影响,由多途径调节,是一个复杂的过程。
对于海洋硫酸多糖的免疫调节活性及机制研究,仍存在以下问题:a.多数研究仅局限于海洋硫酸多糖对免疫细胞的影响,与海洋硫酸多糖互作的靶细胞位点研究较少,作用机制研究不够深入。b.与免疫调节活性相关的结构特征包括单糖和糖苷键的组成、构象、分子量、官能团和分支结构,但是只有经过纯化的均一性多糖并进行选择性结构修饰,才能建立海洋硫酸多糖-免疫调节活性间的构效关系,这方面研究相对较少。c.海洋硫酸多糖、肠道微生物及免疫调节三者之间复杂的联系已成为热点问题之一,海洋硫酸多糖具有调节肠道菌群组成、增加肠道有益菌的功能,这反映了海洋硫酸多糖调节肠道微生物群作用的共性。但共性作用之外,海洋硫酸多糖对肠道微生物群的差异调节机制仍不清楚,未来可以针对海洋硫酸多糖对肠道微生物的特异性调节开展研究,进一步明确海洋硫酸多糖通过肠道微生物发挥免疫调节的作用机制。d.现有研究缺乏临床数据支撑,海洋硫酸多糖在人体内的安全性、有效性和药代动力学研究有待进一步考察。此外,海洋硫酸多糖的产业化应用还需要建立规模化提取纯化技术。未来研究可以从以上角度入手,开发基于海洋硫酸多糖的针对免疫力低下、肿瘤和自身免疫性病的功能性食品或特殊医学用途食品,为海洋硫酸多糖的精深利用提供理论基础和技术支撑。
-
表 1 海洋硫酸多糖的主要来源及结构特征
Table 1 Main sources and structural characteristics of marine sulfated polysaccharides
来源 种类 相对分子质量
(106 Da)硫酸基团含量
(%)糖苷键连接 刺参[8]
(Apostichopus japonicus)FUC 1.970 22.3 α-L-Fucp2(OSO3−)-1→3,(α-L-Fucp-1→4-α-L-Fucp-1→)4-α-L-Fucp2
(OSO3−)-1→3-α-L-Fucp2(OSO3−)褐藻[9]
(Algae brunas)FCS 0.095~0.418 35.0 →4)-β-D-GlcA-(1→3)-β-D-GalNAc-(1→ 红藻[10]
(Algae rubri)CGN 0.500~1.000 26.5 α-(1→4)-D-DD; 3,6-DA; β-(1→3)-D-DD 绿藻[11]
(Conglobata kjellman)ULV 0.068 30.3 →4)-α/β-L-Rhap-(1→; →4)-β-D-Xylp-(1→; →4)-β-D-GlcAp-(1→ 施氏鲟鱼[12]
(Acipenser schrenckii)CS 0.300 48.9 →4)-β-D-GlcA-(1→3)-β-D-GalNAcp4(OSO3−)-(1→; →4)-β-D-GlcA-(1→3)-β-D-GalNAc-(1→ 鲨鱼[13]
(Shark cartilage)KS 0.045 26.1 →3)-β-D-Gal-(1→4)-β-D-GlcNAc-(1→ 注:FUC:岩藻聚糖硫酸酯;CGN:卡拉胶;ULV:石莼聚糖;CS:硫酸软骨素;FCS:岩藻糖基化硫酸软骨素;KS:硫酸可拉坦;Fuc:岩藻糖;Xyl:木糖;Gal:半乳糖;GlcA:葡萄糖醛酸;GalNAc:N-乙酰半乳糖胺;Rha:鼠李糖;DD:吡喃半乳糖;DA:脱水半乳糖。 -
[1] 李俊慧, 李珊, 胡亚芹, 等. 食源性海洋硫酸多糖的神经保护构效机理研究进展[J]. 中国食品学报,2017,17(4):155−164. [LI Junhui, LI Shan, HU Yaqin, et al. Research progress on the neuroprotective structure-activity mechanism of foodborne marine sulfate polysaccharides[J]. Journal of Chinese Institute of Food Science and Technology,2017,17(4):155−164.] LI Junhui, LI Shan, HU Yaqin, et al. Research progress on the neuroprotective structure-activity mechanism of foodborne marine sulfate polysaccharides[J]. Journal of Chinese Institute of Food Science and Technology, 2017, 17(4): 155−164.
[2] LI Y, QIN J, CHENG Y, et al. Marine sulfated polysaccharides:Preventive and therapeutic effects on metabolic syndrome:A review[J]. Marine Drugs,2021,19(11):608. doi: 10.3390/md19110608
[3] MUTHUKUMAR J, CHIDAMBARAM R, SUKUMARAN S. Sulfated polysaccharides and its commercial applications in food industries—A review[J]. Journal of Food Science Technology,2021,58(7):2453−2466. doi: 10.1007/s13197-020-04837-0
[4] FERREIRA S S, PASSOS C P, MADUREIRA P, et al. Structure-function relationships of immunostimulatory polysaccharides:A review[J]. Carbohydrate Polymers,2015,132:378−396. doi: 10.1016/j.carbpol.2015.05.079
[5] 谷福蝶, 周钰, 陈慧莹, 等. “蓝色食物”来源多糖的免疫调节活性研究进展[J]. 食品科学,2023,44(13):272−280. [GU Fudie, ZHOU Yu, CHEN Huiying, et al. Research progress on the immunomodulatory activity of polysaccharides derived from "blue food"[J]. Food Science,2023,44(13):272−280.] doi: 10.7506/spkx1002-6630-20220730-343 GU Fudie, ZHOU Yu, CHEN Huiying, et al. Research progress on the immunomodulatory activity of polysaccharides derived from "blue food"[J]. Food Science, 2023, 44(13): 272−280. doi: 10.7506/spkx1002-6630-20220730-343
[6] HUANG L, SHEN M, MORRIS G A, et al. Sulfated polysaccharides:Immunomodulation and signaling mechanisms[J]. Trends in Food Science & Technology,2019,92:1−11.
[7] JIANG J L, ZHANG W Z, NI W X, et al. Insight on structure-property relationships of carrageenan from marine red algal:A review[J]. Carbohydrate Polymers,2021,257:117642. doi: 10.1016/j.carbpol.2021.117642
[8] YU L, XUE C, CHANG Y, et al. Structure and rheological characteristics of fucoidan from sea cucumber Apostichopus japonicus[J]. Food Chemistry,2015,180:71−76. doi: 10.1016/j.foodchem.2015.02.034
[9] ZHU Z, DONG X, YAN C, et al. Structural features and digestive behavior of fucosylated chondroitin sulfate from sea cucumbers Stichopus japonicus[J]. Journal of Agricultural Food Chemistry,2019,67(37):10534−10542. doi: 10.1021/acs.jafc.9b04996
[10] KHOTIMCHENKO M, TIASTO V, KALITNIK A, et al. Antitumor potential of carrageenans from marine red algae[J]. Carbohydrate Polymers,2020,246:116568. doi: 10.1016/j.carbpol.2020.116568
[11] CAO S, YANG Y, LIU S, et al. Immunomodulatory activity in vitro and in vivo of a sulfated polysaccharide with novel structure from the green alga ulva Conglobata kjellman[J]. Marine Drugs,2022,20(7):447. doi: 10.3390/md20070447
[12] WANG T, ZHANG S, REN S, et al. Structural characterization and proliferation activity of chondroitin sulfate from the sturgeon, Acipenser schrenckii[J]. International Journal of Biological Macromolecules,2020,164:3005−3011. doi: 10.1016/j.ijbiomac.2020.08.110
[13] SHANG Q, LI Q, ZHANG M, et al. Dietary keratan sulfate from shark cartilage modulates gut microbiota and increases the abundance of Lactobacillus spp[J]. Marine Drugs,2016,14(12):224. doi: 10.3390/md14120224
[14] 王莹莹, 张振坤, 李亚, 等. 岩藻多糖在肿瘤治疗中的作用[J]. 郑州大学学报(医学版),2021,56(1):47−52. [WANG Yingying, ZHANG Zhenkun, LI Ya, et al. The role of fucoidan in tumor treatment[J]. Journal of Zhengzhou University (Medical Edition),2021,56(1):47−52.] WANG Yingying, ZHANG Zhenkun, LI Ya, et al. The role of fucoidan in tumor treatment[J]. Journal of Zhengzhou University (Medical Edition), 2021, 56(1): 47−52.
[15] SENTHILKUMAR K, MANIVASAGAN P, VENKATESAN J, et al. Brown seaweed fucoidan:Biological activity and apoptosis, growth signaling mechanism in cancer[J]. International Journal of Biological Macromolecules,2013,60:366−374. doi: 10.1016/j.ijbiomac.2013.06.030
[16] YUGUCHI Y, TRAN V T T, BUI L M, et al. Primary structure, conformation in aqueous solution, and intestinal immunomodulating activity of fucoidan from two brown seaweed species Sargassum crassifolium and Padina australis[J]. Carbohydrate Polymers,2016,147:69−78. doi: 10.1016/j.carbpol.2016.03.101
[17] 安子哲, 张朝辉, 刘梦阳, 等. 海参硫酸多糖化学组成与结构的研究进展[J]. 食品科学,2022,43(7):289−297. [AN Zizhe, ZHANG Chaohui, LIU Mengyang, et al. Research progress on the chemical composition and structure of sulfated polysaccharides from sea cucumber[J]. Food Science,2022,43(7):289−297.] doi: 10.7506/spkx1002-6630-20210315-189 AN Zizhe, ZHANG Chaohui, LIU Mengyang, et al. Research progress on the chemical composition and structure of sulfated polysaccharides from sea cucumber[J]. Food Science, 2022, 43(7): 289−297. doi: 10.7506/spkx1002-6630-20210315-189
[18] KARIYA Y, MULLOY B, IMAI K, et al. Isolation and partial characterization of fucan sulfates from the body wall of sea cucumber Stichopus japonicus and their ability to inhibit osteoclastogenesis[J]. Carbohydrate Research,2004,339(7):1339−1346. doi: 10.1016/j.carres.2004.02.025
[19] YANG W, CAI Y, YIN R, et al. Structural analysis and anticoagulant activities of two sulfated polysaccharides from the sea cucumber Holothuria coluber[J]. International Journal of Biological Macromolecules,2018,115:1055−1062. doi: 10.1016/j.ijbiomac.2018.04.175
[20] 徐元庆, 王哲奇, 张静, 等. 岩藻多糖的抗氧化功能研究进展[J]. 天然产物研究与开发,2020,32(10):1782−1793. [XU Yuanqing, WANG Zheqi, ZHANG Jing, et al. Research progress on the antioxidant function of fucoidan[J]. Research and Development of Natural Products,2020,32(10):1782−1793.] XU Yuanqing, WANG Zheqi, ZHANG Jing, et al. Research progress on the antioxidant function of fucoidan[J]. Research and Development of Natural Products, 2020, 32(10): 1782−1793.
[21] BARBOSA A I, COUTINHO A J, COSTA LIMA S A, et al. Marine polysaccharides in pharmaceutical applications:Fucoidan and chitosan as key players in the drug delivery match field[J]. Marine Drugs,2019,17(12):654. doi: 10.3390/md17120654
[22] PRADHAN B, KI J S. Biological activity of algal derived carrageenan:A comprehensive review in light of human health and disease[J]. International Journal of Biological Macromolecules,2023,238:124085. doi: 10.1016/j.ijbiomac.2023.124085
[23] 沈伟. 低分子量κ-卡拉胶烷氧基化衍生物合成及生物活性的研究[D]. 汕头:汕头大学, 2008. [SHEN Wei. Low molecular weight κ- Study on the synthesis and biological activity of alkoxylated derivatives of carrageenan[D]. Shantou:Shantou University, 2008.] SHEN Wei. Low molecular weight κ- Study on the synthesis and biological activity of alkoxylated derivatives of carrageenan[D]. Shantou: Shantou University, 2008.
[24] 田秀芳. 低分子量κ-卡拉胶O-琥珀酰基化衍生物的合成、表征及生物活性的研究[D]. 汕头:汕头大学, 2006. [TIAN Xiufang. Low molecular weight κ- Synthesis, characterization, and biological activity of O-succinylated derivatives of carrageenan[D]. Shantou:Shantou University, 2006.] TIAN Xiufang. Low molecular weight κ- Synthesis, characterization, and biological activity of O-succinylated derivatives of carrageenan[D]. Shantou: Shantou University, 2006.
[25] CHEN H, WU D, MA W, et al. Strong fish gelatin hydrogels enhanced by carrageenan and potassium sulfate[J]. Food Hydrocolloids,2021,119:106841. doi: 10.1016/j.foodhyd.2021.106841
[26] SINTHUSAMRAN S, BENJAKUL S, SWEDLUND P J, et al. Physical and rheological properties of fish gelatin gel as influenced by κ-carrageenan[J]. Food Bioscience,2017,20:88−95. doi: 10.1016/j.fbio.2017.09.001
[27] 高鑫, 山珊, 曾德永, 等. 石莼属绿藻多糖的生物活性研究进展[J]. 食品工业科技, 2021, 42(2):364−369. [GAO Xin, SHAN Shan, ZENG Deyong, et al. Research progress on the biological activity of polysaccharides from Ulva genus green algae[J]. Food Industry Technology 2021, 42 (2):364−369.] GAO Xin, SHAN Shan, ZENG Deyong, et al. Research progress on the biological activity of polysaccharides from Ulva genus green algae[J]. Food Industry Technology 2021, 42 (2): 364−369.
[28] TZIVELEKA L A, IOANNOU E, ROUSSIS V. Ulvan, a bioactive marine sulphated polysaccharide as a key constituent of hybrid biomaterials:A review[J]. Carbohydrate Polymers,2019,218:355−370. doi: 10.1016/j.carbpol.2019.04.074
[29] MOU J, LI Q, QI X, et al. Structural comparison, antioxidant and anti-inflammatory properties of fucosylated chondroitin sulfate of three edible sea cucumbers[J]. Carbohydrate Polymers,2018,185:41−47. doi: 10.1016/j.carbpol.2018.01.017
[30] LI Q, HU F, ZHU B, et al. Insights into ulvan lyase:Review of source, biochemical characteristics, structure and catalytic mechanism[J]. Critical Reviews in Biotechnology,2020,40(3):432−441. doi: 10.1080/07388551.2020.1723486
[31] WANG H, CAO Z, YAO L, et al. Insights into the edible and biodegradable ulvan-based films and coatings for food packaging[J]. Foods,2023,12(8):1622. doi: 10.3390/foods12081622
[32] 王宏玲, 罗盛, 杨劲松. 硫酸软骨素二糖重复片段的合成[J]. 华西药学杂志,2022,37(1):15−18. [WANG Hongling, LUO Sheng, YANG Jinsong. Synthesis of chondroitin sulfate disaccharide repeat fragments[J]. Huaxi Pharmaceutical Journal,2022,37(1):15−18.] WANG Hongling, LUO Sheng, YANG Jinsong. Synthesis of chondroitin sulfate disaccharide repeat fragments[J]. Huaxi Pharmaceutical Journal, 2022, 37(1): 15−18.
[33] YANG J, SHEN M, WEN H, et al. Recent advance in delivery system and tissue engineering applications of chondroitin sulfate[J]. Carbohydrate Polymers,2020,230:115650. doi: 10.1016/j.carbpol.2019.115650
[34] 左格格, 钟赛意, 陈菁, 等. 罗非鱼加工副产物不同部位硫酸软骨素的制备、理化性质及结构表征[J]. 食品科学,2022,43(24):67−73. [ZUO Gege, ZHONG Saiyi, CHEN Jing, et al. Preparation, physicochemical properties, and structural characterization of chondroitin sulfate from different parts of tilapia processing by-products[J]. Food Science,2022,43(24):67−73.] doi: 10.7506/spkx1002-6630-20211013-119 ZUO Gege, ZHONG Saiyi, CHEN Jing, et al. Preparation, physicochemical properties, and structural characterization of chondroitin sulfate from different parts of tilapia processing by-products[J]. Food Science, 2022, 43(24): 67−73. doi: 10.7506/spkx1002-6630-20211013-119
[35] REGINSTER J Y, VERONESE N. Highly purified chondroitin sulfate:A literature review on clinical efficacy and pharmacoeconomic aspects in osteoarthritis treatment[J]. Aging Clinical and Experimental Research,2021,33(1):37−47. doi: 10.1007/s40520-020-01643-8
[36] 高洁, 赵玲, 马丽曼, 等. 鱼源硫酸软骨素的研究进展[J]. 食品安全质量检测学报,2020,11(22):8166−8172. [GAO Jie, ZHAO Ling, MA Liman, et al. Research progress on fish derived chondroitin sulfate[J]. Journal of Food Safety and Quality Testing,2020,11(22):8166−8172.] GAO Jie, ZHAO Ling, MA Liman, et al. Research progress on fish derived chondroitin sulfate[J]. Journal of Food Safety and Quality Testing, 2020, 11(22): 8166−8172.
[37] 白雪, 高昕, 赵雪, 等. 鲟鱼软骨硫酸软骨素的制备及结构分析[J]. 中国海洋药物,2022,41(2):28−36. [BAI Xue, GAO Xin, ZHAO Xue, et al. Preparation and structural analysis of chondroitin sulfate from sturgeon cartilage[J]. China Marine Medicine,2022,41(2):28−36.] BAI Xue, GAO Xin, ZHAO Xue, et al. Preparation and structural analysis of chondroitin sulfate from sturgeon cartilage[J]. China Marine Medicine, 2022, 41(2): 28−36.
[38] GONG P X, LI Q Y, WU Y C, et al. Structural elucidation and antidiabetic activity of fucosylated chondroitin sulfate from sea cucumber Stichopus japonicas[J]. Carbohydrate Polymers,2021,262:117969. doi: 10.1016/j.carbpol.2021.117969
[39] MOU J, LI Q, SHI W, et al. Chain conformation, physicochemical properties of fucosylated chondroitin sulfate from sea cucumber Stichopus chloronotus and its in vitro fermentation by human gut microbiota[J]. Carbohydrate Polymers,2020,228:115359. doi: 10.1016/j.carbpol.2019.115359
[40] USTYUZHANINA N E, BILAN M I, DMITRENOK A S, et al. Fucosylated chondroitin sulfates from the sea cucumbers Paracaudina chilensis and Holothuria hilla:Structures and anticoagulant activity[J]. Marine Drugs,2020,18(11):540. doi: 10.3390/md18110540
[41] UCHIMURA K. Keratan sulfate:Biosynthesis, structures, and biological functions[J]. Methods in Molecular Biology,2015,1229:389−400.
[42] MELROSE J. Keratan sulfate (KS)-proteoglycans and neuronal regulation in health and disease:The importance of KS-glycodynamics and interactive capability with neuroregulatory ligands[J]. Journal of Neurochemistry,2019,149(2):170−194. doi: 10.1111/jnc.14652
[43] 国家药典委员会. 中华人民共和国药典[M]. 第二部. 北京:中国医药科技出版社, 2015. [National Pharmacopoeia Committee. Pharmacopoeia of the People's Republic of China[M]. Part II. Beijing:China Medical Science & Technology Press, 2015.] National Pharmacopoeia Committee. Pharmacopoeia of the People's Republic of China[M]. Part II. Beijing: China Medical Science & Technology Press, 2015.
[44] SONG S, PENG H, WANG Q, et al. Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2[J]. Food Function,2020,11(9):7415−7420. doi: 10.1039/D0FO02017F
[45] KOLLINIATI O, IERONYMAKI E, VERGADI E, et al. Metabolic regulation of macrophage activation[J]. Journal of Innate Immunity,2022,14(1):48−64.
[46] 张祺, 李学敏, 李兆杰, 等. 海参岩藻聚糖硫酸酯对巨噬细胞的调节作用及信号通路研究[J]. 中国药理学通报,2015,31(1):87−92. [ZHANG Qi, LI Xuemin, LI Zhaojie, et al. The regulatory effect and signaling pathway of sea cucumber fucoidan sulfate on macrophages[J]. Chinese Pharmacological Bulletin,2015,31(1):87−92.] doi: 10.3969/j.issn.1001-1978.2015.01.019 ZHANG Qi, LI Xuemin, LI Zhaojie, et al. The regulatory effect and signaling pathway of sea cucumber fucoidan sulfate on macrophages[J]. Chinese Pharmacological Bulletin, 2015, 31(1): 87−92. doi: 10.3969/j.issn.1001-1978.2015.01.019
[47] KIDGELL J T, GLASSON C R, Magnusson M, et al. The molecular weight of ulvan affects the in vitro inflammatory response of a murine macrophage[J]. International Journal of Biological Macromolecules,2020,150:839−848. doi: 10.1016/j.ijbiomac.2020.02.071
[48] JIANG S, YIN H, QI X, et al. Immunomodulatory effects of fucosylated chondroitin sulfate from Stichopus chloronotus on RAW 264.7 cells[J]. Carbohydrate Polymers,2021,251:117088. doi: 10.1016/j.carbpol.2020.117088
[49] 萨仁娜. 海带岩藻聚糖分级纯化及对肉仔鸡巨噬细胞免疫调节的研究[D]. 北京:中国农业科学院, 2008. [SA Renna. Classification and purification of kelp fucoidan and its effect on immune regulation of broiler macrophages[D]. Beijing:Chinese Academy of Agricultural Sciences, 2008.] SA Renna. Classification and purification of kelp fucoidan and its effect on immune regulation of broiler macrophages[D]. Beijing: Chinese Academy of Agricultural Sciences, 2008.
[50] JAYAWARDENA T U, SANJEEWA K A, Nagahawatta D, et al. Anti-Inflammatory effects of sulfated polysaccharide from Sargassum swartzii in macrophages via blocking TLR/NF-Kb signal transduction[J]. Marine Drugs,2020,18(12):601. doi: 10.3390/md18120601
[51] XU H P, HITOSHI K, TOMOMI I, et al. The keratan sulfate disaccharide gal(6S03)β 1, 4-GlcNAc(6S03) modulates interleukin 12 production by macrophages in murine Thy-1 type autoimmune disease[J]. The Journal of Biological Chemistry,2005(21):280−284.
[52] BAE S, YIM J, LEE H, et al. Activation of murine peritoneal macrophages by sulfated exopolysaccharide from marine microalga Gyrodinium impudicum (strain KG03):Involvement of the NF-κB and JNK pathway[J]. International Immunopharmacology,2021,99:107981. doi: 10.1016/j.intimp.2021.107981
[53] KIM J K, CHO M L, KARNJANAPRATUM S, et al. In vitro and in vivo immunomodulatory activity of sulfated polysaccharides from Enteromorpha prolifera[J]. International Journal of Biological Macromolecules,2011,49(5):1051−1058. doi: 10.1016/j.ijbiomac.2011.08.032
[54] TAN L H. Sulfation of a polysaccharide obtained from Phellinus ribis and potential biological activities of the sulfated derivatives[J]. Carbohydrate Polymers:Scientific and Technological Aspects of Industrially Important Polysaccharides,2009,77(2):370−375.
[55] DI T, CHEN G, SUN Y, et al. Antioxidant and immunostimulating activities in vitro of sulfated polysaccharides isolated from Gracilaria rubra[J]. Journal of Functional Foods,2017,28:64−75. doi: 10.1016/j.jff.2016.11.005
[56] CRABTREE G R, CLIPSTONE N A. Signal transmission between the plasma membrane and nucleus of T lymphocytes[J]. Annual Review of Biochemistry,1994,63:1045−1083. doi: 10.1146/annurev.bi.63.070194.005145
[57] HAN S B, PARK S K, AHN H J, et al. Characterization of B cell membrane receptors of polysaccharide isolated from the root of Acanthopanax koreanum[J]. International Immunopharmacology,2003,3(5):683−691. doi: 10.1016/S1567-5769(03)00056-0
[58] AHN G, BING S J, KANG S-M, et al. The JNK/NFκB pathway is required to activate murine lymphocytes induced by a sulfated polysaccharide from Ecklonia cava[J]. Biochimica et Biophysica Acta-General Subjects,2013,1830(3):2820−2829. doi: 10.1016/j.bbagen.2012.12.008
[59] JANG J Y, MOON S Y, JOO H G. Differential effects of fucoidans with low and high molecular weight on the viability and function of spleen cells[J]. Food and Chemical Toxicology:An International Journal Published for the British Industrial Biological Research Association,2014,68:234−238. doi: 10.1016/j.fct.2014.03.024
[60] MOREIRA A S P, GASPAR D, FERREIRA S S, et al. Water-soluble Saccharina latissima polysaccharides and relation of their structural characteristics with in vitro immunostimulatory and hypocholesterolemic activities[J]. Marine Drugs,2023,21(3):183. doi: 10.3390/md21030183
[61] ZHAO X, JIAO G, YANG Y, et al. Structure and immunomodulatory activity of a sulfated agarose with pyruvate and xylose substitutes from Polysiphonia senticulosa Harvey[J]. Carbohydrate Polymers,2017,176:29−37. doi: 10.1016/j.carbpol.2017.08.065
[62] PARK H B, HWANG J, ZHANG W, et al. Polysaccharide from Codium fragile induces anti-cancer immunity by activating natural killer cells[J]. Marine Drugs,2020,18(12):626. doi: 10.3390/md18120626
[63] SURAYOT U, LEE S, YOU S. Effects of sulfated fucan from the sea cucumber Stichopus japonicus on natural killer cell activation and cytotoxicity[J]. International Journal of Biological Macromoleculesm,2018,108:177−184. doi: 10.1016/j.ijbiomac.2017.11.102
[64] BRUSILOVSKY M, CORDOBA M, ROSENTAL B, et al. Genome-wide siRNA screen reveals a new cellular partner of NK cell receptor KIR2DL4:Heparan sulfate directly modulates KIR2DL4-mediated responses[J]. Journal of Immunology,2013,191(10):5256−5267. doi: 10.4049/jimmunol.1302079
[65] ZHANG W, AN E K, PARK H B, et al. Ecklonia cava fucoidan has potential to stimulate natural killer cells in vivo[J]. International Journal of Biological Macromolecules,2021,185:111−121. doi: 10.1016/j.ijbiomac.2021.06.045
[66] MERLE N S, CHURCH S E, FREMEAUX-BACCHI V, et al. Complement system part I - molecular mechanisms of activation and regulation[J]. Frontiers in Immunology,2015,6:262.
[67] REID K B, TURNER M W. Mammalian lectins in activation and clearance mechanisms involving the complement system; Proceedings of the Springer seminars in immunopathology[J]. Springer Seminars in Immunopathology,1994,15:307−26. doi: 10.1007/BF01837363
[68] ZVYAGINTSEVA T N, SHEVCHENKO N M, NAZAROVA I V, et al. Inhibition of complement activation by water-soluble polysaccharides of some far-eastern brown seaweeds[J]. Comparative Biochemistry and Physiology Part C Toxicology & Pharmacology,2000,126(3):209−215.
[69] BLONDIN C, FISCHER E, BOISSON-VIDAL C, et al. Inhibition of complement activation by natural sulfated polysaccharides (fucans) from brown seaweed[J]. Molecular Immunology,1994,31(4):247−253. doi: 10.1016/0161-5890(94)90121-X
[70] TISSOT B, MONTDARGENT B, CHEVOLOT L, et al. Interaction of fucoidan with the proteins of the complement classical pathway[J]. Acta Biochimica Et Biophysica Sinica,2003,1651(1-2):5−16. doi: 10.1016/S1570-9639(03)00230-9
[71] COFRANCSCO E, RADAELLI F, POGLIANIA E, et al. Correlation of sulfate content and degree of carboxylation of heparin and related glycosaminoglycans with anticomplement activity. Relationships to the anticoagulant and platelet-aggregating activities[J]. Thrombosis Research,1979,14(1):179−187. doi: 10.1016/0049-3848(79)90036-7
[72] SHARATH M D, MERCHANT Z M, KIM Y S, et al. Small heparin fragments regulate the amplification pathway of complement[J]. Immunopharmacology,1985,9(2):73−80. doi: 10.1016/0162-3109(85)90002-5
[73] LI L, LI Y, IJAZ M, et al. Review on complement analysis method and the roles of glycosaminoglycans in the complement system[J]. Carbohydrate Polymers,2015,134:590−597. doi: 10.1016/j.carbpol.2015.08.028
[74] TISSOT B, DANIEL R, PLACE C. Interaction of the C1 complex of complement with sulfated polysaccharide and DNA probed by single molecule fluorescence microscopy[J]. European Journal of Biochemistry,2003,270(23):4714−4720. doi: 10.1046/j.1432-1033.2003.03870.x
[75] LI Y, LIU S, DING Y, et al. Structure, in vitro digestive characteristics and effect on gut microbiota of sea cucumber polysaccharide fermented by Bacillus subtilis Natto[J]. Food Research International,2023,169:112872. doi: 10.1016/j.foodres.2023.112872
[76] QIN J, LI R, RAES J, et al. A human gut microbial gene catalogue established by metagenomic sequencing[J]. Nature,2010,464(7285):59−65. doi: 10.1038/nature08821
[77] ZHU Z, HAN Y, DING Y, et al. Health effects of dietary sulfated polysaccharides from seafoods and their interaction with gut microbiota[J]. Comprehensive Reviews in Food Science and Food Safety,2021,20(3):2882−2913. doi: 10.1111/1541-4337.12754
[78] LIU X, ZHANG Y, LI W, et al. Fucoidan ameliorated dextran sulfate sodium-induced ulcerative colitis by modulating gut microbiota and bile acid metabolism[J]. Journal of Agricultural and Food Chemistry,2022,70(47):14864−14876. doi: 10.1021/acs.jafc.2c06417
[79] ZHU Z, ZHU B, SUN Y, et al. Sulfated polysaccharide from sea cucumber modulates the gut microbiota and its metabolites in normal mice[J]. International Journal of Biological Macromolecules, 2018, 120(Pt A):502−512.
[80] HU S, WANG J, XU Y, et al. Anti-inflammation effects of fucosylated chondroitin sulphate from Acaudina molpadioides by altering gut microbiota in obese mice[J]. Food Function,2019,10(3):1736−1746. doi: 10.1039/C8FO02364F
[81] BISSON-BOUTELLIEZ C, MASSIN F, DUMAS D, et al. Desulfovibrio spp. survive within KB cells and modulate inflammatory responses[J]. Molecular Oral Microbiology,2010,25(3):226−235. doi: 10.1111/j.2041-1014.2009.00550.x
[82] ZHU Z, ZHU B, SUN Y, et al. Sulfated polysaccharide from sea cucumber and its depolymerized derivative prevent obesity in association with modification of gut microbiota in high-fat diet-fed mice[J]. Molecular Nutrition & Food Research,2018,62(23):1800446.





 下载:
下载:
 下载:
下载:
