Sustained Release Properties of Acid Resistant Carboxymethyl Chitosan Gel Microspheres Loaded with Anthocyanins
-
摘要: 为拓宽壳聚糖基水凝胶的应用范围,构筑可食用的花色苷(ACNs)递送体系,采用反相乳液法,以玉米油为连续相,水杨醛(SA)为交联剂,制备了具有抗酸性能的载ACNs羧甲基壳聚糖(CMCS)凝胶微球(ACNs/CMCS-SA),表征了其结构和形貌,研究了其稳定性、溶胀性能和缓释性能。结果表明,ACNs/CMCS-SA具有微米级粒径,表面光滑呈圆形;ACNs/CMCS-SA在酸性条件下稳定性良好,溶胀和缓释过程展现pH响应性;ACNs的释放行为符合Weibull模型,pH7和9时为扩散和骨架溶蚀复合释放机制控制,pH3和5时为菲克扩散和CaseⅡ transport(0级-溶胀依赖性释放)联合释放机制控制。ACNs/CMCS-SA制备方法简单,过程绿色;对ACNs展现良好的胃酸保护和肠道释放性能,为环境友好型药物包封材料的开发和ACNs的应用提供了理论和实践参考。Abstract: This study was aimed at broadening the application range of chitosan hydrogels and constructing an edible anthocyanins (ACNs) delivery system. The acid resistant carboxymethyl chitosan (CMCS) gel microspheres (ACNs/CMCS-SA) loaded with ACNs were prepared by inversion-phase emulsion method. During the process, corn oil was used as continuous phase and salicylaldehyde (SA) was used as crosslinking agent. The structure and morphology of ACNs/CMCS-SA were characterized. And the stability, swelling ability and sustained release properties were studied. The results showed that ACNs/CMCS-SA had a micron particle size with smooth and round surface. The gel microspheres showed good acid resistance and pH responsiveness during swelling and sustained release experiments. The sustained release behavior of ACNs conformed to Weibull model. In pH7 and pH9 media, the release process of ACNs was controlled by a combination mechanism of diffusion and skeleton dissolution. In pH3 and 5 media, it was controlled by a combination of Fick diffusion and Case Ⅱ transport (order 0 grade-swelling dependent release). The preparation method of ACNs/CMCS-SA was simple and green, and the sample exhibited good gastric acid protection and intestinal release performance for ACNs. Therefore, this study lays a theoretical and experimental foundation for the development of environmentally friendly drug encapsulation materials and the application of ACNs.
-
花色苷(anthocyanins,ACNs)是花青素与糖以糖苷键结合形成的一种类黄酮化合物,具有抗氧化、抗癌等多种生理功能[1−2],在食品、保健品及生物医药领域具有广阔的应用前景。ACNs通常由小肠上皮细胞吸收[3],由于其多酚羟基结构,导致其易受酶、pH、温度等因素影响,在胃消化过程中快速降解[4−5],无法在生理消化过程中保持稳定。药代动力学研究表明,仅有0.005%~1.2%的ACNs被吸收到血液中[6]。因此,保证ACNs在消化过程中不被提前降解,并能稳定输送到肠道中非常关键。
设计ACNs的生物递送体系,如水凝胶[7]、脂质体[8]、微胶囊[9]等是提高其稳定性的有效方法。脂质体本身稳定性较差,长期贮存易聚集、泄露,且胃肠道半衰期较短,限制了其应用[10];微胶囊的性能受制备条件影响显著,其中喷雾干燥法受限于ACNs的热稳定性[11],分子包埋法受限于ACNs与壁材分子疏水区容纳能力的匹配度[12]。水凝胶具有三维网络结构,通过物理或化学交联形成,具有溶胀性能和多孔结构[13],制备方法简单、性能优良。水解氧化淀粉基多孔微凝胶能够显著延长ACNs的稳定性,37 ℃储存30 d后ACNs保存率为31%,为相同条件下溶液中游离ACNs残留率的约5倍[14]。一种新型肽基凝胶能够提高ACNs不同离子环境如K+、Na+以及Cu2+中的稳定性能;且在常温暴露于光线72 h,凝胶中ACNs的保留率最高为81.69%±1.71%,而溶液中游离ACNs的保留率仅为17.73%±2.85%[15]。上述水凝胶体系虽能提高ACNs的稳定性能,但其制备过程中均无法避免地使用次氯酸钠、乙腈等物质,由于食品药品领域对安全性和生物相容性的严格要求,开发全可食用的递送体系对于花色苷的应用至关重要。壳聚糖基亚胺水凝胶具有低细胞毒性和良好的细胞相容性[16],适合作为ACNs的递送体系,已有研究表明,其能够显著提高ACNs的热稳定性能,并展现pH响应释放性能[17]。但壳聚糖水溶性较差,通常需要在酸性条件下才能溶解,限制了壳聚糖基亚胺水凝胶的应用;此外,亚胺键在酸性条件下不稳定,pH<2时亚胺键断裂,凝胶溶胶[18],因此壳聚糖基亚胺水凝胶无法在强酸条件下使用。
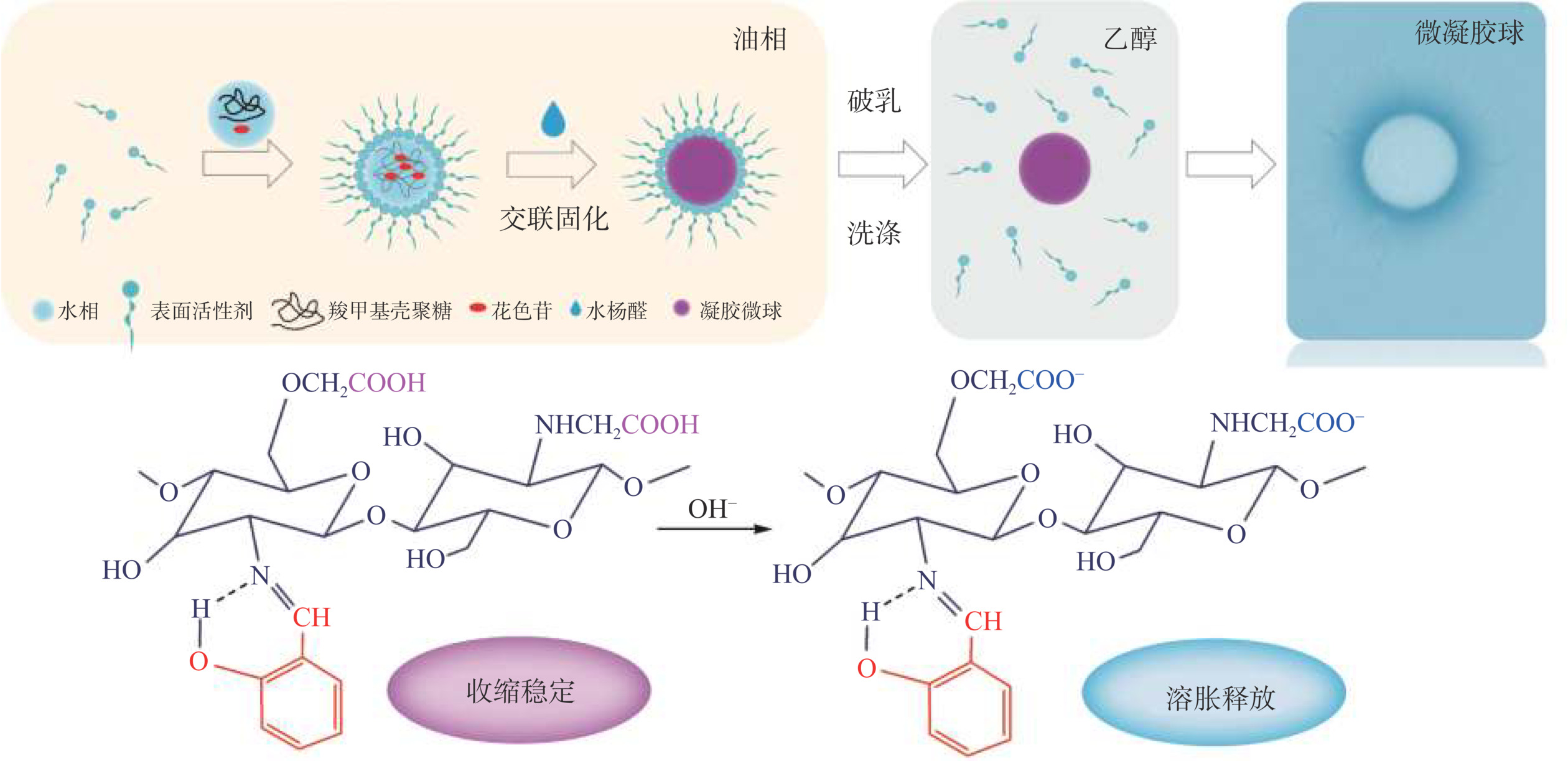
羧甲基壳聚糖(carboxymethyl chitosan,CMCS)是壳聚糖羧甲基化产物,水溶性优于壳聚糖,具有生物相容性和可生物降解性[19],其分子链中的氨基能够与醛基化合物发生席夫碱反应交联形成水凝胶[20]。CMCS分子中的羧基在碱性介质中去质子化,分子链延展[21];在酸性条件下质子化,分子链收缩维持凝胶稳定性,有望解决由于亚胺键断裂造成的凝胶不稳定问题,扩大凝胶在酸性条件下的应用范围。凝胶微球是水凝胶的一种特殊形态,其纳微米尺度的粒径,使其在靶向治疗[22]、经皮给药[23]、黏膜给药[24]中具有潜在应用价值,且在相同条件下更易被机体吸收[25]。为拓宽壳聚糖基水凝胶的应用范围,构筑全可食用的ACNs递送体系,采用反相乳液法,选择生物安全性能较好的天然醛水杨醛(salicylaldehyde,SA)为交联剂,玉米油为连续相,制备负载ACNs的羧甲基壳聚糖凝胶微球(组装机制见图1),并对其缓释性能、稳定性能、溶胀性能进行评价,以期利用CMCS的两性性能提高凝胶的稳定性能,并为壳聚糖基可食用缓释材料的开发提供新的思路。
1. 材料与方法
1.1 材料与仪器
羧甲基壳聚糖(总取代度80%) 上海笛柏生物科技有限公司;水杨醛(98%) 分析纯,北京伊诺凯科技有限公司;玉米油 中粮福临门食品营销有限公司;蓝莓提取物冻干粉(总花色苷含量25%,矢车菊素-3-O-葡萄糖苷质量分数6.5%) 西安隆择生物工程有限责任公司;矢车菊-3-O-葡萄糖苷标准品(>95%) 色谱纯,上海阿拉丁生化科技股份有限公司;磷酸二氢钾、无水乙醇、冰醋酸、氯化钾、无水碳酸钠、醋酸钠、吐温80 分析纯,天津市永大化学试剂有限公司;磷酸氢二钠 分析纯,天津市化学试剂六厂;浓盐酸 分析纯,天津标准科技有限公司。
FA2004N型电子天平 上海精密科学仪器有限公司;PHSJ-3F型实验室pH计 上海雷磁仪器厂;RCT basic型IKA磁力加热搅拌器 艾卡(广州)仪器设备有限公司;is5型傅里叶变换红外光谱仪 赛默飞世尔科技(中国)有限公司;UV-2600型紫外可见分光光度计 岛津企业管理(中国)有限公司;TM3030型台式扫描电镜 日本株式会社日立高新技术那珂事业所;TD3000型X-射线衍射仪 丹东通达科技有限公司;S90型纳米粒度及Zeta电位仪 英国马尔文仪器有限公司。
1.2 实验方法
1.2.1 凝胶微球的制备
采用反相乳液法[26]制备凝胶微球。
CMCS-SA的制备:取15 mL玉米油,加入0.6 mL吐温80,室温下磁力搅拌8 min,转速1500 r/min。滴入15 mL 1.0wt%的羧甲基壳聚糖(CMCS)水溶液,滴加速度2 d/s,滴加完成后继续搅拌10 min。继续加入0.6 mL水杨醛(SA),室温下磁力搅拌1.5 h,充分交联。然后在上述乳液中加入70 mL无水乙醇,在冰水浴中破乳30 min,转速1500 r/min。离心,保留凝胶层,用无水乙醇洗涤3次,得到羧甲基壳聚糖-水杨醛凝胶微球(CMCS-SA)。
ACNs/CMCS-SA的制备:称取0.0700 g蓝莓提取物冻干粉,溶解于15 mL 1.0wt%的CMCS水溶液中,制得含有ACNs的CMCS溶液。取15 mL玉米油,加入0.6 mL吐温80,室温下磁力搅拌8 min,转速1500 r/min。滴入含有ACNs的CMCS溶液,滴加速度2 d/s,滴加完成后继续搅拌10 min。继续加入0.6 mL SA,室温下磁力搅拌1.5 h,充分交联。然后在上述乳液中加入70 mL无水乙醇,在冰水浴中破乳30 min,转速1500 r/min。离心,保留凝胶层,用无水乙醇洗涤3次,得到载花色苷羧甲基壳聚糖-水杨醛凝胶微球(ACNs/CMCS-SA)。
1.2.2 ACNs包埋率的测定
按1.2.1中方法,保持其他制备条件不变,制得油水比(v:v)分别为0.5:1、1:1、1.5:1、2:1、2.5:1的ACNs/CMCS-SA,按式(1)计算ACNs包埋率[27]。
包埋率(%)=(1−C上×V上m×n)×100 (1) 式中:C上为第一次破乳、离心后的上清液中矢车菊-3-O-葡萄糖苷的浓度,mg/mL;V上为第一次破乳、离心后的上清液体积,mL;m为蓝莓提取物冻干粉添加质量,mg;n为蓝莓提取物冻干粉中矢车菊-3-O-葡萄糖苷的质量分数(6.5%);其中C上利用标准曲线测定。
标准曲线的绘制方法:将0.005 g的矢车菊-3-O-葡萄糖粉末溶于无水乙醇,定容于25 mL容量瓶,分别稀释到0.02、0.04、0.08、0.12、0.20 mg/mL。使用紫外分光光度计进行紫外-可见光扫描,得到矢车菊-3-O-葡萄糖苷最大吸收波长为536 nm;在此波长下进行标准曲线的绘制。标准曲线回归方程式为y=−0.20976+20.33876x,R2=0.99965。
1.2.3 表征方法
SEM表征:分别取适量不同油水比的ACNs/CMCS-SA均匀分散在无水乙醇中,使用硅片分别蘸取上述凝胶微球样品,自然干燥,粘贴到导电胶上,喷金60 s,观察形貌。
粒度及Zata电位测定:取适量ACNs/CMCS-SA均匀分散在蒸馏水中,测定平均粒径和Zata电位,测试温度25 ℃,分散相为水(折射率1.33),每个样品自动测定3次。
FT-IR表征:分别取适量CMCS-SA和ACNs/CMCS-SA,加入少量无水乙醇,用磁力搅拌器搅拌至均匀溶液后倒入聚四氟乙烯模具,自然干燥成薄膜[17];将ACNs、CMCS、SA分别与KBr粉末以1:100的质量比混合研磨至均匀粉末,装入模具,15 MPa下保持30 s压成薄片。在4000~500 cm−1范围内扫描,分辨率4 cm−1,测绘上述样品的FT-IR图谱。
UV-Vis表征:分别取适量CMCS-SA、ACNs/CMCS-SA滴入0.1 mm石英比色皿中;取2 mL ACNs水溶液于1 cm石英比色皿中,在波长200~700 nm范围测绘上述样品UV-Vis图谱。
XRD表征:分别取适量CMCS-SA、ACNs/CMCS-SA,少量多次均匀涂于载玻片上,自然干燥成膜[18];取适量CMCS充分研磨、压片。采用Cu-ka(λ=1.54 Å)辐射,扫描范围3°~50°,电压30 kV,电流20 mA,扫描速率0.01º/s,测绘上述样品XRD图谱。
1.2.4 稳定性能测定方法
分别取适量CMCS-SA和ACNs/CMCS-SA均匀分散在蒸馏水中,常温、日常光照条件,每间隔5 d测定粒径和Zata电位。
1.2.5 溶胀性能测定方法
将ACNs/CMCS-SA置于培养皿中,自然干燥48 h,制得ACNs/CMCS-SA干凝胶。分别取20 mL pH3、5、7、9的溶液于烧杯中,准确称取一定质量的ACNs/CMCS-SA干凝胶,分别放入上述不同pH溶液中,每隔一段时间取出,用滤纸擦干表面水分,称重,按式(2)计算溶胀率SR。
SR(%)=mt−m0m0×100 (2) 式中:m0为干凝胶质量,g;mt为溶胀之后凝胶质量,g。
1.2.6 缓释性能测定方法及释放动力学研究
分别取多份ACNs/CMCS-SA干凝胶,称重;将干凝胶分别浸于50 mL pH3、5、7、9的溶液中,取2 mL溶液于石英比色皿中,测定释放时间为0~1440 min内的溶液吸光度,并利用标准曲线(见表1),计算溶液浓度,按式(3)计算ACNs累计释放率Q。
表 1 标准曲线及线性相关度Table 1. Standard curve and linear correlationpH 标准曲线 R2 3 y=34.7385x+0.0321 0.99927 5 y=3.189x+0.0320 0.99938 7 y=4.437x+0.0146 0.99903 9 y=5.841x+0.0129 0.99959 Q(%)=cn×Vm×100 (3) 式中:cn为第n次溶液中ACNs的浓度,mg/mL;V为浸泡溶液的体积,mL;m为凝胶微球中负载的ACNs质量,mg。
释放动力学方程拟合:分别利用不同方程拟合释放过程,见式(4)~(8),研究不同pH对ACNs/CMCS-SA中ACNs释放行为的影响。
准一级动力学方程:ln(1−Q/Qe)=−kt (4) 准二级动力学方程:tQ=1kQe2+tQe (5) Higuchi方程:Q=kt1/2+b (6) Weibull方程:Q/Qe=1−exp(−ktd) (7) Pepaas方程:Q=ktn (8) 式中:Q为t时刻ACNs的累计释放率;Qe为平衡时ACNs的累计释放率;t为释放时间,min;k为动力学常数;b为参数;d为形状参数;n为表征扩散机制的指数。
1.3 数据处理
性能测定实验重复3次,数据结果用“平均值±标准差”表示;通过SPSS 27软件采用事后比较Duncan法进行单因素方差分析,P<0.05,认为差异显著;采用Origin 2018软件绘图。
2. 结果与分析
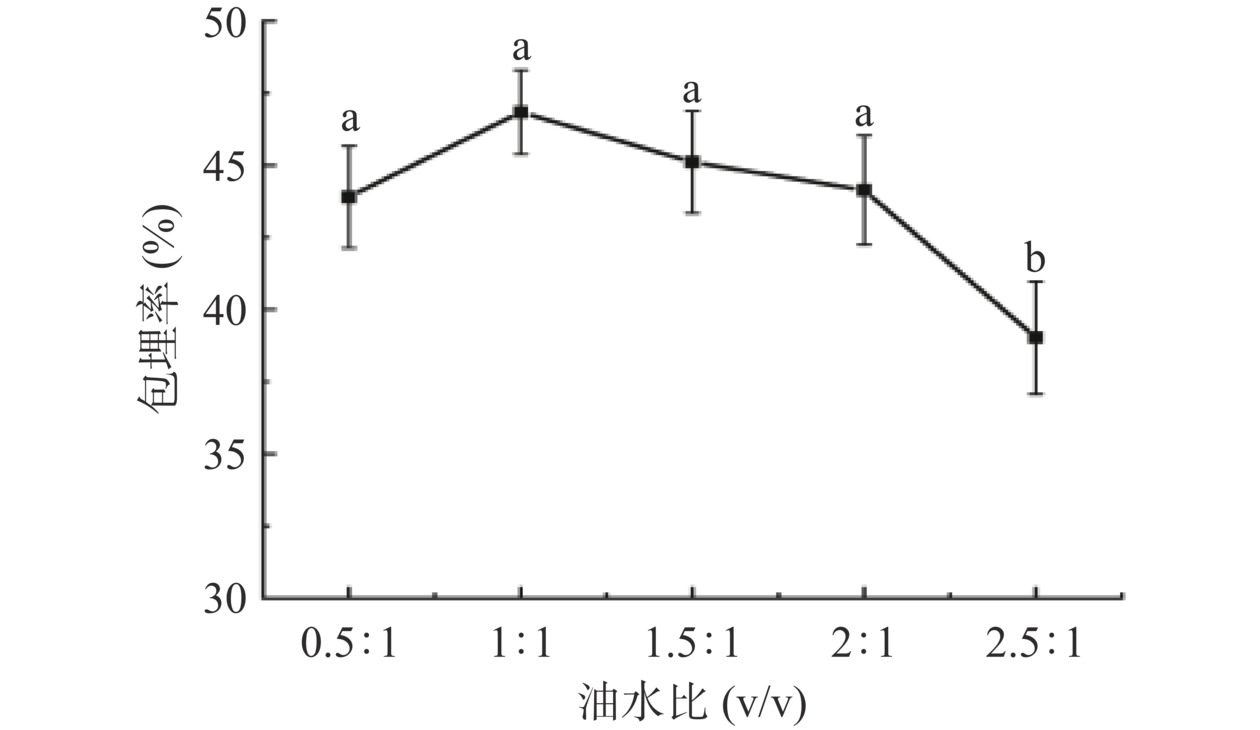
2.1 油水比对凝胶包埋率的影响
油水比对ACNs包埋率的影响如图2所示,油水比对ACNs包埋率有显著影响(P<0.004)。随油水比增加,ACNs包埋率呈先增加后下降的趋势,油水比1:1时ACNs包埋率最高,为46.84%±1.46%。
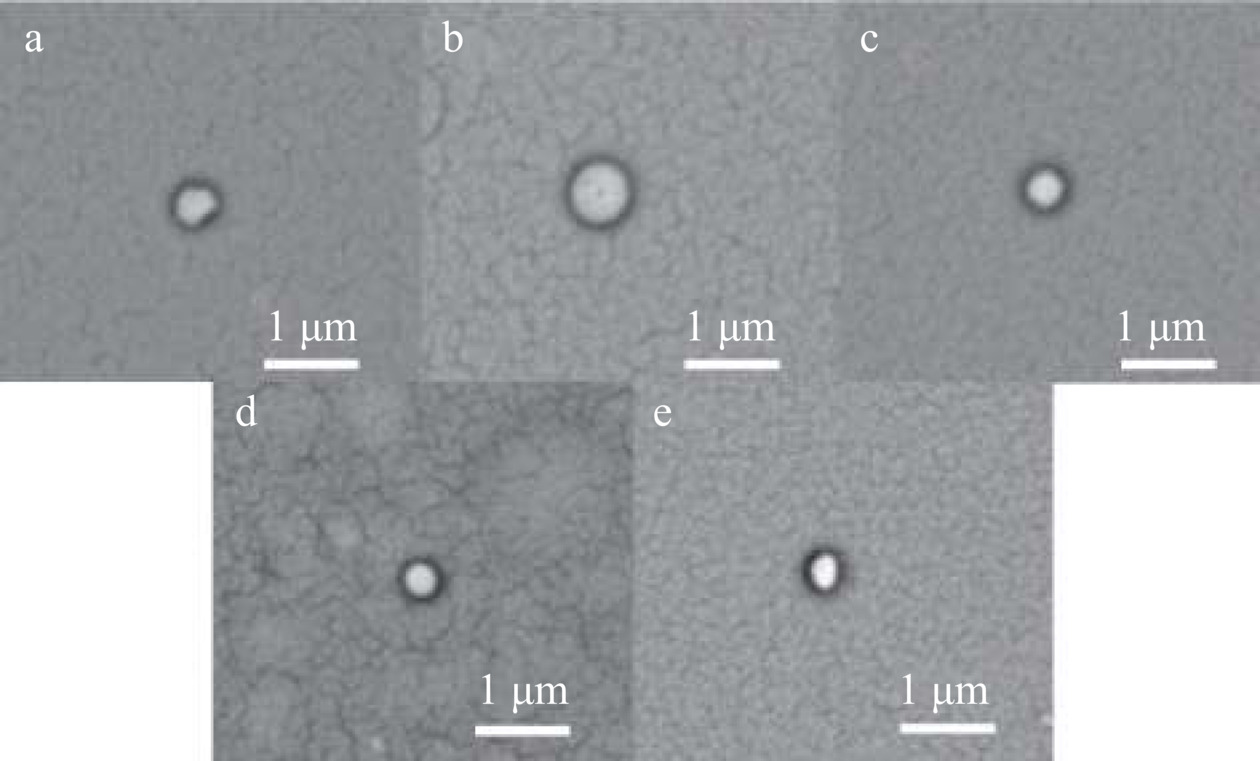
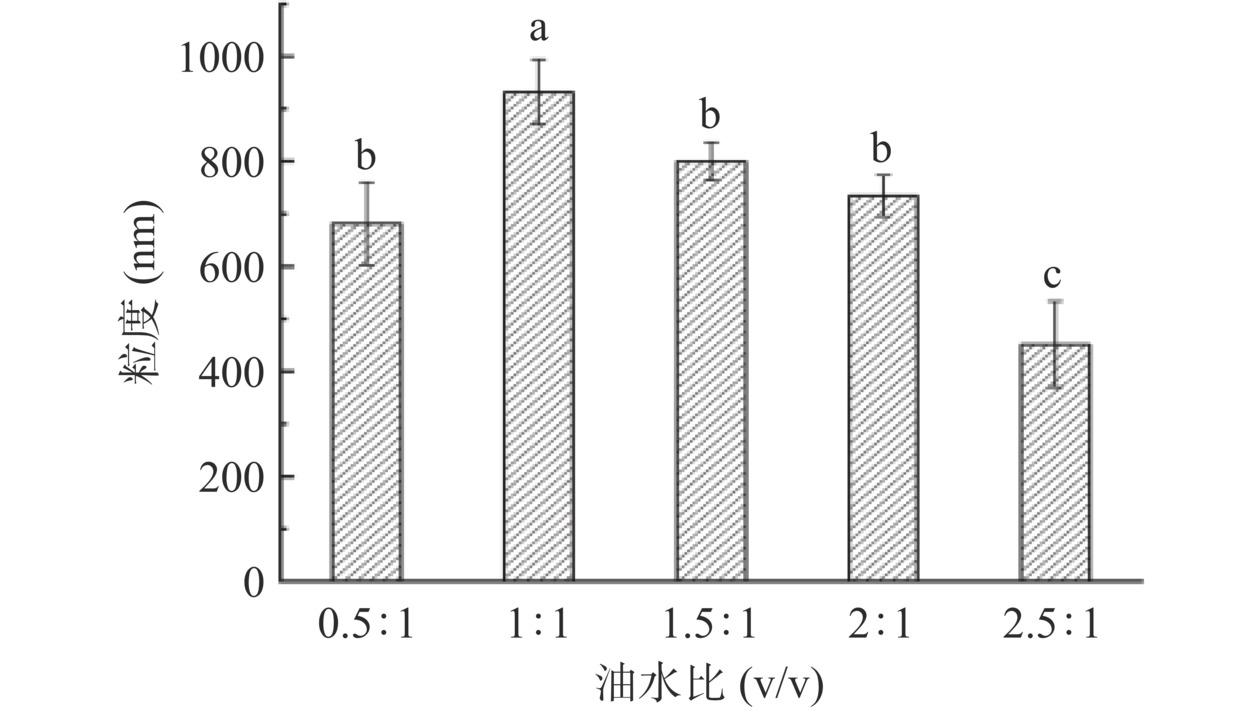
2.2 油水比对凝胶微球形态的影响
不同油水比ACNs/CMCS-SA的形态表征结果如图3、图4所示。图3中ACNs/CMCS-SA均呈现圆球形态,尺寸较为均匀,油水比1:1、1.5:1、2:1时ACNs/CMCS-SA表面更加光滑、饱满。由图4可以看出,油水比对ACNs/CMCS-SA粒径有高度显著影响(P<0.001)。油水比为0.5:1时,由于油相体积较小,无法实现对水相微滴的很好包覆;油水比>1:1时,随油相体积的增加,单个水相微滴越小,水相微滴碰撞长大的机会也越少[28]。油水比1:1时ACNs/CMCS-SA粒径最大,其大小为931.80±61.67 nm,可能为ACNs的包埋提供了更大的空间,正如2.1中油水比1:1时ACNs/CMCS-SA具有最高的包埋率。
2.3 FT-IR表征
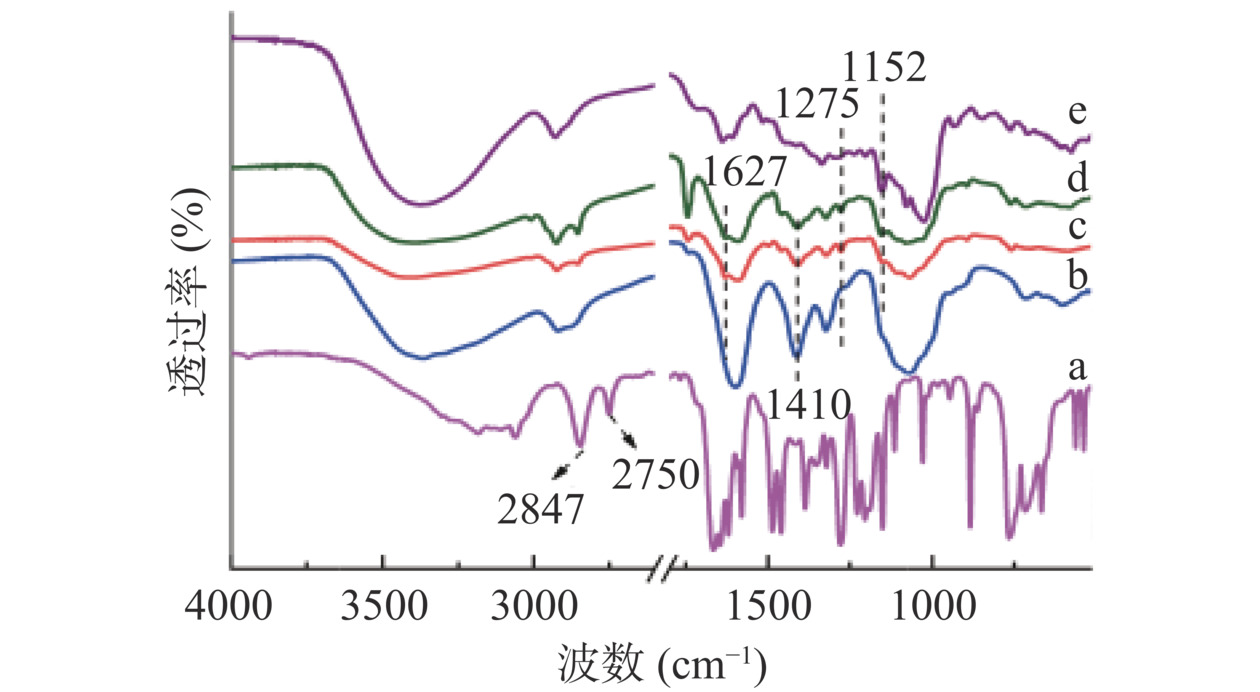
SA、CMCS、CMCS-SA、ACNs/CMCS-SA、ACNs的FT-IR见图5。图5a中2847 cm−1和2750 cm−1处为SA醛基C–H伸缩振动峰。由CMCS-SA、ACNs/CMCS-SA的FT-IR表征(图5c、d)可知,形成凝胶微球后,醛基C-H伸缩振动峰消失,1627 cm−1处出现亚胺键C=N伸缩振动峰,说明SA与CMCS发生缩合反应生成了亚胺键。图5d中1275 cm−1和1152 cm−1处特征峰分别归于酚羟基面内弯曲振动特征峰和ACNs上C-O-C对称伸缩振动特征峰[29],说明ACNs被有效包埋在CMCS-SA中。此外图5b、c、d上1410 cm−1处羧基O-H面内弯曲振动特征峰逐渐减小,是由于凝胶微球的形成和ACNs的负载使体系中羧基含量减少的结果。
2.4 UV-Vis表征
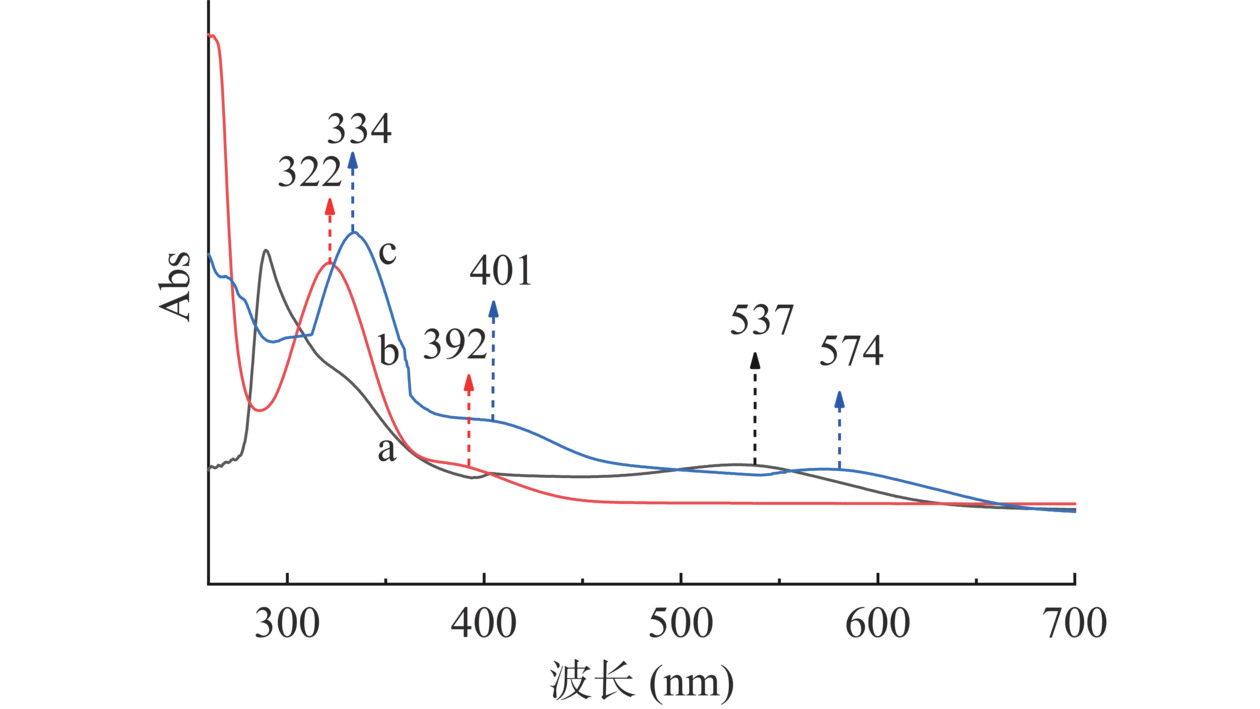
图6a中,ACNs在波长537 nm处出现多羟基黄酮类化合物特征吸收峰[30]。图6b中CMCS-SA在322 nm处吸收峰归属于苯环,392 nm处吸收峰归属于SA-亚胺的酮胺。图6c中上述特征峰均出现红移,说明CMCS-SA负载ACNs过程中,SA结构中的苯环和ACNs黄烊盐发色团(糖苷配基发色团)错位平行排布,即发生了J-聚集[14]。
2.5 XRD测定
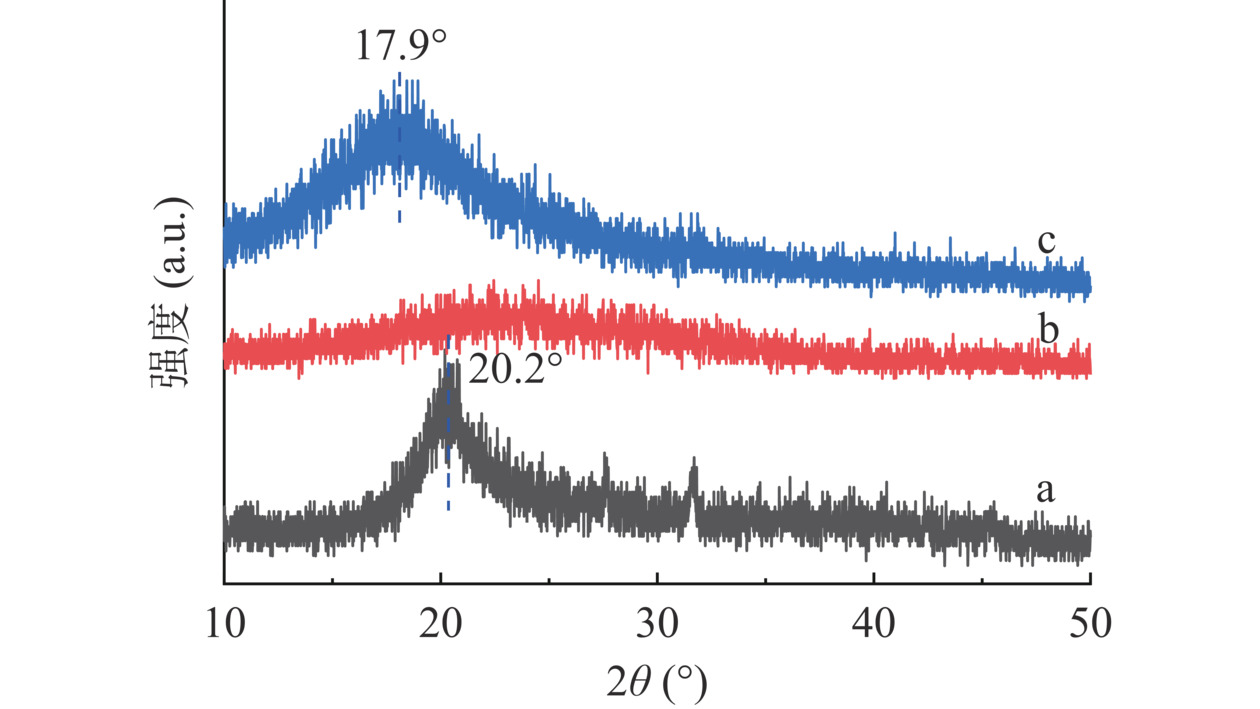
由CMCS的XRD图谱(图7a)可知,2θ=20.2°处衍射峰对应相邻糖链的规则排布。由CMCS-SA的XRD图谱(图7b)可知,凝胶微球形成后,SA的苯环进入糖链间,影响了CMCS分子链排布,衍射峰消失。由ACNs/CMCS-SA的XRD图谱(图7c)可知,包埋ACNs后,在2θ=17.9°处出现新的相邻糖链衍射峰,这是由于花色苷母核苯并吡喃环与SA的苯环间隔有序排布,且ACNs的包埋使得糖链间的距离增大,正如UV-Vis表征(图6)发现,SA结构中的苯环和ACNs黄烊盐发色团发生了J-聚集。
2.6 胶体稳定性能
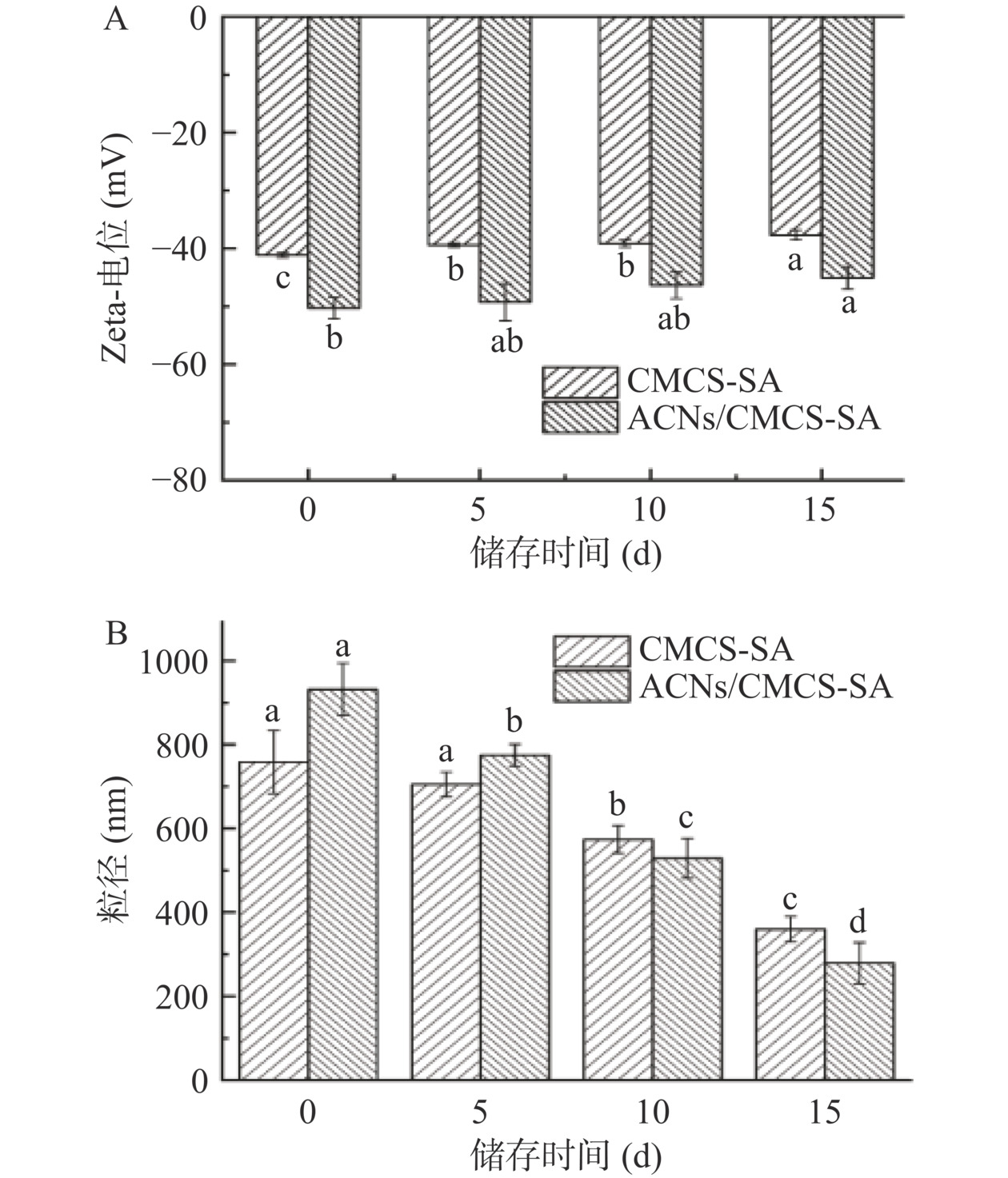
CMCS-SA和ACNs/CMCS-SA的Zeta-电位和粒径随储存时间变化情况见图8。由图8可知,随储存时间增加,分散在蒸馏水中的CMCS-SA和ACNs/CMCS-SA Zeta-电位绝对值和平均粒径均呈现下降趋势。由图8A可知,新制CMCS-SA和ACNs/CMCS-SA的Zeta-电位分别为−41.1±0.4 mV和−50.3±1.9 mV;15 d后降低为−37.7±0.86 mV和−45.1±1.90 mV,呈负电体系,说明凝胶微球在水中稳定存在且分散均匀。由图8B可知,新制CMCS-SA和ACNs/CMCS-SA的平均粒径分别为758.57±75.37 nm和931.80±61.67 nm,储存15 d后显著降低(P<0.05)为360.07±30.25 nm和278.60±49.21 nm,这可能由于凝胶球在水溶液长期储存溶胀破裂。此外,CMCS-SA在储存过程中没有明显颜色变化,呈乳黄色,但ACNs/CMCS-SA的颜色随储存时间增加逐渐褪色,呈现由粉色到淡黄色的变化,这可能由于ACNs在光照条件下不稳定,凝胶微球在水中逐渐破裂对ACNs保护作用减弱,导致ACNs逐渐降解所致[31]。
2.7 溶胀性能
凝胶微球的溶胀性能与其稳定性能密切相关,且是其应用于食药领域的关键性能指标之一。在pH3、5、7、9介质环境中对ACNs/CMCS-SA进行溶胀性能测定,见图9。由图9可知,ACNs/CMCS-SA的溶胀性能展现pH响应性,ACNs/CMCS-SA在pH3、5、7、9介质环境中132 min的溶胀率分别为20.16%±8.08%、26.68%±3.71%、46.85%±10.08%和350.96%±79.76%。ACNs/CMCS-SA的溶胀性能受亚胺键的pH响应性和CMCS的两性作用共同影响。碱性介质(pH9)中,亚胺键保持凝胶微球结构稳定,CMCS分子中的羧基去质子化,凝胶网络膨胀,此时溶胀率较大[21];强酸性介质(pH3)中,亚胺键部分断裂,但CMCS分子中的羧基质子化,分子链收缩,疏水作用占主导,保持凝胶微球的结构稳定,此时溶胀率最小;中性介质(pH7)中,亚胺键稳定性较强,且CMCS分子中的羧基难以电离出氢离子,因此,此时凝胶稳定性较强,溶胀率较小。
2.8 缓释性能
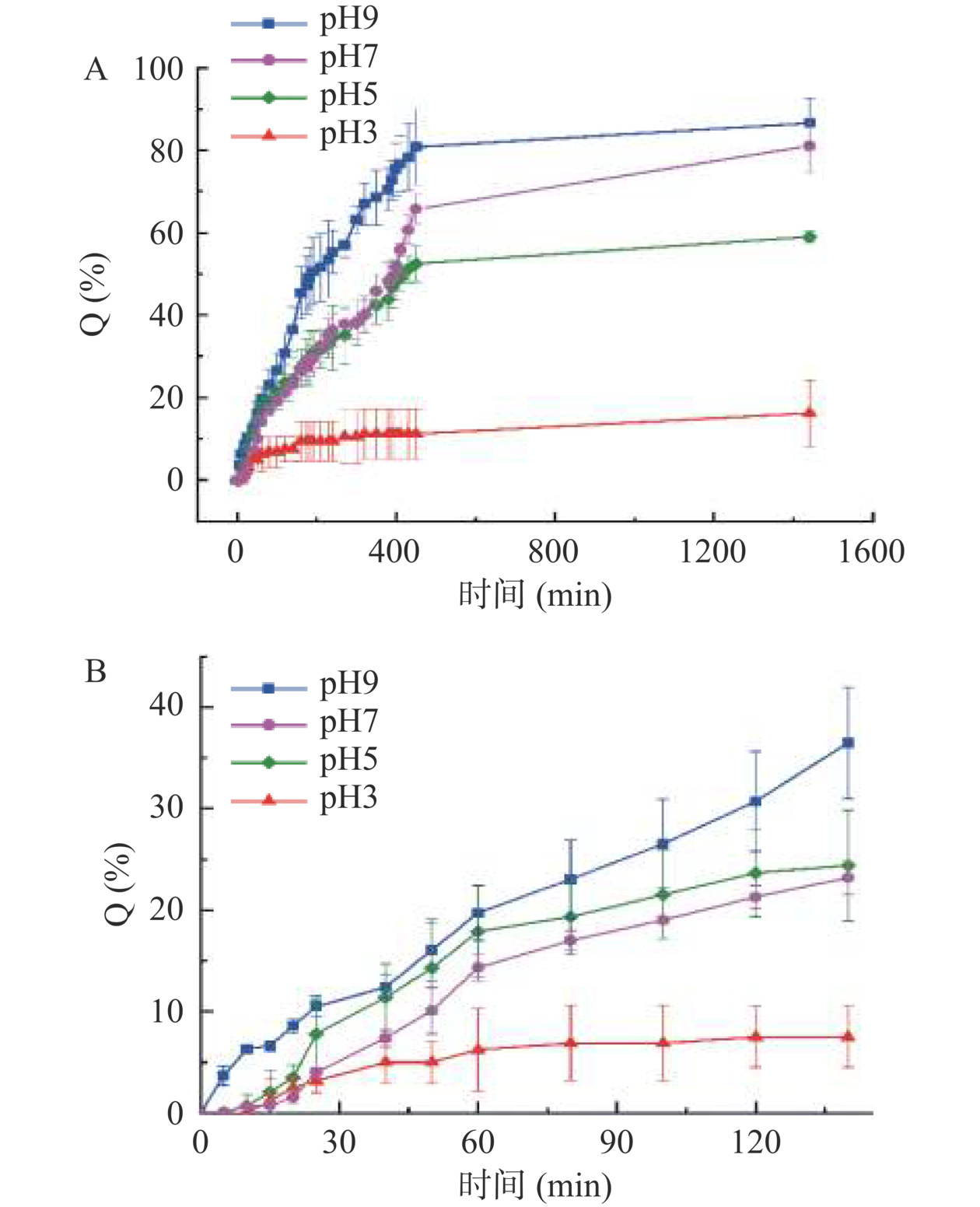
ACNs/CMCS-SA的缓释性能通过ACNs的累计释放率表征,见图10。由图10A可知,ACNs/CMCS-SA累计释放率随pH增加而增加,在pH3、5、7和9介质中,24 h累计释放率分别为16.21%±8.06%、58.99%±1.25%、81.07%±6.52%和86.58%±6.05%,展现了pH响应性能。随时间延长,ACNs累计释放率呈现先增大后趋于平衡的规律。考虑到胃肠中的食物留存时间,比较了不同介质中140 min内ACNs/CMCS-SA累计释放率,见图10B。pH3介质中ACNs 2 h累计释放率较低,说明ACNs/CMCS-SA在胃pH条件下能够较好保护ACNs。pH9介质中ACNs 140 min快速释放,说明ACNs在肠pH条件下能够较好释放,对ACNs的肠道吸收有利。此外,pH7时ACNs/CMCS-SA的缓释性能较强,而溶胀率仅为46.85%±10.08%(图9),说明ACNs/CMCS-SA在中性条件下稳定存在的同时,具有较强的释放性能。
为进一步阐明ACNs/CMCS-SA中ACNs的释放机制,采用准一级动力学方程、准二级动力学方程、Higuchi模型、Weibull模型以及Peppas模型对不同pH条件下ACNs/CMCS-SA中ACNs释放行为进行拟合,结果见表2。其中,Weibull模型方程对于不同pH介质条件下ACNs/CMCS-SA的释放行为有较好拟合结果(R2=0.96~0.98),这说明Weibull模型最适合描述ACNs/CMCS-SA的释放行为。Weibull模型具有扩展指数的形式,常用于药物释放和溶出研究[32],其释放机制与d值相关。d值大于1.0时,说明包埋药物为扩散和骨架溶蚀复合释放机制;d值在0.75~1.0时,说明包埋药物为菲克扩散和Case Ⅱ transport联合释放机制[33],其中Case Ⅱ transport表示药物释放为0级-溶胀依赖性释放[34]。
表 2 不同pH介质中ACNs/CMCS-SA的释放动力学拟合Table 2. Fitting of release kinetics of ACNs/CMCS-SA in different pH media模型 pH 动力学方程 R2 准一级
动力学3 Q=16.42859×[1−exp(−0.00341t)] 0.85529 5 Q=60.10736×[1−exp(−0.00375t)] 0.98782 7 Q=87.09679×[1−exp(−0.00225t)] 0.96131 9 Q=92.63497×[1−exp(−0.00385t)] 0.98620 准二级
动力学3 Q=1/(0.06522+8.20357/t) 0.97157 5 Q=1/(0.013127+3.60852/t) 0.98274 7 Q=1/(0.01095+5.5337/t) 0.95342 9 Q=1/(0.01093+2.8222/t) 0.92891 Higuchi 3 Q=0.49999x1/2+1.27631 0.88889 5 Q=2.13893x1/2−0.51062 0.90484 7 Q=2.72027x1/2−6.9491 0.93998 9 Q=3.3087x1/2−0.48994 0.87031 Weibull 3 Q=16.02433[1−exp(−0.00383×t0.75938)] 0.96857 5 Q=61.23770[1−exp(−0.00361×t0.95239)] 0.98795 7 Q=83.63356[1−exp(−0.00249×t1.10303)] 0.98388 9 Q=88.78055[1−exp(−0.00419×t1.2779)] 0.98854 Peppas 3 Q=1.20615t0.37321 0.93407 5 Q=2.80277t0.45177 0.91248 7 Q=1.6066t0.56213 0.92322 9 Q=4.46135t0.44780 0.87933 pH7和9时,ACNs/CMCS-SA的Weibull模型方程d值均大于1.0,说明此时ACNs的释放为扩散和骨架溶蚀复合释放机制控制,正如图9中所示,pH7和9介质中凝胶球溶胀率较大。此时影响ACNs释放的主要因素包括凝胶微球表面ACNs的释放扩散、凝胶微球内外浓度差引起的以渗透压为驱动力的扩散;以及由于凝胶微球骨架松弛、溶胀破裂导致的ACNs由内向外转移。
pH 3和5时,ACNs/CMCS-SA的Weibull模型方程d值均在0.75~1.0范围之间,说明此时ACNs的释放为菲克扩散和CaseⅡtransport(0级-溶胀依赖性释放)联合释放机制控制。此时ACNs以均匀的速率稳定释放,其主要影响因素为介质渗入凝胶微球导致其松弛溶胀[35]和凝胶微球内外浓度差。
此外,通过Weibull模型也可求出平衡时ACNs的累计释放率,在pH3、5、7和9介质中,平衡时ACNs的累计释放率分别为16.02%±1.15%、61.24%±2.03%、83.63%±3.36%和88.78%±2.64%。
有研究表明,ACNs水凝胶在给药系统中的应用几乎没有毒性[36];ACNs抗炎、促进伤口愈合等生理活性在其负载于水凝胶体系时依然存在,且有动物实验表明,ACNs水凝胶在细胞或动物模型中表现出比游离ACNs更好的生物活性[37]。在课题组的前期研究中构筑了壳聚糖-水杨醛pH响应水凝胶包埋ACNs[17],凝胶在酸性条件下不稳定,ACNs的释放主要受扩散作用影响。本文中ACNs/CMCS-SA在较宽pH范围内稳定,尤其对ACNs展现良好的胃酸保护和肠道释放性能,有望为ACNs应用提供新思路。
3. 结论
采用反相乳液法,制备了抗酸性载ACNs凝胶微球ACNs/CMCS-SA。ACNs/CMCS-SA具有均匀的微米级粒径,通过物理作用实现对ACNs的有效包埋;在酸性条件下稳定性良好,溶胀和缓释过程展现pH响应性;ACNs/CMCS-SA的溶胀性能受亚胺键的pH响应性和CMCS的两性作用共同影响;ACNs/CMCS-SA对ACNs的释放行为符合Weibull模型,pH7和9时,ACNs的释放为扩散和骨架溶蚀复合释放机制控制,其主要影响因素既包括凝胶微球表面ACNs的释放扩散、凝胶微球内外浓度差引起的以渗透压为驱动力的扩散,又包括由于凝胶微球骨架松弛、溶胀破裂导致的ACNs由内向外转移;pH3和5时,ACNs的释放为菲克扩散和Case Ⅱ transport(0级-溶胀依赖性释放)联合释放机制控制,其主要影响因素为介质渗入凝胶微球导致其松弛溶胀和凝胶微球内外浓度差。
ACNs/CMCS-SA展现了良好的胃酸保护和肠道释放性能,解决了壳聚糖基亚胺水凝胶耐酸性能差的问题;制备过程绿色,所用原料无毒、易得,为环境友好型药物包封材料的开发和ACNs的应用提供了理论和实践参考。但本研究仅对ACNs/CMCS-SA在胃、肠pH条件下的释放性能进行了探索,其体内释放性能仍需进一步研究。
-
表 1 标准曲线及线性相关度
Table 1 Standard curve and linear correlation
pH 标准曲线 R2 3 y=34.7385x+0.0321 0.99927 5 y=3.189x+0.0320 0.99938 7 y=4.437x+0.0146 0.99903 9 y=5.841x+0.0129 0.99959 表 2 不同pH介质中ACNs/CMCS-SA的释放动力学拟合
Table 2 Fitting of release kinetics of ACNs/CMCS-SA in different pH media
模型 pH 动力学方程 R2 准一级
动力学3 Q=16.42859×[1−exp(−0.00341t)] 0.85529 5 Q=60.10736×[1−exp(−0.00375t)] 0.98782 7 Q=87.09679×[1−exp(−0.00225t)] 0.96131 9 Q=92.63497×[1−exp(−0.00385t)] 0.98620 准二级
动力学3 Q=1/(0.06522+8.20357/t) 0.97157 5 Q=1/(0.013127+3.60852/t) 0.98274 7 Q=1/(0.01095+5.5337/t) 0.95342 9 Q=1/(0.01093+2.8222/t) 0.92891 Higuchi 3 Q=0.49999x1/2+1.27631 0.88889 5 Q=2.13893x1/2−0.51062 0.90484 7 Q=2.72027x1/2−6.9491 0.93998 9 Q=3.3087x1/2−0.48994 0.87031 Weibull 3 Q=16.02433[1−exp(−0.00383×t0.75938)] 0.96857 5 Q=61.23770[1−exp(−0.00361×t0.95239)] 0.98795 7 Q=83.63356[1−exp(−0.00249×t1.10303)] 0.98388 9 Q=88.78055[1−exp(−0.00419×t1.2779)] 0.98854 Peppas 3 Q=1.20615t0.37321 0.93407 5 Q=2.80277t0.45177 0.91248 7 Q=1.6066t0.56213 0.92322 9 Q=4.46135t0.44780 0.87933 -
[1] CHEN L Z, ZHONG J J, LIN Y Y, et al. Microwave and enzyme co-assisted extraction of anthocyanins from purple-heart radish:Process optimization, composition analysis and antioxidant activity[J]. LWT,2023,187:115312. doi: 10.1016/j.lwt.2023.115312
[2] 李煦, 白雪晴, 刘长霞, 等. 天 然花青素的抗氧化机制及功能活性研究进展[J]. 食品安全质量检测学报,2021,12(20):8163−8171. [LI X, BAI X Q, LIU C X, et al. Research progress on antioxidant mechanism and functional activity of natural anthocyanins[J]. Journal of Food Safety and Quality,2021,12(20):8163−8171.] LI X, BAI X Q, LIU C X, et al. Research progress on antioxidant mechanism and functional activity of natural anthocyanins[J]. Journal of Food Safety and Quality, 2021, 12(20): 8163−8171.
[3] FARIA A, PESTANA D, AZEVEDO J, et al. Absorption of anthocyanins through intestinal epithelial cells-putative involvement of GLUT2[J]. Molecular Nutrition & Food Research,2009,53(11):1430−1437.
[4] HUANG Y X, ZHOU S Y, ZHAO G H, et al. Destabilisation and stabilisation of anthocyanins in purple-fleshed sweet potatoes:A review[J]. Trends in Food Science & Technology,2021,116:1141−1154.
[5] YUAN Y T, FAN Q, XU X Y, et al. Nanocarriers based on polysaccharides for improving the stability and bioavailability of anthocyanins:A review[J]. Carbohydrate Polymer Technologies and Applications,2023,6:100346. doi: 10.1016/j.carpta.2023.100346
[6] HE J, MAGNUSON B A, LALA G, et al. Intact anthocyanins and metabolites in rat urine and plasma after 3 months of anthocyanin supplementation[J]. Nutrition and Cancer,2006,54(1):3−12. doi: 10.1207/s15327914nc5401_2
[7] ZHOU X, NIE S, LIU L, et al. Compound hydrogels derived from gelatin and gellan gum regulates the release of anthocyanins in simulated digestion[J]. Food Hydrocolloids,2022,127:107487. doi: 10.1016/j.foodhyd.2022.107487
[8] GHREAGHAJLOU N, HALLAJ-NEZHADI S, GHASEMPOUR Z. Nano-liposomal system based on lyophilization of monophase solution technique for encapsulating anthocyanin-rich extract from red cabbage[J]. Dyes Pigments,2022,202:110263. doi: 10.1016/j.dyepig.2022.110263
[9] THIECLA K O R, SILVA M P D, LOURENO F R, et al. Nanoencapsulation of anthocyanins from blackberry (Rubus spp) through pectin and lysozyme self-assembling[J]. Food Hydrocolloids,2021,114:106563. doi: 10.1016/j.foodhyd.2020.106563
[10] IRMAK O S, NESLIHAN A D, KUBRA U, et al. Lyophilized nano-liposomal system for red onion (Allium cepa L.) peel anthocyanin:Characterization, bioaccessibility and release kinetics[J]. Food Bioscience,2023,53:102702. doi: 10.1016/j.fbio.2023.102702
[11] SANTIAGOGARCÍA A P, LEÓNMARTÍNEZ M F, GUTIÉRREZ C M, et al. Microencapsulation of strawberry juice in Agave angustifolia fructans:Effect of spray-drying conditions on the anthocyanin content and physicochemical properties[J]. International Journal of Food Science Technology,2023,58(12):6725−6735. doi: 10.1111/ijfs.16529
[12] LIU R R, WANG X H, YANG L X, et al. Coordinated encapsulation by β-cyclodextrin and chitosan derivatives improves the stability of anthocyanins[J]. International Journal of Biological Macromolecules,2023,242:125060. doi: 10.1016/j.ijbiomac.2023.125060
[13] WU C L, JULIAN M D, MA B H, et al. Composite hydrogels formed from okara cellulose nanofibers and carrageenan:Fabrication and characterization[J]. International Journal of Biological Macromolecules,2023,258(P2):129079.
[14] YING J. Synthesis of porous starch microgels for the encapsulation, delivery and stabilization of anthocyanins[J]. Journal of Food Engineering,2021,302:110552. doi: 10.1016/j.jfoodeng.2021.110552
[15] LI W J, LINLI F Z, YANG W Y, et al. Enhancing the stability of natural anthocyanins against environmental stressors through encapsulation with synthetic peptide-based gels[J]. International Journal of Biological Macromolecules,2023,253:127133. doi: 10.1016/j.ijbiomac.2023.127133
[16] 曹亚婵, 刘晓坤, 党奇峰, 等. 基于壳聚糖的抗菌可注射自愈性水凝胶的制备及其生物相容性研究[J]. 中国海洋大学学报(自然科学版),2024,54(3):60−69. [CAO Y C, LIU X K, DANG Q F, et al. Preparation and biocompatibility of antibacterial injectable chitosan-based hydrogel for self-healing biomateria[J]. Periodical of Ocean University of China,2024,54(3):60−69.] CAO Y C, LIU X K, DANG Q F, et al. Preparation and biocompatibility of antibacterial injectable chitosan-based hydrogel for self-healing biomateria[J]. Periodical of Ocean University of China, 2024, 54(3): 60−69.
[17] 李煦, 董翠芳, 刘长霞, 等. 负载花色苷的壳聚糖-水杨醛水凝胶的制备及性能[J]. 食品工业科技,2023,44(9):111−118. [LI X, DONG C F, LIU C X, et al. Preparation and properties of chitosan salicylaldehyde hydrogel loaded with anthocyanins[J]. Science and Technology of Food Industry,2023,44(9):111−118.] LI X, DONG C F, LIU C X, et al. Preparation and properties of chitosan salicylaldehyde hydrogel loaded with anthocyanins[J]. Science and Technology of Food Industry, 2023, 44(9): 111−118.
[18] LIU C X, DONG C F, LIU S H, et al. Multiple chiroptical switches and logic circuit based on salicyl- imine- chitosan hydrogel[J]. Carbohydrate Polymers,2021,257:117534. doi: 10.1016/j.carbpol.2020.117534
[19] FATEMEH K, SAEED S S. Synthesis and characterization of a novel hydrogel based on carboxymethyl chitosan/sodium alginate with the ability to release simvastatin for chronic wound healing[J]. Biomedial Materials,2023,18(2):025001. doi: 10.1088/1748-605X/acb0a3
[20] GUO F B, LIU Y, CHEN S Q, et al. A schiff base hydrogel dressing loading extracts from Periplaneta americana for diabetic wound healing[J]. International Journal of Biological Macromolecules,2023,230:123256. doi: 10.1016/j.ijbiomac.2023.123256
[21] YU R, ZHANG Y, BARBORU M, et al. Biobased pH-responsive and self-healing hydrogels prepared from O-carboxymethyl chitosan and a 3-dimensional dynamer as cartilage engineering scaffold[J]. Carbohydrate Polymers,2020,244:116471. doi: 10.1016/j.carbpol.2020.116471
[22] 李俊杰, 贾鹏, 刘功稳, 等. 一种口服骨靶向微/纳水凝胶微球制备及对雌性去势小鼠骨质量影响的研究[J]. 中国骨质疏松杂志,2023,29(11):1581−1586,1597. [LI J J, JIA P, LIU G W, et al. Preparation of an oral bone-targeting micro/nano hydrogel microsphere and its effect on bone quality in ovariectomized mice[J]. Chinese Journal of Osteoporosis,2023,29(11):1581−1586,1597.] doi: 10.3969/j.issn.1006-7108.2023.11.005 LI J J, JIA P, LIU G W, et al. Preparation of an oral bone-targeting micro/nano hydrogel microsphere and its effect on bone quality in ovariectomized mice[J]. Chinese Journal of Osteoporosis, 2023, 29(11): 1581−1586,1597. doi: 10.3969/j.issn.1006-7108.2023.11.005
[23] GIULBUDAGIAN M, YEALLAND G, HNZHE S, et al. Breaking the barrier-Potent anti-inflammatory activity following efficient topical delivery of etanercept using thermoresponsive nanogels[J]. Theranostics,2018,8(2):450−463. doi: 10.7150/thno.21668
[24] ZHU C, WANG S, WANG D, et al. Novel nano-micro-macro multiple-nested hydrogel with gradient ciliary neurotrophic factor distribution induces directional axon regeneration of retinal ganglion cells[J]. Colloids and Surfaces-A Physicochemical and Engineering Aspects,2023,675:131904. doi: 10.1016/j.colsurfa.2023.131904
[25] LI X, WU X L. The microspheres/hydrogels scaffolds based on the proteins, nucleic acids, or polysaccharides composite as carriers for tissue repair:A review[J]. International Journal of Biological Macromolecules,2023,253:126611. doi: 10.1016/j.ijbiomac.2023.126611
[26] SATOMI T, ANDREA C, SUZUKA S, et al. Preparation of ultrasmall cyclodextrin nanogels by an inverse emulsion method using a cationic surfactant[J]. Chemical Communications,2023,59(27):4071−4074. doi: 10.1039/D3CC00523B
[27] 刘长姣, 郑霞, 熊湘炜, 等. 分光光度法测定黑米花青素方法的建立[J]. 粮食与油脂,2019,32(1):73−77. [LIU C J, ZHENG X, XIONG X W, et al. Establishment of a spectrophotometric method for the determination of anthocyanins in black rice[J]. Food and Oil,2019,32(1):73−77.] doi: 10.3969/j.issn.1008-9578.2019.01.020 LIU C J, ZHENG X, XIONG X W, et al. Establishment of a spectrophotometric method for the determination of anthocyanins in black rice[J]. Food and Oil, 2019, 32(1): 73−77. doi: 10.3969/j.issn.1008-9578.2019.01.020
[28] 戴文, 王晓东, 黄培, 等. 乳液模板法制备聚酰亚胺中空微球及其形貌调控[J]. 高分子材料科学与工程,2023,39(7):25−32. [DAI W, WANG X D, HUANG P, et al. Morphology control and preparation of polyimide hollow microspheres by emulsion template method[J]. Polymer Materials Science & Engineering,2023,39(7):25−32.] DAI W, WANG X D, HUANG P, et al. Morphology control and preparation of polyimide hollow microspheres by emulsion template method[J]. Polymer Materials Science & Engineering, 2023, 39(7): 25−32.
[29] ZHANG B, WANG Q, ZHOU P P, et al. Copigmentation evidence of oenin with phenolic compounds:A comparative study of spectrographic, thermodynamic and theoretical data[J]. Food Chemistry,2020,313:126163. doi: 10.1016/j.foodchem.2020.126163
[30] 薛宏坤, 李鹏程, 钟雪, 等. 高速逆流色谱分离纯化桑葚花色苷及其抗氧化活性[J]. 食品科学,2020,41(15):96−104. [XUE H S, LI P C, ZHONG X, et al. Separation and purification of anthocyanins from mulberry fruit by high-speed counter-current chromatography and their antioxidant activity[J]. Food Science,2020,41(15):96−104.] doi: 10.7506/spkx1002-6630-20190715-193 XUE H S, LI P C, ZHONG X, et al. Separation and purification of anthocyanins from mulberry fruit by high-speed counter-current chromatography and their antioxidant activity[J]. Food Science, 2020, 41(15): 96−104. doi: 10.7506/spkx1002-6630-20190715-193
[31] WU X H, LIN Q W, BELWAL T, et al. Effect of advanced/hybrid oxidation process involving ultrasonication and ultraviolet radiation (sonophotolysis) on anthocyanin stability:Degradation kinetics and mechanism[J]. Food Chemistry,2022,370:131083. doi: 10.1016/j.foodchem.2021.131083
[32] KOSMIDIS K, MACHERAS P. On the dilemma of fractal or fractional kinetics in drug release studies:A comparison between Weibull and Mittag-Leffler functions[J]. International Journal of Pharmaceutics,2018,543(1-2):269−273. doi: 10.1016/j.ijpharm.2018.03.060
[33] PAPADOPOILOU V, KOSMIDIS K, VLACHOU M, et al. On the use of the Weibull function for the discernment of drug release mechanisms[J]. International Journal of Pharmaceutics,2006,309(1−2):44−50. doi: 10.1016/j.ijpharm.2005.10.044
[34] NAZIM N, STEFAN K. Fundamental advances in hydrogels for the development of the next generation of smart delivery systems as biopharmaceuticals[J]. International Journal of Pharmaceutics,2023,633:122634. doi: 10.1016/j.ijpharm.2023.122634
[35] 李凌冰, 谭业邦. 亲水聚合物凝胶系统中药物控制释放两类特殊情况的数学模型[J]. 生物医学工程学杂志,2003,20(1):17−21. [LI L B, TAN Y B. Two sorts of problems on drug controlled release from swellable polymer[J]. Journal of Biomedical Engineering,2003,20(1):17−21.] doi: 10.3321/j.issn:1001-5515.2003.01.006 LI L B, TAN Y B. Two sorts of problems on drug controlled release from swellable polymer[J]. Journal of Biomedical Engineering, 2003, 20(1): 17−21. doi: 10.3321/j.issn:1001-5515.2003.01.006
[36] LIU L Y, ZHANG D D, SONG X X, et al. Compound hydrogels derived from gelatin and gellan gum regulates the release of anthocyanins in simulated digestion[J]. Food Hydrocolloids,2022,127(6):107487.
[37] GHAZAL S, KORDESTANI S S, TAHRIRI M, et al. Evaluation of L929 cell morphology on anthocyanin-containing gelatin-based hydrogel for early detection of infection[J]. Bio-Design and Manufacturing,2019,2(3):181−186. doi: 10.1007/s42242-019-00047-6
-
期刊类型引用(1)
1. 朱越,李治宽,高翔,杨静丽,何美军,彭诗琴. 黄精精深加工研究现状. 农业与技术. 2025(04): 35-40 .  百度学术
百度学术
其他类型引用(0)





 下载:
下载:

 下载:
下载:



