Analysis of Taste and Differential Metabolites of Different Ganoderma lucidum Strains Based on Electronic Tongue and Non-targeted Metabolomics
-
摘要: 为探究不同灵芝菌株的滋味和代谢物差异性,以不同灵芝菌株的子实体为样品,采用电子舌测定21个不同灵芝菌株水提液滋味,使用超高效液相色谱和质谱的非靶向代谢组学技术,结合主成分分析(Principal component analysis,PCA)、正交偏最小二乘判别分析(Orthogonal partial least squares-discriminant analysis,OPLS-DA)等统计学方法对滋味聚类分析的结果选取出3个灵芝菌株(GL229、GL219、GL236)水提液进行代谢物鉴定分析。结果表明,不同灵芝菌株水提液的酸味、鲜味、苦味和咸味响应值差异比较大。3个不同灵芝菌株水提液样品共鉴定出6057个代谢物,GL219与GL229、GL236与GL229、GL219与GL236之间的差异代谢物分别有1282、1311、1966个。其中,3个对比组间共有差异代谢产物有123个,组间共有差异代谢产物包括有机酸及其衍生物、苯环类化合物、有机氧化合物、有机杂环化合物、脂类和类脂类化合物等,主要差异代谢物有Jamaicamide C、2-异丙基苹果酸(2-Isopropylmalic acid)、1-萘胺(1-Naphthylamine)、对羟基苯乙胺(Tyramine)、京尼平(Genipin)等。差异代谢物L-谷氨酰胺(L-Glutamine)、谷氨酰胺(Glutamine)、辛可宁(Cinchonine)、龙葵次碱(Solanidine)、苯乙醛(Phenylacetaldehyde)和去氢胆酸(Dehydrocholic acid)是滋味物质,对不同灵芝菌株水提液的滋味差异有一定的贡献作用。本研究说明代谢组学可用于分析不同滋味的灵芝的代谢物差异,可为灵芝滋味物质基础提供一定的理论依据。Abstract: In order to explore the differences of taste and metabolites of different Ganoderma lucidum strains, the fruiting bodies of different Ganoderma lucidum strains were taken as samples, the taste of aqueous extracts of 21 different Ganoderma lucidum strains were determined by electronic tongue. The non-targeted metabonomics techniques of ultra high performance liquid chromatography and mass spectrometry were used. Combined with principal component analysis (PCA) and orthogonal partial least square discriminant analysis (OPLS-DA) and other statistical methods based on the results of taste cluster analysis, three different Ganoderma lucidum strains (GL229, GL219, GL236) were selected for metabolite identification and analysis. The results showed that the response values of sour, umami, bitter and salty taste of water extracts of different Ganoderma lucidum strains were quite different. A total of 6057 metabolites were identified from the water extracts of three different Ganoderma lucidum strains. There were 1282, 1311 and 1966 differential metabolites between GL219 and GL229, GL236 and GL229, GL219 and GL236, respectively. There were 123 differential metabolites among the three control groups, including organic acids and their derivatives, benzene ring compounds, organic oxygen compounds, organic heterocyclic compounds, lipids and lipid compounds. The main differential metabolites were Jamaicamide C, 2-isopropylmalicacid, 1-naphthylamine, tyramine, genipin and so on. The differential metabolites L-glutamine, glutamine, cinchonine, solanidine, phenylacetaldehyde and dehydrocholicacid were flavor substances, which contributed to the taste differences of water extracts of different Ganoderma lucidum strains. This study showed that metabonomics could be used to analyze the compositions differences of Ganoderma lucidum with different taste. Therefore, it could provide a theoretical basis for the material basis of Ganoderma lucidum taste.
-
Keywords:
- Ganoderma lucidum /
- strains /
- taste /
- non-targeted metabonomics /
- different metabolites
-
灵芝在2020年版《中华人民共和国药典》中被收录为多孔菌科真菌赤芝Ganoderma lucidum(Leyss. ex Fr.)Karst.或紫芝Ganoderma sinense Zhao,Xu et Zhang的干燥子实体,其中赤芝味苦,是一种药食同源中药材[1],亦是一种带苦味的食用菌[2]。灵芝中含有多糖、三萜、多酚、甾醇、腺苷、氨基酸、蛋白质等活性成分,具有抗肿瘤、抗炎、调节免疫力、降血糖、降血压等药理作用,因此在医药、保健品、食品方面均具有良好的前景[3−5]。有研究表明不同灵芝菌株之间及同一菌株经过驯化前后,它们的多糖、三萜、蛋白质、氨基酸等成分含量和抗氧化活性存在明显差异[6−7]。目前已报道的灵芝菌种繁多,不同灵芝菌株会在性状、化学成分和活性上存在差异,而灵芝成为药食同源中药材后,在食品中的应用需求将会增多,更加注重感官品质和营养成分,因此研究在食品中适用的灵芝菌株可以让丰富的灵芝菌种资源得到充分利用,促进其在食品行业的发展。
电子舌为现代电子感官技术,可模拟人体味觉,传感器相当于生物系统中的舌头,可感受不同化学物质的刺激,将其转化为信号传递给检测系统,从而表达液体样品的整体滋味特征[8−9]。Feng等[10]研究发现电子舌对食用菌感官质量的定性和定量分析方面具有很大的潜力。田婧[11]利用电子舌分析不同产地灵芝的滋味。电子舌用来区分牛肝菌、灰树花、金钱菇、姬松茸和猴头菇5种食用菌,可以识别不同食用菌的风味特征[12]。也有研究利用电子舌评估韩国市售17种食用菌的鲜味,发现金针菇在电子舌鲜味测量中获得最高分[13]。
代谢组学是继基因组学、转录组学及蛋白组学后的一门新兴学科[14−15]。代谢组学中的非靶向代谢组学借助色谱、质谱等仪器对生物系统中小分子代谢物进行系统的定性定量分析,通过数据处理和分析准确找出样本间差异代谢物[16]。目前代谢组学技术已逐渐应用于食用菌,如研究白灵菇(Pleurotus tuoliensis)的菌丝成熟潜在指标[17]、金针菇的生长发育[18]、环境温度胁迫对食用菌的代谢物影响[19−20]、不同栽培条件对食用菌的代谢物变化[21]、食用菌不同生长阶段代谢物的变化[22]等方面。
灵芝除了食用菌特有的鲜味,还带有一定的苦味,研究灵芝的味道及其造成味道差异的物质将会促进灵芝在食品的应用,而不同灵芝菌株的味道特征及造成味道差异的物质未有研究报道,因此本研究以21个不同灵芝菌株子实体为试验材料,使用电子舌对其水提液进行滋味测定。基于聚类分析,通过电子舌滋味响应值选出开展非靶向代谢组学分析的灵芝菌株水提液样品。探究不同灵芝菌株水提液代谢物的差异,分析可能造成灵芝水提液滋味特征差异的原因,以期为灵芝滋味物质基础的研究提供理论依据。
1. 材料与方法
1.1 材料与仪器
21个灵芝菌株样品 由四川省中医药科学院菌类药材研究所驯化的赤芝(Ganoderma lucidum(Leyss.ex Fr.)Karst.)干燥子实体,所有样品均为代料栽培,编号为GL154、GL171、GL215、GL216、GL217、GL219、GL221、GL222、GL224、GL226、GL227、GL229、GL230、GL231、GL232、GL233、GL234、GL235、GL236、GL237、GL240;乙腈 色谱纯,德国Merck公司;乙酸铵、甲酸、甲醇等质谱试剂 色谱纯,美国Sigma公司;实验用水均为超纯水。
UPR-II-40L超纯水器 四川优普超纯科技有限公司;AL104万分之一电子天平 梅特勒托利多仪器上海有限公司;ASTREE电子舌 法国Alpha MOS公司;5430R低温高速离心机 德国Eppendorf有限公司;Dionex Ultimate 3000超高效液相色谱系统 美国Thermo Fisher Scientific公司;QE HF-X质谱仪 美国Thermo公司;ACQUITY UPLC BEH Amide色谱柱(1.7 µm,2.1 mm×100 mm) 美国Waters公司。
1.2 实验方法
1.2.1 21个不同灵芝菌株水提液制备
21个不同菌株灵芝子实体粉末(过10目筛)均精密称取6.00 g,以料液比1:50(g/mL)、浸泡1 h和提取1 h进行回流提取1次,滤液定容至300 mL。
1.2.2 感官评价
参考刘瑞新等[23]的方法并加以修改,由经过培训的10名感官评价员(4名男生,6名女生,年龄22~30岁)对样品的滋味进行评分,评分标准为:几乎没有苦味,略有鲜味[0.5,1.5),略有苦味和鲜味[1.5,2.5),苦味适中,可尝到微微的鲜味[2.5,3.5),苦味明显,无法尝出其他味道[3.5,4.5],不能忍受的苦味[4.5,5.5]。取10名评价员评分的平均值作为最终结果。
1.2.3 电子舌检测
参考姚月凤等[24]的方法并加以修改。ASTREE型电子舌主要配备7根传感器,分别是ANS(甜)、SCS(苦)、AHS(酸)、CTS(咸)、NMS(鲜)、CPS(通用)和PKS(通用)。取1.2.1制备的样品滤液80 mL,放入电子舌检测专用烧杯。在检测样品前,先清洗传感器120 s,然后检测120 s。每个样品检测6次,取后3次在100~120 s之间的稳定值作为检测结果,进行分析。
1.2.4 非挥发性化合物分析
1.2.4.1 样本前处理
样本前处理、色谱与质谱条件参考Ivanisevic等[25]的方法并略作修改。称取一定量1.2.1制备的样品,加入400 μL预冷甲醇/乙腈/水溶液(4:4:2,v/v),涡旋混合,−20 ℃静置60 min,14000×g 4℃离心20 min,取上清真空干燥,质谱分析时加入100 μL乙腈水溶液(乙腈:水=1:1,v/v)复溶,涡旋,14000×g 4 ℃离心15 min,取2 μL上清液进样分析。为测定进样前仪器状态及平衡色谱-质谱系统,并用于评价整个实验过程中系统稳定性,样品等量混合用于制备质控样本(QC)。
1.2.4.2 色谱条件
整个分析过程中样品置于8 ℃自动进样器中,样品经超高效液相色谱系统,使用HSS T3色谱柱进行分离。其中进样量2 μL,柱温40 ℃,流速0.3 mL/min;色谱流动相A:0.1%甲酸水,B:0.1%甲酸甲醇,C:0.05%乙酸水,D:0.05%乙酸甲醇。样本队列中插入QC样品,用于监测和评价系统的稳定性及实验数据的可靠性。
1.2.4.3 Q-EXACTIVE 质谱条件
每例样品分别采用电喷雾电离(ESI)进行正离子和负离子模式检测。样品经UHPLC分离后用Thermo QE HF-X质谱仪进行质谱分析。质谱条件如下:鞘气流速:50 arb;辅助气:13 arb;喷雾电压:2.5 kV(+)/2.5 kV(−);S-Lens RF:50%;毛细管温度:325 ℃;辅助气温度:300 ℃;二级碰撞能量(NCE):30 eV;Top N=10。扫描范围:70~1050 m/z,扫描方式:正、负离子分别扫描。分析软件:Xcalibur 4.1版本。
1.3 数据处理
将电子舌检测得到的数据采用SPSS 19.0软件进行聚类分析。采用Origin软件绘图。将质谱采集得到的原始数据经过TidyMass包进行峰提取、峰对齐、峰校正、标准化等数据前处理。输出由样本名称、谱峰信息(包括保留时间和分子量)、及峰面积组成的三维数据矩阵。代谢物结构鉴定采用精确质量数匹配(<25 ppm)和二级谱图匹配的方式,检索实验室自建数据库以及HMDB、Massbank、KEGG、snyder、mona等数据库。采用软件SIMCA-P 14.1(Umetrics,Umea,Sweden)进行模式识别,数据经Pareto-scaling预处理后,进行无监督主成分分析(PCA)分析、正交偏最小二乘法判别分析(OPLS-DA)等多维统计分析。单维统计分析包括Student's-test和变异倍数分析。
2. 结果与分析
2.1 不同灵芝菌株水提液滋味分析
2.1.1 感官评价滋味结果
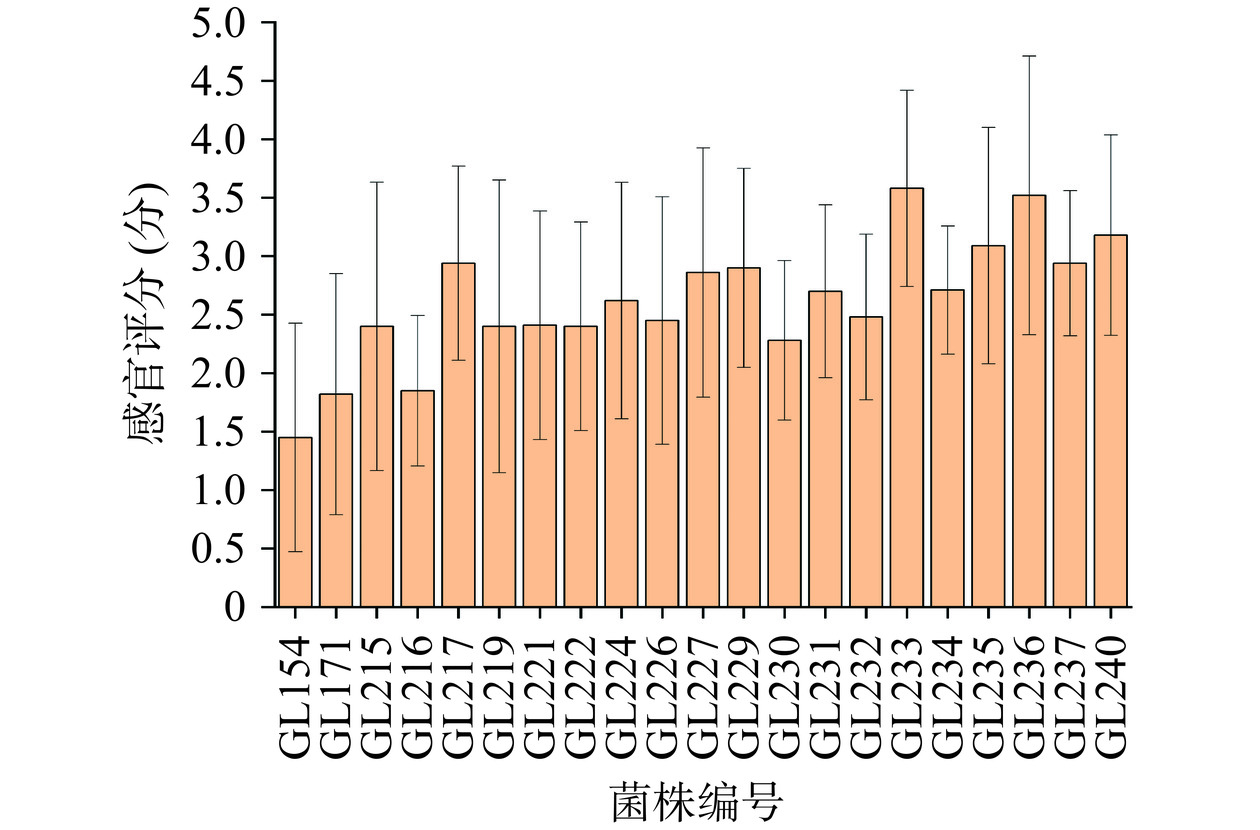
21个灵芝菌株水提液感官评价结果如图1所示,结果显示不同灵芝菌株的水提液滋味评分存在差异,感官评价分数越高表明样品中苦味越重,大部分样品的苦味明显,也因此无法尝出食用菌特有的鲜味。由于感官评价未能尝出滋味中的其它味道以及存在个体差异,因此采用电子舌进一步了解不同灵芝菌株水提液的滋味差异。
2.1.2 电子舌传感器响应值的雷达图
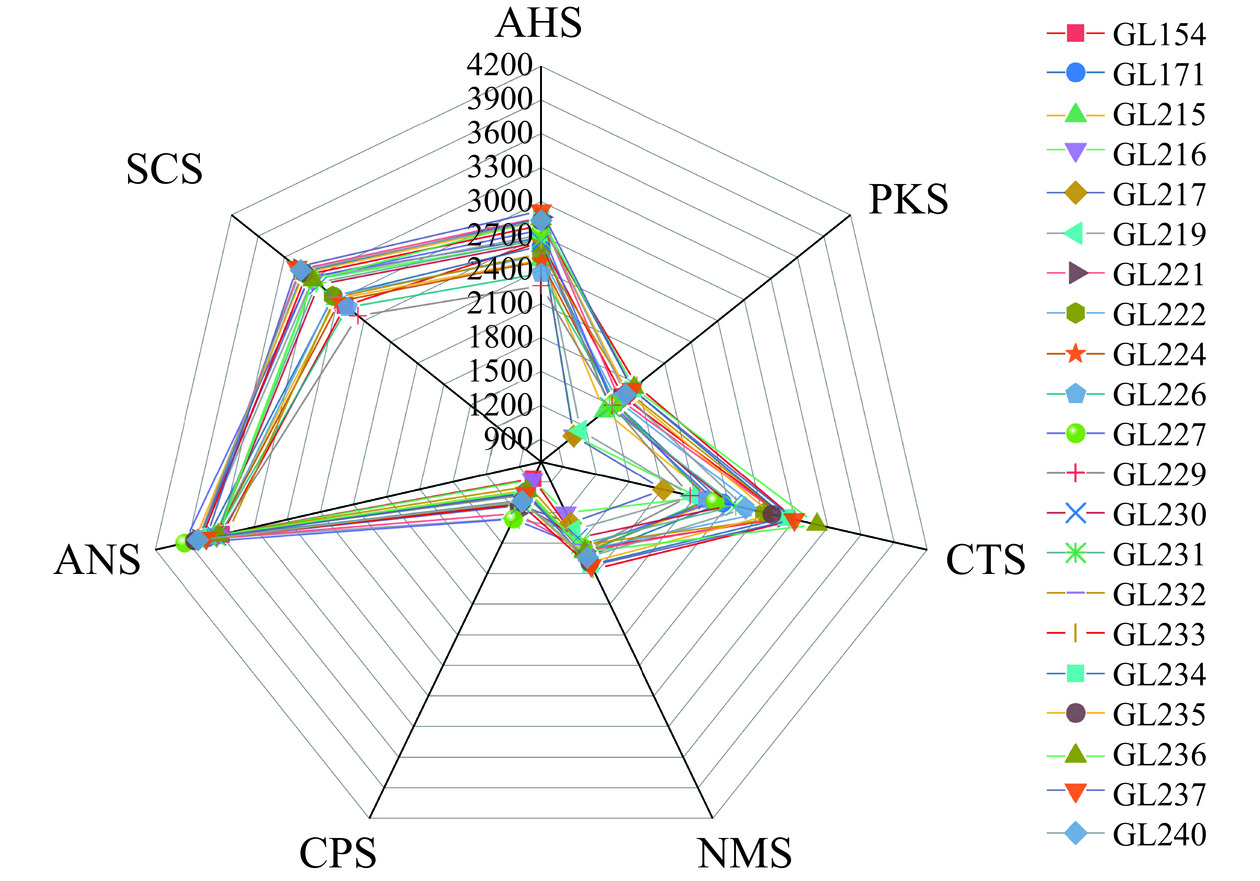
根据图2可知,不同灵芝菌株水提液滋味轮廓不同,即滋味特征不同。所有灵芝水提液样品的甜味(ANS)、苦味(SCS)、酸味(AHS)、咸味(CTS)响应值较高,酸味(AHS)、鲜味(NMS)、苦味(SCS)和咸味(CTS)响应值差异比较大。
2.1.3 电子舌传感器响应值的聚类分析
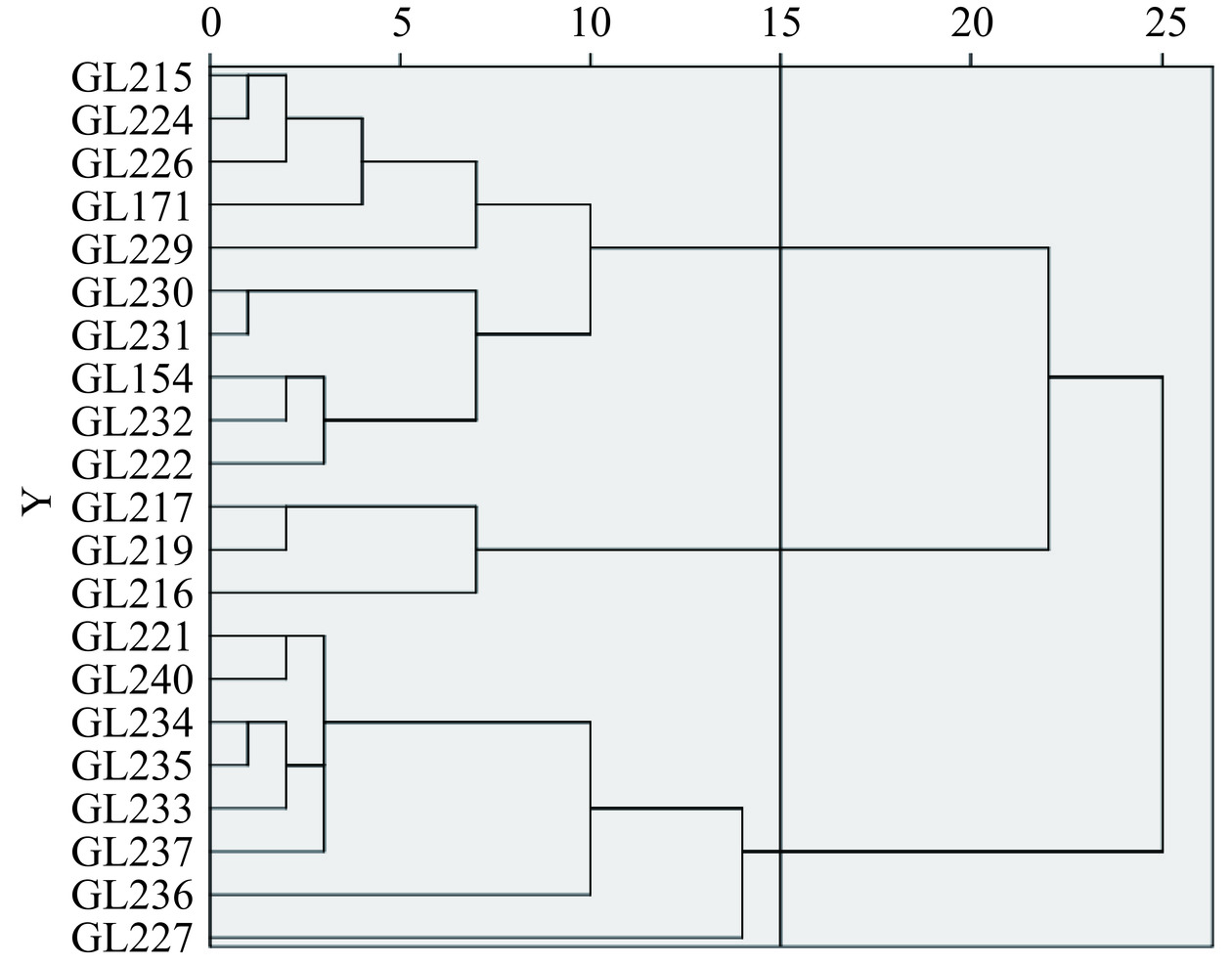
使用SPSS19.0分析软件,聚类方法为组间联接,度量标准为平方欧氏距离,对21个不同灵芝菌株水提液的电子舌传感器酸味、鲜味、甜味、咸味、苦味响应值的平均值进行聚类分析。聚类可用于衡量不同样品滋味特征间的相似性,将不同样品按照滋味品质相似程度聚合在一起进行分类,相似度最大的优先聚合在一起[26−27]。由图3可知,当类间距为15时,将21个不同灵芝菌株水提液分为了3类,第1类为样品GL215、GL224、GL226、GL171、GL229、GL230、GL231、GL154、GL232、GL222;第2类为样品GL217、GL219、GL216;第3类为样品GL221、GL240、GL234、GL235、GL233、GL237、GL236、GL227。这3个聚类样品在滋味的差异主要体现在酸味、咸味、苦味和鲜味响应值,第1聚类样品具有酸味和苦味响应值低、咸味和鲜味响应值居中的特点;第2聚类样品具有酸味和鲜味响应值低、苦味响应值高、咸味响应值居中的特点;第3聚类样品比另外两个聚类样品的酸味、咸味、苦味和鲜味响应值高的特点。根据聚类分析结果,随机从每类中选择样品GL229、GL219、GL236进行非挥发性化合物分析。
2.2 3个不同灵芝菌株水提液代谢组学差异分析
2.2.1 质控分析
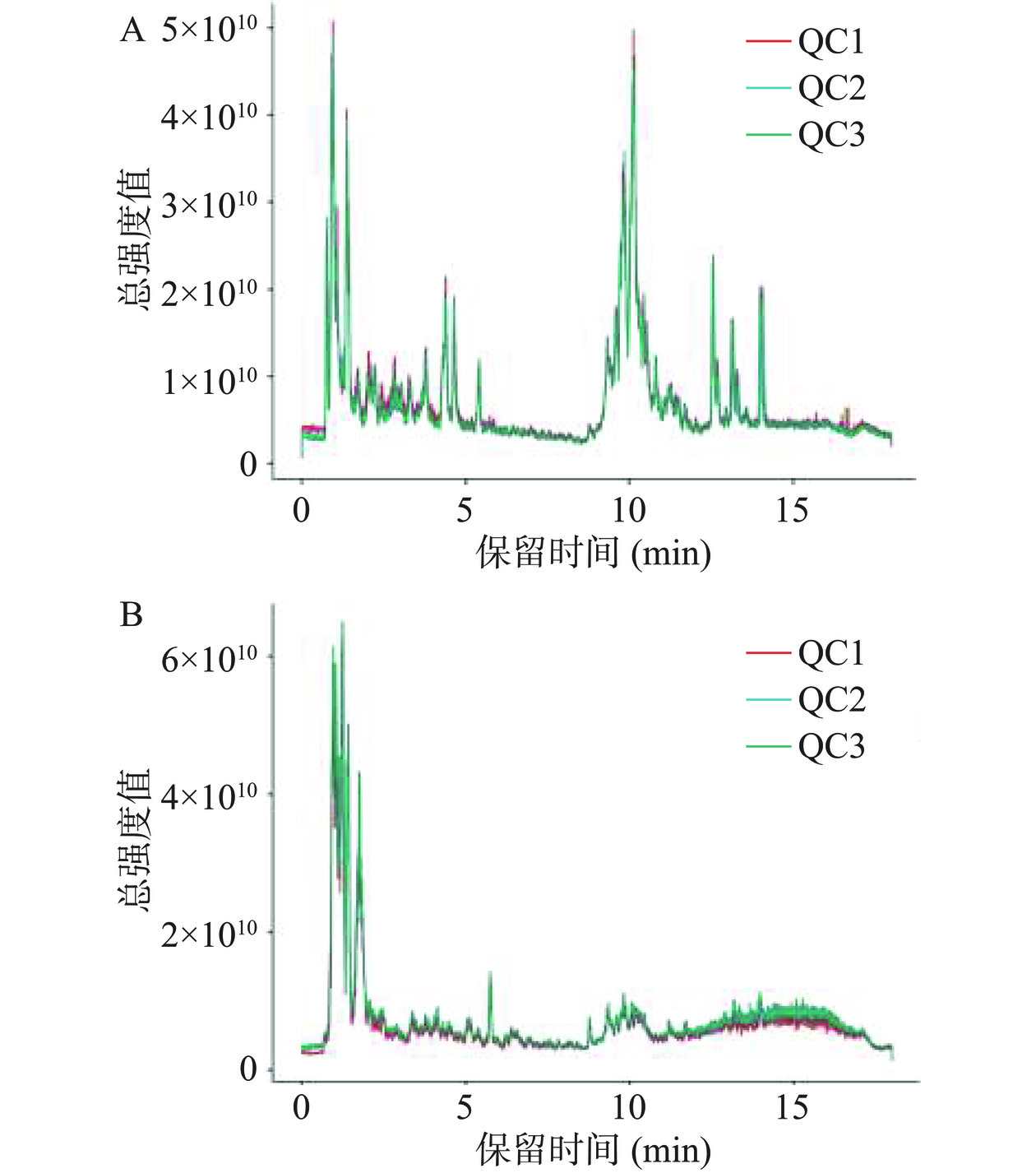
质控分析(Quality controlled,QC)对于监测和评价系统的稳定性及实验数据的可靠性至关重要。将QC样本总离子流图(TIC),进行谱图重叠比较,见图4,结果表明各色谱峰的响应强度和保留时间基本重叠,说明在整个实验过程中仪器误差引起的变异较小,结果可靠。
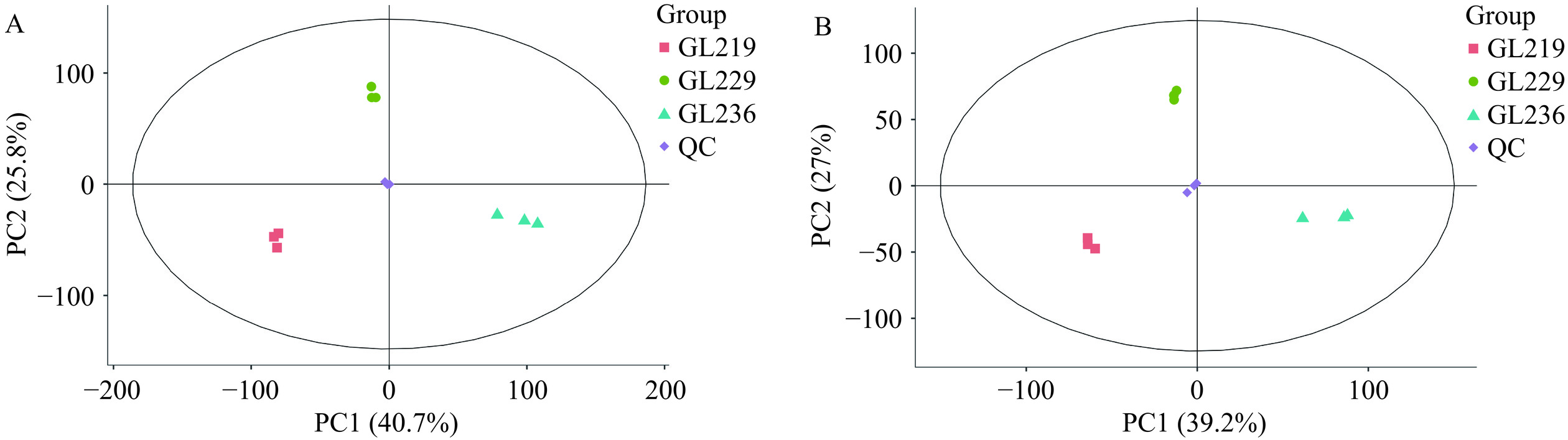
2.2.2 主成分分析
主成分分析(PCA)结果如图5所示,3组样本在PCA图上表现出明显的分离趋势,可以从总体上反映出3组样品之间的代谢物差异。根据PCA图可知组内差异不明显,组间差异明显,表明样品组内代谢物差异小,组间代谢物差异明显。
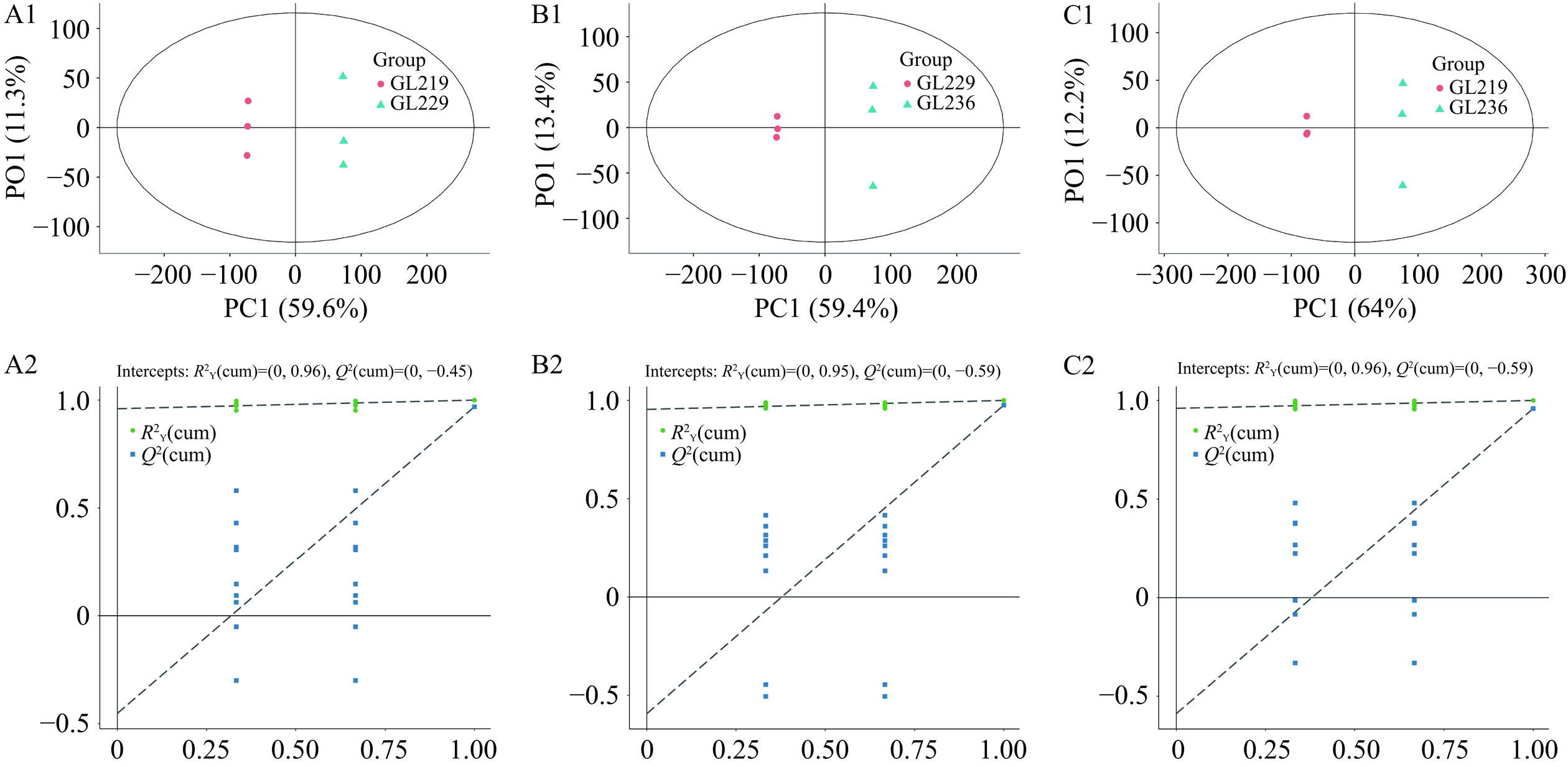
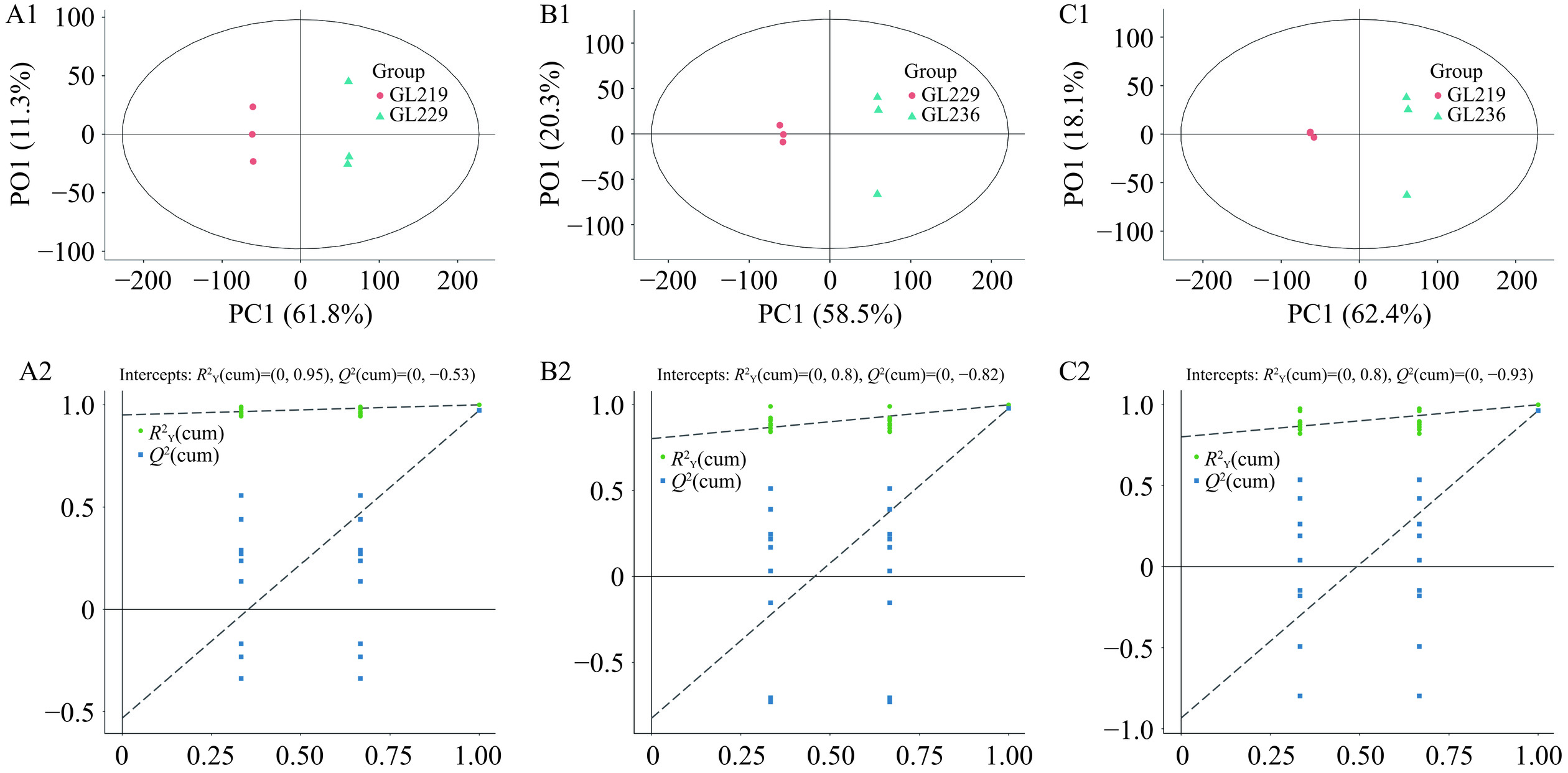
2.2.3 正交偏最小二乘判别分析
正交偏最小二乘判别分析(OPLS-DA)模型得分图中横坐标代表组间差异分量,将组间差异最大化的反映在横坐标上,纵坐标代表组内差异分量,反映了组内的变异[28]。三个样品OPLS-DA得分图和置换检验结果如图6和图7所示,样品GL219与GL229、样品GL236与GL229、样品GL219与GL236均能明显的分开,说明不同菌株的样本存在较大差异,而组内3次生物学重复散点较集中,表明组内差异较小。
置换检验能够判断OPLS-DA模型是否过拟合,对于OPLS-DA模型导出的VIP值、P值和FC才更具说服力[29]。采用R2Y和Q2对OPLS-DA模型进行了评估,其中,R2Y代表模型的拟合能力,Q2代表预测能力[30]。采用200次响应的置换检验对模型进行验证,正、负离子模式下的6个原模型R2Y均接近1,说明模型拟合效果较好,结果稳定可靠。
2.2.4 差异代谢物筛选及分析
从3个样品中共鉴定出6057个代谢物,其中正离子模式下3331个,负离子模式下2726个。根据一般的差异代谢物筛选标准:VIP>1、P<0.05、FC≥2或≤0.5,筛选出各组间的差异代谢物,结果正离子模式下GL219 vs GL229组有722个、GL236 vs GL229组有698个、GL219 vs GL236组有1074个,负离子模式下各组分别有560个、613个、892个,由检测结果可知,不同灵芝菌株的显著差异代谢物数量较多,因此,在此基础上进一步对各组进行共有差异代谢物的筛选,最终得到正离子模式下共有差异代谢物67个(表1),负离子模式下共有差异代谢物56个(表2)。FC值大,上调就越显著,FC值小,下调就越显著。
表 1 正离子模式下共有差异代谢物及变化趋势Table 1. Common differential metabolites and trends in positive ion mode序号 类别 化合物名称 保留时间(s) FC GL219 vs GL229 FC GL236 vs GL229 FC GL219 vs GL236 1 有机酸及其
衍生物N-Cyclohexylformamide 55.03 0.22 down 2.75 up 0.08 down 2 Homoarginine 56.62 0.47 down 3.29 up 0.14 down 3 Seryllysine 395.36 0.31 down 0.15 down 2.03 up 4 Arginylglutamic acid 401.22 3.42 up 0.40 down 8.61 up 5 Glutaminylphenylalanine 588.15 10.59 up 2.12 up 5.01 up 6 Dihydroceramide 665.64 0.42 down 6.39 up 0.07 down 7 苯环类化合物 Dibenz[a,h]anthracene 61.21 0.47 down 2.21 up 0.21 down 8 Fospropofol 96.79 0.49 down 2.46 up 0.20 down 9 Tyramine 121.09 0.13 down 2.19 up 0.06 down 10 Phenylacetaldehyde 121.09 0.14 down 2.11 up 0.07 down 11 [3-(4-methoxyphenyl)propoxy]sulfonic acid 134.00 0.47 down 4.54 up 0.10 down 12 4-Chloro-3,5-dimethoxybenzaldehyde 195.33 0.23 down 0.50 down 0.47 down 13 1-Naphthylamine 324.41 0.12 down 6.54 up 0.02 down 14 Rotigotine 325.09 0.32 down 2.04 up 0.16 down 15 Ioxynil 363.48 0.41 down 3.13 up 0.13 down 16 Di-n-heptyl phthalate 548.46 3.53 up 0.47 down 7.43 up 17 3,17-Androstanediol glucuronide 549.79 0.29 down 7.98 up 0.04 down 18 有机氧化合物 cis-Hydroxy perhexiline 65.12 0.49 down 2.61 up 0.19 down 19 Cyclohexanone 78.25 0.32 down 2.54 up 0.13 down 20 Cymorcin monoglucoside 288.27 0.33 down 2.44 up 0.14 down 21 Validamycin A 349.21 0.38 down 2.38 up 0.16 down 22 1-Hydroxyepiacorone 370.82 0.47 down 2.14 up 0.22 down 23 Adlupone 394.72 2.04 up 0.48 down 4.27 up 24 有机杂环
化合物Marcanine A 68.53 5.13 up 2.37 up 2.16 up 25 Immepip 255.25 0.50 down 2.46 up 0.20 down 26 Erythroskyrin 269.00 0.40 down 0.13 down 3.17 up 27 2-Benzimidazolylguanidine 334.21 7.36 up 2.36 up 3.12 up 28 N-Ethylammelide 358.52 2.07 up 0.27 down 7.59 up 29 Pentobarbital 437.41 0.49 down 2.91 up 0.17 down 30 6-(Hydroxymethyl)-7,8-dihydropterin 468.00 0.42 down 2.63 up 0.16 down 31 Benzimidazole 515.53 7.62 up 2.36 up 3.23 up 32 1-Methyl-4-phenylpyridinium 537.84 2.49 up 0.29 down 8.57 up 33 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanol 560.16 2.46 up 0.33 down 7.51 up 34 有机氮化合物 N-methylphenylethanolaminium 71.37 0.41 down 2.67 up 0.15 down 35 4-Methylthiobutanaldoxime 284.54 0.43 down 5.47 up 0.08 down 36 Minoxidil 587.45 3.48 up 0.48 down 7.20 up 37 脂类和类脂类化合物 Dethiobiotin 75.85 2.34 up 0.29 down 7.99 up 38 N,2,3-Trimethyl-2-(1-methylethyl)butanamide 204.80 2.02 up 0.46 down 4.39 up 39 Nandrolone phenpropionate 258.00 0.47 down 2.09 up 0.23 down 40 Hydroxybutyrylcarnitine 291.83 0.31 down 0.13 down 2.42 up 41 2-Isopropylmalic acid 407.77 0.03 down 0.10 down 0.34 down 42 Galactosylsphingosine 426.92 0.27 down 2.33 up 0.12 down 43 Mevalonic acid 436.43 0.33 down 0.09 down 3.59 up 44 PA(16:1(9Z)/14:1(9Z)) 445.96 0.25 down 2.44 up 0.10 down 45 2-Linoleoyl-sn-glycero-3-phosphocholine 557.48 0.38 down 2.88 up 0.13 down 46 10-Deoxymethymycin 566.93 4.92 up 0.23 down 21.54 up 47 萜类 Kalihinol A 83.11 0.50 down 2.61 up 0.19 down 48 Trichodermin 373.30 0.17 down 0.37 down 0.45 down 49 Glycosyl-4,4'-diaponeurosporenoate 430.47 0.50 down 2.38 up 0.21 down 50 trans-Geranylgeranylbixin 511.69 0.43 down 2.60 up 0.17 down 51 核苷、核苷酸和类似物 Clofarabine 134.95 0.45 down 2.22 up 0.20 down 52 生物碱 Cinchonine 233.62 2.32 up 6.07 up 0.38 down 53 Tubulosine 496.03 0.49 down 3.46 up 0.14 down 54 Cycloprotobuxine C 538.41 3.33 up 0.49 down 6.78 up 55 Solanidine 583.75 2.22 up 10.09 up 0.22 down 56 有机硫化合物 3-Methyl-2-butene-1-thiol 426.12 2.22 up 0.50 down 4.49 up 57 苯丙素类
和聚酮类Dehydrocarpaine I 457.25 0.23 down 0.50 down 0.47 down 58 其他类 2-({[(4-Methoxyphenyl)methyl](methyl)amino}methyl)-2-methylpropane-1,3-diol 76.81 0.43 down 2.24 up 0.19 down 59 1,3-Diphenylpropan-1-ol 211.82 0.44 down 2.64 up 0.17 down 60 Prednisolone 21-All-cis-farnesylate 337.56 0.45 down 2.58 up 0.17 down 61 2Alpha,3alpha-(difluoromethylene)-5alpha-androstan-17beta-ol acetate 358.02 0.36 down 6.12 up 0.06 down 62 4,4'-(Diphenylethenylidene)bis[N,N-dimethylbenzenamine] 368.70 0.45 down 0.18 down 2.53 up 63 Cytarabine ocfosphate 427.70 0.39 down 2.63 up 0.15 down 64 8-(1,2-dihydroxypropan-2-yl)-9-hydroxy-2H,8H,9H-furo[2,3-h]chromen-2-one 439.27 0.40 down 0.10 down 2.52 up 65 6-[4-(2-carboxy-2-hydroxyethyl)-2-(3-methylbut-2-en-1-yl)phenoxy]-3,4,5-trihydroxyoxane-2-carboxylic acid 538.73 0.43 down 0.21 down 2.03 up 66 Blue pigment 623.99 0.31 down 4.34 up 0.07 down 67 Dimefox 664.12 0.28 down 8.64 up 0.03 down 表 2 负离子模式下共有差异代谢物及变化趋势Table 2. Common differential metabolites and trends in negative ion mode序号 类别 化合物名称 保留时间(s) FC up/down
GL219 vs GL229FC up/down
GL236 vs GL229FC up/down
GL219 vs GL2361 有机酸及其
衍生物Glutamine 55.45 0.42 down 3.14 up 0.14 down 2 Aminoparathion 289.08 0.14 down 0.29 down 0.47 down 3 L-Glutamine 377.33 0.34 down 0.13 down 2.71 up 4 Vignatic acid A 401.72 0.44 down 2.23 up 0.20 down 5 苯环类化合物 3,4-Dihydroxymandelic acid 60.47 3.11 up 0.45 down 6.91 up 6 Tolylacetate 459.19 2.78 up 0.48 down 5.81 up 7 3-Phenoxybenzoic acid 537.90 2.60 up 0.49 down 5.27 up 8 有机氧化合物 Xanthurenate-8-O-beta-D-glucoside 58.28 4.00 up 0.36 down 11.08 up 9 3,5-Dihydroxyphenyl 1-O-(6-O-galloyl-beta-D-glucopyranoside) 63.16 2.50 up 0.24 down 10.53 up 10 Paederoside 160.36 0.42 down 2.01 up 0.21 down 11 有机杂环
化合物1,9-Dimethyluric acid 60.70 9.97 up 2.13 up 4.68 up 12 Imipenem 73.66 2.08 up 0.24 down 8.66 up 13 Lophophorine 83.61 0.31 down 2.99 up 0.11 down 14 N6-(Delta2-Isopentenyl)-adenine 128.28 4.10 up 0.20 down 20.50 up 15 Nnal-N-oxide 290.74 7.02 up 2.19 up 3.21 up 16 Dulciol C 329.77 0.45 down 3.50 up 0.13 down 17 Nicardipine 331.87 0.32 down 2.45 up 0.13 down 18 Dihydroxyfumitremorgin C 420.20 0.37 down 2.59 up 0.14 down 19 6-Benzylaminopurine 477.26 7.87 up 2.72 up 2.90 up 20 苯丙素类
和聚酮类trans-3,3',4',5,5',7-Hexahydroxyflavanone 63.17 0.48 down 0.20 down 2.41 up 21 脂类和类脂类化合物 Glycerophosphocholine 116.40 0.28 down 2.94 up 0.09 down 22 5a,11a-Dehydrooxytetracycline 128.75 5.29 up 2.22 up 2.38 up 23 Hydroxypropionylcarnitine 312.45 0.35 down 2.06 up 0.17 down 24 7C-aglycone 440.66 0.32 down 0.13 down 2.42 up 25 Tetradecanedioic acid 478.17 10.38 up 2.21 up 4.69 up 26 PA(8:0/18:0) 528.02 0.41 down 2.16 up 0.19 down 27 Sulfocholic acid 546.12 0.32 down 3.00 up 0.11 down 28 Sphinganine 1-phosphate 548.20 3.41 up 0.44 down 7.72 up 29 Dehydrocholic acid 548.27 3.41 up 0.46 down 7.40 up 30 10-Deoxymethymycin 550.31 2.19 up 9.34 up 0.23 down 31 Petromyzonol 24-sulfate 563.31 0.49 down 3.40 up 0.14 down 32 Novapikromycin 648.45 0.49 down 4.12 up 0.12 down 33 核苷、核苷酸和类似物 6-Mercaptopurine ribonucleoside 5'-diphosphate 148.92 0.46 down 0.22 down 2.04 up 34 萜类 Genipin 221.60 0.26 down 3.65 up 0.07 down 35 Acidissiminol 464.65 0.47 down 3.20 up 0.15 down 36 木脂素、新木脂素及相关
化合物Podophyllotoxone 264.72 0.33 down 2.74 up 0.12 down 37 有机金属
化合物Dimethicone 321.49 0.44 down 2.21 up 0.20 down 38 有机硫化合物 (E)-S-1-Propenyl thiosulfate 343.75 0.36 down 3.12 up 0.11 down 39 EPTC 474.48 0.39 down 2.29 up 0.17 down 40 生物碱 Veprisinium 425.77 0.45 down 3.14 up 0.14 down 41 Apovincamine 639.80 0.24 down 2.12 up 0.11 down 42 其他类 6,7-dihydroxy-3-(2,4,5-trihydroxyphenyl)-4H-chromen-4-one 63.55 0.42 down 0.17 down 2.43 up 43 3,4,5-trihydroxy-6-(1H-indole-3-carbonyloxy)oxane-2-carboxylic acid 64.00 0.18 down 0.46 down 0.39 down 44 Sulpyrine 68.11 3.13 up 6.75 up 0.46 down 45 1,8-Dinitropyrene 68.78 7.90 up 3.47 up 2.27 up 46 Silicic acid 69.25 2.10 up 7.35 up 0.29 down 47 p-Chlorobenzhydrol 72.15 0.12 down 0.46 down 0.26 down 48 2-Pyrone-4,6-dicarboxylate 120.09 0.42 down 2.32 up 0.18 down 49 Ditalimfos 331.49 0.45 down 3.05 up 0.15 down 50 (5,7-dihydroxy-6,8-dimethyl-2-phenyl-3,4-dihydro-2H-1-benzopyran-4-yl)oxidanesulfonic acid 423.82 0.46 down 0.19 down 2.50 up 51 Dihydrorhizobitoxine 492.55 0.24 down 10.97 up 0.02 down 52 RSL3 506.93 0.37 down 0.18 down 2.06 up 53 Jamaicamide C 540.79 5.38 up 0.04 down 140.34 up 54 3-Hydroxy-2-(4-morpholinylmethyl)estra-1,3,5(10)-trien-17-one 558.07 0.22 down 0.49 down 0.45 down 55 Procarbazine hydrochloride 606.69 0.33 down 2.95 up 0.11 down 56 3alpha-Hydroxy-3,5-dihydromonacolin L acid 737.68 0.43 down 2.74 up 0.16 down 正离子模式下共有差异代谢物占比较多的是脂类和类脂类化合物、苯环类化合物、有机杂环化合物、有机酸及其衍生物和有机氧化合物。GL219 vs GL229组中上调物质多为有机杂环化合物和生物碱,主要差异化合物为谷氨酰苯丙氨酸(Glutaminylphenylalanine,FC=10.59)、2-胍啶苯并咪唑(2-Benzimidazolylguanidine)、苯并咪唑(Benzimidazole)等,下调物质多为脂类和类脂类化合物、苯环类化合物和有机氧化合物,主要化合物为2-异丙基苹果酸(2-Isopropylmalic acid,FC=0.03)、1-萘胺(1-Naphthylamine)、对羟基苯乙胺(Tyramine)等。GL236 vs GL229组中上调物质多为苯环类化合物、有机杂环化合物和有机氧化合物,主要差异化合物为龙葵次碱(Solanidine,FC=10.09)、3,17-Androstanediol glucuronide、双氢神经酰胺(Dihydroceramide)等,下调物质多为脂类和类脂类化合物,主要化合物为3,5-二羟基-3-甲基戊酸(Mevalonic acid,FC=0.09)、2-异丙基苹果酸、羟基丁酰肉碱(Hydroxybutyrylcarnitine)等。GL219 vs GL236组中上调物质多为有机杂环化合物,主要差异化合物为10-Deoxymethymycin(FC=21.54)、Arginylglutamic acid、D-脱硫生物素(Dethiobiotin)等,下调物质多为苯环类化合物和有机氧化合物,主要化合物为1-萘胺(FC=0.02)、对羟基苯乙胺、苯乙醛(Phenylacetaldehyde)等。2-异丙基苹果酸在GL219 vs GL229组和GL236 vs GL229组中是明显下调的物质,是一种亮氨酸生物合成的中间体。葡萄酒中可检测出2-异丙基苹果酸,有机酸是葡萄酒中酸味的主要成分之一[31],因此,2-异丙基苹果酸可能贡献了灵芝水提液的酸味。1-萘胺、对羟基苯乙胺在GL219 vs GL229组和GL219 vs GL236组中是明显下调的物质。
负离子模式下共有差异代谢物占比较多的是脂类和类脂类化合物、有机杂环化合物,GL219 vs GL229组中上调物质多为有机杂环化合物,主要差异化合物为十四烷二酸(Tetradecanedioic acid,FC=10.38)、1,9-二甲基-2,6,8-三羟基嘌呤(1,9-Dimethyluric acid)、6-苄氨基嘌呤(6-Benzylaminopurine)等,下调物质多为脂类和类脂类化合物、有机酸及其衍生物,主要化合物为4-氯二苯甲醇(p-Chlorobenzhydrol,FC=0.12)、京尼平(Genipin)、阿扑长春胺(Apovincamine)等。GL236 vs GL229组中上调物质多为脂类和类脂类化合物、有机杂环化合物,主要差异化合物为Dihydrorhizobitoxine(FC=10.97)、10-Deoxymethymycin、安乃近(Sulpyrine)等,下调物质多为苯环类化合物和有机氧化合物,主要为Jamaicamide C(FC=0.04)、L-谷氨酰胺(L-Glutamine)、7C-aglycone等。GL219 vs GL236组中上调物质多为有机杂环化合物、苯环类化合物和有机氧化合物,主要差异化合物为Jamaicamide C(FC=140.34)、N6-异戊烯基腺嘌呤(N6-(Delta2-Isopentenyl)-adenine)、Xanthurenate-8-O-beta-D-glucoside等,下调物质多为脂类和类脂类化合物、有机酸及其衍生物,主要化合物为Dihydrorhizobitoxine(FC=0.02)、京尼平、甘油磷酰胆碱(Glycerophosphocholine)等。京尼平在GL219 vs GL229组和GL219 vs GL236组中是明显下调的物质,是环烯醚萜类化合物,具有较好的治疗糖尿病肾病作用[32]。Jamaicamide C在GL236 vs GL229组中是明显下调的物质,而在GL219 vs GL236组中是明显上调的物质,表明Jamaicamide C在3个样品中的含量差异比较大,Jamaicamide C是从巨大鞘丝藻中发现的天然钠通道阻滞剂[33]。
食物滋味特征不同主要与含有的非挥发性物质有关,包括有机酸、生物碱、萜类、酚类、氨基酸、核苷酸等滋味成分[34−35]。有研究表明不同梨汁的酸味强度值差异与梨汁的有机酸含量呈显著正相关[36],木瓜酸味值与总有机酸含量呈正相关[37]。因此有机酸化合物及其表达情况可能是3个样品的酸味响应值存在差异的原因,GL236 vs GL229组相对于GL219 vs GL229组,有机酸及其衍生物中上调物质多而下调物质少,这可能是GL236的酸味响应值大于GL219的原因。无机盐类等是引起咸味的主要物质,以氨基酸、核苷酸和小分子多肽为代表的化合物也可以刺激咸味,天冬氨酸钠和谷氨酸钠这类氨基酸钠盐可以呈现出咸味[38],因此,3个样品在咸味响应值上存在差异的原因可能是氨基酸代谢水平的不同。氨基酸是鲜味的主要来源,L-谷氨酰胺和谷氨酰胺呈鲜味[39−40] 和甜味[41−42],有研究表明白茶中鲜味氨基酸与鲜度呈显著正相关[40],因此L-谷氨酰胺和谷氨酰胺对灵芝水提液的鲜味和甜味有一定的贡献,这两个物质在3个样品中的表达情况不同可能造成了鲜味和甜味的响应值有差异,样品GL219的鲜味值小于GL229,这可能是因为L-谷氨酰胺和谷氨酰胺在GL219 vs GL229组中是下调物质。共有差异代谢物中生物碱辛可宁(Cinchonine)和龙葵次碱(Solanidine),苯环类化合物苯乙醛(Phenylacetaldehyde),脂类和类脂类化合物去氢胆酸(Dehydrocholic acid)在Bitter DB数据库(https://bitterdb.agri.huji.ac.il/dbbitter.php)中查出是呈苦味的物质,辛可宁在加香饮料苦味的形成中有着重要的作用[43],龙葵次碱在绿竹笋中是形成苦味的关键化学物质,其含量随着竹笋的发育而增加[44],酱香型白酒的苦味与苯乙醛呈正相关[45],因此,这些苦味物质的表达情况可能是3个灵芝水提液苦味响应值不同的原因,GL219和GL236的苦味响应值均比GL229的高,推测是这些苦味物质在GL219 vs GL229组和GL236 vs GL229组中上调占比较多的原因。
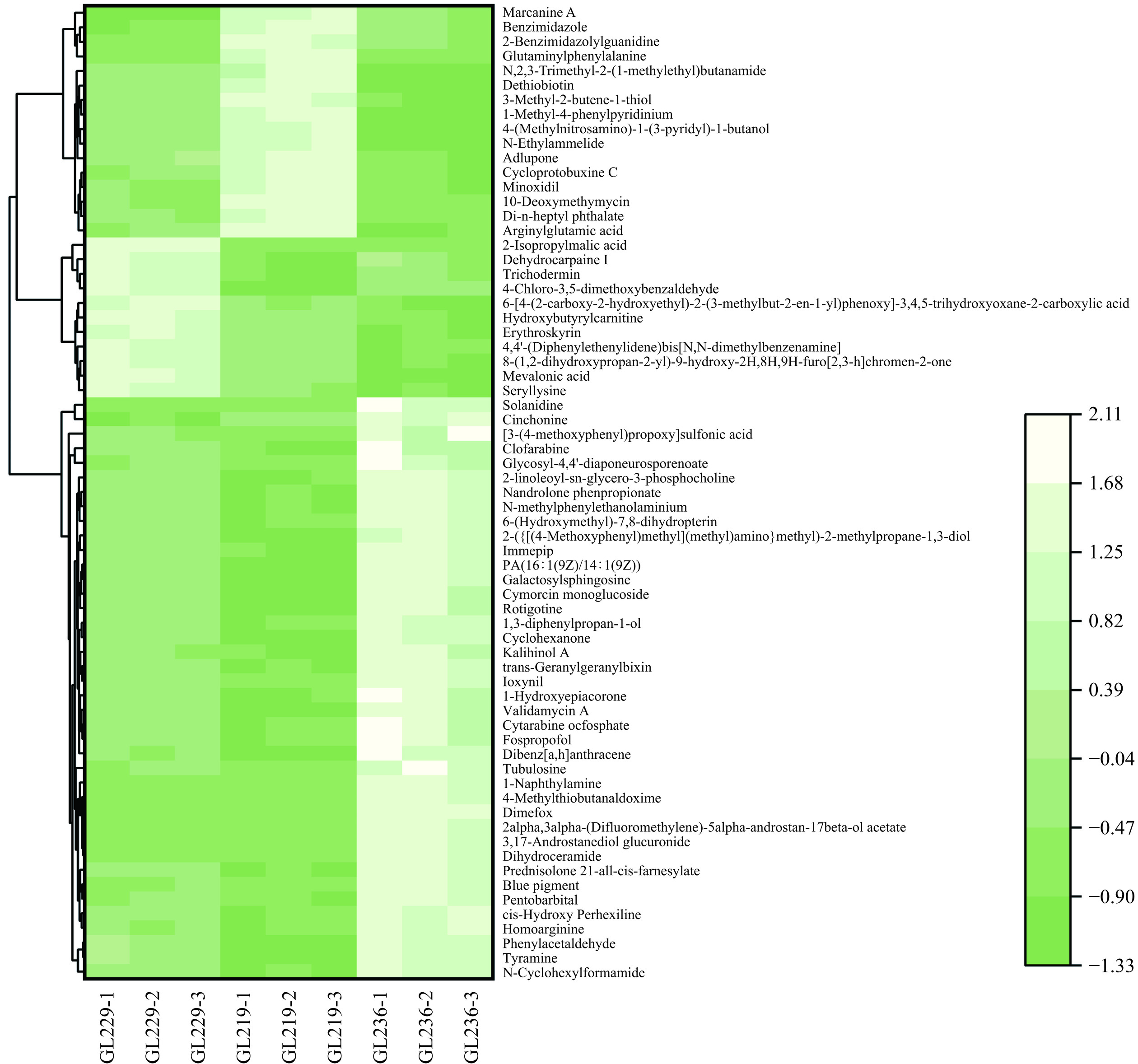
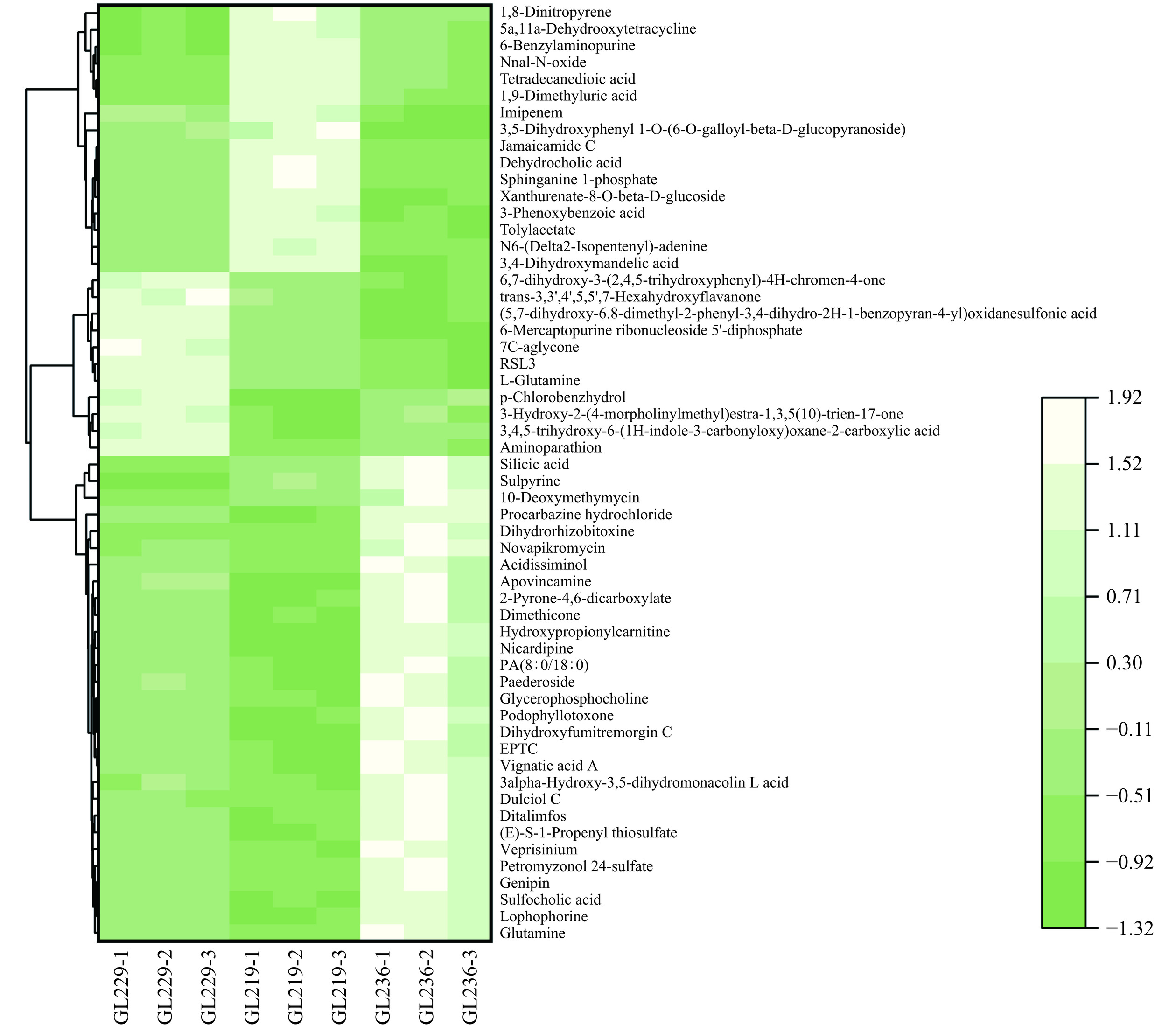
为了更直观地了解不同灵芝菌株水提液之间差异代谢物的变化,对正离子、负离子模式下3个对比组间共有差异代谢物的峰面积进行聚类分析热图(图8~图9),每行各代表一种差异代谢产物,列代表一个灵芝水提液样本。正、负离子模式下浅色代表高表达区域,深色代表低表达区域。从图中可以看出不同灵芝菌株水提液之间对比有着明确的高表达或低表达的区域。样品GL236主要集中在浅色高表达区域,而样品GL229、GL219主要集中在深色低表达区域,说明共有差异代谢物中样品GL236的表达量普遍高于其他两个菌株样品,这也可能是样品GL236滋味的整体响应值比另外两个样品高的原因。不同灵芝菌株水提液样品的共有差异代谢物具有较大差距。
2.2.5 KEGG通路分析
不同代谢物之间的相互协调作用使得生物体具有相应的功能,通过对生物体 KEGG 通路的详细分析更加有助于了解不同生物体在代谢组水平上的生物学功能[30]。正离子、负离子模式下3个对比组间共有差异代谢物的代谢通路分析结果显示共涉及22条代谢通路,如表3所示,22个代谢通路所涉及共有差异代谢物13个,其中酪氨酸代谢(Tyrosine metabolism)和鞘脂代谢(Sphingolipid metabolism)涉及的差异代谢物各有2个,这两个代谢通路的生理作用为氨基酸代谢和脂类代谢。L-谷氨酰胺为呈味氨基酸,参与了共10条代谢通路,这些代谢通路的生理作用为氨基酸代谢、核苷酸代谢、其他氨基酸的代谢、碳水化合物代谢、辅因子和维生素的代谢、能量代谢和膜运输,说明其在灵芝的滋味品质中起到一定的作用。
表 3 差异代谢通路及其差异代谢物Table 3. Differential metabolic pathways and the involved differential metabolites代谢通路 参与差异代谢物数目 参与的差异代谢物 生物素代谢(Biotin metabolism) 1 D-脱硫生物素 酪氨酸代谢(Tyrosine metabolism) 2 对羟基苯乙胺,3,4-二羟基扁桃酸 甲烷代谢(Methane metabolism) 1 对羟基苯乙胺 阿特拉津降解(Atrazine degradation) 1 N-乙基氰尿酰胺 缬氨酸、亮氨酸和异亮氨酸的生物合成(Valine, leucine and isoleucine biosynthesis) 1 2-异丙基苹果酸 丙酮酸代谢(Pyruvate metabolism) 1 2-异丙基苹果酸 鞘脂代谢(Sphingolipid metabolism) 2 Dihydroceramide,Sphinganine 1-phosphate 萜类化合物骨架合成(Terpenoid backbone biosynthesis) 1 甲戊二羟酸 叶酸生物合成(Folate biosynthesis) 1 6-(Hydroxymethyl)-7,8-dihydropterin 烟酸和烟酰胺代谢(Nicotinate and nicotinamide metabolism) 1 Blue pigment 甘油磷脂代谢(Glycerophospholipid metabolism) 1 甘油磷酰胆碱 醚脂代谢(Ether lipid metabolism) 1 甘油磷酰胆碱 精氨酸生物合成(Arginine biosynthesis) 1 L-谷氨酰胺 嘌呤代谢(Purine metabolism) 1 L-谷氨酰胺 嘧啶代谢(Pyrimidine metabolism) 1 L-谷氨酰胺 丙氨酸、天冬氨酸和谷氨酸代谢(Alanine, aspartate and glutamate metabolism) 1 L-谷氨酰胺 D-氨基酸代谢(D-Amino acid metabolism) 1 L-谷氨酰胺 乙醛酸和二羧酸代谢(Glyoxylate and dicarboxylate metabolism) 1 L-谷氨酰胺 维生素B6代谢(Vitamin B6 metabolism) 1 L-谷氨酰胺 氮素代谢(Nitrogen metabolism) 1 L-谷氨酰胺 氨酰tRNA生物合成(Aminoacyl-tRNA biosynthesis) 1 L-谷氨酰胺 ABC转运蛋白(ABC transporters) 1 L-谷氨酰胺 3. 结论
本研究利用电子舌检测了21个灵芝菌株水提液的滋味,不同灵芝菌株水提液的酸味、鲜味、苦味和咸味响应值差异比较大,21个灵芝菌株水提液响应值(酸味、鲜味、甜味、咸味、苦味)的聚类分析分为了不同滋味特点的3类。根据聚类分析结果,从每类中各选取1个样品进行UHPLC-Q-EXACTIVE MS(超高效液相色谱-四级杆-静电场轨道阱高分辨质谱仪-质谱)的非靶向代谢组学分析,3个样品(GL229、GL219、GL236)共鉴定出6057个代谢产物,以VIP>1、P<0.05、FC≥2或≤0.5筛选出GL219与GL229、GL236与GL229、GL219 与GL236之间的差异代谢物,得到3个对比组间共有差异代谢物123个,其中有机酸及其衍生物(占比8.13%)、脂类和类脂类化合物(占比17.89%)、苯环类化合物(占比11.38%)、有机氧化合物(占比7.31%)和有机杂环化合物(占比15.45%)的占比较大。经分析发现,各组间上调物质中有机杂环化合物占比较多,下调物质中脂类和类脂类化合物占比较多,共有差异代谢物中的有机酸及其衍生物、鲜味和甜味代谢物(L-谷氨酰胺、谷氨酰胺)和苦味代谢物(辛可宁、龙葵次碱、苯乙醛、去氢胆酸)对灵芝水提液的滋味品质有一定的贡献作用,这些物质在GL236 vs GL229组中上调占比较多,而在GL219 vs GL229组和GL219 vs GL236组中下调占比较多,这可能是样品GL236的滋味指标(酸味、鲜味、甜味、咸味、苦味)响应值比另外两个样品都较高的原因。因此,酸味和苦味响应值低的样品GL229更易开发为消费者接受度高的食品。本研究说明代谢组学可用于分析不同滋味的灵芝的代谢物差异,可为灵芝滋味物质基础提供一定的理论依据,但呈味物质的含量以及对样品的滋味贡献有待下一步深入研究。
-
表 1 正离子模式下共有差异代谢物及变化趋势
Table 1 Common differential metabolites and trends in positive ion mode
序号 类别 化合物名称 保留时间(s) FC GL219 vs GL229 FC GL236 vs GL229 FC GL219 vs GL236 1 有机酸及其
衍生物N-Cyclohexylformamide 55.03 0.22 down 2.75 up 0.08 down 2 Homoarginine 56.62 0.47 down 3.29 up 0.14 down 3 Seryllysine 395.36 0.31 down 0.15 down 2.03 up 4 Arginylglutamic acid 401.22 3.42 up 0.40 down 8.61 up 5 Glutaminylphenylalanine 588.15 10.59 up 2.12 up 5.01 up 6 Dihydroceramide 665.64 0.42 down 6.39 up 0.07 down 7 苯环类化合物 Dibenz[a,h]anthracene 61.21 0.47 down 2.21 up 0.21 down 8 Fospropofol 96.79 0.49 down 2.46 up 0.20 down 9 Tyramine 121.09 0.13 down 2.19 up 0.06 down 10 Phenylacetaldehyde 121.09 0.14 down 2.11 up 0.07 down 11 [3-(4-methoxyphenyl)propoxy]sulfonic acid 134.00 0.47 down 4.54 up 0.10 down 12 4-Chloro-3,5-dimethoxybenzaldehyde 195.33 0.23 down 0.50 down 0.47 down 13 1-Naphthylamine 324.41 0.12 down 6.54 up 0.02 down 14 Rotigotine 325.09 0.32 down 2.04 up 0.16 down 15 Ioxynil 363.48 0.41 down 3.13 up 0.13 down 16 Di-n-heptyl phthalate 548.46 3.53 up 0.47 down 7.43 up 17 3,17-Androstanediol glucuronide 549.79 0.29 down 7.98 up 0.04 down 18 有机氧化合物 cis-Hydroxy perhexiline 65.12 0.49 down 2.61 up 0.19 down 19 Cyclohexanone 78.25 0.32 down 2.54 up 0.13 down 20 Cymorcin monoglucoside 288.27 0.33 down 2.44 up 0.14 down 21 Validamycin A 349.21 0.38 down 2.38 up 0.16 down 22 1-Hydroxyepiacorone 370.82 0.47 down 2.14 up 0.22 down 23 Adlupone 394.72 2.04 up 0.48 down 4.27 up 24 有机杂环
化合物Marcanine A 68.53 5.13 up 2.37 up 2.16 up 25 Immepip 255.25 0.50 down 2.46 up 0.20 down 26 Erythroskyrin 269.00 0.40 down 0.13 down 3.17 up 27 2-Benzimidazolylguanidine 334.21 7.36 up 2.36 up 3.12 up 28 N-Ethylammelide 358.52 2.07 up 0.27 down 7.59 up 29 Pentobarbital 437.41 0.49 down 2.91 up 0.17 down 30 6-(Hydroxymethyl)-7,8-dihydropterin 468.00 0.42 down 2.63 up 0.16 down 31 Benzimidazole 515.53 7.62 up 2.36 up 3.23 up 32 1-Methyl-4-phenylpyridinium 537.84 2.49 up 0.29 down 8.57 up 33 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanol 560.16 2.46 up 0.33 down 7.51 up 34 有机氮化合物 N-methylphenylethanolaminium 71.37 0.41 down 2.67 up 0.15 down 35 4-Methylthiobutanaldoxime 284.54 0.43 down 5.47 up 0.08 down 36 Minoxidil 587.45 3.48 up 0.48 down 7.20 up 37 脂类和类脂类化合物 Dethiobiotin 75.85 2.34 up 0.29 down 7.99 up 38 N,2,3-Trimethyl-2-(1-methylethyl)butanamide 204.80 2.02 up 0.46 down 4.39 up 39 Nandrolone phenpropionate 258.00 0.47 down 2.09 up 0.23 down 40 Hydroxybutyrylcarnitine 291.83 0.31 down 0.13 down 2.42 up 41 2-Isopropylmalic acid 407.77 0.03 down 0.10 down 0.34 down 42 Galactosylsphingosine 426.92 0.27 down 2.33 up 0.12 down 43 Mevalonic acid 436.43 0.33 down 0.09 down 3.59 up 44 PA(16:1(9Z)/14:1(9Z)) 445.96 0.25 down 2.44 up 0.10 down 45 2-Linoleoyl-sn-glycero-3-phosphocholine 557.48 0.38 down 2.88 up 0.13 down 46 10-Deoxymethymycin 566.93 4.92 up 0.23 down 21.54 up 47 萜类 Kalihinol A 83.11 0.50 down 2.61 up 0.19 down 48 Trichodermin 373.30 0.17 down 0.37 down 0.45 down 49 Glycosyl-4,4'-diaponeurosporenoate 430.47 0.50 down 2.38 up 0.21 down 50 trans-Geranylgeranylbixin 511.69 0.43 down 2.60 up 0.17 down 51 核苷、核苷酸和类似物 Clofarabine 134.95 0.45 down 2.22 up 0.20 down 52 生物碱 Cinchonine 233.62 2.32 up 6.07 up 0.38 down 53 Tubulosine 496.03 0.49 down 3.46 up 0.14 down 54 Cycloprotobuxine C 538.41 3.33 up 0.49 down 6.78 up 55 Solanidine 583.75 2.22 up 10.09 up 0.22 down 56 有机硫化合物 3-Methyl-2-butene-1-thiol 426.12 2.22 up 0.50 down 4.49 up 57 苯丙素类
和聚酮类Dehydrocarpaine I 457.25 0.23 down 0.50 down 0.47 down 58 其他类 2-({[(4-Methoxyphenyl)methyl](methyl)amino}methyl)-2-methylpropane-1,3-diol 76.81 0.43 down 2.24 up 0.19 down 59 1,3-Diphenylpropan-1-ol 211.82 0.44 down 2.64 up 0.17 down 60 Prednisolone 21-All-cis-farnesylate 337.56 0.45 down 2.58 up 0.17 down 61 2Alpha,3alpha-(difluoromethylene)-5alpha-androstan-17beta-ol acetate 358.02 0.36 down 6.12 up 0.06 down 62 4,4'-(Diphenylethenylidene)bis[N,N-dimethylbenzenamine] 368.70 0.45 down 0.18 down 2.53 up 63 Cytarabine ocfosphate 427.70 0.39 down 2.63 up 0.15 down 64 8-(1,2-dihydroxypropan-2-yl)-9-hydroxy-2H,8H,9H-furo[2,3-h]chromen-2-one 439.27 0.40 down 0.10 down 2.52 up 65 6-[4-(2-carboxy-2-hydroxyethyl)-2-(3-methylbut-2-en-1-yl)phenoxy]-3,4,5-trihydroxyoxane-2-carboxylic acid 538.73 0.43 down 0.21 down 2.03 up 66 Blue pigment 623.99 0.31 down 4.34 up 0.07 down 67 Dimefox 664.12 0.28 down 8.64 up 0.03 down 表 2 负离子模式下共有差异代谢物及变化趋势
Table 2 Common differential metabolites and trends in negative ion mode
序号 类别 化合物名称 保留时间(s) FC up/down
GL219 vs GL229FC up/down
GL236 vs GL229FC up/down
GL219 vs GL2361 有机酸及其
衍生物Glutamine 55.45 0.42 down 3.14 up 0.14 down 2 Aminoparathion 289.08 0.14 down 0.29 down 0.47 down 3 L-Glutamine 377.33 0.34 down 0.13 down 2.71 up 4 Vignatic acid A 401.72 0.44 down 2.23 up 0.20 down 5 苯环类化合物 3,4-Dihydroxymandelic acid 60.47 3.11 up 0.45 down 6.91 up 6 Tolylacetate 459.19 2.78 up 0.48 down 5.81 up 7 3-Phenoxybenzoic acid 537.90 2.60 up 0.49 down 5.27 up 8 有机氧化合物 Xanthurenate-8-O-beta-D-glucoside 58.28 4.00 up 0.36 down 11.08 up 9 3,5-Dihydroxyphenyl 1-O-(6-O-galloyl-beta-D-glucopyranoside) 63.16 2.50 up 0.24 down 10.53 up 10 Paederoside 160.36 0.42 down 2.01 up 0.21 down 11 有机杂环
化合物1,9-Dimethyluric acid 60.70 9.97 up 2.13 up 4.68 up 12 Imipenem 73.66 2.08 up 0.24 down 8.66 up 13 Lophophorine 83.61 0.31 down 2.99 up 0.11 down 14 N6-(Delta2-Isopentenyl)-adenine 128.28 4.10 up 0.20 down 20.50 up 15 Nnal-N-oxide 290.74 7.02 up 2.19 up 3.21 up 16 Dulciol C 329.77 0.45 down 3.50 up 0.13 down 17 Nicardipine 331.87 0.32 down 2.45 up 0.13 down 18 Dihydroxyfumitremorgin C 420.20 0.37 down 2.59 up 0.14 down 19 6-Benzylaminopurine 477.26 7.87 up 2.72 up 2.90 up 20 苯丙素类
和聚酮类trans-3,3',4',5,5',7-Hexahydroxyflavanone 63.17 0.48 down 0.20 down 2.41 up 21 脂类和类脂类化合物 Glycerophosphocholine 116.40 0.28 down 2.94 up 0.09 down 22 5a,11a-Dehydrooxytetracycline 128.75 5.29 up 2.22 up 2.38 up 23 Hydroxypropionylcarnitine 312.45 0.35 down 2.06 up 0.17 down 24 7C-aglycone 440.66 0.32 down 0.13 down 2.42 up 25 Tetradecanedioic acid 478.17 10.38 up 2.21 up 4.69 up 26 PA(8:0/18:0) 528.02 0.41 down 2.16 up 0.19 down 27 Sulfocholic acid 546.12 0.32 down 3.00 up 0.11 down 28 Sphinganine 1-phosphate 548.20 3.41 up 0.44 down 7.72 up 29 Dehydrocholic acid 548.27 3.41 up 0.46 down 7.40 up 30 10-Deoxymethymycin 550.31 2.19 up 9.34 up 0.23 down 31 Petromyzonol 24-sulfate 563.31 0.49 down 3.40 up 0.14 down 32 Novapikromycin 648.45 0.49 down 4.12 up 0.12 down 33 核苷、核苷酸和类似物 6-Mercaptopurine ribonucleoside 5'-diphosphate 148.92 0.46 down 0.22 down 2.04 up 34 萜类 Genipin 221.60 0.26 down 3.65 up 0.07 down 35 Acidissiminol 464.65 0.47 down 3.20 up 0.15 down 36 木脂素、新木脂素及相关
化合物Podophyllotoxone 264.72 0.33 down 2.74 up 0.12 down 37 有机金属
化合物Dimethicone 321.49 0.44 down 2.21 up 0.20 down 38 有机硫化合物 (E)-S-1-Propenyl thiosulfate 343.75 0.36 down 3.12 up 0.11 down 39 EPTC 474.48 0.39 down 2.29 up 0.17 down 40 生物碱 Veprisinium 425.77 0.45 down 3.14 up 0.14 down 41 Apovincamine 639.80 0.24 down 2.12 up 0.11 down 42 其他类 6,7-dihydroxy-3-(2,4,5-trihydroxyphenyl)-4H-chromen-4-one 63.55 0.42 down 0.17 down 2.43 up 43 3,4,5-trihydroxy-6-(1H-indole-3-carbonyloxy)oxane-2-carboxylic acid 64.00 0.18 down 0.46 down 0.39 down 44 Sulpyrine 68.11 3.13 up 6.75 up 0.46 down 45 1,8-Dinitropyrene 68.78 7.90 up 3.47 up 2.27 up 46 Silicic acid 69.25 2.10 up 7.35 up 0.29 down 47 p-Chlorobenzhydrol 72.15 0.12 down 0.46 down 0.26 down 48 2-Pyrone-4,6-dicarboxylate 120.09 0.42 down 2.32 up 0.18 down 49 Ditalimfos 331.49 0.45 down 3.05 up 0.15 down 50 (5,7-dihydroxy-6,8-dimethyl-2-phenyl-3,4-dihydro-2H-1-benzopyran-4-yl)oxidanesulfonic acid 423.82 0.46 down 0.19 down 2.50 up 51 Dihydrorhizobitoxine 492.55 0.24 down 10.97 up 0.02 down 52 RSL3 506.93 0.37 down 0.18 down 2.06 up 53 Jamaicamide C 540.79 5.38 up 0.04 down 140.34 up 54 3-Hydroxy-2-(4-morpholinylmethyl)estra-1,3,5(10)-trien-17-one 558.07 0.22 down 0.49 down 0.45 down 55 Procarbazine hydrochloride 606.69 0.33 down 2.95 up 0.11 down 56 3alpha-Hydroxy-3,5-dihydromonacolin L acid 737.68 0.43 down 2.74 up 0.16 down 表 3 差异代谢通路及其差异代谢物
Table 3 Differential metabolic pathways and the involved differential metabolites
代谢通路 参与差异代谢物数目 参与的差异代谢物 生物素代谢(Biotin metabolism) 1 D-脱硫生物素 酪氨酸代谢(Tyrosine metabolism) 2 对羟基苯乙胺,3,4-二羟基扁桃酸 甲烷代谢(Methane metabolism) 1 对羟基苯乙胺 阿特拉津降解(Atrazine degradation) 1 N-乙基氰尿酰胺 缬氨酸、亮氨酸和异亮氨酸的生物合成(Valine, leucine and isoleucine biosynthesis) 1 2-异丙基苹果酸 丙酮酸代谢(Pyruvate metabolism) 1 2-异丙基苹果酸 鞘脂代谢(Sphingolipid metabolism) 2 Dihydroceramide,Sphinganine 1-phosphate 萜类化合物骨架合成(Terpenoid backbone biosynthesis) 1 甲戊二羟酸 叶酸生物合成(Folate biosynthesis) 1 6-(Hydroxymethyl)-7,8-dihydropterin 烟酸和烟酰胺代谢(Nicotinate and nicotinamide metabolism) 1 Blue pigment 甘油磷脂代谢(Glycerophospholipid metabolism) 1 甘油磷酰胆碱 醚脂代谢(Ether lipid metabolism) 1 甘油磷酰胆碱 精氨酸生物合成(Arginine biosynthesis) 1 L-谷氨酰胺 嘌呤代谢(Purine metabolism) 1 L-谷氨酰胺 嘧啶代谢(Pyrimidine metabolism) 1 L-谷氨酰胺 丙氨酸、天冬氨酸和谷氨酸代谢(Alanine, aspartate and glutamate metabolism) 1 L-谷氨酰胺 D-氨基酸代谢(D-Amino acid metabolism) 1 L-谷氨酰胺 乙醛酸和二羧酸代谢(Glyoxylate and dicarboxylate metabolism) 1 L-谷氨酰胺 维生素B6代谢(Vitamin B6 metabolism) 1 L-谷氨酰胺 氮素代谢(Nitrogen metabolism) 1 L-谷氨酰胺 氨酰tRNA生物合成(Aminoacyl-tRNA biosynthesis) 1 L-谷氨酰胺 ABC转运蛋白(ABC transporters) 1 L-谷氨酰胺 -
[1] 国家药典委员会. 中华人民共和国药典(一部)[S]. 北京:中国医药科技出版社, 2020:195. [National Pharmacopoeia Committee. Pharmacopoeia of the People's Republic of China, Volume I[S]. Beijing:China Medical Science and Technology Press, 2020:195.] National Pharmacopoeia Committee. Pharmacopoeia of the People's Republic of China, Volume I[S]. Beijing: China Medical Science and Technology Press, 2020: 195.
[2] BATRA P, SHARMA A K, KHAJURIA R. Probing Lingzhi or Reishi medicinal mushroom Ganoderma lucidum (higher Basidiomycetes):A bitter mushroom with amazing health benefits[J]. International Journal of Medicinal Mushrooms,2013,15(2):127−143. doi: 10.1615/IntJMedMushr.v15.i2.20
[3] TONG T, YAN R, KANG J, et al. Chemical components of ganoderma[J]. Adv Exp Med Biol,2019,1181:59−106.
[4] 钱坤, 武冬梅, 王豪, 等. 野生四川灵芝的生物学特性和抗氧化活性[J]. 菌物学报,2022,41(4):601−617. [QIAN K, WU D M, WANG H, et al. Biological characteristics and antioxidant activities of wild Ganoderma sichuanense[J]. Mycosystema,2022,41(4):601−617.] QIAN K, WU D M, WANG H, et al. Biological characteristics and antioxidant activities of wild Ganoderma sichuanense[J]. Mycosystema, 2022, 41(4): 601−617.
[5] 李玲, 孙元章, 李刚. 灵芝生物活性成分及其药理作用研究进展[J]. 南方农业,2019,13(4):50−55. [LI L, SUN Y Z, LI G. Research progress of Ganoderma lucidum bioactive components and their pharmacological effects[J]. South China Agriculture,2019,13(4):50−55.] LI L, SUN Y Z, LI G. Research progress of Ganoderma lucidum bioactive components and their pharmacological effects[J]. South China Agriculture, 2019, 13(4): 50−55.
[6] 张彬彬, 王慧真, 刘晓雪, 等. 野生灵芝驯化前后子实体活性成分比较[J]. 食品研究与开发,2022,43(20):164−171. [ZHANG B B, WANG H Z, LIU X X, et al. Comparative of active components of wild ganoderma lucidum fruiting bodies before and after domestication[J]. Food Research and Development,2022,43(20):164−171.] doi: 10.12161/j.issn.1005-6521.2022.20.021 ZHANG B B, WANG H Z, LIU X X, et al. Comparative of active components of wild ganoderma lucidum fruiting bodies before and after domestication[J]. Food Research and Development, 2022, 43(20): 164−171. doi: 10.12161/j.issn.1005-6521.2022.20.021
[7] 李学龙, 李跃, 张鹏, 等. 不同灵芝菌株主要农艺性状及活性成分比较分析[J]. 北方园艺,2022(13):119−125. [LI X L, LI Y, ZHANG P, et al. Comparative analysis of main agronomic characters and active components of different Ganoderma lucidum strains[J]. Northern Horticulture,2022(13):119−125.] LI X L, LI Y, ZHANG P, et al. Comparative analysis of main agronomic characters and active components of different Ganoderma lucidum strains[J]. Northern Horticulture, 2022(13): 119−125.
[8] XU Y, JIN Y M, SU J J, et al. Changes in the nutritional value, flavor, and antioxidant activity of brown glutinous rice during fermentation[J]. Food Bioscience,2021,43:101273. doi: 10.1016/j.fbio.2021.101273
[9] 韩腾, 陆波, 韩永斌, 等. 怀远县糯米加工甜酒酿适用性研究[J]. 食品工业科技,2024,45(14):271−281. [HAN T, LU B, HAN Y B, et al. Study on the applicability of glutinous rice varieties from Huaiyuan county to sweet rice wine production[J]. Science and Technology of Food Industry,2024,45(14):271−281.] HAN T, LU B, HAN Y B, et al. Study on the applicability of glutinous rice varieties from Huaiyuan county to sweet rice wine production[J]. Science and Technology of Food Industry, 2024, 45(14): 271−281.
[10] FENG T, BING F L, YANG Y, et al. Discrimination of edible fungi varieties and evaluation of their umami intensities by using an electronic tongue method[J]. International Journal of Food Science & Technology,2016,51(6):1393−1400.
[11] 田婧. 基于电子鼻和电子舌技术的灵芝风味物质快速测定及产地鉴别[D]. 开封:河南大学, 2023. [TIAN J. Rapid determination of the flavor substances and origin identification of Ganoderma lucidum based on electronic nose and electronic tongue technology[D]. Kaifeng:Henan University, 2023.] TIAN J. Rapid determination of the flavor substances and origin identification of Ganoderma lucidum based on electronic nose and electronic tongue technology[D]. Kaifeng: Henan University, 2023.
[12] 邴芳玲, 冯涛, 杨焱, 等. 食用菌鲜味味觉定性定量方法的电子舌研究[J]. 现代食品科技,2017,33(8):224−229. [BING F L, FENG T, YANG Y, et al. Quantification of the umami taste of edible fungi using electronic tongue[J]. Modern Food Science and Technology,2017,33(8):224−229.] BING F L, FENG T, YANG Y, et al. Quantification of the umami taste of edible fungi using electronic tongue[J]. Modern Food Science and Technology, 2017, 33(8): 224−229.
[13] PHAT C, MOON B, LEE C. Evaluation of umami taste in mushroom extracts by chemical analysis, sensory evaluation, and an electronic tongue system[J]. Food Chemistry,2016,192:1068−1077. doi: 10.1016/j.foodchem.2015.07.113
[14] 孟媛, 程卓, 林锋科, 等. 民族药用植物代谢组学研究进展[J]. 植物资源与环境学报,2022,31(2):73−81. [MENG Y, CHENG Z, LIN F K, et al. Research progress on metabolomics of ethnomedicinal plants[J]. Journal of Plant Resources and Environment,2022,31(2):73−81.] MENG Y, CHENG Z, LIN F K, et al. Research progress on metabolomics of ethnomedicinal plants[J]. Journal of Plant Resources and Environment, 2022, 31(2): 73−81.
[15] 王杰, 隗鑫, 陈威, 等. 代谢组学技术在中药复方配伍规律研究中的应用[J]. 中草药,2022,53(5):1528−1539. [WANG J, WEI X, CHEN W, et al. Application on metabolomics techniques in compatibility law of traditional Chinese medicine formulae[J]. Chinese Traditional and Herbal Drugs,2022,53(5):1528−1539.] WANG J, WEI X, CHEN W, et al. Application on metabolomics techniques in compatibility law of traditional Chinese medicine formulae[J]. Chinese Traditional and Herbal Drugs, 2022, 53(5): 1528−1539.
[16] PENG Y, HONG J W, RAFTERY D, et a1. Metabolomic-based clinical studies and murine models for acute pancreatitis disease:A review[J]. Biochim Biophys Acta Mol Basis Dis, 2021, 1867(7):166123.
[17] DU F, ZOU Y J, HU Q X, et al. Metabolic profiling of Pleurotus tuoliensis during mycelium physiological maturation and exploration on a potential indicator of mycelial maturation[J]. Frontiers in Microbiology,2019,9:3274. doi: 10.3389/fmicb.2018.03274
[18] 姚森, 刘媛媛, 赵琛, 等. 不饱和脂肪酸参与调控金针菇菌柄伸长的潜在分析[J]. 菌物学报,2019,38(12):2232−2240. [YAO S, LIU Y Y, ZHAO C, et al. Unsaturated fatty acids as potential regulator involved in stipe elongation of Flammulina filiformis[J]. Mycosystema,2019,38(12):2232−2240.] YAO S, LIU Y Y, ZHAO C, et al. Unsaturated fatty acids as potential regulator involved in stipe elongation of Flammulina filiformis[J]. Mycosystema, 2019, 38(12): 2232−2240.
[19] ZHAO X, CHEN M J, LI Z P, et al. The response of Volvariella volvacea to low-temperature stress based on metabonomics[J]. Front Microbiol,2020,11:1787. doi: 10.3389/fmicb.2020.01787
[20] ZHAO X, CHEN M J, ZHAO Y, et al. GC-MS-based nontargeted and targeted metabolic profiling identifies changes in the Lentinula edodes mycelial metabolome under high-temperature stress[J]. International Journal of Molecular Sciences,2019,20(9):2330. doi: 10.3390/ijms20092330
[21] SATO M, MIYAGI A, YONEYAMA S, et al. CE-MS-based metabolomics reveals the metabolic profile of maitake mushroom (Grifola frondosa) strains with different cultivation characteristics[J]. Bioscience, Biotechnology, and Biochemistry,2017,81(12):2314−2322. doi: 10.1080/09168451.2017.1387049
[22] SATRIA D, TAMRAKAR S, SUHARA H, et al. Mass spectrometry-based untargeted metabolomics and α-glucosidase inhibitory activity of Lingzhi (Ganoderma lingzhi) during the developmental stages[J]. Molecules,2019,24(11):2044. doi: 10.3390/molecules24112044
[23] 刘瑞新, 张杏芬, 李学林, 等. 3种口尝评价方法用于药物苦度评价的比较[J]. 中国实验方剂学杂志,2013,19(20):118−122. [LIU R X, ZHANG X F, LI X L, et al. Drug evaluation of bitterness intensity by three kinds of THTPM[J]. Chinese Journal of Experimental Traditional Medical Formulae,2013,19(20):118−122.] LIU R X, ZHANG X F, LI X L, et al. Drug evaluation of bitterness intensity by three kinds of THTPM[J]. Chinese Journal of Experimental Traditional Medical Formulae, 2013, 19(20): 118−122.
[24] 姚月凤, 王家勤, 滑金杰, 等. 电子舌在工夫红茶甜纯滋味特征评价中的应用[J]. 食品科学,2019,40(18):236−241. [YAO Y F, WANG J Q, HUA J J, et al. Application of electronic tongue in the evaluation of sweet taste quality of congou black tea[J]. Food Science,2019,40(18):236−241.] doi: 10.7506/spkx1002-6630-20181012-100 YAO Y F, WANG J Q, HUA J J, et al. Application of electronic tongue in the evaluation of sweet taste quality of congou black tea[J]. Food Science, 2019, 40(18): 236−241. doi: 10.7506/spkx1002-6630-20181012-100
[25] IVANISEVIC J, ZHU Z, PLATE L, et al. Toward 'omic scale metabolite profiling:A dual separation–mass spectrometry approach for coverage of lipid and central carbon metabolism[J]. Analytical Chemistry,2013,85(14):6876−6684. doi: 10.1021/ac401140h
[26] 张桂英, 张喜文, 杨斌, 等. 不同品种小米淀粉理化特性的主成分分析与聚类分析[J]. 现代食品科技, 2017, 33(11):224−229. [ZHANG G Y, ZHANG X W, YANG B, et al. Principal components analysis and cluster analysis of physicochemical properties of starch from different cultivars of millet[J]. Modern Food Science and Technology, 2020, 36(5):310−318.] ZHANG G Y, ZHANG X W, YANG B, et al. Principal components analysis and cluster analysis of physicochemical properties of starch from different cultivars of millet[J]. Modern Food Science and Technology, 2020, 36(5): 310−318.
[27] 黄盼, 周改莲, 王倩, 等. 基于主成分和聚类分析评价国产不同批次肉豆蔻挥发油的质量[J]. 现代食品科技,2020,36(5):310−318. [HUANG P, ZHOU G L, WANG Q, et al. Evaluation of the quality of domestically produced different batches of nutmeg volatile oil based on principal component and cluster analysis[J]. Modern Food Science and Technology,2020,36(5):310−318.] HUANG P, ZHOU G L, WANG Q, et al. Evaluation of the quality of domestically produced different batches of nutmeg volatile oil based on principal component and cluster analysis[J]. Modern Food Science and Technology, 2020, 36(5): 310−318.
[28] 唐佳代, 冉光耀, 陈诺, 等. 基于非靶向代谢组学分析不同陈化时间老鹰茶代谢产物的差异[J]. 中国酿造,2023,42(9):115−119. [TANG J D, RAN G Y, CHEN N, et al. Difference analysis ofmetabolites in hawk tea with different aging time based on non-targeted metabolomics technology[J]. China Brewing,2023,42(9):115−119.] TANG J D, RAN G Y, CHEN N, et al. Difference analysis ofmetabolites in hawk tea with different aging time based on non-targeted metabolomics technology[J]. China Brewing, 2023, 42(9): 115−119.
[29] 刘含, 郭银萍, 穆兴燕, 等. 刺梨不同提取物的非靶向代谢组学比较与分析[J]. 食品与发酵工业,2024,50(2):312−320. [LIU H, GUO Y P, MU X Y, et al. Comparison and analysis of non-targeted metabolomics of different extracts of Rosa roxburghii Tratt J]. Food and Fermentation Industries,2024,50(2):312−320.
[30] 祝晓云, 蒋永梅, 余家奇, 等. 非靶向代谢组学分析不同品种青花椒的化学成分差异[J]. 种子,2023,42(9):49−61. [ZHU X Y, JIANG Y M, YU J Q, et al. Analysis of chemical composition differences in different varieties of Zanthoxylum schinifolium based on non-targeted metabonomics[J]. Seed,2023,42(9):49−61.] ZHU X Y, JIANG Y M, YU J Q, et al. Analysis of chemical composition differences in different varieties of Zanthoxylum schinifolium based on non-targeted metabonomics[J]. Seed, 2023, 42(9): 49−61.
[31] RICCIUTELLI M, MORETTI S, GALARINI R, et al. Identification and quantification of new isomers of isopropyl-malic acid in wine by LC-IT and LC-Q-Orbitrap[J]. Food Chem,2019,294:390−396. doi: 10.1016/j.foodchem.2019.05.068
[32] WANG M X, WANG M M, LIU C, et al. A geniposide-phospholipid complex ameliorates posthyperuricemia chronic kidney disease induced by inflammatory reactions and oxidative stress[J]. Eur J Pharmacol,2022,930:175157. doi: 10.1016/j.ejphar.2022.175157
[33] GRAF K M, TABOR M G, BROWN M L, et al. Synthesis of (S)-jamaicamide C carboxylic acid[J]. Org Lett,2009,11(23):5382−5385. doi: 10.1021/ol9021222
[34] SUM L B, ZHANG Z Y, XIN G, et al. Advances in umami taste and aroma of edible mushrooms[J]. Trends in Food Science & Technology,2020,96:176−187.
[35] 游兴勇, 许杨, 李燕萍. 食用菌非挥发性呈味物质的研究[J]. 中国调味品,2008(8):32−35,47. [YOU X Y, XU Y, LI Y P. The studies of nonvolatile taste compounds of edible fungi[J]. China Condiment,2008(8):32−35,47.] doi: 10.3969/j.issn.1000-9973.2008.08.004 YOU X Y, XU Y, LI Y P. The studies of nonvolatile taste compounds of edible fungi[J]. China Condiment, 2008(8): 32−35,47. doi: 10.3969/j.issn.1000-9973.2008.08.004
[36] 易岸威, 程红, 程玉豆, 等. ‘巴梨’和‘早红考密斯’梨汁品质特性及其抗氧化活性分析[J/OL]. 食品与发酵工业, 1−18[2024-07-29]. https://doi.org/10.13995/j.cnki.11-1802/ts.037156. [YI A W, CHENG H, CHENG Y D, et al. Quality properties and antioxidant activities of pear juice made by ‘Bartlett’ and ‘Doyenne du Comice’ pear[J/OL]. Food and Fermentation Industries, 1−18[2024-07-29]. https://doi.org/10.13995/j.cnki.11-1802/ts.037156.] YI A W, CHENG H, CHENG Y D, et al. Quality properties and antioxidant activities of pear juice made by ‘Bartlett’ and ‘Doyenne du Comice’ pear[J/OL]. Food and Fermentation Industries, 1−18[2024-07-29]. https://doi.org/10.13995/j.cnki.11-1802/ts.037156.
[37] 张玲, 李宗金, 张亚莉, 等. 木瓜“酸味”与有机酸成分的相关性研究[J]. 中成药,2023,45(2):476−482. [ZHANG L, LI Z J, ZHANG Y L, et al. Correlation between sour taste and organic acids of Chaenomelis Fructus[J]. Chinese Traditional Patent Medicine,2023,45(2):476−482.] doi: 10.3969/j.issn.1001-1528.2023.02.023 ZHANG L, LI Z J, ZHANG Y L, et al. Correlation between sour taste and organic acids of Chaenomelis Fructus[J]. Chinese Traditional Patent Medicine, 2023, 45(2): 476−482. doi: 10.3969/j.issn.1001-1528.2023.02.023
[38] 汪少芸, 黄心澄, 高婷婷, 等. 咸味感知与咸味肽的研究进展[J]. 食品科学,2023,44(1):1−13. [WANG S Y, HUANG X C, GAO T T, et al. Progress in research on saltiness perception and salty peptides[J]. Food Science,2023,44(1):1−13.] doi: 10.7506/spkx1002-6630-20221103-030 WANG S Y, HUANG X C, GAO T T, et al. Progress in research on saltiness perception and salty peptides[J]. Food Science, 2023, 44(1): 1−13. doi: 10.7506/spkx1002-6630-20221103-030
[39] 李文亚, 班雨函, 于宏伟, 等. 基于GC-MS的低盐虾酱低温发酵过程中代谢组学分析[J]. 食品科学,2022,43(8):166−174. [[LI W Y, BAN Y H, YU H W, et al. Metabolomic analysis of low-salt shrimp paste during fermentation at low temperature based on gas chromatography-mass spectrometry[J]. Food Science,2022,43(8):166−174.] [LI W Y, BAN Y H, YU H W, et al. Metabolomic analysis of low-salt shrimp paste during fermentation at low temperature based on gas chromatography-mass spectrometry[J]. Food Science, 2022, 43(8): 166−174.
[40] 龚淑英, 谷兆骐, 范方媛, 等. 浙江省主栽茶树品种工艺白茶的滋味成分研究[J]. 茶叶科学, 2016, 36(3):277−284. [GONG S Y, GU Z Q, FAN F Y, Research on taste compounds in white tea processed from cultivars in Zhejiang Province[J]. Journal of Tea Science, 2016, 36(3):277−284.] GONG S Y, GU Z Q, FAN F Y, Research on taste compounds in white tea processed from cultivars in Zhejiang Province[J]. Journal of Tea Science, 2016, 36(3): 277−284.
[41] 游小妹, 韩奥迪, 李鑫磊, 等. 黄化茶树新品种‘茗冠’多茶类品质差异分析[J]. 食品工业科技,2023,44(23):287−297. [YOU X M, HAN A D, LI X L, et al. Analysis of metabolites difference of the albino tea tree variety 'Ming Guan'[J]. Science and Technology of Food Industry,2023,44(23):287−297.] YOU X M, HAN A D, LI X L, et al. Analysis of metabolites difference of the albino tea tree variety 'Ming Guan'[J]. Science and Technology of Food Industry, 2023, 44(23): 287−297.
[42] 邓慧莉, 李鑫磊, 毛贻帆, 等. 不同做青温度对乌龙茶滋味与香气品质的影响[J]. 食品安全质量检测学报,2021,12(14):5766−5771. [DENG H L, LI X L, MAO Y F, et al. Effect of different turning-over temperatures on the taste and aroma quality of Oolong tea[J]. Journal of Food Safety & Quality,2021,12(14):5766−5771.] DENG H L, LI X L, MAO Y F, et al. Effect of different turning-over temperatures on the taste and aroma quality of Oolong tea[J]. Journal of Food Safety & Quality, 2021, 12(14): 5766−5771.
[43] 徐安康. 加香葡萄酒的生产技术[J]. 酿酒科技,1989(4):39−42. [XU A K. Production technology of flavored wine[J]. Liquor-Making Science & Technology,1989(4):39−42.] XU A K. Production technology of flavored wine[J]. Liquor-Making Science & Technology, 1989(4): 39−42.
[44] JIAO Y L. Transcriptomic and metabolomic analyses reveal the flavor of bitterness in the tip shoots of Bambusa oldhamii Munro.[J]. Sci Rep,2023,13(1):14853. doi: 10.1038/s41598-023-40918-8
[45] 孙优兰, 骆红波, 王金龙, 等. 酱香型白酒不同轮次基酒风味特征分析[J/OL]. 食品与发酵工业, 1−11[2024-07-29]. https://doi.org/10.13995/j.cnki.11-1802/ts.037173. [SUN Y L, LUO H B, WANG J L, et al. Analysis of flavor characteristics of different rounds base liquor for Jiangxiangxing Baijiu[J/OL]. Food and Fermentation Industries, 1−11[2024-07-29]. https://doi.org/10.13995/j.cnki.11-1802/ts.037173.] SUN Y L, LUO H B, WANG J L, et al. Analysis of flavor characteristics of different rounds base liquor for Jiangxiangxing Baijiu[J/OL]. Food and Fermentation Industries, 1−11[2024-07-29]. https://doi.org/10.13995/j.cnki.11-1802/ts.037173.





 下载:
下载:
 下载:
下载:



