Establishment of Fermentation Kinetic Model of Streptococcus thermophilus JM108
-
摘要: 本研究以嗜热链球菌JM108的生长和代谢为研究对象,以建立随时间变化模型,模拟细菌的生长、产物合成以及底物消耗的动力学变化。在M17培养基中接种嗜热链球菌JM108,每隔2 h对发酵体系内的嗜热链球菌JM108活菌数、乳酸含量以及葡萄糖含量进行测定,采用3种经典模型Logistic模型、Boltzmann模型和SGompertz模型对实验值进行非线性拟合。非线性拟合结果表明,Logistic模型最适合用于描述细菌生长动力学、乳酸生成动力学和葡萄糖消耗动力学,其R2值分别为0.9974、0.9947和0.9964,均大于0.99,拟合值与实验值的误差均小于15%,拟合良好,表明所建立的动力学模型能够较好地预测发酵过程的动态变化。嗜热链球菌JM108生长量动力学方程为:y=8.59+−2.391+(x2.32)0.02;乳酸生成量动力学方程为:y=1.05+−1.061+(x3.67)3.23;葡萄糖消耗量动力学方程为:y=0.02+0.151+(x3.47)3.90。建立嗜热链球菌JM108的发酵动力学模型,为描述发酵的动力学特征提供了理论支持。
-
关键词:
- 嗜热链球菌JM108 /
- 发酵 /
- 发酵过程 /
- 非线性拟合 /
- 发酵动力学模型
Abstract: This study examined the growth and metabolism of Streptococcus thermophilus JM108 to establish a time-varying model that simulated the dynamics of bacterial growth, product synthesis, and substrate consumption. Streptococcus thermophilus JM108 was inoculated into M17 medium. The viable bacteria count, lactic acid content, and glucose content of Streptococcus thermophilus JM108 in the fermentation system were measured every 2 h. The measured values were nonlinearly fitted using the three classical models: Logistic model, Boltzmann model, and SGompertz model. The results of the nonlinear fitting indicated that the Logistic model was the most appropriate for describing the kinetics of bacterial growth, lactic acid generation, and glucose consumption. The R2 values for the three cases were 0.9974, 0.9947, and 0.9964, respectively, all greater than 0.99. The errors between the fitted and experimental values were less than 15%, indicating a good fit. This suggested that the established dynamic model could predict the dynamic changes of the fermentation process. The growth kinetics equation of Streptococcus thermophilus JM108 was y=8.59+−2.391+(x2.32)0.02. The kinetic equation for lactic acid production was y=1.05+−1.061+(x3.67)3.23. The kinetic equation for glucose consumption was y=0.02+0.151+(x3.47)3.90. A fermentation kinetics model was established for Streptococcus thermophilus JM108 to provide theoretical support for describing the fermentation process. -
由于现代消费者对天然、无添加剂且有益健康的食品越来越感兴趣,使得酸奶和其他发酵乳制品的消费量正逐年上升[1]。嗜热链球菌是一种在工业上用于生产发酵乳制品的重要乳酸菌[2],它在乳制品发酵过程中的主要功能是产生乳酸,导致快速酸化并抑制其他微生物[3]。一些嗜热链球菌菌株被视为益生菌,食用后对人类健康有益[4−5],具有良好的益生特性,比如抗氧化能力和调节肠道菌群等[6]。除此之外,它还被报道具有缓解乳糖不耐症、慢性胃炎、腹泻以及对病原体的抑菌作用[7−9]。最近,越来越多的研究关注发酵产品中的嗜热链球菌[10−11]。
嗜热链球菌在发酵过程中的生长与代谢变化之间存在着密切的联系[2]。通过将嗜热链球菌纳入发酵动力学模型的研究框架中,人们能够更全面地了解嗜热链球菌发酵过程中的生物学特性,并预测其在发酵过程中的行为[12]。通过发酵动力学的研究,可以深入了解微生物的生长、底物的消耗和产物的形成规律[13−14],并建立相应的数学模型,以便更好地掌握发酵过程的规律,优化发酵工艺,最终提升发酵质量[15]。发酵动力学模型建立的主流方法是利用软件的非线性拟合功能来实现,通过选用合适的拟合曲线进行操作,最后选取R2较高的模型为发酵动力学模型。Logistic模型、Boltzmann模型和SGompertz模型是三种用于研究发酵动力学的经典模型,已经被广泛用于酵母菌发酵酒类的发酵动力学的建立[16−18],如吕明珊等[19]应用Logistic模型、SGompertz模型和Boltzmann模型对新疆药桑葚酵素发酵中乳酸菌生长量、总酸生成量及还原糖基质的消耗量进行非线性拟合,拟合结果良好。然而,关于嗜热链球菌的发酵动力学没有被研究报道过。
本文以嗜热链球菌JM108作为原始菌株进行研究,在M17培养基中进行发酵。采用Logistic模型、Boltzmann模型、SGompertz模型对葡萄糖含量、乳酸生成和菌体生长量进行非线性拟合,建立发酵动力学模型。对发酵过程中的嗜热链球菌JM108的生长量、葡萄糖的消耗及产物乳酸的生成量进行测定,分析其动态变化规律并探究三者之间的相关性,以期为以嗜热链球菌JM108的发酵产品的控制发酵提供理论基础,对产业化生产和经济发展具有重要意义。
1. 材料与方法
1.1 材料与仪器
嗜热链球菌JM108(Streptococcus thermophilus JM108) 东北农业大学乳品重点实验室自主分离鉴定保藏;M17肉汤培养基、M17固体培养基 青岛海博生物技术有限公司;PBS缓释片 上海生工生物工程有限公司;葡萄糖(GOPOD氧化酶法)含量试剂盒、乳酸含量试剂盒 苏州格锐思生物科技有限公司。
ME204E/02精密电子天平(0.0001 g) 瑞士梅特勒-托利多有限公司;PB-10 pH计 德国赛多利斯公司;DH-101恒温鼓风干燥箱 青岛海尔集团公司;BCN1360型生物洁净工作台 北京东联哈尔仪器有限公司;SPL-150微生物培养箱 上海龙跃仪器设备有限公司;GR85DA高压灭菌锅 美国ZEALWAY公司;Spectra Max iD3/iD5多功能酶标仪 美谷分子仪器(上海)有限公司;Sigma 1-14K冷冻微离心机 德国Sigma有限公司。
1.2 实验方法
1.2.1 嗜热链球菌JM108的活化及传代
采用Korcz等[20]描述的方法,稍作修改。从−80 ℃冰箱取出实验所用菌株,以4%的接种量接种于无菌M17液体培养基中,37 ℃培养至浑浊状态,完成活化。将活化种子液按4%(体积分数)的接种量转接到无菌M17液体培养基,在37 ℃,24 h的条件下传两代。
1.2.2 发酵
将活化扩培后的菌种按4%(体积分数)的接种量接种到灭菌冷却后的发酵液(M17液体培养基)中,置于37 ℃下发酵18 h,发酵过程中每隔2 h取发酵样液供后续实验使用。
1.2.3 菌体生长量的测定
发酵过程中菌体生长量的测定采用Veselá等[21]描述的方法,稍作修改。取5 mL发酵样液,加入到45 mL PBS缓冲盐溶液中进行第一次梯度稀释,再从中取0.5 mL稀释液样液加入到4.5 mL PBS缓冲盐溶液中,依次稀释到一定倍数后,选择两个适合的梯度进行涂布。嗜热链球菌JM108用M17固体培养基培养,于37 ℃恒温培养箱中培养48 h后计其活菌数。
1.2.4 乳酸含量的测定
发酵过程中乳酸含量采用乳酸含量试剂盒法测定。参照试剂盒推荐说明进行操作,测定发酵样液中乳酸含量。
1.2.5 葡萄糖质量浓度的测定
发酵过程中葡萄糖质量浓度采用葡萄糖氧化酶试剂盒法,参照试剂盒推荐说明进行操作,测定发酵样液中葡萄糖质量浓度。
1.2.6 pH的测定
从培养箱中取出发酵样液,用pH计测定发酵样液的pH。
1.2.7 动力学模型的建立
动力学模型的建立采用张阳阳等[22]描述的方法,稍作修改,拟合方程见表1。利用嗜热链球菌JM108发酵样液,每隔2 h测定其活菌数、乳酸生成量及葡萄糖消耗量,计算出动力学模型的参数,建立嗜热链球菌JM108的菌体生长、产物生成和底物消耗动力学模型,预测其发酵过程变化。
表 1 三种经典的发酵动力学模型Table 1. Three classical models of fermentation kinetics模型 模型方程 Logistic y=A2+(A1−A2)1+(xx0)p SGompertz y=a×e−e[−k×(x−xc)] Boltzmann y=A2+(A1−A2)1+exp(x−x0dx) 1.3 数据处理
所有实验均重复三次。采用OriginPro2022软件对数据进行统计与作图分析,选取合适的动力学模型对发酵过程中嗜热链球菌JM108生长量、乳酸含量、葡萄糖含量变化情况进行非线性拟合,并选取拟合效果最佳的动力学模型对发酵过程中微生物代谢活动进行定量分析描述。通过发酵过程曲线对嗜热链球菌JM108生长量、乳酸含量、葡萄糖含量建立发酵动力学模型。通过决定系数与模型理论值和实验值的误差对模型进行评价,误差由以下公式计算得出:误差(%)=[(实验值−拟合值)/拟合值]×100,结果以绝对值呈现。
2. 结果与分析
2.1 嗜热链球菌JM108生长量、乳酸含量、葡萄糖含量及pH的变化情况
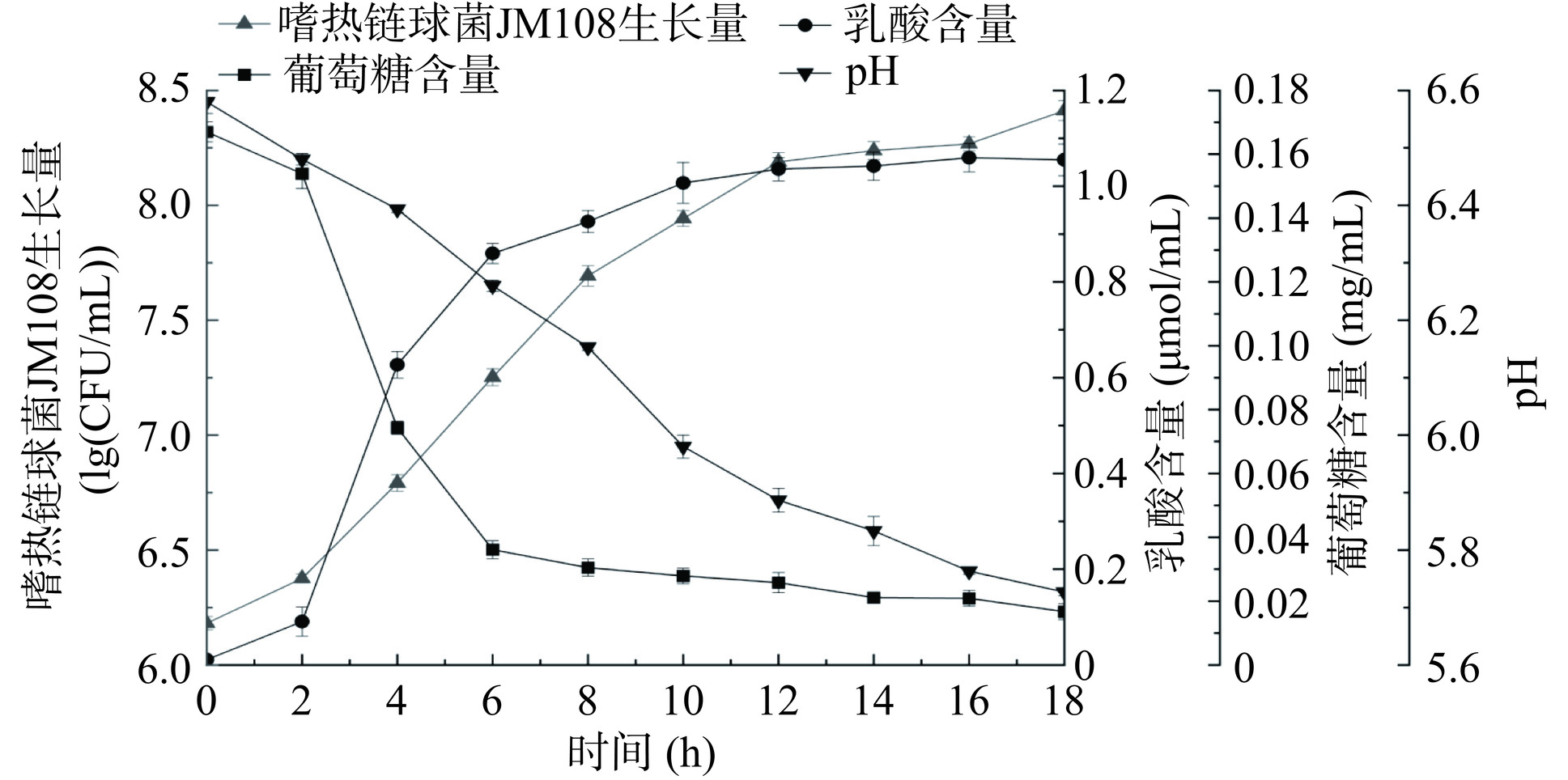
本实验对嗜热链球菌JM108在M17培养基中连续发酵18 h过程中的菌体生长量、乳酸含量、葡萄糖含量及pH进行测定,嗜热链球菌JM108发酵过程中主要指标的变化情况见图1。嗜热链球菌JM108生长量和乳酸含量在发酵过程中呈现出上升趋势,在前2 h中,乳酸含量和菌体生长量都处于较低水平并缓慢上升,其原因是乳酸菌需要一定的时间适应新环境,处于适应阶段,菌体不能快速生长,乳酸生成量低。此变化趋势与李皖光等[23]对蜂蜜酸奶发酵过程中乳酸菌生长情况相似。在2~10 h处于快速上升阶段,10 h之后变化趋于平缓,在18 h测得嗜热链球菌JM108生长量为8.4122 lg(CFU/mL),乳酸含量为1.0549 μmol/mL。发酵过程中葡萄糖含量和pH呈下降趋势,葡萄糖含量在0~2 h变化较为缓慢,与嗜热链球菌JM108生长量和乳酸含量在前2 h的变化趋势相对应。在发酵过程的前2~10 h,葡萄糖含量经历了迅速的下降阶段,随后进入平缓阶段。这是由于嗜热链球菌JM108处于对数生长期,其对于营养物质的需求显著增加,从而加速了葡萄糖的消耗和利用[24]。在18 h葡萄糖含量为0.0167 mg/mL。嗜热链球菌JM108生长量、乳酸含量、葡萄糖含量的变化曲线符合发酵过程中菌体形成、产物形成和底物消耗三者间的对应关系。Li等[25]研究了嗜热链球菌JM905发酵过程中的活菌数,乳酸含量与pH的变化情况,本实验的研究结果显示出了相似的变化趋势。在发酵过程中,葡萄糖是嗜热链球菌JM108生长的主要能量来源。因此,在JM108生长迅速的阶段,葡萄糖的消耗速度相应加快。随着大量消耗和分解M17培养基中的葡萄糖及其他营养物质,乳酸的产量也迅速增加。然而,在发酵的第12~18 h,JM108的生长速度明显放缓,这标志着其生长状态从对数生长期逐渐过渡到稳定期,其消耗葡萄糖的能力和生产乳酸的作用均开始减弱[26],乳酸生成曲线和葡萄糖消耗曲线呈现出变缓的趋势。结果表明嗜热链球菌JM108在发酵过程中乳酸的生成与菌体生长和底物葡萄糖糖消耗相对应。
2.2 嗜热链球菌JM108的生长动力学模型
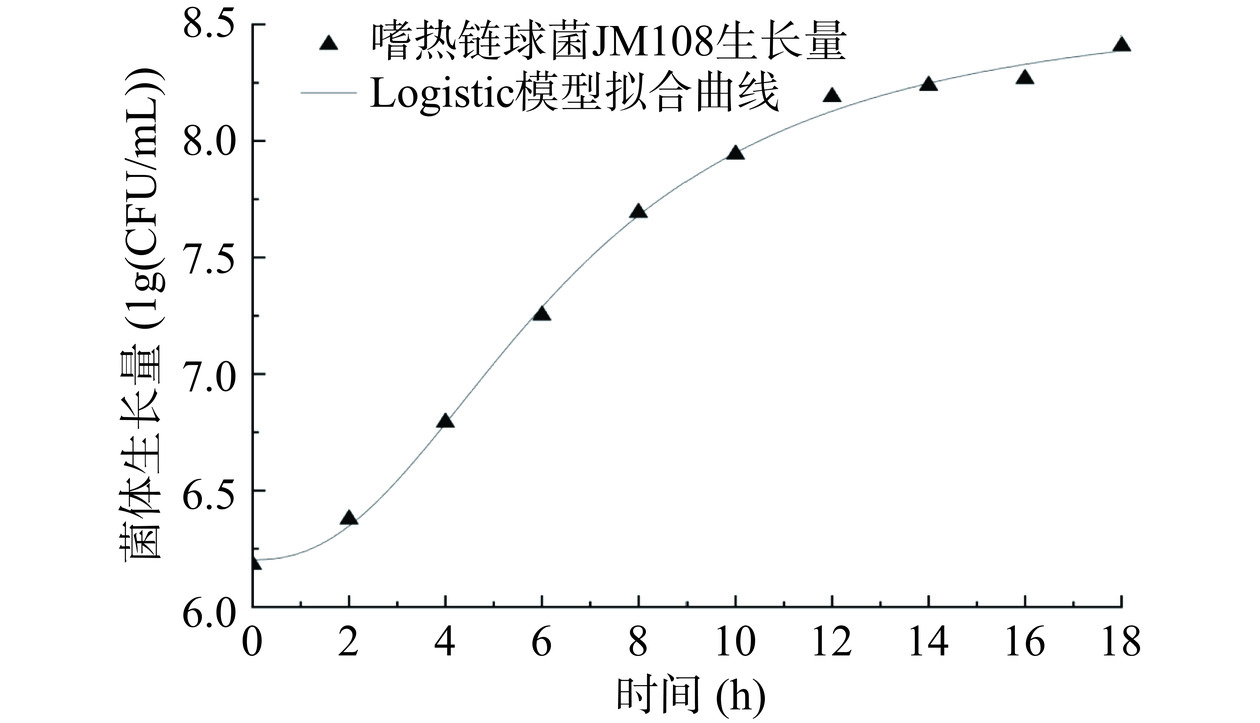
根据图1可以发现,在0~10 h内,M17培养基培养环境中的营养物质充足,乳酸含量相对较低,嗜热链球菌JM108处于生长阶段。然而,10 h后,该培养环境中的嗜热链球菌JM108生长逐渐进入稳定期。这种变化可能是由于营养物质的消耗以及培养环境中乳酸的增加导致pH下降,这些因素共同对微生物的生长产生了抑制作用[27]。图1中嗜热链球菌JM108生长曲线显示出为“S”型曲线的特性,故采用呈现“S”型趋势的Logistic、SGompertz、Boltzmann三种经典模型对嗜热链球菌JM108生长量进行非线性拟合[28]。通常决定系数R2>0.99被认定为拟合良好[29]。表2展示了Logistic、SGompertz、Boltzmann三种模型对嗜热链球菌JM108生长量拟合的决定系数与模型方程,Logistic与Boltzmann的决定系数均大于0.99,为0.9974、0.9970,表明可以对生长量良好拟合。Logistic模型的决定系数更大,故采取Logistic模型对嗜热链球菌JM108生长量进行非线性拟合。郝丽粉等[30]应用Logistic模型对百香果酒中菌体生长量进行了非线性拟合,决定系数R2>0.99,本文的结果与其相似。嗜热链球菌JM108生长量的Logistic模型非线性拟合曲线见图2。如图2拟合曲线所示,嗜热链球菌JM108在发酵过程中生长曲线呈“S”型,在发酵0~2 h,嗜热链球菌JM108生长速率均较慢,处于生长适应期;在2~10 h的时间中处于对数生长期,此时嗜热链球菌JM108生长速率最快,乳酸含量逐渐增加。
表 2 嗜热链球菌JM108生长量的模型方程及其决定系数Table 2. Model equation of Streptococcus thermophilus JM108 growth and its correlation coefficient模型 模型方程 决定系数R2 Logistic y=8.59+−2.391+(x2.32)0.02 0.9974 SGompertz y=8.93×e−e[−0.11×(x+8.83)] 0.9759 Boltzmann y=8.37+−2.701+exp(x−5.452.70) 0.9970 2.3 乳酸生成量动力学模型
乳酸含量的Logistic、SGompertz、Boltzmann非线性拟合模型的决定系数见表3。结果显示决定系数:Logistic>SGompertz>0.99>Boltzmann,表明应用Logistic和SGompertz模型可以较好拟合乳酸生成量动力学模型,其中Logistic的拟合系数最好,为0.9947,故采取Logistic模型对乳酸生成量进行非线性拟合。经验证,此模型可有效展示嗜热链球菌JM108在M17培养基中发酵乳酸的动态变化。基于该模型,可以精准预测发酵过程中任意时间点的乳酸浓度。此外,通过建立pH与乳酸含量的关联,能够通过监测pH来预测乳酸含量,为实际操作提供了便利。嗜热链球菌JM108乳酸生成量Logistic模型非线性拟合曲线见图3。其趋势与曾美端等[31]对丙酸生成动力学的研究结果一致。乳酸含量在0~10 h的时间段中快速积累,原因可能是嗜热链球菌JM108具有在短时间内快速产酸的能力,其在适应期结束后,快速利用培养基中的葡萄糖进行同型发酵产生乳酸,随着乳酸含量的增加,培养环境中的pH开始降低[32]。在10 h后,乳酸含量生成逐渐变缓,进入平稳期,其原因可能是嗜热链球菌JM108快速消耗底物导致底物大量减少和乳酸产生导致环境pH降低,抑制了嗜热链球菌JM108的生长代谢[33]。
表 3 乳酸生成量动力学模型方程及其决定系数Table 3. Kinetic model equation and correlation coefficients of lactate production模型 模型方程 决定系数R2 Logistic y=1.05+−1.061+(x3.67)3.23 0.9947 SGompertz y=1.03×e−e[−0.66×(x−3.14)] 0.9916 Boltzmann y=1.02+−1.091+exp(x−3.601.18) 0.9815 2.4 葡萄糖消耗量动力学模型的建立
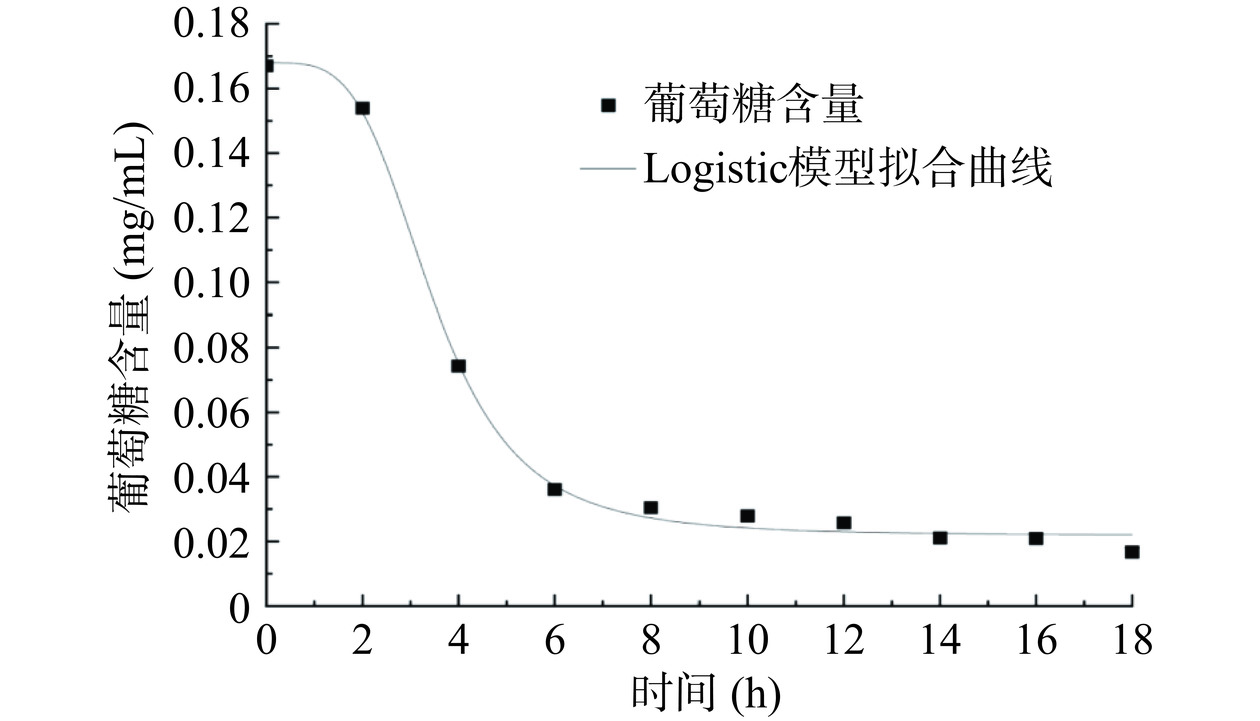
葡萄糖消耗量动力学模型建立的拟合方程、决定系数见表4。对比Logistic、SGompertz、Boltzmann三种模型的决定系数,可知Logistic和Boltzmann模型均可以较好反映嗜热链球菌JM108在发酵过程中葡萄糖含量的变化情况,Logistic的决定系数最大,因此其具有最好的拟合效果。而SGompertz的决定系数仅为0.16,远远小于0.99,结果表明SGompertz几乎不能用来拟合嗜热链球菌JM108的葡萄糖消耗动力学模型。嗜热链球菌JM108葡萄糖消耗量的Logistic模型非线性拟合曲线见图4。经过毛亚玲等[34]的研究,在共接种酒球菌和酿酒酵母的发酵体系下,Logistic模型对还原糖含量变化的拟合效果最佳。本研究结果与毛亚玲等[34]的结论一致。葡萄糖的消耗与乳酸的生成呈对应关系,嗜热链球菌JM108生长代谢过程中,乳酸快速积累,葡萄糖含量快速下降。在0~2 h内,葡萄糖含量的下降速度相对缓慢,此趋势与嗜热链球菌JM108的生长情况曲线相吻合。出现这种情况的原因可能是嗜热链球菌JM108数量较少,导致其利用葡萄糖的能力受到限制[35]。随着嗜热链球菌JM108快速增殖,葡萄糖被急剧消耗。10 h之后葡萄糖含量趋于稳定,与嗜热链球菌JM108的生长动力学模型与乳酸生成量动力学模型有较好的对应关系。
表 4 葡萄糖消耗量动力学模型方程及其决定系数Table 4. Kinetic model equation and correlation coefficients of glucose consumption模型 模型方程 决定系数R2 Logistic y=0.02+0.151+(x3.47)3.90 0.9964 SGompertz y=0.16×e−0 0.16 Boltzmann y=0.02+0.151+exp(x−3.520.81) 0.9913 2.5 模型验证
经过比较决定系数,当R2值大于0.99时,表明模型能够实现对数据的良好拟合。研究结果显示,Logistic与Boltzmann模型在预测生长量方面表现出色;而Logistic和SGompertz模型则对乳酸生成量的预测具有显著优势;Logistic和Boltzmann模型能够精确模拟葡萄糖含量的变化。误差低于15%证明模型可以良好拟合。对表5与表6的数据进行分析后,发现模型的预测值与实验值之间的相对误差均低于15%,证明拟合值与实验值之间的高度一致性[36]。杨清清等[29]使用Logistic模型对鼠李糖乳杆菌发酵过程中的菌体生长量进行拟合,结果显示相对误差<15%,拟合效果良好。其中,Logistic模型在模拟细菌生长动力学、乳酸生成动力学和葡萄糖消耗动力学方面均表现出色。Logistic模型的拟合值与实验值之间的误差更小,进一步验证了模型的良好拟合效果,并证明其能够准确反映实际的发酵过程。
表 5 Logistic模型的拟合值和实验值的比较Table 5. Comparison of fitted and experimental values of Logistic models时间(h) 菌体生长量(lg(CFU/mL)) 乳酸含量(μmol/mL) 葡萄糖含量(mg/mL) 实验值 拟合值 误差(%) 实验值 拟合值 误差(%) 实验值 拟合值 误差(%) 0 6.2819 6.2023 1.28 0.0149 0.0153 2.61 0.1670 0.1679 0.56 2 6.3766 6.3480 0.45 0.1195 0.1225 2.47 0.1539 0.1526 0.86 4 6.7921 6.7876 0.07 0.6270 0.5946 5.45 0.0743 0.0751 1.06 6 7.2524 7.2869 0.47 0.8594 0.8711 1.34 0.0356 0.0373 4.58 8 7.6927 7.6800 0.16 0.9262 0.9716 4.67 0.0285 0.0273 4.47 10 7.9428 7.9495 0.08 1.0069 1.0107 0.37 0.0267 0.0252 5.95 12 8.1891 8.1281 0.75 1.0355 1.0281 0.72 0.0258 0.0246 4.88 14 8.2382 8.2474 0.11 1.0420 1.0368 0.50 0.0211 0.0225 6.14 16 8.2674 8.3291 0.74 1.0597 1.0416 1.74 0.0209 0.0222 5.98 18 8.3122 8.3866 0.89 1.0549 1.0444 1.00 0.0208 0.0221 5.84 表 6 SGompertz和Boltzmann模型的拟合值和实验值的比较Table 6. Comparison of fitted and experimental values of SGompertz and Boltzmann models时间(h) 菌体生长量(lg(CFU/mL)) 乳酸含量(μmol/mL) 葡萄糖含量(mg/mL) 实验值 拟合值 误差(%) 实验值 拟合值 误差(%) 实验值 拟合值 误差(%) 0 6.2819 6.1599 1.98 0.0149 0.0147 0.01 0.1670 0.1688 1.08 2 6.3766 6.4115 0.54 0.1195 0.1240 0.12 0.1539 0.1512 1.82 4 6.7921 6.7890 0.05 0.6270 0.5826 0.63 0.0743 0.0767 3.08 6 7.2524 7.2440 0.12 0.8594 0.8840 0.86 0.0356 0.0340 4.71 8 7.6927 7.6675 0.33 0.9262 0.9891 0.93 0.0285 0.0279 2.16 10 7.9428 7.9770 0.43 1.0069 1.0195 1.01 0.0277 0.0254 2.30 12 8.1891 8.1652 0.29 1.0355 1.0278 1.03 0.0258 0.0243 6.08 14 8.2382 8.2673 0.35 1.0420 1.0301 1.04 0.0211 0.0223 5.41 16 8.2674 8.3193 0.62 1.0597 1.0307 1.06 0.0209 0.0223 6.28 18 8.3122 8.3448 0.39 1.0549 1.0308 1.05 0.0208 0.0223 6.73 注:Boltzmann模型拟合菌体生长量和葡萄糖消耗量,SGompertz模型拟合乳酸生成量。 3. 结论
本文对嗜热链球菌JM108在发酵期间的菌体生长量、葡萄糖消耗量及乳酸生成量的动力学进行了初步的探索,从变化趋势来看,三者相互关联。模型拟合结果表明,Logistic模型对嗜热链球菌JM108生长量、乳酸生成量及葡萄糖消耗的拟合情况较好,决定系数R2>0.99,拟合值与实验值误差均<15%。本研究建立的发酵动力学模型能够客观地描述发酵过程中微生物生长和代谢产物转化的动力学特征,对于了解嗜热链球菌JM108在发酵过程中的变化规律具有重要意义,并有助于预测和优化控制嗜热链球菌JM108发酵生产中的关键指标。通过运用数学模型,可以在特定的发酵时间内,精确预测发酵过程中的菌体数量、乳酸含量、葡萄糖含量及pH。目前,发酵产品中嗜热链球菌已成为研究的热点,对其在发酵过程中的生长动力学参数进行深入探究,可以为嗜热链球菌发酵的工业化生产提供有力的理论支撑和参考依据。未来需要深入研究嗜热链球菌JM108对发酵制品的影响,以提供特色化定向发酵制品、改善生产效率和促进本地菌株产业化应用。
-
表 1 三种经典的发酵动力学模型
Table 1 Three classical models of fermentation kinetics
模型 模型方程 Logistic y=A2+(A1−A2)1+(xx0)p SGompertz y=a×e−e[−k×(x−xc)] Boltzmann y=A2+(A1−A2)1+exp(x−x0dx) 表 2 嗜热链球菌JM108生长量的模型方程及其决定系数
Table 2 Model equation of Streptococcus thermophilus JM108 growth and its correlation coefficient
模型 模型方程 决定系数R2 Logistic y=8.59+−2.391+(x2.32)0.02 0.9974 SGompertz y=8.93×e−e[−0.11×(x+8.83)] 0.9759 Boltzmann y=8.37+−2.701+exp(x−5.452.70) 0.9970 表 3 乳酸生成量动力学模型方程及其决定系数
Table 3 Kinetic model equation and correlation coefficients of lactate production
模型 模型方程 决定系数R2 Logistic y=1.05+−1.061+(x3.67)3.23 0.9947 SGompertz y=1.03×e−e[−0.66×(x−3.14)] 0.9916 Boltzmann y=1.02+−1.091+exp(x−3.601.18) 0.9815 表 4 葡萄糖消耗量动力学模型方程及其决定系数
Table 4 Kinetic model equation and correlation coefficients of glucose consumption
模型 模型方程 决定系数R2 Logistic y=0.02+0.151+(x3.47)3.90 0.9964 SGompertz y=0.16×e−0 0.16 Boltzmann y=0.02+0.151+exp(x−3.520.81) 0.9913 表 5 Logistic模型的拟合值和实验值的比较
Table 5 Comparison of fitted and experimental values of Logistic models
时间(h) 菌体生长量(lg(CFU/mL)) 乳酸含量(μmol/mL) 葡萄糖含量(mg/mL) 实验值 拟合值 误差(%) 实验值 拟合值 误差(%) 实验值 拟合值 误差(%) 0 6.2819 6.2023 1.28 0.0149 0.0153 2.61 0.1670 0.1679 0.56 2 6.3766 6.3480 0.45 0.1195 0.1225 2.47 0.1539 0.1526 0.86 4 6.7921 6.7876 0.07 0.6270 0.5946 5.45 0.0743 0.0751 1.06 6 7.2524 7.2869 0.47 0.8594 0.8711 1.34 0.0356 0.0373 4.58 8 7.6927 7.6800 0.16 0.9262 0.9716 4.67 0.0285 0.0273 4.47 10 7.9428 7.9495 0.08 1.0069 1.0107 0.37 0.0267 0.0252 5.95 12 8.1891 8.1281 0.75 1.0355 1.0281 0.72 0.0258 0.0246 4.88 14 8.2382 8.2474 0.11 1.0420 1.0368 0.50 0.0211 0.0225 6.14 16 8.2674 8.3291 0.74 1.0597 1.0416 1.74 0.0209 0.0222 5.98 18 8.3122 8.3866 0.89 1.0549 1.0444 1.00 0.0208 0.0221 5.84 表 6 SGompertz和Boltzmann模型的拟合值和实验值的比较
Table 6 Comparison of fitted and experimental values of SGompertz and Boltzmann models
时间(h) 菌体生长量(lg(CFU/mL)) 乳酸含量(μmol/mL) 葡萄糖含量(mg/mL) 实验值 拟合值 误差(%) 实验值 拟合值 误差(%) 实验值 拟合值 误差(%) 0 6.2819 6.1599 1.98 0.0149 0.0147 0.01 0.1670 0.1688 1.08 2 6.3766 6.4115 0.54 0.1195 0.1240 0.12 0.1539 0.1512 1.82 4 6.7921 6.7890 0.05 0.6270 0.5826 0.63 0.0743 0.0767 3.08 6 7.2524 7.2440 0.12 0.8594 0.8840 0.86 0.0356 0.0340 4.71 8 7.6927 7.6675 0.33 0.9262 0.9891 0.93 0.0285 0.0279 2.16 10 7.9428 7.9770 0.43 1.0069 1.0195 1.01 0.0277 0.0254 2.30 12 8.1891 8.1652 0.29 1.0355 1.0278 1.03 0.0258 0.0243 6.08 14 8.2382 8.2673 0.35 1.0420 1.0301 1.04 0.0211 0.0223 5.41 16 8.2674 8.3193 0.62 1.0597 1.0307 1.06 0.0209 0.0223 6.28 18 8.3122 8.3448 0.39 1.0549 1.0308 1.05 0.0208 0.0223 6.73 注:Boltzmann模型拟合菌体生长量和葡萄糖消耗量,SGompertz模型拟合乳酸生成量。 -
[1] MARKAKIOU S, GASPAR P, JOHANSEN E, et al. Harnessing the metabolic potential of Streptococcus thermophilus for new biotechnological applications[J]. Current Opinion in Biotechnology,2020,61:142−152. doi: 10.1016/j.copbio.2019.12.019
[2] TIAN H, LIANG H, HUO G, et al. Research progress on the property and application of Streptococcus thermophilus[J]. Biotechnology Bulletin,2015,31(9):38.
[3] HAN M, WU Y, GUO X, et al. Milk fermentation by monocultures or co-cultures of Streptococcus thermophilus strains[J]. Frontiers in Bioengineering and Biotechnology,2022,10:1097013. doi: 10.3389/fbioe.2022.1097013
[4] CUI Y, JIANG X, HAO M, et al. New advances in exopolysaccharides production of Streptococcus thermophilus[J]. Archives of Microbiology,2017,199:799−809. doi: 10.1007/s00203-017-1366-1
[5] URSHEV Z, NINOVA-NIKOLOVA N, ISHLIMOVA D, et al. Selection and characterization of naturally occurring high acidification rate Streptococcus thermophilus strains[J]. Biotechnology & Biotechnological Equipment,2014,28(5):899−903.
[6] MARCO M L, HEENEY D, BINDA S, et al. Health benefits of fermented foods:Microbiota and beyond[J]. Current Opinion in Biotechnology,2017,44:94−102. doi: 10.1016/j.copbio.2016.11.010
[7] 赵洁. 自然发酵乳中嗜热链球菌群体遗传学和功能基因组学研究[D]. 呼和浩特:内蒙古农业大学, 2018. [ZHAO Jie. Population genetics and functional genomics of Streptococcus thermophilus in naturally fermented milk[D]. Hohhot:Inner Mongolia Agricultural University, 2018.] ZHAO Jie. Population genetics and functional genomics of Streptococcus thermophilus in naturally fermented milk[D]. Hohhot: Inner Mongolia Agricultural University, 2018.
[8] 靳汝霖. 具有优良发酵特性嗜热链球菌的筛选及其发酵乳中关键性风味物质的研究[D]. 呼和浩特:内蒙古农业大学, 2018. [JIN Rulin. Screening of Streptococcus thermophilus with excellent fermentation characteristics and study of key flavor substances in fermented milk[D]. Hohhot:Inner Mongolia Agricultural University, 2018.] JIN Rulin. Screening of Streptococcus thermophilus with excellent fermentation characteristics and study of key flavor substances in fermented milk[D]. Hohhot: Inner Mongolia Agricultural University, 2018.
[9] WASILEWSKA E, ZLOTKOWSKA D, WROBLEWSKA B. Yogurt starter cultures of Streptococcus thermophilus and Lactobacillus bulgaricus ameliorate symptoms and modulate the immune response in a mouse model of dextran sulfate sodium-induced colitis[J]. Journal of Dairy Science,2019,102(1):37−53. doi: 10.3168/jds.2018-14520
[10] HAN M, LIAO W Y, WU S M, et al. Use of Streptococcus thermophilus for the in situ production of γ-aminobutyric acid-enriched fermented milk[J]. Journal of Dairy Science,2020,103(1):98−105. doi: 10.3168/jds.2019-16856
[11] LI J, ZHAO W T, PAN X, et al. Improvement of antioxidant properties of jujube puree by biotransformation of polyphenols via Streptococcus thermophilus fermentation[J]. Food Chemistry X,2022,13:100214. doi: 10.1016/j.fochx.2022.100214
[12] HUANG Y Y, LU Y H, LIU X T, et al. Metabolic properties, functional characteristics, and practical application of Streptococcus thermophilus[J]. Food Reviews International,2023,40(2):792−813.
[13] 后立琼. 苗族酸汤中乳酸菌的分离鉴定及发酵动力学模型研究[D]. 成都:四川农业大学, 2012. [HOU Liqiong. Isolation and identification of lactic acid bacteria in Miao sour soup and study on fermentation kinetic model[D]. Chengdu:Sichuan Agricultural University, 2012.] HOU Liqiong. Isolation and identification of lactic acid bacteria in Miao sour soup and study on fermentation kinetic model[D]. Chengdu: Sichuan Agricultural University, 2012.
[14] 欧红艳, 赵良忠, 刘婷, 等. 豆清饮料发酵及贮藏过程中品质变化及动力学研究[J]. 大豆科学,2021,40(4):528−538. [OU Hongyan, ZHAO Liangzhong, LIU Ting, et al. Study on quality change and kinetics of bean clear beverage during fermentation and storage[J]. Soybean Science,2021,40(4):528−538.] OU Hongyan, ZHAO Liangzhong, LIU Ting, et al. Study on quality change and kinetics of bean clear beverage during fermentation and storage[J]. Soybean Science, 2021, 40(4): 528−538.
[15] 王园园, 王康, 金令凯, 等. 阿维拉霉素发酵工艺优化及动力学模型建立[J]. 高校化学工程学报,2019,33(5):1156−63. [WANG Yuanyuan, WANG Kang, JIN Lingkai, et al. Optimization of avilramycin fermentation process and establishment of kinetic model[J]. Journal of Chemical Engineering of Chinese Universities,2019,33(5):1156−63.] doi: 10.3969/j.issn.1003-9015.2019.05.017 WANG Yuanyuan, WANG Kang, JIN Lingkai, et al. Optimization of avilramycin fermentation process and establishment of kinetic model[J]. Journal of Chemical Engineering of Chinese Universities, 2019, 33(5): 1156−63. doi: 10.3969/j.issn.1003-9015.2019.05.017
[16] 熊亚, 李敏杰, 姜少娟. 芒果酒发酵动力学模型及抗氧化性研究[J]. 食品工业科技,2019,40(19):7−12. [XIONG Ya, LI Minjie, JIANG Shaojuan. Research on fermentation kinetics model and antioxidant activity of mango wine[J]. Science and Technology of Food Industry,2019,40(19):7−12.] XIONG Ya, LI Minjie, JIANG Shaojuan. Research on fermentation kinetics model and antioxidant activity of mango wine[J]. Science and Technology of Food Industry, 2019, 40(19): 7−12.
[17] 李雪, 白新鹏, 曹君, 等. 仙人掌果酒发酵动力学及其抗氧化性[J]. 食品科学,2017,38(4):87−92. [LI Xue, BAI Xinpeng, CAO Jun, et al. Fermentation kinetics and antioxidant activity of cactus wine[J]. Food Science,2017,38(4):87−92.] doi: 10.7506/spkx1002-6630-201704015 LI Xue, BAI Xinpeng, CAO Jun, et al. Fermentation kinetics and antioxidant activity of cactus wine[J]. Food Science, 2017, 38(4): 87−92. doi: 10.7506/spkx1002-6630-201704015
[18] 李静雯, 张东亚, 陆洋, 等. 低度米酒发酵工艺优化及发酵动力学模型建立[J]. 酿酒科技,2021(8):58−64. [LI Jingwen, ZHANG Dongya, LU Yang, et al. Optimization of fermentation process and establishment of fermentation kinetic model of low-grade rice wine[J]. Brewing Science and Technology,2021(8):58−64.] LI Jingwen, ZHANG Dongya, LU Yang, et al. Optimization of fermentation process and establishment of fermentation kinetic model of low-grade rice wine[J]. Brewing Science and Technology, 2021(8): 58−64.
[19] 吕明珊, 袁艺洋, 邢军, 等. 新疆药桑葚酵素发酵动力学模型及其发酵比速率的研究[J]. 中国调味品,2022,47(3):39−43. [LÜ Mingshan, YUAN Yiyang, XING Jun, et al. Study on fermentation kinetics model and fermentation specific rate of morula in Xinjiang[J]. Chinese Condiments,2022,47(3):39−43.] doi: 10.3969/j.issn.1000-9973.2022.03.007 LÜ Mingshan, YUAN Yiyang, XING Jun, et al. Study on fermentation kinetics model and fermentation specific rate of morula in Xinjiang[J]. Chinese Condiments, 2022, 47(3): 39−43. doi: 10.3969/j.issn.1000-9973.2022.03.007
[20] KORCZ E, VARGA L, KERÉNYI Z. Relationship between total cell counts and exopolysaccharide production of Streptococcus thermophilus T9 in reconstituted skim milk[J]. LWT,2021,148:111775. doi: 10.1016/j.lwt.2021.111775
[21] VESELÁ K, KUMHEROVÁ M, KLOJDOVÁ I, et al. Selective culture medium for the enumeration of Lactobacillus plantarum in the presence of Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus[J]. LWT,2019,114:108365. doi: 10.1016/j.lwt.2019.108365
[22] 张阳阳, 靳羽慧, 王荣荣, 等. 蓝莓猕猴桃酒发酵动力学模型的研究[J]. 食品科技,2023,48(7):79−84. [ZHANG Yangyang, JIN Yuhui, WANG Rongrong, et al. Blueberry dynamic model of the fermentation of kiwi wine[J]. Journal of Food Science and Technology,2023,48(7):79−84.] ZHANG Yangyang, JIN Yuhui, WANG Rongrong, et al. Blueberry dynamic model of the fermentation of kiwi wine[J]. Journal of Food Science and Technology, 2023, 48(7): 79−84.
[23] 李皖光, 王新文, 马艳, 等. 蜂蜜酸奶发酵过程中乳酸菌生长动力学的初步研究[J]. 食品与发酵科技,2018,54(2):4−7. [LI Wanguang, WANG Xinwen, MA Yan, et al. Preliminary study on the growth kinetics of lactic acid bacteria during the fermentation of honey yogurt[J]. Food and Fermentation Science and Technology,2018,54(2):4−7.] LI Wanguang, WANG Xinwen, MA Yan, et al. Preliminary study on the growth kinetics of lactic acid bacteria during the fermentation of honey yogurt[J]. Food and Fermentation Science and Technology, 2018, 54(2): 4−7.
[24] 唐凯伟, 黄晓英, 易宇文, 等. 保加利亚乳杆菌和嗜热链球菌单菌发酵与复配发酵对酸奶品质的影响[J]. 食品工业科技,2022,43(23):127−132. [TANG Kaiwei, HUANG Xiaoying, YI Yuwen, et al. Effects of single and combined fermentation of Lactobacillus bulgaricus and Streptococcus thermophilus on yoghurt quality[J]. Science and Technology of Food Industry,2022,43(23):127−132.] TANG Kaiwei, HUANG Xiaoying, YI Yuwen, et al. Effects of single and combined fermentation of Lactobacillus bulgaricus and Streptococcus thermophilus on yoghurt quality[J]. Science and Technology of Food Industry, 2022, 43(23): 127−132.
[25] LI Y, WANG Y, LI B, et al. Streptococcus thermophilus JM905—strain carbon source utilization and its fermented milk metabolic profile at different fermentation stages[J]. Foods,2023,12(19):3690. doi: 10.3390/foods12193690
[26] LI K, DUAN Z, ZHANG J, et al. Growth kinetics, metabolomics changes, and antioxidant activity of probiotics in fermented highland barley-based yogurt[J]. LWT,2023,173:114239. doi: 10.1016/j.lwt.2022.114239
[27] LI D, PENG J, KWOK L, et al. Metabolomic analysis of Streptococcus thermophilus S10-fermented milk[J]. LWT,2022,161:113368. doi: 10.1016/j.lwt.2022.113368
[28] 李敏杰, 熊亚. 嘉宝果果酒发酵动力学模型的建立[J]. 食品科技,2022,47(9):83−87. [LI Minjie, XIONG Ya. Gerber blended wine fermentation kinetics model[J]. Journal of Food Science and Technology,2022,47(9):83−87.] LI Minjie, XIONG Ya. Gerber blended wine fermentation kinetics model[J]. Journal of Food Science and Technology, 2022, 47(9): 83−87.
[29] 杨清清, 雷霜江, 包善思, 等. 鼠李糖乳杆菌产胞外多糖发酵条件优化及发酵动力学模型构建[J]. 中国酿造,2023,42(5):206−211. [YANG Qingqing, LEI Shuangjiang, BAO Shansi, et al. Optimization of fermentation conditions and fermentation kinetic model of exopolysaccharides produced by Lactobacillus rhamnosus[J]. China Brewing,2023,42(5):206−211.] YANG Qingqing, LEI Shuangjiang, BAO Shansi, et al. Optimization of fermentation conditions and fermentation kinetic model of exopolysaccharides produced by Lactobacillus rhamnosus[J]. China Brewing, 2023, 42(5): 206−211.
[30] 郝丽粉, 叶晓芳, 张静进, 等. 百香果酒发酵动力学及抗氧化活性研究[J]. 中国酿造,2023,42(12):219−225. [HAO Lifen, YE Xiaofang, ZHANG Jingjin, et al. Study on fermentation kinetics and antioxidant activity of passion fruit wine[J]. China Brewing,2023,42(12):219−225.] HAO Lifen, YE Xiaofang, ZHANG Jingjin, et al. Study on fermentation kinetics and antioxidant activity of passion fruit wine[J]. China Brewing, 2023, 42(12): 219−225.
[31] 曾美端, 洪艺萍, 蔡玉凤, 等. 产酸丙酸杆菌FS1171产丙酸发酵动力学研究[J]. 微生物学杂志,2022,42(6):32−38. [ZENG Meiduan, HONG Yiping, CAI Yufeng, et al. Kinetics of propionic acid-producing fermentation of Propionibacterium acid-producing FS1171[J]. Chinese Journal of Microbiology,2022,42(6):32−38.] ZENG Meiduan, HONG Yiping, CAI Yufeng, et al. Kinetics of propionic acid-producing fermentation of Propionibacterium acid-producing FS1171[J]. Chinese Journal of Microbiology, 2022, 42(6): 32−38.
[32] 刘清霞, 林伟锋, 陈中. 嗜热链球菌在脱脂乳中发酵特性的研究[J]. 食品工业科技,2017,38(3):122−126. [LIU Qingxia, LIN Weifeng, CHEN Zhong. Study on the fermentation characteristics of Streptococcus thermophilus in buttermilk[J]. Science and Technology of Food Industry,2017,38(3):122−126.] LIU Qingxia, LIN Weifeng, CHEN Zhong. Study on the fermentation characteristics of Streptococcus thermophilus in buttermilk[J]. Science and Technology of Food Industry, 2017, 38(3): 122−126.
[33] TARRAH A, NOAL V, GIARETTA S, et al. Effect of different initial pH on the growth of Streptococcus macedonicus and Streptococcus thermophilus strains[J]. International Dairy Journal,2018,86:65−68. doi: 10.1016/j.idairyj.2018.07.003
[34] 毛亚玲, 李俊娥, 于静, 等. 酒酒球菌和酿酒酵母共接种发酵动力学模型建立[J]. 食品科学,2023,44(2):156−164. [MAO Yaling, LI June, YU Jing, et al. Establishment of co-inoculation fermentation kinetic model of Oenococcus dinococcus and Saccharomyces cerevisiae[J]. Food Science,2023,44(2):156−164.] MAO Yaling, LI June, YU Jing, et al. Establishment of co-inoculation fermentation kinetic model of Oenococcus dinococcus and Saccharomyces cerevisiae[J]. Food Science, 2023, 44(2): 156−164.
[35] SONG X, HOU C, YANG Y, et al. Effects of different carbon sources on metabolic profiles of carbohydrates in Streptococcus thermophilus during fermentation[J]. Journal of the Science of Food and Agriculture,2022,102(11):4820−4829. doi: 10.1002/jsfa.11845
[36] 姚妞妞, 常春晖, 于宏伟, 等. 适合奶醋发酵的酵母菌发酵动力学[J]. 食品与发酵工业,2020,46(22):106−112. [YAO Niuniu, CHANG Chunhui, YU Hongwei, et al. Fermentation kinetics of yeast suitable for milk vinegar fermentation[J]. Food and Fermentation Industry,2020,46(22):106−112.] YAO Niuniu, CHANG Chunhui, YU Hongwei, et al. Fermentation kinetics of yeast suitable for milk vinegar fermentation[J]. Food and Fermentation Industry, 2020, 46(22): 106−112.





 下载:
下载:

 下载:
下载:
